false
0001849380
0
0
0001849380
2023-01-01
2023-09-30
0001849380
dei:BusinessContactMember
2023-01-01
2023-09-30
0001849380
2023-09-30
0001849380
2022-12-31
0001849380
us-gaap:RelatedPartyMember
2023-09-30
0001849380
us-gaap:RelatedPartyMember
2022-12-31
0001849380
us-gaap:CommonClassAMember
2023-09-30
0001849380
us-gaap:CommonClassAMember
2022-12-31
0001849380
us-gaap:CommonClassBMember
2023-09-30
0001849380
us-gaap:CommonClassBMember
2022-12-31
0001849380
2023-07-01
2023-09-30
0001849380
2022-07-01
2022-09-30
0001849380
2022-01-01
2022-09-30
0001849380
ONMD:CommonClassASubjectToRedemptionMember
2023-07-01
2023-09-30
0001849380
ONMD:CommonClassASubjectToRedemptionMember
2022-07-01
2022-09-30
0001849380
ONMD:CommonClassASubjectToRedemptionMember
2023-01-01
2023-09-30
0001849380
ONMD:CommonClassASubjectToRedemptionMember
2022-01-01
2022-09-30
0001849380
ONMD:ClassAAndClassBCommonStockNotSubjectToRedemptionMember
2023-07-01
2023-09-30
0001849380
ONMD:ClassAAndClassBCommonStockNotSubjectToRedemptionMember
2022-07-01
2022-09-30
0001849380
ONMD:ClassAAndClassBCommonStockNotSubjectToRedemptionMember
2023-01-01
2023-09-30
0001849380
ONMD:ClassAAndClassBCommonStockNotSubjectToRedemptionMember
2022-01-01
2022-09-30
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-12-31
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-12-31
0001849380
us-gaap:AdditionalPaidInCapitalMember
2022-12-31
0001849380
us-gaap:RetainedEarningsMember
2022-12-31
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-03-31
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-03-31
0001849380
us-gaap:AdditionalPaidInCapitalMember
2023-03-31
0001849380
us-gaap:RetainedEarningsMember
2023-03-31
0001849380
2023-03-31
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-06-30
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-06-30
0001849380
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001849380
us-gaap:RetainedEarningsMember
2023-06-30
0001849380
2023-06-30
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2021-12-31
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2021-12-31
0001849380
us-gaap:AdditionalPaidInCapitalMember
2021-12-31
0001849380
us-gaap:RetainedEarningsMember
2021-12-31
0001849380
2021-12-31
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-06-30
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-06-30
0001849380
us-gaap:AdditionalPaidInCapitalMember
2022-06-30
0001849380
us-gaap:RetainedEarningsMember
2022-06-30
0001849380
2022-06-30
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-01-01
2023-03-31
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-01-01
2023-03-31
0001849380
us-gaap:AdditionalPaidInCapitalMember
2023-01-01
2023-03-31
0001849380
us-gaap:RetainedEarningsMember
2023-01-01
2023-03-31
0001849380
2023-01-01
2023-03-31
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-04-01
2023-06-30
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-04-01
2023-06-30
0001849380
us-gaap:AdditionalPaidInCapitalMember
2023-04-01
2023-06-30
0001849380
us-gaap:RetainedEarningsMember
2023-04-01
2023-06-30
0001849380
2023-04-01
2023-06-30
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-07-01
2023-09-30
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-07-01
2023-09-30
0001849380
us-gaap:AdditionalPaidInCapitalMember
2023-07-01
2023-09-30
0001849380
us-gaap:RetainedEarningsMember
2023-07-01
2023-09-30
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-01-01
2022-06-30
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-01-01
2022-06-30
0001849380
us-gaap:AdditionalPaidInCapitalMember
2022-01-01
2022-06-30
0001849380
us-gaap:RetainedEarningsMember
2022-01-01
2022-06-30
0001849380
2022-01-01
2022-06-30
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-07-01
2022-09-30
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-07-01
2022-09-30
0001849380
us-gaap:AdditionalPaidInCapitalMember
2022-07-01
2022-09-30
0001849380
us-gaap:RetainedEarningsMember
2022-07-01
2022-09-30
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2023-09-30
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2023-09-30
0001849380
us-gaap:AdditionalPaidInCapitalMember
2023-09-30
0001849380
us-gaap:RetainedEarningsMember
2023-09-30
0001849380
us-gaap:CommonClassAMember
us-gaap:CommonStockMember
2022-09-30
0001849380
us-gaap:CommonClassBMember
us-gaap:CommonStockMember
2022-09-30
0001849380
us-gaap:AdditionalPaidInCapitalMember
2022-09-30
0001849380
us-gaap:RetainedEarningsMember
2022-09-30
0001849380
2022-09-30
0001849380
2021-02-08
2021-02-08
0001849380
us-gaap:IPOMember
2021-05-11
2021-05-11
0001849380
us-gaap:PrivatePlacementMember
2021-05-11
2021-05-11
0001849380
us-gaap:PrivatePlacementMember
2021-05-11
0001849380
2021-05-11
2021-05-11
0001849380
2021-05-11
0001849380
us-gaap:IPOMember
2023-09-30
0001849380
us-gaap:IPOMember
2023-01-01
2023-09-30
0001849380
ONMD:DataKnightsLLCMember
2023-09-30
0001849380
2022-11-11
0001849380
2022-11-11
2022-11-11
0001849380
2023-08-11
2023-08-11
0001849380
2023-08-11
0001849380
us-gaap:OverAllotmentOptionMember
2023-09-30
0001849380
2022-01-01
2022-12-31
0001849380
ONMD:ClassCommonStockNotSubjectToPossibleRedemptionMember
2023-09-30
0001849380
ONMD:CommonClassSubjectToRedemptionMember
2023-09-30
0001849380
ONMD:CommonClassASubjectToRedemptionMember
2022-12-31
0001849380
ONMD:CommonClassASubjectToRedemptionMember
2021-12-31
0001849380
ONMD:CommonClassASubjectToRedemptionMember
2023-09-30
0001849380
ONMD:CommonClassASubjectToRedemptionMember
2022-09-30
0001849380
us-gaap:IPOMember
2021-05-11
0001849380
us-gaap:IPOMember
ONMD:PublicWarrantsMember
2021-05-11
2021-05-11
0001849380
ONMD:PublicWarrantsMember
us-gaap:IPOMember
2021-05-11
0001849380
us-gaap:PrivatePlacementMember
2023-09-30
0001849380
us-gaap:PrivatePlacementMember
2023-01-01
2023-09-30
0001849380
ONMD:ArcGroupLimitedMember
us-gaap:RelatedPartyMember
ONMD:IntroducingAdvisorAgreementWithArcGroupLimitedMember
2021-06-26
2021-06-26
0001849380
ONMD:ArcGroupLimitedMember
us-gaap:RelatedPartyMember
ONMD:IntroducingAdvisorAgreementWithArcGroupLimitedMember
2022-03-22
2022-03-22
0001849380
ONMD:ArcGroupLimitedMember
us-gaap:RelatedPartyMember
ONMD:SecondAmendmentToIntroducingAdvisorAgreementWithArcGroupLimitedMember
us-gaap:CommonClassBMember
2022-01-01
2022-12-31
0001849380
srt:ChiefExecutiveOfficerMember
ONMD:FounderSharesMember
us-gaap:CommonClassBMember
2021-02-25
2021-02-25
0001849380
srt:ChiefFinancialOfficerMember
ONMD:FounderSharesMember
us-gaap:CommonClassBMember
2021-02-25
2021-02-25
0001849380
ONMD:TwoIndependentDirectorMember
ONMD:FounderSharesMember
us-gaap:CommonClassBMember
2021-02-25
2021-02-25
0001849380
ONMD:ThirdIndependentDirectorMember
ONMD:FounderSharesMember
us-gaap:CommonClassBMember
2021-03-23
2021-03-23
0001849380
ONMD:ArcGroupLimitedMember
ONMD:FounderSharesMember
us-gaap:RelatedPartyMember
us-gaap:CommonClassBMember
2022-01-01
2022-12-31
0001849380
ONMD:ArcGroupLimitedMember
ONMD:FounderSharesMember
us-gaap:RelatedPartyMember
us-gaap:CommonClassBMember
2022-12-31
0001849380
us-gaap:RelatedPartyMember
ONMD:PromissoryNoteWithRelatedPartyMember
2021-02-08
0001849380
us-gaap:RelatedPartyMember
ONMD:PromissoryNoteWithRelatedPartyMember
2021-06-01
2021-06-01
0001849380
us-gaap:RelatedPartyMember
ONMD:PromissoryNoteWithRelatedPartyMember
2023-09-30
0001849380
us-gaap:RelatedPartyMember
ONMD:PromissoryNoteWithRelatedPartyMember
2022-12-31
0001849380
ONMD:ArcGroupLimitedMember
ONMD:AdministrativeSupportAgreementMember
us-gaap:RelatedPartyMember
2023-01-01
2023-09-30
0001849380
ONMD:AdministrativeSupportAgreementMember
us-gaap:RelatedPartyMember
2023-07-01
2023-09-30
0001849380
ONMD:AdministrativeSupportAgreementMember
us-gaap:RelatedPartyMember
2022-07-01
2022-09-30
0001849380
ONMD:AdministrativeSupportAgreementMember
us-gaap:RelatedPartyMember
2023-01-01
2023-09-30
0001849380
ONMD:AdministrativeSupportAgreementMember
us-gaap:RelatedPartyMember
2022-01-01
2022-09-30
0001849380
us-gaap:OverAllotmentOptionMember
2023-01-01
2023-09-30
0001849380
ONMD:FirstAmendmentToIntroducingAdvisorAgreementWithArcGroupLimitedMember
ONMD:ArcGroupLimitedMember
2022-03-22
2022-03-22
0001849380
ONMD:ArcGroupLimitedMember
us-gaap:CommonClassBMember
ONMD:SecondAmendmentToIntroducingAdvisorAgreementWithArcGroupLimitedMember
2022-01-01
2022-12-31
0001849380
ONMD:ArcGroupLimitedMember
ONMD:FounderSharesMember
us-gaap:CommonClassBMember
2022-01-01
2022-12-31
0001849380
ONMD:PublicWarrantsMember
2023-01-01
2023-09-30
0001849380
ONMD:PublicWarrantsMember
2023-09-30
0001849380
ONMD:PublicWarrantsMember
us-gaap:CommonClassAMember
2023-09-30
0001849380
us-gaap:WarrantMember
2023-09-30
0001849380
us-gaap:WarrantMember
2023-01-01
2023-09-30
0001849380
us-gaap:WarrantMember
2022-01-01
2022-12-31
0001849380
ONMD:PublicWarrantsMember
2022-01-01
2022-12-31
0001849380
ONMD:PlacementWarrantsMember
2022-01-01
2022-12-31
0001849380
us-gaap:CommonClassAMember
2023-01-01
2023-09-30
0001849380
us-gaap:CommonClassBMember
2023-01-01
2023-09-30
0001849380
ONMD:FounderSharesMember
ONMD:ArcGroupLimitedMember
us-gaap:CommonClassBMember
ONMD:SecondAmendmentToIntroducingAdvisorAgreementWithArcGroupLimitedMember
2022-12-31
2022-12-31
0001849380
ONMD:FounderSharesMember
ONMD:ArcGroupLimitedMember
us-gaap:CommonClassBMember
ONMD:SecondAmendmentToIntroducingAdvisorAgreementWithArcGroupLimitedMember
2022-12-31
0001849380
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2023-09-30
0001849380
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2023-09-30
0001849380
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2023-09-30
0001849380
us-gaap:FairValueInputsLevel1Member
us-gaap:FairValueMeasurementsRecurringMember
2022-12-31
0001849380
us-gaap:FairValueInputsLevel2Member
us-gaap:FairValueMeasurementsRecurringMember
2022-12-31
0001849380
us-gaap:FairValueInputsLevel3Member
us-gaap:FairValueMeasurementsRecurringMember
2022-12-31
0001849380
us-gaap:USTreasurySecuritiesMember
2023-09-30
0001849380
us-gaap:USTreasurySecuritiesMember
2022-12-31
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputExpectedDividendRateMember
2023-09-30
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputExercisePriceMember
2023-09-30
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputExercisePriceMember
2022-12-31
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputSharePriceMember
2023-09-30
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputSharePriceMember
2022-12-31
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputExpectedTermMember
2023-09-30
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputExpectedTermMember
2022-12-31
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
ONMD:MeasurementInputProbabilityOfCompletingBusinessCombinationMember
2023-09-30
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
ONMD:MeasurementInputProbabilityOfCompletingBusinessCombinationMember
2022-12-31
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputPriceVolatilityMember
2023-09-30
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputPriceVolatilityMember
2022-12-31
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputRiskFreeInterestRateMember
2023-09-30
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputRiskFreeInterestRateMember
2022-12-31
0001849380
ONMD:PrivateWarrantMember
us-gaap:FairValueInputsLevel3Member
us-gaap:MeasurementInputExpectedDividendRateMember
2022-12-31
0001849380
ONMD:PrivateWarrantMember
2022-12-31
0001849380
ONMD:PublicWarrantsMember
2022-12-31
0001849380
ONMD:WarrantLiabilityMember
2022-12-31
0001849380
ONMD:PrivateWarrantMember
2023-01-01
2023-09-30
0001849380
ONMD:WarrantLiabilityMember
2023-01-01
2023-09-30
0001849380
ONMD:PrivateWarrantMember
2023-09-30
0001849380
ONMD:WarrantLiabilityMember
2023-09-30
0001849380
ONMD:PrivateWarrantMember
2021-12-31
0001849380
ONMD:PublicWarrantsMember
2021-12-31
0001849380
ONMD:WarrantLiabilityMember
2021-12-31
0001849380
ONMD:PrivateWarrantMember
2022-01-01
2022-09-30
0001849380
ONMD:PublicWarrantsMember
2022-01-01
2022-09-30
0001849380
ONMD:WarrantLiabilityMember
2022-01-01
2022-09-30
0001849380
ONMD:PrivateWarrantMember
2022-09-30
0001849380
ONMD:PublicWarrantsMember
2022-09-30
0001849380
ONMD:WarrantLiabilityMember
2022-09-30
0001849380
us-gaap:SubsequentEventMember
2023-10-12
2023-10-12
0001849380
us-gaap:SubsequentEventMember
2023-10-17
2023-10-17
0001849380
us-gaap:SubsequentEventMember
us-gaap:CommonClassAMember
2023-10-17
2023-10-17
0001849380
us-gaap:SubsequentEventMember
us-gaap:IPOMember
2023-10-17
2023-10-17
0001849380
us-gaap:SubsequentEventMember
us-gaap:IPOMember
2023-10-17
0001849380
us-gaap:SubsequentEventMember
2023-10-17
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
ONMD:Integer
As
filed with the Securities and Exchange Commission on December 15, 2023
Registration
No. 333-[__]
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT
UNDER THE SECURITIES ACT OF 1933
ONEMEDNET
CORPORATION
(Exact
name of registrant as specified in its charter)
| Delaware |
|
3721 |
|
86-2049355 |
(State
or other jurisdiction
of
incorporation or organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
Number) |
OneMedNet
Corporation
6385
Old Shady Oak Road, Suite 250
Eden Prairie, MN 55344
Telephone:
800-918-7189
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
The
Corporation Trust Company
Corporation
Trust Center
1209
Orange Street
Wilmington,
DE 19801
Telephone:
302-658-7581
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Debbie
A. Klis, Esq.
Rimon
P.C.
1990
K. Street, NW, Suite 420
Washington,
DC 20006
Telephone:
(202) 935-3390
Approximate
date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large-accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company, or an emerging growth company. See the definitions of “large-accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large-accelerated
filer ☐ |
Accelerated
filer ☐ |
| |
|
| Non-accelerated
filer ☒ |
Smaller
reporting company ☒ |
| |
|
| |
Emerging
growth company ☒ |
If
an emerging growth company, indicate by check market if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective
in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date
as the Commission acting pursuant to said section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration
statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities
and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY
PROSPECTUS |
|
SUBJECT
TO COMPLETION |
|
DATED
DECEMBER 15, 2023 |

ONEMEDNET
CORPORATION
Up
to [*] shares of Common Stock
Pre-Funded
Warrants to Purchase up to [*] Shares of Common Stock
Warrants
to Purchase [*] shares of Common Stock
Shares
of Common Stock underlying the Pre-Funded Warrants and Warrants
We
are offering up to [*] shares of common stock, par value $0.0001 per share (“Common Stock”) of OneMedNet Corporation, a Delaware
corporation (referred to herein as the “Company,” “we,” “our,” “us,” or other similar
pronouns), together with warrants to purchase [*] shares of our Common Stock (and the shares of Common Stock that are issuable from time
to time upon exercise of the warrants). We are also offering to certain purchasers whose purchase of shares of Common Stock in this offering
would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99%
(or, at the election of the purchaser, 9.99%) of our outstanding Common Stock immediately following the consummation of this offering,
the opportunity to purchase, if any such purchaser so chooses, pre-funded warrants to purchase shares of our Common Stock, in lieu of
shares of Common Stock that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election
of the purchaser, 9.99%) of our outstanding Common Stock. Each pre-funded warrant will be exercisable for one share of our Common Stock.
The purchase price of each pre-funded warrant and accompanying warrant will be equal to the price at which a share of Common Stock and
accompanying warrant are sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will be
$0.0001 per share. The pre-funded warrants will be immediately exercisable and may be exercised at any time until all of the pre-funded
warrants are exercised in full. This offering also relates to the shares of Common Stock issuable upon exercise of any pre-funded warrants
sold in this offering. Each share of Common Stock and pre-funded warrant is being sold together with a warrant to purchase one share
of our Common Stock at an exercise price of $[*] per share (representing 100% of the price at which a share of Common Stock and accompanying
warrant are sold to the public in this offering).
The
warrants will be exercisable immediately and will expire five years from the date of issuance. For each pre-funded warrant we sell, the
number of shares of Common Stock we are offering will be decreased on a one-for-one basis. Because we will issue a warrant for each share
of our Common Stock and for each pre-funded warrant to purchase one share of our Common Stock sold in this offering, the number of warrants
sold in this offering will not change as a result of a change in the mix of the shares of our Common Stock and pre-funded warrants sold.
The shares of Common Stock and pre-funded warrants, and the accompanying warrants, can only be purchased together in this offering but
will be issued separately and will be immediately separable upon issuance.
This prospectus also relates
to the resale of shares of our Common Stock and warrants to purchase shares of our Common Stock of the Company pursuant to the securities
purchase agreement (the “PIPE SPA”) executed on June 28, 2023. The securities offered hereunder include 95,745
warrant shares of our Common Stock exercisable at a price per share of $10.00, subject to adjustment as provided in the agreements
governing the PIPE warrants (the “PIPE Warrants) for PIPE financing in the aggregate original
principal amount of $1,595,744.70 and the purchase price of $1.5 million. Pursuant to the PIPE SPA, the Company issued and sold to each
of Thomas Kosasa, Dr. Jeffrey Yu, Aaron Green and Steve Kester (the “PIPE Investors” or “selling security holders”),
a new series of senior secured convertible notes (the “PIPE Notes”), which PIPE Notes are convertible into shares of Common
Stock at the PIPE Investors election at a conversion price equal to the lower of (i) $10.00 per share, and (ii) 92.5% of the lowest volume
weighted average trading price for the ten (10) Trading Days immediately preceding the Conversion Date, subject to a minimum of $2.50,
which would convert to a maximum of 638,298 PIPE Notes shares of our Common Stock. The PIPE Investors’ $1.5 million investment
in the Notes closed and funded contemporaneous to the Closing of the Merger Agreement (collectively, the “Business Combination”).
At
the Closing of the Business Combination and funding of the PIPE, the PIPE Investors each executed a PIPE Note and a PIPE Warrant in the
amount corresponding to each PIPE Investor’s investment amount and in accordance with the terms set forth in the PIPE SPA as well
as a registration rights agreement (the “PIPE Registration Rights Agreement”).
We are registering the offer
and sale of these securities to satisfy certain registration rights we have granted. We will not receive any of the proceeds from the
sale of the securities by the PIPE Investors as the “selling securityholders.” We received the proceeds from the issuance
of the PIPE Notes and would receive proceeds from the Warrants exercised in the event that such Warrants are exercised for cash. We will
pay the expenses associated with registering the sales by the selling securityholders, as described in more detail in the section titled
“Use of Proceeds” appearing elsewhere in this prospectus.
The selling securityholders
may sell the securities described in this prospectus in a number of different ways and at varying prices. We provide more information
about how the selling securityholders may sell their securities in the section titled “Plan of Distribution” appearing
elsewhere in this prospectus.
The selling securityholders
may sell any, all or none of the securities and we do not know when or in what amount the selling securityholders may sell their securities
hereunder following the effective date of this registration statement.
Of the 734,043 shares of Common
Stock that may be offered or sold by selling securityholders identified in this prospectus, pursuant to the PIPE Notes and PIPE Warrants,
612,312 of those shares of Common Stock are subject to certain lock-up restrictions. The 612,312 shares of Common Stock, which are subject
to certain lock-up restrictions, include 79,788 shares of Common Stock issuable upon the exercise of the PIPE Warrants and 532,524 shares
of Common Stock issuable upon the conversion of the PIPE Notes.
Our
Common Stock and warrants are listed on The Nasdaq Stock Market LLC (“Nasdaq”) under the symbols, “ONMD”
and “ONMDW.” As of December 12, 2023, we had approximately 23,572,232 shares of Common
Stock and 12,181,019 warrants outstanding. On December 14, 2023, the last reported closing price of our of
Common Stock and warrants as reported on Nasdaq was $[0.9521] per share and $[0.0106] per warrant.
The
public offering price per share of Common Stock and accompanying warrant and any pre-funded warrant and accompanying warrant, as the
case may be, will be determined by us at the time of pricing, may be at a discount to the current market price, and the recent market
price used throughout this prospectus may not be indicative of the final offering price.
We
are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, have
elected to comply with certain reduced public company reporting requirements.
Investing
in our securities involves a high degree of risk. See “Risk Factors” beginning on page 31 of this prospectus for a
discussion of information that should be considered in connection with an investment in our securities.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | |
Per Share and Accompanying Warrant (2) | | |
Per
Pre-Funded
Warrant and Accompanying Warrant (2) | | |
Total | |
| Price to the public | |
$ | | | |
$ | | | |
| | |
| Underwriting discounts and commissions(1) | |
$ | | | |
$ | | | |
| | |
| Proceeds to us, before expenses | |
$ | | | |
$ | | | |
| | |
| (1) |
Represents an underwriting discount equal to (i) 7% per share of Common Stock, which is the underwriting discount
we have agreed to pay for sales to investors in this offering identified by the underwriter. The registration statement,
of which this prospectus is a part, also registers for sale warrants to purchase [*] shares of Common Stock to be issued to the representatives
of the underwriter in connection with this offering. We have agreed to issue the warrants to the representatives of the underwriter
as a portion of the underwriting compensation payable to the underwriters in connection with this offering. See “Underwriting”
for a description of compensation payable to the underwriters. |
| (2) |
The
public offering corresponds to a public offering price per share of common stock (or pre-funded warrant) of $[_], and a public offering
price per accompanying warrant of $0.0001. |
We
anticipate that delivery of the securities against payment will be made on or about ______, 2023.
EF
Hutton
division
of Benchmark Investments, LLC
Prospectus
dated ______, 2023.
TABLE
OF CONTENTS
You
should rely only on the information contained in this prospectus or any supplement. Neither we nor the Selling Securityholders have authorized
anyone else to provide you with different information. The securities offered by this prospectus are being offered only in jurisdictions
where the offer is permitted. You should not assume that the information in this prospectus or any supplement is accurate as of any date
other than the date on the front of each document. Our business, financial condition, results of operations and prospects may have changed
since that date.
Except
as otherwise set forth in this prospectus, neither we nor the Selling Securityholders have taken any action to permit a public offering
of these securities outside the United States or to permit the possession or distribution of this prospectus outside the United States.
Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions
relating to the offering of these securities and the distribution of this prospectus outside the United States.
ABOUT
THIS PROSPECTUS
Except
where the context otherwise requires or where otherwise indicated, the terms “OneMedNet,” the “Company,” “we,”
“us,” “our,” “our company,” and “our business” refer to OneMedNet Corporation and, where
appropriate, its consolidated subsidiaries.
We
incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without
charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus
as well as additional information described under “Information Incorporated by Reference,” before deciding to invest in our
securities.
Neither
we nor the underwriters have authorized anyone to provide you with additional information or information different from that contained
or incorporated by reference in this prospectus filed with the Securities and Exchange Commission (the “SEC”). We take no
responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. The underwriters
are offering to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted. The information
contained in this prospectus, or any document incorporated by reference in this prospectus, is accurate only as of the date of those
respective documents, regardless of the time of delivery of this prospectus or any sale of our securities. Our business, financial condition,
results of operations and prospects may have changed since that date.
The
information incorporated by reference or provided in this prospectus contains estimates and other statistical data made by independent
parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this
prospectus from our own research as well as from industry and general publications, surveys and studies conducted by third parties. This
data involves a number of assumptions and limitations and contains projections and estimates of the future performance of the industries
in which we operate that are subject to a high degree of uncertainty, including those discussed in “Risk Factors.” We caution
you not to give undue weight to such projections, assumptions, and estimates. Further, industry and general publications, studies and
surveys generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy
or completeness of such information. While we believe that these publications, studies, and surveys are reliable, we have not independently
verified the data contained in them. In addition, while we believe that the results and estimates from our internal research are reliable,
such results and estimates have not been verified by any independent source.
For
investors outside the United States (“U.S.”): We and the underwriters have not done anything that would permit this offering
or the possession or distribution of this prospectus in any jurisdiction where action for those purposes is required, other than in the
U.S. Persons outside the U.S. who come into possession of this prospectus must inform themselves about, and observe any restrictions
relating to, the offering of the securities and the distribution of this prospectus outside of the U.S.
MARKET
AND INDUSTRY DATA
Unless
otherwise indicated, information contained in this prospectus concerning our industry and the regions in which we operate, including
our general expectations and market position, market opportunity, market share and other management estimates, is based on information
obtained from various independent publicly available sources and other industry publications, surveys and forecasts. While we believe
that the market data, industry forecasts and similar information included in this prospectus are generally reliable, such information
is inherently imprecise. In addition, assumptions and estimates of our future performance and growth objectives and the future performance
of our industry and the markets in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety
of factors, including those discussed under the headings “Risk Factors,” “Cautionary Note Regarding Forward-Looking
Statements” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this
prospectus.
Information
contained in this prospectus concerning our industry, market and competitive position data in this prospectus from our own internal estimates
and research as well as from publicly available information, industry and general publications and research, surveys and studies conducted
by third parties.
Industry
publications and forecasts generally state that the information they contain has been obtained from sources believed to be reliable,
but such information is inherently imprecise. Forecasts and other forward-looking information obtained from these sources are subject
to the same qualifications and uncertainties as the other forward-looking statements in this prospectus. These forecasts and forward-looking
information are subject to uncertainty and risk due to a variety of factors, including those described under “Risk Factors.”
These and other factors could cause results to differ materially from those expressed in any forecasts or estimates.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements that involve risks and uncertainties. You should not place undue reliance on these forward-looking
statements. All statements other than statements of historical facts contained in this prospectus are forward-looking statements. The
forward-looking statements in this prospectus are only predictions. We have based these forward-looking statements largely on our current
expectations and projections about future events and financial trends that we believe may affect our business, financial condition, and
results of operations. In some cases, you can identify these forward-looking statements by terms such as “anticipate,” “believe,”
“continue,” “could,” “depends,” “estimate,” “expects,” “intend,”
“may,” “ongoing,” “plan,” “potential,” “predict,” “project,”
“should,” “will,” “would” or the negative of those terms or other similar expressions, although not
all forward-looking statements contain those words. We have based these forward-looking statements on our current expectations and projections
about future events and trends that we believe may affect our financial condition, results of operations, strategy, short- and long-term
business operations and objectives, and financial needs. These forward-looking statements include, but are not limited to, statements
concerning the following:
| |
● |
our
projected financial position and estimated cash burn rate; |
| |
|
|
| |
● |
our
estimates regarding expenses, future revenues and capital requirements; |
| |
|
|
| |
● |
our
ability to continue as a going concern; |
| |
|
|
| |
● |
our
need to raise substantial additional capital to fund our operations; |
| |
|
|
| |
● |
our
ability to compete in the global space industry; |
| |
|
|
| |
● |
our
ability to obtain and maintain intellectual property protection for our current products and services; |
| |
|
|
| |
● |
our
ability to protect our intellectual property rights and the potential for us to incur substantial costs from lawsuits to enforce
or protect our intellectual property rights; |
| |
|
|
| |
● |
the
possibility that a third party may claim we have infringed, misappropriated or otherwise violated their intellectual property rights
and that we may incur substantial costs and be required to devote substantial time defending against these claims; |
| |
|
|
| |
● |
our
reliance on third-party suppliers and manufacturers; |
| |
|
|
| |
● |
the
success of competing products or services that are or become available; |
| |
|
|
| |
● |
our
ability to expand our organization to accommodate potential growth and our ability to retain and attract key personnel; and |
| |
|
|
| |
● |
the
potential for us to incur substantial costs resulting from lawsuits against us and the potential for these lawsuits to cause us to
limit our commercialization of our products and services. |
These
forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk
Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is
not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which
any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements
we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus
may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You
should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected
in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events
and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither
we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation
to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual
results or to changes in our expectations.
You
should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration
statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and
events and circumstances may be materially different from what we expect. As a result of a number of known and unknown risks and uncertainties,
our actual results or performance may be materially different from those expressed or implied by these forward-looking statements including
those described in the “Risk Factors” section beginning on page 31 and elsewhere in this prospectus.
TRADEMARKS
AND COPYRIGHTS
We
own or have rights to trademarks or trade names that we use in connection with the operation of our business, including our corporate
names, logos and website names. In addition, we own or have the rights to copyrights, trade secrets and other proprietary rights that
protect the content of our products and the formulations for such products. This prospectus may also contain trademarks, service marks
and trade names of other companies, which are the property of their respective owners. Our use or display of third parties’ trademarks,
service marks, trade names or products in this prospectus is not intended to, and should not be read to, imply a relationship with or
endorsement or sponsorship of us. Solely for convenience, some of the copyrights, trade names and trademarks referred to in this prospectus
are listed without their ©, ® and ™ symbols, but we will assert, to the fullest extent under applicable law, our rights
to our copyrights, trade names and trademarks. All other trademarks are the property of their respective owners.
IMPLICATIONS
OF BEING AN EMERGING GROWTH COMPANY AND
A
SMALLER REPORTING COMPANY
We
qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”).
For so long as we remain an emerging growth company, we are permitted, and currently intend, to rely on the following provisions of the
JOBS Act that contain exceptions from disclosure and other requirements that otherwise are applicable to public companies and file periodic
reports with the SEC. These provisions include, but are not limited to:
| |
● |
being
permitted to present only two years of audited financial statements and selected financial data and only two years of related “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” in our periodic reports and registration statements,
including this prospectus, subject to certain exceptions; |
| |
|
|
| |
● |
not
being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended (“SOX”); |
| |
|
|
| |
● |
reduced
disclosure obligations regarding executive compensation in our periodic reports, proxy statements, and registration statements, including
in this prospectus; |
| |
|
|
| |
● |
not
being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board (the “PCAOB”)
regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the
audit and the financial statements; and |
| |
|
|
| |
● |
exemptions
from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute
payments not previously approved. |
We
will remain an emerging growth company until the earliest to occur of:
| |
● |
December
31, 2028 (the last day of the fiscal year that follows the fifth anniversary of the completion of our initial public offering); |
| |
● |
the
last day of the fiscal year in which we have total annual gross revenue of at least $1.235 billion; |
| |
|
|
| |
● |
the
date on which we are deemed to be a “large-accelerated filer,” as defined in the U.S. Securities Exchange Act of 1934,
as amended (the “Exchange Act”); and |
| |
|
|
| |
● |
the
date on which we have issued more than $1 billion in non-convertible debt over a three-year period. |
We
have elected to take advantage of certain of the reduced disclosure obligations in this prospectus and may elect to take advantage of
other reduced reporting requirements in our future filings with the SEC. As a result, the information that we provide to holders of our
Common Stock may be different than what you might receive from other public reporting companies in which you hold equity interests. We
have elected to avail ourselves of the provision of the JOBS Act that permits emerging growth companies to take advantage of an extended
transition period to comply with new or revised accounting standards applicable to public companies. As a result, we will not be subject
to new or revised accounting standards at the same time as other public companies that are not emerging growth companies.
We
are also a “smaller reporting company” as defined in the Exchange Act. We may continue to be a smaller reporting company
even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller
reporting companies until the fiscal year following the determination that our voting and non-voting common stock held by non-affiliates
is $250 million or more measured on the last business day of our second fiscal quarter, or our annual revenues are less than $100 million
during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is $700 million or more
measured on the last business day of our second fiscal quarter.
PROSPECTUS
SUMMARY
This
summary of the prospectus highlights material information concerning our business and this offering. This summary does not contain all
of the information that you should consider before making your investment decision. You should carefully read the entire prospectus,
including the information presented under the section entitled “Risk Factors” and the financial data and related notes, before
making an investment decision. It does not contain all the information that may be important to you and your investment decision. You
should carefully read this entire prospectus, including the matters set forth under “Risk Factors,” “Management’s
Discussion and Analysis of Financial Condition and Results of Operations,” and our financial statements and related notes included
elsewhere in this prospectus. This summary contains forward-looking statements that involve risks and uncertainties. Our actual results
may differ significantly from future results contemplated in the forward-looking statements as a result of factors such as those set
forth in “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements.”
In
this prospectus, unless the context indicates otherwise, “OneMedNet,” the “Company,” “we,” “our,”
“ours” or “us” refer to OneMedNet Corporation a Delaware corporation, and its direct and indirect subsidiaries,
including, but not limited to, OneMedNet Solutions Corporation, a Delaware corporation and its wholly-owned subsidiary, OneMedNet Technologies
(Canada) Inc., incorporated on October 16, 2015 under the provisions of the Business Corporations Act of British Columbia whose functional
currency is the Canadian dollar.
We
have not authorized anyone to provide you with different information and you must not rely on any unauthorized information or representation.
We are not making an offer to sell these securities in any jurisdiction where an offer or sale is not permitted. This document may only
be used where it is legal to sell these securities. You should assume that the information appearing in this prospectus is accurate only
as of the date on the front of this prospectus, regardless of the time of delivery of this prospectus, or any sale of our Common Stock.
Our business, financial condition and results of operations may have changed since the date on the front of this prospectus. We urge
you to carefully read this prospectus before deciding whether to invest in any of the Common Stock being offered.
The
Company
OneMedNet
Corporation a Delaware corporation (the “Company,” “we,” “us,” or “OneMedNet”) together
with its wholly-owned subsidiary OneMedNet Solutions Corporation, a Delaware corporation, founded in 2009 and incorporated in November
20, 2015, and its wholly-owned subsidiary, OneMedNet Technologies (Canada) Inc., incorporated on October 16, 2015 under the provisions
of the Business Corporations Act of British Columbia whose functional currency is the Canadian dollar. All refences in this prospectus
to the “Company,” “we,” “us,” or “OneMedNet” include OneMedNet Solutions Corporation.
Corporate
History
We
were originally incorporated in Delaware on February 8, 2021 under the name “Data Knights Acquisition Corp” as a special
purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase,
reorganization or similar business combination with one or more businesses.
On
May 11, 2021, we consummated an initial public offering (the “Initial Public Offering”). The registration statement for the
Company’s Initial Public Offering was declared effective on May 6, 2021. The Company’s Initial Public Offering of 10,000,000
units (the “Units” and, with respect to the Common Stock included in the Units being offered, the “Public Shares”),
at $10.00 per Unit, generated gross proceeds of $100,000,000. The Company granted the underwriter a 45-day option to purchase up to an
additional 1,500,000 Units at the Initial Public Offering price to cover over-allotments, which the underwriters exercised the over-allotment
option in full, and the closing of the issuance and sale of the additional Units occurred (the “Over-allotment Option Units”).
The total aggregate issuance by the Company of 1,500,000 units at a price of $10.00 per unit resulted in total gross proceeds of $15,000,000.
Simultaneously with the consummation of the closing of the Offering, the Company consummated the private placement of an aggregate of
585,275 units (the “Placement Units”) to the Sponsor at a price of $10.00 per Placement Unit, generating total gross proceeds
of $5,852,750 (the “Private Placement”).
The
Placement Units were issued pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended, as the transactions did not involve
a public offering. A total of $117,300,000, comprised of the proceeds from the Offering and the proceeds of private placements that closed
on May 11, 2021, net of the underwriting commissions, discounts, and offering expenses, was deposited in a trust account established
for the benefit of the Company’s public stockholders. On June 22, 2021, the Common Stock and Public Warrant included in the Units
began separate trading.
No
payments for our expenses were made in the offering described above directly or indirectly to (i) any of our directors, officers or their
associates, (ii) any person(s) owning 10% or more of any class of our equity securities or (iii) any of our affiliates, except in connection
with the repayment of outstanding loans and pursuant to the administrative support agreement disclosed herein which we entered into with
our sponsor.
On
April 25, 2022, the Company, Data Knights Merger Sub, Inc., a Delaware corporation (“Merger Sub”), and Data Knights, LLC,
the Company’s sponsor (the “Sponsor”), entered into a definitive Agreement and Plan of Merger (the “Merger Agreement”)
with OneMedNet Corporation, Inc., a Delaware corporation (the “Target,” which has since been renamed “OneMedNet Solutions
Corporation”), and together with the Company and Merger Sub, the “Parties”) and Paul Casey, as seller representative
(“Casey”). Pursuant to the Merger Agreement, upon the closing (the “Closing”) of the transactions contemplated
in the Merger Agreement (collectively, the “Business Combination”), the Parties would consummate the merger of Merger Sub
with and into the Target, with the Target continuing as the surviving entity (the “Merger”), which would result in all of
the issued and outstanding capital stock of the Target being exchanged for shares of the Company’s Common Stock upon the terms
set forth in the Merger Agreement. The Merger and Merger Agreement and the related transactions were approved unanimously by the boards
of directors of each of the Company and the Target.
On
December 31, 2022, substantially all of the assets held in the Trust Account were held in mutual funds.
On
June 28, 2023, the Company executed a Securities Purchase Agreement for PIPE financing in the aggregate original principal amount of
$1,595,744.70 and a purchase price of $1.5 million. Pursuant to the Securities Purchase Agreement, the Company agreed to issue and sell
to each of Thomas Kosasa, Dr. Jeffrey Yu, Aaron Green and Steve Kester (the “PIPE Investors”), a new series of senior secured
convertible notes (the “PIPE Notes”), which Notes shall be convertible into shares of Common Stock at the PIPE Investors
election at the conversion price (rounded to the nearest 1/100th of one cent) which shall be computed as the lesser of:
(a)
with respect to a conversion pursuant to Section 4.1 of the Securities Purchase Agreement (discussed below), the lesser of: (i) a price
per share equal to the product of (x) 100% less the Discount and (y) the lowest per share purchase price of the Equity Securities issued
in the Next Equity Financing; and (ii) $2.50 per share; and
(b)
with respect to a conversion pursuant to Section 4.2 (discussed below), (relating to payment at maturity) or Section 4.3, $2.50 per share.
The Securities Purchase agreement provided that the PIPE Investors’ $1.5 million investment in the PIPE Notes would close and fund
contemporaneous to the Closing of the Business Combination.
Section
4.1 of the Securities Purchase Agreement provides that the principal balance and unpaid accrued interest on each Note will automatically
convert into the PIPE Conversion Shares upon the closing of the Next Equity Financing (“Next Equity Financing” means the
next sale or series of related sales by the Company of its Common Stock in one or more offerings relying on Section 4(a)(2) of the Securities
Act or Regulation D thereunder for exemption from the registration requirements of Section 5 of the Securities Act, from which the Company
receives gross proceeds of not less than US$5,000,000 (excluding, for the avoidance of doubt, the aggregate principal amount of the Notes).
Section
4.2 of the Securities Purchase Agreement provides that in the event of a Corporate Transaction or the repayment of such Note, at the
closing of a corporate transaction, the holder of each Note may elect that either: (a) the Company will pay the holder of such Note an
amount equal to the sum of (x) the outstanding principal balance of such Note, and (y) a premium equal to 20% of the outstanding principal
balance of such Note (which premium, is in lieu of all accrued and unpaid interest due on such Note); or (b) such Note will convert into
that number of Conversion Shares equal to the quotient (rounded down to the nearest whole share) obtained by dividing (x) the outstanding
principal balance and unpaid accrued interest of such Note on a date that is no more than five days prior to the closing of such corporate
transaction by (y) the applicable Conversion Price.
Notwithstanding
the foregoing, any sale (or series of related sales) of the Company’s Equity Securities to a special purpose acquisition company
will not be deemed a “Next Equity Financing. Notwithstanding the foregoing, the Company may, at its option, pay any unpaid accrued
interest on each Note in cash at the time of conversion. The number of PIPE Conversion Shares the Company issues upon such conversion
will equal the quotient (rounded down to the nearest whole share) obtained by dividing (x) the outstanding principal balance and unpaid
accrued interest under each converting Note on a date that is no more than five days prior to the closing of the Next Equity Financing
by (y) the applicable Conversion Price. At least five days prior to the closing of the Next Equity Financing, the Company will notify
the holder of each Note in writing of the terms of the Equity Securities that are expected to be issued in such financing. The issuance
of PIPE Conversion Shares pursuant to the conversion of each Note will be on, and subject to, the same terms and conditions applicable
to the Equity Securities issued in the Next Equity Financing.
Also
on June 28, 2023, EF Hutton, division of Benchmark Investments, LLC (“EF Hutton”) waived $3,025,000 of the $4,025,000
cash deferred underwriting commission do to it at the Closing of the Business Combination, pursuant to a Satisfaction and Discharge Agreement.
In accordance with the Satisfaction and Discharge Agreement, EF Hutton accepted, in lieu of the cash deferred underwriting commission
due at closing (i) a one-time cash payment of $500,000 at the time of the Closing; (ii) a $500,000 promissory note executed by the Company
on June 30, 2023 in which it is obligated to make six monthly payments to EF Hutton in the cash amount of $83,333.33 commencing after
the Closing; and (iii) 277,778 shares of common stock of the Company (the “Common Stock”) at $10.89 per share, for an aggregate
value of $3,025,000.
On
September 21, 2023, the Securities and Exchange Commission (the “SEC”) declared effective the Company’s registration
statement and proxy statement/prospectus on Form S-4 (the “Definitive Proxy”).
As
of the close of business on September 20, 2023 (the “Record Date”), 5,172,973 shares of common stock of the Company (the
“Common Stock”) were issued and outstanding and entitled to vote at the Special Meeting. On October 17, 2023, the Company
held a special meeting of its stockholders (the “Stockholders”) in lieu of its 2023 annual meeting of Stockholders (the “Special
Meeting”) in connection with the transactions contemplated by the Merger Agreement. At the Special Meeting, the Stockholders were
asked to consider and vote on the proposals identified in the Definitive Proxy; 4,690,565 shares of Common Stock were represented in
person or by proxy at the Special Meeting, and, therefore, a quorum was present and all proposal were approved.
On
November 7, 2023, we held the Closing of the previously announced Merger whereby Merger Sub merged with and into OneMedNet Solutions
Corporation (formerly named OneMedNet Corporation), with OneMedNet Solutions Corporation continuing as the surviving entity, which resulted
in all of the issued and outstanding capital stock of OneMedNet Solutions Corporation being exchanged for shares of the Company’s
Common Stock upon the terms set forth in the Merger Agreement. The Merger and other transactions that closed on November 7, 2023, pursuant
to the Merger Agreement, led to Data Knights changing its name to “OneMedNet Corporation” and the business of the Company
became the business of OneMedNet Solutions Corporation.
Pursuant
to the terms of the Merger Agreement, the total consideration for the Business Combination and related transactions (the “Merger
Consideration”) was approximately $200 million. In connection with the Special Meeting, certain public holders (the “Redeeming
Stockholders”) holding 1,600,741 shares of Common Stock exercised their right to redeem such shares for a pro rata portion of the
funds held by Continental Stock Transfer & Trust Company, as trustee (“Continental”) in the trust account established
in connection with Data Knights’ initial public offering (the “Trust Account”).
Effective
November 7, 2023, Data Knights’ units ceased trading, and effective November 8, 2023, OneMedNet’s common stock began trading
on the Nasdaq Capital Market under the symbol “ONMD” and the warrants began trading on the Nasdaq Capital Market under the
symbol “ONMDW.”
As
a result of the Merger and the Business Combination, holders of Data Knights common stock automatically received common stock of OneMedNet,
and holders of Data Knights warrants automatically received warrants of OneMedNet with substantively identical terms. At the Closing
of the Business Combination, all shares of Data Knights owned by the Sponsor (consisting of shares of Common Stock and shares of Class
B common stock, which we refer to as the founder shares), automatically converted into an equal number of shares of OneMedNet’s
Common Stock, and the Private Placement Warrants held by the Sponsor, automatically converted into warrants to purchase one share of
OneMedNet Common Stock with substantively identical terms.
Effective
as of the Closing on November 7, 2023, among other holders, public stockholders own 98,178 shares of OneMedNet Common Stock approximately
representing 0.35% of the outstanding shares of OneMedNet Common Stock; the Sponsor and its affiliates own approximately 15.1% of the
outstanding shares of OneMedNet common stock (inclusive of shares received upon conversion of the Sponsor’s loan); OneMedNet’s
former security holders own approximately 61.992% of the outstanding shares of OneMedNet common stock from the conversion of their shares;
PIPE investors own 0.46% of the outstanding shares of OneMedNet Common Stock and former convertible note holders own approximately 16.24%
of the outstanding shares of OneMedNet Common Stock resulting from the issuance of 5,238,800 shares of Common Stock upon conversion of
their notes.
Who
We Are
We
are OneMedNet, and our goal is to be a leader in the future of regulatory-grade Imaging Real Word Data (“RWD”) through
our intelligent, thoughtful and inclusive iRWDTM solution. We strive to revolutionize access fast and secure access to curated
medical images with our strategic thinking, successful market entry and execution to continue to open new possibilities for providing
clinical imaging evidence.
We
are bold, decisive and eager to advance a global platform for our OneMedNet iRWD™ solution.
We
aim to constantly push boundaries in our approach to technology, service innovation, customer engagement and curation excellence,
all for the sake of delivering an exceptional customer experience.
Our
mission is affect a material positive impact on the lives of tens of millions of people while improving our customers’ business
productivity. First and foremost, OneMedNet’s iRWDTM offering plays a significant role in enabling Life Science
companies to bring safer and more effective patient care to market sooner. Using our highly curated de-identified clinical data in our
iRWDTM offering in Life Science product development, validation, and regulatory approval processes, they contribute to patient
care advancements in more meaningful ways, which Life Sciences industry can improve their product development and validation processes,
which benefits all parties.
At
OneMedNet, our motto is to “Unlock the Value in Imaging ArchivesTM”. By utilizing OneMedNet’s iRWDTM
offering, providers can greatly improve their research efforts with streamlined data access. Health care providers such as hospitals,
clinics, and imaging centers can also accelerate life science patient care innovations by sharing de-identified data in a well-defined
and de-identified and secure manner. In return for doing so, income is generated and applied to critical and possibly unfunded provider
projects. In that spirit, we are breaking boundaries by focusing on the future, constantly innovating from a technology and user experience
perspective and are ready to push forward. With that said, we recognize that we cannot do this alone, and we urge those who share this
desire to unite with us on our journey to a brighter and greener future.
Come
join the charge with us.
Our
Business
OneMedNet
is a global provider of clinical imaging innovation and curator of regulatory-grade Imaging Real-World Data3 or iRWDTM. OneMedNet’s
innovative solutions connect healthcare providers and patients satisfying a crucial need within the Life Sciences field offering direct
access to clinical images and the associated contextual patient record. OneMedNet’s innovative technology proved the commercial
and regulatory viability of imaging Real-World Data, an emerging market, and provides regulatory-grade image-centric iRWDTM
that exactly matches OMN’s Life Science partners Case Selection Protocols and paves the way for Real World Evidence.
OneMedNet
was founded to solve a deficiency in how clinical images were shared between healthcare providers. This resulted in OMN’s initial
product BEAMTM image exchange that enabled the successful sharing of images for more than a decade with OMN’s largest
customer being the Country of Ireland.
OneMedNet
continued to innovate by responding to the demand for and utilization of Real-World Data and Real-World Evidence, specifically data that
focused on clinical images with its associated contextual clinical record. We were able to leverage internal technological competencies
along with OneMedNet’s formidable healthcare provider installed base from its first product with BEAMTM to become the
first RWD solution for Life Science companies with its launch of iRWDTM in 2019.
OneMedNet
provides innovative solutions that unlock the significant value contained within clinical image archives. With a growing federated network
of 95+ healthcare facilities, OneMedNet has the immediate ability to quickly search and extensively curate multi-layer data from a Federated
group of healthcare facilities. The term “healthcare facilities” refers specifically to the hospitals, integrated delivery
networks (“IDNs”) and imaging centers that provide imaging to OneMedNet, which represent the core source of our data. At
present, OneMedNet works with more than 95 facilities who provide regulatory grade imaging to us. OneMedNet has access to these more
than 95 facilities because these 95+ contracted facilities have more than 200 locations among them including offices and clinics, which
in total generates regulatory grade imaging from more than 200 customers. Among these customers, all are data providers and some are
data purchasers.
OneMedNet
is ahead of the curve when it comes to providing fast and secure access to curated medical images. Initially, it was all about solving
the diverse access needs of patient care providers. This focus systematically evolved to addressing the rapidly growing needs of image
analysis and researchers, clinicians, regulators, scientists and more.

Real-world
data is any data that is collected in the context of the routine delivery of care, in contrast to data collected within a clinical trial
where study design controls variability in ways that are not representative of real-world care and outcomes.
A
key component driving its mission is that OneMedNet believes we have a unique opportunity to affect a material positive impact on the
lives of tens of millions of people while improving our customers’ business productivity. First and foremost, OneMedNet’s
iRWDTM offering plays a significant role in enabling Life Science companies to bring safer and more effective patient care
to market sooner. Using our highly curated de-identified clinical data in our iRWDTM offering in Life Science product development,
validation, and regulatory approval processes, they contribute to patient care advancements in more meaningful ways. Moreover, Life Sciences
improve their product development and validation processes, which benefits all parties.
Significant
documentation exists that shows that Real-World Data can provide expanded insights across broader and more representative patient populations.1
For this reason, the Food and Drug Administration (“FDA”) has instituted Real-World Data guidelines for regulatory
approvals. Utilization of highly reliable and quality Real-World Data that strictly adheres to all of the very specific data stratification
requirements can supplement or supplant clinical trials.
OneMedNet
covers the complete value chain in imaging Real-World Data; it begins with our 10+ year federated network of providers and is supported
by a multi-faceted data curation process managed by an expert in-house clinical team. Additionally, we work hand-in-hand with our Life
Science partners regarding the Case Selection Protocol and when required producing Case Report Forms for regulatory clearance. We are
focused on delivering value by supporting Life Science Advancements with OneMedNet’s iRWDTM which holds the key to unlocking
boundless patient care advances. We unleash the power of research-grade image-centric iRWDTM that is highly curated to painstakingly
meet every cohort requirement and stand up to all of the rigors of prospective clinical trials.
Today,
life science companies, including pharmaceutical companies, artificial intelligence (AI) developers, medical device businesses, and clinical
research organizations share the same widespread challenge in obtaining insight-rich, high-quality patient data that explicitly matches
their precise cohort specifications. A substantial portion of patient diagnosis involves clinical imaging and approximately 90% of healthcare
data, by size, is associated with imaging. Historically, much of imaging value has been derived from its initial review and further gains
from the image archives have been very limited.
1
See https://www.fda.gov/media/120060/download
We
help providers to “Unlock the Value in Imaging Archives”.TM By utilizing OneMedNet’s iRWDTM offering,
providers can greatly improve their research efforts with streamlined data access. Health care providers such as hospitals, clinics,
and imaging centers can also accelerate life science patient care innovations by sharing de-identified data in a well-defined and de-identified
and secure manner. In return for doing so, income is generated and applied to critical and possibly unfunded provider projects.
The
OneMedNet Difference
OneMedNet
has been a leader in the business of extracting, securing, and transferring medical data for 12+ years. Doing so requires specialized
expertise in:
| |
● |
Compliancy
(HIPAA, GDPR, 21 Part11) |
| |
|
|
| |
● |
Advanced
privacy & security measures |
| |
|
|
| |
● |
Clinical
patient condition(s) and hospital processes |
| |
|
|
| |
● |
Radiology
interpretation |
| |
|
|
| |
● |
AI/ML
technology |
Attaining
in-house expertise in all essential elements is quite a challenge and deters many organizations from even attempting such a venture.
We take pride in this ambitious achievement – while continually working to maintain state-of-the-art expertise. OneMedNet strictly
adheres to the highest level of professional and ethical standards and applicable regulations throughout all interactions and activities.
We
believe there is a reason OneMedNet is the leader in an uncrowded field of regulatory-grade imaging RWD curators. Doing so requires specialized
expertise in AI/ML technology, data privacy/security, as well as expertise in clinical patient condition(s) and healthcare record keeping.
Having, or achieving, expertise in all essential disciplines is a challenging achievement. OneMedNet had a significant head start with
our clinical image exchange solution which served to launch the company nearly a decade ago. All data remains “native” within
the federated OneMedNet iRWDTM provider network – meaning all the data remains locally onsite until specific de-identified
data is licensed for a particular Life Science research opportunity.
OneMedNet’s
Competitive Advantages
We
believe that OneMedNet iRWDTM offers the best of advanced technology, clinical expert curation, and service. Medical imaging
and associated clinical data is indexed at each network site using state-of-the-art AI/ML technology. This typically includes electronic
health records (“EHR”), radiology, cardiology, lab, path and more. Our in-house clinical team performs intensive curation
of the data ensuring that results meet the exact specification and requirements of Life Science Data Collection Protocol (“DCP”)
– regardless of the complexity.
We
believe that OneMedNet unlocks the value in imaging and electronic health records data in the following three principal ways:
| |
● |
Regulatory
Grade — Our imaging results serve as proof of effectiveness for regulatory agencies, meeting requirements for quality &
diversity; |
| |
|
|
| |
● |
On
Demand — Our powerful indexing platform access and harmonizes complete patient profiles across fragmented data silos, delivering
images and records on-demand; |
| |
|
|
| |
● |
Expertly
Curated — We curate to the most stringent multi-level stratified requirements, providing unmatched data accuracy and completeness. |
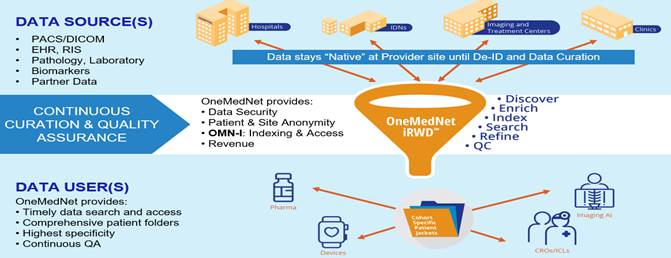
OneMedNet’s
data is fully de-identified using a multi-step quality control process and goes beyond PHI to include PII (personally identifiable information),
SII (Site Identifiable Information), and more. Importantly, Life Science users receive the data in the exact format that they require.
No data sifting or manipulation is needed. The data is simply ready for use. Moreover, OneMedNet has the unique combination of knowledge,
tools, and experience to:
| |
● |
Access
and harmonize complete patient profiles across fragmented data silos; |
| |
|
|
| |
● |
Provide
unmatched data accuracy and completeness; |
| |
|
|
| |
● |
Ensure
the security and privacy of patients’ Protected Health Information (PHI)Imaging RWD is our singular passion and focus and no
one does it better. |
Finally,
OneMedNet has the most experienced and clinically trained data curators in the industry. This team appreciates the complexity and criticality
of clinical data and can effectively communicate with both Provider and Life Science specialists.
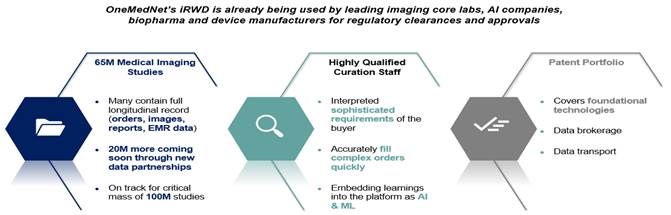
Industry
Background
A
2016 analysis published in the Journal of Health Economics and authored by the Tufts Center for the Study of Drug Development placed
the cost of bringing a drug to market, including post-approval research and development, at a staggering $2.87 billion.2 Meanwhile,
a 2018 study from the Tufts Center noted that the timeline for new drug development ranged from 12.8 years for the average drug to 17.2
years for ultra-orphan drugs that only affect several hundred patients.3 This places the onus on life science organizations
to find ways to deliver treatments to patients faster — especially those who cannot wait 17 years for a potentially
life-saving treatment. Knowing how a medicinal product is actually used by patients can help stakeholders across the healthcare ecosystem
make important and potentially life-saving real-time decisions.
Real-World
Data is observational data typically gathered when an approved medical product is on the market and used by “real” patients
in real life, as opposed to clinical trials or real world images for real patients. The FDA cites several potential sources of Real-World
Data, including electronic health records (“EHRs”), claims, disease and product registries, there are multiple types of data
including structured and unstructured data, clinical and billing data, transactional and claims data, patient-generated data, and data
gathered from additional sources that can shed light on a patient’s health status and more. As reliance on healthcare data grows
exponentially, OneMedNet has observed that the reliance on information has increased coming from multiple additional sources including
EHRs, claims, registries, clinical trials, patient and provider surveys, wearable devices and more. These additional sources include
the internet of things (“IoT”), social media forums and blogs. Real-World Data has the potential to break down inefficiencies
and fill gaps in information silos among stakeholders throughout the healthcare ecosystem of providers, payers, manufacturers, government
entities and patients. This information sharing, in turn, enables all parties to derive new insights, support value-based care and deliver
better health outcomes.
Commercializing
a drug requires its developer to harness various sources of Real-World Data to identify patient populations and refine sales and marketing
strategies for those populations among many other undertakings. Historically, this practice involved purchasing large amounts of data
from data aggregators or data platforms, if not directly from the source itself, sometimes without much knowledge about the quality of
the data. Preparing this data for analysis is both expensive and time-consuming thus many organizations would outsource the process to
consultants or third-party vendors; moreover, the process of preparing this data for analysis by untrained consultants can yield a static
analysis that is difficult to modify or rerun in response to follow-up questions or potential discrepancies.
2
https://www.outsourcing-pharma.com/Article/2016/03/14/Tufts-examines-2.87bn-drug-development-cost
3
https://www.hcplive.com/view/new-data-reinforces-difficulty-orphan-drug-development
Definitions
of Real-World Data and Real-World Evidence
Real-World
Data has become a powerful tool in the life sciences industry. After decades of relying on clinical data as the gold standard for decision
making, industry leaders now recognize how data collected in the real world adds valuable context and insight to their efforts. From
identifying unmet medical needs and defining the patient journey, to supporting regulatory submissions, proving value to payers, and
shaping market strategies, Real-World Data adds value at every stage of the drug development lifecycle. Real-World Data also sets the
foundation for Real World Evidence, and while the terms are often used interchangeably, they are distinct and they are changing health
care. Here’s how it happens:
| 1.
|
First,
Real-World Data are data relating to patient health status and/or the delivery of health care routinely collected from a variety
of sources. Real-World Data is aggregated and transformed such as through OneMedNet’s robust analytics. Real-World Data are
the data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources. There
are many different types, sources and uses of Real-World Data, for example: |
| |
● |
Clinical
Data — For example, clinical data from EHRs and case report forms (“eCRF”) including biopsies and other
pathology tests, diagnostic imaging, social determinants of health, cancer organoids, that provide patient demographics, family history,
comorbidities, procedure and treatment history, and outcomes. |
| |
|
|
| |
● |
Patient
Generated Data — For example, patient-generated data from patient-reported outcome surveys, which data provide insights
directly from the patient, and they help researchers understand what happens outside of clinic visits, procedures, and hospital stays. |
| |
|
|
| |
● |
Cost
and Utilization Data (Qualitative Studies) — For example, cost and utilization data from claims and public
datasets, which data provides information regarding healthcare services utilization, population coverage, and prescribing patterns. |
| |
|
|
| |
● |
Public
Health Data — For example, public health data from various government data sources. These add critical information
to enable stakeholders to best serve the needs of the populations they serve. |
The
availability of medical imaging in Real-World Data such as that provided by OneMedNet is facilitated by the development of digital image
analysis to increase the accuracy of diagnostics and conduct passive screening on large databases of medical images using artificial-intelligence
(“AI”) algorithms such as those applied by OneMedNet. Algorithms can also help identify additional diagnostic tests of value
from medical images with pathology.
Real-World
Evidence is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of Real-World
Data, as defined by the Food and Drug Administration. Real-World Evidence can be generated by different study designs or analyses, including
but not limited to, randomized trials, including large simple trials, pragmatic trials, and observational studies (prospective and/or
retrospective). The difference in Real World Evidence and Real World Data focuses on the end use case. Real World Data can take the form
of claims, electronic health records, labs, data etc. Often this insight is used to better understand a patient’s journey or a
natural history of a disorder (how does a disease progress if left untreated.)

Real
World Evidence in contrast builds upon many of these data sets and prepares them for submission, as part of regulatory review such as
to the Food and Drug Administration or the European Medicines Agency, for example, in support of a customer’s clinical trial application.
When data and in particular imaging data is submitted to the FDA the agency requires the following:
| |
● |
Guard
against biased — evidence must align with the patient population being study — expectations
focus on the similar patient demographics, comorbidities, disease severity, etc.; |
| |
|
|
| |
● |
Traceability — confirm
the chain of custody, the source of the data is known and can be validated if required; and |
| |
|
|
| |
● |
Go
forward basis — regulatory agencies seek evidence that aligns with the trials timeframe and when possible collect
evidence that mirrors the clinical trials timeline. |
One
area where Real World Evidence has been relief on heavily relates to oncology approvals. Food and Drug Administration’s Oncology
Center of Excellence actually presented an analysis of this at American Society of Clinical Oncology in 2021, looking at oncology applications
containing Real-World Data and Real-World Evidence. That analysis looked at 94 applications that were submitted from 2011-2020, and showed
that inclusion of Real-World Data to support regulatory decision-making has increased dramatically over that period. In 2020 alone, there
were 28 submissions for oncology products that contained Real-World Data. Outside of the oncology context, probably the most notable
recent example of an approval relying on Real World Evidence is the July 2021 approval of a new indication for Astellas’ drug Prograf
(or tacrolimus) for the prevention of organ rejection in lung transplant patients. The approval there was based on a non-interventional
study providing Real-World Evidence of effectiveness.4 FDA’s press release announcing the approval noted that the approval
was “significant because it reflects how a well-designed, non-interventional study relying on fit-for-purpose real-world data,
when compared to a suitable control, can be considered adequate and well-controlled under FDA regulations.”
4See https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-new-use-transplant-drug-based-real-world-evidence
An
additional recent approval of note was the December 2021 approval of the supplemental BLA for Orencia to prevent graft versus host disease.5 The application included data from a randomized clinical trial, with additional evidence of effectiveness provided by a
registry-based clinical study that was conducted using real-world data from the Center for International Blood and Marrow Transplant
Research.6 And that registry study analyzed outcomes of 54 patients treated with Orencia for the prevention of graft versus
host disease, in combination with standard immunosuppressive drugs, versus 162 patients treated with the standard immunosuppressive drugs
alone, and showed efficacy in that indication.
AI
is employed in Real-World Data to enhance data anomaly detection, standardization, and quality checking at the pre-processing stage.
AI is expected to offer pharma and biotech companies the ability to increase meaningful Real World Evidence output, decrease time to
insights, and make the most of the available vast data sources. A Real World Evidence technology platform that delivers smart data processing,
analysis, and outcomes offers an unparalleled opportunity to capitalize on these computing advancements.
When
used as part of an overall comprehensive Real World Evidence strategy, AI innovations can enhance drug development, improve patient treatment
and access, and drive valuable new business opportunities.
In
post-marketing studies, adverse events reporting is an area where AI is used, creating greater automation and efficiency in historical
data sets. Techniques like natural language processing (“NLP”) enable AI to scan tens of thousands of records and quickly
find adverse event details. AI integrated analytics and automation provide access to crucial insights from historical clinical trial
Real-World Data and Real World Evidence, expanding end-to-end clinical trial capabilities:
| |
● |
Data
ingestion — publicly/historical available Real-World Data |
| |
|
|
| |
● |
Text
extraction — NLP used to extract key entities from clinical trial documents |
| |
|
|
| |
● |
Data
transformation & standardization — data standardization using pre-built models |
| |
|
|
| |
● |
AI
model deployment — predicting trial design impacts on costs, feasibility, cycle times, and quality risk |
AI
is driving ground-breaking leaps in protein structure identification, and advances in regulations are providing healthcare research organizations
with access to real-world data to accelerate clinical trial processes. We believe that AI-enabled technologies have unparalleled potential
to offer innovative trial design and collection, organizing, and analyzing the increasing amount of data generated by clinical trials.
AI has many applications in clinical trials, both short and long-term. AI technologies make possible innovations crucial for transforming
clinical trials, such as seamlessly combining Phases I and II, developing novel patient-centered endpoints, and collecting and analyzing
Real-World Data.
OneMedNet
believes that AI tools also have wider benefits for hospitals and health systems. Professor Alexander Wong, University of Waterloo Canada
Research Chair in AI and Medical Imaging, points out that AI benefits include the potential to ease the burden on radiology departments
in terms of assessing scans and predicting upcoming demand for general hospital and intensive care beds, and demand for equipment such
as respirators and ventilators, medicines, masks, and ventilator mouthpieces, as well as aiding workforce planning.7
Across
a diverse set of imaging modalities, digital images typically include metadata and/or annotations that may include protected health information
(e.g., patient name, date of birth). Although diagnostic images generally do not warrant the same level of privacy concerns as
genomic data, researchers must also remove facial characteristics or other features that could identify a patient.
Digital
image analysis can be used to support research and development by analyzing large volumes of tissue specimens or other medical images
to run molecular screens that model biomarkers and treatment responses by transplanting a portion of a patient’s tumor into humanized
mice or 3D tissue cultures derived from stem cells that resemble miniature organs. These models allow researchers to conduct controlled
laboratory experiments that can inform treatment approaches and link predicted treatment response to actual clinical outcomes by linking
this data to EHR, claims, and other sources of Real-World Data. Similarly, preclinical studies can be informed by safety assessments
conducted in animal models or studies of animal molecular biomarkers or anatomic abnormalities to minimize the burden on human study
participants. Findings can also inform clinical trial optimization by stratifying participants according to predicted response and determining
appropriate eligibility criteria.
5
See https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-prevent-graft-versus-host-disease
6
See https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-orencia-abatacept-prophylaxis-acute-graft-versus-host-disease
7
See https://www.eurekalert.org/news-releases/936861
| 2.
|
Second,
Real-World Evidence is the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis
of Real-World Data. Real World Evidence provides clinically-rich insights into what actually happens in everyday practice and why. The
FD&C Act defines Real-World Evidence as “data regarding the usage, or the potential benefits or risks, of a drug derived
from sources other than traditional clinical trials.”8 In developing its Real-World Evidence program, FDA believes
it is helpful to distinguish between the sources of Real-World Data and the evidence derived from that data. |
Evaluating
Real-World Evidence in the context of regulatory decision-making depends not only on the evaluation of the methodologies used to generate
the evidence but also on the reliability and relevance of the underlying Real-World Data; these constructs may raise different types
of considerations. Real-World Evidence refers to evidence about the risks and benefits of a product derived from analysis of the Real-World
Data. For example, the FDA has used Real-World Data and Real-World Evidence, derived from its Sentinel system for monitoring the safety
of regulated products, in place of post-marketing studies. It has carried this out for nine potential safety issues involving five products.
Real-World
Evidence is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of Real-World
Data. Real World Evidence can be generated by different study designs or analysis, including but not limited to, randomized trials, including
large simple trials, pragmatic trials, and observational studies (prospective and/or retrospective).
Unlike
traditional clinical trials, where necessary data elements can be curated and collection mandated, the creation of Real World Evidence
requires assessing, validating and aggregating various, often disparate, sources of data available through routine clinical practice.
Real-world evidence is used by different stakeholders in many different ways.
|
● |
It
gives life sciences companies insight into how their drugs are being used. |
|
● |
It
helps providers improve the delivery of care. |
|
● |
It
enables regulatory authorities to monitor post-market safety and adverse events. |
|
● |
It
helps payers assess outcomes from treatments. |
From
Real-World Data to Real World Evidence
The
creation of Real World Evidence requires a combination of high-powered analytics, a validated approach and a robust knowledge of available
Real-World Data sources (e.g., what data is captured within existing quality registries, what data can be captured through electronic
health records and case report forms or claims, which patient organizations capture data on relevant patient cohorts). This process includes
several steps, which are summarized here:
| |
1. |
Defining
a study protocol answering relevant clinical questions. |
| |
|
|
| |
2.
|
Defining
which data elements can be collected from which Real-World Data sources. |
| |
|
|
| |
3.
|
Establishing
data capture arrangements and protocols with existing Real-World Data sources. |
| |
|
|
| |
4.
|
Blending
disparate data sources through probabilistic record matching algorithms. |
| |
|
|
| |
5.
|
Validating
and supplementing blended data through editable eCRFs. |
| |
|
|
| |
6.
|
Defining
and calculating clinically relevant outcomes and measures. |
| |
|
|
| |
7.
|
Appropriately
assessing and controlling for variability in data quality, availability and confounding patient factors affecting measured outcomes. |
| |
|
|
| |
8.
|
Real
World Evidence can provide a holistic view of patients that in many cases cannot be studied through traditional clinical trials. |
8
See 21 U.S.C. 355(g)(b).
Real
World Evidence has been proven to fill a gap between research (what we learn) and everyday practice (what we do) in healthcare, and it
creates a difference between what is expected to happen and what really happens. Driving measurable improvements in healthcare requires
us all to be rooted in the reality of what actually happens before, during, and after clinical procedures, interventions, and office
visits. Real World Evidence fill those gaps and documents the truth by establishing definitively what really happens when doctors treat
a wide range of patients that do not look like the homogeneous patient groups in a clinical trial. Because of this, Real World Evidence
serves many uses and provides many benefits across the healthcare ecosystem.
As
more countries battle to contain healthcare costs, and as the population ages and the number of patients with chronic diseases increases,
the need to remove inefficiencies and upgrade the delivery of coordinated care that improves outcomes is more pressing. At the same time,
life sciences companies are facing tumultuous times. Industry globalization, the end of the blockbuster era, and an increasingly complex
regulatory environment all add to the difficulty of bringing products to market. And across the board, companies are moving toward a
patient-centric and outcome-focused model. In this environment, Real World Evidence can be transformative for the industry when Real-World
Data is combined with the right technology framework and the regulatory intelligence to make sense of it. As data is consumed across
life sciences in different ways and by different stakeholders, it can provide valuable insights and “evidence” across the
product life cycle. In addition, stakeholders across the healthcare ecosystem use this new knowledge to support decision-making and improve
safety and effectiveness, and ultimately, patient outcomes.
Uses
of Real World Evidence in Life Sciences, Among Regulators, Clinicians, Researchers and Healthcare Systems
According
to repeated studies by Deloitte,9 the importance of Real World Evidence continues to rise as it promises to accelerate regulatory
decision-making and support the approval of new indications for drugs already on the market. Life Sciences, pharmaceutical and medical
device companies are significant consumers of Real World Evidence because it can provide value across the entire product lifecycle from
pre-trial design to clinical studies and trials to post-market surveillance. Medical product developers are using Real World Evidence
to support clinical trial designs (e.g., large simple trials, pragmatic clinical trials) and observational studies to generate
innovative, new treatment approaches.
Real
World Evidence can be used to make clinical trials more effective and efficient, for example in patient recruitment or label extension,
Real World Evidence gathered from other studies or from currently marketed products in a similar category, for example, can have a positive
effect on the product portfolio by exposing positive side effects as new potential indications. The most famous example is Viagra, which
was initially studied as a drug to lower blood pressure, but an unexpected side effect led to the drug ultimately being approved for
erectile dysfunction.
The
benefits of Real World Evidence derived from Real-World Data are increasingly being recognized by regulatory authorities. The FDA released
a framework for using Real World Evidence to support the process of drug regulation and submission. This is a major step toward recognizing
that clinical trials, while still relevant, are not the only way to assess the efficacy and safety of a product. Indeed, the FDA is soon
expected to conduct its first full post-market safety approval using only Real World Evidence.
Real
World Evidence is now accepted as a reliable source of information for regulatory decision making in certain circumstances. A primary
rationale for the FDA to use Real World Evidence E is to help support the approval of a new or extended use for a drug approved under
the FD&C Act and to help support or satisfy post-approval study requirements always with the condition that the data quality is up
to the standard required. In a recent statement, the FDA even noted how new tools for capturing data in the post-market period, including
more sophisticated use of Real-World Data and Real-World Evidence are providing new approaches to address important questions about the
safety and benefits of new drugs in real world settings and that these approaches have the potential to do to so more rapidly and with
greater efficiency than traditional methods.10
9
See https://www2.deloitte.com/content/dam/insights/us/articles/4354_Real-World-Evidence/DI_Real-World-Evidence.pdf
10
See https://www.fda.gov/science-research/science-and-research-special-topics/real-world- evidence
Why
Do We Need Real-World Evidence?
There’s
a gap between research (what we learn) and everyday practice (what we do) in healthcare, and it creates a difference between what is
expected to happen and what really happens. But it’s what really happens that matters. Driving measurable improvements in healthcare
requires us all to be rooted in the reality of what actually happens before, during, and after clinical procedures, interventions, and
office visits. Real-World Evidence is here to fill those gaps and root us in truth. It tells us what really happens when doctors treat
a wide range of patients that don’t look like the homogeneous patient groups in a clinical trial. Because of this, Real-World Evidence
serves many uses and provides many benefits across the healthcare ecosystem.
Uses
of Real-World Evidence in Pharmaceutical and Device Companies
Pharmaceutical
and medical device companies are major consumers of Real-World Evidence, as it can provide value across the entire product lifecycle.
Real-World Evidence plays an important role for research across the product lifecycle for both pharmaceutical and device companies. It
can inform pre-trial study design by helping researchers identify potential patients and create proper inclusion criteria for clinical
trials. Much of medical innovation is driven by traditional clinical trials, where new pharmaceuticals and devices are rigorously studied
and tracked before they can be sold and widely distributed.
Although
clinical trials are incredibly important to determine the safety and efficacy of new technologies, when compared to real-world evidence
they do have some limitations. For example, traditional clinical trials can have strict inclusion criteria that makes it challenging
for providers to accurately extrapolate the results of a clinical trial to a broader population. Clinical trial participation is often
limited by who the study administrators are able to recruit, and various demographics are often not able to participate. This again challenges
the generalizability of clinical trial results across patient populations. Real-world evidence can help overcome the limitations of clinical
trials by providing information about a broader cross-section of society. This can help clinicians, researchers, and industry partners
better understand their products and how they work.
Once
a product is approved and marketed, Real-World Evidence assists pharmaceutical or medical device company understand their products’
relative safety, effectiveness, value, off-label use and more. This post-market surveillance, or post-marketing surveillance, is valuable
to stakeholders across the healthcare industry.
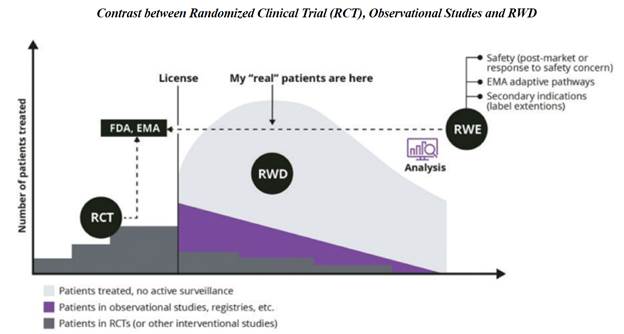
The
AI-enabled patient enrichment and recruitment process can improve suitable cohorts and increase clinical trial effectiveness, data management,
analysis, and interpretation of multiple Real-World Data sources, including EHRs and medical imaging data. This presents a unique opportunity
for NLP to perform the sophisticated analysis necessary to combine genomic data with electronic medical records (“EMRs”)
and other patient data, present in various locations, owners, and formats — from handwritten paper copies to digital
medical images — to surface biomarkers that lead to endpoints that can be more efficiently measured, and thereby
identify and characterize appropriate patient subpopulations. AI-enabled systems can help to improve patient cohort composition and aid
with patient recruitment.
AI
technologies can help biopharma companies identify target locations, qualified investigators, and priority candidates and collect and
collate evidence to satisfy regulators that the trial process complies with good clinical practice (“GMP”) requirements.
One of the most important elements of a clinical trial is a selection of high-functioning investigator sites. Site qualities such as
resource availability, administrative procedures, and experienced clinicians with in-depth knowledge and understanding of the disease
can shape study timelines and data quality, accuracy, completeness, and consistency.
AI
integrated clinical trial programs can help monitor and manage patients by automating real-world data capture, sharing data across systems,
and digitalizing standard clinical assessments. AI technologies and wearable technologies can help enable continuous patient monitoring
and generate real-time insights into the safety and effectiveness of treatment while predicting the possible risk of dropouts, thereby
enhancing patient engagement and retention. To comply with trial adherence criteria, patients must keep detailed records of their medication
intake and other data points related to their bodily functions, response to medication, and daily protocols. This can be an overwhelming
and tedious task, leading to 40% of patients becoming non-adherent after 150 days into a clinical trial. Wearable devices/sensors and
video monitoring are used to collect patient data automatically and continuously, thereby relieving the patient of this task. In combination
with wearable technology, AI techniques offer new approaches to developing real-time, power-efficient, mobile, and personalized patient
monitoring systems.
Among
regulators, clinicians, academic researchers and healthcare systems, the reliance on curated Real World Evidence has grown significantly
because of the value it can provide, which is unique relative to each parties’ objectives and mandates. It also helps that the
FDA has also sharpened its focus on Real-World Data and Real World Evidence. For example, late last year, the FDA published proposed
guidance related to data standards for product submissions with Real-World Data and also weighed in on the use of Real-World Data and
Real World Evidence to support regulatory decision-making for drugs and biological products with specific advice for data from electronic
health records and medical claims.11 In addition, the FDA uses Real-World Data and Real World Evidence to monitor post-market
safety and adverse events and to make regulatory decisions. The health care community is using these data to support coverage decisions
and to develop guidelines and decision support tools for use in clinical practice.
11See
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-use-real-world-data-and-real-world-evidence-support-regulatory-decision-making-drug
AI
with deep-learning capability is also helpful in organizing and translating a vast amount of structured and unstructured data to RWE.12
The human mind can possibly manage 4-5 variables, therefore, AI-enabled data mapping and integration and their normalization into
a common data model according to disease pathway and workflow will likely be useful for both quality management in clinical trials and
generating meaningful insight for human disease by providing a broader perspective based on real-world data.13
Market
Size
The
global real world evidence solutions market is projected to grow from $16.13 billion in 2023 to $36.24 billion by 2030, at a CAGR of
12.3%.14 The drug development and approvals segment accounted for the highest revenue share of around 28.9% in 2020. Real-world
evidence solutions services allow pharmaceutical companies and healthcare providers as well as payers for efficient management of operations
and accelerate the process of drug development and its approval, which fuels market growth. Support from regulatory bodies for using
Real World Evidence solutions and an increase in research and development spending are anticipated to boost the market growth.
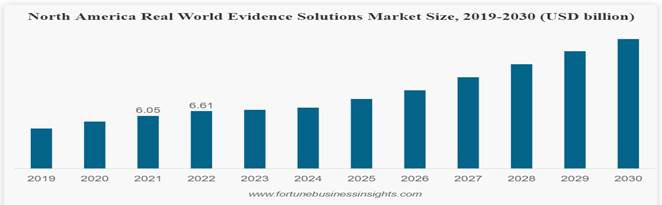
Based
on end user, the global Real World Evidence solutions market is segmented into pharmaceutical, biotechnology, and medical device companies;
healthcare payers; healthcare providers; and other end-users (academic research institutions, patient advocacy groups, regulators, and
health technology assessment agencies). In 2021, the pharmaceutical, biotechnology, and medical device companies segment is estimated
to account for the largest share of 36.5% of the global real-world evidence solutions market. The large share of this segment is primarily
attributed to the increasing importance of Real World Evidence studies in drug development and approvals and the growing need to avoid
costly drug recalls and assess drug performance in real-world settings.
With
the growing need for evidence generated from Real-World Data, the increasing importance of epidemiological data in decision making, and
a shift from volume to value-based care, there has been an increased focus on patient registries, a rise in the adoption of EMR in hospitals,
and exponential growth in mobile health data and social media which have resulted in the generation of huge amounts of medical data.
In 2021, the real-world datasets segment is estimated to account for the larger share of 51.2% of the global real-world evidence solutions
market.15 According to Coherent Market Insights, the global real-World Data market is
estimated to be valued at $1.59 billion in 2023 and is expected to exhibit a CAGR of 14.4% during the forecast period (2023-2030).16
12
Franklin JM, Schneeweiss S. When and how can real world data analyses substitute for randomized controlled trials? Clin Pharmacol
Ther. 2017;102(6):924-33.
13
From real-world data to real-world evidence: An interregional perspective | RAPS, July 30, 2021
14
https://www.fortunebusinessinsights.com/real-world-evidence-solutions-market-107676
15
Meticulous Research® Analysis, which derived its analysis after a detailed assessment of various quantitative and qualitative
factors, such as the historical revenue growth trends of leading players, major market growth drivers, restraints, and challenges, along
with their impacts over the forecast period and relevant macroeconomic and microeconomic indicators.
16
Real-world Data (RWD) Market to reach $4.07 billion by (globenewswire.com)
Our
Long-Term Growth Strategies
Our
long-term growth strategy is anchored on the following key pillars:
|
● |
Increase
Global Reach to Meet Demand: Our strategy is to continue growing our global footprint into areas where we expect high demand
growth in the global real world evidence solutions
market, which is projected to grow from $16.13 billion in 2023 to $36.24 billion by 2030, at a CAGR of 12.3%. There
is a rise in emphasis on evidence-based medicine that relies on Real-World Evidence, which comes from Real-World Data. Market players
in healthcare industries, including regulators, healthcare providers, and payers are becoming more aware of the importance of using
Real-World Data for making informed decisions regarding comparative effectiveness, treatment effectiveness, cost-effectiveness, and
safety. As a result, the demand for real world data solutions is increasing rapidly, which is further driving growth of the market. |
| |
|
|
| |
|
Regulatory
agencies such as the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) are making use of real-world
evidence in regulatory decision making processes. These regulatory authorities have frameworks and guidelines for using Real-World
Evidence and Real-World Data in regulatory submissions, post-market surveillance, and drug approvals. As a result, the demand for
real-world data is rising, which in turn is expected to support growth of the market in the coming future.17 |
| |
|
|
| |
|
The
use of Real-World Evidence derived from Real-World Data demonstrates value and cost-effectiveness of medical devices and drugs for
healthcare technology assessment agencies and payers. With this Real-World Evidence, market access becomes easier and it also enables
reimbursement negotiations. This further facilities the inclusion of new therapies in the coverage of healthcare, which in turn creates
major opportunities in the global market.18 |
| |
|
|
|
● |
Innovate
Our Commercial Approach to Drive Incremental Market Share: We intend to rapidly expand our sales network across the globe,
while simultaneously building out our sales infrastructure. We intend to focus on our target markets, which include (i) Imaging
AI; (ii) medical device companies; and (iii) pharmaceutical companies, as summarized here: |

17
Id.
18
Id.
| |
● |
Enhance
and Refine Our Service Offering: Building on our customer-centric mindset throughout our development, curation and commercial
processes, we plan to continue expanding and improving our service offering. As we continue to expand into additional geographies
globally, we plan to build upon these three pillars: |
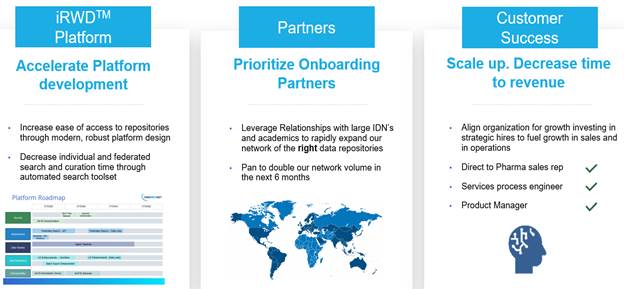
|
● |
Expand
Our Product Offering: We plan to continually evaluate the benefits of expanding our portfolio into other high-growth, high-demand
Real-World Data and Real-World Evidence solutions in the future. |
Corporate
Information
We
were originally incorporated in Delaware on February 8, 2021 under the name “Data Knights Acquisition Corp” as a special
purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase,
reorganization or similar business combination with one or more businesses. On November 7, 2023, we held the Closing of the previously
announced Merger whereby Merger Sub merged with and into OneMedNet Solutions Corporation (formerly named OneMedNet Corporation), with
OneMedNet Solutions Corporation continuing as the surviving entity, which resulted in all of the issued and outstanding capital stock
of OneMedNet Solutions Corporation being exchanged for shares of the Company’s Common Stock upon the terms set forth in the Merger
Agreement.
The
Merger and other transactions that closed on November 7, 2023, pursuant to the Merger Agreement, led to Data Knights changing its name
to “OneMedNet Corporation” and the business of the Company became the business of OneMedNet Solutions Corporation. We are
located at 6385 Old Shady Oak Road, Suite 250, Eden Prairie, MN 55344 and reachable by telephone on 800-918-7189.
The
information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information
contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our Common Stock.
OneMedNet
Corporation a Delaware corporation (the “Company,” “we,” “us,” or “OneMedNet”) together
with its wholly-owned subsidiary OneMedNet Solutions Corporation, a Delaware corporation, founded on October 13, 2009 in the State of
Hawaii and later incorporated in the State of Delaware on November 20, 2015 and its wholly-owned subsidiary, OneMedNet Technologies (Canada)
Inc., incorporated on October 16, 2015 under the provisions of the Business Corporations Act of British Columbia whose functional currency
is the Canadian dollar. All refences in this prospectus to the “Company,” “we,” “us,” or “OneMedNet”
include OneMedNet Solutions Corporation and its wholly-owned subsidiary, OneMedNet Technologies (Canada) Inc., incorporated on October
16, 2015 under the provisions of the Business Corporations Act of British Columbia whose functional currency is the Canadian dollar.
Recent
Developments
Closing
of Business Combination
OneMedNet
Corporation, a Delaware corporation (the “Company,” “we,” “us” or “OneMedNet”) together
with its wholly-owned subsidiary, OneMedNet Solutions Corporation, a Delaware corporation, and its wholly-owned subsidiary, OneMedNet
Technologies (Canada) Inc., incorporated under the provisions of the Business Corporations Act of British Columbia whose functional currency
is the Canadian dollar. All references in this prospectus to the “Company,” “we,” “us,” or “OneMedNet”
include OneMedNet Corporation and both OneMedNet Solutions Corporation and OneMedNet Technologies (Canada) Inc., except that references
to the “Company” “we,” “us,” or “Data Knights” in this Item 7 refer to OneMedNet Corporation
f/k/a Data Knights Acquisition Corp.
We
were originally incorporated in Delaware on February 8, 2021 under the name “Data Knights Acquisition Corp” as a special
purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase,
reorganization or similar business combination with one or more businesses. On May 11, 2021, we consummated an initial public offering.
On
November 7, 2023, following the approval at the special meeting of the shareholders of Data Knights Acquisition Corp., a Delaware corporation
held on October 17, 2023 (the “Special Meeting”), Data Knights Merger Sub, Inc., a Delaware corporation (“Merger Sub”)
and a wholly-owned subsidiary of Data Knights Acquisition Corp., a Delaware corporation (“Data Knights”), consummated a merger
(the “Merger”) with and into OneMedNet Solutions Corporation (formerly named OneMedNet Corporation), a Delaware corporation
(“OneMedNet”) pursuant to an agreement and plan of merger, dated as of April 25, 2022 (the “Merger Agreement”),
by and among Data Knights, Merger Sub, OneMedNet, Data Knights, LLC, a Delaware limited liability company (“Sponsor” or “Purchaser
Representative”) in its capacity as the representative of the stockholders of Data Knights, and Paul Casey in his capacity as the
representative of the stockholders of OneMedNet (“Seller Representative”). Accordingly, the Merger Agreement was adopted,
and the Merger and other transactions contemplated thereby (collectively, the “Business Combination”) were approved and completed.
At
the closing, on November 7, 2023, of the Business Combination pursuant to the Merger Agreement, Merger Sub merged with and into OneMedNet
with OneMedNet surviving the Merger, as a wholly-owned subsidiary of Data Knights, and Data Knights changed its name to “OneMedNet
Corporation.”
The
Business Combination was accounted for as a reverse recapitalization in accordance with U.S. GAAP. Under this method of accounting, Data
Knights was treated as the acquired company and OneMedNet Corporation was treated as the acquirer for financial statement reporting purposes.
At
the closing of the Merger, holders of Data Knights common stock automatically received common stock of OneMedNet, and holders of Data
Knights warrants automatically received warrants of OneMedNet with substantively identical terms. At the Closing of the Business Combination,
all shares of Data Knights owned by the Sponsor (consisting of shares of Class A common stock and shares of Class B common stock), which
we refer to as the founder shares, automatically converted into an equal number of shares of OneMedNet’s common stock, and Private
Placement Warrants held by the Sponsor, automatically converted into warrants to purchase one share of OneMedNet common stock with substantively
identical terms.
On
June 28, 2023, the Company and Data Knights entered into a Securities Purchase Agreement (the “PIPE SPA”) with
certain investors (collectively referred to herein as the “Purchasers”) for PIPE financing in the aggregate original
principal amount of $1,595,744.70 and the purchase price of $1.5 million. Pursuant to the Securities Purchase Agreement, Data
Knights will issue and sell to each of the Purchasers, a new series of senior secured convertible notes (the “PIPE
Notes”), which are convertible into shares of Common Stock at the Purchasers election at a conversion price equal to the lower
of (i) $10.00 per share, and (ii) 92.5% of the lowest volume weighted average trading price for the ten (10) Trading Days
immediately preceding the Conversion Date. The Purchasers’ $1.5 million investment in the PIPE Notes closed and funded
contemporaneous to the Closing of the Business Combination. Effective immediately prior to the Closing, Data Knights issued the PIPE
Notes to the Purchasers pursuant to the private offering rules under the Securities Act of 1933, as amended (the “Securities
Act”).
As
of the Closing, among other holders, public stockholders own 98,178 shares of OneMedNet common stock approximately representing 0.35%
of the outstanding shares of OneMedNet common stock; the Sponsor and its affiliates own approximately 15.1% of the outstanding shares
of OneMedNet common stock (inclusive of shares received upon conversion of the Sponsor’s loan); OneMedNet’s former security
holders own approximately 61.992% of the outstanding shares of OneMedNet common stock from the conversion of their shares; PIPE investors
own 0.46% of the outstanding shares of OneMedNet common stock and former convertible note holders own approximately 16.24% of the outstanding
shares of OneMedNet common stock resulting from the issuance of 5,238,800 shares of Common Stock upon conversion of their notes.
In
addition, at the closing of the Merger, the Company issued an aggregate of 277,778 shares of the Company’s Company Stock at $10.89
per share, for an aggregate value of $3,025,000 (the “Compensation Shares”) to EF Hutton, LLC, division of Benchmark Investments,
LLC (“EF Hutton”) win partial satisfaction of fees due to them in connection with the Merger.
Lock-up
Agreements
Effective
April 25, 2022, in connection with the execution of the Merger Agreement, certain stockholders of OneMedNet and certain of OneMedNet’s
officers and directors (such stockholders, the “Company Holders”) entered into a lock-up agreement (the “Lock-up Agreement”)
pursuant to which the Company Holders will be contractually restricted, during the Lock-up Period (as defined below), from selling or
transferring any of (i) their shares of OneMedNet common stock held immediately following the Closing and (ii) any of their shares of
OneMedNet common stock that result from converting securities held immediately following the Closing (the “Lock-up Shares”).
Effective November 7, 2023, the newly appointed officers and directors of OneMedNet Corporation have entered into a Lock-Up Agreement.
The
“Lock-up Period” means the period commencing at Closing and end the earliest of: (a) six months from the Closing, and (b)
the date after the Closing on which the Purchaser consummates a liquidation, merger, capital stock exchange, reorganization, or other
similar transaction with an unaffiliated third party that results in all of the Purchaser’s stockholders having the right to exchange
their shares of the Purchaser Common Stock for cash, securities, or other property: (i) lend, offer, pledge, hypothecate, encumber, donate,
assign, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right
or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any Restricted Securities, (ii) enter into any swap
or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the Restricted
Securities, or (iii) publicly disclose the intention to do any of the foregoing, whether any such transaction described in clauses (i),
(ii), or (iii) above is to be settled by delivery of Restricted Securities or other securities, in cash or otherwise (any of the foregoing
described in clauses (i), (ii), or (iii), a “Prohibited Transfer”).
In
addition, the Sponsor is subject to a lock-up pursuant to a letter agreement (the “Sponsor Lock-up Agreement”), entered into
on May 6, 2021, at the time of the IPO (as defined below), among Data Knights, the Sponsor and each of the individuals who were a member
of Data Knights’ board of directors and/or management team (each, an “Insider” and collectively, the “Insiders”),
who agreed that it, he or she shall not transfer any founder shares which means the 2,875,000 shares of Data Knights Class B common stock,
par value $0.0001 per share, initially held by the Sponsor, or shares of OneMedNet’s Common Stock issuable upon conversion thereof)
until the earlier of (A) six months after the date of Data Knights’ initial Business Combination or (B) subsequent to the initial
Business Combination, (x) if the reported last sale price of the Common Stock equals or exceeds $12.00 per share (as adjusted for stock
splits, stock dividends, right issuances, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading
day period commencing at least 150 days after the Company’s initial Business Combination, or (y) the date on which the Company
completes a liquidation, merger, capital stock exchange, reorganization or other similar transaction that results in all of our stockholders
having the right to exchange their shares of common stock for cash, securities or other property. Further, the Sponsor and each of the
Insiders agreed further in the Sponsor Lock-Up Agreement that he, she or it shall not transfer any private placement units, the private
placement shares, the private placement warrants or shares of Common Stock issued or issuable upon the exercise of the private placement
warrants, until 30 days after the completion of the initial Business Combination.
Registration
Rights Agreements
At
the Closing of the Business Combination and funding of the PIPE, the PIPE Investors each executed a PIPE Note and a PIPE Warrant in the
amount corresponding to each PIPE Investor’s investment amount and in accordance with the terms set forth in the PIPE SPA as well
as a registration rights agreement (the “PIPE Registration Rights Agreement”). We are registering the offer and sale of these
securities to satisfy the registration rights we have granted in the PIPE Registration Rights Agreement.
At the Closing of the Business Combination, OneMedNet,
Data Knights and the Sponsor entered into a registration rights agreement (the “Registration Rights Agreement”), pursuant
to which, among other things, the Company is obligated to file a registration statement to register the resale of certain securities
of the Company held by the holders, as defined in the Registration Rights Agreement and the Sponsor. The Registration Rights Agreement
also provides the holders and the Sponsor with “piggy-back” registration rights, subject to certain requirements and customary
conditions.
Voting
Agreement and Sponsor Support Agreement
In
connection with entry into the Merger Agreement, the Company entered into voting agreements (the “Voting Agreements”) with
certain stockholders of OneMedNet representing approximately 55% of the outstanding voting power of OneMedNet’s equity securities
(the “OneMedNet Stockholders”) pursuant to which OneMedNet Stockholders agreed to vote their securities in favor of the approval
of the Merger Agreement and the Business Combination, be bound by certain covenants and agreements related to the Business Combination
and to take other customary actions to cause the Business Combination to occur.
In
connection with entry into the Merger Agreement, the Company, the Sponsor and OneMedNet entered into a sponsor support agreement (the
“Sponsor Support Agreement”) pursuant to which the Sponsor agreed to vote its Data Knights securities in favor of the approval
of the Merger Agreement and the Business Combination and to take other customary actions to cause the Business Combination to occur.
Executive
Employment Agreements
In
connection with the Closing of the Business Combination, the Company has entered into employment agreements (the “Employment Agreements”)
with executive officers: Aaron Green (President), Lisa Embree (Chief Financial Officer), and Paul Casey (Chief Executive Officer). The
Employment Agreements provide for at-will employment that may be terminated by the Company with or without cause, by the executive with
or without good reason, or mutually terminated by the parties.
The
Employment Agreement for Mr. Green provides for $350,000 annual salary, eligibility to receive an annual cash performance bonus of $175,000
upon his achievement of the performance goals set by the Company’s CEO and Board of Directors, and eligibility to receive 600,000
of the Company’s outstanding shares at closing, as part of the Company’s Restricted Stock Unit Plan, subject to the approval
of the Company’s Board of Directors. In the event that his employment is terminated by the Company without Cause (as defined in
the Employment Agreement), or is terminated by Mr. Green for Good Reason (as defined in the Employment Agreement), after six months of
employment, and he signs and does not revoke a standard release of claims with the Company in a form reasonably satisfactory to the Company’s
Board of Directors (a “Release”), which Release becomes irrevocable no later than sixty (60) days (the “Release Deadline”),
after the date of his termination of employment (the “Termination Date”) he will be entitled to the following severance payment,
as follows: (a) if the Termination Date is after six (6) months’ of employment, but before he has completed 12 months’ of
employment, he will receive three (3) months’ salary; and (b) if the Termination Date is after 12 months’ employment he will
receive six (6) months’ salary. If the Release does not become effective and irrevocable by the Release Deadline, he will forfeit
any right to severance.
The
Employment Agreement for Ms. Embree provides for $225,000 annual salary, eligibility to receive an annual cash performance bonus of twenty-five
percent (25%) of her annual salary upon her achievement of the performance goals set by the Company’s CEO and Board of Directors,
and eligibility to receive 260,000 of the Company’s outstanding shares, as part of the Company’s Restricted Stock Unit Plan,
subject to the approval of the Company’s Board of Directors. In the event that her employment with the Company is terminated by
the Company without Cause (as defined in the Employment Agreement) or is terminated by Ms. Embree for Good Reason (as defined in the
Employment Agreement) she will receive six (6) months’ salary as a Severance Payment.
The
Employment Agreement for Mr. Casey provides for $144,000 annual salary, eligible to receive 147,000 shares of stock upon the successful
fundraising of an amount equal to or greater than $5,000,000 and, as part of the Company’s Restricted Stock Unit Plan, further
equity will be rewarded to Mr. Casey subject to the approval of the Company’s Board of Directors. In the event that his employment
with the Company is terminated by the Company without Cause (as defined in the Employment Agreement) or is terminated by Mr. Casey for
Good Reason (as defined in the Employment Agreement) he will receive six (6) months’ salary as a Severance Payment.
Stock
Purchase Agreement
On
June 28, 2023, the Company and Data Knights entered into a Securities Purchase Agreement (the “PIPE SPA”) with certain
investors (collectively referred to herein as the “Purchasers”) for PIPE financing in the aggregate original principal amount
of $1,595,744.70 and the purchase price of $1.5 million. Pursuant to the Securities Purchase Agreement, Data Knights will issue and sell
to each of the Purchasers, a new series of senior secured convertible notes (the “PIPE Notes”), which are convertible into
shares of Common Stock at the Purchasers election at a conversion price equal to the lower of (i) $10.00 per share, and (ii) 92.5% of
the lowest volume weighted average trading price for the ten (10) Trading Days immediately preceding the Conversion Date. The Purchasers’
$1.5 million investment in the PIPE Notes closed and funded contemporaneous to the Closing of the Business Combination. Effective immediately
prior to the Closing, Data Knights issued the PIPE Notes to the Purchasers pursuant to the private offering rules under the Securities
Act of 1933, as amended (the “Securities Act”).
Government
Regulation
Many
aspects of our businesses are regulated by federal and state laws, rules and regulations. Accordingly, we maintain a robust compliance
program aimed at ensuring we operate our business in compliance with all existing legal requirements material to the operation of our
businesses. There are, however, occasionally uncertainties involving the application of various legal requirements, the violation of
which could result in, among other things, fines or other sanctions. See “Risk Factors” for additional detail.
Regulation
of Patient Information. Our information management services relate to the processing of information regarding patient diagnosis
and treatment of disease and are, therefore, subject to substantial governmental regulation. In addition, the confidentiality of patient-specific
information and the circumstances under which such patient-specific records may be released for inclusion in our databases or used in
other aspects of our business is heavily regulated. Federal, state and foreign governments are contemplating or have proposed or adopted
additional legislation governing the possession, use and dissemination of personal data, such as personal health information and personal
financial data, as well as security breach notification rules for loss or theft of such data. Additional legislation or regulation of
this type might, among other things, require us to implement additional security measures and processes or bring within the legislation
or regulation deidentified health or other data, each of which may require substantial expenditures or limit our ability to offer some
of our services.
In
particular, personal health information is recognized as a special, sensitive category of personal information, subject to additional
mandatory protections. Violations of data protection regulations are subject to administrative penalties, civil money penalties and criminal
prosecution, including corporate fines and personal liability.
Data
Privacy
Patient
health information is among the most sensitive of personal information, and it is critically important that information about an individual’s
healthcare is properly protected from inappropriate access, use and disclosure. Real world evidence — information
that allows us to examine actual practices and outcomes — is essential to increase access to care, improve outcomes,
and lower costs.
OneMedNet
uses a wide variety of privacy-enhancing technologies and safeguards to protect individual privacy while generating and analyzing information
on a scale that helps healthcare stakeholders identify disease patterns and correlate with the precise treatment path and therapy needed
for better outcomes. We employ a wide variety of methods to manage privacy requirements, including:
| |
● |
governance,
frameworks, models and training to promote good decision making and accountability; |
| |
|
|
| |
● |
a
layered approach to privacy and security management to avoid a single point of failure; |
| |
|
|
| |
● |
ongoing
evaluation of privacy and security practices to promote continuous improvement; |
| |
|
|
| |
● |
use
of technical, administrative, physical and organizational safeguards and controls; |
| |
|
|
| |
● |
collaboration
with data suppliers and trusted third parties for our syndicated market research and analytics offerings to remove identifiable information
or employ effective encryption or other techniques to render information non-identified before data is delivered to us; and |
| |
|
|
| |
● |
work
with leading researchers, policy makers, thought leaders and others in a variety of fields relevant to the application of effective
privacy and security practices, including statistical, epidemiological and cryptographic sciences, legal, information security and
compliance, and privacy. |
We
have relied on expertise in the industry with de-identifying data. Our capabilities allow us to render data non-identified while still
maintaining data utility, thus protecting privacy while still advancing innovation. Not only do we make use of de-identification techniques
with respect to the data we hold, but we also share our expertise in this area with policymakers, regulators and others to help them
understand de-identification methodologies and practical considerations to avoid re-identification risk. We operate in more than 100
countries around the world, many of which have data protection and privacy laws and regulations based on similar core principles (e.g.,
openness, accountability, security safeguards, etc.). We apply those principles globally and augment our practices to address local
laws, contractual obligations and other data privacy requirements.
