As
filed with the Securities and Exchange Commission on December 23, 2024.
Registration
No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
GT
BIOPHARMA, INC.
(Exact
Name of Registrant as Specified in Its Charter)
| Delaware |
|
2834 |
|
94-1620407 |
(State
or Other Jurisdiction of
Incorporation
or Organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
Number) |
315
Montgomery Street, 10th Floor
San
Francisco, CA 94104
(415)
919-4040
(Address,
Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Michael
Breen
Interim
Chief Executive Officer
315
Montgomery Street, 10th Floor
San
Francisco, CA 94104
(415)
919-4040
(Name,
Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
Copies
to:
Roger
W. Bivans
Baker
& McKenzie LLP
1900
N. Pearl Street, Suite 1500
Dallas,
TX 75201, USA
(214)
978 3000 |
|
Charles
Phillips
Ellenoff
Grossman & Schole LLP
1345
Avenue of Americas
New
York, NY
(212)
370 1300 |
Approximate
date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
Accelerated filer |
☐ |
Accelerated
filer |
☐ |
| |
|
|
|
| Non-accelerated
filer |
☒ |
Smaller
reporting company |
☒ |
| |
|
|
|
| |
|
Emerging
growth company |
☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
registrant files a further amendment which specifically states that this registration statement shall thereafter become effective in
accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on
such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration
statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell
these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT
TO COMPLETION DATED DECEMBER 23, 2024
PRELIMINARY
PROSPECTUS
Up
to Shares of
Common Stock
Pre-Funded
Warrants to Purchase up to Shares of Common Stock
Common Warrants to Purchase up to
Shares of Common Stock
Placement Agent Warrants to Purchase up to
Shares of Common Stock
Up
to
Shares of Common Stock underlying Pre-Funded Warrants,
Common
Warrants and Placement Agent Warrants

This
is a reasonable best efforts public offering of up to shares (the
“shares”) of our common stock, par value $0.001 per share (“common stock”) together with up to
common warrants to purchase up to
shares of common stock at an assumed combined public offering price of
$ per share and common warrant (the last reported sale price per share
of our common stock on the Nasdaq Capital Market, on ). Each share
of common stock is being offered together with one common warrant to purchase one share of common stock. The shares of common stock
and common warrants will be separately issued. This prospectus also covers the shares of common stock issuable from time to time
upon the exercise of the common warrants. See “Description of Securities we are Offering – Common
Warrants.”
We
are also offering pre-funded warrants to those purchasers, whose purchase of shares of common stock in this offering would result in
the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of
the purchaser, 9.99%) of our outstanding common stock following the consummation of this offering in lieu of the shares of our common
stock that would result in ownership in excess of 4.99% (or, at the election of the purchaser, 9.99%). Each pre-funded warrant will be
exercisable for one share of common stock at an exercise price of $0.0001 per share. Each pre-funded warrant is being offered together
with the same common warrant to purchase one share of common stock described above that is being offered with each share of common stock.
The purchase price of each pre-funded warrant will equal the combined public offering price per share of common stock and common warrants
being sold in this offering, less the $0.0001 per share exercise price of each such pre-funded warrant. Each pre-funded warrant will
be exercisable upon issuance and will expire when exercised in full. The pre-funded warrants and common warrants will be separately issued.
For each pre-funded warrant that we sell, the number of shares of common stock that we are selling will be decreased on a one-for-one
basis. This prospectus also covers the shares of common stock issuable from time to time upon the exercise of the pre-funded warrants.
See “Description of Securities we are Offering – Pre-Funded Warrants.”
We
have engaged Roth Capital Partners, LLC, or the placement agent, to act as our exclusive placement agent in connection with this offering.
The placement agent has agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus.
The placement agent is not purchasing or selling any of the securities we are offering and the placement agent is not required to arrange
the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay to the placement agent the placement
agent fees set forth in the table below, which assumes that we sell all of the securities offered by this prospectus. There is no arrangement
for funds to be received in escrow, trust or similar arrangement. There is no minimum number of shares of securities or minimum aggregate
amount of proceeds that is a condition for this offering to close. We may sell fewer than all of the securities offered hereby, which
may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund if we do not
sell all of the securities offered hereby. Because there is no escrow account and no minimum number of securities or amount of proceeds,
investors could be in a position where they have invested in us, but we have not raised sufficient proceeds in this offering to adequately
fund the intended uses of the proceeds as described in this prospectus. We will bear all costs associated with the offering. See “Plan
of Distribution” on page 29 of this prospectus for more information regarding these arrangements. This offering will terminate
no later than , 2025, unless we decide to terminate the offering (which we may do at any time
in our discretion) prior to that date. The securities will be offered at a fixed price and are expected to be issued in a single closing.
Investors purchasing securities offered hereby will have the option to execute a securities purchase agreement with us. We expect that
the closing of the offering will occur one trading day after we price the securities offered hereby. When we price the securities, we
will simultaneously enter into securities purchase agreements relating to the offering with those investors who so choose. The offering
will settle delivery versus payment (“DVP”)/receipt versus payment (“RVP”). That is, on the closing date, we
will issue the shares of common stock directly to the account(s) at the placement agent identified by each purchaser; upon receipt of
such shares, the placement agent shall promptly electronically deliver such shares to the applicable purchaser, and payment therefor
shall be made by the placement agent (or its clearing firm) by wire transfer to us.
Our
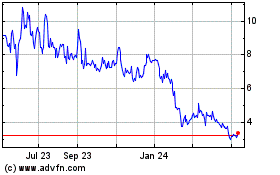
common stock is listed on the Nasdaq Capital Market under the symbol “GTBP.” On December 20, 2024, the last reported
sale price of our common stock on the Nasdaq Capital Market was $1.75 per share. All share, common warrant and pre-funded warrant
numbers are based on an assumed combined public offering price of $ per share or pre-funded
warrant, as applicable, and common warrants. The actual combined public offering price per share and common warrants and the actual combined
public offering price per common warrants and pre-funded warrants will be determined between us and investors based on market conditions
at the time of pricing, and may be at a discount to the current market price of our common stock. Therefore, the assumed public offering
price used throughout this prospectus may not be indicative of the final public offering price.
You
should read this prospectus, together with additional information described under the headings “Incorporation of Certain Information
By Reference” and “Where You Can Find More Information,” carefully before you invest in any of our securities.
Investing
in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 9
of this prospectus and in the documents incorporated by reference into this prospectus for a discussion of risks that should be considered
in connection with an investment in our securities.
| | |
| Per
Share and Common Warrant | | |
| Per Common Pre-Funded Warrant and
-Funded Warrant | | |
| Total | |
| Public offering price | |
$ | | | |
$ | | | |
$ | | |
| Placement Agent fees(1) | |
$ | | | |
$ | | | |
$ | | |
| Proceeds to us, before expenses(2) | |
$ | | | |
$ | | | |
$ | | |
|
(1) Includes a cash fee up to 6% of the gross proceeds raised in this offering; provided,
however, the cash fee shall equal 3% of the gross proceeds raised in this offering for securities purchased by
certain excluded investors, to be paid to the placement agent. We have also agreed to reimburse the placement agent for
certain of its offering-related expenses in an aggregate amount up to $100,000. In addition, we have agreed to issue the placement
agent or its designees warrants to purchase up to shares of common stock (equal to
6% of the aggregate number of securities sold in this offering, subject to a partial adjustment in the event certain
investors participate) at an exercise price of $ per share, which represents 125% of the public offering price per share of
common stock and common warrants. We refer to these warrants in this prospectus as the “placement agent
warrants.” See “Plan of Distribution” for a complete description of the compensation to be received by the
placement agent. |
| |
|
| |
(2) Because there is no minimum number of securities or amount of proceeds required as a condition
to closing in this offering, the actual public offering amount, placement agent fees, and proceeds to us, if any, are not presently
determinable and may be substantially less than the total maximum offering amounts set forth above. We estimate the total expenses
of this offering payable by us, excluding the placement agent fee, will be approximately $ . |
The
delivery of the shares of common stock any pre-funded warrants and common warrants to purchasers is expected to be made no later
than , 2025, subject to the satisfaction of customary closing conditions.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed
upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Roth
Capital Partners
The
date of this prospectus is , 2024.
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
We
incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without
charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus
as well as additional information described under “Incorporation of Certain Information by Reference,” before deciding
to invest in our securities.
We
have not, and the placement agent has not, authorized anyone to provide any information or to make any representations other than those
contained in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We
take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This
prospectus is an offer to sell only the securities offered hereby, and only under circumstances and in jurisdictions where it is lawful
to do so. The information contained in this prospectus or in any applicable free writing prospectus is current only as of its date, regardless
of its time of delivery or any sale of our securities. Our business, financial condition, results of operations and prospects may have
changed since that date.
The
information incorporated by reference or provided in this prospectus contains statistical data and estimates, including those relating
to market size and competitive position of the markets in which we participate, that we obtained from our own internal estimates and
research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Industry publications,
studies and surveys generally state that they have been obtained from sources believed to be reliable. While we believe our internal
company research is reliable and the definitions of our market and industry are appropriate, neither this research nor these definitions
have been verified by any independent source.
For
investors outside the United States: We have not, and the placement agent has not, done anything that would permit this offering or possession
or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons
outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating
to, the offering of the securities and the distribution of this prospectus outside the United States.
This
prospectus and the information incorporated by reference into this prospectus contain references to our trademarks and to trademarks
belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus and the information incorporated
by reference into this prospectus, including logos, artwork, and other visual displays, may appear without the ® or TM symbols, but
such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights
or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’
trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other company.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some
of the statements in this prospectus are “forward-looking statements” within the meaning of the safe harbor from liability
established by the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding our current
beliefs, goals and expectations about matters such as our expected financial performance and condition, operating results, our business
strategy and our financing plans. The forward-looking statements in this prospectus are not based on historical facts, but rather reflect
the current expectations of our management concerning future results and events. The forward-looking statements generally can be identified
by the use of terms such as “believe,” “expect,” “anticipate,” “intend,” “plan,”
“foresee,” “may,” “guidance,” “estimate,” “potential,” “outlook,”
“target,” “forecast,” “likely” or other similar words or phrases. Similarly, statements that describe
our objectives, plans or goals are, or may be, forward-looking statements.
Forward-looking
statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements
to be different from any future results, performance and achievements expressed or implied by these statements. We cannot guarantee that
our forward-looking statements will turn out to be correct or that our beliefs and goals will not change. Our actual results could be
very different from and worse than our expectations for various reasons. The risk factors and cautionary language discussed in this prospectus
provide examples of risks, uncertainties and events that may cause actual results to differ materially from the expectations described
by us in such forward-looking statements, including among other things:
| |
● |
our
ability to develop and advance our current product candidates and programs into, and successfully complete, clinical trials; |
| |
|
|
| |
● |
our financial condition raises substantial doubt as
to our ability to continue as a going concern; |
| |
|
|
| |
● |
our
financial performance and our ability to effectively manage our anticipated growth; |
| |
|
|
| |
● |
the
period over which we estimate our existing cash and cash equivalents will be sufficient to fund our future operating expenses and
capital expenditure requirements; |
| |
|
|
| |
● |
the
impact of laws and regulations; |
| |
|
|
| |
● |
general
economic conditions; |
| |
|
|
| |
● |
the
effects of the coronavirus and other communicable diseases on the continued or resumed disruption of supply chains, the global economy,
on the global financial markets and on our business; |
| |
|
|
| |
● |
the
timing, scope and likelihood of regulatory filings and approvals; |
| |
|
|
| |
● |
our
continued reliance on third parties to conduct additional clinical trials of our product candidates; |
| |
|
|
| |
● |
our
manufacturing, commercialization, and marketing capabilities and strategy; |
| |
|
|
| |
● |
our
intellectual property position, including the scope of protection we are able to establish and maintain for intellectual property
rights covering product candidates we may develop, including the validity of intellectual property rights held by third parties,
and our ability not to infringe, misappropriate or otherwise violate any third-party intellectual property rights; |
| |
|
|
| |
● |
the
rate and degree of market acceptance and clinical utility of our product candidates we may develop; |
| |
● |
our
ability to hire additional qualified personnel and attract and retain key employees; and |
| |
|
|
| |
● |
the
result of any future financing efforts; and |
| |
|
|
| |
● |
our
failure to maintain compliance with the Nasdaq Capital Market’s (“Nasdaq”) continued listing requirements could
result in the delisting of our common stock. |
These
risks, among others, could cause actual results to differ materially from those implied by the forward-looking statements contained in
this prospectus. You should review carefully all information, including the discussion under “Risk Factors” in this
prospectus and in the documents incorporated by reference herein. Any forward-looking statements in this prospectus are made only as
of the date hereof and, except as may be required by law, we do not have any obligation to publicly update any forward-looking statements
contained in this prospectus to reflect subsequent events or circumstances.
You
should read this prospectus with the understanding that our actual future results may be materially different from what we expect. We
do not assume any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise,
except as required by applicable law.
PROSPECTUS
SUMMARY
This
summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information that you
should consider before deciding to invest in our securities. You should read this entire prospectus carefully, including the “Risk
Factors” section in this prospectus and under similar captions in the documents incorporated by reference into this prospectus.
In this prospectus, unless otherwise stated or the context otherwise requires, references to the terms “GTBP,” “the
Company,” “we,” “us” and “our” refer to GT Biopharma, Inc., together with its subsidiaries,
unless the context otherwise requires. This prospectus and the information incorporated herein by reference include trademarks, service
marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference
into this prospectus and the information incorporated herein by reference are the property of their respective owners.
Overview
We
are a clinical stage biopharmaceutical company focused on the development and commercialization of novel immuno-oncology products based
on our proprietary Tri-specific Killer Engager (TriKE®), and Tetra-specific Killer Engager (Dual Targeting TriKE®)
fusion protein immune cell engager technology platforms. Our TriKE® and Dual Targeting TriKE® platforms generate
proprietary therapeutics designed to harness and enhance the cancer killing abilities of a patient’s own natural killer cells,
or NK cells. Once bound to an NK cell, our moieties are designed to activate the NK cell to direct it to one or more specifically targeted
proteins expressed on a specific type of cancer cell or virus infected cell, resulting in the targeted cell’s death. TriKE®s
can be designed to target any number of tumor antigens on hematologic malignancies or solid tumors and do not require patient-specific
customization.
We
are using our TriKE® platform with the intent to bring to market immuno-oncology products that can treat a range of hematologic
malignancies, solid tumors, and potentially autoimmune disorders. The platform is scalable, and we are implementing processes to produce
investigational new drug (IND) ready moieties in a timely manner after a specific TriKE® conceptual design. Specific drug
candidates can then be advanced into the clinic on our own or through potential collaborations with partnering companies. We believe
our TriKE®s may have the ability, if approved for marketing, to be used as both monotherapy and in combination with other standard-of-care
therapies.
Our
initial work was conducted in collaboration with the Masonic Cancer Center at the University of Minnesota under a program led by Dr.
Jeffrey Miller, Professor of Medicine, and the Deputy Director at the Center. Dr. Miller is a recognized key opinion leader in the field
of NK cell and IL-15 biology and their therapeutic potential. We have exclusive rights to the TriKE® platform and are
generating additional intellectual property for specific moieties.
Product
Pipeline
Our
current product candidate pipeline is summarized in the table below

GTB-3550
GTB-3550
was our first TriKE® product candidate and its clinical development was suspended so that we could focus resources on second-generation
TriKEs®. GTB-3550 is a tri-specific killer engager (TriKE) comprised of two single-chain variable fragments
(“scFv”) composed of the variable regions of the heavy and light chains of anti-CD16
and anti-CD33 antibodies and a modified form of IL-15. We studied this anti-CD16-IL-15-anti-CD33 TriKE® in CD33 positive leukemias,
a marker expressed on tumor cells in acute myelogenous leukemia, or AML, and myelodysplastic syndrome, or MDS. The anti-CD33 antibody
fragment in GTB-3550 was derived from the M195 humanized anti-CD33 scFv We believe the approval of the antibody-drug conjugate gemtuzumab
validates the targeting of CD33.
GTB-3550
was replaced by a more potent next-generation camelid nanobody TriKE®, GTB-3650, that similarly targets CD33 on relapsed/refractory
AML and high-risk MDS. A key difference between GTB-3550 and GTB-3650 is the incorporation of camelid antibody technology instead of
a scFv; our preclinical experience showed markedly enhanced potency of TriKEs® comprised of camelid components.
This is illustrated below by better tumor control of AML bearing animals with GTB-3650 (purple dots) compared to GTB-3550 (blue dots).
This provided the rationale for pausing further development of GTB-3550 and moving over to solely develop the second-generation, camelid-based
TriKE® platform.

Second
Generation TriKE®s Utilize Camelid Nanobody Technology
Our
goal is to be a leader in immuno-oncology therapies targeting a broad range of indications including hematological malignancies and solid
tumors. A key element of our strategy includes introducing a next-generation camelid nanobody platform. Camelid antibodies (often referred
as nanobodies) are smaller than human immunoglobulin, consisting of two heavy chains instead of two heavy and two light chains. These
nanobodies have the potential to have greater affinity to target antigens, potentially resulting in greater potency. We are utilizing
this camelid antibody structure for all of our new TriKE® product candidates.
To
develop second generation TriKE®s, we designed a new humanized CD16 engager derived from a single-domain antibody. While scFvs consist
of a heavy and a light variable chain joined by a linker, single-domain antibodies consist of a single variable heavy chain capable of
engaging without the need of a light chain counterpart (see figure below).

