false
0001583107
0001583107
2024-05-13
2024-05-13
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
Current Report Pursuant
to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event Reported):
May 13, 2024
THERAVANCE BIOPHARMA, INC.
(Exact Name of Registrant as Specified in its
Charter)
| Cayman Islands |
|
001-36033 |
|
98-1226628 |
| (State
or Other Jurisdiction of |
|
(Commission
File Number) |
|
(I.R.S.
Employer Identification |
| Incorporation) |
|
|
|
Number) |
C/O Theravance Biopharma US, Inc.
901 Gateway Boulevard
South San Francisco, CA
94080
(650)
808-6000
(Addresses, including zip code, and telephone
numbers, including area code, of principal executive offices)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General
Instruction A.2. below):
¨
Written communications pursuant
to Rule 425 under the Securities Act (17 CFR 230.425)
¨
Soliciting material pursuant to
Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨
Pre-commencement communications
pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨
Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Ordinary
Share $0.00001 Par Value |
|
TBPH |
|
NASDAQ
Global Market |
Indicate by check mark whether the registrant is
an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of
the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company ¨
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 2.02. Results of Operations and Financial
Condition.
On May 13, 2024, Theravance Biopharma, Inc. (the “Company”)
issued a press release and is holding a conference call regarding its financial results for the quarter ended March 31, 2024 and a business
update. A copy of the press release is furnished as Exhibit 99.1 to this Current Report and a copy of materials that will accompany the
call is furnished as Exhibit 99.2 to this Current Report.
The information in Item 2.02 and in Item 9.01 of this Current Report
on Form 8-K, including Exhibits 99.1 and 99.2, is being furnished and shall not be deemed “filed” for the purposes of Section
18 of the Securities Exchange Act of 1934, as amended (the “Securities Exchange Act of 1934”), or otherwise subject to the
liabilities of that Section, nor shall it be incorporated by reference in any filing under the Securities Act of 1933, as amended, or
the Securities Exchange Act of 1934, except as expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| 104 | Cover Page Interactive
Data File (cover page XBRL tags embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
|
|
| |
THERAVANCE BIOPHARMA, INC. |
| |
|
|
| Date: May 13, 2024 |
By: |
/s/ Aziz Sawaf |
| |
|
Aziz Sawaf |
| |
|
Senior Vice President and Chief Financial Officer |
Exhibit 99.1

Theravance Biopharma, Inc. Reports First
Quarter 2024
Financial Results and Provides Business Update
| • | Q1 2024 YUPELRI® (revefenacin) net sales of $55.2 million,
recognized by Viatris, increased 18% from Q1 20231 |
| • | Viatris collaboration revenue of $14.5 million, increased 39% versus Q1
2023, reflecting margin improvement |
| • | Continued progress for ampreloxetine CYPRESS enrollment |
| • | Virtual ampreloxetine KOL event scheduled for May 23, 2024 |
| • | Q1 2024 ending cash balance of $100 million |
DUBLIN, IRELAND
– MAY 13, 2024 – Theravance Biopharma, Inc. (“Theravance
Biopharma” or the “Company”) (NASDAQ: TBPH) today announced financial and operational results for the first quarter
of 2024.
“Building
on recent momentum, the Theravance commercial team again delivered a solid quarter of YUPELRI hospital sales execution in the first quarter,
setting us on a path to contribute significantly to the product’s overall growth in 2024”, said Rick E Winningham,
Chief Executive Officer. “We remain laser focused on YUPELRI growth and CYPRESS execution and are looking forward to sharing more
about our progress and plans for ampreloxetine at a Key Opinion Leader-led virtual investor event scheduled for May 23rd.”
First Quarter Highlights
YUPELRI®
(revefenacin) inhalation solution, the first and only once-daily, nebulized LAMA (long- acting muscarinic agent) bronchodilator approved
in the US for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD):
| • | Achieved total net sales of $55.2 million for the quarter, increasing 18%
year-over-year (Q1 2024 vs Q1 2023).1 |
| • | Grew doses sold into the hospital channel by 31% year-over-year (Q1 2024
vs Q1 2023).2 |
| • | Increased share within the long-acting nebulized segment of the COPD market.
During the quarter, share within the community and hospital settings increased year-over-year to 30.5% and 16.6%, respectively, from 28.0%
and 15.0% in Q1 2023.3 |
Ampreloxetine, an investigational,
once-daily norepinephrine reuptake inhibitor in development for the treatment of symptomatic neurogenic orthostatic hypotension (nOH)
in patients with multiple system atrophy (MSA):
| • | Virtual ampreloxetine KOL event scheduled for May 23, 2024. |
| • | Enrollment continues globally for the CYPRESS study, with footprint expanded
to include active sites in Latin America and Asia Pacific. |
| • | CYPRESS enrollment into the open-label period of the study expected to be
completed in the second half of 2024. |
1
In the US, Viatris is leading the commercialization of YUPELRI, and the Company co-promotes the product under a profit and loss sharing
arrangement (65% to Viatris; 35% to the Company).
2
Source: IQVIA DDD, HDS, VA and Non-Reporting Hospital through Mar’24.
3
Hospital LA-NEB Market Share - IQVIA DDD through 12/31/2023. Community LA-NEB Market Share includes Retail + DME / Med B FFS through
Nov ’23.

TRELEGY Update:
| • | GSK posted first quarter 2024 global net sales of $749 million (up 32% from
$567 million reported in the first quarter of 2023). As of January 1, 2024, Theravance Biopharma is eligible to receive a total of
$200 million in milestone payments from Royalty Pharma, should TRELEGY achieve certain sales thresholds. The next milestone payment of
$25 million will be achieved if TRELEGY global net sales are approximately $2.9 billion in 2024 (an increase of 5% compared with 2023).
A second milestone payment of another $25 million (for a total of $50 million) can be achieved if TRELEGY global net sales exceed approximately
$3.2 billion in 2024 (an increase of 17% compared with 2023).4 |
First Quarter Financial
Results
| • | Revenue: Total revenue for the first quarter of 2024 was $14.5 million,
consisting entirely of Viatris collaboration revenue. Viatris collaboration revenue increased by $4.1 million, or 39%, in the first quarter
compared to the same period in 2023 due primarily to higher net sales and lower costs incurred by Viatris. The Viatris collaboration revenue
represents amounts receivable from Viatris and comprises the Company’s 35% share of net sales of YUPELRI, as well as its proportionate
amount of the total shared costs incurred by the two companies. The non-shared YUPELRI costs incurred by Theravance Biopharma are recorded
within operating expenses. While Viatris records the total net sales of YUPELRI within its financial statements, Theravance Biopharma’s
implied 35% share of net sales of YUPELRI for the first quarter of 2024 was $19.3 million which represents a 18% increase compared to
the same period in 2023. |
| • | Research and Development (R&D) Expenses: R&D expenses for
the first quarter of 2024 were $9.0 million, compared to $14.6 million in the same period in 2023. First quarter R&D expenses included
total non-cash share-based compensation of $1.5 million. |
| • | Selling, General and Administrative (SG&A) Expenses: SG&A
expenses for the first quarter of 2024 were $16.7 million, compared to $19.2 million in the same period in 2023. First quarter SG&A
expenses included total non-cash share-based compensation of $3.7 million. |
| • | Share-Based Compensation: Share-based compensation expenses for the
first quarter of 2024 was $5.2 million, compared to $7.0 million in the same period in 2023. Share-based compensation expenses consisted
of $1.5 million for R&D and $3.7 million for SG&A in the first quarter of 2024, compared to $2.4 million and $4.2 million, respectively,
in the same period in 2023. In the first quarter of 2023, we also incurred $0.4 million in restructuring-related share-based compensation
expenses. |
4
The next milestone payment of $25.0 million will be triggered if Royalty Pharma receives $240.0 million or more in royalty payments
from GSK with respect to 2024 TRELEGY global net sales, which we would expect to occur in the event TRELEGY global net sales reach approximately
$2.863 billion. Another milestone payment of $25.0 million will be received if Royalty Pharma receives $275.0 million or more in royalty
payments from GSK with respect to 2024 TRELEGY global net sales, which we would expect to occur in the event TRELEGY global net sales
reach approximately $3.213 billion. Royalties payable from GSK to Royalty Pharma are upward tiering from 6.5% to 10%.

| • | Net Loss and Non-GAAP Net Loss from Operations5:
Net loss was $11.7 million in the first quarter of 2024 compared to $22.1 million in the same period in 2023, and non-GAAP net loss from
operations was $4.5 million in the first quarter 2024 compared to a non-GAAP net loss from operations of $14.9 million in the same period
in 2023. See the section titled "Non-GAAP Financial Measures" for more information. |
| • | Cash Position: Cash, cash equivalents and marketable securities totaled
$100.0 million as of March 31, 2024. |
2024 Financial Guidance
| • | Operating Expenses (excluding share-based
compensation): The Company continues to expect full year 2024 R&D expenses of $30 million to $36 million and SG&A expenses
of $45 million to $55 million, in each case excluding share-based compensation. |
| • | Share-Based Compensation: The Company continues to expect full year
share-based compensation expenses of $18 million to $22 million. |
| • | Non-GAAP Net Profit / Loss: The Company continues to expect Non-GAAP
net loss in the first half of 2024 and to approach non-GAAP breakeven in the second half of 2024; limited cash burn expected in 2024. |
Conference Call and Live Webcast Today at 5:00 pm ET
Theravance Biopharma will hold a conference
call and live webcast accompanied by slides today at 5:00 pm ET / 2:00 pm PT / 10:00 pm IST. To participate in the live call
by telephone, please register here. Those interested in listening to the conference call live via the internet may do so by visiting Theravance
Biopharma’s website at www.theravance.com, under the Investors section, Events and Presentations.
A replay of the webcast will be available on Theravance Biopharma’s
website for 30 days through June 12, 2024.
About Ampreloxetine
Ampreloxetine, an investigational, once-daily norepinephrine reuptake
inhibitor in development for the treatment of symptomatic neurogenic orthostatic hypotension (nOH) in patients with multiple system atrophy
(MSA). The unique benefits of ampreloxetine treatment reported in MSA patients from Study 0170 included an increase in norepinephrine
levels, a favorable impact on blood pressure, clinically meaningful and durable symptom improvement, and no signal for supine hypertension.
In the US, the Company has been granted an Orphan Drug Designation for ampreloxetine for the treatment of symptomatic nOH in patients
with MSA and, if results from the ongoing Phase 3 CYPRESS study are supportive, plans to file an NDA for full approval in this indication.
5
Non-GAAP profit (loss) consists of GAAP net income (loss) before taxes less share-based compensation expense and non-cash interest expense.
See the section titled "Non-GAAP Financial Measures" for more information.

About CYPRESS (Study 0197), a Phase 3 Study
Study 0197 (NCT05696717)
is currently enrolling. This is a registrational Phase 3, multi-center, randomized withdrawal study to evaluate the efficacy and durability
of ampreloxetine in participants with MSA and symptomatic nOH after 20 weeks of treatment; the primary endpoint of the study is change
in the Orthostatic Hypotension Symptom Assessment (OHSA) composite score. The Study includes four periods: screening, open label (12-week
period, participants will receive a single daily 10 mg dose of ampreloxetine), randomized withdrawal (eight-week period, double-blind,
placebo-controlled, participants will receive a single daily 10 mg dose of placebo or ampreloxetine), and a long-term treatment extension.
Secondary outcome measures include change from baseline in Orthostatic Hypotension Daily Activity Scale (OHDAS) item 1 (activities that
require standing for a short time) and item 3 (activities that require walking for a short time).
About Multiple System Atrophy (MSA) and Symptomatic Neurogenic Orthostatic
Hypotension (nOH)
MSA is a progressive brain disorder that affects movement and balance
and disrupts the function of the autonomic nervous system. The autonomic nervous system controls body functions that are mostly involuntary.
One of the most frequent autonomic symptoms associated with MSA is a sudden drop in blood pressure upon standing (nOH).6
There are approximately 50,000 MSA patients in the US7 and 70-90% of MSA patients experience nOH symptoms.8
Despite available therapies, many MSA patients remain symptomatic with nOH.
Neurogenic orthostatic
hypotension (nOH) is a rare disorder defined as a fall in systolic blood pressure of ≥20 mm Hg or diastolic blood pressure
of ≥10 mm Hg, within 3 minutes of standing. Severely affected patients are unable to stand for more
than a few seconds because of their decrease in blood pressure, leading to cerebral hypoperfusion and syncope. A debilitating condition,
nOH results in a range of symptoms including dizziness, lightheadedness, fainting, fatigue, blurry vision, weakness, trouble concentrating,
and head and neck pain.
About Theravance Biopharma
Theravance Biopharma, Inc.’s focus
is to deliver Medicines that Make a Difference® in people’s lives. In pursuit of its purpose, Theravance Biopharma
leverages decades of expertise, which has led to the development of FDA-approved YUPELRI® (revefenacin) inhalation solution
indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). Ampreloxetine, its late-stage investigational
once-daily norepinephrine reuptake inhibitor in development for symptomatic neurogenic orthostatic hypotension (nOH) in patients with
Multiple System Atrophy (MSA), has the potential to be a first in class therapy effective in treating a constellation of cardinal symptoms
in MSA patients. The Company is committed to creating/driving shareholder value.
For more information,
please visit www.theravance.com.
6
https://medlineplus.gov/genetics/condition/multiple-system-atrophy/
7
UCSD Neurological Institute (25K-75K, with ~10K new cases per year); NIH National Institute of Neurological Disorders and Stroke (15K-50K).
8
Delveinsight MSA Market Forecast (2023); Symptoms associated with orthostatic hypotension in pure autonomic failure and multiple systems
atrophy, CJ Mathias (1999).

THERAVANCE BIOPHARMA®, THERAVANCE®
and the Cross/Star logo are registered trademarks of the Theravance Biopharma group of companies (in the U.S. and
certain other countries).
YUPELRI® is a registered trademark
of Mylan Specialty L.P., a Viatris company. Trademarks, trade names or service marks of other companies appearing in this press
release are the property of their respective owners.
Forward-Looking Statements
This press release and the conference call will
contain certain "forward-looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995
regarding, among other things, statements relating to goals, plans, objectives, expectations and future events. Theravance Biopharma intends
such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E
of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995. Examples of such statements
include statements relating to: the Company’s expectations regarding its future profitability, expenses and uses of cash, the Company’s
goals, designs, strategies, plans and objectives, future growth of YUPELRI sales, future royalty payments, the ability to provide value
to shareholders, the Company’s regulatory strategies and timing of clinical studies, possible safety, efficacy or differentiation
of our investigational therapy, the status of patent infringement litigation initiated by the Company and its partner against certain
generic companies in federal district courts; contingent payments due to the Company from the sale of the Company’s TRELEGY ELLIPTA
royalty interests to Royalty Pharma, and expectations around the use of OHSA scores as endpoints for clinical trials. These statements
are based on the current estimates and assumptions of the management of Theravance Biopharma as of the date of this press release and
the conference call and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the
actual results of Theravance Biopharma to be materially different from those reflected in the forward-looking statements. Important factors
that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks
related to: factors that could increase the Company’s cash requirements or expenses beyond its expectations and any factors that
could adversely affect its profitability, whether the milestone thresholds can be achieved, delays or difficulties in commencing, enrolling
or completing clinical studies, the potential that results from clinical or non-clinical studies indicate the Company’s product
candidates or product are unsafe, ineffective or not differentiated, risks of decisions from regulatory authorities that are unfavorable
to the Company, dependence on third parties to conduct clinical studies, delays or failure to achieve and maintain regulatory approvals
for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture and commercialize products,
and risks associated with establishing and maintaining sales, marketing and distribution capabilities with appropriate technical expertise
and supporting infrastructure, the ability of the Company to protect and to enforce its intellectual property rights, volatility and fluctuations
in the trading price and volume of the Company’s shares, and general economic and market conditions. Other risks affecting Theravance
Biopharma are in the Company's Form 10-K filed with the SEC on March 1, 2024, and other periodic reports filed with the SEC.
In addition to the risks described above and in Theravance Biopharma’s filings with the SEC, other unknown or unpredictable factors
also could affect Theravance Biopharma’s results. No forward-looking statements can be guaranteed, and actual results may differ
materially from such statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance
Biopharma assumes no obligation to update its forward-looking statements on account of new information, future events or otherwise, except
as required by law.

Non-GAAP Financial Measures
Theravance Biopharma provides a non-GAAP profitability target and a
non-GAAP metric in this press release. Theravance Biopharma believes that the non-GAAP profitability target and non-GAAP net profit (loss)
from operations provide meaningful information to assist investors in assessing prospects for future performance and actual performance
as they provide better metrics for analyzing the performance of its business by excluding items that may not be indicative of core operating
results and the Company's cash position. Because non-GAAP financial targets and metrics, such as non-GAAP profitability and non-GAAP net
loss from continuing operations, are not standardized, it may not be possible to compare these measures with other companies' non-GAAP
targets or measures having the same or a similar name. Thus, Theravance Biopharma's non-GAAP measures should be considered in addition
to, not as a substitute for, or in isolation from, the Company's actual GAAP results and other targets.
Please see the appendix attached to this press release for a reconciliation
of non-GAAP net profit (loss) from operations to its corresponding measure, net profit (loss) from operations. A reconciliation of non-GAAP
net profit (loss) from operations to its corresponding GAAP measure is not available on a forward-looking basis without unreasonable effort
due to the uncertainty regarding, and the potential variability of, expenses and other factors in the future.
Contact:
investor.relations@theravance.com
650-808-4045

| THERAVANCE BIOPHARMA, INC. |
| CONDENSED CONSOLIDATED BALANCE SHEETS |
| (In thousands) |
| | |
March 31, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| | |
(Unaudited) | | |
(1) | |
| Assets | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents and short-term marketable securities | |
$ | 99,975 | | |
$ | 102,426 | |
| Receivables from collaborative arrangements | |
| 14,664 | | |
| 17,474 | |
| Prepaid clinical and development services | |
| 2,086 | | |
| 2,038 | |
| Other prepaid and current assets | |
| 7,569 | | |
| 11,603 | |
| Total current assets | |
| 124,294 | | |
| 133,541 | |
| Property and equipment, net | |
| 8,717 | | |
| 9,068 | |
| Operating lease assets | |
| 35,364 | | |
| 36,287 | |
| Future contingent milestone and royalty assets | |
| 194,200 | | |
| 194,200 | |
| Restricted cash | |
| 836 | | |
| 836 | |
| Other assets | |
| 7,896 | | |
| 8,067 | |
| Total assets | |
$ | 371,307 | | |
$ | 381,999 | |
| | |
| | | |
| | |
| Liabilities and Shareholders' Equity | |
| | | |
| | |
| Current liabilities | |
$ | 21,662 | | |
$ | 24,767 | |
| Long-term operating lease liabilities | |
| 43,840 | | |
| 45,236 | |
| Future royalty payment contingency | |
| 28,417 | | |
| 27,788 | |
| Unrecognized tax benefits | |
| 67,075 | | |
| 65,294 | |
| Other long-term liabilities | |
| 5,445 | | |
| 5,919 | |
| Shareholders' equity | |
| 204,868 | | |
| 212,995 | |
| Total liabilities and shareholders’ equity | |
$ | 371,307 | | |
$ | 381,999 | |
| (1) | The condensed consolidated balance
sheet as of December 31, 2023 has been derived from the audited consolidated financial statements included in the Company's Annual Report
on Form 10-K for the year ended December 31, 2023. |

THERAVANCE BIOPHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| | |
Three Months Ended March 31, | |
| | |
2024 | | |
2023 | |
| | |
| | |
| |
| | |
(Unaudited) | |
| Revenue: | |
| | | |
| | |
| Viatris collaboration agreement (1) | |
$ | 14,503 | | |
$ | 10,411 | |
| Collaboration revenue | |
| - | | |
| 6 | |
| Total revenue | |
| 14,503 | | |
| 10,417 | |
| | |
| | | |
| | |
| Costs and expenses: | |
| | | |
| | |
| Research and development (2) | |
| 8,968 | | |
| 14,572 | |
| Selling, general and administrative (2) | |
| 16,742 | | |
| 19,183 | |
| Restructuring and related expenses (2) | |
| - | | |
| 1,574 | |
| Total costs and expenses | |
| 25,710 | | |
| 35,329 | |
| Loss from operations | |
| (11,207 | ) | |
| (24,912 | ) |
| Interest expense (non-cash) | |
| (629 | ) | |
| (550 | ) |
| Interest income and other income (expense), net | |
| 1,434 | | |
| 2,979 | |
| Loss before income taxes | |
| (10,402 | ) | |
| (22,483 | ) |
| Provision for income tax (expense) benefit | |
| (1,262 | ) | |
| 395 | |
| Net loss | |
$ | (11,664 | ) | |
$ | (22,088 | ) |
| | |
| | | |
| | |
| Net loss per share: | |
| | | |
| | |
| Basic and diluted net loss per share | |
$ | (0.24 | ) | |
$ | (0.35 | ) |
| | |
| | | |
| | |
| Shares used to compute basic and diluted net loss per share | |
| 48,283 | | |
| 62,934 | |
| | |
| | | |
| | |
| Non-GAAP net loss | |
$ | (4,544 | ) | |
$ | (14,912 | ) |
| (1) | While Viatris, Inc. records the
total YUPELRI net sales, the Company is entitled to a 35% share of the net profit (loss) pursuant
to a co-promotion agreement with Viatris as presented below: |
| | |
Three Months Ended March 31, | |
| (In thousands) | |
2024 | | |
2023 | |
| YUPELRI net sales (100% recorded by Viatris) | |
$ | 55,226 | | |
$ | 46,955 | |
| YUPELRI net sales (Theravance Biopharma implied 35%) | |
| 19,329 | | |
| 16,434 | |
| (2) | Amounts include share-based compensation
expense as follows: |
| | |
Three Months Ended March 31, | |
| (In thousands) | |
2024 | | |
2023 | |
| Research and development | |
$ | 1,465 | | |
$ | 2,441 | |
| Selling, general and administrative | |
| 3,764 | | |
| 4,223 | |
| Restructuring and related expenses | |
| - | | |
| 357 | |
| Total share-based compensation expense | |
$ | 5,229 | | |
$ | 7,021 | |

THERAVANCE BIOPHARMA, INC.
Reconciliation of GAAP to Non-GAAP Net Loss
(In thousands)
| | |
Three Months Ended March 31, | |
| | |
2024 | | |
2023 | |
| | |
| | |
| |
| | |
(Unaudited) | |
| GAAP net loss | |
$ | (11,664 | ) | |
$ | (22,088 | ) |
| Adjustments: | |
| | | |
| | |
| Share-based compensation expense | |
| 5,229 | | |
| 7,021 | |
| Non-cash interest expense | |
| 629 | | |
| 550 | |
| Income tax expense (benefit) | |
| 1,262 | | |
| (395 | ) |
| Non-GAAP net loss | |
$ | (4,544 | ) | |
$ | (14,912 | ) |
Exhibit 99.2

THERAVANCE BIOPHARMA ® , THERAVANCE ® , the Cross/Star logo and MEDICINES THAT MAKE A DIFFERENCE ® are registered trademarks of the Theravance Biopharma group of companies (in the U.S. and certain other countries). All third party trademarks used herein are the property of their respective owners. First Quarter 2024 Financial Results and Business Update May 13 , 2024 © 2024 Theravance Biopharma. All rights reserved.

