0001862150
false
0001862150
2023-10-17
2023-10-17
0001862150
CING:CommonStockParValue0.0001PerShareMember
2023-10-17
2023-10-17
0001862150
CING:WarrantsExercisableForOneShareOfCommonStockMember
2023-10-17
2023-10-17
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported):
October
17, 2023
CINGULATE
INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
001-40874 |
|
86-3825535 |
| (State
or other jurisdiction |
|
(Commission |
|
(IRS
Employer |
| of
incorporation) |
|
File
Number) |
|
Identification
No.) |
1901
W. 47th Place
Kansas
City, KS 66205
(Address
of principal executive offices) (Zip Code)
(913)
942-2300
(Registrant’s
telephone number, including area code)
(Former
name or former address, if changed since last report.)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions (see General Instruction A.2. below):
| ☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| |
|
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of exchange on which registered |
| Common
Stock, par value $0.0001 per share |
|
CING |
|
The
Nasdaq Stock Market LLC
(Nasdaq
Capital Market) |
| Warrants,
exercisable for one share of common stock |
|
CINGW |
|
The
Nasdaq Stock Market LLC
(Nasdaq
Capital Market) |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405)
or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR §240.12b-2).
Emerging
growth company ☒
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item
7.01. Regulation FD Disclosure.
On
October 17, 2023, Cingualte Inc. (the “Company”) issued a press release announcing a key opinion leader event on October
23, 2023 at which ADHD, anxiety and the Company’s leading, late-stage asset CTx-1301 (dexmethylphenidate), including the
results from the Company’s Phase 3 adult efficacy and safety trial of CTx-1301 for ADHD, will be discussed. A copy of the press
release is attached hereto as Exhibit 99.1.
The
Company updated its investor presentation to be used at investor conferences and in investor meetings.