Our
Compliance team, led by our Chief Compliance Officer, is comprised of privacy professionals and privacy law experts who drive our strategy
and develop and manage our policies and standards. The Compliance team provides subject matter expertise related to the proper management
of all data types. In addition, our Compliance team liaises with our Legal, IT, Information Security and other teams so that privacy
requirements are addressed in technology, contracting, offerings and other business activities.
The
OneMedNet Privacy Policy (the “Privacy Policy”) is our foundational privacy policy. It explains how, when applicable, we
collect, hold, use and disclose personal information, including that of our personnel, consumers, healthcare professionals, patients,
medical research subjects, clinical investigators, customers, suppliers, vendors, business partners and investors.
Cybersecurity
We
employ an array of data security technologies, processes, and methods across our infrastructure to protect systems and sensitive information
from unauthorized access. OneMedNet maintains comprehensive identity and access management practices (e.g., roles and access privileges
for each user; multi-factor authentication, privileged user accounts, single sign-on, user lifecycle management) and employs a variety
of security information and event management tools. We developed, maintain and utilize a global integrated information security framework
to guide our practices, based on relevant industry frameworks and laws, including, but not limited to NIST, GxP, HITRUST, the ISO 27000
family, COBIT, GDPR, and HIPAA.
The
framework consists of policies, standards, procedures, work Instructions and documentation. Information is classified into four categories
to help individuals apply the right level of controls and safeguards to information, applications and systems. Our cybersecurity program
focuses on all areas of our business, including cloud-based environments, data centers, devices used by employees and contractors, facilities,
networks, applications, vendors, disaster recovery / business continuity and controls and safeguards enabled through business processes
and tools. We continuously monitor for threats and unauthorized access.
We
draw on the knowledge and insight of external cybersecurity experts and vendors, and internally employ dedicated, certified, cybersecurity
staff, such as but not limited to, CISSP, CISM, CISA, CSSP or other equivalent certifications, that leverage an array of third-party
tools to secure OneMedNet information infrastructure and protect systems and information from unauthorized access. Non-technical safeguards
also play an important role in our cybersecurity program. We provide various training programs and tools to employees so they can avoid
risky practices and help us promptly identify potential or actual issues. We also have global incident response procedures, global service
tools to log incidents and issues for investigation, and an ethics line to report concerns and follow-up on matters already reported.
The Compliance team, led by our Chief Compliance Officer, develops and implements our strategy, as well as monitors systems and devices
for risks and threats.
Regulatory
Quality Compliance (FDA 21 CFR Part 11)
OneMedNet
provides high-quality, de-identified, regulatory-grade imaging and clinical data; as such OneMedNet adheres to all applicable local and
Federal regulatory quality requirements, including but not limited to FDA 21 CFR Part 11. OneMedNet maintains a rigorous and ongoing
internal quality management system to enable the organization to produce the highest quality regulatory compliant clinical data for our
clients and consumers. This program includes:
| |
● |
Ongoing
internal audits, policy reviews, and procedure testing to ensure validation, audit trails, legacy systems, and record handling and
retention adhere to the latest regulatory guidelines and best practices. |
| |
|
|
| |
● |
Regular
third-party or client initiated external audits to assess the compliance of OneMedNet to ensure operations are in accordance with,
but not limited to the applicable regulations, standards, policies, and standard operation procedures. |
Organizational
Structure
The
following is a current organizational chart of our Company:

Employees
Prior
to the closing of the Business Combination, we had four
officers: Paul Casey, Chief Executive Officer and Director; Dr. Jeffrey Yu, Chairman of the Board and Chief Medical Officer; Aaron Green,
President; and Lisa Embree, Vice President — Finance and Chief Financial Officer; two executives, Brad Overby and Doug Arent; a
director of marketing, Del Coufal, and 18 employees. Contemporaneous to the closing of the Business
Combination, we appointed two additional executives, Betty Cosgrove as Area Vice President, and Steven
Chartier, as Director Product Management, Head of Data. Presently, we have 20 employees.
Facilities
Prior
to the closing of the Business Combination, the Company’s executive offices were located at Unit G6, Frome Business Park, Manor
Road, Frome, United Kingdom, BA11 4FN and its telephone number was +44 203 833 4000. The Company agreed to pay ARC Group Ltd., an affiliate
of the Sponsor, up to an amount of $10,000 per month for office space, secretarial and administrative support. For the nine months ended
September 30, 2023 and 2022, we had incurred $60,000 in fees under this agreement, respectively. Upon completion of our Business Combination,
the Company ceased paying these monthly fees.
After
the closing of the Business Combination, our headquarters is located at 6385 Old Shady Oak Road, Suite 250, Eden Prairie, MN 55344 and
our telephone number is (800) 918-7189, where we lease and occupy our office space with an aggregate floor area of approximately 67 square
feet from unrelated third parties under operating lease agreements. We consider our current office space adequate for our current operations
and needs and that, should it be needed, suitable additional or alternative space will be available to accommodate our operations.
Implications
of Being an Emerging Growth Company
As
a company with less than $1.235 billion in revenues during our last fiscal year, we qualify as an emerging growth company as defined
in the Jumpstart Our Business Startups Act (“JOBS Act”) enacted in 2012. As an emerging growth company, we expect to take
advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not
limited to:
| |
● |
being
permitted to present only two years of audited financial statements, in addition to any required unaudited interim financial statements,
with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
disclosure in this prospectus; |
| |
|
|
| |
● |
not
being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended (“Sarbanes-Oxley
Act”); |
| |
|
|
| |
● |
reduced
disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and
|
| |
|
|
| |
● |
exemptions
from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute
payments not previously approved. |
THE
OFFERING
| Common
Stock offered by us |
[*] shares based on the sale of our Common Stock at an assumed combined public offering price of $ [*] per share of Common Stock and accompanying warrant, which is the last reported sale price of our Common Stock on The Nasdaq Capital Market on December __, 2023, and no sale of any pre-funded warrants.
In
addition, an aggregate of 734,043 outstanding shares of Common Stock, which include 95,745
warrant shares pursuant to the PIPE SPA and each of the PIPE Warrants, and a maximum of up
to 638,298 shares of our Common Stock issuable upon the conversion of the PIPE Notes by the
selling securityholders identified in this prospectus, at a conversion price equal to the
lower of (i) $10.00 per share, and (ii) 92.5% of the lowest volume weighted average trading
price for the ten (10) Trading Days immediately preceding the Conversion Date, subject to
a minimum of $2.50, which would convert to a maximum of 638,298 PIPE Notes shares of our
Common Stock.
|
| |
|
| Pre-funded
warrants offered by us |
We
are also offering to certain purchasers whose purchase of shares of Common Stock in this offering would otherwise result in the purchaser,
together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser,
9.99%) of our outstanding Common Stock immediately following the consummation of this offering, the opportunity to purchase, if such
purchasers so choose, pre-funded warrants to purchase shares of Common Stock, in lieu of shares of Common Stock that would otherwise
result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding
Common Stock. Each pre-funded warrant will be exercisable for one share of our Common Stock. The purchase price of each pre-funded
warrant and accompanying warrant will equal the price at which the share of Common Stock and accompanying warrant are being sold
to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will be $0.0001 per share. |
| |
|
| |
The
pre-funded warrants will be exercisable immediately and may be exercised at any time until all of the pre-funded warrants are exercised
in full. This offering also relates to the shares of Common Stock issuable upon exercise of any pre-funded warrants sold in this
offering. For each pre-funded warrant we sell, the number of shares of Common Stock we are offering will be decreased on a one-for-one
basis. Because we will issue a warrant for each share of our Common Stock and for each pre-funded warrant to purchase one share of
our Common Stock sold in this offering, the number of warrants sold in this offering will not change as a result of a change in the
mix of the shares of our Common Stock and pre-funded warrants sold. |
| |
|
| Warrants
offered by us |
Warrants
to purchase an aggregate of [*] shares of our Common Stock based on the sale of our Common Stock at an assumed combined public offering
price of $ [*] per share of Common Stock and accompanying warrant, which is the last reported sale price of our common stock on The
Nasdaq Capital Market on December [*], 2023. Each share of our Common Stock and each pre-funded warrant to purchase one share
of our Common Stock is being sold together with a warrant to purchase one share of our common stock. |
| |
Each
warrant will have an exercise price of $ [*] per share (representing 100% of the price at which a share of Common Stock and accompanying
warrant are sold to the public in this offering), will be immediately exercisable and will expire on the fifth anniversary of the
original issuance date. Each Warrant is exercisable for one share of Common Stock, subject to adjustment in the event of stock dividends,
stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock. |
| |
|
| |
On
or after the thirty (30) day anniversary of the date of this prospectus, a holder of the Warrants may also provide notice and elect
an “alternative cashless exercise” pursuant to which they would receive an aggregate number of shares equal to the product
of (x) the aggregate number of shares of the Company’s common stock that would be issuable upon a cash exercise and (y) [*].
|
| |
The
shares of Common Stock and pre-funded warrants, and the accompanying warrants, as the case may be, can only be purchased together
in this offering but will be issued separately and will be immediately separable upon issuance. This prospectus also relates to the
offering of the shares of common stock issuable upon exercise of the warrants. |
| |
|
| Warrants Offered by the Selling Securityholders Hereunder |
95,745 PIPE
Warrants exercisable for one share of Common Stock by the selling security holders at an exercise price of $10.00 per share, subject
to adjustment as set forth in the PIPE Warrants. |
| |
|
| Shares Offered by the Selling Securityholders Pursuant to the PIPE Notes |
638,298
shares of Common Stock that are the PIPE Note shares issuable
upon the conversion of the PIPE Notes by the selling securityholders identified in
this prospectus, at a conversion price equal to the lower
of (i) $10.00 per share, and (ii) 92.5% of the lowest volume weighted average trading price
for the ten (10) Trading Days immediately preceding the Conversion Date, subject to a minimum
of $2.50.
|
| |
|
| Shares
of Common Stock outstanding immediately prior to this offering(1) |
23,572,232
shares |
| |
|
| Common
Stock outstanding immediately after this offering(1) |
[*] shares (assuming no exercise of the warrants and pre-funded warrants issued in connection with this offering).
638,298 shares of Common Stock that are the PIPE
Note shares issuable upon the conversion of the PIPE Notes.
95,745 shares of Common Stock that
are the PIPE Warrants shares assuming an exercise price of $10.00 per share.
|
| |
|
| Use
of proceeds |
We
estimate that the net proceeds from this offering will be approximately $[ ] million at an assumed public offering price of $[*] per share and accompanying
warrant, which was the closing price of our Class A common stock on The Nasdaq Capital Market on December [*], 2023, after
deducting the underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds
from this offering for (i) sales and marketing, (ii) operational costs, (iii) product development, (iv) manufacturing expansion and
(v) working capital and other general corporate purposes. We may also use a portion of the net proceeds to in-license, acquire or
invest in complementary businesses or products, however, we have no current commitments or obligations to do so.
We will not receive any proceeds from the sale of our securities offered by the selling securityholders under
this prospectus (the “PIPE Securities”). We will receive up to an aggregate of approximately $957,450
from the exercise of all PIPE Warrants, assuming the exercise in full of all of the PIPE Warrants for cash. We expect to use the net
proceeds from the exercise of the PIPE Warrants for general corporate purposes. See the section titled “Use of Proceeds”
appearing elsewhere in this prospectus for more information.
|
| |
|
| Risk
Factors |
See
“Risk Factors” on page 31 and other information included in this prospectus for a discussion of factors to consider carefully
before deciding to invest in shares of our Common Stock. |
| |
|
| Nasdaq
Capital Market symbol |
Shares
of our Common Stock are listed on The Nasdaq Capital Market under the symbol “ONMD.” There is no established
trading market for the warrants, and we do not expect a trading market to develop. We do not intend to list the warrants on any securities
exchange or other trading market. Without a trading market, the liquidity of the warrants will be extremely limited. |
| |
|
| Lock-Up Restrictions |
Of the shares of Common Stock that may be offered or
sold by selling securityholders identified in this prospectus, pursuant
to the PIPE Notes and PIPE Warrants, 612,312 of those shares of Common Stock are subject to certain lock-up restrictions, which include
79,788 shares of Common Stock issuable upon the exercise of the PIPE Warrants and 532,524 shares of Common Stock issuable upon the
conversion of the PIPE Notes. |
(1)
The number of shares of Common Stock that will be outstanding after this offering is based on approximately 23,572,232 shares
of Common Stock outstanding as of December [12], 2023, and excludes 1,685,881 shares of Common Stock reserved for
future issuance under our 2022 Equity Incentive Plan.
RISK
FACTORS
An
investment in our securities involves a high degree of risk. This prospectus contains a discussion of the risks applicable to an investment
in our securities. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not
presently known to us or that we currently deem immaterial may also affect our operations. The occurrence of any of these known or unknown
risks might cause you to lose all or part of your investment in the offered securities. We may not be successful in preventing
the material adverse effects that any of the following risks and uncertainties may cause. You could lose all or a significant portion
of your investment due to any of these risks and uncertainties.
You
should carefully consider the following risks, as well as the other information contained in this prospectus, including our historical
financial statements and related notes included elsewhere in this prospectus before you decide to purchase our securities. Any one of
these risks and uncertainties has the potential to cause material adverse effects on our business, prospects, financial condition and
operating results which could cause actual results to differ materially from any forward-looking statements expressed by us and a significant
decrease in the value of our Common Stock shares and warrants. Refer to “Cautionary Statement Regarding Forward-Looking Statements.”
Risks
Related to this Offering and Our Common Stock
Our
stock price may be volatile, and purchasers of our Common Stock could incur substantial losses.
The
stock market in general has experienced significant price and volume fluctuations that have often been unrelated or disproportionate
to operating performance of individual companies, particularly following a public offering of a company with a small public float. There
is the potential for rapid and substantial price volatility of our Common Stock following this offering. These broad market factors may
seriously harm the market price of our Common Stock, regardless of our actual or expected operating performance and financial condition
or prospects, which may make it difficult for investors to assess the rapidly changing value of our Common Stock.
We
are currently listed on The Nasdaq Capital Market. If we are unable to maintain listing of our securities on Nasdaq or any stock exchange,
our stock price could be adversely affected and the liquidity of our stock and our ability to obtain financing could be impaired and
it may be more difficult for our stockholders to sell their securities.
Although
our Common Stock is currently listed on The Nasdaq Capital Market, we may not be able to continue to meet the exchange’s minimum
listing requirements or those of any other national exchange. If we are unable to maintain listing on Nasdaq or if a liquid market for
our Common Stock does not develop or is sustained, our Common Stock may remain thinly traded.
The
listing rules of Nasdaq require listing issuers to comply with certain standards in order to remain listed on its exchange. If, for any
reason, we should fail to maintain compliance with these listing standards and Nasdaq should delist our securities from trading on its
exchange and we are unable to obtain listing on another national securities exchange, a reduction in some or all of the following may
occur, each of which could have a material adverse effect on our stockholders:
| |
● |
the
liquidity of our Common Stock; |
| |
|
|
| |
● |
the
market price of our Common Stock; |
| |
|
|
| |
● |
our
ability to obtain financing for the continuation of our operations; |
| |
|
|
| |
● |
the
number of institutional and general investors that will consider investing in our Common Stock; |
| |
|
|
| |
● |
the
number of investors in general that will consider investing in our Common Stock; |
| |
|
|
| |
● |
the
number of market makers in our Common Stock; |
| |
|
|
| |
● |
the
availability of information concerning the trading prices and volume of our Common Stock; and |
| |
|
|
| |
● |
the
number of broker-dealers willing to execute trades in shares of our Common Stock. |
Our
principal stockholders will continue to have significant influence over the election of our board of directors and approval of any significant
corporate actions, including any sale of the company.
Our
founders, executive officers, directors, and other principal stockholders, in the aggregate, beneficially own a majority of our outstanding
stock. These stockholders currently have, and likely will continue to have, significant influence with respect to the election of our
board of directors and approval or disapproval of all significant corporate actions. The concentrated voting power of these stockholders
could have the effect of delaying or preventing an acquisition of the company or another significant corporate transaction.
We
have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our
management will have broad discretion in the application of the net proceeds from this offering, including for any of the currently intended
purposes described in the section entitled “Use of Proceeds.” Because of the number and variability of factors that will
determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use.
Our management may not apply our cash from this offering in ways that ultimately increase the value of any investment in our securities
or enhance stockholder value. The failure by our management to apply these funds effectively could harm our business. Pending their use,
we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may
not yield a favorable return to our stockholders. If we do not invest or apply our cash in ways that enhance stockholder value, we may
fail to achieve expected financial results, which may result in a decline in the price of our shares of Common Stock, and, therefore,
may negatively impact our ability to raise capital, invest in or expand our business, acquire additional products or licenses, commercialize
our product, or continue our operations.
We
could be subject to securities class action litigation.
In
the past, securities class action litigation has often been brought against companies following a decline in the market price of their
securities. In 2020, 22% of securities class action litigation filings
were against defendants in the health technology and services sector, which accounted for 22% of new filings. If we face such
litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our
business.
If
securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the market
price for the shares and trading volume could decline.
The
trading market for our Common Stock will depend in part on the research and reports that securities or industry analysts publish about
us or our business. If research analysts do not establish and maintain adequate research coverage or if one or more of the analysts who
covers us downgrades our Common Stock or publishes inaccurate or unfavorable research about our business, the market price for our Common
Stock would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly,
we could lose visibility in the financial markets, which, in turn, could cause the market price or trading volume for our common stock
to decline.
We
do not expect to pay dividends in the foreseeable future, and you must rely on price appreciation of your shares of Common Stock for
return on your investment.
We
have paid no cash dividends on any class of our stock to date, and we do not anticipate paying cash dividends in the near term. For the
foreseeable future, we intend to retain any earnings to finance the development and expansion of our business, and we do not anticipate
paying any cash dividends on our stock. Accordingly, investors must be prepared to rely on sales of their shares after price appreciation
to earn an investment return, which may never occur. Investors seeking cash dividends should not purchase our shares. Any determination
to pay dividends in the future will be made at the discretion of our board of directors and will depend on our results of operations,
financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board deems relevant.
As
the public offering price is substantially higher than our net tangible book value per share, you will experience immediate and substantial
dilution.
If
you purchase Common Stock in this offering, you will pay more for your shares of Common Stock than the amount paid by our existing stockholders
for their shares on a per share basis. As a result, you will experience immediate and substantial dilution in net tangible book value
per share in relation to the price that you paid for your shares. We expect the dilution as a result of the offering to be $_____ per
share to new investors purchasing our shares of Common Stock in this offering. In addition, you will experience further dilution to the
extent that our shares are issued upon the exercise of any warrants or exercise of stock options under any stock incentive plans. See
“Dilution” for a more complete description of how the value of your investment in our shares will be diluted upon completion
of this offering.
Future
sales of substantial amounts of our Common Stock or securities convertible into or exchangeable or exercisable for shares of Common Stock,
either by us or by our existing stockholders, or the possibility that such sales could occur, could adversely affect the market price
of our Common Stock.
Future
sales in the public market of shares of our Common Stock or securities convertible into or exchangeable or exercisable for shares of
Common Stock, shares held by our existing stockholders or shares issued upon exercise of our outstanding stock options or warrants, or
the perception by the market that these sales could occur, could lower the market price of our Common Stock or make it difficult for
us to raise additional capital.
There
is no public market for the pre-funded warrants or warrants being offered in this offering.
There
is no established public trading market for the pre-funded warrants or warrants being offered in this offering, and we do not expect
a market to develop. In addition, we do not intend to apply to list the pre-funded warrants or warrants on any securities exchange or
nationally recognized trading system, including The Nasdaq Capital Market. Without an active market, the liquidity of the pre-funded
warrants and warrants will be limited.
Holders
of pre-funded warrants and warrants purchased in this offering will have no rights as common stockholders until such holders exercise
such warrants and acquire our Common Stock.
Until
holders of pre-funded warrants or warrants acquire shares of our Common Stock upon exercise of such warrants, holders of pre-funded warrants
or warrants will have no rights with respect to the shares of our Common Stock underlying such warrants. Upon exercise of the pre-funded
warrants or warrants, the holders will be entitled to exercise the rights of a Common Stockholder only as to matters for which the record
date occurs after the exercise date.
We
will incur increased costs as a public company, and our management will be required to devote substantial time to new compliance initiatives
and corporate governance practices.
As
a public company, and particularly after we no longer qualify as an emerging growth company, we will incur significant legal, accounting,
and other expenses that we did not incur previously. The Sarbanes-Oxley Act of 2002 (“SOX”), the Dodd-Frank Wall Street Reform
and Consumer Protection Act, the listing requirements of Nasdaq, and other applicable securities rules and regulations impose various
requirements on U.S. reporting public companies, including the establishment and maintenance of effective disclosure and financial controls
and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance
initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities
more time-consuming and costly. For example, we expect that these rules and regulations may make it more expensive for us to obtain director
and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified senior management
personnel or members for our board of directors. In addition, these rules and regulations are often subject to varying interpretations,
and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies.
This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure
and governance practices. Pursuant to Section 404 of SOX (“Section 404”), we will be required to furnish a report by our
senior management on our internal control over financial reporting.
While
we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting
issued by our independent registered public accounting firm. To prepare for eventual compliance with Section 404, once we no longer qualify
as an emerging growth company, we will be engaged in a process to document and evaluate our internal control over financial reporting,
which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside
consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue
steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement
a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that
we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective
as required by Section 404.
We
are an “emerging growth company,” and the reduced reporting requirements applicable to emerging growth companies may make
our common stock less attractive to investors.
We
are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act (“the JOBS Act”).
For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements
that are applicable to other public companies that are not emerging growth companies, including exemption from compliance with the
auditor attestation requirements of Section 404, reduced disclosure obligations regarding executive compensation and exemptions from
the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute
payments not previously approved. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year
(a) following the fifth anniversary of the closing of our initial public offering, (b) in which we have total annual gross revenue
of at least $1.235 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common
stock held by non-affiliates exceeds $700 million as of the end of our prior second fiscal quarter, and (2) the date on which we
have issued more than $1 billion in non-convertible debt during the prior three-year period.
In
addition, under the JOBS Act, emerging growth companies may delay adopting new or revised accounting standards until such time as those
standards apply to private companies. We may elect not to avail ourselves of this exemption from new or revised accounting standards
and, therefore, may be subject to the same new or revised accounting standards as other public companies that are not emerging growth
companies. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some
investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our share
price may be more volatile.
Anti-takeover
provisions contained in our certificate of incorporation and bylaws as well as provisions of Delaware law, could impair a takeover attempt.
Our
certificate of incorporation, bylaws and Delaware law contain provisions which could have the effect of rendering more difficult, delaying
or preventing an acquisition deemed undesirable by our board of directors. Our corporate governance documents include provisions:
| |
● |
authorizing
“blank check” preferred stock, which could be issued by our board of directors without stockholder approval and may contain
voting, liquidation, dividend, and other rights superior to our common stock; |
| |
|
|
| |
● |
limiting
the liability of, and providing indemnification to, our directors and officers; |
| |
|
|
| |
● |
limiting
the ability of our stockholders to call and bring business before special meetings; |
| |
|
|
| |
● |
requiring
advance notice of stockholder proposals for business to be conducted at meetings of our stockholders and for nominations of candidates
for election to our board of directors; |
| |
|
|
| |
● |
controlling
the procedures for the conduct and scheduling of board of directors and stockholder meetings; and |
| |
|
|
| |
● |
providing
our board of directors with the express power to postpone previously scheduled annual meetings and to cancel previously scheduled
special meetings. |
These
provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. As a Delaware
corporation, we are also subject to provisions of Delaware law, including Section 203 of the Delaware General Corporation law, which
prevents some stockholders holding more than 15% of our outstanding common stock from engaging in certain business combinations without
approval of the holders of substantially all of our outstanding common stock.
Any
provision of our certificate of incorporation, bylaws or Delaware law that has the effect of delaying or deterring a change in control
could limit the opportunity for our stockholders to receive a premium for their shares of our Common Stock and could also affect the
price that some investors are willing to pay for our Common Stock.
Our
amended and restated certificate of incorporation, as amended, designates the Court of Chancery of the State of Delaware as the sole
and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’
ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or other employees.
Our
certificate of incorporation requires that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery
of the State of Delaware will, to the fullest extent permitted by law, be the sole and exclusive forum for each of the following:
| |
● |
any
derivative action or proceeding brought on our behalf; |
| |
|
|
| |
● |
any
action asserting a claim for breach of any fiduciary duty owed by any director, officer, or other employee of ours to the Company
or our stockholders, creditors or other constituents; |
| |
|
|
| |
● |
any
action asserting a claim against us or any director or officer of ours arising pursuant to, or a claim against us or any of our directors
or officers, with respect to the interpretation or application of any provision of, the DGCL, our certificate of incorporation or
bylaws; or |
| |
|
|
| |
● |
any
action asserting a claim governed by the internal affairs doctrine; |
provided,
that, if and only if the Court of Chancery of the State of Delaware dismisses any of the foregoing actions for lack of subject matter
jurisdiction, any such action or actions may be brought in another state court sitting in the State of Delaware.
The
exclusive forum provision is limited to the extent permitted by law, and it will not apply to claims arising under the Securities Exchange
Act of 1934, as amended (the “Exchange Act”), the Securities Act of 1933, as amended (the “Securities Act”),
or for any other federal securities laws which provide for exclusive federal jurisdiction.
Our
Amended and Restated Certificate of Incorporation, as amended, provides that unless we consent in writing to the selection of an alternative
forum, the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting
a cause of action arising under the Securities Act or the Securities Exchange Act of 1934, as amended. Any person or entity purchasing
or otherwise acquiring any interest in shares of our capital stock are deemed to have notice of and consented to this provision.
Furthermore,
Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly,
both state and federal courts have jurisdiction to entertain such claims. To prevent having to litigate claims in multiple jurisdictions
and the threat of inconsistent or contrary rulings by different courts, among other considerations, our second amended and restated certificate
of incorporation provides that the federal district courts of the United States of America will be the exclusive forum for resolving
any complaint asserting a cause of action arising under the Securities Act. While the Delaware courts have determined that such choice
of forum provisions are facially valid, a stockholder may nevertheless seek to bring such a claim arising under the Securities Act against
us, our directors, officers, or other employees in a venue other than in the federal district courts of the United States of America.
In such instance, we would expect to vigorously assert the validity and enforceability of the exclusive forum provisions of our second
amended and restated certificate of incorporation.
Although
we believe this provision benefits us by providing increased consistency in the application of Delaware law in the types of lawsuits
to which it applies, this provision may limit or discourage a stockholder’s ability to bring a claim in a judicial forum that it
finds favorable for disputes with us or our directors, officers, or other employees, which may discourage such lawsuits against us and
our directors, officers and other employees. Alternatively, if a court were to find the choice of forum provision contained in our certificate
of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action
in other jurisdictions, which could adversely affect our business and financial condition.
We
note that there is uncertainty as to whether a court would enforce the provision and that investors cannot waive compliance with the
federal securities laws and the rules and regulations thereunder. Although we believe this provision benefits us by providing increased
consistency in the application of Delaware law in the types of lawsuits to which it applies, the provision may have the effect of discouraging
lawsuits against our directors and officers.
Our
Business Risks
We
have a history of operating losses and may never achieve profitability in the future.
We
have experienced significant recurring operating losses and negative cash flows from operating activities since inception. For example,
for the years ended December 31, 2020, 2021 and 2022, we had net losses of approximately $2.8 million, $2.9 million and $6.6 million,
respectively. As of December 31, 2022, we had accumulated losses of approximately $31.9 million.
We
expect to continue to incur significant losses in the development, marketing, sale and delivery of our services. If we do not grow our
revenues or if we lose existing customers, we expect to continue to incur losses from operations for the foreseeable future. Because
of the numerous risks and uncertainties associated with the development, marketing, sale and delivery of our imaging real world data
(“iRWDTM”) services, we may experience larger than expected future losses and may never become profitable. Moreover,
there is a substantial risk that we may not be able to successfully commercialize our iRWDTM services, which would make it
unlikely that we would ever achieving profitability.
OneMedNet
believes it has demonstrated its quality and responsiveness in clinical imaging and curation of RWD based upon success in compiling one
of the largest networks of imaging centers (comprised of hospitals, imaging centers and clinics) throughout the United States covering
more than 15 million patients to date. On the global front, OneMedNet works with hospitals and life science companies around the world
including Ireland, United Kingdom, Ghana, Denmark and South Korea and growing. We base these claims on our understanding of our competition
in the United States and globally. However, if we were to lose these relationships with our network of imaging centers or lose our customers
or our competitors’ technology surpasses ours, our competitors could claim a greater market share domestically or abroad, which
could reduce our growth and our profits, which could harm our business, financial position, results of operations and prospects.
Two
significant customers represented 31%, 28% and 53% of our revenues for 2020, 2021 and 2022, respectively, and is expected to continue
to represent a significant portion of our forecasted revenue for 2023.
Change
Healthcare and Siemens Medical Solutions USA, collectively represented 53% and 28% of our revenues in 2022 and 2021, respectively. Change
Healthcare and Imaging, LLC, collectively represented 31% of our revenues in 2020. Change Healthcare is expected to continue to represent
a significant portion of our forecasted revenue for 2023. If we fail to maintain and grow our relationships with Change Healthcare, we
could lose a significant portion of our revenue for 2023, which would materially adversely affect our results of operations and our business.
Change
Healthcare and OneMedNet have a seven-year relationship, which we expect to continue and to grow. Potential growth of this relationship
could involve several paths potentially. The first, Change Healthcare continues as a valued customer and partner of OneMedNet in connection
to which, Change Healthcare automatically renews its contract every year in March. The second relates to the sale that OneMedNet made
in the Country of Ireland three years ago, and began implementation of in 2020, whereby OneMedNet has successfully installed its proprietary
BEAM Image Exchange technology in all 46 public hospitals as well as the 24 private hospitals. Thus, the entire country of Ireland is
using OneMedNet’s BEAM Image Exchange technology. It is anticipated that this relationship will continue well into the future.
The subscription revenue anticipated from this relationship in Ireland is expected to generate approximately $1 million per year for
the next four years at a minimum. OneMedNet believes the revenue for BEAM will continue to be flat over the next four years with the
exception of volume increases from the clients increasing their image volume and adjustments for inflation.
Siemens
Medical Solutions USA and its affiliated entities are new clients of OneMedNet since 2021 (collectively, “Siemens”). In 2022,
OneMedNet’s revenue from contracts with Siemens was more than double its revenue earned from contracts with Siemens in 2021. OneMedNet
is optimistic to continue to maintain this customer’s contracts and potential grow it further in 2023 but there can be no assurance
of this outcome. If OneMedNet were to lose one or more of its significant customers, its revenue may significantly decline. In addition,
revenue from significant customers may vary from period to period depending on the timing of renewing existing agreements or entering
into new agreements for additional OneMedNet products as well as other unforeseen risks and variables discussed in this proxy statement/prospectus.
The loss of one or more of OneMedNet’s significant customers could adversely affect its business, results of operations and financial
condition.
We
believe Siemen’s will continue to demand the highest quality imaging services across numerous therapies, diseases, and disorders.
Siemens Healthineers has a large demand for imaging studies to create artificial Intelligence driven algorithms to increase diagnosis
precision and accuracy. OneMedNet has been asked to supply Siemens numerous disease specific studies in the near future including: Chest
Radiography – 4,000+ studies; Brain MRI – 400 studies; Pelvic MR Imaging – 500 studies; Training data CR – 200,000
studies, among others. We expect the number and size of the orders to grow to fuel Siemens aggressive growth strategy however there can
be no assurance of such growth. Failure to continue our relationships with Change Healthcare and Siemens would have a material adverse
effect on our business, financial condition and results of operations. You should not rely on our historical relationship with these
companies as an indication of our future performance.
We
may encounter difficulties in managing our attempted growth of our business, which could negatively impact our operations.
As
we expand, market, sell and deliver our service offerings, we anticipate that we will need to increase our service development, sales
and marketing and administrative headcount. Such an evolution may impact our strategic focus and our deployment and allocation of resources.
Our
ability to manage our operations and growth effectively depends upon the continual improvement of our procedures, reporting systems and
operational, financial and management controls. We may not be able to implement administrative and operational improvements in an efficient
or timely manner and may discover deficiencies in existing systems and controls. If we do not meet these challenges, we may be unable
to execute our business strategies and may be forced to expend more resources than anticipated addressing these issues.
We
may acquire additional technology and complementary businesses in the future. Acquisitions involve many risks, any of which could materially
harm our business, including the diversion of management’s attention from core business concerns, failure to effectively exploit
acquired technologies, failure to successfully integrate the acquired business or realize expected synergies or the loss of key employees
from either our business or the acquired businesses.
In
addition, in order to continue to meet our obligations as a public listed company in both Australia and the United States and to support
our anticipated long-term growth, we will need to increase our general and administrative capabilities. Our management, personnel and
systems may not be adequate to support this future growth. If we are unable to successfully manage our growth and the increased complexity
of our operations, our business, financial position, results of operations and prospects may be harmed.
Our
recent growth rates may not be sustainable, or indicative of future growth.
Our
historical revenue is extremely low in absolute dollars. While our revenues grew significantly on a percentage basis from 2020 to 2022,
this rate of growth may not be sustainable or indicative of our future rate of growth. We believe that our continued revenue growth,
as well as our ability to reach profitability, will depend upon, among other factors, our ability to address the challenges, risks and
difficulties described elsewhere in this proxy statement/prospectus. We cannot provide assurance that we will be able to successfully
manage any such challenges or risks to our future growth. Any of these factors could cause our revenue growth to decline and may adversely
affect our margins and reaching profitability. Failure to continue our revenue growth or improve margins would have a material adverse
effect on our business, financial condition and results of operations. You should not rely on our historical rate of revenue growth as
an indication of our future performance.
We
may be unable to execute our business objectives and growth strategies successfully or sustain our growth and, as a result, this could
have a material adverse effect on our operating results.
The
highly complex nature of our industry requires that we effectively execute and manage our business objectives and growth strategies,
such as expanding our marketing and commercialization of our services in the U.S. and internationally, adding new customers, and increasing
our service delivery capacity. However, we may not be able to execute on these strategies as effectively as anticipated. Our ability
to execute on these strategies depends on a number of factors, including, without limitation:
| ● | our
ability to obtain adequate capital resources to complete execute our growth plans; |
| | | |
| ● | our
ability to hire, train and retain skilled managers and
personnel, including quality and production personnel, and marketing and commercial specialists; |
| | | |
| ● | our
ability to protect our existing and new services by registering and defending our intellectual
property rights; and |
| | | |
| ● | our
ability to successfully add new customers. |
To
the extent we are unable to execute on our growth strategies in accordance with our expectations, this could have a material adverse
effect on our business, financial condition, and future results of operations.
The
real-world data and real-world evidence business market continues to evolve, is highly competitive, and we may not be successful in competing
in this industry or establishing and maintaining confidence in our long-term business prospects among current and future partners and
customers.
The
real-world data and real-world evidence business market in which we compete continues to evolve and is highly competitive. To date, we
have focused our efforts on its expertise in clinical imaging innovation solutions that connects healthcare providers and patients and
satisfies a crucial need with the life sciences. We offer direct access to clinical images and associated contextual patient record.
OneMedNet proved the commercial and regulatory viability of imaging Regulatory Grade Real-World Data (“iRWDTM”),
a promising emerging market, that exactly matches OneMedNet’s life science partners’ case selection protocol. OneMedNet has
the immediate ability to quickly search and extensively curate multi-layer data from a federated group of healthcare facilities and to
provide fast access to curated medical images that has proved the commercial and regulatory viability of imaging RWD and covers the complete
value chain in imaging RWD, validated by an increasing federated network of providers. However, real-world data and real-world evidence
has been increasingly adopted and our current competitors have, and future competitors may have, greater resources than we do and may
also be able to devote greater resources to the development of their current and future technologies. These competitors also may have
greater access to customers and may be able to establish cooperative or strategic relationships amongst themselves or with third parties
that may further enhance their resources and competitive positioning.
Developments
in improvements in real-world data and real-world evidence curation by competitors may materially adversely affect the sales, pricing
and gross margins of our business. If a competing technology or process is developed that has superior operational or price performance,
our business will be harmed. Similarly, if we fail to accurately predict and ensure that our real-world data and real-world evidence
offering can address customers’ changing needs or emerging technological trends, or if our customers fail to achieve the benefits
expected from our real-world data and real-world evidence offering, our business will be harmed.
We
must continue to commit resources to develop our real-world data and real-world evidence technology in order to establish a competitive
position, and these commitments will be made without knowing whether such investments will result in products potential customers will
accept. There is no assurance we will successfully identify new customer requirements, develop and bring our real-world data and real-world
evidence to market on a timely basis, or that products and technologies developed by others will not render our real-world data and real-world
evidence obsolete or noncompetitive, any of which would adversely affect our business and operating results.
If
we are unable to attract and retain key employees and qualified personnel, our ability to compete could be harmed.
We
depend on the talents and continued efforts of our senior management and key employees. The loss of members of our management or key
employees may disrupt our business and harm our results of operations. Further, our ability to manage further expansion will require
us to continue to attract, motivate and retain additional qualified personnel. Competition for this type of personnel is intense, and
we may not be successful in attracting, integrating and retaining the personnel required to grow and operate our business effectively.
There can be no assurance that our current management team or any new members of our management team will be able to successfully execute
our business and operating strategies.
Our
operating and financial results forecast relies in large part upon assumptions and analyses developed by us. If these assumptions or
analyses prove to be incorrect, our actual operating results may be materially different from our forecasted results.
The
projected financial and operating information appearing elsewhere in this prospectus reflect current estimates of future performance.
Whether actual operating and financial results and business developments will be consistent with our expectations and assumptions as
reflected in our forecasts depends on a number of factors, many of which are outside our control, including, but not limited to:
| |
● |
success
and timing of development activity; |
| |
● |
customer
acceptance of our products; |
| |
● |
competition,
including from established and future competitors; |
| |
● |
whether
we can obtain sufficient capital to sustain and grow our business; |
| |
● |
our
ability to manage our growth; |
| |
● |
whether
we can manage relationships with key suppliers; |
| |
● |
our
ability to retain existing key management, integrate recent hires and attract, retain and motivate qualified personnel; and |
| |
● |
the
overall strength and stability of domestic and international economies. |
Unfavorable
changes in any of these or other factors, most of which are beyond our control, could materially and adversely affect our business, results
of operations and financial results.
Our
operations could be damaged or adversely affected as a result of natural disasters and other catastrophic events.
Our
operations could be adversely affected by events outside of our control, such as natural disasters, wars, health epidemics such as the
ongoing COVID-19 pandemic, and other calamities. We cannot assure you that any backup systems will be adequate to protect us from
the effects of fire, floods, typhoons, earthquakes, power loss, telecommunications failures, break-ins, war, riots, terrorist attacks
or similar events. Any of the foregoing events may give rise to interruptions, breakdowns, system failures, technology platform failures
or internet failures, which could cause the loss or corruption of data or malfunctions of software or hardware as well as adversely affect
our ability to provide services.
Any
financial or economic crisis, or perceived threat of such a crisis, including a significant decrease in consumer confidence, may materially
and adversely affect our business, financial condition, and results of operations.
In
recent years, the United States and global economies suffered dramatic downturns as the result of the COVID-19 pandemic, a deterioration
in the credit markets and related financial crisis as well as a variety of other factors including, among other things, extreme volatility
in security prices, severely diminished liquidity and credit availability, ratings downgrades of certain investments and declining valuations
of others. The United States and certain foreign governments have taken unprecedented actions in an attempt to address and rectify these
extreme market and economic conditions by providing liquidity and stability to the financial markets. If the actions taken by these governments
are not successful, the return of adverse economic conditions may negatively impact the demand for iRWDTM offering and may
negatively impact our ability to raise capital, if needed, on a timely basis and on acceptable terms or at all.
Our
ability to utilize our net operating loss and tax credit carryforwards to offset future taxable income may be subject to certain limitations.
In
general, under Section 382 of the Code, a corporation that undergoes an “ownership change” is subject to limitations on its
ability to use its pre-change net operating loss carryforwards (“NOLs”), to offset future taxable income. The limitations
apply if a corporation undergoes an “ownership change,” which is generally defined as a greater than 50 percentage point
change (by value) in its equity ownership by certain stockholders over a three-year period. If we have experienced an ownership change
at any time since our incorporation, we may already be subject to limitations on our ability to utilize our existing NOLs and other tax
attributes to offset taxable income or tax liability. In addition, the Business Combination and future changes in our stock ownership,
which may be outside of our control, may trigger an ownership change. Similar provisions of state tax law may also apply to limit our
use of accumulated state tax attributes. As a result, even if we earn net taxable income in the future, our ability to use these or our
pre-change NOL carryforwards and other tax attributes to offset such taxable income or tax liability may be subject to limitations, which
could potentially result in increased future income tax liability to us.
There
is also a risk that changes in law or regulatory changes made in response to the need for some jurisdictions to raise additional revenue
to help counter the fiscal impact from unforeseen reasons, including suspensions on the use of net operating losses or tax credits, possibly
with retroactive effect, may result in our existing net operating losses or tax credits expiring or otherwise being unavailable to offset
future income tax liabilities.
We
are subject to many hazards and operational risks that can disrupt our business, some of which may not be insured or fully covered by
insurance.
Our
operations are subject to many hazards and operational risks inherent to our business, including: (a) general business risks; (b) warranty
liability; and (c) damage to third parties (e.g., our vendors), our infrastructure or properties caused by fires, floods and other natural
disasters, power losses, telecommunications failures, terrorist attacks, riots, cyberattacks, public health crises such as the current
COVID-19 pandemic (and other future pandemics or epidemics), human errors and similar events. As a result of the COVID-19 outbreak, or
similar pandemics, we have and may in the future experience disruptions that could severely impact our business and the business of our
customers.
Our
insurance coverage may be inadequate to cover our liabilities related to such hazards or operational risks. For example, we do not currently
maintain cybersecurity insurance and our insurance providers may take the position that our coverage, under present circumstances, does
not extend to business interruptions as they relate to the COVID-19 pandemic. In addition, we may not be able to maintain adequate insurance
in the future at rates we consider reasonable and commercially justifiable, and insurance may not continue to be available on terms as
favorable as our current arrangements. The occurrence of a significant uninsured claim or a claim in excess of the insurance coverage
limits maintained by us could have a material adverse effect on our business, financial condition and results of operations.
Our
management has limited experience in operating a public company.
Our
executive officers have limited experience in the management of a publicly traded company. Our management team may not successfully or
effectively manage our transition to a public company that will be subject to significant regulatory oversight and reporting obligations
under federal securities laws. Their limited experience in dealing with the increasingly complex laws pertaining to public companies
could be a significant disadvantage in that it is likely that an increasing amount of their time may be devoted to these activities which
will result in less time being devoted to the management and growth of our Company. We may not have adequate personnel with the appropriate
level of knowledge, experience, and training in the accounting policies, practices or internal controls over financial reporting required
of public companies in the United States. The development and implementation of the standards and controls necessary for us to achieve
the level of accounting standards required of a public company in the United States may require costs greater than expected. It is possible
that we will be required to expand our employee base and hire additional employees to support our operations as a public company which
will increase our operating costs in future periods.
We
will incur significant increased expenses and administrative burdens as a public company, which could have an adverse effect on our business,
financial condition and results of operations.
We
will face increased legal, accounting, administrative and other costs and expenses as a public company that legacy OneMedNet Corporation
did not incur as a private company. The Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), including the requirements
of Section 404, as well as rules and regulations subsequently implemented by the SEC, the Dodd-Frank Wall Street Reform and Consumer
Protection Act of 2010 and the rules and regulations promulgated and to be promulgated thereunder, the PCAOB and the securities exchanges,
impose additional reporting and other obligations on public companies. Compliance with public company requirements will increase costs
and make certain activities more time-consuming. A number of those requirements will require us to carry out activities we have not done
previously. For example, we have created new Board committees and adopted new internal controls and disclosure controls and procedures.
In addition, expenses associated with SEC reporting requirements will be incurred. Furthermore, if any issues in complying with those
requirements are identified (for example, if the auditors identify a material weakness or significant deficiency in the internal control
over financial reporting), we could incur additional costs rectifying those issues, and the existence of those issues could adversely
affect our reputation or investor perceptions of it. It may also be more expensive to obtain director and officer liability insurance.
Risks associated with our status as a public company may make it more difficult to attract and retain qualified persons to serve on our
Board or as executive officers. The additional reporting and other obligations imposed by these rules and regulations will increase legal
and financial compliance costs and the costs of related legal, accounting and administrative activities. These increased costs will require
us to divert a significant amount of money that could otherwise be used to expand the business and achieve strategic objectives. Advocacy
efforts by stockholders and third parties may also prompt additional changes in governance and reporting requirements, which could further
increase costs.
If
securities or industry analysts do not publish or cease publishing research or reports about us, our business, or the market in which
we operate, or if they change their recommendations regarding our securities adversely, the price and trading volume of our securities
could decline.
The
trading market for our securities will be influenced by the research and reports that industry or securities analysts may publish about
us, our business, market or competitors. Securities and industry analysts do not currently, and may never, publish research on us. If
no securities or industry analysts commence coverage of us, our share price and trading volume would likely be negatively impacted. If
any of the analysts who may cover us change their recommendation regarding our shares of Common Stock adversely, or provide more favorable
relative recommendations about our competitors, the price of our shares of Common Stock would likely decline. If any analyst who may
cover us were to cease our coverage of us or fail to regularly publish reports on it, we could lose visibility in the financial markets,
which in turn could cause our share price or trading volume to decline.
Risks
Related to Ownership of Our Common Stock, this Offering, and Our Certificate of Incorporation and Bylaws Provisions
Our
Common Stock has been and may in the future continue to be subject to extreme volatility.
The
trading price of our Common Stock may be subject to extreme volatility. We cannot predict the magnitude of future fluctuations in the
trading price of our Common Stock. The trading price of our Common Stock may be affected by a number of factors, including events described
in the risk factors set forth in this prospectus and in our periodic reports filed with the SEC from time to time, as well as our operating
results, financial condition and other events or factors. Any of the factors listed below could have a material adverse effect on your
investment in our securities. Factors affecting the trading price of our securities may include:
| |
● |
announcements
by us or our competitors regarding technical developments and levels of performance achieved by our or their real-world data and
real-world evidence offering; |
| |
● |
announcements
by us regarding developments in our relationship with existing and future key customers; |
| |
● |
our
ability to bring our products and technologies to market on a timely basis, or at all; |
| |
● |
our
operating results or development efforts failing to meet the expectation of securities analysts or investors in a particular period; |
| |
● |
actual
or anticipated fluctuations in our quarterly financial results or the quarterly financial results of companies perceived to be similar
to it; |
| |
● |
changes
in the market’s expectations about our operating results or the real-world data and real-world evidence industry; |
| |
● |
success
of competitors actual or perceived development efforts; |
| |
● |
changes
in financial estimates and recommendations by securities analysts concerning the Company or the real-world data and real-world evidence
industry in general; |
| |
● |
operating
and share price performance of other companies that investors deem comparable to the Company; |
| |
● |
disputes
or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain intellectual
property protection for our technologies; |
| |
● |
changes
in laws and regulations affecting our business; |
| |
● |
our
ability to meet compliance requirements; |
| |
● |
commencement
of, or involvement in, litigation involving the Company; |
| |
● |
changes
in our capital structure, such as future issuances of securities or the incurrence of additional debt; |
| |
● |
the
volume of shares of Common Stock available for public sale; |
| |
● |
the
level of demand for our Common Stock, including the amount of short interest in our stock; |
| |
● |
any
major change in our Board or management; |
| |
● |
sales
of substantial amounts of the shares of Common Stock by our directors, executive officers or significant stockholders or the perception
that such sales could occur; |
| |
● |
the
expiration of contractual lock-up agreements with our executive officers, directors and stockholders, which we have entered into
and may enter into in the future from time to time; and |
| |
● |
general
economic and political conditions such as recessions, interest rates, fuel prices, international currency fluctuations and acts of
war or terrorism. |
Broad
market and industry factors may materially harm the market price of our securities irrespective of our operating performance. The stock
market in general, and the Nasdaq in particular, have experienced price and volume fluctuations that have often been unrelated or disproportionate
to the operating performance of the particular companies affected. The trading prices and valuations of these stocks, and of our securities,
may not be predictable. A loss of investor confidence in the market for retail stocks or the stocks of other companies which investors
perceive to be similar to the Company could depress our share price regardless of our business, prospects, financial conditions or results
of operations. A decline in the market price of our securities also could adversely affect our ability to issue additional securities
and our ability to obtain additional financing in the future.
Following
certain periods of volatility in the market price of our securities, we may become subject of securities litigation. We have experienced,
and may in the future experience additional litigation following periods of volatility. This type of litigation may result in substantial
costs and a diversion of management’s attention and resources.
Sales
of substantial amounts of our Common Stock in the public markets, or the perception that such sales could occur, could reduce the price
that our Common Stock might otherwise attain.
Sales
of a substantial number of shares of our Common Stock in the public market after this offering, or the perception that such sales could
occur, could adversely affect the market price of our Common Stock and may make it more difficult for you to sell your Common Stock at
a time and price that you deem appropriate. Based on the total number of outstanding shares of our capital stock as of December [*],
2023, we will have approximately [*] shares of Common Stock outstanding upon completion of this offering. All of the shares of Common
Stock sold in this offering will be freely tradable without restrictions or further registration under the Securities Act, except for
any shares held by the majority of our PIPE Investors.
In
connection with the transactions contemplated by the Merger Agreement (the “Transactions”), certain holders of the Company’s
securities entered into a Lock-Up Agreement pursuant to which they agreed to certain restrictions on the transfer of their Common Stock.
The
PIPE Investors are entitled to require us to register shares underlying the PIPE Notes and the PIPE Warrants owned by them for public
sale in the United States, and are filing this proxy statement/prospectus, to register those shares. Subject to the satisfaction of applicable
exercise periods and expiration of the lock-up agreements referred to above, the shares issued upon exercise of the PIPE Notes and the
PIPE Warrants will be available for immediate resale in the United States in the open market. See the section titled “Shares
Eligible for Future Sale” for additional information.
We
have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our
management has broad discretion in the application of the net proceeds from this offering, including to provide sufficient funding to
grow our customer base and for working capital and general corporate purposes, and we may spend or invest these proceeds in a way with
which our stockholders disagree. Because of the number and variability of factors that will determine our use of the net proceeds from
this offering, their ultimate use may vary substantially from their currently intended use. The failure by our management to apply these
funds effectively could harm our business. Pending their use, we may invest such proceeds in a manner that does not produce income or
that loses value, which may negatively impact the market price of our common stock.
Our
business model is capital-intensive, and we may not be able to raise additional capital on attractive terms, if at all, which could be
dilutive to stockholders. If we cannot raise additional capital when needed, our operations and prospects could be materially and adversely
affected.
We
can be expected to continue to sustain substantial operating expenses without generating sufficient revenues to cover expenditures. Over
time, we expect that we will need to raise additional funds, including through the issuance of equity, equity-related or debt securities
or through obtaining credit from financial institutions to fund, together with our principal sources of liquidity, ongoing costs, any
significant unplanned or accelerated expenses, and new strategic investments. We cannot be certain that additional capital will be available
on attractive terms, if at all, when needed, which could be dilutive to stockholders, and our financial condition, results of operations,
business and prospects could be materially and adversely affected.
We
do not expect to declare any dividends in the foreseeable future.
We
do not anticipate declaring any cash dividends to holders of our Common Stock in the foreseeable future. Consequently, investors may
need to rely on sales of their shares after price appreciation, which may never occur, as the only way to realize any future gains on
their investment.
There
can be no assurance that we will be able to comply with the continued listing standards of the Nasdaq.
Our
Common Stock and public Warrants are listed on the Nasdaq under the symbols “ONMD” and “ONMDW”, respectively.
If the Nasdaq delists our securities from trading on its exchange for failure to meet the listing standards and we are not able to list
such securities on another national securities exchange, we expect such securities could be quoted on an over-the-counter market. If
this were to occur, we and our stockholders could face significant material adverse consequences including:
| |
● |
a
limited availability of market quotations for our securities; |
| |
● |
reduced
liquidity for our securities; |
| |
● |
a
limited amount of news and analyst coverage; and |
| |
● |
a
decreased ability to issue additional securities or obtain additional financing in the future. |
Risks
Related to Our Warrants
We
may redeem unexpired Warrants prior to their exercise at a time that is disadvantageous to Warrantholders.
Our
public Warrants are currently exercisable for one share of Common Stock at a price of $11.50 per share. We have the ability to redeem
outstanding Warrants at any time prior to their expiration, at a price of $0.01 per Warrant, provided that the last reported sales price
of Common Stock equals or exceeds $18.00 per share for any 20 trading days within a 30-trading day period ending on the third trading
day prior to the date we send the notice of redemption to Warrantholders and provided certain other conditions are met. If and when the
Warrants become redeemable by us, we may exercise our redemption rights even if we are unable to register or qualify the underlying securities
for sale under all applicable state securities laws. As a result, we may redeem the Warrants, as set forth above even if the holders
are otherwise unable to exercise the Warrants.
Redemption
of the outstanding Warrants could force Warrantholders (i) to exercise their Warrants and pay the exercise price therefor at a time when
it may be disadvantageous for them to do so, (ii) to sell their Warrants at the then-current market price when they might otherwise wish
to hold their Warrants or (iii) to accept the nominal redemption price which, at the time the outstanding Warrants are called for redemption,
we expect would be substantially less than the market value of their Warrants. None of the private placement Warrants will be redeemable
by us so long as they are held by the Sponsor or its permitted transferees.
USE
OF PROCEEDS
We
estimate that the net proceeds from our issuance and sale of shares of our Common Stock in this offering will be approximately $[*]
million based on an assumed public offering price of $[*] per share and accompanying warrant, which was the closing price
of our Common Stock on The Nasdaq Capital Market on December ___, 2023, after deducting estimated underwriting discounts and commissions
and estimated offering expenses payable by us.
The
foregoing discussion assumes no sale of pre-funded warrants, which if sold, would reduce the number of shares of common stock that we
are offering on a one-for-one basis.
We
intend to use the net proceeds from this offering for (i) sales and marketing, (ii) operational costs, (iii) product development, (iv)
manufacturing expansion and (v) working capital and other general corporate purposes. We may also use a portion of the net proceeds to
in-license, acquire or invest in complementary businesses or products, however, we have no current commitments or obligations to do so.
A
$[*] increase or decrease in the assumed public offering price of $[*] per share would increase or decrease the net proceeds
from this offering by approximately $[*] million, assuming that the number of shares offered by us, as set forth on the cover
page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions.
An
increase (decrease) of _____ million shares in the number of shares offered by us, as set forth on the cover page of this prospectus,
would increase (decrease) the net proceeds from this offering by approximately $_____, assuming no change in the assumed public offering
price per share and after deducting estimated underwriting discounts and commissions.
We will not receive any proceeds from the sale
of our securities offered by the PIPE Investors (as selling securityholders) under this prospectus (the “PIPE Securities”).
We will receive up to an aggregate of approximately $957,450
from the exercise of all PIPE Warrants, assuming the exercise in full of all of the PIPE Warrants for cash. We expect to use the net
proceeds from the exercise of the PIPE Warrants, if any, for general corporate purposes.
This
expected use of the net proceeds from this offering and our existing cash represents our intentions based upon our current plans, financial
condition and business conditions. Predicting the cost necessary to develop product candidates can be difficult and the amounts and timing
of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development and commercialization
efforts, any collaborations that we may enter into with third parties for our product candidates and any unforeseen cash needs. As a
result, our management will retain broad discretion over the allocation of the net proceeds from this offering and our existing cash.
In
the ordinary course of our business, we expect to from time to time evaluate the acquisition of, or investment in, complementary products,
technologies or businesses, and we could use a portion of the net proceeds from this offering for such activities. We currently do not
have any agreements, arrangements, or commitments with respect to any potential acquisition, investment or license.
Pending
our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments,
including short-term, investment-grade, interest-bearing instruments, and government securities.
DILUTION
If
you invest in our securities, your ownership interest will be diluted to the extent of the difference between public offering price per
share of our Common Stock and the as adjusted net tangible book value per share of our Common Stock immediately after this offering.
As
of September 30, 2023 we had a historical net tangible book value of $(14,738,338), or $(0.87) per share of Common
Stock, based on 16,858,811 shares
of Common Stock outstanding at November 29, 2023.
To
the extent that stock options or warrants are exercised, new stock options are issued under our equity incentive plan, or we issue additional
common stock in the future, there will be further dilution to investors participating in this offering. In addition, we may choose to
raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for
our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the
issuance of these securities could result in further dilution to our stockholders.
DESCRIPTION
OF SECURITIES WE ARE OFFERING
Common
Stock
The
description of our Common Stock under the section “Description of Capital Stock” in this prospectus is incorporated herein
by reference.
Warrants
General
- The following is a brief summary of certain terms and conditions of the warrants being offered by us. The following description
is subject in all respects to the provisions contained in the form of warrant, the form of which will be filed as an exhibit to the registration
statement of which this prospectus forms a part.
Duration
and Exercise Price - The warrants offered hereby will have an exercise price of $[*] per share. The warrants will be immediately
exercisable and may be exercised at any time on or after the initial exercise date and on or before the five-year anniversary of the
date of issuance. The exercise prices and numbers of shares of Common Stock issuable upon exercise are subject to appropriate adjustment
in the event of stock dividends, stock splits, reorganizations or similar events affecting our Common Stock. Warrants will be issued
in certificated form only.
Exercisability
- The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise
notice accompanied by payment in full for the number of shares of our Common Stock purchased upon such exercise (except in the case of
a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s
warrants to the extent that the holder would own more than 4.99% (or 9.99%, at the holder’s election) of our outstanding Common
Stock immediately after exercise, except that upon notice from the holder to us, the holder may decrease or increase the limitation of
ownership of outstanding stock after exercising the holder’s warrants up to 9.99% of the number of shares of our Common Stock outstanding
immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants,
provided that any increase in such limitation shall not be effective until 61 days following notice to us.
Cashless
Exercise - If, at the time a holder exercises its warrants, a registration statement registering the issuance of the shares of
Common Stock underlying the warrants under the Securities Act, is not then effective or available for the issuance of such shares, then
in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price,
the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of Common Stock determined
according to a formula set forth in the warrant.
Alternative
Cashless Exercise - On or after the thirty (30) day anniversary of the date of the underwriting agreement, a holder of common
warrants may also provide notice and elect an “alternative cashless exercise” pursuant to which they would receive an aggregate
number of shares equal to the product of (x) the aggregate number of shares of common stock that would be issuable upon a cash exercise
and (y) [*].
Transferability
- A warrant may be transferred at the option of the holder upon surrender of the warrant to us together with the appropriate
instruments of transfer.
Fractional
Shares - No fractional shares of Common Stock will be issued upon the exercise of the warrants. Rather, the number of shares
of Common Stock to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment
in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading
Market - There is no established trading market for any of the warrants, and we do not expect a market to develop. We do not
intend to apply for a listing for any of the warrants on any securities exchange or other nationally recognized trading system. Without
an active trading market, the liquidity of the warrants will be limited.
Rights
as a Shareholder - Except as otherwise provided in the warrants or by virtue of the holders’ ownership of shares of our
Common Stock, the holders of warrants do not have the rights or privileges of holders of our Common Stock, including any voting rights,
until such warrant holders exercise their warrants.
Fundamental
Transaction - In the event of a fundamental transaction, as described in the warrants and generally including any reorganization,
recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our
properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding Common
Stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the
holders of the warrants will be entitled to receive upon exercise of the warrants the kind and amount of securities, cash or other property
that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction. In the event
of a Change of Control (as defined in each common warrant) approved by our Board of Directors, the holders of the common warrants have
the right to require us or a successor entity to redeem the common warrants for cash in the amount of the Black-Scholes Value (as defined
in each common warrant) of the unexercised portion of the common warrants on the date of the consummation of the Change of Control. In
the event of a Change of Control which is not approved by our Board of Directors, the holders of the common warrants have the right to
require us or a successor entity to redeem the common warrants for the consideration paid in the Change of Control in the amount of the
Black-Scholes Value of the unexercised portion of the common warrants on the date of the consummation of the Change of Control.
Waivers
and Amendments - No term of the warrants may be amended or waived without the written consent of the holder of such warrant.
Pre-Funded
Warrants
The
following summary of certain terms and provisions of the pre-funded warrants that are being offered hereby is not complete and is subject
to, and qualified in its entirety by, the provisions of the pre-funded warrant, the form of which is filed as an exhibit to the registration
statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of
pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Duration
and Exercise Price - Each pre-funded warrant offered hereby will have an initial exercise price per share equal to $0.0001. The
pre-funded warrants will be immediately exercisable and may be exercised at any time until the pre-funded warrants are exercised in full.
The exercise price and number of shares of Common Stock issuable upon exercise is subject to appropriate adjustment in the event of stock
dividends, stock splits, reorganizations or similar events affecting our Common Stock and the exercise price.
Exercisability
- Each pre-funded warrant may be exercised, in cash or by a cashless exercise at the election of the holder at any time following
the date of issuance and from time to time thereafter until the pre-funded warrants are exercised in full. The pre-funded warrants will
be exercisable in whole or in part by delivering to the Company a completed instruction form for exercise and complying with the requirements
for exercise set forth in the pre-funded warrant. Payment of the exercise price may be made in cash or pursuant to a cashless exercise,
in which case the holder would receive upon such exercise the net number of shares of Common Stock determined according to the formula
set forth in the pre-funded warrant.
Cashless
Exercise - At the time a holder exercises its pre-funded warrants, in lieu of making the cash payment otherwise contemplated
to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise
(either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants.
Exercise
Limitation - In general, a holder will not have the right to exercise any portion of a pre-funded warrant if the holder (together
with its Attribution Parties (as defined in the pre-funded warrant)) would beneficially own in excess of 4.99% or 9.99%, at the election
of the holder, of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage
ownership is determined in accordance with the terms of the pre-funded warrant. However, any holder may increase or decrease such percentage
to any other percentage not in excess of 9.99% upon notice to us, provided, that any increase in this limitation will not be effective
until 61 days after such notice from the holder to us and such increase or decrease will apply only to the holder providing such notice.
Transferability
- Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded
warrant to us together with the appropriate instruments of transfer.
Fractional
Shares - No fractional shares of Common Stock will be issued upon the exercise of the pre-funded warrants. Rather, the number
of shares of Common Stock to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash
adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading
Market - There is no trading market available for the pre-funded warrants on any securities exchange or nationally recognized
trading system.
Right
as a Stockholder - Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares
of our Common Stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our Common Stock, including
any voting rights, until they exercise their pre-funded warrants.
SHARES
ELIGIBLE FOR FUTURE SALE
Our
Common Stock is listed on NASDAQ under the symbol “ONMD”. Future sales of substantial amounts of our Common Stock in the
public market could adversely affect market prices prevailing from time to time and future distributions of the shares owned by the
PIPE Investors could also adversely affect the market price of our common stock.
Sale
of Restricted Shares
As
of September 30, 2023, we had approximately 16,858,811 Common Stock equivalents issued and outstanding, excluding
shares awarded in exchange for convertible notes and pursuant
to equity award arrangements with directors and employees. Of these shares, all of the shares of our Common Stock received in the
distribution are, and all of the shares sold pursuant to this registration statement of which this prospectus forms a part will be
freely transferable without restriction under the Securities Act, unless purchased by our “affiliates” as that term is
defined in Rule 144 under the Securities Act.
Rule
144
In
general, under Rule 144 as currently in effect, a person (or persons whose shares are aggregated) who is not deemed to be or have been
one of our affiliates for purposes of the Securities Act at any time during 90 days preceding a sale and who has beneficially owned the
shares proposed to be sold for at least six months, including the holding period of any prior owner other than an affiliate, is entitled
to sell such shares without registration, subject to compliance with the public information requirements of Rule 144. If such a person
has beneficially owned the shares proposed to be sold for at least one year, including the holding period of a prior owner other than
an affiliate, then such person is entitled to sell such shares without complying with any of the requirements of Rule 144.
In
general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates, who have met the
six-month holding period for beneficial ownership of “restricted shares” of our Common Stock, are entitled to sell within
any three-month period, a number of shares that does not exceed the greater of:
| |
● |
1.0%
of our then-outstanding shares of Common Stock, which will equal approximately [*] shares immediately after this offering; and |
| |
|
|
| |
● |
the
average weekly trading volume of our Common Stock on NASDAQ during the four calendar weeks preceding the filing of a notice of the
sale on Form 144. |
Sales
under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions
and notice requirements and to the availability of current public information about us. The sale of these shares, or the perception that
sales will be made, could adversely affect the price of our common stock after this offering because a greater supply of shares would
be, or would be perceived to be, available for sale in the public market.
Registration
Statement on Form S-8
In
the near future, we intend to file with the SEC a Registration Statement on Form S-8 registering an aggregate of [*] shares of
common stock underlying equity awards we have made and will make to our employees and certain other qualifying individuals, and the resale
of those shares of common stock. The Form S-8 became effective upon filing and shares of common stock so registered will become freely
tradable when issued.
Registration
Rights
We
have entered into the stockholder and registration
rights agreement with the PIPE Investors. This prospectus is part of the registration statement filed pursuant to
the stockholder and registration rights agreement. We do not have any other contractual obligations to register our common stock.
UNDERWRITING
We
have entered into an underwriting agreement with EF Hutton, division of Benchmark Investments, LLC (the “Representatives”)
as the representatives of the underwriters named below, with respect to the offering of shares of our Common Stock and warrants. Subject
to the terms and conditions of an underwriting agreement between us and the Representatives, we have agreed to sell to the underwriters,
and the underwriters have agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page
of this prospectus, the number of shares of Common Stock and warrants listed next to its name in the following table:
| | |
Number of Shares and Accompanying Warrants | |
| | |
| |
| EF Hutton, division of Benchmark Investments, LLC | |
| | |
| Total | |
| | |
The
underwriters are committed to purchase all of the securities offered by us. The obligations of the underwriters may be terminated upon the occurrence of certain events specified
in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to
customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriters of
officers’ certificates and legal opinions.
We
have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute
to payments the underwriters may be required to make in respect thereof.
The
underwriters are offering the above securities, subject to prior sale, when, as and if issued to and accepted by it, subject to approval
of legal matters by its counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw,
cancel or modify offers to the public and to reject orders in whole or in part.
Discounts
The
Representative has advised that the underwriters propose to offer the shares of Common Stock (and pre-funded warrants) and accompanying
warrants directly to the public at the public offering price per share and accompanying warrant set forth on the cover page of this prospectus.
After
the offering to the public, the offering prices and other selling terms may be changed by the underwriters without changing the proceeds
we will receive from the underwriters. Any shares and accompanying warrants sold by the underwriters to securities dealers will be sold
at the public offering price less a concession not in excess of $____ per share.
The
following table summarizes the public offering price, underwriting commissions, and proceeds before expenses to us:
| | |
| | |
Per Pre- Funded | | |
Total | |
| | |
Per share and accompanying warrant | | |
Warrant
and accompanying warrant | | |
Without Over- Allotment Option | | |
With Over- Allotment Option | |
| Public offering price | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| Underwriting discounts and commissions (__.0%) | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| Proceeds, before expenses, to us | |
$ | | | |
$ | | | |
$ | | | |
$ | | |
| (1) |
We
have agreed to pay a non-accountable expense allowance to the Representative equal to 1% of the gross proceeds received
in this offering which is not included in the underwriting discounts and commission. |
We
have agreed to reimburse the Representatives for all reasonable out-of-pocket costs and expenses relating to the offering, including,
without limitation, (a) all fees, expenses and disbursements relating to FINRA background checks of our officers, directors and
entities in an amount not to exceed $5,000 in the aggregate, (b) the fees and expenses of the underwriters’ legal counsel
not to exceed $45,000; (d) the approximate $25,000 cost associated with Blue-Sky
notice filings; (e) $10,000 for data services and communications expenses; (f) up to $5,000 of the Representative’s actual
accountable “road show” expenses; and (g) up to [$30,000] of the Representative’s
market making and trading, and clearing firm settlement expenses for the offering.
We
expect that the total expenses of the offering payable by us, excluding underwriting discount and commissions, will be approximately
$[*].
Discretionary
Accounts
The
underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which it has discretionary authority.
Lock-Up
Agreements
We
will not, without the prior written consent of the Representatives, from the date of execution of the Underwriting Agreement and continuing
for a period of 3 months from such date (the “Lock-Up Period”), (a) offer, pledge, sell, contract to sell, sell any option
or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise
transfer or dispose of, directly or indirectly, any shares of our capital stock or any securities convertible into or exercisable or
exchangeable for shares of our capital stock; (b) file or caused to be filed any registration statement with the Commission relating
to the offering of any shares of our capital stock or any securities convertible into or exercisable or exchangeable for shares of our
capital stock; (c) complete any offering of our debt securities, other than entering into a line of credit with a traditional bank or
(d) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership
of our capital stock, whether any such transaction described in clause (a), (b), (c) or (d) above is to be settled by delivery of shares
of our capital stock or such other securities, in cash or otherwise.
Our
executive officers and directors have agreed pursuant to “lock-up” agreements not to, without the prior written consent of
the Representatives, directly or indirectly, offer to sell, sell, pledge or otherwise transfer or dispose of any of shares of (or enter
into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any
time in the future of) our common stock, enter into any swap or other derivatives transaction that transfers to another, in whole or
in part, any of the economic benefits or risks of ownership of shares of our common stock, make any demand for or exercise any right
or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common
stock or securities convertible into or exercisable or exchangeable for shares of common stock or any other of our securities or publicly
disclose the intention to do any of the foregoing, subject to customary exceptions, for a period of six months from the date of this
prospectus. The Representatives may, in their sole discretion and at any time or from time to time before the termination of the lock-up
period release all or any portion of the securities subject to lock-up agreements; provided, however, that, subject to limited exceptions,
at least three business days before the release or waiver or any lock-up agreement, the Representatives must notify us of the impending
release or waiver and we will be required to announce the impending release or waiver through a major news service at least two business
days before the release or waiver.
Indemnification
We
have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute
to payments that the underwriters may be required to make for these liabilities.
Price
Stabilization, Short Positions, and Penalty Bids
Until
the distribution of the Common Stock offered by this prospectus is completed, rules of the SEC may limit the ability of the underwriters
to bid for and to purchase our Common Stock. As an exception to these rules, the underwriters may engage in transactions effected in
accordance with Regulation M under the Exchange Act that are intended to stabilize, maintain or otherwise affect the price of our Common
Stock. The underwriters may engage in over-allotment sales, syndicate covering transactions, stabilizing transactions and penalty bids
in accordance with Regulation M.
|
● |
Stabilizing
transactions consist of bids or purchases made by the managing underwriter for the purpose of preventing or slowing a decline in
the market price of our securities while this offering is in progress. |
| |
|
|
| |
● |
Short
sales and over-allotments occur when the managing underwriter, on behalf of the underwriting syndicate, sells more of our shares
than they purchase from us in this Offering. In order to cover the resulting short position, the managing underwriter may exercise
the over-allotment option described above and/or may engage in syndicate covering transactions. There is no contractual limit on
the size of any syndicate covering transaction. The underwriters will deliver a prospectus in connection with any such short sales.
Purchasers of shares sold short by the underwriters are entitled to the same remedies under the federal securities laws as any other
purchaser of units covered by the registration statement. |
| |
|
|
| |
● |
Syndicate
covering transactions are bids for or purchases of our securities on the open market by the managing underwriter on behalf of the
underwriters in order to reduce a short position incurred by the managing underwriter on behalf of the underwriters. |
| |
|
|
| |
● |
A
penalty bid is an arrangement permitting the managing underwriter to reclaim the selling concession that would otherwise accrue to
an underwriter if the Common Stock originally sold by the underwriter were later repurchased by the managing underwriter and therefore
were not effectively sold to the public by such underwriter. |
Stabilization,
syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our Common Stock or
preventing or delaying a decline in the market price of our Common Stock. As a result, the price of our Common Stock may be higher than
the price that might otherwise exist in the open market.
Neither
we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the
prices of our Common Stock. These transactions may occur on Nasdaq or on any trading market. If any of these transactions are commenced,
they may be discontinued without notice at any time. See also “Plan of Distribution” elsewhere in this proxy statement/prospectus.
Electronic
Distribution
This
prospectus in electronic format may be made available on websites or through other online services maintained by the underwriters, or
by their affiliates. Other than this prospectus in electronic format, the information on the underwriters’ websites and any information
contained in any other websites maintained by an underwriter is not part of this prospectus or the registration statement of which this
prospectus forms a part, has not been approved and/or endorsed by us or the underwriters in their capacity as underwriter, and should
not be relied upon by investors.
Other
than the prospectus in electronic or printed format, the information on the underwriters’ website and any information contained
in any other website maintained by an underwriter is not part of the prospectus or the registration statement of which this prospectus
forms a part, has not been approved and/or endorsed by us or the underwriters in their capacity as underwriters and should not be relied
upon by investors.
Certain
Relationships
The
Representative and its affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings
in the ordinary course of business with us or our affiliates. The Representative has received, or may in the future receive, customary
fees and commissions for these transactions.
Offer
Restrictions Outside of the United States
Other
than in the United States, no action has been taken that would permit a public offering of our common stock in any jurisdiction where
action for the purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor
may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be
distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and
regulations of that country or jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about
and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute
an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an
offer or a solicitation is unlawful.
Australia
This
prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian
Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter
6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to
whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions
set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available
in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance
that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless
permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to
the offeree within 12 months after its transfer to the offeree under this prospectus.
Canada
The
shares of Common Stock may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors,
as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted
clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale
of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements
of applicable securities laws.
Securities
legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus
(including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by
the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser
should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars
of these rights or consult with a legal advisor.
China
The
information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s
Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region
and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly
to “qualified domestic institutional investors.”
European
Economic Area—Belgium, Germany, Luxembourg and Netherlands
The
information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under
the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a
“Relevant Member State”), from the requirement to produce a prospectus for offers of securities. An offer to the public of
securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under
the Prospectus Directive as implemented in that Relevant Member State:
| |
● |
to
legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or
regulated, whose corporate purpose is solely to invest in securities; |
| |
● |
to
any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance
sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial
statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or
consolidated financial statements); |
| |
● |
to
fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive)
subject to obtaining the prior consent of the Company or any underwriter for any such offer;
or |
| |
● |
in
any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities
shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus
Directive. |
France
This
document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers)
in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code Monétaire et Financier) and Articles
211-1 et seq. of the General Regulation of the French Autorité de marchés financiers (“AMF”). The securities
have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This
document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval
in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such
offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés)
acting for their own account, as defined in and in accordance with Articles L.411-2-II-2 and D.411-1 to D.411- 3, D.744-1, D.754-1 ;and
D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors
(cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2 and
D.411-4, D.744-1, D.754-1; and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant
to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly
or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3
of the French Monetary and Financial Code.
Hong
Kong
Neither
the information in this document nor any other document relating to the offer has been delivered for registration to the Registrar of
Companies in Hong Kong, and its contents have not been reviewed or approved by any regulatory authority in Hong Kong, nor have we been
authorized by the Securities and Futures Commission in Hong Kong. This document does not constitute an offer or invitation to the public
in Hong Kong to acquire securities. Accordingly, unless permitted by the securities laws of Hong Kong, no person may issue or have in
its possession for the purpose of issue, this document or any advertisement, invitation or document relating to the securities, whether
in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong
other than in relation to securities which are intended to be disposed of only to persons outside Hong Kong or only to “professional
investors” (as such term is defined in the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (“SFO”)
and the subsidiary legislation made thereunder) or in circumstances which do not result in this document being a “prospectus”
as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance of Hong Kong (Cap. 32 of the Laws of Hong Kong) (the
“CO”) or which do not constitute an offer or an invitation to the public for the purposes of the SFO or the CO. The offer
of the securities is personal to the person to whom this document has been delivered by or on behalf of our company, and a subscription
for securities will only be accepted from such person.
No
person to whom a copy of this document is issued may issue, circulate or distribute this document in Hong Kong or make or give a copy
of this document to any other person. You are advised to exercise caution in relation to the offer. If you are in any doubt about any
of the contents of this document, you should obtain independent professional advice. No document may be distributed, published or reproduced
(in whole or in part), disclosed by or to any other person in Hong Kong or to any person to whom the offer of sale of the securities
would be a breach of the CO or SFO.
Ireland
The
information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed
with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities
in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”).
The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of
a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than
100 natural or legal persons who are not qualified investors.
Israel
The
securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the ISA), nor have such
securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel,
absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing
the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion
as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered
by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities
laws and regulations.
Italy
The
offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione
Nazionale per le Società e la Borsa, or “CONSOB”) pursuant to the Italian securities legislation and, accordingly,
no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in
a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other
than:
| |
● |
to
Italian qualified investors, as defined in Article 100 of Decree no.58 by reference to Article 34-ter of CONSOB
Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
| |
|
|
| |
● |
in
other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58
and Article 34-ter of Regulation No. 11971 as amended. |
| |
|
|
| |
● |
Any
offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements
where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must
be: |
| |
|
|
| |
● |
made
by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative
Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No.
16190 of 29 October 2007 and any other applicable laws; and |
| |
|
|
| |
● |
in
compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any
subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules
provided under Decree No. 58 and the Regulation No. 11971 as amended unless an exception from those rules applies. Failure to comply
with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring
the securities for any damages suffered by the investors.
Japan
The
securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan
(Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a
private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of
the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly,
in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional
Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition
by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This
document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários)
in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The
securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document
and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market
Commission (Comissão Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed
or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify
as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to
persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this
document and they may not distribute it or the information contained in it to any other person.
Sweden
This
document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority).
Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances
that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel
med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as
defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the
information contained in it to any other person.
Switzerland
The
securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any
other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards
for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses
under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland.
Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly
available in Switzerland.
Neither
this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory
authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial
Market Supervisory Authority (FINMA). This document is personal to the recipient only and not for general circulation in Switzerland.
United
Arab Emirates
Neither
this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates
or any other governmental authority in the United Arab Emirates, nor have we received authorization or licensing from the Central Bank
of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the
United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating
to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the
United Arab Emirates by us.
United
Kingdom
Neither
the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services
Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as
amended (“FSMA”) has been published or is intended to be published in respect of the securities. This document is issued
on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and
the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document,
except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not
be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in
the United Kingdom.
Any
invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the
issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be
communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to the Company.
In
the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters
relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial
Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high
net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together
“relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement
to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document
or any of its contents.
Pursuant
to section 3A.3 of National Instrument 33-105 Underwriting Conflicts, or NI 33-105, the underwriters are not required to comply with
the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
CERTAIN
U.S. FEDERAL INCOME AND ESTATE TAX CONSEQUENCES
TO
NON-U.S. HOLDERS
The
following is a summary of certain U.S. federal income and estate tax consequences of the purchase, ownership and disposition of our common
stock as of the date hereof. Except where noted, this summary deals only with common stock that is held as a capital asset by a non-U.S.
holder (as defined below).
A
“non-U.S. holder” means a beneficial owner of our common stock (other than an entity treated as a partnership for U.S. federal
income tax purposes) that is not, for U.S. federal income tax purposes, any of the following:
| |
● |
an
individual citizen or resident of the United States; |
| |
● |
a
corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under
the laws of the United States, any state thereof or the District of Columbia; |
| |
● |
an
estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| |
● |
a
trust if it (1) is subject to the primary supervision of a court within the United States and one or more United States persons have
the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable United States
Treasury regulations to be treated as a United States person. |
This
summary is based upon provisions of the Code, and regulations, rulings and judicial decisions as of the date hereof. Those authorities
may be changed, perhaps retroactively, so as to result in U.S. federal income and estate tax consequences different from those summarized
below. This summary does not address all aspects of U.S. federal income and estate taxes and does not deal with foreign, state, local
or other tax considerations that may be relevant to non-U.S. holders in light of their particular circumstances. In addition, it does
not represent a detailed description of the U.S. federal income and estate tax consequences applicable to you if you are subject to special
treatment under the U.S. federal income tax laws (including if you are a United States expatriate, foreign pension fund, “controlled
foreign corporation,” “passive foreign investment company” or a partnership or other pass-through entity for U.S. federal
income tax purposes). We cannot assure you that a change in law will not alter significantly the tax considerations that we describe
in this summary.
If
a partnership (or other entity treated as a partnership for U.S. federal income tax purposes) holds our common stock, the tax treatment
of a partner will generally depend upon the status of the partner and the activities of the partnership. If you are a partner of a partnership
holding our common stock, you should consult your tax advisors.
If
you are considering the purchase of our Common Stock, you should consult your own tax advisors concerning the particular U.S. federal
income and estate tax consequences to you of the purchase, ownership and disposition of our Common Stock, as well as the consequences
to you arising under other U.S. federal tax laws and the laws of any other taxing jurisdiction.
Dividends
In
the event that we make a distribution of cash or other property (other than certain pro rata distributions of our stock) in respect of
our common stock, the distribution generally will be treated as a dividend for U.S. federal income tax purposes to the extent it is paid
from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Any portion of a distribution
that exceeds our current and accumulated earnings and profits generally will be treated first as a tax-free return of capital, causing
a reduction in the adjusted tax basis of a non-U.S. holder’s common stock, and to the extent the amount of the distribution exceeds
a non-U.S. holder’s adjusted tax basis in our common stock, the excess will be treated as gain from the disposition of our common
stock (the tax treatment of which is discussed below under “—Gain on Disposition of Common Stock”).
Dividends
paid to a non-U.S. holder generally will be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may
be specified by an applicable income tax treaty. However, dividends that are effectively connected with the conduct of a trade or business
by the non-U.S. holder within the United States (and, if required by an applicable income tax treaty, are attributable to a United States
permanent establishment) are not subject to the withholding tax, provided certain certification and disclosure requirements are satisfied.
Instead, such dividends are subject to U.S. federal income tax on a net income basis in the same manner as if the non-U.S. holder were
a United States person as defined under the Code. Any such effectively connected dividends received by a foreign corporation may be subject
to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
A
non-U.S. holder who wishes to claim the benefit of an applicable treaty rate and avoid backup withholding, as discussed below, for dividends
will be required (a) to provide the applicable withholding agent with a properly executed IRS Form W-BEN or Form W-8BEN-E (or other applicable
form) certifying under penalty of perjury that such holder is not a United States person as defined under the Code and is eligible for
treaty benefits or (b) if our common stock is held through certain foreign intermediaries, to satisfy the relevant certification requirements
of applicable United States Treasury regulations. Special certification and other requirements apply to certain non-U.S. holders that
are pass-through entities rather than corporations or individuals.
A
non-U.S. holder eligible for a reduced rate of U.S. federal withholding tax pursuant to an income tax treaty may obtain a refund of any
excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
Gain
on Disposition of Common Stock
Subject
to the discussion of backup withholding below, any gain realized by a non-U.S. holder on the sale or other disposition of our common
stock generally will not be subject to U.S. federal income tax unless:
| |
● |
the
gain is effectively connected with a trade or business of the non-U.S. holder in the United States (and, if required by an applicable
income tax treaty, is attributable to a United States permanent establishment of the non-U.S. holder); |
| |
● |
the
non-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of that disposition,
and certain other conditions are met; or |
| |
● |
we
are or have been a “United States real property holding corporation” for U.S. federal income tax purposes and certain
other conditions are met. |
A
non-U.S. holder described in the first bullet point immediately above will be subject to tax on the gain derived from the sale or other
disposition in the same manner as if the non-U.S. holder were a United States person as defined under the Code. In addition, if any non-U.S.
holder described in the first bullet point immediately above is a foreign corporation, the gain realized by such non-U.S. holder may
be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income
tax treaty. An individual non-U.S. holder described in the second bullet point immediately above will be subject to a 30% (or such lower
rate as may be specified by an applicable income tax treaty) tax on the gain derived from the sale or other disposition, which gain may
be offset by United States source capital losses even though the individual is not considered a resident of the United States.
Generally,
a corporation is a “United States real property holding corporation” if the fair market value of its United States real property
interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests and its other assets used
or held for use in a trade or business (all as determined for U.S. federal income tax purposes). We believe we are not and do not anticipate
becoming a “United States real property holding corporation” for U.S. federal income tax purposes.
Federal
Estate Tax
Common
Stock held by an individual non-U.S. holder at the time of death will be included in such holder’s gross estate for U.S. federal
estate tax purposes, unless an applicable estate tax treaty provides otherwise.
Information
Reporting and Backup Withholding
Distributions
paid to a non-U.S. holder and the amount of any tax withheld with respect to such distributions generally will be reported to the IRS.
Copies of the information returns reporting such distributions and any withholding may also be made available to the tax authorities
in the country in which the non-U.S. holder resides under the provisions of an applicable income tax treaty.
A
non-U.S. holder will not be subject to backup withholding on dividends received if such holder certifies under penalty of perjury that
it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that such holder is a United States person as
defined under the Code), or such holder otherwise establishes an exemption.
Information
reporting and, depending on the circumstances, backup withholding will apply to the proceeds of a sale or other disposition of our common
stock made within the United States or conducted through certain United States-related financial intermediaries, unless the beneficial
owner certifies under penalty of perjury that it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know
that the beneficial owner is a United States person as defined under the Code), or such owner otherwise establishes an exemption.
Backup
withholding is not an additional tax and any amounts withheld under the backup withholding rules will be allowed as a refund or a credit
against a non-U.S. holder’s U.S. federal income tax liability provided the required information is timely furnished to the IRS.
Additional
Withholding Requirements
Under
Sections 1471 through 1474 of the Code (such Sections commonly referred to as “FATCA”), a 30% U.S. federal withholding tax
may apply to any dividends paid on our common stock to (i) a “foreign financial institution” (as specifically defined in
the Code) which does not provide sufficient documentation, typically on IRS Form W-8BEN-E, evidencing either (x) an exemption from FATCA,
or (y) its compliance (or deemed compliance) with FATCA (which may alternatively be in the form of compliance with an intergovernmental
agreement with the United States) in a manner which avoids withholding, or (ii) a “non-financial foreign entity” (as specifically
defined in the Code) which does not provide sufficient documentation, typically on IRS Form W-8BEN-E, evidencing either (x) an exemption
from FATCA, or (y) adequate information regarding certain substantial United States beneficial owners of such entity (if any). If a dividend
payment is both subject to withholding under FATCA and subject to the withholding tax discussed above under “—Dividends,”
the withholding under FATCA may be credited against, and therefore reduce, such other withholding tax. You should consult your own tax
advisors regarding these requirements and whether they may be relevant to your ownership and disposition of our common stock.
PLAN
OF DISTRIBUTION
The
selling stockholders (i.e., the PIPE Investors) identified in this prospectus may offer, from time to time, shares of our Common
Stock. We are registering such shares under the terms of the stockholder and registration rights agreement between us and the selling
stockholder. We will not receive any of the proceeds from the sale of shares of our Common Stock by the selling stockholder. Registration
of the shares of Common Stock covered by this prospectus does not mean, however, that those shares of common stock necessarily will be
offered or sold.
The
selling stockholders and its successors, including its transferees, may sell all or a portion of the shares of our Common Stock directly
to purchasers or through underwriters, broker-dealers or agents, who may receive compensation in the form of discounts, concessions or
commissions from the selling stockholders or the purchasers of the shares. These discounts, concessions or commissions as to any particular
underwriter, broker-dealer or agent may be in excess of those customary in the types of transactions involved.
The
shares of our Common Stock may be sold in one or more transactions on any national securities exchange or quotation service on which
the shares may be listed or quoted at the time of sale, in the over-the-counter market or in transactions otherwise than on these exchanges
or systems or in the over-the-counter market and in one or more transactions at fixed prices, at prevailing market prices at the time
of sale, at varying prices determined at the time of sale or at negotiated prices. These sales may be effected in transactions, which
may involve crosses or block transactions. Additionally, the selling stockholders may enter into derivative transactions with third parties,
or sell securities not covered by this prospectus to third parties in privately negotiated transactions. The selling stockholders may
use any one or more of the following methods when selling shares:
| |
● |
on
any national securities exchange or quotation service on which the shares may be listed or quoted at the time of sale, including
Nasdaq; |
| |
● |
in
the over-the-counter market; |
| |
● |
in
transactions otherwise than on these exchanges or services or in the over-the-counter market; |
| |
● |
through
the writing or settlement of options or other hedging transactions, whether the options are listed on an options exchange or otherwise; |
| |
● |
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
● |
block
trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as
principal to facilitate the transaction; |
| |
● |
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
● |
an
exchange distribution in accordance with the rules of the applicable exchange; |
| |
● |
a
debt-for-equity exchange; |
| |
● |
privately
negotiated transactions; |
| |
● |
settlement
of short sales entered into after the effective date of the registration statement of which this prospectus forms a part; |
| |
● |
broker-dealers
may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share; |
| |
● |
a
combination of any such methods of sale; and |
| |
● |
any
other method permitted pursuant to applicable law. |
The
selling stockholders may offer our Common Stock to the public through underwriting syndicates represented by managing underwriters or
through underwriters without an underwriting syndicate. If underwriters are used for the sale of our Common Stock, the securities will
be acquired by the underwriters for their own account. The underwriters may resell the common stock in one or more transactions, including
in negotiated transactions at a fixed public offering price or at varying prices determined at the time of sale. In connection with any
such underwritten sale of common stock, underwriters may receive compensation from the selling stockholder, for whom they may act as
agents, in the form of discounts, concessions or commissions. Underwriters may sell Common Stock to or through dealers, and the dealers
may receive compensation in the form of discounts, concessions or commissions from the underwriters or commissions from the purchasers
for whom they may act as agents. Such compensation may be in excess of customary discounts, concessions or commissions.
If
underwriters are used for the sale of our Common Stock, to the extent required by law, the names of the underwriters will be set forth
in the prospectus or prospectus supplement used by the underwriters to sell those securities. In addition, the selling stockholders may
use underwriters with whom we or the selling stockholders have a material relationship. We will describe the nature of such relationship
in any applicable prospectus supplement naming the underwriter or underwriters.
If
underwriters are used for the sale of our Common Stock, unless otherwise indicated in the prospectus or prospectus supplement relating
to a particular offering of Common Stock, the obligations of any underwriters to purchase the securities will be subject to customary
conditions precedent, and the underwriters will be obligated to purchase all of the securities offered if any of the securities are purchased.
If
underwriters are used for the sale of our Common Stock, in connection with such offering, the underwriters may advise us that they may
engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common stock in the open market
for the purpose of preventing or retarding a decline in the market price of the common stock while this offering is in progress. These
stabilizing transactions may include making short sales of the common stock, which involves the sale by the underwriters of a greater
number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open
market to cover positions created by short sales. The underwriters may close out any covered short position by purchasing shares in the
open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure
on the price of the Common Stock in the open market that could adversely affect investors who purchase in this offering. To the extent
that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The
anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of the shares of our Common Stock pursuant to this
prospectus and any applicable prospectus supplement and to the activities of the selling stockholder. In addition, we will make copies
of this prospectus and any applicable prospectus supplement available to the selling stockholders for the purpose of satisfying the prospectus
delivery requirements of the Securities Act. To the extent applicable, Regulation M may also restrict the ability of any person engaged
in the distribution of the common stock to engage in market-making activities with respect to the common stock. All of the foregoing
may affect the marketability of the common stock and the ability of any person or entity to engage in market-making activities with respect
to the Common Stock.
In
addition, any securities that qualify for sale pursuant to Rule 144 or Regulation S under the Securities Act or under Section 4(1) under
the Securities Act may be sold under such rules rather than pursuant to this prospectus or a prospectus supplement.
The
selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of
our Common Stock in the course of hedging the positions they assume. The selling stockholders may also sell short the shares and deliver
Common Stock to close out short positions, or loan or pledge the shares to broker-dealers that in turn may sell these shares. The selling
stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of
one or more derivative securities that require the delivery to such broker-dealer or other financial institution of shares offered by
this prospectus and any applicable prospectus supplement, which shares such broker-dealer or other financial institution may resell pursuant
to this prospectus and any applicable prospectus supplement. The selling stockholders also may transfer and donate the shares in other
circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for
purposes of this prospectus and any applicable prospectus supplement.
The
aggregate proceeds to the selling stockholders from the sale of the shares of our Common Stock will be the purchase price of the shares
less discounts and commissions, if any.
In
offering the shares of our Common Stock covered by this prospectus and any applicable prospectus supplement, the selling stockholders
and any broker-dealers who execute sales for the selling stockholders may be deemed to be “underwriters” within the meaning
of Section 2(a)(11) of the Securities Act in connection with such sales. Any profits realized by the selling stockholders and the compensation
of any broker-dealer may be deemed to be underwriting discounts and commissions. Selling stockholders who are “underwriters”
within the meaning of Section 2(a)(11) of the Securities Act will be subject to the prospectus delivery requirements of the Securities
Act and may be subject to certain statutory and regulatory liabilities, including liabilities imposed pursuant to Sections 11, 12 and
17 of the Securities Act and Rule 10b-5 under the Exchange Act.
In
order to comply with the securities laws of certain states, if applicable, the shares of our common stock must be sold in such jurisdictions
only through registered or licensed brokers or dealers. In addition, in certain states the shares may not be sold unless the shares are
registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available
and is complied with.
There
can be no assurance that the selling stockholders will sell any or all of the common stock registered pursuant to the registration statement
of which this prospectus forms a part.
At
the time a particular offering of the shares is made, a prospectus supplement, if required, will be distributed, which will set forth
the name of the selling stockholders, the aggregate amount of shares being offered by the selling stockholders and the terms of the offering,
including, to the extent required, (1) the name or names of any underwriters, broker-dealers or agents, (2) any discounts, commissions
and other terms constituting compensation from the selling stockholders, and (3) any discounts, commissions or concessions allowed or
reallowed to be paid to broker-dealers.
Pursuant
to the PIPE Registration Rights Agreement, we have agreed to indemnify the selling stockholders against certain liabilities, including
certain liabilities under the Securities Act. Agents and underwriters will be entitled to indemnification by us and the selling stockholders
against certain liabilities, including liabilities under the Securities Act, or to contribution with respect to payments which the agents
or underwriters may be required to make in respect thereof.
Agents
and underwriters and their respective affiliates may engage in transactions with, or perform services for us in the ordinary course of
business for which they may receive customary fees and reimbursement of expenses.
LEGAL
MATTERS
The
validity of the issuance of the shares of Common Stock (or pre-funded warrants) and accompanying warrants offered by us in this offering
will be passed upon for us by Rimon P.C., Washington DC. Nelson Mullins Riley & Scarborough LLP (Washington, DC), has acted
as counsel for the underwriters in connection with certain legal matters related to this offering.
EXPERTS
The
financial statements of OneMedNet Corporation (fka Date Knights Acquisition Corp), as of December 31, 2022 and 2021 and
for each of the years then ended incorporated by reference in this Registration Statement, of which this prospectus forms a part, have
been so included in reliance on the report of BF Borgers CPA PC, an independent registered public accounting firm, appearing elsewhere
herein, given on the authority of said firm as experts in auditing and accounting.
LEGAL
PROCEEDINGS
From
time to time and in the course of business, we may become involved in various legal proceedings seeking monetary damages and other relief.
The amount of the ultimate liability, if any, from such claims cannot be determined. As of the date hereof, there are no legal claims
currently pending or, to our knowledge, threatened against us or any of our officers or directors in their capacity as such or against
any of our properties that, in the opinion of our management, would be likely to have a material adverse effect on our financial position,
results of operations or cash flows.
UNAUDITED
PRO FORMA CONDENSED COMBINED BALANCE SHEET
AS OF JUNE 30, 2023
(in thousands)
| | |
OneMedNet (Historical) | | |
Data Knights (Historical) | | |
Data Knights 11-Aug-23 | | |
Pro Forma Adjustments Assuming Minimum Redemption | | |
| | |
Pro Forma Combined Assuming Minimum Redemption | | |
Pro Forma Adjustment Assuming Maximum Redemption | | |
| | |
Pro Forma Combined Assuming Maximum Redemption | |
| ASSETS | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Current Assets: | |
| 312 | | |
| 3 | | |
| | | |
| 18,644 | | |
| A | | |
| 19,355 | | |
| (18,603 | ) | |
| I | | |
| 752 | |
| Cash and Cash Equivalents | |
| | | |
| | | |
| | | |
| 1,500 | | |
| J | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| (1,000 | ) | |
| B | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| (104 | ) | |
| M | | |
| | | |
| | | |
| | | |
| | |
| Accounts Receivable | |
| 17 | | |
| | | |
| | | |
| | | |
| | | |
| 17 | | |
| | | |
| | | |
| 17 | |
| Prepaid expenses and other current assets | |
| 1,674 | | |
| | | |
| | | |
| | | |
| | | |
| 1,674 | | |
| | | |
| | | |
| 1,674 | |
| Total current assets | |
| 2,003 | | |
| 3 | | |
| | | |
| 19,040 | | |
| | | |
| 21,046 | | |
| (18,603 | ) | |
| | | |
| 2,443 | |
| Non-current assets | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Prepaid expenses | |
| | | |
| | | |
| | | |
| | | |
| | | |
| — | | |
| | | |
| | | |
| — | |
| Cash and marketable securities held in Trust Account | |
| | | |
| 29,979 | | |
| (11,335 | ) | |
| (18,644 | ) | |
| A | | |
| 0 | | |
| | | |
| | | |
| 0 | |
| Property and equipment, net | |
| 77 | | |
| | | |
| | | |
| | | |
| | | |
| 77 | | |
| | | |
| | | |
| 77 | |
| Total non-current assets | |
| 77 | | |
| 29,979 | | |
| (11,335 | ) | |
| (18,644 | ) | |
| | | |
| 77 | | |
| | | |
| | | |
| 77 | |
| TOTAL ASSETS | |
$ | 2,080 | | |
$ | 29,982 | | |
$ | (11,335 | ) | |
$ | 396 | | |
| | | |
$ | 21,123 | | |
$ | (18,603 | ) | |
| | | |
$ | 2,520 | |
| LIABILITIES, TEMPORARY EQUITY AND STOCKHOLDER’S EQUITY (DEFICIT) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Accounts Payable and accrued expenses | |
| 1,412 | | |
| 2,039 | | |
| | | |
| 1,000 | | |
| C | | |
| 4,451 | | |
| | | |
| | | |
| 4,451 | |
| Canada Energy Business Loan Act | |
| 45 | | |
| | | |
| | | |
| | | |
| | | |
| 45 | | |
| | | |
| | | |
| 45 | |
| Amount due to related party | |
| | | |
| 11 | | |
| | | |
| | | |
| | | |
| 11 | | |
| | | |
| | | |
| 11 | |
| Income taxes payable | |
| | | |
| 32 | | |
| | | |
| | | |
| | | |
| 32 | | |
| | | |
| | | |
| 32 | |
| Franchise tax payable | |
| | | |
| 8 | | |
| | | |
| | | |
| | | |
| 8 | | |
| | | |
| | | |
| 8 | |
| Short term debt | |
| | | |
| | | |
| | | |
| 1,500 | | |
| J | | |
| 1,500 | | |
| | | |
| | | |
| 1,500 | |
| Deferred revenues | |
| 354 | | |
| | | |
| | | |
| | | |
| | | |
| 354 | | |
| | | |
| | | |
| 354 | |
| Convertible promissory notes | |
| 10,590 | | |
| | | |
| | | |
| (10,590 | ) | |
| K | | |
| — | | |
| | | |
| | | |
| — | |
| Total current liabilities | |
| 12,401 | | |
| 2,090 | | |
| | | |
| (8,090 | ) | |
| | | |
| 6,401 | | |
| | | |
| | | |
| 6,401 | |
| Non-current liabilities | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Accrued interest, related party | |
| 1,007 | | |
| | | |
| | | |
| (1,007 | ) | |
| K | | |
| — | | |
| | | |
| | | |
| — | |
| Loan, related party | |
| 604 | | |
| | | |
| | | |
| (104 | ) | |
| M | | |
| 500 | | |
| | | |
| | | |
| 500 | |
| Derivative liability | |
| | | |
| 363 | | |
| | | |
| | | |
| | | |
| 363 | | |
| | | |
| | | |
| 363 | |
| Deferred underwriting fee payable | |
| | | |
| 4,025 | | |
| | | |
| (4,025 | ) | |
| B | | |
| — | | |
| | | |
| | | |
| — | |
| Extension loan | |
| | | |
| 3,284 | | |
| | | |
| (3,284 | ) | |
| L | | |
| — | | |
| | | |
| | | |
| — | |
| Working capital loan | |
| | | |
| 368 | | |
| | | |
| | | |
| | | |
| 368 | | |
| | | |
| | | |
| 368 | |
| Convertible promissory notes | |
| 1,500 | | |
| | | |
| | | |
| (1,500 | ) | |
| J | | |
| — | | |
| | | |
| | | |
| — | |
| Total non-current liabilities | |
| 3,111 | | |
| 8,040 | | |
| | | |
| (9,920 | ) | |
| | | |
| 1,231 | | |
| — | | |
| | | |
| 1,231 | |
| Total liabilities | |
| 15,512 | | |
| 10,130 | | |
| | | |
| (18,010 | ) | |
| | | |
| 7,632 | | |
| — | | |
| | | |
| 7,632 | |
| COMMITMENTS AND CONTINGENCIES | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Temporary equity | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Class A and Class B common stock subject to possible redemption | |
| | | |
| 29,938 | | |
| (11,335 | ) | |
| (18,603 | ) | |
| D | | |
| 0 | | |
| | | |
| | | |
| 0 | |
| Stockholders’ equity (deficit) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Preferred Series A-2 | |
| | | |
| | | |
| | | |
| — | | |
| E | | |
| | | |
| | | |
| | | |
| | |
| Preferred Series A-1 | |
| | | |
| | | |
| | | |
| — | | |
| E | | |
| | | |
| | | |
| | | |
| | |
| Common stock | |
| | | |
| | | |
| | | |
| 2 | | |
| F | | |
| 3 | | |
| (1 | ) | |
| I | | |
| 2 | |
| | |
| | | |
| | | |
| | | |
| 1 | | |
| D | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| — | | |
| E | | |
| | | |
| | | |
| | | |
| | |
| Class A common stock | |
| | | |
| — | | |
| | | |
| — | | |
| G | | |
| | | |
| | | |
| | | |
| | |
| Class B common stock | |
| | | |
| — | | |
| | | |
| — | | |
| G | | |
| | | |
| | | |
| | | |
| | |
| Additional paid-in capital | |
| 21,712 | | |
| 1,638 | | |
| | | |
| 18,602 | | |
| D | | |
| 48,632 | | |
| (18,062 | ) | |
| I | | |
| 30,030 | |
| | |
| | | |
| | | |
| | | |
| (2 | ) | |
| F | | |
| | | |
| | | |
| | | |
| — | |
| | |
| | | |
| | | |
| | | |
| (11,724 | ) | |
| H | | |
| | | |
| | | |
| | | |
| — | |
| | |
| | | |
| | | |
| | | |
| (1,000 | ) | |
| C | | |
| | | |
| | | |
| | | |
| — | |
| | |
| | | |
| | | |
| | | |
| 3,284 | | |
| L | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| 13,097 | | |
| K | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| 3,025 | | |
| B | | |
| | | |
| | | |
| | | |
| | |
| Accumulated deficit | |
$ | (35,144 | ) | |
| (11,724 | ) | |
| | | |
| 11,724 | | |
| H | | |
| (35,144 | ) | |
| | | |
| | | |
| (35,144 | ) |
| Total shareholders’ equity (deficit) | |
$ | (13,432 | ) | |
| (10,086 | ) | |
| — | | |
| 37,009 | | |
| | | |
| 13,491 | | |
| (18,603 | ) | |
| | | |
| (5,112 | ) |
| TOTAL LIABILITES, TEMPORARY EQUITY AND STOCKHOLDERS’ DEFECIT | |
$ | 2,080 | | |
$ | 29,982 | | |
$ | (11,335 | ) | |
$ | 396 | | |
| | | |
$ | 21,123 | | |
$ | (18,603 | ) | |
| | | |
$ | 2,520 | |
UNAUDITED
PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE SIX MONTHS ENDED JUNE 30, 2023
(in thousands, except share and per share data)
| | |
OneMedNet
(Historical) | | |
Data
Knights (Historical) | | |
Pro
Forma Adjustments Assuming Minimum and Maximum Redemption | | |
| |
Pro
Forma Combined Assuming Minimum and Maximum Redemption | |
| Revenue | |
$ | 355 | | |
$ | — | | |
$ | — | | |
| |
$ | 355 | |
| Cost
of revenue | |
| 519 | | |
| — | | |
| — | | |
| |
| 519 | |
| Gross
profit | |
| (164 | ) | |
| — | | |
| — | | |
| |
| (164 | ) |
| Operating
costs and expenses: | |
| | | |
| | | |
| | | |
| |
| | |
| Selling,
general and administrative expenses | |
| 2,751 | | |
| 639 | | |
| 1,000 | | |
AA | |
| 4,390 | |
| Total
operating costs and expenses | |
| 2,751 | | |
| 639 | | |
| 1,000 | | |
| |
| 4,390 | |
| Loss
from operations | |
| (2,915 | ) | |
| (639 | ) | |
| (1,000 | ) | |
| |
| (4,554 | ) |
| Other
income (expense): | |
| | | |
| | | |
| | | |
| |
| | |
| Interest
expense | |
| (316 | ) | |
| | | |
| (23 | ) | |
CC | |
| (339 | ) |
| Other
income (expense) | |
| (36 | ) | |
| | | |
| (500 | ) | |
| |
| (536 | ) |
| Realized
and unrealized gains on Trust Account | |
| | | |
| 670 | | |
| (670 | ) | |
BB | |
| — | |
| Total
other income (expense) | |
| (352 | ) | |
| 670 | | |
| (1,193 | ) | |
| |
| (875 | ) |
| Net
income (loss) before income tax provision | |
| (3,267 | ) | |
| 31 | | |
| (2,193 | ) | |
| |
| (5,429 | ) |
| Income
tax provision | |
| | | |
| (122 | ) | |
| | | |
| |
| (122 | ) |
| Net
income (loss) | |
| (3,267 | ) | |
| (91 | ) | |
| (2,193 | ) | |
| |
| (5,551 | ) |
| | |
OneMedNet
(Historical) | | |
Data
Knights (Historical) | | |
Assuming
Minimum Redemption | | |
Assuming
Maximum Redemption | |
| Weighted
average shares outstanding – Common stock | |
| 4,550,166 | | |
| — | | |
| — | | |
| — | |
| Basic
and diluted net income (loss) per share – Common stock | |
| (0.27 | ) | |
| — | | |
| — | | |
| — | |
| Weighted
average shares outstanding – Class A and Class B common stock subject to redemption | |
| — | | |
| 2,731,544 | | |
| 34,102,468 | | |
| 32,299,770 | |
| Basic
and diluted net income (loss) per share – Class A and Class B common stock subject to redemption | |
| — | | |
| (0.01 | ) | |
| (0.16 | ) | |
| (0.17 | ) |
| Weighted
average shares outstanding – Class A and Class B non-redeemable common stock | |
| — | | |
| 4,838,792 | | |
| — | | |
| — | |
| Basic
and diluted net income (loss) per share – Class A and Class B non-redeemable common stock | |
| — | | |
| (0.01 | ) | |
| — | | |
| — | |
UNAUDITED
PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2022
(in thousands, except share and per share data)
| | |
OneMedNet
(Historical) | | |
Data Knights
(Historical) | | |
Pro Forma
Adjustments
Assuming
Minimum and
Maximum
Redemption | | |
| |
Pro Forma
Combined
Assuming
Minimum and
Maximum
Redemption | |
| Revenues | |
$ | 1,153 | | |
$ | — | | |
$ | — | | |
| |
$ | 1,153 | |
| Cost of revenue | |
| 1,513 | | |
| — | | |
| — | | |
| |
| 1,513 | |
| Gross profit | |
| (360 | ) | |
| — | | |
| — | | |
| |
| (360 | ) |
| Operating costs and expenses: | |
| | | |
| | | |
| | | |
| |
| | |
| Selling, general and administrative expenses | |
| 5,755 | | |
| 5,309 | | |
| 1,000 | | |
AA | |
| 12,064 | |
| Total operating costs and expenses | |
| 5,755 | | |
| 5,309 | | |
| 1,000 | | |
| |
| 12,064 | |
| Loss from operations | |
| (6,115 | ) | |
| (5,309 | ) | |
| (1,000 | ) | |
| |
| (12,424 | ) |
| Other income (expense): | |
| | | |
| | | |
| | | |
| |
| | |
| Interest expense | |
| (403 | ) | |
| — | | |
| (90 | ) | |
| |
| (493 | ) |
| Change in fair value of derivative liability | |
| | | |
| 4,489 | | |
| — | | |
| |
| 4,489 | |
| Other income (expense) | |
| (47 | ) | |
| | | |
| | | |
| |
| (47 | ) |
| Realized and unrealized gains on Trust Account | |
| | | |
| 1,372 | | |
| (1,372 | ) | |
BB | |
| — | |
| Total other income (expense) | |
| (450 | ) | |
| 5,861 | | |
| (1,462 | ) | |
| |
| 3,949 | |
| Net income (loss) before income tax provision | |
| (6,565 | ) | |
| 552 | | |
| (2,462 | ) | |
| |
| (8,475 | ) |
| Income tax provision | |
| — | | |
| (215 | ) | |
| — | | |
| |
| (215 | ) |
| Net income (loss) | |
| (6,565 | ) | |
| 337 | | |
| (2,462 | ) | |
| |
| (8,690 | ) |
| | |
OneMedNet
(Historical) | | |
Data Knights
(Historical) | | |
Assuming
Minimum
Redemption | | |
Assuming
Maximum
Redemption | |
| Weighted average shares outstanding – Common stock | |
| 4,342,666 | | |
| — | | |
| — | | |
| — | |
| Basic and diluted net income per share – Common stock | |
| (0.68 | ) | |
| — | | |
| — | | |
| — | |
| Weighted average shares outstanding – Class A and Class B common stock subject to redemption | |
| — | | |
| 8,667,969 | | |
| 34,102,468 | | |
| 32,299,770 | |
Basic and diluted net income per share – Class A and
Class B common stock subject to redemption | |
| — | | |
| 0.03 | | |
| (0.25 | ) | |
| (0.27 | ) |
| Weighted average shares outstanding – Class A and Class B non-redeemable common stock | |
| — | | |
| 3,464,052 | | |
| — | | |
| — | |
Basic and diluted net income per share – Class A and
Class B non-redeemable common stock | |
| — | | |
| 0.03 | | |
| — | | |
| — | |
NOTES
TO THE UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTS
Note
1 — Description of the Merger
On
April 25, 2022, Data Knights, Data Knights Merger Sub, Inc., a Delaware corporation (“Merger Sub”), and Data Knights, LLC,
the Company’s sponsor (the “Sponsor”), entered into a definitive Agreement and Plan of Merger (the “Merger Agreement”)
with OneMedNet (together with the Company and Merger Sub, the “Parties”) and Paul Casey, as seller representative (“Casey”).
