Form 8-K - Current report
December 04 2023 - 9:01AM
Edgar (US Regulatory)
false 0001826892 0001826892 2023-12-04 2023-12-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 4, 2023
BIOATLA, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-39787 |
|
85-1922320 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
|
|
| 11085 Torreyana Road San Diego, California |
|
|
|
92121 |
| (Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: 858 558-0708
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common Stock, $0.0001 par value per share |
|
BCAB |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 8.01 |
Other Information. |
As previously announced, BioAtla, Inc. (the “Company”) will host a virtual Key Opinion Leader Event (“KOL Event”) on Monday, December 4, 2023 at 11:00 a.m. Eastern Time. The KOL Event will feature Carl M. Gay, MD, PhD (MD Anderson Cancer Center), who will review the Phase 2 clinical trial data of mecbotamab vedotin (BA3011), a CAB-AXL-ADC, in refractory non-small cell lung cancer (NSCLC) and include a discussion of AXL as a contributor of therapeutic resistance and a marker of poor prognosis in NSCLC.
Select slides included in the presentation materials to be used at the KOL Event are filed as Exhibit 99.1 hereto and incorporated by reference herein.
Forward-looking statements
Statements in this Current Report on Form 8-K (this “Current Report”) contain “forward-looking statements” that are subject to substantial risks and uncertainties. Forward-looking statements contained in this Current Report may be identified by the use of words such as “anticipate,” “expect,” “believe,” “will,” “may,” “should,” “estimate,” “project,” “outlook,” “forecast” or other similar words. Examples of forward-looking statements include, among others, statements the Company makes regarding its business plans and prospects and whether its clinical trials will support registration; achievement of milestones; results, conduct, progress and timing of its research and development programs and clinical trials; expectations with respect to enrollment and dosing in its clinical trials, plans and expectations regarding future data updates, clinical trials, regulatory meetings and regulatory submissions; plans to form collaborations or other strategic partnerships for selected assets; the potential regulatory approval path for its product candidates; expectations about the sufficiency of its cash and cash equivalents to fund planned operations, which includes plans to not explore additional dosing regimens, delaying certain pre-clinical development programs and to prioritize and focus development on selected assets and indications; and expected R&D and G&A expenses. Forward-looking statements are based on the Company’s current expectations and are subject to inherent uncertainties, risks and assumptions, many of which are beyond the Company’s control, difficult to predict and could cause actual results to differ materially from what the Company expects. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. Factors that could cause actual results to differ include, among others: potential delays in clinical and pre-clinical trials; the uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for clinical trials, regulatory submission dates, or regulatory approval dates, as well as the possibility of unfavorable new clinical data and further analyses of existing clinical data; whether regulatory authorities will be satisfied with the design of and results from the clinical studies or take favorable regulatory actions based on results from the clinical studies; the Company’s dependence on the success of its CAB technology platform; the Company’s ability to enroll patients in its ongoing and future clinical trials; the successful selection and prioritization of assets to focus development on selected product candidates and indications; the Company’s ability to form collaborations and partnerships with third parties and the success of such collaborations and partnerships; the Company’s reliance on third parties for the manufacture and supply of its product candidates for clinical trials; the Company’s reliance on third parties to conduct its clinical trials and some aspects of its research and preclinical testing; potential adverse impacts due to any resurgence of COVID-19 and its variants and those other risks and uncertainties described in the section titled “Risk Factors” in the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on March 23, 2023, in its Quarterly Reports on Form 10-Q filed with the SEC on May 11, 2023, August 1, 2023 and November 7, 2023 and its other reports as filed with the SEC. Forward-looking statements contained in this Current Report are made as of this date, and the Company undertakes no duty to update such information except as required under applicable law.
