TABLE OF CONTENTS
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14D-9
(Rule 14d-101)
SOLICITATION/RECOMMENDATION STATEMENT
UNDER SECTION 14(d)(4) OF THE SECURITIES EXCHANGE ACT OF 1934
Zynerba Pharmaceuticals, Inc.
(Name of Subject Company)
Zynerba Pharmaceuticals, Inc.
(Name of Persons Filing Statement)
COMMON STOCK, PAR VALUE $0.001 PER SHARE
(Title of Class of Securities)
98986X109
(CUSIP Number of Class of Securities)
Armando Anido
Zynerba Pharmaceuticals, Inc.
Chairman and Chief Executive Officer
80 W. Lancaster Avenue, Suite 300
Devon, Pennsylvania 19333
(484) 581-7505
(Name, address, and telephone numbers of person authorized to receive notices and communications
on behalf of the persons filing statement)
With copies to:
Rachael M. Bushey, Esq.
Jennifer L. Porter, Esq.
Laura K. Umbrecht, Esq.
Goodwin Procter LLP
One Commerce Square
2005 Market St., 32nd Floor
Philadelphia, PA 19103
(445) 207-7800
☐
| Check the box if the filing relates solely to preliminary communications made before the commencement of a tender offer. |
TABLE OF CONTENTS
| SUBJECT COMPANY INFORMATION |
Name and Address
The name of the subject company to which this Solicitation/Recommendation Statement on Schedule 14D-9 (together with any exhibits and annexes attached hereto, this “Schedule 14D-9”) relates is Zynerba Pharmaceuticals, Inc., a Delaware corporation (“Zynerba” or the “Company”). The address of Zynerba’s principal executive office is 80 W. Lancaster Avenue, Suite 300, Devon, Pennsylvania 19333, and its telephone number is (484) 581-7505.
Securities
The title of the class of equity securities to which this Schedule 14D-9 relates is Zynerba’s common stock, par value $0.001 per share (the “Shares”). As of the close of business on August 24, 2023, there were 53,939,431 Shares issued and outstanding.
TABLE OF CONTENTS
| IDENTITY AND BACKGROUND OF FILING PERSON |
Name and Address
The name, business address and business telephone number of Zynerba, which is both the person filing this Schedule 14D-9 and the subject company, are set forth above in “Item 1. Subject Company Information—Name and Address.”
Tender Offer
This Schedule 14D-9 relates to a tender offer by Xylophone Acquisition Corp., a Delaware corporation (“Purchaser”), a wholly owned subsidiary of Harmony Biosciences Holdings, Inc., a Delaware corporation (“Harmony Biosciences”), to acquire all of the issued and outstanding Shares for (i) $1.1059 per Share (the “Closing Amount”), in cash, subject to any applicable withholding of taxes and without interest, plus (ii) one contingent value right (each, a “CVR”) per Share (the “CVR Amount”), which represents the right to receive contingent payments, in cash, subject to any applicable withholding of taxes and without interest, upon the achievement of the milestones set forth in, and subject to and in accordance with the terms and conditions of, the CVR Agreement (as defined in Item 3. Past Contacts, Transactions, Negotiations and Agreements—Arrangements with Harmony Biosciences and Purchaser and their Affiliates—CVR Agreement below) (the Closing Amount plus the CVR Amount, collectively, being the “Offer Price”), upon the terms and subject to the conditions set forth in the Offer to Purchase, dated August 28, 2023 (as amended or supplemented from time to time, the “Offer to Purchase”), and in the related Letter of Transmittal (as amended or supplemented from time to time, the “Letter of Transmittal,” which, together with the Offer to Purchase, constitute the “Offer”).
The Offer is described in a Tender Offer Statement on Schedule TO (as amended or supplemented from time to time, and together with the exhibits thereto, the “Schedule TO”), filed by Harmony Biosciences and Purchaser with the U.S. Securities and Exchange Commission (the “SEC”) on August 28, 2023. Copies of the Offer to Purchase and form of Letter of Transmittal are filed as Exhibits (a)(1)(A) and (a)(1)(B) hereto, respectively, and are incorporated herein by reference. The Offer to Purchase and form of Letter of Transmittal are being mailed to Zynerba’s stockholders together with this Schedule 14D-9.
The Offer is being made pursuant to an Agreement and Plan of Merger, dated as of August 14, 2023 (as it may be amended or supplemented, the “Merger Agreement”), by and among Zynerba, Purchaser and Harmony Biosciences, pursuant to which, among other matters, after the completion of the Offer and the satisfaction or waiver of certain conditions set forth in the Merger Agreement, Purchaser will merge with and into Zynerba (the “Merger”) pursuant to Section 251(h) of the General Corporation Law of the State of Delaware, as amended (the “DGCL”), with Zynerba continuing as the surviving corporation in the Merger (the “Surviving Corporation”) and as a wholly owned subsidiary of Harmony Biosciences, without a meeting or vote of Zynerba’s stockholders in accordance with Section 251(h) of the DGCL. Upon the closing of the Merger and filing of the certificate of merger with the Secretary of State of the State of Delaware (the “Effective Time”), each Share (other than Shares that (a) as of immediately prior to the Effective Time were held in the treasury of Zynerba, (b) owned by Harmony Biosciences or Purchaser as of the commencement of the Offer, (c) irrevocably accepted for payment in the Offer and/or (d) Shares held by a holder (or beneficial owner, as the case may be) who is entitled to demand and properly exercises and perfects appraisal rights in accordance with Section 262 of the DGCL with respect to such Shares) (collectively, the “Excluded Shares”) will be automatically converted into the right to receive the Offer Price (the “Merger Consideration”), subject to any applicable withholding of taxes and without interest.
In addition, immediately prior to the Effective Time, by virtue of the Merger and without any action on the part of any holder thereof:
• | Each option to purchase a Share (a “Company Option”), whether vested or unvested, that has a per share exercise price that is less than the Closing Amount that is outstanding and unexercised immediately prior to the Effective Time will be cancelled and automatically converted into the right to receive for each Share underlying such Company Option, without interest and subject to deduction for any required withholding under applicable tax law, (i) an amount in cash equal to the excess of the Closing Amount over the per share exercise price of such Company Option and (ii) one CVR; |
• | Each Company Option that has a per share exercise price that is equal to or greater than the Closing Amount but less than $2.71, whether vested or unvested, that is outstanding and unexercised immediately prior to the Effective Time will be cancelled and automatically converted into the right to receive, for each Share underlying such Company Option, without interest and subject to deduction for any required withholding |
TABLE OF CONTENTS
under applicable tax law, upon the occurrence of any Milestone Payment (as defined in the CVR Agreement) a cash payment, if any, equal to (i) the amount, if any, by which (A) the Closing Amount plus the applicable Milestone Payment plus any Milestone Payment that was previously paid in respect of a Share exceeds (B) the per share exercise price of such Company Option, minus (ii) the gross amount of Milestone Payments previously paid with respect to such Share underlying such Company Option;
• | Each Company Option that has a per share exercise price equal to or greater than $2.71 will be cancelled at the Effective Time without any consideration payable therefore (whether in the form of cash or a CVR or otherwise); and |
• | Each restricted stock award of the Company subject to vesting conditions based solely on continued employment or service to the Company and its subsidiary (a “Company RSA”) that is outstanding immediately prior to the Effective Time, whether vested or unvested, will be cancelled and automatically converted into the right to receive for each Share subject to a Company RSA, without interest and subject to deduction for any required withholding under applicable tax law, (i) an amount in cash equal to the Closing Amount and (ii) one CVR. |
The treatment of equity awards under Zynerba’s benefit plans, including stock options, is discussed below in “Item 3. Past Contacts, Transactions, Negotiations and Agreements—Arrangements Between Zynerba and its Executive Officers, Directors and Affiliates.” A copy of the Merger Agreement is filed as Exhibit (e)(1) hereto and is incorporated herein by reference.
The obligation of Purchaser to purchase the Shares tendered in the Offer is subject to the satisfaction or waiver of a number of conditions set forth in Annex I to the Merger Agreement, including (i) that there will have been validly tendered (and “received” as defined in Section 251(h) of the DGCL) and not validly withdrawn prior to the expiration date of the Offer; (ii) that number of Shares that, considered together with all other Shares (if any) beneficially owned by Harmony Biosciences and its controlled affiliates, represent one more than 50% of the total number of Shares outstanding at the time of the expiration of the Offer (the “Minimum Condition”); and (iii) those other conditions set forth in Annex I to the Merger Agreement and further summarized in Section 15 of the Offer to Purchase (collectively, the “Offer Conditions”).
The Offer will initially expire at 5:00 p.m. (New York City time) on September 26, 2023 (the “Expiration Date”). The expiration date may be extended: (i) if on the then scheduled Expiration Date, the Minimum Condition has not been satisfied or any of the other Offer Conditions has not been satisfied, or waived by Harmony Biosciences or Purchaser if permitted under the Merger Agreement, then Purchaser may, and at the request of Zynerba, shall, extend the Offer for one (1) or more occasions in consecutive increments of up to ten (10) business days (or such longer period as may be agreed to by Zynerba and Harmony Biosciences) in order to permit the satisfaction of such Offer Conditions (subject to the right of Harmony Biosciences or Purchaser to waive any Offer Condition to the extent permitted under the Merger Agreement) and (ii) Purchaser shall (and Harmony Biosciences shall cause Purchaser to) extend the Offer from time to time to the minimum extent required by any applicable law, any interpretation or position of the SEC, the staff thereof or Nasdaq applicable to the Offer, except that Purchaser shall not (A) be required to extend the Offer and the then-scheduled Expiration Date beyond the earlier to occur of (the “Extension Deadline”) (1) the valid termination of the Merger Agreement or (2) the first business day following the “End Date” under the Merger Agreement, which is November 13, 2023, or (B) be permitted to extend the Offer beyond the Extension Deadline without the prior written consent of Zynerba; and (ii) Purchaser shall extend the Offer for the minimum period required by applicable law, requirement, interpretation or position of the SEC or its staff or Nasdaq Capital Market (“Nasdaq”) or its staff.
As set forth in the Schedule TO, the address of the principal executive office of Harmony Biosciences is 630 W. Germantown Pike, Suite 215, Plymouth Meeting, PA 19462 and its telephone number is (484) 539-9800. The address of the principal executive office of Purchaser is c/o Harmony Biosciences Holdings, Inc., 630 W. Germantown Pike, Suite 215, Plymouth Meeting, PA 19462, and its telephone number is (484) 539-9800.
TABLE OF CONTENTS
| PAST CONTACTS, TRANSACTIONS, NEGOTIATIONS AND AGREEMENTS |
Except as set forth or incorporated by reference in this Schedule 14D-9, to the knowledge of Zynerba, as of the date hereof, there are no material agreements, arrangements or understandings, or any actual or potential conflicts of interest between Zynerba or its affiliates, on the one hand, and (i) its executive officers, directors or affiliates, or (ii) Harmony Biosciences, Purchaser, or their respective executive officers, directors or affiliates, on the other hand. The board of directors of Zynerba (the “Zynerba Board”) was aware of the agreements and arrangements described in this Item 3 during its deliberations of the merits of the Merger Agreement and in determining to make the recommendation set forth in this Schedule 14D-9.
Arrangements with Harmony Biosciences and Purchaser and Their Affiliates
Merger Agreement
On August 14, 2023, Zynerba, Purchaser and Harmony Biosciences entered into the Merger Agreement. The summary of the material provisions of the Merger Agreement contained in Section 11 of the Offer to Purchase, and the description of the conditions of the Offer contained in Section 15 of the Offer to Purchase are incorporated herein by reference. Such summary and description are qualified in their entirety by reference to the full text of the Merger Agreement.
The Merger Agreement governs the contractual rights among Harmony Biosciences, Purchaser and Zynerba in relation to the Offer and the Merger. The Merger Agreement has been included as an exhibit to this Schedule 14D-9 to provide Zynerba’s stockholders with information regarding the terms of the Merger Agreement. The Merger Agreement contains representations and warranties made by Zynerba to Harmony Biosciences and Purchaser and representations and warranties made by Harmony Biosciences and Purchaser to Zynerba. Neither the inclusion of the Merger Agreement nor the summary of the Merger Agreement is intended to modify or supplement any factual disclosures about Zynerba, Harmony Biosciences or Purchaser in Zynerba’s public reports filed with the SEC. In particular, the assertions embodied in these representations and warranties are qualified by information in a confidential disclosure schedule provided by Zynerba to Harmony Biosciences and Purchaser in connection with the signing of the Merger Agreement. This disclosure schedule contains information that modifies, qualifies and creates exceptions to the representations and warranties set forth in the Merger Agreement. In addition, the representations and warranties in the Merger Agreement were negotiated with the principal purpose of allocating risk among Zynerba, Harmony Biosciences and Purchaser, rather than establishing matters of fact. Additionally, such representations and warranties may also be subject to a contractual standard of materiality that is different from what may be viewed as material by holders of Shares or from the standard of materiality generally applicable to reports or documents filed with the SEC. Accordingly, the representations and warranties in the Merger Agreement may not constitute the actual state of facts about Zynerba, Harmony Biosciences or Purchaser. Zynerba’s stockholders are not third-party beneficiaries of the Merger Agreement, except with respect to their right to receive the Offer Price, subject to any applicable withholding of taxes, following the time Purchaser accepts for payment of Shares tendered and not validly withdrawn pursuant to the Offer (the “Offer Acceptance Time”) or to receive the Merger Consideration at and after the Effective Time, and should not rely on the representations, warranties and covenants or any descriptions thereof as characterizations of the actual state of facts or conditions of Zynerba, Harmony Biosciences, Purchaser or any of their respective subsidiaries or affiliates. Information concerning the subject matter of such representations, warranties and covenants, which do not purport to be accurate as of the date of this Schedule 14D-9, may have changed since the date of the Merger Agreement, which subsequent information may or may not be fully reflected in the public disclosures of Zynerba or Harmony Biosciences.
The summary of the material terms of the Merger Agreement and the descriptions of the conditions to the Offer contained in the Offer to Purchase and incorporated herein by reference do not purport to be complete and are qualified in their entirety by reference to the full text of the Merger Agreement, which is filed as Exhibit (e)(1) hereto and is incorporated herein by reference.
CVR Agreement
Pursuant to the Merger Agreement, at or prior to the Offer Acceptance Time, Harmony Biosciences and Computershare Limited or one or more of its Affiliates (collectively, the “Rights Agent”) will enter into a Contingent Value Rights Agreement (the “CVR Agreement”) governing the terms of the CVRs to be issued pursuant to the Merger Agreement. The Rights Agent will maintain an up-to-date register of the holders of CVRs. Such holders shall not be permitted to transfer CVRs (subject to certain limited exceptions specified in the CVR Agreement).
TABLE OF CONTENTS
Each CVR represents the non-transferable right to receive the following Milestone Payments, if any, without interest thereon and less any applicable withholding taxes, with each Milestone Payment conditioned upon the achievement of the applicable milestone as follows (each, a “Milestone”):
• | Milestone 1: An aggregate milestone payment of $15,000,000, or approximately $0.2747 per Share, payable upon the completion of Zynerba’s ongoing RECONNECT (ZYN2-CL-033) clinical trial (the “Pivotal Study”) for Zynerba’s lead product candidate, Zygel™ (ZYN002) (“Zygel” or the “Product”). |
• | Milestone 2: Upon the completion of the Pivotal Study for the Product and a finding that the data from such Pivotal Study meet the primary end point(s) with statistical significance as set forth in the protocol of such Pivotal Study (“Milestone 2”), an aggregate milestone payment of: |
○ | $30,000,000, or approximately $0.5494 per Share, with respect to the achievement of Milestone 2 by or before December 31, 2024; |
○ | $20,000,000, or approximately $0.3663 per Share, with respect to the achievement of Milestone 2 between January 1, 2025 and June 30, 2025; or |
○ | $10,000,000, or approximately $0.1831 per Share, with respect to the achievement of Milestone 2 on or after July 1, 2025. |
• | Milestone 3: An aggregate milestone payment of $35,000,000, or approximately $0.6389 per Share, payable upon the achievement of FDA approval respect to the Product in the FXS indication (“Milestone 3”). |
• | Milestone 4: An aggregate milestone payment of $15,000,000, or approximately $0.2707 per Share, payable upon the achievement of FDA approval with respect to the Product in any second indication. |
• | Milestone 5: An aggregate milestone payment of $15,000,000, or approximately $0.2702 per Share, payable upon the achievement of worldwide aggregate Product net sales of at least $250,000,000, so long as Milestone 3 is achieved by or before December 31, 2030 (“Milestone 5”). |
• | Milestone 6: An aggregate milestone payment of $30,000,000, or approximately $0.5405 per Share, payable upon the achievement of worldwide aggregate Product net sales of at least $500,000,000, so long as Milestone 3 is achieved by or before December 31, 2030. |
Harmony Biosciences has the right, in its sole and absolute discretion, to direct and control the research, development, commercialization and other exploitation of Zygel in all respects, except that Harmony Biosciences is obligated to use Commercially Reasonable Efforts (as defined and described in the CVR Agreement) to achieve the foregoing Milestones. The CVR Agreement will terminate on December 31, 2040, and no Milestone may be achieved following such date. There can be no assurance that any of the Milestones will be achieved and that any of the resulting Milestone Payments will be required to be paid by Harmony Biosciences. It is not possible to predict whether a payment will become payable with respect to the CVRs. Whether any Milestone required for payment of any Milestone Payment is met will depend on many factors, some within the control of Harmony Biosciences and its subsidiaries and others outside the control of Harmony Biosciences and its subsidiaries.
The summary of the material provisions of the CVR Agreement contained in Section 11 of the Offer to Purchase is incorporated herein by reference. The foregoing summary and description of the material terms of the CVR Agreement does not purport to be complete and is qualified in its entirety by reference to the full text of the CVR Agreement, a form of which is included as Exhibit B to the Merger Agreement filed as Exhibit (e)(2) to this Schedule 14D-9, and is incorporated herein by reference.
Tender and Support Agreements
On August 14, 2023, in connection with the execution of the Merger Agreement, certain directors and executive officers of Zynerba (the “Supporting Stockholders”), entered into Tender and Support Agreements with Harmony Biosciences and Purchaser (the “Support Agreements”). Under the terms of the Support Agreements, each Supporting Stockholder has agreed, among other things, to tender their Shares (other than unvested Company RSAs) in the Offer and, subject to certain exceptions, not to transfer any of the Shares prior to the consummation or expiration of the Offer.
TABLE OF CONTENTS
As of August 24, 2023, the Supporting Stockholders collectively held shares representing approximately 6.9% of the outstanding Shares. The Support Agreements will terminate upon termination of the Merger Agreement and certain other specified events.
The foregoing summary and description of the material terms of the Support Agreements does not purport to be complete and is qualified in its entirety by reference to the full text of the form of Support Agreement, a copy of which is included as Exhibit A to the Merger Agreement filed as Exhibit (e)(3) to this Schedule 14D-9, and is incorporated herein by reference.
Confidentiality Agreement
Harmony Biosciences, LLC, a wholly owned subsidiary of Harmony Biosciences, and Zynerba entered into a Confidentiality and Nondisclosure Agreement, effective November 17, 2021 (the “Confidentiality Agreement”), in connection with Harmony Biosciences, LLC’s consideration of a potential negotiated business relationship with Zynerba. Under the terms of the Confidentiality Agreement, Harmony Biosciences, LLC agreed, subject to certain exceptions, to keep confidential certain confidential and non-public information relating to Zynerba for a period lasting two (2) years from the date of the Confidentiality Agreement. The Confidentiality Agreement did not contain a standstill or similar provision. For further discussion, see “Item 4. The Solicitation or Recommendation—Background of the Offer and the Merger.”
The foregoing summary and description of the material terms of the Confidentiality Agreement does not purport to be complete and is qualified in its entirety by reference to the full text of the Confidentiality Agreement, a copy of which is filed as Exhibit (e)(4) to this Schedule 14D-9, and is incorporated herein by reference.
Arrangements Between Zynerba and its Executive Officers, Directors and Affiliates
Certain Zynerba executive officers and directors have financial interests in the transactions contemplated by the Merger Agreement, including the Offer and the Merger (the “Transactions”) that are different from, or in addition to, the interests of stockholders generally. The Zynerba Board was aware of these potentially differing interests and considered them, among other matters, in evaluating and negotiating the Merger Agreement and in reaching its decision to approve the Merger Agreement and the Transactions, as more fully discussed below in “Item 4. The Solicitation or Recommendation—Recommendation of the Zynerba Board” and “Item 4. The Solicitation or Recommendation—Reasons for the Recommendation.”
For further information with respect to the arrangements between Zynerba and its named executive officers, see the information included under “Item 3. Past Contacts, Transactions, Negotiations and Agreements—Treatment of Equity Awards in the Transaction—Golden Parachute Compensation.”
Zynerba’s executive officers are as follows:
Armando Anido | | | Chairman and Chief Executive Officer |
James E. Fickenscher | | | Chief Financial Officer and VP, Corporate |
Terri B. Sebree | | | President |
Albert P. Parker | | | Chief Legal Officer and Corporate Secretary |
Brian Rosenberger | | | Vice President, Commercial and Business Development |
Kenneth Jones | | | Interim Chief Financial Officer |
Effect of the Transactions on Company Equity Awards
Consideration for Company Options and Company RSAs in the Merger
Immediately prior to the Effective Time, and consistent with the terms of the Zynerba Pharmaceuticals, Inc. Amended and Restated 2014 Omnibus Incentive Compensation Plan (as amended from time to time, the “2014 Plan”), by virtue of the Merger and without any action on the part of any holder thereof:
• | Each Company Option, whether vested or unvested, that has a per share exercise price that is less than the Closing Amount that is outstanding and unexercised immediately prior to the Effective Time will be |
TABLE OF CONTENTS
cancelled and automatically converted into the right to receive, for each Share underlying such Company Option, without interest and subject to deduction for any required withholding under applicable tax law, (i) an amount in cash equal to the excess of the Closing Amount over the per share exercise price of such Company Option and (ii) one CVR.
• | Each Company Option that has a per share exercise price that is equal to or more than the Closing Amount but less than $2.71, whether vested or unvested, that is outstanding and unexercised immediately prior to the Effective Time will be cancelled and automatically converted into the right to receive, for each Share underlying such Company Option, without interest and subject to deduction for any required withholding under applicable tax law, upon the occurrence of any Milestone Payment, a cash payment, if any, equal to (A) the amount, if any, by which (i) the Closing Amount plus the applicable Milestone Payment plus any Milestone Payment that was previously paid in respect of a Share exceeds (ii) the per share exercise price of such Company Option, minus (B) the gross amount of Milestone Payments previously paid with respect to such Share underlying such Company Option. |
• | At the Effective Time, each Company Option that has a per share exercise price equal to or greater than $2.71 shall be canceled without any consideration payable therefor whether before or after the Effective Time. |
• | Each Company RSA that is outstanding immediately prior to the Effective Time, whether vested or unvested, will be cancelled and automatically converted into the right to receive, for each Share subject to such Company RSA, without interest and subject to deduction for any required withholding under applicable tax law, (A) an amount in cash from Harmony Biosciences or the Surviving Corporation equal to the Closing Amount and (B) one CVR. |
For an estimate of the amounts that would be payable to each of our executive officers and directors with respect to his or her outstanding equity awards, see the section entitled “—Consideration Payable for Outstanding Shares and Company Options Pursuant to the Offer or the Merger” below.
Consideration Payable for Outstanding Shares and Company Options Pursuant to the Offer or the Merger
Immediately prior to the Effective Time, by virtue of the Merger and without any action on the part of any holder thereof, each Company Option and Company RSA, whether vested or unvested, that is outstanding immediately prior thereto, will be cancelled and converted into the right to receive the amount, if any, described above in the section entitled “—Effect of the Transactions on Company Equity Awards.”
Effect of the Offer and the Merger on Outstanding Company Options
Company Options. The following table identifies, for each of the Company’s executive officers and directors, the number of Shares subject to his or her In-the-Money Company Options or Out-of-the-Money Company Options, as applicable, that were outstanding and vested and exercisable as of August 24, 2023 that will be cancelled in exchange for consideration in connection with the Merger. No Company executive officer holds any In-the-Money Company Option. The following table assumes that no Company Stock Options will be exercised between August 24, 2023 and the closing of the Merger, and no awards will be exercised or forfeited, between the date of the Merger Agreement and the Effective Time. All dollar amounts have been rounded to the nearest whole dollar.
Executive Officers:
| | | | | | | | | |
Armando Anido(2) | | | — | | | 190,000 | | | 235,657 |
James E. Fickenscher | | | — | | | 125,000 | | | 155,038 |
Terri B. Sebree | | | — | | | 125,000 | | | 155,038 |
Albert P. Parker | | | — | | | 200,000 | | | 190,060 |
Brian Rosenberger | | | — | | | 90,000 | | | 111,627 |
Kenneth Jones | | | — | | | 21,250 | | | 26,356 |
| | | | | | | | | |
TABLE OF CONTENTS
Directors:
| | | | | | | | | |
John P. Butler | | | 106,399 | | | — | | | 328,264 |
Warren D. Cooper, MB, BS, BSc, MFPM | | | 106,399 | | | — | | | 328,264 |
William J. Federici | | | 106,399 | | | — | | | 328,264 |
Daniel L. Kisner, MD | | | 106,399 | | | — | | | 328,264 |
Kenneth I. Moch | | | 106,399 | | | — | | | 328,264 |
Pamela Stephenson | | | 106,399 | | | — | | | 328,264 |
(1)
| To estimate the aggregate amount payable in respect of an individual’s In-the-Money Company Options, (a) the aggregate number of Shares subject to In-the-Money Company Options was multiplied by (b) the excess of the Closing Amount over the per share exercise price per share of such In-the-Money Company Stock Options. The estimated amount payable in respect of an individual’s Out-of-the-Money Company Stock Options, equals (A) the amount, if any, by which (i) the Closing Amount plus the applicable Milestone Payment plus any Milestone Payment that was previously paid in respect of such Share underlying such Company Option exceeds (ii) the per share exercise price of such Company Option, minus (B) the gross amount of Milestone Payments previously paid with respect to such Share underlying such Company Option. |
(2)
| Armando Anido is also a director of the Company. |
Effect of the Offer and the Merger on Outstanding Shares
If the executive officers and directors of Zynerba tender Shares for purchase pursuant to the Offer, they will receive the same consideration on the same terms and conditions as the other stockholders of Zynerba. If such executive officers and directors do not tender their Shares for purchase pursuant to the Offer, but the conditions to the Offer are otherwise satisfied or waived in accordance with the terms of the Merger Agreement and the Merger is consummated, such executive officers and directors will also receive the same consideration on the same terms and conditions as the other stockholders of Zynerba. As of August 24, 2023, excluding the Shares underlying Company Options (whether or not currently exercisable) and including the Shares subject to Company RSAs, the executive officers and directors of Zynerba beneficially own 3,837,525 Shares.
The following table sets forth the number of Shares beneficially owned as of August 24, 2023 by each of Zynerba’s executive officers and directors (excluding Shares underlying Company Options (whether or not currently exercisable) and including Shares subject to Company RSAs) and the approximate aggregate Closing Amount and maximum value of CVRs in respect of such Shares and the approximate maximum aggregate consideration that would be payable for such Shares pursuant to the Offer or the Merger (i.e., $3.6503 per share, which represents the Closing Amount plus the maximum amount payable for one CVR), respectively.
TABLE OF CONTENTS
Executive Officers:
| | | | | | | | | | | | |
Armando Anido(1) | | | 1,234,444 | | | 1,365,171.62 | | | 3,140,919.31 | | | 4,506,090.93 |
James E. Fickenscher | | | 557,085 | | | 616,080.30 | | | 1,417,447.07 | | | 2,033,527.37 |
Terri B. Sebree | | | 778,642 | | | 861,100.19 | | | 1,981,176.70 | | | 2,842,276.89 |
Albert P. Parker | | | 312,018 | | | 345,060.71 | | | 793,898.60 | | | 1,138,959.31 |
Brian Rosenberger | | | 375,970 | | | 415,785.22 | | | 956,618.07 | | | 1,372,403.29 |
Kenneth Jones | | | 119,711 | | | 132,388.40 | | | 304,592.67 | | | 436,981.07 |
| | | | | | | | | | | | |
Directors:
| | | | | | | | | | | | |
John P. Butler | | | 80,765 | | | 89,318.01 | | | 205,498.47 | | | 294,816.48 |
Warren D. Cooper, MB, BS, BSc, MFPM | | | 75,778 | | | 83,802.89 | | | 192,809.54 | | | 276,612.43 |
William J. Federici | | | 75,778 | | | 83,802.89 | | | 192,809.54 | | | 276,612.43 |
Daniel L. Kisner, MD | | | 75,778 | | | 83,802.89 | | | 192,809.54 | | | 276,612.43 |
Kenneth I. Moch | | | 75,778 | | | 83,802.89 | | | 192,809.54 | | | 276,612.43 |
Pamela Stephenson | | | 75,778 | | | 83,802.89 | | | 192,809.54 | | | 276,612.43 |
All of the current executive officers and directors as a group (12 persons) | | | 3,837,525 | | | 4,243,918.90 | | | 9,764,198.59 | | | 14,008,117.49 |
(1)
| Armando Anido is also a director of Zynerba. |
Arrangements with Zynerba’s Executive Officers
Zynerba is party to preexisting employment agreements with each of its executive officers. Zynerba may terminate the executive officers’ employment with the Company at any time, with or without cause. The employment agreements confirm the executive officers’ titles, compensation arrangements and eligibility for benefits made available to employees generally. These agreements, together with the indemnification agreement for certain executive officers executed upon commencing employment with the Company, set forth the rights and responsibilities of each party.
To incentivize Zynerba’s executive officers to remain with Zynerba through the closing of the Merger, and to bring alignment to each executive officer’s employment agreement so that these agreements have similar terms, and following discussion by the Compensation Committee, the Compensation Committee recommended to the Zynerba Board in late June 2023 that the Employment Agreement Amendments (as defined below) for Mr. Anido and Ms. Sebree be approved. In August 2023, the Zynerba Board approved, and the Company amended, the employment agreements previously entered into with each of the following executive officers: (i) Armando Anido, its Chief Executive Officer, (ii) Terri Sebree, its President, (iii) James Fickenscher, its Chief Financial Officer, Vice President, Corporate Development, (iv) Albert Parker, its Chief Legal Officer and Corporate Secretary, and (v) Brian Rosenberger, its Vice President, Commercial and Business Development (the “Employment Agreements” and, such amendments, the “Employment Agreement Amendments”). The Employment Agreement Amendments were entered into concurrently with the signing of the Merger Agreement. Each Employment Agreement Amendment revises the severance benefits payable to the applicable executive pursuant to his or her employment agreement in the event of such executive’s termination by Zynerba (or Harmony Biosciences) without “cause” or executive’s resignation for “good reason” (each, as defined in the applicable Employment Agreement Amendment, and each such termination, collectively, a “Qualifying Termination”) within the ninety (90) day period preceding a “change of control” (as defined therein) or within twelve (12) months following a change of control (such period, the “Change of Control Period”) to provide for (i) a lump sum cash payment equal to the full monthly COBRA premium applicable to such executive multiplied by the number of months in such executive’s applicable severance period (whereas prior to such Employment Agreement Amendment, the applicable employment agreement provided for a monthly COBRA continuation subsidy during the applicable severance period equal to 90% of the total COBRA premium applicable
TABLE OF CONTENTS
thereto) and (ii) vesting of such executive’s performance awards at target (if such award specifies a target). In addition, with respect to the Employment Agreement Amendments for each of Mr. Anido and Ms. Sebree, the minimum share price related to their target bonus severance payment was removed, such that each of Mr. Anido and Ms. Sebree will be entitled to receive 100% of his or her target bonus in the event of a Qualifying Termination during the Change of Control Period, which is consistent with the provisions set forth in the employment agreements for the other executive officers. Mr. Anido’s and Ms. Sebree’s target bonuses are 60% of their respective base salaries. The estimated value of Mr. Anido’s and Ms. Sebree’s target bonus amounts is set forth below in the table entitled “—Golden Parachute Compensation.” The Zynerba Board determined to remove the minimum share price related to payment of target bonus following a change of control from Mr. Anido’s and Ms. Sebree’s employment agreements, which minimum share price was included in their employment agreements prior to the Company’s initial public offering in 2015, in recognition of their long service to the Company, to incentivize them to stay with the Company through Closing and to align their agreements with the employment agreements of all other executive officers, which do not contain such a minimum share price.
Pursuant to the Employment Agreement Amendments, if an executive officer’s termination of employment is due to a Qualifying Termination, in each case within the Change of Control Period, and subject to the executive officer’s execution of an effective general release of potential claims against the Company and compliance with all of the terms and provisions of his or her employment agreement that survive the executive’s termination of employment, each executive officer will be entitled to:
• | An amount equal to fifteen (15) months in the case of Mr. Rosenberger, eighteen (18) months in the case of Ms. Sebree and Messrs. Fickenscher and Parker, or twenty-four (24) months in the case of Mr. Anido, of the executive officer’s base salary; |
• | A lump sum cash payment equal to the full monthly COBRA premium applicable to the executive officer multiplied by: fifteen (15) months in the case of Mr. Rosenberger, eighteen (18) months in the case of Ms. Sebree and Messrs. Fickenscher and Parker, or twenty-four (24) months in the case of Mr. Anido; |
• | One hundred percent (100%) of all outstanding unvested stock options and other equity-based awards held by the executive as of the termination date shall become fully vested and exercisable (to the extent applicable) as of the termination date; |
• | Each performance-based stock option and other equity-based awards will vest at target (if such award specifies a target); |
• | All outstanding stock options and other equity-based awards that become vested upon a Qualifying Termination in connection with a change in control shall remain exercisable until the earlier of (x) the three (3) year anniversary of the date of termination and (y) the applicable expiration date; and |
• | One hundred percent (100%) of the applicable executive officer’s target annual bonus for the year in which the executive officer’s Qualifying Termination occurs. |
As described in the section titled “—Effect of the Transactions on Company Equity Awards” above, the vesting of all outstanding Company Stock Options and Company RSAs is being accelerated in full and such outstanding equity awards will be paid out in accordance with the terms of the Merger Agreement as described above in “Item 3. Past Contacts, Transactions, Negotiations and Agreements—Treatment of Equity Awards in the Transaction.”