Forward Looking Statements This presentation contains certain "forward - looking" statements as that term is defined in the Private Securities Litigation Ref orm Act of 1995 regarding, among other things, statements relating to goals, plans, objectives, expectations and future events. Theravance Biopharma, Inc. (the “Company”) intends such forward - looking state ments to be covered by the safe harbor provisions for forward - looking statements contained in Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Re form Act of 1995. Examples of such statements include statements relating to: the Company’s expectations regarding its future profitability, ex pen ses and uses of cash, the Company’s goals, designs, strategies, plans and objectives, future growth of YUPELRI sales, future royalty payments, the ability to provide value to shareholders, the Compan y’s regulatory strategies and timing of clinical studies, possible safety, efficacy or differentiation of our investigational therapy, the status of patent infringement litigation initiated by the Company and its pa rtner against certain generic companies in federal district courts; contingent payments due to the Company from the sale of the Company’s TRELEGY ELLIPTA royalty interests to Royalty Pharma, and expectati ons around the use of OHSA scores as endpoints for clinical trials. These statements are based on the current estimates and assumptions of the management of Theravance Biopharma as of the date of thi s p ress release and the conference call and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance Biopha rma to be materially different from those reflected in the forward - looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward - looking statements include, among others, risks related to: factors that could increase the Company’s cash requirements or expenses beyond its expectations and any factors that could adversely affect its profitabi lit y, whether the milestone thresholds can be achieved, delays or difficulties in commencing, enrolling or completing clinical studies, the potential that results from clinical or non - clinical studies indicate the Company’s product candidates or product are unsafe, ineffective or not differentiated, risks of decisions from regulatory authorities that are unfavorable to the Company, dependence on third parti es to conduct clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture a nd commercialize products, and risks associated with establishing and maintaining sales, marketing and distribution capabilities with appropriate technical expertise and supporting infrastructure, the abilit y o f the Company to protect and to enforce its intellectual property rights, volatility and fluctuations in the trading price and volume of the Company’s shares, and general economic and market conditions. Other risks affecting the Company are in the Company’s Form 10 - K filed with the SEC on March 1, 2024, and other periodic reports filed with the SEC. In addition to the risks described above and in Theravance Biopharma's filings with the SEC, other unknown or unpredictable factors also could affect Theravance Biopharma’s res ults. No forward - looking statements can be guaranteed, and actual results may differ materially from such statements. Given these uncertainties, you should not place undue reliance on these forward - look ing statements. Theravance Biopharma assumes no obligation to update its forward - looking statements on account of new information, future events or otherwise, except as required by law. Non - GAAP Financial Measures Theravance Biopharma provides a non - GAAP profitability target and a non - GAAP metric in this press release. Theravance Biopharma believes that the non - GAAP profitability target and non - GAAP net profit (loss) from continuing operations provide meaningful information to assist investors in assessing prospects for future perfor man ce and actual performance as they provide better metrics for analyzing the performance of its business by excluding items that may not be indicative of core operating results and the Company's cash po sit ion. Because non - GAAP financial targets and metrics, such as non - GAAP profitability and non - GAAP net loss from continuing operations, are not standardized, it may not be possible to compare these me asures with other companies' non - GAAP targets or measures having the same or a similar name. Thus, Theravance Biopharma's non - GAAP measures should be considered in addition to, not as a substitute for, or in isolation from, the Company's actual GAAP results and other targets. Please see the appendix attached to this presentation for a reconciliation of non - GAAP net profit (loss) from continuing operati ons to its corresponding measure, net profit (loss) from continuing operations. A reconciliation of non - GAAP net profit (loss) from continuing operations to its corresponding GAAP measure is not available on a forward - looking basis without unreasonable effort due to the uncertainty regarding, and the potential variability of, expenses and other factors in the future. 2

Introduction Rick Winningham Chief Executive Officer Ampreloxetine Update Áine Miller Senior Vice President, Head of Development YUPELRI ® Update Rhonda Farnum Senior Vice President, Chief Business Officer Financial Update Aziz Sawaf Senior Vice President, Chief Financial Officer Closing Remarks Rick Winningham Chief Executive Officer Agenda 3
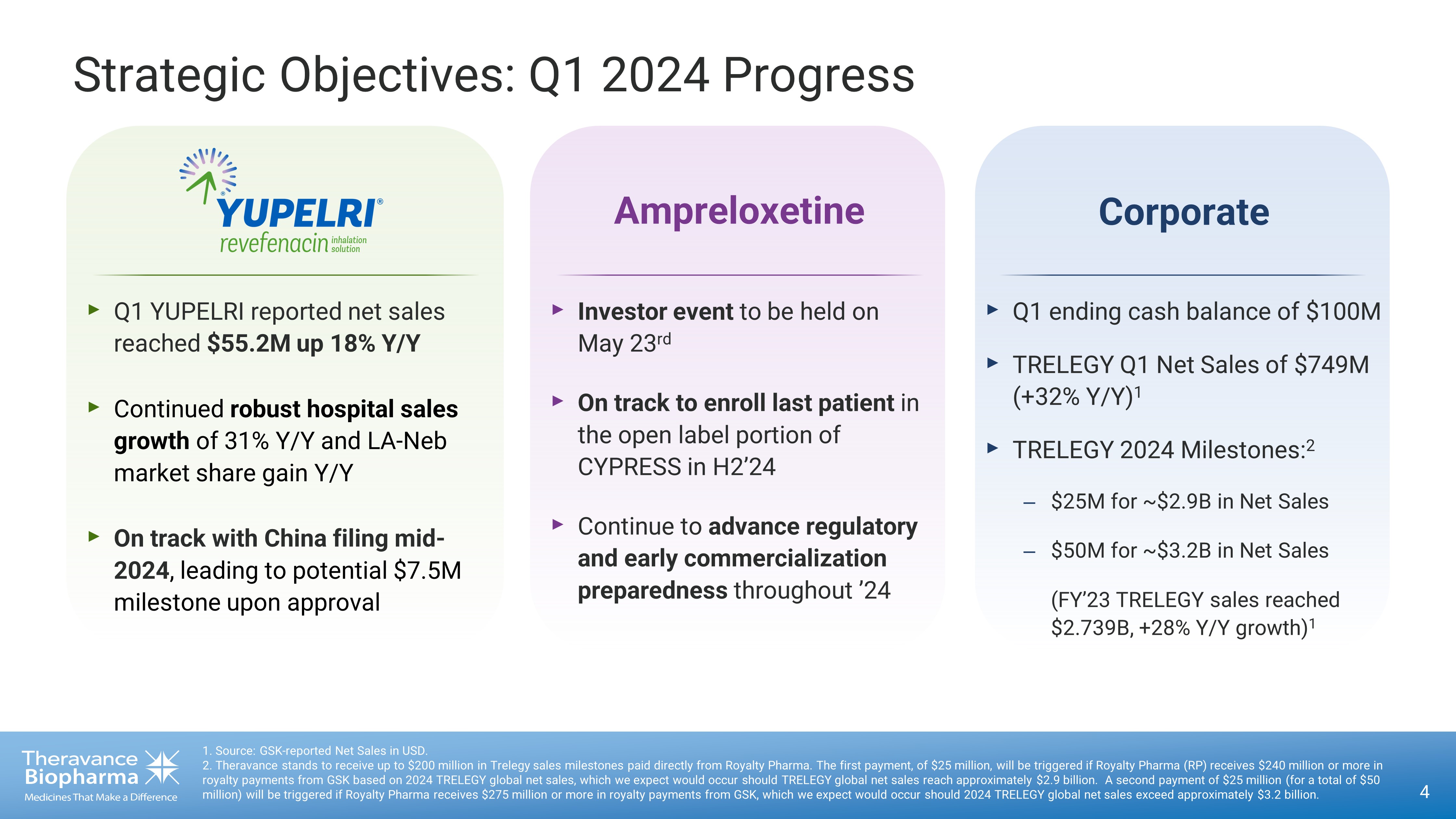
Strategic Objectives: Q1 2024 Progress 4 1. Source: GSK - reported Net Sales in USD. 2. Theravance stands to receive up to $200 million in Trelegy sales milestones paid directly from Royalty Pharma. The first p aym ent, of $25 million, will be triggered if Royalty Pharma (RP) receives $240 million or more in royalty payments from GSK based on 2024 TRELEGY global net sales, which we expect would occur should TRELEGY global net sales re ach approximately $2.9 billion. A second payment of $25 million (for a total of $50 million) will be triggered if Royalty Pharma receives $275 million or more in royalty payments from GSK, which we expect woul d o ccur should 2024 TRELEGY global net sales exceed approximately $3.2 billion. Corporate ‣ Q1 ending cash balance of $100M ‣ TRELEGY Q1 Net Sales of $749M (+32% Y/Y) 1 ‣ TRELEGY 2024 Milestones: 2 – $25M for ~$2.9B in Net Sales – $50M for ~$3.2B in Net Sales (FY’23 TRELEGY sales reached $2.739B, +28% Y/Y growth) 1 ‣ Investor event to be held on May 23 rd ‣ On track to enroll last patient in the open label portion of CYPRESS in H2’24 ‣ Continue to advance regulatory and early commercialization preparedness throughout ’24 Ampreloxetine ‣ Q1 YUPELRI reported net sales reached $55.2M up 18% Y/Y ‣ Continued robust hospital sales growth of 31% Y/Y and LA - Neb market share gain Y/Y ‣ On track with China filing mid - 2024 , leading to potential $7.5M milestone upon approval
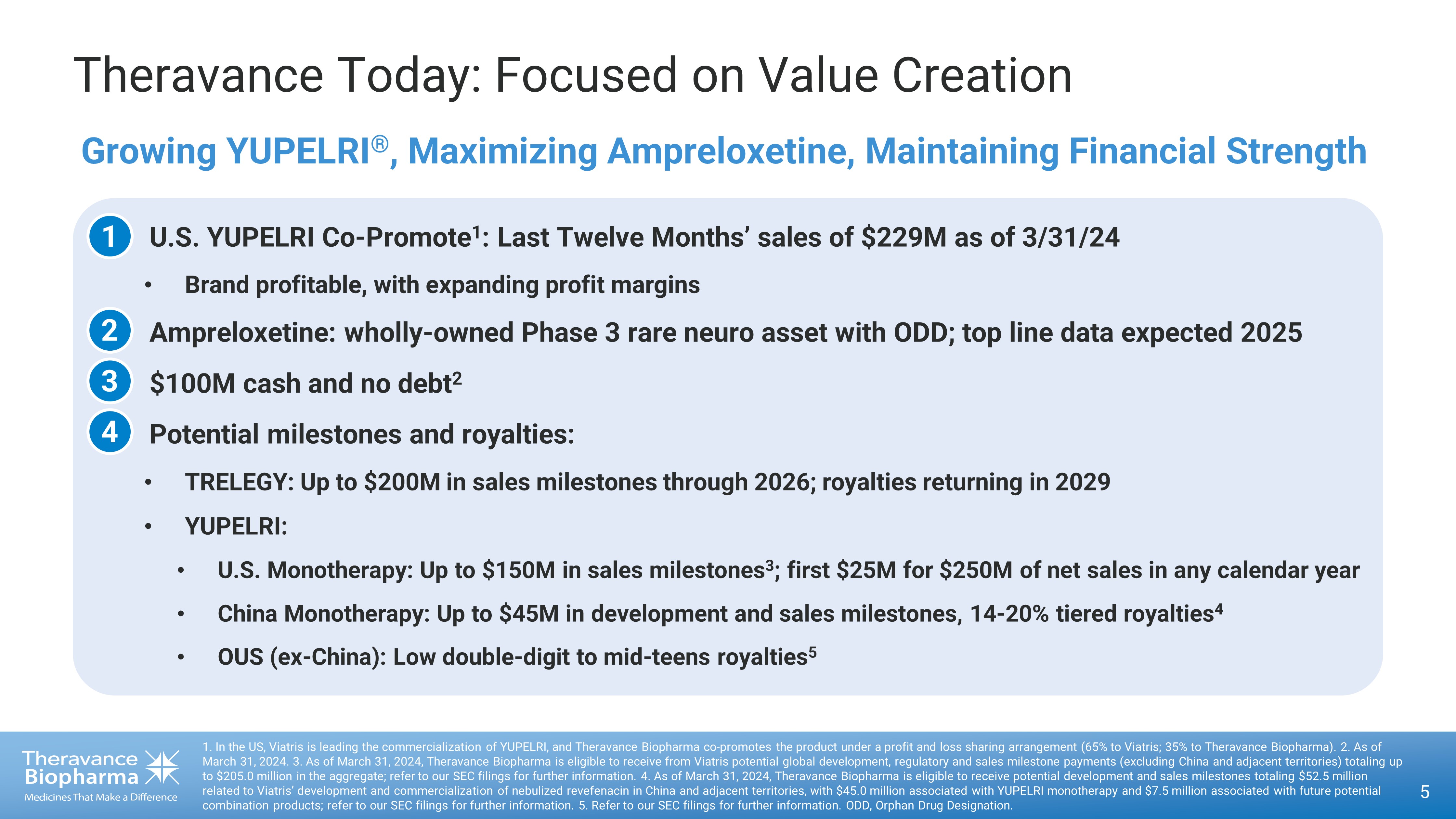
5 1. In the US, Viatris is leading the commercialization of YUPELRI, and Theravance Biopharma co - promotes the product under a prof it and loss sharing arrangement (65% to Viatris; 35% to Theravance Biopharma). 2 . As of March 31, 2024 . 3 . As of March 31, 2024, Theravance Biopharma is eligible to receive from Viatris potential global development, regulatory and sa les milestone payments (excluding China and adjacent territories) totaling up to $205.0 million in the aggregate; refer to our SEC filings for further information. 4. As of March 31, 2024 , Theravance Biopharma is eligible to receive potential development and sales milestones totaling $52.5 million related to Viatris’ development and commercialization of nebulized revefenacin in China and adjacent territories, with $45.0 mil lion associated with YUPELRI monotherapy and $7.5 million associated with future potential combination products; refer to our SEC filings for further information. 5 . Refer to our SEC filings for further information. ODD, Orphan Drug Designation. Theravance Today: Focused on Value Creation • U.S. YUPELRI Co - Promote 1 : Last Twelve Months’ sales of $229M as of 3/31/24 • Brand profitable, with expanding profit margins • Ampreloxetine: wholly - owned Phase 3 rare neuro asset with ODD; top line data expected 2025 • $100M cash and no debt 2 • Potential milestones and royalties: • TRELEGY: Up to $200M in sales milestones through 2026; royalties returning in 2029 • YUPELRI: • U.S. Monotherapy: Up to $150M in sales milestones 3 ; first $25M for $250M of net sales in any calendar year • China Monotherapy: Up to $45M in development and sales milestones, 14 - 20% tiered royalties 4 • OUS (ex - China): Low double - digit to mid - teens royalties 5 Growing YUPELRI ® , Maximizing Ampreloxetine, Maintaining Financial Strength 1 2 3 4

Ampreloxetine Investigational once - daily norepinephrine reuptake inhibitor For symptomatic neurogenic orthostatic hypotension (nOH) in multiple system atrophy (MSA) patients

Phase 3 CYPRESS Study Update Maximizing the Probability of Clinical and Regulatory Success 7 1. Kaufmann H. (2023, November 15 - 18). Evaluating clinically meaningful changes in the Orthostatic Hypotension Symptom Assessmen t domain of the Orthostatic Hypotension Questionnaire. [Poster presentation]. KOL, key opinion leader; LatAm, Latin America; NA; North America; OHSA, orthostatic hypotension symptom assessment. Program Alignment Derisks Regulatory Path CYPRESS Study Management • Careful site selection • Informed by Study 0169/170 experience, internal data analytics • Includes leading KOLs and many of the same sites from Studies 0169 and 0170 • To - date enrollment metrics consistent with expectations and Study 170 • Patient - centered design • Infrastructure in place to support remote visits • High standards for training and conduct • Sites actively recruiting in NA, Europe, LatAm • Aligned with FDA on CYPRESS design, and OHSA composite as primary endpoint • FDA - supported, Anchor - Based Analysis included to establish clinically meaningful thresholds for patient - reported outcomes measures • ~1 point change in OHSA Composite identified as clinically meaningful 1 • NDA authoring underway • CMC, non - clinical pharmacology/toxicology, and clinical pharmacology programs complete • Successful CYPRESS study fulfills requirement for a full approval 1 3 2 1 2 3 4 4
VIRTUAL KOL EVENT To Discuss Ampreloxetine’s Promise for the Treatment of Symptomatic Neurogenic Orthostatic Hypotension (nOH) in Patients with Multiple System Atrophy (MSA) Horacio Kaufmann, MD, FAAN (New York University) Thursday, May 23, 2024 | 10:00 AM ET Italo Biaggioni, MD (Vanderbilt University) Featuring:

