UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 6-K
REPORT OF
FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR
15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the Month of August 2023
Commission File Number: 001-39621
OPTHEA LIMITED
(Translation of registrant’s name into English)
Level 4
650 Chapel Street
South Yarra, Victoria 3141
Australia
(Address of
principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
OPTHEA LIMITED |
|
|
|
|
| Date: August 24, 2023 |
|
|
|
By: |
|
/s/ Megan Baldwin |
|
|
|
|
|
|
Megan Baldwin, Ph.D. |
|
|
|
|
|
|
Chief Executive Officer and Managing Director |

Equity Raising Presentation
Institutional Placement and Accelerated Non-Renounceable Entitlement Offer (“ANREO”) August 2023 OPTHEA.COM | @OptheaLimited | ASX (OPT.AX); NASDAQ: OPT Exhibit 99.1

Important Notice and Disclaimer
IMPORTANT: You must read the following before continuing or making use of the information contained in this presentation. By attending an investor presentation, or accepting or viewing this presentation you represent and warrant that you are
entitled to receive or access the presentation in accordance with the restrictions below and agree to be bound by the limitations contained within it. This presentation is dated 24 August 2023 and has been prepared by Opthea Limited (ABN32 006 340
567) (“Company” or “Opthea”) in relation to: a placement of new fully paid ordinary shares in the Company (“New Shares”) to certain eligible institutional investors (“Placement”); and an accelerated
non-renounceable entitlement offer of New Shares to be made to existing eligible shareholders of Opthea (“Entitlement Offer”), the Placement and Entitlement Offer together, the “Offer”. For every two (2) New Shares issued
under the Offer, one (1) option will be issued. The option will have an exercise price of A$0.80 and an expiry date of 31 August 2025 (“New Options”). Application will be made for the options to be quoted on ASX. The Company reserves the
right to withdraw, or vary the timetable for, the Offer without notice. Summary information This presentation contains summary information about the Company and its activities and is current as at the date of this presentation. The information in
this presentation is of a general nature and does not purport to be complete nor does it provide or contain all the information that would be required in a prospectus or other disclosure document prepared in accordance with the requirements of the
Corporations Act 2001 (Cth) (“Corporations Act”), or that an investor should consider when making an investment decision. No representation or warranty, express or implied, is provided in relation to the accuracy or completeness of the
information. Statements in this presentation are made only as of the date of this presentation unless otherwise stated and the information in this presentation remains subject to change without notice. The Company is not responsible for updating,
nor undertakes to update, this presentation. It should be read in conjunction with the Company’s other periodic and continuous disclosure announcements lodged with the Australian Securities Exchange (“ASX”), which are available at
www.asx.com.au, and with the U.S. Securities and Exchange Commission (the “SEC”), which are available at www.sec.gov. The Company has also prepared a target market determination in respect of the New Options which is available on the
Company’s website at https://opthea.com/. This presentation may also include information derived from public or third-party sources, including public filings, research, surveys or studies conducted by third parties, including industry or
general publications and other publicly available information, that has not been independently verified. Neither Opthea nor any of its subsidiaries or any of the respective directors, officers, employees, representatives, agents or advisers of
Opthea or its subsidiaries (“Opthea Related Persons”) makes any representation or warranty with respect to the fairness, accuracy, completeness or adequacy of such information. Not financial product advice This presentation is for
information purposes only and is not a prospectus, product disclosure statement or other offer document under Australian law or the law of any other jurisdiction. This presentation has not been, nor will it be, lodged with the Australian Securities
and Investments Commission (“ASIC”). This presentation is not a financial product or investment advice, a recommendation to acquire New Shares, New Options or accounting, legal or tax advice. It has been prepared without taking into
account the objectives, financial or tax situation or needs of individuals. Any references to, or explanations of, legislation, regulatory issues or any other legal commentary (if any) are indicative only, do not summarize all relevant issues and
are not intended to be a full explanation of a particular matter. You are solely responsible for forming your own opinions and conclusions on such matters and the market, and for making your own independent assessment of the information in this
presentation. Before making an investment decision, prospective investors should consider the appropriateness of the information having regard to their own objectives, financial and tax situation and needs, and seek legal, taxation and other
professional advice appropriate to their circumstances. The Company is not licensed to provide financial product advice in respect of its securities. Cooling off rights do not apply to the acquisition of New Shares or New Options. Effect of rounding
A number of figures, amounts, percentages, estimates, calculations of value and fractions in this presentation are subject to the effect of rounding. Accordingly, the actual calculation of these figures may differ from the figures set out in this
presentation. Past performance Information relating to past performance and activities included in this presentation is given for illustrative purposes only and should not be relied upon as (and is not) an indication of the Company’s views on
its future performance or condition. Investors should note that past performance, including the past share price performance of Opthea, cannot be relied upon as an indicator of (and provides no guidance as to) future performance, including future
share price performance. The historical information included in this presentation is, or is based on, information that has previously been released to the market and is not represented as being indicative of Opthea’s views on its future
financial condition and/or performance. Trademarks and trade names This presentation may contain trademarks and trade names of third parties, which are the property of their respective owners. Third party trademarks and trade names used in this
presentation belong to the relevant owners and use is not intended to represent sponsorship, approval or association by or with any of Opthea or the Extended Parties (defined below). 2

Important Notice and Disclaimer
Financial data All dollar values are in United States dollars ($ or US$) unless stated otherwise. This presentation includes pro forma financial information which is provided for illustrative purposes only and is not represented as being indicative
of the Company’s (or anyone else's) views on the Company’s future financial position or performance. The pro-forma financial information included in this presentation is for illustrative purposes and does not purport to be in compliance
with Article 11 of Regulation S-X of the rules and regulations of the U.S. Securities and Exchange Commission. Any conversion of amounts in US$ to amounts in A$ has been conducted at the exchange rate of 0.64. Preliminary Financial Information
Throughout this presentation, we have presented certain preliminary estimated unaudited financial results and other data as of and for the fiscal year ended June 30, 2023, including preliminary estimated cash and cash equivalent amounts as of June
30, 2023. The estimated results are not a comprehensive statement of our results as of and for the fiscal year ended June 30, 2023, and our actual results may differ materially from these preliminary estimated results. Our actual results remain
subject to the completion of management’s and our audit and risk committee’s reviews and our other financial closing processes. During the course of the preparation of our consolidated financial statements and related notes and the
completion of the audit for the fiscal year ended June 30, 2023, additional adjustments to the preliminary estimated financial information presented in this presentation may be identified, and our final results for these periods may vary from these
preliminary estimates. The preliminary estimated unaudited financial and other data contained in this presentation have been prepared in good faith by, and are the responsibility of, management based upon our internal reporting as of and for the
fiscal year ended June 30, 2023. Deloitte Touche Tohmatsu, our independent registered public accounting firm, has not audited, reviewed, compiled or performed any procedures with respect to such preliminary data. Accordingly, Deloitte Touche
Tohmatsu does not express an opinion or any other form of assurance with respect thereto. Future performance / forward-looking statements This presentation contains certain forward-looking statements. The words "expect", "anticipate", "estimate",
"intend", "believe", "guidance", "should", "could", "may", "will", "predict", "plan" and other similar expressions are intended to identify forward-looking statements. Indications of, and guidance on, future financial position and performance
including the preliminary estimated unaudited financial information and pro forma data, are also forward-looking statements. Forward-looking statements in this presentation include statements regarding the timetable, conduct and outcome of the Offer
and the use of the proceeds thereof, the therapeutic and commercial potential and size of estimated market opportunity of the Company’s product in development, the viability of future opportunities, future market supply and demand, the
expected receipt of payments (including the additional potential increase of US$50 million of funding under the Development Funding Agreement (“DFA”)) and the timing of such payments, the expected cash runway, the expected timing of
completion of patient enrollment under the clinical trials and timing of top-line data, expectations about topline data based on masked pooled data, the financial condition, results of operations and businesses of Opthea, certain plans, objectives
and strategies of the management of Opthea, including with respect to the current and planned clinical trials of its product candidate, and the future performance of Opthea. Forward-looking statements, opinions and estimates provided in this
presentation are based on assumptions and contingencies which are subject to change without notice, as are statements about market and industry trends, which are based on interpretations of current conditions. Forward-looking statements, including
projections, guidance on the future financial position of the Company including the preliminary estimated unaudited financial information and pro forma data, are provided as a general guide only and should not be relied upon as an indication or
guarantee of future performance. They involve known and unknown risks and uncertainties and other factors, many of which are beyond the control of Opthea and its directors and management and may involve significant elements of subjective judgment
and assumptions as to future events that may or may not be correct. These statements may be affected by a range of variables which could cause actual results or trends to differ materially, including but not limited to the risks described in this
presentation under “Key Risks”, including risks associated with: the availability of funding, the receipt of funding under the DFA (including the additional potential increase of US$50 million of funding under the DFA), future capital
requirements, the development, testing, production, marketing and sale of drug treatments, regulatory risk and potential loss of regulatory approvals, ongoing clinical studies to demonstrate OPT-302 safety, tolerability and therapeutic efficacy,
additional analysis of data from Opthea’s Phase 3 clinical trials once unmasked, timing of completion of Phase 3 clinical trial patient enrollment and CRO and labor costs, intellectual property protections, the successful completion of the
Offer, completion of management’s and the Company’s audit and risk committee’s review and the Company’s other closing processes, and other factors that are of a general nature which may affect the future operating and
financial performance of the Company. No representation, warranty or assurance (express or implied) is given or made in relation to any forward-looking statement by any person (including the Company and Opthea Related Persons). In particular, no
representation, warranty or assurance (express or implied) is given that the occurrence of the events expressed or implied in any forward-looking statements in this presentation will actually occur. Actual results, performance or achievement may
vary materially from any projections and forward-looking statements and the assumptions on which those statements are based. The forward-looking statements in this presentation speak only as of the date of this presentation. Subject to any
continuing obligations under applicable law or any relevant ASX listing rules, the Company and Opthea Related Persons disclaim any obligation or undertaking to provide any updates or revisions to any forward-looking statements in this presentation
to reflect any change in expectations in relation to any forward-looking statements or any change in events, conditions or circumstances on which any such statement is based. Nothing in this presentation will create an implication that there has
been no change in the affairs of Opthea since the date of this presentation.

Important Notice and Disclaimer
Diagram, charts, graphs and tables Any diagrams, charts, graphs and tables appearing in this presentation are illustrative only and may not be drawn to scale. Investment risk An investment in New Shares and New Options is subject to investment and
other known and unknown risks, many of which are beyond the control of Opthea. Opthea does not guarantee any particular rate of return on the New Shares, New Options or their respective performance, nor does it guarantee any particular tax
treatment. In considering an investment in New Shares and New Options, investors should have regard to (among other things) the risks and disclosures outlined in this presentation, including the “Key Risks” section of this presentation,
before making an investment decision, and should consult their professional adviser(s) if they are in any doubt about what to do. Not an offer This presentation is not a disclosure document and should not be considered as investment advice. This
presentation is for information purposes only and should not be considered an offer or an invitation to acquire, or a solicitation or recommendation in relation to the subscription, purchase or sale of Company securities (including the New Shares
and New Options to be offered and sold in the Offer) or any other financial products and does not and will not form any part of any contract for the acquisition of New Shares or New Options. This presentation does not constitute an offer to sell, or
the solicitation of an offer to buy, any securities in the United States or any other jurisdiction in which such an offer would be illegal or impermissible. The New Shares and New Options to be issued in the Offer have not been, and will not be,
registered under the U.S. Securities Act of 1933, as amended (the “Securities Act”) or the securities laws of any state or other jurisdiction of the United States. The New Shares and New Options to be issued in the Offer may not be
offered and sold to, directly or indirectly, any person in the United States except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the U.S. Securities Act and applicable U.S. state securities
laws. The release, publication or distribution of this presentation (including an electronic copy) in other jurisdictions outside Australia may also be restricted by law and any such restrictions should be observed. If you come into possession of
this presentation, you should observe such restrictions and should seek your own advice on such restrictions. Any failure to comply with such restrictions may constitute a violation of applicable securities laws. Refer to the “Foreign Selling
Restrictions” section of this presentation for more information. By accepting this presentation you represent and warrant that you are entitled to receive such presentation in accordance with the above restrictions and agree to be bound by the
limitations contained herein. Disclaimer The lead manager and underwriter of the Offer (“Lead Manager”), nor any of the Company’s advisers or any of their respective affiliates, related bodies corporate, directors, officers,
partners, employees, agents and associates, have authorised, permitted or caused the issue, submission, dispatch or provision of this presentation and, except to the extent referred to in this presentation, none of them makes or purports to make any
statement in this presentation and there is no statement in this presentation which is based on any statement by any of them. To the maximum extent permitted by law, the Company, the Lead Manager, and their respective affiliates, related bodies
corporate, directors, officers, partners, employees, agents and advisers (the “Extended Parties”): (i) exclude and disclaim all liability, for any expenses, losses, damages or costs incurred by you as a result of your participation in
any offer of the New Shares and New Options and the information in this presentation being inaccurate or incomplete in any way for any reason, whether by negligence or otherwise; and (ii) make no representation or warranty, express or implied, as to
the currency, accuracy, reliability, timeliness or completeness of information in this presentation, or likelihood of fulfilment of any forward-looking statement or any event or results expressed or implied in any forward-looking statement. Pursuant
to ASX Listing Rule 7.2, the directors of the Company give notice that they reserve the right to issue any New Shares (and New Options) not issued in the Entitlement Offer (“Shortfall Shares”) to new investors or existing shareholders
within 3 months of the close of the offer at a price no less than the Offer Price. The allocation of Shortfall Shares will be within the complete discretion of the Company, having regard to factors such as the Company’s desire for an informed
and active trading market, its desire to establish a wide spread of shareholders, the size and type of funds under management of particular investors, the likelihood that particular investors will be long-term shareholders, and any other factors the
Company considers appropriate. You acknowledge and agree that determination of eligibility of investors for the purposes of the Offer is determined by reference to a number of matters, including legal requirements and the discretion of the Company
and the Lead Manager and each of the Company and the Lead Manager (and their respective Extended Parties) disclaim any duty or liability (including for negligence) in respect of the exercise or otherwise of that discretion, to the maximum extent
permitted by law. Further, you acknowledge and agree that any allocation of New Shares (and New Options) (other than pursuant to an entitlement under the Entitlement Offer) is at the sole discretion of the Company and the Lead Manager and each of
the Company and the Lead Manager (and their respective Extended Parties) disclaim any duty or liability (including for negligence) in respect of the exercise or otherwise of that discretion, to the maximum extent permitted by law. The Company and
the Lead Manager reserve the right to change the timetable in their absolute discretion including by closing the Offer early, withdrawing the Offer entirely or extending the Offer closing time (generally or for particular investor(s)) in their
absolute discretion (but have no obligation to do so), without recourse to them or notice to you. Acceptance By attending a presentation or briefing, or accepting, accessing or reviewing this presentation, you acknowledge and agree to the terms set
out in this important notice and disclaimer, including any modifications to them.
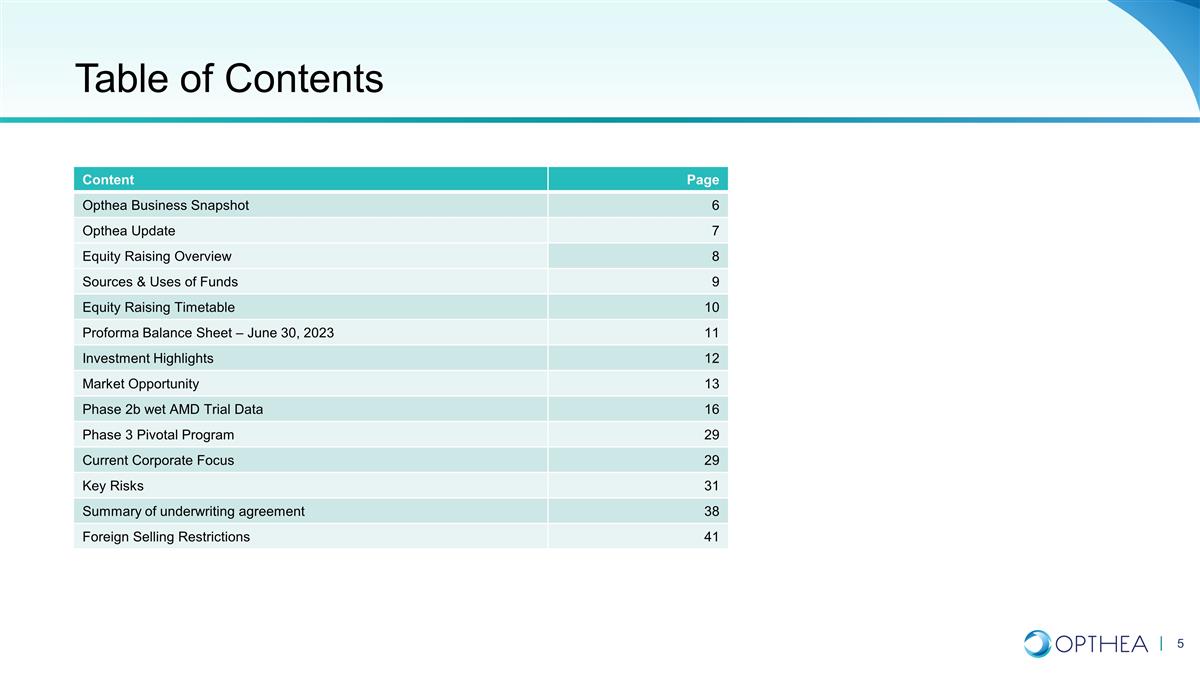
Table of Contents Content Page Opthea
Business Snapshot 6 Opthea Update 7 Equity Raising Overview 8 Sources & Uses of Funds 9 Equity Raising Timetable 10 Proforma Balance Sheet – June 30, 2023 11 Investment Highlights 12 Market Opportunity 13 Phase 2b wet AMD Trial Data 16
Phase 3 Pivotal Program 29 Current Corporate Focus 29 Key Risks 31 Summary of underwriting agreement 38 Foreign Selling Restrictions 41

Opthea Business Snapshot Opthea
Limited Public company listed on ASX (ASX:OPT; Nasdaq:OPT) developing sozinibercept (OPT-302) for wet Age-related Macular Degeneration (“AMD”) Market capitalization prior to capital raise of approximately A$280M at 23 August 2023 and
cash on hand of US$89M at 30 June 2023 (unaudited) OPT-302 has a novel mechanism of action OPT-302 is a ‘trap’ inhibitor of VEGF-C and VEGF-D designed specifically for the eye In combination with anti-VEGF-A therapies, blocks VEGFR-2 and
VEGFR-3 activity Targets mechanisms of resistance and sub-optimal clinical response to existing therapies Large commercial potential Current and growing market opportunity estimated at >US$8B+ worldwide for wet AMD OPT-302 being developed for use
in combination with any of the existing anti-VEGF-A agents, biosimilars or novel therapies in development for wet AMD A novel approach seeking to provide additional visual acuity benefit over standard of care Potential future extension to Diabetic
Macular Edema (“DME”), Retinal Vein Occlusion (“RVO”) and other retinal clinical pathologies Primary endpoint met in Phase 2b study of OPT-302 in wet AMD OPT-302 combination therapy demonstrated superiority in visual acuity
over ranibizumab (Lucentis®) alone at 24 weeks in an international, randomized, controlled, double-masked trial of 366 patients Secondary endpoint results also supportive of primary outcome Pre-specified sub-group analyses suggest greater
activity of OPT-302 in lesion-types considered more difficult to treat with anti-VEGF-A therapy and highest unmet need Intellectual property covering OPT-302 to 2034 Granted patents in the USA (2), Europe (validated in all countries), Australia,
Brazil, Canada, China, Columbia, Indonesia, Israel, India, Japan, Korea, Mexico, Malaysia, New Zealand, Russia, Singapore & South Africa Divisional applications pending in Europe, Malaysia & USA Application pending in Philippines
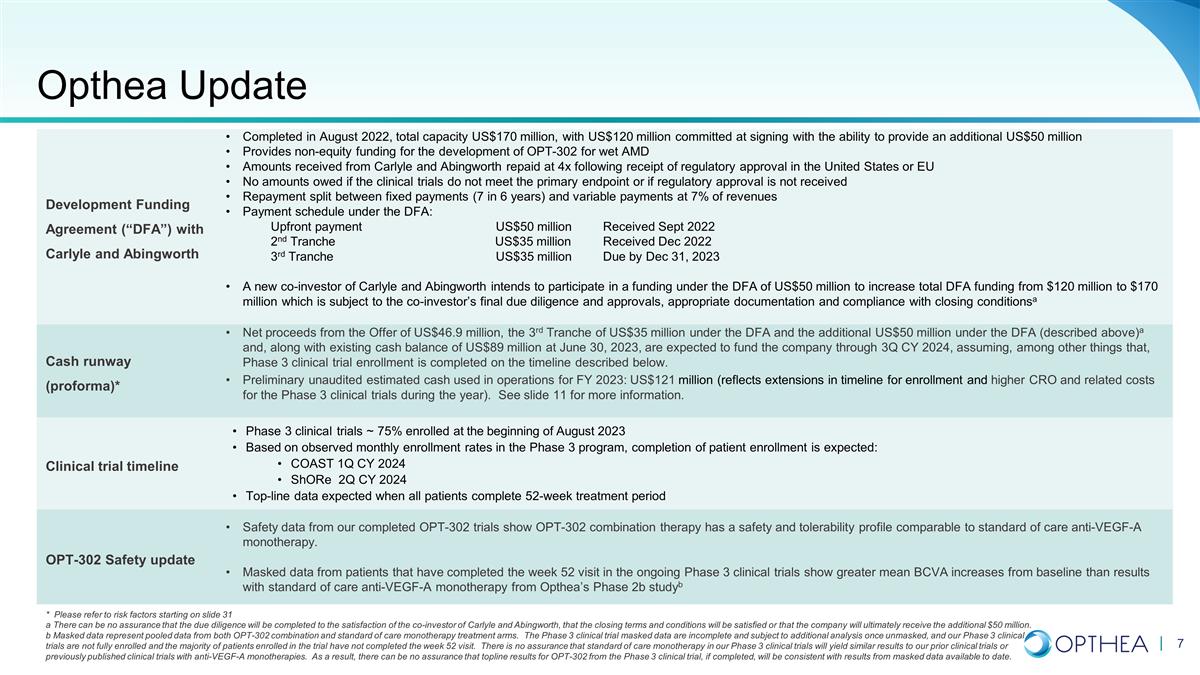
Opthea Update Development Funding
Agreement (“DFA”) with Carlyle and Abingworth Completed in August 2022, total capacity US$170 million, with US$120 million committed at signing with the ability to provide an additional US$50 million Provides non-equity funding for the
development of OPT-302 for wet AMD Amounts received from Carlyle and Abingworth repaid at 4x following receipt of regulatory approval in the United States or EU No amounts owed if the clinical trials do not meet the primary endpoint or if regulatory
approval is not received Repayment split between fixed payments (7 in 6 years) and variable payments at 7% of revenues Payment schedule under the DFA: Upfront paymentUS$50 million Received Sept 2022 2nd Tranche US$35 million Received Dec 2022 3rd
TrancheUS$35 million Due by Dec 31, 2023 A new co-investor of Carlyle and Abingworth intends to participate in a funding under the DFA of US$50 million to increase total DFA funding from $120 million to $170 million which is subject to the
co-investor’s final due diligence and approvals, appropriate documentation and compliance with closing conditionsa Cash runway (proforma)* Net proceeds from the Offer of US$46.9 million, the 3rd Tranche of US$35 million under the DFA and the
additional US$50 million under the DFA (described above)a and, along with existing cash balance of US$89 million at June 30, 2023, are expected to fund the company through 3Q CY 2024, assuming, among other things that, Phase 3 clinical trial
enrollment is completed on the timeline described below. Preliminary unaudited estimated cash used in operations for FY 2023: US$121 million (reflects extensions in timeline for enrollment and higher CRO and related costs for the Phase 3 clinical
trials during the year). See slide 11 for more information. Clinical trial timeline Phase 3 clinical trials ~ 75% enrolled at the beginning of August 2023 Based on observed monthly enrollment rates in the Phase 3 program, completion of patient
enrollment is expected: COAST 1Q CY 2024 ShORe 2Q CY 2024 Top-line data expected when all patients complete 52-week treatment period OPT-302 Safety update Safety data from our completed OPT-302 trials show OPT-302 combination therapy has a safety
and tolerability profile comparable to standard of care anti-VEGF-A monotherapy. Masked data from patients that have completed the week 52 visit in the ongoing Phase 3 clinical trials show greater mean BCVA increases from baseline than results
with standard of care anti-VEGF-A monotherapy from Opthea’s Phase 2b studyb * Please refer to risk factors starting on slide 31 a There can be no assurance that the due diligence will be completed to the satisfaction of the co-investor of
Carlyle and Abingworth, that the closing terms and conditions will be satisfied or that the company will ultimately receive the additional $50 million. b Masked data represent pooled data from both OPT-302 combination and standard of care
monotherapy treatment arms. The Phase 3 clinical trial masked data are incomplete and subject to additional analysis once unmasked, and our Phase 3 clinical trials are not fully enrolled and the majority of patients enrolled in the trial have
not completed the week 52 visit. There is no assurance that standard of care monotherapy in our Phase 3 clinical trials will yield similar results to our prior clinical trials or previously published clinical trials with anti-VEGF-A
monotherapies. As a result, there can be no assurance that topline results for OPT-302 from the Phase 3 clinical trial, if completed, will be consistent with results from masked data available to date.

Assumes AUD/USD exchange rate of
A$0.64 Last closing share price as at Wednesday 23 August 2023 5-day Volume Weighted Average Price (VWAP) to Wednesday 23 August 2023 TERP means the ‘theoretical ex-right price’ at which OPT shares should trade immediately after the
ex-date of the Offer and is adjusted for Placement Shares. TERP is a theoretical calculation only and the actual price at which OPTs shares trade at that time will depend on many factors and may not be equal to the TERP Equity Raising Overview Offer
Structure and Size Opthea is seeking to raise up to approximately A$80.0 million (US$51.2 million1) via the issue of up to approximately 173.9 million new fully paid ordinary shares (“New Shares”) An institutional Placement to raise up
to approximately A$10.0 million (US$6.4) million1 (“Placement”) A 1 for 3.07 pro-rata accelerated non-renounceable entitlement offer (“ANREO”) to raise approximately A$70.0 (US$44.8) million1 (“Entitlement Offer”)
(together, the “Equity Raising” or “Offer”) The Company in its sole discretion reserves the right to raise additional funds under the Placement (“Oversubscriptions”). Any New Shares and New Options issued as a
result of Oversubscriptions, will be issued within Opthea’s available placement capacity under LR 7.1. Options For every two (2) New Shares issued under the Offer, one (1) option will be issued. The option will have an exercise price of A$0.80
and an expiry date of 31 August 2025. The options will be issued post completion of the Retail Entitlement Offer (“New Options”). Offer Price The Equity Raising will be conducted at A$0.46 per New Share representing a 23.3% discount to
the last traded price of $0.6002 23.2% discount to the 5-day VWAP price of $0.5993 18.1% discount to TERP of A$0.5624 Use of Proceeds To continue advancing the clinical development of OPT-302 for the treatment of wet AMD, including to progress the
Phase 3 clinical program and for general corporate purposes Placement and Institutional Entitlement Offer The Placement and institutional Offer will be conducted by way of a bookbuild process on Thursday, 24 August 2023 Entitlements under the
Institutional Entitlement Offer that are not taken-up, entitlements of ineligible institutional shareholders and ineligible retail shareholders under the Entitlement Offer will also be sold in the bookbuild process. Retail Entitlement Offer The
Retail Entitlement Offer will open on Thursday, 31 August 2023 and close on Thursday, 14 September 2023 Eligible existing retail shareholders in Australia and New Zealand have the opportunity to apply for additional New Shares up to 25.0% of their
entitlement under a “Top-up Facility” (subject to scale back at the Company’s discretion) Ranking Each New Share issued under the Equity Raising will rank equally with existing fully paid ordinary shares on issue Underwriting The
Entitlement Offer is fully underwritten by MST Financial Services Pty Ltd “MST”
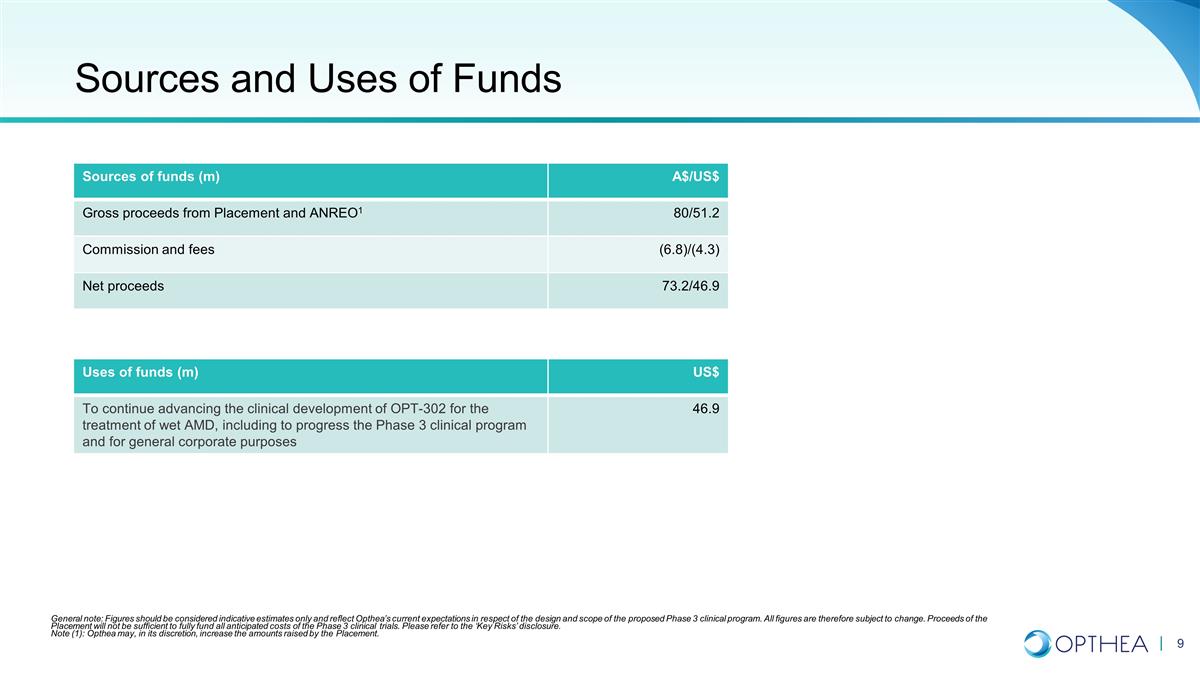
Sources and Uses of Funds Sources of
funds (m) A$/US$ Gross proceeds from Placement and ANREO1 80/51.2 Commission and fees (6.8)/(4.3) Net proceeds 73.2/46.9 Uses of funds (m) US$ To continue advancing the clinical development of OPT-302 for the treatment of wet AMD, including to
progress the Phase 3 clinical program and for general corporate purposes 46.9 General note: Figures should be considered indicative estimates only and reflect Opthea’s current expectations in respect of the design and scope of the proposed
Phase 3 clinical program. All figures are therefore subject to change. Proceeds of the Placement will not be sufficient to fully fund all anticipated costs of the Phase 3 clinical trials. Please refer to the ‘Key Risks’ disclosure. Note
(1): Opthea may, in its discretion, increase the amounts raised by the Placement.
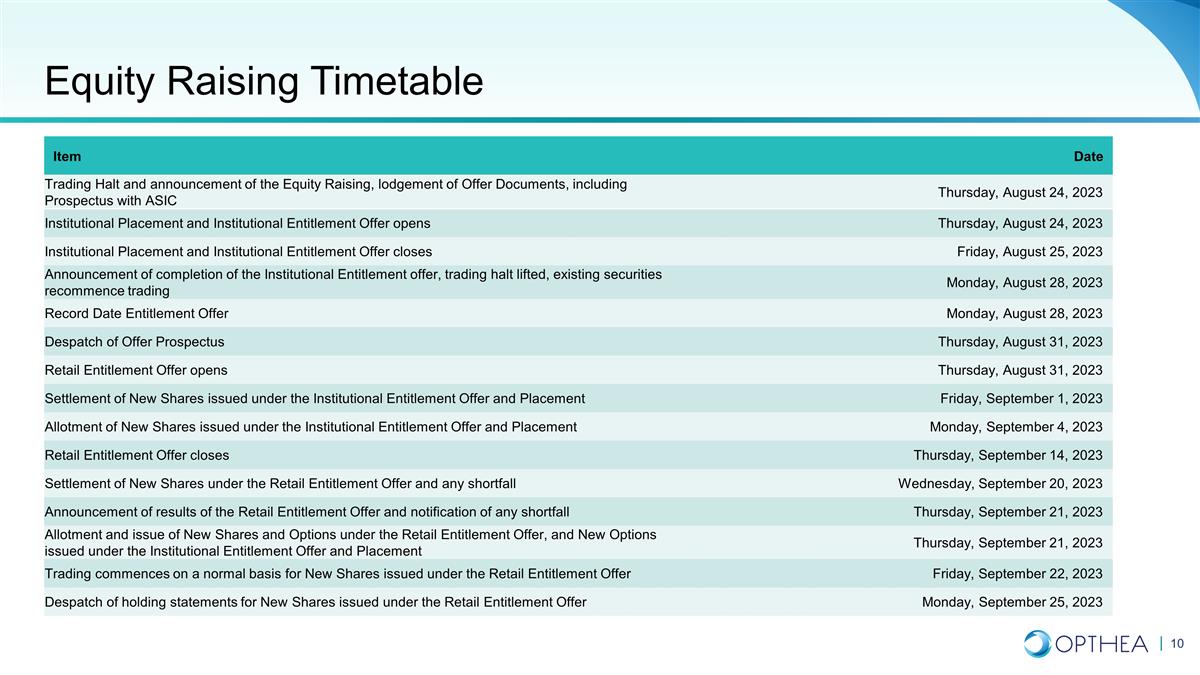
Equity Raising Timetable Item Date
Trading Halt and announcement of the Equity Raising, lodgement of Offer Documents, including Prospectus with ASIC Thursday, August 24, 2023 Institutional Placement and Institutional Entitlement Offer opens Thursday, August 24, 2023 Institutional
Placement and Institutional Entitlement Offer closes Friday, August 25, 2023 Announcement of completion of the Institutional Entitlement offer, trading halt lifted, existing securities recommence trading Monday, August 28, 2023 Record Date
Entitlement Offer Monday, August 28, 2023 Despatch of Offer Prospectus Thursday, August 31, 2023 Retail Entitlement Offer opens Thursday, August 31, 2023 Settlement of New Shares issued under the Institutional Entitlement Offer and Placement Friday,
September 1, 2023 Allotment of New Shares issued under the Institutional Entitlement Offer and Placement Monday, September 4, 2023 Retail Entitlement Offer closes Thursday, September 14, 2023 Settlement of New Shares under the Retail Entitlement
Offer and any shortfall Wednesday, September 20, 2023 Announcement of results of the Retail Entitlement Offer and notification of any shortfall Thursday, September 21, 2023 Allotment and issue of New Shares and Options under the Retail Entitlement
Offer, and New Options issued under the Institutional Entitlement Offer and Placement Thursday, September 21, 2023 Trading commences on a normal basis for New Shares issued under the Retail Entitlement Offer Friday, September 22, 2023 Despatch of
holding statements for New Shares issued under the Retail Entitlement Offer Monday, September 25, 2023
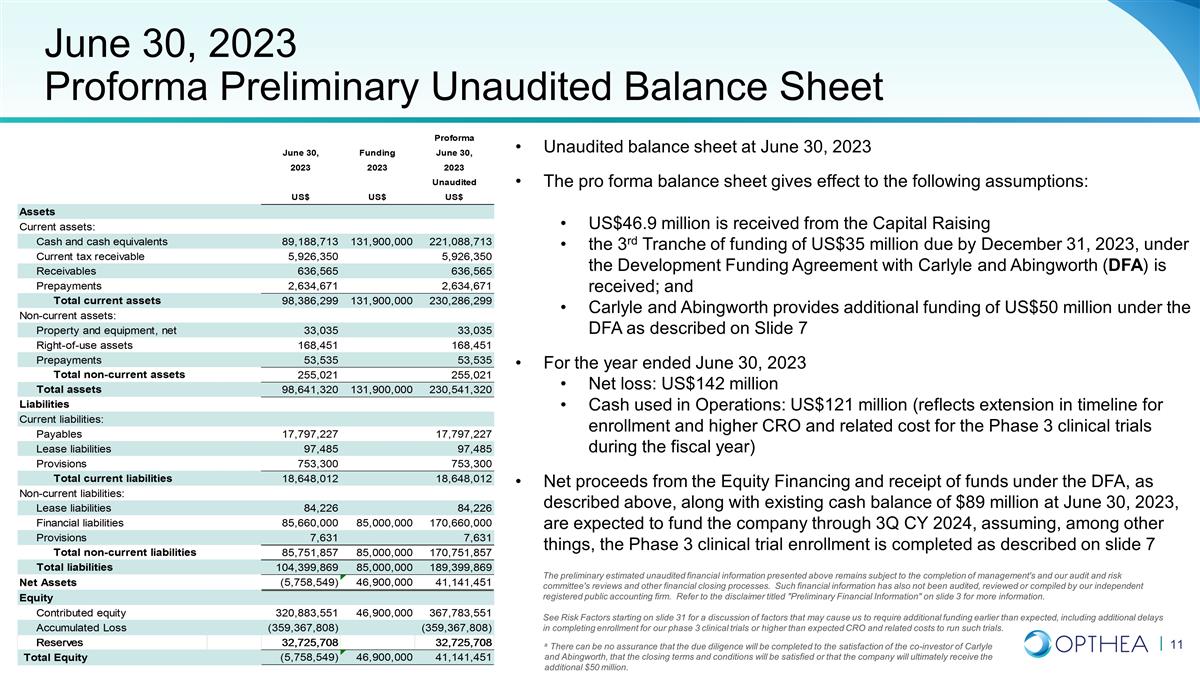
June 30, 2023 Proforma Preliminary
Unaudited Balance Sheet Unaudited balance sheet at June 30, 2023 The pro forma balance sheet gives effect to the following assumptions: US$46.9 million is received from the Capital Raising the 3rd Tranche of funding of US$35 million due by December
31, 2023, under the Development Funding Agreement with Carlyle and Abingworth (DFA) is received; and Carlyle and Abingworth provides additional funding of US$50 million under the DFA as described on Slide 7 For the year ended June 30, 2023 Net loss:
US$142 million Cash used in Operations: US$121 million (reflects extension in timeline for enrollment and higher CRO and related cost for the Phase 3 clinical trials during the fiscal year) Net proceeds from the Equity Financing and receipt of funds
under the DFA, as described above, along with existing cash balance of $89 million at June 30, 2023, are expected to fund the company through 3Q CY 2024, assuming, among other things, the Phase 3 clinical trial enrollment is completed as described
on slide 7 The preliminary estimated unaudited financial information presented above remains subject to the completion of management's and our audit and risk committee's reviews and other financial closing processes. Such financial information has
also not been audited, reviewed or compiled by our independent registered public accounting firm. Refer to the disclaimer titled "Preliminary Financial Information" on slide 3 for more information. See Risk Factors starting on slide 31 for a
discussion of factors that may cause us to require additional funding earlier than expected, including additional delays in completing enrollment for our phase 3 clinical trials or higher than expected CRO and related costs to run such trials. a
There can be no assurance that the due diligence will be completed to the satisfaction of the co-investor of Carlyle and Abingworth, that the closing terms and conditions will be satisfied or that the company will ultimately receive the additional
$50 million.
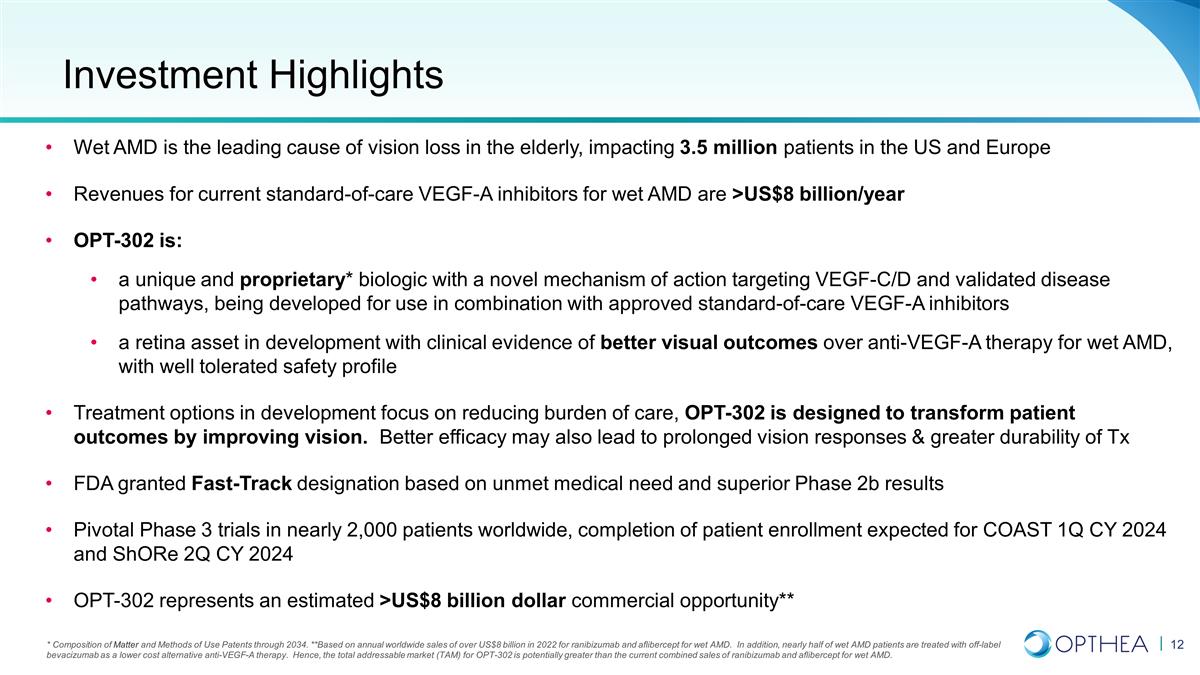
* Composition of Matter and Methods
of Use Patents through 2034. **Based on annual worldwide sales of over US$8 billion in 2022 for ranibizumab and aflibercept for wet AMD. In addition, nearly half of wet AMD patients are treated with off-label bevacizumab as a lower cost
alternative anti-VEGF-A therapy. Hence, the total addressable market (TAM) for OPT-302 is potentially greater than the current combined sales of ranibizumab and aflibercept for wet AMD. Wet AMD is the leading cause of vision loss in the
elderly, impacting 3.5 million patients in the US and Europe Revenues for current standard-of-care VEGF-A inhibitors for wet AMD are >US$8 billion/year OPT-302 is: a unique and proprietary* biologic with a novel mechanism of action targeting
VEGF-C/D and validated disease pathways, being developed for use in combination with approved standard-of-care VEGF-A inhibitors a retina asset in development with clinical evidence of better visual outcomes over anti-VEGF-A therapy for wet AMD,
with well tolerated safety profile Treatment options in development focus on reducing burden of care, OPT-302 is designed to transform patient outcomes by improving vision. Better efficacy may also lead to prolonged vision responses & greater
durability of Tx FDA granted Fast-Track designation based on unmet medical need and superior Phase 2b results Pivotal Phase 3 trials in nearly 2,000 patients worldwide, completion of patient enrollment expected for COAST 1Q CY 2024 and ShORe 2Q CY
2024 OPT-302 represents an estimated >US$8 billion dollar commercial opportunity** Investment Highlights
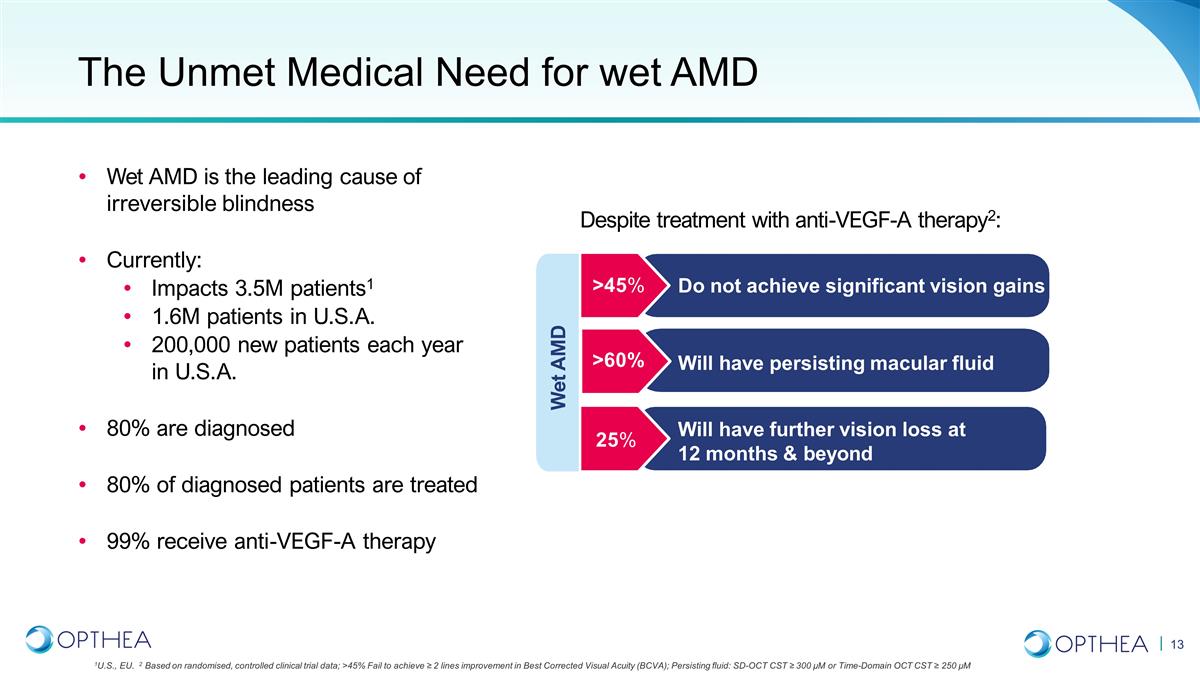
The Unmet Medical Need for wet AMD
>45% >60% 25% Do not achieve significant vision gains Will have persisting macular fluid Will have further vision loss at 12 months & beyond Wet AMD Wet AMD is the leading cause of irreversible blindness Currently: Impacts 3.5M patients1
1.6M patients in U.S.A. 200,000 new patients each year in U.S.A. 80% are diagnosed 80% of diagnosed patients are treated 99% receive anti-VEGF-A therapy Despite treatment with anti-VEGF-A therapy2: 1U.S., EU. 2 Based on randomised, controlled
clinical trial data; >45% Fail to achieve ≥ 2 lines improvement in Best Corrected Visual Acuity (BCVA); Persisting fluid: SD-OCT CST ≥ 300 µM or Time-Domain OCT CST ≥ 250 µM

