false 0001845337 0001845337 2024-06-17 2024-06-17
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 17, 2024
Month
DAY ONE BIOPHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-40431 |
|
83-2415215 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
|
|
| 2000 Sierra Point Parkway, Suite 501 Brisbane, California |
|
94005 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (650) 484-0899
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
DAWN |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 1.01 |
Entry into a Material Definitive Agreement. |
On June 17, 2024, Day One Biopharmaceuticals, Inc. (the “Company”) and MabCare Therapeutics (“MabCare”) entered into an Exclusive License Agreement (the “License Agreement”) pursuant to which MabCare will license to the Company, on an exclusive basis, the right to develop, manufacture and commercialize MTX-13 (which going forward will be identified as DAY301), a novel anti-body drug conjugate targeting protein-tyrosine kinase 7 (PTK7), worldwide, excluding the MabCare Territory which covers Greater China. All capitalized terms herein have the definitions assigned to them in the License Agreement unless otherwise defined herein.
In consideration for the rights and licenses granted by MabCare to the Company in the License Agreement, the Company will pay MabCare an upfront license fee in the amount of $55.0 million. Further, pursuant to the License Agreement, MabCare is eligible to receive up to an additional $1.15 billion in development, regulatory and commercial milestones and tiered royalty payments ranging from low-to-mid single digit percentages of Net Sales of Licensed Products in the Day One Territory, subject to the certain adjustments specified in the License Agreement.
The royalty payment obligations under the License Agreement expire on a Licensed Product-by-Licensed Product and country-by-country basis no earlier than ten years following the first commercial sale of such product in the applicable country. The License Agreement contains customary termination provisions, including that either party may terminate the License Agreement (a) upon the material breach of the other party or (b) in the event the other party experiences an insolvency event. Additionally, the Company may terminate the License Agreement for convenience and MabCare may terminate the License Agreement if the Company or any of its Affiliates or Sublicensees challenge any claim in any MabCare Patent as being invalid, unenforceable or otherwise unpatentable.
The above description of the License Agreement does not purport to be complete and is qualified in its entirety by reference to the License Agreement, which will be filed as an exhibit to the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ending June 30, 2024.
| Item 7.01. |
Regulation FD Disclosure. |
On June 18, 2024, the Company issued a press release announcing the entry into the License Agreement with MabCare, a copy of which is attached hereto as Exhibit 99.1.
On June 18, 2024, the Company also updated its corporate presentation. A copy of the presentation is attached hereto as Exhibit 99.2.
The information furnished in this Item 7.01, including Exhibit 99.1 and Exhibit 99.2 to this Current Report on Form 8-K, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”). The information contained in this Current Report on Form 8-K and in the accompanying Exhibits 99.1 and 99.2 shall not be incorporated by reference into any other filing under the Exchange Act or under the Securities Act, except as shall be expressly set forth by specific reference in such filing.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
DAY ONE BIOPHARMACEUTICALS, INC. |
|
|
|
|
| Date: June 18, 2024 |
|
|
|
By: |
|
/s/ Charles N. York II, M.B.A. |
|
|
|
|
|
|
Charles N. York II, M.B.A. |
|
|
|
|
|
|
Chief Operating Officer and Chief Financial Officer |
Exhibit 99.1

Day One Expands Pipeline with Potential
First-in-Class Clinical-Stage Antibody
Drug
Conjugate (ADC) Targeting PTK7 in Solid Tumors for Adult and Pediatric
Cancers
Day One receives exclusive license for development and commercialization of MTX-13 (DAY301), which
received IND clearance by the FDA in April 2024
Targets PTK7, highly expressed in broad range of adult and pediatric solid tumors
BRISBANE, Calif., June 18, 2024 – Day One Biopharmaceuticals (Nasdaq: DAWN) (“Day One” or the “Company”), a
commercial-stage biopharmaceutical company dedicated to developing and commercializing targeted therapies for people of all ages with life-threatening diseases, today announced it has entered into an exclusive licensing agreement (the Agreement)
with MabCare Therapeutics (MabCare) for MTX-13, a novel ADC targeting protein-tyrosine kinase 7 (PTK7). Pursuant to the terms of the Agreement, Day One has exclusive rights to develop, manufacture, and
commercialize MTX-13 worldwide, excluding Greater China.
In April 2024, the U.S. Food and Drug Administration
(FDA) cleared the investigational new drug (IND) application for MTX-13, which going forward will be identified as DAY301. In pre-clinical studies, DAY301 showed
antitumor activity in a wide range of solid tumors.
“Our priorities for 2024 are to successfully launch OJEMDATM (tovorafenib), to advance our existing programs and to expand our pipeline by in-licensing clinical-stage assets that have the potential to transform outcomes
for patients of all ages living with cancers,” said Jeremy Bender, Ph.D., chief executive officer of Day One. “We are excited by the opportunity presented by DAY301, and we believe we have the right team in place to develop the program to
its full potential.”
DAY301 targets PTK7, a highly-conserved, catalytically inactive transmembrane protein that is overexpressed in multiple adult
cancers, including esophageal, ovarian, lung, and endometrial cancer, as well as pediatric cancers such as neuroblastoma, rhabdomyosarcoma and osteosarcoma. PTK7 has limited expression in normal tissues or organs, making it an attractive target for
therapeutic development.
“The addition of DAY301 to our pipeline strategically fits our mission of advancing both pediatric and adult medicines in
areas of unmet need with equal urgency,” said Dr. Samuel Blackman, co-founder and head of research and development at Day One. “We believe the linker-payload technology embodied in DAY301 will
overcome the limitations of earlier PTK7-targeted ADCs, giving us a potential first-in-class drug against a clinically-validated target. We are excited to add this
program to Day One and will look to enter the clinic in the coming months.”
Under the terms of the licensing agreement, MabCare will receive $55 million upfront, and is eligible
to receive an additional $1.152 billion in development, regulatory and commercial success-based milestones, plus low-to-mid single-digit royalties on net sales
outside of Greater China. Day One expects the first patient to be dosed in the Phase I study in the fourth quarter of 2024 or first quarter of 2025.
About Day One Biopharmaceuticals
Day One
Biopharmaceuticals is a commercial-stage biopharmaceutical company that believes when it comes to pediatric cancer, we can do better. The Company was founded to address a critical unmet need: the dire lack of therapeutic development in pediatric
cancer. Inspired by “The Day One Talk” that physicians have with patients and their families about an initial cancer diagnosis and treatment plan, Day One aims to re-envision cancer drug development
and redefine what’s possible for all people living with cancer—regardless of age—starting from Day One.
Day One partners with leading
clinical oncologists, families, and scientists to identify, acquire, and develop important targeted cancer treatments. The Company’s pipeline includes tovorafenib (OJEMDA™) and
pimasertib.
Day One is based in Brisbane, California. For more information, please visit www.dayonebio.com or find the Company
on LinkedIn or X.
Cautionary Note Regarding Forward-Looking Statements
This press release contains “forward-looking” statements within the meaning of the “safe harbor” provisions of the Private Securities
Litigation Reform Act of 1995, including, but not limited to: Day One’s plans to develop cancer therapies, including DAY301, expectations regarding planned and current clinical trials and the ability of tovorafenib to treat pLGG or related
indications.
Statements including words such as “believe,” “plan,” “continue,” “expect,” “will,”
“develop,” “signal,” “potential,” or “ongoing” and statements in the future tense are forward-looking statements. These forward-looking statements involve risks and uncertainties, as well as assumptions,
which, if they do not fully materialize or prove incorrect, could cause our results to differ materially from those expressed or implied by such forward-looking statements.
Forward-looking statements are subject to risks and uncertainties that may cause Day One’s actual activities or results to differ significantly from
those expressed in any forward-looking statement, including risks and uncertainties in this press release and other risks set forth in our filings with the Securities and Exchange Commission, including Day One’s ability to develop, obtain
regulatory approval for or commercialize any product candidate, Day One’s ability to protect intellectual property, the potential impact of global business or macroeconomic conditions, including as a result of inflation, rising interest rates,
instability in the global banking system, geopolitical conflicts and the sufficiency of Day One’s cash, cash equivalents and investments to fund its operations. These forward-looking statements speak only as of the date hereof and Day One
specifically disclaims any obligation to update these forward-looking statements or reasons why actual results might differ, whether as a result of new information, future events or otherwise, except as required by law.
#####
DAY ONE MEDIA
Laura Cooper, Head of Communications
media@dayonebio.com
DAY ONE INVESTORS
LifeSci Advisors, PJ Kelleher
pkelleher@lifesciadvisors.com

Exhibit 99.2 Day One Biopharmaceuticals Targeted Therapies for People of
All Ages June 2024 1

Disclaimer This presentation and the accompanying oral commentary
contain forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements are inherently subject to risks and uncertainties, some of which
cannot be predicted or quantified. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expect,” “plan,”
anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential,” “would,” “continue,” “ongoing” or the negative of these terms or other
comparable terminology. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, including the sufficiency of our
cash, cash equivalents and short-term investments to fund our operations, business plans and objectives, timing and success of our commercialization and marketing efforts, timing and success of our planned nonclinical and clinical development
activities, the results of any of our strategic collaborations, including the potential achievement of milestones and provision of royalty payments thereunder, timing and results of nonclinical studies and clinical trials, efficacy and safety
profiles of our products and product candidates, the ability of OJEMDA™ (tovorafenib) to treat pediatric low-grade glioma (pLGG) or related indications, the potential therapeutic benefits and economic value of our products and product
candidates, potential growth opportunities, competitive position, industry environment and potential market opportunities, our ability to protect intellectual property and the impact of global business or macroeconomic conditions, including as a
result of inflation, changing interest rates, cybersecurity incidents, potential instability in the global banking system, uncertainty with respect to the federal debt ceiling and budget and potential government shutdowns related thereto and global
regional conflicts, on our business and operations. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors. It is not possible for our management to predict all risks, nor can we assess the
impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those
that are described under the heading “Risk Factors” contained in our most recent Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) and other documents we file from time to time with the SEC, may cause
our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs
and opinions on the relevant subject. These statements are based upon information available to us as of the date of this presentation, and although we believe such information forms a reasonable basis for such statements, such information may be
limited or incomplete, and our statements should not be read to indicate that we have conducted a thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are
cautioned not to unduly rely upon these statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not
regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements,
whether as a result of new information, future events or otherwise, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other
data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future
performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. 2

Cancer Therapies for People of All Ages Our Approach • Develop
medicines for genomically-defined cancers • Establish first-in-class position through rapid registration pathways • Expand to adolescent and adult populations in parallel and pursue those opportunities with the same commitment we do for
children IPO: 2021 Financial Position: Runway into 2026 Founded: 2018 Nasdaq: DAWN 3
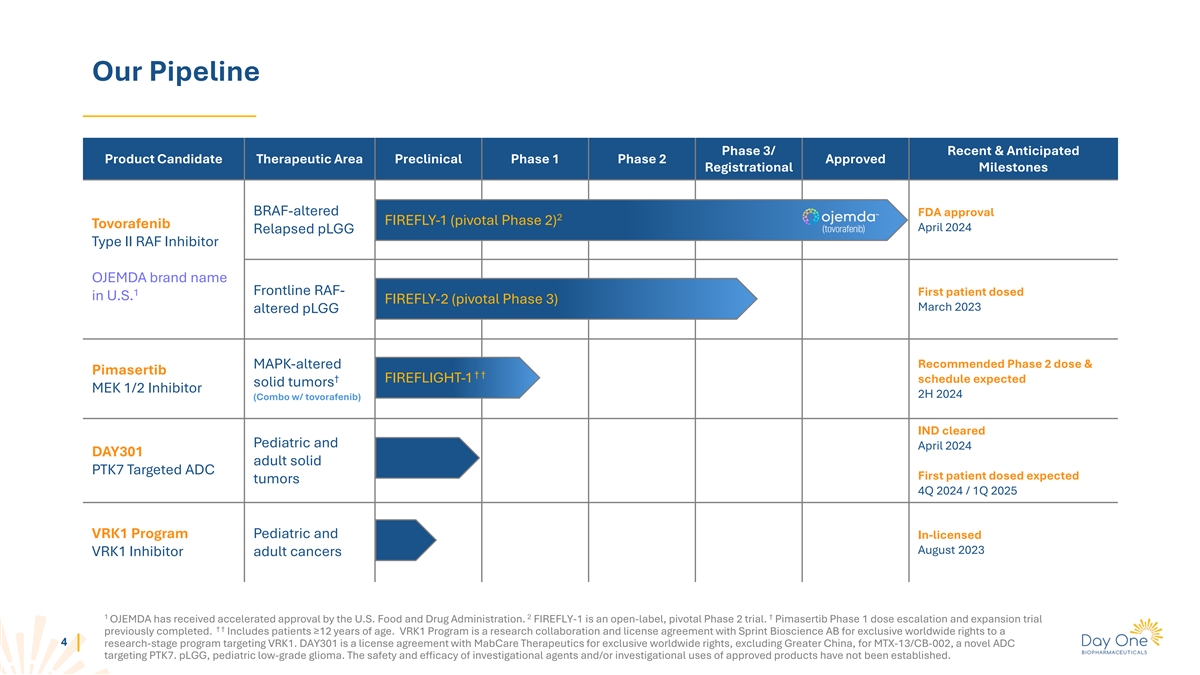
Our Pipeline Phase 3/ Recent & Anticipated Product Candidate
Therapeutic Area Preclinical Phase 1 Phase 2 Approved Registrational Milestones BRAF-altered FDA approval 2 FIREFLY-1 (pivotal Phase 2) Tovorafenib April 2024 Relapsed pLGG Type II RAF Inhibitor OJEMDA brand name Frontline RAF- 1 First patient dosed
in U.S. FIREFLY-2 (pivotal Phase 3) March 2023 altered pLGG Recommended Phase 2 dose & MAPK-altered Pimasertib † † † FIREFLIGHT-1 schedule expected solid tumors MEK 1/2 Inhibitor 2H 2024 (Combo w/ tovorafenib) IND cleared
Pediatric and April 2024 DAY301 adult solid PTK7 Targeted ADC First patient dosed expected tumors 4Q 2024 / 1Q 2025 VRK1 Program Pediatric and In-licensed August 2023 VRK1 Inhibitor adult cancers 1 2 † OJEMDA has received accelerated approval
by the U.S. Food and Drug Administration. FIREFLY-1 is an open-label, pivotal Phase 2 trial. Pimasertib Phase 1 dose escalation and expansion trial † † previously completed. Includes patients ≥12 years of age. VRK1 Program is a
research collaboration and license agreement with Sprint Bioscience AB for exclusive worldwide rights to a 4 research-stage program targeting VRK1. DAY301 is a license agreement with MabCare Therapeutics for exclusive worldwide rights, excluding
Greater China, for MTX-13/CB-002, a novel ADC targeting PTK7. pLGG, pediatric low-grade glioma. The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established.

