Dyne Therapeutics Gets New FDA Orphan Drug Designation
September 20 2023 - 4:51PM
Dow Jones News
By Sabela Ojea
Dyne Therapeutics said the U.S. Food and Drug Administration
granted orphan drug designation to its DYNE-101 treatment, which
targets the multisystem disorder Myotonic Dystrophy Type 1.
The biotechnology company on Wednesday said that DYNE-101 is
being evaluated in the Phase 1/2 global achieve clinical trial with
initial data on safety expected for the second half of the
year.
The orphan drug designation is granted to drugs or biologics
intended for treatment, prevention or diagnosis of a rare disease
that affects less than 200,000 people in the U.S.
Write to Sabela Ojea at sabela.ojea@wsj.com
(END) Dow Jones Newswires
September 20, 2023 16:36 ET (20:36 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
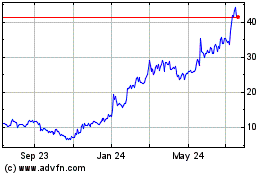
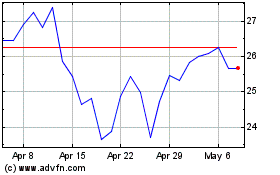
Dyne Therapeutics (NASDAQ:DYN)
Historical Stock Chart
From Apr 2024 to May 2024
Dyne Therapeutics (NASDAQ:DYN)
Historical Stock Chart
From May 2023 to May 2024