Pursuant to the Merger Agreement, upon the closing (the “Closing”) of the Business Combination, the Parties will affect the
merger of Merger Sub with and into OneMedNet, with OneMedNet continuing as the surviving entity (the “Merger”), as a result
of which all of the issued and outstanding capital stock of OneMedNet shall be exchanged shares of the Class A Common Stock of the Company
upon the terms set forth in the Merger Agreement. Per the Merger Agreement all of the issued and outstanding capital stock of OneMedNet
shall be exchanged shares of the Class A Common Stock of Data Knights. OneMedNet’s shareholders collectively shall be entitled
to receive from Data Knights, in the aggregate, a number of Data Knights’ securities with an aggregate value equal to $200,000,000,
subject to certain adjustments.
Note
2 — Basis of Presentation
The
unaudited pro forma condensed combined financial information was prepared in accordance with Article 11 of SEC Regulation S-X as amended
by the final rule, Release No. 33-10786 “Amendments to Financial Disclosures about Acquired and Disposed Businesses.” The
historical financial information of Data Knights and OneMedNet include transaction accounting adjustments to illustrate the estimated
effect of the Business Combination and certain other adjustments to provide relevant information necessary for an understanding of the
Combined Entity upon consummation of the transactions described herein.
The
Business Combination between Data Knights and OneMedNet under both the minimum and maximum redemption scenarios is expected to be accounted
for as a reverse recapitalization with OneMedNet as the accounting acquirer.
The
unaudited pro forma condensed combined financial information has been prepared using both the Minimum Redemption and Maximum Redemption
scenarios with respect to the potential redemption of Public Shares into cash. The public stockholder redemptions are expected to be
within the parameters described by the two scenarios. However, there can be no assurance regarding which scenario will be closest to
the actual results.
The
unaudited pro forma condensed combined financial information does not reflect the income tax effects of the transaction accounting adjustments
as any change in the deferred tax balance would be offset by an increase in the valuation allowance given the Companies’ incurred
losses during the historical period presented.
Note
3 — Transaction Accounting Adjustments to the Data Knights and OneMedNet Unaudited Pro Forma Condensed Combined Balance
Sheet as of June 30, 2023
The
transaction accounting adjustments included in the unaudited pro forma condensed combined balance sheet as of June 30, 2023 are as follows:
| |
(A) |
Reflects
the reclassification of $18.6 million of cash and cash equivalents held in the Trust Account at the balance sheet date that becomes
available to fund expenses in connection with the Business Combination or future cash needs of the Company. |
| |
|
|
| |
(B) |
Reflects
the payment of approximately $1.0 million of deferred underwriting fee and the remainder converted to stock at $10.89 per share. |
| |
|
|
| |
(C) |
Represents
transaction costs totaling $1.0 million. |
| | |
| | | |
| OMN | | |
| DK | |
| Total estimated | |
$ | 3.2 million | | |
| 1.8 million | | |
| 1.4 million | |
| Total incurred | |
$ | 2.2 million | | |
| 1.1 million | | |
| 1.1 million | |
| Net | |
$ | 1.0
million | | |
| 0.7 million | | |
| 0.3 million | |
| |
(D) |
Reflects
the reclassification of approximately $19 million of Class A shares subject to possible redemption to permanent equity. |
| |
|
|
| |
(E) |
Reflects
the conversion of convertible preferred stock to common stock. |
| |
|
|
| |
(F) |
Represents
the issuance of 20 million shares of the post-combination company’s Class A Common Stock to OneMedNet equity holders as consideration
for the reverse recapitalization. |
| |
|
|
| |
(G) |
Reflects
the conversion of Class B shares held by the initial shareholders to Class A shares. |
| |
|
|
| |
(H) |
Reflects
the reclassification of Data Knights’ historical accumulated deficit. |
| |
|
|
| |
(I) |
Reflects
the maximum redemption of 1.7 million shares for $19 million. |
| |
|
|
| |
(J) |
$1.5
million PIPE — 12 month notes with 6% interest. |
| |
|
|
| |
(K) |
Reflects
the conversion of convertibles notes and accrued interest at a rate of $2.50 per share. |
| |
|
|
| |
(L) |
Reflects
the conversion of Sponsor loans at a rate of $10.00 per share. |
| |
|
|
| |
(M) |
Reflects
partial payment of related party loan. |
Note
4 — Transaction Accounting Adjustments to the Data Knights and OneMedNet Unaudited Pro Forma Condensed Combined Statement
of Operations for the six months ended June 30, 2023
The
transaction accounting adjustments included in the unaudited pro forma condensed combined statement of operations for the six months
ended June 30, 2023 are as follows:
(AA)
Transaction costs.
(BB)
Elimination of realized and unrealized gains in the trust.
(CC)
Interest on short debt.
Note
5 — Loss Per Share
Net
loss per share calculated using the historical weighted average shares outstanding, and the issuance of additional shares in connection
with the Business Combination assuming the shares were outstanding since January 1, 2021. As the Business Combination are being reflected
as if they had occurred at the beginning of the periods presented, the calculation of weighted average shares outstanding for basic and
diluted net loss per share assumes that the shares issuable relating to the Business Combination have been outstanding for the entire
period presented. If the maximum number of shares are redeemed, this calculation is retroactively adjusted to eliminate such shares for
the entire periods. Warrants have been excluded from the calculation as they are anti-dilutive.
The
unaudited pro forma condensed combined financial information has been prepared assuming five alternative levels of redemption for the
six months ended June 30, 2023 and year ended December 31, 2022:
| | |
OneMedNet Historical | | |
Data Knights Historical | | |
Minimum Redemption | | |
33.33% of Maximum Redemption | | |
50% of Maximum Redemption | | |
66.67% of Maximum Redemption | | |
Maximum Redemption | |
| June 30, 2023 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Book value per diluted share | |
| (2.95 | ) | |
| (1.33 | ) | |
| 0.40 | | |
| 0.22 | | |
| 0.13 | | |
| 0.03 | | |
| (0.16 | ) |
| Weighted average shares outstanding – Common stock | |
| 4,550,166 | | |
| — | | |
| 34,102,468 | | |
| 33,502,170 | | |
| 33,201,119 | | |
| 32,900,158 | | |
| 32,299,770 | |
| Basic and diluted net income per share – Common stock | |
| (0.27 | ) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding – Class A and Class B common stock subject to redemption | |
| | | |
| 2,731,544 | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted net income per share – Class A and Class B common stock subject to redemption | |
| | | |
| (0.01 | ) | |
| (0.16 | ) | |
| (0.17 | ) | |
| (0.17 | ) | |
| (0.17 | ) | |
| (0.17 | ) |
| Weighted average shares outstanding – Class A and Class B non-redeemable common stock | |
| | | |
| 4,838,792 | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted net income per share – Class A and Class B non-redeemable | |
| | | |
| (0.01 | ) | |
| | | |
| | | |
| | | |
| | | |
| | |
| December 31, 2022 | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding – Common stock | |
| 4,342,666 | | |
| | | |
| 34,102,468 | | |
| 33,502,170 | | |
| 33,201,119 | | |
| 32,900,158 | | |
| 32,299,770 | |
| Basic and diluted net income per share – Common stock | |
| (0.68 | ) | |
| | | |
| (0.25 | ) | |
| (0.26 | ) | |
| (0.26 | ) | |
| (0.26 | ) | |
| (0.27 | ) |
| Weighted average shares outstanding – Class A and Class B common stock subject to redemption | |
| | | |
| 8,667,969 | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted net income per share – Class A and Class B common stock subject to redemption | |
| | | |
| 0.03 | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding – Class A and Class B non-redeemable common stock | |
| | | |
| 3,464,052 | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted net income per share – Class A and Class B non-redeemable | |
| | | |
| 0.03 | | |
| | | |
| | | |
| | | |
| | | |
| | |
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The
following discussion and analysis of OneMedNet Corporation (formerly named Data Knights Acquisition Corp.) financial condition and results
of operations should be read in conjunction with its audited consolidated financial statements and the notes related thereto which are
included elsewhere in this prospectus. Certain information contained in the discussion and analysis set forth below includes forward-looking
statements. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors,
including those set forth under “Special Note Regarding Forward-Looking Statements,” “ Risk Factors” and elsewhere
in this prospectus.
Company
Overview
OneMedNet
Corporation, a Delaware corporation (the “Company,” “we,” “us” or “OneMedNet”) together
with its wholly-owned subsidiary, OneMedNet Solutions Corporation, a Delaware corporation, and its wholly-owned subsidiary,
OneMedNet Technologies (Canada) Inc., incorporated under the provisions of the Business Corporations Act of British Columbia whose functional
currency is the Canadian dollar. All references in this prospectus to the “Company,” “we,” “us,”
or “OneMedNet” include OneMedNet Corporation and both OneMedNet Solutions Corporation and OneMedNet
Technologies (Canada) Inc., except that references to the “Company” “we,” or “us,” refer to
OneMedNet Corporation f/k/a Data Knights Acquisition Corp.
We
were originally incorporated in Delaware on February 8, 2021 under the name “Data Knights Acquisition Corp” as a special
purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase,
reorganization or similar business combination with one or more businesses. On May 11, 2021, we consummated an initial public offering.
On
November 7, 2023, following the approval at the special meeting of the shareholders of Data Knights Acquisition Corp., a Delaware corporation
held on October 17, 2023 (the “Special Meeting”), Data Knights Merger Sub, Inc., a Delaware corporation (“Merger Sub”)
and a wholly-owned subsidiary of Data Knights Acquisition Corp., a Delaware corporation (“Data Knights”), consummated a merger
(the “Merger”) with and into OneMedNet Solutions Corporation (formerly named OneMedNet Corporation), a Delaware corporation
(“OneMedNet”) pursuant to an agreement and plan of merger, dated as of April 25, 2022 (the “Merger Agreement”),
by and among Data Knights, Merger Sub, OneMedNet, Data Knights, LLC, a Delaware limited liability company (“Sponsor” or “Purchaser
Representative”) in its capacity as the representative of the stockholders of Data Knights, and Paul Casey in his capacity as the
representative of the stockholders of OneMedNet (“Seller Representative”). Accordingly, the Merger Agreement was adopted,
and the Merger and other transactions contemplated thereby (collectively, the “Business Combination”) were approved and completed.
At
the closing, on November 7, 2023, of the Business Combination pursuant to the Merger Agreement, Merger Sub merged with and into OneMedNet
with OneMedNet surviving the Merger, as a wholly-owned subsidiary of Data Knights, and Data Knights changed its name to “OneMedNet
Corporation.”
The
Business Combination was accounted for as a reverse recapitalization in accordance with U.S. GAAP. Under this method of accounting, Data
Knights was treated as the acquired company and OneMedNet Corporation was treated as the acquirer for financial statement reporting purposes.
At
the closing of the Merger, holders of Data Knights common stock automatically received common stock of OneMedNet, and holders of Data
Knights warrants automatically received warrants of OneMedNet with substantively identical terms. At the Closing of the Business Combination,
all shares of Data Knights owned by the Sponsor (consisting of shares of Class A common stock and shares of Class B common stock), which
we refer to as the founder shares, automatically converted into an equal number of shares of OneMedNet’s common stock, and Private
Placement Warrants held by the Sponsor, automatically converted into warrants to purchase one share of OneMedNet common stock with substantively
identical terms.
On
June 28, 2023, the Company and Data Knights entered into a Securities Purchase Agreement (the “SPA”) with certain investors
(collectively referred to herein as the “Purchasers”) for PIPE financing in the aggregate original principal amount of $1,595,744.70
and the purchase price of $1.5 million. Pursuant to the Securities Purchase Agreement, Data Knights will issue and sell to each of the
Purchasers, a new series of senior secured convertible notes (the “PIPE Notes”), which are convertible into shares of Common
Stock at the Purchasers election at a conversion price equal to the lower of (i) $10.00 per share, and (ii) 92.5% of the lowest volume
weighted average trading price for the ten (10) Trading Days immediately preceding the Conversion Date. The Purchasers’ $1.5 million
investment in the PIPE Notes closed and funded contemporaneous to the Closing of the Business Combination. Effective immediately prior
to the Closing, Data Knights issued the PIPE Notes to the Purchasers pursuant to the private offering rules under the Securities Act
of 1933, as amended (the “Securities Act”).
As
of the Closing, among other holders, public stockholders own 98,178 shares of OneMedNet common stock approximately representing 0.35%
of the outstanding shares of OneMedNet common stock; the Sponsor and its affiliates own approximately 15.1% of the outstanding shares
of OneMedNet common stock (inclusive of shares received upon conversion of the Sponsor’s loan); OneMedNet’s former security
holders own approximately 61.992% of the outstanding shares of OneMedNet common stock from the conversion of their shares; PIPE investors
own 0.46% of the outstanding shares of OneMedNet common stock and former convertible note holders own approximately 16.24% of the outstanding
shares of OneMedNet common stock resulting from the issuance of 5,238,800 shares of Common Stock upon conversion of their notes.
In
addition, at the closing of the Merger, the Company issued an aggregate of 277,778 shares of the
Company’s Company Stock at $10.89 per share, for an aggregate value of $3,025,000 (the “Compensation Shares”)
to EF Hutton, LLC, division of Benchmark Investments, LLC (“EF
Hutton”) win partial satisfaction of fees due to them in connection with the
Merger.
The
Business Combination is a subsequent event that occurred after the periods for which the financial information herein is presented. The
financial information included in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
reflects the historical operations of OneMedNet prior to the Business Combination, unless otherwise noted. For additional information
on the Business Combination please see the section titled “Recent Developments” on page 22 of this prospectus. For
additional information on the corporate history of our Company please see the section titled “Corporate History” on
page 4 of this prospectus.
Business
Overview
OneMedNet
Corporation (“OneMedNet” or “OMN”) is a provider of leader in clinical imaging innovation and experienced curator
of regulatory-grade Imaging Real-World DataTM
or iRWDTM.
Our innovation solutions connect healthcare providers and patients satisfying a crucial need within the Life Sciences field offering
direct access to clinical images and associated contextual patient record. Our innovative technology proved the commercial and regulatory
viability of imaging Real-World Data, a promising emerging market, and provides regulatory-grade image-centric iRWDTM
that exactly matches our
Life Science partners’ Case Selection Protocol.
OneMedNet
was founded in 2006 to solve a deficiency in how clinical images were shared between healthcare providers. This resulted in our initial
product BEAMTM
image exchange that enabled
the successful sharing of images for more than a decade. OneMedNet continued to innovate which responded to the demand for and utilization
of Real-World Data, specifically data that focused on clinical images. We were able to leverage internal technological competencies along
with a formidable healthcare provider installed database from our first product with BEAMTM
to become the first RWD
solution for Life Science companies with our launch of iRWDTM
in 2019.
OneMedNet
provides innovative solutions that unlock the significant value contained within clinical image archives. With a growing federated network
of 90+ healthcare providers, OneMedNet has the immediate ability to quickly search and extensively curate multi-layer data from a Federated
group of healthcare facilities. OneMedNet is ahead of the curve when it comes to providing fast and secure access to curated medical
images. Initially, it was all about solving the diverse access needs of patient care providers. This focus systematically evolved to
addressing the rapidly growing needs of image analysis and researchers, clinicians, regulators, scientists and more.
Beyond
the economic opportunity, we believe we have a unique opportunity to affect a material positive impact on the lives of tens of millions
of people, while also improving business productivity. First and foremost, OneMedNet’s iRWDTM
offering plays a significant
role in enabling Life Science companies to bring safer and more effective patient care to market sooner. Using our highly curated de-identified
clinical data in our iRWDTM
offering in healthcare
providers’ product development, validation, and regulatory approval processes, they contribute to patient care advancements in
more meaningful ways. Moreover, healthcare providers improve their product development and validation processes, which benefits all parties.
Significant
documentation exists that shows that Real-World Data can provide expanded insights across broader and more representative patient populations
and holds great potential for
generating real-world evidence for designing and conducting confirmatory trials and answering questions that may not be addressed otherwise,
according to a November 5, 2022
article by BMC Medical Research Methodology.
For this reason, the Food and Drug Administration (“FDA”) is instituting Real-World Data usage guidelines for regulatory
approvals, which can supplement or supplant clinical trials that would be premised on the utilization of highly reliable and quality
Real-World Data that strictly adheres to all of the very specific data stratification requirements.
The
increasing accessibility of Real-World
Data and the fast development
of artificial intelligence (AI) and machine learning (ML) techniques, together with rising costs and recognized limitations of traditional
trials, has spurred great interest in the use of Real-World
Data to enhance the efficiency
of clinical research and discoveries and bridge the evidence gap between clinical research and practice. OneMedNet
covers the complete value chain in imaging Real-World Data; it begins with our 10+ year federated network of providers and is supported
by a multi-faceted data curation process managed by an expert in-house clinical team.
Additionally,
we work hand-in-hand with our Life Science partners regarding the Case Selection Protocol and when required producing Case Report Forms
for regulatory clearance. We are focused on delivering value in by supporting Life Science Advancements with OneMedNet’s iRWDTM
that holds the key to unlocking
boundless patient care advances. We unleash the power of research-grade image-centric iRWDTM
that is highly curated
to painstakingly meet every cohort requirement and stand up to all of the rigors of prospective clinical trials.
Today,
life science companies, typically pharmaceutical companies, artificially intelligence (AI) developers, medical device businesses, and
clinical research organizations share the same widespread challenge in obtaining insight-rich, high-quality patient data that explicitly
matches their precise cohort specifications. A substantial portion of patient care diagnosis involves clinical imaging and approximately
90% of healthcare data, by size, is associated with imaging. Historically, much of imaging value has been derived from its initial review
and further gains from the image archives have been very limited.
We
help providers to “Unlock the Value in Imaging Archives”.TM
By utilizing OneMedNet’s
iRWDTM
offering, providers can
greatly improve their research efforts with streamlined data access. Health care providers can also accelerate life science patient care
innovations by sharing de-identified data in a well-defined and de-identified and secure manner. In return for doing so, income is generated
and applied to critical and possibly unfunded provider projects. OneMedNet believes that access to high quality data with advanced analytics
and AI companions enable healthcare providers to make smarter decisions:
| |
● |
Longitudinal
patient and cohort data from imaging, laboratory and genomics can help to improve clinical decisions in single interventions, along
patient pathways or across whole populations. |
| |
● |
Enterprise-wide,
real-time, operational data is needed for better operational decisions targeting single asset and fleets, workflows and workforce or
the overall enterprise performance. |
| |
● |
Consumer
decision-making benefits from self-scheduling, health informatics or even predictive wellness coaches. |
Our
Business Model
Clinical
images remain one of the most important tools used in delivering and improving patient care. For healthcare providers, timely access — no
matter the source, directly impacts patient lives. OneMedNet first responded to this need with the BEAM™
Image Exchange solution offering
a unique combination of simplicity, security, and low cost. Pharmaceutical companies, Imaging AI developers, Device organizations, Clinical
Research Organizations, and Core Labs are all challenged to find the volume and specificity of imaging real world data to fulfill their
respective needs.
OneMedNet’s
innovative technology which Unlocks the Value in Imaging Data® for both providers and aforementioned industry members in our
iRWD™ solution
goes far beyond simply sourcing de-identified images. Frankly, other organizations can do that. OneMedNet has the clinical expertise
to understand and anticipate the nuances and depth of the most stringent and specific imaging cohort request. By quickly tapping into
our large federated healthcare provider network, we are able to adeptly move from request to fulfillment — with comprehensive
regulatory-grade patient data. And if additional patient insights are required, OneMedNet is setup and ready to support progressive needs.
WHERE
YOU CAN FIND MORE INFORMATION
This
prospectus is part of a registration statement we filed with the SEC. This prospectus does not contain all of the information set forth
in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities
we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the
registration statement. You should rely only on the information contained in this prospectus or incorporated by reference into this prospectus.
We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any jurisdiction
where the offer is not permitted. You should assume that the information contained in this prospectus, or any document incorporated by
reference in this prospectus, is accurate only as of the date of those respective documents, regardless of the time of delivery of this
prospectus or any sale of our securities.
We
file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the
public from commercial document retrieval services and over the Internet at the SEC’s website at http://www.sec.gov. We maintain
a website at www.sonnetbio.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form
8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge
at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information
contained in, or that can be accessed through, our website is not incorporated by reference into, and is not part of, this prospectus.
INCORPORATION
OF DOCUMENTS BY REFERENCE
This
prospectus is part of the registration statement but the registration statement includes and incorporates by reference additional information
and exhibits. The SEC permits us to “incorporate by reference” the information contained in documents we file with the SEC,
which means that we can disclose important information to you by referring you to those documents rather than by including them in this
prospectus. Information that is incorporated by reference is considered to be part of this prospectus and you should read it with the
same care that you read this prospectus. Information that we file later with the SEC will automatically update and supersede the information
that is either contained, or incorporated by reference, in this prospectus, and will be considered to be a part of this prospectus from
the date those documents are filed. We have filed with the SEC, and incorporate by reference in this prospectus:
| |
● |
Annual
Report on Form 10-K for the year ended December 31, 2022 filed with the SEC on March 31,
2023 and for the year ended December 31, 2021 filed with the SEC on April 1,
20232; |
| |
● |
Current
Report on Form 8-K, filed with the SEC on November 13, 2023; and |
| |
● |
The
description of our Common Stock contained in our Registration Statement on Form 8-A12b filed with
the SEC on May 5, 2021, and any amendments or reports filed updating such description. |
Notwithstanding
the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information
that we have “furnished” to the SEC pursuant to the Securities Exchange Act of 1934, as amended shall be incorporated by
reference into this prospectus. We will furnish without charge to you, on written or oral request, a copy of any or all of the documents
incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to:
OneMedNet
Corporation
6385
Old Shady Oak Road, Suite 250
Eden Prairie, MN 55344
Telephone:
800-918-7189
You
also may access these filings on our website at http://www.sidusspace.com. We do not incorporate the information on our website into
this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through,
our website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically
incorporate by reference into this prospectus or any supplement to this prospectus).
Any
statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified,
superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes
or replaces such statement. Any statement contained herein or in any document incorporated or deemed to be incorporated by reference
shall be deemed to be modified or superseded for purposes of the registration statement of which this prospectus forms a part to the
extent that a statement contained in any other subsequently filed document which also is or is deemed to be incorporated by reference
modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed to constitute a part of the registration
statement of which this prospectus forms a part, except as so modified or superseded.
PART
I - FINANCIAL INFORMATION
Item
1. Financial Statements
ONEMEDNET
CORPORATION
(F/K/A
DATA KNIGHTS ACQUISITION CORP.)
CONDENSED
CONSOLIDATED BALANCE SHEETS
| | |
September 30, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| | |
(Unaudited) | | |
(Audited) | |
| ASSETS | |
| | | |
| | |
| Current assets | |
| | | |
| | |
| Cash | |
$ | 1,537 | | |
$ | 30,870 | |
| Prepaid expense | |
| 21,600 | | |
| - | |
| Total Current Assets | |
| 23,137 | | |
| 30,870 | |
| | |
| | | |
| | |
| Investments held in Trust Account | |
| 19,308,261 | | |
| 29,029,416 | |
| Total assets | |
$ | 19,331,398 | | |
$ | 29,060,286 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDER’S EQUITY | |
| | | |
| | |
| Current Liabilities | |
| | | |
| | |
| Accrued expenses | |
$ | 2,835,755 | | |
$ | 1,679,821 | |
| Amount due to related parties | |
| 11,200 | | |
| 11,500 | |
| Income tax payable | |
| 120,017 | | |
| 214,850 | |
| Franchise tax payable | |
| - | | |
| 69,966 | |
| Excise tax liability | |
| 113,353 | | |
| - | |
| Total Current Liabilities | |
| 3,080,325 | | |
| 1,976,137 | |
| | |
| | | |
| | |
| Warrant liabilities | |
| 604,849 | | |
| 362,558 | |
| Deferred underwriter fee payable | |
| 4,025,000 | | |
| 4,025,000 | |
| Extension loan | |
| 3,556,278 | | |
| 207,081 | |
| Working capital loans | |
| 477,548 | | |
| 2,545,838 | |
| Total liabilities | |
| 11,744,000 | | |
| 9,116,614 | |
| | |
| | | |
| | |
| Commitments and Contingencies | |
| - | | |
| - | |
| Class A Common Stock subject to possible redemption;1,712,698 and 2,731,544 shares at redemption value of $11.22 and $10.53 per share as of September 30, 2023 and December 31, 2022, respectively | |
| 19,209,844 | | |
| 28,750,110 | |
| | |
| | | |
| | |
| Stockholders’ Deficit | |
| | | |
| | |
| Preferred shares, $0.0001 par value; 1,000,000 shares authorized; none issued and outstanding | |
| - | | |
| - | |
| Class A Common Stock, $0.0001 par value; 100,000,000 shares authorized; 585,275 issued and outstanding, excluding 1,712,698 and 2,731,544 shares subject to redemption as of September 30, 2023 and December 31, 2022, respectively | |
| 59 | | |
| 59 | |
| Class B Common Stock, par value $0.0001; 10,000,000 shares authorized; 4,253,517 issued and outstanding as of September 30, 2023 and December 31, 2022, respectively | |
| 425 | | |
| 425 | |
| Common Stock, value | |
| 425 | | |
| 425 | |
| Additional paid-in capital | |
| 917,476 | | |
| 2,825,823 | |
| Accumulated deficit | |
| (12,540,406 | ) | |
| (11,632,745 | ) |
| Total Stockholders’ Deficit | |
| (11,622,446 | ) | |
| (8,806,438 | ) |
| Total Liabilities and Stockholders’ Deficit | |
$ | 19,331,398 | | |
$ | 29,060,286 | |
The
accompanying notes are an integral part of the condensed consolidated financial statements.
ONEMEDNET
CORPORATION
(F/K/A
DATA KNIGHTS ACQUISITION CORP.)
CONDENSED
CONSOLIDATED STATEMENTS OF OPERATIONS
(UNAUDITED)
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
For the | | |
For the | |
| | |
Three Months Ended | | |
Nine months ended | |
| | |
September 30, | | |
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Formation and operating costs | |
$ | 908,077 | | |
$ | 467,223 | | |
$ | 1,455,434 | | |
$ | 1,771,902 | |
| Franchise tax expense | |
| 10,400 | | |
| 52,802 | | |
| 102,281 | | |
| 152,802 | |
| Loss from operation costs | |
| (918,477 | ) | |
| (520,025 | ) | |
| (1,557,715 | ) | |
| (1,924,704 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | |
| Dividends, realized and unrealized gain in Trust Account | |
| 431,961 | | |
| 587,303 | | |
| 1,102,363 | | |
| 779,706 | |
| Change in fair value of warrant liabilities | |
| (242,291 | ) | |
| (113,829 | ) | |
| (242,291 | ) | |
| 4,243,893 | |
| Provision for income taxes | |
| (87,713 | ) | |
| (106,724 | ) | |
| (210,017 | ) | |
| (106,724 | ) |
| Net income (loss) | |
$ | (816,520 | ) | |
$ | (153,275 | ) | |
$ | (907,660 | ) | |
$ | 2,992,171 | |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding of Class A Common Stock subject to redemption | |
| 2,166,749 | | |
| 11,500,000 | | |
| 2,541,210 | | |
| 11,500,000 | |
| Basic and diluted net income (loss) per common stock | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average shares outstanding of Class A and Class B non-redeemable common stock | |
| 4,838,792 | | |
| 3,460,275 | | |
| 4,838,792 | | |
| 3,460,275 | |
| Basic and diluted net income (loss) per common stock | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
The
accompanying notes are an integral part of the condensed consolidated financial statements.
ONEMEDNET
CORPORATION
(F/K/A
DATA KNIGHTS ACQUISITION CORP.)
CONDENSED
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
FOR
THE THREE AND NINE MONTHS ENDED SEPTEMBER 30, 2023
(UNAUDITED)
| | |
Shares | | |
Amounts | | |
Shares | | |
Amounts | | |
Capital | | |
Deficit | | |
Deficit | |
| | |
Class A | | |
Class B | | |
Additional | | |
| | |
Total | |
| | |
Common Stock | | |
Common Stock | | |
Paid in | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amounts | | |
Shares | | |
Amounts | | |
Capital | | |
Deficit | | |
Deficit | |
| Balance — January 1, 2023 | |
| 585,275 | | |
$ | 59 | | |
| 4,253,517 | | |
$ | 425 | | |
$ | 2,242,253 | | |
$ | (11,632,745 | ) | |
$ | (8,806,438 | ) |
| Re-measurement of Class A Common Stock Subject to Possible Redemption | |
| — | | |
| — | | |
| — | | |
| — | | |
| (583,570 | ) | |
| — | | |
| (583,570 | ) |
| Net Loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (85,983 | ) | |
| (85,983 | ) |
| Balance — March 31, 2023 (unaudited) | |
| 585,275 | | |
$ | 59 | | |
| 4,253,517 | | |
$ | 425 | | |
$ | 2,242,253 | | |
$ | (11,718,728 | ) | |
$ | (9,475,991 | ) |
| Re-measurement of Class A Common Stock Subject to Possible Redemption | |
| — | | |
| — | | |
| — | | |
| — | | |
| (604,656 | ) | |
| — | | |
| (604,656 | ) |
| Net Loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (5,158 | ) | |
| (5,158 | ) |
| Balance — June 30, 2023 (unaudited) | |
| 585,275 | | |
$ | 59 | | |
| 4,253,517 | | |
$ | 425 | | |
$ | 1,637,597 | | |
$ | (11,723,886 | ) | |
$ | (10,085,805 | ) |
| Re-measurement of Class A Common Stock Subject to Possible Redemption | |
| — | | |
| — | | |
| — | | |
| — | | |
| (606,768 | ) | |
| — | | |
| (606,768 | ) |
| Excise tax liability | |
| — | | |
| — | | |
| — | | |
| — | | |
| (113,353 | ) | |
| — | | |
| (113,353 | ) |
| Net Loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (816,520 | ) | |
| (816,520 | ) |
| Balance — September 30, 2023 (unaudited) | |
| 585,275 | | |
$ | 59 | | |
| 4,253,517 | | |
$ | 425 | | |
$ | 917,476 | | |
$ | (12,540,406 | ) | |
$ | (11,622,446 | ) |
FOR
THE THREE AND NINE MONTHS ENDED SEPTEMBER 30, 2022
(UNAUDITED)
| | |
Class A | | |
Class B | | |
Additional | | |
| | |
Total | |
| | |
Common Stock | | |
Common Stock | | |
Paid in | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amounts | | |
Shares | | |
Amounts | | |
Capital | | |
Deficit | | |
Deficit | |
| Balance — January 1, 2022 | |
| 585,275 | | |
$ | 59 | | |
| 2,875,000 | | |
$ | 288 | | |
$ | — | | |
$ | (8,609,810 | ) | |
$ | (8,609,463 | ) |
| Re-measurement of carrying value of Class A redeemable stock to redemption value | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (1,150,000 | ) | |
| (1,150,000 | ) |
| Net income | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 3,145,445 | | |
| 3,145,455 | |
| Balance — June 30, 2022 (unaudited) | |
| 585,275 | | |
$ | 59 | | |
| 2,875,000 | | |
$ | 288 | | |
$ | — | | |
$ | (6,614,365 | ) | |
$ | (6,614,018 | ) |
| Re-measurement of carrying value of Class A redeemable stock to redemption value | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (1,150,000 | ) | |
| (1,150,000 | ) |
| Accretion of Class A common stocks at redemption value | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (533,869 | ) | |
| (533,869 | ) |
| Net income | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (153,275 | ) | |
| (153,275 | ) |
| Net income (loss) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (153,275 | ) | |
| (153,275 | ) |
| Balance — September 30, 2022 (unaudited) | |
| 585,275 | | |
$ | 59 | | |
| 2,875,000 | | |
$ | 288 | | |
$ | — | | |
$ | (8,451,509 | ) | |
$ | (8,451,162 | ) |
| Balance | |
| 585,275 | | |
$ | 59 | | |
| 2,875,000 | | |
$ | 288 | | |
$ | — | | |
$ | (8,451,509 | ) | |
$ | (8,451,162 | ) |
The
accompanying notes are an integral part of the condensed consolidated financial statements.
ONEMEDNET
CORPORATION
(F/K/A
DATA KNIGHTS ACQUISITION CORP.)
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| | |
2023 | | |
2022 | |
| | |
For the Nine Months | |
| | |
Ended | |
| | |
September 30, | |
| | |
2023 | | |
2022 | |
| Cash flow from operating activities: | |
| | | |
| | |
| Net income (loss) | |
$ | (907,660 | ) | |
$ | 2,992,171 | |
| Adjustments to reconcile net income to net cash used in operating activities: | |
| | | |
| | |
| Dividends, realized and unrealized gain in Trust Account | |
| (1,102,363 | ) | |
| (779,706 | ) |
| Change in fair value of warrant liability | |
| 242,291 | | |
| (4,243,893 | ) |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Prepaid expense | |
| (21,600 | ) | |
| 83,061 | |
| Accrued expenses | |
| 1,155,934 | | |
| 1,159,114 | |
| Franchise tax payable | |
| (69,966 | ) | |
| (114,008 | ) |
| Income tax payable | |
| (94,833 | ) | |
| 106,724 | |
| Net cash used in operating activities | |
| (798,197 | ) | |
| (796,537 | ) |
| | |
| | | |
| | |
| Cash flow from investing activities: | |
| | | |
| | |
| Investment of cash in Trust Account | |
| (1,010,440 | ) | |
| (2,300,000 | ) |
| Cash withdrawn from Trust Account in connection with redemption | |
| 11,335,260 | | |
| - | |
| Interest withdraw from Trust Account | |
| 498,697 | | |
| 226,809 | |
| Net cash used in investing activities | |
| 10,823,517 | | |
| (2,033,191 | ) |
| | |
| | | |
| | |
| Cash flow from financing activities: | |
| | | |
| | |
| Advances from related parties | |
| (300 | ) | |
| — | |
| Proceeds from working capital loan | |
| 270,467 | | |
| 300,000 | |
| Redemption of Class A common stock | |
| (11,335,260 | ) | |
| - | |
| Amount due to related parties | |
| - | | |
| 11,500 | |
| Proceeds from extension loans | |
| 1,010,440 | | |
| 2,300,000 | |
| Net cash provided by financing activities | |
| (10,054,653 | ) | |
| 2,611,500 | |
| | |
| | | |
| | |
| Net change in cash | |
| (29,333 | ) | |
| (218,228 | ) |
| Cash at the beginning of the period | |
| 30,870 | | |
| 453,151 | |
| Cash at the end of the period | |
$ | 1,537 | | |
$ | 234,923 | |
| | |
| | | |
| | |
| Supplemental disclosure of non-cash financing activities: | |
| | | |
| | |
| Re-measurement of Class A common stock subject to possible redemption | |
$ | 1,794,994 | | |
$ | 2,833,869 | |
| Excise tax liability accrued for Class A Common Stock subject to redemption | |
$ | 113,353 | | |
$ | - | |
The
accompanying notes are an integral part of the condensed consolidated financial statements.
ONEMEDNET
CORPORATION
(F/K/A
DATA KNIGHTS ACQUISITION CORP.)
NOTES
TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS
OneMedNet
Corporation (f/k/a Data Knights Acquisition Corp.) (the “Company”) was incorporated in Delaware on February 8, 2021. The
Company was formed for the purpose of effectuating a merger, capital stock exchange, asset acquisition, stock purchase, reorganization
or other similar business combination with one or more businesses (the “Business Combination”). The Company is an early stage
and emerging growth company and, as such, the Company is subject to all of the risks associated with early stage and emerging growth
companies.
As
of September 30, 2023, the Company had not yet commenced any operations. All activity for the period February 8, 2021 (inception) through
September 30, 2023, relates to the Company’s formation and the initial public offering (the “Initial Public Offering”),
and, since the closing of the initial public offering, the Company has entered into a merger agreement (as described below), and continued
a search for a Business Combination candidate. The Company has selected December 31 as its fiscal year end.
The
registration statement for the Company’s Initial Public Offering was declared effective on May 6, 2021. On May 11, 2021, the Company
consummated the Initial Public Offering of 11,500,000 units (“Units” and, with respect to the shares of Class A Common Stock
included in the Units offered, the “Public Shares”), generating gross proceeds of $115,000,000, which is described in Note
3.
Simultaneously
with the closing of the Initial Public Offering, the Company consummated the sale of 585,275 private placement units (the “Private
Placement Units”) at a price of $10.00 per unit in a private placement to the Sponsor, generating gross proceeds of $5,852,750,
which is described in Note 4.
Following
the closing of the Initial Public Offering on May 11, 2021, an amount of $117,300,000 ($10.00 per Unit) from the net proceeds of the
sale of the Units in the Initial Public Offering and the Private Placement Units was placed in a trust account (“Trust Account”)
which may be invested in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act of
1940, as amended (the “Investment Company Act”), with a maturity of 185 days or less or in any open-ended investment company
that holds itself out as a money market fund meeting the conditions of Rule 2a-7 of the Investment Company Act, as determined by the
Company, until the earlier of: (i) the consummation of a Business Combination or (ii) the distribution of the Trust Account to the Company’s
stockholders, as described below.
Transaction
costs of the Initial Public Offering amounted to $6,771,112, of which $2,300,000 was for underwriting fees paid at the time of the IPO,
$4,025,000 was for deferred underwriting commissions, and $446,112 was for other offering costs.
Following
the closing of the Initial Public Offering $959,560 of cash was held outside of the Trust Account available for working capital purposes.
As of September 30, 2023, the Company has $1,537 of cash and a working capital deficit of $2,943,835.
The
Company’s management has broad discretion with respect to the specific application of the net proceeds of the Initial Public Offering
and the sale of the Private Placement Units, although substantially all of the net proceeds are intended to be applied generally toward
consummating a Business Combination. NASDAQ rules provide that the Business Combination must be with one or more target businesses that
together have a fair market value equal to at least 80% of the balance in the Trust Account (as defined below) (less any deferred underwriting
commissions and taxes payable on interest earned on the Trust Account) at the time of the signing of a definitive agreement to enter
a Business Combination. The Company will only complete a Business Combination if the post-Business Combination company owns or acquires
50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target sufficient
for it not to be required to register as an investment company under the Investment Company Act. There is no assurance that the Company
will be able to successfully affect a Business Combination.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
On
April 25, 2022, the Company, Data Knights Merger Sub, Inc., a Delaware corporation (“Merger Sub”), and Data Knights, LLC,
the Company’s sponsor (the “Sponsor”), entered into a definitive Agreement and Plan of Merger (the “Merger Agreement”)
with OneMedNet Corporation, Inc., a Delaware corporation (the “Target”, and together with the Company and Merger Sub, the
“Parties”) and Paul Casey, as seller representative (“Casey”). Pursuant to the Merger Agreement, upon the closing
(the “Closing”) of the Business Combination, the Parties will effect the merger of Merger Sub with and into the Target, with
the Target continuing as the surviving entity (the “Merger”), as a result of which all of the issued and outstanding capital
stock of the Target shall be exchanged shares of the Class A Common Stock of the Company upon the terms set forth in the Merger Agreement.
On
May 5, 2022, the Company extended the date by which the Company has to consummate a business combination from May 11, 2022 to August
11, 2022 (the “First Extension”). The First Extension was the first of two three-month extensions permitted under the Company’s
governing documents.
On
August 10, 2022, the Company extended the date by which the Company has to consummate a business combination from August 11, 2022 to
November 11, 2022 (the “Second Extension”). The Second Extension was the second of two three-month extensions permitted under
the Company’s governing documents.
On
October 27, 2022, the Company filed a definitive proxy statement with the SEC in connection with the Company’s solicitation of
proxies for the vote by the stockholders of the Company at a special meeting of the Company’s stockholders to be held on November
11, 2022 (the “Special Meeting”).
On
November 11, 2022, at 10:00 a.m. ET, the Company held a virtual special meeting of its stockholders. At the special meeting, Company
stockholders entitle to vote at the special meeting cast their votes and approved the Trust Amendment Proposal, pursuant to which
the Trust Agreement was amended to extend the date on which Continental must liquidate the Trust Account established in connection
with the IPO if the Company has not completed its initial business combination, from November 11, 2022 to August 11, 2023 (or such
earlier date after November 11, 2022, as determined by the Company Board). As a part of Special Meeting, the Company’s
stockholders approved amendments to its second amended and restated certificate of incorporation (the “Extension
Amendment”) and the investment management trust agreement (the “Trust Agreement”) between Continental Stock
Transfer & Trust Company, as trustee (“Continental”), and the Company governing the trust account (the “Trust
Account”) established in connection with the Company’s initial public offering dated May 11, 2021 (the “Trust
Amendment”), which together allow the Company to extend the deadline by which it must complete its initial business
combination by up to nine one-month periods. In connection with each such extension, Data Knights, LLC, the Company’s sponsor,
shall cause $0.045
per outstanding share of the Company’s Class A Common Stock, or approximately $122,920,
to be deposited in the Trust Account. On July 12, 2023, the Company elected to exercise its ninth of nine one-month extension to the
Termination Date, which extended its deadline to complete its initial business combination to August 11, 2023, by depositing $0.045
per share for each Public Share outstanding after giving effect to the redemptions disclosed above, or approximately $122,920,
was deposited in the Trust Account.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
In
connection with the proposed Business Combination with the Target, the Company will provide its public stockholders with the opportunity
to redeem all or a portion of their Class A Common Stock upon the completion of such Business Combination in connection with a stockholder
meeting called to approve such Business Combination. In the event the proposed Business Combination with the Target is not consummated,
in connection with an alternative proposed initial business combination, the Company will provide its public stockholders with the opportunity
to redeem all or a portion of their Public Shares upon the completion of a Business Combination either (i) in connection with a stockholder
meeting called to approve the Business Combination or (ii) by means of a tender offer. In connection with a proposed Business Combination,
the Company may seek stockholder approval of a Business Combination at a meeting called for such purpose at which stockholders may seek
to redeem their shares, regardless of whether they vote for or against a Business Combination. The Company will proceed with a Business
Combination only if the Company has net tangible assets of at least $5,000,001 either immediately prior to or upon such consummation
of a Business Combination and, if the Company seeks stockholder approval, a majority of the outstanding shares voted are voted in favor
of the Business Combination. In connection with the voting on the Extension Amendment Proposal and the Trust Amendment Proposal at the
special meeting, holders of 8,768,456 shares of Class A Common Stock exercised their right to redeem those shares for cash at an approximate
price of $10.42 per share, for an aggregate of approximately $91.4 million. Following the payment of the redemptions, the Trust Account
had a balance of approximately $28.5 million.
On
August 11, 2023, at 1:00 p.m. ET, the Company held a virtual special meeting of its stockholders. At the Special Meeting, the Company
stockholders entitled to vote at the Special Meeting cast their votes and approved the proposal (the “Trust Amendment Proposal”)
to authorize the Company to enter into Amendment No. 2 to the Trust Agreement (the “Trust Agreement Amendment”) to amend
the Trust Agreement to allow the Company to extend beyond August 11, 2023 the date by which either the Company must have completed its
initial business combination or Continental must liquidate the Trust Account established in connection with the IPO (the “Trust
Account”). Following approval of the Trust Amendment Proposal by the Stockholders, the Company and Continental promptly entered
into the Trust Agreement Amendment. At the Special Meeting, the Stockholders approved the Extension Amendment Proposal to amend
Company’s Charter and authorized the Company to adopt and file the Amended Charter with the Secretary of State of Delaware, which
the Company promptly filed following the Stockholders’ approval. The stockholders approved amendments to the Company’s charter
and trust agreement allowing the Company to extend its termination date in a series of up to nine (9) one-month extensions until May
11, 2024 in exchange for depositing into its trust account (the “Trust Account”) with Continental Stock Transfer and Trust
Company the lesser of $75,000 or $0.045 per share for each public share outstanding (the “Extension Amount”). In
connection with the voting on the Extension Amendment Proposal and the Trust Amendment Proposal at the Special Meeting, holders of 1,018,846
shares of Class A ordinary shares exercised the right to redeem such shares for cash.
On
August 11, 2023, the Company deposited $75,000 into the Company’s trust account, allowing the Company to extend the period of time
it has to consummate its initial business combination by one month from August 11, 2023 to September 11, 2023 (the “Extension”).
The Extension is the first of nine (9) one-month extensions permitted under the Company’s governing documents. As of September
30, 2023, the Company has executed two one-month extensions, out of the nine, resulting in deposits of approximately $150,000 into the
Trust Account.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
Based
on the above, the Company will have until May 11, 2024 to consummate a Business Combination. If the Company is unable to complete a Business
Combination on May 11, 2024 at the election of the Company subject to satisfaction of certain conditions, including the deposit of up
$2,300,000 since the underwriters’ over-allotment option is exercised in full ($0.10 per unit), into the Trust Account, or as extended
by the Company’s stockholders in accordance with the Company’s amended and restated certificate of incorporation) (the “Combination
Period”), the Company will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible
but no more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate
amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released
to the Company to pay taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding
public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to
receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following
such redemption, subject to the approval of the remaining stockholders and the Company’s board of directors, proceed to commence
a voluntary liquidation and thereby a formal dissolution of the Company, subject in each case to its obligations under Delaware law to
provide for claims of creditors and the requirements of applicable law. The underwriter has agreed to waive its rights to the deferred
underwriting commission held in the Trust Account in the event the Company does not complete a Business Combination within the Combination
Period and, in such event, such amounts will be included with the funds held in the Trust Account that will be available to fund the
redemption of the Public Shares. In the event of such distribution, it is possible that the per share value of the assets remaining available
for distribution will be less than the Initial Public Offering price per Unit ($10.00). There will be no redemption rights or liquidating
distributions with respect to the Founder Shares (as defined below) or the shares of Class A Common Stock and the warrants that are included
as components of the Private Placement Units. Such warrants will expire worthless if the Company fails to complete a Business Combination
within the Combination Period.
The
Sponsor has agreed that it will be liable to the Company if and to the extent any claims by a third party for services rendered or products
sold to the Company, or a prospective target business with which the Company has entered into a written letter of intent, confidentiality
or similar agreement or Business Combination agreement, reduce the amount of funds in the Trust Account to below the lesser of (i) $10.20
per Public Share and (ii) the actual amount per Public Share held in the Trust Account as of the day of liquidation of the Trust Account,
if less than $10.20 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability will
not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to monies held in
the Trust Account (whether or not such waiver is enforceable) nor will it apply to any claims under the Company’s indemnity of
the underwriter of Initial Public Offering against certain liabilities, including liabilities under the Securities Act of 1933, as amended
(the “Securities Act”). However, the Company has not asked the Sponsor to reserve for such indemnification obligations, nor
has the Company independently verified whether the Sponsor has sufficient funds to satisfy its indemnity obligations and believe that
the Sponsor’s only assets are securities of the Company. Therefore, the Company cannot assure its stockholders that the Sponsor
would be able to satisfy those obligations. None of the Company’s officers or directors will indemnify the Company for claims by
third parties including, without limitation, claims by vendors and prospective target businesses. The Company will seek to reduce the
possibility that the Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have all vendors,
service providers, prospective target businesses or other entities with which the Company does business, execute agreements with the
Company waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account.
Business
Combination with OneMedNet
On
November 7, 2023, the Company consummated its initial business combination (the “Business Combination”) with OneMedNet Solutions
Corporation (formerly named OneMedNet Corporation), a Delaware corporation (“OneMedNet”), pursuant to an agreement and plan
of merger, dated as of April 25, 2022 (the “Merger Agreement”), by and among the Company, Data Knights Merger Sub, Inc.,
a Delaware corporation and a wholly-owned subsidiary of the Company (“Merger Sub”), OneMedNet, Data Knights, LLC, a Delaware
limited liability company (“Sponsor” or “Purchaser Representative”) in its capacity as the representative of
the stockholders of the Company, and Paul Casey in his capacity as the representative of the stockholders of OneMedNet (“Seller
Representative”).
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
On
July 22, 2022, the Company filed a registration statement on Form S-4 (File No. 333-266274) (the “Form S-4”, as amended on
September 20, 2022, November 10, 2022, January 3, 2023, February 3, 2023, April 11, 2023, July 5, 2023, August 2, 2023, August 8, 2023,
August 29, 2023 and September 21, 2023) with the SEC, which includes the proxy statement/prospectus/information statement distributed
to holders of the Company’s ordinary shares in connection with the Company’s solicitation for proxies for the vote by the
Company’s stockholders in connection with the Business Combination and other matters as described in the Form S-4. The Form S-4
was declared effective by the SEC on September 22, 2023, and the definitive proxy statement/prospectus/information statement was mailed
to the Company’s shareholders of record on the record date for voting on the Business Combination. The Business Combination closed
on November 13, 2023. Effective November 7, 2023, the Company’s units ceased trading, and
effective November 8, 2023, the Company’s common stock began trading on the Nasdaq Capital Market under the symbol “ONMD”
and warrants began trading on the Nasdaq Capital Market under the symbol “ONMDW.”
The
Business Combination was accounted for as a reverse recapitalization in accordance with GAAP, whereby the Company is treated as the acquired
company and OneMedNet is treated as the acquirer. Accordingly, for accounting purposes, the Business Combination was treated as the equivalent
of OneMedNet issuing stock for the net assets of the Company, accompanied by a recapitalization. The net assets of the Company were stated
at historical cost, with no goodwill or other intangible assets recorded. Subsequent presentations of the results of operations presented
for the period prior to the Business Combination will be for those of OneMedNet.
OneMedNet
has been determined to be the accounting acquirer in the Business Combination based on the following predominate factors:
| |
● |
OneMedNet’s
existing stockholders have the greatest voting interest in the combined entity; |
| |
● |
OneMedNet
has the ability to nominate a majority of the initial members of the OneMedNet Board; |
| |
● |
OneMedNet’s
senior management is the senior management of the combined entity; and |
| |
● |
OneMedNet
is the larger entity based on historical operating activity and has the larger employee base. |
Going
Concern, Liquidity and Capital Resources
As
of September 30, 2023 and December 31, 2022, the Company had cash held outside of the Trust Account of $1,537 and $30,870, respectively.
We intend to use the funds held outside the Trust Account primarily to identify and evaluate target businesses, perform business due
diligence on prospective target businesses, travel to and from the offices, plants or similar locations of prospective target businesses
or their representatives or owners, review corporate documents and material agreements of prospective target businesses, and structure,
negotiate and complete our initial business combination. As of September 30, 2023 and December 31,2022, the Company had working capital
deficit of $3,057,188 and $1,945,267, respectively.
The
Company’s liquidity needs prior to the consummation of its IPO were satisfied through the proceeds of $ from the sale of
the Founder Shares and proceed from the promissory note from sponsor of $, which was repaid upon closure of the IPO. Subsequent
to the IPO, the Company’s liquidity will be satisfied through a portion of the net proceeds from IPO held outside of the Trust
Account.
As
of September 30, 2023 and December 31, 2022, we had investments of $19,308,261 and $29,029,416 held in the Trust Account, respectively.
We intend to use substantially all of the funds held in the Trust Account, including any amounts representing interest earned on the
Trust Account (less taxes paid and deferred underwriting commissions) to complete our initial business combination. We may withdraw interest
to pay taxes. For the nine months ended September 30, 2023, we withdraw $498,697 of interest earned on the Trust Account pay Delaware
Franchise Tax and Income Tax. During the period ended December 31, 2022, we withdraw $299,601 interest earned on the Trust Account to
pay Delaware Franchise Tax. To the extent that our capital stock or debt is used, in whole or in part, as consideration to complete our
initial business combination, the remaining proceeds held in the Trust Account will be used as working capital to finance the operations
of the target business or businesses, make other acquisitions and pursue our growth strategies.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
The
accompanying consolidated financial statements have been prepared in conformity with U.S. GAAP, which contemplates the continuation of
the Company as a going concern and the realization of assets and the satisfaction of liabilities in the normal course of business. The
financial statements do not include any adjustments that might result from the outcome of this uncertainty. Further, we have incurred
and expect to continue to incur significant costs in pursuit of our financing and acquisition plans. Management plans to address this
uncertainty during the period leading up to the business combination, however this cannot be guaranteed. The Company will have until
May 11, 2024 to consummate a Business Combination. If our initial business combination is not consummated by May 11, 2024, less than
one year after the date the financial statements are issued, then our existence will terminate, and we will distribute all amounts in
the trust account. The Company intends to complete a business combination before the liquidation date and no adjustments have been made
to the carrying amounts of assets or liabilities should the company be required to liquidate after such date. There can be no assurance
that the Company will be able to consummate an initial business combination by May 11, 2024 and/or have sufficient working capital and
borrowing capacity to meet its needs. Based upon the above analysis, management determined that these conditions raise substantial doubt
about the Company’s ability to continue as a going concern.
In
order to fund working capital deficiencies or finance transaction costs in connection with our initial Business Combination, our Sponsor
or an affiliate of our Sponsor or certain of our officers and directors may, but are not obligated to, loan us funds as may be required.
If we complete our initial Business Combination, we would repay such loaned amounts. In the event that our initial Business Combination
does not close, we may use a portion of the working capital held outside the Trust Accounts to repay such loaned amounts but no proceeds
from our Trust Accounts would be used for such repayment. Up to $1,500,000 of such loans may be convertible into units identical to the
Placement Units, at a price of $10.00 per unit at the option of the lender.
Risks
and Uncertainties
Management
is currently evaluating the impact of the COVID-19 pandemic and has concluded that while it is reasonably possible that the virus could
have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific
impact is not readily determinable as of the date of the financial statement. The financial statements do not include any adjustments
that might result from the outcome of this uncertainty.
On
August 16, 2022, the Inflation Reduction Act of 2022 (the “IR Act”) was signed into law. The IR Act provides for, among other
measures, a new 1% U.S. federal excise tax on certain repurchases (including redemptions) of stock by publicly traded domestic (i.e.,
U.S.) corporations. The excise tax is imposed on the repurchasing corporation itself, not its shareholders from whom the shares are repurchased.
The amount of the excise tax is generally 1% of the fair market value of the shares repurchased. For purposes of calculating the excise
tax, however, repurchasing corporations are permitted to net the fair market value of certain new stock issuances against the fair market
value of stock repurchases during the same taxable year. In addition, certain exceptions apply to the excise tax. The U.S. Department
of the Treasury (the “Treasury Department”) has been given authority to provide regulations and other guidance to carry out,
and prevent the abuse or avoidance of, the excise tax. The IR Act applies only to repurchases that occur after December 31, 2022.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
Any
redemption or other repurchase effected by us that occurs after December 31, 2022, in connection with a Business Combination or otherwise,
may be subject to this excise tax. Whether and to what extent we would be subject to the excise tax in connection with a Business Combination
will depend on a number of factors, including (i) the fair market value of the redemptions and repurchases in connection with the Business
Combination, (ii) the nature and amount of any PIPE financing or other equity issuances in connection with the Business Combination (or
any other equity issuances within the same taxable year of the Business Combination) and (iii) the content of any regulations and other
guidance issued by the Treasury Department and/or the Internal Revenue Service. In addition, because the excise tax would be payable
by us and not by the redeeming holder, it could cause a reduction in the value of our stock. The foregoing could cause a reduction in
the cash available on hand to complete a business Combination in the required time and redeem 100% of our public shares in accordance
with our amended and restated certificate of incorporation) could be subject to the excise tax, in which case the amount that would otherwise
be received by our stockholders in connection with our liquidation may be reduced.
Inflation
Reduction Act of 2022
On
August 16, 2022, the Inflation Reduction Act of 2022 (the “IR Act”) was signed into federal law. The IR Act provides for,
among other things, a new U.S. federal 1% excise tax on certain repurchases (including redemptions) of stock by publicly traded domestic
(i.e., U.S.) corporations and certain domestic subsidiaries of publicly traded foreign corporations. The excise tax is imposed on the
repurchasing corporation itself, not its shareholders from which shares are repurchased. The amount of the excise tax is generally 1%
of the fair market value of the shares repurchased at the time of the repurchase. However, for purposes of calculating the excise tax,
repurchasing corporations are permitted to net the fair market value of certain new stock issuances against the fair market value of
stock repurchases during the same taxable year. In addition, certain exceptions apply to the excise tax. The U.S. Department of the Treasury
(the “Treasury”) has been given authority to provide regulations and other guidance to carry out and prevent the abuse or
avoidance of the excise tax. The IR Act applies only to repurchases that occur after December 31, 2022.
Any
redemption or other repurchase that occurs after December 31, 2022, in connection with a Business Combination, extension vote or otherwise,
may be subject to the excise tax. Whether and to what extent the Company would be subject to the excise tax in connection with a Business
Combination, extension vote or otherwise would depend on a number of factors, including (i) the fair market value of the redemptions
and repurchases in connection with the Business Combination, extension or otherwise, (ii) the structure of a Business Combination, (iii)
the nature and amount of any “PIPE” or other equity issuances in connection with a Business Combination (or otherwise issued
not in connection with a Business Combination but issued within the same taxable year of a Business Combination) and (iv) the content
of regulations and other guidance from the Treasury. In addition, because the excise tax would be payable by the Company and not by the
redeeming holders, the mechanics of any required payment of the excise tax have not been determined. The foregoing could cause a reduction
in the cash available on hand to complete a Business Combination and in the Company’s ability to complete a Business Combination.
At
this time, it has been determined that the IR Act tax provisions would have an impact to the Company’s fiscal 2023 tax provision
as there were redemptions by the public stockholders in August 2023; as a result, the Company recorded $113,353 excise tax liability
as of September 30, 2023. The Company will continue to monitor for updates to the Company’s business along with guidance issued
with respect to the IR Act to determine whether any adjustments are needed to the Company’s tax provision in future periods.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of presentation
The
accompanying financial statements are presented in U.S. Dollars and conformity with accounting principles generally accepted in the United
States of America (“GAAP”) and pursuant to the rules and regulations of the SEC.
Principles
of Consolidation
The
accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All significant intercompany
balances and transactions have been eliminated in consolidation.
Emerging
growth company
The
Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our
Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements
that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required
to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding
executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory
vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further,
Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting
standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do
not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting
standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements
that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of
such extended transition period which means that when a standard is issued or revised and it has different application dates for public
or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies
adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which
is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult
or impossible because of the potential differences in accounting standards used.
Use
of estimates
The
preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported
amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the
reported amounts of revenues and expenses during the reporting period.
Making
estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of
a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating
its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ
significantly from those estimates.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. Cash
equivalents are carried at cost, which approximates fair value. The Company had $1,537 and $30,870 in cash and no cash equivalents as
of September 30, 2023 and December 31, 2022, respectively.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Trust
Account
Upon
the closing of the Initial Public Offering and the Private Placement, $117,300,000 ($10.00 per Unit) of the net proceeds of the Initial
Public Offering and certain of the proceeds of the Private Placement was held in a trust account (“Trust Account”) located
in the United States with Continental Stock Transfer & Trust Company acting as trustee, and invested only in U.S. government treasury
obligations with a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 under the Investment
Company Act 1940, as amended (the “Investment Company Act”), which will be invested only in direct U.S. government treasury
obligations, as determined by the Company, until the earlier of: (i) the completion of a Business Combination and (ii) the distribution
of the Trust Account as described below.
Offering
Costs Associated with the Initial Public Offering
Offering
costs consisted of legal, accounting, underwriting fees and other costs incurred that were directly related to the Initial Public Offering.
Offering costs are allocated to the separable financial instruments issued in the Initial Public Offering based on a relative fair value
basis, compared to total proceeds received. Offering costs associated with warrant liabilities are expensed as incurred, presented as
offering costs allocated to warrants in the consolidated statements of operations. Offering costs associated with the Public Shares were
charged to stockholders’ equity upon the completion of the Initial Public Offering.
Class
A Common Stock Subject to Possible Redemption
The
Company accounts for its shares subject to possible redemption in accordance with the guidance in Accounting Standards Codification (“ASC”)
Topic 480 “Distinguishing Liabilities from Equity.” Shares subject to mandatory redemption (if any) is classified as a liability
instrument and is measured at fair value. Conditionally redeemable shares of common stock (including shares of common stock that feature
redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not
solely within the Company’s control) is classified as temporary equity. At all other times, shares are classified as stockholders’
equity. The Company’s Class A Common Stock features certain redemption rights that are considered to be outside of the Company’s
control and subject to occurrence of uncertain future events.
On
September 30, 2023, there are 585,275 shares of Class A Common Stock related to the Private Placement Units (Note 8) outstanding, which
are not subject to redemption, and 1,712,698 shares of Class A Common Stock outstanding, which are subject to possible redemption.
If
it is probable that the equity instrument will become redeemable, the Company has the option to either accrete changes in the redemption
value over the period from the date of issuance (or from the date that it becomes probable that the instrument will become redeemable,
if later) to the earliest redemption date of the instrument or to recognize changes in the redemption value immediately as they occur
and adjust the carrying amount of the instrument to equal the redemption value at the end of each reporting period. The Company has elected
to recognize the changes immediately. The accretion or remeasurement is treated as a deemed dividend (i.e., a reduction to retained earnings,
or in absence of retained earnings, additional paid-in capital).
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
As
of September 30, 2023 and 2022, the Class A Common Stock reflected on the balance sheet are reconciled in the following table:
SCHEDULE
OF RECONCILED CLASS A COMMON STOCK
| | |
2023 | | |
2022 | |
| | |
For the Nine Months Ended | |
| | |
September 30, | |
| | |
2023 | | |
2022 | |
| Contingently redeemable Class A Common Stock – Opening Balance | |
$ | 28,750,110 | | |
$ | 117,300,000 | |
| Less: | |
| | | |
| | |
| Redemption of Class A Common Stock, including interest | |
| (11,335,260 | ) | |
| | |
| Plus: | |
| | | |
| | |
| Re-measurement of carrying value to redemption value | |
| 1,794,994 | | |
| 2,833,869 | |
| Contingently redeemable Class A Common Stock - Ending Balance | |
| 19,209,844 | | |
| 120,133,869 | |
Net
income (loss) per share
Net
income (loss) per share is computed by dividing net income (loss) by the weighted average number of common stock shares outstanding for
the period. The calculation of diluted income (loss) per share does not consider the effect of the warrants issued in connection with
the Initial Public Offering and warrants issued as components of the Private Placement Units (the “Placement Warrants”) since
the exercise of the warrants are contingent upon the occurrence of future events and the inclusion of such warrants would be anti-dilutive.
The
Company applies the two-class method in calculating earnings per share. The contractual formula utilized to calculate the redemption
amount approximates fair value. The Class feature to redeem at fair value means that there is effectively only one class of stock. Changes
in fair value are not considered a dividend for the purposes of the numerator in the earnings per share calculation. Net income per common
share is computed by dividing the pro rata net income (loss) between the redeemable shares and the non-redeemable shares by the weighted
average number of common shares outstanding for each of the periods. The calculation of diluted income (loss) per common stock does not
consider the effect of the warrants issued in connection with the IPO since the exercise of the warrants are contingent upon the occurrence
of future events and the inclusion of such warrants would be anti-dilutive. The warrants are exercisable for 12,085,275 shares of common
stock in the aggregate.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The
following table reflects the calculation of basic and diluted net income (loss) per common share:
RECONCILIATION
OF NET LOSS PER COMMON SHARE
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
For the Three Months Ended | | |
For the Nine months ended | |
| | |
September 30, | | |
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Redeemable Class A common shares | |
| | | |
| | | |
| | | |
| | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) allocable to common stock subject to possible redemption | |
$ | (252,542 | ) | |
$ | (117,823 | ) | |
$ | (312,541 | ) | |
$ | 2,300,089 | |
| | |
| | | |
| | | |
| | | |
| | |
| Denominator: weighted average number of redeemable common share | |
| 2,166,749 | | |
| 11,500,000 | | |
| 2,541,210 | | |
| 11,500,000 | |
| Basic and diluted net income (loss) per redeemable common share | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
| | |
| | | |
| | | |
| | | |
| | |
| Non-redeemable Class A and Class B common shares | |
| | | |
| | | |
| | | |
| | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) allocable to common stock not subject to redemption | |
$ | (563,978 | ) | |
$ | (35,452 | ) | |
$ | (595,119 | ) | |
$ | 692,082 | |
| | |
| | | |
| | | |
| | | |
| | |
| Denominator: weighted average number of non-redeemable common shares | |
| 4,838,792 | | |
| 3,460,275 | | |
| 4,838,792 | | |
| 3,460,275 | |
| Basic and diluted net income (loss) per non-redeemable common share | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
Concentration
of credit risk
Financial
instruments that potentially subject the Company to concentration of credit risk consist of a cash account in a financial institution
which, at times may exceed the Federal depository insurance coverage of $250,000. At September 30, 2023 and 2022, the Company had not
experienced losses on this account and management believes the Company is not exposed to significant risks on such account.
Fair
value of financial instruments
The
fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC Topic 820, “Fair Value
Measurements and Disclosures,” approximates the carrying amounts represented in the accompanying consolidated balance sheets, primarily
due to their short-term nature, except warrant liabilities (See Note 9).
Derivative
Financial Instruments
The
Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded
derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. For derivative financial instruments that are accounted
for as liabilities, the derivative instrument is initially recorded at its fair value on the grant date and is then re-valued at each
reporting date, with changes in the fair value reported in the statements of operations. The classification of derivative instruments,
including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period.
Derivative liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement or conversion
of the instrument could be required within 12 months of the balance sheet date.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Income
Taxes
The
Company complies with the accounting and reporting requirements of ASC Topic 740, “Income Taxes,” which requires an asset
and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed
for differences between the financial statement and tax bases of assets and liabilities that will result in future taxable or deductible
amounts, based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income.
Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC
Topic 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax
positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not
to be sustained upon examination by taxing authorities. The Company’s management determined that the United States is the Company’s
only major tax jurisdiction. The Company recognizes accrued interest and penalties related to unrecognized tax benefits, if any, as income
tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of September 30, 2023 and 2022,
respectively. The Company is currently not aware of any issues under review that could result in significant payments, accruals, or material
deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Our
effective tax rate was (12.15)% and 0% for the three months ended September 30, 2023 and 2022, respectively. Our effective tax rate was
(30.10)% and 0% for the nine months ended September 30, 2023 and 2022, respectively. The effective tax rate differs from the statutory
tax rate of 21% for the three months ended September 30, 2023 and 2022, due to transaction costs and the valuation allowance on the deferred
tax assets.
Recently
Issued Accounting Standards
In
August 2020, the FASB issued Accounting Standards Update (“ASU”) 2020-06, “Debt — Debt with Conversion and Other
Options” (Subtopic 470-20) and “Derivatives and Hedging — Contracts in Entity’s Own Equity” (Subtopic 815-40)
(“ASU 2020-06”) to simplify accounting for certain financial instruments. ASU 2020-06 eliminates the current models that
require separation of beneficial conversion and cash conversion features from convertible instruments and simplifies the derivative scope
exception guidance pertaining to equity classification of contracts in an entity’s own equity. The new standard also introduces
additional disclosures for convertible debt and freestanding instruments that are indexed to and settled in an entity’s own equity.
ASU 2020-06 amends the diluted earnings per share guidance, including the requirement to use the if-converted method for all convertible
instruments. ASU 2020-06 is effective for the Company for the fiscal year beginning after December 15, 2023, including interim periods
within those fiscal years.
Management
does not believe that any recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material
effect on the Company’s financial statements.
NOTE
3. INITIAL PUBLIC OFFERING
Pursuant
to the Initial Public Offering, the Company sold 11,500,000 Units at a purchase price of $10.00 per Unit. Each Unit consists of one share
of the Company’s Class A Common Stock, $0.0001 par value, and one redeemable warrant (“Public Warrant”). Each Public
Warrant entitles the holder to purchase one share of Class A Common Stock at an exercise price of $11.50 per whole share (see Note 9).
NOTE
4. PRIVATE PLACEMENT
Simultaneously
with the Initial Public Offering, the Sponsor purchased an aggregate of 585,275 Private Placement Units at a price of $10.00 per Private
Placement Unit for an aggregate purchase price of $5,852,750.
The
Private Placement Units are identical to the Units, except that (a) the Private Placement Units and their component securities will not
be transferable, assignable or saleable until 30 days after the consummation of the Company’s initial business combination except
to permitted transferees and (b) the Placement Warrants, so long as they are held by the Sponsor or its permitted transferees, (i) may
be exercised by the holders on a cashless basis and (ii) will be entitled to registration rights.
NOTE
5. RELATED PARTY TRANSACTIONS
Introducing
Advisor Agreement
On
June 26, 2021, the Company entered into an introducing advisor agreement (the “Introducing Advisor Agreement”) with ARC Group
Limited, the Company’s financial advisor (“ARC”), pursuant to which ARC will make strategic introductions to the Company
of potential target companies and/or their subsidiaries, affiliates, or representatives (each an “Advisor Target”) who may
be interested in potential business combinations with the Company. In consideration for ARC’s services under the Introducing Advisor
Agreement, we agreed to (i) pay to ARC (a) a retainer of $50,000 upon execution of the Introducing Advisor Agreement and (b) a success
fee of $100,000 upon the closing our initial business combination, and (ii) cause to be issued to ARC equity interests in the post-combination
company representing a five-percent (5%) ownership interest in the post-combination company, if at any time prior to June 25, 2022 (the
“Termination Date”), or within six (6) months after the consummation of an initial business combination or any financing
with any Advisor Target or any affiliate of an Advisor Target (the “Equity Issuance”).
On
March 22, 2022, the Company and ARC entered into the First Amendment to the Introducing Advisor Agreement, pursuant to which both parties
agreed that the Company would pay to ARC an additional success fee equivalent to five percent (5%) on any PIPE that was brought by ARC
in connection with the Company’s initial business combination upon the closing of the Company’s initial business combination.
On
December 31, 2022, the Company and ARC entered into the Second Amendment to the Introducing Advisor Agreement, pursuant to which both
parties agreed to extend the Termination Date to December 31, 2024, and to change the performance condition for the Equity Issuance from
the closing of an initial business combination to the execution of a business combination agreement. On December 31, 2022, following
the execution of the Second Amendment to the Introducing Advisor Agreement, the performance condition for the Equity Issuance was deemed
to have been met, and ARC was issued 1,378,517 shares of the Company’s Class B Common Stock, up to 143,766 shares of which are
subject to forfeiture if the public stockholders exercise redemption rights with respect to any of the remaining outstanding shares of
Class A Common Stock.
Founder
Shares
On
February 25, 2021, the Company issued an aggregate of shares of Class B Common Stock (the “Founder Shares”) to
the Sponsor for an aggregate purchase price of $. On February 25, 2021, the Sponsor transferred 15,000 shares to the Company’s
Chief Executive Officer, 15,000 shares to the Company’s Chief Financial Officer and 5,000 shares to two of the Company’s
independent directors. Following the determination of the Company’s third independent director, on March 23, 2021, the Sponsor
transferred 5,000 shares to such independent director. The Founder Shares which the Sponsor and its permitted transferees will collectively
own, on an as-converted basis, represent 20% of the Company’s issued and outstanding shares after the Initial Public Offering.
In connection with the Introducing Advisors Agreement, on December 31, 2022, ARC was granted 1,378,517 shares of Class B common stock,
$0.0001 par value per share, up to 143,766 of which are subject to forfeiture by ARC if the Company’s public shareholders exercise
redemption rights with respect to any of outstanding shares of Class A common stock.
NOTE
5. RELATED PARTY TRANSACTIONS (Continued)
The
Sponsor has agreed not to transfer, assign or sell any of its Founder Shares until the earlier to occur of: (A) after the
completion of a Business Combination or (B) the date on which the Company completes a liquidation, merger, capital stock exchange or
similar transaction that results in the Company’s stockholders having the right to exchange their shares of common stock for cash,
securities or other property. Notwithstanding the foregoing, if the last reported sale price of the Company’s Class A Common Stock
equals or exceeds $ per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for
any 20 trading days within any 30-trading day period commencing at least 150 days after the Business Combination, the Founder Shares
will be released from the lock-up.
Promissory
Note — Related Party
On
February 8, 2021, the Sponsor committed to loan the Company an aggregate of up to $300,000 to cover expenses related to the Initial Public
Offering pursuant to a promissory note (the “Note”). The Note was non-interest bearing and was payable on the earlier of
July 31, 2021 or the completion of the Initial Public Offering. On June 1, 2021, the $78,925 outstanding under the promissory note was
repaid in full. On September 30, 2023 and December 31, 2022, there is no amount outstanding under the promissory note.
Related
Party Loans
In
order to finance transaction costs in connection with a Business Combination, the Company’s Sponsor, an affiliate of the Sponsor,
or the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (the “Working
Capital Loans”). Such Working Capital Loans would be evidenced by promissory notes. The notes would either be repaid upon consummation
of a Business Combination, without interest, or, at the lender’s discretion, up to $ of notes may be converted upon consummation
of a Business Combination into units at a price of $ per unit. The Units will be identical to the Private Placement Units. In the
event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust Account to repay the
Working Capital Loans but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. On September 30, 2023
and December 31, 2022, there is $ and $ outstanding under the Working Capital Loans, respectively.
NOTE
5. RELATED PARTY TRANSACTIONS (Continued)
The
Company’s second amended and restated certificate of incorporation provides that, if the Company anticipates that it may not be
able to consummate a Business Combination within 12 months from the closing of the Company’s initial public offering, the Company
may, by resolution of the Company’s board if requested by the Sponsor, extend the period of time to consummate a Business Combination
up to two times, each by an additional three months (for a total of up to 18 months to complete a Business Combination), subject to the
Sponsor depositing additional funds into the Trust Account as set out below. Pursuant to the terms of the Company’s second amended
and restated certificate of incorporation and the trust agreement entered into between the Company and Continental Stock Transfer &
Trust Company, in order for the time available for the Company to consummate the initial Business Combination to be extended, the Sponsor
or its affiliates or designees, upon five business days advance notice prior to the applicable deadline, must deposit into the Trust
Account $ since the underwriters’ over-allotment option is exercised in full ($ per unit), on or prior to the date
of the applicable deadline, for each of the available three month extensions, providing a total possible Business Combination period
of 18 months at a total payment value of $ since the underwriters’ over-allotment option is exercised in full ($ per
unit) (the “Extension Loans”). Any such payments would be made in the form of non-interest-bearing loans. If the Company
completes its initial Business Combination, the Company will, at the option of the Sponsor, repay the Extension Loans out of the proceeds
of the Trust Account released to the Company or convert a portion or all of the total loan amount into units at a price of $ per
unit, which units will be identical to the Private Placement Units. If the Company does not complete a Business Combination, the Company
will repay such loans only from funds held outside of the Trust Account. Furthermore, the letter agreement among the Company and the
Company’s officers, directors, and the Sponsor contains a provision pursuant to which the Sponsor will agree to waive its right
to be repaid for such loans to the extent there is insufficient funds held outside of the Trust Account in the event that the Company
does not complete a Business Combination. The Sponsor and its affiliates or designees are not obligated to fund the Trust Account to
extend the time for the Company to complete the initial Business Combination. The public stockholders will not be afforded an opportunity
to vote on the extension of time to consummate an initial Business Combination from 12 months to 18 months described above or redeem
their shares in connection with such extensions. Pursuant to the foregoing, on May 5, 2022, the Company extended the date by which the
Company had to consummate a business combination from May 11, 2022 to August 11, 2022. On August 10, 2022, the Company extended the date
by which the Company had to consummate a business combination from August 11, 2022 to November 11, 2022.
As
described in Note 1, on August 11, 2023, the Stockholders of the Company approved the Extension Amendment and the Trust Amendment to
allow the Company to extend the deadline by which it must complete its initial business combination by up to nine one-month periods from
August 11, 2023. In connection with each such extension, Data Knights, LLC, the Company’s sponsor, caused approximately $,
deposited in the Trust Account in connection with the exercise of the monthly extension. In connection with each such extension, the
Company will have until May 11, 2023 to consummate a Business Combination(see Note 10). On September 30, 2023 and December 31, 2022,
there is $ and $ outstanding under the Extension Loan, respectively.
Administrative
Services Arrangement
Commencing
on the date of the prospectus and until completion of the Company’s Business Combination or liquidation, the Company may reimburse
ARC Group Ltd., an affiliate of the Sponsor, up to an amount of $10,000 per month for office space, secretarial and administrative support.
For the three months ended September 30, 2023 and 2022, we have incurred $30,000 in fees under this agreement, respectively. For the
nine months ended September 30, 2023 and 2022, we have incurred $90,000 in fees under this agreement, respectively.
NOTE
6. COMMITMENTS AND CONTINGENCIES
Registration
Rights
Pursuant
to a registration rights agreement entered into on May 6, 2021, the holders of the Founder Shares, Private Placement Units (including
the securities contained therein), the units (including the securities contained therein) that may be issued upon conversion of the Working
Capital Loans, and any shares of Class A Common Stock issuable upon the exercise of the Placement Warrants and any shares of Class A
Common Stock, warrants (and underlying Class A Common Stock) that may be issued upon conversion of the units issued as part of the working
capital loans and Class A Common Stock issuable upon conversion of the founder shares are entitled to registration rights. The holders
of a majority of these securities are entitled to make up to three demands, excluding short form demands, that the Company register such
securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements
filed subsequent to the completion of a Business Combination and rights to require the Company to register for resale such securities
pursuant to Rule 415 under the Securities Act. However, the registration rights agreement provides that the Company will not permit any
registration statement filed under the Securities Act to become effective until termination of the applicable lock-up period. The Company
will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriters
Agreement
The
Company granted the underwriter a 45-day option to purchase up to 1,500,000 additional Units to cover over-allotments at the Initial
Public Offering price, less the underwriting discounts and commissions. The aforementioned option was exercised on May 11, 2021.
The
underwriter was paid a cash underwriting discount of two percent (2.00%) of the gross proceeds of the Initial Public Offering, or $2,300,000.
In addition, the underwriter is entitled to a deferred fee of three and a half percent (3.50%) of the gross proceeds of the Initial Public
Offering, or $4,025,000. The deferred fee was placed in the Trust Account and will be paid in cash upon the closing of a Business Combination,
subject to the terms of the underwriting agreement.
Right
of First Refusal
For
a period beginning on May 7, 2021 and ending 12 months from the closing of a business combination, we have granted the underwriters a
right of first refusal to act as lead-left book running manager and lead left manager for any and all future private or public equity,
convertible and debt offerings during such period. In accordance with FINRA Rule 5110(f)(2)(E)(i), such right of first refusal shall
not have a duration of more than three years from the effective date of our Registration Statement.
First
Amendment to the Introducing Advisor Agreement
On
March 22, 2022, the Company and ARC entered into the First Amendment to the Introducing Advisor Agreement, pursuant to which both parties
agreed that the Company would pay to ARC an additional success fee equivalent to five percent (5%) on any PIPE that was brought by ARC
in connection with an initial business combination upon the closing of an initial business combination.
Second
Amendment to the Introducing Advisor Agreement
On
December 31, 2022, the Company and ARC entered into the Second Amendment to the Introducing Advisor Agreement, pursuant to which both
parties agreed to extend the Termination Date to December 31, 2024, and to change the performance condition for the Equity Issuance from
the closing of an initial business combination to the execution of a business combination agreement. On December 31, 2022, following
the execution of the Second Amendment to the Introducing Advisor Agreement, the performance condition for the Equity Issuance was deemed
to have been met, and ARC was issued 1,378,517 shares of the Company’s Class B Common Stock, up to 143,766 shares of which are
subject to forfeiture if the public stockholders exercise redemption rights with respect to any of the remaining outstanding shares of
Class A Common Stock.
NOTE
7. WARRANT LIABILITY
Public
Warrants may only be exercised for a whole number of shares. No fractional warrants will be issued upon separation of the Units and only
whole warrants will trade. The Public Warrants will become exercisable 30 days after the completion of a Business Combination and will
expire five years after the completion of a Business Combination or earlier upon redemption or liquidation.
The
Company will not be obligated to deliver any shares of Class A Common Stock pursuant to the exercise of a warrant and will have no obligation
to settle such warrant exercise unless a registration statement under the Securities Act covering the issuance of the shares of Class
A Common Stock issuable upon exercise of the warrants is then effective and a current prospectus relating to those shares of Class A
Common Stock is available, subject to the Company satisfying its obligations with respect to registration. No warrant will be exercisable
for cash or on a cashless basis, and the Company will not be obligated to issue any shares to holders seeking to exercise their warrants,
unless the issuance of the shares upon such exercise is registered or qualified under the securities laws of the state of the exercising
holder, or an exemption from registration is available.
The
Company has agreed that as soon as practicable, but in no event later than 20 business days after the closing of its initial Business
Combination, it will use its commercially reasonable efforts to file with the SEC a post-effective amendment to the registration statement
or a new registration statement covering the shares of Class A Common Stock issuable upon exercise of the warrants, to cause such registration
statement to become effective and to maintain a current prospectus relating to those shares of Class A Common Stock until the warrants
expire or are redeemed, as specified in the warrant agreement. If a registration statement covering the shares of Class A Common Stock
issuable upon exercise of the warrants is not effective by the 60th business day after the closing of the Company’s initial business
combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company
will have failed to maintain an effective registration statement, exercise warrants on a “cashless basis” in accordance with
Section 3(a)(9) of the Securities Act or another exemption.. Notwithstanding the above, if the Company’s shares of Class A Common
Stock are at the time of any exercise of a warrant not listed on a national securities exchange such that they satisfy the definition
of a “covered security” under Section 18(b)(1) of the Securities Act, it may, at its option, require holders of Public Warrants
who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and,
in the event the Company so elects, it will not be required to file or maintain in effect a registration statement, and in the event
it does not so elect, it will use its commercially reasonable efforts to register or qualify the shares under applicable blue sky laws
to the extent an exemption is not available. If that exemption, or another exemption, is not available, holders will not be able to exercise
their warrants on a cashless basis.
Redemption
of warrants when the price per Class A Common Stock equals or exceeds $18.00. Once the warrants become exercisable, the Company may redeem
the Public Warrants:
| ● | in
whole and not in part; |
| | | |
| ● | at
a price of $0.01 per Public Warrant; |
| | | |
| ● | upon
not less than 30 days’ prior written notice of redemption to each warrant holder; and |
| | | |
| ● | if,
and only if, the reported last sale price of the Class A Common Stock equals or exceeds $18.00
per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations
and the like and certain issuances of Class A Common Stock and equity-linked securities)
for any 20 trading days within a 30-trading day period commencing no earlier than the date
the warrants become exercisable and ending on the third business day before the date on which
the Company sends the notice of redemption to the warrant holders. |
If
and when the warrants become redeemable by the Company, the Company may exercise its redemption right even if it is unable to register
or qualify the underlying securities for sale under all applicable state securities laws.
NOTE
7. WARRANT LIABILITY (Continued)
If
the Company calls the Public Warrants for redemption, management will have the option to require all holders that wish to exercise the
Public Warrants to do so on a “cashless basis,” as described in the warrant agreement. The exercise price and number of shares
of Class A Common Stock issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock
dividend, or recapitalization, reorganization, merger or consolidation. However, the warrants will not be adjusted for issuance of Class
A Common Stock at a price below its exercise price. Additionally, in no event will the Company be required to net cash settle the warrants.
If the Company is unable to complete a Business Combination within the Combination Window and the Company liquidates the funds held in
the Trust Account, holders of warrants will not receive any of such funds with respect to their warrants, nor will they receive any distribution
from the Company’s assets held outside of the Trust Account with the respect to such warrants. Accordingly, the warrants may expire
worthless. The Placement Warrants were identical to the Public Warrants underlying the Units being sold in the Proposed Public Offering,
except that the Placement Warrants and the Class A Common Stock issuable upon the exercise of the Placement Warrants will not be transferable,
assignable or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions. Additionally,
the Placement Warrants will be exercisable on a cashless basis and be non-redeemable so long as they are held by the initial purchasers
or their permitted transferees. If the Placement Warrants are held by someone other than the initial purchasers or their permitted transferees,
the Placement Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
In
addition, if (x) the Company issues additional shares of Class A Common Stock or equity-linked securities for capital raising purposes
in connection with the closing of its initial Business Combination at an issue price or effective issue price of less than $9.20 per
share of Class A Common Stock (with such issue price or effective issue price to be determined in good faith by the Company’s board
of directors and, in the case of any such issuance to the Sponsor or its affiliates, without taking into account any Founder Shares held
by the Sponsor or such affiliates, as applicable, prior to such issuance) (the “Newly Issued Price”), (y) the aggregate gross
proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of
the Company’s initial Business Combination on the date of the consummation of such initial Business Combination (net of redemptions),
and (z) the volume weighted average trading price of the Company’s common stock during the 20 trading day period starting on the
trading day prior to the day on which the Company consummates its initial Business Combination (such price, the “Market Value”)
is below $9.20 per share, the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the higher
of the Market Value and the Newly Issued Price, the $18.00 per share redemption trigger price described above will be adjusted (to the
nearest cent) to be equal to 180% of the greater of the Market Value and the Newly Issued Price and the $10.00 per share redemption trigger
price described above will be adjusted (to the nearest cent) to be equal to the greater of the Market Value and the Newly Issued Price.
The
Placement Warrants will be identical to the Public Warrants underlying the Units being sold in the Proposed Public Offering, except that
the Placement Warrants and the Class A Common Stock issuable upon the exercise of the Placement Warrants will not be transferable, assignable
or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions. Additionally, the Placement
Warrants will be exercisable on a cashless basis and be non-redeemable so long as they are held by the initial purchasers or their permitted
transferees. If the Placement Warrants are held by someone other than the initial purchasers or their permitted transferees, the Placement
Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
At
September 30, 2022 and December 31, 2022, the Company accounted for the aggregate 12,085,275 warrants issued in connection with the Initial
Public Offering (the 11,500,000 Public Warrants and the 585,275 Placement Warrants) in accordance with the guidance contained in ASC
815-40. Such guidance provides that because the warrants do not meet the criteria for equity treatment thereunder, each warrant must
be recorded as a liability. Accordingly, the Company will classify each warrant as a liability at its fair value, with the change in
fair value recognized in the Company’s statement of operations.
NOTE
8. STOCKHOLDER’S EQUITY
Preferred
Shares — The Company is authorized to issue 1,000,000 preferred shares with a par value of $0.0001 per share with such
designation, rights and preferences as may be determined from time to time by the Company’s Board of Directors. At September 30,
2023 and December 31, 2022, there were no preferred shares issued or outstanding.
Class
A Common Stock — The Company is authorized to issue 100,000,000 shares of Class A Common Stock with a par value of $0.0001
per share. Holders of the Company’s Class A Common Stock are entitled to one vote for each share. At September 30, 2023 and December
31, 2022, there were 1,712,698 shares of Class A Common Stock issued and outstanding that were subject to possible redemption and 585,275
shares of non-redeemable Class A Common Stock issued and outstanding that were issued in connection with the private placement (Note
4).
Class
B Common Stock — The Company is authorized to issue up to 10,000,000 shares of Class B Common Stock with a par value of
$0.0001 per share. Holders of the Company’s Class B Common Stock are entitled to one vote for each share. On February 25, 2021,
the Sponsor transferred shares to the Company’s Chief Executive Officer, shares to the Company’s Chief Financial
Officer and shares to two of the Company’s independent directors. Following the determination of the Company’s third
independent director, on March 23, 2021, the Sponsor transferred shares to such independent director.
On
December 31, 2022, ARC Group Limited, the Company’s Financial Advisor, was granted 1,378,517 shares of Class B common stock with
a par value of $0.0001 per share, up to 143,766 of which are subject to forfeiture if the Company’s public stockholders exercise
redemption rights with respect to any of the Company’s remaining outstanding shares of Class A common stock.
Accordingly,
at September 30, 2023 and December 31, 2022, there were 4,253,517 shares of Class B Common Stock issued and outstanding.
Holders
of Class A Common Stock and Class B Common Stock will vote together as a single class on all other matters submitted to a vote of stockholders,
except as required by law.
The
shares of Class B Common Stock will automatically convert into shares of Class A Common Stock at the time of the Business Combination
on a one-for-one basis, subject to adjustment for stock splits, stock dividends, reorganizations, recapitalizations and the like. In
the case that additional shares of Class A Common Stock, or equity linked securities, are issued or deemed issued in excess of the amounts
offered in the Initial Public Offering and related to the closing of a Business Combination, the ratio at which shares of Class B Common
Stock shall convert into shares of Class A Common Stock will be adjusted (unless the holders of a majority of the outstanding shares
of Class B Common Stock agree to waive such adjustment with respect to any such issuance or deemed issuance) so that the number of shares
of Class A Common Stock issuable upon conversion of all shares of Class B Common Stock will equal, in the aggregate, on an as converted
basis, 20% of the sum of the total number of all shares of common stock outstanding upon the completion of the Initial Public Offering
plus all shares of Class A Common Stock and equity linked securities issued or deemed issued in connection with a Business Combination
(excluding any shares or equity linked securities issued, or to be issued, to any seller in a Business Combination, and any private placement-equivalent
units and its underlying securities issued to the Sponsor or its affiliates upon conversion of loans made to the Company).
The
Company may issue additional common stock or preferred stock to complete its Business Combination or under an employee incentive plan
after completion of its Business Combination.
NOTE
9. FAIR VALUE MEASUREMENTS
The
following table presents information about the Company’s assets and derivative warrant liabilities that are measured at fair value
on a recurring basis as of September 30, 2023 and December 31, 2022 and indicates the fair value hierarchy of the valuation techniques
that the Company utilized to determine such fair value:
SCHEDULE
OF FAIR VALUE HIERARCHY OF VALUATION TECHNIQUES
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| | |
September 30, 2023 | |
| | |
Quoted Prices in | | |
Significant Other | | |
Significant Other | |
| | |
Active Markets | | |
Observable Inputs | | |
Unobservable Inputs | |
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| Asset: | |
| | | |
| | | |
| | |
| Investments held in Trust Account | |
$ | 19,308,261 | | |
$ | — | | |
$ | — | |
| | |
| | | |
| | | |
| | |
| Warrant Liabilities: | |
| | | |
| | | |
| | |
| Public Warrants | |
$ | 575,000 | | |
$ | — | | |
$ | — | |
| Private Placement Warrants | |
$ | — | | |
$ | — | | |
$ | 29,849 | |
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| | |
December 31, 2022 | |
| | |
Quoted Prices in | | |
Significant Other | | |
Significant Other | |
| | |
Active Markets | | |
Observable Inputs | | |
Unobservable Inputs | |
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| Asset: | |
| | | |
| | | |
| | |
| Investments held in Trust Account | |
$ | 29,029,416 | | |
$ | — | | |
$ | — | |
| | |
| | | |
| | | |
| | |
| Warrant Liabilities: | |
| | | |
| | | |
| | |
| Public Warrants | |
$ | 345,000 | | |
$ | — | | |
$ | — | |
| Private Placement Warrants | |
$ | — | | |
$ | — | | |
$ | 17,558 | |
The
Warrants are measured at fair value on a recurring basis. The Public Warrants were valued initially and at each reporting period that
the warrants were not actively traded, using a Monte Carlo simulation. As of September 30, 2023 and December 31, 2022, the Public Warrants
were valued using the instrument’s publicly listed trading price, which is considered to be a Level 1 measurement due to the use
of an observable market quote in an active market. Private Placement Warrants were valued using a Monte Carlo valuation model using level
3 inputs at initial valuation and as of September 30, 2023 and December 31, 2022.
At
September 30, 2023 and December 31, 2022, assets held in the Trust Account were invested solely in Morgan Stanley Bank cash, bank deposit
program and money market fund and BlackRock US Treasury mutual fund of $19,308,261 and $29,029,416, respectively.
The
Warrants were accounted for as liabilities in accordance with ASC 815-40 and are presented within warrant liabilities in the accompanying
consolidated balance sheets. The warrant liabilities are measured at fair value at inception and on a recurring basis, with changes in
fair value presented within the consolidated statement of operations.
The
accounting treatment of derivative financial instruments requires that the Company record a derivative liability upon the closing of
the Initial Public Offering. Accordingly, the Company classified each warrant as a liability at its fair value and the warrants were
allocated a portion of the proceeds from the issuance of the Units equal to its fair value determined by the Monte Carlo simulation.
This liability is subject to remeasurement at each balance sheet date. With each such re-measurement, the warrant liability will be adjusted
to fair value, with the change in fair value recognized in the Company’s statement of operations. The Company will reassess the
classification at each balance sheet date. If the classification changes as a result of events during the period, the warrants will be
reclassified as of the date of the event that causes the reclassification.
NOTE
9. FAIR VALUE MEASUREMENTS (Continued)
The
Company utilized a Monte Carlo simulation to estimate the fair value of the Public warrants at each reporting period for its warrants
that are not actively traded. Inherent in a Monte Carlo simulation are assumptions related to expected stock-price volatility, expected
life, risk-free interest rate and dividend yield. The Company estimates the volatility of its common stock based on historical volatility
of select peer companies that matches the expected remaining life of the warrants. The risk-free interest rate is based on the U.S. Treasury
zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of
the warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which
the Company anticipates remaining at zero. On June 22, 2021, the Public Warrants surpassed the threshold waiting period to be publicly
traded. Once publicly traded, the observable input qualifies the liability for treatment as a Level 1 liability. As such, as of September
30, 2023 and December 31, 2022, the Company classified the Public Warrants as Level 1.
The
estimated fair value of the Private Placement Warrants is determined using Level 3 inputs. Inherent in a Monte Carlo model are assumptions
related to expected share-price volatility, expected life, risk-free interest rate and dividend yield. The Company estimates the volatility
of its warrants based on implied volatility from the Company’s traded warrants and from historical volatility of select peer company’s
common stock that matches the expected remaining life of the Warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon
yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of the warrants
is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which the Company
anticipates remaining at zero.
Transfers
to/from Levels 1, 2 and 3 are recognized at the end of the reporting period in which a change in valuation technique or methodology occurs.
For the three months ended September 30, 2023 and December 31, 2022, there were no transfers between levels.
The
following table provides quantitative information regarding Level 3 fair value measurements inputs as their measurement dates:
SCHEDULE
OF FAIR VALUE MEASUREMENTS INPUTS AS THEIR MEASUREMENT DATES
| | |
September 30, 2023 | | |
December 31, 2022 | |
| | |
(Private Warrants) | | |
(Private Warrants) | |
| Exercise price | |
$ | 11.50 | | |
$ | 11.50 | |
| Share price | |
$ | 11.26 | | |
$ | 10.44 | |
| Expected term (years) | |
| 5.61 | | |
| 5.12 | |
| Probability of Acquisition | |
| 2.10 | % | |
| 2.75 | % |
| Volatility | |
| 7.9 | % | |
| 4.2 | % |
| Risk-free rate | |
| 4.50 | % | |
| 3.91 | % |
| Dividend yield (per share) | |
$ | 0.00 | | |
$ | 0.00 | |
The
change in the fair value of the derivative warrant liabilities for the three months ended September 30, 2023 and 2022 is as follows:
SCHEDULE
OF CHANGES IN FAIR VALUE OF DERIVATIVE WARRANT LIABILITIES
| | |
Private Warrants | | |
Public Warrants | | |
Total Warrant Liability | |
| Fair value as of December 31, 2022 | |
$ | 17,558 | | |
$ | 345,000 | | |
$ | 362,558 | |
| Change in valuation inputs or other assumptions(1) | |
| 12,291 | | |
| 230,000 | | |
| 242,291 | |
| Fair value as of September 30, 2023 | |
$ | 29,849 | | |
$ | 575,000 | | |
$ | 604,849 | |
| | |
Private Warrants | | |
Public Warrants | | |
Total Warrant Liability | |
| Fair value as of December 31, 2021 | |
$ | 251,668 | | |
$ | 4,600,000 | | |
$ | 4,851,668 | |
| Fair value, beginning balance | |
$ | 251,668 | | |
$ | 4,600,000 | | |
$ | 4,851,668 | |
| Change in valuation inputs or other assumptions(1) | |
| (117,640 | ) | |
| (2,645,000 | ) | |
| (2,762,640 | ) |
| Fair value as of September 30, 2022 | |
$ | 134,028 | | |
$ | 1,955,000 | | |
$ | 2,089,028 | |
| Fair value, ending balance | |
$ | 134,028 | | |
$ | 1,955,000 | | |
$ | 2,089,028 | |
NOTE
10. SUBSEQUENT EVENTS
In
accordance with ASC Topic 855, “Subsequent Events”, which establishes general standards of accounting for and disclosure
of events that occur after the balance sheet date but before financial statements are issued, the Company has evaluated all events or
transactions that occurred after the balance sheet date up to the date that the financial statements were issued. Based upon this review,
the Company did not identified any subsequent events that would have required adjustment or disclosure in the financial statements other
than as described below.
