UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN
PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934
September 11, 2023

Commission File Number: 001-39363
IMMATICS N.V.
Paul-Ehrlich-Straße 15
72076 Tübingen, Federal Republic of Germany
(Address of principal executive office)
Indicate by check mark whether the registrant files
or will file annual reports under cover of Form 20-F or Form 40-F:
INFORMATION CONTAINED IN THIS REPORT ON FORM
6-K
On September 7, 2023, Immatics Biotechnologies
GmbH, a subsidiary of Immatics N.V. (the “Company” or “Immatics”), entered into a Master Collaboration and License
Agreement (the “Master Collaboration and License Agreement”) with ModernaTX, Inc., a subsidiary of Moderna, Inc. (“Moderna”),
relating to three research programs for the development and commercialization of products employing Immatics’ and Moderna’s
technologies: (i) a collaboration to discover and develop mRNA-based TCER therapeutics against targets of interest to Moderna (the “TCER
Program”); (ii) the validation, generation and application of data useful for the research and development of cancer vaccines (the
“Database/Vaccine Program”); and (iii) a combination therapy clinical trial with respect to IMA203 and a Moderna mRNA-based
cancer vaccine (the “Clinical Combo Program”). Each research program will be governed by the Master Collaboration and License
Agreement and a project agreement as described below.
Pursuant to the Master Collaboration and License
Agreement, following Hart-Scott-Rodino Antitrust Improvements Act clearance, Moderna will pay to Immatics a $120 million upfront payment. In addition, as described below, Immatics may be eligible to receive development, regulatory and commercial milestone payments that could exceed $1.7 billion.
With respect to the TCER Program, pursuant to the
Master Collaboration and License Agreement and the TCER Collaboration Project Agreement between the parties (the “TCER Project Agreement”),
the parties will conduct the TCER Program for the research and development of TCERs with respect to HLA-presented peptide targets derived
from an agreed upon number of proteins selected by Moderna. Immatics will be responsible for, and be reimbursed the cost of, TCER identification, validation
and engineering to generate the applicable TCER sequence and preclinical studies in accordance with the applicable mutually agreed research
plan, while Moderna will be responsible for, and bear the cost of, developing, manufacturing and commercializing the applicable products
containing or comprising such TCERs; provided that Immatics has a right to co-fund the development and commercialization of certain products
by making an opt-in payment in exchange for profit and loss sharing on such products. Immatics will grant to Moderna an exclusive, worldwide
sublicensable license to develop, manufacture and commercialize any product (or that contain any product) developed under the TCER Project
Agreement. For each target, depending on certain product characteristics, Immatics may be eligible to receive milestone payments of up
to a mid-eight-digit amount upon the achievement of certain development milestones and up to a mid-nine-digit amount upon the achievement
of certain regulatory and commercial milestones. In addition, during the royalty term (as described below) and depending on certain product
characteristics, Immatics will be eligible to receive tiered, mid-single-digit to low-double-digit percentage royalties on worldwide net
sales of the applicable product, which royalty percentages are subject to reduction in a given country under certain circumstances. A
royalty term with respect to a product under the TCER Program in a given country begins upon the first commercial sale of such product
in such country and terminates on the latest of the expiration of regulatory exclusivity, the expiration of valid patent claims covering
such product, and 10 years after first commercial sale of the product in a given country. The TCER Project Agreement will expire upon
expiration of the last royalty term contemplated by the TCER Project Agreement. During the term of the TCER Program, Immatics has certain
exclusivity and notification obligations to Moderna, and its ability to develop, manufacture and commercialize certain cell therapy products
that bind to the targets subject to the TCER Project Agreement is limited by the TCER Project Agreement.
With respect to the Database/Vaccine Program, pursuant
to the Master Collaboration and License Agreement and the Database/Vaccine Collaboration Project Agreement between the parties (the “Database/Vaccine
Project Agreement”), the parties will use Immatics’ XPRESIDENT platform to (i) generate reports for proteins or cancer vaccine
candidates and validate cancer vaccine candidates (the “Database Query Program”), (ii) select peptides for respect to specific
tumor types selected by Moderna for the development of cancer vaccines (the “Shared Vaccine Program”), and (iii) provide certain
epitope prediction data for potential development and validation of cancer vaccines (the “Optimized Vaccine Program”). The
term of these programs can be up to approximately five years. Immatics will grant to Moderna an exclusive, worldwide sublicensable license
to develop, manufacture and commercialize any Shared Vaccine product or Optimized Vaccine product developed under the Database/Vaccine
Project Agreement. Immatics may be eligible to receive (i) depending on the characteristics of the cancer vaccine, certain milestone payments
under the Database Query Program, (ii) for each resulting cancer vaccine in the Shared Vaccine Program and the Optimized Vaccine Program,
depending on certain product characteristics, up to a low-eight-digit amount upon the achievement of certain development milestones and
up to a low-nine-digit amount upon the achievement of certain regulatory and commercial milestones, and (iii) for each resulting cancer
vaccine in the Shared Vaccine Program, during the royalty term (as described below) and depending on certain product characteristics,
tiered, low- to mid-single-digit percentage royalties on worldwide net sales of such product. A royalty term with respect to a cancer
vaccine in the Shared Vaccine Program and the Optimized Vaccine Program in a given country begins upon the first commercial sale of such
product in such country and
terminates
on the latest of the expiration of regulatory exclusivity, the expiration of valid patent claims covering such product, and 10 years
after first commercial sale of the product in a given country. During the term of the Database/Vaccine Program, Immatics has certain
exclusivity obligations to Moderna, and its ability to develop certain cancer vaccines is limited by the Database/Vaccine Project Agreement.
With respect to the Clinical Combo Program, pursuant
to the Master Collaboration and License Agreement and the Combination Collaboration Project Agreement between the parties (the “Clinical
Combo Project Agreement”), the parties will collaborate to develop a combination therapy of IMA203 (or IMA203CD8) and a Moderna
mRNA-based cancer vaccine. Immatics will be responsible for, and the parties will share the cost of, development activities in accordance
with the applicable mutually agreed research plan. For so long as the parties are conducting the combination therapy clinical trial, Immatics
has certain exclusivity obligations to Moderna, and its ability to develop, manufacture and commercialize combination products that involve
a cancer vaccine and a cell therapy product that binds to the target of IMA203 is limited by the Clinical Combo Project Agreement.
The foregoing
descriptions of the Master Collaboration and License Agreement and the project agreements thereunder do not
purport to be complete and are qualified in their entirety by reference to the full text of the applicable agreements, which will
be filed as an exhibit to the Company’s Annual Report on Form 20-F for the year ended December 31, 2023 or a Report on Form 6-K.
In connection with the foregoing, the Company issued
a press release, a copy of which is attached hereto as Exhibit 99.1, and made available an updated investor presentation on its website,
a copy of which is attached hereto as Exhibit 99.2.
INCORPORATION BY REFERENCE
This Report on Form 6-K (other than Exhibit 99.1
and 99.2 hereto) shall be deemed to be incorporated by reference into the registration statements on Form F-3 (Registration Nos. 333-258351,
333-240260 and 333-274218) of Immatics N.V. and to be a part thereof from the date on which this report is filed, to the extent not superseded
by documents or reports subsequently filed or furnished.
EXHIBIT INDEX
| Exhibit No. |
Description |
| 99.1 |
Press release dated September 11, 2023 |
| 99.2 |
Presentation dated September 11, 2023 |
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
IMMATICS N.V. |
| Date: September 11, 2023 |
|
| |
By: |
/s/ Harpreet Singh |
| |
Name: |
Harpreet Singh |
| |
Title: |
Chief Executive Officer |
Exhibit 99.1
PRESS RELEASE
Moderna
and Immatics Announce Strategic Multi-Platform Collaboration
to Develop Innovative Oncology Therapeutics
| · | Collaboration combines leading technologies to develop breakthrough, mRNA-enabled in vivo expressed TCER® molecules |
| · | Companies to leverage Immatics’ XPRESIDENT® target discovery platform and Moderna’s mRNA technology for the development
of novel cancer vaccines |
| · | Collaboration to include evaluation of Immatics’ investigational IMA203 PRAME TCR-T in combination with Moderna’s investigational
PRAME mRNA cancer vaccine |
| · | Immatics to receive $120 million upfront cash payment plus research funding with the potential for additional milestone and royalty
payments |
Cambridge,
Massachusetts and Tuebingen, Germany, September 11,
2023 – Moderna, Inc. (NASDAQ: MRNA, “Moderna”) and Immatics N.V. (NASDAQ: IMTX, “Immatics”),
a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, today
announced a strategic research and development collaboration to pioneer novel and transformative therapies for cancer patients with high
unmet medical need. This broad multi-platform collaboration will leverage the deep scientific expertise and core operational capabilities
of both companies, combining Immatics’ TCR platform with Moderna’s cutting-edge mRNA technology, and span various therapeutic
modalities including bispecifics, cell therapy and cancer vaccines.
The strategic R&D collaboration between Moderna
and Immatics focuses on three pillars:
| · | Applying Moderna’s mRNA technology for in vivo expression of Immatics’ next-generation,
half-life extended TCR bispecifics (TCER®) targeting cancer-specific HLA-presented peptides. |
| · | Enabling the discovery and development of novel mRNA-based cancer vaccines by leveraging Moderna’s
deep knowledge of mRNA science and customized information from Immatics’ wealth of tumor and normal tissue data included in the
target discovery platform XPRESIDENT® and its bioinformatics and AI platform XCUBE™. |
| · | Evaluating Immatics’ IMA203 TCR-T therapy targeting PRAME in combination with Moderna’s PRAME
mRNA-based cancer vaccine. The collaboration contemplates conducting preclinical studies and a Phase 1 clinical trial evaluating the safety
and efficacy of the combination with the objective of further enhancing IMA203 T cell responses. |
| Immatics Press Release September 11, 2023 | 1 | 5 |
“We are excited to embark on this strategic
collaboration with Immatics, a pioneer in developing innovative cancer immunotherapies. This partnership presents a groundbreaking opportunity
to leverage our mRNA technology alongside Immatics' TCR platform, potentially diversifying and augmenting the way we approach cancer treatment.
We believe this collaboration will accelerate the development of novel oncology therapies and bring us one step closer to providing significant
benefits for patients with high unmet medical needs,” said Rose Loughlin, Ph.D., Moderna's Senior Vice President for Research and
Early Development.
“We are thrilled to join forces with Moderna
in our quest to pioneer innovative and transformative therapies to combat cancer. We believe Immatics’ cancer target and TCR platforms,
along with Moderna's cutting-edge mRNA technology, represent a powerful combination that has the potential to deliver meaningful benefits
to cancer patients,” said Toni Weinschenk, PhD, Chief Innovation Officer at Immatics. “The rapid advancement of our first
2 TCER® programs into the clinic, with additional TCER® compounds fueling our pre-clinical pipeline, underscores our commitment
to develop innovative therapeutics. We are confident that we can explore the optimal delivery of TCER® molecules through this collaboration
to maximize clinical benefit in a broad patient population,” added Carsten Reinhardt, MD, PhD, Chief Development Officer of Immatics.
About the Collaboration
Under the terms of the agreement, Immatics will
receive an upfront payment of $120 million. Immatics will also receive research funding and is eligible to receive development, regulatory,
and commercial milestone payments that could exceed $1.7 billion. Immatics is also eligible to receive tiered royalties on global net
sales of TCER® products and certain vaccine products that are commercialized under the agreement. Under the agreement, Immatics has
an option to enter into a global profit and loss share arrangement for the most advanced TCER®.
Moderna will lead the clinical development and
commercialization of cancer vaccines and TCER® therapeutics resulting from the collaboration. Immatics will be responsible for conducting
the preclinical studies and a potential Phase 1 clinical trial investigating IMA203 TCR-T in combination with the PRAME mRNA vaccine to
further enhance IMA203 T cell responses. Each party will retain full ownership of its investigational PRAME compound, and the parties
will fund the clinical study on a cost sharing basis.
Within the collaboration, preclinical activities
conducted by Immatics will be managed by the Immatics Discovery Unit, a recently created internal division at Immatics integrating its
| Immatics Press Release September 11, 2023 | 2 | 5 |
technology platforms into one interdisciplinary
team focused on all early-stage preclinical pipeline and collaboration programs.
The collaboration is subject to customary antitrust
clearance in the United States.
– END –
About Moderna
In over 10 years since its inception, Moderna has
transformed from a research-stage company advancing programs in the field of messenger RNA (mRNA), to an enterprise with a diverse clinical
portfolio of vaccines and therapeutics across seven modalities, a broad intellectual property portfolio in areas including mRNA and lipid
nanoparticle formulation, and an integrated manufacturing plant that allows for both clinical and commercial production at scale. Moderna
maintains alliances with a broad range of domestic and overseas government and commercial collaborators, which has allowed for the pursuit
of both groundbreaking science and rapid scaling of manufacturing. Most recently, Moderna’s capabilities have come together to allow
the authorized use and approval of one of the earliest and most effective vaccines against the COVID-19 pandemic.
Moderna’s mRNA platform builds on continuous
advances in basic and applied mRNA science, delivery technology, and manufacturing, and has allowed the development of therapeutics and
vaccines for infectious diseases, immune-oncology, rare diseases, cardiovascular diseases, and autoimmune diseases. Moderna Genomics was
created to leverage the recent advancement in both the RNA delivery platform and the genomic medicines to create the next generation of
in vivo gene editing therapeutics. Moderna has been named a top biopharmaceutical employer by Science for the past eight years. To learn
more, visit www.modernatx.com.
Moderna Forward-Looking Statements:
This press release contains forward-looking statements
within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including regarding: the agreement between Moderna
and Immatics to develop innovative oncology therapeutics; the opportunity presented by the collaboration to leverage Moderna’s mRNA
technology alongside Immatics’ TCR platform to potentially diversify and augment the way we approach cancer treatment; the potential
for the collaboration to accelerate the development of novel oncology therapies; and the antitrust clearance process in the United States.
The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these
forward-looking statements because they involve known and unknown risks, uncertainties, and
| Immatics Press Release September 11, 2023 | 3 | 5 |
other factors, many of which are beyond Moderna’s
control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements.
These risks, uncertainties, and other factors include those other risks and uncertainties described under the heading “Risk Factors”
in Moderna’s Annual Report on Form 10-K for the year ended December 31, 2022, filed with the U.S. Securities and Exchange Commission
(SEC), and in subsequent filings made by Moderna with the SEC, which are available on the SEC’s website at www.sec.gov. Except as
required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in
this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna’s
current expectations and speak only as of the date of this press release.
About Immatics
Immatics combines the discovery of true targets
for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response
against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as
our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking
new avenues for patients in their fight against cancer.
Immatics intends to use its website www.immatics.com
as a means of disclosing material non-public information. For regular updates you can also follow us on Twitter, Instagram and LinkedIn.
Immatics Forward-Looking
Statements:
Certain statements in
this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’
future financial or operating performance. For example, statements concerning the timing of product candidates and Immatics’ focus
on partnerships to advance its strategy are forward-looking statements. In some cases, you can identify forward-looking statements by
terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”,
“anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives
of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and
other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements.
These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management,
are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties.
Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors
beyond management's control
| Immatics Press Release September 11, 2023 | 4 | 5 |
including general economic
conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this press release should be regarded
as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated
results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which
speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements.
Moderna Contacts:
| Media Contact |
|
| Chris Ridley |
|
| Vice President, Communications |
|
| Phone: +1 617-800-3651 |
|
| Chris.Ridley@modernatx.com |
|
| Investor Relations Contact |
|
| Lavina Talukdar |
|
| Senior Vice President & Head of Investor Relation |
|
| Phone: +1 617-209-5834 |
|
| Lavina.Talukdar@modernatx.com |
|
Immatics Contacts:
| Media and Investor Relations Contact |
|
| Eva Mulder or Charlotte Spitz |
|
| Trophic Communications |
|
| Phone: +31 6 52 33 15 79 |
|
| immatics@trophic.eu |
|
| Immatics N.V. |
|
| Anja Heuer |
Sabrina Schecher, Ph.D. |
| Senior Director, Corporate Communications |
Senior Director, Investor Relations |
| Phone: +49 89 540415-606 |
Phone: +49 89 262002433 |
| media@immatics.com |
InvestorRelations@immatics.com |
| Immatics Press Release September 11, 2023 | 5 | 5 |
Exhibit 99.2