TABLE OF CONTENTS
In lieu of the payments and benefits described above, under the terms of the employment agreements with the executive officers, if an executive’s employment is terminated by the Company without “Cause” or by such executive officer for Good Reason, in each case outside of the Change of Control period, and subject to the executive officer’s execution of an effective general release of potential claims against the Company and compliance with all of the terms and provisions of his or her employment agreement that survive the executive’s termination of employment, each executive officer will be entitled to:
• | An amount equal to nine (9) months in the case of Mr. Rosenberger, twelve (12) months in the case of Ms. Sebree and Messrs. Fickenscher and Parker, or eighteen (18) months in the case of Mr. Anido, of the executive officer’s base salary; |
• | Continued medical and dental coverage at the same level in effect at the time of termination (or generally comparable coverage) for nine (9) months in the case of Mr. Rosenberger, twelve (12) months in the case of Ms. Sebree and Messrs. Fickenscher and Parker, or eighteen (18) months in the case of Mr. Anido, at the same premium rates as may be charged from time to time for employees generally; |
• | Pro rata vesting of all outstanding unvested Company Stock Options and other equity-based awards held by the executive officer that would have vested had the executive remained employed for twelve (12) months following the termination date; and |
• | Exercise of all vested equity awards by executive at the termination of employment, except on the account of death or disability, shall be governed by the terms of the appliable equity plan governing such equity awards. |
For purposes of the executive officers’ employment agreements, “Good Reason” shall mean the following actions by Company, in each case without executive’s consent: (i) a material reduction in the executive’s duties and responsibilities, which means the assignment to the executive of any duties or responsibilities which are materially inconsistent with or adverse to the executive’s then current duties, responsibilities, positions and/or titles with the Company; (ii) a material reduction in the executive officer’s then-current base salary or target bonus opportunity; (iii) requirement that the executive regularly report to work at a location that is more than fifty (50) miles from the location of the executive’s employment as of the effective date of the employment agreement; (iv) a material breach of the employment agreement by the Company; (v) in the event of the assignment of employment agreement to a third party, the failure of the assignee or successor entity to agree to be bound to the terms of the agreement, in each case, subject to the executive officer’s written notification of his or her intention to resign within ninety (90) days after such executive officer first knows or has reason to know of the occurrence of any such event or condition, and Zynerba must have thirty (30) business days to cure such event or condition.
The estimated value of the severance payments and benefits for each of the Company’s named executive officers is set forth below in the table entitled “—Golden Parachute Compensation.” If there is a Qualifying Termination within the Change of Control Period, the estimated aggregate cash severance payment for all of the Company’s executive officers who are not named executive officers, excluding any cash payment in lieu of applicable COBRA premiums, is $1,540,956.
Future Arrangements
It is possible that Zynerba employees, including the executive officers, will enter into new compensation arrangements with Harmony Biosciences or its affiliates. Such arrangements may include agreements regarding future terms of employment and/or retention awards. As of the date of this Schedule 14D-9, no compensation arrangements between such persons and Harmony Biosciences and/or its affiliates have been established.
Rule 14d-10(d) Matters
Pursuant to the Merger Agreement, prior to the acceptance of the Shares in the Offer, the Compensation Committee of the Zynerba Board (the “Compensation Committee”) will cause each employment compensation, severance or other employee benefit arrangement pursuant to which consideration is payable to any officer, director or employee who is a holder of any security of the Company to be approved by the Compensation Committee (comprised solely of “independent directors”) in accordance with the requirements of Rule 14d-10(d)(2) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the instructions thereto as an “employment compensation, severance or other employee benefit arrangement” within the meaning of Rule 14d-10(d)(2) under the Exchange Act and satisfy the requirements of the non-exclusive safe harbor set forth in Rule 14d-10(d) of the Exchange Act.
TABLE OF CONTENTS
Director and Officer Exculpation, Indemnification and Insurance
Section 145 of the DGCL permits a Delaware corporation to include in its charter documents and in agreements between the corporation and its directors and officers, provisions expanding the scope of indemnification beyond that specifically provided by current law.
Zynerba’s sixth amended and restated certificate of incorporation includes provisions that limit the liability of its directors for monetary damages for breach of their fiduciary duty as directors, except for liability that cannot be eliminated under the DGCL. Accordingly, Zynerba’s directors will not be personally liable for monetary damages for breach of their fiduciary duty as Zynerba directors, except for liabilities:
• | for any breach of the director’s duty of loyalty to Zynerba or its stockholders; |
• | for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
• | for unlawful payments of dividends or unlawful stock repurchases or redemptions; or |
• | for any transaction from which the director derived an improper personal benefit. |
Zynerba’s sixth amended and restated certificate of incorporation and amended and restated bylaws provide for the indemnification of Zynerba’s directors and officers to the fullest extent permitted under the DGCL.
Pursuant to the terms of the Merger Agreement, Zynerba’s directors and executive officers will be entitled to certain ongoing indemnification and coverage under directors’ and officers’ liability insurance policies from the Surviving Corporation as follows.
For six (6) years after the Offer Acceptance Time, Harmony Biosciences shall, and shall cause the Surviving Corporation to, maintain directors’ and officers’ liability insurance in respect of any acts, errors, omissions, facts or events occurring on or before the Offer Acceptance Time, including in respect of the Transactions, covering each such person currently covered by Zynerba’s directors’ and officers’ liability insurance policies on terms with respect to coverage and amount no less favorable than those of such policies in effect on the date of the Merger Agreement, except that that neither Harmony Biosciences nor the Surviving Corporation shall be obligated to pay annual premiums in excess of 300% of the annual premium most recently paid by Zynerba prior to the date of the Offer Acceptance Time for such insurance (the “Current Premium”) and if such premiums for such insurance would at any time exceed 300% of the Current Premium, then Harmony Biosciences shall, and shall cause the Surviving Corporation to, maintain policies of insurance that, in Harmony Biosciences’ and the Surviving Corporation’s good faith judgment, provide the maximum coverage available at an annual premium equal to 300% of the Current Premium. The provisions of the immediately preceding sentence shall be deemed to have been satisfied if prepaid “tail” or “runoff” policies have been obtained by Zynerba in its discretion prior to the Offer Acceptance Time, which policies provide such persons currently covered by such policies with coverage for an aggregate period of six (6) years with respect to claims arising from any acts, errors, omissions, facts or events that occurred on or before the Offer Acceptance Time (including matters that continue after the Offer Acceptance Time that are interrelated to claims arising on or before the Offer Acceptance Time), including in respect of the Transactions, except that the amount paid for such prepaid policies does not exceed 300% of the Current Premium. If any such prepaid policies have been obtained prior to the Offer Acceptance Time, the Surviving Corporation shall (and Harmony Biosciences shall cause the Surviving Corporation to) maintain any and all such policies in full force and effect for their full term, and continue to honor the obligations thereunder.
In addition, for six (6) years from and after the Offer Acceptance Time, each of Harmony Biosciences and the Surviving Corporation shall:
(i)
| indemnify and hold harmless each individual who at the Offer Acceptance Time is, or at any time prior to the Offer Acceptance Time was, a director or officer of Zynerba or of a subsidiary of Zynerba (each an “Indemnified Party”) for any and all costs and expenses (including fees and expenses of legal counsel, which shall be advanced as they are incurred), judgments, fines, penalties or liabilities (including amounts paid in settlement or compromise) imposed upon or reasonably incurred by such Indemnified Party in connection with or arising out of any demand, action, suit or other legal proceeding (whether civil or criminal) in which such Indemnified Party may be involved or with which he, she or they may be threatened (regardless of whether as a named party or as a participant other than as a named party, including as a witness) (an “Indemnified Party Proceeding”) arising out of such Indemnified Party’s service in |
TABLE OF CONTENTS
connection with any other corporation or organization for which he, she or they serves or has served as a director, officer, employee, agent, trustee, or fiduciary at the request of Zynerba (including in any capacity with respect to any employee benefit plan) whether or not the Indemnified Party continues in such position at the time such Indemnified Party Proceeding is brought or threatened and at, or at any time prior to, the Offer Acceptance Time (including any Indemnified Party Proceeding relating in whole or in part to the Transactions or relating to the enforcement of this provision or any other indemnification or advancement right of any Indemnified Party), to the fullest extent permitted under applicable law; and
(ii)
| fulfill and honor in all respects the obligations of Zynerba pursuant to: (x) each indemnification agreement in effect as of the date of the Merger Agreement between Zynerba and any Indemnified Party; and (y) any indemnification provision (including advancement of expenses) and any exculpation provision set forth in the Zynerba sixth amended and restated certificate of incorporation or Zynerba amended and restated bylaws as in effect on the date of the Merger Agreement. |
The Surviving Corporation’s obligations under the foregoing clauses (i) and (ii) shall continue in full force and effect for a period of six (6) years from the Offer Acceptance Time.
Section 16 Matters
Prior to the Effective Time, Zynerba and the Zynerba Board will take all such steps as may reasonably be necessary to cause the Transactions, including any dispositions of Shares (including any Company Options or Company RSAs) by each person who is or will be subject to the reporting requirements of Section 16(a) of the Exchange Act with respect to Zynerba, to be exempt under Rule 16b-3 under the Exchange Act.
TABLE OF CONTENTS
| THE SOLICITATION OR RECOMMENDATION |
Recommendation of the Zynerba Board
At a meeting of the Zynerba Board held on August 12, 2023, the Zynerba Board unanimously (i) approved, adopted and declared advisable the execution, delivery and performance by Zynerba of the Merger Agreement and the transactions contemplated thereby, including the Offer and the Merger; (ii) determined that the transactions contemplated by the Merger Agreement, including the Offer and the Merger, are in the best interests of Zynerba and its stockholders; (iii) resolved that the Merger Agreement will be governed by and effected under Section 251(h) of the DGCL; and (iv) recommended that the Zynerba’s stockholders accept the Offer and tender their Shares to Purchaser pursuant to the Offer.
Accordingly, and for other reasons described in more detail below, the Zynerba Board unanimously recommends that Zynerba’s stockholders accept the Offer and tender their Shares to Purchaser pursuant to the Offer.
On August 14, 2023, Zynerba and Harmony Biosciences issued a joint press release announcing the Offer. The joint press release of Zynerba and Harmony Biosciences is included as Exhibit (a)(5)(B) hereto, and is incorporated herein by reference.
Background of the Offer and the Merger
The following chronology summarizes the key meetings and events that led to the signing of the Merger Agreement. The chronology does not purport to catalogue every conversation among the Zynerba Board or committees thereof or the representatives of Zynerba and other parties.
The Zynerba Board, together with Zynerba’s management team, regularly reviews its research and development programs, business opportunities and potential transactions to protect and enhance the value of its business and enhance stockholder value. Zynerba considers its strategic options in light of the totality of the circumstances, including current and anticipated business and industry trends, regulatory conditions, future growth prospects, the current and expected financing environment and overall strategic direction of its business, in each case, with the goal of maximizing short-term and long-term value for its stockholders.
In particular, in arriving at the transactions contemplated by the Merger Agreement, Zynerba followed a careful process assisted by experienced outside financial and legal advisors to rigorously examine potential transactions and transaction candidates through broad outreach to life sciences companies and a thorough process of evaluation of prospective strategic partners and strategic alternatives. The terms of the Merger Agreement are the result of extensive arm’s-length negotiations among members of the management teams of Zynerba and Harmony Biosciences along with their respective advisors and under the guidance and direction of each of the Zynerba Board and the board of directors of Harmony Biosciences, respectively.
During the third quarter of 2021 and into the first half of 2022, representatives of Zynerba and Harmony Biosciences engaged in intermittent informal discussions regarding Harmony Biosciences’ interest in Zynerba’s Zygel program and a potential strategic partnership transaction between the two companies. These discussions on occasion involved exchanging confidential information pursuant to a mutual confidentiality agreement executed on November 17, 2021. This confidentiality agreement did not include a standstill obligation.
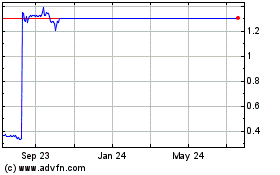
On May 16, 2022, Zynerba publicly announced its financial results for the three months ended March 31, 2022. The closing price per Share on Nasdaq on that date was $0.93, which represented a 57% decline from the closing price of $2.16 on March 1, 2022 (the day on which Zynerba announced its financial results for the twelve months ended December 31, 2021).
During the second half of 2022, the Zynerba Board held meetings at which members of senior management were present and discussed the strategic, financial and operational challenges of operating Zynerba’s business in the then-current environment, including the macro-economic, industry and market conditions negatively impacting late-stage clinical biopharmaceutical companies such as Zynerba. The Zynerba Board discussed the need for meaningful capital investment to advance Zygel expeditiously in Fragile X Syndrome (“FXS”) and chromosome 22q11.2 deletion syndrome (“22q”), and explore additional indications, fund its research and development efforts, expand its organization, and operate as a public company, as well as the risks and uncertainties associated with conducting clinical trials for Zygel, pursuing related regulatory approvals and, if successful, launching and commercializing Zygel. The Zynerba Board also discussed the risks and uncertainties associated with
TABLE OF CONTENTS
the timeline for completion of the RECONNECT trial, Zynerba’s cash requirements and its ability to raise sufficient capital through equity financing, as well as developments in the biotechnology and pharmaceutical industries, including the financing challenges associated with the weakness in biotechnology and pharmaceutical stock prices. The Zynerba Board also discussed the potential delisting of Zynerba’s common stock from the Nasdaq Global Select Market due to failure to comply with applicable listing standards, and the dilution that Zynerba’s stockholders would likely experience in connection with the implementation of Zynerba’s long-term strategic plan, including planned equity capital fundraising in order to finance Zynerba’s ongoing and planned clinical trials evaluating Zygel in the treatment of behavioral symptoms of FXS and 22q and other preclinical and clinical development activities.
During this period, Harmony Biosciences and Zynerba continued to exchange due diligence information and engage in periodic informational discussions between members of their respective senior management. The financial terms of any potential transaction were not discussed during any of these meetings.
On August 9, 2022, the Zynerba Board held a meeting at which members of Zynerba senior management were also present. Armando Anido, Zynerba’s Chief Executive Officer, provided a status update regarding discussions to date with Harmony Biosciences. Following discussion, the Zynerba Board requested that Mr. Anido continue to provide timely updates regarding any meaningful developments as appropriate.
On August 10, 2022, Zynerba publicly announced its financial results for the three months ended June 30, 2022. The closing price per Share on Nasdaq on that date was $1.25, which represented a 42% decline from the closing price of $2.16 on March 1, 2022 (the day on which Zynerba announced its financial results for the twelve months ended December 31, 2021).
On August 26, 2022, Harmony Biosciences sent an initial indication of interest to acquire Zynerba at a valuation that could be in excess of $100,000,000, with transaction consideration that could be comprised of both cash and Harmony Biosciences stock.
On August 29, 2022, members of Zynerba’s and Harmony Biosciences’ senior management met by teleconference to discuss the indication of interest. Zynerba management indicated that Zynerba would be interested in exploring a potential transaction with Harmony Biosciences and requested that Harmony Biosciences provide a more specific proposal.
On September 15, 2022, Mr. Anido provided the Zynerba Board with a written update concerning, among other things, the status of discussions with Harmony Biosciences.
During September and October of 2022, Zynerba and Harmony Biosciences continued to exchange due diligence information and meet informally to discuss regulatory and other matters related to Zynerba’s business.
On November 1, 2022, Zynerba received a deficiency letter from the Listing Qualifications Department of the Nasdaq Stock Market LLC notifying Zynerba that, for the last 30 consecutive business days, the closing bid price for Zynerba’s common stock has been below the minimum $1.00 per share required for continued listing on The Nasdaq Global Market.
On November 8 and 9, 2022, the Zynerba Board held a meeting at which members of Zynerba senior management and representatives of Troutman Pepper Hamilton Sanders LLP (“Troutman”), Zynerba’s outside legal counsel, were also present. At the meeting, among other things, Zynerba management provided an update on recent communications and due diligence activities regarding a potential transaction with Harmony Biosciences and other strategic business development opportunities, as well as the legal developments related to the Nasdaq delisting notice and related next steps. The Zynerba Board and senior management then discussed Zynerba’s long-term strategic plans and priorities. As part of this long-term strategic discussion, the Zynerba Board discussed with senior management potential cases for long-term revenue projections and reviewed a preliminary long-term financial projection prepared by senior management and related assumptions. Following discussion, the Zynerba Board instructed senior management to refine such projections for further review by the Zynerba Board in light of management’s assessment of the timing for completion of the RECONNECT trial, progress of patient enrollment in the RECONNECT trial and Zynerba’s cash position. Representatives of Troutman then provided an overview of the Zynerba Board’s fiduciary duties in connection with the evaluation of acquisition proposals and the Zynerba Board’s potential consideration of various strategic opportunities.
TABLE OF CONTENTS
On November 14, 2022, Zynerba publicly announced its financial results for the three months ended September 30, 2022. The closing price per Share on Nasdaq on that date was $0.63, which represented a 71% decline from the closing price of $2.16 on March 1, 2022 (the day on which Zynerba announced its financial results for the twelve months ended December 31, 2021).
Throughout the third quarter of 2022 and into the first quarter of 2023, Zynerba and Harmony Biosciences continued to exchange due diligence information and engage in discussions concerning Zynerba’s business, operations, product candidates and clinical programs.
On January 30, 2023, Harmony Biosciences and Zynerba senior management met via videoconference, during which Harmony Biosciences senior management indicated its interest in proceeding with a potential business combination transaction. In a follow up call on February 1, 2023 between Harmony Biosciences and Zynerba senior management, Zynerba senior management requested that Harmony Biosciences provide a letter of intent containing proposed transaction terms, including the specific CVR milestones that Harmony Biosciences contemplated. Zynerba management noted that it would not expect Harmony Biosciences’ valuation proposal to be inclusive of Zynerba’s cash position.
On February 23, 2023, Harmony Biosciences sent a revised non-binding indication of interest reiterating Harmony Biosciences’ interest in a potential transaction with Zynerba at a valuation of in excess of $100,000,000, not taking into account Zynerba’s cash position or material liabilities, with transaction consideration consisting of an unspecified combination of cash, Harmony Biosciences stock and unregistered CVRs based upon the achievement of future development milestones (the “February 23 Proposal”). Later that day, senior management of Harmony Biosciences and Zynerba exchanged email correspondence in which Zynerba management indicated that it was not prepared to respond to the terms of the February 23 Proposal as written, and would require a more specific proposal with respect to the potential business combination. Nonetheless, Zynerba agreed to continue facilitating Harmony Bioscience’s due diligence activities.
On March 7, 2023, the Zynerba Board held a meeting at which members of Zynerba senior management and representatives of MTS Health were also present. At the meeting, management reviewed and the Zynerba Board discussed Zynerba’s financial results for the fourth quarter of 2022 and the fiscal year 2023 budget, including matters related to the cash runway and the timing of topline data from the RECONNECT trial, as well as potential ex-U.S. partnering opportunities for Zygel, reviewing the approach for engaging with potential partners, as well as timing and financial objectives. The Zynerba Board engaged in discussions of strategic business development priorities, including a potential out-license of Zygel outside of the United States, the impact of RECONNECT timelines on partnering discussions, analogs for recent transactions, and other related topics. The Zynerba Board then met in executive session with selected members of senior management and representatives of MTS Health to further discuss the overall strategy with respect to the February 23 Proposal and Zynerba’s evaluation of other strategic opportunities. The Zynerba Board then directed senior management and MTS Health to continue discussions with Harmony Biosciences and simultaneously initiate a market-check process to determine if other third parties might be interested in pursuing a potential business combination transaction with Zynerba and to seek additional indications of value in response to Harmony Biosciences’ proposal.
On March 8, 2023, Zynerba granted access to a virtual data room, which was established by Zynerba in connection with the pending market-check process and in light of the ongoing discussions with Harmony Biosciences, to members of Harmony Biosciences senior management. Over the course of that month, Zynerba and Harmony Biosciences continued to exchange due diligence materials and meet informally to discuss Zynerba’s business.
On March 10, 2023, Zynerba and MTS Health entered into an amendment to Zynerba’s existing engagement letter with MTS Health setting forth the terms and conditions of the engagement by MTS Health as financial advisor with respect to a potential M&A transaction.
On March 28, 2023, Zynerba publicly announced its financial results for the twelve (12) months ended December 31, 2022. The closing price per Share on Nasdaq on that date was $0.41, which represented a 81% decline from the closing price of $2.16 on March 1, 2022 (the day on which Zynerba announced its financial results for the twelve months ended December 31, 2021).
Throughout March and April of 2023, representatives of MTS Health, at the direction of Zynerba, initiated outreach to representatives of 48 additional biotechnology and pharmaceutical companies identified by MTS Health as companies whose technologies and/or pipeline products could potentially be complementary to Zynerba’s portfolio
TABLE OF CONTENTS
and which had the relevant expertise necessary to continue developing and obtaining regulatory approval for Zygel, for the purpose of gauging such companies’ potential interest in pursuing a transaction with Zynerba. During this period, Zynerba received preliminary exploratory responses concerning such potential strategic transaction with approximately 13 parties, and exchanged preliminary information with five such parties pursuant to new or existing confidentiality agreements, including (i) a publicly traded global biopharmaceutical company, referred to herein as “Party A,” (ii) a mid-sized privately-held global biopharmaceutical company referred to herein as “Party B,” (iii) a global publicly traded pharmaceutical company referred to herein as “Party C,” (iv) a privately held clinical stage biotechnology company referred to herein as “Party D,” and (v) a publicly traded international pharmaceutical company referred to herein as “Party E”. None of the confidentiality agreements executed or otherwise in effect with such parties at this time contained a standstill provision. These discussions focused on exploring such parties’ potential interest in pursuing a business combination with Zynerba. These discussions did not address the value or terms of any potential business combination transaction, nor did these contacts progress to the point of substantive discussions with respect to a potential business combination at that time. After preliminary exploratory discussions, each of Party A, Party B, Party C, Party D and Party E declined to pursue any potential business combination transaction with Zynerba.
On April 5, 2023, members of Zynerba and Harmony Biosciences’ senior management held an in-person meeting during which, among other things, Harmony Biosciences presented an overview of its business and operations and introduced key department leads to Zynerba management. No financial or other terms of a potential transaction were discussed at this meeting.
Later that day, the Zynerba Board held a meeting at which members of Zynerba’s senior management and representatives of MTS Health were also present. Members of Zynerba management provided an overview of recent business development and strategic discussions, including the recent meeting with Harmony Biosciences and the status of the potential transaction with Harmony Biosciences as well as discussion of potential out-licensing opportunities for Zygel outside of the United States. MTS Health provided an update on the status of outreach activities by representatives of MTS Health soliciting interest from other third parties in a potential transaction with Zynerba and other strategic opportunities.
On April 14, 2023, Harmony Biosciences provided Zynerba with an addendum to its February 23, 2023 non-binding indication of interest (the “April 14 Proposal”), which provided for a valuation of Zynerba of up to $150,000,000, or $2.81 per Share, with transaction consideration comprised of (i) upfront cash consideration of $75,000,000, or $1.41 per Share, and (ii) up to $75,000,000 in CVRs based on obtaining FDA approval for Zygel in FXS, 22q and Autism Spectrum Disorder.
On April 19, 2023, the Zynerba Board held a meeting at which representatives of MTS Health and members of Zynerba senior management were also present. Mr. Anido and representatives of MTS Health led a discussion regarding recent activities concerning a potential business combination with Harmony Biosciences, including the terms of the April 14 Proposal. A representative of MTS Health then provided an overview of current public market conditions and reviewed the fundamental valuation analysis of Zynerba given certain assumptions under various scenarios and analysis frameworks. Members of Zynerba’s senior management then reviewed the Management Projections, summarizing Zynerba’s long-range forecasts for 2023 through 2031 with the Zynerba Board (as more fully described under the heading titled “— Certain Financial Projections”), which the Zynerba Board agreed should be used by MTS Health in connection with its evaluation of the terms of a proposed transaction with Harmony Biosciences. Members of Zynerba’s senior management also described the most recent communications with Harmony Biosciences. A representative of MTS Health then provided a summary financial analysis of the April 14 Proposal. The Zynerba Board, members of senior management and representatives of MTS Health engaged in extended discussion regarding next steps with Harmony Biosciences and broader strategic considerations. Following that discussion, the Zynerba Board instructed management to continue to provide timely updates and to reconvene the Zynerba Board as needed to review any material developments with Harmony Biosciences.
On April 24, 2023, members of Harmony Biosciences and Zynerba senior management met by videoconference to discuss the terms of the April 14 Proposal. Zynerba management communicated that the CVRs should include a potential milestone payment based on positive data resulting from the RECONNECT trial and include commercial milestone triggers with respect to future sales of Zygel.
On April 28, 2023, Harmony Biosciences provided Zynerba with an updated addendum to its February 23, 2023 non-binding indication of interest (the “April 28 Proposal”), which provided for the same upfront and aggregate
TABLE OF CONTENTS
consideration as the April 14 Proposal but revised the CVRs to cover positive data resulting from the RECONNECT trial, FDA approval of Zygel in FXS, FDA approval of Zygel in any second indication, and achievement of at least $500,000,000 in aggregate net sales of Zygel. Later that day, representatives of Harmony Biosciences and Zynerba senior management engaged in telephonic discussions to clarify the terms of the April 28 Proposal.
On May 2, 2023, the Zynerba Board held a meeting at which members of Zynerba management and representatives of MTS Health were also present. Mr. Anido summarized the recent correspondence received from Harmony Biosciences indicating potential interest in a strategic transaction, and members of Zynerba senior management summarized recent communications with Harmony Biosciences following receipt of the April 28 Proposal. Following discussion by the Zynerba Board regarding the April 28 Proposal, a representative of MTS Health reviewed the valuation analyses conducted by MTS Health under various scenarios and assumptions, provided a summary of market comparators, and answered numerous questions from the Zynerba Board. Mr. Anido and a representative of MTS Health then reviewed potential options for responding to the April 28 Proposal, and the Zynerba Board engaged in extended discussion regarding each of the options presented. The Zynerba Board directed Zynerba management to continue to pursue discussions with Harmony Biosciences and keep the Zynerba Board appraised of material updates as those discussions continued to develop.
On May 10, 2023, Zynerba delivered to Harmony Biosciences a response letter (the “May 10 Counterproposal”) indicating that the April 28 Proposal required meaningful improvement in order to obtain the support of the Zynerba Board to proceed with a transaction between the parties and containing a counterproposal to the terms of the April 28 Proposal. The May 10 Counterproposal set forth a proposal for aggregate merger consideration valued at a maximum of $245,000,000, or approximately $4.50 per Share, consisting of (i) upfront cash consideration of $110,000,000, or $2.04 per Share, plus (ii) up to $135,000,000, or approximately $2.46 per Share, in CVR consideration based on the achievement of clinical milestones set out in the April 28 Proposal plus additional CVR milestones based on the achievement of at least $300,000,000 and $600,000,000 in cumulative net revenue from sales of Zygel, respectively.
On May 18, 2023, members of senior management of Harmony Biosciences and Zynerba corresponded by e-mail and teleconference concerning the terms of the May 10 Counterproposal. Later that day, Harmony Biosciences delivered to Zynerba a revised addendum to its February 23, 2023 non-binding indication of interest (the “May 18 Proposal”), setting forth transaction terms consisting of aggregate merger consideration valued at a maximum of $200,000,000, or approximately $3.73 per Share, consisting of (i) upfront cash consideration of $80,000,000, or approximately $1.68 per Share, and (ii) up to $120,000,000, or approximately $2.05 per share, in CVR consideration, based on the achievement of the clinical and commercial milestones set forth in the May 10 Counterproposal, with scaling levels of merger consideration based upon the timing of achievement of certain regulatory events.
On May 24, 2023, the Zynerba Board convened a meeting at which members of senior management of Zynerba and representatives of MTS Health were also present. A representative of MTS Health provided an update to the prior reports prepared by MTS Health summarizing the process overview and valuation analyses conducted by MTS Health under various scenarios and assumptions, and discussing other related items with the Zynerba Board. Mr. Anido and a representative of MTS Health then reviewed the May 18 Proposal with the Zynerba Board and engaged in extended discussion and analysis with the Zynerba Board regarding the terms contemplated in the letter and potential counters. At the conclusion of that discussion, Mr. Anido reviewed the proposed next steps with Harmony Biosciences and potential responses to the May 18 Proposal. The Zynerba Board authorized Mr. Anido to continue negotiating the terms of a potential transaction with Harmony Biosciences in order to obtain the most favorable terms reasonably available.
On June 1, 2023, members of senior management of Zynerba and Harmony Biosciences met to discuss the status of Zynerba’s ongoing RECONNECT clinical trial and other clinical and preclinical programs.
On June 8, 2023, Mr. Anido met with Dr. Jeffrey Dayno, M.D., Chief Executive Officer of Harmony Biosciences, and certain other members of senior management of Zynerba and Harmony Biosciences held an in person meeting to discuss the May 18 Proposal. During the course of that discussion, the parties came to an agreement in principal on the proposed terms of the Offer, consisting of a valuation for Zynerba of up to $205 million, or approximately $3.82 per Share, consisting of (i) an upfront payment of $80,000,000, or approximately $1.49 per Share and (ii) up to $125 million, or approximately $2.33 per Share, in CVR milestones, payable upon the achievement of the regulatory events set forth in the April 28 Proposal plus additional CVR milestones based on the achievement of at least $250,000,000 and $500,000,000 in cumulative net revenue from sales of Zygel, respectively.
TABLE OF CONTENTS
On June 11, 2023, the parties entered into a non-binding letter of intent reflecting the agreement reached by Zynerba and Harmony Biosciences senior management during their meeting on June 8, 2023 (the “June 11 Letter of Intent”).
On June 13, 2023, the Zynerba Board held a meeting at which members of Zynerba management and representatives of MTS Health were also present. Mr. Anido provided the Zynerba Board with a summary of recent discussions with Harmony Biosciences and the terms of the June 8 Proposal, and discussion ensued. Members of Zynerba’s senior management provided an overview of other business development opportunities to date, including discussions with third parties concerning a potential out-license of Zygel outside of the United States. A representative of MTS Health then summarized the preliminary valuation materials and offer analysis materials prepared by MTS Health, Zynerba’s stock performance over the past year, and the valuation methodologies utilized in connection with the preliminary analysis performed by MTS Health with respect to the terms of the June 11 Letter of Intent, including discounted cash flow, comparable publicly traded companies, and precedent transactions.
On June 15, 2023, representatives of Goodwin Procter LLP, outside legal counsel to Zynerba (“Goodwin”), met by videoconference with members of Zynerba’s senior management and representatives of MTS Health to discuss the initial draft of the Merger Agreement prepared by Goodwin, which contemplated, among other things, (i) a “no-shop” provision requiring Zynerba to cease solicitation of alternative proposals following the execution of the Merger Agreement, (ii) the ability of Zynerba to negotiate with counterparties in connection with an unsolicited superior proposal and/or terminate the Merger Agreement to accept a superior proposal and (iii) a termination fee of $1,600,000 in favor of Harmony Biosciences if Zynerba terminated the Merger Agreement under certain circumstances (the “Termination Fee”). The draft Merger Agreement also provided, among other things, that the parties would not be permitted to consider the outcome of any clinical data or the timing or results of any clinical trial or any regulatory filings or approvals with respect to any of Zynerba’s product candidates in determining whether a “Company Material Adverse Effect” (as defined in the Merger Agreement) has occurred. Later that day, representatives of Goodwin, on behalf of Zynerba, distributed to representatives of Hogan Lovells US LLP, counsel to Harmony Biosciences (“Hogan Lovells”), the draft Merger Agreement.
On June 19, 2023, representatives of Goodwin met by videoconference with members of Zynerba’s senior management and representatives of MTS Health to discuss the initial draft of the CVR Agreement prepared by Goodwin, which contemplated, among other things, the inclusion of a “commercially reasonable efforts” standard with respect to Harmony Biosciences’ obligation to develop and commercialize Zygel. The draft CVR Agreement did not include a deadline by which the CVR milestones must be achieved.
On June 20, 2023, representatives of Goodwin, on behalf of Zynerba, distributed to representatives of Hogan Lovells the draft CVR Agreement.
On June 22, 2023, representatives of Goodwin and representatives of Hogan Lovells met by videoconference to discuss certain material terms of the Merger Agreement and the CVR Agreement as well as certain diligence items.
Also on June 22, 2023, the Compensation Committee of the Zynerba Board (the “Compensation Committee”) met to discuss certain executive compensation matters, including the establishment of a “change in control” policy and certain amendments to the terms of certain executive employment agreements intended to, among other reasons, bring the terms of those employment agreements in line with other Zynerba executives’ employment agreements, as further described in the section under the caption “— Arrangements Between Zynerba and its Executive Officers, Directors and Affiliates.” Members of senior management of Zynerba were also present at the meeting. Following discussion, the Compensation Committee approved the “change in control” policy and reviewed the proposed employment agreement amendments and recommended that such proposals be submitted to the Zynerba Board for approval. Interested members of Zynerba management did not participate in the discussions and deliberations of the Compensation Committee regarding the employment agreement amendments.
On June 26, 2023, representatives of Hogan Lovells, on behalf of Harmony Biosciences, distributed to representatives of Goodwin a due diligence request list soliciting certain information concerning Zynerba and its business in connection with its legal due diligence review. Zynerba management continued to provide due diligence information in response to such requests.
