Aurora Spine Receives IRB Approval To Commence Multicenter Study for its DEXA-C™ Cervical Interbody System
March 02 2023 - 7:15AM

Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV:
ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative
medical devices that improve spinal surgery outcomes, today
announced it has received Institutional Review Board (IRB) approval
for its new multicenter study of its DEXA-C Cervical Interbody
System, which is indicated for anterior cervical interbody fusion
procedures in skeletally mature patients with degenerative disc
disease (DDD) of the cervical spine with accompanying radicular
symptoms at one or two contiguous levels from C2-T1.
The DEXA-C system is the first product on the market using
Aurora’s patented DEXA technology platform, which creates a series
of implants that are color-coded and manufactured with varying
densities in order to match with a patient’s bone density and DEXA
T-Score. DEXA-C is intended for use on patients who require
anterior cervical discectomy and fusion surgery. The system
implants an interbody spacer(s) into the cervical intervertebral
body space(s) to stabilize and fuse the level(s). Allograft will be
used in the spacer and the spinal segment(s) are fixed with an
anterior cervical plate.
The DEXA-C study will be conducted at up to 10 investigative
sites in the United States and it is anticipated that data from at
least 40 single level subjects and at least 40 multiple level
subjects will be entered into the study.
Mr. Trent Northcutt, Aurora’s President, CEO and co-founder,
stated, "Aurora has been in the beta-testing phase with our DEXA-C
series of implants and initial response has been very positive. As
we build out our DEXA franchise, the clinical data around DEXA-C
usage will help us advance this product and future DEXA products.
We appreciate the doctors and patients participating in this study
and look forward to receiving and reviewing their outcomes in the
future.”
Dr. Nitin N. Bhatia, Chairman, Orthopaedic Surgery, Professor of
Orthopaedic Surgery and Neurosurgery, Chief, Spine Service at
University of California, Irvine, stated, “Cervical interbody
implants matched to a patient's underlying bone density may help
solve some of the challenges in anterior cervical surgery including
subsidence and nonunion. Our surgeons at the UC Irvine Spine Center
are excited to be part of this study.”
Dr. Steven Falowski, a Functional Neurosurgeon in Lancaster, PA,
commented, “The Dexa-C technology marks a milestone in the
development of spinal fusion products to not only help ensure, but
also further improve great patient outcomes. A patient’s bone
density and quality is a very well known factor in fusion results
and patient outcomes. Aurora has also shown its dedication to not
only bringing these innovative landmark products to market, but
also ensuring the production of strong clinical data to support
their use."
Sebastian Koga, MD, FAANS, a neurosurgeon with Forrest Health
Institute of Neurosciece, and Director of Koga Neurosurgery in
Covington, Louisiana, said, “DEXA is a huge step in biomaterials
innovation, and the first step in personalized spine medicine. Like
all great inventions it is an elegant and simple concept. Aurora
Spine has demonstrated that artificial implants can match and mimic
human biology. I have used DEXA implants in nearly 200 cases
already and I cannot imagine going back to a conventional cage. Any
spine surgeon operating on patients with osteoporosis will
understand the great advantage of this new material. I can also
envision this new material expanding from spinal procedures to
other bone implants and dental implants, and further advancing to
replace most orthopedic implants.”
The primary outcomes of interest for this study will be fusion
assessment with patient follow-up visits at 3 months, 6 months and
12 months post-surgery. Included in the data collection will be
fusion assessment from Static and Dynamic X-Ray (AP/LAT/Flex/Ex)
using the following criteria: bridging bone inside or outside of
graft; no lucencies at the graft-vertebral body junction; and
motion < 1mm.
The secondary outcome measures will include subsidence and
alignment assessments. Patient reported outcomes (NDI and VAS) will
be collected at follow up visits and assessed compared to
baseline.
About Aurora Spine
Aurora Spine is focused on bringing new solutions to the spinal
implant market through a series of innovative, minimally invasive,
regenerative spinal implant technologies. Additional information
can be accessed at www.aurora-spine.com or
www.aurorapaincare.com.
Neither TSX Venture Exchange nor its Regulation Services
Provider (as that term is defined in the policies of the TSX
Venture Exchange) accepts responsibility for the adequacy or
accuracy of this release.
Forward-Looking Statements
This news release contains forward-looking information that
involves substantial known and unknown risks and uncertainties,
most of which are beyond the control of Aurora Spine, including,
without limitation, those listed under "Risk Factors" and
"Cautionary Statement Regarding Forward-Looking Information" in
Aurora Spine's final prospectus (collectively, "forward-looking
information"). Forward-looking information in this news release
includes information concerning the proposed use and success of the
company’s products in surgical procedures. Aurora Spine cautions
investors of Aurora Spine's securities about important factors that
could cause Aurora Spine's actual results to differ materially from
those projected in any forward-looking statements included in this
news release. Any statements that express, or involve discussions
as to, expectations, beliefs, plans, objectives, assumptions or
future events or performance are not historical facts and may be
forward-looking and may involve estimates, assumptions and
uncertainties which could cause actual results or outcomes to
differ unilaterally from those expressed in such forward-looking
statements. No assurance can be given that the expectations set out
herein will prove to be correct and, accordingly, prospective
investors should not place undue reliance on these forward-looking
statements. These statements speak only as of the date of this
press release and Aurora Spine does not assume any obligation to
update or revise them to reflect new events or circumstances.
Contact:
Aurora Spine Corporation
Trent Northcutt
President and Chief Executive Officer
(760) 424-2004
Chad Clouse
Chief Financial Officer
(760) 424-2004
www.aurora-spine.com
Adam Lowensteiner
LYTHAM PARTNERS, LLC
Phoenix | New York
Telephone: 646-829-9700
asapf@lythampartners.com
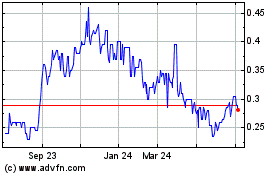
Aurora Spine (TSXV:ASG)
Historical Stock Chart
From Oct 2024 to Nov 2024
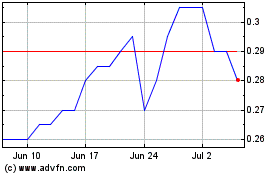
Aurora Spine (TSXV:ASG)
Historical Stock Chart
From Nov 2023 to Nov 2024