As
filed with the Securities and Exchange Commission on May 21, 2024
Registration
No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
S-3
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
Processa
Pharmaceuticals, Inc.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
45-1539785 |
(State
or other jurisdiction of
incorporation or organization) |
|
(I.R.S.
Employer
Identification No.) |
7380
Coca Cola Drive, Suite 106
Hanover,
Maryland 21076
(443)
776-3133
(Address,
including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
George
Ng
Chief
Executive Officer
Processa
Pharmaceuticals, Inc.
7380
Coca Cola Drive, Suite 106
Hanover,
Maryland 21076
(443)
776-3133
(Name,
address, including zip code, and telephone number,
including
area code, of agent for service)
with
a copy to:
Michael
B. Kirwan
John
J. Wolfel, Jr.
Foley
& Lardner LLP
One
Independent Drive, Suite 1300
Jacksonville,
Florida 32202
(904)
359-2000
Approximate
date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If
the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check
the following box: ☐
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following
box: ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective
upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If
this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional
securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting
company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer
☐ |
|
Accelerated
filer
☐ |
|
Non-accelerated filer
☐ |
|
Smaller reporting company
☒ |
|
Emerging growth company
☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act or until the registration statement shall become effective on such date as the
Securities and Exchange Commission acting pursuant to said Section 8(a), may determine.
EXPLANATORY
NOTE
This
Registration Statement contains two prospectuses:
| |
● |
A
base prospectus for up to $50,000,000 in the aggregate of the securities identified in the base prospectus from time to time
in one or more offerings; and |
| |
|
|
| |
● |
A
prospectus covering the offering, issuance and sale by us of up to a maximum aggregate offering price of $2,400,000 of our common
stock that may be issued and sold under a sales agreement with A.G.P./Alliance Global Partners (“A.G.P”). |
The
base prospectus immediately follows this explanatory note. The specific terms of any securities to be offered pursuant to the base prospectus
will be specified in a prospectus supplement to the base prospectus. The sales agreement prospectus immediately follows the base
prospectus. The $2,400,000 of common stock that may be offered, issued and sold under the sales agreement prospectus is included
in the $50,000,000 of securities that may be offered, issued and sold by us under the base prospectus, and if no shares are sold
under the sales agreement, the full $50,000,000 of securities may be sold in other offerings pursuant to the base prospectus
and a corresponding prospectus supplement.
The
information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement
filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities nor a solicitation
of an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY
PROSPECTUS
SUBJECT
TO COMPLETION, DATED MAY 21, 2024
Common
Stock
Preferred
Stock
Warrants
Units
We
may offer and sell from time to time up to $50,000,000 of any combination of the securities described in this prospectus, in one
or more classes or series and in amounts, at prices and on terms that we will determine at the times of the offerings.
This
prospectus describes the general manner in which our securities may be offered using this prospectus. We will provide specific terms
of the securities, including the offering prices, in one or more supplements to this prospectus. The supplements may also add, update
or change information contained in this prospectus. You should read this prospectus and the prospectus supplement relating to the specific
issue of securities carefully before you invest. This prospectus may be used to offer and sell any of the securities for the account
of persons other than us as provided in an applicable prospectus supplement.
We
may offer the securities independently or together in any combination for sale directly to purchasers or through underwriters, dealers
or agents to be designated at a future date. The supplements to this prospectus will provide the specific terms of the plan of distribution.
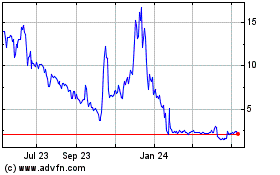
Our
common stock is traded on the Nasdaq Capital Market under the symbol “PCSA.” On May 17, 2024, the closing price of
our common stock on the Nasdaq Capital Market was $1.96 per share.
Investment
in our securities involves a high degree of risk. Before making an investment decision, please read the information in the section titled
“Risk Factors” on page 4 of this prospectus. Please read carefully and consider these risk factors, as well as those
included in reports we file under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), such as our most
recent Annual Report on Form 10-K, and those included in any applicable prospectus supplement and/or other offering material we file
with the Securities and Exchange Commission (the “SEC”).
Neither
the SEC nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful
or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is [●], 2024.
TABLE
OF CONTENTS
ABOUT THIS PROSPECTUS
This
prospectus is part of a registration statement that we filed with the SEC using a “shelf” registration process. Under this
shelf registration process, we may sell any combination of the securities described in this prospectus in one or more offerings up to
a total dollar amount of $50,000,000. This prospectus sets forth certain terms of the securities that we may offer.
This
prospectus provides a general description of the securities we may offer. Each time we offer securities, we will, to the extent required
by law, provide a prospectus supplement to this prospectus. The prospectus supplement will contain the specific description of the securities
we are then offering and the terms of the offering. The prospectus supplement will supersede this prospectus to the extent it contains
information that is different from, or that conflicts with, the information contained in this prospectus. For a more complete understanding
of the offering of the securities, you should refer to the registration statement, including its exhibits. You should read this prospectus,
any prospectus supplement and any other offering material together with the documents incorporated herein by reference and the
additional information described herein under the heading “Where You Can Find More Information” before making an investment
decision.
You
should rely only on the information contained or incorporated by reference in this prospectus and any accompanying prospectus supplement.
We have not authorized anyone else to provide you with different or additional information. We are offering to sell these securities
and seeking offers to buy these securities only in jurisdictions where offers and sales are permitted. This prospectus and any accompanying
supplement to this prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the
registered securities to which they relate.
You
should not assume that the information contained in this prospectus and any accompanying supplement to this prospectus is accurate on
any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is correct
on any date subsequent to the date of the document incorporated by reference, even though this prospectus and any accompanying supplement
to this prospectus is delivered or securities are sold on a later date.
This
prospectus may not be used by us to consummate sales of our securities unless it is accompanied by a prospectus supplement. To the extent
there are inconsistencies between any prospectus supplement, this prospectus and any documents incorporated by reference, the document
with the most recent date will control.
As
used in this prospectus, unless the context indicates or otherwise requires, “the Company,” “our Company,” “we,”
“us,” and “our” refer to Processa Pharmaceuticals, Inc., a Delaware corporation, and its consolidated subsidiary.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents incorporated by reference herein contain certain “forward-looking statements” within the meaning
of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act, and Section 21E of the Exchange Act. All
statements other than statements of historical facts are forward-looking statements. In some cases, you can identify forward-looking
statements by words such as “anticipate,” “believe,” “contemplate,” “continue,” “could,”
“estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,”
“project,” “seek,” “should,” “target,” “will,” “would,” or the
negative of these words or other comparable terminology. We have based these forward-looking statements on our current expectations and
projections about future events and trends that we believe may affect our financial condition, results of operations, strategy, short-
and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks,
uncertainties and assumptions. Moreover, we operate in a very competitive and rapidly changing environment, and new risks emerge from
time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business
or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any
forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances
discussed in this prospectus may not occur, and actual results could differ materially and adversely from those anticipated or implied
in the forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
Forward-looking statements include, but are not limited to, statements about:
| |
● |
our
ability to obtain funding for our future clinical trials, preclinical activities and our operations; |
| |
|
|
| |
● |
our
ability to obtain and maintain regulatory approval of our product candidates; |
| |
|
|
| |
● |
our
ability to contract with third-party suppliers, manufacturers and other service providers and their ability to perform adequately; |
| |
|
|
| |
● |
our
ability to meet obligations under our license agreements; |
| |
|
|
| |
● |
the
potential market size, opportunity and growth potential for our product candidates, if approved; |
| |
|
|
| |
● |
our
ability to build our own sales and marketing capabilities, or seek collaborative partners, to commercialize our product candidates,
if approved; |
| |
|
|
| |
● |
our
ability to retain the continued service of our key professionals and to identify, hire and retain additional qualified professionals; |
| |
|
|
| |
● |
our
ability to advance product candidates into, and successfully complete, clinical trials; |
| |
|
|
| |
● |
our
ability to recruit and enroll suitable patients in our clinical trials; |
| |
|
|
| |
● |
the
initiation, timing, progress and results of clinical trials and pre-clinical studies for our drug candidates; |
| |
|
|
| |
● |
the
timing or likelihood of the accomplishment of various scientific, clinical, regulatory filings and approvals and other product development
objectives; |
| |
|
|
| |
● |
the
pricing and reimbursement of our product candidates, if approved; |
| |
|
|
| |
● |
the
rate and degree of market acceptance of our product candidates by physicians, patients, third-party payors and others in the medical
community, if approved; |
| |
|
|
| |
● |
the
implementation of our business model, strategic plans for our business, product candidates and technology; |
| |
|
|
| |
● |
the
scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology; |
| |
|
|
| |
● |
developments
relating to our competitors and our industry; |
| |
|
|
| |
● |
the
accuracy of our estimates regarding expenses, capital requirements and needs for additional financing; and |
| |
|
|
| |
● |
our
financial performance. |
Forward-looking
statements reflect our management’s expectations or predictions of future conditions, events or results based on various assumptions
and management’s estimates of trends and economic factors in the markets in which we are active, as well as our business plans.
They are not guarantees of future performance. By their nature, forward-looking statements are subject to risks and uncertainties. Our
actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated
in these forward-looking statements. There are a number of factors that could cause actual conditions, events or results to differ materially
from those described in the forward-looking statements contained in this prospectus and the documents incorporated by reference into
this prospectus.
See
an additional discussion under “Risk Factors” in any applicable prospectus supplement and any related free writing prospectus,
and in our most recent Annual Report on Form 10-K and any subsequently filed quarterly reports on Form 10-Q. These forward-looking statements
are representative only as of the date they are made, and we undertake no obligation to update any forward-looking statement as a result
of new information, future events or otherwise.
PROSPECTUS
SUMMARY
Overview
We
are a clinical-stage biopharmaceutical company focused on utilizing our “regulatory science” approach, including the principles
associated with U.S. Food and Drug Administration’s (FDA) Project Optimus Oncology initiative and the related FDA Draft Guidance,
in the development of Next Generation Chemotherapy oncology drug products. Our mission is to provide better treatment options than those
that presently exist by extending a patient’s survival and/or improving a patient’s quality of life. This is achieved by
improving upon FDA-approved, widely used oncology drugs or the cancer-killing metabolites of these drugs by altering how they are metabolized
and/or distributed in the body, including how they are distributed to the actual cancer cells.
Our
Annual Report on Form 10-K for the year ended December 31, 2023 and subsequently filed Quarterly Reports on Form 10-Q provide additional
information about our business, operations and financial condition.
Corporate
Information
We
were incorporated under the laws of the State of Delaware on March 29, 2011. Our principal executive office is located at 7380 Coca Cola
Drive, Suite 106, Hanover, MD 21076. Our telephone number is (443) 776-3133. Our website is www.processapharmaceuticals.com. The information
found on, or otherwise accessible through, our website is not incorporated into, and does not form a part of, this prospectus or any
other report or document we file with or furnish to the SEC. We have included our website address in this prospectus solely as an inactive
textual reference. Investors should not rely on any such information in deciding whether to purchase our securities.
RISK
FACTORS
Investing
in our securities involves a high degree of risk. You should carefully consider the specific risks set forth under the caption “Risk
Factors” in our most recent Annual Report on Form 10-K and any subsequently filed quarterly reports on Form 10-Q, incorporated
into this prospectus by reference, as updated by any future filings we make under the Exchange Act. You should consider carefully those
risk factors together with all of the other information included and incorporated by reference in this prospectus before investing in
any securities offered by this prospectus. For more information, see “Where You Can Find More Information.” These risks are
not the only ones faced by us. Additional risks not known, or that are deemed immaterial could also materially and adversely affect our
financial condition, results of operations, our drug candidates, business and prospects. Any of these risks might cause you to lose all
or part of your investment.
USE
OF PROCEEDS
Unless
we indicate otherwise in the applicable prospectus supplement, the net proceeds from the sale of the securities will be used for continued
research and development for our portfolio of drug candidates, especially our oncology products, and working capital and general corporate
purposes. We may also use a portion of the net proceeds, together with our existing cash and cash equivalents, to in-license, acquire,
or invest in complementary businesses, technologies, products or assets; however, we have no current commitments or obligations to do
so.
We
have not determined the amounts we plan to spend on any of the areas listed above or the timing of these expenditures. As a result, our
management will have broad discretion to allocate the net proceeds, if any, we receive in connection with securities offered pursuant
to this prospectus for any purpose. Pending application of the net proceeds as described above, we may initially invest the net proceeds
in short-term, investment-grade, interest-bearing securities or apply them to the reduction of short-term liabilities.
DESCRIPTION
OF SECURITIES
The
following descriptions are summaries of the material terms of our amended and restated certificate of incorporation and amended and restated
bylaws. Reference is made to the more detailed provisions of, and the descriptions are qualified in their entirety by reference to, our
Certificate of Incorporation and Bylaws, forms of which are filed with the SEC as exhibits to the registration statement of which this
prospectus is a part, and applicable law.
General
We,
directly or through one or more underwriters, dealers and agents designated from time to time, or directly to purchasers, or through
a combination of these methods, may offer, issue and sell, together or separately, in one or more offerings, the following securities:
| |
● |
shares
of our common stock; |
| |
|
|
| |
● |
shares
of our preferred stock, in one or more series; |
| |
|
|
| |
● |
warrants
to purchase shares of our common stock or preferred stock; |
| |
|
|
| |
● |
units
consisting of any combination of the securities listed above, each on terms to be determined at the time of sale. |
The
preferred stock may also be exchangeable for and/or convertible into shares of common stock, another series of preferred stock, or other
securities. The common stock, preferred stock, warrants, and units are collectively referred to in this prospectus as the “securities.”
When a particular series of securities is offered, a supplement to this prospectus will be delivered with this prospectus, which will
set forth the terms of the offering and sale of the offered securities.
We
have the authority to issue an aggregate of 100,000,000 shares of $0.0001 par value common stock and 1,000,000 shares of $0.0001 par
value preferred stock. Our common stock is listed on the Nasdaq Capital Market under the symbol “PCSA.” As of May 17,
2024, we had 2,858,007 shares of our common stock outstanding; no shares of preferred stock outstanding; warrants to purchase 1,778,284
shares of our common stock for an average exercisable price of $6.17 on terms expiring on or prior to January 30, 2029; and stock options
to purchase 6,992 shares of our common stock, for an average exercise price of $364.72 on terms expiring on or prior to October 1, 2028.
Common
Stock
Dividend
Rights. Subject to the rights of holders of preferred stock of any series that may be issued and outstanding from time to time,
holders of our common stock are entitled to receive such dividends and other distributions as may be declared by our Board of Directors
from time to time.
Voting
Rights. Each outstanding share of our common stock is entitled to one vote on all matters submitted to a vote of stockholders
generally. In the event we issue one or more series of preferred or other securities in the future such preferred stock or other securities
may be given rights to vote, either together with the common stock or as a separate class on one or more types of matters. The holders
of our common stock do not have cumulative voting rights.
Liquidation
Rights. In the event of any liquidation, dissolution or winding up of the Company, the holders of our common stock will be entitled,
subject to any preferential or other rights of any then outstanding preferred stock, to receive all assets of the Company available for
distribution to stockholders.
Preemptive
Rights. As of the date hereof, the holders of our common stock have no preemptive rights in their capacities as such holders.
Board
of Directors. Holders of common stock do not have cumulative voting rights with respect to the election of directors. At any
meeting to elect directors by holders of our common stock, the presence, in person or by proxy, of the holders of a majority of the voting
power of shares of our capital stock then outstanding will constitute a quorum for such election. Directors may be elected by a plurality
of the votes of the shares present and entitled to vote on the election of directors, except for directors whom the holders of any then
outstanding preferred stock have the right to elect, if any.
Preferred
Stock
Our
Board is authorized, subject to certain limitations prescribed by law, without further stockholder approval, to issue from time to time
up to an aggregate of 1,000,000 shares of preferred stock in one or more series and to fix or alter the designations, preferences, rights
and any qualifications, limitations or restrictions of the shares of each such series thereof, including the dividend rights, dividend
rates, conversion rights, voting rights and terms of redemption of shares constituting any series or designations of such series. The
rights of holders of our common stock may be subject to, and adversely affected by, the rights of the holders of any preferred stock
that may be issued in the future. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change of
control and may adversely affect the voting and other rights of holders of our common stock. As of the date of this prospectus, there
were no shares of preferred stock outstanding.
Warrants
We
may issue warrants, in one or more series, for the purchase of our common stock or preferred stock. Warrants may be issued independently
or together with our common stock or preferred stock and may be attached to or separate from any offered securities.
A
prospectus supplement accompanying this prospectus relating to a particular series of warrants will describe the terms of those warrants,
including:
| |
● |
the
title and the aggregate number of warrants; |
| |
|
|
| |
● |
the
security for which each warrant is exercisable; |
| |
|
|
| |
● |
the
date or dates on which the right to exercise such warrants commence and expire; |
| |
|
|
| |
● |
the
price or prices at which such warrants are exercisable; |
| |
|
|
| |
● |
the
periods during which and places at which such warrants are exercisable; |
| |
|
|
| |
● |
the
terms of any mandatory or optional call provisions; |
| |
|
|
| |
● |
the
price or prices, if any, at which the warrants may be redeemed at the option of the holder or will be redeemed upon expiration; |
| |
|
|
| |
● |
the
identity of the warrant agent; and |
| |
|
|
| |
● |
the
exchanges, if any, on which such warrants may be listed. |
You
should read the particular terms of the documents pursuant to which the warrants will be issued, which will be described in more detail
in the applicable prospectus supplement.
As
of the date of this prospectus, we have outstanding warrants to purchase shares of our common stock to various persons and entities,
under which we could be obligated to issue up to 1,778,284 shares of common stock, including:
| |
● |
158,007
shares of common stock issuable upon the exercise of outstanding warrants allowing the holders to purchase shares of common stock
at an exercise price of $20.40 per share through April 17, 2026 and |
| |
|
|
| |
● |
1,555,555
shares of common stock issuable upon the exercise of outstanding warrants allowing the holders to purchase shares of common stock
at an exercise price of $4.50 per share through January 30, 2029. |
Units
We
may issue units consisting of one or more warrants, shares of preferred stock, shares of common stock or any combination of such securities.
The applicable prospectus supplement will describe the terms of the units and of the securities comprising the units, including whether
and under what circumstances the securities comprising the units may be traded separately. You should read the particular terms of the
documents pursuant to which the units will be issued, which will be described in more detail in the applicable prospectus supplement.
Indemnification
of Directors and Officers
Our
amended and restated certificate of incorporation provides that, to the fullest extent permitted by the Delaware General Corporate Law
(“DGCL”) as it may hereafter be amended, none of our directors will be personally liable to us or our stockholders for monetary
damages for breach of fiduciary duty as a director. Under the DGCL as it now reads, such limitation of liability is not permitted:
| |
● |
for
any breach of the director’s duty of loyalty to us or our stockholders; |
| |
|
|
| |
● |
for
acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| |
|
|
| |
● |
for
payments of unlawful dividends or unlawful stock purchases or redemptions under Section 174 of the DGCL; or |
| |
|
|
| |
● |
for
any transaction from which the director derived an improper personal benefit. |
These
provisions will have no effect on the availability of equitable remedies such as an injunction or rescission based on a director’s
breach of his or her duty of care.
Our
amended and restated certificate of incorporation and our amended and restated bylaws include provisions that require us to indemnify
and advance expenses, to the fullest extent allowable under the DGCL as it now exists or may hereafter be amended, to our directors or
officers for actions taken as a director or officer of us, or for serving at our request as a director or officer at another corporation
or enterprise, as the case may be.
Section
145 of the DGCL provides that a corporation may indemnify directors and officers, as well as other employees and individuals, against
expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement, that are incurred in connection with various
actions, suits or proceedings, whether civil, criminal, administrative or investigative, other than an action by or in the right of the
corporation, known as a derivative action, if they acted in good faith and in a manner they reasonably believed to be in or not opposed
to the best interests of the corporation, and, with respect to any criminal action or proceeding, if they had no reasonable cause to
believe their conduct was unlawful. A similar standard is applicable in the case of derivative actions, except that indemnification only
extends to expenses, including attorneys’ fees, incurred in connection with the defense or settlement of such actions, and the
statute requires court approval before there can be any indemnification if the person seeking indemnification has been found liable to
the corporation. The statute provides that it is not exclusive of other indemnification that may be granted by a corporation’s
bylaws, disinterested director vote, stockholder vote, agreement or otherwise.
Our
amended and restated bylaws require us to indemnify any person who was or is a party or is threatened to be made a party to, or was otherwise
involved in, a legal proceeding by reason of the fact that he or she is or was a director or officer of the Company or is or was serving
at our request as a director or officer of another corporation or enterprise, as the case may be, to the fullest extent authorized by
the DGCL as it now exists or may hereafter be amended, against all expense, liability and loss (including attorneys’ fees, judgments,
fines, Employee Retirement Income Security Act excise taxes or penalties and amounts paid in settlement) reasonably incurred or suffered
by such director or officer in connection with such service. The right to indemnification in our amended and restated bylaws includes
the right to be paid by the Company the expenses incurred in defending any proceeding for which indemnification may be sought in advance
of the final disposition of such proceeding, subject to certain limitations. We carry directors’ and officers’ insurance
protecting us, any director, officer, employee or agent of ours or who was serving at the request of the Company as a director, officer,
employee or agent of another corporation or enterprise, as the case may be, against any expense, liability or loss, whether or not we
would have the power to indemnify the person under the DGCL.
The
limitation of liability and indemnification and advancement provisions in our amended and restated certificate of incorporation and our
amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of fiduciary duty. These
provisions also may reduce the likelihood of derivative litigation against our directors and officers, even though such an action, if
successful, might otherwise benefit us and our stockholders. In addition, your investment in our common stock may be adversely affected
to the extent we pay the costs of settlement and damage awards under these indemnification provisions.
Certain
Anti-Takeover Effects
Provisions
of Delaware Law. We are a Delaware corporation and Section 203 of the DGCL applies to us. It is an anti-takeover statute that
is designed to protect stockholders against coercive, unfair or inadequate tender offers and other abusive tactics and to encourage any
person contemplating a business combination with us to negotiate with our Board of Directors for the fair and equitable treatment of
all stockholders.
Under
Section 203 of the DGCL, a Delaware corporation is not permitted to engage in a “business combination” with an “interested
stockholder” for a period of three years following the date that the stockholder became an interested stockholder. As defined for
this purpose, the term “business combination” includes a merger, consolidation, asset sale or other transaction resulting
in a financial benefit to the interested stockholder. The term “interested stockholder” is defined to mean a person who,
together with affiliates and associates, owns, or within three years did own, 15% or more of the corporation’s outstanding voting
stock. This prohibition does not apply if:
| |
● |
prior
to the time that the stockholder became an interested stockholder, the Board of Directors of the corporation approved either the
business combination or the transaction resulting in the stockholder becoming an interested stockholder; |
| |
|
|
| |
● |
upon
completion of the transaction resulting in the stockholder becoming an interested stockholder, the stockholder owns at least 85%
of the outstanding voting stock of the corporation, excluding voting stock owned by directors who are also officers and by certain
employee stock plans; or |
| |
|
|
| |
● |
at
or subsequent to the time that the stockholder became an interested stockholder, the business combination is approved by the Board
and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least two-thirds
of the outstanding voting stock that the interested stockholder does not own. |
A
Delaware corporation may elect not to be governed by these restrictions. We have not opted out of Section 203.
Advance
Notice Procedures. Our bylaws establish an advance notice procedure for stockholder nominations of persons for election to our
Board of Directors and for any proposals to be presented by stockholders at an annual meeting. Stockholders at an annual meeting will
only be able to consider nominations and other proposals specified in the notice of meeting or brought before the meeting by or at the
direction of our Board of Directors or by a stockholder who was a stockholder of record on the record date for the meeting, who is entitled
to vote at the meeting and who has given our corporate secretary timely written notice, in proper form, of the stockholder’s intention
to nominate a person for election as a director or to bring a proposal for action at the meeting.
Potential
Effects of Authorized but Unissued Stock
Pursuant
to our amended and restated certificate of incorporation, we have shares of common stock and preferred stock available for future issuance
without stockholder approval. We may utilize these additional shares for a variety of corporate purposes, including future public offerings
to raise additional capital, to facilitate corporate acquisitions or payment as a dividend on the capital stock.
The
existence of unissued and unreserved common stock and preferred stock may enable our Board of Directors to issue shares to persons friendly
to current management or to issue preferred stock with terms that could render more difficult or discourage a third-party attempt to
obtain control of us by means of a merger, tender offer, proxy contest or otherwise, thereby protecting the continuity of our management.
In addition, the Board of Directors has the discretion to determine designations, rights, preferences, privileges and restrictions, including
voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences of each series of preferred stock,
all to the fullest extent permissible under the Delaware General Corporation Law and subject to any limitations set forth in our certificate
of incorporation. The purpose of authorizing the Board of Directors to issue preferred stock and to determine the rights and preferences
applicable to such preferred stock is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred
stock, while providing desirable flexibility in connection with possible financings, acquisitions and other corporate purposes, could
have the effect of making it more difficult for a third-party to acquire, or could discourage a third-party from acquiring, a majority
of our outstanding voting stock.
