NASDAQ false 0001746466 0001746466 2023-10-16 2023-10-16
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 16, 2023
Equillium, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-38692 |
|
82-1554746 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
| 2223 Avenida de la Playa Suite 105 |
|
|
| La Jolla, California |
|
92037 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (858) 412-5302
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock, par value $0.0001 per share |
|
EQ |
|
Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On October 16, 2023, Equillium, Inc. (the “Company”) will virtually host its previously announced analyst and investor day commencing at 9 a.m. PT. During the event, representatives of the Company will, among other things, provide an update on the Company’s pipeline and a review of the Company’s Multi-Cytokine Platform and clinical stage Multi-Cytokine Inhibitors. A copy of the presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 7.01 of this Current Report on Form 8-K and the presentation attached hereto as Exhibit 99.1, are deemed to be “furnished” and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section. The information set forth in this Item 7.01, including Exhibit 99.1, shall not be deemed incorporated by reference into any filing under the Exchange Act or the Securities Act of 1933, as amended, except to the extent that the Company specifically incorporates it by reference.
Item 8.01 Other Events.
On October 16, 2023, the Company announced a presentation highlighting EQ302, a second generation orally deliverable multi-cytokine inhibitor in development to target IL-15 and IL-21.
The presentation outlines the origins of EQ302 from its parent peptide, EQ102, which was originally modeled from the D-helix of the IL-2 family of cytokines and binds directly to CD132 to inhibit the signaling of IL-15 and IL-21. Stable peptide derivatives of EQ102, including EQ302, are being developed by adding hydrocarbon staples to confer proteolytic resistance and increase stability in the gastrointestinal (“GI”) tract while retaining cytokine inhibitory properties, making it a potential candidate for clinical testing in a variety of GI diseases via oral administration.
EQ302 was administered to mice via oral delivery in a pre-clinical formulation designed to enhance permeability of the intestine. Following oral administration to mice challenged with human IL-15, small intestinal tissue was harvested and shown to contain significant amounts of EQ302. To demonstrate the local efficacy of the peptide, IL-15-induced Interferon-gamma (“IFNg”) levels within the intestinal tissue were measured and shown to be significantly decreased in EQ302-treated animals versus vehicle control animals.
The data illustrates that:
| |
• |
|
Adding hydrocarbon staples to a peptide can confer increased stability in the GI tract while retaining its cytokine inhibitory properties. |
| |
• |
|
Utilizing a pre-clinical formulation for enhanced epithelial permeability, EQ302 achieved meaningful concentration levels for localized cytokine inhibition in the small intestine. |
| |
• |
|
EQ302 delivered to mice via oral gavage can inhibit IL-15 induced IFNg transcription locally in the GI tract indicating potential utility for Celiac Disease and inflammatory bowel disease. |
Forward Looking Statements
Statements contained in this Current Report on Form 8-K regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words such as “anticipate”, “believe”, “could”, “continue”, “expect”, “estimate”, “may”, “plan”, “outlook”, “future” and “project” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. Because such statements are subject to risks and uncertainties, many of which are outside of the Company’s control, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: the Company’s ability to execute its plans and strategies; risks related to performing clinical and pre-clinical studies; whether the results from clinical and pre-clinical studies will validate and support the safety and efficacy of the Company’s product candidates. These and other risks and uncertainties are described more fully under the caption “Risk Factors” and elsewhere in the Company’s filings and reports, which may be accessed for free by visiting the Securities and Exchange Commission’s website at and on the Company’s website under the heading “Investors.” Investors should take such risks into account and should not rely on forward-looking statements when making investment decisions. All forward-looking statements contained in this Current Report on Form 8-K speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Equillium, Inc. |
|
|
|
|
| Dated: October 16, 2023 |
|
|
|
By: |
|
/s/ Jason A. Keyes |
|
|
|
|
|
|
Jason A. Keyes |
|
|
|
|
|
|
Chief Financial Officer |

Exhibit 99.1 Analyst & Investor Day With Dr. Arash Mostaghimi
October 16, 2023

Forward-Looking Statements This presentation contains forward-looking
statements about Equillium, Inc. (the Actual results or events could differ materially from the plans, intentions and “Company”). In some cases, you can identify forward-looking statements by expectations disclosed or implied in the
forward-looking statements the the words “will,” “expect,” “intend,” “plan,” “objective,” “believe,” “estimate,” Company makes due to the risks and uncertainties
inherent in the Company’s “potential,” “continue” and “ongoing,” or the negative of these terms, or other business, including without limitation, the risks described in the Company’s filings comparable
terminology intended to identify statements about the future. with the Securities and Exchange Commission (“SEC”). You are cautioned not These statements are based on Company management’s current beliefs and to place undue reliance
on these forward-looking statements, which represent expectations. These statements include but are not limited to statements the Company’s views as of the date of this presentation. The Company regarding the Company’s business strategy,
the Company’s plans to develop anticipates that subsequent events and developments will cause its views to and commercialize its product candidates, the safety and efficacy of the change. However, while the Company may elect to update these
forward- Company’s product candidates, the Company’s plans and expected timing looking statements at some point in the future, the Company has no current with respect to regulatory filings and approvals, size and growth potential of
intention of doing so except to the extent required by applicable law. These the markets for the Company’s product candidates and cash runway. These and other risks and uncertainties are described more fully under the caption statements
involve known and unknown risks, uncertainties and other factors “Risk Factors” and elsewhere in the Company’s filings and reports, which may that may cause the Company’s actual results, levels of activity, performance be
accessed for free by visiting EDGAR on the SEC web site at www.sec.gov or achievements to be materially different from the information expressed or and on the Company’s website under the heading “Investors.” All forward- implied by
these forward-looking statements. The Company may not actually looking statements are qualified in their entirety by this cautionary statement. achieve the plans, intentions or expectations disclosed in its forward-looking This caution is made under
the “safe harbor” provisions of Section 21E of the statements, and you should not place undue reliance on the Company’s Securities Exchange Act of 1934, as amended. forward-looking statements. Trademarks This presentation may
contain trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this
presentation may be listed without the TM, SM © or ® symbols, but Equillium will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to these trademarks, service marks, trade names and
copyrights. 2