| Item 9.01 |
Financial Statements and Exhibits. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
BioAtla, Inc. |
|
|
|
|
| Date: December 4, 2023 |
|
|
|
By: |
|
/s/ Richard Waldron |
|
|
|
|
|
|
Richard Waldron Chief Financial Officer |

Exhibit 99.1 Phase 2 Trial of Mecbotamab Vedotin (BA3011), CAB-AXL-ADC,
Alone or in Combination with Nivolumab in Patients with Non- Squamous NSCLC BA3011 AXL NSCLC December 4, 2023 BioAtla | Overview 1 confidential

Important Notices & Disclaimers This presentation (the
“Presentation”) by BioAtla, Inc. (“we”, “us”, “our”, “BioAtla”, or the “Company”) contains “forward-looking statements” within the meaning of the Private Securities
Litigation Reform Act of 1995 relating to our business, operations and financial conditions, including but not limited to statements regarding business plans and prospects and whether our clinical trials will support registration; achievement of
milestones; results, conduct, progress and timing of our research and development programs and clinical trials; expectations with respect to enrollment and dosing in our clinical trials, plans and expectations regarding future data updates, clinical
trials, regulatory meetings and regulatory submissions; plans to form collaborations or other strategic partnerships for selected assets; the potential regulatory approval path for our product candidates; expectations about the sufficiency of our
cash and cash equivalents and plans to prioritize and focus development on selected assets and indications. Words such as, but not limited to, “anticipate”, “believe”, “could”, “estimate”,
“expect”, “intend”, “may”, “plan”, “potential”, “predict”, “project”, “should”, “will”, “would” or the negative of those terms,
and similar expressions that convey uncertainty of future events or outcomes, identify forward-looking statements. These forward-looking statements reflect management’s beliefs and views with respect to future events and are based on estimates
and assumptions as of the date of this Presentation and are subject to risks and uncertainties, including those described in the Company's filings with the SEC, including but not limited to the Company's latest Quarterly Report on Form 10-Q.
Moreover, the Company operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for management to predict all risks, nor can the Company assess the impact of all factors on its business
or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on these
forward-looking statements. The Company qualifies all the forward-looking statements in this Presentation by these cautionary statements. Except as required by law, the Company undertakes no obligation to publicly update any forward-looking
statements, whether as a result of new information, future events or otherwise. Statements contained herein are made as of the date of this Presentation unless stated otherwise, and this Presentation shall not under any circumstances create an
implication that the information contained herein is correct as of any time after such date or that the information will be updated or revisited to reflect information that subsequently becomes available or changes occurring after that date hereof.
Certain information contained in this Presentation relates to or is based on statistical and other industry and market data obtained from independent industry publications and research, surveys and studies conducted by independent third parties as
well as the Company’s own estimates of the prevalence of certain diseases and conditions. The market data used in this Presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data.
Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information.
The Company’s estimates of the patient population with the potential to benefit from treatment with any product candidates the Company may develop include several key assumptions based on its industry knowledge, industry publications and
third-party research, which may be based on a small sample size and may fail to accurately reflect the addressable patient population. While the Company believes that its internal assumptions are reasonable, no independent source has verified such
assumptions. This Presentation may contain trademarks, trade names, or service marks belonging to other entities. The Company does not intend the use or display of other parties’ trade names, trademarks or service marks to imply a relationship
with, or endorsement or sponsorship of, or by these other parties. None of the Company or any of its directors, officers, employees, contractors, agents, consultants, advisors or other representatives makes any representation or warranty, express or
implied, as to the accuracy or completeness of the information contained in this Presentation.