Choice
of Forum
Unless
we consent in writing to the selection of an alternative forum, the Delaware Court of Chancery shall be the sole and exclusive forum
for any stockholder to bring (i) any derivative action or proceeding brought on behalf of the Company, (ii) any action asserting a claim
of breach of fiduciary duty owed by any director, officer or other employee of the Company or the Company’s stockholders, (iii)
any action asserting a claim against the Company or any director or officer of the Company arising pursuant to, or a claim against the
Company or any director or officer of the Company, with respect to the interpretation or application of any provision of the DGCL, our
certificate of incorporation or bylaws, or (iv) any action asserting a claim governed by the internal affairs doctrine, except for, in
each of the aforementioned actions, any claims to which the Delaware Court of Chancery determines it lacks jurisdiction. This provision
will not apply to claims arising under the Exchange Act, or for any other federal securities laws which provide for exclusive federal
jurisdiction. However, the exclusive forum provision provides that unless we consent in writing to the selection of an alternative forum,
the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting
a cause of action arising under the Securities Act. Therefore, this provision could apply to a suit that falls within one or more of
the categories enumerated in the exclusive forum provision and that asserts claims under the Securities Act, inasmuch as Section 22 of
the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability
created by the Securities Act or the rules and regulations thereunder. There is uncertainty as to whether a court would enforce such
an exclusive forum provision with respect to claims under the Securities Act.
We
note that there is uncertainty as to whether a court would enforce the provision and that investors cannot waive compliance with the
federal securities laws and the rules and regulations thereunder. Although we believe this provision benefits us by providing increased
consistency in the application of Delaware law in the types of lawsuits to which it applies, the provision may have the effect of discouraging
lawsuits against our directors and officers.
Listing
Our
common stock is listed on the Nasdaq Capital Market under the symbol “PCSA.”
Transfer
Agent and Registrar
Our
transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company, LLC.
PLAN
OF DISTRIBUTION
We
may sell the securities on a delayed or continuous basis through one or more agents, underwriters or dealers, directly to one or more
purchasers, through a combination of any of these methods of sale, in negotiated transactions (including block trades), in transactions
that are deemed to be “at the market” offerings as defined in Rule 415 of the Securities Act or in any other manner, as provided
in the applicable prospectus supplement. We will identify the specific plan of distribution, including any underwriters, dealers, agents
or direct purchasers and their compensation in a prospectus supplement.
We
may distribute the securities from time to time in one or more transactions:
| |
● |
at
a fixed price or prices, which may be changed; |
| |
|
|
| |
● |
at
market prices prevailing at the time of sale; |
| |
|
|
| |
● |
at
prices related to prevailing market prices; or |
| |
|
|
| |
● |
at
negotiated prices. |
In
connection with the sale of the securities, underwriters may be deemed to have received compensation from us in the form of underwriting
discounts or commissions and may also receive commissions from purchasers of the securities for whom they may act as agent. Underwriters
may sell the securities to or through dealers, and dealers may receive compensation in the form of discounts, concessions or commissions
from the underwriters and/or commissions from the purchasers for whom they may act as agent.
If
we use an underwriter in the sale of the securities being offered by this prospectus, we will execute an underwriting agreement with
the underwriter at the time of sale and we will provide the name of any underwriter in the applicable prospectus supplement. We will
describe in the applicable prospectus supplement any underwriting compensation we pay to underwriters or agents in connection with the
offering of the securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers. Dealers and
agents participating in the distribution of the securities may be deemed to be underwriters, and any discounts and commissions received
by them and any profit realized by them on resale of the securities may be deemed to be underwriting discounts and commissions. We may
enter into agreements with any underwriters, dealers and agents which may entitle them to indemnification against and contribution toward
certain civil liabilities, including liabilities under the Securities Act, and to reimburse for certain expenses.
Unless
we specify otherwise in the related prospectus supplement, each series of securities offered will be a new issue with no established
trading market. We may elect to list any series of securities on any exchange, but we are not obligated to do so. It is possible that
one or more underwriters or agents may make a market in a series of offered securities, but will not be obligated to do so and may discontinue
any market making at any time without notice. Therefore, we cannot assure you as to the liquidity of the trading market for the securities.
If
indicated in the applicable prospectus supplement, we may authorize underwriters, dealers or other persons acting as our agents to solicit
offers by certain institutions or other suitable persons to purchase the securities from us at the public offering price set forth in
the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on the date or dates stated in the
prospectus supplement. We may make delayed delivery with various institutions, including commercial and savings banks, insurance companies,
pension funds, investment companies and educational and charitable institutions. Delayed delivery contracts will be subject to the condition
that the purchase of the securities covered by the delayed delivery contracts will not at the time of delivery be prohibited under the
laws of any jurisdiction in the United States to which the purchaser is subject. The underwriters and agents will not have any responsibility
with respect to the validity or performance of these contracts.
To
facilitate an offering of a series of the securities, certain persons participating in the offering may engage in transactions that stabilize,
maintain, or otherwise affect the price of the securities. This may include over-allotments or short sales of the securities, which involves
the sale by persons participating in the offering of more securities than we sold to them. In these circumstances, these persons would
cover the over-allotments or short positions by making purchases in the open market or by exercising their over-allotment option. In
addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market
or by imposing penalty bids, whereby selling concessions allowed to dealers participating in the offering may be reclaimed if securities
sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be to stabilize or maintain
the market price of the securities at a level above that which might otherwise prevail in the open market. These transactions may be
discontinued at any time.
LEGAL
MATTERS
The
validity of the securities offered by this prospectus will be passed upon for us by Foley & Lardner LLP.
EXPERTS
The
consolidated financial statements of the Company as of December 31, 2023 and 2022 and for the years ended December 31, 2023 and 2022
appearing in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, have been audited by BD & Company, Inc.,
an independent registered public accounting firm, as set forth in their report thereon and incorporated herein by reference. Such consolidated
financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts
in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
We
file annual, quarterly and current reports, proxy statements and other information with the SEC. We also filed a registration statement
on Form S-3, including exhibits, under the Securities Act with respect to the securities offered by this prospectus. This prospectus
is a part of the registration statement, but does not contain all of the information included in the registration statement or the exhibits.
The SEC maintains a web site, www.sec.gov, which contains reports, proxy and information statements and other information regarding
issuers that file electronically with the SEC. You may review the registration statement and any other document we file on the SEC’s
web site. Our SEC filings are also available to the public on our website, www.processapharmaceuticals.com. The information on
our website, however, is not, and should not be deemed to be, a part of this prospectus.
INCORPORATION
OF CERTAIN DOCUMENTS BY REFERENCE
We
are “incorporating by reference” specified documents that we file with the SEC, which means:
| |
● |
incorporated
documents are considered part of this prospectus; |
| |
|
|
| |
● |
we
are disclosing important information to you by referring you to those documents; and |
| |
|
|
| |
● |
information
we file with the SEC will automatically update and supersede information contained in this prospectus. |
We
incorporate by reference the documents listed below and any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d)
of the Exchange Act after the date we filed the registration statement of which this prospectus is a part and before the effective date
of the registration statement and any future filings we will make with the SEC under those sections, except to the extent that any information
in such filing is deemed “furnished” in accordance with rules of the SEC:
| |
● |
our
Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 29, 2024; |
| |
|
|
| |
● |
our
Proxy Statement on Schedule 14A for our Annual Meeting of Stockholders, filed April 29, 2024; |
| |
|
|
| |
● |
our
Quarterly Report on Form 10-Q for the quarter ended March 31, 2024, filed on May 10, 2024; |
| |
|
|
| |
● |
our
Current Reports on Form 8-K, filed with the SEC on January 18, 2024, January 25, 2024, January 30, 2024 and February 6, 2024; and |
| |
|
|
| |
● |
the
description of our common stock contained in or incorporated into our Registration Statement on Form 8-A, filed September 17, 2020,
and any amendment or report updating that description. |
Notwithstanding
the foregoing, documents or portions thereof containing information furnished under Items 2.02 and 7.01 of any Current Report on Form
8-K, including the related exhibits under Item 9.01, are not incorporated by reference in this prospectus.
Any
statement contained in this prospectus or in a document incorporated or deemed to be incorporated by reference into this prospectus will
be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or
any other subsequently filed document that is deemed to be incorporated by reference into this prospectus modifies or supersedes the
statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this
prospectus.
You
may request a copy of any of these filings, at no cost, by request directed to us at the following address or telephone number:
Processa
Pharmaceuticals, Inc.
7380
Coca Cola Drive, Suite 106
Hanover,
Maryland 21076
(443)
776-3133
Attention: Wendy Guy
You
should not assume that the information in this prospectus or any prospectus supplement, as well as the information we file or previously
filed with the SEC that we incorporate by reference in this prospectus or any prospectus supplement, is accurate as of any date other
than the respective date of such documents. Our business, financial condition, results of operations and prospects may have changed since
that date.
Filed
Pursuant to Rule 424(b)(5)
Registration No. 333-
PROSPECTUS
SUPPLEMENT
(To
Prospectus dated [●●], 2024)
$2,400,000
Common
Stock
We
have entered into a sales agreement (the “Sales Agreement”) with A.G.P./Alliance Global Partners (“A.G.P”) (the
“Sales Agent”), dated May 21, 2024, relating to the sale of shares of our common stock, par value $0.0001 per share (the
“common stock”), offered by this prospectus supplement. In accordance with the terms of the Sales Agreement, we may offer
and sell shares of our common stock, having an aggregate offering price of up to $2,400,000 from time to time through the Sales Agent,
acting as agent.
Sales
of our common stock, if any, under this prospectus supplement may be made by any method deemed to be an “at the market offering”
as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended (the “Securities Act”). The Sales Agent
is not required to sell any specific amount, but will act as our sales agent using commercially reasonable efforts, consistent with its
normal trading and sales practices. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
The
Sales Agent will be entitled to compensation at a commission rate of up to 3.0% of the gross sales price per share sold under the Sales
Agreement. See “Plan of Distribution” beginning on page S-9 for additional information regarding the compensation to be paid
to the Sales Agent. In connection with the sale of the common stock on our behalf, the Sales Agent will be deemed to be an “underwriter”
within the meaning of the Securities Act and the compensation of the Sales Agent will be deemed to be underwriting commissions or discounts.
We also have agreed to provide indemnification and contribution to the Sales Agent with respect to certain liabilities, including liabilities
under the Securities Act.
Our
common stock is listed on the Nasdaq Capital Market under the trading symbol “PCSA.” On May 17, 2024, the last reported
sale price of our common stock on the Nasdaq Capital Market was $1.96 per share. The aggregate market value of our outstanding
common stock held by non-affiliates as of the date of this prospectus was approximately $7.7 million, based on 2,858,007
shares of common stock outstanding, 2,574,477 of which were held by non-affiliates, and a per share price of $2.985 based
on the closing sale price of our common stock on April 8, 2024. We have sold no securities pursuant to General Instructions I.B.6
of Form S-3 during the prior 12 calendar month period that ends on, and includes, the date of this prospectus supplement.
INVESTING
IN OUR COMMON STOCK INVOLVES RISKS. SEE “RISK FACTORS” BEGINNING ON PAGE S-5
OF THIS PROSPECTUS SUPPLEMENT AND IN OUR ANNUAL REPORT ON FORM 10-K FOR THE YEAR ENDED DECEMBER
31, 2023 AND OUR OTHER PERIODIC REPORTS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION (THE “SEC”), AND INCORPORATED BY
REFERENCE HEREIN.
Neither
the SEC nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of
this prospectus supplement. Any representation to the contrary is a criminal offense.
A.G.P.
The
date of this prospectus supplement is , 2024.
TABLE
OF CONTENTS
PROSPECTUS
SUPPLEMENT
PROSPECTUS
For
further information regarding us and our financial information, you should refer to our recent filings with the SEC. See the sections
titled “Where You Can Find More Information” in the accompanying prospectus “Incorporation of Certain Documents by
Reference” in this prospectus supplement.
You
should rely only on the information contained in or incorporated by reference into this prospectus supplement, the accompanying prospectus
or any applicable free writing prospectus in making a decision about whether to invest in our common stock. We have not, and the Sales
Agent has not, authorized any other person to provide you with different or additional information. If anyone provides you with different
or additional information, you should not rely on it. This prospectus supplement and the accompanying prospectus do not constitute an
offer to sell, or a solicitation of an offer to purchase, any securities in any jurisdiction where it is unlawful to make such an offer
or solicitation. You should assume that the information appearing in this prospectus supplement, the accompanying prospectus, any applicable
free writing prospectus and the documents incorporated by reference herein or therein is accurate only as of their respective dates or
on the date or dates which are specified in these documents. Our business, financial condition, liquidity, results of operations and
prospects may have changed since those dates.
We
are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The
distribution of this prospectus supplement and the accompanying prospectus and the offering of our common stock in certain jurisdictions
may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying
prospectus must inform themselves about, and observe any restrictions relating to, the offering of our common stock and the distribution
of this prospectus supplement and the accompanying prospectus outside the United States. This prospectus supplement and the accompanying
prospectus does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities
offered by this prospectus supplement and the accompanying prospectus to or by any person in any jurisdiction in which it is unlawful
for such person to make such an offer or solicitation.
ABOUT
THIS PROSPECTUS SUPPLEMENT
This
document is in two parts. The first part is this prospectus supplement, including the documents incorporated by reference, which describes
the specific terms of this offering. The second part, the accompanying prospectus dated [●], 2024, gives more general information,
some of which may not apply to this offering.
To
the extent the information contained in this prospectus supplement differs or varies from the information contained in the accompanying
prospectus or documents incorporated by reference, the information in this prospectus supplement will supersede such information.
This
prospectus supplement does not contain all of the information that is important to you. You should read the accompanying prospectus as
well as the documents incorporated by reference in this prospectus supplement and the accompanying prospectus. See “Incorporation
of Certain Documents by Reference” in this prospectus supplement and “Where You Can Find More Information” in the accompanying
prospectus.
Processa’s
name and logo are either registered trademarks or trademarks of Processa Pharmaceuticals, Inc. in the United States and/or other countries.
All other trademarks, service marks or other tradenames appearing in this prospectus supplement and the accompanying prospectus are the
property of their respective owners.
Unless
otherwise mentioned or unless the context requires otherwise, all references in this prospectus supplement to the “Company,”
“Processa,” “we,” “us,” “our,” or similar references mean Processa Pharmaceuticals, Inc.,
a Delaware corporation, and its wholly owned subsidiary.
FORWARD-LOOKING
STATEMENTS
This
prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein contain certain “forward-looking
statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act, and
Section 21E of the Exchange Act. All statements other than statements of historical facts are forward-looking statements. In some cases,
you can identify forward-looking statements by words such as “anticipate,” “believe,” “contemplate,”
“continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,”
“potential,” “predict,” “project,” “seek,” “should,” “target,”
“will,” “would,” or the negative of these words or other comparable terminology. We have based these forward-looking
statements on our current expectations and projections about future events and trends that we believe may affect our financial condition,
results of operations, strategy, short- and long-term business operations and objectives, and financial needs. These forward-looking
statements are subject to a number of risks, uncertainties and assumptions. Moreover, we operate in a very competitive and rapidly changing
environment, and new risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the
impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ
materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions,
the forward-looking events and circumstances discussed in this prospectus may not occur, and actual results could differ materially and
adversely from those anticipated or implied in the forward-looking statements. Given these uncertainties, you should not place undue
reliance on these forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| |
● |
our
ability to obtain funding for our future clinical trials, preclinical activities and our operations; |
| |
|
|
| |
● |
our
ability to obtain and maintain regulatory approval of our product candidates; |
| |
|
|
| |
● |
our
ability to contract with third-party suppliers, manufacturers and other service providers and their ability to perform adequately; |
| |
|
|
| |
● |
our
ability to meet obligations under our license agreements; |
| |
|
|
| |
● |
the
potential market size, opportunity and growth potential for our product candidates, if approved; |
| |
|
|
| |
● |
our
ability to build our own sales and marketing capabilities, or seek collaborative partners, to commercialize our product candidates,
if approved; |
| |
|
|
| |
● |
our
ability to retain the continued service of our key professionals and to identify, hire and retain additional qualified professionals; |
| |
|
|
| |
● |
our
ability to advance product candidates into, and successfully complete, clinical trials; |
| |
|
|
| |
● |
our
ability to recruit and enroll suitable patients in our clinical trials; |
| |
|
|
| |
● |
the
initiation, timing, progress and results of clinical trials and pre-clinical studies for our drug candidates; |
| |
|
|
| |
● |
the
timing or likelihood of the accomplishment of various scientific, clinical, regulatory filings and approvals and other product development
objectives; |
| |
|
|
| |
● |
the
pricing and reimbursement of our product candidates, if approved; |
| |
|
|
| |
● |
the
rate and degree of market acceptance of our product candidates by physicians, patients, third-party payors and others in the medical
community, if approved; |
| |
|
|
| |
● |
the
implementation of our business model, strategic plans for our business, product candidates and technology; |
| |
● |
the
scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology; |
| |
|
|
| |
● |
developments
relating to our competitors and our industry; |
| |
|
|
| |
● |
the
accuracy of our estimates regarding expenses, capital requirements and needs for additional financing; and |
| |
|
|
| |
● |
our
financial performance. |
Forward-looking
statements reflect our management’s expectations or predictions of future conditions, events or results based on various assumptions
and management’s estimates of trends and economic factors in the markets in which we are active, as well as our business plans.
They are not guarantees of future performance. By their nature, forward-looking statements are subject to risks and uncertainties. Our
actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated
in these forward-looking statements. There are a number of factors that could cause actual conditions, events or results to differ materially
from those described in the forward-looking statements contained in this prospectus supplement, the accompanying prospectus and the documents
incorporated by reference herein.
See
an additional discussion under “Risk Factors” in any applicable prospectus supplement and any related free writing prospectus,
and in our most recent Annual Report on Form 10-K and any subsequently filed quarterly reports on Form 10-Q. These forward-looking statements
are representative only as of the date they are made, and we undertake no obligation to update any forward-looking statement as a result
of new information, future events or otherwise.
PROSPECTUS
SUPPLEMENT SUMMARY
Overview
We
are a clinical-stage biopharmaceutical company focused on utilizing our “regulatory science” approach, including the principles
associated with U.S. Food and Drug Administration’s (FDA) Project Optimus Oncology initiative and the related FDA Draft Guidance,
in the development of Next Generation Chemotherapy oncology drug products. Our mission is to provide better treatment options than those
that presently exist by extending a patient’s survival and/or improving a patient’s quality of life. This is achieved by
improving upon FDA-approved, widely used oncology drugs or the cancer-killing metabolites of these drugs by altering how they are metabolized
and/or distributed in the body, including how they are distributed to the actual cancer cells.
Our
Annual Report on Form 10-K for the year ended December 31, 2023 and subsequently filed Quarterly Reports on Form 10-Q provide additional
information about our business, operations and financial condition.
Corporate
Information
We
were incorporated under the laws of the State of Delaware on March 29, 2011. Our principal executive office is located at 7380 Coca Cola
Drive, Suite 106, Hanover, MD 21076. Our telephone number is (443) 776-3133. Our website is www.processapharmaceuticals.com. The information
found on, or otherwise accessible through, our website is not incorporated into, and does not form a part of, this prospectus supplement
or any other report or document we file with or furnish to the SEC. We have included our website address in this prospectus supplement
solely as an inactive textual reference. Investors should not rely on any such information in deciding whether to purchase our common
stock.
THE
OFFERING
For
a more complete description of the terms of the common stock being offered by this prospectus supplement and the accompanying prospectus,
see “Description of Securities” in the accompanying prospectus.
| Common
stock offered by us: |
Common
stock having an aggregate offering price of up to $2,400,000. |
| |
|
| Manner
of offering: |
“At
the market offering” that may be made from time to time through our Sales Agent, A.G.P. See “Plan of Distribution”
beginning on page S-9. |
| |
|
| Use
of proceeds: |
We
intend to use the net proceeds for continued research and development for our portfolio of drug candidates, especially our oncology
products, and working capital and general corporate purposes. We may also use a portion of the net proceeds, together with our existing
cash and cash equivalents, to in-license, acquire, or invest in complementary businesses, technologies, products or assets; however,
we have no current commitments or obligations to do so. |
| |
|
| Risk
factors: |
An
investment in our common stock involves a high degree of risk. See “Risk Factors” beginning on page S-5 of this
prospectus supplement and other information included in this prospectus supplement, the accompanying prospectus and the documents
incorporated by references in this prospectus supplement and the accompanying prospectus for a discussion of factors you should carefully
consider before deciding to invest in our common stock. |
| |
|
| Nasdaq
Capital Market symbol: |
“PCSA” |
RISK
FACTORS
An
investment in our common stock involves a high degree of risk. Prior to making a decision about investing in our common stock, you should
consider carefully the specific risk factors discussed in the sections entitled “Risk Factors” contained in our most recent
Annual Report on Form 10-K for the year ended December 31, 2023, and subsequent Quarterly Reports on Form 10-Q which are incorporated
into this prospectus supplement and the accompanying prospectus by reference in their entirety, as updated or superseded by the risks
and uncertainties described under similar headings in the other documents that are filed after the date hereof and incorporated by reference
into this prospectus supplement and the accompanying prospectus, together with other information in this prospectus supplement and the
accompanying prospectus, the documents incorporated by reference and any free writing prospectus that we may authorize for use in connection
with this offering. These risks and uncertainties are not the only risks and uncertainties we face. Additional risks and uncertainties
not presently known to us, or that we currently view as immaterial, may also impair our business. Past financial performance may not
be a reliable indicator of future performance, and historical trends should not be unduly relied upon to anticipate results or trends
in future periods. If any of the risks or uncertainties described in our SEC filings or any additional risks and uncertainties actually
occur, our business, financial condition, results of operations and cash flow could be materially and adversely affected. In that case,
the trading price of our common stock could decline and you might lose all or part of your investment. Please also read carefully the
section above titled “Forward-Looking Statements.”
Risks
Relating to this Offering
Our
management will have broad discretion to use the net proceeds from this offering, and our investment of these proceeds pending any such
use may not yield a favorable return.
Our
management will have broad discretion as to the use of the net proceeds from this offering by us and could use them for purposes other
than those contemplated at the time of this offering. Accordingly, you will be relying on the judgment of our management with regard
to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds
are being used appropriately. It is possible that the proceeds will be invested in a way that does not yield a favorable, or any, return
for us.
Investors
in this offering may suffer immediate and substantial dilution in the net tangible book value per share of our common stock.
The
shares sold in this offering, if any, will be sold from time to time at various prices. However, the offering price of our common stock
in this offering could be higher than the net tangible book value per share of our outstanding common stock. Therefore, if you purchase
shares of our common stock in this offering, you may pay a price per share that substantially exceeds our net tangible book value per
share after this offering. To the extent outstanding options or warrants are exercised, you may incur further dilution.
You
may experience future dilution as a result of future issuances of common stock, including through equity offerings or acquisition.
To
raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable
for our common stock at prices that may not be the same as the price per share in this offering. We may sell shares or other securities
in any other offering at a price per share that is less than the price per share paid by investors in this offering. The prices per share
at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions
may be higher or lower than the price per share paid by investors in this offering.
Future
issuances of common stock could further depress the market for our common stock. We expect to continue to incur drug development and
selling, general and administrative costs, and to satisfy our funding requirements, we will need to sell additional equity securities,
which may include sales of significant amounts of common stock to strategic investors, and which common stock may be subject to registration
rights. The sale or the proposed sale of substantial amounts of our common stock or other equity securities in the public markets or
in private transactions may adversely affect the market price of our common stock and our stock price may decline substantially. Also,
new equity securities issued may have greater rights, preferences or privileges than our existing common stock.
If
we make one or more significant acquisitions in which the consideration includes common stock or other securities, our stockholders’
holdings may be significantly diluted. In addition, stockholders’ holdings may also be diluted if we enter into arrangements with
third parties permitting us to issue shares of common stock in lieu of certain cash payments upon the achievement of milestones.
Sales
of a significant number of shares of our common stock in the public markets, or the perception that such sales could occur, could depress
the market price of our common stock.
Sales
of a substantial number of shares of our common stock in the public markets could depress the market price of our common stock, which
could impair your ability to sell any shares of common stock that you purchase in this offering at prices above the price you pay in
this offering and impair our ability to raise capital through the sale of additional equity securities. We cannot predict the effect
that future sales of our common stock would have on the market price of our common stock.
The
actual number of shares we will issue in this offering under the Sales Agreement with our Sales Agent, at any one time or in total, is
uncertain.
Subject
to certain limitations set forth in the Sales Agreement with our Sales Agent and compliance with applicable law, we have the discretion
to deliver placement notices to our Sales Agent at any time throughout the term of the Sales Agreement. The number of shares that are
sold by our Sales Agent after we deliver a placement notice will fluctuate based on the market price of our common stock during the sales
period and the limits we set with our Sales Agent.
The
common stock offered hereby will be sold in “at the market offerings,” and investors who buy shares at different times will
likely pay different prices.
Investors
who purchase shares in this offering at different times will likely pay different prices, and so may experience different outcomes in
their investment results. We will have discretion, subject to market demand, to vary the timing, prices, and numbers of shares sold,
and there is no minimum or maximum sales price. Investors may experience a decline in the value of their shares as a result of share
sales made at prices lower than the prices they paid.
