0001764013FALSE00017640132023-09-262023-09-26
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 26, 2023
IMMUNOVANT, INC.
(Exact name of Registrant as specified in its Charter)
| | | | | | | | | | | |
| Delaware | 001-38906 | 83-2771572 |
(State or other jurisdiction of incorporation or organization) | (Commission File Number) | (IRS Employer Identification No.) |
| | |
| 320 West 37th Street | | |
| New York, | NY | | 10018 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code: (917) 580-3099
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | |
| Common Stock, $0.0001 par value per share | | IMVT | | The Nasdaq Stock Market LLC | |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD Disclosure.
On September 26, 2023, Immunovant, Inc. (the “Company”) issued a press release announcing initial data from its Phase 1 clinical trial of IMVT-1402 for the treatment of IgG-mediated autoimmune diseases. A copy of the press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information furnished under this Item 7.01, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, or the Exchange Act, or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, or the Securities Act. The information in this Item 7.01, including Exhibit 99.1, shall not be deemed incorporated by reference into any other filing with the U.S. Securities Exchange Commission, or the SEC, made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 8.01. Other Events.
As described in the press release, the Company will host a conference call and webcast to discuss the results of the IMVT-1402 trial at 8:00 a.m. ET on September 26, 2023. A copy of the presentation to be used by the Company during the conference call is filed as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
| | | | | | | | | | | |
| Exhibit No. | | Description | |
| 99.1 | | | |
| 99.2 | | | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | |
| | | |
| IMMUNOVANT, INC. |
| | |
| By: | | /s/ Eva Renee Barnett |
| | | Eva Renee Barnett |
| | | Chief Financial Officer |
| Date: September 26, 2023 | | | |
Exhibit 99.1
Immunovant Announces Positive Initial IMVT-1402 Phase 1 SAD and 300 mg Subcutaneous MAD Results
•IMVT-1402 subcutaneous (SC) doses achieved peak Immunoglobulin G (IgG) reductions that are similar to those previously observed with batoclimab
•No decrease in serum albumin below baseline or increase in low-density lipoprotein cholesterol (LDL-C) above baseline was observed after 4 weeks of dosing in the 300 mg multiple-ascending dose (MAD) SC cohort
•IMVT-1402 is being developed as a simple SC injection
NEW YORK, September 26, 2023 – Immunovant, Inc. (Nasdaq: IMVT), a clinical-stage immunology company dedicated to enabling normal lives for people with autoimmune diseases, today announced that subcutaneously administered doses of IMVT-1402 produced dose-dependent reductions in IgG in initial data from a Phase 1 clinical trial in healthy adults, with no dose-related changes in serum albumin or LDL-C, bolstering IMVT-1402 as a potential best-in-class neonatal fragment crystallizable receptor (FcRn) inhibitor.
“We are encouraged by the strong pharmacodynamic data observed to date with IMVT-1402,” said Pete Salzmann, M.D., chief executive officer of Immunovant. “These first-in-human results are consistent with those observed in prior non-human primate studies, and we look forward to sharing additional MAD data in November.”
This Phase 1 clinical trial is a randomized, double-blind, placebo-controlled ascending dose study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of IMVT-1402 in healthy adults.
In the single-ascending dose (SAD) portion of the study, subcutaneously administered IMVT-1402 demonstrated a consistent reduction in IgG with potency that was similar to or greater than that of batoclimab. The safety data were generally favorable, with all adverse events (AEs) mild or moderate, and no significant reduction from baseline in serum albumin or increase in LDL-C observed at any timepoint measured (all p>0.05).
Immunovant is also pleased to announce that initial MAD study results for the 300 mg cohort were released ahead of schedule today. These data represent all the MAD data currently available. Dosing for the 600 mg cohort has recently begun. After four weekly 300 mg SC doses of IMVT-1402, the mean total IgG reduction from baseline in this MAD cohort was 63%, with no decrease in serum albumin below baseline and no increase in LDL-C above baseline observed. Treatment-emergent adverse events were observed to be mild or moderate in severity. IMVT-1402 was delivered subcutaneously in seconds to participants in this cohort as a simple 2 mL injection at a concentration of 150 mg/mL.
Conference Call & Webcast:
Immunovant will host a conference call with accompanying slides and a simultaneous webcast today, September 26, 2023 at 8:00 a.m. EDT to discuss the initial single-ascending dose and multiple-ascending dose data. To participate in the conference call, please register in advance here. To access the live and archived webcast, please visit Immunovant’s website at https://www.immunovant.com/investors/news-events. The archived webcast will be available for a limited time on the Company’s website.
About IMVT-1402
IMVT-1402 is designed to be a potentially best-in-class anti-FcRn antibody for the treatment of IgG-mediated autoimmune diseases. In the initial results of a Phase 1 clinical trial in healthy volunteers, IMVT-1402 demonstrated favorable pharmacodynamic and safety data. These attributes, combined with a convenient route of administration that may enable patient self-administration, position IMVT-1402 well as a potential treatment for a variety of autoimmune diseases associated with patient unmet need.
About Immunovant, Inc.
Immunovant, Inc. is a clinical-stage immunology company dedicated to enabling normal lives for people with autoimmune diseases. As a trailblazer in anti-FcRn technology, the Company is developing innovative, targeted therapies to meet the complex and variable needs of people with autoimmune diseases. For additional information on the Company, please visit www.immunovant.com.
Forward-Looking Statements
This press release contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "can," “may,” “might,” “will,” “would,” “should,” “expect,” “believe,” “estimate,” “design,” “plan,” "intend," and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include Immunovant’s expectations regarding the timing and results of Immunovant’s clinical trials of IMVT-1402; and the potential benefits of IMVT-1402’s unique product attributes and its best-in-class potential. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive final trial results or of the results of later clinical trials; results of animal studies may not be predictive of results in humans; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidates, including the timing of the commencement of additional clinical trials and resumption of current trials; Immunovant’s scientific approach, clinical trial design, indication selection and general development progress; future clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this press release; any product candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant’s product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the potential impact of global factors, such as the post-COVID-19 environment, geopolitical tensions, and adverse macroeconomic conditions on Immunovant’s business operations and supply chain, including its clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of batoclimab and IMVT-1402; Immunovant is at an early stage of development for IMVT-1402 and in various stages of clinical development for batoclimab; and Immunovant will require additional capital to fund its operations and advance batoclimab and IMVT-1402 through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s Form 10-Q filed with the SEC on August 10, 2023, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
Contact:
Chau Cheng, PhD, MBA
Vice President, Investor Relations
Immunovant, Inc.
info@immunovant.com

Targeted science, Tailored solutions for people with autoimmune disease IMVT-1402 Initial First-in-human Data Presentation September 26, 2023 Exhibit 99.2

Forward-Looking Statements 2 This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "can," “may,” “might,” “will,” “would,” “should,” “expect,” “believe,” “estimate,” “design,” “plan,” "intend," and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include the timing and results of Immunovant’s clinical trials of IMVT–1402; expectations with respect to the safety and monitoring plan and size of the safety database for these planned clinical trials; the timing of discussions with regulatory agencies; the size and growth of the potential markets for Immunovant's product candidates and indication selections; Immunovant’s plan to explore in subsequent study periods follow-on treatment with alternative dosing regimens; Immunovant's beliefs regarding the potential benefits of IMVT-1402's unique product attributes; and Immunovant's expectations regarding the issuance and term of any pending patents. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive of final trial results or of the results of later clinical trials; results of animal studies may not be predictive of results in humans; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidates, including the timing of the commencement of additional clinical trials and resumption of current trials; Immunovant’s scientific approach, clinical trial design, indication selection, and general development progress; future clinical trials may not confirm any safety, potency, or other product characteristics described or assumed in this presentation; any product candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant's pending composition of matter patent for IMVT-1402 may not be issued; Immunovant’s product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the effect of global factors such as the ongoing COVID-19 pandemic, geopolitical tensions, and adverse macroeconomic conditions on Immunovant’s business operations and supply chains, including its clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of batoclimab and IMVT-1402; Immunovant is at an early stage in development for IMVT-1402 and in various stages of clinical development for batoclimab; and Immunovant will require additional capital to fund its operations and advance batoclimab and IMVT-1402 through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2023, filed with the SEC on August 10, 2023, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. All trademarks, trade names, service marks, and copyrights appearing in this presentation are the property of their respective owners. Dates used in this presentation refer to the applicable calendar year unless otherwise noted.