Today’s Agenda Speakers Welcome: Pipeline Overview Guest Speaker
Bruce Steel, CEO Arash Mostaghimi, M.D., M.P.A., M.P.H. Associate Professor of Dermatology Powerful Multi-Cytokine Platform Harvard Medical School Steve Connelly, CSO Director, Inpatient Consultation Service, Department of Dermatology Brigham and
Women’s Hospital EQ101: A First-in-Class Tri-Specific Inhibitor of IL-2, IL-9 & IL-15 Equillium Management Steve Connelly, CSO EQ101: Clinical Program Targeting Alopecia Areata Bruce Steel, C.F.A. Chief Executive Officer Maple Fung, CMO
Arash Mostaghimi, Harvard University & Brigham and Women’s Hospital Closing Remarks Steve Connelly, Ph.D. Bruce Steel, CEO Chief Scientific Officer Q&A Maple Fung, M.D. Chief Medical Officer 3

Equillium Snapshot & Investment Highlights First-in-Class Immunology
Assets Leaders in Novel Multi-Cytokine Partnership for Inhibitors immunobiology Itolizumab Developing high-impact, novel therapeutics to Multiple Multiple treat autoimmune and Clinical Stage Near-term Programs Catalysts inflammatory disorders
Expected Cash Runway Into 2025 4

Diversified Pipeline of First-In-Class Immunology Assets Drugs
Indication Pre-Clinical Phase 1 Phase 2 Phase 3 Partners Anticipated Milestones EQ101 Q4 2023 initial data PoC data & FDA/EMA Orphan Worldwide IL-2/9/15 alopecia areata rights Drug Designations for CTCL Mid-2024 topline data inhibitor Q4 2023
SAD/MAD data Potential EQ102 Worldwide IL-15/21 celiac disease expansion into other 2024 celiac disease rights inhibitor GI indications patient data Multi- opportunities in autoimmunity, Worldwide Cytokine inflammation & oncology rights Platform
acute graft-versus-host FDA Fast Track & 2024 interim review disease Orphan Drug Designations EQ001 FDA Fast Track systemic lupus erythematosus itolizumab Early 2024 topline data (SLE) / lupus nephritis (LN) Designation for LN & anti-CD6
ulcerative colitis Conducted by Biocon in India 5

Anticipated Milestones EQ101 – Phase 2 Clinical Study Alopecia
Areata – Initial Data Q4 2023 Alopecia Areata – Topline Data Mid-2024 EQ102 – Phase 1 Clinical Study SAD / MAD (healthy volunteers) – Data Q4 2023 Celiac Disease – Data 2024 Itolizumab – Phase 1b / Phase 3
Clinical Studies Lupus Nephritis EQUALISE Study (Phase 1b) – Topline Data Early 2024 aGVHD EQUATOR Study (Phase 3) – Interim Review 2024 6

Itolizumab First-in-Class immune- modifying mAb targeting the CD6-ALCAM
signaling pathway 7

Ono Pharmaceutical: High Value Strategic Partnership Equillium receives
upfront payment and full funding of itolizumab R&D in exchange for Ono exclusive option to purchase Equillium’s rights to itolizumab Closing payments*: $38.2M Option exercise payment**: $35.1M Milestone payments: $101.4M Option period
expires three Itolizumab development and funding: months following delivery of: • Equillium to continue conducting all itolizumab R&D • topline data from the EQUALISE • Ono to fund all R&D expenses from July 1, 2022 study
in lupus nephritis, and through the option period • interim data from the EQUATOR • R&D budget approximately $8M per quarter study in acute GVHD Milestone Payments up to $101.4M: • Clinical • Regulatory • First sale
* Includes upfront payment of $26.4M and R&D expense reimbursement for the period July 1 through December 31, 2022. 8 ** ¥5.0B subject to currency exchange rate at the time of payment (USD amount above is based on the exchange rate as of
August 8, 2023); milestone payments and R&D reimbursement are based in USD.

Multi-Cytokine Platform & Therapeutic Candidates 9

The Multi-Cytokine Problem Cytokines leverage shared receptors
exhibiting overlapping or synergistic biological activities which presents unique drug development challenges when treating disease Cytokines often exhibit… Cytokines signal via… Upstream shared receptor ‘Overlapping
signaling’ signaling hubs overlapping biological Targets include Dupixent™ activities Downstream divergent ‘Synergistic signaling’ kinase cascades combine to create biological activities Targets include JAK inhibitors
10

Challenges in Targeting Cytokine Signaling Ideal Therapeutic Approach
mAb JAK inhibitor Selective inhibition of disease driving cytokines Inhibits Only One Cytokine Inhibits 50+ Cytokines Inhibiting the right Upstream combination of signaling cytokines is key for optimal therapeutic effect, balancing both Downstream
potent activity and signaling potential toxicities Inhibition too Narrow Inhibition too Broad Optimal Therapeutic Effect Other Cytokines Involved, Non-selective cytokine targeting leads leads to insufficient activity to broad immunosuppression and
safety risks 11

