
ASSET PURCHASE AGREEMENT between CLEVER LEAVES PORTUGAL UNIPESSOAL, Lda. (As Seller), and TERRA VERDE, Lda. (as Purchaser) and Clever Leaves Holdings Inc. (as Guarantor)

02 CONTENTS 1. DEFINITIONS AND INTERPRETATION ___________________________________ 4 2. TRANSFER OF ASSETS ________________________________________________ 7 3. PURCHASE PRICE ___________________________________________________ 8 4. TAXES ____________________________________________________________ 8 5. DELIVERIES BY THE PARTIES __________________________________________ 9 6. CONTRACTS ____________________________________ Error! Bookmark not defined. 7. RECORDS _________________________________________________________ 10 8. WARRANTIES _____________________________________________________ 10 9. BREACH __________________________________________________________ 11 10. ANNOUNCEMENTS AND CONFIDENTIALITY ______________________________ 12 11. DISPUTE RESOLUTION ______________________________________________ 13 12. GENERAL _________________________________________________________ 13 13. ADDRESSES FOR LEGAL PROCESS AND NOTICES __________________________ 16 SCHEDULE 1 __________________________________________________________ 18 SCHEDULE 2 __________________________________________________________ 27

03 THIS AGREEMENT is made BETWEEN: (1) CLEVER LEAVES PORTUGAL UNIPESSOAL, Lda., a private company incorporated under the laws of Portugal, with registered offices at Avenida da Liberdade 144, 2.º Direito, Lisbon, Portugal, registered in the Lisbon Commercial Registry with the sole identification and taxpayer number 515371009, duly represented by Marta Maria Reynaud Pinto Leite de Areia, acting in the capacity of legal representant, duly empowered for this act as evidenced by the commercial registry certificate with the access code 0836-8281-8378(“Clever Leaves Portugal”, or the Seller); And (2) Terra Verde, Lda., a private company incorporated under the laws of Portugal, with registered offices at Rua Castilho, n.13, D, 7o A, 1250-066 Lisbon, Portugal, registered at the Commercial Registry Office with the sole identification and taxpayer number 513079688, duly represented by Nuno Pedro Cristovão Martins Mendonça and Zafrin Abzal Samsudin , acting in the capacity of Directors, duly empowered for this act as evidenced by the commercial registry certificate with the access code 6378-8342-2809 (“Terra Verde”, or the Purchaser); And (3) Clever Leaves Holdings Inc., a public company incorporated under the laws of British Columbia, Canada, with registered offices at Bodega 19-B Parque Industrial Tibitoc P.H Tocancipá, Cundinamarca, 251017, Colombia, duly represented by Andres Fajardo Luna, acting in the capacity of legal representant duly empowered for this act as evidenced by the notice of articles sent to Purchaser, (“Clever Leaves Holdings”, or the Guarantor), Seller and Purchaser hereinafter jointly referred to as the “Parties” and each, individually, as a “Party”. Guarantor is considered as included in the expression “Parties” for the purposes of sections 8 through 12 below, as well as other sections or clauses where the Guarantor acts and is involved. BACKGROUND: (A) The Seller and Purchaser are companies engaged in the production of medical cannabis; (B) The Seller is the legitimate owner of the Company Assets (as defined below), used in the connection with the Division (as defined below);

04 (C) In view of the above, the Seller wishes to sell and transfer to the Purchaser, and the Purchaser wishes to purchase from the Seller, all Company Assets along with other rights (the Sale Assets, as defined below), pursuant to the terms and subject to the conditions set out hereinafter. (D) As part of the sale process arranged by the Seller, the Purchaser, with the assistance of advisers of its choice, conducted a due diligence review (the "Due Diligence") during which the Purchaser and its advisers has had access to the Information, which the Purchaser has had the opportunity to review prior to the Signature Date; (E) Additionally, during 2023 the Purchaser and its advisers visited the Division, where they conducted on-site due diligence visits of the Division prior to the Signature Date, and have had the time and conditions to assess and evaluate the apparent characteristics and external appearance of the Company Assets but not including a verification of their operation and proper functioning; (F) Having made the above-referred assessment and evaluation of the Sale Assets and having confirmed the verification of all conditions and requirements on its offer of 28 April 2023, the Purchaser confirmed its interest in acquiring the Sale Assets, and the Seller and the Purchaser have negotiated the final terms and conditions for the sale and purchase of the Sale Assets, which are the ones established herein. IT IS AGREED as follows: 1. DEFINITIONS AND INTERPRETATION 1.1. Unless inconsistent with the context, in this Agreement the words and expressions set forth below shall bear the following meanings and cognate expressions shall bear corresponding meanings: Affiliate means, with respect to any juristic person, any other person that, directly or indirectly, through one or more intermediaries, Controls, or is Controlled by, or is Under Common Control with, such person; Agreed Form means, in relation to any document, the form of that document which has been initialled for the purpose of identification by or on behalf of the Seller and the Purchaser; Agreement means this Sale of Assets agreement including all the Schedules hereto; Business Day means any day other than a Saturday, Sunday or an official public holiday in Portugal; Company Assets means the items owned or used by the Seller in connection with the Division and listed in Schedule 1; Division means the manufacturing medical cannabis site identified as PH1 and conducted by the Seller located in Edifício 7, Blue Biz Parque, Estrada da Rosa, in Setubal, Portugal;

05 Due Diligence means the Due Diligence investigation that was conducted by the Purchaser in relation to the Company Assets, through the analysis of the documents that form the Information; Encumbrance means any impairment of a right, including, without limitation, rights of ownership (whether such encumbrance sounds in money or not) and all other rights exercisable by third parties in relation to such right or rights, and unencumbered has a corresponding meaning; Equipment means the set of tools and machinery as defined in Schedule 1; EUR or Euro means Euro, the official currency of the Eurozone; Excluded Assets means any item owned or used by the Seller in connection with the Division and not included within the definition of Sale Assets, including but not limited to: (a) the manufacturing and GMP license issued by Infarmed to the Seller in connection with the Division; (b) any documents and the respective copies of the facilities certification issued by the Police; (c) any documents and the respective copies regarding the environmental and industrial licenses; (d) any vehicles which ownership or use belongs to the Seller; (e) any account balances held by or for the Seller in connection with the Division as at the Signature Date; the Lease and Services Agreement with Parque Blue Biz; and (f) any agreements concluded by the Seller with third parties in respect of the Division that are not being assigned to the Purchaser, that have been terminated or will terminate on or before the Signature Date, for which the Seller retains all liability. Information means the information on the Division and the Sale Assets provided by the Seller or its advisers to the Purchaser or its representatives and advisors before the date of this Agreement, and which includes (i) the information that is included in the non-rewritable DVD- ROM attached hereto, and (ii) the information contained in this Agreement and its Schedules. Intellectual Property means all right, title and interest in and to, under the applicable law, all copyright, know-how or trade secrets used by the Sellers in relation with the Quality Documental System as at the Signature Date; GMP Inspection means the inspection carried out by the competent regulatory authority in Portugal, Infarmed, in 2022, following which the GMP License (as defined below) has been awarded by Infarmed to the Seller. “GMP License” means the cannabis manufacturing license complying with the Guidelines on Good Manufacturing Practices (GMP), as issued by the competent regulatory authority in Portugal, Infarmed, in accordance with any applicable laws and regulations.

06 “Losses” means any direct damage, loss, loss of use due to malfunctioning of equipment for its purposes, liability, penalties, fines, judgments, awards, together with any costs including interests, court fees, expenses (including reasonable attorney’s fees) and disbursements, except that “Losses” shall not include any loss of future profits or revenues, loss of goodwill, loss of an opportunity or exemplary or punitive damages, nor any consequential loss. Parties means the Purchaser and the Seller, and Party shall, as the context requires, be a reference to any one of them. The Guarantor is considered as included in the expression “Parties” for the purposes of sections 8 through 12 below, as well as other sections or clauses where the Guarantor acts and is involved; Purchase Price means the purchase price payable by the Purchaser for the Sale Assets under the terms of clause 3; Quality Documental System means all the documents integrating the facility’s Quality Management System, including Site Master File; Standard Operational Procedures; available validation protocols and reports; Batch records masters; Manufacturing instructions, operating and instruction manuals and other supplier or manufacturer documentation; Analytical methods and specifications; available IQ/OQ/PQ protocols and reports; available Supplier Qualification; CAPA Plans; Deviation Reports; available Calibration and Maintenance Protocols. Sale Assets means all the assets of the Seller being sold to the Purchaser under this Agreement, including but not limited to: (a) the Company Assets, as described in Schedule 1; and (b) the Intellectual Property Rights regarding, in accordance with the applicable law, the facility’s Quality Management System, including, but not limited to, Site Master File; Standard Operational Procedures; available validation protocols and reports; Batch records masters; available Manufacturing instructions, operating and instruction manuals and other supplier or manufacturer documentation; Analytical methods and specifications; IQ/OQ/PQ protocols and reports; available supplier Qualification; CAPA Plans; Deviation Reports; available calibration and Maintenance Protocols; in respect of the Business and, for the avoidance of doubt, Sale Assets shall exclude the Excluded Assets; Sellers’ Bank Account means the following bank account: Account in the name of: Cleaver Leaves Bank: Bank of Montreal Account number: 4630-065 CC Code: 000100022 SWIFT/BIC: BOFMCAM2

