UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
March 4, 2024
Commission File Number: 0-29374
EDAP TMS S.A.
Parc Activite La Poudrette Lamartine
4/6 Rue du Dauphine
69120 Vaulx-en-Velin - France
Indicate by check mark whether the registrant files or will file annual reports under cover
of Form 20-F or Form 40-F.
Form 20-F [ x] Form 40-F [ ]
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by
Regulation S-T Rule 101(b)(1): ____
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by
Regulation S-T Rule 101(b)(7): ____
SIGNATURES
Pursuant to the requirements of the Securities Act of 1934, the registrant has duly caused this
report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: March 4, 2024
EDAP TMS S.A.
/s/ KEN MOBECK
KEN MOBECK
CHIEF FINANCIAL OFFICER

EDAP Announces FDA Breakthrough Device Designation
for Focal One® in the Treatment of Deep
Infiltrating Rectal Endometriosis
Focal One HIFU has potential to address large market impacting thousands
of women each year
LYON, France, March 4, 2024 - EDAP TMS SA (Nasdaq:
EDAP), the global leader in robotic energy-based therapies, announced today that its Focal
One platform has been granted Breakthrough Device designation by the US Food and Drug Administration (FDA) for the treatment of deep infiltrating
endometriosis (DIE). In June 2018, the FDA cleared Focal One Robotic Focal HIFU for the ablation of prostatic tissue.
“Receiving Breakthrough Device designation
from the FDA represents a major milestone and reinforces our commitment to expand the use of Focal One Robotic HIFU technology to treat
other patient conditions beyond prostate disease,” said Ryan Rhodes, Chief Executive Officer of EDAP TMS. “This designation
reflects the FDA’s recognition that deep infiltrating endometriosis remains a significant unmet medical need in women’s health
with few treatment alternatives. By expanding our proprietary robotic HIFU technology, we aim to provide women with a safe and effective
treatment option that is significantly less invasive and less morbid than conventional surgical approaches.”
By definition, the FDA’s Breakthrough Device
designation is granted to products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating
diseases or conditions. This unique program is intended to provide patients and health care providers with timely access to medical devices
by speeding up development, assessment, and review. The Focal One system being granted a Breakthrough Device designation underscores the
significance of this innovative development for DIE patients.
Rectal endometriosis induces lesions associated
with painful symptoms that can seriously alter quality of life for many women. Focal One HIFU is a non-invasive, robotic ablative procedure
using a high-intensity ultrasound probe to deliver tissue devitalization through use of acoustic cavitation and thermal ablation.
In January 2022, EDAP reported positive results
from the Phase 2 Endo-HIFU-1R study (N=60) evaluating Focal One HIFU for the treatment of deep infiltrating rectal endometriosis, and
this data was included in EDAP’s submission in consideration for receiving Breakthrough Device designation from the FDA. The study
evaluated the effect of HIFU treatment on endometriosis symptoms and Quality of Life (QoL). Results showed a significant decrease of the
evaluated symptoms (acute pelvic pain, dyspareunia, diarrhea, constipation, rectal bleeding, false urges, tenesmus, rectal spams, posterior
pelvic pain and asthenia) from the first post-treatment evaluation (at one month), and the reduction of symptoms was maintained at three
and six months following HIFU treatment. With respect to QoL measurement, a significant improvement was also observed from the first month
after HIFU treatment and maintained at three and six months after treatment for almost all evaluated criteria: physical functioning, role
limitation due to emotional problems, energy – fatigue, emotional well-being, social functioning, bodily pain, general health, and
on physical and mental global score components. The study also blindly evaluated the evolution of nodule volume via MRI, noting a
significant reduction of the volume of lesions observed at six months. Results from the study also showed a positive safety profile with
96.7% of patients with no or non-significant adverse events (Clavien 1), 3.3% of treated patients presenting Clavien 2 complications and
zero patients presenting Clavien 3 complications.
In February
2024, EDAP announced the completion of enrollment in a Phase 3 study evaluating Focal One HIFU therapy for the treatment of deep infiltrating
rectal endometriosis. The ongoing Phase 3 study (NCT05755958) is a comparative, randomized, double
blind trial, with the primary objective of evaluating acute pelvic pain levels in 60 patients. Patients enrolled in the study are being
followed for three months comparing HIFU treatment to a sham group. The last patient was treated in January 2024. Study results are expected
in the second half of 2024.
