UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
For the Month of July 2023
Commission File Number: 001-38104
IMMURON LIMITED
(Name of Registrant)
Level 3, 62 Lygon Street, Carlton South,
Victoria, 3053, Australia
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form
40-F ☐
Indicate by check mark whether by furnishing the information contained
in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities
Exchange Act of 1934.
Yes ☐ No
☒
If “Yes” is marked, indicate below the file number assigned
to the registrant in connection with Rule 12g3-2(b): 82-
IMMURON LIMITED
EXPLANATORY NOTE
Immuron Limited (the “Company”) published
one announcement (the “Public Notices”) to the Australian Securities Exchange on July 24, 2023 titled:
| - | “Immuron
CEO, Steven Lydeamore to present at Bioshares” |
A copy of the Public Notice is attached as an exhibit to this report
on Form 6-K.
This report on Form 6-K (including the exhibit
hereto) shall not be deemed to be “filed” for purposes of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”) and shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, except as shall be
expressly set forth by specific reference in such filing.
EXHIBITS
SIGNATURE
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
IMMURON LIMITED |
| |
|
|
| |
 |
| |
|
|
| Date: July 24, 2023 |
By: |
/s/ Phillip Hains |
| |
|
Phillip Hains |
| |
|
Company Secretary |
Exhibit 99.1

Immuron
CEO, Steven Lydeamore to present at Bioshares
Melbourne, Australia, July 24, 2023: Immuron Limited
(ASX: IMC; NASDAQ: IMRN), an Australian based and globally integrated biopharmaceutical company is pleased to advise our Chief Executive
Officer, Steven Lydeamore will be presenting at the 17th Bioshares Biotech Summit in Hobart on July 24th.
A copy of the presentation being made at the 17th
Bioshares Biotech Summit is included below.
This release has been authorised by the directors
of Immuron Limited.
- - - END - - -
COMPANY CONTACT:
Steven Lydeamore
Chief Executive Officer
Ph: +61 (0)3 9824 5254
info@immuron.com
About Travelan®
Travelan® is an
orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting traveller’s diarrhoea, a
digestive tract disorder that is commonly caused by pathogenic bacteria and the toxins they produce. Travelan® is a highly
purified tabletised preparation of hyperimmune bovine antibodies and other factors, which when taken with meals bind to diarrhoea-causing
bacteria and prevent colonisation and the pathology associated with traveller’s diarrhoea. In Australia, Travelan® is a listed
medicine on the Australian Register for Therapeutic Goods (AUST L 106709) and is indicated to reduce the risk of Traveller’s Diarrhoea,
reduce the risk of minor gastrointestinal disorders and is antimicrobial. In Canada, Travelan® is a licensed natural health product
(NPN 80046016) and is indicated to reduce the risk of Traveller’s Diarrhoea. In the U.S., Travelan® is sold as a dietary supplement
for digestive tract protection.
About Traveller’s Diarrhoea
Traveller’s Diarrhoea
is a gastrointestinal infection with symptoms that include loose, watery (and occasionally bloody) stools, abdominal cramping, bloating,
and fever, Enteropathogenic bacteria are responsible for most cases, with enterotoxigenic Escherichia coli (ETEC) playing a dominant
causative role. Campylobacter spp. are also responsible for a significant proportion of cases. The more serious infections with Salmonella
spp. the bacillary dysentery organisms belonging to Shigella spp. and Vibrio spp. (the causative agent of cholera) are often confused
with Traveller’s Diarrhoea as they may be contracted while travelling and initial symptoms are often indistinguishable.
About Immuron
Immuron Limited (ASX:
IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercialising orally delivered targeted polyclonal
antibodies for the treatment of infectious diseases.
For more information
visit: http://www.immuron.com


FORWARD-LOOKING STATEMENTS:
This press release may contain “forward-looking
statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934,
each as amended. Such statements include, but are not limited to, any statements relating to our growth strategy and product development
programs and any other statements that are not historical facts. Forward-looking statements are based on management’s current expectations
and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock
value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to our growth
strategy; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; risks relating to the
results of research and development activities; risks relating to the timing of starting and completing clinical trials; uncertainties
relating to preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key
personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent and
intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation
or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in
our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law.