Our Vision: Normal Lives for People with Autoimmune Disease 3 Love Trailblazing All Voices Bolder, Faster What we do: We are developing targeted therapies that are designed to address the complex and variable needs of people with autoimmune diseases.
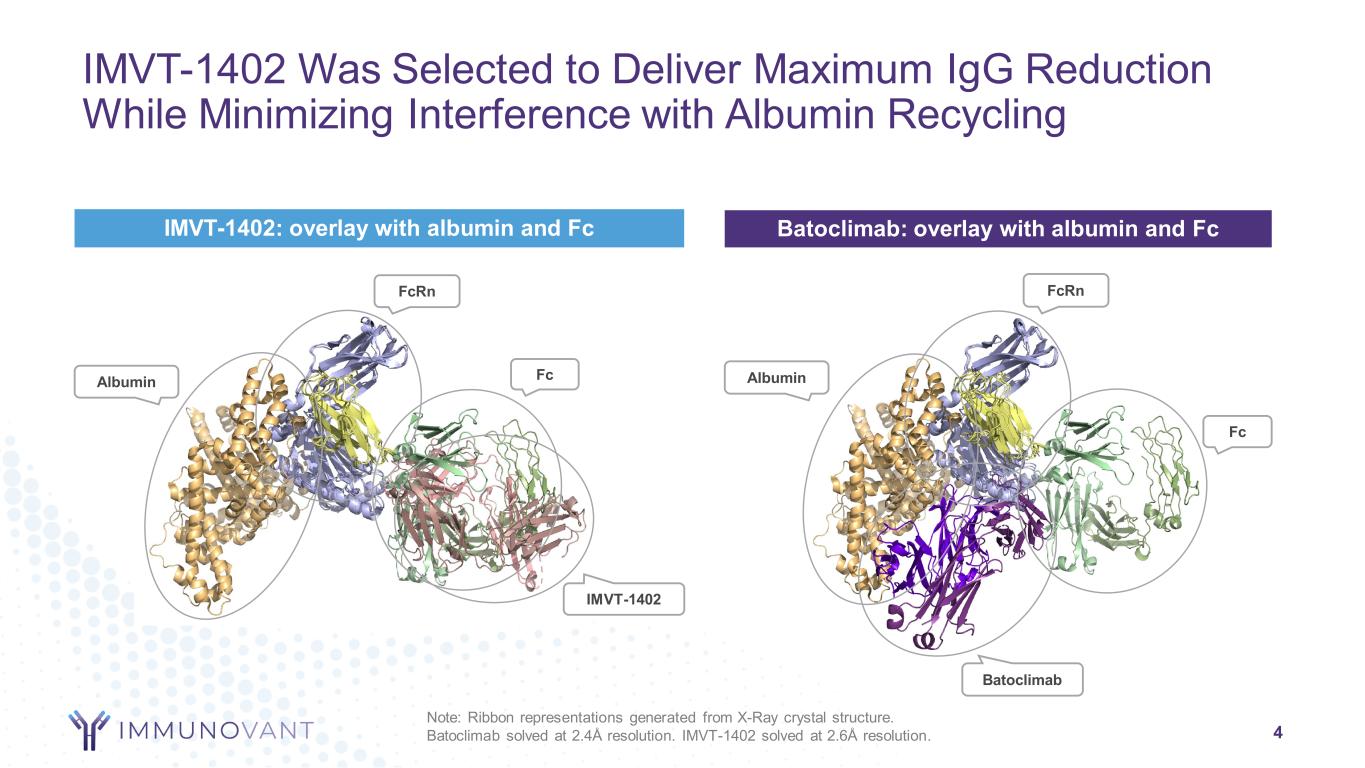
4 Note: Ribbon representations generated from X-Ray crystal structure. Batoclimab solved at 2.4Å resolution. IMVT-1402 solved at 2.6Å resolution. Fc Albumin FcRn Batoclimab FcAlbumin FcRn IMVT-1402 Batoclimab: overlay with albumin and FcIMVT-1402: overlay with albumin and Fc IMVT-1402 Was Selected to Deliver Maximum IgG Reduction While Minimizing Interference with Albumin Recycling
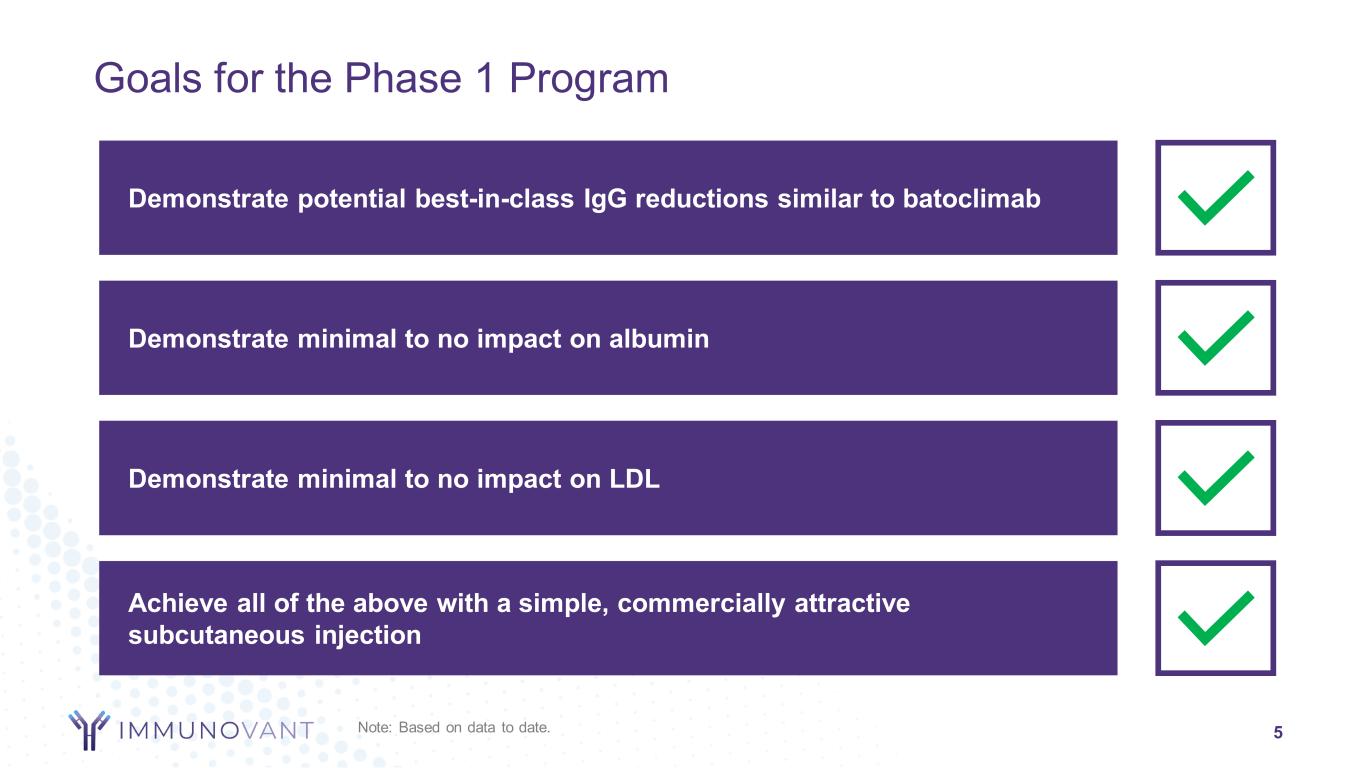
Achieve all of the above with a simple, commercially attractive subcutaneous injection Demonstrate minimal to no impact on LDL Demonstrate minimal to no impact on albumin 5 Goals for the Phase 1 Program Demonstrate potential best-in-class IgG reductions similar to batoclimab Note: Based on data to date.