07 Signature Date means the date of the signature of the Party last signing this Agreement in time; Standard Operational Procedures (SoP) means the written procedures listed in Schedule 1 related to the activities performed by the entity; Transaction means the sale and purchase of the Sale Assets between the Seller and the Purchaser pursuant to and subject to the conditions set out in this Agreement; VAT means value-added tax (Imposto sobre o Valor Acrescentado) including any similar tax which may be imposed in place thereof from time to time; VAT Code means the Código do Imposto sobre o Valor Acrescentado, approved by the Decree- Law 102/2008, of 20 June, as amended from time to time; Warranties means the warranties listed in Schedule 2 to this Agreement, and Warranty shall have a corresponding meaning, as the context may require. 1.2. In this Agreement any reference, express or implied, to an enactment (which includes any legislation in any jurisdiction) includes: (a) that enactment as amended, extended or applied by or under any other enactment (before, on or after the date of this Agreement); (b) any enactment which that enactment re-enacts (with or without modification); and (c) any subordinate legislation (including regulations) made (before, on or after the date of this Agreement) under that enactment, including (where applicable) that enactment as amended, extended or applied as described in paragraph (a), or under any enactment which it re-enacts as described in paragraph (b). 1.3. In this Agreement: (a) the headings do not affect its interpretation; (b) words denoting persons include bodies corporate and unincorporated associations of persons; (c) references to an individual or a natural person include his estate and personal representatives; (d) unless the contrary intention appears, a reference to a clause, subclause or schedule is a reference to a clause, subclause or schedule of or to this Agreement. The schedules form part of this Agreement; (e) general words used in this Agreement shall not be given a restrictive meaning by reason of the fact that they are followed by particular examples intended to be embraced by the general words. The word including shall mean including without limitation; (f) references to a party to this Agreement include the successors or assigns (immediate or otherwise) of that party; and (g) any reference to time is to Lisbon, Portugal time. 2. TRANSFER OF ASSETS

08 2.1. Subject to the terms, conditions and exclusions of this Agreement, on the Signature Date, the Seller sells and transfers to the Purchaser, and the Purchaser purchases and acquires from the Seller, free and clear of any Encumbrances, the full ownership of, title to and interest in the Sale Assets. 2.2. The Seller shall not sell or transfer to the Purchaser, and the Purchaser shall not purchase and acquire the Excluded Assets. 2.3. The Seller is not transferring any liabilities to the Purchaser and nothing in this Agreement shall be construed as a transfer of a company, an economic unit or a business. 3. PURCHASE PRICE 3.1. As consideration for the purchase and transfer of the Sale Assets, the Purchaser shall pay to the Seller an amount equal to EUR 2,500,000.00 (two million and five hundred thousand euros) (the “Purchase Price”), excluding VAT, if any. 3.2. The Purchase Price is paid in full, without any kind of withholding, retention, or deduction due to applicable taxes, related costs and expenses or similar concepts, by wire transfer of immediately available funds to the Sellers’ Bank Account. 4. TAXES 4.1. Value Added Tax (a) VAT payable in connection with the transfer of the Assets to the Purchaser and the Transaction shall be borne and paid solely by the Purchaser when due in compliance with applicable Tax laws; if any such amount shall be incurred by the Sellers, the Purchaser must, subject to receipt of satisfactory evidence of the Sellers’ payment thereof, promptly reimburse the Sellers. (b) The Purchaser will pay the VAT due to Sellers not later than 7 (seven) business days prior to the date that Sellers are required to pay such VAT amount to the Portuguese Tax Authorities or alternatively, within thirty (30) days after the invoice date of the invoice issued by the Sellers, whichever date comes first. If a request for a VAT refund by the Purchaser has been denied due to an invalid invoice, the Sellers will cooperate to ensure that the Purchaser will be provided with a valid invoice by the Sellers. (c) Should the Portuguese Tax Authorities determine that VAT was applied and paid in error, then (i) the Sellers shall provide Purchaser with a valid credit note or any other appropriate document according to Portuguese law; and (ii) if the VAT applied in error was paid by the Purchaser to the Sellers, the Sellers shall repay to Purchaser any such VAT under the condition that Purchaser cooperates in ensuring, where possible, that the Sellers will be able to correct this error under the applicable VAT regularization procedure and, if applicable, upon liaising with its competent tax office and obtain a VAT credit/refund from the Portuguese Tax Authorities for the VAT that was charged in error. 4.2. Stamp Tax

09 (a) In case the Portuguese Tax Authorities consider that this Agreement is subject to Stamp Tax, this tax (or any similar tax replacing or increasing it) shall be borne by the Purchaser. (b) All tax penalties, administrative charges or compensatory or default interest arising from the non-prompt payment of Stamp Tax shall be borne by the Purchaser. 5. DELIVERIES BY THE PARTIES 5.1. On the Signature Date or except as otherwise stated in the relevant sub-clause below, in addition to any other action to be taken and any other instruments to be executed and/or delivered for the purposes of the transactions contemplated in this Agreement: a) The Purchaser delivers to the Seller: (i) Certified true copy of the relevant resolution or appropriate corporate powers of the Purchaser authorizing the execution of this Agreement and the completion of any relevant transactions contemplated hereunder; and (ii) A copy of the irrevocable SWIFT order for the transfer of the Purchase Price to the Seller’s Bank Account, identifying the name of the bank from which the relevant amount has been transferred, the Purchaser as the account holder, the IBAN and, if applicable, the SWIFT code. b) The Seller and the Guarantor shall deliver to the Purchaser: (i) As soon as reasonably practicable after the Signature Date, a certified true copy of the relevant resolution or appropriate corporate powers of the Seller and of the Guarantor authorizing the execution of this Agreement and the completion of any relevant transactions contemplated hereunder; (ii) The Company Assets, which shall be considered as delivered by being made available to the Seller at the premises identified in section 5 of Schedule 2; and (iii) The originals or copies of any documents pertaining to the Sale Assets. 5.2. The Parties agree to execute and deliver all documents and take all actions as may be appropriate or necessary to give full effect to the transfer of the Sale Assets. With the taking of possession of the Sale Assets by the Purchaser on Signature Date at the premises identified in section 5 of Schedule 2, the Purchaser shall take legal possession of the Sale Assets, and all risk and benefit shall pass from the Seller to the Purchaser. 5.3. The assignment of the relevant Intellectual Property under the applicable law shall be valid and effective as of the Signature Date. The Seller agrees, upon receiving the Purchase Price and at the request and expense of the Purchaser, to sign all documentation and do all other things which may be necessary to give effect to the assignment of the relevant Intellectual Property, including the filing of such assignment at the relevant intellectual property offices. Further, upon payment of the Purchase Price and at the request and expense of the Purchaser, the Seller shall deliver to the Purchaser all information and documentation necessary for the Purchaser to take

10 possession of, edit, and otherwise manage and control the relevant Intellectual Property, including all know-how relating to the Division. From and after the Signature Date, Purchaser shall be responsible, in its discretion, for the management, prosecution and maintenance of all Intellectual Property Rights assigned under this Agreement. 6. RECORDS Where the Seller is required by law or regulations to retain the originals of any of records of the Sale Assets, then the Seller shall retain such records but shall grant access to the Purchaser to review and provide Purchaser with copies of all such records. In respect of all other records, the Seller shall only be entitled to retain copies of records which refer to periods prior to the Signature Date and which are required by the Seller to meet its obligations arising prior to the Signature Date. 7. WARRANTIES 7.1. The Parties hereby make the representations and Warranties set forth in Schedule 2 which are true, accurate and not misleading in all respects as at Signature Date. 7.2. The Seller’s obligation to indemnify the Purchaser for any Losses due to any breach, inaccuracy (including due to omission) or falsity of any of the Seller’s Warranties shall be subject to the following limitations (except in relation to any claim which arises out of any fraud or willful misconduct (dolo) by the Seller, where these limitations shall not apply): a) Seller’s indemnification obligation shall remain in force for a period of 12 (twelve) months following the Signature Date, provided that any claim is sent to the Seller prior to the expiry of the above-mentioned period, in which case it will lead to the claims surviving the above mentioned deadlines until their full and final resolution; b) The maximum amount of the Seller’s liability shall be equivalent to the Purchase Price; c) The Seller shall not be liable in respect of any individual claim regarding a Loss of an amount lower than EUR 10,000.00 (ten thousand euros), provided, however, that in the event of a series of claims based on the same or related facts, events or circumstances, such series of claims will be treated as a single claim; d) The Purchaser shall not be entitled to recover damages or otherwise claim reimbursement or restitution more than once in respect of the same Loss; for the avoidance of doubt, a Loss that is ongoing or occurs in stages shall not be considered the same Loss and may be subject to additional recovery; and e) The Seller shall not be liable to indemnify any contingent or threatened Losses, but only Losses that have effectively been incurred by the Purchaser (i.e. once the Purchaser has made a disbursement or payment in connection with such Losses) or that are or become due and payable by the Purchaser; however, if a claim, which may lead to an effective Loss, is sent to the Seller prior to the expiry of the 12-months period mentioned in paragraph a) above, such claim shall survive the above mentioned deadline until its full and final resolution.

