Form
10-K
x
ANNUAL REPORT PURSUANT TO SECTION 13
OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For
the Fiscal Year Ended July 31, 2009
¨
TRANSITION REPORT PURSUANT TO SECTION
13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For
the Transition Period from _____ to _____
Commission
File Number 333-130295
WELLSTAR
INTERNATIONAL, INC.
(Exact
name of small business issuer as specified in its charter)
|
Nevada
|
|
20-1834908
|
|
(State
or other jurisdiction of incorporation
or
organization)
|
|
(IRS
Employer Identification
No.)
|
|
6911
Pilliod Road
Holland,
Ohio
|
|
43528
|
|
419-865-0069
|
|
(Address
of principal executive office)
|
|
(Postal
Code)
|
|
(Issuer's
telephone
number)
|
Securities
registered under Section 12(b) of the Exchange Act: None
Securities
registered under Section 12(g) of the Exchange Act: None
Indicate
by check mark if the registrant is a wll-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes
¨
No
x
Indicate
by check by mark if the registrant is not required to file reports pursuant to
Section 13 or 15(d) of the Exchange Act. Yes
¨
No
x
Indicate
by check mark whether the registrant (1) filed all reports required to be filed
by Section 13 or 15(d) of the Exchange Act during the past 12 months (or for
such shorter period that the registrant was required to file such reports), and
(2) has been subject to such filing requirements for the past 90 days. Yes
x
No
¨
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files). Yes
x
No
¨
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not
be contained, to the best of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in Part III of this Form 10-K
or any amendment to this Form 10-K.
¨
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting company. See
the definitions of “large accelerated filer,” “accelerated filer” and “smaller
reporting company” in Rule 12b-2 of the Exchange Act.
|
Large
accelerated filer
¨
|
Accelerated
filer
¨
|
|
|
|
|
Non-accelerated
filer (Do not check if a smaller reporting company)
¨
Smaller
reporting company
x
|
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act). Yes
¨
No
x
State
issuer's revenues for its most recent fiscal year: $0.
The
aggregate market value of the voting stock held by non-affiliates as of November
12, 2009 was $1,434,665.
Number of
outstanding shares of the registrant's par value $0.001 common stock as of
November 12, 2009: 14,346,647,175
WELLSTAR
INTERNATIONAL, INC.
FORM
10-K
For
the Fiscal Year Ended July 31, 2009
|
|
|
Page
|
|
|
|
|
|
Part
I
|
|
2
|
|
|
|
|
|
Item
1. Description of Business.
|
|
2
|
|
|
|
|
|
Item
1A. Risk Factors
|
|
11
|
|
|
|
|
|
Item
1B. Unresolved Staff Comments
|
|
11
|
|
|
|
|
|
Item
2. Description of Property.
|
|
11
|
|
|
|
|
|
Item
3. Legal Proceedings.
|
|
11
|
|
|
|
|
|
Item
4. Submission of Matters to a Vote of Security Holders.
|
|
11
|
|
|
|
|
|
Part
II
|
|
12
|
|
|
|
|
|
Item
5. Market for Common Equity and Related Stockholder
Matters.
|
|
12
|
|
|
|
|
|
Item
6. Selected Financial Data
|
|
14
|
|
|
|
|
|
Item
7. Management's Discussion and Analysis or Plan of
Operation.
|
|
14
|
|
|
|
|
|
Item
7A. Quantitative and Qualitative Disclosures about Market
Risk
|
|
21
|
|
|
|
|
|
Item
8. Financial Statements and Supplementary Data
|
|
21
|
|
|
|
|
|
Item
9. Changes In and Disagreements with Accountants on Accounting and
Financial Disclosure.
|
|
21
|
|
|
|
|
|
Item
9A. Controls and Procedures.
|
|
22
|
|
|
|
|
|
Item
9A(T). Controls and Procedures.
|
|
22
|
|
|
|
|
|
Item
9B. Other Information.
|
|
22
|
|
|
|
|
|
Part
III
|
|
Page
|
|
|
|
|
|
Item
10. Directors, Executive Officers and Corporate
Governance.
|
|
23
|
|
|
|
|
|
Item
11. Executive Compensation.
|
|
24
|
|
|
|
|
|
Item
12. Security Ownership of Certain Beneficial Owners and Management and
Related Stockholder Matters
|
|
26
|
|
|
|
|
|
Item
13. Certain Relationships and Related Transactions.
|
|
27
|
|
|
|
|
|
Item
14. Principal Accountant Fees and Services.
|
|
27
|
|
|
|
|
|
Item
15 Exhibits, Financial Statement Schedules
|
|
28
|
|
|
|
|
|
Signatures.
|
|
31
|
|
|
|
|
|
Financial
Statements
|
|
F-1
|
PART I
FORWARD-LOOKING
INFORMATION
This
Annual Report on Form 10-K (including the section regarding Management's
Discussion and Analysis of Financial Condition and Results of Operations)
contains forward-looking statements regarding our business, financial condition,
results of operations and prospects. Words such as "expects," "anticipates,"
"intends," "plans," "believes," "seeks," "estimates" and similar expressions or
variations of such words are intended to identify forward-looking statements,
but are not deemed to represent an all-inclusive means of identifying
forward-looking statements as denoted in this Annual Report on Form 10-K.
Additionally, statements concerning future matters are forward-looking
statements.
Although
forward-looking statements in this Annual Report on Form 10-K reflect the good
faith judgment of our management, such statements can only be based on facts and
factors currently known by us. Consequently, forward-looking statements are
inherently subject to risks and uncertainties and actual results and outcomes
may differ materially from the results and outcomes discussed in or anticipated
by the forward-looking statements. Factors that could cause or contribute to
such differences in results and outcomes include, without limitation, those
specifically addressed under the heading "Risks Factors” that may be included in
our reports from time to time, as well as those discussed elsewhere in this
Annual Report on Form 10-K. Readers are urged not to place undue reliance on
these forward-looking statements, which speak only as of the date of this Annual
Report on Form 10-K. We file reports with the Securities and Exchange Commission
("SEC"). We make available on our Web site under "Investor Relations/SEC
Filings," free of charge, our annual reports on Form 10-K, quarterly reports on
Form 10-QSB, current reports on Form 8-K and amendments to those reports as soon
as reasonably practicable after we electronically file such materials with or
furnish them to the SEC. You can also read and copy any materials we file with
the SEC at the SEC's Public Reference Room at 100 F Street, N.E., Washington, DC
20549. You can obtain additional information about the operation of the Public
Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC
maintains an Internet site (www.sec.gov) that contains reports, proxy and
information statements, and other information regarding issuers that file
electronically with the SEC, including us.
We
undertake no obligation to revise or update any forward-looking statements in
order to reflect any event or circumstance that may arise after the date of this
Annual Report on Form 10-K. Readers are urged to carefully review and consider
the various disclosures made throughout the entirety of this Annual Report,
which attempt to advise interested parties of the risks and factors that may
affect our business, financial condition, results of operations and
prospects.
|
ITEM 1.
|
DESCRIPTION
OF BUSINESS
|
Corporate
History
Wellstar
International, Inc. ("Wellstar" or the "Company") was formed in the State of
Nevada on December 5, 1997. Wellstar was a development stage company with no
operating activities. On July 12, 2005, Wellstar entered into a share exchange
agreement with Trillennium Medical Imaging, Inc. ("Trillennium" or "TMI"), a
development stage company formed in June of 2005. As a result of the share
exchange agreement, Trillennium became a subsidiary of Wellstar.
Wellstar
International, Inc., through its Trillennium subsidiary, is dedicated to
developing and licensing the use of advanced thermal imaging technology (TMI
SYSTEM) in the consumer health care markets throughout the World.
Introduction
Trillennium
Medical Imaging, Inc. (TMI) is dedicated to placing cameras for a monthly charge
in hospitals, clinical and long care facilities throughout the United States.
The company’s current competitive advantage is derived from the state of the art
technology of the camera and software it is utilizing, combined with an intimate
knowledge and relationships in the markets. It plans to grow in these markets by
expanding its imaging capabilities by the constant acquisitions of new imaging
technologies.
The TMI
Infrared Technology and software is approved by the FDA as an Adjunctive
Diagnostic screening procedure for early breast cancer detection, differential
diagnosis of pain dysfunctions, (such as Reflex Sympathetic Dystrophy,
Neuromuscular Skeletal Syndromes and Neurological disorders), detection of
pressure ulcers, deep tissue injuries, and bed sores, as well as orthopedic
applications. This screening modality provides a differential diagnosis to
justify additional screening procedures to ensure successful patient outcome
assessment. Thermal Imaging is a low cost, non-contact, non-radioactive
diagnostic screening procedure designed for clinical evaluation. In addition
thermal imaging provides an ability to track the progress of therapies being
utilized in a low cost, non-invasive manner.
The TMI
Infrared Technology fills a need that currently exists in diagnostic medicine
today. Thermal imaging provides information that is specific to the
physiological and functional activity in the body. This information becomes
invaluable in that conventional diagnostic screening utilizes tests such as
X-Ray, MRI and CT Scans. All aspects of these tests are concerned with the
anatomical and structural problems of the body. Many problems arise from not
combining both diagnostic modalities of structure and physiology, in that people
are missed diagnosed and considered healthy and well when the physiological
information provides an earlier outcome or risk assessment of future health
predictors than the structural modalities.
Major
markets such as University Research Centers, hospitals, multidisciplinary
physician practices, pain centers, long term care facilities, home health care,
and rehabilitation centers support the need for this type of screening
modality.
In
summary, Trillennium Medical Imaging has created an innovative new business
model that gives it early market advantage.
History
and Future of Medical Infrared Applications
The
detection and estimation or measurement of heat released by the body has been a
cornerstone of medicine since its beginning. The earliest physicians knew the
presence of excessive heat signaled illness. For centuries the measurement of
heat, either present in or being released by the body, required contact.
Temperature was physically felt and much later was measured with various devices
such as the thermometer.
In the
1800’s Herschel demonstrated the presence of heat energy as an invisible wave,
much like light. Heat could be reflected and refracted under the right
conditions. Understanding of this heat energy wave would require years of
investigation. Much later, as all with all scientific advances, these studies
would provide more finite measurements of heat energy.
In the
1940’s, the electronic detector of infrared energy was developed for use in
military applications. Night vision scopes were the first applications. The
science of infrared detection owes its current development and sophistication to
demands from military and industrial applications.
Medical
use of infrared detection lagged behind military application due to government
classification of the infrared detectors and technologies. Only when scientific
advances are released from such government restrictions are they open to general
use and applications. Once available, the scientific/medical application of the
heat detector was made simultaneously by the British, the Canadians and
Americans. These scientists and physicians used thermal imaging as a measurement
of human physiology; the most common applications being the evaluation of
cancerous tumors. The level of heat emitted was correlated to the presence and
type of disease, as well as a prognostic indicator. Many tumors were hot, and
the hotter the tumor the worse the outcome.
These
first infrared applications were both costly and cumbersome, therefore the
infrared detection units were found mostly in research. Like all early
electronics the infrared detection systems were physically large, costly to
produce and challenging to operate. These restrictions meant exposure to general
medicine use was limited.
Today,
through the advances in all levels of science, and driven by military funding,
infrared detection devices are state-of-the art electronics. Like all electronic
components, the infrared detector has come down in cost, size and has
significantly improved in general applicability.
The
continued refinement of infrared systems, both in image acquisition and data
collection, has brought the use of infrared energy analysis to a new level.
Together with a better understanding of human and animal physiological
thermoregulatory mechanisms, the use of infrared detection in health and illness
provides a new mechanism for early disease detection.
Trillennium
Medical Imaging recognized the medical need for cost-effective, non-contact
physiological monitoring – and embraced the use of infrared imaging. Trillennium
is dedicated to the advancement of infrared detection in medicine. Trillennium
has forged relationships with well-known manufacturers of infrared detection
systems, recognizable entities in multiple medical specialties, and highly
skilled computer analysts – and together are developing state-of the art
infrared imaging systems, as well as investigating new uses/applications in
medicine.
The focus
of medicine and health care today means technology must be “faster, better, and
more cost-effective.” Trillennium Medical Imaging and its partners are working
to make infrared detection systems the easiest to use, applicable in the
every-day clinical setting and cost-effective in its application.
The use
of infrared analysis has historically been limited in clinical application. With
improved science comes improve equipment development and with computerization –
the analytical evaluation of infrared information is moving to new levels.
Today’s computerization of infrared systems is overcoming the limitations of the
past applications. Trillennium Medical Imaging systems incorporate, not only
objective temperature data analysis, but TMI has also taken data collection to a
new level. Today, medical device development and use is based on evidence of
efficacy. Application data collected from all TMI systems is compiled into a
single database that will continue to provide the statistical analysis needed to
demonstrate the efficacy of this technology in all current and future
applications. TMI believes that it is currently the only company to incorporate
this data collection component into their systems.
The first
records of physical observation demonstrate that physiological temperature
variations have signaled the presence of disease. Today the importance of this
vital physical parameter is of no less significance. Together, scientific
advancements and technological improvements will demonstrate that temperature
shifts can signal the earliest stages of physiological change. Through
non-contact temperature analysis, Trillennium Medical Imaging and partners will
continue to demonstrate the efficacy of infrared analysis in disease detection
and to drive the use of infrared analysis in new medical
applications.
The
Future of Disease Detection
Trillennium
Medical Imaging sees the future of infrared imaging moving to critical positions
in early phase disease detection and monitoring. Although currently not a
diagnostic modality, infrared analysis still provides key information in health
monitoring. The development of objective data analysis and proof of efficacy
will catapult infrared detection to key positions and prominence. Trillennium’s
completed research project conducted at Duke University for early detection of
tissue breakdown, provides the initial data demonstrating the TMI Systems
ability to detect damage prior to visible evidence. This capability – in a
non-contact device – will save patients from potential disabling conditions, as
well as generate a sizeable cost-savings in health care.
Trillennium
believes translation of the ‘early tissue damage detection’ application to other
previously untried medical areas- such as early detection of infection – will
reduce the associated costs and potential spread of treatment-resistant super
bugs. Super drugs have created super bugs and today the spread of
treatment-resistant disease is a major concern. Both community spread and
in-hospital presence of these organisms is a detection nightmare. In spite of
improved hygiene – transmission and containment of various diseases is still of
critical importance.
Cost-effective,
non-contact detection of circulatory compromise in surgical applications will
improve the success of many tissue transplants, reducing the need for additional
surgeries. And non-contact evaluation of tissue changes may help categorize
potentially malignant conditions demanding greater intervention from those that
are currently benign. However, these arenas are all in the invention phase, and
as all research, requires large capital investments to translate the research
findings to clinical application. Trillennium believes these areas hold huge
potential in both dollars spent in health care delivery as well as corporate
return-on-investment and strives to keep these investigations
on-going.
Markets
Skin
Assessment
The early
visualization of tissue damage that can lead to development of pressure ulcers
and or deep tissue injury is paramount to the reduction of open sores and the
deadly complications that accompany them. Currently no method of detection is
used that can identify tissue breakdown prior to the visible and palpable
changes that take place.
Trillennium
Medical Imaging began investigation of this health care issue shortly after the
company was formed. The ability of infrared to detect blood flow changes is well
documented and the key benefit of the technology. Association of blood flow
changes to both pressure ulcers and deep tissue injury has been documented since
the 1970’s when early imaging units were used to image suspicious damage. One of
the original studies of pressure sores by thermal detection was conducted by
Barton and Barton in 1973. Studying existing ulcers they determined the
temperature variations present with their classification of ‘indolent’ and
normal pressure sores. The category of indolent was associated with a longer
healing time (approximately four months) and a temperature difference of 1°C or
less. A classification of normal demonstrated a higher temperature differential
of approximately 2.5ºC and healed more quickly than indolent sores and the
patients generally displayed a better outcome. This ‘normal’ classification of
ulcer was generally attributed to the ‘otherwise healthy person’ whose pressure
sore was either post operative from extended times in one position or from
incorrect use of a prosthetic device. This wound usually resolved in six weeks.
This thermal evaluation has been substantiated by Hansen et al in a 1996
study.
The size,
cost and availability of infrared was a limiting factor in its use for many
years. The most cost effective detection was contact thermography, and the
question was if the contact alone was increasing the possible artifact of
damage. Electronic imaging provided the benefit of non-contact evaluation, but
thermal artifact induced in uncontrolled environments was still a
question.
Trillennium
Medical Imaging began the process of incorporating computerized analysis of
images to eliminate the subjective influence of previous infrared analysis.
Changes introduced in the analytical phase reduced the influence of
environmental shifts. This proprietary approach has been evaluated through a
completed study at Duke University and the data / results will be released and
published in the very near future.
Market
Opportunities
Functional
or physiological evaluation conducted with infrared imaging has applications in
many areas of medicine. Initially, Trillennium Medical Imaging focused on three
key markets with our current imaging system: Breast Health Monitoring, Pain
Evaluation; and Sports Medicine applications. However recent developments
indicate significant economic gains can be realized with a revised
plan.
Trillennium
Medical has begun investigating the use of infrared detection in early stage
pressure ulcer development. Trillennium is proposing a unique approach for early
detection of tissue changes that will significantly impact the wound care
market. TMI is in the initial phase for design and application of a new imaging
device that incorporates software and data collection that is separate and apart
from its current clinical markets.
While
Trillennium Medical Imaging will continue to prove the viability of IR through
its ongoing trials in existing markets (outlined under existing market profile),
it is evident that a method of early detection of tissue changes related to
pressure ulcers is not currently available.
Pressure
ulcers have always been an area of concern for healthcare providers due to the
degradation of the patient’s condition and the increased care required when
pressure ulcers occur. Pressure ulcers are responsible for over 65,000 deaths
per year in the United States alone. Aside from the human toll, the costs
involved in treating this problem are enormous and escalating at an alarming
rate annually. Taking into consideration all direct, indirect and peripheral
costs, it is conservatively estimated that currently over $25 billion is spent
annually on wound care in the United States (Columbia Surgery, Department of
Surgery, H. Brem, M.D., 2006). The urgency of this situation is not lost on
those in the medical field, associated industries and the government as
well.
Each year
the government spends on average over $3 billion on wound treatment and care for
pressure sores and bedsores. Insurance companies pay out millions annually for
this same treatment, directly affecting premiums to increase annually for
healthcare facilities. Inflationary effects add to this escalation.
New
regulation recently imposed by the Center for Medicare and Medicaid will reshape
the approach by all health care facilities and practitioners. In October 2008,
reimbursement for the care of individuals incurring pressure ulcers during
hospitalization (secondary diagnosis) will no longer be reimbursed. This edict
from the government has been followed by third party payers. This loss of
revenue can cripple many health care facilities if a procedure or validated
system is not put in place.
Additionally,
the potential of fines incurred for the occurrence of pressure ulcers in the
long term care facilities will drive these markets with burden of
proof.
Trillennium
Medical Imaging will be ideally positioned to provide a cost-effective,
non-contact method to identify the presence of early or existing tissue damage.
The next step for TMI is to implement the system within the marketplace with a
Beta Test. TMI believes that the Beta Test will begin the early part of 2010 as
we are currently in discussions.
Breast Health
Monitoring
Current
Testing/Screening Methods
The
medically accepted tool (gold standard) for breast cancer screening is x-ray
mammography. Mammography detects structural changes present in the breast
tissue, indicating either the presence of dead cell structures (calcifications)
or a shift in tissue density. Mammography was established as the test of choice
following the Breast Cancer Detection Demonstration Project (BCDDP) study and as
a result of the Health Insurance Plan (HIP) study of 1974.
According
to the National Cancer Institute Fact Sheet on Mammography, “Several large
studies conducted around the world show that breast cancer screening with
mammograms reduces the number of deaths from breast cancer for women ages 40 to
69, especially those over age 50. Studies conducted to date have not shown a
benefit from regular screening mammograms, or from a baseline screening
mammogram (a mammogram used for comparison), in women under age 40.” Regardless,
it is the only medically accepted screening test to-date.
With the
evolution in technology, proposals have been made to implement other modalities
in the screening process. Due to associated cost, and or sensitivity and
specificity of the exams (Ultrasound/MRI/PET) recommendations for screening have
not changed. These tests are used as ‘adjunctive’ or in addition to mammography
following a positive or questionable test.
Limitations
of Current Methods
Contact,
compression, ionizing radiation, limited or no options for screening other than
mammography (physician restricted access) and cost are some of the primary
reasons women list for limited or non-participation in breast health
evaluation.
Current
statistics demonstrating the number of women who participate in mammography are
not readily available. 2002 studies estimated approx 60% of women over the age
of 40 participated in mammography within the year. Studies show that for the
uninsured it (screening mammography) is a low priority procedure, because few
programs exist to assist in the payment for or toward mammography for women less
than 50 years of age as are for those who are older (Medicare).
“Women
over the ages of 50 and 60 are not getting mammograms as frequently as women in
their 40s, yet mammograms are more effective in reducing deaths from breast
cancer as women age.”
Women
reject or delay mammography for many reasons. Fear appears to be the primary
factor. Fear of discomfort, fear of radiation and fear of finding cancer are
only a few reasons that women site.
Many
women falsely believe that if there is no known breast cancer within their
family that there is no reason to be concerned. Few women know that
approximately 75% of breast cancers found occur in women who have no known
history.