On
October 12, 2023, the Company elected to exercise its eighth of third one-month extension to the Termination Date, which extended its
deadline to complete its initial business combination to November 11, 2023, by depositing $75,000 into the Trust Account.
On
October 17, 2023, the Company held a special meeting of its stockholders (the “Stockholders”) in lieu of the 2023 annual
meeting of stockholders (the “Special Meeting”) in connection with the transactions contemplated by that certain Agreement
and Plan of Merger dated April 25, 2022 (the “Merger Agreement”), by and among the Company, Data Knights Merger Sub, Inc.,
a Delaware corporation (“Merger Sub”), Data Knights, LLC, the Company’s sponsor, OneMedNet Solutions Corporation (formerly
named OneMedNet Corporation), a Delaware corporation, (“OneMedNet”), and Paul Casey, as seller representative (such transactions,
collectively, the “Business Combination”). At the Special Meeting, the Stockholders were asked to consider and vote on the
proposals identified in the definitive proxy statement/prospectus that the Company filed with the U.S. Securities and Exchange Commission
(the “SEC”) on September 21, 2023 (the “Definitive Proxy”). Proposals 1 through 7 set forth below were approved:
| - | Proposal
1: To approve an amendment to the Second Amendment to the Second Amended and Restated
Certificate of Incorporation (the “Charter”) effective prior to the consummation
of the Business Combination, to remove from the Charter the redemption limitation contained
under Section 9.2(a) preventing the Company from closing a business combination if it would
have less than $5,000,0001 of net tangible assets (the “NTA Proposal”). |
| | | |
| - | Proposal
2: To approve and adopt the Merger Agreement and approve the transactions contemplated
thereby (the “Business Combination Proposal”). |
| | | |
| - | Proposal
3: To approve, for purposes of complying with Nasdaq Listing Rules 5635(a) and (b), the
issuance of more than 20% of the issued and outstanding Class A common stock and the resulting
change in control in connection with the Business Combination (the “Nasdaq Proposal”). |
NOTE 10. SUBSEQUENT
EVENTS (Continued)
| - | Proposal
4: To approve the Third Amended and Restated Certificate of Incorporation, which shall
become effective upon the closing of the Business Combination (the “Charter Amendment
Proposal”), including (i) the addition of a supermajority voting requirement to amend
the Surviving Corporation’s Bylaws, and (ii) the addition of a supermajority voting
requirement to amend Articles V (Board of Directors), VI (Stockholders), VII (Liability and
Indemnification; Corporate Opportunity), VIII (Business Combinations), IX (Exclusive Forum),
and Article X (Amendments) to the Third Amended and Restated Certificate of Incorporation. |
| | | |
| - | Proposal
4A: To approve the addition to Company’s Certificate of Incorporation of a super
majority voting requirement to amend the Surviving Corporation’s Bylaws (the “Bylaw
Amendment Requirement”), as contained in the Third Amended and Restated Certificate
of Incorporation. |
| | | |
| - | Proposal
4B: To approve the addition to the Company’s Certificate of Incorporation of a
super majority voting requirement (the “Article Amendment Requirement”) to amend
Articles V (Board of Directors), VI (Stockholders), VII (Liability and Indemnification; Corporate
Opportunity), VIII (Business Combinations), IX (Exclusive Forum), and Article X (Amendments)
of the Third Amended and Restated Certificate of Incorporation. |
| | | |
| - | Proposal
5: To elect eight directors to serve on the Company’s board of directors following
the consummation of the Business Combination until the 2024 annual meeting of Stockholders,
in the case of Class I directors, the 2025 annual meeting of Stockholders, in the case of
Class II directors, and the 2026 annual meeting of Stockholders, in the case of Class III
directors, and, in each case, until their respective successors are duly elected and qualified
(the “Director Election Proposal”). |
| | | |
| - | Proposal
6: To approve the 2022 Equity Incentive Plan (the “Incentive Plan Proposal”). |
| - | Proposal
7: To approve adjourning the Special Meeting to a later date or dates, if necessary to
permit further solicitation and vote of proxies if it is determined by the Company that more
time is necessary or appropriate to approve one or more Proposals at the Special Meeting
(the “Adjournment Proposal”). |
In
connection with the Special Meeting, certain Stockholders (the “Redeeming Stockholders”) holding 1,614,148 shares of Class
A Common Stock (after giving effect to withdrawals of redemptions) exercised their right to redeem such shares for a pro rata portion
of the funds held by Continental Stock Transfer & Trust Company, as trustee (“Continental”) in the trust account established
in connection with the Company’s initial public offering (the “Trust Account”). As a result, approximately $17.9 million
(approximately $11.12 per share) will be removed from the Trust Account to pay the Redeeming Stockholders, and approximately $1.1 million
will remain in the Trust Account.
On
November 7, 2023, the Company consummated the Business Combination with OneMedNet pursuant to Merger Agreement, following the approval
of the Business Combination at the Special Meeting. On November 7, 2023, at the closing of the Business Combination pursuant to the Merger
Agreement, Merger Sub merged with and into OneMedNet with OneMedNet surviving the Merger, as a wholly-owned subsidiary of the Company,
and the Company changed its name to “OneMedNet Corporation.”
PART
II—INFORMATION NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution
The
following table sets forth all expenses, other than the underwriting discounts and commissions, payable by the registrant in connection
with the sale of the securities being registered. All the amounts shown are estimates except the SEC registration fee and the FINRA filing
fee.
| |
Amount
to be paid | |
| SEC
registration fee | |
$ | 1,476 | |
| FINRA
filing fee | |
$ | 5,000 | |
| Accounting
fees and expenses | |
$ | 20,000 | |
| Legal
fees and expenses | |
$ | 100,000 | |
| Printing
and engraving expenses | |
$ | 2,000 | |
| Miscellaneous | |
$ | 21,524 | |
| | |
| | |
| Total | |
$ | 150,000 | |
Item
14. Indemnification of Directors and Officers
Section
102 of the General Corporation Law of the State of Delaware (the “DGCL”) permits a corporation to eliminate the personal
liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as
a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or
knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law
or obtained an improper personal benefit. Our Amended and Restated Certificate of Incorporation, as amended, provides that no director
of the Company shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director,
notwithstanding any provision of law imposing such liability, except to the extent that the DGCL prohibits the elimination or limitation
of liability of directors for breaches of fiduciary duty.
Section
145 of the DGCL provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or
a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in
related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably
incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party
to any threatened, ending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and
in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding,
had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation,
no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable
to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the
adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity
for such expenses which the Court of Chancery or such other court shall deem proper.
Our
Amended and Restated Certificate of Incorporation, as amended, and Amended and Restated Bylaws provide indemnification for our directors
and officers to the fullest extent permitted by the DGCL. We will indemnify each person who was or is a party or threatened to be made
a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of us) by reason
of the fact that he or she is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at
our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership,
joint venture, trust or other enterprise (all such persons being referred to as an “Indemnitee”), or by reason of any action
alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and
amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom,
if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests,
and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful.
Our Amended and Restated Certificate of Incorporation, as amended, and Amended and Restated Bylaws will provide that we will indemnify
any Indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the
fact that the Indemnitee is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at
our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership,
joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against
all expenses (including attorneys’ fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably
incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a
manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with
respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines
that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding
the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us
against all expenses (including attorneys’ fees) actually and reasonably incurred in connection therewith. Expenses must be advanced
to an Indemnitee under certain circumstances.
We
have entered into separate indemnification agreements with each of our directors and executive officers. Each indemnification agreement
provide, among other things, for indemnification to the fullest extent permitted by law and our Amended and Restated Certificate of Incorporation,
as amended, and Amended and Restated Bylaws against any and all expenses, judgments, fines, penalties and amounts paid in settlement
of any claim. The indemnification agreements provide for the advancement or payment of all expenses to the indemnitee and for the reimbursement
to us if it is found that such indemnitee is not entitled to such indemnification under applicable law and our Amended and Restated Certificate
of Incorporation, as amended, and Amended and Restated Bylaws.
We
also have a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out
of claims based on acts or omissions in their capacities as directors or officers.
Item
15. Recent Sales of Unregistered Securities
On
June 28, 2023, the Company (formerly as Data Knights Acquisition Corp) and OneMedNet Solutions Corporation (formerly as OneMedNet Corporate)
entered into a Securities Purchase Agreement (the “SPA”) with certain investors (collectively referred to herein as the “Purchasers”)
for PIPE financing in the aggregate original principal amount of $1,595,744.70 and the purchase price of $1.5 million.
Effective
immediately prior to the Closing on September 7, 2023, as provided under the SPA, the Company issued and sold to each of the Purchasers,
a new series of senior secured convertible notes (the “PIPE Notes”), which are convertible into shares of Common Stock at
the Purchasers election at a conversion price equal to the lower of (i) $10.00 per share, and (ii) 92.5% of the lowest volume weighted
average trading price for the ten (10) Trading Days immediately preceding the Conversion Date. We deemed the offer, sale and issuance
of such securities to be exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act, including
Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving a public offering.
Item
16. Exhibits and Financial Statement Schedules
EXHIBIT
INDEX
| Exhibit
No. |
|
Title
of Document |
| 2.1† |
|
Agreement
and Plan of Merger, dated April 25, 2022, by and among Data Knights, Merger Sub, Sponsor, OneMedNet, and Paul Casey (incorporated
by reference to Exhibit 2.1 to the Company’s Form 8-K, filed with the SEC on April 25, 2022). |
| |
|
| 3.1* |
|
Third Amended and Restated Certificate of Incorporation of OneMedNet Corporation. |
| |
|
| 3.2* |
|
Amended and Restated Bylaws of OneMedNet Corporation. |
| |
|
| 4.1 |
|
Warrant Agreement, dated May 6, 2021, by and between Continental Stock Transfer & Trust Company and the Company (incorporated by reference to Exhibit 4.3 to the Company’s Form S-1/A, filed with the SEC on April 7, 2021). |
| |
|
| 4.2 |
|
Specimen
Unit Certificate (incorporated by reference to Exhibit 4.1 to the Company’s Form S-1/A, filed with the SEC on April 7, 2021). |
| |
|
| 4.3 |
|
Specimen
Class A Common Stock Certificate (incorporated by reference to Exhibit 4.2 to the Company’s Form S-1/A, filed with the SEC
on April 7, 2021). |
| 4.4 |
|
Specimen
Warrant Certificate (incorporated by reference to Exhibit 4.3 to the Company’s Form S-1/A, filed with the SEC on April 7, 2021). |
| |
|
| 10.1+ |
|
Form of OneMedNet Corporation 2022 Equity Incentive Plan (incorporated by reference to Annex D to the proxy statement/prospectus which is part of the Registration Statement on Form S-4 declared effective by the SEC on September 22, 2023). |
| |
|
|
| 10.2* |
|
Form of Registration Rights Agreement by certain OneMedNet equity holders (included as Exhibit G to Annex B to the proxy statement/prospectus). |
| |
|
| 10.3* |
|
Lockup Agreement by certain OneMedNet equity holders (included as Exhibit C to Annex B to the proxy statement/ prospectus). |
| |
|
| 10.4 |
|
Sponsor
Lock-up Agreement (as incorporated by reference to Exhibit B of Exhibit 2.1 to the Company’s Form 8-K, filed with the SEC on
April 25, 2022). |
| |
|
| 10.5 |
|
Letter
Agreement, dated May 6, 2021, by and between Data Knights, the initial security holders and the officers and directors of the Data
Knights (incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K, filed with the SEC on May 11, 2021) |
| |
|
| 10.6 |
|
Voting
Agreement incorporated by reference to Form 8-K filed April 25, 2022 which is included as Appendix A to Exhibit 2.2. |
| |
|
| 10.7 |
|
Sponsor Support Agreement Voting Agreement. (incorporated by reference to Exhibit B to Annex B to the proxy statement/ prospectus filed by Data Knights Acquisition Corp.). |
| |
|
| 10.8*+ |
|
Employment Agreement between OneMedNet Corporation and Aaron Green, President. |
| |
|
| 10.9*+ |
|
Employment Agreement between OneMedNet Corporation and Lisa Embree, Chief Financial Officer. |
| |
|
|
| 10.10*+ |
|
Employment Agreement between OneMedNet Corporation and Paul Casey, Chief Executive Officer. |
| |
|
| 10.11* |
|
Securities Purchase Agreement dated June 28, 2023 with OneMedNet Corporation. |
| |
|
| 14.1* |
|
Code of Ethics |
| |
|
| 21.1 |
|
Subsidiaries of the Registrant incorporated by reference to Exhibit 21.1 to the Company’s Form S-4, filed with the SEC on September 21, 2023). |
| |
|
| 23.1* |
|
Consent of BF Borgers CPA PC. |
| |
|
|
| 24 |
|
Power of Attorney (included on signature page hereto). |
| |
|
|
| 107 |
|
Filing Fee Table |
| * |
Filed herewith |
| + |
Indicates
a management or compensatory plan. |
| † |
Schedules
to this exhibit have been omitted pursuant to Item 601(b)(2) of Registration S-K. The Registrant hereby agrees to furnish a copy
of any omitted schedules to the SEC upon request. |
Item
17. Undertakings
The
undersigned registrant hereby undertakes:
| |
(1) |
To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| |
(i) |
To
include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| |
(ii) |
To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end
of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b)
if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set
forth in the “Calculation of Registration Fee” table in the effective registration statement. |
| |
(iii) |
To
include any material information with respect to the plan of distribution not previously
disclosed in the registration statement or any material change to such information in the
registration statement; provided, however, that paragraphs (i), (ii) and (iii) do not apply
if the registration statement is on Form S-1 and the
information required to be included in a post-effective amendment by those paragraphs is
contained in reports filed with or furnished to the Commission by the registrant pursuant
to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated
by reference in the registration statement, or is contained in a form of prospectus filed
pursuant to Rule 424(b) that is part of the registration statement; |
| |
(2) |
That,
for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof. |
| |
(3) |
To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering. |
| |
(4) |
That,
for the purpose of determining liability under the Securities Act of 1933 to any purchaser: each prospectus filed pursuant to Rule
424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other
than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of
the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior
to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the
registration statement or made in any such document immediately prior to such date of first use; and |
| |
(5) |
That,
for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of the securities: the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant
to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller
to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| |
(i) |
Any
preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule
424; |
| |
(ii) |
Any
free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by
the undersigned registrant; |
| |
(iii) |
The
portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant
or its securities provided by or on behalf of the undersigned registrant; and |
| |
(iv) |
Any
other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| |
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling
persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion
of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933
and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment
by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense
of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities
being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed
in the Act and will be governed by the final adjudication of such issue. |
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement on Form S-1
to be signed on its behalf by the undersigned, thereunto duly authorized in the State of Hawaii, on the 15th day of December
2023.
| |
ONEMEDNET
CORPORATION |
| |
|
| |
By: |
/s/
Paul J. Casey |
| |
|
Paul
J. Casey |
| |
|
Chief
Executive Officer and Director |
KNOW
ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Carol Craig, his or her true and lawful
attorney-in-fact and agent with full power of substitution and re-substitution, for him or her and in his or her name, place and stead,
in any and all capacities to sign any or all amendments (including, without limitation, post-effective amendments) to this Registration
Statement, any related Registration Statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and any or
all pre- or post-effective amendments thereto, and to file the same, with all exhibits thereto, and all other documents in connection
therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do
and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully for all intents and purposes
as he or she might or could do in person, hereby ratifying and confirming that said attorney-in-fact and agent, or any substitute or
substitutes for him, may lawfully do or cause to be done by virtue hereof. Pursuant to the requirements of the Securities Act of 1933,
as amended, the following persons in the capacities and on the dates indicated have signed this Registration Statement below.
Pursuant
to the requirements of the Securities Act of 1933, as amended, this Registration Statement on Form S-1 has been signed by the following
persons in the capacities and on the dates indicated below.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Paul J. Casey |
|
Chief
Executive Officer (Principal Executive Officer) and Director |
|
December
15, 2023 |
| Paul
J. Casey |
|
|
|
|
| |
|
|
|
|
| /s/
Lisa Embree |
|
Chief
Financial Officer |
|
December
15, 2023 |
| Lisa
Embree |
|
(Principal
Financial and Accounting Officer) |
|
|
| |
|
|
|
|
| /s/
Aaron Green |
|
President |
|
December
15, 2023 |
| Aaron
Green |
|
|
|
|
| |
|
|
|
|
| /s/
Dr. Jeffrey Yu |
|
Chairman
of the Board of Directors, Founder, Chief Medical Officer, Vice President |
|
December
15, 2023 |
| Dr.
Jeffrey Yu |
|
|
|
|
| |
|
|
|
|
| /s/
Erkan Akyuz |
|
Director |
|
December
15, 2023 |
| Erkan
Akyuz |
|
|
|
|
| |
|
|
|
|
| /s/
Eric Casaburi |
|
Director |
|
December
15, 2023 |
| Eric
Casaburi |
|
|
|
|
| |
|
|
|
|
| /s/
Robert Golden |
|
Director |
|
December
15, 2023 |
| Robert
Golden |
|
|
|
|
| |
|
|
|
|
| /s/
Dr. Julianne Huh |
|
Director |
|
December
15, 2023 |
| Dr.
Julianne Huh |
|
|
|
|
| |
|
|
|
|
| /s/
Dr. Thomas Kosasa |
|
Director |
|
December
15, 2023 |
| Dr.
Thomas Kosasa |
|
|
|
|
| |
|
|
|
|
| /s/
R. Scott Holbrook |
|
Director |
|
December
15, 2023 |
| R.
Scott Holbrook |
|
|
|
|
Exhibit
3.1
THIRD
AMENDED AND RESTATED
CERTIFICATE
OF INCORPORATION
OF
ONEMEDNET CORPORATION
November
8, 2023
OneMedNet
Corporation, a corporation organized and existing under the laws of the State of Delaware (the “Corporation”),
DOES HEREBY CERTIFY AS FOLLOWS:
1.
The name of the Corporation is “Data Knights Acquisition Corp” and hereby changes its name to “OneMedNet Corporation”
The original certificate of incorporation of the Corporation was filed with the Secretary of State of the State of Delaware on February
8, 2021, was amended and restated on March 8, 2021 by the First Amended and Restated Certificate, and was amended and restated on April
6, 2021 by the Second Amended and Restated Certificate, which Second Amended and Restated Certificate was further amended by the First
Amendment to the Second Amended and Restated Certificate on November 11, 2022 and the Second Amendment to the Second Amended and Restated
Certificate on August 11, 2023 (the “Certificate”).
2.
This Third Amended and Restated Certificate of Incorporation (this “Third Amended and Restated Certificate”),
which both restates and amends the provisions of the Certificate, was duly adopted in accordance with Sections 228, 242 and 245 of the
General Corporation Law of the State of Delaware, as amended from time to time (the “DGCL”).
3.
This Third Amended and Restated Certificate shall become effective on the date of filing with Secretary of State of Delaware.
4.
The texts of the Certificate, as amended to the date hereof, is hereby restated and amended in their entirety to read as follows:
ARTICLE
I
NAME
The
name of the corporation is OneMedNet Corporation (the “Corporation”).
ARTICLE
II
PURPOSE
The
purpose of the Corporation is to engage in any lawful act or activity for which a corporation may be organized under the General Corporation
Law of the State of Delaware (the “DGCL”).
ARTICLE
III
REGISTERED
OFFICE AND AGENT
The
address of the Corporation’s registered office in the State of Delaware is 1209 Orange Street, Corporation Trust Center, City of
Wilmington, County of New Castle, 19801. The name of its registered agent at that address is The Corporation Trust Company.
ARTICLE
IV
CAPITAL
STOCK
Section
4.1 The total number of shares of all classes of capital stock, each with a par value of $0.0001 per share, which the Corporation
is authorized to issue is 101,000,000 shares, consisting of (a) 100,000,000 shares of common stock (the “Common Stock”),
(b) 1,000,000 shares of preferred stock (the “Preferred Stock”).
Section
4.2 Shares of Preferred Stock may be issued from time to time in one or more classes or series. The Board of Directors of the Corporation
(the “Board of Directors”) is hereby authorized to provide from time to time by resolution or resolutions for
the creation and issuance, out of the authorized and unissued shares of Preferred Stock, of one or more classes or series of Preferred
Stock by filing a certificate (a “Certificate of Designation”) pursuant to the DGCL, setting forth such resolution
and, with respect to each such series, establishing the designation of such class or series and the number of shares to be included in
such class or series and fixing the voting powers (full or limited, or no voting power), preferences and relative, participating, optional
or other special rights, and the qualifications, limitations and restrictions thereof, of the shares of each such class or series. Without
limiting the generality of the foregoing, the resolution or resolutions providing for the establishment of any class or series of Preferred
Stock may, to the extent permitted by law, provide that such class or series shall be superior to, rank equally with or be junior to
the Preferred Stock of any other class or series. The powers, preferences and relative, participating, optional and other special rights
of each class or series of Preferred Stock, and the qualifications, limitations, or restrictions thereof, if any, may be different from
those of any and all other classes or series at any time outstanding. Except as otherwise expressly provided in the resolution or resolutions
providing for the establishment of any class or series of Preferred Stock, no vote of the holders of shares of Preferred Stock or Common
Stock shall be a prerequisite to the issuance of any shares of any class or series of the Preferred Stock so authorized in accordance
with this Third Amended and Restated Certificate. Unless otherwise provided in the Certificate of Designation establishing a class or
series of Preferred Stock, the Board of Directors may, by resolution or resolutions, increase or decrease (but not below the number of
shares of such class or series then outstanding) the number of shares of such class or series and, if the number of shares of such class
or series shall be so decreased, the shares constituting such decrease shall resume the status that they had prior to the adoption of
the resolution originally fixing the number of shares of such class or series.
Section
4.3 The Corporation has the authority to create and issue rights, warrants, and options entitling the holders thereof to purchase
shares of any class or series of the Corporation’s capital stock or other securities of the Corporation, and such rights, warrants
and options shall be evidenced by or in instrument(s) approved by the Board of Directors. The Board of Directors is empowered to set
the exercise price, duration, times for exercise and other terms and conditions of such rights, warrants or options; provided, however,
that the consideration to be received for any shares of capital stock subject thereto may not be less than the par value thereof.
Section
4.4
(a)
Except as otherwise required by law or this Third Amended and Restated Certificate (or any Certificate of Designation made hereunder),
the holders of Common Stock shall exclusively possess all voting power with respect to the Corporation. The holders of shares of Common
Stock shall be entitled to one vote for each such share on each matter properly submitted to the stockholders on which the holders of
the Common Stock are entitled to vote. The holders of shares of the Common Stock shall at all times vote together as one class on all
matters submitted to a vote of the stockholders of the Corporation.
(b)
Except as otherwise required by law or this Third Amended and Restated Certificate (or any Certificate of Designation made hereunder),
at any annual or special meetings of the stockholders of the Corporation, the holders of the Common Stock shall have the exclusive right
to vote for the election of directors and on all other matters properly submitted to a vote of the stockholders. Notwithstanding the
foregoing, except as otherwise required by law or this Third Amended and Restated Certificate (or any Certificate of Designation made
hereunder), the holders of the Common Stock shall not be entitled to vote on any amendment to this Third Amended and Restated Certificate
(or any Certificate of Designation made hereunder) that relates solely to the terms of one or more outstanding class or series of the
Preferred Stock if the holders of such affected class or series of Preferred Stock are entitled, either separately or together with the
holders of one or more other such class or series, to vote thereon pursuant to this Third Amended and Restated Certificate (or any Certificate
of Designation made hereunder) or the DGCL.
(c)
Subject to applicable law and the rights, if any, of the holders of any outstanding class or series of the Preferred Stock, the holders
of the shares of the Common Stock shall be entitled to receive such dividends and other distributions (payable in cash, property or capital
stock of the Corporation) when, as and if declared thereon by the Board from time to time out of any assets or funds of the Corporation
legally available therefor, and shall share equally on a per share basis in such dividends and distributions.
(d)
Subject to applicable law and the rights, if any, of the holders of any outstanding class or series of the Preferred Stock, in the event
of any voluntary or involuntary liquidation, dissolution or winding-up of the Corporation, after payment or provision for payment of
the debts and other liabilities of the Corporation, the holders of the shares of the Common Stock shall be entitled to receive all the
remaining assets of the Corporation available for distribution to its stockholders, ratably in proportion to the number of shares of
the Common Stock held by them.
ARTICLE
V
BOARD
OF DIRECTORS
For
the management of the business and for the conduct of the affairs of the Corporation it is further provided that:
Section
5.1
(a)
The management of the business and the conduct of the affairs of the Corporation shall be vested in the Board of Directors. The number
of directors of the Corporation shall be nine (9), which number may be increased or decreased by one or more resolutions adopted from
time to time by the Board of Directors, subject to Sections 5.1(c) and 5.1(d). The Board of Directors shall be classified, with respect
to the term for which the directors hold office, into three classes, one class serving for a term expiring at the annual meeting of stockholders
to be held in 2023, the second class serving for a term expiring at the annual meeting of stockholders to be held in 2024, and the third
class serving for a term expiring at the annual meeting of stockholders to be held in 2025. At each annual meeting of stockholders, directors
elected to succeed those directors whose terms expire shall be elected for a term of office to expire at the third succeeding annual
meeting of stockholders after their election. Notwithstanding the foregoing, the directors elected to each class shall hold office until
their successors are duly elected and qualified or until their earlier resignation, death, or removal. If the number of directors is
hereafter changed, any newly created directorships or decrease in directorships shall be so apportioned among the classes as to make
all classes as nearly equal in number as is practicable, provided that no decrease in the number of directors constituting the entire
Board shall shorten the term of any incumbent director. No reduction in the number of directors shall cause the removal of any director
from office prior to the expiration of his or her term.
(b)
Except as may otherwise be provided in the terms of any Preferred Stock issued by the Corporation, the Board of Directors or any individual
director may be removed from office at any time but only by the affirmative vote of the holders of at least a majority of the voting
power of all of the then outstanding shares of voting stock of the Corporation with the power to vote at an election of directors (the
“Voting Stock”) and, in addition to and without limitation of the foregoing only for cause.
(c)
Except as may otherwise be provided in the terms of any Preferred Stock issued by the Corporation and subject to Section 5.1(d) below,
any vacancies on the Board of Directors resulting from death, resignation, disqualification, retirement, removal or other causes and
any newly created directorships resulting from any increase in the number of directors shall, unless the Board of Directors determines
by resolution that any such vacancies or newly created directorships shall be filled by the stockholders, and except as otherwise provided
by law, be filled only by the affirmative vote of a majority of the directors then in office, even though less than a quorum, or by a
sole remaining director, and shall not be filled by the stockholders. Any director appointed in accordance with the preceding sentence
shall hold office for a term that shall coincide with the remaining term of the vacancy to which the director shall have been appointed
and until such director’s successor shall have been elected and qualified or until his or her earlier death, resignation, disqualification,
retirement, or removal.
(d)
Effective as of the date of filing of this Third Amended and Restated Certificate, the Board of Directors of the Corporation shall consist
of nine (9) members, constituted as follows:
(i)
in the director class whose term expires at the annual meeting held in 2023, three (3) directors nominated by OneMedNet Technologies
Corporation, f/k/a OneMedNet Corporation, a Delaware corporation (“OneMedNet Technologies”), one of whom shall qualify as
an independent director under the rules of The Nasdaq Stock Market (an “Independent Director”);
(ii)
in the director class whose term expires at the annual meeting held in 2024, (A) two (2) directors designated by OneMedNet Technologies,
one (1) of whom shall be an Independent Director, and (B) one (1) director designated by Data Knights, LLC, a Delaware limited liability
company and the Corporation’s sponsor (the “Sponsor”), who shall be an Independent Director; and
(iii)
in the director class whose term expires at the annual meeting held in 2025, (A) two (2) directors designated by OneMedNet Technologies,
one (1) of whom shall be an Independent Director, and (B) one (1) director designated by the Sponsor.
Section
5.2
(a)
In furtherance and not in limitation of the powers conferred by statute, the Board of Directors is expressly authorized to adopt, amend,
alter, or repeal the Bylaws of the Corporation. In addition to any vote of the holders of any class or series of stock of the Corporation
required by applicable law or by this Third Amended and Restated Certificate (including any Certificate of Designation in respect of
one or more classes or series of Preferred Stock), the adoption, amendment or repeal of the Bylaws of the Corporation by the stockholders
of the Corporation shall require the affirmative vote of the holders of at least sixty-six and two-thirds percent (66-2/3%) of the voting
power of all the then-outstanding shares of the Voting Stock, voting together as a single class; provided that no Bylaws hereafter adopted
by the stockholders of the Corporation shall invalidate any prior act of the Board of Directors that would have been valid if such Bylaws
had not been adopted.
(b)
The directors of the Corporation need not be elected by written ballot unless the Bylaws so provide.
ARTICLE
VI
STOCKHOLDERS
Section
6.1 Subject to the special rights of the holders of one or more classes or series of Preferred Stock, any action required or permitted
to be taken by the stockholders of the Corporation must be effected at a duly called annual or special meetings of the stockholders of
the Corporation, and the taking of any action by written consent of the stockholders in lieu of a meeting of the stockholders is specifically
denied.
Section
6.2 Subject to the special rights of the holders of one or more classes or series of Preferred Stock, special meetings of the stockholders
of the Corporation may be called, for any purpose or purposes as is a proper matter for stockholder action under the DGCL, by (i) the
Chairperson of the Board of Directors, (ii) the Chief Executive Officer, or (iii) the Board of Directors pursuant to a resolution adopted
by a majority of the total number of authorized directors (whether or not there exist any vacancies in previously authorized directorships
at the time any such resolution is presented to the Board of Directors for adoption). Such special meetings may not be called by stockholders
or any other person or persons.
Section
6.3 Advance notice of stockholder nominations for the election of directors and of other business proposed to be brought by stockholders
before any meeting of the stockholders of the Corporation shall be given in the manner provided in the Bylaws of the Corporation.
ARTICLE
VII
LIABILITY
AND INDEMNIFICATION; CORPORATE OPPORTUNITY
Section
7.1 To the fullest extent permitted by the DGCL, as the same exists or as may hereafter be amended, a director of the Corporation
shall not be personally liable to the Corporation or its stockholders for monetary damages for breach of fiduciary duty as a director.
If the DGCL is amended after approval by the stockholders of this Article VII to authorize corporate action further eliminating or limiting
the personal liability of directors, then the liability of a director of the Corporation shall be eliminated or limited to the fullest
extent permitted by the DGCL as so amended, automatically and without further action, upon the date of such amendment.
Section
7.2 The Corporation, to the fullest extent permitted by law, shall indemnify and advance expenses to any person made or threatened
to be made a party to an action, suit or proceeding, whether criminal, civil, administrative or investigative, by reason of the fact
that he or she, or his or her testator or intestate, is or was a director or officer of the Corporation or any predecessor of the Corporation,
or serves or served at any other enterprise as a director or officer at the request of the Corporation or any predecessor to the Corporation.
Section
7.3 The Corporation, to the fullest extent permitted by law, may indemnify and advance expenses to any person made or threatened
to be made a party to an action, suit or proceeding, whether criminal, civil, administrative or investigative, by reason of the fact
that he or she, or his or her testator or intestate, is or was an employee or agent of the Corporation or any predecessor of the Corporation,
or serves or served at any other enterprise as an employee or agent at the request of the Corporation or any predecessor to the Corporation.
Section
7.4 Neither any amendment nor repeal of this Article VII, nor the adoption by amendment of this Third Amended and Restated Certificate
of any provision inconsistent with this Article VII, shall eliminate or reduce the effect of this Article VII in respect of any matter
occurring, or any action or proceeding accruing or arising (or that, but for this Article VII, would accrue or arise) prior to such amendment
or repeal or adoption of an inconsistent provision.
ARTICLE
VIII
BUSINESS
COMBINATIONS
Section
8.1 The Corporation will be subject to Section 203 of the DGCL.
Section
8.2 Notwithstanding Section 8.1, the Corporation shall not engage in any business combination (as defined below), at any point in
time at which the Corporation’s common stock is registered under Section 12(b) or 12(g) of the Exchange Act, with any interested
stockholder (as defined below) for a period of three years following the time that such stockholder of the Corporation became an interested
stockholder, unless:
(a)
prior to such time, the Board approved either the business combination or the transaction which resulted in the stockholder becoming
an interested stockholder, or
(b)
upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder
owned at least 85% of the voting stock (as defined below) of the Corporation outstanding at the time the transaction commenced, excluding
for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those
shares owned by (i) persons who are directors and also officers of the Corporation or (ii) employee stock plans in which employee participants
do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer,
or
(c)
at or subsequent to such time, the business combination is approved by the Board and authorized at an annual or special meeting of stockholders,
and not by written consent, by the affirmative vote of at least 662∕3% of the outstanding voting stock of the Corporation which
is not owned by the interested stockholder.
Section
8.3 For the purposes of this Article VIII:
(a)
“affiliate” means a person that directly, or indirectly through one or more intermediaries, controls, or is controlled by,
or is under common control with, another person.
(b)
“associate,” when used to indicate a relationship with any person, means: (i) any corporation, partnership, unincorporated
association or other entity of which such person is a director, officer or partner or is, directly or indirectly, the owner of 20% or
more of any class of voting stock; (ii) any trust or other estate in which such person has at least a 20% beneficial interest or as to
which such person serves as trustee or in a similar fiduciary capacity; and (iii) any relative or spouse of such person, or any relative
of such spouse, who has the same residence as such person.
(c)
“business combination,” when used in reference to the Corporation and any interested stockholder, means:
(i)
any merger or consolidation of the Corporation or any direct or indirect majority-owned subsidiary of the Corporation (A) with the interested
stockholder, or (B) with any other corporation, partnership, unincorporated association or other entity if the merger or consolidation
is caused by the interested stockholder and as a result of such merger or consolidation Article XI is not applicable to the surviving
entity;
(ii)
any sale, lease, exchange, mortgage, pledge, transfer or other disposition (in one transaction or a series of transactions), except proportionately
as a stockholder of the Corporation, to or with the interested stockholder, whether as part of a dissolution or otherwise, of assets
of the Corporation or of any direct or indirect majority-owned subsidiary of the Corporation which assets have an aggregate market value
equal to 10% or more of either the aggregate market value of all the assets of the Corporation determined on a consolidated basis or
the aggregate market value of all the outstanding stock of the Corporation;
(iii)
any transaction which results in the issuance or transfer by the Corporation or by any direct or indirect majority-owned subsidiary of
the Corporation of any stock of the Corporation or of such subsidiary to the interested stockholder, except: (A) pursuant to the exercise,
exchange or conversion of securities exercisable for, exchangeable for or convertible into stock of the Corporation or any such subsidiary
which securities were outstanding prior to the time that the interested stockholder became such; (B) pursuant to a merger under Section
251(g) of the DGCL; (C) pursuant to a dividend or distribution paid or made, or the exercise, exchange or conversion of securities exercisable
for, exchangeable for or convertible into stock of the Corporation or any such subsidiary which security is distributed, pro rata to
all holders of a class or series of stock of the Corporation subsequent to the time the interested stockholder became such; (D) pursuant
to an exchange offer by the Corporation to purchase stock made on the same terms to all holders of said stock; or (E) any issuance or
transfer of stock by the Corporation; provided, however, that in no case under items (C)-(E) of this subsection (iii) shall there be
an increase in the interested stockholder’s proportionate share of the stock of any class or series of the Corporation or of the
voting stock of the Corporation (except as a result of immaterial changes due to fractional share adjustments);
(iv)
any transaction involving the Corporation or any direct or indirect majority-owned subsidiary of the Corporation which has the effect,
directly or indirectly, of increasing the proportionate share of the stock of any class or series, or securities convertible into the
stock of any class or series, of the Corporation or of any such subsidiary which is owned by the interested stockholder, except as a
result of immaterial changes due to fractional share adjustments or as a result of any purchase or redemption of any shares of stock
not caused, directly or indirectly, by the interested stockholder; or
(v)
any receipt by the interested stockholder of the benefit, directly or indirectly (except proportionately as a stockholder of the Corporation),
of any loans, advances, guarantees or pledges (other than those expressly permitted in subsections (i)-(iv) above) provided by or through
the Corporation or any direct or indirect majority-owned subsidiary.
(d)
“control,” including the terms “controlling,” “controlled by” and “under common control with,”
means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of a person,
whether through the ownership of voting stock, by contract, or otherwise. A person who is the owner of 20% or more of the outstanding
voting stock of the Corporation, partnership, unincorporated association or other entity shall be presumed to have control of such entity,
in the absence of proof by a preponderance of the evidence to the contrary. Notwithstanding the foregoing, a presumption of control shall
not apply where such person holds voting stock, in good faith and not for the purpose of circumventing this Article VIII, as an agent,
bank, broker, nominee, custodian or trustee for one or more owners who do not individually or as a group have control of such entity.
(e)
“interested stockholder” means any person (other than the Corporation or any direct or indirect majority-owned subsidiary
of the Corporation) that (i) is the owner of 15% or more of the outstanding voting stock of the Corporation, or (ii) is an affiliate
or associate of the Corporation and was the owner of 15% or more of the outstanding voting stock of the Corporation at any time within
the three year period immediately prior to the date on which it is sought to be determined whether such person is an interested stockholder,
and the affiliates and associates of such person; provided, however, that the term “interested stockholder” shall not include
(A) the Principal Stockholder or Principal Stockholder Transferees or any “group” (within
the meaning of Rule 13d-5 of the Exchange Act) that includes any Principal Stockholder or Principal Stockholder Transferee or (B) any
person whose ownership of shares in excess of the 15% limitation set forth herein is the result of any action taken solely by the Corporation;
provided that such person specified in this clause (B) shall be an interested stockholder if thereafter such person acquires additional
shares of voting stock of the Corporation, except as a result of further corporate action not caused, directly or indirectly, by such
person. For the purpose of determining whether a person is an interested stockholder, the voting stock of the Corporation deemed to be
outstanding shall include stock deemed to be owned by the person through application of the definition of “owner” below but
shall not include any other unissued stock of the Corporation which may be issuable pursuant to any agreement, arrangement or understanding,
or upon exercise of conversion rights, warrants or options, or otherwise.
(f)
“owner,” including the terms “own” and “owned,” when used with respect to any stock, means a person
that individually or with or through any of its affiliates or associates:
(i)
beneficially owns such stock, directly or indirectly; or
(ii)
has (A) the right to acquire such stock (whether such right is exercisable immediately or only after the passage of time) pursuant to
any agreement, arrangement or understanding, or upon the exercise of conversion rights, exchange rights, warrants or options, or otherwise;
provided, however, that a person shall not be deemed the owner of stock tendered pursuant to a tender or exchange offer made by such
person or any of such person’s affiliates or associates until such tendered stock is accepted for purchase or exchange; or (B)
the right to vote such stock pursuant to any agreement, arrangement or understanding; provided, however, that a person shall not be deemed
the owner of any stock because of such person’s right to vote such stock if the agreement, arrangement or understanding to vote
such stock arises solely from a revocable proxy or consent given in response to a proxy or consent solicitation made to ten or more persons;
or
(iii)
has any agreement, arrangement or understanding for the purpose of acquiring, holding, voting (except voting pursuant to a revocable
proxy or consent as described in item (B) of subsection (ii) above), or disposing of such stock with any other person that beneficially
owns, or whose affiliates or associates beneficially own, directly or indirectly, such stock.
(g)
“person” means any individual, corporation, partnership, unincorporated association or other entity.
(h)
“stock” means, with respect to any corporation, capital stock and, with respect to any other entity, any equity interest.
(i)
“Principal Stockholder” means, collectively, (i) the Sponsor and (ii) any affiliate or successor of a person referenced in
clauses (i) and (ii) of this definition.
(j)
“Principal Stockholder Transferee” means any Person who acquires voting stock of the Corporation from the Principal Stockholder
(other than in connection with a public offering) and who is designated in writing by the Principal Stockholder as a “Principal
Stockholder Transferee.”
(k)
“voting stock” means stock of any class or series entitled to vote generally in matters submitted for stockholders’
approval other than the election of directors.
ARTICLE
IX
EXCLUSIVE
FORUM
Section
9.1 Unless the Corporation consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware
shall, to the fullest extent permitted by law, be the sole and exclusive forum for (a) any derivative action or proceeding brought on
behalf of the Corporation, (b) any action asserting a claim of breach of fiduciary duty owed by any director, officer, employee, agent
or stockholder of the Corporation to the Corporation or the Corporation’s stockholders, creditors or other constituents, (c) any
action asserting a claim arising pursuant to any provision of the DGCL or this Third Amended and Restated Certificate or the Bylaws of
the Corporation, or (d) any action asserting a claim governed by the internal affairs doctrine, in each case subject to said Court of
Chancery having personal jurisdiction over the indispensable parties named as defendants therein; provided that suits brought to enforce
any liability or duty created by the Securities Act of 1933, as amended, the Securities Exchange Act of 1934, as amended, or any other
claim for which the federal courts have exclusive jurisdiction, shall be brought in the federal district court for the district of Delaware;
and provided further that, if and only if the Court of Chancery of the State of Delaware dismisses any such action for lack of subject
matter jurisdiction, such action may be brought in another state or federal court sitting in the State of Delaware. To the fullest extent
permitted by applicable law, any person or entity purchasing or otherwise acquiring or holding any interest in shares of capital stock
of the Corporation shall be deemed to have notice of and consented to the provisions of this Article VIII. Notwithstanding any other
provisions of law, this Third Amended and Restated Certificate or the Bylaws of the Corporation, and notwithstanding the fact that a
lesser percentage may be specified by law, the affirmative vote of the holders of at least two-thirds in voting power of the outstanding
shares of capital stock of the Corporation entitled to vote thereon shall be required to amend or repeal, or to adopt any provision inconsistent
with, this Article VIII. If any provision or provisions of this Article VIII shall be held to be invalid, illegal or unenforceable as
applied to any person or entity or circumstance for any reason whatsoever, then, to the fullest extent permitted by law, the validity,
legality and enforceability of such provisions in any other circumstance and of the remaining provisions of this Article VIII (including,
without limitation, each portion of any sentence of this Article VIII containing any such provision held to be invalid, illegal or unenforceable
that is not itself held to be invalid, illegal or unenforceable) and the application of such provision to other persons or entities and
circumstances shall not in any way be affected or impaired thereby.
Section
9.2 If any action the subject matter of which is within the scope of Section 9.1 is filed in a court other than within the State
of Delaware (a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have consented
to (i) the personal jurisdiction of the state and federal courts of the State of Delaware in connection with any action brought in any
such court to enforce Section 9.1 (an “FSC Enforcement Action”) and (ii) having service of process made upon
such stockholder in any such FSC Enforcement Action by service upon such stockholder’s counsel in the Foreign Action as agent for
such stockholder.
Section
9.3 If any provision or provisions of this Article VIII shall be held to be invalid, illegal or unenforceable as applied to any person
or entity or circumstance for any reason whatsoever, then, to the fullest extent permitted by law, the validity, legality and enforceability
of such provisions in any other circumstance and of the remaining provisions of this Article VIII (including, without limitation, each
portion of any sentence of this Article VIII containing any such provision held to be invalid, illegal or unenforceable that is not itself
held to be invalid, illegal or unenforceable) and the application of such provision to other persons or entities and circumstances shall
not in any way be affected or impaired thereby. Any person or entity purchasing or otherwise acquiring any interest in shares of capital
stock of the Corporation shall be deemed to have notice of and consented to the provisions of this Article VIII.
ARTICLE
X
AMENDMENTS
Notwithstanding
any other provisions of this Third Amended and Restated Certificate or any provision of law which might otherwise permit a lesser vote
or no vote, but in addition to any affirmative vote of the holders of any particular class or series of the Voting Stock required by
law or by this Third Amended and Restated Certificate (including any Certificate of Designation in respect of one or more series of Preferred
Stock), the affirmative vote of the holders of at least sixty-six and two-thirds percent (66-2/3%) of the voting power of all of the
then-outstanding shares of the Voting Stock, voting together as a single class, shall be required to alter, amend or repeal Articles
V, VI, VII, VIII, IX and this Article X.
*****
IN
WITNESS WHEREOF, OneMedNet Corporation has caused this Amended and Restated Certificate to be duly executed and acknowledged in its name
and on its behalf by an authorized officer as of the date first set forth above.
| |
ONEMEDNET
CORPORATION |
| |
|
|
| |
By: |
/s/
Paul Casey |
| |
Name:
|
Paul
Casey |
| |
Title:
|
Chief
Executive Officer |
Exhibit
3.2

AMENDED
AND RESTATED BYLAWS
OF
ONEMEDNET
CORPORATION
(THE
“CORPORATION”)
(Adopted
November 8, 2023)
ARTICLE
I
OFFICES
Section
1.1. Registered Office. The registered office of the Corporation within the State of Delaware shall be located at either (a) the
principal place of business of the Corporation in the State of Delaware or (b) the office of the corporation or individual acting as
the Corporation’s registered agent in Delaware.
Section
1.2. Additional Offices. The Corporation may, in addition to its registered office in the State of Delaware, have such other offices
and places of business, both within and outside the State of Delaware, as the Board of Directors of the Corporation (the “Board”)
may from time to time determine or as the business and affairs of the Corporation may require.
ARTICLE
II
STOCKHOLDERS
MEETINGS
Section
2.1. Annual Meetings. The annual meeting of stockholders shall be held at such place, either within or without the State of Delaware,
and time and on such date as shall be determined by the Board and stated in the notice of the meeting, provided that the Board may in
its sole discretion determine that the meeting shall not be held at any place, but may instead be held solely by means of remote communication
pursuant to Section 9.5(a). At each annual meeting, the stockholders entitled to vote on such matters shall elect those directors
of the Corporation to fill any term of a directorship that expires on the date of such annual meeting and may transact any other business
as may properly be brought before the meeting.
Section
2.2. Special Meetings. Subject to the rights of the holders of any outstanding series of the preferred stock of the Corporation (“Preferred
Stock”), and to the requirements of applicable law, special meetings of stockholders, for any purpose or purposes, may
be called only by the Chairman of the Board, Chief Executive Officer, or the Board pursuant to a resolution adopted by a majority of
the Board, and may not be called by any other person. Special meetings of stockholders shall be held at such place, either within or
without the State of Delaware, and at such time and on such date as shall be determined by the Board and stated in the Corporation’s
notice of the meeting, provided that the Board may in its sole discretion determine that the meeting shall not be held at any place,
but may instead be held solely by means of remote communication pursuant to Section 9.5(a).
Section
2.3. Notices. Written notice of each stockholders meeting stating the place, if any, date, and time of the meeting, and the means
of remote communication, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting
and the record date for determining the stockholders entitled to vote at the meeting, if such date is different from the record date
for determining stockholders entitled to notice of the meeting, shall be given in the manner permitted by Section 9.3 to each
stockholder entitled to vote thereat as of the record date for determining the stockholders entitled to notice of the meeting, by the
Corporation not less than 10 nor more than 60 days before the date of the meeting unless otherwise required by the General Corporation
Law of the State of Delaware (the “DGCL”). If said notice is for a stockholders meeting other than an annual
meeting, it shall in addition state the purpose or purposes for which the meeting is called, and the business transacted at such meeting
shall be limited to the matters so stated in the Corporation’s notice of meeting (or any supplement thereto). Any meeting of stockholders
as to which notice has been given may be postponed, and any meeting of stockholders as to which notice has been given may be cancelled,
by the Board upon public announcement (as defined in Section 2.7(c)) given before the date previously scheduled for such meeting.
Section
2.4. Quorum. Except as otherwise provided by applicable law, the Corporation’s Certificate of Incorporation, as the same may
be amended or restated from time to time (the “Certificate of Incorporation”) or these By Laws, the presence,
in person or by proxy, at a stockholders meeting of the holders of shares of outstanding capital stock of the Corporation representing
a majority of the voting power of all outstanding shares of capital stock of the Corporation entitled to vote at such meeting shall constitute
a quorum for the transaction of business at such meeting, except that when specified business is to be voted on by a class or series
of stock voting as a class, the holders of shares representing a majority of the voting power of the outstanding shares of such class
or series shall constitute a quorum of such class or series for the transaction of such business. If a quorum shall not be present or
represented by proxy at any meeting of the stockholders of the Corporation, the chairman of the meeting may adjourn the meeting from
time to time in the manner provided in Section 2.6 until a quorum shall attend. The stockholders present at a duly convened meeting
may continue to transact business until adjournment, notwithstanding the withdrawal of enough stockholders to leave less than a quorum.
Shares of its own stock belonging to the Corporation or to another corporation, if a majority of the voting power of the shares entitled
to vote in the election of directors of such other corporation is held, directly or indirectly, by the Corporation, shall neither be
entitled to vote nor be counted for quorum purposes; provided, however, that the foregoing shall not limit the right of the Corporation
or any such other corporation to vote shares held by it in a fiduciary capacity.
Section
2.5. Voting of Shares.
(a)
Voting Lists. The Secretary of the Corporation (the “Secretary”) shall prepare, or shall cause the officer
or agent who has charge of the stock ledger of the Corporation to prepare and make, at least 10 days before every meeting of stockholders,
a complete list of the stockholders of record entitled to vote at such meeting; provided, however, that if the record date for determining
the stockholders entitled to vote is less than 10 days before the meeting date, the list shall reflect the stockholders entitled to vote
as of the tenth day before the meeting date, arranged in alphabetical order and showing the address and the number and class of shares
registered in the name of each stockholder. Nothing contained in this Section 2.5(a) shall require the Corporation to include electronic
mail addresses or other electronic contact information on such list. Such list shall be open to the examination of any stockholder, for
any purpose germane to the meeting, during ordinary business hours for a period of at least 10 days prior to the meeting: (i) on a reasonably
accessible electronic network, provided that the information required to gain access to such list is provided with the notice of the
meeting, or (ii) during ordinary business hours, at the principal place of business of the Corporation. In the event that the Corporation
determines to make the list available on an electronic network, the Corporation may take reasonable steps to ensure that such information
is available only to stockholders of the Corporation. If the meeting is to be held at a place, then the list shall be produced and kept
at the time and place of the meeting during the whole time thereof, and may be inspected by any stockholder who is present. If a meeting
of stockholders is to be held solely by means of remote communication as permitted by Section 9.5(a), the list shall be open to the examination
of any stockholder during the whole time of the meeting on a reasonably accessible electronic network, and the information required to
access such list shall be provided with the notice of meeting. The stock ledger shall be the only evidence as to who are the stockholders
entitled to examine the list required by this Section 2.5(a) or to vote in person or by proxy at any meeting of stockholders.
(b)
Manner of Voting. At any stockholders meeting, every stockholder entitled to vote may vote in person or by proxy. If authorized
by the Board, the voting by stockholders or proxy holders at any meeting conducted by remote communication may be effected by a ballot
submitted by electronic transmission (as defined in Section 9.3), provided that any such electronic transmission must either set
forth or be submitted with information from which the Corporation can determine that the electronic transmission was authorized by the
stockholder or proxy holder. The Board, in its discretion, or the chairman of the meeting of stockholders, in such person’s discretion,
may require that any votes cast at such meeting shall be cast by written ballot.
(c)
Proxies. Each stockholder entitled to vote at a meeting of stockholders or to express consent or dissent to corporate action in
writing without a meeting may authorize another person or persons to act for such stockholder by proxy, but no such proxy shall be voted
or acted upon after three years from its date, unless the proxy provides for a longer period. Proxies need not be filed with the Secretary
until the meeting is called to order, but shall be filed with the Secretary before being voted. Without limiting the manner in which
a stockholder may authorize another person or persons to act for such stockholder as proxy, either of the following shall constitute
a valid means by which a stockholder may grant such authority. No stockholder shall have cumulative voting rights.
(i)
A stockholder may execute a writing authorizing another person or persons to act for such stockholder as proxy. Execution may be accomplished
by the stockholder or such stockholder’s authorized officer, director, employee or agent signing such writing or causing such person’s
signature to be affixed to such writing by any reasonable means, including, but not limited to, by facsimile signature.
(ii)
A stockholder may authorize another person or persons to act for such stockholder as proxy by transmitting or authorizing the transmission
of an electronic transmission to the person who will be the holder of the proxy or to a proxy solicitation firm, proxy support service
organization or like agent duly authorized by the person who will be the holder of the proxy to receive such transmission, provided that
any such electronic transmission must either set forth or be submitted with information from which it can be determined that the electronic
transmission was authorized by the stockholder. Any copy, facsimile telecommunication or other reliable reproduction of the writing or
transmission authorizing another person or persons to act as proxy for a stockholder may be substituted or used in lieu of the original
writing or transmission for any and all purposes for which the original writing or transmission could be used; provided that such copy,
facsimile telecommunication or other reproduction shall be a complete reproduction of the entire original writing or transmission.
(d)
Required Vote. Subject to the rights of the holders of one or more series of Preferred Stock, voting separately by class or series,
to elect directors pursuant to the terms of one or more series of Preferred Stock, at all meetings of stockholders at which a quorum
is present, the election of directors shall be determined by a plurality of the votes cast by the stockholders present in person or represented
by proxy at the meeting and entitled to vote thereon. All other matters presented to the stockholders at a meeting at which a quorum
is present shall be determined by the vote of a majority of the votes cast by the stockholders present in person or represented by proxy
at the meeting and entitled to vote thereon, unless the matter is one upon which, by applicable law, the Certificate of Incorporation,
these By Laws or applicable stock exchange rules, a different vote is required, in which case such provision shall govern and control
the decision of such matter.
(e)
Inspectors of Election. The Board may, and shall if required by law, in advance of any meeting of stockholders, appoint one or
more persons as inspectors of election, who may be employees of the Corporation or otherwise serve the Corporation in other capacities,
to act at such meeting of stockholders or any adjournment thereof and to make a written report thereof. The Board may appoint one or
more persons as alternate inspectors to replace any inspector who fails to act. If no inspectors of election or alternates are appointed
by the Board, the chairman of the meeting shall appoint one or more inspectors to act at the meeting. Each inspector, before discharging
his or her duties, shall take and sign an oath faithfully to execute the duties of inspector with strict impartiality and according to
the best of his or her ability. The inspectors shall ascertain and report the number of outstanding shares and the voting power of each;
determine the number of shares present in person or represented by proxy at the meeting and the validity of proxies and ballots; count
all votes and ballots and report the results; determine and retain for a reasonable period a record of the disposition of any challenges
made to any determination by the inspectors; and certify their determination of the number of shares represented at the meeting and their
count of all votes and ballots. No person who is a candidate for an office at an election may serve as an inspector at such election.
Each report of an inspector shall be in writing and signed by the inspector or by a majority of them if there is more than one inspector
acting at such meeting. If there is more than one inspector, the report of a majority shall be the report of the inspectors.
Section
2.6. Adjournments. Any meeting of stockholders, annual or special, may be adjourned by the chairman of the meeting, from time to
time, whether or not there is a quorum, to reconvene at the same or some other place. Notice need not be given of any such adjourned
meeting if the date, time, and place, if any, thereof, and the means of remote communication, if any, by which stockholders and proxy
holders may be deemed to be present in person and vote at such adjourned meeting are announced at the meeting at which the adjournment
is taken. At the adjourned meeting the stockholders, or the holders of any class or series of stock entitled to vote separately as a
class, as the case may be, may transact any business that might have been transacted at the original meeting. If the adjournment is for
more than 30 days, notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting. If after
the adjournment a new record date for stockholders entitled to vote is fixed for the adjourned meeting, the Board shall fix a new record
date for notice of such adjourned meeting in accordance with Section 9.2, and shall give notice of the adjourned meeting to each
stockholder of record entitled to vote at such adjourned meeting as of the record date fixed for notice of such adjourned meeting.
Section
2.7. Advance Notice for Business.
(a)
Annual Meetings of Stockholders. No business may be transacted at an annual meeting of stockholders, other than business that
is either (i) specified in the Corporation’s notice of meeting (or any supplement thereto) given by or at the direction of the
Board, (ii) otherwise properly brought before the annual meeting by or at the direction of the Board or (iii) otherwise properly brought
before the annual meeting by any stockholder of the Corporation (x) who is a stockholder of record entitled to vote at such annual meeting
on the date of the giving of the notice provided for in this Section 2.7(a) and on the record date for the determination of stockholders
entitled to vote at such annual meeting and (y) who complies with the notice procedures set forth in this Section 2.7(a). Notwithstanding
anything in this Section 2.7(a) to the contrary, only persons nominated for election as a director to fill any term of a directorship
that expires on the date of the annual meeting pursuant to Section 3.5 will be considered for election at such meeting.
(i)
In addition to any other applicable requirements, for business (other than nominations) to be properly brought before an annual meeting
by a stockholder, such stockholder must have given timely notice thereof in proper written form to the Secretary and such business must
otherwise be a proper matter for stockholder action. Subject to Section 2.7(a)(iii), a stockholder’s notice to the Secretary
with respect to such business, to be timely, must be received by the Secretary at the principal executive offices of the Corporation
not later than the close of business on the 90th day nor earlier than the opening of business on the 120th day before the anniversary
date of the immediately preceding annual meeting of stockholders; provided, however, that in the event that the annual meeting is more
than 30 days before or more than 60 days after such anniversary date, notice by the stockholder to be timely must be so delivered not
earlier than the close of business on the 120th day before the meeting and not later than the later of (x) the close of business on the
90th day before the meeting or (y) the close of business on the 10th day following the day on which public announcement of the date of
the annual meeting is first made by the Corporation. The public announcement of an adjournment or postponement of an annual meeting shall
not commence a new time period (or extend any time period) for the giving of a stockholder’s notice as described in this Section
2.7(a).
(ii)
To be in proper written form, a stockholder’s notice to the Secretary with respect to any business (other than nominations) must
set forth as to each such matter such stockholder proposes to bring before the annual meeting (A) a brief description of the business
desired to be brought before the annual meeting, the text of the proposal or business (including the text of any resolutions proposed
for consideration and in the event such business includes a proposal to amend these By Laws, the language of the proposed amendment)
and the reasons for conducting such business at the annual meeting, (B) the name and record address of such stockholder and the name
and address of the beneficial owner, if any, on whose behalf the proposal is made, (C) the class or series and number of shares of capital
stock of the Corporation that are owned beneficially and of record by such stockholder and by the beneficial owner, if any, on whose
behalf the proposal is made, (D) a description of all arrangements or understandings between such stockholder and the beneficial owner,
if any, on whose behalf the proposal is made and any other person or persons (including their names) in connection with the proposal
of such business by such stockholder, (E) any material interest of such stockholder and the beneficial owner, if any, on whose behalf
the proposal is made in such business and (F) a representation that such stockholder (or a qualified representative of such stockholder)
intends to appear in person or by proxy at the annual meeting to bring such business before the meeting.
(iii)
The foregoing notice requirements of this Section 2.7(a) shall be deemed satisfied by a stockholder as to any proposal (other
than nominations) if the stockholder has notified the Corporation of such stockholder’s intention to present such proposal at an
annual meeting in compliance with Rule 14a-8 (or any successor thereof) of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”), and such stockholder has complied with the requirements of such Rule for inclusion of such proposal in a proxy statement
prepared by the Corporation to solicit proxies for such annual meeting. No business shall be conducted at the annual meeting of stockholders
except business brought before the annual meeting in accordance with the procedures set forth in this Section 2.7(a), provided,
however, that once business has been properly brought before the annual meeting in accordance with such procedures, nothing in this Section
2.7(a) shall be deemed to preclude discussion by any stockholder of any such business. If the Board or the chairman of the annual
meeting determines that any stockholder proposal was not made in accordance with the provisions of this Section 2.7(a) or that
the information provided in a stockholder’s notice does not satisfy the information requirements of this Section 2.7(a),
such proposal shall not be presented for action at the annual meeting. Notwithstanding the foregoing provisions of this Section 2.7(a),
if the stockholder (or a qualified representative of the stockholder) does not appear at the annual meeting of stockholders of the Corporation
to present the proposed business, such proposed business shall not be transacted, notwithstanding that proxies in respect of such matter
may have been received by the Corporation.
(iv)
In addition to the provisions of this Section 2.7(a), a stockholder shall also comply with all applicable requirements of the
Exchange Act and the rules and regulations thereunder with respect to the matters set forth herein. Nothing in this Section 2.7(a)
shall be deemed to affect any rights of stockholders to request inclusion of proposals in the Corporation’s proxy statement
pursuant to Rule 14a-8 under the Exchange Act.
(b)
Special Meetings of Stockholders. Only such business shall be conducted at a special meeting of stockholders as shall have been
brought before the meeting pursuant to the Corporation’s notice of meeting. Nominations of persons for election to the Board may
be made at a special meeting of stockholders at which directors are to be elected pursuant to the Corporation’s notice of meeting
only pursuant to Section 3.5.
(c)
Public Announcement. For purposes of these By Laws, “public announcement” shall mean disclosure in a
press release reported by the Dow Jones News Service, Associated Press or comparable national news service or in a document publicly
filed by the Corporation with the Securities and Exchange Commission pursuant to Sections 13, 14 or 15(d) of the Exchange Act (or any
successor thereto).
Section
2.8. Conduct of Meetings. The chairman of each annual and special meeting of stockholders shall be the Chairman of the Board or,
in the absence (or inability or refusal to act) of the Chairman of the Board, any Chief Executive Officer (if he or she shall be a director)
or, in the absence (or inability or refusal to act of a Chief Executive Officer or if a Chief Executive Officer is not a director, the
President (if he or she shall be a director) or, in the absence (or inability or refusal to act) of the President or if the President
is not a director, such other person as shall be appointed by the Board. The date and time of the opening and the closing of the polls
for each matter upon which the stockholders will vote at a meeting shall be announced at the meeting by the chairman of the meeting.
The Board may adopt such rules and regulations for the conduct of the meeting of stockholders as it shall deem appropriate. Except to
the extent inconsistent with these By Laws or such rules and regulations as adopted by the Board, the chairman of any meeting of stockholders
shall have the right and authority to convene and to adjourn the meeting, to prescribe such rules, regulations and procedures and to
do all such acts as, in the judgment of such chairman, are appropriate for the proper conduct of the meeting. Such rules, regulations
or procedures, whether adopted by the Board or prescribed by the chairman of the meeting, may include, without limitation, the following:
(a) the establishment of an agenda or order of business for the meeting; (b) rules and procedures for maintaining order at the meeting
and the safety of those present; (c) limitations on attendance at or participation in the meeting to stockholders of record of the Corporation,
their duly authorized and constituted proxies or such other persons as the chairman of the meeting shall determine; (d) restrictions
on entry to the meeting after the time fixed for the commencement thereof; and (e) limitations on the time allotted to questions or comments
by participants. Unless and to the extent determined by the Board or the chairman of the meeting, meetings of stockholders shall not
be required to be held in accordance with the rules of parliamentary procedure. The secretary of each annual and special meeting of stockholders
shall be the Secretary or, in the absence (or inability or refusal to act) of the Secretary, an Assistant Secretary so appointed to act
by the chairman of the meeting. In the absence (or inability or refusal to act) of the Secretary and all Assistant Secretaries, the chairman
of the meeting may appoint any person to act as secretary of the meeting.
Section
2.9. Consents in Lieu of Meeting. Unless otherwise provided by the Certificate of Incorporation, until the Corporation consummates
an initial public offering (“Offering”), any action required to be taken at any annual or special meeting of
stockholders, or any action which may be taken at any annual or special meeting of such stockholders, may be taken without a meeting,
without prior notice and without a vote, if a consent in writing, setting forth the action so taken, shall be signed by the holders of
outstanding stock entitled to vote thereon having not less than the minimum number of votes that would be necessary to authorize or take
such action at a meeting at which all shares entitled to vote thereon were present and voted, and shall be delivered to the Corporation
by delivery to its registered office in the State of Delaware, its principal place of business, or an officer or agent of the Corporation
having custody of the book in which proceedings of meetings of stockholders are recorded. Delivery made to the Corporation’s registered
office shall be by hand or by certified or registered mail, return receipt requested.
Every
written consent shall bear the date of signature of each stockholder who signs the consent, and no written consent shall be effective
to take the corporate action referred to therein unless, within 60 days of the earliest dated consent delivered in the manner required
by this section and the DGCL to the Corporation, written consents signed by a sufficient number of holders entitled to vote to take action
are delivered to the Corporation by delivery to its registered office in Delaware, its principal place of business or an officer or agent
of the Corporation having custody of the book in which proceedings of meetings of stockholders are recorded. Delivery made to the Corporation’s
registered office shall be by hand or by certified or registered mail, return receipt requested.
ARTICLE
III
DIRECTORS
Section
3.1. Powers; Number. The business and affairs of the Corporation shall be managed by or under the direction of the Board, which may
exercise all such powers of the Corporation and do all such lawful acts and things as are not by statute or by the Certificate of Incorporation
or by these By Laws required to be exercised or done by the stockholders. Directors need not be stockholders or residents of the State
of Delaware. Subject to the Certificate of Incorporation, the number of directors shall be fixed exclusively by resolution of the Board.
Each director shall hold office until a successor is duly elected and qualified or until the director’s earlier death, resignation,
disqualification, or removal.
Section
3.2. Newly Created Directorships and Vacancies. Any newly created directorships resulting from an increase in the authorized number
of directors and any vacancies occurring in the Board of Directors, may be filled by the affirmative votes of a majority of the remaining
members of the Board of Directors, although less than a quorum, or by a sole remaining director. A director so elected shall be elected
to hold office until the earlier of the expiration of the term of office of the director whom he or she has replaced, a successor is
duly elected and qualified, or the earlier of such director’s death, resignation, or removal.
Section
3.3. Resignations. Any director may resign at any time by notice given in writing or by electronic transmission to the Corporation.
Such resignation shall take effect at the date of receipt of such notice by the Corporation or at such later effective date or upon the
happening of an event or events as is therein specified.
Section
3.4. Removals. Except as prohibited by applicable law or the Certificate of Incorporation, the stockholders holding a majority of
the shares then entitled to vote at an election of directors may remove any director from office with or without cause.
Section
3.5. Advance Notice for Nomination of Directors.
(a)
Only persons who are nominated in accordance with the following procedures shall be eligible for election as directors of the Corporation,
except as may be otherwise provided by the terms of one or more series of Preferred Stock with respect to the rights of holders of one
or more series of Preferred Stock to elect directors. Nominations of persons for election to the Board at any annual meeting of stockholders,
or at any special meeting of stockholders called for the purpose of electing directors as set forth in the Corporation’s notice
of such special meeting, may be made (i) by or at the direction of the Board or (ii) by any stockholder of the Corporation (x) who is
a stockholder of record entitled to vote in the election of directors on the date of the giving of the notice provided for in this Section
3.5 and on the record date for the determination of stockholders entitled to vote at such meeting and (y) who complies with the notice
procedures set forth in this Section 3.5.
(b)
In addition to any other applicable requirements, for a nomination to be made by a stockholder, such stockholder must have given timely
notice thereof in proper written form to the Secretary. To be timely, a stockholder’s notice to the Secretary must be received
by the Secretary at the principal executive offices of the Corporation (i) in the case of an annual meeting, not later than the close
of business on the 90th day nor earlier than the opening of business on the 120th day before the anniversary date of the immediately
preceding annual meeting of stockholders; provided, however, that in the event that the annual meeting is more than 30 days before or
more than 60 days after such anniversary date, notice by the stockholder to be timely must be so received not earlier than the opening
of business on the 120th day before the meeting and not later than the later of (x) the close of business on the 90th day before the
meeting or (y) the close of business on the 10th day following the day on which public announcement of the date of the annual meeting
was first made by the Corporation; and (ii) in the case of a special meeting of stockholders called for the purpose of electing directors,
not later than the close of business on the 10th day following the day on which public announcement of the date of the special meeting
is first made by the Corporation. In no event shall the public announcement of an adjournment or postponement of an annual meeting or
special meeting commence a new time period (or extend any time period) for the giving of a stockholder’s notice as described in
this Section 3.5.
(c)
Notwithstanding anything in paragraph (b) to the contrary, in the event that the number of directors to be elected to the Board at an
annual meeting is greater than the number of directors whose terms expire on the date of the annual meeting and there is no public announcement
by the Corporation naming all of the nominees for the additional directors to be elected or specifying the size of the increased Board
before the close of business on the 90th day prior to the anniversary date of the immediately preceding annual meeting of stockholders,
a stockholder’s notice required by this Section 3.5 shall also be considered timely, but only with respect to nominees for
the additional directorships created by such increase that are to be filled by election at such annual meeting, if it shall be received
by the Secretary at the principal executive offices of the Corporation not later than the close of business on the 10th day following
the date on which such public announcement was first made by the Corporation.
(d)
To be in proper written form, a stockholder’s notice to the Secretary must set forth (i) as to each person whom the stockholder
proposes to nominate for election as a director (A) the name, age, business address and residence address of the person, (B) the principal
occupation or employment of the person, (C) the class or series and number of shares of capital stock of the Corporation that are owned
beneficially or of record by the person and (D) any other information relating to the person that would be required to be disclosed in
a proxy statement or other filings required to be made in connection with solicitations of proxies for election of directors pursuant
to Section 14 of the Exchange Act and the rules and regulations promulgated thereunder; and (ii) as to the stockholder giving the notice
(A) the name and record address of such stockholder as they appear on the Corporation’s books and the name and address of the beneficial
owner, if any, on whose behalf the nomination is made, (B) the class or series and number of shares of capital stock of the Corporation
that are owned beneficially and of record by such stockholder and the beneficial owner, if any, on whose behalf the nomination is made,
(C) a description of all arrangements or understandings relating to the nomination to be made by such stockholder among such stockholder,
the beneficial owner, if any, on whose behalf the nomination is made, each proposed nominee and any other person or persons (including
their names), (D) a representation that such stockholder (or a qualified representative of such stockholder) intends to appear in person
or by proxy at the meeting to nominate the persons named in its notice and (E) any other information relating to such stockholder and
the beneficial owner, if any, on whose behalf the nomination is made that would be required to be disclosed in a proxy statement or other
filings required to be made in connection with solicitations of proxies for election of directors pursuant to Section 14 of the Exchange
Act and the rules and regulations promulgated thereunder. Such notice must be accompanied by a written consent of each proposed nominee
to being named as a nominee and to serve as a director if elected.
(e)
If the Board or the chairman of the meeting of stockholders determines that any nomination was not made in accordance with the provisions
of this Section 3.5, or that the information provided in a stockholder’s notice does not satisfy the information requirements
of this Section 3.5, then such nomination shall not be considered at the meeting in question. Notwithstanding the foregoing provisions
of this Section 3.5, if the stockholder (or a qualified representative of the stockholder) does not appear at the meeting of stockholders
of the Corporation to present the nomination, such nomination shall be disregarded, notwithstanding that proxies in respect of such nomination
may have been received by the Corporation.
(f)
In addition to the provisions of this Section 3.5, a stockholder shall also comply with all of the applicable requirements of
the Exchange Act and the rules and regulations thereunder with respect to the matters set forth herein. Nothing in this Section 3.5
shall be deemed to affect any rights of the holders of Preferred Stock to elect directors pursuant to the Certificate of Incorporation.
Section
3.6. Compensation. Unless otherwise restricted by the Certificate of Incorporation or these By Laws, the Board shall have the authority
to fix the compensation of directors, including for service on a committee of the Board, and may be paid either a fixed sum for attendance
at each meeting of the Board or other compensation as director. The directors may be reimbursed their expenses, if any, of attendance
at each meeting of the Board. No such payment shall preclude any director from serving the Corporation in any other capacity and receiving
compensation therefor. Members of committees of the Board may be allowed like compensation and reimbursement of expenses for service
on the committee.
ARTICLE
IV
BOARD
MEETINGS
Section
4.1. Annual Meetings. The Board shall meet as soon as practicable after the adjournment of each annual stockholders meeting at the
place of the annual stockholders meeting unless the Board shall fix another time and place and give notice thereof in the manner required
herein for special meetings of the Board. No notice to the directors shall be necessary to legally convene this meeting, except as provided
in this Section 4.1.
Section
4.2. Regular Meetings. Regularly scheduled, periodic meetings of the Board may be held without notice at such times, dates and places
(within or without the State of Delaware) as shall from time to time be determined by the Board.
Section
4.3. Special Meetings. Special meetings of the Board (a) may be called by the Chairman of the Board or President and (b) shall be
called by the Chairman of the Board, President or Secretary on the written request of at least a majority of directors then in office,
or the sole director, as the case may be, and shall be held at such time, date and place (within or without the State of Delaware) as
may be determined by the person calling the meeting or, if called upon the request of directors or the sole director, as specified in
such written request. Notice of each special meeting of the Board shall be given, as provided in Section 9.3, to each director
(i) at least 24 hours before the meeting if such notice is oral notice given personally or by telephone or written notice given by hand
delivery or by means of a form of electronic transmission and delivery; (ii) at least two days before the meeting if such notice is sent
by a nationally recognized overnight delivery service; and (iii) at least five days before the meeting if such notice is sent through
the United States mail. If the Secretary shall fail or refuse to give such notice, then the notice may be given by the officer who called
the meeting or the directors who requested the meeting. Any and all business that may be transacted at a regular meeting of the Board
may be transacted at a special meeting. Except as may be otherwise expressly provided by applicable law, the Certificate of Incorporation,
or these By Laws, neither the business to be transacted at, nor the purpose of, any special meeting need be specified in the notice or
waiver of notice of such meeting. A special meeting may be held at any time without notice if all the directors are present or if those
not present waive notice of the meeting in accordance with Section 9.4.
Section
4.4. Quorum; Required Vote. A majority of the Board shall constitute a quorum for the transaction of business at any meeting of the
Board, and the act of a majority of the directors present at any meeting at which there is a quorum shall be the act of the Board, except
as may be otherwise specifically provided by applicable law, the Certificate of Incorporation or these By Laws. If a quorum shall not
be present at any meeting, a majority of the directors present may adjourn the meeting from time to time, without notice other than announcement
at the meeting, until a quorum is present.
Section
4.5. Consent In Lieu of Meeting. Unless otherwise restricted by the Certificate of Incorporation or these By Laws, any action required
or permitted to be taken at any meeting of the Board or any committee thereof may be taken without a meeting if all members of the Board
or committee, as the case may be, consent thereto in writing or by electronic transmission, and the writing or writings or electronic
transmission or transmissions (or paper reproductions thereof) are filed with the minutes of proceedings of the Board or committee. Such
filing shall be in paper form if the minutes are maintained in paper form and shall be in electronic form if the minutes are maintained
in electronic form.
Section
4.6. Organization. The chairman of each meeting of the Board shall be the Chairman of the Board or, in the absence (or inability
or refusal to act) of the Chairman of the Board, any Chief Executive Officer (if he or she shall be a director) or, in the absence (or
inability or refusal to act) of a Chief Executive Officer or if a Chief Executive Officer is not a director, the President (if he or
she shall be a director) or in the absence (or inability or refusal to act) of the President or if the President is not a director, a
chairman elected from the directors present. The Secretary shall act as secretary of all meetings of the Board. In the absence (or inability
or refusal to act) of the Secretary, an Assistant Secretary shall perform the duties of the Secretary at such meeting. In the absence
(or inability or refusal to act) of the Secretary and all Assistant Secretaries, the chairman of the meeting may appoint any person to
act as secretary of the meeting.
ARTICLE
V
COMMITTEES
OF DIRECTORS
Section
5.1. Establishment. The Board may by resolution of the Board designate one or more committees, each committee to consist of one or
more of the directors of the Corporation. Each committee shall keep regular minutes of its meetings and report the same to the Board
when required by the resolution designating such committee. The Board shall have the power at any time to fill vacancies in, to change
the membership of, or to dissolve any such committee.
Section
5.2. Available Powers. Any committee established pursuant to Section 5.1 hereof, to the extent permitted by applicable law
and by resolution of the Board, shall have and may exercise all of the powers and authority of the Board in the management of the business
and affairs of the Corporation, and may authorize the seal of the Corporation to be affixed to all papers that may require it.
Section
5.3. Alternate Members. The Board may designate one or more directors as alternate members of any committee, who may replace any
absent or disqualified member at any meeting of such committee. In the absence or disqualification of a member of the committee, the
member or members thereof present at any meeting and not disqualified from voting, whether or not he, she or they constitute a quorum,
may unanimously appoint another member of the Board to act at the meeting in place of any such absent or disqualified member.
Section
5.4. Procedures. Unless the Board otherwise provides, the time, date, place, if any, and notice of meetings of a committee shall
be determined by such committee. At meetings of a committee, a majority of the number of members of the committee (but not including
any alternate member, unless such alternate member has replaced any absent or disqualified member at the time of, or in connection with,
such meeting) shall constitute a quorum for the transaction of business. The act of a majority of the members present at any meeting
at which a quorum is present shall be the act of the committee, except as otherwise specifically provided by applicable law, the Certificate
of Incorporation, these By Laws or the Board. If a quorum is not present at a meeting of a committee, the members present may adjourn
the meeting from time to time, without notice other than an announcement at the meeting, until a quorum is present. Unless the Board
otherwise provides and except as provided in these By Laws, each committee designated by the Board may make, alter, amend and repeal
rules for the conduct of its business. In the absence of such rules each committee shall conduct its business in the same manner as the
Board is authorized to conduct its business pursuant to Article III and Article IV of these By Laws.
ARTICLE
VI
OFFICERS
Section
6.1. Officers. The officers of the Corporation elected by the Board shall be one or more Chief Executive Officers, a Chief Financial
Officer, a Secretary and such other officers (including without limitation, a Chairman of the Board, Presidents, Vice Presidents, Assistant
Secretaries and a Treasurer) as the Board from time to time may determine. Officers elected by the Board shall each have such powers
and duties as generally pertain to their respective offices, subject to the specific provisions of this Article VI. Such officers
shall also have such powers and duties as from time to time may be conferred by the Board. Any Chief Executive Officer or President may
also appoint such other officers (including without limitation one or more Vice Presidents and Controllers) as may be necessary or desirable
for the conduct of the business of the Corporation. Such other officers shall have such powers and duties and shall hold their offices
for such terms as may be provided in these By Laws or as may be prescribed by the Board or, if such officer has been appointed by any
Chief Executive Officer or President, as may be prescribed by the appointing officer.
(a)
Chairman of the Board. The Chairman of the Board shall preside when present at all meetings of the stockholders and the Board.
The Chairman of the Board shall have general supervision and control of the acquisition activities of the Corporation subject to the
ultimate authority of the Board, and shall be responsible for the execution of the policies of the Board with respect to such matters.
In the absence (or inability or refusal to act) of the Chairman of the Board, any Chief Executive Officer (if he or she shall be a director)
shall preside when present at all meetings of the stockholders and the Board. The powers and duties of the Chairman of the Board shall
not include supervision or control of the preparation of the financial statements of the Corporation (other than through participation
as a member of the Board). The position of Chairman of the Board and Chief Executive Officer may be held by the same person and may be
held by more than one person.
(b)
Chief Executive Officer. One or more Chief Executive Officers shall be the chief executive officer(s) of the Corporation, shall
have general supervision of the affairs of the Corporation and general control of all of its business subject to the ultimate authority
of the Board, and shall be responsible for the execution of the policies of the Board with respect to such matters, except to the extent
any such powers and duties have been prescribed to the Chairman of the Board pursuant to Section 6.1(a) above. In the absence
(or inability or refusal to act) of the Chairman of the Board, any Chief Executive Officer (if he or she shall be a director) shall preside
when present at all meetings of the stockholders and the Board. The position of Chief Executive Officer and President may be held by
the same person and may be held by more than one person.
(c)
President. The President shall make recommendations to any Chief Executive Officer on all operational matters that would normally
be reserved for the final executive responsibility of any Chief Executive Officer. In the absence (or inability or refusal to act) of
the Chairman of the Board and a Chief Executive Officer, the President (if he or she shall be a director) shall preside when present
at all meetings of the stockholders and the Board. The President shall also perform such duties and have such powers as shall be designated
by the Board. The position of President and Chief Executive Officer may be held by the same person.
(d)
Vice Presidents. In the absence (or inability or refusal to act) of the President, the Vice President (or in the event there be
more than one Vice President, the Vice Presidents in the order designated by the Board) shall perform the duties and have the powers
of the President. Any one or more of the Vice Presidents may be given an additional designation of rank or function.
(e)
Secretary.
(i)
The Secretary shall attend all meetings of the stockholders, the Board and (as required) committees of the Board and shall record the
proceedings of such meetings in books to be kept for that purpose. The Secretary shall give, or cause to be given, notice of all meetings
of the stockholders and special meetings of the Board and shall perform such other duties as may be prescribed by the Board, the Chairman
of the Board, any Chief Executive Officer or President. The Secretary shall have custody of the corporate seal of the Corporation and
the Secretary, or any Assistant Secretary, shall have authority to affix the same to any instrument requiring it, and when so affixed,
it may be attested by his or her signature or by the signature of such Assistant Secretary. The Board may give general authority to any
other officer to affix the seal of the Corporation and to attest the affixing thereof by his or her signature.
(ii)
The Secretary shall keep, or cause to be kept, at the principal executive office of the Corporation or at the office of the Corporation’s
transfer agent or registrar, if one has been appointed, a stock ledger, or duplicate stock ledger, showing the names of the stockholders
and their addresses, the number and classes of shares held by each and, with respect to certificated shares, the number and date of certificates
issued for the same and the number and date of certificates cancelled.
(f)
Assistant Secretaries. The Assistant Secretary or, if there be more than one, the Assistant Secretaries in the order determined
by the Board shall, in the absence (or inability or refusal to act) of the Secretary, perform the duties and have the powers of the Secretary.
(g)
Chief Financial Officer. The Chief Financial Officer shall perform all duties commonly incident to that office (including, without
limitation, the care and custody of the funds and securities of the Corporation, which from time to time may come into the Chief Financial
Officer’s hands and the deposit of the funds of the Corporation in such banks or trust companies as the Board, any Chief Executive
Officer or the President may authorize).
(h)
Treasurer. The Treasurer shall, in the absence (or inability or refusal to act) of the Chief Financial Officer, perform the duties
and exercise the powers of the Chief Financial Officer.
Section
6.2. Term of Office; Removal; Vacancies. The elected officers of the Corporation shall be appointed by the Board and shall hold office
until their successors are duly elected and qualified by the Board or until their earlier death, resignation, retirement, disqualification,
or removal from office. Any officer may be removed, with or without cause, at any time by the Board. Any officer appointed by any Chief
Executive Officer or President may also be removed, with or without cause, by any Chief Executive Officer or President, as the case may
be, unless the Board otherwise provides. Any vacancy occurring in any elected office of the Corporation may be filled by the Board. Any
vacancy occurring in any office appointed by any Chief Executive Officer or President may be filled by any Chief Executive Officer, or
President, as the case may be, unless the Board then determines that such office shall thereupon be elected by the Board, in which case
the Board shall elect such officer.
Section
6.3. Other Officers. The Board may delegate the power to appoint such other officers and agents, and may also remove such officers
and agents or delegate the power to remove same, as it shall from time to time deem necessary or desirable.
Section
6.4. Multiple Officeholders; Stockholder and Director Officers. Any number of offices may be held by the same person unless the Certificate
of Incorporation or these By Laws otherwise provide. Officers need not be stockholders or residents of the State of Delaware.
ARTICLE
VII
SHARES
Section
7.1. Certificated and Uncertificated Shares. The shares of the Corporation may be certificated or uncertificated, subject to the
sole discretion of the Board and the requirements of the DGCL.
Section
7.2. Multiple Classes of Stock. If the Corporation shall be authorized to issue more than one class of stock or more than one series
of any class, the Corporation shall (a) cause the powers, designations, preferences and relative, participating, optional or other special
rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights
to be set forth in full or summarized on the face or back of any certificate that the Corporation issues to represent shares of such
class or series of stock or (b) in the case of uncertificated shares, within a reasonable time after the issuance or transfer of such
shares, send to the registered owner thereof a written notice containing the information required to be set forth on certificates as
specified in clause (a) above; provided, however, that, except as otherwise provided by applicable law, in lieu of the foregoing requirements,
there may be set forth on the face or back of such certificate or, in the case of uncertificated shares, on such written notice a statement
that the Corporation will furnish without charge to each stockholder who so requests the powers, designations, preferences and relative,
participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions
of such preferences or rights.
Section
7.3. Signatures. Each certificate representing capital stock of the Corporation shall be signed by or in the name of the Corporation
by (a) the Chairman of the Board, any Chief Executive Officer, the President or a Vice President and (b) the Treasurer, an Assistant
Treasurer, the Secretary or an Assistant Secretary of the Corporation. Any or all the signatures on the certificate may be a facsimile.
In case any officer, transfer agent or registrar who has signed or whose facsimile signature has been placed upon a certificate shall
have ceased to be such officer, transfer agent or registrar before such certificate is issued, such certificate may be issued by the
Corporation with the same effect as if such person were such officer, transfer agent or registrar on the date of issue.
Section
7.4. Consideration and Payment for Shares.
(a)
Subject to applicable law and the Certificate of Incorporation, shares of stock may be issued for such consideration, having in the case
of shares with par value a value not less than the par value thereof, and to such persons, as determined from time to time by the Board.
The consideration may consist of any tangible or intangible property or any benefit to the Corporation including cash, promissory notes,
services performed, contracts for services to be performed or other securities, or any combination thereof.
(b)
Subject to applicable law and the Certificate of Incorporation, shares may not be issued until the full amount of the consideration has
been paid, unless upon the face or back of each certificate issued to represent any partly paid shares of capital stock or upon the books
and records of the Corporation in the case of partly paid uncertificated shares, there shall have been set forth the total amount of
the consideration to be paid therefor and the amount paid thereon up to and including the time said certificate representing certificated
shares or said uncertificated shares are issued.
Section
7.5. Lost, Destroyed or Wrongfully Taken Certificates.
(a)
If an owner of a certificate representing shares claims that such certificate has been lost, destroyed or wrongfully taken, the Corporation
shall issue a new certificate representing such shares or such shares in uncertificated form if the owner: (i) requests such a new certificate
before the Corporation has notice that the certificate representing such shares has been acquired by a protected purchaser; (ii) if requested
by the Corporation, delivers to the Corporation a bond sufficient to indemnify the Corporation against any claim that may be made against
the Corporation on account of the alleged loss, wrongful taking or destruction of such certificate or the issuance of such new certificate
or uncertificated shares; and (iii) satisfies other reasonable requirements imposed by the Corporation.
(b)
If a certificate representing shares has been lost, apparently destroyed or wrongfully taken, and the owner fails to notify the Corporation
of that fact within a reasonable time after the owner has notice of such loss, apparent destruction or wrongful taking and the Corporation
registers a transfer of such shares before receiving notification, the owner shall be precluded from asserting against the Corporation
any claim for registering such transfer or a claim to a new certificate representing such shares or such shares in uncertificated form.
Section
7.6. Transfer of Stock.
(a)
If a certificate representing shares of the Corporation is presented to the Corporation with an endorsement requesting the registration
of transfer of such shares or an instruction is presented to the Corporation requesting the registration of transfer of uncertificated
shares, the Corporation shall register the transfer as requested if:
(i)
in the case of certificated shares, the certificate representing such shares has been surrendered;
(ii)
(A) with respect to certificated shares, the endorsement is made by the person specified by the certificate as entitled to such shares;
(B) with respect to uncertificated shares, an instruction is made by the registered owner of such uncertificated shares; or (C) with
respect to certificated shares or uncertificated shares, the endorsement or instruction is made by any other appropriate person or by
an agent who has actual authority to act on behalf of the appropriate person;
(iii)
the Corporation has received a guarantee of signature of the person signing such endorsement or instruction or such other reasonable
assurance that the endorsement or instruction is genuine and authorized as the Corporation may request;
(iv)
the transfer does not violate any restriction on transfer imposed by the Corporation that is enforceable in accordance with Section
7.8(a); and
(v)
such other conditions for such transfer as shall be provided for under applicable law have been satisfied.
(b)
Whenever any transfer of shares shall be made for collateral security and not absolutely, the Corporation shall so record such fact in
the entry of transfer if, when the certificate for such shares is presented to the Corporation for transfer or, if such shares are uncertificated,
when the instruction for registration of transfer thereof is presented to the Corporation, both the transferor and transferee request
the Corporation to do so.
Section
7.7. Registered Stockholders. Before due presentment for registration of transfer of a certificate representing shares of the Corporation
or of an instruction requesting registration of transfer of uncertificated shares, the Corporation may treat the registered owner as
the person exclusively entitled to inspect for any proper purpose the stock ledger and the other books and records of the Corporation,
vote such shares, receive dividends or notifications with respect to such shares and otherwise exercise all the rights and powers of
the owner of such shares, except that a person who is the beneficial owner of such shares (if held in a voting trust or by a nominee
on behalf of such person) may, upon providing documentary evidence of beneficial ownership of such shares and satisfying such other conditions
as are provided under applicable law, may also so inspect the books and records of the Corporation.
Section
7.8. Effect of the Corporation’s Restriction on Transfer.
(a)
A written restriction on the transfer or registration of transfer of shares of the Corporation or on the amount of shares of the Corporation
that may be owned by any person or group of persons, if permitted by the DGCL and noted conspicuously on the certificate representing
such shares or, in the case of uncertificated shares, contained in a notice, offering circular or prospectus sent by the Corporation
to the registered owner of such shares within a reasonable time prior to or after the issuance or transfer of such shares, may be enforced
against the holder of such shares or any successor or transferee of the holder including an executor, administrator, trustee, guardian
or other fiduciary entrusted with like responsibility for the person or estate of the holder.
(b)
A restriction imposed by the Corporation on the transfer or the registration of shares of the Corporation or on the amount of shares
of the Corporation that may be owned by any person or group of persons, even if otherwise lawful, is ineffective against a person without
actual knowledge of such restriction unless: (i) the shares are certificated and such restriction is noted conspicuously on the certificate;
or (ii) the shares are uncertificated and such restriction was contained in a notice, offering circular or prospectus sent by the Corporation
to the registered owner of such shares within a reasonable time prior to or after the issuance or transfer of such shares.
Section
7.9. Regulations. The Board shall have power and authority to make such additional rules and regulations, subject to any applicable
requirement of law, as the Board may deem necessary and appropriate with respect to the issue, transfer or registration of transfer of
shares of stock or certificates representing shares. The Board may appoint one or more transfer agents or registrars and may require
for the validity thereof that certificates representing shares bear the signature of any transfer agent or registrar so appointed.
ARTICLE
VIII
INDEMNIFICATION
Section
8.1. Right to Indemnification. To the fullest extent permitted by applicable law, as the same exists or may hereafter be amended,
the Corporation shall indemnify and hold harmless each person who was or is made a party or is threatened to be made a party to or is
otherwise involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative
(hereinafter a “proceeding”), by reason of the fact that he or she is or was a director or officer of the Corporation
or, while a director or officer of the Corporation, is or was serving at the request of the Corporation as a director, officer, employee
or agent of another corporation or of a partnership, joint venture, trust, other enterprise or nonprofit entity, including service with
respect to an employee benefit plan (hereinafter an “Indemnitee”), whether the basis of such proceeding is
alleged action in an official capacity as a director, officer, employee or agent, or in any other capacity while serving as a director,
officer, employee or agent, against all liability and loss suffered and expenses (including, without limitation, attorneys’ fees,
judgments, fines, ERISA excise taxes and penalties and amounts paid in settlement) reasonably incurred by such Indemnitee in connection
with such proceeding; provided, however, that, except as provided in Section 8.3 with respect to proceedings to enforce rights
to indemnification, the Corporation shall indemnify an Indemnitee in connection with a proceeding (or part thereof) initiated by such
Indemnitee only if such proceeding (or part thereof) was authorized by the Board.
Section
8.2. Right to Advancement of Expenses. In addition to the right to indemnification conferred in Section 8.1, an Indemnitee
shall also have the right to be paid by the Corporation to the fullest extent not prohibited by applicable law the expenses (including,
without limitation, attorneys’ fees) incurred in defending or otherwise participating in any such proceeding in advance of its
final disposition (hereinafter an “advancement of expenses”); provided, however, that, if the DGCL requires,
an advancement of expenses incurred by an Indemnitee in his or her capacity as a director or officer of the Corporation (and not in any
other capacity in which service was or is rendered by such Indemnitee, including, without limitation, service to an employee benefit
plan) shall be made only upon the Corporation’s receipt of an undertaking (hereinafter an “undertaking”),
by or on behalf of such Indemnitee, to repay all amounts so advanced if it shall ultimately be determined that such Indemnitee is not
entitled to be indemnified under this Article VIII or otherwise.
Section
8.3. Right of Indemnitee to Bring Suit. If a claim under Section 8.1 or Section 8.2 is not paid in full by the Corporation
within 60 days after a written claim therefor has been received by the Corporation, except in the case of a claim for an advancement
of expenses, in which case the applicable period shall be 20 days, the Indemnitee may at any time thereafter bring suit against the Corporation
to recover the unpaid amount of the claim. If successful in whole or in part in any such suit, or in a suit brought by the Corporation
to recover an advancement of expenses pursuant to the terms of an undertaking, the Indemnitee shall also be entitled to be paid the expense
of prosecuting or defending such suit. In (a) any suit brought by the Indemnitee to enforce a right to indemnification hereunder (but
not in a suit brought by an Indemnitee to enforce a right to an advancement of expenses) it shall be a defense that, and (b) in any suit
brought by the Corporation to recover an advancement of expenses pursuant to the terms of an undertaking, the Corporation shall be entitled
to recover such expenses upon a final judicial decision from which there is no further right to appeal (hereinafter a “final
adjudication”) that, the Indemnitee has not met any applicable standard for indemnification set forth in the DGCL. Neither
the failure of the Corporation (including its directors who are not parties to such action, a committee of such directors, independent
legal counsel, or its stockholders) to have made a determination prior to the commencement of such suit that indemnification of the Indemnitee
is proper in the circumstances because the Indemnitee has met the applicable standard of conduct set forth in the DGCL, nor an actual
determination by the Corporation (including a determination by its directors who are not parties to such action, a committee of such
directors, independent legal counsel, or its stockholders) that the Indemnitee has not met such applicable standard of conduct, shall
create a presumption that the Indemnitee has not met the applicable standard of conduct or, in the case of such a suit brought by the
Indemnitee, shall be a defense to such suit. In any suit brought by the Indemnitee to enforce a right to indemnification or to an advancement
of expenses hereunder, or by the Corporation to recover an advancement of expenses pursuant to the terms of an undertaking, the burden
of proving that the Indemnitee is not entitled to be indemnified, or to such advancement of expenses, under this Article VIII
or otherwise shall be on the Corporation.
Section
8.4. Non-Exclusivity of Rights. The rights provided to any Indemnitee pursuant to this Article VIII shall not be exclusive
of any other right, which such Indemnitee may have or hereafter acquire under applicable law, the Certificate of Incorporation, these
By Laws, an agreement, a vote of stockholders or disinterested directors, or otherwise.
Section
8.5. Insurance. The Corporation may maintain insurance, at its expense, to protect itself and/or any director, officer, employee
or agent of the Corporation or another corporation, partnership, joint venture, trust or other enterprise against any expense, liability
or loss, whether or not the Corporation would have the power to indemnify such person against such expense, liability or loss under the
DGCL.
Section
8.6. Indemnification of Other Persons. This Article VIII shall not limit the right of the Corporation to the extent and in
the manner authorized or permitted by law to indemnify and to advance expenses to persons other than Indemnitees. Without limiting the
foregoing, the Corporation may, to the extent authorized from time to time by the Board, grant rights to indemnification and to the advancement
of expenses to any employee or agent of the Corporation and to any other person who is or was serving at the request of the Corporation
as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust or other enterprise, including
service with respect to an employee benefit plan, to the fullest extent of the provisions of this Article VIII with respect to
the indemnification and advancement of expenses of Indemnitees under this Article VIII.
Section
8.7. Amendments. Any repeal or amendment of this Article VIII by the Board or the stockholders of the Corporation or by changes
in applicable law, or the adoption of any other provision of these By Laws inconsistent with this Article VIII, will, to the extent
permitted by applicable law, be prospective only (except to the extent such amendment or change in applicable law permits the Corporation
to provide broader indemnification rights to Indemnitees on a retroactive basis than permitted prior thereto), and will not in any way
diminish or adversely affect any right or protection existing hereunder in respect of any act or omission occurring prior to such repeal
or amendment or adoption of such inconsistent provision; provided however, that amendments or repeals of this Article VIII shall
require the affirmative vote of the stockholders holding at least 66.7% of the voting power of all outstanding shares of capital stock
of the Corporation.
Section
8.8. Certain Definitions. For purposes of this Article VIII, (a) references to “other enterprise”
shall include any employee benefit plan; (b) references to “fines” shall include any excise taxes assessed
on a person with respect to an employee benefit plan; (c) references to “serving at the request of the Corporation”
shall include any service that imposes duties on, or involves services by, a person with respect to any employee benefit plan, its participants,
or beneficiaries; and (d) a person who acted in good faith and in a manner such person reasonably believed to be in the interest of the
participants and beneficiaries of an employee benefit plan shall be deemed to have acted in a manner “not opposed to the best interest
of the Corporation” for purposes of Section 145 of the DGCL.
Section
8.9. Contract Rights. The rights provided to Indemnitees pursuant to this Article VIII shall be contract rights and such rights
shall continue as to an Indemnitee who has ceased to be a director, officer, agent or employee and shall inure to the benefit of the
Indemnitee’s heirs, executors and administrators.
Section
8.10. Severability. If any provision or provisions of this Article VIII shall be held to be invalid, illegal or unenforceable
for any reason whatsoever: (a) the validity, legality and enforceability of the remaining provisions of this Article VIII shall
not in any way be affected or impaired thereby; and (b) to the fullest extent possible, the provisions of this Article VIII (including,
without limitation, each such portion of this Article VIII containing any such provision held to be invalid, illegal or unenforceable)
shall be construed so as to give effect to the intent manifested by the provision held invalid, illegal or unenforceable.
ARTICLE
IX
MISCELLANEOUS
Section
9.1. Place of Meetings. If the place of any meeting of stockholders, the Board or committee of the Board for which notice is required
under these By Laws is not designated in the notice of such meeting, such meeting shall be held at the principal business office of the
Corporation; provided, however, if the Board has, in its sole discretion, determined that a meeting shall not be held at any place, but
instead shall be held by means of remote communication pursuant to Section 9.5 hereof, then such meeting shall not be held at
any place.
Section
9.2. Fixing Record Dates.
(a)
In order that the Corporation may determine the stockholders entitled to notice of any meeting of stockholders or any adjournment thereof,
the Board may fix a record date, which shall not precede the date upon which the resolution fixing the record date is adopted by the
Board, and which record date shall not be more than 60 nor less than 10 days before the date of such meeting. If the Board so fixes a
date, such date shall also be the record date for determining the stockholders entitled to vote at such meeting unless the Board determines,
at the time it fixes such record date, that a later date on or before the date of the meeting shall be the date for making such determination.
If no record date is fixed by the Board, the record date for determining stockholders entitled to notice of and to vote at a meeting
of stockholders shall be at the close of business on the business day next preceding the day on which notice is given, or, if notice
is waived, at the close of business on the business day next preceding the day on which the meeting is held. A determination of stockholders
of record entitled to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however,
that the Board may fix a new record date for the adjourned meeting, and in such case shall also fix as the record date for stockholders
entitled to notice of such adjourned meeting the same or an earlier date as that fixed for determination of stockholders entitled to
vote in accordance with the foregoing provisions of this Section 9.2(a) at the adjourned meeting.
(b)
In order that the Corporation may determine the stockholders entitled to receive payment of any dividend or other distribution or allotment
of any rights or the stockholders entitled to exercise any rights in respect of any change, conversion or exchange of stock, or for the
purpose of any other lawful action, the Board may fix a record date, which record date shall not precede the date upon which the resolution
fixing the record date is adopted, and which record date shall be not more than 60 days prior to such action. If no record date is fixed,
the record date for determining stockholders for any such purpose shall be at the close of business on the day on which the Board adopts
the resolution relating thereto.
Section
9.3. Means of Giving Notice.
(a)
Notice to Directors. Whenever under applicable law, the Certificate of Incorporation or these By Laws notice is required to be
given to any director, such notice shall be given either (i) in writing and sent by mail, or by a nationally recognized delivery service,
(ii) by means of facsimile telecommunication or other form of electronic transmission, or (iii) by oral notice given personally or by
telephone. A notice to a director will be deemed given as follows: (i) if given by hand delivery, orally, or by telephone, when actually
received by the director, (ii) if sent through the United States mail, when deposited in the United States mail, with postage and fees
thereon prepaid, addressed to the director at the director’s address appearing on the records of the Corporation, (iii) if sent
for next day delivery by a nationally recognized overnight delivery service, when deposited with such service, with fees thereon prepaid,
addressed to the director at the director’s address appearing on the records of the Corporation, (iv) if sent by facsimile telecommunication,
when sent to the facsimile transmission number for such director appearing on the records of the Corporation, (v) if sent by electronic
mail, when sent to the electronic mail address for such director appearing on the records of the Corporation, or (vi) if sent by any
other form of electronic transmission, when sent to the address, location or number (as applicable) for such director appearing on the
records of the Corporation.
(b)
Notice to Stockholders. Whenever under applicable law, the Certificate of Incorporation or these By Laws notice is required to
be given to any stockholder, such notice may be given (i) in writing and sent either by hand delivery, through the United States mail,
or by a nationally recognized overnight delivery service for next day delivery, or (ii) by means of a form of electronic transmission
consented to by the stockholder, to the extent permitted by, and subject to the conditions set forth in Section 232 of the DGCL. A notice
to a stockholder shall be deemed given as follows: (i) if given by hand delivery, when actually received by the stockholder, (ii) if
sent through the United States mail, when deposited in the United States mail, with postage and fees thereon prepaid, addressed to the
stockholder at the stockholder’s address appearing on the stock ledger of the Corporation, (iii) if sent for next day delivery
by a nationally recognized overnight delivery service, when deposited with such service, with fees thereon prepaid, addressed to the
stockholder at the stockholder’s address appearing on the stock ledger of the Corporation, and (iv) if given by a form of electronic
transmission consented to by the stockholder to whom the notice is given and otherwise meeting the requirements set forth above, (A)
if by facsimile transmission, when directed to a number at which the stockholder has consented to receive notice, (B) if by electronic
mail, when directed to an electronic mail address at which the stockholder has consented to receive notice, (C) if by a posting on an
electronic network together with separate notice to the stockholder of such specified posting, upon the later of (1) such posting and
(2) the giving of such separate notice, and (D) if by any other form of electronic transmission, when directed to the stockholder. A
stockholder may revoke such stockholder’s consent to receiving notice by means of electronic communication by giving written notice
of such revocation to the Corporation. Any such consent shall be deemed revoked if (1) the Corporation is unable to deliver by electronic
transmission two consecutive notices given by the Corporation in accordance with such consent and (2) such inability becomes known to
the Secretary or an Assistant Secretary or to the Corporation’s transfer agent, or other person responsible for the giving of notice;
provided, however, the inadvertent failure to treat such inability as a revocation shall not invalidate any meeting or other action.