We
have never paid and do not intend to pay cash dividends in the foreseeable future. As a result, capital appreciation, if any, will be
your sole source of gain.
We
have never paid cash dividends on any of our capital stock and we currently intend to retain future earnings, if any, to fund the development
and growth of our business. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable
future.
USE
OF PROCEEDS
We
may issue and sell shares of our common stock having aggregate sales proceeds of up to $2,400,000 from time to time. Because there is
no minimum offering amount required as a condition to close this offering, the actual total public offering amount, commissions and proceeds
to us, if any, are not determinable at this time.
We
intend to use the net proceeds we will receive from this offering for continued research and development for our portfolio of drug candidates,
especially our oncology products, and working capital and general corporate purposes. We may also use a portion of the net proceeds,
together with our existing cash and cash equivalents, to in-license, acquire, or invest in complementary businesses, technologies, products
or assets; however, we have no current commitments or obligations to do so.
Our
management will have broad discretion to allocate the net proceeds, if any, we receive in connection with securities offered pursuant
to this prospectus supplement for any purpose. Pending application of the net proceeds as described above, we may initially invest the
net proceeds in short-term, investment-grade, interest-bearing securities or apply them to the reduction of short-term liabilities.
DIVIDEND
POLICY
We
have never declared or paid cash dividends on our capital stock. We currently intend to retain our future earnings, if any, for use in
our business and therefore do not anticipate paying cash dividends in the foreseeable future. Payment of future dividends, if any, will
be at the discretion of our Board after taking into account various factors, including our financial condition, operating results, current
and anticipated cash needs and plans for expansion.
DILUTION
If
you invest in our common stock in this offering, your ownership interest may be diluted to the extent of the difference between the public
offering price per share of our common stock in this offering and the as adjusted net tangible book value per share of our common stock
immediately after this offering.
As
of March 31, 2024, we had net tangible book value of $8.9 million, or $3.11 per share, based on an aggregate of 2,855,981
shares of our common stock outstanding as of that date. Historical net tangible book value per share represents the amount of total tangible
assets, less total liabilities, divided by the outstanding number of shares of our common stock. Dilution in net tangible book value
per share to new investors represents the difference between the amount per share paid by purchasers of shares of our common stock in
this offering and the net tangible book value per share of our common stock immediately afterwards.
Without
taking into account any other changes in net tangible book value after March 31, 2024, after giving effect to the assumed sale by us
of shares of our common stock in the aggregate amount of $2.4 million at an assumed public offering price of $1.96 per share,
which was the last reported sale price of our common stock on The Nasdaq Capital Market on May 17, 2024, and after deducting offering
commissions and estimated offering expenses payable by us, our as adjusted net tangible book value at March 31, 2024 would have been
approximately $11.1 million, or $2.72 per share. The following table illustrates this per share:
| Assumed public offering price per share | |
| | | |
$ | 1.96 | |
| Historical net tangible book value per share as of March 31, 2024 | |
| 3.11 | | |
| | |
| Decrease in net tangible book value per share attributable to new investors | |
| (0.39 | ) | |
| | |
| As adjusted net tangible book value per share after this offering | |
| | | |
$ | 2.72 | |
| Increase in per share price to new investors purchasing shares in this offering | |
| | | |
$ | 0.76 | |
The
number of shares of our common stock to be outstanding immediately after this offering is based on 2,855,981 shares of common stock outstanding
as of March 31, 2024, and excludes:
| |
● |
6,992
shares of our common stock issuable upon exercise of outstanding options, which have a weighted average exercise price of $364.72
per share; |
| |
|
|
| |
● |
206,006
shares of common stock issuable for restricted stock units (RSUs) (124,529 of which are vested and issuable upon meeting distribution
restrictions); |
| |
|
|
| |
● |
1,778,284
shares of common stock issuable upon exercise of outstanding common warrants at a weighted-average exercise price of $6.17 per share
(of which 1,555,555 are exercisable at $4.50 per share); and |
| |
|
|
| |
● |
35,508
shares of common stock reserved for issuance and available for future grant under our 2019 Omnibus Incentive Plan. |
To
the extent that any of these outstanding options or warrants are exercised at prices per share below the public offering price per share
in this offering or we issue additional shares under our equity incentive plans at prices below the public offering price per share in
this offering, there will be further dilution to new investors.
In
addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient
funds for our current or future operating plans. To the extent that any options or warrants are exercised, new securities are issued
under our equity incentive plans, or we otherwise raise additional capital through the sale of equity or convertible debt securities,
the issuance of these securities could result in further dilution to new investors.
PLAN
OF DISTRIBUTION
We
have entered into a sales agreement (the “Sales Agreement”) with A.G.P (the “Sales Agent”), under which we may
offer and sell up to $2,400,000 of our shares of common stock from time to time through the Sales Agent. Sales of our shares of common
stock, if any, under this prospectus supplement will be made by any method that is deemed to be an “at the market offering”
as defined in Rule 415(a)(4) under the Securities Act or, if expressly authorized by us, in privately negotiated transactions.
Each
time we wish to issue and sell our shares of common stock under the Sales Agreement, we will notify our Sales Agent of the number of
shares to be issued, the dates on which such sales are anticipated to be made, any limitation on the number of shares to be sold in any
one day and any minimum price below which sales may not be made. Once we have instructed our Sales Agent, unless our Sales Agent declines
to accept the terms of such notice, our Sales Agent has agreed to use its commercially reasonable efforts consistent with its normal
trading and sales practices to sell such shares up to the amount specified on such terms. The obligations of our Sales Agent under the
Sales Agreement to sell our shares of common stock are subject to a number of conditions that we must meet.
Until
May 28, 2024, settlement for sales of common stock will occur, unless the parties agree otherwise, on the second business day that is
also a trading day following the date on which any sales were made. After May 28, 2024, settlement for sales of common stock will occur,
unless the parties agree otherwise, on the first business day that is also a trading day following the date on which any sales were made.
Sales of our shares of common stock as contemplated in this prospectus supplement will be settled through the facilities of The Depository
Trust Company or by such other means as we and our Sales Agent may agree upon. There is no arrangement for funds to be received in an
escrow, trust or similar arrangement.
We
will pay our Sales Agent a commission of up to 3.0% of the aggregate gross proceeds we receive from each sale of shares of common
stock. Because there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount,
commissions and proceeds to us, if any, are not determinable at this time. In addition, we have agreed to reimburse our Sales Agent for
the fees and disbursements of its counsel, payable upon execution of the Sales Agreement, in an amount not to exceed $50,000, in addition
to certain ongoing disbursements of its legal counsel. We estimate that the total expenses for the offering, excluding any commissions
or expense reimbursement payable to our Sales Agent under the terms of the Sales Agreement, will be approximately $75,000. The
remaining sale proceeds, after deducting any other transaction fees, will equal our net proceeds from the sale of such shares.
We
will report at least quarterly the number of shares of common stock sold through our Sales Agent under the Sales Agreement, the net proceeds
to us and the compensation paid by us to our Sales Agent in connection with the sales of common stock.
Our
Sales Agent will provide written confirmation to us before the open on The Nasdaq Capital Market on the day following each day on which
our shares of common stock are sold by our Sales Agent under the Sales Agreement. Each confirmation will include the number of shares
sold on that day, the aggregate gross proceeds of such sales and the proceeds to us.
In
connection with the sale of our shares of common stock on our behalf, our Sales Agent will be deemed to be an “underwriter”
within the meaning of the Securities Act, and the compensation of our Sales Agent will be deemed to be underwriting commissions or discounts.
We have agreed to indemnify our Sales Agent against certain civil liabilities, including liabilities under the Securities Act. We have
also agreed to contribute to payments our Sales Agent may be required to make in respect of such liabilities.
The
offering of our shares of common stock pursuant to the Sales Agreement will terminate upon the earlier of (i) the sale of all shares
of common stock subject to the Sales Agreement and (ii) the termination of the sales agreement as permitted therein. We and our Sales
Agent may each terminate the Sales Agreement at any time upon ten trading days’ prior notice.
This
summary of the material provisions of the Sales Agreement does not purport to be a complete statement of its terms and conditions. A
copy of the Sales Agreement is filed as an exhibit to a report filed under the Exchange Act, with the SEC, and is incorporated by reference
into this prospectus supplement and the accompanying prospectus.
The
Sales Agent and its affiliates may in the future provide various investment banking, commercial banking, financial advisory and other
financial services for us and our affiliates, for which services they may in the future receive customary fees. In the course of its
business, the Sales Agent may actively trade our securities for its own accounts or for the accounts of its respective customers, and,
accordingly the Sales Agent may at any time hold long or short positions in such securities.
To
the extent required by Regulation M, the Sales Agent will not engage in any market making activities involving our common stock while
the offering is ongoing under this prospectus supplement.
A
prospectus supplement in electronic format may be made available on a website maintained by our Sales Agent, and our Sales Agent may
distribute the prospectus supplement electronically.
LEGAL
MATTERS
The
validity of the common stock offered by this prospectus supplement will be passed upon for us by Foley & Lardner LLP, Jacksonville,
Florida. Our Sales Agent is being represented by Duane Morris LLP, New York, New York.
EXPERTS
The
consolidated financial statements incorporated in this prospectus supplement by reference from the Company’s Annual Report on Form
10-K for the year ended December 31, 2023 and 2022 have been audited by BD & Company, Inc., an independent registered public accounting
firm, as set forth in their report thereon, included therein, and incorporated herein by reference. Such consolidated financial statements
are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
INCORPORATION
OF CERTAIN DOCUMENTS BY REFERENCE
The
SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information
to you by referring you to those documents. The information that is incorporated by reference is considered to be part of this prospectus
supplement, and the information that we file later with the SEC will automatically update and supersede this information. We incorporate
by reference any future filings we make with the SEC under Section 13(a), 13(c), 14, or 15(d) of the Exchange Act prior to the termination
of this offering and the following documents (other than information in documents that is deemed not to be filed, including the portions
of these documents that are furnished under items 2.02 or Item 7.01 of a Current Report on Form 8-K, including any exhibits included
with such Items):
| |
● |
our
Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 29, 2024; |
| |
|
|
| |
● |
our
Proxy Statement on Schedule 14A for our Annual Meeting of Stockholders, filed April 29, 2024; |
| |
|
|
| |
● |
our
Quarterly Report on Form 10-Q for the quarter ended March 31, 2024, filed on May 10, 2024; |
| |
|
|
| |
● |
our
Current Reports on Form 8-K, filed with the SEC on January 18, 2024, January 25, 2024, January 30, 2024 and February 6, 2024; and |
| |
|
|
| |
● |
the
description of our common stock contained in or incorporated into our Registration Statement on Form 8-A, filed September 17, 2020,
and any amendment or report updating that description. |
Notwithstanding
the foregoing, documents or portions thereof containing information furnished under Items 2.02 and 7.01 of any Current Report on Form
8-K, including the related exhibits under Item 9.01, are not incorporated by reference in this prospectus.
$2,400,000
Common
Stock
PROSPECTUS
SUPPLEMENT
___
__, 2024
A.G.P.
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
| Item
14. |
Other
Expenses of Issuance and Distribution |
Set
forth below are the expenses (other than underwriting discounts and commissions) expected to be incurred in connection with the offering
of the securities registered hereby. Underwriting and other selling discounts and commissions in connection with this offering will be
payable by the selling stockholders. However, the selling stockholders will not bear any portion of the below expenses. With the exception
of the SEC registration fee, the amounts set forth below are estimates.
| SEC registration fee | |
$ | 7,380 | |
| Accounting fees and expenses | |
| * | |
| Legal fees and expenses | |
| * | |
| Miscellaneous | |
| * | |
| Total | |
$ | * | |
| * |
The
above expenses cannot currently be estimated because we have not yet determined the terms or amounts of any securities to be offered
and sold under this registration statement or the number of offerings that will be made under this registration statement. |
| Item
15. |
Indemnification
of Directors and Officers |
Processa
Pharmaceuticals, Inc. is incorporated under the laws of the State of Delaware.
Section
102(b)(7) of the General Corporation Law of the State of Delaware, or the “DGCL,” permits a Delaware corporation to include
a provision in its certificate of incorporation eliminating or limiting the personal liability of directors to the corporation or its
stockholders for monetary damages for breach of fiduciary duty as a director. This provision, however, may not eliminate or limit a director’s
liability (1) for breach of the director’s duty of loyalty to the corporation or its stockholders, (2) for acts or omissions not
in good faith or involving intentional misconduct or a knowing violation of law, (3) under Section 174 of the DGCL, or (4) for any transaction
from which the director derived an improper personal benefit. The amended and restated certificate of incorporation of Processa contains
such a provision.
Section
145(a) of the DGCL provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to
any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than
an action by or in the right of the corporation), by reason of the fact that the person is or was a director, officer, employee or agent
of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation,
partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts
paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding if the person acted
in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with
respect to any criminal action or proceeding, had no reasonable cause to believe the person’s conduct was unlawful.
Section
145(b) of the DGCL provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to
any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason
of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request
of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise,
against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement
of such action or suit if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the
best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue or matter as to which
such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Delaware Court of Chancery
or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but
in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the
Delaware Court of Chancery or such other court shall deem proper.
Section
145(c) of the DGCL provides that to the extent that a present or former director or officer of a corporation has been successful on the
merits or otherwise in defense of any action, suit or proceeding referred to in subsections (a) and (b) of Section 145 of the DGCL, or
in defense of any claim, issue or matter therein, such person shall be indemnified against expenses (including attorneys’ fees)
actually and reasonably incurred by such person in connection therewith.
Section
145(e) of the DGCL permits a Delaware corporation to advance litigation expenses, including attorneys’ fees, incurred by present
and former directors and officers prior to the final disposition of the relevant proceedings. The advancement of expenses to a present
director or officer is conditioned upon receipt of an undertaking by or on behalf of such director or officer to repay the advancement
if it is ultimately determined that such director or officer is not entitled to be indemnified by the corporation. Advancement to former
officers and directors may be conditioned upon such terms and conditions, if any, as the corporation may deem appropriate.
Section
145(g) of the DGCL specifically allows a Delaware corporation to purchase liability insurance on behalf of its directors and officers
and to insure against potential liability of such directors and officers regardless of whether the corporation would have the power to
indemnify such directors and officers under Section 145 of the DGCL.
The
amended and restated certificate of incorporation and the amended and restated bylaws of Processa authorize the corporation to indemnify
its directors and officers to the fullest extent permitted by law.
The
foregoing summaries are necessarily subject to the complete text of the DGCL and Processa’s amended and restated certificate of
incorporation and amended and restated bylaws.
The
exhibits listed in the accompanying Exhibit Index are filed or incorporated by reference as part of this Registration Statement.
| |
a) |
The
undersigned Registrant hereby undertakes: |
| |
1) |
To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| |
(i) |
To
include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| |
|
|
| |
(ii) |
To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end
of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission
(the “Commission”) pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than
20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in
the effective registration statement; and |
| |
|
|
| |
(iii) |
To
include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement; |
provided,
however, that paragraphs (i), (ii) and (iii) do not apply if the information required to be included in a post-effective amendment
by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or Section
15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form
of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
| |
2) |
That,
for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof. |
| |
|
|
| |
3) |
To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering. |
| |
|
|
| |
4) |
That,
for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
| |
(i) |
If
the Registrant is relying on Rule 430B: |
| |
(A) |
Each
prospectus filed by the Registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the
date the filed prospectus was deemed part of and included in the registration statement; and |
| |
|
|
| |
(B) |
Each
prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5) or (b)(7) as part of a registration statement in reliance on Rule
430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii) or (x) for the purpose of providing the information required
by Section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the
earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities
in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is
at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities
in the registration statement to which the prospectus relates, and the offering of such securities at that time shall be deemed to
be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior
to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part
of the registration statement or made in any such document immediately prior to such effective date. |
| |
(ii) |
If
the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating
to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A,
shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided,
however, that no statement made in a registration statement or prospectus that is part of the registration statement or made
in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the
registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement
that was made in the registration statement or prospectus that was part of the registration statement or made in any such document
immediately prior to such date of first use. |
| |
5) |
That,
for the purpose of determining liability of the Registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of the securities: |
| |
(i) |
The
undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this registration
statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold
to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to the purchaser and
will be considered to offer or sell such securities to such purchaser: |
| |
(A) |
Any
preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule
424; |
| |
|
|
| |
(B) |
Any
free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by
the undersigned Registrant; |
| |
|
|
| |
(C) |
The
portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant
or its securities provided by or on behalf of the undersigned Registrant; and |
| |
|
|
| |
(D) |
Any
other communication that is an offer in the offering made by the undersigned Registrant to the purchaser. |
| |
(ii) |
The
undersigned Registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing
of its annual report pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing
of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated
by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered
therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| |
|
|
| |
(iii) |
Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling
persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion
of the Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred
or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding)
is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will,
unless in the opinion of its counsel the issue has been settled by controlling precedent, submit to a court of appropriate jurisdiction
the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed
by the final adjudication of such issue. |
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all
of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned,
thereunto duly authorized, in Hanover, Maryland, on May 21, 2024.
| |
Processa
Pharmaceuticals, Inc. |
| |
|
|
| |
By: |
/s/
George Ng |
| |
|
George
Ng |
| |
|
Chief
Executive Officer |
KNOW
ALL MEN BY THESE PRESENTS, that each of the undersigned hereby constitutes and appoints David Young and George Ng and each of them, the
true and lawful attorneys-in-fact of the undersigned, with full power of substitution and resubstitution, for him or her and in his or
her name, place and stead, in any and all capacities, to sign this registration statement and any or all amendments to this registration
statement, including post-effective amendments, and registration statements filed pursuant to 462(b) under the Securities Act, and to
file or cause to be filed the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange
Commission, granting unto said attorneys-in-fact full power and authority to do and perform each and every act and thing requisite and
necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby
ratifying and confirming all that said attorneys or attorneys-in-fact or any of them or their substitute or substitutes may lawfully
do or cause to be done by virtue hereof.
Pursuant
to the requirements of the Securities Act, this registration statement has been signed by the following persons on May 21, 2024
in the capacities indicated.
| Signature |
|
Title |
| |
|
|
| /s/
George Ng |
|
Chief
Executive Officer (Principal Executive Officer) |
| George
Ng |
|
| |
|
|
| /s/ James Stanker |
|
Chief
Financial Officer |
| James
Stanker |
|
(Principal
Financial Officer and Principal |
| |
|
Accounting
Officer) |
| |
|
|
| /s/
Justin Yorke |
|
Director |
| Justin
Yorke |
|
| |
|
|
| /s/
David Young, Pharm.D, Ph.D. |
|
President
of Research and Development and Director |
| David
Young, Pharm.D, Ph.D. |
|
| |
|
|
| /s/
Khoso Baluch |
|
Director |
| Khoso
Baluch |
|
| |
|
|
| /s/
James Neal |
|
Director |
| James
Neal |
|
| |
|
|
| /s/
Geraldine Pannu |
|
Director |
| Geraldine
Pannu |
|
EXHIBIT
INDEX
| Exhibit
Number |
|
Exhibit
Description |
| 1.1** |
|
Form
of Underwriting Agreement |
| |
|
|
| 1.2* |
|
Sales Agreement between the Company and A.G.P./Alliance Global Partners |
| |
|
|
| 3.1 |
|
Fourth Amended and Restated Certificate of Incorporation of Heatwurx, Inc. (incorporated herein by reference to Exhibit 3.1 to the Form S-1/A filed with the SEC on September 16, 2020) |
| |
|
|
| 3.1.1 |
|
Amendment to Fourth Amended and Restated Certificate of Incorporation of Heatwurx, Inc. (incorporated herein by reference to Exhibit 3.1.1 to the Form S-1/A filed with the SEC on September 16, 2020) |
| |
|
|
| 3.1.2 |
|
Certificate of Amendment to Fourth Amended and Restated Certificate of Incorporation dated August 8, 2019 (incorporated herein by reference to Exhibit 3 to Form 10-Q filed on August 14, 2019)
|
| |
|
|
| 3.1.3 |
|
Certificate of Amendment to Fourth Amended and Restated Certificate of Incorporation of Processa Pharmaceuticals, Inc. dated June 25, 2020 (incorporated by reference to Exhibit 3.1.4 to Form S-1 filed on September 17, 2020) |
| |
|
|
| 3.1.4 |
|
Certificate of Amendment to Fourth Amended and Restated Certificate of Incorporation dated January 1, 2022 (incorporated by reference to Exhibit 3.1 to Form 8-K filed on January 6, 2022) |
| |
|
|
| 3.1.5 |
|
Certificate of Amendment to the Fourth Amended and Restated Certificate of Incorporation of Processa Pharmaceuticals, Inc. (incorporated by reference to Exhibit 3.1 to Form 8-K filed on June 29, 2023) |
| |
|
|
| 3.1.6 |
|
Certificate of Amendment to the Fourth Amended and Restated Certificate of Incorporation of Processa Pharmaceuticals, Inc. (incorporated by reference to Exhibit 3.1.6 to Form S-1/A filed on January 22, 2024) |
| |
|
|
| 3.2 |
|
Amended and Restated Bylaws (incorporated herein by reference to Exhibit 3.2 to the Form S-1/A filed with the SEC on September 16, 2020) |
| |
|
|
| 4.1 |
|
Description of Securities Registered under Section 12 of the Securities Exchange Act of 1934, as amended (incorporated herein by reference to Exhibit 4.5 to the Form 10-K filed with the SEC on March 29, 2024) |
| |
|
|
| 4.2 |
|
Specimen of Common Stock Certificate (incorporated herein by reference to Exhibit 4.1 to the Form S-1/A filed with the SEC on September 16, 2020) |
| |
|
|
| 4.3 |
|
Form of Common Warrant (incorporated by reference to Exhibit 4.1 to Exhibit 8-K Filed on January 30, 2024) |
| |
|
|
| 4.4 |
|
Form of Placement Agent Warrant (incorporated by reference to Exhibit 4.3 to Form 8-K filed on January 30, 2024) |
| |
|
|
| 4.5 |
|
Warrant issued to Spartan Capital, Inc. (incorporated herein by reference to Exhibit 4.4 to the Form 10-K filed with the SEC on March 29, 2024) |
| |
|
|
| 4.6** |
|
Form
of Certificate of Designations |
| |
|
|
| 4.7** |
|
Form
of Unit |
| |
|
|
| 4.8** |
|
Form
of Warrant |
| |
|
|
| 5.1* |
|
Legal Opinion of Foley & Lardner LLP |
| |
|
|
| 5.2* |
|
Legal Opinion of Foley & Lardner LLP as to the sale agreement prospectus |
| |
|
|
| 23.1* |
|
Consent of BD & Company, Inc. |
| |
|
|
| 23.2* |
|
Consent of Foley & Lardner LLP (included in Exhibit 5.1) |
| |
|
|
| 23.3* |
|
Consent of Foley & Lardner LLP as to the sales agreement prospectus (included in Exhibit 5.2) |
| |
|
|
| 24.1* |
|
Powers of Attorney (included on signature page hereto) |
| |
|
|
| 107* |
|
Filing Fee Table |
| |
|
|
| * |
Filed
herewith. |
| ** |
To
be filed by amendment or as an exhibit to a document incorporated by reference or deemed to be incorporated by reference in this
registration statement, including a current report on Form 8-K, in connection with the offering of any securities, as appropriate. |
Exhibit
1.2
PROCESSA
PHARMACEUTICALS, INC.
COMMON
STOCK
SALES
AGREEMENT
May
21, 2024
A.G.P./Alliance
Global Partners
590
Madison Avenue
New
York, NY 10022
Ladies
and Gentlemen:
Processa
Pharmaceuticals, Inc., a Delaware corporation (the “Company”), confirms its agreement (this “Agreement”)
with A.G.P./Alliance Global Partners (the “Sales Agent”), as follows:
1.
Issuance and Sale of Shares. The Company agrees that, from time to time during the term of this Agreement, on the terms and subject
to the conditions set forth herein, it may issue and sell to or through the Sales Agent, acting as agent or principal, shares of the
Company’s common stock, par value $0.0001 per share (the “Common Stock”), subject to the limitations
set forth in Section 3(b) hereof. The issuance and sale of shares of Common Stock to or through the Sales Agent will be effected
pursuant to the Registration Statement (as defined below) filed by the Company, when such Registration Statement is declared effective
under the Securities Act (as defined below) by the U.S. Securities and Exchange Commission (the “Commission”).
On
the date of this Agreement, the Company has filed, or will file, in accordance with the provisions of the Securities Act of 1933, as
amended, and the rules and regulations thereunder (collectively, the “Securities Act”), with the Commission,
a shelf registration statement on Form S-3, including a base prospectus, relating to certain securities, including the shares of Common
Stock, to be issued from time to time by the Company, and which incorporates by reference documents that the Company has filed or will
file in accordance with the provisions of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder (collectively,
the “Exchange Act”). The Company has prepared a prospectus supplement specifically relating to the offering
of Common Stock pursuant to this Agreement included as part of such registration statement (the “ATM Prospectus”).