Multi-Cytokine Platform Modular platform generates products that
modulate the natural biological redundancy or synergy of cytokines affording greater therapeutic benefit Validated Disease • Technology originating from • Products can be flexibly Targets the National Institutes of Health modified to
optimize Computational Functional therapeutic profile and tissue Biology Fusions • Uniquely targets upstream targeting properties shared receptors to selectively Drug modulate multiple cytokine • Broad IP portfolio covering Products
pathways in a single novel the platform, methods of product treatment and composition Structure In-silico Activity of matter design Relationships • Intravenous, subcutaneous and oral delivery Protein Engineering 12
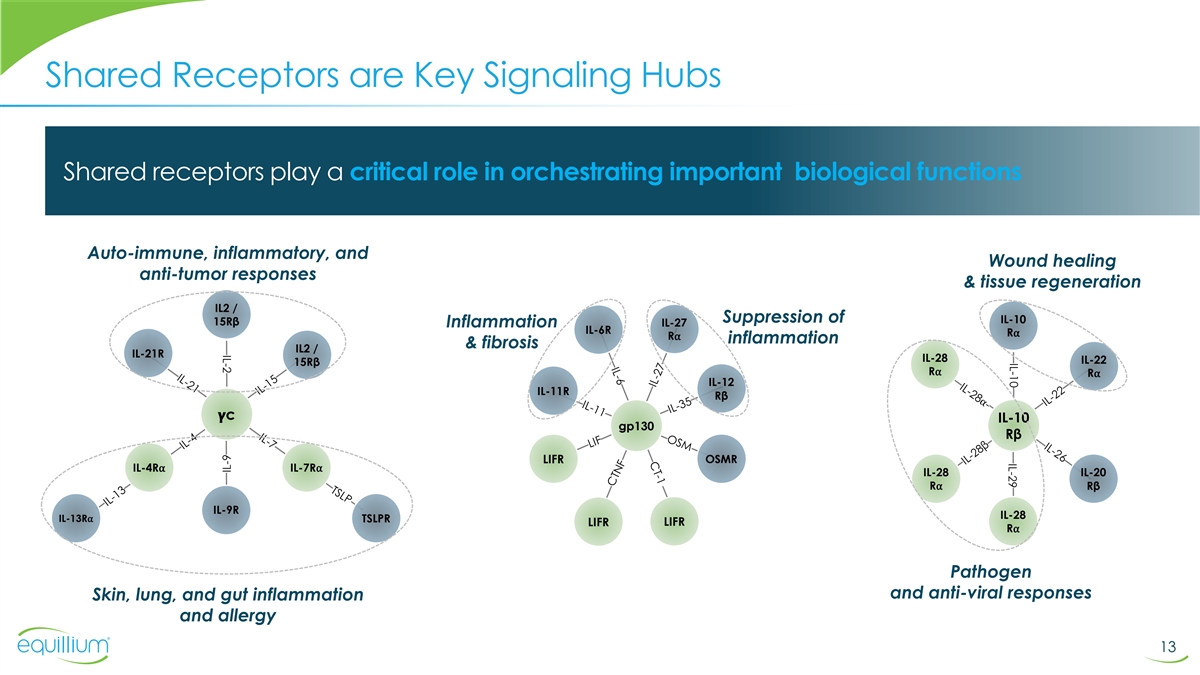
IL-10 IL-29 IL-2 Shared Receptors are Key Signaling Hubs Shared
receptors play a critical role in orchestrating important biological functions Auto-immune, inflammatory, and Wound healing anti-tumor responses & tissue regeneration IL2 / Suppression of IL-10 15Rβ IL-27 Inflammation IL-6R R⍺
R⍺ inflammation & fibrosis IL2 / IL-21R IL-28 IL-22 15Rβ R⍺ R⍺ IL-12 IL-11R Rβ ���� c IL-10 gp130 Rβ LIFR OSMR IL-4R⍺ IL-7R⍺ IL-28 IL-20 R⍺ Rβ IL-9R IL-28
IL-13R⍺ TSLPR LIFR LIFR R⍺ Pathogen and anti-viral responses Skin, lung, and gut inflammation and allergy 13 IL-9

Products of the Multi-Cytokine Platform Shared receptors offer
opportunity to more effectively •MULTI-CYTOKINE ACTIVATOR (MCa) modulate biology Engineered proteins that engage multiple signaling pathways to modulate biological function •MULTI-CYTOKINE INHIBITOR (MCi) Engineered peptides or proteins
that block multiple signaling pathways to modulate biological function 14

γC Receptor: A Key Shared Cytokine Signaling Hub Cytokines of the
γC receptor are critical regulators of immune responses thus, making it an attractive drug target for modulating T, NK and B cells Synergistic signaling Overlapping Signaling on CD8 cytotoxic T cells on CD8 & NK T cells αα
γαγαγαγγαγ β β Private Shared Receptor Receptor 15

IL-15 at the Apex of Tissue Specific Immuno-inflammation Approaches to
Targeting IL-15 EQ101 & EQ102 • Initiates proinflammatory cascade preceding expression of TNFα and other key cytokines • IL-15 play pathogenic roles in diverse organ-specific autoimmune disorders • IL-15 stimulates
generation of NK, NK-T, γδ, ILC1, and memory CD8 T cells • Diverse approaches in development to block IL-15 16 Waldmann et al., JEM., 2019