6 Best-in-Class Potential for IMVT-1402 as FcRn Inhibitor Highlighted by Initial Phase 1 Safety and Pharmacodynamic Data Initial SAD and 300 mg MAD data demonstrated deep and rapid IgG reduction, similar to batoclimab, with 63% mean IgG reduction in the 300 mg MAD cohort after four doses Initial 300 mg MAD data after four doses showed a favorable analyte profile of no decrease in albumin and no increase in LDL relative to baseline levels Simple subcutaneous formulation designed to enable patient self-administration and provide additional differentiation beyond depth of IgG reduction
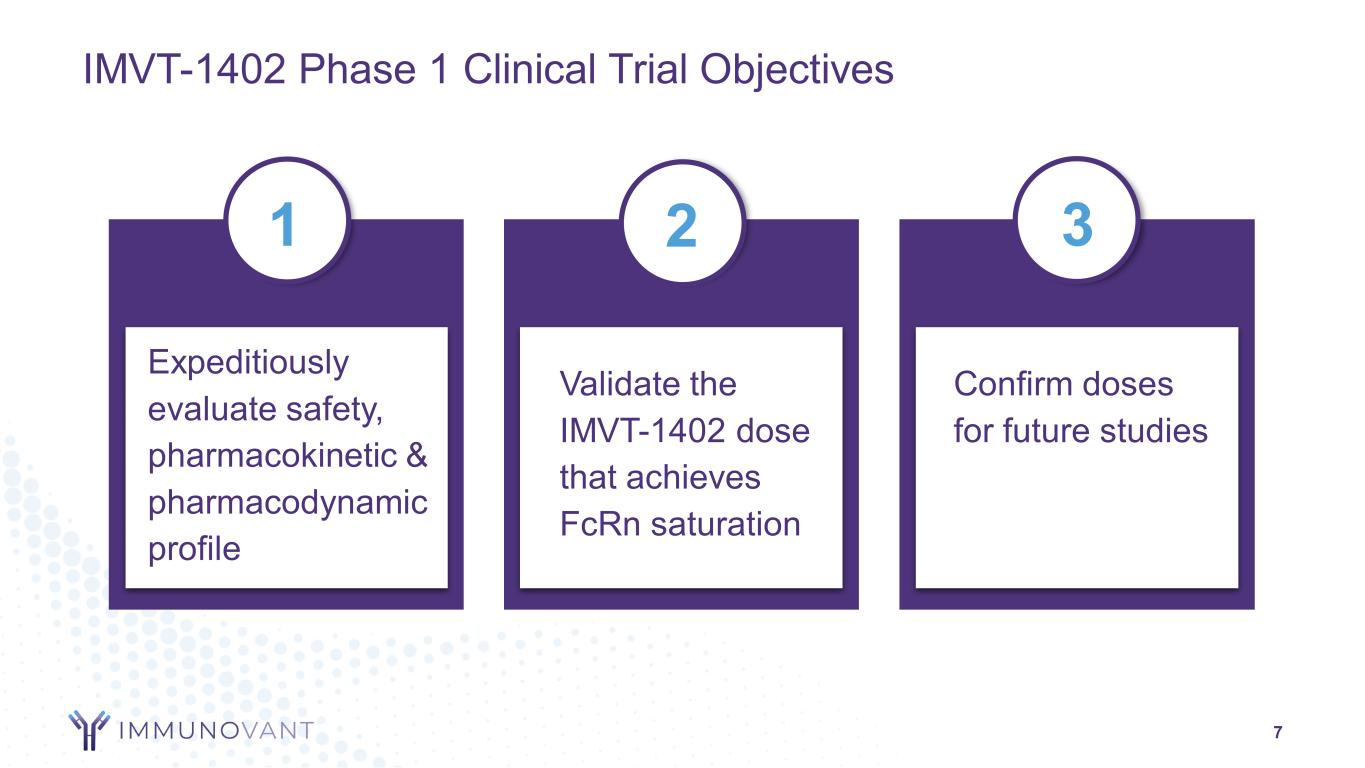
7 Expeditiously evaluate safety, pharmacokinetic & pharmacodynamic profile Validate the IMVT-1402 dose that achieves FcRn saturation Confirm doses for future studies 2 31 IMVT-1402 Phase 1 Clinical Trial Objectives
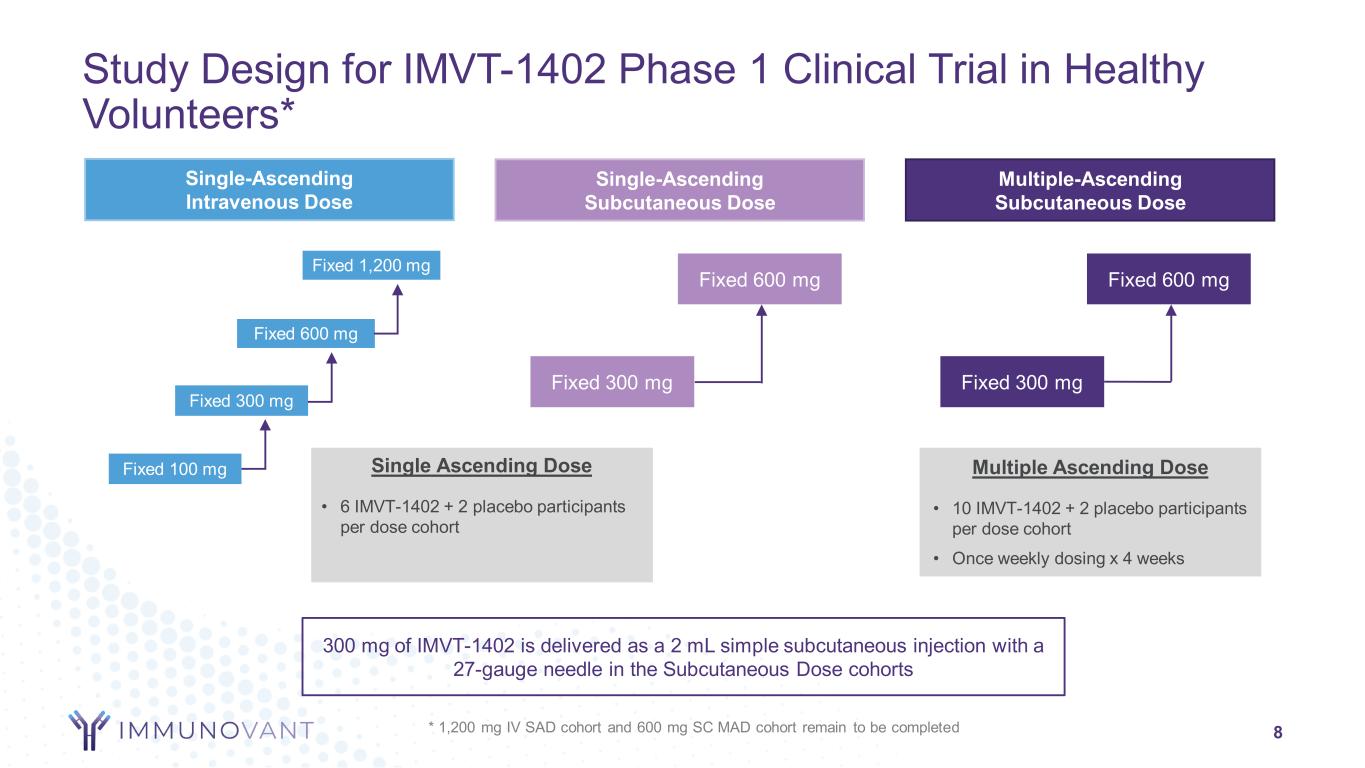
Study Design for IMVT-1402 Phase 1 Clinical Trial in Healthy Volunteers* 8 Single-Ascending Intravenous Dose Single-Ascending Subcutaneous Dose Multiple-Ascending Subcutaneous Dose Fixed 300 mg Fixed 100 mg Fixed 600 mg Fixed 300 mg Fixed 600 mg Fixed 300 mg Fixed 600 mg Single Ascending Dose • 6 IMVT-1402 + 2 placebo participants per dose cohort Multiple Ascending Dose • 10 IMVT-1402 + 2 placebo participants per dose cohort • Once weekly dosing x 4 weeks 300 mg of IMVT-1402 is delivered as a 2 mL simple subcutaneous injection with a 27-gauge needle in the Subcutaneous Dose cohorts Fixed 1,200 mg * 1,200 mg IV SAD cohort and 600 mg SC MAD cohort remain to be completed

Single-Ascending Subcutaneous Doses 9
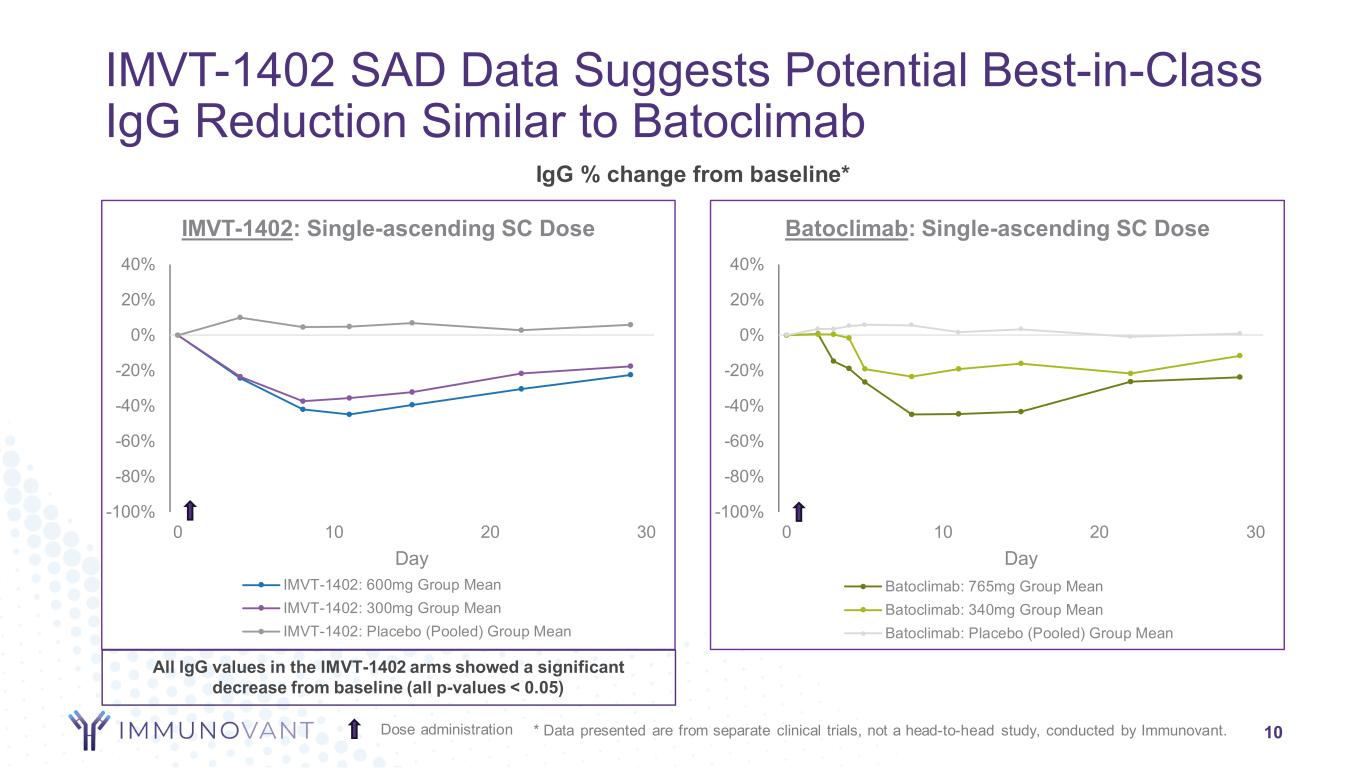