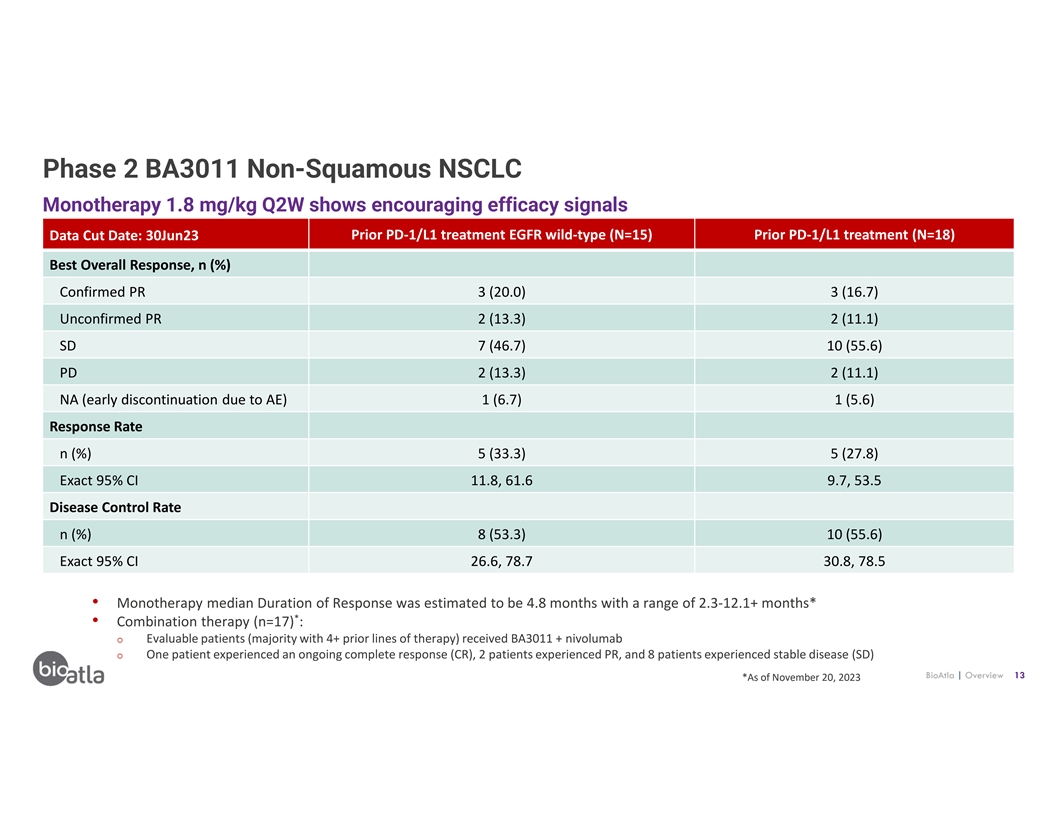
Phase 2 BA3011 Non-Squamous NSCLC Monotherapy 1.8 mg/kg Q2W shows
encouraging efficacy signals Data Cut Date: 30Jun23 Prior PD-1/L1 treatment EGFR wild-type (N=15) Prior PD-1/L1 treatment (N=18) Best Overall Response, n (%) Confirmed PR 3 (20.0) 3 (16.7) Unconfirmed PR 2 (13.3) 2 (11.1) SD 7 (46.7) 10 (55.6) PD 2
(13.3) 2 (11.1) NA (early discontinuation due to AE) 1 (6.7) 1 (5.6) Response Rate n (%) 5 (33.3) 5 (27.8) Exact 95% CI 11.8, 61.6 9.7, 53.5 Disease Control Rate n (%) 8 (53.3) 10 (55.6) Exact 95% CI 26.6, 78.7 30.8, 78.5 • Monotherapy median
Duration of Response was estimated to be 4.8 months with a range of 2.3-12.1+ months* * • Combination therapy (n=17) : o Evaluable patients (majority with 4+ prior lines of therapy) received BA3011 + nivolumab o One patient experienced an
ongoing complete response (CR), 2 patients experienced PR, and 8 patients experienced stable disease (SD) BioAtla | Overview 13 *As of November 20, 2023
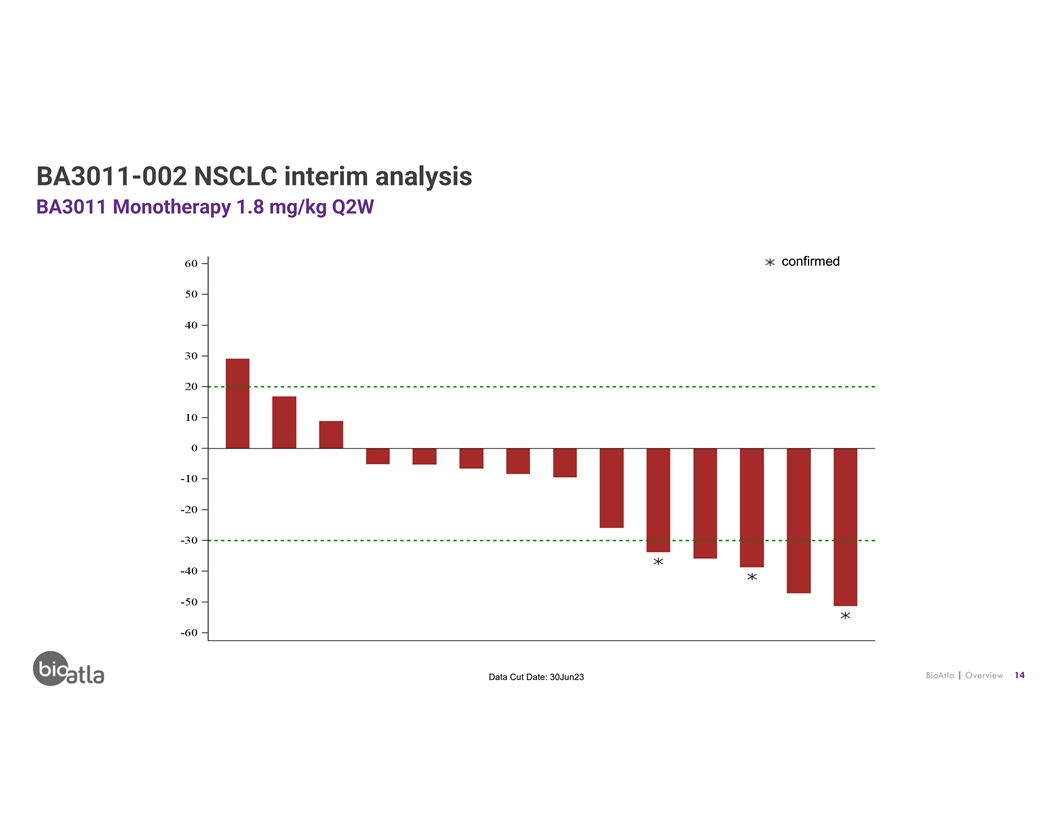
BA3011-002 NSCLC interim analysis BA3011 Monotherapy 1.8 mg/kg Q2W