On July 7, 2023, representatives of Hogan Lovells, on behalf of Harmony Biosciences, distributed to representatives of Goodwin a revised draft of the Merger Agreement, reflecting, among other things, (i) a revised “no shop” covenant that did not permit Zynerba to terminate the Merger Agreement in response to a superior proposal, (ii) a company termination fee of $3,200,000, (iii) a revised definition of “Company Material Adverse Effect” which did not exclude
TABLE OF CONTENTS
the timing or outcome of Zynerba’s preclinical or clinical studies or the timing or results of regulatory filings or approvals for Zynerba’s product candidates in determining whether a “Company Material Adverse Effect” has occurred, and (iv) a post-closing right of offset against the CVR consideration for any losses incurred by Harmony Biosciences due to breaches of Zynerba’s representations, warranties and covenants under the Merger Agreement.
On July 10, 2023, representatives of Goodwin and MTS Health met by videoconference with members of Zynerba senior management to discuss the revised drafts of the Merger Agreement and CVR Agreement and proposed responses to Harmony Biosciences.
On July 12, 2023, representatives of Goodwin, on behalf of Zynerba, distributed to representatives of Hogan Lovells a revised draft of the Merger Agreement, which draft reflected, among other things, (i) the reinstatement of Zynerba’s right to terminate the Merger Agreement in response to a superior proposal, (ii) a proposed Termination Fee of $2,400,000, (iii) the reinstatement of Zynerba’s proposal to exclude certain preclinical, clinical and other regulatory events related to Zynerba’s product candidates from consideration in the determination of whether a “Company Material Adverse Effect” has occurred, and (iv) the removal of Harmony Biosciences’ right of offset for breaches of Zynerba’s representations, warranties and covenants under the Merger Agreement.
On July 13, 2023, Mr. Albert Parker, Zynerba’s Chief Legal Officer, met telephonically with Mr. Christian Ulrich, Harmony Biosciences’ Senior Vice President and General Counsel, and Mr. Andrew Serafin, Harmony Biosciences’ Chief Strategy Offer, to inform them of the proposed amendments to certain of Zynerba’s executive employment agreements previously approved by the Compensation Committee, as further described in the section under the caption “— Arrangements Between Zynerba and its Executive Officers, Directors and Affiliates” to, among other reasons, bring the terms of those employment agreements in line with other Zynerba executives’ employment agreements.
On July 19, 2023, representatives of Hogan Lovells, on behalf of Harmony Biosciences, distributed a revised draft of the Merger Agreement to representatives of Goodwin, on behalf of Zynerba. The revised Merger Agreement reflected, among other things, (i) the removal of Zynerba’s right to terminate the Merger Agreement in response to a superior proposal, (ii) a proposed Termination Fee of $3,200,000, (iii) the removal of Zynerba’s proposal to exclude certain preclinical, clinical and other regulatory events related to Zynerba’s product candidates from consideration in the determination of whether a “Company Material Adverse Effect” has occurred, and (iv) the re-insertion of Harmony Biosciences’ post-closing right of offset against the CVR consideration for any losses incurred by Harmony Biosciences due to breaches of Zynerba’s representations, warranties and covenants under the Merger Agreement.
Also on July 19, 2023, representatives of Hogan Lovells, on behalf of Harmony Biosciences, distributed a revised draft of the CVR Agreement to representatives of Goodwin, on behalf of Zynerba, reflecting, among other things, (i) the removal of Harmony Biosciences’ obligation to use diligent efforts to develop and commercialize Zygel, and (ii) certain deadlines by which CVR milestones must be achieved, including requirement that all milestones be achieved by December 31, 2032.
On July 21, 2023, representatives of Goodwin and MTS Health met by videoconference with members of Zynerba senior management to discuss the revised drafts of the Merger Agreement and CVR Agreement and proposed responses to Harmony Biosciences, including Zynerba’s position on the size of the proposed Termination Fee and “fiduciary out” protections and the removal of the diligent efforts covenant in the CVR Agreement.
On July 24, 2023, Mr. Anido met virtually with Dr. Dayno to discuss the results of Harmony Biosciences’ financial due diligence to date and certain proposed revisions to the terms of Harmony Biosciences’ June 11 Proposal resulting from Harmony Biosciences’ review of additional information concerning cost of goods and supply chain expenses and the enrollment pace and timing of the RECONNECT trial. Later that day, representatives of Harmony Biosciences distributed to Zynerba management a revised letter of intent (the “July 24 Proposal”) revising the terms of the June 11 Proposal to provide for up to $200,000,000 in aggregate merger consideration, consisting of (i) $60,000,000 in aggregate upfront merger consideration and (ii) an additional CVR milestone payment of $15,000,000, payable upon completion of the RECONNECT trial on or prior to June 30, 2026, in addition to up to $125,000,000 in CVR milestone payments as described in the June 11 Proposal.
On July 27, 2023, the Zynerba Board held a meeting at which members of Zynerba’s senior management and representatives of MTS Health were also present. Mr. Anido summarized his recent conversations with Harmony Biosciences management and reviewed the terms of the July 24 Proposal. A representative of MTS Health provided a detailed analysis of the terms of the July 24 Proposal, and discussion ensued concerning the financial terms, valuation impact, and other related topics. Mr. Parker then reviewed certain legal issues and key drafting provisions
TABLE OF CONTENTS
under consideration, and the Zynerba Board discussed at length various items concerning the response to the July 24 Proposal, including potential business and legal terms, timing, and other matters. The Zynerba Board directed Zynerba management to continue negotiations concerning the potential transaction with Harmony Biosciences and to keep the Zynerba Board apprised of the timing, status and terms thereof.
On July 28, 2023, representatives of Zynerba distributed to Harmony Biosciences management a written response to the July 24 Proposal indicating Zynerba’s acceptance of the terms of the July 24 Proposal subject to agreement on certain outstanding key points in the Merger Agreement and the CVR Agreement, including (i) the re-insertion of Zynerba’s right to terminate the Merger Agreement in response to a superior proposal, (ii) the removal of any post-closing right of offset against the CVR Agreement due to breaches of Zynerba’s representations, warranties and covenants under the Merger Agreement, (iii) the inclusion of a “commercially reasonable efforts” standard with respect to Harmony Biosciences’ efforts to achieve the CVR milestones, and (iv) the removal of milestone deadlines under the CVR Agreement (collectively, the “July 28 Key Terms”).
On August 1, 2023, the Zynerba Board convened a meeting at which members of Zynerba’s senior management and representatives of MTS Health were also present. At the meeting, Mr. Anido provided an update regarding his recent discussions with Harmony Biosciences management and the terms of the July 24 Proposal. Mr. Parker then provided additional information regarding recent discussions with Harmony Biosciences’ legal counsel, and reviewed the anticipated timeline and expected response from Harmony Biosciences. Following discussion, the Zynerba Board directed Mr. Anido and Zynerba management to continue updating the Zynerba Board on the progress of the potential transaction with Harmony Biosciences.
On August 2, 2023, representatives of Hogan Lovells, on behalf of Harmony Biosciences, distributed revised drafts of the Merger Agreement and the CVR Agreement to representatives of Goodwin, on behalf of Zynerba. The revised Merger Agreement reflected, among other things, (i) the acceptance of the July 28 Key Terms, (ii) the re-insertion of Harmony Biosciences’ previous position permitting Harmony Biosciences to consider clinical, regulatory and related events in determining whether a “Company Material Adverse Effect” has occurred, and (iii) a proposed increase in the size of the Termination Fee to $8,000,000. The revised CVR Agreement reflected, among other things, a commercially reasonable efforts standard with respect to the achievement by Harmony Biosciences of the CVR milestones.
On August 4, 2023, representatives of Goodwin and MTS Health met by videoconference with members of Zynerba senior management to discuss Harmony Biosciences’ revised draft of the Merger Agreement and the CVR Agreement. Later that day, following such discussion among Zynerba management and its legal and financial advisors, representatives of Goodwin, on behalf of Zynerba, distributed to representatives of Hogan Lovells a revised draft of the Merger Agreement, reflecting, among other things, (i) a proposed Termination Fee of $2,400,000 and (ii) the reinstatement of a more narrowly targeted proposal to exclude certain preclinical, clinical and other regulatory events related to Zynerba’s product candidates from the definition of “Company Material Adverse Effect.” Also on August 4, 2023, Mr. Anido met with Dr. Dayno by teleconference to discuss the outstanding items in the Merger Agreement and the CVR Agreement.
On August 5, 2023, representatives of Goodwin, on behalf of Zynerba, distributed to representatives of Hogan Lovells a revised draft of the CVR Agreement.
On August 7, 2023, representatives of Hogan Lovells, on behalf of Harmony Biosciences, distributed revised drafts of the Merger Agreement and the CVR Agreement to representatives of Goodwin, on behalf of Zynerba. The revised Merger Agreement reiterated Harmony Biosciences’ previous positions with respect to (i) Harmony Biosciences’ ability to consider clinical, regulatory and related events in the determining whether a “Company Material Adverse Effect” has occurred and (ii) an $8,000,000 proposed Termination Fee.
On the morning of August 8, 2023, representatives of Goodwin met with members of Zynerba management and representatives of MTS Health to discuss the revised terms of the Merger Agreement and the CVR Agreement and proposed responses to Harmony Biosciences. Later that day, representatives of Goodwin, on behalf of Zynerba, distributed to representatives of Hogan Lovells an issues list setting forth counterproposals of Zynerba with respect to certain key open issues in the draft Merger Agreement and the draft CVR Agreement, including (i) a proposed Termination Fee of $3,600,000, (ii) a more limited carve-out to the definition of “Company Material Adverse Effect” for certain regulatory and clinical events and (iii) a deadline of December 31, 2040 for the achievement of all milestones under the CVR Agreement.
TABLE OF CONTENTS
In the evening of August 8, 2023, representatives of Goodwin, on behalf of Zynerba, met with representatives of Hogan Lovells, on behalf of Harmony Biosciences, to discuss Zynerba’s counterproposals and expectations with respect to the timeline to execution of the Merger Agreement.
On August 9, 2023, representatives of Goodwin, on behalf of Zynerba, distributed to representatives of Hogan Lovells a revised draft of the Merger Agreement reflecting Zynerba’s counterproposals as discussed during the August 8, 2023 meeting between representatives of Goodwin and Hogan Lovells.
Throughout the morning and afternoon of August 10, 2023, representatives of Hogan Lovells and Goodwin met by videoconference to discuss certain outstanding diligence items and open issues in the draft Merger Agreement and the draft CVR Agreement. Later that day, representatives of Hogan Lovells, on behalf of Harmony Biosciences, distributed a revised draft of the Merger Agreement to representatives of Goodwin, on behalf of Zynerba, reflecting, among other things, a proposed Termination Fee of $6,000,000. Following telephonic discussions between representatives of Goodwin, representatives of MTS Health and members of Zynerba senior management, representatives of Goodwin, on behalf of Zynerba, distributed to representatives of Hogan Lovells a revised Merger Agreement proposing a proposed Termination Fee of $4,200,000. In making such proposal, Zynerba management considered the size of the proposed Termination Fee relative to the upfront transaction equity value plus near-term milestone payments. Representatives of Goodwin, on behalf of Zynerba, also delivered a revised draft of the CVR Agreement reflecting the December 31, 2040 deadline for the achievement of the CVR milestones.
During the course of the day on August 11, 2023, representatives of Goodwin and representatives of Hogan Lovells engaged in discussions by videoconference and exchanged drafts of the Merger Agreement, the CVR Agreement and the Support Agreements. During this time, Dr. Dayno and Mr. Anido also exchanged emails concerning certain outstanding financial due diligence questions.
On the morning of August 12, 2023, the Zynerba Board held a virtual meeting at which representatives of Goodwin and representatives of MTS Health, as well as members of Zynerba’s senior management, were also present, to consider approval of the proposed transaction with Harmony Biosciences. Representatives of MTS Health reviewed with the Zynerba Board MTS Health’s financial analyses of the Offer Price, and rendered to the Zynerba Board MTS Securities’ oral opinion, which was subsequently confirmed by delivery of a written opinion dated August 12, 2023, that, as of such date and based upon and subject to various assumptions made, procedures followed, matters considered, and qualifications and limitations upon the review undertaken in preparing its opinion, the Offer Price to be paid in the Offer and the Merger to the holders of Shares (other than Excluded Shares) pursuant to the Merger Agreement was fair, from a financial point of view, to such holders. For a detailed discussion of MTS Securities’ opinion, please see below under the caption “— Opinion of Zynerba’s Financial Advisor.” Representatives of Goodwin then reviewed the terms of the final proposed transaction documentation with Harmony Biosciences, including the Merger Agreement and the CVR Agreement. Representatives of Goodwin and the Zynerba Board also discussed certain amendments to certain executive employment agreements, as further described in the section under the caption “— Arrangements Between Zynerba and its Executive Officers, Directors and Affiliates.” After discussion, the independent members of the Zynerba Board unanimously approved the amendments to such executive employment agreements in accordance with the recommendation of the Compensation Committee. Following additional discussion and consideration of the Merger Agreement and the Offer, the Merger, the CVR Agreement and the other transactions contemplated by the Merger Agreement (including the factors described in “—Reasons for the Recommendation”), the Zynerba Board unanimously adopted resolutions (1) approving, adopting and declaring advisable the Merger Agreement and the transactions contemplated thereby, including the Offer and the Merger, (2) determining that the transactions contemplated by the Merger Agreement, including the Offer and the Merger, were in the best interests of Zynerba and its stockholders, (3) resolving that the Merger Agreement will be governed by and effected under Section 251(h) of the DGCL, and (4) recommending that the Zynerba stockholders accept the Offer and tender their Shares to Merger Sub pursuant to the Offer. The approval of the Zynerba Board was contingent upon the parties reaching agreement on the size of the Termination Fee, which the Zynerba Board authorized to be up to $4,500,000.
Later in the afternoon on August 12, 2023, Mr. Anido, Dr. Dayno and other representatives of senior management of Zynerba and Harmony Biosciences, as well as representatives of Goodwin, Hogan Lovells and MTS Health, met by videoconference to discuss certain confirmatory financial due diligence questions with respect to Zynerba.
On the morning of August 13, 2023, Mr. Anido and Dr. Dayno met by teleconference to discuss the size of the company termination fee and anticipated timing for signing the Merger Agreement, during which they agreed to a
TABLE OF CONTENTS
Termination Fee of $4,500,000 and to proceed with finalizing the transaction documents and executing the Merger Agreement as of 12:01 a.m. Eastern Time on August 14, 2023. Over the course of the afternoon and evening on August 13, 2023, Mr. Anido and Dr. Dayno exchanged emails concerning the transaction, and representatives of Goodwin and Hogan Lovells engaged in discussions telephonically and by videoconference and exchanged comments to the draft Merger Agreement and the draft CVR Agreement. At 12:01 a.m. Eastern Time on August 14, 2023, Harmony Biosciences, Merger Sub and Zynerba entered into the Merger Agreement, and Harmony Biosciences, Merger Sub and the Supporting Stockholders entered into the Support Agreements.
Before the opening of trading of the U.S. stock markets on August 14, 2023, Harmony Biosciences and Zynerba issued a joint press release announcing the execution of the Merger Agreement.
Reasons for the Recommendation
In evaluating the Merger Agreement and the Transactions, including the Offer and the Merger, the Zynerba Board consulted with Zynerba’s senior management and legal and financial advisors. In the course of reaching its determination that the Transactions, including the Offer and the Merger, are in the best interests of Zynerba and its stockholders, and unanimously recommending that Zynerba’s stockholders accept the Offer and tender all of their Shares in the Offer, the Zynerba Board reviewed, evaluated, and considered a wide and complex range of factors discussed below. The Zynerba Board also consulted with its legal advisors regarding their fiduciary duties, legal due diligence matters and the terms of the Merger Agreement. Based on these consultations, considerations and analyses, and the factors discussed below, the Zynerba Board approved and declared advisable the execution, delivery, and performance by the Company of the Merger Agreement with Harmony Biosciences and Purchaser and the consummation of the Transactions and determined that the Transactions are in the best interests of Zynerba’s stockholders.
Offer Price. The Zynerba Board considered:
• | the historical market prices, volatility and trading information with respect to the Shares; |
• | the recent historical trading prices of the Shares, as compared to the Closing Amount, including the fact that the Closing Amount of $1.1059 per Share represents a 226% premium to the closing price of $0.339 per Share on August 11, 2023, the last full trading day prior to the public announcement of the execution of the Merger Agreement; and |
• | that in its view it had obtained Harmony Biosciences’ best and final offer, and that, as of the date of the Merger Agreement, the Offer Price and Merger Consideration represented the highest per-Share consideration reasonably obtainable. |
Zynerba’s Operating and Financial Condition and Prospects. The Zynerba Board considered Zynerba’s historical and projected ability to execute on its long-term stand-alone plan, operating and financial performance and its prospects, including certain prospective forecasts for Zynerba prepared by Zynerba’s senior management, which reflect an application of various assumptions of senior management. The Zynerba Board considered the inherent uncertainty of achieving management’s prospective forecasts, as set forth under the heading titled “— Certain Financial Projections,” and that, as a result, Zynerba’s actual financial results in future periods could differ materially from senior management’s forecasts. The Zynerba Board considered, among other factors, that the holders of the Shares would continue to be subject to the risks and uncertainties of Zynerba executing on its short and long-range plan if it remained independent. These risks and uncertainties included risks relating to the macro-economic, industry and market conditions negatively impacting valuations of clinical pharmaceutical and biotechnology companies such as Zynerba, the need for meaningful capital investment to fund Zynerba’s continued operations, the uncertainty in obtaining marketing approval for Zygel, the need to successfully commercialize and gain market acceptance of Zygel, the need to build a sales and marketing infrastructure, the risks and costs associated with partnerships to assist in the global development and commercialization of Zygel outside of the United States, the development by other companies of competitive products, the dependence on the success of a single lead product candidate, the dependence on key personnel and compliance with government regulations, the risk that Zynerba is unable to satisfy the continued listing requirements of Nasdaq in order to permit Zynerba to maintain the listing of its common stock on Nasdaq, and the general risk of market conditions that could reduce Zynerba’s stock price. The Zynerba Board also considered the risks and challenges facing Zynerba as a result of its current cash position and that, as a standalone company, Zynerba would need to seek substantial additional funding through future equity, royalty and/or debt financings or additional collaborations or strategic partnerships, and any such fundraising could have a highly dilutive effect on Zynerba’s existing stockholders, could require Zynerba to enter into restrictive covenants, might only be
TABLE OF CONTENTS
available on unfavorable terms or might not be available at all. The Zynerba Board weighed the certainty of realizing a substantial value for Shares in the Offer and the Merger compared to the uncertainty that trading values would approach the Offer Price in the foreseeable future and the substantial risk and uncertainty associated with Zynerba and its business as a clinical-stage pharmaceutical company (including the risk factors set forth in Zynerba’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, and subsequent quarterly reports on Form 10-Q).
Potential Strategic Alternatives. The Zynerba Board reviewed the possible alternatives to the Offer and the Merger, including the execution of management’s stand-alone plan. The Zynerba Board considered the risks inherent in the development of drug products, the risks related to designing, conducting and compiling data from clinical trials, the risks related to seeking approval for marketing from the FDA and other regulatory authorities, competition, and other factors affecting the revenues and profitability of pharmaceutical and biotechnology companies generally. The Zynerba Board also considered the fact that Zygel has not yet been approved for marketing by the FDA or any similar non-U.S. regulatory body in any indication, as well as the status and prospects for the RECONNECT trial and Zynerba’s other clinical and pre-clinical development programs.
Product Commercialization and Development Risks; Existing Resources. The Zynerba Board considered the status and prospects for Zynerba’s current pipeline, including the fact that Zynerba is heavily dependent on the regulatory approval and commercial success of Zygel, its lead product candidate. The Zynerba Board considered the risk of failure of Zynerba’s ongoing clinical trials, the risk that Zygel will not be approved or, if approved, successfully commercialized for any indication. The Zynerba Board considered the fact that Zynerba will require significant additional capital in order to complete the remaining clinical development for Zygel in the treatment of behavioral symptoms of Fragile X syndrome and chromosome 22q11.2 deletion syndrome, expand development to additional indications to maximize the value of Zygel, obtain marketing approval for and commercialize Zygel, as well as fund other clinical and pre-clinical programs and otherwise execute on its business plan. The Zynerba Board also considered that, while Zynerba may seek additional funding through future debt and equity financing or additional collaborations or strategic partnerships, any such fundraising could have a highly dilutive effect on Zynerba’s existing stockholders, might only be available on unfavorable terms, or might not be available at all.
Negotiation Process. The Zynerba Board considered the fact that the terms of the Offer and Merger were the result of robust arm’s-length negotiations conducted by Zynerba with the knowledge and at the direction of the Zynerba Board and with the assistance of its financial and legal advisors. The Zynerba Board also considered the enhancements that Zynerba and its advisors were able to obtain as a result of robust arm’s-length negotiations with Harmony Biosciences, including the increase in the valuation offered by Harmony from the time of its initial expression of interest to the end of the negotiations and the inclusion of provisions in the Merger Agreement that increase the likelihood of completing the Offer and consummating the Merger and allow the Zynerba Board to consider unsolicited superior acquisition proposals from third parties.
Potentially Interested Counterparties. The Zynerba Board, with the assistance of members of its senior management and its legal and financial advisors, considered the process conducted by Zynerba, with the assistance of representatives of MTS Health Partners, L.P. (“MTS Health Partners”), to identify potential buyers taking into account the expected interest of parties in Zygel generally, their financial capability to consummate a transaction of this size, and their ability to move quickly and efficiently in a process, the outcome of those discussions, and the fact that none of these parties, other than Harmony Biosciences, had expressed interest in a strategic transaction such as the Offer and the Merger. In connection with the foregoing, the Zynerba Board reviewed potential strategic alternatives for Zynerba in light of its current and projected financial position and results of operations, the challenges it faces in growing its core business operations as a stand-alone company (including its projected need to raise additional capital in the near term), and its historical and projected ability to execute on its long-term stand-alone plan, in order to identify the course of action that would, in the Zynerba Board’s opinion, create the most value for its stockholders. The Zynerba Board believes, after such review of potential strategic alternatives and Zynerba’s prospects and challenges as a stand-alone company, that the Offer and the Merger represent a superior alternative to the other alternatives available to Zynerba, including remaining a stand-alone public company, considering the potential stockholder value that might result from such alternatives, the feasibility of such alternatives and the risks and uncertainties associated with pursuing such alternatives relative to the Offer and the Merger.
Opinion of Financial Advisor. The Zynerba Board considered the opinion of MTS Securities, LLC rendered to the Zynerba Board on August 12, 2023, which was subsequently confirmed by delivery of a written opinion dated August 12, 2023 that, as of such date and based upon and subject to the assumptions made, procedures followed, matters considered, and qualifications and limitations upon the review undertaken by MTS Securities, LLC in
TABLE OF CONTENTS
preparing its opinion, the Offer Price to be paid in the Offer and the Merger to the holders of Shares (other than Excluded Shares) pursuant to the Merger Agreement, was fair, from a financial point of view, to such holders, as more fully described below under the caption “— Opinion of Zynerba’s Financial Advisor.” The full text of the written opinion, dated August 12, 2023, of MTS Securities, LLC has been included as Annex I to this Schedule 14D-9 and are incorporated herein by reference.
The Merger Agreement; Ability to Consider, Receive and Respond to Unsolicited Proposals. The Zynerba Board considered the provisions of the Merger Agreement, including:
• | the agreed exclusions of certain events and conditions from the definition of “Company Material Adverse Effect”, including certain regulatory events related to Zynerba’s product candidates; |
• | that Zynerba and Harmony have agreed to use their respective commercially reasonable efforts to complete the Offer and the Merger and, if applicable, obtain the consents and approvals required under applicable antitrust laws; |
• | the ability of Zynerba under certain circumstances to entertain unsolicited proposals for an acquisition that constitutes or could reasonably be expected to lead to a superior proposal; |
• | the ability of the Zynerba Board under certain circumstances to withdraw or modify its recommendation that the holders of Shares accept the Offer and tender their Shares in the Offer, including in connection with a superior proposal and certain reasonably unforeseeable events or developments; |
• | Zynerba’s right to terminate the Merger Agreement under certain circumstances in order to accept a superior proposal and enter into an agreement with respect to such superior proposal; and |
• | the fact that the deal protections set forth in the merger agreement do not preclude a third party from making an acquisition proposal that is superior to the terms of the merger agreement, including the reasonableness of the size of the termination fee and the related termination rights of the parties. |
Conditions to the Consummation of the Offer and Merger; Likelihood of Completion. The Zynerba Board considered the likelihood of completing the Offer and the Merger, particularly in light of the terms of the Merger Agreement, including (1) the conditions to the Offer and the Merger being specific and limited and (2) the exceptions contained within the “Company Material Adverse Effect” definition, which generally defines the standard for closing risk. The Zynerba Board also considered the fact that there is no financing condition to the completion of the Offer and consummation of the Merger. The Zynerba Board also considered the Support Agreements, pursuant to which certain directors and executive officers of Zynerba have agreed, solely in their capacity as stockholders of Zynerba, to tender their shares in the Offer.
Tender Offer Structure; Timing of Completion. The Zynerba Board considered the anticipated timing of the consummation of the Transactions, and the structure of the Transactions as a cash tender offer for all outstanding Shares, with the anticipated result of allowing Zynerba’s stockholders to receive the Offer Price in a relatively short time frame, followed by the Merger (to be effected pursuant to Section 251(h) of the DGCL) in which Zynerba’s stockholders who do not validly exercise appraisal rights will receive the same consideration received by those stockholders who tender their Shares in the Offer. The Zynerba Board noted that Harmony Biosciences would require no additional financing and, instead, would fund the purchase price from existing cash resources. The Zynerba Board also considered that the potential for closing in a relatively short timeframe could reduce the amount of time in which Zynerba’s business would be subject to the potential uncertainty of closing and related disruption.
Extension of Offer Period. The Zynerba Board considered that, under certain circumstances set forth in the Merger Agreement, Purchaser is required to extend the Offer beyond the initial expiration date of the Offer or, if applicable, subsequent expiration dates, if the conditions to the consummation of the Offer are not satisfied or waived as of such date.
End Date. The Zynerba Board considered the termination date under the Merger Agreement on which either Harmony Biosciences or Zynerba, subject to certain exceptions, can terminate the Merger Agreement, which is anticipated to allow for sufficient time to consummate the Offer and the Merger while minimizing the length of time during which Zynerba would be required to operate subject to the restrictions on interim operations set forth in the Merger Agreement.
Appraisal Rights. The Zynerba Board considered the availability of statutory appraisal rights to Zynerba’s stockholders (and certain beneficial owners, as the case may be) who do not tender their Shares in the Offer and otherwise comply with all required procedures under the DGCL.
TABLE OF CONTENTS
Business Reputation of Harmony Biosciences. The Zynerba Board considered the business reputation and capabilities of Harmony Biosciences and its management and the substantial financial resources of Harmony Biosciences and, by extension, Purchaser, which the Zynerba Board believed supported the conclusion that a transaction with Harmony Biosciences and Purchaser could be completed relatively quickly and in an orderly manner.
Certainty of Consideration. The Zynerba Board considered the nature of the consideration to be paid in the Offer and the Merger, which allows holders of Shares to realize immediate value, in cash, for their investment in Zynerba, while avoiding Zynerba’s regulatory, commercialization and other business risks, and while also providing such holders of Shares with certainty of value and liquidity for their Shares.
Opportunity to Realize Additional Value. The Zynerba Board considered that in addition to the Closing Amount, the holders of Shares will receive a CVR, which provides the holders of Shares with opportunities to realize additional value through additional cash payments to the extent that the Milestones are achieved within the time periods described therein. The Zynerba Board also took into account the fact that, while Harmony has the right, in its discretion, to direct and control the research, development, commercialization and other exploitation of Zygel™, Harmony Biosciences must use “Commercially Reasonable Efforts” (as described in the CVR Agreement) to achieve the Milestones and the experience and resources of Harmony Biosciences in pharmaceutical product development and commercialization, particularly as such commercial experience and resources relate to the potential achievement of the Milestones.
In the course of its deliberations, the Zynerba Board also considered a variety of material risks and other countervailing factors related to entering into the Merger Agreement, including, but not limited to, the following:
• | the fact that Zynerba would no longer exist as an independent, publicly traded company, and Zynerba’s stockholders will not be entitled to participate in any potential future benefit from Zynerba’s execution of management’s stand-alone strategic business plan, except to the extent Milestone Payments are made pursuant to the CVR Agreement; |
• | the risk that the Milestones may not be achieved at all or during the period required by the CVR Agreement for the holders of Shares to receive the payments due with respect to each Milestone pursuant to the CVRs; |
• | the effect of the public announcement of the Merger Agreement, including effects on Zynerba’s clinical and pre-commercialization activities, Zynerba’s relationship with its partners and other business relationships, and Zynerba’s ability to attract and retain key management and personnel; |
• | the fact that the Merger Agreement precludes Zynerba from actively soliciting alternative takeover proposals and requires payment by Zynerba of a $4,500,000 termination fee under certain circumstances, including in the event that the Merger Agreement is terminated by Zynerba to accept a superior proposal. Although the Zynerba board believed that the termination fee is reasonable in light of the benefits of the Offer and the Merger, it is possible that such termination fee, either alone or together with other terms of the Merger Agreement, could discourage other potential interested third parties, if any, from making a competing offer for Zynerba. In addition, Zynerba will generally be required to pay its own expenses associated with the Offer and the Merger, and in certain circumstances will be required to reimburse Harmony for up to $600,000 of its expenses if the Merger Agreement is terminated under certain circumstances; |
• | the possibility that the Transactions, including the Offer and the Merger, might not be consummated, and the fact that if the Offer and the Merger are not consummated, Zynerba’s directors, management and other employees will have expended extensive time and effort and will have experienced significant distractions from their work during the pendency of the Transactions, Zynerba will have incurred significant transaction costs, the trading price of the Shares could be adversely affected and Zynerba’s relationships with its partners, employees and other third parties may be adversely affected; |
• | the fact that there are restrictions in the Merger Agreement on Zynerba’s ability to solicit competing bids to acquire it and to entertain other acquisition proposals, unless certain conditions are satisfied, and the fact that the Zynerba Board may not, under the Merger Agreement, unilaterally terminate the Merger Agreement to accept an alternative proposal; |
• | the restrictions imposed on the conduct of Zynerba’s business prior to completion of the Offer due to pre-closing covenants in the Merger Agreement, whereby Zynerba agreed that it will carry on its business |
TABLE OF CONTENTS
in the ordinary course of business consistent with past practice and, subject to specified exceptions, will not take a number of actions related to the conduct of its business without the prior written consent of Harmony Biosciences, which could delay or prevent Zynerba from undertaking some business opportunities that may arise during that time, or taking other actions with respect to the operations and strategy of Zynerba that the Zynerba Board and Zynerba’s management might otherwise believe were appropriate or desirable;
• | the risk of litigation related to the Offer or the Merger; |
• | the fact that significant costs have been and will continue to be incurred in connection with negotiating and entering into the Merger Agreement and completing the Offer and the Merger, and substantial time and effort of Zynerba’s management will be required, potentially resulting in disruptions to the operation of Zynerba’s business; |
• | the interests that certain directors and executive officers of Zynerba may have with respect to the Transaction that may be different from, or in addition to, their interests as Zynerba’s stockholders or the interests of Zynerba’s other stockholders generally, as described in “Item 3. Past Contacts, Transactions, Negotiations and Agreements—Arrangements Between Zynerba and its Executive Officers, Directors and Affiliates”; and |
• | the treatment of the consideration to be received by the holders of Shares in the Offer and the Merger as taxable to the holders of Shares for U.S. federal income tax purposes. |
The foregoing discussion of the information and factors considered by the Zynerba Board in reaching its conclusions and recommendations is intended to be illustrative and not exhaustive, but includes the material reasons and factors considered by the Zynerba Board. In view of the wide variety of reasons and factors considered, the Zynerba Board did not find it practicable to, and did not, quantify, rank or otherwise assign any relative or specific weights to the various specific factors considered in reaching its determination and making its recommendation. In addition, the Zynerba Board did not reach any specific conclusion with respect to any of the factors or reasons considered. Instead, the Zynerba Board conducted an overall review of the factors and reasons described above and determined that, in the aggregate, the potential benefits considered outweighed the potential risks or possible negative consequences of the Offer and the Merger.
The foregoing discussion of the reasoning of the Zynerba Board and certain information presented in this section is forward-looking in nature and, therefore, the information should be read in light of the factors discussed in “Item 8. Additional Information—Cautionary Note Regarding Forward-Looking Statements.” For the reasons described above, and in light of other factors that the Zynerba Board believed were appropriate to consider, the Zynerba Board approved the Merger Agreement and the transactions contemplated thereby, including the Offer and the Merger, and unanimously recommends that Zynerba’s stockholders tender their Shares to Purchaser pursuant to the Offer.
Intent to Tender
To Zynerba’s knowledge, after making reasonable inquiry, all of Zynerba’s executive officers and directors currently intend to tender or cause to be tendered pursuant to the Offer all of their Shares (other than unvested Company RSAs) held of record or beneficially owned by such persons immediately prior to the expiration of the Offer, as it may be extended (other than Shares for which such holder does not have discretionary authority). The foregoing does not include any Shares over which, or with respect to which, any such executive officer or director acts in a fiduciary or representative capacity or is subject to the instructions of a third party with respect to such tender.
In addition, Harmony Biosciences and Purchaser entered into Support Agreements with certain Zynerba directors and executive officers pursuant to which each such director and executive officer has agreed to tender all of their Shares in the Offer.
