- Troriluzole 200 mg dosed orally, once daily, in patients with
SCA met the study's primary endpoint on the change from baseline in
the modified functional Scale for the Assessment and Rating of
Ataxia (f-SARA) at 3 years in all study population genotypes.
- Troriluzole also showed statistically significant superiority
after both 1 and 2 years of treatment.
- Troriluzole achieved statistically significant superiority on 9
consecutive, prespecified primary and secondary endpoints.
- SCA patients treated with troriluzole showed a 50-70% slowing
of disease progression, representing 1.5-2.2 years delay in disease
progression over the 3-year study period.
- Biohaven plans to submit a New Drug Application (NDA) to the US
Food and Drug Administration (FDA) for troriluzole in the treatment
of all SCA genotypes in 4Q 2024. The application is eligible for a
priority review given orphan drug and fast-track designations
previously granted by FDA.
- Conference call and webcast to be held today at 8:30am ET
NEW
HAVEN, Conn., Sept. 23,
2024 /PRNewswire/ -- Biohaven Ltd. (NYSE: BHVN)
(Biohaven or the Company), today announced positive topline
results from pivotal Study BHV4157-206-RWE (NCT06529146)
demonstrating the efficacy of troriluzole on the mean change from
baseline in the f-SARA after 3 years of treatment. The study
achieved the primary endpoint and showed statistically significant
improvements on the f-SARA at years 1 and 2 (Figure 1). SCA is a
rare, progressively debilitating neurodegenerative disease that
affects approximately 15,000 people in the United States and 24,000 in Europe and the United Kingdom. There are no FDA approved
treatments for SCA.
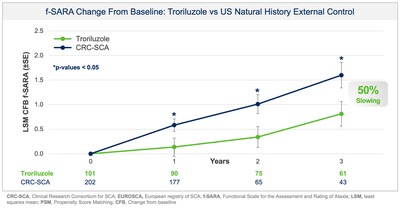
Collectively, data across multiple analyses demonstrate a robust
and clinically meaningful slowing of disease progression in SCA
patients. These treatment benefits translate into a 50-70% slower
rate of decline compared to untreated patients, representing
1.5-2.2 years delay in disease progression over the 3-year study
period. Additionally, in a responder sensitivity analysis, disease
progression when defined by a 2 point or greater worsening on the
f-SARA at 3 years showed an odds ratio (OR) of 4.1 (95% CI: 2.1,
8.1) for the untreated external control arm versus troriluzole
treated subjects (p < 0.0001; pooled analysis).
Dr. Susan Perlman, Director of
Ataxia Clinic and Neurogenetics Clinical Trials at the David Geffen
School of Medicine at UCLA stated, "SCA
is a debilitating, relentlessly progressive disease that destroys
quality of life, leaving patients unable to care for themselves,
walk, or speak. Troriluzole is the very first treatment to show a
delay in disease progression that can give patients additional
years of independence, where they can walk without assistance,
continue to work, play with their children, and participate in
daily activities. This is an exciting and hopeful moment for the
SCA community."
Study BHV4157-206-RWE was designed, in discussion with the US
Food and Drug Administration (FDA), to assess the effectiveness of
troriluzole in SCA after 3 years of treatment as measured by the
change from baseline in the f-SARA. The study utilized Phase 3 data
and an external control of matched, untreated SCA subjects from the
US Clinical Research Consortium for the Study of Cerebellar Ataxia
(CRC-SCA) in accordance with FDA's Guidance on Real-World Evidence
(RWE) of effectiveness. All endpoints were prespecified, and both
the study protocol and statistical analysis plan were submitted to,
and reviewed by, FDA prior to topline data analysis. The new
analysis doubled the previously available 3 year data with 63
subjects now completing 3 years of treatment with troriluzole and
matched to the external control arm. Propensity Score Matching
(PSM) was used to ensure that untreated patients from the CRC-SCA
study were rigorously matched to treated patients from Study
BHV4157-206 on baseline characteristics. The primary
objective was to examine the treatment effects of troriluzole for
up to 3 years, by comparing data on the f-SARA from patients
treated with troriluzole in Study BHV4157-206 to untreated patients
from the natural history study. Troriluzole-treated patients
demonstrated statistically significant and sustained benefits at
years 1, 2 and 3 on the f-SARA compared to a rigorously matched
natural history control.
