0001494650false00014946502024-08-082024-08-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________________________________________________________________
FORM 8-K
____________________________________________________________________________________________
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 8, 2024
__________________________________________________________________________________________
OPTINOSE, INC.
(Exact Name of Registrant as Specified in its Charter)
____________________________________________________________________________________________ | | | | | | | | |
| Delaware | 001-38241 | 42-1771610 |
| (State or Other Jurisdiction of Incorporation or Organization) | (Commission File No.) | (I.R.S. Employer Identification No.) |
777 Township Line Road, Suite 300
Yardley, Pennsylvania 19067
(Address of principal executive offices and zip code)
(267) 364-3500
(Registrant’s telephone number, including area code)
(Former name or former address, if changed from last report)
____________________________________________________________________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below): | | | | | |
| |
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| | |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| | |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| | |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-14(c)) |
| |
| Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). |
| |
| ☐ | Emerging growth company |
| |
| ☐ | If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. |
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | | | | | | | |
| Title of each class | | Trading symbol(s) | | Name of each exchange on which registered |
| Common stock, par value $0.001 per share | | OPTN | | Nasdaq Global Select Market |
Item 2.02 Results of Operations and Financial Condition.
On August 8, 2024, OptiNose, Inc. (the “Company”) issued a press release announcing its financial results for the quarter ended June 30, 2024. A copy of the press release is attached as Exhibit 99.1 to this report and is incorporated herein by reference.
* * *
The information included in Item 2.02 (including Exhibit 99.1) of this Form 8-K, shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed to be incorporated by reference in any Company filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 8.01 Other Events.
On August 8, 2024, the Company will present an updated Corporate Presentation during its financial results and corporate updates call. A copy of the presentation is attached as Exhibit 99.2 to this report and is incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits | | | | | | | | |
|
| | |
| Exhibit No. | | Description |
| 99.1 | | |
| 99.2 | | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| | | | | | | | |
|
| | |
| | | |
| | | OptiNose, Inc. |
| | By: /s/ Anthony J. Krick |
| | | Anthony J. Krick |
| | | Chief Accounting Officer |
Date: August 8, 2024
Optinose Reports Second Quarter 2024 Financial Results and Recent Operational Highlights
Company reports Q2 2024 XHANCE net revenue of $20.5 million, an increase of 5% compared to Q2 2023
XHANCE has been added to Express Scripts’ national formularies, among the largest commercial formularies in the U.S. with more than 24 million lives.
Company narrows full year 2024 XHANCE net revenue guidance to be between $85.0 to $90.0 million and increases expected average net revenue per prescription guidance to be at least $250
Conference call and webcast to be held today at 8:00 a.m. Eastern Time
YARDLEY, Pa., Aug. 8, 2024 Optinose (NASDAQ:OPTN), a pharmaceutical company focused on patients treated by ear, nose and throat (ENT) and allergy specialists, today reported financial results for the quarter ended June 30, 2024, and provided recent operational highlights.
“This is the first quarter in which we are executing on the launch of our new first-and-only label indication for chronic sinusitis, also called chronic rhinosinusitis without nasal polyps, which gives us access to a greatly expanded total addressable market,” stated CEO Ramy Mahmoud, MD, MPH. “We believe the addition of XHANCE to Express Scripts preferred formularies late in the second quarter is an example of gradually improving insurance barriers with our new first-and-only approval. Improving insurance barriers, in conjunction with consistent efforts to disseminate highly differentiated clinical results to both old and new prescribers, are important enablers of our future revenue growth trajectory."
Second Quarter 2024 and Recent Highlights
Improved Formulary Access
In June 2024, the Company announced that XHANCE® has been added to Express Scripts’ national formularies, including the National Preferred, Flex, and Basic formularies, among the largest commercial formularies in the U.S. with more than 24 million lives.
$55 Million Registered Direct Offering
On May 10, 2024, the Company completed a registered direct offering of its common stock and pre-funded common stock warrants to a group of existing and new institutional investors that resulted in approximately $55 million of net proceeds to the Company. The Company expects that its current cash and cash equivalents will be sufficient to fund its operations and debt service obligations through 2025.
Second Quarter 2024 Financial Results
Total revenues
The Company reported $20.5 million in net revenue from sales of XHANCE during the three-month period ended June 30, 2024, an increase of 5% compared to $19.5 million during the three-month period ended June
30, 2023. For the six-month period ended June 30, 2024, the Company reported $35.4 million in net revenue from sales of XHANCE, an increase of 13% compared to the six-month period ended June 30, 2023.
Costs and expenses and net loss
For the three-month and six-month periods ended June 30, 2024, research and development expenses were $0.9 million and $2.1 million, respectively. Selling, general and administrative expenses were $24.1 million and $44.6 million respectively for the three-month and six-month periods ended June 30, 2024. The increase of $4.0 million for the three-month period ended June 30, 2024 when compared to the three-month period ended June 30, 2023, is primarily attributable to an increase in sales and marketing expenses related to the launch of XHANCE as the first and only FDA-approved drug treatment for chronic rhinosinusitis without nasal polyps (CRSsNP) and increased stock-based compensation expense.
The net loss for the three-month period ended June 30, 2024 was $7.6 million, or $0.07 per share (diluted). The net loss for the six-month period ended June 30, 2024 was $21.6 million, or $0.17 per share (diluted).
Balance Sheet
The Company had cash and cash equivalents of $91.4 million as of June 30, 2024.
Financial Guidance
XHANCE Net Revenue
The Company expects XHANCE net revenues for the full year of 2024 to be between $85.0 to $90.0 million. Previously the Company expected XHANCE net revenues for the full year of 2024 to be between $85.0 to $95.0 million.