-100% -80% -60% -40% -20% 0% 20% 40% 0 10 20 30 Day Batoclimab: Single-ascending SC Dose Batoclimab: 765mg Group Mean Batoclimab: 340mg Group Mean Batoclimab: Placebo (Pooled) Group Mean -100% -80% -60% -40% -20% 0% 20% 40% 0 10 20 30 Day IMVT-1402: Single-ascending SC Dose IMVT-1402: 600mg Group Mean IMVT-1402: 300mg Group Mean IMVT-1402: Placebo (Pooled) Group Mean IMVT-1402 SAD Data Suggests Potential Best-in-Class IgG Reduction Similar to Batoclimab 10 IgG % change from baseline* Dose administration * Data presented are from separate clinical trials, not a head-to-head study, conducted by Immunovant. All IgG values in the IMVT-1402 arms showed a significant decrease from baseline (all p-values < 0.05)
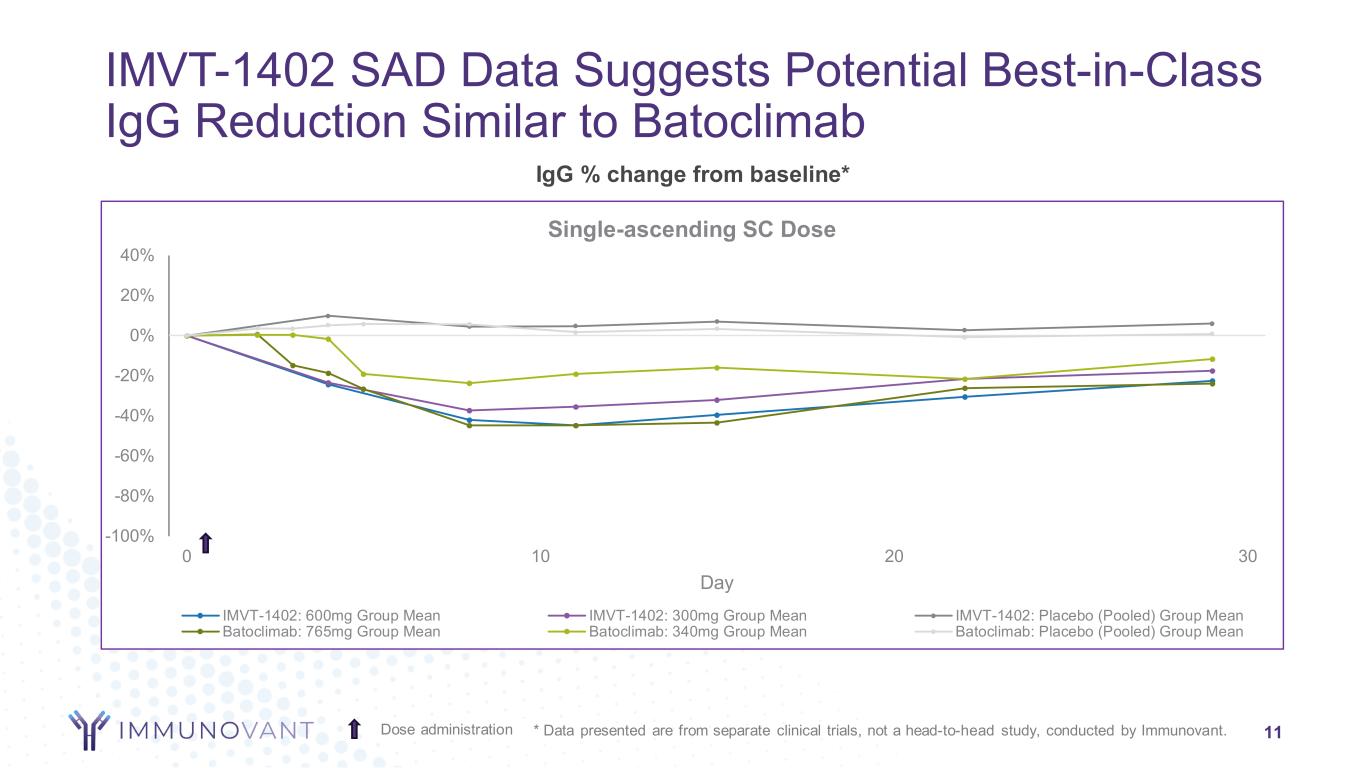
-100% -80% -60% -40% -20% 0% 20% 40% 0 10 20 30 Day Single-ascending SC Dose IMVT-1402: 600mg Group Mean IMVT-1402: 300mg Group Mean IMVT-1402: Placebo (Pooled) Group Mean Batoclimab: 765mg Group Mean Batoclimab: 340mg Group Mean Batoclimab: Placebo (Pooled) Group Mean IMVT-1402 SAD Data Suggests Potential Best-in-Class IgG Reduction Similar to Batoclimab 11Dose administration * Data presented are from separate clinical trials, not a head-to-head study, conducted by Immunovant. IgG % change from baseline*
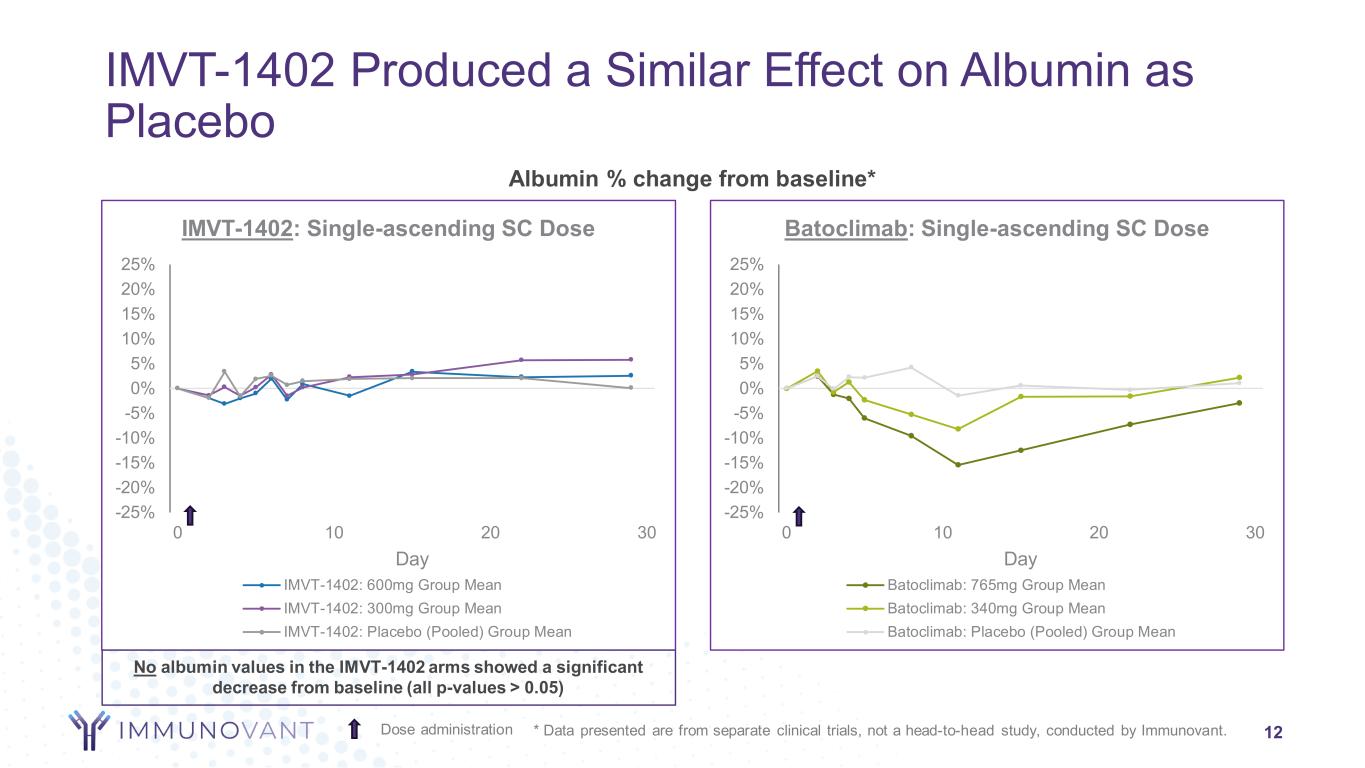
-25% -20% -15% -10% -5% 0% 5% 10% 15% 20% 25% 0 10 20 30 Day Batoclimab: Single-ascending SC Dose Batoclimab: 765mg Group Mean Batoclimab: 340mg Group Mean Batoclimab: Placebo (Pooled) Group Mean -25% -20% -15% -10% -5% 0% 5% 10% 15% 20% 25% 0 10 20 30 Day IMVT-1402: Single-ascending SC Dose IMVT-1402: 600mg Group Mean IMVT-1402: 300mg Group Mean IMVT-1402: Placebo (Pooled) Group Mean IMVT-1402 Produced a Similar Effect on Albumin as Placebo 12 Albumin % change from baseline* Dose administration * Data presented are from separate clinical trials, not a head-to-head study, conducted by Immunovant. No albumin values in the IMVT-1402 arms showed a significant decrease from baseline (all p-values > 0.05)