Certain Financial Projections
Zynerba does not yet have any marketed products and does not, as a matter of course, publicly disclose forecasts or projections as to future performance, earnings or other results due to the inherent unpredictability of the underlying assumptions, estimates and projections. However, in connection with its strategic planning process and at the direction of the Zynerba Board in connection with its evaluation of the proposed transaction with Harmony Biosciences, Zynerba management prepared long-range projections of revenue and costs for fiscal years 2023 through 2031 based on its view of the prospects for Zynerba on a stand-alone basis with respect to Zynerba’s
TABLE OF CONTENTS
programs for Zygel (“Management Projections”), and directed MTS Health Partners to extrapolate such projections for fiscal years 2032 through 2038 (holding earnings before interest and taxes, or EBIT, in fiscal year 2031 constant through fiscal year 2038) and to adjust such projections based on probabilities of success provided by Zynerba management (such projections for fiscal year 2023 through 2038, as more fully described below are referred to as the “Forecasts”). The Forecasts reflect a non-risk-adjusted outlook and risk-adjusted outlook, as applicable, and were based on certain internal assumptions about the probability of technical success and regulatory approval, timing of completion of clinical studies and commercial launch, a successful equity capital raise of up to $17 million to sustain the company through completion of its current phase 3 trial of Zygel in patients with Fragile X Syndrome, sales ramp, commercial reach, market size and prevalence, diagnosis rates, market share, compliance and persistence, pricing, market access, expected cash burn rate, competition, partnering and licensing arrangements, market exclusivity, estimated costs and expenses, effective tax rate and utilization of net operating losses, ability to raise future capital, and the impact of future capital raises on current stockholders, and other relevant factors relating to Zynerba and Zygel. The Forecasts were developed solely using the information available to Zynerba management at the time they were created.
The Management Projections were presented to and considered by the Zynerba Board in connection with their evaluations of the Transactions in comparison to Zynerba’s other strategic alternatives. The Management Projections also were provided to MTS Health Partners, and the Zynerba Board directed MTS Health Partners to use the Forecasts, including the Management Projections, in its financial analyses and opinion (as summarized above under “— Opinion of Zynerba’s Financial Advisor”). The Forecasts, including the Management Projections, were not provided to Harmony Biosciences.
The summaries of the Forecasts are not being included in this Schedule 14D-9 to influence any stockholder’s decision whether to tender his, her or its Shares in the Offer. The summaries of the Forecasts are being included in this Schedule 14D-9 because the Forecasts were presented to the Zynerba Board to evaluate strategic transactions considered by the Zynerba Board, including the transactions contemplated by the Offer and the Merger Agreement and to MTS Health Partners for its financial analyses and MTS Securities’ opinion. The Forecasts may differ from publicized analyst estimates and forecasts and, in each instance, do not take into account any events or circumstances after the date they were prepared, including the announcement of the Offer and Merger.
Each of the Forecasts, although presented with numerical specificity, are necessarily based on numerous variables, estimates and assumptions that are inherently uncertain, including as a result of Zynerba not currently having any marketed products, and many of which are beyond Zynerba’s control. Because the Forecasts cover multiple years, by their nature they will become subject to greater uncertainty with each successive year and are unlikely to anticipate each circumstance that will have an effect on Zynerba’s business and its results of operations. Each of the Forecasts was prepared by Zynerba’s management, or extrapolated by MTS Health Partners as described above, based on certain estimates and assumptions with respect to general business, economic, competitive, regulatory, reimbursement and other market and financial conditions and other future events, all of which are difficult to predict and many of which are beyond Zynerba’s control. As a result, there can be no assurance that any of the Forecasts accurately reflect future trends or accurately estimate the future market for Zygel. Each of the Forecasts was developed solely using the information available to Zynerba management at the time they were created and reflect assumptions as to certain business decisions that are subject to change. Important factors that may affect actual results or that may result in any of the Forecasts not being achieved include, but are not limited to: (1) the success of clinical trials (including the funding therefor, anticipated patient enrollment, clinical outcomes, timing or associated costs); (2) regulatory approvals and related timelines; (3) the market acceptance of Zynerba’s potential products; (4) Zynerba’s development of potential products and product candidates for different indications; (5) the availability of third-party reimbursement; (6) the impact of competitive products and pricing; (7) the effect of regulatory actions; (8) the availability of partnering arrangements on favorable terms or at all; (9) the effect of global economic conditions; (10) conditions in the financing markets and access to sufficient capital; (11) changes in applicable laws, rules and regulations; (12) accuracy of certain accounting assumptions; (13) changes in actual or projected cash flows; and (14) other risk factors described in Zynerba’s annual report on Form 10-K for the fiscal year ended December 31, 2022, subsequent quarterly reports on Form 10-Q, and current reports on Form 8-K, as well as the section titled “Cautionary Note Regarding Forward-Looking Statements” in this Schedule 14D-9. In addition, the Forecasts may be affected by Zynerba’s ability to achieve strategic goals, objectives and targets over the applicable period. Accordingly, there can be no assurance that any of the Forecasts will be realized, and actual results may vary materially from those shown.
TABLE OF CONTENTS
Modeling and forecasting the future development and commercialization of drug candidates by a clinical-stage company is a highly speculative endeavor. In addition to the various limitations described above, there can be no assurance of the approval, or timing of approval, of Zynerba’s lead product candidate, and it is possible that other therapeutic scenarios will be preferable. There also can be no assurance that Zynerba will obtain the regulatory approvals necessary for the commercialization of Zygel, or that Zynerba’s competitors will not commercialize products that are safer, more effective or more successfully marketed and sold than any product that Zynerba may market or commercialize. Since the Forecasts cover a long period of time, the Forecasts by their nature are unlikely to anticipate each circumstance that will have an effect on Zynerba’s product candidate.
The Forecasts were not prepared with a view toward complying with U.S. generally accepted accounting principles (“GAAP”), the published guidelines of the SEC regarding projections or the guidelines established by the American Institute of Certified Public Accountants for preparation and presentation of prospective financial information. Neither Zynerba’s independent registered public accounting firm nor any other independent accountant, has audited, reviewed, compiled or performed any procedures with respect to any of the Forecasts or expressed any opinion or any form of assurance related thereto.
The Forecasts were not prepared with a view toward public disclosure. The inclusion of the Forecasts in this Schedule 14D-9 should not be regarded as an indication that any of Zynerba, Harmony Biosciences, Purchaser or any of their respective affiliates, officers, directors, advisors or other representatives considered or consider any of the Forecasts necessarily predictive of actual future events, and none of the Forecasts should be relied upon as such or construed as financial guidance. None of Zynerba, Harmony Biosciences, Purchaser or any of their respective affiliates, officers, directors, advisors or other representatives assumes any responsibility for the accuracy of this information. None of Zynerba, Harmony Biosciences, Purchaser or any of their respective affiliates, officers, directors, advisors or other representatives can give any assurance that actual results will not differ from any of the Forecasts, and none of them undertakes any obligation to update or otherwise revise or reconcile any of the Forecasts to reflect circumstances existing after the date any of the Forecasts were generated or to reflect the occurrence of future events even in the event that any or all of the assumptions underlying such Forecasts are shown to be in error. None of Zynerba, Harmony Biosciences, Purchaser or any of their respective affiliates, officers, directors, advisors or other representatives has made or makes any representation or warranty to any Zynerba stockholders or Harmony Biosciences stockholders regarding the ultimate performance of Zynerba compared to the information contained in any of the Forecasts, the likelihood that the Forecasts will be achieved consistent with any of the Forecasts or at all, the results of Zynerba’s clinical trials, the potential timing and approval of commercial launch of any of Zynerba’s future products, the effectiveness or marketability of Zygel, or the overall future performance of Zynerba. The Forecasts are subjective in many respects and, thus, are subject to interpretation. Accordingly, there can be no assurance that any of the Forecasts will be realized, and actual results may vary materially from those shown. None of Zynerba, Harmony Biosciences, Purchaser or any of their respective affiliates, officers, directors, advisors or other representatives can give any assurance that actual results will not differ materially from any of the Forecasts.
The Forecasts were reasonably prepared by Zynerba management and extrapolated by MTS Health Partners at the direction of Zynerba management on bases reflecting the best currently available estimates and judgments of Zynerba management as to the matters covered thereby. The Forecasts were prepared assuming Zynerba’s continued operation as a stand-alone, publicly traded company, and therefore do not give effect to the Offer or the Merger or any changes to Zynerba’s operations or strategy that may be implemented following the consummation of the Offer and the Merger or to any costs incurred in connection with the Offer or the Merger, including the potential synergies that may be achieved by the combined company as a result of the Merger or the effect of any business or strategic decision or action that has been or will be taken as a result of the execution of the Merger Agreement. Zynerba management believed the assumptions used in the preparation of these Forecasts to be reasonable at the time they were made, including, but not limited to, assumptions relating to the probability of technical success and regulatory approval, timing of completion of clinical studies and commercial launch, sales ramp commercial reach, market size and prevalence, diagnosis rates, market share, compliance and persistence, pricing, market access, expected cash burn rate, competition, partnering and licensing arrangements, market exclusivity, estimated costs and expenses, effective tax rate and utilization of net operating losses, ability to raise future capital, and the impact of future capital raises on current stockholders, and other relevant factors relating to Zynerba and Zygel. The foregoing is a summary of certain key assumptions and does not purport to be a comprehensive overview of all assumptions reflected in each of the Forecasts.
TABLE OF CONTENTS
Certain of the measures included in the Forecasts, including EBIT and unlevered free cash flow in the Forecasts, are financial measures that are not calculated in accordance with GAAP. Such non-GAAP financial measures should not be viewed as a substitute for GAAP financial measures, and may be different from non-GAAP financial measures used by other companies. Furthermore, there are limitations inherent in non-GAAP financial measures, because they exclude charges and credits that are required to be included in a GAAP presentation. Accordingly, non-GAAP financial measures should be considered together with, and not as an alternative to, financial measures prepared in accordance with GAAP. EBIT and unlevered free cash flow should not be considered as an alternative to operating income or net income or as a measure of operating performance, cash flow or liquidity. The summary of the Forecasts below is included solely to give Zynerba’s stockholders access to the information that was made available to the Zynerba Board and MTS Health Partners, and is not included in this Schedule 14D-9 in order to influence any Zynerba stockholder to make any investment decision with respect to Offer and the Merger or as to whether or not such holder should tender shares in connection with the Offer or otherwise act with respect to the Transaction or any other matter.
Financial measures provided to a financial advisor are excluded from the definition of non-GAAP financial measures and, therefore, are not subject to SEC rules regarding disclosures of non-GAAP financial measures, which would otherwise require a reconciliation of a non-GAAP financial measure to a GAAP financial measure. Reconciliations of non-GAAP financial measures were not relied upon by MTS Health Partners for purposes of its financial analysis as described above in the section titled “—Opinion of Zynerba’s Financial Advisor” or by the Zynerba Board in connection with its consideration of the Offer and the Merger. Accordingly, Zynerba has not provided a reconciliation of any financial measures included in any of the Forecasts.
Zynerba undertakes no obligation to update or otherwise revise or reconcile any of the Forecasts to reflect circumstances existing after the date such Forecasts were generated or to reflect the occurrence of future events even in the event that any or all of the assumptions underlying such Forecasts are shown to be in error. None of Zynerba, or, to the knowledge of Zynerba, Harmony Biosciences or Purchaser, intends to make publicly available any update or other revisions to any of the Forecasts, except as otherwise required by law.
The Forecasts below vary based on the assumed completion date of the RECONNECT trial and such Forecasts should not be understood as an indication of when the RECONNECT trial will be completed or if it will be completed all.
Q1 Results Forecasts
Set forth below is a summary of the Management Projections, which are selected projected financial information for Zynerba for fiscal years 2023 through 2031 and reflect the following key assumptions: (1) the completion of the RECONNECT trial, a pivotal, multi-national, confirmatory Phase 3 trial of Zygel in patients with Fragile X Syndrome (“FXS”) by March of fiscal year 2024, (2) the commercial launch of Zygel for patients with FXS in the first quarter of fiscal year 2026, (3) the initiation of the Phase 3 clinical trial of Zygel in patients with chromosome 22q11.2 deletion syndrome (“22q”) (“22Q Phase 3 Trial”), contingent upon the success of the completed RECONNECT trial, (4) completion of the 22q Phase 3 Trial in the first quarter of fiscal year 2027, (5) the commercial launch of Zygel for patients with 22q in the third quarter of 2028, (6) no further development of Zygel with respect to autism spectrum disorder (“ASD”), and (7) upfront licensing revenue from outside the U.S. of $25.0 million prior to commercial launch of Zygel in patients with FXS and ongoing licensing milestone and royalty revenues from 2026 through 2031 (the “Q1 Results Forecasts”).
The Q1 Results Forecasts vary based on three different assumptions with respect to Zygel’s expected pricing as more fully described below.
TABLE OF CONTENTS
Q1 Results (Comparable Pricing) Forecast
The Q1 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced at a slight premium to Epidiolex ®, an FDA-approved cannabidiol developed and marketed by Jazz Pharmaceuticals.
Revenue | | | $— | | | $15 | | | $10 | | | $41 | | | $120 | | | $186 | | | $321 | | | $466 | | | $591 |
Cost of Goods Sold | | | — | | | — | | | — | | | (6) | | | (14) | | | (24) | | | (39) | | | (50) | | | (55) |
R&D Expenses | | | (26) | | | (27) | | | (41) | | | (43) | | | (41) | | | (42) | | | (48) | | | (54) | | | (60) |
Commercial Expenses | | | (1) | | | (4) | | | (19) | | | (32) | | | (34) | | | (40) | | | (44) | | | (51) | | | (52) |
General & Administrative Expenses | | | (11) | | | (13) | | | (18) | | | (19) | | | (20) | | | (21) | | | (22) | | | (23) | | | (23) |
EBIT(1) | | | (38) | | | (29) | | | (68) | | | (59) | | | 11 | | | 58 | | | 169 | | | 289 | | | 401 |
Unlevered Free Cash Flow(2) | | | $(35) | | | $(26) | | | $(66) | | | $(64) | | | $(1) | | | $41 | | | $146 | | | $212 | | | $268 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Q1 Results (Mid-Pricing) Forecast
The Q1 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced higher than Epidiolex ®, but lower than a rare disease drug.
Revenue | | | $— | | | $15 | | | $10 | | | $49 | | | $136 | | | $215 | | | $372 | | | $542 | | | $688 |
Cost of Goods Sold | | | — | | | — | | | — | | | (5) | | | (12) | | | (21) | | | (31) | | | (41) | | | (47) |
R&D Expenses | | | (26) | | | (27) | | | (41) | | | (43) | | | (41) | | | (42) | | | (48) | | | (54) | | | (60) |
Commercial Expenses | | | (1) | | | (4) | | | (21) | | | (35) | | | (37) | | | (44) | | | (47) | | | (55) | | | (56) |
General & Administrative Expenses | | | (11) | | | (13) | | | (18) | | | (19) | | | (20) | | | (21) | | | (22) | | | (23) | | | (23) |
EBIT(1) | | | (38) | | | (29) | | | (70) | | | (53) | | | 27 | | | 87 | | | 224 | | | 369 | | | 501 |
Unlevered Free Cash Flow(2) | | | $(35) | | | $(26) | | | $(68) | | | $(60) | | | $15 | | | $66 | | | $197 | | | $236 | | | $337 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Q1 Results (Rare Disease Pricing) Forecast
The Q1 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced as a rare disease drug.
Revenue | | | $— | | | $15 | | | $10 | | | $52 | | | $141 | | | $223 | | | $386 | | | $577 | | | $700 |
Cost of Goods Sold | | | — | | | — | | | — | | | (5) | | | (10) | | | (19) | | | (27) | | | (38) | | | (41) |
R&D Expenses | | | (26) | | | (27) | | | (41) | | | (43) | | | (41) | | | (42) | | | (48) | | | (54) | | | (60) |
Commercial Expenses | | | (1) | | | (4) | | | (22) | | | (37) | | | (38) | | | (44) | | | (48) | | | (56) | | | (58) |
General & Administrative Expenses | | | (11) | | | (13) | | | (18) | | | (19) | | | (20) | | | (21) | | | (22) | | | (23) | | | (23) |
EBIT(1) | | | (38) | | | (29) | | | (71) | | | (51) | | | 32 | | | 96 | | | 241 | | | 406 | | | 518 |
Unlevered Free Cash Flows(2) | | | $(35) | | | $(26) | | | $(69) | | | $(58) | | | $20 | | | $76 | | | $209 | | | $254 | | | $349 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
TABLE OF CONTENTS
Adjusted Q1 Results Forecasts
The Q1 Results Forecasts were extrapolated by MTS Health Partners, at the direction of Zynerba management, through fiscal year 2038 (holding EBIT in fiscal year 2031 constant through fiscal year 2038) and risk-adjusted to reflect Zynerba management’s assessment of the probability of: (1) positive results of the RECONNECT trial (56.3%), (2) approval by the FDA of the NDA for Zygel in highly methylated patients with FXS (91.2%), (3) positive results of the 22q Phase 3 Trial (56.3%), which trial is dependent on the positive results of the RECONNECT trial, and (4) approval by the FDA of the NDA for Zygel in patients with 22q (91.2%) (such adjustments, the “Probability Adjustments” and such Forecasts, the “Adjusted Q1 Results Forecasts”). In arriving at the Probability Adjustments, Zynerba management consulted various industry publications containing aggregate risk adjustment statistics and applied their professional judgement based on their familiarity with Zygel’s existing clinical data.
The Adjusted Q1 Results Forecasts vary based on three different assumptions with respect to Zygel’s expected pricing as more fully described below.
Adjusted Q1 Results (Comparable Pricing) Forecast
The Adjusted Q1 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced at a slight premium to Epidiolex ® (the “Adjusted Q1 Results (Comparable Pricing) Forecast”). (The Adjusted Q1 Results (Comparable Pricing) Forecast is referred to as “Case 1” in the section titled “—Opinion of Zynerba’s Financial Advisor”).
Revenue | | | $— | | | $8 | | | $6 | | | $21 | | | $62 | | | $92 | | | $153 | | | $214 | | | $264 | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— |
Cost of Goods Sold | | | — | | | — | | | — | | | (3) | | | (7) | | | (12) | | | (18) | | | (23) | | | (24) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
R&D Expenses | | | (26) | | | (15) | | | (23) | | | (23) | | | (20) | | | (22) | | | (25) | | | (28) | | | (31) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Commercial Expenses | | | (1) | | | (2) | | | (11) | | | (17) | | | (17) | | | (21) | | | (23) | | | (26) | | | (27) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
General and Administrative Expense | | | (11) | | | (7) | | | (10) | | | (10) | | | (10) | | | (11) | | | (11) | | | (12) | | | (12) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
EBIT(1) | | | (38) | | | (16) | | | (38) | | | (31) | | | 7 | | | 27 | | | 76 | | | 126 | | | 171 | | | 171 | | | 171 | | | 171 | | | 171 | | | 171 | | | 171 | | | 171 |
Unlevered Free Cash Flow(2) | | | $(36) | | | $(13) | | | $(36) | | | $(33) | | | $(0) | | | $17 | | | $60 | | | $103 | | | $142 | | | $110 | | | $110 | | | $110 | | | $110 | | | $110 | | | $110 | | | $110 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Adjusted Q1 Results (Mid-Pricing) Forecast
The Adjusted Q1 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced higher than Epidiolex ®, but lower than a rare disease drug (the “Adjusted Q1 Results (Mid-Pricing) Forecast”). (The Adjusted Q1 Results (Mid-Pricing) Forecast is referred to as “Case 2” in the section titled “—Opinion of Zynerba’s Financial Advisor”).
Revenue | | | $— | | | $8 | | | $6 | | | $25 | | | $70 | | | $106 | | | $176 | | | $247 | | | $305 | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— |
Cost of Goods Sold | | | — | | | — | | | — | | | (3) | | | (6) | | | (11) | | | (15) | | | (19) | | | (21) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
R&D Expenses | | | (26) | | | (15) | | | (23) | | | (23) | | | (20) | | | (22) | | | (25) | | | (28) | | | (31) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Commercial Expenses | | | (1) | | | (2) | | | (12) | | | (18) | | | (19) | | | (22) | | | (24) | | | (28) | | | (29) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
General and Administrative Expense | | | (11) | | | (7) | | | (10) | | | (10) | | | (10) | | | (11) | | | (11) | | | (12) | | | (12) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
EBIT(1) | | | (38) | | | (16) | | | (39) | | | (28) | | | 14 | | | 41 | | | 101 | | | 161 | | | 213 | | | 213 | | | 213 | | | 213 | | | 213 | | | 213 | | | 213 | | | 213 |
Unlevered Free Cash Flow(2) | | | $(36) | | | $(13) | | | $(37) | | | $(31) | | | $7 | | | $28 | | | $81 | | | $134 | | | $150 | | | $138 | | | $138 | | | $138 | | | $138 | | | $138 | | | $138 | | | $138 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
TABLE OF CONTENTS
Adjusted Q1 Results (Rare Disease Pricing) Forecast
The Adjusted Q1 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced as a rare disease drug (the “Adjusted Q1 Results (Rare Disease Pricing) Forecast”). (The Adjusted Q1 Results (Rare Disease Pricing) Forecast is referred to as “Case 3” in the section titled “—Opinion of Zynerba’s Financial Advisor”).
Revenue | | | $— | | | $8 | | | $6 | | | $27 | | | $72 | | | $109 | | | $181 | | | $263 | | | $308 | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— |
Cost of Goods Sold | | | — | | | — | | | — | | | (2) | | | (5) | | | (9) | | | (13) | | | (17) | | | (18) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
R&D Expenses | | | (26) | | | (15) | | | (23) | | | (23) | | | (20) | | | (22) | | | (25) | | | (28) | | | (31) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Commercial Expenses | | | (1) | | | (2) | | | (12) | | | (19) | | | (19) | | | (23) | | | (25) | | | (29) | | | (30) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
General and Administrative Expense | | | (11) | | | (7) | | | (10) | | | (10) | | | (10) | | | (11) | | | (11) | | | (12) | | | (12) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
EBIT(1) | | | (38) | | | (16) | | | (40) | | | (27) | | | 17 | | | 45 | | | 108 | | | 178 | | | 218 | | | 218 | | | 218 | | | 218 | | | 218 | | | 218 | | | 218 | | | 218 |
Unlevered Free Cash Flow(2) | | | $(36) | | | $(13) | | | $(37) | | | $(30) | | | $10 | | | $32 | | | $86 | | | $149 | | | $145 | | | $141 | | | $141 | | | $141 | | | $141 | | | $141 | | | $141 | | | $141 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Q2 Results Forecasts
Set forth below is a summary of the Management Projections, which are selected projected financial information for Zynerba for fiscal years 2023 through 2031 and reflect the following key assumptions: (1) the completion of the RECONNECT trial by June of fiscal year 2024, (2) the commercial launch of Zygel for patients with FXS in the second quarter of fiscal year 2026, (3) the initiation of the 22Q Phase 3 Trial, contingent upon the success of the completed RECONNECT trial, (4) completion of the 22q Phase 3 Trial in the second quarter of fiscal year 2027, (5) the commercial launch of Zygel for patients with 22q in the fourth quarter of 2028, (6) no further development of Zygel with respect to autism spectrum disorder (“ASD”), and (7) upfront licensing revenue from outside the U.S. of $25.0 million prior to commercial launch of Zygel in patients with FXS and ongoing licensing milestone and royalty revenues from 2026 through 2031(the “Q2 Results Forecasts”).
The Q2 Results Forecasts vary based on three different assumptions with respect to Zygel’s expected pricing as more fully described below.
Q2 Results (Comparable Pricing) Forecast
The Q2 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced at a slight premium to Epidiolex ®.
Revenue | | | $— | | | $15 | | | $10 | | | $30 | | | $108 | | | $163 | | | $293 | | | $432 | | | $571 |
Cost of Goods Sold | | | — | | | — | | | — | | | (4) | | | (12) | | | (22) | | | (35) | | | (48) | | | (54) |
R&D Expenses | | | (25) | | | (25) | | | (44) | | | (48) | | | (45) | | | (47) | | | (53) | | | (59) | | | (65) |
Commercial Expenses | | | (1) | | | (2) | | | (13) | | | (27) | | | (32) | | | (39) | | | (42) | | | (49) | | | (51) |
General & Administrative Expenses | | | (11) | | | (12) | | | (17) | | | (20) | | | (20) | | | (21) | | | (22) | | | (23) | | | (23) |
EBIT(1) | | | (37) | | | (24) | | | (64) | | | (69) | | | (1) | | | 34 | | | 141 | | | 254 | | | 379 |
Unlevered Free Cash Flow(2) | | | $(34) | | | $(23) | | | $(63) | | | $(71) | | | $(11) | | | $20 | | | $118 | | | $213 | | | $249 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
TABLE OF CONTENTS
Q2 Results (Mid-Pricing) Forecast
The Q2 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced higher than Epidiolex ®, but lower than a rare disease drug.
Revenue | | | $— | | | $15 | | | $10 | | | $36 | | | $122 | | | $188 | | | $338 | | | $502 | | | $663 |
Cost of Goods Sold | | | — | | | — | | | — | | | (4) | | | (10) | | | (19) | | | (28) | | | (39) | | | (46) |
R&D Expenses | | | (25) | | | (25) | | | (44) | | | (48) | | | (45) | | | (47) | | | (53) | | | (59) | | | (65) |
Commercial Expenses | | | (1) | | | (2) | | | (15) | | | (30) | | | (34) | | | (42) | | | (45) | | | (53) | | | (55) |
General & Administrative Expenses | | | (11) | | | (12) | | | (17) | | | (20) | | | (20) | | | (21) | | | (22) | | | (23) | | | (23) |
EBIT(1) | | | (37) | | | (24) | | | (66) | | | (65) | | | 12 | | | 59 | | | 190 | | | 329 | | | 475 |
Unlevered Free Cash Flow(2) | | | $(34) | | | $(23) | | | $(64) | | | $(68) | | | $1 | | | $43 | | | $163 | | | $236 | | | $314 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Q2 Results (Rare Disease Pricing) Forecast
The Q2 Results Forecast summarized immediately below reflect the assumption that Zygel will be priced as a rare disease drug.
Revenue | | | $— | | | $15 | | | $10 | | | $39 | | | $126 | | | $193 | | | $350 | | | $520 | | | $689 |
Cost of Goods Sold | | | — | | | — | | | — | | | (3) | | | (9) | | | (16) | | | (24) | | | (35) | | | (40) |
R&D Expenses | | | (25) | | | (25) | | | (44) | | | (48) | | | (45) | | | (47) | | | (53) | | | (59) | | | (65) |
Commercial Expenses | | | (1) | | | (2) | | | (16) | | | (31) | | | (35) | | | (42) | | | (46) | | | (54) | | | (56) |
General & Administrative Expenses | | | (11) | | | (12) | | | (17) | | | (20) | | | (20) | | | (21) | | | (22) | | | (23) | | | (23) |
EBIT(1) | | | (37) | | | (24) | | | (67) | | | (63) | | | 17 | | | 66 | | | 205 | | | 349 | | | 504 |
Unlevered Free Cash Flow(2) | | | $(34) | | | $(23) | | | $(65) | | | $(67) | | | $5 | | | $50 | | | $177 | | | $242 | | | $334 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Adjusted Q2 Results Forecasts
The Q2 Results Forecasts were extrapolated by MTS Health Partners, at the direction of Zynerba management, through fiscal year 2038 (holding earnings before interest and taxes, or EBIT, in fiscal year 2031 constant through fiscal year 2038) and risk-adjusted to reflect the Probability Adjustments (such Forecasts, the “Adjusted Q2 Results Forecasts”).
The Adjusted Q2 Results Forecasts vary based on three different assumptions with respect to Zygel’s expected pricing as more fully described below.
TABLE OF CONTENTS
Adjusted Q2 Results (Comparable Pricing) Forecast
The Adjusted Q2 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced at a slight premium to Epidiolex ® (the “Adjusted Q2 Results (Comparable Pricing) Forecast”). (The Adjusted Q2 Results (Comparable Pricing) Forecast is referred to as “Case 4” in the section titled “—Opinion of Zynerba’s Financial Advisor”).
Revenue | | | $— | | | $8 | | | $6 | | | $16 | | | $56 | | | $82 | | | $140 | | | $199 | | | $257 | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— |
Cost of Goods Sold | | | — | | | — | | | — | | | (2) | | | (6) | | | (11) | | | (17) | | | (22) | | | (24) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
R&D Expenses | | | (25) | | | (14) | | | (25) | | | (25) | | | (23) | | | (24) | | | (27) | | | (30) | | | (33) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Commercial Expenses | | | (1) | | | (1) | | | (7) | | | (14) | | | (16) | | | (20) | | | (22) | | | (25) | | | (26) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
General and Administrative Expense | | | (11) | | | (7) | | | (10) | | | (10) | | | (10) | | | (11) | | | (11) | | | (12) | | | (12) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
EBIT(1) | | | (37) | | | (13) | | | (36) | | | (36) | | | — | | | 16 | | | 64 | | | 111 | | | 162 | | | 162 | | | 162 | | | 162 | | | 162 | | | 162 | | | 162 | | | 162 |
Unlevered Free Cash Flow(2) | | | $(35) | | | $(12) | | | $(33) | | | $(37) | | | $(5) | | | $8 | | | $48 | | | $89 | | | $142 | | | $106 | | | $102 | | | $102 | | | $102 | | | $102 | | | $102 | | | $102 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Adjusted Q2 Results (Mid-Pricing) Forecast
The Adjusted Q2 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced higher than Epidiolex ®, but lower than a rare disease drug (the “Adjusted Q2 Results (Mid-Pricing) Forecast”). (The Adjusted Q2 Results (Mid-Pricing) Forecast is referred to as “Case 5” in the section titled “—Opinion of Zynerba’s Financial Advisor”).
Revenue | | | $— | | | $8 | | | $6 | | | $19 | | | $63 | | | $94 | | | $161 | | | $230 | | | $297 | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— |
Cost of Goods Sold | | | — | | | — | | | — | | | (2) | | | (5) | | | (9) | | | (13) | | | (18) | | | (20) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
R&D Expenses | | | (25) | | | (14) | | | (25) | | | (25) | | | (23) | | | (24) | | | (27) | | | (30) | | | (33) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Commercial Expenses | | | (1) | | | (1) | | | (8) | | | (15) | | | (18) | | | (21) | | | (23) | | | (27) | | | (28) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
General and Administrative Expense | | | (11) | | | (7) | | | (10) | | | (10) | | | (10) | | | (11) | | | (11) | | | (12) | | | (12) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
EBIT(1) | | | (37) | | | (13) | | | (37) | | | (34) | | | 7 | | | 28 | | | 86 | | | 144 | | | 203 | | | 203 | | | 203 | | | 203 | | | 203 | | | 203 | | | 203 | | | 203 |
Unlevered Free Cash Flow(2) | | | $(35) | | | $(12) | | | $(34) | | | $(35) | | | $1 | | | $18 | | | $67 | | | $118 | | | $153 | | | $129 | | | $129 | | | $129 | | | $129 | | | $129 | | | $129 | | | $129 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
TABLE OF CONTENTS
Adjusted Q2 Results (Rare Disease Pricing) Forecast
The Adjusted Q2 Results Forecast summarized immediately below reflect the assumption that Zygel will be priced as a rare disease drug (the “Adjusted Q2 Results (Rare Disease Pricing) Forecast”). (The Adjusted Q2 Results (Rare Disease Pricing) Forecast is referred to as “Case 6” in the section titled “—Opinion of Zynerba’s Financial Advisor”).
Revenue | | | $— | | | $8 | | | $6 | | | $20 | | | $65 | | | $97 | | | $166 | | | $238 | | | $307 | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— |
Cost of Goods Sold | | | — | | | — | | | — | | | (2) | | | (5) | | | (8) | | | (12) | | | (16) | | | (18) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
R&D Expenses | | | (25) | | | (14) | | | (25) | | | (25) | | | (23) | | | (24) | | | (27) | | | (30) | | | (33) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Commercial Expenses | | | (1) | | | (1) | | | (9) | | | (16) | | | (18) | | | (22) | | | (24) | | | (28) | | | (29) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
General and Administrative Expense | | | (11) | | | (7) | | | (10) | | | (10) | | | (10) | | | (11) | | | (11) | | | (12) | | | (12) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
EBIT(1) | | | (37) | | | (13) | | | (37) | | | (33) | | | 9 | | | 32 | | | 92 | | | 152 | | | 215 | | | 215 | | | 215 | | | 215 | | | 215 | | | 215 | | | 215 | | | 215 |
Unlevered Free Cash Flow(2) | | | $(35) | | | $(12) | | | $(34) | | | $(35) | | | $3 | | | $21 | | | $72 | | | $126 | | | $156 | | | $137 | | | $137 | | | $137 | | | $137 | | | $137 | | | $137 | | | $137 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Q3 Results Forecasts
Set forth below is a summary of the Management Projections, which are selected projected financial information for Zynerba for fiscal years 2023 through 2031 and reflect the following key assumptions: (1) the completion of the RECONNECT trial by September of fiscal year 2024, (2) the commercial launch of Zygel for patients with FXS in the third quarter of fiscal year 2026, (3) the initiation of the 22Q Phase 3 Trial, contingent upon the success of the completed RECONNECT trial, (4) completion of the 22q Phase 3 Trial in the third quarter of fiscal year 2027, (5) the commercial launch of Zygel for patients with 22q in the first quarter of 2029, (6) no further development of Zygel with respect to autism spectrum disorder (“ASD”), and (7) upfront licensing revenue from outside the U.S. of $25.0 million prior to commercial launch of Zygel in patients with FXS and ongoing licensing milestone and royalty revenues from 2026 through 2031 (the “Q3 Results Forecasts”).
The Q3 Results Forecasts vary based on three different assumptions with respect to Zygel’s expected pricing as more fully described below.
Q3 Results (Comparable Pricing) Forecast
The Q3 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced at a slight premium to Epidiolex ®.