(c)
Electronic Transmission. “Electronic transmission” means any form of communication, not directly involving
the physical transmission of paper, that creates a record that may be retained, retrieved and reviewed by a recipient thereof, and that
may be directly reproduced in paper form by such a recipient through an automated process, including but not limited to transmission
by telex, facsimile telecommunication, electronic mail, telegram and cablegram.
(d)
Notice to Stockholders Sharing Same Address. Without limiting the manner by which notice otherwise may be given effectively by
the Corporation to stockholders, any notice to stockholders given by the Corporation under any provision of the DGCL, the Certificate
of Incorporation or these By Laws shall be effective if given by a single written notice to stockholders who share an address if consented
to by the stockholders at that address to whom such notice is given. A stockholder may revoke such stockholder’s consent by delivering
written notice of such revocation to the Corporation. Any stockholder who fails to object in writing to the Corporation within 60 days
of having been given written notice by the Corporation of its intention to send such a single written notice shall be deemed to have
consented to receiving such single written notice.
(e)
Exceptions to Notice Requirements. Whenever notice is required to be given, under the DGCL, the Certificate of Incorporation or
these By Laws, to any person with whom communication is unlawful, the giving of such notice to such person shall not be required and
there shall be no duty to apply to any governmental authority or agency for a license or permit to give such notice to such person. Any
action or meeting that shall be taken or held without notice to any such person with whom communication is unlawful shall have the same
force and effect as if such notice had been duly given. In the event that the action taken by the Corporation is such as to require the
filing of a certificate with the Secretary of State of Delaware, the certificate shall state, if such is the fact and if notice is required,
that notice was given to all persons entitled to receive notice except such persons with whom communication is unlawful. Whenever notice
is required to be given by the Corporation, under any provision of the DGCL, the Certificate of Incorporation or these By Laws, to any
stockholder to whom (1) notice of two consecutive annual meetings of stockholders and all notices of stockholder meetings or of the taking
of action by written consent of stockholders without a meeting to such stockholder during the period between such two consecutive annual
meetings, or (2) all, and at least two payments (if sent by first-class mail) of dividends or interest on securities during a 12-month
period, have been mailed addressed to such stockholder at such stockholder’s address as shown on the records of the Corporation
and have been returned undeliverable, the giving of such notice to such stockholder shall not be required. Any action or meeting that
shall be taken or held without notice to such stockholder shall have the same force and effect as if such notice had been duly given.
If any such stockholder shall deliver to the Corporation a written notice setting forth such stockholder’s then current address,
the requirement that notice be given to such stockholder shall be reinstated. In the event that the action taken by the Corporation is
such as to require the filing of a certificate with the Secretary of State of Delaware, the certificate need not state that notice was
not given to persons to whom notice was not required to be given pursuant to Section 230(b) of the DGCL. The exception in subsection
(1) of the first sentence of this paragraph to the requirement that notice be given shall not be applicable to any notice returned as
undeliverable if the notice was given by electronic transmission.
Section
9.4. Waiver of Notice. Whenever any notice is required to be given under applicable law, the Certificate of Incorporation, or these
By Laws, a written waiver of such notice, signed by the person or persons entitled to said notice, or a waiver by electronic transmission
by the person entitled to said notice, whether before or after the time stated therein, shall be deemed equivalent to such required notice.
All such waivers shall be kept with the books of the Corporation. Attendance at a meeting shall constitute a waiver of notice of such
meeting, except where a person attends for the express purpose of objecting to the transaction of any business on the ground that the
meeting was not lawfully called or convened.
Section
9.5. Meeting Attendance via Remote Communication Equipment.
(a)
Stockholder Meetings. If authorized by the Board in its sole discretion, and subject to such guidelines and procedures as the
Board may adopt, stockholders entitled to vote at such meeting and proxy holders not physically present at a meeting of stockholders
may, by means of remote communication:
(i)
participate in a meeting of stockholders; and
(ii)
be deemed present in person and vote at a meeting of stockholders, whether such meeting is to be held at a designated place or solely
by means of remote communication, provided that (A) the Corporation shall implement reasonable measures to verify that each person deemed
present and permitted to vote at the meeting by means of remote communication is a stockholder or proxy holder, (B) the Corporation shall
implement reasonable measures to provide such stockholders and proxy holders a reasonable opportunity to participate in the meeting and,
if entitled to vote, to vote on matters submitted to the applicable stockholders, including an opportunity to read or hear the proceedings
of the meeting substantially concurrently with such proceedings, and (C) if any stockholder or proxy holder votes or takes other action
at the meeting by means of remote communication, a record of such votes or other action shall be maintained by the Corporation.
(b)
Board Meetings. Unless otherwise restricted by applicable law, the Certificate of Incorporation or these By Laws, members of the
Board or any committee thereof may participate in a meeting of the Board or any committee thereof by means of conference telephone or
other communications equipment by means of which all persons participating in the meeting can hear each other. Such participation in
a meeting shall constitute presence in person at the meeting, except where a person participates in the meeting for the express purpose
of objecting to the transaction of any business on the ground that the meeting was not lawfully called or convened.
Section
9.6. Dividends. The Board may from time to time declare, and the Corporation may pay, dividends (payable in cash, property or shares
of the Corporation’s capital stock) on the Corporation’s outstanding shares of capital stock, subject to applicable law and
the Certificate of Incorporation.
Section
9.7. Reserves. The Board may set apart out of the funds of the Corporation available for dividends a reserve or reserves for any
proper purpose and may abolish any such reserve.
Section
9.8. Contracts and Negotiable Instruments. Except as otherwise provided by applicable law, the Certificate of Incorporation or these
By Laws, any contract, bond, deed, lease, mortgage or other instrument may be executed and delivered in the name and on behalf of the
Corporation by such officer or officers or other employee or employees of the Corporation as the Board may from time to time authorize.
Such authority may be general or confined to specific instances as the Board may determine. The Chairman of the Board, any Chief Executive
Officer, the President, the Chief Financial Officer, the Treasurer or any Vice President may execute and deliver any contract, bond,
deed, lease, mortgage or other instrument in the name and on behalf of the Corporation. Subject to any restrictions imposed by the Board,
the Chairman of the Board , any Chief Executive Officer, President, the Chief Financial Officer, the Treasurer or any Vice President
may delegate powers to execute and deliver any contract, bond, deed, lease, mortgage or other instrument in the name and on behalf of
the Corporation to other officers or employees of the Corporation under such person’s supervision and authority, it being understood,
however, that any such delegation of power shall not relieve such officer of responsibility with respect to the exercise of such delegated
power.
Section
9.9. Fiscal Year. The fiscal year of the Corporation shall be fixed by the Board.
Section
9.10. Seal. The Board may adopt a corporate seal, which shall be in such form as the Board determines. The seal may be used by causing
it or a facsimile thereof to be impressed, affixed or otherwise reproduced.
Section
9.11. Books and Records. The books and records of the Corporation may be kept within or outside the State of Delaware at such place
or places as may from time to time be designated by the Board.
Section
9.12. Resignation. Any director, committee member or officer may resign by giving notice thereof in writing or by electronic transmission
to the Chairman of the Board, any Chief Executive Officer, the President or the Secretary. The resignation shall take effect at the time
it is delivered unless the resignation specifies a later effective date or an effective date determined upon the happening of an event
or events. Unless otherwise specified therein, the acceptance of such resignation shall not be necessary to make it effective.
Section
9.13. Surety Bonds. Such officers, employees and agents of the Corporation (if any) as the Chairman of the Board, any Chief Executive
Officer, President or the Board may direct, from time to time, shall be bonded for the faithful performance of their duties and for the
restoration to the Corporation, in case of their death, resignation, retirement, disqualification or removal from office, of all books,
papers, vouchers, money and other property of whatever kind in their possession or under their control belonging to the Corporation,
in such amounts and by such surety companies as the Chairman of the Board, any Chief Executive Officer, President or the Board may determine.
The premiums on such bonds shall be paid by the Corporation and the bonds so furnished shall be in the custody of the Secretary.
Section
9.14. Securities of Other Corporations. Powers of attorney, proxies, waivers of notice of meeting, consents in writing and other
instruments relating to securities owned by the Corporation may be executed in the name of and on behalf of the Corporation by the Chairman
of the Board, any Chief Executive Officer, President, any Vice President or any officers authorized by the Board. Any such officer, may,
in the name of and on behalf of the Corporation, take all such action as any such officer may deem advisable to vote in person or by
proxy at any meeting of security holders of any corporation in which the Corporation may own securities, or to consent in writing, in
the name of the Corporation as such holder, to any action by such corporation, and at any such meeting or with respect to any such consent
shall possess and may exercise any and all rights and power incident to the ownership of such securities and which, as the owner thereof,
the Corporation might have exercised and possessed. The Board may from time to time confer like powers upon any other person or persons.
Section
9.15. Amendments. The Board shall have the power to adopt, amend, alter or repeal the By Laws. The affirmative vote of a majority
of the Board shall be required to adopt, amend, alter or repeal the By Laws. The By Laws also may be adopted, amended, altered or repealed
by the stockholders; provided, however, that in addition to any vote of the holders of any class or series of capital stock of the Corporation
required by applicable law or the Certificate of Incorporation, the affirmative vote of the holders of at least a majority of the voting
power (except as otherwise provided in Section 8.7) of all outstanding shares of capital stock of the Corporation entitled to vote generally
in the election of directors, voting together as a single class, shall be required for the stockholders to adopt, amend, alter or repeal
the By Laws.
Exhibit
10.2
FORM
OF REGISTRATION RIGHTS AGREEMENT
THIS
REGISTRATION RIGHTS AGREEMENT (this “Agreement”) is made and entered into as of November 7, 2023 (the “Effective
Date”) by and among (i) Data Knights Acquisition Corp., a Delaware corporation (including its successors, the “Purchaser”),
and (ii) and the undersigned parties listed on Exhibit A hereto (each such party, together with any person or entity who hereafter
becomes a party to this Agreement pursuant to Section 6.2 of this Agreement, a “Holder” and collectively
the “Holders”).
WHEREAS,
on April 25, 2022, Purchaser, Data Knights Merger Sub, Inc., a Delaware corporation and a wholly-owned subsidiary of the Purchaser (“Merger
Sub”), Data Knights, LLC, a Delaware limited liability company (the “Purchaser Representative”),
Paul Casey (the “Seller Representative”), and (v) OneMedNet Corporation, a Delaware corporation (the “Company”),
entered into that certain Agreement and Plan of Merger (as amended from time to time in accordance with the terms thereof, the “Merger
Agreement”);
WHEREAS,
pursuant to the Merger Agreement, subject to the terms and conditions thereof, upon the consummation of the transactions contemplated
thereby (the “Closing”), among other matters, Merger Sub will merge with and into the Company, with the Company
continuing as the surviving entity and a wholly-owned subsidiary of the Purchaser, and with the Holders, as stockholders of the Purchaser,
receiving shares of the Purchaser’s Class A common stock (the “Merger Shares”), all upon the terms and
subject to the conditions set forth in the Merger Agreement and in accordance with the provisions of applicable law;
WHEREAS,
the Company is a party to that certain Investors’ Rights Agreement dated as of December 31, 2009, entered into with certain investors
listed therein (the “Company Investors”) holding shares of Company capital stock to be exchanged into shares
of Purchaser’s Class A common stock in the Merger (the “Prior Agreement”), and that certain Founders
Registration Rights Agreement with the Founders with regard to the Founder Securities (each as such term is defined below) (together
with the Prior Agreement, the “Existing Agreements”); and
WHEREAS,
the parties desire to enter into this Agreement, and terminate the Existing Agreements, to provide the Holders and Founders with certain
rights relating to the registration of the Merger Shares and Founder Securities.
NOW,
THEREFORE, in consideration of the mutual covenants and agreements set forth herein, and for other good and valuable consideration,
the receipt and sufficiency of which are hereby acknowledged, the parties hereto agree as follows:
1. DEFINITIONS.
The following capitalized terms used herein have the following meanings:
“Affiliate”
means, with respect to any specified Person, any Person that, directly or indirectly through one or more entities, controls or is controlled
by, or is under common control with, such specified Person. The term “control” (including the terms “controlled by”
and “under common control with”) means the possession, directly or indirectly, of the power to direct or cause the direction
of the management and policies of a Person, whether through the ownership of voting securities, by Contract or otherwise.
“Agreement”
means this Agreement, as amended, restated, supplemented, or otherwise modified from time to time.
“Business
Combination” means the acquisition of direct or indirect ownership through a merger, share exchange, asset acquisition,
share purchase, recapitalization, reorganization or other similar type of transaction, of one or more businesses or entities.
“Commission”
means the Securities and Exchange Commission, or any other Federal agency then administering the Securities Act or the Exchange Act.
“Common
Stock” means the Class A common stock, par value $0.000001 per share, of the Purchaser and the Class B common stock, par
value $0.000001 per share of the Purchaser, along with any equity securities paid as dividends or distributions after the Closing with
respect to such shares or into which such shares are exchanged or converted after the Closing.
“Company”
is defined in the preamble to this Agreement.
“Demand
Registration” is defined in Section 2.2.1.
“Demanding
Holder” is defined in Section 2.2.1.
“Effectiveness
Date” means, with respect to the Initial Registration Statement, the 90th calendar day following the Filing Date (or in
the event the Registration Statement receives a “full review” by the Commission, the 120th day following the Filing Date)
and with respect to any additional Registration Statements which may be required pursuant to Sections 2.2 and 2.3, the 90th calendar
day following the date on which an additional Registration Statement is required to be filed hereunder; provided, however, that in the
event the Purchaser is notified by the Commission that one or more of the above Registration Statements will not be reviewed or is no
longer subject to further review and comments, the Effectiveness Date as to such Registration Statement shall be the fifth Business Day
following the date on which the Purchaser is so notified if such date precedes the dates otherwise required above; provided, further,
that, if the Effectiveness Date falls on a Saturday, Sunday or any other day which shall be a legal holiday or a day on which the Commission
is authorized or required by law or other government actions to close, the Effectiveness Date shall be the following Business Day.
“Effectiveness
Period” shall have the meaning set forth in Section 2.1.1
“Exchange
Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the Commission promulgated
thereunder, all as the same shall be in effect at the time.
“Filing
Date” means, with respect to the Initial Registration Statement required hereunder, the 30th calendar day following the
date hereof and, with respect to any additional Registration Statements which may be required pursuant to Sections 2.2 and 2.3, the earliest
practical date on which the Purchaser is permitted by Commission Guidance to file such additional Registration Statement related to the
Registrable Securities; provided, however, that, if the Filing Date falls on a Saturday, Sunday or any other day which shall be a legal
holiday or a day on which the Commission is authorized or required by law or other government actions to close, the Filing Date shall
be the following Business Day.
“Form
S-3” is defined in Section 2.4.
“Founder
Registration Rights Agreement” means that certain Registration Rights Agreement, dated as of May 6, 2021, by and among
Purchaser, Data Knights, LLC and certain directors of Purchaser.
“Founder
Securities” means all shares of Common Stock, and all securities convertible into or exercisable for shares of Common Stock,
held by the Founders as of the Effective Date of this Agreement.
“Founders”
means Data Knights, LLC, Barry Anderson, Firdauz Edmin Bin Mokhtar, Syed Musheer Ahmed, Julianne Huh and Annie Damit Undikai, and any
successors in interest thereto with respect to any Founder Securities.
“Holder”
is defined in the preamble to this Agreement.
“Indemnified
Party” is defined in Section 4.3.
“Indemnifying
Party” is defined in Section 4.3.
“Initial
Registration Statement” means the Registration Statement required to be filed pursuant to Section 2.1.
“Holder
Indemnified Party” is defined in Section 4.1.
“Maximum
Number of Shares” is defined in Section 2.2.4.
“Merger
Shares” means the shares of Common Stock of the Purchaser issued or issuable to the Holders pursuant to the terms of the
Merger Agreement.
“Notices”
is defined in Section 6.3.
“Piggy-Back
Registration” is defined in Section 2.3.1.
“Pro
Rata” is defined in Section 2.2.4.
“Purchaser”
is defined in the preamble to this Agreement.
“Register,”
“Registered” and “Registration” mean a registration effected by preparing and filing
a registration statement or similar document in compliance with the requirements of the Securities Act, and the applicable rules and
regulations promulgated thereunder, and such registration statement becoming effective.
“Registrable
Securities” means (i) the Shares and any shares of Common Stock held by the Holders and Founders immediately following
the closing of the Business Combination, and (ii) any warrants, shares of capital stock or other securities of the Purchaser acquired
by a Holder or Founder after the Closing, or issued as a dividend or other distribution with respect to or in exchange for or in replacement
of such Shares. As to any particular Registrable Securities, such securities shall cease to be Registrable Securities when: (a) a Registration
Statement with respect to the sale of such securities shall have become effective under the Securities Act and such securities shall
have been sold, transferred, disposed of or exchanged in accordance with such Registration Statement; (b) such securities shall have
been otherwise transferred, new certificates for them not bearing a legend restricting further transfer shall have been delivered by
the Purchaser and subsequent public distribution of them shall not require registration under the Securities Act; (c) such securities
shall have ceased to be outstanding; or (d) the Registrable Securities are freely saleable under Rule 144 without volume limitations.
“Registration
Statement” means a registration statement filed by the Purchaser with the Commission in compliance with the Securities
Act and the rules and regulations promulgated thereunder for a public offering and sale of equity securities, or securities or other
obligations exercisable or exchangeable for, or convertible into, equity securities (other than a registration statement on Form S-4
or Form S-8, or their successors, or any registration statement covering only securities proposed to be issued in exchange for securities
or assets of another entity).
“Securities
Act” means the Securities Act of 1933, as amended, and the rules and regulations of the Commission promulgated thereunder,
all as the same shall be in effect at the time.
“Shares”
means the Merger Shares and the Founder Securities.
“Underwriter”
means, solely for the purposes of this Agreement, a securities dealer who purchases any Registrable Securities as principal in an underwritten
offering and not as part of such dealer’s market-making activities.
2. REGISTRATION
RIGHTS.
2.1 Shelf
Registration.
2.1.1
On or prior to each Filing Date, the Purchaser shall prepare and file with the Commission a Registration Statement covering the resale
of all or such maximum portion of the Registrable Securities as permitted by SEC Guidance (provided that, the Purchaser shall use diligent
efforts to advocate with the Commission for the registration of all of the Registrable Securities in accordance with the SEC Guidance,
including without limitation, the Manual of Publicly Available Telephone Interpretations D.29) that are not then registered on an effective
Registration Statement for an offering to be made on a continuous basis pursuant to Rule 415. Each Registration Statement filed hereunder
shall be on Form S-1 (except if the Purchaser is then eligible to register for resale the Registrable Securities on Form S-3, such registration
shall be on Form S-3 in accordance herewith). Subject to the terms of this Agreement, the Purchaser shall use its commercially reasonable
efforts to cause a Registration Statement to be declared effective under the Securities Act as promptly as practicable after the filing
thereof, but in any event prior to the applicable Effectiveness Date, and shall use its commercially reasonable efforts to keep such
Registration Statement continuously effective under the Securities Act until all Registrable Securities covered by such Registration
Statement have been sold, or may be sold without volume or manner-of-sale restrictions pursuant to Rule 144, without the requirement
for the Purchaser to be in compliance with the current public information requirement under Rule 144, as determined by the counsel to
the Purchaser pursuant to a written opinion letter to such effect, addressed and acceptable to the Transfer Agent and the affected Holders
(the “Effectiveness Period”). The Purchaser shall telephonically request effectiveness of a Registration Statement
as of 5:00 p.m. New York City time on a Business Day. The Purchaser shall promptly notify the Holders by e-mail of the effectiveness
of a Registration Statement on the same Business Day that the Purchaser telephonically confirms effectiveness with the Commission. The
Purchaser shall, no later than the second Business Day after the effective date of such Registration Statement, file a final Prospectus
with the Commission as required by Rule 424.
2.1.2 Notwithstanding
any other provision of this Agreement, if any SEC Guidance sets forth a limitation on the number of Registrable Securities permitted
to be registered on a particular Registration Statement (and notwithstanding that the Purchaser used diligent efforts to advocate with
the Commission for the registration of all or a greater portion of Registrable Securities), the number of Registrable Securities to be
registered shall be reduced on a on a pro rata basis based on the total number of Registrable Securities held by such Holders (such proportion
is referred to herein as “Pro Rata”). In the event of a reduction hereunder, the Purchaser shall give the Holder
and Founders, as applicable, at least five (5) Business Days prior written notice along with the calculations as to such Holder’s
or Founder’s allotment. Promptly after such SEC Guidance is no longer applicable with respect to some or all of the remaining unregistered
Registrable Securities, the Purchaser shall file an additional Registration Statement in accordance with this Section 2 to with respect
to such shares.
2.1.3 Each
Holder agrees to furnish to the Purchaser a completed Selling Stockholder Questionnaire within five (5) Business Days following the date
of this Agreement. Each Holder further acknowledges and agrees that it shall not be entitled to be named as a selling security holder
in the Registration Statement or use the Prospectus for offers and resales of Registrable Securities at any time unless such Holder has
returned to the Purchaser a completed and signed Selling Stockholder Questionnaire. If a Holder of Registrable Securities returns a Selling
Stockholder Questionnaire after the deadline specified in the previous sentence, the Purchaser shall use its commercially reasonable
efforts to take such actions as are required to name such Holder as a selling security holder in the Registration Statement or any pre-effective
or post-effective amendment thereto and to include (to the extent not theretofore included) in the Registration Statement the Registrable
Securities identified in such late Selling Stockholder Questionnaire; provided that the Purchaser shall not be required to file an additional
Registration Statement solely for such shares. Each Holder acknowledges and agrees that the information in the Selling Stockholder Questionnaire
will be used by the Purchaser in the preparation of the Registration Statement and hereby consents to the inclusion of such information
in the Registration Statement.
2.2 Demand
Registration.
2.2.1 Request
for Registration. At any time and from time to time on or after the date of this Agreement, the Holders of twenty-five percent (25%)
of the Registrable Securities may make a written demand for registration under the Securities Act of all or part of their Registrable
Securities, as the case may be (a “Demand Registration”). Any demand for a Demand Registration shall specify
the number of shares of Registrable Securities proposed to be sold and the intended method(s) of distribution thereof. The Purchaser
will within twenty (20) days of the Purchaser’s receipt of the Demand Registration notify all holders of Registrable Securities
of the demand, and each holder of Registrable Securities who wishes to include all or a portion of such holder’s Registrable Securities
in the Demand Registration (each such holder including shares of Registrable Securities in such registration, a “Demanding
Holder”) shall so notify the Purchaser within ten (10) days after the receipt by the holder of the notice from the Purchaser.
Upon any such request, the Demanding Holders shall be entitled to have their Registrable Securities included in the Demand Registration,
subject to Section 2.1.4 and the provisos set forth in Section 3.1.1. The Purchaser shall not be obligated to effect no more than an
aggregate of three (3) Demand Registrations under this Section 2.1.1 in respect of all Registrable Securities.
2.2.2 Effective
Registration. A registration will not count as a Demand Registration until the Registration Statement filed with the Commission with
respect to such Demand Registration has been declared effective and the Purchaser has complied with all of its obligations under this
Agreement with respect thereto; provided, however, that if, after such Registration Statement has been declared effective, the offering
of Registrable Securities pursuant to a Demand Registration is interfered with by any stop order or injunction of the Commission or any
other governmental agency or court, the Registration Statement with respect to such Demand Registration will be deemed not to have been
declared effective, unless and until, (i) such stop order or injunction is removed, rescinded or otherwise terminated, and (ii) a majority-in-interest
of the Demanding Holders thereafter elect to continue the offering; provided, further, that the Purchaser shall not be obligated to file
a second Registration Statement until a Registration Statement that has been filed is counted as a Demand Registration or is terminated.
2.2.3 Underwritten
Offering. If a majority-in-interest of the Demanding Holders so elect and such holders so advise the Purchaser as part of their written
demand for a Demand Registration, the offering of such Registrable Securities pursuant to such Demand Registration shall be in the form
of an underwritten offering; provided that the total offering price is reasonably expected to exceed, in the aggregate, $50 million.
In such event, the right of any holder to include its Registrable Securities in such registration shall be conditioned upon such holder’s
participation in such underwriting and the inclusion of such holder’s Registrable Securities in the underwriting to the extent
provided herein. All Demanding Holders proposing to distribute their Registrable Securities through such underwriting shall enter into
an underwriting agreement in customary form with the Underwriter or Underwriters selected for such underwriting by a majority-in-interest
of the holders initiating the Demand Registration.
2.2.4 Reduction
of Offering. If the managing Underwriter or Underwriters for a Demand Registration that is to be an underwritten offering, in good
faith, advises the Purchaser and the Demanding Holders in writing that the dollar amount or number of shares of Registrable Securities
which the Demanding Holders desire to sell, taken together with all other shares of Common Stock or other securities which the Purchaser
desires to sell and the shares of Common Stock, if any, as to which registration has been requested pursuant to written contractual piggy-back
registration rights held by other shareholders of the Purchaser who desire to sell, exceeds the maximum dollar amount or maximum number
of shares that can be sold in such offering without adversely affecting the proposed offering price, the timing, the distribution method,
or the probability of success of such offering (such maximum dollar amount or maximum number of shares, as applicable, the “Maximum
Number of Shares” ), then the Purchaser shall include in such registration: (i) first, the Registrable Securities as to
which Demand Registration has been requested by the Demanding Holders (Pro Rata in accordance with the number of shares that each such
Person has requested be included in such registration, regardless of the number of shares held by each such Person) that can be sold
without exceeding the Maximum Number of Shares; (ii) second, to the extent that the Maximum Number of Shares has not been reached under
the foregoing clause (i), the Registrable Securities of Holders exercising their rights to register their Registrable Securities that
can be sold without exceeding the Maximum Number of Shares; and (iii) third, to the extent that the Maximum Number of Shares has not
been reached under the foregoing clauses (i) and (ii), shares of Common Stock or other securities that the Purchaser desires to sell
that can be sold without exceeding the Maximum Number of Shares.
2.2.5 Withdrawal.
If, prior to filing of the applicable “red herring prospectus” or prospectus supplement used for marketing such registration,
a majority-in-interest of the Demanding Holders disapprove of the terms of any underwriting or are not entitled to include all of their
Registrable Securities in any offering, such majority-in-interest of the Demanding Holders may elect to withdraw from such offering by
giving written notice to the Purchaser and the Underwriter or Underwriters of their request to withdraw prior to the effectiveness of
the Registration Statement filed with the Commission with respect to such Demand Registration. If the majority-in-interest of the Demanding
Holders withdraws from a proposed offering relating to a Demand Registration, then such registration shall not count as a Demand Registration
provided for in Section 2.1.
2.3 Piggy-Back
Registration.
2.3.1 Piggy-Back
Rights. If at any time on or after the date of this Agreement the Purchaser proposes to file a Registration Statement under the Securities
Act with respect to an offering of equity securities, or securities or other obligations exercisable or exchangeable for, or convertible
into, equity securities, by the Purchaser for its own account or for shareholders of the Purchaser for their account (or by the Purchaser
and by shareholders of the Purchaser including, without limitation, pursuant to Section 2.1), other than a Registration Statement (i)
filed in connection with any employee stock option or other benefit plan, (ii) for an exchange offer or offering of securities solely
to the Purchaser’s existing shareholders, (iii) for an offering of debt that is convertible into equity securities of the Purchaser
or (iv) for a dividend reinvestment plan, then the Purchaser shall (x) give written notice of such proposed filing to the holders of
Registrable Securities as soon as practicable but in no event less than twenty (20) days before the anticipated filing date, which notice
shall describe the amount and type of securities to be included in such offering, the intended method(s) of distribution, and the name
of the proposed managing Underwriter or Underwriters, if any, of the offering, and (y) offer to the holders of Registrable Securities
in such notice the opportunity to register the sale of such number of shares of Registrable Securities as such holders may request in
writing within ten (10) days following receipt of such notice (a “Piggy-Back Registration” ). The Purchaser
shall, in good faith, cause such Registrable Securities to be included in such registration and shall use its best efforts to cause the
managing Underwriter or Underwriters of a proposed underwritten offering to permit the Registrable Securities requested to be included
in a Piggy-Back Registration on the same terms and conditions as any similar securities of the Purchaser and to permit the sale or other
disposition of such Registrable Securities in accordance with the intended method(s) of distribution thereof. All holders of Registrable
Securities proposing to distribute their securities through a Piggy-Back Registration that involves an Underwriter or Underwriters shall
enter into an underwriting agreement in customary form with the Underwriter or Underwriters selected for such Piggy-Back Registration.
Notwithstanding the provisions set forth in the immediately preceding sentences, the right to a Piggy-Back Registration set forth under
this Section 2.2.1 with respect to the Registrable Securities shall terminate on the seventh anniversary of the Effective Date.
2.3.2 Reduction
of Offering. If the managing Underwriter or Underwriters for a Piggy-Back Registration that is to be an underwritten offering advises
the Purchaser and the holders of Registrable Securities in writing that the dollar amount or number of shares of Common Stock which the
Purchaser desires to sell, taken together with the shares of Common Stock, if any, as to which registration has been demanded pursuant
to written contractual arrangements with persons other than the holders of Registrable Securities hereunder, the Registrable Securities
as to which registration has been requested under this Section 2.2, and the shares of Common Stock, if any, as to which registration
has been requested pursuant to the terms hereof exceeds the Maximum Number of Shares, then the Purchaser shall include in any such registration:
a) If
the registration is undertaken for the Purchaser’s account: (A) first, the shares of Common Stock or other securities that the
Purchaser desires to sell that can be sold without exceeding the Maximum Number of Shares; and (B) second, to the extent that the Maximum
Number of Shares has not been reached under the foregoing clause (A), the shares of Common Stock or other securities, if any, comprised
of Registrable Securities as to which registration has been requested pursuant to the terms hereof, Pro Rata, that can be sold without
exceeding the Maximum Number of Shares; and (C) third, to the extent that the Maximum Number of Shares has not been reached under the
foregoing clauses (A) and (B), the shares of Common Stock or other securities for the account of other persons that the Purchaser is
obligated to register pursuant to written contractual piggy-back registration rights with such persons and that can be sold without exceeding
the Maximum Number of Shares;
b) If
the registration is a “demand” registration undertaken at the demand of persons other than the holders of Registrable Securities,
(A) first, the shares of Common Stock or other securities for the account of the demanding persons that can be sold without exceeding
the Maximum Number of Shares; (B) second, to the extent that the Maximum Number of Shares has not been reached under the foregoing clause
(A), the shares of Common Stock or other securities comprised of Registrable Securities, Pro Rata, as to which registration has been
requested pursuant to the terms hereof, that can be sold without exceeding the Maximum Number of Shares; and (C) third, to the extent
that the Maximum Number of Shares has not been reached under the foregoing clauses (A) and (B), the shares of Common Stock or other securities
that the Purchaser desires to sell that can be sold without exceeding the Maximum Number of Shares; and (D) fourth, to the extent that
the Maximum Number of Shares has not been reached under the foregoing clauses (A), (B) and (C), the shares of Common Stock or other securities
for the account of other persons that the Purchaser is obligated to register pursuant to written contractual arrangements with such persons,
that can be sold without exceeding the Maximum Number of Shares.
2.3.3 Withdrawal.
Any holder of Registrable Securities may elect to withdraw such holder’s request for inclusion of Registrable Securities in any
Piggy-Back Registration by giving written notice to the Purchaser of such request to withdraw prior to the effectiveness of the Registration
Statement. The Purchaser (whether on its own determination or as the result of a withdrawal by persons making a demand pursuant to written
contractual obligations) may withdraw a Registration Statement at any time prior to the effectiveness of such Registration Statement.
Notwithstanding any such withdrawal, the Purchaser shall pay all expenses incurred by the holders of Registrable Securities in connection
with such Piggy-Back Registration as provided in Section 3.3.
2.3.4 Unlimited
Piggy-Back Registration Rights. For purposes of clarity, any Registration effected pursuant to Section 2.2 hereof shall not be counted
as a Registration pursuant to a Demand Registration effected under Section 2.1 hereof. The Holders shall have unlimited Piggy-Back Registration
Rights.
2.3.5 Registrations
on Form S-3. The holders of Registrable Securities may at any time and from time to time, request in writing that the Purchaser register
the resale of any or all of such Registrable Securities on Form S-3 or any similar short-form registration which may be available at
such time (“Form S-3”); provided, however, that the Purchaser shall not be obligated to effect such request
through an underwritten offering. Upon receipt of such written request, the Purchaser will promptly give written notice of the proposed
registration to all other holders of Registrable Securities, and, as soon as practicable thereafter, effect the registration of all or
such portion of such holder’s or holders’ Registrable Securities as are specified in such request, together with all or such
portion of the Registrable Securities or other securities of the Purchaser, if any, of any other holder or holders joining in such request
as are specified in a written request given within fifteen (15) days after receipt of such written notice from the Purchaser; provided,
however, that the Purchaser shall not be obligated to effect any such registration pursuant to this Section 2.3: (i) if Form S-3 is not
available for such offering; or (ii) if the holders of the Registrable Securities, together with the holders of any other securities
of the Purchaser entitled to inclusion in such registration, propose to sell Registrable Securities and such other securities (if any)
at any aggregate price to the public of less than $500,000. Registrations effected pursuant to this Section 2.3 shall not be counted
as Demand Registrations effected pursuant to Section 2.1.
2.4 Block
Trades; Other Coordinated Offerings.
2.4.1 Notwithstanding
any other provision of this Section 2,4, at any time and from time to time when an effective Shelf Registration is on file with the Commission,
if a Demanding Holder wishes to engage in (a) an underwritten registered offering (whether firm commitment or otherwise) not involving
a “road show” or other substantial marketing efforts prior to pricing (commonly referred to as a “Block Trade”)
or (b) an otherwise coordinated “at the market” or similar registered offering through a broker, sales agent or distribution
agent, whether as agent or principal (an “Other Coordinated Offering”), in each case, with a total offering
price reasonably expected to exceed, in the aggregate, either (x) $10 million or (y) all remaining Registrable Securities held by the
Demanding Holder, then such Demanding Holder shall notify the Purchaser of the Block Trade or Other Coordinated Offering at least five
(5) business days prior to the day such offering is expected to commence, and the Purchaser shall as expeditiously as possible use its
commercially reasonable efforts to facilitate such Block Trade or Other Coordinated Offering; provided that the Demanding Holders representing
a majority of the Registrable Securities wishing to engage in the Block Trade or Other Coordinated Offering shall use commercially reasonable
efforts to work with the Purchaser and any Underwriters, brokers, sales agents or placement agents prior to making such request in order
to facilitate preparation of the registration statement, prospectus and other offering documentation related to the Block Trade or Other
Coordinated Offering.
2.4.2 The
Purchaser may facilitate a Block Trade or Other Coordinated Offering if it determines that sufficient shares shall be traded by any Holder
or Holders that would be more efficiently traded as a block trade.
2.4.3 Prior
to the filing of the applicable “red herring” prospectus or prospectus supplement used in connection with a Block Trade or
Other Coordinated Offering, a majority-in-interest of the Demanding Holders initiating such Block Trade or Other Coordinated Offering
shall have the right to submit a notice to the Purchaser and the Underwriter(s) if any, of their intention to withdraw from such Block
Trade or Other Coordinated Offering. Notwithstanding anything to the contrary in this Agreement, the Purchaser shall be responsible for
the Registration Expenses incurred in connection with a block trade prior to its withdrawal under this Section 2.4.3.
2.4.4 Notwithstanding
anything to the contrary in this Agreement, Section 2.3 shall not apply to a Block Trade or Other Coordinated Offering initiated by a
Demanding Holder pursuant to this Section 2.4.
2.4.5 The
Purchaser shall have the right to select the Underwriters, and brokers, sale agents or placement agents (if any) for such Block Trade
or Other Coordinated Offering, in each case, which shall consist of one or more reputable nationally recognized investment bank.
2.4.6 A
Holder in the aggregate may demand no more than two (2) Block Trades or Other Coordinated Offerings pursuant to this Section 2.4 in any
twelve (12) month period.
3. REGISTRATION
PROCEDURES.
3.1 Filings;
Information. Whenever the Purchaser is required to effect the registration of any Registrable Securities pursuant to Section 2, the
Purchaser shall use its best efforts to effect the registration and sale of such Registrable Securities in accordance with the intended
method(s) of distribution thereof as expeditiously as practicable, and in connection with any such request:
3.1.1 Filing
Registration Statement. The Purchaser shall use its best efforts to, as expeditiously as possible after receipt of a request for
a Demand Registration pursuant to Section 2.1, prepare and file with the Commission a Registration Statement on any form for which the
Purchaser then qualifies or which counsel for the Purchaser shall deem appropriate and which form shall be available for the sale of
all Registrable Securities to be registered thereunder in accordance with the intended method(s) of distribution thereof, and shall use
its best efforts to cause such Registration Statement to become effective and use its best efforts to keep it effective for the period
required by Section 3.1.3; provided, however, that the Purchaser shall have the right to defer any Demand Registration for up to ninety
(90) days, and any Piggy-Back Registration for such period as may be applicable to deferment of any demand registration to which such
Piggy-Back Registration relates, in each case if the Purchaser shall furnish to the holders a certificate signed by Chief Executive Officer
or Chairman of the Purchaser stating that, in the good faith judgment of the Board of Directors of the Purchaser, it would be materially
detrimental to the Purchaser and its shareholders for such Registration Statement to be effected at such time; provided further, however,
that the Purchaser shall not have the right to exercise the right set forth in this provision more than once in any 365-day period in
respect of a Demand Registration hereunder.
3.1.2 Copies.
The Purchaser shall, prior to filing a Registration Statement or prospectus, or any amendment or supplement thereto, furnish without
charge to the holders of Registrable Securities included in such registration, and such holders’ legal counsel, copies of such
Registration Statement as proposed to be filed, each amendment and supplement to such Registration Statement (in each case including
all exhibits thereto and documents incorporated by reference therein), the prospectus included in such Registration Statement (including
each preliminary prospectus), and such other documents as the holders of Registrable Securities included in such registration or legal
counsel for any such holders may request in order to facilitate the disposition of the Registrable Securities owned by such holders.
3.1.3 Amendments
and Supplements. The Purchaser shall prepare and file with the Commission such amendments, including post-effective amendments, and
supplements to such Registration Statement and the prospectus used in connection therewith as may be necessary to keep such Registration
Statement effective and in compliance with the provisions of the Securities Act until all Registrable Securities and other securities
covered by such Registration Statement have been disposed of in accordance with the intended method(s) of distribution set forth in such
Registration Statement or such securities have been withdrawn.
3.1.4 Notification.
After the filing of a Registration Statement, the Purchaser shall promptly, and in no event more than two (2) business days after such
filing, notify the holders of Registrable Securities included in such Registration Statement of such filing, and shall further notify
such holders promptly and confirm such advice in writing in all events within two (2) business days of the occurrence of any of the following:
(i) when such Registration Statement becomes effective; (ii) when any post-effective amendment to such Registration Statement becomes
effective; (iii) the issuance or threatened issuance by the Commission of any stop order (and the Purchaser shall take all actions required
to prevent the entry of such stop order or to remove it if entered); and (iv) any request by the Commission for any amendment or supplement
to such Registration Statement or any prospectus relating thereto or for additional information or of the occurrence of an event requiring
the preparation of a supplement or amendment to such prospectus so that, as thereafter delivered to the purchasers of the securities
covered by such Registration Statement, such prospectus will not contain an untrue statement of a material fact or omit to state any
material fact required to be stated therein or necessary to make the statements therein not misleading, and promptly make available to
the holders of Registrable Securities included in such Registration Statement any such supplement or amendment; except that before filing
with the Commission a Registration Statement or prospectus or any amendment or supplement thereto, including documents incorporated by
reference, the Purchaser shall furnish to the holders of Registrable Securities included in such Registration Statement and to the legal
counsel for any such holders, copies of all such documents proposed to be filed sufficiently in advance of filing to provide such holders
and legal counsel with a reasonable opportunity to review such documents and comment thereon, and the Purchaser shall not file any Registration
Statement or prospectus or amendment or supplement thereto, including documents incorporated by reference, to which such holders or their
legal counsel shall object.
3.1.5 State
Securities Laws Compliance. The Purchaser shall use its best efforts to (i) register or qualify the Registrable Securities covered
by the Registration Statement under such securities or “blue sky” laws of such jurisdictions in the United States as the
holders of Registrable Securities included in such Registration Statement (in light of their intended plan of distribution) may request
and (ii) take such action necessary to cause such Registrable Securities covered by the Registration Statement to be registered with
or approved by such other governmental authorities as may be necessary by virtue of the business and operations of the Purchaser and
do any and all other acts and things that may be necessary or advisable to enable the holders of Registrable Securities included in such
Registration Statement to consummate the disposition of such Registrable Securities in such jurisdictions; provided, however, that the
Purchaser shall not be required to qualify generally to do business in any jurisdiction where it would not otherwise be required to qualify
but for this paragraph or subject itself to taxation in any such jurisdiction.
3.1.6 Agreements
for Disposition. The Purchaser shall enter into customary agreements (including, if applicable, an underwriting agreement in customary
form) and take such other actions as are reasonably required in order to expedite or facilitate the disposition of such Registrable Securities.
The representations, warranties and covenants of the Purchaser in any underwriting agreement which are made to or for the benefit of
any Underwriters, to the extent applicable, shall also be made to and for the benefit of the holders of Registrable Securities included
in such registration statement. No holder of Registrable Securities included in such registration statement shall be required to make
any representations or warranties in the underwriting agreement except, if applicable, with respect to such holder’s organization,
good standing, authority, title to Registrable Securities, lack of conflict of such sale with such holder’s material agreements
and organizational documents, and with respect to written information relating to such holder that such holder has furnished in writing
expressly for inclusion in such Registration Statement.
3.1.7 Cooperation.
The principal executive officer of the Purchaser, the principal financial officer of the Purchaser, the principal accounting officer
of the Purchaser and all other officers and members of the management of the Purchaser shall cooperate fully in any offering of Registrable
Securities hereunder, which cooperation shall include, without limitation, the preparation of the Registration Statement with respect
to such offering and all other offering materials and related documents, and participation in meetings with Underwriters, attorneys,
accountants and potential Holders.
3.1.8 Records.
The Purchaser shall make available for inspection by the holders of Registrable Securities included in such Registration Statement, any
Underwriter participating in any disposition pursuant to such registration statement and any attorney, accountant or other professional
retained by any holder of Registrable Securities included in such Registration Statement or any Underwriter, all financial and other
records, pertinent corporate documents and properties of the Purchaser, as shall be necessary to enable them to exercise their due diligence
responsibility, and cause the Purchaser’s officers, directors and employees to supply all information requested by any of them
in connection with such Registration Statement.
3.1.9 Opinions
and Comfort Letters. Upon request, the Purchaser shall furnish to each holder of Registrable Securities included in any Registration
Statement a signed counterpart, addressed to such holder, of (i) any opinion of counsel to the Purchaser delivered to any Underwriter
and (ii) any comfort letter from the Purchaser’s independent public accountants delivered to any Underwriter. In the event no legal
opinion is delivered to any Underwriter, the Purchaser shall furnish to each holder of Registrable Securities included in such Registration
Statement, at any time that such holder elects to use a prospectus, an opinion of counsel to the Purchaser to the effect that the Registration
Statement containing such prospectus has been declared effective and that no stop order is in effect.
3.1.10 Earnings
Statement. The Purchaser shall comply with all applicable rules and regulations of the Commission and the Securities Act, and make
available to its shareholders, as soon as practicable, an earnings statement covering a period of twelve (12) months, which earnings
statement shall satisfy the provisions of Section 11(a) of the Securities Act and Rule 158 thereunder.
3.1.11 Listing.
The Purchaser shall use its best efforts to cause all Registrable Securities included in any registration to be listed on such exchanges
or otherwise designated for trading in the same manner as similar securities issued by the Purchaser are then listed or designated or,
if no such similar securities are then listed or designated, in a manner satisfactory to the holders of a majority of the Registrable
Securities included in such registration.
3.1.12 Road
Show. If the registration involves the registration of Registrable Securities involving gross proceeds in excess of $50,000,000,
the Purchaser shall use its reasonable efforts to make available senior executives of the Purchaser to participate in customary “road
show” presentations that may be reasonably requested by the Underwriter in any underwritten offering.
3.2 Obligation
to Suspend Distribution. Upon receipt of any notice from the Purchaser of the happening of any event of the kind described in Section
3.1.4(iv), or, in the case of a resale registration on Form S-3 pursuant to Section 2.3 hereof, upon any suspension by the Purchaser,
pursuant to a written insider trading compliance program adopted by the Purchaser’s Board of Directors, of the ability of all “insiders”
covered by such program to transact in the Purchaser’s securities because of the existence of material non-public information,
each holder of Registrable Securities included in any registration shall immediately discontinue disposition of such Registrable Securities
pursuant to the Registration Statement covering such Registrable Securities until such holder receives the supplemented or amended prospectus
contemplated by Section 3.1.4(iv) or the restriction on the ability of “insiders” to transact in the Purchaser’s securities
is removed, as applicable, and, if so directed by the Purchaser, each such holder will deliver to the Purchaser all copies, other than
permanent file copies then in such holder’s possession, of the most recent prospectus covering such Registrable Securities at the
time of receipt of such notice.
3.3 Registration
Expenses. The Purchaser shall bear all costs and expenses incurred in connection with any Demand Registration pursuant to Section
2.2, any Piggy-Back Registration pursuant to Section 2.3, and any registration on Form S-3 effected pursuant to Section 2.4, and all
expenses incurred in performing or complying with its other obligations under this Agreement, whether or not the Registration Statement
becomes effective, including, without limitation: (i) all registration and filing fees; (ii) fees and expenses of compliance with securities
or “blue sky” laws (including fees and disbursements of counsel in connection with blue sky qualifications of the Registrable
Securities); (iii) printing expenses; (iv) the fees and expenses incurred in connection with the listing of the Registrable Securities
as required by Section 3.1.11; (v) Financial Industry Regulatory Authority fees; (vi) fees and disbursements of counsel for the Purchaser
and fees and expenses for independent certified public accountants retained by the Purchaser (including the expenses or costs associated
with the delivery of any opinions or comfort letters requested pursuant to Section 3.1.9); and (viii) the reasonable fees and expenses
of any special experts retained by the Purchaser in connection with such registration. The Purchaser shall have no obligation to pay
(i) any underwriting discounts or selling commissions attributable to the Registrable Securities being sold by the holders thereof, which
underwriting discounts or selling commissions shall be borne by such holders, or (ii) the fees and expenses of any legal counsel representing
any Holders. Additionally, in an underwritten offering, all selling shareholders and the Purchaser shall bear the expenses of the Underwriter
pro rata in proportion to the respective amount of shares each is selling in such offering.
3.4 Information.
The holders of Registrable Securities shall provide such information as may reasonably be requested by the Purchaser, or the managing
Underwriter, if any, in connection with the preparation of any Registration Statement, including amendments and supplements thereto,
in order to effect the registration of any Registrable Securities under the Securities Act pursuant to Section 2 and in connection with
the Purchaser’s obligation to comply with Federal and applicable state securities laws. In addition, the holders of Registrable
Securities shall comply with all prospectus delivery requirements under the Securities Act and applicable SEC regulations.
3.5 Legend
Removal Obligations. In connection with the written request of any Holder, the Purchaser shall remove any restrictive legend included
on the certificates (or, in the case of book-entry shares, any other instrument or record) representing such Holder’s and/or its
affiliates’ or permitted transferee’s ownership of Registrable Securities, and promptly issue a certificate (or evidence
of the issuance of securities in book-entry form) without such restrictive legend or any other restrictive legend to the holder of the
applicable shares of Registrable Securities upon which it is stamped, if (i) such Registrable Securities are registered for resale under
the Securities Act and such Registration Statement for such Registrable Securities has not been suspended under the Securities Act, the
Exchange Act or the rules and regulations of the Commission promulgated thereunder, (ii) such Registrable Securities are sold or transferred
pursuant to Rule 144, or (iii) such Registrable Securities are eligible for sale pursuant to Section 4(a)(1) of the Securities Act or
Rule 144 without volume or manner-of-sale restrictions. Following the earlier of (A) the effective date of a Registration Statement registering
such Registrable Securities or (B) Rule 144 becoming available for the resale of such Registrable Securities without volume or manner-of-sale
restrictions, the Purchaser upon the written request of the Holder or its permitted transferee, shall instruct the Purchaser’s
transfer agent to remove the legend from such Registrable Securities (in whatever form) and shall cause the Purchaser’s counsel
to issue any legend removal opinion required by the transfer agent. Any reasonable and documented fees (with respect to the transfer
agent, the Purchaser’s counsel, or otherwise) associated with the removal of such legend shall be borne by the Purchaser. If a
legend is no longer required pursuant to the foregoing, the Purchaser will, as soon as practicable following the delivery by any Holder
or its permitted transferee to the Purchaser or the transfer agent (with notice to the Purchaser) of a legended certificate (if applicable)
representing such Registrable Securities and, to the extent such sale is not pursuant to an effective registration statement, such other
documentation as reasonably requested by the Purchaser, deliver or cause to be delivered to the holder of such Registrable Securities
a certificate representing such Registrable Securities (or evidence of the issuance of such Registrable Securities in book-entry form)
that is free from all restrictive legends; provided that, notwithstanding the foregoing, the Purchaser will not be required to deliver
any opinion, authorization, certificate or direction to remove the restrictive legend pursuant to this Section 3.5 if (x) removal of
the legend would result in or facilitate transfer of securities in violation of applicable law or (y) following receipt of instruction
from the Purchaser, the transfer agent refuses to remove the legend.
4. INDEMNIFICATION
AND CONTRIBUTION.
4.1 Indemnification
by the Purchaser. The Purchaser agrees to indemnify and hold harmless each Holder and each other holder of Registrable Securities,
and each of their respective officers, employees, affiliates, directors, partners, members, attorneys and agents, and each person, if
any, who controls an Holder and each other holder of Registrable Securities (within the meaning of Section 15 of the Securities Act or
Section 20 of the Exchange Act) (each, an “Holder Indemnified Party” ), from and against any expenses, losses,
judgments, claims, damages or liabilities, whether joint or several, arising out of or based upon any untrue statement (or allegedly
untrue statement) of a material fact contained in any Registration Statement under which the sale of such Registrable Securities was
registered under the Securities Act, any preliminary prospectus, final prospectus or summary prospectus contained in the Registration
Statement, or any amendment or supplement to such Registration Statement, or arising out of or based upon any omission (or alleged omission)
to state a material fact required to be stated therein or necessary to make the statements therein not misleading, or any violation by
the Purchaser of the Securities Act or any rule or regulation promulgated thereunder applicable to the Purchaser and relating to action
or inaction required of the Purchaser in connection with any such registration; and the Purchaser shall promptly reimburse the Holder
Indemnified Party for any legal and any other expenses reasonably incurred by such Holder Indemnified Party in connection with investigating
and defending any such expense, loss, judgment, claim, damage, liability or action; provided, however, that the Purchaser will not be
liable in any such case to the extent that any such expense, loss, claim, damage or liability arises out of or is based upon any untrue
statement or allegedly untrue statement or omission or alleged omission made in such Registration Statement, preliminary prospectus,
final prospectus, or summary prospectus, or any such amendment or supplement, in reliance upon and in conformity with information furnished
to the Purchaser, in writing, by such selling Holder expressly for use therein. The Purchaser also shall indemnify any Underwriter of
the Registrable Securities, their officers, affiliates, directors, partners, members and agents and each person who controls such Underwriter
on substantially the same basis as that of the indemnification provided above in this Section 4.1.
4.2 Indemnification
by Holders of Registrable Securities. Each selling Holder will, in the event that any registration is being effected under the Securities
Act pursuant to this Agreement of any Registrable Securities held by such selling Holder, indemnify and hold harmless the Purchaser,
each of its directors and officers and each Underwriter (if any), and each other selling Holder and each other person, if any, who controls
another selling Holder or such Underwriter within the meaning of the Securities Act, against any losses, claims, judgments, damages or
liabilities, whether joint or several, insofar as such losses, claims, judgments, damages or liabilities (or actions in respect thereof)
arise out of or are based upon any untrue statement or allegedly untrue statement of a material fact contained in any Registration Statement
under which the sale of such Registrable Securities was registered under the Securities Act, any preliminary prospectus, final prospectus
or summary prospectus contained in the Registration Statement, or any amendment or supplement to the Registration Statement, or arise
out of or are based upon any omission or the alleged omission to state a material fact required to be stated therein or necessary to
make the statement therein not misleading, if the statement or omission was made in reliance upon and in conformity with information
furnished in writing to the Purchaser by such selling Holder expressly for use therein, and shall reimburse the Purchaser, its directors
and officers, and each other selling holder or controlling person for any legal or other expenses reasonably incurred by any of them
in connection with investigation or defending any such loss, claim, damage, liability or action. Each selling Holder’s indemnification
obligations hereunder shall be several and not joint and shall be limited to the amount of any net proceeds actually received by such
selling Holder.
4.3 Conduct
of Indemnification Proceedings. Promptly after receipt by any person of any notice of any loss, claim, damage or liability or any
action in respect of which indemnity may be sought pursuant to Section 4.1 or 4.2, such person (the “Indemnified Party”)
shall, if a claim in respect thereof is to be made against any other person for indemnification hereunder, notify such other person (the
“Indemnifying Party” ) in writing of the loss, claim, judgment, damage, liability or action; provided, however,
that the failure by the Indemnified Party to notify the Indemnifying Party shall not relieve the Indemnifying Party from any liability
which the Indemnifying Party may have to such Indemnified Party hereunder, except and solely to the extent the Indemnifying Party is
actually prejudiced by such failure. If the Indemnified Party is seeking indemnification with respect to any claim or action brought
against the Indemnified Party, then the Indemnifying Party shall be entitled to participate in such claim or action, and, to the extent
that it wishes, jointly with all other Indemnifying Parties, to assume control of the defense thereof with counsel satisfactory to the
Indemnified Party. After notice from the Indemnifying Party to the Indemnified Party of its election to assume control of the defense
of such claim or action, the Indemnifying Party shall not be liable to the Indemnified Party for any legal or other expenses subsequently
incurred by the Indemnified Party in connection with the defense thereof other than reasonable costs of investigation; provided, however,
that in any action in which both the Indemnified Party and the Indemnifying Party are named as defendants, the Indemnified Party shall
have the right to employ separate counsel (but no more than one such separate counsel) to represent the Indemnified Party and its controlling
persons who may be subject to liability arising out of any claim in respect of which indemnity may be sought by the Indemnified Party
against the Indemnifying Party, with the fees and expenses of such counsel to be paid by such Indemnifying Party if, based upon the written
opinion of counsel of such Indemnified Party, representation of both parties by the same counsel would be inappropriate due to actual
or potential differing interests between them. No Indemnifying Party shall, without the prior written consent of the Indemnified Party,
consent to entry of judgment or effect any settlement of any claim or pending or threatened proceeding in respect of which the Indemnified
Party is or could have been a party and indemnity could have been sought hereunder by such Indemnified Party, unless such judgment or
settlement includes an unconditional release of such Indemnified Party from all liability arising out of such claim or proceeding.
4.4 Contribution.
4.4.1 If
the indemnification provided for in the foregoing Sections 4.1, 4.2 and 4.3 is unavailable to any Indemnified Party in respect of any
loss, claim, damage, liability or action referred to herein, then each such Indemnifying Party, in lieu of indemnifying such Indemnified
Party, shall contribute to the amount paid or payable by such Indemnified Party as a result of such loss, claim, damage, liability or
action in such proportion as is appropriate to reflect the relative fault of the Indemnified Parties and the Indemnifying Parties in
connection with the actions or omissions which resulted in such loss, claim, damage, liability or action, as well as any other relevant
equitable considerations. The relative fault of any Indemnified Party and any Indemnifying Party shall be determined by reference to,
among other things, whether the untrue or alleged untrue statement of a material fact or the omission or alleged omission to state a
material fact relates to information supplied by such Indemnified Party or such Indemnifying Party and the parties’ relative intent,
knowledge, access to information and opportunity to correct or prevent such statement or omission.
4.4.2 The
parties hereto agree that it would not be just and equitable if contribution pursuant to this Section 4.4 were determined by pro rata
allocation or by any other method of allocation which does not take account of the equitable considerations referred to in the immediately
preceding Section 4.4.1.
4.4.3 The
amount paid or payable by an Indemnified Party as a result of any loss, claim, damage, liability or action referred to in the immediately
preceding paragraph shall be deemed to include, subject to the limitations set forth above, any legal or other expenses incurred by such
Indemnified Party in connection with investigating or defending any such action or claim. Notwithstanding the provisions of this Section
4.4, no holder of Registrable Securities shall be required to contribute any amount in excess of the dollar amount of the net proceeds
(after payment of any underwriting fees, discounts, commissions or taxes) actually received by such holder from the sale of Registrable
Securities which gave rise to such contribution obligation. No person guilty of fraudulent misrepresentation (within the meaning of Section
11(f) of the Securities Act) shall be entitled to contribution from any person who was not guilty of such fraudulent misrepresentation.
4.5 Survival.
The indemnification provided for under this Agreement shall remain in full force and effect regardless of any investigation made by or
on behalf of the Indemnified Party or any officer, director or controlling person of such Indemnified Party and shall survive the transfer
of securities.
5. RULE
144.
5.1 Rule
144. The Purchaser covenants that it shall file any reports required to be filed by it under the Securities Act and the Exchange
Act and shall take such further action as the holders of Registrable Securities may reasonably request, all to the extent required from
time to time to enable such holders to sell Registrable Securities without registration under the Securities Act within the limitation
of the exemptions provided by Rule 144 under the Securities Act, as such Rules may be amended from time to time, or any similar rule
or regulation hereafter adopted by the Commission.
6. MISCELLANEOUS.
6.1 Other
Registration Rights. The Purchaser represents and warrants that no person, other than the holders of the Registrable Securities,
has any right to require the Purchaser to register any shares of the Purchaser’s capital stock for sale or to include shares of
the Purchaser’s capital stock in any registration filed by the Purchaser for the sale of shares of capital stock for its own account
or for the account of any other person. Further, the Purchaser represents and warrants that this Agreement supersedes any other registration
rights agreement or agreement with similar terms and conditions and in the event of a conflict between any such agreement or agreements
and this Agreement, the terms of this Agreement shall prevail.
6.2 Assignment;
No Third Party Beneficiaries. This Agreement and the rights, duties and obligations of the Purchaser hereunder may not be assigned
or delegated by the Purchaser in whole or in part. This Agreement and the rights, duties and obligations of the holders of Registrable
Securities hereunder may be freely assigned or delegated by such holder of Registrable Securities in conjunction with and to the extent
of any transfer of Registrable Securities by any such holder. This Agreement and the provisions hereof shall be binding upon and shall
inure to the benefit of each of the parties, to the permitted assigns of the Holders or holder of Registrable Securities or of any assignee
of the Holders or holder of Registrable Securities. This Agreement is not intended to confer any rights or benefits on any persons that
are not party hereto other than as expressly set forth in Article 4 and this Section 6.2.
6.3 Notices.
All notices, demands, requests, consents, approvals or other communications (collectively, “Notices” ) required
or permitted to be given hereunder or which are given with respect to this Agreement shall be in writing and shall be personally served,
delivered by reputable air courier service with charges prepaid, or transmitted by hand delivery, telegram, telex or facsimile, addressed
as set forth below, or to such other address as such party shall have specified most recently by written notice. Notice shall be deemed
given on the date of service or transmission if personally served or transmitted by telegram, telex or facsimile; provided, that if such
service or transmission is not on a business day or is after normal business hours, then such notice shall be deemed given on the next
business day. Notice otherwise sent as provided herein shall be deemed given on the next business day following timely delivery of such
notice to a reputable air courier service with an order for next-day delivery.
To
the Purchaser after the Closing:
OneMedNet
Corporation
6385
Old Shady Oak Road
Suite
250
Eden
Prairie, Minnesota 55344
Attn:
Paul Casey
Telephone
No.: (808) 228-5998
E-mail:
paul.casey@onemednet.com
with
a copy to:
Rimôn,
P.C.
1990
K Street NW, Suite 420
Washington,
D.C. 20006
Attn:
Debbie Klis
Telephone:
(202) 971-9494
E-mail:
debbie.klis@rimonlaw.com
To
a Holder, to the address set forth below such Holder’s name on Exhibit A hereto.
6.4 Severability.
This Agreement shall be deemed severable, and the invalidity or unenforceability of any term or provision hereof shall not affect the
validity or enforceability of this Agreement or of any other term or provision hereof. Furthermore, in lieu of any such invalid or unenforceable
term or provision, the parties hereto intend that there shall be added as a part of this Agreement a provision as similar in terms to
such invalid or unenforceable provision as may be possible that is valid and enforceable.
6.5 Counterparts.
This Agreement may be executed in multiple counterparts, each of which shall be deemed an original, and all of which taken together shall
constitute one and the same instrument.
6.6 Entire
Agreement. This Agreement (including all agreements entered into pursuant hereto and all certificates and instruments delivered pursuant
hereto and thereto) constitute the entire agreement of the parties with respect to the subject matter hereof and supersede all prior
and contemporaneous agreements, representations, understandings, negotiations and discussions between the parties, whether oral or written,
including but not limited to the Existing Agreements.
6.7 Modifications
and Amendments; Termination. No amendment, modification or termination of this Agreement shall be binding upon the Purchaser unless
executed in writing by the Purchaser. No amendment, modification or termination of this Agreement shall be binding upon the holders of
the Registrable Securities unless executed in writing by the holders of the majority Registrable Securities. This Agreement shall terminate
with respect to any Holder on the date that such Holder no longer holds any Registrable Securities. The provisions of Article IV shall
survive any termination.
6.8 Titles
and Headings. Titles and headings of sections of this Agreement are for convenience only and shall not affect the construction of
any provision of this Agreement.
6.9 Waivers
and Extensions. Any party to this Agreement may waive any right, breach or default which such party has the right to waive, provided
that such waiver will not be effective against the waiving party unless it is in writing, is signed by such party, and specifically refers
to this Agreement. Waivers may be made in advance or after the right waived has arisen or the breach or default waived has occurred.
Any waiver may be conditional. No waiver of any breach of any agreement or provision herein contained shall be deemed a waiver of any
preceding or succeeding breach thereof nor of any other agreement or provision herein contained. No waiver or extension of time for performance
of any obligations or acts shall be deemed a waiver or extension of the time for performance of any other obligations or acts.
6.10 Governing
Law. This Agreement shall be governed by, interpreted under, and construed in accordance with the internal laws of the State of Delaware
applicable to agreements made and to be performed within the State of Delaware, without giving effect to any choice-of-law provisions
thereof that would compel the application of the substantive laws of any other jurisdiction.
6.11 Waiver
of Trial by Jury. Each party hereby irrevocably and unconditionally waives the right to a trial by jury in any action, suit, counterclaim
or other proceeding (whether based on contract, tort or otherwise) arising out of, connected with or relating to this Agreement, the
transactions contemplated hereby, or the actions of the Holder in the negotiation, administration, performance or enforcement hereof.
6.12 Termination
of Existing Agreements. The Existing Agreements are hereby terminated in their entirety.
[REMAINDER
OF PAGE INTENTIONALLY LEFT BLANK]
IN
WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be executed and delivered by their duly authorized representatives
as of the date first written above.
| |
PURCHASER: |
| |
|
| |
DATA
KNIGHTS ACQUISITION CORP. |
| |
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
| |
|
| |
HOLDERS: |
| |
|
| |
[_______________________] |
Exhibit
A
Schedule
of Holders
Exhibit
10.3
FORM
OF LOCK-UP AGREEMENT
THIS
LOCK-UP AGREEMENT (this “Agreement”) is made and entered into as of the Closing Date (as defined in the Merger
Agreement, as defined below) by and between (i) Data Knights Acquisition Corp., a Delaware corporation (including any successor
entity thereto, the “Purchaser”), and (ii) _______________ (the “Subject Party”).
Any capitalized term used but not defined in this Agreement will have the meaning ascribed to such term in the Merger Agreement.
WHEREAS,
on April 25, 2022, (i) the Purchaser, (ii) Data Knights Merger Sub, Inc., a Delaware corporation and a wholly-owned subsidiary of the
Purchaser (“Merger Sub”), (iii) Data Knights, LLC, a Delaware limited liability company (the “Purchaser
Representative”), (iv) Paul Casey (the “Seller Representative”), and (v) OneMedNet Corporation,
a Delaware corporation (the “Company”) entered into that certain Agreement and Plan of Merger (as amended from
time to time in accordance with the terms thereof, the “Merger Agreement”), pursuant to which the parties thereto
intend to effect the merger of Merger Sub with and into the Company, with the Company continuing as the surviving entity (the “Merger”),
as a result of which all of the issued and outstanding capital stock of the Company immediately prior to the Effective Time shall be
exchanged for the Stockholder Merger Consideration, all upon the terms and subject to the conditions set forth in this Agreement;
WHEREAS,
pursuant to the Merger Agreement, and in view of the valuable consideration to be received by the Subject Party thereunder, the parties
desire to enter into this Agreement, pursuant to which the Purchaser Common Stock received by the Subject Party in the Merger (all such
securities, together with any securities paid as dividends or distributions with respect to such securities or into which such securities
are exchanged or converted, the “Restricted Securities”) shall become subject to limitations on disposition
as set forth herein.
NOW,
THEREFORE, in consideration of the premises set forth above, which are incorporated into this Agreement as if fully set forth below,
and intending to be legally bound hereby, the parties hereby agree as follows:
1.
Lock-Up Provisions.
(a)
The Subject Party hereby agrees not to, during the period commencing from the Closing and ending on the earliest of (x) six (6) months
after the date of the Closing and (y) the date after the Closing on which the Purchaser consummates a liquidation, merger, capital stock
exchange, reorganization, or other similar transaction with an unaffiliated third party that results in all of the Purchaser’s
stockholders having the right to exchange their shares of the Purchaser Common Stock for cash, securities, or other property (the “Lock-Up
Period”): (i) lend, offer, pledge, hypothecate, encumber, donate, assign, sell, contract to sell, sell any option or contract
to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose
of, directly or indirectly, any Restricted Securities, (ii) enter into any swap or other arrangement that transfers to another, in whole
or in part, any of the economic consequences of ownership of the Restricted Securities, or (iii) publicly disclose the intention to do
any of the foregoing, whether any such transaction described in clauses (i), (ii), or (iii) above is to be settled by delivery of Restricted
Securities or other securities, in cash or otherwise (any of the foregoing described in clauses (i), (ii), or (iii), a “Prohibited
Transfer”).
(b)
The foregoing shall not apply to the transfer of any or all of the Restricted Securities (I) to any Permitted Transferee or (II) pursuant
to a court order or settlement agreement related to the distribution of assets in connection with the dissolution of marriage or civil
union; provided, however, that in either of cases (I) or (II), it shall be a condition to such transfer that such transfer complies with
the Securities Act of 1933, as amended, and other applicable law, and that the transferee executes and delivers to the Purchaser an agreement
stating that the transferee is receiving and holding the Restricted Securities subject to the provisions of this Agreement applicable
to the Subject Party, and there shall be no further transfer of such Restricted Securities except in accordance with this Agreement.
As used in this Agreement, the term “Permitted Transferee” shall mean: (1) the members of the Subject Party’s
immediate family (for purposes of this Agreement, “immediate family” shall mean with respect to any natural person, any of
the following: such person’s spouse or domestic partner, the siblings of such person and his or her spouse or domestic partner,
and the direct descendants and ascendants (including adopted and step children and parents) of such person and his or her spouses or
domestic partners and siblings), (2) any trust for the direct or indirect benefit of the Subject Party or the immediate family of the
Subject Party, (3) if the Subject Party is a trust, to the trustor or beneficiary of such trust or to the estate of a beneficiary of
such trust, (4) in the case of an entity, officers, directors, general partners, limited partners, members, or stockholders of such entity
that receive such transfer as a distribution, or related investment funds or vehicles controlled or managed by such persons or their
respective affiliates, (5) to any affiliate of the Subject Party, and (6) any transferee whereby there is no change in beneficial ownership.
The Subject Party further agrees to execute such agreements as may be reasonably requested by the Purchaser that are consistent with
the foregoing or that are necessary to give further effect thereto.
(c)
If any Prohibited Transfer is made or attempted contrary to the provisions of this Agreement, such purported Prohibited Transfer shall
be null and void ab initio, and the Purchaser shall refuse to recognize any such purported transferee of the Restricted Securities as
one of its equity holders for any purpose, and shall refuse to record any such purported transfer of the Restricted Securities in the
books of the Company. In order to enforce this Section 1, the Purchaser may impose stop-transfer instructions with respect to
the Restricted Securities of the Subject Party (and Permitted Transferees and assigns thereof) until the end of the Lock-Up Period.
(d)
During the Lock-Up Period, each certificate evidencing any Restricted Securities shall be stamped or otherwise imprinted with a legend
in substantially the following form, in addition to any other applicable legends:
“THE
SECURITIES REPRESENTED BY THIS CERTIFICATE ARE SUBJECT TO RESTRICTIONS ON TRANSFER SET FORTH IN A LOCK-UP AGREEMENT, DATED AS OF [●],
2022, BY AND AMONG THE ISSUER OF SUCH SECURITIES (THE “ISSUER”) AND THE ISSUER’S SECURITY HOLDER NAMED THEREIN, AS
AMENDED. A COPY OF SUCH LOCK-UP AGREEMENT WILL BE FURNISHED WITHOUT CHARGE BY THE ISSUER TO THE HOLDER HEREOF UPON WRITTEN REQUEST.”
(e)
For the avoidance of any doubt, the Subject Party shall retain all of its rights as a stockholder of the Purchaser during the Lock-Up
Period, including the right to vote any Restricted Securities.
(f)
The foregoing notwithstanding, to the extent any Subject Party is granted a release or waiver from the restrictions contained in this
Section 1 prior to the expiration of the Lock-Up Period, then all Subject Parties shall be automatically granted a release or waiver
from the restrictions contained in this Section to the same extent, on substantially the same terms as and on a pro rata basis with,
the Subject Party to which such release or waiver is granted.
2.
Miscellaneous; No Third-Party Beneficiaries.
(a)
Binding Effect; Assignment. This Agreement and all of the provisions herein shall be binding upon and inure to the benefit of
the parties hereto and their respective permitted successors and assigns. This Agreement and all rights and obligations of a party are
personal and may not be transferred or delegated at any time. Notwithstanding the foregoing, the Purchaser may freely assign any or all
of its rights under this Agreement, in whole or in part, to any successor entity (whether by merger, consolidation, equity sale, asset
sale, or otherwise) without obtaining the consent or approval of the Subject Party. This Agreement is intended for the benefit of the
parties hereto and their respective successors and permitted assigns and is not for the benefit of, nor may any provision herein be enforced
by, any other person.
(b)
Third Parties. Nothing contained in this Agreement or in any instrument or document executed by any party in connection with the
transactions contemplated hereby shall create any rights in, or be deemed to have been executed for the benefit of, any person or entity
that is not a party hereto or thereto or a successor or permitted assign of such a party.
(c)
Governing Law; Jurisdiction. This Agreement and any dispute or controversy arising out of or relating to this Agreement shall
be governed by and construed in accordance with the laws of the State of Delaware, without regard to the conflict of law principles thereof.
All Actions arising out of or relating to this Agreement shall be heard and determined exclusively in any state or federal court located
in Wilmington, Delaware (or in any appellate courts thereof) (the “Specified Courts”). Each party hereto hereby
(i) submits to the exclusive jurisdiction of any Specified Court for the purpose of any Action arising out of or relating to this Agreement
brought by any party hereto and (ii) irrevocably waives, and agrees not to assert by way of motion, defense, or otherwise, in any such
Action, any claim that it is not subject personally to the jurisdiction of the above-named courts, that its property is exempt or immune
from attachment or execution, that the Action is brought in an inconvenient forum, that the venue of the Action is improper, or that
this Agreement or the transactions contemplated hereby may not be enforced in or by any Specified Court. Each party agrees that a final
judgment in any Action shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner
provided by Law. Each party irrevocably consents to the service of the summons and complaint and any other process in any other action
or proceeding relating to the transactions contemplated by this Agreement, on behalf of itself, or its property, by personal delivery
of copies of such process to such party at the applicable address set forth in Section 2(f). Nothing in this Section shall affect
the right of any party to serve legal process in any other manner permitted by applicable law.
(d)
WAIVER OF JURY TRIAL. EACH OF THE PARTIES HERETO HEREBY WAIVES TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW ANY RIGHT IT
MAY HAVE TO A TRIAL BY JURY WITH RESPECT TO ANY ACTION DIRECTLY OR INDIRECTLY ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT
OR THE TRANSACTIONS CONTEMPLATED HEREBY. EACH PARTY HERETO (i) CERTIFIES THAT NO REPRESENTATIVE OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY
OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF ANY ACTION, SEEK TO ENFORCE THAT FOREGOING WAIVER AND (ii) ACKNOWLEDGES
THAT IT AND THE OTHER PARTIES HERETO HAVE BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS
IN THIS SECTION.
(e)
Interpretation. The titles and subtitles used in this Agreement are for convenience only and are not to be considered in construing
or interpreting this Agreement. In this Agreement, unless the context otherwise requires: (i) any pronoun used in this Agreement shall
include the corresponding masculine, feminine, or neuter forms, and the singular form of nouns, pronouns, and verbs shall include the
plural and vice versa; (ii) “including” (and with correlative meaning “include”) means including without limiting
the generality of any description preceding or succeeding such term and shall be deemed in each case to be followed by the words “without
limitation”; (iii) the words “herein,” “hereto,” and “hereby” and other words of similar import
in this Agreement shall be deemed in each case to refer to this Agreement as a whole and not to any particular section or other subdivision
of this Agreement; and (iv) the term “or” means “and/or”. The parties have participated jointly in the negotiation
and drafting of this Agreement. Consequently, in the event an ambiguity or question of intent or interpretation arises, this Agreement
shall be construed as if drafted jointly by the parties hereto, and no presumption or burden of proof shall arise favoring or disfavoring
any party by virtue of the authorship of any provision of this Agreement.
(f)
Notices. All notices, consents, waivers, and other communications hereunder shall be in writing and shall be deemed to have been
duly given when delivered (i) in person, (ii) by facsimile or other electronic means, with affirmative confirmation of receipt, (iii)
one Business Day after being sent, if sent by reputable, nationally recognized overnight courier service, or (iv) three (3) Business
Days after being mailed, if sent by registered or certified mail, pre-paid and return receipt requested, in each case to the applicable
party at the following addresses (or at such other address for a party as shall be specified by like notice):
| If
to the Purchaser before the Closing, to: |
with
copies to (which shall not constitute notice): |
| |
|
| Data
Knights Acquisition Corp. |
Nelson
Mullins Riley & Scarborough LLP |
| Unit
G6, Frome Business Park, |
101
Constitution Avenue, NW, Suite 900 |
| Manor
Road, Frome, BA11 4FN, |
Washington,
D.C. 20001 |
| United
Kingdom |
Attn:
Andrew M. Tucker, Esq.
|
| Tel:
+44 203 833 4000 |
Facsimile No.: (202) 689-2860 |
| Email:
barry@dataknightsacuk.com |
Telephone
No.: (202) 689-2987 |
| |
Email:
andy.tucker@nelsonmullins.com |
| |
|
| If
to the Company (or to the Purchaser after the Closing), to: |
with
copies to (which shall not constitute notice): |
| |
|
| OneMedNet
Corporation |
|
| 6385
Old Shady Oak Road |
Rimôn,
P.C. |
| Suite
250 |
1990
K Street NW, Suite 420 |
| Eden
Prairie, Minnesota 55344 |
Washington,
D.C. 20006 |
| Attn:
Paul Casey |
Attn:
Debbie Klis; Debra Vernon |
| Telephone
No.: (808) 228-5998 |
Telephone:
(202) 971-9494; (650) 292-5910 |
| E-mail:
paul.casey@onemednet.com |
E-mail:
debbie.klis@rimonlaw.com; debra.vernon@rimonlaw.com |
If
to the Subject Party, to: the address set forth below the Subject Party’s name on the signature page to this Agreement.
(g)
Amendments and Waivers. Any term of this Agreement may be amended and the observance of any term of this Agreement may be waived
(either generally or in a particular instance, and either retroactively or prospectively) only with the written consent of the Purchaser
and the Subject Party. No failure or delay by a party in exercising any right hereunder shall operate as a waiver thereof. No waivers
of or exceptions to any term, condition, or provision of this Agreement, in any one or more instances, shall be deemed to be or construed
as a further or continuing waiver of any such term, condition, or provision.
(h)
Authorization on Behalf of the Purchaser. The parties acknowledge and agree that notwithstanding anything to the contrary contained
in this Agreement, any and all determinations, actions, or other authorizations under this Agreement on behalf of the Purchaser, including
enforcing the Purchaser’s rights and remedies under this Agreement, or providing any waivers with respect to the provisions hereof,
shall solely be made, taken, and authorized by majority of the disinterested independent directors of the Purchaser’s board of
directors. In the event that the Purchaser at any time does not have any disinterested directors, so long as the Subject Party has any
remaining obligations under this Agreement, the Purchaser will promptly appoint one in connection with this Agreement. Without limiting
the foregoing, in the event that an affiliate of a Subject Party serves as a director, officer, employee, or other authorized agent of
the Purchaser or any of its current or future affiliates, neither the Subject Party nor its affiliate shall have authority, express or
implied, to act or make any determination on behalf of the Purchaser or any of its current or future affiliates in connection with this
Agreement or any dispute or Action with respect hereto.
(i)
Severability. In case any provision in this Agreement shall be held invalid, illegal, or unenforceable in a jurisdiction, such
provision shall be modified or deleted, as to the jurisdiction involved, only to the extent necessary to render the same valid, legal,
and enforceable, and the validity, legality, and enforceability of the remaining provisions hereof shall not in any way be affected or
impaired thereby nor shall the validity, legality, or enforceability of such provision be affected thereby in any other jurisdiction.
Upon such determination that any term or other provision is invalid, illegal, or incapable of being enforced, the parties will substitute
for any invalid, illegal, or unenforceable provision a suitable and equitable provision that carries out, so far as may be valid, legal,
and enforceable, the intent and purpose of such invalid, illegal, or unenforceable provision.
(j)
Specific Performance. Each party acknowledges that its obligations under this Agreement are unique, recognizes and affirms that,
in the event of a breach of this Agreement, money damages will be inadequate and there will be no adequate remedy at law, and agrees
that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with their
specific terms or were otherwise breached. Accordingly, the adversely affected party or parties shall be entitled to an injunction or
restraining order to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof, without the requirement
to post any bond or other security, this being in addition to any other right or remedy available under this Agreement, at law or in
equity.
(k)
Entire Agreement. This Agreement constitutes the full and entire understanding and agreement among the parties with respect to
the subject matter hereof, and any other written or oral agreement relating to the subject matter hereof existing between the parties
is expressly canceled; provided, that, for the avoidance of doubt, the foregoing shall not affect the rights and obligations of
the parties under the Merger Agreement or any Ancillary Document. Notwithstanding the foregoing, nothing in this Agreement shall limit
any of the rights or remedies or any of the obligations of the parties hereto under any other agreement between a Subject Party and the
Purchaser or any certificate or instrument delivered in connection with the Purchase, and nothing in any other agreement, certificate,
or instrument shall limit any of the rights or remedies or any of the obligations under this Agreement.
(l)
Further Assurances. From time to time, at another party’s request and without further consideration (but at the requesting
party’s reasonable cost and expense), each party shall execute and deliver such additional documents and take all such further
action as may be reasonably necessary to consummate the transactions contemplated by this Agreement.
(m)
Counterparts; Facsimile. This Agreement may also be executed and delivered by facsimile signature or by email in portable document
format in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the
same instrument.
[Remainder
of Page Intentionally Left Blank; Signature Pages Follow]
IN
WITNESS WHEREOF, the parties have executed this Lock-Up Agreement as of the date first written above.
| |
The
Purchaser: |
| |
|
|
| |
DATA
KNIGHTS ACQUISITION CORP. |
| |
|
|
| |
By: |
|
| |
Name: |
|
| |
Title: |
|
{Additional
Signatures on the Following Pages}
| The
Subject Party: |
|
| |
|
|
| The
Baba One Irrevocable Trust dated March 26, 2021 |
|
| |
|
|
| By: |
|
|
| Name:
|
Bradley
Overby |
|
| Title:
|
Trustee |
|
| Number of Shares and Type of Purchaser Common Stock: |
|
| |
|
|
| Purchaser
Common Stock: |
|
|
| Address for Notice: |
|
| |
|
|
| Address: |
|
|
| |
|
| |
|
| Attention: |
|
|
| Email: |
|
|
| with a copy (which will not constitute notice) to: |
|
| |
|
| |
|
| |
|
| |
|
| Attn: |
|
|
| Telephone
No.: |
|
|
| Email: |
|
|
| The Subject Party: |
|
| |
|
|
| The Soph One Irrevocable Trust dated March 26, 2021 |
|
| |
|
|
| By: |
|
|
| Name: |
Bradley Overby |
|
| Title: |
Trustee |
|
| Number of Shares and Type of Purchaser Common Stock: |
|
| |
|
|
| Purchaser
Common Stock: |
|
|
| Address for Notice: |
|
| |
|
|
| Address: |
|
|
| |
|
| |
|
| Attention: |
|
|
| Email: |
|
|
| with a copy (which will not constitute notice) to: |
|
| |
|
|
| |
|
| |
|
| |
|
| Attn: |
|
|
| Telephone
No.: |
|
|
| Email: |
|
|
The
Subject Party:
Jeffrey
Yu
| Number
of Shares and Type of Purchaser Common Stock: |
|
| |
|
|
| Purchaser
Common Stock: |
|
|
| Address
for Notice: |
|
| |
|
|
| Address: |
|
|
| |
|
| |
|
| Attention: |
|
|
| Email: |
|
|
| with
a copy (which will not constitute notice) to: |
|
| |
|
| |
|
| |
|
| |
|
| Attn: |
|
|
| Telephone
No.: |
|
|
| Email: |
|
|
| The
Subject Party: |
|
| |
|
|
| The
Revocable Trust of Jerry M. Hiatt dated March 17, 1997, as amended |
|
| |
|
|
| By: |
|
|
| Name: |
Jerry M. Hiatt |
|
| Title: |
Trustee |
|
| Number of Shares and Type of Purchaser Common Stock: |
|
| |
|
|
| Purchaser
Common Stock: |
|
|
| Address
for Notice: |
|
| |
|
|
| Address: |
|
|
| |
|
| |
|
| Attention: |
|
|
| Email: |
|
|
| with
a copy (which will not constitute notice) to: |
|
| |
|
| |
|
| |
|
| |
|
| Attn: |
|
|
| Telephone
No.: |
|
|
| Email: |
|
|
The
Subject Party:
Paul
Casey
| Number
of Shares and Type of Purchaser Common Stock: |
| |
|
|
| Purchaser
Common Stock: |
|
|
| Address
for Notice: |
|
| |
|
|
| Address: |
|
|
| |
|
| |
|
| Attention: |
|
|
| Email: |
|
|
| with
a copy (which will not constitute notice) to: |
|
| |
|
| |
|
| |
|
| |
|
| Attn: |
|
|
| Telephone
No.: |
|
|
| Email: |
|
|
The
Subject Party:
Thomas
Kosasa
| Number
of Shares and Type of Purchaser Common Stock: |
| |
|
|
| Purchaser
Common Stock: |
|
|
| Address
for Notice: |
|
| |
|
|
| Address: |
|
|
| |
|
| |
|
| Attention: |
|
|
| Email: |
|
|
| with
a copy (which will not constitute notice) to: |
|
| |
|
| |
|
| |
|
| |
|
| Attn: |
|
|
| Telephone
No.: |
|
|
| Email: |
|
|
| The
Subject Party: |
|
| |
|
|
| The
Revocable Trust of Jerry M. Hiatt dated March 17, 1997, |
|
| as
amended, as Tenants in Common |
|
| |
|
|
| By: |
|
|
| Name: |
Thomas Kosasa |
|
| Title: |
Trustee |
|
| By: |
|
|
| Name: |
Jerry M. Hiatt |
|
| Title: |
Trustee |
|
| Number
of Shares and Type of Purchaser Common Stock: |
| |
|
|
| Purchaser
Common Stock: |
|
|
| Address
for Notice: |
|
| |
|
|
| Address: |
|
|
| |
|
| |
|
| Attention: |
|
|
| Email: |
|
|
| with
a copy (which will not constitute notice) to: |
|
| |
|
| |
|
| |
|
| |
|
| Attn: |
|
|
| Telephone
No.: |
|
|
| Email: |
|
|
The
Subject Party:
Erkan
Akyuz
| Number
of Shares and Type of Purchaser Common Stock: |
| |
|
|
| Purchaser
Common Stock: |
|
|
| Address
for Notice: |
|
| |
|
|
| Address: |
|
|
| |
|
| |
|
| Attention: |
|
|
| Email: |
|
|
| with
a copy (which will not constitute notice) to: |
|
| |
|
| |
|
| |
|
| |
|
| Attn: |
|
|
| Telephone
No.: |
|
|
| Email: |
|
|
The
Subject Party:
Lisa
Embree
| Number
of Shares and Type of Purchaser Common Stock: |
| |
|
|
| Purchaser
Common Stock: |
|
|
| Address
for Notice: |
|
| |
|
|
| Address: |
|
|
| |
|
| |
|
| Attention: |
|
|
| Email: |
|
|
| with
a copy (which will not constitute notice) to: |
|
| |
|
| |
|
| |
|
| |
|
| Attn: |
|
|
| Telephone
No.: |
|
|
| Email: |
|
|
Exhibit
10.8
OneMedNet
Corporation
April
18, 2023
Dear
Aaron Green,
OneMedNet
Corp. (the “Company”) is pleased to offer you employment on the following terms:
1.
Position. Your title will be President and will have dual reporting to the CEO and the Chairman of the Board. This is a full-time,
exempt position. While you render services to the Company, you will not engage in any other employment, consulting or other business
activity (whether full-time or part-time) that would create a conflict of interest with the Company. By signing this letter agreement,
you confirm to the Company that you have no contractual commitments or other legal obligations that would prohibit you from performing
your duties for the Company.
2.
Cash Compensation. The Company will pay you a starting salary at the rate of $350,000 USD per year, payable in accordance with
the Company’s standard payroll schedule and subject to applicable deductions and withholdings. This salary will be subject to periodic
review and adjustments at the Company’s discretion.
3.
Annual Bonus. Beginning in fiscal year 2023, as pro-rated for the amount of the fiscal year that you are employed, you will also
be eligible to receive an annual cash performance bonus of $175,000 USD upon the achievement of the perfo1mance goals set by the Company’s
CEO and Board of Directors (the “Target Bonus”). The Target Bonus, or any portion thereof, will be paid as soon as
practicable after the Company’s CEO and Board of Directors determines that the applicable performance goals have been achieved,
provided that you must remain an employee of the Company through the date the Target Bonus is paid out in order to receive the Target
Bonus.
4.
Employee Benefits. As an employee of the Company, you will be eligible to receive certain employee benefits. Refer to benefit
plan for further detail. You should note that the Company may modify job titles, salaries and benefits from time to time as it deems
necessary.
5.
Equity. Subject to the approval of the Company’s Board of Directors, you are eligible to receive 600,000 of the company’s
outstanding shares at closing, as part of the Company’s Restricted Stock Unit Plan.
6.
Proprietary Information and Inventions Agreement. Like all Company employees, you will be required, as a condition of your employment
with the Company, to sign the Company’s standard Proprietary Information and Inventions Agreement, a copy of which is attached
hereto as Exhibit A, requires, among other provisions, the assignment of patent rights to any invention made during your employment
at the Company.
Aaron
Green
Page
2
7.
Employment Relationship. Employment with the Company is for no specific period of time. Your employment with the Company will
be “at will,” meaning that either you or the Company may terminate your employment at any time and for any reason, with or
without cause. Any contrary representations that may have been made to you are superseded by this letter agreement. This is the full
and complete agreement between you and the Company on this term. Although your job duties, title, compensation and benefits, as well
as the Company’s personnel policies and procedures, may change from time to time, the “at will” nature of your employment
may only be changed in an express written agreement signed by you and a duly authorized officer of the Company (other than you).
8.
Termination.
a.
In the event that your employment with the Company is terminated by the Company without Cause (as defined below)), or is terminated
by you for Good Reason (as defined below), after six months of employment, and you sign and do not revoke a standard release of claims
with the Company in a form reasonably satisfactory to the Company’s Board of Directors (a “Release”), which
Release becomes irrevocable no later than sixty (60) days (the “Release Deadline”) after the date of your termination
of employment (the “Termination Date”) you will be entitled to the following severance payment (“Severance”):
i.
If the Termination Date is after 6 months’ of employment, but before you have completed 12 months’ of employment, you will
receive 3 months’ salary;
ii.
If the Termination Date is after 12 months’ employment you will receive 6 months’ salary.
If
the Release does not become effective and irrevocable by the Release Deadline, you will forfeit any right to Severance. In no event will
Severance be paid or provided hereunder until the Release actually becomes effective and irrevocable.
The
following terms referred to in this offer letter shall have the following meanings:
“Cause”
shall mean: (i) your material failure to perform your stated duties, and your continued failure to cure such failure to the reasonable
satisfaction of the Company within ten (10) days following written notice of such failure to you from the Company’s Board of Directors;
(ii) your material violation of a written Company policy (including any insider trading policy) or any written agreement or covenant
with the Company; (iii) your conviction of, or entry of a plea of guilty or nolo contendere to, a felony (other than motor vehicle
offenses the effect of which do not materially impair your performance of your employment duties); (iv) a willful act by you that constitutes
gross misconduct and which is injurious to the Company; (v) your commission of any act of fraud, embezzlement, dishonesty or any other
willful misconduct that has caused or is reasonably expected to result in material injury to the Company; (vi) the unauthorized use or
disclosure by you of any proprietary information or trade secrets of the Company or any other party to whom you owe an obligation of
nondisclosure as a result of your relationship with the Company; or your willful failure to cooperate with an investigation by a governmental
authority.
Aaron
Green
Page
3
“Good
Reason” shall mean your voluntary termination, within thirty (30) days following the expiration of any Company cure period
(discussed below) following the occurrence of one or more of the following, without your consent: (i) a material reduction of your duties,
authority, or responsibilities, relative to your title, duties, authority, or responsibilities as in effect immediately prior to such
reduction; provided that your title, duties, authority and responsibilities will not be deemed to be materially reduced if you have reasonably
comparable duties, authorities and responsibilities as an employee with respect to the Company’s business following a Change of
Control, regardless of any change in title or whether you provide services to a subsidiary, affiliate, business unit, division or otherwise;
(ii) a material reduction by the Company of your annual base salary, except to the extent the base salaries of all other senior executives
of the Company are similarly reduced; (iii) a material change in the location of the performance of your work, required by the Company,
of more than fifty (50) miles from your principal place of residence. You may not resign for Good Reason without first providing the
Company with written notice within ninety (90) days of the initial existence of the condition that you believe constitutes Good Reason
specifically identifying the acts or omissions constituting the grounds for Good Reason and a reasonable cure period of not less than
thirty (30) days following the date of such notice.
9.
Tax Matters.
a.
Withholding. All forms of compensation referred to in this letter agreement are subject to reduction to reflect applicable withholding
and payroll taxes and other deductions required by law.
b.
Tax Advice. You are encouraged to obtain your own tax advice regarding your compensation from the Company. You agree that the Company
does not have a duty to design its compensation policies in a manner that minimizes your tax liabilities, and you will not make any claim
against the Company or its Board of Directors related to tax liabilities arising from your compensation.
10.
Interpretation, Amendment and Enforcement. This letter agreement and Exhibit A constitute the complete agreement between you and
the Company, contain all of the terms of your employment with the Company and supersede any prior agreements, representations or understandings
(whether written, oral or implied) between you and the Company. This letter agreement may not be amended or modified, except by an express
written agreement signed by both you and a duly authorized officer of the Company. The terms of this letter agreement and the resolution
of any disputes as to the meaning, effect, performance or validity of this letter agreement or arising out of, related to, or in any
way connected with, this letter agreement, your employment with the Company or any other relationship between you and the Company (the
“Disputes”) will be governed by Delaware law, excluding laws relating to conflicts or choice of law. You and the Company
submit to the exclusive personal jurisdiction of the federal and state courts located in Delaware in connection with any Dispute or any
claim related to any Dispute.
Aaron
Green
Page
4
11.
We also ask that, if you have not already done so, you disclose to the Company any and all agreements relating to your prior employment
that may affect your eligibility to be employed by the Company or limit the manner in which you may be employed. It is the Company’s
understanding that any such agreements will not prevent you from performing the duties of your position and you represent that such is
the case. Moreover, you agree that, during the term of your employment with the Company, you will not engage in any other employment,
occupation, consulting or other business activity directly related to the business in which the Company is now involved or becomes involved
during the term of your employment, nor will you engage in any other activities that conflict with your obligations to the Company. Similarly,
you agree not to bring any third-party confidential information to the Company, including that of your former employer, and that in performing
your duties for the Company you will not in any way utilize any such information.
12.
As a condition of your employment, you are also required to sign and comply with the Company’s standard Employee Non-Disclosure
Agreement.
We
hope that you will accept our offer to join the Company. You may indicate your agreement with these terms and accept this offer by signing
and dating both the enclosed duplicate original of this letter agreement and the enclosed Proprietary Information and Inventions Agreement
and returning them to me. This offer, if not accepted, will expire at the close of business on April 30, 2023. As required by
law, your employment with the Company is contingent upon your providing legal proof of your identity and authorization to work in the
United States. Your employment is also contingent upon successful background check and your starting work with the Company on May 23,
2023.
If
you have any questions, please let me know.
Very
truly yours,
| /s/ Paul
Casey |
|
|
| Paul Casey |
|
|
| Chief Executive Officer |
|
|
I
have read and accept this employment offer:
| /s/ Aaron Green |
|
|
| Aaron Green |
|
|
| Dated: May 7, 2023 |
|
|
Attachment
Exhibit
A: Proprietary Information and Inventions Agreement
Aaron
Green
Page
6
Exhibit
A
Proprietary
Information and Inventions Agreement
Aaron
Green
Page
7
PROPRIETARY
INFORMATION AND INVENTIONS AGREEMENT
The
following confirms and memorializes an agreement that COMPANY NAME INC, Inc., a Delaware corporation (the “Company”)
and I have had since the commencement of my employment (which term, for purposes of this agreement, shall be deemed to include any relationship
of service to the Company that I may have had prior to actually becoming an employee) with the Company in any capacity and that is and
has been a material part of the consideration for my employment by Company:
1.
I have not entered into, and I agree I will not enter into, any agreement either written or oral in conflict with this Agreement or my
employment with Company. I will not violate any agreement with or rights of any third party or, except as expressly authorized by Company
in writing hereafter, use or disclose my own or any third party’s confidential information or intellectual property when acting
within the scope of my employment or otherwise on behalf of Company. Further, I have not retained anything containing any confidential
information of a prior employer or other third party, whether or not created by me.
2.
Company shall own all right, title and interest (including patent rights, copyrights, trade secret rights, mask work rights, sui generis
database 1ights and all other intellectual property rights of any sort throughout the world) relating to any and all inventions (whether
or not patentable), works of authorship, mask works, designs, know-how, ideas and information made or conceived or reduced to practice,
in whole or in part, by me during the term of my employment with Company to and only to the fullest extent allowed (which is attached
as Appendix A) (collectively “Inventions”) and I will promptly disclose all Inventions to Company. Without disclosing
any third party confidential information, I will also disclose anything I believe is excluded so that the Company can make an independent
assessment. I hereby make all assignments necessary to accomplish the foregoing. I shall further assist Company, at Company’s expense,
to further evidence, record and perfect such assignments, and to perfect, obtain, maintain, enforce, and defend any rights specified
to be so owned or assigned. I hereby irrevocably designate and appoint Company as my agent and attorney-in-fact, coupled with an interest
and with full power of substitution, to act for and in my behalf to execute and file any document and to do all other lawfully permitted
acts to further the purposes of the foregoing with the same legal force and effect as if executed by me. If I wish to clarify that something
created by me prior to my employment that relates to Company’s actual or proposed business is not within the scope of the foregoing
assignment, I have listed it on Appendix B in a manner that does not violate any third party rights or disclose any confidential
information. Without limiting Section 1 or Company’s other rights and remedies, if, when acting within the scope of my employment
or otherwise on behalf of Company, I use or (except pursuant to this Section 2) disclose my own or any third party’s confidential
information or intellectual property (or if any Invention cannot be fully made, used, reproduced, distributed and otherwise exploited
without using or violating the foregoing), Company will have and I hereby grant Company a perpetual, i1Tevocable, worldwide, royalty-free,
non-exclusive, sublicensable right and license to exploit and exercise all such confidential information and intellectual property rights.
Aaron
Green
Page
8
3.
To the extent allowed by law, paragraph 2 includes all rights of paternity, integrity, disclosure and withdrawal and any other rights
that may be known as or referred to as “moral rights,” “artist’s rights,” “droit moral,” or
the like (collectively “Moral Rights”). To the extent I retain any such Moral Rights under applicable law, I hereby ratify
and consent to any action that may be taken with respect to such Moral Rights by or authorized by Company and agree not to assert any
Moral Rights with respect thereto. I will confirm any such ratifications, consents and agreements from time to time as requested by the
Company.
4.
I agree that all Inventions and all other business, technical and financial information (including, without limitation, the identity
of and information relating to customers or employees) I develop, learn or obtain during the term of my employment that relate to Company
or the business or demonstrably anticipated business of Company or that are received by or for Company in confidence, constitute “Proprietary
Information.” I will hold in confidence and not disclose or, except within the scope of my employment, use any Proprietary Information.
However, I shall not be obligated under this paragraph with respect to information I can document is or becomes readily publicly available
without restriction through no fault of mine. Upon termination of my employment, I will promptly return to Company all items containing
or embodying Proprietary Information (including all copies), except that I may keep my personal copies of (i) my compensation records,
(ii) materials distributed to shareholders generally and (iii) this Agreement. I also recognize and agree that I have no expectation
of privacy with respect to Company’s telecommunications, networking or infom1ation processing systems (including, without limitation,
stored computer files, embedded messages and voice messages) and that my activity and any files or messages on or using any of those
systems may be monitored at any time without notice.
5.
Until one year after the term of my employment, I will not encourage or solicit any employee or consultant of Company to leave
Company for any reason (except for the bona fide firing of Company personnel within the scope of my employment).
6.
I agree that during the term of my employment with Company (whether or not during business hours), I will not engage in any activity
that is in any way competitive with the business or demonstrably anticipated business of Company, and I will not assist any other person
or organization in competing or in preparing to compete with any business or demonstrably anticipated business of Company.
7.
I agree that this Agreement is not an employment contract for any particular term and that I have the right to resign and the Company
has the right to terminate my employment at will, at any time, for any or no reason, with or without cause. In addition, this Agreement
does not purport to set forth all of the terms and conditions of my employment, and, as an employee of Company, I have obligations to
Company which are not set forth in this Agreement. However, the terms of this Agreement govern over any inconsistent terms and can only
be changed by a subsequent written agreement signed by the President of the Company.
8.
I agree that my obligations under paragraphs 2, 3, 4 and 5 of this Agreement shall continue in effect after termination of my employment,
regardless of the reason or reasons for termination, and whether such termination is voluntary or involuntary on my part, and that Company
is entitled to communicate my obligations under this Agreement to any future employer or potential employer of mine. My obligations under
paragraphs 2, 3 and 4 also shall be binding upon my heirs, executors, assigns, and administrators and shall insure to the benefit of
Company, its subsidiaries, successors and assigns.
Aaron
Green
Page
9
9.
Any dispute in the meaning, effect or validity of this Agreement shall be resolved in accordance with the laws of the State of
Delaware without regard to the conflict of laws provisions thereof. I further agree that if one or more provisions of this Agreement
are held to be illegal or unenforceable under applicable law, such illegal or unenforceable portion(s) shall be limited or excluded
from this Agreement to the minimum extent required so that this Agreement shall otherwise remain in full force and effect and
enforceable in accordance with its te1ms. This Agreement is fully assignable and transferable by Company, but any purported
assignment or transfer by me is void. I also understand that any breach of this Agreement will cause irreparable harm to Company for
which damages would not be an adequate remedy, and, therefore, Company will be entitled to injunctive relief with respect thereto in
addition to any other remedies and without any requirement to post bond.
10.
I acknowledge receipt of the following notice under 18 U.S.C § 1833(b)(l): “An individual shall not be held criminally
or civilly liable under any Federal or State trade secret law for the disclosure of a trade secret that (A) is made (i) in confidence
to a Federal, State, or local government official, either directly or indirectly, or to an attorney; and (ii) solely for the purpose
of reporting or investigating a suspected violation of law; or (B) is made in a complaint or other document filed in a lawsuit or other
proceeding, if such filing is made under seal.”
Remainder
of Page Intentionally Left Blank
Aaron
Green
Page
10
I
HAVE READ THIS AGREEMENT CAREFULLY AND I UNDERSTAND AND ACCEPT THE OBLIGATIONS WHICH IT IMPOSES UPON ME WITHOUT RESERVATION. NO PROMISES
OR REPRESENTATIONS HAVE BEEN MADE TO ME TO INDUCE ME TO SIGN THIS AGREEMENT. I SIGN THIS AGREEMENT VOLUNTARILY AND FREELY, IN
DUPLICATE, WITH THE UNDERSTANDING THAT THE COMPANY WILL RETURN ONE COUNTERPART AND THE OTHER COUNTERPART WILL BE RETAINED BYME.
Employee
| /s/ Aaron Green |
|
|
| Signature |
|
|
| May 7, 2023 |
|
|
| Aaron Green |
|
|
| Accepted and Agreed to: OneMedNet Corp. |
|
|
| |
|
|
|
| By |
/s/
Paul Casey |
|
|
| |
Paul Casey |
|
|
Aaron
Green
Page
11
APPENDIX
A
Application
of provision providing that employee shall assign or offer to assign rights in invention to employer.
(a)
Any provision in an employment agreement which provides that an employee shall assign, or offer to assign, any of his or her rights in
an invention to his or her employer shall not apply to an invention that the employee developed entirely on his or her own time without
using the employer’s equipment, supplies, facilities, or trade secret information except for those inventions that either:
1.
Relate at the time of conception or reduction to practice of the invention to the employer’s business, or actual or demonstrably
anticipated research or development of the employer; or
2.
Result from any work performed by the employee for his employer.
(b)
To the extent a provision in an employment agreement purports to require an employee to assign an invention otherwise excluded from being
required to be assigned under subdivision (a), the provision is against the public policy of this state and is unenforceable.
Aaron
Green
Page
12
APPENDIX
B
PRIOR
MATTER
Exhibit
10.9
OneMedNet
Corporation
October
19, 2023
Dear
Lisa Embree,
OneMedNet
Corp. (the “Company”) is pleased to offer you employment on the following terms:
1.
Position. Your title will be Chief Financial Officer, reporting to the CEO. This is a full-time, exempt position. While you render
services to the Company, you will not engage in any other employment, consulting or other business activity (whether full-time or part-time)
that would create a conflict of interest with the Company. By signing this letter agreement, you confirm to the Company that you have
no contractual commitments or other legal obligations that would prohibit you from performing your duties for the Company.
2.
Cash Compensation. The Company will pay you a starting salary at the rate of $225,000 USO per year, payable in accordance with
the Company’s standard payroll schedule and subject to applicable deductions and withholdings. This salary will be subject to periodic
review and adjustments at the Company’s discretion.
3.