XHANCE Average Net Revenue per Prescription
The Company expects full year 2024 XHANCE average net revenue per prescription to exceed $250. Previously the Company expected full year 2024 XHANCE average net revenue per prescription to exceed $230.
Operating Expenses
The Company expects total GAAP operating expenses (selling, general & administrative expenses and research & development expenses) for 2024 to be between $95.0 to $101.0 million, of which the Company expects stock-based compensation to be approximately $6.0 million.
Company to Host Conference Call
Members of the Company’s leadership team will host a conference call and presentation to discuss financial results and corporate updates beginning at 8:00 a.m. Eastern Time today.
Participants may access the conference call live via webcast by visiting the Investors section of Optinose’s website at http://ir.optinose.com/presentations. To participate via telephone, please register in advance at this link. Upon registration, all telephone participants will receive a confirmation email detailing how to join the conference call, including the dial-in number and a personal PIN that can be used to access the call. In addition, a replay of the webcast will be available on the Company website for 60 days following the event.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| OptiNose, Inc. |
| Condensed Consolidated Statement of Operations |
| (in thousands, except share and per share data) |
| (Unaudited) |
| | | | | | | | |
| | Three Months Ended | | Six Months Ended |
| | June 30, | | June 30, |
| | 2024 | | 2023 | | 2024 | | 2023 |
| Revenues: | | | | | | | | |
| Net product revenues | | $ | 20,490 | | | $ | 19,454 | | | $ | 35,370 | | | $ | 31,299 | |
| | | | | | | | |
| Total revenues | | 20,490 | | | 19,454 | | | 35,370 | | | 31,299 | |
| Costs and expenses: | | | | | | | | |
| Cost of product sales | | $ | 1,981 | | | $ | 2,571 | | | $ | 3,212 | | | $ | 4,277 | |
| Research and development | | 928 | | 951 | | 2,134 | | | 2,736 | |
| Selling, general and administrative | | 24,129 | | | 20,104 | | | 44,647 | | | 42,828 | |
| Total costs and expenses | | 27,038 | | | 23,626 | | | 49,993 | | | 49,841 | |
| Loss from operations | | (6,548) | | | (4,172) | | | (14,623) | | | (18,542) | |
| Other (income) expense | | 1,033 | | | (6,798) | | | 7,026 | | | (2,318) | |
| Net (loss) income | | $ | (7,581) | | | $ | 2,626 | | | $ | (21,649) | | | $ | (16,224) | |
| | | | | | | | |
| Less: undistributed earnings to participating shareholders | | — | | | (53) | | | — | | | — | |
| Net (loss) income - basic | | $ | (7,581) | | | $ | 2,573 | | | $ | (21,649) | | | $ | (16,224) | |
| Net income (loss) per share of common stock - basic | | $ | (0.05) | | | $ | 0.02 | | | $ | (0.17) | | | $ | (0.15) | |
| Weighted average common shares outstanding - basic | | 147,455,374 | | | 111,979,778 | | | 130,025,113 | | | 111,877,669 | |
| | | | | | | | |
| Net (loss) income - basic | | $ | (7,581) | | | $ | 2,573 | | | $ | (21,649) | | | $ | (16,224) | |
| | | | | | | | |
| Add: Unrealized gain on the fair value of warrants | | (3,100) | | | — | | | (1,800) | | | — | |
| Net (loss) income - diluted | | $ | (10,681) | | | $ | 2,573 | | | $ | (23,449) | | | $ | (16,224) | |
| Net income (loss) per share of common stock - diluted | | $ | (0.07) | | | $ | 0.02 | | | $ | (0.17) | | | $ | (0.15) | |
| Weighted average common shares outstanding - diluted | | 150,698,374 | | | 112,042,097 | | | 136,918,539 | | | 111,877,669 | |
| | | | | | | | | | | | | | |
| OptiNose, Inc. |
| Condensed Consolidated Balance Sheet Data |
| (in thousands) |
| | | | |
| | June 30, | | December 31, |
| | 2024 | | 2023 |
| | (unaudited) | | |
| Cash and cash equivalents | | $ | 91,358 | | $ | 73,684 |
| Other assets | | 40,514 | | 34,045 |
| Total assets | | $ | 131,872 | | $107,729 |
| | | | |
Total current liabilities (1) | | $ | 32,700 | | $ | 176,524 |
Long term liabilities (1) | | 142,025 | | 17,811 |
| Total stockholders' equity | | (42,853) | | (86,606) |
| Total liabilities and stockholders' equity | | $ | 131,872 | | $ | 107,729 |
| | | | |
| (1) – All outstanding debt principal and fees payable upon debt maturity have been classified as a long term liability at June 30, 2024. All outstanding debt principal and fees payable upon debt maturity were classified as a current liability at December 31, 2023. Please refer to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, which will be filed after the issuance of this press release for additional information. |
About Optinose
Optinose is a specialty pharmaceutical company focused on serving the needs of patients cared for by ear, nose and throat (ENT) and allergy specialists. To learn more, please visit www.optinose.com or follow us on Twitter and LinkedIn.
About XHANCE
XHANCE is a drug-device combination product that uses the Exhalation Delivery System™ (also referred to as the EDS®) designed to deliver a topical steroid to the high and deep regions of the nasal cavity where sinuses ventilate and drain. XHANCE is approved by the U.S. Food and Drug Administration for both the treatment of chronic rhinosinusitis without nasal polyps (also called chronic sinusitis) and chronic rhinosinusitis with nasal polyps (also called nasal polyps) in patients 18 years of age or older.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS: Hypersensitivity to any ingredient in XHANCE.
WARNINGS AND PRECAUTIONS:
•Local nasal adverse reactions, including epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing, can occur. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.
•Glaucoma and cataracts may occur with long-term use. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use XHANCE long-term.
•Hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, contact dermatitis, rash, hypotension, and bronchospasm) have been reported after administration of fluticasone propionate. Discontinue XHANCE if such reactions occur.
•Immunosuppression and infections can occur, including potential increased susceptibility to or worsening of infections (e.g., existing tuberculosis; fungal, bacterial, viral, or parasitic infection; ocular herpes simplex).
Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients.
•Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue XHANCE slowly.
•Assess for decrease in bone mineral density initially and periodically thereafter.
ADVERSE REACTIONS:
•Chronic rhinosinusitis without nasal polyps: The most common adverse reactions (incidence ≥3%) are epistaxis, headache, and nasopharyngitis.
•Chronic rhinosinusitis with nasal polyps: The most common adverse reactions (incidence ≥3%) are epistaxis, nasal septal ulceration, nasopharyngitis, nasal mucosal erythema, nasal mucosal ulcerations, nasal congestion, acute sinusitis, nasal septal erythema, headache, and pharyngitis.
DRUG INTERACTIONS: Strong cytochrome P450 3A4 inhibitors (e.g., ritonavir, ketoconazole): Use not recommended. May increase risk of systemic corticosteroid effects.
USE IN SPECIFIC POPULATIONS: Hepatic impairment. Monitor patients for signs of increased drug exposure.
Please see full Prescribing Information, including Instructions for Use
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. All statements that are not historical facts are hereby identified as forward-looking statements for this purpose and include, among others, statements relating to the potential benefits of XHANCE as the first FDA-approved drug treatment for chronic rhinosinusitis without nasal polyps (also referred to as chronic sinusitis) and expanded market opportunities relating thereto; the potential benefits of XHANCE for the treatment of chronic sinusitis; potential for gradually improving insurance barriers and other enablers of future XHANCE net revenue growth; the potential benefits of the Exhalation Delivery System; the Company’s expectation for XHANCE net revenue and average net revenue per prescription for full year 2024; the Company’s expectations for GAAP operating expenses (selling, general and administrative expenses and research & development expenses) and stock-based compensation for 2024; the Company's expectation that its current cash and cash equivalents will be sufficient to fund its operations and debt service obligations through 2025; and other statements regarding the Company's future operations, financial performance, financial position, prospects, objectives, strategies and other future events. Forward-looking statements are based upon management’s current expectations and assumptions and are subject to a number of risks, uncertainties and other factors that could cause actual results and events to differ materially and adversely from those indicated by such forward-looking statements including, among others: physician and patient acceptance of XHANCE for its new indication; the Company’s ability to maintain adequate third-party reimbursement for XHANCE (including its new indication); the prevalence of chronic sinusitis and market opportunities for XHANCE may be smaller than expected; the Company’s ability to efficiently generate XHANCE prescriptions and net revenues; unanticipated costs and expenses; the Company's ability to achieve its financial guidance; potential for varying interpretation of the results from the ReOpen program; the Company’s ability to comply with the covenants and other terms of its Amended and Restated Note Purchase Agreement; risks and uncertainties relating to intellectual property and competitive products; and the risks, uncertainties and other factors discussed under the caption "Item 1A. Risk Factors" and elsewhere in the Company’s most recent Form 10-K and Form 10-Q filings with the Securities and Exchange Commission - which are available at www.sec.gov. As a result, you are cautioned not to place undue reliance on any forward-looking statements. Any forward-looking statements made in this press release speak only as of the date of this press release, and the Company undertakes no obligation to update such forward-looking statements, whether as a result of new information, future developments or otherwise.
Optinose Investor Contact
Jonathan Neely
jonathan.neely@optinose.com
267.521.0531
###

Building a Leading ENT / Allergy Specialty Company Corporate Presentation August 8, 2024

2 Forward-Looking Statements This presentation and our accompanying remarks contain “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. All statements that are not historical facts are hereby identified as forward-looking statements for this purpose and include, among others, statements relating to: the potential benefits of the recent FDA approval of XHANCE for the treatment of chronic rhinosinusitis without nasal polyps (also referred to as chronic sinusitis) and expanded market and growth opportunities related thereto; the benefit of XHANCE for treatment of chronic sinusitis; our commercial plans and expectations for the launch of XHANCE for its new indication; the generation of future XHANCE prescriptions and net revenues; projected GAAP operating expenses (selling, general and administrative expenses and research and development expenses) and stock-based compensation for 2024; projected XHANCE net revenues for 2024; projected XHANCE average net revenue per prescription for 2024; expectation for positive income from operations (GAAP) for full year 2025; expectation that current sales footprint of 75 territories and phased modest incremental commercial investment can grow XHANCE peak year net revenues to more than $300 million; expectation that cash and cash equivalents will be sufficient to fund operations and debt service obligations through 2025; impact of changes to XHANCE co-pay assistance program; and other statements regarding to our future operations, financial performance, prospects, intentions, strategies, objectives and other future events. Forward-looking statements are based upon management’s current expectations and assumptions and are subject to a number of risks, uncertainties and other factors that could cause actual results and events to differ materially and adversely from those indicated by such forward-looking statements including, among others: impact of, and the uncertainties caused by, physician and patient acceptance of XHANCE (including its new indication); our ability to maintain adequate third party reimbursement for XHANCE (including for its new indication); our ability to efficiently generate XHANCE prescriptions and net revenues; the prevalence of chronic sinusitis and market opportunities for XHANCE may be smaller than expected; unexpected costs and expenses; our ability to achieve our financial guidance; potential for varying interpretation of the results from the ReOpen Program; our ability to comply with the covenants and other terms of the A&R Pharmakon Note Purchase Agreement; risks and uncertainties relating to intellectual property and competition; and the risks, uncertainties and other factors discussed in the “Risk Factors” section and elsewhere in our most recent Form 10-K and Form 10-Q filings with the Securities and Exchange Commission (SEC) (including our Form 10-K to be filed with the SEC on March 7, 2024) – which are available at http://www.sec.gov. As a result, you are cautioned not to place undue reliance on any forward-looking statements. Any forward-looking statements made in this presentation speak only as of the date of this presentation, and we undertake no obligation to update such forward-looking statements, whether as a result of new information, future developments or otherwise. Market, Industry and Other Data This presentation and our accompanying remarks contain estimates, projections, market research and other information concerning markets for XHANCE and the size of those markets, the prevalence of certain medical conditions, XHANCE market access, and other physician, patient, payor and prescription data. Unless otherwise expressly stated, we obtain this information from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources, as well as from our own internal estimates and research.