BIOSHARES PRESENTATION 2 4 J U L Y , 2023 S teven Lydeamore NASD A Q: I M RN ASX: IMC - C E O

SA F E HARB O R STAT E M E NT Certain statements made in this presentation are forward - looking statements and are based on Immuron’s current expectations, estimates and projections. Words such as “anticipates,” “expects,” “ intends,” “ plans,” “ believes,” “seeks,” “estimates,” “ guidance” and s imilar expressions are intended to identify forward - looking statements. Although Immuron believes the forward - looking statements are based on reasonable assumptions, they are subject to certain r i sks and uncertainties, some of which are beyond Immuron’s control, including those r i sks or uncertainties inherent in the process of both developing and commercializing technology. As a result, actual results could materially differ f rom those e x p r e s s e d o r fo r e c a s t e d i n th e fo r w a r d - l oo k i n g s t a t e m e nt s . The forward - looking statements made in this presentation relate only to events as of the date on which the statements are made. Immuron will not undertake any obligation to release publicly any revisions or updates to these forward - looking statements to reflect events, c i rcumstances or unanticipated events occurring after the date of this presentation except as required by law or by any appropriate regulatory authority. FY 2023 results in this presentation are subject to audit review. 2

T O P I C S 1. Immuron Overview 2. US Department of Defense interest in Immuron Technology 3. Immuron’s DoD partnered programs 4. Commercial path forward if Travelan® Phase 2 study is successful 5. Partnering with the US Department of Defense 3
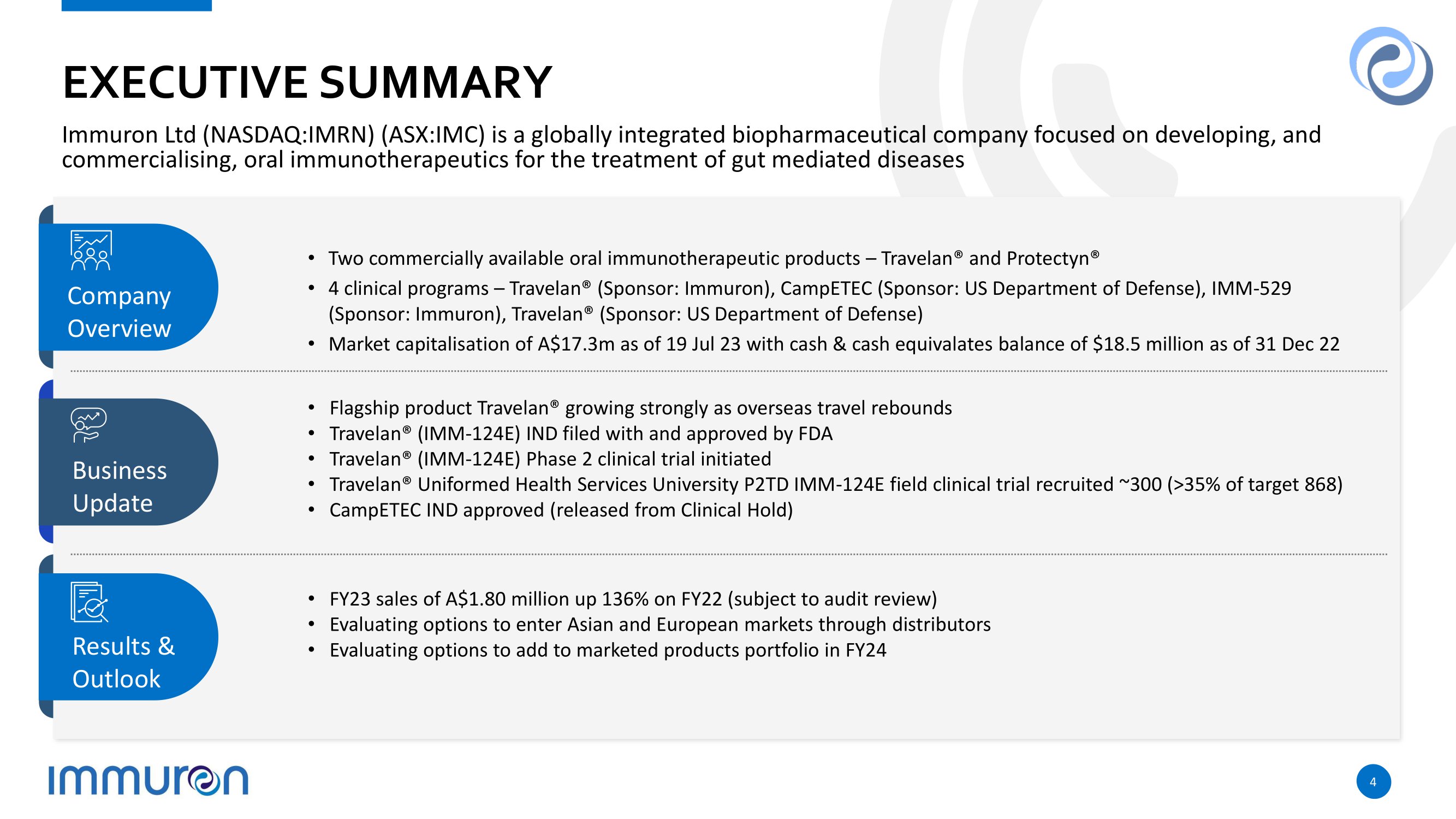
EX E CU T I V E SU M MARY Immuron Ltd (NASDAQ:IMRN) (ASX:IMC) is a globally integrated biopharmaceutical company focused on developing, and commercialising, oral immunotherapeutics for the treatment of gut mediated diseases C o m p a n y O v e rv i e w Bu s i nes s Update R esu l ts & Outlook • Two commercially available oral immunotherapeutic products – Travelan® and Protectyn® • 4 clinical programs – Travelan® (Sponsor: Immuron), CampETEC (Sponsor: US Department of Defense), IMM - 529 (Sponsor: Immuron), Travelan® (Sponsor: US Department of Defense) • Market capitalisation of A$17.3m as of 19 Jul 23 with cash & cash equivalates balance of $18.5 million as of 31 Dec 22 • Flagship