SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
of the Securities Exchange Act of 1934
For the month of June 2024
Commission File Number 001-38512
Oncolytics Biotech Inc.
(Translation of registrant's name into English)
Suite 804, 322 11th Avenue SW
Calgary, Alberta, Canada T2R 0C5
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): o
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): o
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant's “home country”), or under the rules of the home country exchange on which the registrant's securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant's security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
| | | | | | | | |
EXHIBIT
NUMBER | | DESCRIPTION |
| | | |
| 99.1 | | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | | | | |
| | | Oncolytics Biotech Inc. (Registrant) |
| | | | | |
| | | | | |
| | | By: | | /s/ Kirk Look |
| Date: June 20, 2024 | | | | Kirk Look
Chief Financial Officer |
Oncolytics Biotech® Doses First Patient in Study of Pelareorep/FOLFIRINOX Combination Therapy in Pancreatic Cancer
Demonstrating pelareorep’s synergy with modified FOLFIRINOX +/- atezolizumab in pancreatic cancer could expand the number of patients it may benefit
Funding for the study comes from the US$5 million Therapeutic Accelerator Award from PanCAN
SAN DIEGO, CA, and CALGARY, AB, June 20, 2024 -- Oncolytics Biotech® Inc. (NASDAQ: ONCY) (TSX: ONC), a leading clinical-stage company specializing in immunotherapeutics for oncology, announced the dosing of the first patient in the new GOBLET study cohort evaluating pelareorep and modified FOLFIRINOX (mFOLFIRINOX) with or without atezolizumab (Tecentriq®) in newly diagnosed metastatic pancreatic ductal adenocarcinoma (PDAC) patients. The co-primary endpoints of the cohort are objective response rate (ORR) and safety. It is supported by the US$5M Pancreatic Cancer Action Network (PanCAN) Therapeutic Accelerator Award, an innovative program established to accelerate the development of new treatments for pancreatic cancer patients. It will be conducted in collaboration with AIO-Studien-gGmbH (AIO), a clinical trial group within the German Cancer Society, as part of GOBLET, a Phase 1/2 multiple indication study evaluating pelareorep-based combinations in gastrointestinal cancers.
“Initiation of dosing in the mFOLFIRINOX cohort of the GOBLET study is an important milestone for Oncolytics, and we’re excited to begin evaluating another pelareorep combination therapy that could result in a second pancreatic cancer registration program for the company,” said Thomas Heineman, M.D., Ph.D., Chief Medical Officer at Oncolytics. “The combination of pelareorep, atezolizumab, gemcitabine, and nab-paclitaxel in pancreatic cancer patients more than doubled tumor response rates compared to earlier trials of chemotherapy alone. That combination received Fast Track Designation from the FDA and is expected to be evaluated in an adaptive registration-enabling trial through the Global Coalition for Adaptive Research (GCAR). If the combination of pelareorep and mFOLFIRINOX also demonstrates a promising efficacy signal, we could have two pancreatic cancer treatment regimens on the path to registration. I want to highlight PanCAN’s important support for this program with gratitude. The US$5M Therapeutic Accelerator Award has made it possible for us to broaden our evaluation of potential therapies that have the potential to improve outcomes for pancreatic cancer patients.”
Anna Berkenblit, MD, MMSc, Chief Scientific and Medical Officer at PanCAN said, “Working toward our vision to create a world in which all patients with pancreatic cancer will thrive, PanCAN launched the Therapeutic Accelerator Award to speed the drug development process and bring new options to patients faster. Dosing the first patient in this new cohort of the GOBLET study is an important step toward further evaluation of this investigational immunotherapeutic approach.”
Dirk Arnold, M.D., Ph.D., Director of Asklepios Tumorzentrum Hamburg and primary investigator of the GOBLET trial commented, “I have been pleased to observe the strength of the clinical response data for pelareorep in multiple cohorts of the GOBLET gastrointestinal study, especially in pancreatic and anal cancer. mFOLFIRINOX is currently considered the best treatment option for many pancreatic cancer patients. Therefore, the evaluation of pelareorep and mFOLFIRINOX, with or without atezolizumab, presents an important opportunity to identify a novel therapeutic approach that may broaden the population of metastatic pancreatic cancer patients who could benefit from pelareorep-based therapies.”
“Oncolytics is in a favorable position as we prepare to advance multiple pelareorep programs toward registration track studies and continue to expand pelareorep’s potential as a backbone immunotherapy that
can impact various tumor types. The collaboration with GCAR on a registration-enabling study for the combination of pelareorep, atezolizumab, gemcitabine, and nab-paclitaxel in pancreatic cancer, meeting with the FDA to align on next steps for our breast cancer program, expanded enrollment in the GOBLET anal cancer cohort, and now the initiation of dosing in the mFOLFIRINOX cohort of GOBLET, announced today, are all important elements of our corporate plan,” said Dr. Matt Coffey, President and Chief Executive Officer of Oncolytics. “The ability to improve the lives of cancer patients is something that motivates everyone at Oncolytics, and beginning to treat pancreatic cancer patients in the mFOLFIRINOX cohort of GOBLET is hopefully yet another step towards that goal.”