Wet AMD Large and Growing Market
Opportunity in Wet AMD OPT-302 is Anti-VEGF-A and Durability Agnostic >US$8B ~50% treated patients receive Lucentis® or Eylea ® Potential Addressable Market Wet AMD Off-label use Implied Total Addressable Market for OPT-302 in wet AMD
(Captures Lucentis, Eylea, and Avastin or biosimilar-treated patients worldwide) >$16B OPT-302 is positioned to tap into the entire VEGF-A inhibitor market Total global revenue for Lucentis and Eylea ~50% treated patients receive Avastin ®
*Eye drug Vabysmo blasts off as Roche's biggest growth driver (fiercepharma.com) New entrant trending to $2B* in revenues shows willingness to switch and impact of commercial investment

OPT-302 Combination Therapy
Achieves Broad Blockade of the Validated Pathway in Wet AMD *Bevacizumab is used ’off-label’ for the treatment of neovascular (wet) AMD. # As assessed in cell-based bioassays. VEGF: Vascular Endothelial Growth Factor; VEGFR: Vascular
Endothelial Growth Factor Receptor Used in combination with any VEGF-A inhibitor, OPT-302 blocks# VEGFR-2 and VEGFR-3 signaling, inhibiting the most important pathways driving angiogenesis and vascular leakage VEGF-A inhibition elevates VEGF-C and
VEGF-D which may contribute to sub-optimal clinical efficacy of anti-VEGF-A treatments VEGF-A inhibition elevates VEGF-C and VEGF-D which may contribute VEGF-B VEGF-A VEGF-C OPT-302 VEGFR**-1 VEGFR-2 VEGFR-3 PIGF VEGF-D **Vascular endothelial growth
factor receptor Ranibizumab (Lucentis®) Brolucizumab (Beovu®) Bevacizumab (Avastin®)* Aflibercept (Eylea®) VEGF-B PIGF VEGF-A

OPT-302 Combination Therapy
Clinical Program ShORe Phase 3 wet AMD (n=990) Comparator Ranibizumab once every month COAST Phase 3 wet AMD (n=990) Comparator Aflibercept once every two months after three monthly doses Standard Dosing OPT-302 once every month Monthly dosing
Extended Dosing OPT-302 once every two months after three monthly doses Every two months dosing Treatment naïve patients Standard Dosing OPT-302 once every month Monthly dosing Extended Dosing OPT-302 once every two months after three monthly
doses Every two months dosing Treatment naïve patients Completed Phase 1/2a wet AMD (n=51) Comparator Ranibizumab once every month OPT-302 once every month 3 x monthly dosing Treatment naïve / Prior-treated Comparator Aflibercept once
every month OPT-302 once every month 3 x monthly dosing Prior-treated Completed Phase 1b/2a DME (n=153) Comparator Ranibizumab once every month OPT-302 once every month 6 x monthly dosing Treatment naïve Completed Phase 2b wet AMD (n=366) Now
Recruiting OPT-302 pivotal registrational Phase 3 wet AMD program designed to maximize outcomes with flexible standard of care dosing regimens Note: Ranibizumab (Lucentis®); Aflibercept (Eylea®)
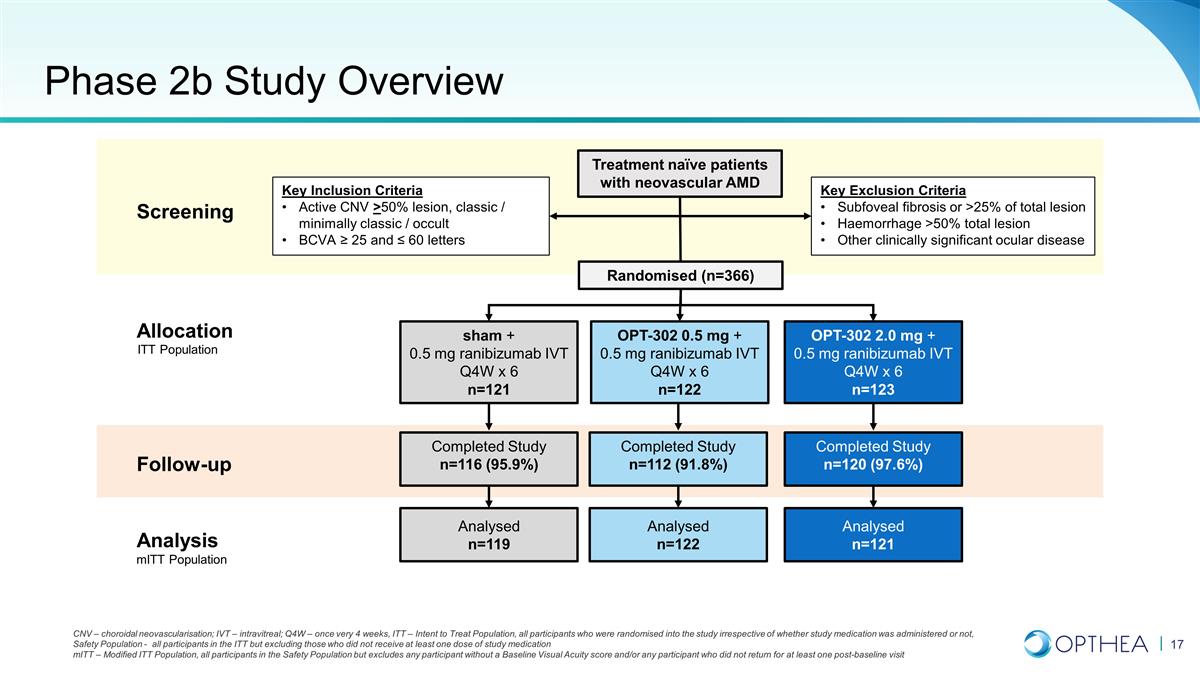
Phase 2b Study Overview Screening
Treatment naïve patients with neovascular AMD Key Exclusion Criteria Subfoveal fibrosis or >25% of total lesion Haemorrhage >50% total lesion Other clinically significant ocular disease Key Inclusion Criteria Active CNV >50% lesion,
classic / minimally classic / occult BCVA ≥ 25 and ≤ 60 letters CNV – choroidal neovascularisation; IVT – intravitreal; Q4W – once very 4 weeks, ITT – Intent to Treat Population, all participants who were
randomised into the study irrespective of whether study medication was administered or not, Safety Population - all participants in the ITT but excluding those who did not receive at least one dose of study medication mITT – Modified ITT
Population, all participants in the Safety Population but excludes any participant without a Baseline Visual Acuity score and/or any participant who did not return for at least one post-baseline visit Allocation Follow-up Analysis Randomised (n=366)
OPT-302 0.5 mg + 0.5 mg ranibizumab IVT Q4W x 6 n=122 sham + 0.5 mg ranibizumab IVT Q4W x 6 n=121 OPT-302 2.0 mg + 0.5 mg ranibizumab IVT Q4W x 6 n=123 Completed Study n=112 (91.8%) Completed Study n=116 (95.9%) Completed Study n=120 (97.6%)
Analysed n=122 Analysed n=119 Analysed n=121 mITT Population ITT Population
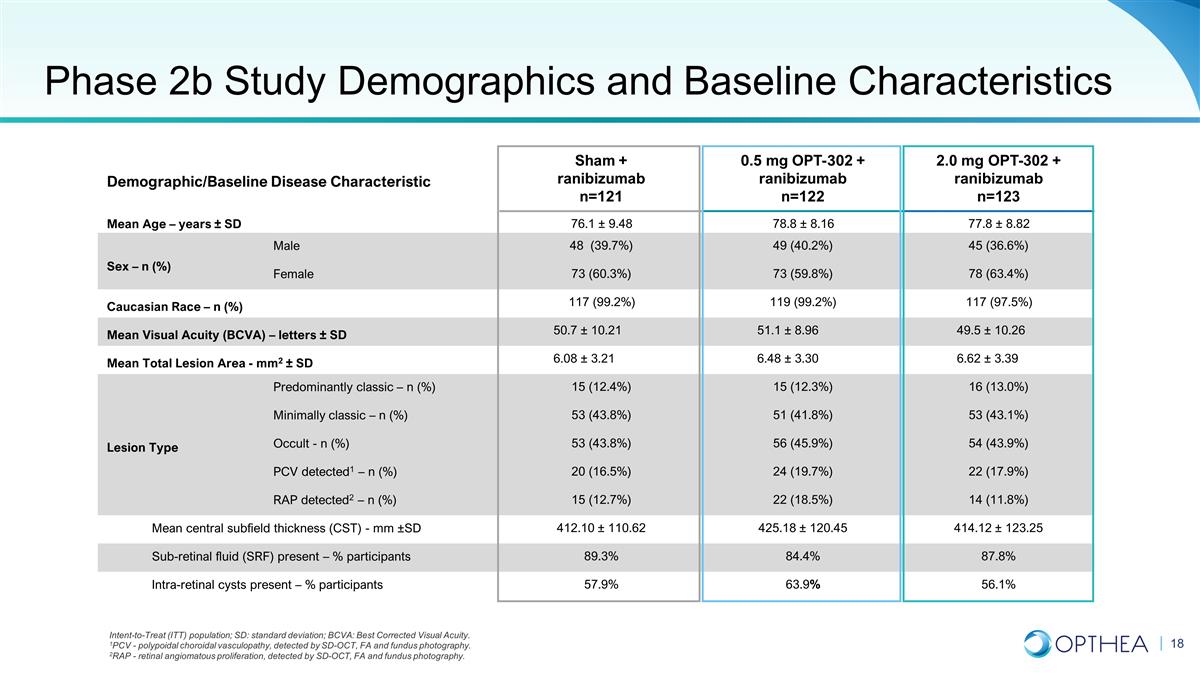
Demographic/Baseline Disease
Characteristic Sham + ranibizumab n=121 0.5 mg OPT-302 + ranibizumab n=122 2.0 mg OPT-302 + ranibizumab n=123 Mean Age – years ± SD 76.1 ± 9.48 78.8 ± 8.16 77.8 ± 8.82 Sex – n (%) Male 48 (39.7%) 49 (40.2%) 45 (36.6%)
Female 73 (60.3%) 73 (59.8%) 78 (63.4%) Caucasian Race – n (%) 117 (99.2%) 119 (99.2%) 117 (97.5%) Mean Visual Acuity (BCVA) – letters ± SD 50.7 ± 10.21 51.1 ± 8.96 49.5 ± 10.26 Mean Total Lesion Area - mm2 ± SD
6.08 ± 3.21 6.48 ± 3.30 6.62 ± 3.39 Lesion Type Predominantly classic – n (%) 15 (12.4%) 15 (12.3%) 16 (13.0%) Minimally classic – n (%) 53 (43.8%) 51 (41.8%) 53 (43.1%) Occult - n (%) 53 (43.8%) 56 (45.9%) 54 (43.9%) PCV
detected1 – n (%) 20 (16.5%) 24 (19.7%) 22 (17.9%) RAP detected2 – n (%) 15 (12.7%) 22 (18.5%) 14 (11.8%) Mean central subfield thickness (CST) - mm ±SD 412.10 ± 110.62 425.18 ± 120.45 414.12 ± 123.25 Sub-retinal
fluid (SRF) present – % participants 89.3% 84.4% 87.8% Intra-retinal cysts present – % participants 57.9% 63.9% 56.1% Intent-to-Treat (ITT) population; SD: standard deviation; BCVA: Best Corrected Visual Acuity. 1PCV - polypoidal
choroidal vasculopathy, detected by SD-OCT, FA and fundus photography. 2RAP - retinal angiomatous proliferation, detected by SD-OCT, FA and fundus photography. Phase 2b Study Demographics and Baseline Characteristics
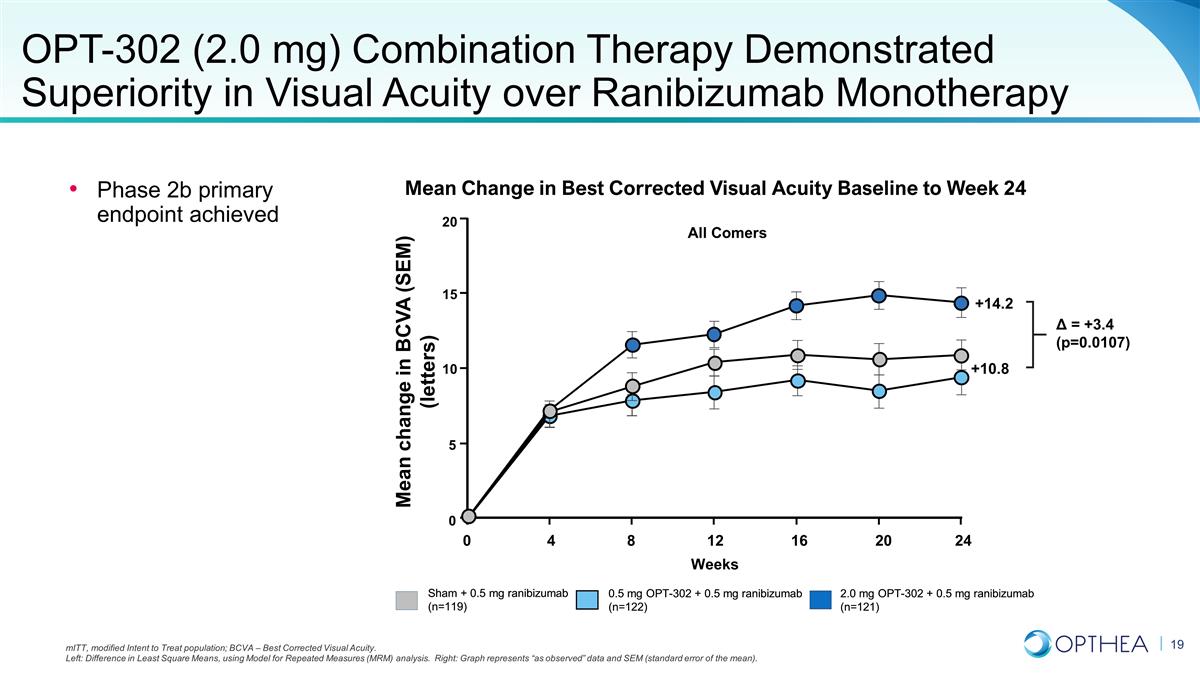
mITT, modified Intent to Treat
population; BCVA – Best Corrected Visual Acuity. Left: Difference in Least Square Means, using Model for Repeated Measures (MRM) analysis. Right: Graph represents “as observed” data and SEM (standard error of the mean). Sham + 0.5
mg ranibizumab (n=119) 2.0 mg OPT-302 + 0.5 mg ranibizumab (n=121) 0.5 mg OPT-302 + 0.5 mg ranibizumab (n=122) Phase 2b primary endpoint achieved OPT-302 (2.0 mg) Combination Therapy Demonstrated Superiority in Visual Acuity over Ranibizumab
Monotherapy Mean Change in Best Corrected Visual Acuity Baseline to Week 24 Sham + 0.5 mg ranibizumab (n=119) 2.0 mg OPT-302 + 0.5 mg ranibizumab (n=121) 0.5 mg OPT-302 + 0.5 mg ranibizumab (n=122) Mean change in BCVA (SEM) (letters) Δ = +3.4
(p=0.0107) 20 15 10 5 0 0 4 8 12 16 20 24 Weeks All Comers +14.2 +10.8
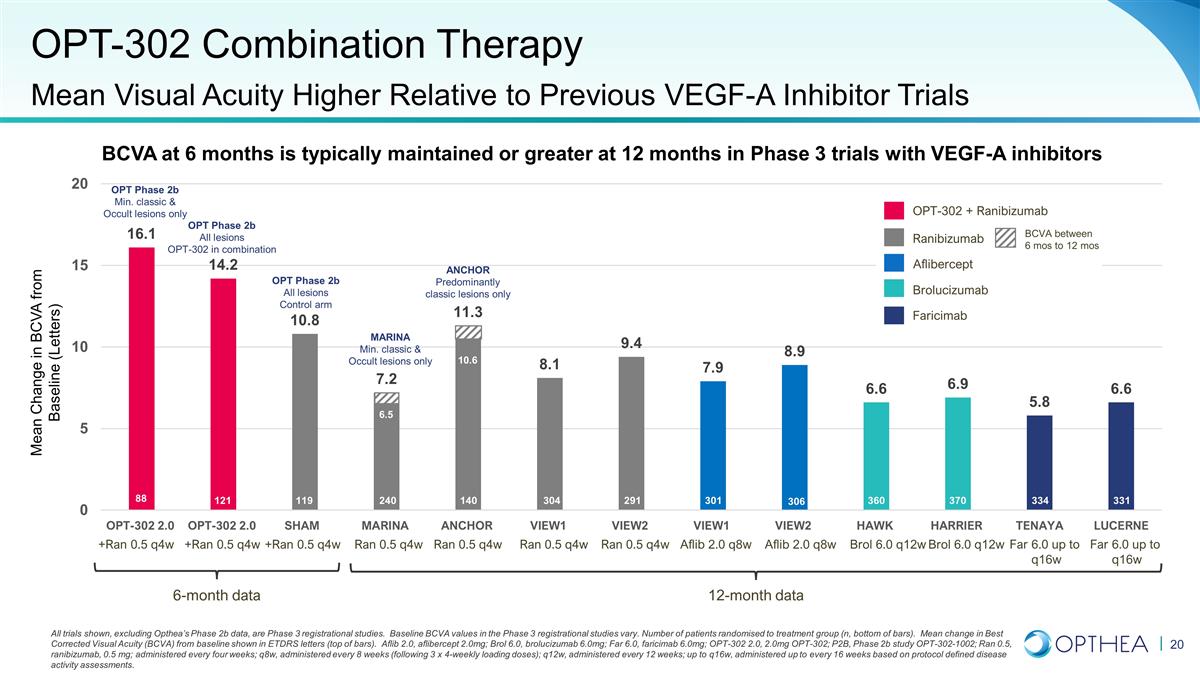
BCVA at 6 months is typically
maintained or greater at 12 months in Phase 3 trials with VEGF-A inhibitors OPT-302 Combination Therapy Mean Visual Acuity Higher Relative to Previous VEGF-A Inhibitor Trials 121 119 240 140 304 291 301 306 360 370 6-month data 12-month data MARINA
Min. classic & Occult lesions only ANCHOR Predominantly classic lesions only OPT Phase 2b Min. classic & Occult lesions only 88 +Ran 0.5 q4w +Ran 0.5 q4w +Ran 0.5 q4w Ran 0.5 q4w Ran 0.5 q4w Aflib 2.0 q8w Aflib 2.0 q8w Ran 0.5 q4w Ran 0.5
q4w Brol 6.0 q12w Brol 6.0 q12w OPT-302 + Ranibizumab Ranibizumab Aflibercept Brolucizumab BCVA between 6 mos to 12 mos Faricimab 10.6 6.5 OPT Phase 2b All lesions OPT-302 in combination OPT Phase 2b All lesions Control arm Mean Change in BCVA from
Baseline (Letters) 334 331 Far 6.0 up to q16w Far 6.0 up to q16w All trials shown, excluding Opthea’s Phase 2b data, are Phase 3 registrational studies. Baseline BCVA values in the Phase 3 registrational studies vary. Number of patients
randomised to treatment group (n, bottom of bars). Mean change in Best Corrected Visual Acuity (BCVA) from baseline shown in ETDRS letters (top of bars). Aflib 2.0, aflibercept 2.0mg; Brol 6.0, brolucizumab 6.0mg; Far 6.0, faricimab 6.0mg; OPT-302
2.0, 2.0mg OPT-302; P2B, Phase 2b study OPT-302-1002; Ran 0.5, ranibizumab, 0.5 mg; administered every four weeks; q8w, administered every 8 weeks (following 3 x 4-weekly loading doses); q12w, administered every 12 weeks; up to q16w, administered up
to every 16 weeks based on protocol defined disease activity assessments.
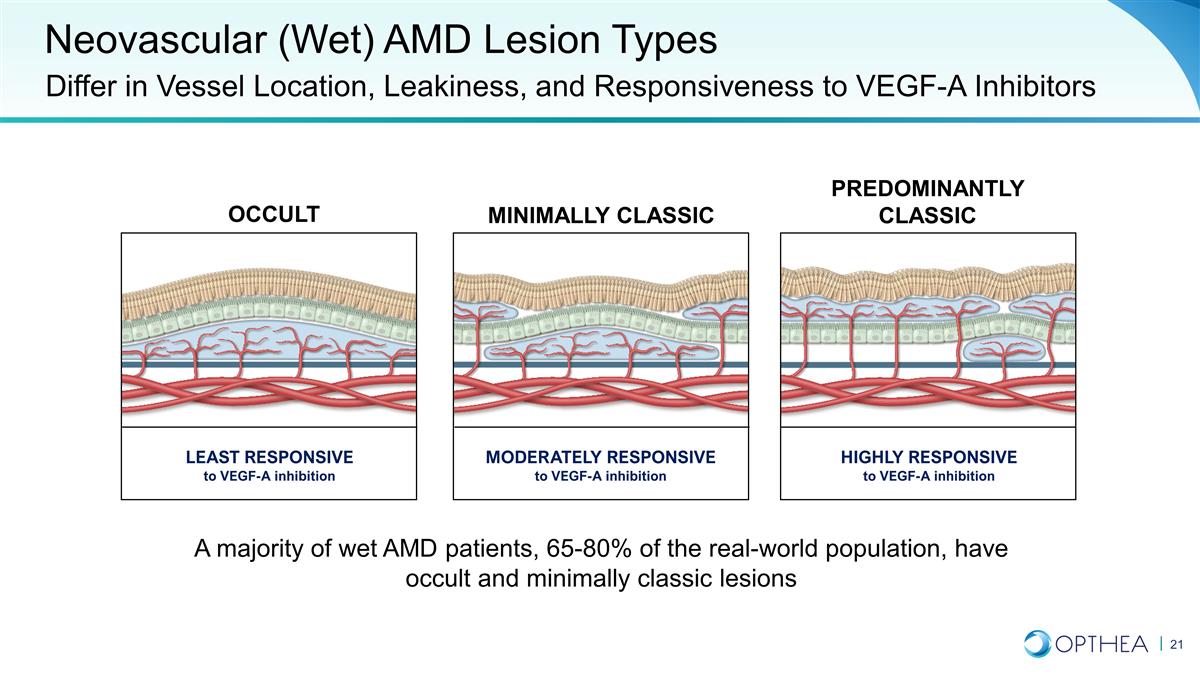
Neovascular (Wet) AMD Lesion Types
Differ in Vessel Location, Leakiness, and Responsiveness to VEGF-A Inhibitors LEAST RESPONSIVE to VEGF-A inhibition MODERATELY RESPONSIVE to VEGF-A inhibition HIGHLY RESPONSIVE to VEGF-A inhibition Predominantly Classic MINIMALLY CLASSIC OCCULT A
majority of wet AMD patients, 65-80% of the real-world population, have occult and minimally classic lesions
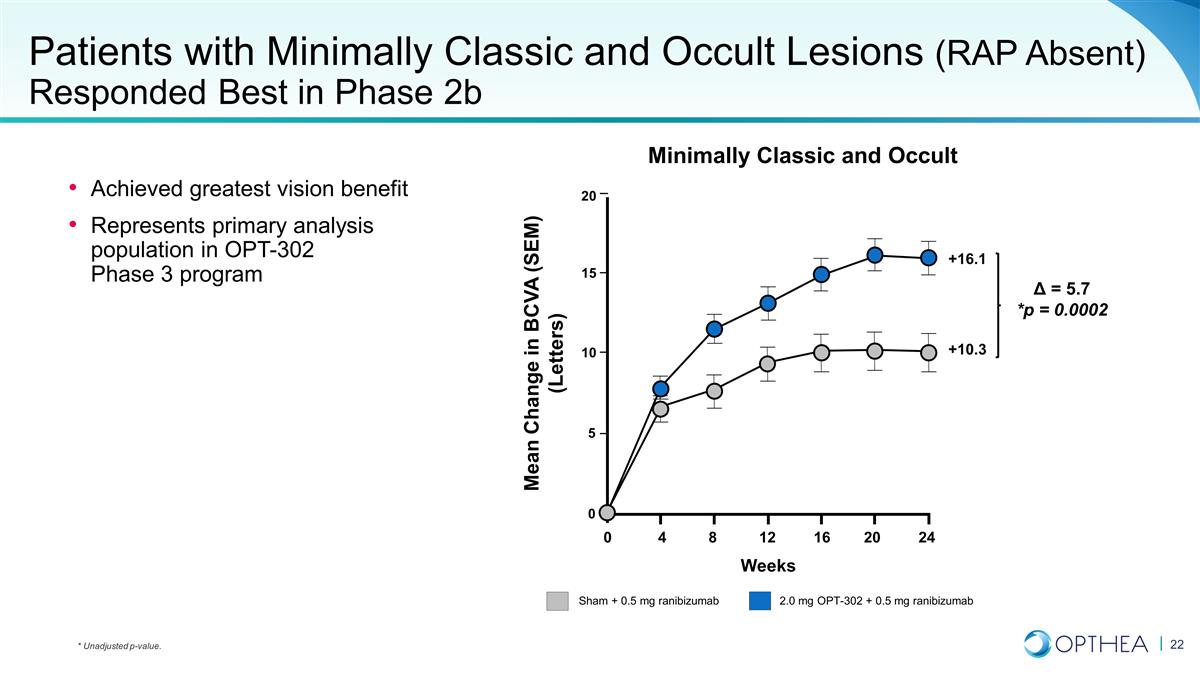
Patients with Minimally Classic and
Occult Lesions (RAP Absent) Responded Best in Phase 2b Achieved greatest vision benefit Represents primary analysis population in OPT-302 Phase 3 program Minimally Classic and Occult Sham + 0.5 mg ranibizumab 2.0 mg OPT-302 + 0.5 mg ranibizumab
+10.3 +16.1 Δ = 5.7 *p = 0.0002 Weeks 20 15 10 5 0 0 4 8 12 16 20 24 Mean Change in BCVA (SEM) (Letters) * Unadjusted p-value.
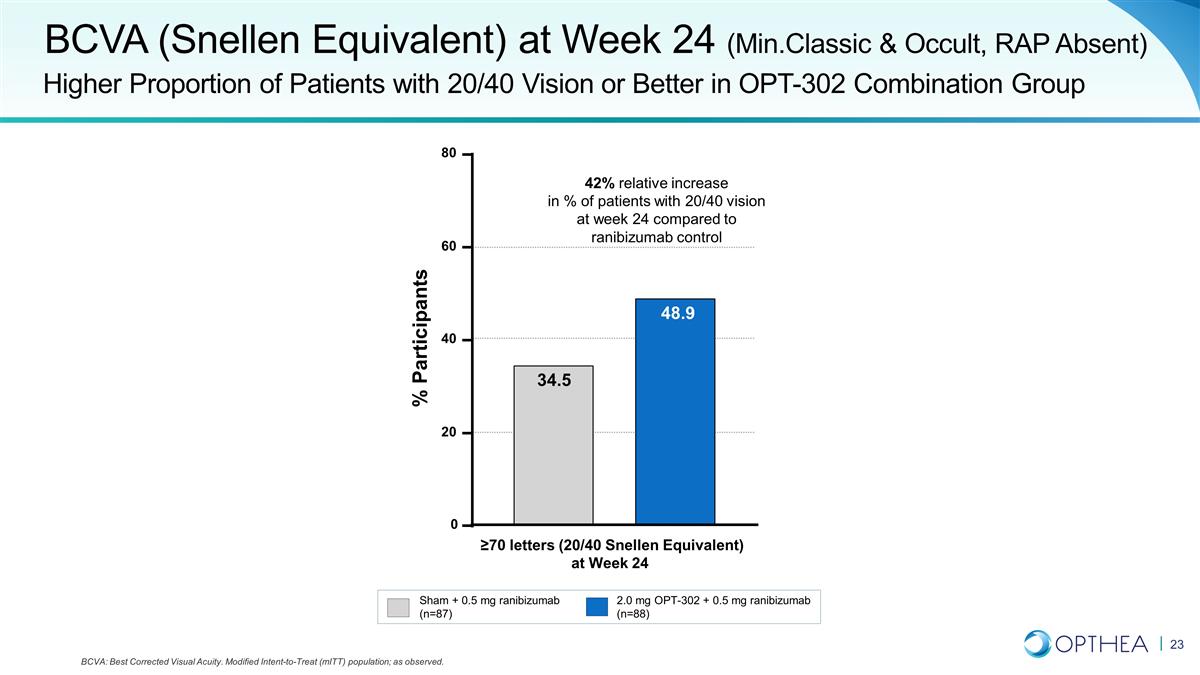
BCVA (Snellen Equivalent) at Week
24 (Min.Classic & Occult, RAP Absent) Higher Proportion of Patients with 20/40 Vision or Better in OPT-302 Combination Group Sham + 0.5 mg ranibizumab (n=87) 2.0 mg OPT-302 + 0.5 mg ranibizumab (n=88) 42% relative increase in % of patients with
20/40 vision at week 24 compared to ranibizumab control BCVA: Best Corrected Visual Acuity. Modified Intent-to-Treat (mITT) population; as observed. 0 20 40 60 80 48.9 34.5 % Participants ≥70 letters (20/40 Snellen Equivalent) at Week 24
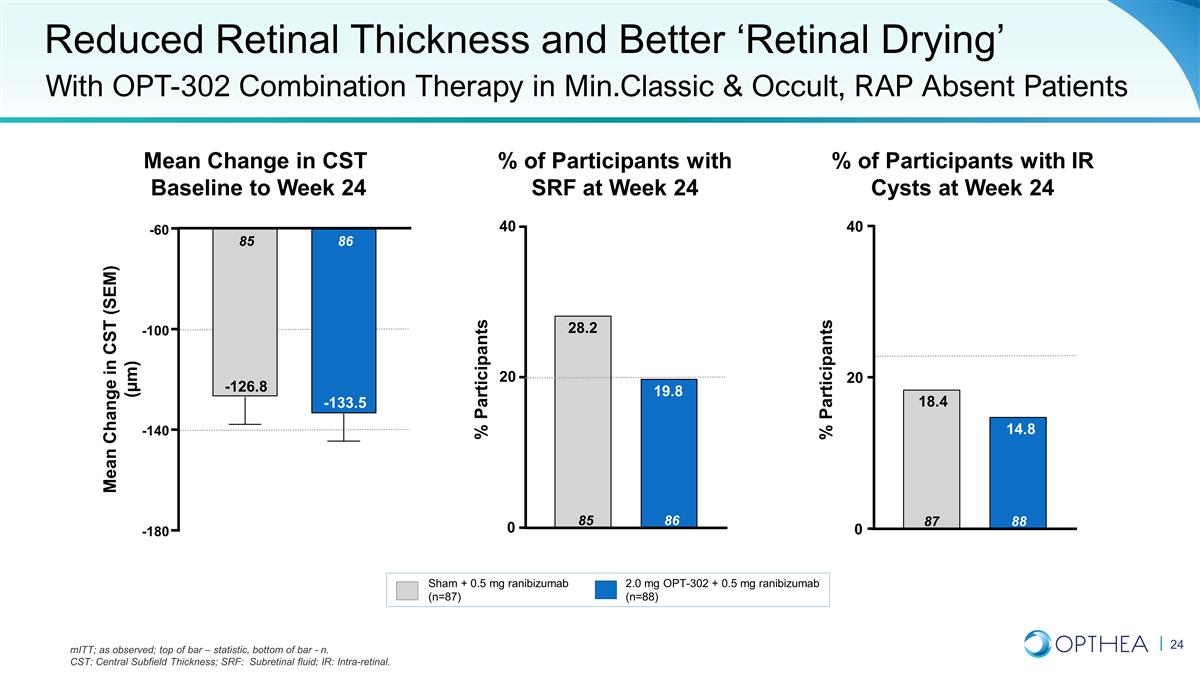
Reduced Retinal Thickness and
Better ‘Retinal Drying’ With OPT-302 Combination Therapy in Min.Classic & Occult, RAP Absent Patients Mean Change in CST Baseline to Week 24 Sham + 0.5 mg ranibizumab (n=87) 2.0 mg OPT-302 + 0.5 mg ranibizumab (n=88) % of
Participants with IR Cysts at Week 24 % of Participants with SRF at Week 24 mITT; as observed; top of bar – statistic, bottom of bar - n. CST: Central Subfield Thickness; SRF: Subretinal fluid; IR: Intra-retinal. % Participants -180 -140 -100
-60 86 -133.5 85 -126.8 Mean Change in CST (SEM) (µm) 0 20 40 88 14.8 87 18.4 0 20 40 86 19.8 85 28.2 % Participants
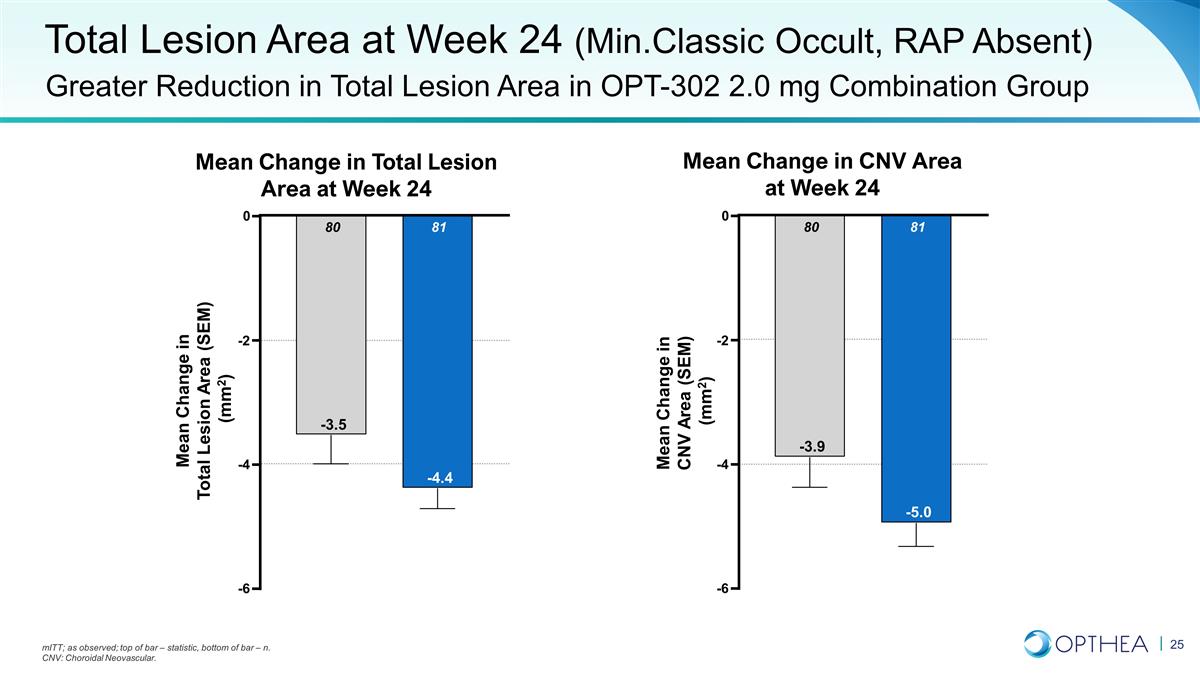
Total Lesion Area at Week 24
(Min.Classic Occult, RAP Absent) Greater Reduction in Total Lesion Area in OPT-302 2.0 mg Combination Group mITT; as observed; top of bar – statistic, bottom of bar – n. CNV: Choroidal Neovascular. Mean Change in Total Lesion Area at
Week 24 Mean Change in CNV Area at Week 24 -6 -4 -2 0 81 -4.4 80 -3.5 Mean Change in Total Lesion Area (SEM) (mm2) -6 -4 -2 0 81 -5.0 80 -3.9 Mean Change in CNV Area (SEM) (mm2)

