Orphan Drug Designation
June 22 2006 - 3:01AM
UK Regulatory
RNS Number:9615E
VASTox plc
22 June 2006
VASTOX RECEIVES POSITIVE ORPHAN DRUG DESIGNATION OPINION FROM THE EMEA FOR ITS
DUCHENNE MUSCULAR DYSTROPHY THERAPY
Oxford, UK, 22 June 2006 - VASTox (AIM: VOX), a leading chemical genomics
company, announces today that the European Medicines Agency (EMEA) has
recommended awarding orphan drug designation for the company's initial compound
for the treatment of Duchenne muscular dystrophy (DMD).
The orphan drug application has received a positive opinion from the Committee
for Orphan Medicinal Products (COMP) of EMEA. VASTox expects the formal approval
from the European Commission within the next 30 days. The European Commission
has never failed to ratify a positive opinion from EMEA.
Orphan drug designation in Europe provides VASTox with ten years of market
exclusivity following market approval. This protection is in addition to any
intellectual property rights that cover the product which is marketed. In
addition, the designation provides VASTox with support and assistance throughout
the drug development process, including waiving significant regulatory costs
before a drug reaches the market.
EMEA grants orphan drug designation to medical products that satisfy a number of
criteria, including the severity of the disease for which they will be used, the
prevalence of the disease in Europe and the lack of alternative treatments.
Duchenne muscular dystrophy satisfies these criteria and VASTox was able to show
that the company's approach to treat this condition (i.e. the up-regulation of
utrophin) could be of significant benefit to these patients for which there are
at present very limited treatment options.
DMD is the company's most advanced drug development programme. At present the
company is working on selecting a lead drug candidate. This important milestone
is expected to be reached by Q1 2007, with clinical trials forecast to start a
year afterwards.
Steven Lee, PhD, CEO of VASTox said: "I am very pleased that VASTox has received
this positive opinion from the Committee for Orphan Medicinal Products.
Receiving orphan drug designation is an important step for the company; it
validates our unique approach to the treatment of Duchenne muscular dystrophy
while at the same time it will reduce the costs of developing what we hope will
be a major advance in the treatment of this devastating neuromuscular disease."
- ends -
For more information please contact:
VASTox
Steven Lee, PhD, Chief Executive Officer Tel: +44 (0)1235 443910
Darren Millington, Chief Financial Officer
Citigate Dewe Rogerson Tel: +44 (0)207 638 9571
David Dible / Mark Swallow / Valerie Auffray
About VASTox plc
VASTox is a chemical genomics technology company that discovers and develops
proprietary novel drugs and provides services to the pharmaceutical industry.
The company's most advanced drug development programme is focused on developing
a new treatment for Duchenne muscular dystrophy based on the up-regulation of
utrophin. A second drug development programme for spinal muscular atrophy is
also progressing rapidly. VASTox has three additional programmes focused on
osteoarthritis, cancer and tuberculosis that are expected to be out-licensed
prior to entering the clinic.
The company's technology platform, which uses zebrafish and fruitflies, has the
potential to dramatically decrease the time and cost of drug discovery and
development. This is because using whole organisms allows it to carry out high
volume, high content screening that delivers data which is highly predictive of
the efficacy and toxicity of potential drug compounds in humans. VASTox is
growing revenues based on marketing its unique technology platform and its
chemistry expertise. The company listed on the AIM market of the London Stock
Exchange in October 2004.
Further information about the company is available at www.vastox.com.
This document contains "forward-looking statements" within the meaning of the
U.S. Private Securities Litigation Reform Act of 1995. Forward-looking
statements can be identified by words such as "anticipates", "intends", "plans",
"seeks", "believes", "estimates", "expects" and similar references to future
periods, or by the inclusion of forecasts or projections.
Forward-looking statements are based on the Company's current expectations and
assumptions regarding our business, the economy and other future conditions.
Because forward-looking statements relate to the future, by their nature, they
are subject to inherent uncertainties, risks and changes in circumstances that
are difficult to predict. The Company's actual results may differ materially
from those contemplated by the forward-looking statements. The Company cautions
you therefore that you should not rely on any of these forward-looking
statements as statements of historical fact or as guarantees or assurances of
future performance. Important factors that could cause actual results to differ
materially from those in the forward-looking statements include (factors
included in this presentation) and regional, national, global political,
economic, business, competitive, market and regulatory conditions.
This information is provided by RNS
The company news service from the London Stock Exchange
END
MSCSEDFLFSMSELM
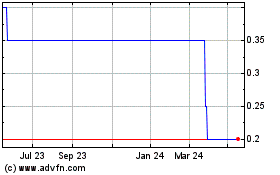
Vox Valor Capital (LSE:VOX)
Historical Stock Chart
From Jun 2024 to Jul 2024
Vox Valor Capital (LSE:VOX)
Historical Stock Chart
From Jul 2023 to Jul 2024