FDA - approved for maintenance treatment of COPD First and only once - daily, LAMA (long - acting muscarinic agent) nebulized maintenance medicine for COPD Co - promotion agreement with VIATRIS TM (35% / 65% Profit Share)
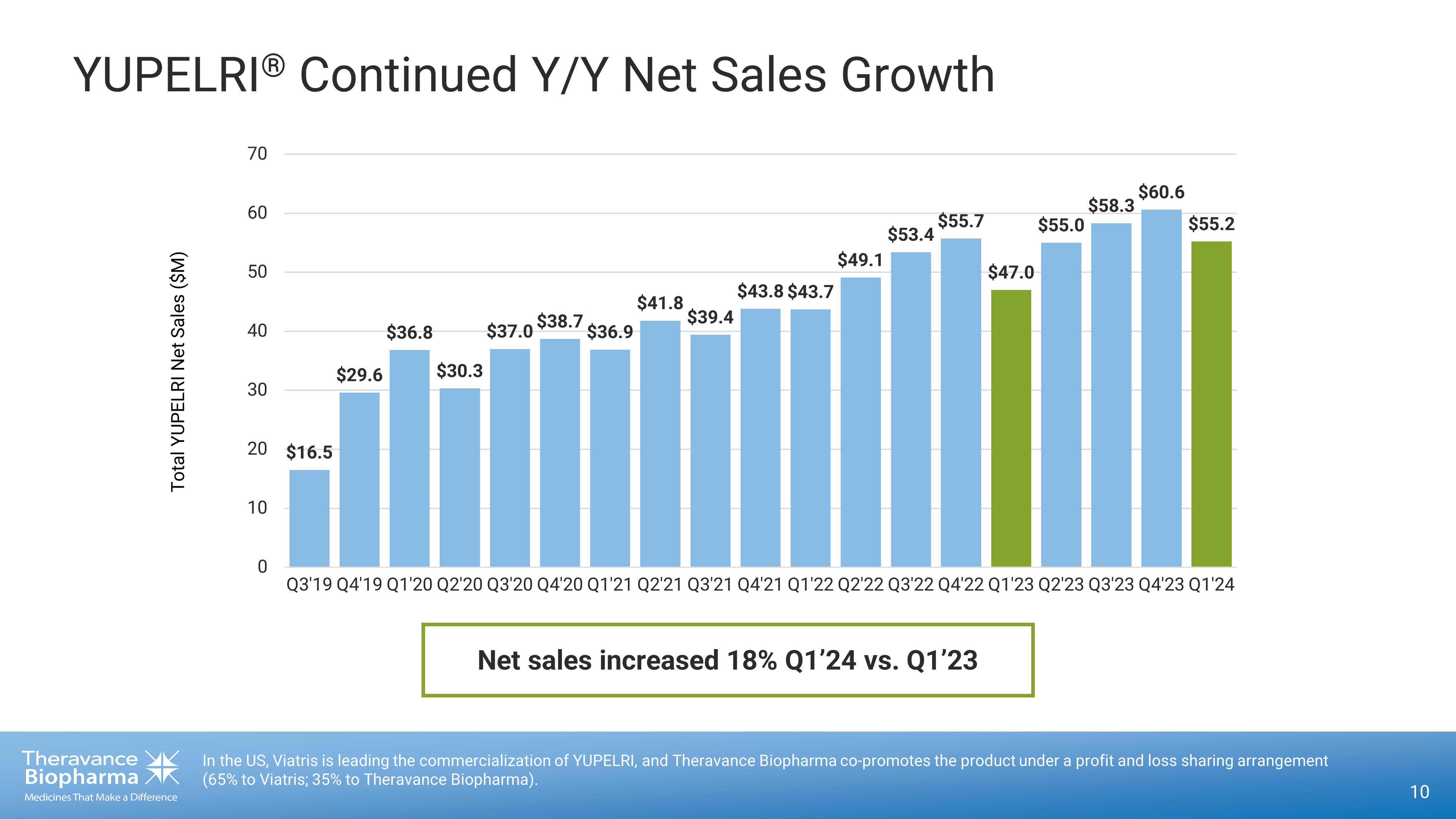
YUPELRI ® Continued Y/Y Net Sales Growth 10 In the US, Viatris is leading the commercialization of YUPELRI, and Theravance Biopharma co - promotes the product under a profit and loss sharing arrangement (65% to Viatris; 35% to Theravance Biopharma). $16.5 $29.6 $36.8 $30.3 $37.0 $38.7 $36.9 $41.8 $39.4 $43.8 $43.7 $49.1 $53.4 $55.7 $47.0 $55.0 $58.3 $60.6 $55.2 0 10 20 30 40 50 60 70 Q3'19 Q4'19 Q1'20 Q2'20 Q3'20 Q4'20 Q1'21 Q2'21 Q3'21 Q4'21 Q1'22 Q2'22 Q3'22 Q4'22 Q1'23 Q2'23 Q3'23 Q4'23 Q1'24 Total YUPELRI Net Sales ($M) Net sales increased 18% Q1’24 vs. Q1’23
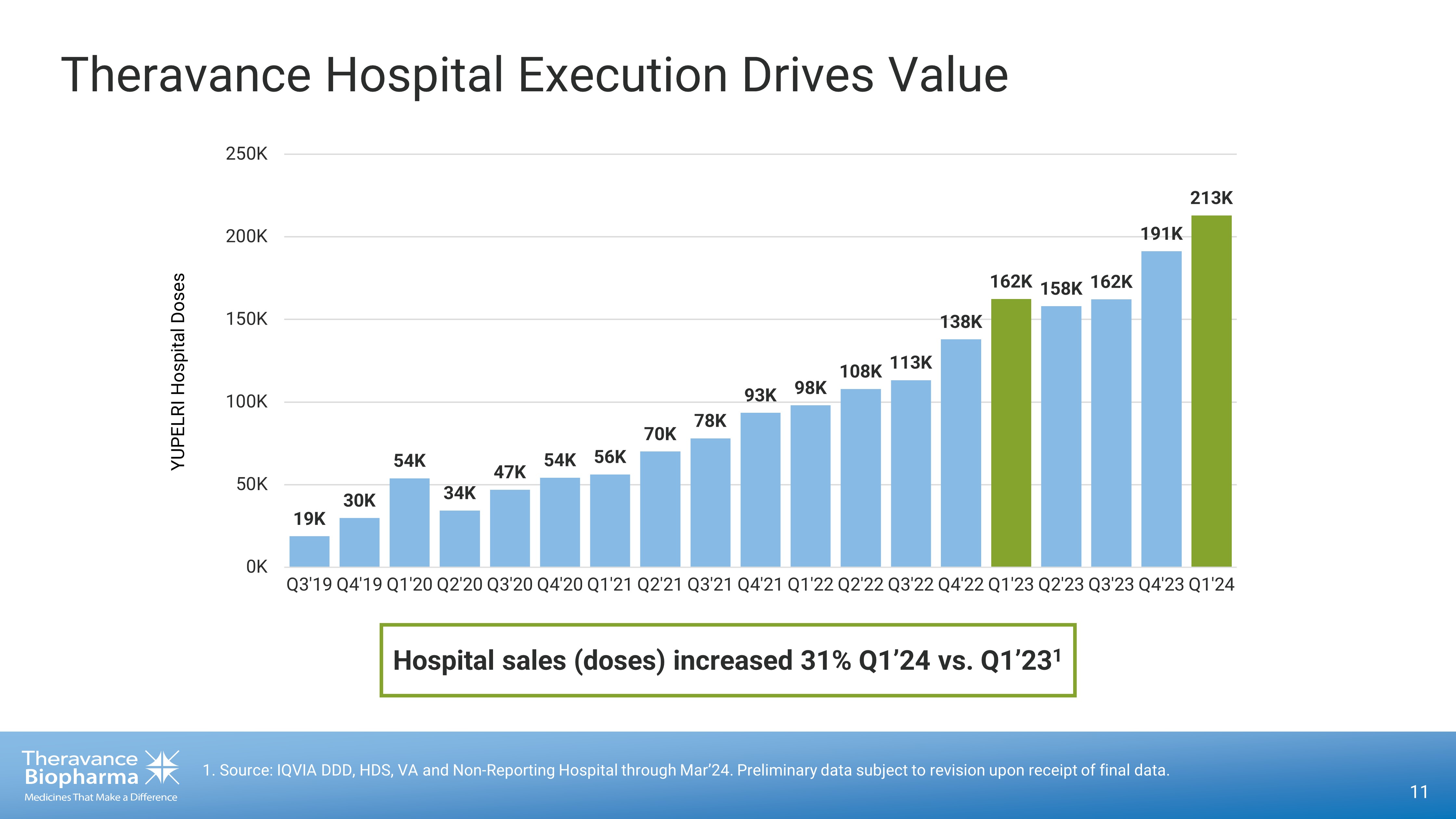
Theravance Hospital Execution Drives Value 11 1. Source: IQVIA DDD, HDS, VA and Non - Reporting Hospital through Mar’24. Preliminary data subject to revision upon receipt of fi nal data. 19K 30K 54K 34K 47K 54K 56K 70K 78K 93K 98K 108K 113K 138K 162K 158K 162K 191K 213K 0K 50K 100K 150K 200K 250K Q3'19 Q4'19 Q1'20 Q2'20 Q3'20 Q4'20 Q1'21 Q2'21 Q3'21 Q4'21 Q1'22 Q2'22 Q3'22 Q4'22 Q1'23 Q2'23 Q3'23 Q4'23 Q1'24 YUPELRI Hospital Doses Hospital sales (doses) increased 31% Q1’24 vs. Q1’23 1
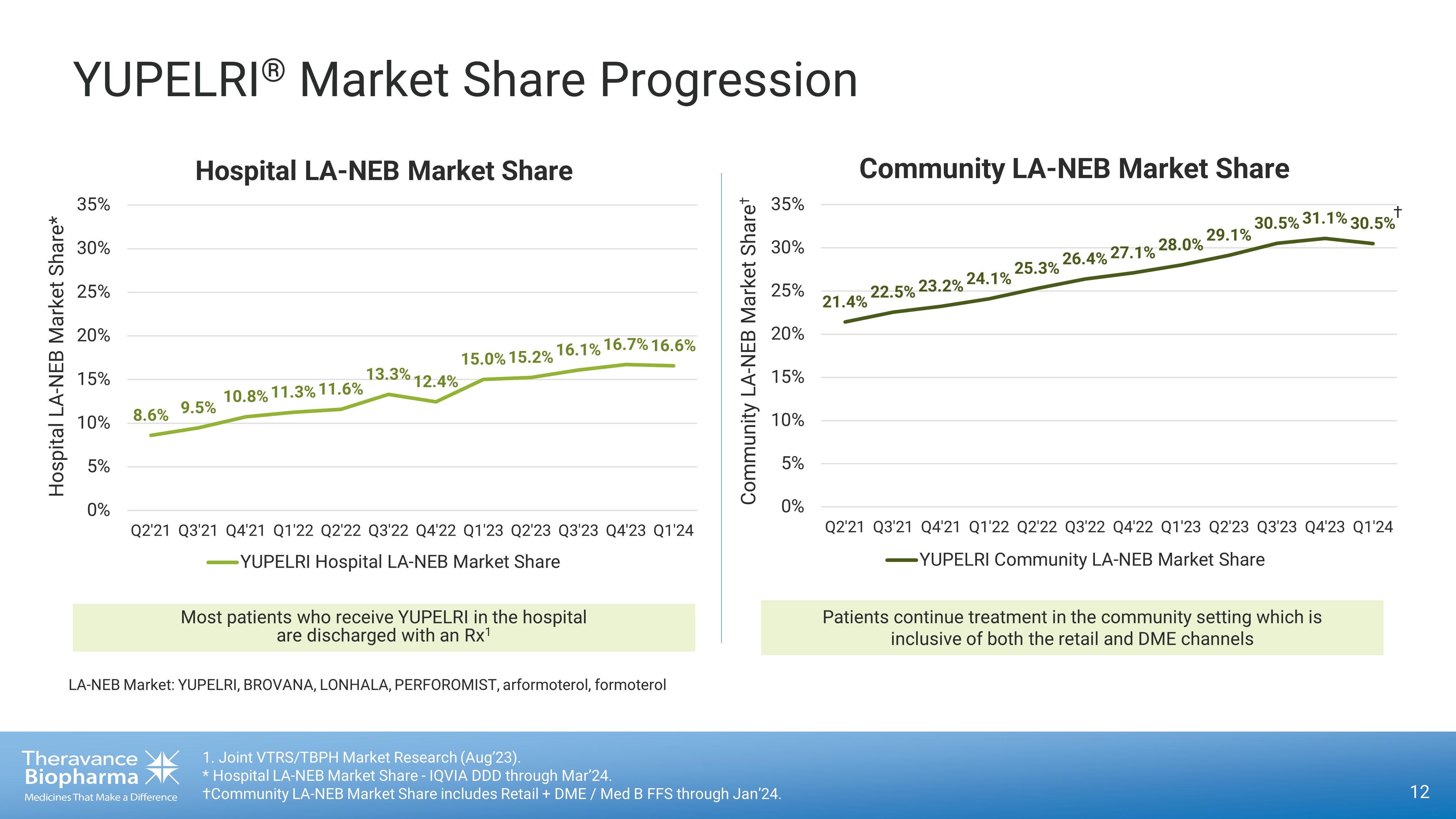
YUPELRI ® Market Share Progression 12 1. Joint VTRS/TBPH Market Research (Aug’23). * Hospital LA - NEB Market Share - IQVIA DDD through Mar’24. †Community LA - NEB Market Share includes Retail + DME / Med B FFS through Jan’24. 8.6% 9.5% 10.8% 11.3% 11.6% 13.3% 12.4% 15.0% 15.2% 16.1% 16.7% 16.6% 0% 5% 10% 15% 20% 25% 30% 35% Q2'21 Q3'21 Q4'21 Q1'22 Q2'22 Q3'22 Q4'22 Q1'23 Q2'23 Q3'23 Q4'23 Q1'24 Hospital LA - NEB Market Share* YUPELRI Hospital LA-NEB Market Share Patients continue treatment in the community setting which is inclusive of both the retail and DME channels 21.4% 22.5% 23.2% 24.1% 25.3% 26.4% 27.1% 28.0% 29.1% 30.5% 31.1% 30.5% 0% 5% 10% 15% 20% 25% 30% 35% Q2'21 Q3'21 Q4'21 Q1'22 Q2'22 Q3'22 Q4'22 Q1'23 Q2'23 Q3'23 Q4'23 Q1'24 Community LA - NEB Market Share † YUPELRI Community LA-NEB Market Share Most patients who receive YUPELRI in the hospital are discharged with an Rx 1 LA - NEB Market: YUPELRI, BROVANA, LONHALA, PERFOROMIST, arformoterol, formoterol Hospital LA - NEB Market Share Community LA - NEB Market Share †
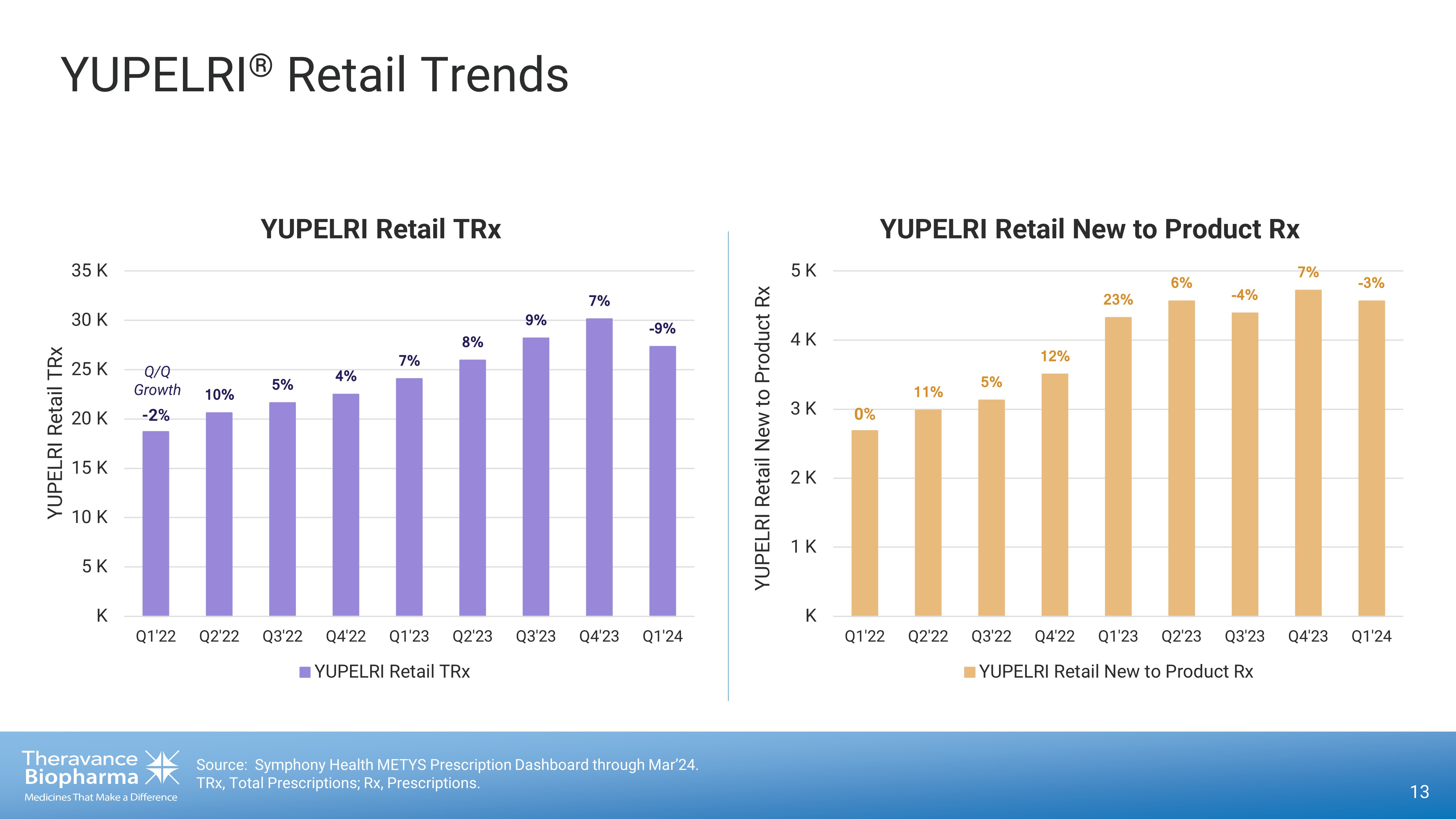
13 Source: Symphony Health METYS Prescription Dashboard through Mar’24. TRx, Total Prescriptions; Rx, Prescriptions. - 2% ÛÚ Ï ß Ï Þ Ï á Ï â Ï ã Ï á Ï × ã Ï K 5 K 10 K 15 K 20 K 25 K 30 K 35 K Q1'22 Q2'22 Q3'22 Q4'22 Q1'23 Q2'23 Q3'23 Q4'23 Q1'24 YUPELRI Retail TRx YUPELRI Retail TRx YUPELRI Retail TRx Q/Q Growth 0% ÛÛ Ï ß Ï ÛÜ Ï ÜÝ Ï à Ï × Þ Ï á Ï × Ý Ï K 1 K 2 K 3 K 4 K 5 K Q1'22 Q2'22 Q3'22 Q4'22 Q1'23 Q2'23 Q3'23 Q4'23 Q1'24 YUPELRI Retail New to Product Rx YUPELRI Retail New to Product Rx YUPELRI Retail New to Product Rx YUPELRI ® Retail Trends
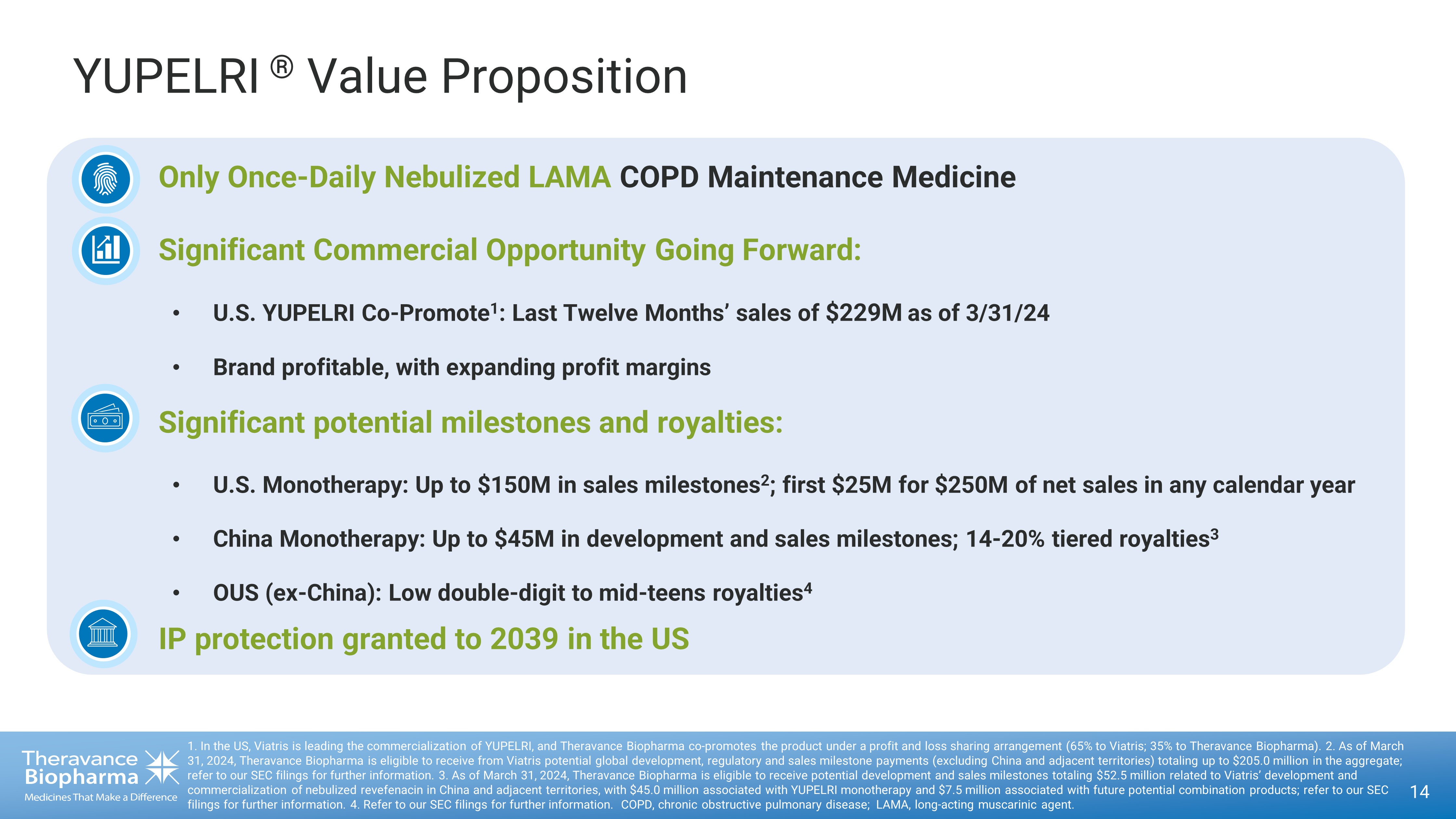
YUPELRI ® Value Proposition 14 1. In the US, Viatris is leading the commercialization of YUPELRI, and Theravance Biopharma co - promotes the product under a prof it and loss sharing arrangement (65% to Viatris; 35% to Theravance Biopharma). 2. As of March 31, 2024, Theravance Biopharma is eligible to receive from Viatris potential global development, regulatory and sales milestone payment s (excluding China and adjacent territories) totaling up to $205.0 million in the aggregate; refer to our SEC filings for further information. 3. As of March 31, 2024, Theravance Biopharma is eligible to receive potent ial development and sales milestones totaling $52.5 million related to Viatris’ development and commercialization of nebulized revefenacin in China and adjacent territories, with $45.0 million associated with YUPELRI mono the rapy and $7.5 million associated with future potential combination products; refer to our SEC filings for further information . 4. Refer to our SEC filings for further information. COPD, chronic obstructive pulmonary disease; LAMA, long - acting muscarinic agent . • Only Once - Daily Nebulized LAMA COPD Maintenance Medicine • Significant Commercial Opportunity Going Forward: • U.S. YUPELRI Co - Promote 1 : Last Twelve Months’ sales of $229M as of 3/31/24 • Brand profitable, with expanding profit margins • Significant potential milestones and royalties: • U.S. Monotherapy: Up to $150M in sales milestones 2 ; first $25M for $250M of net sales in any calendar year • China Monotherapy: Up to $45M in development and sales milestones; 14 - 20% tiered royalties 3 • OUS (ex - China): Low double - digit to mid - teens royalties 4 • IP protection granted to 2039 in the US