Revenue | | | $— | | | $15 | | | $— | | | $31 | | | $96 | | | $136 | | | $251 | | | $405 | | | $552 |
Cost of Goods Sold | | | — | | | — | | | — | | | (3) | | | (11) | | | (20) | | | (31) | | | (43) | | | (53) |
R&D Expenses | | | (24) | | | (27) | | | (36) | | | (45) | | | (44) | | | (43) | | | (48) | | | (54) | | | (60) |
Commercial Expenses | | | (1) | | | (3) | | | (11) | | | (25) | | | (31) | | | (37) | | | (42) | | | (48) | | | (50) |
General & Administrative Expenses | | | (11) | | | (11) | | | (15) | | | (20) | | | (19) | | | (21) | | | (21) | | | (22) | | | (23) |
EBIT(1) | | | (36) | | | (26) | | | (62) | | | (62) | | | (9) | | | 15 | | | 109 | | | 237 | | | 366 |
Unlevered Free Cash Flow(2) | | | $(33) | | | $(24) | | | $(63) | | | $(62) | | | $(19) | | | $3 | | | $86 | | | $213 | | | $234 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
TABLE OF CONTENTS
Q3 Results (Mid-Pricing) Forecast
The Q3 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced higher than Epidiolex ® and lower than a rare disease drug.
Revenue | | | $— | | | $15 | | | $— | | | $34 | | | $108 | | | $156 | | | $292 | | | $470 | | | $640 |
Cost of Goods Sold | | | — | | | — | | | — | | | (2) | | | (9) | | | (16) | | | (25) | | | (37) | | | (44) |
R&D Expenses | | | (24) | | | (27) | | | (36) | | | (45) | | | (44) | | | (43) | | | (48) | | | (54) | | | (60) |
Commercial Expenses | | | (1) | | | (3) | | | (13) | | | (28) | | | (33) | | | (39) | | | (45) | | | (52) | | | (54) |
General & Administrative Expenses | | | (11) | | | (11) | | | (16) | | | (20) | | | (20) | | | (21) | | | (22) | | | (23) | | | (23) |
EBIT(1) | | | (36) | | | (26) | | | (64) | | | (61) | | | 2 | | | 36 | | | 152 | | | 304 | | | 459 |
Unlevered Free Cash Flow(2) | | | $(33) | | | $(24) | | | $(64) | | | $(62) | | | $(9) | | | $23 | | | $126 | | | $235 | | | $297 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Q3 Results (Rare Disease Pricing) Forecast
The Q3 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced as a rare disease drug.
Revenue | | | $— | | | $15 | | | $— | | | $36 | | | $111 | | | $160 | | | $303 | | | $488 | | | $664 |
Cost of Goods Sold | | | — | | | — | | | — | | | (2) | | | (8) | | | (14) | | | (22) | | | (32) | | | (39) |
R&D Expenses | | | (24) | | | (27) | | | (36) | | | (45) | | | (44) | | | (43) | | | (48) | | | (54) | | | (60) |
Commercial Expenses | | | (1) | | | (3) | | | (14) | | | (29) | | | (34) | | | (40) | | | (46) | | | (54) | | | (55) |
General & Administrative Expenses | | | (11) | | | (12) | | | (16) | | | (20) | | | (20) | | | (21) | | | (22) | | | (23) | | | (23) |
EBIT(1) | | | (36) | | | (27) | | | (65) | | | (60) | | | 6 | | | 42 | | | 165 | | | 326 | | | 487 |
Unlevered Free Cash Flow(2) | | | $(33) | | | $(24) | | | $(65) | | | $(61) | | | $(6) | | | $27 | | | $139 | | | $249 | | | $317 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
Adjusted Q3 Results Forecasts
The Q3 Results Forecasts were extrapolated by MTS Health Partners, at the direction of Zynerba management, through fiscal year 2038 (holding earnings before interest or taxes, or EBIT, in fiscal year 2031 constant through fiscal year 2038) and risk-adjusted to reflect the Probability Adjustments (the “Adjusted Q3 Results Forecasts”).
TABLE OF CONTENTS
The Adjusted Q3 Results Forecasts vary based on three different assumptions with respect to Zygel’s expected pricing as more fully described below.
Adjusted Q3 Results (Comparable Pricing) Forecast
The Adjusted Q3 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced at a slight premium to Epidiolex ® (the “Adjusted Q3 Results (Comparable Pricing) Forecast”). (The Adjusted Q3 Results (Comparable Pricing) Forecast is referred to as “Case 7” in the section titled “—Opinion of Zynerba’s Financial Advisor”).
Revenue | | | $— | | | $8 | | | $— | | | $16 | | | $49 | | | $70 | | | $122 | | | $190 | | | $250 | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— |
Cost of Goods Sold | | | — | | | — | | | — | | | — | | | (6) | | | (10) | | | (15) | | | (20) | | | (24) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
R&D Expenses | | | (24) | | | (15) | | | (20) | | | (24) | | | (21) | | | (22) | | | (25) | | | (28) | | | (31) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Commercial Expenses | | | (1) | | | (2) | | | (6) | | | (13) | | | (16) | | | (19) | | | (22) | | | (25) | | | (26) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
General and Administrative Expense | | | (11) | | | (6) | | | (9) | | | (10) | | | (10) | | | (11) | | | (11) | | | (11) | | | (12) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
EBIT(1) | | | (36) | | | (15) | | | (35) | | | (31) | | | (3) | | | 8 | | | 50 | | | 106 | | | 158 | | | 158 | | | 158 | | | 158 | | | 158 | | | 158 | | | 158 | | | 158 |
Unlevered Free Cash Flow(2) | | | $(34) | | | $(13) | | | $(34) | | | $(31) | | | $(8) | | | $1 | | | $35 | | | $87 | | | $135 | | | $106 | | | $97 | | | $97 | | | $97 | | | $97 | | | $97 | | | $97 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, incentive and tax receivables, prepaid and other current assets. |
Adjusted Q3 Results (Mid-Pricing) Forecast
The Adjusted Q3 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced higher than Epidiolex ® and lower than a rare disease drug (the “Adjusted Q3 Results (Mid-Pricing) Forecast”). (The Adjusted Q3 Results (Mid-Pricing) Forecast is referred to as “Case 8” in the section titled “—Opinion of Zynerba’s Financial Advisor”).
Revenue | | | $— | | | $8 | | | $— | | | $18 | | | $55 | | | $80 | | | $141 | | | $219 | | | $289 | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— |
Cost of Goods Sold | | | — | | | — | | | — | | | — | | | (4) | | | (8) | | | (12) | | | (17) | | | (20) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
R&D Expenses | | | (24) | | | (15) | | | (20) | | | (24) | | | (21) | | | (22) | | | (25) | | | (28) | | | (31) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Commercial Expenses | | | (1) | | | (2) | | | (7) | | | (14) | | | (17) | | | (20) | | | (23) | | | (27) | | | (28) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
General and Administrative Expense | | | (11) | | | (6) | | | (9) | | | (10) | | | (10) | | | (11) | | | (11) | | | (12) | | | (12) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
EBIT(1) | | | (36) | | | (15) | | | (36) | | | (31) | | | 2 | | | 19 | | | 70 | | | 136 | | | 199 | | | 199 | | | 199 | | | 199 | | | 199 | | | 199 | | | 199 | | | 199 |
Unlevered Free Cash Flow(2) | | | $(34) | | | $(13) | | | $(35) | | | $(31) | | | $(4) | | | $11 | | | $52 | | | $112 | | | $156 | | | $123 | | | $123 | | | $123 | | | $123 | | | $123 | | | $123 | | | $123 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
TABLE OF CONTENTS
Adjusted Q3 Results (Rare Disease Pricing) Forecast
The Adjusted Q3 Results Forecast summarized immediately below reflects the assumption that Zygel will be priced as a rare disease drug (the “Adjusted Q3 Results (Rare Disease Pricing) Forecast”). (The Adjusted Q3 Results (Rare Disease Pricing) Forecast is referred to as “Case 9” in the section titled “—Opinion of Zynerba’s Financial Advisor”).
Revenue | | | $— | | | $8 | | | $— | | | $19 | | | $57 | | | $82 | | | $145 | | | $226 | | | $298 | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— | | | $— |
Cost of Goods Sold | | | — | | | — | | | — | | | — | | | (4) | | | (7) | | | (10) | | | (15) | | | (17) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
R&D Expenses | | | (24) | | | (15) | | | (20) | | | (24) | | | (21) | | | (22) | | | (25) | | | (28) | | | (31) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Commercial Expenses | | | (1) | | | (2) | | | (8) | | | (15) | | | (17) | | | (20) | | | (24) | | | (27) | | | (28) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
General and Administrative Expense | | | (11) | | | (7) | | | (9) | | | (10) | | | (10) | | | (11) | | | (11) | | | (12) | | | (12) | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
EBIT(1) | | | (36) | | | (15) | | | (37) | | | (30) | | | 4 | | | 22 | | | 75 | | | 145 | | | 210 | | | 210 | | | 210 | | | 210 | | | 210 | | | 210 | | | 210 | | | 210 |
Unlevered Free Cash Flow(2) | | | $(34) | | | $(13) | | | $(35) | | | $(31) | | | $(2) | | | $13 | | | $57 | | | $120 | | | $158 | | | $131 | | | $131 | | | $131 | | | $131 | | | $131 | | | $131 | | | $131 |
(1)
| “EBIT” refers to Zynerba’s revenue, less cost of goods sold, research and development cost, commercialization expenses and general and administrative expenses. |
(2)
| “Unlevered Free Cash Flow” refers to EBIT, plus interest income, less income taxes, plus depreciation, plus net change in accounts payable and accrued expenses, plus net change in Australian tax credits and goods and services taxes, less working capital charge and less capital investments. “Working capital charge” refers to the change in Zynerba’s accounts receivable, inventories, prepaid and other current assets. |
In light of the foregoing factors and the uncertainties inherent in each of the Forecasts, stockholders are cautioned not to place undue, if any, reliance on the Forecasts.
Opinion of Zynerba’s Financial Advisor
Zynerba retained MTS Health Partners as its financial advisor in connection with the Transactions. On August 12, 2023, MTS Securities, LLC (“MTS Securities”), an affiliate of MTS Health Partners, rendered to the Zynerba Board its oral opinion, subsequently confirmed in a written opinion, dated August 12, 2023, that, as of such date and based upon and subject to the various assumptions made, procedures followed, matters considered, and qualifications and limitations upon the review undertaken by MTS Securities in preparing its opinion, the Offer Price to be paid in the Offer and the Merger to the holders of Shares (other than Excluded Shares) pursuant to the Merger Agreement, was fair, from a financial point of view, to such holders.
The full text of MTS Securities’ written opinion (the “MTS Opinion”), dated August 12, 2023, which describes the various assumptions made, procedures followed, matters considered, and qualifications and limitations upon the review undertaken by MTS Securities in preparing its opinion, is attached as Annex I to this Schedule 14D-9 and is incorporated herein by reference. The summary of the MTS Opinion set forth below is qualified in its entirety by the full text of the MTS Opinion attached hereto as Annex I. MTS Securities’ opinion was provided for the information and assistance of the Zynerba Board (in its members’ capacity as directors and not in any other capacity) in connection with and for purposes of the Zynerba Board’s consideration of the Transactions, and the MTS Opinion only addressed the fairness, from a financial point of view, as of the date thereof, to the holders of Shares (other than the Excluded Shares) of the Offer Price to be paid in the Offer and the Merger to such holders pursuant to the Merger Agreement. The MTS Opinion did not address any other term or aspect of the Merger Agreement or the Transactions and does not constitute a recommendation to any stockholder of Zynerba as to whether such holder should tender its Shares in connection with the Offer, or otherwise act with respect to the Transactions or any other matter.
The full text of the MTS Opinion should be read carefully in its entirety for a description of the various assumptions made, procedures followed, matters considered, and qualifications and limitations upon the review undertaken by MTS Securities in preparing its opinion.
TABLE OF CONTENTS
In connection with rendering the opinion described above and performing its related financial analyses, MTS Securities, among other things:
i.
| reviewed the financial terms of a draft copy of the Merger Agreement delivered to it on August 12, 2023, which was the most recent draft available to it (the “Draft Merger Agreement”), and the financial terms of a draft copy of the CVR Agreement delivered to it on August 12, 2023, which was the most recent draft made available to it (the “Draft CVR Agreement”); |
ii.
| reviewed certain publicly available business and financial information concerning Zynerba and the industry in which it operates; |
iii.
| reviewed certain internal financial analyses and forecasts prepared by and provided to it by the management of Zynerba relating to its business (the “Company Projections”); |
iv.
| conducted discussions with members of senior management and representatives of Zynerba concerning the matters described in clauses ii and iii above and any other matters it deemed relevant; |
v.
| reviewed and analyzed the reported current and historical prices and trading history of Shares; |
vi.
| reviewed and analyzed, based on the Company Projections, the projected cash flows of Zynerba to determine the present value of Zynerba’s discounted cash flows; |
vii.
| reviewed and analyzed the financial performance of Zynerba as compared to publicly available information for certain other publicly-traded companies that it deemed relevant; |
viii.
| reviewed and analyzed the proposed financial terms of the Transactions as compared to the financial terms of certain selected business combinations that it deemed relevant and the consideration paid in such transactions; and |
ix.
| performed such other financial studies, analyses and investigations and considered such other information as it deemed appropriate for the purposes of the opinion set forth below. |
In arriving at the MTS Opinion, MTS Securities assumed and relied upon, without assuming liability or responsibility for independent verification, the accuracy and completeness of all of the financial, legal, regulatory, tax, accounting and other information that was publicly available or was provided to, discussed with or reviewed by it and upon the assurances of the management of Zynerba that it was not aware of any material relevant developments or matters related to Zynerba or that may affect any of the Transactions that have been omitted or were not disclosed to MTS Securities. The MTS Opinion does not address any legal, regulatory, tax, accounting or financial reporting matters, as to which MTS Securities understood that Zynerba obtained such advice as it deemed necessary from other advisors, and MTS Securities relied with Zynerba’s consent on any assessments made by such other advisors to Zynerba with respect to such matters. Without limiting the foregoing, MTS Securities did not consider any tax effects of the Transactions or the form or transaction structure of the Transactions on any person or entity. MTS Securities did not conduct any independent verification of the Company Projections, and MTS Securities expresses no view as to the Company Projections or the assumptions on which they are based. Without limiting the generality of the foregoing, with respect to the Company Projections, MTS Securities assumed, with Zynerba’s consent and based upon discussions with Zynerba’s management, that they were reasonably prepared in good faith and that the Company Projections reflect the best currently available estimates and judgments of the management of Zynerba of the future results of operations and financial performance of the Company.
In arriving at the MTS Opinion, MTS Securities made no analysis of, and expressed no opinion as to, the adequacy of the reserves of Zynerba and relied upon information supplied to MTS Securities by Zynerba as to such adequacy. In addition, MTS Securities did not make any independent evaluations or appraisals of the assets or liabilities (including any contingent, derivative or off-balance-sheet assets or liabilities) of Zynerba or its subsidiary, and MTS Securities was not furnished with any such evaluations or appraisals, nor did MTS Securities evaluate the solvency of Zynerba or any other entity under any state or federal law relating to bankruptcy, insolvency or similar matters. The analyses performed by MTS Securities in connection with the MTS Opinion were going-concern analyses. MTS Securities expresses no opinion regarding the liquidation value of Zynerba or any other entity. MTS Securities assumed that there has been no material change in the assets, financial condition, business or prospects of Zynerba or its subsidiary since the date of the most recent relevant financial statements or financial information made available to MTS Securities. Without limiting the generality of the foregoing, MTS Securities undertook no independent analysis of any pending or threatened litigation, regulatory action, possible unasserted claims or other contingent liabilities to which Zynerba or any of its affiliates is a party or may be
TABLE OF CONTENTS
subject, and, at the direction of Zynerba and with the Zynerba Board’s consent, the MTS Opinion makes no assumption concerning, and therefore does not consider, the possible assertion of claims, outcomes or damages arising out of any such matters. MTS Securities also assumed that neither Zynerba, Harmony Biosciences nor any of their respective subsidiaries, is party to any material pending transaction that had not been disclosed to it, including any financing, recapitalization, acquisition or merger, divestiture or spin-off, other than the Transactions. MTS Securities did not conduct, nor assume any obligation to conduct, any physical inspection of the properties or facilities of Zynerba or its subsidiary. MTS Securities also did not consider any potential legislative or regulatory changes currently being considered or that may be adopted by any governmental or regulatory bodies or any potential changes in accounting methods or generally accepted accounting principles that may be adopted.
MTS Securities assumed that the representations and warranties of each party contained in each of the Merger Agreement, the CVR Agreement and in all other related documents and instruments that are referred to therein are and will be true and correct as of the date or the dates made or deemed made, that each party thereto will fully and timely perform all of the covenants and agreements required to be performed by it under the Merger Agreement, the CVR Agreement and any other agreement contemplated thereby, that all conditions to the consummation of the Transactions will be satisfied without waiver thereof and that the Transactions will be consummated in accordance with the terms of the Merger Agreement and the CVR Agreement without waiver, modification or amendment of any term, condition or agreement thereof. MTS Securities assumed that the final form of each of the Merger Agreement and the CVR Agreement would be, in all respects relevant to its analysis, identical to the Draft Merger Agreement and the Draft CVR Agreement, respectively. MTS Securities also assumed that any governmental, regulatory and other consents and approvals contemplated in connection with the Transactions will be obtained and that, in the course of obtaining any of those consents and approvals, no restrictions will be imposed or waivers made that would have an adverse effect on Zynerba or the benefits contemplated to be realized as a result of the Transactions. For purposes of the MTS Opinion, MTS Securities assumed, at Zynerba’s direction and with its consent, that achievement of the conditions for each Milestone Payment will occur as and to the extent contemplated by the Company Projections and that the corresponding Milestone Payments will be paid to each holder of a CVR to the extent thereof.
The MTS Opinion is necessarily based on economic, market, financial and other conditions as they existed, and on the information made available to MTS Securities, as of the date of MTS Opinion. It should be understood that, although subsequent developments may affect the conclusion reached in the MTS Opinion, MTS Securities does not have any obligation to update, revise or reaffirm the MTS Opinion.
The MTS Opinion addresses solely the fairness, from a financial point of view, to the holders of Shares (other than Excluded Shares) of the Offer Price to be received by such holders pursuant to the Transactions, and does not address any other terms in the Merger Agreement, the CVR Agreement or any other agreement relating to the Transactions or any other aspect or implication of the Transactions, including any financing arrangements to be entered into in connection with the Transactions. The MTS Opinion does not address Zynerba’s underlying business decision to proceed with the Transactions or the relative merits of the Transactions compared to other alternatives available to Zynerba. MTS Securities expressed no opinion as to the prices or ranges of prices at which shares or other securities of any person, including the Shares, will trade at any time, including following the announcement or consummation of the Transactions. MTS Securities was not requested to opine as to, and the MTS Opinion does not in any manner address, the amount or nature of compensation to any of the officers, directors or employees of any party to any of the Transactions, or any class of such persons, relative to the consideration to be received by the holders of Shares in connection with the Transactions or with respect to the fairness of any such compensation.
The MTS Opinion does not constitute a recommendation to the Zynerba Board, any committee thereof or any of Zynerba’s stockholders as to how to vote or take any other action in connection with any of the Transactions, including whether any stockholder should tender his, her or its shares in the Offer.
The MTS Opinion was reviewed and approved by a fairness committee of MTS Securities.
Summary of MTS Securities Financial Analysis
The following is a summary of the material financial analyses prepared and reviewed with the Zynerba Board in connection with the MTS Opinion. The summary set forth below does not purport to be a complete description of the financial analyses performed or factors considered by, and underlying the opinion of, MTS Securities, nor does the order of the financial analyses described represent the relative importance or weight given to those financial analyses by MTS Securities. MTS Securities may have deemed various assumptions more or less probable than other assumptions, so the reference ranges resulting from any particular portion of the
TABLE OF CONTENTS
analyses summarized below should not be understood as MTS Securities’ view of the actual value of Zynerba. Some of the summaries of the financial analyses set forth below include information presented in tabular format. In order to fully understand the financial analyses, the tables must be read together with the text of each summary, as the tables alone do not constitute a complete description of the financial analyses performed by MTS Securities. Considering the data in the tables below without considering all financial analyses or factors or the full narrative description of such analyses or factors, including the methodologies and assumptions underlying such analyses or factors, could create a misleading or incomplete view of the processes underlying MTS Securities’ financial analyses and its opinion. In performing its analyses, MTS Securities made numerous assumptions with respect to industry performance, general business and economic conditions and other matters, many of which are beyond the control of Zynerba or any other parties to the Transactions. None of Zynerba, the Purchaser or MTS Securities or any other person assumes responsibility if future results are materially different from those discussed. Any estimates contained in these analyses are not necessarily indicative of actual values or predictive of future results or values, which may be significantly more or less favorable than as set forth below. In addition, analyses relating to the value of Zynerba do not purport to be appraisals or reflect the prices at which Zynerba may actually be sold. Accordingly, the assumptions and estimates used in, and the results derived from, the financial analyses are inherently subject to substantial uncertainty. Except as otherwise noted, the following quantitative information, to the extent that it is based on market data, is based on market data as it existed on or before August 9, 2023 and is not necessarily indicative of current market conditions.
Discounted Cash Flow Analysis
MTS Securities performed a discounted cash flow analysis of Zynerba based on the Company Projections (for more details, including the unlevered free cash flows used in MTS Securities’ analysis, please see the section titled “ – Certain Financial Projections”). A discounted cash flow analysis is a traditional valuation methodology used to derive a valuation of an asset or set of assets by calculating the “present value” of estimated future cash flows of the asset or set of assets. “Present value” refers to the current value of future cash flows and is obtained by discounting those future cash flows by a discount rate that takes into account macroeconomic assumptions and estimates of risk, the opportunity cost of capital, expected returns and other appropriate factors.
In performing this analysis, MTS Securities calculated an implied per share equity value range for the Shares, by:
(a)
| discounting to present value as of October 1, 2023 at 18% (reflecting MTS Securities’ analysis of the Company’s weighted average cost of capital) based upon the weighted average cost of capital calculated for the publicly traded comparable companies in the second set of comparable companies identified below under the section titled “—Summary of MTS Securities Financial Analysis – Public Trading Comparable Companies Analysis” and using the mid-year convention: |
(i)
| the forecasted risk-adjusted, after-tax unlevered free cash flows of the Company over the period beginning on January 1, 2023 and ending on December 31, 2038, in Cases 1 through 9, utilized by MTS Securities at the direction of Zynerba management and approved by the Zynerba Board for use by MTS Securities as set forth in the section titled “—Certain Financial Projections”; and |
(ii)
| tax savings from usage of federal net operating losses and future losses as set forth in the Company Projections; |
(b)
| and adding to the foregoing results Zynerba’s estimated net cash of approximately $27 million as of October 1, 2023, plus the cash from an assumed capital raise (the “Capital Raise”), as set forth in the Company Projections and described in the section titled “ – Certain Financial Projections”. In Cases 1 through 6, MTS Securities, at the direction of Zynerba management and as approved by the Zynerba Board, assumed a $10 million Capital Raise issued at $0.46 per unit. It was assumed that each unit would consist of one share and one warrant and priced at Zynerba’s closing share price on August 9, 2023, plus $0.125, per Nasdaq rules. In Cases 7 through 9, MTS Securities, at the direction of Zynerba management and as approved by the Zynerba Board, assumed a $17 million Capital Raise issued at $0.46 per unit. In this scenario, it was assumed that each unit would consist of one share and two warrants and priced at Zynerba’s closing share price on August 9, 2023, plus $0.125 per warrant, per Nasdaq rules. |
TABLE OF CONTENTS
MTS Securities divided the results of the foregoing calculations by Zynerba’s fully diluted Shares outstanding (determined using the treasury stock method and taking into account in-the-money options, warrants, and restricted stock units) as of August 9, 2023 and taking into account the expected dilutive effect of the shares and warrants issued in the Capital Raise, as directed by Zynerba’s management. In Cases 1-6, Zynerba’s fully diluted Shares considers the expected dilutive effect of 21.8 million shares and 21.8 million warrants. In Cases 7-9, the Zynerba’s fully diluted Shares considers the expected dilutive effect of 37.1 million shares and 74.1 million warrants.
At the direction of Zynerba, MTS Securities conducted certain sensitivity analysis for the purposes of its discounted cash flow analysis using ranges of (i) revenue achievement of 85% to 115%, as provided by Zynerba management, and (ii) weighted average cost of capital of 17% to 19%, reflecting estimates of Zynerba’s average cost of capital, based upon MTS Securities’ analysis of the cost of capital for Zynerba’s comparable company universe.
The following table reflects the ranges of implied price per Share of Zynerba implied by this discounted cash flow analysis for Cases 1-9.
Case 1 | | | $1.57 – $2.48 |
Case 2 | | | $2.06 – $3.23 |
Case 3 | | | $2.16 – $3.37 |
Case 4 | | | $1.37 – $2.21 |
Case 5 | | | $1.83 – $2.91 |
Case 6 | | | $1.96 – $3.11 |
Case 7 | | | $0.90 – $1.35 |
Case 8 | | | $1.16 – $1.74 |
Case 9 | | | $1.23 – $1.85 |
Subsequent to delivery of the MTS Opinion, MTS Securities discovered that the fully-diluted number of shares relating to its discounted cash flow analysis based upon Zynerba’s September 2024 projections was overstated. Using the correct number of shares, the per share range of values for the Shares would have been $1.00 to $2.15. MTS Securities advised Zynerba that using this range would not have had any impact on its view of fairness of the Offer to the holders or Shares (other than Excluded Shares).
Public Trading Comparable Companies Analysis
MTS Securities reviewed and compared the projected operating performance of Zynerba with publicly available information concerning other publicly traded comparable companies and reviewed the current market price of certain publicly traded securities of such other companies that MTS Securities deemed relevant. MTS Securities created two sets of comparable companies. In Set 1, MTS Securities selected the publicly traded companies identified below based on late-stage clinical status, a single lead asset driving the majority of value, and low or negative enterprise value. In Set 2, MTS Securities selected the publicly traded companies identified below based on late-stage clinical status, a focus on orphan / rare indication(s), and a single lead asset driving the majority of value.
Set 1
Fulcrum Therapeutics, Inc. | | | $246 | | | ($32) | | | NA |
Spruce Biosciences, Inc. | | | $97 | | | ($17) | | | 0.152x |
Relmada Therapeutics, Inc. | | | $82 | | | ($37) | | | 0.044x |
Immunic, Inc. | | | $81 | | | $4 | | | 0.061x |
InDex Pharmaceuticals Holding AB (publ) | | | $39 | | | ($1) | | | 0.064x |
Synlogic, Inc. | | | $33 | | | ($24) | | | 0.030x |
Cyclo Therapeutics, Inc. | | | $32 | | | $25 | | | 0.051x |
Akari Therapeutics, Plc | | | $20 | | | $7 | | | 0.018x |
Palisade Bio, Inc. | | | $5 | | | $11 | | | 0.015x |
TABLE OF CONTENTS
Set 2
Savara Inc. | | | $689 | | | $526 | | | 1.227x |
Aerovate Therapeutics, Inc. | | | $400 | | | $281 | | | 0.191x |
KalVista Pharmaceuticals, Inc. | | | $377 | | | $228 | | | 0.520x |
Fulcrum Therapeutics, Inc. | | | $246 | | | ($32) | | | NA |
Reneo Pharmaceuticals, Inc. | | | $239 | | | $77 | | | 0.068x |
Soleno Therapeutics, Inc. | | | $126 | | | $106 | | | 0.265x |
Egetis Therapeutics AB (publ) | | | $103 | | | $79 | | | 0.178x |
Spruce Biosciences, Inc. | | | $97 | | | ($17) | | | (0.027x) |
Akari Therapeutics, Plc | | | $20 | | | $7 | | | 0.006x |
Although none of the selected companies is directly comparable to Zynerba, MTS Securities included these companies in its analysis because they are publicly traded companies with certain characteristics including those discussed above that, for purposes of analysis, may be considered similar to certain characteristics of Zynerba. MTS Securities calculated the market capitalization for each of the selected companies, as of August 9, 2023, by multiplying the closing price per share of common stock of such company on such date by the number of such company’s fully diluted outstanding shares, using the treasury stock method. MTS Securities calculated the enterprise value by deducting such company’s total cash and cash equivalents and adding book value of debt from the calculated market capitalization. For Set 1, MTS Securities calculated market capitalization as a multiple of the estimated unadjusted peak sales, using consensus equity research estimates as of August 9, 2023. For Set 2, MTS Securities calculated enterprise value as a multiple of the estimated unadjusted peak sales, using consensus equity research estimates as of August 9, 2023.
For Set 1, MTS Securities considered a range of market capitalizations based on the first and third quartiles of the comparable companies data set. MTS Securities then derived implied per Share prices by the fully diluted Shares outstanding of Zynerba at the implied per Share price. MTS Securities also derived a range of market capitalization to unadjusted peak sales multiples based on the first and third quartiles of the comparable companies data set. MTS Securities applied the range of multiples to Zynerba management’s estimated unadjusted peak sales, calculated as the average of unadjusted peak revenues in Cases 4 and 6, to calculate the implied market capitalization of Zynerba. MTS Securities then derived implied per Share prices by the fully diluted Shares outstanding of Zynerba at the implied per Share price. For avoidance of doubt, MTS Securities did not adjust the fully diluted Shares outstanding for the contemplated Capital Raise. The following table reflects the ranges of implied price per Share of Zynerba implied by this analysis, rounded to the nearest $0.05:
Market Capitalization | | | $26mm - $89mm | | | $0.50 - $1.65 |
Market Capitalization / Unadjusted Peak Sales | | | 0.021x – 0.063x | | | $0.25 – $0.70 |
For Set 2, MTS Securities considered a range of enterprise values based on the first and third quartiles of the comparable companies data set. MTS Securities then derived implied per Share prices by subtracting Zynerba’s net debt and then dividing by the fully diluted Shares outstanding of Zynerba at the implied per Share price. MTS Securities also derived a range of enterprise value to unadjusted peak sales multiples based on the first and third quartiles of the comparable companies data set. MTS Securities applied the range of multiples to Zynerba management’s estimated unadjusted peak sales, calculated as the average of unadjusted peak revenues in Cases 4 and 6, to calculate the implied enterprise value of Zynerba. MTS Securities then derived implied per Share prices by subtracting Zynerba’s net debt and then dividing by the fully diluted Shares outstanding of Zynerba at the implied per Share price. For avoidance of doubt, MTS Securities did not adjust the fully diluted Shares outstanding for the contemplated Capital Raise. The following table reflects the ranges of implied price per Share of Zynerba implied by this analysis, rounded to the nearest $0.05:
Enterprise Value | | | ($5mm)-$254mm | | | $0.40 - $5.05 |
Enterprise Value / Unadjusted Peak Sales | | | 0.022x – 0.456x | | | $0.75 - $5.30 |
TABLE OF CONTENTS
Precedent Transactions Analysis
MTS Securities reviewed and analyzed the proposed financial terms of the Transactions as compared to the financial terms of certain selected business combinations that MTS Securities deemed relevant and the consideration paid in such transactions. MTS Securities examined selected business combinations or asset transactions since 2017 involving companies where the publicly traded target had a pivotal stage (pre-data) lead asset(s) in Orphan / Rare indications. MTS Securities reviewed and analyzed certain publicly available information for the following business combinations:
05/22/23 | | | VectivBio Holding AG | | | Ironwood Pharmaceuticals, Inc. | | | $862 | | | 0.690x |
08/01/22 | | | Forma Therapeutics Holdings, Inc. | | | Novo Nordisk A/S | | | $638 | | | 0.426x |
06/01/20 | | | Censa GmbH | | | PTC Therapeutics, Inc. | | | $538 | | | NA |
03/04/19 | | | Nightstar Therapeutics Limited | | | Biogen Inc.. | | | $725 | | | 0.731x |
02/24/19 | | | Clementia Pharmaceuticals Inc. | | | Ipsen Biopharmaceuticals, Inc. | | | $1,112 | | | 1.390x |
04/11/18 | | | Wilson Therapeutics AB (publ) | | | Alexion Pharmaceuticals, Inc. | | | $798 | | | 1.916x |
05/18/17 | | | River Vision Development Corp. | | | Horizon Pharma plc | | | $471 | | | 1.882x |
03/31/17 | | | Vtesse, Inc. | | | SUCAMPO PHARMACEUTICALS, INC. | | | $200 | | | 1.333x |
Although none of the selected transactions are directly comparable to the Transactions, the target companies in the selected transactions are companies with certain characteristics including those described above that, for the purposes of analysis, may be considered similar to those of Zynerba, and as such, for purposes of analysis, the selected transactions may be considered similar to the Transactions. The reasons for and the circumstances surrounding each of the selected transactions analyzed were diverse and there are inherent differences in the business, operations, financial condition and prospects of Zynerba and the companies included in the selected transactions analysis.
Financial data for the precedent transactions was based on publicly available information at the time of the announcement of the relevant transactions that MTS Securities obtained from SEC filings, relevant press releases, S&P Capital IQ, Cortellis, and company websites as of August 9, 2023. Using this information, MTS Securities calculated for each selected transaction the total transaction enterprise value as a multiple of the target company’s unadjusted peak sales.