As soon as practicable following the date that such registration statement is declared effective, the Company will furnish to the Sales
Agent, for use by the Sales Agent, copies of the ATM Prospectus included as part of such registration statement, relating to the Placement
Shares (as defined below). Except where the context otherwise requires, such registration statement, as amended when it becomes effective,
and any post-effective amendment thereto, including all documents filed as part thereof or incorporated by reference therein, and including
any information contained in a Prospectus (as defined below) subsequently filed with the Commission pursuant to Rule 424(b) under the
Securities Act or deemed to be a part of such registration statement pursuant to Rule 430B or 462(b) of the Securities Act, is herein
called the “Registration Statement.” The base prospectus, including all documents incorporated therein by reference
(to the extent such information has not been superseded or modified in accordance with Rule 412 under the Securities Act (as qualified
by Rule 430B(g) of the Securities Act), and the ATM Prospectus, including all documents incorporated therein by reference (to the extent
such information has not been superseded or modified in accordance with Rule 412 under the Securities Act (as qualified by Rule 430B(g)
of the Securities Act), each of which is included in the Registration Statement, as it or they may be supplemented by any additional
prospectus supplement, in the form in which such prospectus and/or ATM Prospectus have most recently been filed by the Company with the
Commission pursuant to Rule 424(b) under the Securities Act, together with any “issuer free writing prospectus” (“Issuer
Free Writing Prospectus”), as defined in Rule 433 of the Securities Act (“Rule 433”), relating
to the Placement Shares that (i) is required to be filed with the Commission by the Company or (ii) is exempt from filing pursuant to
Rule 433(d)(5)(i), in each case in the form filed or required to be filed with the Commission or, if not required to be filed, in the
form retained in the Company’s records pursuant to Rule 433(g), is herein called the “Prospectus.” Any
reference herein to the Registration Statement, the Prospectus or any amendment or supplement thereto shall be deemed to refer to and
include the documents incorporated by reference therein, and any reference herein to the terms “amend,” “amendment”
or “supplement” with respect to the Registration Statement or the Prospectus shall be deemed to refer to and include the
filing after the execution hereof of any document with the Commission deemed to be incorporated by reference therein. For purposes of
this Agreement, all references to the Registration Statement, the Prospectus or to any amendment or supplement thereto shall be deemed
to include any copy filed with the Commission pursuant to either the Electronic Data Gathering Analysis and Retrieval System, or if applicable,
the Interactive Data Electronic Applications (collectively “EDGAR”).
2.
Placements. Each time that the Company wishes to issue and sell the Common Stock through the Sales Agent, as agent, hereunder
(each, a “Placement”), it will notify the Sales Agent by email notice (or other method mutually agreed to in
writing by the parties) (a “Placement Notice”) containing the parameters in accordance with which it desires
the Common Stock to be sold, which shall at a minimum include the number of shares of Common Stock to be issued (the “Placement
Shares”), the time period during which sales are requested to be made, any limitation on the number of shares of Common
Stock that may be sold in any one Trading Day (as defined in Section 3) and any minimum price below which sales may not be made,
a form of which containing such minimum sales parameters necessary is attached hereto as Schedule 1. The Placement Notice
shall originate from any of the individuals from the Company set forth on Schedule 2 (with a copy to each of the other
individuals from the Company listed on such schedule), and shall be addressed to each of the individuals from the Sales Agent set forth
on Schedule 2, as such Schedule 2 may be amended from time to time. The Placement Notice shall be effective
upon receipt by the Sales Agent unless and until (i) in accordance with the notice requirements set forth in Section 4, the Sales
Agent declines to accept the terms contained therein for any reason, in its sole discretion, (ii) the entire amount of the Placement
Shares have been sold, (iii) in accordance with the notice requirements set forth in Section 4, the Company suspends or terminates
the Placement Notice, (iv) the Company issues a subsequent Placement Notice with parameters superseding those on the earlier dated Placement
Notice, or (v) the Agreement has been terminated under the provisions of Section 11. The amount of any discount, commission or
other compensation to be paid by the Company to the Sales Agent in connection with the sale of the Placement Shares through the Sales
Agent, as agent, shall be as set forth in Schedule 3. It is expressly acknowledged and agreed that neither the Company
nor the Sales Agent will have any obligation whatsoever with respect to a Placement or any Placement Shares unless and until the Company
delivers a Placement Notice to the Sales Agent and the Sales Agent does not decline such Placement Notice pursuant to the terms set forth
above, and then only upon the terms specified therein and herein. In the event of a conflict between the terms of this Agreement and
the terms of a Placement Notice, the terms of the Placement Notice will control.
3.
Sale of Placement Shares by the Sales Agent.
(a)
Subject to the terms and conditions herein set forth, upon the Company’s issuance of a Placement Notice, and unless the sale of
the Placement Shares described therein has been declined, suspended, or otherwise terminated in accordance with the terms of this Agreement,
the Sales Agent, as agent for the Company, will use its commercially reasonable efforts consistent with its normal trading and sales
practices and applicable state and federal laws, rules and regulations and the rules of The Nasdaq Capital Market (the “Exchange”),
for the period specified in the Placement Notice, to sell such Placement Shares up to the amount specified by the Company in, and otherwise
in accordance with the terms of such Placement Notice. If acting as agent hereunder, the Sales Agent will provide written confirmation
to the Company (including by email correspondence to each of the individuals of the Company set forth on Schedule 2, if
receipt of such correspondence is actually acknowledged by any of the individuals to whom the notice is sent, other than via auto-reply)
no later than the opening of the Trading Day (as defined below) immediately following the Trading Day on which it has made sales of Placement
Shares hereunder setting forth the number of Placement Shares sold on such day, the volume-weighted average price of the Placement Shares,
the compensation payable by the Company to the Sales Agent pursuant to Section 2 with respect to such sales, and the Net Proceeds
(as defined below) payable to the Company, with an itemization of the deductions made by the Sales Agent (as set forth in Section
5(a)) from the gross proceeds that it receives from such sales. Subject to the terms of the Placement Notice, the Sales Agent may
sell Placement Shares by any method permitted by law deemed to be an “at the market” offering as defined in Rule 415 under
the Securities Act. The Company acknowledges and agrees that (i) there can be no assurance that the Sales Agent will be successful in
selling Placement Shares, (ii) the Sales Agent will incur no liability or obligation to the Company or any other person or entity if
it does not sell Placement Shares for any reason other than a failure by the Sales Agent to use its commercially reasonable efforts consistent
with its normal trading and sales practices and applicable law and regulations to sell such Placement Shares as required under this Agreement
and (iii) the Sales Agent shall be under no obligation to purchase Placement Shares on a principal basis pursuant to this Agreement,
except as otherwise agreed by the Sales Agent and the Company in writing and expressly set forth in a Placement Notice. For the purposes
hereof, “Trading Day” means any day on which the Company’s Common Stock is purchased and sold on the
principal market on which the Common Stock is listed or quoted.
(b)
Under no circumstances shall the Company cause or request the offer or sale of any Placement Shares if, after giving effect to the sale
of such Placement Shares, the aggregate number or gross sales proceeds of Placement Shares sold pursuant to this Agreement would exceed
the lesser of: (i) the number or dollar amount of shares of Common Stock registered pursuant to the Registration Statement pursuant to
which the offering hereunder is being made, (ii) the number of authorized but unissued and unreserved shares of Common Stock, (iii) the
number or dollar amount of shares of Common Stock permitted to be offered and sold by the Company under Form S-3 (including General Instruction
I.B.6. of Form S-3, if and for so long as applicable), (iv) the number or dollar amount of shares of Common Stock authorized from time
to time to be issued and sold under this Agreement by the Company’s board of directors, a duly authorized committee thereof or
a duly authorized executive committee, and notified to the Sales Agent in writing, or (v) the number or dollar amount of shares of Common
Stock for which the Company has filed the ATM Prospectus or other prospectus supplement specifically relating to the offering of the
Placement Shares pursuant to this Agreement. Under no circumstances shall the Company cause or request the offer or sale of any Placement
Shares pursuant to this Agreement at a price lower than the minimum price authorized from time to time by the Company’s board of
directors, a duly authorized committee thereof or a duly authorized executive committee, and notified to the Sales Agent in writing.
Notwithstanding anything to the contrary contained herein, the parties hereto acknowledge and agree that compliance with the limitations
set forth in this Section 3(b) on the number or dollar amount of Placement Shares that may be issued and sold under this Agreement
from time to time shall be the sole responsibility of the Company, and that the Sales Agent shall have no obligation in connection with
such compliance.
(c)
During the term of this Agreement, neither the Sales Agent nor any of its affiliates or subsidiaries shall engage in (i) any short sale
of any security of the Company or (ii) any sale of any security of the Company that the Sales Agent does not own or any sale which is
consummated by the delivery of a security of the Company borrowed by, or for the account of, the Sales Agent. During the term of this
Agreement and notwithstanding anything to the contrary herein, the Sales Agent agrees that in no event will the Sales Agent or its affiliates
engage in any market making, bidding, stabilization or other trading activity with regard to the Common Stock or related derivative securities
if such activity would be prohibited under Regulation M or other anti-manipulation rules under the Exchange Act.
4.
Suspension of Sales.
(a)
The Company or the Sales Agent may, upon notice to the other party in writing (including by email correspondence to each of the individuals
of the other party set forth on Schedule 2, if receipt of such correspondence is actually acknowledged by any of the individuals
to whom the notice is sent, other than via auto-reply) or by telephone (confirmed immediately by verifiable facsimile transmission or
email correspondence to each of the individuals of the other party set forth on Schedule 2), suspend any sale of Placement
Shares for a period of time (a “Suspension Period”); provided, however, that such suspension
shall not affect or impair either party’s obligations with respect to any Placement Shares sold hereunder prior to the receipt
of such notice. Each of the parties agrees that no such notice under this Section 4 shall be effective against the other unless
it is made to one of the individuals named on Schedule 2 hereto, as such schedule may be amended from time to time. During
a Suspension Period, the Company shall not issue any Placement Notices and the Sales Agent shall not sell any Placement Shares hereunder.
The party that issued a suspension notice shall notify the other party in writing of the Trading Day on which the Suspension Period shall
expire not later than twenty-four (24) hours prior to such Trading Day.
(b)
Notwithstanding any other provision of this Agreement, during any period in which the Company is in possession of material non-public
information, the Company and the Sales Agent agree that (i) no sale of Placement Shares will take place, (ii) the Company shall not request
the sale of any Placement Shares, and (iii) the Sales Agent shall not be obligated to sell or offer to sell any Placement Shares.
5.
Settlement.
(a)
Settlement of Placement Shares. Unless otherwise specified in the applicable Placement Notice, settlement for sales of Placement
Shares will occur (i) prior to May 28, 2024, on the second Trading Day following the date on which such sales are made and (ii) beginning
May 28, 2024, on the first Trading Day following the date on which such sales are made (each such day, in (i) and (ii), a “Settlement
Date”). The amount of proceeds to be delivered to the Company on a Settlement Date against receipt of the Placement Shares
sold (the “Net Proceeds”) will be equal to the aggregate sales price received by the Sales Agent at which such
Placement Shares were sold, after deduction for (i) the Sales Agent’s discount, commission or other compensation for such sales
payable by the Company pursuant to Section 2 hereof, and (ii) any transaction fees, trading expenses or execution fees imposed
by any clearing organization or any governmental or self-regulatory organization and any other fees or expenses incurred by the Sales
Agent in respect of such sales.
(b)
Delivery of Placement Shares. On or before each Settlement Date, the Company will, or will cause its transfer agent to, electronically
transfer the Placement Shares being sold by crediting the Sales Agent’s or its designee’s account (provided the Sales Agent
shall have given the Company written notice of such designee prior to the Settlement Date) at The Depository Trust Company through its
Deposit and Withdrawal at Custodian System or by such other means of delivery as may be mutually agreed upon by the parties hereto which
in all cases shall be freely tradable, transferable, registered shares in good deliverable form. On each Settlement Date, the Sales Agent
will deliver the related Net Proceeds in same day funds to an account designated by the Company on, or prior to, the Settlement Date.
The Company agrees that if the Company, or its transfer agent (if applicable), defaults in its obligation to deliver duly authorized
Placement Shares on a Settlement Date, through no fault of the Sales Agent, the Company agrees that in addition to and in no way limiting
the rights and obligations set forth in Section 9(a) (Indemnification and Contribution) hereto, the Company will (i) hold the
Sales Agent, its directors, officers, members, partners, employees and agents of the Sales Agent, each broker dealer affiliate of the
Sales Agent, and each person, if any, who (A) controls the Sales Agent within the meaning of Section 15 of the Securities Act or Section
20 of the Exchange Act or (B) is controlled by or is under common control with the Sales Agent (each, a “Sales Agent Affiliate”),
and the Sales Agent’s clearing organization, harmless against any loss, claim, damage, or reasonable and documented expense (including
reasonable legal fees and expenses), as incurred, arising out of or in connection with such default by the Company or its transfer agent
(if applicable) and (ii) pay to the Sales Agent any commission, discount, or other compensation to which it would otherwise have been
entitled absent such default.
6.
Representations and Warranties of the Company. The Company represents and warrants to, and agrees with, the Sales Agent that as
of each Applicable Time (as defined in Section 22(a)), unless such representation, warranty or agreement specifies a different
time or times:
(a)
Compliance with Registration Requirements. As of each Applicable Time other than the date of this Agreement, the Registration
Statement and any Rule 462(b) Registration Statement have been declared effective by the Commission under the Securities Act. The Company
has complied to the Commission’s satisfaction with all requests of the Commission for additional or supplemental information related
to the Registration Statement and the Prospectus. No stop order suspending the effectiveness of the Registration Statement or any Rule
462(b) Registration Statement is in effect and no proceedings for such purpose have been instituted or are pending or, to the knowledge
of the Company, are contemplated or threatened by the Commission. The Registration Statement and, assuming no act or omission on the
part of the Sales Agent that would make such statements untrue, the offer and sale of the Placement Shares as contemplated hereby meet
the requirements of Rule 415 under the Securities Act and comply in all material respects with said Rule. In the section entitled “Plan
of Distribution” in the ATM Prospectus, the Company has named A.G.P./Alliance Global Partners as an agent that the Company has
engaged in connection with the transactions contemplated by this Agreement. The Company was not and is not an “ineligible issuer”
as defined in Rule 405 under the Securities Act.
(b)
No Misstatement or Omission. The Registration Statement and any post-effective amendment thereto, at the time it became or becomes
effective, complied or will comply in all material respects with the Securities Act. The Prospectus, and any amendment or supplement
thereto, on the date of such Prospectus or amendment or supplement, complied or will comply in all material respects with the Securities
Act. The Registration Statement and any post-effective amendment thereto, at the time it became or becomes effective, did not and will
not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make
the statements therein not misleading. The Prospectus, as amended or supplemented, as of its date, did not and, as of each Point of Sale
and each Settlement Date, will not contain any untrue statement of a material fact or omit to state a material fact necessary in order
to make the statements therein, in the light of the circumstances under which they were made, not misleading. The representations and
warranties set forth in the two immediately preceding sentences do not apply to statements in or omissions from the Registration Statement
or any post-effective amendment thereto, or the Prospectus, or any amendments or supplements thereto, made in reliance upon and in conformity
with information relating to the Sales Agent furnished to the Company in writing by the Sales Agent expressly for use therein. “Point
of Sale” means, for a Placement, the time at which an acquiror of Placement Shares entered into a contract, binding upon
such acquiror, to acquire such Placement Shares.
(c)
Offering Materials Furnished to the Sales Agent. Copies of the Registration Statement, the Prospectus, and all amendments or supplements
thereto and all documents incorporated by reference therein that were filed with the Commission on or prior to the date of this Agreement,
have been delivered, or are publicly available through EDGAR, to the Sales Agent. Each Prospectus delivered to the Sales Agent for use
in connection with the sale of the Placement Shares pursuant to this Agreement will be identical to the version of such Prospectus filed
with the Commission via EDGAR, except to the extent permitted by Regulation S-T.
(d)
Distribution of Offering Material By the Company. The Company has not distributed and will not distribute, prior to the completion
of the Sales Agent’s distribution of the Placement Shares, any offering material in connection with the offering and sale of the
Placement Shares other than the Prospectus or the Registration Statement.
(e)
The Sales Agreement. This Agreement has been duly authorized, executed and delivered by the Company, and constitutes a valid,
legal, and binding obligation of the Company, enforceable against the Company in accordance with its terms, except as rights to indemnity
hereunder may be limited by federal or state securities laws and except as such enforceability may be limited by bankruptcy, insolvency,
reorganization, moratorium or similar laws affecting the rights of creditors generally, and subject to general principles of equity.
The Company has full corporate power and authority to enter into this Agreement and to authorize, issue and sell the Placement Shares
as contemplated by this Agreement. This Agreement conforms in all material respects to the descriptions thereof in the Registration Statement
and the Prospectus.
(f)
Authorization of the Placement Shares. The Placement Shares, when issued and paid for as contemplated herein, will be validly
issued, fully paid and nonassessable, will be issued in compliance with all applicable securities laws, and will be free of preemptive,
registration or similar rights, and will conform to the description of the Common Stock contained in the Registration Statement and the
Prospectus.
(g)
No Applicable Registration or Other Similar Rights. There are no persons with registration or other similar rights to have any
equity or debt securities registered for sale under the Registration Statement or included in the offering contemplated by this Agreement,
except for such rights as have been duly waived.
(h)
No Material Adverse Change. Except as otherwise disclosed in the Prospectus, subsequent to the respective dates as of which information
is given in the Prospectus: (i) there has been no material adverse change in the business, properties, prospects, operations, condition
(financial or otherwise) or results of operations of the Company (any such change is called a “Material Adverse Change”),
which, individually or in the aggregate, has had or would reasonably be expected to result in a Material Adverse Change; (ii) the Company
has not incurred any material liability or obligation, indirect, direct or contingent, not in the ordinary course of business nor entered
into any material transaction or agreement not in the ordinary course of business; (iii) there has been no dividend or distribution of
any kind declared, paid or made by the Company; (iv) no officer or director of the Company has resigned from any position with the Company;
and (v) there has not been any Material Adverse Change in the Company’s long-term debt.
(i)
Independent Accountants. To the knowledge of the Company, BD & Company, Inc., whose report is filed with the Commission and
included or incorporated by reference in the Registration Statement and the Prospectus, is an independent registered public accounting
firm as required by the Securities Act and the Public Company Accounting Oversight Board.
(j)
Financial Statements. The financial statements filed with the Commission as a part of the Registration Statement and included
in the Prospectus, together with the related notes and schedules, present fairly, in all material respects, the consolidated financial
position of the Company and its subsidiaries as of and at the dates indicated and the results of their operations and cash flows for
the periods specified. Such financial statements and supporting schedules have been prepared in conformity, in all material respects,
with U.S. generally accepted accounting principles (“GAAP”) applied on a consistent basis throughout the periods involved,
except as may be expressly stated in the related notes thereto. No other financial statements or supporting schedules are required to
be included in or incorporated in the Registration Statement.
(k)
Forward-Looking Statements. No forward-looking statement (within the meaning of Section 27A of the Securities Act and Section
21E of the Exchange Act) contained in the Registration Statement or the Prospectus has been made or reaffirmed by the Company without
a reasonable basis or has been disclosed by the Company other than in good faith.
(l)
Statistical and Marketing-Related Data. The statistical and market-related data included in each of the Registration Statement
and the Prospectus are based on or derived from sources that the Company reasonably and in good faith believes are reliable and accurate
or represent the Company’s good faith estimates that are made on the basis of data derived from such sources.
(m)
XBRL. The interactive data in eXtensible Business Reporting Language included or incorporated by reference in the Registration
Statement fairly presents the information called for in all material respects and has been prepared in accordance with the Commission’s
rules and guidelines applicable thereto.
(n)
Incorporation and Good Standing of the Company. The Company is a corporation duly incorporated and validly existing under the
laws of the State of Delaware. The Company has requisite corporate power to carry on its business as described in the Prospectus. The
Company is duly qualified to transact business and is in good standing in all jurisdictions in which the conduct of its business requires
such qualification; except where the failure to be so qualified or to be in good standing would not result in a Material Adverse Change.
The Company does not own or control, directly or indirectly, any corporation, association or other entity other than the subsidiaries
listed in Exhibit 21.1 to the Company’s Annual Report on Form 10-K for the most recently ended fiscal year and other than (i) those
subsidiaries not required to be listed on Exhibit 21.1 by Item 601 of Regulation S-K under the Exchange Act and (ii) those subsidiaries
formed since the last day of the most recently ended fiscal year.
(o)
Capital Stock Matters. All issued and outstanding securities of the Company issued prior to the transactions contemplated by this
Agreement have been duly authorized and validly issued and are fully paid and non-assessable; the holders thereof have no rights of rescission
with respect thereto, and are not subject to personal liability by reason of being such holders; and none of such securities were issued
in violation of the preemptive rights of any holders of any security of the Company or similar contractual rights granted by the Company.
The authorized shares of Common Stock conform in all material respects to all statements relating thereto contained in the Registration
Statement and the Prospectus. The offers and sales of the outstanding shares of Common Stock were at all relevant times either registered
under the Securities Act and the applicable state securities or “blue sky” laws or, based in part on the representations
and warranties of the purchasers of such shares, exempt from such registration requirements. The description of the Company’s stock
option, stock bonus and other stock plans or arrangements, and the options or other rights granted thereunder, as described in the Registration
Statement and the Prospectus, accurately and fairly present, in all material respects, the information required to be shown with respect
to such plans, arrangements, options and rights.
(p)
Non-Contravention of Existing Instruments; No Further Authorizations or Approvals Required. The Company’s execution, delivery
and performance of this Agreement and consummation of the transactions contemplated hereby or by the Registration Statement and the Prospectus
(including the issuance and sale of the Placement Shares and the use of the proceeds from the sale of the Placement Shares as described
in the Prospectus under the caption “Use of Proceeds”) will not (A) result in a material violation of any existing applicable
law, rule, regulation, judgment, order or decree of any Governmental Entity as of the date hereof, (B) conflict with, result in any violation
or breach of, or constitute a default (or an event that with notice or lapse of time or both would become a default) under, or give to
others any right of termination, amendment, acceleration or cancellation (with or without notice, lapse of time or both) (a “Default
Acceleration Event”) of, any agreement, lease, credit facility, debt, note, bond, mortgage, indenture or other instrument
(“Contract”) or obligation or other understanding to which the Company is a party or by which any property
or asset of the Company is bound or affected, except to the extent that such conflict, default, or Default Acceleration Event is not
reasonably likely to result in a Material Adverse Change, or (C) result in a breach or violation of any of the terms and provisions of,
or constitute a default under, the Company’s certificate of incorporation (as the same may be amended or restated from time to
time) or bylaws (as the same may be amended or restated from time to time). The Company is not in violation, breach or default under
its certificate of incorporation (as the same may be amended or restated from time to time) or bylaws (as the same may be amended or
restated from time to time). Except as otherwise disclosed in the Prospectus, neither the Company nor, to its knowledge, any other party
is in violation, breach or default of any Contract that has resulted in or could reasonably be expected to result in a Material Adverse
Change. Each approval, consent, order, authorization, designation, declaration or filing by or with any regulatory, administrative or
other governmental body necessary in connection with the execution and delivery by the Company of this Agreement and the performance
of the Company of the transactions herein contemplated has been obtained or made and is in full force and effect, except (i) with respect
to any Applicable Time at which the Sales Agent would not be able to rely on Rule 5110(h)(1)(C) of the Financial Industry Regulatory
Authority, Inc. (“FINRA”), such additional steps as may be required by FINRA, (ii) filings with the Commission
required under the Securities Act or the Exchange Act, or filings with the Exchange pursuant to the rules and regulations of the Exchange,
in each case that are contemplated by this Agreement to be made after the date of this Agreement, and (iii) such additional steps as
may be necessary to qualify the Common Stock for sale by the Sales Agent under state securities or Blue Sky laws.
(q)
No Material Actions or Proceedings. Except as otherwise disclosed in the Prospectus, there is no action, suit, proceeding, inquiry,
arbitration, investigation, litigation or governmental proceeding pending or, to the Company’s knowledge, threatened against, or
involving the Company or, to the Company’s knowledge, any executive officer or director, which has not been disclosed in the Registration
Statement and the Prospectus which is required to be disclosed, except as would not, individually or in the aggregate, reasonably be
expected to have a Material Adverse Change.
(r)
Labor Disputes. No labor dispute with the employees of the Company exists or, to the knowledge of the Company, is imminent. The
Company is not aware that any key employee or significant group of employees of the Company plans to terminate employment with the Company,
except that the Company’s Chief Financial Officer has expressed an intent to retire but has not made any definitive plans or arrangements
with respect thereto.