Financial Update
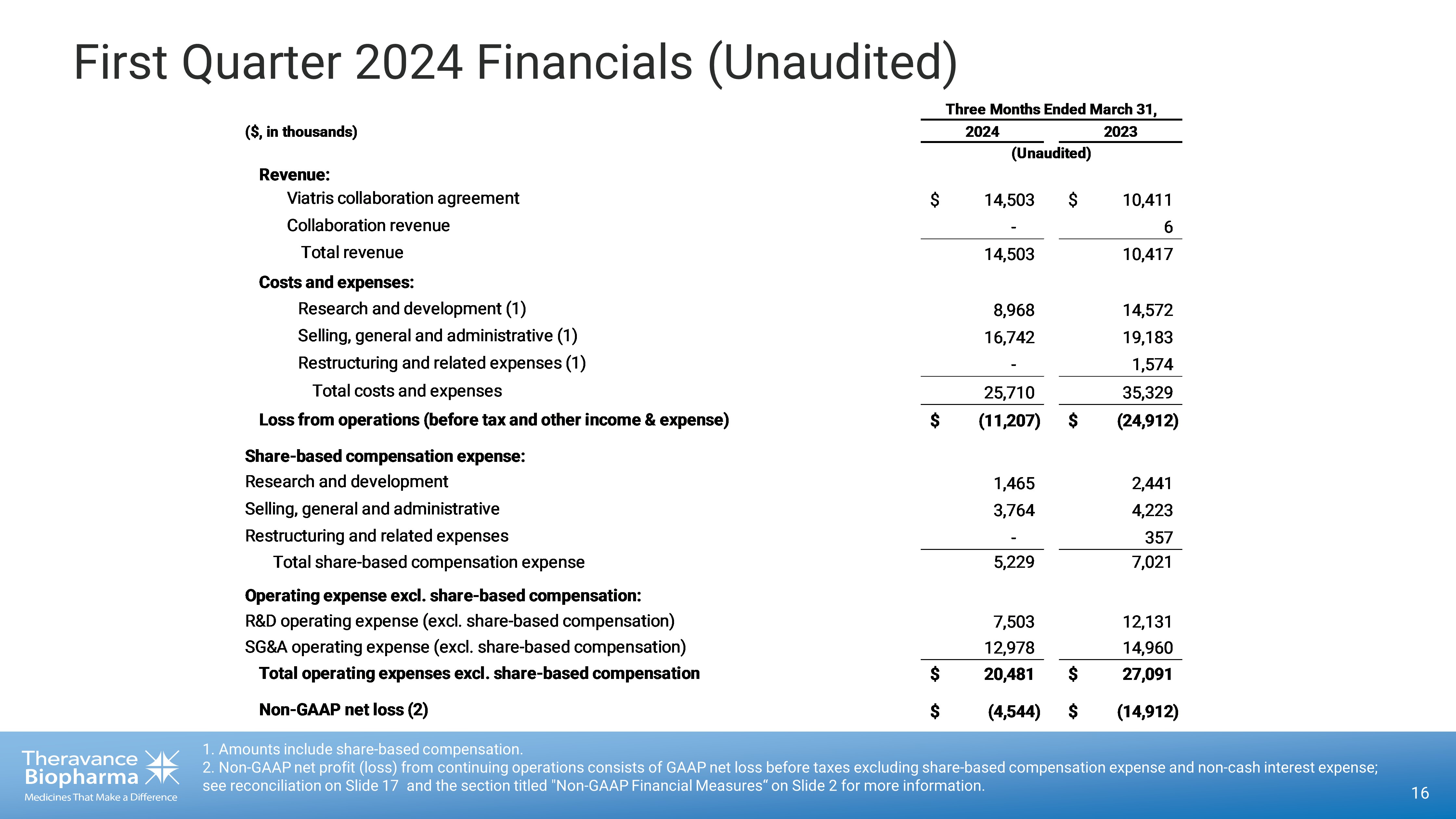
16 1. Amounts include share - based compensation. 2. Non - GAAP net profit (loss) from continuing operations consists of GAAP net loss before taxes excluding share - based compensati on expense and non - cash interest expense; see reconciliation on Slide 17 and the section titled "Non - GAAP Financial Measures“ on Slide 2 for more information. First Quarter 2024 Financials (Unaudited) ($, in thousands) Revenue: Viatris collaboration agreement $ 14,503 $ 10,411 Collaboration revenue - 6 Total revenue 14,503 10,417 Costs and expenses: Research and development (1) 8,968 14,572 Selling, general and administrative (1) 16,742 19,183 Restructuring and related expenses (1) - 1,574 Total costs and expenses 25,710 35,329 Loss from operations (before tax and other income & expense) $ (11,207) $ (24,912) Share-based compensation expense: Research and development 1,465 2,441 Selling, general and administrative 3,764 4,223 Restructuring and related expenses - 357 Total share-based compensation expense 5,229 7,021 Operating expense excl. share-based compensation: R&D operating expense (excl. share-based compensation) 7,503 12,131 SG&A operating expense (excl. share-based compensation) 12,978 14,960 Total operating expenses excl. share-based compensation $ 20,481 $ 27,091 Non-GAAP net loss (2) $ (4,544) $ (14,912) Three Months Ended March 31, 2024 2023 (Unaudited)
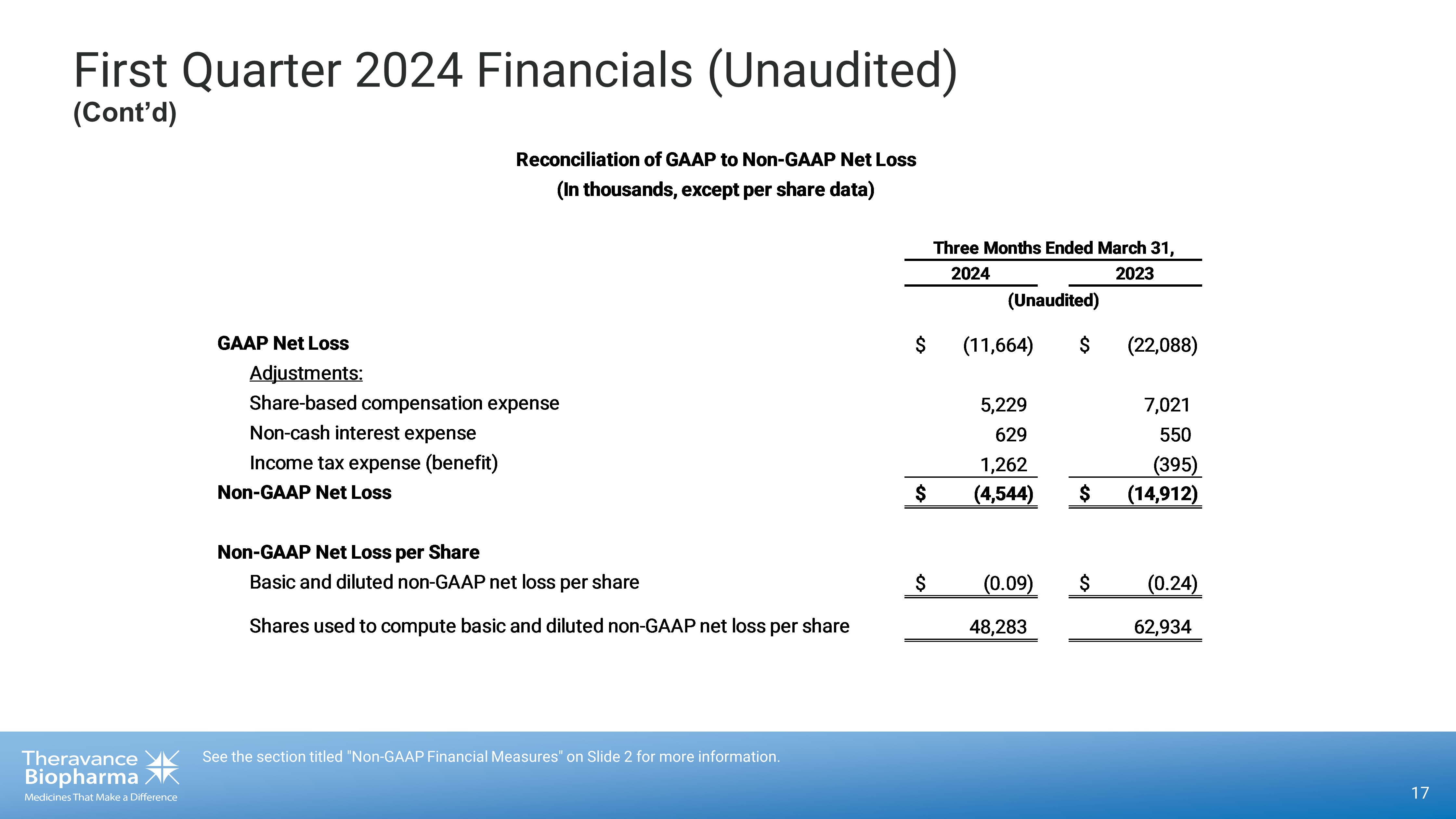
First Quarter 2024 Financials (Unaudited) (Cont’d) 17 See the section titled "Non - GAAP Financial Measures" on Slide 2 for more information. GAAP Net Loss $ (11,664) $ (22,088) Adjustments: Share-based compensation expense 5,229 7,021 Non-cash interest expense 629 550 Income tax expense (benefit) 1,262 (395) Non-GAAP Net Loss $ (4,544) $ (14,912) Non-GAAP Net Loss per Share Basic and diluted non-GAAP net loss per share $ (0.09) $ (0.24) Shares used to compute basic and diluted non-GAAP net loss per share 48,283 62,934 (Unaudited) Reconciliation of GAAP to Non-GAAP Net Loss (In thousands, except per share data) Three Months Ended March 31, 2024 2023
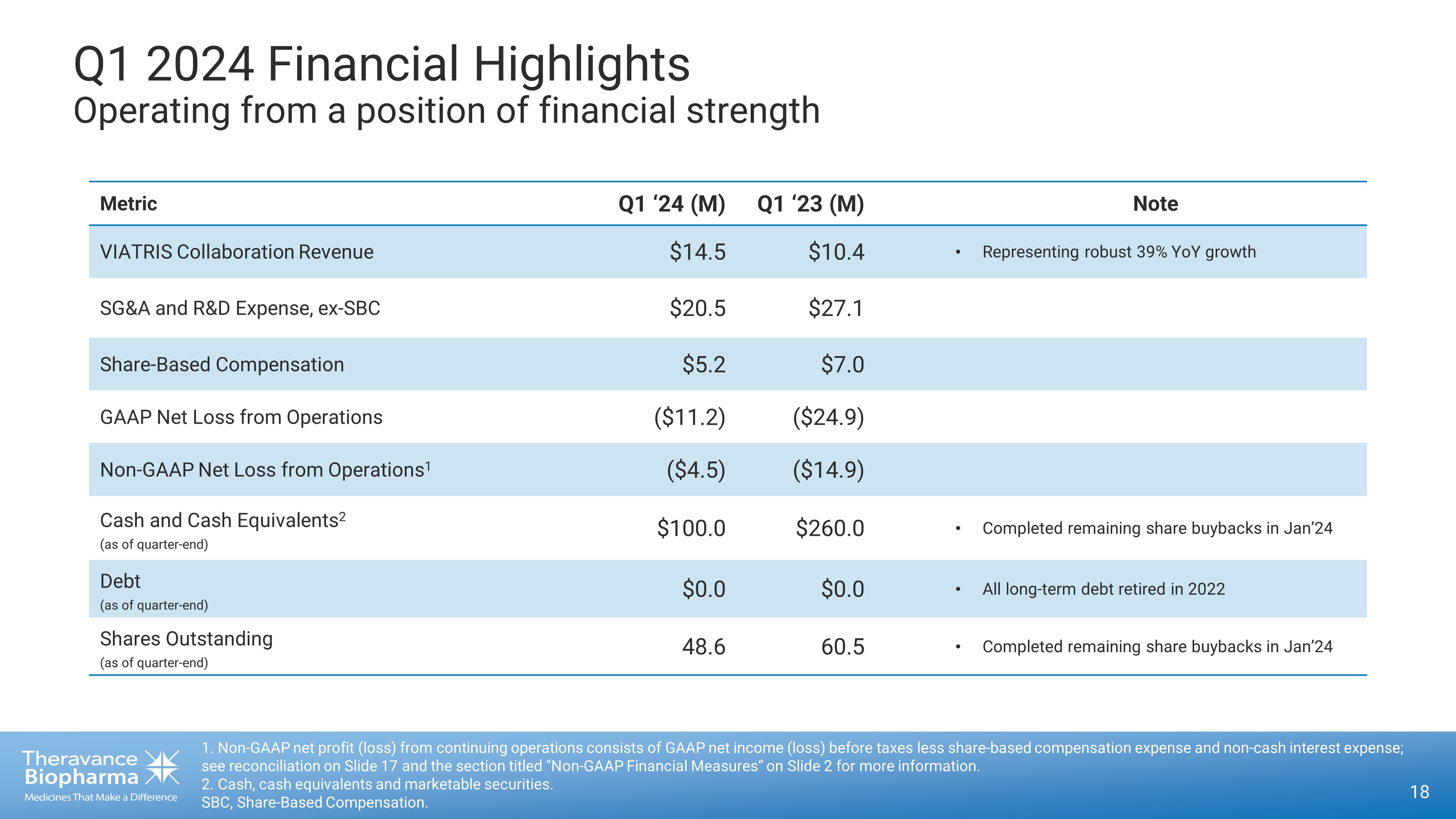
Q1 2024 Financial Highlights Operating from a position of financial strength 18 1. Non - GAAP net profit (loss) from continuing operations consists of GAAP net income (loss) before taxes less share - based compen sation expense and non - cash interest expense; see reconciliation on Slide 17 and the section titled "Non - GAAP Financial Measures“ on Slide 2 for more information. 2. Cash, cash equivalents and marketable securities. SBC, Share - Based Compensation. Metric Q1 ‘24 (M) Q1 ‘23 (M) Note VIATRIS Collaboration Revenue $14.5 $10.4 • Representing robust 39% YoY growth SG&A and R&D Expense, ex - SBC $20.5 $27.1 Share - Based Compensation $5.2 $7.0 GAAP Net Loss from Operations ($11.2) ($24.9) Non - GAAP Net Loss from Operations 1 ($4.5) ($14.9) Cash and Cash Equivalents 2 (as of quarter - end) $100.0 $260.0 • Completed remaining share buybacks in Jan’24 Debt (as of quarter - end) $0.0 $0.0 • All long - term debt retired in 2022 Shares Outstanding (as of quarter - end) 48.6 60.5 • Completed remaining share buybacks in Jan’24
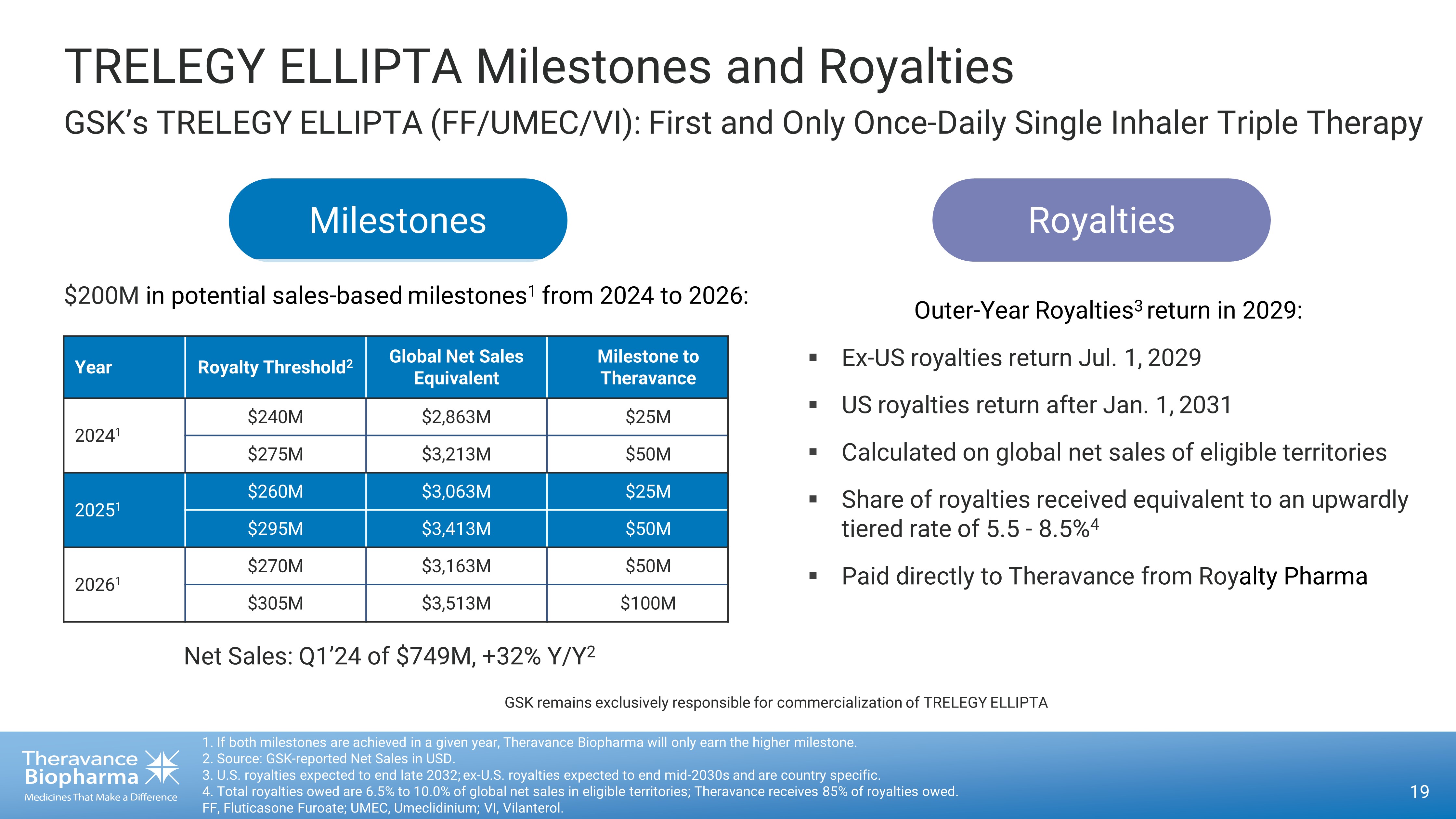
Net Sales: Q1’24 of $749M, +32% Y/Y 2 Outer - Year Royalties 3 return in 2029: ▪ Ex - US royalties return Jul. 1, ௗ 2029 ▪ US royalties return after Jan. 1, ௗ 2031 ▪ Calculated on global net sales of eligible territories ▪ Share of royalties received equivalent to an upwardly tiered rate of 5.5 - 8.5% 4 ▪ Paid directly to Theravance from Roy alty Pharma TRELEGY ELLIPTA Milestones and Royalties GSK’s TRELEGY ELLIPTA (FF/UMEC/VI): First and Only Once - Daily Single Inhaler Triple Therapy 19 1. If both milestones are achieved in a given year, Theravance Biopharma will only earn the higher milestone. 2. Source: GSK - reported Net Sales in USD. 3. U.S. royalties expected to end late 2032; ex - U.S. royalties expected to end mid - 2030s and are country specific. 4. Total royalties owed are 6.5% to 10.0% of global net sales in eligible territories; Theravance receives 85% of royalties o wed . FF, Fluticasone Furoate; UMEC, Umeclidinium; VI, Vilanterol. Milestones Royalties $200M in potential s ales - based milestones 1 from 2024 to 2026: GSK remains exclusively responsible for commercialization of TRELEGY ELLIPTA Year Royalty Threshold 2 Global Net Sales Equivalent Milestone to Theravance 2024 1 $240M $2,863M $25M $275M $3,213M $50M 2025 1 $260M $3,063M $25M $295M $3,413M $50M 2026 1 $270M $3,163M $50M $305M $3,513M $100M
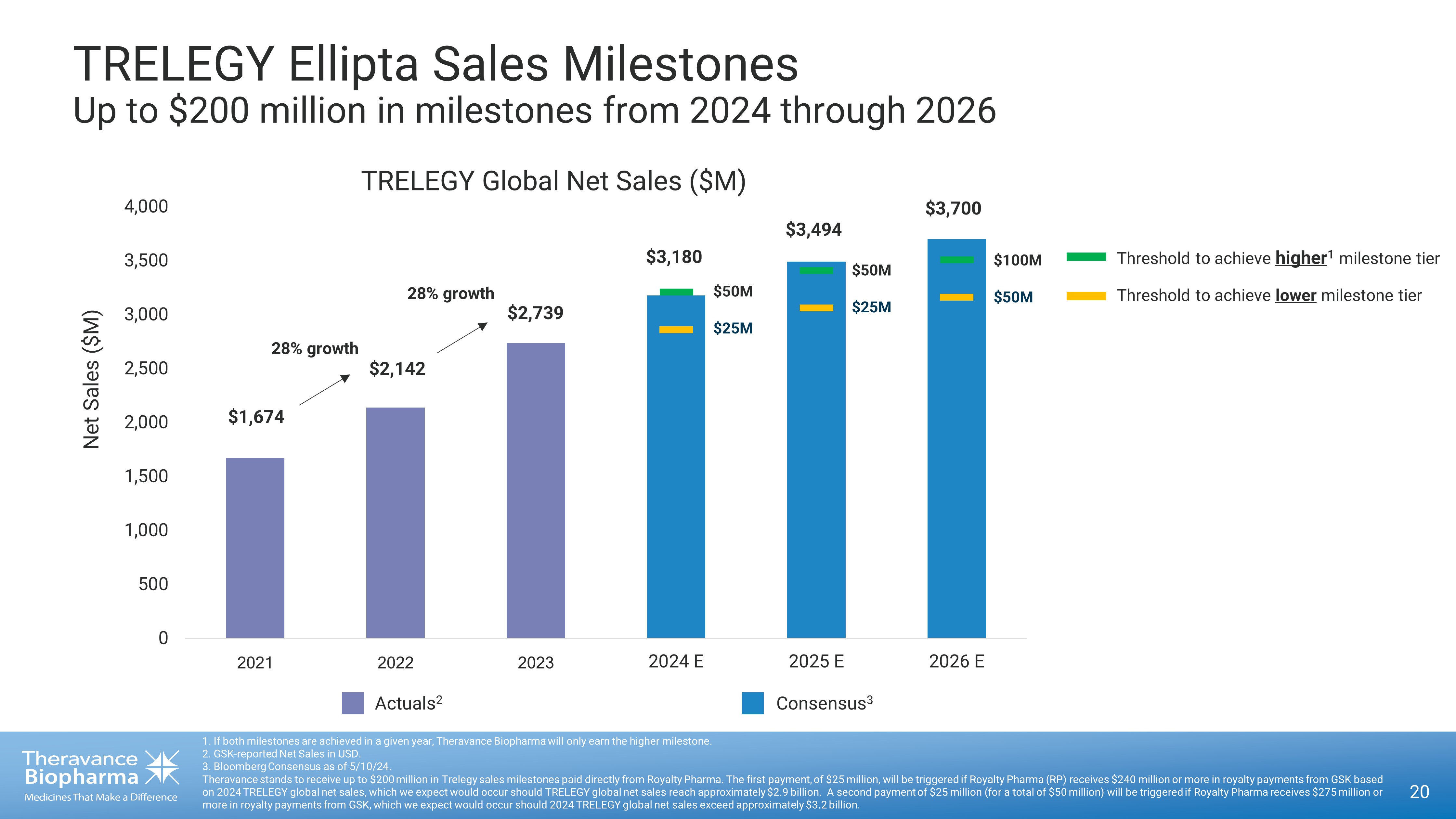
20 1. If both milestones are achieved in a given year, Theravance Biopharma will only earn the higher milestone. 2. GSK - reported Net Sales in USD. 3. Bloomberg Consensus as of 5/10/24. Theravance stands to receive up to $200 million in Trelegy sales milestones paid directly from Royalty Pharma. The first paym ent , of $25 million, will be triggered if Royalty Pharma (RP) receives $240 million or more in royalty payments from GSK based on 2024 TRELEGY global net sales, which we expect would occur should TRELEGY global net sales reach approximately $2.9 billio n. A second payment of $25 million (for a total of $50 million) will be triggered if Royalty Pharma receives $275 million or more in royalty payments from GSK, which we expect would occur should 2024 TRELEGY global net sales exceed approximately $3.2 bi llion. TRELEGY Ellipta Sales Milestones Up to $200 million in milestones from 2024 through 2026 0 500 1,000 1,500 2,000 2,500 3,000 3,500 4,000 ÜÚÜÛ ÜÚÜÜ ÜÚÜÝ 2024 E 2025 E 2026 E Consensus 3 Actuals 2 Net Sales ($M) $1,674 $2,142 $2,739 $3,180 $3,494 $3,700 Threshold to achieve higher 1 milestone tier Threshold to achieve lower milestone tier TRELEGY Global Net Sales ($M) $50M $25M $50M $25M $100M $50M 28% growth 28% growth
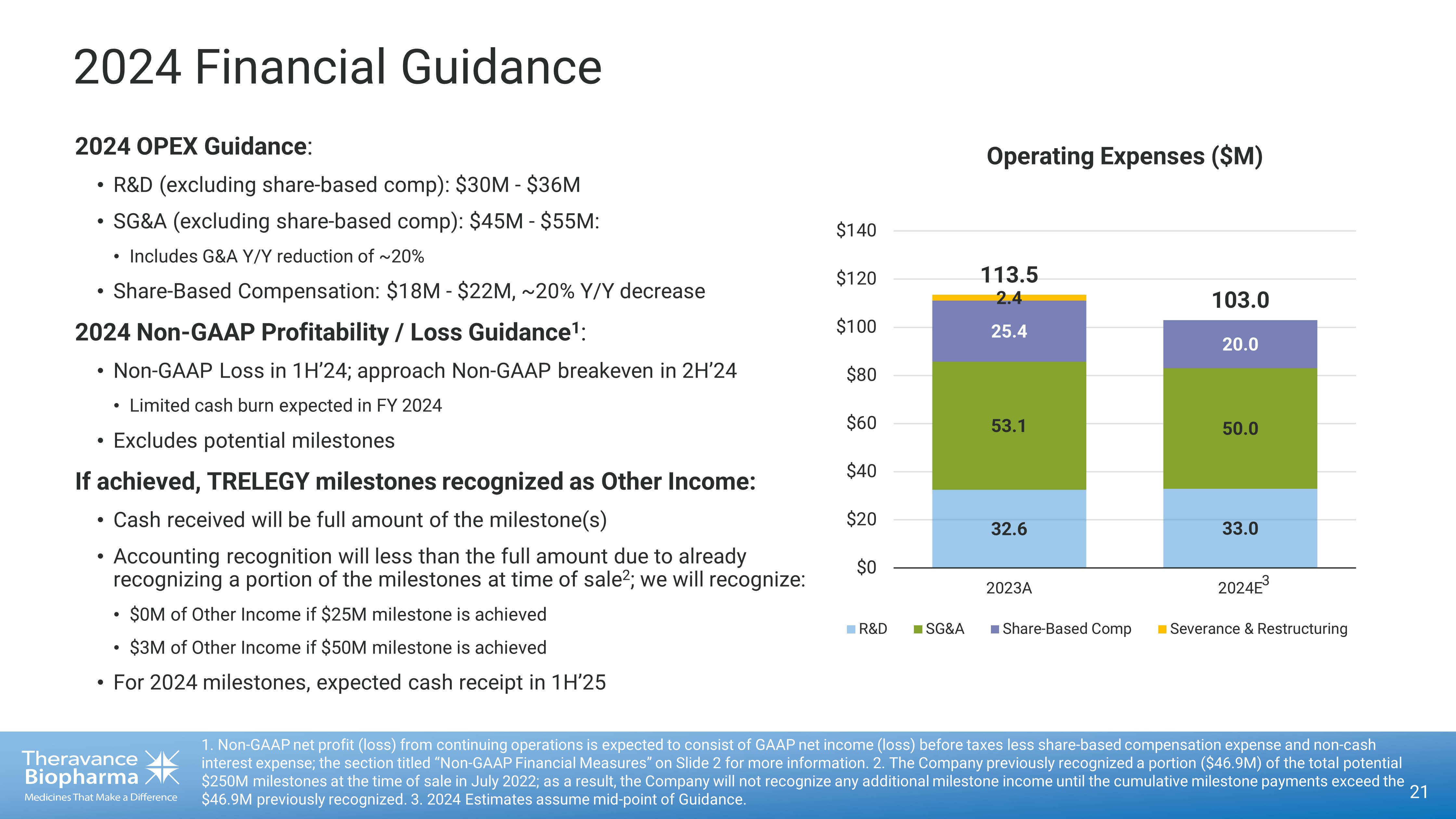
2024 Financial Guidance 21 1. Non - GAAP net profit (loss) from continuing operations is expected to consist of GAAP net income (loss) before taxes less shar e - based compensation expense and non - cash interest expense; the section titled “Non - GAAP Financial Measures” on Slide 2 for more information. 2. The Company previously re cognized a portion ($46.9M) of the total potential $250M milestones at the time of sale in July 2022; as a result, the Company will not recognize any additional milestone incom e u ntil the cumulative milestone payments exceed the $46.9M previously recognized. 3. 2024 Estimates assume mid - point of Guidance. 32.6 33.0 53.1 50.0 25.4 20.0 2.4 113.5 103.0 $0 $20 $40 $60 $80 $100 $120 $140 2023A 2024E R&D SG&A Share-Based Comp Severance & Restructuring Operating Expenses ($M) 2024 OPEX Guidance : • R&D (excluding share - based comp): $30M - $36M • SG&A (excluding share - based comp): $45M - $55M: • Includes G&A Y/Y reduction of ~20% • Share - Based Compensation: $18M - $22M, ~20% Y/Y decrease 2024 Non - GAAP Profitability / Loss Guidance 1 : • Non - GAAP Loss in 1H’24; approach Non - GAAP breakeven in 2H’24 • Limited cash burn expected in FY 2024 • Excludes potential milestones If achieved, TRELEGY milestones recognized as Other Income: • Cash received will be full amount of the milestone(s) • Accounting recognition will less than the full amount due to already recognizing a portion of the milestones at time of sale 2 ; we will recognize: • $0M of Other Income if $25M milestone is achieved • $3M of Other Income if $50M milestone is achieved • For 2024 milestones, expected cash receipt in 1H’25 3

