Lexicon Pharmaceuticals Reports on Clinical Program Status and 2011 Second Quarter Results
August 08 2011 - 6:00PM
PR Newswire (US)
THE WOODLANDS, Texas,
Aug. 8, 2011 /PRNewswire/ -- Lexicon
Pharmaceuticals, Inc. (Nasdaq: LXRX), a biopharmaceutical company
focused on discovering and developing breakthrough treatments for
human disease, today updated its drug development progress and
reported financial results for the three and six months ended
June 30, 2011.
Progress in Clinical Pipeline
- Lexicon commenced a Phase 2b clinical trial for LX4211, a dual
inhibitor of sodium-glucose cotransporters 1 and 2. The
company also presented data at the 71st Scientific Sessions of the
American Diabetes Association from a mechanistic study designed to
investigate the effect of LX4211 on peptide hormones in the
intestinal tract in patients with type 2 diabetes. The data
from the study demonstrated that a single dose of LX4211
significantly increased GLP-1 (total and active) and PYY, important
mediators of glycemic and appetite control.
- Lexicon successfully completed a Phase 1 study of LX1033, a
serotonin synthesis inhibitor being developed as a potential
treatment for irritable bowel syndrome (IBS). Results from
the study demonstrated that LX1033 was well tolerated at all doses
and produced a statistically significant reduction in serotonin
synthesis compared to placebo, as measured by both plasma (p
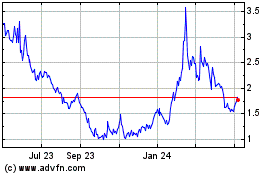
Lexicon Pharmaceuticals (NASDAQ:LXRX)
Historical Stock Chart
From Jun 2024 to Jul 2024
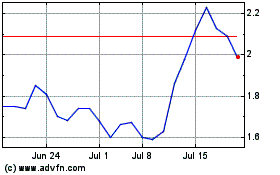
Lexicon Pharmaceuticals (NASDAQ:LXRX)
Historical Stock Chart
From Jul 2023 to Jul 2024