11 7.3. The Seller shall also not be liable to the Purchaser in the following circumstances: a) Where the liability originates from acts or omissions by the Seller acting in compliance with this Agreement, or as requested or authorized in writing by the Purchaser; b) Where the circumstances or the events that give rise to the obligation to indemnify in relation to the Seller’s Warranties have been Fairly Disclosed in the Information. For the purposes of this Agreement, “Fairly Disclosed” shall mean, that the Purchaser, acting reasonably and diligently, as a sophisticated buyer with adequate knowledge and expertise in the business of the Division, could properly identify and assess the nature, scope and impact of the relevant disclosed facts, data and information on the basis of the Information, without the need to make enquiries to third parties; c) Where the Loss is covered by an insurance policy and full payment is made under such policy to the Purchaser. In the event that any Loss is covered and paid partially by said insurance policy, the Seller shall only be liable for the remaining amount; d) Where the damages, costs or Losses are effectively recovered from third parties under a third party action directly by the Purchaser. In that event, the Purchaser shall pay to the Seller on a Euro per Euro basis the amount actually and effectively recovered by the Purchaser (net of any recovery cost and Taxes); or e) Where the Loss arises as a result of actions taken, or transactions carried out, or omissions, by the Purchaser or by any of its directors, officers, agents and employees (or approved by such persons) after the Signature Date, other than acting in compliance with or to receive the benefit of this Agreement, or as requested or authorized in writing by the Seller. 7.4. Notwithstanding any other provisions in this Agreement, the Parties must cooperate diligently and in good faith with each other and procure that all commercially reasonable steps are taken and all commercially reasonable assistance is given to avoid or mitigate the scope and extent of any Losses suffered by them. 7.5. With the limitations provided for in this Clause 7, the Seller shall indemnify the Purchaser within a maximum period of 14 (fourteen) days after a claim that has been sent by the Purchaser to the Seller under this Agreement is definitely settled. Late interest shall be calculated as of the 15th day following the sending of the claim by the Purchaser to the Seller. 7.6. The Parties agree that the rights of the Purchaser to be indemnified by the Seller under this Agreement shall (i) not be transferable to any third party who acquires the Sale Assets from the Purchaser or any other third party, except with the prior written consent of the Seller and (ii) be the sole remedy available to the Purchaser as against the Seller in respect of any breach, inaccuracy (including due to omission) or falsity of any of the Seller’s Warranties, (except in case of dolus or fraud). 8. BREACH

12 8.1. Should any Party commit a breach of any of the provisions of this Agreement and fail to remedy that breach within fourteen (14) Business Days after having been called upon to do so by the other Party then, the non-defaulting Party shall have the right to claim damages for any Losses, except where the breach relates to late payment in which case late interest (juros de mora) shall be due at the applicable statutory rate. 8.2. The Guarantor declares that it guarantees the obligations of the Seller under this Agreement, exclusively relative to (i) liabilities under clause 6.4 of Schedule 2, and only for the period of 12 (twelve) months as of the Signature Date and (ii) verified malfunction of Equipment listed in Schedule 1 for the period of 6 (six) months as of the Signature Date. The total amount of the guarantee will not exceed, in any circumstances, 500.000 Euros (five hundred thousand euros) and undertakes the obligation of principal payor (principal pagador) renouncing to the benefit of prior foreclosure of the Seller’s assets (renunciando ao benefício da excussão previa). 9. ANNOUNCEMENTS AND CONFIDENTIALITY 9.1. Subject to clauses 9.3 and 9.4, the Parties shall: (a) not make any announcement concerning the sale and purchase of the Sale Assets or any related or ancillary matter; and (b) keep confidential the provisions and subject matter of, and the negotiations relating to, this Agreement. 9.2. The provisions of clause 9.1 shall apply before, on and after the Signature Date. 9.3. Nothing in clause 9.1 prevents any announcement being made or any confidential information being disclosed: (a) where such announcement is in the Agreed Form or the confidential information disclosed comprises only information set out in an announcement in the Agreed Form; (b) with the written approval of the Parties, which in the case of any announcement shall not be unreasonably withheld or delayed; or (c) to the extent required by Portuguese law or any other law indirectly or directly applicable to the Seller, Purchaser or its respective Affiliates, any court of competent jurisdiction or any competent regulatory body, but if a person is so required to make any announcement or to disclose any confidential information, the relevant party shall promptly notify the other Parties, where practicable and lawful to do so, before the announcement is made or disclosure occurs (as the case may be) and shall co-operate with the other Parties regarding the timing and content of such announcement or disclosure (as the case may be) or any action which the other Parties may reasonably elect to take to challenge the validity of such requirement. 9.4. Nothing in clause 9.1 prevents any confidential information being disclosed to the extent: (a) required to enable any person to enforce its rights under this Agreement or for the purpose of any judicial proceedings;

13 (b) that the information is disclosed on a strictly confidential basis by a person to its professional advisers, auditors or bankers; (c) that the information is disclosed by the Seller on a strictly confidential and need to know basis to an Affiliate of the Seller or by the Purchaser on a strictly confidential and need to know basis to an Affiliate of the Purchaser; (d) that the information is disclosed on a strictly confidential and need to know basis in order to comply with any other obligation under the terms of this Agreement; or (e) that the information is in or comes into the public domain. 10. DISPUTE RESOLUTION 10.1. In the event of there being any dispute or difference between the Parties arising out of this Agreement (including but not limited to any dispute as to the validity or otherwise of this Agreement, or as to the enforceability of this Agreement), such dispute shall be submitted to arbitration under the Rules of Arbitration of the Arbitration Centre of the Portuguese Chamber of Commerce and Industry, upon written demand by either Party. 10.2. Within 10 (ten) calendar days after receipt of a notice of intention to arbitrate sent by one Party, the Seller (and or the Guarantor, in case the Guarantor is involved in the dispute), on one side, and the Purchaser, on the other, shall each designate in writing 1 (one) arbitrator to resolve the dispute, which 2 (two) arbitrators shall, in turn, jointly select a 3rd (third) arbitrator within 20 (twenty) calendar days of their designation, failing which the 3rd (third) arbitrator shall be appointed by the Arbitration Centre of the Portuguese Chamber of Commerce and Industry. 10.3. The outcome of the arbitral tribunal’s decision shall be final and binding on the Parties. The arbitral tribunal shall decide in accordance with the applicable Portuguese law. 10.4. The place of arbitration proceedings shall be in Lisbon and the language of the arbitration proceedings and hearings shall be English. 10.5. The Parties shall treat as confidential details of the dispute submitted to arbitration, the conduct of the arbitration proceedings and the outcome of the arbitration. 10.6. This clause 10 will continue to be binding on the Parties notwithstanding any termination or cancellation of the Agreement. 10.7. The Parties declare that it is their intention that this clause 10 will regulate the manner in which they will resolve any dispute or difference regarding the validity or otherwise of this Agreement, regardless of the fact that one of the Parties may dispute the validity or enforceability of the Agreement. 10.8. Notwithstanding the provisions of this clause 10, the Parties shall be entitled to approach a court of competent jurisdiction for interim relief in respect of any dispute arising from this Agreement or for enforcement of an arbitral decision reached under this clause. 11. GENERAL 11.1. Communications between the Parties

14 All notices, demands and other oral or written communications given or made by or on behalf of any of the Parties to any other Party shall be in English. 11.2. Severance If any provision of this Agreement is rendered void, illegal or unenforceable in any respect under any law, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby. 11.3. Costs Each Party shall bear its own costs incurred by its attorneys and other professional advisers for the preparation and signing of this Agreement and its schedules. 11.4. Termination and survival of rights, duties and obligations Termination of this Agreement for any cause shall not release a Party from any liability which at the time of termination has already accrued to such Party or which thereafter may accrue in respect of any act or omission prior to such termination. 11.5. Entire Agreement (a) This Agreement, together with the schedules, constitutes the entire agreement between the Parties and save as otherwise expressly provided, no modification, amendment or waiver of any of the provisions of this Agreement or any agreement to cancel or terminate it shall be effective unless made in writing specifically referring to this Agreement and duly signed by the Parties. (b) For purposes of clause 11.5(a) in writing shall exclude any electronic communication made in relation to the modification, amendment, waiver, cancellation or termination of this Agreement. 11.6. Variations No agreement to vary, add to or cancel this Agreement shall be of any force or effect unless recorded in writing and signed by or on behalf of all of the Parties. 11.7. Assignment This Agreement shall be binding on the Parties hereto and their respective successors and assignees. The Purchaser shall be entitled to cede, assign or delegate this Agreement or any of its rights and/or obligations hereunder to any other person without the consent of the Seller. The Seller shall not be entitled to cede, assign or delegate this Agreement or any of its rights and/or obligations hereunder to any other person without the prior written consent of the Purchaser. 11.8. No partnership Nothing in this Agreement shall be deemed to constitute a partnership between the Parties or, except as specifically provided for in this Agreement, constitute either Party the agent of the other Party for any purpose. 11.9. Further assurance Each Party shall co-operate in good faith with the other Party and execute and deliver to the other Party such other instruments and documents and take such other actions as may be