These
single-domain antibodies are thought to have certain attractive features for antibody engineering, including physical stability, ability
to bind deep grooves, and increased production yields, amongst others. Pre-clinical studies demonstrated increased NK cell activation
against CD33+ targets including enhanced NK cell degranulation (% CD107a+) and IFNγ with the single-domain CD16 TriKE®
(cam 16-wt15-33; GTB-3650) compared to the original TriKE® (scFv16-m 15-33; GTB-3550) (see figure below). This data was published
by Dr. Felices M et al (2020) in Cancer Immunol Res.
CD33+
HL60 Targets in Killing Assays
The
purple line represents the GTB-3650 and the blue line represents GTB-3550.

GTB-3650
GTB-3650
is a TriKE® which targets CD33 on the surface of myeloid leukemias and an agonistic camelid engager to the potent activating receptor
on NK cells, CD16. Use of this engager enhances the activity of wild type IL-15 included in GTB-3650. The TriKE® approach provides
a novel way to specifically target these tumors by leveraging NK cells, which have been shown to mediate relapse protection in this setting,
in an anti-CD33-targeted fashion. We are advancing GTB-3650 to clinical studies based on pre-clinical data showing a marked increase
in potency compared to GTB-3550, which we anticipate could lead to an enhanced efficacy signal in AML and MDS. We advanced GTB-3650 through
requisite preclinical studies and filed an Investigational New Drug (IND) application with the U.S. Food and Drug Administration (FDA)
in December 2023. In late June 2024, the FDA cleared our IND Application for GTB-3650. We expect to start study enrollment targeting
patients with relapsed/refractory AML and high grade MDS early in the first quarter of 2025. This initial study will test
GTB-3650 as monotherapy testing administration 2 weeks on and two weeks off (to prevent NK cell exhaustion) for at least 2 cycles of
therapy. The design of the trial has been agreed on with the FDA.

GTB-5550
GTB-5550
is a B7-H3 targeted TriKE® which targets B7-H3 on the surface of advanced solid tumors (figure above). GTB-5550 is our first
dual camelid TriKE®. B7-H3 is expressed on a broad spectrum of solid tumor malignancies, allowing our team to target these
malignancies through GTB-5550. Pre-clinical work has shown that this molecule has NK-cell targeted activity against a variety of
solid tumors, including head and neck cancer squamous cell carcinoma (figure below), prostate cancer, breast cancer, ovarian cancer,
glioblastoma, and lung cancer (amongst others). We are advancing GTB-5550 through preclinical studies and initiated a GMP
manufacturing campaign in anticipation of filing an IND in the first half of 2025. A pre-IND packet was submitted to the FDA in
October 2023 with a written response from the FDA in December 2023. The main question from the FDA was regarding pre-clinical
toxicology and a pivot to subcutaneous dosing. The initial trial expected in 2025 is designed as a basket trial for patients with
B7-H3+ solid tumors using Monday through Friday dosing (2 weeks on and 2 weeks off to prevent immune exhaustion), and is dependent
on manufacturing of clinical materials.

GTB-7550
GTB-7550
TriKE® is a product candidate in development for the treatment of lupus and other autoimmune disorders. GTB-7550 TriKE® is a
tri-specific molecule composed of a camelid nanobody that binds the CD16 receptor on NK cells, a scFv engager against CD19 on malignant
and normal B cells, and a human IL-15 sequence between them.
Implications
of Being a Smaller Reporting Company
We
are a “smaller reporting company” as defined in the Securities Exchange Act of 1934, as amended, or the Exchange Act, and
have elected to take advantage of certain of the scaled disclosures available to smaller reporting companies. Accordingly, we may provide
less public disclosure than larger public companies, including the inclusion of only two years of audited consolidated financial statements
and only two years of management’s discussion and analysis of financial condition and results of operations disclosure and the
inclusion of reduced disclosure about our executive compensation arrangements. As a smaller reporting company, we are also exempt from
compliance with the auditor attestation requirements pursuant to the Sarbanes-Oxley Act. As a result, the information that we provide
to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
We will continue to be a “smaller reporting company” until we have $250 million or more in public float (based on our common
stock) measured as of the last business day of our most recently completed second fiscal quarter or, in the event we have no public float
or a public float (based on our common stock) that is less than $700 million, annual revenues of $100 million or more during the most
recently completed fiscal year.
Corporate
Information
Our
common stock currently trades on the Nasdaq under the symbol “GTBP.” Our principal executive offices are located at
315 Montgomery Street, 10th Floor, San Francisco, CA 94104, and our telephone number is (415) 919-4040. We maintain a website
at www.gtbiopharma.com. Information contained on or accessible through our website is not, and should not be considered, part
of, or incorporated by reference into, this prospectus.
| THE
OFFERING |
| |
| Common
Stock to be Offered |
|
Up
to shares of common stock. |
| Common
Warrants to be Offered |
|
Common warrants
to purchase up to shares of our common stock. Each common warrant has an exercise
price of $ per share of common stock. Common warrants will become exercisable immediately after issuance and will expire five years
from the date of issuance.
The
shares of common stock and pre-funded warrants, and the accompanying common warrant, as the
case may be, can only be purchased together in this offering but will be issued separately
and will be immediately separable upon issuance. This prospectus also relates to the offering
of the shares of common stock issuable upon exercise of the common warrants.
|
| Pre-funded
Warrants to be Offered |
|
We
are also offering to certain purchasers whose purchase of shares of common stock in this
offering would otherwise result in the purchaser, together with its affiliates and certain
related parties, beneficially owning more than 4.99% (or, at the election of the purchaser,
9.99%) of our outstanding common stock immediately following the consummation of this offering,
the opportunity to purchase, if such purchasers so choose, pre-funded warrants to purchase
shares of common stock, in lieu of shares of common stock that would otherwise result in
any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the
purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant will be exercisable
for one share of our common stock. The purchase price of each pre-funded warrant and accompanying
common warrants will equal the price at which the share of common stock and accompanying
common warrants are being sold to the public in this offering, minus $0.0001, and the exercise
price of each pre-funded warrant will be $0.0001 per share. The pre-funded warrants will
be exercisable immediately and may be exercised at any time until all of the pre-funded warrants
are exercised in full. This offering also relates to the shares of common stock issuable
upon exercise of any pre-funded warrants sold in this offering. For each pre-funded warrant
we sell, the number of shares of common stock we are offering will be decreased on a one-for-one
basis. Because we will also issue one common warrant to purchase one share of common stock
for each share of our common stock and for each pre-funded warrant to purchase one share
of our common stock sold in this offering, the number of common warrants sold in this offering
will not change as a result of a change in the mix of the shares of our common stock and
pre-funded warrants sold.
|
| Common
Stock to be Outstanding Before this Offering |
|
2,234,328
shares. |
| |
|
|
| Common
Stock to be Outstanding Immediately After this Offering |
|
shares, (assuming full exercise of the pre-funded warrants and assuming no exercise of the common warrants). |
| |
|
|
| Use
of Proceeds |
|
We
estimate that the net proceeds from this offering will be approximately $ million, excluding the proceeds, if any, from the cash
exercise of the common warrants in this offering. We currently intend to use the net proceeds from this offering for working capital
and general corporate purposes, including the further development of our product candidates that are currently undergoing clinical
trials, our product candidates that we expect to submit an investigational new drug application for in the near term, and our product
candidates that are pre-clinical. See “Use of Proceeds” for additional information. |
| |
|
|
| Risk
Factors |
|
An
investment in our securities involves a high degree of risk. See “Risk Factors” beginning on page 9 of
this prospectus and the other information included and incorporated by reference in |
| |
|
this
prospectus for a discussion of the risk factors you should carefully consider before deciding to invest in our securities. |
| |
|
|
| Nasdaq
Symbol |
|
Our
common stock is listed on the Nasdaq Capital Market under the symbol “GTBP.” There is no established public trading market
for the common warrants or pre-funded warrants, and we do not expect a market to develop. In addition, we do not intend to apply
to list the common warrants or pre-funded warrants on any national securities exchange or other nationally recognized trading system.
Without an active trading market, the liquidity of the common warrants and pre-funded warrants will be limited. |
Unless
otherwise stated in this prospectus, the number of shares of our common stock to be outstanding as of the date of this prospectus and
after this offering is based on 2,234,328 shares outstanding as of September 30, 2024, and excludes (a) placement agent warrants
to purchase up to shares of our common stock issuable upon the exercise of warrants
to be issued to the placement agent in connection with this offering having an exercise price of $
per share, (b) the shares of common stock issuable upon exercise of the common warrants and pre-funded warrants being offered by us in
this offering, (c) previously issued and outstanding warrants to purchase up to 1,133,762 shares of our common stock, and (d)
outstanding stock options to purchase up to 101,264 shares of our common stock. See “Note 7 – Common Stock Warrants
and Options” to our Third Quarter Report filed on Form 10-Q that is incorporated by reference into this prospectus.
RISK
FACTORS
Investing
in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below in addition
to the other information contained in this prospectus and any prospectus supplement before deciding whether to invest in shares of our
common stock. The risks summarized below and others are discussed more fully in the section titled “Risk Factors” in our
Annual Report on Form 10-K for the year ended December 31, 2023, which is incorporated herein by reference. If any of the following risks
or the risks incorporated by reference occur, our business, financial condition or operating results could be harmed. In that case, the
trading price of our common stock could decline and you may lose part or all of your investment. In the opinion of management, the risks
discussed below and incorporated by reference represent the material risks known to us. Additional risks and uncertainties not currently
known to us or that we currently deem immaterial may also impair our business, financial condition and operating results and adversely
affect the market price of our common stock..
Risks
Related to Our Business
Risks
related to our business risks include, but are not limited to, the following:
| |
● |
Our
financial condition raises substantial doubt as to our ability to continue as a going concern. |
| |
|
|
| |
● |
Our
business is at an early stage of development and we may not develop therapeutic products that can be commercialized. |
| |
|
|
| |
● |
We
have a history of operating losses and we expect to continue to incur losses for the foreseeable future. We may never generate revenue
or achieve profitability. |
| |
|
|
| |
● |
We
will need additional capital to conduct our operations and develop our products, and our ability to obtain the necessary funding
is uncertain. |
| |
|
|
| |
● |
Our
current and future indebtedness may impose significant operating and financial restrictions on us and affect our ability to access
liquidity. |
| |
|
|
| |
● |
The
cost of our research and development programs may be significantly higher than expected, and there is no assurance that they will
successful in a timely manner, or at all. |
| |
|
|
| |
● |
If
our efforts to protect the proprietary nature of the intellectual property related to our technologies are not adequate, we may not
be able to compete effectively in our market and our business would be harmed. |
| |
|
|
| |
● |
Claims
that we infringe the intellectual property rights of others may prevent or delay our drug discovery and development efforts. |
| |
|
|
| |
● |
We
may desire, or be forced, to seek additional licenses to use intellectual property owned by third parties, and such licenses may
not be available on commercially reasonable terms, or at all. |
| |
|
|
| |
● |
If
we are unsuccessful in obtaining or maintaining patent protection for intellectual property in development or licensed from third
parties, our business and competitive position would be harmed. |
| |
|
|
| |
● |
If
we fail to meet our obligations under our license agreements, we may lose our rights to key technologies on which our business depends. |
| |
|
|
| |
● |
Our
reliance on the activities of our non-employee consultants, research institutions and scientific contractors, whose activities are
not wholly within our control, may lead to delays in development of our proposed products. |
| |
|
|
| |
● |
Clinical
drug development is costly, time-consuming and uncertain, and we may suffer setbacks in our clinical development program that could
harm our business. |
| |
|
|
| |
● |
If
we experience delays or difficulties in the enrollment of patients in clinical trials, those clinical trials could take longer than
expected to complete and our receipt of necessary regulatory approvals could be delayed or prevented. |
| |
|
|
| |
● |
Obtaining
regulatory approval, even after clinical trials that are believed to be successful, is an uncertain process. |
| |
|
|
| |
● |
We
will continue to be subject to extensive FDA regulation following any product approvals, and if we fail to comply with these regulations,
we may suffer a significant setback in our business. |
| |
|
|
| |
● |
Many
of our business practices are subject to scrutiny and potential investigation by regulatory and government enforcement authorities,
as well as to lawsuits brought by private citizens under federal and state laws. We could become subject to investigations, and our
failure to comply with applicable law or an adverse decision in lawsuits may result in adverse consequences to us. If we fail to
comply with U.S. healthcare laws, we could face substantial penalties and financial exposure, and our business, operations and financial
condition could be adversely affected. |
| |
|
|
| |
● |
Our
product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval,
limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if
any. |
| |
|
|
| |
● |
We
may expend our limited resources to pursue a particular product candidate or indication that does not produce any commercially viable
products and may fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater
likelihood of success. |
| |
|
|
| |
● |
Our
products may be expensive to manufacture, and they may not be profitable if we are unable to control the costs to manufacture them. |
| |
|
|
| |
● |
We
currently lack manufacturing capabilities to produce our therapeutic product candidates at commercial-scale quantities and do not
have an alternate manufacturing supply, which would negatively impact our ability to meet any demand for the product. |
| |
|
|
| |
● |
Our
business is based on novel technologies that are inherently expensive and risky and may not be understood by or accepted in the marketplace,
which could adversely affect our future value. |
| |
|
|
| |
● |
We
could be subject to product liability lawsuits based on the use of our product candidates in clinical testing or, if obtained, following
marketing approval and commercialization. If product liability lawsuits are brought against us, we may incur substantial liabilities
and may be required to cease clinical testing or limit commercialization of our product candidates. |
| |
|
|
| |
● |
We
rely on third parties to supply candidates for clinical testing and to conduct preclinical and clinical trials of our product candidates.
If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain
regulatory approval for or commercialize our product candidates. As a result, our business could be substantially harmed. |
| |
|
|
| |
● |
Our
failure to maintain compliance with the Nasdaq Capital Market’s (“Nasdaq”) continued listing requirements could
result in the delisting of our common stock. |
These
risks are described more fully in our Annual Report on Form 10-K for the year ended December 31, 2023, which is incorporated herein by
reference.
Risks
Related to our Business
Our
financial condition raises substantial doubt as to our ability to continue as a going concern.
As
of September 30, 2024, we had approximately $6.5 million in cash and cash equivalents and restricted cash, and working capital of $2.1
million, and we have incurred and expect to continue to incur significant costs in pursuit of our drug candidates. For
the nine months ended September 30, 2024, we recorded a net loss of approximately $9.4 million and used cash in operations of approximately
$10.4 million. Our unaudited consolidated condensed financial statements for the nine month period ended September 30, 2024 have
been prepared assuming that we will continue to operate as a going concern, which contemplates the realization of assets and the satisfaction
of liabilities in the normal course of business. To date, we have not generated substantial product revenues from our activities and
have incurred substantial operating losses. We expect that we will continue to generate substantial operating losses for the foreseeable
future until we complete development and approval of our product candidates. We will continue to fund our operations primarily through
utilization of our current financial resources and additional raises of capital.
These
conditions raise substantial doubt about our ability to continue as a going concern. For further information, please see Note 1 to our
third quarter unaudited condensed consolidated financial statements for the nine-months ended September 30, 2024. The Company has evaluated
the significance of the uncertainty regarding the Company’s financial condition in relation to its ability to meet its obligations,
which has raised substantial doubt about the Company’s ability to continue as a going concern. While it is very difficult to estimate
the Company’s future liquidity requirements, the Company believes if it is unable to obtain additional financing, existing cash
resources will not be sufficient to enable it to fund the anticipated level of operations through one year from the date the accompanying
unaudited condensed consolidated financial statements are issued. There can be no assurances that the Company will be able to secure
additional financing on acceptable terms. In the event the Company does not secure additional financing, the Company will be forced to
delay, reduce, or eliminate some or all of its discretionary spending, which could adversely affect the Company’s business prospects,
ability to meet long-term liquidity needs and the ability to continue operations.
Risks
Related to this Offering and Our Common Stock
There
has been a limited public market for our common stock, and we do not know whether one will develop to provide you adequate liquidity.
Furthermore, the trading price for our common stock, should an active trading market develop, may be volatile and could be subject to
wide fluctuations in per-share price.
Our
common stock is now listed on the Nasdaq Capital Market under the trading symbol “GTBP”; historically, however, there has
been a limited public market for our common stock. We cannot assure you that an active trading market for our common stock will develop
or be sustained. The liquidity of any market for the shares of our common stock will depend on a number of factors, including:
| |
● |
the
number of stockholders;
|
| |
|
|
| |
● |
our
operating performance and financial condition;
|
| |
|
|
| |
● |
the
market for similar securities;
|
| |
|
|
| |
● |
the
extent of coverage of us by securities or industry analysts; and
|
| |
|
|
| |
● |
the
interest of securities dealers in making a market in the shares of our common stock. |
Even
if an active trading market develops, the market price for our common stock may be highly volatile and could be subject to wide fluctuations.
In addition, the price of shares of our common stock could decline significantly if our future operating results fail to meet or exceed
the expectations of market analysts and investors and actual or anticipated variations in our quarterly operating results could negatively
affect our share price.
The
volatility of the price of our common stock may also be impacted by the risks discussed under this “Risk Factors” section,
in addition to other factors, including:
| |
● |
developments
in the financial markets and worldwide or regional economies;
|
| |
|
|
| |
● |
announcements
of innovations or new products or services by us or our competitors;
|
| |
|
|
| |
● |
announcements
by the government relating to regulations that govern our industry;
|
| |
|
|
| |
● |
significant
sales of our common stock or other securities in the open market;
|
| |
|
|
| |
● |
variations
in interest rates;
|
| |
|
|
| |
● |
changes
in the market valuations of other comparable companies; and
|
| |
|
|
| |
● |
changes
in accounting principles. |
Our
outstanding warrants and options may affect the market price of our common stock
As
of the date of this prospectus, we had 2,234,328 shares of common stock outstanding
and issued and had outstanding warrants for the purchase of up to 1,120,429 additional shares of common stock at a weighted average
exercise price of $18.85 per share, all of which are exercisable as of the date of this prospectus (subject to certain beneficial ownership
limitations). In addition, we had outstanding options for the purchase of up to 124,600 additional shares of common stock at a
weighted average exercise price of $32.69 per share, 105,154 of which are exercisable as of the date of this prospectus. The amount of
common stock reserved for issuance may have an adverse impact on our ability to raise capital and may affect the price and liquidity
of our common stock in the public market. In addition, the issuance of these shares of common stock will have a dilutive effect on current
stockholders’ ownership.