Annual Bonus. You will also be eligible to receive an annual cash performance bonus of 25% of your annual salary based on achievement
of the performance goals set by the Company’s CEO. The Target Bonus, or any portion thereof, will be paid as soon as practicable
after the Company’s CEO and Board of Directors determines that the applicable performance goals have been achieved, provided that
you must remain an employee of the Company through the date the Target Bonus is paid out in order to receive the Target Bonus.
4.
Employee Benefits. As an employee of the Company, you will be eligible to receive certain employee benefits. Refer to benefit
plan for further detail. You should note that the Company may modify job titles, salaries and benefits from time to time as it deems
necessary.
5.
Equity. Subject to the approval of the Company’s Board of Directors, you are eligible to receive 260,000 of the company’s
outstanding shares at closing, as part of the Company’s Restricted Stock Unit Plan.
6.
Proprietary Information and Inventions Agreement. Like all Company employees, you will be required, as a condition of your employment
with the Company, to sign the Company’s standard Proprietary Information and Inventions Agreement, a copy of which is attached
hereto as Exhibit A, requires, among other provisions, the assignment of patent rights to any invention made during your employment
at the Company.
Lisa
Embree
Page
2
7.
Employment Relationship. Employment with the Company is for no specific period of time. Your employment with the Company will
be “at will,” meaning that either you or the Company may terminate your employment at any time and for any reason, with or
without cause. Any contrary representations that may have been made to you are superseded by this letter agreement. This is the full
and complete agreement between you and the Company on this term. Although your job duties, title, compensation and benefits, as well
as the Company’s personnel policies and procedures, may change from time to time, the “at will” nature of your employment
may only be changed in an express written agreement signed by you and a duly authorized officer of the Company (other than you).
8.
Termination.
In
the event that your employment with the Company is terminated by the Company without Cause (as defined below) or is terminated by you
for Good Reason (as defined below). After the date of your termination of employment (the “Termination Date”) you
will receive 6 months’ salary as a Severance Payment.
If
the Release does not become effective and irrevocable by the Release Deadline, you will forfeit any right to Severance. In no event will
Severance be paid or provided hereunder until the Release becomes effective and irrevocable.
The
following terms referred to in this offer letter shall have the following meanings:
“Cause”
shall mean: (i) your material failure to perform your stated duties, and your continued failure to cure such failure to the reasonable
satisfaction of the Company within ten (10) days following written notice of such failure to you from the Company’s Board of Directors;
(ii) your material violation of a written Company policy (including any insider trading policy) or any written agreement or covenant
with the Company; (iii) your conviction of, or entry of a plea of guilty or nolo contendere to, a felony (other than motor vehicle
offenses the effect of which do not materially impair your performance of your employment duties); (iv) a willful act by you that constitutes
gross misconduct and which is injurious to the Company; (v) your commission of any act of fraud, embezzlement, dishonesty or any other
willful misconduct that has caused or is reasonably expected to result in material injury to the Company; (vi) the unauthorized use or
disclosure by you of any proprietary information or trade secrets of the Company or any other party to whom you owe an obligation of
nondisclosure as a result of your relationship with the Company; or your willful failure to cooperate with an investigation by a governmental
authority.
Lisa
Embree
Page
3
“Good
Reason” shall mean your voluntary termination, within thirty (30) days following the expiration of any Company cure period
(discussed below) following the occurrence of one or more of the following, without your consent: (i) a material reduction of your duties,
authority, or responsibilities, relative to your title, duties, authority, or responsibilities as in effect immediately prior to such
reduction; provided that your title, duties, authority and responsibilities will not be deemed to be materially reduced if you have reasonably
comparable duties, authorities and responsibilities as an employee with respect to the Company’s business following a Change of
Control, regardless of any change in title or whether you provide services to a subsidiary, affiliate, business unit, division or otherwise;
(ii) a material reduction by the Company of your annual base salary, except to the extent the base salaries of all other senior executives
of the Company are similarly reduced; (iii) a material change in the location of the performance of your work, required by the Company,
of more than fifty (50) miles from your principal place of residence. You may not resign for Good Reason without first providing the
Company with written notice within ninety (90) days of the initial existence of the condition that you believe constitutes Good Reason
specifically identifying the acts or omissions constituting the grounds for Good Reason and a reasonable cure period of not less than
thirty (30) days following the date of such notice.
9.
Tax Matters.
a.
Withholding. All forms of compensation referred to in this letter agreement are subject to reduction to reflect applicable withholding
and payroll taxes and other deductions required by law.
b.
Tax Advice. You are encouraged to obtain your own tax advice regarding your compensation from the Company. You agree that the
Company does not have a duty to design its compensation policies in a manner that minimizes your tax liabilities, and you will not make
any claim against the Company or its Board of Directors related to tax liabilities arising from your compensation.
10.
Interpretation, Amendment and Enforcement. This letter agreement and Exhibit A constitute the complete agreement between you and
the Company, contain all of the terms of your employment with the Company and supersede any prior agreements, representations or understandings
(whether written, oral or implied) between you and the Company. This letter agreement may not be amended or modified, except by an express
written agreement signed by both you and a duly authorized officer of the Company. The terms of this letter agreement and the resolution
of any disputes as to the meaning, effect, performance or validity of this letter agreement or arising out of, related to, or in any
way connected with, this letter agreement, your employment with the Company or any 0th.er relationship between you and the Company (the
“Disputes”) will be governed by Delaware law, excluding laws relating to conflicts or choice of law. You and the Company
submit to the exclusive personal jurisdiction of the federal and state courts located in Delaware in connection with any Dispute or any
claim related to any Dispute.
Lisa
Embree
Page
4
11.
We also ask that, if you have not already done so, you disclose to the Company any and all agreements relating to your prior employment
that may affect your eligibility to be employed by the Company or limit the manner in which you may be employed. It is the Company’s
understanding that any such agreement will not prevent you from performing the duties of your position and you represent that such is
the case. Moreover, you agree that, during the term of your employment with the Company, you will not engage in any other employment,
occupation, consulting or other business activity directly related to the business in which the Company is now involved or becomes involved
during the term of your employment, nor will you engage in any other activities that conflict with your obligations to the Company. Similarly,
you agree not to bring any third-party confidential information to the Company, including that of your former employer, and that in performing
your duties for the Company you will not in any way utilize any such information.
12.
As a condition of your employment, you are also required to sign and comply with the Company’s standard Employee Non-Disclosure
Agreement.
We
hope that you will accept our offer to join the Company. You may indicate your agreement with these terms and accept this offer by signing
and dating both the enclosed duplicate original of this letter agreement and the enclosed Proprietary Information and Inventions Agreement
and returning them to me. This offer, if not accepted, will expire at the close of business on October 31, 2023. As required by
law, your employment with the Company is contingent upon your providing legal proof of your identity and authorization to work in the
United States.
If
you have any questions, please let me know. Very truly
yours,
| /s/
Paul Casey |
|
| Paul
Casey |
|
| Chief
Executive Officer |
|
I
have read and accept this employment offer:
| /s/
Lisa Embree |
|
| Lisa
Embree |
|
Lisa
Embree
Page
5
PROPRIETARY
INFORMATION AND INVENTIONS AGREEMENT
The
following confirms and memorializes an agreement that COMPANY NAME INC, Inc., a Delaware corporation (the “Company”)
and I have had since the commencement of my employment (which term, for purposes of this agreement, shall be deemed to include any relationship
of service to the Company that I may have had prior to actually becoming an employee) with the Company in any capacity and that is and
has been a material part of the consideration for my employment by Company:
1.
I have not entered into, and I agree I will not enter into, any agreement either written or oral in conflict with this Agreement or my
employment with Company. I will not violate any agreement with or rights of any third party or, except as expressly authorized by Company
in writing hereafter, use or disclose my own or any third party’s confidential information or intellectual property when acting
within the scope of my employment or otherwise on behalf of Company. Further, I have not retained anything containing any confidential
information of a prior employer or other third party, whether or not created by me.
2.
Company shall own all right, title and interest (including patent rights, copyrights, trade secret rights, mask work rights, sui generis
database rights and all other intellectual property rights of any sort throughout the world) relating to any and all inventions (whether
or not patentable), works of authorship, mask works, designs, know-how, ideas and information made or conceived or reduced to practice,
in whole or in part, by me during the term of my employment with Company to and only to the fullest extent allowed (which is attached
as Appendix A) (collectively “Inventions”) and I will promptly disclose all Inventions to Company. Without disclosing
any third party confidential information, I will also disclose anything I believe is excluded so that the Company can make an independent
assessment. I hereby make all assignments necessary to accomplish the foregoing. I shall further assist Company, at Company’s expense,
to further evidence, record and perfect such assignments, and to perfect, obtain, maintain, enforce, and defend any rights specified
to be so owned or assigned. I hereby irrevocably designate and appoint Company as my agent and attorney-in-fact, coupled with an interest
and with full power of substitution, to act for and in my behalf to execute and file any document and to do all other lawfully permitted
acts to further the purposes of the foregoing with the same legal force and effect as if executed by me. If I wish to clarify
that something created by me prior to my employment that relates to Company’s actual or proposed business is not within the scope
of the foregoing assignment, I have listed it on Appendix B in a manner that does not violate any third party rights or disclose
any confidential information. Without limiting Section 1 or Company’s other rights and remedies, if, when acting within the scope
of my employment or otherwise on behalf of Company, I use or (except pursuant to this Section 2) disclose my own or any third party’s
confidential information or intellectual property (or if any Invention cannot be fully made, used, reproduced, distributed and otherwise
exploited without using or violating the foregoing), Company will have and I hereby grant Company a perpetual, irrevocable, worldwide,
royalty-free, non-exclusive, sublicensable right and license to exploit and exercise all such confidential information and intellectual
property rights.
Lisa
Embree
Page
6
3.
To the extent allowed by law, paragraph 2 includes all rights of paternity, integrity, disclosure and withdrawal and any other rights
that may be known as or referred to as “moral rights,” “artist’s rights,” “droit moral,” or
the like (collectively “Moral Rights”). To the extent I retain any such Moral Rights under applicable law, I hereby ratify
and consent to any action that may be taken with respect to such Moral Rights by or authorized by Company and agree not to assert any
Moral Rights with respect thereto. I will confirm any such ratifications, consents and agreements from time to time as requested by the
Company.
4.
I agree that all Inventions and all other business, technical and financial information (including, without limitation, the identity
of and information relating to customers or employees) I develop, learn or obtain during the term of my employment that relate to Company
or the business or demonstrably anticipated business of Company or that are received by or for Company in confidence, constitute “Proprietary
Information.” I will hold in confidence and not disclose or, except within the scope of my employment, use any Proprietary Information.
However, I shall not be obligated under this paragraph with respect to information I can document is or becomes readily publicly available
without restriction through no fault of mine. Upon termination of my employment, I will promptly return to Company all items containing
or embodying Proprietary Information (including all copies), except that I may keep my personal copies of (i) my compensation records,
(ii) materials distributed to shareholders generally and (iii) this Agreement. I also recognize and agree that I have no expectation
of privacy with respect to Company’s telecommunications, networking or information processing systems (including, without limitation,
stored computer files, embedded messages and voice messages) and that my activity and any files or messages on or using any of those
systems may be monitored at any time without notice.
5.
Until one year after the term of my employment, I will not encourage or solicit any employee or consultant of Company to leave Company
for any reason (except for the bona fide firing of Company personnel within the scope of my employment).
6.
I agree that during the term of my employment with Company (whether or not during business hours), I will not engage in any activity
that is in any way competitive with the business or demonstrably anticipated business of Company, and I will not assist any other person
or organization in competing or in preparing to compete with any business or demonstrably anticipated business of Company.
7.
I agree that this Agreement is not an employment contract for any particular term and that I have the right to resign a11d the Company
has the right to terminate my employment at will, at any time, for any or no reason, with or without cause. In addition, this Agreement
does not purport to set forth all of the terms and conditions of my employment, and, as an employee of Company, I have obligations to
Company which are not set forth in this Agreement. However, the terms of this Agreement govern over any inconsistent terms and can only
be changed by a subsequent written agreement signed by the President of the Company.
Lisa
Embree
Page
7
8.
I agree that my obligations under paragraphs 2, 3, 4 and 5 of this Agreement shall continue in effect after termination of my employment,
regardless of the reason or reasons for termination, and whether such termination is voluntary or involuntary on my part, and that Company
is entitled to communicate my obligations under this Agreement to any future employer or potential employer of mine. My obligations under
paragraphs 2, 3 and 4 also shall be binding upon my heirs, executors, assigns, and administrators and shall insure to the benefit of
Company, its subsidiaries, successors and assigns.
9.
Any dispute in the meaning, effect or validity of this Agreement shall be resolved in accordance with the laws of the State of Delaware
without regard to the conflict of laws provisions thereof. I further agree that if one or more provisions of this Agreement are held
to be illegal or unenforceable under applicable law, such illegal or unenforceable portion(s) shall be limited or excluded from this
Agreement to the minimum extent required so that this Agreement shall otherwise remain in full force and effect and enforceable in accordance
with its terms. This Agreement is fully assignable and transferable by Company, but any purported assignment or transfer by me is void.
I also understand that any breach of this Agreement will cause irreparable harm to Company for which damages would not be an adequate
remedy, and, therefore, Company will be entitled to injunctive relief with respect thereto in addition to any other remedies and without
any requirement to post bond.
10.
I acknowledge receipt of the following notice under 18 U.S.C § 1833(b)(l): “An individual shall not be held criminally or
civilly liable under any Federal or State trade secret law for the disclosure of a trade secret that (A) is made (i) in confidence to
a Federal, State, or local government official, either directly or indirectly, or to an attorney; and (ii) solely for the purpose of
reporting or investigating a suspected violation of law; or (B) is made in a complaint or other document filed in a lawsuit or other
proceeding, if such filing is made under seal.”
Remainder
of Page Intentionally Left Blank
Lisa
Embree
Page
8
I
HAVE READ THIS AGREEMENT CAREFULLY AND I UNDERSTAND AND ACCEPT THE OBLIGATIONS WHICH IT IMPOSES UPON ME WITHOUT RESERVATION. NO PROMISES
OR REPRESENTATIONS HAVE BEEN MADE TO ME TO INDUCE ME TO SIGN THIS AGREEMENT. I SIGN THIS AGREEMENT VOLUNTARILY AND FREELY, IN DUPLICATE,
WITH THE UNDERSTANDING THAT THE COMPANY WILL RETURN ONE COUNTERPART AND THE OTHER COUNTERPART WILL BE RETAINED BY ME.
Employee
| /s/
Lisa Embree |
|
| Lisa
Embree |
|
| /s/
Paul Casey |
|
| Paul
Casey, CEO ONEMEDNET |
|
APPENDIX
A
Application
of provision providing that employee shall assign or offer to assign rights in invention to employer.
(a)
Any provision in an employment agreement which provides that an employee shall assign, or offer to assign, any of his or her rights in
an invention to his or her employer shall not apply to an invention that the employee developed entirely on his or her own time
without using the employer’s equipment, supplies, facilities, or trade secret information except for those inventions that either:
1.
Relate at the time of conception or reduction to practice of the invention to the employer’s business, or actual or demonstrably
anticipated research or development of the employer; or
2.
Result from any work performed by the employee for his employer.
(b)
To the extent a provision in an employment agreement purports to require an employee to assign an invention otherwise excluded from being
required to be assigned under subdivision (a), the provision is against the public policy of this state and is unenforceable.
Exhibit
10.10
OneMedNet
Corporation
March
28, 2022
Paul
Casey
1010
Wilder Avenue
Apt.
1301
Honolulu,
HI 96822
Dear
Paul,
OneMedNet
Corp. (the “Company”) is pleased to offer you employment on the following terms:
1.
Position. Your title will be CEO and you will report to the Board of Directors. This is a full-time, exempt position. While you
render services to the Company, you will not engage in any other employment, consulting or other business activity (whether full-time
or part- time) that would create a conflict of interest with the Company. By signing this letter agreement, you confirm to the Company
that you have no contractual commitments or other legal obligations that would prohibit you from performing your duties for the Company.
2.
Cash Compensation. The Company will pay you a starting salary at the rate of $144,000 per year, payable in accordance with the
Company’s standard payroll schedule and subject to applicable deductions and withholdings. This salary will be subject to periodic
review and adjustments at the Company’s discretion.
3.
Employee Benefits. As an employee of the Company, you will be eligible to receive certain employee benefits. Refer to benefit
plan for further detail. You should note that the Company may modify job titles, salaries and benefits from time to time as it deems
necessary.
4.
Equity. You will be awarded 147,000 shares of stock upon the successful fundraising of an amount equal to or greater than $5,000,000.
As part of the Company’s Restricted Stock Unit Plan, further equity will be rewarded subject to the approval of the Company’s
Board of Directors.
5.
Proprietary Information and Inventions Agreement. Like all Company employees, you will be required, as a condition of your employment
with the Company, to sign the Company’s standard Proprietary Information and Inventions Agreement, a copy of which is attached
hereto as Exhibit A, requires, among other provisions, the assignment of patent rights to any invention made during your employment
at the Company.
6.
Employment Relationship. Employment with the Company is for no specific period of time. Your employment with the Company will
be “at will,” meaning that either you or the Company may terminate your employment at any time and for any reason, with or
without cause. Any contrary representations that may have been made to you are superseded by this letter agreement. This is the full
and complete agreement between you and the Company on this term. Although your job duties, title, compensation and benefits, as well
as the Company’s personnel policies and procedures, may change from time to time, the “at will” nature of your employment
may only be changed in an express written agreement signed by you and a duly authorized officer of the Company (other than you).
Paul
Casey
Page
2
7.
Termination.
a.
In the event that your employment with the Company is terminated by the Company without Cause (as defined below)), or is terminated by
you for Good Reason (as defined below), after six months of employment, and you sign and do not revoke a standard release of claims with
the Company in a form reasonably satisfactory to the Company’s Board of Directors (a “Release”), which Release
becomes irrevocable no later than sixty (60) days (the “Release Deadline”) after the date of your termination of employment
(the “Termination Date”) you will be entitled to the following severance payment (“Severance”):
i.
If the Termination Date is after 6 months’ of employment, but before you have completed 12 months’ of employment, you will
receive 3 months’ salary;
ii.
If the Termination Date is after 12 months’ employment you will receive 6 months’ salary.
If
the Release does not become effective and irrevocable by the Release Deadline, you will forfeit any right to Severance. In no event will
Severance be paid or provided hereunder until the Release actually becomes effective and irrevocable.
The
following terms referred to in this offer letter shall have the following meanings:
“Cause”
shall mean: (i) your material failure to perform your stated duties, and your continued failure to cure such failure to the reasonable
satisfaction of the Company within ten (10) days following written notice of such failure to you from the Company’s Board of Directors;
(ii) your material violation of a written Company policy (including any insider trading policy) or any written agreement or covenant
with the Company; (iii) your conviction of, or entry of a plea of guilty or nolo contendere to, a felony (other than motor vehicle
offenses the effect of which do not materially impair your performance of your employment duties); (iv) a willful act by you that constitutes
gross misconduct and which is injurious to the Company; (v) your commission of any act of fraud, embezzlement, dishonesty or any other
willful misconduct that has caused or is reasonably expected to result in material injury to the Company; (vi) the unauthorized use or
disclosure by you of any proprietary information or trade secrets of the Company or any other party to whom you owe an obligation of
nondisclosure as a result of your relationship with the Company; or your willful failure to cooperate with an investigation by a governmental
authority.
“Good
Reason” shall mean your voluntary termination, within thirty (30) days following the expiration of any Company cure period
(discussed below) following the occurrence of one or more of the following, without your consent: (i) a material reduction of your duties,
authority, or responsibilities, relative to your title, duties, authority, or responsibilities as in effect immediately prior to such
reduction; provided that your title, duties, authority and responsibilities will not be deemed to be materially reduced if you have reasonably
comparable duties, authorities and responsibilities as an employee with respect to the Company’s business following a Change of
Control, regardless of any change in title or whether you provide services to a subsidiary, affiliate, business unit, division or otherwise;
(ii) a material reduction by the Company of your annual base salary, except to the extent the base salaries of all other senior executives
of the Company are similarly reduced; (iii) a material change in the location of the performance of your work, required by the Company,
of more than fifty (50) miles from your principal place of residence. You may not resign for Good Reason without first providing the
Company with written notice within ninety (90) days of the initial existence of the condition that you believe constitutes Good Reason
specifically identifying the acts or omissions constituting the grounds for Good Reason and a reasonable cure period of not less than
thirty (30) days following the date of such notice.
Paul
Casey
Page
3
8.
Tax Matters.
a.
Withholding. All forms of compensation referred to in this letter agreement are subject to reduction to reflect applicable withholding
and payroll taxes and other deductions required by law.
b.
Tax Advice. You are encouraged to obtain your own tax advice regarding your compensation from the Company. You agree that the
Company does not have a duty to design its compensation policies in a manner that minimizes your tax liabilities, and you will not make
any claim against the Company or its Board of Directors related to tax liabilities arising from your compensation.
9.
Interpretation, Amendment and Enforcement. This letter agreement and Exhibit A constitute the complete agreement between you and
the Company, contain all of the terms of your employment with the Company and supersede any prior agreements, representations or understandings
(whether written, oral or implied) between you and the Company. This letter agreement may not be amended or modified, except by an express
written agreement signed by both you and a duly authorized officer of the Company. The terms of this letter agreement and the resolution
of any disputes as to the meaning, effect, performance or validity of this letter agreement or arising out of, related to, or in any
way connected with, this letter agreement, your employment with the Company or any other relationship between you and the Company (the
“Disputes”) will be governed by Delaware law, excluding laws relating to conflicts or choice of law. You and the Company
submit to the exclusive personal jurisdiction of the federal and state courts located in Delaware in connection with any Dispute or any
claim related to any Dispute.
10.
For purposes of federal immigration law, you will be required to provide to the Company documentary evidence of your identity and eligibility
for employment in the United States. Such documentation must be provided to us within three (3) business days of your date of hire, or
our employment relationship with you may be terminated.
11.
We also ask that, if you have not already done so, you disclose to the Company any and all agreements relating to your prior employment
that may affect your eligibility to be employed by the Company or limit the manner in which you may be employed. It is the Company’s
understanding that any such agreements will not prevent you from performing the duties of your position and you represent that such is
the case. Moreover, you agree that, during the term of your employment with the Company, you will not engage in any other employment,
occupation, consulting or other business activity directly related to the business in which the Company is now involved or becomes involved
during the term of your employment, nor will you engage in any other activities that conflict with your obligations to the Company. Similarly,
you agree not to bring any third-party confidential information to the Company, including that of your former employer, and that in performing
your duties for the Company you will not in any way utilize any such information.
12.
As a condition of your employment, you are also required to sign and comply with the Company’s standard Employee Non-Disclosure
Agreement.
*
* * * *
Paul
Casey
Page
4
We
hope that you will accept our offer to join the Company. You may indicate your agreement with these terms and accept this offer by signing
and dating both the enclosed duplicate original of this letter agreement and the enclosed Proprietary Information and Inventions Agreement
and returning them to me.
If
you have any questions, please let me know.
Very
truly yours,
| /s/ Dr.
Jeffrey Yu |
|
| Dr.
Jeffrey Yu Chairman of the Board |
|
I
have read and accept this employment offer:
| /s/
Paul Casey |
|
| Paul
Casey |
|
Attachment
Exhibit
A: Proprietary Information and Inventions Agreement
Paul
Casey
Page
5
Exhibit
A
Proprietary
Information and Inventions Agreement
Paul
Casey
Page
6
PROPRIETARY
INFORMATION AND INVENTIONS AGREEMENT
The
following confirms and memorializes an agreement that COMPANY NAME INC, Inc., a Delaware corporation (the “Company”)
and I have had since the commencement of my employment (which term, for purposes of this agreement, shall be deemed to include any relationship
of service to the Company that I may have had prior to actually becoming an employee) with the Company in any capacity and that is and
has been a material part of the consideration for my employment by Company:
1.
I have not entered into, and I agree I will not enter into, any agreement either written or oral in conflict with this Agreement or my
employment with Company. I will not violate any agreement with or rights of any third party or, except as expressly authorized by Company
in writing hereafter, use or disclose my own or any third party’s confidential information or intellectual property when acting
within the scope of my employment or otherwise on behalf of Company. Further, I have not retained anything containing any confidential
information of a prior employer or other third party, whether or not created by me.
2.
Company shall own all right, title and interest (including patent rights, copyrights, trade secret rights, mask work rights, sui generis
database rights and all other intellectual property rights of any sort throughout the world) relating to any and all inventions (whether
or not patentable), works of authorship, mask works, designs, know-how, ideas and information made or conceived or reduced to practice,
in whole or in part, by me during the term of my employment with Company to and only to the fullest extent allowed (which is attached
as Appendix A) (collectively “Inventions”) and I will promptly disclose all Inventions to Company. Without disclosing
any third party confidential information, I will also disclose anything I believe is excluded so that the Company can make an independent
assessment. I hereby make all assignments necessary to accomplish the foregoing. I shall further assist Company, at Company’s expense,
to further evidence, record and perfect such assignments, and to perfect, obtain, maintain, enforce, and defend any rights specified
to be so owned or assigned. I hereby irrevocably designate and appoint Company as my agent and attorney-in-fact, coupled with an interest
and with full power of substitution, to act for and in my behalf to execute and file any document and to do all other lawfully permitted
acts to further the purposes of the foregoing with the same legal force and effect as if executed by me. If I wish to clarify that something
created by me prior to my employment that relates to Company’s actual or proposed business is not within the scope of the foregoing
assignment, I have listed it on Appendix B in a manner that does not violate any third party rights or disclose any confidential
information. Without limiting Section 1 or Company’s other rights and remedies, if, when acting within the scope of my employment
or otherwise on behalf of Company, I use or (except pursuant to this Section 2) disclose my own or any third party’s confidential
information or intellectual property (or if any Invention cannot be fully made, used, reproduced, distributed and otherwise exploited
without using or violating the foregoing), Company will have and I hereby grant Company a perpetual, irrevocable, worldwide, royalty-free,
non-exclusive, sublicensable right and license to exploit and exercise all such confidential information and intellectual property rights.
3.
To the extent allowed by law, paragraph 2 includes all rights of paternity, integrity, disclosure and withdrawal and any other rights
that may be known as or referred to as “moral rights,” “artist’s rights,” “droit moral,” or
the like (collectively “Moral Rights”). To the extent I retain any such Moral Rights under applicable law, I hereby ratify
and consent to any action that may be taken with respect to such Moral Rights by or authorized by Company and agree not to assert any
Moral Rights with respect thereto. I will confirm any such ratifications, consents and agreements from time to time as requested by the
Company.
Paul
Casey
Page
7
4.
I agree that all Inventions and all other business, technical and financial information (including, without limitation, the identity
of and information relating to customers or employees) I develop, learn or obtain during the term of my employment that relate to Company
or the business or demonstrably anticipated business of Company or that are received by or for Company in confidence, constitute “Proprietary
Information.” I will hold in confidence and not disclose or, except within the scope of my employment, use any Proprietary Information.
However, I shall not be obligated under this paragraph with respect to information I can document is or becomes readily publicly available
without restriction through no fault of mine. Upon termination of my employment, I will promptly return to Company all items containing
or embodying Proprietary Information (including all copies), except that I may keep my personal copies of (i) my compensation records,
(ii) materials distributed to shareholders generally and (iii) this Agreement. I also recognize and agree that I have no expectation
of privacy with respect to Company’s telecommunications, networking or information processing systems (including, without limitation,
stored computer files, embedded messages and voice messages) and that my activity and any files or messages on or using any of those
systems may be monitored at any time without notice.
5.
Until one year after the term of my employment, I will not encourage or solicit any employee or consultant of Company to leave Company
for any reason (except for the bona fide firing of Company personnel within the scope of my employment).
6.
I agree that during the term of my employment with Company (whether or not during business hours), I will not engage in any activity
that is in any way competitive with the business or demonstrably anticipated business of Company, and I will not assist any other person
or organization in competing or in preparing to compete with any business or demonstrably anticipated business of Company.
7.
I agree that this Agreement is not an employment contract for any particular term and that I have the right to resign and the Company
has the right to terminate my employment at will, at any time, for any or no reason, with or without cause. In addition, this Agreement
does not purport to set forth all of the terms and conditions of my employment, and, as an employee of Company, I have obligations to
Company which are not set forth in this Agreement. However, the terms of this Agreement govern over any inconsistent terms and can only
be changed by a subsequent written agreement signed by the President of the Company.
8.
I agree that my obligations under paragraphs 2, 3, 4 and 5 of this Agreement shall continue in effect after termination of my employment,
regardless of the reason or reasons for termination, and whether such termination is voluntary or involuntary on my part, and that Company
is entitled to communicate my obligations under this Agreement to any future employer or potential employer of mine. My obligations under
paragraphs 2, 3 and 4 also shall be binding upon my heirs, executors, assigns, and administrators and shall insure to the benefit of
Company, its subsidiaries, successors and assigns.
Paul
Casey
Page
8
9.
Any dispute in the meaning, effect or validity of this Agreement shall be resolved in accordance with the laws of the State of Delaware
without regard to the conflict of laws provisions thereof. I further agree that if one or more provisions of this Agreement are held
to be illegal or unenforceable under applicable law, such illegal or unenforceable portion(s) shall be limited or excluded from this
Agreement to the minimum extent required so that this Agreement shall otherwise remain in full force and effect and enforceable in accordance
with its terms. This Agreement is fully assignable and transferable by Company, but any purported assignment or transfer by me is void.
I also understand that any breach of this Agreement will cause irreparable harm to Company for which damages would not be an adequate
remedy, and, therefore, Company will be entitled to injunctive relief with respect thereto in addition to any other remedies and without
any requirement to post bond.
10.
I acknowledge receipt of the following notice under 18 U.S.C § 1833(b)(1): “An individual shall not be held criminally or
civilly liable under any Federal or State trade secret law for the disclosure of a trade secret that (A) is made (i) in confidence to
a Federal, State, or local government official, either directly or indirectly, or to an attorney; and (ii) solely for the purpose of
reporting or investigating a suspected violation of law; or (B) is made in a complaint or other document filed in a lawsuit or other
proceeding, if such filing is made under seal.”
Remainder
of Page Intentionally Left Blank
Paul
Casey
Page
9
I
HAVE READ THIS AGREEMENT CAREFULLY AND I UNDERSTAND AND ACCEPT THE OBLIGATIONS WHICH IT IMPOSES UPON ME WITHOUT RESERVATION. NO PROMISES
OR REPRESENTATIONS HAVE BEEN MADE TO ME TO INDUCE ME TO SIGN THIS AGREEMENT. I SIGN THIS AGREEMENT VOLUNTARILY AND FREELY, IN DUPLICATE,
WITH THE UNDERSTANDING THAT THE COMPANY WILL RETURN ONE COUNTERPART AND THE OTHER COUNTERPART WILL BE RETAINED BY ME.
Employee
| /s/
Paul Casey |
|
| Signature |
|
| |
|
| /s/
Paul Casey |
|
| Paul
Casey |
|
Accepted
and Agreed to:
OneMedNet
Corp.
| Dr.
Jeffrey Yu |
|
| Chairman
of the Board |
|
Paul
Casey
Page
10
APPENDIX
A
Application
of provision providing that employee shall assign or offer to assign rights in invention to employer.
(a)
Any provision in an employment agreement which provides that an employee shall assign, or offer to assign, any of his or her rights in
an invention to his or her employer shall not apply to an invention that the employee developed entirely on his or her own time without
using the employer’s equipment, supplies, facilities, or trade secret information except for those inventions that either:
1.
Relate at the time of conception or reduction to practice of the invention to the employer’s business, or actual or demonstrably
anticipated research or development of the employer; or
2.
Result from any work performed by the employee for his employer.
(b)
To the extent a provision in an employment agreement purports to require an employee to assign an invention otherwise excluded from being
required to be assigned under subdivision (a), the provision is against the public policy of this state and is unenforceable.
Paul
Casey
Page
11
APPENDIX
B
PRIOR MATTER
Exhibit
10.11
SECURITIES
PURCHASE AGREEMENT
This
SECURITIES PURCHASE AGREEMENT (the “Agreement”), dated as of this 28th day of June 2023, is by and among Data
Knights Acquisition Corp, a Delaware corporation with offices located at Unit G6, Frome Business Park, Manor Road, Frome, United Kingdom,
BA11 4FN (the “Company”), and each of the investors listed on the Schedule of Buyers attached hereto (individually, a “Buyer”
and collectively, the “Buyers”).
RECITALS
A.
The Company and each Buyer is executing and delivering this Agreement in reliance upon the exemption from securities registration afforded
by Section 4(a)(2) of the Securities Act of 1933, as amended (the “1933 Act”), and Rule 506(b) of Regulation D (“Regulation
D”) as promulgated by the United States Securities and Exchange Commission (the “SEC”) under the 1933 Act.
B.
The Company has authorized a new series of senior secured convertible notes of the Company, in the aggregate original principal amount
of $1,595,744.70, substantially in the form attached hereto as Exhibit A (the “Notes”), which Notes shall be convertible
into shares of Common Stock (as defined below) (the shares of Common Stock issuable pursuant to the terms of the Notes, including, without
limitation, upon conversion, as Pre-Delivery Shares (as defined in the Notes) or otherwise, collectively, the “Conversion Shares”),
in accordance with the terms of the Notes. Each Buyer wishes to purchase, and the Company wishes to sell, upon the terms and conditions
stated in this Agreement, a Note in the aggregate original principal amount set forth opposite such Buyer’s name in column (2)
on the Schedule of Buyers.
C.
The Company has authorized a new series of Common Stock purchase warrants of the Company, substantially in the form attached hereto as
Exhibit B (the “Warrants”), which Warrants shall be exercisable for up to 95,744.70 shares of Common Stock (as defined
below) in the aggregate (the shares of Common Stock issuable pursuant to the terms of the Warrants, collectively, the “Warrant
Shares”), in accordance with the terms of the Warrants. Each Buyer wishes to purchase, and the Company wishes to sell, upon the
terms and conditions stated in this Agreement, the number of Warrants set forth opposite such Buyer’s name in column (3) on the
Schedule of Buyers.
D.
At the Closing, the parties hereto shall execute and deliver a Registration Rights Agreement, in the form attached hereto as Exhibit
C (the “Registration Rights Agreement”), pursuant to which the Company has agreed to provide certain registration rights
with respect to the Registrable Securities (as defined in the Registration Rights Agreement), under the 1933 Act and the rules and regulations
promulgated thereunder, and applicable state securities laws.
E.
The Notes, the Warrants, the Warrant Shares and the Conversion Shares are collectively referred to herein as the “Securities.”
F.
The Notes will be secured by a security interest in the existing intellectual property assets of the Company, as evidenced by a security
agreement in the form attached hereto as Exhibit D (the “Security Agreement” and the other security documents and
agreements entered into in connection with this Agreement, as each may be amended or modified from time to time, collectively, the “Security
Documents”), executed by OneMedNet Solutions Corporation, a Delaware corporation and its subsidiary (the “OneMedNet”),
which are prospective subsidiaries of the Company upon the closing of the Merger Agreement (as defined below).
AGREEMENT
NOW,
THEREFORE, in consideration of the premises and the mutual covenants contained herein and for other good and valuable consideration,
the receipt and sufficiency of which are hereby acknowledged, the Company and each Buyer hereby agree as follows:
1.
PURCHASE AND SALE OF NOTES AND WARRANTS.
(a)
Purchase of Notes. Subject to the satisfaction (or waiver) of the conditions set forth in Sections 6 and 7 below, the Company
shall issue and sell to each Buyer, and each Buyer severally, but not jointly, agrees to purchase from the Company on the applicable
Closing Date (as defined below) a Note in the original principal amount as is set forth opposite such Buyer’s name in column (2)
on the Schedule of Buyers.
(b)
Purchase of Warrants. Subject to the satisfaction (or waiver) of the conditions set forth in Sections 6 and 7 below, the Company
shall issue and sell to each Buyer, and each Buyer severally, but not jointly, agrees to purchase from the Company on the applicable
Closing Date (as defined below) the number of Warrants as is set forth opposite such Buyer’s name in column (3) on the Schedule
of Buyers.
(c)
Closing. Following the execution of this Agreement, the closing and payment (the “Closing”) of the Purchase Price
(as defined below) of the Notes and Warrants by the Buyers shall occur at the offices of Rimon P.C., 1990 K. Street, NW, Suite 420, Washington,
DC 20006. The date and time of the Closing (the “Closing Date”) shall occur at 10:00 a.m., EST, on the closing date of the
Merger Agreement (or such other date, time or place (including remotely) as is mutually agreed to by the Company and each Buyer).
As used herein “Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City
of New York are authorized or required by law to remain closed; provided, however, for clarification, commercial banks
shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”,
“non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations at the
direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial
banks in The City of New York generally are open for use by customers on such day.
(d)
Purchase Price. The aggregate purchase price for the Notes and Warrants to be purchased by each Buyer (the “Purchase Price”)
shall be the amount set forth opposite such Buyer’s name in column (4) on the Schedule of Buyers.
(e)
Payment. On the Closing Date, (i) each Buyer shall pay its respective Purchase Price to the Company for the Notes and Warrants
to be issued and sold to such Buyer at such Closing, by wire transfer of immediately available funds, and (ii) the Company shall deliver
to each Buyer (1) a Note in the aggregate original principal amount as is set forth opposite such Buyer’s name in column (2) of
the Schedule of Buyers, duly executed on behalf of the Company and registered in the name of such Buyer or its designee and (2) a Warrant
in the amount as is set forth opposite such Buyer’s name in column (3) of the Schedule of Buyers, duly executed on behalf of the
Company and registered in the name of such Buyer or its designee.
2.
BUYER’S REPRESENTATIONS AND WARRANTIES.
Each
Buyer, severally and not jointly, represents and warrants to the Company with respect to only itself that, as of the date hereof and
as of the Closing Date:
(a)
Organization; Authority. Such Buyer, if an entity and thus applicable, is an entity duly organized, validly existing and in good
standing under the laws of the jurisdiction of its organization with the requisite power and authority to enter into and to consummate
the transactions contemplated by the Transaction Documents to which it is a party and otherwise to carry out its obligations hereunder
and thereunder.
(b)
No Public Sale or Distribution. Such Buyer (i) is acquiring its Note and its Warrant, and (ii) upon conversion of its Note will
acquire the Conversion Shares, and upon exercise of its Warrant will acquire the Warrant Shares, in each case, for its own account and
not with a view towards, or for resale in connection with, the public sale or distribution thereof in violation of applicable securities
laws, except pursuant to sales registered or exempted under the 1933 Act; provided, however, by making the representations herein, such
Buyer does not agree, or make any representation or warranty, to hold any of the Securities for any minimum or other specific term and
reserves the right to dispose of the Securities at any time in accordance with or pursuant to a registration statement or an exemption
from registration under the 1933 Act. Such Buyer does not presently have any agreement or understanding, directly or indirectly, with
any Person to distribute any of the Securities in violation of applicable securities laws. For purposes of this Agreement, “Person”
means an individual, a limited liability company, a partnership, a joint venture, a corporation, a trust, an unincorporated organization,
any other entity and any Governmental Entity or any department or agency thereof.
(c)
Accredited Investor Status. Such Buyer is an “accredited investor” as that term is defined in Rule 501(a) of Regulation
D.
(d)
Reliance on Exemptions. Such Buyer understands that the Securities are being offered and sold to it in reliance on specific exemptions
from the registration requirements of United States federal and state securities laws and that the Company is relying in part upon the
truth and accuracy of, and such Buyer’s compliance with, the representations, warranties, agreements, acknowledgments and understandings
of such Buyer set forth herein in order to determine the availability of such exemptions and the eligibility of such Buyer to acquire
the Securities.
(e)
Information. Such Buyer and its advisors, if any, have been furnished with all materials relating to the business, finances and
operations of the Company and its Subsidiaries and potential Subsidiaries and materials relating to the offer and sale of the Securities
that have been requested by such Buyer and its Subsidiaries and potential Subsidiaries. Such Buyer and its advisors, if any, have been
afforded the opportunity to ask questions of the Company and its Subsidiaries and potential Subsidiaries. Neither such inquiries nor
any other due diligence investigations conducted by such Buyer or its advisors, if any, or its representatives shall modify, amend or
affect such Buyer’s right to rely on the Company’s representations and warranties contained herein. Such Buyer understands
that its investment in the Securities involves a high degree of risk. Such Buyer has sought such accounting, legal and tax advice as
it has considered necessary to make an informed investment decision with respect to its acquisition of the Securities.
(f)
No Governmental Review. Such Buyer understands that no United States federal or state agency or any other government or governmental
agency has passed on or made any recommendation or endorsement of the Securities or the fairness or suitability of the investment in
the Securities nor have such authorities passed upon or endorsed the merits of the offering of the Securities.
(g)
Transfer or Resale. Such Buyer understands that except as provided in the Registration Rights Agreement: (i) the Securities have
not been and are not being registered under the 1933 Act or any state securities laws, and may not be offered for sale, sold, assigned
or transferred unless (A) subsequently registered thereunder, (B) such Buyer shall have delivered to the Company (if requested by the
Company) an opinion of counsel, in a form reasonably acceptable to the Company, to the effect that such Securities to be sold, assigned
or transferred may be sold, assigned or transferred pursuant to an exemption from such registration, or (C) such Buyer provides the Company
with reasonable assurance that such Securities can be sold, assigned or transferred pursuant to Rule 144 or Rule 144A promulgated under
the 1933 Act (or a successor rule thereto) (collectively, “Rule 144”); (ii) any sale of the Securities made in reliance on
Rule 144 may be made only in accordance with the terms of Rule 144, and further, if Rule 144 is not applicable, any resale of the Securities
under circumstances in which the seller (or the Person through whom the sale is made) may be deemed to be an underwriter (as that term
is defined in the 1933 Act) may require compliance with some other exemption under the 1933 Act or the rules and regulations of the SEC
promulgated thereunder; and (iii) neither the Company nor any other Person is under any obligation to register the Securities under the
1933 Act or any state securities laws or to comply with the terms and conditions of any exemption thereunder. Notwithstanding the foregoing,
the Securities may be pledged in connection with a bona fide margin account or other loan or financing arrangement secured by the Securities
and such pledge of Securities shall not be deemed to be a transfer, sale or assignment of the Securities hereunder, and no Buyer effecting
a pledge of Securities shall be required to provide the Company with any notice thereof or otherwise make any delivery to the Company
pursuant to this Agreement or any other Transaction Document (as defined in Section 3(b)), including, without limitation, this Section
2(g).
(h)
Validity; Enforcement. This Agreement and the Registration Rights Agreement have been duly and validly authorized, executed and
delivered on behalf of such Buyer and shall constitute the legal, valid and binding obligations of such Buyer enforceable against such
Buyer in accordance with their respective terms, except as such enforceability may be limited by general principles of equity or to applicable
bankruptcy, insolvency, reorganization, moratorium, liquidation and other similar laws relating to, or affecting generally, the enforcement
of applicable creditors’ rights and remedies.
(i)
No Conflicts. The execution, delivery and performance by such Buyer of this Agreement and the Registration Rights Agreement and
the consummation by such Buyer of the transactions contemplated hereby and thereby will not, where applicable, (i) result in a violation
of the organizational documents of such Buyer, or (ii) conflict with, or constitute a default (or an event which with notice or lapse
of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of,
any agreement, indenture or instrument to which such Buyer is a party, or (iii) result in a violation of any law, rule, regulation, order,
judgment or decree (including federal and state securities laws) applicable to such Buyer, except in the case of clauses (ii) and (iii)
above, for such conflicts, defaults, rights or violations which could not, individually or in the aggregate, reasonably be expected to
have a material adverse effect on the ability of such Buyer to perform its obligations hereunder.
3.
REPRESENTATIONS AND WARRANTIES OF THE COMPANY.
The
Company represents and warrants to each of the Buyers that, as of the date hereof and as of the Closing Date:
(a)
Organization and Qualification. The Company is an entity duly organized and validly existing and in good standing under the laws
of the jurisdiction in which it is formed, and has the requisite power and authority to own its properties and to carry on its business
as now being conducted and as presently proposed to be conducted. The Company is duly qualified as a foreign entity to do business and
is in good standing in every jurisdiction in which its ownership of property or the nature of the business conducted by it makes such
qualification necessary, except to the extent that the failure to be so qualified or be in good standing would not reasonably be expected
to have a Material Adverse Effect (as defined below). As used in this Agreement, “Material Adverse Effect” means any material
adverse effect on (i) the business, properties, assets, liabilities, operations (including results thereof), condition (financial or
otherwise) or prospects of the Company or any Subsidiary, individually or taken as a whole, (ii) the transactions contemplated hereby
or in any of the other Transaction Documents or any other agreements or instruments to be entered into in connection herewith or therewith
or (iii) the authority or ability of the Company or any of its Subsidiaries to perform any of their respective obligations under any
of the Transaction Documents.
(b)
Authorization; Enforcement; Validity. The Company has the requisite power and authority to enter into and perform its obligations
under this Agreement and the other Transaction Documents and to issue the Securities in accordance with the terms hereof and thereof.
Each Subsidiary has the requisite power and authority to enter into and perform its obligations under the Transaction Documents to which
it is a party. The execution and delivery of this Agreement and the other Transaction Documents by the Company and its Subsidiaries,
and the consummation by the Company and its Subsidiaries of the transactions contemplated hereby and thereby (including, without limitation,
the issuance of the Notes and Warrants, respectively, and the reservation for the Conversion Shares issuable upon conversion of the Notes
and the Warrant Shares issuable upon exercise of the Warrants, respectively) have been duly authorized by the Company’s board of
directors and each of its Subsidiaries’ board of directors or other governing body, as applicable, and (other than the filing with
the SEC of one or more Registration Statements in accordance with the requirements of the Registration Rights Agreement, a Form D with
the SEC and any other filings as may be required by any state securities agencies) no further filing, consent or authorization is required
by the Company, its Subsidiaries, their respective boards of directors or their stockholders or other governing body. This Agreement
has been, and the other Transaction Documents to which it is a party will be prior to the Closing, duly executed and delivered by the
Company, and each constitutes the legal, valid and binding obligations of the Company, enforceable against the Company in accordance
with its respective terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency,
reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of applicable creditors’
rights and remedies and except as rights to indemnification and to contribution may be limited by federal or state securities law. Prior
to the Closing, the Transaction Documents to which each Subsidiary is a party will be duly executed and delivered by each such Subsidiary,
and shall constitute the legal, valid and binding obligations of each such Subsidiary, enforceable against each such Subsidiary in accordance
with their respective terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency,
reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of applicable creditors’
rights and remedies and except as rights to indemnification and to contribution may be limited by federal or state securities law. “Transaction
Documents” means, collectively, this Agreement, the Notes, the Warrants, the Registration Rights Agreement, and the Guaranty, and
each of the other agreements and instruments entered into or delivered by any of the parties hereto in connection with the transactions
contemplated hereby and thereby, as may be amended from time to time.
(c)
Issuance of Securities. The issuance of the Notes and Warrants are duly authorized and upon issuance in accordance with the terms
of the Transaction Documents shall be validly issued, fully paid and non-assessable and free from all preemptive or similar rights, mortgages,
defects, claims, liens, pledges, charges, taxes, rights of first refusal, encumbrances, security interests and other encumbrances (collectively
“Liens”) with respect to the issuance thereof. As of the Closing, the Company shall have reserved from its duly authorized
capital stock not less than 200% of the maximum number of Conversion Shares issuable upon conversion of the Notes (assuming for purposes
hereof that (x) the Notes are convertible at the Alternate Conversion Price (as defined in the Notes) assuming an Alternate Conversion
Date (as defined in the Note) as of the date hereof, (y) interest on the Notes shall accrue through the first anniversary of the Closing
Date and will be converted in shares of Common Stock at a conversion price equal to the Alternate Conversion Price assuming an Alternate
Conversion Date as of the date hereof and (z) any such conversion shall not take into account any limitations on the conversion of the
Notes set forth in the Notes) and Warrant Shares issuable upon exercise of the Warrants (assuming for purposes hereof that any such exercise
shall not take into account any limitations on the exercise of the Warrants set forth in the Warrants). Upon issuance or conversion in
accordance with the Notes, the Conversion Shares, when issued, will be validly issued, fully paid and nonassessable and free from all
preemptive or similar rights or Liens with respect to the issue thereof, with the holders being entitled to all rights accorded to a
holder of Common Stock. Upon exercise in accordance with the Warrants, the Warrant Shares, when issued, will be validly issued, fully
paid and nonassessable and free from all preemptive or similar rights or Liens with respect to the issue thereof, with the holders being
entitled to all rights accorded to a holder of Common Stock. Subject to the accuracy of the representations and warranties of the Buyers
in this Agreement, the offer and issuance by the Company of the Securities is exempt from registration under the 1933 Act.
(d)
No Conflicts. The execution, delivery and performance of the Transaction Documents by the Company and its Subsidiaries and the
consummation by the Company and its Subsidiaries of the transactions contemplated hereby and thereby (including, without limitation,
the issuance of the Notes and the Conversion Shares, the issuance of the Warrants and the Warrant Shares, and the reservation for issuance
of the Conversion Shares and Warrant Shares, will not (i) result in a violation of the Certificate of Incorporation (as defined below)
(including, without limitation, any certificate of designation contained therein), Bylaws (as defined below), certificate of formation,
memorandum of association, articles of association, bylaws or other organizational documents of the Company or any of its Subsidiaries,
or any capital stock or other securities of the Company or any of its Subsidiaries, (ii) conflict with, or constitute a default (or an
event which with notice or lapse of time or both would become a default) in any respect under, or give to others any rights of termination,
amendment, acceleration or cancellation of, any agreement, indenture or instrument to which the Company or any of its Subsidiaries is
a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including, without limitation, foreign,
federal and state securities laws and regulations and the rules and regulations of the Nasdaq Capital Market (the “Principal Market”)
and including all applicable foreign, federal and state laws, rules and regulations) applicable to the Company or any of its Subsidiaries
or by which any property or asset of the Company or any of its Subsidiaries is bound or affected.
(e)
Consents. Neither the Company nor any Subsidiary is required to obtain any consent from, authorization or order of, or make any
filing or registration with (other than the filing with the SEC of one or more Registration Statements in accordance with the requirements
of the Registration Rights Agreement, a Form D with the SEC and any other filings as may be required by any state securities agencies),
any Governmental Entity (as defined below) or any regulatory or self-regulatory agency or any other Person in order for it to execute,
deliver or perform any of its respective obligations under or contemplated by the Transaction Documents, in each case, in accordance
with the terms hereof or thereof. All consents, authorizations, orders, filings and registrations which the Company or any Subsidiary
is required to obtain pursuant to the preceding sentence have been or will be obtained or effected on or prior to the Closing Date, and
neither the Company nor any of its Subsidiaries are aware of any facts or circumstances which might prevent the Company or any of its
Subsidiaries from obtaining or effecting any of the registration, application or filings contemplated by the Transaction Documents. The
Company is not in violation of the requirements of the Principal Market and has no knowledge of any facts or circumstances which could
reasonably lead to delisting or suspension of the Common Stock in the foreseeable future.
(f)
Acknowledgment Regarding Buyer’s Purchase of Securities. The Company acknowledges and agrees that each Buyer is acting solely
in the capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated hereby
and thereby and that no Buyer is (i) an officer or director of the Company or any of its Subsidiaries, (ii) an “affiliate”
(as defined in Rule 144) of the Company or any of its Subsidiaries or (iii) to its knowledge, a “beneficial owner” of more
than 10% of the shares of Common Stock (as defined for purposes of Rule 13d-3 of the Securities Exchange Act of 1934, as amended (the
“1934 Act”)). The Company further acknowledges that no Buyer is acting as a financial advisor or fiduciary of the Company
or any of its Subsidiaries (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated hereby
and thereby, and any advice given by a Buyer or any of its representatives or agents in connection with the Transaction Documents and
the transactions contemplated hereby and thereby is merely incidental to such Buyer’s purchase of the Securities. The Company further
represents to each Buyer that the Company’s and each Subsidiary’s decision to enter into the Transaction Documents to which
it is a party has been based solely on the independent evaluation by the Company, each Subsidiary and their respective representatives.
(g)
No General Solicitation; Placement Agent’s Fees. Neither the Company, nor any of its Subsidiaries or affiliates, nor any
Person acting on its or their behalf, has engaged in any form of general solicitation or general advertising (within the meaning of Regulation
D) in connection with the offer or sale of the Securities. The Company shall be responsible for the payment of any placement agent’s
fees, financial advisory fees, or brokers’ commissions (other than for Persons engaged by any Buyer or its investment advisor)
relating to or arising out of the transactions contemplated hereby. The Company shall pay, and hold each Buyer harmless against, any
liability, loss or expense (including, without limitation, attorney’s fees and out-of-pocket expenses) arising in connection with
any such claim. The Company acknowledges that neither the Company nor any of its Subsidiaries has engaged any placement agent or other
agent in connection with the offer or sale of the Securities.
(h)
No Integrated Offering. None of the Company, its Subsidiaries or any of their affiliates, nor any Person acting on their behalf
has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances
that would require registration of the issuance of any of the Securities under the 1933 Act, whether through integration with prior offerings
or otherwise, or cause this offering of the Securities to require approval of stockholders of the Company for purposes of the 1933 Act
or under any applicable stockholder approval provisions, including, without limitation, under the rules and regulations of any exchange
or automated quotation system on which any of the securities of the Company are listed or designated for quotation. None of the Company,
its Subsidiaries, their affiliates nor any Person acting on their behalf will take any action or steps that would require registration
of the issuance of any of the Securities under the 1933 Act or cause the offering of any of the Securities to be integrated with other
offerings of securities of the Company.
(i)
Dilutive Effect. The Company understands and acknowledges that the number of Conversion Shares and Warrant Shares will increase
in certain circumstances. The Company further acknowledges that its obligation to issue the Conversion Shares pursuant to the terms of
the Notes and its obligation to issue the Warrant Shares pursuant to the terms of the Warrants in accordance with this Agreement is,
in each case, absolute and unconditional regardless of the dilutive effect that such issuance may have on the ownership interests of
other stockholders of the Company.
(j)
Application of Takeover Protections; Rights Agreement. The Company and its board of directors have taken all necessary action,
if any, in order to render inapplicable any control share acquisition, interested stockholder, business combination, poison pill (including,
without limitation, any distribution under a rights agreement), stockholder rights plan or other similar anti-takeover provision under
the Certificate of Incorporation, Bylaws or other organizational documents or the laws of the jurisdiction of its incorporation or otherwise
which is or could become applicable to any Buyer as a result of the transactions contemplated by this Agreement, including, without limitation,
the Company’s issuance of the Securities and any Buyer’s ownership of the Securities. The Company and its board of directors
have taken all necessary action, if any, in order to render inapplicable any stockholder rights plan or similar arrangement relating
to accumulations of beneficial ownership of shares of Common Stock or a change in control of the Company or any of its Subsidiaries.
(k)
SEC Documents; Financial Statements. During the twelve (12) months prior to the date hereof, the Company has timely filed all
reports, schedules, forms, proxy statements, statements and other documents pursuant to the reporting requirements of the 1934 Act (all
of the foregoing filed prior to the date hereof and all exhibits and appendices included therein and financial statements, notes and
schedules thereto and documents incorporated by reference therein being hereinafter referred to as the “SEC Documents”).
The Company has delivered or has made available to the Buyers or their respective representatives true, correct and complete copies of
each of the SEC Documents not available on the EDGAR system. As of their respective dates, the SEC Documents complied in all material
respects with the requirements of the 1934 Act and the rules and regulations of the SEC promulgated thereunder applicable to the SEC
Documents, and none of the SEC Documents, at the time they were filed with the SEC, contained any untrue statement of a material fact
or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light
of the circumstances under which they were made, not misleading. As of their respective dates, the financial statements of the Company
included in the SEC Documents complied in all material respects with applicable accounting requirements and the published rules and regulations
of the SEC with respect thereto as in effect as of the time of filing. Such financial statements have been prepared in accordance with
generally accepted accounting principles (“GAAP”), consistently applied, during the periods involved (except (i) as may be
otherwise indicated in such financial statements or the notes thereto, or (ii) in the case of unaudited interim statements, to the extent
they may exclude footnotes or may be condensed or summary statements) and fairly present in all material respects the financial position
of the Company as of the dates thereof and the results of its operations and cash flows for the periods then ended (subject, in the case
of unaudited statements, to normal year-end audit adjustments which will not be material, either individually or in the aggregate). No
information provided by or on behalf of the Company to any of the Buyers which is not included in the SEC Documents contains any untrue
statement of a material fact or omits to state any material fact necessary in order to make the statements therein not misleading, in
the light of the circumstance under which they are or were made. The Company is not currently contemplating to amend or restate any of
the financial statements (including, without limitation, any notes or any letter of the independent accountants of the Company with respect
thereto) included in the SEC Documents (the “Financial Statements”), nor is the Company currently aware of facts or circumstances
which would require the Company to amend or restate any of the Financial Statements, in each case, in order for any of the Financials
Statements to be in compliance with GAAP and the rules and regulations of the SEC. The Company has not been informed by its independent
accountants that they recommend that the Company amend or restate any of the Financial Statements or that there is any need for the Company
to amend or restate any of the Financial Statements.
(l)
Absence of Certain Changes. Since the date of the Company’s most recent audited financial statements contained in a Form
10-K, there has been no material adverse change and no material adverse development in the business, assets, liabilities, properties,
operations (including results thereof), condition (financial or otherwise) or prospects of the Company or any of its Subsidiaries. Since
the date of the Company’s most recent audited financial statements contained in a Form 10-K, neither the Company nor any of its
Subsidiaries has (i) declared or paid any dividends, (ii) sold any assets, individually or in the aggregate, outside of the ordinary
course of business or (iii) made any capital expenditures, individually or in the aggregate, outside of the ordinary course of business.
Neither the Company nor any of its Subsidiaries has taken any steps to seek protection pursuant to any law or statute relating to bankruptcy,
insolvency, reorganization, receivership, liquidation or winding up, nor does the Company or any Subsidiary have any knowledge or reason
to believe that any of their respective creditors intend to initiate involuntary bankruptcy proceedings or any actual knowledge of any
fact which would reasonably lead a creditor to do so. The Company and its Subsidiaries, individually and on a consolidated basis, are
not as of the date hereof, and after giving effect to the transactions contemplated hereby to occur at the Closing, will not be Insolvent
which means, (i) with respect to the Company and its Subsidiaries, on a consolidated basis, (A) the present fair saleable value of the
Company’s and its Subsidiaries’ assets is less than the amount required to pay the Company’s and its Subsidiaries’
total Indebtedness (as defined below), (B) the Company and its Subsidiaries are unable to pay their debts and liabilities, subordinated,
contingent or otherwise, as such debts and liabilities become absolute and matured or (C) the Company and its Subsidiaries intend to
incur or believe that they will incur debts that would be beyond their ability to pay as such debts mature; and (ii) with respect to
the Company and each Subsidiary, individually, (A) the present fair saleable value of the Company’s or such Subsidiary’s
(as the case may be) assets is less than the amount required to pay its respective total Indebtedness, (B) the Company or such Subsidiary
(as the case may be) is unable to pay its respective debts and liabilities, subordinated, contingent or otherwise, as such debts and
liabilities become absolute and matured or (C) the Company or such Subsidiary (as the case may be) intends to incur or believes that
it will incur debts that would be beyond its respective ability to pay as such debts mature.
(m)
No Undisclosed Events, Liabilities, Developments or Circumstances. No event, liability, development or circumstance has occurred
or exists, or is reasonably expected to exist or occur with respect to the Company, any of its Subsidiaries or any of their respective
businesses, properties, liabilities, prospects, operations (including results thereof) or condition (financial or otherwise), that (i)
would be required to be disclosed by the Company under applicable securities laws on a registration statement on Form S-1 filed with
the SEC relating to an issuance and sale by the Company of its Common Stock and which has not been publicly announced, (ii) could have
a material adverse effect on any Buyer’s investment hereunder or (iii) could have a Material Adverse Effect.
(n)
Conduct of Business; Regulatory Permits. Neither the Company nor any of its Subsidiaries is in violation of any term of or in
default under its Certificate of Incorporation, any certificate of designation, preferences or rights of any other outstanding series
of preferred stock of the Company or any of its Subsidiaries or Bylaws or their organizational charter, certificate of formation, memorandum
of association, articles of association, Certificate of Incorporation or certificate of incorporation or bylaws, respectively. Neither
the Company nor any of its Subsidiaries is in violation of any judgment, decree or order or any statute, ordinance, rule or regulation
applicable to the Company or any of its Subsidiaries, and neither the Company nor any of its Subsidiaries will conduct its business in
violation of any of the foregoing, except in all cases for possible violations which could not, individually or in the aggregate, have
a Material Adverse Effect. Without limiting the generality of the foregoing, the Company is not in violation of any of the rules, regulations
or requirements of the Principal Market and has no knowledge of any facts or circumstances that could reasonably lead to delisting or
suspension of the Common Stock by the Principal Market in the foreseeable future. As applicable, during the two years prior to the date
hereof, (i) the Common Stock has been listed or designated for quotation on the Principal Market, (ii) trading in the Common Stock has
not been suspended by the SEC or the Principal Market and (iii) the Company has received no communication, written or oral, from the
SEC or the Principal Market regarding the suspension or delisting of the Common Stock from the Principal Market. The Company and each
of its Subsidiaries possess all certificates, authorizations and permits issued by the appropriate regulatory authorities necessary to
conduct their respective businesses, except where the failure to possess such certificates, authorizations or permits would not have,
individually or in the aggregate, a Material Adverse Effect, and neither the Company nor any such Subsidiary has received any notice
of proceedings relating to the revocation or modification of any such certificate, authorization or permit. There is no agreement, commitment,
judgment, injunction, order or decree binding upon the Company or any of its Subsidiaries or to which the Company or any of its Subsidiaries
is a party which has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice
of the Company or any of its Subsidiaries, any acquisition of property by the Company or any of its Subsidiaries or the conduct of business
by the Company or any of its Subsidiaries as currently conducted other than such effects, individually or in the aggregate, which have
not had and would not reasonably be expected to have a Material Adverse Effect on the Company or any of its Subsidiaries.
(o)
Foreign Corrupt Practices. Neither the Company, the Company’s subsidiary or any director, officer, agent, employee, nor
any other person acting for or on behalf of the foregoing (individually and collectively, a “Company Affiliate”) have violated
the U.S. Foreign Corrupt Practices Act (the “FCPA”) or any other applicable anti-bribery or anti-corruption laws, nor has
any Company Affiliate offered, paid, promised to pay, or authorized the payment of any money, or offered, given, promised to give, or
authorized the giving of anything of value, to any officer, employee or any other person acting in an official capacity for any Governmental
Entity to any political party or official thereof or to any candidate for political office (individually and collectively, a “Government
Official”) or to any person under circumstances where such Company Affiliate knew or was aware of a high probability that all or
a portion of such money or thing of value would be offered, given or promised, directly or indirectly, to any Government Official, for
the purpose of:
(i)
(A) influencing any act or decision of such Government Official in his/her official capacity, (B) inducing such Government Official to
do or omit to do any act in violation of his/her lawful duty, (C) securing any improper advantage, or (D) inducing such Government Official
to influence or affect any act or decision of any Governmental Entity, or
(ii)
assisting the Company or its Subsidiaries in obtaining or retaining business for or with, or directing business to, the Company or its
Subsidiaries.
(p)
Sarbanes-Oxley Act. The Company and each Subsidiary is in compliance with any and all applicable requirements of the Sarbanes-Oxley
Act of 2002, as amended, and any and all applicable rules and regulations promulgated by the SEC thereunder.
(q)
Equity Capitalization.
(i)
Definitions:
(A)
“Common Stock” means (x) the Company’s shares of Class A common stock, $0.0001 par value per share, and (y) any capital
stock into which such common stock shall have been changed or any share capital resulting from a reclassification of such common stock.
(B)
“Preferred Stock” means (x) the Company’s blank check preferred stock, $0.0001 par value per share, the terms of which
may be designated by the board of directors of the Company in a certificate of designations and (y) any capital stock into which such
preferred stock shall have been changed or any share capital resulting from a reclassification of such preferred stock (other than a
conversion of such preferred stock into Common Stock in accordance with the terms of such certificate of designations).
(ii)
Authorized and Outstanding Capital Stock. As of the date hereof, the authorized capital stock of the Company consists of (A) 100,000,000
shares of Common Stock, of which, 3,316,819 shares of Class A Common Stock and 4,253,517 shares of Class B Common Stock are issued and
outstanding and 1,908,080 shares are reserved for issuance pursuant to Convertible Securities exercisable or exchangeable for, or convertible
into, shares of Common Stock and (B) 1,000,000 shares of Preferred Stock, none of which are issued and outstanding. “Convertible
Securities” means any capital stock or other security of the Company or any of its Subsidiaries that is directly or indirectly
convertible into, exercisable or exchangeable for, or which otherwise entitles the holder thereof to acquire, any capital stock or other
security of the Company (including, without limitation, Common Stock) or any of its Subsidiaries.
(iii)
Valid Issuance. All of such outstanding shares are duly authorized and have been, or upon issuance will be, validly issued and
are fully paid and nonassessable.
(iv)
Existing Securities; Obligations. Except as disclosed in the SEC Documents: (A) none of the Company’s or any Subsidiary’s
shares, interests or capital stock is subject to preemptive rights or any other similar rights or Liens suffered or permitted by the
Company or any Subsidiary; (B) there are no outstanding options, warrants, scrip, rights to subscribe to, calls or commitments of any
character whatsoever relating to, or securities or rights convertible into, or exercisable or exchangeable for, any shares, interests
or capital stock of the Company or any of its Subsidiaries, or contracts, commitments, understandings or arrangements by which the Company
or any of its Subsidiaries is or may become bound to issue additional shares, interests or capital stock of the Company or any of its
Subsidiaries or options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities
or rights convertible into, or exercisable or exchangeable for, any shares, interests or capital stock of the Company or any of its Subsidiaries;
(C) there are no agreements or arrangements under which the Company or any of its Subsidiaries is obligated to register the sale of any
of their securities under the 1933 Act (except pursuant to the Registration Rights Agreement); (D) there are no outstanding securities
or instruments of the Company or any of its Subsidiaries which contain any redemption or similar provisions, and there are no contracts,
commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to redeem a security
of the Company or any of its Subsidiaries; (E) there are no securities or instruments containing anti-dilution or similar provisions
that will be triggered by the issuance of the Securities; and (F) neither the Company nor any Subsidiary has any stock appreciation rights
or “phantom stock” plans or agreements or any similar plan or agreement.
(v)
Organizational Documents. The Company has furnished to the Buyers true, correct and complete copies of the Company’s Certificate
of Incorporation, as amended and as in effect on the date hereof (the “Certificate of Incorporation”), and the Company’s
bylaws, as amended and as in effect on the date hereof (the “Bylaws”), and the terms of all Convertible Securities and the
material rights of the holders thereof in respect thereto.
(r)
Indebtedness and Other Contracts. Neither the Company nor any of its Subsidiaries, (i) is a party to any contract, agreement or
instrument, the violation of which, or default under which, by the other party(ies) to such contract, agreement or instrument could reasonably
be expected to result in a Material Adverse Effect, (ii) has any financing statements securing obligations in any amounts filed in connection
with the Company or any of its Subsidiaries; (iii) is in violation of any term of, or in default under, any contract, agreement or instrument
relating to any Indebtedness, except where such violations and defaults would not result, individually or in the aggregate, in a Material
Adverse Effect, or (iv) is a party to any contract, agreement or instrument relating to any Indebtedness, the performance of which, in
the judgment of the Company’s officers, has or is expected to have a Material Adverse Effect. Neither the Company nor any of its
Subsidiaries have any liabilities or obligations required to be disclosed in the SEC Documents which are not so disclosed in the SEC
Documents, other than those incurred in the ordinary course of the Company’s or its Subsidiaries’ respective businesses and
which, individually or in the aggregate, do not or could not have a Material Adverse Effect. For purposes of this Agreement: (x) “Indebtedness”
of any Person means, without duplication all indebtedness for borrowed money.
(s)
Litigation. There is no action, suit, arbitration, proceeding, inquiry or investigation before or by the Principal Market, any
court, public board, other Governmental Entity, self-regulatory organization or body pending or, to the knowledge of the Company, threatened
against or affecting the Company or any of its Subsidiaries, the Common Stock or any of the Company’s or its Subsidiaries’
officers or directors, whether of a civil or criminal nature or otherwise, in their capacities as such. No director, officer or employee
of the Company or any of its subsidiaries has willfully violated 18 U.S.C. §1519 or engaged in spoliation in reasonable anticipation
of litigation. Without limitation of the foregoing, there has not been, and to the knowledge of the Company, there is not pending or
contemplated, any investigation by the SEC involving the Company, any of its Subsidiaries or any current or former director or officer
of the Company or any of its Subsidiaries. The SEC has not issued any stop order or other order suspending the effectiveness of any registration
statement filed by the Company under the 1933 Act or the 1934 Act. After reasonable inquiry of its employees, the Company is not aware
of any fact which might result in or form the basis for any such action, suit, arbitration, investigation, inquiry or other proceeding.
Neither the Company nor any of its Subsidiaries is subject to any order, writ, judgment, injunction, decree, determination or award of
any Governmental Entity.
(t)
Insurance. The Company and each of its Subsidiaries are insured by insurers of recognized financial responsibility against such
losses and risks and in such amounts as management of the Company believes to be prudent and customary in the businesses in which the
Company and its Subsidiaries are engaged. Neither the Company nor any such Subsidiary has been refused any insurance coverage sought
or applied for, and neither the Company nor any such Subsidiary has any reason to believe that it will be unable to renew its existing
insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue
its business at a cost that would not have a Material Adverse Effect.
(u)
Employee Relations. Neither the Company nor any of its Subsidiaries is a party to any collective bargaining agreement or employs
any member of a union. The Company and its Subsidiaries believe that their relations with their employees are good. No executive officer
(as defined in Rule 501(f) promulgated under the 1933 Act) or other key employee of the Company or any of its Subsidiaries has notified
the Company or any such Subsidiary that such officer intends to leave the Company or any such Subsidiary or otherwise terminate such
officer’s employment with the Company or any such Subsidiary. No current (or former) executive officer or other key employee of
the Company or any of its Subsidiaries is, or is now expected to be, in violation of any material term of any employment contract, confidentiality,
disclosure or proprietary information agreement, non-competition agreement, or any other contract or agreement or any restrictive covenant,
and the continued employment of each such executive officer or other key employee (as the case may be) does not subject the Company or
any of its Subsidiaries to any liability with respect to any of the foregoing matters. The Company and its Subsidiaries are in compliance
with all federal, state, local and foreign laws and regulations respecting labor, employment and employment practices and benefits, terms
and conditions of employment and wages and hours, except where failure to be in compliance would not, either individually or in the aggregate,
reasonably be expected to result in a Material Adverse Effect.
(v)
Title.
(i)
Real Property. Each of the Company and its Subsidiaries holds good title to all real property, leases in real property, facilities
or other interests in real property owned or held by the Company or any of its Subsidiaries (the “Real Property”) owned by
the Company or any of its Subsidiaries (as applicable). The Real Property is free and clear of all Liens and is not subject to any rights
of way, building use restrictions, exceptions, variances, reservations, or limitations of any nature except for (a) Liens for current
taxes not yet due and (b) zoning laws and other land use restrictions that do not impair the present or anticipated use of the property
subject thereto. Any Real Property held under lease by the Company or any of its Subsidiaries are held by them under valid, subsisting
and enforceable leases with such exceptions as are not material and do not interfere with the use made and proposed to be made of such
property and buildings by the Company or any of its Subsidiaries.
(ii)
Fixtures and Equipment. Each of the Company and its Subsidiaries (as applicable) has good title to, or a valid leasehold interest
in, the tangible personal property, equipment, improvements, fixtures, and other personal property and appurtenances that are used by
the Company or its Subsidiary in connection with the conduct of its business (the “Fixtures and Equipment”). The Fixtures
and Equipment are structurally sound, are in good operating condition and repair, are adequate for the uses to which they are being put,
are not in need of maintenance or repairs except for ordinary, routine maintenance and repairs and are sufficient for the conduct of
the Company’s and/or its Subsidiaries’ businesses (as applicable) in the manner as conducted prior to the Closing. Each of
the Company and its Subsidiaries owns all of its Fixtures and Equipment free and clear of all Liens except for (a) liens for current
taxes not yet due and (b) zoning laws and other land use restrictions that do not impair the present or anticipated use of the property
subject thereto.
(w)
Intellectual Property Rights. The Company and its Subsidiaries own or possess adequate rights or licenses to use all trademarks,
trade names, service marks, service mark registrations, service names, original works of authorship, patents, patent rights, copyrights,
inventions, licenses, approvals, governmental authorizations, trade secrets and other intellectual property rights and all applications
and registrations therefor (“Intellectual Property Rights”) necessary to conduct their respective businesses as now conducted
and presently proposed to be conducted. None of the Company’s Intellectual Property Rights have expired or terminated or have been
abandoned or are expected to expire or terminate or are expected to be abandoned, within three years from the date of this Agreement.
The Company does not have any knowledge of any infringement by the Company or its Subsidiaries of Intellectual Property Rights of others.
There is no claim, action or proceeding being made or brought, or to the knowledge of the Company or any of its Subsidiaries, being threatened,
against the Company or any of its Subsidiaries regarding its Intellectual Property Rights. Neither the Company nor any of its Subsidiaries
is aware of any facts or circumstances which might give rise to any of the foregoing infringements or claims, actions or proceedings.
The Company and its Subsidiaries have taken reasonable security measures to protect the secrecy, confidentiality and value of all of
their Intellectual Property Rights.
(x)
Environmental Laws. (i) The Company and its Subsidiaries (A) are in compliance with any and all Environmental Laws (as defined
below), (B) have received all permits, licenses or other approvals required of them under applicable Environmental Laws to conduct their
respective businesses and (C) are in compliance with all terms and conditions of any such permit, license or approval where, in each
of the foregoing clauses (A), (B) and (C), the failure to so comply could be reasonably expected to have, individually or in the aggregate,
a Material Adverse Effect. The term “Environmental Laws” means all federal, state, local or foreign laws relating to pollution
or protection of human health or the environment (including, without limitation, ambient air, surface water, groundwater, land surface
or subsurface strata), including, without limitation, laws relating to emissions, discharges, releases or threatened releases of chemicals,
pollutants, contaminants, or toxic or hazardous substances or wastes (collectively, “Hazardous Materials”) into the environment,
or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous
Materials, as well as all authorizations, codes, decrees, demands or demand letters, injunctions, judgments, licenses, notices or notice
letters, orders, permits, plans or regulations issued, entered, promulgated or approved thereunder.
(i)
No Hazardous Materials:
(A)
have been disposed of or otherwise released from any Real Property of the Company or any of its Subsidiaries in violation of any Environmental
Laws; or
(B)
are present on, over, beneath, in or upon any Real Property or any portion thereof in quantities that would constitute a violation of
any Environmental Laws. No prior use by the Company or any of its Subsidiaries of any Real Property has occurred that violates any Environmental
Laws, which violation would have a material adverse effect on the business of the Company or any of its Subsidiaries.
(ii)
Neither the Company nor any of its Subsidiaries knows of any other person who or entity which has stored, treated, recycled, disposed
of or otherwise located on any Real Property any Hazardous Materials, including, without limitation, such substances as asbestos and
polychlorinated biphenyls.
(iii)
None of the Real Properties are on any federal or state “Superfund” list or Liability Information System (“CERCLIS”)
list or any state environmental agency list of sites under consideration for CERCLIS, nor subject to any environmental related Liens.
(y)
Subsidiary Rights. The Company or one of its Subsidiaries has the unrestricted right to vote, and (subject to limitations imposed
by applicable law) to receive dividends and distributions on, all capital securities of its Subsidiaries as owned by the Company or such
Subsidiary.
(z)
Tax Status. The Company and each of its Subsidiaries (i) has timely made or filed all foreign, federal and state income and all
other tax returns, reports and declarations required by any jurisdiction to which it is subject, and (ii) has timely paid all taxes and
other governmental assessments and charges that are material in amount, shown or determined to be due on such returns, reports and declarations,
except those being contested in good. There are no unpaid taxes in any material amount claimed to be due by the taxing authority of any
jurisdiction, and the officers of the Company and its Subsidiaries know of no basis for any such claim. The Company is not operated in
such a manner as to qualify as a passive foreign investment company, as defined in Section 1297 of the Internal Revenue Code of 1986,
as amended (the “Code”). The net operating loss carryforwards (“NOLs”) for United States federal income tax purposes
of the consolidated group of which the Company is the common parent, if any, shall not be adversely effected by the transactions contemplated
hereby. The transactions contemplated hereby do not constitute an “ownership change” within the meaning of Section 382 of
the Code, thereby preserving the Company’s ability to utilize such NOLs.
(aa)
Internal Accounting and Disclosure Controls. The Company and each of its Subsidiaries maintains internal control over financial
reporting (as such term is defined in Rule 13a-15(f) under the 1934 Act) that is effective to provide reasonable assurance regarding
the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally
accepted accounting principles, including that (i) transactions are executed in accordance with management’s general or specific
authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP and
to maintain asset and liability accountability, (iii) access to assets or incurrence of liabilities is permitted only in accordance with
management’s general or specific authorization and (iv) the recorded accountability for assets and liabilities is compared with
the existing assets and liabilities at reasonable intervals and appropriate action is taken with respect to any difference. The Company
maintains disclosure controls and procedures (as such term is defined in Rule 13a-15(e) under the 1934 Act) that are effective in ensuring
that information required to be disclosed by the Company in the reports that it files or submits under the 1934 Act is recorded, processed,
summarized and reported, within the time periods specified in the rules and forms of the SEC, including, without limitation, controls
and procedures designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under
the 1934 Act is accumulated and communicated to the Company’s management, including its principal executive officer or officers
and its principal financial officer or officers, as appropriate, to allow timely decisions regarding required disclosure. Neither the
Company nor any of its Subsidiaries has received any notice or correspondence from any accountant, Governmental Entity or other Person
relating to any potential material weakness or significant deficiency in any part of the internal controls over financial reporting of
the Company or any of its Subsidiaries.
(bb)
Off Balance Sheet Arrangements. There is no transaction, arrangement, or other relationship between the Company or any of its
Subsidiaries and an unconsolidated or other off balance sheet entity that is required to be disclosed by the Company in its 1934 Act
filings and is not so disclosed or that otherwise could be reasonably likely to have a Material Adverse Effect.
(cc)
Investment Company Status. The Company is not, and upon consummation of the sale of the Securities will not be, an “investment
company,” an affiliate of an “investment company,” a company controlled by an “investment company” or an
“affiliated person” of, or “promoter” or “principal underwriter” for, an “investment company”
as such terms are defined in the Investment Company Act of 1940, as amended.
(dd)
Acknowledgement Regarding Buyers’ Trading Activity. It is understood and acknowledged by the Company that (i) following
the public disclosure of the transactions contemplated by the Transaction Documents, in accordance with the terms thereof, none of the
Buyers have been asked by the Company or any of its Subsidiaries to agree, nor has any Buyer agreed with the Company or any of its Subsidiaries,
to desist from effecting any transactions in or with respect to (including, without limitation, purchasing or selling, long and/or short)
any securities of the Company, or “derivative” securities based on securities issued by the Company or to hold any of the
Securities for any specified term; (ii) any Buyer, and counterparties in “derivative” transactions to which any such Buyer
is a party, directly or indirectly, presently may have a “short” position in the Common Stock which was established prior
to such Buyer’s knowledge of the transactions contemplated by the Transaction Documents; (iii) each Buyer shall not be deemed to
have any affiliation with or control over any arm’s length counterparty in any “derivative” transaction; and (iv) each
Buyer may rely on the Company’s obligation to timely deliver shares of Common Stock upon conversion or exchange, as applicable,
of the Securities as and when required pursuant to the Transaction Documents for purposes of effecting trading in the Common Stock of
the Company. The Company further understands and acknowledges that following the public disclosure of the transactions contemplated by
the Transaction Documents pursuant to the Press Release (as defined below) one or more Buyers may engage in hedging and/or trading activities
(including, without limitation, the location and/or reservation of borrowable shares of Common Stock) at various times during the period
that the Securities are outstanding, including, without limitation, during the periods that the value and/or number of the Conversion
Shares and Warrant Shares deliverable with respect to the Securities are being determined and such hedging and/or trading activities
(including, without limitation, the location and/or reservation of borrowable shares of Common Stock), if any, can reduce the value of
the existing stockholders’ equity interest in the Company both at and after the time the hedging and/or trading activities are
being conducted. The Company acknowledges that such aforementioned hedging and/or trading activities do not constitute a breach of this
Agreement, the Notes, the Warrants, or any other Transaction Document or any of the documents executed in connection herewith or therewith.
(ee)
Manipulation of Price. Neither the Company nor any of its Subsidiaries has, and, to the knowledge of the Company, no Person acting
on their behalf has, directly or indirectly, (i) taken any action designed to cause or to result in the stabilization or manipulation
of the price of any security of the Company or any of its Subsidiaries to facilitate the sale or resale of any of the Securities, (ii)
sold, bid for, purchased, or paid any compensation for soliciting purchases of, any of the Securities (other than the Placement Agent),
(iii) paid or agreed to pay to any Person any compensation for soliciting another to purchase any other securities of the Company or
any of its Subsidiaries or (iv) paid or agreed to pay any Person for research services with respect to any securities of the Company
or any of its Subsidiaries.
(ff)
U.S. Real Property Holding Corporation. Neither the Company nor any of its Subsidiaries is, or has ever been, and so long as any
of the Securities are held by any of the Buyers, shall become, a U.S. real property holding corporation within the meaning of Section
897 of the Code, and the Company and each Subsidiary shall so certify upon any Buyer’s request.
(gg)
Registration Eligibility. The Company is currently eligible to register the Registrable Securities (defined in the Registration
Rights Agreement) for resale by the Buyers using Form S-1 promulgated under the 1933 Act.
(hh)
Transfer Taxes. On the Closing Date, all stock transfer or other taxes (other than income or similar taxes) which are required
to be paid in connection with the issuance, sale and transfer of the Securities to be sold to each Buyer hereunder will be, or will have
been, fully paid or provided for by the Company, and all laws imposing such taxes will be or will have been complied with.
(ii)
Bank Holding Company Act. Neither the Company nor any of its Subsidiaries is subject to the Bank Holding Company Act of 1956,
as amended (the “BHCA”) and to regulation by the Board of Governors of the Federal Reserve System (the “Federal Reserve”).
Neither the Company nor any of its Subsidiaries or affiliates owns or controls, directly or indirectly, five percent (5%) or more of
the outstanding shares of any class of voting securities or twenty-five percent (25%) or more of the total equity of a bank or any entity
that is subject to the BHCA and to regulation by the Federal Reserve. Neither the Company nor any of its Subsidiaries or affiliates exercises
a controlling influence over the management or policies of a bank or any entity that is subject to the BHCA and to regulation by the
Federal Reserve.
(jj)
Shell Company Status. The Company is not, and has never been, an issuer identified in, or subject to, Rule 144(i).
(kk)
Illegal or Unauthorized Payments; Political Contributions. Neither the Company nor any of its Subsidiaries nor, to the best of
the Company’s knowledge (after reasonable inquiry of its officers and directors), any of the officers, directors, employees, agents
or other representatives of the Company or any of its Subsidiaries or any other business entity or enterprise with which the Company
or any Subsidiary is or has been affiliated or associated, has, directly or indirectly, made or authorized any payment, contribution
or gift of money, property, or services, whether or not in contravention of applicable law, (i) as a kickback or bribe to any Person
or (ii) to any political organization, or the holder of or any aspirant to any elective or appointive public office except for personal
political contributions not involving the direct or indirect use of funds of the Company or any of its Subsidiaries.
(ll)
Money Laundering. The Company and its Subsidiaries are in compliance with, and have not previously violated, the USA Patriot Act
of 2001 and all other applicable U.S. and non-U.S. anti-money laundering laws and regulations, including, without limitation, the laws,
regulations and Executive Orders and sanctions programs administered by the U.S. Office of Foreign Assets Control, including, but not
limited, to (i) Executive Order 13224 of September 23, 2001 entitled, “Blocking Property and Prohibiting Transactions With Persons
Who Commit, Threaten to Commit, or Support Terrorism” (66 Fed. Reg. 49079 (2001)); and (ii) any regulations contained in 31 CFR,
Subtitle B, Chapter V.
(mm)
Management. During the past five year period, no current or former officer or director or, to the knowledge of the Company, no
current ten percent (10%) or greater stockholder of the Company or any of its Subsidiaries has been the subject of:
(i)
a petition under bankruptcy laws or any other insolvency or moratorium law or the appointment by a court of a receiver, fiscal agent
or similar officer for such Person, or any partnership in which such person was a general partner at or within two years before the filing
of such petition or such appointment, or any corporation or business association of which such person was an executive officer at or
within two years before the time of the filing of such petition or such appointment;
(ii)
a conviction in a criminal proceeding or a named subject of a pending criminal proceeding (excluding traffic violations that do not relate
to driving while intoxicated or driving under the influence);
(iii)
any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or
temporarily enjoining any such person from, or otherwise limiting, the following activities:
(A)
Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage
transaction merchant, any other person regulated by the United States Commodity Futures Trading Commission or an associated person of
any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director
or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct
or practice in connection with such activity;
(B)
Engaging in any particular type of business practice; or
(C)
Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of
securities laws or commodities laws;
(iv)
any order, judgment or decree, not subsequently reversed, suspended or vacated, of any authority barring, suspending or otherwise limiting
for more than sixty (60) days the right of any such person to engage in any activity described in the preceding sub paragraph, or to
be associated with persons engaged in any such activity;
(v)
a finding by a court of competent jurisdiction in a civil action or by the SEC or other authority to have violated any securities law,
regulation or decree and the judgment in such civil action or finding by the SEC or any other authority has not been subsequently reversed,
suspended or vacated; or
(vi)
a finding by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any
federal commodities law, and the judgment in such civil action or finding has not been subsequently reversed, suspended or vacated.
(nn)
Stock Option Plans. Each stock option granted by the Company was granted (i) in accordance with the terms of the applicable stock
option plan of the Company and (ii) with an exercise price at least equal to the fair market value of the Common Stock on the date such
stock option would be considered granted under GAAP and applicable law. No stock option granted under the Company’s stock option
plan has been backdated. The Company has not knowingly granted, and there is no and has been no policy or practice of the Company to
knowingly grant, stock options prior to, or otherwise knowingly coordinate the grant of stock options with, the release or other public
announcement of material information regarding the Company or its Subsidiaries or their financial results or prospects.
(oo)
No Disagreements with Accountants and Lawyers. There are no material disagreements of any kind presently existing, or reasonably
anticipated by the Company to arise, between the Company and the accountants and lawyers formerly or presently employed by the Company
and the Company is current with respect to any fees owed to its accountants and lawyers which could affect the Company’s ability
to perform any of its obligations under any of the Transaction Documents. In addition, on or prior to the date hereof, the Company had
discussions with its accountants about its financial statements previously filed with the SEC. Based on those discussions, the Company
has no reason to believe that it will need to restate any such financial statements or any part thereof.
(pp)
No Disqualification Events. With respect to Securities to be offered and sold hereunder in reliance on Rule 506(b) under the 1933
Act (“Regulation D Securities”), none of the Company, any of its predecessors, any affiliated issuer, any director, executive
officer, other officer of the Company participating in the offering contemplated hereby, any beneficial owner of 20% or more of the Company’s
outstanding voting equity securities, calculated on the basis of voting power, nor any promoter (as that term is defined in Rule 405
under the 1933 Act) connected with the Company in any capacity at the time of sale (each, an “Issuer Covered Person” and,
together, “Issuer Covered Persons”) is subject to any of the “Bad Actor” disqualifications described in Rule
506(d)(1)(i) to (viii) under the 1933 Act (a “Disqualification Event”), except for a Disqualification Event covered by Rule
506(d)(2) or (d)(3). The Company has exercised reasonable care to determine whether any Issuer Covered Person is subject to a Disqualification
Event. The Company has complied, to the extent applicable, with its disclosure obligations under Rule 506(e), and has furnished to the
Buyers a copy of any disclosures provided thereunder.
(qq)
Other Covered Persons. The Company is not aware of any Person (other than the Placement Agent) that has been or will be paid (directly
or indirectly) remuneration for solicitation of Buyers or potential purchasers in connection with the sale of any Regulation D Securities.
(rr)
No Additional Agreements. The Company does not have any agreement or understanding with any Buyer with respect to the transactions
contemplated by the Transaction Documents other than as specified in the Transaction Documents.
(ss)
Public Utility Holding Act. None of the Company nor any of its Subsidiaries is a “holding company,” or an “affiliate”
of a “holding company,” as such terms are defined in the Public Utility Holding Act of 2005.
(tt)
Federal Power Act. None of the Company nor any of its Subsidiaries is subject to regulation as a “public utility”
under the Federal Power Act, as amended.
(uu)
Ranking of Notes. No Indebtedness of the Company, at the Closing, will be senior to the Notes in right of payment, whether with
respect to payment or redemptions, interest, damages, upon liquidation or dissolution or otherwise, unless waived by the Buyers.
(vv)
Cybersecurity. The Company and its Subsidiaries’ information technology assets and equipment, computers, systems, networks,
hardware, software, websites, applications, and databases (collectively, “IT Systems”) are adequate for, and operate and
perform in all material respects as required in connection with the operation of the business of the Company and its subsidiaries as
currently conducted, free and clear of all material bugs, errors, defects, Trojan horses, time bombs, malware and other corruptants that
would reasonably be expected to have a Material Adverse Effect on the Company’s business. The Company and its Subsidiaries have
implemented and maintained commercially reasonable physical, technical and administrative controls, policies, procedures, and safeguards
to maintain and protect their material confidential information and the integrity, continuous operation, redundancy and security of all
IT Systems and data, including “Personal Data,” used in connection with their businesses. “Personal Data” means
(i) a natural person’s name, street address, telephone number, e-mail address, photograph, social security number or tax identification
number, driver’s license number, passport number, credit card number, bank information, or customer or account number; (ii) any
information which would qualify as “personally identifying information” under the Federal Trade Commission Act, as amended;
(iii) “personal data” as defined by the European Union General Data Protection Regulation (“GDPR”) (EU 2016/679);
(iv) any information which would qualify as “protected health information” under the Health Insurance Portability and Accountability
Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act (collectively, “HIPAA”);
and (v) any other piece of information that allows the identification of such natural person, or his or her family, or permits the collection
or analysis of any data related to an identified person’s health or sexual orientation. There have been no breaches, violations,
outages or unauthorized uses of or accesses to same, except for those that have been remedied without material cost or liability or the
duty to notify any other person or such, nor any incidents under internal review or investigations relating to the same except in each
case, where such would not, either individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect. The
Company and its Subsidiaries are presently in compliance with all applicable laws or statutes and all judgments, orders, rules and regulations
of any court or arbitrator or governmental or regulatory authority, internal policies and contractual obligations relating to the privacy
and security of IT Systems and Personal Data and to the protection of such IT Systems and Personal Data from unauthorized use, access,
misappropriation or modification except in each case, where such would not, either individually or in the aggregate, reasonably be expected
to result in a Material Adverse Effect.
(ww)
Compliance with Data Privacy Laws. The Company and its Subsidiaries are, and at all prior times were, in compliance with all applicable
state and federal data privacy and security laws and regulations, including without limitation HIPAA, and the Company and its Subsidiaries
have taken commercially reasonable actions to prepare to comply with, and since May 25, 2018, have been and currently are in compliance
with, the GDPR (EU 2016/679) (collectively, the “Privacy Laws”) except in each case, where such would not, either individually
or in the aggregate, reasonably be expected to result in a Material Adverse Effect. To ensure compliance with the Privacy Laws, the Company
and its Subsidiaries have in place, comply with, and take appropriate steps reasonably designed to ensure compliance in all material
respects with their policies and procedures relating to data privacy and security and the collection, storage, use, disclosure, handling,
and analysis of Personal Data (the “Policies”). The Company and its Subsidiaries have at all times made all disclosures to
users or customers required by applicable laws and regulatory rules or requirements, and none of such disclosures made or contained in
any Policy have, to the knowledge of the Company, been inaccurate or in violation of any applicable laws and regulatory rules or requirements
in any material respect. The Company further certifies that neither it nor any Subsidiary: (i) has received notice of any actual or potential
liability under or relating to, or actual or potential violation of, any of the Privacy Laws, and has no knowledge of any event or condition
that would reasonably be expected to result in any such notice; (ii) is currently conducting or paying for, in whole or in part, any
investigation, remediation, or other corrective action pursuant to any Privacy Law; or (iii) is a party to any order, decree, or agreement
that imposes any obligation or liability under any Privacy Law.
(xx)
Disclosure. The Company confirms that neither it nor any other Person acting on its behalf has provided any of the Buyers or their
agents or counsel with any information that constitutes or could reasonably be expected to constitute material, non-public information
concerning the Company or any of its Subsidiaries, other than the existence of the transactions contemplated by this Agreement and the
other Transaction Documents. The Company understands and confirms that each of the Buyers will rely on the foregoing representations
in effecting transactions in securities of the Company. All disclosure provided to the Buyers regarding the Company and its Subsidiaries,
their businesses and the transactions contemplated hereby, including the schedules to this Agreement, furnished by or on behalf of the
Company or any of its Subsidiaries is true and correct and does not contain any untrue statement of a material fact or omit to state
any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made,
not misleading.
4.
COVENANTS.
(a)
Best Efforts. Each Buyer shall use its best efforts to timely satisfy each of the covenants hereunder and conditions to be satisfied
by it as provided in Section 6 of this Agreement. The Company shall use its best efforts to timely satisfy each of the covenants hereunder
and conditions to be satisfied by it as provided in Section 7 of this Agreement.
(b)
Form D and Blue Sky. The Company shall file a Form D with respect to the Securities as required under Regulation D and to provide
a copy thereof to each Buyer promptly after such filing. The Company shall, on or before the Closing Date, take such action as the Company
shall reasonably determine is necessary in order to obtain an exemption for, or to, qualify the Securities for sale to the Buyers at
the Closing pursuant to this Agreement under applicable securities or “Blue Sky” laws of the states of the United States
(or to obtain an exemption from such qualification), and shall provide evidence of any such action so taken to the Buyers on or prior
to the Closing Date. Without limiting any other obligation of the Company under this Agreement, the Company shall timely make all filings
and reports relating to the offer and sale of the Securities required under all applicable securities laws (including, without limitation,
all applicable federal securities laws and all applicable “Blue Sky” laws), and the Company shall comply with all applicable
foreign, federal, state and local laws, statutes, rules, regulations and the like relating to the offering and sale of the Securities
to the Buyers.
(c)
Reporting Status. Until the date on which the Buyers shall have sold all of the Registrable Securities (the “Reporting Period”),
the Company shall timely file all reports required to be filed with the SEC pursuant to the 1934 Act, and the Company shall not terminate
its status as an issuer required to file reports under the 1934 Act even if the 1934 Act or the rules and regulations thereunder would
no longer require or otherwise permit such termination. From the time Form S-3 is available to the Company for the registration of the
Registrable Securities, the Company shall take all actions necessary to maintain its eligibility to register the Registrable Securities
for resale by the Buyers on Form S-3.
(d)
Use of Proceeds. The Company will use the proceeds from the sale of the Securities for working capital, but not, directly or indirectly,
for the redemption or repurchase of any securities of the Company or any of its Subsidiaries.
(e)
Financial Information. The Company agrees to send the following to each Investor (as defined in the Registration Rights Agreement)
during the Reporting Period (i) unless the following are filed with the SEC through EDGAR and are available to the public through the
EDGAR system, within one (1) Business Day after the filing thereof with the SEC, a copy of its Annual Reports on Form 10-K and Quarterly
Reports on Form 10-Q, any interim reports or any consolidated balance sheets, income statements, stockholders’ equity statements
and/or cash flow statements for any period other than annual, any Current Reports on Form 8-K and any registration statements (other
than on Form S-8) or amendments filed pursuant to the 1933 Act, (ii) unless the following are either filed with the SEC through EDGAR
or are otherwise widely disseminated via a recognized news release service (such as PR Newswire), on the same day as the release thereof,
e-mail copies of all press releases issued by the Company or any of its Subsidiaries and (iii) unless the following are filed with the
SEC through EDGAR, copies of any notices and other information made available or given to the stockholders of the Company generally,
contemporaneously with the making available or giving thereof to the stockholders.
(f)
Listing. The Company shall promptly secure the listing or designation for quotation (as the case may be) of all of the Registrable
Securities upon each national securities exchange and automated quotation system, if any, upon which the Common Stock is then listed
or designated for quotation (as the case may be) (subject to official notice of issuance) and shall maintain such listing or designation
for quotation (as the case may be) of all Registrable Securities from time to time issuable under the terms of the Transaction Documents
on such national securities exchange or automated quotation system. The Company shall maintain the Common Stock’s listing or authorization
for quotation (as the case may be) on the Principal Market, The New York Stock Exchange, the Nasdaq American, the Nasdaq Global
Market or the Nasdaq Global Select Market (each, an “Eligible Market”). Neither the Company nor any of its Subsidiaries shall
take any action which could be reasonably expected to result in the delisting or suspension of the Common Stock on an Eligible Market.
The Company shall pay all fees and expenses in connection with satisfying its obligations under this Section 4(f).
(g)
Additional Registration Statements. Until the Applicable Date (as defined below) and at any time
(h)
Dilutive Issuances. For so long as any Notes and Warrants remain outstanding, the Company shall not, in any manner, enter into
or affect any Dilutive Issuance (as defined in the Notes) if the effect of such Dilutive Issuance is to cause the Company to be required
to issue upon conversion of any Notes or exercise of any Warrants any shares of Common Stock in excess of that number of shares of Common
Stock which the Company may issue upon conversion of the Notes or exercise of the Warrants without breaching the Company’s obligations
under the rules or regulations of the Principal Market.
(i)
Passive Foreign Investment Company. The Company shall conduct its business, and shall cause its Subsidiaries to conduct their
respective businesses, in such a manner as will ensure that the Company will not be deemed to constitute a passive foreign investment
company within the meaning of Section 1297 of the Code.
(j)
Restriction on Redemption and Cash Dividends. So long as any Notes and Warrants are outstanding, the Company shall not, directly
or indirectly, redeem, or declare or pay any cash dividend or distribution on, any securities of the Company without the prior express
written consent of the Buyers.
(k)
Corporate Existence. So long as any Buyer beneficially owns any Notes or Warrants, the Company shall not be party to any Fundamental
Transaction (as defined in the Notes) unless the Company is in compliance with the applicable provisions governing Fundamental Transactions
set forth in the Notes and Warrants.
(l)
Stock Splits. Until the Notes and Warrants are no longer outstanding, the Company shall not effect any stock combination, reverse
stock split or other similar transaction (or make any public announcement or disclosure with respect to any of the foregoing) without
the prior written consent of a majority of the Buyers.
(m)
Conversion Procedures. Each of the form of Conversion Notice and Exercise Notice (as defined in the Notes and Warrants, respectively)
included in the Notes and Warrants set forth the totality of the procedures required of the Buyers in order to convert the Notes and
exercise the Warrants. Except as provided in Section 5(d), no additional legal opinion, other information or instructions shall be required
of the Buyers to convert their Notes or exercise their Warrants. The Company shall honor conversions of the Notes and exercises of the
Warrants and shall deliver the Conversion Shares and Warrant Shares in accordance with the terms, conditions and time periods set forth
in the Notes and Warrants.
(n)
Regulation M. The Company will not take any action prohibited by Regulation M under the 1934 Act, in connection with the distribution
of the Securities contemplated hereby.
(o)
General Solicitation. None of the Company, any of its affiliates (as defined in Rule 501(b) under the 1933 Act) or any person
acting on behalf of the Company or such affiliate will solicit any offer to buy or offer or sell the Securities by means of any form
of general solicitation or general advertising within the meaning of Regulation D, including: (i) any advertisement, article, notice
or other communication published in any newspaper, magazine or similar medium or broadcast over television or radio; and (ii) any seminar
or meeting whose attendees have been invited by any general solicitation or general advertising.
(p)
Integration. None of the Company, any of its affiliates (as defined in Rule 501(b) under the 1933 Act), or any person acting on
behalf of the Company or such affiliate will sell, offer for sale, or solicit offers to buy or otherwise negotiate in respect of any
security (as defined in the 1933 Act) which will be integrated with the sale of the Securities in a manner which would require the registration
of the Securities under the 1933 Act or require stockholder approval under the rules and regulations of the Principal Market and the
Company will take all action that is appropriate or necessary to assure that its offerings of other securities will not be integrated
for purposes of the 1933 Act or the rules and regulations of the Principal Market, with the issuance of Securities contemplated hereby.
(q)
Notice of Disqualification Events. The Company will notify the Buyers in writing, prior to the Closing Date of (i) any Disqualification
Event relating to any Issuer Covered Person and (ii) any event that would, with the passage of time, become a Disqualification Event
relating to any Issuer Covered Person.
(r)
Stockholder Approval. The Company shall either (x) if the Company shall have obtained the prior written consent of the requisite
stockholders (the “Stockholder Consent”) to obtain the Stockholder Approval (as defined below), inform the stockholders of
the Company of the receipt of the Stockholder Consent by preparing and filing with the SEC, as promptly as practicable after the date
hereof.
(s)
No Sales of Pre-Delivery Shares; Return of Pre-Delivery Shares. Each Buyer, severally, hereby covenants and agrees (i) not to
sell any Pre-Delivery Shares issued to such Buyer until such time as such Pre-Delivery Shares shall become Delivery Shares (as defined
in the Notes) and (ii) that if such Buyer holds any Pre-Delivery Shares after such date that no Notes are then held by such Buyer (whether
following the conversion or redemption, as applicable, of all Notes then held by such Buyer, but after any applicable Delivery Share
Applications (as defined in the Notes) related thereto), such remaining Pre-Delivery Shares shall be promptly returned by such Buyer
to the Company for cancellation.
(t)
Holding Period. For the purposes of Rule 144, the Company acknowledges that the holding period of the Pre-Delivery Shares may
be tacked onto the holding period of the Notes and shall, consequently, be deemed to have been issued as of the Closing Date for purposes
of Rule 144 and the Company agrees not to take a position contrary to this Section 4(dd).
(u)
Closing Documents. On or prior to fourteen (14) calendar days after the Closing Date, the Company agrees to deliver, or cause
to be delivered, to each Buyer and Rimon P.C., a complete closing set of the executed Transaction Documents, Securities and any other
document required to be delivered to any party pursuant to Section 7 hereof or otherwise.
5.
REGISTER; TRANSFER AGENT INSTRUCTIONS; LEGEND.
(a)
Register. The Company shall maintain at its principal executive offices (or such other office or agency of the Company as it may
designate by notice to each holder of Securities), a register for the Notes and Warrants in which the Company shall record the name and
address of the Person in whose name the Notes and Warrants have been issued (including the name and address of each transferee), the
principal amount of the Notes and Warrants held by such Person and the number of Conversion Shares issuable pursuant to the terms of
the Notes and the number of Warrant Shares exercisable pursuant to the terms of the Warrants. The Company shall keep the register open
and available at all times during business hours for inspection of any Buyer or its legal representatives.
