|
PROSPECTUS SUPPLEMENT
To Prospectus, dated February 13, 2019
|
|
Filed pursuant to Rule 424(b)(5)
Registration File No. 333-226905
|
PALATIN
TECHNOLOGIES, INC.
Up
to $40,000,000
Common
Stock
We have entered
into an equity distribution agreement with Canaccord Genuity LLC,
or Canaccord, as sales agent, relating to shares of our common
stock, $0.01 par value per share, offered by this prospectus
supplement and the accompanying prospectus. In accordance with the
terms of the equity distribution agreement, we may offer and sell
shares of our common stock from time to time up to an aggregate
offering price
of $40,000,000 through Canaccord.
Upon our delivery
of a placement notice and subject to the terms and conditions of
the equity distribution agreement, Canaccord may sell the common
stock by methods deemed to be an “at the market”
offering as defined in Rule 415 promulgated under the Securities
Act of 1933, as amended, or the Securities Act, including sales
made directly on the NYSE American, on any other existing trading
market for the common stock or to or through a market maker other
than on an exchange. In addition, with our prior written approval,
Canaccord may also sell the common stock by any other method
permitted by law, including in privately negotiated transactions.
Canaccord is not required to sell any specific number or dollar
amount of our common stock, but will use its commercially
reasonable efforts, as our sales agent and subject to the terms of
the equity distribution agreement, to sell the shares of common
stock offered, as instructed by us and applicable state and federal
laws, rules and regulations and the rules of the NYSE American.
There is no arrangement for funds to be received in any escrow,
trust or similar arrangement.
We will pay
Canaccord a fixed commission, or allow a discount, for its services
in acting as agent in the sale of common stock equal to 3.0% of the
gross sales price per share of all shares sold through it as agent
under the equity distribution agreement. See “Plan of
Distribution” for information relating to certain expenses of
the sales agent to be reimbursed by us.
In connection with
the sale of common stock on our behalf, Canaccord may be deemed to
be an “underwriter” within the meaning of the
Securities Act and the compensation to Canaccord will be deemed to
be underwriting commissions or discounts. We have also agreed to
provide indemnification and contribution to Canaccord with respect
to certain liabilities, including liabilities under the Securities
Act.
The net proceeds we
receive from any sales under this prospectus supplement will be the
gross proceeds from such sales less the commissions and any other
costs we may incur in offering the common stock. See “Use of
Proceeds” and “Plan of Distribution” for
additional information.
Our common stock is
traded on the NYSE American under the symbol “PTN.” On
June 18, 2019, the reported closing price of the common stock was $
1.31 per share.
Investing
in our common stock involves a high degree of risk. You should
purchase our common stock only if you can afford a complete loss of
your investment. See “Risk Factors” beginning on page
S-5 of this prospectus supplement and page 5 of the accompanying
prospectus, as well as the information under the caption
“Risk Factors” in our Annual Report on Form 10-K for
the year ended June 30, 2018 and in the other documents
incorporated by reference into this prospectus supplement and the
accompanying prospectus for a discussion of the factors you should
carefully consider before investing in our common
stock.
Neither
the U.S. Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these
securities or determined if this prospectus supplement and the
accompanying prospectus is truthful or complete. Any representation
to the contrary is a criminal offense.
Canaccord Genuity
The
date of this prospectus supplement is June 21,
2019
TABLE OF CONTENTS
|
|
|
|
Prospectus Supplement
|
|
|
Page
|
|
About
this Prospectus Supplement
|
S-i
|
|
Prospectus
Supplement Summary
|
S-1
|
|
Risk
Factors
|
S-5
|
|
Cautionary
Note Concerning Forward-Looking Statements
|
S-8
|
|
Use of
Proceeds
|
S-10
|
|
Dilution
|
S-11
|
|
Dividend
Policy
|
S-12
|
|
Material
U.S. Federal Income Tax Consequences to Non-U.S.
Holders
|
S-13
|
|
Plan of
Distribution
|
S-16
|
|
Legal
Matters
|
S-17
|
|
Experts
|
S-17
|
|
Where
You Can Find More Information
|
S-17
|
|
Incorporation
of Information by Reference
|
S-18
|
|
|
|
|
Prospectus
|
|
|
Page
|
|
Prospectus
Summary
|
1
|
|
Risk
Factors
|
5
|
|
Note
Concerning Forward-Looking Statements
|
6
|
|
Incorporation
of Information by Reference
|
8
|
|
Where
You Can Find More Information
|
9
|
|
Use of
Proceeds
|
9
|
|
Dilution
|
9
|
|
Market
Information and Related Stockholder Matters
|
9
|
|
Description
of Securities
|
10
|
|
Anti-Takeover
Effects of Provisions of Delaware Law and Our Charter
Documents
|
16
|
|
Plan of
Distribution
|
17
|
|
Legal
Matters
|
18
|
|
Experts
|
18
|
We
are responsible for the information contained and incorporated by
reference in this prospectus supplement, in any accompanying
prospectus, and in any related free writing prospectus we prepare
or authorize. You should rely only on the information contained in
this prospectus supplement, the accompanying prospectus and
information incorporated by reference herein. We have not
authorized anyone to provide you with information different from
that contained in this prospectus supplement, the accompanying
prospectus or any authorized free writing prospectus, and we take
no responsibility for any other information that others may give
you. We are offering to sell, and seeking offers to buy, common
stock only in jurisdictions where offers and sales are permitted.
The information contained in this prospectus supplement, the
accompanying prospectus and any authorized free writing prospectus
is accurate only as of the date of this prospectus supplement, the
accompanying prospectus and any such authorized free writing
prospectus, regardless of the time of delivery of this prospectus
supplement, the accompanying prospectus, any such authorized free
writing prospectus or of any sale of our common stock. Our
business, financial condition, results of operations and prospects
may have changed since those dates. You should read this prospectus
supplement, the accompanying prospectus, the documents incorporated
by reference in this prospectus supplement and any free writing
prospectus that we have authorized for use in connection with this
offering, in their entirety before making an investment decision.
You should also read and consider the information in the documents
to which we have referred you in the sections of this prospectus
supplement entitled “Where You Can Find More
Information” and “Incorporation of Documents by
Reference.”
ABOUT THIS PROSPECTUS SUPPLEMENT
This
prospectus supplement is part of a registration statement that we
have filed with the U.S. Securities and Exchange Commission (the
“SEC”) utilizing a “shelf” registration
process. Before buying any shares of our common stock offered
hereby, we urge you to carefully read this prospectus supplement
and the accompanying prospectus, together with the information
incorporated herein and therein by reference as described under the
headings “Where You Can Find More Information” and
“Incorporation of Certain Documents by Reference.”
These documents contain important information that you should
consider when making your investment decision. Under the shelf
registration process, we are offering to sell shares of our common
stock, which we also refer herein collectively as the securities,
using this prospectus supplement and the accompanying
prospectus.
In this
prospectus supplement, we provide you with specific information
about the securities that we are selling in this offering. Both
this prospectus supplement and the accompanying prospectus include
important information about us, our securities being offered and
other information you should know before investing. This prospectus
supplement also adds updates and changes information contained in
the accompanying prospectus. You should read both this prospectus
supplement and the accompanying prospectus as well as additional
information described under “Incorporation of Information by
Reference” elsewhere in this prospectus supplement and in the
accompanying prospectus before investing in our securities. To the
extent there is a conflict between the information contained in
this prospectus supplement, on the one hand, and the information
contained in any document incorporated by reference filed with the
SEC before the date of this prospectus supplement, on the other
hand, you should rely on the information in this prospectus
supplement.
We
further note that the representations, warranties and covenants
made by us in any agreement that is filed as an exhibit to any
document that is incorporated by reference in the prospectus
supplement or the accompanying prospectus were made solely for the
benefit of the parties to such agreement, including, in some cases,
for the purpose of allocating risk among the parties to such
agreements, and should not be deemed to be a representation,
warranty or covenant to you. Moreover, such representations,
warranties or covenants were accurate only as of the date when
made. Accordingly, such representations, warranties and covenants
should not be relied on as accurately representing the current
state of our affairs.
This
prospectus supplement, the accompanying prospectus and the
documents incorporated by reference herein contain market data and
industry statistics and forecasts that are based on independent
industry publications and other publicly available information.
Although we believe that these sources are reliable, we do not
guarantee the accuracy or completeness of this information and we
have not independently verified this information. Although we are
not aware of any misstatements regarding the market and industry
data presented or incorporated by reference in this prospectus,
these estimates involve risks and uncertainties and are subject to
change based on various factors, including those discussed under
the heading “Risk Factors” and any related free writing
prospectus. Accordingly, investors should not place undue reliance
on this information.
Unless
we have indicated otherwise or the context otherwise requires
references in the prospectus supplement and the accompanying
prospectus to “
Palatin
,” the “
Company
,” “
we
,” “
us
” and “
our
” or similar terms are to
Palatin Technologies, Inc. and its subsidiary.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights certain information appearing elsewhere in
this prospectus supplement and in the accompanying prospectus and
in the documents we incorporate by reference. This summary is not
complete and does not contain all of the information you should
consider prior to investing. After you read this summary, you
should read and consider carefully the more detailed information
and financial statements and related notes that we include in
and/or incorporate by reference into this prospectus supplement and
the accompanying prospectus, especially the section entitled
“Risk Factors.” If you invest in our securities, you
are assuming a high degree of risk.
Overview
We are
a specialized biopharmaceutical company developing first-in-class
medicines based on molecules that modulate the activity of the
melanocortin and natriuretic peptide receptor systems. Our approved
product and product candidates are targeted, receptor-specific
therapeutics for the treatment of diseases with significant unmet
medical need and commercial potential. Our approved product is
Vyleesi™, the trade name for bremelanotide, a peptide
melanocortin receptor 4 (“
MC4r
”) agonist, for the treatment
of premenopausal women with acquired, generalized hypoactive sexual
desire disorder (“
HSDD
”), which is a type of female
sexual dysfunction (“
FSD
”), defined as low desire with
associated distress or interpersonal difficulty.
Vyleesi.
Vyleesi is a subcutaneous
injectable product for the treatment of HSDD in premenopausal
women. Vyleesi is a synthetic peptide analog of the naturally
occurring hormone alpha-MSH (melanocyte-stimulating hormone). In
March 2018, our exclusive North American licensee for Vyleesi, AMAG
Pharmaceuticals, Inc. (“
AMAG
”), submitted a New Drug
Application (“
NDA
”) to the U.S. Food and Drug
Administration (“
FDA
”) for Vyleesi for the
treatment of HSDD in premenopausal women, which was accepted for
filing and review by the FDA. On June 21, 2019 the FDA granted
marketing approval. We have also licensed rights to bremelanotide
to Shanghai Fosun Pharmaceutical Industrial Development Co. Ltd.
(“Fosun”) for the territories of the People’s
Republic of China, Taiwan, Hong Kong S.A.R. and Macau S.A.R.
(collectively, “
Chinese
Territories
”), and Kwangdong Pharmaceutical Co., Ltd.
(“
Kwangdong
”)
for the Republic of Korea (“
Korea
”).
The
FDA’s approval of the NDA triggers a $60 million milestone
payment to us under our North American license agreement with AMAG.
We will also receive tiered royalties on net sales ranging from
high single-digit to low double-digit percentages and up to $300
million contingent upon meeting certain annual sales milestones.
AMAG is expected to launch Vyleesi in the United States during the
third quarter of calendar year 2019.
Our
Phase 3 studies for HSDD in premenopausal women, called the
RECONNECT studies, consisted of two double-blind
placebo-controlled, randomized parallel group studies comparing the
on-demand use of 1.75 mg of Vyleesi versus placebo, in each case,
delivered via a subcutaneous auto-injector. Each trial consisted of
more than 600 patients randomized in a 1:1 ratio to either the
treatment arm or placebo with a 24-week evaluation period. In both
clinical trials, Vyleesi met the pre-specified co-primary efficacy
endpoints of improvement in desire and decrease in distress
associated with low sexual desire as measured using validated
patient-reported outcome instruments.
After
completing the studies, patients had the option to continue in an
open-label safety extension study for an additional 52 weeks.
Nearly 80% of patients who completed the randomized portion of the
study elected to remain in the open-label portion of the study. In
the Phase 3 clinical trials, the most frequent adverse events were
nausea, flushing, and headache, which were generally
mild-to-moderate in intensity and were transient.
We
retain worldwide rights for Vyleesi for HSDD and all other
indications outside North America, Korea and the Chinese
Territories. We are actively seeking potential partners for
marketing and commercialization rights for Vyleesi for HSDD outside
the licensed territories. However, we may not be able to enter into
suitable agreements with potential partners on acceptable terms, if
at all.
Melanocortin Receptor Systems.
There are
five melanocortin receptors, MC1r through MC5r. Modulation of these
receptors, through use of receptor-specific agonists, which
activate receptor function, or receptor-specific antagonists, which
block receptor function, can have significant pharmacological
effects. Our new product development activities primarily focus on
MC1r agonists, with potential to treat a number of inflammatory and
autoimmune diseases such as dry eye disease, also known as
keratoconjunctivitis sicca, uveitis, diabetic retinopathy and
inflammatory bowel disease. We believe that MC1r agonists,
including the MC1r agonist peptides we are developing, have broad
anti-inflammatory effects and appear to utilize mechanisms engaged
by the endogenous melanocortin system in regulation of the immune
system and resolution of inflammatory responses. We are also
developing peptides that are active at more than one melanocortin
receptor, and MC4r agonists, with potential utility in a number of
obesity and metabolic-related disorders, including rare disease and
orphan indications.
●
PL-8177, a
selective MC1r agonist peptide, is our lead clinical development
candidate for inflammatory bowel diseases, including ulcerative
colitis, with potential applicability for a number of other
diseases. We filed an Investigational New Drug (“
IND
”) application on PL-8177 in
late 2017 and have completed subcutaneous dosing of human subjects
in a Phase 1 single and multiple ascending dose clinical safety
study, with favorable results announced in a press release issued
November 8, 2018. We completed a clinical study with oral dosing of
PL-8177 in human subjects in the fourth quarter of calendar year
2018, with positive results announced in a press release issued
April 4, 2019. Phase 2 clinical trials with oral PL-8177 in
ulcerative colitis patients are anticipated to commence in the
fourth quarter of calendar year 2019. A phase 2 clinical trial with
systemic PL-8177 in non-infectious uveitis, an ocular indication,
is also planned to start in the fourth quarter of calendar
2019.