A copy of the investor presentation is furnished as Exhibit 99.2 and incorporated by reference.
The
information in this Current Report on Form 8-K under Item 7.01, including the information contained in Exhibits 99.1 and 99.2, is being
furnished to the Securities and Exchange Commission, and shall not be deemed to be “filed” for the purposes of Section 18
of the Exchange Act, or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference
into any filing under the Securities Act or the Exchange Act, except as shall be expressly set forth by a specific reference in such
filing.
Item
8.01. Other Events.
On
October 17, 2023, the Company issued a press release announcing a key opinion leader event on October 23, 2023 at which ADHD, anxiety
and the Company’s leading, late-stage asset CTx-1301 (dexmethylphenidate), including the results from the Company’s
Phase 3 adult efficacy and safety trial of CTx-1301 for ADHD, will be discussed.
Top-line
results for the Phase 3 adult trial were released on July 11, 2023 and included in the Company’s Quarterly Report on Form 10-Q
for the period ended June 30, 2023 and detailed trial results were presented on September 8, 2023
at the 36th Annual Psych Congress in Nashville, Tennessee and included in
the Company’s Current Report on Form 8-K filed with the SEC on September 11, 2023.
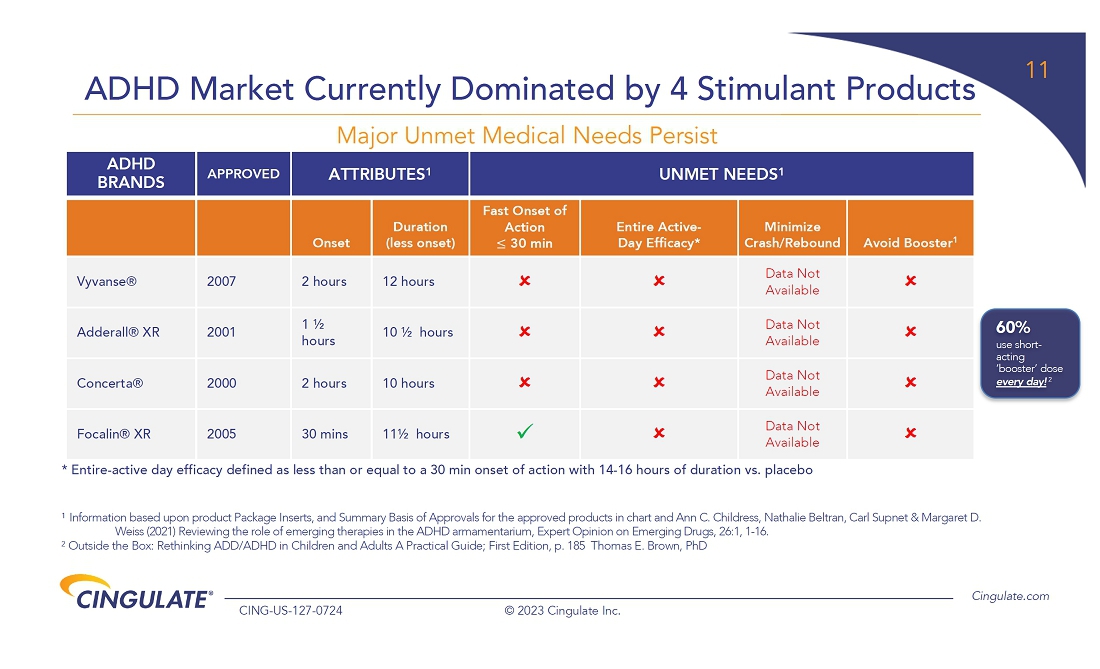
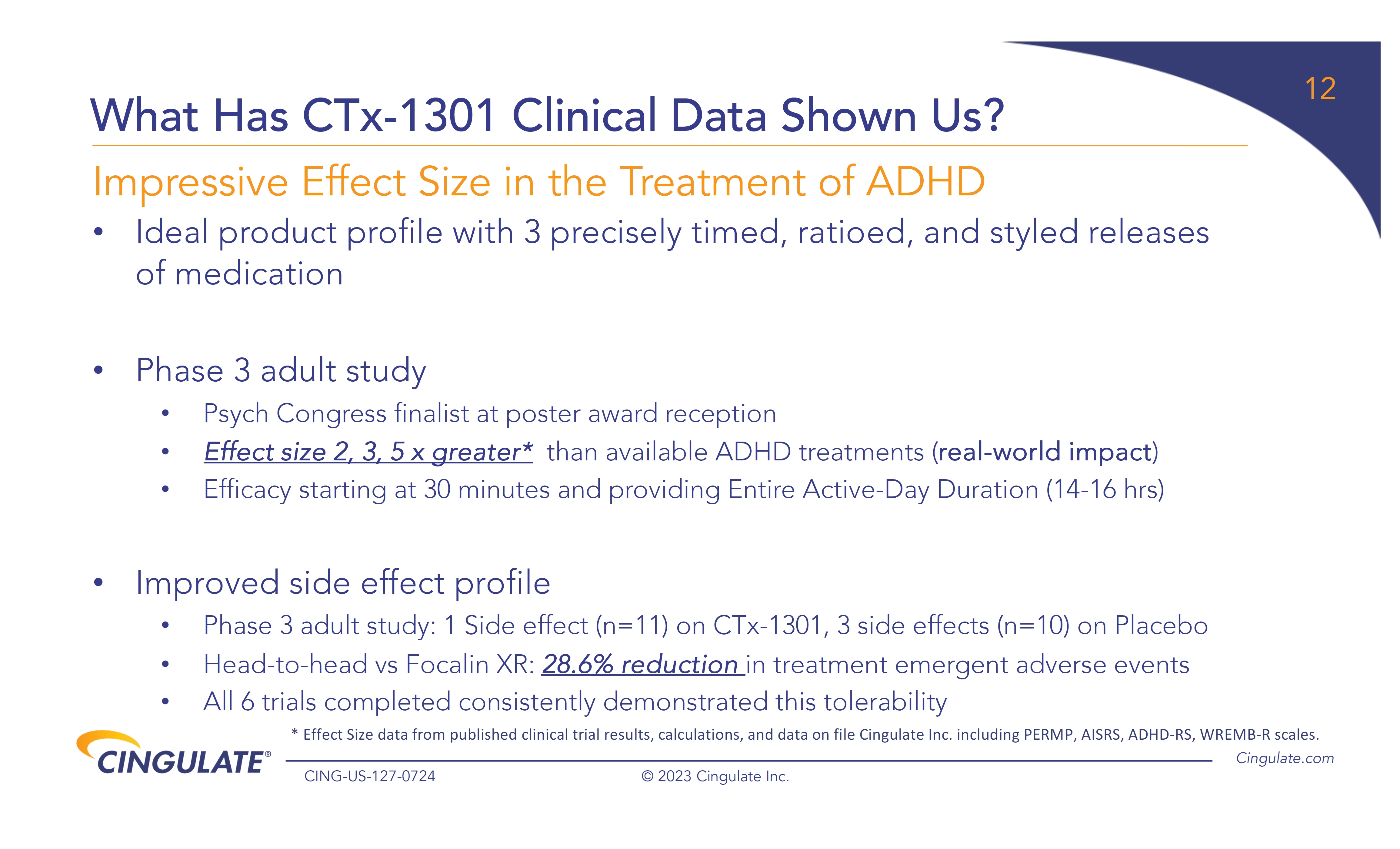
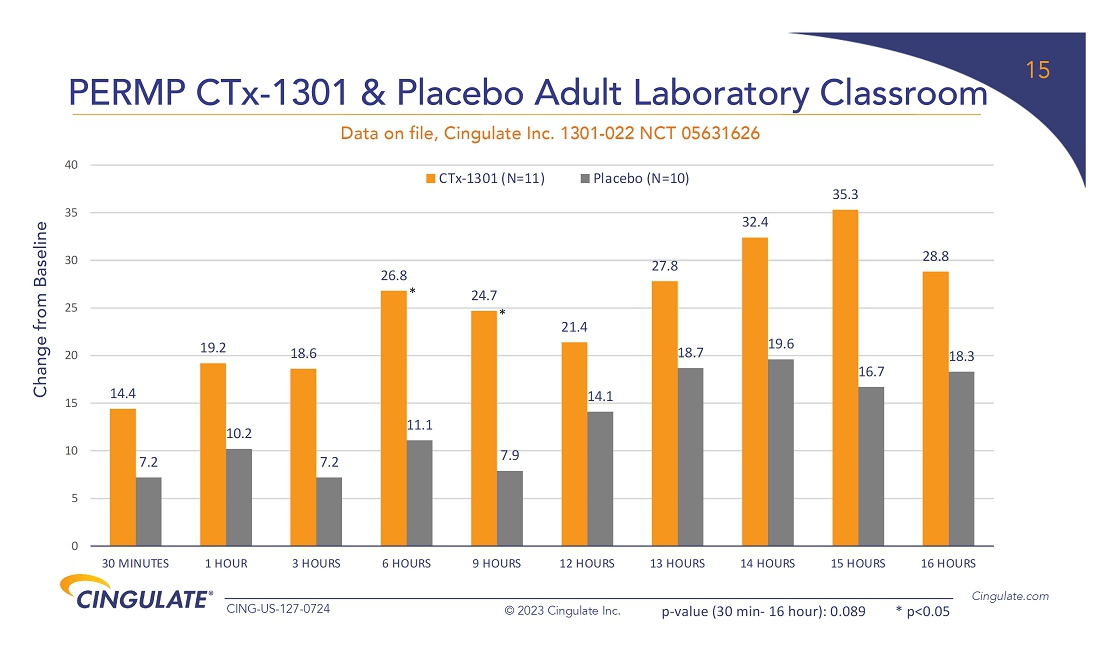
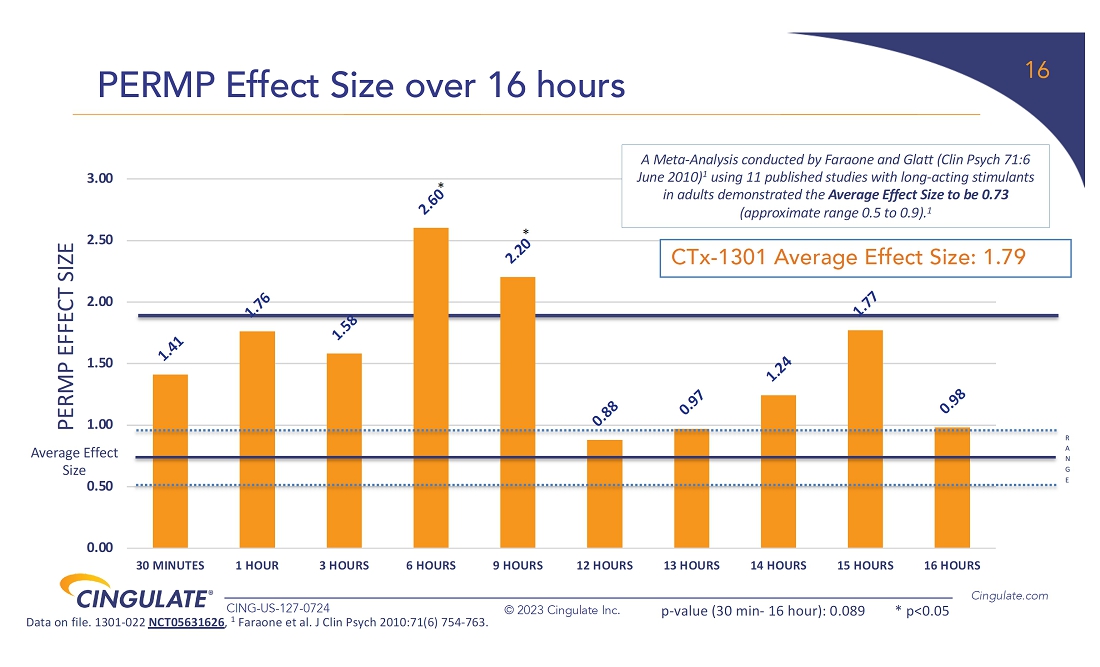
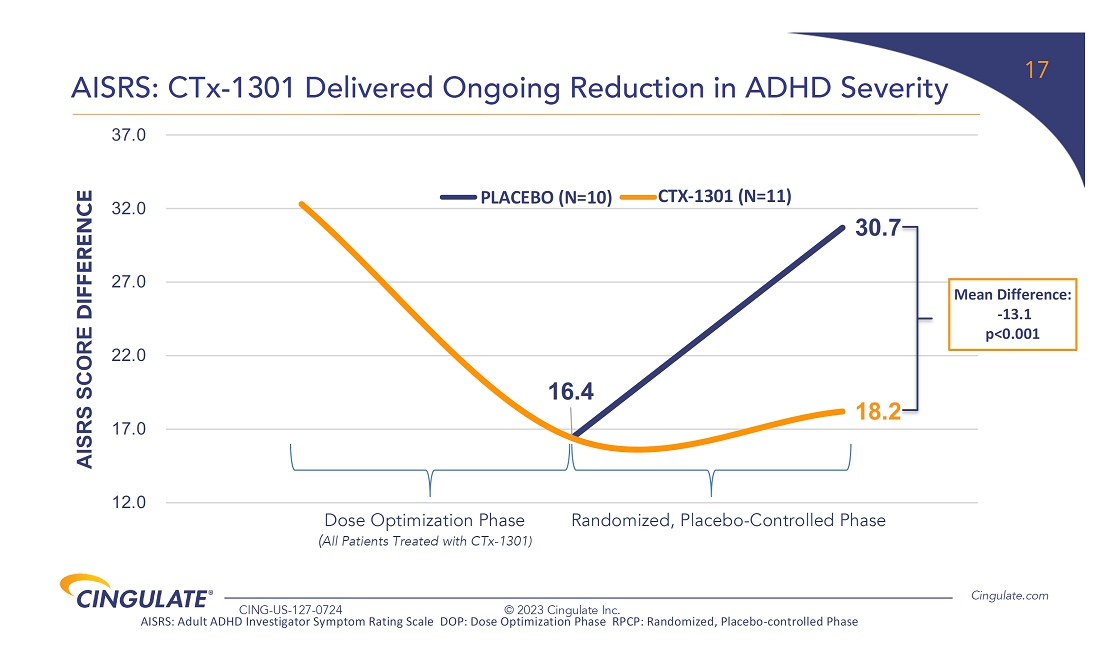
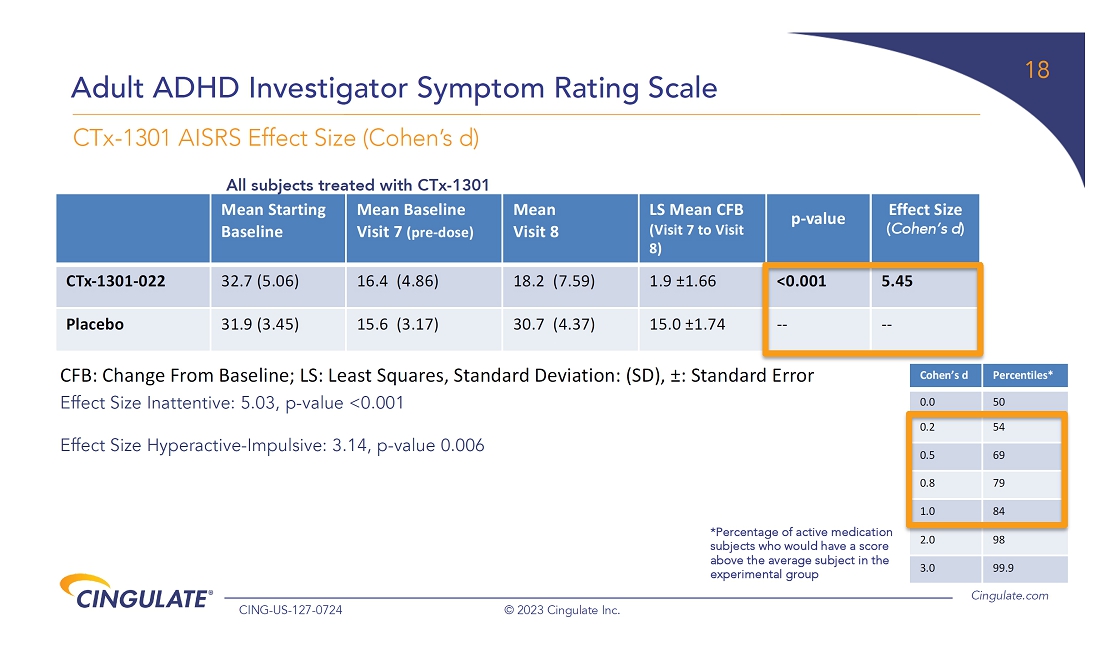
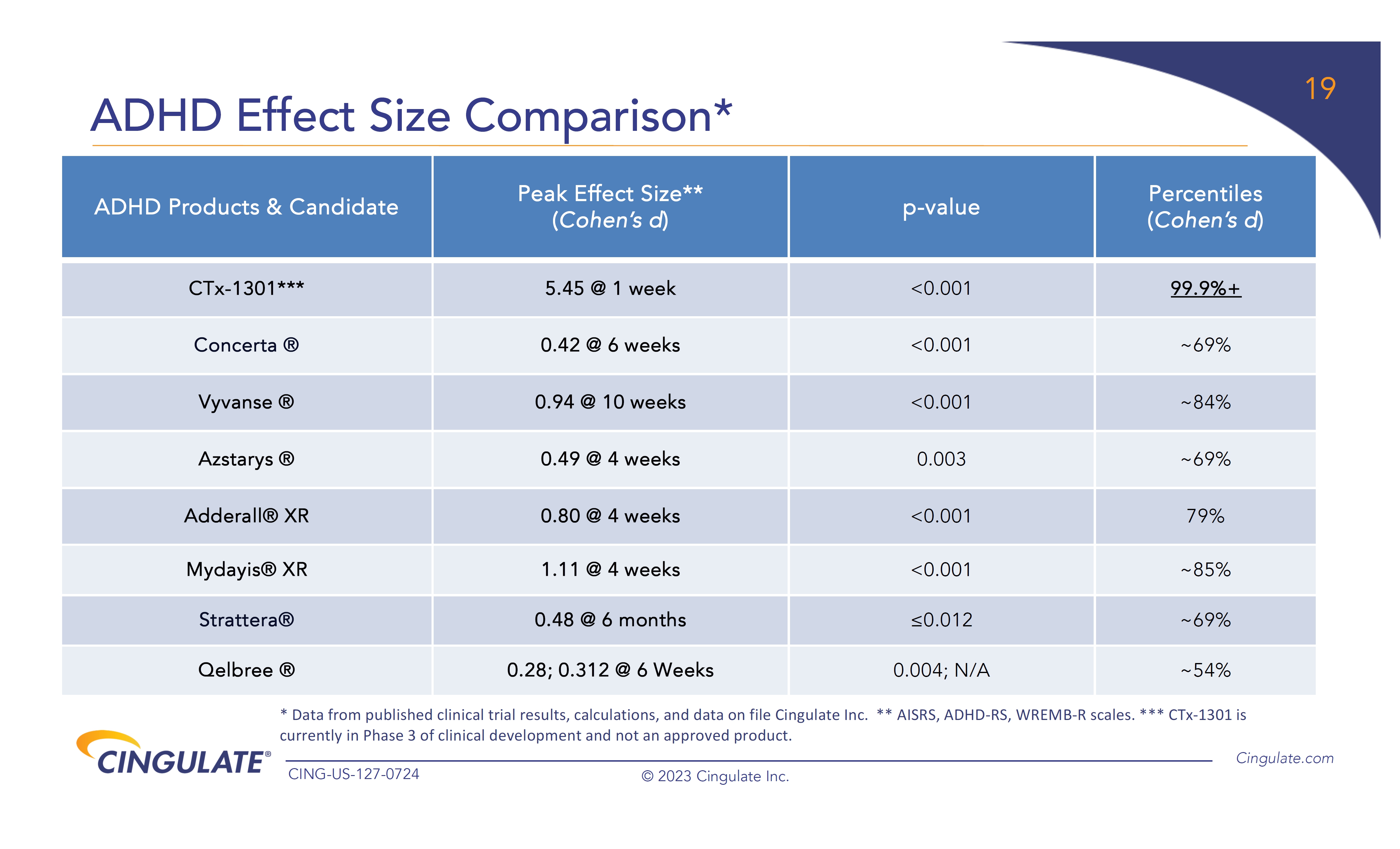
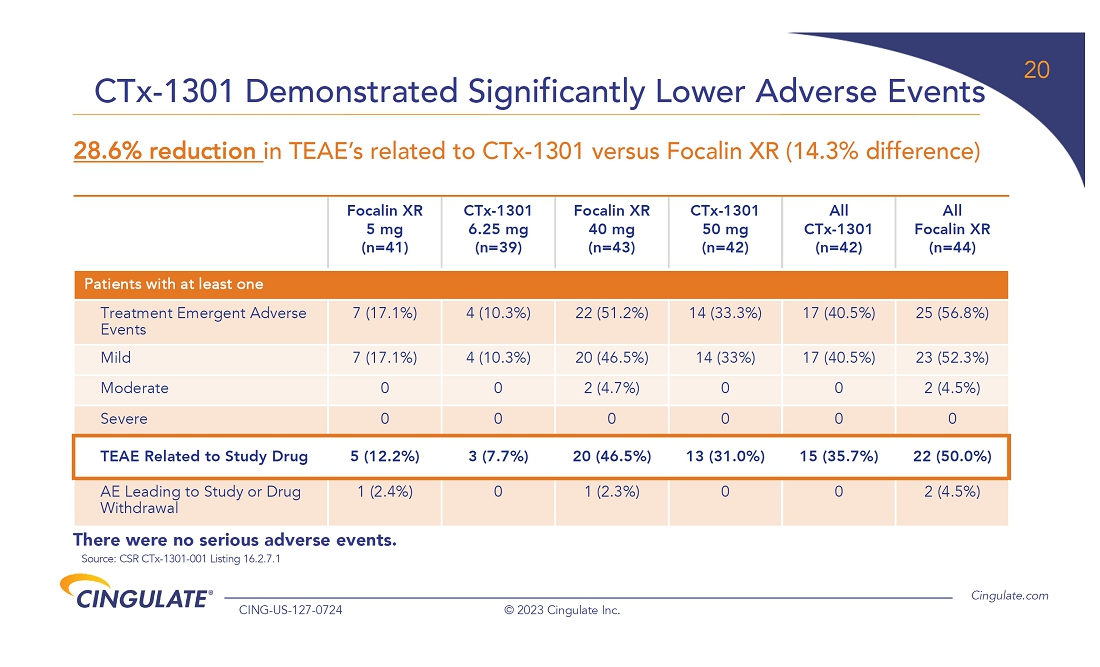
CTx-1301
demonstrated a reduction in Adult ADHD Investigator Symptom Rating Scale (AISRS) scores (-16.3) during the dose-optimization period.
During the randomized placebo controlled period, CTx-1301 demonstrated a reduction in AISRS scores (mean difference: -13.1) with
an effect size of 5.45 and a p-value of <0.001.