Generation of Selective Multi-Cytokine Inhibitors (MCi)
•CYTOKINE-RECEPTOR COMPLEX •CYTOKINE D-HELICES •FUNCTIONAL FUSIONS Cytokine Shared PK extension strategies receptor Private PEGylation receptor Lipidation FC-fused peptibodies native sequences Oral delivery Peptide Stapling
Multi-specific molecules proprietary composite sequence Fc-fusions – peptibodies B mAb-fusions – bi/tri-specific Helices A & C A interact with the private receptor, D while helix D C interacts with shared receptor •OPTIMIZED
MCi THERAPEUTIC engineered multi-cytokine inhibitor 17

MCi MoA: Disrupting Assembly of the Receptor Complex Selective
inhibition of cytokines A, D & F Cytokines E A D C F Multi-cytokine B A Inhibitor (MCi) A No Signaling Private Shared Receptors Receptor A B C D E F Cytokines E Control +MCi Cytokines first bind to private receptor MCi binds to γc receptor
with high affinity, then transiently dimerize with and selectively blocks the γc shared receptor at lower affinity that signaling of only selected leads to signaling cytokines e.g., A, D & F Signaling 18 % Inhibition

The Multi-Cytokine Inhibitor Advantage Addressing limitations of
single-target biologics and broadly immunosuppressive kinase inhibitors by selectively inhibiting only those cytokines driving disease mAb JAK inhibitor MCi Improved Therapeutic Profile Inhibits One Cytokine Inhibits 50+ Cytokine Inhibits selected
cytokines ü Targets undruggable sites not possible with mAb or small molecules Upstream ü Optimized targeting – not signaling too narrow / not too broad ü Increased selectivity over JAKi can afford improved safety profile
Downstream ü Greater potency by signaling inhibiting downstream signaling of targeted cytokines 19 Insufficient Activity Safety Liabilities Optimal therapeutic effect

Differentiated Pipeline of First-in-Class Multi-cytokine Agents EQ101
EQ102 EQ302 EQ400 Series Phase 2/3 In Phase 1 Pre-clinical Pre-clinical PEGylated peptide inhibits: PEGylated peptide inhibits: Stabilized peptide inhibits: Protein activates: IL-X + IL-Y IL-15 + IL-21 IL-2 + IL-9 + IL-15 IL-15 + IL-21 Celiac
Disease Oncology Cutaneous T Cell Celiac Disease Lymphoma Inflammatory Bowel Disease Vaccine Adjuvants Inflammatory Bowel Disease Alopecia Areata Type 1 Diabetes Potential use in combination Orally-delivered, with checkpoint inhibitors, Vitiligo SLE
locally-acting peptide ADC or cell therapy Rheumatoid Arthritis Hepatic disease approaches both in-vivo and ex-vivo Myositis Administered by Interstitial Lung Disease sub-Q injection IV injection and sub-Q IV injection with sub-Q delivery in
development 20 SLE = systemic lupus erythematosus; ADC = antibody drug conjugate

EQ101 First-in-Class Tri-specific Inhibitor of IL-2/IL-9/IL-15
21

EQ101: A First-in-Class Tri-Specific Cytokine Inhibitor Inhibition of
the key cytokines IL-2, IL-9 and IL-15 by EQ101 translates from preclinical models into humans IL-9 IL-15 IL-2 • IL-2 & IL-15 are important • PEGylated peptide • As effective as ruxolitinib • Significant clinical to CD8
and NK cell (based on the D-helix) in inhibiting multiple experience in >100 biology, IL-9 contributes to selectively inhibiting IL-2, lymphoproliferative or subjects, demonstrated 1 2,3 inflammation in the skin IL-9 & IL-15 signaling
leukemic T-cell lines favorable safety and 5,6 tolerability • Important to inhibit both • Administered as a low • More effective than IL-2 and IL-15 due to volume intravenous push, ruxolitinib in mouse • Positive proof-of-
redundancy in signaling with a sub-cutaneous model of immune- concept achieved on cytotoxic CD8 T cells formulation in mediated hair loss / in cutaneous T cell 4 6 development alopecia areata lymphoma patients 22 1) Nata et al., J. Biol. Chem.,
2015; 2) Massoud et al., PNAS, 2015; 3) Wang et al., Leukemia, 2018; 4) Azimi et al., AHRS, Orlando, Florida, 20185) Frohna et al., J. Clin. Pharm., 2019 6) Querfeld et al., Blood, 2019

EQ101: More Complete Downstream Signal Inhibition Versus JAKi JAKi
suppression of cytokine signaling is incomplete, while EQ101 inhibits the multiple downstream pathways for complete suppression of cytokine signaling Ruxolitinib blocks EQ101 blocks all JAK/STAT pathway only major pathways α +IL-2 +IL-2 + IL-2
- + IL-2 - + rux β γ + EQ101 γ β pSTAT-3 pSTAT-3 pSTAT-1 pSTAT-1 pSTAT-5 pSTAT-5 pERK pERK pPI3K pPI3K Vinculin Vinculin 23 In-house data and Frohna et al., Alopecia Areata Research Summit, New York, New York, 2018

EQ101 Performs Similarly to JAK Inhibition in IL-15 Driven ATL Model
IL-2, IL-9 & IL-15 are overexpressed in adult T cell leukemia (ATL) and cutaneous T cell lymphoma 1,2,3,4,5 (CTCL) where they drive cancer cell proliferation and increased inflammation in the skin Blocking IL-2 or IL-15 alone was ineffective in
treating large granular lymphocytic 6 7 leukemia (LGLL) and ATL control PBS EQ101 EQ101 ruxolitinib 40mg/kg, IV, BIW, 4 weeks ruxolitinib continuous administration, 50mg/kg/d 24 1) Dobbeling et al., Blood,1998; 2) Mishra et al., Clin. Cancer Res.,
2014; 3) Qin et al., 2001; 4) Garcia et al., 2016; 5) Waldmann et al., 1993; 6) Waldmann et al., 2013; 7) Berkowitz et al., 2014; 8) Wang et al., 2018