Studies
indicate that 40% or more of the population eligible for screening mammography
do not participate in testing. Reasons listed are economic, cultural and
perception of increased harm (radiation / compression) from the exam – whether
real or imagined. These factors contribute to late-stage
Importance
of Infrared Imaging for Breast Cancer
Management
defines risk as the likelihood that harm will arise coupled with consequence.
Medical assessment of ‘risk’ for developing breast cancer is linked with
time/cost and effort to arrive at the benefit of risk assessment. Risk/benefit
studies determine that screening for breast cancer should begin at 40 years of
age. The cost of screening the female population younger than 40 years of age
provides no reduction in life-years saved compared to costs
incurred.
Although
mammography screening is an effective tool for detecting the presence of breast
cancer, it has inherent and well described limitations. In addition,
mathematical models such as the Gail Model have been developed to predict
breast cancer
risk
, but such models are
better at assessing population-based risk rather than individual
risk.
Monitoring
individuals with infrared imaging provides for improved individual assessment to
determine the ‘at-risk’ person as indicated by physiology (versus mathematical),
and therefore in a screening scenario, the ‘at-risk’ population.
Infrared
imaging is a non-contact, non-invasive, non-ionizing imaging tool and poses no
physical risk. It displays information regarding breast blood flow. Temperature
assessment of breast tissue provides for a profile as it relates to homeostasis
within the breast tissue. Any alterations are attributed to imbalance. The
presence of structural changes or tissue is not visible on the infrared image.
But neither is the vascular information displayed on the mammogram. Screening
the breast with infrared provides the individual and practitioner with
information not currently available as a screening modality. Other functional
imaging is prohibited by availability and cost. (fMRI/ PET/f CT). Additionally
other functional modalities are most often incorporated into structural exams
such as computed tomography and MRI.
Infrared
study parameters have been identified as consistent repeatable and independent
markers that identify those patients at risk for breast disease. Ease of
implementation (non-ionizing), low comparative cost and appeal to the general
population (non-contact) make it an ideal test for screening in the younger
patient (not a candidate for mammography), the woman with dense breasts or
implants, those who have surgically altered breasts that add to mammography
distortion (biopsies, lumpectomies, mastectomies) and those that refuse to
participate in screening mammography.
Market
Opportunities
Breast
Health Monitoring/Screening
Today the
U.S population is roughly 300 million (projection based on 2000 Census Data) and
of this 150 million individuals are female. According to the Census Bureau,
currently the largest segments of females are 40-44 (11.35M), 35-39 (11.34M) and
45-49 (10.27M) years of age respectively. These are closely followed by the
30-34 (10.21M) and the 10-14 (10M) years of age.
These
numbers become significant when we consider the disease of breast cancer. It is
the second leading cause of cancer deaths in the female population. 1 in 8 women
will be diagnosed with cancer of the breast during their lifetime.
The
probability of breast cancer occurrence increases with age. Fifty percent of all
breast cancers will occur in women under 61 — the median age of occurrence. But
roughly 1/3 of all breast cancers will occur in women under the age of 50 (20 to
50 years of age.) In the total population of breast cancers the largest
percentage are found in women over 50 years of age. The average projected growth
rate (disease development prior to detection) for breast cancer is approximately
8 to 10 years. For this reason, mammography is recommended as a screening
procedure beginning at 40 years of age.
U.S
Government statistics in 2005, show an estimated 2 million women diagnosed with
or living with breast cancer. 2006 estimates projected 212,920 new cases of
invasive breast cancer diagnosed, along with 61,980 new cases of non-invasive
breast cancer. The current medical paradigm with breast imaging is demonstrated
in the inability of mammography to reduce the mortality statistics of those
women found with invasive breast lesions. Structural studies, and mammography in
particular, are detecting the presence of tumors and tissue changes, but missing
the functional or metabolic activity presented by the disease. Infrared has
continued to demonstrate underlying metabolic changes present in tissue years in
advance of mammography. Slow growing, metabolically inactive cancers such as
those with low IR signals can easily be detected with mammography. The issue of
early detection is key for the women with dense breasts, especially the younger
woman who would be missed by never participating in mammography until a palpable
lesion is found.
Sports
Medicine
Sports
medicine or sport medicine is an interdisciplinary subspecialty of medicine
which deals with the treatment and preventive care of athletes, both amateur and
professional. The team includes specialty physicians and surgeons, athletic
trainers, physical therapists, coaches, other personnel, and, of course, the
athlete.
There has
been a tendency for many to assume that sport-related problems are by default
musculoskeletal and that sports medicine is an orthopedic specialty. It is
commonly understood that there is much more to sports medicine than just
musculoskeletal diagnosis and treatment. Illness or injury in sport can be
caused by many factors – from environmental to physiological and psychological.
Consequently, sports medicine
can encompass an array of specialties, including cardiology, pulmonology,
orthopedic surgery, exercise physiology, biomechanics, and
traumatology.
The risk
of injury in athletic practice will never be entirely eliminated, but
modifications in training techniques, equipment, sports venues and rules, based
on outcomes of meaningful research have shown that it can be lowered. For these
reasons sports medicine will make its most significant future contributions in
the area of prevention.
Market
Opportunities
When all
the inclusive markets are considered, the total revenue projections for the
sports medicine market are vast but illusive. Costs are measured in billions of
dollars when both treatment cost and indirect costs of loss (wage and
productivity) are considered.
Differential
diagnosis related to sports injuries is complicated by the fact that soft tissue
injuries are some of the most common and clinically challenging musculoskeletal
disorders in patients presenting for treatment. Therefore, establishing
clear-cut diagnostic and therapeutic objectives for these injuries is important.
Current imaging methods of evaluation are structural. Flat plane x-ray, computed
tomography (CT), ultrasound, and MRI are the most common examinations. This
leaves the assessment of soft tissue injury to visual, palpable and indirect
visualization via the structural image.
Complication
of evaluation (structure vs. tissue) results in inappropriate or ill-timed care.
Oversight of the magnitude of soft tissue injuries may result in a failure to
expeditiously consider vascular injury or compartment syndrome and its resultant
complications, including loss of a limb. Misdiagnosis or mismanagement of damage
may lead to chronic problems with subsequent development of degenerative joint
disease and/or loss of function, including but not limited to an inability to
bear weight or ambulate.
Like all
areas of pain, the number of those afflicted by joint and connective tissue
disorders seems to be increasing rather than under control.
Statistics
taken from the U.S. government website on arthritis provides the following
numbers related to occurrence.An estimated 46 million adults in the United
States reported being told by a doctor that they have some form of arthritis,
rheumatoid arthritis, gout, lupus, or fibromyalgia. By 2030, an estimated 67
million of Americans aged 18 years or older are projected to have
doctor-diagnosed arthritis. Arthritis & Rheumatism 2006;54(1):226-229 [Data
Source: 2003 NHIS]
Importance
of TMI Infrared Monitoring System
Until
recently, sports medicine has been a little-explored market for infrared
technology as an adjunctive to diagnostics. Infrared imaging provides an
indirect measurement of skin blood flow and a temperature measurement of the
physiological response controlled by the body’s autonomic nervous system. This
information has broad application in the world of sports medicine. Infrared
evaluation can provide a new dimension to diagnostic capabilities in an
adjunctive capacity (physiology vs. anatomy). Some of these applications are:
providing a monitor for hyperthermic and hypothermic responses associated with
nerve irritation, acute injuries, swelling, inflammation, infection, and
atrophy. Provide physicians and trainers with a visual and measurable reference
for differential diagnosis, and a visual and objective reference relating to
treatment efficacy.
TMI
Solution in Sports Medicine Applications
TMI is
currently involved in a pilot study with Duke University Sports Medicine
evaluating infrared technology for spot-checking of core body temperatures of
athletes in the field. The preliminary study of this non-invasive technology has
demonstrated temperature readings that are directly correlated with the core
body temperature in a small population. A more extensive study is being planned.
The value will be an easy to use, non-invasive tool for monitoring heat
exhaustion in athletics that can be easily used in the field. All levels and
types of sports organizations (schools, teams and professionals) could all
benefit from using this technology. TMI is working to develop a portable,
cost-effective, handheld solution that will fit the need of this extensive
market.
TMI is
also exploring the use of this same assessment tool for immediate trauma
evaluation on the field or in emergency rooms for monitoring deep tissue injury
and assist with fast evaluation of potential compartment syndrome.
TMI
Market Approach
With significant relationships in the
medical industry, our initial marketing approach and main thrust will be to
hospitals and long term care facilities for the early detection of pressure
ulcers. The study at Duke Medical Center has been completed and was successful.
The data and the publishing of the data will be released in the near
future.
Rheumatology
For over
forty years infrared measurement has been used in the evaluation of
rheumatological disease. Initially identified and analyzed in the 1970’s by
Abernathy, Ring, and many others, joint and connective tissue disorders were an
undisputed area in which infrared detection provided a non-ionizing and
non-contact method of detection.
Infrared
evaluation provides both an indication of abnormal blood flow in the area of
pain, and as a monitoring device for the efficacy of various therapies.
Additionally infrared imaging is an ideal tool for evaluation of this peripheral
blood flow and vasospastic disorders of peripheral digital vessels is often an
indication of connective tissue disorders.
Research and
Development
Equipment
Development
We
continue to interface with equipment manufacturers and medical device research
and development firms to seek out state of the art thermal imaging
technology.
In
addition, we have created a Data Collection division. This will enable us in the
future to keep data and compare results of images taken from all of our cameras.
This will then lead to definitive conclusions for the medical community. Through
a HIPPA-compliant database we plan to gather a statistically-verifiable data set
from our patient population from all of our participating facilities. This
information will be compared to currently known statistics and provide
additional verification regarding the efficacy of infrared imaging in a variety
of applications. The medical community requires "evidence" to back the use or
implementation of all technologies. TMI expects that its thermal imaging
operations will yield significant data. This will supply the data needed to
support evidenced-based practice. This data can then be compared to the patient
outcome-information held by each practitioner.
Future
Market Research/Medical
Grafts
and Flaps
The use
of skin grafts and local flaps can help solve some difficult wound closure
problems. Postoperative flap failure is a devastating complication that surgeons
occasionally encounter when using plastic surgery techniques.
Intraoperative
monitoring of flap perfusion has shown to prevent flap failure. Infrared imaging
can be used to evaluate and monitor reperfusion, and is a fast, non-contact,
reliable method.
Infrared
can be used preoperatively to trace perforators in the skin to be excised for
reconstruction, as well as postoperatively to monitor the rewarming following
attachment.
Amputation
Infrared
has proven to be valuable in the area of amputation of tissue as well.
Evaluation of digits or limbs of persons undergoing amputation has improved the
success by proving or disproving the viability of tissue present. In instances
of lower limb amputation due to peripheral vascular disease, amputation can
often be preformed below the knee, improving the rehabilitation and mobility of
the patient and therefore quality of life issues. Selection of amputation level
made on the basis of laboratory criteria using skin blood flow and infrared
thermography data proves to be more successful.
Diabetic
Neuropathy
Diabetic
peripheral neuropathy (DPN) is a condition secondary to the disease and can
result in ulceration. Of the diabetic population 0.6 % eventually require lower
limb amputation. However of those with peripheral neuropathy roughly 40% require
amputation of a toe, 12% a foot, and 47% The total annual cost of DPN and its
complications in the U.S. was estimated to be between $4.6 and $13.7 billion. Up
to 27% of the direct medical cost of diabetes
Dermatology
“The skin
is the largest organ in the body, and it is therefore not surprising that cancer
of the skin is the most common of all cancers. Basal cell carcinoma and squamous
cell carcinoma make up the vast majority of cases. Melanoma is the least common
but the most deadly skin cancer, accounting for only about 4% of all cases but
79% of skin cancer deaths. For 2002, the American Cancer Society estimates there
will be 53,600 new cases of melanoma in the United States and 7,400 deaths from
the disease. The United States has experienced a dramatic increase in the number
of melanoma cases over the past few decades. According to the American Cancer
Society, the incidence rate for melanoma (number of new cases of melanoma per
100,000 people each year) has more than doubled since 1973. The mortality rate
for melanoma (number of deaths per 100,000 people each year) has increased at a
much slower pace and has remained stable over the past 10 years. During this
same time period, there has been a significant rise in overall five-year
survival in patients with melanoma. This may be due to thinner depth of tumors
at time of diagnosis and improved surgical techniques to treat the disease.
“
The study
of skin and skin lesions is an area of infrared that has waxed and waned since
the early studies of thermography conducted in the 1980’s by the French.
Evaluation of the thermal flare presented by the increased vascularity present
in melanomas was thermally mapped prior to radiotherapy. Infrared mapping
provided information that demonstrated that the underlying vascular bed related
to the visible lesion was usually significantly larger than assumed, and if not
included in the area of radiation, may remain as a pathway of
metastasis.
With the
increase in melanoma occurrence, the use of infrared imaging in screening
evaluations could add to number of cases found in the early stages.
Additionally, the use to map the underlying vascularity could again aid in
mapping the underlying angiogenic blood supply to the tumor.
Pharmaceuticals/Vaccines
The use
of infrared detection for adverse reactions resulting from administration of
drugs and vaccines is an area of potential investigation.
Trillennium
Medical Imaging anticipates that investigation of adverse reactions with
infrared imaging may provide a non-contact, cost-effective way to monitor for
adverse reactions prior to their occurrence. Through is affiliation with Duke
University, TMI plans to participate in research projects that are first
isolating these drug-reaction potentials in mice. Participation in this and
other research projects will require capital investment to provide various lens
and other adaptations to the existing infrared imaging device, as well as the
possible development of other specific IR optical imaging devices that could
revolutionize this arena.
Trillennium,
through its advisors and affiliates will continue to investigate areas of
medical concern where infrared imaging will possibly benefit the
survival-outcome, the cost-effective delivery of non-invasive diagnostic and
adjunctive imaging, as well the return-on-investment from development of such
products.
FDA
Regulations
Changes
in Approved Devices - The FD&C Act requires device manufacturers to obtain a
new FDA 510(k) clearance when there is a substantial change or modification in
the intended use of a legally marketed device or a change or modification,
including product enhancements and, in some cases, manufacturing changes, to a
legally marketed device that could significantly affect its safety or
effectiveness. Supplements for approved PMA devices are required for device
changes, including some manufacturing changes that affect safety or
effectiveness. For devices marketed pursuant to 510(k) determinations of
substantial equivalence, the manufacturer must obtain FDA clearance of a new
510(k) notification prior to marketing the modified device. For devices marketed
with PMA, the manufacturer must obtain FDA approval of a supplement to the PMA
prior to marketing the modified device.
Good
Manufacturing Practices and Reporting. TMI does not manufacture the infrared
cameras used in its thermal imaging system. Nevertheless, the FD&C Act
requires device manufacturers to comply with Good Manufacturing Practices
regulations. The regulations require that medical device manufacturers comply
with various quality control requirements pertaining to design controls,
purchasing contracts, organization and personnel, including device and
manufacturing process design, buildings, environmental control, cleaning and
sanitation; equipment and calibration of equipment; medical device components;
manufacturing specifications and processes; reprocessing of devices; labeling
and packaging; in-process and finished device inspection and acceptance; device
failure investigations; and record keeping requirements including complaint
files and device tracking. We are in compliance with the above
requirements.
Current
Regulatory Status- The FDA found that the cameras utilized in the TMI thermal
imaging system are substantially equivalent to an existing legally marketed
device, thus permitting them to be marketed as an adjunct (supplemental)
screening/diagnostic device. The cameras utilized in our system first received
510(k) clearance in September 2001 and subsequently in March 2003. Together with
our software we, are marketing the TMI thermal imaging system as an adjunct
(supplemental) method for the diagnosis of breast cancer and other diseases
affecting the perfusion or reperfusion of blood in tissue or organs in the
foreign markets, and exclusively promoting the early detection of Pressure
Ulcers in the US market.
Permits
and Inspections
The
manufacturer of the thermal imaging cameras utilized in the TMI thermal imaging
system is subject to compliance with Good Manufacturing Practices under the
FD&C Act, as described above. The manufacturer is subject to government
inspection and failure to comply with all applicable standards can result in
suspension or termination of its ability to manufacture cameras for use in our
system. In that event we would be compelled to seek thermal imaging cameras from
other manufacturers.
Users of
the TMI thermal imaging system are required to utilize protocols for accurate
thermal imaging such as the "Technical Protocols for High Resolution Infrared
Imaging" approved by the ACCII 5-15-99. Failure to comply with these
requirements can result in suspension or termination of the facilities'
authority to utilize our thermal imaging system. In that event we would
experience a disruption in revenue while we removed our equipment and sought to
install it in another location.
As a
Smaller Reporting Company, the Company is not required to include the disclosure
under this Item 1A. Risk Factors.
ITEM
1B. UNRESOLVED STAFF COMMENTS
As a
Smaller Reporting Company, the Company is not required to include the disclosure
under this Item 1B. Unresolved Staff Comments.
ITEM
2. DESCRIPTION OF PROPERTY.
We
maintain our principal office at 6911 Pilliod Road, Holland OH 43528. Our
telephone number at that office is (419) 865-0069 and our facsimile number is
(419) 867-0829. We currently occupy this facility on a month to month basis at a
no cost arrangement with the building owner. The facilities are in good
condition.
ITEM 3. LEGAL PROCEEDINGS.
From time
to time, we may become involved in various lawsuits and legal proceedings, which
arise in the ordinary course of business. However, litigation is subject to
inherent uncertainties, and an adverse result in these or other matters may
arise from time to time that may harm our business. We are currently not aware
of any such legal proceedings or claims that we believe will have, individually
or in the aggregate, a material adverse affect on our business, financial
condition or operating results.
None of
our directors, officers or affiliates are involved in a proceeding adverse to
our business or have a material interest adverse to our business.
ITEM
4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS.
None.
PART II
ITEM
5. MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS.
MARKET INFORMATION
Our
common stock is quoted on the OTC Bulletin Board under the symbol "WLSI".
For the
periods indicated, the following table sets forth the high and low bid prices
per share of common stock. These prices represent inter-dealer quotations
without retail markup, markdown, or commission and may not necessarily represent
actual transactions.
|
|
|
Fiscal
2008
|
|
|
Fiscal
2009
|
|
|
|
|
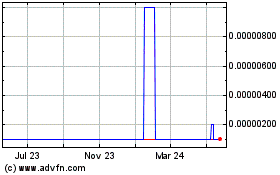
High
|
|
|
Low
|
|
|
High
|
|
|
Low
|
|
|
First
Quarter
|
|
$
|
0.02
|
|
|
$
|
0.004
|
|
|
$
|
0.004
|
|
|
$
|
0.0038
|
|
|
Second
Quarter
|
|
$
|
0.0016
|
|
|
$
|
0.001
|
|
|
$
|
0.0047
|
|
|
$
|
0.001
|
|
|
Third
Quarter
|
|
$
|
0.0009
|
|
|
$
|
0.0007
|
|
|
$
|
.002
|
|
|
$
|
.0005
|
|
|
Fourth
Quarter
|
|
$
|
0.022
|
|
|
$
|
0.016
|
|
|
$
|
.001
|
|
|
$
|
.0001
|
|
Holders
As of
November 12, 2009, there were approximately 14,346,647,175 shares of common
stock outstanding.
As of
November 12, 2009, there were approximately 99 active holders of our common
stock. The number of record holders was determined from the records of our
transfer agent and does not include beneficial owners of common stock whose
shares are held in the names of various security brokers, dealers, and
registered clearing agencies. The transfer agent of our common stock is Pacific
Stock Transfer.
Dividends
There are
no restrictions in our articles of incorporation or bylaws that prevent us from
declaring dividends. The Nevada Revised Statutes, however, do prohibit us from
declaring dividends where, after giving effect to the distribution of the
dividend:
|
·
|
we
would not be able to pay our debts as they become due in the usual course
of business; or
|
|
|
|
|
·
|
our
total assets would be less than the sum of our total liabilities plus the
amount that would be needed to satisfy the rights of shareholders who have
preferential rights superior to those receiving the
distribution.
|
We have
not declared any dividends, and we do not plan to declare any dividends in the
foreseeable future.
We have
never declared or paid any cash dividends on our common stock. We do not
anticipate paying any cash dividends to stockholders in the foreseeable future.
In addition, any future determination to pay cash dividends will be at the
discretion of the Board of Directors and will be dependent upon our financial
condition, results of operations, capital requirements, and such other factors
as the Board of Directors deem relevant.
Recent
Sales of Unregistered Securities
Convertible Debenture
Financing
On May
15, 2009, the Company entered into a Securities Purchase Agreement with AJW
Partners, LLC ("Partners"), AJW Partners II, LLC ("Partners II "), AJW Master
Fund, Ltd. ("Master"), AJW Master Fund II, Ltd. ("Master II") and New Millennium
Capital Partners, II, LLC ("Millennium" and collectively with Partners, Partners
II, Master and Maser II, the “Purchasers”) for the sale of 13% secured
convertible notes in an aggregate principal amount of up to $79,500 (the
"Notes"). The Purchasers closed on $22,000 in Notes on May 18,
2009.
The Notes
bear interest at the rate of 13% per annum. Interest is payable monthly, unless
the Company's common stock is greater than $0.045 per share for each trading day
of a month, in which event no interest is payable during such month. Any
interest not paid when due shall bear interest of 15% per annum from the date
due until the same is paid. The Notes mature three years from the date of
issuance, and are convertible into common stock, at the Purchasers' option, at
the lesser of (i) $0.12 or (ii) a 75% discount to the average of the three
lowest trading prices of the common stock during the 20 trading day period prior
to conversion. The Notes contain a call option whereby, if the Company's stock
price is below $0.045, the Company may prepay the outstanding principal amount
of the Notes, subject to the conditions set forth in the call option. The Notes
also contain a partial call option whereby, if the Company's stock price is
below $0.045, the Company may prepay a portion of the outstanding principal
amount of the Note, subject to the conditions set forth in the partial call
option.