22 Theravance’s Strategic Focus • Grow YUPELRI in the United States; realize value through China expansion: • Drive U.S. hospital growth as part of overall brand maximization strategy • Achieve up to $150M in U.S. monotherapy sales milestones; first $25M for $250M of net sales in any given year • Realize up to $45M in China monotherapy development and sales milestones, 14 - 20% tiered royalties • Successfully develop and commercialize ampreloxetine globally: • Retain U.S. rights, Partner ex - US • Achieve Up to $200M in TRELEGY sales milestones, beginning in 2024, with royalties returning in 2029 • Maintain financial strength Grow YUPELRI ® , Maximize Ampreloxetine, Optimize Financial Returns 1 2 3 4

Rick Winningham Chairman and Chief Executive Officer Aziz Sawaf, CFA Senior Vice President, Chief Financial Officer Rhonda Farnum Senior Vice President, Chief Business Officer Áine Miller Senior Vice President, Development Q&A Session

YUPELRI ® (revefenacin) Inhalation Solution YUPELRI ® inhalation solution is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD) . Important Safety Information (US) YUPELRI is contraindicated in patients with hypersensitivity to revefenacin or any component of this product . YUPELRI should not be initiated in patients during acutely deteriorating or potentially life - threatening episodes of COPD, or for the relief of acute symptoms, i . e . , as rescue therapy for the treatment of acute episodes of bronchospasm . Acute symptoms should be treated with an inhaled short - acting beta 2 - agonist . As with other inhaled medicines, YUPELRI can produce paradoxical bronchospasm that may be life - threatening . If paradoxical bronchospasm occurs following dosing with YUPELRI, it should be treated immediately with an inhaled, short - acting bronchodilator . YUPELRI should be discontinued immediately and alternative therapy should be instituted . YUPELRI should be used with caution in patients with narrow - angle glaucoma . Patients should be instructed to immediately consult their healthcare provider if they develop any signs and symptoms of acute narrow - angle glaucoma, including eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema . Worsening of urinary retention may occur . Use with caution in patients with prostatic hyperplasia or bladder - neck obstruction and instruct patients to contact a healthcare provider immediately if symptoms occur . Immediate hypersensitivity reactions may occur after administration of YUPELRI . If a reaction occurs, YUPELRI should be stopped at once and alternative treatments considered . The most common adverse reactions occurring in clinical trials at an incidence greater than or equal to 2 % in the YUPELRI group, and higher than placebo, included cough, nasopharyngitis, upper respiratory infection, headache and back pain . Coadministration of anticholinergic medicines or OATP 1 B 1 and OATP 1 B 3 inhibitors with YUPELRI is not recommended . YUPELRI is not recommended in patients with any degree of hepatic impairment . 24 OATP, organic anion transporting polypeptide.
About YUPELRI ® (revefenacin) Inhalation Solution YUPELRI ® (revefenacin) inhalation solution is a once - daily nebulized LAMA approved for the maintenance treatment of COPD in the US . Market research by Theravance Biopharma indicates approximately 9 % of the treated COPD patients in the US use nebulizers for ongoing maintenance therapy . 1 LAMAs are a cornerstone of maintenance therapy for COPD and YUPELRI ® is positioned as the first once - daily single - agent bronchodilator product for COPD patients who require, or prefer, nebulized therapy . YUPELRI ® ’s stability in both metered dose inhaler and dry powder device formulations suggest that this LAMA could also serve as a foundation for novel handheld combination products . 25 1. TBPH market research (N=160 physicians); refers to US COPD patients. COPD, chronic obstructive pulmonary disease; LAMA, long - acting muscarinic antagonist.

Appendix

Appendix I: YUPELRI ®
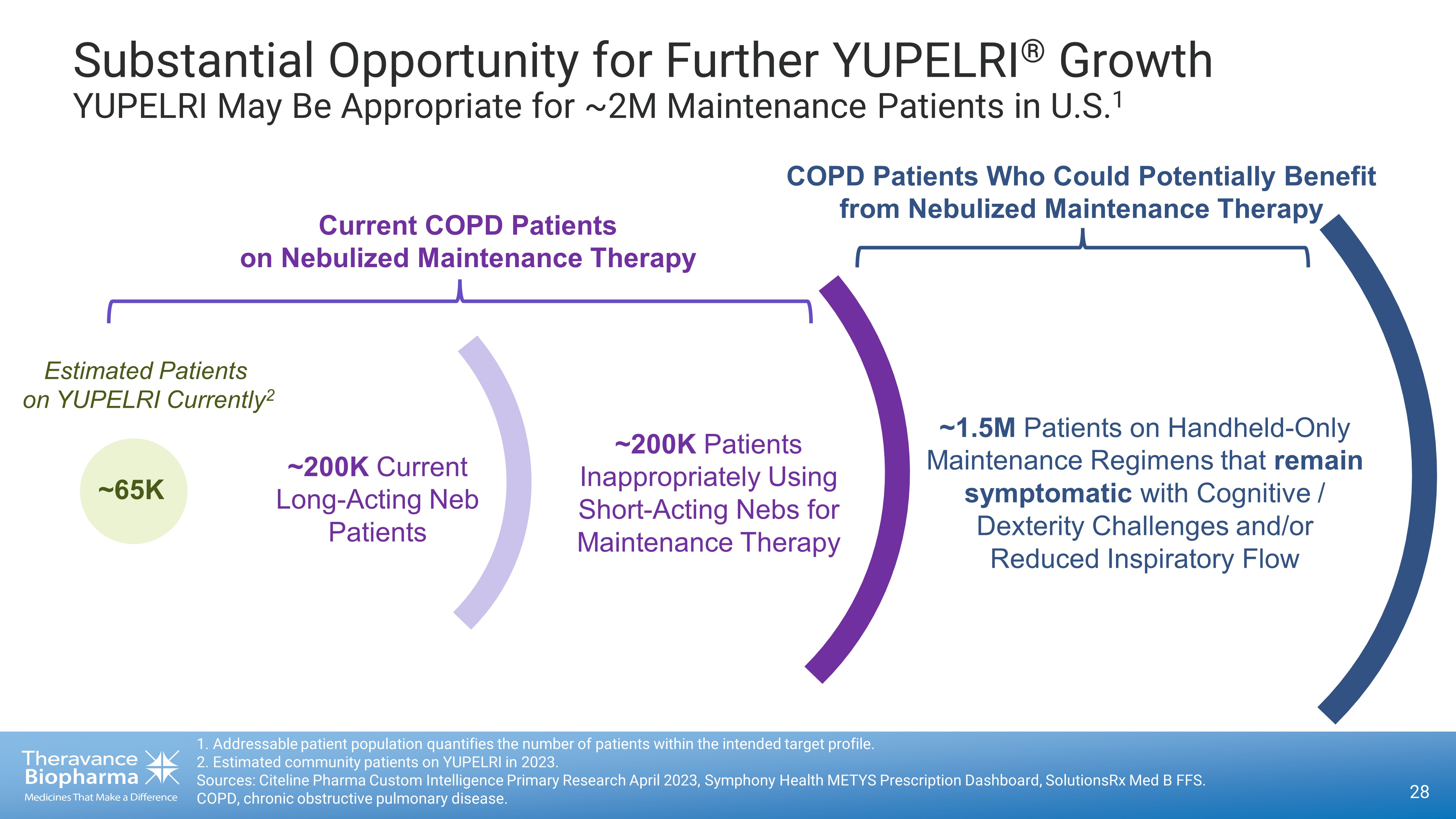
28 Substantial Opportunity for Further YUPELRI ® Growth YUPELRI May Be Appropriate for ~2M Maintenance Patients in U.S. 1 1. Addressable patient population quantifies the number of patients within the intended target profile. 2. Estimated community patients on YUPELRI in 2023. Sources: Citeline Pharma Custom Intelligence Primary Research April 2023, Symphony Health METYS Prescription Dashboard, Solut ion sRx Med B FFS. COPD, chronic obstructive pulmonary disease . Current COPD Patients on Nebulized Maintenance Therapy COPD Patients Who Could Potentially Benefit from Nebulized Maintenance Therapy Estimated Patients on YUPELRI Currently 2 ~65K ~200K Current Long - Acting Neb Patients ~200K Patients Inappropriately Using Short - Acting Nebs for Maintenance Therapy ~1.5M Patients on Handheld - Only Maintenance Regimens that remain symptomatic with Cognitive / Dexterity Challenges and/or R educed Inspiratory Flow
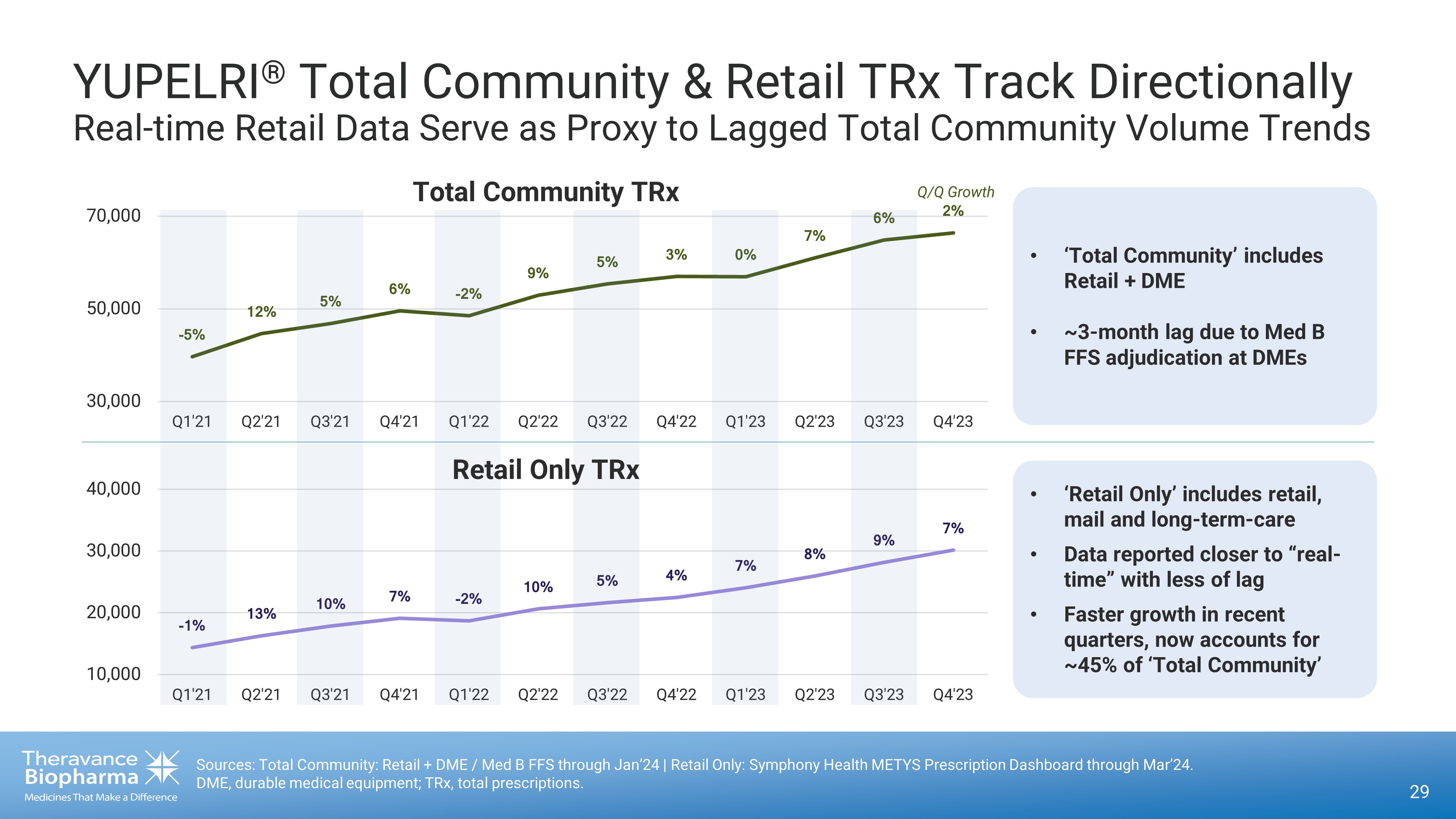
29 × ß Ï ÛÜ Ï ß Ï à Ï × Ü Ï ã Ï ß Ï Ý Ï Ú Ï á Ï à Ï Ü Ï 30,000 50,000 70,000 Q1'21 Q2'21 Q3'21 Q4'21 Q1'22 Q2'22 Q3'22 Q4'22 Q1'23 Q2'23 Q3'23 Q4'23 Total Community TRx × Û Ï ÛÝ Ï ÛÚ Ï á Ï × Ü Ï ÛÚ Ï ß Ï Þ Ï á Ï â Ï ã Ï á Ï 10,000 20,000 30,000 40,000 Q1'21 Q2'21 Q3'21 Q4'21 Q1'22 Q2'22 Q3'22 Q4'22 Q1'23 Q2'23 Q3'23 Q4'23 • ‘Total Community’ includes Retail + DME • ~3 - month lag due to Med B FFS adjudication at DMEs Retail Only TRx • ‘Retail Only’ includes retail, mail and long - term - care • Data reported closer to “real - time” with less of lag • Faster growth in recent quarters, now accounts for ~45% of ‘Total Community’ Q /Q Growth Sources: Total Community: Retail + DME / Med B FFS through Jan’24 | Retail Only: Symphony Health METYS Prescription Dashboard through Mar’24. DME, durable medical equipment; TRx, total prescriptions. YUPELRI ® Total Community & Retail TRx Track Directionally Real - time Retail Data Serve as Proxy to Lagged Total Community Volume Trends