confirmed BioAtla | Overview 14 Data Cut Date: 30Jun23

BA3011-002 NSCLC interim analysis BA3011 Monotherapy 1.8 mg/kg Q2W
BioAtla | Overview 15 Data Cut Date: 30Jun23
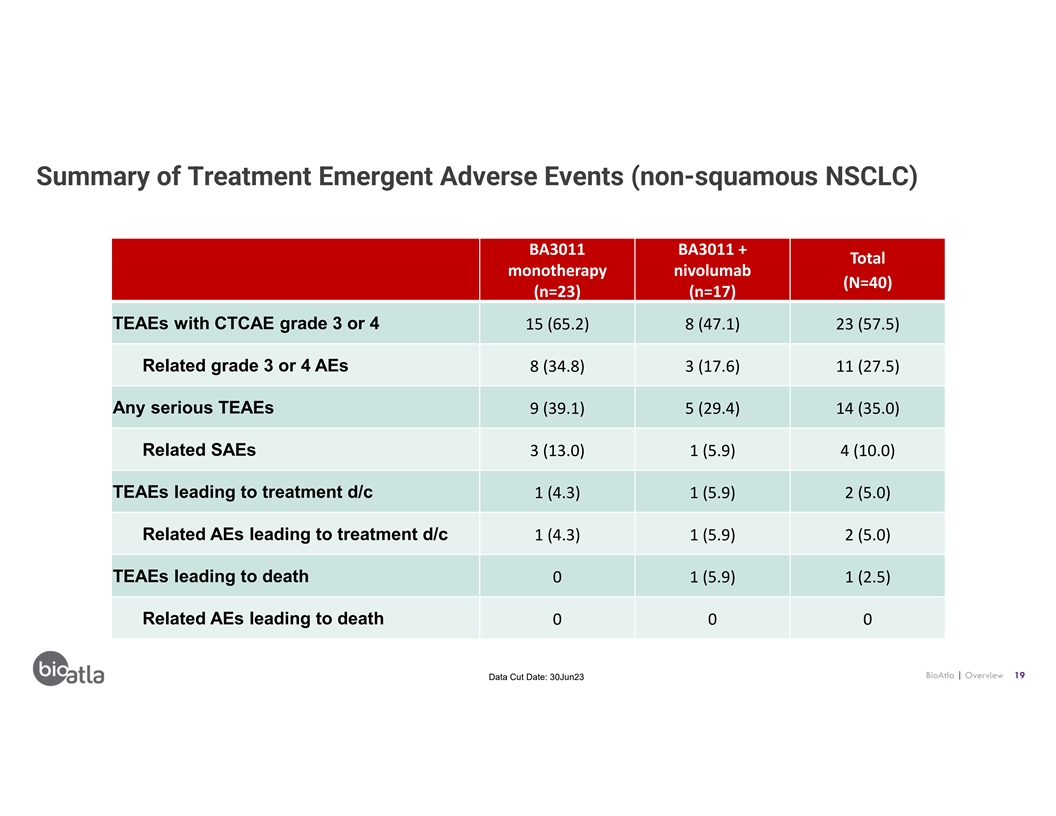
Summary of Treatment Emergent Adverse Events (non-squamous NSCLC) BA3011
BA3011 + Total monotherapy nivolumab (N=40) (n=23) (n=17) TEAEs with CTCAE grade 3 or 4 15 (65.2) 8 (47.1) 23 (57.5) Related grade 3 or 4 AEs 8 (34.8) 3 (17.6) 11 (27.5) Any serious TEAEs 9 (39.1) 5 (29.4) 14 (35.0) Related SAEs 3 (13.0) 1 (5.9) 4
(10.0) TEAEs leading to treatment d/c 1 (4.3) 1 (5.9) 2 (5.0) Related AEs leading to treatment d/c 1 (4.3) 1 (5.9) 2 (5.0) TEAEs leading to death 0 1 (5.9) 1 (2.5) Related AEs leading to death 0 0 0 BioAtla | Overview 19 Data Cut Date:
30Jun23

Treatment Emergent Adverse Events (Non-Squamous NSCLC) * Any grade
(≥15% of patients) OR grade ≥3 (≥3% of patients) in the study population Preferred term TEAEs of any grade, n (%) TEAEs of grade 3, n (%) Fatigue 14 (35.0) 1 (2.5) Diarrhea 10 (25.0) 1 (2.5) Constipation 9 (22.5) 0 Decreased