Because
our common stock may be deemed a low-priced “penny” stock, an investment in our common stock should be considered high-risk
and subject to marketability restrictions.
Historically,
the trading price of our common stock has been $5.00 per share or lower, and deemed a penny stock, as defined in Rule 3a51-1 under the
Exchange Act, and subject to the penny stock rules of the Exchange Act specified in rules 15g-1 through 15g-100. Those rules require
broker–dealers, before effecting transactions in any penny stock, to:
| ● |
deliver
to the customer, and obtain a written receipt for, a disclosure document; |
| |
|
| ● |
disclose
certain price information about the stock; |
| |
|
| ● |
disclose
the amount of compensation received by the broker-dealer or any associated person of the broker-dealer; |
| |
|
| ● |
send
monthly statements to customers with market and price information about the penny stock; and |
| |
|
| ● |
in
some circumstances, approve the purchaser’s account under certain standards and deliver written statements to the customer
with information specified in rules. |
Consequently,
the penny stock rules may restrict the ability or willingness of broker-dealers to sell the common stock and may affect the ability of
holders to sell their common stock in the secondary market and the price at which such holders can sell any such securities. These additional
procedures could also limit our ability to raise additional capital in the future.
Financial
Industry Regulatory Authority (“FINRA”) sales practice requirements may also limit a stockholder’s ability to buy and
sell our common stock, which could depress the price of our common stock.
In
addition to the “penny stock” rules described above, FINRA has adopted rules that require a broker-dealer to have reasonable
grounds for believing that the investment is suitable for that customer before recommending an investment to a customer. Prior to recommending
speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information
about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these
rules, FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some
customers. Thus, the FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock,
which may limit your ability to buy and sell our shares of common stock, have an adverse effect on the market for our shares of common
stock, and thereby depress our price per share of common stock.
If
securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion
regarding our stock, our stock price and trading volume could decline.
The
trading market for our common stock may be influenced by the research and reports that industry or securities analysts publish about
us or our business. We currently have research coverage by only one securities analyst, and we may never obtain research coverage by
additional analysts. If no or few securities or industry analysts commence coverage of us, the trading price for our common stock may
be negatively affected. In the event that we receive additional securities or industry analyst coverage, if any of the analysts who cover
us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if
our operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts
cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could
cause our stock price or trading volume to decline.
Anti-takeover
provisions may limit the ability of another party to acquire us, which could cause our stock price to decline.
Delaware
law and our restated certificate of incorporation (“certificate of incorporation”), our restated bylaws (“bylaws”)
and other governing documents contain provisions that could discourage, delay or prevent a third party from acquiring us, even if doing
so may be beneficial to our stockholders, which could cause our stock price to decline. In addition, these provisions could limit the
price investors would be willing to pay in the future for shares of our common stock.
We
do not currently or for the foreseeable future intend to pay dividends on our common stock.
We
have never declared or paid any cash dividends on our common stock. We currently anticipate that we will retain future earnings for the
development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable
future. As a result, any return on your investment in our common stock will be limited to the appreciation in the price of our common
stock, if any.
Purchasers
of our common stock in this offering will experience immediate and substantial dilution in the book value of their investment.
The
offering price per share in this offering is substantially higher than the net tangible book value per share of our ordinary shares before
giving effect to this offering. Accordingly, purchasers of our securities in this offering will incur immediate dilution of approximately
$ per share, representing the difference between the public offering price per share and our as-adjusted net tangible book value
per share as of September 30, 2024. Furthermore, if outstanding options or warrants are exercised, purchasers could experience further
dilution. For more information, including how these amounts were calculated, see “Dilution.”
Our
management will have broad discretion as to the use of the proceeds from this offering, and may not use the proceeds effectively.
Our
management will have broad discretion as to the application of the net proceeds from this offering. Currently, we intend to use the net
proceeds from this offering for working capital and general corporate purposes, including the further development of our product candidates
that are currently undergoing clinical trials, our product candidates that we expect to submit an investigational new drug application
for in the near term, and our product candidates that are pre-clinical. See “Use of Proceeds.” Purchasers will not have
the opportunity, as part of their investment decision, to assess whether these proceeds are being used appropriately. Our management
may use the net proceeds for corporate purposes that may not improve our financial condition or market value, which could cause the price
of our securities to decline.
We
will need additional capital to conduct our operations and develop our products, and our ability to obtain the necessary funding is uncertain.
We
have used a significant amount of cash since inception to finance the continued development and testing of our product candidates, and
we expect to need substantial additional capital resources to develop our product candidates going forward and launch and commercialize
any product candidates for which we receive regulatory approval.
We
may not be successful in generating and/or maintaining operating cash flow, and the timing of our capital expenditures and other expenditures
may not result in cash sufficient to sustain our operations through the commercialization of our product candidates. If financing is
not sufficient and additional financing is not available or available only on terms that are detrimental to our long-term survival, it
could have a material adverse effect on our ability to continue to function. The timing and degree of any future capital requirements
will depend on many factors, including:
| ● |
accuracy
of the assumptions underlying our estimates for capital needs in 2025 and beyond;
|
| |
|
| ● |
scientific
and clinical progress in our research and development programs;
|
| |
|
| ● |
the
magnitude and scope of our research and development programs and our ability to establish,
enforce and maintain strategic arrangements for research, development, clinical testing,
manufacturing and marketing;
|
| |
|
| ● |
our
progress with pre-clinical development and clinical trials;
|
| |
|
| ● |
the
time and costs involved in obtaining regulatory approvals;
|
| |
|
| ● |
the
costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent
claims; and
|
| |
|
| ● |
the
number and type of product candidates that we pursue. |
Additional
financing through strategic collaborations, public or private equity or debt financings or other financing sources may not be available
on acceptable terms, or at all. Additional equity financing could result in significant dilution to our stockholders, and any debt financings
will likely involve covenants restricting our business activities. Further, if we obtain additional funds through arrangements with collaborative
partners, these arrangements may require us to relinquish rights to some of our technologies, product candidates or products that we
would otherwise seek to develop and commercialize on our own.
If
sufficient capital is not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or product
development initiatives, any of which could have a material adverse effect on our financial condition or business prospects.
There
is no public market for the common warrants or pre-funded warrants being offered by us in this offering.
There
is no established public trading market for the common warrants or the pre-funded warrants, and we do not expect a market to develop.
In addition, we do not intend to apply to list the common warrants or pre-funded warrants on any national securities exchange or other
nationally recognized trading system. Without an active market, the liquidity of the common warrants and pre-funded warrants will be
limited.
Holders of warrants purchased in this
offering will have no rights as common stockholders until such holders exercise their warrants and acquire our common stock.
Until holders of warrants
acquire shares of our common stock upon exercise thereof, holders of warrants will have no rights with respect to the shares of our common
stock underlying such warrants. Upon exercise of the warrants, the holders will be entitled to exercise the rights of a common stockholder
only as to matters for which the record date occurs after the exercise date.
This
is a reasonable best efforts offering, no minimum amount of securities is required to be sold, and we may not raise the amount of capital
we believe is required for our business plans, including our near-term business plans.
The
placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities in this offering. The placement
agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar
amount of the securities. There is no required minimum number of securities that must be sold as a condition to completion of this offering.
Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, placement
agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above.
We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and
investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to support our
continued operations, including our near-term continued operations. Thus, we may not raise the amount of capital we believe is required
for our operations in the short-term and may need to raise additional funds, which may not be available or available on terms acceptable
to us.
Purchasers who purchase our securities
in this offering pursuant to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit
of a securities purchase agreement.
In addition to rights
and remedies available to all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities
purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract
provides those investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement including,
but not limited to: (i) timely delivery of shares; (ii) agreement to not issue any shares or securities convertible into shares for a
period of days from closing of the offering, subject to certain exceptions; and (iii) indemnification for breach of contract.
Our
common stock may be at risk for delisting from the Nasdaq Capital Market in the future if we do not maintain compliance with Nasdaq’s
continued listing requirements. Delisting could adversely affect the liquidity of our common stock and the market price of our common
stock could decrease.
Our
common stock is currently listed on Nasdaq. Nasdaq has minimum requirements that a company must meet in order to remain listed on Nasdaq,
including corporate governance standards and a requirement that we maintain a stockholders’ equity above $2,500,000 as set forth
in Nasdaq Listing Rule 5550(b)(1).
On
November 21, 2024, the Company received a letter from Nasdaq notifying the Company that its amount of stockholders’ equity has
fallen below the $2,500,000 required minimum for continued listing set forth in Nasdaq Listing Rule 5550(b)(1).
Nasdaq’s
Letter has no immediate effect on the listing of the Company’s common stock, and its common stock will continue to trade on The
Nasdaq Capital Market under the symbol “GTBP” at this time. Under Nasdaq Listing Rules, the Company has until January 6,
2025 to provide Nasdaq with a plan to achieve and sustain compliance. If Nasdaq accepts the Company’s plan to regain compliance,
Nasdaq may grant an extension of up to 180 calendar days from the date of the Letter to evidence compliance. If Nasdaq does not accept
the Company’s plan to regain compliance, the Company will have the opportunity to appeal the decision to a Nasdaq Hearings Panel.
The Company intends to submit to Nasdaq, within the requisite time period, a plan to regain compliance with Listing Rule 5550(b)(1).
There can be no assurance that Nasdaq will accept the Company’s plan, that the Company will be able to regain compliance with Listing
Rule 5550(b)(1) or that the Company will be able meet the continued listing requirements during any compliance period that may be granted
by Nasdaq.
In
the future, if we fail to maintain such minimum requirements and a final determination is made by Nasdaq that our common stock must be
delisted, the liquidity of our common stock would be adversely affected and the market price of our common stock could decrease. In addition,
if delisted, we would no longer be subject to Nasdaq rules, including rules requiring us to have a certain number of independent directors
and to meet other corporate governance standards. Our failure to be listed on Nasdaq or another established securities market would have
a material adverse effect on the value of your investment in us.
USE
OF PROCEEDS
We
estimate that the net proceeds from the offering will be approximately $
million, based on the assumed public offering price of $ per share and common warrant,
which is the last reported sales price of our common stock on Nasdaq on , 2024 after deducting
the placement agent fees and estimated offering expenses payable by us, assuming no sale of any fixed combinations of warrants and pre-funded
warrants offered hereunder. We may only receive additional proceeds from the exercise of the common warrants issuable in connection with
this offering and, if such common warrants are exercised in full for cash, the estimated net proceeds will increase to $ million. However,
because this is a reasonable best-efforts offering and there is no minimum offering amount required as a condition to the closing of
this offering, the actual offering amount, the placement agent’s fees and net proceeds to us are not presently determinable and
may be substantially less than the maximum amounts set forth on the cover page of this prospectus. Based on the assumed public offering
price set forth above, we estimate that our net proceeds from the sale of 75%, 50%, and 25% of the securities offered in this offering
would be approximately $ million, $ million,
and $ million, respectively, after deducting the estimated placement agent fees and
estimated offering expenses payable by us.
We
currently intend to use the net proceeds from this offering for working capital to fund our clinical programs and general corporate purposes,
including the further development of: our product candidates that are currently undergoing clinical trials, our product candidates that
we expect to submit an investigational new drug application for in the near term, and our product candidates that are pre-clinical. This
expected use of proceeds from this offering represents our intentions based upon our current plans and prevailing business conditions,
which could change in the future as our plans and prevailing business conditions evolve. The amounts and timing of our use of proceeds
will vary depending on a number of factors, including the amount of cash generated or used by our operations. As a result, we will retain
broad discretion in the allocation of the net proceeds of this offering.
CAPITALIZATION
The
following table presents a summary of our cash and cash equivalents and capitalization as of September 30, 2024:
| ● |
on
an actual basis; and |
| |
|
| ● |
on
an as adjusted basis to reflect the issuance and sale of common stock and accompanying common warrants in this offering, after deducting
placement agent fees and estimated offering expenses payable by us. The as adjusted basis assumes no pre-funded warrants are
sold in this offering and excludes the proceeds, if any, from the exercise of any common warrants issued in this offering. |
The
unaudited as adjusted information below is prepared for illustrative purposes only and our capitalization following the completion of
this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You
should read the following table in conjunction with Management’s Discussion and Analysis of Financial Condition and Results of
Operations and the historical financial statements and related notes in the Company’s Annual Report on Form 10-K for the fiscal
year ended December 31, 2023 and our Quarterly Report on Form 10-Q for the period ended September 30, 2024, incorporated herein by reference.
| | |
As of September 30, 2024 | |
| (in thousands) | |
Actual | | |
As adjusted | |
| Cash and cash equivalents | |
$ | 6,418,000 | | |
$ | | |
| | |
| | | |
| | |
| Stockholders’ equity: | |
| | | |
| | |
| Convertible preferred stock, $0.01 par value,
15,000,000 shares authorized Series C, 96,230 shares issued and outstanding | |
$ | 1,000 | | |
$ | 1,000 | |
| Common stock: $0.001 par value; 250,000,000
shares authorized; 2,234,328 shares issued and outstanding, actual; shares issued and outstanding, pro forma as adjusted | |
$ | 2,000 | | |
$ | | |
| Additional paid-in capital | |
$ | 693,546,000 | | |
$ | | |
| Accumulated deficit | |
$ | (691,452,000 | ) | |
$ | | |
| Total stockholders’
equity | |
$ | 2,097,000 | | |
$ | | |
Each
$0.25 increase (decrease) in the assumed public offering price of $ per share would increase (decrease) each of cash and cash equivalents,
additional paid-in capital and total shareholders’ equity by approximately $ , assuming the number of shares of common stock and
common warrants offered, as set forth on the cover page of this prospectus, remains the same, and after deducting estimated placement
agent fees and estimated offering expenses. Similarly, each increase (decrease) of 100,000 shares in the number of shares of common stock
and common warrants offered would increase (decrease) cash and cash equivalents, additional paid-in capital and total shareholders’
equity by approximately $ , assuming the assumed public offering price remains the same, and after deducting estimated placement agent
fees and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will
be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
Unless
otherwise stated in this prospectus, the number of shares of our common stock to be outstanding as of the date of this prospectus and
after this offering is based on 2,234,328 shares outstanding as of September 30, 2024, and excludes (a) placement agent warrants
to purchase up to shares of our common stock issuable upon the exercise of warrants
to be issued to the placement agent in connection with this offering having an exercise price of $
per share, (b) the shares of common stock issuable upon exercise of the common warrants and pre-funded warrants being offered by us in
this offering, (c) previously issued and outstanding warrants to purchase up to 1,133,762 shares of our common stock, and
(d) outstanding stock options to purchase up to 101,264 shares of our common stock. See “Note 7 – Common Stock
Warrants and Options” to our Third Quarter Report filed on Form 10-Q that is incorporated by reference into this prospectus.
DILUTION
If
you purchase shares of our common stock, your interest will be diluted immediately to the extent of the difference between the offering
price per share you will pay in this offering and the as adjusted net tangible book value per share of our common stock after this offering.
Net tangible book value per share represents our total tangible assets less total liabilities, divided by the number of shares of our
common stock outstanding.
As
of September 30, 2024, our net tangible book value was $2,097,000, or $0.94 per share of Common Stock.
After
giving effect to the foregoing pro forma adjustments and the sale by us of shares of common stock and/or pre-funded warrants at
an assumed public offering price of $ per share and accompanying common warrant,
and after deducting the placement agent fees and estimated offering expenses payable by us, our as adjusted net tangible book value as
of September 30, 2024, would have been $ , or $ per share. This represents an immediate increase in as adjusted net tangible book value
of approximately $ per share to our existing stockholders, and an immediate dilution of $ per share to purchasers of shares in this offering,
as illustrated in the following table. The information below is illustrative only and will change based on actual pricing and other
terms of this offering determined at pricing. The final public offering price will be determined through negotiation between us, the
placement agent and the investors in the offering and may be at a discount to the current market price. Therefore, the assumed public
offering price used throughout this prospectus may not be indicative of the final public offering price.
| Assumed
public offering price |
|
$ |
|
|
| Net
tangible book value per share as of September 30, 2024 |
|
$ |
0.94 |
|
| Net
increase in net tangible book value per share attributable to existing shareholders |
|
$ |
|
|
| As
adjusted net tangible book value per share after this offering |
|
$ |
|
|
| Dilution
in net tangible book value per share to new investors in the offering |
|
$ |
( |
) |
Unless
otherwise stated in this prospectus, the number of shares of our common stock to be outstanding as of the date of this prospectus and
after this offering is based on 2,234,328 shares outstanding as of September 30, 2024, and excludes (a) placement agent warrants
to purchase up to shares of our common stock issuable upon the exercise of warrants
to be issued to the placement agent in connection with this offering having an exercise price of $
per share, (b) the shares of common stock issuable upon exercise of the common warrants and pre-funded warrants being offered by us in
this offering, (c) previously issued and outstanding warrants to purchase up to 1,133,762 shares of our common stock, and (d)
outstanding stock options to purchase up to 101,264 shares of our common stock. See “Note 7 – Common Stock Warrants
and Options” to our Third Quarter Report filed on Form 10-Q that is incorporated by reference into this prospectus.