●
PL-9643, a
pan-melanocortin peptide agonist, is a preclinical development
candidate for treating ocular inflammation. We have ongoing
IND-enabling preclinical activities with PL-9643, and if results
continue to be favorable, we anticipate filing an IND
.
●
We have initiated
preclinical programs with MC4r peptides and orally active small
molecules for treatment of rare genetic metabolic and obesity
disorders, and if results are favorable, anticipate selecting a
lead clinical development candidate and completing IND-enabling
activities in calendar year 2019.
Natriuretic Peptide Receptor Systems.
The natriuretic
peptide receptor (“
NPR
”) system has numerous
cardiovascular functions, and therapeutic agents modulating this
system may be useful in treatment of cardiovascular diseases,
including reducing cardiac hypertrophy and fibrosis, heart failure,
acute asthma, other pulmonary diseases and hypertension. While the
therapeutic potential of modulating this system is well
appreciated, development of therapeutic agents has been difficult
due, in part, to the short biological half-life of native peptide
agonists. We have designed and are developing potential candidate
drugs that are selective for one or more different natriuretic
peptide receptors, including natriuretic peptide receptor-A
(“
NPR-A
”),
natriuretic peptide receptor B (“
NPR-B
”), natriuretic peptide
receptor C (“
NPR-C
”).
●
PL-3994 is an NPR-A
agonist we developed which has completed Phase 1 clinical safety
studies. It has potential utility in treatment of a number of
cardiovascular diseases, including genetic and orphan diseases
resulting from a deficiency of endogenous active NPR-A. We have
ongoing academic collaborations with several institutions with
PL-3994, and seek to enter into a development partnership by the
end of calendar year 2019.
●
PL-5028, a dual
NPR-A and NPR-C agonist we developed, is in preclinical development
for cardiovascular diseases, including reducing cardiac hypertrophy
and fibrosis. We have ongoing academic collaborations with several
institutions with PL-5028, and seek to enter into a development
partnership by the end of calendar year 2019.
The
following chart illustrates the status of our drug development
programs.
Our Strategy
Key
elements of our business strategy include:
●
Using our
technology and expertise to develop and commercialize products in
our active drug development programs;
●
Entering into
strategic alliances and partnerships with pharmaceutical companies
to facilitate the development, manufacture, marketing, sale and
distribution of product candidates that we are
developing;
●
Partially funding
our product development programs with the cash flow generated from
existing license agreements, as well as any future research,
collaboration or license agreements; and
●
Completing
development and seeking regulatory approval of certain of our other
product candidates.
At
March 31, 2019, we had an accumulated deficit of approximately $348
million. We expect to incur substantial operating losses in future
periods. We do not expect to generate significant product revenue,
sales-based milestones or royalties until we successfully complete
development and obtain marketing approval for our product
candidates. While our North American licensee, AMAG, has received
marketing approval for Vyleesi for HSDD, our other product
candidates are in their early stages and must successfully complete
clinical trials before marketing approval can be sought, which will
take a number of years to complete. In order to commercialize our
product candidates, we need to complete clinical development and to
comply with comprehensive regulatory requirements.
We
believe that our existing capital resources, together with the $60
million milestone payment due from AMAG on approval of Vyleesi and
proceeds we receive from the sale of shares of our common stock in
the “at-the-market” program (if any), will be adequate
to fund our planned operations through at least calendar year 2020.
Following this offering we will need additional funding to complete
required clinical trials for our product candidates other than
bremelanotide, and, assuming those clinical trials are successful,
as to which there can be no assurance, to complete submission of
required regulatory applications to the FDA. It is possible that we
will not achieve the progress that we expect because the actual
costs and timing of clinical development activities are difficult
to predict and are subject to substantial risks and delays.
Accordingly, we will be required to obtain further funding through
public or private equity offerings, debt financings, collaborations
and licensing arrangements or other sources. Financing may not be
available to us in the necessary timeframe, in the amounts that we
need, on terms acceptable to us, or at all. Our failure to raise
capital as and when needed would have a negative impact on our
financial condition and our ability to pursue our business
strategy.
Our cash and
cash equivalents balance as of March 31, 2019 was approximately
$19.8 million.
Between April 1,
2019 and June 18, 2019, a total of 12,573,643 shares of the
Company’s common stock were sold through Canaccord in an
at-the-market offering under an equity distribution agreement
entered into with Canaccord on April 20, 2018, for net proceeds of
$17,343,286.
Corporate Information
Our
corporate offices are located at 4B Cedar Brook Drive, Cedar Brook
Corporate Center, Cranbury, NJ 08512. Our telephone number is (609)
495-2200. Our internet address is www.palatin.com. The information
on our website is not incorporated by reference into this
prospectus supplement and should not be considered to be part of
this prospectus supplement. Our website address is included in this
prospectus supplement as an inactive textual reference
only.
The Offering
Issuer
Palatin
Technologies, Inc.
Securities offered
by us
Shares of our
common stock having an aggregate offering price of up to
$40,000,000.
Shares outstanding
after offering
246,090,573 shares,
assuming sale of $40,000,000 of shares of common stock at $1.31,
the closing sale price on June 18, 2019 (30,534,351
shares).
Manner of
offering
An
“at-the-market” offering that may be made from time to
time through our sales agent. See “Plan of
Distribution.”
Use of
proceeds
We intend to use
the proceeds from this offering for research and further
development of our product candidates, working capital, capital
expenditures, general and administrative expenses and other general
corporate purposes. See the section of this prospectus supplement
entitled “Use of Proceeds.”
NYSE American
symbol
“PTN”
Risk
factors
You should read the
section of this prospectus supplement entitled “Risk
Factors”, including the information incorporated by
reference, and the other information included in this prospectus
supplement for a discussion of factors that you should consider
before deciding to invest in our securities.
The
number of shares of our common stock to be outstanding after this
offering is based on 215,556,222 shares outstanding as of June 18,
2019.
Unless
otherwise indicated, all information in this prospectus supplement,
including the number of shares of our common stock to be
outstanding after this offering, excludes the
following:
●
61,335 shares of
common stock reserved as of May 31, 2019 for issuance upon any
conversion of our Series A Convertible Preferred Stock outstanding
as of June 18, 2019;
●
12,481,399 shares
of common stock issuable upon the exercise of stock options at a
weighted-average exercise price of $0.75 per share outstanding as
of June 18, 2019;
●
4,863,758 shares of
common stock issuable upon the vesting of outstanding restricted
stock units as of June 18, 2019 which vest on dates between June
11, 2019 and June 26, 2022, subject to the fulfillment of service
or performance conditions, some of which are subject to provisions
to delay delivery upon vesting;
●
3,950,125 shares of
common stock which have vested under restricted stock unit
agreements as of June 18, 2019 which are subject to provisions to
delay delivery; and
●
23,122,046 shares
of common stock issuable upon the exercise of warrants at a
weighted-exercise exercise price of $0.77 per share outstanding as
of June 18, 2019.
RISK FACTORS
You should carefully consider the risks described below and
discussed under the section entitled “Risk Factors” in
Part I, Item 1A of our Annual Report on Form 10-K for the fiscal
year ended June 30, 2018, which are incorporated by reference in
this prospectus supplement and the accompanying prospectus in their
entirety, together with other information in this prospectus
supplement, the accompanying prospectus and the documents
incorporated by reference before deciding to invest in our
securities. These risks should be considered in conjunction with
any other information included or incorporated by reference herein,
including in conjunction with forward-looking statements made
herein. See the section of this prospectus supplement entitled
“Where You Can Find More Information.” If any of the
following risks actually occur, they could materially adversely
affect our business, financial condition, operating results or
prospects.
Risks Related to this Offering
Our stock price is volatile and may fluctuate in a way that is
disproportionate to our operating performance and we expect it to
remain volatile, which could limit investors’ ability to sell
stock at a profit.
The
volatile price of our stock makes it difficult for investors to
predict the value of their investment, to sell shares at a profit
at any given time or to plan purchases and sales in advance. A
variety of factors may affect the market price of our common stock.
These include, but are not limited to:
●
publicity regarding
actual or potential clinical results relating to products under
development by our competitors or us;
●
delay or failure in
initiating, completing or analyzing preclinical or clinical trials
or unsatisfactory designs or results of these trials;
●
interim decisions
by regulatory agencies, including the FDA, as to clinical trial
designs, acceptable safety profiles and the benefit/risk ratio of
products under development;
●
achievement or
rejection of regulatory approvals by our competitors or by
us;
●
announcements of
technological innovations or new commercial products by our
competitors or by us;
●
developments
concerning proprietary rights, including patents;
●
developments
concerning our collaborations;
●
regulatory
developments in the United States and foreign
countries;
●
economic or other
crises and other external factors;
●
period-to-period
fluctuations in our revenue and other results of
operations;
●
changes in the
structure of healthcare payment systems or other actions that
affect the effective reimbursement rates for treatment regimens
containing our products;
●
changes in
financial estimates and recommendations by securities analysts
following our business or our industry;
●
sales of our common
stock, or the perception that such sales could occur;
and
●
the other factors
described in this “Risk Factors” section and
in
the section entitled
“Risk Factors” in Part I, Item 1A of our Annual Report
on Form 10-K for the fiscal year ended June 30, 2018.
We will
not be able to control many of these factors, and we believe that
period-to-period comparisons of our financial results will not
necessarily be indicative of our future performance. If our
revenues, if any, in any particular period do not meet
expectations, we may not be able to adjust our expenditures in that
period, which could cause our operating results to suffer further.
If our operating results in any future period fall below the
expectations of securities analysts or investors, our stock price
may fall by a significant amount.
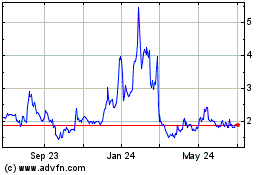
For the
12-month period ended June 30, 2018, the price of our stock has
been volatile, ranging from a high of $1.59 per share to a low of
$0.38 per share. For the eleven-month period ended May 31, 2019,
the price of our stock has been volatile, ranging from a high of
$1.74 per share to a low of $0.59 per share. In addition, the stock
market in general, and the market for biotechnology companies in
particular, has experienced extreme price and volume fluctuations
that may have been unrelated or disproportionate to the operating
performance of individual companies. These broad market and
industry factors may seriously harm the market price of our common
stock, regardless of our operating performance.
You will experience immediate and substantial dilution in the net
tangible book value per share of the common stock you
purchase.
The
offering price per share in this offering may exceed the net
tangible book value per share of our common stock outstanding prior
to this offering. Assuming that an aggregate of 30,534,351 shares
of our common stock are sold at the assumed offering price of $1.31
per share (the last reported sale price of our common stock on the
NYSE American on June 18, 2019), and after deducting commissions
and estimated aggregate offering expenses payable by us, you will
experience immediate dilution of $1.08 per share, representing the
difference between our as adjusted net tangible book value per
share as of March 31, 2019 after giving effect to this offering and
the assumed offering price. In addition, we are not restricted from
issuing additional securities in the future, including shares of
common stock, securities that are convertible into or exchangeable
for, or that represent the right to receive, common stock or
substantially similar securities. The issuance of these securities
may cause further dilution to our stockholders. The exercise of
outstanding stock options, the vesting of outstanding restricted
stock units and the delivery of shares under restricted stock unit
agreements containing provisions to delay delivery may also result
in further dilution of your investment. See the section entitled
“Dilution” on page S-11 below for a more detailed
illustration of the dilution you may incur if you participate in
this offering.
You may experience future dilution as a result of future equity
offerings.
In
order to raise additional capital, we may in the future offer
additional shares of our common stock or other securities
convertible into or exchangeable for our common stock. We cannot
assure you that we will be able to sell shares or other securities
in any other offering at a price per share that is equal to or
greater than the price per share paid by investors in this
offering, and investors purchasing shares or other securities in
the future could have rights superior to existing stockholders. The
price per share at which we sell additional shares of our common
stock or other securities convertible into or exchangeable for our
common stock in future transactions may be higher or lower than the
price per share in this offering. As of June 18, 2019, an aggregate
total of approximately 29.3 million shares of common stock are
either subject to outstanding options or restricted stock unit
grants or reserved for future issuance under our equity incentive
plans. To the extent we grant additional awards under our equity
incentive plans, you could experience dilution, and, as a result,
the market price of our common stock may decline.
Resales of our common stock in the public market by our
stockholders during this offering may cause the market price of our
common stock to fall.
We may
issue common stock from time to time in connection with this
offering. The issuance from time to time of these new shares of our
common stock, or our ability to issue new shares of common stock in
this offering, could result in resales of our common stock by our
current stockholders concerned about the potential dilution of
their holdings. In turn, these resales could have the effect of
depressing the market price for our common stock.
We will have broad discretion over the use of the proceeds of this
offering and may not realize a return.
Our
management will have broad discretion over the use of our net
proceeds from this offering, and you will be relying on the
judgment of our management regarding the application of these
proceeds. Our management might not apply our net proceeds in ways
that ultimately increase the value of your investment and we might
not be able to yield a significant return, if any, on any
investment of these net proceeds. Our failure to apply these funds
effectively could have a material adverse effect on our business,
delay the development of our products and cause the price of our
common stock to decline.
Because we do not intend to declare cash dividends on our shares of
common stock in the foreseeable future, stockholders must rely on
appreciation of the value of our common stock for any return on
their investment.
We have
never declared or paid cash dividends on our common stock. We
currently anticipate that we will retain future earnings for the
development, operation and expansion of our business and do not
anticipate declaring or paying any cash dividends in the
foreseeable future. In addition, the terms of any existing or
future debt agreements may preclude us from paying dividends. As a
result, we expect that only appreciation of the price of our common
stock, if any, will provide a return to investors in this offering
for the foreseeable future.
Investing in our common stock may involve a high degree of
risk.
The
investments that we make in accordance with our investment
objectives may result in a high amount of risk, resulting in a
complete loss of principal, when compared to alternative investment
options. Our investments may be highly speculative and aggressive,
and therefore an investment in our common stock may not be suitable
for someone with lower risk tolerance.
It is not possible to predict the aggregate proceeds resulting from
sales made under the equity distribution agreement.