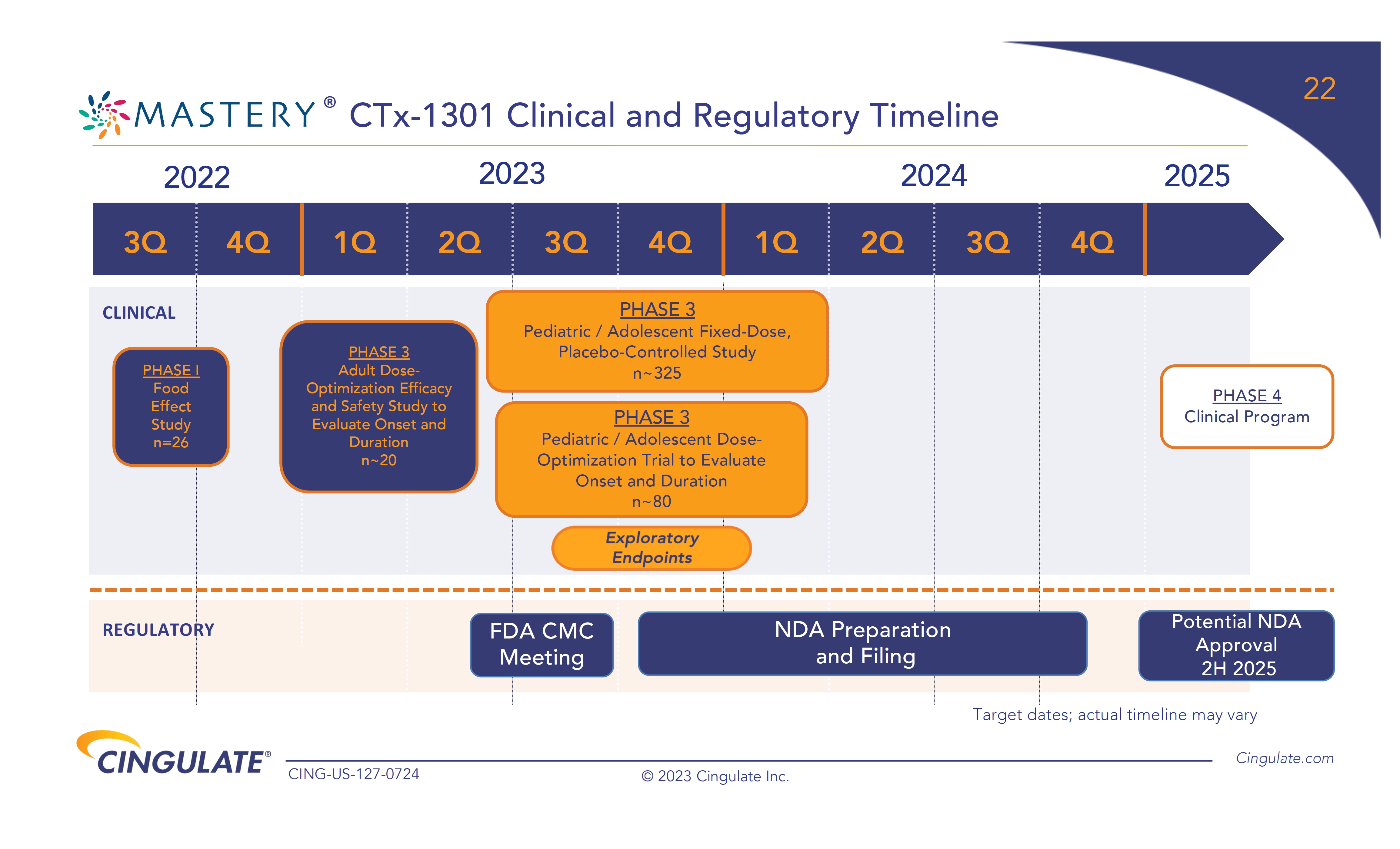
The
pivotal Phase 3 fixed-dose pediatric and adolescent safety and efficacy study and a Phase 3 pediatric dose-optimization onset and duration
study of CTx-1301 have commenced, with results expected in the first half of 2024.
Assuming
the Company receives positive clinical results from its Phase 3 trials, the Company expects to submit the NDA for CTx-1301 in the second
half of 2024 under the Section 505(b)(2) pathway.
The Company plans to initiate a Phase 1/2 bioavailability
study in ADHD patients for CTx-1302 (dextroamphetamine), its second investigational asset for the treatment of ADHD, in the second half
of 2024 and, if the results from this study are successful, subsequently initiate pivotal Phase 3 clinical trials in all patient segments
in 2025.
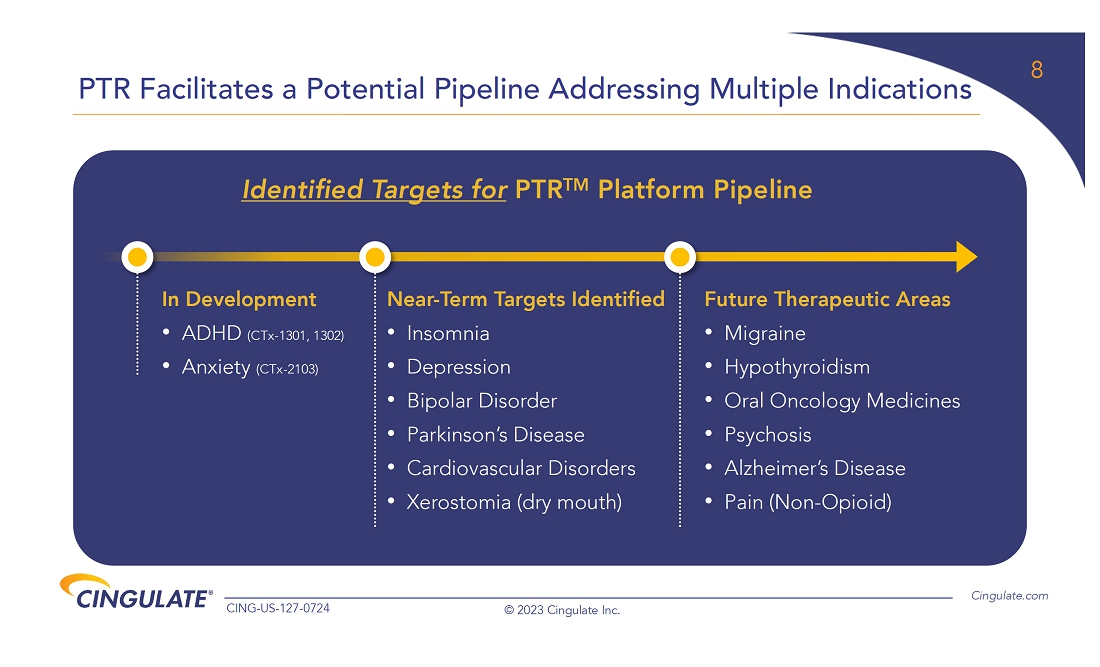
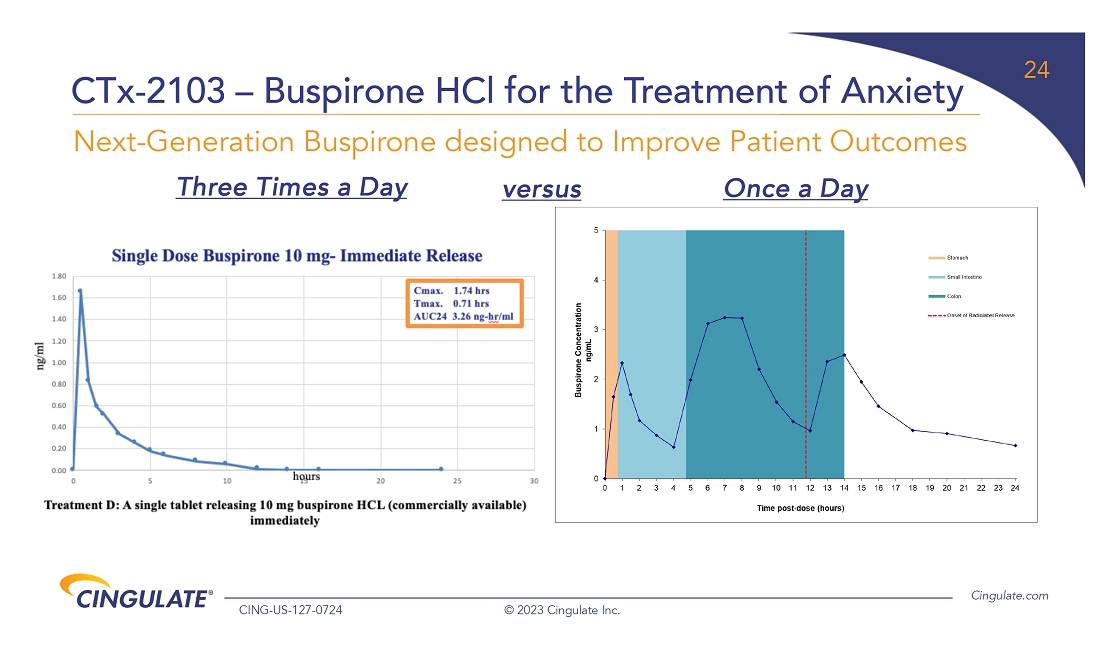
The Company has embarked on a program to develop CTx-2103 (buspirone) for
the treatment of anxiety, which is the most common mental health concern in the U.S. The Company completed a formulation study in which
the pharmacokinetics were evaluated for this trimodal tablet providing three precisely timed doses of buspirone versus one immediate release
dose. In addition, scintigraphic imaging visualized transit of the tablets through the gastrointestinal tract to confirm both the site
and onset of release, which will then be correlated with pharmacokinetic data to establish the full release profile of the CTx-2103 formulation.
