Q10001527599--12-31false0001527599us-gaap:RetainedEarningsMember2024-03-310001527599us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-3100015275992023-09-272023-09-270001527599sybx:UnderwrittenPublicOfferingMembersybx:CommonStockAndWarrantsMember2023-10-030001527599sybx:PurchaseWarrantsMember2023-01-012023-03-310001527599us-gaap:WarrantMember2023-01-012023-12-310001527599us-gaap:AdditionalPaidInCapitalMember2022-12-3100015275992022-12-310001527599sybx:AtTheMarketMembersybx:JefferiesLLCMember2021-07-012021-07-310001527599sybx:UnvestedRestrictedStockAwardsMember2024-01-012024-03-310001527599sybx:RocheCollaborationAndOptionAgreementMember2024-01-012024-03-310001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:WarrantMember2024-01-012024-03-310001527599us-gaap:CommercialPaperMember2024-03-310001527599sybx:USGovernmentAgenciesDebtSecuritiesAndTreasuriesMember2023-12-310001527599us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-310001527599us-gaap:EmployeeStockOptionMember2024-01-012024-03-310001527599us-gaap:RetainedEarningsMember2023-12-310001527599sybx:LabEquipmentMember2024-01-012024-03-310001527599sybx:PreFundedWarrantsMemberus-gaap:CommonStockMember2024-01-012024-03-310001527599us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-03-3100015275992024-03-310001527599us-gaap:AdditionalPaidInCapitalMember2023-12-310001527599us-gaap:CommonStockMember2023-01-012023-03-310001527599sybx:UnderwrittenPublicOfferingMembersybx:CommonStockAndWarrantsMembersybx:October2023FinancingMember2023-10-030001527599us-gaap:EmployeeStockOptionMember2023-01-012023-03-310001527599sybx:GinkgoBioworksIncMember2019-06-300001527599sybx:AtTheMarketMembersybx:JefferiesLLCMember2024-01-012024-03-310001527599sybx:USTreasuryAndGovernmentLongTermDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599sybx:GinkgoBioworksIncMember2024-03-310001527599sybx:OptionsExercisableToPurchaseCommonStockMember2024-03-310001527599sybx:USTreasuryAndGovernmentLongTermDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:RetainedEarningsMember2024-01-012024-03-3100015275992023-12-310001527599sybx:PreFundedWarrantsMember2019-06-300001527599us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599sybx:October2023FinancingMember2024-01-012024-03-310001527599us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599sybx:AtTheMarketOfferingMember2024-01-012024-03-310001527599us-gaap:CommonStockMember2019-06-012019-06-300001527599us-gaap:AdditionalPaidInCapitalMember2024-03-310001527599us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:RetainedEarningsMember2023-03-310001527599us-gaap:CommercialPaperMember2023-12-310001527599us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599sybx:October2023FinancingMember2023-10-032023-10-030001527599us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:EmployeeStockMember2023-01-012023-03-310001527599sybx:UnderwrittenPublicOfferingMemberus-gaap:CommonStockMember2023-10-030001527599sybx:LongTermIncentivePlan2015Member2024-01-012024-01-0100015275992024-01-012024-03-310001527599sybx:RestructuringChargesExpenseAccelerationMember2024-01-012024-03-310001527599us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:MoneyMarketFundsMember2023-12-310001527599us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-310001527599sybx:UnderwrittenPublicOfferingMemberus-gaap:CommonStockMember2023-10-032023-10-030001527599us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599sybx:GinkgoBioworksIncMembersrt:MaximumMembersybx:PreFundedWarrantsMember2019-06-300001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:CommonStockMember2023-12-310001527599sybx:RocheCollaborationAndOptionAgreementMember2023-01-012023-12-310001527599sybx:PurchaseWarrantsMember2024-03-310001527599us-gaap:CommonStockMember2024-03-310001527599sybx:AtTheMarketOfferingMember2023-01-012023-03-310001527599us-gaap:RestrictedStockMember2024-01-012024-03-310001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:EmployeeStockOptionMember2023-01-012023-03-310001527599us-gaap:RetainedEarningsMember2022-12-310001527599sybx:GinkgoBioworksIncMembersybx:PreFundedWarrantsMember2019-06-300001527599us-gaap:TreasuryStockCommonMember2022-12-310001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:PreferredStockMember2024-01-012024-03-310001527599us-gaap:EmployeeStockMember2024-01-012024-01-0100015275992023-10-030001527599us-gaap:EmployeeStockOptionMember2024-01-012024-03-310001527599sybx:USTreasuryAndGovernmentLongTermDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMember2024-03-310001527599us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-03-310001527599us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001527599sybx:PurchaseWarrantsMember2024-01-012024-03-310001527599us-gaap:RestrictedStockMember2024-03-310001527599us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:StockCompensationPlanMember2024-01-012024-03-310001527599us-gaap:RestrictedStockMember2023-01-012023-03-310001527599us-gaap:WarrantMembersybx:October2023FinancingMember2024-03-310001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMember2023-12-3100015275992024-05-070001527599us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:EmployeeStockMember2024-01-012024-03-310001527599sybx:October2023FinancingMembersrt:MinimumMember2023-10-032023-10-0300015275992023-09-270001527599us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001527599sybx:UnderwrittenPublicOfferingMembersybx:CommonStockAndWarrantsMembersybx:October2023FinancingMember2023-10-032023-10-030001527599sybx:USTreasuryAndGovernmentLongTermDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:TreasuryStockCommonMember2023-12-310001527599sybx:GinkgoBioworksIncMembersybx:PreFundedWarrantsMember2024-03-310001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:CommonStockMember2023-03-310001527599sybx:RestructuringChargesExpenseAccelerationMember2023-01-012023-03-310001527599us-gaap:RetainedEarningsMember2023-01-012023-03-310001527599sybx:TwoThousandSeventeenAndTwoThousandFifteenEquityIncentiveAwardPlanMember2024-03-310001527599us-gaap:CommonStockMember2022-12-310001527599us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599sybx:GinkgoBioworksIncMemberus-gaap:CommonStockMember2019-06-012019-06-300001527599us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001527599sybx:UnderwrittenPublicOfferingMembersybx:CommonStockAndWarrantsMember2023-10-032023-10-030001527599us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:MoneyMarketFundsMember2024-03-310001527599sybx:PreFundedWarrantsMember2024-03-310001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599sybx:PreFundedWarrantsMember2023-01-012023-03-310001527599us-gaap:CommonStockMember2024-01-012024-03-310001527599us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599sybx:GinkgoBioworksIncMember2019-06-012019-06-300001527599us-gaap:StockCompensationPlanMember2023-01-012023-03-310001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-03-310001527599sybx:PreFundedWarrantsMember2024-01-012024-03-310001527599sybx:UnvestedRestrictedStockAwardsMember2023-01-012023-03-310001527599sybx:GinkgoBioworksIncMembersybx:PreFundedWarrantsMember2023-09-300001527599us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310001527599us-gaap:AdditionalPaidInCapitalMember2023-03-3100015275992023-01-012023-03-310001527599us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:EmployeeStockMember2024-03-310001527599us-gaap:WarrantMember2024-03-310001527599srt:MaximumMembersybx:October2023FinancingMember2023-10-032023-10-030001527599us-gaap:WarrantMember2023-12-310001527599us-gaap:TreasuryStockCommonMember2023-03-310001527599us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:CommercialPaperMemberus-gaap:CashAndCashEquivalentsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001527599us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-3100015275992023-03-310001527599us-gaap:GeneralAndAdministrativeExpenseMember2023-01-012023-03-310001527599us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-03-310001527599us-gaap:TreasuryStockCommonMember2024-03-310001527599us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2024-03-31xbrli:puresybx:Securityxbrli:sharessybx:Investmentiso4217:USD
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
|
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2024
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number 001-37566
SYNLOGIC, INC.
(Exact name of Registrant as specified in its Charter)
|
|
|
Delaware (State or other jurisdiction of incorporation or organization) |
|
26-1824804 (I.R.S. Employer Identification No.) |
|
|
|
301 Binney St., Suite 402 Cambridge, MA (Address of principal executive offices) |
|
02142 (Zip Code) |
(617) 401-9975 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: |
|
|
|
Title of each class |
Trading Symbol |
Name of exchange on which registered |
Common Stock, par value $0.001 per share |
SYBX |
The Nasdaq Capital Market |
Preferred Stock Purchase Rights |
N/A |
The Nasdaq Capital Market |
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b–2 of the Exchange Act.
|
|
|
|
|
Large accelerated filer |
☐ |
|
Accelerated filer |
☐ |
Non-accelerated filer |
☒ |
|
Smaller reporting company |
☒ |
|
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of May 7, 2024, there were 11,707,011 shares of the registrant’s common stock, par value $0.001 per share, outstanding.
FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking statements that involve risks and uncertainties. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. All statements other than statements of historical facts contained herein are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue” or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about:
•our evaluation of strategic alternatives with a goal to enhance stockholder value, including the possibility of a merger or a sale of the Company;
•the success of our research and development efforts;
•the initiation, progress, timing, costs and results of clinical trials for our product candidates;
•the time and costs involved in obtaining regulatory approvals for our product candidates;
•the success of our collaborations with third parties;
•the progress, timing and costs involved in developing manufacturing processes and in manufacturing products, as well as agreements with third-party manufacturers;
•the rate of progress and cost of our commercialization activities;
•the expenses we incur in marketing and selling our product candidates, if approved;
•the revenue generated by sales of our product candidates, if approved;
•the emergence of competing or complementary technological developments;
•the terms and timing of any additional collaborative, licensing or other arrangements that we may establish;
•the acquisition of businesses, products and technologies;
•our need to implement additional infrastructure and internal systems;
•our need to add personnel and financial and management information systems to support our product development and potential future commercialization efforts, and to enable us to operate as a public company;
•the extent to which our business is adversely impacted by the effects of the coronavirus outbreak (COVID-19) or by other health epidemics or pandemics; and
•other risks and uncertainties, including those listed under Part II, Item 1A. “Risk Factors."
Any forward-looking statements in this Quarterly Report on Form 10-Q reflect our current views with respect to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under Part II, Item 1A. “Risk Factors” and elsewhere in this Quarterly Report on Form 10-Q. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
This Quarterly Report on Form 10-Q also contains estimates, projections and other information concerning our industry, our business, and the markets for certain diseases, including data regarding the incidence and prevalence of certain medical conditions. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources.
SYNLOGIC, INC.
QUARTERLY REPORT ON FORM 10-Q
TABLE OF CONTENTS
SYNlogic, Inc. and SUBSIDIARIES
Unaudited Consolidated Balance Sheets
(In thousands, except share amounts)
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
2024 |
|
|
2023 |
|
Assets |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
24,822 |
|
|
$ |
23,960 |
|
Short-term marketable securities |
|
|
7,489 |
|
|
|
23,786 |
|
Prepaid expenses and other current assets |
|
|
2,429 |
|
|
|
2,161 |
|
Assets held for sale |
|
|
206 |
|
|
|
— |
|
Total current assets |
|
|
34,946 |
|
|
|
49,907 |
|
Property and equipment, net |
|
|
— |
|
|
|
5,603 |
|
Right of use asset - operating lease |
|
|
— |
|
|
|
12,102 |
|
Restricted cash |
|
|
1,097 |
|
|
|
1,097 |
|
Prepaid research and development, net of current portion |
|
|
— |
|
|
|
6,825 |
|
Other assets |
|
|
14 |
|
|
|
16 |
|
Total assets |
|
$ |
36,057 |
|
|
$ |
75,550 |
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
719 |
|
|
$ |
1,457 |
|
Accrued expenses |
|
|
3,116 |
|
|
|
3,000 |
|
Lease liability - operating lease |
|
|
2,671 |
|
|
|
4,780 |
|
Finance lease obligations |
|
|
1 |
|
|
|
4 |
|
Purchase warrant liability |
|
|
4,433 |
|
|
|
11,163 |
|
Total current liabilities |
|
|
10,940 |
|
|
|
20,404 |
|
Long-term liabilities: |
|
|
|
|
|
|
Lease liability - operating lease, net of current portion |
|
|
11,792 |
|
|
|
12,491 |
|
Total long-term liabilities |
|
|
11,792 |
|
|
|
12,491 |
|
Commitments and contingencies (Note 13) |
|
|
|
|
|
|
Stockholders' equity |
|
|
|
|
|
|
Common stock, $0.001 par value |
|
|
|
|
|
|
250,000,000 shares authorized as of March 31, 2024 and December 31, 2023;
11,907,004 shares issued and 11,627,212 shares outstanding as of March 31, 2024 and 9,465,949 shares issued and 9,186,157 shares outstanding as of December 31, 2023 |
|
|
12 |
|
|
|
10 |
|
Additional paid-in capital |
|
|
461,696 |
|
|
|
459,458 |
|
Accumulated other comprehensive income |
|
|
— |
|
|
|
6 |
|
Accumulated deficit |
|
|
(445,865 |
) |
|
|
(414,301 |
) |
Treasury stock, at cost (279,792 shares at March 31, 2024 and at December 31, 2023) |
|
|
(2,518 |
) |
|
|
(2,518 |
) |
Total stockholders' equity |
|
|
13,325 |
|
|
|
42,655 |
|
Total liabilities and stockholders' equity |
|
$ |
36,057 |
|
|
$ |
75,550 |
|
The accompanying notes are an integral part of the unaudited consolidated financial statements.
Synlogic, INC. aND SUBSIDIARIES
Unaudited Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended |
|
|
|
2024 |
|
|
2023 |
|
Revenue |
|
$ |
8 |
|
|
$ |
174 |
|
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
|
7,680 |
|
|
|
12,450 |
|
General and administrative |
|
|
2,884 |
|
|
|
3,967 |
|
Restructuring and other charges |
|
|
28,289 |
|
|
|
— |
|
Total operating expenses |
|
|
38,853 |
|
|
|
16,417 |
|
Loss from operations |
|
|
(38,845 |
) |
|
|
(16,243 |
) |
Other income (expense): |
|
|
|
|
|
|
Interest and investment income |
|
|
553 |
|
|
|
628 |
|
Interest expense |
|
|
— |
|
|
|
(1 |
) |
Change in fair value of purchase warrant liability |
|
|
6,730 |
|
|
|
— |
|
Other expense |
|
|
(2 |
) |
|
|
(6 |
) |
Other income (expense), net |
|
|
7,281 |
|
|
|
621 |
|
Net loss |
|
$ |
(31,564 |
) |
|
$ |
(15,622 |
) |
|
|
|
|
|
|
|
Net loss per share - basic and diluted |
|
$ |
(2.60 |
) |
|
$ |
(3.39 |
) |
Weighted-average common stock outstanding - basic and diluted |
|
|
12,131,461 |
|
|
|
4,604,682 |
|
Comprehensive loss: |
|
|
|
|
|
|
Net loss |
|
$ |
(31,564 |
) |
|
$ |
(15,622 |
) |
Net unrealized gain (loss) on marketable securities |
|
|
(6 |
) |
|
|
131 |
|
Comprehensive loss |
|
$ |
(31,570 |
) |
|
$ |
(15,491 |
) |
The accompanying notes are an integral part of the unaudited consolidated financial statements.
Synlogic, INC. AND SUBSIDIARIES
Unaudited Consolidated Statements of Stockholders’ Equity
(In thousands, except share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock |
|
|
Additional |
|
|
Accumulated
other |
|
|
|
|
|
Treasury Stock |
|
|
|
|
|
|
$0.001 par value |
|
|
paid-in |
|
|
comprehensive |
|
|
Accumulated |
|
|
|
|
|
Total |
|
|
|
Shares |
|
|
Amount |
|
|
capital |
|
|
income (loss) |
|
|
deficit |
|
|
Shares |
|
|
Amount |
|
|
equity |
|
Balance at December 31, 2022 |
|
|
4,728,874 |
|
|
$ |
5 |
|
|
$ |
442,303 |
|
|
$ |
(161 |
) |
|
$ |
(357,019 |
) |
|
|
(279,792 |
) |
|
$ |
(2,518.00 |
) |
|
$ |
82,610 |
|
Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs |
|
|
68,893 |
|
|
|
— |
|
|
|
816 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
816 |
|
Issuance of restricted stock |
|
|
10,803 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Issuance of common stock under employee stock purchase plan |
|
|
7,322 |
|
|
|
— |
|
|
|
59 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
59 |
|
Equity-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
718 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
718 |
|
Unrealized gain (loss) on securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
131 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
131 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(15,622 |
) |
|
|
— |
|
|
|
— |
|
|
|
(15,622 |
) |
Balance at March 31, 2023 |
|
|
4,815,892 |
|
|
$ |
5 |
|
|
$ |
443,896 |
|
|
$ |
(30 |
) |
|
$ |
(372,641 |
) |
|
|
(279,792 |
) |
|
$ |
(2,518 |
) |
|
$ |
68,712 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2023 |
|
|
9,465,949 |
|
|
$ |
10 |
|
|
$ |
459,458 |
|
|
$ |
6 |
|
|
$ |
(414,301 |
) |
|
|
(279,792 |
) |
|
$ |
(2,518 |
) |
|
$ |
42,655 |
|
Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs |
|
|
7,839 |
|
|
|
— |
|
|
|
13 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
13 |
|
Exercise of options |
|
|
2,494 |
|
|
|
— |
|
|
|
4 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4 |
|
Exercise of pre-funded warrants |
|
|
2,251,000 |
|
|
|
2 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2 |
|
Issuance of restricted stock |
|
|
362,700 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Cancellation of restricted stock |
|
|
(182,978 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Equity-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
2,221 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,221 |
|
Unrealized gain (loss) on securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(6 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(6 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(31,564 |
) |
|
|
— |
|
|
|
— |
|
|
|
(31,564 |
) |
Balance at March 31, 2024 |
|
|
11,907,004 |
|
|
|
12 |
|
|
|
461,696 |
|
|
|
— |
|
|
|
(445,865 |
) |
|
|
(279,792 |
) |
|
|
(2,518 |
) |
|
|
13,325 |
|
The accompanying notes are an integral part of the unaudited consolidated financial statements.
Synlogic, INC. AND SUBSIDIARIES
Unaudited Consolidated Statements of Cash Flows
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
|
Three Months Ended |
|
|
|
March 31, 2024 |
|
|
March 31, 2023 |
|
Cash flows from operating activities: |
|
|
|
|
|
|
Net loss |
|
$ |
(31,564 |
) |
|
|
(15,622 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
Depreciation |
|
|
347 |
|
|
|
576 |
|
Gain on disposal of property and equipment |
|
|
(103 |
) |
|
|
(11 |
) |
Impairment loss on fixed assets |
|
|
5,393 |
|
|
|
— |
|
Impairment loss on ROU assets |
|
|
9,571 |
|
|
|
— |
|
Gain on lease termination |
|
|
(43 |
) |
|
|
— |
|
Impairment of prepaid research and development |
|
|
5,219 |
|
|
|
— |
|
Equity-based compensation expense |
|
|
2,221 |
|
|
|
718 |
|
Change in fair value of warrant liability |
|
|
(6,730 |
) |
|
|
— |
|
Accretion/amortization of investment securities |
|
|
(231 |
) |
|
|
(327 |
) |
Reduction in carrying amount of operating lease right of use asset |
|
|
948 |
|
|
|
809 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
Prepaid expenses and other current assets |
|
|
(568 |
) |
|
|
(2,838 |
) |
Prepaid research and development, net of current portion |
|
|
1,906 |
|
|
|
(1,233 |
) |
Other assets |
|
|
2 |
|
|
|
(15 |
) |
Accounts payable and accrued expenses |
|
|
(622 |
) |
|
|
(2,378 |
) |
Deferred revenue |
|
|
— |
|
|
|
(190 |
) |
Operating lease liabilities |
|
|
(1,182 |
) |
|
|
(994 |
) |
Net cash used in operating activities |
|
|
(15,436 |
) |
|
|
(21,505 |
) |
Cash flows from investing activities: |
|
|
|
|
|
|
Purchases of marketable securities |
|
|
(1,477 |
) |
|
— |
|
Proceeds from maturity of marketable securities |
|
|
17,999 |
|
|
|
30,397 |
|
Purchases of property and equipment |
|
|
(395 |
) |
|
|
(143 |
) |
Proceeds from the sale of property and equipment |
|
|
155 |
|
|
|
16 |
|
Net cash provided by investing activities |
|
|
16,282 |
|
|
|
30,270 |
|
Cash flows from financing activities: |
|
|
|
|
|
|
Payments on finance lease obligations |
|
|
(3 |
) |
|
|
(2 |
) |
Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs |
|
|
13 |
|
|
|
856 |
|
Proceeds from employee stock purchases and exercise of stock options |
|
|
4 |
|
|
|
59 |
|
Proceeds from exercise of pre-funded warrants |
|
|
2 |
|
|
|
— |
|
Net cash provided by financing activities |
|
|
16 |
|
|
|
913 |
|
Net increase in cash, cash equivalents and restricted cash |
|
|
862 |
|
|
|
9,678 |
|
Cash, cash equivalents and restricted cash at beginning of period |
|
|
25,057 |
|
|
|
16,958 |
|
Cash, cash equivalents and restricted cash at end of period |
|
$ |
25,919 |
|
|
$ |
26,636 |
|
Supplemental disclosure of non-cash investing activities: |
|
|
|
|
|
|
Decrease in right-of-use asset and operating lease liabilities due to lease termination |
|
$ |
1,626 |
|
|
$ |
— |
|
Supplemental disclosure of non-cash financing activities: |
|
|
|
|
|
|
Issuance costs included in accounts payable and accrued expenses |
|
$ |
— |
|
|
$ |
40 |
|
The accompanying notes are an integral part of the unaudited consolidated financial statements.
SYNLOGIC, INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements
Organization
Synlogic, Inc., together with its wholly owned and consolidated subsidiaries (Synlogic or the Company), is a clinical-stage biopharmaceutical company applying synthetic biology to the discovery and development of Synthetic Biotics. Synthetic Biotics are generated from Synlogic’s proprietary platform, leveraging a reproducible, modular approach to the generation of novel drug candidates that perform or deliver critical therapeutic functions. Synthetic Biotics are designed to metabolize a toxic substance, compensate for missing or damaged metabolic pathways or deliver combinations of therapeutic factors. Synlogic’s goal is to discover, develop and ultimately commercialize Synthetic Biotics. Since incorporation, the Company has devoted substantially all of its efforts to the research and development of its product candidates.
In February 2024, the Company and its board of directors decided to discontinue the Synpheny-3 trial and to conduct a comprehensive review of strategic alternatives. The Company also announced a corporate restructuring that resulted in a reduction in its workforce by 90% that was substantially completed in the first quarter of 2024 (see Note 8).
Going Concern and Liquidity
The Company’s consolidated financial statements have been prepared assuming it will continue as a going concern. The going concern assumption contemplates the continuity of operations, and the realization of assets and the satisfaction of liabilities in the ordinary course of business. The Company has historically generated negative cash flows from operations and has an accumulated deficit of $445.9 million at March 31, 2024. At March 31, 2024, the Company had $32.3 million in unrestricted cash, cash equivalents and short-term marketable securities. The Company believes the conditions and events raising substantial doubt about its ability to continue as a going concern no longer exist following the execution of the corporate restructuring (see Note 8) that was substantially completed in the first quarter of 2024. Accordingly, its current cash and cash equivalents as of March 31, 2024 will be sufficient to fund its operations at the current levels for at least the next 12 months from the date of this filing. The Company expects to continue to incur costs and expenditures in connection with the process of evaluating strategic alternatives.
(2)Summary of Significant Accounting Policies
The significant accounting policies described in the Company’s audited financial statements as of and for the year ended December 31, 2023, and the notes thereto, which are included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the Securities and Exchange Commission (SEC) on March 19, 2024 (the 2023 Annual Report), have had no material changes during the three months ended March 31, 2024.
Reverse Stock Split
On September 27, 2023, the Company effected a one-for-fifteen reverse stock split of its issued and outstanding common stock, which also adjusted all outstanding warrants. Accordingly, all share and per share amounts for all periods presented in the accompanying consolidated financial statements and notes thereto have been adjusted retroactively, where applicable, to reflect this stock split. All fractional shares resulting from the reverse stock split were paid in cash.
Basis of Presentation
The accompanying consolidated financial statements and the related disclosures as of March 31, 2024 and for the three months ended March 31, 2024 and 2023 are unaudited and have been prepared in accordance with accounting principles generally accepted in the United States (GAAP) and the rules and regulations of the SEC for interim financial statements. Accordingly, they do not include all of the information and notes required by GAAP for complete financial statements. These interim consolidated financial statements should be read in conjunction with the Company’s 2023 and 2022 audited consolidated financial statements and notes included in the 2023 Annual Report. The consolidated balance sheet as of December 31, 2023 included herein was derived from the audited financial statements as of that date but does not include all disclosures including notes required by GAAP for complete financial statements. In the opinion of management, the unaudited interim consolidated financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for the fair presentation of the Company’s financial position and results of operations for the three months ended March 31, 2024 and 2023. The results of operations for the interim periods are not necessarily indicative of the results to be expected for the year ending December 31, 2024 or any other interim period or future year or period.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Synlogic and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (FASB) or other accounting standard setting boards that the Company adopts as of the effective date. Unless otherwise discussed below, recently issued pronouncements that are or will be applicable to the Company did not have, or are not expected to have, a material impact on the Company’s present or future financial statements.
(3)Fair Value of Financial Instruments
The tables below present information about the Company’s assets and liabilities that are measured at fair value on a recurring basis and indicate the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value, as described under Note 2, Summary of Significant Accounting Policies, in the audited financial statements included in the 2023 Annual Report.
The Company’s investment portfolio includes many fixed income securities that do not always trade on a daily basis. As a result, the pricing services used by the Company applied other available information as applicable through processes such as benchmark yields, benchmarking of like securities, sector groupings and matrix pricing to prepare evaluations. In addition, model processes were used to assess interest rate impact and develop prepayment scenarios. These models take into consideration relevant credit information, perceived market movements, sector news and economic events. The inputs into these models may include benchmark yields, reported trades, broker-dealer quotes, issuer spreads and other relevant data.
The Company accounts for issued warrants either as derivative liabilities or as equity instruments depending on the specific terms of the agreement. Warrants that are equity-classified instruments and recorded in additional paid-in capital at issuance are not subject to remeasurement. The purchase warrants issued in October 2023 are liability classified and recorded at fair value using the Black-Scholes option-pricing model at issuance, with any subsequent changes in fair value recognized in the consolidated statements of operations. We periodically evaluate changes in facts and circumstances that could impact the classification of warrants. None of the purchase warrants have been exercised since their issuance.
At March 31, 2024 and December 31, 2023, the Company has classified assets and liabilities measured at fair value on a recurring basis as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements at Reporting Date Using |
|
|
|
March 31, |
|
|
Quoted Prices in Active
Markets for Identical
Assets |
|
|
Significant Other
Observable Inputs |
|
|
Significant
Unobservable Inputs |
|
Description |
|
2024 |
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
22,825 |
|
|
$ |
22,825 |
|
|
$ |
— |
|
|
$ |
— |
|
Commercial paper (included in cash and cash equivalents) |
|
|
1,997 |
|
|
|
— |
|
|
|
1,997 |
|
|
|
— |
|
Commercial paper |
|
|
7,489 |
|
|
|
— |
|
|
|
7,489 |
|
|
|
— |
|
Total |
|
$ |
32,311 |
|
|
$ |
22,825 |
|
|
$ |
9,486 |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Purchase warrant liability |
|
$ |
4,433 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
4,433 |
|
Total |
|
$ |
4,433 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
4,433 |
|
|
|
Fair Value Measurements at Reporting Date Using |
|
|
|
December 31, |
|
|
Quoted Prices in Active
Markets for Identical
Assets |
|
|
Significant Other
Observable Inputs |
|
|
Significant
Unobservable Inputs |
|
Description |
|
2023 |
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
15,476 |
|
|
$ |
15,476 |
|
|
$ |
— |
|
|
$ |
— |
|
Commercial paper (included in cash and cash equivalents) |
|
|
8,484 |
|
|
|
— |
|
|
|
8,484 |
|
|
|
— |
|
Commercial paper |
|
|
14,342 |
|
|
|
— |
|
|
|
14,342 |
|
|
|
— |
|
U.S. government agency securities and treasuries |
|
|
9,444 |
|
|
|
6,956 |
|
|
|
2,488 |
|
|
|
— |
|
Total |
|
$ |
47,746 |
|
|
$ |
22,432 |
|
|
$ |
25,314 |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Purchase warrant liability |
|
$ |
11,163 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,163 |
|
Total |
|
$ |
11,163 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,163 |
|
Cash equivalents, prepaid expenses and other current assets, accounts payable and accrued expenses at March 31, 2024 and December 31, 2023 are carried at amounts that approximate fair value due to their short-term maturities. Finance lease obligations at March 31, 2024 and December 31, 2023 approximate fair value as they bear interest at a rate approximating a market interest rate.