TM OJEMDA (tovorafenib) Relapsed or Refractory BRAF-altered pLGG
5
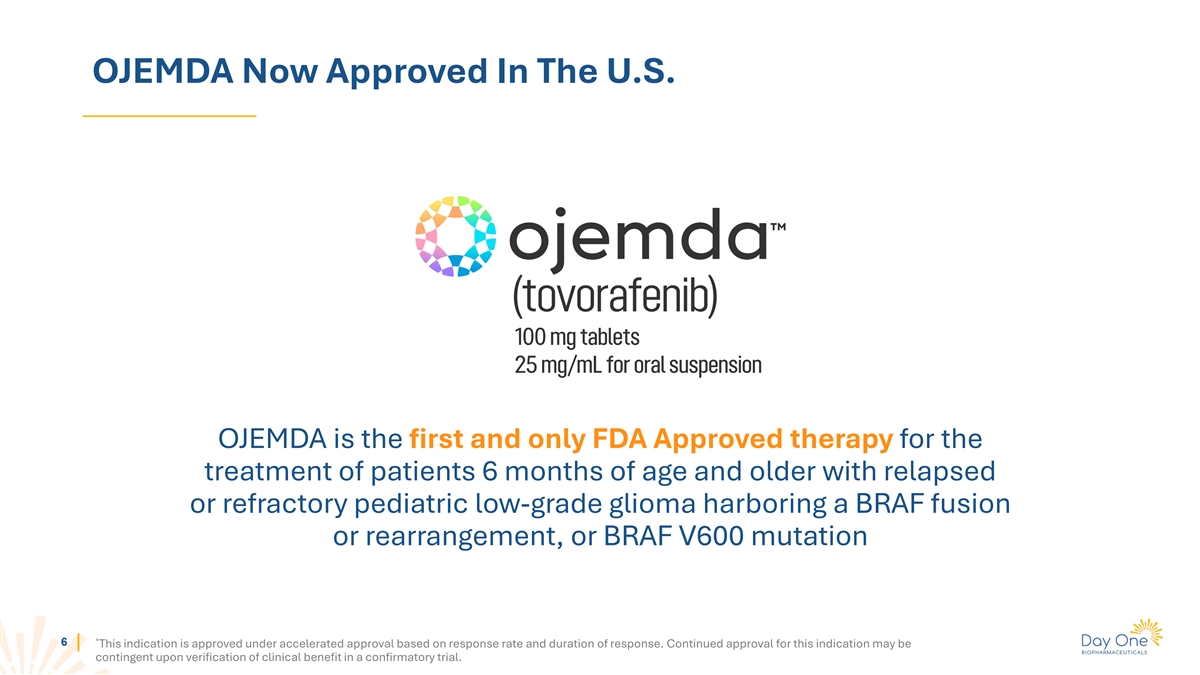
OJEMDA Now Approved In The U.S. OJEMDA is the first and only FDA
Approved therapy for the treatment of patients 6 months of age and older with relapsed or refractory pediatric low-grade glioma harboring a BRAF fusion or rearrangement, or BRAF V600 mutation * 6 This indication is approved under accelerated
approval based on response rate and duration of response. Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial.
pLGG Impact On Patients’ Lives Lily was diagnosed with an operable
brain tumor at 5 months of age 7 Lily, lives with pLGG.
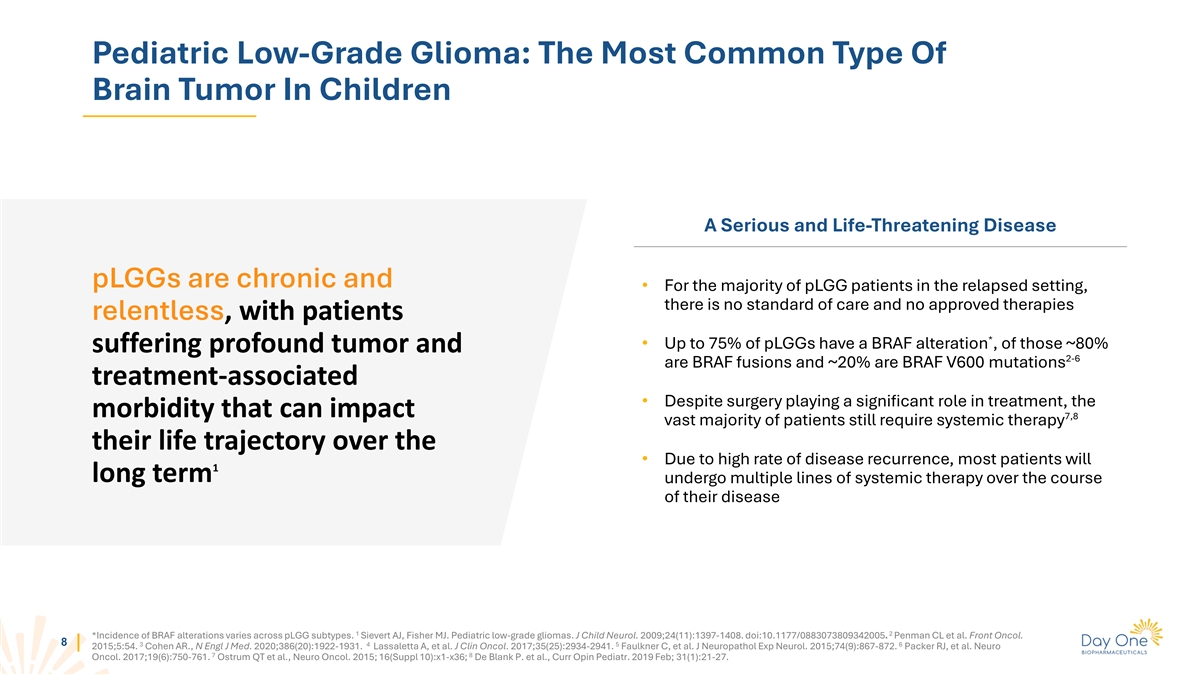
Pediatric Low-Grade Glioma: The Most Common Type Of Brain Tumor In
Children A Serious and Life-Threatening Disease pLGGs are chronic and • For the majority of pLGG patients in the relapsed setting, there is no standard of care and no approved therapies relentless, with patients * • Up to 75% of pLGGs
have a BRAF alteration , of those ~80% suffering profound tumor and 2-6 are BRAF fusions and ~20% are BRAF V600 mutations treatment-associated • Despite surgery playing a significant role in treatment, the morbidity that can impact 7,8 vast
majority of patients still require systemic therapy their life trajectory over the • Due to high rate of disease recurrence, most patients will 1 long term undergo multiple lines of systemic therapy over the course of their disease 1 2
*Incidence of BRAF alterations varies across pLGG subtypes. Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24(11):1397-1408. doi:10.1177/0883073809342005. Penman CL et al. Front Oncol. 8 3 4 5 6 2015;5:54. Cohen AR., N Engl
J Med. 2020;386(20):1922-1931. Lassaletta A, et al. J Clin Oncol. 2017;35(25):2934-2941. Faulkner C, et al. J Neuropathol Exp Neurol. 2015;74(9):867-872. Packer RJ, et al. Neuro 7 8 Oncol. 2017;19(6):750-761. Ostrum QT et al., Neuro Oncol. 2015;
16(Suppl 10):x1-x36; De Blank P. et al., Curr Opin Pediatr. 2019 Feb; 31(1):21-27.
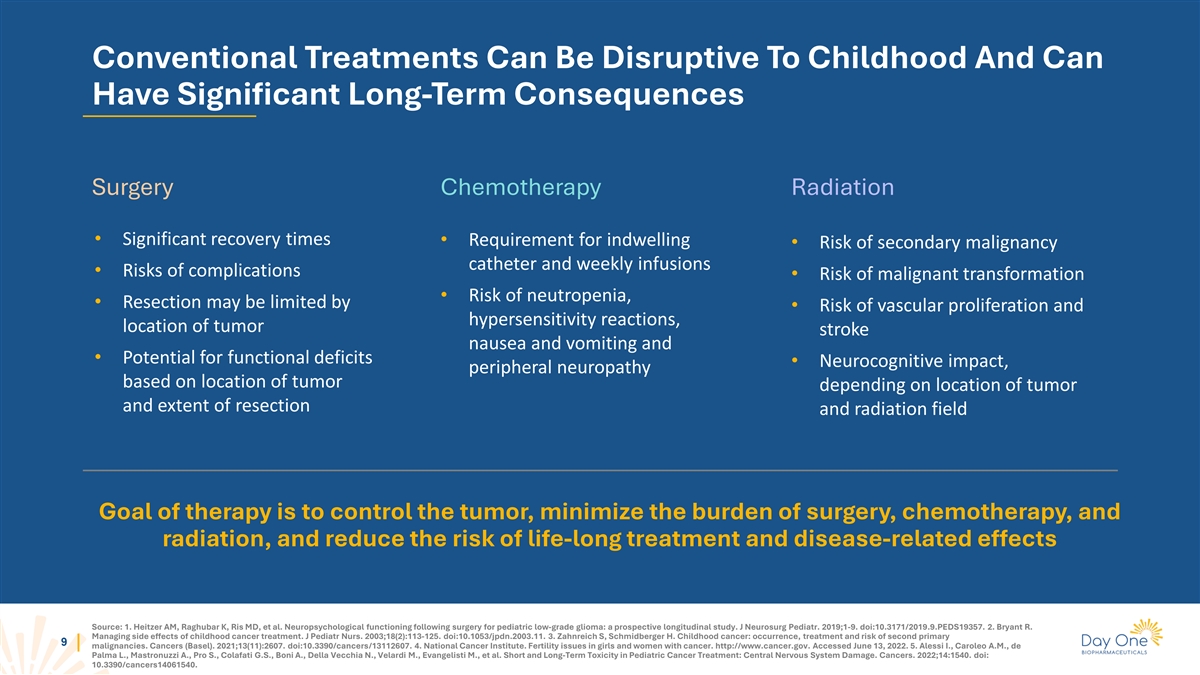
Conventional Treatments Can Be Disruptive To Childhood And Can Have
Significant Long-Term Consequences Surgery Chemotherapy Radiation • Significant recovery times • Requirement for indwelling • Risk of secondary malignancy catheter and weekly infusions • Risks of complications • Risk of
malignant transformation • Risk of neutropenia, • Resection may be limited by • Risk of vascular proliferation and hypersensitivity reactions, location of tumor stroke nausea and vomiting and • Potential for functional
deficits • Neurocognitive impact, peripheral neuropathy based on location of tumor depending on location of tumor and extent of resection and radiation field Goal of therapy is to control the tumor, minimize the burden of surgery,
chemotherapy, and radiation, and reduce the risk of life-long treatment and disease-related effects Source: 1. Heitzer AM, Raghubar K, Ris MD, et al. Neuropsychological functioning following surgery for pediatric low-grade glioma: a prospective
longitudinal study. J Neurosurg Pediatr. 2019;1-9. doi:10.3171/2019.9.PEDS19357. 2. Bryant R. Managing side effects of childhood cancer treatment. J Pediatr Nurs. 2003;18(2):113-125. doi:10.1053/jpdn.2003.11. 3. Zahnreich S, Schmidberger H.
Childhood cancer: occurrence, treatment and risk of second primary 9 malignancies. Cancers (Basel). 2021;13(11):2607. doi:10.3390/cancers/13112607. 4. National Cancer Institute. Fertility issues in girls and women with cancer. http://www.cancer.gov.
Accessed June 13, 2022. 5. Alessi I., Caroleo A.M., de Palma L., Mastronuzzi A., Pro S., Colafati G.S., Boni A., Della Vecchia N., Velardi M., Evangelisti M., et al. Short and Long-Term Toxicity in Pediatric Cancer Treatment: Central Nervous System
Damage. Cancers. 2022;14:1540. doi: 10.3390/cancers14061540.