3 Today’s Agenda Introduction and Key Takeaways Ramy Mahmoud, MD, MPH CS Launch Update Paul Spence Q2 2024 Performance and FY 2024 Financial Guidance Jonathan Neely Closing Remarks Ramy Mahmoud, MD, MPH
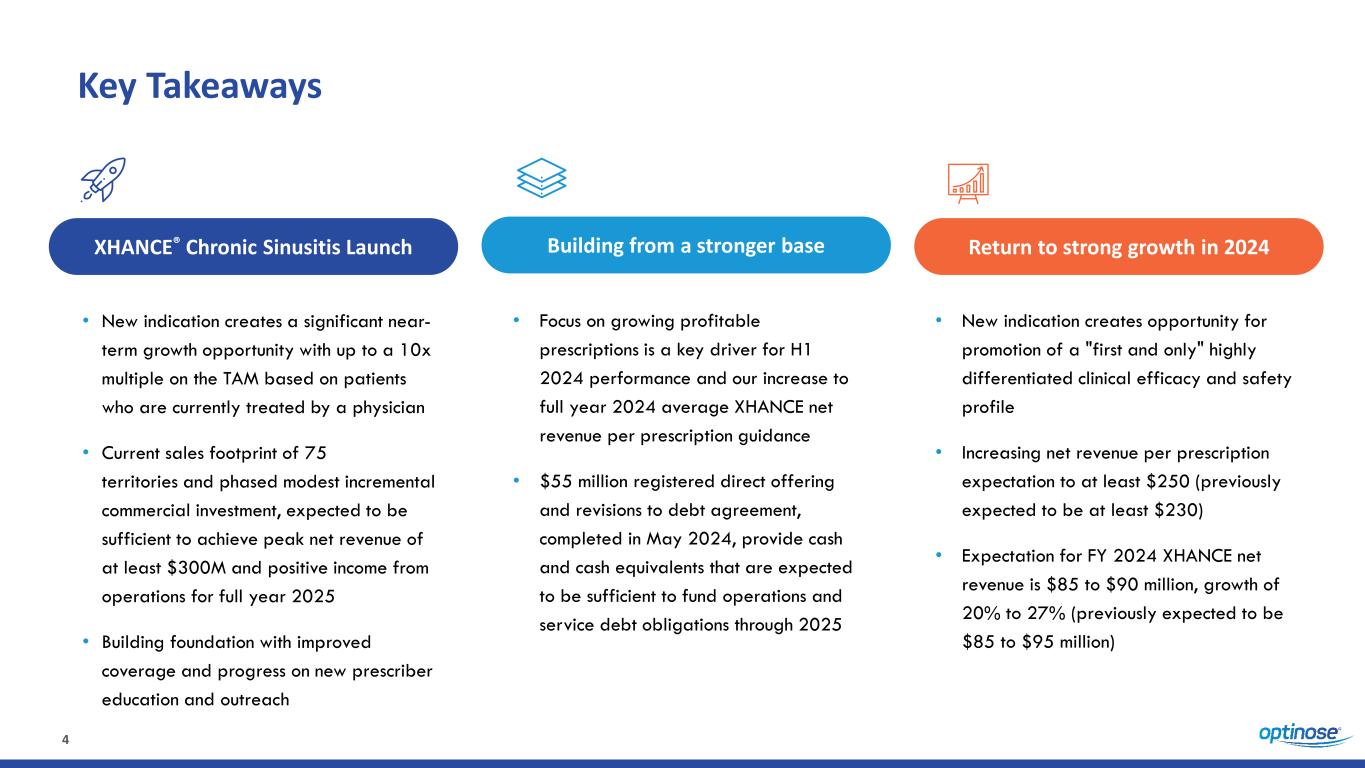
4 Key Takeaways • New indication creates a significant near- term growth opportunity with up to a 10x multiple on the TAM based on patients who are currently treated by a physician • Current sales footprint of 75 territories and phased modest incremental commercial investment, expected to be sufficient to achieve peak net revenue of at least $300M and positive income from operations for full year 2025 • Building foundation with improved coverage and progress on new prescriber education and outreach XHANCE® Chronic Sinusitis Launch Building from a stronger base Return to strong growth in 2024 • Focus on growing profitable prescriptions is a key driver for H1 2024 performance and our increase to full year 2024 average XHANCE net revenue per prescription guidance • $55 million registered direct offering and revisions to debt agreement, completed in May 2024, provide cash and cash equivalents that are expected to be sufficient to fund operations and service debt obligations through 2025 • New indication creates opportunity for promotion of a "first and only" highly differentiated clinical efficacy and safety profile • Increasing net revenue per prescription expectation to at least $250 (previously expected to be at least $230) • Expectation for FY 2024 XHANCE net revenue is $85 to $90 million, growth of 20% to 27% (previously expected to be $85 to $95 million)