The following tables summarize the estimated fair value of the assets presented within cash and cash equivalents measured at fair value and the gross unrealized holding gains and losses (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
Amortized Cost |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair Value |
|
Money market funds (included in cash and cash equivalents) |
|
$ |
22,825 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
22,825 |
|
Commercial paper (included in cash and cash equivalents) |
|
|
1,997 |
|
|
|
— |
|
|
|
— |
|
|
|
1,997 |
|
Total |
|
$ |
24,822 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
24,822 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
Amortized Cost |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair Value |
|
Money market funds (included in cash and cash equivalents) |
|
$ |
15,476 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
15,476 |
|
Commercial paper (included in cash and cash equivalents) |
|
|
8,482 |
|
|
|
2 |
|
|
|
— |
|
|
|
8,484 |
|
Total |
|
$ |
23,958 |
|
|
$ |
2 |
|
|
$ |
— |
|
|
$ |
23,960 |
|
Assumptions Used in Determining Fair Value of Warrants
The assumptions used in the Black-Scholes option-pricing model for the purchase warrants on the consolidated balance sheets at March 31, 2024 and December 31, 2023 are included below:
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
|
December 31, 2023 |
|
Expected Term |
|
4.5 years |
|
|
4.75 years |
|
Weighted-average, risk free interest rate |
|
|
4.3 |
% |
|
|
3.9 |
% |
Expected volatility |
|
|
98.2 |
% |
|
|
94.0 |
% |
Dividend yield |
|
|
— |
|
|
|
— |
|
Strike price |
|
$ |
3.41 |
|
|
$ |
3.41 |
|
Stock price |
|
$ |
1.79 |
|
|
$ |
3.85 |
|
(4)Available-for-Sale Securities
The following tables summarize the available-for-sale securities held at March 31, 2024 and December 31, 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
Amortized cost |
|
|
Gross unrealized
gains |
|
|
Gross unrealized
losses |
|
|
Fair Value |
|
Commercial paper |
|
$ |
7,489 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
7,489 |
|
Total |
|
$ |
7,489 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
7,489 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
Amortized cost |
|
|
Gross unrealized
gains |
|
|
Gross unrealized
losses |
|
|
Fair Value |
|
Commercial paper |
|
$ |
14,338 |
|
|
$ |
4 |
|
|
$ |
— |
|
|
$ |
14,342 |
|
U.S. government agency securities and treasuries |
|
|
9,444 |
|
|
|
1 |
|
|
|
(1 |
) |
|
|
9,444 |
|
Total |
|
$ |
23,782 |
|
|
$ |
5 |
|
|
$ |
(1 |
) |
|
$ |
23,786 |
|
The contractual maturity of all securities held at March 31, 2024 was two months or less. There was one investment in an unrealized loss position at March 31, 2024. The aggregate fair value of the security in an unrealized loss position at March 31, 2024 was $1.5 million. There were two investments in an unrealized loss position at December 31, 2023, none of which had been in an unrealized loss position for more than twelve months. The aggregate fair value of the securities in an unrealized loss position at December 31, 2023 was $5.4 million. The Company reviews its investments for other-than-temporary impairment whenever the fair value of an investment is less than amortized cost and evidence indicates that an investment’s carrying amount is not recoverable within a reasonable period of time. To determine whether an impairment is other-than-temporary, the Company considers whether it has the ability and intent to hold the investment until a market price recovery and considers whether evidence indicating the cost of the investment is recoverable outweighs evidence to the contrary. The Company did not hold any securities with an other-than-temporary impairment at March 31, 2024.
Gross realized gains and losses on the sales of investments have not been material to the Company’s consolidated statement of operations.
In February 2024, the Company committed to a plan to sell its remaining lab equipment and therefore has classified the amount as assets held for sale on the consolidated balance sheet as of March 31, 2024. The assets held for sale were reported at the lower of the carrying amount or fair value, less costs to sell. Accordingly, during the three months ended March 31, 2024, the Company recorded an impairment charge, which was included in restructuring and other charges, of $0.8 million related to the lab equipment classified as assets held for sale.
(6)Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
2024 |
|
|
2023 |
|
Prepaid insurance |
|
$ |
432 |
|
|
$ |
691 |
|
Prepaid research and development |
|
|
1,384 |
|
|
|
788 |
|
Other prepaid expenses |
|
|
502 |
|
|
|
536 |
|
Other current assets |
|
|
111 |
|
|
|
146 |
|
Total prepaid expenses and other current assets |
|
$ |
2,429 |
|
|
$ |
2,161 |
|
Accrued expenses consist of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
2024 |
|
|
2023 |
|
Payroll related |
|
$ |
15 |
|
|
$ |
2,556 |
|
Professional fees |
|
|
392 |
|
|
|
290 |
|
Research and development |
|
|
507 |
|
|
|
91 |
|
Restructuring costs |
|
|
2,079 |
|
|
|
— |
|
Other |
|
|
123 |
|
|
|
63 |
|
Total accrued expenses |
|
$ |
3,116 |
|
|
$ |
3,000 |
|
(8)Restructuring and Other Charges
In February 2024, the Company and its board of directors decided to discontinue the Synpheny-3 trial and as a result are currently evaluating strategic options for the Company with a goal to enhance stockholder value, including the possibility of a merger or sale of the Company. Due to this decision there has been an interim evaluation of impairment of long-lived and other assets which is described in detail below.
The Company also announced a corporate restructuring that included a reduction in its workforce by more than 90% that was substantially completed during the three months ended March 31, 2024. In connection with the corporate restructuring, the Company recorded a restructuring charge for severance and related costs of $5.6 million during the three months ended March 31, 2024. The Company also executed consulting agreements with a select number of former employees in which their equity continues to vest under the terms of the original award. The consulting services were determined to be non-substantive and as a result, the Company has accelerated the related stock compensation expense and recorded an additional $1.8 million to stock compensation included in restructuring charges for the three months ended March 31, 2024.
Restructuring and other charges also includes impairment of the right-of-use assets associated with the Company's existing leased spaces of $9.6 million and impairment of property and equipment of $5.3 million, primarily related to leasehold improvements in connection with the lease impairment.
Other charges relating to the restructuring include a $5.2 million charge to impairment for prepaid research and development in relation to the Ginkgo collaboration (see Note 11) and $0.8 million for various costs related to the restructuring including legal fees, banking fees and lab decommissioning fees.
As of March 31, 2024, approximately $2.1 million of the total restructuring charges remain unpaid and were included in accrued restructuring charges.
Reverse Stock Split
On September 27, 2023, the Company effected a reverse stock split of its shares of common stock, pursuant to which every fifteen (15) shares of the its issued and outstanding common stock was automatically converted into one (1) issued and outstanding share of common stock without any change in the par value of $0.001 per share. The reverse stock split was approved by the stockholders on September 21, 2023 at a special meeting of stockholders.
October 2023 Financing
On October 3, 2023, the Company issued and sold, through an underwritten public offering:
•3,921,928 shares of its common stock at a price of $2.84 per share less underwriting discounts and commissions;
•pre-funded warrants to purchase up to 3,472,435 shares of its common stock at a price of $2.839 immediately following the consummation of the offering, and;
•accompanying common stock warrants to purchase up to 7,394,363 shares of its common stock at a price of $3.408 per share exercisable immediately after issuance and expires five years from the date of issuance.
Each share of its common stock and each pre-funded warrant was sold together with a common warrant to purchase one share of its common stock. A holder of pre-funded warrants may not exercise the warrant if the holder, together with its affiliates, would beneficially own more than 4.99% (or, upon election by a holder prior to the issuance of any warrants, 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. The net proceeds to the Company from the sale of common stock and pre-funded warrants through the offering, after deducting the underwriting discounts and commissions and offering expenses payable by the Company, were approximately $19.6 million.
The common stock and pre-funded warrants met the criteria for equity classification. The purchase warrants met the definition of a derivative instrument. Accordingly, upon issuance, the purchase warrants were recorded as a liability at fair value using the Black-Scholes option-pricing model in the amount of $7.1 million. Any subsequent changes in fair value of the purchase warrants is recognized in the consolidated statements of operations. The residual proceeds were allocated between the common stock and pre-funded warrants based on their relative fair values at the time of issuance. The amount allocated to the pre-funded warrants was recorded as a component of stockholders’ equity within additional paid-in capital.
At March 31, 2024, the fair value of the purchase warrants was $4.4 million. Accordingly, a gain on remeasurement of the purchase warrant liability of $6.7 million was recorded in the three months ended March 31, 2024. Subsequent to their issuance and through March 31, 2024, 2,920,126 pre-funded warrants have been exercised. None of the purchase warrants have been exercised since their issuance.
At-the-Market (ATM) Offering Program
In July 2021, the Company entered into a sales agreement with Jefferies, LLC (Jefferies) with respect to an ATM, under which the Company may offer and sell, from time to time at its sole discretion, shares of its common stock having aggregate sales proceeds of up to $50.0 million. Jefferies is not required to sell any specific amount but acts as the Company’s sales agent using commercially reasonable efforts consistent with its normal trading and sales practices. During the three months ended March 31, 2024, 7,839 shares of common stock were sold pursuant to the sales agreement with Jefferies, resulting in net proceeds of approximately $0.01 million.
Ginkgo Warrants
In June 2019, the Company issued to Ginkgo Bioworks, Inc. (Ginkgo) an aggregate of 422,718 shares of common stock at a purchase price per share of $135, and pre-funded warrants (the Ginkgo pre-funded warrants) to purchase up to an aggregate of 169,874 shares of common stock at an exercise price of $135 per share, with $134.85 of such exercise price paid at the closing of the offering. The net proceeds to the Company were approximately $79.9 million. None of the Ginkgo pre-funded warrants have been exercised as of March 31, 2024. (See Note 9, Collaboration Agreements: Ginkgo Collaboration).
The Company has reserved for future issuance the following shares of common stock related to the potential exercise of Ginkgo pre-funded warrants, exercise of stock options, and the employee stock purchase plan:
|
|
|
|
|
March 31, 2024 |
|
Common stock issuable under pre-funded warrants |
|
552,309 |
|
Common stock issuable under purchase warrants |
|
3,921,928 |
|
Common stock issuable under Ginkgo pre-funded warrants |
|
169,874 |
|
Options exercisable to purchase common stock |
|
334,081 |
|
Employee Stock Purchase Plan |
|
- |
|
Total |
|
4,978,192 |
|
(10)Equity-based Compensation
On January 1, 2024, the number of shares of common stock available for issuance under the 2015 Equity Incentive Award Plan (the 2015 Plan) and the 2015 Employee Stock Purchase Plan (ESPP) was increased by 459,392 shares and 91,878 shares, respectively, due to the annual evergreen provision to increase shares available under the 2015 Plan and the ESPP. As of March 31, 2024, there were an aggregate of 604,072 shares available for future grant under the 2017 Stock Incentive Plan (the 2017 Plan) and the 2015 Plan, and 187,012 shares available for future grant under the ESPP.
The following table summarizes equity‑based compensation expense within the Company’s consolidated statements of operations and comprehensive loss for the three months ended March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Three months ended 31, |
|
|
|
2024 |
|
|
2023 |
|
Research and development |
|
$ |
115 |
|
|
$ |
277 |
|
General and administrative |
|
|
306 |
|
|
|
441 |
|
Restructuring charges: expense acceleration |
|
|
1,800 |
|
|
|
— |
|
|
|
$ |
2,221 |
|
|
$ |
718 |
|
The following table summarizes equity‑based compensation expense by type of award for the three months ended March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Three months ended March 31, |
|
|
|
2024 |
|
|
2023 |
|
Stock options |
|
$ |
1,480 |
|
|
$ |
607 |
|
Restricted stock awards |
|
|
763 |
|
|
|
91 |
|
ESPP |
|
|
(22 |
) |
|
|
20 |
|
|
|
$ |
2,221 |
|
|
$ |
718 |
|
During the three months ended March 31, 2024, the Company granted 44,701 stock options with a weighted average exercise price of $4.20 per share. As of March 31, 2024, there was $0.7 million of unrecognized share-based compensation related to unvested stock option grants which is expected to be recognized over a weighted average period of 1.93 years. The total unrecognized share-based compensation cost will be adjusted for actual forfeitures as they occur.
During the three months ended March 31, 2024, the Company granted 362,700 restricted stock awards with a weighted average grant date fair value per share of $4.44. As of March 31, 2024, there was approximately $0.5 million of unrecognized share-based compensation related to restricted stock awards granted, which is expected to be recognized over a weighted average period of 1.8 years. The total unrecognized share-based compensation cost will be adjusted for actual forfeitures as they occur.
For a full description of the Company’s equity plans, refer to Note 9, Equity-based Compensation and Equity Incentive Plans in the 2023 Annual Report.
(11)Collaboration Agreements
Roche Collaboration
In June 2021, the Company entered into a Pilot Collaboration and Option Agreement (the Roche Collaboration and Option Agreement) with F. Hoffmann-La Roche Ltd (Roche Basel) and Hoffmann-La Roche Inc. (Roche US, and together with Roche Basel, Roche). Under the terms of the Roche Collaboration and Option Agreement, the Company and Roche will seek to collaborate to research and pre-clinically develop Synthetic Biotics for addressing an undisclosed novel target for the treatment of inflammatory bowel disease.
During the three months ended March 31, 2024 and 2023, the Company recognized $0.01 million and $0.2 million, respectively, as collaboration revenue associated with the Roche Collaboration and Option Agreement. The Roche Collaboration and Option Agreement concluded after the last milestone was achieved by the Company in October 2023. Subsequently, Roche did not exercise its exclusive option to enter into a licensing and collaboration agreement for further development and commercialization of the product candidate
For a full description of the Roche Collaboration and Option Agreement, refer to Note 10, Collaboration Agreements in the 2023 Annual Report.
Ginkgo Collaboration
In 2017, the Company established a technology collaboration with Ginkgo. In June 2019, the Company expanded its collaboration and entered into an agreement with Ginkgo for the research and development of engineered microbial therapeutic products (See Note 9). Under the 2019 expanded agreement, the Company made a prepayment to Ginkgo of $30.0 million for its foundry services that was to be provided to the Company over an initial term of five years, which could be extended for an additional three (3) years, subject to the satisfaction of specified conditions. Upon the expiration of such initial term and, if applicable, an additional term, any portion of the prepayment that has not been used to purchase services from Ginkgo will be retained by Ginkgo. In February 2024, the Company and its board of directors decided to discontinue the Synpheny-3 trial and significantly reduce its workforce and have made the decision to evaluate strategic options for the Company. As a result of this decision, the Company would no longer be purchasing services from Ginkgo. At March 31, 2024, the remaining $5.2 million of prepaid expenses related to this collaboration that were historically recorded in prepaid expenses and other current assets and prepaid research and development, net of current portion on the consolidated balance sheet, were recorded as restructuring and other charges on the consolidated statements of operations and comprehensive loss.
Basic net loss per share is computed using the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share is computed using the sum of the weighted-average number of shares of common stock outstanding during the period and if dilutive, the weighted-average number of potential shares of common stock, including unvested restricted common stock and outstanding stock options. In June 2019, the Company sold 422,718 shares of common stock and pre-funded warrants to purchase an aggregate of 169,874 shares of common stock at an exercise price of $135 per share, with $134.85 of such exercise price paid at the closing of the offering (see Note 8, Stockholder's Equity and Note 10, Collaborations: Ginkgo Collaboration, in the audited financial statements included in the 2023 Annual Report). The shares of common stock into which the warrants may be exercised are considered outstanding for the purposes of computing net loss per share.
The Company’s potentially dilutive shares, which include outstanding stock options, unvested restricted common stock and potential shares issuable under the ESPP, are considered to be common share equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive.
The following potential common shares, presented based on amounts outstanding at each period end, were excluded from the calculation of the diluted net loss per share attributable to common stockholders for the period indicated because including them would have had an anti-dilutive effect.
|
|
|
|
|
|
|
|
|
|
|
As of March 31, |
|
|
|
2024 |
|
|
2023 |
|
Purchase warrants |
|
|
3,921,928 |
|
|
|
— |
|
Unvested restricted common stock awards |
|
|
213,204 |
|
|
|
65,616 |
|
Outstanding options to purchase common stock |
|
|
504,390 |
|
|
|
740,474 |
|
Potential shares issuable under the ESPP |
|
|
— |
|
|
|
— |
|
(13)Commitments and Contingencies
In the ordinary course of business, the Company may be subject to legal proceedings, claims and litigation as the Company operates in an industry susceptible to patent legal claims. The Company accounts for estimated losses with respect to legal proceedings and claims when such losses are probable and estimable. Legal costs associated with these matters are expensed when incurred. The Company is not currently a party to any material legal proceedings.
The Company’s commitments described in the Company’s consolidated financial statements as of and for the year ended December 31, 2023 and the notes thereto included in the 2023 Annual Report have had no material changes during the three months ended March 31, 2024.
(14) Related-Party Transactions
In June 2019, the Company expanded its collaboration and entered into an agreement with Ginkgo for the research and development of engineered microbial therapeutic products. As of March 31, 2024, Ginkgo owned 422,718 shares of the Company’s outstanding common stock. See Note 10, Ginkgo Collaboration, in the audited financial statements included in the 2023 Annual Report.
Under the agreement the Company made a prepayment to Ginkgo of $30.0 million for its foundry services that would be provided to the Company over an initial term of five years. Upon the expiration of such initial term and, if applicable, an additional term, any portion of the prepayment that has not been used to purchase services from Ginkgo will be retained by Ginkgo. In February 2024, the Company and its board of directors decided to discontinue the Synpheny-3 trial, significantly reduce its workforce and to evaluate strategic options for the Company. As a result of this decision, the Company would no longer be purchasing services from Ginkgo. At March 31, 2024, the remaining $5.2 million of prepaid expenses related to this collaboration that were historically recorded in prepaid expenses and other current assets and prepaid research and development, net of current portion on the consolidated balance sheet, were recorded as restructuring and other charges on the consolidated statements of operations and comprehensive loss.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Forward-Looking Information
The interim financial statements and this Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read together with our audited financial statements and accompanying notes for the years ended December 31, 2023 and 2022 included in our Annual Report on Form 10-K filed with the SEC on March 19, 2024 (the "2023 Annual Report"). In addition to historical information, this discussion and analysis contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). Please see “Risk Factors” beginning on page [24] of this Quarterly Report on Form 10-Q for a discussion of certain risk factors applicable to our business, financial condition, and results of operations. Operating results are not necessarily indicative of results that may occur for the full fiscal year or any other future period. On August 28, 2017, Synlogic, Inc., formerly known as Mirna Therapeutics, Inc. (NASDAQ: MIRN) (Mirna), completed a business combination with Synlogic, a private company, pursuant to the Agreement and Plan of Merger and Reorganization, dated as of May 15, 2017 (the Merger Agreement), pursuant to which the private Synlogic entity survived as a wholly owned subsidiary of Mirna (the Merger). Immediately after completion of the Merger, Mirna changed its name to “Synlogic, Inc.” (NASDAQ: SYBX). The term “Private Synlogic” refers to Synlogic Operating Company, Inc. (formerly known as Synlogic, Inc.) prior to the consummation of the Merger. Unless otherwise indicated, references to the terms the "combined company”, “Synlogic”, the "Company”, “we”, “our” and “us” refer to Private Synlogic prior to the consummation of the Merger and Synlogic, Inc. (formerly known as Mirna Therapeutics, Inc.) and its subsidiaries upon the consummation of the Merger described herein. The term "Mirna" refers to the Mirna Therapeutics, Inc. and its subsidiaries prior to the Merger.
Overview
We are a biopharmaceutical company advancing novel therapeutics to transform the care of serious diseases. We focus on rare metabolic disorders, with our lead program, labafenogene marselecobac (SYNB1934), studied in Synpheny-3, a global, pivotal Phase 3 study for patients with phenylketonuria (PKU), and SYNB1353, a potential treatment for homocystinuria (HCU). Both PKU and HCU are caused by inborn errors of metabolism, and present significant need for innovation due to limitations of both efficacy and safety in the currently available medical treatment options.
In February 2024, we made the decision to discontinue Synpheny-3, our pivotal study of our lead product candidate, labafenogene marselecobac (SYNB1934), as a potential treatment for PKU. The decision to end Synpheny-3 is based on results of an internal review in advance of an upcoming independent Data Monitoring Committee (DMC) assessment, which indicated the trial was unlikely to meet its primary endpoint. The decision was not based on concerns regarding safety or tolerability. We have completed the discontinuation with all of the Synpheny-3 clinical trial sites. As a result, our current corporate strategy is focused on pursuing strategic initiatives to enhance stockholder value, including but not limited to, a merger or the sale of the Company. Our strategic process is both active and ongoing and includes a range of interactions with transaction counterparties. Thus, we believe it is in our stockholders’ best interest to allow sufficient opportunity to pursue and consummate one or more such transactions and to consider additional alternatives that may materialize in the future. However, there can be no assurance that such activities will result in any agreements or transactions that will enhance shareholder value. Further, any strategic transaction that is completed ultimately may not deliver the anticipated benefits or enhance shareholder value.
Our early-stage pipeline includes product candidates for enteric hyperoxaluria, gout, and cystinuria, and has been fueled by a reproducible, proprietary approach that creates GI-restricted, oral medicines with new enzymatic pathways designed to consume or produce specific biological targets. We design, develop and manufacture these drug candidates, which are produced by applying genetic engineering to well-characterized probiotics.
Our drug candidates are designed through precise engineering to target validated biological pathways in the pathophysiology of a given disease. By using a probiotic to deliver these new enzymatic pathways, the activity is restricted to the gastrointestinal (GI) tract, avoiding systemic exposure and associated risks that limit the success of other modalities. Our pipeline programs are all based on the same probiotic Escherichia coli Nissle 1917, which provides synergies across programs, as well as more than one hundred years of human dosing experience. Our drug candidates are engineered to be non-colonizing, and fully reversible via GI clearance. These potential biopharmaceuticals are all orally administered, conducive to straightforward shipping, distribution and storage. For manufacturing, our platform leverages processes with familiar foundations, including fermentation and lyophilization, facilitating process design and scale-up, combined with unique and proprietary innovations tailored to our unique products.
Since our founding, based upon technology from the Massachusetts Institute of Technology (MIT) in 2014, we have progressed a pipeline of multiple drug candidates across different stages, including:
•Labafenogene marselecobac (SYNB1934), which was being studied in Synpheny-3, a pivotal, Phase 3 study for the treatment of patients with PKU;
•SYNB1353, a potential treatment for HCU, has achieved proof of mechanism in a Phase 1 study in healthy volunteers;
•Preclinical research activities on a potential drug candidate for cystinuria, a rare, genetic cause of recurrent kidney stones which is also caused by an underlying metabolic disorder;
•SYNB2081, a drug candidate for gout which was in IND-enabling studies; and
•Preclinical research focused on novel, locally-acting, GI-restricted biotherapeutics for indications in inflammatory bowel disease (IBD).
Our Pipeline: Synthetic Biotics In Clinical Development
Our product pipeline consists of drug candidates targeting significant medical needs caused by an underlying metabolic disorder. These include labafenogene marselecobac, which was being evaluated in a pivotal, Phase 3 study in PKU, and drug candidates designed to treat HCU, enteric hyperoxaluria, and gout. Our preclinical work includes additional metabolic disease research, including cystinuria, target exploration, and focused research efforts in IBD.
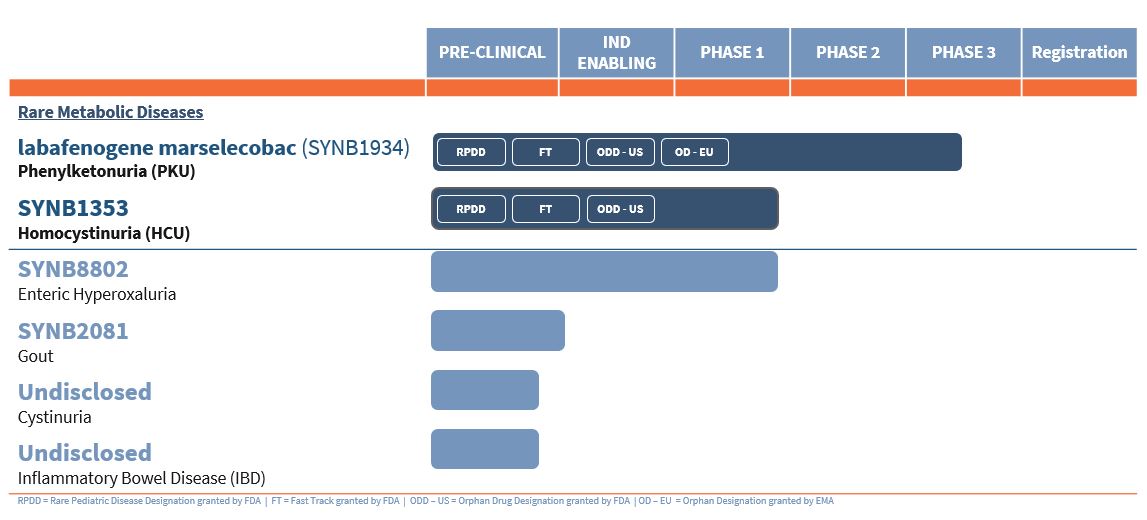
Business Overview
We currently operate in one reportable business segment—the discovery and development of Synthetic Biotics. To date, we have dedicated substantially all of our activities to the research and development of our product candidates. We have funded our operations to date primarily with proceeds from the sale of preferred stock, common stock, preferred units, warrants, payments received under the Roche Collaboration and Option agreement, prior collaborations, interest earned on investments, and cash received in the Merger.
We have not generated any revenue to date from product sales and have incurred significant operating losses since our inception. We have incurred net losses of approximately $31.6 million and $15.6 million for the three months ended March 31, 2024 and 2023, respectively. As of March 31, 2024, we had an accumulated deficit of approximately $445.9 million, and we expect to incur losses for the foreseeable future as we develop our product candidates and explore strategic alternatives. Historically, our expenses and capital requirements have increased substantially in connection with our research and development activities, as we:
•Completed preclinical studies, initiated and completed clinical trials for product candidates;
•Contracted to manufacture product candidates;
•Advanced research and development related activities to expand our product pipeline;
•Sought regulatory approval for our product candidates;
•Maintained, expanded and protected our intellectual property portfolio;
•Hired additional staff, including clinical, scientific, commercial, and management personnel;
•Expanded our existing infrastructure and secure space in a facility to support continued growth in our research and development efforts; and
•Added operational and finance personnel to support product development efforts and to support operating as a public company.
We do not expect to generate product revenue unless and until we successfully complete clinical development and obtain regulatory approvals for our product candidates, either alone or in collaboration with third parties. Additionally, we expect to utilize third-party contract research organizations (CROs) and contract manufacturing organizations (CMOs) to carry out our clinical development and manufacturing activities, and we do not yet have a commercial organization. If we obtain regulatory approval for any of our product candidates, we expect to incur significant expenses related to developing our internal commercialization capability to support product sales, marketing and distribution. Accordingly, we anticipate that we will seek to fund our operations through public or private equity or debt financings, collaborations or licenses, finance lease transactions or other available financing transactions. However, we may be unable to raise additional funds through these or other means when needed. Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when or if it will be able to achieve or maintain profitability. Even if we are able to generate product revenue, we may not become profitable.
Effects of Inflation
We do not believe that inflation has had a material impact on our business or operating results during the periods presented. However, inflation, has had, and may continue to have, an impact on the labor costs we incur to attract and retain qualified personnel, costs to conduct clinical trials and other operational costs. Inflationary costs could adversely affect our business, financial condition and results of operations. In addition, increased inflation has had, and may continue to have, an effect on interest rates. Increased interest rates may adversely affect our borrowing rate and our ability to obtain, or the terms under which we can obtain, any potential additional funding.
Financial Overview
Revenue
Revenue was previously generated through a collaboration and option agreement with Roche for the development and commercialization of product candidates. The Roche Collaboration and Option Agreement concluded after we achieved the last milestone under the Roche Collaboration and Option Agreement in October 2023. Subsequently, Roche did not exercise its exclusive option to enter into a licensing and collaboration agreement for further development and commercialization of the product candidate. See Note 11, Collaboration Agreements: Roche Collaboration in the notes to the unaudited consolidated financial statements appearing elsewhere in this Quarterly Report on Form 10-Q for a full discussion of the arrangement.
Research and Development Expense
Research and development expense consists of expenses incurred in connection with the discovery and development of our product candidates, including the conduct of preclinical and clinical studies and product development, which are expensed as they are incurred. These expenses consist primarily of:
•compensation, benefits and other employee related expenses;
•supplies to support our internal research and development efforts;
•research and development related facility and depreciation costs;
•leased manufacturing space; and
•third-party contract costs relating to research, process and formulation development, preclinical and clinical studies and regulatory operations.
The lengthy process of securing regulatory approvals for new drugs requires the expenditure of substantial resources. Any delay or failure to obtain regulatory approvals would materially adversely affect our product candidate development efforts and our business overall. Given the inherent uncertainties of pharmaceutical product development, we cannot estimate with any degree of certainty the likelihood, timing or cost of obtaining regulatory approval and marketing our product candidates and thus, when, if ever, our product candidates will generate revenues and cash flows.
The successful development of our product candidates is highly uncertain and subject to a number of risks. Refer to the risk factors under the heading Risks Related to the Development of Our Product Candidates in Part II, Item 1A, found elsewhere in this Quarterly Report on Form 10-Q.
Research and development activities account for a significant portion of our operating expenses. We expect our research and development expenses to decrease in the near future as we have discontinued our Synpheny-3 clinical trial and are evaluating strategic options for the Company.
We track direct research and development expenses, consisting principally of external costs, such as costs associated with contract research organizations and manufacturing of preclinical and clinical drug product and other outsourced research and development expenses to specific product programs. Costs related to specific product candidates are tracked upon the selection of a product candidate. We do not allocate employee and consulting-related costs, costs associated with our platform and facility expenses, including depreciation or other indirect costs, to specific product candidate programs because these costs are deployed across multiple product candidate programs under research and development and, as such, are separately classified.
General and Administrative Expense
General and administrative expenses consist primarily of compensation, benefits and other employee-related expenses for personnel in our administrative, finance, legal, information technology, investor relations, business development and human resource functions. Other general and administrative costs include the legal costs of pursuing patent protection of our intellectual property, facility and information technology infrastructure costs, directors’ and officers’ insurance, and professional fees for accounting, tax, legal and consulting services. We anticipate that our general and administrative expenses may increase in the future as we explore strategic alternatives, including potential legal, accounting and advisory expenses and other related charges. We also anticipate that we will continue to incur accounting, legal, regulatory, compliance and director and officer insurance costs as well as investor and public relations expenses associated with being a public company.
Restructuring and Other Charges
In February 2024, we made the decision to discontinue Synpheny-3, our pivotal study of our lead product candidate, labafenogene marselecobac (SYNB1934), as a potential treatment for PKU. As a result, we started to undertake certain operational and organizational steps in connection with a strategic reorganization plan and related cost-saving measures. We initiated a plan to review strategic alternatives in which we substantially reduced our operations, which included implementing a reduction in workforce by more than 90%.
Other Income (Expense)
Interest and investment income consists of income earned on investments. Interest expense consists of expense related to our finance leases. Other expense consists primarily of gains and losses on foreign currency invoices and losses on remeasurement of the purchase warrant liability.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements prepared in accordance with generally accepted accounting principles in the U.S. (GAAP). The preparation of these financial statements requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities, the reported amounts of revenues and expenses during the reported periods and related disclosures.
Our critical accounting policies are described in our 2023 Annual Report. During the three months ended March 31, 2024, there were no new or material changes to our existing critical accounting policies. We believe that these identified policies are critical to fully understanding and evaluating our financial condition and results of operations.
Our estimates and assumptions, including those related to revenue recognition and research and development expenses are monitored and analyzed by us for changes in facts and circumstances, and material changes in these estimates could occur in the future. The estimates and assumptions involved in our revenue recognition policy, particularly (a) assessing the number of performance obligations; (b) determination of transaction price; and (c) determining the pattern over which performance obligations are satisfied, including estimates to complete performance obligations, and those estimates and assumptions involved in our contract research accrual process, particularly estimates of work completed to date, involve a greater degree of judgment, and therefore we consider revenue recognition and research and development expenses to be our critical accounting policies. We evaluate our estimates and assumptions on an ongoing basis. Actual results may differ from our estimates under different assumptions or conditions.