TM Overview U.S. Prescribing Information For OJEMDA (tovorafenib)
Available in tablet formulation and pediatric-friendly powder for oral suspension INDICATION OJEMDA is indicated for the treatment of pediatric patients 6 months of age and older with relapsed or refractory pediatric low-grade glioma harboring a
BRAF fusion or rearrangement, or BRAF V600 mutation RECOMMENDED DOSE 2 380 mg/m administered orally once weekly (not to exceed a dose of 600mg once weekly); OJEMDA can be taken with or without food For full prescribing information, visit
dayonebio.com * 10 This indication is approved under accelerated approval based on response rate and duration of response. Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory
trial.
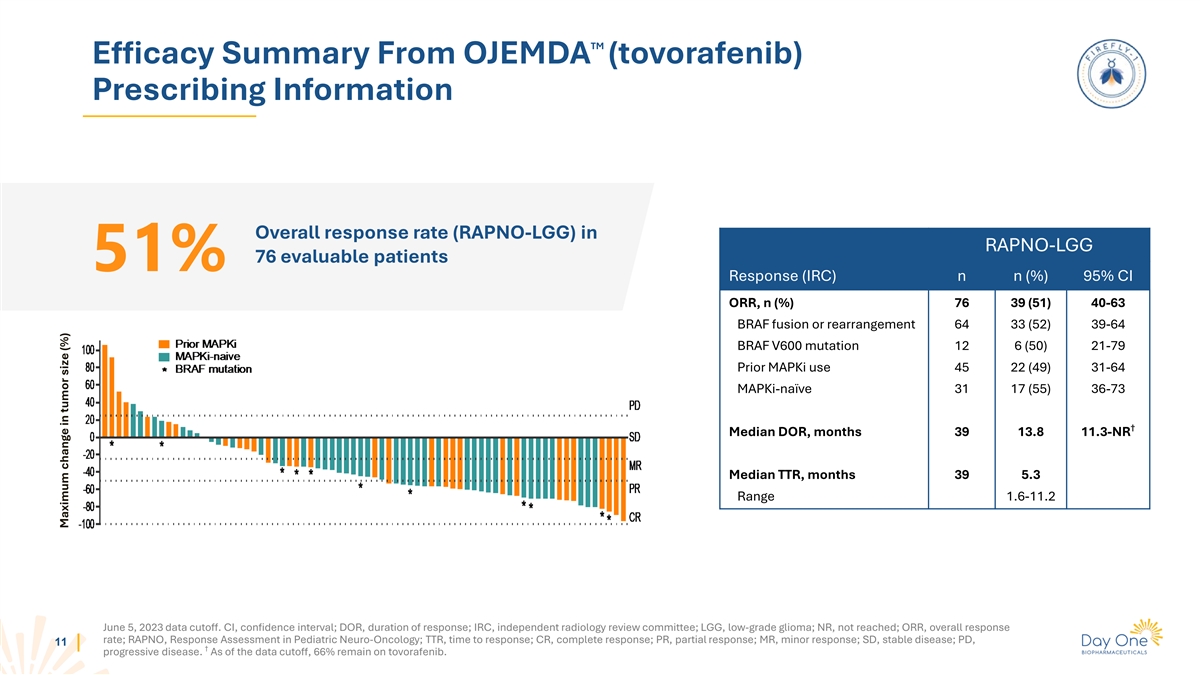
TM Efficacy Summary From OJEMDA (tovorafenib) Prescribing Information
Overall response rate (RAPNO-LGG) in RAPNO-LGG 76 evaluable patients 51% Response (IRC) n n (%) 95% CI ORR, n (%) 76 39 (51) 40-63 BRAF fusion or rearrangement 64 33 (52) 39-64 BRAF V600 mutation 12 6 (50) 21-79 Prior MAPKi use 45 22 (49) 31-64
MAPKi-naïve 31 17 (55) 36-73 † Median DOR, months 39 13.8 11.3-NR Median TTR, months 39 5.3 Range 1.6-11.2 June 5, 2023 data cutoff. CI, confidence interval; DOR, duration of response; IRC, independent radiology review committee; LGG,
low-grade glioma; NR, not reached; ORR, overall response rate; RAPNO, Response Assessment in Pediatric Neuro-Oncology; TTR, time to response; CR, complete response; PR, partial response; MR, minor response; SD, stable disease; PD, 11 †
progressive disease. As of the data cutoff, 66% remain on tovorafenib. Maximum change in tumor size (%)
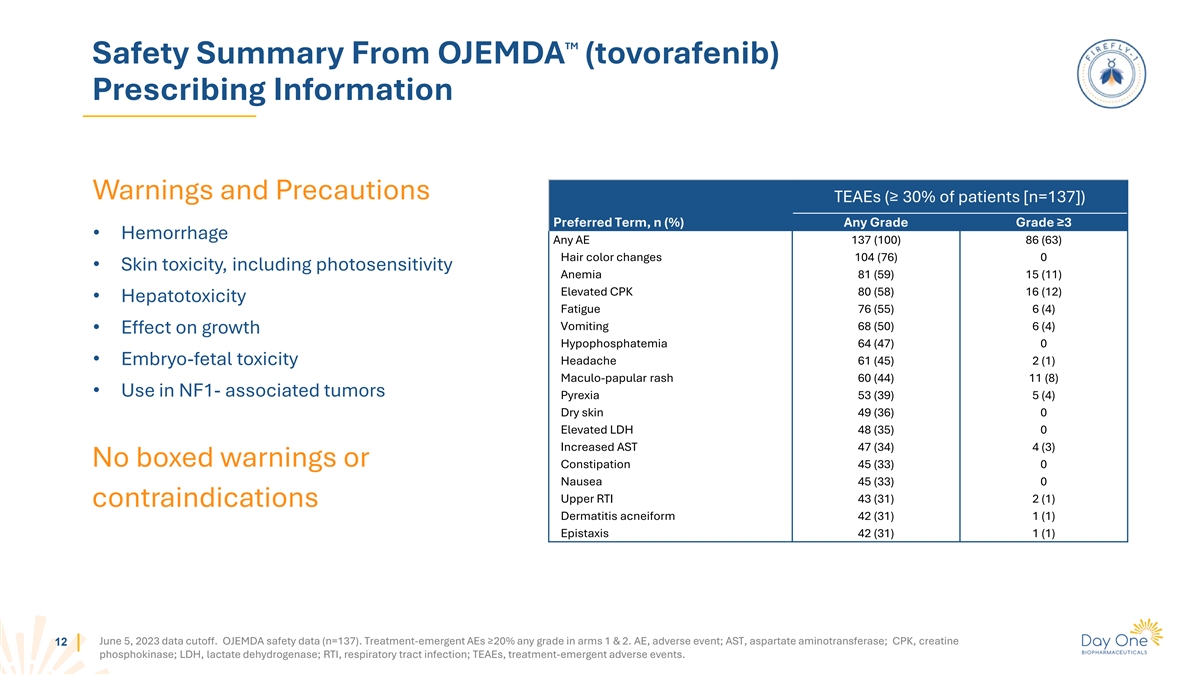
TM Safety Summary From OJEMDA (tovorafenib) Prescribing Information
Warnings and Precautions TEAEs (≥ 30% of patients [n=137]) Preferred Term, n (%) Any Grade Grade ≥3 • Hemorrhage Any AE 137 (100) 86 (63) Hair color changes 104 (76) 0 • Skin toxicity, including photosensitivity Anemia 81
(59) 15 (11) Elevated CPK 80 (58) 16 (12) • Hepatotoxicity Fatigue 76 (55) 6 (4) Vomiting 68 (50) 6 (4) • Effect on growth Hypophosphatemia 64 (47) 0 • Embryo-fetal toxicity Headache 61 (45) 2 (1) Maculo-papular rash 60 (44) 11 (8)
• Use in NF1- associated tumors Pyrexia 53 (39) 5 (4) Dry skin 49 (36) 0 Elevated LDH 48 (35) 0 Increased AST 47 (34) 4 (3) No boxed warnings or Constipation 45 (33) 0 Nausea 45 (33) 0 Upper RTI 43 (31) 2 (1) contraindications Dermatitis
acneiform 42 (31) 1 (1) Epistaxis 42 (31) 1 (1) June 5, 2023 data cutoff. OJEMDA safety data (n=137). Treatment-emergent AEs ≥20% any grade in arms 1 & 2. AE, adverse event; AST, aspartate aminotransferase; CPK, creatine 12 phosphokinase;
LDH, lactate dehydrogenase; RTI, respiratory tract infection; TEAEs, treatment-emergent adverse events.
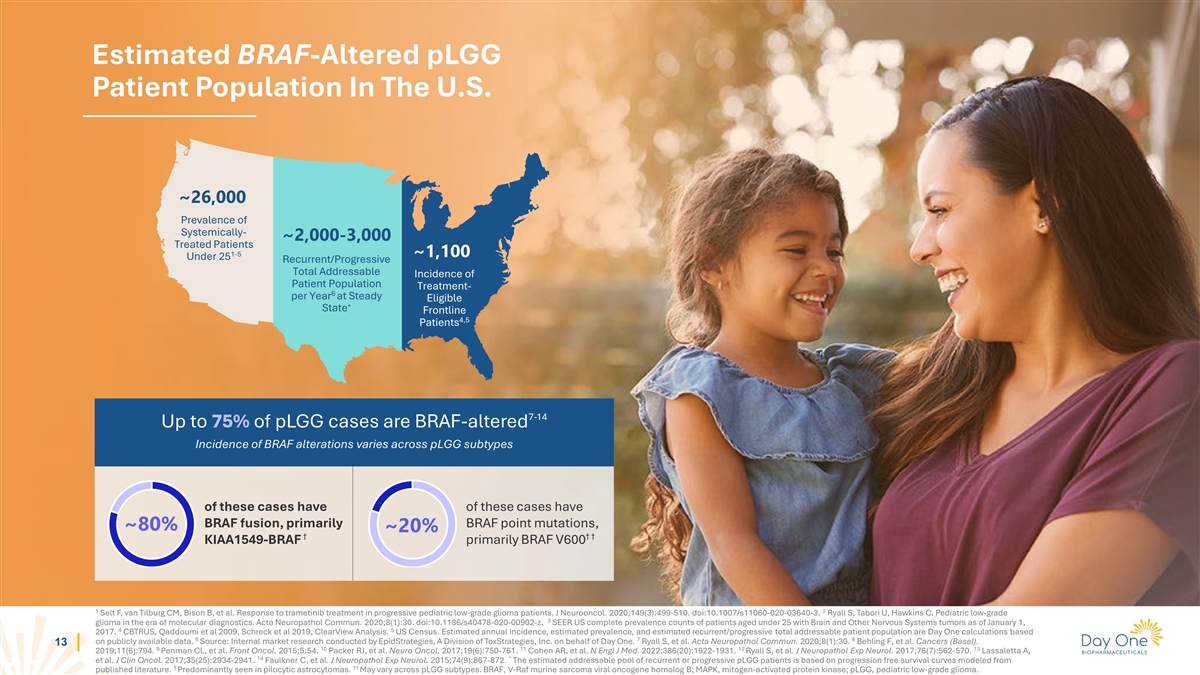
Estimated BRAF-Altered pLGG Patient Population In The U.S. ~26,000
Prevalence of Systemically- ~2,000-3,000 Treated Patients 1-5 ~1,100 Under 25 Recurrent/Progressive Total Addressable Incidence of Patient Population Treatment- 6 per Year at Steady Eligible * State Frontline 4,5 Patients 7-14 Up to 75% of pLGG
cases are BRAF-altered Incidence of BRAF alterations varies across pLGG subtypes of these cases have of these cases have BRAF fusion, primarily BRAF point mutations, ~80% ~20% † † † KIAA1549-BRAF primarily BRAF V600 1 2 Selt F, van
Tilburg CM, Bison B, et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neurooncol. 2020;149(3):499-510. doi:10.1007/s11060-020-03640-3. Ryall S, Tabori U, Hawkins C. Pediatric low-grade 3 glioma in the era
of molecular diagnostics. Acta Neuropathol Commun. 2020;8(1):30. doi:10.1186/s40478-020-00902-z. SEER US complete prevalence counts of patients aged under 25 with Brain and Other Nervous Systems tumors as of January 1, 4 5 2017. CBTRUS, Qaddoumi et
al 2009, Schreck et al 2019, ClearView Analysis. US Census. Estimated annual incidence, estimated prevalence, and estimated recurrent/progressive total addressable patient population are Day One calculations based 6 7 8 on publicly available data.
Source: Internal market research conducted by EpidStrategies, A Division of ToxStrategies, Inc. on behalf of Day One. Ryall S, et al. Acta Neuropathol Commun. 2020;8(1):30. Behling F, et al. Cancers (Basel). 13 9 10 11 12 13 2019;11(6):794. Penman
CL, et al. Front Oncol. 2015;5:54. Packer RJ, et al. Neuro Oncol. 2017;19(6):750-761. Cohen AR, et al. N Engl J Med. 2022;386(20):1922-1931. Ryall S, et al. J Neuropathol Exp Neurol. 2017;76(7):562-570. Lassaletta A, 14 * et al. J Clin Oncol.
2017;35(25):2934-2941. Faulkner C, et al. J Neuropathol Exp Neurol. 2015;74(9):867-872. The estimated addressable pool of recurrent or progressive pLGG patients is based on progression free survival curves modeled from † ††
published literature. Predominantly seen in pilocytic astrocytomas. May vary across pLGG subtypes. BRAF, V-Raf murine sarcoma viral oncogene homolog B; MAPK, mitogen-activated protein kinase; pLGG, pediatric low-grade glioma.

What Physicians & Caregivers Are Looking For In A Therapy What
HCP’s are Seeking What Caregivers are Seeking Effective in stopping or shrinking tumors Live as normal of a childhood as possible Manageable safety profile Minimal impact from the disease Minimal disruption to child’s life Minimal
disruption to child’s life “Our time with our kids is precious and not guaranteed, so the less time with meds and “The goal is not interfering with the child’s life.” doctors the better.” – Ped Onc, Chicago
Ad Board – Caregiver for a child under 5 yrs 14
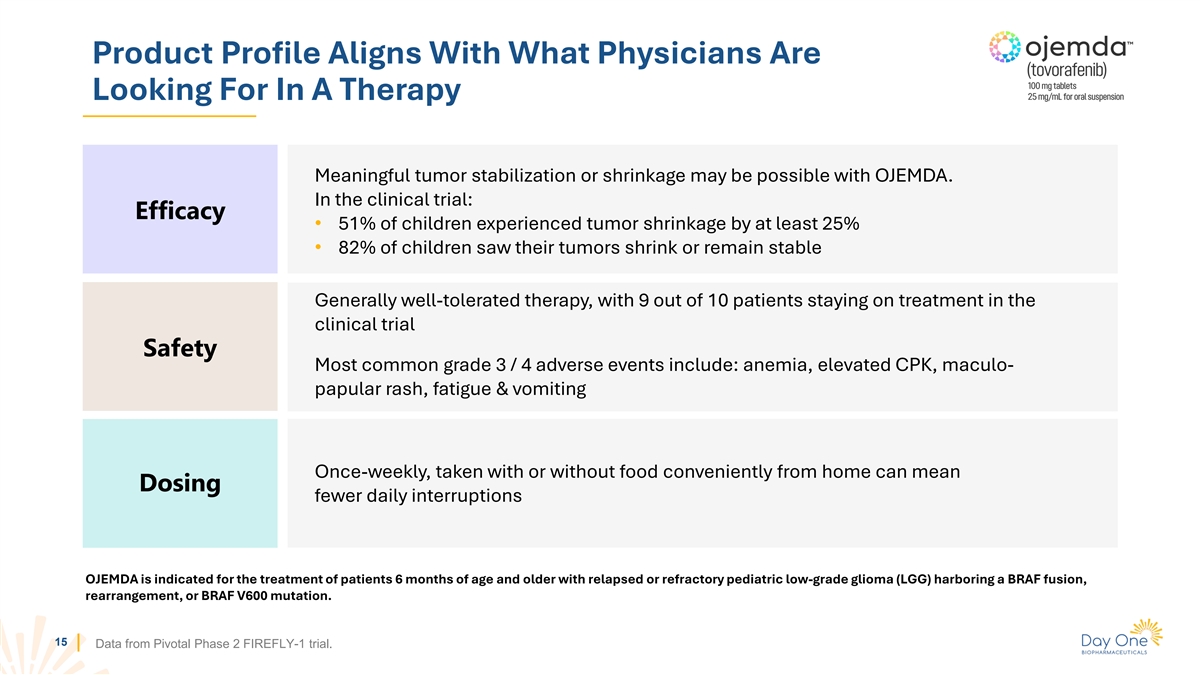
Product Profile Aligns With What Physicians Are Looking For In A
Therapy Meaningful tumor stabilization or shrinkage may be possible with OJEMDA. In the clinical trial: Efficacy • 51% of children experienced tumor shrinkage by at least 25% • 82% of children saw their tumors shrink or remain stable
Generally well-tolerated therapy, with 9 out of 10 patients staying on treatment in the clinical trial Safety Most common grade 3 / 4 adverse events include: anemia, elevated CPK, maculo- papular rash, fatigue & vomiting Once-weekly, taken with
or without food conveniently from home can mean Dosing fewer daily interruptions OJEMDA is indicated for the treatment of patients 6 months of age and older with relapsed or refractory pediatric low-grade glioma (LGG) harboring a BRAF fusion,
rearrangement, or BRAF V600 mutation. 15 Data from Pivotal Phase 2 FIREFLY-1 trial.
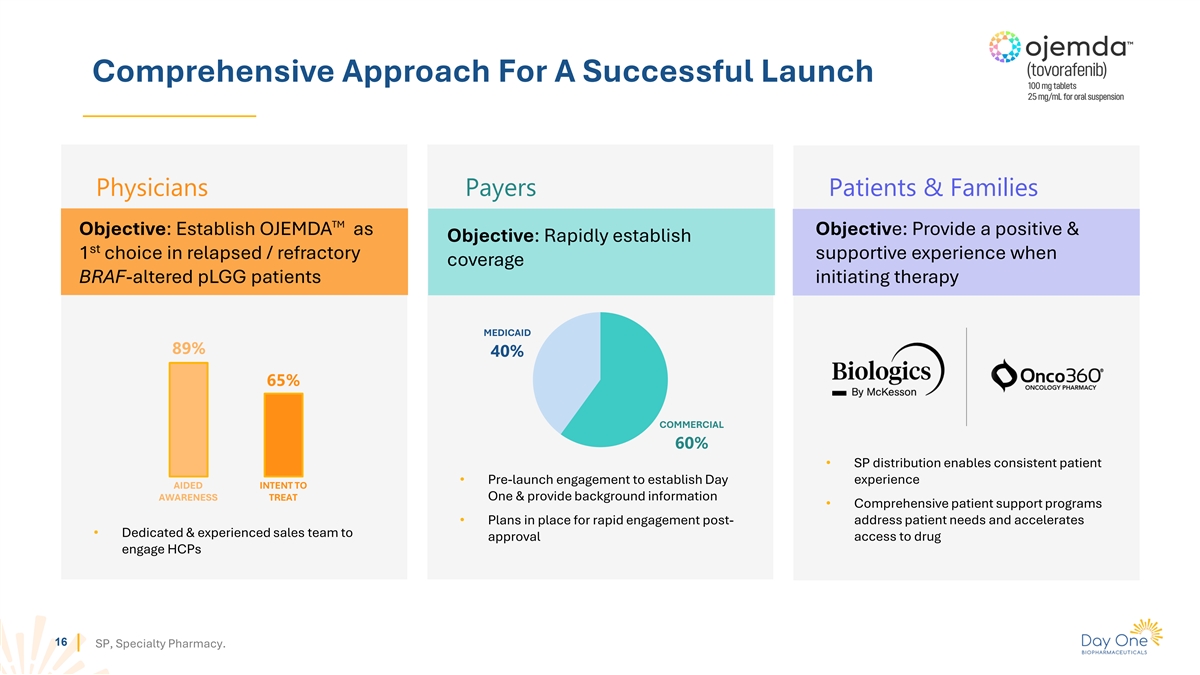
Comprehensive Approach For A Successful Launch Physicians Payers
Patients & Families TM Objective: Establish OJEMDA as Objective: Provide a positive & Objective: Rapidly establish st 1 choice in relapsed / refractory supportive experience when coverage BRAF-altered pLGG patients initiating therapy
MEDICAID 89% 40% 65% COMMERCIAL 60% • SP distribution enables consistent patient • Pre-launch engagement to establish Day experience AIDED INTENT TO One & provide background information AWARENESS TREAT • Comprehensive patient
support programs • Plans in place for rapid engagement post- address patient needs and accelerates • Dedicated & experienced sales team to approval access to drug engage HCPs 16 SP, Specialty Pharmacy.
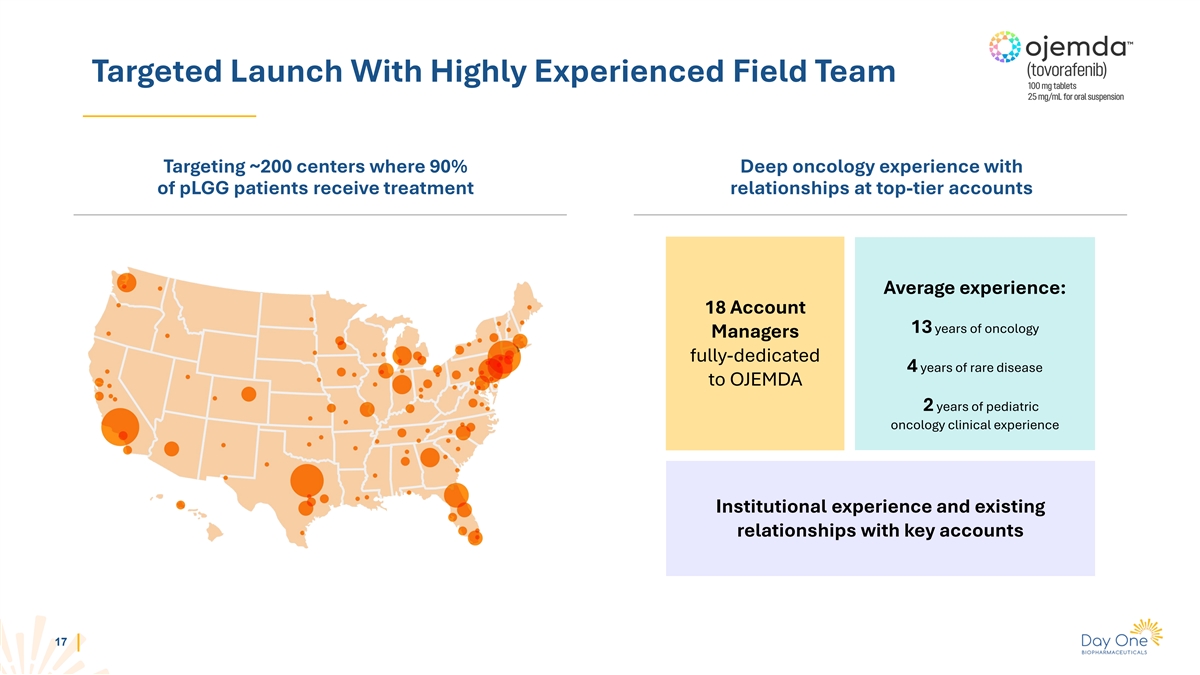
Targeted Launch With Highly Experienced Field Team Targeting ~200
centers where 90% Deep oncology experience with of pLGG patients receive treatment relationships at top-tier accounts Average experience: 18 Account 13 years of oncology Managers fully-dedicated 4 years of rare disease to OJEMDA 2 years of pediatric
oncology clinical experience Institutional experience and existing relationships with key accounts 17