© Immatics. Not for further reproduction or distribution. Delivering the Power of T cells to Cancer Patients © Immatics. Not for further reproduction or distribution. Immatics Corporate Presentation September 11, 2023

Forward - Looking Statement This presentation (“Presentation”) is provided by Immatics N . V . (“Immatics” or the “Company”) for informational purposes only . The information contained herein does not purport to be all - inclusive and none of Immatics, any of its affiliates, any of its or their respective control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation . Forward - Looking Statements . Certain statements in this presentation may be considered forward - looking statements . Forward - looking statements generally relate to future events or the Company’s future financial or operating performance . For example, statements concerning timing of data read - outs for product candidates, the timing of IND or CTA filing for pre - clinical stage product candidates, the Company’s focus on partnerships to advance its strategy, and other metrics are forward - looking statements . In some cases, you can identify forward - looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology . Such forward - looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements . These forward - looking statements are based upon estimates and assumptions that, while considered reasonable, Immatics and its management, are inherently uncertain . New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties . Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management's control including general economic conditions and other risks, uncertainties and factors set forth in the Company’s Annual report on Form 20 - F and other filings with the Securities and Exchange Commission (SEC) . Nothing in this presentation should be regarded as a representation by any person that the forward - looking statements set forth herein will be achieved or that any of the contemplated results of such forward - looking statements will be achieved . You should not place undue reliance on forward - looking statements, which speak only as of the date they are made . The Company undertakes no duty to update these forward - looking statements . No Offer or Solicitation . This communication is for informational purposes only and does not constitute, or form a part of, an offer to sell or the solicitation of an offer to sell or an offer to buy or the solicitation of an offer to buy any securities, and there shall be no sale of securities, in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction . No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933 , as amended, or in an offering exempt from registration . Certain information contained in this Presentation relates to or is based on studies, publications, surveys and the Company’s own internal estimates and research . In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions . Finally, while the Company believes its internal research is reliable, such research has not been verified by any independent source . All the scientific and clinical data presented within this presentation are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification . 2
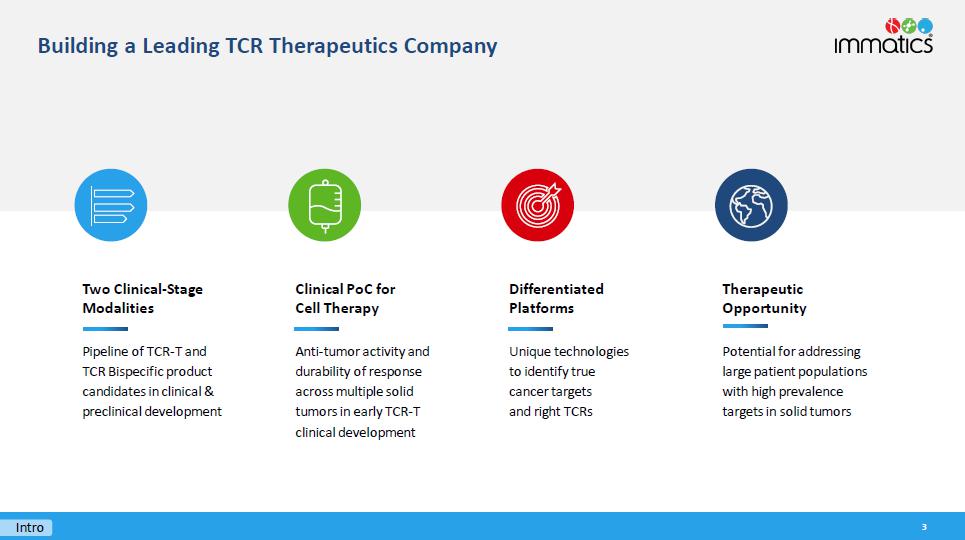
Therapeutic Opportunity Potential for addressing large patient populations with high prevalence targets in solid tumors Two Clinical - Stage Modalities Pipeline of TCR - T and TCR Bispecific product candidates in clinical & preclinical development Building a Leading TCR Therapeutics Company 3 Intro Differentiated Platforms Unique technologies to identify true cancer targets and right TCRs Clinical PoC for Cell Therapy Anti - tumor activity and durability of response across multiple solid tumors in early TCR - T clinical development

4 Three IMA203 Ph1b cohorts • IMA203 monotherapy • Checkpoint combo • IMA203CD8 monotherapy Next update on all three IMA203 cohorts and clinical development path for PRAME TCR - T monotherapy towards registration - directed trials is planned for 4Q 2023 ACTengine® IMA203 (PRAME) Advance ongoing Phase 1 clinical trial Establish clinical PoC TCER® IMA401 (MAGEA4/8) Phase 1/2 clinical trial started in Aug 2023 First clinical data planned in 2024 TCER® IMA402 (PRAME) Intro Projected cash runway well into 2026 to reach multiple value inflections points across our portfolio Our Near - Term Focus – Clinical Development of Our Lead Assets from Our Autologous TCR - T (ACTengine®) and TCR Bispecifics (TCER®) Pipeline 1 Clinical Trial Application (CTA) is the European equivalent of an Investigational New Drug (IND) application

Our TCR - based Approaches Leverage the Full Target Space beyond the Cancer Cell Surface 5 Intro

Two Distinct TCR - based Therapeutic Modalities in Clinical Development 6 Differentiated positioning of ACTengine® vs. TCER® based on patient population and medical need Intro 1 Interim data update from the ACTengine® IMA203 TCR - T monotherapy Phase 1b Cohort A (published May 02, 2023) with a 64% (7/11) OR R and 67% (6/9) confirmed ORR; 2 Initial manufacturing may provide sufficient quantity for potential repeat dosing. Autologous TCR - T (ACTengine®) TCR Bispecifics (TCER®) • Strong clinical activity in patients with high tumor burden 1 • Single dose 2 • Proprietary manufacturing process for enhanced potency of T cells • Specialized medical centers • Target requirements: stringent tumor selectivity, low, medium, high copy numbers • Off - the - shelf biologic for immediate treatment • Repeat dosing • All hospitals and out - patient, opportunity for larger patient reach • Favorable commercial characteristics • Target requirements: strong tumor association, median to high copy numbers
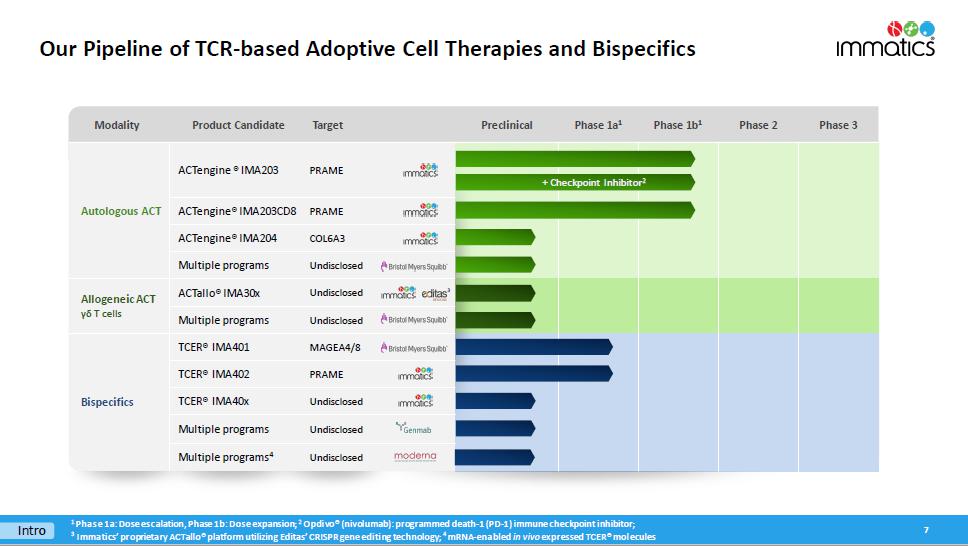
Modality Product Candidate Target Preclinical Phase 1a 1 Phase 1b 1 Phase 2 Phase 3 Autologous ACT ACTengine ® IMA203 PRAME ACTengine® IMA203CD8 PRAME ACTengine® IMA204 COL6A3 Multiple programs Undisclosed Allogeneic ACT γδ T cells ACTallo® IMA30x Undisclosed Multiple programs Undisclosed Bispecifics TCER® IMA401 MAGEA4/8 TCER® IMA402 PRAME TCER® IMA40x Undisclosed Multiple programs Undisclosed Multiple programs 4 Undisclosed Our Pipeline of TCR - based Adoptive Cell Therapies and Bispecifics 7 Intro 1 Phase 1a: Dose escalation, Phase 1b: Dose expansion; 2 Opdivo ® (nivolumab): programmed death - 1 (PD - 1) immune checkpoint inhibitor; 3 I mmatics ’ proprietary ACTallo® platform utilizing Editas’ CRISPR gene editing technology; 4 mRNA - enabled in vivo expressed TCER® molecules 3 + Checkpoint Inhibitor 2