About GOBLET cohort 5
The mFOLFIRINOX cohort of the Phase 1/2 GOBLET study is designed to evaluate newly diagnosed PDAC patients treated with pelareorep + mFOLFIRINOX with or without atezolizumab. There will be a three-patient safety run-in to evaluate the tolerability of each treatment arm: pelareorep + mFOLFIRINOX + atezolizumab and pelareorep + mFOLFIRINOX. A total of fifteen evaluable patients will be randomized to each arm in Stage 1 of this Simon two-stage study. The co-primary endpoints are objective response rate and safety. If Stage 1 success criteria are met, one or both treatment arms may be expanded to Stage 2 in which 17 additional evaluable patients per arm will be enrolled. Blood and tumor samples will also be collected for translational evaluations.
About GOBLET
The GOBLET (Gastrointestinal tumOrs exploring the treatment comBinations with the oncolytic reovirus peLarEorep and anTi-PD-L1) study is a phase 1/2 multiple indication study in advanced or metastatic gastrointestinal tumors. The study is being conducted at 17 centers in Germany and is being managed by AIO-Studien-gGmbH. The co-primary endpoints of the study are objective response rate (ORR) and/or disease control rate assessed at week 16 and safety. Key secondary and exploratory endpoints include additional efficacy assessments and evaluation of potential biomarkers. The study comprises five treatment groups:
1.Pelareorep in combination with atezolizumab, gemcitabine, and nab-paclitaxel in 1st line advanced/metastatic pancreatic cancer patients;
2.Pelareorep in combination with atezolizumab in 1st line MSI (microsatellite instability)-high metastatic colorectal cancer patients;
3.Pelareorep in combination with atezolizumab and TAS-102 in 3rd line metastatic colorectal cancer patients
4.Pelareorep in combination with atezolizumab in 2nd line advanced and unresectable anal cancer patients; and
5.Pelareorep in combination with mFOLFIRINOX with and without atezolizumab in newly diagnosed metastatic PDAC patients.
Any cohort meeting pre-specified efficacy criteria in Stage 1 may be advanced to Stage 2 and enroll additional patients.
About AIO
AIO-Studien-gGmbH (AIO) emerged from the study center of the medical oncology working group within the German Cancer Society (DKG). AIO operates with a non-profit purpose of promoting
science and research with a focus on medical oncology. Since its foundation, AIO has become a successful sponsor and study management company and has established itself both nationally and internationally.
About Oncolytics Biotech Inc.
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an intravenously delivered immunotherapeutic agent. Pelareorep has demonstrated promising results in two randomized Phase 2 studies in metastatic breast cancer and Phase 1 and 2 studies in pancreatic cancer. It acts by inducing anti-cancer immune responses and promotes an inflamed tumor phenotype -- turning "cold" tumors "hot" -- through innate and adaptive immune responses to treat a variety of cancers.
Pelareorep has demonstrated synergies with multiple approved oncology treatments. Oncolytics is currently conducting and planning combination clinical trials with pelareorep in solid and hematological malignancies as it advances towards registrational studies in metastatic breast cancer and pancreatic cancer, both of which have received Fast Track designation from the FDA. For further information, please visit: www.oncolyticsbiotech.com or follow the company on social media on LinkedIn and on X @oncolytics.
This press release contains forward-looking statements, within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements contained in this press release include Oncolytics’ belief as to the potential, mechanism of action and benefits of pelareorep as a cancer therapeutic; the design, intent and potential benefits of the mFOLFIRINOX cohort of the GOBLET study; our belief that if the combination of pelareorep and mFOLFIRINOX also demonstrates a promising efficacy signal, the Company could have two pancreatic cancer treatment regimens on the path to registration; our corporate plan; the potential for expansion to Stage 2 if Stage 1 success criteria are met; our plans to advance towards registrational studies in metastatic breast cancer and pancreatic cancer; and other statements related to anticipated developments in Oncolytics’ business and technologies. In any forward-looking statement in which Oncolytics expresses an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. Such forward-looking statements involve known and unknown risks and uncertainties, which could cause Oncolytics’ actual results to differ materially from those in the forward-looking statements. Such risks and uncertainties include, among others, the availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, Oncolytics’ ability to successfully commercialize pelareorep, uncertainties related to the research and development of pharmaceuticals, uncertainties related to the regulatory process and general changes to the economic environment. In particular, we may be impacted by business interruptions resulting from COVID-19 coronavirus, including operating, manufacturing supply chain, clinical trial and project development delays and disruptions, labour shortages, travel and shipping disruption, and shutdowns (including as a result of government regulation and prevention measures). We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition. Investors should consult Oncolytics’ quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Investors are cautioned against placing undue reliance on forward-looking statements. The Company does not undertake any obligation to update these forward-looking statements, except as required by applicable laws.
| | | | | |
Company Contact Jon Patton Director of IR & Communication jpatton@oncolytics.ca | Investor Relations for Oncolytics Timothy McCarthy LifeSci Advisors +1-917-679-9282 tim@lifesciadvisors.com |
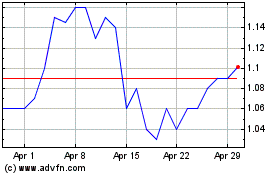
Oncolytics Biotech (NASDAQ:ONCY)
Historical Stock Chart
From May 2024 to Jun 2024
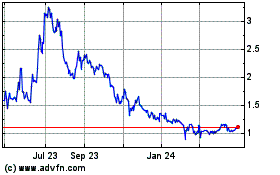
Oncolytics Biotech (NASDAQ:ONCY)
Historical Stock Chart
From Jun 2023 to Jun 2024