15 reasonably requested from time to time in order to carry out, evidence and confirm their rights and the intended purpose of this Agreement. 11.10. Survival of Rights, Duties and Obligations Termination of this Agreement for any cause shall not release any Party from any liability which at the time of termination has already accrued to any other Party or which thereafter may accrue in respect of any act or omission prior to such termination. 11.11. Conflicts with other Agreements If there is any conflict between the terms of this Agreement and any other agreement, this Agreement shall prevail (as between the Parties to this Agreement and as between the Sellers and their Affiliates) unless (a) such other agreement expressly states that it overrides this Agreement in the relevant respect and (b) the Parties are either also Parties to that other agreement or otherwise expressly agree in writing that such other agreement shall override this Agreement in that respect. 11.12. Governing Law and Jurisdiction This Agreement and the rights and obligations of the Parties, including all non-contractual obligations arising under or in connection with this Agreement, shall be governed by, and construed and enforced in accordance with, the laws of the Republic of Portugal. 11.13. Rights of Third Parties Unless expressly provided otherwise in this Agreement, a person who is not a party to this Agreement shall have no right under any applicable legislation giving rights to such persons or on any other basis, to enforce any of its terms, and the consent of such persons shall not be needed in respect of any amendment or termination of this Agreement. 11.14. Specific performance The Parties agree that irreparable damage would occur if any provision of this Agreement were not performed in accordance with the terms hereof and that the Parties shall be entitled to an order to prevent breaches of this Agreement or to enforce specifically (execução específica) the performance of the terms and provisions hereof, in addition to any other remedy to which they are entitled at law. 11.15. Completion of Agreement a) This Agreement is to be signed by the Parties in a digital support by means of DocuSign or other electronic signature platform accepted by the Parties. b) The Parties expressly accept and acknowledge that the signatures made in accordance with the previous paragraph of this Clause, represent full evidence of the authorship and integrity of electronic documents, in accordance with the provisions of no. 9 of article 3.º of the Decree Law no. 12/2021, of 9 February, and article 376.º of the Civil Code, guaranteeing the authenticity and integrity of said signatures. c) The Parties expressly acknowledge, for all legal effects, that the electronic signatures made in accordance with this Clause possess the technical characteristics of a qualified signature, except for the intervention of a certifying entity, namely its authenticity,

16 integrity and stability therefore undertaking not to reject the Contract on the basis of this mutually agreed signing method. d) The Parties agree to complete this Agreement in counterparts signed by the method provided for herein, for all due legal effects. e) Additionally, the Parties shall sign this Agreement by hand (including initials on all pages) and send the respective signed counterpart to the other Party in PDF format via e-mail and subsequently the original via courier. 12. ADDRESSES FOR LEGAL PROCESS AND NOTICES 12.1. The Parties choose for the purposes of this Agreement the following addresses, email addresses and, for the purpose of any notices, designated officers: (a) Seller (i) address: Bodega 19-B Parque Industrial Tibitoc P.H Tocancipá, Cundinamarca, 251017, Colombia (ii) e-mail: andres.fajardo@cleverleaves.com (iii) attention: Andres Fajardo, CEO (b) Purchaser (i) address: Rua Castilho, n.13, D, 7o A, 1250-066 Lisbon, Portugal (ii) e-mail: nuno.mendonca@curaleafint.com; luis.teixeira@curaleaf.com (iii) attention: Nuno Mendonca, General Manager (Iberia); Luis Teixeira, VP, Operations, Curaleaf International (c) Guarantor (i) address: Bodega 19-B Parque Industrial Tibitoc P.H Tocancipá, Cundinamarca, 251017, Colombia (ii) e-mail: andres.fajardo@cleverleaves.com (iii) attention: Andres Fajardo, CEO Any legal process to be served on any of the Parties may be served on it at the physical address specified for it in this Clause 12.1 and it chooses that address as its domicilium citandi et executandi for all purposes under this Agreement. 12.2. Any notice or other communication to be given to any of the Parties in terms of this Agreement shall be valid and effective only if it is given in writing. 12.3. A notice to any of the Parties which is sent by recorded or special delivery or courier using an internationally recognised courier company in a correctly addressed envelope to the postal address specified for it in Clause 12.1 shall be deemed to have been received within 3 (three) days from the date it was posted, or which is delivered to the Party by hand at the physical