About Endometriosis
Endometriosis is a chronic, progressive disease
affecting nearly 10-12% of women of reproductive age. The disease is characterized by tissue resembling the lining of the uterus growing
outside the uterine cavity. This extraneous endometrial tissue may commonly occur in the peritoneum or in pelvic and extra-pelvic organs
such as the bowels, appendix, bladder, diaphragm muscle and thoracic cavity. The space between the uterus and the rectum, known as the
Douglas pouch, is one of the most frequent and symptomatic sites of endometriosis leading to rectal endometriosis.1
1 Source: https://drseckin.com/rectal-endometriosis/
About EDAP TMS SA
A recognized leader in the global therapeutic
ultrasound market, EDAP TMS develops, manufactures, promotes and distributes worldwide minimally invasive medical devices for various
pathologies using ultrasound technology. By combining the latest technologies in imaging and treatment modalities in its complete range
of Robotic HIFU devices, EDAP TMS introduced the Focal One® in Europe and in the U.S. as an answer to all requirements
for ideal prostate tissue ablation. With the addition of the ExactVu™ Micro-Ultrasound device, EDAP TMS is now the only company
offering a complete solution from diagnostics to focal treatment of Prostate Cancer. EDAP TMS also produces and distributes other medical
equipment including the Sonolith® i-move lithotripter and lasers for the treatment of urinary tract stones using extra-corporeal
shockwave lithotripsy (ESWL). For more information on the Company, please visit http://www.edap-tms.com, us.hifu-prostate.com and www.focalone.com.
Forward-Looking Statements
In addition to historical information, this press
release contains forward-looking statements within the meaning of applicable federal securities laws, including Section 27A of the U.S.
Securities Act of 1933 (the “Securities Act”) or Section 21E of the U.S. Securities Exchange Act of 1934, which may be identified
by words such as “believe,” “can,” “contemplate,” “could,” “plan,” “intend,”
“is designed to,” “may,” “might,” “potential,” “objective,” “target,”
“project,” “predict,” “forecast,” “ambition,” “guideline,” “should,”
“will,” “estimate,” “expect” and “anticipate,” or the negative of these and similar expressions,
which reflect our views about future events and financial performance. Such statements are based on management's current expectations
and are subject to a number of risks and uncertainties, including matters not yet known to us or not currently considered material by
us, and there can be no assurance that anticipated events will occur or that the objectives set out will actually be achieved. Important
factors that could cause actual results to differ materially from the results anticipated in the forward-looking statements include, among
others, the clinical status and market acceptance of our HIFU devices and the continued market potential for our lithotripsy and distribution
divisions, as well as risks associated with the current worldwide inflationary environment, the uncertain worldwide economic, political
and financial environment, geopolitical instability, climate change and pandemics like the COVID 19 pandemic, or other public health crises,
and their related impact on our business operations, including their impacts across our businesses or demand for our devices and services.
Other factors that may cause such a difference
may also include, but are not limited to, those described in the Company's filings with the Securities and Exchange Commission and in
particular, in the sections "Cautionary Statement on Forward-Looking Information" and "Risk Factors" in the Company's
Annual Report on Form 20-F.
Forward-looking statements speak only as of the
date they are made. Other than required by law, we do not undertake any obligation to update them in light of new information or future
developments. These forward-looking statements are based upon information, assumptions and estimates available to us as of the date of
this press release, and while we believe such information forms a reasonable basis for such statements, such information may be limited
or incomplete.
Company Contact
Blandine Confort
Investor Relations / Legal Affairs
EDAP TMS SA
+33 4 72 15 31 50
bconfort@edap-tms.com
Investor Contact
John Fraunces
LifeSci Advisors, LLC
(917) 355-2395
jfraunces@lifesciadvisors.com
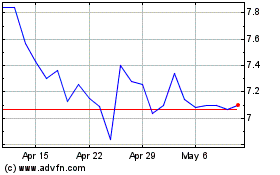
EDAP TMS (NASDAQ:EDAP)
Historical Stock Chart
From Jul 2024 to Aug 2024
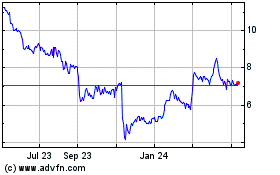
EDAP TMS (NASDAQ:EDAP)
Historical Stock Chart
From Aug 2023 to Aug 2024