EQ101: Summary of Historical Studies to Date Study BNZ-CT-101, Phase 1
Study in Healthy Volunteers (n=18) First-in-Human study to investigate the safety, tolerability and PK of single ascending doses of BNZ132-1-40 • 3 subjects per treatment group • Single IV dose of 0.2 mg/kg, 0.4 mg/kg, 0.8 mg/kg, 1.6
mg/kg, 3.2 mg/kg, 6.4 mg/kg Study BNZ-CT-102, Phase 1 Study in Healthy Volunteers (n = 25, treated) Single center, randomized, single-blind, placebo controlled, multiple-dose study to characterize the safety, tolerability & PK/PD of IV
BNZ132-1-40 • Subjects dosed weekly (QW) for 4 doses, or once every other week (QOW) for 3 doses • 3 QW cohorts of 0.5 mg/kg, 1.0 mg/kg and 1.5 mg/kg • 2 QOW cohorts of 2 mg/kg and 3.0 mg/kg Study BNZ1-CT-201, Phase 1/2 Dose
Ranging Study in Patients with LGLL or CTCL (n = 50) Open-label, multi-center study to characterize the safety, tolerability, preliminary efficacy, and PK/PD of up to four dose levels of BNZ-1 administered QW by IV infusion • Treatment on Days
1, 8, 15, and 22 followed by 3-month treatment extension period, during which subjects received up to 13 doses administered QW for a maximum of 17 weekly doses of BNZ-1 • 4 cohorts dosed between 0.5 mg/kg and 4 mg/kg 25 Froha et al., Journal
of Clinical Pharmacology 2020; Querfeld et al., presentation at ASH 2020; Brammer et al Blood 2023 BNZ-1 = EQ101, PK = pharmacokinetic, PD = pharmacodynamic, IV = intravenous, LGLL = Large Granular Lymphocytic Leukemia, CTCL = Cutaneous T Cell
Lymphoma

EQ101: Summary of Experience in Normal Healthy Volunteers ü ü
ü No AE >= Grade 3, most common AE Dose proportional PK with Observed to be safe and • SAD: mild headache in 3 of 18 subjects (17%) single dose half-life in well tolerated, no DLT and • MAD: sore throat and headache in 4 of 24
approximately 5 days no clinically significant lab subjects (16%) abnormalities ü ü ü Dose-dependent changes in Multiple doses of EQ101 ≥1.5mg/kg No detectable anti-drug IL-2/IL-15 dependent cells led to prolonged PD effects
>7 days antibodies suggesting QW to QOW dosing 26 Froha et al., Journal of Clinical Pharmacology 2020 QW = Weekly Dosing QOW = Every Other Week Dosing

EQ101: Phase 1/2 Proof-of-Concept Study in CTCL Clinical validation and
favorable safety profile positions EQ101 for further study 1 Phase 1/2 MAD study in Cutaneous T Cell Lymphoma patients (n=30) • Heavily pre-treated population (median of 5 prior systemic treatments) • Dose-dependent PK/PD relationship in
key cellular markers • Observed to be safe and well-tolerated with no drug related SAEs, no DLTs and no clinically significant laboratory abnormalities • Dose-dependent reductions of IL-2 & IL-15 dependent cells and improvements in
mSWAT • 2mg/kg chosen for dose expansion Duration of study up to 18 months Duration of study up to 8 months* 27 (1) Querfeld et al., ASH 2020; * study closed to prepare for EOP2 meeting

EQ101: Ready for Late-Stage Advancement in CTCL EQ101 reduces IL-2
& IL-15 dependent cells and inflammation with improvements in skin lesions and ORR comparing favorably with benchmark drugs MAVORIC Phase 3 trial evaluating mogamulizumab and vorinostat provides the most recent and reliable 1 estimates for
benchmarking EQ-101 CTCL disease stage IB/II Mogamulizumab (n=68) Vorinostat (n=72) EQ101 (n=19) Response Rate 2 27.9% 19.4% 42.1% (mSWAT, CR + PR) EQ101, 2mg/kg Baseline Week 16 Baseline Week 16 Week 31 28 1) Kim et al. 2018. 2) Two additional
patients achieved PR but are not included since they did not reach 4 weeks of response due to termination of the study. Including those patients response rate =52.6% 3) Patients shown were entered into the long-term extension portion of BNZ1-CT-201
and continued to be dosed for over 70 weeks. mSWAT = modified severity-weighted assessment tool

EQ101: Positive Proof-of-Concept Opens Up Indication Expansion ü
ü ü >100 subjects dosed, Compelling clinical activity Attractive target product demonstrated favorable observed in cutaneous T cell profile for the treatment of lymphoma (CTCL) patients CTCL patients safety and tolerability ü
ü ü IND open and Use of JAKi in medical dermatology Now in ongoing Phase 2/3 ready opens up opportunity for proof-of-concept study in in CTCL indication expansion alopecia areata 29