17 address specified for it in Clause 12.1 shall be deemed to have been received on the day of delivery, provided it was delivered to a responsible person during ordinary Business hours. 12.4. Notwithstanding anything to the contrary in this Clause 12, a written notice or other communication actually received by any of the Parties (and for which written receipt has been obtained) shall be adequate written notice or communication to it notwithstanding that the notice was not sent to or delivered at its chosen address. 12.5. Any Party may by written notice to the other Parties change its physical or postal address or email address for the purposes of Clause 12.1 to any other physical or postal address or email address provided the physical or postal address is in Portugal and the change shall become effective on the 7th (seventh) day after the receipt of the notice. 12.6. The Parties acknowledge that whilst they may correspond via email during the execution of this Agreement for operational reasons, no formal notice, legal processes nor any amendment or variation to this Agreement may be given or concluded via email.
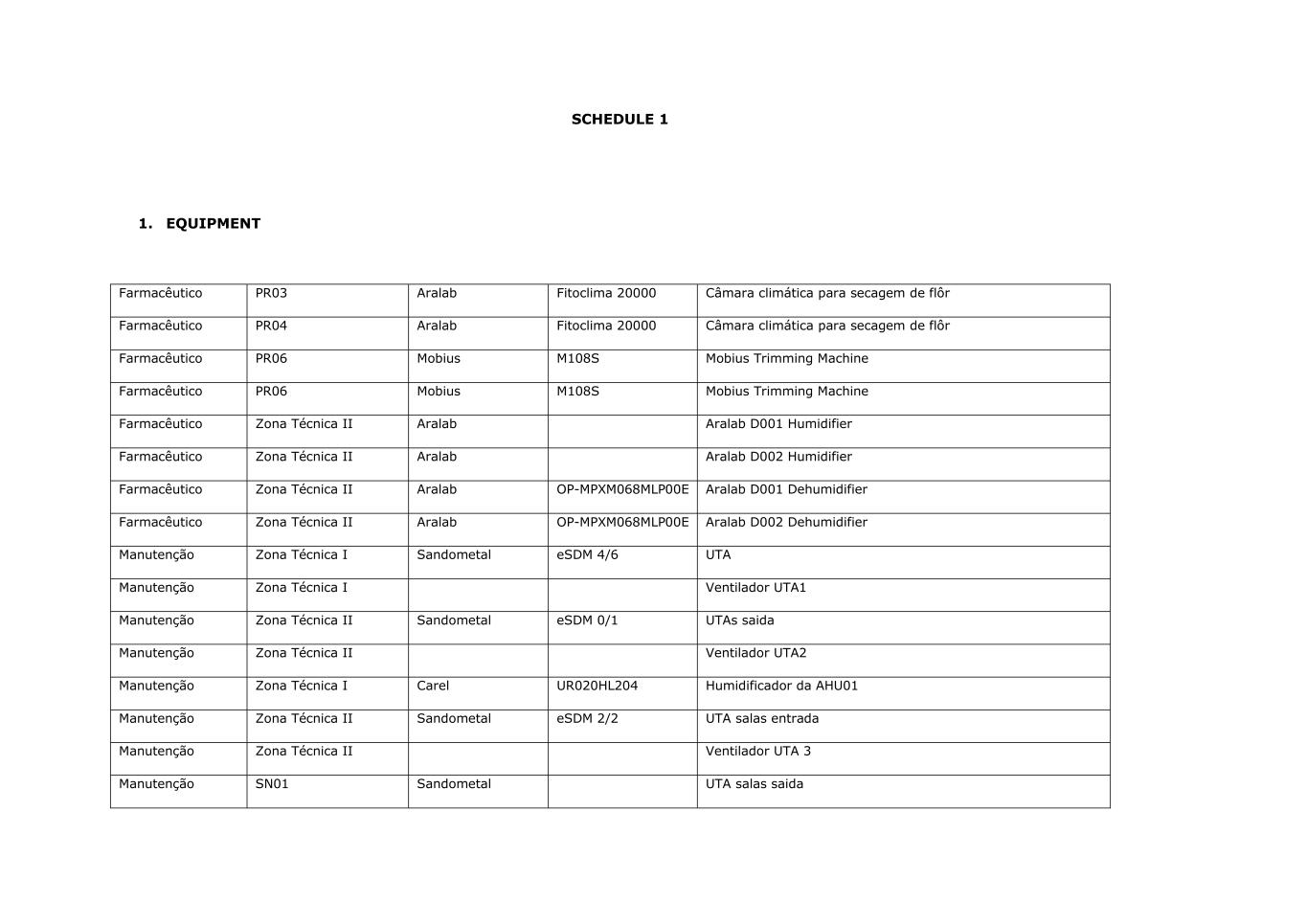
SCHEDULE 1 1. EQUIPMENT Farmacêutico PR03 Aralab Fitoclima 20000 Câmara climática para secagem de flôr Farmacêutico PR04 Aralab Fitoclima 20000 Câmara climática para secagem de flôr Farmacêutico PR06 Mobius M108S Mobius Trimming Machine Farmacêutico PR06 Mobius M108S Mobius Trimming Machine Farmacêutico Zona Técnica II Aralab Aralab D001 Humidifier Farmacêutico Zona Técnica II Aralab Aralab D002 Humidifier Farmacêutico Zona Técnica II Aralab OP-MPXM068MLP00E Aralab D001 Dehumidifier Farmacêutico Zona Técnica II Aralab OP-MPXM068MLP00E Aralab D002 Dehumidifier Manutenção Zona Técnica I Sandometal eSDM 4/6 UTA Manutenção Zona Técnica I Ventilador UTA1 Manutenção Zona Técnica II Sandometal eSDM 0/1 UTAs saida Manutenção Zona Técnica II Ventilador UTA2 Manutenção Zona Técnica I Carel UR020HL204 Humidificador da AHU01 Manutenção Zona Técnica II Sandometal eSDM 2/2 UTA salas entrada Manutenção Zona Técnica II Ventilador UTA 3 Manutenção SN01 Sandometal UTA salas saida
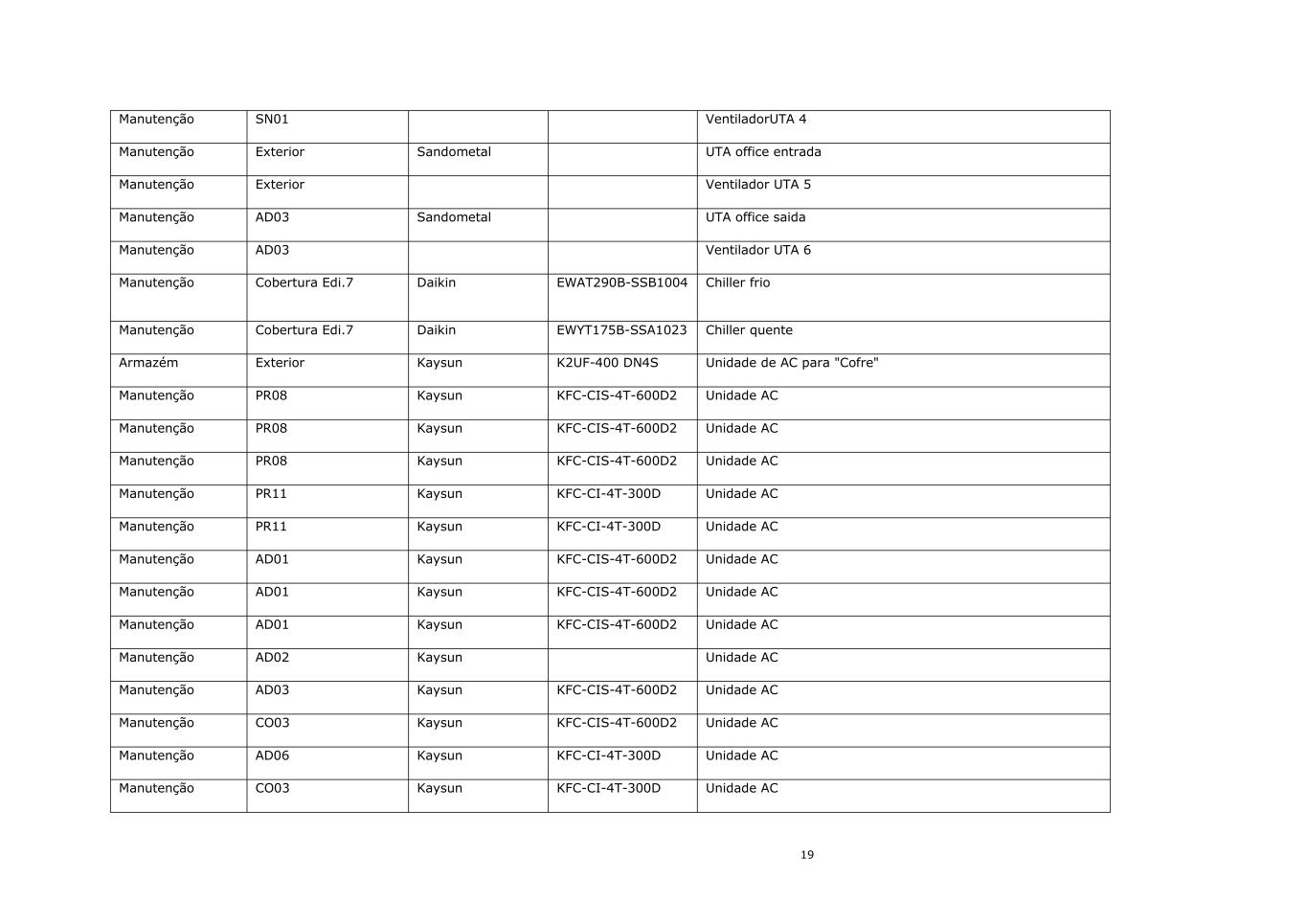
19 Manutenção SN01 VentiladorUTA 4 Manutenção Exterior Sandometal UTA office entrada Manutenção Exterior Ventilador UTA 5 Manutenção AD03 Sandometal UTA office saida Manutenção AD03 Ventilador UTA 6 Manutenção Cobertura Edi.7 Daikin EWAT290B-SSB1004 Chiller frio Manutenção Cobertura Edi.7 Daikin EWYT175B-SSA1023 Chiller quente Armazém Exterior Kaysun K2UF-400 DN4S Unidade de AC para "Cofre" Manutenção PR08 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção PR08 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção PR08 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção PR11 Kaysun KFC-CI-4T-300D Unidade AC Manutenção PR11 Kaysun KFC-CI-4T-300D Unidade AC Manutenção AD01 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção AD01 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção AD01 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção AD02 Kaysun Unidade AC Manutenção AD03 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção CO03 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção AD06 Kaysun KFC-CI-4T-300D Unidade AC Manutenção CO03 Kaysun KFC-CI-4T-300D Unidade AC
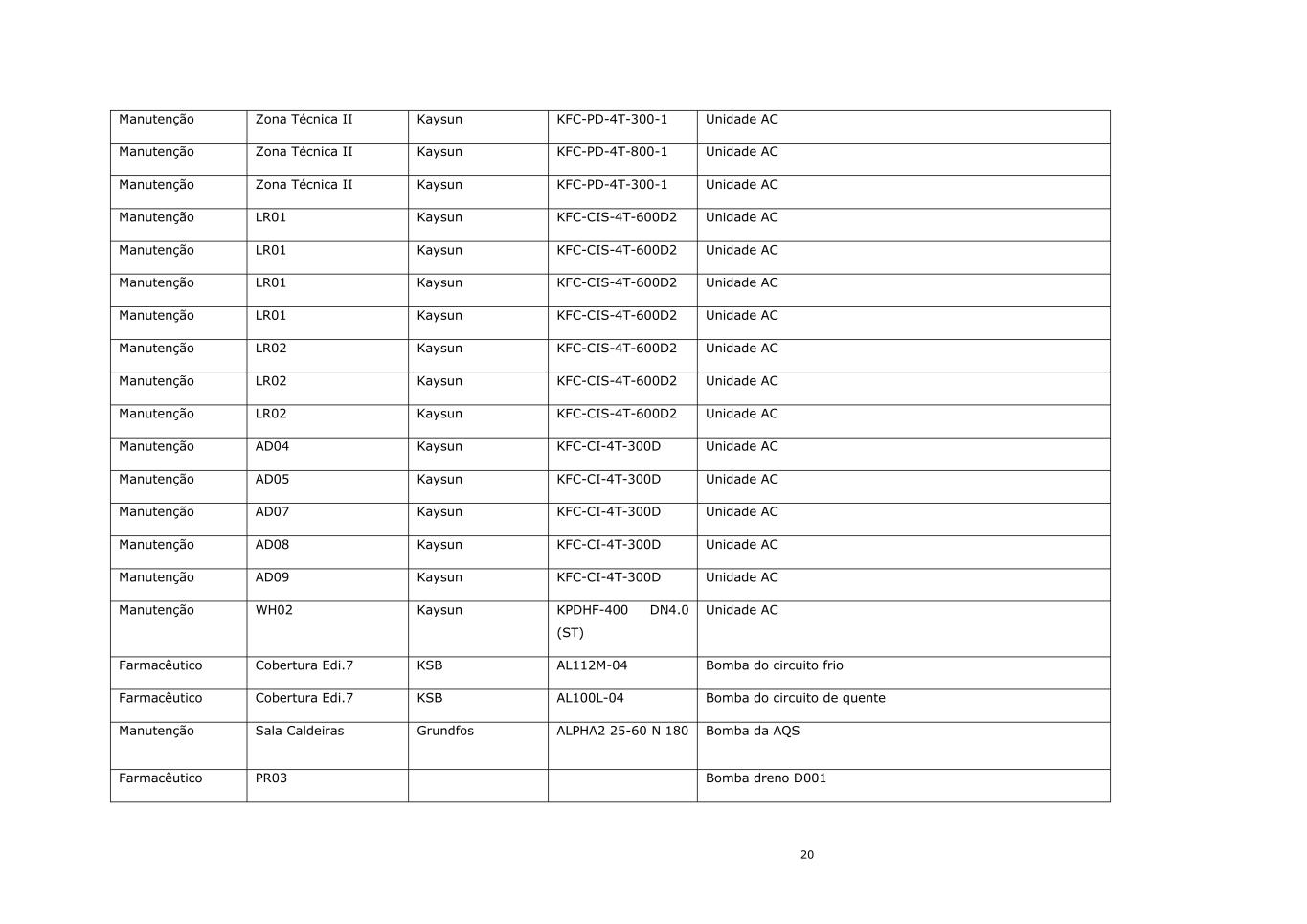
20 Manutenção Zona Técnica II Kaysun KFC-PD-4T-300-1 Unidade AC Manutenção Zona Técnica II Kaysun KFC-PD-4T-800-1 Unidade AC Manutenção Zona Técnica II Kaysun KFC-PD-4T-300-1 Unidade AC Manutenção LR01 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção LR01 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção LR01 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção LR01 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção LR02 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção LR02 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção LR02 Kaysun KFC-CIS-4T-600D2 Unidade AC Manutenção AD04 Kaysun KFC-CI-4T-300D Unidade AC Manutenção AD05 Kaysun KFC-CI-4T-300D Unidade AC Manutenção AD07 Kaysun KFC-CI-4T-300D Unidade AC Manutenção AD08 Kaysun KFC-CI-4T-300D Unidade AC Manutenção AD09 Kaysun KFC-CI-4T-300D Unidade AC Manutenção WH02 Kaysun KPDHF-400 DN4.0 (ST) Unidade AC Farmacêutico Cobertura Edi.7 KSB AL112M-04 Bomba do circuito frio Farmacêutico Cobertura Edi.7 KSB AL100L-04 Bomba do circuito de quente Manutenção Sala Caldeiras Grundfos ALPHA2 25-60 N 180 Bomba da AQS Farmacêutico PR03 Bomba dreno D001
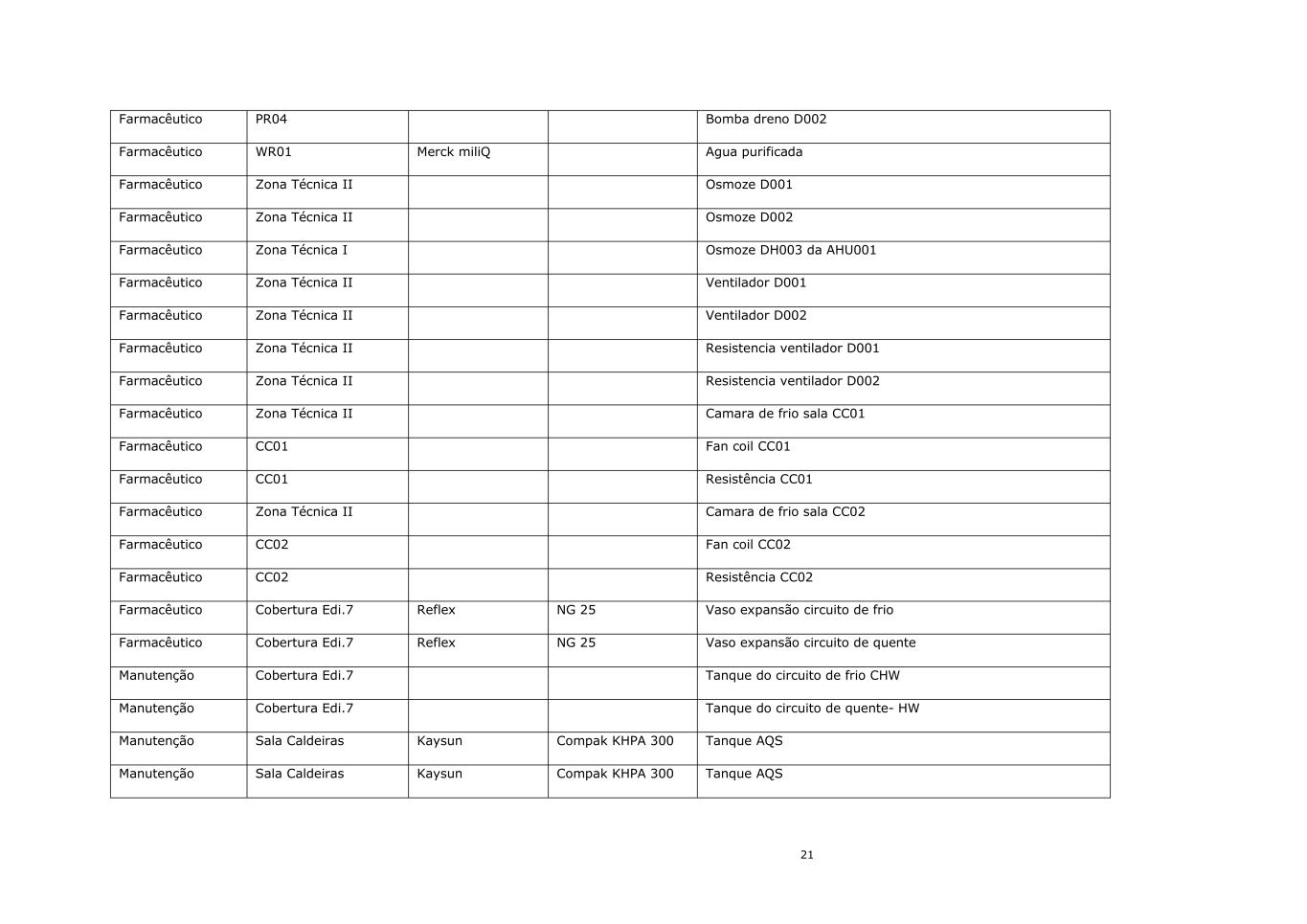
21 Farmacêutico PR04 Bomba dreno D002 Farmacêutico WR01 Merck miliQ Agua purificada Farmacêutico Zona Técnica II Osmoze D001 Farmacêutico Zona Técnica II Osmoze D002 Farmacêutico Zona Técnica I Osmoze DH003 da AHU001 Farmacêutico Zona Técnica II Ventilador D001 Farmacêutico Zona Técnica II Ventilador D002 Farmacêutico Zona Técnica II Resistencia ventilador D001 Farmacêutico Zona Técnica II Resistencia ventilador D002 Farmacêutico Zona Técnica II Camara de frio sala CC01 Farmacêutico CC01 Fan coil CC01 Farmacêutico CC01 Resistência CC01 Farmacêutico Zona Técnica II Camara de frio sala CC02 Farmacêutico CC02 Fan coil CC02 Farmacêutico CC02 Resistência CC02 Farmacêutico Cobertura Edi.7 Reflex NG 25 Vaso expansão circuito de frio Farmacêutico Cobertura Edi.7 Reflex NG 25 Vaso expansão circuito de quente Manutenção Cobertura Edi.7 Tanque do circuito de frio CHW Manutenção Cobertura Edi.7 Tanque do circuito de quente- HW Manutenção Sala Caldeiras Kaysun Compak KHPA 300 Tanque AQS Manutenção Sala Caldeiras Kaysun Compak KHPA 300 Tanque AQS