Patient Support Program Supporting Access DEDICATED PATIENT NAVIGATOR
COPAY CARD PHONE & VIDEO PROGRAM CONSULTATIONS COVERAGE PATIENT DELAY ASSISTANCE PROGRAM PROGRAM COVERAGE INTERRUPTION SUPPORT 18

FIREFLY-2 / LOGGIC Pivotal Phase 3 Trial of Tovorafenib in Frontline
pLGG 19
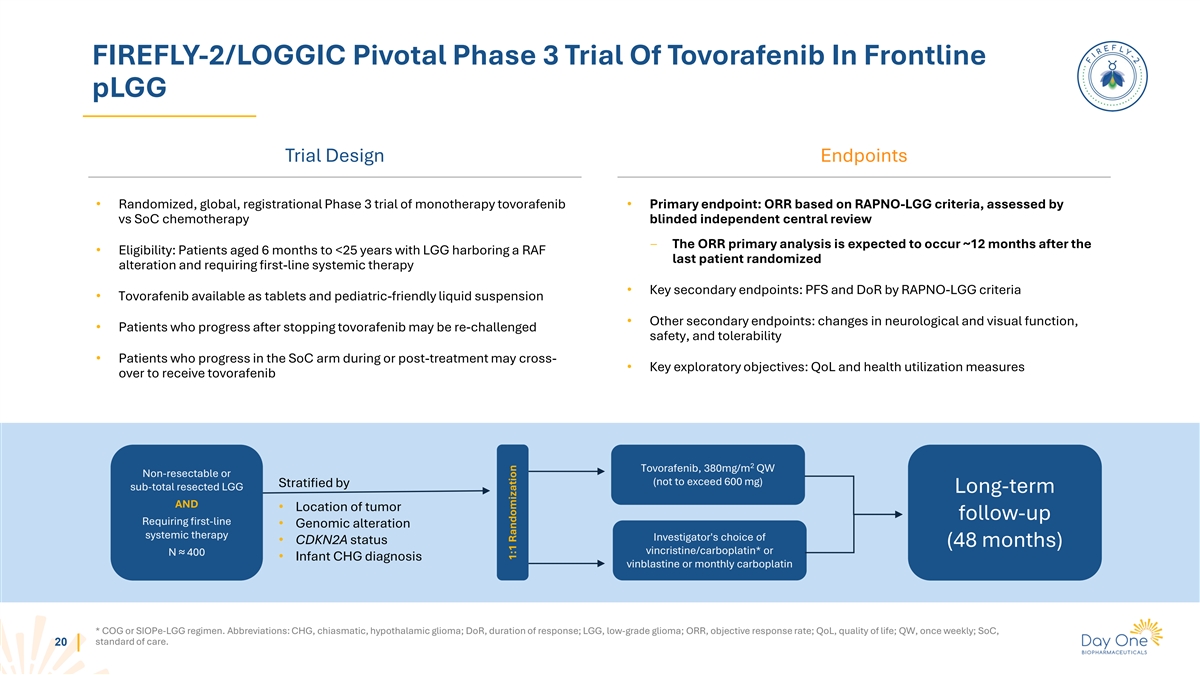
FIREFLY-2/LOGGIC Pivotal Phase 3 Trial Of Tovorafenib In Frontline pLGG
Trial Design Endpoints • Randomized, global, registrational Phase 3 trial of monotherapy tovorafenib • Primary endpoint: ORR based on RAPNO-LGG criteria, assessed by vs SoC chemotherapy blinded independent central review ‒ The ORR
primary analysis is expected to occur ~12 months after the • Eligibility: Patients aged 6 months to <25 years with LGG harboring a RAF last patient randomized alteration and requiring first-line systemic therapy • Key secondary
endpoints: PFS and DoR by RAPNO-LGG criteria • Tovorafenib available as tablets and pediatric-friendly liquid suspension • Other secondary endpoints: changes in neurological and visual function, • Patients who progress after
stopping tovorafenib may be re-challenged safety, and tolerability • Patients who progress in the SoC arm during or post-treatment may cross- • Key exploratory objectives: QoL and health utilization measures over to receive tovorafenib 2
Tovorafenib, 380mg/m QW Non-resectable or (not to exceed 600 mg) Stratified by sub-total resected LGG Long-term AND • Location of tumor follow-up Requiring first-line • Genomic alteration systemic therapy Investigator's choice of •
CDKN2A status (48 months) vincristine/carboplatin* or N ≈ 400 • Infant CHG diagnosis vinblastine or monthly carboplatin * COG or SIOPe-LGG regimen. Abbreviations: CHG, chiasmatic, hypothalamic glioma; DoR, duration of response; LGG,
low-grade glioma; ORR, objective response rate; QoL, quality of life; QW, once weekly; SoC, standard of care. 20 1:1 Randomization

FIRELIGHT-1 Phase 1b/2 Trials Evaluating Tovorafenib as a Combination
with Pimasertib 21

Pimasertib: Investigational Allosteric MEK1/2 Inhibitor With
Demonstrated Activity In MAPK-Driven Solid Tumors • Pimasertib is an investigational orally-bioavailable, selective, non- competitive MEK1/2 inhibitor in-licensed from Merck KGaA in February 2021 • Extensive non-clinical and clinical
development work through Phase 2, including a solid tumor trial in Japan and combinations with other MOAs • Main AEs typical for all in-class allosteric MEK inhibitors (GI, CPK elevation, skin rash, visual disturbances) • Nearly
three-fold higher CNS penetration than other MEKi inhibitors (trametinib or selumetinib) • Pimasertib showed monotherapy clinical activity, including an improvement in median PFS versus dacarbazine in NRAS mutant melanoma • Combination
with tovorafenib and other targeted therapies may unlock the full value of pimasertib in advanced solid tumors 22 Sources: Pimasertib Investigator Brochure, v12, 2019; de Gooijer et al., Int J Cancer, 2018; Shaw et al., AACR LB-456, 2012; Lebbe et
al., Cancers, 2020.
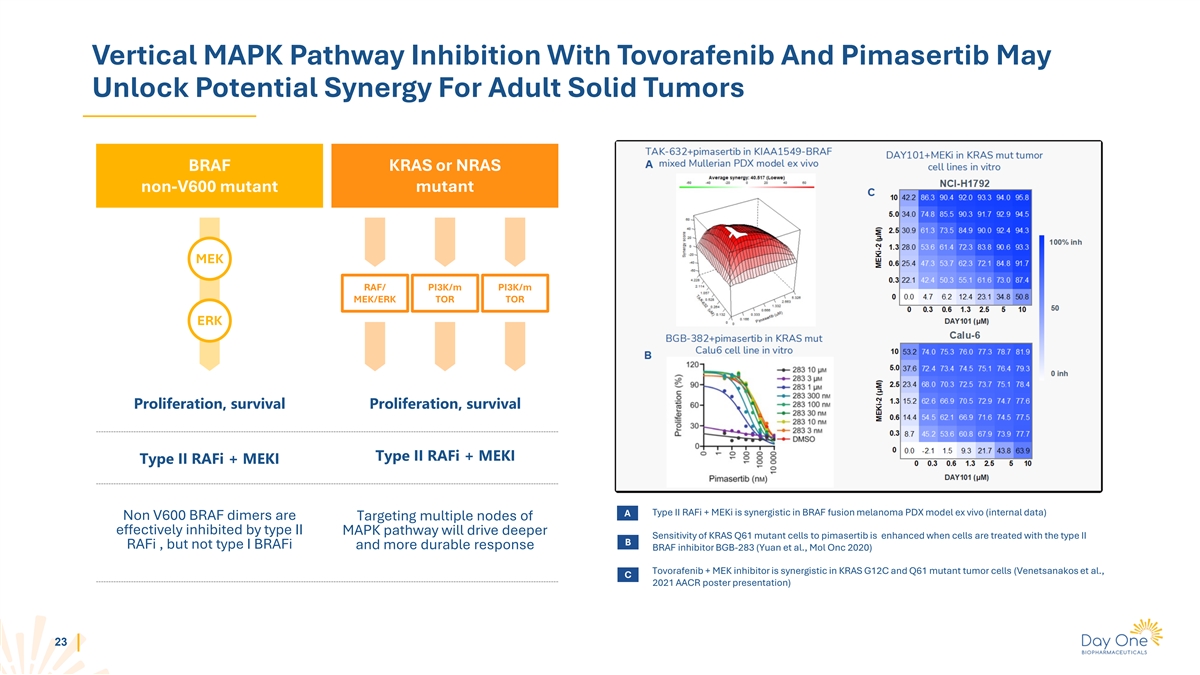
Vertical MAPK Pathway Inhibition With Tovorafenib And Pimasertib May
Unlock Potential Synergy For Adult Solid Tumors BRAF KRAS or NRAS non-V600 mutant mutant MEK RAF/ PI3K/m PI3K/m MEK/ERK TOR TOR ERK Proliferation, survival Proliferation, survival Type II RAFi + MEKI Type II RAFi + MEKI A Type II RAFi + MEKi is
synergistic in BRAF fusion melanoma PDX model ex vivo (internal data) Non V600 BRAF dimers are Targeting multiple nodes of effectively inhibited by type II MAPK pathway will drive deeper Sensitivity of KRAS Q61 mutant cells to pimasertib is enhanced
when cells are treated with the type II B RAFi , but not type I BRAFi and more durable response BRAF inhibitor BGB-283 (Yuan et al., Mol Onc 2020) Tovorafenib + MEK inhibitor is synergistic in KRAS G12C and Q61 mutant tumor cells (Venetsanakos et
al., C 2021 AACR poster presentation) 23
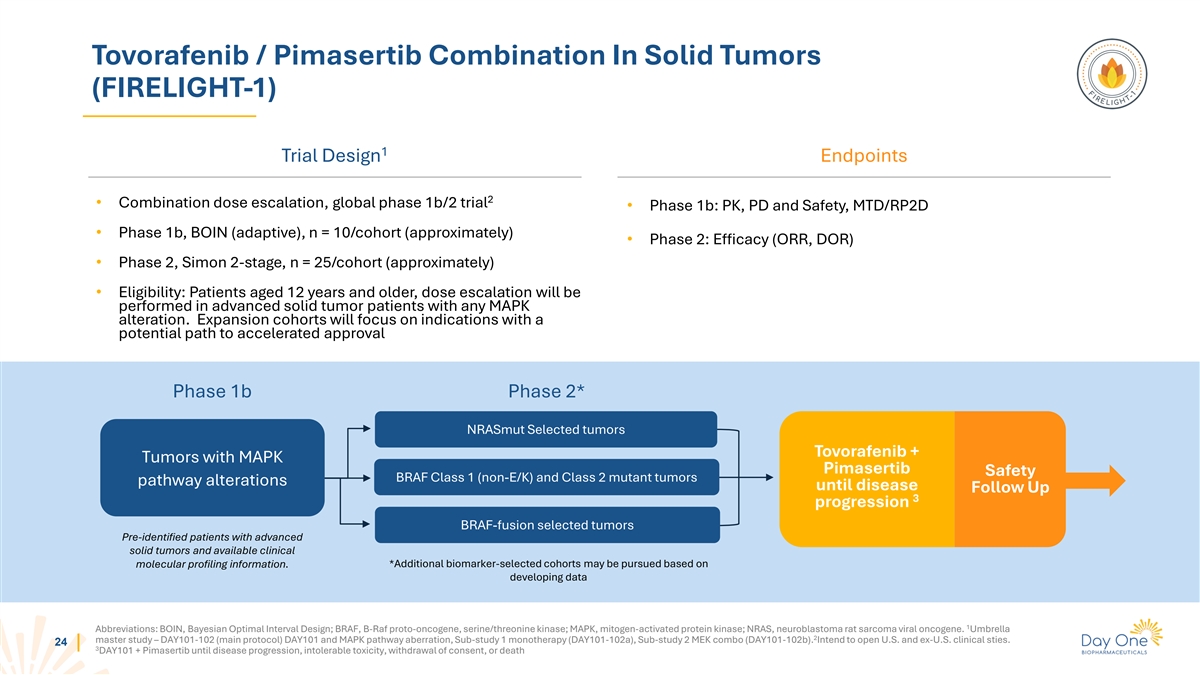
Tovorafenib / Pimasertib Combination In Solid Tumors (FIRELIGHT-1) 1
Trial Design Endpoints 2 • Combination dose escalation, global phase 1b/2 trial • Phase 1b: PK, PD and Safety, MTD/RP2D • Phase 1b, BOIN (adaptive), n = 10/cohort (approximately) • Phase 2: Efficacy (ORR, DOR) • Phase
2, Simon 2-stage, n = 25/cohort (approximately) • Eligibility: Patients aged 12 years and older, dose escalation will be performed in advanced solid tumor patients with any MAPK alteration. Expansion cohorts will focus on indications with a
potential path to accelerated approval Phase 1b Phase 2* NRASmut Selected tumors Tovorafenib + Tumors with MAPK Pimasertib Safety BRAF Class 1 (non-E/K) and Class 2 mutant tumors pathway alterations until disease Follow Up 3 progression BRAF-fusion
selected tumors Pre-identified patients with advanced solid tumors and available clinical molecular profiling information. *Additional biomarker-selected cohorts may be pursued based on developing data 1 Abbreviations: BOIN, Bayesian Optimal
Interval Design; BRAF, B-Raf proto-oncogene, serine/threonine kinase; MAPK, mitogen-activated protein kinase; NRAS, neuroblastoma rat sarcoma viral oncogene. Umbrella 2 master study – DAY101-102 (main protocol) DAY101 and MAPK pathway
aberration, Sub-study 1 monotherapy (DAY101-102a), Sub-study 2 MEK combo (DAY101-102b). Intend to open U.S. and ex-U.S. clinical sties. 24 3 DAY101 + Pimasertib until disease progression, intolerable toxicity, withdrawal of consent, or
death