EQ101: Valuable Medical Dermatology Franchise Multi-Billion dollar
market opportunity for EQ101 in medical dermatology Large need exists for safe and effective therapies 6.6M US 250,000 US 24,000 adult 1.5M+ US moderate-severe moderate- 1 3 US Patients Patients 2 4 Patients severe Patients Cutaneous T cell Alopecia
Areata Vitiligo Atopic Dermatitis Lymphoma PoC Achieved Phase 2 Study ongoing Future Opportunity Future Opportunity Phase 2/3 Ready • Only two FDA approvals: JAKi • Only one FDA approval: JAKi • 3 FDA approved JAKi Open IND •
Late-stage Industry pipeline • Late-stage Industry pipeline • Large refractory • Unmet need for safe predominantly JAKi predominantly JAKi population in need of and effective therapies safe treatment alternatives 1) Kx Advisors 2)
Benigno, Clin Cosmet Investig Dermatol, 2020 3) Bergqvist C, Ezzedine K. Vitiligo: A Review. Dermatology. 2020 30 4) Chiesa Fuxench ZC, J Invest Dermatol. 2019

EQ101: Targeted Treatment Approach for Alopecia Areata IL-2, IL-9 and
IL-15 are key drivers of disease in alopecia areata, promoting a cycle of increased IFN-γ production and immune cell attack of the hair follicle Hair follicle bulb IFN-γ CD4 IL-2 Drives inflammation IL-15 & hair loss IL-9 NK CD8 IL-15
31 Xing et al., Nat. Med., 2014; Bertolini et al., PLOS, 2014; Suarez-Farinas et al., J Allergy Clin Immunol, 2015; Dai et al., JCI Insight, 2021

EQ101 Reverses Immune-Mediated Hair Loss in a Mouse Model EQ101 is more
effective than ruxolitinib at hair regrowth and suppression of cytotoxic CD8+ T cells in humanized alopecia model EQ101 Reduces CD8+ Cytotoxic EQ101 Improves Hair Regrowth T-cell Activation Marker NKG2D 140 EQ101 2 mg/kg 120 IV 2x/week 100 80 Day 0
Day 14 Day 21 60 40 Ruxolitinib 30 mg/kg 20 2x/day 0 PBS Ruxolitinib EQ101 Day 0 Day 14 Day 21 32 In-house data and Azimi et al., AHRS, Orlando, Florida 2018; Frohna, et al., Alopecia Areata Research Summit, New York, New York, 2018 Fold over
control

EQ101: Alopecia Areata Clinical Review
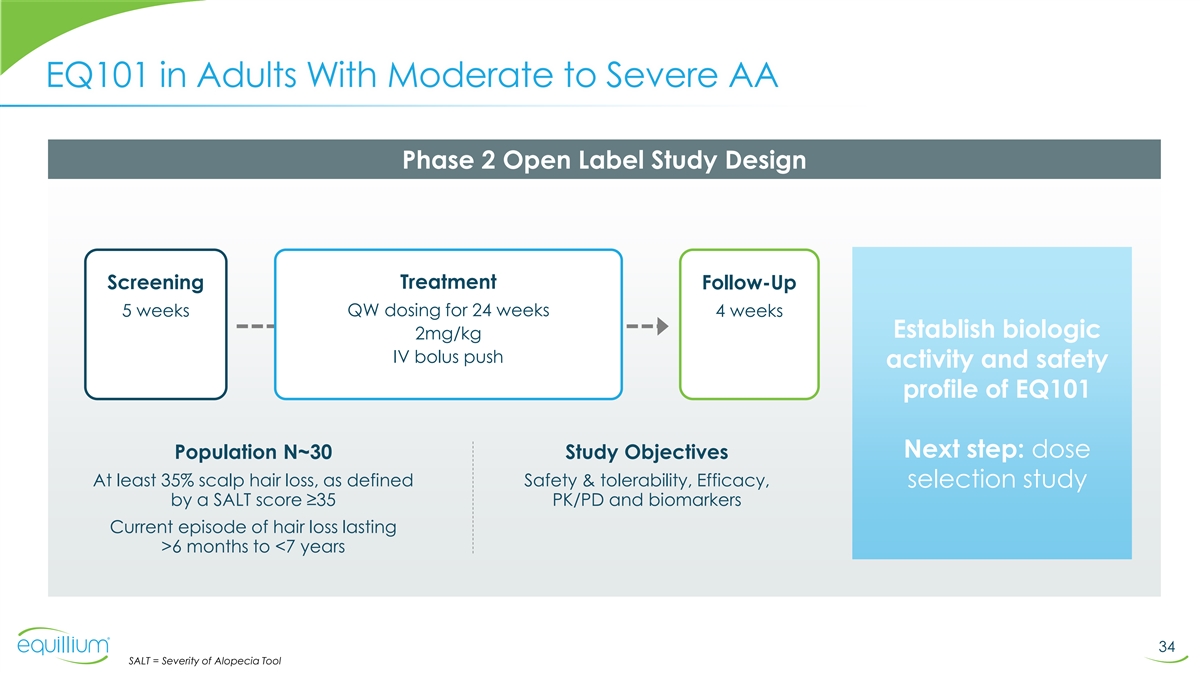
EQ101 in Adults With Moderate to Severe AA Phase 2 Open Label Study
Design Treatment Screening Follow-Up 5 weeks QW dosing for 24 weeks 4 weeks Establish biologic 2mg/kg IV bolus push activity and safety profile of EQ101 Next step: dose Population N~30 Study Objectives At least 35% scalp hair loss, as defined Safety
& tolerability, Efficacy, selection study by a SALT score ≥35 PK/PD and biomarkers Current episode of hair loss lasting >6 months to <7 years 34 SALT = Severity of Alopecia Tool

