Intercept Pharma Discontinues NASH-Related Investment, Will Focus on Liver Diseases
June 22 2023 - 6:36PM
Dow Jones News
By Stephen Nakrosis
Intercept Pharmaceuticals said it will discontinue its
NASH-related investment and restructure operations to strengthen
the company's focus on rare and serious liver diseases.
The decision comes after the company received a complete
response letter from the Food and Drug Administration pertaining to
its new drug application for obeticholic acid as a treatment of
pre-cirrhotic fibrosis due to nonalcoholic steatohepatitis, or
NASH. The FDA said the application can't be approved in its present
form, according to Intercept.
The company expects to achieve profitability in 2024 as a result
of its planned actions.
Jerry Durso, the company's president and chief executive, said,
"we believe that taking decisive action to reshape Intercept will
improve our long-term ability to grow our business, innovate for
patients and create value for shareholders."
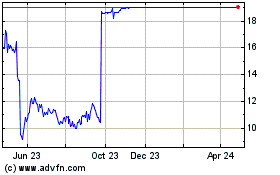
The company's stock was halted for trading in Thursday's
after-hours market. The stock finished the day's regular session
with a 1.62% loss at $11.55 per share.
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
June 22, 2023 18:21 ET (22:21 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Intercept Pharmaceuticals (NASDAQ:ICPT)
Historical Stock Chart
From May 2024 to Jun 2024
Intercept Pharmaceuticals (NASDAQ:ICPT)
Historical Stock Chart
From Jun 2023 to Jun 2024