Appendix II: Ampreloxetine
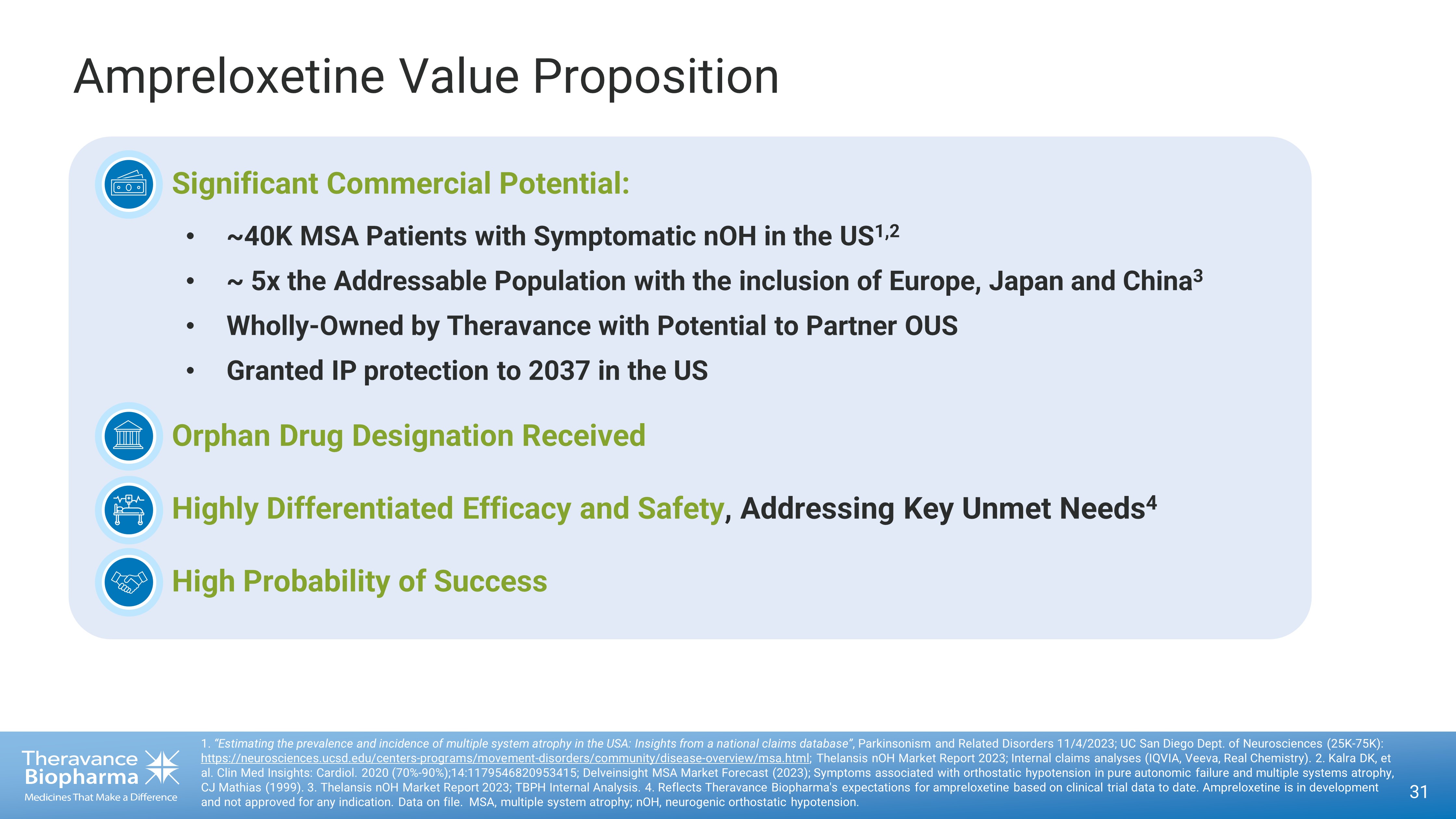
Ampreloxetine Value Proposition 31 1. “Estimating the prevalence and incidence of multiple system atrophy in the USA: Insights from a national claims database” , Parkinsonism and Related Disorders 11/4/2023; UC San Diego Dept. of Neurosciences (25K - 75K): https://neurosciences.ucsd.edu/centers - programs/movement - disorders/community/disease - overview/msa.html ; Thelansis nOH Market Report 2023 ; Internal claims analyses (IQVIA, Veeva, Real Chemistry). 2. Kalra DK, et al. Clin Med Insights: Cardiol. 2020 (70% - 90%);14:1179546820953415; Delveinsight MSA Market Forecast (2023); Symptoms associated with orthostatic hypotension in pure autonomic failure and multiple systems atrophy, CJ Mathias (1999). 3. Thelansis nOH Market Report 2023; TBPH Internal Analysis. 4. Reflects Theravance Biopharma's expectations for ampreloxetine based on clinical trial data to date. Ampreloxetine is in d eve lopment and not approved for any indication. Data on file. MSA, multiple system atrophy; nOH, neurogenic orthostatic hypotension. • Significant Commercial Potential: • ~40K MSA Patients with Symptomatic nOH in the US 1,2 • ~ 5x the Addressable Population with the inclusion of Europe, Japan and China 3 • Wholly - Owned by Theravance with Potential to Partner OUS • Granted IP protection to 2037 in the US • Orphan Drug Designation Received • Highly Differentiated Efficacy and Safety , Addressing Key Unmet Needs 4 • High Probability of Success
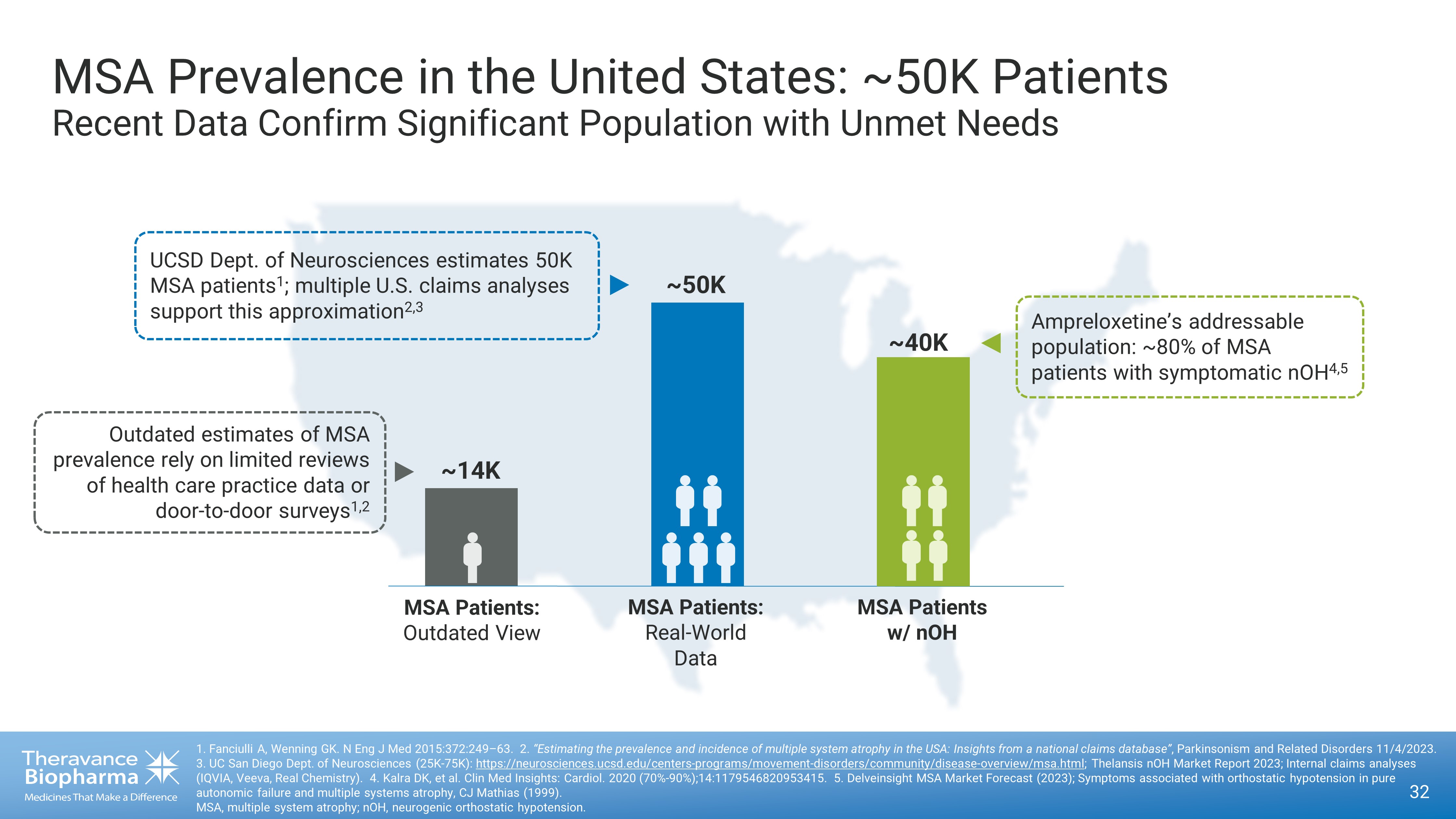
MSA Prevalence in the United States: ~50K Patients Recent Data C onfirm S ignificant P opulation with Unmet N eeds 1. Fanciulli A, Wenning GK. N Eng J Med 2015:372:249 – 63. 2. “Estimating the prevalence and incidence of multiple system atrophy in the USA: Insights from a national claims database” , Parkinsonism and Related Disorders 11/4/2023. 3. UC San Diego Dept. of Neurosciences (25K - 75K): https://neurosciences.ucsd.edu/centers - programs/movement - disorders/community/disease - overview/msa.html ; Thelansis nOH Market Report 2023 ; Internal claims analyses (IQVIA, Veeva, Real Chemistry). 4. Kalra DK, et al. Clin Med Insights: Cardiol. 2020 (70% - 90%);14:1179546820953415. 5. Delveinsight MSA Market Forecast (2023); Sy mptoms associated with orthostatic hypotension in pure autonomic failure and multiple systems atrophy, CJ Mathias (1999). MSA, multiple system atrophy; nOH, neurogenic orthostatic hypotension. ~14K MSA Patients: Outdated View MSA Patients: Real - World Data ~50K Outdated estimates of MSA prevalence rely on limited reviews of health care practice data or door - to - door surveys 1,2 UCSD Dept. of Neurosciences estimates 50K MSA patients 1 ; multiple U.S. claims analyses support this approximation 2,3 MSA Patients w/ nOH ~40K Ampreloxetine’s addressable p opulation: ~80% of MSA patients with symptomatic nOH 4,5 32
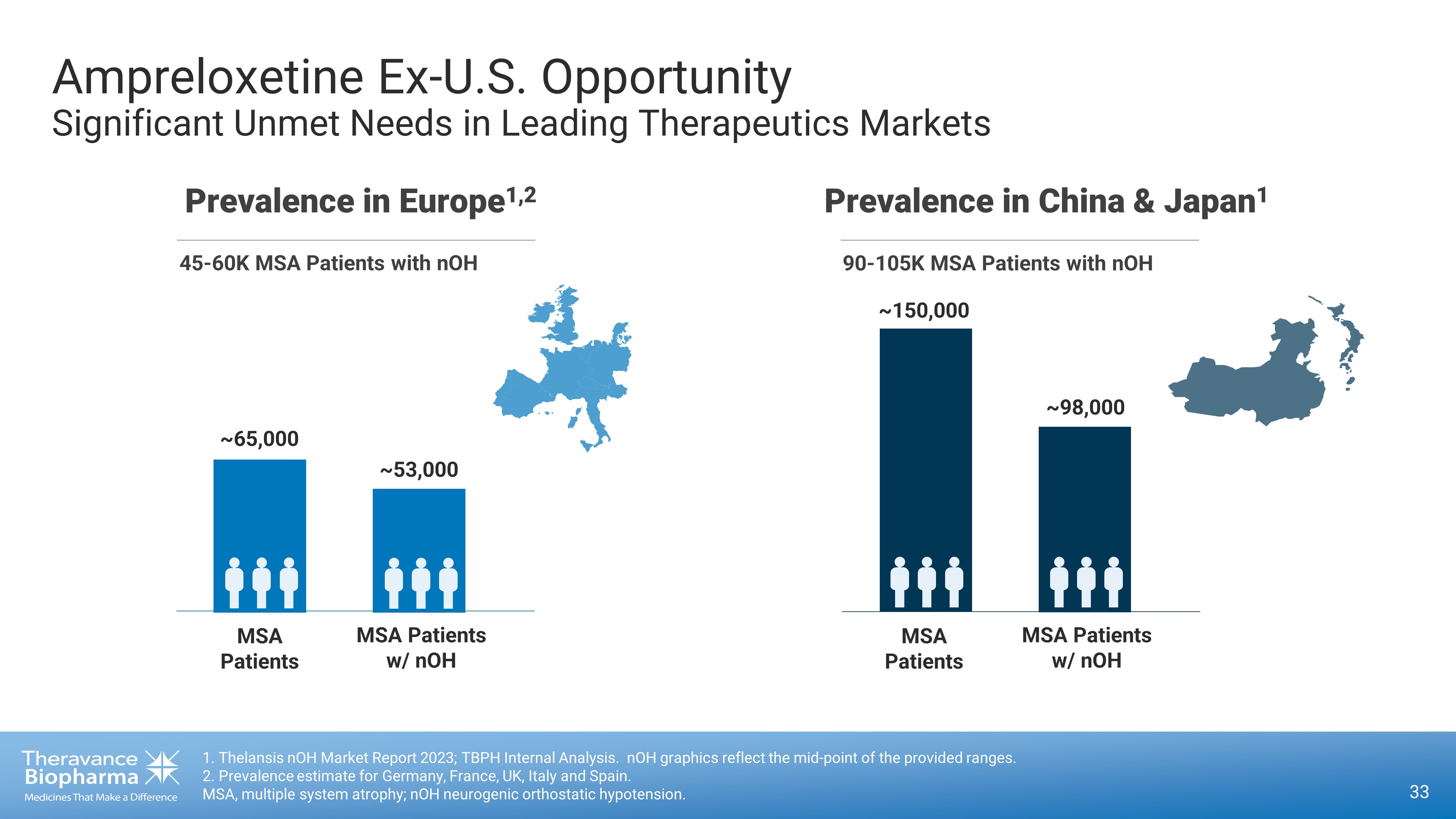
33 1. Thelansis nOH Market Report 2023; TBPH Internal Analysis. nOH graphics reflect the mid - point of the provided ranges. 2. Prevalence estimate for Germany, France, UK, Italy and Spain. MSA, multiple system atrophy; nOH neurogenic orthostatic hypotension. Ampreloxetine Ex - U.S. Opportunity Significant Unmet Needs in Leading Therapeutics Markets Prevalence in Europe 1,2 ~65,000 ~53 ,000 MSA Patients MSA Patients w/ nOH 45 - 60K MSA Patients with nOH Prevalence in China & Japan 1 ~ 150,000 ~98 ,000 MSA Patients MSA Patients w/ nOH 90 - 105K MSA Patients with nOH
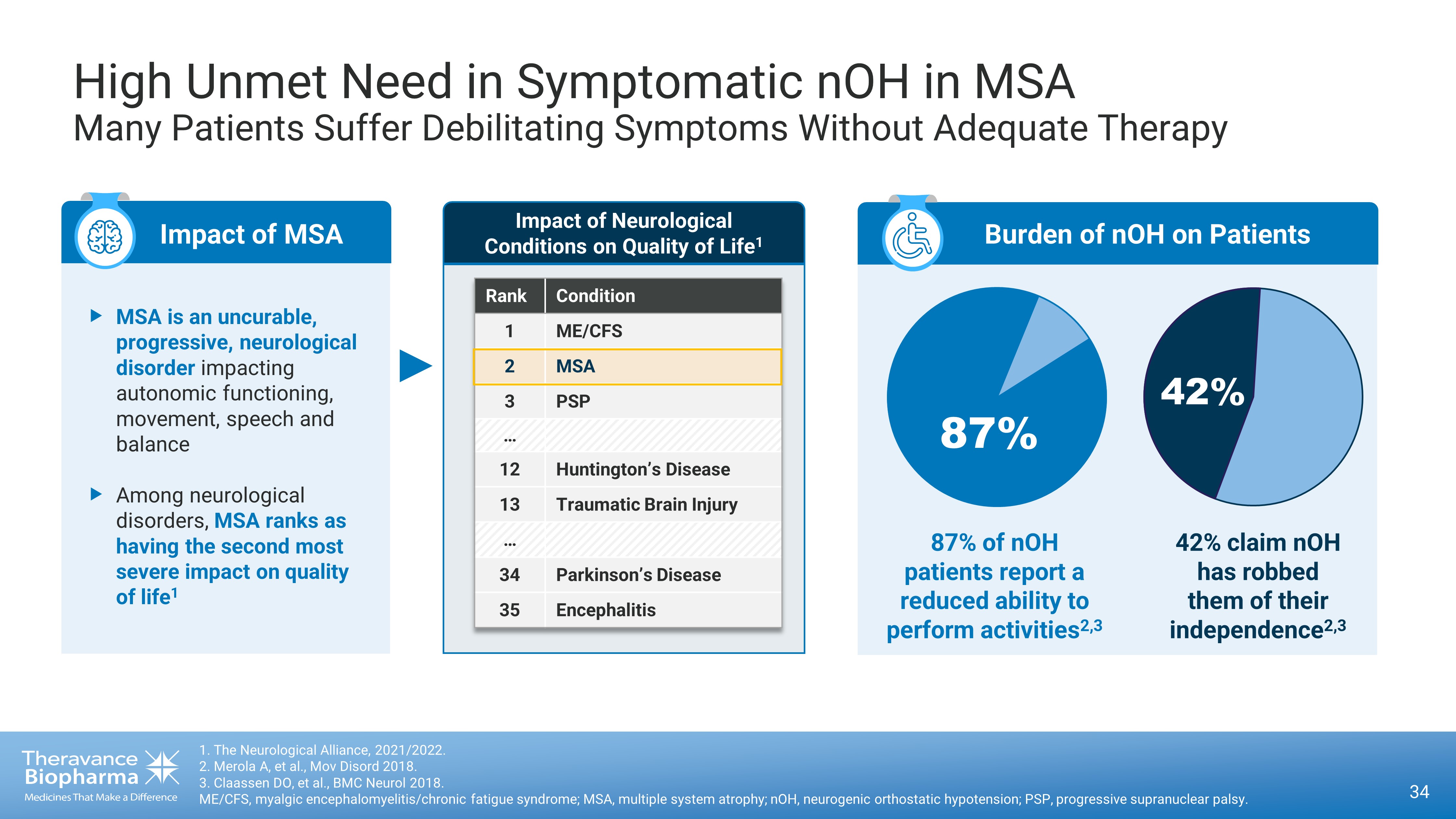
High Unmet Need in Symptomatic nOH in MSA Many Patients Suffer Debilitating Symptoms Without Adequate Therapy 1 . The Neurological Alliance, 2021/2022. 2 . Merola A, et al., Mov Disord 2018 . 3. Claassen DO, et al., BMC Neurol 2018. ME/CFS, m yalgic encephalomyelitis/chronic fatigue syndrome; MSA, multiple system atrophy; nOH, neurogenic orthostatic hypotension; PSP, progressive supranuclear palsy. 34 Impact of MSA ‣ MSA is an uncurable, progressive, neurological disorder impacting autonomic functioning, movement, speech and balance ‣ Among neurological disorders, MSA ranks as having the second most severe impact on quality of life 1 Impact of Neurological Conditions on Quality of Life 1 Rank Condition 1 ME/CFS 2 MSA 3 PSP … 12 Huntington’s Disease 13 Traumatic Brain Injury … 34 Parkinson’s Disease 35 Encephalitis Burden of nOH on Patients 87% 42% 87% of nOH patients report a reduced ability to perform activities 2,3 42% claim nOH has robbed them of their independence 2,3
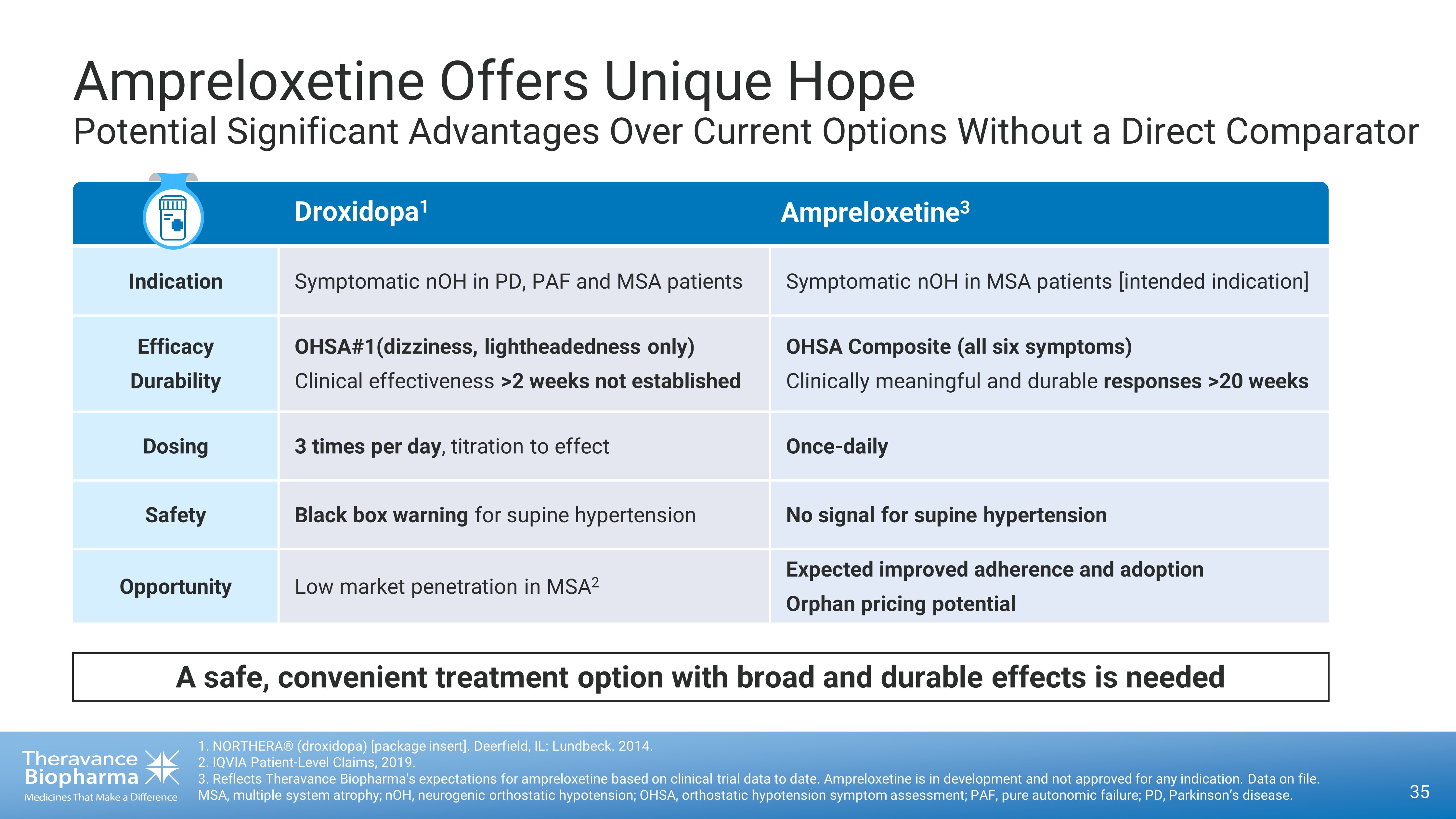
Droxidopa Ampreloxetine Indication Symptomatic nOH in PD, PAF and MSA patients Symptomatic nOH in MSA patients [intended indication] Efficacy Durability OHSA#1(dizziness, lightheadedness only) Clinical effectiveness >2 weeks not established OHSA Composite (all six symptoms) Clinically meaningful and durable responses >20 weeks Dosing 3 times per day , titration to effect Once - daily Safety Black box warning for supine hypertension No signal for supine hypertension Opportunity Low market penetration in MSA 2 Expected improved adherence and adoption Orphan pricing potential Ampreloxetine Offers Unique Hope Potential Significant Advantages Over Current Options Without a Direct Comparator 35 1. NORTHERA® (droxidopa) [package insert]. Deerfield, IL: Lundbeck. 2014. 2. IQVIA Patient - Level Claims, 2019. 3. Reflects Theravance Biopharma's expectations for ampreloxetine based on clinical trial data to date. Ampreloxetine is in d eve lopment and not approved for any indication. Data on file. MSA, multiple system atrophy; nOH, neurogenic orthostatic hypotension; OHSA, orthostatic hypotension symptom assessment; PAF, pure autonomic failure; PD, Parkinson’s disease. Droxidopa 1 Ampreloxetine 3 A safe, convenient treatment option with broad and durable effects is needed
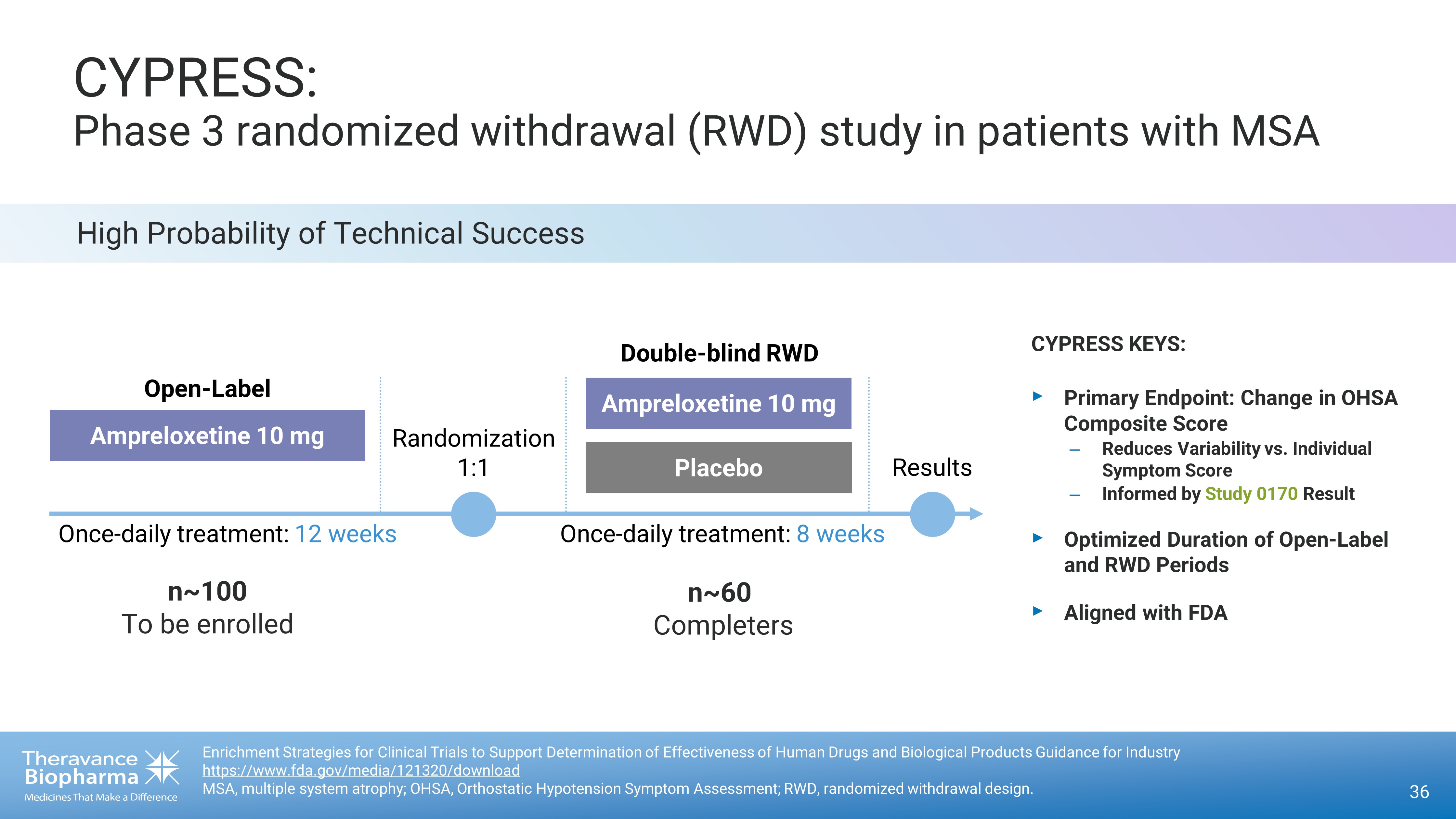
High Probability of Technical Success CYPRESS: Phase 3 randomized withdrawal (RWD) study in patients with MSA 36 Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products Gu ida nce for Industry https://www.fda.gov/media/121320/download MSA, multiple system atrophy; OHSA, Orthostatic Hypotension Symptom Assessment; RWD, randomized withdrawal design. CYPRESS KEYS: ‣ Primary Endpoint: Change in OHSA Composite Score – Reduces Variability vs. Individual Symptom Score – Informed by Study 0170 Result ‣ Optimized D uration of Ope n - Label and RWD Periods ‣ Aligned with FDA Randomization 1:1 Results Ampreloxetine 10 mg Placebo Once - daily treatment: 8 weeks Ampreloxetine 10 mg Open - Label Once - daily treatment: 12 weeks Double - blind RWD n~100 To be enrolled n~60 Completers
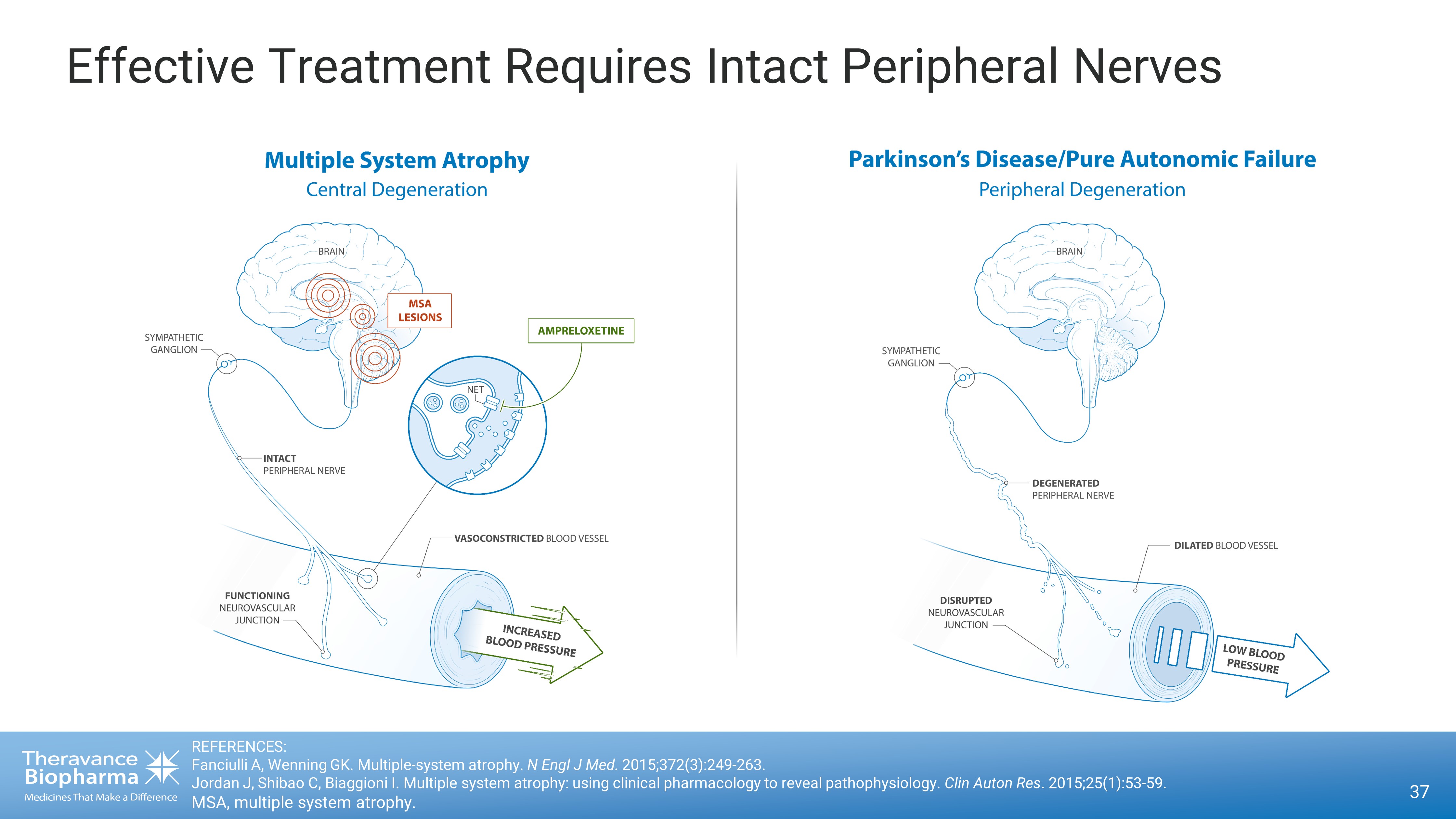
37 Effective Treatment Requires Intact Peripheral Nerves REFERENCES: Fanciulli A, Wenning GK. Multiple - system atrophy. N Engl J Med. 2015;372(3):249 - 263. Jordan J, Shibao C, Biaggioni I. Multiple system atrophy: using clinical pharmacology to reveal pathophysiology. Clin Auton Res . 2015;25(1):53 - 59. MSA, multiple system atrophy.
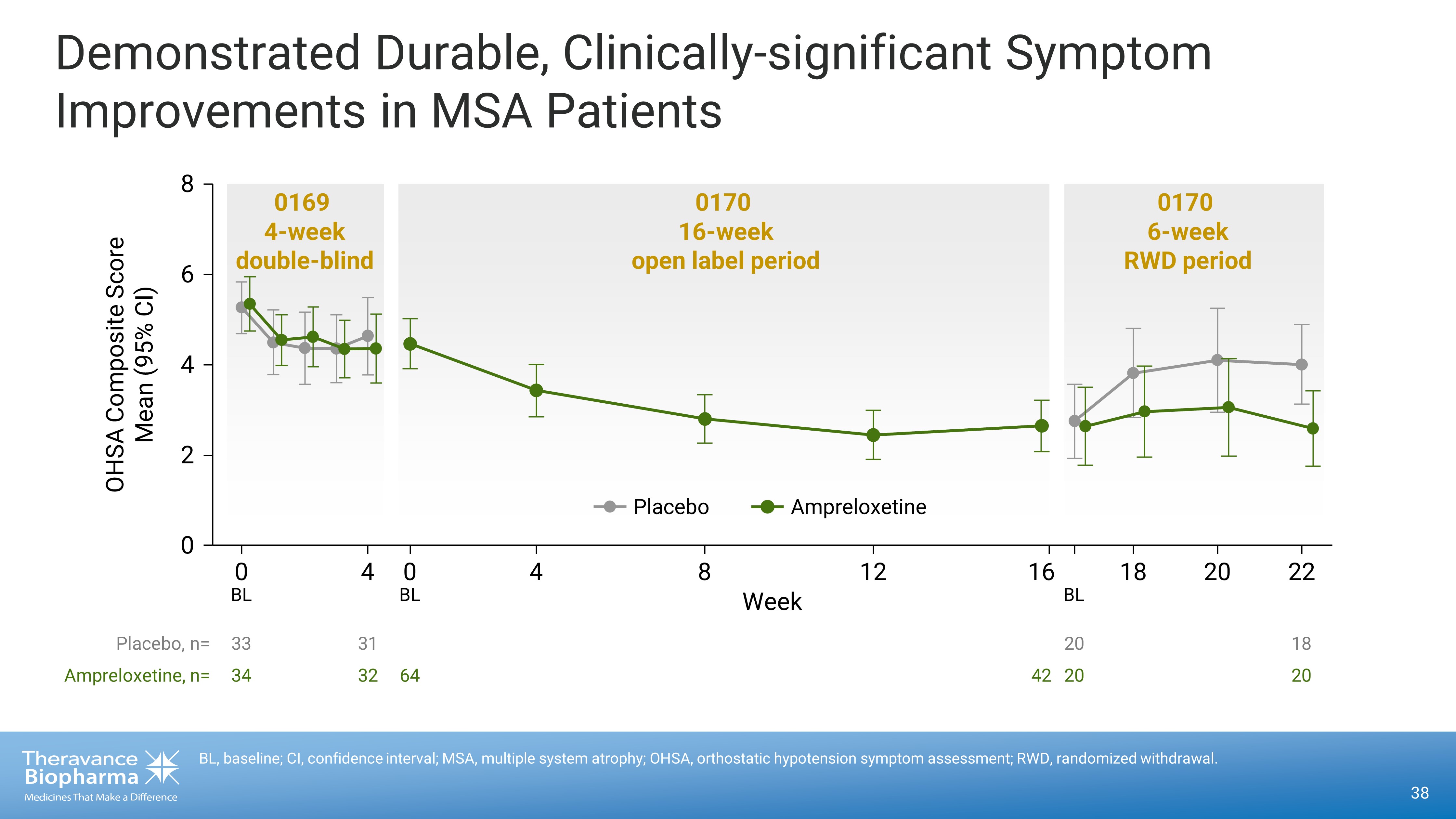
38 BL, baseline; CI, confidence interval; MSA, multiple system atrophy; OHSA, orthostatic hypotension symptom assessment; RWD, r and omized withdrawal. Demonstrated Durable, Clinically - significant Symptom Improvements in MSA Patients 33 34 31 32 64 42 20 20 18 20 0 0 4 0 4 8 12 16 18 20 22 2 4 6 8 0169 4 - week double - blind 0170 6 - week RWD period 0170 16 - week open label period Placebo, n= Ampreloxetine, n= Week OHSA Composite Score Mean (95% CI) BL BL BL Ampreloxetine Placebo
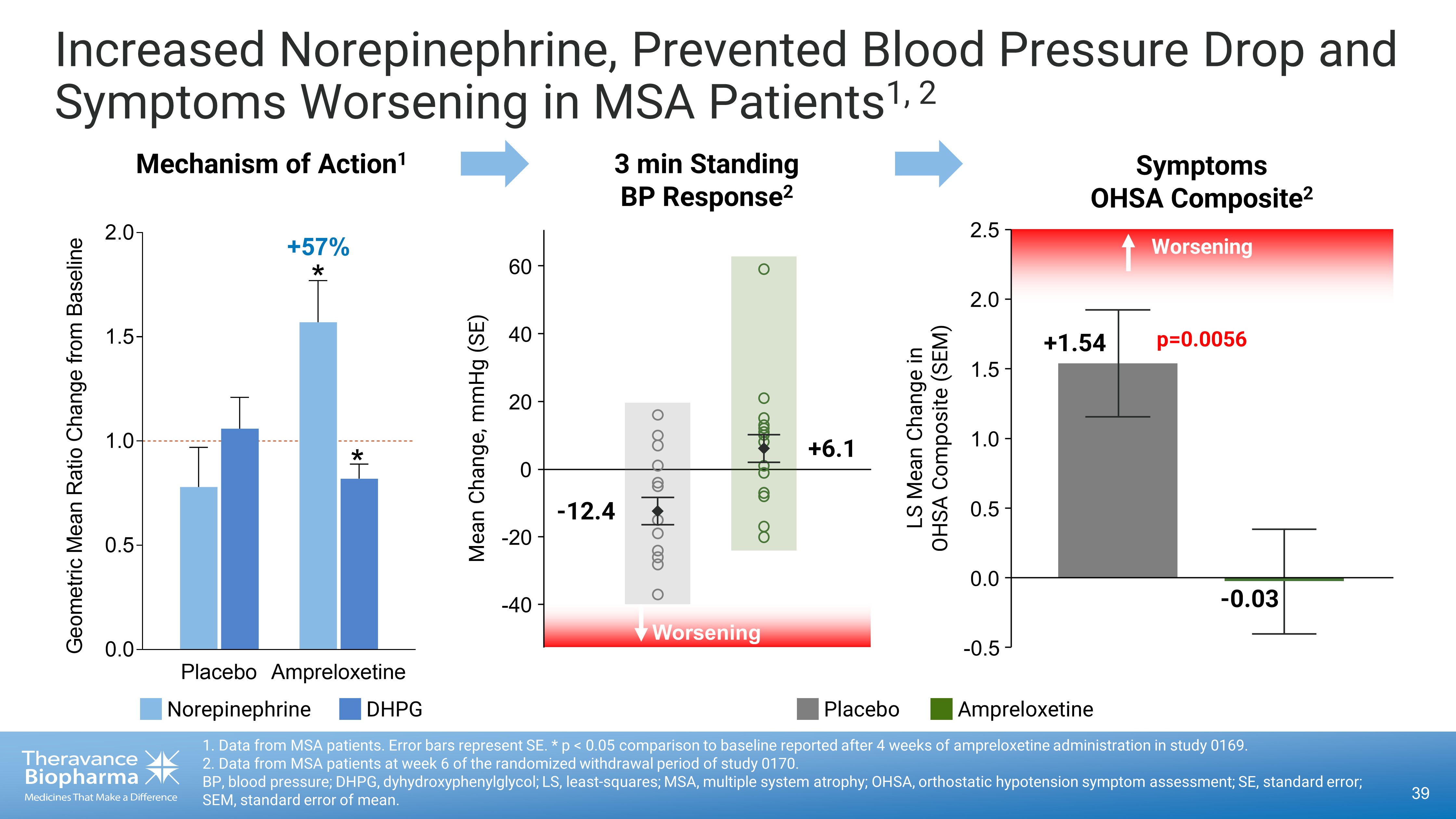
Increased Norepinephrine, Prevented Blood Pressure Drop and Symptoms Worsening in MSA Patients 1, 2 39 1. Data from MSA patients. Error bars represent SE. * p < 0.05 comparison to baseline reported after 4 weeks of ampreloxetine ad ministration in study 0169. 2. Data from MSA patients at week 6 of the randomized withdrawal period of study 0170. BP, blood pressure; DHPG, dyhydroxyphenylglycol; LS, least - squares; MSA, multiple system atrophy; OHSA, orthostatic hypotension symptom assessment; SE, standard error; SEM, standard error of mean. Placebo Ampreloxetine 0.0 0.5 1.0 1.5 2.0 G e o m e t r i c M e a n R a t i o C h a n g e f r o m B a s e l i n e Mechanism of Action 1 3 min Standing BP Response 2 * * +57% - 0.03 p=0.0056 LS Mean Change in OHSA Composite (SEM) Worsening +1.54 Symptoms OHSA Composite 2 0.0 - 0.5 0.5 1.0 1.5 2.0 2.5 - 40 - 20 0 20 40 60 Worsening - 12.4 +6.1 Mean Change, mmHg (SE) Placebo Ampreloxetine Norepinephrine DHPG
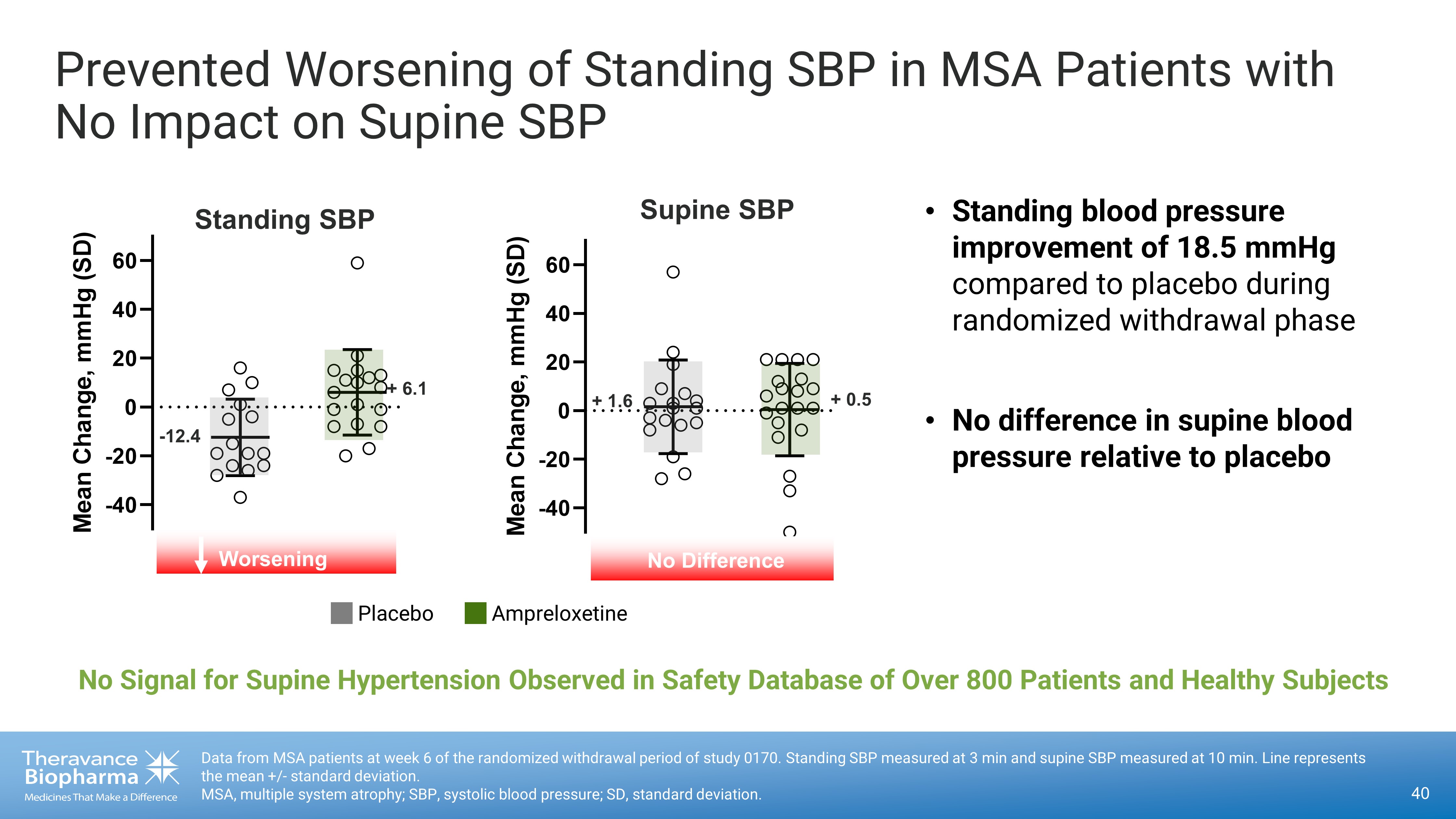
Data from MSA patients at week 6 of the randomized withdrawal period of study 0170. Standing SBP measured at 3 min and supine SB P measured at 10 min. Line represents the mean +/ - standard deviation. MSA, multiple system atrophy; SBP, systolic blood pressure; SD, standard deviation. Prevented Worsening of Standing SBP in MSA Patients with No Impact on Supine SBP Placebo Ampreloxetine -40 -20 0 20 40 60 M e a n C h a n g e , m m H g ( S D ) Supine SBP No Difference + 1.6 + 0.5 • Standing blood pressure improvement of 18.5 mmHg compared to placebo during randomized withdrawal phase • No difference in supine blood pressure relative to placebo -40 -20 0 20 40 60 M e a n C h a n g e , m m H g ( S D ) - 12.4 + 6.1 Standing SBP Worsening No Signal for Supine Hypertension Observed in Safety Database of Over 800 Patients and Healthy Subjects 40