Results of Operations
The following discussion summarizes the key factors our management believes are necessary for an understanding of our consolidated financial results.
Three Months Ended March 31, 2024 Compared to Three Months Ended March 31, 2023
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended
March 31, |
|
|
|
2024 |
|
|
2023 |
|
|
|
(in thousands) |
|
Revenue |
|
$ |
8 |
|
|
$ |
174 |
|
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
|
7,680 |
|
|
|
12,450 |
|
General and administrative |
|
|
2,884 |
|
|
|
3,967 |
|
Restructuring and other charges |
|
|
28,289 |
|
|
|
— |
|
Total operating expenses |
|
|
38,853 |
|
|
|
16,417 |
|
Loss from operations |
|
|
(38,845 |
) |
|
|
(16,243 |
) |
Other income (expense): |
|
|
|
|
|
|
Interest and investment income |
|
|
553 |
|
|
|
628 |
|
Interest expense |
|
|
— |
|
|
|
(1 |
) |
Loss on purchase warrant liability |
|
|
6,730 |
|
|
|
— |
|
Other expense |
|
|
(2 |
) |
|
|
(6 |
) |
Other income (expense), net |
|
|
7,281 |
|
|
|
621 |
|
Net loss |
|
$ |
(31,564 |
) |
|
$ |
(15,622 |
) |
Revenue
Revenue was $0.01 million for the three months ended March 31, 2024, as compared to $0.2 million for the corresponding period in 2023. Revenue for the three months ended March 31, 2024 was related to a material transfer and consulting agreement, under which work was completed in March 2024. Revenue for the three months ended March 31, 2023 was related to services performed under the Roche collaboration that we entered into in June 2021. The Roche Collaboration concluded after the last milestone was achieved by the Company in October 2023. Subsequently, Roche did not exercise its exclusive option to enter into a licensing and collaboration agreement for further development and commercialization of the product candidate.
Operating Expenses
Research and Development Expense
Research and development expense was $7.7 million for the three months ended March 31, 2024 compared to $12.5 million in the corresponding period in 2023, an decrease of $4.8 million. The following table summarizes our research and development expense for the three months ended March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended
March 31, |
|
|
|
2024 |
|
|
2023 |
|
Labafenogene marselecobac (SYNB1934) |
|
$ |
3,518 |
|
|
$ |
2,324 |
|
SYNB1618 |
|
|
— |
|
|
|
737 |
|
SYNB8802 |
|
|
126 |
|
|
|
902 |
|
SYNB1353 |
|
|
— |
|
|
|
173 |
|
SYNB1891 |
|
|
— |
|
|
|
(139 |
) |
External pre-development candidate costs and unallocated costs |
|
|
62 |
|
|
|
1,600 |
|
Total external costs |
|
|
3,706 |
|
|
|
5,597 |
|
Internal costs: |
|
|
|
|
|
|
Employee compensation and benefits (including equity-based compensation expense) |
|
|
2,084 |
|
|
|
4,677 |
|
Facility and other |
|
|
1,890 |
|
|
|
2,176 |
|
Total internal costs |
|
|
3,974 |
|
|
|
6,853 |
|
Total research and development expense |
|
$ |
7,680 |
|
|
$ |
12,450 |
|
The decrease in research and development expense was primarily due to the following:
•A decrease in external research and development costs of $1.9 million, which primarily consisted of:
o$1.6 million decrease in clinical, manufacturing and professional costs associated with SYNB1618 (earlier generation for Labafenogene marselecobac) for the Phase 2 Synpheny-1 study, SYNB8802 (achieved proof of concept in 2022), SYNB1353 (demonstrated proof of mechanism in 2022) and SYNB1891 (completed enrollment in November 2021);
o$1.5 million decrease in other early development candidates and unallocated costs; and
o$1.2 million increase in clinical, manufacturing and professional costs associated with Labafenogene marselecobac (SYNB1934). The decision to discontinue Synpheny-3, our pivotal study of our lead product candidate, labafenogene marselecobac (SYNB1934), as a potential treatment for PKU, was made in February 2024. We expect these costs to decrease in future periods due to the discontinuation of the clinical trial.
•$2.9 million decrease in internal research and development costs, which was primarily driven by the corporate restructuring and reduction in its workforce by more than 90% during the three months ended March 31, 2024.
General and Administrative Expense
General and administrative expense was $2.9 million for the three months ended March 31, 2024, compared to $4.0 million for the corresponding period in 2023. The decrease was primarily due to lower compensation, benefits and other employee-related expenses due to reduced headcount and decreased professional services costs.
Restructuring and Other Charges
In February 2024, the Company and its board of directors decided to discontinue the Synpheny-3 trial and to conduct a comprehensive review of strategic alternatives. The Company also announced a corporate restructuring that resulted in a reduction in its workforce by 90% that was substantially completed in the first quarter of 2024. As a result of this decision, restructuring and other charges were $28.3 million for the three months ended March 31, 2024. The restructuring charges were primarily due to impairment charges related to our leases, property and equipment, and prepaid research and development, severance payments relating to the reduction in workforce, and accelerated stock compensation expense.
Other Income (Expense)
Other income was $7.3 million for the three months ended March 31, 2024, compared to $0.6 million for the corresponding period in 2023. The increase in other income of $6.7 million was primarily related to the change in fair value of the purchase warrants classified as liabilities on the consolidated balance sheet.
Liquidity and Capital Resources
We have incurred losses since our inception on March 14, 2014 and, as of March 31, 2024, we had an accumulated deficit of $445.9 million. We have financed our operations to date primarily through the sale of preferred stock, common stock, preferred units and warrants, payments received under collaboration agreements, including the technology collaboration with Ginkgo, the Roche Collaboration and Option agreement, and prior collaborations, interest earned on investments, and cash received in the Merger. At March 31, 2024, we had $32.3 million in cash, cash equivalents and short-term marketable securities. Our cash and cash equivalents include amounts held in money market funds and commercial paper, stated at cost plus unrealized gain and loss, which approximates fair market value. During the three months ended March 31, 2024 our available-for-sale securities include amounts held in commercial paper. We invest cash in excess of immediate requirements in accordance with our investment policy, which limits the amounts we may invest in any one type of investment and requires all investments held by us to maintain minimum ratings from Nationally Recognized Statistical Rating Organizations so as to primarily achieve liquidity and capital preservation.
During the three months ended March 31, 2024, our cash, cash equivalents and short-term marketable securities balance decreased by $15.4 million. This decrease was primarily due to the cash used to operate our business. In February 2024, we implemented a strategic reduction of our workforce by over 90%. In March 2024, we recorded restructuring charges and one-time impairment charges in connection with the restructuring.
The following table sets forth the major sources and uses of cash, cash equivalents and restricted cash for each of the periods below:
|
|
|
|
|
|
|
|
|
|
|
Three months ended March 31, |
|
|
|
2024 |
|
|
2023 |
|
|
|
(in thousands) |
|
Net cash, cash equivalents and restricted cash (used in) provided by |
|
|
|
|
|
|
Operating activities |
|
$ |
(15,436 |
) |
|
$ |
(21,505 |
) |
Investing activities |
|
|
16,282 |
|
|
|
30,270 |
|
Financing activities |
|
|
16 |
|
|
|
913 |
|
Net increase in cash, cash equivalents and restricted cash |
|
$ |
862 |
|
|
$ |
9,678 |
|
Cash Flows from Operating Activities
Net cash, cash equivalents and restricted cash used in operating activities was $15.4 million for the three months ended March 31, 2024. The primary use of cash was our net loss of $31.6 million, changes in our assets and liabilities of $0.4 million, partially offset by $16.6 million of non-cash items primarily including the impairment of fixed assets and the right of use asset, change in fair value of purchase warrants, depreciation, equity-based compensation, and the right of use asset. The changes in our assets and liabilities include increases in prepaid expenses and other current assets, and decreases in prepaid research and development expenses, the operating lease liability, and accounts payable and accrued expenses. Net cash, cash equivalents and restricted cash used in operating activities for each of the remaining three quarters of the fiscal year ending December 31, 2024 is expected to be lower than the amount reported for the three months ended March 31, 2024, primarily due to the discontinuation of the Synpheny-3 trial and the reduction in workforce.
Net cash, cash equivalents and restricted cash used in operating activities was $21.5 million for the three months ended March 31, 2023. The primary use of cash was our net loss of $15.6 million, changes in our assets and liabilities of $7.7 million, partially offset by $1.8 million of non-cash items primarily including depreciation, equity-based compensation, and the right of use asset. The changes in our assets and liabilities include increases in prepaid research and development expenses, increases in prepaid expenses and other current assets, and decreases in the operating lease liability, accounts payable and accrued expenses, and deferred revenue.
Cash Flows from Investing Activities
Net cash provided by investing activities for the three months ended March 31, 2024 was $16.3 million and resulted primarily from the proceeds from maturity of marketable securities of $18.0 million and $0.2 million from sales of property and equipment. This was offset by the purchases of marketable securities of $1.5 million, and the purchases of property and equipment of $0.4 million.
Net cash provided by investing activities for the three months ended March 31, 2023 was $30.3 million and resulted primarily from the proceeds from maturity of marketable securities of $30.4 million. This was offset by the purchases of property and equipment of $0.1 million.
Cash Flows from Financing Activities
Net cash provided by financing activities for the three months ended March 31, 2024 totaled $0.02 million, primarily related to net proceeds from the sale of our common stock in the ATM offering program.
Net cash provided by financing activities for the three months ended March 31, 2023 totaled $0.9 million, primarily related to net proceeds from the sale of our common stock in the ATM offering program.
Funding Requirements
We currently expect our expenses to decrease in the near term due to our decision to discontinue our Synpheny-3 clinical trial and conduct workforce reductions while we explore strategic alternatives. Pending the outcome of our review of strategic alternatives, and should we decide to continue to advance the clinical development of our product candidates, we expect to incur additional costs in connection with such activities.
We have generated revenue from our prior collaboration with Roche and other collaborations, but have not generated any product revenue since our inception and do not expect to generate any product revenue unless we receive regulatory approval for our product candidates. The Company believes that its current cash and cash equivalents as of March 31, 2024 will be sufficient to fund its operations at the current levels for at least the next 12 months from the date of this filing.
Our funding requirements will depend on many factors, including, but not limited to, the following:
•the outcome, success, timing and cost of any strategic transactions, business combinations or divestiture;
•the success of our research and development efforts;
•the initiation, progress, timing, costs and results of clinical trials for our product candidates;
•the time and costs involved in obtaining regulatory approvals for our product candidates;
•the progress, timing and costs involved in developing manufacturing processes and agreements with third-party manufacturers;
•the rate of progress and cost of our commercialization activities;
•the expenses we incur in marketing and selling our product candidates;
•the revenue generated by sales of our product candidates;
•the emergence of competing or complementary technological developments;
•the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
•the terms and timing of any additional collaborative, licensing or other arrangements that we may establish;
•the acquisition of businesses, products and technologies;
•our need to implement additional infrastructure and internal systems;
•our need to add personnel and financial and management information systems to support our product development and potential future commercialization efforts, and to enable us to operate as a public company;
•the extent to which our business is adversely impacted by the effects of the coronavirus outbreak or by other health epidemics or pandemics; and
•other risks and uncertainties, including those listed under Part I, Item 1A. “Risk Factors”.
Contractual Commitments and Obligations
There have been no material changes to our contractual obligations and commitments set forth under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Contractual Obligations and Commitments” in our 2023 Annual Report.
Related Party Transactions
For a description of transactions with related parties which may fall outside of the reporting period of this section, please see the section entitled “Certain Relationships and Related Person Transactions” in Amendment No. 1 to our 2023 Annual Report on Form 10-K/A filed with the SEC on April 29, 2024.
Recently Issued Accounting Pronouncements
For detailed information regarding recently issued accounting pronouncements and the expected impact on our consolidated financial statements, see Note 2, Summary of Significant Accounting Policies in the notes to the unaudited consolidated financial statements appearing elsewhere in this Quarterly Report on Form 10-Q.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide this information required under this item.
Item 4. Controls and Procedures
Definition and limitations of disclosure controls
Our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed under the Exchange Act, such as this report, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures are also designed to ensure that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. Our management evaluates these controls and procedures on an ongoing basis.
There are inherent limitations to the effectiveness of any system of disclosure controls and procedures. These limitations include the possibility of human error, the circumvention or overriding of the controls and procedures and reasonable resource constraints. In addition, because we have designed our system of controls based on certain assumptions, which we believe are reasonable, about the likelihood of future events, our system of controls may not achieve its desired purpose under all possible future conditions. Accordingly, our disclosure controls and procedures provide reasonable assurance, but not absolute assurance, of achieving their objectives.
Evaluation of Disclosure Controls and Procedures
Our principal executive officer and principal financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of the end of the period covered by this Form 10-Q, have concluded that, based on such evaluation, our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control
There have not been any changes in our internal controls over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) identified in connection with the evaluation of such internal control that occurred during our fiscal quarter ended March 31, 2024 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
PART II – OTHER INFORMATION
Item 1. Legal Proceedings.
We are not currently a party to any material legal proceedings.
1A. Risk Factors.
Investing in our common stock involves a high degree of risk. Our business, prospects, financial condition or operating results could be materially adversely affected by the risks identified below, as well as other risks not currently known to us or that we currently consider immaterial. Furthermore, these factors represent risks and uncertainties that could cause actual results to differ materially from those implied by forward-looking statements. Accordingly, in evaluating our business, we encourage you to consider the following discussion of risk factors, in its entirety, in addition to other information contained in this Quarterly Report on Form 10-Q and our other public filings with the SEC. The following risk factors may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future.
In the following discussion of risk factors, References to “we”, “us”, “our” and similar terms refer to the combined business of Synlogic, Inc. after the Merger on August 28, 2017.
Summary of Risk Factors
Our business is subject to numerous risks and uncertainties, including those highlighted in this section below, that represent challenges that we face in connection with the successful implementation of our strategy. The occurrence of one or more of the events or circumstances described in more detail in the risk factors below, alone or in combination with other events or circumstances, may have an adverse effect on our business, cash flows, financial condition and results of operations. Such risks include, but are not limited to:
•Our business to date has been almost entirely dependent on the success of SYNB1934, and we have decided to discontinue further development of SYNB1934 and devote significant time and resources to identifying and evaluating strategic alternatives, which may not be successful.
•If we do not successfully consummate a strategic transaction, our board of directors may decide to pursue a dissolution and liquidation of our company. In such an event, the amount of cash available for distribution to our stockholders will depend heavily on the timing of such liquidation as well as the amount of cash that will need to be reserved for commitments and contingent liabilities.
•We are a biopharmaceutical company with a history of losses, and we expect to continue to incur losses for the foreseeable future, and we may never achieve or maintain profitability.
•We will require substantial additional funding, which may not be available on acceptable terms, or at all.
•Our quarterly and annual operating results may fluctuate in the future. As a result, we may fail to meet the expectations of research analysts or investors, which could cause our stock price to decline.
•Our stock price is volatile, and our stockholders may not be able to resell shares of our common stock at or above the price they paid.
•Our short operating history may make it difficult for stockholders to evaluate the success of our business to date and to assess our future viability.
•Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults or non-performance by financial institutions or transactional counterparties, could adversely affect our current and projected business operations and its financial condition and results of operations.
•Clinical trials are costly, time consuming and inherently risky, and we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
•The approach we are taking to discover and develop novel therapeutics using synthetic biology to create novel medicines is unproven and may never lead to marketable products.
•Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial viability of an approved label, or result in significant negative consequences following marketing approval, if any.
•Product development involves a lengthy and expensive process with an uncertain outcome, and results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results.
•If we experience delays or difficulties in the enrollment of patients in clinical trials, our costs might be higher than expected and our receipt of necessary regulatory approvals could be delayed or prevented.
•We may face potential product liability claims, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use or misuse of our product candidates harms patients or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals, if any, could be revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims. If we are unable to obtain adequate insurance or are required to pay for liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage, such liability could adversely affect our financial condition.
•A pandemic, epidemic, or outbreak of an infectious disease, such as COVID-19, or geopolitical tensions, such as the armed conflict between Russia and Ukraine, or the conflict in the Middle East, may materially and adversely affect our business and our financial results.
•Even if we obtain regulatory approval for a product candidate, we will remain subject to ongoing regulatory requirements.
•We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
•We may be subject to, or may in the future become subject to, U.S. federal and state, and foreign laws and regulations imposing obligations on how we collect, use, disclose, store and process personal information. Our actual or perceived failure to comply with such obligations could result in liability or reputational harm and could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our customer base, and thereby decrease our revenue.
•If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business, financial condition or results of operations.
•Our internal computer systems, or those of our collaborators or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
•Ethical, legal and social concerns about synthetic biology and genetic engineering could limit or prevent the use of our technologies and limit our revenues.
•We may not be successful in obtaining or maintaining necessary rights to Synthetic Biotics, product candidates and processes for our development pipeline through acquisitions and in-licenses.
•We may not have sufficient patent term protections for our product candidates to effectively protect our business.
•If we are unable to maintain effective proprietary rights for our product candidates or any future product candidates, we may not be able to compete effectively in our proposed markets.
•Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
•We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming, and unsuccessful.
•We may be subject to claims challenging the inventorship of our patents and other intellectual property.
•We may not be able to protect our intellectual property rights throughout the world.
•We have historically relied on and may in the future rely, on third parties to conduct some aspects of our product formulation, research, preclinical, and clinical studies, and those third parties may not perform satisfactorily, including by failing to meet deadlines for the completion of such formulation, research or testing.
•We have historically relied on and may in the future rely on third-party supply and manufacturing partners for drug supplies for our late-stage clinical activities and may do the same for any commercial supplies of our product candidates.
•We may attempt to form collaborations in the future with respect to our product candidates, but we may not be able to do so, which may cause us to alter our development and commercialization plans.
•If the market opportunities for our product candidates are smaller than we believe they are, we may not meet our revenue expectations and, assuming approval of a product candidate, our business may suffer. Because the patient populations in the market for our product candidates may be small, we must be able to successfully identify patients and acquire a significant market share to achieve profitability and growth.
•Our principal stockholders and management own a significant percentage of our stock and are able to exert significant control over matters subject to stockholder approval.
•Provisions of our charter documents or Delaware law could delay or prevent an acquisition of us, even if the acquisition would be beneficial to our stockholders and could make it more difficult for you to change management.
•If we fail to comply with the continued listing requirements of the Nasdaq Capital Market, our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
Risks Related to Our Evaluation of Strategic Alternatives
Our business to date has been significantly dependent on the success of SYNB1934, and we have decided to discontinue further development of SYNB1934 and devote significant time and resources to identifying and evaluating strategic alternatives, which may not be successful.
To date, we have invested significant efforts and financial resources in the research and development of SYNB1934, which was our lead product candidate in clinical trials. In February 2024, we voluntarily halted Synpheny-3 based on results of an internal review in advance of an upcoming independent Data Monitoring Committee (DMC) assessment, which indicated the trial was unlikely to meet its primary endpoint. The decision was not based on concerns regarding safety or tolerability. Beginning in February 2024, we started to reduce operating expenses while we evaluate our strategic alternatives with a goal to enhance stockholder value, including the possibility of a merger or sale of the Company. There can be no assurance that our process to identify and evaluate potential strategic alternatives will result in any definitive offer to consummate a strategic transaction, or if made what the terms thereof will be or that any transaction will be approved or consummated. If any definitive offer to consummate a strategic transaction is received, there can be no assurance that a definitive agreement will be executed or that, if a definitive agreement is executed, the transaction will be consummated. In addition, there can be no assurance that any transaction, involving our company and/or assets, that is consummated would enhance shareholder value. There also can be no assurance that we will conduct further drug research or development activities in the future.
Any such strategic transaction may require us to incur non-recurring or other charges, may increase our near-and long-term expenditures and may pose significant integration challenges or disrupt our management or business, which could adversely affect our operations and financial results. For example, these transactions may entail numerous operational and financial risks, including:
•exposure to unknown liabilities;
•incurrence of substantial debt or dilutive issuances of equity securities to pay for acquisitions;
•higher-than-expected acquisition and integration costs;
•write-downs of assets or goodwill or impairment charges;
•increased amortization expenses;
•difficulty and cost in combining the operations and personnel of any acquired businesses with our operations and personnel;
•impairment of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and
•inability to retain key employees of our company or any acquired businesses.
If we do not successfully consummate a strategic transaction, our board of directors may decide to pursue a dissolution and liquidation of our company. In such an event, the amount of cash available for distribution to our stockholders will depend heavily on the timing of such liquidation as well as the amount of cash that will need to be reserved for commitments and contingent liabilities.
There can be no assurance that the process to identify a strategic transaction will result in a successfully consummated transaction. If no transaction is completed, our board of directors may decide to pursue a dissolution and liquidation of our company. In such an event, the amount of cash available for distribution to our stockholders will depend heavily on the timing of such decision and, ultimately, such liquidation, since the amount of cash available for distribution continues to decrease as we fund our operations while we evaluate our strategic alternatives. In addition, if our board of directors were to approve and recommend, and our
stockholders were to approve, a dissolution and liquidation of our company, we would be required under Delaware corporate law to pay our outstanding obligations, as well as to make reasonable provision for contingent and unknown obligations, prior to making any distributions in liquidation to our stockholders. Our commitments and contingent liabilities may include (i) obligations under our employment and related agreements with certain employees that provide for severance and other payments following a termination of employment occurring for various reasons, including a change in control of our company; (ii) potential litigation against us, and other various claims and legal actions arising in the ordinary course of business; and (iii) non-cancelable facility lease obligations. As a result of this requirement, a portion of our assets may need to be reserved pending the resolution of such obligations. In addition, we may be subject to litigation or other claims related to a dissolution and liquidation of our company. If a dissolution and liquidation were pursued, our board of directors, in consultation with its advisors, would need to evaluate these matters and make a determination about a reasonable amount to reserve. Accordingly, holders of our common stock could lose all or a significant portion of their investment in the event of a liquidation, dissolution or winding up of our company.
Risks Related to Our Financial Condition, Capital Requirements and Operating Results
We are a biopharmaceutical company with a history of losses, and we expect to continue to incur losses for the foreseeable future, and we may never achieve or maintain profitability.
We are a biopharmaceutical company developing Synthetic Biotics and we have incurred significant operating losses since our inception. Our net loss was approximately $31.6 million for the three months ended March 31, 2024 and $15.6 million for the three months ended March 31, 2023, respectively. As of March 31, 2024, we had an accumulated deficit of approximately $445.9 million. To date, we have not generated any product revenue. Substantially all of our losses have resulted from expenses incurred in connection with our research and development programs and from general and administrative costs associated with our operations. We have no products on the market.
We have not generated, and do not expect to generate, any product revenue for the foreseeable future, and we expect to continue to incur significant operating losses for the foreseeable future due to the cost of research and development, preclinical studies and clinical trials, the regulatory review process for product candidates, and the development of manufacturing and marketing capabilities for any product candidates approved for commercial sale. The amount of our potential future losses is uncertain. To achieve profitability, we must successfully develop product candidates, obtain regulatory approvals to market and commercialize product candidates, manufacture any approved product candidates on commercially reasonable terms, establish a sales and marketing organization or suitable third-party alternatives for any approved product candidates and raise sufficient funds to finance our business activities. We may never succeed in these activities and, even if we do, may never generate revenues that are significant or large enough to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease our value and could impair our ability to raise capital, maintain our research and development efforts, expand our business or continue our operations. A decline in our value could also cause our stockholders to lose all or part of their investment.
We will require substantial additional funding, which may not be available on acceptable terms, or at all.
We have used substantial funds to discover and develop our programs and proprietary drug development platform in synthetic biology and will require substantial additional funds to conduct further research and development, including preclinical studies and clinical trials of our product candidates, seek regulatory approvals for our product candidates and manufacture and market any products that are approved for commercial sale. Our future capital requirements and the period for which we expect our existing resources to support our operations may vary significantly from what we expect. Our monthly spending levels vary based on new and ongoing research and development and corporate activities. Because we cannot be certain of the length of time or activities associated with successful development and commercialization of our product candidates, we are unable to estimate the actual funds we will require to develop and commercialize them.
We do not expect to realize any appreciable revenue from product sales or royalties in the foreseeable future, if at all. Our revenue sources will remain very limited unless and until our product candidates complete clinical development and are approved for commercialization and successfully marketed or we enter into third-party arrangements with collaborators. To date, we have primarily financed our operations through sales of our securities, our third-party collaborations and the Merger. We intend to seek additional funding in the future through collaborations, equity or debt financings, credit or loan facilities or a combination of one or more of these financing sources. Our ability to raise additional funds will depend on financial, economic and other factors, many of which are beyond our control. Additional funds may not be available to us on acceptable terms or at all. If we raise additional funds by issuing equity or convertible debt securities, our stockholders will suffer dilution and the terms of any financing may adversely affect the rights of our stockholders. In addition, as a condition to providing additional funds to us, future investors may demand, and may be granted, rights superior to those of existing stockholders. Debt financing, if available, may involve restrictive covenants limiting our flexibility in conducting future business activities, and, in the event of insolvency, debt holders would be repaid before holders of equity securities received any distribution of corporate assets.
If we are unable to obtain funding on a timely basis or on acceptable terms, or at all, we may have to delay, limit or terminate our research and development programs and preclinical studies or clinical trials, if any, limit strategic opportunities or undergo
reductions in our workforce or other corporate restructuring activities. We also could be required to seek funds through arrangements with collaborators or others that may require us to relinquish rights to some of our product candidates or technologies that we would otherwise pursue on our own.
Our quarterly and annual operating results may fluctuate in the future. As a result, we may fail to meet the expectations of research analysts or investors, which could cause our stock price to decline.
Our financial condition and operating results may fluctuate from quarter to quarter and year to year in the future due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include the following factors, as well as factors described elsewhere in this Quarterly Report on Form 10-Q and others:
•Our ability to achieve or maintain profitability;
•Our ability to develop and maintain Synthetic Biotic technologies;
•Our ability to manage our growth;
•The outcomes of research programs, clinical trials, or other product development and approval processes;
•Our ability to accurately report our financial results in a timely manner;
•Our dependence on, and the need to attract and retain, key management and other personnel;
•Our ability to obtain, protect and enforce our intellectual property rights;
•Our ability to prevent the theft or misappropriation of our intellectual property, know-how or technologies;
•Our ability to achieve milestones with our collaborators;
•Potential advantages that our competitors and potential competitors may have in securing funding or developing competing technologies or products;
•Our ability to obtain additional capital that may be necessary to expand our business; and
•Other macro-economic factors outside of our control.
Due to the various factors mentioned above, and others, the results of any prior quarterly or annual periods should not be relied upon as indications of our future operating performance.
Our stock price is volatile, and our stockholders may not be able to resell shares of our common stock at or above the price they paid.
The trading price of our common stock is highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, such as reports by industry analysts, investor perceptions, general industry trends, macro-economic conditions, or negative announcements by other companies involving similar technologies or diseases. These factors also include those discussed in this “Risk Factors” section of this Quarterly Report on Form 10-Q and others such as:
•Announcements relating to collaborations that we may enter into with respect to the development or commercialization of our product candidates;
•Announcements relating to the receipt, modification or termination of government contracts or grants;
•Termination or delay of a development program;
•Product liability claims related to our clinical trials or product candidates;
•Prevailing economic conditions;
•Perspectives on synthetic biology and genetic engineering;
•Perspectives on the programs of competitors;
•Additions or departures of key personnel;
•Business disruptions caused by natural disasters;
•Disputes concerning our intellectual property or other proprietary rights;
•FDA or other U.S. or foreign regulatory actions affecting us or our industry;
•Sales of our common stock by the company, our executive officers and directors or our stockholders in the future;
•Future sales or issuances of equity or debt securities by us;
•Lack of an active, liquid and orderly market in our common stock;
•Fluctuations in our quarterly operating results; and
•The issuance of new or changed securities analysts’ reports or recommendations regarding us.
In addition, the stock markets in general, and the markets for pharmaceutical, biopharmaceutical and biotechnology stocks in particular, have experienced extreme volatility that have been often unrelated to the operating performance of the issuer. These broad market fluctuations may adversely affect the trading price or liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our management would be diverted from the operation of our business.
Our short operating history may make it difficult for stockholders to evaluate the success of our business to date and to assess our future viability.
We are a biopharmaceutical company with a limited operating history. We commenced active operations in 2014. Our operations to date have been limited to organizing and staffing our company, research and development activities, business planning and raising capital. In February 2024, we voluntarily halted Synpheny-3 based on results of an internal review in advance of an upcoming independent Data Monitoring Committee (DMC) assessment, which indicated the trial was unlikely to meet its primary endpoint. The decision was not based on concerns regarding safety or tolerability. We have not yet demonstrated our ability to successfully complete large-scale, pivotal clinical trials, obtain marketing approvals, manufacture a commercial-scale product, or arrange for a third-party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Typically, it takes many years to develop one new product candidate from the time it is discovered to the time that it becomes available for treating patients. We may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors that may hinder our success in commercializing one or more of our product candidates. Further, drug development is a capital-intensive and highly speculative undertaking that involves a substantial degree of risk. You should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development and clinical trials. Any forward-looking statements regarding our future prospects, plans or viability may not be as accurate as they may be if we had a longer operating history or a history of successfully developing and commercializing pharmaceutical products.
Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults or non-performance by financial institutions or transactional counterparties, could adversely affect our current and projected business operations and its financial condition and results of operations.
Actual events involving limited liquidity, defaults, non-performance or other adverse developments that affect financial institutions, transactional counterparties or other companies in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds or other similar risks, have in the past and may in the future lead to market-wide liquidity problems. For example, on March 10, 2023, Silicon Valley Bank (SVB), was closed by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation (the FDIC) as receiver. Similarly, on March 12, 2023, Signature Bank and Silvergate Capital Corp. were each swept into receivership and on May 1, 2023, First Republic Bank was swept into receivership. Although a statement by the Department of the Treasury, the Federal Reserve and the FDIC stated that all depositors of SVB would have access to all of their money after only one business day of closure, including funds held in uninsured deposit accounts, uncertainty remains over liquidity concerns in the broader financial services industry. Similar impacts have occurred in the past, such as during the 2008-2010 financial crisis.