MTS Securities derived a range of enterprise value to unadjusted peak sales multiples based on the first and third quartiles of the comparable companies data set. MTS Securities applied the range of multiples to Zynerba management’s estimated unadjusted peak sales, calculated as the average of unadjusted peak revenues in Cases 4 and 6, to calculate the implied enterprise value of Zynerba. MTS Securities then derived implied per Share prices by subtracting Zynerba’s net debt and then dividing by the fully diluted Shares outstanding of Zynerba at the implied per Share price. For avoidance of doubt, MTS Securities did not adjust the fully diluted Shares outstanding for the contemplated Capital Raise. The following table reflects the ranges of implied price per Share of Zynerba implied by this analysis, rounded to the nearest $0.05:
Enterprise Value | | | $488mm - $846mm | | | $9.05 - $15.15 |
Enterprise Value / Unadjusted Peak Sales | | | 0.690x – 1.882x | | | $7.60 - $19.35 |
TABLE OF CONTENTS
MTS Securities also derived a range of enterprise value to unadjusted peak sales multiples based on the first and third quartiles of the comparable companies data set excluding transactions with program failures. Transactions with program failures include the NightstarTx/Biogen, Clementia/Ipsen, and Wilson/Alexion transactions. MTS Securities applied the range of multiples to Zynerba management’s estimated unadjusted peak sales, calculated as the average of unadjusted peak revenues in Cases 4 and 6, to calculate the implied enterprise value of Zynerba. MTS Securities then derived implied per Share prices by subtracting Zynerba’s net debt and then dividing by the fully diluted Shares outstanding of Zynerba at the implied per Share price. For avoidance of doubt, MTS Securities did not adjust the fully diluted Shares outstanding for the contemplated Capital Raise. The following table reflects the ranges of implied price per Share of Zynerba implied by this analysis, rounded to the nearest $0.05:
Enterprise Value | | | $335mm - $750mm | | | $6.45 - $13.55 |
Enterprise Value / Unadjusted Peak Sales | | | 0.492x – 1.745x | | | $5.60 - $18.00 |
Other Factors
MTS Securities noted for the Zynerba Board certain additional factors solely for informational purposes, including, among other things, the following:
• | Historical Stock Price Trading Analysis. MTS Securities reviewed historical closing trading prices of the Shares during the 52-week period ending on August 9, 2023, which reflected low and high closing prices for the Shares during such period of $0.25 to $1.35 per Share, respectively. |
General
The preparation of a fairness opinion is a complex analytical process involving various determinations as to the most appropriate and relevant methods of financial analysis and the application of those methods to the particular circumstances and, therefore, a fairness opinion is not readily susceptible to summary description. In arriving at its opinion, MTS Securities did not draw, in isolation, conclusions from or with regard to any factor or analysis that it considered. Rather, MTS Securities made its determination as to fairness on the basis of its experience and professional judgment after considering the results of all of the analyses.
MTS Securities’ financial analyses and opinion were only one of many factors taken into consideration by the Zynerba Board in its evaluation of the Transactions. Consequently, the analyses described above should not be viewed as determinative of the views of the Zynerba Board or Zynerba management with respect to the Offer Price or as to whether the Zynerba Board would have been willing to determine that a different consideration was fair. The consideration for the Transactions was determined through arm’s-length negotiations between Zynerba and Harmony Biosciences and was approved by the Zynerba Board. MTS Health Partners provided advice to Zynerba during these negotiations. MTS Health Partners did not, however, recommend any specific amount of consideration to Zynerba or the Zynerba Board or that any specific amount of consideration constituted the only appropriate consideration for the Transactions.
MTS Securities has consented to the use of the MTS Opinion and disclosures regarding the MTS Opinion in this Schedule 14D-9; however, neither MTS Health Partners nor MTS Securities have assumed any responsibility for the form or content of this Schedule 14D-9. MTS Health Partners and its affiliates, as part of their investment banking services, are regularly engaged in the valuation of businesses and securities in connection with mergers and acquisitions, and for other purposes. As noted above, MTS Health Partners acted as financial advisor to Zynerba in connection with the Transactions and advised on certain of the negotiations leading to the Merger Agreement and the CVR Agreement. Zynerba selected MTS Health Partners as its financial advisor because it is nationally recognized in the healthcare and biotechnology industries as having investment banking professionals with significant experience in healthcare and biotechnology investment banking and merger and acquisition transactions, including transactions similar to the Transactions.
Pursuant to an engagement letter agreement, dated as of November 6, 2015, and engagement letter amendment, dated as of March 10, 2023, by and between Zynerba and MTS Health Partners, Zynerba engaged MTS Health Partners to act as its financial advisor in connection with Zynerba’s consideration, evaluation and/or exploration of certain potential merger and acquisition transactions or similar transactions. As permitted by the terms of the engagement letter and pursuant to MTS Health Partners’ internal policies, MTS Securities, a wholly-owned subsidiary of MTS
TABLE OF CONTENTS
Health Partners, delivered the MTS Opinion. As compensation for MTS Health Partners’ and its affiliates’ financial advisory services to the Zynerba Board, Zynerba paid a nonrefundable retainer of $100,000 upon execution of the engagement letter (the “Retainer Fee”) and paid MTS Securities a fee of $375,000 for rendering the MTS Opinion in connection with Zynerba’s Board’s consideration of the proposed Transactions with Harmony Biosciences, which fee was not contingent upon the successful completion of the Offer or the Merger or the conclusion reached in the MTS Opinion (the “Opinion Fee”). Upon the consummation of the Transactions, Zynerba will be obligated to pay to MTS Health Partners a fee up to approximately $4.375 million, of which the Retainer Fee and the Opinion Fee will be credited, and $2.375 million of which is contingent upon the achievement of milestones under the CVR Agreement. In addition, Zynerba has agreed to reimburse to MTS Health Partners and its affiliates for their out-of-pocket expenses incurred, and to indemnify MTS Health Partners and its related persons for certain liabilities that may arise, in each case, in connection with any of the matters contemplated by the engagement letter. MTS Health Partners and its affiliates have not, in the past two years, provided investment banking or financial advisory services to Harmony Biosciences or any of its affiliates for which it has received compensation.
TABLE OF CONTENTS
| PERSONS/ASSETS, RETAINED, EMPLOYED, COMPENSATED OR USED |
MTS Health Partners
Pursuant to MTS Health Partners’ engagement letter with Zynerba, as amended, Zynerba retained MTS Health Partners and its affiliates as its financial advisor in connection with the Offer and the Merger and, in connection with such engagement, MTS Securities, LLC provided to the Zynerba Board the MTS Opinion as further described in the section above titled “Item 4. The Solicitation or Recommendation—Opinion of Zynerba’s Financial Advisor,” which is filed as Annex I hereto and incorporated herein by reference. In connection with MTS Health Partners’ services as the financial advisor to the Zynerba Board, Zynerba has agreed to pay MTS Health Partners an aggregate fee of up to approximately $4.375 million, which consists of (i) the Retainer Fee, (ii) the Opinion Fee and (iii) approximately $1.525 million that is contingent upon the consummation and closing of the Transactions and an additional $2.375 million of which is contingent upon the achievement of milestones under the CVR Agreement. In addition, Zynerba has agreed to reimburse certain of MTS Health Partners’ and its affiliates’ expenses arising, and to indemnify MTS Health Partners and its affiliates against certain liabilities that may arise, out of MTS Health Partners’ engagement. MTS Health Partners and its affiliates have not, in the past two years, provided investment banking or financial advisory services to Harmony Biosciences or any of its affiliates for which it has received compensation.
Additional information pertaining to the retention of MTS Health Partners by the Company is set forth under “Item 4. Solicitation or Recommendation–Background of the Merger.”
MacKenzie Partners
Harmony Biosciences has retained MacKenzie Partners, Inc. (“MacKenzie Partners”) to provide advisory, consulting and solicitation services in connection with, among other things, the Offer, the Merger and related matters. Harmony Biosciences expects to pay MacKenzie Partners approximately $9,500, plus additional fees in the event of any extensions of the Offer, reimbursement of out-of-pocket expenses, including reasonable attorneys’ fees, and agreed to indemnify MacKenzie and related persons against various liabilities.
Except as set forth above, neither Zynerba nor any person acting on its behalf has or currently intends to employ, retain or compensate any person to make solicitations or recommendations to the Zynerba’s stockholders on its behalf with respect to the Offer or the Merger.
| INTEREST IN SECURITIES OF THE SUBJECT COMPANY |
Other than the scheduled vesting of Company Options, no transactions with respect to the Shares have been effected by Zynerba or, to the knowledge of Zynerba after making reasonable inquiry, by any of its executive officers, directors, affiliates or subsidiaries during the sixty (60) days prior to the date of this Schedule 14D-9.
| PURPOSES OF THE TRANSACTION AND PLANS OR PROPOSALS |
Except as set forth in this Schedule 14D-9 or as incorporated in this Schedule 14D-9 by reference, Zynerba is not undertaking or engaged in any negotiations in response to the Offer that relate to:
• | a tender offer or other acquisition of Zynerba’s securities by Zynerba or any other person; |
• | any extraordinary transaction, such as a merger, reorganization or liquidation, involving Zynerba; |
• | any purchase, sale or transfer of a material amount of assets of Zynerba; or |
• | any material change in the present dividend rate or policy or indebtedness or capitalization of Zynerba. |
Except as set forth in this Schedule 14D-9 or as incorporated in this Schedule 14D-9 by reference, there are no transactions, resolutions of the Zynerba Board, agreements in principle or signed contracts entered into in response to the Offer that relate to one or more of the matters referred to in the preceding paragraph.
TABLE OF CONTENTS
The information set forth under “Item 3. Past Contacts, Transactions, Negotiations and Agreements—Arrangements Between Zynerba and its Executive Officers, Directors and Affiliates” is incorporated herein by reference.
Golden Parachute Compensation
This section sets forth the information required by Item 402(t) of Regulation S-K regarding the compensation that is based on or otherwise related to the Offer and the Merger for each of Zynerba’s executive officers who were designated as “named executive officers” in the Company’s Definitive Proxy Statement filed with the SEC on April 21, 2023. This compensation is referred to as “golden parachute” compensation by the applicable SEC disclosure rules, and in this section we use such term to describe the Merger-related compensation that will or may be payable to our named executive officers. The amounts set forth in the table below are based on multiple assumptions that may or may not actually prove correct, including assumptions described in the footnotes to the table below. As a result, the actual amounts, if any, to be received by a named executive officer in connection with the Merger may differ materially from the amounts set forth below.
The table below sets forth, for the purposes of this golden parachute disclosure, the amount of payments and benefits that each of Zynerba’s named executive officers would receive, assuming that (i) the Effective Time occurs on August 24, 2023 (which is the assumed date solely for purposes of this golden parachute compensation disclosure); (ii) each of Zynerba’s named executive officers experiences a qualifying termination of employment at the Effective Time; (iii) Company Options with exercise prices that are less than $2.71 per share, and Company RSAs, outstanding as of August 24, 2023 are cancelled in exchange for the consideration described above under “Item 3. Past Contacts, Transactions, Negotiations and Agreements—Treatment of Equity Awards in the Transaction”; (iv) no named executive officer receives any additional equity grants or retention awards on or prior to the Effective Time; (v) the Milestones set forth in the CVR Agreement are achieved and holders of Company Options and Company RSAs receive the Closing Amount plus the maximum amount payable for one CVR (i.e., approximately $3.65), less the applicable exercise price in the case of Company Options, or in the case of Company Options with an exercise price greater than the Closing Amount (i.e., $1.1059 per share), the maximum amount payable for one CVR, less the portion of the exercise price that exceeds the Closing Amount; and (vi) no named executive officer enters into any new agreement with Zynerba or Harmony Biosciences or becomes entitled to, prior to the Effective Time.
Potential Change in Control Payments to Named Executive Officers
Armando Anido | | | 1,719,861 | | | 2,698,686 | | | 122,616 | | | 4,541,163 |
James E. Fickenscher | | | 893,698 | | | 1,820,305 | | | 68,902 | | | 2,782,905 |
Terri B. Sebree | | | 1,061,940 | | | 1,870,679 | | | 33,465 | | | 2,966,084 |
(1)
| Pursuant to Mr. Anido’s employment agreement, upon a termination of his employment by Zynerba or any successor without “Cause” or by Mr. Anido for “Good Reason” (each, as defined in his employment agreement) within one year following the Effective Time, Mr. Anido is entitled to (i) continuation of his annual base salary for 24 months; and (ii) one hundred percent (100%) of Mr. Anido’s target annual bonus for the year in which such termination occurs. |
Pursuant to Mr. Fickenscher and Ms. Sebree’s employment agreements, as amended by the Employment Agreement Amendments, upon a termination of employment by Zynerba without “Cause” or by Mr. Fickenscher and Ms. Sebree for “Good Reason” (each, as defined in the applicable employment agreement) within one year following the Effective Time, Mr. Fickenscher and Ms. Sebree are each entitled to (i) continuation of their respective annual base salaries for 18 months; and (ii) one hundred percent (100%) of the applicable executive officer’s target annual bonus for the year in which such termination occurs. Such severance arrangements constitute “double trigger” arrangements and are payable subject to delivery by the named executive officer of an effective general release of claims within sixty (60) days after the date of termination and compliance with all terms and provisions of the employment agreement. The amount of each type of payment is shown in the following table:
Armando Anido | | | 1,322,970 | | | 396,891 | | | 1,719,861 |
James E. Fickenscher | | | 705,551 | | | 188,147 | | | 893,698 |
Terri B. Sebree | | | 758,529 | | | 303,411 | | | 1,061,940 |
(2)
| Represents the value of accelerated vesting of Company Options (i.e., Out-of-the-Money Company Options) and Company RSAs held by each of the named executive officers as of August 24, 2023 assuming the maximum consideration payable for Shares pursuant to the Offer or the Merger (i.e., $3.65 per Share), which represents a cash payment, if any, equal to (A) the amount, if any, by which (i) the Closing |
TABLE OF CONTENTS
Amount plus the applicable Milestone Payment that was previously paid in respect of such Share underlying such Company Option exceeds (ii) the per share exercise price of such Company Option, minus (B) the gross amount of Milestone Payments previously paid with respect to such Share underlying such Company Option. None of the named executive officers hold any In-the-Money Company Option. Pursuant to their employment agreements, each of our named executive officers is entitled to “double trigger” accelerated vesting of his Company Options upon a termination by Zynerba or any successor thereto without “Cause” or for “Good Reason” within one year following the Effective Time. However, as described in the section titled “— Effect of the Transactions on Company Equity Awards” above, the vesting of all outstanding equity awards, including those held by our named executive officers, is “single-trigger,” in that they are being accelerated in full and such outstanding equity awards will be paid out in accordance with the terms of the Merger Agreement upon the Effective Time, regardless of whether our named executive officers’ employment is terminated following the Effective Time. The amount of each type of payment is shown in the following table:
Armando Anido | | | 235,657 | | | 2,463,029 | | | 2,698,686 |
James E. Fickenscher | | | 155,038 | | | 1,665,267 | | | 1,820,305 |
Terri B. Sebree | | | 155,038 | | | 1,715,641 | | | 1,870,679 |
(3)
| Pursuant to the named executive officers’ respective employment agreements, as amended by the Employment Agreement Amendments, upon a termination other than a resignation without “Good Reason” or termination for “Cause” (within one year following the Effective Time), the named executive officer is entitled to receive a lump sum cash payment equal to the full monthly COBRA premium applicable to the named executive officer multiplied by eighteen (18) months, or twenty-four (24) months in the case of Mr. Anido. |
Employee Arrangements Following the Merger
Pursuant to the Merger Agreement, for a period of not less than one (1) year after the Closing Date, Harmony Biosciences shall, or shall cause the Surviving Corporation to, provide to each employee of Zynerba who is employed as of immediately prior to the Effective Time and continues employment with Harmony Biosciences, the Surviving Corporation or any subsidiary of Harmony Biosciences or the Surviving Corporation following the closing date of the Merger (each, a “Continuing Employee”) with (i) (A) base salary or base hourly wage rate (as applicable) in an amount at least equal to the level that was provided to each such Continuing Employee as of immediately prior to the Effective Time and (B) short-term (i.e., annual or shorter) and long-term incentive compensation opportunities (including bonuses and commissions and the value of equity awards) and employee benefits that are no less favorable in the aggregate than the lesser of the incentive compensation opportunities provided to such Continuing Employee as of immediately prior to the Effective Time or to those provided to similarly-situated employees of Harmony Biosciences, and (ii) employee benefits that are no less favorable in the aggregate than the lesser of what was provided to the Continuing Employee as of immediately prior to the Effective Time and those provided to similarly-situated employees of Harmony Biosciences.
From and after the Effective Time, Harmony Biosciences shall, or shall cause the Surviving Corporation to, use commercially reasonable efforts to provide each Continuing Employee, subject to applicable law and applicable tax qualification requirements, with full credit for purposes of eligibility to participate (but not for purposes of benefit accruals, vacation entitlement and severance benefits) for service with Zynerba (or predecessor employers to the extent that Zynerba provides such past service credit) under the comparable employee benefit plans, programs and policies of Harmony Biosciences or the Surviving Corporation, as applicable, in which such employees become participants; provided, however, that such service shall not be credited to the extent that such service was not credited under the corresponding Zynerba benefit plan; provided, further, that the foregoing shall not apply with respect to benefit accrual under any defined benefit pension plan or to the extent that its application would result in a duplication of benefits. As of the Effective Time, Harmony Biosciences shall, or shall cause the Surviving Corporation to, credit to Continuing Employees the amount of vacation time that such employees had accrued but not used under any Zynerba benefit plan as of the Effective Time, which shall be subject to accrual limits or forfeiture to the same extent as provided under the applicable Zynerba benefit plan as of immediately prior to the Effective Time.
From and after the Effective Time, with respect to each Parent Plan that is an “employee welfare benefit plan”, as defined in Section 3(2) of the Employee Retirement Income Security Act of 1974, as amended (each, a “Parent Welfare Plan”), in which any Continuing Employee becomes eligible to participate, Harmony Biosciences shall use commercially reasonable efforts to (i) cause each such Parent Welfare Plan to waive all limitations as to pre-existing conditions, waiting periods, required physical examinations and exclusions with respect to participation and coverage requirements applicable under such Parent Welfare Plan for such Continuing Employees and their eligible dependents to the same extent that such pre-existing conditions, waiting periods, required physical examinations and exclusions would not have applied or would have been waived under the corresponding Zynerba benefit plan in which such Continuing Employee was a participant immediately prior to his or her commencement of participation in such Parent
TABLE OF CONTENTS
Welfare Plan, and (ii) at Harmony Biosciences’ sole discretion either (A) provide each Continuing Employee and their eligible dependents with credit for any co-payments and deductibles paid in the calendar year that, and prior to the date that, such Continuing Employee commences participation in such Parent Welfare Plan in satisfying any applicable co-payment or deductible requirements or out-of-pocket maximums under such Parent Welfare Plan for such calendar year, to the extent that such expenses were recognized for such purposes under the comparable Zynerba benefit plan or (B) provide such Continuing Employee with benefits that are no less favorable in the aggregate than any such credits described in (A) above.
Vote Required to Approve the Merger
The Zynerba Board has approved the Merger Agreement and the Transactions, including the Offer and the Merger, in accordance with the DGCL. If the Offer is consummated, Zynerba does not anticipate seeking the approval of Zynerba’s remaining stockholders before effecting the Merger. Section 251(h) of the DGCL provides that, following consummation of a successful tender offer for a public corporation, and subject to certain statutory provisions, if the acquiring corporation holds at least the amount of shares of each class of stock of the target corporation that would otherwise be required to approve a merger for the target corporation, and the other stockholders receive the same consideration for their stock in the merger as was payable in the tender offer, the acquiror can effect a merger without the action of the other stockholders of the target corporation. Accordingly, if the Offer is consummated, Zynerba, Harmony Biosciences and Purchaser intend to effect the closing of the Merger without a vote of the stockholders of Zynerba in accordance with Section 251(h) of the DGCL, upon the terms and subject to the satisfaction or waiver of the conditions to the Merger, as soon as practicable after the consummation of the Offer.
Anti-Takeover Statutes
Delaware
Zynerba is incorporated under the laws of the State of Delaware. In general, Section 203 of the DGCL prevents an “interested stockholder” (defined generally to include a person who, together with such person’s affiliates and associates, owns or has the right to acquire 15% or more of a corporation’s outstanding voting stock) from engaging in a “business combination” (defined to include mergers and certain other actions and transactions) with a Delaware corporation whose stock is publicly traded or held of record by more than 2,000 stockholders for a period of three (3) years following the date such person became an interested stockholder unless:
• | the transaction in which the stockholder became an interested stockholder or the business combination was approved by the board of directors of the corporation before such party to the business combination became an interested stockholder; |
• | upon completion of the transaction that made it an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the commencement of the transaction (excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) the voting stock owned by directors who are also officers or held in employee stock plans in which the employees do not have a right to determine confidentially whether to tender stock held by the plan); or |
• | at or following such time at which such person became an interested stockholder, the business combination was approved by the board of directors of the corporation and authorized at a meeting of stockholders by the affirmative vote of the holders of at least 66 2/3% of the outstanding voting stock that the interested stockholder did not own. |
Neither Harmony Biosciences nor Purchaser, nor any of its “affiliates” or “associates” is, or has been within the past three (3) years an “interested stockholder” of Zynerba as defined in Section 203 of the DGCL. In addition, in accordance with the provisions of Section 203, the Zynerba Board has approved the Merger Agreement and the Transactions contemplated thereby, including the Offer and the Merger, as described in “Item 4. The Solicitation or Recommendation” above and, therefore, the restrictions of Section 203 are inapplicable to the Offer, the Merger and the Transactions.
Appraisal Rights
No appraisal rights are available to Zynerba’s stockholders (or beneficial owners, as the case may be) in connection with the Offer. However, if the Offer is successful and the Merger is consummated, Zynerba stockholders (and certain beneficial owners of Zynerba stock) who: (i) did not tender their Shares in the Offer; (ii) properly demand appraisal
TABLE OF CONTENTS
of their Shares; (iii) continuously hold (or beneficially own, as the case may be) their Shares from the date of making the demand through the Effective Time; (iv) otherwise comply with the applicable requirements and procedures of Section 262 of the DGCL; and (v) do not thereafter withdraw their demand for appraisal of such Shares or otherwise lose their appraisal rights, in each case in accordance with the DGCL, will be entitled to demand appraisal of their Shares and receive, in lieu of the consideration payable in the Merger, a cash payment equal to the “fair value” of their Shares, exclusive of any elements of value arising from the accomplishment or expectation of the Merger, together with interest, if any, as determined by the Delaware Court of Chancery, in accordance with Section 262 of the DGCL. Stockholders and beneficial owners seeking appraisal should be aware that the fair value of their Shares as so determined by the Delaware Court of Chancery could be more than, the same as, or less than the consideration to be received pursuant to the Merger if they did not seek appraisal of their Shares, and that an investment banking opinion as to the fairness, from a financial point of view, of the consideration payable in a sale transaction, such as the Offer and the Merger, is not an opinion as to, and does not otherwise address, fair value under Section 262 of the DGCL. Any stockholder contemplating the exercise of such appraisal rights should review carefully the provisions of Section 262 of the DGCL, particularly the procedural steps required to properly demand and perfect such rights. Unless the context requires otherwise, all references in this summary to a (i) “stockholder” are to the holder of Shares as to which appraisal rights are asserted, unless otherwise expressly noted herein, (ii) “beneficial owner” are to a person who is the beneficial owner of Shares held either in voting trust or by a nominee on behalf of such person, and (iii) “person” are to an individual, corporation, partnership, unincorporated association or other entity.
The following is a summary of the procedures to be followed by stockholders and beneficial owners that wish to exercise their appraisal rights under Section 262 of the DGCL, the full text of which is attached to this Schedule 14D-9 as Annex II and incorporated herein by reference. This summary does not purport to be a complete statement of, and is qualified in its entirety by reference to, Section 262 of the DGCL and to any amendments to such section adopted or otherwise made effective after the date of this Schedule 14D-9. Failure to follow any of the procedures of Section 262 of the DGCL may result in termination or waiver of appraisal rights under Section 262 of the DGCL. Stockholders and beneficial owners should assume that Zynerba will take no action to perfect any appraisal rights of any stockholder.
Any person who desires to exercise his, her or its appraisal rights should review carefully Section 262 of the DGCL and is urged to consult his, her or its legal advisor before electing or attempting to exercise such rights. The following summary does not constitute any legal or other advice nor does it constitute a recommendation that the Zynerba’s stockholders or beneficial owners exercise appraisal rights under Section 262 of the DGCL.
Under Section 262 of the DGCL, where a merger is approved under Section 251(h), either a constituent corporation before the effective date of the merger, or the surviving corporation within ten days thereafter, shall notify each of the holders of any class or series of stock of such constituent corporation who are entitled to appraisal rights of the approval of the merger, consolidation or conversion and that appraisal rights are available for any or all shares of such class or series of stock of such constituent corporation, and shall include in such notice a copy of Section 262 or information directing the stockholders and beneficial owners to a publicly available electronic resource at which Section 262 may be accessed without subscription or cost. Any stockholder or beneficial owner entitled to appraisal rights may, within the later of the consummation of the Offer and twenty (20) days after the date of giving such notice, demand in writing from Zynerba the appraisal of such holder’s or beneficial owner’s Shares, as applicable. THIS SCHEDULE 14D-9 CONSTITUTES THE FORMAL NOTICE OF APPRAISAL RIGHTS UNDER SECTION 262 OF THE DGCL. ANY HOLDER OR BENEFICIAL OWNER OF SHARES WHO WISHES TO EXERCISE SUCH APPRAISAL RIGHTS OR WHO WISHES TO PRESERVE HIS, HER OR ITS RIGHT TO DO SO SHOULD REVIEW THE FOLLOWING DISCUSSION AND ANNEX II CAREFULLY BECAUSE FAILURE TO TIMELY AND PROPERLY COMPLY WITH THE PROCEDURES SPECIFIED IN SECTION 262 OF THE DGCL MAY RESULT IN THE LOSS OF APPRAISAL RIGHTS UNDER THE DGCL.
If a holder of record or beneficial owner of Shares elects to exercise appraisal rights under Section 262 of the DGCL, such stockholder or beneficial owner must do ALL of the following:
• | prior to the later of the consummation of the Offer and twenty (20) days after the mailing of this Schedule 14D-9, deliver to Zynerba at 80 W. Lancaster Avenue, Suite 300, Devon, Pennsylvania 19333, Attention: Albert P. Parker, Chief Legal Officer, a written demand for appraisal of the Shares held or beneficially owned, which demand must reasonably inform Zynerba of the identity of the stockholder and that the stockholder intends to demand appraisal for such stockholder’s Shares (or, in the case of a written demand |
TABLE OF CONTENTS
for appraisal made by a beneficial owner, the demand must reasonably identify the record holder of the Shares for which the demand is made, be accompanied by documentary evidence of such beneficial owner’s beneficial ownership of such Shares and a statement that such documentary evidence is a true and correct copy of what it purports to be, and provide an address at which such beneficial owner consents to receive notices given by Zynerba and to be set forth on the Verified List (as defined below));
• | not tender his, her or its Shares in the Offer; |
• | continuously hold of record or beneficially own the Shares from the date on which the written demand for appraisal is made through the Effective Time (a person demanding appraisal will lose appraisal rights if after demanding appraisal and before the Effective Time, in the case of a record holder, transfers, or in the case of a beneficial owner, ceases to beneficially own, such Shares); and |
• | comply with the procedures of Section 262 of the DGCL for perfecting appraisal rights thereafter. |
If a holder of record or beneficial owner of Shares fails to comply with any of these conditions and the Merger is completed, such person will be entitled to receive the Merger Consideration, subject to any applicable withholding taxes and without interest, but will have no appraisal rights with respect to such person’s Shares.
Written Demand
All written demands for appraisal should be addressed to Zynerba, 80 W. Lancaster Avenue, Suite 300, Devon, Pennsylvania 19333, Attention: Albert P. Parker Chief Legal Officer. A demand for appraisal must (i) be executed by or for the stockholder of record, fully and correctly, as such stockholder’s name appears on the stockholder’s certificates (whether in book entry or on physical certificates) evidencing such stockholder’s Shares and (ii) state that the stockholder intends thereby to demand appraisal of his, her or its shares in connection with the Merger. If the Shares are owned of record in a fiduciary or representative capacity, such as by a trustee, guardian or custodian, the demand must be executed by or on behalf of the stockholder in such capacity, and if the Shares are owned of record by more than one person, as in a joint tenancy or tenancy in common, the demand must be made by or for all owners of record. An authorized agent, including an authorized agent for one or more joint owners, may execute the demand for appraisal for a stockholder of record; provided, however, that such agent must identify the record owner or owners and expressly disclose in such demand that the agent is acting as agent for the record owner or owners of such Shares.
A stockholder, such as a brokerage firm, bank, trust or other nominee, who holds Shares as a nominee or intermediary for one or more beneficial owners, some or all of whom desire to demand appraisal, may exercise rights on behalf of such beneficial owners with respect to the Shares held for such beneficial owners while not exercising appraisal rights for other beneficial owners. In such case, the written demand for appraisal must set forth the number of Shares covered by such demand. Unless a demand for appraisal specifies a number of Shares, such demand will be presumed to cover all the Shares held in the name of such record owner. Alternatively, a beneficial owner may demand appraisal, in his, her or its own name, of such beneficial owner’s Shares, provided that (i) such beneficial owner continuously owns such Shares through the Effective Time and (ii) the demand made by such beneficial owner reasonably identifies the holder of record of the Shares for which the demand is made, and is accompanied by documentary evidence of such beneficial owner’s beneficial ownership of Shares and a statement that such documentary evidence is a true and correct copy of what it purports to be, and provides an address at which such beneficial owner consents to receive notices given by the Surviving Corporation under Section 262 and to be set forth on the Verified List (defined below).
Filing a Petition for Appraisal
If the Merger is completed, within ten (10) days after the Effective Time, the Surviving Corporation will notify each stockholder and beneficial owner who has made a written demand for appraisal pursuant to Section 262. At any time within sixty (60) days after the Effective Time, any person entitled to appraisal rights who has not commenced an appraisal proceeding or joined that proceeding as a named party may withdraw such person’s demand for appraisal and accept the consideration offered pursuant to the Merger Agreement, subject to any applicable withholding taxes and without interest, by delivering to Zynerba a written withdrawal of the demand for appraisal. Within one hundred twenty (120) days after the Effective Time, but not thereafter, the Surviving Corporation, or any person who has complied with Section 262 of the DGCL and is entitled to appraisal rights under Section 262 of the DGCL may commence an appraisal proceeding by filing a petition (a “Petition”) in the Delaware Court of Chancery (the “Delaware Court”), with a copy served on the Surviving Corporation in the case of a Petition filed by any person
TABLE OF CONTENTS
other than the Surviving Corporation, demanding a determination of the fair value of the Shares held or owned by all holders who did not tender in the Offer and demanded appraisal. If no such Petition is filed within that one hundred and twenty (120) day period, appraisal rights will be lost for all holders and beneficial owners of Shares who had previously demanded appraisal of their Shares. The Surviving Corporation is under no obligation to and has no present intention to file a Petition and no person should assume that Surviving Corporation will file a Petition or that it will initiate any negotiations with respect to the fair value of the Shares. Accordingly, it is the obligation of the stockholders and beneficial owners to initiate all necessary action to perfect their appraisal rights in respect of the Shares within the period prescribed in Section 262 of the DGCL.
No appraisal proceeding in the Delaware Court will be dismissed as to any person without the approval of the Delaware Court, and such approval may be conditioned upon such terms as the Delaware Court deems just; provided, however, that this shall not affect the right of any person who has not commenced an appraisal proceeding or joined that proceeding as a named party to withdraw such person’s demand for appraisal and to accept the Merger Consideration, subject to any applicable withholding taxes and without interest, within 60 days after the Effective Time.
Within one hundred and twenty (120) days after the Effective Time, any person who has complied with the requirements for exercise of appraisal rights will be entitled, upon request given in writing, to receive from the Surviving Corporation a statement setting forth the aggregate number of Shares not tendered into, and accepted for purchase or exchange in, the Offer and with respect to which demands for appraisal have been received and the aggregate number of stockholders and beneficial owners holding or owning such Shares (provided that, where a beneficial owner makes a demand pursuant to paragraph (d)(3) of Section 262, the record holders of such Shares shall not be considered a separate stockholder holding such Shares for purposes of such aggregate number). Such statement must be given to the requesting person within ten (10) days after a request by such person for the information has been received by the Surviving Corporation or within ten (10) days after the expiration of the period for delivery of demands for appraisal, whichever is later.
Upon the filing of such Petition by any such stockholder or beneficial owner (a “Dissenting Stockholder”), service of a copy thereof must be made upon the Surviving Corporation, which will then be obligated within twenty (20) days after such service to file with the office of the Delaware Register in Chancery in which the Petition was filed a duly verified list (the “Verified List”) containing the names and addresses of all persons who have demanded appraisal for their Shares and with whom agreements as to the value of their Shares have not been reached. If a Petition is filed by the Surviving Corporation, the Petition must be accompanied by the Verified List. Upon the filing of a Petition by a Dissenting Stockholder, the Delaware Court may order a hearing and that notice of the time and place fixed for the hearing on the Petition be mailed to the Surviving Corporation and all the Dissenting Stockholders. The forms of the notices by mail and by publication shall be approved by the Delaware Court, and the costs relating to these notices will be borne by the Surviving Corporation.
After notice is provided to the applicable persons as required by the Delaware Court, at the hearing on such Petition, the Delaware Court is empowered to determine which Dissenting Stockholders have complied with the provisions of Section 262 of the DGCL and are entitled to an appraisal of their Shares. The Delaware Court may require that Dissenting Stockholders submit the certificates evidencing their Shares, as applicable, for notation thereon of the pendency of the appraisal proceedings. The Delaware Court is empowered to dismiss the proceedings as to any Dissenting Stockholder who does not comply with such requirement. Accordingly, Dissenting Stockholders are cautioned to retain the certificates evidencing their Shares pending resolution of the appraisal proceedings. In addition, because immediately before the Effective Time, the Shares were listed on a national securities exchange, the Delaware Court shall dismiss the proceedings as to all holders of such Shares who are otherwise entitled to appraisal rights unless (1) the total number of Shares for which appraisal rights have been pursued or effected exceeds 1% of the outstanding Shares eligible for appraisal as measured in accordance with subsection (g) of Section 262, or (2) the value of the Merger Consideration in respect of such Shares exceeds $1 million.