Based on the pharmacokinetic profile seen in the data, CTx-2103 achieved the desired triple release of buspirone. These results provided
the critical information required to allow the Company to request a Pre-IND meeting with the FDA to discuss the design of its clinical
and regulatory program for CTx-2103, and the Company expects to receive feedback from the FDA in the fourth quarter of 2023 to allow for
a potential IND filing in the first half of 2024.
Item 9.01. Financial Statements and Exhibits.
(d)
Exhibits
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
| |
CINGULATE
INC. |
| |
|
|
| Dated:
October 19, 2023 |
By: |
/s/
Shane J. Schaffer |
| |
Name: |
Shane
J. Schaffer |
| |
Title: |
Chief
Executive Officer |
Exhibit
99.1
Cingulate
to Host CNS Key Opinion Leader Panel in New York City
Expert
Analysis of Cingulate, it’s Phase 3 ADHD Adult Data, Anxiety, and PTR™ Drug Delivery Platform Innovations
KANSAS
CITY, Kan., October 17, 2023 — Cingulate Inc. (NASDAQ: CING), a biopharmaceutical company utilizing its proprietary
Precision Timed Release™ (PTR™) drug delivery platform technology to build and advance a pipeline of next-generation pharmaceutical
products, announced today that it will be hosting a key opinion leader event on the morning of October 23, 2023, at the Union
League Club in New York City.
The
live-streamed event will be held from 10:00am-11:30am EST at the Union League Club in Midtown Manhattan, and will focus on the
Company’s leading, late-stage asset CTx-1301(dexmethylphenidate), along with ADHD and anxiety-related disorders. For those in the
New York area who would like to attend, there will be space for approximately 25-30 guests in the club’s Grant Room. Those who
wish to watch the event virtually may do so through the link provided here, with the passcode 429018.
What: Cingulate
Key Opinion Leader Panel
Where: Union
League Club (Grant Room)
39
East 37th Street, New York, NY 10016
When: Monday
October 23, 2023, 10am-11:30am EST
Attire:
The Union League Club requests that in-person attendees abide by club dress guidelines. For more information, please visit the club’s
website.
The
event may be added to calendars through the following links:
Add
to Calendar
Add
to Google Calendar
Cingulate’s
Chief Medical Officer, Matthew Brams, M.D., and Chief Science Officer, Raul Silva, M.D., will moderate the discussion.
The
panel will include Ann Childress, M.D., President, Center for Psychiatry and Behavior Medicine, Inc., and lead investigator of Cingulate’s
recently completed Phase 3 adult dose-optimization study, as well as Greg Mattingly, M.D., Founding Partner, St. Charles Psychiatric
Associates.
“The
Cingulate team thanks Dr. Childress and Dr. Mattingly for their participation in this event. We believe this will be an excellent opportunity
for healthcare providers, patients, advocacy groups, and the investor community to hear the Cingulate story and take a deeper look at
our most recent Phase 3 data with two of the top key opinion leaders in ADHD and CNS disorders,” said Cingulate Chairman &
CEO Shane J. Schaffer.
About
Attention Deficit/Hyperactivity Disorder (ADHD)
ADHD
is a chronic neurobiological and developmental disorder that affects millions of children often continues into adulthood. The condition
is marked by an ongoing pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning or development. In the
U.S., approximately 6.4 million children and adolescents (11 percent) aged under the age of 18 have been diagnosed with ADHD. Among this
group, approximately 80 percent receive treatment, with 65-90 percent demonstrating clinical ADHD symptoms that persist into adulthood.
Adult ADHD prevalence is estimated at approximately 11 million patients (4.4 percent), almost double the size of the child and adolescent
segment combined, however, only an estimated 20 percent receive treatment.
About
Anxiety
Anxiety
disorders are the most common mental health concern in the U.S.1 Anxiety is the feeling of fear that occurs when faced
with threatening or stressful situations or can be endogenous and not have an identified stressor. It can be a normal response when confronted
with danger, but, if severe and chronic and affects functioning, it could be regarded as an anxiety disorder. An estimated 31 percent
of U.S. adults experience an anxiety disorder at some time in their lives.2 People may live with anxiety for years before
they are diagnosed or treated. The global COVID-19 crisis has exacerbated the diagnosis and treatment of anxiety and anxiety related
disorders and as a result is a priority within the class of unmet medical needs in mental health.
About
CTx-1301
Cingulate’s
lead candidate, CTx-1301, utilizes Cingulate’s proprietary PTR drug delivery platform to create a breakthrough, multi-core formulation
of the active pharmaceutical ingredient dexmethylphenidate, a compound approved by the FDA for the treatment of ADHD. Dexmethylphenidate
is part of the stimulant class of medicines and increases norepinephrine and dopamine activity in the brain to affect attention and behavior.