DESCRIPTION
OF CAPITAL STOCK
The
following summary of the rights of our capital stock is not complete and is subject to and qualified in its entirety by reference to
our Charter and Bylaws, copies of which are filed as exhibits to our Annual Report on Form 10-K for the year ended December 31, 2023,
filed with the SEC on March 26, 2024, and the Certificate of Designations and forms of securities, copies of which are filed as exhibits
to the registration statement of which this prospectus forms a part , which are incorporated by reference herein.
The
following summary describes the material terms of our capital stock. The summary is qualified in its entirety by reference to our certificate
of incorporation and our bylaws.
Authorized
Shares
Our
authorized shares consists of 250,000,000 shares of common stock and 15,000,000 shares of preferred stock, $0.001 par value per
share (the “Preferred Stock”). Our common stock is registered under Section 12(b) of the Exchange Act and is listed
on the Nasdaq under the trading symbol “GTBP.”
On
February 2, 2024, the Company effectuated a reverse stock-split of its common stock, par value $0.001 per share, at a ratio of 1 for
30. The Company’s common stock began trading on a reverse stock-split-adjusted basis on The Nasdaq Capital Market on February 5,
2024 under the existing trading symbol “GTBP.”
As
a result of the reverse stock-split, every thirty (30) shares of issued and outstanding common stock were automatically combined into
one issued and outstanding share of common stock, without any change in the par value per share. No fractional shares will be issued
in connection with the reverse stock split. Stockholders who otherwise would be entitled to receive fractional shares of common stock
will be entitled to receive their pro-rata portion of the net proceeds obtained from the aggregation and sale by the exchange agent of
the fractional shares resulting from the reverse stock-split (reduced by any customary brokerage fees, commission and other expenses).
The reverse stock split reduced the number of shares of common stock outstanding on the effective date of the reverse stock-split from
41,419,000 shares to 1,380,633 shares, subject to minor adjustments due to the treatment of fractional shares. The number of authorized
shares of common stock remains unchanged at 250,000,000 shares.
Proportionate
adjustments have been made to the per share exercise price and the number of shares of common stock that may be purchased upon exercise
of outstanding stock options and warrants for the Company’s common stock, and to the number of shares of common stock reserved
for future issuance pursuant to the Company’s 2022 Omnibus Incentive Plan.
All
share and per share information within this report have been adjusted to retroactively reflect the reverse stock-split as of the earliest
period presented.
Common
Stock
Holders
of our common stock are entitled to one vote for each share of common stock held of record for the election of directors and on all matters
submitted to a vote of stockholders. Holders of our common stock are entitled to receive dividends ratably, if any, as may be declared
by the board of directors out of legally available funds, subject to any preferential dividend rights of any preferred stock then outstanding.
In the event of our dissolution, liquidation or winding up, holders of our common stock are entitled to share ratably in our net assets
legally available after the payment of all of our debts and other liabilities, subject to the liquidation preferences of any preferred
stock then outstanding. Holders of our common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences
and privileges of holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any
series of preferred stock currently outstanding or that we may designate and issue in the future. All outstanding shares of our common
stock are fully paid and non-assessable. Except as described below in “Anti-Takeover Provisions Under Our Charter and Bylaws and
Delaware Law,” holders of a majority of the outstanding shares of stock entitled to vote shall constitute a quorum for the transaction
of business, and a vote of the majority of the voting power represented at such meeting at which a quorum is generally required to take
action under our certificate of incorporation and bylaws.
Preferred
Stock
Our
Board is authorized, without action by the stockholders, to designate and issue up to 15,000,000 shares of preferred stock in one or
more series. In the past the Board has designated series lettered A through K and issued shares in those series (other than Series K).
As of the date of this prospectus, only preferred shares in the series designated C have shares issued and outstanding. Our Board can
fix or alter the rights, preferences and privileges of the shares of each series and any of its qualifications, limitations or restrictions,
including dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences and the number of shares constituting
a class or series. The issuance of preferred stock could, under certain circumstances, result in one or more of the following adverse
effects:
| ● | decreasing
the market price of our common stock; |
| | |
| ● | restricting
dividends on our common stock; |
| | |
| ● | diluting
the voting power of our common stock; |
| | |
| ● | impairing
the liquidation rights of our common stock; or |
| | |
| ● | delaying
or preventing a change in control of us without further action by our shareholders. |
Our
Board will make any determination to issue such shares based on its judgment as to our best interests and the best interests of our stockholders.
Series
C Preferred Stock
For
a discussion of the terms of our Series C Preferred Stock, see Note 6 to our audited financial statements, Stockholders’ Equity,
incorporated in this document by reference.
Warrants
Common
warrants for the purchase of up to 740,000 shares of common stock (the “2024 Common Warrants”) were issued pursuant
to a securities purchase agreement between us and certain institutional investors, dated as of May 21, 2024 (the “2024 Purchase
Agreement”) in registered form and entitle the registered holder to purchase one share of our common stock at a price equal to
$4.35 per share, subject to adjustment as discussed below, terminating at 5:00 p.m., New York City time, on the fifth anniversary of
the date of issuance. Also pursuant to the 2024 Purchase Agreement, we had an additional number of outstanding placement agent warrants
for the purchase of up to 88,800 shares of common stock at an exercise price of $5.4375. (the “2024 Placement Agent Warrants,”
and together with the 2024 Common Warrants, the “2024 Warrants”).
Common
warrants to purchase up to an aggregate of 216,667 shares of the Company’s common stock (the “2023 Common Warrants”),
pre-funded warrants to purchase up to 96,667 shares of the Company’s common stock (the “Pre-Funded Warrants”), and
placement agent warrants to purchase up to 13,000 of the Company’s common stock (the “2023 Placement Agents Warrants,”
and together with the 2023 Common Warrants and Pre-Funded Warrants, the “2024 Warrants”) were issued pursuant to a purchase
agreement dated December 30, 2022. The 2023 Common Warrants have an exercise price equal to $30.00 per share, are exercisable commencing
six months following issuance, and have a term of exercise equal to five years following the initial issuance date. The Pre-Funded Warrants
had an exercise price of $0.003 per share, are immediately exercisable and could be exercised at any time after their original issuance
until such Pre-Funded Warrants were exercised in full. The 2023 Placement Agents Warrants have an exercise price equal to $37.50 per
share, are exercisable commencing six months following issuance, and have a term of exercise equal to five years following the initial
issuance date. The 2023 Shares and 2023 Common Warrants were sold at an offering price of $30.00 per share and accompanying 2023 Common
Warrant and the Pre-Funded Warrants and 2023 Common Warrants were sold at an offering price of $29.997 per Pre-Funded Warrant and accompanying
2023 Common Warrant.
Holders
of 2023 Warrants and 2024 Warrants (together, the “Warrants”) may exercise such warrants on a “cashless” basis
if an effective registration statement is not available with respect to the offering of shares of common stock upon exercise of
such Warrant. In such event, the Holder may elect instead to receive upon such exercise (either in whole or in part) the net number of
shares of common stock determined according to a formula set forth in the Warrants. The exercise price and number of shares of common
stock issuable upon exercise of the Warrants may be adjusted in certain circumstances, including in the event of a stock dividend, extraordinary
dividend on or recapitalization, reorganization, merger or consolidation. The Warrants may be exercised by delivery of a notice of exercise
and the aggregate exercise price (assuming no cashless exercise has been elected if an effective registration statement is not available
with respect to the offering of shares of common stock upon exercise of such Warrant) to us as specified in such Warrant. Holders
of Warrants do not have the rights or privileges of holders of common stock and any voting rights until they exercise their Warrants
and receive shares of common stock. After the issuance of shares of common stock upon exercise of the Warrants, each holder will be entitled
to one vote for each share held of record on all matters to be voted on by stockholders.
Anti-Takeover
Provisions Under Our Charter and Bylaws and Delaware Law
Certain
provisions of Delaware law, our certificate of incorporation and our bylaws contain provisions that could have the effect of delaying,
deferring or discouraging another party from acquiring control of us. These provisions, which are summarized below, may have the effect
of discouraging coercive takeover practices and inadequate takeover bids. These provisions are also designed, in part, to encourage persons
seeking to acquire control of us to first negotiate with our Board. We believe that the benefits of increased protection of our potential
ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because
negotiation of these proposals could result in an improvement of their terms.
Amended
and Restated Certificate of Incorporation
Undesignated
Preferred Stock. Our Board has the ability to issue preferred stock with voting or other rights or preferences that could impede
the success of any attempt to change control of us. These and other provisions may have the effect of deferring hostile takeovers or
delaying changes in control or management of our company.
Special
Meetings of Stockholders. Our bylaws provide that special meetings of our stockholders may be called only by our Chairman, President
or a majority of the entire Board of Directors, thus prohibiting a stockholder from calling a special meeting. This provision might delay
the ability of our stockholders to force consideration of a proposal or for stockholders controlling a majority of our capital stock
to take any action, including the removal of directors.
Board
Vacancies Filled Only by Majority of Directors. Vacancies and newly created seats on our Board may be filled only by a majority of
the directors then in office. Only our Board may determine the number of directors on our board. The inability of stockholders to determine
the number of directors or to fill vacancies or newly created seats on our Board makes it more difficult to change the composition of
our Board, but these provisions promote a continuity of existing management.
No
Cumulative Voting. The DGCL provides that stockholders are not entitled to the right to cumulate votes in the election of directors
unless our certificate of incorporation provides otherwise. Our certificate of incorporation and bylaws do not expressly provide for
cumulative voting.
Directors
Removed Only by Special Meeting of Stockholders. A director can be removed only by the affirmative vote of a majority of the votes
of the issued and outstanding stock entitled to vote for the election of directors of the corporation given at a special meeting of the
stockholders called and held for this purpose.
Amendment
of Charter Provisions. In order to amend certain of the above provisions in our certificate of incorporation and our bylaws, the
Board is expressly authorized to adopt, alter or repeal the bylaws, subject to the rights of the stockholders entitled to vote. Stockholders
can vote at any stockholder meeting and repeal, alter, or amend the bylaws by the affirmative vote of a majority of the stockholders
entitled to vote in such meeting.
Delaware
Anti-takeover Statute
We
are subject to Section 203 of the DGCL. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business
combination” with an “interested stockholder” for a period of three years after the date of the transaction in which
the person became an interest stockholder, unless the business combination is approved in a prescribed manner. A “business combination”
includes mergers, asset sales and other transactions in which the interested stockholder receives or could receive a financial benefit
on other than a pro rata basis with other stockholders. An “interested stockholder” is a person who, together with
affiliates and associates, owns, or within three years did own, 15% or more of the corporation’s outstanding voting stock. This
provision has an anti-takeover effect with respect to transactions not approved in advance by our Board, including discouraging takeover
attempts that might result in a premium over the market price for the shares of our market price. With approval of our stockholders,
we could amend our amended and restated certificate of incorporation in the future to avoid the restrictions imposed by this anti-takeover
law.
The
provisions of Delaware law and our amended and restated certificate of incorporation could have the effect of discouraging others from
attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock
that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in
our management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise
deem to be in their best interests.
Transfer
Agent and Registrar
Our
transfer agent and registrar for our capital stock is Computershare. The transfer agent’s address is 8742 Lucent Blvd., Suite 225,
Highland Ranch, CO 80129, and its telephone number is (303) 262-0600.
Existing
Trading Markets
Our
common stock is listed on the Nasdaq Capital Market under the trading symbol “GTBP.” The closing sale price of our common
stock on the Nasdaq Capital Market on December 20, 2024, was $1.75 per share.
Listing
on the Nasdaq Capital Market
Our
common stock is listed on the Nasdaq Capital Market under the symbol “GTBP.”
DESCRIPTION
OF SECURITIES WE ARE OFFERING
We
are offering up to shares of our common stock (or pre-funded warrants in lieu of)
and up to common warrants to purchase up to shares of common stock. Each share of
common stock is being offered together with one common warrant to purchase one share of common stock. We are also offering
pre-funded warrants to those purchasers whose purchase of shares of common stock in this offering would result in the purchaser,
together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser,
9.99%) of our outstanding shares of common stock following the consummation of this offering in lieu of the shares of common stock
that would result in such excess ownership. Each pre-funded warrant will be exercisable for one share of common stock. Each
pre-funded warrant is being offered together with one common warrant to purchase one share of common stock. No warrant for
fractional shares of common stock will be issued, rather warrants will be issued only for whole shares of common stock. We are also
registering the shares of common stock issuable from time to time upon exercise of the pre-funded warrants and common warrants
offered hereby.
Common
Stock
The
material terms and provisions of our common stock are described under the caption “Description of Capital Stock” in
this prospectus and are incorporated herein by reference.
Common
Warrants
General
The
following is a summary of certain terms and provisions of the common warrants that are being offered hereby is not complete and is subject
to, and qualified in its entirety by, the provisions of the common warrant, the form of which will be filed as an exhibit to the registration
statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of
common warrant for a complete description of the terms and conditions of the common warrants.
Duration,
Exercise Price and Form
Each
common warrant offered hereby has an initial exercise price per share equal to $ . Each common warrant is exercisable for one share of
common stock. The common warrants are exercisable immediately following issuance, and have a term of exercise equal to five years following
the initial exercise date. The exercise price and number of shares issuable upon exercise is subject to appropriate adjustment in the
event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The common
warrants will be issued in certificated form only.
Exercisability
The
common warrants are exercisable immediately after issuance, at the option of each holder, in whole or in part, by delivering to us a
duly-executed exercise notice accompanied by payment in full for the number of shares purchased upon such exercise (except in the case
of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s
common warrants to the extent that the holder would own more than 4.99% (or 9.99%, at the holder’s election) of our outstanding
common stock immediately after exercise, except that upon notice from the holder to us, the holder may decrease or increase the limitation
of ownership of outstanding stock after exercising the holder’s common warrants up to 9.99% of the number of shares of our common
stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the
terms of the common warrants, provided that any increase in such limitation shall not be effective until 61 days following notice to
us. No fractional shares will be issued in connection with the exercise of a common warrant. In lieu of fractional shares, we will either
pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
Cashless
Exercise
If,
at the time a holder exercises its common warrants, a registration statement registering the issuance of the shares of common stock underlying
the common warrants under the Securities Act, is not then effective or available for the resale of such shares, then in lieu of making
the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may
elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according
to a formula set forth in the common warrant.
Fundamental
Transactions
In
the event of any fundamental transaction, as described in the common warrants and generally including any merger with or into another
entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our shares of common stock,
then upon any subsequent exercise of a common warrant, the holder will have the right to receive as alternative consideration, for each
share of common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction,
the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation,
and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of common stock
for which the common warrant is exercisable immediately prior to such event. In addition, upon a fundamental transaction, the holder
will have the right to require us to repurchase its common warrant at its fair value using the Black Scholes option pricing formula in
the common warrants; provided, however, that, if the fundamental transaction is not within our control, including not approved by our
board of directors, then the holder shall only be entitled to receive the same type or form of consideration (and in the same proportion),
at the Black Scholes value of the unexercised portion of the common warrant, that is being offered and paid to the holders of our common
stock in connection with the fundamental transaction.
Transferability
Subject
to applicable laws, a common warrant may be transferred at the option of the holder upon surrender of the common warrant to us together
with the appropriate instruments of transfer.
Exchange
Listing
There
is no trading market available for the common warrants on any securities exchange or nationally recognized trading system. We do not
intend to list the common warrants on any securities exchange or nationally recognized trading system. Without an active market, the
liquidity of the common warrants will be limited.
Right
as a Stockholder
Except
as otherwise provided in the common warrants or by virtue of such holder’s ownership of our common stock, the holders of the common
warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their
common warrants.
Pre-Funded
Warrants
General
The
following is a summary of certain terms and
provisions of the pre-funded warrants that are being offered hereby is not complete and is subject to, and qualified in
its entirety by, the provisions of the pre-funded warrant, the form of which will be filed as an exhibit to the
registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of pre-funded warrant for a complete
description of the terms and conditions of the pre-funded warrants.
Duration,
Exercise Price and Form
Each
pre-funded warrant offered hereby has an initial exercise price per share equal to $0.0001. Each pre-funded warrant is exercisable for
one share of common stock. The pre-funded warrants are exercisable immediately, and will be exercisable until exercised in full. The
exercise price and number of shares issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock
splits, reorganizations or similar events affecting our common stock and the exercise price. The pre-funded warrants will be issued in
certificated form only.
Exercisability
The
pre-funded warrants are exercisable immediately, at the option of each holder, in whole or in part, by delivering to us a duly-executed
exercise notice accompanied by payment in full for the number of shares purchased upon such exercise (except in the case of a cashless
exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s pre-funded
warrants to the extent that the holder would own more than 4.99% (or 9.99%, at the holder’s election) of our outstanding common
stock immediately after exercise, except that upon notice from the holder to us, the holder may decrease or increase the limitation of
ownership of outstanding stock after exercising the holder’s pre-funded warrants up to 9.99% of the number of shares of our common
stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the
terms of the pre-funded warrants, provided that any increase in such limitation shall not be effective until 61 days following notice
to us. No fractional shares will be issued in connection with the exercise of a pre-funded warrant. In lieu of fractional shares, we
will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next
whole share.
Cashless
Exercise
A
holder, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise
price, may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined
according to a formula set forth in the pre-funded warrant.
Fundamental
Transactions
In
the event of any fundamental transaction, as described in the pre-funded warrants and generally including any merger with or into another
entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our shares of common stock,
then upon any subsequent exercise of a pre-funded warrant, the holder will have the right to receive as alternative consideration, for
each share of common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction,
the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation,
and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of common stock
for which the pre-funded warrant is exercisable immediately prior to such event.