DAY301 PTK7 Targeted Antibody Drug Conjugate (ADC) 25
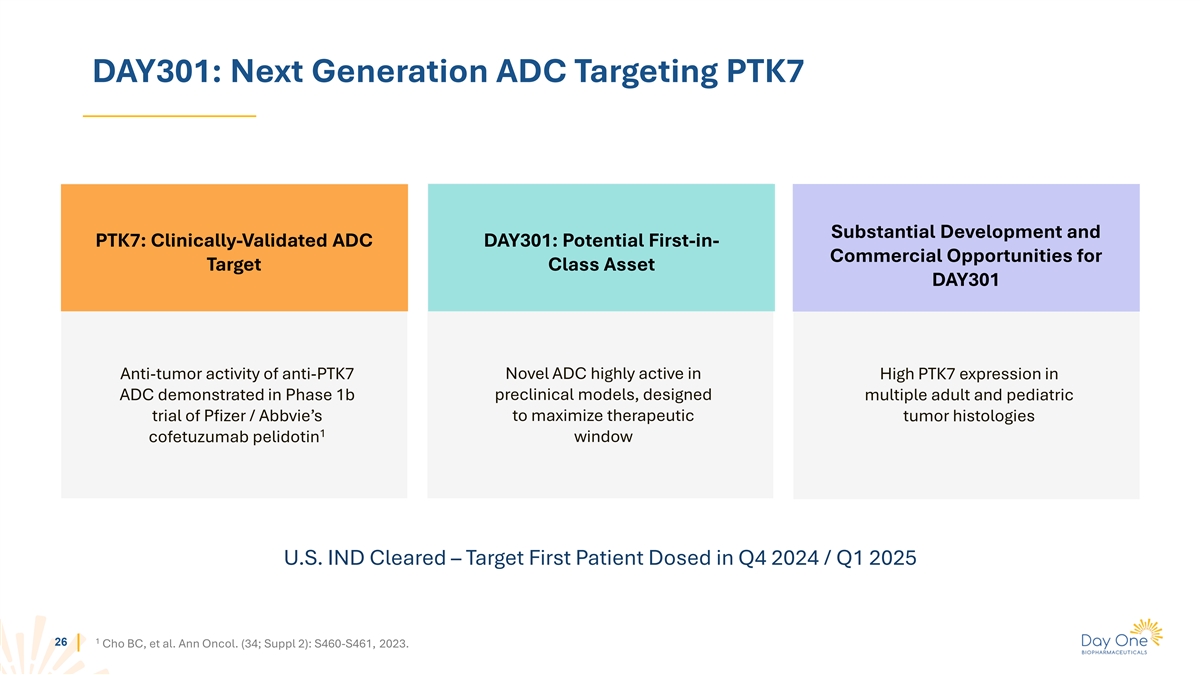
DAY301: Next Generation ADC Targeting PTK7 Substantial Development and
PTK7: Clinically-Validated ADC DAY301: Potential First-in- Commercial Opportunities for Target Class Asset DAY301 Novel ADC highly active in Anti-tumor activity of anti-PTK7 High PTK7 expression in ADC demonstrated in Phase 1b preclinical models,
designed multiple adult and pediatric trial of Pfizer / Abbvie’s to maximize therapeutic tumor histologies 1 cofetuzumab pelidotin window U.S. IND Cleared – Target First Patient Dosed in Q4 2024 / Q1 2025 1 26 Cho BC, et al. Ann Oncol.
(34; Suppl 2): S460-S461, 2023.
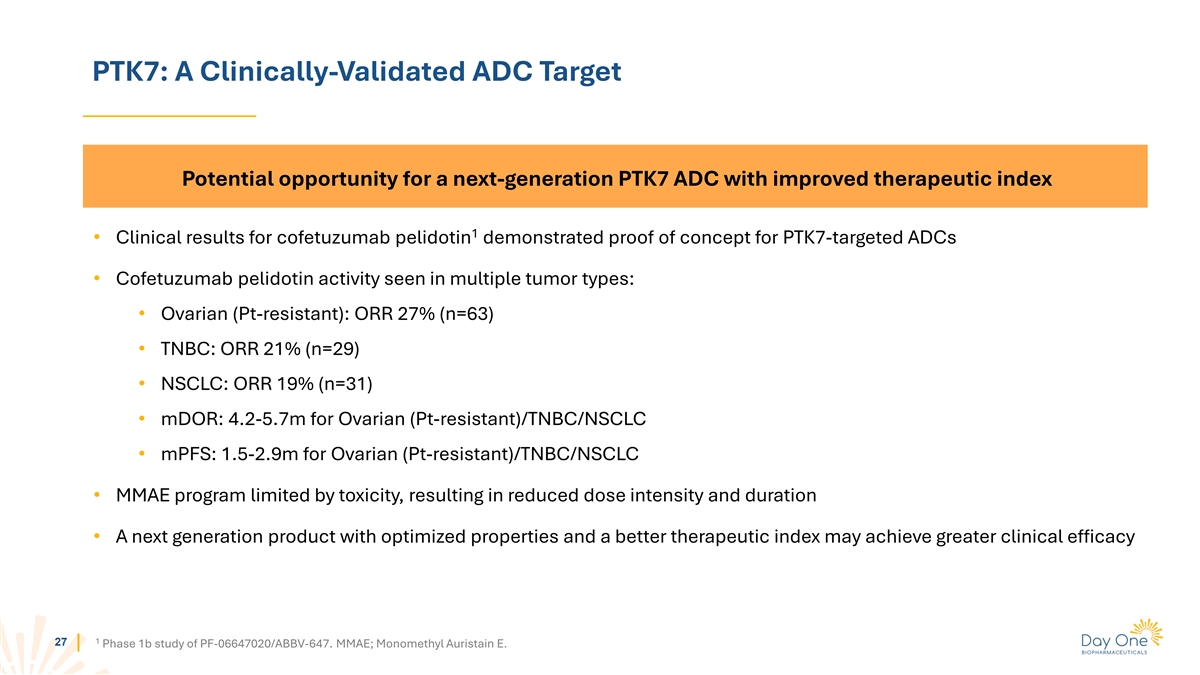
PTK7: A Clinically-Validated ADC Target Potential opportunity for a
next-generation PTK7 ADC with improved therapeutic index 1 • Clinical results for cofetuzumab pelidotin demonstrated proof of concept for PTK7-targeted ADCs • Cofetuzumab pelidotin activity seen in multiple tumor types: • Ovarian
(Pt-resistant): ORR 27% (n=63) • TNBC: ORR 21% (n=29) • NSCLC: ORR 19% (n=31) • mDOR: 4.2-5.7m for Ovarian (Pt-resistant)/TNBC/NSCLC • mPFS: 1.5-2.9m for Ovarian (Pt-resistant)/TNBC/NSCLC • MMAE program limited by
toxicity, resulting in reduced dose intensity and duration • A next generation product with optimized properties and a better therapeutic index may achieve greater clinical efficacy 1 27 Phase 1b study of PF-06647020/ABBV-647. MMAE; Monomethyl
Auristain E.
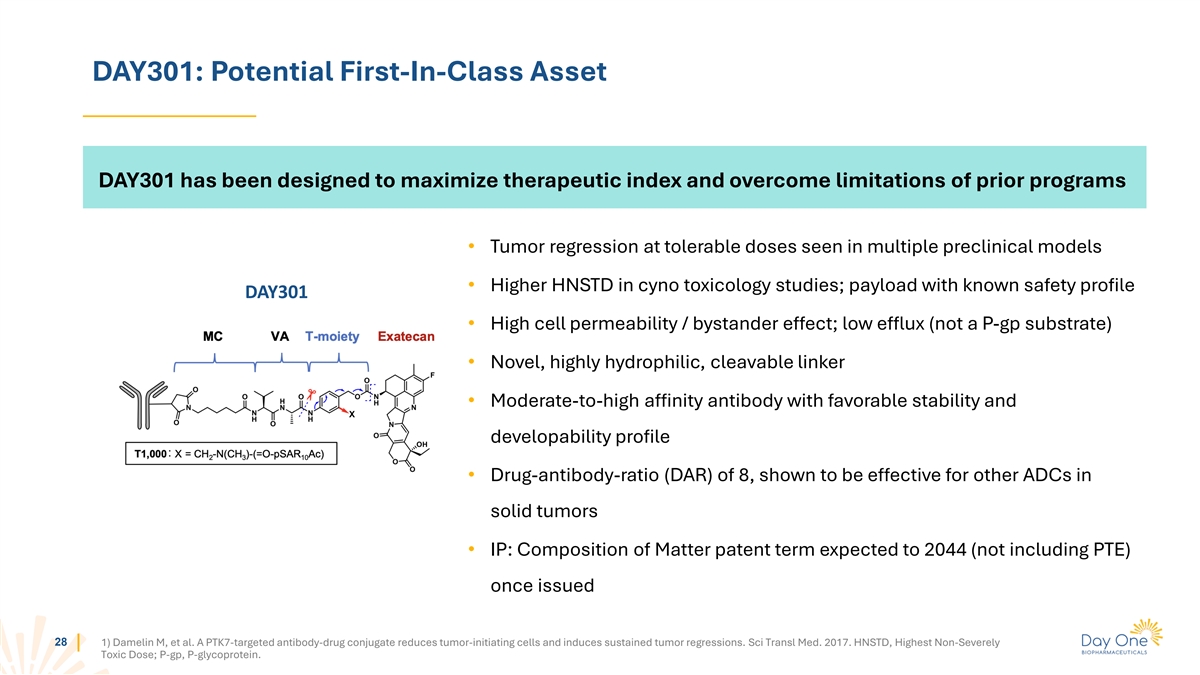
DAY301: Potential First-In-Class Asset DAY301 has been designed to
maximize therapeutic index and overcome limitations of prior programs • Tumor regression at tolerable doses seen in multiple preclinical models • Higher HNSTD in cyno toxicology studies; payload with known safety profile DAY301 •
High cell permeability / bystander effect; low efflux (not a P-gp substrate) • Novel, highly hydrophilic, cleavable linker • Moderate-to-high affinity antibody with favorable stability and developability profile •
Drug-antibody-ratio (DAR) of 8, shown to be effective for other ADCs in solid tumors • IP: Composition of Matter patent term expected to 2044 (not including PTE) once issued 28 1) Damelin M, et al. A PTK7-targeted antibody-drug conjugate
reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med. 2017. HNSTD, Highest Non-Severely Toxic Dose; P-gp, P-glycoprotein.
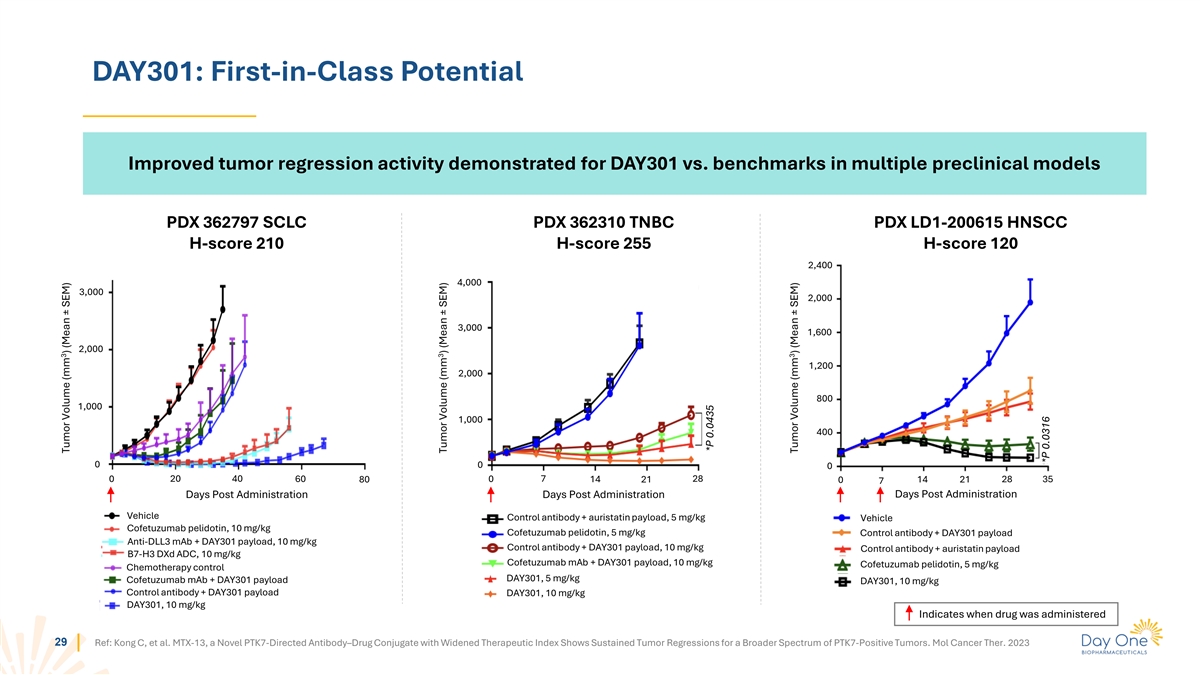
DAY301: First-in-Class Potential Improved tumor regression activity
demonstrated for DAY301 vs. benchmarks in multiple preclinical models PDX 362797 SCLC PDX 362310 TNBC PDX LD1-200615 HNSCC H-score 210 H-score 255 H-score 120 2,400 4,000 3,000 2,000 3,000 1,600 2,000 1,200 2,000 800 1,000 1,000 400 0 0 0 0 20 40 60
80 0 7 14 21 28 14 21 28 35 0 7 Days Post Administration Days Post Administration Days Post Administration Vehicle Control antibody + auristatin payload, 5 mg/kg Vehicle Cofetuzumab pelidotin, 10 mg/kg Cofetuzumab pelidotin, 5 mg/kg Control antibody
+ DAY301 payload Anti-DLL3 mAb + DAY301 payload, 10 mg/kg Control antibody + DAY301 payload, 10 mg/kg Control antibody + auristatin payload B7-H3 DXd ADC, 10 mg/kg Cofetuzumab mAb + DAY301 payload, 10 mg/kg Cofetuzumab pelidotin, 5 mg/kg
Chemotherapy control DAY301, 5 mg/kg Cofetuzumab mAb + DAY301 payload DAY301, 10 mg/kg Control antibody + DAY301 payload DAY301, 10 mg/kg DAY301, 10 mg/kg Indicates when drug was administered 29 Ref: Kong C, et al. MTX-13, a Novel PTK7-Directed
Antibody–Drug Conjugate with Widened Therapeutic Index Shows Sustained Tumor Regressions for a Broader Spectrum of PTK7-Positive Tumors. Mol Cancer Ther. 2023 3 Tumor Volume (mm ) (Mean ± SEM) 3 Tumor Volume (mm ) (Mean ± SEM) *P
0.0435 3 Tumor Volume (mm ) (Mean ± SEM) *P 0.0316
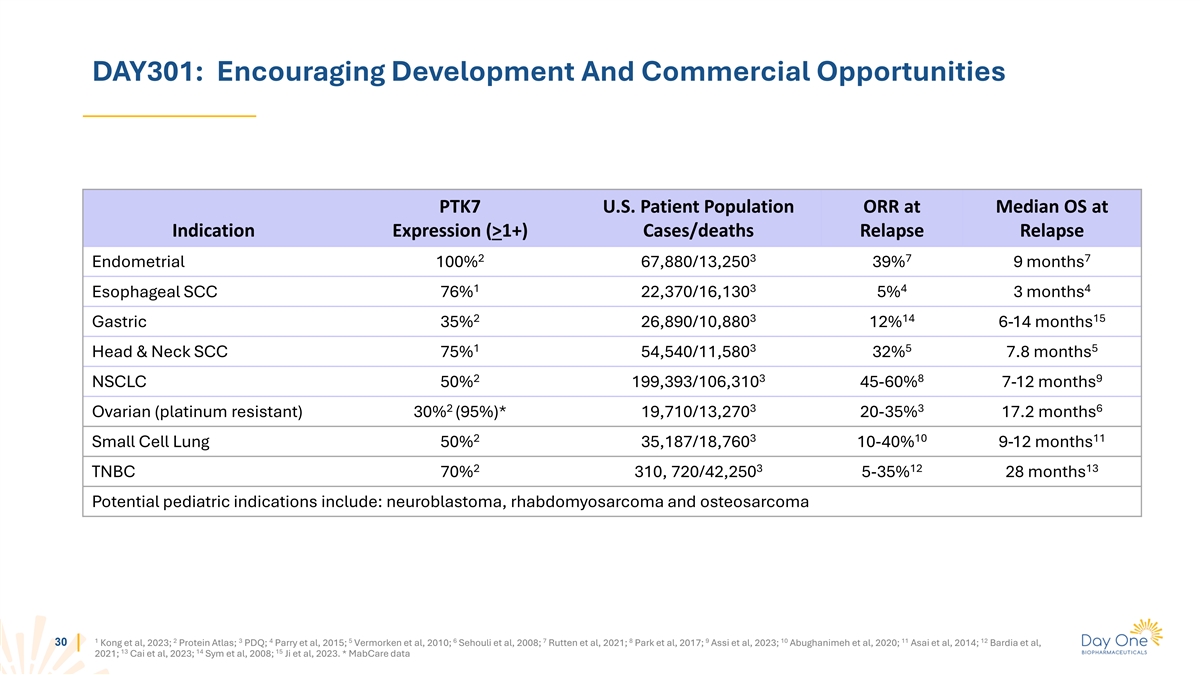
DAY301: Encouraging Development And Commercial Opportunities PTK7 U.S.
Patient Population ORR at Median OS at Indication Expression (>1+) Cases/deaths Relapse Relapse 2 3 7 7 Endometrial 100% 67,880/13,250 39% 9 months 1 3 4 4 Esophageal SCC 76% 22,370/16,130 5% 3 months 2 3 14 15 Gastric 35% 26,890/10,880 12% 6-14
months 1 3 5 5 Head & Neck SCC 75% 54,540/11,580 32% 7.8 months 2 3 8 9 NSCLC 50% 199,393/106,310 45-60% 7-12 months 2 3 3 6 Ovarian (platinum resistant) 30% (95%)* 19,710/13,270 20-35% 17.2 months 2 3 10 11 Small Cell Lung 50% 35,187/18,760
10-40% 9-12 months 2 3 12 13 TNBC 70% 310, 720/42,250 5-35% 28 months Potential pediatric indications include: neuroblastoma, rhabdomyosarcoma and osteosarcoma 1 2 3 4 5 6 7 8 9 10 11 12 30 Kong et al, 2023; Protein Atlas; PDQ; Parry et al, 2015;
Vermorken et al, 2010; Sehouli et al, 2008; Rutten et al, 2021; Park et al, 2017; Assi et al, 2023; Abughanimeh et al, 2020; Asai et al, 2014; Bardia et al, 13 14 15 2021; Cai et al, 2023; Sym et al, 2008; Ji et al, 2023. * MabCare data
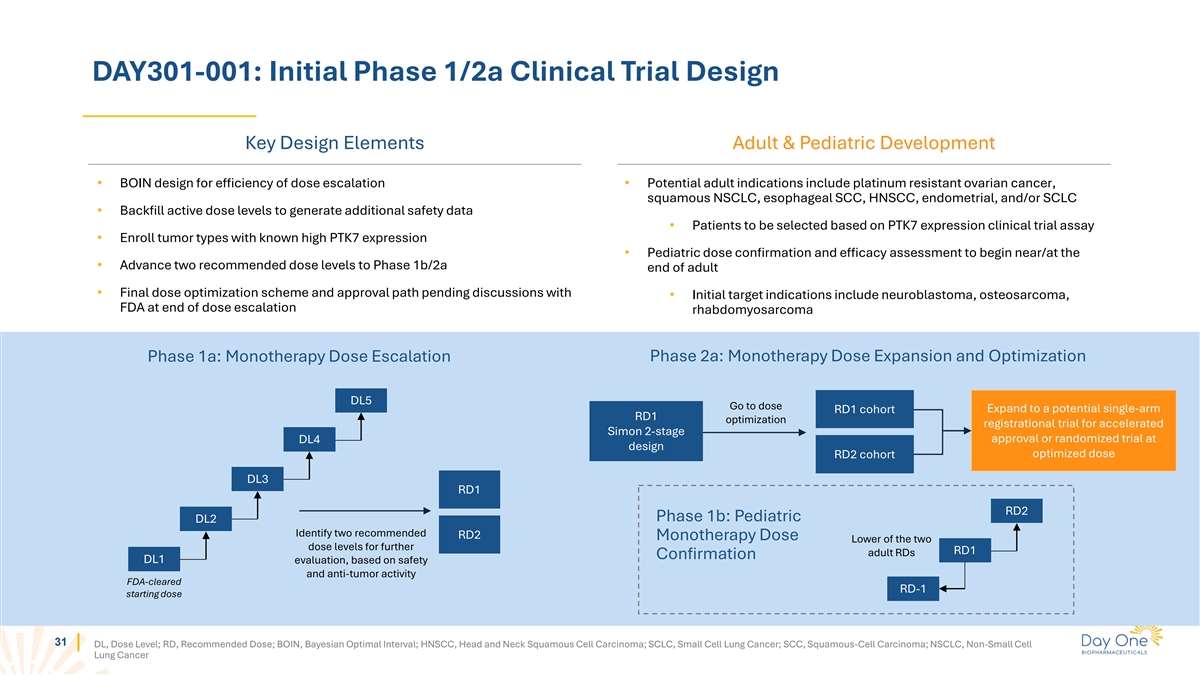
DAY301-001: Initial Phase 1/2a Clinical Trial Design Key Design
Elements Adult & Pediatric Development • BOIN design for efficiency of dose escalation • Potential adult indications include platinum resistant ovarian cancer, squamous NSCLC, esophageal SCC, HNSCC, endometrial, and/or SCLC •
Backfill active dose levels to generate additional safety data • Patients to be selected based on PTK7 expression clinical trial assay • Enroll tumor types with known high PTK7 expression • Pediatric dose confirmation and efficacy
assessment to begin near/at the • Advance two recommended dose levels to Phase 1b/2a end of adult • Final dose optimization scheme and approval path pending discussions with • Initial target indications include neuroblastoma,
osteosarcoma, FDA at end of dose escalation rhabdomyosarcoma Phase 2a: Monotherapy Dose Expansion and Optimization Phase 1a: Monotherapy Dose Escalation DL5 Go to dose Expand to a potential single-arm RD1 cohort RD1 optimization registrational trial
for accelerated Simon 2-stage approval or randomized trial at DL4 design optimized dose RD2 cohort DL3 RD1 RD2 Phase 1b: Pediatric DL2 Identify two recommended RD2 Monotherapy Dose Lower of the two dose levels for further RD1 adult RDs Confirmation
DL1 evaluation, based on safety and anti-tumor activity FDA-cleared RD-1 starting dose 31 DL, Dose Level; RD, Recommended Dose; BOIN, Bayesian Optimal Interval; HNSCC, Head and Neck Squamous Cell Carcinoma; SCLC, Small Cell Lung Cancer; SCC,
Squamous-Cell Carcinoma; NSCLC, Non-Small Cell Lung Cancer