Inflation and rapid increases in interest rates have led to a decline in the trading value of previously issued government securities with interest rates below current market interest rates. Although the U.S. Department of Treasury, FDIC and Federal Reserve Board have announced a program to provide up to $25 billion of loans to financial institutions secured by certain of such government securities held by financial institutions to mitigate the risk of potential losses on the sale of such instruments, widespread demands for customer withdrawals or other liquidity needs of financial institutions for immediate liquidity may exceed the capacity of such program. There is no guarantee that the U.S. Department of Treasury, FDIC and Federal Reserve Board will provide access to uninsured funds in the future in the event of the closure of other banks or financial institutions, or that they would do so in a timely fashion.
Although we review our banking relationships as we believe appropriate, our access to funding sources and other credit arrangements in amounts adequate to finance or capitalize our current and projected future business operations could be significantly impaired by factors that affect us, the financial institutions with which we have arrangements directly, or the financial services industry or economy in general. These factors could include, among others, events such as liquidity constraints or failures, the ability to perform obligations under various types of financial, credit or liquidity agreements or arrangements, disruptions or instability in the financial services industry or financial markets, or concerns or negative expectations about the prospects for companies in the financial services industry. These factors could involve financial institutions or financial services industry companies with which we have financial or business relationships, but could also include factors involving financial markets or the financial services industry generally.
The results of events or concerns that involve one or more of these factors could include a variety of material and adverse impacts on our current and projected business operations and our financial condition and results of operations. These could include, but may not be limited to, the following:
•delayed access to deposits or other financial assets or the uninsured loss of deposits or other financial assets;
•loss of access to working capital sources;
•potential or actual breach of contractual obligations that require us to maintain letters or credit or other credit support arrangements; or
•termination of cash management arrangements and/or delays in accessing or actual loss of funds subject to cash management arrangements.
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any decline in available funding or access to our cash and liquidity resources could, among other risks, adversely impact our ability to meet our operating expenses, financial obligations or fulfill our other obligations, result in breaches of our financial and/or contractual obligations or result in violations of federal or state wage and hour laws. Any of these impacts, or any other impacts resulting from the factors described above or other related or similar factors not described above, could have material adverse impacts on our liquidity and our current and/or projected business operations and financial condition and results of operations.
In addition, any further deterioration in the macroeconomic economy or financial services industry could lead to losses or defaults by parties with whom we conduct business, which in turn, could have a material adverse effect on our current and/or projected business operations and results of operations and financial condition.
Risks Related to the Development of Our Product Candidates
Clinical trials are costly, time consuming and inherently risky, and we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Clinical development of a product candidate is expensive, time consuming and involves significant risk. We cannot guarantee that any clinical trials we undertake to conduct will be conducted as planned or completed on schedule or at all. A failure of one or more clinical trials can occur at any stage of development. For example, in February 2024, we voluntarily halted Synpheny-3 based on results of an internal review in advance of an upcoming independent DMC assessment, which indicated the trial was unlikely to meet its primary endpoint. The decision was not based on concerns regarding safety or tolerability. Events that may prevent successful or timely completion of clinical development of our product candidates include but are not limited to:
•Inability to generate satisfactory preclinical or other nonclinical data, including, toxicology, or other in vivo or in vitro data or diagnostics to support the initiation or continuation of clinical trials;
•Delays in reaching agreement on acceptable terms with CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites;
•Delays in obtaining required institutional review board approval at each clinical trial site;
•Failure to permit the conduct of a clinical trial by regulatory authorities, after review of an investigational new drug or equivalent foreign application or amendment;
•Delays in recruiting qualified patients in our clinical trials;
•Failure by clinical sites or CROs or other third parties to adhere to clinical trial requirements;
•Failure by us, clinical sites, CROs or other third parties to perform in accordance with the good clinical practices requirements of the FDA or applicable foreign regulatory guidelines;
•Patients dropping out of the clinical trials;
•Occurrence of adverse events, unacceptable side effects or toxicity issues associated with our product candidates;
•Imposition by the FDA of a clinical hold or the requirement by other similar regulatory agencies that one or more clinical trials be delayed or halted;
•Changes in regulatory requirements and guidance that require amending or submitting new clinical protocols or performing additional nonclinical studies;
•The ultimate affordability of the cost of clinical trials of our product candidates;
•Negative or inconclusive results from our clinical trials that may result in us deciding, or regulators requiring us, to conduct additional clinical trials or abandon such clinical trials and/or clinical trials or development programs in other ongoing or planned indications for a product candidate; and
•Delays in identifying or reaching agreement on acceptable terms with third-party manufacturers, delays in developing and transferring a reproducible, scalable manufacturing process, or delays or failure in manufacturing sufficient quantities of our product candidates for use in clinical trials.
Any inability to successfully complete clinical development and obtain regulatory approval for our product candidates could result in additional costs to us or impair our ability to raise additional capital or generate revenue. In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional nonclinical studies and/or clinical trials, the results obtained from such new formulation may not be consistent with previous results obtained, or the regulatory authorities may need to further review and approve our process before we can proceed. Clinical trial delays could also shorten any anticipated periods of patent exclusivity for our product candidates and may allow competitors to develop and bring products to market before we do which could impair our ability to successfully commercialize our product candidates and may harm our business and results of operations.
The approach we are taking to discover and develop novel therapeutics using synthetic biology to create novel medicines is unproven and may never lead to marketable products.
The scientific discoveries that form the basis for our efforts to generate and develop our product candidates are relatively recent. The scientific evidence to support the feasibility of developing drugs based on our approach is both preliminary and limited. Synthetic Biotics represent a novel therapeutic modality and their successful development by us may require additional studies and efforts to optimize their therapeutic potential. Any product candidates that we develop may not demonstrate in patients the therapeutic properties ascribed to them in laboratory and other preclinical studies, and they may interact with human biological systems in unforeseen, ineffective or even harmful ways. We have also not yet succeeded and may never succeed in demonstrating efficacy and safety for our current or any future product candidates in a pivotal clinical trial. If we are not able to successfully develop and commercialize product candidates based upon this technological approach, we may never become profitable, and the value of our capital stock may decline. Additionally, the FDA or other regulatory authorities may lack experience in evaluating the safety and efficacy of novel product candidates based on synthetic biology, which could result in a longer than expected regulatory review process, increase our expected development costs and delay or prevent commercialization of our product candidates.
Our Synthetic Biotic product candidates are based on a relatively novel technology, which makes it difficult to predict the time and cost of development and of subsequently obtaining regulatory approval, if at all.
We have concentrated our research and development efforts to date on a limited number of product candidates based on our Synthetic Biotic therapeutic platform. Our future success depends on our successful development of viable product candidates. There can be no assurance that we will not experience problems or delays in developing our product candidates and that such problems or delays will not cause unanticipated costs, or that any such development problems can be solved. For example, in February 2024, we voluntarily halted Synpheny-3 based on results of an internal review in advance of an upcoming independent DMC assessment, which indicated the trial was unlikely to meet its primary endpoint. The decision was not based on concerns regarding safety or tolerability.
The clinical trial and manufacturing requirements of the FDA, the EMA and other regulatory authorities, and the criteria these regulators use to determine the safety and efficacy of a product candidate, vary substantially according to the type, complexity, novelty and intended use and market of the product candidate. The regulatory approval process for novel product candidates such as Synthetic Biotics may be more expensive and take longer than for other, better known or more extensively studied therapeutic modalities. It is difficult to determine how long it will take or how much it will cost to obtain regulatory approvals for our product candidates in either the United States or the European Union or how long it will take to commercialize our product candidates, even if approved for marketing. Approvals by the EMA or national regulatory agencies may not be indicative of what the FDA, and vice versa, may require for approval and different or additional nonclinical studies or clinical trials may be required to support regulatory
approval in each respective jurisdiction. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a potential product candidate to market could decrease our ability to generate sufficient product revenue, and our business, financial condition, results of operations and prospects may be harmed.
We may not be successful in our efforts to use and expand our development platform to build a pipeline of product candidates.
A key element of our strategy is to create Synthetic Biotics that can be deployed against a broad range of human diseases in order to build a pipeline of product candidates. Although our research and development efforts to date have resulted in potential product candidates, we may not be able to continue to identify and develop additional product candidates. Even if we are successful in continuing to build our pipeline, the potential product candidates that we identify may not be suitable for clinical development. For example, these potential product candidates may be shown to have harmful side effects or other characteristics that indicate that they are unlikely to be drugs that will receive marketing approval and achieve market acceptance. If we do not successfully develop and commercialize product candidates based upon our approach, we will not be able to obtain product revenue in future periods, which likely would result in significant harm to our financial position. There is no assurance that we will be successful in our preclinical and clinical development, and the process of obtaining regulatory approvals will, in any event, require the expenditure of substantial time and financial resources.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial viability of an approved label, or result in significant negative consequences following marketing approval, if any.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or terminate our clinical trials or result in a restrictive label or delay regulatory approval by the FDA or comparable foreign authorities. Undesirable side effects and negative results for other indications may negatively impact the development and potential for approval of our product candidates for their proposed indications.
Additionally, even if one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such products, potentially significant negative consequences could result, including but not limited to:
•Regulatory authorities may withdraw approvals of or revoke licenses for such products;
•Regulatory authorities may require additional warnings on the labels of such products;
•We may be required to create a REMS plan, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers, and/or other elements to assure safe use;
•We may be required to conduct additional clinical trials or costly post-marketing testing and surveillance to monitor the safety of the product;
•We could be sued and held liable for harm caused to patients; and
•Our reputation may suffer.
Any of these events could prevent us from achieving or maintaining market acceptance of a product candidate, even if approved, and could significantly harm our business, results of operations, and prospects.
Our product development program may not uncover all possible adverse events that patients who take our product candidates may experience. The number of subjects exposed to our product candidates during clinical trials and the average exposure time in the clinical development program may be inadequate to detect rare adverse events, or chance findings, that may only be detected once the product is administered to more patients and for greater periods of time.
Clinical trials by their nature use a sample of the potential patient population. However, with a limited number of patients and limited duration of exposure, we cannot be fully assured that uncommon or severe side effects of our product candidates will be uncovered. Such side effects may only be uncovered with a significantly larger number of patients exposed to the drug. If such safety problems occur or are identified after a product candidate reaches the market, the FDA may require that we amend the labeling of the product or recall the product or may even withdraw approval for the product. Any of these events could prevent us from achieving or maintaining market acceptance of a product candidate, even if approved, and could significantly harm our business, results of operations, and prospects.
If we fail to obtain or maintain orphan drug exclusivity for some of our products, our competitors may obtain approval to sell competing drugs to treat the same conditions and our revenues will be reduced.
As part of our business strategy, we have developed and may in the future develop product candidates that may be eligible for FDA and European Commission orphan drug designation. In May 2023, the FDA granted orphan drug designation to labafenogene marselecobac (SYNB1934) for the treatment of PKU. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is intended to treat, diagnose or prevent rare diseases or conditions that affect fewer than 200,000 people in the United States. In the EU, orphan drug designation may be granted to drugs intended to treat, diagnose or prevent a life-threatening or chronically debilitating disease having a prevalence of no more than five in 10,000 people in the EU. The company that first obtains FDA approval for a designated orphan drug for the associated rare disease receives marketing exclusivity for use of that drug for the stated condition for a period of seven years. Orphan drug exclusive marketing rights may be lost under several circumstances, including a later determination by the FDA that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug. Similar regulations are in effect in the EU with a ten-year period of market exclusivity.
Because the extent and scope of patent protection for some of our product candidates may be limited, obtaining orphan drug exclusivity is especially important for any product candidates that may be eligible for orphan drug designation. For eligible products, we plan to rely on the exclusivity period under the Orphan Drug Act to maintain a competitive position. If we do not obtain orphan drug designation for our product candidates that do not have broad patent protection, our competitors may then seek to sell a competing drug to treat the same condition and our revenues, if any, may be adversely affected thereby.
Even though we have obtained orphan drug designation for some of our product candidates and intend to seek orphan drug designation for other product candidates, there is no assurance that we will be the first to obtain marketing approval for any particular orphan indication. Further, even though we have obtained orphan drug designation for certain of our product candidates, or even if we obtain orphan drug designation for other potential product candidates, and obtain approval for such products, orphan product exclusivity may not effectively protect us from competition because different drugs can be approved for the same condition and the same drug can be approved for different conditions and potentially used off-label in the orphan indication. In addition, after an orphan drug is approved, the FDA can subsequently approve a competing drug for the same condition for several reasons, including, if the FDA concludes that the later drug is safer or more effective or makes a major contribution to patient care. Orphan drug designation neither shortens the development time or regulatory review time of a drug, nor gives the drug any advantage in the regulatory review or approval process.
We may seek a Rare Pediatric Disease Designation, or RPDD, for one or more of our product candidates. However, a BLA for one or more of our product candidates may not meet the eligibility criteria for a priority review voucher upon approval.
In January 2023, labafenogene marselecobac received RPDD (Rare Pediatric Drug Designation) for phenylketonuria and in December 2022, SYNB1353 received RPDD for homocystinuria. With enactment of the Food and Drug Administration Safety and Innovation Act in 2012, Congress authorized the FDA to award priority review vouchers to sponsors of certain rare pediatric disease product applications that meet the criteria specified in the law. This provision is designed to encourage development of new drug and biological products for prevention and treatment of certain rare pediatric diseases. Specifically, under this program, a sponsor who receives an approval for a drug or biologic for a “rare pediatric disease” may qualify for a voucher that can be redeemed to receive a priority review of a subsequent marketing application for a different product. The sponsor of a rare pediatric disease drug product receiving a priority review voucher may transfer (including by sale) the voucher to another sponsor. The voucher may be further transferred any number of times before the voucher is used, as long as the sponsor making the transfer has not yet submitted the application. The FDA may also revoke any priority review voucher if the rare pediatric disease drug for which the voucher was awarded is not marketed in the U.S. within one year following the date of approval.
For the purposes of this program, a “rare pediatric disease” is a (a) serious or life-threatening disease in which the serious or life-threatening manifestations primarily affect individuals aged from birth to 18 years, including age groups often called neonates, infants, children, and adolescents; and (b) rare disease or conditions within the meaning of the Orphan Drug Act. The FDA may determine that a BLA for one or more of our product candidates does not meet the eligibility criteria for a priority review voucher upon approval. Moreover, due to the current statutory authority for the RPDD and voucher program, the FDA may not award the voucher to sponsors of marketing applications unless either (i) the drug has received rare pediatric disease designation as of September 30, 2024, and is then approved by the FDA no later than September 30, 2026; or (ii) Congress reauthorizes the program. Even if legislation is enacted that extends the date by which approval of the rare pediatric disease-designated drug must obtain approval to receive a priority review voucher, we may not obtain approval by that date, and even if we do, we may not obtain a priority review voucher.
Product development involves a lengthy and expensive process with an uncertain outcome, and results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results.
The results from preclinical studies or early clinical trials of a product candidate may not predict the results that will be obtained in subsequent subjects or in later stage clinical trials of that product candidate or any other product candidate. Flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We have limited experience in designing clinical trials and we may be unable to design and execute clinical trials to support regulatory approval of our product candidates. In addition, preclinical study and clinical trial data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain regulatory authority approval. Product candidates that seemingly perform satisfactorily in preclinical studies and clinical trials may nonetheless fail to obtain regulatory approval. There is a high failure rate for drugs proceeding through clinical trials. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway, or safety or efficacy observations made in preclinical studies and clinical trials, including previously unreported adverse events. Any such setbacks in our clinical development could negatively affect our business and operating results.
If we experience delays or difficulties in the enrollment of patients in clinical trials, our costs might be higher than expected and our receipt of necessary regulatory approvals could be delayed or prevented.
Clinical trials of a new product candidate require the enrollment of a sufficient number of healthy volunteers or patients suffering from the disease or condition the product candidate is intended to treat and who meet other eligibility criteria. The timing of our clinical trials depends on our ability to recruit eligible subjects to participate as well as the completion of required follow-up evaluations. Patients and healthy volunteers may be unwilling to participate in our clinical trials because of negative publicity from adverse events related to novel therapeutic approaches, competitive clinical trials for similar patient populations, the existence of current treatments or for other reasons including due to concerns posed by local or global health emergencies. Rates of patient enrollment are affected by many factors, including the size of the potential patient population, the age and condition of the patients, the stage and severity of disease or condition, the nature and requirements of the protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease or condition, the perceived risks, the clinical trial administration practices of the contract research organization (CRO) or clinical trial sites, labor shortages at the CRO or clinical trial sites, benefits and convenience of administration of the product candidate being studied, the patient referral practices of physicians, the amount of attention provided to our trial by clinical trial sites, our efforts and the CRO efforts, our efforts to facilitate timely enrollment in clinical trials, and the eligibility criteria for the clinical trial. Delays or difficulties in patient enrollment or difficulties retaining trial participants, including as a result of the availability of existing or other investigational treatments, can result in increased costs, longer development times or termination of a clinical trial.
In addition, our success may depend, in part, on our ability to identify patients who qualify for our clinical trials or are likely to benefit from any product candidate that we may develop, which will require those potential patients to undergo a screening assay for the presence or absence of a particular genetic sequence or clinical trait. Genetically defined diseases generally, and especially those for which our current product candidates are targeted, may have relatively low prevalence. If we, or any third parties that we engage to assist us, are unable to successfully identify patients with these diseases, or experience delays in doing so, then we may not realize the full commercial potential of any product candidate we develop.
Congress also recently amended the FDCA to require sponsors of a Phase 3 clinical trial, or other “pivotal study” of a new drug or biologic to support marketing authorization, to design and submit a diversity action plan for such clinical trial. The action plan must describe appropriate diversity goals for enrollment, as well as a rationale for the goals and a description of how the sponsor will meet them. For any future Phase 3 trials we plan to conduct, we must submit a diversity action plan to the FDA by the time we submit plans for such Phase 3, or pivotal study, protocol to the agency for review as part of an IND, unless we are able to obtain a waiver for some or all of the requirements for a diversity action plan. It is unknown at this time how the diversity action plan may affect the planning and timing of any future Phase 3 trial for our product candidates or what specific information FDA will expect in such plans. However, initiation of such trials may be delayed if the FDA objects to our proposed diversity action plans for any future Phase 3 trial for our product candidates, and we may experience difficulties recruiting a diverse population of patients in attempting to fulfill the requirements of any approved diversity action plan.
Interim “ top-line” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in final data.
From time to time, we may publicly disclose interim, top-line or preliminary data from our nonclinical studies and clinical trials, which is based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the top-line or preliminary results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results once additional data have been received and fully
evaluated. Top-line or preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the top-line or preliminary data that we previously published. As a result, top-line and preliminary data should be viewed with caution until final data are available. Adverse differences between interim data and final data could significantly harm our business prospects. For example, in February 2024, we voluntarily halted Synpheny-3 based on results of an internal review in advance of an upcoming independent DMC assessment, which indicated the trial was unlikely to meet its primary endpoint. The decision was not based on concerns regarding safety or tolerability. Further, disclosure of interim data by us or by our competitors could result in volatility in the price of our common stock.
Further, others, including regulatory authorities, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our company in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure.
If the interim, top-line or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed, which could harm our business, operating results, prospects or financial condition.
We may face potential product liability claims, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use or misuse of our product candidates harms patients or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals, if any, could be revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims. If we are unable to obtain adequate insurance or are required to pay for liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage, such liability could adversely affect our financial condition.
The use or misuse of our product candidates in clinical trials and the sale of any products for which we may obtain marketing approval exposes us to the risk of potential product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our product candidates and approved products, if any. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. Patients with the diseases targeted by our product candidates may already be in severe and advanced stages of disease and have both known and unknown significant pre-existing and potentially life-threatening health risks. During the course of treatment, patients may suffer adverse events, including death, for reasons that may be related to our product candidates. Such events could subject us to costly litigation, require us to pay substantial amounts of money to injured patients, delay, negatively impact or end our opportunity to receive or maintain regulatory approval to market our products, or require us to suspend or abandon our commercialization efforts. Even in a circumstance in which an adverse event is unrelated to our product candidates, the investigation into the circumstance may be time-consuming or inconclusive. These investigations may delay our regulatory approval process or impact and limit the type of regulatory approvals our product candidates receive or maintain. As a result of these factors, a product liability claim, even if successfully defended, could have a material adverse effect on our business, financial condition or results of operations.
Although we have product liability insurance, which covers any clinical trial we may conduct in the United States or internationally, our insurance may be insufficient to reimburse us for any expenses or losses we may suffer. We will also likely be required to increase our product liability insurance coverage for the advanced clinical trials that we plan to initiate. If we obtain marketing approval for any of our product candidates, we will need to expand our insurance coverage to include the sale of commercial products. There is no way to know if we will be able to continue to obtain product liability coverage and obtain expanded coverage we may require, in sufficient amounts to protect us against losses due to liability, on acceptable terms, or at all. We may not have sufficient resources to pay for any liabilities resulting from a claim excluded from, or beyond the limits of, our insurance coverage. Where we have provided indemnities in favor of third parties under our agreements with them, there is also a risk that these third parties could incur liability and bring a claim under such indemnities. An individual may bring a product liability claim against us alleging that one of our product candidates or products causes, or is claimed to have caused, an injury or is found to be unsuitable for consumer use. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts. Any product liability claim brought against us, with or without merit, could result in:
•Withdrawal of clinical trial volunteers, investigators, patients or trial sites or limitations on approved indications;
•The inability to commercialize, or if commercialized, decreased demand for, our product candidates;
•If commercialized, product recalls, withdrawals of labeling, marketing or promotional restrictions or the need for product modification;
•Initiation of investigations by regulators;
•Substantial costs of litigation, including monetary awards to patients or other claimants;
•Liabilities that substantially exceed our product liability insurance, which we would then be required to pay ourselves;
•An increase in our product liability insurance rates or the inability to maintain insurance coverage in the future on acceptable terms, if at all;
•The diversion of management’s attention from our business;
•Loss of key employees; and
•Damage to our reputation and the reputation of our products and our technology.
Product liability claims may subject us to the foregoing and other risks, which could have a material adverse effect on our business, financial condition or results of operations.
We or the third parties upon which we depend may be adversely affected by natural disasters and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
Natural disasters could severely disrupt our operations and have a material adverse effect on our business, results of operations, financial condition and prospects. If a natural disaster, power outage or other event occurred that prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure, such as the manufacturing facilities of our third-party contract manufacturers, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity plans we have in place may prove inadequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which could have a material adverse effect on our business.
A pandemic, epidemic, or outbreak of an infectious disease, such as COVID-19, or geopolitical tensions, such as the armed conflict between Russia and Ukraine, or the conflict in the Middle East, may materially and adversely affect our business and our financial results.
Over the past several years, COVID-19 has affected segments of the global economy and it may materially affect our operations again, including potentially significant interruption of our clinical trial activities. In addition, there could be a continuing effect of COVID-19 to the business at FDA or other health authorities, which could result in delays of reviews and approvals, including with respect to our product candidates. The COVID-19 pandemic, including surges in cases, could also have a material adverse effect on our clinical trial operations in the United States and elsewhere, including our ability to recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19 if an outbreak occurs in their geography.
COVID-19 may also affect employees of third-party contract research organizations and contract manufacturing organizations located in affected geographies that we rely upon to carry out our clinical trials.
While the potential economic impact brought by and the duration of COVID-19 may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global financial markets, potentially reducing our ability to access capital, which could in the future negatively affect our liquidity. Similarly, the current conflicts between Ukraine and Russia and in the Middle East have created extreme volatility in the global capital markets and is expected to have further global economic consequences, including disruptions of the global supply chain and energy markets. In addition, we have previously announced our intention to open a clinical trial site in Israel and this could be impacted by the events in the Middle East. A recession or market correction resulting from the continued spread of COVID-19 or the geopolitical tensions in Russia and Ukraine, and in the Middle East, could materially affect our business and the price of our common stock.
Risks Related to Regulatory Approval of Our Product Candidates and Other Legal Compliance Matters
The regulatory approval processes of the FDA and other comparable foreign regulatory authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
The time required to obtain marketing approval for a novel therapeutic product from the FDA and other comparable foreign regulatory authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, laws or regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for commercialization, of any product
candidate and it is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain that approval.
Our product candidates could fail to receive regulatory approval for many reasons, including the following:
•The FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials;
•We may be unable to demonstrate to the satisfaction of the FDA or other comparable foreign regulatory authorities that our product candidates are safe, pure and potent or effective for their proposed indications;
•The results of clinical trials may not meet the level of statistical significance required by the FDA or other comparable foreign regulatory authorities in order to support approval;
•We may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks;
•The FDA or other comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials;
•The data collected from clinical trials of our product candidates may not be sufficient to support the submission of a BLA, to the FDA or other equivalent marketing authorization application submissions to obtain regulatory approval in the United States or elsewhere;
•Upon review of our clinical trial sites and data, the FDA or comparable foreign regulatory authorities may find our record keeping or the record keeping of our clinical trial sites or investigators to be inadequate;
•The FDA or other comparable foreign regulatory authorities may find deficiencies with or fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and
•The approval policies or regulations of the FDA or other comparable foreign regulatory authorities or the laws they enforce may significantly change in a manner rendering our clinical data insufficient for approval.
This lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market any of our product candidates, which would significantly harm our business, financial condition and results of operations. The FDA and other comparable foreign regulatory authorities have substantial discretion in the approval process and determining when or whether to grant regulatory approval will be obtained for any of our product candidates, and whether to impose any conditions on such marketing approvals as described below. Even if we believe the data collected from clinical trials of our product candidates are promising, such data may not be sufficient to support approval by the FDA or other comparable foreign regulatory authorities.
In addition, even if we were to obtain approval, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, if any, they may grant approval contingent on the performance of costly post-marketing clinical trials, or they may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate or with restrictive risk mitigation measures or warning language or contraindications that make the approved product more difficult or costly to commercialize. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
The regulatory landscape related to clinical trials in the European Union (EU) recently evolved. The EU Clinical Trials Regulation (CTR) which was adopted in April 2014 and repeals the EU Clinical Trials Directive, became applicable on January 31, 2022. The CTR introduced a centralized process and only requires the submission of a single application to all member states concerned the competent authority and an ethics committee in each member state, leading to a single decision per member state. The assessment procedure of the CTA has been harmonized as well, including a joint assessment by all member states concerned, and a separate assessment by each member state with respect to specific requirements related to its own territory, including ethics rules. Each member state’s decision is communicated to the sponsor via the centralized EU portal or CTIS. Once the CTA is approved, clinical study development may proceed. The extent to which ongoing and new clinical trials will be governed by the CTR varies. Clinical trials for which an application was submitted (i) prior to January 31, 2022 under the Clinical Trials Directive, which the CTR replaced, or (ii) between January 31, 2022 and January 31, 2023 and for which the sponsor has opted for the application of the Clinical Trials Directive remain governed by said Directive until January 31, 2025. After this date, all clinical trials, including those that are ongoing, will become subject to the provisions of the CTR. Compliance with the CTR requirements by us, our collaborators and third-party service providers, such as CROs, may impact our development plans.
This lengthy approval process, as well as the unpredictability of the results of clinical trials, may result in our failing to obtain regulatory approval to market any of our product candidates, which would significantly harm our business, results of operations and prospects.
We may seek breakthrough therapy designation for one or more of our product candidates, but we might not receive such designation, and even if we do, such designation may not lead to a faster development or regulatory review or approval process, and it does not increase the likelihood that our product candidates will receive marketing approval.
We may seek a breakthrough therapy designation from the FDA for some of our product candidates. A breakthrough therapy is defined as a drug or biological product that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and for which preliminary clinical evidence indicates that the drug or biological product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For drugs or biological products that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development. A drug or biologic designated as breakthrough therapies by the FDA could also be eligible for accelerated approval.
Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if we believe one of our product candidates meets the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determine not to grant such designation. In any event, the receipt of a breakthrough therapy designation for a product candidate may not result in a faster development process, review or approval compared to drugs considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more of our product candidates qualify and are designated as breakthrough therapies, the FDA may later decide that the drugs or biological products no longer meet the conditions for designation and the designation may be rescinded.
We have received Fast Track designation for three of our product candidates and may seek such designation for one or more of our other product candidates, but we might not receive such designation, and even if we do, such designation may not actually lead to a faster development or regulatory review or approval process.
If a product candidate is intended for the treatment of a serious condition and nonclinical or clinical data demonstrate the potential to address unmet medical need for the condition, a product sponsor may apply for Fast Track designation. We were awarded Fast track designation for SYNB1618 (an earlier generation of SYNB1934:labafenogene marselecobac) in April 2018, for labofenogene marselecobac in July 2023, and for SYNB1353 in August 2022. Fast track designation does not ensure that we will receive marketing approval for the product candidate or that approval will be granted within any particular timeframe. We may not experience a faster development or regulatory review or approval process with fast track designation compared to conventional FDA procedures. In addition, the FDA may withdraw fast track designation if it believes that the designation is no longer supported by data from our clinical development program. Fast track designation alone does not guarantee qualification for the FDA’s priority review procedures.
Even if we obtain regulatory approval for a product candidate, we will remain subject to ongoing regulatory requirements.
If any of our product candidates are approved for marketing, we will be subject to ongoing regulatory requirements, including with respect to manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing clinical trials, and submission of safety, efficacy and other post-approval information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities are required to continuously comply with FDA and comparable foreign regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to current good manufacturing practices (cGMP) regulations and corresponding foreign regulatory manufacturing requirements. As such, we and our contract manufacturers will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any BLA or marketing authorization application.
Any regulatory approvals that we receive for our product candidates may be subject to limitations on the approved indicated uses for which the product candidate may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. We will be required to report adverse reactions and production problems, if any, to the FDA and comparable foreign regulatory authorities. Any new legislation addressing drug safety issues could result in delays in product development or commercialization, or increased costs to ensure compliance. If our original marketing approval for a product candidate were obtained through an accelerated approval pathway, we could be required to conduct a successful post-marketing clinical trial in order to confirm the clinical benefit for
our products. An unsuccessful post-marketing clinical trial or failure to complete such a trial could result in the withdrawal of marketing approval.
If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of a product, the regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
•Issue untitled or warning letters;
•Impose civil or criminal penalties;
•Suspend or withdraw regulatory approval or revoke a license;
•Suspend any of our ongoing clinical trials;
•Refuse to approve pending applications or supplements to approved applications submitted by us;
•Impose restrictions on our operations, including closing our contract manufacturers’ facilities; or
•Require a product recall.
Any government investigation of alleged violations of law would be expected to require us to expend significant time and resources in response and could generate adverse publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to develop and commercialize our products and our value and operating results would be adversely affected.