OPT-302 Combination Therapy:
Demonstrated potential to improve vision outcomes in patients with PCV lesions Polypoidal Choroidal Vasculopathy (PCV) is a difficult-to-treat wet AMD subtype with a large unmet need In Phase 2b, OPT-302 combination therapy demonstrated potential to
improve vision outcomes for patients with PCV Sham + 0.5 mg ranibizumab (n=20) 2.0 mg OPT-302 + 0.5 mg ranibizumab (n=22) 0.5 mg OPT-302 + 0.5 mg ranibizumab (n=24) Δ = 6.7 *p = 0.0253 PCV is highly prevalent in Asian populations (up to ~60%)
Described as the most prevalent form of wet AMD worldwide 0 5 10 15 20 22 13.5 24 10.9 20 6.9 Mean Change in BCVA (SEM) (Letters) *Unadjusted p-value

Pooled Safety for Completed OPT-302
Trials Combination therapy well-tolerated and comparable to standard of care monotherapy N Participants (%) OPT-302 Any dose* N=399 (N=1,842 injections) OPT-302 2.0 mg N=263 (N=1,121 injections) Sham + anti-VEGF-A control N=169 (N=854 injections)
Ocular TEAEs - Study Eye – related to study product(s) 41 (10.2%) 22 (8.4%) 20 (11.8%) Ocular TEAEs - Study Eye – Severe 4 (1.0%) 2 (0.8%) 2 (1.2%) Intraocular inflammation – Study Eye 71,2,3 (1.8%) 31 (1.1%) 31 (1.8%) Participants
with AEs leading to treatment discontinuation 42,4-6 (1.0%) 14 (0.4%) 27,8 (1.2%) Any APTC event 44,5,9,10 (1.0%) 35,9,10(1.1%) 211,12 (1.2%) Deaths 210,13 (0.5%) 210,13 (0.8%) 214,15 (1.2%) 1Transient anterior chamber cell (trace 1-4 cells); 2 SAE
of endophthalmitis, with AE’s of hypopyon and anterior chamber cell (n=1; 0.5 mg); 3 SAE of vitritis (n=1; 0.5 mg); 4Non-fatal myocardial infarction; 5Cerebrovascular accident; 6Enteritis; 7Abdominal pain; 8Increased IOP; 9 Non-fatal angina
pectoris; 10Fatal congestive heart failure/myocardial infarction; 11Non-fatal arterial embolism; 12Embolic stroke; 13Metatstaic ovarian cancer; 14 Pneumonia; 15 infective endocarditis. * Any dose (OPT-302 0.3 mg, 0.5 mg, 1 mg or 2 mg) ** Masked data
represent pooled data from both OPT-302 combination and standard of care monotherapy treatment arms. The Phase 3 clinical trial masked data are incomplete and subject to additional analysis once unmasked, and our Phase 3 clinical trials are
not fully enrolled and the majority of patients enrolled in the trial have not completed the week 52 visit. There is no assurance that standard of care monotherapy in our Phase 3 clinical trials will yield similar results to our prior clinical
trials or previously published clinical trials with anti-VEGF-A monotherapies. As a result, there can be no assurance that topline results for OPT-302 from the Phase 3 clinical trial, if completed, will be consistent with results from masked
data available to date. Pooled safety analysis of 399 patients for completed OPT-302 trials Data Monitoring Committee (“DMC”) regularly reviews data from ongoing Phase 3 COAST and ShORe studies Safety data from our completed OPT-302
trials show OPT-302 combination therapy has a safety and tolerability profile comparable to standard of care anti-VEGF-A monotherapy. Masked data from patients that have completed the week 52 visit in the ongoing Phase 3 clinical trials show greater
mean BCVA increases from baseline than results with standard of care anti-VEGF-A monotherapy from Opthea’s Phase 2b study**
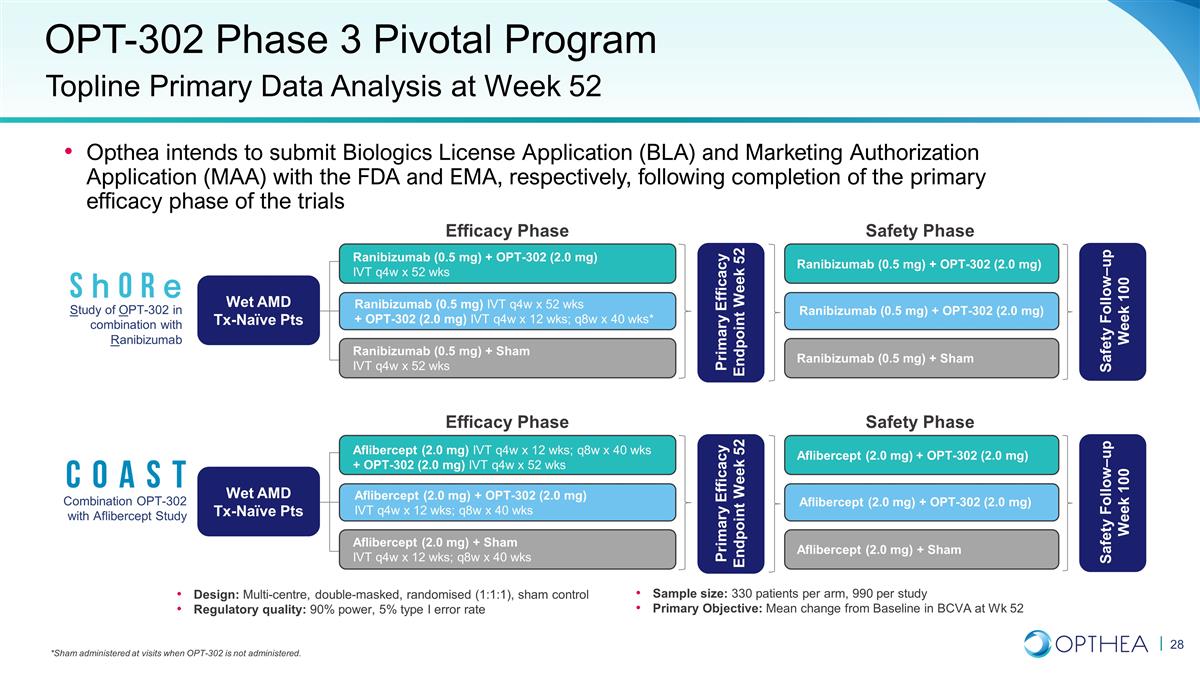
OPT-302 Phase 3 Pivotal Program
Opthea intends to submit Biologics License Application (BLA) and Marketing Authorization Application (MAA) with the FDA and EMA, respectively, following completion of the primary efficacy phase of the trials Topline Primary Data Analysis at Week 52
Sample size: 330 patients per arm, 990 per study Primary Objective: Mean change from Baseline in BCVA at Wk 52 Design: Multi-centre, double-masked, randomised (1:1:1), sham control Regulatory quality: 90% power, 5% type I error rate *Sham
administered at visits when OPT-302 is not administered. Study of OPT-302 in combination with Ranibizumab Primary Efficacy Endpoint Week 52 Wet AMD Tx-Naïve Pts Ranibizumab (0.5 mg) + OPT-302 (2.0 mg) IVT q4w x 52 wks Ranibizumab (0.5 mg) +
Sham IVT q4w x 52 wks Efficacy Phase Ranibizumab (0.5 mg) IVT q4w x 52 wks + OPT-302 (2.0 mg) IVT q4w x 12 wks; q8w x 40 wks* Safety Phase Ranibizumab (0.5 mg) + OPT-302 (2.0 mg) Ranibizumab (0.5 mg) + Sham Ranibizumab (0.5 mg) + OPT-302 (2.0 mg)
Safety Follow–up Week 100 Combination OPT-302 with Aflibercept Study Primary Efficacy Endpoint Week 52 Wet AMD Tx-Naïve Pts Aflibercept (2.0 mg) IVT q4w x 12 wks; q8w x 40 wks + OPT-302 (2.0 mg) IVT q4w x 52 wks Aflibercept (2.0 mg) +
Sham IVT q4w x 12 wks; q8w x 40 wks Efficacy Phase Aflibercept (2.0 mg) + OPT-302 (2.0 mg) IVT q4w x 12 wks; q8w x 40 wks Safety Phase Aflibercept (2.0 mg) + OPT-302 (2.0 mg) Aflibercept (2.0 mg) + Sham Aflibercept (2.0 mg) + OPT-302 (2.0 mg) Safety
Follow–up Week 100
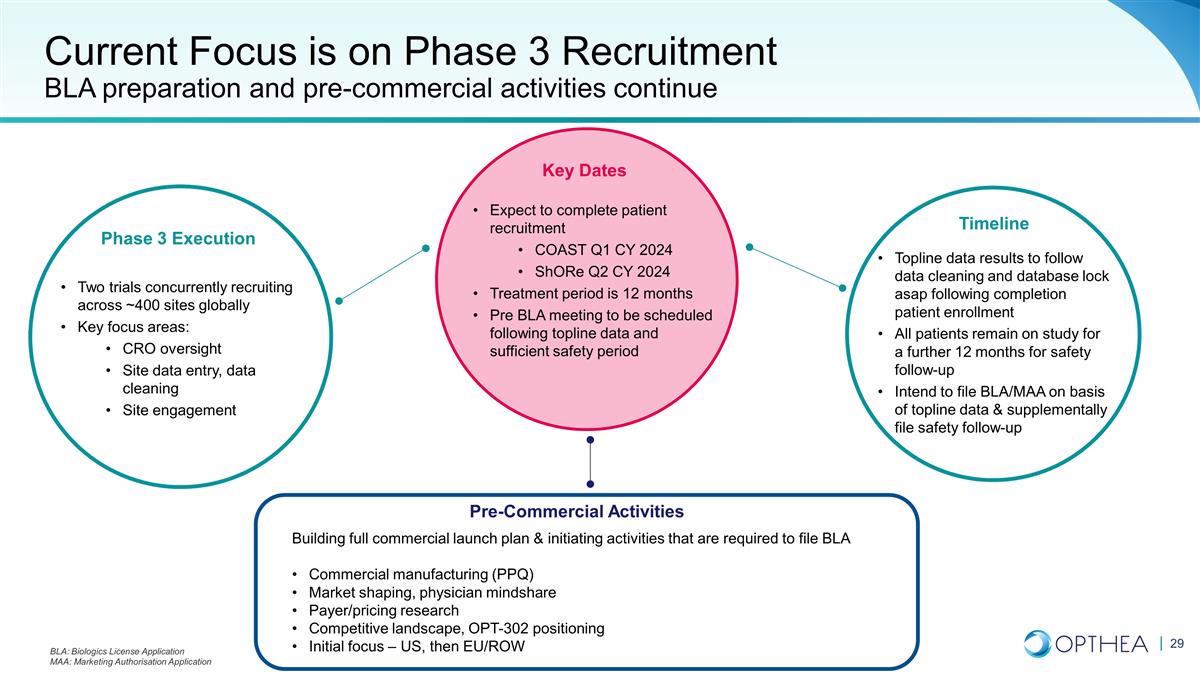
Current Focus is on Phase 3
Recruitment BLA preparation and pre-commercial activities continue Key Dates Pre-Commercial Activities Building full commercial launch plan & initiating activities that are required to file BLA Commercial manufacturing (PPQ) Market shaping,
physician mindshare Payer/pricing research Competitive landscape, OPT-302 positioning Initial focus – US, then EU/ROW Phase 3 Execution Two trials concurrently recruiting across ~400 sites globally Key focus areas: CRO oversight Site data
entry, data cleaning Site engagement Timeline Expect to complete patient recruitment COAST Q1 CY 2024 ShORe Q2 CY 2024 Treatment period is 12 months Pre BLA meeting to be scheduled following topline data and sufficient safety period Topline data
results to follow data cleaning and database lock asap following completion patient enrollment All patients remain on study for a further 12 months for safety follow-up Intend to file BLA/MAA on basis of topline data & supplementally file safety
follow-up BLA: Biologics License Application MAA: Marketing Authorisation Application
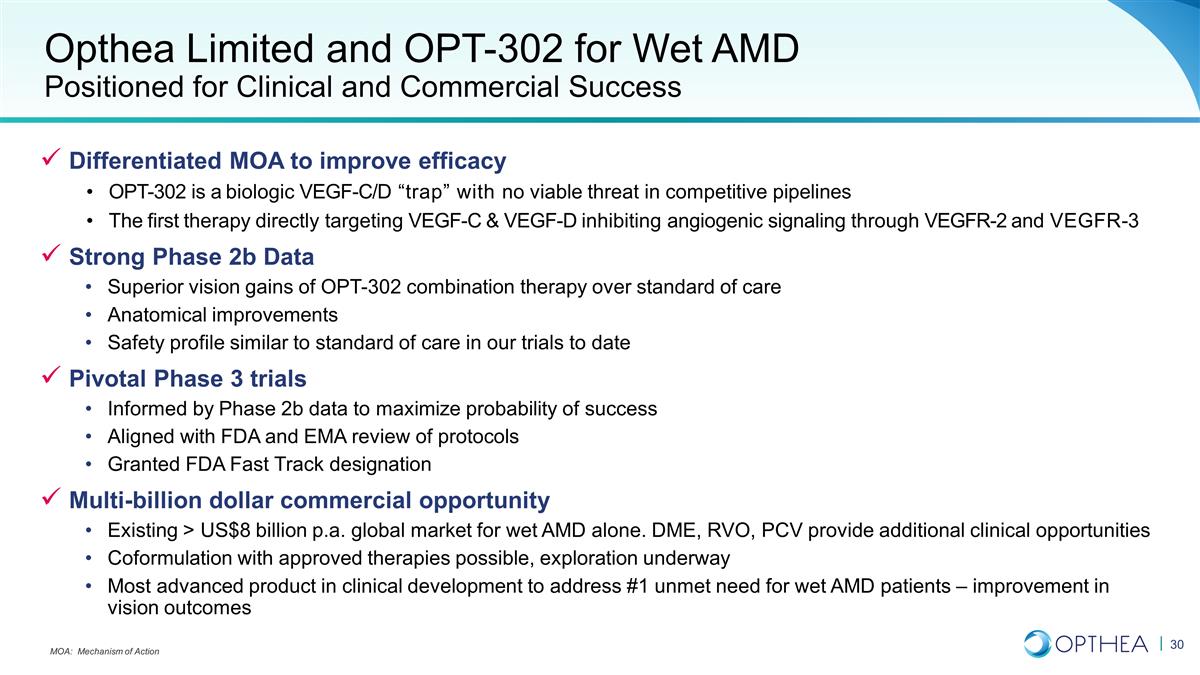
Summary Opthea’s OPT-302 for
wet AMD Opthea Limited and OPT-302 for Wet AMD Positioned for Clinical and Commercial Success Differentiated MOA to improve efficacy OPT-302 is a biologic VEGF-C/D “trap” with no viable threat in competitive pipelines The first therapy
directly targeting VEGF-C & VEGF-D inhibiting angiogenic signaling through VEGFR-2 and VEGFR-3 Strong Phase 2b Data Superior vision gains of OPT-302 combination therapy over standard of care Anatomical improvements Safety profile similar to
standard of care in our trials to date Pivotal Phase 3 trials Informed by Phase 2b data to maximize probability of success Aligned with FDA and EMA review of protocols Granted FDA Fast Track designation Multi-billion dollar commercial opportunity
Existing > US$8 billion p.a. global market for wet AMD alone. DME, RVO, PCV provide additional clinical opportunities Coformulation with approved therapies possible, exploration underway Most advanced product in clinical development to address #1
unmet need for wet AMD patients – improvement in vision outcomes MOA: Mechanism of Action

Key Risks 48 Future capital
requirements Opthea’s activities will require substantial expenditures. Opthea’s losses from operations, including from clinical trial activities, and negative cash flows, raise substantial doubt about the ability for Opthea to
continue as a going concern without additional capital raising activities. While Opthea expects that the proceeds of the Offer, together with additional funding expected under the DFA of US$35 million due under the DFA by December 31, 2023,
the possible increased funding under the DFA of US$50m milliona and cash on hand, will provide funding to progress the activities set out in this presentation, such proceeds will not be sufficient to fully fund all anticipated costs of the Phase 3
clinical trials. In addition, Opthea’s forecast of its cash runway, following receipt of the proceeds from the Offer and under the DFA, is subject to a number of assumptions, including the timing of completion of Phase 3 clinical trial patient
enrollment and CRO and labor costs. Although the co-investor of Carlyle and Abingworth have indicated their intent to provide Opthea additional funding of US$50 million under the DFA, this is subject to final due diligence and the satisfaction
of closing conditions and there is no assurance that this funding will be received or the timing of receipt of such funding. Estimated patient enrollment timing set forth in this presentation is primarily based on Opthea’s monthly enrollment
rates for its Phase 3 clinical trials, which timing has in the past significantly fluctuated from prior estimates, including due to factors outside Opthea’s control. CRO and related costs for the Phase 3 clinical trials have also significantly
fluctuated from estimates in the past, including factors outside Opthea’s control. If patient enrollment continues to be delayed in the future, or if any additional factors cause the Phase 3 clinical trials to be further delayed or more
costly, then Opthea will need to obtain additional financing earlier than the third quarter of calendar year 2024. In addition, if Opthea is unable to complete the Offer as contemplated in this presentation, or obtain the increased US$50
million of funding under the DFA, then Opthea will need to seek additional capital from other sources, which may not be available on a timely basis or at all. In such case, Opthea could be forced to delay, limit or terminate its operations,
liquidate all or a portion of its assets and/or seek insolvency protection in the near term. Opthea’s failure to raise capital, if and when needed, could delay or suspend Opthea’s business strategy and could have a material adverse
effect on Opthea’s activities. If additional funds are raised by issuing equity, this may result in additional dilution to the Shareholders. The pricing of future security issues will also depend on the results of Opthea's scientific research
projects, market factors, demand for securities and the need for capital. If Opthea is unable to secure funding in the short term, there is a risk that Opthea will not be able to continue operating. Underwriting risk Opthea has entered into an
underwriting agreement with MST Financial Services Pty Ltd (“Lead Manager”). The Lead Manager has agreed to act as sole lead manager in relation to the Placement, and sole lead manager and underwriter in relation to the Entitlement
Offer, subject to certain terms and conditions. Details of the fees payable to the Lead Manager are included in the Appendix 3B released to ASX on the date of this presentation. If certain customary conditions are not satisfied or certain customary
termination events occur, then the Lead Manager may terminate the underwriting agreement. A summary of the underwriting agreement including events which may trigger termination of the underwriting agreement is set out in “Summary of
underwriting agreement” below. If the underwriting agreement is terminated by the Lead Manager, Opthea would need to find alternative financing to meet its future funding requirements. There is no guarantee that alternative funding could be
sourced, either at all or on satisfactory terms and conditions. See also the ‘Future capital requirements’ disclosure above. Termination of the underwriting agreement could materially adversely affect Opthea’s business, cash flow,
financial condition and results of operations. This section outlines some of the key risks associated with an investment in Opthea, together with the risks relating to participation in the Offer. Opthea’s business is subject to a number of
risk factors both specific to its business and of a general nature which may impact on its future performance and forecasts. This is not an exhaustive list of the relevant risks and the risks set out below are not presented in order of importance.
The risks set out below and other risks not specifically referred to may in the future materially adversely affect the value of Opthea shares and their performance. Accordingly, no assurance or guarantee of future performance or profitability is
given by Opthea in respect of Opthea shares. Before subscribing for Opthea shares, prospective investors should carefully consider and evaluate Opthea and its business and whether Opthea shares are suitable to acquire having regard to their own
investment objectives and financial circumstances and taking into consideration the material risk factors, as set out below. The risk factors set out below are not exhaustive, and many of them are outside the control of Opthea and its directors. In
deciding whether to participate in the Offer, you should read this presentation in its entirety and carefully consider the risks outlined in this section. Prospective investors should also consider publicly available information on Opthea, examine
the full content of this presentation and consult their financial, tax and other professional advisers before making an investment decision. Business Risks a There can be no assurance that the due diligence will be completed to the satisfaction of
the co-investor of Carlyle and Abingworth, that the closing terms and conditions will be satisfied or that the company will ultimately receive the additional $50 million.

Development Funding Agreement We
are highly reliant on the funding under the DFA including the third tranche of $35 million expected before December 31, 2023, and the potential increase in funding of US$50 million by a new co-investor of Carlyle and Abingworth (see below for
further details). The DFA contains several terms that require compliance by the company in conduct of the study including the governance by a Joint Steering Committee (“JSC”) for changes in the original protocols, study design or
timelines. Modifications require JSC approval and it will be difficult for the company to make modifications on their own. The DFA contains terms that require compliance by the company to maintain a minimum cash balance and to provide a notice to
Ocelot (the SPV established by Carlyle and Abingworth for the purposes of providing funding to Opthea) in the event it anticipates a “Going Concern” opinion in its annual financial statements or that it does not have sufficient cash to
fund its operations for the next six months. The termination provisions in the DFA on the part of Ocelot are extensive and give Ocelot a wide range of conditions to terminate the agreement. In the event of termination, unless mutual or for
breach by Carlyle and Abingworth, amounts owed by the company will be multiples of the invested capital to date. As of June 30, 2023, Ocelot has invested $85 million. A new co-investor of Carlyle and Abingworth intends to participate in
a funding under the DFA of US$50 million to increase total DFA funding from $120 million to $170 million, which is subject to the co-investor’s final due diligence and approvals, appropriate documentation and compliance with closing
conditions. There can be no assurance that a new co-investor of Carlyle and Abingworth will increase the funding by $50 million. The third tranche of $35 million is due under the terms of the DFA before December 31, 2023, however, in the event
the $35 million is not paid it would be considered a Fundamental Material Breach of the DFA by Carlyle and Abingworth. Under a Fundamental Material Breach of the DFA, Opthea has limited recourse but would have the ability to terminate the DFA.
Termination by Opthea for lack of payment of the $35 million would relieve Opthea from any repayments under the DFA. Failure to receive the third tranche of $35 million or the increased funding of $50 million would have a negative impact on our cash
runway and our ability to complete enrollment in the ongoing trials. See Risks noted above under Future Capital Requirements on slide 31. Access to capital The Opthea business model requires ongoing re-investment into clinical trials with no
revenues currently contracted. As such, Opthea will continue to rely upon cash, raised through equity or debt, to fund the business as an on-going concern, including in respect of its Phase 3 clinical trials. Any unforeseen events which restrict the
ability of Opthea to access capital is likely to affect Opthea’s ability to become profitable in future. Clinical development Clinical trials are inherently risky, and may prove unsuccessful or non-efficacious, impracticable or costly, which
may impact profitability and commercial potential. Difficulties in enrolling patients in clinical trials may cause delays to clinical trial schedules. Enrollment has been challenged, and may be challenged in the future, in part by the Covid-19
pandemic, supply chain issues, global and regional inflation, national and local recessions, hiring qualified staff at sites, Opthea’s CRO and distribution locations, local regulatory approvals, importation and custom requirements and
administrative delays. Failure, or negative or inconclusive results, can occur at many stages in development, and the results of earlier clinical trials are not necessarily predictive of future results and data from clinical trials to date may not
be indicative of results obtained when these trials are completed or in later-stage trials. Further, masked data from patients in Opthea’s Phase 3 clinical trials may not be consistent with topline results which are subject to additional
analysis once unmasked. A critical trial may fail to meet its primary or secondary endpoints and as a result inhibit product development, prevent regulatory requirements being met for marketing approval and restrict successful commercialisation. In
addition, data obtained from trials is susceptible to varying interpretations, and regulators may not interpret the data as favourably as Opthea, which may delay, limit or prevent regulatory approval. OPT-302 may fail to demonstrate a safety profile
or sufficient evidence of therapeutic efficacy in future clinical studies to support its ongoing clinical development. In addition, the ability to recruit wet AMD patients into future clinical studies, or secure clinical locations in which to
conduct those studies, may not occur at a sufficient rate to maintain program timelines. Clinical data Opthea maintains sensitive clinical data. Opthea may be subject to a cyber security attack or data breaches by employees or external parties with
either permitted or unauthorized access. There is therefore a risk that sensitive data may be exposed to the public or be permanently lost. A cyber security attack or data breach may also have implications for Opthea’s obligations under any
relevant data protection or privacy legislation. Failure to comply with such legislation or regulations can result in penalties, negative publicity and damage to its brand and reputation. Key Risks – Business Risks (cont’d)

Research and development activities
Opthea’s future success is dependent on the performance of OPT-302 in clinical trials and whether it proves to be a safe and effective treatment. OPT-302 is an experimental product in clinical development and product commercialisation
resulting in potential product sales and revenues is likely to be years away, if ever, and there is no guarantee that it will be successful. OPT-302 requires additional research and development, including ongoing clinical evaluation of safety and
efficacy in clinical trials and regulatory approval prior to marketing authorisation. Drug development is associated with a high failure rate and until Opthea is able to provide further clinical evidence of OPT-302’s ability to improve
outcomes in patients with eye disease, the future success of the product developed remains speculative. Research and development risks include uncertainty of the outcome of results, difficulties or delays in development and general uncertainty
around the scientific development of novel pharmaceutical products and any of these risks, if they were to materialize, could impact Opthea’s progress and could have a material adverse effect on Opthea’s future financial performance.
Regulatory approval Opthea operates within a highly regulated industry, relating to the manufacture, distribution and supply of pharmaceutical products. There is no guarantee that Opthea will obtain or maintain the required approvals, licenses and
registrations from all relevant regulatory authorities in all jurisdictions in which it operates. Further clinical trials may be delayed and Opthea may incur further costs if the Food and Drug Administration (FDA) and other regulatory agencies
observe deficiencies that require resolution or request additional studies be conducted in addition to those that are currently planned. Furthermore, Opthea is exposed to the risk of changes to existing, or the introduction of new, government
policies, regulations and legislation in all jurisdictions in which it operates. A failure to obtain or maintain any required approvals, licenses and registrations or any change in regulation may adversely affect Opthea’s ability to
commercialise and manufacture its treatments. Commercial risk Opthea may, from time to time, consider acquisition, licensing, partnership or other corporate opportunities for Opthea’s development programs. There can be no assurance that any
such acquisition, licensing, partnership or corporate opportunities can be concluded on terms that are, or are believed by Opthea to be, commercially acceptable. In the case of licensing and partnership opportunities, even if such terms are agreed
there is a risk that the performance of distributors and the delivery of contracted outcomes by collaborators will not occur due to a range of unforeseen factors relating to environment, technology and market conditions. Future success will also
depend on Opthea’s ability to achieve market acceptance and attract and retain customers, which includes convincing potential consumers and partners of the efficacy of Opthea’s products and Opthea’s ability to manufacture a
sufficient quantity and quality of products at a satisfactory price. Information technology Opthea relies on effective information technology, software, data centres and communication systems. There is a risk that these systems may be adversely
affected by disruption, failure, service outages or data corruption that could occur as a result of computer viruses, “bugs” or “worms”, malware, internal or external misuse by websites, cyber-attacks or other disruptions
including natural disasters, power outages or other similar events. Opthea may be significantly impacted by disruption to any of these systems or platforms. Key Risks – Business Risks (cont’d)
50 Competition The biotechnology
and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change, in Australia, the United States and elsewhere, and there are no guarantees about Opthea’s ability to successfully compete.
Opthea's products may compete with existing alternative treatments that are already available to customers. In addition, a number of companies, both in Australia and internationally, are pursuing the development of products that target wet AMD and
DME. Some of these companies may have, or may develop, technologies superior to Opthea’s own technology. Some competitors of Opthea may have substantially greater financial, technical and human resources than Opthea does, as well as broader
product offerings and greater market and brand presence. Opthea’s services, expertise or products may be rendered obsolete or uneconomical or decrease in attractiveness or value by advances or entirely different approaches developed by either
Opthea or its competitors. Intellectual property Securing rights in technology and patents is an integral part of securing potential product value in the outcomes of biotechnology research and development. Competition in retaining and sustaining
protection of technology and the complex nature of technologies can lead to patent disputes. Opthea’s success depends, in part, on its ability to obtain patents, maintain trade secret protection and operate without infringing the proprietary
rights of third parties. Because the patent position of biotechnology companies can be highly uncertain and frequently involves complex legal and factual questions, neither the breadth of claims allowed in biotechnology patents nor their
enforceability can be predicted. There can be no assurance that any patents which Opthea may own, access or control will afford Opthea commercially significant protection of its technology or its products or have commercial application, or that
access to these patents will mean that Opthea will be free to commercialize its drug candidates. The granting of a patent does not guarantee that the rights of others are not infringed or that competitors will not develop technology or products to
avoid Opthea's patented technology. Patenting strategies do not cover all countries which may lead to generic competition arising in those markets. Manufacturing Scale-up of OPT-302 manufacture to support Phase 3 clinical studies has been completed
but the process is required to be validated. Process performance qualification or “PPQ” will need to be completed as part of the filing for marketing approval. As such, there is a risk that the PPQ may present technical difficulties.
Technical difficulties could include the inability to generate material that meets regulatory specifications for human administration or the product yield from manufacturing batches may be insufficient to conduct the clinical studies and support
commercialization as currently planned. Any unforeseen difficulty relating to manufacturing, including changes in methods of product candidate manufacturing or formulation, disruption to supply, shortages of input materials or changes to
arrangements with, or capacity of, any third-party manufacturers, may negatively impact Opthea’s ability to generate profit in the future. Commercialization OPT-302 has not been approved for commercial sale, and Opthea expects it to be several
years before OPT-302 is approved, if ever, and Opthea is able to commence sales of OPT-302. If OPT-302 is approved for commercial sale, Opthea’s commercialization expenses will increase significantly as it establishes sales, marketing,
distribution, manufacturing, supply chain and other commercial infrastructure. In addition, OPT-302 may not achieve adequate market acceptance among physicians, patients, healthcare payors and others in the medical community necessary for commercial
success. Covid-19 Opthea’s business was and may in the future be negatively affected by the effects of health epidemics in regions where Opthea or third parties on which Opthea relies have significant manufacturing facilities, concentrations
of clinical trial sites or other business operations. Health epidemics in regions where Opthea has concentrations of clinical trial sites or other business operations could negatively affect its business, including by causing significant disruption
in the operations of third-party manufacturers and CROs upon whom Opthea relies (for example, Covid-19 negatively impacted Opthea’s ability to initiate clinical trial sites, maintain patient enrollment and enroll new patients. Key Risks
– Business Risks (cont’d)
51 Joint venture parties, agents,
suppliers, distributors and contractors Opthea is unable to predict the risk of financial failure or default by a participant in any joint venture to which Opthea may become a party or the insolvency or managerial failure by any of the contractors
used by Opthea in any of its activities or the insolvency or other managerial failure by any of the other service providers used by Opthea for any activity. Opthea may engage with various third parties to assist with different stages of the research
and development process, including agents, suppliers, distributors and contractors, and there is no guarantee that these third parties will comply with their respective contractual obligations. Transition of certain of these third parties could
cause delay or disruption in the clinical trials. This could adversely impact Opthea’s progress and cause delays in or impede research or production, or result in cost increases. Reliance on key personnel Opthea is reliant on key personnel it
employs or engages. Loss of such personnel may have a material adverse impact on the performance of Opthea. In addition, recruiting qualified personnel is critical to Opthea’s success. This includes attracting and retaining staff with
sufficient skills to develop intellectual property. As Opthea’s business grows and progresses to Phase 3 development, it will require additional key staff for clinical development operations as well as additional key financial and
administrative personnel. There can be no assurance that Opthea will be successful in attracting and retaining qualified personnel. The loss of key personnel or the inability to attract suitably qualified additional personnel could have a material
adverse effect on Opthea’s financial performance. Insurance and uninsured risks Although Opthea maintains insurance to protect against certain risks in such amounts as it considers to be reasonable, its insurance will not cover all the
potential risks associated with its operations and insurance coverage may not continue to be available, commercially acceptable, or may not be adequate to cover any resulting liability. It is not always possible to obtain insurance against all such
risks and Opthea may decide not to insure against certain risks because of high premiums or other reasons. Product safety and efficacy Serious or unexpected health, safety or efficacy concerns with products may expose Opthea to reputational harm or
reduced market acceptance of its products, and may lead to product recalls and/or product liability claims and resulting liability, and increased regulatory reporting. There can be no guarantee that unforeseen adverse events or manufacturing defects
will not occur. Opthea may seek to obtain product liability insurance at the appropriate time in order to seek to minimise its liability to such claims, however there can be no assurance that adequate insurance coverage will be available at an
acceptable cost. Any health, safety or efficacy concerns are likely to lead to reduced customer demand and impact on the potential future profitability of Opthea. Litigation In the ordinary course of conducting its business, Opthea is exposed to
potential litigation and other proceedings, including through claims of breach of agreements, intellectual property infringement or in relation to employees (through personal injuries, occupational health and safety or otherwise). If such
proceedings were brought against Opthea, it could incur considerable defence costs (even if successful), with the potential for damages and costs awards against Opthea if it were unsuccessful, which could have a significant adverse financial impact
on Opthea’s business. Changes in laws can heighten litigation risk (for example, antitrust and intellectual property). Circumstances may also arise in which Opthea, having received legal advice, considers that it is reasonable or necessary to
initiate litigation or other proceedings, including for example to protect its intellectual property rights. There has been substantial litigation and other proceedings in the pharmaceutical industry, including class actions from purchasers and end
users of pharmaceutical products. Key Risks – Business Risks (cont’d)