CS Launch Updates
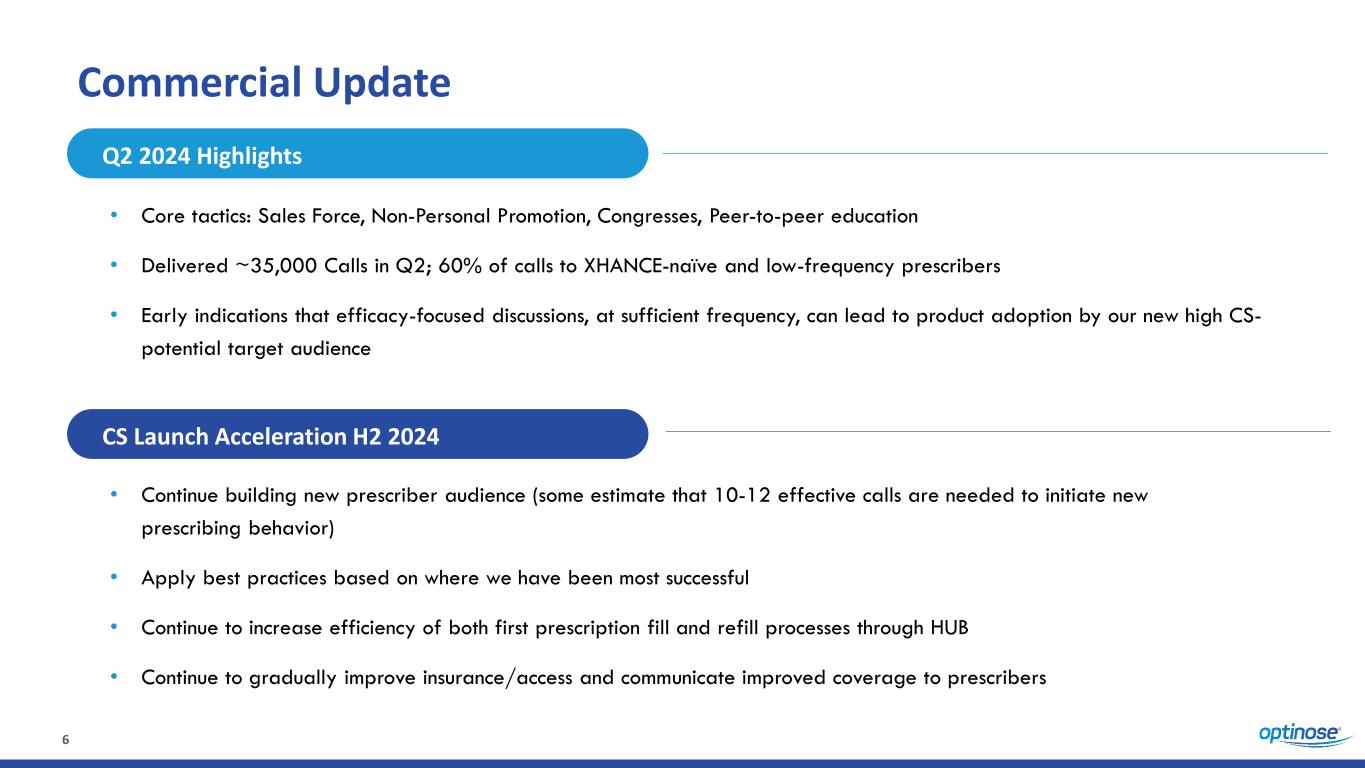
6 Commercial Update • Core tactics: Sales Force, Non-Personal Promotion, Congresses, Peer-to-peer education • Delivered ~35,000 Calls in Q2; 60% of calls to XHANCE-naïve and low-frequency prescribers • Early indications that efficacy-focused discussions, at sufficient frequency, can lead to product adoption by our new high CS- potential target audience • Continue building new prescriber audience (some estimate that 10-12 effective calls are needed to initiate new prescribing behavior) • Apply best practices based on where we have been most successful • Continue to increase efficiency of both first prescription fill and refill processes through HUB • Continue to gradually improve insurance/access and communicate improved coverage to prescribers Q2 2024 Highlights CS Launch Acceleration H2 2024

7 Addition of XHANCE to National Commercial Formularies As announced at the end of June, XHANCE was added to Express Scripts’ national formularies including the National Preferred, Flex, and Basic formularies These formularies are among the largest commercial formularies in the U.S. with more than 24 million lives First time with preferred coverage on a national formulary for XHANCE

Q2 2024 Performance
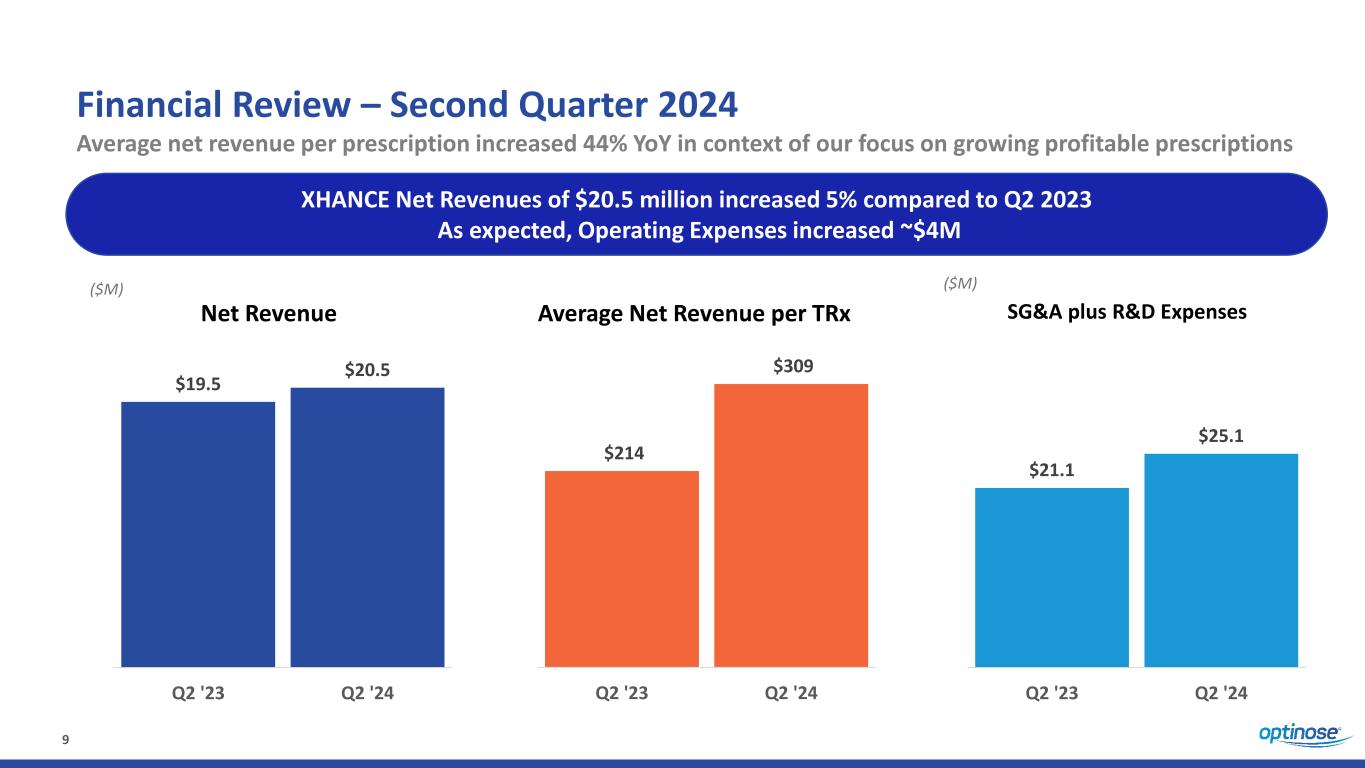
9 Financial Review – Second Quarter 2024 Average net revenue per prescription increased 44% YoY in context of our focus on growing profitable prescriptions XHANCE Net Revenues of $20.5 million increased 5% compared to Q2 2023 As expected, Operating Expenses increased ~$4M $21.1 $25.1 Q2 '23 Q2 '24 ($M) SG&A plus R&D Expenses $19.5 $20.5 Q2 '23 Q2 '24 ($M) Net Revenue Average Net Revenue per TRx $214 $309 Q2 '23 Q2 '24