Obtaining and maintaining marketing approval of our current and future product candidates in one jurisdiction does not mean that we will be successful in obtaining marketing approval of our current and future therapeutic product candidates in other jurisdictions.
Obtaining and maintaining marketing approval of our current and future product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain marketing approval in any other jurisdiction, while a failure or delay in obtaining marketing approval in one jurisdiction may have a negative effect on the marketing approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable foreign regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our future products will also be subject to approval.
We may submit marketing applications in other countries in addition to the United States. Regulatory authorities in jurisdictions outside of the United States have requirements for approval of product candidates with which we must comply prior to marketing in those jurisdictions. Obtaining foreign marketing approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we fail to comply with the regulatory requirements in international markets and/or receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
The FDA and other comparable ex-U.S. regulatory authorities may not accept data from trials conducted in locations outside of their jurisdiction.
We may choose to conduct international clinical trials in the future. The acceptance of study data by the FDA or other comparable ex-U.S. regulatory authority from clinical trials conducted outside of their respective jurisdictions may be subject to certain conditions. The acceptance of study data from clinical trials conducted outside the United States or another jurisdiction by the FDA or comparable ex-U.S. regulatory authority may be subject to certain conditions or may not be accepted at all. In cases where data from ex-U.S. clinical trials are intended to serve as the sole basis for marketing approval in the United States, the FDA will generally not approve the application on the basis of ex-U.S. data alone unless (i) the data are applicable to the U.S. population and U.S. medical practice; (ii) the trials were performed by clinical investigators of recognized competence and pursuant to current GCP requirements; and (iii) the FDA is able to validate the data through an on-site inspection or other appropriate means. Additionally, the FDA's clinical trial requirements, including the adequacy of the patient population studied and statistical powering, must be met.
Furthermore, even where the ex-U.S. study data are not intended to serve as the sole basis for approval, the FDA will not accept the data as support for an application for marketing approval unless the study is well-designed and well-conducted in accordance with GCP requirements and the FDA is able to validate the data from the study through an onsite inspection if deemed necessary. Many ex-U.S. regulatory authorities have similar approval requirements. In addition, such ex-U.S. trials would be subject to the applicable local laws of the ex-U.S. jurisdictions where the trials are conducted. There can be no assurance that the FDA or any comparable ex-U.S. regulatory authority will accept data from trials conducted outside of its applicable jurisdiction. If the FDA or any comparable ex-U.S. regulatory authority does not accept such data, it would result in the need for additional trials.
Inadequate funding for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, increases in workload, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In 2023, for example, members of Congress wrote to officials at the FDA expressing their concern that clinical holds should not be a means for FDA to gain additional time to review a clinical protocol. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. The coronavirus pandemic has also adversely affected the operations of necessary government agencies. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations. In addition, competing demands from other companies or issues can affect the timeliness for which the FDA can review and process our regulatory submissions.
Healthcare legislative reform measures may have a material adverse effect on our business, financial condition or results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the “ACA”), was passed, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly impacted the U.S. pharmaceutical industry. The ACA, among other things, subjected biological products to potential competition by lower-cost biosimilars, addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs and biologics that are inhaled, infused, instilled, implanted or injected, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extended the rebate program to individuals enrolled in Medicaid managed care organizations, established annual fees and taxes on manufacturers of certain branded prescription drugs and biologics, and created a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 70% (increased from 50% pursuant to the Bipartisan Budget Act of 2018, effective as of 2019) point-of-sale discounts off negotiated prices of applicable brand drugs and biologics to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs or biologics to be covered under Medicare Part D.
Further legislative and regulatory changes under the ACA remain possible, although it is unknown what form any such changes or any law would take, and how or whether it may affect the biopharmaceutical industry as a whole or our business in the future. We expect that changes or additions to the ACA, the Medicare and Medicaid programs, such as changes allowing the federal government to directly negotiate drug prices, and changes stemming from other healthcare reform measures, especially with regard to healthcare access, financing or other legislation in individual states, could have a material adverse effect on the healthcare industry in the United States.
Further, over the past several years there has been heightened governmental scrutiny over the manner in which biopharmaceutical manufacturers set prices for their marketed products, which has resulted in several U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. The probability of success of these newly announced policies, many of which have been subjected to
legal challenge in the federal court system, and their potential impact on the U.S. prescription drug marketplace is unknown. There are likely to be continued political and legal challenges associated with implementing these reforms as they are currently envisioned.
Most recently, in August 2022, President Biden signed into the law the Inflation Reduction Act of 2022, or the IRA. Among other things, the IRA has multiple provisions that may impact the prices of drug products that are both sold into the Medicare program and throughout the United States. Starting in 2023, a manufacturer of a drug or biological product covered by Medicare Parts B or D must pay a rebate to the federal government if the product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting in payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease. Additional state and federal healthcare reform measures are expected to be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for certain biopharmaceutical products or additional pricing pressures.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. For example, California requires pharmaceutical manufacturers to notify certain purchasers, including health insurers and government health plans at least 60 days before any scheduled increase in the wholesale acquisition cost (WAC), of their product if the increase exceeds 16%, and further requires pharmaceutical manufacturers to explain whether a change or improvement in the product necessitates such an increase. Similarly, Vermont requires pharmaceutical manufacturers to disclose price information on certain prescription drugs, and to provide notification to the state if introducing a new drug with a WAC in excess of the Medicare Part D specialty drug threshold. In addition, in the last few years, several states have formed prescription drug affordability boards (PDABs) with the authority to implement upper payment limits (UPLs) on drugs sold in their respective jurisdictions. However, there are several pending federal lawsuits challenging the authority of states to impose UPLs.
In December 2020, the U.S. Supreme Court also held unanimously that federal law does not preempt the states’ ability to regulate pharmaceutical benefit managers, or PBMs, and other members of the healthcare and pharmaceutical supply chain, an important decision that appears to be leading to further and more aggressive efforts by states in this area. The Federal Trade Commission in mid-2022 also launched sweeping investigations into the practices of the PBM industry that could lead to additional federal and state legislative or regulatory proposals targeting such entities’ operations, pharmacy networks, or financial arrangements. Significant efforts to change the PBM industry as it currently exists in the United States may affect the entire pharmaceutical supply chain and the business of other stakeholders, including biopharmaceutical developers like us. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for our product candidates, if approved, or put pressure on our product pricing, which could negatively affect our business, results of operations, financial condition and prospects.
In the European Union, similar political, economic and regulatory developments may affect our ability to profitably commercialize our products. In addition to continuing pressure on prices and cost containment measures, legislative developments at the European Union or EU member state level may result in significant additional requirements or obstacles that may increase our operating costs.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action. We expect that additional federal and state healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in limited coverage and reimbursement and reduced demand for our products, once approved, or additional pricing pressures.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
If we obtain FDA approval for any of our product candidates and begin commercializing those products in the United States, our operations may be subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and physician payments sunshine laws and regulations. These laws may impact, among other
things, our proposed sales, marketing, and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
•The federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs;
•Federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other government payors that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
•The federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters;
•HIPAA, as amended by the Health Information Technology and Clinical Health Act, and its implementing regulations, which imposes specified requirements relating to the privacy, security, and transmission of individually identifiable health information;
•The federal physician payments sunshine requirements under the ACA require manufacturers of drugs, devices, biologics, and medical supplies to report annually to CMS information related to payments and other transfers of value to physicians, certain advanced non-physician healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members; and
•State law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including governmental and private payors, to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, and state laws governing the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent healthcare reform legislation has strengthened these laws. For example, the ACA, among other things, amended the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. Moreover, the ACA provides that the U.S. government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We may be subject to, or may in the future become subject to, U.S. federal and state, and international laws and regulations imposing obligations on how we collect, use, disclose, store and process personal information. Our actual or perceived failure to comply with such obligations could result in liability or reputational harm and could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our customer base, and thereby decrease our revenue.
In many activities, including the conduct of clinical trials, we are subject to domestic and international laws and regulations governing data privacy, data security, and the protection of health-related and other personal information. The regulatory framework for collecting, using, safeguarding, sharing, transferring and other processing of personal information worldwide is rapidly evolving and in recent years there has been an increasing focus on privacy and data security issues with the potential to affect our business.
In the U.S., numerous federal and state laws and regulations, including state data breach notification laws, and federal and state consumer protection laws govern the collection, use, disclosure and protection of health-related and other personal information. Failure to comply with data protection laws and regulations, where applicable, could result in government enforcement actions, which could include civil or criminal penalties, private litigation and/or adverse publicity and could negatively affect our operating results
and business. For example, California enacted the California Consumer Privacy Act (the CCPA), which took effect on January 1, 2020, and gives California residents expanded rights to access and delete their personal information, opt out of certain personal information sharing and receive detailed information about how their personal information is used by requiring covered companies to provide new disclosures to California consumers (as that term is broadly defined) and provide such consumers new ways to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. Further, in 2020, California voters passed the California Privacy Rights Act (the CPRA), which became effective as of January 1, 2023. The CPRA significantly amends the CCPA and imposes additional data protection obligations on companies doing business in California, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It also creates a new regulatory entity, the California Privacy Protection Agency, which is authorized to issue substantive regulations under the CPRA and could result in increased privacy and information security enforcement. Although the CCPA currently exempts certain health-related information, including clinical trial data, the CCPA and the CPRA may increase our compliance costs and potential liability. In addition to California, more U.S. states are enacting similar legislation, increasing compliance complexity and increasing risks of failures to comply. In 2023, comprehensive privacy laws in Virginia, Colorado, Connecticut, and Utah all took effect, and laws in Montana, Oregon, and Texas will take effect in 2024. In addition, laws in other U.S. states are set to take effect beyond 2024, and additional U.S. states have proposals under consideration, all of which are likely to increase our regulatory compliance costs and risks, exposure to regulatory enforcement action and other liabilities.
Additionally, the collection, use, disclosure, transfer, or other processing of personal data in the European Union, including personal health data, is subject to the General Data Protection Regulation, or GDPR, which took effect in 2018 and applies to companies within and outside of the European Union. The GDPR, which is wide-ranging in scope, imposes several requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals, the security and confidentiality of the personal data, data breach notification and the use of third-party processors in connection with the processing of the personal data. The GDPR also enhances enforcement authority and imposes large penalties for noncompliance, including the potential for fines of up to €20 million or 4% of the annual global revenues of the infringer, whichever is greater. Additionally, the GDPR confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies, and obtain compensation for damages resulting from violations of the GDPR. In addition, the withdrawal of the United Kingdom from the European Union and the subsequent separation of the data protection regimes of these territories mean we are required to also comply with similar data protection laws in the United Kingdom, which may lead to additional compliance costs and could increase our overall risk.
Laws in the European Economic Area (EEA), Switzerland, and the UK on data export are also evolving. For example, the GDPR only permits exports of data outside the EU where there is a suitable data transfer solution in place to safeguard personal data (e.g. the EU Commission approved Standard Contractual Clauses or certification under the recently-adopted Data Privacy Framework). If we have to rely on third parties to carry out services for us, including processing personal data on our behalf, we are required under GDPR and similar laws in Switzerland and the UK to enter into contractual arrangements to help ensure that these third parties only process such data according to our instructions and have sufficient security measures in place. Any security breach or non-compliance with our contractual terms or breach of applicable law by such third parties could result in enforcement actions, litigation, fines and penalties or adverse publicity and could cause customers to lose trust in us, which would have an adverse impact on our reputation and business. Future actions of EU, Swiss, and UK data protection authorities are difficult to predict. Some customers or other may respond to these evolving laws and regulations by asking us to make certain privacy or data-related contractual commitments that we are unable or unwilling to make. This could lead to the loss of current or prospective customers or other business relationships.
Numerous international, federal and state laws and regulations govern collection, dissemination, use and confidentiality of personally identifiable health information, including state privacy and confidentiality laws (including state laws requiring disclosure of breaches); federal and state consumer protection and employment laws; HIPAA; and European and other international data protection laws. These laws and regulations are increasing in complexity and number, may change frequently and sometimes conflict.
HIPAA establishes a set of national privacy and security standards for the protection of individually identifiable health information, including protected health information, or PHI, by health plans, certain healthcare clearinghouses and healthcare providers that submit certain covered transactions electronically, or covered entities, and their ‘‘business associates,’’ which are persons or entities that perform certain services for, or on behalf of, a covered entity that involve creating, receiving, maintaining or transmitting PHI. While we are not currently a covered entity or business associate under HIPAA, we may receive identifiable information from these entities. Failure to receive this information properly could subject us to HIPAA’s criminal penalties, which may include fines up to $50,000 per violation and/or imprisonment. In addition, responding to government investigations regarding alleged violations of these and other laws and regulations, even if ultimately concluded with no findings of violations or no penalties imposed, can consume company resources and impact our business and, if public, harm our reputation.
In addition, various states, such as California and Massachusetts, have implemented similar privacy laws and regulations, such as the California Confidentiality of Medical Information Act, that impose restrictive requirements regulating the use and disclosure of
health information and other personally identifiable information. In addition to fines and penalties imposed upon violators, some of these state laws also afford private rights of action to individuals who believe their personal information has been misused. California’s patient privacy laws, for example, provide for penalties of up to $250,000 and permit injured parties to sue for damages.
Other states have implemented similar laws protecting identifiable health and personal information, and most such laws differ from each other in significant ways and may not be preempted by HIPAA, thus complicating compliance efforts.
The interplay of federal and state laws may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and our clients and potentially exposing us to additional expense, adverse publicity and liability. Further, as regulatory focus on privacy issues continues to increase and laws and regulations concerning the protection of personal information expand and become more complex, these potential risks to our business could intensify.
In addition, the interpretation and application of consumer, health-related, and data protection laws are often uncertain, contradictory, and in flux.
The legislative and regulatory landscape for privacy and data security continues to evolve, and there has been an increasing focus on privacy and data security issues which may affect our business. Despite our efforts, we may not have fully complied in the past and may not in the future. Failure to comply with current and future laws and regulations could result in government enforcement actions (including the imposition of significant penalties), criminal and civil liability for us and our officers and directors, private litigation and/or adverse publicity that negatively affects our business.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business, financial condition or results of operations.
Our research and development activities and our third-party manufacturers’ and suppliers’ activities involve the controlled storage, use, and disposal of hazardous materials, including the components of our product candidates and other hazardous compounds. We and our manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling, and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our and our manufacturers’ facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause an interruption of our research and development efforts, commercialization efforts and business operations and environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by us and our third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of specified materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently, and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. Given the nature of the research and development work conducted by us, we do not currently carry biological or hazardous waste insurance coverage.
Laws and regulations governing international operations may preclude us from developing, manufacturing and selling certain products outside of the United States and require us to develop, implement and maintain costly compliance programs.
To develop, manufacture and sell certain products outside the United States, we must dedicate resources to comply with numerous laws and regulations in each jurisdiction in which we operate. The Foreign Corrupt Practices Act (FCPA), prohibits any United States individual or business from paying, offering, authorizing payment or offering anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the FCPA presents particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees may be considered government employees or foreign officials. In other circumstances, certain payments to hospitals in connection with clinical trials and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions.
Various laws, regulations and executive orders also restrict the use and dissemination outside of the United States, or the sharing with certain non-United States nationals, of information classified for national security purposes, as well as certain products and
technical data relating to those products. These laws may preclude us from developing, manufacturing, or selling certain products and product candidates outside of the U.S., which could limit our growth potential and increase our development costs.
The failure to comply with laws governing international business practices may result in substantial civil and criminal penalties and suspension or debarment from government contracting. The SEC also may suspend or bar issuers from trading securities on U.S. exchanges for violations of the FCPA’s accounting provisions and export control laws.
Our internal computer systems, or those of our collaborators or other contractors or consultants, may fail or suffer cybersecurity incidents, which could result in a material disruption of our product development programs.
Our internal information technology, or IT, systems and those of our current and any future collaborators and other contractors, consultants, or clinical trial sites are vulnerable to damage from cyberattacks, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Cyberattacks are increasing in their frequency, sophistication and intensity, and have become increasingly difficult to detect. Cyberattacks could include the deployment of harmful malware, ransomware, denial-of-service attacks, unauthorized access to or deletion of files, social engineering, phishing and other means to affect service reliability and threaten the confidentiality, integrity and availability of information and IT systems. If any of the above events were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other proprietary information or other similar disruptions. For example, the loss of preclinical or clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or cybersecurity incident were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability, our competitive position could be harmed, and the further development and commercialization of our product candidates could be delayed. The market perception of the effectiveness of our security measures could be harmed and our reputation and credibility could be damaged. Although we develop and maintain systems and controls designed to prevent these events from occurring, and we have processes to identify and mitigate threats, the development and maintenance of these systems, controls and processes is costly and requires ongoing monitoring and updating as technologies evolve and efforts to overcome security measures become increasingly sophisticated. Moreover, despite our efforts, the possibility of these events occurring cannot be eliminated entirely. In addition, there can be no assurance that we will promptly detect any such disruption or cybersecurity incident, if at all. As we outsource more of our information systems to vendors, engage in more electronic transactions with payors and patients, and rely more on cloud-based information systems, the related security risks will increase and we will need to expend additional resources to protect our technology and information systems. In addition, there can be no assurance that our internal IT systems or those of our third-party contractors, or our consultants’ efforts to implement adequate security and control measures, will be sufficient to protect us against breakdowns, service disruption, data deterioration or loss in the event of a system malfunction, or prevent data from being stolen or corrupted in the event of a cyberattack, cybersecurity incident, industrial espionage attacks or insider threat attacks which could result in financial, legal, business or reputational harm, as well as loss of competitive advantage or loss of consumer confidence.
Ethical, legal and social concerns about synthetic biology and genetic engineering could limit or prevent the use of our technologies and limit our revenues.
Our technologies involve the use of synthetic biology and genetic engineering. Public perception about the safety and environmental hazards of, and ethical concerns over, synthetic biology and genetic engineering could influence public acceptance of our technologies, product candidates and processes. If we and our collaborators are not able to overcome the ethical, legal and social concerns relating to synthetic biology and genetic engineering, our technologies, product candidates and processes may not be accepted. These concerns could result in increased expenses, regulatory scrutiny and increased regulation, trade restrictions on imports of Synthetic Biotics, delays or other impediments to our programs or the public acceptance and commercialization of Synthetic Biotics. Further, there is a risk that Synthetic Biotics made using our technologies could result in adverse health effects or other adverse events, which could also lead to negative publicity. We design and produce product candidates with characteristics comparable or disadvantaged to those found in naturally occurring organisms or enzymes in a controlled laboratory; however, the release of such organisms into uncontrolled environments could have unintended consequences. Any adverse effect resulting from such a release could have a material adverse effect on our business, financial condition or results of operations and we may have exposure to liability for any resulting harm.
Risks Related to Our Intellectual Property
We may not be successful in obtaining or maintaining necessary rights to Synthetic Biotics, product candidates and processes for our development pipeline through acquisitions and in-licenses.
Presently, we have rights to certain intellectual property, through licenses from third parties and under patents and patent applications owned by us. The growth of our business will likely depend in part on our ability to obtain, maintain or enforce our and
our licensors’ intellectual property rights and to acquire or in-license additional proprietary rights. For example, our programs may involve additional product candidates or delivery systems that may require the use of additional proprietary rights held by third parties.
In addition, our product candidates may require specific formulations to work effectively and efficiently, and these rights may be held by other third parties. We may be unable to develop, acquire or in-license compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of other companies may also be pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These companies could have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, we have previously and may continue to collaborate with academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such right of first negotiation for intellectual property, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to it. If we are unable to do so, the institution may offer intellectual property rights to other parties, potentially blocking our ability to pursue our program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.
We intend to rely on patent rights and the status of our product candidates, if approved, as biologics eligible for exclusivity under the Biologics Price Competition and Innovation Act (BPCIA). If Synlogic is unable to obtain or maintain exclusivity from the combination of these approaches, Synlogic may not be able to compete effectively in our markets.
We rely or will rely upon a combination of patents, trade secret protection, and confidentiality agreements to protect the intellectual property related to our technologies and product candidates. Our success depends in large part on our and our licensors’ ability to obtain regulatory exclusivity and maintain patent and other intellectual property protection in the United States and in other countries with respect to our proprietary technology and products.
We have sought to protect our proprietary position by filing patent applications in the United States and abroad related to our product candidates that are important to our business. This process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unsolved. The patent applications that we own, or in-license may fail to result in issued patents with claims that cover our product candidates in the United States or in other foreign countries. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from being issued from a pending patent application. Even if patents do successfully issue, and even if such patents cover our product candidates, third parties may challenge their validity, enforceability, or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our product candidates, or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
We, independently or together with our licensors or collaborators, have filed several patent applications covering various aspects of our product candidates. We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patent or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Any successful opposition to these patents or any other patents owned by or licensed to us after patent issuance could deprive us of rights necessary for the successful commercialization of any product candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product candidate under patent protection could be reduced.
Even if we cannot obtain and maintain effective protection of exclusivity from our regulatory efforts and intellectual property rights, including patent protection, data exclusivity or orphan drug exclusivity, for our product candidates, we believe that our product candidates will be protected by exclusivity that prevents approval of a biosimilar in the United States for a period of twelve years from the time the product to which it claims similarity was first approved. However, The Biologics Price Competition and Innovation Act of 2009, Title VII, Subtitle A of the Patent Protection and Affordable Care Act, Pub.L.No.111-148, 124 Stat.119, Sections 7001-02
signed into law March 23, 2010, and codified in 42 U.S.C. §262 (the BPCIA), created an elaborate and complex patent dispute resolution mechanism for biosimilars that could prevent us from launching our product candidates in the United States or could substantially delay such launches. Current biosimilars litigation is addressing certain requirements of the BPCIA which is creating uncertainty over how certain terms of the BPCIA should be construed and this presents uncertainty for both the biologics innovator and biosimilar party. The BPCIA mechanism required for biosimilar applicants may pose greater risk that patent infringement litigation will disrupt our activities and add increased expenses as well as divert management’s attention. If a biosimilar version of one of our product candidates were approved in the United States, it could have a negative effect on our business.
We may not have sufficient patent term protections for our product candidates to effectively protect our business.
Patents have a limited term. In the United States, the statutory expiration of a patent is generally 20 years after it is filed. Although various extensions may be available, the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired for a product candidate, we may be open to competition. In addition, upon issuance in the United States any patent term can be adjusted based on specified delays caused by the applicant(s) or the USPTO.
Patent term extensions under the Hatch-Waxman Act in the United States and under supplementary protection certificates in Europe may be available to extend the patent or data exclusivity terms of our product candidates. We will likely seek patent term extensions, and we cannot provide any assurances that any such patent term extensions will be obtained and, if so, for how long. As a result, we may not be able to maintain exclusivity for our product candidates for an extended period after regulatory approval, if any, which would negatively impact our business, financial condition, results of operations and prospects. If we do not have sufficient patent terms or regulatory exclusivity to protect our product candidates, our business and results of operations will be adversely affected.
Changes in U.S. and foreign patent law could diminish the value of patents in general, thereby impairing our ability to protect our products, and recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
As is the case with other biotechnology companies, our success is heavily dependent on patents. Obtaining and enforcing patents in the biotechnology industry involves both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in specified circumstances and weakened the rights of patent owners in specified situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
European patent practice is expected to change now that the European Unitary Patent (UP) and Unified Patent Court (UPC) went into force on June 1, 2023. The new system will impact both pending European applications and granted European patents, and uncertainty remains about long-term implications of this change. The UPC may particularly present uncertainties for our ability to protect and enforce our patent rights against competitors in Europe. While the UPC is being implemented to provide more certainty and efficiency to patent enforcement throughout Europe, it will also provide our competitors with a new forum to use to centrally revoke our European patents. It will be several years before we will understand the scope of patent rights that will be recognized and the strength of patent remedies that will be provided by the UPC. We will have the right to opt our patents out of the UPC system over the first seven years, but doing so may preclude us from realizing the benefits of the new unified court.
Obtaining And Maintaining Our Patent Protection Depends on Compliance with Various Procedural, Document Submission, Fee Payment and Other Requirements Imposed by Governmental Patent Agencies, And Our Patent Protection Could be Reduced or Eliminated for Non-Compliance with These Requirements.
Periodic maintenance fees on any issued patents are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. Although an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to official actions within prescribed time limits, non-payment of fees, and failure to properly legalize and submit formal documents. In any such event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
If we are unable to maintain effective proprietary rights for our product candidates or any future product candidates, we may not be able to compete effectively in our proposed markets.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent. We also utilize processes for which patents are difficult to enforce. In addition, other elements of our products, and many elements of our product candidate discovery and development processes involve proprietary know-how, information or technology that is not covered by patents. Trade secrets may be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, collaborators, advisors, independent contractors or other third parties. We also seek to preserve the integrity and confidentiality of our data and trade secrets, including by maintaining physical and electronic security of our premises and our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, competitors may otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Furthermore, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent unauthorized material disclosure of our intellectual property to third parties, or misappropriation of our intellectual property by third parties, we may not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, operating results, and financial condition.
Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, collaborators, advisors, independent contractors and any third parties who have access to our proprietary know-how, information, or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business, financial condition or results of operations. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our ability to develop, manufacture, market and sell our product candidates and use our proprietary technology without infringing the patent rights of third parties. Numerous third-party U.S. and non-U.S. issued patents and pending applications exist in the area of synthetic biology. We may become aware of U.S. and foreign patents and pending patent applications owned by third parties that cover similar therapeutic uses as the product candidates we are developing and we may in the future pursue available proceedings in the U.S. and foreign patent offices to challenge the validity of such patents and patent applications. In addition, or alternatively, we may consider whether to seek to negotiate a license of rights to technology covered by one or more of such patents and patent applications. If any patents or patent applications cover our product candidates or technologies, we may not be free to manufacture or market our product candidates as planned, absent such a license, which may not be available to us on commercially reasonable terms, or at all.
It is possible that we have failed to identify relevant third-party patents or applications. For example, applications filed before November 29, 2000 and applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Moreover, it is difficult for industry participants, including us, to identify all third-party patent rights that may be relevant to our product candidates and technologies because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. We may fail to identify relevant patents or patent applications or may identify pending patent applications of potential interest but incorrectly predict the likelihood that such patents may issue with claims of relevance to our technology. In addition, we may be unaware of one or more issued patents that would be infringed by the manufacture, sale or use of a current or future product candidate, or we may incorrectly conclude that a third-party patent is invalid, unenforceable or not infringed by our activities. Additionally, pending patent applications that have been published can, subject to specified limitations, be later amended in a manner that could cover our technologies, our product candidates or the use of our product candidates.
There have been many lawsuits and other proceedings filed by third parties involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, and reexamination, post-grant review and equivalent proceedings before the USPTO and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a
successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
We depend, in part, on our licensors to file, prosecute, maintain, defend and enforce patents and patent applications that are material to our business.
While we normally seek and gain the right to fully prosecute the patent applications relating to our product candidates, there may be times when the patent applications enabling our product candidates are controlled by our licensors. If any of our existing or future licensors fail to appropriately and broadly prosecute and maintain patent protection for patents covering any of our product candidates, our ability to develop and commercialize those product candidates may be adversely affected and we may not be able to prevent competitors from making, using, importing, and selling competing products. In addition, even where we now have the right to control patent prosecution of patents and patent applications we have licensed from third parties, we may still be adversely affected or prejudiced by actions or inactions of our licensors in effect from actions prior to us assuming control over patent prosecution.
If we fail to comply with obligations in the agreements under which we license intellectual property and other rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
We are a party to certain intellectual property license agreements and may enter into additional license agreements in the future. Our existing agreements impose, and future license agreements may impose, certain obligations, including the payment of milestones and royalties based on revenues from sales of our products utilizing the technologies licensed from our licensors, and such obligations could adversely affect the overall profitability for us of any products that we may seek to commercialize. In addition, we will need to outsource and rely on third parties for many aspects of the clinical development, sales and marketing of our product candidates covered under our license agreements. Delay or failure by these third parties could adversely affect the continuation of our license agreements with our third-party licensors. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, these agreements may be subject to termination by the licensor which could have a material adverse effect on our business.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming, and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To cease such infringement or unauthorized use, we or one of our licensing partners may be required to file patent infringement claims against a third-party to enforce one of our patents which can be expensive, time-consuming and unpredictable. In addition, in an infringement proceeding or a declaratory judgment action against us, a court may decide that one or more of our patents is not valid or is unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly and could put our patent applications at risk of not issuing. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business.
If we or one of our licensing partners were to initiate legal proceedings against a third-party to enforce a patent covering one of our product candidates, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace, and there are numerous grounds upon which a third-party can assert invalidity or unenforceability of a patent. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, written description, clarity or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or other jurisdictions, even outside the context of litigation. Such mechanisms include re-examination, inter partes review, post-grant review and equivalent proceedings in foreign jurisdictions, such as opposition or derivation proceedings. Such proceedings could result in revocation or amendment to our patents in such a way that they no longer cover and protect our product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity of our patents, for example, we cannot be certain that there is no invalidating prior art of which we, our patent counsel, and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity, unpatentability and/or unenforceability, we may lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection could have a material adverse impact on our business.
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions or correct inventorship with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could result in a loss of our current patent rights and could require us to cease using the related
technology or to attempt to license rights to us from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation, derivation or interference proceedings may result in a decision adverse to our interests and, even if successful, may result in substantial costs and distract our management and other employees. In addition, we may be unable to raise the funds necessary to conduct our clinical trials, continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring our product candidates to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. Any disclosure of confidential information could adversely affect our business. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We may in the future be subject to claims that former employees, consultants, collaborators, advisors, independent contractors or other third parties have an interest in our patents or other intellectual property as an inventor or co-inventor or other claims challenging the inventorship of our patents or ownership of our intellectual property (including patents and intellectual property that we in-license). Therefore, our rights to these patents may not be exclusive and third parties, including competitors, may have access to intellectual property that is important to our business. In addition, co-owners from whom we do not yet have a license or assignment may raise claims surrounding inventorship or ownership of patents that ultimately issue from this patent family, potentially resulting in issued patents to which we would not have rights under our existing license agreements. Further, in jurisdictions outside the United States, a license may not be enforceable unless all the owners of the intellectual property agree or consent to the license. In addition, we may have inventorship disputes arising from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship of our patents. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may be subject to claims that our employees, consultants, collaborators, advisors, independent contractors or other third parties have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We have received confidential and proprietary information from third parties. In addition, we employ individuals who were previously employed at universities, academic research institutions and at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we have written agreements with and make every effort to ensure that our employees, consultants, collaborators, advisors, independent contractors or other third parties do not use the proprietary information or intellectual property rights of others in their work for us, we may in the future be subject to claims that our employees, consultants, collaborators, advisors, independent contractors or other third parties have inadvertently or intentionally used or disclosed confidential information of these third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may not be able to protect our intellectual property rights throughout the world.