Immatics & Moderna – A Strategic Cross - platform R&D Collaboration Combining Immatics’ Target and TCR Platforms with Moderna’s mRNA Technology 8 TCER® mRNA Approach Development of mRNA - enabled in vivo expressed half - life extended TCER® molecules targeting cancer - specific HLA - presented peptides Option for global P&L sharing for most advanced TCER® program TCR - T + mRNA Vaccine Combo mRNA Cancer Vaccines Development of mRNA cancer vaccines by leveraging Moderna’s mRNA technology and Immatics’ target discovery platform XPRESIDENT® and bioinformatics and AI platform XCUBE Ρ Economics • $ 120 million upfront cash payment plus research funding • >$1.7 billion potential development, regulatory & commercial milestones • Potential for tiered royalties on global net sales of TCER® products and certain cancer vaccine products commercialized under the agreement Evaluation of Immatics’ IMA203 TCR - T therapy targeting PRAME in combination with Moderna’s PRAME mRNA - based cancer vaccine 1 Within the collaboration, preclinical activities conducted by Immatics will be managed by the Immatics Discovery Unit, a rece ntl y created internal division at Immatics integrating its technology platforms into one interdisciplinary team focused on all early - stage preclinical pipeline and collaboration programs.; 1 Each Party will retain full ownership of its investigational compound Intro

Strategic Collaborations Synergistic Expertise that Can Foster Transformative Innovations across Various Modalities Research collaboration to develop bispecific immunotherapies $54 M upfront, up to $550 M aggregated milestone payments per program, up to double - digit tiered royalties; Co - promotion option Research collaboration to develop autologous TCR - T therapies $75 M (2019) + $20 M (2022) upfront , up to $505 M aggregated milestone payments per program, tiered royalties; Co - development/Co - fund option; Opt - in right for 1 st program exercised by BMS in 2Q 2023 for $15 M option exercise fee Research collaboration to develop off - the - shelf allogeneic γδ - based TCR - T/ CAR - T programs $60 M upfront up to $700 M milestone payments per program, low double - digit tiered royalties Clinical co - development collaboration to develop Immatics’ TCR Bispecific program TCER® IMA401 $150 M upfront , up to $770 M aggregated milestone payments, double - digit tiered royalties; Co - promotion option in the US 2022 2021 2018 2019 9 Intro 2023 Multi - platform R&D collaboration t o develop in vivo expressed TCER® molecules, mRNA cancer vaccines and combo of TCR - T + mRNA vaccine $120 M upfront , >$1.7 B potential aggregated milestone payments, tiered royalties; Option for global P&L sharing for most advanced TCER®
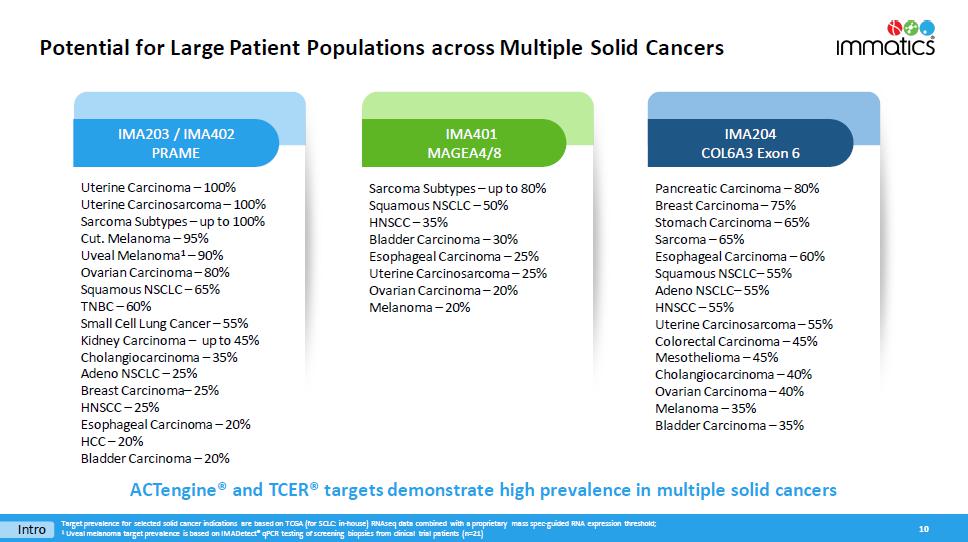
Potential for Large Patient Populations across Multiple Solid Cancers 10 Uterine Carcinoma – 100% Uterine Carcinosarcoma – 100% Sarcoma Subtypes – up to 100% Cut. Melanoma – 95% Uveal Melanoma 1 – 90% Ovarian Carcinoma – 80% Squamous NSCLC – 65% TNBC – 60% Small Cell Lung Cancer – 55% Kidney Carcinoma – up to 45% Cholangiocarcinoma – 35% Adeno NSCLC – 25% Breast Carcinoma – 25% HNSCC – 25% Esophageal Carcinoma – 20% HCC – 20% Bladder Carcinoma – 20% Sarcoma Subtypes – up to 80% Squamous NSCLC – 50% HNSCC – 35% Bladder Carcinoma – 30% Esophageal Carcinoma – 25% Uterine Carcinosarcoma – 25% Ovarian Carcinoma – 20% Melanoma – 20% I MA203 / IMA402 PRAME IMA401 MAGEA4/8 IMA204 COL6A3 Exon 6 Intro Pancreatic Carcinoma – 80% Breast Carcinoma – 75% Stomach Carcinoma – 65% Sarcoma – 65% Esophageal Carcinoma – 60% Squamous NSCLC – 55% Adeno NSCLC – 55% HNSCC – 55% Uterine Carcinosarcoma – 55% Colorectal Carcinoma – 45% Mesothelioma – 45% Cholangiocarcinoma – 40% Ovarian Carcinoma – 40% Melanoma – 35% Bladder Carcinoma – 35% ACTengine® and TCER® targets demonstrate high prevalence in m ultiple s olid cancers Target prevalence for selected solid cancer indications are based on TCGA (for SCLC: in - house) RNAseq data combined with a propr ietary mass spec - guided RNA expression threshold ; 1 Uveal melanoma target prevalence is based on IMADetect® qPCR testing of screening biopsies from clinical trial patients (n=21 )

Realizing the Full Multi - Cancer Opportunity of PRAME ACTengine® IMA203 (TCR - T) and TCER® IMA402 (TCR Bispecific) 11 Indication % PRAME positive patients 1 Uterine Carcinoma Uterine Carcinosarcoma Sarcoma Subtypes Cut. Melanoma Uveal Melanoma 2 Ovarian Carcinoma Squamous NSCLC TNBC Small Cell Lung Cancer Kidney Carcinoma Cholangiocarcinoma Adeno NSCLC Breast Carcinoma HNSCC Esophageal Carcinoma HCC Bladder Carcinoma 100% 100% up to 100% 95% 90% 80% 65% 60% 55% up to 45% 35% 25% 25% 25% 20% 20% 20% ACTengine® IMA203 (TCR - T) Cancer Cell Death PRAME is one of the most promising and most prevalent, clinically validated solid tumor targets known to date Leverage the full potential of targeting PRAME by continued evaluation of the best suited therapeutic modality (ACTengine® vs. TCER® or both) for each cancer type 1 PRAME target prevalence is based on TCGA (for SCLC: in - house) RNAseq data combined with a proprietary mass spec - guided RNA expre ssion threshold; 2 Uveal melanoma target prevalence is based on IMADetect® qPCR testing of screening biopsies from clinical trial patients (n=21); NSCLC: Non - small cell lung cancer, TNBC: Triple - negative breast cancer, HNSCC: Head and neck squamous cell carcinoma; HCC: Hepatocellular carcinoma Intro Phase 1b dose expansion ongoing Initiation of Phase 1/2 trial Aug 2023 TCER® IMA402 (TCR Bispecific)

ACTengine® IMA203 – TCR - T Targeting PRAME 12

ACTengine® IMA203 Targeting PRAME – Mechanism of Action Immatics’ Leading TCR - T Approach 13 IMA203

Key Pillars of Developing a Successful TCR - T Product Candidate Summary of Interim Update on IMA203 TCR - T Phase 1b Cohort A as of April 2023 14 Safety Anti - Tumor Activity Durability Product Quality Broad Reach Manageable tolerability at doses as high as ~9x10 9 TCR - T cells High rate of objective responses: 64% (7/11) ORR 1 67% (6/9) cORR 2 Ongoing durable responses at 9+ months mDOR: Not reached min 1.3+, max 8.8+ mFU : 8.5 months Rapid manufacturing time of 7 days (+ 7 - day release testing), manufacturing success rate of 94% Confirmed objective responses in broad range of solid cancer types at low, medium and high PRAME levels above threshold 1 Initial ORR: Objective response rate according to RECIST 1.1 at first scan post infusion at ~week 6; 2 Confirmed ORR ( cORR ): Confirmed objective response rate according to RECIST 1.1 for patients with available second scan post infusion at ~month 3 or patients with progressive disease (PD) at any timepoint before this s can ; mDOR: median duration of response; mFU : median follow - up Data cut - off Apr 04, 2023 IMA203

The Multi - Cancer Opportunity of PRAME One of the Most Promising Solid Tumor Targets for TCR - based Therapies Known To Date 15 High prevalence High target density Homogeneous expression “Clean” expression profile Clinical proof - of - concept s qNSCLC Ovarian Cancer PRAME fulfills all properties of an ideal target for TCR - based therapies PRAME RNA detection in tumor samples (ISH) ISH: in situ hybridization, sqNSCLC : squamous n on - small cell lung cancer IMA203 78025�&(// 7�&(// ,> � Ͳ � Ύ ϬϮ ͗ Ϭϭ WZ�D�� d �Z WZ�D�� W Ğ Ɖ ƟĚĞ

