Disc Medicine Polycythemia Vera Treatment MWTX-003 Gets FDA Fast Track Designation
September 20 2023 - 5:18PM
Dow Jones News
By Denny Jacob
Disc Medicine's MWTX-003, a treatment for patients with
polycythemia vera, was granted fast track designation by the U.S.
Food and Drug Administration.
Chief Executive John Quisel said the clinical-stage
biopharmaceutical company plans to initiate a Phase 1 trial in the
coming months.
MWTX-003 is an investigational monoclonal antibody designed to
increase hepcidin production and suppress serum iron.
Polycythemia vera is a chronic and rare myeloproliferative
neoplasm characterized by the abnormal proliferation of red blood
cells.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
September 20, 2023 17:03 ET (21:03 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
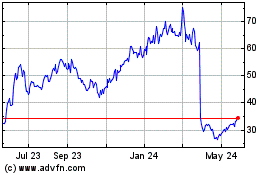
Disc Medicine (NASDAQ:IRON)
Historical Stock Chart
From Apr 2024 to May 2024
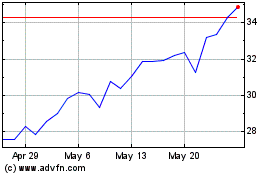
Disc Medicine (NASDAQ:IRON)
Historical Stock Chart
From May 2023 to May 2024