The full
principal amount of Notes are due upon a default under the terms of the secured
convertible notes. In addition, the Company granted the Purchasers a security
interest in substantially all of the Company's assets and intellectual property.
The Company is required to file a registration statement with the Securities and
Exchange Commission upon demand, which will include the common stock underlying
the Notes.
The
conversion price of the Notes may be adjusted in certain circumstances such as
if the Company pays a stock dividend, subdivides or combines outstanding shares
of common stock into a greater or lesser number of shares, or takes such other
action as would otherwise result in dilution of the selling stockholder's
position.
The
Purchasers have agreed to restrict their ability to convert their Notes and
receive shares of common stock such that the number of shares of common stock
held by them in the aggregate and their affiliates after such conversion or
exercise does not exceed 4.99% of the then issued and outstanding shares of
common stock.
Series A Preferred
Stock
On May
20, 2009, the Company entered into a conversion agreement with John Antonio
(“Antonio”) and Kenneth McCoppen (“McCoppen”), both executive officers and
directors of the Company, pursuant to which the Company agreed to convert
$30,000 in outstanding wages owed to each McCoppen and Antonio into a total of
60,000 shares of Series A Preferred Stock.
The above
transactions were approved by the Board of Directors of the Company. The Series
A Preferred Stock does not pay dividends but each holder of Series A Preferred
Stock shall be entitled to 100 votes for each share of common stock that the
Series A Preferred Stock shall be convertible into. The Series A Preferred Stock
has a conversion price of $0.0014 (the “Conversion Price”) and a stated value of
$1.00 (the “Stated Value”). Each share of Series A Preferred Stock is
convertible, at the option of the holder, into such number of shares of common
stock of the Company as determined by dividing the Stated Value by the
Conversion Price. The Series A Preferred Stock has no liquidation
preference.
Series B Preferred
Stock
On
October 1, 2009, the Company entered into a conversion agreement with John
Antonio (“Antonio”) and Kenneth McCoppen (“McCoppen”), both executive officers
and directors of the Company, pursuant to which the Company agreed to convert
$50,000 in outstanding wages owed to each McCoppen and Antonio into a total of
100,000 shares of Series B Preferred Stock.
The above
transactions were approved by the Board of Directors of the Company. The Series
B Preferred Stock does not pay dividends but each holder of Series B Preferred
Stock shall be entitled to 100 votes for each share of common stock that the
Series B Preferred Stock shall be convertible into. The Series B Preferred Stock
has a conversion price of $0.001 (the “Conversion Price”) and a stated value of
$1.00 (the “Stated Value”). Each share of Series B Preferred Stock is
convertible, at the option of the holder, into such number of shares of common
stock of the Company as determined by dividing the Stated Value by the
Conversion Price. The Series B Preferred Stock has no liquidation
preference.
JMJ
Financing
On May
22, 2009, the Company issued a Convertible Promissory Note to JMJ Financial
(“JMJ”) in aggregate principal amounts of $575,000 (the “Initial JMJ Note”). In
consideration for Wellstar’s issuing of the Initial JMJ Note, JMJ issued
Wellstar a Secured and Collateralized Promissory Note in the principle amount of
$500,000 (the “Initial Wellstar Note”).
In
addition, on August 19, 2009 Wellstar issued a Convertible Promissory Note to
JMJ in aggregate principal amounts of $1,150,000 (the “Second JMJ Note” and
together with the Initial JMJ Note, the “JMJ Notes”). In consideration for
Wellstar’s issuing of the Second JMJ Note, JMJ issued Wellstar a Secured and
Collateralized Promissory Note in the principle amouns of $1,000,000 (the
“Second Wellstar Note” and together with the Initial Wellstar Note, the
“Wellstar Notes”).
The JMJ
Notes bear interest at 12%, mature three years from the date of issuance, and
are convertible into our common stock, at JMJ’s option, at a conversion price,
equal to 70% of the lowest trade for our common stock during the 20 trading days
prior to the conversion. Prior to the conversion of the JMJ Notes, JMJ must make
a payment to Wellstar reducing the amount owed to Wellstar under the Wellstar
Notes. As of October 7, 2009, the lowest trade for our common stock during the
20 trading days as reported on the Over-The-Counter Bulletin Board was $.0001
and, therefore, the conversion price for the JMJ Notes was $.00007. Based on
this conversion price, the JMJ Notes in the aggregate amount of $1,725,000,
excluding interest, are convertible into 24.6 billion shares of our common
stock.
JMJ has
agreed to restrict their ability to convert the JMJ Notes and receive shares of
common stock such that the number of shares of common stock held by them in the
aggregate and their affiliates after such conversion or exercise does not exceed
4.99% of the then issued and outstanding shares of common stock.
The
Wellstar Notes bear interest at the rate of 13.8% per annum and mature three
years from the date of issuance. No interest or principal payments are required
until the maturity date, but both principal and interest may be prepaid prior to
Maturity Date. The Wellstar Notes are secured by units of STIC AIM Liquidity
Portfolio Select Investment Select Investment Fund (the “JMJ Collateral”). On
each of the Wellstar Notes, JMJ has agreed to pay down the principal of the
Wellstar Notes commencing 210 days after the original issuance of the Wellstar
Notes, However, JMJ may adjust the payment schedule within its sole discretion.
In the event that JMJ defaults on the Wellstar Notes, Wellstar may take
possession of the JMJ Collateral.
* All of
the above offerings and sales were deemed to be exempt under Rule 506 of
Regulation D and/or Section 4(2) of the Securities Act of 1933, as amended. No
advertising or general solicitation was employed in offering the securities. The
offerings and sales were made to a limited number of persons, all of whom were
accredited investors, business associates of the Company or executive officers
of the Company, and transfer was restricted by the Company in accordance with
the requirements of the Securities Act of 1933. In addition to representations
by the above-referenced persons, we have made independent determinations that
all of the above-referenced persons were accredited or sophisticated investors,
and that they were capable of analyzing the merits and risks of their
investment, and that they understood the speculative nature of their investment.
Furthermore, all of the above-referenced persons were provided with access to
our Securities and Exchange Commission filings.
ITEM
6. SELECTED FINANCIAL DATA
As a
Smaller Reporting Company, the Company is not required to include the disclosure
under this Item 6. Selected Financial Data.
ITEM
7. MANAGEMENT’S DISCUSSION AND ANALYSIS OR PLAN OF OPERATION.
The
following information should be read in conjunction with the consolidated
financial statements and the notes thereto contained elsewhere in this report.
The Private Securities Litigation Reform Act of 1995 provides a "safe harbor"
for forward-looking statements. Information in this Item 6, "Management's
Discussion and Analysis or Plan of Operation," and elsewhere in this 10-K that
does not consist of historical facts, are "forward-looking statements."
Statements accompanied or qualified by, or containing words such as "may,"
"will," "should," "believes," "expects," "intends," "plans," "projects,"
"estimates," "predicts," "potential," "outlook," "forecast," "anticipates,"
"presume," and "assume" constitute forward-looking statements, and as such, are
not a guarantee of future performance. The statements involve factors, risks and
uncertainties including those discussed in the “Risk Factors” section contained
elsewhere in this report, the impact or occurrence of which can cause actual
results to differ materially from the expected results described in such
statements. Risks and uncertainties can include, among others, fluctuations in
general business cycles and changing economic conditions; changing product
demand and industry capacity; increased competition and pricing pressures;
advances in technology that can reduce the demand for the Company's products, as
well as other factors, many or all of which may be beyond the Company's control.
Consequently, investors should not place undue reliance on forward-looking
statements as predictive of future results. The Company disclaims any obligation
to update the forward-looking statements in this report.
Plan
of Operation and Financing Needs
We are
seeking financing in different amounts and up to 12 million dollars. While we
have signed definitive agreements with JMJ Financial for up to 1.2 million
dollars, there is no guarantee that this amount will be fully funded as per the
agreements and explained in the company’s last 8K filing. As the funding is
released to the company, we will be moving forward with implementing a Beta Test
in long term care facilities and possibly a hospital as well.
Once the
Beta Test is completed, the company will have demonstrated to the market that
the system works within the environment and the company has the ability to
execute the installations and operation of the systems. The company’s goal is to
have the Beta test start in January of 2010. This test will be run for
approximately 3 months. Upon conclusion of the test, we feel confident that we
should have multiple orders for the system and should be in a position to raise
any additional capital that would be needed to retire the company debt as well
as fund the complete roll out of our system.
If We Are
Unable to Obtain Additional Funding, Our Business Operations Will be Harmed. In
Addition, Section 4e of the October 2005 Securities Purchase Agreements Contains
Certain Restrictions and Limitations on Our Ability to Seek Additional
Financing. If We Do Obtain Additional Financing, Our Then Existing Shareholders
Will Suffer Substantial Dilution.
Results
of Operations
Year
Ended July 31, 2009 compared to Year Ended July 31, 2008 (all references are to
the Year Ended July 31)
Revenue:
We did not have revenue during the years ended July 31, 2009 and 2008.
Cost of
Sales and Gross Profit: There was no Cost of Sales for the years ended July 31,
2009 and 2008 as we did not generate revenue during these periods.
Operating,
Selling, General and Administrative Expenses: Operating, selling, general and
administrative expenses decreased by $203,031, or 9% in the 2009 fiscal year to
$2,163,673 from $2,366,704 in 2008. This decrease reflects increases in
stockholder relations expenses by $377,550. In addition, salaries decreased by
$157,272 from $892,772 to $735,500, professional fees decreased by $62,379 from
$319,319 to $256,940, research and development expense decreased by 259,200 from
$259,200 to none and travel decreased by $83,107 from $141,054 to $57,947for the
same period in 2008.
Loss from
Operations: Loss from operations for the 2009 fiscal year was $2,163,673, a
decrease of $203,031 or 9% from the loss from operations in 2008 of $2,366,704
as a result of the aforementioned decrease in operating, sales and
administrative expenses.
Other
Income and Expense: Total other expenses of $39,668,848 in the 2009 fiscal year
represent an increase of $36,322,465 from the expense of $3,346,383 in 2008 as a
result of a greater expense from derivative instrument expense for the period
related to a decrease in derivative instrument liabilities caused by lower stock
prices.
Net
Income: Net loss of $41,832,521 for the 2009 fiscal year was $36,119,434 greater
than the net loss of $5,713,087 for the same period in 2008 due to the greater
amount of derivative instrument expense.
Liquidity
and Capital Resources
It is
Managements opinion that the current financial position of the company is in
dire straits and the Company will need to obtain additional funding to continue
operations. The Company expects that it will be able to continue operating
through January 2010. If the Company does not obtain financing at this time, it
will be required to cease operations.
As of
July 31, 2009, we had a working capital deficit of approximately $30,631,304,
and cash of $29,564. We do not have the funds necessary to maintain our
operations for the coming fiscal year, and will need to raise additional
funding.
The
liquidity impact of our outstanding debt is as follows:
Our
secured convertible note with Andrew W. Thompson (the "Thompson Note"), in the
principal amount of $400,000, matured on April 11, 2006 and remains outstanding.
We are in default pursuant to the terms of the Thompson Note, although we have
not received a notice of default from Mr. Thompson, nor has Mr. Thompson
indicated to the Company that he intends to place the Company in default under
the loan agreement. Interest on the Thompson Note is at the rate of 8% plus the
prevailing margin rate charged to the lender, which is currently 7.625%. In
addition to the outstanding principal, we also owe accrued interest in the
amount of $233,364. The lender has the option of converting the loan into fully
registered common stock at a discount of 40% on the day of conversion, which is
the prepayment date or the due date, whichever occurs first. Additionally, the
lender also received warrants to purchase 1,000,000 shares of the company's
fully registered common stock at an exercise price of $0.50 per share. If the
lender converts, the Company will issue the appropriate number of shares and
will not be required to use cash to liquidate the debt. Additionally, the
Company will receive the cash proceeds in the amount of $500,000 if the lender
exercises the $0.50 warrants. On November 10, 2006, the Thompson Note was
amended to include a provision stipulating that the holder may not convert the
secured convertible note if such conversion or exercise would cause him to own
more than 9.99% of our outstanding common stock. However, this restriction does
not prevent the holder from converting a portion of the note and then converting
the rest of the note. In this way, the holder could sell more than this limit
while never holding more than this limit.
Our
unsecured demand note with Michael Sweeney (the "Sweeney Note"), in the
principal amount of $150,000, matured on August 1, 2006 and remains outstanding.
In addition to the outstanding principal, we also owe accrued interest in the
amount of $36,500. We are in default pursuant to the terms of the Sweeney Note
and we have not received a notice of default from Mr. Sweeney, nor has Mr.
Sweeney indicated to the Company that he intends to place the Company in default
under the note.
Our
unsecured demand note with Micro Health Systems (the "MHS Note"), dated December
21, 2005 in the principal amount of $200,000, with interest at 8% per annum, has
two maturity dates: at the 180th day and the 365th day following issuance. A
payment of $100,000.00 is due at each maturity date. We did not make the first
or second payment. There is an acceleration provision in the MHS Note
stipulating that the entire $200,000.00 was due upon non-payment of the first
$100,000. The interest rate then goes to the highest rate allowed by Florida
law. We received a notice of default from MHS on November 28, 2006 but no
further action has been taken. The MHS Note is secured by a pledge of 1.5
million shares of the Company's treasury stock.
To obtain
funding for our ongoing operations, we entered into a Securities Purchase
Agreement with four accredited investors - AJW Partners, LLC, AJW Qualified
Partners, LLC, AJW Offshore, Ltd. and New Millennium Partners II, LLC on October
31, 2005 for the sale of (i) $3,000,000 in secured convertible notes and (ii)
warrants to buy 5,000,000 shares of our common stock. The gross financing
proceeds were paid to the Company in three separate tranches of $1,000,000 each.
The first tranche of the financing, in the amount of $1,000,000, was received by
the Company upon closing. The second tranche was received on January 20, 2006.
The third tranche was received as follows:
$500,000
in July 2006 and $500,000 in August 2006.
The
secured convertible notes issued pursuant to our October 2005 through June 2008
Securities Purchase Agreements bear interest originally at 8% but increasing to
13% effective September 8, 2009, mature three years from the date of issuance,
and are convertible into our common stock, at the selling stockholders' option,
at the lower of (i) $0.12 or (ii) generally a 75% discount to the average of the
three lowest intraday trading prices for the common stock on a principal market
for the 20 trading days before but not including the conversion date. As of July
28, 2009, the average of the three lowest intraday trading prices for our common
stock during the preceding 20 trading days as reported on the Over-The-Counter
Bulletin Board was $ .00016 and, therefore, the conversion price for the secured
convertible notes was $ .00004. Based on this conversion price, the $5,219,855
outstanding principal amount of the secured convertible notes, excluding
interest, were convertible into approximately 130,496,375,000 shares of our
common stock. The stock purchase warrants have an exercise price of $0.0001 and
$0.50 per share. If the lender converts, the Company will issue the appropriate
number of shares and will not be required to use cash to liquidate the debt.
Additionally, the Company will receive cash proceeds in the amount of $3,055,000
if the lender exercises the warrants. If the lender converts, the Company will
issue the appropriate number of shares and will not be required to use the cash
to liquidate the debt.
The
registration statement we filed to register the shares underlying the
convertible notes and warrants was declared effective by the Securities &
Exchange Commission on August 4, 2006 (File No. 333-130295).
To obtain
additional funding for our ongoing operations, we entered into a loan agreement
with JMJ Financial a loan in the principal sum of $ 575,000, of which $ 75,000
is a loan acquisition cost. The note provides for a one time 12% interest charge
on the principal sum. The convertible note is convertible into our common stock,
at the selling stockholders' option, at 70% of the average of the three lowest
intraday trading prices for the common stock on a principal market for the 20
trading days before but not including the conversion date. As of July 31, 2009
the principal balance of the loan is $ 340,000.
On May 15, 2009, the Company entered
into a Securities Purchase Agreement with AJW Partners, LLC ("Partners"), AJW
Partners II, LLC ("Partners II "), AJW Master Fund, Ltd. ("Master"), AJW Master
Fund II, Ltd. ("Master II") and New Millennium Capital Partners, II, LLC
("Millennium" and collectively with Partners, Partners II, Master and Maser II,
the “Purchasers”) for the sale of 13% secured convertible notes in an aggregate
principal amount of up to $79,500 (the "Notes"). The Purchasers closed on
$22,000 in Notes on May 18, 2009.
The Notes
bear interest at the rate of 13% per annum. Interest is payable monthly, unless
the Company's common stock is greater than $0.045 per share for each trading day
of a month, in which event no interest is payable during such month. Any
interest not paid when due shall bear interest of 15% per annum from the date
due until the same is paid. The Notes mature three years from the date of
issuance, and are convertible into common stock, at the Purchasers' option, at
the lesser of (i) $0.12 or (ii) a 75% discount to the average of the three
lowest trading prices of the common stock during the 20 trading day period prior
to conversion. The Notes contain a call option whereby, if the Company's stock
price is below $0.045, the Company may prepay the outstanding principal amount
of the Notes, subject to the conditions set forth in the call option. The Notes
also contain a partial call option whereby, if the Company's stock price is
below $0.045, the Company may prepay a portion of the outstanding principal
amount of the Note, subject to the conditions set forth in the partial call
option.
The full
principal amount of Notes are due upon a default under the terms of the secured
convertible notes. In addition, the Company granted the Purchasers a security
interest in substantially all of the Company's assets and intellectual property.
The Company is required to file a registration statement with the Securities and
Exchange Commission upon demand, which will include the common stock underlying
the Notes.
The
conversion price of the Notes may be adjusted in certain circumstances such as
if the Company pays a stock dividend, subdivides or combines outstanding shares
of common stock into a greater or lesser number of shares, or takes such other
action as would otherwise result in dilution of the selling stockholder's
position.
The
Purchasers have agreed to restrict their ability to convert their Notes and
receive shares of common stock such that the number of shares of common stock
held by them in the aggregate and their affiliates after such conversion or
exercise does not exceed 4.99% of the then issued and outstanding shares of
common stock.
JMJ
Financing
On May
22, 2009, the Company issued a Convertible Promissory Note to JMJ Financial
(“JMJ”) in aggregate principal amounts of $575,000 (the “Initial JMJ Note”). In
consideration for Wellstar’s issuing of the Initial JMJ Note, JMJ issued
Wellstar a Secured and Collateralized Promissory Note in the principle amount of
$500,000 (the “Initial Wellstar Note”).
In
addition, on August 19, 2009 Wellstar issued a Convertible Promissory Note to
JMJ in aggregate principal amounts of $1,150,000 (the “Second JMJ Note” and
together with the Initial JMJ Note, the “JMJ Notes”). In consideration for
Wellstar’s issuing of the Second JMJ Note, JMJ issued Wellstar a Secured and
Collateralized Promissory Note in the principle amouns of $1,000,000 (the
“Second Wellstar Note” and together with the Initial Wellstar Note, the
“Wellstar Notes”).
The JMJ
Notes bear interest at 12%, mature three years from the date of issuance, and
are convertible into our common stock, at JMJ’s option, at a conversion price,
equal to 70% of the lowest trade for our common stock during the 20 trading days
prior to the conversion. Prior to the conversion of the JMJ Notes, JMJ must make
a payment to Wellstar reducing the amount owed to Wellstar under the Wellstar
Notes. As of October 7, 2009, the lowest trade for our common stock during the
20 trading days as reported on the Over-The-Counter Bulletin Board was $.0001
and, therefore, the conversion price for the JMJ Notes was $.00007. Based on
this conversion price, the JMJ Notes in the aggregate amount of $1,725,000,
excluding interest, are convertible into 24.6 billion shares of our common
stock.
JMJ has
agreed to restrict their ability to convert the JMJ Notes and receive shares of
common stock such that the number of shares of common stock held by them in the
aggregate and their affiliates after such conversion or exercise does not exceed
4.99% of the then issued and outstanding shares of common stock.
The
Wellstar Notes bear interest at the rate of 13.8% per annum and mature three
years from the date of issuance. No interest or principal payments are required
until the maturity date, but both principal and interest may be prepaid prior to
Maturity Date. The Wellstar Notes are secured by units of STIC AIM Liquidity
Portfolio Select Investment Select Investment Fund (the “JMJ Collateral”). On
each of the Wellstar Notes, JMJ has agreed to pay down the principal of the
Wellstar Notes commencing 210 days after the original issuance of the Wellstar
Notes, However, JMJ may adjust the payment schedule within its sole discretion.
In the event that JMJ defaults on the Wellstar Notes, Wellstar may take
possession of the JMJ Collateral.
We
presently do not have any additional available credit, bank financing or other
external sources of liquidity. Due to our brief operating history as a start up
company, our operations have not been a source of liquidity. We will need to
obtain additional capital in order to maintain and expand our operations. We are
currently investigating other financial alternatives, including additional
equity and/or debt financing. In order to obtain capital, we may need to sell
additional shares of our common stock or borrow funds from private lenders.
However, there can be no assurance that that any additional financing will
become available to us, and if available, on terms acceptable to
us.