(s)
Compliance with Certain Applicable Laws. The Company: (A) is and at all times has been in material compliance with all statutes,
rules and regulations applicable to the ownership, testing, development, manufacture, packaging, processing, use, distribution, marketing,
labeling, promotion, sale, offer for sale, storage, import, export or disposal of any product under development, manufactured or distributed
by the Company (“Applicable Laws”), (b) have not received any Form 483 from the United States Food and Drug
Administration of the U.S. Department of Health and Human Services (the “FDA”), notice of adverse finding,
warning letter, or other written correspondence or notice from the FDA, the European Medicines Agency (the “EMA”),
or any other federal, state, local or foreign governmental or regulatory authority alleging or asserting material noncompliance with
any Applicable Laws or any licenses, certificates, approvals, clearances, authorizations, permits and supplements or amendments thereto
required by any such Applicable Laws (“Authorizations”), which would, individually or in the aggregate, result
in a Material Adverse Change; (C) possess all material Authorizations and such Authorizations are valid and in full force and effect
and neither the Company nor the Subsidiaries is in material violation of any term of any such Authorizations; (D) have not received written
notice of any claim, action, suit, proceeding, hearing, enforcement, investigation, arbitration or other action from the FDA, the EMA,
or any other federal, state, local or foreign governmental or regulatory authority or third party alleging that any Company product,
operation or activity is in material violation of any Applicable Laws or Authorizations and has no knowledge that the FDA, the EMA, or
any other federal, state, local or foreign governmental or regulatory authority or third party is considering any such claim, litigation,
arbitration, action, suit, investigation or proceeding against the Company; (E) have not received written notice that the FDA, EMA, or
any other federal, state, local or foreign governmental or regulatory authority has taken, is taking or intends to take action to limit,
suspend, modify or revoke any material Authorizations and has no knowledge that the FDA, EMA, or any other federal, state, local or foreign
governmental or regulatory authority is considering such action; and (F) have filed, obtained, maintained or submitted all reports, documents,
forms, notices, applications, records, claims, submissions and supplements or amendments as required by any Applicable Laws or Authorizations
except where the failure to file such reports, documents, forms, notices, applications, records, claims, submissions and supplements
or amendments would not result in a Material Adverse Change, and that all such reports, documents, forms, notices, applications, records,
claims, submissions and supplements or amendments were materially complete and correct on the date filed (or were corrected or supplemented
by a subsequent submission).
(t)
Compliance Program. The Company has established and administers a compliance program applicable to the Company, to assist the
Company and the directors, officers and employees of the Company in complying with applicable regulatory guidelines (including, without
limitation, those administered by the FDA, the EMA, and any other foreign, federal, state or local governmental or regulatory authority
performing functions similar to those performed by the FDA or EMA); except the Company does not have an independent QA/compliance function
as party of its compliance program and except where such noncompliance would not reasonably be expected to have a Material Adverse Change.
(u)
Clinical Studies. The animal and other preclinical studies and clinical trials conducted by the Company or on behalf of the Company
were, and, if still pending are, to the Company’s knowledge, being conducted in all material respects in compliance with all Applicable
Laws and in accordance with experimental protocols, procedures and controls generally used by qualified experts in the preclinical study
and clinical trials of new drugs and biologics as applied to comparable products to those being developed by the Company; the descriptions
of the results of such preclinical studies and clinical trials contained in the Registration Statement and the Prospectus are accurate
and complete in all material respects, and, except as set forth in the Registration Statement and the Prospectus, the Company has no
knowledge of any other clinical trials or preclinical studies, the results of which reasonably call into question the clinical trial
or preclinical study results described or referred to in the Registration Statement and the Prospectus when viewed in the context in
which such results are described; and the Company has not received any written notices or correspondence from the FDA, the EMA, or any
other domestic or foreign governmental agency requiring the termination, suspension or modification of any preclinical studies or clinical
trials conducted by or on behalf of the Company that are described in the Registration Statement and the Prospectus or the results of
which are referred to in the Registration Statement and the Prospectus.
(v)
Tax Law Compliance. The Company has filed all returns (as hereinafter defined) required to be filed with taxing authorities prior
to the date hereof or has duly obtained extensions of time for the filing thereof. The Company has paid all taxes (as hereinafter defined)
shown as due on such returns that were filed and has paid all taxes imposed on or assessed against the Company. The provisions for taxes
payable, if any, shown on the financial statements filed with or as part of or incorporated by reference in the Registration Statement
are sufficient for all accrued and unpaid taxes, whether or not disputed, and for all periods to and including the dates of such consolidated
financial statements. Other than as disclosed in the Registration Statement and the Prospectus, (i) no issues have been raised (and are
currently pending) by any taxing authority in connection with any of the returns or taxes asserted as due from the Company, and (ii)
no waivers of statutes of limitation with respect to the returns or collection of taxes have been given by or requested from the Company.
There are no tax liens against the assets, properties or business of the Company. The term “taxes” means all
federal, state, local, foreign and other net income, gross income, gross receipts, sales, use, ad valorem, transfer, franchise, profits,
license, lease, service, service use, withholding, payroll, employment, excise, severance, stamp, occupation, premium, property, windfall
profits, customs, duties or other taxes, fees, assessments or charges of any kind whatever, together with any interest and any penalties,
additions to tax or additional amounts with respect thereto. The term “returns” means all returns, declarations,
reports, statements and other documents required to be filed in respect to taxes.
(w)
Company Not an “Investment Company”. The Company is not, and will not be, either after receipt of payment for the
Placement Shares or after the application of the proceeds therefrom as described under “Use of Proceeds” in the Registration
Statement or the Prospectus, required to register as an “investment company” under the Investment Company Act of 1940, as
amended (the “Investment Company Act”).
(x)
Insurance. The Company carries or is entitled to the benefits of insurance, with insurers, in such amounts and covering such risks
which the Company believes are adequate, and all such insurance is in full force and effect. The Company has no reason to believe that
it will not be able (i) to renew its existing insurance coverage as and when such policies expire or (ii) to obtain comparable coverage
from similar institutions as may be necessary or appropriate to conduct its business as now conducted and at a cost that would not result
in a Material Adverse Change.
(y)
No Price Stabilization or Manipulation. The Company has not taken, directly or indirectly (without giving any effect to the activities
of the Sales Agent), any action designed to or that might cause or result in stabilization or manipulation of the price of the Common
Stock or of any “reference security” (as defined in Rule 100 of Regulation M under the Exchange Act (“Regulation
M”)) with respect to the Common Stock, whether to facilitate the sale or resale of the Placement Shares or otherwise, and
has taken no action which would directly or indirectly violate Regulation M.
(z)
Related Party Transactions. There are no business relationships or related party transactions involving the Company or any other
person required to be described in the Registration Statement and the Prospectus that have not been described as required pursuant to
the Securities Act.
(aa)
Exchange Act Compliance. The documents incorporated or deemed to be incorporated by reference in the Registration Statement, the
Prospectus or any amendment or supplement thereto, at the time they were or hereafter are filed with the Commission under the Exchange
Act, complied and will comply in all material respects with the requirements of the Exchange Act, and, when read together with the other
information in the Prospectus, at each Point of Sale and each Settlement Date, will not contain an untrue statement of a material fact
or omit to state a material fact required to be stated therein or necessary to make the fact required to be stated therein or necessary
to make the statements therein, in the light of the circumstances under which they were made, not misleading.
(bb)
Conformity of Issuer Free Writing Prospectus. Each Issuer Free Writing Prospectus conformed or will conform in all material respects
to the requirements of the Securities Act on the date of first use, and the Company has complied or will comply with any filing requirements
applicable to such Issuer Free Writing Prospectus pursuant to the Securities Act. Each Issuer Free Writing Prospectus, as of its issue
date and at all subsequent times through the completion of the public offer and sale of the Placement Shares, did not, does not and will
not include any information that conflicted, conflicts or will conflict with the information contained in the Registration Statement
or the Prospectus, including any document incorporated by reference therein that has not been superseded or modified. The Company has
not made any offer relating to the Placement Shares that would constitute an Issuer Free Writing Prospectus without the prior written
consent of the Sales Agent. The Company has retained in accordance with the Securities Act all Issuer Free Writing Prospectuses that
were not required to be filed pursuant to the Securities Act.
(cc)
Compliance with Environmental Laws. The Company is in compliance with all foreign, federal, state and local rules, laws and regulations
relating to the use, treatment, storage and disposal of hazardous or toxic substances or waste and protection of health and safety or
the environment which are applicable to its business (“Environmental Laws”), except where the failure to comply
would not, singularly or in the aggregate, result in a Material Adverse Change. There has been no storage, generation, transportation,
handling, treatment, disposal, discharge, emission, or other release of any kind of toxic or other wastes or other hazardous substances
by, due to, or caused by the Company (or, to the Company’s knowledge, any other entity for whose acts or omissions the Company
is or may otherwise be liable) upon any of the property now or previously owned or leased by the Company, or upon any other property,
in violation of any law, statute, ordinance, rule, regulation, order, judgment, decree or permit or which would, under any law, statute,
ordinance, rule (including rule of common law), regulation, order, judgment, decree or permit, give rise to any liability, except for
any violation or liability which would not have, singularly or in the aggregate with all such violations and liabilities, a Material
Adverse Change; and there has been no disposal, discharge, emission or other release of any kind onto such property or into the environment
surrounding such property of any toxic or other wastes or other hazardous substances with respect to which the Company has knowledge,
except for any such disposal, discharge, emission, or other release of any kind which would not have, singularly or in the aggregate
with all such discharges and other releases, a Material Adverse Change. In the ordinary course of business, the Company conducts periodic
reviews of the effect of Environmental Laws on its business and assets, in the course of which it identifies and evaluates associated
costs and liabilities (including, without limitation, any capital or operating expenditures required for clean-up, closure of properties
or compliance with Environmental Laws or governmental permits issued thereunder, any related constraints on operating activities and
any potential liabilities to third parties). On the basis of such reviews, the Company has reasonably concluded that such associated
costs and liabilities would not have, singularly or in the aggregate, a Material Adverse Change.
(dd)
Intellectual Property. The Company owns or possesses or has valid rights to use all patents, patent applications, trademarks,
service marks, trade names, trademark registrations, service mark registrations, copyrights, licenses, inventions, trade secrets and
similar rights (“Intellectual Property Rights”) necessary for the conduct of the business of the Company as
currently carried on and as described in the Registration Statement and the Prospectus, except as disclosed in the Prospectus or except
as would not be reasonably likely to result in a Material Adverse Change. To the knowledge of the Company, no action or use by the Company
necessary for the conduct of its business as currently carried on and as described in the Registration Statement and the Prospectus will
involve or give rise to any infringement of, or license or similar fees for, any Intellectual Property Rights of others, except where
such action, use, license or fee is not reasonably likely to result in a Material Adverse Change. The Company has not received any notice
alleging any such infringement, fee or conflict with asserted Intellectual Property Rights of others. Except as would not reasonably
be expected to result, individually or in the aggregate, in a Material Adverse Change (A) to the knowledge of the Company, there is no
infringement, misappropriation or violation by third parties of any of the Intellectual Property Rights owned by the Company; (B) except
as disclosed in the Prospectus, there is no pending or, to the knowledge of the Company, threatened action, suit, proceeding or claim
by others challenging the rights of the Company in or to any such Intellectual Property Rights, and the Company is unaware of any facts
which would form a reasonable basis for any such claim, that would, individually or in the aggregate, together with any other claims
in this Section 6(bb), reasonably be expected to result in a Material Adverse Change; (C) the Intellectual Property Rights owned
by the Company and, to the knowledge of the Company, the Intellectual Property Rights licensed to the Company have not been adjudged
by a court of competent jurisdiction invalid or unenforceable, in whole or in part, and there is no pending or, to the Company’s
knowledge, except as disclosed in the Prospectus, threatened action, suit, proceeding or claim by others challenging the validity or
scope of any such Intellectual Property Rights, and the Company is unaware of any facts which would form a reasonable basis for any such
claim that would, individually or in the aggregate, together with any other claims in this Section 6(bb), reasonably be expected
to result in a Material Adverse Change; (D) there is no pending or, to the Company’s knowledge, threatened action, suit, proceeding
or claim by others that the Company infringes, misappropriates or otherwise violates any Intellectual Property Rights or other proprietary
rights of others, the Company has not received any written notice of such claim and the Company is unaware of any other facts which would
form a reasonable basis for any such claim that would, individually or in the aggregate, together with any other claims in this Section
6(bb), reasonably be expected to result in a Material Adverse Change; and (E) to the Company’s knowledge, no employee of the
Company is in or has ever been in violation in any material respect of any term of any employment contract, patent disclosure agreement,
invention assignment agreement, non-competition agreement, non-solicitation agreement, nondisclosure agreement or any restrictive covenant
to or with a former employer where the basis of such violation relates to such employee’s employment with the Company, or actions
undertaken by the employee while employed with the Company and could reasonably be expected to result, individually or in the aggregate,
in a Material Adverse Change. To the Company’s knowledge, all material technical information developed by and belonging to the
Company which has not been patented has been kept confidential. The Company is not a party to or bound by any options, licenses or agreements
with respect to the Intellectual Property Rights of any other person or entity that are required to be set forth in the Registration
Statement and the Prospectus and are not described therein. The Registration Statement and the Prospectus contain in all material respects
the same description of the matters set forth in the preceding sentence. None of the technology employed by the Company has been obtained
or is being used by the Company in violation of any contractual obligation binding on the Company or, to the Company’s knowledge,
any of its officers, directors or employees, or otherwise in violation of the rights of any persons.
(ee)
Brokers. The Company is not a party to any contract, agreement or understanding with any person (other than as contemplated by
this Agreement) that would give rise to a valid claim against the Company or the Sales Agent for a brokerage commission, finder’s
fee or like payment in connection with the offering and sale of the Placement Shares by the Sales Agent under this Agreement.
(ff)
No Outstanding Loans or Other Indebtedness. There are no outstanding loans, advances (except normal advances for business expenses
in the ordinary course of business) or guarantees or indebtedness by the Company to or for the benefit of any of the officers or directors
of the Company, or any of their respective family members, except as disclosed in the Registration Statement and the Prospectus.
(gg)
No Reliance. The Company has not relied upon the Sales Agent or legal counsel for the Sales Agent for any legal, tax or accounting
advice in connection with the offering and sale of the Placement Shares.
(hh)
Broker-Dealer Status. Neither the Company nor any of its related entities (i) is required to register as a “broker”
or “dealer” in accordance with the provisions of the Exchange Act or (ii) directly or indirectly through one or more intermediaries,
controls or is a “person associated with a member” or “associated person of a member” (within the meaning of
Article I of the NASD Manual administered by FINRA). To the Company’s knowledge, there are no affiliations or associations between
any member of FINRA and any of the Company’s officers, directors or 5% or greater security holders, except as set forth in the
Registration Statement.
(ii)
Public Float Calculation. At the time the Registration Statement and any Rule 462(b) Registration Statement was or will be filed
with the Commission, at the time the Registration Statement and any Rule 462(b) Registration Statement was or will be declared effective
by the Commission, and at the time the Company’s most recent Annual Report on Form 10-K was filed with the Commission, the Company
met or will meet the then applicable requirements for the use of Form S-3 under the Securities Act. As of the close of trading on the
Exchange on May 16, 2024, the aggregate market value of the outstanding voting and non-voting common equity (as defined in Rule 405)
of the Company held by persons other than affiliates of the Company (pursuant to Rule 144 of the Securities Act, those that directly,
or indirectly through one or more intermediaries, control, or are controlled by, or are under common control with, the Company) (the
“Non-Affiliate Shares”), was approximately $5.1 million (calculated by multiplying (x) the price at which the
common equity of the Company was last sold on the Exchange on May 16, 2024 by (y) the number of Non-Affiliate Shares outstanding on May
16, 2024). The Company is not a shell company (as defined in Rule 405) and has not been a shell company for at least 12 calendar months
previously and if it has been a shell company at any time previously, has filed current Form 10 information (as defined in Instruction
I.B.6. of Form S-3) with the Commission at least 12 calendar months previously reflecting its status as an entity that is not a shell
company.
(jj)
FINRA Matters. All of the information provided to the Sales Agent or to counsel for the Sales Agent by the Company, its counsel,
its officers and directors and, to the Company’s knowledge, the holders of any securities (debt or equity) or options to acquire
any securities of the Company in connection with the offering of the Placement Shares is true, complete, correct and compliant with FINRA’s
rules in all material respects and any letters, filings or other supplemental information provided to FINRA pursuant to FINRA Rules or
NASD Conduct Rules is true, complete and correct in all material respects. Except as disclosed in the Registration Statement and the
Prospectus, to the Company’s knowledge, there is no (i) officer or director of the Company, (ii) beneficial owner of 5% or more
of any class of the Company’s securities or (iii) beneficial owner of the Company’s unregistered equity securities that were
acquired during the 180-day period immediately preceding the date of this Agreement that is an affiliate or associated person of a FINRA
member participating in the offer, issuance and sale of the Placement Shares as contemplated by this Agreement and the Registration Statement
and the Prospectus (as determined in accordance with the rules and regulations of FINRA).
(kk)
Compliance with Orders. The Company is not in violation of any material judgment, decree, or order of any court, arbitrator or
other governmental authority.
(ll)
Sarbanes–Oxley Act. The Company is in material compliance with any and all applicable requirements of the Sarbanes-Oxley
Act of 2002 and the rules and regulations promulgated in connection therewith (the “Sarbanes-Oxley Act”) that
are effective as of the date hereof, and any and all applicable rules and regulations promulgated by the Commission thereunder that are
effective as of the date hereof and as of the date hereof.
(mm)
Disclosure Controls and Procedures. Except as set forth in the Registration Statement and the Prospectus, the Company maintains
systems of “internal control over financial reporting” (as defined under Rules 13a-15 and 15d-15 under the Exchange Act)
that comply with the requirements of the Exchange Act and have been designed by, or under the supervision of, their respective principal
executive and principal financial officers, or persons performing similar functions, to provide reasonable assurance regarding the reliability
of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP, including, but not
limited to, internal accounting controls sufficient to provide reasonable assurance that (i) transactions are executed in accordance
with management’s general or specific authorizations; (ii) transactions are recorded as necessary to permit preparation of financial
statements in conformity with GAAP and to maintain asset accountability; (iii) access to assets is permitted only in accordance with
management’s general or specific authorization; (iv) the recorded accountability for assets is compared with the existing assets
at reasonable intervals and appropriate action is taken with respect to any differences; and (v) the interactive data in eXtensible Business
Reporting Language included or incorporated by reference in the Registration Statement and the Prospectus fairly present the information
called for in all material respects and are prepared in accordance with the Commission’s rules and guidelines applicable thereto.
Since the date of the latest audited financial statements included in the Registration Statement and the Prospectus, there has been no
change in the Company’s internal control over financial reporting that has materially affected, or is reasonably likely to materially
affect, the Company’s internal control over financial reporting.
(nn)
ERISA. The Company and any “employee benefit plan” (as defined under the Employee Retirement Income Security Act of
1974, as amended, and the regulations and published interpretations thereunder (collectively, “ERISA”)) established
or maintained by the Company or its “ERISA Affiliates” (as defined below) are in compliance in all material respects with
ERISA. No “reportable event” (as defined under ERISA) has occurred or is reasonably expected to occur with respect to any
“employee benefit plan” established or maintained by the Company or any of its ERISA Affiliates. No “employee benefit
plan” established or maintained by the Company or any of its ERISA Affiliates, if such “employee benefit plan” were
terminated, would have any “amount of unfunded benefit liabilities” (as defined under ERISA). Neither the Company nor any
of its ERISA Affiliates has incurred or reasonably expects to incur any material liability under (i) Title IV of ERISA with respect to
termination of, or withdrawal from, any “employee benefit plan” or (ii) Sections 412, 4971, 4975 or 4980B of the Code. Each
“employee benefit plan” established or maintained by the Company or any of its ERISA Affiliates that is intended to be qualified
under Section 401(a) of the Code is so qualified and, to the knowledge of the Company, nothing has occurred, whether by action or failure
to act, which would cause the loss of such qualification. “ERISA Affiliate” means, with respect to the Company,
any member of any group of organizations described in Sections 414(b),(c),(m) or (o) of the Internal Revenue Code of 1986, as amended,
and the regulations and published interpretations thereunder (the “Code”) of which the Company is a member.
(oo)
Contracts and Agreements. The agreements and documents described in the Registration Statement and the Prospectus conform in all
material respects to the descriptions thereof contained therein and there are no agreements or other documents required by the Securities
Act to be described in the Registration Statement and the Prospectus or to be filed with the Commission as exhibits to the Registration
Statement, that have not been so described or filed. Each agreement or other instrument (however characterized or described) to which
the Company is a party or by which it is or may be bound or affected and (i) that is referred to in the Registration Statement and the
Prospectus, or (ii) is material to the Company’s business, has been duly authorized and validly executed by the Company, is in
full force and effect in all material respects and is enforceable against the Company and, to the Company’s knowledge, the other
parties thereto, in accordance with its terms, except as disclosed in the Prospectus or (x) as such enforceability may be limited by
bankruptcy, insolvency, reorganization or similar laws affecting creditors’ rights generally, (y) as enforceability of any indemnification
or contribution provision may be limited under the federal and state securities laws, and (z) that the remedy of specific performance
and injunctive and other forms of equitable relief may be subject to the equitable defenses and to the discretion of the court before
which any proceeding therefor may be brought. None of such agreements or instruments has been assigned by the Company, and neither the
Company nor, to the Company’s knowledge, any other party is in default thereunder and, to the Company’s knowledge, no event
has occurred that, with the lapse of time or the giving of notice, or both, would constitute a default thereunder. To the best of the
Company’s knowledge, performance by the Company of the material provisions of such agreements or instruments will not result in
a violation of any existing applicable law, rule, regulation, judgment, order or decree of any governmental agency or court, domestic
or foreign, having jurisdiction over the Company or any of its assets or businesses (each, a “Governmental Entity”),
including, without limitation, those relating to environmental laws and regulations.
(pp)
Title to Properties. Except as set forth in the Registration Statement and the Prospectus, the Company has good and marketable
title in fee simple to, or has valid rights to lease or otherwise use, all items of real or personal property which are material to the
business of the Company, in each case free and clear of all liens, encumbrances, security interests, claims and defects that do not,
singly or in the aggregate, materially affect the value of such property and do not interfere with the use made and proposed to be made
of such property by the Company; and all of the leases and subleases material to the business of the Company, and under which the Company
holds properties described in the Registration Statement and the Prospectus, are in full force and effect, and the Company has not received
any notice of any material claim of any sort that has been asserted by anyone adverse to the rights of the Company under any of the leases
or subleases mentioned above, or affecting or questioning the rights of the Company to the continued possession of the leased or subleased
premises under any such lease or sublease, which would result in a Material Adverse Change.
(qq)
No Unlawful Contributions or Other Payments. No payments or inducements have been made or given, directly or indirectly, to any
federal or local official or candidate for, any federal or state office in the United States or foreign offices by the Company or any
of its officers or directors, or, to the knowledge of the Company, by any of its employees or agents or any other person in connection
with any opportunity, contract, permit, certificate, consent, order, approval, waiver or other authorization relating to the business
of the Company, except for such payments or inducements as were lawful under applicable laws, rules and regulations. Neither the Company,
nor, to the knowledge of the Company, any director, officer, agent, employee or other person associated with or acting on behalf of the
Company, (i) has used any corporate funds for any unlawful contribution, gift, entertainment or other unlawful expense relating to political
activity; (ii) made any direct or indirect unlawful payment to any government official or employee from corporate funds; or (iii) made
any bribe, unlawful rebate, payoff, influence payment, kickback or other unlawful payment in connection with the business of the Company.
(rr)
Foreign Corrupt Practices Act. None of the Company or, to the knowledge of the Company, any director, officer, agent, employee,
affiliate or other person acting on behalf of the Company, is aware of or has taken any action, directly or indirectly, that would result
in a violation by such persons of the Foreign Corrupt Practices Act of 1977, as amended, and the rules and regulations thereunder (collectively,
the “FCPA”), including, without limitation, making use of the mails or any means or instrumentality of interstate
commerce corruptly in furtherance of an offer, payment, promise to pay or authorization of the payment of any money, or other property,
gift, promise to give, or authorization of the giving of anything of value to any “foreign official” (as such term is defined
in the FCPA) or any foreign political party or official thereof or any candidate for foreign political office, in contravention of the
FCPA. The Company has conducted its business in compliance with the FCPA and has instituted and maintains policies and procedures designed
to ensure, and which are reasonably expected to continue to ensure, continued compliance therewith.
(ss)
Money Laundering Laws. The operations of the Company are and have been conducted at all times in compliance with applicable financial
recordkeeping and reporting requirements of the Currency and Foreign Transactions Reporting Act of 1970, as amended, the money laundering
statutes of all jurisdictions, the rules and regulations thereunder and any related or similar rules, regulations or guidelines, issued,
administered or enforced by any governmental agency (collectively, the “Money Laundering Laws”) and no action,
suit or proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company with respect
to the Money Laundering Laws is pending or, to the knowledge of the Company, threatened.
(tt)
OFAC. None of the Company or, to the knowledge of the Company, any director, officer, agent, employee, affiliate or person acting
on behalf of the Company is currently subject to any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S.
Treasury Department (“OFAC”); and the Company will not directly or indirectly use the proceeds of the offering,
or lend, contribute or otherwise make available such proceeds to any subsidiary, joint venture partner or other person or entity, for
the purpose of financing the activities of any person currently subject to any U.S. sanctions administered by OFAC.
(uu)
Exchange Listing. The Common Stock is registered pursuant to Section 12(b) of the Exchange Act and is currently listed on the
Exchange under the trading symbol “PCSA”. Except as disclosed in the Registration Statement and the Prospectus, there is
no action pending by the Company or, to the Company’s knowledge, the Exchange to delist the Common Stock from the Exchange, nor
has the Company received any notification that the Exchange is contemplating terminating such listing. The Company has no intention to
delist the Common Stock from the Exchange or to deregister the Common Stock under the Exchange Act, in either case, at any time during
the period commencing on the date of this Agreement through and including the 90th calendar day after the termination of this Agreement.