Summary 32
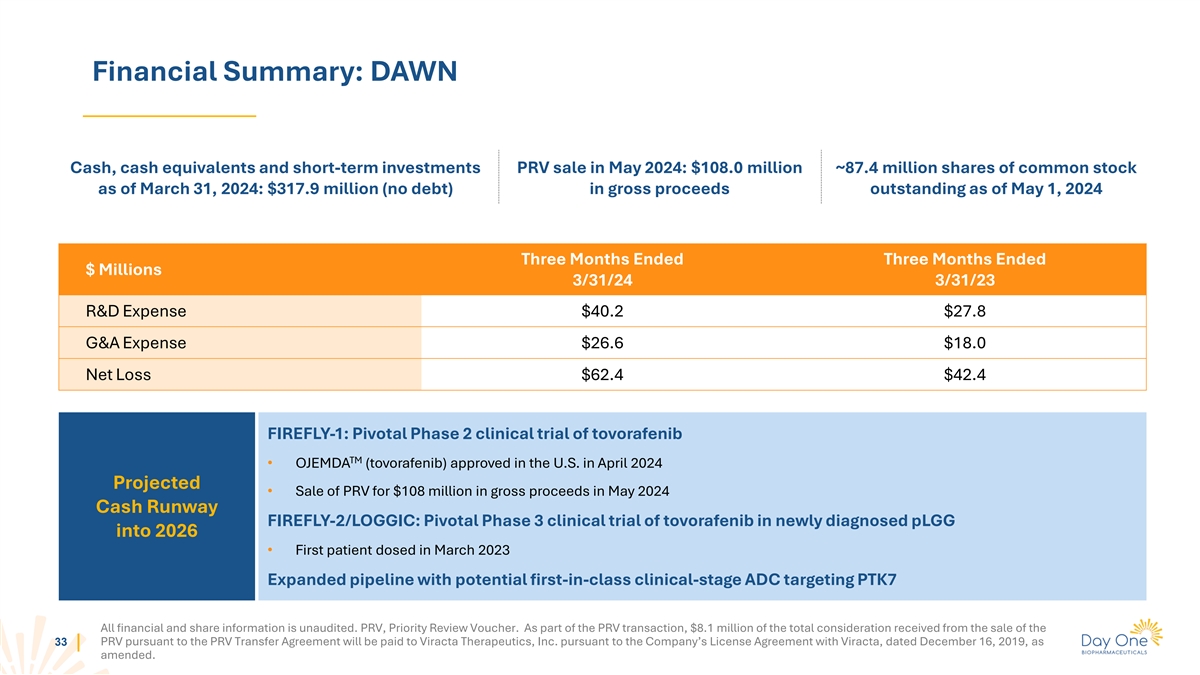
Financial Summary: DAWN Cash, cash equivalents and short-term
investments PRV sale in May 2024: $108.0 million ~87.4 million shares of common stock as of March 31, 2024: $317.9 million (no debt) in gross proceeds outstanding as of May 1, 2024 Three Months Ended Three Months Ended $ Millions 3/31/24 3/31/23
R&D Expense $40.2 $27.8 G&A Expense $26.6 $18.0 Net Loss $62.4 $42.4 FIREFLY-1: Pivotal Phase 2 clinical trial of tovorafenib TM • OJEMDA (tovorafenib) approved in the U.S. in April 2024 Projected • Sale of PRV for $108 million
in gross proceeds in May 2024 Cash Runway FIREFLY-2/LOGGIC: Pivotal Phase 3 clinical trial of tovorafenib in newly diagnosed pLGG into 2026 • First patient dosed in March 2023 Expanded pipeline with potential first-in-class clinical-stage ADC
targeting PTK7 All financial and share information is unaudited. PRV, Priority Review Voucher. As part of the PRV transaction, $8.1 million of the total consideration received from the sale of the PRV pursuant to the PRV Transfer Agreement will be
paid to Viracta Therapeutics, Inc. pursuant to the Company’s License Agreement with Viracta, dated December 16, 2019, as 33 amended.
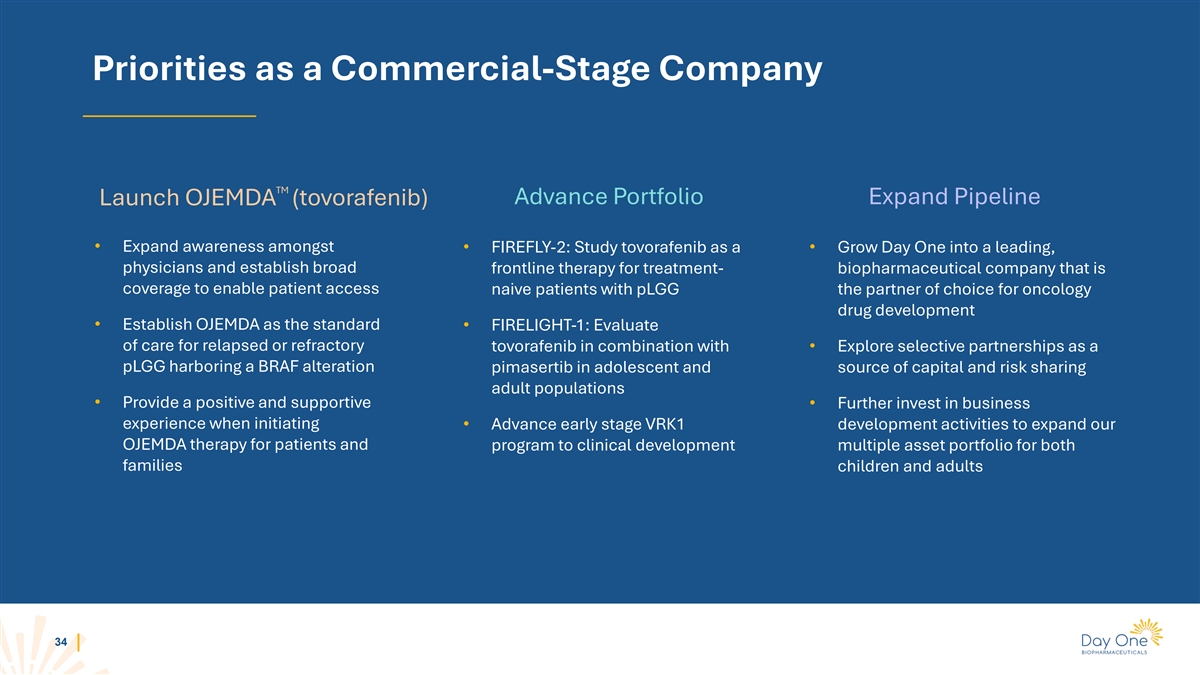
Priorities as a Commercial-Stage Company TM Launch OJEMDA (tovorafenib)
Advance Portfolio Expand Pipeline • Expand awareness amongst • FIREFLY-2: Study tovorafenib as a • Grow Day One into a leading, physicians and establish broad frontline therapy for treatment- biopharmaceutical company that is
coverage to enable patient access naive patients with pLGG the partner of choice for oncology drug development • Establish OJEMDA as the standard • FIRELIGHT-1: Evaluate of care for relapsed or refractory tovorafenib in combination with
• Explore selective partnerships as a pLGG harboring a BRAF alteration pimasertib in adolescent and source of capital and risk sharing adult populations • Provide a positive and supportive • Further invest in business experience
when initiating • Advance early stage VRK1 development activities to expand our OJEMDA therapy for patients and program to clinical development multiple asset portfolio for both families children and adults 34