Alopecia Areata Trials Illustrate Efficacy Bar & Low Placebo Rates
Ritlecitinib results from Ph 2 and ALLEGRO-2b/3 studies: reduction in SALT scores in placebo subjects at 24 weeks was less than 6% Percent change from baseline in SALT Score baricitinib deuruxolitinib Results from THRIVE AA1 Results from BRAVE AA1
deuruxolitinib deuruxolitinib SALT = Severity of Alopecia Tool Efficacy and Safety of Baricitinib in Patients with Severe Alopecia Areata Source: Eli Lilly & Concert Pharmaceuticals and Pfizer 35 King et al J Am Acad Dermatol 2021; King et al
Lancet 2023 Mean % Relative Change in SALT Score Mean % Relative Change in SALT Score

SALT Response Based on Baseline Severity in Baricitinib Studies SALT
Score ≤20 Response Rate SALT Score ≤20 Response Rate baricitinib 2-mg baricitinib 4-mg EQ101 study EQ101 study 24 wk treatment 24 wk treatment Placebo response rates are very low for alopecia areata patients: at 36 weeks of treatment
<1% for patients with very severe disease and ~7% for patients with severe disease a Severe : baseline SALT score of 50-94; b Very severe : baseline SALT Score >95 36 Taylor et al. 2022 JAAD, presented at AAD, P35766

Alopecia Areata Pipeline: Late-Stage Assets ® ® ®
OLUMIANT LITFULO SOTYKTU LY3009104 PF-06651600 CTP-543 SHR0302 BMS-986165 ASLAN003 ADX-914 HXN-7734 baracitinib ritlecitinib deuruxolitinib jaktinib ivarmacitinib deucravacitinib etrasimod farudostat bempikibart daxdilimab Approved Approved P3 Data:
Key Dates NDA: H1 2023 P3 Data: 2024 P2 Data: Q3 2024 P2 Data: Q4 2023 P2 Data: Q1 2024 P2 Data: 2024 P2 Data: Q3 2023 June 2022 July 2023 2024 MoA JAK1/2 JAK3/TEC JAK1/2 JAK1/2 JAK1 TYK2 S1P antagonist DHODH Anti-IL7Ra mAb Anti-ILT7 mAb Phase 3
Phase 3 Phase 3 Status MARKETED MARKETED Phase 2 Phase 2 Phase 2 Phase 2 Phase 2 completed (China only) (China only) US Approvals AA, RA, COVID AA NONE NONE NONE Psoriasis NONE NONE NONE NONE P2/3 (n=764, 2yr 4 P3 (n=706, 1yr mo, 75 sites) P2b/3
(n=718) 5mo, 72 sites) Trials/Size P3 (n=420) P3 (n=330) P2 (n=90) P2 (n=80) P2 (n=60) P2 (n=40) P2 (n=30) P3 (=546, 1yr 6mo, P3 LT ongoing P3 (n=517, 1yr 98 sites) 1 mo, 63 sites) SALT ≥ 50 SALT ≥ 50 SALT ≥ 50 SALT ≥ 50 SALT
≥ 50 SALT ≥ 50 SALT ≥ 25 to < 95 SALT ≥ 50 SALT ≥ 50 SALT ≥ 50 to ≤ 95 Patients placebo placebo placebo placebo placebo placebo open label placebo placebo open label AT: 15% AA: 42-43% Alopecia AU: 45-47%
AU: 27% AT: 16-22% pending pending pending pending pending pending pending type for 200 mg +50 mg AU: 27-37% SALT ≤ 20 at 24 SALT ≤ 20 at SALT ≤ 20 at % change SALT % change SALT % change SALT % change SALT % change SALT Endpoints
SALT ≤ 20 at 36 wks SALT ≤ 20 at 24 wks wks 24 wks 24 wks at 24 wks at 24 wks at 24 wks at 24 wks at 24 wks BRAVE-AA1: THRIVE-AA1: Allegro 2b/3 4 mg: 35%; 41% 52 w 12 mg 42% 200 mg LD 2 mg: 22%; 21% 52 w; 8 mg 30% ; +50 mg: 31% +30 pbo:
3.3% pbo 1% No Results No Results No Results No Results No Results No Results Outcome mg: 22% No Results BRAVE-AA2: THRIVE-AA2: Available Available Available Available Available Available 50 mg LD: 24% Available 4mg: 32%; 37% 52w 12 mg 38% 30 mg
LD:14.5% 2mg: 17%; 24% 52w; 8 mg 33.0%; 10 mg LD: --- pbo: 6.2% pbo 1% 2 mg or 2 SC at Dosing 50 mg Oral QD Oral BID Oral BID Oral QD Oral QD Oral QD Oral BID SC Q2W 4 mg Oral QD 3.5 wks OLUMIANT (baricitinib) List price: $2,497.20 for 30-day supply
of 2mg, and $4,994.40 for 30-day supply of 4mg (lillypricinginfo.com) 37

Current Concerns in the Alopecia Areata Treatment Landscape EQ101 is
well positioned to be a better-tolerated and effective alternative to JAK inhibitors ** Currently approved and late-stage Blackbox warning “I am concerned WARNING: SERIOUS INFECTIONS, MORTALITY, pipeline drugs are all JAK inhibitors about the
safety of MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), and THROMBOSIS oral JAK inhibitors See full prescribing information for complete boxed warning. There remains a chronic need, * • Increased risk of serious bacterial, fungal,
viral and for my AA patients” opportunistic infections leading to hospitalization or death, without a safe chronic treatment including tuberculosis (TB). Interrupt treatment with OLUMIANT if serious infection occurs until the infection is % of
respondents, n = 101 controlled. OLUMIANT should not be given to patients with Dermatologists indicate safety active tuberculosis. Test for latent TB before and during therapy, except for COVID-19; treat latent TB prior to use. Monitor all patients
for active TB during treatment, even concerns are the primary barriers 13% patients with initial negative, latent TB test. (5.1) • Higher rate of all-cause mortality, including sudden to JAKi use in AA patients cardiovascular death with
another Janus kinase inhibitor (JAK) vs. TNF blockers in rheumatoid arthritis (RA) patients. (5.2) 21% • Malignancies have occurred in patients treated with OLUMIANT. Higher rate of lymphomas and lung cancers with of physicians surveyed,
another JAK inhibitor vs. TNF blockers in RA patients. (5.3) • Higher rate of MACE (defined as cardiovascular death, 66% believe there is an extremely % myocardial infarction, and stroke) with another JAK inhibitor vs. TNF blockers in RA
patients. (5.4) * high unmet medical need 79 • Thrombosis has occurred in patients treated with OLUMIANT. Increased incidence of pulmonary embolism, venous and arterial thrombosis with another JAK inhibitor vs. TNF blockers. (5.5) n
Disagree n Neutral n Agree 38 * Spherix Global Insights; ** Olumiant Package Insert

