Autolus Therapeutics to Present Three Clinical Data Updates on obecabtagene autoleucel (obe-cel) in relapsed/refractory (r/r) B-Cell acute lymphoblastic leukemia (ALL) patients at the 2024 European Hematology Association (EHA) Congress
May 14 2024 - 10:00AM

Autolus Therapeutics plc (Nasdaq: AUTL), a clinical-stage
biopharmaceutical company developing next-generation programmed T
cell therapies, today announces the online publication of three
abstracts submitted to the European Hematology Association (EHA)
Congress, to be held June 13-16, 2024.
Oral presentation:
- Title: obecabtagene
autoleucel in adult relapsed/refractory B Cell acute lymphoblastic
leukemia: Survival and potential impact of CAR-T cell persistence
and stem cell transplantation in the FELIX
studySession Title: s419 Acute
lymphoblastic leukemia - Clinical 1: Immunotherapy: antibodies and
CAR-T cellsSession date and time: Friday, June 14
from 14:45 - 16:00 CESTSession room:
N104Final Abstract Code: S114Presenting
Author: Dr. Claire Roddie
Poster presentations:
- Title: obecabtagene autoleucel
(obe-cel, AUTO1) for relapsed/refractory adult B-Cell acute
lymphoblastic leukemia (R/R B-ALL): The impact of
inotuzumab-containing bridging therapy on treatment
outcomesSession Title: Poster
sessionSession date and time: Friday, June 14 from
18:00 - 19:00 CESTFinal Abstract Code:
P418Presenting Author: Dr. Jae H. Park
- Title: Droplet digital PCR and flow cytometry
sensitivity for measuring CAR-T cell kinetics in adult patients
with relapsed/refractory B Cell acute lymphoblastic leukemia
treated with obecabtagene autoleucelSession
Title: Poster sessionSession date and
time: Friday, June 14 from 18:00 - 19:00 CESTFinal
Abstract Code: P1469Presenting Author:
Dr. Claire Roddie
About Autolus Therapeutics
plcAutolus is a clinical-stage biopharmaceutical company
developing next-generation, programmed T cell therapies for the
treatment of cancer and autoimmune disease. Using a broad suite of
proprietary and modular T cell programming technologies, Autolus is
engineering precisely targeted, controlled and highly active T cell
therapies that are designed to better recognize target cells, break
down their defense mechanisms and eliminate these cells. Autolus
has a pipeline of product candidates in development for the
treatment of hematological malignancies, solid tumors and
autoimmune diseases. For more information, please visit
www.autolus.com
About
obe-cel (AUTO1)Obe-cel is a CD19 CAR T cell
investigational therapy designed to overcome the limitations in
clinical activity and safety compared to current CD19 CAR T cell
therapies. Obe-cel is designed with a fast target binding
off-rate to minimize excessive activation of the programmed T
cells. In clinical trials of obe-cel, this “fast off-rate” profile
reduced toxicity and T cell exhaustion, resulting in improved
persistence and leading to high levels of durable remissions in r/r
Adult ALL patients. The results of the FELIX trial, a pivotal trial
for adult ALL, have been submitted and accepted by the FDA with a
PDUFA target action date of November 16, 2024. A regulatory
submission to the EMA was made in the first half of 2024. In
collaboration with Autolus’ academic partner, UCL, obe-cel is
currently being evaluated in a Phase 1 clinical trials for
B-NHL.
About obe-cel
FELIX clinical trialAutolus’ Phase 1b/2 clinical
trial of obe-cel enrolled adult patients with relapsed / refractory
B-precursor ALL. The trial had a Phase 1b component prior to
proceeding to the single arm, Phase 2 clinical trial. The primary
endpoint was overall response rate, and the secondary endpoints
included duration of response, MRD negative CR rate and safety. The
trial enrolled over 100 patients across 30 of the leading academic
and non-academic centers in the United States, United
Kingdom and Europe. [NCT04404660]
Contact:
Olivia Manser+44 (0) 7780 471
568o.manser@autolus.com
Julia Wilson+44 (0) 7818
430877j.wilson@autolus.com
Susan A. NoonanS.A. Noonan
Communications+1-917-513-5303susan@sanoonan.com
Autolus Therapeutics (NASDAQ:AUTL)
Historical Stock Chart
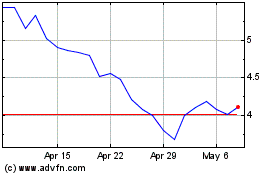
From Apr 2024 to May 2024
Autolus Therapeutics (NASDAQ:AUTL)
Historical Stock Chart
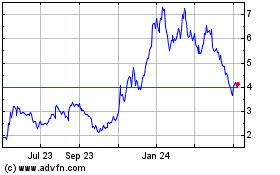
From May 2023 to May 2024