Subject
to certain limitations in the equity distribution agreement and
compliance with applicable law, we have the discretion to deliver a
placement notice to Canaccord at any time throughout the term of
the equity distribution agreement. The number of shares that are
sold through Canaccord after delivering a placement notice will
fluctuate based on a number of factors, including the market price
of our common stock during the sales period, the limits we set with
Canaccord in any applicable placement notice, and the demand for
our common stock during the sales period. Because the price per
share of each share sold will fluctuate during the sales period, it
is not currently possible to predict the aggregate proceeds to be
raised in connection with those sales.
The common
stock offered hereby will be sold in “at the market
offerings,” and investors who buy shares at different times
will likely pay different prices.
Investors
who purchase shares in this offering at different times will likely
pay different prices, and so may experience different levels of
dilution and different outcomes in their investment results. We
will have discretion, subject to market demand, to vary the timing,
prices, and number of shares sold in this offering. In addition,
subject to the final determination by our board of directors, there
is no minimum or maximum sales price for shares to be sold in this
offering. Investors may experience a decline in the value of the
shares they purchase in this offering as a result of sales made at
prices lower than the prices they paid.
CAUTIONARY NOTE CONCERNING FORWARD-LOOKING
STATEMENTS
This
prospectus supplement and the accompanying prospectus, including
the information that we incorporate by reference, contains
forward-looking statements within the meaning of the Private
Securities Litigation Reform Act of 1995 that involve substantial
risks and uncertainties. All statements other than statements of
historical facts contained in this prospectus, including statements
regarding our future financial condition, business strategy and
plans and objectives of management for future operations, are
forward-looking statements. In some cases, you can identify
forward-looking statements by terminology such as
“believe,” “will,” “may,”
“estimate,” “continue,”
“anticipate,” “intend,”
“should,” “plan,” “expect,”
“predict,” “could,”
“potentially” or the negative of these terms or other
similar expressions. We have based these forward-looking statements
largely on our current expectations and projections about future
events and financial trends that we believe may affect our
financial condition, results of operations, business strategy and
financial needs. These forward-looking statements include, but are
not limited to, statements concerning the following:
●
estimates of our
expenses, future revenue and capital requirements;
●
our ability to
achieve and maintain profitability;
●
our ability to
obtain additional financing on terms acceptable to us, or at
all;
●
our ability to
advance product candidates into, and successfully complete,
clinical trials;
●
the initiation,
timing, progress and results of future preclinical studies and
clinical trials, and our research and development
programs;
●
the timing or
likelihood of regulatory filings and approvals;
●
our expectations
regarding market acceptance and sales of Vyleesi™ (the trade
name for bremelanotide) for the treatment of premenopausal women
with hypoactive sexual desire disorder (“
HSDD
”), which is a type of female
sexual dysfunction (“FSD”);
●
our expectation
regarding the timing of our regulatory submissions for approval of
Vyleesi for HSDD in certain other jurisdictions outside the United
States;
●
our expectation
regarding performance of our exclusive licensees of Vyleesi,
including;
o
AMAG
Pharmaceuticals, Inc. (“
AMAG
”) for North
America,
o
Shanghai Fosun
Pharmaceutical Industrial Development Co. Ltd. (“
Fosun
”), a subsidiary of Shanghai
Fosun Pharmaceutical (Group) Co., Ltd., for the territories of the
People’s Republic of China, Taiwan, Hong Kong S.A.R. and
Macau S.A.R. (collectively, the “
Chinese Territories
”),
and
o
Kwangdong
Pharmaceutical Co., Ltd. (“
Kwangdong
”) for the Republic of
Korea (“
Korea
”);
●
the potential for
commercialization of Vyleesi for HSDD in North America by AMAG and
other product candidates, if approved, by us;
●
our expectations
regarding the potential market size and market acceptance for
Vyleesi for HSDD and our other product candidates, if approved for
commercial use;
●
our ability to
compete with other products and technologies similar to our product
candidates;
●
the ability of our
third-party collaborators to timely carry out their duties under
their agreements with us;
●
the ability of our
contract manufacturers to perform their manufacturing activities
for us in compliance with applicable regulations;
●
our ability to
recognize the potential value of our licensing arrangements with
third parties;
●
the potential to
achieve revenues from the sale of our product
candidates;
●
our ability to
obtain adequate reimbursement from Medicare, Medicaid, private
insurers and other healthcare payers;
●
our ability to
maintain product liability insurance at a reasonable cost or in
sufficient amounts, if at all;
●
the performance of
our management team, senior staff professionals, and third-party
contractors and consultants;
●
the retention of
key management, employees and third-party contractors;
●
the scope of
protection we are able to establish and maintain for intellectual
property rights covering our product candidates and technology in
the United States and throughout the world;
●
our compliance with
federal and state laws and regulations;
●
the timing and
costs associated with obtaining regulatory approval for our product
candidates;
●
the impact of
fluctuations in foreign exchange rates;
●
the impact of
legislative or regulatory healthcare reforms in the United
States;
●
our ability to
adapt to changes in global economic conditions as well as competing
products and technologies; and
●
our ability to
remain listed on the NYSE American stock exchange.
These
forward-looking statements are subject to a number of risks,
uncertainties and assumptions described under the section titled
“Risk Factors” and elsewhere in this prospectus
supplement, the accompanying base prospectus, and in the reports
with file with the SEC. We also operate in a very competitive and
rapidly changing environment. New risks emerge from time to time
and it is not possible for our management to predict all risks, nor
can we assess the impact of all factors on our business or the
extent to which any factor, or combination of factors, may cause
actual results to differ materially from those contained in, or
implied by, any forward-looking statements. In light of these
risks, uncertainties and assumptions, the forward-looking events
and circumstances described in this prospectus may not occur and
actual results could differ materially and adversely from those
anticipated or implied in the forward-looking statements contained
or incorporated by reference in this prospectus.
You
should not rely upon forward-looking statements as predictions of
future events. Although we believe that the expectations reflected
in the forward-looking statements are reasonable, we cannot
guarantee that the future results, levels of activity, performance,
events, circumstances or achievements reflected in the
forward-looking statements will ever be achieved or occur. Except
as required by law, we undertake no obligation to update any
forward-looking statements for any reason after the date of this
prospectus to conform these statements to actual results or to
changes in our expectations.
You
should read this prospectus, together with the information
incorporated herein by reference as described under the section
entitled “Incorporation of Information by Reference,”
and the documents that we reference in this prospectus and have
filed with the SEC as exhibits to the registration statement on
Form S-3, of which this prospectus is a part, with the
understanding that our actual future results, levels of activity,
performance and achievements may be materially different from what
we expect. We qualify all of our forward-looking statements by
these cautionary statements.
All
forward-looking statements attributable to us, or to persons acting
on our behalf, are expressly qualified in their entirety by these
cautionary statements.
USE OF PROCEEDS
The
proceeds from this offering may vary if we choose to raise less
than, or are unable to raise up to, the maximum $40,000,000 in
gross offering proceeds permitted by this prospectus supplement.
The number of shares that we offer and the offering price per share
also may vary.
We will
retain broad discretion over the use of the net proceeds from the
sale of the securities offered hereby. We currently intend to use
the net proceeds from the sale of the securities offered hereby for
research and further development of our product candidates and for
general corporate purposes, capital expenditures, working capital
and general and administrative expenses. We may also use a portion
of the net proceeds to acquire or invest in businesses, products
and technologies that are complementary to our own, although we
have no current plans, commitments or agreements with respect to
any acquisitions as of the date of this prospectus
supplement.
We have
not determined the amounts we plan to spend on any of the areas
listed above or the timing of these expenditures. As a result, our
management will have broad discretion to allocate the net proceeds
from this offering. Pending application of the net proceeds as
described above, we intend to invest the net proceeds of the
offering in short-term, investment-grade, interest-bearing
securities.
DILUTION
If
you invest in our common stock, your ownership interest will be
diluted immediately to the extent of the difference between the
offering price per share of our common stock and the as adjusted
net tangible book value per share of our common stock after this
offering.
As of
March 31, 2019, our net tangible book value was approximately $16.1
million, or $0.08 per share of common stock. Such net tangible book
value per share represents the amount of our total tangible assets
less total liabilities, divided by the number of shares of common
stock outstanding on March 31, 2019.
After
giving effect to the sale of 30,534,351 shares of common stock in
this offering at an assumed public offering price of $1.31 per
share (which was the last reported sale price on June 18, 2019),
after deducting estimated offering expenses and after deducting
estimated sales agent discounts payable by us, our pro forma net
tangible book value as of March 31, 2019 would have been
approximately $54.7 million, or $0.23 per share of common stock.
This would represent an immediate increase in pro forma net
tangible book value of $0.15 per share to existing stockholders and
an immediate dilution of $1.08 per share to new investors
purchasing shares of common stock in this offering, assuming
30,534,351 shares are sold at the assumed public offering price of
$1.31 per share.
The
following table illustrates this dilution on a per share
basis:
|
Assumed public
offering price per share
|
|
$
1.31
|
|
Historical net book
value per share as of March 31, 2019
|
$
0.08
|
|
|
As adjusted
increase in net book value per share attributable to new investors
in this offering
|
$
0.15
|
|
|
As adjusted net
book value per share of our common stock after this
offering
|
|
$
0.23
|
|
Dilution of as
adjusted net book value per share to new investors
|
|
$
1.08
|
The
foregoing table is based on 203,063,429 shares of our common stock
outstanding as of March 31, 2019 and assumes the conversion of all
then convertible preferred stock and excludes:
●
12,481,399
shares issuable on the exercise of stock options, at exercise
prices ranging from $0.37 to $2.80 per share;
●
4,872,333 shares
issuable under restricted stock units which vest on dates between
June 11, 2019 and June 26, 2022, subject to the fulfillment of
service or performance conditions;
●
3,952,875 shares of
common stock which have vested under restricted stock unit
agreements, but are subject to provisions to delay delivery;
and
●
23,404,046 shares
issuable on the exercise of warrants at exercise prices ranging
from $0.70 to $0.91 per share.
To the
extent that options or warrants are exercised, you will experience
further dilution. In addition, we may choose to raise additional
capital due to market conditions or strategic considerations even
if we believe we have sufficient funds for our current or future
operating plans. To the extent that additional capital is raised
through the sale of equity or convertible debt securities, the
issuance of these securities may result in further dilution to our
stockholders.
DIVIDEND POLICY
We have
not paid cash dividends on our common stock and do not anticipate
paying dividends in the foreseeable future. Our outstanding Series
A Preferred Stock, consisting of 4,030 shares on June 18, 2019,
provides that we may not pay a dividend or make any distribution to
holders of any class of stock unless we first pay a special
dividend or distribution of $100 per share to the holders of the
Series A Preferred Stock. Our board of directors currently intends
to retain any future earnings for reinvestment in our growing
business. Any future determination to pay dividends will also be at
the discretion of our board of directors and will be dependent upon
our results of operations and cash flows, our financial position
and capital requirements, general business conditions, legal, tax,
regulatory and any contractual restrictions on the payment of
dividends, and any other factors our board of directors deems
relevant. We currently intend to retain all available funds and any
future earnings to fund the development and expansion of our
business, and we do not anticipate paying any cash dividends in the
foreseeable future.
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S.
HOLDERS
The
following discussion describes the material U.S. federal income tax
consequences of the acquisition, ownership and disposition of our
common stock acquired in this offering by Non-U.S. Holders (as
defined below). This discussion does not address all aspects of
U.S. federal income taxes, does not discuss the potential
application of the Medicare Contribution tax, and does not deal
with state, local or non-U.S. tax consequences that may be relevant
to Non-U.S. Holders in light of their particular circumstances, nor
does it address U.S. federal tax consequences other than income
taxes (except to the limited extent set forth below). Rules
different from those described below may apply to certain Non-U.S.
Holders that are subject to special treatment under the Internal
Revenue Code of 1986, as amended, or the Code, such as financial
institutions, insurance companies, tax-exempt organizations,
“foreign governments,” international organizations,
broker-dealers and traders in securities, U.S. expatriates,
“controlled foreign corporations,” “passive
foreign investment companies,” corporations that accumulate
earnings to avoid U.S. federal income tax, persons that hold our
common stock as part of a “straddle,” “conversion
transaction,” or other risk reduction strategy, partnerships
and other pass-through entities, and investors in such pass-through
entities or entities that are treated as disregarded entities for
U.S. federal income tax purposes (regardless of their places of
organization or formation). Such Non-U.S. Holders are urged to
consult their tax advisors to determine the U.S. federal, state,
local and other tax consequences that may be relevant to them.
Furthermore, the discussion below is based upon the provisions of
the Code, and U.S. Treasury Regulations, rulings and judicial
decisions thereunder as of the date hereof, and such authorities
may be repealed, revoked or modified, perhaps retroactively, so as
to result in U.S. federal income tax consequences different from
those discussed below. We have not requested a ruling from the
Internal Revenue Service, or IRS, with respect to the statements
made and the conclusions reached in the following summary. This
discussion assumes that the Non-U.S. Holder holds our common stock
as a “capital asset” within the meaning of Section 1221
of the Code (generally, property held for investment).
The
following discussion is for general information only and is not tax
advice for Non-U.S. Holders under their particular circumstances.
Persons considering the purchase of our common stock pursuant to
this offering should consult their tax advisors concerning the U.S.
federal income tax consequences of acquiring, owning and disposing
of our common stock in light of their particular situations as well
as any consequences arising under the laws of any other taxing
jurisdiction, including any state, local and non-U.S. tax
consequences and any U.S. federal non-income tax
consequences.
For the
purposes of this discussion, a “Non-U.S. Holder” is,
for U.S. federal income tax purposes, a beneficial owner of common
stock that is not a U.S. Holder. A “U.S. Holder” means
a beneficial owner of our common stock that is for U.S. federal
income tax purposes (a) an individual who is a citizen or resident
of the United States, (b) a corporation or other entity treated as
a corporation created or organized in or under the laws of the
United States, any state thereof or the District of Columbia, (c)
an estate the income of which is subject to U.S. federal income
taxation regardless of its source or (d) a trust if it (1) is
subject to the primary supervision of a court within the United
States and one or more U.S. persons have the authority to control
all substantial decisions of the trust or (2) has a valid election
in effect under applicable U.S. Treasury Regulations to be treated
as a U.S. person. Also, partnerships, or other entities that are
treated as partnerships for U.S. federal income tax purposes
(regardless of their place of organization or formation) and
entities that are treated as disregarded entities for U.S. federal
income tax purposes (regardless of their place of organization or
formation) are not addressed by this discussion and are, therefore,
not considered to be Non-U.S. Holders for the purposes of this
discussion.