Dr. Arash Mostaghimi Alopecia Areata Fireside Chat Arash Mostaghimi,
M.D., M.P.A., M.P.H. Associate Professor of Dermatology Harvard Medical School Director, Inpatient Consultation Service, Department of Dermatology Brigham and Women’s Hospital

Question 1 How are you treating patients with alopecia areata of
different severities (SALT scores), particularly given the approval of the JAKi for AA this past year? What treatments do patients prefer? 40

ALOPECIA AREATA TREATMENT • Major problem: safe, effective,
consistent drug delivery • Limited disease (95% of patients) • No treatment • Topical therapies (very limited efficacy) • Injectable steroids • Extensive disease • Systemic immunosuppressive agents
(antimetabolites, prednisone) • Potential role for some biologic agents (dupilumab) • JAK inhibitors (baricitinib, ritlecitinib, deuruxolitinib) 41 Slides from Dr. Arash Mostaghimi, Associate Professor, Harvard Medical School

PROFOUND DISSATISFACTION WITH CURRENT THERAPIES DOI:
10.4103/ijt.ijt_53_17 42 Slides from Dr. Arash Mostaghimi, Associate Professor, Harvard Medical School

PATIENT IMPACT • Variable, but often profound impact on quality
of life • Higher rates of depression, anxiety, emotional behaviors, acute stress • Impacts performance in school, work, and relationships • Traumatic, unpredictable disease with 1/3 meeting PTSD criteria Source: DOI:
10.1007/s13555-021-00512-0 43 Slides from Dr. Arash Mostaghimi, Associate Professor, Harvard Medical School

Question 2 Does there remain an unmet need in the treatment of alopecia
areata with the approval of JAKi? Are there concerns about long term use of JAKi? 44

WHERE WE ARE TODAY 45 Slides from Dr. Arash Mostaghimi, Associate
Professor, Harvard Medical School

NEXT STEP: TARGETED THERAPIES • JAK inhibitors are a huge
advance, but not enough • Targeted therapy designed for AA is critical • Better outcomes • Improve risk/benefit of treatment • Localized therapy is an unsolved problem! • Addresses need of 95% of those who do not have
an FDA approved treatment • May be used as an adjunct therapy • Even better risk/benefit 46 Slides from Dr. Arash Mostaghimi, Associate Professor, Harvard Medical School

DERMATOLOGISTS ARE LOOKING FOR SAFER ALTERNATIVES TO JAKI FOR AA
Statement Agreement: Unmet Needs for New Treatment Options % of respondents (n=103) I am concerned about the safety of oral JAK inhibitors for my AA patients % of respondents (n=101) 6.4 8.1 9.0 3.8 Remaining Unmet Needs for AA Treatment Options %
of respondents (n=103) Mild Moderate Severe 47 Spherix Global Insights, Market Dynamix Alopecia Areata (US) 2022

Question 3 Can you share your thoughts on EQ101’s MOA and the
potential use in AA patients? What are you, as a clinician, hoping to observe in this study? 48

HOW SHOULD WE MEASURE SUCCESS? • For a proof of concept study,
would like to see early activity and then follow on studies to optimize dosing and administration • Not worried about taking an injectable treatment, but able to grow hair consistently and feel confident • Speed might be less important
• We want patients to live full and happy lives 49 Slides from Dr. Arash Mostaghimi, Associate Professor, Harvard Medical School

FINAL THOUGHTS • AA has unique properties as a disease—we
cannot treat it the same way as psoriasis and atopic dermatitis • Need a safe chronic long term treatment, physicians and patients are looking beyond JAKI • Systemic treatment is in the early innings—please innovate in this space
50 Slides from Dr. Arash Mostaghimi, Associate Professor, Harvard Medical School

THANK YOU DR. MOSTAGHIMI AVAILABLE FOR Q&A Arash Mostaghimi, M.D.,
M.P.A., M.P.H. Associate Professor of Dermatology Harvard Medical School Director, Inpatient Consultation Service, Department of Dermatology Brigham and Women’s Hospital

Equillium: Short Term Forecast 1. EQ101 initial data from alopecia
areata study expected before end of year, followed by topline data expected mid-2024 2. EQ102 SAD/MAD data expected before end of year 3. Itolizumab: • lupus nephritis data from EQUALISE study to be presented at ASN & ACR in November,
followed by topline data expected early next year • Interim review of aGVHD data from EQUATOR study expected in 2024 • Ono exercise decision and potential option payment 52

Thank you www.equilliumbio.com
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Equillium (NASDAQ:EQ)
Historical Stock Chart
From Jun 2024 to Jul 2024
Equillium (NASDAQ:EQ)
Historical Stock Chart
From Jul 2023 to Jul 2024