-50% -40% -30% -20% -10% 0% 10% 20% 30% 40% 50% 0 10 20 30 Day IMVT-1402: Single-ascending SC Dose IMVT-1402: 600mg Group Mean IMVT-1402: 300mg Group Mean IMVT-1402: Placebo (Pooled) Group Mean LDL % change from baseline* IMVT-1402 Produced a Similar Effect on LDL as Placebo 13Dose administration No LDL values in the IMVT-1402 arms showed a significant increase from baseline (all p-values > 0.05) * Batoclimab phase 1 study did not measure LDL, so no comparison provided

Multiple-Ascending Subcutaneous Doses (Once-weekly dosing x 4 weeks) 14
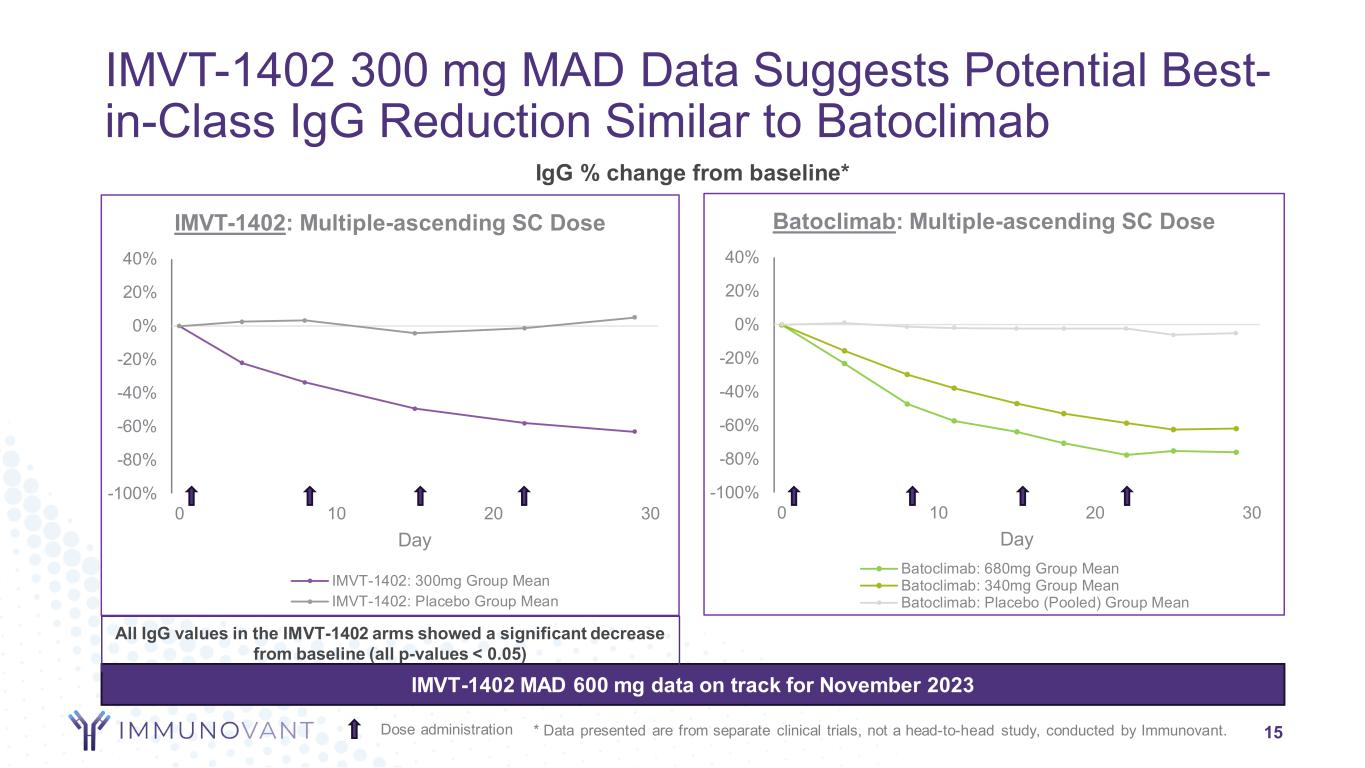
-100% -80% -60% -40% -20% 0% 20% 40% 0 10 20 30 Day Batoclimab: Multiple-ascending SC Dose Batoclimab: 680mg Group Mean Batoclimab: 340mg Group Mean Batoclimab: Placebo (Pooled) Group Mean -100% -80% -60% -40% -20% 0% 20% 40% 0 10 20 30 Day IMVT-1402: Multiple-ascending SC Dose IMVT-1402: 300mg Group Mean IMVT-1402: Placebo Group Mean IgG % change from baseline* IMVT-1402 300 mg MAD Data Suggests Potential Best- in-Class IgG Reduction Similar to Batoclimab 15Dose administration IMVT-1402 MAD 600 mg data on track for November 2023 * Data presented are from separate clinical trials, not a head-to-head study, conducted by Immunovant. All IgG values in the IMVT-1402 arms showed a significant decrease from baseline (all p-values < 0.05)
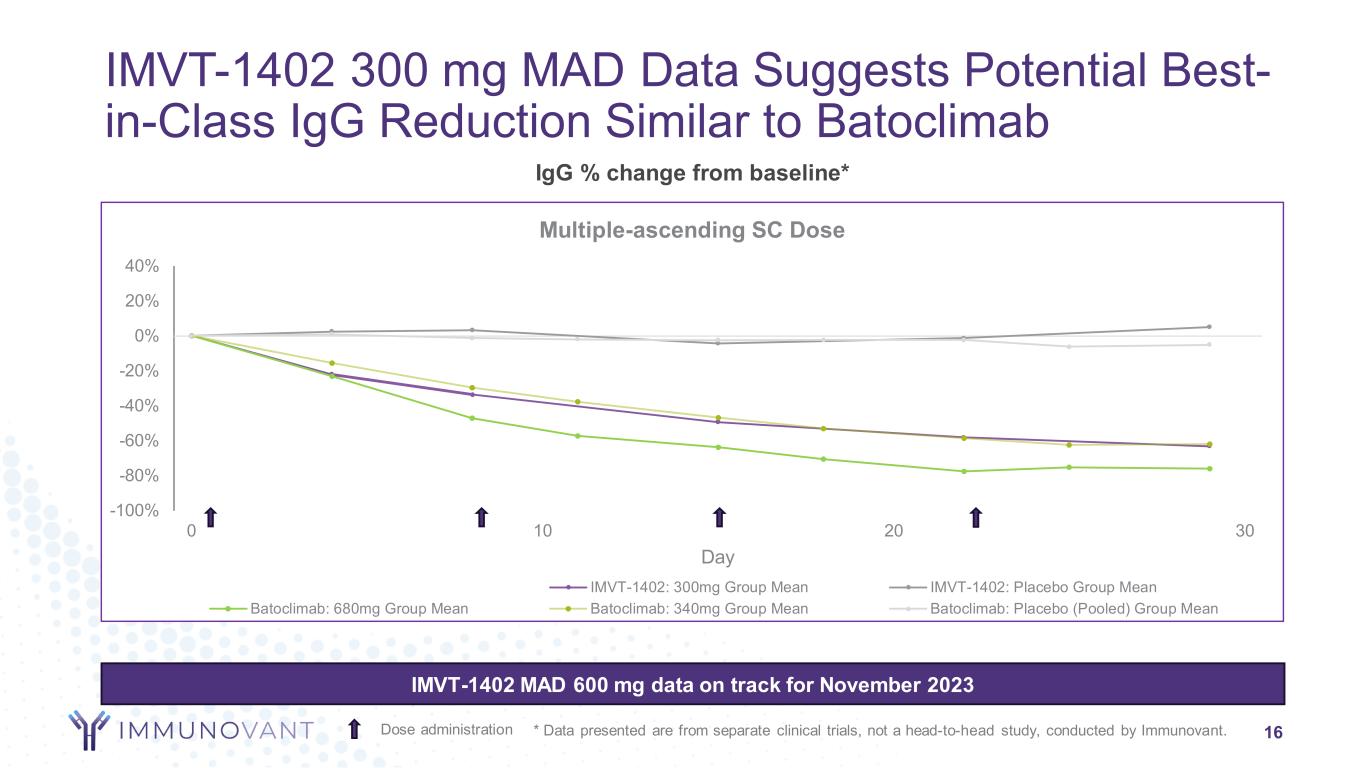
IMVT-1402 300 mg MAD Data Suggests Potential Best- in-Class IgG Reduction Similar to Batoclimab 16Dose administration IMVT-1402 MAD 600 mg data on track for November 2023 * Data presented are from separate clinical trials, not a head-to-head study, conducted by Immunovant. -100% -80% -60% -40% -20% 0% 20% 40% 0 10 20 30 Day Multiple-ascending SC Dose 600 placeholder IMVT-1402: 300mg Group Mean IMVT-1402: Placebo Group Mean Batoclimab: 680mg Group Mean Batoclimab: 340mg Group Mean Batoclimab: Placebo (Pooled) Group Mean IgG % change from baseline*