The Placement Shares have been approved for listing on the Exchange. The issuance and sale of the Placement Shares under this Agreement
does not contravene the rules and regulations of the Exchange.
(vv)
Margin Rules. The Company owns no “margin securities” as that term is defined in Regulation U of the Board of Governors
of the Federal Reserve System (the “Federal Reserve Board”), and none of the proceeds from the issuance, sale
and delivery of the Placement Shares as contemplated by this Agreement and as described in the Registration Statement and the Prospectus
will be used, directly or indirectly, for the purpose of purchasing or carrying any margin security, for the purpose of reducing or retiring
any indebtedness which was originally incurred to purchase or carry any margin security or for any other purpose which might cause any
of the shares of Common Stock to be considered a “purpose credit” within the meanings of Regulation T, U or X of the Federal
Reserve Board.
(ww)
Underwriter Agreements. The Company is not a party to any agreement with an agent or underwriter for any other “at-the-market”
or continuous equity transaction.
(xx)
Board of Directors. The qualifications of the persons serving as board members of the Company and the overall composition of the
Company’s Board of Directors comply with the applicable requirements of the Exchange Act and the Sarbanes-Oxley Act and the listing
rules of the Exchange applicable to the Company. At least one member of the Audit Committee of the Board of Directors of the Company
qualifies as an “audit committee financial expert,” as such term is defined under Regulation S-K and the listing rules of
the Exchange. In addition, at least a majority of the persons serving on the Board of Directors of the Company qualify as “independent,”
as defined under the listing rules of the Exchange.
(yy)
No Integration. Neither the Company, nor any of its affiliates, nor any person acting on its or their behalf has, directly or
indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would cause
the offer and sale of the Placement Shares hereunder to be integrated with prior offerings by the Company for purposes of the Securities
Act that would require the registration of any such securities under the Securities Act.
(zz)
No Material Defaults. The Company has not defaulted on any installment on indebtedness for borrowed money or on any rental on
one or more long-term leases, which defaults, individually or in the aggregate, could reasonably be expected to result in a Material
Adverse Change. The Company has not filed a report pursuant to Section 13(a) or 15(d) of the Exchange Act since the filing of its last
Annual Report on Form 10-K, indicating that it (i) has failed to pay any dividend or sinking fund installment on preferred stock or (ii)
has defaulted on any installment on indebtedness for borrowed money or on any rental on one or more long-term leases, which defaults,
individually or in the aggregate, could reasonably be expected to result in a Material Adverse Change.
(aaa)
Books and Records. The minute books of the Company have been made available to the Sales Agent and counsel for the Sales Agent,
and such books (i) contain a substantially complete summary of all meetings and material actions of the board of directors (including
each board committee) and stockholders of the Company (or analogous governing bodies and interest holders, as applicable) since 2023
through the date of the latest meeting and action, and (ii) accurately in all material respects reflect all transactions referred to
in such minutes.
(bbb)
Regulations. The disclosures in the Registration Statement and the Prospectus concerning the effects of federal, state, local
and all foreign regulation on the Company’s business in the past and as currently contemplated are correct in all material respects
and no other such regulations are required to be disclosed in the Registration Statement and the Prospectus which are not so disclosed.
(ccc)
Confidentiality and Non-Competitions. To the Company’s knowledge, no director, officer, key employee or consultant of the
Company is subject to any confidentiality, non-disclosure, non-competition agreement or non-solicitation agreement with any employer
or prior employer that could reasonably be expected to materially affect his ability to be and act in his respective capacity of the
Company or be expected to result in a Material Adverse Change.
Any
certificate signed by an officer of the Company and delivered to the Sales Agent or to counsel for the Sales Agent pursuant to or in
connection with this Agreement shall be deemed to be a representation and warranty by the Company to the Sales Agent as to the matters
set forth therein.
The
Company acknowledges that the Sales Agent and, for purposes of the opinions to be delivered pursuant to Section 7 hereof, counsel
to the Company and counsel to the Sales Agent, will rely upon the accuracy and truthfulness of the foregoing representations and hereby
consents to such reliance.
7.
Covenants of the Company. The Company covenants and agrees with the Sales Agent that:
(a)
Registration Statement Amendments. After the date of this Agreement and during any period in which a Prospectus relating to any
Placement Shares is required to be delivered by the Sales Agent under the Securities Act (including in circumstances where such requirement
may be satisfied pursuant to Rule 153 or Rule 172 under the Securities Act), (i) the Company will notify the Sales Agent promptly of
the time when any subsequent amendment to the Registration Statement, other than documents incorporated by reference, has been filed
with the Commission and/or has become effective or any subsequent supplement to the Prospectus has been filed and of any request by the
Commission for any amendment or supplement to the Registration Statement or Prospectus or for additional information; (ii) the Company
will prepare and file with the Commission, promptly upon the Sales Agent’s reasonable request, any amendments or supplements to
the Registration Statement or Prospectus that, in the Sales Agent’s reasonable opinion, may be necessary or advisable in connection
with the distribution of the Placement Shares by the Sales Agent (provided, however, that the failure of the Sales Agent
to make such request shall not relieve the Company of any obligation or liability hereunder, or affect the Sales Agent’s right
to rely on the representations and warranties made by the Company in this Agreement, and provided, further, that the only
remedy the Sales Agent shall have with respect to the failure to make such filing shall be to cease making sales under this Agreement
until such amendment or supplement is filed); (iii) the Company will not file any amendment or supplement to the Registration Statement
or Prospectus, other than documents incorporated by reference, relating to the Placement Shares or a security convertible into the Placement
Shares unless a copy thereof has been submitted to the Sales Agent within a reasonable period of time before the filing and the Sales
Agent has not reasonably objected thereto (provided, however, that the failure of the Sales Agent to make such objection
shall not relieve the Company of any obligation or liability hereunder, or affect the Sales Agent’s right to rely on the representations
and warranties made by the Company in this Agreement, and provided, further, that the only remedy the Sales Agent shall
have with respect to the failure by the Company to obtain such consent shall be to cease making sales under this Agreement); (iv) the
Company will furnish to the Sales Agent at the time of filing thereof a copy of any document that upon filing is deemed to be incorporated
by reference into the Registration Statement or Prospectus, except for those documents available via EDGAR; and (v) the Company will
cause each amendment or supplement to the Prospectus, other than documents incorporated by reference, to be filed with the Commission
as required pursuant to the applicable paragraph of Rule 424(b) of the Securities Act (without reliance on Rule 424(b)(8) of the Securities
Act) or, in the case of any documents incorporated by reference, to be filed with the Commission as required pursuant to the Exchange
Act, within the time period prescribed.
(b)
Notice of Commission Stop Orders. The Company will advise the Sales Agent, promptly after it receives notice or obtains knowledge
thereof, of the issuance by the Commission of any stop order suspending the effectiveness of the Registration Statement or any notice
objecting to, or other order preventing or suspending the use of, the Prospectus, of the suspension of the qualification of the Placement
Shares for offering or sale in any jurisdiction, or of the initiation of any proceeding for any such purpose or any examination pursuant
to Section 8(e) of the Securities Act, or if the Company becomes the subject of a proceeding under Section 8A of the Securities Act in
connection with the offering of the Placement Shares; and it will promptly use its commercially reasonable efforts to prevent the issuance
of any stop order or to obtain its withdrawal if such a stop order should be issued. Until such time as any stop order is lifted, the
Sales Agent shall cease making offers and sales under this Agreement.
(c)
Delivery of Prospectus; Subsequent Changes. During any period in which a Prospectus relating to the Placement Shares is required
to be delivered by the Sales Agent under the Securities Act with respect to a pending sale of the Placement Shares (including in circumstances
where such requirement may be satisfied pursuant to Rule 153 or Rule 172 under the Securities Act), the Company will comply in all material
respects with all requirements imposed upon it by the Securities Act, as from time to time in force, and to file on or before their respective
due dates all reports and any definitive proxy or information statements required to be filed by the Company with the Commission pursuant
to Sections 13(a), 13(c), 14, 15(d) or any other provision of or under the Exchange Act. If during such period any event occurs as a
result of which the Prospectus as then amended or supplemented would include an untrue statement of a material fact or omit to state
a material fact necessary to make the statements therein, in the light of the circumstances then existing, not misleading, or if during
such period it is necessary to amend or supplement the Registration Statement or Prospectus to comply with the Securities Act, the Company
will promptly notify the Sales Agent to suspend the offering of Placement Shares during such period and the Company will promptly amend
or supplement the Registration Statement or Prospectus (at the expense of the Company) so as to correct such statement or omission or
effect such compliance; provided, however, that the Company may delay any such amendment or supplement if, in the reasonable
judgment of the Company, it is in the best interests of the Company to do so.
(d)
Listing of Placement Shares. During any period in which the Prospectus relating to the Placement Shares is required to be delivered
by the Sales Agent under the Securities Act with respect to a pending sale of the Placement Shares (including in circumstances where
such requirement may be satisfied pursuant to Rule 153 or Rule 172 under the Securities Act), the Company will use its commercially reasonable
efforts to cause the Placement Shares to be listed on the Exchange and to qualify the Placement Shares for sale under the securities
laws of such jurisdictions as the Sales Agent reasonably designates and to continue such qualifications in effect so long as required
for the distribution of the Placement Shares; provided, however, that the Company shall not be required in connection therewith
to qualify as a foreign corporation or dealer in securities or file a general consent to service of process in any jurisdiction.
(e)
Delivery of Registration Statement and Prospectus. The Company will furnish to the Sales Agent and its counsel (at the expense
of the Company) electronic copies of the Registration Statement, the Prospectus (including all documents incorporated by reference therein)
and all amendments and supplements to the Registration Statement or Prospectus that are filed with the Commission during any period in
which a Prospectus relating to the Placement Shares is required to be delivered under the Securities Act (including all documents filed
with the Commission during such period that are deemed to be incorporated by reference therein), in each case as soon as reasonably practicable
and in such quantities as the Sales Agent may from time to time reasonably request and, at the Sales Agent’s request, will also
furnish electronic copies of the Prospectus to each exchange or market on which sales of the Placement Shares may be made; provided,
however, that the Company shall not be required to furnish any document (other than the Prospectus) to the Sales Agent to the
extent such document is available on EDGAR.
(f)
Earnings Statement. The Company will make generally available to its security holders as soon as practicable, but in any event
not later than 15 months after the end of the Company’s current fiscal quarter, an earnings statement of the Company (which need
not be audited) covering a 12-month period that complies with Section 11(a) and Rule 158 of the Securities Act. The terms “earnings
statement” and “make generally available to its security holders” shall have the meanings set forth in Rule 158 under
the Securities Act.
(g)
Expenses. The Company, whether or not the transactions contemplated hereunder are consummated or this Agreement is terminated
in accordance with the provisions of Section 11 hereunder, will pay the following expenses all incident to the performance of
its obligations hereunder, including, but not limited to, expenses relating to (i) the preparation and filing of the Registration Statement
and each amendment and supplement thereto, of each Prospectus and of each amendment and supplement thereto, (ii) the preparation, issuance
and delivery of the Placement Shares, including any stock or other transfer taxes and any stamp or other duties payable upon the sale,
issuance or delivery of the Placement Shares to the Sales Agent, (iii) the fees and disbursements of the counsel, accountants and other
advisors to the Company in connection with the transactions contemplated by this Agreement; (iv) the qualification of the Placement Shares
under securities laws in accordance with the provisions of Section 7(d) of this Agreement, including filing fees (provided,
however, that any fees or disbursements of counsel for the Sales Agent in connection therewith shall be paid by the Sales Agent
except as set forth in (ix) below), (v) the printing and delivery to the Sales Agent of copies of the Prospectus and any amendments or
supplements thereto, and of this Agreement, (vi) the fees and expenses incurred in connection with the listing or qualification of the
Placement Shares for trading on the Exchange, (vii) the fees and expenses of the transfer agent or registrar for the Common Stock; (viii)
filing fees and expenses, if any, of the Commission and the FINRA Corporate Financing Department (provided, however, that
any fees or disbursements of counsel for the Sales Agent in connection therewith shall be paid by the Sales Agent except as set forth
in (ix) below), (ix) the Company shall reimburse the Sales Agent for its reasonable and documented out-of-pocket expenses (including
but not limited to the Sales Agent’s transaction costs and the reasonable and documented fees and expenses of counsel to the Sales
Agent) in an amount not to exceed $50,000 (the “Sales Agent Expenses”) which Sales Agent Expenses shall be due and
payable prior to the first Placement pursuant to this Agreement, provided further that the Company shall reimburse the Sales Agent
for its reasonable and documented out-of-pocket expenses related to annual maintenance of the Agreement (including but not limited to
the Sales Agent’s transaction costs and the reasonable and documented fees and expenses of counsel to the Sales Agent) on an annual
basis in an amount not to exceed $10,000 which shall be due and payable prior to each Representation Date following the filing of an
annual report on Form 10-K.
(h)
Use of Proceeds. The Company will use the Net Proceeds as described in the Prospectus in the section entitled “Use of Proceeds.”
(i)
Notice of Other Sales. The Company (I) shall provide the Sales Agent notice as promptly as reasonably possible before it offers
to sell, contracts to sell, sells, grants any option to sell or otherwise disposes of any shares of Common Stock (other than Placement
Shares offered pursuant to the provisions of this Agreement) or securities convertible into or exchangeable for Common Stock, or warrants
or any rights to purchase or acquire Common stock, during the period beginning on the fifth (5th) Trading Day immediately
prior to the date on which any Placement Notice is delivered to the Sales Agent hereunder and ending on the fifth (5th) Trading
Day immediately following the final Settlement Date with respect to Placement Shares sold pursuant to such Placement Notice (or, if the
Placement Notice has been terminated or suspended prior to the sale of all Placement Shares covered by a Placement Notice, the fifth
(5th) Trading Day immediately following the date of such suspension or termination), and (II) will not directly or indirectly
engage in any other “at-the-market” or continuous equity transaction offer to sell, sell, contract to sell, grant any option
to sell or otherwise dispose of any shares of Common Stock (other than the Placement Shares offered pursuant to this Agreement) or securities
convertible into or exchangeable for shares of Common Stock, warrants or any rights to purchase or acquire, shares of Common Stock prior
to the termination of this Agreement without the prior written consent of the Sales Agent; provided, however, that such
notice requirements or restrictions, as the case may be, will not be required in connection with the Company’s issuance or sale
of (i) shares of Common Stock, options to purchase shares of Common Stock, other equity awards or shares of Common Stock issuable upon
the exercise of options or other equity awards, pursuant to any employee or director stock option or benefits plan, stock ownership plan
or dividend reinvestment plan of the Company whether now in effect or hereafter implemented, (ii) shares of Common Stock issuable upon
exchange, conversion or redemption of securities or the exercise of warrants, options or other rights in effect or outstanding, and disclosed
in filings by the Company available on EDGAR or otherwise in writing (including by email correspondence) to the Sales Agent and (iii)
shares of Common Stock or securities convertible into or exchangeable for shares of Common Stock as consideration for mergers, acquisitions,
sale or purchase of assets or other business combinations or strategic alliances occurring after the date of this Agreement which are
not issued for capital raising purposes. Notwithstanding the foregoing, the Company shall provide the Sales Agent notice at least two
(2) days prior to pursuing any private or public offerings of equity and/or other securities (including debt securities) in one or more
transactions.
(j)
Change of Circumstances. The Company will, at any time during a fiscal quarter in which the Company intends to tender a Placement
Notice or sell Placement Shares, advise the Sales Agent promptly after it shall have received notice or obtained knowledge thereof, of
any information or fact that would alter or affect in any material respect any opinion, certificate, letter or other document provided
to the Sales Agent pursuant to this Agreement.
(k)
Due Diligence Cooperation. The Company will cooperate with any reasonable due diligence review conducted by the Sales Agent or
its agents in connection with the transactions contemplated hereby, including, without limitation, providing information and making available
documents and senior corporate officers, during regular business hours and at the Company’s principal offices, as the Sales Agent
may reasonably request.
(l)
Required Filings Relating to Placement of Placement Shares. The Company shall set forth in each Annual Report on Form 10-K and
Quarterly Report on Form 10-Q filed by the Company with the Commission in respect of any quarter in which sales of Placement Shares were
made by or through the Sales Agent under this Agreement, with regard to the relevant period, the amount of Placement Shares sold to or
through the Sales Agent, the Net Proceeds to the Company and the compensation payable by the Company to the Sales Agent with respect
to such sales of Placement Shares. To the extent that the filing of a prospectus supplement with the Commission with respect to any sales
of Placement Shares becomes required under Rule 424(b) under the Securities Act, the Company agrees that, on or before such dates as
the Securities Act shall require, the Company will (i) file a prospectus supplement with the Commission under the applicable paragraph
of Rule 424(b) under the Securities Act, which prospectus supplement will set forth, with regard to the relevant period, the amount of
Placement Shares sold to or through the Sales Agent, the Net Proceeds to the Company and the compensation payable by the Company to the
Sales Agent with respect to such Placement Shares, and (ii) deliver such number of copies of each such prospectus supplement to each
exchange or market on which such sales were effected as may be required by the rules or regulations of such exchange or market. The Company
shall afford the Sales Agent and its counsel with a reasonable opportunity to review and comment upon, shall consult with the Sales Agent
and its counsel on the form and substance of, and shall give due consideration to all such comments from the Sales Agent or its counsel
on, any such filing prior to the issuance, filing or public disclosure thereof; provided, however, that the Company shall not be required
to submit for review (A) any portion of any periodic reports filed with the Commission under the Exchange Act other than the specific
disclosure relating to any sales of Placement Shares and (B) any disclosure contained in periodic reports filed with the Commission under
the Exchange Act if it shall have previously provided the same disclosure for review in connection with a previous filing.
(m)
Representation Dates; Certificate. On or prior to the date the first Placement Notice is given hereunder and each time the Company
(i) files the Prospectus relating to the Placement Shares or amends or supplements the Registration Statement or the Prospectus relating
to the Placement Shares (other than (A) a prospectus supplement filed in accordance with Section 7(l) of this Agreement or (B)
a supplement or amendment that relates to an offering of securities other than the Placement Shares) by means of a post-effective amendment,
sticker, or supplement but not by means of incorporation of document(s) by reference to the Registration Statement or the Prospectus
relating to the Placement Shares; (ii) files an annual report on Form 10-K under the Exchange Act (including any Form 10-K/A containing
amended financial information or a material amendment to the previously filed Form 10-K); (iii) files a quarterly report on Form 10-Q
under the Exchange Act; or (iv) files a current report on Form 8-K containing amended financial information (other than an earnings release,
to “furnish” information pursuant to Items 2.02 or 7.01 of Form 8-K or to provide disclosure pursuant to Item 8.01 of Form
8-K relating to the reclassification of certain properties as discontinued operations in accordance with Statement of Financial Accounting
Standards No. 144) under the Exchange Act (each date of filing of one or more of the documents referred to in clauses (i) through (iv)
shall be a “Representation Date”), the Company shall furnish the Sales Agent within three (3) Trading Days
after each Representation Date with a certificate, in the form attached hereto as Exhibit 7(m). The requirement to provide a certificate
under this Section 7(m) shall be waived for any Representation Date occurring at a time at which no Placement Notice is pending,
which waiver shall continue until the earlier to occur of the date the Company delivers a Placement Notice hereunder (which for such
calendar quarter shall be considered a Representation Date) and the next occurring Representation Date; provided, however,
that such waiver shall not apply for any Representation Date on which the Company files its annual report on Form 10-K. Notwithstanding
the foregoing, if the Company subsequently decides to sell Placement Shares following a Representation Date when the Company relied on
such waiver and did not provide the Sales Agent with a certificate under this Section 7(m), then before the Company delivers the
Placement Notice or the Sales Agent sells any Placement Shares, the Company shall provide the Sales Agent with a certificate, in the
form attached hereto as Exhibit 7(m), dated the date of the Placement Notice.
(n)
Legal Opinion. On or prior to the date the first Placement Notice is given hereunder, the Company shall cause to be furnished
to the Sales Agent (i) the written opinion and negative assurance of Foley & Lardner LLP, as counsel to the Company, or other counsel
reasonably satisfactory to the Sales Agent (“Company Counsel”). Thereafter, within three (3) Trading Days after
each Representation Date with respect to which the Company is obligated to deliver a certificate pursuant to Section 7(m) for
which no waiver is applicable pursuant to Section 7(m), the Company shall cause to be furnished to the Sales Agent the written
opinion and negative assurance of Company Counsel substantially in the forms previously agreed between the Company and the Sales Agent,
modified, as necessary, to relate to the Registration Statement and the Prospectus as then amended or supplemented; provided,
however, that if Company Counsel has previously furnished to the Sales Agent such written opinion and negative assurance of such
counsel, in each case substantially in the forms previously agreed between the Company and the Sales Agent, then each Company Counsel
may, in respect of any future Representation Date, furnish the Sales Agent with a letter signed by such counsel (each, a “Reliance
Letter”) in lieu of such opinion and negative assurance of such counsel to the effect that the Sales Agent may rely on
the prior opinion and negative assurance of such counsel delivered pursuant to this Section 7(n) to the same extent as if it were
dated the date of such Reliance Letter (except that statements in such prior opinion and negative assurance shall be deemed to relate
to the Registration Statement and the Prospectus as amended or supplemented to the date of such Reliance Letter).
(o)
Comfort Letter. On or prior to the date the first Placement Notice is given hereunder and within three (3) Trading Days after
each subsequent Representation Date with respect to which the Company is obligated to deliver a certificate pursuant to Section 7(m)
for which no waiver is applicable pursuant to Section 7(m), other than a Representation Date under Section 7(m)(iii) or Section
7(m)(iv) unless with respect to a Representation Date under Section 7(m)(iv) the Sales Agent reasonably requests delivery
thereof, the Company shall cause its independent accountants to furnish the Sales Agent letters (the “Comfort Letters”),
dated the date that the Comfort Letter is delivered, in form and substance satisfactory to the Sales Agent, (i) confirming that they
are an independent registered public accounting firm within the meaning of the Securities Act, the Exchange Act and the rules and regulations
of the PCAOB and are in compliance with the applicable requirements relating to the qualification of accountants under Rule 2-01 of Regulation
S-X of the Commission, (ii) stating, as of such date, the conclusions and findings of such firm with respect to the financial information
and other matters ordinarily covered by accountants’ “comfort letters” to the Sales Agent in connection with registered
public offerings (the first such letter, the “Initial Comfort Letter”) and (iii) updating the Initial Comfort
Letter with any information that would have been included in the Initial Comfort Letter had it been given on such date and modified as
necessary to relate to the Registration Statement and the Prospectus, as amended and supplemented to the date of such letter.
(p)
Market Activities. The Company will not, directly or indirectly, (i) take any action designed to cause or result in, or that constitutes
or might reasonably be expected to constitute, the stabilization or manipulation of the price of any security of the Company to facilitate
the sale or resale of the Common Stock or (ii) sell, bid for, or purchase shares of Common Stock in violation of Regulation M, or pay
anyone any compensation for soliciting purchases of the Placement Shares other than the Sales Agent.
(q)
Insurance. The Company shall maintain insurance in such amounts and covering such risks as it believes to be reasonable and customary
for the business in which it is engaged.
(r)
Investment Company Act. The Company will conduct its affairs in such a manner so as to reasonably ensure that it is not and, after
giving effect to the offering and sale of the Placement Shares and the application of proceeds therefrom as described in the Prospectus,
will not be, an “investment company” within the meaning of such term under the Investment Company Act.
(s)
Securities Act and Exchange Act. The Company will use its reasonable best efforts to comply with all requirements imposed upon
it by the Securities Act and the Exchange Act as from time to time in force, so far as necessary to permit the continuance of sales of,
or dealings in, the Placement Shares as contemplated by the provisions hereof and the Prospectus.
(t)
No Offer to Sell. Other than the Prospectus and an Issuer Free Writing Prospectus approved in advance by the Company and the Sales
Agent in its capacity as principal or agent hereunder, neither the Sales Agent nor the Company (including its agents and representatives,
other than the Sales Agent in its capacity as such) will make, use, prepare, authorize, approve or refer to any written communication
(as defined in Rule 405 under the Securities Act), required to be filed with the Commission, that constitutes an offer to sell or solicitation
of an offer to buy Placement Shares hereunder.
(u)
Sarbanes-Oxley Act. The Company will use its reasonable best efforts to comply with all effective applicable provisions of the
Sarbanes-Oxley Act.
(v)
Transfer Agent. The Company shall maintain, at its sole expense, a registrar and transfer agent for the Common Stock.
8.
Conditions to the Sales Agent’s Obligations. The obligations of the Sales Agent hereunder with respect to a Placement will
be subject to the continuing accuracy and completeness of the representations and warranties made by the Company herein, to the due performance
by the Company of its obligations hereunder, to the completion by the Sales Agent of a due diligence review satisfactory to the Sales
Agent in its reasonable judgment, and to the continuing satisfaction (or waiver by the Sales Agent in its sole discretion) of the following
additional conditions:
(a)
Registration Statement Effective. The Registration Statement shall be effective and shall be available for the sale of all Placement
Shares contemplated to be issued by any Placement Notice.
