0001534120false00015341202023-11-092023-11-09
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 9, 2023
AVALO THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
Delaware
(State or other jurisdiction of incorporation)
| | | | | | | | |
| 001-37590 | | 45-0705648 |
| (Commission File Number) | | (IRS Employer Identification No.) |
540 Gaither Road, Suite 400, Rockville, Maryland 20850
(Address of principal executive offices) (Zip Code)
Registrant’s Telephone Number, Including Area Code: (410) 522-8707
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, $0.001 Par Value | AVTX | Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging Growth Company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02. Results of Operations and Financial Condition.
On November 9, 2023, Avalo Therapeutics, Inc. (the “Company”) issued a press release announcing its financial results for the quarter ended September 30, 2023. A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated herein in its entirety by reference.
Information in this Item 2.02 (including Exhibit 99.1) shall not be deemed “filed” for purposes of Section 18 of the Securities and Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits:
| | | | | | | | |
| Exhibit No. | | Description |
| | |
| 99.1 | | |
| | |
| 104 | | The cover pages of this Current Report on Form 8-K, formatted in Inline XBRL. |
| | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | |
| | | |
| | | AVALO THERAPEUTICS, INC. |
| | | |
| Date: November 9, 2023 | | By: | /s/ Christopher Sullivan |
| | | Christopher Sullivan |
| | | Chief Financial Officer |
Avalo Reports Third Quarter 2023 Financial Results and Provides Business Updates
•Successfully eliminated $35 million debt paving the way for future growth and innovation
•Divested AVTX-800 series for potential milestone payments of $45 million, fully focusing the pipeline on Avalo’s promising immunology assets
•Disclosed improved cash of approximately $10.2 million as of September 30, 2023
WAYNE, PA AND ROCKVILLE, MD, November 9, 2023 — Avalo Therapeutics, Inc. (Nasdaq: AVTX), today announced business updates and financial results for the third quarter of 2023.
Dr. Garry Neil, Chief Executive Officer and Chairman of the Board remarked, “We made significant progress in the third quarter, highlighted by the full debt payoff and divestiture of the 800 series. Our strengthened balance sheet and focused pipeline underscores our unwavering commitment to execute our strategy to progress our promising immunology drug candidates and positions us to consider collaborations, pursue funding for research and development, and bring innovative treatments to market.”
Dr. Neil continued, “I am excited to potentially kick off a randomized placebo-controlled trial of quisovalimab, our anti-LIGHT mAb, in patients with ulcerative colitis or another inflammatory indication, subject to funding. This drug candidate has previously shown strong target engagement in both acute and chronic inflammatory diseases, and I remain optimistic that it could transform the lives of patients with immunological diseases and address unmet medical needs. Additionally, we look forward to progressing AVTX-008, our BTLA agonist fusion protein with high-binding affinity and serum stability, to IND. Targeting BTLA represents a promising and increasingly recognized avenue for developing therapies that can effectively modulate the immune response in autoimmune diseases while minimizing the risk of systemic immunosuppression. We believe AVTX-008 is unique in this class because it is a fusion protein that utilizes the natural ligand thus avoiding potential problems with agonist mAbs. Finally, we continue to evaluate new opportunities to further augment our immunology pipeline.”
Corporate Updates:
•In September of 2023, Avalo paid off the remaining $14.3 million of its original $35 million debt owed to Horizon Technology Finance Corporation (Nasdaq: HRZN). As a result, Avalo’s obligations under the debt agreement were deemed satisfied.
•On October 27, 2023, Avalo completed the divestiture of its rights, title and interest in, assets relating to AVTX-801, AVTX-802 and AVTX-803 (collectively, the 800 Series) to AUG Therapeutics, LLC (AUG). The Company is entitled to up to $45 million of contingent milestone payments. The Company previously announced it entered into a purchase agreement with AUG to divest the 800 Series on September 12, 2023.
Program Updates:
•Quisovalimab (AVTX-002): Anti-LIGHT monoclonal antibody (mAb) targeting immune-inflammatory diseases.