While stimulants are the gold-standard of ADHD treatment due to their efficacy and safety, the long-standing challenge continues to be
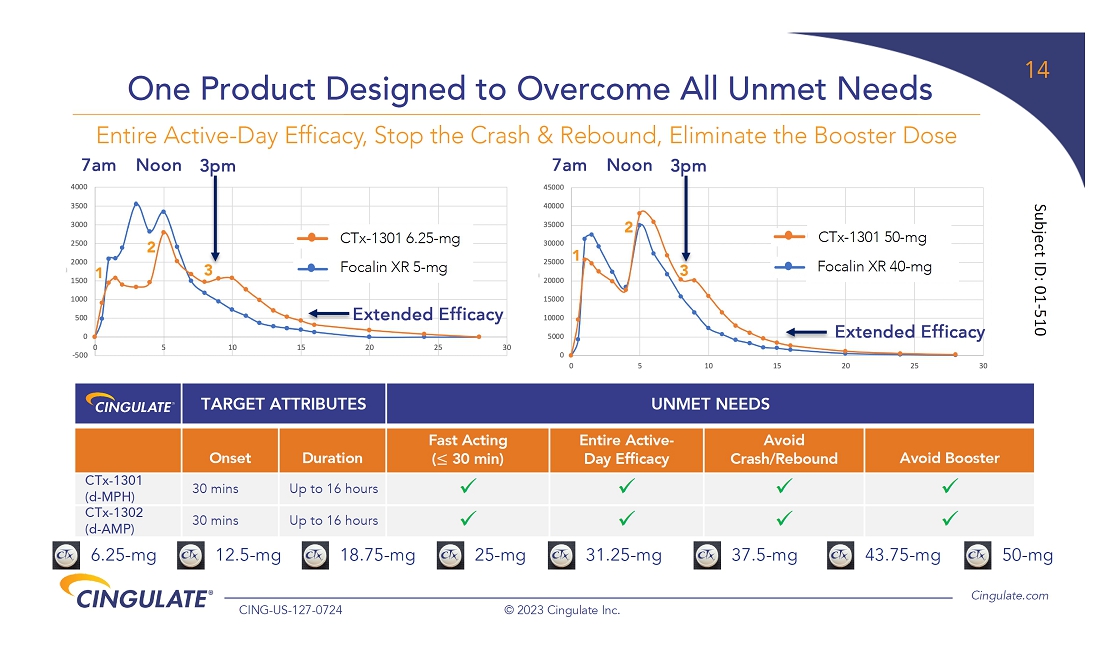
providing patients entire active-day duration of action. CTx-1301 is designed to precisely deliver three releases of medication at the
predefined time, ratio, and style of release to optimize patient care in one tablet. The result is a rapid onset and entire active-day
efficacy, with the third dose being released around the time when other extended-release stimulant products begin to wear off.
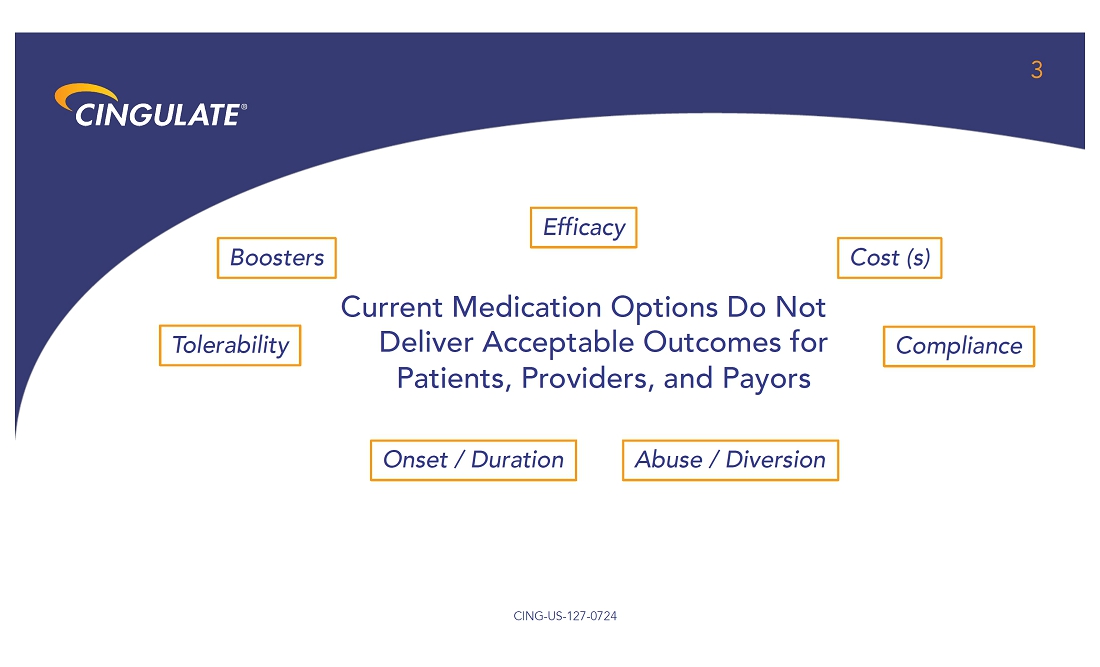
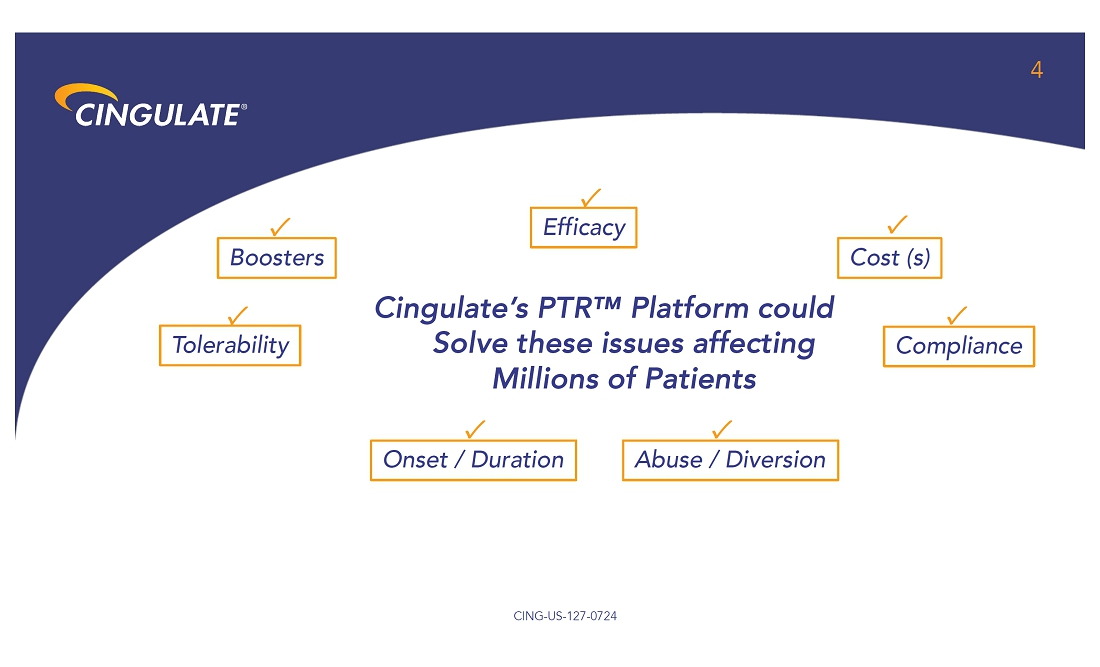
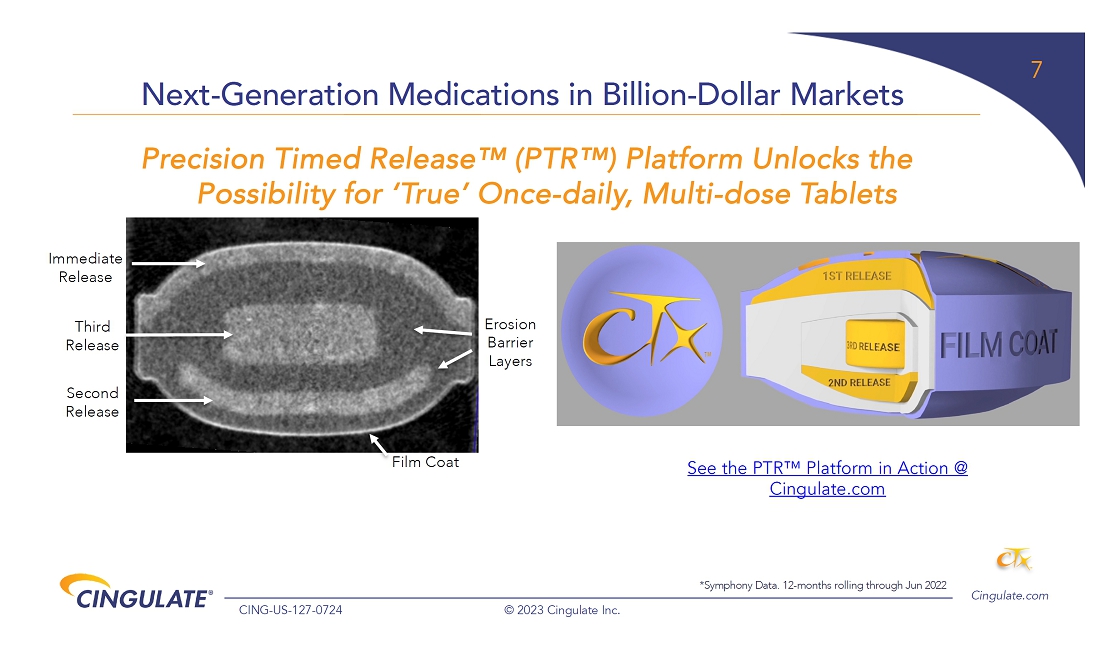
About
Precision Timed Release™ (PTR™) Platform Technology
Cingulate
is developing ADHD and anxiety disorder product candidates capable of achieving true once-daily dosing using Cingulate’s innovative
PTR drug delivery platform technology. It incorporates a proprietary Erosion Barrier Layer (EBL) providing control of drug release at
precise, pre-defined times with no release of drug prior to the intended release. The EBL technology is enrobed around a drug-containing
core to give a tablet-in-tablet dose form. It is designed to erode at a controlled rate until eventually the drug is released from the
core tablet. The EBL formulation, Oralogik™, is licensed from BDD Pharma. Cingulate intends to utilize its PTR technology to expand
and augment its clinical-stage pipeline by identifying and developing additional product candidates in other therapeutic areas in addition
to Anxiety and ADHD where one or more active pharmaceutical ingredients need to be delivered several times a day at specific, predefined
time intervals and released in a manner that would offer significant improvement over existing therapies. To see Cingulate’s PTR
Platform click here.
About
Cingulate Inc.
Cingulate
Inc. (NASDAQ: CING), is a biopharmaceutical company utilizing its proprietary PTR drug delivery platform technology to build and advance
a pipeline of next-generation pharmaceutical products, designed to improve the lives of patients suffering from frequently diagnosed
conditions characterized by burdensome daily dosing regimens and suboptimal treatment outcomes. With an initial focus on the treatment
of ADHD, Cingulate is identifying and evaluating additional therapeutic areas where PTR technology may be employed to develop future
product candidates, including to treat anxiety disorders. Cingulate is headquartered in Kansas City. For more information visit Cingulate.com.
Forward-Looking
Statements
This
press release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended,
and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include all statements, other than
statements of historical fact, regarding our current views and assumptions with respect to future events regarding our business, including