(b)
Securities Act Filings Made. All filings with the Commission required by Rule 424(b) or Rule 433 under the Securities Act to have
been filed prior to the issuance of any Placement Notice hereunder shall have been made within the applicable time period prescribed
for such filing by Rule 424(b) (without reliance on Rule 424(b)(8) of the Securities Act) or Rule 433, as applicable.
(c)
No Material Notices. None of the following events shall have occurred and be continuing: (i) receipt by the Company of any request
for additional information from the Commission or any other federal or state governmental authority during the period of effectiveness
of the Registration Statement, the response to which would require any post-effective amendments or supplements to the Registration Statement
or the Prospectus; (ii) the issuance by the Commission or any other federal or state governmental authority of any stop order suspending
the effectiveness of the Registration Statement or the initiation of any proceedings for that purpose; (iii) receipt by the Company of
any notification with respect to the suspension of the qualification or exemption from qualification of any of the Placement Shares for
sale in any jurisdiction or the initiation or threatening of any proceeding for such purpose; (iv) the occurrence of any event that makes
any material statement made in the Registration Statement or the Prospectus or any material document incorporated or deemed to be incorporated
therein by reference untrue in any material respect or that requires the making of any changes in the Registration Statement, related
Prospectus or such documents so that, in the case of the Registration Statement, it will not contain any materially untrue statement
of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein not misleading
and, that in the case of the Prospectus, it will not contain any materially untrue statement of a material fact or omit to state any
material fact required to be stated therein or necessary to make the statements therein, in the light of the circumstances under which
they were made, not misleading.
(d)
No Misstatement or Material Omission. The Sales Agent shall not have advised the Company that the Registration Statement or Prospectus,
or any amendment or supplement thereto, contains an untrue statement of fact that in the Sales Agent’s reasonable opinion is material,
or omits to state a fact that in the Sales Agent’s reasonable opinion is material and is required to be stated therein or is necessary
to make the statements therein not misleading.
(e)
Material Changes. Except as contemplated in the Prospectus, or disclosed in the Company’s reports filed with the Commission,
there shall not have been any material adverse change in the authorized capital stock of the Company or any Material Adverse Change or
any development that could reasonably be expected to result in a Material Adverse Change, the effect of which, in the case of any such
action by a rating organization described above, in the reasonable judgment of the Sales Agent (without relieving the Company of any
obligation or liability it may otherwise have), is so material as to make it impracticable or inadvisable to proceed with the offering
of the Placement Shares on the terms and in the manner contemplated by this Agreement and the Prospectus.
(f)
Representation Certificate. The Sales Agent shall have received the certificate required to be delivered pursuant to Section
7(m) on or before the date on which delivery of such certificate is required pursuant to Section 7(m).
(g)
Legal Opinion. The Sales Agent shall have received the opinion and negative assurance of Company Counsel required to be delivered
pursuant Section 7(n) on or before the date on which such delivery of such opinion and negative assurance is required pursuant
to Section 7(n).
(h)
Comfort Letter. The Sales Agent shall have received the Comfort Letter required to be delivered pursuant Section 7(o) on
or before the date on which such delivery of such Comfort Letter is required pursuant to Section 7(o).
(i)
Secretary’s Certificate. On or prior to the date the first Placement Notice is given hereunder, the Sales Agent shall have
received a certificate, signed on behalf of the Company by its corporate secretary, certifying as to (i) the certificate of incorporation
of the Company (as the same may be amended or restated from time to time), (ii) the bylaws of the Company (as the same may be amended
or restated from time to time), (iii) the resolutions of the Board of Directors of the Company (or a committee thereof) authorizing the
execution, delivery and performance of this Agreement and the issuance of the Placement Shares and (iv) the incumbency of the officers
duly authorized to execute this Agreement and the other documents contemplated by this Agreement.
(j)
(j) No Suspension. Trading in the Common Stock shall not have been suspended on the Exchange and the Common Stock shall not have
been delisted from the Exchange.
(k)
Other Materials. On each date on which the Company is required to deliver a certificate pursuant to Section 7(m), the Company
shall have furnished to the Sales Agent such appropriate further opinions, certificates, letters and documents as the Sales Agent may
have reasonably requested. All such opinions, certificates, letters and other documents shall have been in compliance with the provisions
hereof. The Company will furnish the Sales Agent with such conformed copies of such opinions, certificates, letters and other documents
as the Sales Agent shall have reasonably requested.
(l)
Approval for Listing. The Placement Shares shall either have been (i) approved for listing on the Exchange, subject only to notice
of issuance, or (ii) the Company shall have filed an application for listing of the Placement Shares on the Exchange at, or prior to,
the issuance of any Placement Notice.
(m)
No Termination Event. There shall not have occurred any event that would permit the Sales Agent to terminate this Agreement pursuant
to Section 11(a).
(n)
FINRA. The Sales Agent shall have received a letter from the Corporate Financing Department of FINRA confirming that such department
has determined to raise no objection with respect to the fairness or reasonableness of the terms and arrangements related to the sale
of the Placement Shares pursuant to this Agreement.
9.
Indemnification and Contribution.
(a)
Company Indemnification. The Company agrees to indemnify and hold harmless the Sales Agent, the directors, officers, members,
partners, employees and agents of the Sales Agent each broker dealer affiliate of the Sales Agent, and each Sales Agent Affiliate, if
any, from and against any and all losses, claims, liabilities, expenses and damages (including, but not limited to, any and all reasonable
investigative, legal and other expenses incurred in connection with, and any and all amounts paid in settlement (in accordance with Section
9(c)) of, any action, suit or proceeding between any of the indemnified parties and any indemnifying parties or between any indemnified
party and any third party, or otherwise, or any claim asserted), as and when incurred, to which the Sales Agent, or any such person,
may become subject under the Securities Act, the Exchange Act or other federal or state statutory law or regulation, at common law or
otherwise, insofar as such losses, claims, liabilities, expenses or damages arise out of or are based, directly or indirectly, on (x)
any untrue statement or alleged untrue statement of a material fact contained in the Registration Statement or the Prospectus or any
amendment or supplement thereto or in any Issuer Free Writing Prospectus or in any application or other document executed by or on behalf
of the Company or based on written information furnished by or on behalf of the Company filed in any jurisdiction in order to qualify
the Common Stock under the securities laws thereof or filed with the Commission, (y) the omission or alleged omission to state in any
such document a material fact required to be stated in it or necessary to make the statements in it not misleading or (z) any breach
by any of the indemnifying parties of any of their respective representations, warranties and agreements contained in this Agreement;
provided, however, that this indemnity agreement shall not apply to the extent that such loss, claim, liability, expense or damage
arises from the sale of the Placement Shares pursuant to this Agreement and is caused directly by an untrue statement or omission made
in reliance upon and in strict conformity with written information relating to the Sales Agent and furnished to the Company by the Sales
Agent expressly for inclusion in any document as described in clause (x) of this Section 9(a). This indemnity agreement will be
in addition to any liability that the Company might otherwise have.
(b)
The Sales Agent Indemnification. The Sales Agent agrees to indemnify and hold harmless the Company and its directors and each
officer of the Company that signed the Registration Statement, and each person, if any, who (i) controls the Company within the meaning
of Section 15 of the Securities Act or Section 20 of the Exchange Act or (ii) is controlled by or is under common control with the Company
(each, a “Company Affiliate”) from and against any and all losses, claims, liabilities, expenses and damages
(including, but not limited to, any and all reasonable investigative, legal and other expenses incurred in connection with, and any and
all amounts paid in settlement (in accordance with Section 9(c)) of, any action, suit or proceeding between any of the indemnified
parties and any indemnifying parties or between any indemnified party and any third party, or otherwise, or any claim asserted), as and
when incurred, to which any such Company Affiliate, may become subject under the Securities Act, the Exchange Act or other federal or
state statutory law or regulation, at common law or otherwise, insofar as such losses, claims, liabilities, expenses or damages arise
out of or are based, directly or indirectly, on (x) any untrue statement or alleged untrue statement of a material fact contained in
the Registration Statement or the Prospectus or any amendment or supplement thereto, or (y) the omission or alleged omission to state
in any such document a material fact required to be stated in it or necessary to make the statements in it not misleading; provided,
however, that this indemnity agreement shall apply only to the extent that such loss, claim, liability, expense or damage is caused
directly by an untrue statement or omission made in reliance upon and in strict conformity with written information relating to the Sales
Agent and furnished to the Company by the Sales Agent expressly for inclusion in any document as described in clause (x) of this Section
9(b).
(c)
Procedure. Any party that proposes to assert the right to be indemnified under this Section 9 will, promptly after receipt
of notice of commencement of any action against such party in respect of which a claim is to be made against an indemnifying party or
parties under this Section 9, notify each such indemnifying party of the commencement of such action, enclosing a copy of all
papers served, but the omission so to notify such indemnifying party will not relieve the indemnifying party from (i) any liability that
it might have to any indemnified party otherwise than under this Section 9 and (ii) any liability that it may have to any indemnified
party under the foregoing provision of this Section 9 unless, and only to the extent that, such omission results in the forfeiture
of substantive rights or defenses by the indemnifying party. If any such action is brought against any indemnified party and it notifies
the indemnifying party of its commencement, the indemnifying party will be entitled to participate in and, to the extent that it elects
by delivering written notice to the indemnified party promptly after receiving notice of the commencement of the action from the indemnified
party, jointly with any other indemnifying party similarly notified, to assume the defense of the action, with counsel reasonably satisfactory
to the indemnified party, and after notice from the indemnifying party to the indemnified party of its election to assume the defense,
the indemnifying party will not be liable to the indemnified party for any legal or other expenses except as provided below and except
for the reasonable costs of investigation subsequently incurred by the indemnified party in connection with the defense. The indemnified
party will have the right to employ its own counsel in any such action, but the fees, expenses and other charges of such counsel will
be at the expense of such indemnified party unless (1) the employment of counsel by the indemnified party has been authorized in writing
by the indemnifying party, (2) the indemnified party has reasonably concluded (based on advice of counsel) that there may be legal defenses
available to it or other indemnified parties that are different from or in addition to those available to the indemnifying party, (3)
a conflict or potential conflict exists (based on advice of counsel to the indemnified party) between the indemnified party and the indemnifying
party (in which case the indemnifying party will not have the right to direct the defense of such action on behalf of the indemnified
party) or (4) the indemnifying party has not in fact employed counsel to assume the defense of such action within a reasonable time after
receiving notice of the commencement of the action, in each of which cases the reasonable fees, disbursements and other charges of counsel
will be at the expense of the indemnifying party or parties. It is understood that the indemnifying party or parties shall not, in connection
with any proceeding or related proceedings in the same jurisdiction, be liable for the reasonable fees, disbursements and other charges
of more than one separate firm admitted to practice in such jurisdiction at any one time for all such indemnified party or parties. All
such fees, disbursements and other charges will be reimbursed by the indemnifying party promptly as they are incurred. An indemnifying
party will not, in any event, be liable for any settlement of any action or claim effected without its written consent. No indemnifying
party shall, without the prior written consent of each indemnified party, settle or compromise or consent to the entry of any judgment
in any pending or threatened claim, action or proceeding relating to the matters contemplated by this Section 9 (whether or not
any indemnified party is a party thereto), unless such settlement, compromise or consent includes an unconditional release of each indemnified
party from all liability arising or that may arise out of such claim, action or proceeding.
(d)
Contribution. In order to provide for just and equitable contribution in circumstances in which the indemnification provided for
in the foregoing paragraphs of this Section 9 is applicable in accordance with its terms but for any reason is held to be unavailable
from the Company or the Sales Agent, the Company and the Sales Agent will contribute to the total losses, claims, liabilities, expenses
and damages (including any investigative, legal and other expenses reasonably incurred in connection with, and any amount paid in settlement
of, any action, suit or proceeding or any claim asserted, but after deducting any contribution received by the Company from persons other
than the Sales Agent, such as persons who control the Company within the meaning of the Securities Act, officers of the Company who signed
the Registration Statement and directors of the Company, who also may be liable for contribution) to which the Company and the Sales
Agent may be subject in such proportion as shall be appropriate to reflect the relative benefits received by the Company on the one hand
and the Sales Agent on the other. The relative benefits received by the Company on the one hand and the Sales Agent on the other hand
shall be deemed to be in the same proportion as the total Net Proceeds from the sale of the Placement Shares received by the Company
bear to the total compensation received by the Sales Agent from the sale of Placement Shares on behalf of the Company. If, but only if,
the allocation provided by the foregoing sentence is not permitted by applicable law, the allocation of contribution shall be made in
such proportion as is appropriate to reflect not only the relative benefits referred to in the foregoing sentence but also the relative
fault of the Company, on the one hand, and the Sales Agent, on the other, with respect to the statements or omission that resulted in
such loss, claim, liability, expense or damage, or action in respect thereof, as well as any other relevant equitable considerations
with respect to such offering. Such relative fault shall be determined by reference to, among other things, whether the untrue or alleged
untrue statement of a material fact or omission or alleged omission to state a material fact relates to information supplied by the Company
or the Sales Agent, the intent of the parties and their relative knowledge, access to information and opportunity to correct or prevent
such statement or omission. The Company and the Sales Agent agree that it would not be just and equitable if contributions pursuant to
this Section 9(d) were to be determined by pro rata allocation or by any other method of allocation that does not take into account
the equitable considerations referred to herein. The amount paid or payable by an indemnified party as a result of the loss, claim, liability,
expense, or damage, or action in respect thereof, referred to above in this Section 9(d) shall be deemed to include, for the purpose
of this Section 9(d), any legal or other expenses reasonably incurred by such indemnified party in connection with investigating
or defending any such action or claim to the extent consistent with Section 9(c) hereof. Notwithstanding the foregoing provisions
of this Section 9(d), the Sales Agent shall not be required to contribute any amount in excess of the commissions received by
it under this Agreement and no person found guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities
Act) will be entitled to contribution from any person who was not guilty of such fraudulent misrepresentation. For purposes of this Section
9(d), any person who controls a party to this Agreement within the meaning of the Securities Act will have the same rights to contribution
as that party (and any officers, directors, members, partners, employees or agents of the Sales Agent and each broker dealer affiliate
of the Sales Agent will have the same rights to contribution as the Sales Agent), and each officer of the Company who signed the Registration
Statement and each director of the Company will have the same rights to contribution as the Company, subject in each case to the provisions
hereof. Any party entitled to contribution, promptly after receipt of notice of commencement of any action against such party in respect
of which a claim for contribution may be made under this Section 9(d), will notify any such party or parties from whom contribution
may be sought, but the omission to so notify will not relieve that party or parties from whom contribution may be sought from any other
obligation it or they may have under this Section 9(d) except to the extent that the failure to so notify such other party materially
prejudiced the substantive rights or defenses of the party from whom contribution is sought. Except for a settlement entered into pursuant
to the last sentence of Section 9(c) hereof, no party will be liable for contribution with respect to any action or claim settled
without its written consent if such consent is required pursuant to Section 9(c) hereof.
10.
Representations and Agreements to Survive Delivery. The indemnity and contribution agreements contained in Section 9 of
this Agreement and all representations and warranties of the Company herein or in certificates delivered pursuant hereto shall survive,
as of their respective dates, regardless of (i) any investigation made by or on behalf of the Sales Agent, any controlling person of
the Sales Agent, or the Company (or any of their respective officers, directors, members or controlling persons), (ii) delivery and acceptance
of the Placement Shares and payment therefor or (iii) any termination of this Agreement.
11.
Termination.
(a)
The Sales Agent shall have the right by giving notice as hereinafter specified at any time to terminate this Agreement if (i) any Material
Adverse Change, or any development that could reasonably be expected to result in a Material Adverse Change has occurred that, in the
reasonable judgment of the Sales Agent, may materially impair the ability of the Sales Agent to sell the Placement Shares hereunder,
(ii) the Company shall have failed, refused or been unable to perform any agreement on its part to be performed hereunder; provided,
however, in the case of any failure of the Company to deliver (or cause another person to deliver) any certification, opinion,
or letter required under Sections 7(m), 7(n), 7(o) or 7(p), the Sales Agent’s right to terminate shall
not arise unless such failure to deliver (or cause to be delivered) continues for more than thirty (30) days from the date such delivery
was required, (iii) any other condition of the Sales Agent’s obligations hereunder is not fulfilled, or (iv) any suspension or
limitation of trading in the Placement Shares or in securities generally on the Exchange shall have occurred (including automatic halt
in trading pursuant to market-decline triggers, other than those in which solely program trading is temporarily halted), or a major disruption
of securities settlements or clearing services in the United States shall have occurred, or minimum prices for trading have been fixed
on the Exchange. Any such termination shall be without liability of any party to any other party except that the provisions of Section
7(g) (Expenses), Section 9 (Indemnification and Contribution), Section 10 (Representations and Agreements to Survive
Delivery), Section 11(f), Section 16 (Applicable Law; Consent to Jurisdiction) and Section 17 (Waiver of Jury Trial)
hereof shall remain in full force and effect notwithstanding such termination. If the Sales Agent elects to terminate this Agreement
as provided in this Section 11(a), the Sales Agent shall provide the required notice as specified in Section 12 (Notices).
(b)
The Company shall have the right, by giving five (5) days’ notice as hereinafter specified in Section 12, to terminate this
Agreement in its sole discretion at any time after the date of this Agreement. Any such termination shall be without liability of any
party to any other party except that the provisions of Section 7(g), Section 9, Section 10, Section 11(f),
Section 16 and Section 17 hereof shall remain in full force and effect notwithstanding such termination.
(c)
The Sales Agent shall have the right, by giving five (5) days’ notice as hereinafter specified in Section 12, to terminate
this Agreement in its sole discretion at any time after the date of this Agreement. Any such termination shall be without liability of
any party to any other party except that the provisions of Section 7(g), Section 9, Section 10, Section 11(f),
Section 16 and Section 17 hereof shall remain in full force and effect notwithstanding such termination.
(d)
Unless earlier terminated pursuant to this Section 11, this Agreement shall automatically terminate upon the earlier to occur
of (i) issuance and sale of all of the Placement Shares to or through the Sales Agent on the terms and subject to the conditions set
forth herein and (ii) the expiration of the Registration Statement on the third (3rd) anniversary of the initial effective
date of the Registration Statement pursuant to Rule 415(a)(5) under the Securities Act; provided that the provisions of Section
7(g), Section 9, Section 10, Section 11(f), Section 16 and Section 17 hereof shall remain in full
force and effect notwithstanding such termination.
(e)
This Agreement shall remain in full force and effect unless terminated pursuant to Sections 11(a), (b), (c) or (d)
above or otherwise by mutual agreement of the parties; provided, however, that any such termination by mutual agreement
shall in all cases be deemed to provide that Section 7(g), Section 9, Section 10, Section 11(f), Section
16 and Section 17 shall remain in full force and effect.
(f)
Any termination of this Agreement shall be effective on the date specified in such notice of termination; provided, however,
that such termination shall not be effective until the close of business on the date of receipt of such notice by the Sales Agent or
the Company, as the case may be. If such termination shall occur prior to the Settlement Date for any sale of Placement Shares, such
termination shall not become effective until the close of business on such Settlement Date and such Placement Shares shall settle in
accordance with the provisions of this Agreement.
12.
Notices. All notices or other communications required or permitted to be given by any party to any other party pursuant to the
terms of this Agreement shall be in writing, unless otherwise specified, and if sent to the Sales Agent, shall be delivered to:
A.G.P./Alliance
Global Partners
590
Madison Avenue
New
York, NY 10022
Attention:
Tom Higgins
Email:
atm@allianceg.com
with
a copy (which shall not constitute notice) to:
Duane
Morris LLP
1540
Broadway
New
York, NY 10036
Attention:
James T. Seery
Telephone:
(973) 424-2088
Email:
jtseery@duanemorris.com
and
if to the Company, shall be delivered to:
Processa
Pharmaceuticals, Inc.
7380
Coca Cola Drive, Suite 106
Hanover,
Maryland 21076
Attention:
Wendy Guy
Email: wguy@processapharmaceuticals.com
with
a copy (which shall not constitute notice) to:
Foley
& Lardner LLP
One
Independent Drive, Suite 1300
Jacksonville,
Florida 32202
Attention:
Michael Kirwan and John Wolfel
Email:
mkirwan@foley.com; jwolfel@foley.com
Each
party may change such address for notices by sending to the other party to this Agreement written notice of a new address for such purpose.
Each such notice or other communication shall be deemed given (i) when delivered personally on or before 4:30 p.m., New York City time,
on a Business Day or, if such day is not a Business Day, on the next succeeding Business Day, (ii) on the next Business Day after timely
delivery to a nationally-recognized overnight courier and (iii) on the Business Day actually received if deposited in the U.S. mail (certified
or registered mail, return receipt requested, postage prepaid). For purposes of this Agreement, “Business Day”
shall mean any day on which the Exchange and commercial banks in the City of New York are open for business.
An
electronic communication (“Electronic Notice”) shall be deemed written notice for purposes of this Section
12 if sent to the electronic mail address specified by the receiving party under separate cover. Electronic Notice shall be deemed
received at the time the party sending Electronic Notice receives confirmation of receipt by the receiving party (other than pursuant
to auto-reply). Any party receiving Electronic Notice may request and shall be entitled to receive the notice on paper, in a nonelectronic
form (“Nonelectronic Notice”) which shall be sent to the requesting party within ten (10) days of receipt of
the written request for Nonelectronic Notice.
13.
Successors and Assigns. This Agreement shall inure to the benefit of and be binding upon the Company and the Sales Agent and their
respective successors and permitted assigns and, as to Sections 5(b) and 9, the other indemnified parties specified therein.
References to any of the parties contained in this Agreement shall be deemed to include the successors and permitted assigns of such
party. Nothing in this Agreement, express or implied, is intended to confer upon any other person any rights, remedies, obligations or
liabilities under or by reason of this Agreement, except as expressly provided in this Agreement. Neither party may assign its rights
or obligations under this Agreement without the prior written consent of the other party; provided, however, that the Sales
Agent may assign its rights and obligations hereunder to an affiliate of the Sales Agent without obtaining the Company’s consent.
14.
Adjustments for Share Splits. The parties acknowledge and agree that all share-related numbers contained in this Agreement shall
be adjusted to take into account any share split, share dividend or similar event effected with respect to the Common Stock.
15.
Entire Agreement; Amendment; Severability. This Agreement (including all schedules and exhibits attached hereto and Placement
Notices issued pursuant hereto) and any other writing entered into by the parties relating to this Agreement constitutes the entire agreement
and supersedes all other prior and contemporaneous agreements and undertakings, both written and oral, among the parties hereto with
regard to the subject matter hereof. Neither this Agreement nor any term hereof may be amended except pursuant to a written instrument
executed by the Company and the Sales Agent. In the event that any one or more of the provisions contained herein, or the application
thereof in any circumstance, is held invalid, illegal or unenforceable as written by a court of competent jurisdiction, then such provision
shall be given full force and effect to the fullest possible extent that it is valid, legal and enforceable, and the remainder of the
terms and provisions herein shall be construed as if such invalid, illegal or unenforceable term or provision was not contained herein,
but only to the extent that giving effect to such provision and the remainder of the terms and provisions hereof shall be in accordance
with the intent of the parties as reflected in this Agreement.
16.
Applicable Law; Consent to Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the internal laws
of the State of New York, without regard to the principles of conflicts of laws. Each party hereby irrevocably submits to the non-exclusive
jurisdiction of the state and federal courts sitting in the City of New York, Borough of Manhattan, for the adjudication of any dispute
hereunder or in connection with any transaction contemplated hereby, and hereby irrevocably waives, and agrees not to assert in any suit,
action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding
is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives
personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof (certified
or registered mail, return receipt requested) to such party at the address in effect for notices to it under this Agreement and agrees
that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed
to limit in any way any right to serve process in any manner permitted by law.
17.
Waiver of Jury Trial. The Company and the Sales Agent each hereby irrevocably waives any right it may have to a trial by jury
in respect of any claim based upon or arising out of this Agreement or any transaction contemplated hereby.
18.
Absence of Fiduciary Relationship. The Company acknowledges and agrees that:
(a)
the Sales Agent is acting solely as agent in connection with the sale of the Placement Shares contemplated by this Agreement and the
process leading to such transactions, and no fiduciary or advisory relationship between the Company or any of its respective affiliates,
stockholders (or other equity holders), creditors or employees or any other party, on the one hand, and the Sales Agent, on the other
hand, has been or will be created in respect of any of the transactions contemplated by this Agreement, irrespective of whether the Sales
Agent has advised or is advising the Company on other matters, and the Sales Agent has no obligation to the Company with respect to the
transactions contemplated by this Agreement, except the obligations expressly set forth in this Agreement;
(b)
the Company is capable of evaluating and understanding and understands and accepts the terms, risks and conditions of the transactions
contemplated by this Agreement;
(c)
the Sales Agent has not provided any legal, accounting, regulatory or tax advice with respect to the transactions contemplated by this
Agreement, and the Company has consulted its own legal, accounting, regulatory and tax advisors to the extent it has deemed appropriate;
(d)
the Company has been advised and is aware that the Sales Agent and its affiliates are engaged in a broad range of transactions which
may involve interests that differ from those of the Company and that the Sales Agent has no obligation to disclose such interests and
transactions to the Company by virtue of any fiduciary, advisory or agency relationship; and
(e)
the Company waives, to the fullest extent permitted by law, any claims it may have against the Sales Agent, for breach of fiduciary duty
or alleged breach of fiduciary duty and agrees that the Sales Agent shall have no liability (whether direct or indirect, in contract,
tort or otherwise) to the Company in respect of such a fiduciary claim or to any person asserting a fiduciary duty claim on behalf of
or in right of the Company, including stockholders, partners, employees or creditors of the Company.