Transferability
Subject
to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant to us
together with the appropriate instruments of transfer.
Exchange
Listing
There
is no trading market available for the pre-funded warrants on any securities exchange or nationally recognized trading system. We do
not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading system. Without an active market,
the liquidity of the pre-funded warrants will be limited.
Right
as a Stockholder
Except
as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of our common stock, the holders of the
pre-funded warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise
their pre-funded warrants.
Placement
Agent Warrants
The
following is a summary of certain terms and provisions of the placement agent warrants that are being offered hereby is not complete
and is subject to, and qualified in its entirety by, the provisions of the placement agent warrant, the form of which will be filed as
an exhibit to the registration statement of which this prospectus forms a part.
Prospective investors should carefully review the terms and provisions of the form of placement agent warrant for a complete description
of the terms and conditions of the placement agent warrants.
The
placement agent warrants are substantially similar to the common warrants, except that each placement agent warrant will have an exercise
price of $ per share (which represents 125% of the public offering price per share of common
stock and accompanying common warrants sold in this offering) and will expire five years from the commencement of sales of the
offering.
MATERIAL
TAX CONSIDERATIONS
The
following is a discussion of material U.S. federal income tax consequences generally applicable to the acquisition, ownership, and disposition
of common stock, common warrants, and pre-funded warrants issued pursuant to this offering. This discussion does not address tax consequences
other than those pertaining to U.S. federal income taxation. For example, this discussion does not address any consequences relating
to estate or gift taxation, the alternative minimum tax, or the Medicare tax on investment income. Nor does this discussion address any
aspects of U.S. state or local or non-U.S. taxation. This discussion applies only to holders that hold our common stock, common warrants,
and pre-funded warrants as “capital assets” within the meaning of Section 1221 of the U.S. Internal Revenue Code of 1986,
as amended (the “Code”). This discussion does not address all aspects of U.S. federal income taxation that may be relevant
to holders in light of their particular circumstances or status, including:
| ● | financial
institutions or financial services entities; |
| | | |
| ● | broker-dealers; |
| | | |
| ● | S
corporations; |
| | | |
| ● | partnerships
(including entities or arrangements treated as partnerships for U.S. federal income tax purposes); |
| | | |
| ● | taxpayers
that are subject to the mark-to-market accounting rules; |
| | | |
| ● | tax-exempt
entities; |
| | | |
| ● | governments
or agencies or instrumentalities thereof; |
| | | |
| ● | insurance
companies; |
| | | |
| ● | regulated
investment companies or real estate investment trusts; |
| | | |
| ● | expatriates
or former long-term residents or citizens of the United States; |
| | | |
| ● | persons
for whom our common stock or pre-funded warrants constitute “qualified small business
stock” within the meaning of Section 1202 of the Code; |
| | | |
| ● | persons
that hold our securities as part of a straddle, constructive sale, hedging, conversion or
other integrated or similar transaction; |
| | | |
| ● | persons
subject to the alternative minimum tax; |
| | | |
| ● | U.S.
persons whose functional currency is not the U.S. dollar; |
| | | |
| ● | controlled
foreign corporations; |
| | | |
| ● | accrual
method taxpayers that file applicable financial statements as described in Section 451(b)
of the Code; or |
| | | |
| ● | passive
foreign investment companies. |
If
a partnership (or any entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds common stock, common
warrants, or pre-funded warrants, the tax treatment of such partnership and a person treated as a partner of such partnership will generally
depend on the status of the partner and the activities of the partnership. Partnerships holding any common stock, common warrants, or
pre-funded warrants, and persons that are treated as partners of such partnerships, should consult their tax advisors.
This
discussion is based on the Code, Treasury Regulations promulgated under the Code, and judicial and administrative interpretations thereof,
all as of the date hereof. U.S. tax law is subject to change, which change could apply retroactively and could affect the tax considerations
described herein. We have not and do not intend to seek any ruling from the U.S. Internal Revenue Service (the “IRS”) regarding
any U.S. federal income tax considerations described herein. There can be no assurance that the IRS will not take positions inconsistent
with the considerations discussed below or that any such positions would not be sustained by a court.
EACH
PROSPECTIVE INVESTOR SHOULD CONSULT ITS TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH PROSPECTIVE INVESTOR OF THE
ACQUISITION, OWNERSHIP, AND DISPOSITION OF COMMON STOCK, COMMON WARRANTS, AND PRE-FUNDED WARRANTS, INCLUDING THE EFFECTS OF U.S. FEDERAL,
STATE AND LOCAL, AND NON-U.S. TAX LAWS.
CHARACTERIZATION
OF COMMON STOCK, COMMON WARRANTS, AND PRE-FUNDED WARRANTS
Allocation
of Purchase Price Between Common Stock, Common Warrants, and Pre-Funded Warrants
For
U.S. federal income tax purposes, each holder that acquires common stock (or pre-funded warrants) and common warrants pursuant to this
offering should allocate the purchase price paid by such holder between the underlying common stock or pre-funded warrant (as applicable)
and common warrant based on their relative fair market values at the time of issuance. The amount of purchase price allocated to each
share of common stock or pre-funded warrant (as applicable) and each common warrant should establish the applicable holder’s initial
tax basis in such share of common stock or pre-funded warrant (as applicable) and such common warrant. We do not intend to advise holders
with respect to the relative fair market values of common stock, common warrants, and pre-funded warrants. Each holder is accordingly
urged to consult its tax advisor with respect to the allocation of purchase price between common stock, common warrants, and pre-funded
warrants.
Characterization
of Pre-Funded Warrants
Although
the characterization of the pre-funded warrants for U.S. federal income tax purposes is not entirely clear, we intend to take
the position that each pre-funded warrant is treated for U.S. federal income tax purposes as a share of our common stock. This is because
the exercise price of each pre-funded warrant is a nominal amount equal to $0.0001. The remainder of this discussion assumes
that the characterization of pre-funded warrants described above will be respected for U.S. federal income tax purposes.
U.S.
HOLDERS
As
used herein, a “U.S. Holder” is a beneficial owner of our common stock, pre-funded warrant, or common warrant (as applicable)
that is, for U.S. federal income tax purposes:
| ● | an
individual citizen or resident of the United States, |
| | | |
| ● | a
corporation (or other entity that is treated as a corporation for U.S. federal income tax
purposes) that is created or organized (or treated as created or organized) in or under the
laws of the United States or any state thereof or the District of Columbia, |
| | | |
| ● | an
estate whose income is subject to U.S. federal income tax regardless of its source, or |
| | | |
| ● | a
trust if (1) a U.S. court can exercise primary supervision over the administration of such
trust and one or more U.S. persons have the authority to control all substantial decisions
of the trust or (2) it has a valid election in place to be treated as a U.S. person. |
Distributions
on Our Common Stock
We
currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate
declaring or paying any cash dividends for the foreseeable future. If we do make distributions with respect to our common stock, a U.S.
Holder generally should be required to include in gross income as a dividend the amount of any cash distribution or the fair market value
of any other property distributed with respect to shares of our common stock, to the extent the distribution is paid out of our current
or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts treated as a dividend that we pay
to a U.S. Holder that is a taxable corporation may qualify for a dividends received deduction, provided certain holding period and other
requirements are satisfied. Amounts treated as a dividend that we pay to a non-corporate U.S. Holder may be taxed as “qualified
dividend income” at preferential tax rates accorded to long-term capital gains, subject to certain exceptions and provided certain
holding period and other requirements are satisfied. Distributions in excess of current and accumulated earnings and profits should generally
constitute a return of capital that is applied against and that reduces (not below zero) the U.S. Holder’s adjusted tax basis in
its shares of our common stock. Any remaining excess should generally be treated as gain realized on the sale or other disposition of
our common stock and should generally be treated as described below under “—U.S. Holders—Sale, Exchange, or Other
Taxable Disposition of Our Common Stock, Pre-Funded Warrants, or Common Warrants.”
Sale,
Exchange, or Other Taxable Disposition of Our Common Stock, Pre-Funded Warrants, or Common Warrants
Upon
a sale, exchange, or other taxable disposition of our common stock, pre-funded warrants, or common warrants, a U.S. Holder generally
should recognize capital gain or loss equal to the difference between the amount realized on such sale, exchange, or other taxable disposition
and the U.S. Holder’s adjusted tax basis in the applicable shares of our common stock, pre-funded warrants, or common warrants.
Any such capital gain or loss generally should be long-term capital gain or loss if the U.S. Holder’s holding period for the shares
of our common stock, pre-funded warrants, or common warrants so disposed of exceeds one year. Long-term capital gains recognized by non-corporate
U.S. Holders may be eligible to be taxed at reduced rates. The deductibility of capital losses is subject to limitations.
Exercise
of a Pre-Funded Warrant
As
described in “—Characterization of Common Stock, Common Warrants, and Pre-Funded Warrants—Characterization of Pre-Funded
Warrants” above, we intend to take the position that each pre-funded warrant is treated for U.S. federal income tax purposes
as a share of our common stock. Based on this characterization, a U.S. Holder generally should not recognize taxable gain or loss as
a result of the acquisition of shares of our common stock upon exercise of a pre-funded warrant for its nominal exercise price. The U.S.
Holder’s tax basis in the share of our common stock received upon exercise of the pre-funded warrant should be an amount equal
to the sum of the U.S. Holder’s tax basis in the pre-funded warrant and the nominal exercise price of such pre-funded warrant.
The U.S. Holder’s holding period in the share of our common stock received upon exercise of the pre-funded warrant should include
the U.S. Holder’s holding period for the pre-funded warrant.
Exercise
or Lapse of a Common Warrant
A
U.S. Holder generally should not recognize taxable gain or loss as a result of the acquisition of shares of our common stock upon exercise
of a common warrant for cash in the amount of its exercise price. The U.S. Holder’s tax basis in the share of our common stock
received upon exercise of the common warrant generally should be an amount equal to the sum of the U.S. Holder’s tax basis in the
common warrant and the exercise price of such common warrant. It is unclear whether a U.S. Holder’s holding period for the shares
of our common stock received upon exercise of the common warrant will commence on the date of exercise of the common warrant or the day
following the date of exercise of the common warrant. In either case, the holding period should not include the period during which the
U.S. Holder held the common warrant. If a common warrant is allowed to lapse unexercised, a U.S. Holder generally should recognize a
capital loss equal to such U.S. Holder’s tax basis in the common warrant. The deductibility of capital losses is subject to limitations.
The
U.S. federal income tax treatment of a cashless exercise of common warrants is unclear under current law and could differ from the treatment
described above. A cashless exercise could be considered a taxable event. U.S. Holders are urged to consult their own tax advisors regarding
the cashless exercise of a common warrant and the U.S. federal income tax treatment thereof.
Possible
Constructive Distributions
The
terms of each common warrant provide in certain circumstances for an adjustment to the exercise price of the common warrant or an increase
in the shares of our common stock issuable upon exercise. An adjustment made pursuant to a bona fide, reasonable, adjustment formula
which has the effect of preventing dilution generally is not taxable. The U.S. Holders of the common warrant would, however, be treated
as receiving a constructive distribution from us if, for example, the adjustment increases the U.S. Holder’s proportionate interest
in our assets or earnings and profits (e.g., through a decrease to the exercise price or an increase in the number of shares of our common
stock that would be obtained upon exercise) as a result of a distribution of cash or other property with respect to shares of our common
stock. Such a constructive distribution to a U.S. Holder of common warrant would generally be treated as if such U.S. Holder had received
a cash distribution from us in an amount equal to the fair market value of such increased interest, taxed under principles similar to
those described in “—U.S. Holders—Distributions on Our Common Stock.” The rules governing constructive
distributions as a result of certain adjustments with respect to a common warrant are complex. U.S. Holders are urged to consult their
tax advisors on the potential existence and tax consequences of any such constructive distribution with respect to a common warrant.
Information
Reporting and Backup Withholding
U.S.
backup withholding and information reporting requirements may apply to distributions on our common stock, constructive distributions
on common warrants, and the receipt of proceeds from the sale, exchange, or other disposition of our common stock, pre-funded warrants,
or common warrants. Backup withholding generally should not apply, however, to a U.S. Holder who furnishes a correct taxpayer identification
number and makes other required certifications, or who is otherwise exempt from backup withholding and establishes such exempt status.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s U.S.
federal income tax liability, and a U.S. Holder generally may obtain a refund of any excess amounts withheld under the backup withholding
rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
NON-U.S.
HOLDERS
As
used herein, a “non-U.S. Holder” is a beneficial owner of our common stock, pre-funded warrant, or common warrant (as applicable)
that, for U.S. federal income tax purposes, is or is treated as an individual, corporation, estate or trust that is not a U.S. Holder.
Distributions
on Our Common Stock
We
currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate
declaring or paying any cash dividends for the foreseeable future. If we do make distributions with respect to our common stock, any
such distribution made to a non-U.S. Holder with respect to our common stock should generally constitute a dividend for U.S. federal
income tax purposes to the extent paid out of our current or accumulated earnings and profits, as determined under U.S. federal income
tax principles. Provided that any such dividend is not effectively connected with such non-U.S. Holder’s conduct of a trade or
business within the United States (and, if required by an applicable income tax treaty, attributable to a U.S. permanent establishment
or fixed base maintained by such non-U.S. Holder), and subject to the discussion below regarding
backup withholding and FATCA (defined below), such dividend should generally be subject to withholding tax from the gross amount
of the dividend at a rate of 30%, unless such non-U.S. Holder is eligible for a reduced rate of withholding tax under an applicable income
tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN or W-8BEN-E, as
applicable). Any distribution not constituting a dividend should generally be treated first as reducing (not below zero) the non-U.S.
Holder’s adjusted tax basis in our common stock and then, to the extent such distribution exceeds the non-U.S. Holder’s adjusted
tax basis, as gain realized from the sale or other disposition of our common stock, which should generally be treated as described below
under “—Non-U.S. Holders—Sale, Exchange or Other Taxable Disposition of Our Common Stock, Pre-Funded Warrants, or
Common Warrants.”
Dividends
paid by us to a non-U.S. Holder that are effectively connected with such non-U.S. Holder’s conduct of a trade or business within
the United States (and, if required by an applicable income tax treaty, attributable to a U.S. permanent establishment or fixed base
maintained by such non-U.S. Holder) should generally not be subject to U.S. withholding tax, provided such non-U.S. Holder complies with
certain certification and disclosure requirements (usually by providing an IRS Form W-8ECI). Instead, such dividends should generally
be subject to U.S. federal income tax, net of certain deductions, at the same graduated individual or corporate rates applicable to U.S.
Holders. If the non-U.S. Holder is a corporation, dividends that are effectively connected income may also be subject to a “branch
profits tax” at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty).
Sale,
Exchange, or Other Taxable Disposition of Our Common Stock, Pre-Funded Warrants, or Common Warrants
Subject
to the discussion below regarding backup withholding and FATCA, a non-U.S.
Holder generally should not be subject to U.S. federal income tax on gain realized from a sale, exchange, or other disposition of our
common stock, pre-funded warrants, or common warrants unless:
| (i) | such
non-U.S. Holder is an individual who was present in the United States for 183 days or more
in the taxable year of such disposition and certain other requirements are met, in which
case any gain realized will generally be subject to a flat 30% U.S. federal income tax; |
| | | |
| (ii) | the
gain is effectively connected with a trade or business of such non-U.S. Holder in the United
States (and, if required by an applicable income tax treaty, attributable to a U.S. permanent
establishment or fixed base maintained by such non-U.S. Holder), in which case such gain
will be subject to U.S. federal income tax, net of certain deductions, at the same graduated
individual or corporate rates applicable to U.S. Holders, and any such gain of a non-U.S.
Holder that is a corporation may be subject to an additional “branch profits tax”
at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty);
or |
| | | |
| (iii) | subject
to certain exceptions discussed below, we are or have been a U.S. real property holding corporation
(a “USRPHC”) at any time during the shorter of the five-year period preceding
such disposition and such non-U.S. Holder’s holding period, in which case (a) gain
recognized by such non-U.S. holder on the sale, exchange, or other disposition of our common
stock, pre-funded warrants, or common warrants should generally be subject to tax at generally
applicable U.S. federal income tax rates and (b) a buyer of our common stock, pre-funded
warrants, or common warrants from such non-U.S. Holder may be required to withhold U.S. federal
income tax at a rate of 15% of the amount realized upon such disposition. |
For
purposes of item (iii) immediately above, we will generally be classified as a USRPHC if the fair market value of our “United States
real property interests” equals or exceeds 50% of the sum of the fair market value of our worldwide real property interests and
our other assets used or held for use in a trade or business, as determined for U.S. federal income tax purposes. Although there can
be no assurance, we believe that we are not currently a USRPHC and we do not anticipating becoming a USRPHC. Even if we are or become
a USRPHC, a non-US Holder should generally not be subject to U.S. federal income tax under the rules discussed in item (iii) with respect
to gain realized on a sale or other disposition of our common stock if (A) our common stock is considered to be regularly traded on an
established securities market and (B) such non-U.S. Holder has not owned and is not deemed to have owned more than 5% of our common stock
at any time during the shorter of the five-year period preceding such disposition and such non-U.S. Holder’s holding period. There
can be no assurance that shares of our common stock qualify as regularly traded on an established securities market for purposes of these
rules.
Exercise
of a Pre-Funded Warrant
The
U.S. federal income tax treatment of a non-U.S. Holder’s exercise of a pre-funded warrant generally should correspond to the U.S.
federal income tax treatment of the exercise of a pre-funded warrant held by a U.S. Holder, as described above under “—U.S.