Distributions
Distributions, if
any, made on our common stock to a Non-U.S. Holder of our common
stock generally will constitute dividends for U.S. tax purposes to
the extent made out of our current or accumulated earnings and
profits (as determined under U.S. federal income tax principles)
and will be subject to withholding tax at a 30% rate or such lower
rate as may be specified by an applicable income tax treaty. To
obtain a reduced rate of withholding under a treaty, a Non-U.S.
Holder generally will be required to provide us with a properly
executed IRS Form W-8BEN, W-8BEN-E or other appropriate form,
certifying the Non-U.S. Holder’s entitlement to benefits
under that treaty. In the case of a Non-U.S. Holder that is an
entity, U.S. Treasury Regulations and the relevant tax treaty
provide rules to determine whether, for purposes of determining the
applicability of a tax treaty, dividends will be treated as paid to
the entity or to those holding an interest in that entity. If a
Non-U.S. Holder holds stock through a financial institution or
other agent acting on the holder’s behalf, the holder will be
required to provide appropriate documentation to such agent. The
holder’s agent may then be required to provide certification
to us or our paying agent, either directly or through other
intermediaries. If you are eligible for a reduced rate of U.S.
federal withholding tax under an income tax treaty, you should
consult with your tax advisor to determine if you are able to
obtain a refund or credit of any excess amounts withheld by timely
filing an appropriate claim for a refund with the IRS.
Withholding tax is
generally not imposed on dividends paid to a Non-U.S. Holder that
are effectively connected with the Non-U.S. Holder’s conduct
of a trade or business within the United States (and, if required
by an applicable income tax treaty, are attributable to a permanent
establishment that such holder maintains in the United States) if a
properly executed IRS Form W-8ECI, stating that the dividends are
so connected, is furnished to us (or, if stock is held through a
financial institution or other agent, to such agent). In general,
such effectively connected dividends will be subject to U.S.
federal income tax, on a net income basis at the regular graduated
rates, unless a specific treaty exemption applies. A Non-U.S.
Holder that is a corporation for U.S. federal income tax purposes
that receives effectively connected dividends may also be subject
to an additional “branch profits tax,” which is
imposed, under certain circumstances, at a rate of 30% (or such
lower rate as may be specified by an applicable treaty) on the
corporate Non-U.S. Holder’s effectively connected earnings
and profits, subject to certain adjustments.
To the
extent distributions on our common stock, if any, exceed our
current and accumulated earnings and profits, they will first
reduce your adjusted basis in our common stock as a non-taxable
return of capital, but not below zero, and then any excess will be
treated as gain and taxed in the same manner as gain realized from
a sale or other disposition of common stock as described in the
next section.
Distributions on
our common stock will also be subject to the rules discussed below
relating to backup withholding and foreign accounts.
Gain on Disposition of Our Common Stock
Subject
to the discussion below regarding backup withholding and foreign
accounts, a Non-U.S. Holder generally will not be subject to U.S.
federal income tax with respect to gain realized on a sale or other
disposition of our common stock unless (a) the gain is effectively
connected with a trade or business of such holder in the United
States (and, if required by an applicable income tax treaty, is
attributable to a permanent establishment that such holder
maintains in the United States), (b) the Non-U.S. Holder is a
nonresident alien individual and is present in the United States
for 183 or more days in the taxable year of the disposition and
certain other conditions are met, or (c) we are or have been a
“United States real property holding corporation”
within the meaning of Code Section 897(c)(2) at any time within the
shorter of the five-year period preceding such disposition or such
holder’s holding period.
If you
are a Non-U.S. Holder described in (a) above, you will be required
to pay tax on the net gain derived from the sale at regular
graduated U.S. federal income tax rates, unless a specific treaty
exemption applies, and corporate Non-U.S. Holders described in (a)
above may be subject to the additional branch profits tax at a 30%
rate or such lower rate as may be specified by an applicable income
tax treaty. If you are an individual Non-U.S. Holder described in
(b) above, you will be required to pay a flat 30% tax on the gain
derived from the sale, which gain may be offset by U.S. source
capital losses (even though you are not considered a resident of
the United States). With respect to (c) above, in general, we would
be a United States real property holding corporation if interests
in U.S. real estate constituted (by fair market value) at least
half of our total worldwide real property interests plus business
assets. We believe that we are not, and do not anticipate becoming,
a United States real property holding corporation; however, there
can be no assurance that we will not become a U.S. real property
holding corporation in the future. Even if we are treated as a U.S.
real property holding corporation, such treatment will not cause
gain realized by a Non-U.S. Holder on a disposition of our common
stock to be subject to U.S. federal income tax so long as (1) the
Non-U.S. Holder owned, directly, indirectly and constructively, no
more than five percent of our common stock at all times within the
shorter of (i) the five-year period preceding the disposition or
(ii) the holder’s holding period and (2) our common stock is
regularly traded on an established securities market. There can be
no assurance that our common stock will continue to qualify as
regularly traded on an established securities market.
Information Reporting Requirements and Backup
Withholding
Generally, we or
certain financial middlemen must report information to the IRS with
respect to any dividends we pay on our common stock including the
amount of any such dividends, the name and address of the
recipient, and the amount, if any, of tax withheld. A similar
report is sent to the holder to whom any such dividends are paid.
Pursuant to tax treaties or certain other agreements, the IRS may
make its reports available to tax authorities in the
recipient’s country of residence.
Dividends paid by
us (or certain financial middlemen) to a Non-U.S. Holder may also
be subject to U.S. backup withholding. U.S. backup withholding
generally will not apply to a Non-U.S. Holder who provides a
properly executed appropriate IRS Form W-8 or otherwise establishes
an exemption.
Under
current U.S. federal income tax law, U.S. information reporting and
backup withholding requirements generally will apply to the
proceeds of a disposition of our common stock effected by or
through a U.S. office of any broker, U.S. or non-U.S., unless the
holder provides a properly executed IRS Form W-8BEN or IRS Form
W-8BEN-E, as applicable, or otherwise establishes an exemption.
Generally, U.S. information reporting and backup withholding
requirements will not apply to a payment of disposition proceeds to
a Non-U.S. Holder where the transaction is considered effected
outside the United States through a non-U.S. office of a non-U.S.
broker. Information reporting and backup withholding requirements
may, however, apply to a payment of disposition proceeds if the
broker has actual knowledge, or reason to know, that the holder is,
in fact, a U.S. person. For information reporting purposes, certain
brokers with substantial U.S. ownership or operations will
generally be treated in a manner similar to U.S.
brokers.
If
backup withholding is applied to you, you should consult with your
tax advisor to determine if you are able to obtain a tax refund or
credit with respect to the amount withheld.
Foreign Accounts
A U.S.
federal withholding tax of 30% may apply to dividends paid to a
foreign financial institution (as specifically defined by
applicable rules), including when the foreign financial institution
holds our common stock on behalf of a Non-U.S. Holder, unless such
institution enters into an agreement with the U.S. government to
withhold on certain payments and to collect and provide to the U.S.
tax authorities substantial information regarding U.S. account
holders of such institution (which includes certain equity holders
of such institution, as well as certain account holders that are
foreign entities with U.S. owners). This U.S. federal withholding
tax of 30% will also apply to dividends paid to a non-financial
foreign entity unless such entity provides the withholding agent
with either a certification that it does not have any substantial
direct or indirect U.S. owners or provides information regarding
direct and indirect U.S. owners of the entity. The withholding tax
described above will not apply if the foreign financial institution
or non-financial foreign entity otherwise qualifies for an
exemption from the rules. An intergovernmental agreement between
the United States and an applicable foreign country may modify
these requirements. While U.S. federal withholding tax of 30% would
have applied also to the gross proceeds from a sale or other
disposition of our common stock on or after January 1, 2019,
recently proposed Treasury Regulations eliminate this withholding
tax on payments of gross receipts entirely. Non-U.S. Holders
generally may rely on the proposed Treasury Regulations until final
Treasury Regulations are issued.
Under
certain circumstances, a Non-U.S. Holder might be eligible for
refunds or credits of such taxes. Holders are encouraged to consult
with their tax advisors regarding the possible implications of this
withholding tax on their investment in our common
stock.
Federal Estate Tax
An
individual who at the time of death is not a citizen or resident of
the United States and who is treated as the owner of, or has made
certain lifetime transfers of, an interest in our common stock will
be required to include the value thereof in his or her taxable
estate for U.S. federal estate tax purposes, and may be subject to
U.S. federal estate tax. Applicable estate or gift tax treaty may
alter the tax treatment described in the preceding sentence. The
definition of when an individual is a resident of the United States
for U.S. federal estate tax purposes differs from the definition
used for U.S. federal income tax purposes. Some individuals,
therefore, may be “Non-U.S. Holders” for U.S. federal
income tax purposes, but not for U.S. federal estate tax purposes,
and vice versa.
EACH
PROSPECTIVE INVESTOR SHOULD CONSULT HIS, HER OR ITS TAX ADVISOR
REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING
OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED
CHANGE IN APPLICABLE LAW, AS WELL AS TAX CONSEQUENCES ARISING UNDER
ANY STATE, LOCAL, NON-U.S. OR U.S. FEDERAL NON-INCOME TAX
LAWS.
PLAN OF DISTRIBUTION
We have
entered into an equity distribution agreement with Canaccord under
which we may issue and sell from time to time shares of our common
stock having an aggregate gross sales price of up to $40,000,000 of
our common stock through Canaccord, acting as our sales agent for
the offer and sale of the common stock.
Sales of the
common stock, if any, will be made through ordinary brokers’
transactions at market prices by methods deemed to be an
“at-the-market” offering as defined in Rule 415
promulgated under the Securities Act, including sales made directly
on the NYSE American stock exchange, on any other existing trading
market for the common stock, or to or through a market maker other
than on an exchange. Canaccord may also sell our common stock
hereunder by any other method permitted by law, including in
privately negotiated transactions.
Upon
delivery of a placement notice, Canaccord may offer the common
stock subject to the terms and conditions of the equity
distribution agreement on a daily basis or as otherwise agreed upon
by us and Canaccord. We will designate the maximum amount of common
stock to be sold through Canaccord on a daily basis or otherwise
determine such maximum amount together with Canaccord. Subject to
the terms and conditions of the equity distribution agreement,
Canaccord will use its commercially reasonable efforts to sell on
our behalf all of the shares of common stock requested to be sold
by us. We may instruct Canaccord not to sell common stock if the
sales cannot be effected at or above the price designated by us in
any such instruction. We or Canaccord may suspend the offering of
the common stock being made through Canaccord under the equity
distribution agreement upon proper notice to the other party and
subject to other conditions.
We will
pay Canaccord commissions, in cash, for its services in acting as
agent in the sale of our common stock. The aggregate compensation
payable to Canaccord shall be equal to 3.0% of the gross sales
price per share of all shares sold through it as agent under the
equity distribution agreement. Because there is no minimum offering
amount required as a condition to close this offering, the actual
total public offering amount, commissions and proceeds to us, if
any, are not determinable at this time. In addition, we have agreed
to reimburse a portion of the expenses of Canaccord in connection
with this offering up to a maximum of $25,000. We estimate that the
total expenses of the offering payable by us, excluding commissions
payable to Canaccord under the equity distribution agreement, will
be approximately $127,500.
Settlement for
sales of common stock will occur on the second trading day
following the date on which any sales are made (or such earlier day
as is industry practice for regular-way trading), in return for
payment of the net proceeds to us. Sales of our common stock as
contemplated in this prospectus supplement will be settled through
the facilities of The Depository Trust Company or by such other
means as we and Canaccord may agree upon. There is no arrangement
for funds to be received in an escrow, trust or similar
arrangement.
Canaccord will use
its commercially reasonable efforts, consistent with its sales and
trading practices, to solicit offers to purchase the common stock
shares under the terms and subject to the conditions set forth in
the equity distribution agreement. In connection with the sales of
the common stock on our behalf, Canaccord may be deemed to be an
“underwriter” within the meaning of the Securities Act,
and the compensation to Canaccord will be deemed to be underwriting
commissions or discounts. We have also agreed in the equity
distribution agreement to provide indemnification and contribution
to Canaccord with respect to certain liabilities, including
liabilities under the Securities Act.
The
offering of our common stock pursuant to equity distribution
agreement will terminate automatically upon the sale of all shares
of our common stock subject to the equity distribution agreement or
as otherwise permitted therein. We and Canaccord may each terminate
the equity distribution agreement at any time upon ten days’
prior written notice.
Any
portion of the $40,000,000 included in this prospectus supplement
that is not previously sold or included in an active placement
notice pursuant to the equity distribution agreement is available
for sale in other offerings pursuant to the accompanying base
prospectus, and if no shares are sold under the equity distribution
agreement, the full $40,000,000 of securities may be sold in other
offerings pursuant to the accompanying base
prospectus.
Our
common stock is listed on the NYSE American stock exchange under
the trading symbol “PTN.” The transfer agent for our
common stock is American Stock Transfer & Trust Company,
LLC.
Canaccord and its
affiliates have in the past provided, and may in the future
provide, various investment banking, commercial banking and other
financial services for us and our affiliates, for which services
they have received or may in the future receive customary fees. To
the extent required by Regulation M, Canaccord will not engage in
any market making activities involving our common stock while the
offering is ongoing under this prospectus supplement.
Canaccord may
distribute this prospectus supplement and the accompanying
prospectus electronically.
LEGAL MATTERS
The validity of the issuance of the
securities offered by this prospectus supplement and the
accompanying prospectus will be passed upon for us by Thompson Hine
LLP, New York, New York.
Goodwin Procter LLP, New York,
New York, is acting as counsel for Canaccord in connection with
various matters related to the securities offered
hereby.
EXPERTS
The
consolidated financial statements of Palatin Technologies, Inc. and
subsidiary as of June 30, 2018 and 2017, and for each of the years
in the three-year period ended June 30, 2018, and
management’s assessment of the effectiveness of internal
control over financial reporting as of June 30, 2018, have been
incorporated by reference herein and in the registration statement
in reliance upon the reports of KPMG LLP, independent registered
public accounting firm, incorporated by reference herein, and upon
the authority of said firm as experts in accounting and
auditing.