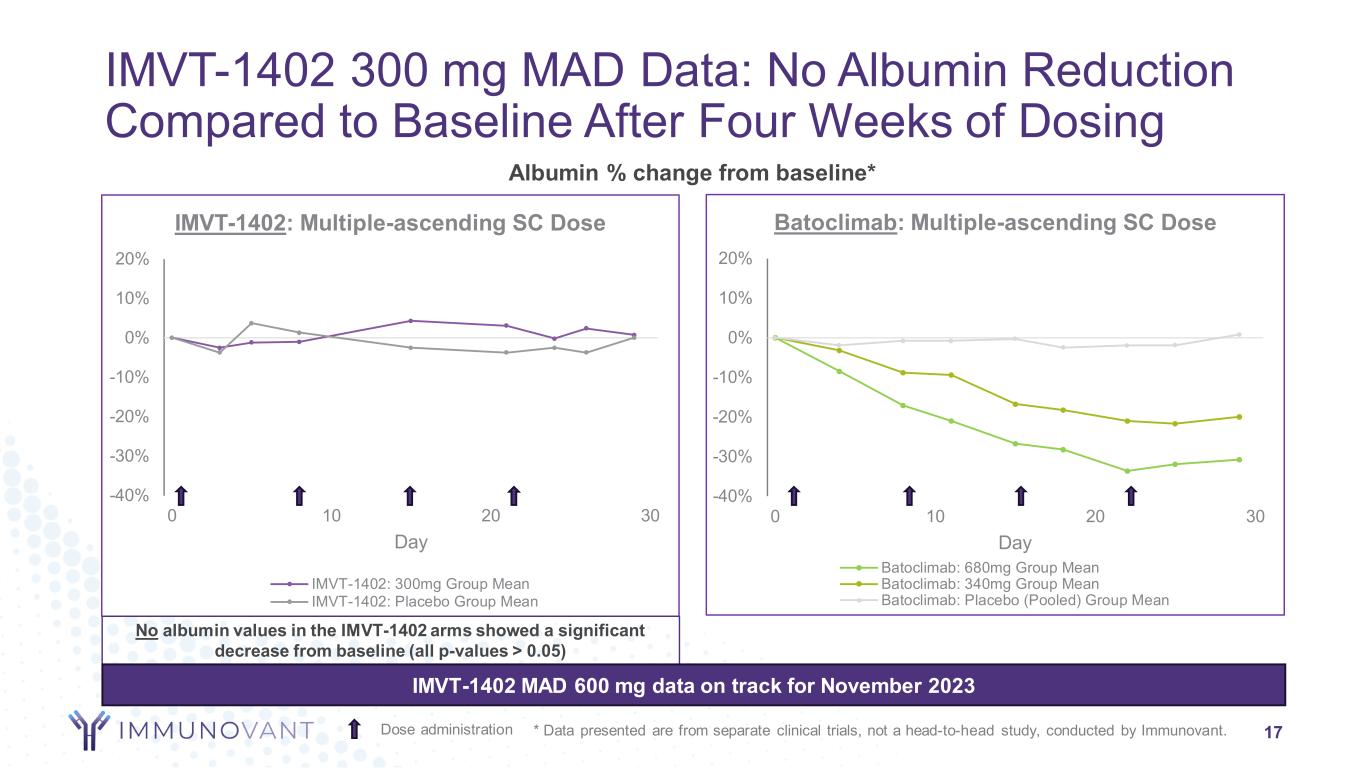
-40% -30% -20% -10% 0% 10% 20% 0 10 20 30 Day IMVT-1402: Multiple-ascending SC Dose IMVT-1402: 300mg Group Mean IMVT-1402: Placebo Group Mean -40% -30% -20% -10% 0% 10% 20% 0 10 20 30 Day Batoclimab: Multiple-ascending SC Dose Batoclimab: 680mg Group Mean Batoclimab: 340mg Group Mean Batoclimab: Placebo (Pooled) Group Mean Albumin % change from baseline* IMVT-1402 300 mg MAD Data: No Albumin Reduction Compared to Baseline After Four Weeks of Dosing 17Dose administration No albumin values in the IMVT-1402 arms showed a significant decrease from baseline (all p-values > 0.05) IMVT-1402 MAD 600 mg data on track for November 2023 * Data presented are from separate clinical trials, not a head-to-head study, conducted by Immunovant.

-50% -40% -30% -20% -10% 0% 10% 20% 30% 40% 50% 0 10 20 30 Day IMVT-1402: Multiple-ascending SC Dose IMVT-1402: 300mg Group Mean IMVT-1402: Placebo Group Mean LDL % change from baseline* IMVT-1402 300 mg MAD Data: No LDL Increase Compared to Baseline After Four Weeks of Dosing 18Dose administration No LDL values in the IMVT-1402 arms showed a significant increase from baseline (all p-values > 0.05) IMVT-1402 MAD 600 mg data on track for November 2023 * Batoclimab phase 1 study did not measure LDL, so no comparison provided
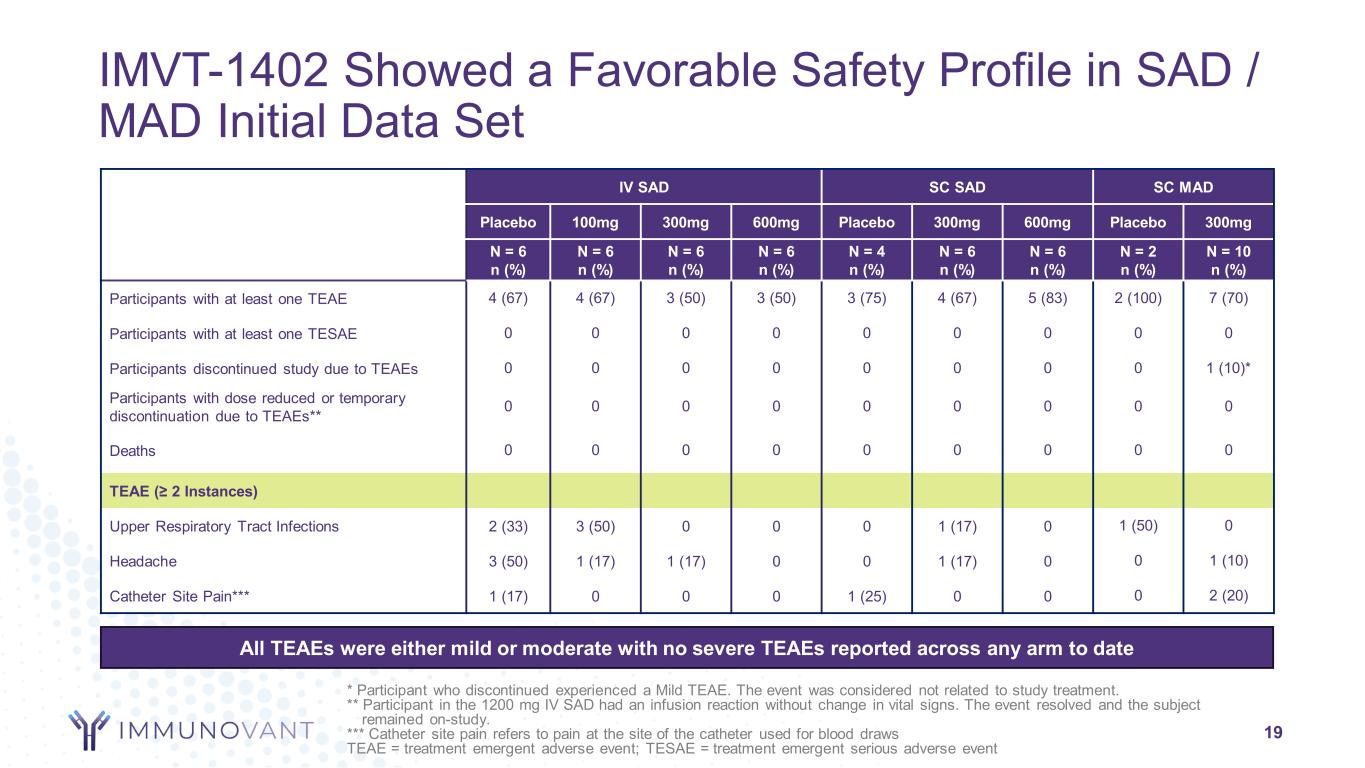