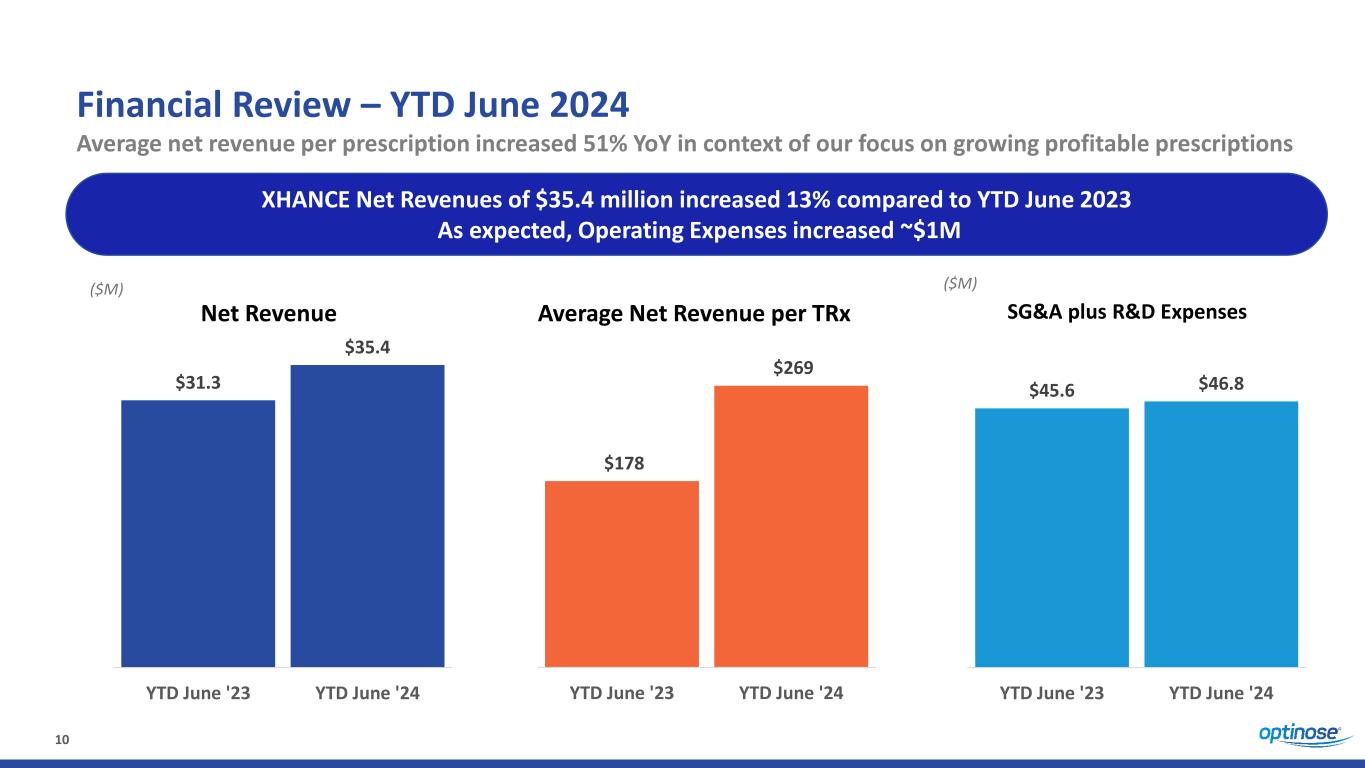
10 Financial Review – YTD June 2024 Average net revenue per prescription increased 51% YoY in context of our focus on growing profitable prescriptions XHANCE Net Revenues of $35.4 million increased 13% compared to YTD June 2023 As expected, Operating Expenses increased ~$1M $45.6 $46.8 YTD June '23 YTD June '24 ($M) SG&A plus R&D Expenses $31.3 $35.4 YTD June '23 YTD June '24 ($M) Net Revenue Average Net Revenue per TRx $178 $269 YTD June '23 YTD June '24

2024 Outlook
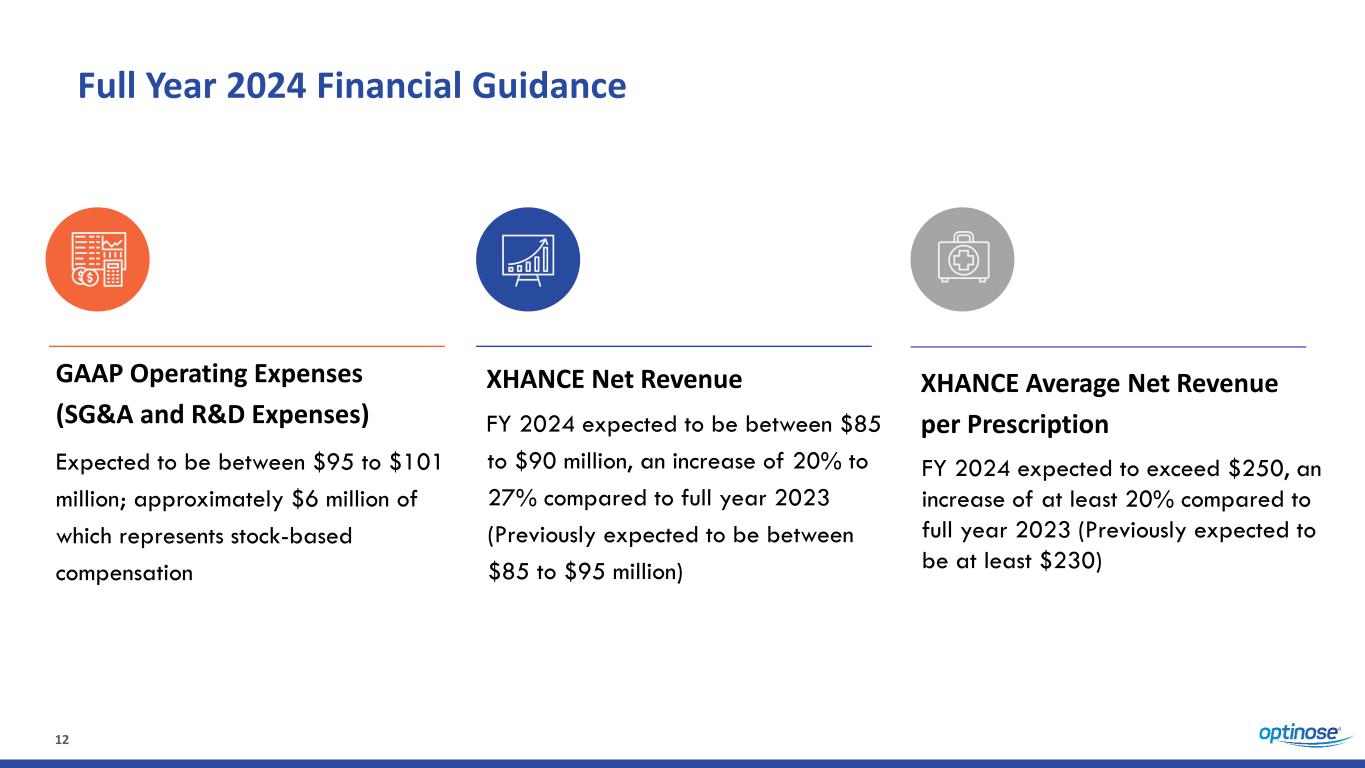
12 Full Year 2024 Financial Guidance GAAP Operating Expenses (SG&A and R&D Expenses) Expected to be between $95 to $101 million; approximately $6 million of which represents stock-based compensation XHANCE Net Revenue FY 2024 expected to be between $85 to $90 million, an increase of 20% to 27% compared to full year 2023 (Previously expected to be between $85 to $95 million) XHANCE Average Net Revenue per Prescription FY 2024 expected to exceed $250, an increase of at least 20% compared to full year 2023 (Previously expected to be at least $230)

Closing Remarks
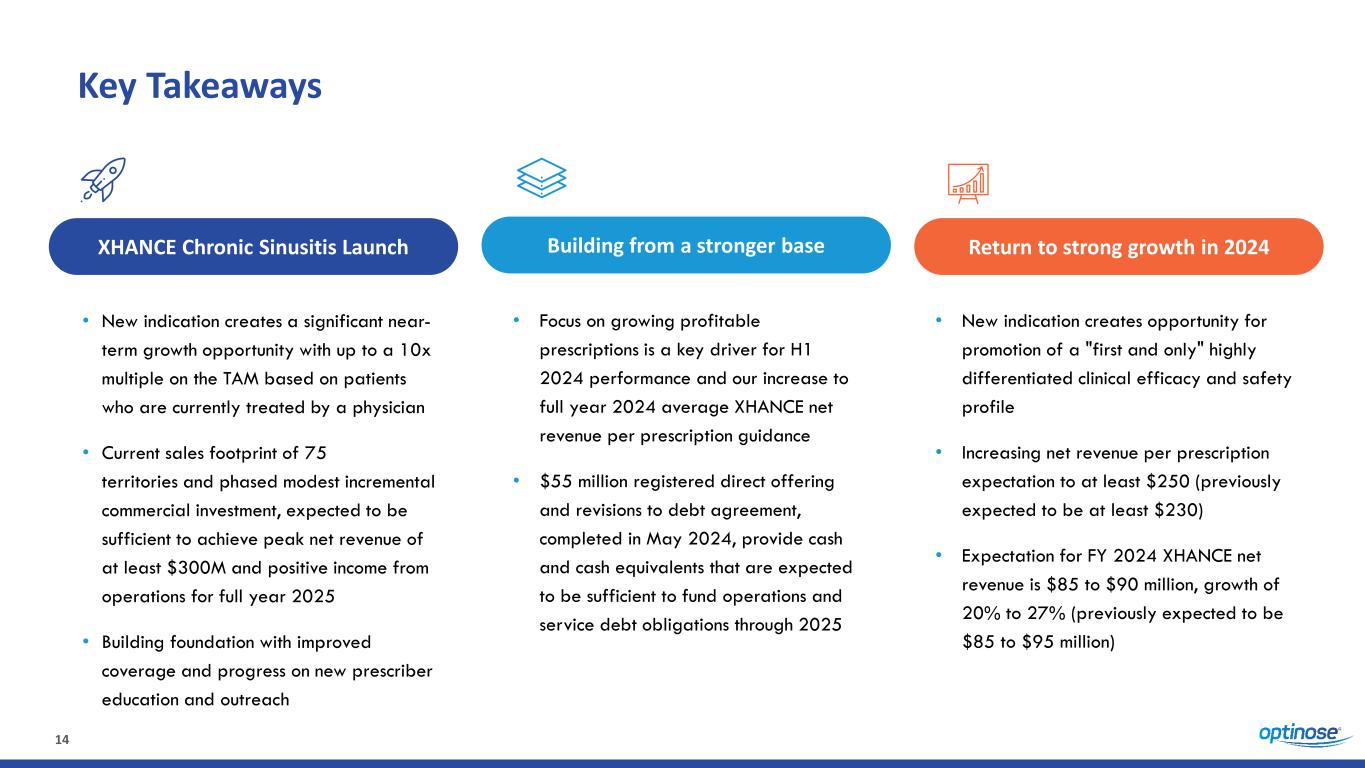