ACTengine® IMA203 TCR - T Monotherapy – Patient Flow 16 HLA - A*02 Testing Blood sample; Central lab Treatment & Observation Phase Long Term Follow - up Screening & Manufacturing Phase Manufacturing by Immatics Infusion of ACTengine® IMA203 TCR - T Product Lymphodepletion * Target Profiling Fresh Tumor Biopsy; IMADetect® Low dose IL - 2 ** Safety and efficacy monitoring for 12 months Leukapheresis x x Expression Antigen 1 3 2 Short process time of 14 days * 30 mg/m 2 Flu darabine and 500 mg/m 2 Cy clophosphamide for 4 days; ** 1m IU daily days 1 - 5 and twice daily days 6 - 10 7 - day rapid manufacturing process 7 - day expedited QC release testing Monocyte depletion process implemented in Phase 1b IMA203
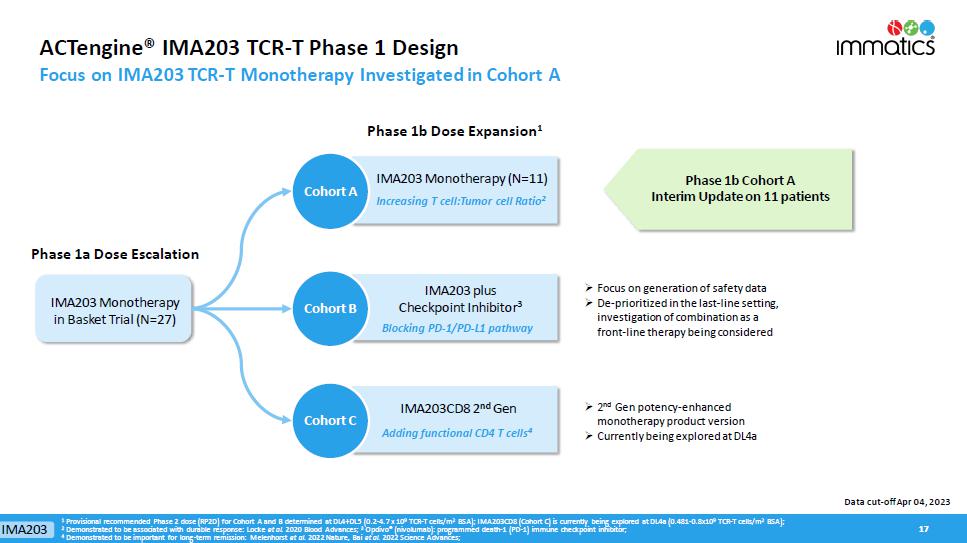
ACTengine® IMA203 TCR - T Phase 1 Design Focus on IMA203 TCR - T Monotherapy Investigated in Cohort A 17 17 Cohort A Phase 1b Cohort A Interim Update on 11 patients » Focus on generation of safety data » D e - prioritized in the last - line setting, investigation of combination as a front - line therapy being considered Phase 1b Dose Expansion 1 Phase 1a Dose Escalation Cohort A IMA203 Monotherapy (N=11) Increasing T cell:Tumor cell Ratio 2 IMA203 Monotherapy in Basket Trial (N=27) » 2 nd Gen potency - enhanced monotherapy product version » Currently being explored at DL4a Data cut - off Apr 04, 2023 Cohort B Cohort C IMA203 Adding functional CD4 T cells 4 IMA203CD8 2 nd Gen IMA203 plus Checkpoint Inhibitor 3 Blocking PD - 1/PD - L1 pathway 1 Provisional recommended Phase 2 dose (RP2D) for Cohort A and B determined at DL4+DL5 (0.2 - 4.7 x 10 9 TCR - T cells/m 2 BSA); IMA203CD8 (Cohort C) is currently being explored at DL4a (0.481 - 0.8x10 9 TCR - T cells/m 2 BSA); 2 Demonstrated to be associated with durable response: Locke et al. 2020 Blood Advances; 3 Opdivo ® (nivolumab): programmed death - 1 (PD - 1) immune checkpoint inhibitor; 4 Demonstrated to be important for long - term remission: Melenhorst et al. 2022 Nature, Bai et al. 2022 Science Advances;

N=11 ACTengine® IMA203 TCR - T Monotherapy – Phase 1b Cohort A Patient and Product Characteristics 18 Data cut - off Apr 04, 2023 Heavily pre - treated, metastatic last - line patients that have exhausted all available standard of care treatments 1 Including ovarian cancer patient A - DL5 - 04 who erroneously received one dose of nivolumab and is part of intent - to - treat (shown h ere) but not per - protocol population; 2 T ransduced viable CD8 T cells; ULN: Upper limit of normal; LDH: Lactate dehydrogenase; BSA: Body surface area; RP2D: Recommended Phase 2 Dose DL5 cleared for safety, updated provisional RP2D comprises DL4 + DL5 : : 0.2 - 4.7 x 10 9 TCR - T cells/m 2 BSA Patients in Phase 1b Cohort A (N=11) 1 Age Mean (min, max) 55.4 (31, 79) Gender Male / Female [% of patients] 45.5 / 54.5 Prior lines of treatment Mean (min, max) 3.7 (1, 10) LDH at baseline >1 x ULN [% of patients] 54.5 Baseline tumor burden Mean target lesion sum of diameter [mm] (min, max) 73.8 (21.0, 207.3) Total infused dose Mean TCR - T cells 2 infused [x10 9 ] (min, max) 3.67 (1.30, 8.84) IMA203
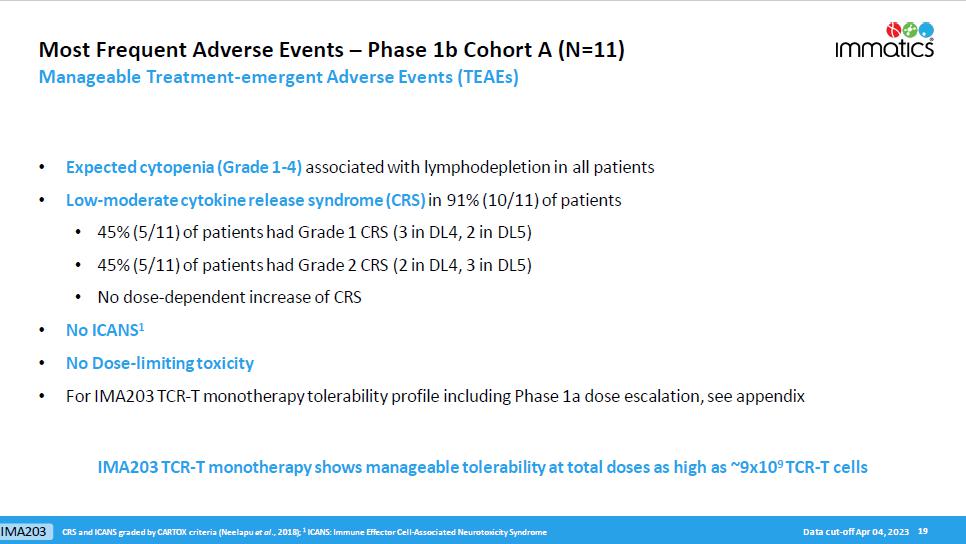
Most Frequent Adverse Events – Phase 1b Cohort A (N=11) Manageable Treatment - emergent Adverse Events (TEAEs) • Expected cytopenia (Grade 1 - 4) associated with lymphodepletion in all patients • Low - moderate cytokine release syndrome (CRS) in 91% (10/11) of patients • 45% (5/11) of patients had Grade 1 CRS (3 in DL4, 2 in DL5) • 45% (5/11) of patients had Grade 2 CRS (2 in DL4, 3 in DL5) • No dose - dependent increase of CRS • No ICANS 1 • No Dose - limiting toxicity • For IMA203 TCR - T monotherapy tolerability profile including Phase 1a dose escalation, see appendix 19 CRS and ICANS graded by CARTOX criteria ( Neelapu et al ., 2018); 1 I CANS: Immune Effector Cell - Associated N eurotoxicity S yndrome IMA203 TCR - T monotherapy shows manageable tolerability at total doses as high as ~9x10 9 TCR - T cells Data cut - off Apr 04, 2023 IMA203

20 Best Overall Response – Phase 1b Cohort A Deep Objective Responses Independent of Tumor Type 1 Ovarian cancer patient A - DL5 - 04 erroneously received one dose of nivolumab and is part of intent - to - treat population (shown here) but not per - protocol population ; 2 Initial ORR: Objective response rate according to RECIST 1.1 at first scan post infusion at ~week 6; 3 Confirmed ORR ( cORR ): Confirmed objective response rate according to RECIST 1.1 for patients with available second scan post infusion at ~month 3 o r patients with progressive disease (PD) at any timepoint before this scan; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR : Confirmed Partial Response; BL: Baseline; BOR: Best Overall Response; NET: Neuroendocrine Tumor ; CPI: Checkpoint Inhibitor • Responses observed in cutaneous and uveal melanoma, synovial sarcoma, head and neck cancer, and ovarian cancer • Initial responses at week 6 were confirmed in all 6 responders with available subsequent 3 - month scan • All cut. melanoma patients were CPI - refractory • All ovarian cancer patients were platinum - resistant ORR (at ~week 6) 2 64% (7/11) cORR (at ~month 3) 3 67% (6/9) Deep objective responses observed across multiple, heavily pre - treated tumor types Data cut - off Apr 04, 2023 1 IMA203

Response over Time – Phase 1b Cohort A Durable Partial Responses 9+ Months after IMA203 TCR - T Treatment 21 ** Ovarian cancer patient A - DL 5 - 04 erroneously received one dose of nivolumab and is part of intent - to - treat population (shown here) but not per - protocol population ; 1 Duration of response (DOR) in confirmed responders is defined as time from first documented response until disease progression/death . Patients with ongoing response will be censored at date of data cut - off . Median DOR is analyzed by using the Kaplan - Meier method ; 2 Median Follow - up is analyzed by using the reverse Kaplan - Meier method ; PD : Progressive Disease ; SD : Stable Disease ; PR : Partial Response ; cPR : Confirmed Partial R esponse ; BL : Baseline Median time from IMA203 TCR - T infusion to onset of response was 1.4 months Ongoing responses in 5 of 7 responders: • 2 cPRs (cut. & uveal melanoma) ongoing at 9+ months • 1 cPR (cut. melanoma) ongoing at 6+ months • 1 cPR (ovarian cancer) ongoing at ~3 months • 1 PR (synovial sarcoma) ongoing at 6+ weeks Median DOR 1 , min, max DOR Not reached, 1.3+, 8.8+ months Median Follow - up 2 8.5 months Scans at approximately week 6, month 3 and then every 3 months Ongoing * ** * Response until 5.7 months post infusion, target lesion response assessment not available (external assessment) Data cut - off Apr 04, 2023 IMA203

Biological Data Consistent with Clinical Data IMA203 TCR - T Levels and Tumor Infiltration across Patients in Phase 1a and Phase 1b Cohort A 22 IMA203 T cells found in all evaluable tumor tissues, level of infiltration associated with objective responses 1 Increased levels of IMA203 T cells in the blood of patients in Cohort A following increase of cell dose and switch to monocyte depletion process Data cut - off Apr 04, 2023 Mann - Whitney U test; 1 T cell infiltration for 21 patients (10 non - responder, 11 responder) with 6 - week post infusion biopsy available (1 patient with ~4 - week, 2 patients with ~13 - week post infusion biopsy); PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR : Confirmed Partial R esponse p=0.0003 Phase 1a Cohort A 0 5×10 5 1×10 6 1.5×10 6 2×10 6 I M A 2 0 3 T c e l l P e a k e x p a n s i o n ( C m a x ) [ c o p i e s / μ g g D N A ] PR cPR PD/SD <0.0001 (n=11)(n=27) Vector copies/µg gDNA p<0.0001 0 4 8 1 2 1 6 1×10 -1 1×10 0 1×10 1 1×10 2 1×10 3 1×10 4 1×10 5 1×10 6 1×10 7 2 0 4 0 6 0 8 0 1 0 0 2 0 0 4 0 0 6 0 0 8 0 0 Days post infusion V e c t o r c o p i e s / μ g g D N A N=38 Phase 1A Cohort A Persistence over time Peak persistence N=38 IMA203

Favorable TCR - T Product Characteristics and High TCR - T Levels in Patients Manufacturing Improvements Implemented in Phase 1b Enhance Key Features of the Cell Product 23 Manufacturing success rate of 94% to reach provisional RP2D ** Mean cell d ose infused in 11 patients in Phase 1b Cohort A was 3.67x10 9 TCR - T cells Prior versions (n=26) Manufacturing process (infused products) MD process (n=12) Prior versions (n=26) MD process (n=12) MD process: Monocyte depletion process; * Unpaired t test; # Mann - Whitney U test; ** Updated provisional RP2D comprises DL4 + DL5: 0.2 - 4.7x10 9 transduced viable CD8 T cells/m 2 BSA; Increased peak TCR - T levels in patients Improved TCR - T product features 0 5×10 5 1×10 6 1.5×10 6 2×10 6 I M A 2 0 3 T c e l l P e a k e x p a n s i o n ( C m a x ) [ c o p i e s / μ g g D N A ] Current Process (n=12) <0.0001 Prior Versions (n=26) IMA203 T cell peak frequency [vector copies/µg gDNA] MD process (n=12) Prior versions (n=26) MD process (n=5) Prior versions (n=7) p<0.0001 # p=0.0025 # DL4 only, normalized to cell dose 0 20 40 60 80 100 % C D 3 + C D 8 + C e l l s ( o f v i a b l e c e l l s ) p=0.0032 0 20 40 60 80 100 % D e x t r a m e r ( o f C D 3 + C D 8 + C e l l s ) p<0.0001 Normalized peak frequency [vector copies per µg gDNA/10 9 TCR - T cells] Data cut - off Apr 04, 2023 * * IMA203