Appendix 35
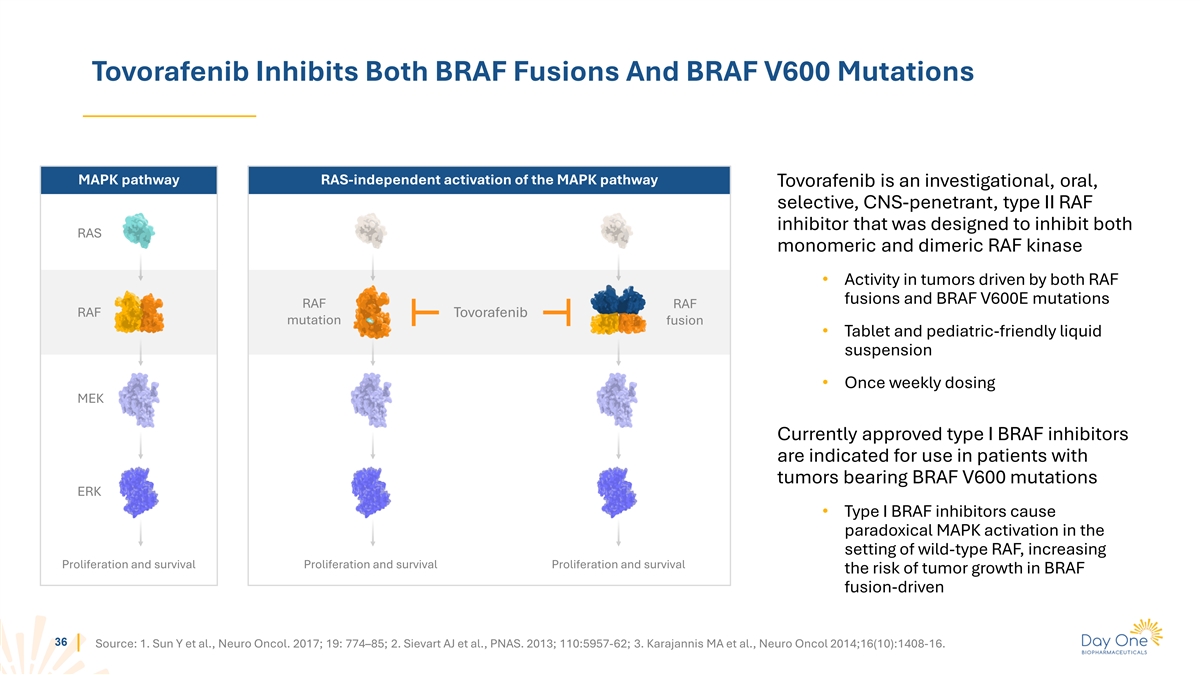
Tovorafenib Inhibits Both BRAF Fusions And BRAF V600 Mutations MAPK
pathway RAS-independent activation of the MAPK pathway Tovorafenib is an investigational, oral, selective, CNS-penetrant, type II RAF inhibitor that was designed to inhibit both RAS monomeric and dimeric RAF kinase • Activity in tumors driven
by both RAF fusions and BRAF V600E mutations RAF RAF RAF Tovorafenib mutation fusion • Tablet and pediatric-friendly liquid suspension • Once weekly dosing MEK Currently approved type I BRAF inhibitors are indicated for use in patients
with tumors bearing BRAF V600 mutations ERK • Type I BRAF inhibitors cause paradoxical MAPK activation in the setting of wild-type RAF, increasing Proliferation and survival Proliferation and survival Proliferation and survival the risk of
tumor growth in BRAF fusion-driven 36 Source: 1. Sun Y et al., Neuro Oncol. 2017; 19: 774–85; 2. Sievart AJ et al., PNAS. 2013; 110:5957-62; 3. Karajannis MA et al., Neuro Oncol 2014;16(10):1408-16.
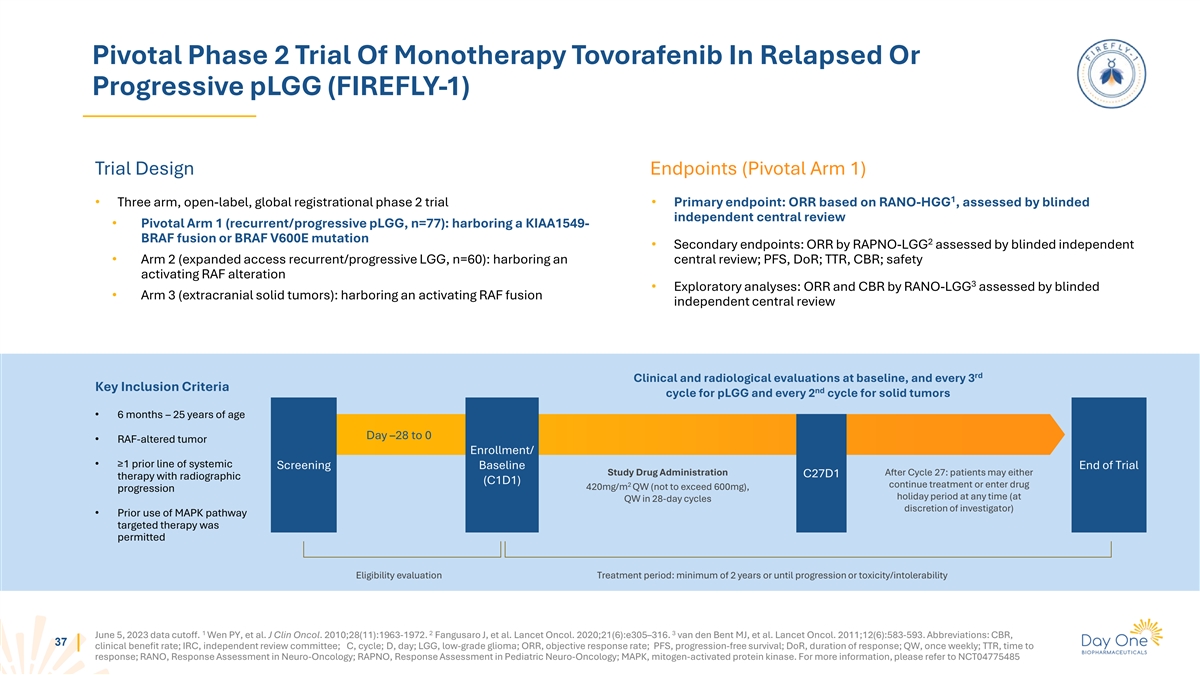
Pivotal Phase 2 Trial Of Monotherapy Tovorafenib In Relapsed Or
Progressive pLGG (FIREFLY-1) Trial Design Endpoints (Pivotal Arm 1) 1 • Three arm, open-label, global registrational phase 2 trial • Primary endpoint: ORR based on RANO-HGG , assessed by blinded independent central review • Pivotal
Arm 1 (recurrent/progressive pLGG, n=77): harboring a KIAA1549- BRAF fusion or BRAF V600E mutation 2 • Secondary endpoints: ORR by RAPNO-LGG assessed by blinded independent • Arm 2 (expanded access recurrent/progressive LGG, n=60):
harboring an central review; PFS, DoR; TTR, CBR; safety activating RAF alteration 3 • Exploratory analyses: ORR and CBR by RANO-LGG assessed by blinded • Arm 3 (extracranial solid tumors): harboring an activating RAF fusion independent
central review rd Clinical and radiological evaluations at baseline, and every 3 Key Inclusion Criteria nd cycle for pLGG and every 2 cycle for solid tumors • 6 months – 25 years of age Day –28 to 0 • RAF-altered tumor
Enrollment/ • ≥1 prior line of systemic Screening Baseline End of Trial Study Drug Administration After Cycle 27: patients may either C27D1 therapy with radiographic (C1D1) 2 continue treatment or enter drug 420mg/m QW (not to exceed
600mg), progression holiday period at any time (at QW in 28-day cycles discretion of investigator) • Prior use of MAPK pathway targeted therapy was permitted Eligibility evaluation Treatment period: minimum of 2 years or until progression or
toxicity/intolerability 1 2 3 June 5, 2023 data cutoff. Wen PY, et al. J Clin Oncol. 2010;28(11):1963-1972. Fangusaro J, et al. Lancet Oncol. 2020;21(6):e305–316. van den Bent MJ, et al. Lancet Oncol. 2011;12(6):583-593. Abbreviations: CBR, 37
clinical benefit rate; IRC, independent review committee; C, cycle; D, day; LGG, low-grade glioma; ORR, objective response rate; PFS, progression-free survival; DoR, duration of response; QW, once weekly; TTR, time to response; RANO, Response
Assessment in Neuro-Oncology; RAPNO, Response Assessment in Pediatric Neuro-Oncology; MAPK, mitogen-activated protein kinase. For more information, please refer to NCT04775485