WHERE YOU CAN FIND MORE INFORMATION
This
prospectus supplement and accompanying prospectus constitute a part
of a registration statement on Form S-3 that we filed with the SEC
under the Securities Act. We refer you to this registration
statement for further information about us and the securities
offered hereby.
We file
annual, quarterly and special reports and other information with
the SEC (Commission File Number 001-15543). These filings contain
important information that does not appear in this prospectus. For
further information about us, you may read and copy any reports,
statements and other information filed by us at the SEC’s
Public Reference Room at 100 F Street, N.E., Washington, D.C.
20549-0102. You may obtain further information on the operation of
the Public Reference Room by calling the SEC at 1-800-SEC-0330. Our
SEC filings are also available on the SEC Internet site at
http://www.sec.gov, which contains periodic reports and other
information regarding issuers that file electronically. You can
find information about Palatin, including our periodic reports and
other information that we file electronically, on our website at
http://www.palatin.com. The reference to our website is an inactive
textual reference only. Information found on our website is not
part of this prospectus. You may also request a copy of any of our
periodic reports filed with the SEC by writing or telephoning us at
the following address:
Stephen
T. Wills
Chief
Financial Officer
Palatin
Technologies, Inc.
4B
Cedar Brook Drive
Cranbury,
New Jersey 08512
Telephone
(609) 495-2200
INCORPORATION OF INFORMATION BY REFERENCE
We
incorporate into this prospectus supplement information contained
in documents which we file with the SEC. We are disclosing
important information to you by referring you to those documents.
The information which we incorporate by reference is an important
part of this prospectus supplement, and certain information that we
file later with the SEC will automatically update and supersede
this information. We incorporate by reference the documents listed
below, and any future filings we make with the SEC under Sections
13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934,
as amended.
●
The Company’s
Annual Report on Form 10-K for the fiscal year ended June 30, 2018,
filed with the SEC on September 13, 2018;
●
The Company’s
Definitive Proxy on Form DEF 14A, filed with the SEC on May 10,
2019;
●
The Company’s
Current Reports on Form 8-K, filed with the SEC on November 13,
2018, January 7, 2019, April 4, 2019 and June 20,
2019;
●
The Company’s
Quarterly Report on Form 10-Q for the quarter ended September 30,
2018, filed with the SEC on November 9, 2018;
●
The Company’s
Quarterly Report on Form 10-Q for the quarter ended December 31,
2018, filed with the SEC on February 11, 2019;
●
The Company’s
Quarterly Report on Form 10-Q for the quarter ended March 31, 2019,
filed with the SEC on May 9, 2019; and
●
The description of
our common stock contained in our registration statement on Form
8-A, filed with the SEC on December 13, 1999, File No. 001-15543,
including any amendment or report filed for the purpose of updating
such description.
This
prospectus supplement may contain information that updates,
modifies or is contrary to information in one or more of the
documents incorporated by reference in this prospectus supplement.
To the extent that any statements contained in a document
incorporated by reference are modified or superseded by any
statements contained in this prospectus supplement, such statements
shall not be deemed incorporated in this prospectus supplement
except as so modified or superseded. Reports we file with the SEC
after the date of this prospectus supplement may also contain
information that updates, modifies or is contrary to information in
this prospectus supplement or in documents incorporated by
reference in this prospectus supplement. Investors should review
these reports as they may disclose a change in our business,
prospectus, financial condition or other affairs after the date of
this prospectus supplement.
You may
obtain a free copy of any or all of the information incorporated by
reference by writing or calling us. Please direct your request
to:
Stephen
T. Wills
Chief
Financial Officer
Palatin
Technologies, Inc.
4B
Cedar Brook Drive
Cranbury,
New Jersey 08512
Telephone
(609) 495-2200
Filed pursuant to
Rule 424(b)(5)
File No.
333-226905
PALATIN
TECHNOLOGIES, INC.
4B Cedar Brook
Drive
Cranbury, New
Jersey 08512
(609)
495-2200
$100,000,000
Common
Stock
Preferred
Stock
Debt
Securities
Warrants
Units
We may offer under
this prospectus from time to time in one or more offerings, at
prices and on terms to be determined by market conditions at the
time we make the offer, up to an aggregate of $100,000,000 of
our:
●
common stock, par
value $0.01 per share;
●
preferred stock,
par value $0.01 per share;
●
warrants to
purchase common or preferred stock, or debt securities;
or
●
any combination of
the above, separately or as units.
This prospectus may
not be used to sell our securities unless accompanied by a
prospectus supplement. The prospectus supplement will provide
specific terms of the securities offered, will describe the
specific manner in which we will offer these securities, and may
also supplement, update or amend information contained in this
prospectus. Before you invest in our securities, you should
carefully read both this prospectus and any prospectus supplement
related to the offering of the securities, together with any
documents incorporated herein or therein.
Our common stock is
listed on the NYSE American under the symbol “PTN.” On
February 11, 2019, the closing price of our common stock as
reported on the NYSE American was $0.74 per share. None of the
other securities that we may offer under this prospectus are
currently publicly traded.
As of February 11,
2019, the aggregate market value of our outstanding common shares
held by non-affiliates was approximately $148,446,337, which was
calculated based on 203,063,429 common shares outstanding as of
that date, of which 200,603,159 common shares were held by
non-affiliates, and a price per share of $0.74, which was the
closing price of our common stock as reported on the NYSE American
on such date.
Investing
in our securities involves a high degree of risk. You should
purchase these securities only if you can afford a complete loss of
your investment. See “Risk Factors” beginning on page
5.
Neither
the United States Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these
securities or passed upon the adequacy or accuracy of this
prospectus. Any representation to the contrary is a criminal
offense.
If we sell
securities through agents or underwriters, we will include their
names and the fees, commissions and discounts they will receive, as
well as the net proceeds to us, in the applicable prospectus
supplement. The underwriters, if any, may over-allot a portion of
the securities.
The date of this
prospectus is February 13, 2019
TABLE OF CONTENTS
|
|
Page
|
|
|
|
|
Prospectus
Summary
|
1
|
|
|
|
|
Risk
Factors
|
5
|
|
|
|
|
Note
Concerning Forward-Looking Statements
|
6
|
|
|
|
|
Incorporation
of Information by Reference
|
8
|
|
|
|
|
Where
You Can Find More Information
|
9
|
|
|
|
|
Use of
Proceeds
|
9
|
|
|
|
|
Dilution
|
9
|
|
|
|
|
Market
Information and Related Stockholder Matters
|
9
|
|
|
|
|
Description
of Securities
|
10
|
|
|
|
|
Anti-Takeover
Effects of Provisions of Delaware Law and Our Charter
Documents
|
16
|
|
|
|
|
Plan of
Distribution
|
17
|
|
|
|
|
Legal
Matters
|
18
|
|
|
|
|
Experts
|
18
|
PROSPECTUS
SUMMARY
This summary highlights certain information appearing elsewhere in
this prospectus and in the information incorporated by reference.
This summary is not complete and does not contain all of the
information you should consider prior to investing in our
securities. After you read this summary, you should read and
consider carefully the more detailed information and financial
statements and related notes that we include in this prospectus or
incorporate by reference, especially the section entitled
“Risk Factors.” If you invest in our securities, you
are assuming a high degree of risk.
Unless we have indicated otherwise or the context otherwise
requires, references in the prospectus to “Palatin,”
the “Company,” “we,” “us” and
“our” or similar terms refer to the operations of
Palatin Technologies, Inc. and its subsidiary.
Overview
We
are a specialized biopharmaceutical company developing
first-in-class medicines based on molecules that modulate the
activity of the melanocortin and natriuretic peptide receptor
systems. Our product candidates are targeted, receptor-specific
therapeutics for the treatment of diseases with significant unmet
medical need and commercial potential. Our most advanced product
candidate is Vyleesi™, the trade name for bremelanotide, a
peptide melanocortin receptor 4 (MC4r) agonist, for the treatment
of premenopausal women with acquired, generalized hypoactive sexual
desire disorder (“HSDD”), which is a type of female
sexual dysfunction (“FSD”), defined as low desire with
associated distress or interpersonal difficulty.
Vyleesi.
Vyleesi is a subcutaneous
injectable product for the treatment of HSDD in premenopausal
women. Vyleesi is a synthetic peptide analog of the naturally
occurring hormone alpha-MSH (melanocyte-stimulating hormone). In
March 2018, our exclusive North American licensee for Vyleesi, AMAG
Pharmaceuticals, Inc. (“AMAG”), submitted a New Drug
Application (“NDA”) to the U.S. Food and Drug
Administration (“FDA”) for Vyleesi for the treatment of
HSDD in premenopausal women, which was accepted for filing and
review by the FDA. In November 2018, AMAG announced that the FDA
requested additional data assessing 24-hour ambulatory blood
pressure with short term daily use of Vyleesi, which study is
ongoing. The Prescription Drug User Fee Act (“PDUFA”)
date for completion of FDA review of the Vyleesi NDA was extended
by three months to June 23, 2019. We have also licensed rights to
bremelanotide to Shanghai Fosun Pharmaceutical Industrial
Development Co. Ltd. (“Fosun”) for the territories of
the People’s Republic of China, Taiwan, Hong Kong S.A.R. and
Macau S.A.R. (collectively, “Chinese Territories”), and
Kwangdong Pharmaceutical Co., Ltd. (“Kwangdong”) for
the Republic of Korea (“Korea”).
Our Phase 3 studies
for HSDD in premenopausal women, called the RECONNECT studies,
consisted of two double-blind placebo-controlled, randomized
parallel group studies comparing the on-demand use of 1.75 mg of
Vyleesi versus placebo, in each case, delivered via a subcutaneous
auto-injector. Each trial consisted of more than 600 patients
randomized in a 1:1 ratio to either the treatment arm or placebo
with a 24-week evaluation period. In both clinical trials, Vyleesi
met the pre-specified co-primary efficacy endpoints of improvement
in desire and decrease in distress associated with low sexual
desire as measured using validated patient-reported outcome
instruments.
After
completing the studies, patients had the option to continue in an
open-label safety extension study for an additional 52 weeks.
Nearly 80% of patients who completed the randomized portion of the
study elected to remain in the open-label portion of the study. In
the Phase 3 clinical trials, the most frequent adverse events were
nausea, flushing, injection site reactions and headache, which were
generally mild-to-moderate in intensity and were
transient.
We retain worldwide
rights for Vyleesi for HSDD and all other indications outside North
America, Korea and the Chinese Territories. We are actively seeking
potential partners for marketing and commercialization rights for
Vyleesi for HSDD outside the licensed territories. However, we may
not be able to enter into suitable agreements with potential
partners on acceptable terms, if at all.
Melanocortin Receptor Systems.
There are
five melanocortin receptors, MC1r through MC5r. Modulation of these
receptors, through use of receptor-specific agonists, which
activate receptor function, or receptor-specific antagonists, which
block receptor function, can have significant pharmacological
effects. Our new product development activities primarily focus on
MC1r agonists, with potential to treat a number of inflammatory and
autoimmune diseases such as dry eye disease, also known as
keratoconjunctivitis sicca, uveitis, diabetic retinopathy and
inflammatory bowel disease. We believe that MC1r agonists,
including the MC1r agonist peptides we are developing, have broad
anti-inflammatory effects and appear to utilize mechanisms engaged
by the endogenous melanocortin system in regulation of the immune
system and resolution of inflammatory responses. We are also
developing peptides that are active at more than one melanocortin
receptor, and MC4r agonists, with potential utility in a number of
obesity and metabolic-related disorders, including rare disease and
orphan indications.
●
PL-8177, a
selective MC1r agonist peptide, is our lead clinical development
candidate for inflammatory bowel diseases, with potential
applicability for a number of other diseases. We filed an
Investigational New Drug (“IND”) application on PL-8177
in late 2017 and have completed subcutaneous dosing of human
subjects in a Phase 1 single and multiple ascending dose clinical
safety study, with favorable results issued in a press release
dated November 8, 2018. We started a clinical study with oral
dosing of PL-8177 in human subjects in the fourth quarter of
calendar year 2018, with data expected in the first quarter of
calendar year 2019.
●
PL-8331, a dual
MC1r and MC5r peptide agonist, is a preclinical development
candidate for treating ocular inflammation. We have initiated
IND-enabling preclinical activities with PL-8331, and if results
are favorable, anticipate filing an IND and initiating clinical
trials for treatment of dry eye disease in the second half of
calendar year 2019.
●
We have initiated
preclinical programs with MC4r peptides and orally-active small
molecules for treatment of rare genetic metabolic and obesity
disorders, and if results are favorable, anticipate selecting a
lead clinical development candidate and completing IND-enabling
activities in calendar year 2019.
Natriuretic Peptide Receptor Systems.
The natriuretic peptide receptor (“NPR”) system has
numerous cardiovascular functions, and therapeutic agents
modulating this system may be useful in treatment of cardiovascular
diseases, including reducing cardiac hypertrophy and fibrosis,
heart failure, acute asthma, other pulmonary diseases and
hypertension. While the therapeutic potential of modulating this
system is well appreciated, development of therapeutic agents has
been difficult due, in part, to the short biological half-life of
native peptide agonists. We have designed and are developing
potential candidate drugs that are selective for one or more
different natriuretic peptide receptors, including natriuretic
peptide receptor-A (“NPR-A”), natriuretic peptide
receptor B (“NPR-B”), natriuretic peptide receptor C
(“NPR-C”).
●
PL-3994 is an NPR-A
agonist we developed which has completed Phase 1 clinical safety
studies. It has potential utility in treatment of a number of
cardiovascular diseases, including genetic and orphan diseases
resulting from a deficiency of endogenous active NPR-A. We have
ongoing academic collaborations with several institutions with
PL-3994, and seek to enter into a development partnership by the
end of calendar year 2019.
●
PL-5028, a dual
NPR-A and NPR-C agonist we developed, is in preclinical development
for cardiovascular diseases, including reducing cardiac hypertrophy
and fibrosis. We have ongoing academic collaborations with several
institutions with PL-5028, and seek to enter into a development
partnership by the end of calendar year 2019.
The following chart
illustrates the status of our drug development
programs.