Responses above Immatics’ PRAME RNA Threshold Independent of Tumor Type Highlighting Tumor Types (left) and Type of Best Overall Response (right) – Phase 1b Cohort A 24 Mann - Whitney U test, p=0.23; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR : Confirmed Partial Response; NET: Neuroendocrine Tumor PRAME RNA expression in pre - treatment biopsies relative to threshold IMA203 has the p otential to provide clinical benefit for all PRAME biomarker - positive cancer patients IMA203 achieved objective responses at all expression levels above Immatics’ mass spectrometry - guided RNA threshold A - DL5 - 01 A - DL4 - 04 A - DL4 - 05 A - DL5 - 02 A - DL5 - 04 A - DL4 - 03 A - DL5 - 05 A - DL4 - 01 A - DL5 - 03 A - DL4 - 02 A - DL5 - 06 Data cut - off Apr 04, 2023 Threshold Threshold IMA203

Potential of IMA203 in Additional Solid Cancer Indications Based on PRAME Expression in IMA203 TCR - T Responders – Phase 1b Cohort A 25 Immatics’ proprietary mass spectrometry - guided mRNA threshold 100% 100% 95% 100% 90% (50% 2 ) 80% 60% 65% 25% % PRAME - positive patients 1 PRAME target expression distribution (blue histogram) based on TCGA RNAseq data, patient data (black dots) based on IMADetect ® q PCR testing of screening biopsies; 1 PRAME target prevalence is based on TCGA RNAseq data combined with a proprietary MS - guided RNA expression threshold; 2 PRAME target prevalence in uveal melanoma based on IMADetect® qPCR testing of screening biopsies from clinical trial patients ( n=21) demonstrates substantial higher prevalence of 90% compared to prevalence based on TCGA data of 50%, TCGA: early & late - stage primary tumor samples, Immatics clinical trials: late - stage/metastatic tumor samples, Role of PRAME in metastasis of uveal melanoma: Field et al. 2016 Clinical Cancer Research; MS: mass spectrometry PRAME mRNA expression in Phase 1b Cohort A responders Data cut - off Apr 04, 2023 Selected indications A - DL4 - 03 A - DL5 - 03 A - DL5 - 06 A - DL5 - 01 A - DL4 - 02 A - DL5 - 05 A - DL4 - 01 IMA203

ACTengine® IMA203 TCR - T Monotherapy Targeting PRAME Summary of Phase 1b Cohort A Interim Data Update • Manageable tolerability with no high - grade CRS, no ICANS in 11 patients in Cohort A 1 • Objective responses observed in heavily pre - treated last - line solid cancer patients including checkpoint - refractory cutaneous melanoma, platinum - resistant ovarian cancer, uveal melanoma, head and neck cancer, synovial sarcoma • High objective response rate (ORR): • 64% (7/11) ORR (at ~week 6) • 67% (6/9) cORR (at ~month 3) • Ongoing durable responses: • Median duration of response not reached at a median follow - up time of 8.5 months • Ongoing PRs 9+ months after IMA203 TCR - T treatment • Objective responses independent of tumor type at low, medium and high PRAME levels above threshold • Manufacturing success rate of 94% to reach current RP2D, rapid 7 - day manufacturing process (+7 - day release testing) 26 Increased confidence in the success and broad potential of targeting PRAME and our product candidate IMA203 TCR - T 1 For IMA203 TCR - T monotherapy tolerability profile including Phase 1a dose escalation, see appendix; CRS: Cytokine Release Syndrome; ICANS: Immune effector cell - associated neurotoxicity syndrome; RP2D: provisional recommended Pha se 2 dose Data cut - off Apr 04, 2023 IMA203

Immatics’ ACTengine® IMA203 TCR - T Development Strategy Two - Pillared Strategy 27 Objective: Expand development to other cancer types • Signal finding in other cancer types with a broad patient reach, such as ovarian cancer, uterine cancer, lung cancer, breast cancer, head and neck cancer GO BROAD Next update on all three IMA203 Phase 1b cohorts including the projected clinical development path for PRAME - targeted TCR - T monotherapy towards registration - directed trials is planned for 4Q 2023 IMA203 Objective: Deliver best - in - class therapy in 1 - 2 last - line solid cancer types as fast as possible • Focus on cutaneous melanoma, uveal melanoma and potentially other tumor types with high PRAME prevalence where clinical proof - of - concept has been demonstrated • Highly modular and scalable manufacturing facility expected to be operational in 2024 to support efforts to maximize speed to market • Planned start of a first Phase 2 trial in 1H 2024 – targeted to be already registration - directed FAST & FOCUSED

ACTengine® IMA203 TCR - T Product Manufacturing Enhancing Manufacturing Process and Capabilities Leukapheresis Rapid Manufacturing Process 1 week Expedited QC Release Testing 1 week Infusion - ready Manufacturing of ACTengine® IMA203 TCR - T & other future autologous /allogeneic candidates Expected to be operational in 2024 Approx. 100,000 sq ft in Houston area, TX – modular and flexible design Early - stage and registration - directed clinical trials as well as initial commercial supply State - of - the - art research & GMP manufacturing facility Short manufacturing turnaround time 28 IMA203

Selected Indications Incidence R/R Incidence PRAME Positive Patient Population Based on R/R Incidence; PRAME and HLA - A*02:01+ Cut. Melanoma 99,800 7,700 95% 2,999 Uveal Melanoma 1,500 800 90% 295 Ovarian Carcinoma 19,900 12,800 80% 4,198 Uterine Carcinoma 62,700 10,700 100% 4,387 Uterine Carcinosarcoma 3,300 1,900 100% 779 Squamous NSCLC 57,000 34,600 65% 9,221 Small Cell Lung Cancer 31,900 19,400 55% 4,375 Adeno NSCLC 91,200 55,300 25% 5,668 HNSCC 66,500 15,100 25% 1,548 Breast Carcinoma 290,600 43,800 25% TNBC: 60% 4,490 Synovial Sarcoma 1,000 400 100% 164 Cholangiocarcinoma 8,000 7,000 35% 1,005 IMA203 TCR - T Has the Potential to Reach a Large Patient Population ~39,000 Patients per Year in the US only 29 Incidences based on public estimates and Immatics internal model; Relapsed/refractory (R/R) or last - line patient population approximated by annual mortality; Estimated 41% HLA - A*02:01 positive population in the US; PRAME target prevalence is based on TCGA (for SCLC: in - house) RNAseq data combined with a proprietary mass spec - guided RNA expression threshold ; Uveal melanoma target prevalence is based on IMADetect® qPCR testing of screening biopsies from clinical trial patients (n= 21) Multiple opportunities to broaden patient reach and patient benefit: » Expand beyond US population » Expand into other indications such as kidney, esophageal, bladder, other liver cancers, other sarcoma subtypes through indication - specific or indication - agonistic label expansion » Move into earlier lines of therapy (R/R Incidence Incidence ) » Inclusion of patients with lower PRAME - threshold TOTAL ~ 39 ,000 annually in the US IMA203

ACTengine® IMA203CD8 – Next - generation TCR - T Building on First - Gen IMA203 Success to Further Improve Anti - Tumor Activity • Engagement of CD4 T cells by CD8 co - transduction reported to boost anti - tumor activity in TCR - T trials • Recent data from leukaemia patients treated with CAR - T suggest a relevant role of engineered CD4 T cells in maintaining durable tumor responses over a long period of time 1 • Functional superiority of the CD8 αβ construct over multiple other CD8 constructs in preclinical experiments • Proprietary 4 - in - 1 lentiviral vector to engineer CD4 and CD8 T cells with the PRAME - specific IMA203 TCR and CD8 αβ construct (IMA203CD8) TUMOR CELL DEATH CD4 T CELL Cytotoxic Activity CD8 T CELL T cell Help Cytotoxic Activity 30 1 M elenhorst et al. 2022 Nature , Bai et al. 2022 Science Advances IMA203CD8

ACTengine® IMA203CD8 – Preclinical Assessment of Anti - Tumor Efficacy Functional CD4 T cells Mediate Longer Anti - Tumor Activity than CD8 T cells in vitro 31 0 50 100 150 200 250 300 350 400 450 0.0 0.5 1.0 1.5 2.0 CD8 Hours after Coculture T u m o r f o l d g r o w t h 0 50 100 150 200 250 300 350 400 450 0.0 0.5 1.0 1.5 2.0 CD4 Hours after Coculture T u m o r f o l d g r o w t h 2 nd addition of tumor cells 3 rd 4 th 5 th 6 th 2 nd addition of tumor cells 3 rd 4 th 5 th 6 th IMA203CD8 Engagement of CD4 T cells may enhance depth and durability of anti - tumor response and clinical outcome of TCR - T in solid cancer patients

ACTengine® IMA204 – TCR - T Targeting COL6A3 Exon 6 32

ACTengine® IMA204 First - in - Class TCR - T Targeting Tumor Stroma Key Features 33 HLA - A*02 - presented peptide derived from COL6A3 exon 6 Naturally and specifically presented on tumors at high target density 1 : 100 - 700 copies/cell Novel tumor stroma target identified and validated by XPRESIDENT® quant. mass spectrometry platform High - affinity, specific TCR targeting COL6A3 exon 6 Affinity - maturated, CD8 - independent TCR High functional avidity 2 : ~0.01ng/ml Identified and characterized by XCEPTOR® TCR discovery and engineering platform CD8 - independent, next - generation TCR engages both, CD8 and CD4 T cells In vitro anti - tumor activity against target - positive cell lines in CD8 and CD4 T cells Complete tumor eradication in in vivo mouse models Pancreatic Carcinoma – 80% Breast Carcinoma – 75% Stomach Carcinoma – 65% Sarcoma – 65% Esophageal Carcinoma – 60% Squamous NSCLC – 55% Adeno NSCLC – 55% HNSCC – 55% Uterine Carcinosarcoma – 55% Colorectal Carcinoma – 45% Mesothelioma – 45% Cholangiocarcinoma – 40% Ovarian Carcinoma – 40% Melanoma – 35% Bladder Carcinoma – 35% 1 Target density: peptide copy number per tumor cell, approximate range representing the majority of tumor samples analyzed; 2 Functional avidity: EC50 half maximal effective concentration; 3 Solid cancer indications with 20% or more target expression, Target prevalence for selected cancer indications based on mRNA expression (TCGA and Immatics inhouse data) TARGET TCR PREC LINICAL DATA PATIENT POPULATION 3 IMA204 provides a promising therapeutic opportunity for a broad patient population as monotherapy or in combination with TCR - T cells directed against tumor targets IMA204

ACTengine® IMA204 – High Affinity, CD8 - independent TCR Complete Tumor Eradication in vitro & in vivo 1 by Affinity - enhanced IMA204 TCR CD8 - independent TCR leads to tumor eradication in all mice treated 34 Control IMA204 TCR D7 D16 D22 D29 Affinity maturated CD8 - independent, next - generation TCR engages both CD4 and CD8 T cells without the need of CD8 co - transduction Stroma cells Tumor cells Stroma Target (COL6A3 exon 6) in Ovarian Cancer sample Example of a Tumor Target in same Ovarian Cancer sample 1 In vivo data in collaboration with Jim Riley, University of Pennsylvania, control: non - transduced T cells. TCR avidity and specificity d ata not shown, available in IMA204 presentation on Immatics website. COL6A3 exon 6 prevalently expressed at high target density in tumor stroma across many solid cancers IMA204