◦Quisovalimab has shown a rapid and sustained reduction of LIGHT levels, as well as a favorable safety and tolerability profile, in all indications studied including COVID-19 Acute Respiratory Distress Syndrome (ARDS), Crohn’s Disease and Non-Eosinophilic Asthma (NEA).
◦Quisovalimab was statistically significant in reducing respiratory failure and mortality in patients hospitalized with COVID-19 ARDS in a randomized placebo-controlled trial. Quisovalimab also demonstrated positive trends in an open-label study in Crohn’s Disease.
◦A post-hoc analyses in the PEAK Trial showed a sub-population of NEA patients with baseline LIGHT levels over 125 pg/mL, which represented over 50% of patients, had an approximate 50% reduction in asthma-related events (AREs) for patients treated with quisovalimab compared to placebo.
◦Avalo is pursuing funding for the program and is considering a randomized placebo-controlled trial in patients with Ulcerative Colitis or other inflammatory conditions.
•AVTX-008: B and T Lymphocyte Attenuator (BTLA) agonist fusion protein targeting immune dysregulation disorders.
◦AVTX-008 is uniquely positioned as a fusion protein with high-binding affinity and serum stability. It utilizes the natural ligand thus it may avoid the potential problems with agonist mAbs.
◦Avalo previously identified a lead molecule, is evaluating several immune dysregulation disorders to pursue and plans to rapidly progress the asset to IND, subject to funding.
Third Quarter 2023 Financial Update:
Avalo had $10.2 million in cash and cash equivalents as of September 30, 2023. The Company fully eliminated its debt with principal payments of $21.2 million, inclusive of the full payoff of the remaining loan in September of 2023. The Company raised $46.2 million of net proceeds from equity financings in the nine months ended September 30, 2023.
Total operating expenses decreased $24.7 million for the nine months ended September 30, 2023 as compared to the same period in 2022. This decrease was primarily driven by decreases to both research and development expenses and selling, general and administrative as a result of cost savings initiatives implemented in the first quarter of 2022 and fewer research and development programs ongoing in the current year.
The net loss and net loss per share for the nine months ended September 30, 2023 was largely driven by operating expenses. The significant decrease in net loss for the nine months ended September 30, 2023 as compared to the prior year period was due to the $24.7 million decrease in operating expenses, partially offset by the $14.5 million of license revenue in the prior year that did not repeat. Net loss per share decreased as a result of the decrease in net loss and due to a significant increase in shares outstanding.
Consolidated Balance Sheets
(In thousands, except share and per share data)
| | | | | | | | | | | | | | |
| | September 30, 2023 | | December 31, 2022 |
| | (unaudited) | | |
| Assets | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | | $ | 10,180 | | | $ | 13,172 | |
| | | | |
| Other receivables | | 1,538 | | | 1,919 | |
| Inventory, net | | — | | | 20 | |
| Prepaid expenses and other current assets | | 940 | | | 1,290 | |
| Restricted cash, current portion | | 1 | | | 15 | |
| Total current assets | | 12,659 | | | 16,416 | |
| Property and equipment, net | | 2,071 | | | 2,411 | |
| Goodwill | | 14,409 | | | 14,409 | |
| Restricted cash, net of current portion | | 131 | | | 131 | |
| Total assets | | $ | 29,270 | | | $ | 33,367 | |
| Liabilities and stockholders’ equity (deficit) | | | | |
| Current liabilities: | | | | |
| Accounts payable | | $ | 789 | | | $ | 2,882 | |
| Deferred revenue | | — | | | 88 | |
| Accrued expenses and other current liabilities | | 5,216 | | | 13,214 | |
| Notes payable, current | | — | | | 5,930 | |
| Total current liabilities | | 6,005 | | | 22,114 | |
| Notes payable, non-current | | — | | | 13,486 | |
| Royalty obligation | | 2,000 | | | 2,000 | |
| Deferred tax liability, net | | 164 | | | 141 | |
| Derivative liability | | 4,950 | | | 4,830 | |
| Other long-term liabilities | | 1,456 | | | 1,711 | |
| Total liabilities | | 14,575 | | | 44,282 | |
| Stockholders’ equity (deficit): | | | | |
| Common stock—$0.001 par value; 200,000,000 shares authorized at September 30, 2023 and December 31, 2022; 192,382,419 and 9,430,535 shares issued and outstanding at September 30, 2023 and December 31, 2022, respectively | | 192 | | | 9 | |
| | | | |
| Additional paid-in capital | | 341,469 | | | 292,900 | |
| Accumulated deficit | | (326,966) | | | (303,824) | |
| Total stockholders’ equity (deficit) | | 14,695 | | | (10,915) | |
| Total liabilities and stockholders’ equity (deficit) | | $ | 29,270 | | | $ | 33,367 | |
The condensed consolidated balance sheets as of September 30, 2023 and December 31, 2022 have been derived from the reviewed and audited financial statements, respectively, but do not include all of the information and footnotes required by accounting principles accepted in the United States for complete financial statements.