appetite 9 (22.5) 1 (2.5) Anemia 8 (20.0) 2 (5.0) Nausea 8 (20.0) 0 Peripheral neuropathy 7 (17.5) 1 (2.5) Increased AST 7 (17.5) 3 (7.5) Dyspnea 6 (15.0) 2 (5.0) Neutropenia 6 (15.0) 2 (5.0) Increased ALT 5 (12.5) 3 (7.5) * No grade 4+ TEAEs among
most frequent. BioAtla | Overview 20 Data Cut Date: 30Jun23

BA3011 NSCLC Randomized Registrational Study Design Two Potentially
Registrational Paths Enabled via the FDA Type C Meeting nd 2 Line + • Open-label; control: docetaxel • Patients with NSCLC who have been previously treated with at least one prior line of therapy for metastatic disease • Dual
primary endpoints: Progression Free Survival and Overall Survival rd 3 Line + • Blinded; control: chemo monotherapy • Patients with NSCLC who have been previously treated with at least two prior lines of therapy for metastatic disease
• Primary endpoint: Overall Survival BioAtla | Overview 23
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
BioAtla (NASDAQ:BCAB)
Historical Stock Chart
From Dec 2024 to Jan 2025
BioAtla (NASDAQ:BCAB)
Historical Stock Chart
From Jan 2024 to Jan 2025