Sources
and Uses of Cash
|
|
|
Year Ended July 31,
|
|
|
(In
thousands)
|
|
2009
|
|
|
2008
|
|
|
Cash
flow data:
|
|
|
|
|
|
|
|
Net
cash provided by (used in) operating activities
|
|
|
(655
|
)
|
|
|
(1,080
|
)
|
|
Net
cash (used in) investing activities
|
|
|
(0
|
)
|
|
|
(52
|
)
|
|
Net
cash provided (used) by financing activities
|
|
|
659
|
|
|
|
1,093
|
|
|
Net
increase (decrease) in cash and cash equivalents
|
|
|
4
|
|
|
|
(39
|
)
|
|
Cash
and cash equivalents, beginning of period
|
|
|
26
|
|
|
|
65
|
|
|
Cash
and cash equivalents, end of period
|
|
|
30
|
|
|
|
26
|
|
Operating
Activities
Net cash
used in operating activities for the year ended July 31, 2009 was $654,584, a
decrease of $425,349 from the same period in 2008 reflecting the decrease in
operating expenses.
Investing
Activities
Cash used
in investing activities for the year ended July 31, 2009 was $344, down $51,454
from the same period in 2008 represented principally in a decrease in the
purchase of imaging equipment.
Financing
Activities
Net cash
provided by financing activities for the year ended July 31, 2009 was $658,932
as compared with $1,092,500 for the same period last year. The decrease is
attributed to a decrease in the proceeds from the issuance of convertible
notes.
As of
July 31, 2009 the Company had cash and cash equivalents in the amount of $29,564
as compared with $25,560 at July 31, 2008.
Critical
Accounting Policies
The
preparation of financial statements in accordance with accounting principles
generally accepted in the United States requires that management make a number
of assumptions and estimates that affect the reported amounts of assets,
liabilities, revenues and expenses in our consolidated financial statements and
accompanying notes. Management bases its estimates on historical information and
assumptions believed to be reasonable. Although these estimates are based on
management's best knowledge of current events and circumstances that may impact
the Company in the future, actual results may differ from these
estimates.
Our
critical accounting policies are those that affect our financial statements
materially and involve a significant level of judgment by
management.
The
Company has adopted the policy of capitalizing the cost of its imaging equipment
and depreciating the cost against earnings over the straight line method using
an estimated useful life of five years. Because the useful life of any new
technology is difficult to estimate due to factors such as competition,
obsolescence, government regulations, etc., this accounting estimate is
reasonably likely to change from period to period with a material impact on our
financial statements. The significance of the accounting estimate to the
Company's financial statements is that the equipment on the balance sheet is
stated at cost less accumulated amortization and the corresponding depreciation
is an expense on the statement of operations. The estimate as to the useful life
of these assets will directly affect the carrying amount on the balance sheet
and the expense for depreciation recorded in the statement of operations.
Accordingly, shareholders' equity and earnings will be materially
affected.
Revenue
Recognition
Revenue
will be recognized as earned per the licensing agreements which provide for a
fixed fee for each thermal imaging camera we install. The revenue is recognized
in the month that the camera is in use at the customer's facility.
Derivative
Instruments
In
connection with the sale of debt or equity instruments, we may sell options or
warrants to purchase our common stock. In certain circumstances, these options
or warrants may be classified as derivative liabilities, rather than as equity.
Additionally, the debt or equity instruments may contain embedded derivative
instruments, such as conversion options, which in certain circumstances may be
required to be bifurcated from the associated host instrument and accounted for
separately as a derivative instrument liability.
The
identification of, and accounting for, derivative instruments is complex. Our
derivative instrument liabilities are re-valued at the end of each reporting
period, with changes in the fair value of the derivative liability recorded as
charges or credits to income, in the period in which the changes occur. For
options, warrants and bifurcated conversion options that are accounted for as
derivative instrument liabilities, we determine the fair value of these
instruments using the Black-Scholes option pricing model. That model requires
assumptions related to the remaining term of the instruments and risk-free rates
of return, our current common stock price and expected dividend yield, and the
expected volatility of our common stock price over the life of the option.
Because of the limited trading history for our common stock, we have estimated
the future volatility of our common stock price based on not only the history of
our stock price but also the experience of other entities considered comparable
to us. The identification of, and accounting for, derivative instruments and the
assumptions used to value them can significantly affect our financial
statements.
Registration
Rights Agreements
In
connection with the sale of debt or equity instruments, we may enter into
registration rights agreements. Generally, these registration rights agreements
require us to file registration statements with the Securities and Exchange
Commission to register common shares that may be issued on conversion of debt or
preferred stock, to permit re-sale of common shares previously sold under an
exemption from registration or to register common shares that may be issued on
exercise of outstanding options or warrants.
The
registration rights agreements usually require us to pay penalties for any time
delay in filing the required registration statements, or in the registration
statements becoming effective, beyond dates specified in the registration rights
agreement. These penalties are usually expressed as a fixed percentage, per
month, of the original amount we received on issuance of the debt or preferred
stock, common shares, options or warrants. We account for these penalties as a
contingent liability and not as a derivative instrument. Accordingly, we
recognize the penalties when it becomes probable that they will be incurred. Any
penalties are expensed over the period to which they relate.
Recent
Accounting Pronouncements
Emerging
Issues Task Force Pronouncement 00-27, relating to certain convertible
instruments, requires the discounting of certain debt instruments when the
conversion feature meets certain criteria. FASB 123R, Stock Options To Employees
And Consultants. This pronouncement relates to employees and consultants who
receive stock based pay.
The
Company will account for the fair value of employee and non-employee options and
warrants in accordance with SFAS No. 123R, "Share-Based Payment", which is
effective for options and warrants during the annual reporting period beginning
after December 15, 2005. The compensation cost will be measured after the grant
date based on the value of the reward and is recognized over the service period.
The fair value of each option grant is estimated on the date of the grant using
the Black-Scholes stock option pricing model. The Company has not yet adopted a
stock option plan but is evaluating the affect of a stock option plan on its
financial position and results of operations in future periods.
In
September, 2006, the Financial Accounting Standards Board (FASB) issued SFAS No.
157, “Fair Value Measurements”. SFAS No. 157 provides a new single authoritative
definition of fair value and enhanced guidance in measuring the fair value of
assets and liabilities. It requires additional disclosures related to the extent
to which companies measure assets and liabilities at fair value, the information
used to measure fair value, and the effect of fair value measurement on
earnings. SFAS No. 157 is effective for fiscal years beginning after November
15, 2007, and interim periods within those fiscal years.
On August
1, 2008, the company adopted SFAS 157, which did not have a material impact on
its financial statements.
On
December 21, 2006, the Financial Accounting Standards Board (FASB) posted FASB
Staff Position (FSP) FSPEITF00-19-2, Accounting For Registration Payment
Arrangements. The FSP specifies that the contingent obligation to make future
payments or otherwise transfer consideration under a registration payment
arrangement, whether issued as a separate agreement or included as a provision
of a financial instrument or other agreement, should be separately recognized
and measured in accordance with FASB Statement No. 5, Accounting For
Contingencies. This FSP further clarifies that the financial instrument subject
to a registration payment arrangement should be accounted for in accordance with
other applicable generally accepted accounting principles (GAAP) without regard
to the contingent obligation to transfer consideration pursuant to the
registration payment arrangement. We have accounted for registration payments as
required under its securities purchase agreement and will follow this
pronouncement effect from date of issue.
In
February 2007, the FASB issued SFAS No. 159, “The Fair Value Option For
Financial Assets And Financial Liabilities” (SFAS 159). SFAS 159 expands the use
of fair value accounting but does not affect the existing standards that require
assets or liabilities to be carried at fair value. Under SFAS 159, a company may
elect to use fair value to measure accounts and loans receivable,
available-for-sale and held-to-maturity securities, accounts payable and issued
debt. If the use of fair value is elected, any up-front costs and fees related
to the item must be recognized in earnings and can not be deferred. The fair
value election is irrevocable and generally made on an instrument-by-instrument
basis, even if a company has similar instruments that it elects not to measure
based on fair value. At the adoption date, unrealized gains and losses on
existing items for which fair value has been elected are reported as a
cumulative adjustment to beginning retained earnings. Subsequent to the adoption
of SFAS 159, changes in fair value are recognized in earnings. SFAS 159 is
effective for fiscal years beginning after November 15, 2007. We are currently
assessing the impact of SFAS 159 will have on our financial position and results
of operations.
|
ITEM 7A
|
QUANTITATIVE
AND QUALITIATIVE DISCLOSURES ABOUT MARKET
RISK
|
As a
Smaller Reporting Company, the Company is not required to include the disclosure
under this Item 7A. Quantitative and Qualitative Disclosures about Market
Risk.
ITEM
8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
See
Consolidated Financial Statements beginning on page F-1.
ITEM
9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE.
None.
ITEM
9A - CONTROLS AND PROCEDURES
Not
applicable.
ITEM
9A(T) - CONTROLS AND PROCEDURES
Evaluation
of Disclosure Controls and Procedures
As of the
end of the period covered by this report, we carried out an evaluation, under
the supervision and with the participation of our management, including our
Chief Executive Officer and Chief Financial Officer, of the effectiveness of the
design and operation of our disclosure controls and procedures, as defined in
Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as
amended. Based upon that evaluation, our Chief Executive Officer and Chief
Financial Officer concluded that our disclosure controls and procedures were
effective as of the end of the applicable period to ensure that the information
required to be disclosed by the Company in reports that it files or submits
under the Exchange Act (i) is recorded, processed, summarized and reported
within the time periods specified in Securities and Exchange Commission rules
and forms, and (ii) is accumulated and communicated to our management, including
our Chief Executive Officer and Chief Financial Officer, as appropriate to allow
timely decisions regarding required disclosures.
Managements
Report on Internal Control over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal
control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f)
under the Securities Exchange Act of 1934. Our internal control over financial
reporting is designed to provide reasonable assurance regarding the reliability
of financial reporting and the preparation of financial statements for external
purposes in accordance with generally accepted accounting principles and
includes those policies and procedures that:
|
|
·
|
Pertain to the maintenance of
records that, in reasonable detail, accurately and fairly reflect the
transactions and disposition of our
assets;
|
|
|
·
|
Provide reasonable assurance that
transactions are recorded as necessary to permit preparation of financial
statements in accordance with U.S. generally accepted accounting
principles, and that our receipts and expenditures are being made only in
accordance with authorizations of our management and directors;
and
|
|
|
·
|
Provide reasonable assurance
regarding prevention or timely detection of unauthorized acquisition, use
or disposition of our assets that could have a material effect on the
financial statements.
|
Because
of its inherent limitations, internal control over financial reporting may not
prevent or detect misstatements. Also, projections of any evaluation of
effectiveness to future periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree of compliance
with the policies or procedures may deteriorate. All internal control systems,
no matter how well designed, have inherent limitations and provide only
reasonable assurance, not absolute assurance, with respect to financial
statement preparation and presentation. The design of an internal control system
reflects resource constraints and the benefits must be considered relative to
the costs of implementing and maintaining the system.
Management
assessed the effectiveness of the Company’s internal control over financial
reporting as of July 31, 2009. This assessment was based on criteria established
in
Internal Control—Integrated
Framework
issued by the Committee of Sponsoring Organizations of the
Treadway Commission (COSO). Based on our assessment, we believe that as of July
31, 2009 the Company’s internal control over financial reporting was effective
based on those criteria.
This
annual report does not include an attestation report of our registered public
accounting firm regarding internal control over financial reporting.
Management’s report was not subject to attestation by the Company’s registered
public accounting firm pursuant to temporary rules of the Securities and
Exchange Commission that permit us to provide only management’s report in this
annual report.
Changes
in Internal Control over Financial Reporting
There
were no changes in the Company’s internal controls over financial reporting
during the fourth quarter of 2009 that materially affected, or are reasonably
likely to materially affect, the Company’s internal control over financial
reporting.
ITEM
9B - Other Information
None.
PART III
ITEM
10. DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS.
The
following are the names and certain other information regarding or current
directors and executive officers.
|
Name
|
|
Position
|
|
Position
Held Since
|
|
John
Antonio
|
|
President,
Chief Executive Officer, Director
|
|
June
2005
|
|
Ken
McCoppen
|
|
Senior
Vice President, Director
|
|
June
2005
|
|
Howard
Bielski
|
|
Chief
Financial Officer
|
|
August
2005
|
|
|
|
|
|
|
|
Dr.
McKinley Boston
|
|
Director
|
|
July 2005
|
For
directors, the term of office is until the next annual meeting of shareholders.
For officers, the term of office is until the next annual meeting of the Board
of Directors, presently scheduled to be held immediately following the annual
meeting of the shareholders.
John
Antonio, President, Chief Executive Officer and Director. John Antonio has
served as our President, Chief Executive Officer, and a Director since June
2005. From March of 2004 until present, Mr. Antonio has been involved in the
establishment of Trillennium Medical Imaging, Inc. and its merger with Wellstar
International, Inc. From January 2003 until present, Mr. Antonio has been the
Vice President of Network Technologies International, Inc. From 1996 to present
Mr. Antonio has been the manager of Intertel LLC, which is engaged in the
distribution of prepaid phone cards in Mexico. Mr. Antonio graduated from
Bowling Green State University with a BLS Degree.
Ken
McCoppen, Senior Vice President and Director. Ken McCoppen has served as our
Senior Vice President and a Director since June 2005. From September 2002 until
September 2004, Mr. McCoppen was Executive Vice President of Sales for Arista
Communications, a provider of telecommunications services. From June 1999 until
September 2002, Mr. McCoppen was Vice President of the National Partners Program
for Qwest Communications. From 1982 to 1999, Mr. McCoppen held sales positions
at the following companies: Toshiba America; Samsung America; Micronics; Mantech
International; and GTE.
Howard
Bielski, Chief Financial Officer. Mr. Bielski has served as our Chief Financial
Officer since August 2005. Mr. Bielski is the managing member of Bielski &
Bielski, LLC, an accounting firm located in West Orange, New Jersey and is a
member of the American Institute of Certified Public Accountants and the New
Jersey Society of Certified Public Accountants.
Dr.
McKinley Boston, Director. Dr. Boston has served as a Director since July 2005.
Since December 2004, Dr. Boston has served as Athletic Director and Vice
President of New Mexico State University. Since September 2000, Dr. Boston has
served as an Education Consultant at the Consortium of Minneapolis Board of
Education, Minneapolis Youth Coordinating Board, and Minneapolis Park
Board.
Audit
Committee
At
present we do not have an audit committee and consequently the entire Board
serves as the audit committee. The Board presently consists of 3 members, one of
whom is independent. We have interviewed several qualified individuals for the
position of Audit Committee Financial Expert on the Board of Directors. All have
declined to serve, with the primary reason being personal liability issues,
especially the perceived view that being the "financial expert" increases the
individual's personal exposure over that of being a regular Board
member.
Code
of Ethics
The Board
of Directors has not adopted a Code of Business Conduct and Ethics that is
applicable to our directors, principal executive and financial officer,
principal accounting officer or controller, and persons performing similar
functions.
Compliance
with Section 16(a) of the Exchange Act
Section
16(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”),
requires executive officers and directors, and persons who beneficially own more
than ten percent of the Company’s common stock, to file initial reports of
ownership and reports of changes in ownership with the SEC for Companies
registered under Section 12 of the Exchange Act (“Section 12”) . Since we are
not registered under Section 12, the executive officers, directors and greater
than ten percent beneficial owners were not required by SEC regulations to file
forms under 16(a).
FAMILY
RELATIONSHIPS
There are
no family relationships between any of our directors or executive
officers.
INVOLVEMENT
IN CERTAIN LEGAL PROCEEDINGS
Other
than as discussed herein, none of our directors, executive officers, promoters
or control persons have been involved in any of the following events during the
past five years:
1. any
bankruptcy petition filed by or against any business of which such person was a
general partner or executive officer either at the time of the bankruptcy or
within two years prior to that time;
2. any
conviction in a criminal proceeding or being subject to a pending criminal
proceeding (excluding traffic violations and other minor offences);
3. being
subject to any order, judgment, or decree, not subsequently reversed, suspended
or vacated, of any court of competent jurisdiction, permanently or temporarily
enjoining, barring, suspending or otherwise limiting his involvement in any type
of business, securities or banking activities; or
4. being
found by a court of competent jurisdiction (in a civil action), the Commission
or the Commodity Futures Trading Commission to have violated a federal or state
securities or commodities law, and the judgment has not been reversed,
suspended, or vacated.
AUDIT
COMMITTEE FINANCIAL EXPERT
We do not
have an Audit Committee, as our board of directors during 2009 performed the
same functions of an Audit Committee, such as: recommending a firm of
independent certified public accountants to audit the annual financial
statements; reviewing the independent auditors independence, the financial
statements and their audit report; and reviewing management's administration of
the system of internal accounting controls. We do not currently have a written
audit committee charter or similar document. As the Company does not have an
Audit Committee, it does not have an Audit Committee Financial
Expert.
ITEM
11. EXECUTIVE COMPENSATION.
The
following table sets forth information concerning the total compensation that we
have paid or that has accrued on behalf of our chief executive officer and our
other executive officers during the fiscal years ended July 31, 2009 and
2008.
|
Name and
principal
position
|
|
Year
|
|
Salary
($)
|
|
|
Bonus
($)
|
|
|
Stock
Awards
($)
|
|
|
Option
Awards
($)
|
|
|
Non-Equity
Incentive Plan
Compensation
($)
|
|
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
|
|
All Other
Compensation
($)
|
|
|
Total
($)
|
|
|
C
John
Antonio
|
|
2009
|
|
$
|
240,000
|
|
|
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
$
|
240,000
|
|
|
|
|
2008
|
|
|
240,000
|
|
|
|
36,000
|
|
|
|
-0-
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
276,000
|
|
|
Ken
McCoppen
|
|
2009
|
|
$
|
240,000
|
|
|
|
|
|
|
|
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
$
|
240,000
|
|
|
|
|
2008
|
|
|
240,000
|
|
|
|
36,000
|
|
|
|
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
$
|
276,000
|
|
Options
Grants in Last Fiscal Year
None.
Aggregate
Option Exercises In Last Fiscal Year and Fiscal Year End Option
Values
None.
Employment
Agreements
As
previously disclosed on, August 5, 2005, we entered into separate employment
agreements with John A. Antonio, our President and CEO; and Kenneth B. McCoppen,
our Vice President and Corporate Secretary. These agreements provide for base
annual salaries of $240,000. Further, each agreement has a base term of five (5)
years.
DIRECTORS
COMPENSATION
Our
directors do not receive any set compensation.
Limitation of Liability of
Directors
Our
Articles of Incorporation, as amended, provide to the fullest extent permitted
by Nevada law, our directors or officers shall not be personally liable to us or
our shareholders for damages for breach of such director's or officer's
fiduciary duty. The effect of this provision of our Articles of Incorporation,
as amended, is to eliminate our rights and our shareholders (through
shareholders' derivative suits on behalf of our company) to recover damages
against a director or officer for breach of the fiduciary duty of care as a
director or officer (including breaches resulting from negligent or grossly
negligent behavior), except under certain situations defined by statute. We
believe that the indemnification provisions in our Articles of Incorporation, as
amended, are necessary to attract and retain qualified persons as directors and
officers.
Election
of Directors and Officers.
Directors
are elected to serve until the next annual meeting of stockholders and until
their successors have been elected and qualified. Officers are appointed to
serve until the meeting of the Board of Directors following the next annual
meeting of stockholders and until their successors have been elected and
qualified.
No
Executive Officer or Director has been the subject of any order, judgment, or
decree of any Court of competent jurisdiction, or any regulatory agency
permanently or temporarily enjoining, barring suspending or otherwise limiting
him from acting as an investment advisor, underwriter, broker or dealer in the
securities industry, or as an affiliated person, director or employee of an
investment company, bank, savings and loan association, or insurance company or
from engaging in or continuing any conduct or practice in connection with any
such activity or in connection with the purchase or sale of any
securities.
No
Executive Officer or Director has been convicted in any criminal proceeding
(excluding traffic violations) or is the subject of a criminal proceeding which
is currently pending.
No
Executive Officer or Director is the subject of any pending legal
proceedings.
Compensation
Discussion and Analysis
Currently,
the Company’s philosophy is to align compensation with its short-term and
long-term goals: short term - development of products, sales, and generating
positive cash flow, and providing expertise to develop long-term development of
markets for the Company to pursue. Our plan was determined by a combination of
upper management, finance and operations departments, based on research
available from NCEO (the National Center for Employee Ownership) and other
research management is familiar with. The current plan has the flexibility to be
changed as the needs of the Company and the circumstances surrounding it change.
The Company has designed its compensation structure to provided its core people
base compensation to insure they maintain (but not necessarily upgrade) the
lifestyle each has built from past business successes. We feel this is
appropriate due to the fact we are in the development stage. Certain consultants
have elected to be paid in shares only.
We may
elect to award a cash bonus to key employees, directors, officers and
consultants based on meeting individual and corporate planned objectives. We
currently have written employment agreements with our officers, and the
following shows additional information, including the annual salaries, bonuses
and stock awards for our officers and executives.
ITEM
12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED
STOCKHOLDER MATTERS.