Our
Strategy
Key elements of our
business strategy include:
●
Using our
technology and expertise to develop and commercialize products in
our active drug development programs;
●
Entering into
strategic alliances and partnerships with pharmaceutical companies
to facilitate the development, manufacture, marketing, sale and
distribution of product candidates that we are
developing;
●
Partially funding
our product development programs with the cash flow generated from
existing license agreements, as well as any future research,
collaboration or license agreements; and
●
Completing
development and seeking regulatory approval of certain of our other
product candidates.
Risks
Related to Our Business
Our business is
subject to numerous risks and uncertainties, including those
incorporated by reference in the section of this prospectus
entitled “Risk Factors,” which you should read
carefully before deciding to invest in our securities. These risks
include, among others, the following:
●
We have incurred
substantial losses since our inception and we anticipate that we
will not attain sustained profitability in the foreseeable future,
if ever. We expect to incur additional losses as we continue our
development of product candidates. Until bremelanotide for HSDD or
other product candidates receive regulatory approval under
applicable regulatory requirements, neither we nor our licensees
can sell products we have developed and we will not have product,
sales milestone or royalty revenues from them;
●
We are
substantially dependent on the clinical and commercial success of
our product candidates, primarily our lead product candidate,
bremelanotide for HSDD, for which AMAG, our North American
licensee, has filed an NDA with FDA. Neither we nor our licensees
may be able to obtain regulatory approval for bremelanotide for
HSDD or our other product candidates under applicable regulatory
requirements. The denial or delay of any such approval would delay
commercialization and have a material adverse effect on our
potential to generate revenue, our business and our results of
operations;
●
Our licensees
control the development and commercialization of bremelanotide in
North America, Chinese Territories and Korea, and as a result we
may not realize a significant portion of the potential value of the
license arrangements. We have limited control over development
activities, including regulatory approvals, and no direct control
over commercialization efforts;
●
Even if
bremelanotide for HSDD or our other product candidates receive
regulatory approval, the products may fail to achieve the level of
market acceptance needed for us to have commercial success. Our
product candidates, if approved, will face significant competition
and our failure, or the failure of our licensees, to effectively
compete may prevent us from achieving significant market
penetration and expansion;
●
We will require
substantial additional funding to achieve our goals, and a failure
to obtain this necessary capital when needed on acceptable terms,
or at all, could force us to delay, limit, reduce or terminate our
product development, other operations or commercialization
efforts;
●
If our efforts to
protect our intellectual property related to bremelanotide for HSDD
or any future product candidates are not adequate, we may not be
able to compete effectively in our market; and
●
We rely on a small
management team and staff as well as various contractors and
consultants to provide critical services to us, including services
related to our clinical programs for bremelanotide, PL-8177 and
PL-3994 and our preclinical programs for other NPR and MC1r and
MC4r peptide or small molecule drug candidates. Such programs
could be adversely affected if we lose the services of existing key
personnel.
Corporate
Information
We were
incorporated under the laws of the State of Delaware on November
21, 1986 and commenced operations in the biopharmaceutical area in
1996. Our corporate offices are located at 4B Cedar Brook Drive,
Cedar Brook Corporate Center, Cranbury, New Jersey 08512 and our
telephone number is (609) 495-2200. Our internet address is
www.palatin.com. The information on our website is not incorporated
by reference into this prospectus and should not be considered to
be part of this prospectus. Our website address is included in this
prospectus as an inactive textual reference only.
“Palatin
Technologies, Inc.” and the Palatin logo are our trademarks.
“Vyleesi” is a trademark of AMAG Pharmaceuticals, Inc.
in North America and of Palatin Technologies, Inc. elsewhere in the
world. All other trademarks and service marks appearing in this
prospectus are the property of their respective
owners.
The
Offering
This prospectus is
part of a registration statement on Form S-3 that we filed with the
U.S. Securities and Exchange Commission (“SEC”)
utilizing a “shelf” registration process. Under this
process, we may sell any combination of the securities described in
this prospectus in one or more offerings up to a total dollar
amount of $100.0 million. This prospectus provides you with a
general description of the securities we may offer. Each time we
offer to sell securities under this prospectus, we will provide a
prospectus supplement containing specific information about the
terms of that offering. The prospectus supplement may also add,
update or change information contained in this prospectus. To the
extent that any information we provide in a prospectus supplement
is inconsistent with information in this prospectus, the
information in the prospectus supplement will modify or supersede
this prospectus. You should read both this prospectus and any
prospectus supplement together with the additional information
described under the headings “Incorporation of Information by
Reference” and “Where You Can Find More
Information.”
You should rely
only on the information contained or incorporated by reference in
this prospectus and in any prospectus supplement. We have not
authorized anyone to provide you with different information. We are
not offering the securities in any jurisdiction where the offering
is prohibited. You should not assume that the information in this
prospectus, any prospectus supplement or any document incorporated
by reference is truthful or complete at any date other than the
date mentioned on the cover page of those documents.
RISK
FACTORS
Investing in our
securities involves risks which you should consider carefully. We
have set forth below risk factors related specifically to this
offering. For risks related to our business operations, see
“Risk Factors” in our annual report, on Form 10-K for
the year ended June 30, 2018 and our quarterly reports on Form 10-Q
for the quarters ended September 30, 2018 and December 31, 2018,
and all subsequent reports that we file with the SEC under Sections
13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934,
as amended (the “Exchange Act”). We have incorporated
those reports by reference into this prospectus. See
“Incorporation of Information by Reference” and
“Where You Can Find More Information”
below.
RISKS
RELATED TO THE OFFERING
We expect to sell additional equity securities, which will cause
dilution.
We expect to sell
more equity securities in the future to obtain operating funds. We
may sell these securities at a discount to the market price. Any
future sales of equity will dilute the holdings of existing
stockholders, possibly reducing the value of their
investment.
Investors in this offering may suffer immediate
dilution.
As of December 31,
2018, we had a net book value of $21.1 million which yields a pro
forma net book value of $0.10 per share of common stock, assuming
the conversion of all then convertible preferred stock and no
exercise of any warrants or options. If you pay more than the net
tangible book value per share for stock in this offering, you will
suffer immediate dilution.
As of February 11, 2019, there were 44,803,050 shares of common
stock underlying outstanding convertible preferred stock, options,
restricted stock units and warrants. Stockholders may experience
dilution from the conversion of preferred stock, exercise of
outstanding options and warrants, vesting of restricted stock units
or delayed delivery of common stock pursuant to restricted stock
unit agreements.
As of February 11,
2019, holders of our outstanding dilutive securities had the right
to acquire the following amounts of underlying common
stock:
●
61,335 shares
issuable on the conversion of immediately convertible Series A
Convertible preferred stock, subject to adjustment, for no further
consideration;
●
12,512,461 shares
issuable on the exercise of stock options, at exercise prices
ranging from $0.37 to $2.80 per share;
●
4,872,333 shares
issuable under restricted stock units which vest on dates between
June 20, 2019 and June 26, 2022, subject either to the fulfillment
of service conditions or attaining defined performance
conditions;
●
3,952,875 shares of
common stock which have vested under restricted stock unit
agreements, but are subject to provisions to delay delivery;
and
●
23,404,046 shares
issuable on the exercise of warrants at exercise prices ranging
from $0.70 to $0.91 per share.
If the holders
convert, exercise or receive these securities, or similar dilutive
securities we may issue in the future, stockholders may experience
dilution in the net tangible book value of their common stock. In
addition, the sale or availability for sale of the underlying
shares in the marketplace could depress our stock price. We have
registered or agreed to register for resale substantially all of
the underlying shares listed above. Holders of registered
underlying shares could resell the shares immediately upon
issuance, which could result in significant downward pressure on
our stock price and could also negatively impact our ability to
raise equity capital.
We will have broad discretion over the use of the proceeds of this
offering and you may not realize a return.
We will have
considerable discretion in the application of the net proceeds of
this offering. We have not determined the amount of net proceeds
that we will apply to various corporate purposes, including
potential acquisitions. We may use the net proceeds for purposes
that do not yield a significant return, if any, for our
stockholders.
NOTE
CONCERNING FORWARD-LOOKING STATEMENTS
In this prospectus,
references to “we”, “our”, “us”
or “Palatin” means Palatin Technologies, Inc. and its
subsidiary.
This prospectus,
and the information that we incorporate by reference, as well as
oral statements that may be made by us or by our officers,
directors, or employees acting on our behalf, that are not
historical facts constitute “forward-looking
statements”, which are made pursuant to the safe harbor
provisions of Section 27A of the Securities Act of 1933, as
amended (the “Securities Act”) and Section 21E of
the Exchange Act. The forward-looking statements in this prospectus
do not constitute guarantees of future performance. Investors are
cautioned that statements that are not strictly historical facts
contained in this prospectus, including, without limitation, the
following are forward looking statements:
●
estimates of our
expenses, future revenue and capital requirements;
●
our ability to
achieve and maintain profitability;
●
our ability to
obtain additional financing on terms acceptable to us, or at
all;
●
our ability to
advance product candidates into, and successfully complete,
clinical trials;
●
the initiation,
timing, progress and results of future preclinical studies and
clinical trials, and our research and development
programs;
●
the timing or
likelihood of regulatory filings and approvals;
●
our expectations
regarding completion of required clinical trials and studies and
validation of methods and controls used to manufacture
Vyleesi™ (the trade name for bremelanotide) for the treatment
of premenopausal women with hypoactive sexual desire disorder
(“HSDD”), which is a type of female sexual dysfunction
(“FSD”);
●
our expectation
regarding the timing of our regulatory submissions for approval of
Vyleesi for HSDD in the United States and in certain other
jurisdictions outside the United States;
●
our expectation
regarding performance of our exclusive licensees of Vyleesi,
including;
o
AMAG
Pharmaceuticals, Inc. (“AMAG”) for North
America,
o
Shanghai Fosun
Pharmaceutical Industrial Development Co. Ltd.
(“Fosun”), a subsidiary of Shanghai Fosun
Pharmaceutical (Group) Co., Ltd., for the territories of the
People’s Republic of China, Taiwan, Hong Kong S.A.R. and
Macau S.A.R. (collectively, the “Chinese Territories”),
and
o
Kwangdong
Pharmaceutical Co., Ltd. (“Kwangdong”) for the Republic
of Korea (“Korea”);
●
the potential for
commercialization of Vyleesi for HSDD in North America by AMAG and
other product candidates, if approved, by us;
●
our expectations
regarding the potential market size and market acceptance for
Vyleesi for HSDD and our other product candidates, if approved for
commercial use;
●
our ability to
compete with other products and technologies similar to our product
candidates;
●
the ability of our
third-party collaborators to timely carry out their duties under
their agreements with us;
●
the ability of our
contract manufacturers to perform their manufacturing activities
for us in compliance with applicable regulations;
●
our ability to
recognize the potential value of our licensing arrangements with
third parties;
●
the potential to
achieve revenues from the sale of our product
candidates;
●
our ability to
obtain adequate reimbursement from Medicare, Medicaid, private
insurers and other healthcare payers;
●
our ability to
maintain product liability insurance at a reasonable cost or in
sufficient amounts, if at all;
●
the performance of
our management team, senior staff professionals, and third-party
contractors and consultants;
●
the retention of
key management, employees and third-party contractors;
●
the scope of
protection we are able to establish and maintain for intellectual
property rights covering our product candidates and technology in
the United States and throughout the world;
●
our compliance with
federal and state laws and regulations;
●
the timing and
costs associated with obtaining regulatory approval for our product
candidates;
●
the impact of
fluctuations in foreign exchange rates;
●
the impact of
legislative or regulatory healthcare reforms in the United
States;
●
our ability to
adapt to changes in global economic conditions as well as competing
products and technologies; and
●
our ability to
remain listed on the NYSE American stock exchange.
These
forward-looking statements involve risks, uncertainties and other
factors that could cause our actual results to be materially
different from our historical results or from any results expressed
or implied by forward-looking statements. Our future operating
results are subject to risks and uncertainties and are dependent
upon many factors, including, without limitation, the risks
identified under the caption “Risk Factors,” and in our
other SEC filings. The statements we make in this prospectus are as
of the date of this prospectus.
Although we believe
that the expectations reflected in the forward-looking statements
are reasonable, we cannot guarantee future results, levels of
activity, performance or achievements. Except as may be required by
law, we do not intend to update any of the forward-looking
statements for any reason after the date of this prospectus to
conform such statements to actual results or if new information
becomes available.
All forward-looking
statements attributable to us, or to persons acting on our behalf,
are expressly qualified in their entirety by these cautionary
statements.
You should read
this prospectus, together with the information incorporated herein
by reference as described under the section entitled
“Incorporation of Information by Reference,” and the
documents that we reference in this prospectus and have filed with
the SEC as exhibits to the registration statement on Form S-3, of
which this prospectus is a part, with the understanding that our
actual future results, levels of activity, performance and
achievements may be materially different from what we expect. We
qualify all of our forward-looking statements by these cautionary
statements.
INCORPORATION
OF INFORMATION BY REFERENCE
We incorporate into
this prospectus information contained in documents which we file
with the SEC. We are disclosing important information to you by
referring you to those documents. The information which we
incorporate by reference is an important part of this prospectus,
and certain information that we file later with the SEC will
automatically update and supersede this information. We incorporate
by reference the documents listed below (other than, in each case,
any documents or information deemed to have been furnished and not
filed in accordance with SEC rules):
●
annual report on
Form 10-K for the fiscal year ended June 30, 2018, filed with the
SEC on September 13, 2018;
●
quarterly report on
Form 10-Q for the quarter ended September 30, 2018, filed with the
SEC on November 9, 2018;
●
quarterly report on
Form 10-Q for the quarter ended December 31, 2018, filed with the
SEC on February 11, 2019;
●
current report on
Form 8-K, filed with the SEC on November 13, 2018;
●
current report on
Form 8-K, filed with the SEC on January 7, 2019; and
●
the description of
our common stock contained in our registration statement on Form
8-A, initially filed with the SEC on December 13, 1999, including
any amendment or report for the purpose of updating such
description.
We also incorporate
by reference any documents that we subsequently file with the SEC
under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, prior
to the termination of the offering (other than, in any case, any
documents or information deemed to have been furnished and not
filed in accordance with SEC rules).
You may obtain a
free copy of any or all of the information incorporated by
reference by writing or calling us. Please direct your request
to:
|
|
Stephen T.
Wills
Executive Vice
President, Chief Financial Officer and Chief Operating
Officer
Palatin
Technologies, Inc.