Additionally, prespecified analyses in the protocol employed a
separate, independent natural history control from the European SCA
natural history study (EUROSCA) for global regulatory purposes.
Results using the EUROSCA patients, in addition to a pooled
analysis using both CRC-SCA and EUROSCA patients, as the external
controls were also statistically significant and consistent with
the primary efficacy analysis at all timepoints (see Figure 2 and
Figure 3). The addition of EUROSCA data increased the external
control sample size and added to the robustness of the
statistically significant treatment differences at years 1, 2, and
3, favoring troriluzole.
Jeremy Schmahmann, M.D.,
Professor of Neurology at Harvard Medical
School and Founding Director of the Ataxia Center at
Massachusetts General Hospital commented, "The stabilization of SCA
symptoms as reflected by the topline data at 3 years along with the
previously reported reductions in falls show the therapeutic
potential of troriluzole. I cannot underscore enough the impact of
a potential treatment that can slow SCA disease progression and the
effect on patients and caregivers who have helplessly watched
generations of family members deteriorate and die from SCA. These
new data provide support for troriluzole as a safe and effective
once daily treatment for patients with SCA."
Spinocerebellar ataxia is a group of dominantly inherited
neurodegenerative disorders characterized by progressive loss of
voluntary motor control and atrophy of the cerebellum, brainstem
and spinal cord. Patients experience significant morbidity,
including progression to a wheelchair, impaired gait leading to
falls, inability to communicate due to speech impairment,
difficulty swallowing, and premature death. While signs and
symptoms can appear anytime from childhood to late adulthood, SCA
typically presents in early adulthood and progresses over a number
of years. Currently, there are no FDA-approved treatments and no
cure for SCA.
Vlad Coric, M.D., Chief Executive
Officer and Chairman of Biohaven stated, "Advancing new therapies
for patients with rare diseases is often a multiyear process of
collaboration across academic, patient advocacy, regulatory and
industry partners. The Biohaven team has always been committed to
rigorously following the science in this area, and through our
partnership with the National Ataxia Foundation and collaboration
with leading SCA experts across the globe, our SCA development
program has provided the first evidence of a clinically meaningful
treatment benefit as well as slowing disease progression in SCA
patients. We were excited to receive the positive topline results
from Study BHV4157-206-RWE, which was designed with FDA input and
pursuant to the principles outlined in the FDA's guidance for the
use of real-world evidence. The need for treatments for this
deadly neurodegenerative disease is urgent. As a company, we remain
committed to developing novel therapies for patients living with
rare disorders with no approved therapies, like SCA. We look
forward to interacting with regulatory agencies to bring
troriluzole to patients with SCA."
Andrew Rosen, Chief Executive
Officer of the National Ataxia Foundation (NAF), shared, "Biohaven
was the first company to join NAF's Drug Development Collaborative
(DDC), a group of pharmaceutical companies dedicated to bringing
together advocates, clinicians, regulatory agencies, and the
patient community to advance research and facilitate the
development of therapies for ataxia. Today's topline results are
the culmination of years dedicated to studying troriluzole as a
treatment for SCA. Patients and families have been waiting for
decades for a treatment that could slow disease progression in this
devastating and relentlessly progressive disorder".
Based upon the topline data from Study BHV4157-206-RWE, and
previous safety and efficacy data from the troriluzole development
program in SCA, Biohaven plans to submit a New Drug Application
(NDA) to the FDA in Q4 2024. The troriluzole development program
has generated the largest clinical trial dataset in SCA and now has
follow-up in some patients treated with troriluzole for over 5
years. Biohaven has previously received both Fast-Track and
Orphan drug designation (ODD) from the FDA, and ODD from the
European Medicines Agency, for troriluzole in SCA. An NDA with ODD
is eligible for priority FDA review. Biohaven will be prepared to
commercialize SCA in the US in 2025, if ultimately approved, based
on potential priority review timelines.
Conference Call and Webcast Details
Biohaven will hold
a live conference call and webcast today at 8:30 a.m. Eastern
Time. The webcast may be accessed via the Investor Relations
portion of Biohaven's website
at https://ir.biohaven.com/events-presentations/events. To
participate in the live conference call via telephone, please
register here. Upon registering, a dial-in number and unique
PIN will be provided to join the conference call.