The
following table indicates beneficial ownership of our common stock as of
November 11, 2009 by each person or entity known by us to beneficially own more
than 5% of the outstanding shares of our common stock; each of our executive
officers and directors; and all of our executive officers and directors as a
group.
|
Name and Address of Beneficial Owner (1)(2)
|
|
Common
Shares
Beneficially
Owned (1)
|
|
|
Percent
of
Class
(3)
|
|
|
|
|
|
|
|
|
|
|
John
A. Antonio (4)
|
|
|
79,128,571
|
(4)
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
Howard
Bielski
|
|
|
3,000,000
|
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
McKinley
Boston
|
|
|
500,000
|
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
Ken
McCoppen
|
|
|
81,541,071
|
(5)
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
All
directors and executive officers
|
|
|
164,169,642
|
|
|
|
|
*
|
*Less
than 1%
|
(1)
|
Beneficial Ownership is
determined in accordance with the rules of the Securities and Exchange
Commission and generally includes voting or investment power with respect
to securities. Shares of common stock subject to options or warrants
currently exercisable or convertible, or exercisable or convertible within
60 days of June 19, 2008 are deemed outstanding for computing the
percentage of the person holding such option or warrant but are not deemed
outstanding for computing the percentage of any other
person.
|
|
(2)
|
Unless otherwise indicated, the
address of each beneficial owner is c/o Wellstar International, Inc., 6911
Pilliod Road, Holland, Ohio
43528.
|
|
(3)
|
Based on 14,346,647,175 shares
issued and outstanding as of November 12,
2009.
|
(4)
Includes (i) 21,428,571 shares of common stock issuable upon conversion of the
Series A Preferred Shares, (ii) 50,000,000 shares of common stock issuable upon
conversion of the Series B Preferred Shares and (iii) 7,700,000.
(5)
Includes (i) 21,428,571 shares of common stock issuable upon conversion of the
Series A Preferred Shares, (ii) 50,000,000 shares of common stock issuable upon
conversion of the Series B Preferred Shares and (iii) 10,112 5000.
Equity
Compensation Plan Information
The
Company does not have an equity compensation plan.
ITEM 13. CERTAIN RELATIONSHIPS AND
RELATED TRANSACTIONS.
Series A Preferred
Stock
On May
20, 2009, the Company entered into a conversion agreement with John Antonio
(“Antonio”) and Kenneth McCoppen (“McCoppen”), both executive officers and
directors of the Company, pursuant to which the Company agreed to convert
$30,000 in outstanding wages owed to each McCoppen and Antonio into a total of
60,000 shares of Series A Preferred Stock.
The above
transactions were approved by the Board of Directors of the Company. The Series
A Preferred Stock does not pay dividends but each holder of Series A Preferred
Stock shall be entitled to 100 votes for each share of common stock that the
Series A Preferred Stock shall be convertible into. The Series A Preferred Stock
has a conversion price of $0.0014 (the “Conversion Price”) and a stated value of
$1.00 (the “Stated Value”). Each share of Series A Preferred Stock is
convertible, at the option of the holder, into such number of shares of common
stock of the Company as determined by dividing the Stated Value by the
Conversion Price. The Series A Preferred Stock has no liquidation
preference.
Series B Preferred
Stock
On
October 1, 2009, the Company entered into a conversion agreement with Antonio
and McCoppen, both executive officers and directors of the Company, pursuant to
which the Company agreed to convert $50,000 in outstanding wages owed to each
McCoppen and Antonio into a total of 100,000 shares of Series B Preferred
Stock.
The above
transactions were approved by the Board of Directors of the Company. The Series
B Preferred Stock does not pay dividends but each holder of Series B Preferred
Stock shall be entitled to 100 votes for each share of common stock that the
Series B Preferred Stock shall be convertible into. The Series B Preferred Stock
has a conversion price of $0.001 (the “Conversion Price”) and a stated value of
$1.00 (the “Stated Value”). Each share of Series B Preferred Stock is
convertible, at the option of the holder, into such number of shares of common
stock of the Company as determined by dividing the Stated Value by the
Conversion Price. The Series B Preferred Stock has no liquidation
preference.
ITEM
14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
Audit
fees
The
aggregate fees billed for professional services rendered by our independent
auditors for the audit of our financial statements, for the reviews of the
financial statements included in our annual report on Form 10K, and for other
services normally provided in connection with statutory filings were $12,852 and
$34,908 for the years ended July 31, 2009 and July 31, 2008,
respectively.
Audit-Related
Fees
No
audit-related fees were incurred for the years ended July 31, 2009 and July 31,
2008.
Tax
Fees
No tax
fees were incurred for the years ended July 31, 2009 and July 31,
2008.
All Other
Fees
We did
not incur any fees for other professional services rendered by our independent
auditors during the years ended July 31, 2009 and July 31, 2008.
Audit
Committee
At
present we do not have an audit committee and consequently the entire Board
serves as the audit committee. The Board presently consists of 4 members, two of
whom are independent. We have interviewed several qualified individuals for the
position of Audit Committee Financial Expert on the Board of Directors. All have
declined to serve, with the primary reason being personal liability issues,
especially the perceived view that being the "financial expert" increases the
individual's personal exposure over that of being a regular Board
member.
ITEM
15. EXHIBITS, Financial Statement Schedules.
Exhibit
|
3.1
|
Articles of Incorporation of
Wellstar International, Inc.
**
|
|
3.2
|
Certificate of Amendment to
Articles of Incorporation of Wellstar International,
Inc.*****
|
|
3.3
|
Bylaws of Wellstar International,
Inc. **
|
3.4
Certificate of Amendment to the
Certificate of Incorporation (Incorporated by reference to the Form 8-K Current
Report filed with the Securities and Exchange Commission filed with Securities
and Exchange Commission on May 27, 2009)
3.5
Amendment to Certificate of Designation
– Series A Preferred Stock
(Incorporated by reference to the Form
8-K Current Report filed with the Securities and Exchange Commission filed with
Securities and Exchange Commission on May 27, 2009)
3.6
Certificate of Correction to the
Amendment to Certificate of Designation – Series A Preferred Stock (Incorporated
by reference to the Form 8-K Current Report filed with the Securities and
Exchange Commission filed with Securities and Exchange Commission on May 27,
2009)
3.7 Certificate
of Amendment to the Certificate of Incorporation (Incorporated by reference to
the Form 8-K Current Report filed with the Securities and Exchange Commission
filed with Securities and Exchange Commission on October 5, 2009)
3.8 Certificate
of Designation – Series B Preferred Stock (Incorporated by reference to the Form
8-K Current Report filed with the Securities and Exchange Commission filed with
Securities and Exchange Commission on October 5, 2009)
4.1
Securities Purchase Agreement, dated May
15, 2009, by and among the Company and the Purchasers (Incorporated by reference
to the Form 8-K Current Report filed with the Securities and Exchange Commission
filed with Securities and Exchange Commission on May 27,
2009)
4.2
Form of Callable Secured Convertible
Note
(Incorporated
by reference to the Form 8-K Current Report filed with the Securities and
Exchange Commission filed with Securities and Exchange Commission on May 27,
2009)
4.3
Registration Rights Agreement
(Incorporated by reference
to the Form 8-K Current Report filed with the Securities and Exchange Commission
filed with Securities and Exchange Commission on May 27, 2009)
4.4 Security
Agreement
(Incorporated by reference to the
Form 8-K Current Report filed with the Securities and Exchange Commission filed
with Securities and Exchange Commission on May 27,
2009)
4.5
Intellectual Property Security
Agreement
(Incorporated by reference to the
Form 8-K Current Report filed with the Securities and Exchange Commission filed
with Securities and Exchange Commission on May 27,
2009)
4.6
Subsidiary Guarantee (Incorporated by reference to the Form 8-K Current Report
filed with the Securities and Exchange Commission filed with Securities and
Exchange Commission on May 27, 2009)
4.7
Form of Convertible Promissory Note
issued by Wellstar International Inc. to JMJ Financial (Incorporated by
reference to the Form 8-K Current Report filed with the Securities and Exchange
Commission filed with Securities and Exchange Commission on October 9,
2009)
4.8
Form of Secured and Collateralized
Promissory Note issued by JMJ Financial to Wellstar International, Inc.
(Incorporated by reference to the Form 8-K Current Report filed with the
Securities and Exchange Commission filed with Securities and Exchange Commission
on October 9, 2009)
|
10.1
|
Securities Purchase Agreement
dated October 31, 2005, by and among Wellstar International Inc. and the
investors named on the signature pages thereto.
****
|
|
10.2
|
Form of Callable Secured
Convertible Note dated October 31, 2005.
**
|
|
10.3
|
Form of Stock Purchase Warrant
dated October 31, 2005. **
|
|
10.4
|
Registration Rights Agreement
dated October 31, 2005, by and among Wellstar International Inc. and the
investors named on the signature pages thereto.
**
|
|
10.5
|
Security Agreement dated October
31, 2005, by and among Wellstar International Inc. and the investors named
on the signature pages thereto.
**
|
|
10.6
|
Intellectual Property Security
Agreement dated October 31, 2005, by and among Wellstar International Inc.
and the investors named on the signature pages thereto.
**
|
|
10.7
|
Technical Services Agreement by
and between Trillennium Medical Imaging, Inc. and Surgicenters of America,
Inc. dated October 4,
2005.**
|
|
10.9
|
Term Loan Agreement dated October
11, 2005. **
|
|
10.10
|
Commercial Cognovit Promissory
Note dated October 17, 2005.
**
|
|
10.11
|
Commercial Cognovit Promissory
Note dated November 28, 2005.
**
|
|
10.12
|
Security Agreement dated October
11, 2005. **
|
|
10.13
|
Demand Note dated August 1, 2005.
**
|
|
10.14
|
Employment Agreement by and
between Wellstar International, Inc. and John A. Antonio *
*
|
|
10.15
|
Employment Agreement by and
between Wellstar International, Inc. and Ken
McCoppen**
|
|
10.16
|
Definitive Agreement dated
December 12, 2005, by and among Wellstar International, Inc., Micro Health
Systems, Robert Barnes and Terri
Cmorey.***
|
|
10.17
|
Marketing Agreement between
Trillennium Medical Imaging, Inc., and Allevia
Medical.*
|
|
10.18
|
Addendum to Commercial Cognovit
Promissory Note between Wellstar International, Inc., Trillennium Medical
Imaging, Inc., and Andrew M.
Thompson.*
|
|
10.19
|
Second Addendum to Commercial
Cognovit Promissory Note between Wellstar International, Inc., Trillennium
Medical Imaging, Inc., and Andrew M. Thompson.
******
|
|
10.20
|
Securities Purchase Agreement, dated November
30, 2006, by and among Wellstar International, Inc.
and
AJW Offshore, Ltd., AJW Qualified
Partners, LLC, AJW Partners, LLC and New Millennium Capital Partners II,
LLC.
|
|
10.21
|
Form of Callable Secured
Convertible Note.*******
|
|
10.22
|
Form of Stock Purchase
Warrant.*******
|
|
10.23
|
Registration Rights Agreement by
and among Wellstar International Inc. and the secured parties signatory
thereto.*******
|
|
10.24
|
Security Agreement by and among
Wellstar International Inc. and the secured parties signatory thereto.
*******
|
|
10.25
|
Intellectual Property Security
Agreement by and among Wellstar International Inc. and the secured parties
signatory thereto. *******
|
|
10.26
|
Limited Technology License
Agreement by and between Trillennium Medical Imaging, Inc. and Maclath
Ltda
|
10.27 Limited
Technology License Agreement dated July 9th, 2007, by and between Trillennium
Medical Imaging, Inc. and Maclath Ltda. (Incorporated by reference to the Form
8-K Current Report filed with the Securities and Exchange Commission filed with
Securities and Exchange Commission on March 19, 2009)
10.28 Notice
of Default dated September 27,2007 sent by Trillennium Medical Imaging, Inc. to
Maclath Ltda (Incorporated by reference to the Form 8-K Current Report filed
with the Securities and Exchange Commission filed with Securities and Exchange
Commission on March 19, 2009)
|
10.29
|
Conversion Agreement between the
Company and John Antonio (Incorporated by reference to the Form 8-K
Current Report filed with the Securities and Exchange Commission filed
with Securities and Exchange Commission on May 27, 2009)
|
|
10.30
|
Conversion Agreement between the
Company and Ken McCoppen (Incorporated by reference to the Form 8-K
Current Report filed with the Securities and Exchange Commission filed
with Securities and Exchange Commission on May 27, 2009)
|
10.31 Conversion
Agreement between the Company and John Antonio – October 2009 (Incorporated by
reference to the Form 8-K Current Report filed with the Securities and Exchange
Commission filed with Securities and Exchange Commission on October 5,
2009)
10.32 Conversion
Agreement between the Company and Ken McCoppen – October 2009 (Incorporated by
reference to the Form 8-K Current Report filed with the Securities and Exchange
Commission filed with Securities and Exchange Commission on October 5,
2009)
|
31.1
|
Certification of Chief Executive
Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the
Securities and Exchange Act of 1934, as amended.
*
|
|
31.2
|
Certification of Chief Financial
Officer pursuant to Rule 13a-14 and Rule 15d-14(a), promulgated under the
Securities and Exchange Act of 1934, as amended.
*
|
|
32.1
|
Certification pursuant to 18
U.S.C. Section 1350, as adopted pursuant to Section 906 of the
Sarbanes-Oxley Act of 2002 (Chief Executive Officer and the Chief
Financial Officer). *
|
* Filed
herewith.
**
Incorporated by reference to Form SB-2 filed with the Commission on December 13,
2005.
***
Incorporated by reference to Form SB-2/A filed with the Commission on February
1, 2006.
****
Incorporated by reference to Form SB-2/A filed with the Commission on April 4,
2006.
*****
Incorporated by reference to Form SB-2/A filed with the Commission on June 20,
2006.
******
Incorporated by reference to Form 8-K filed with the Commission on November 24,
2006
*******
Incorporated by reference to Form 8-K filed with the Commission on December 6,
2006
*******
Incorporated by reference to Form 8-K filed with the Commission on July 12,
2007
SIGNATURES
In
accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the
registrant has duly caused this report to be signed on its behalf by the
undersigned, thereunto duly authorized.
|
|
WELLSTAR
INTERNATIONAL, INC.
|
|
|
|
|
|
|
|
Dated: November
12, 2009
|
By:
|
/s/ John
Antonio
|
|
|
|
|
John
Antonio
|
|
|
|
|
President
and Chief Executive Officer
|
|
|
|
|
(Principal
Executive Officer)
|
|
|
Dated: November
12, 2009
|
By:
|
/s/ Howard
Bielski
|
|
|
|
|
Howard
Bielski
|
|
|
|
|
Chief
Financial Officer
|
|
|
|
|
(Principal
Financial and Accounting Officer)
|
|
PURSUANT
TO THE REQUIREMENTS OF THE SECURITIES EXCHANGE ACT OF 1934, THIS REPORT HAS BEEN
SIGNED BY THE FOLLOWING PERSONS IN THE CAPACITIES AND ON THE DATES
INDICATED:
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/
John Antonio
|
|
President
and Chief Executive Officer, Director
|
|
November
12, 2009
|
|
John
Antonio
|
|
(Principal
Executive Officer)
|
|
|
|
|
|
|
|
|
|
/s/
Ken McCoppen
|
|
Senior
Vice President, Director
|
|
November
12, 2009
|
|
Ken
McCoppen
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/
McKinley Boston
|
|
Director
|
|
November
12, 2009
|
|
McKinley
Boston
|
|
|
|
|
WELLSTAR
INTERNATIONAL, INC.
CONSOLIDATED
FINANCIAL STATEMENTS
JULY
31, 2009 AND 2008
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
CONSOLIDATED
FINANCIAL STATEMENTS
JULY
31, 2009 AND 2008
CONTENTS
|
|
PAGE
|
|
|
|
|
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
|
F-i
|
|
|
|
|
CONSOLIDATED
BALANCE SHEETS
|
F-1
- F-2
|
|
|
|
|
CONSOLIDATED
STATEMENTS OF OPERATIONS
|
F-3
|
|
|
|
|
CONSOLIDATED
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
|
F-4
|
|
|
|
|
CONSOLIDATED
STATEMENTS OF CASH FLOWS
|
F-5,
F-6
|
|
|
|
|
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
|
F-7
- F-30
|
To
The Board of Directors
Wellstar
International, Inc.
Holland,
Ohio 43528
Report of Independent
Registered Public Accounting Firm
We have
audited the accompanying consolidated balance sheets of Wellstar International,
Inc. and Subsidiary as of July 31, 2009 and 2008 and the related
consolidated statements of operations, changes in stockholders’ equity (deficit)
and cash flows for the years ended July 31, 2009 and 2008. The
financial statements are the responsibility of the Company's
management. Our responsibility is to express an opinion on the
financial statements based on our audits.
We
conducted our audits in accordance with the Standards of the Public Company
Accounting Oversite Board (United States). Those standards require
that we plan and perform the audits to obtain reasonable assurance about whether
the financial statements are free of material misstatement. An audit
includes examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements. An audit also includes
assessing the accounting principles used and significant estimates made by
management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis
for our opinion.
In our
opinion, the financial statements referred to above presents fairly, in all
material respects, the financial position of Wellstar International, Inc. and
Subsidiary as of July 31, 2009 and 2008, and the results of their operations and
their cash flows for the years ended July 31, 2009 and 2008 in conformity with
accounting principles generally accepted in the United States of
America.
The
accompanying consolidated financial statements have been prepared assuming that
the Company will continue as a going concern. As discussed in Note 9
to the consolidated financial state-ments, the Company had a net loss of
$41,832,521, negative cash flow from operations of $654,584 negative
working capital of $30,631,304, and a stockholders’ deficiency of $53,131,962 at
July 31, 2009. These matters raise substantial doubt about the
Company’s ability to continue as a going concern. Management’s plan
in regards to these matters is also described in Note 9. The
accompanying consolidated financial statements do not include any adjustments
that might result from the outcome of this uncertainty.
