Icosavax Gets Fast Track Designation for IVX-A12
February 21 2023 - 8:55AM
Dow Jones News
By Chris Wack
Icosavax Inc. said Tuesday the U.S. Food and Drug Administration
granted Fast Track designation for IVX-A12, a bivalent respiratory
syncytial virus and human metapneumovirus virus-like particle
vaccine candidate for older adults 60 years of age and above.
In October 2022, Icosavax said it had begun a Phase 1 study of
IVX-A12 in up to 120 healthy adults aged 60 to 75 years. Icosavax
expects to announce topline interim results from this Phase 1 trial
in mid-2023, with subjects thereafter followed through 12 months
after vaccination. The company plans to start a Phase 2 trial for
IVX-A12 in the second half of 2023.
The FDA's Fast Track is a process designed to facilitate the
development and expedite the review of investigational drugs to
treat serious conditions and fulfill an unmet medical need.
An investigational drug that receives Fast Track designation may
be eligible for more frequent interactions with the FDA to discuss
the candidate's development plan and, if relevant criteria are met,
eligibility for Accelerated Approval and Priority Review.
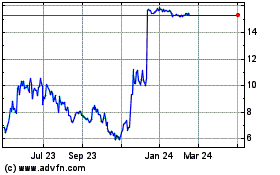
Icosavax shares were up 9% to $9.85 in premarket trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
February 21, 2023 08:40 ET (13:40 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Icosavax (NASDAQ:ICVX)
Historical Stock Chart
From Jun 2024 to Jul 2024
Icosavax (NASDAQ:ICVX)
Historical Stock Chart
From Jul 2023 to Jul 2024