product Travelan® growing strongly as overseas travel rebounds • Travelan® (IMM - 124E) IND filed with and approved by FDA • Travelan® (IMM - 124E) Phase 2 clinical trial initiated • Travelan® Uniformed Health Services University P2TD IMM - 124E field clinical trial recruited ~300 (>35% of target 868) • C a m p E T E C I N D a p p r o v ed ( r e l e as ed f r o m C l i n i c al H o l d ) • FY23 sales of A$1.80 million up 136% on FY22 (subject to audit review) • Evaluating options to enter Asian and European markets through distributors • Evaluating options to add to marketed products portfolio in FY24 4

ADDRESSABLE MARKET & I N D U ST R Y O V ER V I E W Billion Dollar Market Traveller’s diarrhoea treatment market is large and growing at a CAGR of ~7% Indu s t r y tai l w i n d s Travel picking up significantly following C O V I D l o c k d o wn s Frequent Symptom 30% - 70% of travelers experience traveller’s diarrhoea** Chief Commercial Officer has 20+ year’s experience with local and global (Asia, UK) c o mm e r c i al l e a d e r s h i p roles with GSK and P&G U S A Mar ket a m a z o n . c o m s h o p f r o n t launch 1QFY24 Re - entry into retail pharmacies will be explored in FY24 Evaluating options: • fo r e nt r y i n t o As i a and Europe • t o a d d m a r k e t e d products to p o rt fo l i o i n F Y 2 4 $83m Based on US annual travel numbers and a penetration rate of 15%, the market p o t e n ti a l i s e s t im at e d at $83m* $50m Based on EU travel numbers and a penetration rate of 15%, the market p o t e n ti a l i s e s t im at e d at $50m* $1.7b C l os tr i d i o i d es d i ff i c il e infections (CDIs) to grow to almost $1.7 billion by 2026, according to GlobalData * IMC Company Report - Travelan Market Analysis 2019 ** Centers for Disease Control and Prevention Yellow Book 5

T EC HN O L O G Y P LATF O R M Bovine colostrum is the first milk of cows after calving . It is rich in immunoglobulins, lactoferrin, lysozyme, lactoperoxidase, growth factors and bioactive peptides . Colostrum has higher levels of protein, fat, vitamins, and minerals when compared to milk . This enables full development of the newborn calf in addition to immunity against several pathogens . * Immuron’s proprietary technology platform combines the natural human nutrition & health benefits of bovine colostrum with a novel class of specifically targeted oral polyclonal antibodies that