Holders—Exercise of a Pre-Funded Warrant.”
Exercise
or Lapse of a Common Warrant
The
U.S. federal income tax treatment of a non-U.S. Holder’s exercise of a common warrant generally should correspond to the U.S. federal
income tax treatment of the exercise of a common warrant held by a U.S. Holder, as described above under “—U.S. Holders—Exercise
or Lapse of a Common Warrant.” If a common warrant is allowed to lapse unexercised, a non-U.S. Holder generally should recognize
a loss equal to such non-U.S. Holder’s tax basis in the common warrant. However, subject to additional conditions and restrictions,
a non-U.S. Holder generally cannot utilize a loss recognized upon expiration of a common warrant to reduce the non-U.S. Holder’s
U.S. federal income tax liability unless the loss is effectively connected with the non-U.S. Holder’s conduct of a trade or business
within the United States (and, if an income tax treaty applies, is attributable to a U.S. permanent establishment or fixed base maintained
by the non-U.S. Holder) or if the loss is treated as U.S.-source and incurred by a non-U.S. Holder that is an individual during a taxable
year in which the non-U.S. Holder is present in the United States for at least 183 days.
The
U.S. federal income tax treatment of a cashless exercise of common warrants is unclear under current law and could differ from the considerations
described above. A cashless exercise could be considered a taxable event. Non-U.S. Holders are urged to consult their own tax advisors
regarding any cashless exercise of a common warrant and the U.S. federal income tax treatment thereof.
Possible
Constructive Distributions
As
described above under “—U.S. Holders—Possible Constructive Distributions,” certain adjustments with respect
to the common warrants may give rise to a constructive distribution, the consequences of which to a non-U.S. Holder would be similar
to those described above under “—Non-U.S. Holders—Distributions on Our Common Stock.”
Information
Reporting and Backup Withholding
U.S.
backup withholding and information reporting requirements may apply to distributions on our common stock, constructive distributions
on common warrants, and the receipt of proceeds from the sale or disposition of our common stock, pre-funded warrants, or common warrants.
A non-U.S. Holder may have to comply with certification procedures to establish that it is not a U.S. person for U.S. federal income
tax purposes, to otherwise establish an exemption from information reporting and backup withholding requirements, or to claim a reduced
rate of withholding under an applicable income tax treaty. Backup withholding is not an additional tax. Amounts withheld as backup withholding
may be credited against a non-U.S. Holder’s U.S. federal income tax liability, and a non-U.S. Holder generally may obtain a refund
of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the IRS and
furnishing any required information.
Foreign
Account Tax Compliance Act
Sections
1471 through 1474 of the Code and the Treasury Regulations and administrative guidance promulgated thereunder (commonly referred as the
“Foreign Account Tax Compliance Act” or “FATCA”) generally impose withholding at a rate of 30% in certain circumstances
on dividends in respect of securities (including our common stock), and (subject to the proposed
Treasury Regulations discussed below) the gross proceeds derived from the sale or other disposition of our common stock, which
are held by or through certain foreign financial institutions (including investment funds), unless any such institution (i) enters into,
and complies with, an agreement with the IRS to, among other things, comply with specified due diligence, report, on an annual basis,
information with respect to interests in, and accounts maintained by, the institution that are owned by certain U.S. persons and by certain
non-U.S. entities that are wholly or partially owned by U.S. persons and to withhold on certain payments, or (ii) if allowed under an
intergovernmental agreement between the United States and an applicable foreign country, reports such information to its local tax authority,
which may exchange such information with the U.S. authorities. An intergovernmental agreement between the United States and an applicable
foreign country may modify these requirements. Accordingly, the disclosure of the ownership of the entity through which our securities
(including our common stock, pre-funded warrants, and common warrants) is held will affect the determination of whether such withholding
and reporting is required. Withholding agents may, however, rely on proposed U.S. Treasury Regulations
that would no longer require FATCA withholding on payments of gross proceeds. A withholding agent, and not GT BIOPHARMA, INC., will determine
whether or not to implement gross proceeds FATCA withholding. Similarly, dividends in respect of our common stock held by an investor
that is a non-financial non-U.S. entity that does not qualify under certain exceptions will generally be subject to withholding at a
rate of 30%, unless such entity either (i) certifies to the applicable withholding agent that such entity does not have any “substantial
United States owners” or (ii) provides certain information regarding the entity’s “substantial United States owners,”
which may in turn be provided to the U.S. Department of Treasury. All holders should consult their tax advisors regarding the possible
implications of FATCA on their investment in our common stock, pre-funded warrants, and common warrants.
PLAN
OF DISTRIBUTION
We
engaged Roth Capital Partners, LLC (“Roth” or the “placement agent”), to act as our exclusive placement agent
to solicit offers to purchase the securities offered by this prospectus on a reasonable best efforts basis subject to the terms and
conditions of the placement agency agreement dated , 2024. Roth is not purchasing
or selling any securities, nor are they required to arrange for the purchase and sale of any specific number or dollar amount of securities,
other than to use their “reasonable best efforts” to arrange for the sale of the securities by us. Therefore, we may not
sell the entire amount of securities being offered. There is no minimum amount of proceeds that is a condition to closing of this offering.
The placement agent does not guarantee that it will be able to raise new capital in this offering. The terms of this offering were subject
to market conditions and negotiations between us and prospective investors in consultation with the placement agent. The placement agent
will have no authority to bind us. This offering will terminate no later than , 2025,
unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. We will have one closing
for all the securities purchased in this offering. The combined public offering price per share (or pre-funded warrant) and accompanying
common warrant will be fixed for the duration of this offering. Roth may engage one or more sub-placement agents or selected dealers
to assist with the offering.
We
will enter into a securities purchase agreement directly with the institutional investors, at the investor’s option, who purchase
our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus
in connection with the purchase of our securities in this offering.
Placement
Agent Fees and Expenses
The
following table shows the per share and accompanying common warrants, and per pre-funded warrant and accompanying common
warrants, and total placement agent fees we will pay in connection with the sale of the securities in this offering.
| |
|
|
Per
Share and Common Warrant |
|
|
Per Pre-Funded Warrant and
Common Warrant |
|
|
Total |
|
| Public
offering price |
|
$ |
|
|
$ |
|
|
$ |
|
|
| Placement
Agent fees(1) |
|
$ |
|
|
$ |
|
|
$ |
|
|
| Proceeds
to us, before expenses(2) |
|
$ |
|
|
$ |
|
|
$ |
|
|
(1)
Includes a cash fee up to 6% of the gross proceeds raised in this offering; provided, however, the cash fee shall
equal 3% of the gross proceeds raised in this offering for securities purchased by certain excluded investors, to
be paid to the placement agent. We have also agreed to reimburse the placement agent for certain of its offering-related expenses in
an aggregate amount up to $100,000. In addition, we have agreed to issue the placement agent or its designees warrants to purchase
up to shares of common stock (equal to 6% of the aggregate number of securities
sold in this offering, subject to a partial adjustment in the event certain investors participate) at an exercise price of
$ per share, which represents 125% of the public offering price per share of
common stock and common warrants. We refer to these warrants in this prospectus as the “placement agent
warrants.”
(2)
Because there is no minimum number of securities or amount of proceeds required as a condition to closing in this offering, the actual
public offering amount, placement agent fees, and proceeds to us, if any, are not presently determinable and may be substantially less
than the total maximum offering amounts set forth above.
We
estimate that the total expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting
expenses, but excluding placement agent fees, will be approximately $ , all of which are payable by us. This figure includes the placement
agent’s accountable expenses, including, but not limited to, legal fees for placement agent’s legal counsel, that we have
agreed to pay at the closing of the offering up to an aggregate expense reimbursement of $100,000.
Placement
Agent Warrants
In
addition, we have agreed to issue to the placement agent, or its designees, warrants to purchase up to
shares of common stock (equal to 6% of the aggregate number of securities sold in this offering, subject to a partial adjustment in the
event certain investors participate) at an exercise price of $ per share, which represents
125% of the public offering price per share of common stock and accompanying common warrant, and exercisable for five years from the
date of the commencement of sales in this offering. We refer to these warrants in this prospectus as the “placement agent warrants.”
The placement agent warrants are in substantially similar form to the common warrants being issued to investors as part of this offering
other than as described herein. The placement agent warrants and underlying shares of common stock are registered on the registration
statement of which this prospectus is a part. The form of the placement agent warrant will be included as an exhibit to this registration
statement of which this prospectus forms a part.
Other
Relationships
The
placement agent may, from time to time, engage in transactions with or perform services for us in the ordinary course of its business
and may continue to receive compensation from us for such services.
Determination
of Offering Price
The
combined public offering price per share and common warrant and the combined public offering price per pre-funded warrant and
common warrant we are offering and the exercise prices and other terms of the warrants were negotiated between
us and the investors, in consultation with the placement agent based on the trading of our common stock prior to this offering, among
other things. Other factors considered in determining the public offering prices of the securities we are offering and the exercise prices
and other terms of the warrants include the history and prospects of our company, the stage of development of our business, our business
plans for the future and the extent to which they have been implemented, an assessment of our management, general conditions of the securities
markets at the time of the offering and such other factors as were deemed relevant.
Transfer
Agent and Registrar
Our
transfer agent and registrar for our capital stock is Computershare. The transfer agent’s address is 8742 Lucent Blvd., Suite 225,
Highland Ranch, CO 80129, and its telephone number is (303) 262-0600.
The
Nasdaq Capital Market Listing
Our
common stock is currently listed on the Nasdaq Stock Market LLC under the symbol “GTBP.” On December 20, 2024, the
reported closing price per share of our common stock was $1.75. The final public offering price will be determined between us,
the placement agent and the investors in the offering, and may be at a discount to the current market price of our common stock. Therefore,
the assumed public offering price used throughout this prospectus may not be indicative of the final public offering price. There is
no established public trading market for the common warrants or pre-funded warrants, and we do not expect such markets to develop. In
addition, we do not intend to apply for a listing of the common warrants or pre-funded warrants on any national securities exchange or
other nationally recognized trading system.
Indemnification
We
have agreed to indemnify the placement agent against certain liabilities, including certain liabilities arising under the Securities
Act, or to contribute to payments that the placement agent may be required to make for these liabilities.
Lock-up
Restrictions
We have
agreed for a period of days following the closing of this offering not to
issue, enter into an agreement to issue or announce the issuance or proposed issuance of shares of our common stock or any other
securities convertible into, or exercisable or exchangeable for, shares of common stock or file any registration statement or
amendment or supplement thereto, subject to limited exceptions. This agreement does not apply to, in addition to certain customary
exceptions, the issuance by us of equity or debt securities pursuant to acquisitions or strategic transactions approved by a
majority of our disinterested directors, where not for the purpose of raising capital, provided that, in each case, such securities
are issued as “restricted securities” (as defined in Rule 144 under the Securities Act), and carry no registration
rights that require or permit the filing of any registration statement in connection therewith during the -day lock-up period.
We
have also agreed for a period of following the closing date of this offering not to
(i) issue or agree to issue equity or debt securities convertible into, or exercisable or exchangeable for, shares at a conversion price,
exercise price or exchange price which floats with the trading price of our shares or which may be adjusted after issuance upon the occurrence
of certain events or (ii) enter into any agreement, including an equity line of credit, whereby we may issue securities at a future-determined
price, subject to certain conditions and exceptions.
Our
directors and executive officers have also entered into customary lock-up agreements. Under these agreements, these individuals
have agreed, subject to specified exceptions, not to sell or transfer any shares of common stock or securities convertible, exchangeable
or exercisable into, shares of our common stock during a period ending days after the date of this prospectus.
The
placement agent may waive these prohibitions in its sole discretion and without notice.
Regulation
M
The
placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act and any fees received
by it and any profit realized on the sale of the securities by it while acting as principal might be deemed to be underwriting discounts
or commissions under the Securities Act. The placement agent will be required to comply with the requirements of the Securities Act and
the Exchange Act of 1934, as amended (the “Exchange Act”), including, without limitation, Rule 10b-5 and Regulation M under
the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by the placement agent. Under
these rules and regulations, the placement agent may not (i) engage in any stabilization activity in connection with our securities;
and (ii) bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted
under the Exchange Act, until they have completed their participation in the distribution.
Electronic
Distribution
A
prospectus in electronic format may be made available on a website maintained by the placement agent and the placement agent may distribute
prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus
or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent
and should not be relied upon by investors.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information
to you by referring you to those documents instead of having to repeat the information in this prospectus. The information incorporated
by reference is considered to be part of this prospectus, and because we are a smaller reporting company, later information that we file
with the SEC will automatically update and supersede this information. We incorporate by reference the documents listed below and any
future filings (including those made after the initial filing of the registration statement of which this prospectus is a part and prior
to the effectiveness of such registration statement) we will make with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange
Act until the termination of the offering of the shares covered by this prospectus (other than information furnished under Item 2.02
or Item 7.01 of Form 8-K):
| |
● |
our
Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on March 26, 2024; |
| |
● |
our
Quarterly Report on Form 10-Q for the quarter ended March 31, June 30 and September 30, 2024, filed with the SEC on May
15, 2024, August
14, 2024, and November
14, 2024, respectively; |
| |
● |
our
definitive proxy statement on Schedule 14A, filed with the SEC on April 29, 2024; |
| |
● |
our
Current Reports on Form 8-K filed with the SEC on February 1, 2024, April 30, 2024, May 23, 2024, June 7, 2024, June 26, 2024, June 27, 2024; September 16, 2024, November 21, 2024 and November 27, 2024; and |
| |
● |
the
description of our common stock contained in Exhibit
4.3 to our Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on March 26, 2024, including any
amendment or report filed for the purpose of updating such description. |
All
documents the Company subsequently filed with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, except as to
any portion of any report or documents that is not deemed filed under such provisions, (1) on or after the date of filing of the registration
statement containing this prospectus and prior to the effectiveness of the registration statement and (2) on or after the date of this
prospectus until the earlier of the date on which all of the securities registered hereunder have been sold or the registration statement
of which this prospectus is a part has been withdrawn, shall be deemed incorporated by reference in this prospectus and to be a part
of this prospectus from the date of filing of those documents and will be automatically updated and, to the extent described above, supersede
information contained or incorporated by reference in this prospectus and previously filed documents that are incorporated by reference
in this prospectus. Any statements contained in a previously filed document incorporated by reference into this prospectus is deemed
to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus, or in a subsequently
filed document also incorporated by reference herein, modifies or supersedes that statement.
Nothing
in this prospectus shall be deemed to incorporate information furnished but not filed with the SEC pursuant to Item 2.02, 7.01 or 9.01
of Form 8-K.
Upon
written or oral request, we will provide without charge to each person to whom a copy of the prospectus is delivered a copy of the documents
incorporated by reference herein (other than exhibits to such documents, unless such exhibits are specifically incorporated by reference
herein). You may request a copy of these filings, at no cost, by contacting GT Biopharma, Inc.
We
maintain a website at https://ir.gtbiopharma.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current
reports on Form 8-K and other reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of
charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC.
There
have been no material changes to the Company’s affairs that have occurred since December 31, 2023 that have not been described
in a Form 10-Q or Form 8-K filed under the Exchange Act.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed a registration statement on Form S-1 under the Securities Act with the SEC with respect to this offering. This prospectus
was filed as a part of that registration statement but does not contain all of the information contained in the registration statement
and exhibits. Reference is thus made to the omitted information. Statements made in this prospectus are summaries of the material terms
of contracts, agreements and documents and are not necessarily complete; however, all information we considered material has been disclosed.
Reference is made to each exhibit for a more complete description of the matters involved and these statements are qualified in their
entirety by the reference. You can find, copy and inspect information we file at the SEC’s public reference room, which is located
at 100 F Street, N.E., Washington, DC 20549. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC’s
public reference room. The SEC also maintains a web site (http://www.sec.gov) that contains this filed registration statement, reports
and other information regarding us that we have filed electronically with the SEC. For more information pertaining to our company and
this offering, reference is made to the registration statement.
LEGAL
MATTERS
The
validity of the securities offered hereby will be passed upon for us by Baker & McKenzie LLP, Dallas, Texas. The placement agent
is being represented by Ellenoff Grossman & Schole LLP, New York, New York.
EXPERTS
The
financial statements of GT Biopharma, Inc. at December 31, 2023, and for the year in the period ending December 31, 2023, incorporated
by reference into this prospectus, have been audited by Weinberg & Company, P.A., an independent registered public accounting firm,
and the financial statements of GT Biopharma, Inc. at December 31, 2023, and for the year in the period ending December 31, 2023, incorporated
by reference into this prospectus, have been audited by Weinberg & Company, P.A., each as set forth in their report thereon incorporated
by reference herein, and are included in reliance upon such reports given on the authority of such firms as experts in accounting and
auditing.
Up
to Shares of
Common Stock
Pre-Funded
Warrants to Purchase up to Shares of Common Stock
Common
Warrants to Purchase up to Shares of Common Stock
Placement
Agent Warrants to Purchase up to Shares of Common Stock
Up
to
Shares of Common Stock underlying Pre-Funded Warrants,
Common
Warrants and Placement Agent Warrants

PRELIMINARY
PROSPECTUS
Roth
Capital Partners
The
date of this prospectus is , 2024.