We have limited intellectual property rights outside the United States. Filing, prosecuting, and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and intellectual property rights in some countries outside the United States can have a different scope and strength and be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties (including competitors) from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but where enforcement rights are not as strong as those in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries, particularly some developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biopharmaceutical products, which could make it
difficult in those jurisdictions for us to stop the infringement or misappropriation of our patents or other intellectual property rights, or the marketing of competing products in violation of our proprietary rights. Proceedings to enforce our patents and other intellectual property rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business. Furthermore, such proceedings could put our patents at risk of being invalidated, held unenforceable or interpreted narrowly and could put our patent applications at risk of not issuing and could provoke third parties to assert claims of infringement or misappropriation against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Additionally, Europe's Unified Patent Court (UPC) may present uncertainties for our ability to protect and enforce our patent rights against competitors in Europe. Although this new court has been implemented to provide more certainty and efficiency to patent enforcement throughout Europe, it will also provide our competitors with a new forum to use to centrally challenge our patents if opted into the UPC, rather than having to seek invalidity or non-infringement decisions on a country-by-country basis. It will be several years before the scope of patent rights that will be recognized and the strength of patent remedies that will be provided is known.
Some of our intellectual property may be subject to federal regulations such as “march-in” rights, certain reporting requirements and a preference for U.S.-based companies if it is determined that our intellectual property has been discovered through government-funded programs. Compliance with such regulations may limit our exclusive rights, and limit our ability to contract with non-U.S. manufacturers.
Some of the intellectual property rights we have acquired or licensed or may acquire or license in the future may have been generated through the use of U.S. government funding and may therefore be subject to certain federal regulations. These U.S. government rights include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right, under certain limited circumstances, to require us to grant exclusive, partially exclusive, or non-exclusive licenses to any of these inventions to a third party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations (also referred to as “march-in rights”). The U.S. government also has the right to take title to these inventions if the grant recipient fails to disclose the invention to the government or fails to file an application to register the intellectual property within specified time limits. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance with which may require us to expend substantial resources. In addition, the U.S. government requires that any products embodying any of these inventions or produced through the use of any of these inventions be manufactured substantially in the United States. This preference for U.S. industry may be waived by the federal agency that provided the funding if the owner or assignee of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for U.S. industry may limit our ability to contract with non-U.S. product manufacturers for products relating to such intellectual property. To the extent any of our future intellectual property is also generated through the use of U.S. government funding, the provisions of the Bayh-Dole Act may similarly apply.
If our trademarks and trade names are not adequately protected, we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
We have filed for trademark registration of certain marks relating to our current branding. If our trademarks and trade names are not adequately protected, we may not be able to build name recognition in our markets of interest and our business may be adversely affected. Our unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our unregistered trademarks or trade names. Over the long term, if we are unable to successfully register our trademarks and trade names and establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely impact our financial condition or results of operations.
Risks Related to Our Reliance on Third Parties
We have historically relied on and may in the future rely on third parties to conduct some aspects of our product formulation, research, preclinical, and clinical studies, and those third parties may not perform satisfactorily, including by failing to meet deadlines for the completion of such formulation, research or testing.
We have historically relied on and may in the future rely upon third parties, including independent clinical investigators, contracted laboratories and third-party CROs, to conduct our preclinical studies and clinical trials, as well as certain product candidate discovery and development activities, in accordance with applicable regulatory requirements and to monitor and manage data for our ongoing preclinical and clinical programs. We have historically relied on and may in the future rely on these parties for execution of our preclinical studies and clinical trials, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies and trials is conducted in accordance with the applicable protocol, legal and regulatory requirements and scientific standards, and our reliance on these third parties does not relieve us of our regulatory responsibilities. We and any third-party contractors and CROs we engage will be required to comply with GLP, as applicable, and GCP requirements, which are regulations and guidelines enforced by the FDA, the EMA and other comparable foreign regulatory authorities for all of our products in clinical development. Regulatory authorities enforce these GLP and GCP through periodic inspections of laboratories conducting GLP studies, and clinical trial sponsors, principal investigators, CROs, and trial sites when auditing for GCP compliance. If we, our investigators or any of our CROs or contracted laboratories that we engage fail to comply with applicable GLP and GCP, as applicable, the data generated in our preclinical studies and clinical trials may be deemed unreliable and the FDA, the EMA or other comparable foreign regulatory authorities may require us to perform additional preclinical studies or clinical trials before approving our marketing applications for our therapeutic product candidates. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our preclinical studies or clinical trials comply with applicable GLP or GCP regulations. In addition, our clinical trials must be conducted with product produced in compliance with applicable cGMP regulations. Our failure to comply with these regulations may require us to repeat preclinical studies or clinical trials, which would delay the regulatory approval process.
Further, these laboratories, investigators and CROs are not our employees, and we will not be able to control, other than by contract, the amount of resources, including time, which they devote to our product candidates and clinical trials. If independent laboratories, investigators or CROs fail to devote sufficient resources to the development of our product candidates, or if their performance is substandard, it may delay or compromise the prospects for approval and commercialization of any product candidates that we develop. In addition, the use of third-party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated.
There is a limited number of third-party service providers that specialize or have the expertise required to achieve our business objectives. If any of our relationships with these third-party laboratories, CROs or clinical investigators terminate, we may not be able to enter into arrangements with alternative laboratories, CROs or investigators or to do so in a timely manner or on commercially reasonable terms. If laboratories, CROs or clinical investigators do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our preclinical or clinical protocols, regulatory requirements or for other reasons, our preclinical studies or clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates.
Switching or adding additional laboratories or CROs (or investigators) involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new laboratory or CRO commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our contracted laboratories and CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and results of operations.
In addition, clinical investigators may serve as scientific advisors or consultants to us from time to time and may receive cash compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, or the FDA concludes that the financial relationship may have affected the interpretation of the preclinical study or clinical trial, the integrity of the data generated at the applicable preclinical study or clinical trial site may be questioned and the utility of the preclinical study or clinical trial itself may be jeopardized, which could result in the delay or rejection by the FDA. Any such delay or rejection could prevent us from commercializing our clinical-stage product candidate or any future therapeutic product candidates it may develop.
We have historically relied on and may in the future rely on third-party supply and manufacturing partners for drug supplies for our late-stage clinical activities and may do the same for any commercial supplies of our product candidates.
We have historically relied on and may in the future rely on third-party supply and manufacturing partners to supply the materials and components to manufacture parts of the process of late-stage clinical trial drug supplies. We have not yet manufactured or formulated any product candidate on a commercial scale and may not be able to do so for any of our product candidates. We will work to develop and optimize our manufacturing process, and we cannot be sure that the process will result in therapies that are safe, potent, effective, or in an amount that satisfies our commercial needs.
There can be no assurance that our supply of research and development, preclinical and clinical development drugs and other materials will not be limited, interrupted, restricted in certain geographic regions or of satisfactory quality or continue to be available at acceptable prices. In particular, any replacement of any product formulation manufacturer we may engage could require significant effort and expertise because there may be a limited number of qualified replacements.
Synthetic Biotics are complex and difficult to manufacture. We could experience production or technology transfer problems that result in delays in our development or commercialization schedules or otherwise adversely affect our business. Issues with the manufacturing process, even minor deviations from the normal process, could result in insufficient yield, product deficiencies or manufacturing failures that result in lot failures, insufficient inventory, and product recalls.
Many factors common to the manufacturing of most biologics and drugs could also cause production interruptions, including raw materials shortages, raw material failures, growth media failures, equipment malfunctions, facility contamination, labor problems, natural disasters, disruption in utility services, terrorist activities, or acts of God beyond our control. We also may encounter problems in hiring and retaining the experienced specialized personnel needed to operate our manufacturing process, which could result in delays in our production or difficulties in maintaining compliance with applicable regulatory requirements.
Any problems in our manufacturing processes or facilities could make us a less attractive collaborator for academic research institutions and other parties, which could limit our access to additional attractive development programs, result in delays in our clinical development or marketing schedules and harm our business.
The manufacturing process for a product candidate is subject to FDA and foreign regulatory authority review. Suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards, such as cGMP regulations. Although our agreements with our suppliers and manufacturers require them to perform according to certain cGMP requirements such as those relating to quality control, quality assurance and qualified personnel, we have limited control over their conduct to implement and maintain these standards. Any of our suppliers or manufacturers could fail to comply with such requirements or to perform our obligations to us in relation to quality, timing or otherwise, or if our supply of components or other materials could become limited or interrupted for other reasons. Under these circumstances, we may choose or be forced to manufacture the materials ourselves, for which we currently do not have the capabilities or resources, manufacture in collaboration with a third-party at their facilities, or enter into an agreement with another third-party, which we may not be able to do on reasonable terms, if at all. In some cases, the technical skills or technology required to manufacture our product candidates may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from transferring such skills or technology to another third-party and a feasible alternative may not exist. These factors would increase our reliance on such manufacturer or require us to obtain a license from such manufacturer in order to have another third-party manufacture our product candidates. If we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines and may be required to conduct bridging studies or repeat clinical trials to assure comparable safety, purity and potency. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop product candidates in a timely manner or within budget.
In addition, our suppliers and manufacturers are subject to inspection and approval by regulatory authorities before we can commence the manufacture and sale of any of our product candidates, and thereafter are subject to ongoing inspection from time to time. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in regulatory actions, such as the issuance of FDA Form 483 notices of observations, warning letters or sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or drugs, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our products. Any such failure by us or any of our suppliers or manufacturers would significantly impact our ability to develop, obtain regulatory approval for or, if approved, market our product candidates.
We may rely on third-party manufacturers if we receive regulatory approval for any product candidate. To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we will depend on these third parties to perform their obligations in a timely manner consistent with contractual and regulatory requirements, including those related to quality control and assurance. If we are unable to obtain or maintain third-party manufacturing for product candidates, or to do so on commercially
reasonable terms, we may not be able to develop and commercialize our product candidates successfully. Our or a third-party’s failure to execute on our manufacturing requirements could adversely affect our business in a number of ways, including:
•An inability to initiate or continue clinical trials of product candidates under development, which may impact our potential economic benefits;
•Delay in submitting regulatory applications, or receiving regulatory approvals, for product candidates;
•Loss of the cooperation of a collaborator;
•Subjecting our product candidates to additional inspections by regulatory authorities;
•Requirements to cease distribution or to recall batches of our product candidates; and
•In the event of approval to market and commercialize a product candidate, an inability to meet commercial demands for our products.
We enter into various contracts in the normal course of our business in which we indemnify the other party to the contract. In the event we have to perform under these indemnification provisions, it could have a material adverse effect on our business, financial condition and results of operations.
In the normal course of business, we periodically enter into academic, commercial, service, collaboration, licensing, consulting and other agreements that contain indemnification provisions. With respect to our academic and other research agreements, we typically indemnify the institution and related parties from losses arising from claims relating to the products, processes or services made, used, sold or performed pursuant to the agreements for which we have secured licenses, and from claims arising from our or our sublicensees’ exercise of rights under the agreement. With respect to our collaboration agreements, we typically indemnify our collaborators from any third-party product liability claims that could result from the production, use or consumption of the product, as well as for alleged infringements of any patent or other intellectual property right by a third-party. With respect to consulting agreements, we indemnify consultants from claims arising from the good faith performance of their services.
Should our obligation under an indemnification provision exceed applicable insurance coverage or should we be denied insurance coverage, our business, financial condition and results of operations could be adversely affected. Similarly, if we are relying on a collaborator to indemnify us and the collaborator is denied insurance coverage or the indemnification obligation exceeds the applicable insurance coverage, and if the collaborator does not have other assets available to indemnify us, our business, financial condition and results of operations could be adversely affected.
To the extent we are able to enter into collaborative arrangements or strategic alliances, we may be exposed to risks related to those collaborations and alliances.
Biotechnology companies sometimes become dependent upon collaborative arrangements or strategic alliances to complete the development and commercialization of product candidates. If we elect to enter into collaborative arrangements or strategic alliances, these arrangements may place the development of our product candidates outside our control, may require us to relinquish important rights or may otherwise be on terms unfavorable to us.
Dependence on collaborative arrangements or strategic alliances would subject us to a number of risks, including the risk that:
•We may not be able to control the amount and timing of resources that our collaborators may devote to the relevant product candidates;
•Our collaborators may experience financial difficulties;
•We may be required to relinquish important rights, such as marketing and distribution rights;
•Business combinations or significant changes in a collaborator’s business strategy may also adversely affect a collaborator’s willingness or ability to complete our obligations under any arrangement;
•A collaborator could independently move forward with a competing drug candidate developed either independently or in collaboration with others, including our competitors; and
•Collaborative arrangements are often terminated or allowed to expire, which would delay the development and may increase the cost of developing our drug candidates.
We may attempt to form collaborations in the future with respect to our product candidates, but we may not be able to do so, which may cause us to alter our development and commercialization plans.
We may attempt to form strategic collaborations, create joint ventures or enter into licensing arrangements with third parties with respect to our programs or platform that we believe will complement or augment our existing business. We may face significant competition in seeking appropriate strategic collaborators, and the negotiation process to secure appropriate terms is time consuming and complex. We may not be successful in our efforts to establish such a strategic collaboration for any product candidates and programs on terms that are acceptable to us, or at all. This may be because our product candidates and programs may be deemed to be at too early of a stage of development for collaborative effort, our research and development pipeline may be viewed as insufficient, the competitive or intellectual property landscape may be viewed as too intense or risky, the macro-economic conditions may disfavor a collaboration, and/or third parties may not view our product candidates and programs as having sufficient potential for commercialization, including the likelihood of an adequate safety and efficacy profile.
Any delays in identifying suitable collaborators and entering into agreements to develop and/or commercialize our product candidates could delay the development or commercialization of our product candidates, which may reduce their competitiveness even if they reach the market. Absent a strategic collaborator, we would need to undertake development and/or commercialization activities at our own expense. If we elect to fund and undertake development and/or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we are unable to do so, we may not be able to develop our product candidates or bring them to market and our business may be materially and adversely affected.
Risks Related to Commercialization of Our Product Candidates
If any of our product candidates is approved for marketing and commercialization and we are unable to develop sales, marketing and distribution capabilities on our own or enter into agreements with third parties to perform these functions on acceptable terms, we will be unable to successfully commercialize any such future products.
We currently have no sales, marketing or distribution capabilities or experience. If any of our product candidates is approved for marketing and commercialization, we will need to develop internal sales, marketing and distribution capabilities to commercialize such products, which would be expensive and time-consuming, or enter into collaborations with third parties to perform these services. If we decide to market our products directly, we will need to commit significant financial and managerial resources to develop a marketing and sales force with technical expertise and supporting distribution, administration and compliance capabilities. If we rely on third parties with such capabilities to market our products or decide to co-promote products with collaborators, we will need to establish and maintain marketing and distribution arrangements with third parties, and there can be no assurance that we will be able to enter into such arrangements on acceptable terms or at all. In entering into third-party marketing or distribution arrangements, any revenue we receive will depend upon the efforts of third parties and there can be no assurance that such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance of any approved product. If we are not successful in commercializing any product approved for marketing and commercialization in the future, either on our own or through third parties, our business, financial condition, results of operations and prospects may be adversely affected.
If the market opportunities for our product candidates are smaller than we believe they are, we may not meet our revenue expectations and, assuming approval of a product candidate, our business may suffer. Because the patient populations in the market for our product candidates may be small, we must be able to successfully identify patients and acquire a significant market share to achieve profitability and growth.
Our eligible patient population and pricing estimates may differ significantly from the actual market addressable by our product candidates. Our projections of both the number of people who have applicable diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, patient foundations, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. The potentially addressable patient population for each of our product candidates may be limited or may not be amenable to treatment with our product candidates, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect our business, financial condition, results of operations and prospects.
We face substantial competition and our competitors may discover, develop or commercialize products faster or more successfully than us.
The development and commercialization of new products is highly competitive. We face competition from major pharmaceutical companies, specialty pharmaceutical companies, biotechnology companies, universities and other research institutions worldwide with respect to our product candidates that we may seek to develop or commercialize in the future. Our competitors may succeed in developing, acquiring or licensing technologies and products that are more effective or less costly than the product candidates that we are currently developing or that we may develop, which could render our product candidates obsolete and noncompetitive. In addition to the competition we face from alternative therapies for the diseases we intend to target with our product
candidates, we are also aware of several companies that are also working specifically to develop engineered bacteria as cellular drug therapies. Further there are several companies working to develop other similar products. Third-party payors, including governmental and private insurers, may also encourage the use of generic products.
If our competitors obtain marketing approval from the FDA or comparable foreign regulatory authorities for their product candidates more rapidly than us, it could result in our competitors establishing a strong market position before we are able to enter the market.
Many of our competitors have materially greater name recognition and substantially greater financial, manufacturing, marketing, research and drug development resources than we do. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. Large pharmaceutical companies in particular have extensive expertise in preclinical and clinical testing and in obtaining regulatory approvals for drugs. In addition, academic institutions, government agencies, and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies. These organizations may also establish exclusive collaborative or licensing relationships with our competitors. Failure of our product candidates to effectively compete against established treatment options or in the future with new products currently in development would harm our business, financial condition, results of operations and prospects.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we have historically focused and may in the future focus on research programs, therapeutic platforms and product candidates that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other therapeutic platforms or product candidates or for other indications that later prove to have greater commercial potential or a greater likelihood of success. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs, therapeutic platforms and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights.
The commercial success of any of our current or future product candidates will depend upon the degree of market acceptance by physicians, patients, third-party payors, and others in the medical community.
Even with approvals from the FDA and comparable foreign regulatory authorities, the commercial success of our products will depend in part on the healthcare providers, patients, and third-party payors accepting our product candidates as medically useful, cost-effective, and safe. Any product that we bring to the market may not gain market acceptance by physicians, patients and third-party payors. The degree of market acceptance of any of our products will depend on a number of factors, including but not limited to:
•The efficacy of the product as demonstrated in clinical trials and potential advantages over competing treatments;
•The safety and side effect profile of the product as demonstrated in clinical trials and potential advantages over competing treatments;
•The prevalence and severity of the disease targeted;
•The clinical indications for which approval is granted, including any limitations or warnings contained in a product’s approved labeling;
•The convenience and ease of administration;
•The willingness of the patients and physicians to accept products engineered from bacteria and these therapies;
•The perceived ratio of risk and benefit of these therapies by physicians, patients, and payers, and the willingness of physicians to recommend these therapies to patients based on such risks and benefits;
•The marketing, sales and distribution support for the product;
•The publicity concerning the products or competing products and treatments; and
•The pricing and availability of third-party insurance coverage and reimbursement.
Even if a product displays a favorable efficacy and safety profile upon approval, market acceptance of the product remains uncertain. Efforts to educate the medical community and third-party payors on the benefits of the products may require significant investment and resources and may never be successful. If our products fail to achieve an adequate level of acceptance by physicians, patients, third-party payors, and other healthcare providers, we will not be able to generate sufficient revenue to become or remain profitable.
We may not be successful in any efforts to identify, license, discover, develop, or commercialize additional product candidates.
The success of our business is expected to depend largely upon our ability to identify, license, discover, develop, or commercialize additional product candidates. Research programs to identify new product candidates require substantial technical, financial, and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful. Our research programs or licensing efforts may fail to yield additional product candidates for clinical development and commercialization for a number of reasons, including but not limited to the following:
•Our research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates;
•We may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates;
•Our product candidates may not succeed in preclinical or clinical testing;
•Our potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval;
•Competitors may develop alternatives that render our product candidates obsolete or less attractive;
•Product candidates we develop may be covered by third parties’ patents or other exclusive rights;
•The market for a product candidate may change during development or commercialization so that such a product may become unreasonable to continue to develop or commercialize;
•A product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
•A product candidate may not be accepted as safe and effective by patients, the medical community, or third-party payors.
If any of these events occur, we may be forced to abandon our development efforts for one or more product candidates, or we may not be able to identify, license, discover, develop, or commercialize additional product candidates, which would have a material adverse effect on our business, financial condition or results of operations and could potentially cause us to cease operations.
Failure to obtain or maintain adequate reimbursement or insurance coverage for products, if any, could limit our ability to market those products and decrease our ability to generate revenue.
The pricing, coverage, and reimbursement of our approved products, if any, must be sufficient to support our commercial efforts and other development programs and the availability and adequacy of coverage and reimbursement by third-party payors, including governmental and private insurers, are essential for most patients to be able to afford expensive treatments. Sales of our approved products, if any, will depend substantially, both domestically and abroad, on the extent to which the costs of our approved products, if any, will be paid for or reimbursed by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or government payors and private payors. If coverage and reimbursement are not available, or are available only in limited amounts, we may have to subsidize or provide products for free or we may not be able to successfully commercialize our products.
In addition, there is significant uncertainty related to the insurance coverage and reimbursement for newly approved products. In the United States, the principal decisions about coverage and reimbursement for new drugs are typically made by CMS, an agency within DHHS, as CMS decides whether and to what extent a new drug will be covered and reimbursed under Medicare. Private payors tend to follow the coverage reimbursement policies established by CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for novel product candidates such as ours and what reimbursement codes our product candidates may receive if approved. No uniform policy for coverage and reimbursement for drug products exist among third-party payors in the United States. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Furthermore, rules and regulations regarding reimbursement change frequently, in some cases at short notice, and we believe that changes in these rules and regulations are likely.
Third-party payors increasingly are challenging prices charged for pharmaceutical products and services, and many third-party payors may refuse to provide coverage and reimbursement for particular drugs when an equivalent generic/biosimilar drug or a less expensive therapy is available. It is possible that a third-party payor may consider our product candidate and other therapies as substitutable and only offer to reimburse patients for the less expensive product. Even if we show improved efficacy or improved convenience of administration with our product candidate over other available and comparable products, pricing of existing drugs may limit the amount we will be able to charge for its product candidate. These payors may deny or revoke the reimbursement status of a given drug product or establish prices for new or existing marketed products at levels that are too low to enable it to realize an appropriate return on our investment in product development. If coverage and reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our product candidates and may not be able to obtain a satisfactory financial return on products that we may develop.
For products administered under the supervision of a physician, obtaining coverage and adequate reimbursement may be particularly difficult because of the higher prices often associated with such drugs. Additionally, separate reimbursement for the product itself or the treatment or procedure in which the product is used may not be available, which may impact physician utilization. For example, under these circumstances, physicians may limit how much or under what circumstances they will prescribe or administer our products and patients may deliver to purchase such products. This, in turn, could affect our ability to commercialize our products successfully and impact our profitability, results of operations, financial condition, and future success.
Outside the United States, international operations are generally subject to extensive governmental price controls and other price-restrictive regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada, and other countries has and will continue to put pressure on the pricing and usage of products. In many countries, the prices of products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits. Price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our products, if any. Accordingly, in markets outside the United States, the potential revenue from the sale of our products may be insufficient to generate commercially reasonable revenue and profits.
Moreover, increasing efforts by governmental and private payors in the United States and abroad to limit or reduce healthcare costs may result in restrictions on coverage and the level of reimbursement for new products and, as a result, they may not cover or provide adequate payment for our products. We expect to experience pricing pressures in connection with products due to the increasing trend toward managed healthcare, including the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly on prescription drugs has increased and is expected to continue to increase in the future. As a result, profitability of our products, if any, may be more difficult to achieve even if they receive regulatory approval.
Our future product candidates for which we obtain approval may face competition sooner than anticipated.
Even if we are successful in achieving regulatory approval to commercialize a product candidate ahead of our competitors, our product candidates may face competition from biosimilar products. In the United States, Synthetic Biotic products are expected to be regulated by the FDA as biological products and we intend to seek approval for these product candidates pursuant to the BLA pathway. The BPCIA created an abbreviated pathway for FDA approval of biosimilar and interchangeable biological products based on a previously licensed reference product. Under the BPCIA, an application for a biosimilar biological product cannot be approved by the FDA until 12 years after the original reference biological product was approved under a BLA. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty.
We believe that any of our product candidates approved as a biological product under a BLA should qualify for the 12-year period of exclusivity available to reference biological products. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our product candidates to be reference biological products pursuant to its interpretation of the exclusivity provisions of the BPCIA for competing products, potentially creating the opportunity for generic follow-on biosimilar competition sooner than anticipated. Moreover, the extent to which a biosimilar product, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing including whether a future competitor seeks an interchangeability designation for a biosimilar of one of our products. Under the BPCIA as well as state pharmacy laws, only interchangeable biosimilar products are considered substitutable for the reference biological product without the intervention of the healthcare provider who prescribed the original biological product. However, as with all prescribing decisions made in the context of a patient-provider relationship and a patient’s specific medical needs, healthcare providers are not restricted from prescribing biosimilar products in an off-label manner. In addition, a competitor could decide to forego the abbreviated approval pathway available for biosimilar products and to submit a full BLA for product licensure after completing its own preclinical studies and clinical trials. In such a situation, any exclusivity to which we may be eligible under the BPCIA would not prevent the competitor from marketing its biological product as soon as it is approved.
Furthermore, the CREATES Act established a private cause of action that permits a follow-on product developer to sue the brand manufacturer to compel it to furnish necessary samples of a reference product on “commercially reasonable, market-based
terms.” If follow-on product developers request samples of any product candidates for which we receive marketing approval in order to conduct comparative testing to support one or more applications for a follow-on version of our products, and we refuse any such request, we may be subject to litigation under the CREATES Act. Although lawsuits have been filed under the CREATES Act since its enactment, those lawsuits have settled privately; therefore, to date, no federal court has reviewed or opined on the statutory language and there continues to be uncertainty regarding the scope and application of the law.
In Europe, the European Commission has granted marketing authorizations for several biosimilar products pursuant to a set of general and product class-specific guidelines for biosimilar approvals issued over the past few years. In addition, companies may be developing biosimilar products in other countries that could compete with our products, if approved.
If competitors are able to obtain marketing approval for biosimilars referencing our product candidates, if approved, our future products may become subject to competition from such biosimilars, whether or not they are designated as interchangeable, with the attendant competitive pressure and potential adverse consequences. Such competitive products may be able to immediately compete with us in each indication for which our product candidates may have received approval.
Risks Related to Our Business Operations and Employees
Our failure to attract and retain senior management and key scientific personnel may prevent us from successfully developing our product candidates or any future product candidate, conducting our clinical trials and commercializing any products.
Our success depends in part on our ability to attract, retain and motivate highly qualified management, clinical and scientific personnel.
Although we have not historically experienced significant difficulties attracting and retaining qualified employees, we could experience such problems in the future. For example, competition for qualified personnel in the biotechnology and pharmaceuticals field is intense due to the limited number of individuals who possess the skills and experience required by our industry. We will need to hire additional personnel as we expand our clinical development and commercial activities. We may not be able to attract and retain quality personnel on acceptable terms, or at all. Many of the other biotechnology companies that we compete against for qualified personnel have greater financial and other resources, different risk profiles and a longer history in the industry than we do. They also may provide more diverse opportunities and better prospects for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what we have to offer. If we are unable to continue to attract and retain high-quality personnel, the rate and success at which we can discover, develop and commercialize our product candidates will be limited and the potential for successfully growing our business will be harmed.
Our employees, independent contractors, principal investigators, CROs, consultants and collaborators may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading.
We are exposed to the risk that our employees, independent contractors, consultants and collaborators may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or unauthorized activities that violate: (1) regulations of regulatory authorities in jurisdictions where we are performing activities in relation to our product candidates, including those laws requiring the reporting of true, complete and accurate information to such authorities; (2) manufacturing regulations and standards; (3) fraud and abuse and anti-corruption laws and regulations; or (4) laws that require the reporting of true and accurate financial information and data. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, bias, misconduct, kickbacks, self-dealing and other abusive practices, and these laws may differ substantially from country to country. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. These activities also include the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting ourselves from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending itself or asserting our rights, those actions could have a significant impact on our business including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in subsidized healthcare programs in a given country, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Risks Related to Our Common Stock
Our principal stockholders and management own a significant percentage of our stock and are able to exert significant control over matters subject to stockholder approval.
Based on the beneficial ownership of our common stock as of May 7, 2024, our executive officers and directors, together with holders of 5% or more of our common stock outstanding and their respective affiliates, beneficially own the majority of our common
stock. Accordingly, these stockholders have significant influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, consolidation or sale of all or substantially all of our assets or any other significant corporate transaction. The interests of these stockholders may not be the same as or may even conflict with your interests. For example, these stockholders could delay or prevent a change of control, even if such a change of control would benefit our other stockholders, which could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of the company or our assets and might affect the prevailing market price of our common stock. The significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
Future sales of our common stock or securities convertible or exchangeable for our common stock may depress our stock price.
If our existing stockholders or holders of our options sell, or indicate an intention to sell, substantial amounts of our common stock in the public market, the trading price of our common stock could decline. The perception in the market that these sales may occur could also cause the trading price of our common stock to decline. As of May 7, 2024, there were a total of 11,707,011 shares of our common stock outstanding. We have also registered and intend to continue to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and vesting provisions, as applicable.
Our quarterly operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
•Variations in the level of our operating expenses;
•Receipt, modification or termination of government contracts or grants, and the timing of payments we receive under these arrangements;
•Our execution of any collaborative, licensing or similar arrangements, and the timing of payments we may make under these arrangements; and
•Any intellectual property infringement lawsuit or opposition, interference or cancellation proceeding in which we may become involved.
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of the company’s stock to fluctuate substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
Provisions of our charter documents or Delaware law could delay or prevent an acquisition of us, even if the acquisition would be beneficial to our stockholders and could make it more difficult for you to change management.
Provisions in our amended and restated certificate of incorporation and our amended and restated bylaws may discourage, delay or prevent a merger, acquisition or other change in control that our stockholders may consider favorable, including transactions in which our stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempt by our stockholders to replace or remove our current management by making it more difficult to replace or remove our Board of Directors. These provisions include:
•A classified board of directors so that not all directors are elected at one time;
•A prohibition on stockholder action through written consent;
•No cumulative voting in the election of directors;
•The exclusive right of our Board of Directors to elect a director to fill a vacancy created by the expansion of our Board of Directors or the resignation, death or removal of a director;
•A requirement that special meetings of our Stockholders be called only by our Board of Directors, the chairman of our Board of Directors, the chief executive officer or, in the absence of a chief executive officer, the president;
•An advance notice requirement for stockholder proposals and nominations;
•The authority of our Board of Directors to issue preferred stock with such terms as our Board of Directors may determine; and
•A requirement of approval of not less than 66 2/3% of all outstanding shares of our capital stock entitled to vote to amend any bylaws by stockholder action, or to amend specific provisions of our certificate of incorporation.
