Antibe Therapeutics Completes Single Ascending Dose Portion of ATB-346 Phase I Program
October 06 2014 - 7:30AM
Business Wire
Antibe Therapeutics Inc. (“Antibe” or the “Corporation”)
(TSXV:ATE; OTCQX:ATBPF) is pleased to report that the pre-scheduled
single ascending dose portion of its Phase I program has been
completed and has reached its primary objectives of safety and
tolerability of ATB-346, up to the maximum dose tested (1500 mg).
The results of this portion of the Phase I program have met the
corporation’s expectations based on extensive pre-clinical studies,
and support the continuation of the Phase I program into the
multiple ascending dose and food-effect portions, both of which are
underway and are expected to be completed by January 2015.
About Antibe Therapeutics Inc.
Antibe develops safer medicines for pain and inflammation.
Antibe’s technology involves linking a hydrogen sulfide-releasing
molecule to an existing drug to produce a patented, improved
medicine. Antibe’s ATB-346 targets the global need for safer
non-steroidal anti-inflammatory drugs (NSAIDs).
Neither the TSX Venture Exchange nor its Regulation Services
Provider (as that term is defined in the policies of the TSX
Venture Exchange) accepts responsibility for the adequacy or
accuracy of this release.
Antibe Therapeutics Inc.Dan Legault, 416-473-4095Chief Executive
Officerdan.legault@antibethera.comwww.antibethera.com
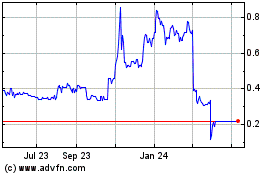
Antibe Therapeutics (CE) (USOTC:ATBPF)
Historical Stock Chart
From Oct 2024 to Nov 2024
Antibe Therapeutics (CE) (USOTC:ATBPF)
Historical Stock Chart
From Nov 2023 to Nov 2024