offer delivery within the gastrointestinal (“GI”) tract and can be used to target viruses or bacteria and neutralize the toxins they produce at mucosal surfaces. S T E P 1 D eve l o p m e n t o f H i g h l y Specific Vaccines S T E P 2 Iso l a ti o n o f H y p er i m mu n e antibody - rich bovine colostrum * Gomes et. al., NFS Journal, Volume 25, November 2021, pages 1 - 11, https://doi.org/10.1016/j.nfs.2021.10.001 S T E P 3 Oral Antimicrobial therapeutics w i t h o u t d r a wb a c k s of antibiotics F IN AL P R O D U C T Toxin Neutralization + C l e a r a n c e o f t a r g e t e d g u t pathogens x Reduce occurrence and reduce/relieve diarrhoea x Reduce/relieve abdominal cramping x Reduce/relieve gastrointestinal pain x Assists repair of ga s t r o i n t es t i na l / g u t w a l l li n i ng x E nhance / pr o m o t e i mmune defence x E nhance / pr o m o t e h e a lt h l i v e r function A u st r a li a n P e r m i t t e d i nd i c a t i on s ; these statements have not been evaluated by the Food and Drug Administration (FDA) 6

U S DE P A R TM E NT O F D E F E N S E • Managed by the Department of Defense collaborating with multiple government agencies • Focuses on development of medical solutions to protect, treat, and optimize health and performance of U.S. military personnel and civilians • Focuses on innovative medical solutions to a range of Force Health Protection and Readiness challenges currently facing U.S. Service Members, along with threats anticipated during future operations • Created a model of vaccine and therapeutic development that is unique, nimble, and responsive to dynamically evolving infectious disease threats of military importance ARMED FORCES RESEARCH INSTITUTE O F M E D I C AL SC I E N C E S • Optimize soldier lethality by developing solutions to infectious diseases capability gaps through product development and surveillance research in Asia N A V A L M E D IC A L RESEARCH COMMAND U N I F O R M E D SERVICES UNIVERSITY • NMRC’s mission is to conduct health and medical research, development, testing, evaluation, and surveillance to enhance deployment readiness of DoD personnel worldwide • NRMC focuses on solutions to operational medical problems such as battlefield neurotrauma and wound infections, decompression sickness, naturally occurring infectious diseases , and biological threat agents ; and is home to the DoD bone marrow registry • Coordinates, initiates, and supports research related to military health needs based on a holistic framework of health that includes prevention, resilience, treatment, recovery, rehabilitation, reintegration, and the overall well - being of the warfighter, as well as dual benefit outcomes that will improve civilian health. Our team advances research and collaborations utilizing several mechanisms, to include grants and a variety of cooperative agreements. The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government 7

US ARMY MEDICAL RESEARCH & DEVELOPMENT COMMAND K ey C o mm e n t a r y • US Military Infectious Diseases Research Program (MIDRP) • The mission of the MIDRP is to plan, coordinate and oversee for the US DOD r e q u i r em e n t s - d ri v en med i c a l s o l u t i o n s t h at P R E V E N T , P R E D I C T, a nd T R EA T i nf ect i ou s diseases threats • Diarrheal disease is the leading infectious threat facing deployed U.S. Military • Effective vaccines are the most suitable preventive measure for infectious diarrheal diseases but there are no licensed products available • Enteric countermeasure products need to provide protection against military - relevant enteric pathogens • The DoD is working with Immuron to develop products with coverage for Shigella , Campylobacter and multiple ETEC phenotypes and is investigating the possibility of increasing coverage to other pathogens including enteroaggregative E . coli (EAEC) Military Infectious Diarrhea Etiological Agents The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government 8

U S DO D R & D C OL L A B O RA T IO N A G R EE M E NT S Established Developing K ey C o mm e n t a r y Australian Packaging U S A P ack ag i n g ▪ Immuron partnered with AFRIMS, NMRC & WRAIR to test Travelan® against Campylobacter, ETEC, Shigella and Vibrio cholera ▪ Walter Reed Army Institute of Research – June 2016 ▪ Armed Forces Research Institute of Medical Sciences (AFRIMS) – June 2016 ▪ Naval Medical Research Command – August 2016 ▪ Travelan® binds 180 pathogenic strains of bacteria from infected personnel deployed in Bhutan, Cambodia, Nepal and Thailand (ETEC, Shigella, Campylobacter) ▪ Travelan® binds to 71 pathogenic strains of Vibrio cholera from infected personnel in Bangladesh, Cambodia, and Thailand ▪ Travelan® prevented the development of Shigellosis in 75% of non - human primates receiving therapy US Department of