The Shares will be appraised by the Delaware Court at the fair value thereof exclusive of any element of value arising from the accomplishment or expectation of the Merger, together with interest, if any, to be paid upon the amount determined to be the fair value. Unless the Delaware Court in its discretion determines otherwise for good cause shown, interest from the Effective Time through the date of payment of the judgment will be compounded quarterly and will accrue at five percent (5%) over the Federal Reserve discount rate (including any surcharge) as established from time to time during the period between the Effective Time and the date of payment of the judgment. In determining the “fair value,” the court is to take into account all relevant factors. At any time before the entry of
TABLE OF CONTENTS
judgment in the proceedings, the Surviving Corporation may pay to each person entitled to appraisal an amount in cash, in which case interest shall accrue thereafter as provided herein only upon the sum of (1) the difference, if any, between the amount so paid and the fair value of the Shares as determined by the Delaware Court, and (2) interest theretofore accrued, unless paid at that time. The Surviving Corporation is under no obligation to make such voluntary cash payment prior to such entry of judgment.
The costs of the appraisal proceedings (which do not include attorneys’ fees or the fees and expenses of experts) may be determined by the Delaware Court and imposed upon the parties as the Delaware Court deems equitable under the circumstances. Upon application of a person whose name appears on the Verified List who participated in the appraisal proceeding and incurred expenses in connection therewith, the Delaware Court may also order that all or a portion of such expenses, including, without limitation, reasonable attorney’s fees and the fees and expenses of experts, be charged pro rata against the value of all the Shares entitled to an appraisal that were not dismissed pursuant to the terms of Section 262 of the DGCL or subject to an award pursuant to a reservation of jurisdiction. In the absence of such determination or assessment, each party bears its own expenses. Determinations by the Delaware Court are subject to appellate review by the Delaware Supreme Court.
The appraisal proceeding will be conducted in accordance with the rules of the Delaware Court, including any rules specifically governing appraisal proceedings. Through such proceeding, the Delaware Court will determine the “fair value” of the Shares, exclusive of any element of value arising from the accomplishment or expectation of the Merger, together with interest, if any, to be paid upon the amount determined to be the fair value. In Weinberger v. UOP, Inc., the Supreme Court of Delaware discussed the factors that could be considered in determining “fair value” in an appraisal proceeding, stating that “proof of value by any techniques or methods which are generally considered acceptable in the financial community and otherwise admissible in court” should be considered, and that “[f]air price obviously requires consideration of all relevant factors involving the value of a company.” The Supreme Court of Delaware stated that, in making this determination of “fair value,” the court must consider market value, asset value, dividends, earnings prospects, the nature of the enterprise and any other facts that could be ascertained as of the date of the Merger that throw any light on future prospects of the merged corporation. Section 262 provides that “fair value” is to be “exclusive of any element of value arising from the accomplishment or expectation of the Merger.” In Cede & Co. v. Technicolor, Inc., the Supreme Court of Delaware stated that such exclusion is a “narrow exclusion [that] does not encompass known elements of value,” but which rather applies only to the speculative elements of value arising from such accomplishment or expectation. In Weinberger, the Supreme Court of Delaware also stated that “elements of future value, including the nature of the enterprise, which are known or susceptible of proof as of the date of the Merger and not the product of speculation, may be considered.”
No representation is made as to the outcome of the appraisal of “fair value” as determined by the Delaware Court, and persons seeking appraisal should recognize that such an appraisal could result in a determination of a value higher or lower than, or the same as, the Merger Consideration. Zynerba does not presently anticipate offering more than the Merger Consideration to any person exercising appraisal rights, and Zynerba reserves the right to assert, in any appraisal proceeding, that for purposes of Section 262, the “fair value” of a Share, as applicable, is less than the Merger Consideration. If a demand for appraisal is duly withdrawn, if a petition for appraisal is not timely filed, if neither of the ownership thresholds described above has been satisfied as to persons seeking appraisal rights, or other requirements imposed by Section 262 to perfect and seek appraisal are not satisfied, then the right to an appraisal will cease.
Any person who has duly demanded and perfected appraisal rights in compliance with Section 262 of the DGCL will not, after the Effective Time, be entitled to vote his, her or its Shares for any purpose or be entitled to the payment of dividends or other distributions thereon, except for dividends or other distributions payable to holders of record of Shares as of a date prior to the Effective Time.
If any person who demands appraisal of Shares under Section 262 of the DGCL fails to perfect, successfully withdraws or loses such holder’s or owner’s right to appraisal, such person’s Shares will be deemed to have been converted at the Effective Time into the right to receive the Merger Consideration, subject to any applicable withholding taxes and without interest. A person will fail to perfect, or effectively lose or withdraw, such person’s right to appraisal if no Petition for appraisal is filed within one hundred and twenty (120) days after the Effective Time.
This summary of appraisal rights under the DGCL is not complete and is qualified in its entirety by reference to Section 262 of the DGCL and the Offer. A copy of Section 262 of the DGCL is included as Annex II to this Schedule
TABLE OF CONTENTS
14D-9. A copy of Section 262 of the DGCL is also provided at the following publicly-available web address, which is maintained on behalf of the state of Delaware and is free to use without subscription or cost: https://delcode.delaware.gov/title8/c001/sc09/index.html#262.
FAILURE TO FOLLOW THE STEPS REQUIRED BY SECTION 262 OF THE DGCL FOR PERFECTING APPRAISAL RIGHTS MAY RESULT IN THE LOSS OF APPRAISAL RIGHTS. IN THAT EVENT, YOU WILL BE ENTITLED TO RECEIVE THE MERGER CONSIDERATION, SUBJECT TO ANY APPLICABLE WITHHOLDING TAXES AND WITHOUT INTEREST, FOR YOUR SHARES IN ACCORDANCE WITH THE MERGER AGREEMENT. IN VIEW OF THE COMPLEXITY OF THE PROVISIONS OF SECTION 262 OF THE DGCL, IF YOU ARE A HOLDER OR BENEFICIAL OWNER OF SHARES AND ARE CONSIDERING EXERCISING YOUR APPRAISAL RIGHTS UNDER THE DGCL, YOU SHOULD CONSULT YOUR OWN LEGAL ADVISOR. Annual and Quarterly Reports
For additional information regarding the business and the financial results of Zynerba, please see Zynerba’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on March 28, 2023, and its Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2023, filed with the SEC on August 14, 2023.
Legal Proceedings
Lawsuits arising out of or relating to the Offer, the Merger or the Transactions may be filed in the future.
Regulatory Approvals
Antitrust in the United States
The Antitrust Division of the U.S. Department of Justice (the “Antitrust Division”) and the Federal Trade Commission (the “FTC”) may review the legality under the antitrust laws of the acquisition of Shares in the Offer at any time before or after Purchaser’s acceptance for payment of Shares pursuant to the Offer, either the Antitrust Division or the FTC could take such action under the antitrust laws of the United States of America as it deems necessary to protect competition in the public interest, including seeking to enjoin the acquisition of Shares in the Offer or seeking divestiture of the Shares so acquired or divestiture of substantial assets of Harmony Biosciences or of its subsidiaries or affiliates or requiring other conduct relief. U.S. state attorneys general and private persons may also bring legal action under the antitrust laws of the United States of America seeking similar relief or seeking conditions to the consummation of the Offer. While Zynerba believes that the consummation of the Offer will not result in a violation of any applicable antitrust laws, there can be no assurance that a challenge to the Offer on antitrust grounds will not be made or, if such a challenge is made, what the result will be. If any such action is commenced by the FTC, the Antitrust Division or any state or any other person, Harmony Biosciences may not be obligated to consummate the Offer or the Merger.
TABLE OF CONTENTS
Cautionary Note Regarding Forward-Looking Statements
This Schedule 14D-9 contains express or implied forward-looking statements related to Harmony Biosciences, Zynerba and the acquisition of Zynerba by Harmony Biosciences, including express or implied forward-looking statements about Zygel, its therapeutic benefits and its regulatory development pathway, and the future operations and performance of Harmony Biosciences and Zynerba. All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including all statements regarding the intent, belief or current expectation of the companies and members of their senior management teams. Words such as “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. Examples of such forward-looking statements include, but are not limited to, express or implied:
• | statements regarding the transaction and related matters, prospective performance and opportunities, post-closing operations and the outlook for the companies’ businesses; |
• | statements of targets, plans, objectives or goals for future operations, including those related to Harmony Biosciences’ and Zynerba’s products, product research, product development, product introductions and product approvals as well as cooperation in relation thereto; |
• | statements containing projections of or targets for revenues, costs, income (or loss), earnings per share, capital expenditures, dividends, capital structure, net financials and other financial measures; |
• | statements regarding future economic performance, future actions and outcome of contingencies such as legal proceedings; and |
• | statements regarding the assumptions underlying or relating to such statements. |
These statements are based on current plans, estimates and projections. By their very nature, forward-looking statements involve inherent risks and uncertainties, both general and specific. Harmony Biosciences and Zynerba each caution that a number of important factors, including those described in this document, could cause actual results to differ materially from those contemplated in any forward-looking statements.
Factors that may affect future results and may cause these forward-looking statements to be inaccurate include, but are not limited to: uncertainties as to the timing of the tender offer and merger; uncertainties as to how many of Zynerba’s stockholders will tender their stock in the offer; the possibility that competing offers will be made; the possibility that various closing conditions for the transaction may not be satisfied or waived, including that a governmental entity may prohibit, delay or refuse to grant approval for the consummation of the transaction (or only grant approval subject to adverse conditions or limitations); the possibility that the proposed transaction may not be completed in the time frame expected by Harmony Biosciences and Zynerba, or at all; failure to realize the anticipated benefits of the proposed transaction in the time frame expected, or at all; the effects of the transaction on relationships with employees, other business partners or governmental entities; potential adverse reactions or changes to business relationships resulting from the announcement or completion of the proposed transaction; significant or unexpected costs, charges or expenses resulting from the proposed transaction; negative effects of this announcement or the consummation of the proposed acquisition on the market price of Harmony Biosciences’ shares or Zynerba’s common stock and/or Harmony Biosciences’ or Zynerba’s operating results; the difficulty of predicting the timing or outcome of regulatory approvals or actions; the risks related to non-achievement of the CVR milestones and that holders of the CVRs will not receive payments in respect of the CVRs; other business effects, including the effects of industry, economic or political conditions outside of the companies’ control; transaction costs; actual or contingent liabilities; risk of litigation and/or regulatory actions related to the proposed acquisition; adverse impacts on business, operating results or financial condition in the future due to pandemics, epidemics or outbreaks, such as COVID-19, and their impact on Harmony Biosciences’ and Zynerba’s respective businesses, operations, supply chain, patient enrollment and retention, clinical trials, strategy, goals and anticipated milestones; government-mandated or market-driven price decreases for Harmony Biosciences’ or Zynerba’s products; introduction of competing products; reliance on information technology; Harmony Biosciences’ or Zynerba’s ability to successfully market current and new products; Harmony Biosciences’, Zynerba’s and their collaborators’ ability to continue to conduct research and clinical programs; exposure to product liability and legal proceedings and investigations; and other risks and uncertainties detailed from time to time in Zynerba’s periodic reports filed with the SEC as well as the Schedule 14D-9 to be filed by Zynerba and the Schedule TO and related tender offer documents to be filed by Harmony Biosciences and Purchaser.
TABLE OF CONTENTS
Any forward-looking statements speak only as of the date of this communication and are made based on the current beliefs and judgments of Harmony Biosciences’ and Zynerba’s management, and the reader is cautioned not to rely on any forward-looking statements made by Harmony Biosciences or Zynerba. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Unless required by law, each of Harmony Biosciences and Zynerba is under no duty and undertakes no obligation to update or revise any forward-looking statement after the distribution of this communication, whether as a result of new information, future events or otherwise.
TABLE OF CONTENTS
| | | Offer to Purchase, dated August 28, 2023 (incorporated herein by reference to Exhibit (a)(1)(A) to the Schedule TO). |
| | | |
| | | Form of Letter of Transmittal (including Guidelines for Certification of Taxpayer Identification Number on IRS Form W-9) (incorporated herein by reference to Exhibit (a)(1)(B) to the Schedule TO). |
| | | |
| | | Form of Letter to Brokers, Dealers, Commercial Banks, Trust Companies and Other Nominees (incorporated herein by reference to Exhibit (a)(1)(C) to the Schedule TO). |
| | | |
| | | Form of Letter to Clients for Use by Brokers, Dealers, Commercial Banks, Trust Companies and Other Nominees (incorporated herein by reference to Exhibit (a)(1)(D) to the Schedule TO). |
| | | |
| | | Form of Summary Advertisement, published August 28, 2023 in The New Yok Times (incorporated herein by reference to Exhibit (a)(1)(F) to the Schedule TO). |
| | | |
| | | Opinion of MTS Securities, LLC, dated August 12, 2023 (included as Annex I to this Schedule 14D-9). |
| | | |
| | | Joint Press Release issued by Harmony Biosciences Holdings, Inc. and Zynerba Pharmaceuticals, Inc., dated August 14, 2023 (incorporated herein by reference to Exhibit 99.2 to the Current Report on Form 8-K filed by Zynerba with the SEC on August 14, 2023). |
| | | |
| | | Email to Zynerba’s 22q11.2 Deletion Syndrome Scientific Advisory Board from Stephen O’Quinn, Vice President, Medical Affairs of Zynerba, first used on August 14, 2023 (incorporated herein by reference to Exhibit 99.1 to the Schedule 14D-9C filed by Zynerba with the SEC on August 14, 2023). |
| | | |
| | | Email to Zynerba’s Fragile X Syndrome Scientific Advisory Board from Stephen O’Quinn, Vice President, Medical Affairs of Zynerba, first used on August 14, 2023 (incorporated herein by reference to Exhibit 99.2 to the Schedule 14D-9C filed by Zynerba with the SEC on August 14, 2023). |
| | | |
| | | Email to Zynerba’s Clinical Trial Investigators from Terri Sebree, President of Zynerba, first used on August 14, 2023 (incorporated herein by reference to Exhibit 99.3 to Schedule 14D-9C filed by Zynerba with the SEC on August 14, 2023). |
| | | |
| | | Email to Zynerba’s Australian Fragile X Syndrome Advocacy Group from Terri Sebree, President of Zynerba, first used on August 14, 2023 (incorporated herein by reference to Exhibit 99.4 to the Schedule 14D-9C filed by Zynerba with the SEC on August 14, 2023). |
| | | |
| | | Email to Zynerba’s Vendors and Partners from Terri Sebree, President of Zynerba, first used on August 14, 2023 (incorporated herein by reference to Exhibit 99.5 to the Schedule 14D-9C filed by Zynerba with the SEC on August 14, 2023). |
| | | |
| | | Email to Zynerba’s General Investigators from Terri Sebree, President of Zynerba, first used on August 14, 2023 (incorporated herein by reference to Exhibit 99.6 to the Schedule 14D-9C filed by Zynerba with the SEC on August 14, 2023). |
| | | |
| | | Email to Zynerba’s United States Fragile X Syndrome Advocacy Group from Stephen O’Quinn, Vice President, Medical Affairs of Zynerba, first used on August 14, 2023 (incorporated by reference to Exhibit 99.7 to the Schedule 14D-9C filed by Zynerba with the SEC on August 14, 2023). |
| | | |
TABLE OF CONTENTS
| | | Email to Zynerba’s Autism Spectrum Disorder Scientific Advisory Board from Stephen O’Quinn, Vice President, Medical Affairs of Zynerba, first used on August 14, 2023 (incorporated herein by reference to Exhibit 99.8 to the Schedule 14D-9C filed by Zynerba with the SEC on August 14, 2023). |
| | | |
| | | Transcript from Harmony Biosciences Holdings, Inc.’s Conference Call regarding the Proposed Acquisition, dated August 14, 2023 (incorporated herein by reference to Exhibit 99.2 to the Schedule TO-C filed by Harmony with the SEC on August 14, 2023). |
| | | |
| | | Agreement and Plan of Merger, dated as of August 14, 2023, by and among Harmony Biosciences Holdings, Inc., Xylophone Acquisition Corp. and Zynerba Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 2.1 to the Current Report on Form 8-K (File No. 001-37526) filed with the SEC on August 14, 2023). |
| | | |
| | | Form of Contingent Value Rights Agreement, by and between Harmony Biosciences Holdings, Inc. and Computershare Limited (incorporated herein by reference to Exhibit (d)(2) to the Schedule TO). |
| | | |
| | | Form of Tender and Support Agreement, dated as of August 14, 2023, by and among Harmony Biosciences Holdings, Inc., Xylophone Acquisition Corp. and the Supporting Stockholder (incorporated herein by reference to Exhibit 99.1 to the Form 8-K (File No. 001-37526) filed with the SEC on August 14, 2023). |
| | | |
| | | Confidentiality and Nondisclosure Agreement, dated as of November 17, 2021, by and between Harmony Biosciences, LLC and Zynerba Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit (d)(4) to the Schedule TO). |
| | | |
| | | Employment Agreement, dated as of September 4, 2014, by and between Zynerba Pharmaceuticals, Inc. and Armando Anido (incorporated herein by reference to Exhibit 10.2(A) to Zynerba’s Registration Statement on Form S-1 (File No. 333-205355) filed with the SEC on June 30, 2015). |
| | | |
| | | Amendment to the Employment Agreement, dated as of October 2, 2014, by and between Zynerba Pharmaceuticals, Inc. and Armando Anido (incorporated herein by reference to Exhibit 10.2(B) to Zynerba’s Registration Statement on Form S-1 (File No. 333-205355) filed with the SEC on June 30, 2015). |
| | | |
| | | Amendment to the Employment Agreement, dated as of August 14, 2023, by and between Zynerba Pharmaceuticals, Inc. and Armando Anido (incorporated herein by reference to Exhibit 10.1 on Zynerba’s Current Report on Form 8-K (File No. 001-37526) filed with the SEC on August 14, 2023). |
| | | |
| | | Employment Agreement, dated as of October 2, 2014, by and between Zynerba Pharmaceuticals, Inc. and Terri B. Sebree (incorporated herein by reference to Exhibit 10.3 to Zynerba’s Registration Statement on Form S-1 (File No. 333-205355) filed with the SEC on June 30, 2015). |
| | | |
| | | Amendment to the Employment Agreement, dated as of August 30, 2019, by and between Zynerba Pharmaceuticals, Inc. and Terri B. Sebree (incorporated herein by reference to Exhibit 10.4 to Zynerba’s Current Report on Form 8-K (File No. 001-37526) filed with the SEC on August 30, 2019). |
| | | |
| | | Amendment to the Employment Agreement, dated as of August 14, 2023, by and between Zynerba Pharmaceuticals, Inc. and Terri B. Sebree (incorporated herein by reference to Exhibit 10.2 on Zynerba’s Current Report on Form 8-K (File No. 001-37526) filed with the SEC on August 14, 2023). |
| | | |
TABLE OF CONTENTS
| | | Employment Agreement, dated as of February 14, 2022, by and between Zynerba Pharmaceuticals, Inc. and Albert P. Parker (incorporated herein by reference to Exhibit 10.3(A) to Zynerba’s Annual Report on Form 10-K for the year ended December 31, 2021 (File No. 001-37526) filed with the SEC on March 1, 2022). |
| | | |
| | | Amendment to the Employment Agreement, dated as of August 14, 2023, by and between Zynerba Pharmaceuticals, Inc. and Albert P. Parker. |
| | | |
| | | Employment Agreement, dated as of August 11, 2016, by and between Zynerba Pharmaceuticals, Inc. and James E. Fickenscher (incorporated herein by reference to Exhibit 10.2 to Zynerba’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2016 (File No. 001-37526) filed with the SEC on November 14, 2016). |
| | | |
| | | Amendment to the Employment Agreement, dated as of August 30, 2019, by and between Zynerba Pharmaceuticals, Inc. and James E. Fickenscher (incorporated herein by reference to Exhibit 10.3 to Zynerba’s Current Report on Form 8-K (File No. 001-37526) filed with the SEC on August 30, 2019). |
| | | |
| | | Amendment to the Employment Agreement, dated as of August 14, 2023, by and between Zynerba Pharmaceuticals, Inc. and James E. Fickenscher (incorporated herein by reference to Exhibit 10.3 to Zynerba’s Current Report on Form 8-K (File No. 001-37526) filed with the SEC on August 14, 2023). |
| | | |
| | | Employment Agreement, dated as of January 18, 2017, by and between Zynerba Pharmaceuticals, Inc. and Brian Rosenberger (incorporated herein by reference to Exhibit 10.7 to Zynerba’s Annual Report on Form 10-K for the year ended December 31, 2016 (File No. 001-37526) filed with the SEC on March 27, 2017). |
| | | |
| | | Amendment to the Employment Agreement, dated as of August 30, 2019, by and between Zynerba Pharmaceuticals, Inc. and Brian Rosenberger (incorporated herein by reference to Exhibit 10.6 to Zynerba’s Quarterly Report on Form 10-Q for the period ended September 30, 2019 (File No. 001-37526) filed with the SEC on November 6, 2019). |
| | | |
| | | Amendment to the Employment Agreement, dated as of August 14, 2023, by and between Zynerba Pharmaceuticals, Inc. and Brian Rosenberger. |
| | | |
| | | Amended and Restated 2014 Omnibus Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.19(A) to Zynerba’s Registration Statement on Form S-1 (File No. 333-205355) filed with the SEC on June 30, 2015). |
| | | |
| | | Form of Amendment to Amended and Restated 2014 Omnibus Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.19(B) to Zynerba’s Registration Statement on Form S-1/A (File No. 333-205355) filed with the SEC on July 23, 2015). |
| | | |
| | | Form of Incentive Stock Option Grant under Amended and Restated 2014 Omnibus Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.19(C) to Zynerba’s Registration Statement on Form S-1 (File No. 333-205355) filed with the SEC on June 30, 2015). |
| | | |
| | | Form of Nonqualified Stock Option Grant under Amended and Restated 2014 Omnibus Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.19(D) to Zynerba’s Registration Statement on Form S-1 (File No. 333-205355) filed with the SEC on June 30, 2015). |
| | | |
TABLE OF CONTENTS
| | | Form of Restricted Stock Grant Agreement under Amended and Restated 2014 Omnibus Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.2 to Zynerba’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2021 (File No. 001-37526) filed with the SEC on August 9, 2021). |
| | | |
| | | Form of Award Agreement for Inducement Awards (incorporated herein by reference to Exhibit 10.17 to Zynerba’s Annual Report on Form 10-K for the year ended December 31, 2016 (File No. 001-37526) filed with the SEC on March 27, 2017). |
| | | |
| | | Zynerba Pharmaceuticals, Inc. Non-Employee Director Compensation Policy (incorporated herein by reference to Exhibit 10.8 to Zynerba’s Annual Report on Form 10-K for the year ended December 31, 2021 (File No. 001-37526) filed with the SEC on March 1, 2022). |
| | | |
| | | Form of Indemnification Agreement between Zynerba Pharmaceuticals, Inc. and each of its directors and officers (incorporated herein by reference to Exhibit 10.20 to Zynerba’s Registration Statement on Form S-1/A (File No. 333-205355) filed with the SEC on July 23, 2015). |
| | | |
| | | Zynerba Pharmaceuticals, Inc. 2023 Stock Option and Incentive Plan, and forms of award agreements thereunder (incorporated herein by reference to Appendix B to the Definitive Proxy Statement on Schedule 14A filed with the SEC on April 21, 2023). |
TABLE OF CONTENTS
After due inquiry and to the best of my knowledge and belief, I certify that the information set forth in this statement is true, complete and correct.
Date: August 28, 2023 | | | |
| | | | |
| | | Zynerba Pharmaceuticals, Inc. |
| | | | |
| | | By: | | | /s/ Armando Anido |
| | | Name: | | | Armando Anido |
| | | Title: | | | Chairman and Chief Executive Officer |
TABLE OF CONTENTS
Opinion of MTS Securities
MTS Securities, LLC
CONFIDENTIAL
August 12, 2023
Board of Directors
Zynerba Pharmaceuticals, Inc.
80 W. Lancaster Avenue, Suite 300
Devon, PA 19333
Members of the Board of Directors (in their capacities as such):
We understand that Zynerba Pharmaceuticals, Inc., a Delaware corporation (the “Company”), proposes to enter into an Agreement and Plan of Merger, to be dated on or about August 14, 2023 (the “Merger Agreement”), by and among Harmony Biosciences Holdings, Inc., a Delaware corporation (“Parent”), Xylophone Acquisition Corp., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”), and the Company, pursuant to which Parent will cause Merger Sub to commence a tender offer (as it may be extended or amended from time to time as permitted under the Merger Agreement, the “Offer”) to acquire all of the issued and outstanding shares of common stock, par value $0.001 per share, of the Company (the “Company Common Stock”) for (a) $1.1059 per share of Company Common Stock, subject to any applicable withholding Taxes and without interest (the “Common Cash Amount”), and (b) one contingent value right (each, a “CVR”) per share of Company Common Stock (the “Common CVR Amount”; the Common Cash Amount plus the Common CVR Amount, collectively, or any different amount per share paid pursuant to the Offer to the extent permitted under the Merger Agreement are referred to as the “Offer Price”). We further understand that, subject to and in accordance with the terms and conditions of a Contingent Value Rights Agreement to be entered into by and between Parent and Computershare Limited, as the rights agent (the “CVR Agreement”), each CVR shall entitle the holder thereof to receive (i) upon the achievement of Milestone 1 (as defined in the CVR Agreement) on or before June 30, 2026, an amount per CVR, rounded to ten decimal places, equal to the quotient obtained by dividing (x) the sum of (A) $15,000,000 plus (B) an aggregate amount, for all Covered Out-of-the-Money Options (as defined in the CVR Agreement), equal to the excess, if any, of the applicable exercise price for each such Covered Out-of-the-Money Option over the Common Cash Amount by (y) the applicable Fully Diluted Share Amount (as defined in the CVR Agreement); (ii)(x) upon the achievement of Milestone 2 (as defined in the CVR Agreement) on or before December 31, 2024, an amount per CVR, rounded to four decimal places, equal to the quotient obtained by dividing (A) the sum of (1) $30,000,000 plus (2) an aggregate amount, for all Covered Out-of-the-Money Options, equal to the excess, if any, of the applicable exercise price for each such Covered Out-of-the-Money Option over the sum of (I) the Common Cash Amount plus (II) the aggregate amount of any Milestone Payments (as defined below) previously paid in respect of a share of Company Common Stock by (B) the applicable Fully Diluted Share Amount, or, alternatively, (y) upon the achievement of Milestone 2 between (and including) January 1, 2025 and June 30, 2025, an amount per CVR, rounded to four decimal places, equal to the quotient obtained by dividing (A) the sum of (1) $20,000,000 plus (2) an aggregate amount, for all Covered Out-of-the-Money Options, equal to the excess, if any, of the applicable exercise price for each such Covered Out-of-the-Money Option over the sum of (I) the Common Cash Amount plus (II) the aggregate amount of any Milestone Payments previously paid in respect of a share of Company Common Stock by (B) the applicable Fully Diluted Share Amount, or, alternatively, (z) upon the achievement of Milestone 2 on or after July 1, 2025, an amount per CVR, rounded to four decimal places, equal to the quotient obtained by dividing (A) the sum of (1) $10,000,000 plus (2) an aggregate amount, for all Covered Out-of-the-Money Options, equal to the excess, if any, of the applicable exercise price for each such Covered Out-of-the-Money Option over the sum of (I) the Common Cash Amount plus (II) the aggregate amount of any Milestone Payments previously paid in respect of a share of Company Common Stock by (B) the applicable Fully Diluted Share Amount; (iii) upon the achievement of Milestone 3 (as defined in the CVR Agreement), an amount per CVR rounded to four decimal places, equal to the quotient obtained by dividing (x) the sum of (A) $35,000,000 plus (B) an aggregate amount, for all Covered Out-of-the-Money Options, equal to the excess, if any, of the applicable exercise price for each such Covered Out-of-the-Money Option over the sum of (1) the Common Cash Amount plus (2) the aggregate amount of any Milestone Payments previously paid in respect of a share of Company Common Stock by (y) the applicable Fully Diluted Share Amount; (iv) upon the achievement
TABLE OF CONTENTS
of Milestone 4 (as defined in the CVR Agreement), an amount per CVR, rounded to four decimal places, equal to the quotient obtained by dividing (x) the sum of (A) $15,000,000 plus (B) an aggregate amount, for all Covered Out-of-the-Money Options, equal to the excess, if any, of the applicable exercise price for each such Covered Out-of-the-Money Option over the sum of (1) the Common Cash Amount plus (2) the aggregate amount of any Milestone Payments previously paid in respect of a share of Company Common Stock by (y) the applicable Fully Diluted Share Amount; (v) upon the achievement of Milestone 5 (as defined in the CVR Agreement), an amount per CVR, rounded to four decimal places, equal to the quotient obtained by dividing (x) the sum of (A) $15,000,000 plus (B) an aggregate amount, for all Covered Out-of-the-Money Options, equal to the excess, if any, of the applicable exercise price for each such Covered Out-of-the- Money Option over the sum of (1) the Common Cash Amount plus (2) the aggregate amount of any Milestone Payments previously paid in respect of a share of Company Common Stock by (y) the applicable Fully Diluted Share Amount; and (vi) upon the achievement of Milestone 6 (as defined in the CVR Agreement), an amount per CVR, rounded to four decimal places, equal to the quotient obtained by dividing (x) the sum of (A) $30,000,000 plus (B) an aggregate amount, for all Covered Out-of-the-Money Options, equal to the excess, if any, of the applicable exercise price for each such Covered Out-of-the-Money Option over the sum of (1) the Common Cash Amount plus (2) the aggregate amount of any Milestone Payments previously paid in respect of a share of Company Common Stock by (y) the applicable Fully Diluted Share Amount (each of the foregoing, a “Milestone Payment”), if any, subject to any applicable withholding Taxes and without interest. We further understand that the Merger Agreement further provides that, as soon as practicable following the consummation of the Offer, upon the terms and conditions set forth in the Merger Agreement and in accordance with the General Corporation Law of the State of Delaware, Merger Sub will be merged with and into the Company (the “Merger” and, together with the Offer, the “Transactions”), with the Company continuing as the surviving corporation, whereby each share of Company Common Stock (other than shares of Company Common Stock (A) held in the treasury of the Company, (B) that at the commencement of the Offer were owned by Parent or Merger Sub, (C) irrevocably accepted for payment in the Offer, or (D) constituting Appraisal Shares (as defined in the Merger Agreement) (collectively, the “Excluded Shares”)) will be converted into the right to receive an amount in cash and CVRs equal to the Offer Price, without interest. The terms and conditions of the Transactions are more fully set forth in the Merger Agreement and the CVR Agreement, and capitalized terms used but not defined herein shall have the meanings ascribed to such terms in the Merger Agreement.
You have requested our opinion, as of the date hereof, as to the fairness, from a financial point of view, to the holders of Company Common Stock (other than Excluded Shares) of the Offer Price to be received by such holders pursuant to the Transactions.
In the course of performing our review and analyses for rendering the opinion set forth below, we have:
(i)
| reviewed the financial terms of a draft copy of the Merger Agreement delivered to us on August 12, 2023, which was the most recent draft available to us (the “Draft Merger Agreement”), and the financial terms of a draft copy of the CVR Agreement delivered to us on August 12, 2023, which was the most recent draft made available to us (the “Draft CVR Agreement”); |
(ii)
| reviewed certain publicly available business and financial information concerning the Company and the industry in which it operates; |
(iii)
| reviewed certain internal financial analyses and forecasts prepared by and provided to us by the management of the Company relating to its business (the “Company Projections”); |
(iv)
| conducted discussions with members of senior management and representatives of the Company concerning the matters described in clauses (ii)-(iii) above and any other matters we deemed relevant; |
(v)
| reviewed and analyzed the reported current and historical prices and trading history of shares of the Company Common Stock; |
(vi)
| reviewed and analyzed, based on the Company Projections, the projected cash flows of the Company to determine the present value of the Company’s discounted cash flows; |
(vii)
| reviewed and analyzed the financial performance of the Company as compared to publicly available information for certain other publicly-traded companies that we deemed relevant; |
TABLE OF CONTENTS
(viii)
| reviewed and analyzed the proposed financial terms of the Transactions as compared to the financial terms of certain selected business combinations that we deemed relevant and the consideration paid in such transactions; and |
(ix)
| performed such other financial studies, analyses and investigations and considered such other information as we deemed appropriate for the purposes of the opinion set forth below. |
In arriving at the opinion set forth below, we have assumed and relied upon, without assuming liability or responsibility for independent verification, the accuracy and completeness of all of the financial, legal, regulatory, tax, accounting and other information that was publicly available or was provided to, discussed with or reviewed by us and upon the assurances of the management of the Company that they are not aware of any material relevant developments or matters related to the Company or that may affect any of the Transactions that has been omitted or that remains undisclosed to us. The opinion set forth below does not address any legal, regulatory, tax, accounting or financial reporting matters, as to which we understand the Company has obtained such advice as it deemed necessary from other advisors, and we have relied with your consent on any assessments made by such other advisors to the Company with respect to such matters. Without limiting the foregoing, we have not considered any tax effects of the Transactions or the form or transaction structure of the Transactions on any person or entity. We have not conducted any independent verification of the Company Projections, and we express no view as to the Company Projections or the assumptions on which they are based. Without limiting the generality of the foregoing, with respect to the Company Projections, we have assumed, with your consent and based upon discussions with the Company’s management, that they have been reasonably prepared in good faith and that the Company Projections reflect the best currently available estimates and judgments of the management of the Company of the future results of operations and financial performance of the Company.
In arriving at the opinion set forth below, we have made no analysis of, and express no opinion as to, the adequacy of the reserves of the Company and have relied upon information supplied to us by the Company as to such adequacy. In addition, we have not made any independent evaluations or appraisals of the assets or liabilities (including any contingent, derivative or off-balance-sheet assets or liabilities) of the Company or its subsidiary, and we have not been furnished with any such evaluations or appraisals, nor have we evaluated the solvency of the Company or any other entity under any state or federal law relating to bankruptcy, insolvency or similar matters. The analyses performed by us in connection with the opinion set forth below were going concern analyses. We express no opinion regarding the liquidation value of the Company or any other entity. We have assumed that there has been no material change in the assets, financial condition, business or prospects of the Company or its subsidiary since the date of the most recent relevant financial statements or financial information made available to us. Without limiting the generality of the foregoing, we have undertaken no independent analysis of any pending or threatened litigation, regulatory action, possible unasserted claims or other contingent liabilities to which the Company or any of its affiliates is a party or may be subject, and, at the direction of the Company and with your consent, the opinion set forth below makes no assumption concerning, and therefore does not consider, the possible assertion of claims, outcomes or damages arising out of any such matters. We have also assumed that neither the Company nor Parent, nor any of their respective subsidiaries, is party to any material pending transaction that has not been disclosed to us, including any financing, recapitalization, acquisition or merger, divestiture or spin-off, other than the Transactions. We have not conducted, nor have we assumed any obligation to conduct, any physical inspection of the properties or facilities of the Company or its subsidiary. We also have not considered any potential legislative or regulatory changes currently being considered or that may be adopted by any governmental or regulatory bodies or any potential changes in accounting methods or generally accepted accounting principles that may be adopted.