Offer and General Risks Key Risks
– Offer and General Risks Share price fluctuations The market price of Opthea shares will fluctuate due to various factors, many of which are non-specific to Opthea and beyond the control of Opthea, including recommendations by brokers and
analysts, Australian and international general economic conditions, inflation rates, interest rates, changes in government, fiscal, monetary and regulatory policies, global geo-political events and hostilities and acts of terrorism, and investor
perceptions. Fluctuations such as these may adversely affect the market price of Opthea shares. Neither Opthea nor the directors warrant the future performance of Opthea or any return on investment in Opthea. New Options Opthea intends to apply for
quotation of the New Options with seven days of the Prospectus being lodged with ASIC. ASX requires Opthea to meet certain conditions for quotation of New Options as a new class on ASX. There is a risk that Opthea may not be able to meet those
requirements. The fact that ASX may agree to grant official quotation of the New Options is not to be taken in any way as an indication of the merits of Opthea or its securities. If Opthea’s application for the New Options to be quoted on ASX
is granted, the trading price of the New Options may be affected by the ongoing performance, financial position, and solvency of Opthea. Should the ASX grant official quotation of the New Options, the liquidity of trading in New Options on the ASX
may be limited at times and may affect an eligible participant’s ability to buy or sell New Options. In addition, Opthea’s share price may not exceed the exercise price of the New Options during the exercise period. In such circumstances
the New Options lapse without any value being realised. Dilution risk Investors who do not participate in the Offer, or do not take up all of their entitlement under the Entitlement Offer, will have their percentage security holding in Opthea
diluted (in addition to the dilution resulting from the Placement). In addition, Opthea’s need to raise additional capital in the future in order to meet its operating or financing requirements, including by way of additional borrowings may
change over time. Future equity raisings or equity funded acquisitions may dilute the holdings of particular shareholders to the extent that such shareholders do not subscribe for additional equity, or are otherwise not invited to subscribe for
additional equity. Economic risks Opthea is exposed to economic factors in the ordinary course of business. A number of economic factors / conditions, both Australian and global, affect the performance of financial markets generally, which could
affect the price at which Opthea shares trade on ASX. Among other things, adverse changes in macroeconomic conditions, including movements on international and domestic stock markets, interest rates, exchange rates, cost and availability of credit,
general consumption and consumer spending, input costs, employment rates and industrial disruptions, inflation and inflationary expectations and overall economic conditions, economic cycles, trade tarrifs and restrictions, investor sentiment,
political events and levels of economic growth, both domestically and internationally, as well as government taxation, fiscal, monetary, regulatory and other policy changes may affect the demand for, and price of, Opthea shares and adversely impact
Opthea’s business, financial position and operating results. Trading prices can be volatile and volatility can be caused by general market risks such as those that have been mentioned. New Shares in Opthea may trade at or below the price at
which they commence trading on ASX including as a result of any of the factors that have been mentioned, and factors such as those mentioned may also affect the income, expenses and liquidity of Opthea. Additionally, the stock market can experience
price and volume fluctuations that may be unrelated or disproportionate to the operating performance of Opthea. Taxation Future changes in Australian taxation law, including changes in interpretation or application of the law by the courts or
taxation authorities in Australia, may affect taxation treatment of an investment in Opthea shares, or the holding and disposal of those shares. Further, changes in tax law, or changes in the way tax law is expected to be interpreted, in the various
jurisdictions in which Opthea operates, may impact the future tax liabilities of Opthea. Opthea projects that it will receive material cash refunds under the Research and Development Tax Incentive scheme (the “Scheme and R&D Tax
Credits”) to offset the costs of its clinical programs and other qualifying expenditure, incurred both in Australia and overseas. The assumptions underlying Opthea’s projected Scheme and R&D Tax Credits are based on actual amounts
received for the 2022 financial year as a proportion of qualifying expenditure under the scheme. The Commonwealth Government and/or the Australian Taxation Office could change the rules of the regulatory regime with the effect that future amounts
paid to Opthea as a proportion of its expenses could be materially lower than assumed in the Company’s projections. Any rule changes made that materially reduce the amount Opthea was able to claim under the scheme would have a material effect
on the cash flows of the Company. Opthea believes that it is not a passive foreign investment company (PFIC) for U.S. federal income tax purposes for its current taxable year and it expects that it will likely not be a PFIC in the foreseeable
future, although there can be no assurance in this regard and this determination depends on legal and factual considerations that cannot be predicted.

53 Accounting standards Opthea
prepares its general purpose financial statements in accordance with Australian International Financial Reporting Standards (AIFRS) and the Corporations Act 2001 (Cth), which may differ significantly from the accounting standards applied by other
companies (such as U.S. GAAP). Australian Accounting Standards are subject to amendment from time to time, and any such changes may impact on Opthea's statement of financial position or statement of financial performance. Forward-looking statements
There can be no guarantee that the assumptions and contingencies on which the forward-looking statements, opinions and estimates are based will ultimately prove to be valid or accurate. The forward-looking statements, opinions and estimates included
in this presentation depend on various factors, including known and unknown risks, many of which are outside the control of Opthea. Actual performance of Opthea may materially differ from expected performance. Dividends No assurances can be given in
relation to the payment of future dividends. Future determinations as to the payment of dividends by Opthea will be at the discretion of Opthea and will depend upon the availability of profits, the operating results and financial conditions of
Opthea, future capital requirements, covenants in relevant financing agreements, general business and financial conditions and other factors considered relevant by Opthea. No assurance can be given in relation to the level of tax deferral of future
dividends. Tax deferred capacity will depend upon the amount of capital allowances available and other factors. Changes in applicable law and regulations Opthea will be subject to changes in laws, regulations and government policy which may affect
its operations and/or financial performance. Such changes may impact income or operational expenditure. Opthea is also subject to changes in taxation regimes and Accounting Standards. There can be no assurance that such changes will not have a
material adverse effect on Opthea’s business, operational performance or financial results or returns to shareholders. As noted above under “Taxation”, adverse changes to tax law may also reduce Opthea’s capacity to claim
research and incentive grants or rebates, thereby increasing expenses and reducing Opthea’s assets. Cost inflation Higher than expected inflation rates generally, or specific to the biotechnology and pharmaceuticals industry in particular,
could be expected to increase operating and development costs and potentially reduce the value of future project developments. While, in some cases, such cost increases might be offset by increased selling prices, there is no assurance that this
would be possible or that Opthea will be in its production and supply phase of its business when this occurs. Key Risks – Offer and General Risks (cont’d)

Summary of underwriting agreement A
summary of the events which may trigger termination of the underwriting agreement include (but are not limited to) the following: (misleading disclosure) a statement contained in the Offer materials is or becomes misleading or deceptive or likely to
mislead or deceive (including by omission) or a matter required to be included is omitted from the Offer Materials; (information) the report of the due diligence committee established for the purposes of the Offer or any information supplied by or
on behalf of Opthea to the Lead Manager for the purposes of due diligence investigations, the Offer materials or the Offer, is false, misleading or deceptive in a material respect; (section 730 notice) a person (other than the Lead Manager) gives a
notice to Opthea under section 730 of the Corporations Act in relation to the Prospectus; (withdrawal of consent) any person (other than the Lead Manager) whose consent to the issue of the Prospectus is required and who has previously consented
withdraws such consent; (supplementary Prospectus) Opthea lodges a supplementary prospectus without the Lead Manager’s consent, or fails to lodge a supplementary prospectus in a form acceptable to the Lead Manager or (in the Lead
Manager’s reasonable opinion) becomes required to lodge a supplementary prospectus; (new circumstance) a new circumstance arises or becomes known which, if known at the time of issue of this presentation or the Prospectus would have been
required to be included in the relevant document; (material adverse change) there occurs any material adverse change, or development involving a prospective material adverse change, in the condition (financial or otherwise) or in the assets,
liabilities, earnings, business, operations, management, profits, losses or prospects of Opthea or the Group; (market fall) the ASX/S&P 300 Index falls by 10% or more at any time from its level at market close on the business day immediately
preceding the date of the underwriting agreement; (future matters) any estimate or expression of opinion, belief, expectation or intention, or statement relating to future matters in any Offer materials is or becomes incapable of being met or, in
the reasonable opinion of the Lead Manager, unlikely to be met in the projected timeframe; (change of law) there is introduced or there is a public announcement of a proposal to introduce, into the Parliament of Australia or any State of Australia a
new law, or the Reserve Bank of Australia, or any Commonwealth or State authority, adopts or announces a proposal to adopt a new policy (other than a law or policy which has been announced before the date of the underwriting agreement), any of which
does or in the reasonable opinion of the Lead Manager is likely to prohibit or adversely affect or regulate the Offer, capital issues or stock markets or the Lead Manager’s ability to promote or market the Offer or enforce contracts to issue
or allot the New Shares or New Options, or adversely affect the taxation treatment of those securities; (unable to proceed) Opthea is or will be prevented from conducting or completing the Offer by or in accordance with the Listing Rules, ASIC, ASX,
any applicable laws or an order of a court of competent jurisdiction, or otherwise are or will become unable or unwilling to do any of these things or a third party applies to a court of competent jurisdiction seeking orders to prevent, or which
will have the effect of preventing any of these things; (force majeure) there is an event or occurrence of any government agency which makes it illegal for the Lead Manager to satisfy an obligation under this document, or to market, promote or
settle the Offer; (listing): Opthea ceases to be admitted to the official list of ASX or Shares (or interests in them) cease trading or are suspended from official quotation or cease to be quoted on the ASX (other than a voluntary suspension
requested by Opthea and consented to by the Lead Manager; or ASX makes any official statement to any person, or indicates to Opthea or the Lead Manager that it will not grant permission for the official quotation of the New Shares or New Options; or
permission for the official quotation of the New Shares or New Options is granted before the date of issue of those Offer Securities, but the approval is subsequently withdrawn, qualified or withheld;

Summary of underwriting agreement
(cont’d) (applications) an application is made by ASIC for an order under Part 9.5 of the Corporations Act in relation to the Offer materials or the Offer or ASIC commences, or gives notice of an intention to hold, any investigation or hearing
in relation to the Offer or any of the Offer materials or prosecutes or commences proceedings against or gives notice of an intention to prosecute or commence proceedings against Opthea and any such application or notice whether or not withdrawn
becomes publicly known or is not withdrawn within two business days after it is made, or where it is made less than two business days before the relevant settlement date under the Offer, it is not withdrawn before that settlement date; or there is
an application to a government agency (including, without limitation, the Takeovers Panel) for an order, declaration or other remedy in connection with the Offer (or any part of it) or any agreement entered into in respect of the Offer (or any part
of it) except where such application does not become public and is withdrawn or dismissed within two business days after it is commenced or where it is commenced less than two business days before the proposed issue date or completion of the Offer
it has not been withdrawn or dismissed by that date; (no misleading or deceptive conduct) Opthea engages in conduct that is misleading or deceptive or which is likely to mislead or deceive in connection with the making of the Offer; (withdrawal)
Opthea withdraws or indicates that it does not intend to proceed with the Offer or any part of the Offer, or withdraws a document forming part of the Offer materials; (market disruption) either of the following occurs: a general moratorium on
commercial banking activities in Australia, the United States of America, Singapore, Hong Kong, the People’s Republic of China, any member state of the European Union or the United Kingdom is declared by the relevant central banking authority
in any of those countries, or there is a material disruption in commercial banking or security settlement or clearance services in any of those countries; or trading in all securities quoted or listed on ASX, the London Stock Exchange, the Hong Kong
Stock Exchange, the Singapore Stock Exchange or the New York Stock Exchange is suspended or limited in a material respect for more than one day on which that exchange is open for trading; (hostilities) hostilities not presently existing commence
(whether war has been declared or not) or a major escalation in existing hostilities occurs (whether war has been declared or not) involving any one or more of Australia, New Zealand, the United States of America, the United Kingdom, any member
state of the European Union, the People’s Republic of China, Hong Kong, Singapore or a major act of terrorism is perpetrated on any of those countries anywhere in the world; (political or economic conditions) the occurrence of any adverse
change or disruption to financial, political or economic conditions, currency exchange rates or controls or financial markets in Australia, New Zealand, any member state of the European Union, the United States of America, the United Kingdom, the
People’s Republic of China, Hong Kong, Singapore or any change or development involving a prospective adverse change in any of those conditions or markets; (pandemic) a pandemic, epidemic or large-scale outbreak of a disease (including without
limitation SARS, swine or avian flu, H5N1, H7N9, COVID-19 or a related or mutated form of these) not presently existing occurs or in respect of which there is a major escalation, involving any one or more of Australia, New Zealand, a member of the
European Union, the United States of America, United Kingdom, Hong Kong, the People’s Republic of China or Singapore; (representations and warranties) a representation and warranty provided by Opthea in the underwriting agreement is untrue or
incorrect when given or taken to be given or becomes untrue or incorrect; (Certificate) any certificate which is required to be furnished by Opthea under the underwriting agreement is not furnished when required or is untrue, incorrect or
misleading; (delay) any event specified in the underwriting agreement is delayed for more than two business days, without the prior written consent of the Lead Manager; (unauthorised change) Opthea or a member of the Group: disposes, or agrees to
dispose, of the whole, or a substantial part, of its business or property other than as contemplated in the Offer materials; ceases or threatens to cease to carry on business; alters its capital structure, other than as contemplated in the Offer
materials or as permitted by the underwriting agreement; or amends its constitution or other constituent document; (breach) Opthea fails to perform or observe any of its obligations under the underwriting agreement;

Summary of underwriting agreement
(cont’d) (compliance): a contravention by Opthea or any member of the Group of the Corporations Act, the Constitution, Listing Rules, any applicable laws, or a requirement, order or request made by or on behalf of the ASIC, ASX or any other
government agency or any agreement entered into by it; or any Offer materials or any aspect of the Offer does not comply with the Corporations Act, Listing Rules or any other applicable law or regulation; (change in directors or management) a change
to Opthea’s chief executive officer, chief financial officer or board of directors occurs, or any such changes are announced (other than a change announced to ASX prior to the date of the underwriting agreement); (prosecution) any of the
following occurs: a director or senior executive of Opthea engages in any fraudulent conduct or activity, or is charged with an indictable offence; any government agency commences any public proceedings against Opthea or any director in their
capacity as a director of Opthea, or announces that it intends to take such action; or any director of Opthea is disqualified from managing a corporation under Part 2D.6 of the Corporations Act; or an investigation, inquiry or other similar
communication is received from a government agency in relation to Opthea; (regulatory approvals) a government agency withdraws, revokes or amends any regulatory approvals required for Opthea to perform its obligations under the underwriting
agreement or to carry out the transactions contemplated by the Offer materials; (Encumbrance) a person encumbers or agrees to encumber, the whole or a substantial part of the business or property of Opthea or the Group; (ASIC Modifications) ASIC
withdraws, revokes or amends any modifications, exemptions or approvals required to enable Opthea to conduct the Offer as described in the Offer materials; (Insolvency) an insolvency event occurs (including the appointment of a receiver, manager,
administrator or controller or any application not withdrawn or dismissed within 7 days for an order to wind up, or an admission that it is insolvent or unable to pay its debts) to a member of the Group or there is an act which has occurred or any
omission made which would result in such an event occurring in respect of any member of the Group. The ability of the Lead Manager to terminate the underwriting agreement in respect of the events set out above, in some cases, is limited to
circumstances where, in the reasonable opinion of the Lead Manager: the event has had (or is likely to have) a material adverse effect on the business operations, assets, liabilities, financial condition, position or performance, profits, losses,
prospects, earning position or results of operations of the Group, the market price of Shares or the success of the Offer; or the Lead Manager will (or is likely to) contravene, be involved in a contravention of, or incur a liability under the
Corporations Act or any other applicable law as a result of that event. Opthea also gives certain representations, warranties and undertakings to the Lead Manager and an indemnity to the Lead Manager and its respective affiliates and related bodies
corporate and their respective directors, officers, employees, partners and agents subject to certain limited exceptions.

Foreign Selling Restrictions This
document does not constitute an offer of New Shares and New Options in any jurisdiction in which it would be unlawful. In particular, this document may not be distributed to any person, and the New Shares and New Options may not be offered or sold,
in any country outside Australia except to the extent permitted below. European Union This document has not been, and will not be, registered with or approved by any securities regulator in the European Union. Accordingly, this document may not be
made available, nor may the New Shares and New Options be offered for sale, in the European Union except in circumstances that do not require a prospectus under Article 1(4) of Regulation (EU) 2017/1129 of the European Parliament and the Council of
the European Union (the “Prospectus Regulation”). In accordance with Article 1(4)(a) of the Prospectus Regulation, an offer of New Shares and New Options in the European Union is limited to persons who are “qualified
investors” (as defined in Article 2(e) of the Prospectus Regulation). Hong Kong WARNING: This document has not been, and will not be, registered as a prospectus under the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32)
of Hong Kong, nor has it been authorised by the Securities and Futures Commission in Hong Kong pursuant to the Securities and Futures Ordinance (Cap. 571) of the Laws of Hong Kong (the “SFO”). Accordingly, this document may not be
distributed, and the New Shares and New Options may not be offered or sold, in Hong Kong other than to “professional investors” (as defined in the SFO and any rules made under that ordinance). No advertisement, invitation or document
relating to the New Shares and New Options has been or will be issued, or has been or will be in the possession of any person for the purpose of issue, in Hong Kong or elsewhere that is directed at, or the contents of which are likely to be accessed
or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to New Shares and New Options that are or are intended to be disposed of only to persons outside Hong Kong or only to
professional investors. No person allotted New Shares and New Options may sell, or offer to sell, such securities in circumstances that amount to an offer to the public in Hong Kong within six months following the date of issue of such securities.
The contents of this document have not been reviewed by any Hong Kong regulatory authority. You are advised to exercise caution in relation to the offer. If you are in doubt about any contents of this document, you should obtain independent
professional advice. Israel The New Shares have not been registered, and no prospectus will be issued, under the Israeli Securities Law, 1968 (the "Securities Law"). Accordingly, the New Shares will only be offered and sold in Israel pursuant to
private placement exemptions, namely to no more than 35 offerees who fall within a category of sophisticated investor as described in the First Addendum of the Securities Law. Neither this document nor any activities related to the Offer shall be
deemed to be the provision of investment advice. If any recipient of this document is not the intended recipient, such recipient should promptly return this document to the Company. This document has not been reviewed or approved by the Israeli
Securities Authority in any way. New Zealand This document has not been registered, filed with or approved by any New Zealand regulatory authority under the Financial Markets Conduct Act 2013 (the “FMC Act”). The New Shares and New
Options are not being offered to the public within New Zealand other than to existing shareholders of the Company with registered addresses in New Zealand to whom the offer of these securities is being made in reliance on the Financial Markets
Conduct (Incidental Offers) Exemption Notice 2021. Other than in the entitlement offer, the New Shares and New Options may only be offered or sold in New Zealand (or allotted with a view to being offered for sale in New Zealand) to a person who:
•is an investment business within the meaning of clause 37 of Schedule 1 of the FMC Act; •meets the investment activity criteria specified in clause 38 of Schedule 1 of the FMC Act; •is large within the meaning of clause 39 of
Schedule 1 of the FMC Act; •is a government agency within the meaning of clause 40 of Schedule 1 of the FMC Act; or •is an eligible investor within the meaning of clause 41 of Schedule 1 of the FMC Act.

Foreign Selling Restrictions
(cont’d) Singapore This document and any other materials relating to the New Shares and New Options have not been, and will not be, lodged or registered as a prospectus in Singapore with the Monetary Authority of Singapore. Accordingly, this
document and any other document or materials in connection with the offer or sale, or invitation for subscription or purchase, of New Shares and New Options, may not be issued, circulated or distributed, nor may the New Shares and New Options be
offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore except pursuant to and in accordance with exemptions in Subdivision (4) Division 1, Part 13 of the
Securities and Futures Act 2001 of Singapore (the “SFA”) or another exemption under the SFA. This document has been given to you on the basis that you are an “institutional investor” or an “accredited investor”
(as such terms are defined in the SFA). If you are not such an investor, please return this document immediately. You may not forward or circulate this document to any other person in Singapore. Any offer is not made to you with a view to the New
Shares or New Options being subsequently offered for sale to any other party in Singapore. On-sale restrictions in Singapore may be applicable to investors who acquire New Shares and New Options. As such, investors are advised to acquaint themselves
with the SFA provisions relating to resale restrictions in Singapore and comply accordingly. South Africa This document does not, nor is it intended to, constitute a prospectus prepared and registered under the South African Companies Act 2008 and
may not be distributed to the public in South Africa. This document has not been registered with nor approved by the South African Companies and Intellectual Property Commission. Any offer of New Shares and New Options in South Africa will be made
by way of a private placement to, and capable of acceptance only by, investors who fall within one of the specified categories listed in section 96(1)(a) of the South African Companies Act. An entity or person resident in South Africa may not
implement participation in the offer unless (i) permitted under the South African Exchange Control Regulations or (ii) a specific approval has been obtained from an authorised foreign exchange dealer in South Africa or the Financial Surveillance
Department of the South African Reserve Bank. United Kingdom Neither this document nor any other document relating to the offer has been delivered for approval to the Financial Conduct Authority in the United Kingdom and no prospectus (within the
meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the New Shares and New Options. The New Shares and New Options may not be offered
or sold in the United Kingdom by means of this document or any other document, except in circumstances that do not require the publication of a prospectus under section 86(1) of the FSMA. This document is issued on a confidential basis in the United
Kingdom to “qualified investors” within the meaning of Article 2(e) of the UK Prospectus Regulation. This document may not be distributed or reproduced, in whole or in part, nor may its contents be disclosed by recipients, to any other
person in the United Kingdom. Any invitation or inducement to engage in investment activity (within the meaning of section 21 of the FSMA) received in connection with the issue or sale of the New Shares and New Options has only been communicated or
caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of the FSMA does not apply to the Company. In the United Kingdom, this document is being distributed
only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005
(“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated
(“relevant persons”). The investment to which this document relates is available only to relevant persons. Any person who is not a relevant person should not act or rely on this document. United States This document does not constitute
an offer to sell, or a solicitation of an offer to buy, securities in the United States. The New Shares and New Options (as well as the shares underlying the New Options) have not been, and will not be, registered under the US Securities Act of 1933
or the securities laws of any state or other jurisdiction of the United States. Accordingly, the New Shares and New Options (as well as the shares underlying the New Options) may not be offered or sold in the United States except in transactions
exempt from, or not subject to, the registration requirements of the US Securities Act and applicable US state securities laws.

Megan Baldwin, PhD CEO and Managing
Director Opthea Limited Level 4, 650 Chapel Street South Yarra 3141 Victoria, Australia P: +61 9826 0399 M: +61 447 788 674 E: megan.baldwin@opthea.com Timothy Morris CFO Opthea Limited 103 Carnegie Center, Suite 300 Princeton, New Jersey, 08540
U.S.A. M: +1 650 400 6874 E: tim.morris@opthea.com
Exhibit 99.2