Defense grant of US$4.45 million to examine a dosing regimen for Travelan® more suited for use by the military • IMM - 124E (Travelan®) IND was approved by the FDA in December 2022 • Initiated clinical trial May 2023: NCT05933525 Travelan® - Uniformed Services University has recruited more than 35% of participants in a randomized clinical trial with Travelan® to evaluate the effectiveness for prophylaxis during deployment or travel to a high t r a v e l e r ’ s d i a rr h ea r i s k r e g i o n • NCT04605783 Naval Medical Research Command Clinical Trials of CampETEC in campylobacter and enterotoxigenic E.coli (ETEC) • Animal ethics approval for Toxicology study – 22 November 2022 • Immuron sponsored Toxicology study - completed 20 December 2022 • FDA removed Clinical Hold on Campylobacter ETEC – 8 May 2023 WRAIR AFRIMS NMRC Travelan®(ETEC) Campylobacter Shigella The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government 9
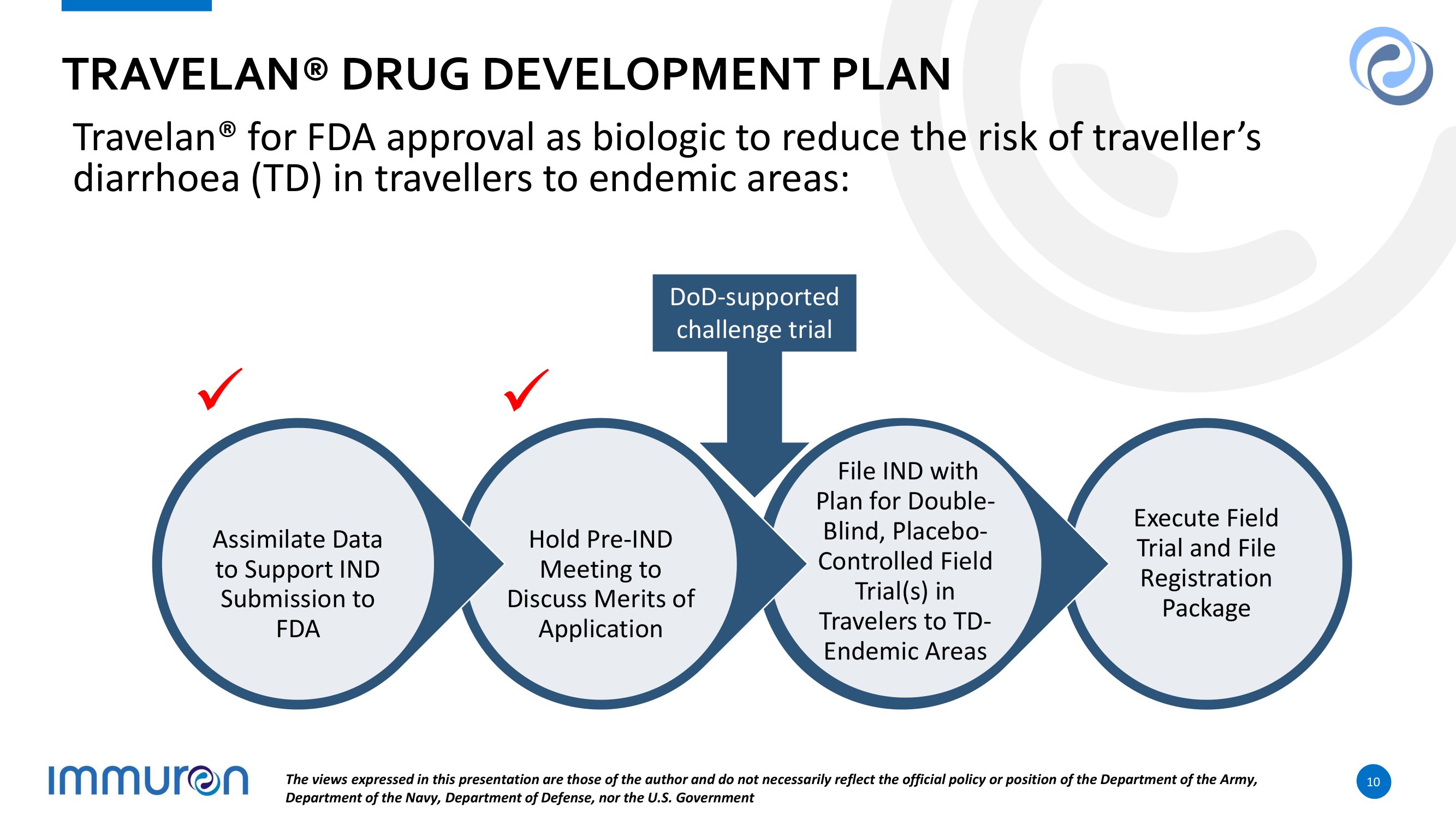
TRAVELAN® DRUG DEVELOPMENT PLAN Travelan® for FDA approval as biologic to reduce the risk of traveller’s diarrhoea (TD) in travellers to endemic areas: D o D - sup p o r t e d challenge trial x x A ss i mi l ate Da t a to Support IND Submission to FDA Hold Pre - IND Meeting to D is c us s M e r i ts o f Application File IND with Plan f o r D o ub le - Blind, Placebo - Controlled Field Trial(s) in Travelers to TD - Endemic Areas E x e c u te F i e l d Trial and File Registration Package The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government 10
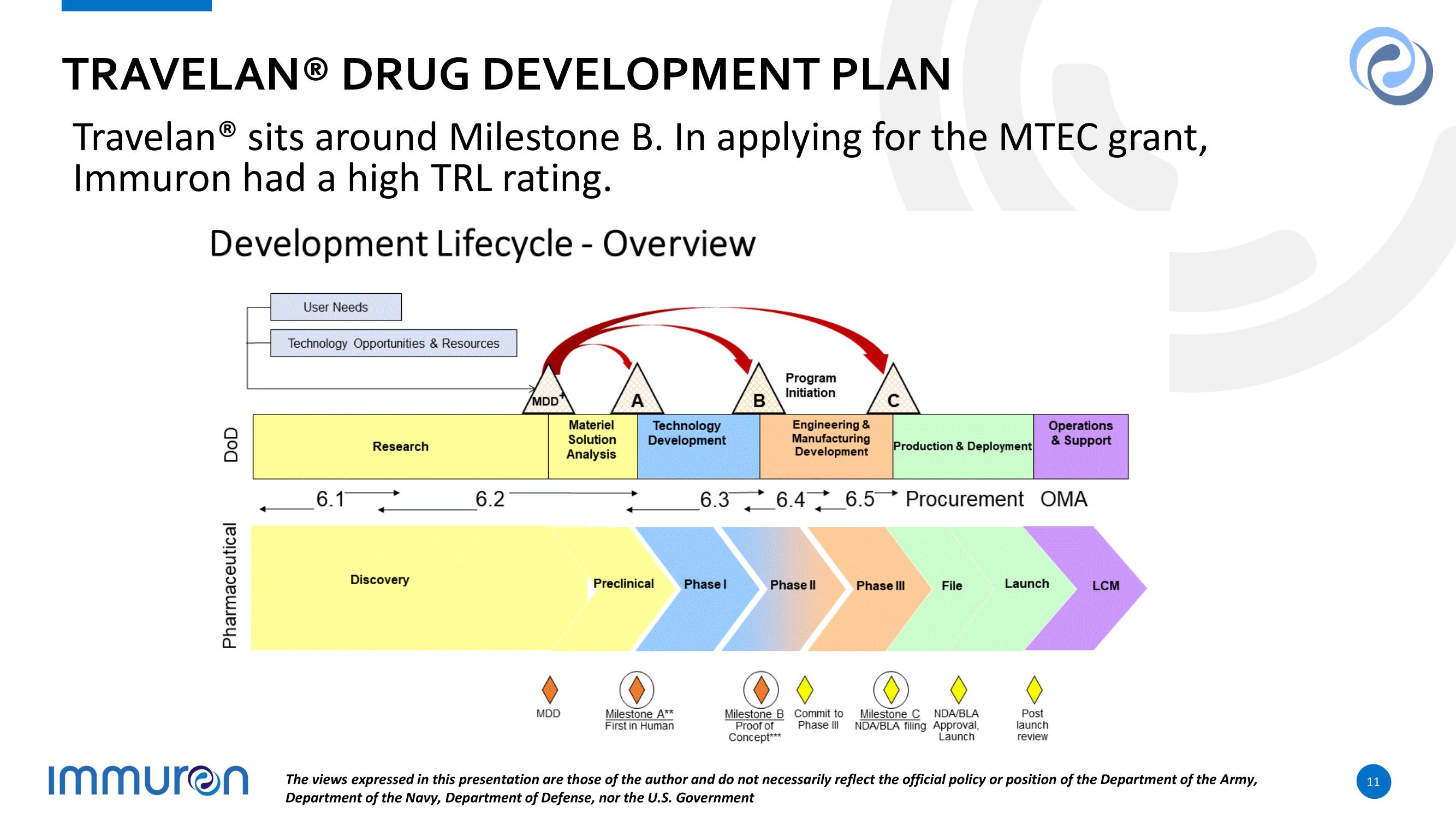
TRAVELAN® DRUG DEVELOPMENT PLAN Travelan® sits around Milestone B. In applying for the MTEC grant, Immuron had a high TRL rating. The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government 11
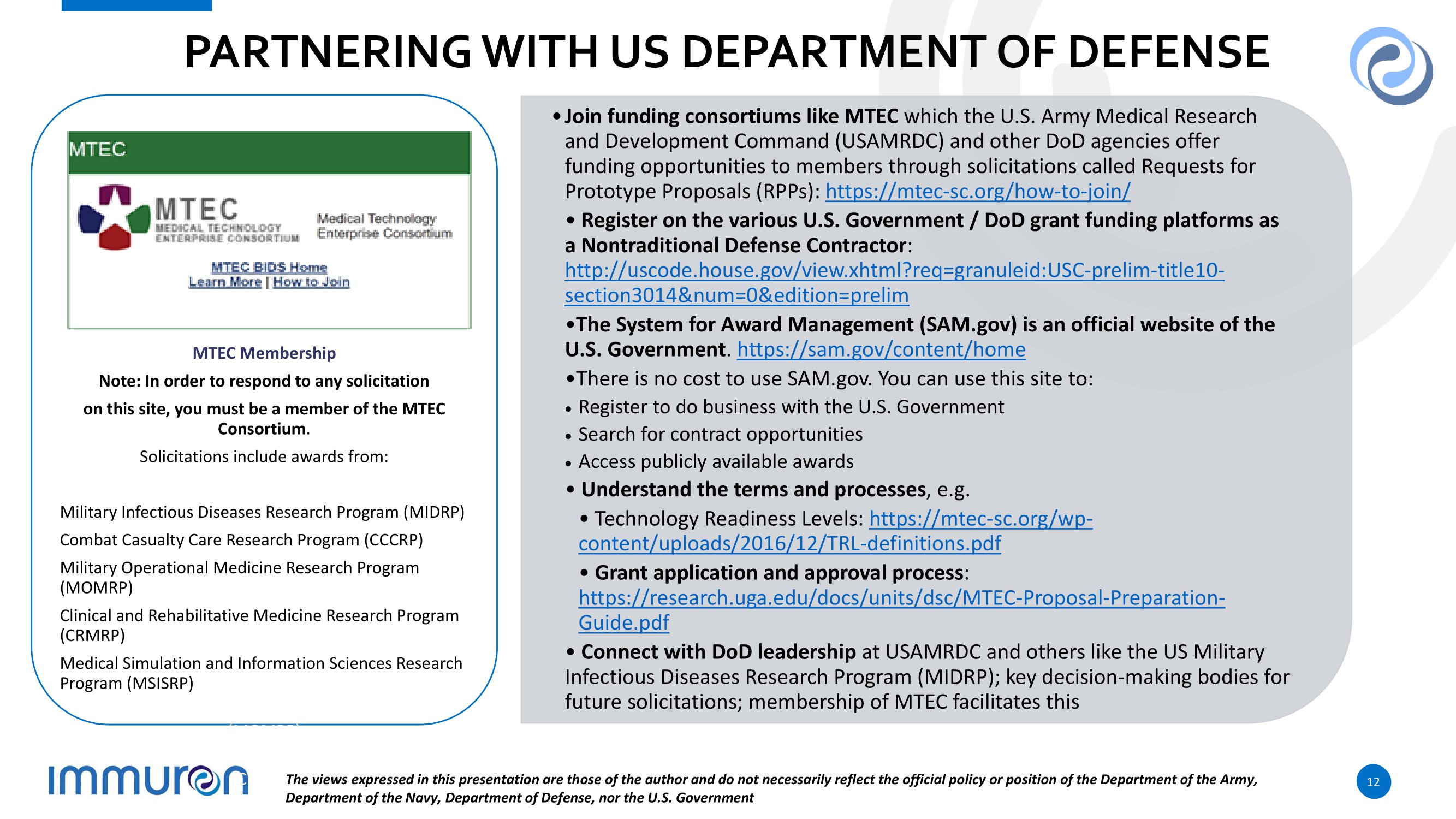
P A R TN E R I N G W I T H U S DE P A R T M E N T O F DE F E N S E • Join funding consortiums like MTEC which the U.S. Army Medical Research and Development Command (USAMRDC) and other DoD agencies offer funding opportunities to members through solicitations called Requests for Prototype Proposals (RPPs): https://mtec - sc.org/how - to - join/ • Register on the various U.S. Government / DoD grant funding platforms as a Nontraditional Defense Contractor : http://uscode.house.gov/view.xhtml?req=granuleid:USC - prelim - title10 - section3014&num=0&edition=prelim • The System for Award