(b)
Transfer Agent Instructions. The Company shall issue irrevocable instructions to its transfer agent and any subsequent transfer
agent (as applicable, the “Transfer Agent”) in a form acceptable to each of the Buyers (the “Irrevocable Transfer Agent
Instructions”) to issue certificates or credit shares to the applicable balance accounts at The Depository Trust Company (“DTC”),
registered in the name of each Buyer or its respective nominee(s), for the Conversion Shares and Warrant Shares in such amounts as specified
from time to time by each Buyer to the Company upon conversion of the Notes and exercise of the Warrants. The Company represents and
warrants that no instruction other than the Irrevocable Transfer Agent Instructions referred to in this Section 5(b), and stop transfer
instructions to give effect to Section 2(g) hereof, will be given by the Company to its transfer agent with respect to the Securities,
and that the Securities shall otherwise be freely transferable on the books and records of the Company, as applicable, to the extent
provided in this Agreement and the other Transaction Documents. If a Buyer effects a sale, assignment or transfer of the Securities in
accordance with Section 2(g), the Company shall permit the transfer and shall promptly instruct its transfer agent to issue one or more
certificates or credit shares to the applicable balance accounts at DTC in such name and in such denominations as specified by such Buyer
to effect such sale, transfer or assignment. In the event that such sale, assignment or transfer involves Conversion Shares or Warrant
Shares sold, assigned or transferred pursuant to an effective registration statement or in compliance with Rule 144, the transfer agent
shall issue such shares to such Buyer, assignee or transferee (as the case may be) without any restrictive legend in accordance with
Section 5(d) below. The Company acknowledges that a breach by it of its obligations hereunder will cause irreparable harm to a Buyer.
Accordingly, the Company acknowledges that the remedy at law for a breach of its obligations under this Section 5(b) will be inadequate
and agrees, in the event of a breach or threatened breach by the Company of the provisions of this Section 5(b), that a Buyer shall be
entitled, in addition to all other available remedies, to an order and/or injunction restraining any breach and requiring immediate issuance
and transfer, without the necessity of showing economic loss and without any bond or other security being required. The Company shall
cause its counsel to issue the legal opinion referred to in the Irrevocable Transfer Agent Instructions to the Company’s transfer
agent on each Effective Date (as defined in the Registration Rights Agreement). Any fees (with respect to the transfer agent, counsel
to the Company or otherwise) associated with the issuance of such opinion or the removal of any legends on any of the Securities shall
be borne by the Company.
(c)
Legends. Each Buyer understands that the Securities have been issued (or will be issued in the case of the Conversion Shares or
Warrant Shares) pursuant to an exemption from registration or qualification under the 1933 Act and applicable state securities laws,
and except as set forth below, the Securities shall bear any legend as required by the “blue sky” laws of any state and a
restrictive legend in substantially the following form (and a stop-transfer order may be placed against transfer of such stock certificates):
[NEITHER
THE ISSUANCE AND SALE OF THE SECURITIES REPRESENTED BY THIS CERTIFICATE NOR THE SECURITIES INTO WHICH THESE SECURITIES ARE CONVERTIBLE
HAVE BEEN][THE SECURITIES REPRESENTED BY THIS CERTIFICATE HAVE NOT BEEN] REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR
APPLICABLE STATE SECURITIES LAWS. THE SECURITIES MAY NOT BE OFFERED FOR SALE, SOLD, TRANSFERRED OR ASSIGNED (I) IN THE ABSENCE OF (A)
AN EFFECTIVE REGISTRATION STATEMENT FOR THE SECURITIES UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR (B) AN OPINION OF COUNSEL TO
THE HOLDER (IF REQUESTED BY THE COMPANY), IN A FORM REASONABLY ACCEPTABLE TO THE COMPANY, THAT REGISTRATION IS NOT REQUIRED UNDER SAID
ACT OR (II) UNLESS SOLD OR ELIGIBLE TO BE SOLD PURSUANT TO RULE 144 OR RULE 144A UNDER SAID ACT. NOTWITHSTANDING THE FOREGOING, THE SECURITIES
MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN OR FINANCING ARRANGEMENT SECURED BY THE SECURITIES.
(d)
Removal of Legends. Certificates evidencing Securities shall not be required to contain the legend set forth in Section 5(c) above
or any other legend (i) while a registration statement (including a Registration Statement) covering the resale of such Securities is
effective under the 1933 Act, (ii) following any sale of such Securities pursuant to Rule 144 (assuming the transferor is not an affiliate
of the Company), (iii) if such Securities are eligible to be sold, assigned or transferred under Rule 144 (provided that a Buyer provides
the Company with reasonable assurances that such Securities are eligible for sale, assignment or transfer under Rule 144 which shall
not include an opinion of Buyer’s counsel), (iv) in connection with a sale, assignment or other transfer (other than under Rule
144), provided that such Buyer provides the Company with an opinion of counsel to such Buyer, in a generally acceptable form, to the
effect that such sale, assignment or transfer of the Securities may be made without registration under the applicable requirements of
the 1933 Act or (v) if such legend is not required under applicable requirements of the 1933 Act (including, without limitation, controlling
judicial interpretations and pronouncements issued by the SEC). If a legend is not required pursuant to the foregoing, the Company shall
no later than two (2) Trading Days (or such earlier date as required pursuant to the 1934 Act or other applicable law, rule or regulation
for the settlement of a trade initiated on the date such Buyer delivers such legended certificate representing such Securities to the
Company) following the delivery by a Buyer to the Company or the Transfer Agent (with notice to the Company) of a legended certificate
representing such Securities (endorsed or with stock powers attached, signatures guaranteed, and otherwise in form necessary to affect
the reissuance and/or transfer, if applicable), together with any other deliveries from such Buyer as may be required above in this Section
5(d), as directed by such Buyer, either: (A) provided that the Company’s Transfer Agent is participating in the DTC Fast Automated
Securities Transfer Program (“FAST”) and such Securities are Conversion Shares or Warrant Shares, credit the aggregate number
of shares of Common Stock to which such Buyer shall be entitled to such Buyer’s or its designee’s balance account with DTC
through its Deposit/Withdrawal at Custodian system or (B) if the Company’s Transfer Agent is not participating in FAST, issue and
deliver (via reputable overnight courier) to such Buyer, a certificate representing such Securities that is free from all restrictive
and other legends, registered in the name of such Buyer or its designee (the date by which such credit is so required to be made to the
balance account of such Buyer’s or such Buyer’s designee with DTC or such certificate is required to be delivered to such
Buyer pursuant to the foregoing is referred to herein as the “Required Delivery Date”, and the date such shares of Common
Stock are actually delivered without restrictive legend to such Buyer or such Buyer’s designee with DTC, as applicable, the “Share
Delivery Date”). The Company shall be responsible for any Transfer Agent fees or DTC fees with respect to any issuance of Securities
or the removal of any legends with respect to any Securities in accordance herewith.
6.
CONDITIONS TO THE COMPANY’S OBLIGATION TO SELL.
(a)
The obligation of the Company hereunder to issue and sell the Notes and Warrants to each Buyer at the Closing is subject to the satisfaction,
at or before the Closing Date, of each of the following conditions, provided that these conditions are for the Company’s sole benefit
and may be waived by the Company at any time in its sole discretion by providing each Buyer with prior written notice thereof:
(i)
Such Buyer shall have executed each of the Transaction Documents to which it is a party and delivered the same to the Company.
(ii)
Such Buyer and each other Buyer shall have delivered to the Company the Purchase Price for the Note and Warrant being purchased by such
Buyer at the Closing by wire transfer of immediately available funds.
(iii)
The representations and warranties of such Buyer shall be true and correct in all material respects as of the date when made and as of
the Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date,
which shall be true and correct as of such specific date), and such Buyer shall have performed, satisfied and complied in all material
respects with the covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by such
Buyer at or prior to the Closing Date.
7.
CONDITIONS TO EACH BUYER’S OBLIGATION TO PURCHASE.
(a)
The obligation of each Buyer hereunder to purchase its Note and Warrant at the Closing is subject to the satisfaction, at or before the
Closing Date, of each of the following conditions, provided that these conditions are for each Buyer’s sole benefit and may be
waived by such Buyer at any time in its sole discretion by providing the Company with prior written notice thereof:
(i)
The Company shall have duly executed and delivered to such Buyer each of the Transaction Documents to which it is a party, and the Company
shall have duly executed and delivered to such Buyer a Note in such original principal amount as is set forth across from such Buyer’s
name in column (2) of the Schedule of Buyers as being purchased by such Buyer at the Closing pursuant to this Agreement.
(ii)
The Company shall have duly executed and delivered to such Buyer each of the Transaction Documents to which it is a party, and the Company
shall have duly executed and delivered to such Buyer a Warrant for such number of Warrant Shares as is set forth across from such Buyer’s
name in column (3) of the Schedule of Buyers as being purchased by such Buyer at the Closing pursuant to this Agreement.
(iii)
Such Buyer shall have received the opinion of Nelson Mullins, the Company’s counsel, dated as of the Closing Date, in the form
acceptable to such Buyer.
(iv)
The Company shall have delivered to such Buyer a certificate evidencing the formation and good standing of the Company in such entity’s
jurisdiction of formation issued by the Secretary of State (or comparable office) of such jurisdiction of formation as of a date within
ten (10) days of the Closing Date.
(v)
The Company shall have delivered to such Buyer a certified copy of the Certificate of Incorporation as certified by the Delaware Secretary
of State within ten (10) days of the Closing Date.
(vi)
Each and every representation and warranty of the Company shall be true and correct as of the date when made and as of the Closing Date
as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true
and correct as of such specific date) and the Company shall have performed, satisfied and complied in all respects with the covenants,
agreements and conditions required to be performed, satisfied or complied with by the Company at or prior to the Closing Date. Such Buyer
shall have received a certificate, duly executed by the Chief Executive Officer of the Company, dated as of the Closing Date, to the
foregoing effect and as to such other matters as may be reasonably requested by such Buyer in the form acceptable to such Buyer.
(vii)
The Company shall have delivered to such Buyer a letter from the Company’s Transfer Agent certifying the number of shares of Common
Stock outstanding on the Closing Date immediately prior to the Closing.
(viii)
The Common Stock (A) shall be designated for quotation or listed (as applicable) on the Principal Market and (B) shall not have been
suspended, as of the Closing Date, by the SEC or the Principal Market from trading on the Principal Market nor shall suspension by the
SEC or the Principal Market have been threatened, as of the Closing Date, either (I) in writing by the SEC or the Principal Market or
(II) by falling below the minimum maintenance requirements of the Principal Market.
(ix)
The Company shall have obtained all governmental, regulatory or third party consents and approvals, if any, necessary for the sale of
the Securities, including without limitation, those required by the Principal Market, if any.
(x)
No statute, rule, regulation, executive order, decree, ruling or injunction shall have been enacted, entered, promulgated or endorsed
by any court or Governmental Entity of competent jurisdiction that prohibits the consummation of any of the transactions contemplated
by the Transaction Documents.
(xi)
Since the date of execution of this Agreement, no event or series of events shall have occurred that reasonably would have or result
in a Material Adverse Effect.
(xii)
Pursuant to an executed Merger Agreement (the “Merger Agreement”), the Company has consummated the merger of
Data Knights Merger Sub, Inc., a Delaware corporation, a wholly-owned subsidiary of the Company, with and into OneMedNet Corporation,
a Delaware corporation (“OneMedNet”), with OneMedNet continuing as the surviving corporation and wholly-owned subsidiary
of the Company (the “Merger”)
8.
TERMINATION.
In
the event that the Closing shall not have occurred with respect to a Buyer by October 1, 2023, then such Buyer shall have the right to
terminate its obligations under this Agreement with respect to itself at any time on or after the close of business on such date without
liability of such Buyer to any other party; provided, however, (i) the right to terminate this Agreement under this Section 8 shall not
be available to such Buyer if the failure of the transactions contemplated by this Agreement to have been consummated by such date is
the result of such Buyer’s breach of this Agreement and (ii) the abandonment of the sale and purchase of the Notes and Warrants
shall be applicable only to such Buyer providing such written notice, provided further that no such termination shall affect any obligation
of the Company under this Agreement to reimburse such Buyer for the expenses described in Section 4(g) above. Nothing contained in this
Section 8 shall be deemed to release any party from any liability for any breach by such party of the terms and provisions of this Agreement
or the other Transaction Documents or to impair the right of any party to compel specific performance by any other party of its obligations
under this Agreement or the other Transaction Documents.
9.
MISCELLANEOUS.
(a)
Governing Law; Jurisdiction; Jury Trial. All questions concerning the construction, validity, enforcement and interpretation of
this Agreement shall be governed by the internal laws of the State of Delaware, without giving effect to any choice of law or conflict
of law provision or rule (whether of the State of Delaware or any other jurisdictions) that would cause the application of the laws of
any jurisdictions other than the State of Delaware. The Company hereby irrevocably submits to the exclusive jurisdiction of the state
and federal courts sitting in The City of Wilmington, Delaware, for the adjudication of any dispute hereunder or in connection herewith
or under any of the other Transaction Documents or with any transaction contemplated hereby or thereby, and hereby irrevocably waives,
and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such
court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding
is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit,
action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that
such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to
limit in any way any right to serve process in any manner permitted by law. Nothing contained herein shall be deemed or operate to preclude
any Buyer from bringing suit or taking other legal action against the Company in any other jurisdiction to collect on the Company’s
obligations to such Buyer or to enforce a judgment or other court ruling in favor of such Buyer. EACH PARTY HEREBY IRREVOCABLY WAIVES
ANY RIGHT IT MAY HAVE TO, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR UNDER ANY OTHER TRANSACTION
DOCUMENT OR IN CONNECTION WITH OR ARISING OUT OF THIS AGREEMENT, ANY OTHER TRANSACTION DOCUMENT OR ANY TRANSACTION CONTEMPLATED HEREBY
OR THEREBY.
(b)
Counterparts. This Agreement may be executed in two or more identical counterparts, all of which shall be considered one and the
same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party. In the event
that any signature is delivered by facsimile transmission or by an e-mail which contains a portable document format (.pdf) file of an
executed signature page, such signature page shall create a valid and binding obligation of the party executing (or on whose behalf such
signature is executed) with the same force and effect as if such signature page were an original thereof.
(c)
Headings; Gender. The headings of this Agreement are for convenience of reference and shall not form part of, or affect the interpretation
of, this Agreement. Unless the context clearly indicates otherwise, each pronoun herein shall be deemed to include the masculine, feminine,
neuter, singular and plural forms thereof. The terms “including,” “includes,” “include” and words
of like import shall be construed broadly as if followed by the words “without limitation.” The terms “herein,”
“hereunder,” “hereof” and words of like import refer to this entire Agreement instead of just the provision in
which they are found.
(d)
Severability; Maximum Payment Amounts. If any provision of this Agreement is prohibited by law or otherwise determined to be invalid
or unenforceable by a court of competent jurisdiction, the provision that would otherwise be prohibited, invalid or unenforceable shall
be deemed amended to apply to the broadest extent that it would be valid and enforceable, and the invalidity or unenforceability of such
provision shall not affect the validity of the remaining provisions of this Agreement so long as this Agreement as so modified continues
to express, without material change, the original intentions of the parties as to the subject matter hereof and the prohibited nature,
invalidity or unenforceability of the provision(s) in question does not substantially impair the respective expectations or reciprocal
obligations of the parties or the practical realization of the benefits that would otherwise be conferred upon the parties. The parties
will endeavor in good faith negotiations to replace the prohibited, invalid or unenforceable provision(s) with a valid provision(s),
the effect of which comes as close as possible to that of the prohibited, invalid or unenforceable provision(s). Notwithstanding anything
to the contrary contained in this Agreement or any other Transaction Document (and without implication that the following is required
or applicable), it is the intention of the parties that in no event shall amounts and value paid by the Company and/or any of its Subsidiaries
(as the case may be), or payable to or received by any of the Buyers, under the Transaction Documents (including without limitation,
any amounts that would be characterized as “interest” under applicable law) exceed amounts permitted under any applicable
law. Accordingly, if any obligation to pay, payment made to any Buyer, or collection by any Buyer pursuant the Transaction Documents
is finally judicially determined to be contrary to any such applicable law, such obligation to pay, payment or collection shall be deemed
to have been made by mutual mistake of such Buyer, the Company and its Subsidiaries and such amount shall be deemed to have been adjusted
with retroactive effect to the maximum amount or rate of interest, as the case may be, as would not be so prohibited by the applicable
law. Such adjustment shall be effected, to the extent necessary, by reducing or refunding, at the option of such Buyer, the amount of
interest or any other amounts which would constitute unlawful amounts required to be paid or actually paid to such Buyer under the Transaction
Documents. For greater certainty, to the extent that any interest, charges, fees, expenses or other amounts required to be paid to or
received by such Buyer under any of the Transaction Documents or related thereto are held to be within the meaning of “interest”
or another applicable term to otherwise be violative of applicable law, such amounts shall be pro-rated over the period of time to which
they relate.
(e)
Entire Agreement; Amendments. This Agreement, the other Transaction Documents and the schedules and exhibits attached hereto and
thereto and the instruments referenced herein and therein supersede all other prior oral or written agreements between the Buyers, the
Company, its Subsidiaries, their affiliates and Persons acting on their behalf, including, without limitation, any transactions by any
Buyer with respect to Common Stock or the Securities, and the other matters contained herein and therein, and this Agreement, the other
Transaction Documents, the schedules and exhibits attached hereto and thereto and the instruments referenced herein and therein contain
the entire understanding of the parties solely with respect to the matters covered herein and therein; provided, however, nothing contained
in this Agreement or any other Transaction Document shall (or shall be deemed to) (i) have any effect on any agreements any Buyer has
entered into with, or any instruments any Buyer has received from, the Company or any of its Subsidiaries prior to the date hereof with
respect to any prior investment made by such Buyer in the Company or (ii) waive, alter, modify or amend in any respect any obligations
of the Company or any of its Subsidiaries, or any rights of or benefits to any Buyer or any other Person, in any agreement entered into
prior to the date hereof between or among the Company and/or any of its Subsidiaries and any Buyer, or any instruments any Buyer received
from the Company and/or any of its Subsidiaries prior to the date hereof, and all such agreements and instruments shall continue in full
force and effect. Except as specifically set forth herein or therein, neither the Company nor any Buyer makes any representation, warranty,
covenant or undertaking with respect to such matters. No waiver shall be effective unless it is in writing and signed by an authorized
representative of the waiving party. No consideration (other than reimbursement of reasonable legal fees) shall be offered or paid to
any Person to amend or consent to a waiver or modification of any provision of any of the Transaction Documents unless the same consideration
also is offered to all of the parties to the Transaction Documents, all holders of the Notes and Warrants. Without limiting the foregoing,
the Company confirms that, except as set forth in this Agreement, no Buyer has made any commitment or promise or has any other obligation
to provide any financing to the Company, any Subsidiary or otherwise.
(f)
Notices. Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement
must be in writing and will be deemed to have been delivered:(i) upon receipt, when delivered personally; (ii) upon receipt, when sent
by electronic mail (provided that such sent email is kept on file (whether electronically or otherwise) by the sending party and the
sending party does not receive an automatically generated message from the recipient’s email server that such e- mail could not
be delivered to such recipient); or (iii) one (1) Business Day after deposit with an overnight courier service with next day delivery
specified, in each case, properly addressed to the party to receive the same. The mailing addresses and e-mail addresses for such communications
shall be:
If
to the Company:
Data
Knights Acquisition Corp
Unit
G6, Frome Business Park
Manor
Road Frome
United
Kingdom, BA11 4FN Telephone:
011-44
203 833 4000 Attention: Barry
Anderson
Email:
barryanderson8@gmail.com
With
a copy (for informational purposes only) to:
Nelson
Mullins Riley & Scarborough LLP 101
Constitution
Avenue, NW, Suite 900
Washington,
DC 20001
Telephone:
(202) 689-2987 Attention:
Andy
Tucker
Email:
andy.tucker@nelsonmullins.com
If
to OneMedNet Corporation:
OneMedNet
Corporation
6385
Old Shady Oak Road, Suite 250
Eden
Prairie, Minnesota 55344
Telephone:
(808) 228-5998
Attention:
Paul Casey
E-Mail:
paul.casey@onemednet.com
With
a copy (for informational purposes only) to:
Rimon
PC
1990
K. Street, NW, Suite 420
Washington
DC 20006
Telephone:
202-935-3390
Attention:
Debbie A. Klis
E-Mail:
debbie.klis@rimonlaw.com
If
to a Buyer, to its mailing address and e-mail address set forth on the Schedule of Buyers, with copies to such Buyer’s representatives
as set forth on the Schedule of Buyers, or to such other mailing address and/or e-mail address and/or to the attention of such other
Person as the recipient party has specified by written notice given. Written confirmation of receipt (A) given by the recipient of such
notice, consent, waiver or other communication, (B) mechanically or electronically generated by the sender’s e-mail containing
the time, date and recipient’s e-mail or (C) provided by an overnight courier service shall be rebuttable evidence of personal
service, receipt by e-mail or receipt from an overnight courier service in accordance with clause (i), (ii) or (iii) above, respectively.
(g)
Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their respective successors
and assigns, including any purchasers of any of the Notes and Warrants. A Buyer may assign some or all of its rights hereunder in connection
with any transfer of any of its Securities without the consent of the Company, in which event such assignee shall be deemed to be a Buyer
hereunder with respect to such assigned rights.
(h)
No Third Party Beneficiaries. This Agreement is intended for the benefit of the parties hereto and their respective permitted
successors and assigns, and is not for the benefit of, nor may any provision hereof be enforced by, any other Person, other than the
Indemnitees referred to herein.
(i)
Survival. The representations, warranties, agreements and covenants shall survive the Closings. Each Buyer shall be responsible
only for its own representations, warranties, agreements and covenants hereunder.
(j)
Further Assurances. Each party shall do and perform, or cause to be done and performed, all such further acts and things, and
shall execute and deliver all such other agreements, certificates, instruments and documents, as any other party may reasonably request
in order to carry out the intent and accomplish the purposes of this Agreement and the consummation of the transactions contemplated
hereby.
(k)
Indemnification. In consideration of each Buyer’s execution and delivery of the Transaction Documents and acquiring the
Securities thereunder and in addition to all of the Company’s other obligations under the Transaction Documents, the Company shall
defend, protect, indemnify and hold harmless each Buyer and each holder of any Securities and all of their stockholders, partners, members,
officers, directors, employees and direct or indirect investors and any of the foregoing Persons’ agents or other representatives
(including, without limitation, those retained in connection with the transactions contemplated by this Agreement) (collectively, the
“Indemnitees”) from and against any and all actions, causes of action, suits, claims, losses, costs, penalties, fees, liabilities
and damages, and expenses in connection therewith (irrespective of whether any such Indemnitee is a party to the action for which indemnification
hereunder is sought), and including reasonable attorneys’ fees and disbursements (the “Indemnified Liabilities”), incurred
by any Indemnitee as a result of, or arising out of, or relating to (i) any misrepresentation or breach of any representation or warranty
made by the Company or any Subsidiary in any of the Transaction Documents, (ii) any breach of any covenant, agreement or obligation of
the Company or any Subsidiary contained in any of the Transaction Documents or (iii) any cause of action, suit, proceeding or claim brought
or made against such Indemnitee by a third party (including for these purposes a derivative action brought on behalf of the Company or
any Subsidiary) or which otherwise involves such Indemnitee that arises out of or results from (A) the execution, delivery, performance
or enforcement of any of the Transaction Documents, (B) any transaction financed or to be financed in whole or in part, directly or indirectly,
with the proceeds of the issuance of the Securities, (C) any disclosure properly made by such Buyer, or (D) the status of such Buyer
or holder of the Securities either as an investor in the Company pursuant to the transactions contemplated by the Transaction Documents
or as a party to this Agreement (including, without limitation, as a party in interest or otherwise in any action or proceeding for injunctive
or other equitable relief). To the extent that the foregoing undertaking by the Company may be unenforceable for any reason, the Company
shall make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities which is permissible under
applicable law. Except as otherwise set forth herein, the mechanics and procedures with respect to the rights and obligations under this
Section 9(k) shall be the same as those set forth in Section 6 of the Registration Rights Agreement.
(l)
Construction. The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual
intent, and no rules of strict construction will be applied against any party. No specific representation or warranty shall limit the
generality or applicability of a more general representation or warranty. Each and every reference to share prices, shares of Common
Stock and any other numbers in this Agreement that relate to the Common Stock shall be automatically adjusted for any stock splits, stock
dividends, stock combinations, recapitalizations or other similar transactions that occur with respect to the Common Stock after the
date of this Agreement. Notwithstanding anything in this Agreement to the contrary, for the avoidance of doubt, nothing contained herein
shall constitute a representation or warranty against, or a prohibition of, any actions with respect to the borrowing of, arrangement
to borrow, identification of the availability of, and/or securing of, securities of the Company in order for such Buyer (or its broker
or other financial representative) to effect short sales or similar transactions in the future.
(m)
Remedies. Each Buyer and in the event of assignment by Buyer of its rights and obligations hereunder, each holder of Securities,
shall have all rights and remedies set forth in the Transaction Documents and all rights and remedies which such holders have been granted
at any time under any other agreement or contract and all of the rights which such holders have under any law. Any Person having any
rights under any provision of this Agreement shall be entitled to enforce such rights specifically (without posting a bond or other security),
to recover damages by reason of any breach of any provision of this Agreement and to exercise all other rights granted by law. Furthermore,
the Company recognizes that in the event that it or any Subsidiary fails to perform, observe, or discharge any or all of its or such
Subsidiary’s (as the case may be) obligations under the Transaction Documents, any remedy at law would inadequate relief to the
Buyers. The Company therefore agrees that the Buyers shall be entitled to specific performance and/or temporary, preliminary and permanent
injunctive or other equitable relief from any court of competent jurisdiction in any such case without the necessity of proving actual
damages and without posting a bond or other security. The remedies provided in this Agreement and the other Transaction Documents shall
be cumulative and in addition to all other remedies available under this Agreement and the other Transaction Documents, at law or in
equity (including a decree of specific performance and/or other injunctive relief).
(n)
Withdrawal Right. Notwithstanding anything to the contrary contained in (and without limiting any similar provisions of) the Transaction
Documents, whenever any Buyer exercises a right, election, demand or option under a Transaction Document and the Company or any Subsidiary
does not timely perform its related obligations within the periods therein provided, then such Buyer may rescind or withdraw, in its
sole discretion from time to time upon written notice to the Company or such Subsidiary (as the case may be), any relevant notice, demand
or election in whole or in part without prejudice to its future actions and rights.
(o)
Independent Nature of Buyers’ Obligations and Rights. The obligations of each Buyer under the Transaction Documents are
several and not joint with the obligations of any other Buyer, and no Buyer shall be responsible in any way for the performance of the
obligations of any other Buyer under any Transaction Document. Nothing contained herein or in any other Transaction Document, and no
action taken by any Buyer pursuant hereto or thereto, shall be deemed to constitute the Buyers as, and the Company acknowledges that
the Buyers do not so constitute, a partnership, an association, a joint venture or any other kind of group or entity, or create a presumption
that the Buyers are in any way acting in concert or as a group or entity, and the Company shall not assert any such claim with respect
to such obligations or the transactions contemplated by the Transaction Documents or any matters, and the Company acknowledges that the
Buyers are not acting in concert or as a group, and the Company shall not assert any such claim, with respect to such obligations or
the transactions contemplated by the Transaction Documents. Each Buyer acknowledges that no other Buyer has acted as agent for such Buyer
in connection with such Buyer making its investment hereunder and that no other Buyer will be acting as agent of such Buyer in connection
with monitoring such Buyer’s investment in the Securities or enforcing its rights under the Transaction Documents. The Company
and each Buyer confirms that each Buyer has independently participated with the Company and its Subsidiaries in the negotiation of the
transaction contemplated hereby with the advice of its own counsel and advisors. Each Buyer shall be entitled to independently protect
and enforce its rights, including, without limitation, the rights arising out of this Agreement or out of any other Transaction Documents,
and it shall not be necessary for any other Buyer to be joined as an additional party in any proceeding for such purpose. The use of
a single agreement to effectuate the purchase and sale of the Securities contemplated hereby was solely in the control of the Company,
not the action or decision of any Buyer, and was done solely for the convenience of the Company and its Subsidiaries and not because
it was required or requested to do so by any Buyer. It is expressly understood and agreed that each provision contained in this Agreement
and in each other Transaction Document is between the Company, each Subsidiary and a Buyer, solely, and not between the Company, its
Subsidiaries and the Buyers collectively and not between and among the Buyers.
[signature
pages follow]
IN
WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Agreement to be duly executed as of the
date first written above.
| |
COMPANY: |
| |
|
|
| |
|
 |
| |
Name: |
Barry Anderson |
| |
Title: |
Chief Executive Officer |
IN
WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Agreement to be duly executed as of the
date first written above.
| |
BUYER: |
| |
|
|
| |
By: |
Thomas
Kosasa |
| |
Name: |
Thomas
Kosasa |
IN
WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Agreement to be duly executed as of the
date first written above.
| |
BUYER: |
| |
|
| |
By: |
Jeffrey
N C Yu |
| |
Name:
|
Revocable
Trust of Jeffrey N C Yu
|
| |
By: |
Dr.
Jeffrey N C Yu |
| |
Title: |
Trustee |
IN
WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Agreement to be duly executed as of the
date first written above.
| |
BUYER: |
| |
|
|
| |
By: |
Aaron
Green |
| |
Name: |
Aaron
Green |
IN
WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Agreement to be duly executed as of the
date first written above.
| |
BUYER: |
| |
|
|
| |
By: |
Steve
Kester |
| |
Name:
|
Iron
Family Holdings LLC
|
| |
By: |
Steve
Kester |
| |
Title: |
Manager |
Schedule of Buyers
Closing
| 1
– Name of Buyer |
|
2
– Original Principal Amount of Notes |
|
3
–
Number
of Warrant Shares |
|
4
–
Purchase
Price |
|
5
– Mailing Address and E-mail Address |
|
6
– Legal Representative’s Mailing Address and E-mail Address |
| Thomas |
|
$531,916.20 |
|
319,16.2 |
|
$500,000 |
|
c/o
OneMedNet |
|
Rimon
PC |
| Kosasa |
|
|
|
|
|
|
|
Corporation |
|
1990
K. Street, NW, Suite |
| |
|
|
|
|
|
|
|
6385
Old Shady Oak |
|
420 |
| |
|
|
|
|
|
|
|
Rd.,
Ste. 250 |
|
Washington
DC 20006 |
| |
|
|
|
|
|
|
|
Eden
Prairie, MN |
|
Tel:
202-935-3390 |
| |
|
|
|
|
|
|
|
55344 |
|
Attn:
Debbie A. Klis |
| |
|
|
|
|
|
|
|
Tel:
(808) 228-5998 |
|
E-Mail: |
| |
|
|
|
|
|
|
|
|
|
debbie.klis@rimonlaw.com |
| |
|
|
|
|
|
|
|
|
|
|
| Dr.
Jeffrey Yu |
|
$531,916.20 |
|
31,916.2 |
|
$500,000 |
|
c/o
OneMedNet |
|
Rimon
PC |
| by
Revocable |
|
|
|
|
|
|
|
Corporation |
|
1990
K. Street, NW, Suite |
| Trust
of |
|
|
|
|
|
|
|
6385
Old Shady Oak |
|
420 |
| Jeffrey
N C |
|
|
|
|
|
|
|
Rd.,
Ste. 250 |
|
Washington
DC 20006 |
| Yu |
|
|
|
|
|
|
|
Eden
Prairie, MN |
|
Tel:
202-935-3390 |
| |
|
|
|
|
|
|
|
55344 |
|
Attn:
Debbie A. Klis |
| |
|
|
|
|
|
|
|
Tel:
(808) 228-5998 |
|
E-Mail: |
| |
|
|
|
|
|
|
|
|
|
debbie.klis@rimonlaw.com |
| |
|
|
|
|
|
|
|
|
|
|
| Aaron
Green |
|
$265,956.15 |
|
15,956.15 |
|
$250,000 |
|
c/o
OneMedNet |
|
Rimon
PC |
| |
|
|
|
|
|
|
|
Corporation |
|
1990
K. Street, NW, Suite |
| |
|
|
|
|
|
|
|
6385
Old Shady Oak |
|
420 |
| |
|
|
|
|
|
|
|
Rd.,
Ste. 250 |
|
Washington
DC 20006 |
| |
|
|
|
|
|
|
|
Eden
Prairie, MN |
|
Tel:
202-935-3390 |
| |
|
|
|
|
|
|
|
55344 |
|
Attn:
Debbie A. Klis |
| |
|
|
|
|
|
|
|
Tel:
(808) 228-5998 |
|
E-Mail: |
| |
|
|
|
|
|
|
|
|
|
debbie.klis@rimonlaw.com |
| |
|
|
|
|
|
|
|
|
|
|
| Steve
Kester |
|
$265,956.15 |
|
15,956.15 |
|
$250,000 |
|
c/o
OneMedNet |
|
Rimon
PC |
| by
Iron |
|
|
|
|
|
|
|
Corporation |
|
1990
K. Street, NW, Suite |
| Family
Holdings LLC |
|
|
|
|
|
|
|
6385
Old Shady Oak
Rd.,
Ste. 250
Eden
Prairie, MN 55344 |
|
420
Washington
DC 20006
Tel:
202-935-3390
Attn:
Debbie A. Klis |
| |
|
|
|
|
|
|
|
Tel:
(808) 228-5998 |
|
E-Mail: |
| |
|
|
|
|
|
|
|
|
|
debbie.klis@rimonlaw.com |
| |
|
|
|
|
|
|
|
|
|
|
| TOTALS; |
|
$1,595,744.70 |
|
95,744.70 |
|
$1,500,000 |
|
|
|
|
Exhibit
A
Note
(Attached)
Exhibit
B
Warrant
(Attached)
Exhibit
C Registration
Rights
Agreement
(Attached)
Exhibit
D
Security
Agreement
(Attached)
Exhibit 14.1

ONEMEDNET
CORPORATION
CODE
OF ETHICS AND BUSINESS CONDUCT POLICY
(Adopted
November 8, 2023)
The
Board of Directors (the “Board”) of OneMedNet Corporation (the “Company”) has adopted this code
of ethics and business conduct policy (this “Code”), as amended from time to time by the Board, and which is applicable
to all of the Company’s directors, officers and employees (to the extent that employees are hired in the future) to:
| |
● |
promote
honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional
relationships; |
| |
|
|
| |
● |
promote
the full, fair, accurate, timely and understandable disclosure in reports and documents that the Company files with, or submits to,
the Securities and Exchange Commission (the “SEC”), as well as in other public communications made by or on behalf
of the Company; |
| |
|
|
| |
● |
promote
compliance with applicable governmental laws, rules and regulations; |
| |
|
|
| |
● |
deter
wrongdoing; and |
| |
|
|
| |
● |
require
prompt internal reporting of breaches of, and accountability for adherence to, this Code. |
In
addition to following this Code in all aspects of business activities, the Company’s directors, officers and employees are expected
to seek guidance in any situation where there is a question regarding compliance issues, whether with the letter or the spirit of the
Company’s policies and applicable laws. Cooperation with this Code is essential to the continued success of the Company’s
business and the cultivation and maintenance of its reputation as a good corporate citizen. Misconduct is never justified, even where
sanctioned or ordered by an officer or other individual in a position of higher management. No individual, regardless of stature or position,
can authorize actions that are illegal, or that jeopardize or violate Company standards. This Code sets forth general principles of conduct
and ethics and is intended to work in conjunction with the policies and procedures that are covered in the Company’s specific policy
statements.
Nothing
in this Code prohibits the Company’s directors, officers or employees from reporting possible violations of federal law or regulation
to any governmental agency or entity, including but not limited to the Department of Justice, the SEC, the Congress, and any agency Inspector
General, or making other disclosures that are protected under the whistleblower provisions of U.S. federal law or regulation. No prior
authorization from the Company is needed to make any such reports or disclosures and there is no duty to notify the Company that any
such reports or disclosures have been made. The Company has a no-tolerance policy for retaliation against persons who raise good faith
compliance, ethics or related issues.
This
Code may be amended and modified by the Board. In this Code, references to the “Company” mean OneMedNet Corporation,
Inc. and, in appropriate context, the Company’s subsidiaries, if any.
| II. |
HONEST,
ETHICAL AND FAIR CONDUCT |
Each
person owes a duty to the Company to act with integrity. Integrity requires, among other things, being honest, fair and candid. Deceit,
dishonesty and subordination of principle are inconsistent with integrity. Service to the Company should never be subordinated to violations
of laws or regulations, unscrupulous dealings or to personal gain and advantage.
Each
person must:
| |
a. |
act
with integrity, including being honest and candid while still maintaining the confidentiality of the Company’s information
where required or when in the Company’s interests; |
| |
|
|
| |
b. |
observe
all applicable governmental laws, rules and regulations; |
| |
|
|
| |
c. |
comply
with the requirements of applicable accounting and auditing standards, as well as Company policies, in order to maintain a high standard
of accuracy and completeness in the Company’s financial records and other business-related information and data; |
| |
|
|
| |
d. |
adhere
to a high standard of business ethics and not seek competitive advantage through unlawful or unethical business practices; |
| |
|
|
| |
e. |
deal
fairly with the Company’s customers, suppliers, competitors, employees and independent contractors; |
| |
|
|
| |
f. |
refrain
from taking advantage of anyone through manipulation, concealment, abuse of privileged information, misrepresentation of material
facts or any other unfair-dealing practice; |
| |
|
|
| |
g. |
protect
the assets of the Company and ensure their proper use; |
| |
|
|
| |
h. |
until
the earliest of (i) the Company’s liquidation, or (ii) such time that such person ceases to be an officer or director of the
Company, in each case, to first present to the Company for the Company’s consideration, prior to presentation to any other
entity, any business opportunity, but only if such opportunity is suitable for the Company, subject to the Company’s certificate
of incorporation and bylaws in effect at such time and subject to any other fiduciary or contractual obligations such officer or
director may have; and |
| |
|
|
| |
i. |
avoid
actual or apparent conflicts between personal, private interests and the interests of the Company, wherever possible, including receiving
improper personal benefits as a result of his or her position, except as may be allowed under guidelines or resolutions approved
by the Board (or the appropriate committee of the Board) or as disclosed in the Company’s public filings with the SEC. Anything
that would be a conflict for a person subject to this Code will also be a conflict for a member of his or her immediate family or
any other close relative. |
Examples
of conflict of interest situations include, but are not limited to, the following:
| |
● |
any
significant ownership interest in any supplier or customer; |
| |
|
|
| |
● |
any
consulting or employment relationship with any supplier or customer; |
| |
|
|
| |
● |
the
receipt of any money, non-nominal gifts or excessive entertainment from any entity with which the Company has current or prospective
business dealings; |
| |
|
|
| |
● |
selling
anything to the Company or buying anything from the Company, except on the same terms and conditions as a third party would buy or
sell a comparable item in an arm’s-length transaction; |
| |
|
|
| |
● |
any
other financial transaction, arrangement or relationship (including any indebtedness or guarantee of indebtedness) involving the
Company; and |
| |
● |
any
other circumstance, event, relationship or situation in which the personal interest of a person subject to this Code interferes,
or even appears to interfere, with the interests of the Company as a whole. |
Any
material transaction or relationship that reasonably could be expected to give rise to a conflict of interest shall be disclosed to the
Board.
The
Company’s directors, officers and employees must maintain and protect the confidentiality of information entrusted to them by the
Company, or that otherwise comes into their possession, while carrying out their duties and responsibilities, except when disclosure
is authorized by the Company or legally mandated.
Confidential
information encompasses all non-public information (including, for example, “inside information” or information that
third-parties have entrusted to the Company) that may be of use to competitors or may otherwise be harmful to the Company or its key
stakeholders, if disclosed. Financial information is of special sensitivity and should under all circumstances be considered confidential,
except where its disclosure is approved by the Company or when the information has been publicly disseminated.
The
Company strives to ensure that the contents of and the disclosures in the reports and documents that the Company files with the SEC and
other public communications shall be full, fair, accurate, timely and understandable in accordance with applicable disclosure standards,
including standards of materiality, where appropriate. Each person must:
| |
● |
not
knowingly misrepresent, or cause others to misrepresent, facts about the Company to others, whether within or outside the Company,
including to the Company’s independent registered public accountants, governmental regulators, self-regulating organizations
and other governmental officials, as appropriate; and |
| |
|
|
| |
● |
in
relation to his or her area of responsibility, properly review and critically analyze proposed disclosure for accuracy and completeness. |
In
addition to the foregoing, the Chief Executive Officer and Chief Financial Officer of the Company and the Chief Executive Officer and
the Chief Financial Officer of each subsidiary of the Company (or persons performing similar functions), if any, and each other person
that typically is involved in the financial reporting of the Company must familiarize himself or herself with the disclosure requirements
applicable to the Company as well as the business and financial operations of the Company.
Each
person must promptly bring to the attention of the Board of Directors any information he or she may have concerning (a) significant deficiencies
in the design or operation of internal and/or disclosure controls that could adversely affect the Company’s ability to record,
process, summarize and report financial data or (b) any fraud that involves management or other employees who have a significant role
in the Company’s financial reporting, disclosures or internal controls.
It
is the Company’s obligation and policy to comply with all applicable governmental laws, rules and regulations. All directors, officers
and employees of the Company are expected to understand, respect and comply with all of the laws, regulations, policies and procedures
that apply to them in their positions with the Company. Employees are responsible for talking to their supervisors to determine which
laws, regulations and Company policies apply to their position and what training is necessary to understand and comply with them.
Directors,
officers and employees are directed to specific policies and procedures available to persons they supervise.
| VI. |
REPORTING
AND ACCOUNTABILITY |
The
Board is responsible for applying this Code to specific situations in which questions are presented to it and has the authority to interpret
this Code in any particular situation. Any person who becomes aware of any existing or potential breach of this Code is required to notify
the Board promptly. Failure to do so is, in and of itself, a breach of this Code.
Specifically,
each person must:
| |
● |
Notify
the Board promptly of any existing or potential violation of this Code. |
| |
● |
Not
retaliate against any other person for reports of potential violations that are made in good faith. |
The
Company will follow the following procedures in investigating and enforcing this Code and in reporting on this Code:
| |
● |
The
Board will take all appropriate action to investigate any breaches reported to it. |
| |
● |
Upon
determination by the Board that a breach has occurred, the Board (by majority decision) will take or authorize such disciplinary
or preventive action as it deems appropriate, after consultation with the Company’s internal or external legal counsel, up
to and including dismissal or, in the event of criminal or other serious violations of law, notification of the SEC or other appropriate
law enforcement authorities. |
No
person following the above procedure shall, as a result of following such procedure, be subject by the Company or any officer or employee
thereof to discharge, demotion suspension, threat, harassment or, in any manner, discrimination against such person in terms and conditions
of employment.
| VII. |
WAIVERS
AND AMENDMENTS |
Any
waiver (defined below) or an implicit waiver (defined below) from a provision of this Code for the principal executive officer, principal
financial officer, principal accounting officer or controller, and persons performing similar functions or any amendment (as defined
below) to this Code is required to be disclosed in a Current Report on Form 8-K filed with the SEC. In lieu of filing a Current Report
on Form 8-K to report any such waivers or amendments, the Company may provide such information on its website, in the event that it establishes
one in the future, and if it keeps such information on the website for at least 12 months and discloses the website address as well as
any intention to provide such disclosures in this manner in its most recently filed Annual Report on Form 10-K.
A
“waiver” means the approval by the Board of a material departure from a provision of this Code. An “implicit
waiver” means the Company’s failure to take action within a reasonable period of time regarding a material departure
from a provision of this Code that has been made known to an executive officer of the Company. An “amendment” means
any amendment to this Code other than minor technical, administrative or other non-substantive amendments hereto.
Any
request for a waiver of any provision of this Code must be in writing and addressed to the Board. All persons should note that it is
not the Company’s intention to grant or to permit waivers from the requirements of this Code. The Company expects full compliance
with this Code.
| VIII. |
INSIDER
INFORMATION AND SECURITIES TRADING |
The
Company’s directors, officers or employees who have access to material, non-public information are not permitted to use that information
for security trading purposes or for any purpose unrelated to the Company’s business. It is also against the law to trade or to
“tip” others who might make an investment decision based on inside company information. For example, using non-public information
to buy or sell the Company securities, options in the Company securities or the securities of any Company supplier, customer, competitor
or potential target is prohibited. The consequences of insider trading violations can be severe. These rules also apply to the use of
material, non-public information about other companies (including, for example, the Company’s customers, competitors, potential
business partners and potential targets). In addition to directors, officers or employees, these rules apply to such person’s spouse,
children, parents and siblings, as well as any other family members living in such person’s home.
| IX. |
FINANCIAL
STATEMENTS AND OTHER RECORDS |
All
of the Company’s books, records, accounts and financial statements must be maintained in reasonable detail, must appropriately
reflect the Company’s transactions and must both conform to applicable legal requirements and to the Company’s system of
internal controls. Unrecorded or “off the books” funds or assets should not be maintained unless permitted by applicable
law or regulation.
Records
should always be retained or destroyed according to the Company’s record retention policies. In accordance with those policies,
in the event of litigation or governmental investigation, please consult the Board or the Company’s internal or external legal
counsel.
Any
and all reports received by the Company of questionable accounting, violations of internal accounting controls, or any other auditing
or financial matters, or the reporting of fraudulent financial information or other questionable conduct that are submitted to an officer
of the Company will be handled as follows:
| |
● |
All
reports received will be logged and include, among other things: (1) the date the report was received, (2) a description of the report,
(3) the reporting party (if provided), and (4) the status and disposition of an investigation of the report. |
| |
● |
The
Secretary of the Company will promptly submit to the Audit Committee of the Board (the “Audit Committee”) all
reports received. The Audit Committee shall direct and oversee an investigation of all reports as it determines to be appropriate.
The Audit Committee may also delegate the oversight and investigation of reports to the appropriate members of the Company’s
management. The Audit Committee may request special treatment for any report and may re-assume the direction and oversight of an
investigation of any report delegated to members of our management. |
| X. |
IMPROPER
INFLUENCE ON CONDUCT OF AUDITS |
No
director or officer, or any other person acting under the direction thereof, shall directly or indirectly take any action to coerce,
manipulate, mislead or fraudulently influence any public or certified public accountant engaged in the performance of an audit or review
of the financial statements of the Company or take any action that such person knows or should know that if successful could result in
rendering the Company’s financial statements materially misleading. Any person who believes such improper influence is being exerted
should report such action to such person’s supervisor, or if that is impractical under the circumstances, to any of the Company’s
directors.
Types
of conduct that could constitute improper influence include, but are not limited to, directly or indirectly:
| |
● |
Offering
or paying bribes or other financial incentives, including future employment or contracts for non-audit services; |
| |
|
|
| |
● |
Providing
an auditor with an inaccurate or misleading legal analysis; |
| |
|
|
| |
● |
Threatening
to cancel or canceling existing non-audit or audit engagements if the auditor objects to the Company’s accounting; |
| |
|
|
| |
● |
Seeking
to have a partner removed from the audit engagement because the partner objects to the Company’s accounting; |
| |
|
|
| |
● |
Blackmailing;
and |
| |
|
|
| |
● |
Making
physical threats. |
The
Company complies with the anti-corruption laws of the countries in which it does business, including the U.S. Foreign Corrupt Practices
Act of 1977 (“FCPA”). Directors, officers, employees and agents, such as third-party sales representatives, shall
not take or cause to be taken any action that would reasonably result in the Company not complying with such anti-corruption laws, including
the FCPA. If you are authorized to engage agents on the Company’s behalf, you are responsible for ensuring they are reputable and
for obtaining a written agreement for them to uphold the Company’s standards in this area. The Company will comply with the Policy
Statement Regarding Compliance and Ethics attached hereto as Schedule A.
The
Board will investigate any reported violations and will oversee an appropriate response, including corrective action and preventative
measures. Any director, officer or employee who violates this Code will face appropriate, case specific disciplinary action, which may
include demotion or discharge. Such action is in addition to any civil or criminal liability which might be imposed by any court or regulatory
agency.
| XIII. |
OTHER
POLICIES AND PROCEDURES |
Any
other policy or procedure set out by the Company in writing or made generally known to employees, officers or directors of the Company
prior to the date hereof or hereafter are separate requirements and remain in full force and effect.
All
inquiries and questions in relation to this Code or its applicability to particular people or situations should be addressed to the Board,
or such other compliance officer as shall be designated from time to time by the Company.
Policy
Statement Regarding Compliance & Ethics
OneMedNet
Corporation, Inc. (the “Company”) is committed to the highest ethical standards, and expects the same of its directors,
officers and employees (to the extent that employees are hired in the future), Third Party (as defined below) representatives, and joint
venture partners. Consistent with this commitment, all Company employees are expected to understand and comply with all applicable laws,
rules, and regulations applicable to their job responsibilities at the Company.
This
Policy Statement sets forth the Company’s general requirements and expectations relating to several key areas of business conduct
and ethics. At the same time, this Policy Statement is not intended to be a comprehensive rulebook and cannot address every situation
that a Company director, officer and employee (to the extent that employees are hired in the future) may face. If any Company director,
officer or employee (to the extent that employees are hired in the future) feels uncomfortable about a situation or has any doubts about
whether a situation or course of conduct is consistent with the Company’s ethical standards, such individuals are advised to seek
help from the Company’s Chief Financial Officer.
| II. |
COMPLIANCE
WITH ALL LAWS INCLUDING BRIBERY AND OTHER CORRUPT PAYMENTS LAWS |
The
Company shall comply with all applicable laws, including applicable laws governing bribery, extortion, kickbacks, and the giving or receiving
of gifts or hospitality to “Government Officials.”1 Consistent with this commitment, Company directors,
officers or employees (to the extent that employees are hired in the future) are prohibited from offering, promising, giving, soliciting,
accepting or authorizing another to offer, promise, give, solicit or accept anything of value, either directly or indirectly, for the
purpose of corruptly influencing the decision of any person, or to otherwise obtain or retain business or a business advantage in connection
with the Company. This includes all local, state and federal corruption laws, as well as international laws such as the Foreign Corrupt
Practices Act, to the extent the Company is conducting business outside of the United States.
| III. |
FACILITATION
PAYMENTS |
It
is the Company’s policy not to make or authorize anyone to make any small, unofficial payment to secure or expedite the performance
of a routine or necessary action by a Government Official (a “Facilitation Payment”)2 in connection with
the Company.
The
Company requires that all gifts and hospitality promised, offered or provided on behalf of the Company be reasonable, related to a legitimate
business purpose, and lawful under applicable laws and regulations, including those relating to the nature and amount of gifts and hospitality
that can be provided to Government Officials.
1
“Government Official” means: (i) any person who is an officer, officeholder, full or part-time employee or representative
of: (1) a national, state, regional, provincial, city, county or other local government; (2) independent agencies of any government;
or (3) state-owned businesses or state-controlled businesses (e.g., a representative of a sovereign wealth fund or public pension fund,
an affiliate of a state-owned company owning distressed assets); (ii) political party officials and candidates for political office;
and (iii) any employees of quasi-public or non-governmental international organizations (sometimes called “NGOs”).
2
A “Facilitation Payment” may take the form of any kind of advantage, and is not limited to cash or cash equivalents.
No
Company employee or Third Party may offer, promise, give, solicit, accept or authorize another to offer, promise, give, solicit or accept
any gift or hospitality for the purpose of corruptly influencing the decision of any person, or to otherwise obtain or retain business
or a business advantage on behalf of the Company.
All
gifts and hospitality are required to be approved by appropriate Company personnel before being provided to any external party, and shall
be documented fairly and accurately in the Company’s books and records.
| V. |
POLITICAL
AND CHARITABLE CONTRIBUTIONS |
It
is the policy of the Company that its funds or assets not be used to make a political or charitable contribution,3 unless
prior approval has been given by the Company’s Chief Financial Officer. The Company shall not reimburse any Company employee or
Third Party for their own personal political or charitable contributions. When Company employees participate in private political affairs,
those employees should be careful to make it clear that their views and actions are their own, and not made on behalf of the Company.
Company
employees are prohibited from making or authorizing any person to make a political or charitable contribution on behalf of the Company
for the purpose of corruptly influencing the decision of any person, or to otherwise obtain or retain business or a business advantage.
| VI. |
RELATIONSHIPS
WITH THIRD PARTIES |
The
Company shall conduct reasonable commercial and risk-based due diligence on all third party vendors, suppliers, agents, representatives,
and intermediaries (each a “Third Party” and collectively “Third Parties”) working on behalf of
the Company.
All
agreements with Third Parties relating to the Company shall be reduced to writing, accurately reflect the actual goods or services to
be provided by the Third Party, and include appropriate anti-corruption compliance representations and warranties.
All
payments to Third Parties made by or on behalf of the Company shall be made transparently, consistent with applicable written agreements,
and reflected accurately in the Company’s books and records.
| VII. |
DISCRIMINATION
& HARASSMENT |
Company
employees shall not discriminate, harass, or authorize any discrimination or harassment on the basis of race, color, religion, sex, sexual
orientation, gender identity or expression, age, disability, marital status, citizenship, genetic information, or any other characteristic
protected by law.
The
Company will not tolerate or authorize any unwelcome sexual advances, requests for sexual favors, or any other verbal or physical conduct
of a sexual nature by any Company employee or Third Party in connection with the Company if and when: (i) submission to such conduct
was made either explicitly or implicitly a term or condition of an individual’s employment; (ii) submission to or rejection of
such conduct by an individual is used as the basis for employment decisions affecting such individual; or (iii) such conduct has the
purpose or effect of unreasonably interfering with an individual’s work performance or creating an intimidating, hostile, or offensive
working environment.
3
For purposes of this provision, the term “political contribution” refers to any contribution of something of
value to an incumbent elected official, a candidate for elected office, a political party, a political committee, a political organization,
a ballot measure committee, an inaugural committee, or a member of the transition team of a successful candidate. The term “charitable
contribution” refers to any contribution of something of value to a charitable or philanthropic cause, including, but not limited
to donations of cash or cash equivalents to registered charities.
It
is the policy of the Company to prohibit and actively prevent money laundering and any activity that could conceivably facilitate money
laundering, the funding of terrorism, or other criminal activity. Consistent with this policy, the local Company partner shall develop
appropriate procedures to help ensure that the Company does not facilitate or engage in any money laundering activities.
| IX. |
HEALTH,
SAFETY, AND ENVIRONMENT |
The
Company is committed to conducting all business activities in a responsible manner, which helps ensure the health, safety, and security
of people, preservation of the environment, quality of our business offerings, security, legal, quality, and regulatory requirements
in all our business activities.
The
Company shall make and maintain records that fairly and accurately reflect the business transactions of the Company.
| XI. |
COOPERATION
WITH AUDITS AND INSPECTIONS |
The
Company, including each Company employee and Third Party, shall cooperate fully with any and all requests made by its shareholders relating
to the review and/or inspection of books and records relating to the Company.
The
Company encourages reporting of suspected violations of this policy statement, violations of law, and perceived incidents of discrimination
or harassment. The Company will not tolerate actions by Company directors, officers or employees (to the extent that employees are hired
in the future) or Third Parties to discourage or authorize discouragement of any kind against any individual who reports a perceived
incident of discrimination or harassment in relation to the Company.
Retaliation
of any kind against any individual who reports any perceived incident of discrimination or harassment, or who cooperates with any investigation
relating to such reports in good faith is prohibited. Engaging in any form of retaliation may subject Company personnel to discipline,
up to and including termination.
Failure
to comply with the guidelines and requirements set forth in this Policy Statement is a serious matter. Violating applicable laws, regulations,
or the policies set forth in this document, or otherwise engaging in illegal, improper, or unethical conduct may result in appropriate
remedial action.
Exhibit
23.1
INDEPENDENT
REGISTERED PUBLIC ACCOUNTING FIRM’S CONSENT
We
hereby consent to the incorporation in this Form S-1 of our report dated March 23, 2023, relating to the financial statements of OneMedNet
Corporation as of December 31, 2022 and 2021 and to all references to our firm included in this registration statement.

BF
Borgers CPA PC
Certified
Public Accountants
Lakewood,
CO
December
15, 2023
Exhibit
107
Calculation
of Filing Fee Tables
FORM
S-1
(Form
Type)
ONEMEDNET
CORPORATION
(Exact
Name of Registrant as Specified in its Charter)
Table
1: Newly Registered Securities
| Title of each Class of Securities to be Registered | |
Amount
Registered | | |
Proposed
Maximum
Offering
Price Per
Share | | |
Maximum
Aggregate
Offering
Price(1) | | |
Amount of
Registration
Fee(2) | |
| Common Stock, $0.0001 par value | |
| - | | |
| - | | |
$ | 6,000,000 | | |
$ | 885.60.00 | |
| Pre-funded Warrants | |
| - | | |
| - | | |
| Included above | | |
| - | |
| Representative’s Warrants(3) | |
| - | | |
| - | | |
| - | | |
| - | |
| Common Stock underlying Pre-funded Warrants | |
| - | | |
| - | | |
| Included above | | |
| - | |
| Registration Fee Previously Paid | |
| | | |
| | | |
| | | |
| - | |
| Registration Fee Paid Herewith | |
| | | |
| | | |
| | | |
$ | 885.60.00 | |
| (1) |
The
registration fee for securities is based on an estimate of the Maximum Aggregate Offering Price of the securities, assuming the sale
of the maximum number of shares at the highest expected offering price, and such estimate is solely for the purpose of calculating
the registration fee (i.e., 0.00014760) pursuant to Rule 457(o). |
| |
|
| (2) |
Calculated
pursuant to Rule 457(a) based on an estimate of the proposed maximum aggregate offering price. |
| |
|
| (3) |
No
registration fee required pursuant to Rule 457(g). |
v3.23.3
Cover
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Entity Addresses [Line Items] |
|
| Document Type |
S-1
|
| Amendment Flag |
false
|
| Entity Registrant Name |
ONEMEDNET
CORPORATION
|
| Entity Central Index Key |
0001849380
|
| Entity Tax Identification Number |
86-2049355
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
6385
Old Shady Oak Road
|
| Entity Address, Address Line Two |
Suite 250
|
| Entity Address, City or Town |
Eden Prairie
|
| Entity Address, State or Province |
MN
|
| Entity Address, Postal Zip Code |
55344
|
| City Area Code |
800
|
| Local Phone Number |
918-7189
|
| Entity Filer Category |
Non-accelerated Filer
|
| Entity Small Business |
true
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| Business Contact [Member] |
|
| Entity Addresses [Line Items] |
|
| Entity Address, Address Line One |
1990
K. Street
|
| Entity Address, Address Line Two |
NW, Suite 420
|
| Entity Address, Address Line Three |
Washington
|
| Entity Address, State or Province |
DC
|
| Entity Address, Postal Zip Code |
20006
|
| City Area Code |
(202)
|
| Local Phone Number |
935-3390
|
| Contact Personnel Name |
Debbie
A. Klis, Esq.
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 3 such as an Office Park
| Name: |
dei_EntityAddressAddressLine3 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
dei_EntityAddressesLineItems |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Condensed Consolidated Balance Sheets - USD ($)
|
Sep. 30, 2023 |
Dec. 31, 2022 |
| Current assets |
|
|
| Cash |
$ 1,537
|
$ 30,870
|
| Prepaid expense |
21,600
|
|
| Total Current Assets |
23,137
|
30,870
|
| Investments held in Trust Account |
19,308,261
|
29,029,416
|
| Total assets |
19,331,398
|
29,060,286
|
| Current Liabilities |
|
|
| Accrued expenses |
2,835,755
|
1,679,821
|
| Income tax payable |
120,017
|
214,850
|
| Franchise tax payable |
|
69,966
|
| Excise tax liability |
113,353
|
|
| Total Current Liabilities |
3,080,325
|
1,976,137
|
| Warrant liabilities |
604,849
|
362,558
|
| Deferred underwriter fee payable |
4,025,000
|
4,025,000
|
| Extension loan |
3,556,278
|
207,081
|
| Working capital loans |
477,548
|
2,545,838
|
| Total liabilities |
11,744,000
|
9,116,614
|
| Commitments and Contingencies |
|
|
| Class A Common Stock subject to possible redemption;1,712,698 and 2,731,544 shares at redemption value of $11.22 and $10.53 per share as of September 30, 2023 and December 31, 2022, respectively |
19,209,844
|
28,750,110
|
| Stockholders’ Deficit |
|
|
| Preferred shares, $0.0001 par value; 1,000,000 shares authorized; none issued and outstanding |
|
|
| Additional paid-in capital |
917,476
|
2,825,823
|
| Accumulated deficit |
(12,540,406)
|
(11,632,745)
|
| Total Stockholders’ Deficit |
(11,622,446)
|
(8,806,438)
|
| Total Liabilities and Stockholders’ Deficit |
19,331,398
|
29,060,286
|
| Common Class A [Member] |
|
|
| Stockholders’ Deficit |
|
|
| Common Stock, value |
59
|
59
|
| Common Class B [Member] |
|
|
| Stockholders’ Deficit |
|
|
| Common Stock, value |
425
|
425
|
| Related Party [Member] |
|
|
| Current Liabilities |
|
|
| Amount due to related parties |
$ 11,200
|
$ 11,500
|
| X |
- DefinitionDeferred offering costs non current.
| Name: |
ONMD_DeferredOfferingCostsNonCurrent |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionWarrant liability non current.
| Name: |
ONMD_WarrantLiabilityNonCurrent |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of cash, securities, or other assets held by a third-party trustee pursuant to the terms of an agreement which assets are available to be used by beneficiaries to that agreement only within the specific terms thereof and which agreement is expected to terminate more than one year from the balance sheet date (or operating cycle, if longer) at which time the assets held-in-trust will be released or forfeited. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_AssetsHeldInTrustNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-21
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 210
-Topic 946
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-20
| Name: |
us-gaap_Cash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of loans payable (with maturities initially due after one year or beyond the operating cycle if longer), excluding current portion. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermLoansPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.15)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_OtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred through that date and payable for statutory sales and use taxes, including value added tax. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_SalesAndExciseTaxPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount, attributable to parent, of an entity's issued and outstanding stock which is not included within permanent equity. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. Includes stock with a put option held by an ESOP and stock redeemable by a holder only in the event of a change in control of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.E.Q2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
| Name: |
us-gaap_TemporaryEquityCarryingAmountAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Condensed Consolidated Balance Sheets (Parenthetical) - $ / shares
|
Sep. 30, 2023 |
Dec. 31, 2022 |
| Common stock, subject to possible redemption |
1,712,698
|
2,731,544
|
| Class A common stock subject to possible redemption, price per share |
$ 11.22
|
$ 10.53
|
| Preferred stock, par value |
$ 0.0001
|
$ 0.0001
|
| Preferred stock, shares authorized |
1,000,000
|
1,000,000
|
| Preferred stock, shares issued |
0
|
0
|
| Preferred stock, shares outstanding |
0
|
0
|
| Common Class A [Member] |
|
|
| Common stock, subject to possible redemption |
1,712,698
|
1,712,698
|
| Common stock, par value |
$ 0.0001
|
$ 0.0001
|
| Common stock, shares authorized |
100,000,000
|
100,000,000
|
| Common stock, shares issued |
585,275
|
585,275
|
| Common stock, shares outstanding |
585,275
|
585,275
|
| Common Class B [Member] |
|
|
| Common stock, par value |
$ 0.0001
|
$ 0.0001
|
| Common stock, shares authorized |
10,000,000
|
10,000,000
|
| Common stock, shares issued |
4,253,517
|
4,253,517
|
| Common stock, shares outstanding |
4,253,517
|
4,253,517
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount to be paid per share that is classified as temporary equity by entity upon redemption. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. If convertible, the issuer does not control the actions or events necessary to issue the maximum number of shares that could be required to be delivered under the conversion option if the holder exercises the option to convert the stock to another class of equity. If the security is a warrant or a rights issue, the warrant or rights issue is considered to be temporary equity if the issuer cannot demonstrate that it would be able to deliver upon the exercise of the option by the holder in all cases. Includes stock with put option held by ESOP and stock redeemable by holder only in the event of a change in control of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 480
-SubTopic 10
-Section S99
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480244/480-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (27)
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TemporaryEquityRedemptionPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of securities classified as temporary equity that have been issued and are held by the entity's shareholders. Securities outstanding equals securities issued minus securities held in treasury. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. If convertible, the issuer does not control the actions or events necessary to issue the maximum number of shares that could be required to be delivered under the conversion option if the holder exercises the option to convert the stock to another class of equity. If the security is a warrant or a rights issue, the warrant or rights issue is considered to be temporary equity if the issuer cannot demonstrate that it would be able to deliver upon the exercise of the option by the holder in all cases. Includes stock with put option held by ESOP and stock redeemable by holder only in the event of a change in control of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TemporaryEquitySharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Condensed Consolidated Statements of Operations (Unaudited) - USD ($)
|
3 Months Ended |
9 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Formation and operating costs |
$ 908,077
|
$ 467,223
|
$ 1,455,434
|
$ 1,771,902
|
| Franchise tax expense |
10,400
|
52,802
|
102,281
|
152,802
|
| Loss from operation costs |
(918,477)
|
(520,025)
|
(1,557,715)
|
(1,924,704)
|
| Other income (expense): |
|
|
|
|
| Dividends, realized and unrealized gain in Trust Account |
431,961
|
587,303
|
1,102,363
|
779,706
|
| Change in fair value of warrant liabilities |
(242,291)
|
(113,829)
|
(242,291)
|
4,243,893
|
| Net income (loss) before provision for income taxes |
(728,807)
|
(46,551)
|
(697,643)
|
3,098,895
|
| Provision for income taxes |
(87,713)
|
(106,724)
|
(210,017)
|
(106,724)
|
| Net income (loss) |
(816,520)
|
(153,275)
|
(907,660)
|
2,992,171
|
| Common Class A Subject To Redemption [Member] |
|
|
|
|
| Other income (expense): |
|
|
|
|
| Net income (loss) |
$ (252,542)
|
$ (117,823)
|
$ (312,541)
|
$ 2,300,089
|
| Weighted average shares outstanding, basic |
2,166,749
|
11,500,000
|
2,541,210
|
11,500,000
|
| Weighted average shares outstanding, diluted |
2,166,749
|
11,500,000
|
2,541,210
|
11,500,000
|
| Basic net income (loss) per common stock |
$ (0.12)
|
$ 0.01
|
$ (0.12)
|
$ 0.20
|
| Diluted net income (loss) per common stock |
$ (0.12)
|
$ 0.01
|
$ (0.12)
|
$ 0.20
|
| Class A And Class B Common Stock Not Subject To Redemption [Member] |
|
|
|
|
| Other income (expense): |
|
|
|
|
| Net income (loss) |
$ (563,978)
|
$ (35,452)
|
$ (595,119)
|
$ 692,082
|
| Weighted average shares outstanding, basic |
4,838,792
|
3,460,275
|
4,838,792
|
3,460,275
|
| Weighted average shares outstanding, diluted |
4,838,792
|
3,460,275
|
4,838,792
|
3,460,275
|
| Basic net income (loss) per common stock |
$ (0.12)
|
$ 0.01
|
$ (0.12)
|
$ 0.20
|
| Diluted net income (loss) per common stock |
$ (0.12)
|
$ 0.01
|
$ (0.12)
|
$ 0.20
|
| X |
- DefinitionRealized and unrealized gain loss.
| Name: |
ONMD_RealizedAndUnrealizedGainLoss |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Excludes Selling, General and Administrative Expense.
| Name: |
us-gaap_OperatingCostsAndExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ONMD_CommonClassASubjectToRedemptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ONMD_ClassAAndClassBCommonStockNotSubjectToRedemptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Condensed Consolidated Statements of Changes in Stockholders' Deficit (Unaudited) - USD ($)
|
Common Class A [Member]
Common Stock [Member]
|
Common Class B [Member]
Common Stock [Member]
|
Additional Paid-in Capital [Member] |
Retained Earnings [Member] |
Total |
| Balance — June 30, 2022 (unaudited) at Dec. 31, 2021 |
$ 59
|
$ 288
|
|
$ (8,609,810)
|
$ (8,609,463)
|
| Balance, shares at Dec. 31, 2021 |
585,275
|
2,875,000
|
|
|
|
| Re-measurement of carrying value of Class A redeemable stock to redemption value |
|
|
|
(1,150,000)
|
(1,150,000)
|
| Net income (loss) |
|
|
|
3,145,445
|
3,145,455
|
| Balance at Jun. 30, 2022 |
$ 59
|
$ 288
|
|
(6,614,365)
|
(6,614,018)
|
| Balance, shares at Jun. 30, 2022 |
585,275
|
2,875,000
|
|
|
|
| Balance — June 30, 2022 (unaudited) at Dec. 31, 2021 |
$ 59
|
$ 288
|
|
(8,609,810)
|
(8,609,463)
|
| Balance, shares at Dec. 31, 2021 |
585,275
|
2,875,000
|
|
|
|
| Net income (loss) |
|
|
|
|
2,992,171
|
| Balance at Sep. 30, 2022 |
$ 59
|
$ 288
|
|
(8,451,509)
|
(8,451,162)
|
| Balance, shares at Sep. 30, 2022 |
585,275
|
2,875,000
|
|
|
|
| Balance — June 30, 2022 (unaudited) at Jun. 30, 2022 |
$ 59
|
$ 288
|
|
(6,614,365)
|
(6,614,018)
|
| Balance, shares at Jun. 30, 2022 |
585,275
|
2,875,000
|
|
|
|
| Re-measurement of carrying value of Class A redeemable stock to redemption value |
|
|
|
(1,150,000)
|
(1,150,000)
|
| Net income (loss) |
|
|
|
(153,275)
|
(153,275)
|
| Accretion of Class A common stocks at redemption value |
|
|
|
(533,869)
|
(533,869)
|
| Balance at Sep. 30, 2022 |
$ 59
|
$ 288
|
|
(8,451,509)
|
(8,451,162)
|
| Balance, shares at Sep. 30, 2022 |
585,275
|
2,875,000
|
|
|
|
| Balance — June 30, 2022 (unaudited) at Dec. 31, 2022 |
$ 59
|
$ 425
|
2,242,253
|
(11,632,745)
|
(8,806,438)
|
| Balance, shares at Dec. 31, 2022 |
585,275
|
4,253,517
|
|
|
|
| Re-measurement of carrying value of Class A redeemable stock to redemption value |
|
|
(583,570)
|
|
(583,570)
|
| Net income (loss) |
|
|
|
(85,983)
|
(85,983)
|
| Balance at Mar. 31, 2023 |
$ 59
|
$ 425
|
2,242,253
|
(11,718,728)
|
(9,475,991)
|
| Balance, shares at Mar. 31, 2023 |
585,275
|
4,253,517
|
|
|
|
| Balance — June 30, 2022 (unaudited) at Dec. 31, 2022 |
$ 59
|
$ 425
|
2,242,253
|
(11,632,745)
|
(8,806,438)
|
| Balance, shares at Dec. 31, 2022 |
585,275
|
4,253,517
|
|
|
|
| Net income (loss) |
|
|
|
|
(907,660)
|
| Balance at Sep. 30, 2023 |
$ 59
|
$ 425
|
917,476
|
(12,540,406)
|
(11,622,446)
|
| Balance, shares at Sep. 30, 2023 |
585,275
|
4,253,517
|
|
|
|
| Balance — June 30, 2022 (unaudited) at Mar. 31, 2023 |
$ 59
|
$ 425
|
2,242,253
|
(11,718,728)
|
(9,475,991)
|
| Balance, shares at Mar. 31, 2023 |
585,275
|
4,253,517
|
|
|
|
| Re-measurement of carrying value of Class A redeemable stock to redemption value |
|
|
(604,656)
|
|
(604,656)
|
| Net income (loss) |
|
|
|
(5,158)
|
(5,158)
|
| Balance at Jun. 30, 2023 |
$ 59
|
$ 425
|
1,637,597
|
(11,723,886)
|
(10,085,805)
|
| Balance, shares at Jun. 30, 2023 |
585,275
|
4,253,517
|
|
|
|
| Re-measurement of carrying value of Class A redeemable stock to redemption value |
|
|
(606,768)
|
|
(606,768)
|
| Net income (loss) |
|
|
|
(816,520)
|
(816,520)
|
| Excise tax liability |
|
|
(113,353)
|
|
(113,353)
|
| Balance at Sep. 30, 2023 |
$ 59
|
$ 425
|
$ 917,476
|
$ (12,540,406)
|
$ (11,622,446)
|
| Balance, shares at Sep. 30, 2023 |
585,275
|
4,253,517
|
|
|
|
| X |
- DefinitionCommon stock subject to possible redemption value.
| Name: |
ONMD_CommonStockSubjectToPossibleRedemptionValue |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionStock issued during period value excise tax liability.
| Name: |
ONMD_StockIssuedDuringPeriodValueExciseTaxLiability |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of other increase (decrease) in additional paid in capital (APIC).
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Condensed Consolidated Statements of Cash Flows (Unaudited) - USD ($)
|
9 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Cash flow from operating activities: |
|
|
| Net income (loss) |
$ (907,660)
|
$ 2,992,171
|
| Adjustments to reconcile net income to net cash used in operating activities: |
|
|
| Dividends, realized and unrealized gain in Trust Account |
(1,102,363)
|
(779,706)
|
| Change in fair value of warrant liability |
242,291
|
(4,243,893)
|
| Changes in operating assets and liabilities: |
|
|
| Prepaid expense |
(21,600)
|
83,061
|
| Accrued expenses |
1,155,934
|
1,159,114
|
| Franchise tax payable |
(69,966)
|
(114,008)
|
| Income tax payable |
(94,833)
|
106,724
|
| Net cash used in operating activities |
(798,197)
|
(796,537)
|
| Cash flow from investing activities: |
|
|
| Investment of cash in Trust Account |
(1,010,440)
|
(2,300,000)
|
| Cash withdrawn from Trust Account in connection with redemption |
11,335,260
|
|
| Interest withdraw from Trust Account |
498,697
|
226,809
|
| Net cash used in investing activities |
10,823,517
|
(2,033,191)
|
| Cash flow from financing activities: |
|
|
| Advances from related parties |
(300)
|
|
| Proceeds from working capital loan |
270,467
|
300,000
|
| Redemption of Class A common stock |
(11,335,260)
|
|
| Amount due to related parties |
|
11,500
|
| Proceeds from extension loans |
1,010,440
|
2,300,000
|
| Net cash provided by financing activities |
(10,054,653)
|
2,611,500
|
| Net change in cash |
(29,333)
|
(218,228)
|
| Cash at the beginning of the period |
30,870
|
453,151
|
| Cash at the end of the period |
1,537
|
234,923
|
| Supplemental disclosure of non-cash financing activities: |
|
|
| Re-measurement of Class A common stock subject to possible redemption |
1,794,994
|
2,833,869
|
| Excise tax liability accrued for Class A Common Stock subject to redemption |
$ 113,353
|
|
| X |
- DefinitionExcise tax liability accrued for class common stock subject to redemption.
| Name: |
ONMD_ExciseTaxLiabilityAccruedForClassCommonStockSubjectToRedemption |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionIncrease decrease franchise tax payable.
| Name: |
ONMD_IncreaseDecreaseFranchiseTaxPayable |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionPayments for investment of cash in trust account.
| Name: |
ONMD_PaymentsForInvestmentOfCashInTrustAccount |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from cash withdrawn from trust account in connection with redemption.
| Name: |
ONMD_ProceedsFromCashWithdrawnFromTrustAccountInConnectionWithRedemption |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from extension loan.
| Name: |
ONMD_ProceedsFromExtensionLoan |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from interest withdrawn from trust account.
| Name: |
ONMD_ProceedsFromInterestWithdrawnFromTrustAccount |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from working capital loan.
| Name: |
ONMD_ProceedsFromWorkingCapitalLoan |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionRealized and unrealized gain loss.
| Name: |
ONMD_RealizedAndUnrealizedGainLoss |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionRemeasurement of class common stock subject to possible redemption.
| Name: |
ONMD_RemeasurementOfClassCommonStockSubjectToPossibleRedemption |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashFlowNoncashInvestingAndFinancingActivitiesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of expenses incurred but not yet paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount of outstanding money paid in advance for goods or services that bring economic benefits for future periods. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow to reacquire common stock during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsForRepurchaseOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS |
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS
OneMedNet
Corporation (f/k/a Data Knights Acquisition Corp.) (the “Company”) was incorporated in Delaware on February 8, 2021. The
Company was formed for the purpose of effectuating a merger, capital stock exchange, asset acquisition, stock purchase, reorganization
or other similar business combination with one or more businesses (the “Business Combination”). The Company is an early stage
and emerging growth company and, as such, the Company is subject to all of the risks associated with early stage and emerging growth
companies.
As
of September 30, 2023, the Company had not yet commenced any operations. All activity for the period February 8, 2021 (inception) through
September 30, 2023, relates to the Company’s formation and the initial public offering (the “Initial Public Offering”),
and, since the closing of the initial public offering, the Company has entered into a merger agreement (as described below), and continued
a search for a Business Combination candidate. The Company has selected December 31 as its fiscal year end.
The
registration statement for the Company’s Initial Public Offering was declared effective on May 6, 2021. On May 11, 2021, the Company
consummated the Initial Public Offering of 11,500,000 units (“Units” and, with respect to the shares of Class A Common Stock
included in the Units offered, the “Public Shares”), generating gross proceeds of $115,000,000, which is described in Note
3.
Simultaneously
with the closing of the Initial Public Offering, the Company consummated the sale of 585,275 private placement units (the “Private
Placement Units”) at a price of $10.00 per unit in a private placement to the Sponsor, generating gross proceeds of $5,852,750,
which is described in Note 4.
Following
the closing of the Initial Public Offering on May 11, 2021, an amount of $117,300,000 ($10.00 per Unit) from the net proceeds of the
sale of the Units in the Initial Public Offering and the Private Placement Units was placed in a trust account (“Trust Account”)
which may be invested in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act of
1940, as amended (the “Investment Company Act”), with a maturity of 185 days or less or in any open-ended investment company
that holds itself out as a money market fund meeting the conditions of Rule 2a-7 of the Investment Company Act, as determined by the
Company, until the earlier of: (i) the consummation of a Business Combination or (ii) the distribution of the Trust Account to the Company’s
stockholders, as described below.
Transaction
costs of the Initial Public Offering amounted to $6,771,112, of which $2,300,000 was for underwriting fees paid at the time of the IPO,
$4,025,000 was for deferred underwriting commissions, and $446,112 was for other offering costs.
Following
the closing of the Initial Public Offering $959,560 of cash was held outside of the Trust Account available for working capital purposes.
As of September 30, 2023, the Company has $1,537 of cash and a working capital deficit of $2,943,835.
The
Company’s management has broad discretion with respect to the specific application of the net proceeds of the Initial Public Offering
and the sale of the Private Placement Units, although substantially all of the net proceeds are intended to be applied generally toward
consummating a Business Combination. NASDAQ rules provide that the Business Combination must be with one or more target businesses that
together have a fair market value equal to at least 80% of the balance in the Trust Account (as defined below) (less any deferred underwriting
commissions and taxes payable on interest earned on the Trust Account) at the time of the signing of a definitive agreement to enter
a Business Combination. The Company will only complete a Business Combination if the post-Business Combination company owns or acquires
50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target sufficient
for it not to be required to register as an investment company under the Investment Company Act. There is no assurance that the Company
will be able to successfully affect a Business Combination.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
On
April 25, 2022, the Company, Data Knights Merger Sub, Inc., a Delaware corporation (“Merger Sub”), and Data Knights, LLC,
the Company’s sponsor (the “Sponsor”), entered into a definitive Agreement and Plan of Merger (the “Merger Agreement”)
with OneMedNet Corporation, Inc., a Delaware corporation (the “Target”, and together with the Company and Merger Sub, the
“Parties”) and Paul Casey, as seller representative (“Casey”). Pursuant to the Merger Agreement, upon the closing
(the “Closing”) of the Business Combination, the Parties will effect the merger of Merger Sub with and into the Target, with
the Target continuing as the surviving entity (the “Merger”), as a result of which all of the issued and outstanding capital
stock of the Target shall be exchanged shares of the Class A Common Stock of the Company upon the terms set forth in the Merger Agreement.
On
May 5, 2022, the Company extended the date by which the Company has to consummate a business combination from May 11, 2022 to August
11, 2022 (the “First Extension”). The First Extension was the first of two three-month extensions permitted under the Company’s
governing documents.
On
August 10, 2022, the Company extended the date by which the Company has to consummate a business combination from August 11, 2022 to
November 11, 2022 (the “Second Extension”). The Second Extension was the second of two three-month extensions permitted under
the Company’s governing documents.
On
October 27, 2022, the Company filed a definitive proxy statement with the SEC in connection with the Company’s solicitation of
proxies for the vote by the stockholders of the Company at a special meeting of the Company’s stockholders to be held on November
11, 2022 (the “Special Meeting”).
On
November 11, 2022, at 10:00 a.m. ET, the Company held a virtual special meeting of its stockholders. At the special meeting, Company
stockholders entitle to vote at the special meeting cast their votes and approved the Trust Amendment Proposal, pursuant to which
the Trust Agreement was amended to extend the date on which Continental must liquidate the Trust Account established in connection
with the IPO if the Company has not completed its initial business combination, from November 11, 2022 to August 11, 2023 (or such
earlier date after November 11, 2022, as determined by the Company Board). As a part of Special Meeting, the Company’s
stockholders approved amendments to its second amended and restated certificate of incorporation (the “Extension
Amendment”) and the investment management trust agreement (the “Trust Agreement”) between Continental Stock
Transfer & Trust Company, as trustee (“Continental”), and the Company governing the trust account (the “Trust
Account”) established in connection with the Company’s initial public offering dated May 11, 2021 (the “Trust
Amendment”), which together allow the Company to extend the deadline by which it must complete its initial business
combination by up to nine one-month periods. In connection with each such extension, Data Knights, LLC, the Company’s sponsor,
shall cause $0.045
per outstanding share of the Company’s Class A Common Stock, or approximately $122,920,
to be deposited in the Trust Account. On July 12, 2023, the Company elected to exercise its ninth of nine one-month extension to the
Termination Date, which extended its deadline to complete its initial business combination to August 11, 2023, by depositing $0.045
per share for each Public Share outstanding after giving effect to the redemptions disclosed above, or approximately $122,920,
was deposited in the Trust Account.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
In
connection with the proposed Business Combination with the Target, the Company will provide its public stockholders with the opportunity
to redeem all or a portion of their Class A Common Stock upon the completion of such Business Combination in connection with a stockholder
meeting called to approve such Business Combination. In the event the proposed Business Combination with the Target is not consummated,
in connection with an alternative proposed initial business combination, the Company will provide its public stockholders with the opportunity
to redeem all or a portion of their Public Shares upon the completion of a Business Combination either (i) in connection with a stockholder
meeting called to approve the Business Combination or (ii) by means of a tender offer. In connection with a proposed Business Combination,
the Company may seek stockholder approval of a Business Combination at a meeting called for such purpose at which stockholders may seek
to redeem their shares, regardless of whether they vote for or against a Business Combination. The Company will proceed with a Business
Combination only if the Company has net tangible assets of at least $5,000,001 either immediately prior to or upon such consummation
of a Business Combination and, if the Company seeks stockholder approval, a majority of the outstanding shares voted are voted in favor
of the Business Combination. In connection with the voting on the Extension Amendment Proposal and the Trust Amendment Proposal at the
special meeting, holders of 8,768,456 shares of Class A Common Stock exercised their right to redeem those shares for cash at an approximate
price of $10.42 per share, for an aggregate of approximately $91.4 million. Following the payment of the redemptions, the Trust Account
had a balance of approximately $28.5 million.
On
August 11, 2023, at 1:00 p.m. ET, the Company held a virtual special meeting of its stockholders. At the Special Meeting, the Company
stockholders entitled to vote at the Special Meeting cast their votes and approved the proposal (the “Trust Amendment Proposal”)
to authorize the Company to enter into Amendment No. 2 to the Trust Agreement (the “Trust Agreement Amendment”) to amend
the Trust Agreement to allow the Company to extend beyond August 11, 2023 the date by which either the Company must have completed its
initial business combination or Continental must liquidate the Trust Account established in connection with the IPO (the “Trust
Account”). Following approval of the Trust Amendment Proposal by the Stockholders, the Company and Continental promptly entered
into the Trust Agreement Amendment. At the Special Meeting, the Stockholders approved the Extension Amendment Proposal to amend
Company’s Charter and authorized the Company to adopt and file the Amended Charter with the Secretary of State of Delaware, which
the Company promptly filed following the Stockholders’ approval. The stockholders approved amendments to the Company’s charter
and trust agreement allowing the Company to extend its termination date in a series of up to nine (9) one-month extensions until May
11, 2024 in exchange for depositing into its trust account (the “Trust Account”) with Continental Stock Transfer and Trust
Company the lesser of $75,000 or $0.045 per share for each public share outstanding (the “Extension Amount”). In
connection with the voting on the Extension Amendment Proposal and the Trust Amendment Proposal at the Special Meeting, holders of 1,018,846
shares of Class A ordinary shares exercised the right to redeem such shares for cash.
On
August 11, 2023, the Company deposited $75,000 into the Company’s trust account, allowing the Company to extend the period of time
it has to consummate its initial business combination by one month from August 11, 2023 to September 11, 2023 (the “Extension”).
The Extension is the first of nine (9) one-month extensions permitted under the Company’s governing documents. As of September
30, 2023, the Company has executed two one-month extensions, out of the nine, resulting in deposits of approximately $150,000 into the
Trust Account.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
Based
on the above, the Company will have until May 11, 2024 to consummate a Business Combination. If the Company is unable to complete a Business
Combination on May 11, 2024 at the election of the Company subject to satisfaction of certain conditions, including the deposit of up
$2,300,000 since the underwriters’ over-allotment option is exercised in full ($0.10 per unit), into the Trust Account, or as extended
by the Company’s stockholders in accordance with the Company’s amended and restated certificate of incorporation) (the “Combination
Period”), the Company will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible
but no more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate
amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released
to the Company to pay taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding
public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to
receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following
such redemption, subject to the approval of the remaining stockholders and the Company’s board of directors, proceed to commence
a voluntary liquidation and thereby a formal dissolution of the Company, subject in each case to its obligations under Delaware law to
provide for claims of creditors and the requirements of applicable law. The underwriter has agreed to waive its rights to the deferred
underwriting commission held in the Trust Account in the event the Company does not complete a Business Combination within the Combination
Period and, in such event, such amounts will be included with the funds held in the Trust Account that will be available to fund the
redemption of the Public Shares. In the event of such distribution, it is possible that the per share value of the assets remaining available
for distribution will be less than the Initial Public Offering price per Unit ($10.00). There will be no redemption rights or liquidating
distributions with respect to the Founder Shares (as defined below) or the shares of Class A Common Stock and the warrants that are included
as components of the Private Placement Units. Such warrants will expire worthless if the Company fails to complete a Business Combination
within the Combination Period.
The
Sponsor has agreed that it will be liable to the Company if and to the extent any claims by a third party for services rendered or products
sold to the Company, or a prospective target business with which the Company has entered into a written letter of intent, confidentiality
or similar agreement or Business Combination agreement, reduce the amount of funds in the Trust Account to below the lesser of (i) $10.20
per Public Share and (ii) the actual amount per Public Share held in the Trust Account as of the day of liquidation of the Trust Account,
if less than $10.20 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability will
not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to monies held in
the Trust Account (whether or not such waiver is enforceable) nor will it apply to any claims under the Company’s indemnity of
the underwriter of Initial Public Offering against certain liabilities, including liabilities under the Securities Act of 1933, as amended
(the “Securities Act”). However, the Company has not asked the Sponsor to reserve for such indemnification obligations, nor
has the Company independently verified whether the Sponsor has sufficient funds to satisfy its indemnity obligations and believe that
the Sponsor’s only assets are securities of the Company. Therefore, the Company cannot assure its stockholders that the Sponsor
would be able to satisfy those obligations. None of the Company’s officers or directors will indemnify the Company for claims by
third parties including, without limitation, claims by vendors and prospective target businesses. The Company will seek to reduce the
possibility that the Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have all vendors,
service providers, prospective target businesses or other entities with which the Company does business, execute agreements with the
Company waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account.
Business
Combination with OneMedNet
On
November 7, 2023, the Company consummated its initial business combination (the “Business Combination”) with OneMedNet Solutions
Corporation (formerly named OneMedNet Corporation), a Delaware corporation (“OneMedNet”), pursuant to an agreement and plan
of merger, dated as of April 25, 2022 (the “Merger Agreement”), by and among the Company, Data Knights Merger Sub, Inc.,
a Delaware corporation and a wholly-owned subsidiary of the Company (“Merger Sub”), OneMedNet, Data Knights, LLC, a Delaware
limited liability company (“Sponsor” or “Purchaser Representative”) in its capacity as the representative of
the stockholders of the Company, and Paul Casey in his capacity as the representative of the stockholders of OneMedNet (“Seller
Representative”).
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
On
July 22, 2022, the Company filed a registration statement on Form S-4 (File No. 333-266274) (the “Form S-4”, as amended on
September 20, 2022, November 10, 2022, January 3, 2023, February 3, 2023, April 11, 2023, July 5, 2023, August 2, 2023, August 8, 2023,
August 29, 2023 and September 21, 2023) with the SEC, which includes the proxy statement/prospectus/information statement distributed
to holders of the Company’s ordinary shares in connection with the Company’s solicitation for proxies for the vote by the
Company’s stockholders in connection with the Business Combination and other matters as described in the Form S-4. The Form S-4
was declared effective by the SEC on September 22, 2023, and the definitive proxy statement/prospectus/information statement was mailed
to the Company’s shareholders of record on the record date for voting on the Business Combination. The Business Combination closed
on November 13, 2023. Effective November 7, 2023, the Company’s units ceased trading, and
effective November 8, 2023, the Company’s common stock began trading on the Nasdaq Capital Market under the symbol “ONMD”
and warrants began trading on the Nasdaq Capital Market under the symbol “ONMDW.”
The
Business Combination was accounted for as a reverse recapitalization in accordance with GAAP, whereby the Company is treated as the acquired
company and OneMedNet is treated as the acquirer. Accordingly, for accounting purposes, the Business Combination was treated as the equivalent
of OneMedNet issuing stock for the net assets of the Company, accompanied by a recapitalization. The net assets of the Company were stated
at historical cost, with no goodwill or other intangible assets recorded. Subsequent presentations of the results of operations presented
for the period prior to the Business Combination will be for those of OneMedNet.
OneMedNet
has been determined to be the accounting acquirer in the Business Combination based on the following predominate factors:
| |
● |
OneMedNet’s
existing stockholders have the greatest voting interest in the combined entity; |
| |
● |
OneMedNet
has the ability to nominate a majority of the initial members of the OneMedNet Board; |
| |
● |
OneMedNet’s
senior management is the senior management of the combined entity; and |
| |
● |
OneMedNet
is the larger entity based on historical operating activity and has the larger employee base. |
Going
Concern, Liquidity and Capital Resources
As
of September 30, 2023 and December 31, 2022, the Company had cash held outside of the Trust Account of $1,537 and $30,870, respectively.
We intend to use the funds held outside the Trust Account primarily to identify and evaluate target businesses, perform business due
diligence on prospective target businesses, travel to and from the offices, plants or similar locations of prospective target businesses
or their representatives or owners, review corporate documents and material agreements of prospective target businesses, and structure,
negotiate and complete our initial business combination. As of September 30, 2023 and December 31,2022, the Company had working capital
deficit of $3,057,188 and $1,945,267, respectively.
The
Company’s liquidity needs prior to the consummation of its IPO were satisfied through the proceeds of $ from the sale of
the Founder Shares and proceed from the promissory note from sponsor of $, which was repaid upon closure of the IPO. Subsequent
to the IPO, the Company’s liquidity will be satisfied through a portion of the net proceeds from IPO held outside of the Trust
Account.
As
of September 30, 2023 and December 31, 2022, we had investments of $19,308,261 and $29,029,416 held in the Trust Account, respectively.
We intend to use substantially all of the funds held in the Trust Account, including any amounts representing interest earned on the
Trust Account (less taxes paid and deferred underwriting commissions) to complete our initial business combination. We may withdraw interest
to pay taxes. For the nine months ended September 30, 2023, we withdraw $498,697 of interest earned on the Trust Account pay Delaware
Franchise Tax and Income Tax. During the period ended December 31, 2022, we withdraw $299,601 interest earned on the Trust Account to
pay Delaware Franchise Tax. To the extent that our capital stock or debt is used, in whole or in part, as consideration to complete our
initial business combination, the remaining proceeds held in the Trust Account will be used as working capital to finance the operations
of the target business or businesses, make other acquisitions and pursue our growth strategies.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
The
accompanying consolidated financial statements have been prepared in conformity with U.S. GAAP, which contemplates the continuation of
the Company as a going concern and the realization of assets and the satisfaction of liabilities in the normal course of business. The
financial statements do not include any adjustments that might result from the outcome of this uncertainty. Further, we have incurred
and expect to continue to incur significant costs in pursuit of our financing and acquisition plans. Management plans to address this
uncertainty during the period leading up to the business combination, however this cannot be guaranteed. The Company will have until
May 11, 2024 to consummate a Business Combination. If our initial business combination is not consummated by May 11, 2024, less than
one year after the date the financial statements are issued, then our existence will terminate, and we will distribute all amounts in
the trust account. The Company intends to complete a business combination before the liquidation date and no adjustments have been made
to the carrying amounts of assets or liabilities should the company be required to liquidate after such date. There can be no assurance
that the Company will be able to consummate an initial business combination by May 11, 2024 and/or have sufficient working capital and
borrowing capacity to meet its needs. Based upon the above analysis, management determined that these conditions raise substantial doubt
about the Company’s ability to continue as a going concern.
In
order to fund working capital deficiencies or finance transaction costs in connection with our initial Business Combination, our Sponsor
or an affiliate of our Sponsor or certain of our officers and directors may, but are not obligated to, loan us funds as may be required.
If we complete our initial Business Combination, we would repay such loaned amounts. In the event that our initial Business Combination
does not close, we may use a portion of the working capital held outside the Trust Accounts to repay such loaned amounts but no proceeds
from our Trust Accounts would be used for such repayment. Up to $1,500,000 of such loans may be convertible into units identical to the
Placement Units, at a price of $10.00 per unit at the option of the lender.
Risks
and Uncertainties
Management
is currently evaluating the impact of the COVID-19 pandemic and has concluded that while it is reasonably possible that the virus could
have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific
impact is not readily determinable as of the date of the financial statement. The financial statements do not include any adjustments
that might result from the outcome of this uncertainty.
On
August 16, 2022, the Inflation Reduction Act of 2022 (the “IR Act”) was signed into law. The IR Act provides for, among other
measures, a new 1% U.S. federal excise tax on certain repurchases (including redemptions) of stock by publicly traded domestic (i.e.,
U.S.) corporations. The excise tax is imposed on the repurchasing corporation itself, not its shareholders from whom the shares are repurchased.
The amount of the excise tax is generally 1% of the fair market value of the shares repurchased. For purposes of calculating the excise
tax, however, repurchasing corporations are permitted to net the fair market value of certain new stock issuances against the fair market
value of stock repurchases during the same taxable year. In addition, certain exceptions apply to the excise tax. The U.S. Department
of the Treasury (the “Treasury Department”) has been given authority to provide regulations and other guidance to carry out,
and prevent the abuse or avoidance of, the excise tax. The IR Act applies only to repurchases that occur after December 31, 2022.
NOTE
1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Continued)
Any
redemption or other repurchase effected by us that occurs after December 31, 2022, in connection with a Business Combination or otherwise,
may be subject to this excise tax. Whether and to what extent we would be subject to the excise tax in connection with a Business Combination
will depend on a number of factors, including (i) the fair market value of the redemptions and repurchases in connection with the Business
Combination, (ii) the nature and amount of any PIPE financing or other equity issuances in connection with the Business Combination (or
any other equity issuances within the same taxable year of the Business Combination) and (iii) the content of any regulations and other
guidance issued by the Treasury Department and/or the Internal Revenue Service. In addition, because the excise tax would be payable
by us and not by the redeeming holder, it could cause a reduction in the value of our stock. The foregoing could cause a reduction in
the cash available on hand to complete a business Combination in the required time and redeem 100% of our public shares in accordance
with our amended and restated certificate of incorporation) could be subject to the excise tax, in which case the amount that would otherwise
be received by our stockholders in connection with our liquidation may be reduced.
Inflation
Reduction Act of 2022
On
August 16, 2022, the Inflation Reduction Act of 2022 (the “IR Act”) was signed into federal law. The IR Act provides for,
among other things, a new U.S. federal 1% excise tax on certain repurchases (including redemptions) of stock by publicly traded domestic
(i.e., U.S.) corporations and certain domestic subsidiaries of publicly traded foreign corporations. The excise tax is imposed on the
repurchasing corporation itself, not its shareholders from which shares are repurchased. The amount of the excise tax is generally 1%
of the fair market value of the shares repurchased at the time of the repurchase. However, for purposes of calculating the excise tax,
repurchasing corporations are permitted to net the fair market value of certain new stock issuances against the fair market value of
stock repurchases during the same taxable year. In addition, certain exceptions apply to the excise tax. The U.S. Department of the Treasury
(the “Treasury”) has been given authority to provide regulations and other guidance to carry out and prevent the abuse or
avoidance of the excise tax. The IR Act applies only to repurchases that occur after December 31, 2022.
Any
redemption or other repurchase that occurs after December 31, 2022, in connection with a Business Combination, extension vote or otherwise,
may be subject to the excise tax. Whether and to what extent the Company would be subject to the excise tax in connection with a Business
Combination, extension vote or otherwise would depend on a number of factors, including (i) the fair market value of the redemptions
and repurchases in connection with the Business Combination, extension or otherwise, (ii) the structure of a Business Combination, (iii)
the nature and amount of any “PIPE” or other equity issuances in connection with a Business Combination (or otherwise issued
not in connection with a Business Combination but issued within the same taxable year of a Business Combination) and (iv) the content
of regulations and other guidance from the Treasury. In addition, because the excise tax would be payable by the Company and not by the
redeeming holders, the mechanics of any required payment of the excise tax have not been determined. The foregoing could cause a reduction
in the cash available on hand to complete a Business Combination and in the Company’s ability to complete a Business Combination.
At
this time, it has been determined that the IR Act tax provisions would have an impact to the Company’s fiscal 2023 tax provision
as there were redemptions by the public stockholders in August 2023; as a result, the Company recorded $113,353 excise tax liability
as of September 30, 2023. The Company will continue to monitor for updates to the Company’s business along with guidance issued
with respect to the IR Act to determine whether any adjustments are needed to the Company’s tax provision in future periods.
|
| X |
- DefinitionThe entire disclosure for the nature of an entity's business, major products or services, principal markets including location, and the relative importance of its operations in each business and the basis for the determination, including but not limited to, assets, revenues, or earnings. For an entity that has not commenced principal operations, disclosures about the risks and uncertainties related to the activities in which the entity is currently engaged and an understanding of what those activities are being directed toward. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
| Name: |
us-gaap_NatureOfOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of presentation
The
accompanying financial statements are presented in U.S. Dollars and conformity with accounting principles generally accepted in the United
States of America (“GAAP”) and pursuant to the rules and regulations of the SEC.
Principles
of Consolidation
The
accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All significant intercompany
balances and transactions have been eliminated in consolidation.
Emerging
growth company
The
Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our
Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements
that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required
to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding
executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory
vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further,
Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting
standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do
not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting
standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements
that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of
such extended transition period which means that when a standard is issued or revised and it has different application dates for public
or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies
adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which
is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult
or impossible because of the potential differences in accounting standards used.
Use
of estimates
The
preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported
amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the
reported amounts of revenues and expenses during the reporting period.
Making
estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of
a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating
its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ
significantly from those estimates.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. Cash
equivalents are carried at cost, which approximates fair value. The Company had $1,537 and $30,870 in cash and no cash equivalents as
of September 30, 2023 and December 31, 2022, respectively.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Trust
Account
Upon
the closing of the Initial Public Offering and the Private Placement, $117,300,000 ($10.00 per Unit) of the net proceeds of the Initial
Public Offering and certain of the proceeds of the Private Placement was held in a trust account (“Trust Account”) located
in the United States with Continental Stock Transfer & Trust Company acting as trustee, and invested only in U.S. government treasury
obligations with a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 under the Investment
Company Act 1940, as amended (the “Investment Company Act”), which will be invested only in direct U.S. government treasury
obligations, as determined by the Company, until the earlier of: (i) the completion of a Business Combination and (ii) the distribution
of the Trust Account as described below.
Offering
Costs Associated with the Initial Public Offering
Offering
costs consisted of legal, accounting, underwriting fees and other costs incurred that were directly related to the Initial Public Offering.
Offering costs are allocated to the separable financial instruments issued in the Initial Public Offering based on a relative fair value
basis, compared to total proceeds received. Offering costs associated with warrant liabilities are expensed as incurred, presented as
offering costs allocated to warrants in the consolidated statements of operations. Offering costs associated with the Public Shares were
charged to stockholders’ equity upon the completion of the Initial Public Offering.
Class
A Common Stock Subject to Possible Redemption
The
Company accounts for its shares subject to possible redemption in accordance with the guidance in Accounting Standards Codification (“ASC”)
Topic 480 “Distinguishing Liabilities from Equity.” Shares subject to mandatory redemption (if any) is classified as a liability
instrument and is measured at fair value. Conditionally redeemable shares of common stock (including shares of common stock that feature
redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not
solely within the Company’s control) is classified as temporary equity. At all other times, shares are classified as stockholders’
equity. The Company’s Class A Common Stock features certain redemption rights that are considered to be outside of the Company’s
control and subject to occurrence of uncertain future events.
On
September 30, 2023, there are 585,275 shares of Class A Common Stock related to the Private Placement Units (Note 8) outstanding, which
are not subject to redemption, and 1,712,698 shares of Class A Common Stock outstanding, which are subject to possible redemption.
If
it is probable that the equity instrument will become redeemable, the Company has the option to either accrete changes in the redemption
value over the period from the date of issuance (or from the date that it becomes probable that the instrument will become redeemable,
if later) to the earliest redemption date of the instrument or to recognize changes in the redemption value immediately as they occur
and adjust the carrying amount of the instrument to equal the redemption value at the end of each reporting period. The Company has elected
to recognize the changes immediately. The accretion or remeasurement is treated as a deemed dividend (i.e., a reduction to retained earnings,
or in absence of retained earnings, additional paid-in capital).
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
As
of September 30, 2023 and 2022, the Class A Common Stock reflected on the balance sheet are reconciled in the following table:
SCHEDULE
OF RECONCILED CLASS A COMMON STOCK
| | |
2023 | | |
2022 | |
| | |
For the Nine Months Ended | |
| | |
September 30, | |
| | |
2023 | | |
2022 | |
| Contingently redeemable Class A Common Stock – Opening Balance | |
$ | 28,750,110 | | |
$ | 117,300,000 | |
| Less: | |
| | | |
| | |
| Redemption of Class A Common Stock, including interest | |
| (11,335,260 | ) | |
| | |
| Plus: | |
| | | |
| | |
| Re-measurement of carrying value to redemption value | |
| 1,794,994 | | |
| 2,833,869 | |
| Contingently redeemable Class A Common Stock - Ending Balance | |
| 19,209,844 | | |
| 120,133,869 | |
Net
income (loss) per share
Net
income (loss) per share is computed by dividing net income (loss) by the weighted average number of common stock shares outstanding for
the period. The calculation of diluted income (loss) per share does not consider the effect of the warrants issued in connection with
the Initial Public Offering and warrants issued as components of the Private Placement Units (the “Placement Warrants”) since
the exercise of the warrants are contingent upon the occurrence of future events and the inclusion of such warrants would be anti-dilutive.