Simontacchi,
Miller & DeAngelis, PA
Rockaway,
New Jersey
November
9, 2009
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
CONSOLIDATED
BALANCE SHEETS
JULY
31, 2009 AND 2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
Assets:
|
|
|
|
|
|
|
|
Cash
|
|
$
|
29,564
|
|
|
$
|
25,560
|
|
|
Accounts
Receivable
|
|
|
-
|
|
|
|
-
|
|
|
Prepaid
Expenses
|
|
|
5,186
|
|
|
|
40
|
|
|
Rent
Refund Receivable
|
|
|
-
|
|
|
|
1,580
|
|
|
Total
Current Assets
|
|
|
34,750
|
|
|
|
27,180
|
|
|
|
|
|
|
|
|
|
|
|
|
Fixed
Assets:
|
|
|
|
|
|
|
|
|
|
Imaging
Equipment
|
|
|
802,169
|
|
|
|
837,874
|
|
|
Office
Equipment and Fixtures
|
|
|
154,884
|
|
|
|
154,540
|
|
|
Subtotal
|
|
|
957,053
|
|
|
|
992,414
|
|
|
|
|
|
|
|
|
|
|
|
|
Less:
Accumulated Depreciation
|
|
|
552,985
|
|
|
|
374,414
|
|
|
Net
Fixed Assets
|
|
|
404,068
|
|
|
|
618,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible
Assets:
|
|
|
|
|
|
|
|
|
|
Covenant
Not To Compete
|
|
|
-
|
|
|
|
20,000
|
|
|
Manufacturing
and Distribution Agreement
|
|
|
700,000
|
|
|
|
700,000
|
|
|
Subtotal
|
|
|
700,000
|
|
|
|
720,000
|
|
|
|
|
|
|
|
|
|
|
|
|
Less:
Accumulated Amortization
|
|
|
459,577
|
|
|
|
352,304
|
|
|
Net
Intangible Assets
|
|
|
240,423
|
|
|
|
367,696
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
Assets:
|
|
|
|
|
|
|
|
|
|
Loan
Acquisition Cost (net of amortization of $261,880 @ 7/31/09 and
$216,861 @ 7/31/08)
|
|
|
110,645
|
|
|
|
80,664
|
|
|
Software
and Manuals (net of amortization of $37,892 @ 7/31/09 and $99,425 @
7/31/08)
|
|
|
17,508
|
|
|
|
35,975
|
|
|
Security
Deposit
|
|
|
4,525
|
|
|
|
4,525
|
|
|
Total
Other Assets
|
|
|
132,678
|
|
|
|
121,164
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Assets
|
|
$
|
811,919
|
|
|
$
|
1,134,040
|
|
See
Accompanying Notes to Consolidated Financial Statements
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
CONSOLIDATED
BALANCE SHEETS
JULY
31, 2009 AND 2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES LESS SHAREHOLDERS’
DEFICIT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
Liabilities:
|
|
|
|
|
|
|
|
Accounts
Payable
|
|
$
|
616,537
|
|
|
$
|
699,295
|
|
|
Accrued
Expenses
|
|
|
2,879,727
|
|
|
|
1,915,925
|
|
|
Note
& Loan Payable - Other
|
|
|
63,382
|
|
|
|
13,000
|
|
|
Notes
Payable
|
|
|
750,000
|
|
|
|
750,000
|
|
|
Derivative
Instrument Liability - Loan
|
|
|
422,243
|
|
|
|
374,952
|
|
|
Derivative
Instrument Liability - Convertible Notes
|
|
|
23,072,247
|
|
|
|
2,525,027
|
|
|
Convertible
Debt
|
|
|
2,861,918
|
|
|
|
530,452
|
|
|
Total
Current Liabilities
|
|
|
30,666,054
|
|
|
|
6,808,651
|
|
|
|
|
|
|
|
|
|
|
|
|
Long
Term Liabilities:
|
|
|
|
|
|
|
|
|
|
Convertible
Debt
|
|
|
2,697,938
|
|
|
|
85,499
|
|
|
Derivative
Instrument Liability - Convertible Notes
|
|
|
20,564,516
|
|
|
|
5,841,505
|
|
|
Derivative
Instrument Liability - Warrants
|
|
|
15,373
|
|
|
|
1,153,454
|
|
|
Total
Long-Term Liabilities
|
|
|
23,277,827
|
|
|
|
7,080,458
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
Liabilities
|
|
|
53,943,881
|
|
|
|
13,889,109
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’
Deficit:
|
|
|
|
|
|
|
|
|
|
Preferred
Stock
|
|
|
|
|
|
|
|
|
|
Series
A, Authorized 10,000,000 Shares, par value .001 per
share
|
|
|
|
|
|
|
|
|
|
Issued
and Outstanding Shares, 60,000
|
|
$
|
60
|
|
|
|
-
|
|
|
Paid
in Surplus
|
|
|
59,940
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
Stock
|
|
|
|
|
|
|
|
|
|
Authorized
10,000,000,000 Shares, par value .001 per share
|
|
|
|
|
|
|
|
|
|
Issued
Shares, 3,949,614,673 - Outstanding Shares, 3,948,114,673 (7/31/09) and
460,040,217 (7/31/08)
|
|
|
3,948,116
|
|
|
|
460,041
|
|
|
Paid
in Surplus
|
|
|
(994,187
|
)
|
|
|
1,098,260
|
|
|
Retained
Earnings (Deficit)
|
|
|
(56,145,891
|
)
|
|
|
(14,313,370
|
)
|
|
Total
Shareholders’ Deficit
|
|
|
(53,131,962
|
)
|
|
|
(12,755,069
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total
Liabilities Less Stockholder’s Deficit
|
|
$
|
811,919
|
|
|
$
|
1,134,040
|
|
See
Accompanying Notes to Consolidated Financial Statements
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
CONSOLIDATED
STATEMENTS OF OPERATIONS
FOR
THE YEARS ENDED JULY 31, 2009 AND 2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
Income
:
|
|
|
|
|
|
|
|
Revenue
from License Agreement
|
|
$
|
- 0
-
|
|
|
$
|
- 0
-
|
|
|
Revenue
from Medical Imaging
|
|
|
- 0 -
|
|
|
|
- 0 -
|
|
|
Total
Revenue
|
|
|
- 0
-
|
|
|
|
- 0
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost
of Sales
|
|
|
- 0 -
|
|
|
|
- 0 -
|
|
|
Gross
Profit (Loss)
|
|
|
- 0 -
|
|
|
|
- 0 -
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating
Expenses:
|
|
|
|
|
|
|
|
|
|
Selling,
General and Administrative
|
|
|
1,774,569
|
|
|
|
1,975,850
|
|
|
Depreciation
and Amortization
|
|
|
389,104
|
|
|
|
390,854
|
|
|
Total
Operating Expenses
|
|
|
2,163,673
|
|
|
|
2,366,704
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
from Operations
|
|
|
(2,163,673
|
)
|
|
|
(2,366,704
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other
Expense (Income):
|
|
|
|
|
|
|
|
|
|
Interest
Income
|
|
|
(182
|
)
|
|
|
(2,313
|
)
|
|
Interest
Expense
|
|
|
778,470
|
|
|
|
477,395
|
|
|
Loss
on Disposal of Furniture
|
|
|
15,931
|
|
|
|
-
|
|
|
Derivative
Instrument (Income) Expense, Net
|
|
|
38,420,029
|
|
|
|
2,471,101
|
|
|
Delinquent
Stock Registration
|
|
|
|
|
|
|
|
|
|
Penalty
- Convertible Debentures
|
|
|
454,600
|
|
|
|
400,200
|
|
|
Total
Other Expenses (Income)
|
|
|
39,668,848
|
|
|
|
3,346,383
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
before Provision for Taxes
|
|
|
(41,832,521
|
)
|
|
|
(5,713,087
|
)
|
|
Provision
for Taxes
|
|
|
-
|
|
|
|
-
|
|
|
Net
Loss
|
|
$
|
(41,832,521
|
)
|
|
$
|
(5,713,087
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss Per Share,
Basic and Diluted
|
|
$
|
(.03
|
)
|
|
$
|
(.02
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
Average Number of Common Shares Outstanding, Basic and
Diluted
|
|
|
1,278,144,365
|
|
|
|
253,954,978
|
|
See
Accountants' Report and Accompanying Notes to Financial Statements
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
CONSOLIDATED
STATEMENTS OF CHANGES IN
STOCKHOLDERS’
EQUITY (DEFICIT)
FOR
THE YEARS ENDED JULY 31, 2009 AND 2008
|
|
|
Preferred
Stock
|
|
|
|
|
|
Common
Stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Paid
in
|
|
|
|
|
|
|
|
|
Paid
in
|
|
|
Accumulated
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
July 31, 2007
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
119,083,975
|
|
|
$
|
119,084
|
|
|
$
|
1,102,545
|
|
|
$
|
(8,600,283
|
)
|
|
$
|
(7,378,654
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
Stock - Issued for Services
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
40,682,142
|
|
|
|
40,682
|
|
|
|
54,400
|
|
|
|
|
|
|
|
95,082
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion
of Debentures
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
300,274,100
|
|
|
|
300,275
|
|
|
|
(58,685
|
)
|
|
|
|
|
|
|
241,590
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
Loss for the Period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(5,713,087
|
)
|
|
|
(5,713,087
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
July 31, 2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
460,040,217
|
|
|
$
|
460,041
|
|
|
$
|
1,098,260
|
|
|
$
|
(14,313,370
|
)
|
|
$
|
(12,755,069
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
August 1, 2008
|
|
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
460,040,217
|
|
|
$
|
460,041
|
|
|
$
|
1,098,260
|
|
|
$
|
(14,313,370
|
)
|
|
$
|
(12,755,069
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred
Stock Issued - For Wages
|
|
|
60,000
|
|
|
|
60
|
|
|
|
59,940
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock
Issued to Consultants and Others for Services and
Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
230,065,339
|
|
|
|
230,065
|
|
|
|
321,307
|
|
|
|
|
|
|
|
551,372
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion
of Debentures
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,070,921,617
|
|
|
|
3,070,922
|
|
|
|
(2,570,216
|
)
|
|
|
|
|
|
|
500,706
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock
Issued for Cash
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
203,200,000
|
|
|
|
203,200
|
|
|
|
140,350
|
|
|
|
|
|
|
|
343,550
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Company
Stock Donated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(16,112,500
|
)
|
|
|
(16,112
|
)
|
|
|
16,112
|
|
|
|
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
(Loss) for the Period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(41,832,521
|
)
|
|
|
(41,832,521
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
July 31, 2009
|
|
|
60,000
|
|
|
$
|
60
|
|
|
$
|
59,940
|
|
|
|
3,948,114,673
|
|
|
$
|
3,948,116
|
|
|
$
|
(994,187
|
)
|
|
$
|
(56,145,891
|
)
|
|
$
|
(53,131,962
|
)
|
See
Accountants' Report and Accompanying Notes to Financial Statements
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
CONSOLIDATED
STATEMENTS OF CASH FLOWS
FOR
THE YEARS ENDED JULY 31, 2009 AND 2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
Cash
Flows from Operating Activities:
|
|
|
|
|
|
|
|
Net
Loss
|
|
$
|
(41,832,521
|
)
|
|
$
|
(5,713,087
|
)
|
|
Adjustments
to Reconcile Net Loss to Net Cash used in Operating
Activities:
|
|
|
|
|
|
|
|
|
|
Depreciation
and Amortization
|
|
|
389,104
|
|
|
|
390,854
|
|
|
Delinquent
Registration Penalty Convertible Debentures
|
|
|
454,600
|
|
|
|
400,200
|
|
|
Services
Paid in Stock
|
|
|
551,372
|
|
|
|
95,082
|
|
|
Issuance
of Preferred Stock in lieu of Salary
|
|
|
60,000
|
|
|
|
-
|
|
|
Derivative
Instrument Expense, Net
|
|
|
38,420,029
|
|
|
|
2,471,101
|
|
|
Loss
on Disposal of Property
|
|
|
15,931
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Changes
in Operating Assets and Liabilities:
|
|
|
|
|
|
|
|
|
|
(Increase)
Decrease Accounts Receivable
|
|
|
-
|
|
|
|
50,000
|
|
|
(Increase)
Decrease Prepaid Expenses
|
|
|
(5,146
|
)
|
|
|
12,584
|
|
|
Decrease
Rent Refund Receivable
|
|
|
1,580
|
|
|
|
-
|
|
|
Increase
Accounts Payable
|
|
|
(82,758
|
)
|
|
|
258,915
|
|
|
Increase
Accrued Expenses
|
|
|
1,373,225
|
|
|
|
954,418
|
|
|
Net
Cash Used in Operating Activities
|
|
|
(654,584
|
)
|
|
|
(1,079,933
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash
Flows from Investing Activities:
|
|
|
|
|
|
|
|
|
|
Purchase
of Equipment
|
|
|
(344
|
)
|
|
|
(36,298
|
)
|
|
Purchase
of Software
|
|
|
-
|
|
|
|
(15,400
|
)
|
|
Security
Deposit
|
|
|
-
|
|
|
|
(100
|
)
|
|
Net
Cash Used in Investing Activities
|
|
|
(344
|
)
|
|
|
(51,798
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash
Flows From Financing Activities:
|
|
|
|
|
|
|
|
|
|
Sale
of Stock for Cash
|
|
|
343,550
|
|
|
|
-
|
|
|
Payments
of Financing Costs
|
|
|
-
|
|
|
|
(60,000
|
)
|
|
Proceeds
from Issuance of Convertible Notes
|
|
|
265,000
|
|
|
|
1,140,000
|
|
|
Other
Loan
|
|
|
50,382
|
|
|
|
12,500
|
|
|
Net
Cash Provided by Financing Activities
|
|
|
658,932
|
|
|
|
1,092,500
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
Increase (Decrease) in Cash
|
|
|
4,004
|
|
|
|
(39,231
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash
at Beginning of Period
|
|
|
25,560
|
|
|
|
64,791
|
|
|
Cash
at End of Period
|
|
$
|
29,564
|
|
|
$
|
25,560
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
Paid for Interest
|
|
$
|
- 0
-
|
|
|
$
|
- 0
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
Paid for Taxes
|
|
$
|
- 0
-
|
|
|
$
|
- 0
-
|
|
See
Accountants' Report and Accompanying Notes to Financial Statements
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
CONSOLIDATED
STATEMENTS OF CASH FLOWS
FOR
THE YEARS ENDED JULY 31, 2009 AND 2008
Supplemental
Disclosure of Non-Cash Investing and Financing Activities:
|
1.
|
During
the year ended July 31, 2009, convertible debentures in the amount of
$500,706 were converted into 3,070,921,617 shares of Common
Stock.
|
|
2.
|
230,065,339
shares of Common Stock was issued for services rendered in the year ended
July 31, 2009. The amount was
$551,372.
|
|
3.
|
During
the year ended July 31, 2009, two officer/stockholders donated 16,112,500
shares of the Company stock they owned to the Company. This
transaction has no effect on cash.
|
|
4.
|
During
the year ended July 31, 2009, two officers were issued Preferred Stock in
lieu of salaries for a value of $60,000, no cash was
expended.
|
|
5.
|
During
the year ended July 31, 2008, stock purchase warrants exercisable for
55,000,000 shares of Common Stock were issued in connection with closings
on $830,000 of convertible notes had no cash
effect.
|
|
6.
|
During
the year ended July 31, 2008, 40,682,142 shares of Common Stock was issued
for services rendered. The amount was
$95,082.
|
|
7.
|
During
the year ended July 31, 2008, convertible debentures in the amount of
$241,590 were converted into 300,274,100 shares of Common
Stock.
|
|
8.
|
During
the year ended July 31, 2008, the Company acquired software for cameras in
the amount of $300,000, which was not paid for, as such, has no effect on
cash in this period.
|
|
9.
|
During
the year ended July 31, 2009 and 2008, delinquent registration penalties
of $454,600 and $400,200, respectively, were recorded, no cash was
expended.
|
See
Accountants' Report and Accompanying Notes to Financial Statements
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
1 Summary
of Significant Accounting Policies and Organization
|
|
a)
|
Organization and Recent Company
History
|
Wellstar
International, Inc. (the “Company”) was incorporated December 15, 1997, under
the laws of the State of Nevada. Through its wholly owned subsidiary,
Trillennium Medical Imaging, Inc. (“TMI”), is developing and licensing the use
of advanced thermal imaging technology.
|
|
b)
|
Principles of
Consolidation
|
The
consolidated financial statements include the accounts of Wellstar
International, Inc. and its wholly owned subsidiary, Trillennium Medical
Imaging, Inc. (collectively, the “Company”).
The
Company recognizes revenues utilizing the accrual method of
accounting. More specifically, the Company enters into licensing
agreements for its advanced thermal imaging technology. Under the
licensing agreements, the Company supplies the camera equipment, related
software and training for each facility. Once the facility is
operational, the licensing agreement provides for a fixed fee monthly fee for
the use of the camera. Accordingly, the revenue is recognized in the
month that the camera is in use at the customer’s facility, which represents the
Company’s right to receive the fixed fee. The Company’s revenue
recognition policy is in compliance with the provisions of EITF
00-21.
The
preparation of financial statements in conformity with generally accepted
accounting principles requires management to make estimates and assumptions that
affect the amounts reported in the financial statements and accompanying
notes. Although these estimates are based on management’s knowledge
of current events and actions it may undertake in the future, they may
ultimately differ from actual results.
For the
purpose of the Statements of Cash Flows, cash is defined as balances held in
corporate checking accounts and money market accounts.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
1 Summary
of Significant Accounting Policies and Organization
(cont’d)
Basic and
diluted net loss per common share for the years ended July 31, 2009 and 2008 are
computed based upon the weighted average number of common shares
outstanding. The assumed conversion of Common Stock equivalents was
not included in the computation of diluted loss per share because the assumed
conversion and exercise would be anti-dilutive due to the net loss
incurred. Based on the conversion formula in the Agreements (see Note
2, 3 and 4) on the conversion of its convertible notes would have resulted in
the issuance of additional common shares in the amount of $164,872,020,106 on
July 31, 2009.
|
|
g)
|
Stock
Based Compensation
|
Stock
based compensation will be valued in accordance with SFAS 123(R) under the Fair
Valued based method. Compensation cost is measured at the grant date
based on the value of the award and is recognized over the service period which
is usually the vesting period. Transactions with non-employees shall
be accounted for based on the Fair Value of the consideration received or Fair
Value of the equity installments issued, whichever is more reliably
measurable.
|
|
h)
|
Derivative
Instruments
|
In
connection with the sale of debt or equity instruments, we may sell options or
warrants to purchase our Common Stock. In certain circumstances,
these options or warrants may be classified as derivative liabilities, rather
than as equity. Additionally, the debt or equity instruments may
contain embedded derivative instruments, such as conversion options, which in
certain circumstances may be required to be bifurcated from the associated host
instrument and accounted for separately as a derivative instrument
liability.
The
identification of, and accounting for, derivative instruments is
complex. Our derivative instrument liabilities are re-valued at the
end of each reporting period, with changes in the fair value of the derivative
liability recorded as charges or credits to income, in the period in which the
changes occur. For options, warrants and bifurcated conversion
options that are accounted for as derivative instrument liabilities, we
determine the fair value of these instruments using the Black -Scholes option
pricing model. That model requires assumptions related to the
remaining term of the instrument and risk-free rates of return, our current
Common Stock price and expected dividend yield, and the expected volatility of
our Common Stock price over the life of the option.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
1 Summary
of Significant Accounting Policies and Organization
(cont’d)
The
Company will provide for income taxes based on the provisions of Financial
Accounting Standards Board (“FASB”) Statements of Financial Accounting Standards
No. 109 (“SFAS No. 109"), “Accounting for Income Taxes”, which requires
recognition of deferred tax assets and liabilities for the expected future tax
consequences of events that have been included in the financial statements and
tax returns in different years. Under this method, deferred income
tax assets and liabilities are determined based on the difference between the
financial statements and tax basis of assets and liabilities using enacted tax
rates in effect for the year in which the differences are expected to
reverse.
|
|
j)
|
Concentration of Credit
Risk
|
Financial
instruments which potentially subject the Company to concentrations of credit
risk consists of a checking account with a financial institution in excess of
insured limits. There was no excess above insured limits at July 31,
2009. The Company does not anticipate non-performance by the
financial institution.
|
|
k)
|
Fair Value of Financial
Instruments
|
Carrying
amounts of certain of the Company’s financial instruments, including cash and
cash equivalents, accounts receivable and accounts payable approximate fair
value because of their short maturities.
Imaging
and office equipment are recorded at cost and depreciated on the straight line
method with an estimated life of five (5) years. Imaging equipment is
at the customers facility where the equipment is used or stored by the Company
until placed in use. The Company retains title to the imaging
equipment while it is at the customers location. Depreciation expense
for the years ended July 31, 2009 and 2008 were $198,345 and $176,493,
respectively.
Loan
acquisition costs are stated at cost and relate to the costs of acquiring the
convertible notes (see Note 2) and to obtaining the $400,000 Note Payable (see
Note 3). Amortization is provided for under the straight line method
over three (3) years, which is the term of the convertible notes and six months
for the original term of the Note Payable. Total amortization for the years
ended July 31, 2009 and 2008 were $ 45,020 and $49,198,
respectively.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
1 Summary
of Significant Accounting Policies and Organization
(cont’d)
m)
Intangible Assets
(cont’d)
Software
and manuals, Covenant Not To Compete and Manufacturing & Distribution
Agreement acquired in the acquisition of Micro Health Systems, Inc. (See Note 3)
with cost of $80,000, $20,000 and $700,000 respectively are being amortized over
a 24 month period for the software and the Covenant and 5 ½ years for the
manufacturing and distribution agreement. The total amortization expense for the
years ended July 31, 2009 and 2008 were $145,739 and $165,163,
respectively.
Estimates
of future cash flows used to test recoverability of long-lived assets shall be
based on the existing service potential of the asset or asset group at the date
it is tested. The testing will be done annually prior to July 31
st
, the
Company’s fiscal year end, but will also be tested whenever events or changes in
circumstances indicate that its carrying value may not be
recoverable.
Management,
in reviewing the value, is of the opinion that its carrying values of its
imaging equipment are not higher than fair value to sell. It also is
of the opinion that it’s exclusive Mikron Manufacture and Distribution Agreement
which is at a carrying approximately 35% of its cost less than four years ago,
is a low value. Current tests of its imaging equipment were recently
completed by a major medical facility and management is of the opinion the tests
will yield positive results to effect future revenue and cash flow.
|
|
o)
|
Derivative
Instruments
|
Because
of the limited trading history of our Common Stock, we have estimated the future
volatility of our Common Stock price based on not only the history of our stock
price but also the experience of other entities considered comparable to
us. The identification of, and accounting for, derivative instruments
and the assumptions used to value them can significantly affect our financial
statements.
p)
Registration Rights
Agreements
In
connection with the sale of debt or equity instruments, we may enter into
Registration Rights Agreements. Generally, these Agreements require
us to file registration statements with the Securities and Exchange Commission
to register common shares that may be issued on conversion of debt or preferred
stock, to permit re-sale of common shares previously sold under an exemption
from registration or to register common shares that may be issued on exercise of
outstanding options or warrants.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
1 Summary
of Significant Accounting Policies and Organization
(cont’d)
p)
Registration Rights Agreements
(cont’d)
The
Agreements usually require us to pay penalties for any time delay in filing the
required registration statements, or in the registration statements becoming
effective, beyond dates specified in the Agreement. These penalties
are usually expressed as a fixed percentage, per month, of the original amount
we received on issuance of the debt or preferred stock, common shares, options
or warrants. We account for these penalties as a contingent liability
and not as a derivative instrument. Accordingly, we recognize the
penalties when it becomes probable that they will be incurred. Any
penalties are expenses over the period to which they relate.
NOTE
2
Convertible Notes
On
September 5, 2008, The Company and AJW partners, LLC and it’s related entities
amended their agreement related to all the notes, which are convertible, into
shares of the Company’s Common Stock. The applicable percentage shall
be 25% of the computed conversion price and the interest rate, as defined in
each of the notes, shall be 13%. All other provisions, as amended
from time to time, shall remain in full force and effect.
On
October 31, 2005, the Company entered into a Securities Purchase Agreement with
AJW Partners, LLC and its related entities for the sale of $3,000,000 of 8%
(amended to 13%) secured convertible notes, each advance is evidenced by a note
which is due three years from the date of the advance, and for stock purchase
warrants exercisable for a total of 5,000,000 shares of Common Stock
each issuance of warrants expiring on the fifth anniversary from the date of
issue. The warrants are issued at the time funds are advanced at
1,666,667 per $1 million advanced. The notes are convertible, at the
holder’s option, into shares of Common Stock, in whole or in part, at any time
after the original issue date. No interest shall be due and payable
for any month in which the Company’s stock trading price is greater than $0.1125
for each trading day of the month.