ASX, Nasdaq and Media Release
24 August 2023
Opthea announces
Placement and fully underwritten Entitlement Offer to raise A$80.0 million (US$51.2 million1)
Melbourne, Australia; 24 August 2023 – Opthea Limited (Opthea or the Company) (ASX:OPT; NASDAQ:OPT), (Opthea,
OPT or the Company) a clinical stage biopharmaceutical company developing novel therapies to treat highly prevalent and progressive retinal diseases, today announced that the Company is seeking to raise A$80.0 million (US$51.2 million1) via a A$10.0 million (US$6.4 million1) private placement (“Placement”) and a $70.0 million (US$44.8 million1) Accelerated Non-Renounceable Entitlement Offer (“ANREO” or “Entitlement Offer”).
The proceeds from the Placement and Entitlement Offer will be used to continue advancing the clinical development of
OPT-302 for the treatment of wet Age-related Macular Degeneration (wet AMD), including to progress the Company’s Phase 3 clinical trials and for general
corporate purposes.
Dr Megan Baldwin, Chief Executive Officer and Managing Director of Opthea commented “This institutional placement and
entitlement offer strengthens Opthea’s cash position as we advance OPT-302 through Phase 3 clinical trials, ShORe and COAST. With the completion of this financing, together with the funding under the
Development Funding Agreement (DFA) with Carlyle and Abingworth, which includes a further US$35.0 million of committed funding and the addition of a new co-investor of Carlyle and Abingworth which
intends to participate in a funding under the DFA of US$50.0 million to increase total DFA funding from $120 million to $170 million which is subject to the co-investor’s final due
diligence and approvals, appropriate documentation and compliance with closing conditions2, the Company expects to fund its operations into the third quarter of calendar year 2024 and through the
important clinical milestones of completion of patient recruitment for both Phase 3 clinical trials.”
With the announcement of the financing, Opthea
provided an update on the progress of the Company’s Phase 3 program. The Phase 3 clinical trials are approximately 75% enrolled, with COAST expected to be fully enrolled in the first quarter calendar 2024 and ShORe in the second quarter of
calendar 2024. Topline data is expected when all patients complete the 52-week treatment period.
| 1. |
Capital raising overview |
Opthea is pleased to announce the launch of a capital raising comprising:
| |
• |
|
a 1 for 3.07 pro-rata accelerated
non-renounceable entitlement offer of new fully paid ordinary shares in OPT (New Shares) to certain eligible shareholders (Entitlement Offer), together with 1 option to acquire 1 fully paid
ordinary share in Opthea (Option) for every 2 New Shares issued under the Entitlement Offer, to raise approximately A$70.0 million (US$44.8 million1); and |
| 1 |
Assumes AUD/USD exchange rate of A$1.00/US$0.64 |
| 2 |
There can be no assurance that the due diligence will be completed to the satisfaction of the co-investor of Carlyle and Abingworth, that the closing terms and conditions will be satisfied or that the company will ultimately receive the additional $50 million. |
| |
• |
|
placement of New Shares to certain institutional and sophisticated investors (Placement), together with 1
Option for every 2 New Shares issued under the Placement, to raise approximately A$10.0 million (US$6.4 million1) |
(collectively, the Offer or Capital Raising)
The
offer price for the Placement and Entitlement Offer will be A$0.46 per New Share (Offer Price), representing a discount of:
| |
• |
|
23.3% to the last closing price on Wednesday 23 August 2023 of A$0.600 per share; |
| |
• |
|
23.2% to the 5 day volume-weighted average price of A$0.599 per share; and |
| |
• |
|
18.1% discount to TERP of A$0.5623. |
Each New Share issued under the Capital Raising will rank equally with existing fully paid ordinary shares in OPT from the date of issue.
MST Financial Services Pty Ltd (MST) will act as sole lead manager to the Offer and sole underwriter to the Entitlement Offer.
| 2. |
Capital Raising Details |
Placement
The Placement is expected to raise up to
approximately A$10.0 million (US$6.4 million1) and is being undertaken utilizing the Company’s existing placement capacity under ASX Listing Rule 7.1. The Placement is not underwritten.
The Company in its sole discretion reserves the right to raise additional funds under the Placement. Any New Shares and New Options issued as a
result of raising such additional funds will be issued within Opthea’s available placement capacity under ASX Listing Rule 7.1
Entitlement Offer
The fully underwritten Entitlement Offer of A$70.0 million (US$44.8 million1) will consist
of a 1 for 3.07 pro-rata accelerated non-renounceable entitlement offer.
The retail component of the Entitlement Offer (Retail Entitlement Offer) is open to eligible shareholders as at 7:00pm (Melbourne time) on Monday,
28 August 2023 (Record Date).
All New Shares issued under the Entitlement Offer will be issued at the Offer Price.
The Entitlement Offer is non-renounceable and accordingly entitlements will not be tradeable on the ASX or be
otherwise transferable. Shareholders who do not take up their full entitlement will not receive any payment in respect of the entitlements they do not take up and their percentage equity interest in Opthea will be diluted.
OPT shares will remain in a trading halt pending completion and announcement of the Placement and the institutional component of the Entitlement Offer
(Institutional Entitlement Offer).
For the avoidance of doubt, holders of the Company’s American Depositary Shares (Nasdaq:OPT) are not
eligible to participate in the Entitlement Offer or receive New Options.
Institutional Entitlement Offer
Eligible institutional and sophisticated shareholders (Institutional Shareholders) will be invited to participate in the Institutional Entitlement Offer
which will commence on Thursday, 24 August 2023.
| 1 |
Assumes AUD/USD exchange rate of A$1.00/US$0.64 |
| 3 |
TERP means that “theoretical ex right price” at which OPT shares should trade immediately after the ex-date of the Offer and is adjusted for Placement Shares. TERP is a theoretical calculation only and the actual price at which OPT’s shares trade at that time will depend on many factors and may not be equal
to the TERP. |
Institutional Shareholders may opt to take up all, part or none of their entitlements. Entitlements not
taken up by Institutional Shareholders together with those entitlements of ineligible institutional and retail shareholders, will be offered to other institutional and sophisticated investors at the Offer Price (Institutional Shortfall
Offer).
Retail Entitlement Offer
Retail
shareholders who have a registered address in Australia or New Zealand on the register as at 7:00pm (Melbourne time) on the Record Date will be invited to participate in the Retail Entitlement Offer at the same Offer Price and offer ratio as under
the Institutional Entitlement Offer.
The Retail Entitlement Offer is expected to open on Thursday, 31 August 2023 and close at 5:00pm (Melbourne
time) on Thursday, 14 September 2023.
The Retail Entitlement Offer will be made under a transaction specific prospectus issued under section 713 of
the Corporations Act 2001 (Cth) (Prospectus). The Prospectus will be lodged with ASIC and the ASX on Thursday, 24 August 2023 and will be dispatched to eligible retail shareholders, along with personalized acceptance forms, on
Thursday, 31 August 2023. The Prospectus will provide details of how to participate in the Retail Entitlement Offer. Eligible retail shareholders may opt to take up all, part or none of their entitlement. Eligible retail shareholders will also
have the opportunity to apply for and be allocated additional New Shares up to 25% of their entitlement (subject to scale back at the sole discretion of Opthea) (Top-Up Facility).
Opthea may (in its absolute discretion) extend the Retail Entitlement Offer to any Institutional Shareholder that was eligible to, but was not invited to
participate in, the Institutional Entitlement Offer (subject to compliance with relevant laws).
New Options
Participants in the Placement and Entitlement Offer will also be offered 1 option, each exercisable at A$0.80 per option and expiring on 31 August 2025
(New Options), for every 2 New Shares issued under the Placement and Entitlement Offer. The offer of New Options is made under the Prospectus.
All
New Options are expected to be issued upon allotment of the Retail Entitlement Offer and, subject to satisfying spread requirements set out in ASX Listing Rule 2.5, condition 6, the Options are intended to be quoted on the ASX.
The full terms and conditions of the New Options will be set out in the Prospectus. Copies of the Prospectus will be available on the ASX
website and at ww.opthea.com
The timetable below is indicative only and subject to change. The Company reserves the right to alter the dates below in its full discretion and without prior
notice, subject to the ASX Listing Rules and the Corporations Act.
|
|
|
| Item |
|
Date |
| Trading Halt and announcement of the Equity Raising, lodgement of Offer Documents, including Prospectus with ASIC |
|
Thursday, August 24, 2023 |
| Institutional Placement and Institutional Entitlement Offer opens |
|
Thursday, August 24, 2023 |
| Institutional Placement and Institutional Entitlement Offer closes |
|
Friday, August 25, 2023 |
| Announcement of completion of the Institutional Entitlement offer, trading halt lifted, existing securities recommence trading |
|
Monday, August 28, 2023 |
| Record Date Entitlement Offer |
|
Monday, August 28, 2023 |
| Despatch of Offer Prospectus |
|
Thursday, August 31, 2023 |
| Retail Entitlement Offer opens |
|
Thursday, August 31, 2023 |
| Settlement of New Shares issued under the Institutional Entitlement Offer and Placement |
|
Friday, September 1, 2023 |
| Allotment of New Shares issued under the Institutional Entitlement Offer and Placement |
|
Monday, September 4, 2023 |
| Retail Entitlement Offer closes |
|
Thursday, September 14, 2023 |
| Settlement of New Shares under the Retail Entitlement Offer and any shortfall |
|
Wednesday, September 20, 2023 |
| Announcement of results of the Retail Entitlement Offer and notification of any shortfall |
|
Thursday, September 21, 2023 |
| Allotment and issue of New Shares and Options under the Retail Entitlement Offer, and New Options issued under the Institutional Entitlement Offer and Placement |
|
Thursday, September 21, 2023 |
| Trading commences on a normal basis for New Shares issued under the Retail Entitlement Offer |
|
Friday, September 22, 2023 |
| Despatch of holding statements for New Shares issued under the Retail Entitlement Offer |
|
Monday, September 25, 2023 |
About Opthea Limited
Opthea (ASX:OPT; Nasdaq:OPT) is a biopharmaceutical company developing novel therapies to address the unmet need in the treatment of highly prevalent and
progressive retinal diseases, including wet age-related macular degeneration (wet AMD) and diabetic macular edema (DME). Opthea’s lead product candidate OPT-302 is
in pivotal Phase 3 clinical trials and being developed for use in combination with anti-VEGF-A monotherapies to achieve broader inhibition of the VEGF family, with the goal of improving overall efficacy and
demonstrating superior vision gains over that which can be achieved by inhibiting VEGF-A alone.
Inherent risks
of Investment in Biotechnology Companies
There are a number of inherent risks associated with the development of pharmaceutical products to a
marketable stage. The lengthy clinical trial process is designed to assess the safety and efficacy of a drug prior to commercialization and a significant proportion of drugs fail one or both of these criteria. Other risks include uncertainty of
patent protection and proprietary rights, whether patent applications and issued patents will offer adequate protection to enable product development, the obtaining of necessary drug regulatory authority approvals and difficulties caused by the
rapid advancements in technology. Companies such as Opthea are dependent on the success of their research and development projects and on the ability to attract funding to support these activities. Investment in research and development projects
cannot be assessed on the same fundamentals as trading and manufacturing enterprises. Therefore, investment in companies specializing in drug development must be regarded as highly speculative. Opthea strongly recommends that professional investment
advice be sought prior to such investments.
Forward-looking statements
This ASX announcement contains certain forward-looking statements, including within the meaning of the U.S. Private Securities Litigation Reform Act of 1995.
The words “expect”, “anticipate”, “estimate”, “intend”, “believe”, “guidance”, “should”, “could”, “may”, “will”, “predict”,
“plan” and other similar expressions are intended to identify forward-looking statements. Indications of, and guidance on, future financial position and performance including the preliminary estimated unaudited financial information and
pro forma data, are also forward-looking statements. Forward-looking statements in this ASX announcement include statements regarding the timetable, conduct and outcome of the Offer and the use of the proceeds thereof, the therapeutic and commercial
potential and size of estimated market opportunity of the Company’s product in development, the viability of future opportunities, future market supply and demand, the expected receipt of payments (including the additional potential increase of
US$50 million of funding under the Development Funding Agreement (“DFA”)) and the timing of such payments, Opthea’s expected cash runway, the expected timing of completion of patient enrollment under the clinical trials
and timing of top-line
data, expectations about topline data and other observations and expectations based on masked pooled data, the financial condition, results of operations and businesses of Opthea, certain plans,
objectives and strategies of the management of Opthea, including with respect to the current and planned clinical trials of its product candidate, and the future performance of Opthea. Forward-looking statements, opinions and estimates provided in
this ASX announcement are based on assumptions and contingencies which are subject to change without notice, as are statements about market and industry trends, which are based on interpretations of current conditions.
Forward-looking statements, including projections, guidance on the future financial position of the Company including the preliminary estimated unaudited
financial information and pro forma data, are provided as a general guide only and should not be relied upon as an indication or guarantee of future performance. They involve known and unknown risks and uncertainties and other factors, many of which
are beyond the control of Opthea and its directors and management and may involve significant elements of subjective judgment and assumptions as to future events that may or may not be correct. These statements may be affected by a range of
variables which could cause actual results or trends to differ materially, including but not limited to the availability of funding, the receipt of funding under the DFA (including the additional potential increase of US$50 million of funding
under the DFA), future capital requirements, the development, testing, production, marketing and sale of drug treatments, regulatory risk and potential loss of regulatory approvals, ongoing clinical studies to demonstrate OPT-302 safety, tolerability and therapeutic efficacy, additional analysis of data from Opthea’s Phase 3 clinical trials once unmasked, timing of completion of Phase 3 clinical trial patient enrollment and CRO
and labor costs, intellectual property protections, the successful completion of the Offer, completion of management’s and the Company’s audit and risk committee’s review and the Company’s other closing processes, and other
factors that are of a general nature which may affect the future operating and financial performance of the Company. Actual results, performance or achievement may vary materially from any projections and forward-looking statements and the
assumptions on which those statements are based. Subject to any continuing obligations under applicable law or any relevant ASX listing rules, Opthea disclaims any obligation or undertaking to provide any updates or revisions to any forward-looking
statements in this ASX announcement to reflect any change in expectations in relation to any forward-looking statements or any change in events, conditions or circumstances on which any such statement is based.
Not an offer
This ASX announcement is not a disclosure
document and should not be considered as investment advice. The information contained in this ASX announcement is for information purposes only and should not be considered an offer or an invitation to acquire Company securities or any other
financial products and does not and will not form part of any contract for the acquisition of New Shares.
In particular, this ASX announcement does not
constitute an offer to sell, or a solicitation of any offer to buy, any securities in the United States or any other jurisdiction in which such an offer would be illegal or impermissible. The securities to be offered and sold in the Placement and
SPP have not been, and will not be, registered under the U.S. Securities Act of 1933, as amended (the “U.S. Securities Act”), or the securities laws of any state or other jurisdiction of the United States. No public offering of securities
is being made in the United States. Accordingly, the securities to be offered and sold in the Placement and SPP may only be offered and sold outside the United States in “offshore transactions” (as defined in Rule 902(h) under Regulation S
of the U.S. Securities Act (“Regulation S”)) in reliance on Regulation S, unless they are offered and sold in a transaction registered under, or exempt from, or in a transaction not subject to, the registration requirements of, the U.S.
Securities Act and applicable U.S. state securities laws.
Authorized for release to ASX by Megan Baldwin, CEO & Managing Director
|
|
|
| Company & Media Enquiries: |
|
|
|
|
| U.S.A. & International: |
|
Australia: |
|
|
| Megan Baldwin, CEO |
|
Rudi Michelson |
| Opthea Limited |
|
Monsoon Communications |
| Tel: +61 447788674 |
|
Tel: +61 (0) 3 9620 3333 |
| Megan.baldwin@opthea.com |
|
|
Media:
Hershel Berry
Blueprint Life Science Group
Tel: +1 415 505 3749
hberry@bplifescience.com
Join our email database to receive program updates:
Tel:
+61 (0) 3 9826 0399 Email: info@opthea.com Web: www.opthea.com
Exhibit 99.3
Opthea Limited
ACN 006
340 567
Prospectus
For the
offer of:
| |
• |
|
approximately 35.9 million New Shares at the Offer Price of $0.46 per New Share and approximately
18.0 million New Options to Eligible Shareholders with an exercise price of $0.80 under the Retail Entitlement Offer on the basis of 1 New Option for every 2 New Shares issued under the Retail Entitlement Offer; and |
| |
• |
|
approximately 69.0 million Institutional Options with an exercise price of $0.80 to participants in the
Placement and the Institutional Entitlement Offer on the basis of 1 Institutional Option for every 2 New Shares issued under the Placement and Institutional Entitlement Offer (as applicable). |
IMPORTANT NOTICE
This Prospectus is a transaction specific prospectus issued in accordance with section 713 of the Corporations Act. This is
an important document that should be read in its entirety. You should read this Prospectus in its entirety before deciding whether to take up Securities under the Offers.
This Prospectus is not for release to US wire services nor distribution in the United States except by
the Company to Applicants that participated in the Placement and the Institutional Entitlement Offer.
TABLE OF CONTENTS
|
|
|
|
|
|
|
| IMPORTANT INFORMATION |
|
|
3 |
|
|
|
| Chairman’s Letter |
|
|
6 |
|
|
|
| Key Dates |
|
|
8 |
|
|
|
|
| 1. |
|
INVESTMENT OVERVIEW |
|
|
9 |
|
|
|
|
| 2. |
|
DETAILS OF THE RETAIL ENTITLEMENT OFFER |
|
|
17 |
|
|
|
|
| 3. |
|
DETAILS OF THE INSTITUTIONAL OPTION OFFER |
|
|
30 |
|
|
|
|
| 4. |
|
PURPOSE AND EFFECT OF THE ENTITLEMENT OFFER AND PLACEMENT |
|
|
33 |
|
|
|
|
| 4.1 |
|
Purpose of the Entitlement Offer and Placement |
|
|
33 |
|
|
|
|
| 5. |
|
RISK FACTORS |
|
|
38 |
|
|
|
|
| 6. |
|
ADDITIONAL INFORMATION |
|
|
46 |
|
|
|
|
| 7. |
|
DIRECTORS’ AUTHORISATION |
|
|
60 |
|
|
|
|
| 8. |
|
DEFINITIONS |
|
|
61 |
|
|
|
| CORPORATE DIRECTORY |
|
|
64 |
|
2
IMPORTANT INFORMATION
General
This Prospectus relates to the Opthea Limited ACN
006 340 567 (Opthea or Company) Retail Entitlement Offer and Institutional Option Offer under which the Company will offer New Shares, New Options and Institutional Options. This Prospectus is dated 24 August 2023 and a copy has
been lodged with ASIC. The expiry date of this Prospectus is 24 September 2024 being 13 months after the date of this Prospectus. No Securities will be issued on the basis of this Prospectus after the expiry date.
The Company has applied or will, within 7 days after the date of this Prospectus, apply for quotation of the Securities on ASX. Neither ASIC nor ASX takes any
responsibility for the contents of this Prospectus nor for the merits of the investment to which this Prospectus relates.
In preparing this Prospectus,
regard has been had to the fact that the Company is a disclosing entity for the purposes of the Corporations Act and that certain matters may reasonably be expected to be known to investors and their professional advisers. This Prospectus is a
transaction specific prospectus for an offer of: (i) continuously quoted securities (as defined in the Corporations Act); and (ii) options to acquire continuously quoted securities, and has been prepared in accordance with section 713 of
the Corporations Act. Section 713 of the Corporations Act allows the issue of a more concise prospectus in relation to: (i) an offer of continuously quoted securities; and (ii) options to acquire continuously quoted securities. This
Prospectus does not include all information that would be included in a prospectus for an initial public offering.
This Prospectus is important and
requires your immediate attention. You should read the entire Prospectus carefully before deciding whether to invest in the Securities under the Offers. In particular you should consider the risk factors that could affect the performance of Opthea
or the value of an investment in Opthea, some of which are outlined in Section 5. However, the information provided in this Prospectus is not investment advice or financial product advice and has been prepared without taking into account your
individual investment objectives, financial situation, tax position or particular needs. Before deciding whether to apply for Securities under the Offers, you should consider whether they are a suitable investment for you in light of your own
investment objectives, financial situation, tax position and
particular needs and having regard to the merits and risks involved. If, after reading this Prospectus, you have any questions about the Offers you should contact your stockbroker, solicitor,
accountant and/or other professional financial adviser. The Company is not licensed to provide financial product advice in relation to Securities or any other financial products. No cooling off regime applies to the acquisition of Securities under
this Prospectus.
The past performance of the price of the Company’s Shares or other securities of the Company provides no guidance or indication as
to how the price of the Securities will perform in the future.
The right to participate in the Offers is not transferable. Please carefully read and
follow the instructions in this Prospectus and on the accompanying personalised Application Form (if applicable) when subscribing for Securities.
Prospectus availability
Eligible Shareholders and
participants in the Placement and Institutional Entitlement Offer will receive a copy of this Prospectus together with an accompanying personalised Application Form (if applicable). Eligible Shareholders and participants in the Placement and the
Institutional Entitlement Offer can obtain a copy of this Prospectus during the Offer Period (free of charge) from the Company’s website at https://opthea.com/.
Any references to documents located on the Company’s website are provided for convenience only, and none of the documents or other information on the
Company’s website are incorporated by reference into this Prospectus.
Target market determination
In accordance with the design and distribution obligations under the Corporations Act, the Company has determined the target market for the offer of Options
issued under this Prospectus. The Company will only distribute this Prospectus to those investors who fall within the target market determination as set out on the Company’s website at https://opthea.com/.
New Zealand
The New Shares and New Options are not being
offered or sold to the public within New Zealand other than to existing Shareholders of the Company with registered addresses in New Zealand
3
to whom the offer of the New Shares is being made in reliance on the Financial Markets Conduct (Incidental Offers) Exemption Notice 2021. The Company is issuing the New Options to existing
Shareholders of the Company for no consideration.
This document has been prepared in compliance with Australian law and has not been registered, filed
with or approved by any New Zealand regulatory authority under the Financial Markets Conduct Act 2013.
This document is not a product disclosure
statement under New Zealand law and is not required to, and may not, contain all the information that a product disclosure statement under New Zealand law is required to contain.
Other jurisdictions
The Retail Entitlement Offer does
not constitute an offer to sell, or the solicitation of any offer to buy, any securities in the United States (or to any person acting for the account or benefit of a person in the United States), or in any place in which, or to any person to whom,
it would not be lawful to make such an offer or invitation.
No action has been taken to register or qualify the Retail Entitlement Offer, or otherwise
permit an offering of the New Shares or New Options, in any jurisdiction other than Australia or New Zealand.
The distribution of this Prospectus
(including an electronic copy) outside Australia may be restricted by law. If you come into possession of this Prospectus, you should observe any such restrictions. Any failure to comply with such restrictions may contravene applicable securities
laws. The Company disclaims all liability to such persons.
By making a payment by BPAY®, you
will be taken to have given the representations and warranties set out in Section 2.11 and represented and warranted that there has been no breach of such laws and that all necessary approvals and consents have been obtained.
The Securities have not been, and will not be, registered under the US Securities Act, or the securities laws of any state or other jurisdiction in the United
States. The Securities may not be offered, sold or resold in the United States or to, or for the account or benefit of, a person in the United States, except in a transaction exempt from, or not subject to, the registration requirements of the US
Securities Act and applicable US state securities laws.
The Retail Entitlement Offer is not being extended to any Shareholder outside Australia and New Zealand. The
Institutional Option Offer is not being extended to any Shareholder outside Australia, New Zealand, the European Union (excluding Austria), Hong Kong, Israel, Singapore, South Africa, the United Kingdom or the United State. For details of the
restrictions that apply to the Securities in New Zealand, the European Union (excluding Austria), Hong Kong, Israel, Singapore, South Africa, the United Kingdom and the United States, please refer to Sections 3.4 and 6.3.
Future performance and forward- looking statements
The
pro forma financial information provided in this Prospectus is for illustrative purposes only and does not represent a forecast or expectation by the Company as to its future financial condition and/or performance. In particular, certain pro forma
financial information and certain other qualitative assessments by Opthea in this Prospectus assume that proceeds of the Offers are received by the Company on the relevant settlement dates under the Offers.
This Prospectus contains forward-looking statements, including statements containing such words as “anticipate”, “estimates”,
“should”, “will”, “expects”, “plans” or similar expressions. “Indications of, and guidance on, future financial position and performance including the preliminary estimated unaudited financial information
and pro forma data, are also forward-looking statements. Forward-looking statements in this Prospectus include statements regarding the timetable, conduct and outcome of the Capital Raising and the Option Offer and the use of the proceeds thereof.
These forward-looking statements are, despite being based on Opthea’s current expectations about future events and on assumptions for which the Directors consider they have reasonable grounds, subject to known and unknown risks and
uncertainties, many of which are outside the control of the Company and its Directors. These known and unknown risks and uncertainties could cause actual results, performance or achievements to differ materially from future results, performance or
achievements expressed or implied by the forward-looking statements. These risks, uncertainties and assumptions include but are not limited to the risks outlined in Section 5. Given these uncertainties, investors are cautioned not to place
undue reliance on such forward-looking statements in this
4
Prospectus. In addition, except as required by law, and then only to the extent required by law, neither the Company nor any other person warrants the future performance of the Company, the
future performance of the Securities, the correctness of the assumptions underlying the forecast financial information or any return on any investment made by you under this Prospectus.
The Company and its Directors, officers and employees disclaim any responsibility to update any risk factors or publicly announce the result of any revisions
to the forward-looking statements contained in this Prospectus to reflect future developments or events, other than where required to do so by the Corporations Act or the ASX Listing Rules.
Electronic Prospectus
In addition to issuing the
Prospectus in printed form, a read-only version of the Prospectus is also available on the Company’s website at https://opthea.com. Any person accessing the electronic version of this Prospectus for the purpose of making an investment in the
Company must be an Australian resident and must only access the Prospectus from within Australia. The Corporations Act prohibits any person passing onto another person an Application Form unless it is included in or accompanied by a hard copy of
this Prospectus or it accompanies the complete and unaltered electronic version of this Prospectus. The Company will not accept a completed Application Form (if applicable) if it has reason to believe that the Applicant has not received a complete
and unaltered copy of the Prospectus. Any person may obtain a hard copy of this Prospectus by contacting the Company prior to the Closing Date.
Risk
factors
Before deciding to invest in the Company, potential investors should read the entire Prospectus. In considering the prospects for the Company,
potential investors should consider the assumptions underlying the prospective financial information and the risk factors that could affect the performance of the Company. Potential investors should carefully consider these factors in light of
personal circumstances (including financial and taxation issues) and seek professional advice from a stockbroker, accountant or other independent financial adviser before deciding to invest. No
person named in this Prospectus, nor any other person, guarantees the performance of the Company, the repayment of capital by the Company or the payment of a return on the Shares.
Publicly available information
Information about the
Company is publicly available and can be obtained from ASIC and ASX (including ASX’s website www.asx.com.au). The contents of any website or ASIC or ASX filing by the Company are not incorporated into this Prospectus and do not constitute part
of the Offers. This Prospectus is intended to be read in conjunction with the publicly available information in relation to the Company which has been notified to ASX. Investors should therefore have regard to the other publicly available
information in relation to the Company before making a decision whether or not to invest in Securities or the Company.
No person is authorised to give
any information or to make any representation in relation to the Offers which is not contained in this Prospectus and any such information may not be relied on as having been authorised by the Directors.
Enquiries
Phone the Offer Information Line:
1300 850 505 (within Australia)
+61 3 9415 5000 (outside
Australia) between 8.30am and 5.00pm (Melbourne time) Monday to Friday (excluding public holidays) during the Offer Period.
If you have questions about
the Offers, please contact your solicitor, stockbroker, accountant and/or other professional financial adviser.
Interpretation
A number of terms and abbreviations used in this Prospectus have defined meanings which are set out in Section 8.
All references in this Prospectus to $, AUD or dollars are references to Australian currency, unless otherwise stated.
Unless otherwise stated, all references to time in this Prospectus relate to the time in Melbourne, Australia.
5
Chairman’s Letter
Thursday, 24 August 2023
Dear Shareholder
As announced by the Company to ASX today, Thursday, 24 August 2023, the Company intends to raise up to approximately $80.0 million by way of:
| • |
|
the Placement, being a placement of approximately 21.7 million new fully paid ordinary shares in the Company
to institutional and sophisticated investors at an issue price of $0.46 per New Share under the Placement to raise up to approximately $10.0 million (with the ability to increase the size of the Placement at the Company’s sole discretion);
and |
| • |
|
the Entitlement Offer, being a fully underwritten pro rata accelerated
non-renounceable entitlement offer of 1 new fully paid ordinary shares in the Company for every 3.07 Existing Shares to: |
| |
• |
|
existing institutional and sophisticated shareholders in Australia and New Zealand and certain other foreign
jurisdictions as approved by the Board under the institutional component of the Entitlement Offer; and |
| |
• |
|
to Eligible Shareholders under the retail component of the Entitlement Offer, |
to raise approximately $70.0 million.
For
every 2 New Shares subscribed for by participants under the Placement and Institutional Entitlement Offer, each participant will also receive, for no additional consideration, 1 Option to acquire 1 Share by way of issue which will be exercisable at
$0.80 per Share. The Company will apply to have the Options quoted on the ASX and the Options will expire at 5.00pm (Melbourne time) on 31 August 2025.
As an Eligible Shareholder, you can choose to take up all, part or none of your Entitlement. If you choose to take up your full Entitlement, you may also
apply for additional New Shares in excess of your Entitlement under a Top-Up Facility. The maximum number of additional New Shares that an Eligible Shareholder may apply for is 25.0% of their Entitlement.
The retail component of the Entitlement Offer will give all Eligible Shareholders an opportunity to apply for 1 New Share for every 3.07 Existing Shares at
the Offer Price of $0.46 per New Share, being the same offer price per New Share under the Placement and Institutional Entitlement Offer, while also receiving, for no additional consideration, 1 option for every 2 New Shares to raise approximately
$70.0 million. The New Options issued under the Retail Entitlement Offer will have the same terms as the Institutional Options to be issued to participants in the Placement and the Institutional Entitlement Offer.
Funds raised through the Capital Raising, are intended to be used by Opthea to continue advancing the clinical development of
OPT-302 for the treatment of wet AMD, including to progress the Phase 3 clinical program and for general corporate purposes, as well as the other purposes described in this Prospectus.
Additionally, funds received by the Company from the exercise of the Options, which are exercisable at any time prior to the Expiry Date, will be primarily
used to provide further funding for the same purposes as listed above.
6
The Retail Entitlement Offer is open to all Shareholders recorded as holding Shares on the Company’s
register of members as at 7.00pm (Melbourne, Australia time) on Monday, 28 August 2023 and who have a registered address in Australia or New Zealand (and who otherwise meet the eligibility criteria set out in Section 2.4).
The issue of New Shares under the Entitlement Offer is fully underwritten by the Underwriter, MST Financial Services Pty Ltd. The Placement is not
underwritten.
Participation in the Retail Entitlement Offer is completely optional, however, a Shareholder’s entitlement to participate in the
Retail Entitlement Offer is non-renounceable meaning that a Shareholder’s right to participate in the Retail Entitlement Offer cannot be transferred to anyone else. If you do not take up your Entitlement,
you will not be allocated New Shares and your Entitlements will lapse. If you do not take up your Entitlement, your proportionate equity interest in Opthea will be diluted as a result of the Entitlement Offer.
If you are eligible and wish to participate in the Retail Entitlement Offer, you should submit a
BPAY® payment in accordance with the instructions on the accompanying personalised Application Form. New Zealand holders will not be able to make a payment using BPAY® and should contact the Share Registry to obtain payment details. Your application and payment must be received by no later than 5.00pm (Melbourne time) on Thursday, 14 September 2023.
You should be aware that your own financial institution may implement earlier cut-off times with regard to
electronic payment, and you should therefore take this into consideration when making payment. It is your responsibility to ensure that Application Monies submitted through BPAY® are
received by 5.00pm (Melbourne time) on Thursday, 14 September 2023.
The Board recommends that you read this Prospectus carefully, and in its
entirety, before you decide whether to participate in the Offers. In particular, you should note the future market price of the Shares is uncertain and may rise or fall. This means the price you pay for New Shares under the Retail Entitlement Offer
may be either higher or lower than the Share price as traded on ASX at the time the New Shares are issued to you under the Retail Entitlement Offer, with the effect that the value of your investment in the Securities could rise or fall. In deciding
whether to participate in the Offers, you should seek your own independent financial, legal and taxation advice in respect of the Offers. No cooling off regime applies to the acquisition of Securities under the Offers.
On behalf of the Directors, I invite you to consider participating in the Offers and thank you for your ongoing support of Opthea.
Yours sincerely,

