Econiche(TM) Vaccine Efficacy Challenge Study Summarized in The Canadian Journal of Veterinary Research
May 05 2011 - 4:00PM
PR Newswire (Canada)
BELLEVILLE, ON, May 5 /CNW/ -- -final study leading to full
Canadian license for the vaccine- BELLEVILLE, ON, May 5 /CNW/ -
Bioniche Life Sciences Inc. (TSX: BNC) (ASX: BNC), a
research-based, technology-driven Canadian biopharmaceutical
company, today announced that the results of a controlled challenge
study with the Company's Escherichia coli (E. coli) O157 cattle
vaccine -Econiche(TM) - have been published in this month's issue
of The Canadian Journal of Veterinary Research (2011;75:98-105), a
peer-reviewed scientific journal. The article, entitled,
"Vaccination with type III secreted proteins leads to decreased
shedding in calves after experimental infection with Escherichia
coli O157", was co-authored by Kevin J. Allen, Dragan Rogan, B.
Brett Finlay, Andrew A. Potter, David J. Asper. This article
summarized a controlled challenge study conducted in early 2008 at
the Vaccine and Infectious Disease Organization (VIDO) - University
of Saskatchewan which was the final study that led to the full
Canadian license for Econiche(TM)( )from the Canadian Food
Inspection Agency (CFIA) in October, 2008. The controlled challenge
study at VIDO involved 30 placebo-treated cows and 30 vaccinated
cows. The vaccinated cows each received three doses of
Econiche(TM). Both groups were then infected with E. coli O157 and
fecal shedding was monitored daily for a two-week period. These
data indicate that Econiche(TM) vaccination had protective effects
through significant reductions in both the number of animals
shedding and the number of challenge organisms shed per animal. The
authors of the article summarized the study by saying it provides
evidence that vaccination with Econiche(TM) is an effective
pre-harvest intervention strategy against E. coli O157. These data
constitute part of the Company's international regulatory dossier
in such jurisdictions as Australasia, Europe, Scandinavia, and
South America. The Company has been actively exploring
opportunities to introduce Econiche(TM) to markets beyond North
America, where it has a full license (Canada) and a pending
conditional license (U.S.). Econiche(TM) will be manufactured in
the Company's newly completed Animal Health and Food Safety Vaccine
Manufacturing Centre in Belleville, Ontario, currently undergoing
validation and commissioning. Full production in the new Centre is
expected to begin by the fall of 2011. About the Bioniche E. coli
O157 Vaccine (Econiche(TM)) The Food Safety Division of Bioniche
Life Sciences Inc. has worked with Canadian university researchers,
in particular, inventor Dr. Brett Finlay and his team at the
University of British Columbia, to develop and license a cattle
vaccine against E. coli O157. This vaccine - trademarked
Econiche(TM) - is meant to reduce the level of the bacterium in
water, food and the environment and, in turn, reduce the potential
infection of humans. Econiche(TM) has the potential to
significantly reduce the amount of E. coli O157 shed into the
environment by beef and dairy cattle. This organism does not cause
illness in cattle, but cattle are the primary reservoir for it.
Vaccination of cattle with Econiche(TM) can help reduce the risk of
food and waterborne contamination with E. coli O157. Econiche(TM)
has been developed by a strategic alliance formed in September,
2000 and composed of the University of British Columbia (UBC), the
Alberta Research Council (ARC), the University of Saskatchewan's
Vaccine and Infectious Disease Organization (VIDO), and Bioniche,
which holds the rights for worldwide commercialization of the
vaccine. More about E. coli O157 The E. coli O157 organism causes
no disease in cattle, but cattle are the primary reservoir for it.
E. coli O157 can cause severe illness and can even be fatal when
ingested by humans from contaminated meat, vegetables, other food
products, or water. Human exposure and infection with E. coli O157
can result in serious health consequences, including abdominal pain
and severe bloody diarrhea. In severe cases, kidney damage can
occur and progress to serious complications and even death.
Lingering, long-term medical conditions can persist in individuals
exposed to the bacterium. These include post-infectious irritable
bowel syndrome (PI-IBS), reduced kidney function, diabetes,
hypertension and reactive arthritis. An estimated 100,000 cases of
human infection with the E. coli O157 organism are reported each
year in North America. Two to seven per cent of those people
develop haemolytic uremic syndrome (HUS), a disease characterized
by kidney failure. Five percent of HUS patients die, many of them
children and senior citizens, whose kidneys are more sensitive to
damage. About Bioniche Life Sciences Inc. Bioniche Life Sciences
Inc. is a research-based, technology-driven Canadian
biopharmaceutical company focused on the discovery, development,
manufacturing, and marketing of proprietary products for human and
animal health markets worldwide. The fully-integrated company
employs more than 200 skilled personnel and has three operating
divisions: Human Health, Animal Health, and Food Safety. The
Company's primary goal is to develop proprietary cancer therapies
supported by revenues from marketed products in human and animal
health. For more information, please visit www.Bioniche.com. Except
for historical information, this news release may contain
forward-looking statements that reflect the Company's current
expectation regarding future events. These forward-looking
statements involve risk and uncertainties, which may cause, but are
not limited to, changing market conditions, the successful and
timely completion of clinical studies, the establishment of
corporate alliances, the impact of competitive products and
pricing, new product development, uncertainties related to the
regulatory approval process, and other risks detailed from time to
time in the Company's ongoing quarterly and annual reporting. To
view this news release in HTML formatting, please use the following
URL:
http://www.newswire.ca/en/releases/archive/May2011/05/c9941.html p
align="justify" Jennifer Shea, Vice-President, Communications,
Investor & Government Relationsbr/ Bioniche Life Sciences
Inc.br/ Telephone: (613) 966-8058; from Australia: 0011 1
613-966-8058br/ Cell: (613) 391-2097; from Australia: 0011 1
613-391-2097br/ a
href="mailto:Jennifer.Shea@Bioniche.com"Jennifer.Shea@Bioniche.com/a
/p
Copyright
Purpose Canadian Financi... (TSX:BNC)
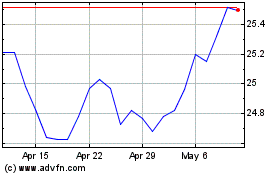
Historical Stock Chart
From Jun 2024 to Jul 2024
Purpose Canadian Financi... (TSX:BNC)
Historical Stock Chart
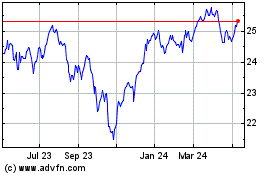
From Jul 2023 to Jul 2024