ACTallo® – Our Next - generation Off - the - shelf TCR - T 35

ACTallo® – Immatics’ Allogeneic Cell Therapy Approach • Off - the - shelf cell therapy , no need for personalized manufacturing reduced logistics and time to application • Potential for hundreds of doses from one single donor leukapheresis lower cost of goods • Use of healthy donor material provides standardized quality and quantity of starting material • Strategic collaborations combining Immatics’ proprietary ACTallo ® platform with Bristol Myers Squibb’s next - gen technologies and Editas Medicine’s CRISPR gene editing technology to develop next - gen allogeneic γδ TCR - T/CAR - T programs 36 ACTallo® γδ T cell Cell Engineering (gene editing & armoring ) γδ T cell Collection from Healthy Donor Expansion Off - the - shelf Products Patient Treatment

Why γδ T cells? γδ T cells Are Well Suited for an Off - the - shelf Cell Therapy Approach 37 γδ T cells x are abundant in the peripheral blood x show intrinsic anti - tumor activity x naturally infiltrate solid tumors & correlate with favorable prognosis x are HLA - independent, thus do not cause graft - vs - host disease in allogeneic setting x can be expanded to high numbers in a cGMP - compatible manner x can be effectively redirected using αβ TCR or CAR constructs In vitro a nti - tumor activity 0 48 96 144 192 0 5 10 15 Hours F o l d G r o w t h ( U 2 0 S - R F P + ) Tumor cells only T cells (NT) T cells IMA203 TCR + T cells (NT) T cells IMA203 TCR + γδ T cells (control) + tumor cells tumor cells only αβ T cells (control) + tumor cells γδ T cells TCR + + tumor cells αβ T cells TCR + + tumor cells ACTallo® 0 5 10 15 20 25 0.001 0.01 0.1 1 10 100 1000 10000 100000 1000000 Day F o l d e x p a n s i o n o f T c e l l s Expansion Fold - growth (target - positive tumor cells)

TCER® – TCR Bispecifics 38

TCER® – Immatics ’ Next - generation, Half - Life Extended Bispecifics Proprietary TCER® Format Consisting of Three Distinct Elements 39 High - affinity TCR domains targeting XPRESIDENT® - selected tumor - specific peptide - HLA molecules Low - affinity T cell recruiter against CD3/TCR Fc part for half - life extension, favorable stability and manufacturability Next - gen, half - life extended TCER® format designed to safely apply high drug doses for activity in a broad range of tumors achieve optimized scheduling 2 1 3 Cytotoxic lytic granules T umor cell killing A ctivated T cell TCER®

TCER® – Immatics’ Next - generation, Half - Life Extended Bispecifics 40 pHLA targeting TCR x High - affinity (single digit nM ) TCR targeting XPRESIDENT® - selected tumor - specific peptide - HLA molecules x Broad therapeutic window through XPRESIDENT® - guided affinity maturation (>1000x) 1 x Complete tumor eradication in mouse xenograft models at low doses T cell recruiting antibody x Low - affinity (triple digit nM ) T cell recruiter against both TCR & CD3 x Optimized biodistribution aiming for enrichment at tumor site and prevention of CRS 2 x Superior anti - tumor activity in mouse models as compared to widely used CD3 recruiters Next - generation TCER® format x Off - the - shelf b iologic with antibody - like manufacturability 3 and low cost of goods x Superior anti - tumor activity 4 compared to six alternative bispecific formats x Half - life of several days expected in humans Our TCER® format is designed to maximize efficacy while minimizing toxicities in patients 1 As compared to natural TCR; 2 Based on literature data for other low - affinity recruiters (e.g. Harber et al ., 2021, Nature; Trinklein et al ., 2019, mAbs ) ; 3 Production in mammalian cells (CHO cells); 4 Based on preclinical testing TCER® 1 2 3

Potency of Our Proprietary TCR Bispecific Format TCER® 41 • Seven different TCR Bispecific formats were evaluated with a pHLA targeting TCR and the identical T cell recruiting antibody • TCER® format had higher combination of potency and specificity 1 than six alternative TCR Bispecific format designs evaluated Flexible Plug - and - play platform: TCER® format successfully validated for different TCRs & different T cell recruiting antibodies TCER® TCER® 2+1 TCR bispecific format: High potency was linked to a significantly reduced specificity profile Killing of target - positive cells by different TCR Bispecifics 1 Preclinical data on specificty not shown

TCER® Format Is Designed for Optimized Efficacy and Safety Superior Tumor Control Using a Novel, Low - Affinity Recruiter 42 Widely used T cell recruiting Ab (3 variants) medium to high affinity (single to double digit nM ) n = 6 mice/treatment group, n = 10 mice in vehicle group, 2 donors/group Dose: 0.025 mg/kg Proprietary, low - affinity T cell recruiting region demonstrates superior tumor control compared to analogous TCER® molecules designed with higher - affinity variants of a widely used recruiter Immatics’ T cell recruiting Ab low affinity (triple digit nM ) TCER® Tumor Model in Mice 1 1 Hs695T xenograft model in NOG m ice , tumor volume of group means shown

TCER® Format Is Designed for Optimized Efficacy and Safety Reduced Target - Unrelated Recruiter - Mediated Cytokine Release using a Low - Affinity Recruiter 43 TCER® Whole blood cytokine release assay N= 3 HLA - A*02 - positive donors N=16 cytokines tested, 4 exemplary cytokines shown

Our TCER® Portfolio Broad Pipeline of Next - Gen Half - Life Extended TCR Bispecifics 44 TCER® • PRAME peptide presented by HLA - A*02:01 • Start of clinical trial in Aug 2023, first clinical data expected 2024 IMA402 Potential for addressing different indications and large patient populations with novel, off - the - shelf TCR Bispecifics • MAGEA4/8 peptide presented by HLA - A*02:01 • Dose escalation ongoing IMA401 • Undisclosed peptides presented by HLA - A*02:01 and other HLA - types • TCER® engineering and preclinical testing ongoing IMA40x Several innovative programs CLINICAL PRECLINICAL

TCER® IMA401 Targeting MAGEA4/8 Homogeneous Expression, Broad Prevalence and High Copy Number Target 45 MAGEA4 RNA detection in tumor samples (ISH) Indications Target p revalence [%] Squamous non - small cell lung carcinoma 50% Head and neck squamous cell carcinoma 35% Bladder carcinoma 30% Uterine carcinosarcoma 25% Esophageal carcinoma 25% Ovarian carcincoma 20% Melanoma 20% plus several further indications MAGEA4/8 target prevalence in selected cancer indications MAGEA4/8 target prevalences are based on TCGA data combined with a XPRESIDENT® - determined target individual MS - based mRNA expression threshold; 1 Copy number per tumor cell (CpC) measured on a paired - sample basis by AbsQuant®, i.e. comparing MAGEA4 vs. MAGEA4/A8 peptide presentation on same sample, 2 Students paired T test IMA401 p<0.001 2 MAGEA4/8 target is presented at >5 - fold higher target density 1 than a commonly used MAGEA4 target peptide

TCER® IMA401 (MAGEA4/8) – Assessment of Anti - Tumor Activity in vitro Patient - Derived Tumor Model 46 NSCLC adenocarcinoma : • M ale, Caucasian, age 58, no therapy prior to surgery • Site of origin: lung, differentiation poor • Date of surgery: 1987, Freiburg Medical Center • Volume doubling time: 7.3 day • Histology: • Stroma content, 4% • Vascularization, high • Grading, undifferentiated • TCER® IMA401 shows high anti - tumor activity in Patient - derived xenograft model of non - small cell lung adenocarcinoma • Remission observed in all mice (3 out of 4 mice with complete remission) LXFA 1012 Tumor Xenograft Model in NOG Mice IMA401

47 TCER® IMA401 (MAGEA4/8) – Pharmacokinetics PK Analysis in NOG Mice • Two different PK assays established to ensure functional integrity of protein domains • Terminal half - life in mice: 10 - 11 days pHLA – V L Assay Fc – V L Assay IMA401

Phase 1 Clinical Trial to Evaluate TCER® IMA401 Targeting MAGEA4/8 48 MTD: maximum tolerated dose, RP2D: recommended phase 2 dose; MABEL: minimum anticipated biological effect level; BLRM: Bayesian logistic regression model; 1 P harmacokinetics data assessed throughout the trial might provide an opportunity to optimize scheduling to a less frequent regimen. 2 Conducted in collaboration with BMS Phase 1a: Dose Escalation Phase 1b: Dose Expansion • Weekly i.v. infusions 1 • Dose escalation decisions based on cohorts of 1 - 6 patients in adaptive design (BLRM model) MTD/ RP2D Adaptive design aimed at accelerating dose escalation • Focus on specific indications planned Potential development option for checkpoint inhibitor combination or other combination therapies 2 Monotherapy expansion cohort Primary Objective • Determine MTD and/or RP2D Secondary Objectives • Safety and tolerability • Initial anti - tumor activity • Pharmacokinetics IMA401

TCER® IMA402 Targeting PRAME – Efficacy Assessment in vitro Tumor Cell Killing at Low Physiological PRAME Peptide Levels 49 0 20 40 60 80 100 120 140 10 -1 10 0 10 1 10 2 10 3 10 4 10 5 IMA402 [pM] Cytotoxicity [%] ~50 PRAME CpCs 0 0 20 40 60 80 100 120 140 10 -1 10 0 10 1 10 2 10 3 10 4 10 5 IMA402 [pM] Cytotoxicity [%] Target-negative 0 0 20 40 60 80 100 120 140 10 -1 10 0 10 1 10 2 10 3 10 4 10 5 IMA402 [pM] Cytotoxicity [%] ~110 PRAME CpCs 0 0 20 40 60 80 100 120 140 10 -1 10 0 10 1 10 2 10 3 10 4 10 5 IMA402 [pM] Cytotoxicity [%] ~250 PRAME CpCs 0 • TCER® IMA402 induces killing of tumor cells with PRAME target copies as low as 50 CpCs • Physiological PRAME levels detected in majority of cancer tissues from patients are 100 – 1000 CpCs • Preclinical activity profile enables targeting of a broad variety of tumor indications, such as lung cancer, breast cancer, ovarian cancer, uterine cancer, melanoma and others IMA402 CpC: Target peptide copy numbers per tumor cell

TCER® IMA402 Achieves Durable Tumor Control of Large Tumors in vivo 50 -29 0 500 1000 1500 2000 2500 0 10 20 30 40 50 60 70 Study day M e d i a n t u m o r v o l u m e [ m m 3 ] Vehicle IMA402 [0.01 mg/kg] IMA402 [0.05 mg/kg] IMA402 [0.25 mg/kg] • Dose - dependent efficacy of IMA402 in cell line - derived in vivo mouse model • Durable shrinkage of large tumors including complete responses over prolonged period • Sufficiently high drug doses are key to achieving desired anti - tumor effect IMA402