The
Company applies the two-class method in calculating earnings per share. The contractual formula utilized to calculate the redemption
amount approximates fair value. The Class feature to redeem at fair value means that there is effectively only one class of stock. Changes
in fair value are not considered a dividend for the purposes of the numerator in the earnings per share calculation. Net income per common
share is computed by dividing the pro rata net income (loss) between the redeemable shares and the non-redeemable shares by the weighted
average number of common shares outstanding for each of the periods. The calculation of diluted income (loss) per common stock does not
consider the effect of the warrants issued in connection with the IPO since the exercise of the warrants are contingent upon the occurrence
of future events and the inclusion of such warrants would be anti-dilutive. The warrants are exercisable for 12,085,275 shares of common
stock in the aggregate.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The
following table reflects the calculation of basic and diluted net income (loss) per common share:
RECONCILIATION
OF NET LOSS PER COMMON SHARE
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
For the Three Months Ended | | |
For the Nine months ended | |
| | |
September 30, | | |
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Redeemable Class A common shares | |
| | | |
| | | |
| | | |
| | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) allocable to common stock subject to possible redemption | |
$ | (252,542 | ) | |
$ | (117,823 | ) | |
$ | (312,541 | ) | |
$ | 2,300,089 | |
| | |
| | | |
| | | |
| | | |
| | |
| Denominator: weighted average number of redeemable common share | |
| 2,166,749 | | |
| 11,500,000 | | |
| 2,541,210 | | |
| 11,500,000 | |
| Basic and diluted net income (loss) per redeemable common share | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
| | |
| | | |
| | | |
| | | |
| | |
| Non-redeemable Class A and Class B common shares | |
| | | |
| | | |
| | | |
| | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) allocable to common stock not subject to redemption | |
$ | (563,978 | ) | |
$ | (35,452 | ) | |
$ | (595,119 | ) | |
$ | 692,082 | |
| | |
| | | |
| | | |
| | | |
| | |
| Denominator: weighted average number of non-redeemable common shares | |
| 4,838,792 | | |
| 3,460,275 | | |
| 4,838,792 | | |
| 3,460,275 | |
| Basic and diluted net income (loss) per non-redeemable common share | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
Concentration
of credit risk
Financial
instruments that potentially subject the Company to concentration of credit risk consist of a cash account in a financial institution
which, at times may exceed the Federal depository insurance coverage of $250,000. At September 30, 2023 and 2022, the Company had not
experienced losses on this account and management believes the Company is not exposed to significant risks on such account.
Fair
value of financial instruments
The
fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC Topic 820, “Fair Value
Measurements and Disclosures,” approximates the carrying amounts represented in the accompanying consolidated balance sheets, primarily
due to their short-term nature, except warrant liabilities (See Note 9).
Derivative
Financial Instruments
The
Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded
derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. For derivative financial instruments that are accounted
for as liabilities, the derivative instrument is initially recorded at its fair value on the grant date and is then re-valued at each
reporting date, with changes in the fair value reported in the statements of operations. The classification of derivative instruments,
including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period.
Derivative liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement or conversion
of the instrument could be required within 12 months of the balance sheet date.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Income
Taxes
The
Company complies with the accounting and reporting requirements of ASC Topic 740, “Income Taxes,” which requires an asset
and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed
for differences between the financial statement and tax bases of assets and liabilities that will result in future taxable or deductible
amounts, based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income.
Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC
Topic 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax
positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not
to be sustained upon examination by taxing authorities. The Company’s management determined that the United States is the Company’s
only major tax jurisdiction. The Company recognizes accrued interest and penalties related to unrecognized tax benefits, if any, as income
tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of September 30, 2023 and 2022,
respectively. The Company is currently not aware of any issues under review that could result in significant payments, accruals, or material
deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Our
effective tax rate was (12.15)% and 0% for the three months ended September 30, 2023 and 2022, respectively. Our effective tax rate was
(30.10)% and 0% for the nine months ended September 30, 2023 and 2022, respectively. The effective tax rate differs from the statutory
tax rate of 21% for the three months ended September 30, 2023 and 2022, due to transaction costs and the valuation allowance on the deferred
tax assets.
Recently
Issued Accounting Standards
In
August 2020, the FASB issued Accounting Standards Update (“ASU”) 2020-06, “Debt — Debt with Conversion and Other
Options” (Subtopic 470-20) and “Derivatives and Hedging — Contracts in Entity’s Own Equity” (Subtopic 815-40)
(“ASU 2020-06”) to simplify accounting for certain financial instruments. ASU 2020-06 eliminates the current models that
require separation of beneficial conversion and cash conversion features from convertible instruments and simplifies the derivative scope
exception guidance pertaining to equity classification of contracts in an entity’s own equity. The new standard also introduces
additional disclosures for convertible debt and freestanding instruments that are indexed to and settled in an entity’s own equity.
ASU 2020-06 amends the diluted earnings per share guidance, including the requirement to use the if-converted method for all convertible
instruments. ASU 2020-06 is effective for the Company for the fiscal year beginning after December 15, 2023, including interim periods
within those fiscal years.
Management
does not believe that any recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material
effect on the Company’s financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the basis of presentation and significant accounting policies concepts. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Accounting policies describe all significant accounting policies of the reporting entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_BasisOfPresentationAndSignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
INITIAL PUBLIC OFFERING
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Initial Public Offering |
|
| INITIAL PUBLIC OFFERING |
NOTE
3. INITIAL PUBLIC OFFERING
Pursuant
to the Initial Public Offering, the Company sold 11,500,000 Units at a purchase price of $10.00 per Unit. Each Unit consists of one share
of the Company’s Class A Common Stock, $0.0001 par value, and one redeemable warrant (“Public Warrant”). Each Public
Warrant entitles the holder to purchase one share of Class A Common Stock at an exercise price of $11.50 per whole share (see Note 9).
|
| X |
- References
+ Details
| Name: |
ONMD_DisclosureInitialPublicOfferingAbstract |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionInitial Public Offering Disclosure [Text Block]
| Name: |
ONMD_InitialPublicOfferingDisclosureTextBlock |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
PRIVATE PLACEMENT
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Private Placement |
|
| PRIVATE PLACEMENT |
NOTE
4. PRIVATE PLACEMENT
Simultaneously
with the Initial Public Offering, the Sponsor purchased an aggregate of 585,275 Private Placement Units at a price of $10.00 per Private
Placement Unit for an aggregate purchase price of $5,852,750.
The
Private Placement Units are identical to the Units, except that (a) the Private Placement Units and their component securities will not
be transferable, assignable or saleable until 30 days after the consummation of the Company’s initial business combination except
to permitted transferees and (b) the Placement Warrants, so long as they are held by the Sponsor or its permitted transferees, (i) may
be exercised by the holders on a cashless basis and (ii) will be entitled to registration rights.
|
| X |
- References
+ Details
| Name: |
ONMD_DisclosurePrivatePlacementAbstract |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrivate Placement [Text Block]
| Name: |
ONMD_PrivatePlacementTextBlock |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
RELATED PARTY TRANSACTIONS
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Related Party Transactions [Abstract] |
|
| RELATED PARTY TRANSACTIONS |
NOTE
5. RELATED PARTY TRANSACTIONS
Introducing
Advisor Agreement
On
June 26, 2021, the Company entered into an introducing advisor agreement (the “Introducing Advisor Agreement”) with ARC Group
Limited, the Company’s financial advisor (“ARC”), pursuant to which ARC will make strategic introductions to the Company
of potential target companies and/or their subsidiaries, affiliates, or representatives (each an “Advisor Target”) who may
be interested in potential business combinations with the Company. In consideration for ARC’s services under the Introducing Advisor
Agreement, we agreed to (i) pay to ARC (a) a retainer of $50,000 upon execution of the Introducing Advisor Agreement and (b) a success
fee of $100,000 upon the closing our initial business combination, and (ii) cause to be issued to ARC equity interests in the post-combination
company representing a five-percent (5%) ownership interest in the post-combination company, if at any time prior to June 25, 2022 (the
“Termination Date”), or within six (6) months after the consummation of an initial business combination or any financing
with any Advisor Target or any affiliate of an Advisor Target (the “Equity Issuance”).
On
March 22, 2022, the Company and ARC entered into the First Amendment to the Introducing Advisor Agreement, pursuant to which both parties
agreed that the Company would pay to ARC an additional success fee equivalent to five percent (5%) on any PIPE that was brought by ARC
in connection with the Company’s initial business combination upon the closing of the Company’s initial business combination.
On
December 31, 2022, the Company and ARC entered into the Second Amendment to the Introducing Advisor Agreement, pursuant to which both
parties agreed to extend the Termination Date to December 31, 2024, and to change the performance condition for the Equity Issuance from
the closing of an initial business combination to the execution of a business combination agreement. On December 31, 2022, following
the execution of the Second Amendment to the Introducing Advisor Agreement, the performance condition for the Equity Issuance was deemed
to have been met, and ARC was issued 1,378,517 shares of the Company’s Class B Common Stock, up to 143,766 shares of which are
subject to forfeiture if the public stockholders exercise redemption rights with respect to any of the remaining outstanding shares of
Class A Common Stock.
Founder
Shares
On
February 25, 2021, the Company issued an aggregate of shares of Class B Common Stock (the “Founder Shares”) to
the Sponsor for an aggregate purchase price of $. On February 25, 2021, the Sponsor transferred 15,000 shares to the Company’s
Chief Executive Officer, 15,000 shares to the Company’s Chief Financial Officer and 5,000 shares to two of the Company’s
independent directors. Following the determination of the Company’s third independent director, on March 23, 2021, the Sponsor
transferred 5,000 shares to such independent director. The Founder Shares which the Sponsor and its permitted transferees will collectively
own, on an as-converted basis, represent 20% of the Company’s issued and outstanding shares after the Initial Public Offering.
In connection with the Introducing Advisors Agreement, on December 31, 2022, ARC was granted 1,378,517 shares of Class B common stock,
$0.0001 par value per share, up to 143,766 of which are subject to forfeiture by ARC if the Company’s public shareholders exercise
redemption rights with respect to any of outstanding shares of Class A common stock.
NOTE
5. RELATED PARTY TRANSACTIONS (Continued)
The
Sponsor has agreed not to transfer, assign or sell any of its Founder Shares until the earlier to occur of: (A) after the
completion of a Business Combination or (B) the date on which the Company completes a liquidation, merger, capital stock exchange or
similar transaction that results in the Company’s stockholders having the right to exchange their shares of common stock for cash,
securities or other property. Notwithstanding the foregoing, if the last reported sale price of the Company’s Class A Common Stock
equals or exceeds $ per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for
any 20 trading days within any 30-trading day period commencing at least 150 days after the Business Combination, the Founder Shares
will be released from the lock-up.
Promissory
Note — Related Party
On
February 8, 2021, the Sponsor committed to loan the Company an aggregate of up to $300,000 to cover expenses related to the Initial Public
Offering pursuant to a promissory note (the “Note”). The Note was non-interest bearing and was payable on the earlier of
July 31, 2021 or the completion of the Initial Public Offering. On June 1, 2021, the $78,925 outstanding under the promissory note was
repaid in full. On September 30, 2023 and December 31, 2022, there is no amount outstanding under the promissory note.
Related
Party Loans
In
order to finance transaction costs in connection with a Business Combination, the Company’s Sponsor, an affiliate of the Sponsor,
or the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (the “Working
Capital Loans”). Such Working Capital Loans would be evidenced by promissory notes. The notes would either be repaid upon consummation
of a Business Combination, without interest, or, at the lender’s discretion, up to $ of notes may be converted upon consummation
of a Business Combination into units at a price of $ per unit. The Units will be identical to the Private Placement Units. In the
event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust Account to repay the
Working Capital Loans but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. On September 30, 2023
and December 31, 2022, there is $ and $ outstanding under the Working Capital Loans, respectively.
NOTE
5. RELATED PARTY TRANSACTIONS (Continued)
The
Company’s second amended and restated certificate of incorporation provides that, if the Company anticipates that it may not be
able to consummate a Business Combination within 12 months from the closing of the Company’s initial public offering, the Company
may, by resolution of the Company’s board if requested by the Sponsor, extend the period of time to consummate a Business Combination
up to two times, each by an additional three months (for a total of up to 18 months to complete a Business Combination), subject to the
Sponsor depositing additional funds into the Trust Account as set out below. Pursuant to the terms of the Company’s second amended
and restated certificate of incorporation and the trust agreement entered into between the Company and Continental Stock Transfer &
Trust Company, in order for the time available for the Company to consummate the initial Business Combination to be extended, the Sponsor
or its affiliates or designees, upon five business days advance notice prior to the applicable deadline, must deposit into the Trust
Account $ since the underwriters’ over-allotment option is exercised in full ($ per unit), on or prior to the date
of the applicable deadline, for each of the available three month extensions, providing a total possible Business Combination period
of 18 months at a total payment value of $ since the underwriters’ over-allotment option is exercised in full ($ per
unit) (the “Extension Loans”). Any such payments would be made in the form of non-interest-bearing loans. If the Company
completes its initial Business Combination, the Company will, at the option of the Sponsor, repay the Extension Loans out of the proceeds
of the Trust Account released to the Company or convert a portion or all of the total loan amount into units at a price of $ per
unit, which units will be identical to the Private Placement Units. If the Company does not complete a Business Combination, the Company
will repay such loans only from funds held outside of the Trust Account. Furthermore, the letter agreement among the Company and the
Company’s officers, directors, and the Sponsor contains a provision pursuant to which the Sponsor will agree to waive its right
to be repaid for such loans to the extent there is insufficient funds held outside of the Trust Account in the event that the Company
does not complete a Business Combination. The Sponsor and its affiliates or designees are not obligated to fund the Trust Account to
extend the time for the Company to complete the initial Business Combination. The public stockholders will not be afforded an opportunity
to vote on the extension of time to consummate an initial Business Combination from 12 months to 18 months described above or redeem
their shares in connection with such extensions. Pursuant to the foregoing, on May 5, 2022, the Company extended the date by which the
Company had to consummate a business combination from May 11, 2022 to August 11, 2022. On August 10, 2022, the Company extended the date
by which the Company had to consummate a business combination from August 11, 2022 to November 11, 2022.
As
described in Note 1, on August 11, 2023, the Stockholders of the Company approved the Extension Amendment and the Trust Amendment to
allow the Company to extend the deadline by which it must complete its initial business combination by up to nine one-month periods from
August 11, 2023. In connection with each such extension, Data Knights, LLC, the Company’s sponsor, caused approximately $,
deposited in the Trust Account in connection with the exercise of the monthly extension. In connection with each such extension, the
Company will have until May 11, 2023 to consummate a Business Combination(see Note 10). On September 30, 2023 and December 31, 2022,
there is $ and $ outstanding under the Extension Loan, respectively.
Administrative
Services Arrangement
Commencing
on the date of the prospectus and until completion of the Company’s Business Combination or liquidation, the Company may reimburse
ARC Group Ltd., an affiliate of the Sponsor, up to an amount of $10,000 per month for office space, secretarial and administrative support.
For the three months ended September 30, 2023 and 2022, we have incurred $30,000 in fees under this agreement, respectively. For the
nine months ended September 30, 2023 and 2022, we have incurred $90,000 in fees under this agreement, respectively.
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
COMMITMENTS AND CONTINGENCIES
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| COMMITMENTS AND CONTINGENCIES |
NOTE
6. COMMITMENTS AND CONTINGENCIES
Registration
Rights
Pursuant
to a registration rights agreement entered into on May 6, 2021, the holders of the Founder Shares, Private Placement Units (including
the securities contained therein), the units (including the securities contained therein) that may be issued upon conversion of the Working
Capital Loans, and any shares of Class A Common Stock issuable upon the exercise of the Placement Warrants and any shares of Class A
Common Stock, warrants (and underlying Class A Common Stock) that may be issued upon conversion of the units issued as part of the working
capital loans and Class A Common Stock issuable upon conversion of the founder shares are entitled to registration rights. The holders
of a majority of these securities are entitled to make up to three demands, excluding short form demands, that the Company register such
securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements
filed subsequent to the completion of a Business Combination and rights to require the Company to register for resale such securities
pursuant to Rule 415 under the Securities Act. However, the registration rights agreement provides that the Company will not permit any
registration statement filed under the Securities Act to become effective until termination of the applicable lock-up period. The Company
will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriters
Agreement
The
Company granted the underwriter a 45-day option to purchase up to 1,500,000 additional Units to cover over-allotments at the Initial
Public Offering price, less the underwriting discounts and commissions. The aforementioned option was exercised on May 11, 2021.
The
underwriter was paid a cash underwriting discount of two percent (2.00%) of the gross proceeds of the Initial Public Offering, or $2,300,000.
In addition, the underwriter is entitled to a deferred fee of three and a half percent (3.50%) of the gross proceeds of the Initial Public
Offering, or $4,025,000. The deferred fee was placed in the Trust Account and will be paid in cash upon the closing of a Business Combination,
subject to the terms of the underwriting agreement.
Right
of First Refusal
For
a period beginning on May 7, 2021 and ending 12 months from the closing of a business combination, we have granted the underwriters a
right of first refusal to act as lead-left book running manager and lead left manager for any and all future private or public equity,
convertible and debt offerings during such period. In accordance with FINRA Rule 5110(f)(2)(E)(i), such right of first refusal shall
not have a duration of more than three years from the effective date of our Registration Statement.
First
Amendment to the Introducing Advisor Agreement
On
March 22, 2022, the Company and ARC entered into the First Amendment to the Introducing Advisor Agreement, pursuant to which both parties
agreed that the Company would pay to ARC an additional success fee equivalent to five percent (5%) on any PIPE that was brought by ARC
in connection with an initial business combination upon the closing of an initial business combination.
Second
Amendment to the Introducing Advisor Agreement
On
December 31, 2022, the Company and ARC entered into the Second Amendment to the Introducing Advisor Agreement, pursuant to which both
parties agreed to extend the Termination Date to December 31, 2024, and to change the performance condition for the Equity Issuance from
the closing of an initial business combination to the execution of a business combination agreement. On December 31, 2022, following
the execution of the Second Amendment to the Introducing Advisor Agreement, the performance condition for the Equity Issuance was deemed
to have been met, and ARC was issued 1,378,517 shares of the Company’s Class B Common Stock, up to 143,766 shares of which are
subject to forfeiture if the public stockholders exercise redemption rights with respect to any of the remaining outstanding shares of
Class A Common Stock.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
WARRANT LIABILITY
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Warrant Liability |
|
| WARRANT LIABILITY |
NOTE
7. WARRANT LIABILITY
Public
Warrants may only be exercised for a whole number of shares. No fractional warrants will be issued upon separation of the Units and only
whole warrants will trade. The Public Warrants will become exercisable 30 days after the completion of a Business Combination and will
expire five years after the completion of a Business Combination or earlier upon redemption or liquidation.
The
Company will not be obligated to deliver any shares of Class A Common Stock pursuant to the exercise of a warrant and will have no obligation
to settle such warrant exercise unless a registration statement under the Securities Act covering the issuance of the shares of Class
A Common Stock issuable upon exercise of the warrants is then effective and a current prospectus relating to those shares of Class A
Common Stock is available, subject to the Company satisfying its obligations with respect to registration. No warrant will be exercisable
for cash or on a cashless basis, and the Company will not be obligated to issue any shares to holders seeking to exercise their warrants,
unless the issuance of the shares upon such exercise is registered or qualified under the securities laws of the state of the exercising
holder, or an exemption from registration is available.
The
Company has agreed that as soon as practicable, but in no event later than 20 business days after the closing of its initial Business
Combination, it will use its commercially reasonable efforts to file with the SEC a post-effective amendment to the registration statement
or a new registration statement covering the shares of Class A Common Stock issuable upon exercise of the warrants, to cause such registration
statement to become effective and to maintain a current prospectus relating to those shares of Class A Common Stock until the warrants
expire or are redeemed, as specified in the warrant agreement. If a registration statement covering the shares of Class A Common Stock
issuable upon exercise of the warrants is not effective by the 60th business day after the closing of the Company’s initial business
combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company
will have failed to maintain an effective registration statement, exercise warrants on a “cashless basis” in accordance with
Section 3(a)(9) of the Securities Act or another exemption.. Notwithstanding the above, if the Company’s shares of Class A Common
Stock are at the time of any exercise of a warrant not listed on a national securities exchange such that they satisfy the definition
of a “covered security” under Section 18(b)(1) of the Securities Act, it may, at its option, require holders of Public Warrants
who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and,
in the event the Company so elects, it will not be required to file or maintain in effect a registration statement, and in the event
it does not so elect, it will use its commercially reasonable efforts to register or qualify the shares under applicable blue sky laws
to the extent an exemption is not available. If that exemption, or another exemption, is not available, holders will not be able to exercise
their warrants on a cashless basis.
Redemption
of warrants when the price per Class A Common Stock equals or exceeds $18.00. Once the warrants become exercisable, the Company may redeem
the Public Warrants:
| ● | in
whole and not in part; |
| | | |
| ● | at
a price of $0.01 per Public Warrant; |
| | | |
| ● | upon
not less than 30 days’ prior written notice of redemption to each warrant holder; and |
| | | |
| ● | if,
and only if, the reported last sale price of the Class A Common Stock equals or exceeds $18.00
per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations
and the like and certain issuances of Class A Common Stock and equity-linked securities)
for any 20 trading days within a 30-trading day period commencing no earlier than the date
the warrants become exercisable and ending on the third business day before the date on which
the Company sends the notice of redemption to the warrant holders. |
If
and when the warrants become redeemable by the Company, the Company may exercise its redemption right even if it is unable to register
or qualify the underlying securities for sale under all applicable state securities laws.
NOTE
7. WARRANT LIABILITY (Continued)
If
the Company calls the Public Warrants for redemption, management will have the option to require all holders that wish to exercise the
Public Warrants to do so on a “cashless basis,” as described in the warrant agreement. The exercise price and number of shares
of Class A Common Stock issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock
dividend, or recapitalization, reorganization, merger or consolidation. However, the warrants will not be adjusted for issuance of Class
A Common Stock at a price below its exercise price. Additionally, in no event will the Company be required to net cash settle the warrants.
If the Company is unable to complete a Business Combination within the Combination Window and the Company liquidates the funds held in
the Trust Account, holders of warrants will not receive any of such funds with respect to their warrants, nor will they receive any distribution
from the Company’s assets held outside of the Trust Account with the respect to such warrants. Accordingly, the warrants may expire
worthless. The Placement Warrants were identical to the Public Warrants underlying the Units being sold in the Proposed Public Offering,
except that the Placement Warrants and the Class A Common Stock issuable upon the exercise of the Placement Warrants will not be transferable,
assignable or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions. Additionally,
the Placement Warrants will be exercisable on a cashless basis and be non-redeemable so long as they are held by the initial purchasers
or their permitted transferees. If the Placement Warrants are held by someone other than the initial purchasers or their permitted transferees,
the Placement Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
In
addition, if (x) the Company issues additional shares of Class A Common Stock or equity-linked securities for capital raising purposes
in connection with the closing of its initial Business Combination at an issue price or effective issue price of less than $9.20 per
share of Class A Common Stock (with such issue price or effective issue price to be determined in good faith by the Company’s board
of directors and, in the case of any such issuance to the Sponsor or its affiliates, without taking into account any Founder Shares held
by the Sponsor or such affiliates, as applicable, prior to such issuance) (the “Newly Issued Price”), (y) the aggregate gross
proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of
the Company’s initial Business Combination on the date of the consummation of such initial Business Combination (net of redemptions),
and (z) the volume weighted average trading price of the Company’s common stock during the 20 trading day period starting on the
trading day prior to the day on which the Company consummates its initial Business Combination (such price, the “Market Value”)
is below $9.20 per share, the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the higher
of the Market Value and the Newly Issued Price, the $18.00 per share redemption trigger price described above will be adjusted (to the
nearest cent) to be equal to 180% of the greater of the Market Value and the Newly Issued Price and the $10.00 per share redemption trigger
price described above will be adjusted (to the nearest cent) to be equal to the greater of the Market Value and the Newly Issued Price.
The
Placement Warrants will be identical to the Public Warrants underlying the Units being sold in the Proposed Public Offering, except that
the Placement Warrants and the Class A Common Stock issuable upon the exercise of the Placement Warrants will not be transferable, assignable
or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions. Additionally, the Placement
Warrants will be exercisable on a cashless basis and be non-redeemable so long as they are held by the initial purchasers or their permitted
transferees. If the Placement Warrants are held by someone other than the initial purchasers or their permitted transferees, the Placement
Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
At
September 30, 2022 and December 31, 2022, the Company accounted for the aggregate 12,085,275 warrants issued in connection with the Initial
Public Offering (the 11,500,000 Public Warrants and the 585,275 Placement Warrants) in accordance with the guidance contained in ASC
815-40. Such guidance provides that because the warrants do not meet the criteria for equity treatment thereunder, each warrant must
be recorded as a liability. Accordingly, the Company will classify each warrant as a liability at its fair value, with the change in
fair value recognized in the Company’s statement of operations.
|
| X |
- References
+ Details
| Name: |
ONMD_DisclosureWarrantLiabilityAbstract |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWarrant Liability [Text Block]
| Name: |
ONMD_WarrantLiabilityTextBlock |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
STOCKHOLDER’S EQUITY
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Equity [Abstract] |
|
| STOCKHOLDER’S EQUITY |
NOTE
8. STOCKHOLDER’S EQUITY
Preferred
Shares — The Company is authorized to issue 1,000,000 preferred shares with a par value of $0.0001 per share with such
designation, rights and preferences as may be determined from time to time by the Company’s Board of Directors. At September 30,
2023 and December 31, 2022, there were no preferred shares issued or outstanding.
Class
A Common Stock — The Company is authorized to issue 100,000,000 shares of Class A Common Stock with a par value of $0.0001
per share. Holders of the Company’s Class A Common Stock are entitled to one vote for each share. At September 30, 2023 and December
31, 2022, there were 1,712,698 shares of Class A Common Stock issued and outstanding that were subject to possible redemption and 585,275
shares of non-redeemable Class A Common Stock issued and outstanding that were issued in connection with the private placement (Note
4).
Class
B Common Stock — The Company is authorized to issue up to 10,000,000 shares of Class B Common Stock with a par value of
$0.0001 per share. Holders of the Company’s Class B Common Stock are entitled to one vote for each share. On February 25, 2021,
the Sponsor transferred shares to the Company’s Chief Executive Officer, shares to the Company’s Chief Financial
Officer and shares to two of the Company’s independent directors. Following the determination of the Company’s third
independent director, on March 23, 2021, the Sponsor transferred shares to such independent director.
On
December 31, 2022, ARC Group Limited, the Company’s Financial Advisor, was granted 1,378,517 shares of Class B common stock with
a par value of $0.0001 per share, up to 143,766 of which are subject to forfeiture if the Company’s public stockholders exercise
redemption rights with respect to any of the Company’s remaining outstanding shares of Class A common stock.
Accordingly,
at September 30, 2023 and December 31, 2022, there were 4,253,517 shares of Class B Common Stock issued and outstanding.
Holders
of Class A Common Stock and Class B Common Stock will vote together as a single class on all other matters submitted to a vote of stockholders,
except as required by law.
The
shares of Class B Common Stock will automatically convert into shares of Class A Common Stock at the time of the Business Combination
on a one-for-one basis, subject to adjustment for stock splits, stock dividends, reorganizations, recapitalizations and the like. In
the case that additional shares of Class A Common Stock, or equity linked securities, are issued or deemed issued in excess of the amounts
offered in the Initial Public Offering and related to the closing of a Business Combination, the ratio at which shares of Class B Common
Stock shall convert into shares of Class A Common Stock will be adjusted (unless the holders of a majority of the outstanding shares
of Class B Common Stock agree to waive such adjustment with respect to any such issuance or deemed issuance) so that the number of shares
of Class A Common Stock issuable upon conversion of all shares of Class B Common Stock will equal, in the aggregate, on an as converted
basis, 20% of the sum of the total number of all shares of common stock outstanding upon the completion of the Initial Public Offering
plus all shares of Class A Common Stock and equity linked securities issued or deemed issued in connection with a Business Combination
(excluding any shares or equity linked securities issued, or to be issued, to any seller in a Business Combination, and any private placement-equivalent
units and its underlying securities issued to the Sponsor or its affiliates upon conversion of loans made to the Company).
The
Company may issue additional common stock or preferred stock to complete its Business Combination or under an employee incentive plan
after completion of its Business Combination.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
FAIR VALUE MEASUREMENTS
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Fair Value Disclosures [Abstract] |
|
| FAIR VALUE MEASUREMENTS |
NOTE
9. FAIR VALUE MEASUREMENTS
The
following table presents information about the Company’s assets and derivative warrant liabilities that are measured at fair value
on a recurring basis as of September 30, 2023 and December 31, 2022 and indicates the fair value hierarchy of the valuation techniques
that the Company utilized to determine such fair value:
SCHEDULE
OF FAIR VALUE HIERARCHY OF VALUATION TECHNIQUES
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| | |
September 30, 2023 | |
| | |
Quoted Prices in | | |
Significant Other | | |
Significant Other | |
| | |
Active Markets | | |
Observable Inputs | | |
Unobservable Inputs | |
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| Asset: | |
| | | |
| | | |
| | |
| Investments held in Trust Account | |
$ | 19,308,261 | | |
$ | — | | |
$ | — | |
| | |
| | | |
| | | |
| | |
| Warrant Liabilities: | |
| | | |
| | | |
| | |
| Public Warrants | |
$ | 575,000 | | |
$ | — | | |
$ | — | |
| Private Placement Warrants | |
$ | — | | |
$ | — | | |
$ | 29,849 | |
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| | |
December 31, 2022 | |
| | |
Quoted Prices in | | |
Significant Other | | |
Significant Other | |
| | |
Active Markets | | |
Observable Inputs | | |
Unobservable Inputs | |
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| Asset: | |
| | | |
| | | |
| | |
| Investments held in Trust Account | |
$ | 29,029,416 | | |
$ | — | | |
$ | — | |
| | |
| | | |
| | | |
| | |
| Warrant Liabilities: | |
| | | |
| | | |
| | |
| Public Warrants | |
$ | 345,000 | | |
$ | — | | |
$ | — | |
| Private Placement Warrants | |
$ | — | | |
$ | — | | |
$ | 17,558 | |
The
Warrants are measured at fair value on a recurring basis. The Public Warrants were valued initially and at each reporting period that
the warrants were not actively traded, using a Monte Carlo simulation. As of September 30, 2023 and December 31, 2022, the Public Warrants
were valued using the instrument’s publicly listed trading price, which is considered to be a Level 1 measurement due to the use
of an observable market quote in an active market. Private Placement Warrants were valued using a Monte Carlo valuation model using level
3 inputs at initial valuation and as of September 30, 2023 and December 31, 2022.
At
September 30, 2023 and December 31, 2022, assets held in the Trust Account were invested solely in Morgan Stanley Bank cash, bank deposit
program and money market fund and BlackRock US Treasury mutual fund of $19,308,261 and $29,029,416, respectively.
The
Warrants were accounted for as liabilities in accordance with ASC 815-40 and are presented within warrant liabilities in the accompanying
consolidated balance sheets. The warrant liabilities are measured at fair value at inception and on a recurring basis, with changes in
fair value presented within the consolidated statement of operations.
The
accounting treatment of derivative financial instruments requires that the Company record a derivative liability upon the closing of
the Initial Public Offering. Accordingly, the Company classified each warrant as a liability at its fair value and the warrants were
allocated a portion of the proceeds from the issuance of the Units equal to its fair value determined by the Monte Carlo simulation.
This liability is subject to remeasurement at each balance sheet date. With each such re-measurement, the warrant liability will be adjusted
to fair value, with the change in fair value recognized in the Company’s statement of operations. The Company will reassess the
classification at each balance sheet date. If the classification changes as a result of events during the period, the warrants will be
reclassified as of the date of the event that causes the reclassification.
NOTE
9. FAIR VALUE MEASUREMENTS (Continued)
The
Company utilized a Monte Carlo simulation to estimate the fair value of the Public warrants at each reporting period for its warrants
that are not actively traded. Inherent in a Monte Carlo simulation are assumptions related to expected stock-price volatility, expected
life, risk-free interest rate and dividend yield. The Company estimates the volatility of its common stock based on historical volatility
of select peer companies that matches the expected remaining life of the warrants. The risk-free interest rate is based on the U.S. Treasury
zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of
the warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which
the Company anticipates remaining at zero. On June 22, 2021, the Public Warrants surpassed the threshold waiting period to be publicly
traded. Once publicly traded, the observable input qualifies the liability for treatment as a Level 1 liability. As such, as of September
30, 2023 and December 31, 2022, the Company classified the Public Warrants as Level 1.
The
estimated fair value of the Private Placement Warrants is determined using Level 3 inputs. Inherent in a Monte Carlo model are assumptions
related to expected share-price volatility, expected life, risk-free interest rate and dividend yield. The Company estimates the volatility
of its warrants based on implied volatility from the Company’s traded warrants and from historical volatility of select peer company’s
common stock that matches the expected remaining life of the Warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon
yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of the warrants
is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which the Company
anticipates remaining at zero.
Transfers
to/from Levels 1, 2 and 3 are recognized at the end of the reporting period in which a change in valuation technique or methodology occurs.
For the three months ended September 30, 2023 and December 31, 2022, there were no transfers between levels.
The
following table provides quantitative information regarding Level 3 fair value measurements inputs as their measurement dates:
SCHEDULE
OF FAIR VALUE MEASUREMENTS INPUTS AS THEIR MEASUREMENT DATES
| | |
September 30, 2023 | | |
December 31, 2022 | |
| | |
(Private Warrants) | | |
(Private Warrants) | |
| Exercise price | |
$ | 11.50 | | |
$ | 11.50 | |
| Share price | |
$ | 11.26 | | |
$ | 10.44 | |
| Expected term (years) | |
| 5.61 | | |
| 5.12 | |
| Probability of Acquisition | |
| 2.10 | % | |
| 2.75 | % |
| Volatility | |
| 7.9 | % | |
| 4.2 | % |
| Risk-free rate | |
| 4.50 | % | |
| 3.91 | % |
| Dividend yield (per share) | |
$ | 0.00 | | |
$ | 0.00 | |
The
change in the fair value of the derivative warrant liabilities for the three months ended September 30, 2023 and 2022 is as follows:
SCHEDULE
OF CHANGES IN FAIR VALUE OF DERIVATIVE WARRANT LIABILITIES
| | |
Private Warrants | | |
Public Warrants | | |
Total Warrant Liability | |
| Fair value as of December 31, 2022 | |
$ | 17,558 | | |
$ | 345,000 | | |
$ | 362,558 | |
| Change in valuation inputs or other assumptions(1) | |
| 12,291 | | |
| 230,000 | | |
| 242,291 | |
| Fair value as of September 30, 2023 | |
$ | 29,849 | | |
$ | 575,000 | | |
$ | 604,849 | |
| | |
Private Warrants | | |
Public Warrants | | |
Total Warrant Liability | |
| Fair value as of December 31, 2021 | |
$ | 251,668 | | |
$ | 4,600,000 | | |
$ | 4,851,668 | |
| Fair value, beginning balance | |
$ | 251,668 | | |
$ | 4,600,000 | | |
$ | 4,851,668 | |
| Change in valuation inputs or other assumptions(1) | |
| (117,640 | ) | |
| (2,645,000 | ) | |
| (2,762,640 | ) |
| Fair value as of September 30, 2022 | |
$ | 134,028 | | |
$ | 1,955,000 | | |
$ | 2,089,028 | |
| Fair value, ending balance | |
$ | 134,028 | | |
$ | 1,955,000 | | |
$ | 2,089,028 | |
| (1) | Changes
in valuation inputs or other assumptions are recognized in the change in fair value of warrant
liability in the consolidated statement of operations. |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
SUBSEQUENT EVENTS
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Subsequent Events [Abstract] |
|
| SUBSEQUENT EVENTS |
NOTE
10. SUBSEQUENT EVENTS
In
accordance with ASC Topic 855, “Subsequent Events”, which establishes general standards of accounting for and disclosure
of events that occur after the balance sheet date but before financial statements are issued, the Company has evaluated all events or
transactions that occurred after the balance sheet date up to the date that the financial statements were issued. Based upon this review,
the Company did not identified any subsequent events that would have required adjustment or disclosure in the financial statements other
than as described below.
On
October 12, 2023, the Company elected to exercise its eighth of third one-month extension to the Termination Date, which extended its
deadline to complete its initial business combination to November 11, 2023, by depositing $75,000 into the Trust Account.
On
October 17, 2023, the Company held a special meeting of its stockholders (the “Stockholders”) in lieu of the 2023 annual
meeting of stockholders (the “Special Meeting”) in connection with the transactions contemplated by that certain Agreement
and Plan of Merger dated April 25, 2022 (the “Merger Agreement”), by and among the Company, Data Knights Merger Sub, Inc.,
a Delaware corporation (“Merger Sub”), Data Knights, LLC, the Company’s sponsor, OneMedNet Solutions Corporation (formerly
named OneMedNet Corporation), a Delaware corporation, (“OneMedNet”), and Paul Casey, as seller representative (such transactions,
collectively, the “Business Combination”). At the Special Meeting, the Stockholders were asked to consider and vote on the
proposals identified in the definitive proxy statement/prospectus that the Company filed with the U.S. Securities and Exchange Commission
(the “SEC”) on September 21, 2023 (the “Definitive Proxy”). Proposals 1 through 7 set forth below were approved:
| - | Proposal
1: To approve an amendment to the Second Amendment to the Second Amended and Restated
Certificate of Incorporation (the “Charter”) effective prior to the consummation
of the Business Combination, to remove from the Charter the redemption limitation contained
under Section 9.2(a) preventing the Company from closing a business combination if it would
have less than $5,000,0001 of net tangible assets (the “NTA Proposal”). |
| | | |
| - | Proposal
2: To approve and adopt the Merger Agreement and approve the transactions contemplated
thereby (the “Business Combination Proposal”). |
| | | |
| - | Proposal
3: To approve, for purposes of complying with Nasdaq Listing Rules 5635(a) and (b), the
issuance of more than 20% of the issued and outstanding Class A common stock and the resulting
change in control in connection with the Business Combination (the “Nasdaq Proposal”). |
NOTE 10. SUBSEQUENT
EVENTS (Continued)
| - | Proposal
4: To approve the Third Amended and Restated Certificate of Incorporation, which shall
become effective upon the closing of the Business Combination (the “Charter Amendment
Proposal”), including (i) the addition of a supermajority voting requirement to amend
the Surviving Corporation’s Bylaws, and (ii) the addition of a supermajority voting
requirement to amend Articles V (Board of Directors), VI (Stockholders), VII (Liability and
Indemnification; Corporate Opportunity), VIII (Business Combinations), IX (Exclusive Forum),
and Article X (Amendments) to the Third Amended and Restated Certificate of Incorporation. |
| | | |
| - | Proposal
4A: To approve the addition to Company’s Certificate of Incorporation of a super
majority voting requirement to amend the Surviving Corporation’s Bylaws (the “Bylaw
Amendment Requirement”), as contained in the Third Amended and Restated Certificate
of Incorporation. |
| | | |
| - | Proposal
4B: To approve the addition to the Company’s Certificate of Incorporation of a
super majority voting requirement (the “Article Amendment Requirement”) to amend
Articles V (Board of Directors), VI (Stockholders), VII (Liability and Indemnification; Corporate
Opportunity), VIII (Business Combinations), IX (Exclusive Forum), and Article X (Amendments)
of the Third Amended and Restated Certificate of Incorporation. |
| | | |
| - | Proposal
5: To elect eight directors to serve on the Company’s board of directors following
the consummation of the Business Combination until the 2024 annual meeting of Stockholders,
in the case of Class I directors, the 2025 annual meeting of Stockholders, in the case of
Class II directors, and the 2026 annual meeting of Stockholders, in the case of Class III
directors, and, in each case, until their respective successors are duly elected and qualified
(the “Director Election Proposal”). |
| | | |
| - | Proposal
6: To approve the 2022 Equity Incentive Plan (the “Incentive Plan Proposal”). |
| - | Proposal
7: To approve adjourning the Special Meeting to a later date or dates, if necessary to
permit further solicitation and vote of proxies if it is determined by the Company that more
time is necessary or appropriate to approve one or more Proposals at the Special Meeting
(the “Adjournment Proposal”). |
In
connection with the Special Meeting, certain Stockholders (the “Redeeming Stockholders”) holding 1,614,148 shares of Class
A Common Stock (after giving effect to withdrawals of redemptions) exercised their right to redeem such shares for a pro rata portion
of the funds held by Continental Stock Transfer & Trust Company, as trustee (“Continental”) in the trust account established
in connection with the Company’s initial public offering (the “Trust Account”). As a result, approximately $17.9 million
(approximately $11.12 per share) will be removed from the Trust Account to pay the Redeeming Stockholders, and approximately $1.1 million
will remain in the Trust Account.
On
November 7, 2023, the Company consummated the Business Combination with OneMedNet pursuant to Merger Agreement, following the approval
of the Business Combination at the Special Meeting. On November 7, 2023, at the closing of the Business Combination pursuant to the Merger
Agreement, Merger Sub merged with and into OneMedNet with OneMedNet surviving the Merger, as a wholly-owned subsidiary of the Company,
and the Company changed its name to “OneMedNet Corporation.”
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Policies)
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of presentation |
Basis
of presentation
The
accompanying financial statements are presented in U.S. Dollars and conformity with accounting principles generally accepted in the United
States of America (“GAAP”) and pursuant to the rules and regulations of the SEC.
|
| Principles of Consolidation |
Principles
of Consolidation
The
accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary. All significant intercompany
balances and transactions have been eliminated in consolidation.
|
| Emerging growth company |
Emerging
growth company
The
Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our
Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements
that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required
to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding
executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory
vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further,
Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting
standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do
not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting
standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements
that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of
such extended transition period which means that when a standard is issued or revised and it has different application dates for public
or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies
adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which
is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult
or impossible because of the potential differences in accounting standards used.
|
| Use of estimates |
Use
of estimates
The
preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported
amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the
reported amounts of revenues and expenses during the reporting period.
Making
estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of
a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating
its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ
significantly from those estimates.
|
| Cash and Cash Equivalents |
Cash
and Cash Equivalents
The
Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. Cash
equivalents are carried at cost, which approximates fair value. The Company had $1,537 and $30,870 in cash and no cash equivalents as
of September 30, 2023 and December 31, 2022, respectively.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
|
| Trust Account |
Trust
Account
Upon
the closing of the Initial Public Offering and the Private Placement, $117,300,000 ($10.00 per Unit) of the net proceeds of the Initial
Public Offering and certain of the proceeds of the Private Placement was held in a trust account (“Trust Account”) located
in the United States with Continental Stock Transfer & Trust Company acting as trustee, and invested only in U.S. government treasury
obligations with a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 under the Investment
Company Act 1940, as amended (the “Investment Company Act”), which will be invested only in direct U.S. government treasury
obligations, as determined by the Company, until the earlier of: (i) the completion of a Business Combination and (ii) the distribution
of the Trust Account as described below.
|
| Offering Costs Associated with the Initial Public Offering |
Offering
Costs Associated with the Initial Public Offering
Offering
costs consisted of legal, accounting, underwriting fees and other costs incurred that were directly related to the Initial Public Offering.
Offering costs are allocated to the separable financial instruments issued in the Initial Public Offering based on a relative fair value
basis, compared to total proceeds received. Offering costs associated with warrant liabilities are expensed as incurred, presented as
offering costs allocated to warrants in the consolidated statements of operations. Offering costs associated with the Public Shares were
charged to stockholders’ equity upon the completion of the Initial Public Offering.
|
| Class A Common Stock Subject to Possible Redemption |
Class
A Common Stock Subject to Possible Redemption
The
Company accounts for its shares subject to possible redemption in accordance with the guidance in Accounting Standards Codification (“ASC”)
Topic 480 “Distinguishing Liabilities from Equity.” Shares subject to mandatory redemption (if any) is classified as a liability
instrument and is measured at fair value. Conditionally redeemable shares of common stock (including shares of common stock that feature
redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not
solely within the Company’s control) is classified as temporary equity. At all other times, shares are classified as stockholders’
equity. The Company’s Class A Common Stock features certain redemption rights that are considered to be outside of the Company’s
control and subject to occurrence of uncertain future events.
On
September 30, 2023, there are 585,275 shares of Class A Common Stock related to the Private Placement Units (Note 8) outstanding, which
are not subject to redemption, and 1,712,698 shares of Class A Common Stock outstanding, which are subject to possible redemption.
If
it is probable that the equity instrument will become redeemable, the Company has the option to either accrete changes in the redemption
value over the period from the date of issuance (or from the date that it becomes probable that the instrument will become redeemable,
if later) to the earliest redemption date of the instrument or to recognize changes in the redemption value immediately as they occur
and adjust the carrying amount of the instrument to equal the redemption value at the end of each reporting period. The Company has elected
to recognize the changes immediately. The accretion or remeasurement is treated as a deemed dividend (i.e., a reduction to retained earnings,
or in absence of retained earnings, additional paid-in capital).
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
As
of September 30, 2023 and 2022, the Class A Common Stock reflected on the balance sheet are reconciled in the following table:
SCHEDULE
OF RECONCILED CLASS A COMMON STOCK
| | |
2023 | | |
2022 | |
| | |
For the Nine Months Ended | |
| | |
September 30, | |
| | |
2023 | | |
2022 | |
| Contingently redeemable Class A Common Stock – Opening Balance | |
$ | 28,750,110 | | |
$ | 117,300,000 | |
| Less: | |
| | | |
| | |
| Redemption of Class A Common Stock, including interest | |
| (11,335,260 | ) | |
| | |
| Plus: | |
| | | |
| | |
| Re-measurement of carrying value to redemption value | |
| 1,794,994 | | |
| 2,833,869 | |
| Contingently redeemable Class A Common Stock - Ending Balance | |
| 19,209,844 | | |
| 120,133,869 | |
|
| Net income (loss) per share |
Net
income (loss) per share
Net
income (loss) per share is computed by dividing net income (loss) by the weighted average number of common stock shares outstanding for
the period. The calculation of diluted income (loss) per share does not consider the effect of the warrants issued in connection with
the Initial Public Offering and warrants issued as components of the Private Placement Units (the “Placement Warrants”) since
the exercise of the warrants are contingent upon the occurrence of future events and the inclusion of such warrants would be anti-dilutive.
The
Company applies the two-class method in calculating earnings per share. The contractual formula utilized to calculate the redemption
amount approximates fair value. The Class feature to redeem at fair value means that there is effectively only one class of stock. Changes
in fair value are not considered a dividend for the purposes of the numerator in the earnings per share calculation. Net income per common
share is computed by dividing the pro rata net income (loss) between the redeemable shares and the non-redeemable shares by the weighted
average number of common shares outstanding for each of the periods. The calculation of diluted income (loss) per common stock does not
consider the effect of the warrants issued in connection with the IPO since the exercise of the warrants are contingent upon the occurrence
of future events and the inclusion of such warrants would be anti-dilutive. The warrants are exercisable for 12,085,275 shares of common
stock in the aggregate.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The
following table reflects the calculation of basic and diluted net income (loss) per common share:
RECONCILIATION
OF NET LOSS PER COMMON SHARE
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
For the Three Months Ended | | |
For the Nine months ended | |
| | |
September 30, | | |
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Redeemable Class A common shares | |
| | | |
| | | |
| | | |
| | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) allocable to common stock subject to possible redemption | |
$ | (252,542 | ) | |
$ | (117,823 | ) | |
$ | (312,541 | ) | |
$ | 2,300,089 | |
| | |
| | | |
| | | |
| | | |
| | |
| Denominator: weighted average number of redeemable common share | |
| 2,166,749 | | |
| 11,500,000 | | |
| 2,541,210 | | |
| 11,500,000 | |
| Basic and diluted net income (loss) per redeemable common share | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
| | |
| | | |
| | | |
| | | |
| | |
| Non-redeemable Class A and Class B common shares | |
| | | |
| | | |
| | | |
| | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) allocable to common stock not subject to redemption | |
$ | (563,978 | ) | |
$ | (35,452 | ) | |
$ | (595,119 | ) | |
$ | 692,082 | |
| | |
| | | |
| | | |
| | | |
| | |
| Denominator: weighted average number of non-redeemable common shares | |
| 4,838,792 | | |
| 3,460,275 | | |
| 4,838,792 | | |
| 3,460,275 | |
| Basic and diluted net income (loss) per non-redeemable common share | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
|
| Concentration of credit risk |
Concentration
of credit risk
Financial
instruments that potentially subject the Company to concentration of credit risk consist of a cash account in a financial institution
which, at times may exceed the Federal depository insurance coverage of $250,000. At September 30, 2023 and 2022, the Company had not
experienced losses on this account and management believes the Company is not exposed to significant risks on such account.
|
| Fair value of financial instruments |
Fair
value of financial instruments
The
fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC Topic 820, “Fair Value
Measurements and Disclosures,” approximates the carrying amounts represented in the accompanying consolidated balance sheets, primarily
due to their short-term nature, except warrant liabilities (See Note 9).
|
| Derivative Financial Instruments |
Derivative
Financial Instruments
The
Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded
derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. For derivative financial instruments that are accounted
for as liabilities, the derivative instrument is initially recorded at its fair value on the grant date and is then re-valued at each
reporting date, with changes in the fair value reported in the statements of operations. The classification of derivative instruments,
including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period.
Derivative liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement or conversion
of the instrument could be required within 12 months of the balance sheet date.
NOTE
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
|
| Income Taxes |
Income
Taxes
The
Company complies with the accounting and reporting requirements of ASC Topic 740, “Income Taxes,” which requires an asset
and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed
for differences between the financial statement and tax bases of assets and liabilities that will result in future taxable or deductible
amounts, based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income.
Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC
Topic 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax
positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not
to be sustained upon examination by taxing authorities. The Company’s management determined that the United States is the Company’s
only major tax jurisdiction. The Company recognizes accrued interest and penalties related to unrecognized tax benefits, if any, as income
tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of September 30, 2023 and 2022,
respectively. The Company is currently not aware of any issues under review that could result in significant payments, accruals, or material
deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Our
effective tax rate was (12.15)% and 0% for the three months ended September 30, 2023 and 2022, respectively. Our effective tax rate was
(30.10)% and 0% for the nine months ended September 30, 2023 and 2022, respectively. The effective tax rate differs from the statutory
tax rate of 21% for the three months ended September 30, 2023 and 2022, due to transaction costs and the valuation allowance on the deferred
tax assets.
|
| Recently Issued Accounting Standards |
Recently
Issued Accounting Standards
In
August 2020, the FASB issued Accounting Standards Update (“ASU”) 2020-06, “Debt — Debt with Conversion and Other
Options” (Subtopic 470-20) and “Derivatives and Hedging — Contracts in Entity’s Own Equity” (Subtopic 815-40)
(“ASU 2020-06”) to simplify accounting for certain financial instruments. ASU 2020-06 eliminates the current models that
require separation of beneficial conversion and cash conversion features from convertible instruments and simplifies the derivative scope
exception guidance pertaining to equity classification of contracts in an entity’s own equity. The new standard also introduces
additional disclosures for convertible debt and freestanding instruments that are indexed to and settled in an entity’s own equity.
ASU 2020-06 amends the diluted earnings per share guidance, including the requirement to use the if-converted method for all convertible
instruments. ASU 2020-06 is effective for the Company for the fiscal year beginning after December 15, 2023, including interim periods
within those fiscal years.
Management
does not believe that any recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material
effect on the Company’s financial statements.
|
| X |
- DefinitionAssets Held In Trust Account Policy [Policy Text Block]
| Name: |
ONMD_AssetsHeldInTrustAccountPolicyPolicyTextBlock |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDerivative Financial Instruments Policy [Policy Text Block]
| Name: |
ONMD_DerivativeFinancialInstrumentsPolicyPolicyTextBlock |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEmerging Growth Company Policy [Policy Text Block]
| Name: |
ONMD_EmergingGrowthCompanyPolicyPolicyTextBlock |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTemporary Equity Policy [Policy Text Block]
| Name: |
ONMD_TemporaryEquityPolicyPolicyTextBlock |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for credit risk. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 825
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480981/942-825-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
| Name: |
us-gaap_ConcentrationRiskCreditRisk |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for deferral and amortization of significant deferred charges. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DeferredChargesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 60
-Paragraph 1
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482053/820-10-60-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Tables)
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| SCHEDULE OF RECONCILED CLASS A COMMON STOCK |
SCHEDULE
OF RECONCILED CLASS A COMMON STOCK
| | |
2023 | | |
2022 | |
| | |
For the Nine Months Ended | |
| | |
September 30, | |
| | |
2023 | | |
2022 | |
| Contingently redeemable Class A Common Stock – Opening Balance | |
$ | 28,750,110 | | |
$ | 117,300,000 | |
| Less: | |
| | | |
| | |
| Redemption of Class A Common Stock, including interest | |
| (11,335,260 | ) | |
| | |
| Plus: | |
| | | |
| | |
| Re-measurement of carrying value to redemption value | |
| 1,794,994 | | |
| 2,833,869 | |
| Contingently redeemable Class A Common Stock - Ending Balance | |
| 19,209,844 | | |
| 120,133,869 | |
|
| RECONCILIATION OF NET LOSS PER COMMON SHARE |
RECONCILIATION
OF NET LOSS PER COMMON SHARE
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
For the Three Months Ended | | |
For the Nine months ended | |
| | |
September 30, | | |
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Redeemable Class A common shares | |
| | | |
| | | |
| | | |
| | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) allocable to common stock subject to possible redemption | |
$ | (252,542 | ) | |
$ | (117,823 | ) | |
$ | (312,541 | ) | |
$ | 2,300,089 | |
| | |
| | | |
| | | |
| | | |
| | |
| Denominator: weighted average number of redeemable common share | |
| 2,166,749 | | |
| 11,500,000 | | |
| 2,541,210 | | |
| 11,500,000 | |
| Basic and diluted net income (loss) per redeemable common share | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
| | |
| | | |
| | | |
| | | |
| | |
| Non-redeemable Class A and Class B common shares | |
| | | |
| | | |
| | | |
| | |
| Numerator: | |
| | | |
| | | |
| | | |
| | |
| Net income (loss) allocable to common stock not subject to redemption | |
$ | (563,978 | ) | |
$ | (35,452 | ) | |
$ | (595,119 | ) | |
$ | 692,082 | |
| | |
| | | |
| | | |
| | | |
| | |
| Denominator: weighted average number of non-redeemable common shares | |
| 4,838,792 | | |
| 3,460,275 | | |
| 4,838,792 | | |
| 3,460,275 | |
| Basic and diluted net income (loss) per non-redeemable common share | |
$ | (0.12 | ) | |
$ | 0.01 | | |
$ | (0.12 | ) | |
$ | 0.20 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of an entity's basic and diluted earnings per share calculations, including a reconciliation of numerators and denominators of the basic and diluted per-share computations for income from continuing operations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of temporary equity. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. If convertible, the issuer does not control the actions or events necessary to issue the maximum number of shares that could be required to be delivered under the conversion option if the holder exercises the option to convert the stock to another class of equity. If the security is a warrant or a rights issue, the warrant or rights issue is considered to be temporary equity if the issuer cannot demonstrate that it would be able to deliver upon the exercise of the option by the holder in all cases. Includes stock with put option held by ESOP and stock redeemable by holder only in the event of a change in control of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 480
-SubTopic 10
-Section S99
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480244/480-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (27)
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TemporaryEquityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
FAIR VALUE MEASUREMENTS (Tables)
|
9 Months Ended |
Sep. 30, 2023 |
|---|
| Fair Value Disclosures [Abstract] |
|
| SCHEDULE OF FAIR VALUE HIERARCHY OF VALUATION TECHNIQUES |
SCHEDULE
OF FAIR VALUE HIERARCHY OF VALUATION TECHNIQUES
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| | |
September 30, 2023 | |
| | |
Quoted Prices in | | |
Significant Other | | |
Significant Other | |
| | |
Active Markets | | |
Observable Inputs | | |
Unobservable Inputs | |
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| Asset: | |
| | | |
| | | |
| | |
| Investments held in Trust Account | |
$ | 19,308,261 | | |
$ | — | | |
$ | — | |
| | |
| | | |
| | | |
| | |
| Warrant Liabilities: | |
| | | |
| | | |
| | |
| Public Warrants | |
$ | 575,000 | | |
$ | — | | |
$ | — | |
| Private Placement Warrants | |
$ | — | | |
$ | — | | |
$ | 29,849 | |
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| | |
December 31, 2022 | |
| | |
Quoted Prices in | | |
Significant Other | | |
Significant Other | |
| | |
Active Markets | | |
Observable Inputs | | |
Unobservable Inputs | |
| Description | |
(Level 1) | | |
(Level 2) | | |
(Level 3) | |
| Asset: | |
| | | |
| | | |
| | |
| Investments held in Trust Account | |
$ | 29,029,416 | | |
$ | — | | |
$ | — | |
| | |
| | | |
| | | |
| | |
| Warrant Liabilities: | |
| | | |
| | | |
| | |
| Public Warrants | |
$ | 345,000 | | |
$ | — | | |
$ | — | |
| Private Placement Warrants | |
$ | — | | |
$ | — | | |
$ | 17,558 | |
|
| SCHEDULE OF FAIR VALUE MEASUREMENTS INPUTS AS THEIR MEASUREMENT DATES |
The
following table provides quantitative information regarding Level 3 fair value measurements inputs as their measurement dates:
SCHEDULE
OF FAIR VALUE MEASUREMENTS INPUTS AS THEIR MEASUREMENT DATES
| | |
September 30, 2023 | | |
December 31, 2022 | |
| | |
(Private Warrants) | | |
(Private Warrants) | |
| Exercise price | |
$ | 11.50 | | |
$ | 11.50 | |
| Share price | |
$ | 11.26 | | |
$ | 10.44 | |
| Expected term (years) | |
| 5.61 | | |
| 5.12 | |
| Probability of Acquisition | |
| 2.10 | % | |
| 2.75 | % |
| Volatility | |
| 7.9 | % | |
| 4.2 | % |
| Risk-free rate | |
| 4.50 | % | |
| 3.91 | % |
| Dividend yield (per share) | |
$ | 0.00 | | |
$ | 0.00 | |
|
| SCHEDULE OF CHANGES IN FAIR VALUE OF DERIVATIVE WARRANT LIABILITIES |
The
change in the fair value of the derivative warrant liabilities for the three months ended September 30, 2023 and 2022 is as follows:
SCHEDULE
OF CHANGES IN FAIR VALUE OF DERIVATIVE WARRANT LIABILITIES
| | |
Private Warrants | | |
Public Warrants | | |
Total Warrant Liability | |
| Fair value as of December 31, 2022 | |
$ | 17,558 | | |
$ | 345,000 | | |
$ | 362,558 | |
| Change in valuation inputs or other assumptions(1) | |
| 12,291 | | |
| 230,000 | | |
| 242,291 | |
| Fair value as of September 30, 2023 | |
$ | 29,849 | | |
$ | 575,000 | | |
$ | 604,849 | |
| | |
Private Warrants | | |
Public Warrants | | |
Total Warrant Liability | |
| Fair value as of December 31, 2021 | |
$ | 251,668 | | |
$ | 4,600,000 | | |
$ | 4,851,668 | |
| Fair value, beginning balance | |
$ | 251,668 | | |
$ | 4,600,000 | | |
$ | 4,851,668 | |
| Change in valuation inputs or other assumptions(1) | |
| (117,640 | ) | |
| (2,645,000 | ) | |
| (2,762,640 | ) |
| Fair value as of September 30, 2022 | |
$ | 134,028 | | |
$ | 1,955,000 | | |
$ | 2,089,028 | |
| Fair value, ending balance | |
$ | 134,028 | | |
$ | 1,955,000 | | |
$ | 2,089,028 | |
| (1) | Changes
in valuation inputs or other assumptions are recognized in the change in fair value of warrant
liability in the consolidated statement of operations. |
|
| X |
- DefinitionTabular disclosure of input and valuation technique used to measure fair value and change in valuation approach and technique for each separate class of asset and liability measured on recurring and nonrecurring basis. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, by class that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueAssetsMeasuredOnRecurringBasisTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the fair value measurement of liabilities using significant unobservable inputs (Level 3), a reconciliation of the beginning and ending balances, separately presenting changes attributable to the following: (1) total gains or losses for the period (realized and unrealized), segregating those gains or losses included in earnings (or changes in net assets), and gains or losses recognized in other comprehensive income (loss) and a description of where those gains or losses included in earnings (or changes in net assets) are reported in the statement of income (or activities); (2) purchases, sales, issues, and settlements (each type disclosed separately); and (3) transfers in and transfers out of Level 3 (for example, transfers due to changes in the observability of significant inputs) by class of liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueLiabilitiesMeasuredOnRecurringBasisUnobservableInputReconciliationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS (Details Narrative)
|
|
|
|
|
9 Months Ended |
12 Months Ended |
|
Aug. 11, 2023
USD ($)
|
Nov. 11, 2022
USD ($)
$ / shares
shares
|
May 11, 2021
USD ($)
$ / shares
shares
|
Feb. 08, 2021
Integer
|
Sep. 30, 2023
USD ($)
$ / shares
|
Sep. 30, 2022
USD ($)
|
Dec. 31, 2022
USD ($)
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
| Condition for future business combination number of businesses minimum | Integer |
|
|
|
1
|
|
|
|
| Proceeds from sale from IPO and Private Placement Units |
|
|
$ 117,300,000
|
|
$ 4,025,000
|
|
|
| Share Price | $ / shares |
|
|
|
|
$ 10.20
|
|
|
| Price per unit | $ / shares |
|
$ 0.045
|
$ 10.00
|
|
|
|
|
| Cash held outside the Trust Account |
|
|
|
|
$ 1,537
|
|
$ 30,870
|
| Working capital deficit |
|
|
|
|
3,057,188
|
|
1,945,267
|
| Amount to be deposited in trust account in connection with the exercise of the first monthly extension |
|
$ 122,920
|
|
|
|
|
|
| Business combination, tangible assets |
|
|
|
|
5,000,001
|
|
|
| Number of shares redeemed | shares |
|
8,768,456
|
|
|
|
|
|
| Common share at redemption value | $ / shares |
|
$ 10.42
|
|
|
|
|
|
| Aggregate amount of shares redeemed |
|
$ 91,400,000
|
|
|
|
|
|
| Assets held in trust |
$ 75,000
|
$ 28,500,000
|
|
|
150,000
|
|
|
| Termination agreement |
At the Special Meeting, the Stockholders approved the Extension Amendment Proposal to amend
Company’s Charter and authorized the Company to adopt and file the Amended Charter with the Secretary of State of Delaware, which
the Company promptly filed following the Stockholders’ approval. The stockholders approved amendments to the Company’s charter
and trust agreement allowing the Company to extend its termination date in a series of up to nine (9) one-month extensions until May
11, 2024 in exchange for depositing into its trust account (the “Trust Account”) with Continental Stock Transfer and Trust
Company the lesser of $75,000 or $0.045 per share for each public share outstanding (the “Extension Amount”). In
connection with the voting on the Extension Amendment Proposal and the Trust Amendment Proposal at the Special Meeting, holders of 1,018,846
shares of Class A ordinary shares exercised the right to redeem such shares for cash.
|
|
|
|
|
|
|
| Maximum net interest to pay dissolution expenses |
|
|
|
|
100,000
|
|
|
| Proceeds from Related Party Debt |
|
|
|
|
|
$ 11,500
|
|
| Investments held in Trust Account |
|
|
|
|
19,308,261
|
|
29,029,416
|
| Proceeds from Interest Withdrawn from Trust Account |
|
|
|
|
498,697
|
|
$ 299,601
|
| Convertible loans |
|
|
|
|
$ 1,500,000
|
|
|
| Units per price | $ / shares |
|
|
|
|
$ 10.00
|
|
|
| Sponsor [Member] |
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
| Proceeds from Related Party Debt |
|
|
|
|
$ 78,925
|
|
|
| Founder Shares [Member] | Sponsor [Member] |
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
| Proceeds from sale of founder shares |
|
|
|
|
$ 25,000
|
|
|
| Data Knights LLC [Member] |
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
| Business combination percentage |
|
|
|
|
80.00%
|
|
|
| Business acquisition voting interest |
|
|
|
|
50.00%
|
|
|
| IPO [Member] |
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
| Number of shares issued | shares |
|
|
11,500,000
|
|
|
|
|
| Proceeds from sale from IPO and Private Placement Units |
|
|
$ 115,000,000
|
|
|
|
|
| Price per unit | $ / shares |
|
|
$ 10.00
|
|
$ 10.00
|
|
|
| Transaction Costs |
|
|
|
|
$ 6,771,112
|
|
|
| Underwriting fees |
|
|
|
|
2,300,000
|
|
|
| Deferred Underwriting Commissions |
|
|
|
|
4,025,000
|
|
|
| Other Offering Costs |
|
|
|
|
446,112
|
|
|
| Cash held outside the Trust Account |
|
|
|
|
959,560
|
|
|
| Working capital deficit |
|
|
|
|
$ 2,943,835
|
|
|
| Private Placement [Member] |
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
| Number of shares issued | shares |
|
|
585,275
|
|
|
|
|
| Proceeds from sale from IPO and Private Placement Units |
|
|
$ 5,852,750
|
|
|
|
|
| Share Price | $ / shares |
|
|
$ 10.00
|
|
|
|
|
| Over-Allotment Option [Member] |
|
|
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
|
|
| Price per unit | $ / shares |
|
|
|
|
$ 0.10
|
|
|
| Condition For Future Business Combination Threshold Net Tangible Assets |
|
|
|
|
$ 2,300,000
|
|
|
| X |
- DefinitionAmount to be deposited in trust account in connection with exercise of first monthly extension.
| Name: |
ONMD_AmountToBeDepositedInTrustAccountInConnectionWithExerciseOfFirstMonthlyExtension |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCondition for future business combination number of businesses minimum.
| Name: |
ONMD_ConditionForFutureBusinessCombinationNumberOfBusinessesMinimum |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCondition for future business combination threshold net tangible assets.
| Name: |
ONMD_ConditionForFutureBusinessCombinationThresholdNetTangibleAssets |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionMaximum net interest to pay dissolution expenses.
| Name: |
ONMD_MaximumNetInterestToPayDissolutionExpenses |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionTermination agreement description.
| Name: |
ONMD_TerminationAgreementDescription |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
ONMD_WorkingCapitalDeficit |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe total amount of cash and securities held by third party trustees pursuant to terms of debt instruments or other agreements as of the date of each statement of financial position presented, which can be used by the trustee only to pay the noncurrent portion of specified obligations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_AssetsHeldInTrust |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of cash, securities, or other assets held by a third-party trustee pursuant to the terms of an agreement which assets are available to be used by beneficiaries to that agreement only within the specific terms thereof and which agreement is expected to terminate more than one year from the balance sheet date (or operating cycle, if longer) at which time the assets held-in-trust will be released or forfeited. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_AssetsHeldInTrustNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of voting equity interests acquired at the acquisition date in the business combination. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 805
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479328/805-10-50-2
| Name: |
us-gaap_BusinessAcquisitionPercentageOfVotingInterestsAcquired |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of equity in the acquiree held by the acquirer immediately before the acquisition date in a business combination.
| Name: |
us-gaap_BusinessCombinationStepAcquisitionEquityInterestInAcquireePercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-21
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 210
-Topic 946
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-20
| Name: |
us-gaap_Cash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe price per share of the conversion feature embedded in the debt instrument. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-5
| Name: |
us-gaap_DebtInstrumentConvertibleConversionPrice1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNet increase or decrease in the carrying amount of the debt instrument for the period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_DebtInstrumentIncreaseDecreaseForPeriodNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe carrying amount of deferred costs.
| Name: |
us-gaap_DeferredCostsCurrentAndNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of interest income earned from interest bearing assets classified as other.
| Name: |
us-gaap_InterestIncomeOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionIncludes offering costs of open-end investment companies, and closed-end funds with a continuous offering period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.14)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NoninterestExpenseOfferingCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of stock bought back by the entity at the exercise price or redemption price. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
| Name: |
us-gaap_StockRedeemedOrCalledDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SubsidiarySaleOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate amount of redemption requirements for each class or type of redeemable stock classified as temporary equity for each of the five years following the latest balance sheet date. The redemption requirement does not constitute an unconditional obligation that will be settled in a variable number of shares constituting a monetary value predominantly indexed to (a) a fixed monetary amount known at inception, (b) an amount inversely correlated with the residual value of the entity, or (c) an amount determined by reference to something other than the fair value of issuer's stock. Does not include mandatorily redeemable stock. The exception is if redemption is required upon liquidation or termination of the reporting entity.
| Name: |
us-gaap_TemporaryEquityAggregateAmountOfRedemptionRequirement |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe difference between the price paid by the public and the contract price less the related expenses. A broker-dealer may underwrite a security offering by contracting to buy the issue either at a fixed price or a price based on selling the offering on a best-effort basis.
| Name: |
us-gaap_UnderwritingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_BusinessAcquisitionAxis=ONMD_DataKnightsLLCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_IPOMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_PrivatePlacementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_OverAllotmentOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
SCHEDULE OF RECONCILED CLASS A COMMON STOCK (Details) - USD ($)
|
9 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Contingently redeemable Class A Common Stock – Opening Balance |
$ 28,750,110
|
|
| Contingently redeemable Class A Common Stock - Ending Balance |
19,209,844
|
|
| Common Class A Subject To Redemption [Member] |
|
|
| Contingently redeemable Class A Common Stock – Opening Balance |
28,750,110
|
$ 117,300,000
|
| Redemption of Class A Common Stock, including interest |
(11,335,260)
|
|
| Re-measurement of carrying value to redemption value |
1,794,994
|
2,833,869
|
| Contingently redeemable Class A Common Stock - Ending Balance |
$ 19,209,844
|
$ 120,133,869
|
| X |
- DefinitionTemporary equity redemption common stock.
| Name: |
ONMD_TemporaryEquityRedemptionCommonStock |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of decrease to net income for accretion of temporary equity to its redemption value to derive net income apportioned to common stockholders.
| Name: |
us-gaap_TemporaryEquityAccretionToRedemptionValueAdjustment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount, attributable to parent, of an entity's issued and outstanding stock which is not included within permanent equity. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. Includes stock with a put option held by an ESOP and stock redeemable by a holder only in the event of a change in control of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.E.Q2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
| Name: |
us-gaap_TemporaryEquityCarryingAmountAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ONMD_CommonClassASubjectToRedemptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
RECONCILIATION OF NET LOSS PER COMMON SHARE (Details) - USD ($)
|
3 Months Ended |
6 Months Ended |
9 Months Ended |
Sep. 30, 2023 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Sep. 30, 2022 |
Jun. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Net income (loss) allocable to common stock not subject to redemption |
$ (816,520)
|
$ (5,158)
|
$ (85,983)
|
$ (153,275)
|
$ 3,145,455
|
$ (907,660)
|
$ 2,992,171
|
| Common Class A Subject To Redemption [Member] |
|
|
|
|
|
|
|
| Net income (loss) allocable to common stock not subject to redemption |
$ (252,542)
|
|
|
$ (117,823)
|
|
$ (312,541)
|
$ 2,300,089
|
| Denominator: weighted average number of non-redeemable common shares |
2,166,749
|
|
|
11,500,000
|
|
2,541,210
|
11,500,000
|
| Net income(loss) per redeemable B common share - basic |
$ (0.12)
|
|
|
$ 0.01
|
|
$ (0.12)
|
$ 0.20
|
| Net income(loss) per redeemable B common share - diluted |
$ (0.12)
|
|
|
$ 0.01
|
|
$ (0.12)
|
$ 0.20
|
| Class A And Class B Common Stock Not Subject To Redemption [Member] |
|
|
|
|
|
|
|
| Net income (loss) allocable to common stock not subject to redemption |
$ (563,978)
|
|
|
$ (35,452)
|
|
$ (595,119)
|
$ 692,082
|
| Denominator: weighted average number of non-redeemable common shares |
4,838,792
|
|
|
3,460,275
|
|
4,838,792
|
3,460,275
|
| Net income(loss) per redeemable B common share - basic |
$ (0.12)
|
|
|
$ 0.01
|
|
$ (0.12)
|
$ 0.20
|
| Net income(loss) per redeemable B common share - diluted |
$ (0.12)
|
|
|
$ 0.01
|
|
$ (0.12)
|
$ 0.20
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ONMD_CommonClassASubjectToRedemptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ONMD_ClassAAndClassBCommonStockNotSubjectToRedemptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Details Narrative) - USD ($)
|
|
3 Months Ended |
9 Months Ended |
|
|
May 11, 2021 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Dec. 31, 2022 |
Nov. 11, 2022 |
| Cash |
|
$ 1,537
|
|
$ 1,537
|
|
$ 30,870
|
|
| Cash equivalents |
|
0
|
|
0
|
|
0
|
|
| Proceeds from sale from IPO and Private Placement Units |
$ 117,300,000
|
|
|
$ 4,025,000
|
|
|
|
| Price per unit |
$ 10.00
|
|
|
|
|
|
$ 0.045
|
| Warrant exercisable |
|
|
|
12,085,275
|
|
|
|
| Unrecognized tax benefits |
|
0
|
|
$ 0
|
|
0
|
|
| Unrecognized tax benefits accrued for interest and penalties |
|
$ 0
|
|
$ 0
|
|
$ 0
|
|
| Effective tax rate |
|
12.15%
|
0.00%
|
30.10%
|
0.00%
|
|
|
| Statutory tax rate |
|
21.00%
|
21.00%
|
|
|
|
|
| Class Common Stock Not Subject To Possible Redemption [Member] |
|
|
|
|
|
|
|
| Common Stock, shares outstanding |
|
585,275
|
|
585,275
|
|
|
|
| Common Class Subject To Redemption [Member] |
|
|
|
|
|
|
|
| Common Stock, shares outstanding |
|
1,712,698
|
|
1,712,698
|
|
|
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-21
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 210
-Topic 946
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-20
| Name: |
us-gaap_Cash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of unrecognized tax benefits. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-15A
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-10B
| Name: |
us-gaap_UnrecognizedTaxBenefits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ONMD_ClassCommonStockNotSubjectToPossibleRedemptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=ONMD_CommonClassSubjectToRedemptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionNumber of warrants issued per unit.
| Name: |
ONMD_NumberOfWarrantsIssuedPerUnit |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUnits issued during period shares new issues.
| Name: |
ONMD_UnitsIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SubsidiarySaleOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_IPOMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=ONMD_PublicWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionClass of warrant or right price of warrants or rights.
| Name: |
ONMD_ClassOfWarrantOrRightPriceOfWarrantsOrRights |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which the class of warrant or right may be converted. For example, but not limited to, 500,000 warrants may be converted into 1,000,000 shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByWarrantsOrRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow from issuance of rights to purchase common shares at predetermined price (usually issued together with corporate debt). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SubsidiarySaleOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_PrivatePlacementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
RELATED PARTY TRANSACTIONS (Details Narrative) - USD ($)
|
|
|
|
|
|
|
3 Months Ended |
9 Months Ended |
12 Months Ended |
|
Nov. 11, 2022 |
Mar. 22, 2022 |
Jun. 26, 2021 |
Jun. 01, 2021 |
Mar. 23, 2021 |
Feb. 25, 2021 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Dec. 31, 2022 |
Feb. 08, 2021 |
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Transfer, assign or sell any shares or warrants after completion of initial business combination, stock price trigger |
|
|
|
|
|
|
$ 10.20
|
|
$ 10.20
|
|
|
|
| Debt instrument, convertible, conversion price |
|
|
|
|
|
|
10.00
|
|
10.00
|
|
|
|
| Sale of stock, price per share |
$ 10.42
|
|
|
|
|
|
|
|
|
|
|
|
| Amount to be deposited in trust account in connection with the exercise of first monthly extension |
$ 122,920
|
|
|
|
|
|
|
|
|
|
|
|
| Common Class B [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Common stock, par value |
|
|
|
|
|
|
$ 0.0001
|
|
$ 0.0001
|
|
$ 0.0001
|
|
| Related Party [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Other liabilities |
|
|
|
|
|
|
$ 11,200
|
|
$ 11,200
|
|
$ 11,500
|
|
| Related Party [Member] | Promissory Note With Related Party [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Maximum borrowing capacity of related party promissory note |
|
|
|
|
|
|
|
|
|
|
|
$ 300,000
|
| Repayments of related party debt |
|
|
|
$ 78,925
|
|
|
|
|
|
|
|
|
| Notes payable |
|
|
|
|
|
|
0
|
|
0
|
|
0
|
|
| Related Party [Member] | Administrative Support Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Administrative fees expense |
|
|
|
|
|
|
$ 30,000
|
$ 30,000
|
90,000
|
$ 90,000
|
|
|
| Sponsor [Member] | Founder Shares [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued during period value new issues |
|
|
|
|
|
|
|
|
$ 25,000
|
|
|
|
| Sponsor [Member] | Founder Shares [Member] | Common Class B [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued during period shares new issues |
|
|
|
|
|
2,875,000
|
|
|
|
|
|
|
| Stock issued during period value new issues |
|
|
|
|
|
$ 25,000
|
|
|
|
|
|
|
| Percentage of issued and outstanding shares after the initial public offering collectively held by initial stockholders |
|
|
|
|
20.00%
|
|
|
|
|
|
|
|
| Restrictions on transfer period of time after business combination completion |
|
|
|
|
|
|
|
|
6 months
|
|
|
|
| Transfer, assign or sell any shares or warrants after completion of initial business combination, stock price trigger |
|
|
|
|
|
|
$ 12.00
|
|
$ 12.00
|
|
|
|
| Sponsor [Member] | Related Party Loans [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related party loans convertible up to consummation of business combination |
|
|
|
|
|
|
|
|
$ 1,500,000
|
|
|
|
| Debt instrument, convertible, conversion price |
|
|
|
|
|
|
$ 10.00
|
|
$ 10.00
|
|
|
|
| Other liabilities |
|
|
|
|
|
|
$ 477,548
|
|
$ 477,548
|
|
207,081
|
|
| Securities deposit held in trust account |
|
|
|
|
|
|
|
|
$ 1,150,000
|
|
|
|
| Sale of stock, price per share |
|
|
|
|
|
|
$ 0.10
|
|
$ 0.10
|
|
|
|
| Securities deposit held in trust account |
|
|
|
|
|
|
|
|
$ 2,300,000
|
|
|
|
| Sponsor [Member] | Extension Loans [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Other liabilities |
|
|
|
|
|
|
$ 3,556,278
|
|
3,556,278
|
|
$ 2,545,838
|
|
| Amount to be deposited in trust account in connection with the exercise of first monthly extension |
$ 75,000
|
|
|
|
|
|
|
|
|
|
|
|
| Chief Executive Officer [Member] | Founder Shares [Member] | Common Class B [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Stock transferred to others during period share |
|
|
|
|
|
15,000
|
|
|
|
|
|
|
| Chief Financial Officer [Member] | Founder Shares [Member] | Common Class B [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Stock transferred to others during period share |
|
|
|
|
|
15,000
|
|
|
|
|
|
|
| Two Independent Director [Member] | Founder Shares [Member] | Common Class B [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Stock transferred to others during period share |
|
|
|
|
|
5,000
|
|
|
|
|
|
|
| Third Independent Director [Member] | Founder Shares [Member] | Common Class B [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Stock transferred to others during period share |
|
|
|
|
5,000
|
|
|
|
|
|
|
|
| Arc Group Limited [Member] | Related Party [Member] | Introducing Advisor Agreement With Arc Group Limited [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Retainer fee upon execution of agreement |
|
|
$ 50,000
|
|
|
|
|
|
|
|
|
|
| Success fee upon closing of initial business combination |
|
|
$ 100,000
|
|
|
|
|
|
|
|
|
|
| Percentage of ownership as consideration for service. |
|
|
5.00%
|
|
|
|
|
|
|
|
|
|
| Threshold period for consideration payable after the Closes on business combination or any financing with any advisor target or any affiliate of an advisor target |
|
|
6 months
|
|
|
|
|
|
|
|
|
|
| Percentage of success fee on any PIPE financing |
|
5.00%
|
|
|
|
|
|
|
|
|
|
|
| Arc Group Limited [Member] | Related Party [Member] | Second Amendment To Introducing Advisor Agreement With Arc Group Limited [Member] | Common Class B [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued during period shares new issues |
|
|
|
|
|
|
|
|
|
|
1,378,517
|
|
| Maximum number of shares subject to forfeiture if public stockholders exercise redemption rights |
|
|
|
|
|
|
|
|
|
|
143,766
|
|
| Arc Group Limited [Member] | Related Party [Member] | Founder Shares [Member] | Common Class B [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued during period shares new issues |
|
|
|
|
|
|
|
|
|
|
1,378,517
|
|
| Maximum number of shares subject to forfeiture if public stockholders exercise redemption rights |
|
|
|
|
|
|
|
|
|
|
143,766
|
|
| Common stock, par value |
|
|
|
|
|
|
|
|
|
|
$ 0.0001
|
|
| Arc Group Limited [Member] | Related Party [Member] | Administrative Support Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Maximum administrative fees expense per month |
|
|
|
|
|
|
|
|
$ 10,000
|
|
|
|
| X |
- DefinitionAmount to be deposited in trust account in connection with exercise of first monthly extension.
| Name: |
ONMD_AmountToBeDepositedInTrustAccountInConnectionWithExerciseOfFirstMonthlyExtension |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionBusiness combination total payments.
| Name: |
ONMD_BusinessCombinationTotalPayments |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionMaximum administrative fees expense per month.
| Name: |
ONMD_MaximumAdministrativeFeesExpensePerMonth |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionMaximum number of shares subject to forfeiture if public stockholders exercise redemption rights.
| Name: |
ONMD_MaximumNumberOfSharesSubjectToForfeitureIfPublicStockholdersExerciseRedemptionRights |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of issued and outstanding shares after initial public offering collectively held by initial stockholders.
| Name: |
ONMD_PercentageOfIssuedAndOutstandingSharesAfterInitialPublicOfferingCollectivelyHeldByInitialStockholders |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of ownership as consideration for service.
| Name: |
ONMD_PercentageOfOwnershipAsConsiderationForService |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of success fee on any PIPE financing.
| Name: |
ONMD_PercentageOfSuccessFeeOnAnyPipeFinancing |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRestrictions on transfer period of time after business combination completion.
| Name: |
ONMD_RestrictionsOnTransferPeriodOfTimeAfterBusinessCombinationCompletion |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRetainer fee upon execution of agreement.
| Name: |
ONMD_RetainerFeeUponExecutionOfAgreement |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionSecurities deposit held intrust account.
| Name: |
ONMD_SecuritiesDepositHeldInTrustAccount |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionStock transferred to others during period share.
| Name: |
ONMD_StockTransferredToOthersDuringPeriodShare |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSuccess fee upon closing of initial business combination.
| Name: |
ONMD_SuccessFeeUponClosingOfInitialBusinessCombination |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThreshold period for consideration payable after the closes on business combination or any financing with any advisor target or any affiliate of an advisor target.
| Name: |
ONMD_ThresholdPeriodForConsiderationPayableAfterClosesOnBusinessCombinationOrAnyFinancingWithAnyAdvisorTargetOrAnyAffiliateOfAdvisorTarget |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for administrative fee from service provided, including, but not limited to, salary, rent, or overhead cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 850
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_AdministrativeFeesExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe price per share of the conversion feature embedded in the debt instrument. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-5
| Name: |
us-gaap_DebtInstrumentConvertibleConversionPrice1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIncluding the current and noncurrent portions, aggregate carrying amount of all types of notes payable, as of the balance sheet date, with initial maturities beyond one year or beyond the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_NotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.15)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_OtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=ONMD_ArcGroupLimitedMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
COMMITMENTS AND CONTINGENCIES (Details Narrative) - USD ($)
|
|
|
|
9 Months Ended |
12 Months Ended |
Dec. 31, 2022 |
Mar. 22, 2022 |
May 11, 2021 |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
| Over allotment option period |
|
|
|
45 days
|
|
| Underwriting discount percentage |
|
|
|
2.00%
|
|
| Underwriting discount paid |
|
|
|
$ 2,300,000
|
|
| Deferred underwriting fees percentage |
|
|
|
3.50%
|
|
| Proceeds from initial public offering |
|
|
$ 117,300,000
|
$ 4,025,000
|
|
| Arc Group Limited [Member] | Common Class B [Member] | Founder Shares [Member] |
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
| Maximum number of shares subject to forfeiture if public stockholders exercise redemption rights |
|
|
|
|
143,766
|
| First Amendment To Introducing Advisor Agreement With Arc Group Limited [Member] | Arc Group Limited [Member] |
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
| Percentage of success fee on any PIPE financing |
|
5.00%
|
|
|
|
| Second Amendment To Introducing Advisor Agreement With Arc Group Limited [Member] | Arc Group Limited [Member] | Common Class B [Member] |
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
| Share-based compensation arrangement by share-based payment award, options, grants in period, gross |
|
|
|
|
1,378,517
|
| Second Amendment To Introducing Advisor Agreement With Arc Group Limited [Member] | Arc Group Limited [Member] | Common Class B [Member] | Founder Shares [Member] |
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
| Share-based compensation arrangement by share-based payment award, options, grants in period, gross |
1,378,517
|
|
|
|
|
| Maximum number of shares subject to forfeiture if public stockholders exercise redemption rights |
143,766
|
|
|
|
|
| Over-Allotment Option [Member] |
|
|
|
|
|
| Subsidiary, Sale of Stock [Line Items] |
|
|
|
|
|
| Units issued during period, shares, new issues |
|
|
|
1,500,000
|
|
| X |
- DefinitionDeferred underwriting fees percentage.
| Name: |
ONMD_DeferredUnderwritingFeesPercentage |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionMaximum number of shares subject to forfeiture if public stockholders exercise redemption rights.
| Name: |
ONMD_MaximumNumberOfSharesSubjectToForfeitureIfPublicStockholdersExerciseRedemptionRights |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionOver allotment option period.
| Name: |
ONMD_OverAllotmentOptionPeriod |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of success fee on any PIPE financing.
| Name: |
ONMD_PercentageOfSuccessFeeOnAnyPipeFinancing |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUnderwriting discount paid.
| Name: |
ONMD_UnderwritingDiscountPaid |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionUnderwriting discount percentage.
| Name: |
ONMD_UnderwritingDiscountPercentage |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUnits issued during period shares new issues.
| Name: |
ONMD_UnitsIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_SubsidiarySaleOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=ONMD_FirstAmendmentToIntroducingAdvisorAgreementWithArcGroupLimitedMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=ONMD_SecondAmendmentToIntroducingAdvisorAgreementWithArcGroupLimitedMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_OverAllotmentOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
WARRANT LIABILITY (Details Narrative) - $ / shares
|
9 Months Ended |
12 Months Ended |
Sep. 30, 2023 |
Dec. 31, 2022 |
| Shares trigger price |
$ 10.20
|
|
| Warrant [Member] |
|
|
| Shares trigger price |
$ 18.00
|
|
| Public Warrants [Member] |
|
|
| Class of warrant or right, minimum threshold written notice period for redemption of warrant |
30 days
|
|
| Warrants and rights outstanding term |
5 years
|
|
| Class of warrant or right redemption of warrants or rights stock price trigger |
$ 18.00
|
|
| Class of warrant or right redemption of warrants or rights stock price trigger |
0.01
|
|
| Shares trigger price |
$ 10.00
|
|
| Percentage of gross proceeds on total equity proceed |
60.00%
|
|
| Class of warrant or right adjustment of exercise price |
115.00%
|
|
| Class of warrant or right adjustment of exercise price |
180.00%
|
|
| Warrants or rights issued |
|
11,500,000
|
| Public Warrants [Member] | Common Class A [Member] |
|
|
| Shares trigger price |
$ 9.20
|
|
| Warrant [Member] |
|
|
| Warrants or rights issued |
12,085,275
|
12,085,275
|
| Placement Warrants [Member] |
|
|
| Warrants or rights issued |
|
585,275
|
| X |
- DefinitionClass of warrant or right adjustment of exercise price.
| Name: |
ONMD_ClassOfWarrantOrRightAdjustmentOfExercisePrice |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionClass of warrant or right minimum threshold written notice period for redemption of warrants.
| Name: |
ONMD_ClassOfWarrantOrRightMinimumThresholdWrittenNoticePeriodForRedemptionOfWarrants |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionClass of warrant or right redemption of warrants or rights stock price trigger.
| Name: |
ONMD_ClassOfWarrantOrRightRedemptionOfWarrantsOrRightsStockPriceTrigger |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionClass of warrant or right redemption price of warrants or rights.
| Name: |
ONMD_ClassOfWarrantOrRightRedemptionPriceOfWarrantsOrRights |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionClass of warrants or right adjustment of exercise price.
| Name: |
ONMD_ClassOfWarrantsOrRightAdjustmentOfExercisePrice |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPercentage of gross proceeds on total equity proceed.
| Name: |
ONMD_PercentageOfGrossProceedsOnTotalEquityProceed |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWarrants or rights issued.
| Name: |
ONMD_WarrantsOrRightsIssued |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod between issuance and expiration of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=ONMD_PublicWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=ONMD_PlacementWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
STOCKHOLDER’S EQUITY (Details Narrative)
|
|
|
|
9 Months Ended |
12 Months Ended |
|
Dec. 31, 2022
$ / shares
shares
|
Mar. 23, 2021
shares
|
Feb. 25, 2021
shares
|
Sep. 30, 2023
$ / shares
shares
|
Dec. 31, 2022
$ / shares
shares
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Preferred stock, shares authorized |
1,000,000
|
|
|
1,000,000
|
1,000,000
|
| Preferred stock, par value | $ / shares |
$ 0.0001
|
|
|
$ 0.0001
|
$ 0.0001
|
| Preferred stock, shares issued |
0
|
|
|
0
|
0
|
| Preferred stock, shares outstanding |
0
|
|
|
0
|
0
|
| Temporary equity subject to possible redemption, outstanding |
2,731,544
|
|
|
1,712,698
|
2,731,544
|
| Common Class A [Member] |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Common stock, shares authorized |
100,000,000
|
|
|
100,000,000
|
100,000,000
|
| Common stock par value per share | $ / shares |
$ 0.0001
|
|
|
$ 0.0001
|
$ 0.0001
|
| Common stock, voting rights |
|
|
|
Holders of the Company’s Class A Common Stock are entitled to one vote for each share
|
|
| Temporary equity subject to possible redemption, issued |
1,712,698
|
|
|
1,712,698
|
1,712,698
|
| Temporary equity subject to possible redemption, outstanding |
1,712,698
|
|
|
1,712,698
|
1,712,698
|
| Common stock, shares issued |
585,275
|
|
|
585,275
|
585,275
|
| Common stock, shares outstanding |
585,275
|
|
|
585,275
|
585,275
|
| Common Class B [Member] |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Common stock, shares authorized |
10,000,000
|
|
|
10,000,000
|
10,000,000
|
| Common stock par value per share | $ / shares |
$ 0.0001
|
|
|
$ 0.0001
|
$ 0.0001
|
| Common stock, voting rights |
|
|
|
Holders of the Company’s Class B Common Stock are entitled to one vote for each share
|
|
| Common stock, shares issued |
4,253,517
|
|
|
4,253,517
|
4,253,517
|
| Common stock, shares outstanding |
4,253,517
|
|
|
4,253,517
|
4,253,517
|
| Common stock, conversion ratio |
|
|
|
1
|
|
| Initial Business combination shares issuable as percent of outstanding Share |
|
|
|
20
|
|
| Common Class B [Member] | Arc Group Limited [Member] | Second Amendment To Introducing Advisor Agreement With Arc Group Limited [Member] |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Number of shares granted |
|
|
|
|
1,378,517
|
| Common Class B [Member] | Arc Group Limited [Member] | Founder Shares [Member] |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Number of shares granted |
|
|
|
|
143,766
|
| Common Class B [Member] | Arc Group Limited [Member] | Founder Shares [Member] | Second Amendment To Introducing Advisor Agreement With Arc Group Limited [Member] |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Common stock par value per share | $ / shares |
$ 0.0001
|
|
|
|
$ 0.0001
|
| Number of shares granted |
1,378,517
|
|
|
|
|
| Number of shares granted |
143,766
|
|
|
|
|
| Common Class B [Member] | Chief Executive Officer [Member] | Sponsor [Member] |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Number of shares transferred |
|
|
15,000
|
|
|
| Common Class B [Member] | Chief Financial Officer [Member] | Sponsor [Member] |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Number of shares transferred |
|
|
15,000
|
|
|
| Common Class B [Member] | Independent Directors [Member] | Sponsor [Member] |
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
| Number of shares transferred |
|
5,000
|
5,000
|
|
|
| X |
- DefinitionInitial business combination shares issuable as percent of outstanding share.
| Name: |
ONMD_InitialBusinessCombinationSharesIssuableAsPercentOfOutstandingShare |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:pureItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionMaximum number of shares subject to forfeiture if public stockholders exercise redemption rights.
| Name: |
ONMD_MaximumNumberOfSharesSubjectToForfeitureIfPublicStockholdersExerciseRedemptionRights |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionStock transferred to others during period share.
| Name: |
ONMD_StockTransferredToOthersDuringPeriodShare |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDescription of voting rights of common stock. Includes eligibility to vote and votes per share owned. Include also, if any, unusual voting rights. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_CommonStockVotingRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRatio applied to the conversion of stock split, for example but not limited to, one share converted to two or two shares converted to one. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 4
-Subparagraph (SAB TOPIC 4.C)
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-4
| Name: |
us-gaap_StockholdersEquityNoteStockSplitConversionRatio1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:pureItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of securities classified as temporary equity that have been sold (or granted) to the entity's shareholders. Securities issued include securities outstanding and securities held in treasury. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. If convertible, the issuer does not control the actions or events necessary to issue the maximum number of shares that could be required to be delivered under the conversion option if the holder exercises the option to convert the stock to another class of equity. If the security is a warrant or a rights issue, the warrant or rights issue is considered to be temporary equity if the issuer cannot demonstrate that it would be able to deliver upon the exercise of the option by the holder in all cases. Includes stock with put option held by ESOP and stock redeemable by holder only in the event of a change in control of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TemporaryEquitySharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of securities classified as temporary equity that have been issued and are held by the entity's shareholders. Securities outstanding equals securities issued minus securities held in treasury. Temporary equity is a security with redemption features that are outside the control of the issuer, is not classified as an asset or liability in conformity with GAAP, and is not mandatorily redeemable. Includes any type of security that is redeemable at a fixed or determinable price or on a fixed or determinable date or dates, is redeemable at the option of the holder, or has conditions for redemption which are not solely within the control of the issuer. If convertible, the issuer does not control the actions or events necessary to issue the maximum number of shares that could be required to be delivered under the conversion option if the holder exercises the option to convert the stock to another class of equity. If the security is a warrant or a rights issue, the warrant or rights issue is considered to be temporary equity if the issuer cannot demonstrate that it would be able to deliver upon the exercise of the option by the holder in all cases. Includes stock with put option held by ESOP and stock redeemable by holder only in the event of a change in control of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TemporaryEquitySharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=ONMD_SecondAmendmentToIntroducingAdvisorAgreementWithArcGroupLimitedMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ChiefExecutiveOfficerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ChiefFinancialOfficerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=ONMD_IndependentDirectorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
SCHEDULE OF FAIR VALUE HIERARCHY OF VALUATION TECHNIQUES (Details) - USD ($)
|
Sep. 30, 2023 |
Dec. 31, 2022 |
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Investments held in Trust Account |
$ 19,308,261
|
$ 29,029,416
|
| Fair Value, Inputs, Level 1 [Member] | Fair Value, Recurring [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Investments held in Trust Account |
19,308,261
|
29,029,416
|
| Public Warrants |
575,000
|
345,000
|
| Private Placement Warrants |
|
|
| Fair Value, Inputs, Level 2 [Member] | Fair Value, Recurring [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Investments held in Trust Account |
|
|
| Public Warrants |
|
|
| Private Placement Warrants |
|
|
| Fair Value, Inputs, Level 3 [Member] | Fair Value, Recurring [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Investments held in Trust Account |
|
|
| Public Warrants |
|
|
| Private Placement Warrants |
$ 29,849
|
$ 17,558
|
| X |
- DefinitionPrivate placement warrants.
| Name: |
ONMD_PrivatePlacementWarrants |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of cash, securities, or other assets held by a third-party trustee pursuant to the terms of an agreement which assets are available to be used by beneficiaries to that agreement only within the specific terms thereof and which agreement is expected to terminate more than one year from the balance sheet date (or operating cycle, if longer) at which time the assets held-in-trust will be released or forfeited. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_AssetsHeldInTrustNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of outstanding derivative securities that permit the holder the right to purchase securities (usually equity) from the issuer at a specified price.
| Name: |
us-gaap_WarrantsAndRightsOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FairValueByMeasurementFrequencyAxis=us-gaap_FairValueMeasurementsRecurringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
SCHEDULE OF FAIR VALUE MEASUREMENTS INPUTS AS THEIR MEASUREMENT DATES (Details) - Private Warrant [Member] - Fair Value, Inputs, Level 3 [Member]
|
Sep. 30, 2023
$ / shares
|
Sep. 30, 2023 |
Dec. 31, 2022
$ / shares
|
| Measurement Input, Exercise Price [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Fair value measurements input |
11.50
|
|
11.50
|
| Measurement Input, Share Price [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Fair value measurements input |
11.26
|
|
10.44
|
| Measurement Input, Expected Term [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Fair value measurements input |
|
5.61
|
5.12
|
| Measurement Input Probability Of Completing Business Combination [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Fair value measurements input |
|
0.0210
|
0.0275
|
| Measurement Input, Price Volatility [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Fair value measurements input |
|
0.079
|
0.042
|
| Measurement Input, Risk Free Interest Rate [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Fair value measurements input |
|
0.0450
|
0.0391
|
| Measurement Input, Expected Dividend Rate [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Fair value measurements input |
0.00
|
0
|
0.00
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=ONMD_PrivateWarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
SCHEDULE OF CHANGES IN FAIR VALUE OF DERIVATIVE WARRANT LIABILITIES (Details) - USD ($)
|
9 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Private Warrant [Member] |
|
|
|
| Class of Warrant or Right [Line Items] |
|
|
|
| Fair value, beginning balance |
|
$ 17,558
|
$ 251,668
|
| Change in valuation inputs or other assumptions |
[1] |
12,291
|
(117,640)
|
| Fair value, ending balance |
|
29,849
|
134,028
|
| Public Warrants [Member] |
|
|
|
| Class of Warrant or Right [Line Items] |
|
|
|
| Fair value, beginning balance |
|
345,000
|
4,600,000
|
| Change in valuation inputs or other assumptions |
[1] |
230,000
|
(2,645,000)
|
| Fair value, ending balance |
|
575,000
|
1,955,000
|
| Warrant Liability [Member] |
|
|
|
| Class of Warrant or Right [Line Items] |
|
|
|
| Fair value, beginning balance |
|
362,558
|
4,851,668
|
| Change in valuation inputs or other assumptions |
[1] |
242,291
|
(2,762,640)
|
| Fair value, ending balance |
|
$ 604,849
|
$ 2,089,028
|
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_ClassOfWarrantOrRightLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=ONMD_PrivateWarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=ONMD_PublicWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=ONMD_WarrantLiabilityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
FAIR VALUE MEASUREMENTS (Details Narrative)
|
3 Months Ended |
12 Months Ended |
|
|
Sep. 30, 2023
USD ($)
|
Dec. 31, 2022
USD ($)
$ / shares
|
Sep. 30, 2023
$ / shares
|
Sep. 30, 2023 |
| Fair Value, off-Balance-Sheet Risks, Disclosure Information [Line Items] |
|
|
|
|
| Assets held in trust account |
$ 19,308,261
|
$ 29,029,416
|
|
|
| Fair value assets and Lliabilities transfers between levels amount |
0
|
$ 0
|
|
|
| Private Warrant [Member] | Fair Value, Inputs, Level 3 [Member] | Measurement Input, Expected Dividend Rate [Member] |
|
|
|
|
| Fair Value, off-Balance-Sheet Risks, Disclosure Information [Line Items] |
|
|
|
|
| Fair value measurements input |
|
0.00
|
0.00
|
0
|
| US Treasury Securities [Member] |
|
|
|
|
| Fair Value, off-Balance-Sheet Risks, Disclosure Information [Line Items] |
|
|
|
|
| Assets held in trust account |
$ 19,308,261
|
$ 29,029,416
|
|
|
| X |
- DefinitionFair value assets and liabilities transfers between levels amount.
| Name: |
ONMD_FairValueAssetsAndLiabilitiesTransfersBetweenLevelsAmount |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of cash, securities, or other assets held by a third-party trustee pursuant to the terms of an agreement which assets are available to be used by beneficiaries to that agreement only within the specific terms thereof and which agreement is expected to terminate more than one year from the balance sheet date (or operating cycle, if longer) at which time the assets held-in-trust will be released or forfeited. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_AssetsHeldInTrustNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=ONMD_PrivateWarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_USTreasurySecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
SUBSEQUENT EVENTS (Details Narrative) - USD ($)
|
|
|
9 Months Ended |
|
|
Oct. 17, 2023 |
Oct. 12, 2023 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Aug. 11, 2023 |
Nov. 11, 2022 |
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Investment of cash in trust account |
|
|
$ 1,010,440
|
$ 2,300,000
|
|
|
| Share Price |
|
|
$ 10.20
|
|
|
|
| Trust account |
|
|
$ 150,000
|
|
$ 75,000
|
$ 28,500,000
|
| Subsequent Event [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Extension period for consummating initial business combination |
|
the Company elected to exercise its eighth of third one-month extension to the Termination Date
|
|
|
|
|
| Investment of cash in trust account |
|
$ 75,000
|
|
|
|
|
| Business combination net of tangible assets description |
preventing the Company from closing a business combination if it would
have less than $5,000,0001 of net tangible assets (the “NTA Proposal”)
|
|
|
|
|
|
| Temporary equity stock redeemed or called during period shares |
1,614,148
|
|
|
|
|
|
| Trust account |
$ 1,100,000
|
|
|
|
|
|
| Subsequent Event [Member] | IPO [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| share issued, value |
$ 17,900,000
|
|
|
|
|
|
| Share Price |
$ 11.12
|
|
|
|
|
|
| Subsequent Event [Member] | Common Class A [Member] |
|
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
|
| Common stock shares issued and outstanding percentage |
20.00%
|
|
|
|
|
|
| X |
- DefinitionBusiness combination contingent consideration asset description.
| Name: |
ONMD_BusinessCombinationContingentConsiderationAssetDescription |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommon stock shares issued and outstanding percentage.
| Name: |
ONMD_CommonStockSharesIssuedAndOutstandingPercentage |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExtension period for consummating initial business combination description.
| Name: |
ONMD_ExtensionPeriodForConsummatingInitialBusinessCombinationDescription |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPayments for investment of cash in trust account.
| Name: |
ONMD_PaymentsForInvestmentOfCashInTrustAccount |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionTemporary equity stock redeemed or called during period shares.
| Name: |
ONMD_TemporaryEquityStockRedeemedOrCalledDuringPeriodShares |
| Namespace Prefix: |
ONMD_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe total amount of cash and securities held by third party trustees pursuant to terms of debt instruments or other agreements as of the date of each statement of financial position presented, which can be used by the trustee only to pay the noncurrent portion of specified obligations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
| Name: |
us-gaap_AssetsHeldInTrust |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDetail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_IPOMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
OneMedNet (NASDAQ:ONMD)
Historical Stock Chart
From Jan 2025 to Feb 2025

OneMedNet (NASDAQ:ONMD)
Historical Stock Chart
From Feb 2024 to Feb 2025