Dr. Jeremy Levin
Board
Chairman
Opthea Limited
7
Key Dates
|
|
|
| Event |
|
Date*
(Melbourne, Australia
Time) |
| Lodgement of this Prospectus and trading halt |
|
Thursday, 24 August 2023 |
|
|
| Record Date for the Retail Entitlement Offer |
|
7.00pm on Monday, 28 August 2023 |
|
|
| Retail Entitlement Offer opens |
|
9.00am on Thursday, 31 August 2023 |
|
|
| Issue of New Shares under the Placement and the Institutional Entitlement Offer |
|
Monday, 4 September 2023 |
|
|
| Retail Entitlement Offer closes |
|
5.00pm on Thursday, 14 September 2023 |
|
|
| Allotment of New Shares under the Retail Entitlement Offer and Options |
|
Thursday, 21 September 2023 |
|
|
| Commencement of trading of New Shares and Options on ASX |
|
Friday, 22 September 2023 |
|
|
| Dispatch of holding statements |
|
Monday, 25 September 2023 |
| * |
The timetable is indicative only and subject to change. The Company retains the discretion, subject to the ASX
Listing Rules and the Corporations Act, to alter any or all of these key dates at its discretion (generally or in particular cases), without prior notice, including extending the Closing Date or to withdraw the Offers without prior notice.
Applicants are encouraged to submit their Application Forms (if applicable) as soon as possible. |
8
The below information is a selective overview of the Retail Entitlement Offer and Institutional Option Offer only. Participants should read the
Prospectus in full before deciding to invest in Securities.
Retail Entitlement Offer
|
|
|
|
|
| Topic |
|
Summary |
|
Where to
find more
information |
| What is the Retail Entitlement Offer? |
|
The Retail Entitlement Offer provides Eligible Shareholders with the opportunity to subscribe for 1 New Share for every 3.07 Existing Shares
held on the Record Date and 1 New Option for every 2 New Shares issued, free of brokerage or other transaction costs.
Eligible Shareholders who take up their full Entitlement may also apply for additional New Shares at the same Offer Price of $0.46 per New Share (Top-Up Facility). The maximum number of additional New Shares that an Eligible Shareholder may apply for is 25.0% of their Entitlement. Further details on how to take up your Entitlement and additional New
Shares under the Top-Up Facility are contained in Section 2.5 and the personalized Entitlement and Acceptance Form.
The Retail Entitlement Offer is non-renounceable. This means that Eligible Shareholders who do not take up their
Entitlements by 5.00pm (Melbourne time) on Thursday, 14 September 2023, will not receive any payment or value for those Entitlements, and their proportionate equity interest in the Company will be diluted.
The Company reserves the right to modify or terminate the Retail Entitlement Offer at
any time including closing the Retail Entitlement Offer early (see Section 2.3). The Company will notify the ASX of any modification to, or termination of, the Retail Entitlement Offer. |
|
Sections 2.1, 2.3, 2.5, 2.7 and 2.19 |
|
|
|
| What is the Offer Price? |
|
The Offer Price is $0.46 per New Share, being the same offer price per New Share under the Placement and Institutional Entitlement Offer.
The Options will be issued for nil consideration. |
|
Section 2.1 and 2.2 |
|
|
|
| Am I eligible to participate in the Retail Entitlement Offer? |
|
Only Eligible Shareholders are entitled to participate in the Retail Entitlement Offer. An Eligible Shareholder is a person:
• who is a registered
holder of Shares as at 7.00pm (Melbourne time) on Monday, 28 August 2023 (being the record date for the Retail Entitlement Offer); |
|
Section 2.4 |
9
|
|
|
|
|
| Topic |
|
Summary |
|
Where to
find more
information |
|
|
• whose registered address is in Australia or New Zealand;
• who is not in the
United States nor acting for the account or benefit of a person in the United States or elsewhere outside Australia or New Zealand; and
• who does not hold Shares on behalf of another person who resides outside Australia or New
Zealand (unless they hold Shares in an eligible capacity). Custodians and nominees
holding Shares on behalf of one or more beneficial holders should refer to Section 2.13.
For the avoidance of doubt, holders of the Company’s American Depositary Shares (Nasdaq:OPT) are not eligible to participate in the Entitlement Offer or
receive New Options. |
|
|
|
|
|
| Is the Retail Entitlement Offer conditional? |
|
The issue of New Shares under the Retail Entitlement Offer is not conditional on Shareholder approval and will not count towards the Company’s placement capacity in ASX Listing Rule 7.1 as it falls under an exemption in ASX
Listing Rule 7.2. |
|
Section 2.1 |
|
|
|
| Is the Retail Entitlement Offer underwritten? |
|
The issue of New Shares under the Retail Entitlement Offer is fully underwritten. |
|
Section 2.5 and 2.12 |
|
|
|
| Do I have to participate in the Retail Entitlement Offer? |
|
No. Participation in the Retail Entitlement Offer is optional. The Retail Entitlement Offer is non-renounceable. This means that Eligible Shareholders who do not take up their Entitlements by
5.00pm (Melbourne time) on Thursday, 14 September 2023, will not receive any payment or value for those Entitlements, and their proportionate equity interest in the Company will be diluted. |
|
Section 2.4 |
|
|
|
| Can I transfer my Entitlement to participate in the Retail Entitlement Offer? |
|
No. You cannot transfer your right to purchase New Shares and receive New Options under the Retail Entitlement Offer to anyone else. |
|
Sections 2.7 and 2.9 |
10
|
|
|
|
|
| Topic |
|
Summary |
|
Where to
find more
information |
| How many New Shares will I receive if I participate in the Retail Entitlement Offer? |
|
Under the Retail Entitlement Offer, Eligible Shareholders may subscribe for 1 New Share for every 3.07 Existing Shares held on the Record Date. |
|
Section 2.1 |
|
|
|
| How many New Options will I receive if I participate in the Retail Entitlement Offer? |
|
You will receive 1 New Option for every 2 New Shares issued to you under the Retail Entitlement Offer.
Eligible Shareholders who take up their full Entitlement may also apply for additional
New Shares at the same Offer Price of $0.46 per New Share (Top-Up Facility). The maximum number of additional New Shares that an Eligible Shareholder may apply for is 25.0% of their Entitlement. Further
details on how to take up your Entitlement and additional New Shares under the Top-Up Facility are contained in Section 2.5 and the personalized Entitlement and Acceptance Form. |
|
Sections 2.1 and 2.5 |
|
|
|
| What are the terms of the New Options? |
|
Each New Option is offered for no additional consideration and is exercisable at a price of $0.80 until the expiry date of 5.00pm (Melbourne
time) on 31 August 2025. The Company will apply for quotation of the New
Options on ASX. The full terms of the New Options are set out in
Section 6.6. |
|
Section 6.6 |
|
|
|
| What is the purpose of the funds raised under the Entitlement Offer? |
|
The Entitlement Offer is being undertaken to raise capital of approximately $70.0 million. The effect of the Entitlement Offer on the Company is set out in Section 4. |
|
Section 4 |
|
|
|
| Do I have to pay brokerage on the New Shares and New Options? |
|
No brokerage, commission or other participation costs are payable by you in respect of the acquisition of New Shares and New Options under the Retail Entitlement Offer. |
|
Section 2.18 |
|
|
|
| What are the risks of subscribing for New Shares and New Options under this Prospectus? |
|
New Shares and New Options offered under this Prospectus should be considered speculative and an investment in the Company is subject to a
range of risks, including (but not limited to):
• future capital requirements; |
|
Section 5 |
11
|
|
|
|
|
| Topic |
|
Summary |
|
Where to
find more
information |
|
|
• underwriting risk;
• development funding
agreement; • access
to capital;
• clinical development;
• clinical data;
• research and
development activities;
• regulatory approval;
• commercial risk;
• information
technology;
• competition;
• intellectual property;
• manufacturing;
• commercialization;
• Covid-19;
• joint venture parties,
agents, suppliers, distributors and contractors;
• reliance on key personnel;
• insurance and
uninsured risks;
• product safety and efficacy; and
• litigation.
Further details on the risks associated with an investment in the Company are set out in
Section 5. |
|
|
|
|
|
| What do I do if I receive more than one Application Form? |
|
Eligible Shareholders who receive more than one Application Form under the Retail Entitlement Offer or who are able to participate in the Retail Entitlement Offer as an underlying beneficial owner of a custodian or nominee (e.g.
where an Eligible Shareholder holds Shares in more than one capacity) may apply on different Application Forms. |
|
Sections 2.7 and 2.13 |
12
|
|
|
|
|
| Topic |
|
Summary |
|
Where to
find more
information |
| How do I participate in the Retail Entitlement Offer? |
|
If you are an Eligible Shareholder and wish to take up New Shares and New Options under the Retail Entitlement Offer, you need to pay by BPAY® so that your payment is received by the Company before 5.00pm (Melbourne time) on the Closing Date.
If you are paying by BPAY® you do not need to submit the accompanying personalised Application Form.
It is the responsibility of the Applicant to ensure that funds submitted through BPAY® are received by the Closing Date. Applicants should be aware that their own financial institution may
implement earlier cut-off times with regards to electronic payment, and should therefore take that into consideration when making payment. New Zealand Shareholders will not be able to make a payment using BPAY® and should contact the Share Registry to obtain payment details. |
|
Section 2.7 |
|
|
|
| When will I receive my New Shares and New Options? |
|
New Shares and New Options are expected to be issued on Thursday, 21 September 2023.Holding statements are expected to be sent to successful Applicants shortly after the issue of the New Shares and New Options. |
|
Section 2.15 |
|
|
|
| When can I trade my New Shares and New Options? |
|
It is expected that New Shares and New Options issued under this Prospectus will commence trading on ASX on Friday, 22 September 2023. You should confirm your shareholding before trading any New Shares and/or New Options you
believe you have acquired under this Prospectus. |
|
Section 2.15 |
|
|
|
| What are the rights and liabilities attaching to the New Shares issued under the Retail Entitlement Offer? |
|
New Shares issued under the Retail Entitlement Offer will rank equally in all respects with Existing Shares. The rights and liabilities attaching to the New Shares are set out in Section 6.5. |
|
Section 6.5 |
|
|
|
| What are the rights and liabilities attaching to the New Options issued under this Prospectus? |
|
The rights and liabilities attaching to the New Options are set out in Section 6.6. |
|
Section 6.6 |
|
|
|
| How can Eligible Shareholders obtain further information? |
|
If you would like further information you can:
• phone the Offer Information Line on 1300 850 505 |
|
N/A |
13
|
|
|
|
|
| Topic |
|
Summary |
|
Where to
find more
information |
|
|
(within Australia) or +61 3 9415 5000 (outside Australia) between 8.30am and 5.00pm (Melbourne time) Monday to Friday (excluding public
holidays) during the Offer Period;
• contact your stockbroker, accountant, solicitor and/or other professional adviser;
and/or • visit the
Company’s website at https://opthea.com |
|
|
14
Institutional Option Offer
|
|
|
|
|
| Topic |
|
Summary |
|
Where to
find more
information |
| What is the Institutional Option Offer? |
|
Participants in the Placement and Institutional Entitlement Offer will receive 1 Institutional Option for every 2 New Shares issued to that participant under the Placement and/or the Institutional Entitlement Offer (as applicable),
free of brokerage or other transaction costs. |
|
Sections 3.1 and 3.2 |
|
|
|
| What is the offer price for the Institutional Option Offer? |
|
The Institutional Options will be issued for nil consideration. |
|
Section 3.2 |
|
|
|
| Am I eligible to participate in the Institutional Option Offer? |
|
Only participants in the Placement and/or the Institutional Entitlement Offer are entitled to participate in the Institutional Option Offer. |
|
Section 3.4 |
|
|
|
| Is the Institutional Option Offer underwritten? |
|
The Institutional Option Offer is not underwritten. |
|
Section 3.7 |
|
|
|
| Can I transfer my entitlement to participate in the Institutional Option Offer? |
|
No. You cannot transfer your right to acquire Institutional Options under the Institutional Option Offer to anyone else. |
|
Section 3.1 |
|
|
|
| How many Institutional Options will I Receive if I Participate in the Institutional Option Offer? |
|
Participants in the Placement and the Institutional Entitlement Offer will receive 1 Institutional Option for every 2 New Shares received under the Placement and/or the Institutional Entitlement Offer (as applicable). |
|
Sections 3.1 and 3.4 |
15
|
|
|
|
|
| Topic |
|
Summary |
|
Where to
find more
information |
| What are the terms of the Institutional Options? |
|
Each Institutional Option is offered for no additional consideration and is exercisable at a price of $0.80 until the expiry date at 5.00pm
(Melbourne time) on 31 August 2025. The Company will apply for quotation of the
Institutional Options on ASX. The full terms of the Institutional Options are set
out in Section 6.6. |
|
Sections 3.1, 3.2 and 6.6 |
|
|
|
| How do I participate in the Institutional Options Offer? |
|
If you participated in the Placement and/or the Institutional Entitlement Offer, no action is required from you to take up the Institutional Options under the Institutional Option Offer. The Institutional Options will be issued to
you on Thursday, 21 September 2023. |
|
Section 3.5 |
|
|
|
| When will I receive my Institutional Options? |
|
Institutional Options are expected to be issued to successful participants in the Institutional Option Offer on Thursday, 21 September 2023. Holding statements are expected to be sent to successful Applicants shortly after the
issue of the Institutional Options. |
|
Section 3.9 |
|
|
|
| What are the rights and liabilities attaching to the Institutional Options? |
|
The rights and liabilities attaching to the Institutional Options are set out in Section 6.6. |
|
Section 6.6 |
|
|
|
| How can participants in the Institutional Options Offer obtain further information? |
|
If you would like further information you can:
• phone the Offer Information Line on 1300 850 505 (within Australia) or +61 3 9415 5000
(outside Australia) between 8.30am and 5.00pm (Melbourne time) Monday to Friday (excluding public holidays) during the Offer Period;
• contact your stockbroker, accountant, solicitor and/or other professional adviser;
and/or • visit the
Company’s website at https://opthea.com |
|
N/A |
16
| 2. |
DETAILS OF THE RETAIL ENTITLEMENT OFFER |
| 2.1 |
The Retail Entitlement Offer |
Under this Prospectus, the Company invites each Eligible Shareholder to subscribe for 1 New Share for every 3.07 Existing Shares held on the
Record Date and 1 New Option for every 2 New Shares issued, free of brokerage or other transaction costs. The Retail Entitlement Offer is non-renounceable. This means that Eligible Shareholders who do not take
up their Entitlements by 5.00pm (Melbourne time) on Thursday, 14 September 2023, will not receive any payment or value for those Entitlements, and their proportionate equity interest in the Company will be diluted.
Eligible Shareholders who take up their full Entitlement may also apply for additional New Shares under the
Top-Up Facility. The maximum number of additional New Shares that an Eligible Shareholder may apply for is 25.0% of their Entitlement. Further details on how to take up your Entitlement and additional New
Shares under the Top-Up Facility are contained in Section 2.5 and the personalized Entitlement and Acceptance Form.
Under the Retail Entitlement Offer, the Company is seeking to raise approximately A$16.5 million through the issuance of approximately
35.9 million New Shares and approximately 18.0 million New Options to Eligible Shareholders.
Any fractional entitlements will be
rounded to the nearest whole number of New Shares or New Options (as applicable).
All New Shares offered under this Prospectus will rank
equally with the Existing Shares on issue as at their date of issue. The material rights and liabilities attaching to the New Shares and New Options are set out in Section 6.5 and Section 6.6 respectively.
The purpose of the Entitlement Offer and the intended use of funds raised are set out in Section 4.
Eligible Shareholders are being offered the opportunity to acquire New Shares at the same price as investors under the Placement and the
Institutional Entitlement Offer.
The Offer Price represents a discount of:
| |
• |
|
23.3% to the closing Share price (as quoted on ASX) of $0.60 on Wednesday, 23 August 2023 (being the last
day on which a trade in Shares occurred prior to the Company’s entry into a trading halt); |
| |
• |
|
23.2% to the 5-day VWAP of Shares up to and including Wednesday 23,
August 2023; and |
| |
• |
|
18.1% to the ‘theoretical ex-right price’ (TERP) at
which Shares should trade immediately after the ex-date of the Capital Raising and is adjusted for Shares to be issued under the Placement. TERP is a theoretical calculation only and the actual price at which
Shares trade at that time will depend on many factors and may not be equal to the TERP. |
The Retail Entitlement Offer opens on 9.00am (Melbourne time) on Thursday, 31 August 2023 and is scheduled to close at 5.00pm (Melbourne
time) on Thursday, 14 September 2023.
17
The Company reserves the right to:
| |
• |
|
extend the Retail Entitlement Offer; |
| |
• |
|
close the Retail Entitlement Offer early; or |
| |
• |
|
withdraw the Retail Entitlement Offer, |
at any time. The Company will announce to ASX any such extension, early closure or withdrawal. Eligible Shareholders who wish to apply for New
Shares and New Options under the Retail Entitlement Offer are encouraged to make their Application as soon as possible.
| 2.4 |
Participation in the Retail Entitlement Offer |
Participation in the Retail Entitlement Offer is optional, subject to the eligibility criteria set out below and the terms and conditions of
this Prospectus.
The Retail Entitlement Offer is only open to Eligible Shareholders. An eligible shareholder is a person who:
| |
• |
|
is registered as the holder of Shares as at 7.00pm (Melbourne time) on the Record Date; |
| |
• |
|
has a registered address in Australia or New Zealand; |
| |
• |
|
is not in the United States nor acting for the account or benefit of a person in the United States or elsewhere
outside Australia or New Zealand; and |
| |
• |
|
does not hold Shares on behalf of another person who resides outside Australia or New Zealand (unless they hold
Shares in another eligible capacity), |
(Eligible Shareholder).
Shareholders who are not Eligible Shareholders are ‘Ineligible Shareholders’. The Company reserves the right to determine
whether a Shareholder is an Eligible Shareholder or an Ineligible Shareholder.
Joint holders of Shares will be taken to be a single
registered holder of Shares for the purposes of determining whether they are an Eligible Shareholder.
The Company has determined that it
is either unlawful or impracticable for holders of Shares with registered addresses in jurisdictions outside Australia (and its external territories) or New Zealand to participate in the Retail Entitlement Offer.
The Company reserves the right to reject any Application for New Shares and New Options under this Prospectus to the extent it considers that
the Application (whether alone or in conjunction with other Applications) does not comply with these requirements.
If you are in any doubt
about the Retail Entitlement Offer, whether you should participate in the Retail Entitlement Offer or how such participation will affect you, you should seek independent financial and taxation advice before making a decision as to whether or not to
take up any New Shares and New Options under the Retail Entitlement Offer.
Eligible Shareholders who take up their Entitlement in full may apply for additional New Shares at the
18
same Offer Price of $0.46 per New Share under the Top-Up Facility. The maximum number of additional New Shares that an Eligible Shareholder may apply for
is 25.0% of their Entitlement. Allocations of New Shares under the Top-Up Facility are at the discretion of Opthea (as is the right to issue any shortfall under the Retail Entitlement Offer). Such New Shares
are to be drawn from the Entitlements (or the parts thereof) that are not taken up by Eligible Shareholders. Accordingly, allocations available to be made under the Top-Up Facility will depend upon the extent
to which Eligible Shareholders take up their Entitlements under the Retail Entitlement Offer. If the number of additional New Shares for which applications are received under the Top-Up Facility exceeds the
number of New Shares available for allocation under the Top-Up Facility, then Opthea may apply any scaleback in its discretion, which may include having regard to the pro rata Entitlement of Eligible
Shareholders who apply for additional New Shares. New Shares allocated under the Top-Up Facility will be issued at the same time as other New Shares under the Retail Entitlement Offer. For the avoidance of
doubt, Eligible Shareholders who are issued additional New Shares will also receive, for no additional consideration, 1 option for every 2 additional New Shares.
The issue of New Shares under the Retail Entitlement Offer is fully underwritten by the Underwriter, MST Financial Services Pty Ltd, subject to
the terms and conditions of the Underwriting Agreement. Please refer to Section 2.12 for further details in relation to the Underwriting Agreement.
It is important to note that the Underwriter will be acting for, and providing services to, the Company in relation to the Retail Entitlement
Offer and will not be acting for or providing services to Shareholders. The Underwriter has been engaged solely as an independent contractor and is acting solely in a contractual relationship on an arm’s length basis with the Company. The
engagement of the Underwriter by the Company is not intended to create any agency or other relationship between the Underwriter and Shareholders.
Under the Retail Entitlement Offer, Eligible Shareholders may subscribe for 1 New Share for every 3.07 Existing Shares held on the Record Date
and 1 New Option for every 2 New Shares issued.
If you are an Eligible Shareholder you may:
| |
• |
|
take up all of your Entitlement (and, if you take up your Entitlement in full, you may apply for additional New
Shares under the Top-Up Facility); |
| |
• |
|
take up part of your Entitlement and allow the balance to lapse; or |
| |
• |
|
decline to exercise your Entitlement, in which case your Entitlement will lapse and you will receive no value for
those lapsed Entitlements. |
If you are an Eligible Shareholder and wish to take up all or part of your Entitlement, or
you wish to also apply for additional New Shares, you should:
| |
• |
|
read this Prospectus and the accompanying personalised Acceptance Form in full; |
| |
• |
|
consider the risks associated with the Retail Entitlement Offer, as summarised in Section 5 of this
Prospectus, in light of your personal circumstances; |
| |
• |
|
decide whether to participate in the Retail Entitlement Offer; and |
| |
• |
|
make payment and apply for New Shares in accordance with Section 2.8. |
19
Any fractional entitlements will be rounded to the nearest whole number of New Shares or New
Options (as applicable).
Any amounts received in excess of the Offer Price multiplied by your Entitlement may be treated as an Application
to apply for as many additional New Shares as your Application Monies will pay for in full. Any Application Monies received for more than an Applicant’s final allocation of New Shares (and only where the amount is $5.00 or greater) will be
refunded, without interest.
You cannot withdraw or revoke your Application once you have paid via BPAY®.
If an Eligible Shareholder holds Shares as a custodian or nominee the Retail
Entitlement Offer is also being made to the custodian or nominee and, subject to certain conditions, the custodian or nominee has the discretion to extend the Offer to the relevant beneficiaries. Please refer to Section 2.13 for further
details.
| 2.8 |
Payment of Application Monies |
For payment by BPAY®, please follow the instructions on the accompanying personalised
Application Form. You can only make a payment via BPAY® if you are the holder of an account with an Australian financial institution that supports BPAY® transactions. Please note that if you pay by BPAY® you do not need to submit the accompanying personalised Application Form but are taken
to have made the declarations in that Application Form.
New Zealand holders will not be able to make a payment using BPAY® and should contact the Share Registry to obtain payment details.
Any amounts
received in excess of the Offer Price multiplied by your Entitlement may be treated as an Application to apply for as many additional New Shares as your Application Monies will pay for in full.
It is your responsibility to ensure that your BPAY® payment is received by the
Share Registry by no later than 5.00pm (Melbourne time) on the Closing Date. You should be aware that your financial institution may implement earlier cut-off times with regards to electronic payment and you
should therefore take this into consideration when making payment. No interest will be paid on any application monies received or refunded.
| 2.9 |
Declining all or part of your Entitlement |
If you decide not to take up all or part of your Entitlement, the Entitlement which is unexercised will lapse and may be taken up by the
Underwriter (or by persons they nominate, including any potential sub-underwriters). Your Entitlement to participate in the Retail Entitlement Offer is non- renounceable
and cannot be traded on the ASX nor any other financial markets, nor can it be privately transferred.
If you decide not to participate in
the Retail Entitlement Offer, you do not need to fill out or return the accompanying personalised Application Form. By allowing your Entitlement to lapse, you will forgo any exposure to increases or decreases in the value of the New Shares or New
Options had you taken up your Entitlement and you will not receive any value for your Entitlement. Your proportionate interest in Opthea will also be diluted by the extent that New Shares are issued under the Retail Entitlement Offer.
| 2.10 |
Ineligible Shareholders |
If you are an Ineligible Shareholder, you may not take up any of, or do anything in relation to, your Entitlement under the Retail Entitlement
Offer.
20
| 2.11 |
Effect of making an Application |
If you apply for New Shares and New Options under the Retail Entitlement Offer or make a payment by BPAY®, you:
| |
• |
|
will be deemed to have represented and warranted (for the benefit of the Company, the Underwriter and their
respective related bodies corporate) that you are an Eligible Shareholder, that you have read and understood the terms and conditions of participating in the Retail Entitlement Offer as set out in this Prospectus and the accompanying personalised
Application Form, that you subscribe for New Shares and New Options in accordance with those terms and conditions and that you agree to be bound by the Constitution as in force from time to time; |
| |
• |
|
declare that all details and statements in the accompanying personalised Application Form (if applicable) are
true, complete and not misleading; |
| |
• |
|
acknowledge that you have not been provided with investment advice or financial product advice by the Company or
its Directors and have made your own enquiries before making an investment decision; |
| |
• |
|
agree that your Application is made on the terms and conditions of the Retail Entitlement Offer set out in this
Prospectus, the accompanying personalised Application Form and the Constitution; |
| |
• |
|
accept that you will not be able to withdraw or revoke your Application or BPAY® payment once you have sent it in (or paid it, as the case may be); |
| |
• |
|
acknowledge that the Company may at any time determine that your Application is valid, in accordance with the
terms and conditions set out in this Prospectus, even if the Application is incomplete, contains errors or is otherwise defective; |
| |
• |
|
accept the risk associated with any refund that may be sent to you by direct credit or cheque to your address
shown on the Company’s register of members; |
| |
• |
|
acknowledge that the Company is not liable for any exercise of its discretions referred to in this Prospectus;
|
| |
• |
|
are in compliance with all relevant laws and regulations (including, without limitation, section 1043A of the
Corporations Act and laws and regulations designed to restrict terrorism financing and/or money laundering); |
| |
• |
|
acknowledge that the market price of the Securities may rise or fall between the date on which the Retail
Entitlement Offer opens and the date of issue of the Securities to you under the Offers and that the price you pay per Security under the Offers may exceed the market price of the Securities at the time the Securities are issued to you under the
Offers; |
| |
• |
|
represent and warrant that that you are not in the United States and you are not acting for the account or
benefit of a person in the United States (or, in the event that you are acting for the account or benefit of a person in the United States, you are not participating in the Retail Entitlement Offer in respect of that person); |
| |
• |
|
acknowledge that the New Shares and New Options have not been, and will not be, registered under the US
Securities Act or the securities laws of any state or other jurisdictions in the United States and accordingly, the New Shares and New Options may not be offered or sold except in accordance with an available exemption from, or in a transaction not
subject to, the registration requirements of the US Securities Act and any other applicable US state securities laws; |
21
| |
• |
|
acknowledge that you have not and will not send this Prospectus or any other document relating to the Retail
Entitlement Offer to any person in the United States or elsewhere outside Australia or New Zealand; |
| |
• |
|
if in the future you decide to sell or otherwise transfer the New Shares (including the ordinary shares acquired
upon exercise of the New Options) acquired under the Retail Entitlement Offer you will only do so in “regular way” transactions on ASX where neither you nor any person acting on your behalf knows, or has reason to know, that the sale has
been pre-arranged with, or that the purchaser is, in the United States; |
| |
• |
|
if you are acting as a nominee or custodian, each beneficial holder on whose behalf you are submitting the
Application Form (i) is resident in Australia or New Zealand, and (ii) is not in the United States; |
| |
• |
|
authorise the Company to register you as the holder(s) of New Shares (including any additional New Shares) and
New Options allotted to you; |
| |
• |
|
if you are a natural person, you declare you are over 18 years of age and have full legal capacity and power to
perform all of your rights and obligations under the accompanying personalised Application Form; |
| |
• |
|
acknowledge that after the Company receives your payment of Application Monies through BPAY®, you may not withdraw your Application or funds provided except as allowed by law; |
| |
• |
|
authorise the Company, the Underwriter, the Share Registry and their respective officers or agents to do anything
on your behalf necessary for New Shares (including any additional New Shares) and New Options to be issued to you, including to act on instructions of the Share Registry on using the contact details set out in your Application Form;
|
| |
• |
|
acknowledge that none of the Company, the Underwriter nor their respective related bodies corporate and
affiliates and their respective directors, officers, partners, employees, representatives, agents, consultants or advisers, guarantees the performance of the Company, nor do they guarantee the repayment of capital; |
| |
• |
|
agree to provide (and direct your custodian or nominee to provide) any requested substantiation of your
eligibility to participate in the Retail Entitlement Offer and of your holding of Existing Shares on the Record Date; and |
| |
• |
|
acknowledge and agree that determination of eligibility of investors for the purposes of the Retail Entitlement
Offer was made by reference to a number of matters, including legal and regulatory requirements, logistical and registry constraints and the discretion of the Company and/or the Underwriter, and each of the Company and the Underwriter and their
respective related bodies corporate and affiliates disclaim any duty or liability (including for negligence) in respect of that determination and the exercise of that discretion to the maximum extent permitted by law. |
| 2.12 |
Underwriting Agreement |
The Company has entered into an underwriting agreement with the Underwriter (Underwriting Agreement). Under the Underwriting Agreement,
the Underwriter was appointed by the Company on an exclusive basis, to act as lead manager for the Capital Raising and as underwriter for the issue of New Shares under the Placement and the Entitlement Offer. The obligations of the Underwriter are
subject to the satisfaction of certain conditions precedent, including (but not limited to):
| |
• |
|
preparation of offer documents; |
22
| |
• |
|
due diligence investigations being undertaken to the satisfaction of the Underwriter (acting reasonably);
|
| |
• |
|
ASX not indicating that it will not admit the New Shares and New Options to quotation; and |
| |
• |
|
receipt by the Underwriter of certain customary opinions and reports from the Company and its advisers.
|
The Company has (subject to certain limitations) agreed to indemnify the Underwriter and its related bodies corporate
and each of any of their respective directors, officers, employees, agents, advisers and representatives against certain losses in connection with the Capital Raising or the performance of the Underwriter’s obligations under the Underwriting
Agreement.
The Company and the Underwriter have given certain representations, warranties and undertakings in connection with (among other
things) the conduct of the Entitlement Offer.
The Underwriter will be remunerated by the Company for providing these underwriting services
at market rates and be reimbursed for certain expenses. The Underwriter has not authorised or caused the issue of, and takes no responsibility for, this Prospectus, and to the maximum extent permitted by law, disclaims all liability in connection
with the Entitlement Offer and this Prospectus.
The Underwriter may (in certain circumstances, including having regard to the materiality
of the relevant event) terminate the Underwriting Agreement and be released from its obligations under it on the occurrence of certain events. A summary of these key termination events is set out below.
Key Underwriting Agreement termination events
The list below is not exhaustive of all of the termination events in the Underwriting Agreement and is a summary of the select events set out
below only.
| |
• |
|
(misleading disclosure) a statement contained in the Offer materials is or becomes misleading or deceptive
or likely to mislead or deceive (including by omission) or a matter required to be included is omitted from the Offer Materials; |
| |
• |
|
(information) the report of the due diligence committee established for the purposes of the Offer or any
information supplied by or on behalf of Opthea to the Underwriter for the purposes of due diligence investigations, the Offer materials or the Offer, is false, misleading or deceptive in a material respect; |
| |
• |
|
(section 730 notice) a person (other than the Underwriter) gives a notice to Opthea under section 730 of
the Corporations Act in relation to the Prospectus; |
| |
• |
|
(withdrawal of consent) any person (other than the Underwriter) whose consent to the issue of the
Prospectus is required and who has previously consented withdraws such consent; |
| |
• |
|
(supplementary Prospectus) Opthea lodges a supplementary prospectus without the Underwriter’s
consent, or fails to lodge a supplementary prospectus in a form acceptable to the Underwriter or (in the Underwriter’s reasonable opinion) becomes required to lodge a supplementary prospectus; |
| |
• |
|
(new circumstance) a new circumstance arises or becomes known which, if known at the time of issue of this
presentation or the Prospectus would have been required to be included in the relevant document; |
23
| |
• |
|
(material adverse change) there occurs any material adverse change, or development involving a prospective
material adverse change, in the condition (financial or otherwise) or in the assets, liabilities, earnings, business, operations, management, profits, losses or prospects of Opthea or the Group; |
| |
• |
|
(market fall) the ASX/S&P 300 Index falls by 10% or more at any time from its level at market close on
the business day immediately preceding the date of the underwriting agreement; |
| |
• |
|
(future matters) any estimate or expression of opinion, belief, expectation or intention, or statement
relating to future matters in any Offer materials is or becomes incapable of being met or, in the reasonable opinion of the Underwriter, unlikely to be met in the projected timeframe; |
| |
• |
|
(change of law) there is introduced or there is a public announcement of a proposal to introduce, into the
Parliament of Australia or any State of Australia a new law, or the Reserve Bank of Australia, or any Commonwealth or State authority, adopts or announces a proposal to adopt a new policy (other than a law or policy which has been announced before
the date of the underwriting agreement), any of which does or in the reasonable opinion of the Underwriter is likely to prohibit or adversely affect or regulate the Offer, capital issues or stock markets or the Underwriter’s ability to promote
or market the Offer or enforce contracts to issue or allot the New Shares or New Options, or adversely affect the taxation treatment of those securities; |
| |
• |
|
(unable to proceed) Opthea is or will be prevented from conducting or completing the Offer by or in
accordance with the Listing Rules, ASIC, ASX, any applicable laws or an order of a court of competent jurisdiction, or otherwise are or will become unable or unwilling to do any of these things or a third party applies to a court of competent
jurisdiction seeking orders to prevent, or which will have the effect of preventing any of these things; |
| |
• |
|
(force majeure) there is an event or occurrence of any government agency which makes it illegal for the
Underwriter to satisfy an obligation under this document, or to market, promote or settle the Offer; |
| |
(i) |
Opthea ceases to be admitted to the official list of ASX or Shares (or interests in them) cease trading or are
suspended from official quotation or cease to be quoted on the ASX (other than a voluntary suspension requested by Opthea and consented to by the Underwriter; or |
| |
(ii) |
ASX makes any official statement to any person, or indicates to Opthea or the Underwriter that it will not
grant permission for the official quotation of the New Shares or New Options; or |
| |
(iii) |
permission for the official quotation of the New Shares or New Options is granted before the date of issue of
those Offer Securities, but the approval is subsequently withdrawn, qualified or withheld; |
| |
(i) |
an application is made by ASIC for an order under Part 9.5 of the Corporations Act in relation to the Offer
materials or the Offer or ASIC commences, or gives notice of an intention to hold, any investigation or hearing in relation to the Offer or any of the |
24
| |
Offer materials or prosecutes or commences proceedings against or gives notice of an intention to prosecute or commence proceedings against Opthea and any such application or notice whether or
not withdrawn becomes publicly known or is not withdrawn within two business days after it is made, or where it is made less than two business days before the relevant settlement date under the Offer, it is not withdrawn before that settlement date;
or |
| |
(ii) |
there is an application to a government agency (including, without limitation, the Takeovers Panel) for an
order, declaration or other remedy in connection with the Offer (or any part of it) or any agreement entered into in respect of the Offer (or any part of it) except where such application does not become public and is withdrawn or dismissed within
two business days after it is commenced or where it is commenced less than two business days before the proposed issue date or completion of the Offer it has not been withdrawn or dismissed by that date; |
| |
• |
|
(no misleading or deceptive conduct) Opthea engages in conduct that is misleading or deceptive or which is
likely to mislead or deceive in connection with the making of the Offer; |
| |
• |
|
(withdrawal) Opthea withdraws or indicates that it does not intend to proceed with the Offer or any part
of the Offer, or withdraws a document forming part of the Offer materials; |
| |
• |
|
(market disruption) either of the following occurs: |
| |
(i) |
a general moratorium on commercial banking activities in Australia, the United States of America, Singapore,
Hong Kong, the People’s Republic of China, any member state of the European Union or the United Kingdom is declared by the relevant central banking authority in any of those countries, or there is a material disruption in commercial banking or
security settlement or clearance services in any of those countries; or |
| |
(ii) |
trading in all securities quoted or listed on ASX, the London Stock Exchange, the Hong Kong Stock Exchange, the
Singapore Stock Exchange or the New York Stock Exchange is suspended or limited in a material respect for more than one day on which that exchange is open for trading; |
| |
• |
|
(hostilities) hostilities not presently existing commence (whether war has been declared or not) or a
major escalation in existing hostilities occurs (whether war has been declared or not) involving any one or more of Australia, New Zealand, the United States of America, the United Kingdom, any member state of the European Union, the People’s
Republic of China, Hong Kong, Singapore or a major act of terrorism is perpetrated on any of those countries anywhere in the world; |
| |
• |
|
(political or economic conditions) the occurrence of any adverse change or disruption to financial,
political or economic conditions, currency exchange rates or controls or financial markets in Australia, New Zealand, any member state of the European Union, the United States of America, the United Kingdom, the People’s Republic of China, Hong
Kong, Singapore or any change or development involving a prospective adverse change in any of those conditions or markets; |
| |
• |
|
(pandemic) a pandemic, epidemic or large-scale outbreak of a disease (including without limitation SARS,
swine or avian flu, H5N1, H7N9, COVID-19 or a related or mutated form of these) not presently existing occurs or in respect of which there is a major escalation, involving any one or more of Australia, New
Zealand, a member of the European Union, the United States of America, United Kingdom, Hong Kong, the People’s Republic of China or Singapore; |
| |
• |
|
(representations and warranties) a representation and warranty provided by Opthea in the underwriting
agreement is untrue or incorrect when given or taken to be given or becomes untrue or incorrect; |
25
| |
• |
|
(Certificate) any certificate which is required to be furnished by Opthea under the underwriting agreement
is not furnished when required or is untrue, incorrect or misleading; |
| |
• |
|
(delay) any event specified in the underwriting agreement is delayed for more than two business days,
without the prior written consent of the Underwriter; |
| |
• |
|
(unauthorised change) Opthea or a member of the Group: |
| |
(i) |
disposes, or agrees to dispose, of the whole, or a substantial part, of its business or property other than as
contemplated in the Offer materials; |
| |
(ii) |
ceases or threatens to cease to carry on business; |
| |
(iii) |
alters its capital structure, other than as contemplated in the Offer materials or as permitted by the
underwriting agreement; or |
| |
(iv) |
amends its constitution or other constituent document; |
| |
• |
|
(breach) Opthea fails to perform or observe any of its obligations under the underwriting agreement;
|
| |
(i) |
a contravention by Opthea or any member of the Group of the Corporations Act, the Constitution, Listing Rules,
any applicable laws, or a requirement, order or request made by or on behalf of the ASIC, ASX or any other government agency or any agreement entered into by it; or |
| |
(ii) |
any Offer materials or any aspect of the Offer does not comply with the Corporations Act, Listing Rules or any
other applicable law or regulation; |
| |
• |
|
(change in directors or management) a change to Opthea’s chief executive officer, chief financial
officer or board of directors occurs, or any such changes are announced (other than a change announced to ASX prior to the date of the underwriting agreement); |
| |
• |
|
(prosecution) any of the following occurs: |
| |
(i) |
a director or senior executive of Opthea engages in any fraudulent conduct or activity, or is charged with an
indictable offence; |
| |
(ii) |
any government agency commences any public proceedings against Opthea or any director in their capacity as a
director of Opthea, or announces that it intends to take such action; or |
| |
(iii) |
any director of Opthea is disqualified from managing a corporation under Part 2D.6 of the Corporations Act; or
|
| |
(iv) |
an investigation, inquiry or other similar communication is received from a government agency in relation to
Opthea; |
| |
• |
|
(regulatory approvals) a government agency withdraws, revokes or amends any regulatory approvals required
for Opthea to perform its obligations under the underwriting agreement or to carry out the transactions contemplated by the Offer materials; |
26
| |
• |
|
(Encumbrance) a person encumbers or agrees to encumber, the whole or a substantial part of the business or
property of Opthea or the Group; |
| |
• |
|
(ASIC Modifications) ASIC withdraws, revokes or amends any modifications, exemptions or approvals required
to enable Opthea to conduct the Offer as described in the Offer materials; |
| |
• |
|
(Insolvency) an insolvency event occurs (including the appointment of a receiver, manager, administrator
or controller or any application not withdrawn or dismissed within 7 days for an order to wind up, or an admission that it is insolvent or unable to pay its debts) to a member of the Group or there is an act which has occurred or any omission made
which would result in such an event occurring in respect of any member of the Group. |
The ability of the Underwriter to
terminate the underwriting agreement in respect of the events set out above, in some cases, is limited to circumstances where, in the reasonable opinion of the Underwriter:
| |
• |
|
the event has had (or is likely to have) a material adverse effect on the business operations, assets,
liabilities, financial condition, position or performance, profits, losses, prospects, earning position or results of operations of the Group; or |
| |
• |
|
the Underwriter will (or is likely to) contravene, be involved in a contravention of, or incur a liability under
the Corporations Act or any other applicable law as a result of that event. |
Underwriting fees
The Underwriter will be paid:
| |
• |
|
a management fee of 3% of the total cash proceeds raised from the Placement; |
| |
• |
|
a selling fee of 3% of the total cash proceeds raised from the Placement; |
| |
• |
|
an underwriting fee of 3.5% of the total cash proceeds raised from the Entitlement Offer; plus
|
| |
• |
|
a management fee of 3% of the total cash proceeds raised from the Entitlement Offer. |
The Underwriter will also be reimbursed for certain expenses, including but not limited to legal, marketing and communication costs, printing,
couriers, postage and distribution, roadshow expenses, accommodation and travel expenses.
Neither the Underwriter nor any of its related
bodies corporate and affiliates, nor any of its directors, officers, partners, employees, representatives or agents have authorised or caused the issue of this Prospectus and they do not take any responsibility for this document or any action taken
by you on the basis of information contained in this document. To the maximum extent permitted by law, the Underwriter and its related bodies corporate and affiliates and each of their respective directors, officers, partners, employees,
representatives or agents exclude and disclaim all liability for any expenses, losses, damages or costs incurred by you as a result of your participation in the Retail Entitlement Offer and this information being inaccurate or incomplete in any way
for any reason, whether by negligence or otherwise. Neither the Underwriter nor any of its related bodies corporate and affiliates, nor any of their respective directors, officers, partners, employees, representatives or agents make any
recommendations as to whether you or your related parties should participate in the Retail Entitlement Offer, nor do they make any representations or warranties to you concerning this Retail Entitlement Offer or any such information, and you
represent, warrant and agree that you have not relied on any statements made by either Underwriter or any of their respective related bodies corporate and affiliates or any of their respective directors, officers, partners, employees,
representatives or agents in relation to the New Shares or the Retail Entitlement Offer generally.
27
| 2.13 |
Custodians and nominees |
Persons who hold Shares as a custodian or nominee must not purport to accept, or make an application under, the Retail Entitlement Offer in
respect of:
| |
• |
|
beneficiaries on whose behalf they hold existing Shares who would not satisfy the criteria for an Eligible
Shareholder; |
| |
• |
|
any Shareholder that is in the United States, including any Shareholder in the United States for whom the
custodian or nominee holds Shares or acts; or |
| |
• |
|
Shareholders who are not eligible under all applicable securities laws to receive an offer under the Retail
Entitlement Offer. |
Persons acting as nominees or custodians for other persons must not take up any Entitlements on
behalf of, or send any documents related to the Entitlement Offer to, any person who is an Ineligible Shareholder or any person in the United States or any person that is acting for the account or benefit of a person in the United States.
Opthea is not required to determine whether or not any registered holder or investor is acting as a nominee or custodian or the identity or
residence of any beneficial owners of existing Shares or Entitlements. Where any person is acting as a nominee or custodian for a foreign person, that person, in dealing with its beneficiary, will need to assess whether indirect participation in the
Entitlement Offer by the beneficiary complies with applicable laws. Opthea is not able to provide legal advice.
By submitting an
Application on behalf of a beneficiary, you certify that you are the custodian or nominee for the beneficiary and the information contained in the Application Form is true and correct as at the date of the Application.
Custodians and nominees holding Shares on behalf of residents outside Australia and New Zealand may not send this Prospectus to persons, or
apply for New Shares and New Options on behalf of beneficial shareholders, resident outside Australia and New Zealand. Payment by BPAY® or such other means will be taken to constitute a
representation and warranty that there has been no breach of this restriction or applicable laws.
Application for official quotation of the New Shares and New Options offered under this Prospectus has been or will be made within 7 days of
the date of this Prospectus.
If the New Shares or New Options are not admitted to official quotation by ASX before the expiration of three
months after the date of this Prospectus, or such period as varied by ASIC, the Company will not issue any New Shares or New Options and will repay all Application Monies for the New Shares within the time prescribed under the Corporations Act,
without interest.
The fact that ASX may grant official quotation to the New Shares and New Options is not to be taken in any way as an
indication of the merits of the Company or the New Shares and New Options now offered for subscription.
| 2.15 |
Issue of New Shares and New Options |
The issue of New Shares and New Options under the Retail Entitlement Offer will take place as soon as practicable after the Closing Date of the
Retail Entitlement Offer. The Company expects that the New Shares and New Options will be issued on Thursday, 21 September 2023. It is expected that New Shares and New Options issued under the Retail Entitlement Offer will
28
commence trading on a normal settlement basis on ASX on Friday, 22 September 2023. Holding statements are expected to be dispatched on or around Monday, 25 September 2023. These dates
are subject to change at the absolute discretion of the Company.
Pending the issue of the New Shares and New Options or payment of refunds
under this Prospectus, all Application Monies will be held by the Company in trust for the Applicants in a separate bank account as required by the Corporations Act. The Company will be entitled to retain all interest that accrues on the bank
account and each Applicant waives the right to claim interest.
| 2.16 |
Defects in Applications |
If an Application is not completed correctly or if the accompanying payment is for the wrong amount, the Company may, in its absolute
discretion, still treat the Application to be valid. The Company’s decision to treat an Application as valid, or how to construe, amend or complete it, will be final.
Refunds under the Retail Entitlement Offer may be paid under various circumstances. If a refund is made, payment will be by cheque mailed to
your address as shown on the Company’s share register or by deposit into your previously nominated bank account. You will not receive any interest on funds refunded to you.
| 2.18 |
Costs of participation |
No brokerage, commissions or other transaction costs will be payable by Eligible Shareholders in respect of the Application for, and allotment
of, New Shares or New Options under the Retail Entitlement Offer.
| 2.19 |
Modification and termination of the Retail Entitlement Offer |
The Company may modify or terminate the Retail Entitlement Offer at any time including closing the Retail Entitlement Offer early. The Company
will notify the ASX of any modification to, or termination of, the Retail Entitlement Offer. The omission to give notice of any modification to, or termination of, the Retail Entitlement Offer or the failure of ASX to receive such notice will not
invalidate the modification or termination.
The Company may settle in any manner it thinks fit, any difficulties, anomalies or disputes
which may arise in connection with, or by reason of, the operation of the Retail Entitlement Offer, whether generally or in relation to any participant or application, and the decision of the Company will be conclusive and binding on all
participants and other persons to whom the determination relates.
The Company reserves the right to waive strict compliance with any
provision of the terms and conditions of this Prospectus. The powers of the Company under this Prospectus may be exercised by the Directors or any delegate of the Directors.
| 2.20 |
Rights and liabilities attaching to New Shares |
The New Shares to be issued under the Retail Entitlement Offer are of the same class and will rank equally in all respects with the Existing
Shares on issue. The rights and liabilities attaching to New Shares are further described in Section 6.5.
29
| 2.21 |
Rights and liabilities attaching to New Options |
Shares issued on exercise of the New Options will rank equally in all respects with the Existing Shares on issue. The rights and liabilities
attaching to New Options are further described in Section 6.6.
| 2.22 |
CHESS and issuer sponsorship |
The Company operates an electronic CHESS sub-register and an electronic issue sponsored sub- register. These two sub-registers will make up the Company’s register of Securities.
The Company will not issue a share certificate to a security holder. Rather, a holding statement (similar to a bank statement) will be
dispatched to security holders as soon as practicable after issue of the New Shares and New Options the subject of the Retail Entitlement Offer. The holding statement will be sent either by CHESS (if the security holder elects to hold the New Shares
and New Options on the CHESS sub-register) or by the Company’s Share Registry (if the security holder elects to hold the New Shares and New Options on the issuer sponsored
sub-register). The statement will set out details of the New Shares and New Options issued under this Prospectus and the Holder Identification Number (if the security holder elects to hold the New Shares and
New Options on the CHESS sub register) or Shareholder Reference Number (if the security holder elects to hold the New Shares and New Options on the issuer sponsored sub-register). Updated holding statements
will also be sent to each security holder following the month in which the balance of their security holding changes, and also as required by the ASX Listing Rules and the Corporations Act.
It is the responsibility of all investors to satisfy themselves of the particular taxation treatment that applies to them in relation to the
Retail Entitlement Offer, by consulting their own professional tax advisors. The Company and the Directors do not accept any liability or responsibility in respect of the taxation consequences of the matters referred to in this Prospectus.
This Prospectus is important and should be read in its entirety. Persons who are in any doubt as to the course of action to be followed should
consult their stockbroker, solicitor, accountant or other professional advisor without delay.
| 3. |
DETAILS OF THE INSTITUTIONAL OPTION OFFER |
| 3.1 |
The Institutional Option Offer |
Under this Prospectus, participants in the Placement and the Institutional Entitlement Offer will be issued 1 Institutional Option for every 2
New Shares to be issued to them under the Placement and/or the Institutional Entitlement Offer (as applicable) on the same terms as the New Options.
The Institutional Options will be issued for nil consideration.
The Institutional Option Offer is scheduled to close at 5.00pm (Melbourne time) on Thursday, 14 September 2023.
30
The Company reserves the right to:
| |
• |
|
extend the Institutional Option Offer; |
| |
• |
|
close the Institutional Option Offer early; or |
| |
• |
|
withdraw the Institutional Option Offer, |
at any time by making an announcement to the ASX.
| 3.4 |
Participation in the Institutional Options Offer |
Participation in the Institutional Option Offer is only open to participants in the Placement and/or the Institutional Entitlement Offer.
Participants will receive 1 Institutional Option for every 2 New Shares issued to them under the Placement and/or the Institutional Entitlement Offer (as applicable).
If you are in any doubt about the Institutional Option Offer, or how participation will affect you, you should seek independent financial and
taxation advice.
To participate in the Institutional Option Offer, an Applicant represents and warrants that:
| |
• |
|
it acknowledges that the Institutional Options (and the underlying ordinary shares) have not been, and will not
be, registered under the US Securities Act or the securities laws of any state or other jurisdictions in the United States and accordingly, the Institutional Options may not be offered or sold in the United States except in accordance with an
available exemption from, or in a transaction not subject to, the registration requirements of the US Securities Act and any other applicable US state securities laws; |
| |
• |
|
it has not and will not send this Prospectus or any other document relating to the Institutional Option Offer to
any person in the United States or elsewhere outside Australia or New Zealand; |
| |
• |
|
if the Applicant is in European Union (exclude Austria), it participated in the Placement and/or the
Institutional Entitlement Offer and it is a “qualified investor” (as defined in Article 2(e) of the Regulation (EU) 2017/1129 of the European Parliament and the Council of the European Union); |
| |
• |
|
if the Applicant is in Hong Kong, it participated in the Placement and/or the Institutional Entitlement
Offer and is a ‘professional investor’ as defined under the Securities and Futures Ordinance of Hong Kong, Chapter 571 of the Laws of Hong Kong; |
| |
• |
|
if the Applicant is in Israel, it participated in the Placement and/or the Institutional Entitlement Offer
and it is a type of sophisticated investor as described in the First Addendum to the Israeli Securities Law, 1968 |
| |
• |
|
if the Applicant is in New Zealand, it participated in the Placement and/or the Institutional Entitlement
Offer and is a person who: |
| |
(v) |
is an investment business within the meaning of clause 37 of Schedule 1 of the Financial Markets Conduct Act
2013 (New Zealand) (the FMC Act); |
| |
(vi) |
meets the investment activity criteria specified in clause 38 of Schedule 1 of the FMC Act;
|
| |
(vii) |
is large within the meaning of clause 39 of Schedule 1 of the FMC Act; |
| |
(viii) |
is a government agency within the meaning of clause 40 of Schedule 1 of the FMC Act; or |
31
| |
(ix) |
is an eligible investor within the meaning of clause 41 of Schedule 1 of the FMC Act (and, if an eligible
investor, have provided the necessary certification); |
| |
• |
|
if the Applicant is in Singapore, it participated in the Placement and/or the Institutional Entitlement
Offer and is an “institutional investor” or an “accredited investor” (as such terms are defined in the Securities and Futures Act 2001 of Singapore; |
| |
• |
|
if the Applicant is in South Africa, it participated in the Placement and/or the Institutional Entitlement
Offer and is included in the categories of persons pertaining to “offers that are not offers to the public” as contained in section 96(1)(a) of the South African Companies Act and, as such, it is not a person in respect of which the
prospectus requirements of the South African Companies Act apply; |
| |
• |
|
if the Applicant is in the United Kingdom, it participated in the Placement and/or the Institutional
Entitlement Offer and is: |
| |
(i) |
a ‘qualified investor’ within the meaning of Article 2(e) of the UK Prospectus Regulation; and
|
| |
(ii) |
within the categories of persons referred to in Article 19(5) (investment professionals) or Article 49(2)(a) to
(d) (high net worth companies, unincorporated associations, etc.) of the UK Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended; and |
| |
• |
|
if the Applicant is in the United States, it participated in the Placement and/or the Institutional
Entitlement Offer and it is either (i) a “qualified institutional buyer” as defined in Rule 144A under the US Securities Act or (ii) a dealer or other professional fiduciary organized or incorporated in the United States that is
acting for a discretionary or similar account (other than an estate or trust) held for the benefit or account of persons that are not US persons and for which it exercises investment discretion, within the meaning of Rule 902(k)(2)(i) of Regulation
S under the US Securities Act. |
Please also see Section 6.3 for further information.
| 3.5 |
Action in relation to Institutional Option Offer |
No action is required from participants in the Placement and the Institutional Entitlement Offer to take up Institutional Options under the
Institutional Option Offer.
| 3.6 |
Effect of receiving Institutional Options |
If you are issued Institutional Options under the Institutional Option Offer, you:
| |
• |
|
acknowledge that you have not been provided with investment advice or financial product advice by the Company or
its Directors and have made your own enquiries before making an investment decision; |
| |
• |
|
agree that your Application is made on the terms and conditions of the Institutional Option Offer set out in this
Prospectus and the Constitution; |
| |
• |
|
acknowledge that the Company is not liable for any exercise of its discretions referred to in this Prospectus;
and |
| |
• |
|
are in compliance with all relevant laws and regulations (including, without limitation, section 1043A of the
Corporations Act and laws and regulations designed to restrict terrorism financing and/or money laundering). |
32
The Institutional Option Offer is not underwritten.
Application for official quotation of the Institutional Options offered under this Prospectus has been or will be made within 7 days of the
date of this Prospectus.
If the Institutional Options are not admitted to Official Quotation by ASX before the expiration of three months
after the date of issue of this Prospectus, or such period as varied by ASIC, the Company may not issue any Institutional Options.
The
fact that ASX may grant Official Quotation to the Institutional Options is not to be taken in any way as an indication of the merits of the Company or the Institutional Options now offered for subscription.
| 3.9 |
Issue of Institutional Options |
The issue of Institutional Options under the Institutional Option Offer will take place as soon as practicable after the Closing Date of the
Retail Entitlement Offer and at the same time as the issue of New Options. The Company expects that the Institutional Options will be issued on Thursday, 21 September 2023. It is expected that Institutional Options issued under the
Institutional Option Offer will commence trading on a normal settlement basis on ASX on Friday, 22 September 2023. Holding statements are expected to be dispatched on or around Monday, 25 September 2023. These dates are subject to change
at the absolute discretion of the Company.
| 3.10 |
Modification of the Institutional Option Offer |
The Company may modify the Institutional Option Offer at any time. The Company will notify the ASX of any modification to the Institutional
Option Offer. The omission to give notice of any modification to the Institutional Option Offer or the failure of ASX to receive such notice will not invalidate the modification.
The Company may settle in any manner it thinks fit, any difficulties, anomalies or disputes which may arise in connection with, or by reason
of, the operation of the Institutional Option Offer, whether generally or in relation to any participant, and the decision of the Company will be conclusive and binding on all participants and other persons to whom the determination relates.
The Company reserves the right to waive strict compliance with any provision of the terms and conditions of this Prospectus. The powers of the
Company under this Prospectus may be exercised by the Directors or any delegate of the Directors.
| 4. |
PURPOSE AND EFFECT OF THE ENTITLEMENT OFFER AND PLACEMENT |
| 4.1 |
Purpose of the Entitlement Offer and Placement |
The primary purpose of the Entitlement Offer is to raise approximately $70.0 million.
The total funds raised from the Entitlement Offer and Placement of up to $80.0 million (noting that the Company has the ability to
increase the size of the Placement in its sole discretion) are intended to be used by Opthea to continue advancing the clinical development of OPT-302 for the treatment of wet AMD, including to progress the
Phase 3 clinical program and for general corporate purposes, as well as the other purposes described in this Prospectus.
33
Please refer to Section 6.10 for further details relating to the estimated expenses of
the Entitlement Offer and the Placement.
| 4.2 |
Effect of the Offers and Placement |
The principal effects of the Entitlement Offer and the Placement, assuming $80.0 million is raised under the Placement and the Entitlement
Offer, will:
| |
• |
|
be to increase the Company’s cash reserves by approximately $73.2 million (after deducting the
estimated expenses of the Entitlement Offer and Placement) immediately after completion of the Retail Entitlement Offer; |
| |
• |
|
be to increase the number of Shares on issue from 467,159,434 (prior to issue of the New Shares under the
Placement and Institutional Entitlement Offer) to up to approximately 641 million Shares; and |
| |
• |
|
be to issue approximately 87.0 million Options. |
Note that no consideration is expected to be received initially by the Company on the issue of the Options. There is no certainty that all or
some of the Options will be exercised and additional Shares issued as a result and, consequently, no certainty that the Company will receive proceeds from the exercise of the Options.
| 4.3 |
Pro forma preliminary unaudited balance sheet |
The pro forma preliminary unaudited balance sheet for the Company as at 30 June 2023 has been prepared based on the accounting policies
normally adopted by the Company. The pro forma preliminary unaudited balance sheet represents preliminary estimated unaudited financial information and remains subject to the completion of management’s and our audit and risk committee’s
reviews and other financial closing processes.
The pro forma preliminary unaudited preliminary balance sheet has not been prepared on a
fully diluted basis meaning that it assumes none of the Options to be issued under this Prospectus have been exercised.
The pro forma
preliminary unaudited balance sheet has been prepared to provide investors with information on the assets and liabilities of the Company and pro forma assets and liabilities of the Company as noted below. The historical and pro forma financial
information is presented in abbreviated form, insofar as it does not include all of the disclosures required by the Australian Accounting Standards applicable to annual financial statements.
34
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
June 30,
2023
US$ |
|
|
Funding
2023
US$ |
|
|
Proforma
June 30,
2023
Unaudited
US$ |
|
| Assets |
|
|
|
|
|
|
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents |
|
|
89,188,713 |
|
|
|
131,900,000 |
|
|
|
221,088,713 |
|
| Current tax receivable |
|
|
5,926,350 |
|
|
|
|
|
|
|
5,926,350 |
|
| Receivables |
|
|
636,565 |
|
|
|
|
|
|
|
636,565 |
|
| Prepayments |
|
|
2,634,671 |
|
|
|
|
|
|
|
2,634,671 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total current assets |
|
|
98,386,299 |
|
|
|
131,900,000 |
|
|
|
230,286,299 |
|
| Non-current assets: |
|
|
|
|
|
|
|
|
|
|
|
|
| Property and equipment, net |
|
|
33,035 |
|
|
|
|
|
|
|
33,035 |
|
| Right-of-use
assets |
|
|
168,451 |
|
|
|
|
|
|
|
168,451 |
|
| Prepayments |
|
|
53,535 |
|
|
|
|
|
|
|
53,535 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total non-current assets |
|
|
255,021 |
|
|
|
|
|
|
|
255,021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total assets |
|
|
98,641,320 |
|
|
|
131,900,000 |
|
|
|
230,541,320 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Payables |
|
|
17,797,227 |
|
|
|
|
|
|
|
17,797,227 |
|
| Lease liabilities |
|
|
97,485 |
|
|
|
|
|
|
|
97,485 |
|
| Provisions |
|
|
753,300 |
|
|
|
|
|
|
|
753,300 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total current liabilities |
|
|
18,648,012 |
|
|
|
|
|
|
|
18,648,012 |
|
| Non-current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
| Lease liabilities |
|
|
84,226 |
|
|
|
|
|
|
|
84,226 |
|
| Financial liabilities |
|
|
85,660,000 |
|
|
|
85,000,000 |
|
|
|
170,660,000 |
|
| Provisions |
|
|
7,631 |
|
|
|
|
|
|
|
7,631 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total non-current liabilities |
|
|
85,751,857 |
|
|
|
85,000,000 |
|
|
|
170,751,857 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total liabilities |
|
|
104,399,869 |
|
|
|
85,000,000 |
|
|
|
189,399,869 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net Assets |
|
|
(5,758,549 |
) |
|
|
46,900,000 |
|
|
|
41,141,451 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Equity |
|
|
|
|
|
|
|
|
|
|
|
|
| Contributed equity |
|
|
320,883,551 |
|
|
|
46,900,000 |
|
|
|
367,783,551 |
|
| Accumulated Loss |
|
|
(359,367,808 |
) |
|
|
|
|
|
|
(359,367,808 |
) |
| Reserves |
|
|
32,725,708 |
|
|
|
|
|
|
|
32,725,708 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Equity |
|
|
(5,758,549 |
) |
|
|
46,900,000 |
|
|
|
41,141,451 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note:
| (1) |
The figures stated in this pro forma preliminary unaudited balance sheet are subject to the effects of rounding
and are in US dollars. |
| (2) |
The preliminary estimated unaudited financial information presented above remains subject to the completion of
management’s and Opthea’s audit and risk committee’s reviews and other financial closing processes. Such financial information has also not been audited, reviewed or compiled by Opthea’s independent registered public accounting
firm (see below for further details). |
| (3) |
See ‘Risk Factors’ in Section 5 for a discussion of factors that may cause Opthea to require
additional funding earlier than expected, including additional delays in completing enrollment for our phase 3 clinical trials or higher than expected CRO and related costs to run such trials. |
The pro forma balance sheet gives effect to the following assumptions:
| |
• |
|
US$46.9 million is received from the Capital Raising; |
35
| |
• |
|
the 3rd Tranche of funding of US$35 million due by
December 31 2023 under the Development Funding Agreement with Carlyle and Abingworth (DFA) is received; and |
| |
• |
|
the co-investor of Carlyle and Abingworth provides additional funding of
US$50 million under the DFA. |
Investors should note that the provision of the additional funding of
US$50 million under the DFA is at the discretion of the co-investor of Carlyle and Abingworth and is subject to their final due diligence and approvals, appropriate documentation, and compliance with