The
number of shares of Common Stock issuable upon a conversion is to be determined
by dividing the outstanding principal amount of the notes to be converted, plus
related accrued interest, by the conversion price. The conversion
price in effect on any conversion date will be at the selling stockholder’s
option, at the lower of (i) $0.12 or (ii) an amended 75% discount to the average
of the three lowest intraday trading prices for the Common Stock on a principal
market for the twenty trading days preceding, but not including, the conversion
date for all notes. The total shares available for conversion at July
31, 2009 were 154,817,030,303.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
2 Convertible
Notes
(cont’d)
The stock
purchase warrants have an exercise price of $0.50 per share.
The
Company has closed on the entire $3,000,000 of convertible notes contemplated by
the Securities Purchase Agreement and issued stock purchase warrants exercisable
for 5,000,000 shares of Common Stock in connection therewith. The
dates of the advance of the funds of $1 million each were October 31, 2005 and
January 20, 2006 and $500,000 each on July 25, 2006 and August 8, 2006. The
stock registration was effective August 4, 2006. $270,500 of the $500,000 Notes
issued August 8, 2006 have been sold to JMJ Financial by the holder in an
agreement dated June 18, 2009 (See Note 4).
On
November 30, 2006, the Company entered into an additional securities purchase
agreement with AJW Partners, LLC and its related entities for the sale of
$400,000 of 8% (amended to 13%) secured convertible notes due November 30, 2009,
and for stock purchase warrants of 4,000,000 shares of Common Stock exercisable
at anytime at $.08 per share, expiring on the seventh anniversary from the date
of issue, November 30, 2013.
The funds
were advanced on November 30, 2006, in the amount of $392,500, less a $7,500
charge as a loan acquisition cost, amortized over the loan period of 36
months. The notes are convertible, at the holders option, into shares
of Common Stock, in whole or in part, at any time after the original issue
date.
No
interest shall be due on any payable for any month in which the Company’s stock
trading price is greater than $.0775 for each trading day of the
month. The notes are secured by all the assets and intellectual
property of the Company.
On March
26, 2007, the Company entered into an additional securities purchase agreement
with AJW Partners, LLC and its related entities for the sale of $165,000 of 8%
(amended to 13%) secured convertible notes due March 26, 2010, and for stock
purchase warrants of 1,000,000 shares of Common Stock exercisable at anytime at
$.03 per share, expiring on the seventh anniversary from the date of issue,
March 26, 2014.
The funds
were advanced on March 26, 2007, in the amount of $150,000, less a $15,000
charge as a loan acquisition cost, amortized over the loan period of 36
months. The notes are convertible, at the holders option, into shares
of Common Stock, in whole or in part, at any time after the original issue
date. No interest shall be due on any payable for any month in
which the Company’s stock trading price is greater than $.0775 for each trading
day of the month. The notes are secured by all the assets and
intellectual property of the Company.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
2 Convertible
Notes
(cont’d)
On May
30, 2007, the Company entered into an additional securities purchase agreement
with AJW Partners, LLC and its related entities for the sale of $435,000 of 8%
(amended to 13%) secured convertible notes due May 30, 2010, and for stock
purchase warrants of 10,000,000 shares of Common Stock exercisable at anytime at
$.02 per share, expiring on the seventh anniversary from the date of issue, May
30, 2014.
The funds
were advanced on May 30, 2007, in the amount of $415,000, less a $20,000 charge
as a loan acquisition cost, amortized over the loan period of 36
months. The notes are convertible, at the holders option, into shares
of Common Stock, in whole or in part, at any time after the original issue
date. No interest shall be due on any payable for any month in
which the Company’s stock trading price is greater than $.0775 for each trading
day of the month. The notes are secured by all the assets and
intellectual property of the Company.
On
October 12, 2007, the Company entered into an additional securities purchase
agreement with AJW Partners, LLC and its related entities for the sale of
$175,000 of 8% (amended to 13%) secured convertible notes due October 12, 2010,
and for stock purchase warrants of 15,000,000 shares of common stock exercisable
at anytime at $ .0001 per share expiring on the seventh anniversary from the
date of issue October 12, 2014.
The funds
were advanced on October 12, 2007 in the amount of $170,000, less a $5,000
charge as loan acquisition cost, amortized over the loan period of 36
months. The notes are convertible, at the holders option, into shares
of common stock, in whole or in part, at any time after the original issue
date. No interest shall be due or payable for any month in which the
Company’s stock trading price is greater than $ .0775 for each trading day of
the month. The notes are secured by all the assets and intellectual
property of the Company.
On
November 15, 2007, the Company entered into an additional securities purchase
agreement with AJW Partners, LLC and its related entities for the sale of
$325,000 of 8% (amended to 13%) secured convertible notes due November 15, 2010,
and for stock purchase warrants of 10,000,000 shares of common stock exercisable
at anytime at $ .0001 per share expiring on the seventh anniversary from the
date of issue November 15, 2014.
The funds
were advanced on November 15, 2007 in the amount of $310,000, less a $15,000
charge as loan acquisition cost, amortized over the loan period of 36
months. The notes are convertible, at the holders option, into shares
of common stock, in whole or in part, at any time after the original issue
date. No interest shall be due or payable for any month in which the
Company’s stock trading price is greater than $ .0775 for each trading day of
the month. The notes are secured by all the assets and intellectual
property of the Company.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2007 AND 2008
NOTE
2 Convertible
Notes
(cont’d)
On
December 14, 2007, the Company entered into an additional securities purchase
agreement with AJW Partners, LLC and its related entities for the sale of
$315,000 of 8% (amended to 13%) secured convertible notes due December 14, 2010,
and for stock purchase warrants of 10,000,000 shares of common stock exercisable
at anytime at $ .0001 per share expiring on the seventh anniversary from the
date of issue December 14, 2014.
The funds
were advanced on December 14, 2007 in the amount of $300,000, less a $15,000
charge as loan acquisition cost, amortized over the loan period of 36
months. The notes are convertible, at the holders option, into shares
of common stock, in whole or in part, at any time after the original issue
date. No interest shall be due or payable for any month in which the
Company’s stock trading price is greater than $ .0775 for each trading day of
the month. The notes are secured by all the assets and intellectual
property of the Company.
On
December 31, 2007, the Lender issued the Company a new note for all accrued
unpaid interest. The Lender applied all of its conversions from
convertible notes into stock to the principal of its original note issued
October 31, 2005. The Company which had been applying the conversions
to interest first then principal made this adjustment to be in agreement with
the Lender and will apply all conversion to principal beginning January 1,
2008. The Callable Secured Convertible Note dated December 31, 2007
in the amount of $427,759.61 bears interest at 2% (amended to 13%) per annum,
payable quarterly. The note is due December 31, 2010. All
of the terms are identical to the above notes, including the conversion
options.
On April
22, 2008, the Company entered into an additional securities purchase agreement
with AJW Partners, LLC and its related entities for the sale of $190,000 of 8%
(amended to 13%) secured convertible notes due April 22, 2011, and for stock
purchase warrants of 20,000,000 shares of common stock exercisable at anytime at
$ .0001 per share expiring on the seventh anniversary from the date of issue
April 22, 2015. On May 19, 2009, $76,000 of these notes were sold to
JMJ Financial by the Holder (See Note 4).
The funds
were advanced on April 22, 2008 in the amount of $185,000, less a $5,000 charge
as loan acquisition cost, amortized over the loan period of 36
months. The notes are convertible, at the holders option, into shares
of common stock, in whole or in part, at any time after the original issue
date. No interest shall be due or payable for any month in which the
Company’s stock trading price is greater than $.0775 for each trading day of the
month. The notes are secured by all the assets and intellectual
property of the Company.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
2 Convertible
Notes
(cont’d)
On June
12, 2008, the Company entered into an additional securities purchase agreement
with AJW Partners, LLC and its related entities for the sale of $135,000 of 8%
(amended to 13%) secured convertible notes due June 12, 2011.
The funds
were advanced on June 12, 2008 in the amount of $105,000, less a $20,000 charge
as loan acquisition cost, amortized over the loan period of 36
months. The notes are convertible, at the holders option, into shares
of common stock, in whole or in part, at any time after the original issue
date. No interest shall be due or payable for any month in which the
Company’s stock trading price is greater than $.0775 for each trading day of the
month. The notes are secured by all the assets and intellectual
property of the Company.
On August
29, 2008, AJW Partners, LLC and its related entities (the Lender) issued the
Company a new Note for all accrued unpaid interest from January 1, 2008 through
August 29, 2008. The accrued interest has been reclassified to a
convertible note payable. The Callable Secured Convertible Note,
dated August 29, 2008, in the amount of $235,113.84, bears interest at 2%
(amended to 13%) per annum, payable quarterly. The Note is due on
August 29, 2011. The conversion price is the average of the three (3)
lowest trading prices in the 20 days prior to conversion (before the conversion
date)
X
32.5% (amended
to
X
25%) = conversion
price. All other terms are identical with the other
Note.
On May
19, 2009, the Company entered into an additional securities purchase agreement
with AJW Partners, LLC and its related entities for the sale of $79,500 of 13%
secured convertible notes due May 15, 2012.
The funds
were advanced on May 15, 2009 in the amount of $79,500. The notes are
convertible, at the holders option, into shares of common stock, in whole or in
part, at any time after the original issue date. No interest shall be
due or payable for any month in which the Company’s stock trading price is
greater than $ .045 for each trading day of the month. The notes are
secured by all the assets and intellectual property of the Company.
On June
30, 2009, AJW Partners, LLC and its related entities (the Lender) issued the
Company a new Note for all accrued unpaid interest from August 30, 2008 through
June 30, 2009. The accrued interest has been reclassified to a convertible note
payable. The Callable Secured Convertible Note, dated June 30, 2009
in the amount of $551,564.55 bears interest at 2% per annum, payable
quarterly. The Note is due on June 30, 2012. The
conversion price is the average of the three (3) lowest trading prices in the 20
days prior to conversion (before the conversion date)
X
25% = conversion
price. All other terms are identical with the other
Note.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
2 Convertible
Notes
(cont’d)
See
Paragraph 2 of this note related to the terms of conversion. The
total shares at July 31, 2009, included in Paragraph 2 above, includes all
additional convertible notes.
All notes
include a Registration Rights Agreement. The Company was required to
register additional shares in relation to all the additional agreements listed
above, this was not done. There is a penalty of 2% per month of the
note amount, a penalty of $1,150,174 was accrued through July 31,
2009.
In
connection with the aforementioned issuance of the $1,000,000 of convertible
notes, on October 31, 2005, the Company granted a first priority security
interest in all the assets of the Company. The issuance of
convertible notes resulted in conversion features being accounted for as
embedded derivative liabilities in accordance with EITF00-19 and SFAS 133 (see
Note 5). The note holder’s have converted notes of $845,904 into
2,160,426,197 shares of Common Stock as of July 31, 2009. The balance
of the notes are $5,001,534 at July 31, 2009. Interest due of $107,429 is
included in Accrued Expenses.
The
classification as short-term and long-term derivative instrument
liabilities-convertible notes, derivative instrument liabilities warrants and
convertible debt is based upon the due date of the notes and the date the
warrants expire. Some of the notes have passed their due dates and
others are due within one year; these are shown as current liabilities, the
other are shown as long-term liabilities. The warrants are shown as
long-term as the expiration dates are over one year.
NOTE
3 Notes
Payable
|
|
a)
|
The
Company has borrowed $150,000 from an unrelated individual. The
Note is dated August 1, 2005. The outstanding balance of the
loan shall bear monetary interest at the fixed rate of six percent (6%)
simple, non-compounding interest payable in arrears per
annum.
|
The
outstanding balance of principal and interest is due and payable on demand on or
after August 1, 2006. All payments shall apply first to interest
accrued and then principal. The Company may prepay all or part
without a pre-payment penalty. The loan was not paid on August 1,
2006 and was extended under the same terms by mutual
agreement. Interest due of $36,500 is included in Accrued
Expenses.
Default
shall occur upon (1) failure to make payment on the note or transfer of stock
when due, (2) Company institutes bankruptcy or solvency proceedings or make an
assignment for the benefit of creditors.
|
Note
Payable - July 31, 2009 and 2008
|
|
$
|
150,000
|
|
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
3 Notes
Payable
(Cont’d)
|
|
b)
|
The
Company has entered into a loan agreement with an unrelated
individual. The note is dated October 11, 2005. The
note provides for a total loan of $400,000, the Company received $190,000
by October 31, 2005. The balance of $210,000 was subsequently
received on November 29, 2005. The note bears interest at a
fixed rate of 8%, plus the prevailing variable margin rate charged to the
lender. As of July 31, 2009, the margin rate was
7.625%. The lender was paid a loan acquisition cost on December
5, 2005, in Common Stock of 1 million
shares.
|
The cost
was recorded at market value at the date of the loan which was $ .12 per share,
for a total of $120,000. The outstanding balance of principal and
accrued interest was due and payable on April 11, 2006. The note has
been extended to February 28, 2007 by addendum under the current terms and
interest is being accrued. The addendum was signed on November 11,
2006. In consideration of the waiver and extension, the Company, with
the signing, paid the lender $20,000. The lender was also issued
additional warrants to purchase 400,000 shares of common stock, 200,000 at $0.10
per share and 200,000 at $0.20 per share, which expire on February 28, 2008. As
of July 31, 2009, the note has not been paid.
At July
31, 2009, $233,385 of interest expense is included in Accrued
Expenses. As security for the loan, the Company has pledged all of
its tangible and intangible assets. Commencing on January 1, 2006,
the Company shall establish an escrow account and shall deposit 25% of all
proceeds generated by the thermal imaging cameras purchased with $210,000 of
proceeds from the loan. The funds shall remain in escrow for use in
paying all sums due to the lender. To July 31, 2009, no funds have
been put into escrow.
In
addition, the lender has the option to convert the loan into fully registered,
unsecured Common Stock of the Company at a conversion price on the day of
conversion, minus 40%. The total shares at July 31, 2009 were
3,518,805,555. The lender shall have the right to convert on the
prepayment date or the due date, whichever occurs first. The issuance
of the notes and warrants resulted in conversion features being accounted for as
embedded derivative liabilities in accordance with EITF00-19 and FASB 133 (see
Note 5).
|
Balance
due at July 31, 2009 and 2008
|
|
$
|
400,000
|
|
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
3 Notes
Payable
(cont’d)
|
|
c)
|
On
December 21, 2005, the Company completed the purchase of certain assets of
Micro Health Systems, Inc. (“MHS”) under a definitive
agreement.
|
Total
consideration paid by the Company was $600,000, plus 2,000,000 shares of
Restricted Common Stock. The Company paid $400,000 at
closing. A promissory note was executed for $200,000 with interest at
8% per annum. $100,000 was due with accrued interest on or before the 180
th
day
following the date of the Note which is June 19, 2006, with the balance of
principal and interest due and payable on or before the 365
th
day
following the date of the note.
The
2,000,000 shares of Restricted Common Stock were issued on December 21, 2005 and
priced at the market price of $ .10 per share for a total value of
$200,000. The cost was allocated as follows:
|
Mikron
Manufacturing Distribution Agreement
|
|
|
|
|
Customer
List and Intangible Assets
|
|
$
|
700,000
|
|
|
Tangible
Assets
|
|
|
80,000
|
|
|
Covenant
Not-To-Compete
|
|
|
20,000
|
|
|
Total
|
|
$
|
800,000
|
|
In
addition, 1,500,000 shares of Restricted Common Stock are being held in escrow
as security for the note payable of $200,000. These shares have been
shown as issued but not outstanding. The Company is in default on
$200,000 of the Note Payable and interest of $4,000 which was due June 19, 2006
on the first $100,000 of notes due. Due to the default, the interest
charged from June 19, 2006 is 18% on the $200,000 Note
Payable. Interest expense of & 114,242 is included in Accrued
Expenses.
On
November 28, 2006, the Company received a letter due to the default, giving it
ten (10) days to pay the note and accrued interest or the 1,500,000 shares held
in escrow will be issued to the shareholder of Micro Health Systems,
Inc. As of July 31, 2009 and through October 7,
2009, nothing has transpired.
|
Balance
due at July 31, 2009 and 2008
|
|
$
|
200,000
|
|
|
|
(d)
|
The
Company has borrowed $16,912 from an unrelated party. The note
is dated January 16, 2009, and was due on February 16, 2009 (maturity
date). The note has an interest rate of 12% per annum. Per the
terms of the note, the Company is in default as it failed to pay the
principal and interest due upon the maturity date. In the event
of default, the lender, by notice given to the borrower, may declare the
unpaid principal and accrued interest owing to be paid. The
Company (borrower) has not received any demand for payment as of October
7, 2009. The note is included as a current liability in Notes
and Loans Payable - Other in the amount of $16,912 and $598 of interest is
included in accrued expenses.
|
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
4
Convertible Notes - Other
|
|
(a)
|
JMJ
Financial purchased certain convertible notes from AJW Partners and
related entities (See Note 2). JMJ Financial purchased $76,000
of Notes on May 19, 2009. The Notes have been converted to
Common in full by July 31, 2009 and no amount is due. On June
18, 2009, JMJ Financial purchased $270,500 of certain Convertible
Notes. Through July 31, 2009, $52,153 of these Notes were
converted to Common Stock. These Notes bear interest at 8% per
annum. The Company has consented to the sale of these
Notes. The Notes bear the same terms as in the hands of the
seller, AJW Partners, and related entities. $3,778 is included in Accrued
Interest at June 30, 2009.
|
|
Balance
Due at July 31, 2009
|
|
$
|
218,347
|
|
|
|
(b)
|
The
Company has entered into an agreement with JMJ Financial, the Lender, and
the Company as the Borrower. They will loan to the Company the
principal sum of $575,000 of which $75,000 has been recorded as a loan
acquisition cost and is being amortized over 36 months. The
loan has a 12% one time interest charge on the principal
sum. No interest or principal payments are required until the
maturity date three (3) years from the effective date (May 27,
2009). Both principal and interest may be included in
conversion prior to maturity date. The conversion formula, the
number of shares issued through conversion, is the conversion amount,
divided by the conversion price which is 70% of the lowest trading price
in the 20 trading days previous to the conversion, as applied to the
Company’s voting Common Stock. Prepayment of the loan is not
permitted, unless approved by
Holder.
|
|
Balance
Due at July 31, 2009
|
|
$
|
340,000
|
|
The
issuance of convertible notes resulted in conversion features being accounted
for as Embedded Derivative Liabilities in accordance with EITF00-19 and SFAS 133
(See Note 5). Total shares available for conversion on all Notes A
& B above, are 6,536,184,248 at July 31, 2009.
NOTE
5 Derivative
Financial Instrument Liabilities
We use
the Black-Scholes option pricing model to value options and warrants, and the
embedded conversion option components of any bifurcated embedded derivative
instruments that are recorded as derivative liabilities. See Note 1,
related to embedded derivative instruments accounting policy.