14 Key Takeaways • New indication creates a significant near- term growth opportunity with up to a 10x multiple on the TAM based on patients who are currently treated by a physician • Current sales footprint of 75 territories and phased modest incremental commercial investment, expected to be sufficient to achieve peak net revenue of at least $300M and positive income from operations for full year 2025 • Building foundation with improved coverage and progress on new prescriber education and outreach XHANCE Chronic Sinusitis Launch Building from a stronger base Return to strong growth in 2024 • Focus on growing profitable prescriptions is a key driver for H1 2024 performance and our increase to full year 2024 average XHANCE net revenue per prescription guidance • $55 million registered direct offering and revisions to debt agreement, completed in May 2024, provide cash and cash equivalents that are expected to be sufficient to fund operations and service debt obligations through 2025 • New indication creates opportunity for promotion of a "first and only" highly differentiated clinical efficacy and safety profile • Increasing net revenue per prescription expectation to at least $250 (previously expected to be at least $230) • Expectation for FY 2024 XHANCE net revenue is $85 to $90 million, growth of 20% to 27% (previously expected to be $85 to $95 million)

15 Investor Relations – NASDAQ: OPTN Optinose Investor ContactAnalyst Coverage 1 Jefferies: Glen Santangelo Lake Street: Thomas Flaten Piper Sandler: David Amsellem H. C. Wainwright: Matthew Caufield 1 - Optinose is followed by the analysts listed above. Please note that any opinions, estimates or forecasts regarding the Company’s performance made by these analysts are theirs alone and do not represent