We have assumed that the representations and warranties of each party contained in each of the Merger Agreement, the CVR Agreement and in all other related documents and instruments that are referred to therein are and will be true and correct as of the date or the dates made or deemed made, that each party thereto will fully and timely perform all of the covenants and agreements required to be performed by it under the Merger Agreement, the CVR Agreement and any other agreement contemplated thereby, that all conditions to the consummation of any of the Transactions will be satisfied without waiver thereof and that the Transactions will be consummated in accordance with the terms of the Merger Agreement and the CVR Agreement without waiver, modification or amendment of any term, condition or agreement thereof. We have assumed that the final form of each of the Merger Agreement and the CVR Agreement will be, in all respects relevant to our analysis, identical to the Draft Merger Agreement and the Draft CVR Agreement, respectively. We have also assumed that any governmental, regulatory and other consents and approvals contemplated in connection with any of the Transactions will be obtained and that, in the course of
TABLE OF CONTENTS
obtaining any of those consents and approvals, no restrictions will be imposed or waivers made that would have an adverse effect on the Company, Parent or the benefits contemplated to be realized as a result of the Transactions. For purposes of the opinion set forth below, we have assumed, at your direction and with your consent, that achievement of the conditions for each Milestone Payment will occur as and to the extent contemplated by the Company Projections and that the corresponding Milestone Payments will be paid to each holder of a CVR to the extent thereof.
The opinion set forth below is necessarily based on economic, market, financial and other conditions as they exist, and on the information made available to us, as of the date of this letter. It should be understood that, although subsequent developments may affect the conclusion reached in such opinion, we do not have any obligation to update, revise or reaffirm the opinion set forth below.
The opinion set forth below addresses solely the fairness, from a financial point of view, to the holders of Company Common Stock (other than Excluded Shares) of the Offer Price to be received by such holders pursuant to the Transactions, and does not address any other terms in the Merger Agreement, the CVR Agreement or any other agreement relating to any of the Transactions or any other aspect or implication of any of the Transactions, including any financing arrangements to be entered into in connection with the Transactions. The opinion set forth below does not address the Company’s underlying business decision to proceed with the Transactions or the relative merits of the Transactions compared to other alternatives available to the Company. We express no opinion as to the prices or ranges of prices at which shares or other securities of any person, including shares of the Company Common Stock, will trade at any time, including following the announcement or consummation of any of the Transactions. We have not been requested to opine as to, and the opinion set forth below does not in any manner address, the amount or nature of compensation to any of the officers, directors or employees of any party to any of the Transactions, or any class of such persons, relative to the consideration to be received by the holders of Company Common Stock in connection with the Transactions or with respect to the fairness of any such compensation.
It is understood that this letter and the opinion set forth below are provided to the Board of Directors of the Company (in its capacity as such) for its information in connection with its consideration of the Transactions and may not be used for any other purpose or disclosed, referred to, or communicated (in whole or in part) to any third party for any purpose whatsoever without our prior written consent, except that a copy of this letter may be included in its entirety in any filing the Company is required to make with the Securities and Exchange Commission in connection with the Transactions if such inclusion is required by applicable law. The opinion set forth below does not constitute a recommendation to the Board of Directors of the Company, any committee thereof or any stockholder of the Company as to how to vote or take any other action in connection with any of the Transactions, including whether any stockholder should tender his, her or its shares in the Offer.
As part of our investment banking services, we are regularly engaged in the valuation of businesses and securities in connection with mergers and acquisitions, and for other purposes. We have acted as the Company’s financial advisor in connection with the Transactions and will receive a fee for our services (the “Transaction Fee”), a significant portion of which is contingent upon consummation of the Transactions. We will receive a fee for rendering the opinion set forth below which is not contingent upon consummation of the Transactions but is creditable against the Transaction Fee. In addition, the Company has agreed to reimburse our expenses and indemnify us for certain liabilities that may arise out of our engagement. We may also seek to provide investment banking and/or financial advisory services to the Company, Parent or their respective affiliates in the future and would expect to receive fees for the rendering of any such services.
The opinion set forth below was reviewed and approved by a fairness committee of MTS Securities, LLC.
Based upon and subject to the foregoing, it is our opinion, as of the date hereof, that the Offer Price to be received by the holders of Company Common Stock (other than Excluded Shares) pursuant to the Transactions is fair, from a financial point of view, to such holders.
Very truly yours,
/s/ MTS Securities, LLC
MTS SECURITIES, LLC
TABLE OF CONTENTS
§ 262. Appraisal rights
(a)
| Any stockholder of a corporation of this State who holds shares of stock on the date of the making of a demand pursuant to subsection (d) of this section with respect to such shares, who continuously holds such shares through the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, who has otherwise complied with subsection (d) of this section and who has neither voted in favor of the merger, consolidation, conversion, transfer, domestication or continuance nor consented thereto in writing pursuant to § 228 of this title shall be entitled to an appraisal by the Court of Chancery of the fair value of the stockholder’s shares of stock under the circumstances described in subsections (b) and (c) of this section. As used in this section, the word “stockholder” means a holder of record of stock in a corporation; the words “stock” and “share” mean and include what is ordinarily meant by those words; the words “depository receipt” mean a receipt or other instrument issued by a depository representing an interest in 1 or more shares, or fractions thereof, solely of stock of a corporation, which stock is deposited with the depository; the words “beneficial owner” mean a person who is the beneficial owner of shares of stock held either in voting trust or by a nominee on behalf of such person; and the word “person” means any individual, corporation, partnership, unincorporated association or other entity. |
(b)
| Appraisal rights shall be available for the shares of any class or series of stock of a constituent, converting, transferring, domesticating or continuing corporation in a merger, consolidation, conversion, transfer, domestication or continuance to be effected pursuant to § 251 (other than a merger effected pursuant to § 251(g) of this title), § 252, § 254, § 255, § 256, § 257, § 258, § 263, § 264, § 266 or § 390 of this title (other than, in each case and solely with respect to a converted or domesticated corporation, a merger, consolidation, conversion, transfer, domestication or continuance authorized pursuant to and in accordance with the provisions of § 265 or § 388 of this title): |
(1)
| Provided, however, that no appraisal rights under this section shall be available for the shares of any class or series of stock, which stock, or depository receipts in respect thereof, at the record date fixed to determine the stockholders entitled to receive notice of the meeting of stockholders, or at the record date fixed to determine the stockholders entitled to consent pursuant to § 228 of this title, to act upon the agreement of merger or consolidation or the resolution providing for the conversion, transfer, domestication or continuance (or, in the case of a merger pursuant to § 251(h) of this title, as of immediately prior to the execution of the agreement of merger), were either: (i) listed on a national securities exchange or (ii) held of record by more than 2,000 holders; and further provided that no appraisal rights shall be available for any shares of stock of the constituent corporation surviving a merger if the merger did not require for its approval the vote of the stockholders of the surviving corporation as provided in § 251(f) of this title. |
(2)
| Notwithstanding paragraph (b)(1) of this section, appraisal rights under this section shall be available for the shares of any class or series of stock of a constituent, converting, transferring, domesticating or continuing corporation if the holders thereof are required by the terms of an agreement of merger or consolidation, or by the terms of a resolution providing for conversion, transfer, domestication or continuance, pursuant to § 251, § 252, § 254, § 255, § 256, § 257, § 258, § 263, § 264, § 266 or § 390 of this title to accept for such stock anything except: |
a.
| Shares of stock of the corporation surviving or resulting from such merger or consolidation, or of the converted entity or the entity resulting from a transfer, domestication or continuance if such entity is a corporation as a result of the conversion, transfer, domestication or continuance, or depository receipts in respect thereof; |
b.
| Shares of stock of any other corporation, or depository receipts in respect thereof, which shares of stock (or depository receipts in respect thereof) or depository receipts at the effective date of the merger, consolidation, conversion, transfer, domestication or continuance will be either listed on a national securities exchange or held of record by more than 2,000 holders; |
c.
| Cash in lieu of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2)a. and b. of this section; or |
d.
| Any combination of the shares of stock, depository receipts and cash in lieu of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2)a., b. and c. of this section. |
TABLE OF CONTENTS
(3)
| In the event all of the stock of a subsidiary Delaware corporation party to a merger effected under § 253 or § 267 of this title is not owned by the parent immediately prior to the merger, appraisal rights shall be available for the shares of the subsidiary Delaware corporation. |
(c)
| Any corporation may provide in its certificate of incorporation that appraisal rights under this section shall be available for the shares of any class or series of its stock as a result of an amendment to its certificate of incorporation, any merger or consolidation in which the corporation is a constituent corporation, the sale of all or substantially all of the assets of the corporation or a conversion effected pursuant to § 266 of this title or a transfer, domestication or continuance effected pursuant to § 390 of this title. If the certificate of incorporation contains such a provision, the provisions of this section, including those set forth in subsections (d), (e), and (g) of this section, shall apply as nearly as is practicable. |
(d)
| Appraisal rights shall be perfected as follows: |
(1)
| If a proposed merger, consolidation, conversion, transfer, domestication or continuance for which appraisal rights are provided under this section is to be submitted for approval at a meeting of stockholders, the corporation, not less than 20 days prior to the meeting, shall notify each of its stockholders who was such on the record date for notice of such meeting (or such members who received notice in accordance with § 255(c) of this title) with respect to shares for which appraisal rights are available pursuant to subsection (b) or (c) of this section that appraisal rights are available for any or all of the shares of the constituent corporations or the converting, transferring, domesticating or continuing corporation, and shall include in such notice either a copy of this section (and, if 1 of the constituent corporations or the converting corporation is a nonstock corporation, a copy of § 114 of this title) or information directing the stockholders to a publicly available electronic resource at which this section (and, § 114 of this title, if applicable) may be accessed without subscription or cost. Each stockholder electing to demand the appraisal of such stockholder’s shares shall deliver to the corporation, before the taking of the vote on the merger, consolidation, conversion, transfer, domestication or continuance, a written demand for appraisal of such stockholder’s shares; provided that a demand may be delivered to the corporation by electronic transmission if directed to an information processing system (if any) expressly designated for that purpose in such notice. Such demand will be sufficient if it reasonably informs the corporation of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such stockholder’s shares. A proxy or vote against the merger, consolidation, conversion, transfer, domestication or continuance shall not constitute such a demand. A stockholder electing to take such action must do so by a separate written demand as herein provided. Within 10 days after the effective date of such merger, consolidation, conversion, transfer, domestication or continuance, the surviving, resulting or converted entity shall notify each stockholder of each constituent or converting, transferring, domesticating or continuing corporation who has complied with this subsection and has not voted in favor of or consented to the merger, consolidation, conversion, transfer, domestication or continuance, and any beneficial owner who has demanded appraisal under paragraph (d)(3) of this section, of the date that the merger, consolidation or conversion has become effective; or |
(2)
| If the merger, consolidation, conversion, transfer, domestication or continuance was approved pursuant to § 228, § 251(h), § 253, or § 267 of this title, then either a constituent, converting, transferring, domesticating or continuing corporation before the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, or the surviving, resulting or converted entity within 10 days after such effective date, shall notify each stockholder of any class or series of stock of such constituent, converting, transferring, domesticating or continuing corporation who is entitled to appraisal rights of the approval of the merger, consolidation, conversion, transfer, domestication or continuance and that appraisal rights are available for any or all shares of such class or series of stock of such constituent, converting, transferring, domesticating or continuing corporation, and shall include in such notice either a copy of this section (and, if 1 of the constituent corporations or the converting, transferring, domesticating or continuing corporation is a nonstock corporation, a copy of § 114 of this title) or information directing the stockholders to a publicly available electronic resource at which this section (and § 114 of this title, if applicable) may be accessed without subscription or cost. Such notice may, and, if given on or after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, shall, also notify such stockholders of the effective date of the merger, consolidation, conversion, transfer, domestication or |
TABLE OF CONTENTS
continuance. Any stockholder entitled to appraisal rights may, within 20 days after the date of giving such notice or, in the case of a merger approved pursuant to § 251(h) of this title, within the later of the consummation of the offer contemplated by § 251(h) of this title and 20 days after the date of giving such notice, demand in writing from the surviving, resulting or converted entity the appraisal of such holder’s shares; provided that a demand may be delivered to such entity by electronic transmission if directed to an information processing system (if any) expressly designated for that purpose in such notice. Such demand will be sufficient if it reasonably informs such entity of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such holder’s shares. If such notice did not notify stockholders of the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, either (i) each such constituent corporation or the converting, transferring, domesticating or continuing corporation shall send a second notice before the effective date of the merger, consolidation, conversion, transfer, domestication or continuance notifying each of the holders of any class or series of stock of such constituent, converting, transferring, domesticating or continuing corporation that are entitled to appraisal rights of the effective date of the merger, consolidation, conversion, transfer, domestication or continuance or (ii) the surviving, resulting or converted entity shall send such a second notice to all such holders on or within 10 days after such effective date; provided, however, that if such second notice is sent more than 20 days following the sending of the first notice or, in the case of a merger approved pursuant to § 251(h) of this title, later than the later of the consummation of the offer contemplated by § 251(h) of this title and 20 days following the sending of the first notice, such second notice need only be sent to each stockholder who is entitled to appraisal rights and who has demanded appraisal of such holder’s shares in accordance with this subsection and any beneficial owner who has demanded appraisal under paragraph (d)(3) of this section. An affidavit of the secretary or assistant secretary or of the transfer agent of the corporation or entity that is required to give either notice that such notice has been given shall, in the absence of fraud, be prima facie evidence of the facts stated therein. For purposes of determining the stockholders entitled to receive either notice, each constituent corporation or the converting, transferring, domesticating or continuing corporation may fix, in advance, a record date that shall be not more than 10 days prior to the date the notice is given, provided, that if the notice is given on or after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, the record date shall be such effective date. If no record date is fixed and the notice is given prior to the effective date, the record date shall be the close of business on the day next preceding the day on which the notice is given.
(3)
| Notwithstanding subsection (a) of this section (but subject to this paragraph (d)(3)), a beneficial owner may, in such person’s name, demand in writing an appraisal of such beneficial owner’s shares in accordance with either paragraph (d)(1) or (2) of this section, as applicable; provided that (i) such beneficial owner continuously owns such shares through the effective date of the merger, consolidation, conversion, transfer, domestication or continuance and otherwise satisfies the requirements applicable to a stockholder under the first sentence of subsection (a) of this section and (ii) the demand made by such beneficial owner reasonably identifies the holder of record of the shares for which the demand is made, is accompanied by documentary evidence of such beneficial owner’s beneficial ownership of stock and a statement that such documentary evidence is a true and correct copy of what it purports to be, and provides an address at which such beneficial owner consents to receive notices given by the surviving, resulting or converted entity hereunder and to be set forth on the verified list required by subsection (f) of this section. |
(e)
| Within 120 days after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, the surviving, resulting or converted entity, or any person who has complied with subsections (a) and (d) of this section and who is otherwise entitled to appraisal rights, may commence an appraisal proceeding by filing a petition in the Court of Chancery demanding a determination of the value of the stock of all such stockholders. Notwithstanding the foregoing, at any time within 60 days after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, any person entitled to appraisal rights who has not commenced an appraisal proceeding or joined that proceeding as a named party shall have the right to withdraw such person’s demand for appraisal and to accept the terms offered upon the merger, consolidation, conversion, transfer, domestication or continuance. Within 120 days after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, any person who has complied with the requirements of subsections (a) and (d) of this section, upon request given in writing (or by electronic transmission directed to an information processing system (if any) expressly designated for that purpose in the notice of appraisal), shall be entitled to receive from the surviving, resulting or converted entity a statement |
TABLE OF CONTENTS
setting forth the aggregate number of shares not voted in favor of the merger, consolidation, conversion, transfer, domestication or continuance (or, in the case of a merger approved pursuant to § 251(h) of this title, the aggregate number of shares (other than any excluded stock (as defined in § 251(h)(6)d. of this title)) that were the subject of, and were not tendered into, and accepted for purchase or exchange in, the offer referred to in § 251(h)(2) of this title), and, in either case, with respect to which demands for appraisal have been received and the aggregate number of stockholders or beneficial owners holding or owning such shares (provided that, where a beneficial owner makes a demand pursuant to paragraph (d)(3) of this section, the record holder of such shares shall not be considered a separate stockholder holding such shares for purposes of such aggregate number). Such statement shall be given to the person within 10 days after such person’s request for such a statement is received by the surviving, resulting or converted entity or within 10 days after expiration of the period for delivery of demands for appraisal under subsection (d) of this section, whichever is later.
(f)
| Upon the filing of any such petition by any person other than the surviving, resulting or converted entity, service of a copy thereof shall be made upon such entity, which shall within 20 days after such service file in the office of the Register in Chancery in which the petition was filed a duly verified list containing the names and addresses of all persons who have demanded appraisal for their shares and with whom agreements as to the value of their shares have not been reached by such entity. If the petition shall be filed by the surviving, resulting or converted entity, the petition shall be accompanied by such a duly verified list. The Register in Chancery, if so ordered by the Court, shall give notice of the time and place fixed for the hearing of such petition by registered or certified mail to the surviving, resulting or converted entity and to the persons shown on the list at the addresses therein stated. The forms of the notices by mail and by publication shall be approved by the Court, and the costs thereof shall be borne by the surviving, resulting or converted entity. |
(g)
| At the hearing on such petition, the Court shall determine the persons who have complied with this section and who have become entitled to appraisal rights. The Court may require the persons who have demanded an appraisal for their shares and who hold stock represented by certificates to submit their certificates of stock to the Register in Chancery for notation thereon of the pendency of the appraisal proceedings; and if any person fails to comply with such direction, the Court may dismiss the proceedings as to such person. If immediately before the merger, consolidation, conversion, transfer, domestication or continuance the shares of the class or series of stock of the constituent, converting, transferring, domesticating or continuing corporation as to which appraisal rights are available were listed on a national securities exchange, the Court shall dismiss the proceedings as to all holders of such shares who are otherwise entitled to appraisal rights unless (1) the total number of shares entitled to appraisal exceeds 1% of the outstanding shares of the class or series eligible for appraisal, (2) the value of the consideration provided in the merger, consolidation, conversion, transfer, domestication or continuance for such total number of shares exceeds $1 million, or (3) the merger was approved pursuant to § 253 or § 267 of this title. |
(h)
| After the Court determines the persons entitled to an appraisal, the appraisal proceeding shall be conducted in accordance with the rules of the Court of Chancery, including any rules specifically governing appraisal proceedings. Through such proceeding the Court shall determine the fair value of the shares exclusive of any element of value arising from the accomplishment or expectation of the merger, consolidation, conversion, transfer, domestication or continuance, together with interest, if any, to be paid upon the amount determined to be the fair value. In determining such fair value, the Court shall take into account all relevant factors. Unless the Court in its discretion determines otherwise for good cause shown, and except as provided in this subsection, interest from the effective date of the merger, consolidation, conversion, transfer, domestication or continuance through the date of payment of the judgment shall be compounded quarterly and shall accrue at 5% over the Federal Reserve discount rate (including any surcharge) as established from time to time during the period between the effective date of the merger, consolidation or conversion and the date of payment of the judgment. At any time before the entry of judgment in the proceedings, the surviving, resulting or converted entity may pay to each person entitled to appraisal an amount in cash, in which case interest shall accrue thereafter as provided herein only upon the sum of (1) the difference, if any, between the amount so paid and the fair value of the shares as determined by the Court, and (2) interest theretofore accrued, unless paid at that time. Upon application by the surviving, resulting or converted entity or by any person entitled to participate in the appraisal proceeding, the Court may, in its discretion, proceed to trial upon the appraisal prior to the final determination of the persons entitled to an appraisal. Any person whose name appears on the list filed by the surviving, resulting or converted entity pursuant to subsection (f) of this section may participate fully in all proceedings until it is finally determined that such person is not entitled to appraisal rights under this section. |
TABLE OF CONTENTS
(i)
| The Court shall direct the payment of the fair value of the shares, together with interest, if any, by the surviving, resulting or converted entity to the persons entitled thereto. Payment shall be so made to each such person upon such terms and conditions as the Court may order. The Court’s decree may be enforced as other decrees in the Court of Chancery may be enforced, whether such surviving, resulting or converted entity be an entity of this State or of any state. |
(j)
| The costs of the proceeding may be determined by the Court and taxed upon the parties as the Court deems equitable in the circumstances. Upon application of a person whose name appears on the list filed by the surviving, resulting or converted entity pursuant to subsection (f) of this section who participated in the proceeding and incurred expenses in connection therewith, the Court may order all or a portion of such expenses, including, without limitation, reasonable attorney’s fees and the fees and expenses of experts, to be charged pro rata against the value of all the shares entitled to an appraisal not dismissed pursuant to subsection (k) of this section or subject to such an award pursuant to a reservation of jurisdiction under subsection (k) of this section. |
(k)
| Subject to the remainder of this subsection, from and after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, no person who has demanded appraisal rights with respect to some or all of such person’s shares as provided in subsection (d) of this section shall be entitled to vote such shares for any purpose or to receive payment of dividends or other distributions on such shares (except dividends or other distributions payable to stockholders of record at a date which is prior to the effective date of the merger, consolidation, conversion, transfer, domestication or continuance). If a person who has made a demand for an appraisal in accordance with this section shall deliver to the surviving, resulting or converted entity a written withdrawal of such person’s demand for an appraisal in respect of some or all of such person’s shares in accordance with subsection (e) of this section, either within 60 days after such effective date or thereafter with the written approval of the corporation, then the right of such person to an appraisal of the shares subject to the withdrawal shall cease. Notwithstanding the foregoing, an appraisal proceeding in the Court of Chancery shall not be dismissed as to any person without the approval of the Court, and such approval may be conditioned upon such terms as the Court deems just, including without limitation, a reservation of jurisdiction for any application to the Court made under subsection (j) of this section; provided, however that this provision shall not affect the right of any person who has not commenced an appraisal proceeding or joined that proceeding as a named party to withdraw such person’s demand for appraisal and to accept the terms offered upon the merger, consolidation, conversion, transfer, domestication or continuance within 60 days after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, as set forth in subsection (e) of this section. If a petition for an appraisal is not filed within the time provided in subsection (e) of this section, the right to appraisal with respect to all shares shall cease. |
(l)
| The shares or other equity interests of the surviving, resulting or converted entity to which the shares of stock subject to appraisal under this section would have otherwise converted but for an appraisal demand made in accordance with this section shall have the status of authorized but not outstanding shares of stock or other equity interests of the surviving, resulting or converted entity, unless and until the person that has demanded appraisal is no longer entitled to appraisal pursuant to this section. |
Exhibit (e) (12)
AMENDMENT TO
EMPLOYMENT AGREEMENT
THIS AMENDMENT (this “Amendment”) to the Employment Agreement by and between Zynerba Pharmaceuticals, Inc., a Delaware corporation (the “Company”), and Albert Parker (“Executive”),
dated February 14, 2022 (the “Employment Agreement”), is made as of August 14, 2023 (the “Effective Date”).
W I T N E S S E T H.
WHEREAS, the Company and the Executive are parties to the Employment Agreement; and
WHEREAS, the Company and the Executive desire to amend the Employment Agreement as set forth herein, effective as of the Effective Date.
NOW, THEREFORE, in consideration of the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as
follows:
1.
The first paragraph of Section 4(e) of the Employment Agreement is amended and restated in its entirety to read as follows:
“(e)
Effect of a Change of Control. Notwithstanding any provision of Section 4(d) to the contrary, if Employee’s employment is terminated by the
Employer in circumstances described in Section 4(d) or Employee resigns for Good Reason (as defined below) pursuant to Section 4(d) within the ninety (90) day period preceding a Change of Control or on or within twelve (12) months following a Change of
Control, upon such termination or resignation, Employee shall be entitled to the same payments and benefits described in Section 4(d) above, subject to execution and nonrevocation of the Release and the Employee’s compliance with all terms and
provisions of this Agreement that survive the termination of the Employee’s employment by the Employer, provided that, Employee shall be entitled to receive (i) in addition to the continuation of base salary set forth in Section 4(d)(iii)(A), an
additional six (6) months of Base Salary continuation (at the rate in effect on the Termination Date) commencing upon the conclusion of the continued Base Salary under Section 4(d)(iii)(A); (ii) in lieu of the benefits provided in Section 4(d)(iii)(B),
a lump sum cash payment equal to the full monthly COBRA premium applicable to the Employee (including any administrative fees permitted by applicable laws) multiplied by eighteen (18) months, and such payments
shall be subject to tax-related deductions and withholdings; (iii) notwithstanding anything to the contrary in any applicable option agreement or other stock-based award agreement, all restricted stock awards, stock options and other stock-based awards
held by the Employee (the “Unvested Equity Awards”) shall immediately accelerate and become fully exercisable or nonforfeitable as of the Termination Date; provided that any termination or forfeiture of
the unvested portion of such Unvested Equity Awards that would otherwise occur on the Termination Date in the absence of this Agreement (or the termination or forfeiture of the vested, but unexercised time-based equity awards that would otherwise occur
at the end of the applicable post-termination exercise period) will be delayed until the later of the date the Release becomes fully effective or the closing of the Change in Control (the “Closing”), and such forfeiture will only occur if the
vesting pursuant to this Agreement does not occur due to the absence of the Release becoming fully effective within the time period set forth therein or the Closing not occurring within three (3) months following the Termination Date; and provided further, that in the case of any performance-based stock option or other stock-based award (a “Performance-Based Award”), full vesting means vesting at target level; and provided further that, if the Termination Date occurs as of or following the last day of the applicable performance period but prior to vesting of any Performance-Based Award, full vesting will mean vesting at the level determined
based on actual performance as of the end of the performance period. Notwithstanding the foregoing, no additional vesting of the Unvested Equity Awards or Performance-Based Awards (other than as contemplated by this Section 4(e)) shall occur during the
period between the Termination Date and the later of the date the Release becomes fully effective or the Closing; (iv) all outstanding stock options and other equity-based awards held by the Employee as of the Termination Date that become vested
pursuant to (iii) above or that are vested as of the Termination Date shall remain exercisable (to the extent applicable) until the earlier of (x) the three (3) year anniversary of the Termination Date and (y) the expiration date of the relevant stock
option or other equity-based award; and (v) Employee shall be entitled to one hundred percent (100%) of Employee’s targeted annual bonus for the year in which the Termination Date occurs, without regard to whether the relevant Employee and Employer
goals have been achieved.”
2.
This Amendment may be executed in any number of counterparts, each of which shall be deemed an original and such counterparts together shall constitute one and the same
instrument.
3.
This Amendment, including the validity, interpretation, construction and performance of this Amendment, shall be governed by and construed in accordance with the laws of the
Commonwealth of Pennsylvania applicable to agreements made and to be performed in such State, without regard to such State’s conflicts of law principles.
4.
This Amendment shall be binding upon and inure to the benefit of and be enforceable by the respective successors and assigns of the parties hereto. The Employment Agreement, as
amended by this Amendment, embodies the entire agreement and understanding between the parties hereto and supersedes all prior agreements and understandings relating to the subject matter hereof.
5.
Except as provided herein, each of the other provisions of the Employment Agreement shall remain in full force and effect.
[remainder of page intentionally left blank; signature page follows]
SIGNATURE PAGE TO AMENDMENT TO EMPLOYMENT AGREEMENT
IN WITNESS WHEREOF, the parties hereto have executed this Amendment as of the Effective Date.
| |
ZYNERBA PHARMACEUTICALS, INC.
|
| |
|
|
| |
By:
|
/s/ Armando Anido
|
| |
Name:
|
Armando Anido
|
| |
Title:
|
Chief Executive Officer
|
| |
|
|
| |
EXECUTIVE
|
| |
|
|
| |
/s/ Albert Parker
|
| |
Albert Parker
|
3
Exhibit (e) (18)
AMENDMENT TO
EMPLOYMENT AGREEMENT
THIS AMENDMENT (this “Amendment”) to the Employment Agreement by and between Zynerba Pharmaceuticals, Inc., a Delaware corporation (the “Company”), and Brian Rosenberger (“Executive”),
originally dated January 18, 2017 and amended on August 30, 2019, (the “Employment Agreement”), is made as of August 14, 2023 (the “Effective Date”).
W I T N E S S E T H.
WHEREAS, the Company and the Executive are parties to the Employment Agreement; and
WHEREAS, the Company and the Executive desire to amend the Employment Agreement as set forth herein, effective as of the Effective Date.
NOW, THEREFORE, in consideration of the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as
follows:
1.
The first paragraph of Section 4(e) of the Employment Agreement is amended and restated in its entirety to read as follows:
“(e)
Effect of a Change of Control. Notwithstanding any provision of Section 4(d) to the contrary, if Employee’s employment is terminated by the
Employer in circumstances described in Section 4(d) or Employee resigns for Good Reason (as defined below) pursuant to Section 4(d) within the ninety (90) day period preceding a Change of Control or on or within twelve (12) months following a Change of
Control, upon such termination or resignation, Employee shall be entitled to the same payments and benefits described in Section 4(d) above, subject to execution and nonrevocation of the Release and the Employee’s compliance with all terms and
provisions of this Agreement that survive the termination of the Employee’s employment by the Employer, provided that, Employee shall be entitled to receive (i) in addition to the continuation of base salary set forth in Section 4(d)(iii)(A), an
additional six (6) months of Base Salary continuation (at the rate in effect on the Termination Date) commencing upon the conclusion of the continued Base Salary under Section 4(d)(iii)(A); (ii) in lieu of the benefits provided in Section 4(d)(iii)(B),
a lump sum cash payment equal to the full monthly COBRA premium applicable to the Employee (including any administrative fees permitted by applicable laws) multiplied by fifteen (15) months, and such payments
shall be subject to tax-related deductions and withholdings; (iii) notwithstanding anything to the contrary in any applicable option agreement or other stock-based award agreement, all restricted stock awards, stock options and other stock-based awards
held by the Employee (the “Unvested Equity Awards”) shall immediately accelerate and become fully exercisable or nonforfeitable as of the Termination Date; provided that any termination or forfeiture of
the unvested portion of such Unvested Equity Awards that would otherwise occur on the Termination Date in the absence of this Agreement (or the termination or forfeiture of the vested, but unexercised time-based equity awards that would otherwise occur
at the end of the applicable post-termination exercise period) will be delayed until the later of the date the Release becomes fully effective or the closing of the Change in Control (the “Closing”), and such forfeiture will only occur if the
vesting pursuant to this Agreement does not occur due to the absence of the Release becoming fully effective within the time period set forth therein or the Closing not occurring within three (3) months following the Termination Date; and provided further, that in the case of any performance-based stock option or other stock-based award (a “Performance-Based Award”), full vesting means vesting at target level; and
provided further that, if the Termination Date occurs as of or following the last day of the applicable performance period but prior to vesting of any Performance-Based Award, full vesting will mean vesting at the level determined based on
actual performance as of the end of the performance period. Notwithstanding the foregoing, no additional vesting of the Unvested Equity Awards or Performance-Based Awards (other than as contemplated by this Section 4(e)) shall occur during the period
between the Termination Date and the later of the date the Release becomes fully effective or the Closing; (iv) all outstanding stock options and other equity-based awards held by the Employee as of the Termination Date that become vested pursuant to
(iii) above or that are vested as of the Termination Date shall remain exercisable (to the extent applicable) until the earlier of (x) the three (3) year anniversary of the Termination Date and (y) the expiration date of the relevant stock option or
other equity-based award; and (v) Employee shall be entitled to one hundred percent (100%) of Employee’s targeted annual bonus for the year in which the Termination Date occurs, without regard to whether the relevant Employee and Employer goals have
been achieved.”
2.
This Amendment may be executed in any number of counterparts, each of which shall be deemed an original and such counterparts together shall constitute one and the same
instrument.
3.
This Amendment, including the validity, interpretation, construction and performance of this Amendment, shall be governed by and construed in accordance with the laws of the
Commonwealth of Pennsylvania applicable to agreements made and to be performed in such State, without regard to such State’s conflicts of law principles.
4.
This Amendment shall be binding upon and inure to the benefit of and be enforceable by the respective successors and assigns of the parties hereto. The Employment Agreement, as
amended by this Amendment, embodies the entire agreement and understanding between the parties hereto and supersedes all prior agreements and understandings relating to the subject matter hereof.
5.
Except as provided herein, each of the other provisions of the Employment Agreement shall remain in full force and effect.
[remainder of page intentionally left blank; signature page follows]
SIGNATURE PAGE TO AMENDMENT TO EMPLOYMENT AGREEMENT
IN WITNESS WHEREOF, the parties hereto have executed this Amendment as of the Effective Date.
| |
ZYNERBA PHARMACEUTICALS, INC.
|
| |
|
|
| |
By:
|
/s/ Armando Anido
|
| |
Name:
|
Armando Anido
|
| |
Title:
|
Chief Executive Officer
|
| |
|
|
| |
EXECUTIVE
|
| |
|
|
| |
/s/ Brian Rosenberger
|
| |
Brian Rosenberger
|
3
Zynerba Pharmaceuticals (NASDAQ:ZYNE)
Historical Stock Chart
From Jun 2024 to Jul 2024
Zynerba Pharmaceuticals (NASDAQ:ZYNE)
Historical Stock Chart
From Jul 2023 to Jul 2024