Data from Pivotal Phase 2 FIREFLY-1 Trial June 5, 2023 data cutoff
38
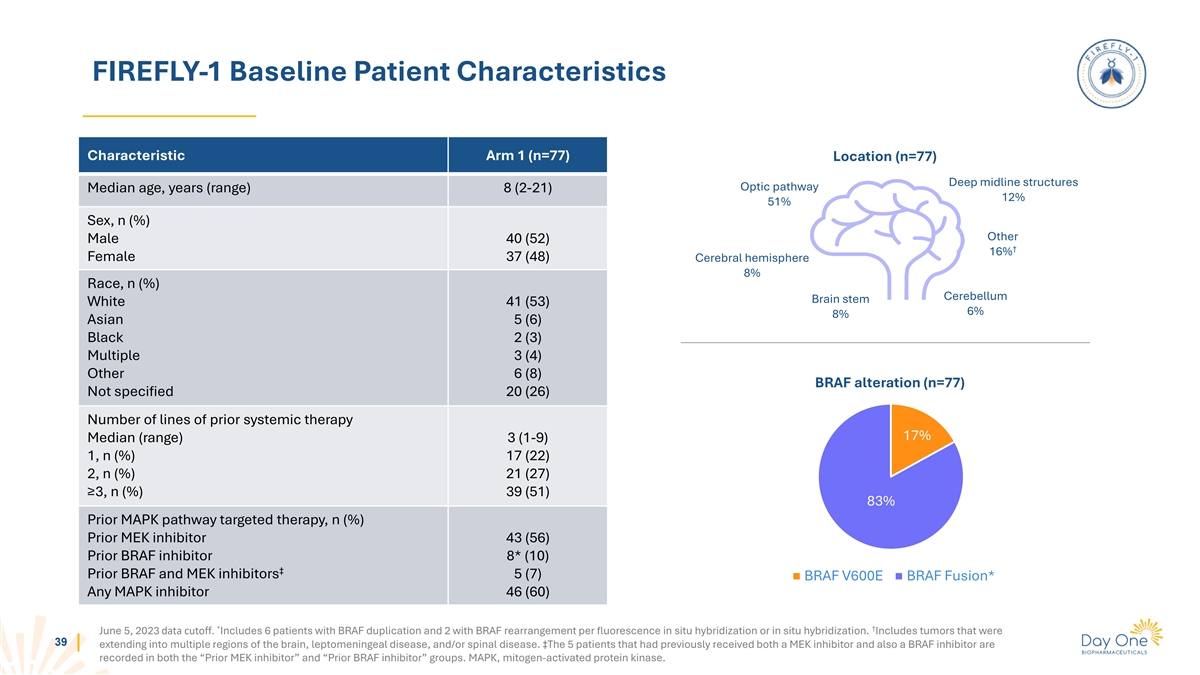
FIREFLY-1 Baseline Patient Characteristics Characteristic Arm 1 (n=77)
Location (n=77) Deep midline structures Optic pathway Median age, years (range) 8 (2-21) 12% 51% Sex, n (%) Other Male 40 (52) † 16% Female 37 (48) Cerebral hemisphere 8% Race, n (%) Cerebellum Brain stem White 41 (53) 6% 8% Asian 5 (6) Black
2 (3) Multiple 3 (4) Other 6 (8) BRAF alteration (n=77) Not specified 20 (26) Number of lines of prior systemic therapy 17% Median (range) 3 (1-9) 1, n (%) 17 (22) 2, n (%) 21 (27) ≥3, n (%) 39 (51) 83% Prior MAPK pathway targeted therapy, n
(%) Prior MEK inhibitor 43 (56) Prior BRAF inhibitor 8* (10) ‡ Prior BRAF and MEK inhibitors 5 (7) BRAF V600E BRAF Fusion* Any MAPK inhibitor 46 (60) * † June 5, 2023 data cutoff. Includes 6 patients with BRAF duplication and 2 with BRAF
rearrangement per fluorescence in situ hybridization or in situ hybridization. Includes tumors that were 39 extending into multiple regions of the brain, leptomeningeal disease, and/or spinal disease. ‡The 5 patients that had previously
received both a MEK inhibitor and also a BRAF inhibitor are recorded in both the “Prior MEK inhibitor” and “Prior BRAF inhibitor” groups. MAPK, mitogen-activated protein kinase.
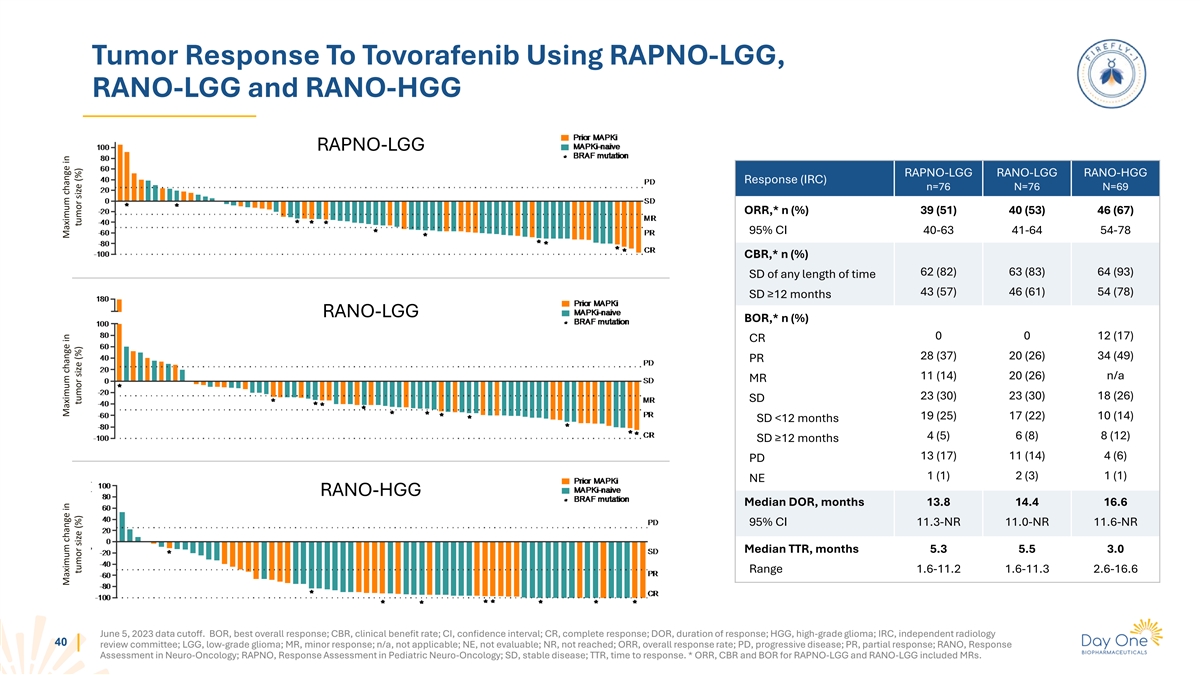
Tumor Response To Tovorafenib Using RAPNO-LGG, RANO-LGG and RANO-HGG
RAPNO-LGG RAPNO-LGG RANO-LGG RANO-HGG Response (IRC) n=76 N=76 N=69 ORR,* n (%) 39 (51) 40 (53) 46 (67) 95% CI 40-63 41-64 54-78 CBR,* n (%) 62 (82) 63 (83) 64 (93) SD of any length of time 43 (57) 46 (61) 54 (78) SD ≥12 months RANO-LGG BOR,*
n (%) 0 0 12 (17) CR 28 (37) 20 (26) 34 (49) PR 11 (14) 20 (26) n/a MR 23 (30) 23 (30) 18 (26) SD 19 (25) 17 (22) 10 (14) SD <12 months 4 (5) 6 (8) 8 (12) SD ≥12 months 13 (17) 11 (14) 4 (6) PD 1 (1) 2 (3) 1 (1) NE RANO-HGG Median DOR,
months 13.8 14.4 16.6 95% CI 11.3-NR 11.0-NR 11.6-NR Median TTR, months 5.3 5.5 3.0 Range 1.6-11.2 1.6-11.3 2.6-16.6 June 5, 2023 data cutoff. BOR, best overall response; CBR, clinical benefit rate; CI, confidence interval; CR, complete response;
DOR, duration of response; HGG, high-grade glioma; IRC, independent radiology 40 review committee; LGG, low-grade glioma; MR, minor response; n/a, not applicable; NE, not evaluable; NR, not reached; ORR, overall response rate; PD, progressive
disease; PR, partial response; RANO, Response Assessment in Neuro-Oncology; RAPNO, Response Assessment in Pediatric Neuro-Oncology; SD, stable disease; TTR, time to response. * ORR, CBR and BOR for RAPNO-LGG and RANO-LGG included MRs. Maximum change
in Maximum change in Maximum change in tumor size (%) tumor size (%) tumor size (%)
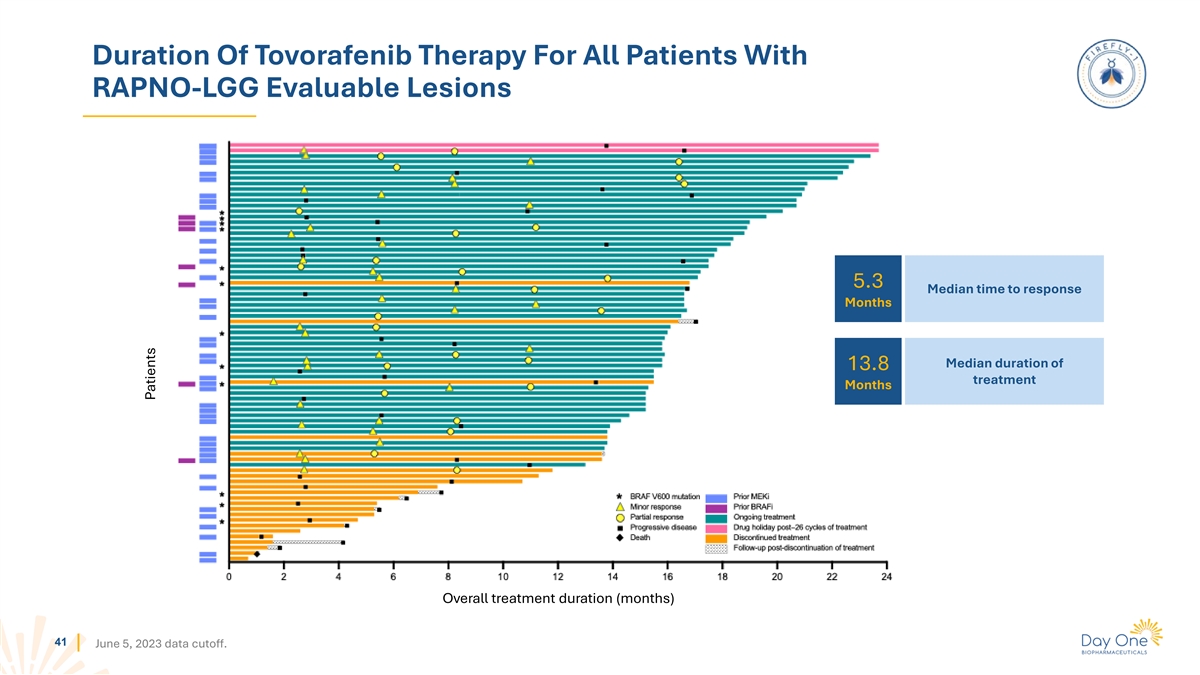
Duration Of Tovorafenib Therapy For All Patients With RAPNO-LGG
Evaluable Lesions 5.3 Median time to response Months Median duration of 13.8 treatment Months Overall treatment duration (months) 41 June 5, 2023 data cutoff. Patients
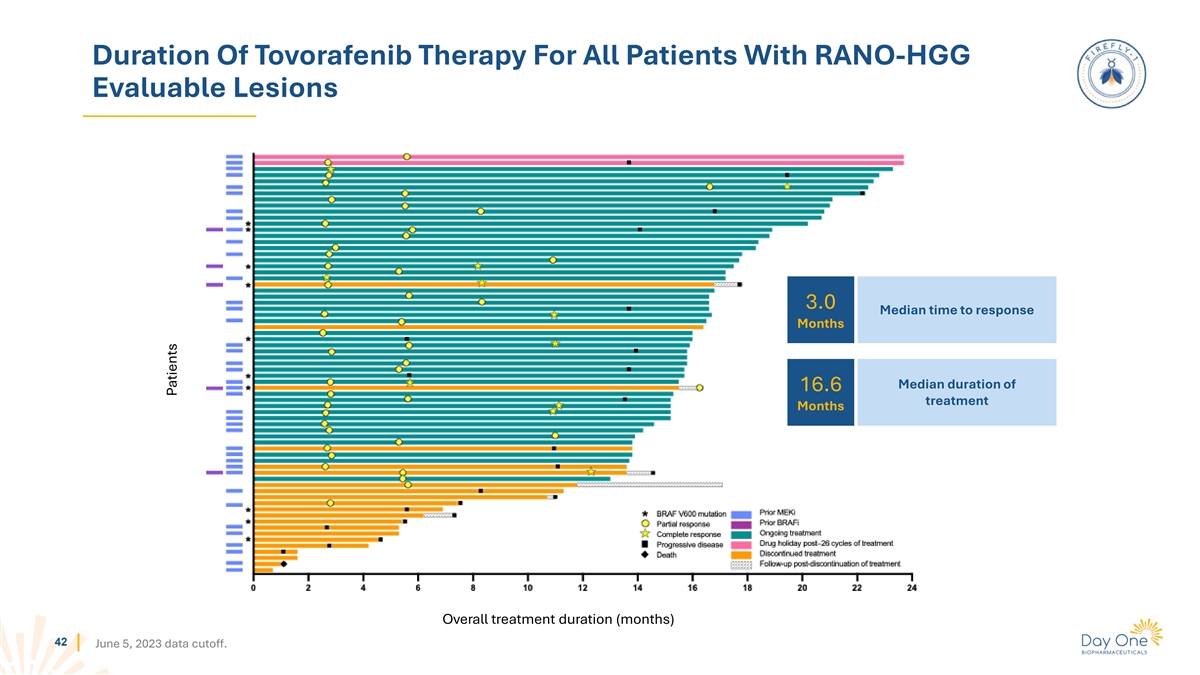
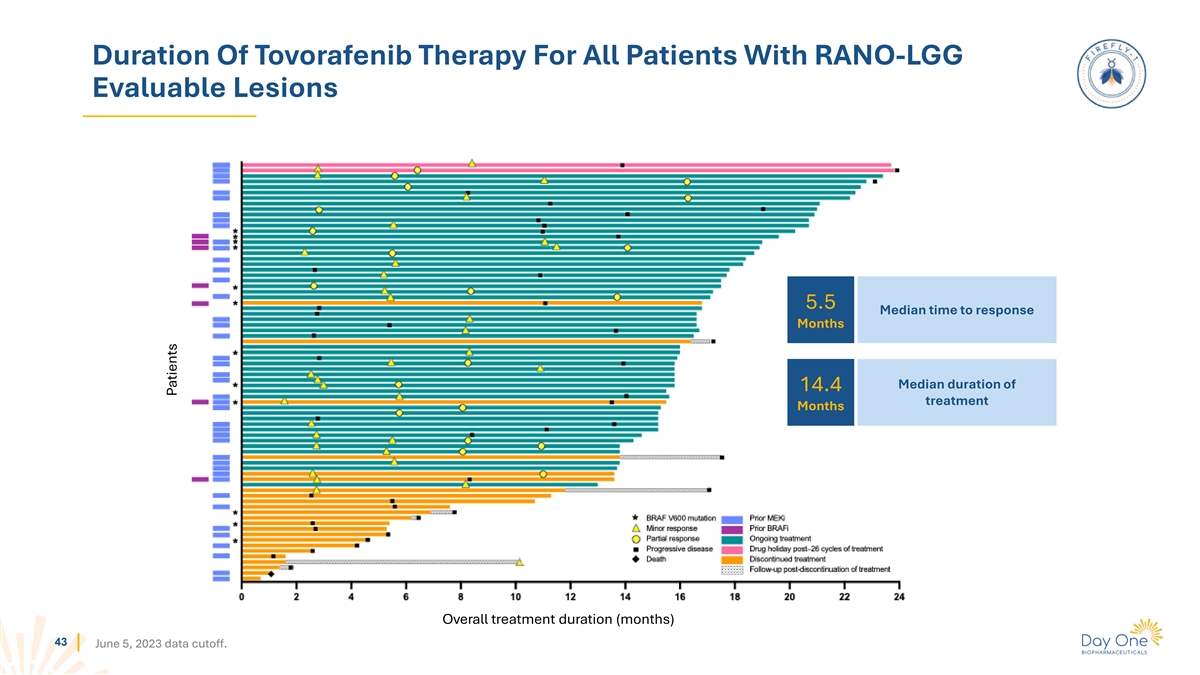
Duration Of Tovorafenib Therapy For All Patients With RANO-HGG
Evaluable Lesions 3.0 Median time to response Months Median duration of 16.6 treatment Months Overall treatment duration (months) 42 June 5, 2023 data cutoff. Patients
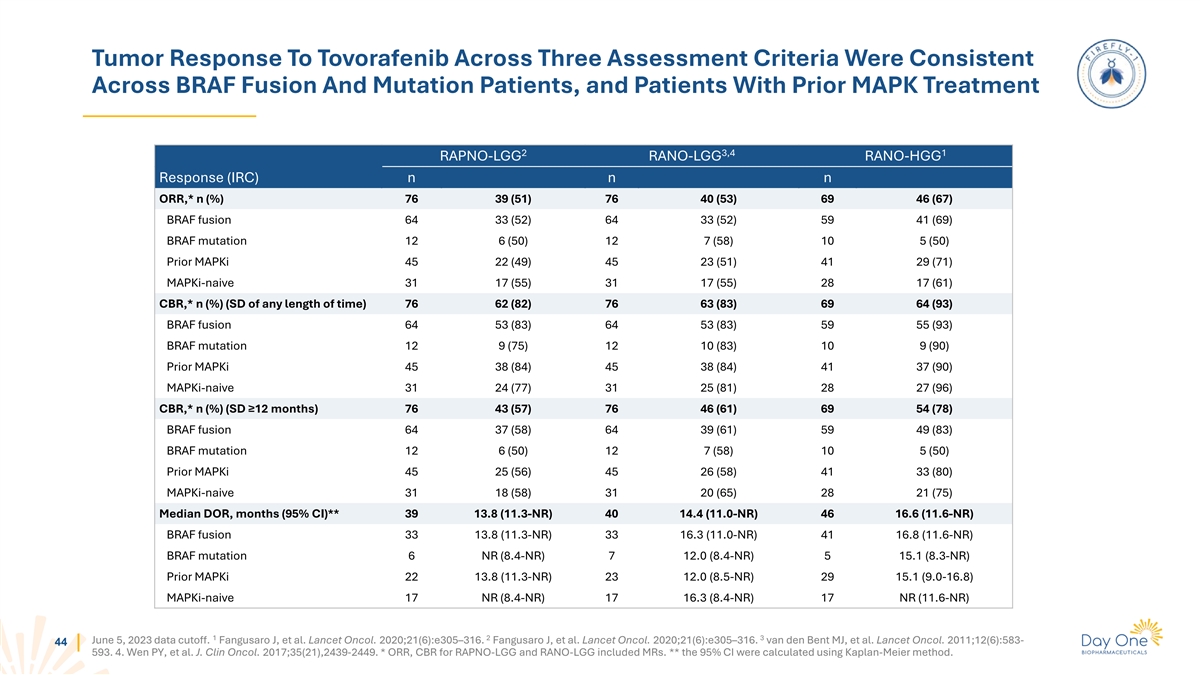
Duration Of Tovorafenib Therapy For All Patients With RANO-LGG
Evaluable Lesions 5.5 Median time to response Months Median duration of 14.4 treatment Months Overall treatment duration (months) 43 June 5, 2023 data cutoff. Patients
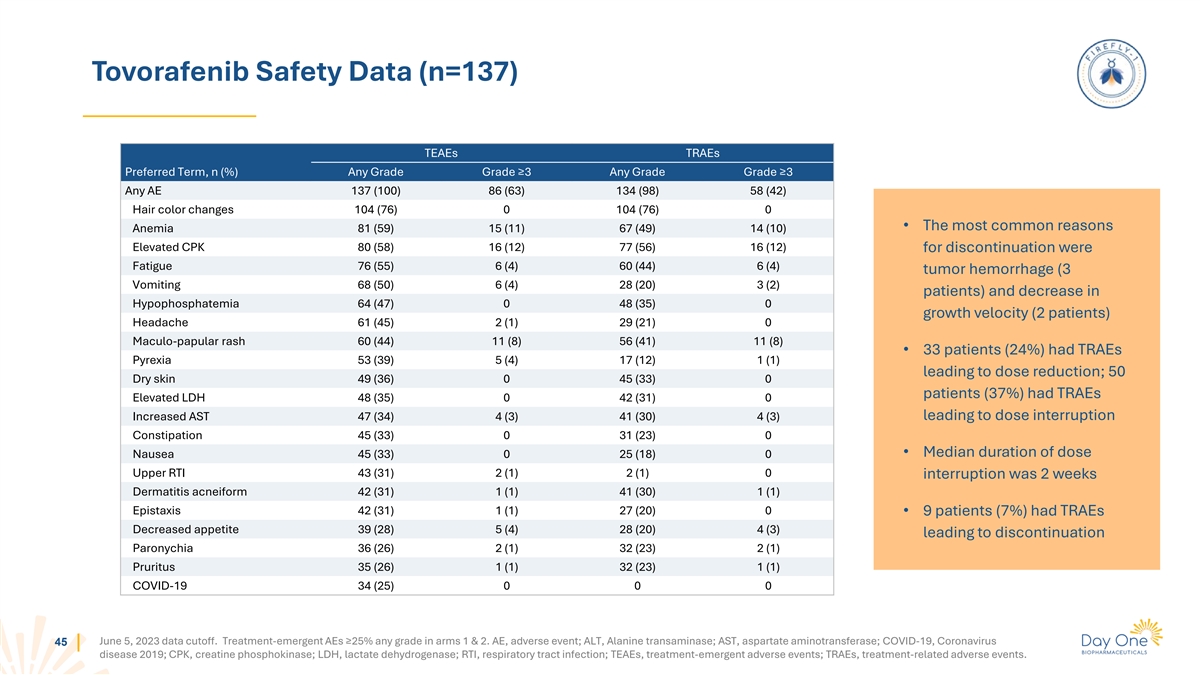
Tumor Response To Tovorafenib Across Three Assessment Criteria Were
Consistent Across BRAF Fusion And Mutation Patients, and Patients With Prior MAPK Treatment 2 3,4 1 RAPNO-LGG RANO-LGG RANO-HGG Response (IRC) n n n ORR,* n (%) 76 39 (51) 76 40 (53) 69 46 (67) BRAF fusion 64 33 (52) 64 33 (52) 59 41 (69) BRAF
mutation 12 6 (50) 12 7 (58) 10 5 (50) Prior MAPKi 45 22 (49) 45 23 (51) 41 29 (71) MAPKi-naive 31 17 (55) 31 17 (55) 28 17 (61) CBR,* n (%) (SD of any length of time) 76 62 (82) 76 63 (83) 69 64 (93) BRAF fusion 64 53 (83) 64 53 (83) 59 55 (93)
BRAF mutation 12 9 (75) 12 10 (83) 10 9 (90) Prior MAPKi 45 38 (84) 45 38 (84) 41 37 (90) MAPKi-naive 31 24 (77) 31 25 (81) 28 27 (96) CBR,* n (%) (SD ≥12 months) 76 43 (57) 76 46 (61) 69 54 (78) BRAF fusion 64 37 (58) 64 39 (61) 59 49 (83)
BRAF mutation 12 6 (50) 12 7 (58) 10 5 (50) Prior MAPKi 45 25 (56) 45 26 (58) 41 33 (80) MAPKi-naive 31 18 (58) 31 20 (65) 28 21 (75) Median DOR, months (95% CI)** 39 13.8 (11.3-NR) 40 14.4 (11.0-NR) 46 16.6 (11.6-NR) BRAF fusion 33 13.8 (11.3-NR)
33 16.3 (11.0-NR) 41 16.8 (11.6-NR) BRAF mutation 6 NR (8.4-NR) 7 12.0 (8.4-NR) 5 15.1 (8.3-NR) Prior MAPKi 22 13.8 (11.3-NR) 23 12.0 (8.5-NR) 29 15.1 (9.0-16.8) MAPKi-naive 17 NR (8.4-NR) 17 16.3 (8.4-NR) 17 NR (11.6-NR) 1 2 3 June 5, 2023 data
cutoff. Fangusaro J, et al. Lancet Oncol. 2020;21(6):e305–316. Fangusaro J, et al. Lancet Oncol. 2020;21(6):e305–316. van den Bent MJ, et al. Lancet Oncol. 2011;12(6):583- 44 593. 4. Wen PY, et al. J. Clin Oncol. 2017;35(21),2439-2449. *
ORR, CBR for RAPNO-LGG and RANO-LGG included MRs. ** the 95% CI were calculated using Kaplan-Meier method.
Tovorafenib Safety Data (n=137) TEAEs TRAEs Preferred Term, n (%) Any
Grade Grade ≥3 Any Grade Grade ≥3 Any AE 137 (100) 86 (63) 134 (98) 58 (42) Hair color changes 104 (76) 0 104 (76) 0 • The most common reasons Anemia 81 (59) 15 (11) 67 (49) 14 (10) Elevated CPK 80 (58) 16 (12) 77 (56) 16 (12) for
discontinuation were Fatigue 76 (55) 6 (4) 60 (44) 6 (4) tumor hemorrhage (3 Vomiting 68 (50) 6 (4) 28 (20) 3 (2) patients) and decrease in Hypophosphatemia 64 (47) 0 48 (35) 0 growth velocity (2 patients) Headache 61 (45) 2 (1) 29 (21) 0
Maculo-papular rash 60 (44) 11 (8) 56 (41) 11 (8) • 33 patients (24%) had TRAEs Pyrexia 53 (39) 5 (4) 17 (12) 1 (1) leading to dose reduction; 50 Dry skin 49 (36) 0 45 (33) 0 patients (37%) had TRAEs Elevated LDH 48 (35) 0 42 (31) 0 Increased
AST 47 (34) 4 (3) 41 (30) 4 (3) leading to dose interruption Constipation 45 (33) 0 31 (23) 0 • Median duration of dose Nausea 45 (33) 0 25 (18) 0 Upper RTI 43 (31) 2 (1) 2 (1) 0 interruption was 2 weeks Dermatitis acneiform 42 (31) 1 (1) 41
(30) 1 (1) Epistaxis 42 (31) 1 (1) 27 (20) 0 • 9 patients (7%) had TRAEs Decreased appetite 39 (28) 5 (4) 28 (20) 4 (3) leading to discontinuation Paronychia 36 (26) 2 (1) 32 (23) 2 (1) Pruritus 35 (26) 1 (1) 32 (23) 1 (1) COVID-19 34 (25) 0 0
0 June 5, 2023 data cutoff. Treatment-emergent AEs ≥25% any grade in arms 1 & 2. AE, adverse event; ALT, Alanine transaminase; AST, aspartate aminotransferase; COVID-19, Coronavirus 45 disease 2019; CPK, creatine phosphokinase; LDH,
lactate dehydrogenase; RTI, respiratory tract infection; TEAEs, treatment-emergent adverse events; TRAEs, treatment-related adverse events.
v3.24.1.1.u2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Day One Biopharmaceuticals (NASDAQ:DAWN)
Historical Stock Chart
From May 2024 to Jun 2024
Day One Biopharmaceuticals (NASDAQ:DAWN)
Historical Stock Chart
From Jun 2023 to Jun 2024