Appendix III: Corporate
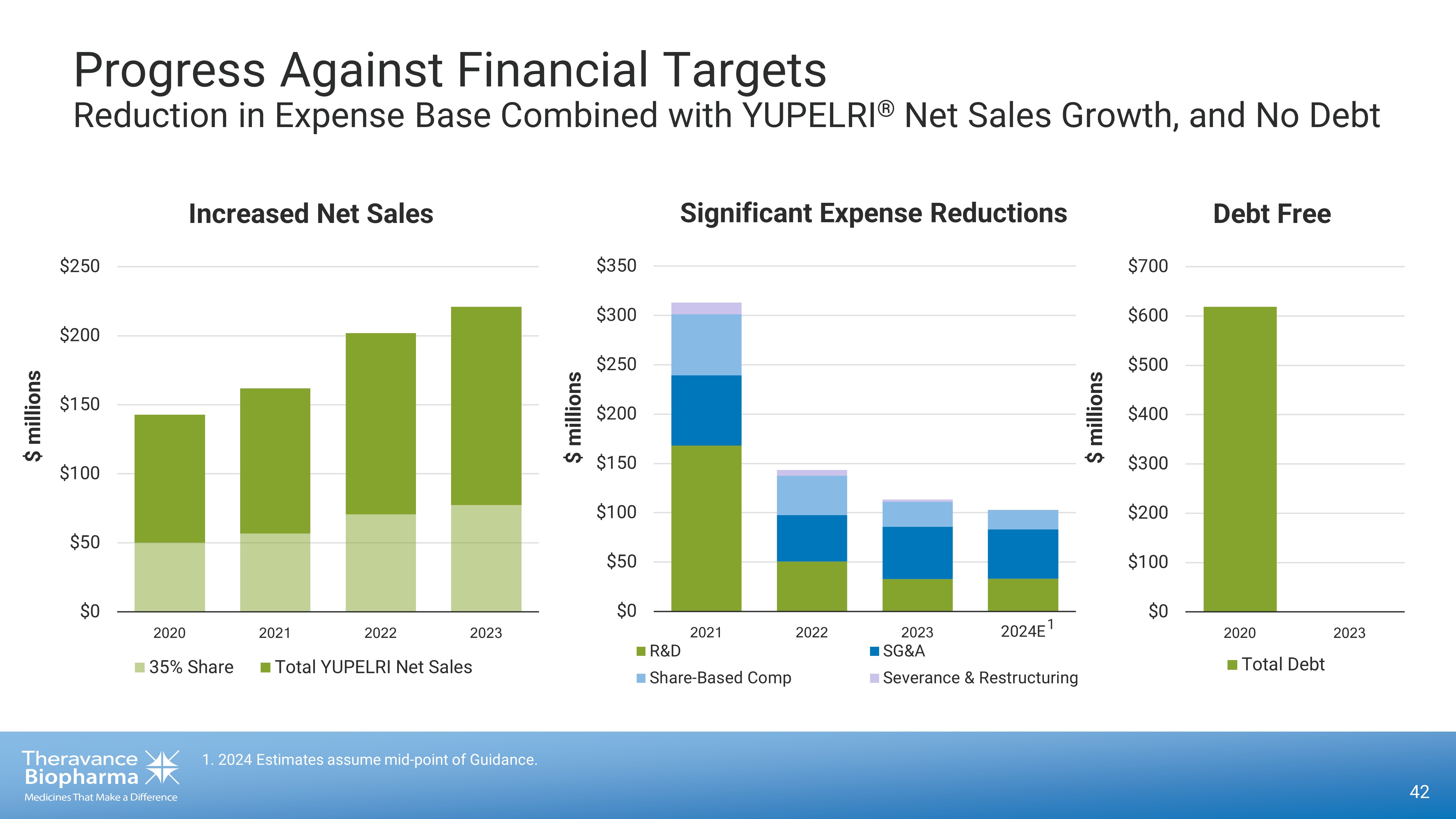
42 1. 2024 Estimates assume mid - point of Guidance. Progress Against Financial Targets Reduction in Expense B ase C ombined with YUPELRI ® Net Sales Growth, and No D ebt $0 $50 $100 $150 $200 $250 $300 $350 ÜÚÜÛ ÜÚÜÜ ÜÚÜÝ 2024E R&D SG&A Share-Based Comp Severance & Restructuring Significant Expense Reductions $ millions $ millions $0 $50 $100 $150 $200 $250 ÜÚÜÚ ÜÚÜÛ ÜÚÜÜ ÜÚÜÝ 35% Share Total YUPELRI Net Sales Increased Net Sales $0 $100 $200 $300 $400 $500 $600 $700 ÜÚÜÚ ÜÚÜÝ Total Debt $ millions Debt Free 1

