Pathway Development Consortium Announces Publication in Human Gene Therapy on the Application of FDA’s Accelerated Approval Pathway for AAV Gene Therapies for Patients with Duchenne Muscular Dystrophy
January 26 2023 - 8:00AM

The Pathway Development Consortium (PDC), a public-private
collaboration founded by REGENXBIO Inc. (Nasdaq: RGNX) and Solid
Biosciences Inc. (Nasdaq: SLDB), today announced the publication of
a peer-reviewed manuscript, Micro-dystrophin expression as a
surrogate endpoint for Duchenne muscular dystrophy clinical trials,
in Human Gene Therapy.
This publication proposes microdystrophin expression levels as a
surrogate endpoint reasonably likely to predict clinical benefit.
The use of surrogate endpoints reasonably likely to predict
clinical benefit could expedite access to therapies for serious
diseases that have demonstrated a meaningful advantage over
available therapy. Improvements in endpoints that are reasonably
likely to provide patients clinical benefit allows patients access
while studies are ongoing to verify and describe the predicted
clinical benefit to patients under the U.S. Food and Drug
Administration (FDA) accelerated approval pathway.
An extended version of the manuscript is available as a white
paper on the PDC website and has been submitted to the FDA. This
white paper clarifies the rationale for use of the accelerated
approval pathway to advance AAV gene therapy development for
patients with Duchenne muscular dystrophy and provides support for
two surrogate endpoints reasonably likely to predict clinical
benefit—muscle fat fraction (FF) obtained by magnetic resonance
(MR) methods and microdystrophin expression levels.
“Multistakeholder collaborative efforts that bring together
expertise from all backgrounds are critical to bringing new
therapeutic options to people with Duchenne,” said Pat Furlong,
Founding President and CEO of Parent Project Muscular Dystrophy
(PPMD). “The PDC’s white paper on the use of the accelerated
approval pathway for AAV gene therapies complements our recent work
to update the Community-led Guidance for Dystrophinopathies to
advance the development of potential therapies.”
This white paper expands on the PDC’s draft framework that
outlined an approach for the use of FDA’s accelerated approval
pathway for different categories of AAV gene therapies that target
the underlying monogenic changes that cause disease.
“The manuscript and white paper are important steps in providing
the scientific rationale that enables use of the accelerated
approval pathway to get new treatment options to patients with
unmet medical needs,” said Jeff Chamberlain, Ph.D., Professor in
the Departments of Neurology, Medicine, and Biochemistry, the McCaw
Endowed Chair in Muscular Dystrophy at the University of Washington
School of Medicine, and Director of the Senator Paul D. Wellstone
Muscular Dystrophy Cooperative Research Center of Seattle. “The
rare disease community needs to work collaboratively to fill
treatment gaps and starting with the science is key to development
of novel therapies,” said Dongsheng Duan, Ph.D., Curators’
Professor and Margaret Proctor Mulligan Professor in Medical
Research at the University of Missouri’s School of Medicine with a
joint appointment in biomedical sciences at the College of
Veterinary Medicine.
About Pathway Development Consortium The
Pathway Development Consortium (PDC) aims to guide the recent
decades of AAV gene therapy research into a future of innovative,
potentially life-saving therapies. The PDC’s goal is to foster
collaboration and partnership among patients, industry, regulators,
academia, payers and other stakeholders. For this reason, REGENXBIO
and Solid Biosciences joined together to launch the PDC with the
vision to construct an ideal pathway to ensure that all born with
serious genetic conditions can find their way to effective AAV gene
therapies. To learn more, visit
https://www.pathwaydevelopmentconsortium.org/.
Pathway Development Consortium Contact:Annie
Ganot, VP, Patient Advocacy, Solid Biosciences Inc.Nina Hunter,
PhD, VP, Corporate Strategy, REGENXBIO
Inc.info@pathwaydevelopmentconsortium.org(202) 503-9060
Media Contact:Tim Palmer, Senior Manager,
Corporate Communications, Solid Biosciences Inc.Dana Cormack,
Director, Corporate Communications, REGENXBIO
Inc.media@pathwaydevelopmentconsortium.org
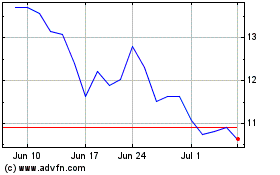
REGENXBIO (NASDAQ:RGNX)
Historical Stock Chart
From Jun 2024 to Jul 2024
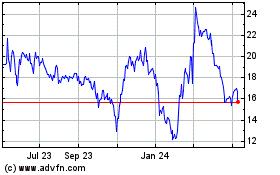
REGENXBIO (NASDAQ:RGNX)
Historical Stock Chart
From Jul 2023 to Jul 2024