Half - life Extended Format of IMA402 Confers Terminal Half - life of >1 Week 51 pHLA – aV L Assay pHLA – aFc Assay • IMA402 shows a terminal serum half - life of ≈ 8 days in mice • IMA402 will be initially dosed weekly in the clinical trial • Dosing frequency may be adapted based on clinical data IMA402
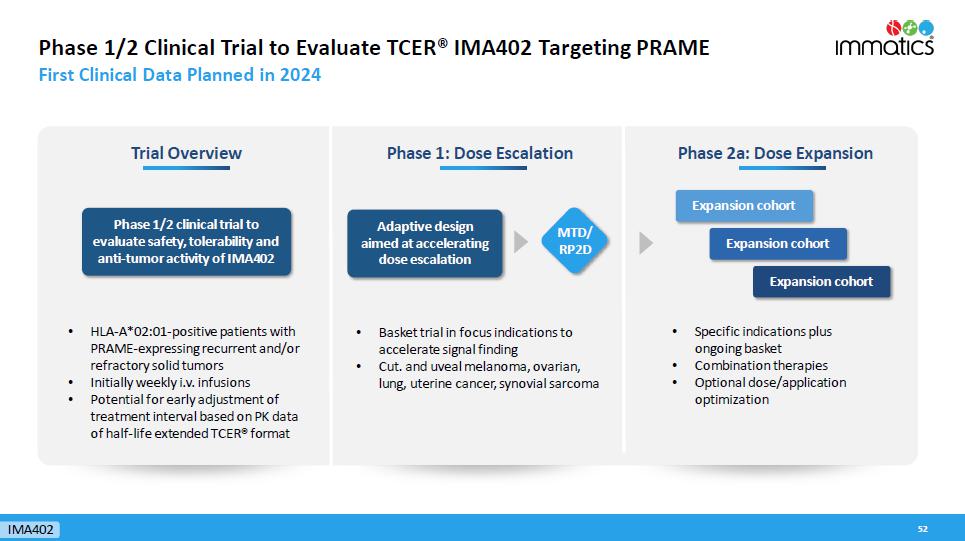
Phase 1/2 Clinical Trial to Evaluate TCER® IMA402 Targeting PRAME First Clinical Data Planned in 2024 52 Phase 1: Dose Escalation Phase 2a: Dose Expansion Adaptive design aimed at accelerating dose escalation • Specific indications plus ongoing basket • Combination therapies • Optional dose/application optimization Expansion cohort Expansion cohort Expansion cohort Trial Overview Phase 1/2 clinical trial to evaluate safety, tolerability and anti - tumor activity of IMA402 • HLA - A*02:01 - positive patients with PRAME - expressing recurrent and/or refractory solid tumors • Initially weekly i.v. infusions • Potential for early adjustment of treatment interval based on PK data of half - life extended TCER® format MTD/ RP2D IMA402 • Basket trial in focus indications to accelerate signal finding • Cut. and uveal melanoma, ovarian, lung, uterine cancer, synovial sarcoma

Immatics’ Proprietary Target and TCR Discovery Platforms 53
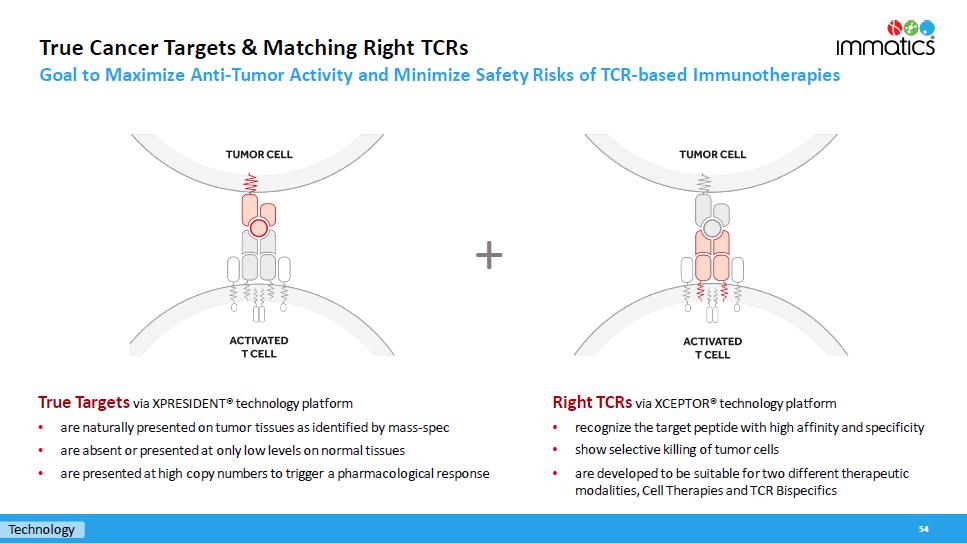
True Cancer Targets & Matching Right TCRs Goal to Maximize Anti - Tumor Activity and Minimize Safety Risks of TCR - based Immunotherapies 54 True Targets via XPRESIDENT® technology platform • are naturally presented on tumor tissues as identified by mass - spec • are absent or presented at only low levels on normal tissues • are presented at high copy numbers to trigger a pharmacological response + Technology Right TCRs via XCEPTOR® technology platform • recognize the target peptide with high affinity and specificity • show selective killing of tumor cells • are developed to be suitable for two different therapeutic modalities, Cell Therapies and TCR Bispecifics

Technology Pool of 200 Prioritized Targets as Foundation for Future Value Generation XPRESIDENT® Target Platform 55 200 Prioritized Targets Grouped in 3 Target Classes: 1. Well known and characterized parent protein (20%) e.g. MAGE family cancer testis antigens 2. Unknown or poorly characterized parent protein (60%) e.g. stroma target COL6A3 exon 6 3. Crypto - targets/Neoantigens (20%) Novel target class which includes RNA - edited peptides & non - classical neoantigens ~50% of our prioritized targets are non - HLA - A*02 restricted, substantially broadening the potential patient reach >2,500 cancer & normal tissues a nalyzed by Quantitative, Ultra - Sensitive Mass Spectrometry pHLA Database based on primary tissues >200 prioritized targets This large data set is leveraged by our bioinformatics & AI - platform XCUBE Ρ – „AI is where the data is ®“

Immatics ’ Unique Capability – Identification of the most Relevant Target Example of MAGEA4/8 Peptide Target 56 1 Copy number per tumor cell (CpC) measured on a paired - sample basis by AbsQuant®, i.e. comparing MAGEA4 vs. MAGEA4/A8 peptide pre sentation on same sample, 2 Students paired T test p<0.001 2 Technology MAGEA4/8 target is presented at >5 - fold higher target density 1 than a commonly targeted MAGEA4 target peptide XPRESIDENT® quantitative information on target density 1 between peptides originating from the same source protein Ranking of pHLA targets Commonly targeted

Development of the Right TCR – XCEPTOR® Technology TCR Discovery and Engineering for ACT and TCR Bispecifics 57 TCR Bispecifics T cell engaging receptor (TCER®) Adoptive Cell Therapy ACTengine® ACTallo ® • Fast, efficient and highly sensitive discovery of highly specific, natural TCRs • Protein engineering capabilities to design and maturate TCRs with increased affinity while retaining specificity • Early de - selection of cross - reactive TCRs by the u nique interplay between Immatics’ target and TCR discovery platforms XPRESIDENT® and XCEPTOR® during TCR discovery 1 and TCR maturation 2 (empowered by our bioinformatics & AI - platform XCUBE Ρ ) Micromolar affinity Nanomolar affinity Technology 1 XPRESIDENT® - guided off - target toxicity screening; 2 XPRESIDENT® - guided similar peptide counterselection

Optimal Target Selection & TCR Specificity for Minimizing Safety Risks Unique Interplay between Technology Platforms Allows Early De - risking for Clinical Development 58 Target peptide presented on tumor cells Selective killing of tumor cells Target peptide presented on normal cells Off - target toxicity On - target (off - tumor) toxicity A different HLA is recognized on normal cells Alloreactivity Similar peptide presented on normal cells 1 XPRESIDENT® - guided screening for on - and off - target toxicities of TCRs based on the extensive database of peptides presented on normal tissues Technology 1 Clinical fatalities have occurred in TCR - T trials using a titin cross - reactive TCR (Cameron et al ., Sci Transl Med)

“AI Is Where the Data Is®” Bioinformatics and AI - Platform XCUBE Ρ 59 Data Engineering Development of data warehouses & user interfaces Data Science Development of statistical & machine learning models Data Processing Processing of mass - spec & next - gen sequencing data 1 THERAPEUTIC KNOWLEDGE XPRESIDENT®/ XCEPTOR® DATA Data Engineering Data Science Data Processing 2 3 1 Cell therapies Bispecifics CDx Therapies Targets Lead Molecules Discovery Characterization Discovery Selection Validation 2 3

Robust IP Portfolio Immatics’ Patent Estate – Territorial Coverage 60 Cancer targets, TCRs and technology protected by: • 5,800 applications and patents filed in all major countries and regions • >115 patent families • >2,400 granted patents, thereof >550 granted patents in the US Technology

Corporate Information & Milestones 61

David Leitner Schuldirektor David Leitner Schuldirektor David Leitner Schuldirektor Harpreet Singh Chief Executive Officer Co - Founder >20 yrs biotech experience Arnd Christ Chief Financial Officer >20 yrs biotech experience ( InflaRx , Medigene , NovImmune , Probiodrug ) Carsten Reinhardt Chief Development Officer >20 yrs pharma & biotech experience ( Micromet , Roche, Fresenius) Cedrik Britten Chief Medical Officer 15 yrs pharma & biotech experience (GSK, BioNTech) Rainer Kramer Chief Business Officer 25 yrs pharma & biotech experience (Amgen, MorphoSys , Jerini , Shire, Signature Dx) Steffen Walter Chief Operating Officer Co - Founder Immatics US >15 yrs biotech experience Edward Sturchio General Counsel >15 yrs pharma & biotech experience ( Abeona Therapeutics, AAA, Novartis, Merck, Schering) ) Jordan Silverstein Head of Strategy >10 yrs biotech experience ( InflaRx , AAA) Toni Weinschenk Chief Innovation Officer Co - Founder >15 yrs biotech experience Experienced Global Leadership Team Across Europe and the US Corporate

Strong, Focused and Highly Integrated Trans - Atlantic Organization 63 Houston, Texas ~ 150 FTEs Cell therapy development & manufacturing Munich, Germany ~ 65 FTEs Various operating functions Tübingen, Germany ~ 215 FTEs Target & TCR discovery and TCR Bispecifics developme nt Corporate FTE status as of December 2022

Delivering s the Power of T cells to Cancer Patients © Immatics. Not for further reproduction or distribution. www.immatics.com Appendix

ACTengine® IMA203 TCR - T 1 st Gen Monotherapy Tolerability Data Focus on IMA203 Phase 1b Cohort A – All ≥Grade 3 Adverse Events (N=11) 65 • IMA203 was well tolerated • No Adverse Event ≥Grade 3 was observed with a frequency ≥10% when excluding expected cytopenias associated with lymphodepletion • No IMA203 - related Grade 5 Adverse Events All treatment - emergent adverse events (TEAEs) with ≥ Grade 3 regardless of relatedness to study treatment that occurred in at least 1 patient (except for CRS and ICANS , where only Grade 1 - 2 occurred ; listed for completeness due to being adverse events of special interest) are presented . Adverse events were coded using the Medical Dictionary for Regulatory Activities . Grades were determined according to National Cancer Institute Common Terminology Criteria of Adverse Events, version 5 . 0 . Grades for CRS and ICANS were determined according to CARTOX criteria (Neelapu et al . , 2018 ) . Patients are counted only once per adverse event and severity classification . Based on interim data extracted from open clinical database ( 04 - Apr - 2023 ) . 1 I CANS : Immune effector cell - associated neurotoxicity syndrome . Data cut - off Apr 04, 2023 Adverse event ( System organ class , Preferred term ) ≥ Grade 3 No . % Patients with any adverse event 11 100.0 Adverse Events of Special Interest Cytokine release syndrome 0 0.0 ICANS 1 0 0.0 Blood and lymphatic system disorders Neutropenia 10 90.9 Lymphopenia 6 54.5 Leukopenia 5 45.5 Anaemia 5 45.5 Thrombocytopenia 4 36.4 Leukocytosis 1 9.1 Lymphocytosis 1 9.1 Adverse event ( System organ class , Preferred term ) ≥ Grade 3 No . % table continued… Investigations Alanine aminotransferase increased 1 9.1 Aspartate aminotransferase increased 1 9.1 Blood alkaline phosphatase increased 1 9.1 Eye disorders Ulcerative keratitis 1 9.1 Gastrointestinal disorders Ileus 1 9.1 Infections and infestations Infection 1 9.1 Nervous system disorders Headache 1 9.1 Respiratory, thoracic and mediastinal disorders Laryngeal inflammation 1 9.1 TEAEs by maximum severity for all patients in Ph1b Cohort A dose expansion ( N= 11 ) IMA203