closing conditions. There can be no assurance that the due diligence will be completed to the satisfaction of the co-investor of Carlyle and Abingworth, that the closing terms and conditions will be satisfied
or that the company will ultimately receive the additional $50 million. Please refer to Section 5, ‘Risk Factors’.
For
the year ended June 30, 2023:
| |
• |
|
net loss: US$142 million; and |
| |
• |
|
cash used in Operations: US$121 million (reflects delays in enrollment and higher CRO and related costs for
the Phase 3 clinical trials during the fiscal year). |
Basis of preparation
The basis of preparation for the historical financial information is in accordance with the Company’s accounting policies, as described in
its financial reports, and the recognition and measurement principles of the Australian Accounting Standards.
The historical financial
information is based on the unaudited balance sheet as of 30 June 2023.
The stated basis of preparation for the pro forma historical
financial Information is in a manner consistent with the recognition and measurement principles of the Australian Accounting Standards applied to the historical financial information and the events or transactions to which the pro forma adjustments
relate, as above below, as if those events or transactions had occurred as of 30 June 2023.
Throughout this Prospectus, we have
presented certain preliminary estimated unaudited financial results and other data as of and for the fiscal year ended June 30, 2023, including preliminary estimated cash and cash equivalent amounts as of June 30, 2023. The estimated
results are not a comprehensive statement of our results as of and for the fiscal year ended June 30, 2023, and our actual results may differ materially from these preliminary estimated results. Our actual results remain subject to the
completion of management’s and our audit and risk committee’s reviews and our other financial closing processes. During the course of the preparation of our consolidated financial statements and related notes and the completion of the
audit for the fiscal year ended June 30, 2023, additional adjustments to the preliminary estimated financial information presented in this presentation may be identified, and our final results for these periods may vary from these preliminary
estimates.
The preliminary estimated unaudited financial and other data contained in this Prospectus have been prepared in good faith by,
and are the responsibility of, management based upon our internal reporting as of and for the fiscal year ended June 30, 2023. Our independent registered public accounting firm, has not audited, reviewed, compiled or performed any procedures
with respect to such preliminary data. Accordingly, our independent registered public accounting firm does not express an opinion or any other form of assurance with respect thereto.
36
| 4.4 |
The effect of the Entitlement Offer and Placement on the capital structure |
The effect of the Entitlement Offer and the Placement (assuming an $80.0 million Capital Raising) on the Company’s capital structure
is set out in the table below.
|
|
|
|
|
| Shares |
|
Number(1) |
|
| Shares on issue as at the date of this Prospectus |
|
|
467,159,434 |
|
| New Shares offered under Entitlement Offer (approximate) |
|
|
152,169,197 |
|
| New Shares offered under the Placement |
|
|
21,739,130 |
|
|
|
|
|
|
| Total Shares on issue after completion of the Capital Raising(2) |
|
|
641,067,761 |
|
|
|
|
|
|
| Options |
|
|
|
|
| Unquoted performance rights on issue as at the date of this Prospectus(3) |
|
|
2,900,000 |
|
| Unquoted options on issue as at the date of this Prospectus(4) |
|
|
22,550,000 |
|
| Unquoted ADS options on issue as at the date of this Prospectus(5) |
|
|
1,505,000 |
|
| New Options offered under the Retail Entitlement Offer, and Institutional Options offered under
the Institutional Option Offer (approximate) |
|
|
86,954,163 |
|
|
|
|
|
|
| Total options/performance rights (quoted or unquoted) after completion of the Capital
Raising(2) |
|
|
113,909,163 |
|
|
|
|
|
|
|
|
|
|
|
| Share capital |
|
|
|
| Share capital after the Capital Raising on an undiluted basis(2) |
|
|
641,067,761 |
|
| Share capital after the Capital Raising on a fully diluted basis |
|
|
765,511,924 |
|
Note:
| (1) |
Ignores impact of rounding. |
| (2) |
Assuming no existing unquoted options/performance rights are exercised. |
|
|
|
|
|
| Number of performance rights |
|
Exercise price |
|
Exercise date |
| 1,300,000 issued 16 November 2022 |
|
Nil exercise price |
|
10-year cliff vesting with accelerated vesting upon change of control, resignation, termination, or Board approval |
|
|
|
| 1,600,000 issued 19 October 2021 |
|
Nil exercise price |
|
• 300,000 Performance Rights vest on a straight-line basis calculated over
3 years; • 150,000
Performance Rights vest upon completion of recruitment for Phase 3 ShORe trial;
• 150,000 Performance Rights vest upon completion of recruitment for Phase 3 COAST trial;
• 400,000 Performance
Rights vest upon the read-out of Phase 3 ShORe trial;
• 400,000 Performance Rights vest upon the read-out
of Phase 3 COAST trial; and
• 200,000 Performance Rights vest upon receipt of FDA approval for OPT-302. |
37
| (4) |
Unquoted options with varying exercise prices and exercise dates. |
| (5) |
ADS options with varying exercise prices and exercise dates. ADS options convert to ordinary shares at an 8:1
ratio. |
| 4.5 |
Details of substantial holders |
Based on publicly available information as at the date of this Prospectus, the following Shareholders (together with their associates) have a
relevant interest in 5% or more of the Shares on issue:
|
|
|
|
|
| Substantial holder |
|
Number of shares |
|
Voting power in the
Company |
| Regal Funds Management Pty Ltd (Regal) |
|
Shares 73,087,499
ADS each
representing 8
Shares: 2,910,178 |
|
20.63%
Shares: 15.65% and ADS: 4.98% |
| 4.6 |
Effect of the Capital Raising on control of the Company |
In summary, section 606 of the Corporations Act provides that a person cannot acquire a ‘relevant interest’ (i.e. a controlling
interest, whether formal or informal) in the issued voting shares of a company which has more than 50 members or is publicly listed if, because of the transaction in relation to securities, a person’s ‘voting power’ in the company
increases from 20% or below to more than 20%, or from a starting point that is above 20% and below 90% unless a relevant exception applies. Item 13 of section 611 of the Corporations Act provides an exception to the takeover restrictions where a
person underwrites or sub-underwrites an issue of securities under a prospectus or other disclosure document, and where the prospectus discloses the effect that this would have on the person’s voting
power in the company.
As at the date of its last substantial shareholder filing, Regal holds 20.63% voting power in the Company. Regal has
entered into a sub-underwriting arrangement with the Underwriter with respect to 81.8% of the New Shares to be issued under the Retail Entitlement Offer
(Sub-underwriting Agreement).
Regal will receive 1.5% of the amount underwritten for sub-underwriting the Retail Entitlement Offer.
There are no significant events that could lead to the Sub-Underwriting Agreement being terminated, other than termination of the Underwriting Agreement between the Company and the Underwriter (in circumstances summarized above).
Through its participation in the Placement and Institutional Entitlement Offer, and any subscription of New Shares as a sub-underwriter of the Retail Entitlement Offer, it is likely that the percentage shareholding of Regal will increase. It is not possible to determine at this stage the extent of that increase and Regal will need to
comply with the provisions of Chapter 6 of the Corporations Act. However, the Company does not expect any increase in the percentage shareholding of Regal to have a material effect on the control of the Company.
As with any share investment, there are risks associated with an investment in the Company. The numerous risk factors are both of a specific
and general nature. Some can be mitigated by the use of safeguards and appropriate systems and controls, but some are outside the control of the Company and cannot be mitigated.
38
This Section 5 identifies and highlights some of the risks that potential investors
should consider prior to entering into the investment opportunity referred to in this Prospectus. However, the following is not, and does not purport to be, a comprehensive statement of all relevant risks and is not listed in order of importance.
Potential investors should seek their own financial or other professional advice in relation to the risks and must make their own assessment regarding an investment in the Company.
| |
(a) |
Future capital requirements |
Opthea’s activities will require substantial expenditures. Opthea’s losses from operations, including from clinical trial
activities, and negative cash flows, raise substantial doubt about the ability for Opthea to continue as a going concern without additional capital raising activities. While Opthea expects that the proceeds of the Offer, together with additional
funding expected under the DFA of US$35 million due under the DFA by December 31, 2023, the possible increased funding under the DFA of US$50m milliona and cash on hand, will provide funding to progress the activities set out in this
Prospectus, such proceeds will not be sufficient to fully fund all anticipated costs of the Phase 3 clinical trials. In addition, Opthea’s forecast of its cash runway, following receipt of the proceeds from the Offer and under the DFA, is
subject to a number of assumptions, including the timing of completion of Phase 3 clinical trial patient enrollment and CRO and labor costs. Although a co-investor of Carlyle and Abingworth has indicated
its intent to provide Opthea additional funding of US$50 million under the DFA, this is subject to final due diligence and approvals, appropriate documentation and compliance with closing conditions and there is no assurance that this funding
will be received or the timing of receipt of such funding. Estimated patient enrollment timing set forth in this Prospectus is primarily based on Opthea’s monthly enrollment rates for its Phase 3 clinical trials, which timing has in the past
significantly fluctuated from prior estimates, including due to factors outside Opthea’s control. CRO and related costs for the Phase 3 clinical trials have also significantly fluctuated from estimates in the past, including factors outside
Opthea’s control. If patient enrollment continues to be delayed in the future, or if any additional factors cause the Phase 3 clinical trials to be further delayed or more costly, then Opthea will need to obtain additional financing
earlier than the third quarter of calendar year 2024. In addition, if Opthea is unable to complete the Offer as contemplated in this Prospectus or obtain the increased US$50 million of funding under the DFA, then Opthea will need to seek
additional capital from other sources, which may not be available on a timely basis or at all. In such case, Opthea could be forced to delay, limit or terminate its operations, liquidate all or a portion of its assets and/or seek insolvency
protection in the near term. Opthea’s failure to raise capital, if and when needed, could delay or suspend Opthea’s business strategy and could have a material adverse effect on Opthea’s activities. If additional funds are raised
by issuing equity, this may result in additional dilution to the Shareholders. The pricing of future security issues will also depend on the results of Opthea’s scientific research projects, market factors, demand for securities and the need
for capital. If Opthea is unable to secure funding in the short term, there is a risk that Opthea will not be able to continue operating.
Opthea has entered into an underwriting agreement with MST Financial Services Pty Ltd (Underwriter). The Underwriter has agreed to act as sole
lead manager in relation to the Placement, and sole lead manager and underwriter in relation to the Entitlement Offer, subject to certain terms and conditions. Details of the fees payable to the Underwriter are included in the Appendix 3B released
to ASX on the date of this Prospectus and above in Section 2.12. If certain customary conditions are not satisfied or certain customary termination events occur, then the Underwriter may terminate the Underwriting Agreement. A summary of the
events which may trigger termination of the Underwriting Agreement is set out in Section 2.12.
39
Opthea also gives certain representations, warranties and undertakings to the Underwriter
and an indemnity to the Underwriter and its respective affiliates and related bodies corporate and their respective directors, officers, employees, partners and agents subject to certain limited exceptions.
If the Underwriting Agreement is terminated by the Underwriter, Opthea would need to find alternative financing to meet its future funding
requirements. There is no guarantee that alternative funding could be sourced, either at all or on satisfactory terms and conditions. See also the ‘Future capital requirements’ disclosure above. Termination of the Underwriting Agreement
could materially adversely affect Opthea’s business, cash flow, financial condition and results of operations.
| |
(c) |
Development Funding Agreement |
We are highly reliant on the funding under the DFA including the third tranche of $35 million expected before December 31, 2023, and
the potential increase in funding of US$50 million by a new co-investor of Carlyle and Abingworth (see below for further details). The DFA contains several terms that require compliance by the
company in conduct of the study including the governance by a Joint Steering Committee (“JSC”) for changes in the original protocols, study design or timelines. Modifications require JSC approval and it will be difficult for the company to
make modifications on their own. The DFA contains terms that require compliance by the company to maintain a minimum cash balance and to provide a notice to Ocelot (the SPV established by Carlyle and Abingworth for the purposes of providing funding
to Opthea) in the event it anticipates a “Going Concern” opinion in its annual financial statements or that it does not have sufficient cash to fund its operations for the next six months. The termination provisions in the DFA on the part
of Ocelot are extensive and give Ocelot a wide range of conditions to terminate the agreement. In the event of termination, unless mutual or for breach by Carlyle and Abingworth, amounts owed by the company will be multiples of the invested
capital to date. As of June 30, 2023, Ocelot has invested $85 million. A new co-investor of Carlyle and Abingworth intends to participate in a funding under the DFA of US$50 million to
increase total DFA funding from $120 million to $170 million, which is subject to the co-investor’s final due diligence and approvals, appropriate documentation and compliance with closing
conditions. There can be no assurance that Carlyle and Abingworth will increase the funding by $50 million. The third tranche of $35 million is due under the terms of the DFA before December 31, 2023, however, in the event the
$35 million is not paid it would be considered a Fundamental Material Breach of the DFA by Carlyle and Abingworth. Under a Fundamental Material Breach of the DFA, Opthea has limited recourse but would have the ability to terminate the DFA.
Termination by Opthea for lack of payment of the $35 million would relieve Opthea from any repayments under the DFA. Failure to receive the third tranche of $35 million or the increased funding of $50 million would have a negative
impact on our cash runway and our ability to complete enrollment in the ongoing trials. See Risks noted above under ‘Future capital requirements’.
The Opthea business model requires ongoing re-investment into clinical trials with no revenues
currently contracted. As such, Opthea will continue to rely upon cash, raised through equity or debt, to fund the business as an on-going concern, including in respect of its Phase 3 clinical trials. Any
unforeseen events which restrict the ability of Opthea to access capital is likely to affect Opthea’s ability to become profitable in future.
Clinical trials are inherently risky, and may prove unsuccessful or non-efficacious, impracticable or
costly, which may impact profitability and commercial potential. Difficulties in enrolling patients in clinical trials may cause delays to clinical trial schedules. Enrollment has been challenged, and may be challenged in the future, in part by the Covid-19 pandemic, supply chain issues, global and regional inflation, national and local recessions, hiring qualified staff at sites, Opthea’s CRO and distribution locations, local regulatory approvals,
importation and custom requirements and administrative delays. Failure, or negative or inconclusive results, can occur at many stages in development, and the results of earlier clinical trials are not necessarily predictive of future results and
data from clinical trials to date may not be indicative of results obtained when these trials are completed or in later-stage trials. Further, masked data from patients in Opthea’s Phase 3 clinical trials may not be consistent with
40
topline results which are subject to additional analysis once unmasked. A critical trial may fail to meet its primary or secondary endpoints and as a result inhibit product development, prevent
regulatory requirements being met for marketing approval and restrict successful commercialisation. In addition, data obtained from trials is susceptible to varying interpretations, and regulators may not interpret the data as favourably as Opthea,
which may delay, limit or prevent regulatory approval.
OPT-302 may fail to demonstrate a safety
profile or sufficient evidence of therapeutic efficacy in future clinical studies to support its ongoing clinical development. In addition, the ability to recruit wet AMD patients into future clinical studies, or secure clinical locations in which
to conduct those studies, may not occur at a sufficient rate to maintain program timelines.
Opthea maintains sensitive clinical data. Opthea may be subject to a cyber security attack or data breaches by employees or external parties
with either permitted or unauthorized access. There is therefore a risk that sensitive data may be exposed to the public or be permanently lost. A cyber security attack or data breach may also have implications for Opthea’s obligations under
any relevant data protection or privacy legislation. Failure to comply with such legislation or regulations can result in penalties, negative publicity and damage to its brand and reputation.
| |
(g) |
Research and development activities |
Opthea’s future success is dependent on the performance of OPT-302 in clinical trials and whether
it proves to be a safe and effective treatment. OPT-302 is an experimental product in clinical development and product commercialisation resulting in potential product sales and revenues is likely to be years
away, if ever, and there is no guarantee that it will be successful. OPT-302 requires additional research and development, including ongoing clinical evaluation of safety and efficacy in clinical trials and
regulatory approval prior to marketing authorisation. Drug development is associated with a high failure rate and until Opthea is able to provide further clinical evidence of OPT-302’s ability to improve
outcomes in patients with eye disease, the future success of the product developed remains speculative. Research and development risks include uncertainty of the outcome of results, difficulties or delays in development and general uncertainty
around the scientific development of novel pharmaceutical products and any of these risks, if they were to materialize, could impact Opthea’s progress and could have a material adverse effect on Opthea’s future financial performance.
Opthea operates within a highly regulated industry, relating to the manufacture, distribution and supply of pharmaceutical products. There is
no guarantee that Opthea will obtain or maintain the required approvals, licenses and registrations from all relevant regulatory authorities in all jurisdictions in which it operates. Further clinical trials may be delayed and Opthea may incur
further costs if the Food and Drug Administration (FDA) and other regulatory agencies observe deficiencies that require resolution or request additional studies be conducted in addition to those that are currently planned. Furthermore Opthea is
exposed to the risk of changes to existing, or the introduction of new, government policies, regulations and legislation in all jurisdictions in which it operates. A failure to obtain or maintain any required approvals, licenses and registrations or
any change in regulation may adversely affect Opthea’s ability to commercialise and manufacture its treatments.
Opthea may, from time to time, consider acquisition, licensing, partnership or other corporate opportunities for Opthea’s development
programs. There can be no assurance that any such acquisition, licensing, partnership or corporate opportunities can be concluded on terms that are, or are believed by Opthea to be, commercially acceptable. In the case of licensing and partnership
opportunities, even if such terms are agreed there is a risk that the performance of distributors and the delivery of contracted outcomes by collaborators will not occur due to a range of unforeseen factors relating to environment, technology and
market conditions.
41
Future success will also depend on Opthea’s ability to achieve market acceptance and
attract and retain customers, which includes convincing potential consumers and partners of the efficacy of Opthea’s products and Opthea’s ability to manufacture a sufficient quantity and quality of products at a satisfactory price.
| |
(j) |
Information technology |
Opthea relies on effective information technology, software, data centres and communication systems. There is a risk that these systems may be
adversely affected by disruption, failure, service outages or data corruption that could occur as a result of computer viruses, “bugs” or “worms”, malware, internal or external misuse by websites, cyber-attacks or other
disruptions including natural disasters, power outages or other similar events. Opthea may be significantly impacted by disruption to any of these systems or platforms.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change, in
Australia, the United States and elsewhere, and there are no guarantees about Opthea’s ability to successfully compete. Opthea’s products may compete with existing alternative treatments that are already available to customers. In
addition, a number of companies, both in Australia and internationally, are pursuing the development of products that target wet AMD and DME. Some of these companies may have, or may develop, technologies superior to Opthea’s own technology.
Some competitors of Opthea may have substantially greater financial, technical and human resources than Opthea does, as well as broader product offerings and greater market and brand presence. Opthea’s services, expertise or products may be
rendered obsolete or uneconomical or decrease in attractiveness or value by advances or entirely different approaches developed by either Opthea or its competitors.
| |
(l) |
Intellectual property |
Securing rights in technology and patents is an integral part of securing potential product value in the outcomes of biotechnology research and
development. Competition in retaining and sustaining protection of technology and the complex nature of technologies can lead to patent disputes.
Opthea’s success depends, in part, on its ability to obtain patents, maintain trade secret protection and operate without infringing the
proprietary rights of third parties. Because the patent position of biotechnology companies can be highly uncertain and frequently involves complex legal and factual questions, neither the breadth of claims allowed in biotechnology patents nor their
enforceability can be predicted. There can be no assurance that any patents which Opthea may own, access or control will afford Opthea commercially significant protection of its technology or its products or have commercial application, or that
access to these patents will mean that Opthea will be free to commercialize its drug candidates.
The granting of a patent does not
guarantee that the rights of others are not infringed or that competitors will not develop technology or products to avoid Opthea’s patented technology. Patenting strategies do not cover all countries which may lead to generic competition
arising in those markets.
Scale-up of OPT-302 manufacture to support Phase 3 clinical
studies has been completed but the process is required to be validated. Process performance qualification or “PPQ” will need to be completed as part of the filing for marketing approval. As such, there is a risk that the PPQ may present
technical difficulties. Technical difficulties could include the inability to generate material that meets regulatory specifications for human administration or the product yield from manufacturing batches may be insufficient to conduct the clinical
studies and support commercialization as currently planned. Any unforeseen difficulty relating to manufacturing, including changes in methods of product candidate manufacturing or formulation, disruption to supply, shortages of input materials or
changes to arrangements with, or capacity of, any third-party manufacturers, may negatively impact Opthea’s ability to generate profit in the future.
42
OPT-302 has not been approved for commercial sale, and Opthea expects it to be several years before OPT-302 is approved, if ever, and Opthea is able to commence sales of OPT-302. If OPT-302 is approved for commercial sale,
Opthea’s commercialization expenses will increase significantly as it establishes sales, marketing, distribution, manufacturing, supply chain and other commercial infrastructure. In addition, OPT-302 may
not achieve adequate market acceptance among physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
Opthea’s business was and may in the future be negatively affected by the effects of health epidemics in regions where Opthea or third
parties on which Opthea relies have significant manufacturing facilities, concentrations of clinical trial sites or other business operations. Health epidemics in regions where Opthea has concentrations of clinical trial sites or other business
operations could negatively affect its business, including by causing significant disruption in the operations of third-party manufacturers and CROs upon whom Opthea relies (for example, Covid-19 negatively
impacted Opthea’s ability to initiate clinical trial sites, maintain patient enrollment and enroll new patients.
| |
(p) |
Joint venture parties, agents, suppliers, distributors and contractors |
Opthea is unable to predict the risk of financial failure or default by a participant in any joint venture to which Opthea may become a party
or the insolvency or managerial failure by any of the contractors used by Opthea in any of its activities or the insolvency or other managerial failure by any of the other service providers used by Opthea for any activity. Opthea may engage with
various third parties to assist with different stages of the research and development process, including agents, suppliers, distributors and contractors, and there is no guarantee that these third parties will comply with their respective
contractual obligations. Transition of certain of these third parties could cause delay or disruption in the clinical trials. This could adversely impact Opthea’s progress and cause delays in or impede research or production, or result in cost
increases.
| |
(q) |
Reliance on key personnel |
Opthea is reliant on key personnel it employs or engages. Loss of such personnel may have a material adverse impact on the performance of
Opthea. In addition, recruiting qualified personnel is critical to Opthea’s success. This includes attracting and retaining staff with sufficient skills to develop intellectual property. As Opthea’s business grows and progresses to Phase 3
development, it will require additional key staff for clinical development operations as well as additional key financial and administrative personnel. There can be no assurance that Opthea will be successful in attracting and retaining qualified
personnel. The loss of key personnel or the inability to attract suitably qualified additional personnel could have a material adverse effect on Opthea’s financial performance.
| |
(r) |
Insurance and uninsured risks |
Although Opthea maintains insurance to protect against certain risks in such amounts as it considers to be reasonable, its insurance will not
cover all the potential risks associated with its operations and insurance coverage may not continue to be available, commercially acceptable, or may not be adequate to cover any resulting liability. It is not always possible to obtain insurance
against all such risks and Opthea may decide not to insure against certain risks because of high premiums or other reasons.
43
| |
(s) |
Product safety and efficacy |
Serious or unexpected health, safety or efficacy concerns with products may expose Opthea to reputational harm or reduced market acceptance of
its products, and may lead to product recalls and/or product liability claims and resulting liability, and increased regulatory reporting. There can be no guarantee that unforeseen adverse events or manufacturing defects will not occur. Opthea may
seek to obtain product liability insurance at the appropriate time in order to seek to minimise its liability to such claims, however there can be no assurance that adequate insurance coverage will be available at an acceptable cost. Any health,
safety or efficacy concerns are likely to lead to reduced customer demand and impact on the potential future profitability of Opthea.
In the ordinary course of conducting its business, Opthea is exposed to potential litigation and other proceedings, including through claims of
breach of agreements, intellectual property infringement or in relation to employees (through personal injuries, occupational health and safety or otherwise). If such proceedings were brought against Opthea, it could incur considerable defence costs
(even if successful), with the potential for damages and costs awards against Opthea if it were unsuccessful, which could have a significant adverse financial impact on Opthea’s business. Changes in laws can heighten litigation risk (for
example, antitrust and intellectual property). Circumstances may also arise in which Opthea, having received legal advice, considers that it is reasonable or necessary to initiate litigation or other proceedings, including for example to protect its
intellectual property rights. There has been substantial litigation and other proceedings in the pharmaceutical industry, including class actions from purchasers and end users of pharmaceutical products.
| |
(a) |
Share price fluctuations |
The market price of Opthea shares will fluctuate due to various factors, many of which are non-specific
to Opthea and beyond the control of Opthea, including recommendations by brokers and analysts, Australian and international general economic conditions, inflation rates, interest rates, changes in government, fiscal, monetary and regulatory
policies, global geo-political events and hostilities and acts of terrorism, and investor perceptions. Fluctuations such as these may adversely affect the market price of Opthea shares.
Neither Opthea nor the directors warrant the future performance of Opthea or any return on investment in Opthea.
Opthea intends to apply for quotation of the Options with seven days of this Prospectus being lodged with ASIC. ASX requires Opthea to meet
certain conditions for quotation of Options as a new class on ASX. There is a risk that Opthea may not be able to meet those requirements. The fact that ASX may agree to grant official quotation of the Options is not to be taken in any way as an
indication of the merits of Opthea or its securities. If Opthea’s application for the Options to be quoted on ASX is granted, the trading price of the Options may be affected by the ongoing performance, financial position, and solvency of
Opthea. Should the ASX grant official quotation of the Options, the liquidity of trading in Options on the ASX may be limited at times and may affect an eligible participant’s ability to buy or sell Options. In addition, Opthea’s share
price may not exceed the exercise price of the Options during the exercise period. In such circumstances the Options lapse without any value being realized.
Investors who do not participate in the Retail Entitlement Offer, or do not take up all of their entitlement under the Retail Entitlement
Offer, will have their percentage security holding in Opthea diluted (in addition to the dilution resulting from the Placement). In addition, Opthea’s need to raise additional capital in the future in order to meet its operating or financing
requirements, including by way of additional borrowings may change over time. Future equity raisings or equity funded acquisitions may dilute the holdings of particular shareholders to the extent that such shareholders do not subscribe for
additional equity, or are otherwise not invited to subscribe for additional equity.
44
Opthea is exposed to economic factors in the ordinary course of business. A number of economic factors / conditions, both Australian and
global, affect the performance of financial markets generally, which could affect the price at which Opthea shares trade on ASX. Among other things, adverse changes in macroeconomic conditions, including movements on international and domestic stock
markets, interest rates, exchange rates, cost and availability of credit, general consumption and consumer spending, input costs, employment rates and industrial disruptions, inflation and inflationary expectations and overall economic conditions,
economic cycles, trade tarrifs and restrictions, investor sentiment, political events and levels of economic growth, both domestically and internationally, as well as government taxation, fiscal, monetary, regulatory and other policy changes may
affect the demand for, and price of, Opthea shares and adversely impact Opthea’s business, financial position and operating results. Trading prices can be volatile and volatility can be caused by general market risks such as those that have
been mentioned. New Shares in Opthea may trade at or below the price at which they commence trading on ASX including as a result of any of the factors that have been mentioned, and factors such as those mentioned may also affect the income, expenses
and liquidity of Opthea. Additionally, the stock market can experience price and volume fluctuations that may be unrelated or disproportionate to the operating performance of Opthea.
Future changes in Australian taxation law, including changes in interpretation or application of the law by the courts or taxation authorities
in Australia, may affect taxation treatment of an investment in Opthea shares, or the holding and disposal of those shares. Further, changes in tax law, or changes in the way tax law is expected to be interpreted, in the various jurisdictions in
which Opthea operates, may impact the future tax liabilities of Opthea.
Opthea projects that it will receive material cash refunds under
the Research and Development Tax Incentive scheme (the “Scheme and R&D Tax Credits”) to offset the costs of its clinical programs and other qualifying expenditure, incurred both in Australia and overseas. The assumptions underlying
Opthea’s projected Scheme and R&D Tax Credits are based on actual amounts received for the 2022 financial year as a proportion of qualifying expenditure under the scheme. The Commonwealth Government and/or the Australian Taxation Office
could change the rules of the regulatory regime with the effect that future amounts paid to Opthea as a proportion of its expenses could be materially lower than assumed in the Company’s projections. Any rule changes made that materially reduce
the amount Opthea was able to claim under the scheme would have a material effect on the cash flows of the Company. Opthea believes that it is not a passive foreign investment company (PFIC) for U.S. federal income tax purposes for its current
taxable year and it expects that it will likely not be a PFIC in the foreseeable future, although there can be no assurance in this regard and this determination depends on legal and factual considerations that cannot be predicted.
Opthea prepares its general purpose financial statements in accordance with Australian International Financial Reporting Standards (AIFRS) and
the Corporations Act 2001 (Cth), which may differ significantly from the accounting standards applied by other companies (such as U.S. GAAP). Australian Accounting Standards are subject to amendment from time to time, and any such changes may impact
on Opthea’s statement of financial position or statement of financial performance.
| |
(g) |
Forward-looking statements |
There can be no guarantee that the assumptions and contingencies on which the forward-looking statements, opinions and estimates are based will
ultimately prove to be valid or accurate. The forward-looking statements, opinions and estimates included in this Prospectus depend on various factors, including known and unknown risks, many of which are outside the control of Opthea. Actual
performance of Opthea may materially differ from expected performance.
45
No assurances can be given in relation to the payment of future dividends. Future determinations as to the payment of dividends by Opthea will
be at the discretion of Opthea and will depend upon the availability of profits, the operating results and financial conditions of Opthea, future capital requirements, covenants in relevant financing agreements, general business and financial
conditions and other factors considered relevant by Opthea. No assurance can be given in relation to the level of tax deferral of future dividends. Tax deferred capacity will depend upon the amount of capital allowances available and other factors.
| |
(i) |
Changes in applicable law and regulations |
Opthea will be subject to changes in laws, regulations and government policy which may affect its operations and/or financial performance. Such
changes may impact income or operational expenditure. Opthea is also subject to changes in taxation regimes and Accounting Standards. There can be no assurance that such changes will not have a material adverse effect on Opthea’s business,
operational performance or financial results or returns to shareholders. As noted above under ‘Taxation’, adverse changes to tax law may also reduce Opthea’s capacity to claim research and incentive grants or rebates, thereby
increasing expenses and reducing Opthea’s assets.
Higher than expected inflation rates generally, or specific to the biotechnology and pharmaceuticals industry in particular, could be expected
to increase operating and development costs and potentially reduce the value of future project developments. While, in some cases, such cost increases might be offset by increased selling prices, there is no assurance that this would be possible or
that Opthea will be in its production and supply phase of its business when this occurs.
| 6. |
ADDITIONAL INFORMATION |
| 6.1 |
Continuous disclosure obligations |
As the Company is admitted to the Official List, the Company is a ‘disclosing entity’ for the purposes of the Corporations Act. As
such, it is subject to regular reporting and disclosure obligations. Specifically, like all listed companies, the Company is required to continuously disclose to the market any information it has which a reasonable person would expect to have a
material effect on the price or the value of the Company’s securities.
Price sensitive information is publicly released through the
ASX before it is disclosed to Shareholders and market participants. Distribution of other information to Shareholders and market participants is also managed through disclosure to the ASX. In addition, the Company posts information on its website
after the ASX confirms an announcement has been made, with the aim of making the information readily accessible to the widest audience.
By
virtue of section 713 of the Corporations Act, the Company is entitled to issue a ‘transaction- specific’ prospectus in respect of the Offers.
In general terms, a ‘transaction-specific prospectus’ is only required to contain information in relation to the effect of the issue
of securities on the Company and the rights and liabilities attaching to the securities. It is not necessary to include general information in relation to all of the assets and liabilities, financial position and performance, profits and losses or
prospects of the issuing company.
46
This Prospectus is intended to be read in conjunction with the publicly available
information in relation to the Company which has been notified to ASX and does not include all of the information that would be included in a prospectus for an initial public offering of securities in an entity that is not already listed on a
securities exchange. Investors should therefore have regard to the other publicly available information in relation to the Company before making a decision whether or not to invest.
Information that is already in the public domain has not been reported in this Prospectus other than that which is considered necessary to make
this Prospectus complete.
As a disclosing entity under the Corporations Act, the Company states that:
| |
(a) |
it is subject to regular reporting and disclosure obligations; |
| |
(b) |
copies of documents lodged with ASIC in relation to the Company may be obtained from, or inspected at, an
office of ASIC; and |
| |
(c) |
it will provide a copy of each of the following documents, free of charge, to any person on request between the
date of issue of this Prospectus and the Closing Date: |
| |
(i) |
the annual financial report of the Company for the financial year ended 30 June 2022;
|
| |
(ii) |
any half-year financial report of the Company lodged with ASIC after the lodgement of the annual financial
report referred to in paragraph (i) above and before the lodgement of this Prospectus with ASIC; and |
| |
(iii) |
all continuous disclosure notices given by the Company after the lodgement of the annual financial report
referred to in paragraph (i) above and before the lodgement of this Prospectus with ASIC (see below). |
There is no
information which has been excluded from a continuous disclosure notice in accordance with the ASX Listing Rules that investors or their professional advisers:
| |
(a) |
would reasonably require for the purpose of making an informed assessment of: |
| |
(i) |
the assets and liabilities, financial position and performance, profits and losses and prospects of the
Company; and |
| |
(ii) |
the rights and liabilities attaching to the securities the subject of this Prospectus; and
|
| |
(b) |
would reasonably expect to find in this Prospectus. |
This Prospectus contains information specific to the Offers. If investors require further information in relation to the Company, they are
recommended to take advantage of the opportunity to inspect or obtain copies of the documents referred to above.
The following
announcements have been lodged with the ASX prior to the date of this Prospectus (and including the announcement and investor presentation relating to his Capital Raising lodged with the ASX on the date of this Prospectus) in respect of the Company
since the lodgment of the annual financial report for the year ended 30 June 2022 with ASX on 30 August 2022.
|
|
|
| Date |
|
Title |
| 24 August 2023 |
|
Opthea Investor Presentation |
| 24 August 2023 |
|
Opthea announces A$80m capital raise |
47
|
|
|
| Date |
|
Title |
| 24 August 2023 |
|
Trading halt |
| 21 August 2023 |
|
Ceasing to be a substantial holder |
| 11 August 2023 |
|
Becoming a substantial holder |
| 9 August 2023 |
|
Ceasing to be a substantial holder |
| 3 August 2023 |
|
Becoming a substantial holder |
| 1 August 2023 |
|
ASRS 2023 – PK and safety of Sozinibercetept (OPT-302) |
| 27 July 2023 |
|
OPT Announces Sozinibercetept as the Drug Name for OPT 302 |
| 25 July 2023 |
|
Opthea Presents at the 2023 Ophthalmology Innovation Summit |
| 14 July 2023 |
|
Opthea to feature at the ASRS 2023 Annual Meeting |
| 11 July 2023 |
|
Opthea Announces Appointment of Snr Medical Advisor |
| 23 June 2023 |
|
Change in substantial holder |
| 9 June 2023 |
|
Initial Director’s Interest Notice AT |
| 9 June 2023 |
|
Opthea Appoints Anshul Thakral as Non-Executive Director |
| 9 June 2023 |
|
Final Director’s Interest Notice MS |
| 2 June 2023 |
|
Ceasing to be a substantial holder |
| 26 May 2023 |
|
Opthea Presents at the Jefferies 2023 Healthcare Conference |
| 18 May 2023 |
|
Opthea participates in EF Hutton hosted webinar on OPT-302 |
| 17 May 2023 |
|
Change in substantial holding |
| 9 May 2023 |
|
Opthea Presents at JMP Securities Life Sciences Conference |
| 28 April 2023 |
|
Opthea to Participate at HC Wainwright BioConnect Conference |
| 20 March 2023 |
|
Becoming a substantial holder |
| 7 March 2023 |
|
Opthea receives A$8.7million R&D Tax Incentive |
| 7 March 2023 |
|
Opthea to participate at Oppenheimer’s Healthcare Conference |
| 23 February 2023 |
|
Half Year Accounts |
| 16 February 2023 |
|
Response to ASX Query |
| 14 February 2023 |
|
Opthea Ph2b Trial Results with OPT-302 published in Journal |
| 10 February 2023 |
|
Appendix 2A |
| 7 February 2023 |
|
Opthea to Present at SVB Leerink Global Biopharma Conference |
| 31 January 2023 |
|
Opthea to Present at Sequire Biotechnology Conference |
| 11 January 2023 |
|
Opthea Corporate Presentation – San Francisco Jan 2023 |
| 13 December 2022 |
|
Opthea Announce MST Access Hosted Conference Call with Professor Jason S. Slakter, MD |
| 7 December 2022 |
|
Opthea to present at the FLORetina 2022 Congress |
| 30 November 2022 |
|
Appendix 2A |
| 30 November 2022 |
|
Cleansing Notice |
| 29 November 2022 |
|
Appendix 2A |
| 29 November 2022 |
|
Cleansing Notice |
| 29 November 2022 |
|
Appendix 3Y MS |
| 29 November 2022 |
|
Appendix 3Y MB |
| 16 November 2022 |
|
Results of Meeting |
| 16 November 2022 |
|
Chairman’s Address to Shareholders |
| 16 November 2022 |
|
Notification regarding unquoted securities - OPT |
| 16 November 2022 |
|
Notification regarding unquoted securities - OPT |
| 16 November 2022 |
|
Notification regarding unquoted securities - OPT |
| 11 November 2022 |
|
Opthea to Present at Jefferies London Healthcare Conference |
48
|
|
|
| Date |
|
Title |
| 28 October 2022 |
|
Cleansing Notice |
| 28 October 2022 |
|
Appendix 2A |
| 25 October 2022 |
|
Opthea Expands Leadership Team with CFO Appointment |
| 17 October 2022 |
|
Notice of General Meeting/Proxy Form |
| 5 October 2022 |
|
Change in substantial holding |
| 5 October 2022 |
|
Change in substantial holding |
| 3 October 2022 |
|
Cleansing Notice |
| 30 September 2022 |
|
Application for quotation of securities - OPT |
| 26 September 2022 |
|
Results of Meeting |
| 26 September 2022 |
|
Application for quotation of securities - OPT |
| 26 September 2022 |
|
Completion of Share Purchase Plan |
| 30 August 2022 |
|
Opthea Appendix 4E and Annual Report 2022 |
| 30 August 2022 |
|
Opthea Appendix 4E Corporate Governance Statement 2022 |
| 30 August 2022 |
|
Opthea Reports Fiscal Year 2022 Financial Results and Corporate Highlights |
| 6.2 |
Design and distribution obligations |
The new product design and distributions obligations under the Corporations Act (DDO Obligations) took effect from 5 October 2021.
The DDO Obligations are intended to help consumers obtain appropriate financial products by requiring issuers and distributors to have a consumer-centric product. The DDO Obligations require product issuers to make publicly available a target market
determination that explains the target market for certain securities, any distribution conditions and any information related to reviewing and monitoring conduct in relation to the target market determination.
The Company has prepared a target market determination in respect of the Options which is available on the Company’s website at
https://opthea.com/. The Options are subject to a distribution condition that retail investors will be provided with a copy of this Prospectus and access to the target market determination before they receive Options. Investors are required
self-confirm that they meet the eligibility criteria of the expected target market outlined in the target market determination.
| 6.3 |
International offer restrictions |
This Prospectus does not constitute an offer of New Shares or Options in any jurisdiction in which it would be unlawful. In particular, this
Prospectus may not be distributed to any person, and the Options may not be offered or sold, in any country outside Australia except to the extent permitted below.
European Union
This
document has not been, and will not be, registered with or approved by any securities regulator in the European Union. Accordingly, this document may not be made available, nor may the New Shares and New Options be offered for sale, in the European
Union except in circumstances that do not require a prospectus under Article 1(4) of Regulation (EU) 2017/1129 of the European Parliament and the Council of the European Union (the “Prospectus Regulation”).
In accordance with Article 1(4)(a) of the Prospectus Regulation, an offer of New Shares and New Options in the European Union is limited to
persons who are “qualified investors” (as defined in Article 2(e) of the Prospectus Regulation).
49
Hong Kong
WARNING: This document has not been, and will not be, registered as a prospectus under the Companies (Winding Up and Miscellaneous Provisions)
Ordinance (Cap. 32) of Hong Kong, nor has it been authorised by the Securities and Futures Commission in Hong Kong pursuant to the Securities and Futures Ordinance (Cap. 571) of the Laws of Hong Kong (the “SFO”). Accordingly, this document
may not be distributed, and the New Shares and New Options may not be offered or sold, in Hong Kong other than to “professional investors” (as defined in the SFO and any rules made under that ordinance).
No advertisement, invitation or document relating to the New Shares and New Options has been or will be issued, or has been or will be in the
possession of any person for the purpose of issue, in Hong Kong or elsewhere that is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong
Kong) other than with respect to New Shares and New Options that are or are intended to be disposed of only to persons outside Hong Kong or only to professional investors. No person allotted New Shares and New Options may sell, or offer to sell,
such securities in circumstances that amount to an offer to the public in Hong Kong within six months following the date of issue of such securities.
The contents of this document have not been reviewed by any Hong Kong regulatory authority. You are advised to exercise caution in relation to
the offer. If you are in doubt about any contents of this document, you should obtain independent professional advice.
Israel
The New Shares have not been registered, and no prospectus will be issued, under the Israeli Securities Law, 1968 (the “Securities
Law”). Accordingly, the New Shares will only be offered and sold in Israel pursuant to private placement exemptions, namely to no more than 35 offerees who fall within a category of sophisticated investor as described in the First Addendum of
the Securities Law.
Neither this document nor any activities related to the Offer shall be deemed to be the provision of investment
advice. If any recipient of this document is not the intended recipient, such recipient should promptly return this document to the Company. This document has not been reviewed or approved by the Israeli Securities Authority in any way.
New Zealand
This
document has not been registered, filed with or approved by any New Zealand regulatory authority under the Financial Markets Conduct Act 2013 (the “FMC Act”).
The New Shares and New Options are not being offered to the public within New Zealand other than to existing shareholders of the Company with
registered addresses in New Zealand to whom the offer of these securities is being made in reliance on the Financial Markets Conduct (Incidental Offers) Exemption Notice 2021.
Other than in the entitlement offer, the New Shares and New Options may only be offered or sold in New Zealand (or allotted with a view to
being offered for sale in New Zealand) to a person who:
| |
• |
|
is an investment business within the meaning of clause 37 of Schedule 1 of the FMC Act; |
| |
• |
|
meets the investment activity criteria specified in clause 38 of Schedule 1 of the FMC Act;
|
50
| |
• |
|
is large within the meaning of clause 39 of Schedule 1 of the FMC Act; |
| |
• |
|
is a government agency within the meaning of clause 40 of Schedule 1 of the FMC Act; or |
| |
• |
|
is an eligible investor within the meaning of clause 41 of Schedule 1 of the FMC Act. |
Singapore
This document
and any other materials relating to the New Shares and New Options have not been, and will not be, lodged or registered as a prospectus in Singapore with the Monetary Authority of Singapore. Accordingly, this document and any other document or
materials in connection with the offer or sale, or invitation for subscription or purchase, of New Shares and New Options, may not be issued, circulated or distributed, nor may the New Shares and New Options be offered or sold, or be made the
subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore except pursuant to and in accordance with exemptions in Subdivision (4) Division 1, Part 13 of the Securities and Futures Act 2001 of
Singapore (the “SFA”) or another exemption under the SFA.
This document has been given to you on the basis that you are an
“institutional investor” or an “accredited investor” (as such terms are defined in the SFA). If you are not such an investor, please return this document immediately. You may not forward or circulate this document to any other
person in Singapore.
Any offer is not made to you with a view to the New Shares or New Options being subsequently offered for sale to any
other party in Singapore. On-sale restrictions in Singapore may be applicable to investors who acquire New Shares and New Options. As such, investors are advised to acquaint themselves with the SFA provisions
relating to resale restrictions in Singapore and comply accordingly.
South Africa
This document does not, nor is it intended to, constitute a prospectus prepared and registered under the South African Companies Act 2008 and
may not be distributed to the public in South Africa. This document has not been registered with nor approved by the South African Companies and Intellectual Property Commission.
Any offer of New Shares and New Options in South Africa will be made by way of a private placement to, and capable of acceptance only by,
investors who fall within one of the specified categories listed in section 96(1)(a) of the South African Companies Act.
An entity or
person resident in South Africa may not implement participation in the offer unless (i) permitted under the South African Exchange Control Regulations or (ii) a specific approval has been obtained from an authorised foreign exchange dealer
in South Africa or the Financial Surveillance Department of the South African Reserve Bank.
United Kingdom
Neither this document nor any other document relating to the offer has been delivered for approval to the Financial Conduct Authority in the
United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the New Shares and New Options.
The New Shares and New Options may not be offered or sold in the United Kingdom by means of this document or any other document, except in
circumstances that do not require the publication of a prospectus under section 86(1) of the FSMA. This document is issued on a
51
confidential basis in the United Kingdom to “qualified investors” within the meaning of Article 2(e) of the UK Prospectus Regulation. This document may not be distributed or reproduced,
in whole or in part, nor may its contents be disclosed by recipients, to any other person in the United Kingdom.
Any invitation or
inducement to engage in investment activity (within the meaning of section 21 of the FSMA) received in connection with the issue or sale of the New Shares and New Options has only been communicated or caused to be communicated and will only be
communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of the FSMA does not apply to the Company.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in
matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in
Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (“relevant persons”). The investment to which this document relates is
available only to relevant persons. Any person who is not a relevant person should not act or rely on this document.
United States
This document does not constitute an offer to sell, or a solicitation of an offer to buy, securities in the United States. The New
Shares and New Options (as well as the shares underlying the New Options) have not, and will not be, been registered under the US Securities Act of 1933 or the securities laws of any state or other jurisdiction of the United States. Accordingly, the
New Shares and New Options (as well as the shares underlying the New Options) may not be offered or sold in the United States except in transactions exempt from, or not subject to, the registration requirements of the US Securities Act and
applicable US state securities laws.
The Company is not currently engaged in litigation and, as at the date of this Prospectus, the Directors are not aware of any legal proceedings
pending or threatened against, or any material legal proceedings affecting, the Company.
| 6.5 |
Rights and liabilities attaching to the New Shares |
The following is a general description of the more significant rights and liabilities attaching to the New Shares. This summary is not
exhaustive. Full details of provisions relating to rights attaching to the New Shares are contained in the Corporations Act, ASX Listing Rules and the Constitution (a copy of which is available for inspection at the Company’s registered office
during normal business hours).
| |
(a) |
Ranking of New Shares |
At the date of this Prospectus, all shares are of the same class and rank equally in all respects. Specifically, the New Shares issued under
this Prospectus will rank equally with the Company’s existing Shares.
Subject to any rights or restrictions, at general meetings:
| |
• |
|
every Shareholder present and entitled to vote may vote in person or by attorney, proxy or representative; and
|
52
| |
• |
|
has one vote on a show of hands; or |
| |
• |
|
has one vote for every fully paid share held, on a poll. |
Shareholders will be entitled to dividends, distributed among members in proportion to the capital paid up or credited as paid up, from the
date of payment. No dividend carries interest against the Company and the declaration of Directors as to the amount to be distributed is conclusive.
Shareholders may be paid interim dividends or bonuses at the discretion of the Directors. The Directors may set aside a sum out of the profits
of the Company, as reserves, before recommending dividends of the profits.
The rights attaching to the New Shares may only be varied by the consent in writing of the holders of three-quarters of the Shares, or with the
sanction of a special resolution passed at a general meeting.
New Shares can be transferred through the financial market operated by the ASX or by a proper instrument of transfer. The instrument of
transfer must be in writing in a usual form or any other form approved by the Directors, and signed by or on behalf of the transferor and the transferee. Except where the operating rules of an applicable CS facility licensee, being the ASTC
Operating Rules provide otherwise, until the transferee has been registered, the transferor is deemed to remain the holder of the Shares, even after signing the instrument of transfer.
In some certain prescribed circumstances, the Directors may refuse to register a transfer of New Shares.
Shareholders are entitled to be present in person, or by proxy, attorney or representative to attend and vote at general meetings of the
Company.
The Directors may convene a general meeting at their discretion.
The Constitution provides for the sale of unmarketable parcels subject to any applicable laws and provided a notice is given to the relevant
Shareholder stating that the Company intends to sell their relevant New Shares unless the relevant Shareholder advises the Company by a specified date that they wish to retain the Shares.
If the Company is wound up, the liquidator may with the sanction of a special resolution, divide the assets of the Company amongst Shareholders
as the liquidator sees fit. The liquidator may not require a Shareholder to accept any New Shares or other securities in respect of which there is any liability.
53
| 6.6 |
Rights and liabilities attaching to the Options |
The Options to be issued under the Offers will be issued on the following terms and conditions:
Each Option entitles the holder to acquire by way of issue one Share on exercise of the Option.
Subject to paragraph (h) below, the exercise price of the Options will be $0.80 (Exercise Price).
Each Option will expire at 5.00pm (Melbourne time) on 31 August 2025 (Expiry Date). An Option not exercised by the Expiry Date will
automatically lapse at that time at that time.
The Options are exercisable at any time on or prior to the Expiry Date (Exercise Period).
A Notice of Exercise is only effective on and from the later of the date of receipt of the Notice of Exercise and the date of receipt of the
payment of the applicable Exercise Price for each Option being exercised in cleared funds (Exercise Date).
A minimum of 3,000
Options may be exercised under each Notice of Exercise. If a Shareholder holds less than 3,000 Options, all of the Options held by them must be exercised in one Notice of Exercise.
| |
(f) |
Timing of issue of Shares on exercise |
As soon as practicable after the Exercise Date, the Company will:
| |
(i) |
issue the number of Shares required under these terms and conditions in respect of the number of Options
specified in the Notice of Exercise and for which cleared funds have been received by the Company; and |
| |
(ii) |
if admitted to the Official List at the time, apply for official quotation on ASX of Shares issued on the
exercise of the Options. |
| |
(g) |
Shares issued on exercise |
Shares issued on exercise of the Options will rank equally in all respects with the then issued Shares.
| |
(h) |
Reconstruction of capital |
If at any time the issued capital of the Company is reconstructed, all rights of a holder of Options are to be changed in a manner consistent
with the Corporations Act and the ASX Listing Rules at the time of the reconstruction.
54
| |
(i) |
Participation in new issues |
There are no participation rights or entitlements inherent in the Options and holders will not be entitled to participate in new issues of
capital offered to Shareholders during the currency of the Options without exercising the Options and unless Shares have been issued in respect of the Options before the record date for determining entitlements to the issue.
| |
(j) |
Change in Exercise Price |
There will be no change to the applicable Exercise Price of an Option or the number of Shares over which an Option is exercisable in the event
of the Company making a pro rata issue of Shares or other securities to the holders of Shares (other than for a Bonus Issue).
If before the expiry of any Options, the Company makes a pro rata issue of Shares to Shareholders for no consideration (Bonus Issue),
the number of Shares over which an Option is exercisable will be increased by the number of Shares which the holder would have received if the Option had been exercised before the record date for the Bonus Issue.
Holders of Options have no voting rights until the Options are exercised and Shares issued on exercise of those Options in accordance with the
ASX Listing Rules.
The Options are transferable and will be quoted on the ASX.
The Options will not give any right to participate in dividends until Shares are issued pursuant to the exercise of the relevant Options.
| 6.7 |
Interests of Directors, experts and advisors |
| |
(a) |
Other than as set out below or elsewhere in this Prospectus, no: |
| |
(i) |
Director or proposed Director; |
| |
(ii) |
person named in this Prospectus as performing a function in a professional, advisory or other capacity in
connection with the preparation or distribution of this Prospectus; |
| |
(iii) |
promoter of the Company; or |
| |
(iv) |
financial services licensee named in this Prospectus as a financial services licensee involved in the Offers,
|
holds, or has held within 2 years before the date of this Prospectus, any interest in the Offers or in the formation or
promotion of, or in any property acquired or proposed to be acquired by, the Company in connection with its formation or promotion or the Offers.
55
| |
(b) |
Other than as set out in Section 6.8 or elsewhere in the Prospectus, no amounts have been paid or agreed
to be paid and no benefits have been given or agreed to be given: |
| |
(i) |
to a Director or proposed Director to induce him or her to become, or to qualify him or her as, a director of
the Company; or |
| |
(ii) |
for services provided in connection with the formation or promotion of the Company or the Offers by any
Director or proposed Director, any person named in this Prospectus as performing a function in a professional, advisory or other capacity in connection with the preparation or distribution of this Prospectus, any promoter of the Company, or any
underwriter or financial services licensee named in this Prospectus as an underwriter or financial services licensee involved in the Offers. |
| |
(c) |
Mr Lawrence Gozlan, a director of the Company, has provided the Company with services associated with managing
and overseeing and coordinating the conduct and implementation of the Capital Raising which, in the opinion of the Directors, are outside the scope of the ordinary duties of a Director. For these services, Mr Gozlan will receive:
|
| |
(i) |
from 1 July 2023, US$75,000 for the first month and US$25,000 per month thereafter until the successful
completion of the Capital Raising; and |
| |
(ii) |
subject to successful closing of the Capital Raising and receipt of shareholder approval (which will be sought
at the 2023 annual general meeting of the Company), 500,000 options (each exercisable for one ordinary share of the Company), with an exercise price of $0.615 (or such otherwise as may be agreed by the parties prior to the issue of options) and
exercisable from the time of issue until the date 3 years after the date of issue of the options. |
In the event the
requisite shareholder approval is not obtained, the Company and Mr Gozlan will agree an appropriate additional amount as an alternative to the proposed issue of options.
56
| |
(a) |
Directors’ security holdings |
The relevant interests of the Directors in securities of the Company as at the date of this Prospectus are as follows:
|
|
|
|
|
|
|
|
|
| Director |
|
Shares |
|
|
Options
/ Rights |
|
| Jeremy Levin
(Non-Executive Director and Chairman) |
|
|
31,496 |
|
|
|
3,000,000 |
|
| Megan Baldwin
(Managing Director and Chief Executive Officer) |
|
|
4,195,299 |
|
|
|
5,100,000 |
|
| Susan Orr
(Non-Executive Director) |
|
|
— |
|
|
|
1,000,000 |
|
| Lawrence Gozlan
(Non-Executive Director) |
|
|
1,877,357 |
|
|
|
4,500,000 |
|
| Daniel Spiegelman
(Non-Executive Director) |
|
|
— |
|
|
|
2,150,000 |
|
| Julia Haller
(Non-Executive Director) |
|
|
— |
|
|
|
2,000,000 |
|
| Quinton Oswald
(Non-Executive Director) |
|
|
— |
|
|
|
1,000,000 |
|
| Anshul Thakral
(Non-Executive Director) |
|
|
— |
|
|
|
— |
|
| |
(b) |
Directors’ remuneration |
As Chair and non-executive Director, Dr. Jeremy Levin is currently paid US$75,000 in directors
fees per annum.
As a Managing Director and Chief Executive Officer, Dr. Megan Baldwin is employed under an ongoing contract and
receives fixed remuneration of A$575,000 per annum.
As a non-executive Director, Dr. Susan
Orr is currently paid US$50,000 in directors fees per annum. Clinical Sub Committee Chair fee of US$10,000 per annum plus US$5,000 per committee fee per annum.
As a non-executive Director, Mr Lawrence Gozlan is currently paid A$65,700 in directors fees per annum.
Nominations Committee Chair fee of A$13,140 per annum plus Committee fees of A$6,570 per committee per annum.
As a non-executive Director, Mr Daniel Spiegelman is currently paid US$50,000 in directors fees per annum. Audit and Risk Committed fee of US$20,000 per annum plus US$5,000 for committee fee per annum.
As a non-executive Director, Dr. Julia Haller is currently paid US$50,000 in directors fees per
annum. Committee fees of US$5,000 per annum per committee.
As a non-executive Director, Mr Quinton
Oswald is currently paid US$50,000 in directors fees per annum. Remuneration Chair fee of US$10,000 per annum plus US$5,000 per committee fee per annum.
57
As a non-executive Director, Mr Anshul Thakral is
currently paid US$50,000 in directors fees per annum. Committee fees of US$5,000 per annum per committee.
| |
(c) |
Related party arrangements |
As announced by Opthea to ASX on 9 June 2023, Opthea appointed Mr. Anshul Thakral to its board of directors. Mr. Thakral is
Chief Executive Officer and Board Member of Launch Therapeutics, a clinical development company backed by funds managed by global investment firm Carlyle (NASDAQ: CG) and its life sciences franchise, Abingworth. Mr Thakral is also a director of
Saama Technologies, LLC. As such, any agreements with Carlyle and Abingworth (and their special purpose vehicle Ocelot), Launch Therapeutics and Saama Technologies are considered related party arrangements.
As described in Section 6.7(c) above, Mr Lawrence Gozlan, a director of the Company, and the Company have entered into a Consultancy
Agreement in respect of the provision services associated with managing and overseeing and coordinating the conduct and implementation of the Capital Raising which, in the opinion of the Directors, are outside the scope of the ordinary duties of a
Director.
Chapter 6D of the Corporations Act imposes a liability regime on the Company (as the offeror of the Securities), the Directors, the persons
named in the Prospectus with their consent as proposed directors, any underwriters, persons named in the Prospectus with their consent having made a statement in the Prospectus and persons involved in a contravention in relation to the Prospectus,
with regard to misleading and deceptive statements made in the Prospectus. Although the Company bears primary responsibility for the Prospectus, the other parties involved in the preparation of the Prospectus can also be responsible for certain
statements made in it.
Gilbert + Tobin has given and has not, before lodgement of this Prospectus, withdrawn its written consent to be named in this Prospectus as
Australian legal adviser to the Company in respect of the Capital Raising in the form and context in which it is named.
Cooley LLP has
given and has not, before lodgement of this Prospectus, withdrawn its written consent to be named in this Prospectus as US legal adviser to the Company in respect of the Capital Raising in the form and context in which it is named.
MST Financial Services Pty Ltd has given and has not, before lodgement of this Prospectus, withdrawn its written consent to be named in this
Prospectus as lead manager of the Capital Raising and underwriter of the issue of New Shares under the Entitlement Offer in the form and context in which it is named.
Regal Funds Management Pty Ltd has given and has not, before lodgement of this Prospectus, withdrawn its written consent to be named in this
Prospectus as lead manager of the Capital Raising and underwriter of the issue of New Shares under the Entitlement Offer in the form and context in which it is named.
Computershare Investor Services Pty Limited has given and has not, before lodgement of this Prospectus, withdrawn its written consent to be
named in this Prospectus as share registry to the Company in respect of the Capital Raising in the form and context in which it is named.
58
Each of the persons named as providing consents above:
| |
(i) |
did not authorise or cause the issue of this Prospectus; |
| |
(ii) |
does not make, or purport to make, any statement in this Prospectus nor is any statement in this Prospectus
based on any statement by any of those parties other than as specified in this Section 6.9; and |
| |
(iii) |
to the maximum extent permitted by law, expressly disclaims any responsibility or liability for any part of
this Prospectus other than a reference to its name and a statement contained in this Prospectus with the consent of that party as specified in this Section 6.9. |
| 6.10 |
Expenses of the Offers |
The total expenses of the Capital Raising are estimated to be approximately $6,770,000 (excluding GST), the table below sets out the breakdown
of these expenses:
|
|
|
|
|
| Item of Expenditure |
|
Amount ($) |
|
| MST Financial Services Pty Ltd underwriter and lead manager fees |
|
$ |
5,050,000 |
|
| Gilbert + Tobin legal fees |
|
$ |
630,000 |
|
| Cooley LLP legal fees |
|
$ |
780,000 |
|
| Miscellaneous, including registry and printing fees |
|
$ |
310,000 |
|
|
|
|
|
|
| TOTAL |
|
$ |
6,770,000 |
|
|
|
|
|
|
The information in this Prospectus, the Offers, and the contracts formed on acceptance of the Application Form are governed by the law
applicable in Victoria, Australia. Any person who applies for Securities under the Offers submits to the non-exclusive jurisdiction of the courts of Victoria, Australia.
59
| 7. |
DIRECTORS’ AUTHORISATION |
This Prospectus is issued by the Company and its issue has been authorised by a resolution of the Directors.
In accordance with section 720 of the Corporations Act, each Director has consented to the lodgement of this Prospectus with ASIC and has not
withdrawn that consent.
Signed for and on behalf of the Company on 24 August 2023.

