Taysha Gets FDA Fast-Track for Rett Syndrome Treatment
August 24 2023 - 8:52AM
Dow Jones News
By Dean Seal
Taysha Gene Therapies said regulators have granted fast-track
designation for TSHA-102, a gene transfer therapy in clinical
evaluation for the neurodevelopmental disorder Rett Syndrome.
The Dallas-based clinical-stage company said the special status
from the U.S. Food and Drug Administration has been granted after
encouraging initial data from the first adult patient in Canada
dosed with TSHA-102. A second patient is set to be dosed in the
current quarter.
The FDA's fast-track designation facilitates the development and
expedites the review of drugs that treat serious or
life-threatening conditions and fill unmet medical needs.
TSHA-102 also has received orphan-drug and
rare-pediatric-disease designations from the FDA, as well as
orphan-drug designation from the European Commission.
Shares climbed 8% to $2.40 in premarket trading.
Write to Dean Seal at dean.seal@wsj.com
(END) Dow Jones Newswires
August 24, 2023 08:37 ET (12:37 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
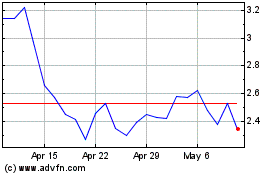
Taysha Gene Therapies (NASDAQ:TSHA)
Historical Stock Chart
From Jun 2024 to Jul 2024
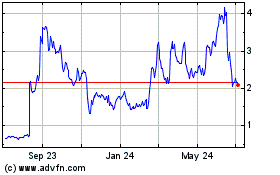
Taysha Gene Therapies (NASDAQ:TSHA)
Historical Stock Chart
From Jul 2023 to Jul 2024