In addition, Delaware law prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person who, together with its affiliates, owns or within the last three years has owned 15% or more of the company’s voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Accordingly, Delaware law may discourage, delay or prevent a change in control of the company.
In addition, our amended and restated certificate of incorporation, to the fullest extent permitted by law, provides that the Court of Chancery of the State of Delaware will be the exclusive forum for: any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, or the DGCL, our amended and restated certificate of incorporation, or our amended and restated bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine. This exclusive forum provision does not apply to suits brought to enforce a duty or liability created by the Exchange Act. It could apply, however, to a suit that falls within one or more of the categories enumerated in the exclusive forum provision and asserts claims under the Securities Act, inasmuch as Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rule and regulations thereunder. There is uncertainty as to whether a court would enforce such provision with respect to claims under the Securities Act, and our stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder.
This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our directors, officers, or other employees, which may discourage lawsuits with respect to such claims. Alternatively, if a court were to find the choice of forum provisions contained in our restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations and financial condition.
Provisions in our charter and other provisions of Delaware law could limit the price that investors are willing to pay in the future for shares of our common stock.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research, about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend, in part, on the research and reports that securities or industry analysts publish about us or our business. If one or more of the analysts who cover us downgrade our common stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. In addition, if our operating results fail to meet the forecast of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our common stock could decrease, which might cause our stock price and trading volume to decline.
Changes in, or interpretations of, accounting rules and regulations could result in unfavorable accounting charges or require us to change our compensation policies.
Accounting methods and policies for biopharmaceutical companies, including policies governing revenue recognition, research and development and related expenses and accounting for stock-based compensation, are subject to further review, interpretation and guidance from relevant accounting authorities, including the SEC. Changes to, or interpretations of, accounting methods or policies may require us to reclassify, restate or otherwise change or revise our financial statements, including those contained in this periodic report.
We will continue to incur costs as a result of being a public company, and our management will continue to devote substantial time to compliance initiatives and corporate governance practices.
As a public company, we have incurred and will continue to incur significant legal, accounting and other expenses. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The Nasdaq Capital Market and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel devote and will need to continue to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations have increased and will continue to increase our legal and financial compliance costs and make some activities more time-consuming and costly. For example, we expect that these rules and regulations will continue to make it more difficult and more expensive for us to maintain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified members of our board of directors.
These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in future uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
If we fail to comply with the continued listing requirements of the Nasdaq Capital Market, our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
Our common stock is currently listed on the Nasdaq Capital Market and was previously listed on the Nasdaq Global Market. To maintain the listing of our common stock on the Nasdaq Global Market, we were required to satisfy minimum financial and other continued listing requirements and standards, including those related to the price of our common stock. On December 6, 2022, we received a written notice from the Listing Qualifications Department of the Nasdaq Stock Market (Nasdaq) notifying us that, based on the closing bid price of our common stock being below $1.00 per share for 30 consecutive business days, we no longer complied with Nasdaq’s minimum bid price requirement in Listing Rule 5450(a)(1) for continued listing on the Nasdaq Global Market.
Pursuant to Nasdaq Listing Rule 5810(c)(3)(A), we were provided an initial compliance period of 180 calendar days from receipt of the Notice, or until June 5, 2023, to regain compliance with the minimum bid price requirement. To regain compliance, the bid price for our common stock would need to close at $1.00 per share or more for a minimum of 10 consecutive business days during this 180-day grace period, among other requirements.
On May 25, 2023, we submitted to the Listing Qualifications Department of Nasdaq an application to transfer the listing of our common stock from The Nasdaq Global Market to The Nasdaq Capital Market. On June 6, 2023, we received a notice (Extension Notice) from the Listing Qualifications Department informing us that Nasdaq granted us an additional 180 calendar days, or until December 4, 2023 to regain compliance with the minimum closing bid price requirement for continued listing on The Nasdaq Capital Market under Nasdaq Marketplace Rule 5550(a)(2). In connection with the Extension Notice, the listing of our common stock was transferred from the Nasdaq Global Market to the Nasdaq Capital Market, effective as of June 7, 2023. The Extension Notice had no other immediate effect on the listing of our common stock.
On September 27, 2023, we implemented a reverse stock split of our shares of common stock, pursuant to which every fifteen (15) shares of our issued and outstanding common stock was automatically converted into one (1) issued and outstanding share of common stock without any change in the par value of $0.001 per share. The reverse stock split was approved by our stockholders on September 21, 2023 at our Special Meeting of stockholders.
On October 13, 2023, we received a written notice from the Listing Qualifications Department of Nasdaq notifying us that we had regained compliance with Listing Rule 5550(a)(2) and that this matter was closed. However, there can be no assurance that we will comply with the minimum bid price requirement in the future nor, is there any assurance that our common stock would never be delisted from Nasdaq at some future date. If our common stock were to be delisted from Nasdaq and is not eligible for quotation or listing on another market or exchange, trading of our common stock could be conducted only in the over-the-counter market or on an electronic bulletin board established for unlisted securities such as the Pink Sheets or the OTC Bulletin Board. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further. Also, it may be difficult for us to raise additional capital if we are not listed on a major exchange.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
Rule 10b5-1 Trading Plans
During the fiscal quarter ended March 31, 2024, none of our directors or executive officers adopted, modified or terminated any contract, instruction or written plan for the purchase or sale of our securities that was intended to satisfy the affirmative defense conditions of Rule 10b5-1 (c) or any "non-Rule 10b5-1 trading arrangement."
Item 6. Exhibits.
EXHIBIT INDEX
|
|
|
|
|
|
|
|
|
|
|
Exhibit Number |
|
Exhibit Description |
|
Filed with this Report |
|
Incorporated by Reference herein from Form or Schedule |
|
Filing Date |
|
SEC File/Reg. Number |
|
|
|
|
|
|
|
|
|
|
|
3.1 |
|
Certificate of Designation of Series A Junior Participating Preferred Stock of Synlogic, Inc., as filed with the Secretary of State of the State of Delaware on February 20, 2024. |
|
|
|
Form 8-K |
|
2/20/2024 |
|
001-37566 |
|
|
|
|
|
|
|
|
|
|
|
4.1 |
|
Rights Agreement, dated as of February 20, 2024, between Synlogic, Inc. and Equiniti Trust Company LLC, as rights agent. |
|
|
|
Form 8-K |
|
2/20/2024 |
|
001-37566 |
|
|
|
|
|
|
|
|
|
|
|
10.1† |
|
Statement of Work dated May 3, 2024 pursuant to Master Contract Services Agreement between Synlogic, Inc. and Azzur Group (d/b/a Azzur of New England LLC), SOW P-Cleanroom Offboarding CR3 |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
Certification of Chief Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a). |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
Certification of Interim Chief Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a). |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1* |
|
Certification required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350). |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.2* |
|
Certification required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350). |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Date File because XBRL tags are embedded within the Inline XBRL document. |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
X |
|
|
|
|
|
|
(*) The certifications attached as Exhibit 32.1 and Exhibit 32.2 that accompany this Quarterly Report on Form 10-Q are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Synlogic, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of such Form 10-Q), irrespective of any general incorporation language contained in such filing.
† Portions of this exhibit (indicated by asterisks) have been omitted in accordance with the rules of the Securities and Exchange Commission.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Date: May 14, 2024
|
|
|
|
|
SYNLOGIC, INC. |
|
|
|
|
|
By: |
|
/s/ ANTOINE AWAD |
|
|
|
Antoine Awad |
|
|
|
Principal Executive Officer |
|
|
|
(principal executive officer) |
|
|
|
|
|
By: |
|
/s/ MARY BETH DOOLEY |
|
|
|
Mary Beth Dooley |
|
|
|
Head of Finance |
|
|
|
(principal financial officer and principal accounting officer) |
Exhibit 10.1
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, HAS BEEN OMITTED BECAUSE THE INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.

Scope of Work P-
Cleanroom Offboarding CR3
Synlogic
COD Waltham

Azzur Cleanrooms on Demand Waltham – Synlogic
CONFIDENTIAL
1.Synlogic Clinical Offboarding
Facility offboarding per SOW P-10558-01 will be priced based on the work both approved and agreed upon by both Synlogic and Azzur in Table 1.
Please see table 1 below.
Table 1: Overall Project Cost Estimate
|
|
|
Synlogic Clinical Offboarding |
Item |
Description |
Price ($) |
1. [***] |
[***] |
[***] |
2. [***] |
[***] |
[***] |
3. [***] |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
[***] |
4. [***] |
[***] |
[***] |
5. [***] |
[***] |
[***] |
[***] |
6.[***] |
[***] |
[***] |
7. [***] |
[***] |
[***] |
8. [***] |
[***] |
[***] |
9. [***] |
[***] |
[***] |
|
Total |
[***] |
Upon execution of this Scope of Work, a PO in the amount of [***] will be required to finalize the contract.
Azzur Cleanrooms on Demand Waltham – Synlogic
CONFIDENTIAL
SOW AGREED TO AND ACCEPTED BY:
AZZUR Cleanrooms on Demand, Waltham
Signature: /s/ Sarah Stevens
Name: Sarah Stevens
Title: President, Azzur Group
Date: 05/03/2024
Signature: /s/ Tony Awad
Name: Tony Awad
Title: Principal Executive Officer and COO
Date: 05/03/2024
EXHIBIT 31.1
CERTIFICATION PURSUANT
TO RULES 13a-14(a) OR 15d-14(a) UNDER
THE SECURITIES EXCHANGE ACT OF 1934
I, Antoine Awad, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of Synlogic, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: May 14, 2024
|
/s/ ANTOINE AWAD |
Antoine Awad |
Principal Executive Officer |
(principal executive officer) |
EXHIBIT 31.2
CERTIFICATION PURSUANT
TO RULES 13a-14(a) OR 15d-14(a) UNDER
THE SECURITIES EXCHANGE ACT OF 1934
I, Mary Beth Dooley, certify that:
1.I have reviewed this Quarterly Report on Form 10-Q of Synlogic, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: May 14, 2024
|
/s/ MARY BETH DOOLEY |
Mary Beth Dooley |
Head of Finance |
(principal financial officer and principal accounting officer) |
EXHIBIT 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of Synlogic, Inc. (the “Company”) on Form 10-Q for the period ended March 31, 2024 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Antoine Awad, Principal Executive Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, to my knowledge that:
(1)The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
/s/ ANTOINE AWAD |
Antoine Awad |
Principal Executive Officer |
(principal executive officer) |
May 14, 2024
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
EXHIBIT 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of Synlogic, Inc. (the “Company”) on Form 10-Q for the period ended March 31, 2024 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Mary Beth Dooley, Head of Finance of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, to my knowledge that:
(1)The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
/s/ MARY BETH DOOLEY |
Mary Beth Dooley |
Head of Finance |
(principal financial officer and principal accounting officer) |
May 14, 2024
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
v3.24.1.1.u2
Document and Entity Information - shares
|
3 Months Ended |
|
Mar. 31, 2024 |
May 07, 2024 |
| Document Information [Table] |
|
|
| Document Type |
10-Q
|
|
| Amendment Flag |
false
|
|
| Document Period End Date |
Mar. 31, 2024
|
|
| Document Fiscal Year Focus |
2024
|
|
| Document Fiscal Period Focus |
Q1
|
|
| Entity Registrant Name |
SYNLOGIC, INC.
|
|
| Entity Central Index Key |
0001527599
|
|
| Entity Current Reporting Status |
Yes
|
|
| Current Fiscal Year End Date |
--12-31
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
| Entity Shell Company |
false
|
|
| Entity Small Business |
true
|
|
| Entity Emerging Growth Company |
false
|
|
| Entity Common Stock, Shares Outstanding |
|
11,707,011
|
| Entity Address, State or Province |
MA
|
|
| Entity File Number |
001-37566
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
| Entity Tax Identification Number |
26-1824804
|
|
| Entity Address, Address Line One |
301 Binney St.
|
|
| Entity Address, Address Line Two |
Suite 402
|
|
| Entity Address, City or Town |
Cambridge
|
|
| Entity Address, Postal Zip Code |
02142
|
|
| City Area Code |
617
|
|
| Local Phone Number |
401-9975
|
|
| Entity Interactive Data Current |
Yes
|
|
| Document Quarterly Report |
true
|
|
| Document Transition Report |
false
|
|
| Common Stock [Member] |
|
|
| Document Information [Table] |
|
|
| Trading Symbol |
SYBX
|
|
| Security Exchange Name |
NASDAQ
|
|
| Title of 12(b) Security |
Common Stock, par value $0.001 per share
|
|
| Preferred Stock [Member] |
|
|
| Document Information [Table] |
|
|
| Security Exchange Name |
NASDAQ
|
|
| Title of 12(b) Security |
Preferred Stock Purchase Rights
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_PreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Unaudited Consolidated Balance Sheets - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Dec. 31, 2023 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 24,822
|
$ 23,960
|
| Short-term marketable securities |
7,489
|
23,786
|
| Prepaid expenses and other current assets |
2,429
|
2,161
|
| Assets held for sale |
206
|
0
|
| Total current assets |
34,946
|
49,907
|
| Property and equipment, net |
0
|
5,603
|
| Right of use asset - operating lease |
0
|
12,102
|
| Restricted cash |
1,097
|
1,097
|
| Prepaid research and development, net of current portion |
0
|
6,825
|
| Other assets |
14
|
16
|
| Total assets |
36,057
|
75,550
|
| Current liabilities: |
|
|
| Accounts payable |
719
|
1,457
|
| Accrued expenses |
3,116
|
3,000
|
| Lease liability - operating lease |
2,671
|
4,780
|
| Finance lease obligations |
1
|
4
|
| Purchase warrant liability |
4,433
|
11,163
|
| Total current liabilities |
10,940
|
20,404
|
| Long-term liabilities: |
|
|
| Lease liability - operating lease, net of current portion |
11,792
|
12,491
|
| Total long-term liabilities |
11,792
|
12,491
|
| Commitments and contingencies (Note 13) |
|
|
| Stockholders' equity |
|
|
| Common stock, $0.001 par value 250,000,000 shares authorized as of March 31, 2024 and December 31, 2023; 11,907,004 shares issued and 11,627,212 shares outstanding as of March 31, 2024 and 9,465,949 shares issued and 9,186,157 shares outstanding as of December 31, 2023 |
12
|
10
|
| Additional paid-in capital |
461,696
|
459,458
|
| Accumulated other comprehensive income |
0
|
6
|
| Accumulated deficit |
(445,865)
|
(414,301)
|
| Treasury stock, at cost (279,792 shares at March 31, 2024 and at December 31, 2023) |
(2,518)
|
(2,518)
|
| Total stockholders' equity |
13,325
|
42,655
|
| Total liabilities and stockholders' equity |
$ 36,057
|
$ 75,550
|
| X |
- DefinitionPrepaid research and development noncurrent.
| Name: |
sybx_PrepaidResearchAndDevelopmentNoncurrent |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionPurchase Warrant Liability
| Name: |
sybx_PurchaseWarrantLiability |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionValue received from shareholders in common stock-related transactions that are in excess of par value or stated value and amounts received from other stock-related transactions. Includes only common stock transactions (excludes preferred stock transactions). May be called contributed capital, capital in excess of par, capital surplus, or paid-in capital. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapitalCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of assets held-for-sale that are not part of a disposal group, expected to be sold within a year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 15
-Paragraph 4
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482309/360-10-15-4
| Name: |
us-gaap_AssetsHeldForSaleNotPartOfDisposalGroupCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481830/320-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtSecuritiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from finance lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_FinanceLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of obligation due after one year or beyond the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 201.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 201.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 23: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 201.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesNoncurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480632/954-210-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount allocated to treasury stock. Treasury stock is common and preferred shares of an entity that were issued, repurchased by the entity, and are held in its treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481520/505-30-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481549/505-30-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29,30)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TreasuryStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.1.1.u2
Unaudited Consolidated Balance Sheets (Parenthetical) - $ / shares
|
Mar. 31, 2024 |
Dec. 31, 2023 |
| Statement of Financial Position [Abstract] |
|
|
| Common stock, par value |
$ 0.001
|
$ 0.001
|
| Common stock, authorized |
250,000,000
|
250,000,000
|
| Common stock, Issued |
11,907,004
|
9,465,949
|
| Common stock, outstanding |
11,627,212
|
9,186,157
|
| Treasury stock, shares |
279,792
|
279,792
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Unaudited Consolidated Statements of Operations and Comprehensive Loss - USD ($)
$ in Thousands |
3 Months Ended |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Income Statement [Abstract] |
|
|
| Revenue |
$ 8
|
$ 174
|
| Operating expenses: |
|
|
| Research and development |
7,680
|
12,450
|
| General and administrative |
2,884
|
3,967
|
| Restructuring and other charges |
28,289
|
0
|
| Total operating expenses |
38,853
|
16,417
|
| Loss from operations |
(38,845)
|
(16,243)
|
| Other income (expense): |
|
|
| Interest and investment income |
553
|
628
|
| Interest expense |
0
|
(1)
|
| Change in fair value of purchase warrant liability |
6,730
|
0
|
| Other expense |
(2)
|
(6)
|
| Total other (expense), net |
7,281
|
621
|
| Net loss |
$ (31,564)
|
$ (15,622)
|
| Net loss per share - basic |
$ (2.6)
|
$ (3.39)
|
| Net loss per share - diluted |
$ (2.6)
|
$ (3.39)
|
| Weighted-average common stock outstanding - basic |
12,131,461
|
4,604,682
|
| Weighted-average common stock outstanding - diluted |
12,131,461
|
4,604,682
|
| Comprehensive loss: |
|
|
| Net loss |
$ (31,564)
|
$ (15,622)
|
| Net unrealized (loss) gain on marketable securities |
(6)
|
131
|
| Comprehensive loss |
$ (31,570)
|
$ (15,491)
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_ComprehensiveIncomeNetOfTaxAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncomeStatementAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income from investments (for example, dividends) not considered a component of the entity's core operations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InvestmentIncomeNonoperating |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after tax and before adjustment, of unrealized holding gain (loss) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Excludes unrealized gain (loss) on investment in debt security measured at amortized cost (held-to-maturity) from transfer to available-for-sale. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-9
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-10A
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
| Name: |
us-gaap_OtherComprehensiveIncomeUnrealizedHoldingGainLossOnSecuritiesArisingDuringPeriodNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (expense) related to nonoperating activities, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses associated with exit or disposal activities pursuant to an authorized plan. Excludes expenses related to a discontinued operation or an asset retirement obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482047/420-10-45-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 5.P.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-1
| Name: |
us-gaap_RestructuringCharges |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Unaudited Consolidated Statements of Stockholders' Equity (Unaudited) - USD ($)
|
Total |
Pre-Funded Warrants |
At-the-market Offering |
Common Stock $0.001 Par Value |
Common Stock $0.001 Par Value
Pre-Funded Warrants
|
Additional Paid-in Capital |
Accumulated Other Comprehensive Income (Loss) |
Accumulated Deficit |
Treasury Stock |
| Balance at Dec. 31, 2022 |
$ 82,610,000
|
|
|
$ 5,000
|
|
$ 442,303,000
|
$ (161,000)
|
$ (357,019,000)
|
$ (2,518,000)
|
| Balance (in Shares) at Dec. 31, 2022 |
|
|
|
4,728,874
|
|
|
|
|
(279,792)
|
| Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs |
|
|
$ 816,000
|
|
|
816,000
|
|
|
|
| Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs, Shares |
|
|
|
68,893
|
|
|
|
|
|
| Issuance of restricted stock, shares |
|
|
|
10,803
|
|
|
|
|
|
| Issuance of common stock under employee stock purchase plan, Shares |
|
|
|
7,322
|
|
|
|
|
|
| Issuance of common stock under employee stock purchase plan |
59,000
|
|
|
|
|
59,000
|
|
|
|
| Equity-based compensation expense |
718,000
|
|
|
|
|
718,000
|
|
|
|
| Unrealized gain (loss) on securities |
131,000
|
|
|
|
|
|
131,000
|
|
|
| Net Income (Loss) |
(15,622,000)
|
|
|
|
|
|
|
(15,622,000)
|
|
| Balance at Mar. 31, 2023 |
68,712,000
|
|
|
$ 5,000
|
|
443,896,000
|
(30,000)
|
(372,641,000)
|
$ (2,518,000)
|
| Balance (in Shares) at Mar. 31, 2023 |
|
|
|
4,815,892
|
|
|
|
|
(279,792)
|
| Balance at Dec. 31, 2023 |
42,655,000
|
|
|
$ 10,000
|
|
459,458,000
|
6,000
|
(414,301,000)
|
$ (2,518,000)
|
| Balance (in Shares) at Dec. 31, 2023 |
|
|
|
9,465,949
|
|
|
|
|
(279,792)
|
| Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs |
|
$ 13,000
|
|
|
|
|
|
|
|
| Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs, Shares |
|
|
|
|
7,839
|
|
|
|
|
| Issuance of restricted stock, shares |
|
|
|
362,700
|
|
|
|
|
|
| Exercise of options |
4,000
|
$ 2,000
|
|
|
$ 2,000
|
4,000
|
|
|
|
| Exercise of options, shares |
|
|
|
2,494
|
2,251,000
|
|
|
|
|
| Cancellation of restricted stock |
|
|
|
(182,978)
|
|
|
|
|
|
| Equity-based compensation expense |
2,221,000
|
|
|
|
|
2,221,000
|
|
|
|
| Unrealized gain (loss) on securities |
(6,000)
|
|
|
|
|
|
$ (6,000)
|
|
|
| Net Income (Loss) |
(31,564,000)
|
|
|
|
|
|
|
(31,564,000)
|
|
| Balance at Mar. 31, 2024 |
$ 13,325,000
|
|
|
$ 12,000
|
|
$ 461,696,000
|
|
$ (445,865,000)
|
$ (2,518,000)
|
| Balance (in Shares) at Mar. 31, 2024 |
|
|
|
11,907,004
|
|
|
|
|
(279,792)
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after tax and before adjustment, of unrealized holding gain (loss) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Excludes unrealized gain (loss) on investment in debt security measured at amortized cost (held-to-maturity) from transfer to available-for-sale. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-9
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-10A
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
| Name: |
us-gaap_OtherComprehensiveIncomeUnrealizedHoldingGainLossOnSecuritiesArisingDuringPeriodNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued during the period as a result of an employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesEmployeeStockPurchasePlans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares related to Restricted Stock Award forfeited during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesRestrictedStockAwardForfeited |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTotal number of shares issued during the period, including shares forfeited, as a result of Restricted Stock Awards. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesRestrictedStockAwardGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate change in value for stock issued during the period as a result of employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueEmployeeStockPurchasePlan |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued as a result of the exercise of stock options. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.1.1.u2
Unaudited Consolidated Statements of Cash Flows - USD ($)
$ in Thousands |
3 Months Ended |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Cash flows from operating activities: |
|
|
| Net loss |
$ (31,564)
|
$ (15,622)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Depreciation |
347
|
576
|
| Gain on disposal of property and equipment |
(103)
|
(11)
|
| Impairment loss on fixed assets |
5,393
|
0
|
| Impairment loss on ROU assets |
9,571
|
0
|
| Gain on lease termination |
(43)
|
0
|
| Impairment of prepaid research and development |
5,219
|
0
|
| Equity-based compensation expense |
2,221
|
718
|
| Change in fair value of warrant liability |
(6,730)
|
0
|
| Accretion/amortization of investment securities |
(231)
|
(327)
|
| Reduction in carrying amount of operating lease right of use asset |
948
|
809
|
| Changes in operating assets and liabilities: |
|
|
| Prepaid expenses and other current assets |
(568)
|
(2,838)
|
| Prepaid research and development, net of current portion |
1,906
|
(1,233)
|
| Other assets |
2
|
(15)
|
| Accounts payable and accrued expenses |
(622)
|
(2,378)
|
| Deferred revenue |
0
|
(190)
|
| Operating lease liabilities |
(1,182)
|
(994)
|
| Net cash used in operating activities |
(15,436)
|
(21,505)
|
| Cash flows from investing activities: |
|
|
| Purchases of marketable securities |
(1,477)
|
0
|
| Proceeds from maturity of marketable securities |
17,999
|
30,397
|
| Purchases of property and equipment |
(395)
|
(143)
|
| Proceeds from the sale of property and equipment |
155
|
16
|
| Net cash provided by investing activities |
16,282
|
30,270
|
| Cash flows from financing activities: |
|
|
| Payments on finance lease obligations |
(3)
|
(2)
|
| Proceeds from employee stock purchases and exercise of stock options |
4
|
59
|
| Net cash provided by financing activities |
16
|
913
|
| Net increase in cash, cash equivalents and restricted cash |
862
|
9,678
|
| Cash, cash equivalents and restricted cash at beginning of period |
25,057
|
16,958
|
| Cash, cash equivalents and restricted cash at end of period |
25,919
|
26,636
|
| Supplemental disclosure of non-cash investing activities: |
|
|
| Decrease in right-of-use asset and operating lease liabilities due to lease termination |
1,626
|
0
|
| Supplemental disclosure of non-cash financing activities: |
|
|
| Issuance costs included in accounts payable and accrued expenses |
0
|
40
|
| Pre-Funded Warrants |
|
|
| Cash flows from financing activities: |
|
|
| Proceeds from exercise of pre-funded warrants |
2
|
0
|
| At-the-market Offering |
|
|
| Cash flows from financing activities: |
|
|
| Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs |
$ 13
|
$ 856
|
| X |
- DefinitionDecrease in right-of-use asset and operating lease liabilities
| Name: |
sybx_DecreaseInRightOfUseAssetAndOperatingLeaseLiabilities |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionImpairment Of In Process Research And Development
| Name: |
sybx_ImpairmentOfInProcessResearchAndDevelopment |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionIncrease (Decrease) In Fair Value Warrant Liability
| Name: |
sybx_IncreaseDecreaseInFairValueWarrantLiability |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionIncrease (decrease) in prepaid research and development net of current portion.
| Name: |
sybx_IncreaseDecreaseInPrepaidResearchAndDevelopmentNetOfCurrentPortion |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionIssuance costs included in accounts payable and accrued expenses.
| Name: |
sybx_IssuanceCostsIncludedInAccountsPayableAndAccruedExpenses |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
sybx_NoncashFinancingItemsAbstract |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
sybx_NoncashInvestingItemsAbstract |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from employee stock purchases and exercise of stock options.
| Name: |
sybx_ProceedsFromEmployeeStockPurchasesAndExerciseOfStockOptions |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionReduction in carrying amount of operating lease right-of-use asset.
| Name: |
sybx_ReductionInCarryingAmountOfOperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe sum of the periodic adjustments of the differences between securities' face values and purchase prices that are charged against earnings. This is called accretion if the security was purchased at a discount and amortization if it was purchased at premium. As a noncash item, this element is an adjustment to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_AccretionAmortizationOfDiscountsAndPremiumsInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before tax of gain (loss) recognized on the sale or disposal of a disposal group. Excludes discontinued operations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-3
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482130/360-10-45-5
| Name: |
us-gaap_DisposalGroupNotDiscontinuedOperationGainLossOnDisposal |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for principal payment on finance lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
| Name: |
us-gaap_FinanceLeasePrincipalPayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of gain (loss) on termination of lease before expiration of lease term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 40
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479092/842-20-40-1
| Name: |
us-gaap_GainLossOnTerminationOfLease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of write-downs for impairments recognized during the period for long lived assets held for use (including those held for disposal by means other than sale). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482130/360-10-45-4
| Name: |
us-gaap_ImpairmentOfLongLivedAssetsHeldForUse |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation to transfer good or service to customer for which consideration has been received or is receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 912
-SubTopic 310
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482312/912-310-45-11
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInContractWithCustomerLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in operating assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherOperatingAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of loss from impairment of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 25
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479365/842-20-25-6
| Name: |
us-gaap_OperatingLeaseImpairmentLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow to acquire investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481830/320-10-45-11
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-11
| Name: |
us-gaap_PaymentsToAcquireAvailableForSaleSecuritiesDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the aggregate amount received by the entity through sale or maturity of marketable securities (held-to-maturity or available-for-sale) during the period.
| Name: |
us-gaap_ProceedsFromSaleAndMaturityOfMarketableSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the sale of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 12
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromSaleOfPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=sybx_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=sybx_AtTheMarketOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 2
-Subparagraph A
| Name: |
ecd_TradingArrByIndTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNon Rule 10b51 Arr Modified Flag
| Name: |
sybx_NonRule10B51ArrModifiedFlag |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRule 10b51 Arr Modified Flag
| Name: |
sybx_Rule10B51ArrModifiedFlag |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Nature of Business
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Nature of Business |
Organization Synlogic, Inc., together with its wholly owned and consolidated subsidiaries (Synlogic or the Company), is a clinical-stage biopharmaceutical company applying synthetic biology to the discovery and development of Synthetic Biotics. Synthetic Biotics are generated from Synlogic’s proprietary platform, leveraging a reproducible, modular approach to the generation of novel drug candidates that perform or deliver critical therapeutic functions. Synthetic Biotics are designed to metabolize a toxic substance, compensate for missing or damaged metabolic pathways or deliver combinations of therapeutic factors. Synlogic’s goal is to discover, develop and ultimately commercialize Synthetic Biotics. Since incorporation, the Company has devoted substantially all of its efforts to the research and development of its product candidates. In February 2024, the Company and its board of directors decided to discontinue the Synpheny-3 trial and to conduct a comprehensive review of strategic alternatives. The Company also announced a corporate restructuring that resulted in a reduction in its workforce by 90% that was substantially completed in the first quarter of 2024 (see Note 8). Going Concern and Liquidity The Company’s consolidated financial statements have been prepared assuming it will continue as a going concern. The going concern assumption contemplates the continuity of operations, and the realization of assets and the satisfaction of liabilities in the ordinary course of business. The Company has historically generated negative cash flows from operations and has an accumulated deficit of $445.9 million at March 31, 2024. At March 31, 2024, the Company had $32.3 million in unrestricted cash, cash equivalents and short-term marketable securities. The Company believes the conditions and events raising substantial doubt about its ability to continue as a going concern no longer exist following the execution of the corporate restructuring (see Note 8) that was substantially completed in the first quarter of 2024. Accordingly, its current cash and cash equivalents as of March 31, 2024 will be sufficient to fund its operations at the current levels for at least the next 12 months from the date of this filing. The Company expects to continue to incur costs and expenditures in connection with the process of evaluating strategic alternatives.