Dr. Jeremy Levin
Non-Executive Director and Chairman
Opthea Limited
60
Definitions used in this Prospectus are as follows:
Applicant means a person who submits an Application.
Application means an application for New Shares and/or Options under this Prospectus.
Application Form means the personalised application form included in or accompanying this Prospectus for participation in the Retail
Entitlement Offer.
Application Monies means monies equal to the value of New Shares at the Offer Price applied for by an Eligible
Shareholder.
ASIC means the Australian Securities and Investments Commission.
ASX Listing Rules means the official listing rules of ASX Listing Rules as amended or waived.
ASX means ASX Limited ACN 008 624 691 or the financial market known as the ‘Australian Securities Exchange’ operated by it, as
the context requires.
ASTC Operating Rules means the operating rules of ASTC in its capacity as a CS facility licensee, except to
the extent of any relief given by ASTC in their application to the Company.
Australian Accounting Standards means the Australian
accounting standards issued by the Australian Accounting Standards Board.
Board means the board of Directors of the Company.
Capital Raising means the Placement and the Entitlement Offer.
Chair means the Chair of the Board.
CHESS means Clearing House Electronic Subregister System operated by ASX Settlement Pty Limited (ABN 49 008 504 532).
Closing Date means the date that the Offers close which is 5.00pm (Melbourne time) on Thursday, 14 September 2023 or such other
time and date as the Directors determine, being the last day on which Applications will be accepted.
Company or Opthea means
Opthea Limited ABN 32 006 340 567.
Constitution means the constitution of the Company.
Corporations Act means the Corporations Act 2001 (Cth).
Director means a director of the Company.
Eligible Shareholder has the meaning given in Section 2.4.
Entitlement means the number of New Shares and New Options each Eligible Shareholder is offered under the Retail Entitlement Offer.
Entitlement Offer means the Retail Entitlement Offer and the Institutional Entitlement Offer.
61
Existing Shares means Shares on issue at the Record Date.
Expiry Date means the expiry date of the New Options offered under this Prospectus, as defined in Section 6.6.
Ineligible Shareholder has the meaning given in Section 2.4.
Institutional Entitlement Offer means the fully underwritten pro-rata accelerated non- renounceable entitlement offer to existing institutional and sophisticated shareholders as announced by the Company to the ASX on Thursday, 24 August 2023.
Institutional Options means the Options offered under the Institutional Option Offer.
Institutional Option Offer means the offer of Institutional Options to participants in the Placement and the Institutional Entitlement
Offer under this Prospectus.
New Options means the Options offered under the Retail Entitlement Offer.
New Shares means the new Shares offered under the Entitlement Offer and the Placement.
Offers means the Retail Entitlement Offer and the Institutional Option Offer.
Offer Period means the period commencing on the Opening Date and ending on the Closing Date.
Offer Price means the offer price of $0.46 per New Share under the Retail Entitlement Offer.
Official List means the official list of the ASX.
Opening Date means the opening date of the Offers.
Option means the right of the holder to be issued one new Share on payment of the applicable exercise price on the terms and conditions
set out in Section 6.6.
Option Offer means the Institutional Option Offer and the offer of New Options under the Retail
Entitlement Offer.
Placement means the issue of up to approximately 21.7 million New Shares to certain sophisticated and
institutional investors under a placement announced by the Company to the ASX on Thursday, 24 August 2023.
Prospectus means
this prospectus dated 24 August 2023 and lodged with ASIC, including any supplementary or replacement prospectus in relation to this prospectus.
Record Date means 7.00pm (Melbourne time) on Monday, 28 August 2023, being the date on which Eligible Shareholders who are
permitted to participate in the Retail Entitlement Offer are determined.
Retail Entitlement Offer means the offer of New Shares
(including, for the avoidance of doubt, the offer of additional New Shares under the Top-Up Facility) and New Options to Eligible Shareholders under this Prospectus.
Section means a section of this Prospectus.
Securities means the New Shares and Options offered under this Prospectus.
Share means a fully paid ordinary share in the capital of the Company.
62
Share Registry means Computershare Investor Services Pty Limited ACN 078 279 277.
Shareholder means a holder of at least one Share.
Top-Up Facility has the meaning given in Section 1.
Underwriter means MST Financial Services Pty Ltd ACN 617 475 180.
US or United States means the United States of America.
US Securities Act means the United States Securities Act of 1933, as amended.
63
CORPORATE DIRECTORY
Directors
Dr. Jeremy Levin (Non-Executive Director and Chairman)
Dr. Megan Baldwin (Managing Director and Chief Executive Officer)
Mr Lawrence Gozlan (Non-Executive Director)
Mr Daniel Spiegelman (Non-Executive Director)
Dr. Julia Haller (Non-Executive Director)
Dr. Susan Orr (Non-Executive Director)
Mr Quinton Oswald (Non-Executive Director)
Mr Anshul Thakral (Non-Executive Director)
Company Secretary
Ms Karen Adams (Vice President Finance
and Company Secretary)
Registered Office
Level 4, 650 Chapel Street
South Yarra, Victoria 3141
Share Registry
Computershare Investor Services Pty
Limited
Yarra Falls, 452 Johnston Street
Abbotsford
Victoria 3067
Underwriter
MST Financial Services
Pty Ltd
Level 13, 14 Martin Place
Sydney, NSW 2000
Australia Legal Adviser
Gilbert + Tobin
Level 25, 101 Collins Street
Melbourne VIC 3000
US Legal Adviser
Cooley LLP
3175 Hanover Street
Palo Alto, CA 94304-1130
64
Opthea (NASDAQ:OPT)
Historical Stock Chart
From Apr 2024 to May 2024
Opthea (NASDAQ:OPT)
Historical Stock Chart
From May 2023 to May 2024