Outlook Therapeutics® to Present at the H.C. Wainwright 25th Annual Global Investment Conference
September 05 2023 - 8:05AM

Outlook Therapeutics, Inc. (Nasdaq: OTLK), a biopharmaceutical
company working to achieve FDA approval for the first ophthalmic
formulation of bevacizumab for the treatment of retinal diseases,
today announced that Russell Trenary, President and CEO of Outlook
Therapeutics will present at the H.C. Wainwright 25th Annual Global
Investment Conference being held in New York, NY on Wednesday,
September 13, 2023 at 9:30 AM ET.
In addition to the presentation, management will
be available to participate in in-person one-on-one meetings with
qualified members of the investor community who are registered to
attend the conference. For more information about the conference,
please visit the conference website.
A live video webcast of the presentation will be
accessible on the Events page in the Investors section of the
Company’s website (outlooktherapeutics.com). A webcast replay will
be archived for 90 days following the event.
About Outlook Therapeutics,
Inc.
Outlook Therapeutics is a biopharmaceutical
company working to achieve FDA approval for the launch of ONS-5010/
LYTENAVA™ (bevacizumab-vikg) as the first FDA-approved ophthalmic
formulation of bevacizumab for use in retinal indications,
including wet AMD, DME and BRVO. The FDA accepted Outlook
Therapeutics’ BLA submission for ONS-5010 to treat wet AMD with an
initial PDUFA goal date of August 29, 2023; FDA did not approve the
BLA during this review cycle and the Company is working with the
FDA to address the issues that have been raised so that the BLA may
be re-submitted. The submission is supported by Outlook
Therapeutics’ wet AMD clinical program, which consists of three
clinical trials: NORSE ONE, NORSE TWO, and NORSE THREE. If ONS-5010
ophthalmic bevacizumab is approved, Outlook Therapeutics expects to
commercialize it as the first and only FDA-approved ophthalmic
formulation of bevacizumab for use in treating retinal diseases in
the United States, United Kingdom, Europe, Japan, and other
markets. As part of the Company’s multi-year commercial planning
process, Outlook Therapeutics and AmerisourceBergen entered into a
strategic commercialization agreement to expand the Company’s reach
for connecting to retina specialists and their patients.
AmerisourceBergen will provide third-party logistics (3PL) services
and distribution, as well as pharmacovigilance services and other
services in the United States. For more information, please visit
www.outlooktherapeutics.com.
CONTACTS:
Media Inquiries:Harriet UllmanVice
PresidentLaVoieHealthScienceT:
617.429.5475hullman@lavoiehealthscience.com
Investor
Inquiries:
Jenene Thomas Chief Executive Officer JTC Team, LLC T: 833.475.8247
OTLK@jtcir.com
Outlook Therapeutics (NASDAQ:OTLK)
Historical Stock Chart
From Jun 2024 to Jul 2024
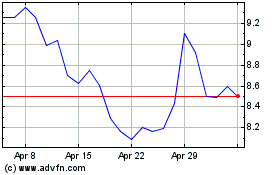
Outlook Therapeutics (NASDAQ:OTLK)
Historical Stock Chart
From Jul 2023 to Jul 2024