19.
Use of Information. The Sales Agent may not provide any information gained in connection with this Agreement and the transactions
contemplated by this Agreement, including due diligence, to any third party other than its legal counsel advising it on this Agreement
unless expressly approved by the Company in writing.
20.
Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of
which together shall constitute one and the same instrument. Delivery of an executed Agreement by one party to the other may be made
by facsimile transmission.
21.
Effect of Headings; Knowledge of the Company. The section and Exhibit headings herein are for convenience only and shall not affect
the construction hereof. All references in this Agreement to the “knowledge of the Company” or the “Company’s
knowledge” or similar qualifiers shall mean the actual knowledge of the directors and officers of the Company, after due inquiry.
22.
Definitions. As used in this Agreement, the following term has the meaning set forth below:
(a)
“Applicable Time” means the date of this Agreement, each Representation Date, each date on which a Placement Notice
is given, each Point of Sale, and each Settlement Date.
[Remainder
of Page Intentionally Blank]
If
the foregoing correctly sets forth the understanding between the Company and the Sales Agent, please so indicate in the space provided
below for that purpose, whereupon this letter shall constitute a binding agreement between the Company and the Sales Agent.
| |
Very
truly yours, |
| |
|
| |
PROCESSA
PHARMACEUTICALS, INC. |
| |
|
|
| |
By: |
/s/
George Ng |
| |
Name:
|
George Ng |
| |
Title:
|
Chief Executive Officer |
| |
|
|
| |
ACCEPTED
as of the date first-above written: |
| |
A.G.P./ALLIANCE
GLOBAL PARTNERS |
| |
|
| |
By: |
/s/
Tom Higgins |
| |
Name: |
Tom Higgins |
| |
Title: |
Managing Director |
SCHEDULE
1
Form
of Placement Notice
| |
From: |
Processa
Pharmaceuticals, Inc. |
| |
|
|
| |
To: |
A.G.P./Alliance
Global Partners |
| |
|
Attention:
[●] |
| |
|
|
| |
Subject: |
Placement
Notice |
| |
|
|
| |
Date: |
[●],
202[●] |
Ladies
and Gentlemen:
Pursuant
to the terms and subject to the conditions contained in the Sales Agreement (the “Sales Agreement”) between
Processa Pharmaceuticals, Inc., a Delaware corporation (the “Company”), and A.G.P./Alliance Global Partners
(the “Sales Agent”), dated May [__], 2024, the Company hereby requests that the Sales Agent sell up to [●]
shares of the Company’s common stock, par value $0.0001 per share (the “Placement Shares”), at a minimum
market price of $[●] per share, during the time period beginning [month, day, time] and ending [month, day, time] [and with no
more than [●] Placement Shares sold in any one Trading Day].
[The
Company may include such other sale parameters as it deems appropriate.]
Capitalized
terms used and not defined herein shall have the respective meanings assigned to them in the Sales Agreement.
SCHEDULE
2
Notice
Parties
THE
COMPANY
George
Ng (gng@processapharmaceuticals.com)
James
Stanker (jstanker@processapharmaceuticals.com)
THE
SALES AGENT
Tom
Higgins (thiggins@allianceg.com)
With
copies to:
atm@allianceg.com
SCHEDULE
3
Compensation
The
Company shall pay to the Sales Agent in cash, upon each sale of Placement Shares through the Sales Agent pursuant to this Agreement,
an amount equal to up to 3.0% of the aggregate gross proceeds from each sale of Placement Shares.*
*
The foregoing rate of compensation shall not apply when the Sales Agent purchases Placement Shares on a principal basis, in which case
the Company may sell the Placement Shares to the Sales Agent as principal at a price to be mutually agreed upon by the Company and the
Sales Agent at the relevant Point of Sale pursuant to the applicable Placement Notice (it being hereby acknowledged and agreed that the
Sales Agent shall be under no obligation to purchase Placement Shares on a principal basis pursuant to the Sales Agreement, except as
otherwise agreed by the Sales Agent and the Company in writing and expressly set forth in a Placement Notice).
Exhibit
7(m)
OFFICER
CERTIFICATE
The
undersigned, the duly qualified and appointed _____________________ of Processa Pharmaceuticals, Inc., a Delaware corporation (the “Company”),
does hereby certify in such capacity and on behalf of the Company, pursuant to Section 7(m) of the Sales Agreement, dated May
[__], 2024 (the “Sales Agreement”), between the Company and A.G.P./Alliance Global Partners, that:
| |
(i) |
the
representations and warranties of the Company in Section 6 of the Sales Agreement (A) to the extent such representations and
warranties are subject to qualifications and exceptions contained therein relating to materiality or Material Adverse Change, are
true and correct on and as of the date hereof with the same force and effect as if expressly made on and as of the date hereof, except
for those representations and warranties that speak solely as of a specific date and which were true and correct as of such date,
and (B) to the extent such representations and warranties are not subject to any qualifications or exceptions, are true and correct
in all material respects as of the date hereof as if made on and as of the date hereof with the same force and effect as if expressly
made on and as of the date hereof except for those representations and warranties that speak solely as of a specific date and which
were true and correct as of such date; and; |
| |
|
|
| |
(ii) |
the
Company has complied with all agreements and satisfied all conditions on its part to be performed or satisfied pursuant to the Sales
Agreement at or prior to the date hereof; |
| |
|
|
| |
(iii) |
as
of the date hereof, (i) the Registration Statement does not contain any untrue statement of a material fact or omit to state a material
fact required to be stated therein or necessary in order to make the statements therein not misleading, (ii) the Prospectus does
not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in
order to make the statements therein, in light of the circumstances under which they were made, not misleading and (iii) no event
has occurred as a result of which it is necessary to amend or supplement the Registration Statement or the Prospectus in order to
make the statements therein not untrue or misleading for clauses (i) and (ii) above, respectively, to be true and correct; |
| |
|
|
| |
(iv) |
there
has been no Material Adverse Change since the date as of which information is given in the Prospectus, as amended or supplemented; |
| |
|
|
| |
(v) |
the
Company will not be in possession of any material non-public information at the time of delivery of any Placement Notice and/or as
long as such Placement Notice is effective; and |
| |
|
|
| |
(vi) |
the
aggregate offering price of the Placement Shares that may be issued and sold pursuant to the Sales Agreement and the maximum number
or amount of Placement Shares that may be sold pursuant to the Sales Agreement have been duly authorized by the Company’s board
of directors or a duly authorized committee thereof. |
Terms
used herein and not defined herein have the meanings ascribed to them in the Sales Agreement.
Exhibit
5.1
 |
ATTORNEYS
AT LAW LLP
ONE
INDEPENDENT DRIVE, SUITE 1300
JACKSONVILLE,
FL 32202-5017
904.359.2000
TEL
904.359.8700
FAX
WWW.FOLEY.COM |
May
21, 2024
Processa
Pharmaceuticals, Inc.
7380
Coca Cola Drive, Suite 106
Hanover,
MD 21076 |
|
| |
Re: |
Registration
Statement on Form S-3 |
Ladies
and Gentlemen:
This
opinion is being furnished in connection with the Registration Statement on Form S-3 of Processa Pharmaceuticals, Inc. a Delaware corporation
(the “Company”), under the Securities Act of 1933, as amended (the “Securities Act”), for the proposed
issuance from time to time, as set forth in the prospectus contained in such Registration Statement (the “Prospectus”)
and as to be set forth in one or more supplements to the Prospectus, of (a) common stock, par value $0.0001 per share (the “Common
Stock”), (b) one or more series of preferred stock, par value $0.0001 per share (the “Preferred Stock”),
(c) warrants to purchase the Company’s common stock or preferred stock (“Warrants”) and (d) units (the “Units”)
that relate to or are comprised of any of the foregoing securities (collectively, the “Offered Securities”).
In
rendering the opinions set forth herein, we have examined and relied upon originals, or copies certified or otherwise identified to our
satisfaction, of the following documents:
(a)
The Registration Statement;
(b)
The Fourth Amended and Restated Certificate of Incorporation of the Company (the “Charter”);
(c)
The amended and restated Bylaws of the Company (the “Bylaws”); and
(d)
Resolutions adopted by the Board of Directors of the Company (the “Board”) relating to the registration of the Offered
Securities.
We
have also examined originals or copies, certified or otherwise identified to our satisfaction, of such records of the Company and such
agreements, certificates and receipts of public officials, certificates of officers or other representatives of the Company and others,
and such other documents as we have deemed necessary or appropriate as a basis for the opinions stated below.
AUSTIN
Boston
CHICAGO
dallas
DENVER |
DETROIT
houston
JACKSONVILLE
LOS
ANGELES
MADISON |
MEXICO
CITY
MIAMI
MILWAUKEE
NEW
YORK
ORLANDO |
SACRAMENTO
Salt
lake city
SAN
DIEGO
SAN
FRANCISCO
SILICON
VALLEY |
Tallahassee
TAMPA
WASHINGTON,
D.C.
BRUSSELS
TOKYO |
May
21, 2024
Page
2
In
our examination, we have assumed the genuineness of all manual and electronic signatures (including, without limitation, signatures delivered
via electronic signature systems such as DocuSign, SecureDocs, or comparable electronic signature methods or systems), including endorsements,
the legal capacity and competency of all natural persons, the authenticity of all documents submitted to us as originals, the conformity
to original documents of all documents submitted to us as facsimile, electronic, certified or photostatic copies, and the authenticity
of the originals of such copies. As to any facts relevant to the opinions stated herein that we did not independently establish or verify,
we have relied upon statements and representations of officers and other representatives of the Company and others and of public officials.
The
opinions stated in paragraphs 1 through 4 below presume that all of the following (collectively, the “general conditions”)
shall have occurred prior to the issuance of the Offered Securities referred to therein: (i) the Registration Statement, as finally amended
(including all necessary post-effective amendments), has become effective under the Securities Act; (ii) an appropriate prospectus supplement
with respect to such Offered Securities has been prepared, delivered and filed in compliance with the Securities Act and the applicable
Rules and Regulations; (iii) the applicable transaction agreements shall have been duly authorized, executed and delivered by the Company
and the other parties thereto, including, if such Offered Securities are to be sold or otherwise distributed pursuant to an underwritten
offering, the underwriting agreement or purchase agreement with respect thereto; (iv) the Board, including any duly authorized committee
thereof, shall have taken all necessary corporate action to approve the issuance and sale of such Offered Securities and related matters
and appropriate officers of the Company have taken all related action as directed by or under the direction of the Board; and (v) the
terms of the applicable transaction agreements and the issuance and sale of such Offered Securities have been duly established in conformity
with the Charter so as not to violate any applicable law, the Charter or the Bylaws, or result in a default under or breach of any agreement
or instrument binding upon the Company, and so as to comply with any requirement or restriction imposed by any court or governmental
body having jurisdiction over the Company, the Warrant Agent and Unit Agent, as applicable.
Based
upon the foregoing and subject to the qualifications and assumptions stated herein, we are of the opinion that:
1.
With respect to any shares of the Common Stock offered by the Company pursuant to the Registration Statement (the “Offered Common
Stock”), when (a) the general conditions shall have been satisfied, (b) if the Offered Common Stock is to be certificated,
certificates in the form required under the Delaware General Corporation Law (“DGCL”) representing the shares of Offered
Common Stock are duly executed and countersigned, and (c) the shares of Offered Common Stock are registered in the Company’s share
registry and delivered upon payment of the agreed-upon consideration therefor, the shares of Offered Common Stock, when issued and sold
or otherwise distributed in accordance with the provisions of the applicable transaction agreements and, if distributed pursuant to an
underwritten offering, in accordance with the provisions of the underwriting agreement or purchase agreement with respect thereto, will
be duly authorized by all requisite corporate action on the part of the Company under the DGCL and validly issued, fully paid and nonassessable,
provided that the consideration therefor is not less than the par value per share of the Common Stock.
May
21, 2024
Page
3
2.
With respect to any shares of the Preferred Stock offered by the Company pursuant to the Registration Statement (the “Offered
Preferred Stock”), when (a) the general conditions shall have been satisfied, (b) the Board, or a duly authorized committee
thereof, has duly adopted articles supplementary for the Offered Preferred Stock in accordance with the DGCL, (c) such articles supplementary
have been duly filed with and accepted for record by the Secretary of the State of Delaware establishing the relative powers, designations,
preferences, rights, qualifications, limitations or restrictions of such Offered Preferred Stock, (d) if the Offered Preferred Stock
is to be certificated, certificates in the form required under the DGCL representing the shares of Offered Preferred Stock are duly executed
and countersigned, and (e) the shares of Offered Preferred Stock are registered in the Company’s share registry and delivered upon
payment of the agreed-upon consideration therefor, the shares of Offered Preferred Stock, when issued and sold or otherwise distributed
in accordance with the provisions of the applicable transaction agreements and, if distributed pursuant to an underwritten offering,
in accordance with the provisions of the underwriting agreement or purchase agreement with respect thereto, will be duly authorized by
all requisite corporate action on the part of the Company under the DGCL and validly issued, fully paid and nonassessable, provided that
the consideration therefor is not less than the par value per share of the Preferred Stock.
3.
With respect to any Warrants offered by the Company pursuant to the Registration Statement (the “Offered Warrants”),
when (a) the general conditions shall have been satisfied, (b) the shares of Common Stock, shares of Preferred Stock or other securities
described in the Registration Statement for which the Offered Warrants are exercisable have been duly authorized for issuance by the
Company, (c) the terms of the Offered Warrants have been established in accordance with the Warrant Agreement, and (d) the Offered Warrants
have been duly executed (if certificated) and delivered in accordance with the provisions of the applicable Warrant Agreement, the Offered
Warrants, when issued and sold or otherwise distributed in accordance with the provisions of the applicable Warrant Agreement and, if
distributed pursuant to an underwritten offering, in accordance with the provisions of the underwriting agreement or purchase agreement
with respect thereto, and upon payment of the agreed-upon consideration therefor, will constitute valid and binding obligations of the
Company, enforceable against the Company in accordance with their respective terms under the laws of the State of Delaware.
4.
With respect to any Units offered by the Company pursuant to the Registration Statement (the “Offered Units”), when
(a) the general conditions shall have been satisfied, (b) the shares of Common Stock, shares of Preferred Stock, Warrants or other securities
or a combination thereof included in such Offered Units have been duly authorized for issuance by the Company, (c) the terms of the Offered
Units have been established in accordance with the applicable Unit Agreement, and (d) the Offered Units have been duly executed (if certificated)
and delivered in accordance with the provisions of the applicable Unit Agreement, the Offered Units, when issued and sold or otherwise
distributed in accordance with the provisions of the applicable Unit Agreement and, if distributed pursuant to an underwritten offering,
in accordance with the provisions of the underwriting agreement or purchase agreement with respect thereto, and upon payment of the agreed-upon
consideration therefor, will constitute valid and binding obligations of the Company, enforceable against the Company in accordance with
their respective terms under the laws of the State of Delaware.
May
21, 2024
Page
4
Our
opinion is limited to the Delaware General Corporation Law and the federal laws of the United States of America to the extent referred
to specifically herein, in each case as are, in our professional judgment, normally applicable to transactions of the type contemplated
by the Registration Statement, without our having made any special investigation as to the applicability of any specific law, rule or
regulation, and we do not express any opinion herein concerning any other laws, statutes, ordinances, rules or regulations. This opinion
letter speaks only as of the date hereof, and we make no undertaking, and expressly disclaim any duty or obligation, to supplement or
update this opinion letter or the opinion expressed herein, if after the date of this opinion letter, facts and/or circumstances come
to our attention, and/or changes in the law occur, which could affect such opinion. This letter is limited to the matters stated herein,
and no opinion is implied or may be inferred beyond the matters expressly stated.
The
opinions stated herein are subject to the following qualifications:
(a)
the opinions stated herein are limited by applicable bankruptcy, insolvency, reorganization, moratorium, fraudulent transfer, preference
and other similar laws affecting creditors’ rights generally, and by general principles of equity (regardless of whether enforcement
is sought in equity or at law);
(b)
we do not express any opinion with respect to any law, rule or regulation that is applicable to any party to any of the transaction agreements
or the transactions contemplated thereby solely because such law, rule or regulation is part of a regulatory regime applicable to any
such party or any of its affiliates as a result of the specific assets or business operations of such party or such affiliates;
(c)
except to the extent expressly stated in the opinions contained herein, we have assumed that each of the transaction agreements constitutes
the valid and binding obligation of each party to such transaction agreement, enforceable against such party in accordance with its terms;
and
(d)
we have assumed that any Warrants and Units that may be issued will be manually authenticated, signed or countersigned, as the case may
be, by duly authorized officers of any Warrant Agent and Unit Agent, as the case may be.
We
hereby consent to the inclusion of this opinion as Exhibit 5.1 in said Registration Statement and to the reference to this firm under
the caption “Legal Matters” in the Prospectus. In giving this consent, we do not hereby admit that we come within the category
of persons whose consent is required under Section 7 of the Securities Act of 1933, as amended, or the rules or regulations of the Securities
and Exchange Commission promulgated thereunder.
| |
Sincerely, |
| |
|
| |
/s/
FOLEY & LARDNER LLP |
Exhibit 5.2
May
21, 2024 |
ATTORNEYS
AT LAW
one
independent drive, suite 1300
Jacksonville,
Florida 32202-5017
904.359.2000
TEL
904.359.8700
FAX
www.foley.com |
| |
|
Processa
Pharmaceuticals, Inc.
7380
Coca Cola Drive, Suite 106
Hanover,
Maryland 21076 |
|
Ladies
and Gentlemen:
We
have acted as counsel to Processa Pharmaceuticals, Inc., a Delaware corporation (the “Company”), in connection with
the Company’s issuance and sale, through A.G.P./Alliance Global Partners (the “Sales Agent”), of up to $2.4
million of shares of the Company’s common stock, par value $0.0001 per share (the “Placement Shares”), from
time to time and at various prices in an “at the market offering” pursuant to (i) that certain Sales Agreement, dated May
21, 2024 (the “Sales Agreement”), by and between the Company and the Sales Agent, and (ii) the Company’s Registration
Statement on Form S-3 filed with the Securities and Exchange Commission (the “Commission”) on the date hereof (the
“Registration Statement”), the base prospectus filed as part of the Registration Statement (the “Base Prospectus”),
and the prospectus supplement contained in the Registration Statement (together with the Base Prospectus, the “Prospectus”).
In
connection with our representation, we have examined: (i) the Sales Agreement, (ii) the Registration Statement and the Prospectus, (iii)
a certified copy of the Fourth Amended and Restated Certificate of Incorporation of the Company, as amended, (iv) a copy of the Amended
and Restated Bylaws of the Company and all amendments thereto certified as true, correct, and complete by the Secretary of the Company,
and (v) the proceedings and actions taken by the Board of Directors of the Company to authorize and approve the transactions contemplated
by the Sales Agreement and the execution and delivery of the Sales Agreement. We have also considered such matters of law and of fact,
including the examination of originals or copies, certified or otherwise identified to our satisfaction, of such records and documents
of the Company, certificates of officers, directors and representatives of the Company, certificates of public officials, and such other
documents as we have deemed appropriate as a basis for the opinions set forth below. In our examination of the above-referenced documents,
we have assumed all natural persons who are signatories to the Agreement or the other documents reviewed by us were legally competent
at the time of execution; all electronic and manual signatures on the Sales Agreement and the other documents reviewed by us (including,
without limitation, signatures delivered via electronic signature systems such as DocuSign, SecureDocs, or comparable electronic signature
methods or systems) are genuine signatures of the purported signatories, executed or adopted with an intent to execute, authenticate,
or adopt such document; the copies of all documents and records submitted to us are accurate and complete, each such document that is
original is authentic, and each such document that is a copy conforms to an authentic original; and the documents executed and delivered
by the parties are in substantially the same form as the forms of those documents that we have reviewed in rendering this.
AUSTIN
Boston
CHICAGO
dallas
DENVER |
DETROIT
houston
JACKSONVILLE
LOS
ANGELES
MADISON |
MEXICO
CITY
MIAMI
MILWAUKEE
NEW
YORK
ORLANDO |
SACRAMENTO
Salt
lake city
SAN
DIEGO
SAN
FRANCISCO
SILICON
VALLEY |
Tallahassee
TAMPA
WASHINGTON,
D.C.
BRUSSELS
TOKYO |
Processa
Pharmaceuticals, Inc.
May
21, 2024
Page
2
Our
opinions expressed herein are limited to the Delaware General Corporation Law, and we express no opinion as to the laws of any other
jurisdiction.
Based
upon, subject to and limited by the foregoing, we are of the opinion that, upon the issuance of the Placement Shares pursuant to the
terms of the Sales Agreement and the receipt by the Company of the consideration for the Placement Shares pursuant to the terms of the
Sales Agreement, the Placement Shares will be validly issued, fully paid, and nonassessable.
This
opinion is issued as of the date hereof, and we assume no obligation to supplement this opinion if any applicable law changes after the
date hereof or if we become aware of any fact that might change the opinion expressed herein after the date hereof. This opinion is limited
to the matters set forth herein, and no other opinion should be inferred beyond the matters expressly stated.
We
consent to the filing of this opinion in accordance with the requirements of Item 601(b)(5) of Regulation S-K under the Securities Act
of 1933, as amended (the “Securities Act”), as Exhibit 5.2 to the Registration Statement and to the references to
our firm therein. In giving our consent, we do not admit that we are “experts” within the meaning of Section 11 of the Securities
Act or within the category of persons whose consent is required by Section 7 of the Securities Act.
| |
Sincerely, |
| |
|
| |
/s/
FOLEY & LARDNER LLP |
Exhibit
23.1
CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We
consent to the incorporation by reference in this Registration Statement on Form S-3 of our report dated March 29, 2024, with respect
to the audited financial statements of Processa Pharmaceuticals, Inc. for the years ended December 31, 2023 and 2022 included in the
Annual Report on Form 10-K of Processa Pharmaceuticals, Inc. for the year ended December 31, 2023. We also consent to the reference to
our firm under the heading “Experts” in such Registration Statement.
/s/
BD & Company, Inc.
Owings
Mills, MD
May
21, 2024
Exhibit
107
Calculation
of Filing Fee Tables
Form
S-3
PROCESSA
PHARMACEUTICALS, INC.
(Exact
Name of Registrant as Specified in its Charter)
Security
Type(1) |
|
Security Class Title |
|
|
Fee
Calculation
Rule |
|
|
Amount
Registered(2) |
|
|
Proposed
Maximum
Offering
Price Per
Share(3) |
|
|
Maximum
Aggregate
Offering
Price(2) |
|
|
Fee Rate |
|
|
Amount of
Registration
Fee |
|
| Equity |
|
|
Common Stock, $0.0001 par value per share |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Equity |
|
|
Preferred Stock, $0.0001 par value per share |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Other |
|
|
Warrants |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Other |
|
|
Units(4) |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
| Unallocated (Universal) Shelf |
|
|
— |
|
|
|
457(o |
) |
|
|
— |
|
|
|
— |
|
|
$ |
50,000,000 |
|
|
|
0.00014760 |
|
|
$ |
7,380 |
|
| Total Offering Amounts |
|
|
$ |
7,380 |
|
| Total Fees Previously Paid |
|
|
|
- |
|
| Net Fee Due |
|
|
$ |
7,380 |
|
(1) Represents
securities that may be offered and sold from time to time in one or more offerings by Processa Pharmaceuticals, Inc.
(2) There
are being registered under this registration statement such indeterminate number of shares of common stock and preferred stock; such
indeterminate number of warrants to purchase common stock and/or preferred stock; and such indeterminate number of units as may be sold
by the registrant from time to time, which together shall have an aggregate initial offering price not to exceed $50.0 million.
Any securities registered hereunder may be sold separately or as units with other securities registered hereunder. In addition, pursuant
to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), the shares being registered hereunder include
such indeterminate number of shares of common stock and preferred stock as may be issuable with respect to the shares being registered
hereunder as a result of stock splits, stock dividends, or similar events.
(3) The
proposed maximum aggregate offering price per class of security will be determined from time to time by the Registrant in connection
with the issuance by the Registrant of the securities registered hereunder and is not specified as to each class of security pursuant
to General Instruction II.D. of Form S-3 under the Securities Act. Separate consideration may or may not be received for securities that
are issuable on exercise, conversion or exchange of other securities, or that are issued in units.
(4) Each
unit will represent an interest in two or more securities, which may or may not be separable from one another.
Processa Pharmaceuticals (NASDAQ:PCSA)
Historical Stock Chart
From May 2024 to Jun 2024
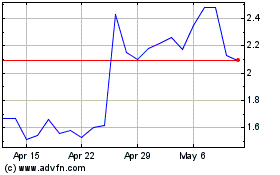
Processa Pharmaceuticals (NASDAQ:PCSA)
Historical Stock Chart
From Jun 2023 to Jun 2024