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distributions
The
following table indicates the expenses to be incurred in connection with the offering described in this registration statement, other
than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimated except the Securities and Exchange
Commission registration fee.
| SEC registration fee | |
$ | 1,975 | |
| FINRA filing fee | |
$ | 2,435 | |
| Accounting fees and expenses | |
$ | 35,000 | |
| Legal fees and expenses | |
$ | 200,000 | |
| Miscellaneous | |
$ | * | |
| Total | |
$ | 239,410 | |
Item
14. Indemnification of Directors and Officers
Section
145 of the DGCL provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses
(including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in
connection with any threatened, pending or completed actions, suits or proceedings in which such person is made a party by reason of
such person being or having been a director, officer, employee or agent to us. The DGCL provides that Section 145 is not exclusive of
other rights to which those seeking indemnification may be entitled under any bylaw, agreement, vote of stockholders or disinterested
directors or otherwise. Our certificate of incorporation provides for indemnification by us of our directors, officers and employees
to the fullest extent permitted by the DGCL.
Section
102(b)(7) of the DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not
be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for
liability (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions
not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) for unlawful payments of dividends or
unlawful stock repurchases, redemptions or other distributions or (iv) for any transaction from which the director derived an improper
personal benefit. Our certificate of incorporation provides for such limitation of liability.
Item
15. Recent Sales of Unregistered Securities
Since
December 2021, the Company made the following issuances of its unregistered securities pursuant exemptions contained in Section 4(a)(2)
or 3(a)(9) of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder:
| |
● |
On
August 24, 2022, the Company entered into a revised agreement with this third-party manufacturer and issued 40,742 shares of common
stock with a fair value of approximately $3.2 million as part of a payment arrangement. |
| |
|
|
| |
● |
During
the year ended December 31, 2022, the Company issued 11,257 shares of common stock upon conversion of notes payable. |
| |
|
|
| |
● |
During
the year ended December 31, 2022, the Company issued 23,654 shares of common stock to officers, employees, and board of directors
with a fair value of approximate $2.5 million. This included 12,602 shares with a fair value of $938,000 that were granted during
the year. |
| |
|
|
| |
● |
During
the year ended December 31, 2022, the Company issued 17,562 shares of common stock to consultants with a fair
value of approximately $2.1 million. |
| |
|
|
| |
● |
During
the year ended December 31, 2022, the Company issued 57,437 shares of common stock to settle approximately $700,000 of vendor payables. |
| |
|
|
| |
● |
During the year ended December 31, 2023, the Company
issued 14,237 shares of common stock to officers, employees, and board of directors with a fair value of approximate $267,000. This
included 13,333 shares with a fair value of $115,000 that were granted during the year. |
| |
|
|
| |
● |
During the year ended December 31, 2023, the Company
issued 1,545 shares of common stock to consultants with a fair value of approximately $162,000. |
| |
|
|
| |
● |
On
January 4, 2023, the Company entered into a purchase agreement signed on December 30, 2022, between the Company and an institutional
investor for the issuance and sale, in a registered direct offering of 120,000 shares of the Company’s common stock,
pre-funded warrants to purchase up to 96,667 shares of the Company’s common stock, warrants to purchase up to an aggregate
of 216,667 shares of the Company’s common stock and placement agent warrants to purchase up to 13,000 of the Company’s
common stock. The Company raised $6.5 million from the offering. |
| |
|
|
| |
● |
On
April 30, 2024, the Company issued 36,018 shares of common stock to settle $278,500 of vendor accounts payable. The shares were valued
at the month-end closing price of the Company’s common stock for the months for which services were provided by the vendor. |
| |
|
|
| |
● |
On
May 23, 2024, the Company issued 740,000 common warrants, each to purchase one share of common
stock at an exercise price equal to $4.35 and are exercisable immediately upon issuance and will expire on the date that is
five years following the date of issuance. |
| |
|
|
| |
● |
On
May 23, 2024, the Company issued placement agent warrants to the placement agent to purchase up to 88,800 shares of common stock as part
of the compensation payable to the placement agent in connection with such offering at an exercise price of $5.4375 per share and will
expire five years from the commencement of sales of the offering. |
| |
|
|
| |
● |
On
June 30, 2024, the Company issued 91,579 shares of common stock to settle $531,300 of vendor accounts payable. The shares were valued
at the month-end closing price of the Company’s common stock for the months for which services were provided by the vendor. |
| |
|
|
| |
● |
Since
December 2021, we have issued 29,935 shares of common stock with a fair value of approximately $1,053,000 in
connection with compensation of the Company’s officers and directors. |
Item
16. Exhibits and Financial Statement Schedules
Exhibit
Index
| Exhibit
Number |
|
Exhibit
Description |
|
Form |
|
Date |
|
Number |
|
File
No. |
|
Filed
Herewith |
| 3.1 |
|
Restated Certificate of Incorporation as filed in Delaware September 10, 1996 and as thereafter amended through March 1, 2002 |
|
10-KSB |
|
04/01/02 |
|
3.A |
|
|
|
|
| 3.2 |
|
Certificate of Amendment to the Restated Certificate of Incorporation of GT Biopharma, Inc., dated February 9, 2011 |
|
10-K |
|
03/31/2011 |
|
3.2 |
|
|
|
|
| 3.3 |
|
Certificate of Amendment to the Restated Certificate of Incorporation of GT Biopharma, Inc., effective as of July 19, 2017 |
|
8-K/A |
|
03/15/2018 |
|
3.1 |
|
|
|
|
| 3.4 |
|
Certificate of Amendment to the Restated Certificate of Incorporation of GT Biopharma, Inc., effective as of February 10, 2021 |
|
8-K |
|
02/11/2021 |
|
3.1 |
|
|
|
|
| 3.5 |
|
Certificate of Amendment to the Restated Certificate of Incorporation of GT Biopharma, Inc., effective June 13, 2022 |
|
10-K |
|
03/30/2023 |
|
3.5 |
|
|
|
|
| 3.6 |
|
Amended and Restated Bylaws of GT Biopharma, Inc., effective November 3, 2022 |
|
8-K |
|
11/09/2022 |
|
3.1 |
|
|
|
|
| 3.7 |
|
Certificate of Amendment of Restated Certificate of Incorporation of GT Biopharma, Inc., effective February 1, 2024 |
|
8-K |
|
02/01/2024 |
|
3.1 |
|
|
|
|
| 4.1 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series J-1 Preferred Stock of GT Biopharma, Inc., dated April 3, 2019 |
|
8-K |
|
04/04/2019 |
|
3.1 |
|
|
|
|
| 4.2 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series K Preferred Stock of GT Biopharma, Inc., dated February 22, 2021 |
|
10-K |
|
04/16/2021 |
|
4.2 |
|
|
|
|
| 4.3 |
|
Description of the Registrant’s Securities Registered pursuant to Section 12 of the Securities Exchange Act of 1934, as Amended |
|
10-K |
|
03/30/2023 |
|
4.3 |
|
|
|
|
| 4.4** |
|
Form
of Common Warrant |
|
|
|
|
|
|
|
|
|
|
| 4.5** |
|
Form
of Pre-Funded Warrant |
|
|
|
|
|
|
|
|
|
|
| 4.6** |
|
Form
of Placement Agent Warrant |
|
|
|
|
|
|
|
|
|
|
| 4.9 |
|
Form of 10% Senior Convertible Debenture (related to Securities Purchase Agreement, dated January 9, 2017) |
|
8-K |
|
01/13/2017 |
|
10.2 |
|
|
|
|
| 4.10 |
|
Form of Common Stock Purchase Warrant (related to Securities Purchase Agreement, dated January 9, 2017) |
|
8-K |
|
01/13/2017 |
|
10.3 |
|
|
|
|
| 4.11 |
|
Registration Rights Agreement, dated January 22, 2018, among GT Biopharma, Inc. and the buyers named therein |
|
8-K |
|
01/23/2018 |
|
10.2 |
|
|
|
|
| 4.12 |
|
Form of Senior Convertible Note (related to Securities Purchase Agreement, dated January 22, 2018) |
|
8-K |
|
01/23/2018 |
|
10.3 |
|
|
|
|
| 4.13 |
|
Form of Warrant to Purchase Common Stock (related to Securities Purchase Agreement, dated January 22, 2018) |
|
8-K |
|
01/23/2018 |
|
10.4 |
|
|
|
|
| 4.14 |
|
Form of 10% Senior Convertible Debenture (related to Securities Purchase Agreement, dated August 2, 2018) |
|
8-K |
|
08/03/2018 |
|
4.1 |
|
|
|
|
| 4.15 |
|
Form of 10% Senior Convertible Debenture (related to Securities Purchase Agreement, dated September 7, 2018) |
|
8-K |
|
09/07/2018 |
|
4.1 |
|
|
|
|
| 4.16 |
|
Form of 10% Senior Convertible Debenture (related to Securities Purchase Agreement, dated September 24, 2018) |
|
8-K |
|
09/28/2018 |
|
4.1 |
|
|
|
|
| Exhibit
Number |
|
Exhibit
Description |
|
Form |
|
Date |
|
Number |
|
File
No. |
|
Filed
Herewith |
| 4.17 |
|
Form of Secured Convertible Note (related to Securities Purchase Agreement, dated February 4, 2019) |
|
8-K |
|
02/06/2019 |
|
4.1 |
|
|
|
|
| 4.18 |
|
Registration Rights Agreement, dated May 22, 2019, among GT Biopharma, Inc. and the purchasers named therein |
|
8-K |
|
05/24/2019 |
|
10.2 |
|
|
|
|
| 4.19 |
|
Form of Convertible Note (related to Securities Purchase Agreement, dated August 20, 2019) |
|
8-K |
|
05/24/2019 |
|
4.1 |
|
|
|
|
| 4.20 |
|
Registration Rights Agreement, dated August 20, 2019, among GT Biopharma, Inc. and the purchasers named therein |
|
8-K |
|
08/20/2019 |
|
10.2 |
|
|
|
|
| 4.21 |
|
Form of Convertible Note (related to Securities Purchase Agreement, dated May 22, 2019) |
|
8-K |
|
05/24/2019 |
|
4.1 |
|
|
|
|
| 4.22 |
|
Registration Rights Agreement, dated January 30, 2020, among GT Biopharma, Inc. and the purchaser named therein |
|
10-Q |
|
05/15/2020 |
|
10.2 |
|
|
|
|
| 4.23 |
|
Registration Rights Agreement, dated January 30, 2020, among GT Biopharma, Inc. and the purchaser named therein |
|
10-Q |
|
05/15/2020 |
|
10.2 |
|
|
|
|
| 4.24 |
|
Form of Convertible Note (related to Securities Purchase Agreement, dated January 30, 2020) |
|
10-Q |
|
05/15/2020 |
|
10.3 |
|
|
|
|
| 4.25 |
|
Form of Registration Rights Agreement among GT Biopharma, Inc. and the purchaser named therein (executed in April/May 2020) |
|
10-Q |
|
05/15/2020 |
|
10.5 |
|
|
|
|
| 4.26 |
|
Form of Convertible Note (related to Securities Purchase Agreement executed in April/May 2020) |
|
10-Q |
|
05/15/2020 |
|
10.6 |
|
|
|
|
| 4.27 |
|
Registration Rights Agreement, dated July 7, 2020, among GT Biopharma, Inc. and the purchaser named therein |
|
8-K |
|
07/09/2020 |
|
10.3 |
|
|
|
|
| 4.28 |
|
Form of Convertible Note (related to Securities Purchase Agreement, dated July 7, 2020) |
|
8-K |
|
07/09/2020 |
|
4.1 |
|
|
|
|
| 4.29 |
|
Form of Convertible Note, dated June 19, 2020 (related to Settlement Agreement, dated June 19, 2020). |
|
8-K |
|
06/19/2020 |
|
10.2 |
|
|
|
|
| 4.30 |
|
Form of Pre-Funded Warrant to Purchase Common Stock, dated June 19, 2020 (related to Settlement Agreement, dated June 19, 2020). |
|
8-K |
|
06/19/2020 |
|
10.3 |
|
|
|
|
| 4.31 |
|
Form of Convertible Note (related to Securities Purchase Agreement, dated September 16, 2020) |
|
8-K |
|
09/22/2020 |
|
4.1 |
|
|
|
|
| 4.32 |
|
Form of Secured Convertible Note |
|
8-K |
|
11/09/2020 |
|
4.1 |
|
|
|
|
| 4.33 |
|
Form of Settlement Note, dated November 9, 2020. |
|
10-Q |
|
11/13/2020 |
|
10.20 |
|
|
|
|
| 4.34 |
|
Settlement Note, dated December 22, 2020, by GT Biopharma Inc. payable to Alto Opportunity Master Fund, SPC - Segregated Master Portfolio B. |
|
8-K |
|
12/28/2020 |
|
10.2 |
|
|
|
|
| 4.35 |
|
Form of Amendment to Convertible Note, dated January 31, 2021 |
|
8-K |
|
02/01/2020 |
|
10.2 |
|
|
|
|
| 4.36 |
|
Form of Common Warrant |
|
8-K |
|
01/03/2023 |
|
4.1 |
|
|
|
|
| 4.37 |
|
Form of Pre-Funded Warrant |
|
8-K |
|
01/03/2023 |
|
4.2 |
|
|
|
|
| 4.38 |
|
Form of Placement Agent Warrant |
|
8-K |
|
01/03/2023 |
|
4.3 |
|
|
|
|
| 4.39 |
|
Form of Common Warrant |
|
8-K |
|
05/23/2024 |
|
4.1 |
|
|
|
|
| 4.40 |
|
GT Biopharma, Inc. 2022 Omnibus Incentive Plan |
|
DEF 14A |
|
04/29/2022 |
|
|
|
|
|
|
| 5.1** |
|
Opinion of Baker & McKenzie LLP |
|
S-1 |
|
|
|
5.1 |
|
|
|
|
| 10.1 |
|
Exclusive License Agreement, dated July 18, 2016, between the Regents of the University of Minnesota and Oxis Biotech, Inc. |
|
10-Q |
|
08/11/2017 |
|
10.3 |
|
|
|
|
| 10.2 |
|
License Agreement, dated September 3, 2015, among Daniel A. Vallera, Jeffrey Lion and Oxis Biotech, Inc. |
|
10-Q |
|
08/11/2017 |
|
10.4 |
|
|
|
|
| 10.3 |
|
Clinical Trial Agreement, dated September 2019, between the Regents of the University of Minnesota and GT Biopharma, Inc. |
|
10-Q |
|
05/15/2020 |
|
10.7 |
|
|
|
|
| Exhibit
Number |
|
Exhibit
Description |
|
Form |
|
Date |
|
Number |
|
File
No. |
|
Filed
Herewith |
| 10.4 |
|
Securities Purchase Agreement, dated January 30, 2020, among GT Biopharma, Inc. and the purchaser named therein |
|
10-Q |
|
05/15/2020 |
|
10.1 |
|
|
|
|
| 10.5 |
|
Form Securities Purchase Agreement among GT Biopharma, Inc. and the purchaser named therein (executed in April/May 2020) |
|
10-Q |
|
05/15/2020 |
|
10.4 |
|
|
|
|
| 10.6 |
|
Securities Purchase Agreement, dated July 7, 2020, among GT Biopharma, Inc. and the purchaser named therein |
|
8-K |
|
07/09/2020 |
|
10.1 |
|
|
|
|
| 10.7 |
|
Form of Standstill and Forbearance Agreement, dated June 23, 2020, between the Company and certain holders of convertible notes and debentures |
|
8-K |
|
06/23/2020 |
|
10.1 |
|
|
|
|
| 10.8 |
|
Settlement Agreement, dated June 19, 2020, among GT Biopharma, Inc., Empery Asset Master Ltd., Empery Tax Efficient, LP and Empery Tax Efficient II, LP, Anthony Cataldo and Paul Kessler. |
|
8-K |
|
06/19/2020 |
|
10.1 |
|
|
|
|
| 10.9 |
|
Executive Employment Agreement, dated October 19, 2018, among GT Biopharma, Inc. and Raymond W. Urbanski |
|
10-Q |
|
11/14/2018 |
|
10.17 |
|
|
|
|
| 10.10 |
|
Consultant Agreement, dated February 14, 2018, among GT Biopharma, Inc., Georgetown Translational Pharmaceuticals, Inc. and Anthony J. Cataldo |
|
8-K |
|
02/21/2018 |
|
10.3 |
|
|
|
|
| 10.11 |
|
Employment agreement with Anthony Cataldo |
|
10-Q |
|
08/14/2020 |
|
10.11 |
|
|
|
|
| 10.12 |
|
Employment agreement with Steven Weldon |
|
10-Q |
|
08/14/2020 |
|
10.12 |
|
|
|
|
| 10.13 |
|
Securities Purchase Agreement, dated September 16, 2020, among GT Biopharma, Inc. and the purchasers named therein |
|
8-K |
|
09/22/2020 |
|
10.1 |
|
|
|
|
| 10.14 |
|
Master Services Agreement, dated October 5, 2020, between Gt Biopharma, Inc. and Cytovance Biologics, Inc. |
|
8-K |
|
10/06/2020 |
|
10.1 |
|
|
|
|
| 10.15 |
|
Form of First Amendment and Extension of Standstill and Forbearance Agreement |
|
8-K |
|
11/04/2020 |
|
10.1 |
|
|
|
|
| 10.16 |
|
Securities Purchase Agreement |
|
8-K |
|
11/09/2020 |
|
10.1 |
|
|
|
|
| 10.17 |
|
Settlement Agreement, dated as of November 9, 2020, by and among Adam Kasower, East Ventures, Inc., A British Virgin Islands company, SV Booth Investments III, LLC, a Delaware limited liability company and Theorem Group, LLC, a California LLC and GT Biopharma Inc., a Delaware corporation. |
|
10-Q |
|
11/13/2020 |
|
10.19 |
|
|
|
|
| 10.18 |
|
Steve Weldon Letter of Resignation, dated November 11, 2020 |
|
10-Q |
|
11/13/2020 |
|
10.21 |
|
|
|
|
| 10.19 |
|
Board Service Agreement with Bruce Wendel, dated November 11, 2020 |
|
10-Q |
|
11/13/2020 |
|
10.22 |
|
|
|
|
| 10.20 |
|
Board Service Agreement with Greg Berk, dated November 11, 2020 |
|
10-Q |
|
11/13/2020 |
|
10.23 |
|
|
|
|
| 10.21 |
|
Consultant Agreement with Michael Handelman, dated November 13, 2020 |
|
10-Q |
|
11/13/2020 |
|
10.24 |
|
|
|
|
| 10.22 |
|
Form of Amendment to Convertible Note & Standstill Agreement |
|
8-K |
|
12/23/2020 |
|
10.1 |
|
|
|
|
| 10.23 |
|
Settlement Agreement, dated as of December 22, 2020, by and among Alto Opportunity Master Fund, SPC - Segregated Master Portfolio B, Anthony Cataldo, Paul Kessler and GT Biopharma Inc., a Delaware corporation. |
|
8-K |
|
12/28/2020 |
|
10.1 |
|
|
|
|
| 10.24 |
|
Form of Second Amendment and Extension of Standstill and Forbearance Agreement. |
|
8-K |
|
02/01/2020 |
|
10.1 |
|
|
|
|
| 10.25 |
|
Board Service Agreement with Rajesh Shrotriya, dated January 12, 2021. |
|
S-1/A |
|
02/08/2021 |
|