4B Cedar Brook
Drive
Cranbury, New
Jersey 08512
Telephone: (609)
495-2200
Fax: (609)
495-2201
|
WHERE
YOU CAN FIND MORE INFORMATION
We file annual,
quarterly and current reports, proxy statements, registration
statements and other information with the SEC. You may read and
copy any materials we file at the SEC’s Public Reference Room
at 100 F St. NE, Washington, DC 20549. You may obtain information
on the operation of the Public Reference Room by calling the SEC at
1-800-SEC-0330. The SEC maintains an Internet site that contains
reports, proxy and information statements, and other information
regarding issuers that file electronically with the SEC. The
address of that website is http://www.sec.gov. You can also access
these documents free of charge and find information about Palatin
on our website at http://www.palatin.com. Information found on our
website is not part of this prospectus or any prospectus
supplement, and investors should not rely on any such information
in deciding whether to invest in our securities.
USE
OF PROCEEDS
Unless we state
otherwise in a prospectus supplement, we intend to use the net
proceeds from the sale of securities under this prospectus for
general corporate purposes, including capital expenditures. From
time to time, we evaluate the possibility of acquiring businesses,
products and technologies, and we may use a portion of the proceeds
as consideration for acquisitions. Until we use net proceeds for
these purposes, we may invest them in interest-bearing
securities.
DILUTION
We may set forth in
a prospectus supplement the following information regarding any
material dilution of the equity interests of purchasers of
securities in an offering under this prospectus:
●
The net tangible
book value per share of our equity securities before and after the
offering;
●
The amount of the
increase in such net tangible book value per share attributable to
the cash payments made by the purchasers in the offering;
and
●
the amount of the
immediate dilution from the public offering price which will be
absorbed by such purchasers.
MARKET
INFORMATION AND RELATED STOCKHOLDER MATTERS
Our common stock
has been listed on NYSE American under the symbol “PTN”
since December 21, 1999. It previously traded on The Nasdaq
SmallCap Market
under the symbol “PLTN.”
Holders of common stock
. On February
11, 2019, we had approximately 145 record holders of common stock
and the closing sales price of our common stock as reported on the
NYSE American was $0.74 per share.
Dividends and dividend policy
. We have
never declared or paid any dividends. We currently intend to retain
earnings, if any, for use in our business. We do not anticipate
paying dividends in the foreseeable future.
Dividend restrictions
. Our outstanding
Series A Preferred Stock, consisting of 4,030 shares on February
11, 2019, provides that we may not pay a dividend or make any
distribution to holders of any class of stock unless we first pay a
special dividend or distribution of $100 per share to the holders
of the Series A Preferred Stock.
DESCRIPTION
OF SECURITIES
General
The following
description of our capital stock is intended as a summary only and
is qualified in its entirety by reference to our amended and
restated certificate of incorporation and bylaws, which are filed
as exhibits to the registration statement of which this prospectus
forms a part. Our authorized capital stock consists
of:
●
300,000,000 shares
of common stock, par value $0.01 per share, and
●
10,000,000 shares
of preferred stock, par value $0.01 per share, of which 9,736,000
shares are undesignated.
As of February 11,
2019, we had outstanding:
●
203,063,429 shares
of our common stock;
●
4,030 shares of
Series A Convertible Preferred Stock, convertible into 61,335
shares of common stock, subject to adjustment, for no further
consideration;
●
stock options to
purchase 12,512,461 shares of common stock at exercise prices
ranging from $0.37 to $2.80 per share;
●
restricted stock
units representing 4,872,333 shares of common stock which vest on
dates between June 20, 2019 and June 26, 2022, subject to the
fulfillment of service conditions or attaining defined
performance conditions;
●
restricted stock
unit agreements representing 3,952,875 shares of common stock which
have vested but are subject to provisions to delay delivery;
and
●
warrants to
purchase 23,404,046 shares of common stock issuable on the exercise
of warrants at exercise prices ranging from $0.70 to $0.91 per
share.
Common
Stock
We have the
authority to issue 300,000,000 shares of common stock, par value
$0.01 per share. As of February 11, 2019, there were 203,063,429
shares of our common stock outstanding, and a maximum of 44,803,050
shares of common stock were issuable on conversion of outstanding
convertible preferred stock, exercise of outstanding options and
warrants, vesting of restricted stock units and delayed delivery
pursuant to restricted stock unit agreements.
Holders of our
common stock are entitled to one vote per share for the election of
directors and on all other matters that require stockholder
approval. Holders of shares of common stock do not have any
cumulative voting rights. Subject to any preferential rights of any
outstanding preferred stock, in the event of our liquidation,
dissolution or winding up, holders of our common stock are entitled
to share ratably in the assets remaining after payment of
liabilities and the liquidation preferences of any outstanding
preferred stock. See “Preferred Stock” and
“Series A Convertible Preferred Stock,” below. Our
common stock does not carry any redemption rights or any preemptive
or preferential rights enabling a holder to subscribe for, or
receive shares of, any class of our common stock or any other
securities convertible into shares of any class of our common
stock. Holders of our common stock have the right to participate
ratably in dividend distributions. Our outstanding Series A
Preferred Stock, consisting of 4,030 shares on February 11, 2019,
provides that we may not pay a dividend or make any distribution to
holders of any class of stock unless we first pay a special
dividend or distribution of $100 per share to the holders of the
Series A Preferred Stock.
Market
Information
Our common stock is
listed on the NYSE American under the symbol “PTN.” On
February 11, 2019, the closing price of the common stock was $0.74
per share. We do not have any other class of securities listed for
trading.
Transfer
Agent and Registrar
The transfer agent
for our common stock is American Stock Transfer & Trust
Company, located at 6201 15
th
Avenue, Brooklyn,
New York 11219. Their telephone number is (800)
937-5449.
Preferred
Stock
We have the
authority to issue 10,000,000 shares of preferred stock. As of
February 11, 2019, 264,000 shares of our preferred stock were
designated as a single class, Series A Convertible Preferred Stock,
of which 4,030 shares were outstanding (see “Series A
Convertible Preferred Stock” below). The description of
preferred stock provisions set forth below is not complete and is
subject to and qualified in its entirety by reference to our
amended and restated certificate of incorporation and the
certificate of designations relating to the Series A Convertible
Preferred Stock.
The board of
directors has the right, without the consent of holders of common
stock, to designate and issue one or more series of preferred
stock, which may be convertible into common stock at a ratio
determined by the board. A series of preferred stock may bear
rights superior to common stock as to voting, dividends,
redemption, distributions in liquidation, dissolution, or winding
up, and other relative rights and preferences. The board may set
the following terms of any series preferred stock (which will be
specified in the applicable prospectus supplement):
●
the number of
shares constituting the series and the distinctive designation of
the series;
●
dividend rates,
whether dividends are cumulative, and, if so, from what date and
the relative rights of priority of payment of
dividends;
●
voting rights and
the terms of the voting rights;
●
conversion
privileges and the terms and conditions of conversion, including
provision for adjustment of the conversion rate;
●
redemption rights
and the terms and conditions of redemption, including the date or
dates upon or after which shares may be redeemable, and the amount
per share payable in case of redemption, which may vary under
different conditions and at different redemption
dates;
●
sinking fund
provisions for the redemption or purchase of shares;
●
rights in the event
of voluntary or involuntary liquidation, dissolution or winding up
of the corporation, and the relative rights of priority of payment;
and
●
any other relative
powers, preferences, rights, privileges, qualifications,
limitations and restrictions of the series.
Dividends on
outstanding shares of preferred stock will be paid or declared and
set apart for payment before any dividends may be paid or declared
and set apart for payment on the common stock with respect to the
same dividend period.
If upon any
voluntary or involuntary liquidation, dissolution or winding up of
the corporation, the assets available for distribution to holders
of preferred stock are insufficient to pay the full preferential
amount to which the holders are entitled, then the available assets
will be distributed ratably among the shares of all series of
preferred stock in accordance with the respective preferential
amounts (including unpaid cumulative dividends, if any) payable
with respect to each series.
Holders of
preferred stock will not be entitled to preemptive rights to
purchase or subscribe for any shares of any class of capital stock
of the corporation. The preferred stock will, when issued, be fully
paid and non-assessable. The rights of the holders of preferred
stock will be subordinate to those of our general
creditors.
Series
A Convertible Preferred Stock
The board of
directors established a series of 264,000 shares of preferred
stock, designated Series A Convertible Preferred Stock, par value
$0.01 per share (the “Series A”). We issued 137,780
shares of Series A in 1997, of which 4,030 shares remain
outstanding as of February 11, 2019, the rest having been converted
into common stock. The Series A has the following rights and
preferences.
Optional conversion.
Each share of
Series A is convertible at any time, at the option of the holder,
into the number of shares of common stock equal to $100 divided by
the conversion price, as defined in the Series A certificate of
designations. The current conversion price is $6.57, so each share
of Series A is currently convertible into approximately 15 shares
of common stock.
Mandatory conversion.
We may, at our
option, cause the conversion of the Series A, in whole or in part,
on a pro rata basis, into common stock, if the closing bid price of
the common stock has exceeded 200% of the conversion price for at
least 20 trading days in any 30 consecutive trading day period,
ending three days prior to the date of mandatory
conversion.
Price protection provisions.
The
conversion price decreases if we sell common stock (or equivalents)
for a price per share less than the conversion price or less than
the market price of the common stock, subject to certain
exceptions. The conversion price is also subject to adjustment upon
the occurrence of a merger, reorganization, consolidation,
reclassification, stock dividend or stock split which results in an
increase or decrease in the number of shares of common stock
outstanding.
Dividend and distribution preference.
We may not pay a dividend or make any distribution to holders of
any other capital stock unless and until we first pay a special
dividend or distribution of $100 per share to the holders of Series
A.
Liquidation preference.
Upon (i)
liquidation, dissolution or winding up of the Company, whether
voluntary or involuntary, (ii) sale or other disposition of all or
substantially all of the assets of the Company, or (iii) any
consolidation, merger, combination, reorganization or other
transaction in which Palatin is not the surviving entity or in
which the shares of common stock constituting in excess of 50% of
the voting power of the Company are exchanged for or changed into
other stock or securities, cash and/or any other property, after
payment or provision for payment of the debts and other liabilities
of the Company, the holders of Series A will be entitled to
receive, pro rata and in preference to the holders of any other
capital stock, an amount per share equal to $100 plus accrued but
unpaid dividends, if any.
Voting rights.
Each holder of Series A
has the number of votes equal to the number of shares of common
stock issuable upon conversion of the holder’s Series A at
the record date for determination of the stockholders entitled to
vote or, if no record date is established, at the date a vote is
taken. Except as provided above or as required by applicable law,
the holders of the Series A are entitled to vote together with the
holders of the common stock and not as a separate
class.
Debt
Securities
As of the date of
this prospectus, we have no debt securities issued and outstanding
other than a four-year senior secured term loan with a group led by
Horizon Technology Finance Corporation for an original total face
amount of $10,000,000 in the aggregate. As of December 31, 2018,
the total of notes payable was $2,321,123 (including unamortized
discounts and issuance costs of $12,210), together with a final
incremental payment of $500,000, all of which was classified as a
current liability.
The following
description, together with the additional information we include in
any applicable prospectus supplement, summarizes the material terms
and provisions of the debt securities that we may offer under this
prospectus. While the terms we have summarized below will apply
generally to any future debt securities we may offer, we will
describe the particular terms of any debt securities that we may
offer in more detail in the applicable prospectus supplement. The
terms of any debt securities we offer under a prospectus supplement
may differ from the terms we describe below.
We will issue notes
under an indenture, which we will enter into with the trustee named
in the indenture. Any indenture will be qualified under the Trust
Indenture Act of 1939. You should read the summary below, the
applicable prospectus supplement and the provisions of the
applicable indenture and any related security documents, if any, in
their entirety before investing in our debt
securities.
We will describe in
each prospectus supplement the following terms relating to a series
of debt securities:
●
the principal
amount being offered, and if a series, the total amount authorized
and the total amount outstanding;
●
any limit on the
amount that may be issued;
●
whether or not we
will issue the series of debt securities in global form, and if so,
the terms and who the depository will be;
●
the principal
amount due at maturity, and whether the debt securities will be
issued with an original issue discount;
●
whether and under
what circumstances, if any, we will pay additional amounts on any
debt securities held by a person who is not a United States person
for tax purposes, and whether we can redeem the debt securities if
we have to pay such additional amounts;
●
the annual interest
rate, which may be fixed or variable, or the method for determining
the rate and the date interest will begin to accrue, the dates
interest will be payable and the regular record dates for interest
payment dates or the method for determining such
dates;
●
whether or not the
debt securities will be secured or unsecured, and the terms of any
secured debt;
●
the terms of the
subordination of any series of subordinated debt;
●
the place where
payments will be payable;
●
restrictions on
transfer, sale or other assignment, if any;
●
our right, if any,
to defer payment of interest and the maximum length of any such
deferral period;
●
the date, if any,
after which the conditions upon which, and the price at which, we
may, at our option, redeem the series of debt securities pursuant
to any optional or provisional redemption provisions and the terms
of those redemptions provisions;
●
the date, if any,
on which, and the price at which we are obligated, pursuant to any
mandatory sinking fund or analogous fund provisions or otherwise,
to redeem, or at the holder’s option to purchase, the series
of debt securities and the currency or currency unit in which the
debt securities are payable;
●
whether the
indenture will restrict our ability to pay dividends, or will
require us to maintain any asset ratios or reserves;
●
whether we will be
restricted from incurring any additional indebtedness, issuing
additional securities, or entering into a merger, consolidation or
sale of our business;
●
a discussion of any
material or special United States federal income tax considerations
applicable to the debt securities;
●
information
describing any book-entry features;
●
provisions for a
sinking fund purchase or other analogous fund, if any;
●
any provisions for
payment of additional amounts for taxes and any provision for
redemption, if we must pay such additional amount with respect to
any debt security;
●
whether the debt
securities are to be offered at a price such that they will be
deemed to be offered at an “original issue discount” as
defined in paragraph (a) of Section 1273 of the Internal Revenue
Code;
●
the denominations
in which we will issue the series of debt securities, if other than
denominations of $1,000 and any integral multiple
thereof;
●
the terms on which
a series of debt securities may be convertible into or exchangeable
for our common stock, any other of our securities or securities of
a third party, and whether conversion or exchange is mandatory, at
the option of the holder or at our option;
●
whether we and/or
the debenture trustee may change an indenture without the consent
of any holders;
●
the form of debt
security and how it may be exchanged and transferred;
●
descriptions of the
debenture trustee and paying agent, and the method of payments;
and
●
any other specific
terms, preferences, rights or limitations of, or restrictions on,
the debt securities, including any additional events of default or
covenants provided with respect to the debt securities, and any
terms which may be required by us or advisable under applicable
laws or regulations.