19 IV SAD SC SAD SC MAD Placebo 100mg 300mg 600mg Placebo 300mg 600mg Placebo 300mg N = 6 n (%) N = 6 n (%) N = 6 n (%) N = 6 n (%) N = 4 n (%) N = 6 n (%) N = 6 n (%) N = 2 n (%) N = 10 n (%) Participants with at least one TEAE 4 (67) 4 (67) 3 (50) 3 (50) 3 (75) 4 (67) 5 (83) 2 (100) 7 (70) Participants with at least one TESAE 0 0 0 0 0 0 0 0 0 Participants discontinued study due to TEAEs 0 0 0 0 0 0 0 0 1 (10)* Participants with dose reduced or temporary discontinuation due to TEAEs** 0 0 0 0 0 0 0 0 0 Deaths 0 0 0 0 0 0 0 0 0 TEAE (≥ 2 Instances) Upper Respiratory Tract Infections 2 (33) 3 (50) 0 0 0 1 (17) 0 1 (50) 0 Headache 3 (50) 1 (17) 1 (17) 0 0 1 (17) 0 0 1 (10) Catheter Site Pain*** 1 (17) 0 0 0 1 (25) 0 0 0 2 (20) IMVT-1402 Showed a Favorable Safety Profile in SAD / MAD Initial Data Set All TEAEs were either mild or moderate with no severe TEAEs reported across any arm to date * Participant who discontinued experienced a Mild TEAE. The event was considered not related to study treatment. ** Participant in the 1200 mg IV SAD had an infusion reaction without change in vital signs. The event resolved and the subject remained on-study. *** Catheter site pain refers to pain at the site of the catheter used for blood draws TEAE = treatment emergent adverse event; TESAE = treatment emergent serious adverse event

Concluding Thoughts 20
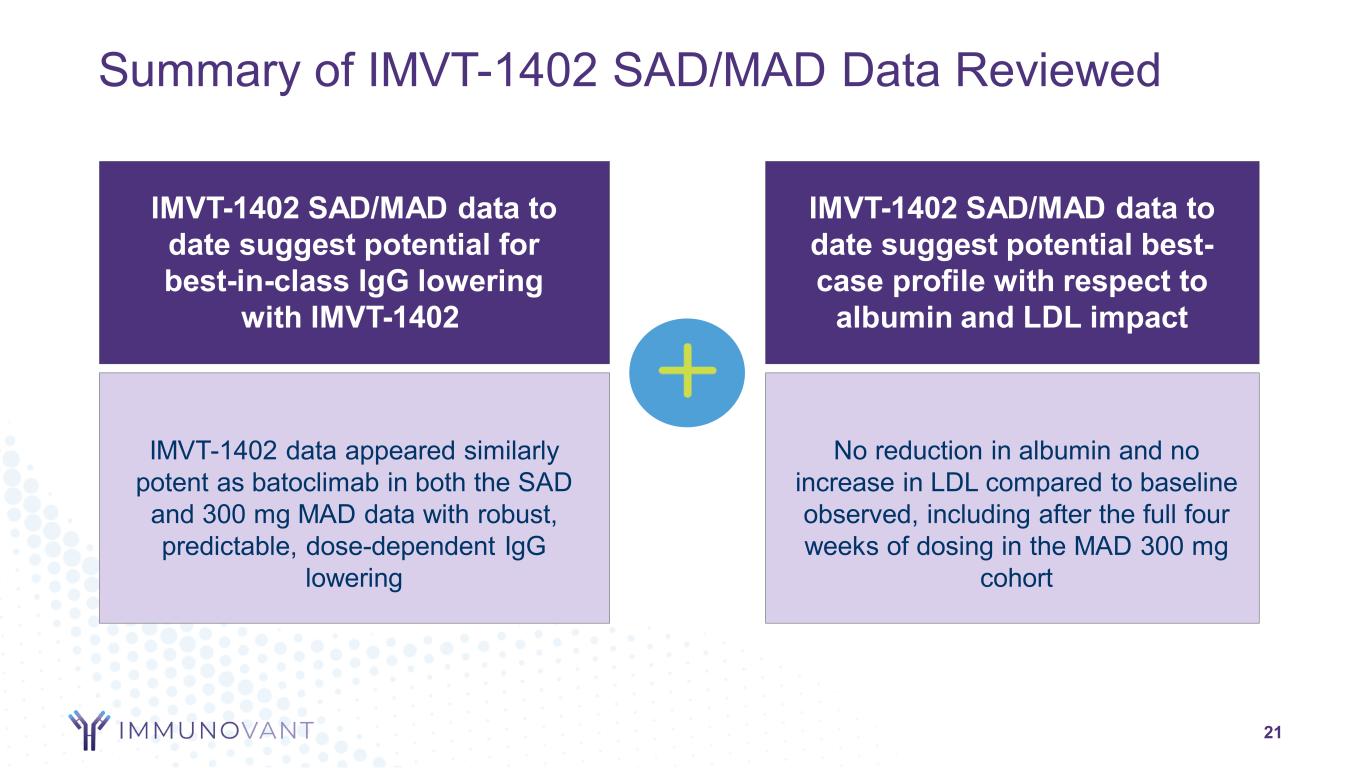
21 Summary of IMVT-1402 SAD/MAD Data Reviewed IMVT-1402 SAD/MAD data to date suggest potential for best-in-class IgG lowering with IMVT-1402 IMVT-1402 SAD/MAD data to date suggest potential best- case profile with respect to albumin and LDL impact IMVT-1402 data appeared similarly potent as batoclimab in both the SAD and 300 mg MAD data with robust, predictable, dose-dependent IgG lowering No reduction in albumin and no increase in LDL compared to baseline observed, including after the full four weeks of dosing in the MAD 300 mg cohort