statements with respect to our plans, assumptions, expectations, beliefs and objectives with respect to product development, clinical
studies, clinical and regulatory timelines, market opportunity, competitive position, business strategies, potential growth opportunities
and other statements that are predictive in nature. These statements are generally identified by the use of such words as “may,”
“could,” “should,” “would,” “believe,” “anticipate,” “forecast,”
“estimate,” “expect,” “intend,” “plan,” “continue,” “outlook,”
“will,” “potential” and similar statements of a future or forward-looking nature. Readers are cautioned that
any forward-looking information provided by us or on our behalf is not a guarantee of future performance. Actual results may differ materially
from those contained in these forward-looking statements as a result of various factors disclosed in our filings with the Securities
and Exchange Commission (SEC), including the “Risk Factors” section of our Annual Report on Form 10-K filed with the SEC
on March 10, 2023. All forward-looking statements speak only as of the date on which they are made, and we undertake no duty to update
or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except to the extent required
by law.
Investor
Relations:
Thomas
Dalton
Vice
President, Investor & Public Relations, Cingulate
tdalton@cingulate.com
(913)
942-2301
Matt
Kreps
Darrow
Associates
mkreps@darrowir.com
(214)
597-8200
Media
Relations
Melyssa
Weible
Elixir
Health Public Relations
mweible@elixirhealthpr.com
(201)
723-5805
Exhibit 99.2













v3.23.3
Cover
|
Oct. 17, 2023 |
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Oct. 17, 2023
|
| Entity File Number |
001-40874
|
| Entity Registrant Name |
CINGULATE
INC.
|
| Entity Central Index Key |
0001862150
|
| Entity Tax Identification Number |
86-3825535
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
1901
W. 47th Place
|
| Entity Address, City or Town |
Kansas
City
|
| Entity Address, State or Province |
KS
|
| Entity Address, Postal Zip Code |
66205
|
| City Area Code |
(913)
|
| Local Phone Number |
942-2300
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
true
|
| Elected Not To Use the Extended Transition Period |
false
|
| Common Stock, par value $0.0001 per share |
|
| Title of 12(b) Security |
Common
Stock, par value $0.0001 per share
|
| Trading Symbol |
CING
|
| Security Exchange Name |
NASDAQ
|
| Warrants, exercisable for one share of common stock |
|
| Title of 12(b) Security |
Warrants,
exercisable for one share of common stock
|
| Trading Symbol |
CINGW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=CING_CommonStockParValue0.0001PerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=CING_WarrantsExercisableForOneShareOfCommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Cingulate (NASDAQ:CING)
Historical Stock Chart
From Oct 2024 to Nov 2024

Cingulate (NASDAQ:CING)
Historical Stock Chart
From Nov 2023 to Nov 2024