Specific indentures
will contain additional important terms and provisions and will be
incorporated by reference as an exhibit to the registration
statement that includes this prospectus, or as an exhibit to a
report filed under the Exchange Act, incorporated by reference in
this prospectus.
Warrants
As of February 11,
2019, warrants for the purchase of 23,404,046 shares of our common
stock were outstanding, exercisable at a weighted average exercise
price of $0.77. The outstanding warrants expire on various dates
from December 23, 2019 through December 6, 2021.
The following
description, together with the additional information we may
include in any applicable prospectus supplement, summarizes the
material terms and provisions of the warrants that we may offer
under this prospectus and the related warrant agreements and
warrant certificates. While the terms summarized below will apply
generally to any warrants that we may offer, we will describe the
particular terms of any series of warrants in more detail in the
applicable prospectus supplement. The terms of any warrants offered
under a prospectus supplement may differ from the terms described
below. Specific warrant agreements will contain additional
important terms and provisions and will be incorporated by
reference as exhibits to the registration statement that includes
this prospectus, or as exhibits to a report filed under the
Exchange Act, incorporated by reference in this
prospectus.
General.
We will describe in the
applicable prospectus supplement the terms of the series of
warrants, including:
●
the offering price
and aggregate number of warrants offered;
●
the currency for
which the warrants may be purchased;
●
if applicable, the
designation and terms of the securities with which the warrants are
issued and the number of warrants issued with each such security or
each principal amount of such security;
●
if applicable, the
date on and after which the warrants and the related securities
will be separately transferable;
●
in the case of
warrants to purchase common stock or preferred stock, the number of
shares of common stock or preferred stock, as the case may be,
purchasable upon the exercise of one warrant and the price at which
these shares may be purchased upon exercise;
●
in the case of
warrants to purchase debt securities, the principal amount of debt
securities purchasable upon exercise of one warrant and the price
at which, and currency in which, this principal amount of debt
securities may be purchased upon exercise;
●
the effect of any
merger, consolidation, sale or other disposition of our business on
the warrant agreement and the warrants;
●
the terms of any
rights to redeem or call the warrants;
●
any provisions for
changes to or adjustments in the exercise price or number of
securities issuable upon exercise of the warrants;
●
the dates on which
the right to exercise the warrants will commence and
expire;
●
the manner in which
the warrant agreement and warrants may be modified;
●
federal income tax
consequences of holding or exercising the warrants;
●
information
relating to book-entry procedures, if any;
●
the terms of the
securities issuable upon exercise of the warrants; and
●
any other specific
terms, preferences, rights or limitations of or restrictions on the
warrants.
Before exercising
their warrants, holders of warrants will not have any of the rights
of holders of the securities purchasable upon such exercise,
including:
●
in the case of
warrants to purchase debt securities, the right to receive payments
of principal of, or premium, if any, or interest on, the debt
securities purchasable upon exercise or to enforce covenants in the
applicable indenture; or
●
in the case of
warrants to purchase common stock or preferred stock, the right to
receive dividends, if any, or, payments upon our liquidation,
dissolution or winding up or to exercise voting rights, if
any.
Exercise of Warrants.
Each warrant will
entitle the holder to purchase the securities that we specify in
the applicable prospectus supplement at the exercise price that we
describe in the applicable prospectus supplement. Unless we
otherwise specify in the applicable prospectus supplement, holders
of the warrants may exercise the warrants at any time up to 5:00
P.M. New York time on the expiration date that we set forth in the
applicable prospectus supplement. After the close of business on
the expiration date, unexercised warrants will become
void.
Holders of the
warrants may exercise the warrants by delivering the warrant
certificate representing the warrants to be exercised together with
specified information, and paying the required amount to the
warrant agent in immediately available funds, as provided in the
applicable prospectus supplement. We will set forth on the reverse
side of the warrant certificate and/or in the applicable prospectus
supplement the information that the holder of the warrant will be
required to deliver to the warrant agent.
Upon receipt of the
required payment and the warrant certificate properly completed and
duly executed at the corporate trust office of the warrant agent or
any other office indicated in the applicable prospectus supplement,
we will issue and deliver the securities purchasable upon such
exercise. If fewer than all of the warrants represented by the
warrant certificate are exercised, then we will issue a new warrant
certificate for the remaining amount of warrants. If we so indicate
in the applicable prospectus supplement, holders of the warrants
may surrender securities as all or part of the exercise price for
the warrants (cashless exercise).
We will describe in
the applicable prospectus supplement exercise procedures for
warrants in a book-entry form, if any.
Enforceability of Rights by Holders of
Warrants.
Each warrant agent will act solely as our agent
under the applicable warrant agreement and will not assume any
obligation or relationship of agency or trust with any holder of
any warrant. A single bank or trust company may act as warrant
agent for more than one issue of warrants. A warrant agent will
have no duty or responsibility in case of any default by us under
the applicable warrant agreement or warrant, including any duty or
responsibility to initiate any proceedings at law or otherwise, or
to make any demand upon us. Any holder of a warrant may, without
the consent of the related warrant agent or the holder of any other
warrant, enforce by appropriate legal action its right to exercise,
and receive the securities purchasable upon exercise of, its
warrants.
ANTI-TAKEOVER
EFFECTS OF PROVISIONS OF DELAWARE LAW
AND
OUR CHARTER DOCUMENTS
Amended
and Restated Certificate of Incorporation
Our amended and
restated certificate of incorporation authorizes the issuance of up
to 10,000,000 shares of preferred stock, par value $.01 per share,
of which 264,000 shares are currently designated as Series A
Convertible Preferred Stock. The board of directors has the
authority, without further approval of the stockholders, to issue
and determine the rights and preferences of other series of
preferred stock, except as limited by the certificate of
designation for the Series A. The board could issue one or more
series of preferred stock with voting, conversion, dividend,
liquidation, or other rights which would adversely affect the
voting power and ownership interest of holders of common stock.
This authority may have the effect of deterring hostile takeovers,
delaying or preventing a change in control, and discouraging bids
for our common stock at a premium over the market
price.
Section
203 of the Delaware General Corporation Law
We are subject to
Section 203 of the Delaware General Corporation Law, which, subject
to certain exceptions, prohibits a Delaware corporation from
engaging in any business combination with any interested
stockholder for a period of three years following the time that
such stockholder became an interested stockholder,
unless:
●
prior to such time,
the board of directors approved either the business combination or
the transaction that resulted in the stockholder becoming an
interested holder;
●
upon consummation
of the transaction that resulted in the stockholder becoming an
interested stockholder, the interested stockholder owned at least
85% of the voting stock of the corporation outstanding at the time
the transaction commenced, excluding for purposes of determining
the number of shares outstanding those shares owned (a) by persons
who are directors and also officers and (b) by employee stock plans
in which employee participants do not have the right to determine
confidentially whether shares held subject to the plan will be
tendered in a tender or exchange offer; or
●
at or subsequent to
such time, the business combination is approved by the board of
directors and authorized at an annual or special meeting of
stockholders, and not by written consent, by the affirmative vote
of at least two thirds of the outstanding voting stock which is not
owned by the interested stockholder.
In general, Section
203 defines “business combination” to include the
following:
●
any merger or
consolidation involving the corporation and the interested
stockholder;
●
any sale, transfer,
pledge or other disposition of 10% or more of the assets of the
corporation involving the interested stockholder;
●
subject to certain
exceptions, any transaction that results in the issuance or
transfer by the corporation of any stock of the corporation to the
interested stockholder;
●
any transaction
involving the corporation that has the effect of increasing the
proportionate share of the stock or any class or series of the
corporation beneficially owned by the interested stockholder;
or
●
the receipt by the
interested stockholder of the benefit of any loans, advances,
guarantees, pledges or other financial benefits provided by or
through the corporation.
In general, Section
203 defines “interested stockholder” as an entity or
person beneficially owning 15% or more of the outstanding voting
stock of the corporation and any entity or person affiliated with
or controlling or controlled by such entity or person.
Indemnification
and Limitation of Liability
Our amended and
restated certificate of incorporation and bylaws require us to
indemnify our directors, officers, employees and agents against the
costs (including fines, judgments and attorney fees) from
involvement in legal proceedings arising from their position or
service, provided that the person seeking indemnification
acted:
●
in a manner he or
she reasonably believed to be in, or not opposed to, the best
interests of the corporation; and,
●
with respect to any
criminal action or proceeding, had no reasonable cause to believe
his or her conduct was unlawful.
The amended and
restated certificate of incorporation and bylaws allow us to buy
indemnification insurance for this purpose.
Our certificate of
incorporation provides that, to the fullest extent permissible
under Delaware law, no director shall be personally liable to the
corporation or its stockholders for monetary damages for breach of
a fiduciary duty as a director. However, this provision does not
eliminate the duty of care, and in appropriate circumstances,
equitable remedies such as injunctive or other forms of
non-monetary relief that will remain available under Delaware law.
In addition, each director will continue to be subject to liability
for (a) breach of the director’s duty of loyalty to us or our
stockholders, (b) acts or omissions not in good faith or which
involve intentional misconduct or a knowing violation of law, (c)
violating Section 174 of the Delaware General Corporation Law, or
(d) any transaction from which the director derived an improper
personal benefit. The provision also does not affect a
director’s responsibilities under any other law, such as the
federal securities laws or state or federal environmental
laws.
PLAN
OF DISTRIBUTION
We may sell
securities under this prospectus in public offerings:
●
through one or more
underwriters or dealers;
●
through other
agents;
●
directly to
investors; or
●
through a
combination of any of these methods.
We may price the
securities we sell under this prospectus:
●
at a fixed public
offering price or prices, which we may change from time to
time;
●
at market prices
prevailing at the times of sale;
●
at prices
calculated by a formula based on prevailing market
prices;
●
at negotiated
prices; or
●
in a combination of
any of the above pricing methods.
If we use
underwriters for an offering, they will acquire securities for
their own account and may resell them from time to time in one or
more transactions at a fixed public offering price or at varying
prices determined at the time of sale. The obligations of the
underwriters to purchase the securities will be subject to the
conditions set forth in the applicable underwriting agreement. We
may offer the securities to the public through underwriting
syndicates represented by managing underwriters or by underwriters
without a syndicate. Subject to certain conditions, the
underwriters will be obligated to purchase all the securities of
the series offered by the prospectus supplement. The public
offering price and any discounts or concessions allowed or
re-allowed or paid to dealers may change from time to time. Only
underwriters named in a prospectus supplement are underwriters of
the securities offered by that prospectus supplement.
We may offer our
securities in “at the market” offerings, with the
meaning of Rule 415(a)(4) of the Securities Act, into an existing
trading market on terms described in the applicable prospectus
supplement. Underwriters and dealers may participate in any
“at the market” offering.
We may also sell
securities directly or through agents. We will name any agent
involved in an offering and we will describe any commissions we
will pay the agent in the prospectus supplement. Unless the
prospectus supplement states otherwise, our agents will act on a
best-efforts basis.
We may authorize
agents or underwriters to solicit offers by certain types of
institutional investors to purchase securities from us at the
public offering price set forth in the prospectus supplement
pursuant to delayed delivery contracts providing for payment and
delivery on a specified date in the future. We will describe the
conditions of these contracts and the commissions we must pay for
solicitation of these contracts in the prospectus
supplement.
We may provide
agents and underwriters with indemnification against certain civil
liabilities, including liabilities under the Securities Act, or
contribution with respect to payments that the agents or
underwriters may make with respect to such liabilities.
Underwriters or agents may engage in transactions with us, or
perform services for us, in the ordinary course of business. We may
also use underwriters or agents with whom we have a material
relationship. We will describe the nature of any such relationship
in the prospectus supplement.
An underwriter may
engage in overallotment, stabilizing transactions, short covering
transactions and penalty bids in accordance with Regulation M under
the Exchange Act . Overallotment involves sales in excess of the
offering size, which create a short position. Stabilizing
transactions permit bids to purchase the underlying security so
long as the stabilizing bids do not exceed a specified maximum.
Short covering transactions involve purchases of the securities in
the open market after the distribution is completed to cover short
positions. Penalty bids permit the underwriter to reclaim a selling
concession from a dealer when the securities originally sold by the
dealer are purchased in a covering transaction to cover short
positions. These activities may cause the price of our securities
to be higher than it would otherwise be on the open market. The
underwriter may discontinue any of these activities at any
time.
All securities we
offer, other than common stock, will be new issues of securities,
with no established trading market. Underwriters may make a market
in these securities, but will not be obligated to do so and may
discontinue market making at any time without notice. We cannot
guarantee the liquidity of the trading markets for any
securities.
LEGAL
MATTERS
Unless otherwise
specified in the applicable prospectus supplement, the validity of
the securities covered by this prospectus will be passed upon for
us by Thompson Hine LLP, New York, New York. In addition, counsel
that will be named in the applicable prospectus supplement will
pass upon the validity of any securities offered under the
applicable prospectus supplement for any underwriters or
agents.
EXPERTS
The consolidated
financial statements of Palatin Technologies, Inc. and subsidiary
as of June 30, 2018 and 2017, and for each of the years in the
three-year period ended June 30, 2018, and management’s
assessment of the effectiveness of internal control over financial
reporting as of June 30, 2018, have been incorporated by reference
herein and in the registration statement in reliance upon the
reports of KPMG LLP, independent registered public accounting firm,
incorporated by reference herein, and upon the authority of said
firm as experts in accounting and auditing.
PALATIN TECHNOLOGIES, INC.
Up to $40,000,000
Common Stock
PROSPECTUS SUPPLEMENT
Canaccord Genuity
June 21, 2019
Palatin Technologies (AMEX:PTN)
Historical Stock Chart
From Oct 2024 to Nov 2024
Palatin Technologies (AMEX:PTN)
Historical Stock Chart
From Nov 2023 to Nov 2024