Consolidated Statements of Operations (Unaudited)
(In thousands, except per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended | | Nine Months Ended |
| | September 30, | | September 30, |
| | 2023 | | 2022 | | 2023 | | 2022 |
| Revenues: | | | | | | | | |
| Product revenue, net | | $ | 236 | | | $ | 432 | | | $ | 1,353 | | | $ | 2,638 | |
| License revenue | | — | | | 14,517 | | | — | | | 14,517 | |
| Total revenues, net | | 236 | | | 14,949 | | | 1,353 | | | 17,155 | |
| | | | | | | | |
| Operating expenses: | | | | | | | | |
| Cost of product sales | | 247 | | | 528 | | | 1,505 | | | 2,814 | |
| Research and development | | 1,249 | | | 7,042 | | | 11,917 | | | 25,136 | |
| Selling, general and administrative | | 2,490 | | | 3,284 | | | 7,624 | | | 17,752 | |
| Amortization expense | | — | | | — | | | — | | | 38 | |
| Total operating expenses | | 3,986 | | | 10,854 | | | 21,046 | | | 45,740 | |
| | (3,750) | | | 4,095 | | | (19,693) | | | (28,585) | |
| Other expense: | | | | | | | | |
| Interest expense, net | | (1,553) | | | (898) | | | (3,498) | | | (3,221) | |
| Change in fair value of derivative liability | | 100 | | | — | | | (120) | | | — | |
| Other expense, net | | (17) | | | — | | | (42) | | | (20) | |
| Total other expense, net | | (1,470) | | | (898) | | | (3,660) | | | (3,241) | |
| (Loss) income before taxes | | (5,220) | | | 3,197 | | | (23,353) | | | (31,826) | |
| Income tax expense | | 8 | | | 5 | | | 23 | | | 20 | |
| Net (loss) income and comprehensive loss | | $ | (5,228) | | | $ | 3,192 | | | $ | (23,376) | | | $ | (31,846) | |
| Net (loss) income per share of common stock, basic and diluted | | $ | (0.11) | | | $ | 0.34 | | | $ | (0.96) | | | $ | (3.39) | |
The unaudited condensed consolidated statements of operations for the three and nine months ended September 30, 2023 and 2022 have been derived from the reviewed financial statements but do not include all of the information and footnotes required by accounting principles generally accepted in the United States for complete financial statements.
About quisovalimab (AVTX-002)
Quisovalimab is a fully human monoclonal antibody (mAb), directed against human LIGHT (Lymphotoxin-like, exhibits Inducible expression, and competes with Herpes Virus Glycoprotein D for Herpesvirus Entry Mediator (HVEM), a receptor expressed by T lymphocytes). There is increasing evidence that the dysregulation of the LIGHT-signaling network which includes LIGHT, its receptors HVEM and LTβR and the downstream checkpoint BTLA, is a disease-driving mechanism in autoimmune and inflammatory reactions in barrier organs. Therefore, we believe reducing LIGHT levels can moderate immune dysregulation in many acute and chronic inflammatory disorders. Quisovalimab previously demonstrated proof of concept in COVID-19 induced acute respiratory distress syndrome including reduction in mortality and respiratory failure, as well as a positive signal in patients with Crohn’s Disease.