Management (SAM.gov) is an official website of the U.S. Government . https://sam.gov/content/home • There is no cost to use SAM.gov. You can use this site to: Register to do business with the U.S. Government S e a r c h fo r c o n tr a c t o pp o rt un iti es A cc e s s pub li cl y a v a il a b l e a w a r d s • Understand the terms and processes , e.g. • Technology Readiness Levels: https://mtec - sc.org/wp - content/uploads/2016/12/TRL - definitions.pdf • Grant application and approval process : https://research.uga.edu/docs/units/dsc/MTEC - Proposal - Preparation - Guide.pdf • Connect with DoD leadership at USAMRDC and others like the US Military Infectious Diseases Research Program (MIDRP); key decision - making bodies for future solicitations; membership of MTEC facilitates this Clini al and ehabili tiv M T E C M e m b e r s h i p Note: In order to respond to any solicitation on this site, you must be a member of the MTEC Consortium . Solicitations include awards from: (C The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government 12 Military Infectious Diseases Research Program (MIDRP) Combat Casualty Care Research Program (CCCRP) Military Operational Medicine Research Program (MOMRP) Clinical and Rehabilitative Medicine Research Program (CRMRP) Medical Simulation and Information Sciences Research Program (MSISRP)

STEVEN LYDEAMORE C HIE F EXE C U T IVE O F FI C ER IMMURON LIMITED C O NT AC T I N FO R MA T IO N : EMAIL: STEVE@IMMURON.COM PHONE: AUSTRALIA: +613 8892 4854 13

S C I E NT I FIC R E F E R E N C E S T r a v e l a n® (I MM - 124 E ) Scandinavian Journal of Gastroenterology, 46:7 - 8, 862 - 868, DOI: 10.3109/00365521.2011.574726 Travelan® has been shown to reduce both the incidence and severity of ETEC - induced diarrhea in up to 90% of volunteers I mm u r o n L i m i t ed, 2 9 A p r il , 2011 T rave l an a s a b r o ad S p e c t r u m a n t i - b a c t e r i a l US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 4 September, 2019 Travelan® demonstrates broad reactivity to Vibrio cholera strains from Southeast Asia indicating broad potential for prevention of traveler’s diarrhea US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 5 September, 2018 Travelan® prevented clinical shigellosis (bacillary dysentery) in 75% of Travelan® treated animals compared to placebo and demonstrated a significant clinical benefit US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 