Granted Patent Protection Into Late 2030s 43 COPD, Chronic obstructive pulmonary disease ; nOH, neurogenic orthostatic hypotension; PTE, patent term extensions. Compound Invention Estimated Patent Expiry YUPELRI ® / revefenacin Composition of Matter 2028 (once PTE awarded) Polymorph 2030 - 2031 Method for the maintenance treatment of COPD patients 2039 Ampreloxetine Composition of Matter 2030 (plus PTE of up to 5 years) Method of Treating nOH 2037
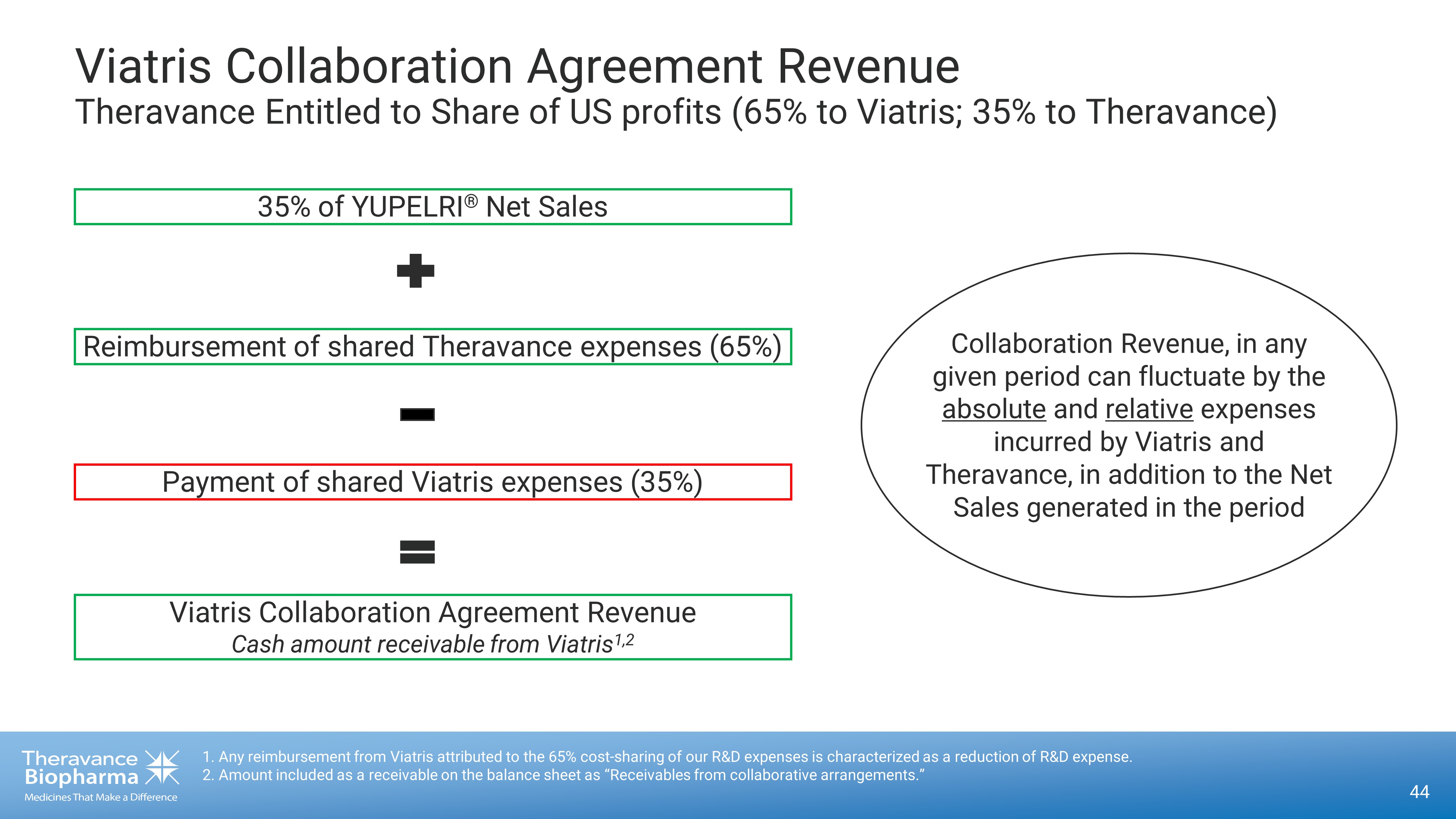
44 Viatris Collaboration Agreement Revenue Theravance Entitled to Share of US profits (65% to Viatris; 35% to Theravance) 1. Any reimbursement from Viatris attributed to the 65% cost - sharing of our R&D expenses is characterized as a reduction of R&D expense. 2. Amount included as a receivable on the balance sheet as “Receivables from collaborative arrangements.” Viatris Collaboration Agreement Revenue Cash amount receivable from Viatris 1,2 Payment of shared Viatris expenses (35%) Reimbursement of shared Theravance expenses (65%) 35% of YUPELRI ® Net Sales Collaboration Revenue, in any given period can fluctuate by the absolute and relative expenses incurred by Viatris and Theravance, in addition to the Net Sales generated in the period
v3.24.1.1.u2
Cover
|
May 13, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
May 13, 2024
|
| Entity File Number |
001-36033
|
| Entity Registrant Name |
THERAVANCE BIOPHARMA, INC.
|
| Entity Central Index Key |
0001583107
|
| Entity Tax Identification Number |
98-1226628
|
| Entity Incorporation, State or Country Code |
E9
|
| Entity Address, Address Line One |
C/O Theravance Biopharma US, Inc.
|
| Entity Address, Address Line Two |
901 Gateway Boulevard
|
| Entity Address, City or Town |
South San Francisco
|
| Entity Address, Country |
CA
|
| Entity Address, Postal Zip Code |
94080
|
| City Area Code |
650
|
| Local Phone Number |
808-6000
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Ordinary
Share $0.00001 Par Value
|
| Trading Symbol |
TBPH
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Theravance Biopharma (NASDAQ:TBPH)
Historical Stock Chart
From Apr 2024 to May 2024
Theravance Biopharma (NASDAQ:TBPH)
Historical Stock Chart
From May 2023 to May 2024