About AVTX-008
AVTX-008 is a fully human B and T Lymphocyte Attenuator (BTLA) agonist fusion protein in the IND-enabling stage. AVTX-008 is differentiated by having specific binding to BTLA, with no binding to LIGHT or CD160. AVTX-008 also has high-serum stability and solubility.
About Avalo Therapeutics
Avalo Therapeutics is a clinical stage biotechnology company focused on the treatment of immune dysregulation by developing therapies that target the LIGHT-signaling network.
LIGHT and its signaling receptors, HVEM (TNFRSF14), and lymphotoxin β receptor (TNFRSF3), form an immune regulatory network with two co-receptors of herpesvirus entry mediator, checkpoint inhibitor B and T Lymphocyte Attenuator (BTLA), and CD160 (the LIGHT-signaling network). Accumulating evidence points to the dysregulation of the LIGHT network as a disease-driving mechanism in autoimmune and inflammatory reactions in barrier organs. Therefore, we believe reducing LIGHT levels can moderate immune dysregulation in many acute and chronic inflammatory disorders.
For more information about Avalo, please visit www.avalotx.com.
Forward-Looking Statements
This press release may include forward-looking statements made pursuant to the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts. Such forward-looking statements are subject to significant risks and uncertainties that are subject to change based on various factors (many of which are beyond Avalo’s control), which could cause actual results to differ from the forward-looking statements. Such statements may include, without limitation, statements with respect to Avalo’s plans, objectives, projections, expectations and intentions and other statements identified by words such as “projects,” “may,” “might,” “will,” “could,” “would,” “should,” “continue,” “seeks,” “aims,” “predicts,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “potential,” or similar expressions (including their use in the negative), or by discussions of future matters such as: the future financial and operational outlook; timing and success of trial results and regulatory review; potential attributes and benefits of product candidates; the development of product candidates or products; and other statements that are not historical. These statements are based upon the current beliefs and expectations of Avalo’s management but are subject to significant risks and uncertainties, including: Avalo's cash position and the need for it to raise additional capital in the near future; the results of our clinical and pre-clinical studies; drug development costs, timing and other risks, including reliance on investigators and enrollment of patients in clinical trials, which might be slowed by the COVID-19 pandemic; reliance on key personnel; regulatory risks; general economic and market risks and uncertainties, including those caused by the COVID-19 pandemic and the war in Ukraine; and those other risks detailed in Avalo’s filings with the SEC. Actual results may differ from those set forth in the forward-looking statements. Except as required by applicable law, Avalo expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Avalo’s expectations with respect thereto or any change in events, conditions or circumstances on which any statement is based.
For media and investor inquiries
Christopher Sullivan, CFO
Avalo Therapeutics, Inc.
ir@avalotx.com
410-803-6793
or
Chris Brinzey
ICR Westwicke
Chris.brinzey@westwicke.com
339-970-2843
v3.23.3
Cover Page Document
|
Nov. 09, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Document Period End Date |
Nov. 09, 2023
|
| Entity Registrant Name |
AVALO THERAPEUTICS, INC.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-37590
|
| Entity Tax Identification Number |
45-0705648
|
| Entity Address, Address Line One |
540 Gaither Road, Suite 400
|
| Entity Address, City or Town |
Rockville
|
| Entity Address, State or Province |
MD
|
| Entity Address, Postal Zip Code |
20850
|
| City Area Code |
410
|
| Local Phone Number |
522-8707
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.001 Par Value
|
| Trading Symbol |
AVTX
|
| Entity Emerging Growth Company |
false
|
| Entity Central Index Key |
0001534120
|
| Amendment Flag |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
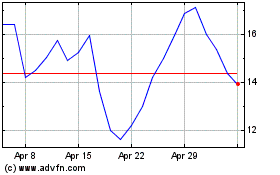
Avalo Therapeutics (NASDAQ:AVTX)
Historical Stock Chart
From Apr 2024 to May 2024
Avalo Therapeutics (NASDAQ:AVTX)
Historical Stock Chart
From May 2023 to May 2024