opinions, forecasts or predictions of Optinose or its management. Optinose does not by its reference above or distribution imply its endorsement of or concurrence with such information, conclusions or recommendations. Jonathan Neely, VP, Investor Relations and Business Development 267-521-0531 Investors@optinose.com As of June 30, 2024: $91.4 million in cash Debt: $130 million 149.7 million common shares o/s 70.5 million options, warrants & RSUs o/s @optinose investors@optinose.com www.optinose.com

Building a Leading ENT / Allergy Specialty Company Corporate Presentation August 8, 2024
v3.24.2.u1
Cover
|
Aug. 08, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Document Period End Date |
Aug. 08, 2024
|
| Entity Registrant Name |
OPTINOSE, INC.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-38241
|
| Entity Tax Identification Number |
42-1771610
|
| Entity Address, Address Line One |
777 Township Line Road
|
| Entity Address, Address Line Two |
Suite 300
|
| Entity Address, City or Town |
Yardley
|
| Entity Address, State or Province |
PA
|
| Entity Address, Postal Zip Code |
19067
|
| City Area Code |
267
|
| Local Phone Number |
364-3500
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
false
|
| Title of 12(b) Security |
Common stock, par value $0.001 per share
|
| Trading Symbol |
OPTN
|
| Entity Central Index Key |
0001494650
|
| Amendment Flag |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
OptiNose (NASDAQ:OPTN)
Historical Stock Chart
From Sep 2024 to Oct 2024
OptiNose (NASDAQ:OPTN)
Historical Stock Chart
From Oct 2023 to Oct 2024