About Troriluzole
Troriluzole is a new chemical entity
(NCE) and third-generation novel prodrug that modulates glutamate,
the most abundant excitatory neurotransmitter in the human body.
The primary mode of action of troriluzole is reducing synaptic
levels of glutamate. Troriluzole increases glutamate uptake from
the synapse, by augmenting the expression and function of
excitatory amino acid transporters located on glial cells that play
a key role in clearing glutamate from the synapse. Troriluzole has
the potential to be developed in a number of other diseases
associated with excessive glutamate. More information about
troriluzole can be found at the Company's website:
https://www.biohaven.com/pipeline/clinical-programs/glutamate/.
About Biohaven
Biohaven is a
biopharmaceutical company focused on the discovery, development,
and commercialization of life-changing treatments in key
therapeutic areas, including immunology, neuroscience, and
oncology. The company is advancing its innovative portfolio of
therapeutics, leveraging its proven drug development experience and
multiple proprietary drug development
platforms. Biohaven's extensive clinical and preclinical
programs include Kv7 ion channel modulation for epilepsy and mood
disorders; extracellular protein degradation for immunological
diseases; TRPM3 antagonism for migraine and neuropathic pain;
TYK2/JAK1 inhibition for neuroinflammatory disorders; glutamate
modulation for OCD and SCA (spinocerebellar ataxia); myostatin
inhibition for neuromuscular and metabolic diseases, including SMA
and obesity; antibody recruiting bispecific molecules and antibody
drug conjugates for cancer. For more information, visit
www.biohaven.com.
Forward-looking Statements
This news release includes
forward-looking statements within the meaning of the Private
Securities Litigation Reform Act of 1995, including statements
about Biohaven Ltd. and our planned and ongoing clinical trials,
the timing of and the availability of data from those trials, the
timing and our decisions to proceed with our planned regulatory
filings (including our plans to submit a NDA to the FDA for
troriluzole in the treatment of all SCA genotypes in 4Q 2024), the
timing of and our ability to obtain regulatory approvals for our
product candidates (including the timing of the regulatory approval
for troriluzole in order to commercialize SCA in the United States in 2025), the clinical
potential utility of our product candidates, alone and as compared
to other existing potential treatment options, and the potential
advancement of our early phase programs. The use of certain words,
including "continue", "plan", "will", "believe", "may", "expect",
"anticipate" and similar expressions, is intended to identify
forward-looking statements. Investors are cautioned that any
forward-looking statements, including statements regarding the
future development, timing and potential marketing approval and
commercialization of our development candidates, are not guarantees
of future performance or results and involve substantial risks and
uncertainties. Actual results, developments and events may differ
materially from those in the forward-looking statements as a result
of various factors including: the expected timing, commencement and
outcomes of Biohaven's planned and ongoing clinical trials; the
timing of planned interactions and filings with the FDA; the timing
and outcome of expected regulatory filings, including the timing
and outcome of the NDA for troriluzole; complying with applicable
U.S. regulatory requirements; the potential commercialization of
Biohaven's product candidates, including the commercialization of
SCA in the United States in 2025;
and the effectiveness and safety of Biohaven's product candidates.
You should, therefore, not rely on these forward-looking statements
as representing our views as of any date subsequent to the date of
this presentation. Additional important factors to be considered in
connection with forward-looking statements are described in the
Company's filings with the Securities and Exchange Commission,
including within the sections titled "Risk Factors" and
"Management's Discussion and Analysis of Financial Condition and
Results of Operations". The forward-looking statements are made as
of the date of this presentation, and Biohaven does not undertake
any obligation to update any forward-looking statements, whether as
a result of new information, future events or otherwise, except as
required by law. This presentation also contains market data and
other information based on industry publications, reports by market
research firms or published independent sources. Some market data
and information is also based on the Company's good faith
estimates, which are derived from management's knowledge of its
industry and such independent sources referred to above.
Investor Contact:
Jennifer
Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Mike
Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/biohaven-achieves-positive-topline-results-in-pivotal-study-of-troriluzole-in-spinocerebellar-ataxia-sca-302255056.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/biohaven-achieves-positive-topline-results-in-pivotal-study-of-troriluzole-in-spinocerebellar-ataxia-sca-302255056.html
SOURCE Biohaven Ltd.