10.69 |
|
|
|
|
| Exhibit
Number |
|
Exhibit
Description |
|
Form |
|
Date |
|
Number |
|
File
No. |
|
Filed
Herewith |
| 10.26 |
|
Board Service Agreement with Michael Breen, dated January 12, 2021. |
|
S-1/A |
|
02/08/2021 |
|
10.70 |
|
|
|
|
| 10.27 |
|
Amendment to Settlement Note with Alto Opportunity Master Fund, SPC - Segregated Master Portfolio B. |
|
S-1/A |
|
02/08/2021 |
|
10.71 |
|
|
|
|
| 10.28 |
|
Form of Securities Purchase Agreement - December 2020 / January 2021 Notes |
|
S-1/A |
|
02/08/2021 |
|
10.72 |
|
|
|
|
| 10.29 |
|
Form of December 2020 / January 2021 Note |
|
S-1/A |
|
02/08/2021 |
|
10.73 |
|
|
|
|
| 10.30 |
|
Amended and Restated Employment Agreement with Anthony Cataldo, dated April 23, 2021. |
|
10-Q |
|
05/17/2021 |
|
10.1 |
|
|
|
|
| 10.31 |
|
Amended and Restated Employment Agreement with Michael Handelman, dated April 23, 2021. |
|
10-Q |
|
05/17/2021 |
|
10.2 |
|
|
|
|
| 10.32 |
|
Amended and Restated Employment Agreement with Dr. Gregory Berk, dated April 23, 2021 |
|
10-Q |
|
05/17/2021 |
|
10.3 |
|
|
|
|
| 10.33 |
|
Exclusive License Agreement with Regents of the University of Minnesota, dated March 26, 2021 |
|
10-K |
|
03/28/2022 |
|
10.73 |
|
|
|
|
| 10.34 |
|
Research Agreement with Regents of the University of Minnesota, dated June 16, 2021. |
|
10-K |
|
03/28/2022 |
|
10.74 |
|
|
|
|
| 10.35 |
|
Sublease Agreement dated November, 2021, between Aimmune Therapeutics, Inc. (Sublandlord) and GT Biopharma, Inc. (Subtenant) |
|
10-K |
|
03/28/2022 |
|
10.75 |
|
|
|
|
| 10.36 |
|
Employment Agreement with Michael Breen, entered into as of December 31, 2021 with an effective date of November 8, 2021 |
|
10-K |
|
03/28/2022 |
|
10.76 |
|
|
|
|
| 10.37 |
|
Amendment No. 1 to Employment Agreement with Michael Breen, dated as of June 17, 2022. |
|
10-K |
|
03/26/2024 |
|
10.77 |
|
|
|
|
| 10.38 |
|
Amendment No. 2 to Services Agreement with Michael Breen, dated as of February 20, 2023. |
|
10-K |
|
03/26/2024 |
|
10.78 |
|
|
|
|
| 10.39 |
|
Board Service Agreement with Michael Breen dated November 11, 2020 |
|
10-Q |
|
05/16/2022 |
|
10.1 |
|
|
|
|
| 10.40 |
|
Employment Agreement with Manu Ohri dated May 15, 2022 |
|
10-Q |
|
05/16/2022 |
|
10.2 |
|
|
|
|
| 10.41 |
|
Amendment No. 1 to Employment Agreement with Manu Ohri, dated as of February 17, 2023 |
|
10-K |
|
03/26/2024 |
|
10.81 |
|
|
|
|
| 10.42 |
|
Settlement and Investment Agreement dated August 24, 2022, by and between GT Biopharma, Inc. and Cytovance Biologics, Inc. |
|
10-Q |
|
10/31/2022 |
|
10.1 |
|
|
|
|
| 10.43 |
|
Form of Securities Purchase Agreement, dated December 2022, by and between GT Biopharma, Inc. and the purchasers named therein. |
|
8-K |
|
01/03/2023 |
|
10.1 |
|
|
|
|
| 10.44 |
|
Amendment No. 1 to Settlement and Investment Agreement, dated as of April 25, 2024, by and between GT Biopharma, Inc. and Cytovance Biologics, Inc. |
|
8-K |
|
04/30/2024 |
|
10.1 |
|
|
|
|
| 10.45 |
|
Amended and Restated Exclusive Patent License Agreement with the Regents of the University of Minnesota, dated May 13, 2024 |
|
10-Q |
|
04/30/2024 |
|
10.2 |
|
|
|
|
| 10.46 |
|
Sponsored Research Agreement with the Regents of the University of Minnesota dated May 20, 2024 |
|
10-Q |
|
04/30/2024 |
|
10.3 |
|
|
|
|
| 10.47 |
|
Form of Securities Purchase Agreement |
|
8-K |
|
05/23/2024 |
|
10.1 |
|
|
|
|
| 10.48 |
|
Form of Placement Agency Agreement |
|
8-K |
|
05/23/2024 |
|
10.2 |
|
|
|
|
| 10.49 |
|
Code of Ethics |
|
10-K |
|
03/31/2015 |
|
14.1 |
|
|
|
|
| 10.50 |
|
Investigator Initiated Clinical Trial Agreement |
|
8-K |
|
11/18/2024 |
|
10.1 |
|
|
|
|
| 10.51** |
|
Form
of Securities Purchase Agreement |
|
|
|
|
|
|
|
|
|
|
| 10.52** |
|
Form
of Placement Agency Agreement |
|
|
|
|
|
|
|
|
|
|
| 10.53 |
|
Employment Agreement between the Company and Alan Urban, dated as of June 7, 2024 |
|
8-K |
|
6/7/2024 |
|
10.1 |
|
|
|
|
| 21.1 |
|
Subsidiaries of GT Biopharma, Inc. |
|
10-K |
|
03/31/2015 |
|
21.1 |
|
|
|
|
| 23.1 |
|
Consent of Weinberg & Company |
|
|
|
|
|
|
|
|
|
X |
| Exhibit
Number |
|
Exhibit
Description |
|
Form |
|
Date |
|
Number |
|
File
No. |
|
Filed
Herewith |
| 23.2** |
|
Consent of Baker McKenzie LLP (incl. in Exhibit 5.1) |
|
|
|
|
|
|
|
|
|
X |
| 24.1 |
|
Power of Attorney |
|
S-1 |
|
06/20/2024
|
|
24.1 |
|
|
|
|
| 101.INS |
|
XBRL
Instance Document |
|
|
|
|
|
|
|
|
|
X |
| 101.SCH |
|
XBRL
Taxonomy Extension Schema Document |
|
|
|
|
|
|
|
|
|
X |
| 101.CAL |
|
XBRL
Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
|
|
|
|
X |
| 101.DEF |
|
XBRL
Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
|
|
X |
| 101.LAB |
|
XBRL
Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
|
|
|
|
X |
| 101.PRE |
|
XBRL
Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
|
|
|
|
X |
| 107 |
|
Filing Fee Table |
|
S-1 |
|
|
|
107 |
|
|
|
X |
| † |
Confidential
treatment granted from the Securities and Exchange Commission as to certain portions, which portions have been omitted and filed
separately with the Securities and Exchange Commission. |
| + |
Schedules
have been omitted pursuant to Item 601(b)(2) of Regulation S-K. GT Biopharma, Inc. will furnish copies of any such schedules to the
Securities and Exchange Commission upon request. |
| |
|
| ** |
To be filed by amendment. |
Item
17. Undertakings
| (1) |
The
undersigned registrant hereby undertakes: |
| |
a. |
To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| |
i. |
To
include any prospectus required by Section 10(a)(3) of the Securities Act; |
| |
|
|
| |
ii. |
To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end
of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if,
in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price
set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| |
|
|
| |
iii. |
To
include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement; provided, however, that paragraphs (1)(i), (1)(ii) and
(1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained
in reports filed with or furnished to the Securities and Exchange Commission by the registrant pursuant to Section 13 or Section
15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement. |
| |
b. |
That,
for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a
new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be
deemed to be the initial bona fide offering thereof. |
| |
|
|
| |
c. |
To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering. |
| |
|
|
| |
d. |
That,
for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule
424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other
than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of
the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior
to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the
registration statement or made in any such document immediately prior to such date of first use. |
| |
|
|
| |
e. |
That,
for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution
of the securities, the undersigned registrant hereby undertakes that in a primary offering of securities of the undersigned registrant
pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the
securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will
be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| |
i. |
Any
preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule
424 (§ 230.424 of this chapter);A |
| |
|
|
| |
ii. |
Any
free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by
the undersigned registrant; |
| |
|
|
| |
iii. |
The
portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant
or its securities provided by or on behalf of the undersigned registrant; and |
| |
|
|
| |
iv. |
Any
other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (2) |
The
undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of
the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Exchange Act (and, where applicable, each
filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference
in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and
the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) |
The
undersigned registrant hereby undertakes that: |
| |
a. |
For
purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part
of this registration statement in reliance on Rule 430A and contained in a form of prospectus filed by the undersigned registrant
pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as
of the time it was declared effective; and |
| |
b. |
For
the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus
shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof. |
| (4) |
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons
of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the
SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event
that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid
by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted
by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in
the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the
question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the
final adjudication of such issue. |
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf
by the undersigned, thereunto duly authorized in the City of Los Angeles, State of California, on December 23, 2024.
| |
GT
Biopharma Inc. |
| |
|
|
| Date:
December 23, 2024 |
By: |
/s/
Michael Breen |
| |
|
Michael
Breen |
| |
|
Interim
Chief Executive Officer |
POWER
OF ATTORNEY
Each
person whose signature appears below hereby constitutes and appoints each of Michael Breen and Alan Urban is
true and lawful attorney-in-fact and agent with full power of substitution and re-substitution, for him and
in his name, place, and stead, in any and all capacities, to sign any amendments to this registration statement, and to sign any
registration statement for the same offering covered by this registration statement, including post-effective amendments or registration
statements filed pursuant to Rule 462(b) under the Securities Act of 1933, and to file the same, with all exhibits thereto and other
documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming that each of said such
attorneys-in-fact and agents or his or her substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant
to the requirements of the Securities Act of 1933, as amended this registration statement has been signed by the following persons in
the capacities dates indicated below.
| /s/ Michael
Breen |
|
| Michael
Breen, Executive Chairman of the Board and |
|
| Interim
Chief Executive Officer |
|
| December
23, 2024 |
|
| |
|
| /s/ Alan Urban |
|
| Alan
Urban, Chief Financial Officer |
|
| December
23, 2024 |
|
| |
|
| /s/ Bruce Wendel |
|
| Bruce
Wendel, Vice Chairman of the Board |
|
| December
23, 2024 |
|
| |
|
| /s/ Rajesh Shrotriva |
|
| Rajesh
Shrotriva, Director |
|
| December
23, 2024 |
|
| |
|
| /s/ Charles J. Casamento |
|
| Charles
J. Casamento, Director |
|
| December
23, 2024 |
|
Exhibit
23.1
Consent
of Independent Registered Public Accounting Firm
We
hereby consent to the incorporation by reference in the foregoing Registration Statement on Form S-1 (File No. 333-________ ) of our
report dated March 26, 2024, relating to the consolidated financial statements of GT Biopharma, Inc. and Subsidiaries as of December
31, 2023 and 2022 which appear in GT Biopharma, Inc.’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023 filed
with the Securities and Exchange Commission on March 26, 2024. We also consent to the reference to our firm under the caption “Experts”
in such Registration Statement and related Prospectus.
| /s/
Weinberg & Company, P.A. |
|
| |
|
| Los
Angeles, California |
|
| December
23, 2024 |
|
Exhibit
107
Calculation
of Filing Fee Tables
Form
S-1
(Form Type)
GT
Biopharma, Inc.
(Exact
Name of Registrant as Specified in its Charter)
Table
1: Newly Registered Securities
| | |
Security Type | |
Security Class Title | |
Fee Calculation or Carry Forward Rule | |
Amount Registered | | |
Proposed Maximum Offering Price Per Unit | | |
Maximum Aggregate Offering Price(1) | | |
Fee Rate | | |
Amount of Registration Fee | |
| Fees to Be Paid | |
Equity | |
Common stock, par value $0.0001 per share(2) | |
457(o) | |
| — | | |
| — | | |
$ | 6,000,000.00 | | |
| 0.0001531 | | |
$ | 918.60 | |
| Fees to Be Paid | |
Equity | |
Pre-funded warrants to purchase common stock, $0.0001 par value per
share(3)(5)(6) | |
457(g) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Fees to Be Paid | |
Equity | |
Common stock, $0.0001 par value per share, underlying the pre-funded
warrants(5)(6) | |
457(o) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Fees to Be Paid | |
Equity | |
Common warrants to purchase common stock(3) | |
457(g) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Fees to Be Paid | |
Equity | |
Common stock, par value $0.0001 per share, underlying common warrants(4) | |
457(o) | |
| — | | |
| — | | |
$ | 6,000,000.00 | | |
| 0.0001531 | | |
$ | 918.60 | |
| Fees to Be Paid | |
Equity | |
Placement Agent Warrants to purchase common stock(3) stock (2) | |
457(g) | |
| — | | |
| — | | |
| — | | |
| — | | |
| | |
| Fees to Be Paid | |
Equity | |
Common stock, par value $0.0001 per share, underlying placement agent warrants(7) | |
457(o) | |
| — | | |
| — | | |
$ | 900,000.00 | | |
| 0.0001531 | | |
$ | 137.79 | |
| | |
Total Offering Amounts | | |
| | | |
$ | 12,900,000.00 | | |
| | | |
$ | 1,974.99 | |
| | |
Total Fees Previously Paid | | |
| | | |
| | | |
| | | |
| — | |
| | |
Total Fee Offsets | | |
| | | |
| | | |
| | | |
| — | |
| | |
Net Fee Due | | |
| | | |
| | | |
| | | |
$ | 1,974.99 | |
| (1) | Estimated
solely for the purpose of computing the registration fee in pursuant to Rule 457(o) under
the Securities Act of 1933, as amended. |
| | |
| (2) | Pursuant
to Rule 416(a) under the Securities Act, this registration statement shall also cover an
indeterminate number of shares that may be issued and resold resulting from stock splits,
stock dividends or similar transactions. |
| | |
| (3) | Pursuant
to Rule 457(g) of the Securities Act, no separate registration fees are payable with respect
to the common warrants, pre-funded warrants or placement agent warrants to purchase shares
of common stock, $0.0001 par value per share, offered hereby since the offering of shares
of common stock underlying the warrants, pre-funded warrants and placement agent warrants
are being registered hereby. |
| | |
| (4) | In
addition to the common stock set forth in this table, pursuant to Rule 416 under the Securities
Act, this registration statement also registers such indeterminate number of common stock
as may become issuable upon exercise of the pre-funded warrants and common warrants. |
| | |
| (5) | The
proposed maximum aggregate offering price of the common stock proposed to be sold in the
offering will be reduced on a dollar-for-dollar basis based on the offering price of any
pre-funded warrants offered and sold in the offering, and as such the proposed maximum offering
price of the common stock and pre-funded warrants (including the common stock issuable upon
exercise of the pre-funded warrants) if any, is $6,000,000. |
| | |
| (6) | The
registrant may issue pre-funded warrants to purchase common stock in the offering. The purchase
price of each pre-funded warrant will equal the price per share at which shares of common
stock are being sold to the public in this offering, minus $0.0001, which constitutes the
pre-funded portion of the exercise price, and the remaining unpaid exercise price of the
pre-funded warrant will equal $0.0001 per share (subject to adjustment as provided for therein). |
| | |
| (7) | The
registrant has agreed to issue upon the closing of this offering, warrants (the “placement
agent warrants”) to the placement agent entitling it to purchase up to 6% of the number
of securities sold in this offering. The exercise price of the placement agent warrants is
equal to 125% of the public offering price of the securities offered hereby. As estimated
solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under
the Securities Act, the proposed maximum aggregate offering price of the placement agent
warrants is $900,000, which is equal to 125% of $720,000 (6% of $12,000,000). |
GT Biopharma (NASDAQ:GTBP)
Historical Stock Chart
From Dec 2024 to Jan 2025
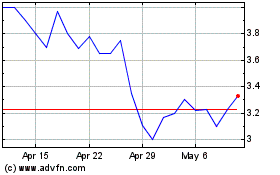
GT Biopharma (NASDAQ:GTBP)
Historical Stock Chart
From Jan 2024 to Jan 2025