22 Manutenção Exterior Compressor de ar comprimido Manutenção Exterior Reservatório de ar comprimido Farmacêutico Gerador de emergência Farmacêutico Zona Técnica II DehuTech DA/DT-800 Desumidificador silica D001 Farmacêutico Zona Técnica II DehuTech DA/DT-800 Desumidificador silica D002 Manutenção Zona Técnica I Controlador do AVAC Farmacêutico PR03 Controlador D001 Farmacêutico PR04 Controlador D002 Manutenção Zona Técnica I Controlador Humidificador Carel AHU1 Farmacêutico Zona Técnica I UPS para equipamentos Farmacêutico PR03 Drenos CC01 Farmacêutico PR04 Drenos CC02 Manutenção Ventilador extracção WR01 Farmacêutico PR05 Máquina de embalagem Swifty Bagger Junior Farmacêutico PR05 Distribuidor de flor_ primo combi Farmacêutico PR05 Alimentador- Vibratory Feed Pan Farmacêutico PR05 Pre-cheq analyzer Farmacêutico PR05 Etiquetadora - Lay flat labeler Farmacêutico PR01 Máquina de Selagem Farmacêutico PR01 Resistencia da maquina de selar MS001 Farmacêutico PR01 Bomba de vácuo da MS001
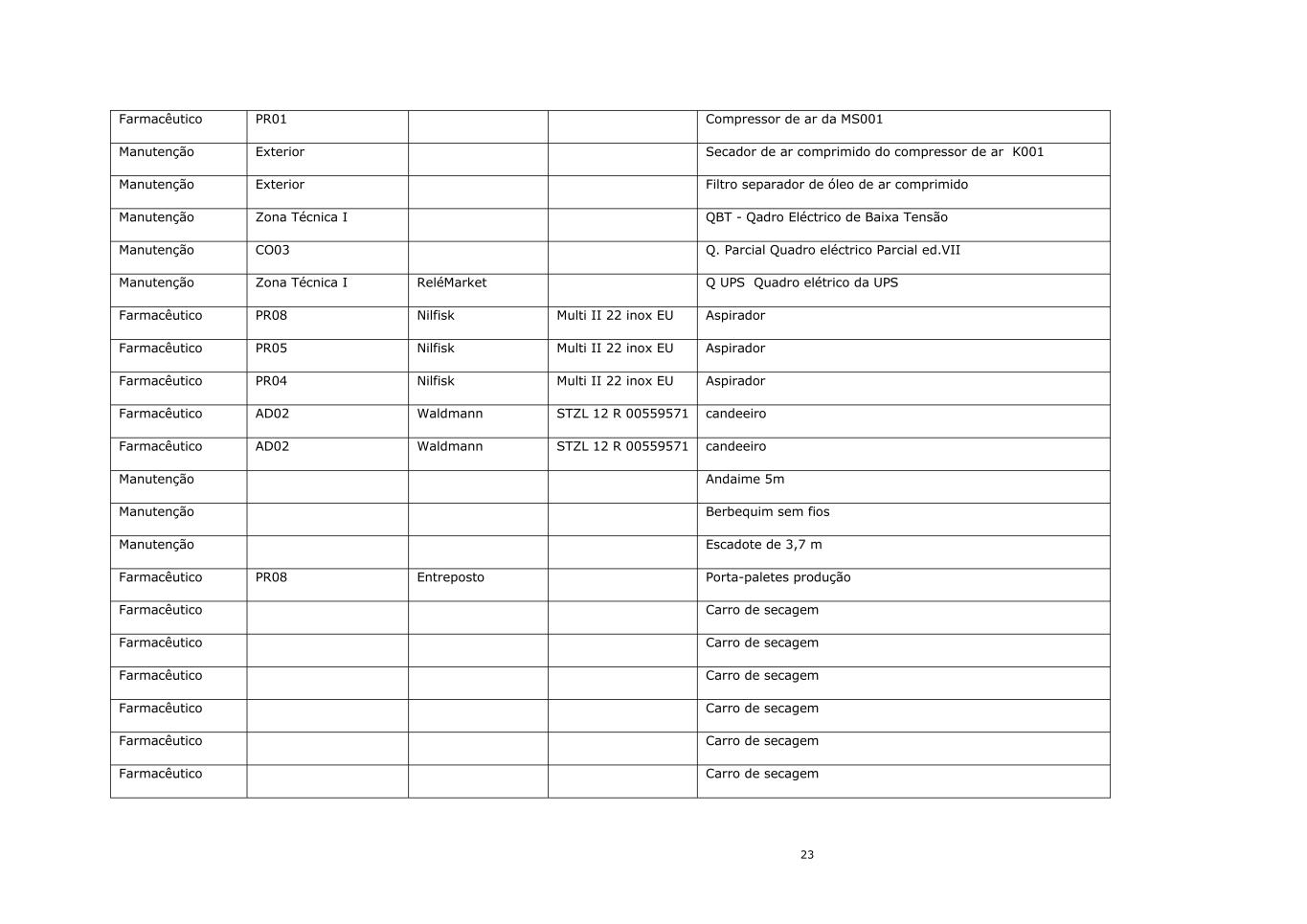
23 Farmacêutico PR01 Compressor de ar da MS001 Manutenção Exterior Secador de ar comprimido do compressor de ar K001 Manutenção Exterior Filtro separador de óleo de ar comprimido Manutenção Zona Técnica I QBT - Qadro Eléctrico de Baixa Tensão Manutenção CO03 Q. Parcial Quadro eléctrico Parcial ed.VII Manutenção Zona Técnica I ReléMarket Q UPS Quadro elétrico da UPS Farmacêutico PR08 Nilfisk Multi II 22 inox EU Aspirador Farmacêutico PR05 Nilfisk Multi II 22 inox EU Aspirador Farmacêutico PR04 Nilfisk Multi II 22 inox EU Aspirador Farmacêutico AD02 Waldmann STZL 12 R 00559571 candeeiro Farmacêutico AD02 Waldmann STZL 12 R 00559571 candeeiro Manutenção Andaime 5m Manutenção Berbequim sem fios Manutenção Escadote de 3,7 m Farmacêutico PR08 Entreposto Porta-paletes produção Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem

24 Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Farmacêutico Carro de secagem Manutenção Zona Técnica II Sandometal eSDM 290/635 UTA extracção WR01 Manutenção Zona Técnica I ReléMarket Quadro Elétrico UPS Manutenção Zona Técnica I ReléMarket Quadro Elétrico Siemens Manutenção Zona Técnica I ReléMarket Quadro Elétrico Desenfumagem Manutenção Sala Caldeiras ReléMarket 025/21 Quadro Elétrico AQS Segurança CO05 Carretel RIA CO05 Segurança CO05 Carretel RIA CO05 Segurança CO03 Carretel RIA CO03 Segurança CO04 Carretel RIA CO04 Segurança WH03 Carretel RIA WH03 Segurança PR12 Carretel RIA PR12

25 Segurança PR12 Carretel RIA PR12 Segurança AD01 Carretel RIA AD01 Segurança Extintores Segurança AD04 Central de Incêndios QC LB01 BOMBA P001 ON QC LB01 BOMBA P002 ON QC LB03 Braço extração (LB03) QC LB04 Braço extração (LB04) QC LB01 Hotte (LB01) QC LB01 Campanula de extração (LB01) Farmacêutico PR01 Marques MSC-LC 5000gr Div 0,01 Balança Farmacêutico PR06 Marques MC SS 40 6/15Kg Balança Farmacêutico PR11 Marques MC SS 40 3/6Kg Balança Farmacêutico PR08 Marques MC SS 40 3/6Kg Balança Farmacêutico PR01 Marques MC SS 40 3/6Kg Balança

SCHEDULE 1 2. QUALITY DOCUMENTAL SYSTEM – LIST OF DOCUMENTS • Site Master File; • Standard Operational Procedures; • available validation protocols and reports; • Batch records masters; • Available Manufacturing instructions, operating and instruction manuals and other supplier or manufacturer documentation; • Analytical methods and specifications; • Available IQ/OQ/PQ protocols and reports; • Available Supplier Qualification; • CAPA Plans; • Deviation Reports; • Available Calibration and Maintenance Protocols. All documents are available at Clever Leaves sharepoint or in paper format at the Division. 3. SOFTWARE BMS system

SCHEDULE 2 REPRESENTATIONS AND WARRANTIES The Seller warrants and represents to the Purchaser the following: 1. Capacity and authority 1.1. Right, power, authority and action The Seller has the right, power and authority, and have taken all action necessary, to execute, deliver and exercise its rights, and perform its obligations, under this Agreement and each document to be executed at the Signature Date. 1.2. Binding agreements The Seller’s obligations under this Agreement and each document to be executed at the Signature Date in terms hereof are, or when the relevant document is executed will be, enforceable in accordance with their terms. 1.3 No Government Authorization Required No consent, approval, authorization or order of, or qualification with, any court, regulatory authority or other governmental body is required for the consummation by Seller of the transaction contemplated by this Agreement. 1.4 Effect of Agreement The execution, delivery and performance of this Agreement and the consummation of the transactions contemplated hereby will not, with or without the giving of notice or the lapse of time or both, (a) violate any provision of law, statute, rule or regulation to which the Seller is subject; (b) violate any judgment, order, writ or decree of any court applicable to the Purchaser; or (c) result in the breach of, or conflict with, any term, covenant, condition or provision of, result in the modification or termination of, constitute a default under, any corporate charter, by-law, commitment, contract or other agreement or instrument to which the Seller is a party. 2. Assets 2.1. Title and condition (a) The rights in respect of the Sale Assets are: (i) legally and beneficially owned solely by the Seller free from any Encumbrance; and (ii) where capable of possession, in the possession or under the control of the Seller. (b) No person or entity has any right (whether pursuant to any option, right of first refusal or otherwise) to purchase or acquire (whether as security or otherwise) the Sale Assets and no person or entity has brought any claim in relation to any of the Sale Assets,

28 including but not limited to any claim that may affect or impact on the transfer of the Sale Assets to the Purchaser, on the Seller’s obligations under this contract, on the full and free enjoyment of the Sale Assets by the Purchaser, and or on the economic and functional value of the Sale Assets; (c) All the Sale Assets being sold in accordance with the terms of this Agreement are the only assets required by the Purchaser; (d) The Seller is aware that the Purchaser intends to use the site and the Sale Assets as a GMP cannabis manufacturing facility and confirms to the Purchaser that no changes or removals took place in the site or were made to the Sales Assets after the GMP Inspection. The equipment included in the Sale Assets is not currently in operation but was appropriately decommissioned by the Seller and was working properly and according to its design specifications before being decommissioned. The equipment is fit for its purpose, functional, complete, and in good condition, and is ready to be used provided it is re-commissioned by the Purchaser in line with the respective operating instructions and specifications. 3. Intellectual property 3.1. Each of the Intellectual Property rights in respect of the Quality Documental System is: (a) valid and enforceable and nothing has been done or omitted to be done by which it may cease to be valid and enforceable; (b) legally and beneficially owned by, and validly granted to, the Seller alone, free from any licence, Encumbrance, restriction on use or disclosure obligation; and (c) not, and will not be, the subject of a claim or opposition from a person (including, without limitation, an employee of the Sellers) as to title, validity, enforceability, entitlement or otherwise. 3.2. The Seller have not infringed any third party’s Intellectual Property or render the Sellers liable to an action in respect of the infringement of any Intellectual Property belonging to a third party or to the Purchaser, and there is and during the two years ending on the date of this Agreement has been, no civil, criminal, arbitration, administrative or other proceeding or dispute in any jurisdiction concerning any of the Intellectual Property. No civil, criminal, arbitration, administrative or other proceeding or dispute concerning any of the Intellectual Property is pending or threatened. To the best of the Sellers’ knowledge, information and belief, no fact or circumstance exists which might give rise to a proceeding of that type. 4. Contracts 4.1. The contracts concluded by the Seller with third parties in respect of the Division, that are not being assigned or novated to the Purchaser have been: (i) disclosed to the Seller as part of the Information; and (b) validly terminated on or prior to the Signature Date such that Purchaser shall have no liability of any kind arising in respect of such contracts. 5. Blue Biz Lease 5.1. The Seller entered into an agreement (“the Blue Biz Lease”) on 17 March 2021 with “Aicep Global Parques - Gestão de Áreas Empresariais e Serviços, S. A.” (“Global Parques”), the owner