In
valuing the options and warrants and the embedded conversion option components
of the bifurcated embedded derivative instruments, at the time they were issued
and at July 31, 2009, we used the market price of our Common Stock on the date
of valuation, an expected dividend yield of 0% and the remaining period to the
expiration date of the options or warrants or repayment date of the convertible
debt instrument. All options, warrants and conversion options can be
exercised by the holder at any time.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
5 Derivative
Financial Instrument Liabilities (cont’d)
Because
of the limited historical trading period of our Common Stock, the expected
volatility of our Common Stock over the remaining life of the options and
warrants has been estimated at 123%, based on a review of the historical
volatility and of entities considered by management as
comparable. The risk-free rates of return used ranged from 0.03% to
0.15%, based on constant maturity rates published by the U.S. Federal Reserve,
applicable to the remaining life of the options or warrants.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
5 Derivative
Financial Instrument Liabilities
(cont’d)
At July
31, 2009, the following derivative liabilities related to Common Stock options
and warrants and embedded derivative instruments were outstanding (see Notes 2,
3 and 4):
|
Issue
|
|
|
|
No. of
|
|
|
|
|
|
|
Value - Issue
|
|
|
Value - July 31,
|
|
|
Date
|
|
Expiry
Date
|
|
Warrants
|
|
Issued
To
|
|
Share
|
|
|
Date
|
|
|
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10/11/05
|
|
04/11/06
|
|
|
1,000,000
|
|
Thompson
|
|
$
|
.50
|
|
|
$
|
41,526
|
|
|
$
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11/19/06
|
|
02/18/08
|
|
|
200,000
|
|
Thompson
|
|
$
|
.10
|
|
|
|
3,845
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11/19/06
|
|
02/18/08
|
|
|
200,000
|
|
Thompson
|
|
$
|
.20
|
|
|
|
2,276
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10/31/05
|
|
10/31/10
|
|
|
1,666,667
|
|
AJW
Partners
|
|
$
|
.50
|
|
|
|
169,629
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
01/20/06
|
|
01/20/11
|
|
|
1,666,667
|
|
AJW
Partners
|
|
$
|
.50
|
|
|
|
81,321
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
07/25/06
|
|
07/25/11
|
|
|
833,333
|
|
AJW
Partners
|
|
$
|
.50
|
|
|
|
146,197
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
08/04/06
|
|
08/04/11
|
|
|
833,333
|
|
AJW
Partners
|
|
$
|
.50
|
|
|
|
102,816
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11/30/06
|
|
11/30/13
|
|
|
4,000,000
|
|
AJW
Partners
|
|
$
|
.08
|
|
|
|
158,741
|
|
|
|
134
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
03/26/07
|
|
03/26/14
|
|
|
1,000,000
|
|
AJW
Partners
|
|
$
|
.03
|
|
|
|
25,433
|
|
|
|
69
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
05/30/07
|
|
05/30/14
|
|
|
10,000,000
|
|
AJW
Partners
|
|
$
|
.02
|
|
|
|
163,409
|
|
|
|
885
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10/12/07
|
|
10/12/14
|
|
|
15,000,000
|
|
AJW
Partners
|
|
$
|
.0001
|
|
|
|
179,353
|
|
|
|
4,104
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11/15/07
|
|
11/15/14
|
|
|
10,000,000
|
|
AJW
Partners
|
|
$
|
.0001
|
|
|
|
39,649
|
|
|
|
2,741
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12/14/07
|
|
12/14/14
|
|
|
10,000,000
|
|
AJW
Partners
|
|
$
|
.0001
|
|
|
|
24,000
|
|
|
|
2,748
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
04/22/08
|
|
04/22/15
|
|
|
20,000,000
|
|
AJW
Partners
|
|
$
|
.0001
|
|
|
|
17,540
|
|
|
|
4,692
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair
value of derivative instrument liabilities for warrants
|
|
|
$
|
1,155,735
|
|
|
$
|
15,373
|
|
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
5 Derivative
Financial Instrument Liabilities
(cont’d)
|
|
|
|
|
|
|
|
|
Exercise
|
|
|
|
|
|
|
|
Issue
|
|
Due
|
|
Note
|
|
|
|
Price Per
|
|
Value - Issue
|
|
|
Value - July
|
|
|
Date
|
|
Date
|
|
Amount
|
|
Instrument
|
|
Share
|
|
Date
|
|
|
31,
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10/11/05
|
|
04/11/06
|
|
$
|
400,000
|
|
Loan
|
|
Various
|
|
$
|
370,189
|
|
|
$
|
422,243
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10/31/05
|
|
10/31/08
|
|
|
1,000,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
2,681,204
|
|
|
|
1,248,182
|
|
|
01/20/06
|
|
01/20/09
|
|
|
1,000,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
1,363,058
|
|
|
|
8,100,000
|
|
|
07/25/06
|
|
07/25/09
|
|
|
500,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
791,994
|
|
|
|
4,050,000
|
|
|
08/04/06
|
|
08/04/09
|
|
|
500,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
616,127
|
|
|
|
1,836,002
|
|
|
11/30/06
|
|
11/30/09
|
|
|
400,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
523,047
|
|
|
|
2,720,216
|
|
|
03/26/07
|
|
03/26/10
|
|
|
165,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
274,500
|
|
|
|
264,443
|
|
|
05/30/07
|
|
05/30/10
|
|
|
435,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
825,801
|
|
|
|
3,106,831
|
|
|
10/12/07
|
|
10/12/10
|
|
|
175,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
711,289
|
|
|
|
1,413,319
|
|
|
11/15/07
|
|
11/15/10
|
|
|
325,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
465,052
|
|
|
|
2,628,300
|
|
|
12/14/07
|
|
12/14/07
|
|
|
315,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
631,254
|
|
|
|
2,552,282
|
|
|
12/31/07
|
|
12/31/10
|
|
|
427,760
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
894,835
|
|
|
|
3,468,288
|
|
|
04/22/08
|
|
04/22/11
|
|
|
190,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
569,394
|
|
|
|
929,704
|
|
|
06/12/08
|
|
06/12/11
|
|
|
135,000
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
555,374
|
|
|
|
1,103,956
|
|
|
08/29/08
|
|
08/29/11
|
|
|
235,114
|
|
Convertible
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
Various
|
|
|
875,919
|
|
|
|
1,930,721
|
|
|
Fair
value of bifurcated embedded derivative instrument liabilities carry
forward
|
|
$
|
12,149,037
|
|
|
$
|
35,774,487
|
|
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
5 Derivative
Financial Instrument Liabilities
(cont’d)
|
|
|
|
|
|
|
|
|
|
Exercise
|
|
|
|
|
|
|
|
|
|
Issue
|
|
Due
|
|
Note
|
|
|
Price Per
|
|
Value -
Issue
|
|
|
Value - July
|
|
|
|
|
Date
|
|
Date
|
|
Amount
|
|
Instrument
|
Share
|
|
Date
|
|
|
|
31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carry
Forward
|
|
|
|
|
|
|
|
|
|
|
$
|
12,149,037
|
|
|
$
|
35,774,487
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
05/15/09
|
|
05/15/12
|
|
$
|
79,500
|
|
Convertible
|
Various
|
|
|
79,500
|
|
|
|
661,611
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
|
|
|
|
|
|
|
|
|
|
|
06/30/09
|
|
06/30/12
|
|
|
551,565
|
|
Convertible
|
Various
|
|
|
551,565
|
|
|
|
4,600,263
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
|
|
|
|
|
|
|
|
|
|
|
08/04/06
|
|
08/04/09
|
|
|
270,500
|
|
Convertible
|
Various
|
|
|
270,500
|
|
|
|
1,746,573
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
|
|
|
|
|
|
|
|
|
|
|
05/27/09
|
|
05/27/12
|
|
|
340,000
|
|
Convertible
|
Various
|
|
|
340,000
|
|
|
|
1,276,072
|
|
|
|
|
|
|
|
|
|
|
|
Notes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair
value of bifurcated embedded derivative instrument
liabilities
|
|
$
|
13,390,602
|
|
|
$
|
44,059,006
|
|
|
|
|
Total
derivative financial instruments
|
|
$
|
14,546,337
|
|
|
$
|
44,074,379
|
|
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
6
Accrued Expenses
The
following are the components of Accrued Expenses:
|
|
|
July 31, 2009
|
|
|
July 31, 2008
|
|
|
|
|
|
|
|
|
|
|
Penalties
- Registrations
|
|
$
|
1,150,174
|
|
|
$
|
695,574
|
|
|
Interest
on Debt
|
|
|
495,313
|
|
|
|
512,488
|
|
|
Payroll
and Payroll Taxes
|
|
|
1,222,486
|
|
|
|
682,133
|
|
|
Professional
Fees
|
|
|
10,000
|
|
|
|
24,530
|
|
|
Accrued
Trade Payables
|
|
|
1,754
|
|
|
|
1,200
|
|
|
|
|
$
|
2,879,727
|
|
|
$
|
1,915,925
|
|
NOTE
7
Stockholders’ Equity (Deficit)
During
the year ended July 31, 2009, the Company issued the following shares of
restricted common stock for services rendered; for Public Relations/Marketing
services 70,900,000 shares, at market value, for computer software design
3,500,000 shares at 70% of market value and for unreimbursed expenses 8,050,000
shares at 70% of market value, for legal fees 5,726,458 and financial consulting
4,000,000 shares at market value and medical consulting 138,888,889 shares at
60% of market value. The total was recorded as common stock $230,065
and additional paid-in capital of $321,307. The total of $551,372 is
reflected as an expense in the Statement of Operations.
On May
19, 2009, by written consent of the Stockholder holding a majority of the
outstanding common stock of the Company, the following was
resolved:
|
|
1.
|
The
prior increase in authorized shares from 200,000,000 to 1,700,000,000 was
ratified and confirmed.
|
|
|
2.
|
The
Certificate of Amendment to the Certificate of Incorporation increasing
the corporation’s authorized share of common stock from 1,700,000,000 to
10,000,000,000 (10 billion) is hereby authorized, ratified and
approved.
|
|
|
3.
|
The
Prior adoption of 10,000,000 shares of blank check preferred stock is
hereby ratified and confirmed, the shares of preferred at $0.001 par value
Series A Preferred Stock.
|
On May
20, 2009, with the unanimous written consent of the Directors, Chief Executive
Officer John Antonio, and the Vice President Ken McCopper, each converted
$30,000 of accrued salary owed to them into shares of the Company’s Series A
Preferred Stock. Each received 30,000 shares of Series A Preferred
$0.001 par value.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
8 Derivative
Instruments Income, Net
Derivative
instruments expense of $38,420,029 and $2,471,101 represents the net unrealized
(non-cash) change during the years ended July 31, 2009 and July 31, 2008,
respectively, in the fair value of our derivative instrument liabilities related
to certain warrants and embedded derivatives in our convertible debt that have
been bifurcated and accounted for separately.
NOTE
9 Going
Concern
The
accompanying consolidated financial statements have been prepared on a going
concern basis, which contemplates the realization of assets and the settlement
of liabilities and commitments in the normal course of business.
As
reflected in the accompanying consolidated financial statements, the Company had
a net loss of $41,832,521 and a negative cash flow from operations of $654,584
for the year ended July 31, 2009, negative working capital of $30,631,304, and a
stockholders’ deficiency of $53,131,962 at July 31, 2009.
The
ability of the Company to continue as a going concern is dependent on the
Company’s ability to raise additional funds and implement its business
plan. The accompanying consolidated financial statements do not
include any adjustments that might be necessary if the Company is unable to
continue as a going concern.
Management’s
plans include the raising of additional capital through private or public
transactions and implementation of its business and marketing plan to increase
revenues.
NOTE
10 Employee
Compensation Plan
In
December 2006, the Company filed a Form S-8 to register 5,000,000 shares of
common stock with a proposed offering price of $ .075 per share related to the
formation of the Wellstar International, Inc. 2006 Employee Compensation
Plan. In August 2007, the Company filed a Form S-8 and registered
8,500,000 shares of Common Stock with a projected offering price of $ .013 per
share related to the formation of the 2007 Employee Compensation
Plan. In August 2008, the Company filed Form S-8 and registered
47,000,000 shares of Common Stock at a proposed maximum offering price of $ .019
per share, issuable pursuant to the Company’s 2008 Compensation Plan, dated
August 7, 2008.
To date,
no shares have been issued under either plan.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
11 Compensated
Absences
There has
been no liability accrued for compensated absences as the Company does not meet
the conditions of SFAS43, Paragraph 6, for accrual. The Company only
has four employees and three officers. There are no retirement plans
and no plans related to future absences.
NOTE
12 Recently
Issued Accounting Pronouncements
In
September 2006, the Financial Accounting Standards Board (FASB) issued SFAS No.
157, “Fair Value Measurements”. SFAS No. 157 provides a new single
authoritative definition of fair value and enhanced guidance on measuring the
fair value of assets and liabilities. It requires additional
disclosures related to the extent to which companies measure assets and
liabilities at fair value, the information used to measure fair value, and the
effect of fair value measurements on earnings. SFAS No. 157 is
effective for fiscal years beginning after November 15, 2007, and interim
periods within those fiscal years. On August 1, 2008, the Company
adopted SFAS 157, which did not have a material impact on its financial
statements.
EITF
Topic D-98, Classification and Measurement of Redeemable Securities, on March
15, 2007, the SEC staff announced its position that requires preferred
securities that are redeemable for cash or other assets to be classified outside
of permanent equity if the stock is: (1) redeemable at a fixed or determinable
price and date, (2) at the option of the holder and (3) conditions outside the
control of the issuer. The pronouncement currently has no effect on
the Company.
On
December 21, 2006, the Financial Accounting Standards Board (FASB) posted FASB
Staff Position (FSP) FSPEIFT00-19-2, Accounting for Registration Payment
Arrangements.
The FSP
specifies that the contingent obligation to make future payments or otherwise
transfer consideration under a registration payment arrangement, whether issued
as a separate agreement or included as a provision of a financial instrument or
other agreement, should be separately recognized and measured in accordance with
FASB Statement No. 5, Accounting for Contingencies. The guidance in
this FSP amends FASB Statement No. 133, Accounting for Derivative Instruments
and Hedging Activities, and No. 150, Accounting for Certain Guarantor’s
Accounting and Disclosure Requirements for Guarantees, including Indirect
Guarantees of Indebtedness of Others, to include scope exceptions for
registration payment arrangements.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
12 Recently
Issued Accounting Pronouncements
(Cont’d)
This FSP
further clarifies that a financial instrument subject to a registration payment
arrangement should be accounted for in accordance with other applicable
generally accepted accounting principles (GAAP) without regard to the contingent
obligation to transfer consideration pursuant to the registration payment
arrangement. The Company has accounted for registration payments as required
under its securities purchase agreement and will follow this pronouncement
effect from date of issue.
In
February 2007, the FASB issued SFAS No. 159
The Fair Value Option for Financial
Assets and Financial Liabilities
(“SFAS 159"). SFAS 159
expands the use of fair value accounting but does not affect existing standards
that require assets or liabilities to be carried at fair value. Under
SFAS 159, a company may elect to use fair value to measure accounts and loans
receivable, available-for-sale and held-to-maturity securities, accounts payable
and issued debt. If the use of fair value is elected, any up-front
costs and fees related to the item must be recognized in earnings and cannot be
deferred. The fair value election is irrevocable and generally made
on an instrument-by-instrument basis, even if a company has similar instruments
that it elects not to measure based on fair value. At the adoption
date, unrealized gains and losses on existing items for which fair value has
been elected are reported as a cumulative adjustment to beginning retained
earnings. Subsequent to the adoption of SFAS 159, changes in fair
value are recognized in earnings.
SFAS 159
is effective for fiscal years beginning after November 15, 2007. SFAS
159 was adopted as of August 1, 2008, and did not have a material impact on the
Company’s financial statements.
In
December 2007, the FASB issued SFAS No. 141 (revised 2007),
Business Combinations
(“SFAS
141R”), which replaces FASB No. 141 and SFAS 160,
Noncontrolling Interests in
Consolidated Financial Statements - an amendment of AFB No. 51,
(“SFAS
160").
SFAS 141R
establishes principles and requirements for how an acquirer recognizes and
measures in its financial statement s the identifiable assets acquired, the
liabilities assumed, any noncontrolling interest in the acquiree and the
goodwill acquired. The Statement also established disclosure
requirements that will easily enable users to evaluate the nature and financial
effects of the business combination. SFAS 160 will change the
accounting and reporting for minority interests, reporting them as equity
separate from the parent entity’s equity, as well as requiring expanded
disclosures.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
12 Recently
Issued Accounting Pronouncements
(Cont’d)
SFAS 141R
and SFAS 160 are effective as of the beginning of an entity’s fiscal year
beginning after December 15, 2008. We have not yet determined the
impact of these pronouncements will have on our results of operations and
financial position.
In March
2008, the FASB issued SFAS No. 161,
Disclosures about Derivative
Instruments and Hedging Activities - an amendment of FASB Statement No.
133
(SFAS No. 161). The standard requires additional
quantitative disclosures (provided in tabular form) and qualitative disclosures
for derivative instruments. The required disclosures include how
derivative instruments and related hedged items affect an entity’s financial
position, financial performance, and cash flows; relative volume of derivative
activity; the objectives and strategies for using derivative instruments; the
accounting treatment for those derivative instruments formally designated as the
hedging instrument in a hedge relationship; and the existence and nature of
credit-related contingent features for derivatives. SFAS No. 161 does
not change the accounting treatment for derivative instruments. SFAS
No 161 is effective for the Company beginning August 1, 2009. The
Company does not expect the adoption of SFAS No. 161 to have a material impact
on their financial condition, results of operations or cash flows.
In May
2008, the FASB released FSP APB 14-1
Accounting For Convertible Debt
Instruments That May Be Settled In Cash Upon Conversion (Including Partial Cash
Settlement)
(FSP APB 14-1) that alters the accounting treatment for
convertible debt instruments that allow for either mandatory or optional cash
settlements.
FSP APB
14-1 specifies that issuers of such instruments should separately account for
the liability and equity components in a manner that will reflect the entity’s
nonconvertible debt borrowing rate when interest cost is recognized in
subsequent periods. This FSP is effective for financial statements issued for
fiscal years beginning after December 15, 2008. The Company is
currently evaluating the materiality of the impact the adoption of FSP APB 14-1
will have on the Company’s consolidated results of operations and financial
conditions.
NOTE
13 Lease
Agreement
On July
17, 2007, Trillenium Medical Imaging, Inc., a wholly owned subsidiary, entered
into a lease agreement with an unrelated party for a facility in New York
City. The lease replaced a prior lease in the same
facility. The lease is for a period of one year with a monthly rent
of $4,435. The lease expired July 16, 2009 and was not
renewed. The Company incurred a rent expense of approximately $55,391
and $50,025 for the years ended July 31, 2009 and 2008,
respectively.
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
14 Subsequent
Events
AJW
Partners, LLC and related entities converted a portion of their notes (See Note
2) into 1,542,669,800 shares of Common Stock during the period August 1, 2009
through October 2, 2009. During the same period, JMJ Financial
converted a portion of their notes in 3,315,500,000 shares of Common Stock (see
Note 4).
The
following resolutions were duly adopted by the Board of Directors of the
Corporation by unanimous written consent on September 10, 2009:
WHEREAS,
the Board of Directors is authorized within the limitations and restrictions
stated in the Articles of Incorporation of the Corporation, to provide by
resolution or resolutions for the issuance of 10,000,000 shares of Preferred
Stock of the Corporation, in such series and with such designations and such
powers, preferences, rights, qualifications, limitations and restrictions
thereof as the Corporation’s Board of Directors shall fix by resolution or
resolutions providing for the issuance thereof duly adopted by the Board of
Directors; and
WHEREAS,
it is the desire of the Board of Directors of the Corporation, pursuant to its
authority as aforesaid, to designate the terms of the Series B Preferred Stock
and the number of shares constituting such series;
NOW,
THEREFORE, BE IT RESOLVED, that the certificate of designation for the Series B
Preferred Stock of the Corporation be amended and restated in its entirety as
follows:
|
|
1.
|
Designation and
Authorized Shares
. The Corporation shall be authorized
to issue 1,000,000 shares of Series B Preferred Stock, par value $.001 per
share (the “
Series B Preferred
Stock
”).
|
|
|
2.
|
Stated
Value
. The stated value of each issued share of Series B
Preferred Stock shall be deemed to be $1.00 (the “
Stated
Value
”).
|
|
|
3.
|
Voting
. Except
as otherwise expressly required by law, each holder of Series B Preferred
Stock shall be entitled to vote on all matters submitted to shareholder of
the Corporation and shall be entitled to one hundred (100) votes for each
share of Common Stock that each holder is entitled to receive upon
conversion of the Series B Preferred Stock in full at the record date for
the determination of shareholders entitled to vote on such matter or, if
no such record date is established, at the date such vote is taken or any
written consent of shareholders is solicited. The holders of
shares of Series B Preferred Stock shall vote together with the holders of
Common Stock on all matters.
|
WELLSTAR
INTERNATIONAL, INC. AND SUBSIDIARY
NOTES
TO CONSOLIDATED FINANCIAL STATEMENTS
AS
OF JULY 31, 2009 AND 2008
NOTE
14 Subsequent
Events
|
|
4.
|
Liquidation
.
The holders of Series B Preferred Stock shall not be entitled to receive
any preference upon the liquidation, dissolution or winding up of the
business of the Corporation, whether voluntary or involuntary, each holder
of Series B Preferred Stock shall share ratably with the holders of the
common stock of the Corporation.
|
|
|
5.
|
Conversion
. The
holder of Series B Preferred Stock shall have the following conversion
rights (the “
Conversion
Rights
”):
|
|
|
5.1
|
Right to
Convert
. Each share of Series B Preferred Stock shall be
convertible at the option of the Holder thereof, at any time and from time
to time from and after the Original Issue Date into that number of shares
of Common Stock determined by dividing the Stated Value by the Conversion
Price. For purposes of this Section, the conversion price for
the Series B Preferred Stock shall equal $0.001 (the “
Conversion
Price
”).
|
On
September 10, 2009, with the unanimous written consent of the Directors, the
Chief Executive Officer John Antonio and the Vice President Ken McCopper, each
converted $50,000 of accrued salary owed to them into shares of the Company’s
Series B Preferred Stock. Each received 50,000 shares of Series B
Preferred Stock, par value $.001 per share, $1.00 stated value.
On
October 1, 2009, with the unanimous written consent of the Directors, the Chief
Executive Officer John Antonio and the Vice President Ken McCopper, each
converted $50,000 of accrued salary owed to them into shares of the Company’s
Series B Preferred Stock. Each received 50,000 shares of Series B
Preferred Stock, par value $.001 per share, $1.00 stated value.
On
October 2, 2009, the Company amended its Certificate of Incorporation to
increase its authorized shares of common stock from 10 billion to 20
billion. The increase amendment was approved by the Board of
Directors as well as the Shareholders holding a majority of the issued and
outstanding voting shares of the Company.
The
Company entered into an additional agreement to borrow up to $1,150000 from JMJ
Financial. The interest rate will be 12% one time interest charge on
the principal sum. No interest or principal payments are required
until the maturity date, but both principal and interest may be included in the
conversion prior to maturity. Maturity is three (3) years from the
effective date. The loan is convertible into voting common stock of
the Company. The conversion formula, the number of shares issued
through conversion is the conversion amount, divided by the conversion price
which is 70% of the lowest trading price in the 20 trading days previous to the
conversion. Prepayment of the loan is not permitted, unless approved
by holder.
Wellstar (CE) (USOTC:WLSI)
Historical Stock Chart
From Apr 2024 to May 2024
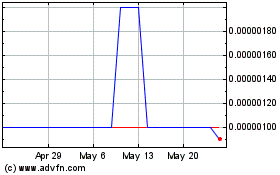
Wellstar (CE) (USOTC:WLSI)
Historical Stock Chart
From May 2023 to May 2024