Favorable Analyte Profile Initial Phase 1 data supports a favorable analyte profile with no or minimal effect on albumin and LDL * Not including any potential patent term extension 22 IMVT-1402 Has Potentially Best-In-Class Attributes to Address Large Unmet Need in Autoimmune Disease Novel, fully human, monoclonal antibody inhibiting FcRn- mediated recycling of IgG + IMVT-1402 ++ + + + Convenient Administration Formulated for simple subcutaneous injection that may enable self-administration at home Compelling Patent Protection Pending composition of matter patent expected for IMVT-1402 to 2043* Deep IgG Lowering Initial Phase 1 data suggests deep dose-dependent IgG lowering similar to batoclimab

Concluding Thoughts 23 Based on SAD / MAD data to date, IMVT-1402 has a potential best-in- class profile MAD 600mg SC cohort just starting with data expected in November 2023 Anti-FcRn market offers many attractive opportunities and a favorable development path
v3.23.3
Cover
|
Sep. 26, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Document Period End Date |
Sep. 26, 2023
|
| Entity Registrant Name |
IMMUNOVANT, INC.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-38906
|
| Entity Tax Identification Number |
83-2771572
|
| Entity Address, Address Line One |
320 West 37th Street
|
| Entity Address, City or Town |
New York,
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10018
|
| City Area Code |
917
|
| Local Phone Number |
580-3099
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.0001 par value per share
|
| Trading Symbol |
IMVT
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| Entity Central Index Key |
0001764013
|
| Amendment Flag |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Immunovant (NASDAQ:IMVT)
Historical Stock Chart
From Dec 2024 to Jan 2025
Immunovant (NASDAQ:IMVT)
Historical Stock Chart
From Jan 2024 to Jan 2025