30 January, 2017 Travelan® able to bind and was reactive to 60 clinical isolates of each bacteria, Campylobacter, ETEC, and Shigella Islam et al., 2020. Submitted to mSphere, American Society for Microbiology Efficacy of hyperimmune bovine colostrum against shigellosis in rhesus macaque (Macaca mulatta), and bioactivity of HBC against common enteric pathogens Clin Vaccine Immunol 24:e00186 - 16. https://doi.org/10.1128/CVI.00186 - 16 B i o a c t i ve I mm un e C o m p o n en t s o f T rav e l a n ® Rachele Gore, Mitra Mohsenipour, Jennifer L Wood, Gayathri K Balasuriya, Elisa L Hill - Yardin, Ashley E Franks Hyperimmune bovine colostrum containing lipopolysaccharide antibodies (IMM - 124E) has a non - detrimental effect on gut microbial communities in unchallenged mice Journal of Crohn's and Colitis, Volume 13, Issue 6, June 2019, Pages 785 – 797, https://doi.org/10.1093/ecco - jcc/jjy213 Administration of the Hyper - immune Bovine Colostrum Extract IMM - 124E Ameliorates Experimental Murine Colitis IMM - 529 Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598 - 017 - 03982 - 5 Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative 14
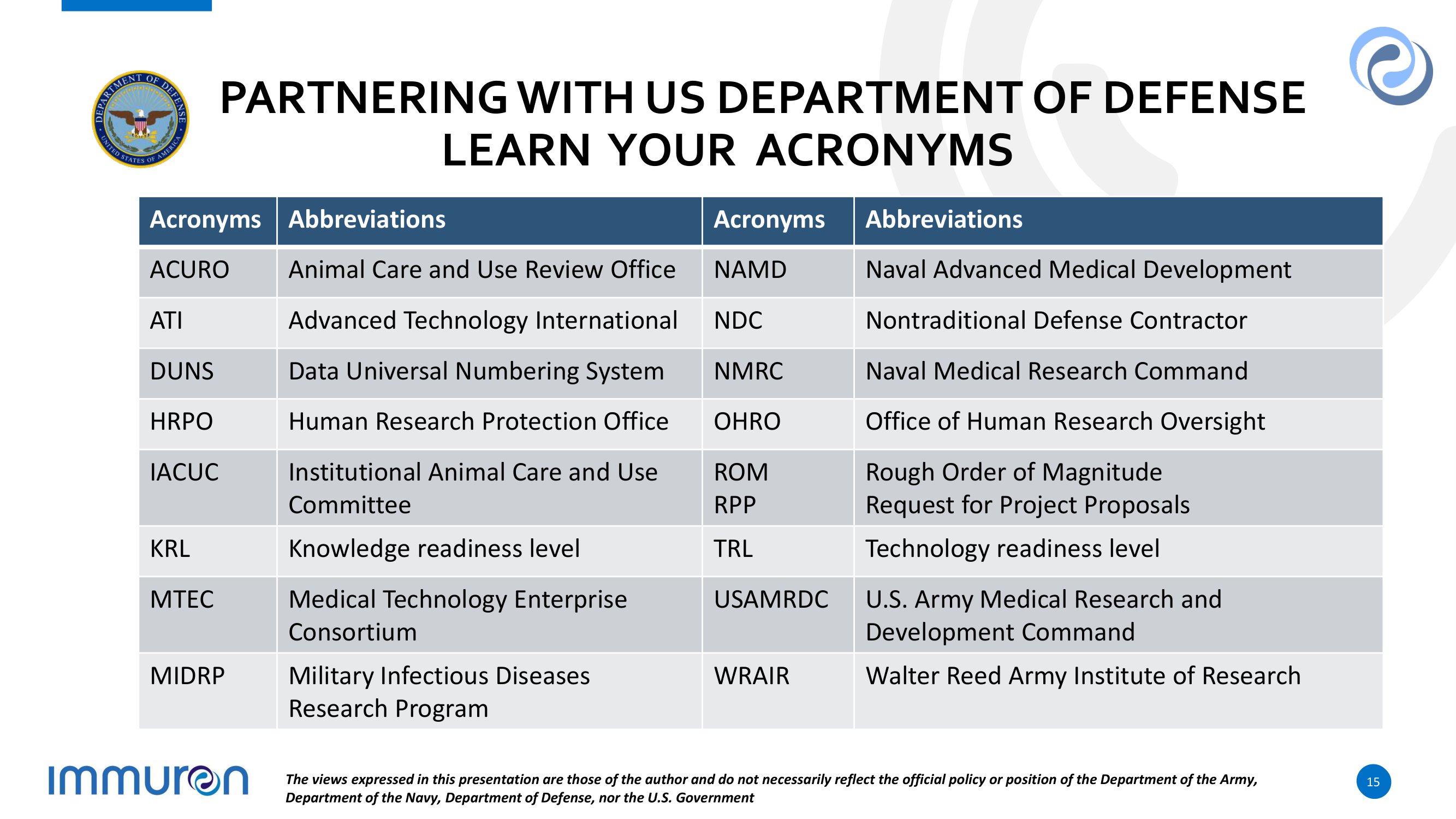
P A R T N ER ING W I T H U S D E P AR T M E N T O F D E F E N S E LEARN YOUR ACRONYMS Abbreviations Acronyms Abbreviations Acronyms Naval Advanced Medical Development NAMD Animal Care and Use Review Office ACURO Nontraditional Defense Contractor NDC A d vanc e d T e c h n o l o g y I n t e r n at i o n al ATI Naval Medical Research Command NMRC Data Universal Numbering System DUNS Office of Human Research Oversight OHRO Human Research Protection Office HRPO Rough Order of Magnitude Request for Project Proposals RO M RPP Institutional Animal Care and Use Committee IACUC T e c hn o l o g y r e a d i n es s l e v e l TRL Knowledge readiness level KRL U. S . A rmy M e d i c al R e s e arch a n d Development Command USAMRDC Medical Technology Enterprise Consortium MTEC Walter Reed Army Institute of Research WRAIR Military Infectious Diseases Research Program MIDRP The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Army, Department of the Navy, Department of Defense, nor the U.S. Government 15
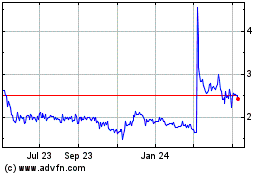
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Jun 2024 to Jul 2024
Immuron (NASDAQ:IMRN)
Historical Stock Chart
From Jul 2023 to Jul 2024