Deep & Durable Responses in Heavily Pre - Treated Patients – Phase 1b Cohort A 66 Patient ID Indication No of prior treatment lines Prior treatments Total infused dose TCR - T cells 1 [x10 9 ] BOR BOR (Max % change of target lesions) Comment A - DL5 - 01 Uveal Melanoma 1 ARRY614/Nivolumab 4.16 cPR - 60.3 Ongoing response 10.1 months post infusion A - DL4 - 03 Cut. Melanoma 7 Dabrafenib/Trametinib, Pembrolizumab, Dabrafenib/Trametinib, Vemurafenib/ Cobimetinib , Dabrafenib/Trametinib, IMCgp - 100, Encorafenib / Binimetinib 1.30 cPR - 73.9 Ongoing response 9.9 months post infusion A - DL5 - 03 Cut. Melanoma 3 Interferon, Pembrolizumab, Nivolumab/Ipilimumab 5.12 cPR - 60.5 Ongoing response 6.2 months post infusion A - DL4 - 01 Head & Neck Cancer 1 Carboplatin/Paclitaxel 1.92 cPR - 33.3 Response until 5.7 months post infusion A - DL4 - 02 Ovarian Cancer 10 Carboplatin/Taxol, Taxol, Gemcitabine/Carboplatin, Olaparib, Letrozole, Rucaparib, UPCC 03118 (CAR - T cell directed folate receptor), Bevacizumab/Cyclophosphamide, Carboplatin, Doxorubicin 1.97 cPR - 41.0 Response until 3.8 months post infusion A - DL5 - 05 Ovarian Cancer 3 Adriamycin/Cytotaxan/Taxol, Carboplatin/Taxol, Carboplatin/Doxil 8.84 cPR - 61.7 Ongoing response 2.5 months post infusion A - DL5 - 06 Synovial Sarcoma 1 Adriamycin/Ifosfamide/Mesna 3.94 P R - 74.8 Initial PR at week 6, 3 - month scan pending A - DL4 - 04 Melanoma (Unk. Primary) 2 Nivolumab/Ipilimumab, Nivolumab 1.73 SD 0.0 Disease stabilization until 5.7 months post infusion A - DL4 - 05 Cut. Melanoma 5 Nivolumab, Nivolumab (re - exposure), Nivolumab/Ipilimumab, Dabrafenib/Trametinib, Nivolumab 1.63 SD 11.4 Ongoing disease stabilization 2.1 months post infusion A - DL5 - 02 Pancreatic Neuroendocrine Tumor 3 Lanreotid, Streptozocin/5 - Fluorouracil, Everolismus 5.12 SD - 21.8 Disease stabilization until 2.3 months post infusion A - DL5 - 04* Ovarian Cancer 5 Paclitaxel/Carboplatin, Niraparib, Doxorubicin/Liposomal/Carpoplatin, 2020 - 0808 ZN - C3/Gemcitabine, 2020 - 0755 COM 701/BMS - 986207/Nivolumab 4.68 PD 50.8 Progressive disease at 1.2 months post infusion 1 Transduced viable CD8 T cells; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR : Confirmed Partial Response; BL: Baseline; BOR: Best Overall Response * Ovarian cancer patient A - DL5 - 04 erroneously received one dose of nivolumab and is part of intent - to - treat population (shown here) but not per - protocol population. Data cut - off Apr 04, 2023 IMA203

ACTengine® IMA203 TCR - T 1 st Gen Monotherapy Tolerability Data Phase 1a and Phase 1b Cohort A – All ≥Grade 3 Adverse Events (N=39) 67 • IMA203 was well tolerated • No Adverse Event ≥Grade 3 was observed with a frequency ≥10% when excluding expected cytopenias associated with lymphodepletion • No IMA203 - related Grade 5 Adverse Events All treatment - emergent adverse events (TEAEs) with ≥ Grade 3 regardless of relatedness to study treatment that occurred in at least 1 patient (except for ICANS, where only Grade 1 - 2 occurred ; listed for completeness due to being an adverse event of special interest) are presented . Adverse events were coded using the Medical Dictionary for Regulatory Activities . Grades were determined according to National Cancer Institute Common Terminology Criteria of Adverse Events, version 5 . 0 . Grades for CRS and ICANS were determined according to CARTOX criteria ( Neelapu et al . , 2018 ) . Patients are counted only once per adverse event and severity classification . Based on interim data extracted from open clinical database ( 04 - Apr - 2023 ) ; 1 Two patients with disease progression after first IMA 203 infusion received exploratory second IMA 203 infusion . They had these ≥ Grade 3 TEAEs only after second infusion, which are included in the table : First patient : Abdominal pain, Cytokine release syndrome, Diarrhoea , Hypokalaemia , Proteinuria ; Second patient : Humerus fracture, Muscle spasms, Neutropenia, Thrombocytopenia ; 2 ICANS : Immune effector cell - associated neurotoxicity syndrome ; 3 DLT : Dose limiting toxicity in phase 1 a at DL 2 reported on March 17 , 2021 ; 4 Fatal Adverse events were not considered related to any study drug ; 5 Patient died from sepsis of unknown origin and did not receive IMA 203 TCR - T cells . Data cut - off Apr 04, 2023 Adverse event ( System organ class , Preferred term ) ≥ Grade 3 No . % Patients with any adverse event 39 100.0 Adverse Events of Special Interest Cytokine release syndrome 2 5.1 ICANS 2 0 0.0 Blood and lymphatic system disorders Neutropenia 32 82.1 Lymphopenia 24 61.5 Leukopenia 22 56.4 Anaemia 20 51.3 Thrombocytopenia 15 38.5 Cytopenia 1 2.6 Leukocytosis 1 2.6 Lymphocytosis 1 2.6 Infections and infestations Appendicitis 1 2.6 COVID - 19 1 2.6 Enterococcal infection 1 2.6 Infection 1 2.6 Orchitis 1 2.6 Sepsis 4,5 1 2.6 Septic shock 4 1 2.6 Respiratory, thoracic and mediastinal disorders Hypoxia 2 5.1 Bronchial obstruction 1 2.6 Laryngeal inflammation 1 2.6 Pleural effusion 1 2.6 Respiratory failure 1 2.6 Investigations Alanine aminotransferase increased 1 2.6 Aspartate aminotransferase increased 1 2.6 Blood alkaline phosphatase increased 1 2.6 Blood creatinine increased 1 2.6 Blood fibrinogen decreased 1 2.6 Gastrointestinal disorders Abdominal pain 1 2.6 Diarrhoea 1 2.6 Ileus 1 2.6 Vomiting 1 2.6 Adverse event ( System organ class , Preferred term ) ≥ Grade 3 No . % table continued… General disorders and administration site conditions Condition aggravated 4 1 2.6 Fatigue 1 2.6 Pyrexia 1 2.6 Swelling face 1 2.6 Vascular disorders Hypertension 3 7.7 Hypotension 1 2.6 Metabolism and nutrition disorders Hypokalaemia 2 5.1 Failure to thrive 1 2.6 Injury, poisoning and procedural complications Humerus fracture 1 2.6 Infusion related reaction 1 2.6 Renal and urinary disorders Acute kidney injury 1 2.6 Proteinuria 1 2.6 Cardiac disorders Atrial fibrillation 3 1 2.6 Endocrine disorders Inappropriate antidiuretic hormone secretion 1 2.6 Eye disorders Ulcerative keratitis 1 2.6 Hepatobiliary disorders Cholangitis 1 2.6 Immune system disorders Contrast media allergy 1 2.6 Musculoskeletal and connective tissue disorders Muscle spasms 1 2.6 Nervous system disorders Headache 1 2.6 Reproductive system and breast disorders Vaginal haemorrhage 1 2.6 Skin and subcutaneous tissue disorders Rash maculo - papular 1 2.6 TEAEs by maximum severity for all patients in Ph1a dose escalation and Ph1b Cohort A dose expansion (N=39) 1 IMA203

Phase 1a and Phase 1b Cohort A – Best Overall Response 68 Confirmed objective responses across a broad spectrum of different tumor types such as cutaneous melanoma, uveal melanoma, head and neck cancer, ovarian cancer, synovial sarcoma * Maximum change of target lesions and RECIST 1.1 BOR at different timepoints ; # Synovial sarcoma patient (DL3) PD at week 6 not shown as t arget lesions were not evaluable; 1 Indication was updated to cutaneous melanoma post data cut - off; PD: Progressive disease; SD: Stable disease; PR: Partial respon se; cPR : Confirmed partial response; BL: Baseline Phase 1a (Dose Escalation) Phase 1b (Cohort A) IMA203 N=27 # N=11 Data cut - off Sept 06, 2022 – presented in Oct 2022 Data cut - off Apr 04, 2023 – presented in May 2023 1

Phase 1a and Phase 1b Cohort A – Responses over Time Improved Durability at Higher Dose and in Phase 1b Patients 69 Phase 1a (Dose Escalation) N=27 # Phase 1b (Cohort A) N=11 Best overall response (RECIST1.1) IMA203 # Synovial sarcoma patient (DL 3 ) PD at week 6 not shown as t arget lesions were not evaluable ; 1 Ovarian cancer patient A - DL 5 - 04 erroneously received one dose of nivolumab and is part of intent - to - treat population (shown here) but not per - protocol population ; PD : Progressive Disease ; SD : Stable Disease ; PR : Partial Response ; cPR : Confirmed Partial R esponse ; BL : Baseline Scans at approximately week 6, month 3 and then every 3 months Ongoing * 1 * Response until 5.7 months post infusion, target lesion response assessment not available (external assessment) Data cut - off Sept 06, 2022 – presented in Oct 2022 Data cut - off Apr 04, 2023 – presented in May 2023

Focus on Melanoma Patients Phase 1a (DL4 only) and Phase 1b Cohort A Continuous Improvement from Phase 1a to Phase 1b Cohort A 70 Patient Characteristics (n=10) Prior lines of treatment Mean (min, max) 4.5 (1, 7) Previous lines of CPI Mean (Min, Max) 2.6 (1, 4) LDH at baseline >1 x ULN [% of patients] 60.0 Baseline tumor burden Mean target lesion sum of diameter [mm] (min, max) 66.9 (21.0, 178.7) Total infused dose Mean TCR - T cells 1 infused [x10 9 ] (min, max) 2.12 (1.07, 5.12) No. of Target - & Non - Target Lesions 60.0% with >3 lesions 40.0% with liver/brain lesions ORR 2 = 70% (7/10) cORR 3 = 56% (5/9) Median DOR 4 , min, max DOR Not reached, 2.4, 8.8+ months Median Follow - up 5 8.5 months * Maximum change of target lesions and RECIST 1 . 1 at different timepoints . 1 Transduced viable CD 8 T cells ; 2 Initial ORR : Objective response rate according to RECIST 1 . 1 at first scan post infusion at ~week 6 ; 3 Confirmed ORR ( cORR ) : Confirmed objective response rate according to RECIST 1 . 1 for patients with available second scan post infusion at ~ 3 months or patients with progressive disease (PD) at any timepoint before this scan ; 4 Duration of response (DOR) in confirmed responders is defined as time from first documented response until disease progression/death . Patients with ongoing response will be censored at date of data cut - off . Median DOR is analyzed by using the Kaplan - Meier method ; 5 Median Follow - up is analyzed by using the reverse Kaplan - Meier method ; PD : Progressive Disease ; SD : Stable Disease ; PR : Partial Response ; cPR : Confirmed P artial Response ; BOR : Best Overall Response ; BL : Baseline ; CPI : Checkpoint inhibitor ; LDH : Lactate dehydrogenase Data cut - off Apr 04, 2023 • Heavily pre - treated melanoma patients after 1 - 4 lines of CPI: Cutaneous (N=8), uveal (N=1) and melanoma of unk . primary (N=1) • Phase 1a (N=5): previous manufacturing process • Phase 1b Cohort A (N=5): new monocyte depletion process, higher dose Phase 1a Phase 1b Cohort A Best Overall Response Response over Time Phase 1a Phase 1b Cohort A IMA203

Delivering s the Power of T cells to Cancer Patients © Immatics. Not for further reproduction or distribution. www.immatics.com
Immatics NV (NASDAQ:IMTX)
Historical Stock Chart
From Sep 2024 to Oct 2024
Immatics NV (NASDAQ:IMTX)
Historical Stock Chart
From Oct 2023 to Oct 2024