29 of the building where the Division is located at Edifício 7, Blue Biz Parque, Estrada da Rosa, in Setubal, Portugal (“the Premises”), for the lease and use of such Premises. The Seller warrants to the Purchaser that (i) the Blue Biz Lease terminated on the day before Signature Date lawfully and with the confirmation of Global Parques; (ii) Global Parques has authorised that the Company Assets remain in the premises so that the Purchaser will take possession of the Company Assets there on Signature Date; (iii) the Seller has fully complied with the Blue Biz Lease terms and the applicable law, has no outstanding obligations in relation to the premises namely, but not limited to, repairs and maintenance, and has paid all amounts due to Global Parques or to any third parties in relation to the premises. 5.2. The Seller expressly undertakes to indemnify and hold harmless the Purchaser in respect of any claim from any person or entity in connection to the premises related to the period prior to Signature Date. Limitations provided for in clause 7.2 shall not apply in this context. 6. Employees 6.1. All employees working for the Seller, of which 17 working at the Division, had their contracts with the Seller lawfully and regularly terminated before signature of this Agreement, except for employees Marta Maria Reynaud Pinto de Leite de Areia and Gonçalo Bruno de Almeida Gonçalves who remaining working for several entities of the Seller’s group. 6.2. No issues have been raised or claims have been brought by the authorities in respect of the termination of the employees’ contracts, or by employees, except for two employees, Ana Paula Mendes Paias and Rodrigo Filipe Rafael João, who were not working at the Division, but rather at the Seller’s agricultural site in Odemira which is unrelated to this Asset Sale Agreement. There is a court case underway brought by these two employees, but Seller warrants to Purchaser that this shall have no impact or consequences to the Purchaser or to the present Asset Sale Agreement. 6.3. This Transaction does not involve any transfer of employees to the Purchaser, given that all employees have been lawfully and regularly dismissed prior to signature of this Agreement in collective dismissal procedures which were fully legal and endorsed by the competent labour authority, all salaries and compensations were fully paid to the employees, all employees have signed the respective agreement and have stated in writing that they renounce to all credits they may have against the employer, and the procedure of transfer of employees provided for in articles 285 ff of the Labour Code is not applicable to the present Transaction considering inter alia that it does not consist in a transfer of a company, economic unit or business, but merely on a transfer of assets, and the employees were dismissed prior and regardless of this Transaction. 6.4. In any case, the Seller expressly undertakes to indemnify and hold harmless the Purchaser in respect of any claim brought against the Purchaser by any person who has been an employee (or service provider equivalent to employee) of the Purchaser or by any authority in respect of any such employee(s) or related to the dismissal or to an alleged right of transfer of such employee(s); the Seller shall indemnify the Purchaser for any Losses in relation thereto and the limitations provided for in clause 7.2 shall not apply. In case any employees have to be

30 reintegrated as employees by the Purchaser, the Parties hereby agree that the appropriate compensation for such damage shall be the payment by the Seller to the Purchaser of an amount of EUR 50,000.00 per employee, in addition to indemnification for all other Losses suffered by the Seller in relation to such employees (including but not limited to salaries and any compensations due to the employee in connection to his employment with the Seller and his dismissal by the Seller). The Purchaser warrants and represents to the Seller the following: 7. Capacity and authority 7.1. Right, power, authority and action (a) The Purchaser has the right, power and authority, and has taken all action necessary, to execute, deliver and exercise its rights, and perform its obligations, under this Agreement and each document to be executed at the Signature Date. (b) The Purchaser’s obligations under this Agreement and each document to be executed at the Signature Date in terms hereof are, or when the relevant document is executed will be, enforceable in accordance with their terms. 7.2. No Government Authorization Required No consent, approval, authorization or order of, or qualification with, any court, regulatory authority or other governmental body is required for the consummation by Purchaser of the transaction contemplated by this Agreement. 7.3. Effect of Agreement The execution, delivery and performance of this Agreement and the consummation of the transactions contemplated hereby will not, with or without the giving of notice or the lapse of time or both, (a) violate any provision of law, statute, rule or regulation to which the Purchaser is subject; (b) violate any judgment, order, writ or decree of any court applicable to the Purchaser; or (c) result in the breach of, or conflict with, any term, covenant, condition or provision of, result in the modification or termination of, constitute a default under, any corporate charter, by-law, commitment, contract or other agreement or instrument to which the Purchaser is a party. 7.4. Disclosure Purchaser acknowledges that it has been provided with the opportunity to visit the Division and assess the apparent characteristics and external appearance of the Sale Assets but not including a verification of their operation and proper functioning. 7.5. Terms of Sale

31 The Sale Assets are being sold to the Purchaser on an “as it was, where it was” basis at the date of the conclusion of the GMP inspection pursuant to which the GMP license has been awarded to the Seller by Infarmed. 7.6. Available funds The Purchaser has and will have available the necessary funds to conclude the Transaction hereunder, namely to the transfer of the Purchase Price to the Sellers’ Bank Account in exchange for the Sale Assets.

32 Stamp Tax provided for in item 10.2 of the General Chart of the Stamp Tax Code is assessed on this date by the Purchaser at the rate of 0.5% on the amount of the guarantee of clause 8.2. hereof. Notwithstanding, as provided by the Stamp Tax Code, the corresponding Stamp Tax burden should be borne by the Seller. SIGNATORIES In witness whereof For and on behalf of Clever Leaves Portugal Unipessoal Lda (as Seller) ______________________________ Signatory: Marta Pinto Leite Capacity: Director For and on behalf of Terra Verde, Lda (as Purchaser) ______________________________ Signatory: Nuno Mendonça Capacity: Director For and on behalf of Terra Verde, Lda (as Purchaser) ______________________________ Signatory: Zafrin Samsudin Capacity: Director Zafrin Samsudin (Jul 1, 2023 11:44 GMT+1) Zafrin Samsudin Nuno Mendonça (Jul 1, 2023 12:11 GMT+1) Nuno Mendonça

33 For and on behalf of Clever Leaves Holdings Inc. (as Guarantor) ______________________________ Signatory: Andres Fajardo Capacity: Director

APA - Clever Leaves - Terra Verde 30062023 FINAL VERSION Final Audit Report 2023-07-01 Created: 2023-06-30 By: Marta Pinto Leite (marta.pintoleite@cleverleaves.com) Status: Signed Transaction ID: CBJCHBCAABAAcxWdOJAcxgd8CKEEaMkBsdwyJD2p7crp "APA - Clever Leaves - Terra Verde 30062023 FINAL VERSION " History Document created by Marta Pinto Leite (marta.pintoleite@cleverleaves.com) 2023-06-30 - 8:15:17 PM GMT- IP address: 81.84.20.90 Document emailed to zafrin.samsudin@curaleafint.com for signature 2023-06-30 - 8:17:44 PM GMT Email viewed by zafrin.samsudin@curaleafint.com 2023-07-01 - 10:06:23 AM GMT- IP address: 104.47.30.126 Signer zafrin.samsudin@curaleafint.com entered name at signing as Zafrin Samsudin 2023-07-01 - 10:44:47 AM GMT- IP address: 78.137.231.100 Document e-signed by Zafrin Samsudin (zafrin.samsudin@curaleafint.com) Signature Date: 2023-07-01 - 10:44:49 AM GMT - Time Source: server- IP address: 78.137.231.100 Document emailed to nuno.mendonca@curaleafint.com for signature 2023-07-01 - 10:44:50 AM GMT Email viewed by nuno.mendonca@curaleafint.com 2023-07-01 - 10:56:07 AM GMT- IP address: 104.47.51.190 Signer nuno.mendonca@curaleafint.com entered name at signing as Nuno Mendonça 2023-07-01 - 11:11:31 AM GMT- IP address: 148.71.18.251 Document e-signed by Nuno Mendonça (nuno.mendonca@curaleafint.com) Signature Date: 2023-07-01 - 11:11:33 AM GMT - Time Source: server- IP address: 148.71.18.251 Agreement completed. 2023-07-01 - 11:11:33 AM GMT