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Summary of Significant Accounting Policies
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Summary of Significant Accounting Policies |
(2)Summary of Significant Accounting Policies The significant accounting policies described in the Company’s audited financial statements as of and for the year ended December 31, 2023, and the notes thereto, which are included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the Securities and Exchange Commission (SEC) on March 19, 2024 (the 2023 Annual Report), have had no material changes during the three months ended March 31, 2024. Reverse Stock Split On September 27, 2023, the Company effected a one-for-fifteen reverse stock split of its issued and outstanding common stock, which also adjusted all outstanding warrants. Accordingly, all share and per share amounts for all periods presented in the accompanying consolidated financial statements and notes thereto have been adjusted retroactively, where applicable, to reflect this stock split. All fractional shares resulting from the reverse stock split were paid in cash. Basis of Presentation The accompanying consolidated financial statements and the related disclosures as of March 31, 2024 and for the three months ended March 31, 2024 and 2023 are unaudited and have been prepared in accordance with accounting principles generally accepted in the United States (GAAP) and the rules and regulations of the SEC for interim financial statements. Accordingly, they do not include all of the information and notes required by GAAP for complete financial statements. These interim consolidated financial statements should be read in conjunction with the Company’s 2023 and 2022 audited consolidated financial statements and notes included in the 2023 Annual Report. The consolidated balance sheet as of December 31, 2023 included herein was derived from the audited financial statements as of that date but does not include all disclosures including notes required by GAAP for complete financial statements. In the opinion of management, the unaudited interim consolidated financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for the fair presentation of the Company’s financial position and results of operations for the three months ended March 31, 2024 and 2023. The results of operations for the interim periods are not necessarily indicative of the results to be expected for the year ending December 31, 2024 or any other interim period or future year or period. Principles of Consolidation The accompanying consolidated financial statements include the accounts of Synlogic and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation. Recently Issued Accounting Pronouncements From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (FASB) or other accounting standard setting boards that the Company adopts as of the effective date. Unless otherwise discussed below, recently issued pronouncements that are or will be applicable to the Company did not have, or are not expected to have, a material impact on the Company’s present or future financial statements. |
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Fair Value of Financial Instruments
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Fair Value of Financial Instruments |
(3)Fair Value of Financial Instruments The tables below present information about the Company’s assets and liabilities that are measured at fair value on a recurring basis and indicate the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value, as described under Note 2, Summary of Significant Accounting Policies, in the audited financial statements included in the 2023 Annual Report. The Company’s investment portfolio includes many fixed income securities that do not always trade on a daily basis. As a result, the pricing services used by the Company applied other available information as applicable through processes such as benchmark yields, benchmarking of like securities, sector groupings and matrix pricing to prepare evaluations. In addition, model processes were used to assess interest rate impact and develop prepayment scenarios. These models take into consideration relevant credit information, perceived market movements, sector news and economic events. The inputs into these models may include benchmark yields, reported trades, broker-dealer quotes, issuer spreads and other relevant data. The Company accounts for issued warrants either as derivative liabilities or as equity instruments depending on the specific terms of the agreement. Warrants that are equity-classified instruments and recorded in additional paid-in capital at issuance are not subject to remeasurement. The purchase warrants issued in October 2023 are liability classified and recorded at fair value using the Black-Scholes option-pricing model at issuance, with any subsequent changes in fair value recognized in the consolidated statements of operations. We periodically evaluate changes in facts and circumstances that could impact the classification of warrants. None of the purchase warrants have been exercised since their issuance. At March 31, 2024 and December 31, 2023, the Company has classified assets and liabilities measured at fair value on a recurring basis as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements at Reporting Date Using |
|
|
|
March 31, |
|
|
Quoted Prices in Active
Markets for Identical
Assets |
|
|
Significant Other
Observable Inputs |
|
|
Significant
Unobservable Inputs |
|
Description |
|
2024 |
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
22,825 |
|
|
$ |
22,825 |
|
|
$ |
— |
|
|
$ |
— |
|
Commercial paper (included in cash and cash equivalents) |
|
|
1,997 |
|
|
|
— |
|
|
|
1,997 |
|
|
|
— |
|
Commercial paper |
|
|
7,489 |
|
|
|
— |
|
|
|
7,489 |
|
|
|
— |
|
Total |
|
$ |
32,311 |
|
|
$ |
22,825 |
|
|
$ |
9,486 |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Purchase warrant liability |
|
$ |
4,433 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
4,433 |
|
Total |
|
$ |
4,433 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
4,433 |
|
|
|
Fair Value Measurements at Reporting Date Using |
|
|
|
December 31, |
|
|
Quoted Prices in Active
Markets for Identical
Assets |
|
|
Significant Other
Observable Inputs |
|
|
Significant
Unobservable Inputs |
|
Description |
|
2023 |
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
15,476 |
|
|
$ |
15,476 |
|
|
$ |
— |
|
|
$ |
— |
|
Commercial paper (included in cash and cash equivalents) |
|
|
8,484 |
|
|
|
— |
|
|
|
8,484 |
|
|
|
— |
|
Commercial paper |
|
|
14,342 |
|
|
|
— |
|
|
|
14,342 |
|
|
|
— |
|
U.S. government agency securities and treasuries |
|
|
9,444 |
|
|
|
6,956 |
|
|
|
2,488 |
|
|
|
— |
|
Total |
|
$ |
47,746 |
|
|
$ |
22,432 |
|
|
$ |
25,314 |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Purchase warrant liability |
|
$ |
11,163 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,163 |
|
Total |
|
$ |
11,163 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,163 |
|
Cash equivalents, prepaid expenses and other current assets, accounts payable and accrued expenses at March 31, 2024 and December 31, 2023 are carried at amounts that approximate fair value due to their short-term maturities. Finance lease obligations at March 31, 2024 and December 31, 2023 approximate fair value as they bear interest at a rate approximating a market interest rate. The following tables summarize the estimated fair value of the assets presented within cash and cash equivalents measured at fair value and the gross unrealized holding gains and losses (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
Amortized Cost |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair Value |
|
Money market funds (included in cash and cash equivalents) |
|
$ |
22,825 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
22,825 |
|
Commercial paper (included in cash and cash equivalents) |
|
|
1,997 |
|
|
|
— |
|
|
|
— |
|
|
|
1,997 |
|
Total |
|
$ |
24,822 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
24,822 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
Amortized Cost |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair Value |
|
Money market funds (included in cash and cash equivalents) |
|
$ |
15,476 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
15,476 |
|
Commercial paper (included in cash and cash equivalents) |
|
|
8,482 |
|
|
|
2 |
|
|
|
— |
|
|
|
8,484 |
|
Total |
|
$ |
23,958 |
|
|
$ |
2 |
|
|
$ |
— |
|
|
$ |
23,960 |
|
Assumptions Used in Determining Fair Value of Warrants The assumptions used in the Black-Scholes option-pricing model for the purchase warrants on the consolidated balance sheets at March 31, 2024 and December 31, 2023 are included below:
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
|
December 31, 2023 |
|
Expected Term |
|
4.5 years |
|
|
4.75 years |
|
Weighted-average, risk free interest rate |
|
|
4.3 |
% |
|
|
3.9 |
% |
Expected volatility |
|
|
98.2 |
% |
|
|
94.0 |
% |
Dividend yield |
|
|
— |
|
|
|
— |
|
Strike price |
|
$ |
3.41 |
|
|
$ |
3.41 |
|
Stock price |
|
$ |
1.79 |
|
|
$ |
3.85 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Available-for-Sale Securities
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Investments, Debt and Equity Securities [Abstract] |
|
| Available-for-Sale Securities |
(4)Available-for-Sale Securities The following tables summarize the available-for-sale securities held at March 31, 2024 and December 31, 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
Amortized cost |
|
|
Gross unrealized
gains |
|
|
Gross unrealized
losses |
|
|
Fair Value |
|
Commercial paper |
|
$ |
7,489 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
7,489 |
|
Total |
|
$ |
7,489 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
7,489 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
Amortized cost |
|
|
Gross unrealized
gains |
|
|
Gross unrealized
losses |
|
|
Fair Value |
|
Commercial paper |
|
$ |
14,338 |
|
|
$ |
4 |
|
|
$ |
— |
|
|
$ |
14,342 |
|
U.S. government agency securities and treasuries |
|
|
9,444 |
|
|
|
1 |
|
|
|
(1 |
) |
|
|
9,444 |
|
Total |
|
$ |
23,782 |
|
|
$ |
5 |
|
|
$ |
(1 |
) |
|
$ |
23,786 |
|
The contractual maturity of all securities held at March 31, 2024 was two months or less. There was one investment in an unrealized loss position at March 31, 2024. The aggregate fair value of the security in an unrealized loss position at March 31, 2024 was $1.5 million. There were two investments in an unrealized loss position at December 31, 2023, none of which had been in an unrealized loss position for more than twelve months. The aggregate fair value of the securities in an unrealized loss position at December 31, 2023 was $5.4 million. The Company reviews its investments for other-than-temporary impairment whenever the fair value of an investment is less than amortized cost and evidence indicates that an investment’s carrying amount is not recoverable within a reasonable period of time. To determine whether an impairment is other-than-temporary, the Company considers whether it has the ability and intent to hold the investment until a market price recovery and considers whether evidence indicating the cost of the investment is recoverable outweighs evidence to the contrary. The Company did not hold any securities with an other-than-temporary impairment at March 31, 2024. Gross realized gains and losses on the sales of investments have not been material to the Company’s consolidated statement of operations.
|
| X |
- DefinitionAvailable for sale securities disclosure.
| Name: |
sybx_AvailableForSaleSecuritiesDisclosureTextBlock |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsDebtAndEquitySecuritiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Assets Held for Sale
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Property, Plant and Equipment Assets Held-for-Sale Disclosure [Abstract] |
|
| Assets Held for Sale |
In February 2024, the Company committed to a plan to sell its remaining lab equipment and therefore has classified the amount as assets held for sale on the consolidated balance sheet as of March 31, 2024. The assets held for sale were reported at the lower of the carrying amount or fair value, less costs to sell. Accordingly, during the three months ended March 31, 2024, the Company recorded an impairment charge, which was included in restructuring and other charges, of $0.8 million related to the lab equipment classified as assets held for sale.
|
| X |
- DefinitionTabular disclosure of long lived assets held for sale. Disclosure may include the description of the facts and circumstances leading to the expected disposal, manner and timing of disposal, the carrying value of the assets held for sale, the gain (loss) recognized in the income statement and the income statement caption that includes that gain (loss). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-3
| Name: |
us-gaap_DisclosureOfLongLivedAssetsHeldForSaleTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAssetsHeldForSaleDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Prepaid Expenses and Other Current Assets
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Text Block [Abstract] |
|
| Prepaid Expenses and Other Current Assets |
(6)Prepaid Expenses and Other Current Assets Prepaid expenses and other current assets consist of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
2024 |
|
|
2023 |
|
Prepaid insurance |
|
$ |
432 |
|
|
$ |
691 |
|
Prepaid research and development |
|
|
1,384 |
|
|
|
788 |
|
Other prepaid expenses |
|
|
502 |
|
|
|
536 |
|
Other current assets |
|
|
111 |
|
|
|
146 |
|
Total prepaid expenses and other current assets |
|
$ |
2,429 |
|
|
$ |
2,161 |
|
|
| X |
- DefinitionPrepaid expenses and other current assets.
| Name: |
sybx_PrepaidExpensesAndOtherCurrentAssetsTextBlock |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Accrued Expenses
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Payables and Accruals [Abstract] |
|
| Accrued Expenses |
Accrued expenses consist of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
2024 |
|
|
2023 |
|
Payroll related |
|
$ |
15 |
|
|
$ |
2,556 |
|
Professional fees |
|
|
392 |
|
|
|
290 |
|
Research and development |
|
|
507 |
|
|
|
91 |
|
Restructuring costs |
|
|
2,079 |
|
|
|
— |
|
Other |
|
|
123 |
|
|
|
63 |
|
Total accrued expenses |
|
$ |
3,116 |
|
|
$ |
3,000 |
|
|
| X |
- DefinitionThe entire disclosure for accounts payable, accrued expenses, and other liabilities that are classified as current at the end of the reporting period.
| Name: |
us-gaap_AccountsPayableAccruedLiabilitiesAndOtherLiabilitiesDisclosureCurrentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Restructuring and Other Charges
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Restructuring and Related Activities [Abstract] |
|
| Restructuring and Other Charges |
(8)Restructuring and Other Charges In February 2024, the Company and its board of directors decided to discontinue the Synpheny-3 trial and as a result are currently evaluating strategic options for the Company with a goal to enhance stockholder value, including the possibility of a merger or sale of the Company. Due to this decision there has been an interim evaluation of impairment of long-lived and other assets which is described in detail below. The Company also announced a corporate restructuring that included a reduction in its workforce by more than 90% that was substantially completed during the three months ended March 31, 2024. In connection with the corporate restructuring, the Company recorded a restructuring charge for severance and related costs of $5.6 million during the three months ended March 31, 2024. The Company also executed consulting agreements with a select number of former employees in which their equity continues to vest under the terms of the original award. The consulting services were determined to be non-substantive and as a result, the Company has accelerated the related stock compensation expense and recorded an additional $1.8 million to stock compensation included in restructuring charges for the three months ended March 31, 2024. Restructuring and other charges also includes impairment of the right-of-use assets associated with the Company's existing leased spaces of $9.6 million and impairment of property and equipment of $5.3 million, primarily related to leasehold improvements in connection with the lease impairment. Other charges relating to the restructuring include a $5.2 million charge to impairment for prepaid research and development in relation to the Ginkgo collaboration (see Note 11) and $0.8 million for various costs related to the restructuring including legal fees, banking fees and lab decommissioning fees. As of March 31, 2024, approximately $2.1 million of the total restructuring charges remain unpaid and were included in accrued restructuring charges.
|
| X |
- DefinitionThe entire disclosure for restructuring and related activities. Description of restructuring activities such as exit and disposal activities, include facts and circumstances leading to the plan, the expected plan completion date, the major types of costs associated with the plan activities, total expected costs, the accrual balance at the end of the period, and the periods over which the remaining accrual will be settled. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//420/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
| Name: |
us-gaap_RestructuringAndRelatedActivitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Stockholders' Equity
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Equity [Abstract] |
|
| Stockholders' Equity |
Reverse Stock Split On September 27, 2023, the Company effected a reverse stock split of its shares of common stock, pursuant to which every fifteen (15) shares of the its issued and outstanding common stock was automatically converted into one (1) issued and outstanding share of common stock without any change in the par value of $0.001 per share. The reverse stock split was approved by the stockholders on September 21, 2023 at a special meeting of stockholders. October 2023 Financing On October 3, 2023, the Company issued and sold, through an underwritten public offering: •3,921,928 shares of its common stock at a price of $2.84 per share less underwriting discounts and commissions; •pre-funded warrants to purchase up to 3,472,435 shares of its common stock at a price of $2.839 immediately following the consummation of the offering, and; •accompanying common stock warrants to purchase up to 7,394,363 shares of its common stock at a price of $3.408 per share exercisable immediately after issuance and expires five years from the date of issuance. Each share of its common stock and each pre-funded warrant was sold together with a common warrant to purchase one share of its common stock. A holder of pre-funded warrants may not exercise the warrant if the holder, together with its affiliates, would beneficially own more than 4.99% (or, upon election by a holder prior to the issuance of any warrants, 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. The net proceeds to the Company from the sale of common stock and pre-funded warrants through the offering, after deducting the underwriting discounts and commissions and offering expenses payable by the Company, were approximately $19.6 million. The common stock and pre-funded warrants met the criteria for equity classification. The purchase warrants met the definition of a derivative instrument. Accordingly, upon issuance, the purchase warrants were recorded as a liability at fair value using the Black-Scholes option-pricing model in the amount of $7.1 million. Any subsequent changes in fair value of the purchase warrants is recognized in the consolidated statements of operations. The residual proceeds were allocated between the common stock and pre-funded warrants based on their relative fair values at the time of issuance. The amount allocated to the pre-funded warrants was recorded as a component of stockholders’ equity within additional paid-in capital. At March 31, 2024, the fair value of the purchase warrants was $4.4 million. Accordingly, a gain on remeasurement of the purchase warrant liability of $6.7 million was recorded in the three months ended March 31, 2024. Subsequent to their issuance and through March 31, 2024, 2,920,126 pre-funded warrants have been exercised. None of the purchase warrants have been exercised since their issuance. At-the-Market (ATM) Offering Program In July 2021, the Company entered into a sales agreement with Jefferies, LLC (Jefferies) with respect to an ATM, under which the Company may offer and sell, from time to time at its sole discretion, shares of its common stock having aggregate sales proceeds of up to $50.0 million. Jefferies is not required to sell any specific amount but acts as the Company’s sales agent using commercially reasonable efforts consistent with its normal trading and sales practices. During the three months ended March 31, 2024, 7,839 shares of common stock were sold pursuant to the sales agreement with Jefferies, resulting in net proceeds of approximately $0.01 million. Ginkgo Warrants In June 2019, the Company issued to Ginkgo Bioworks, Inc. (Ginkgo) an aggregate of 422,718 shares of common stock at a purchase price per share of $135, and pre-funded warrants (the Ginkgo pre-funded warrants) to purchase up to an aggregate of 169,874 shares of common stock at an exercise price of $135 per share, with $134.85 of such exercise price paid at the closing of the offering. The net proceeds to the Company were approximately $79.9 million. None of the Ginkgo pre-funded warrants have been exercised as of March 31, 2024. (See Note 9, Collaboration Agreements: Ginkgo Collaboration). The Company has reserved for future issuance the following shares of common stock related to the potential exercise of Ginkgo pre-funded warrants, exercise of stock options, and the employee stock purchase plan:
|
|
|
|
|
March 31, 2024 |
|
Common stock issuable under pre-funded warrants |
|
552,309 |
|
Common stock issuable under purchase warrants |
|
3,921,928 |
|
Common stock issuable under Ginkgo pre-funded warrants |
|
169,874 |
|
Options exercisable to purchase common stock |
|
334,081 |
|
Employee Stock Purchase Plan |
|
- |
|
Total |
|
4,978,192 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Equity based Compensation
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Equity based Compensation |
(10)Equity-based Compensation On January 1, 2024, the number of shares of common stock available for issuance under the 2015 Equity Incentive Award Plan (the 2015 Plan) and the 2015 Employee Stock Purchase Plan (ESPP) was increased by 459,392 shares and 91,878 shares, respectively, due to the annual evergreen provision to increase shares available under the 2015 Plan and the ESPP. As of March 31, 2024, there were an aggregate of 604,072 shares available for future grant under the 2017 Stock Incentive Plan (the 2017 Plan) and the 2015 Plan, and 187,012 shares available for future grant under the ESPP. The following table summarizes equity‑based compensation expense within the Company’s consolidated statements of operations and comprehensive loss for the three months ended March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Three months ended 31, |
|
|
|
2024 |
|
|
2023 |
|
Research and development |
|
$ |
115 |
|
|
$ |
277 |
|
General and administrative |
|
|
306 |
|
|
|
441 |
|
Restructuring charges: expense acceleration |
|
|
1,800 |
|
|
|
— |
|
|
|
$ |
2,221 |
|
|
$ |
718 |
|
The following table summarizes equity‑based compensation expense by type of award for the three months ended March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Three months ended March 31, |
|
|
|
2024 |
|
|
2023 |
|
Stock options |
|
$ |
1,480 |
|
|
$ |
607 |
|
Restricted stock awards |
|
|
763 |
|
|
|
91 |
|
ESPP |
|
|
(22 |
) |
|
|
20 |
|
|
|
$ |
2,221 |
|
|
$ |
718 |
|
During the three months ended March 31, 2024, the Company granted 44,701 stock options with a weighted average exercise price of $4.20 per share. As of March 31, 2024, there was $0.7 million of unrecognized share-based compensation related to unvested stock option grants which is expected to be recognized over a weighted average period of 1.93 years. The total unrecognized share-based compensation cost will be adjusted for actual forfeitures as they occur. During the three months ended March 31, 2024, the Company granted 362,700 restricted stock awards with a weighted average grant date fair value per share of $4.44. As of March 31, 2024, there was approximately $0.5 million of unrecognized share-based compensation related to restricted stock awards granted, which is expected to be recognized over a weighted average period of 1.8 years. The total unrecognized share-based compensation cost will be adjusted for actual forfeitures as they occur. For a full description of the Company’s equity plans, refer to Note 9, Equity-based Compensation and Equity Incentive Plans in the 2023 Annual Report.
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Collaboration Agreements
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Collaboration Agreements |
(11)Collaboration Agreements Roche Collaboration In June 2021, the Company entered into a Pilot Collaboration and Option Agreement (the Roche Collaboration and Option Agreement) with F. Hoffmann-La Roche Ltd (Roche Basel) and Hoffmann-La Roche Inc. (Roche US, and together with Roche Basel, Roche). Under the terms of the Roche Collaboration and Option Agreement, the Company and Roche will seek to collaborate to research and pre-clinically develop Synthetic Biotics for addressing an undisclosed novel target for the treatment of inflammatory bowel disease. During the three months ended March 31, 2024 and 2023, the Company recognized $0.01 million and $0.2 million, respectively, as collaboration revenue associated with the Roche Collaboration and Option Agreement. The Roche Collaboration and Option Agreement concluded after the last milestone was achieved by the Company in October 2023. Subsequently, Roche did not exercise its exclusive option to enter into a licensing and collaboration agreement for further development and commercialization of the product candidate For a full description of the Roche Collaboration and Option Agreement, refer to Note 10, Collaboration Agreements in the 2023 Annual Report. Ginkgo Collaboration In 2017, the Company established a technology collaboration with Ginkgo. In June 2019, the Company expanded its collaboration and entered into an agreement with Ginkgo for the research and development of engineered microbial therapeutic products (See Note 9). Under the 2019 expanded agreement, the Company made a prepayment to Ginkgo of $30.0 million for its foundry services that was to be provided to the Company over an initial term of five years, which could be extended for an additional three (3) years, subject to the satisfaction of specified conditions. Upon the expiration of such initial term and, if applicable, an additional term, any portion of the prepayment that has not been used to purchase services from Ginkgo will be retained by Ginkgo. In February 2024, the Company and its board of directors decided to discontinue the Synpheny-3 trial and significantly reduce its workforce and have made the decision to evaluate strategic options for the Company. As a result of this decision, the Company would no longer be purchasing services from Ginkgo. At March 31, 2024, the remaining $5.2 million of prepaid expenses related to this collaboration that were historically recorded in prepaid expenses and other current assets and prepaid research and development, net of current portion on the consolidated balance sheet, were recorded as restructuring and other charges on the consolidated statements of operations and comprehensive loss.
|
| X |
- DefinitionThe entire disclosure for collaborative arrangements in which the entity is a participant, including a) information about the nature and purpose of such arrangements; b) its rights and obligations thereunder; c) the accounting policy for collaborative arrangements; and d) the income statement classification and amounts attributable to transactions arising from the collaborative arrangement between participants. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 10
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479402/808-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479402/808-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org//808/tableOfContent
| Name: |
us-gaap_CollaborativeArrangementDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Net Loss per Share
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Earnings Per Share [Abstract] |
|
| Net Loss per Share |
Basic net loss per share is computed using the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share is computed using the sum of the weighted-average number of shares of common stock outstanding during the period and if dilutive, the weighted-average number of potential shares of common stock, including unvested restricted common stock and outstanding stock options. In June 2019, the Company sold 422,718 shares of common stock and pre-funded warrants to purchase an aggregate of 169,874 shares of common stock at an exercise price of $135 per share, with $134.85 of such exercise price paid at the closing of the offering (see Note 8, Stockholder's Equity and Note 10, Collaborations: Ginkgo Collaboration, in the audited financial statements included in the 2023 Annual Report). The shares of common stock into which the warrants may be exercised are considered outstanding for the purposes of computing net loss per share. The Company’s potentially dilutive shares, which include outstanding stock options, unvested restricted common stock and potential shares issuable under the ESPP, are considered to be common share equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive. The following potential common shares, presented based on amounts outstanding at each period end, were excluded from the calculation of the diluted net loss per share attributable to common stockholders for the period indicated because including them would have had an anti-dilutive effect.
|
|
|
|
|
|
|
|
|
|
|
As of March 31, |
|
|
|
2024 |
|
|
2023 |
|
Purchase warrants |
|
|
3,921,928 |
|
|
|
— |
|
Unvested restricted common stock awards |
|
|
213,204 |
|
|
|
65,616 |
|
Outstanding options to purchase common stock |
|
|
504,390 |
|
|
|
740,474 |
|
Potential shares issuable under the ESPP |
|
|
— |
|
|
|
— |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Commitments and Contingencies
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
(13)Commitments and Contingencies In the ordinary course of business, the Company may be subject to legal proceedings, claims and litigation as the Company operates in an industry susceptible to patent legal claims. The Company accounts for estimated losses with respect to legal proceedings and claims when such losses are probable and estimable. Legal costs associated with these matters are expensed when incurred. The Company is not currently a party to any material legal proceedings. The Company’s commitments described in the Company’s consolidated financial statements as of and for the year ended December 31, 2023 and the notes thereto included in the 2023 Annual Report have had no material changes during the three months ended March 31, 2024.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Related-Party Transactions
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Related Party Transactions [Abstract] |
|
| Related-Party Transactions |
Related-Party Transactions In June 2019, the Company expanded its collaboration and entered into an agreement with Ginkgo for the research and development of engineered microbial therapeutic products. As of March 31, 2024, Ginkgo owned 422,718 shares of the Company’s outstanding common stock. See Note 10, Ginkgo Collaboration, in the audited financial statements included in the 2023 Annual Report. Under the agreement the Company made a prepayment to Ginkgo of $30.0 million for its foundry services that would be provided to the Company over an initial term of five years. Upon the expiration of such initial term and, if applicable, an additional term, any portion of the prepayment that has not been used to purchase services from Ginkgo will be retained by Ginkgo. In February 2024, the Company and its board of directors decided to discontinue the Synpheny-3 trial, significantly reduce its workforce and to evaluate strategic options for the Company. As a result of this decision, the Company would no longer be purchasing services from Ginkgo. At March 31, 2024, the remaining $5.2 million of prepaid expenses related to this collaboration that were historically recorded in prepaid expenses and other current assets and prepaid research and development, net of current portion on the consolidated balance sheet, were recorded as restructuring and other charges on the consolidated statements of operations and comprehensive loss.
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Summary of Significant Accounting Policies (Policies)
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of Presentation |
Basis of Presentation The accompanying consolidated financial statements and the related disclosures as of March 31, 2024 and for the three months ended March 31, 2024 and 2023 are unaudited and have been prepared in accordance with accounting principles generally accepted in the United States (GAAP) and the rules and regulations of the SEC for interim financial statements. Accordingly, they do not include all of the information and notes required by GAAP for complete financial statements. These interim consolidated financial statements should be read in conjunction with the Company’s 2023 and 2022 audited consolidated financial statements and notes included in the 2023 Annual Report. The consolidated balance sheet as of December 31, 2023 included herein was derived from the audited financial statements as of that date but does not include all disclosures including notes required by GAAP for complete financial statements. In the opinion of management, the unaudited interim consolidated financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for the fair presentation of the Company’s financial position and results of operations for the three months ended March 31, 2024 and 2023. The results of operations for the interim periods are not necessarily indicative of the results to be expected for the year ending December 31, 2024 or any other interim period or future year or period.
|
| Principles of Consolidation |
Principles of Consolidation The accompanying consolidated financial statements include the accounts of Synlogic and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation.
|
| Recently Issued Accounting Pronouncements |
Recently Issued Accounting Pronouncements From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (FASB) or other accounting standard setting boards that the Company adopts as of the effective date. Unless otherwise discussed below, recently issued pronouncements that are or will be applicable to the Company did not have, or are not expected to have, a material impact on the Company’s present or future financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 810
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1
| Name: |
us-gaap_ConsolidationPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Fair Value of Financial Instruments (Tables)
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Schedule of Company's Classified Assets Measured at Fair Value on Recurring Basis |
At March 31, 2024 and December 31, 2023, the Company has classified assets and liabilities measured at fair value on a recurring basis as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements at Reporting Date Using |
|
|
|
March 31, |
|
|
Quoted Prices in Active
Markets for Identical
Assets |
|
|
Significant Other
Observable Inputs |
|
|
Significant
Unobservable Inputs |
|
Description |
|
2024 |
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
22,825 |
|
|
$ |
22,825 |
|
|
$ |
— |
|
|
$ |
— |
|
Commercial paper (included in cash and cash equivalents) |
|
|
1,997 |
|
|
|
— |
|
|
|
1,997 |
|
|
|
— |
|
Commercial paper |
|
|
7,489 |
|
|
|
— |
|
|
|
7,489 |
|
|
|
— |
|
Total |
|
$ |
32,311 |
|
|
$ |
22,825 |
|
|
$ |
9,486 |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Purchase warrant liability |
|
$ |
4,433 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
4,433 |
|
Total |
|
$ |
4,433 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
4,433 |
|
|
|
Fair Value Measurements at Reporting Date Using |
|
|
|
December 31, |
|
|
Quoted Prices in Active
Markets for Identical
Assets |
|
|
Significant Other
Observable Inputs |
|
|
Significant
Unobservable Inputs |
|
Description |
|
2023 |
|
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds |
|
$ |
15,476 |
|
|
$ |
15,476 |
|
|
$ |
— |
|
|
$ |
— |
|
Commercial paper (included in cash and cash equivalents) |
|
|
8,484 |
|
|
|
— |
|
|
|
8,484 |
|
|
|
— |
|
Commercial paper |
|
|
14,342 |
|
|
|
— |
|
|
|
14,342 |
|
|
|
— |
|
U.S. government agency securities and treasuries |
|
|
9,444 |
|
|
|
6,956 |
|
|
|
2,488 |
|
|
|
— |
|
Total |
|
$ |
47,746 |
|
|
$ |
22,432 |
|
|
$ |
25,314 |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Purchase warrant liability |
|
$ |
11,163 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,163 |
|
Total |
|
$ |
11,163 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,163 |
|
|
| Schedule of Fair Value Assets and Liabilities Measured Gross Unrealized Gains and Losses |
The following tables summarize the estimated fair value of the assets presented within cash and cash equivalents measured at fair value and the gross unrealized holding gains and losses (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
Amortized Cost |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair Value |
|
Money market funds (included in cash and cash equivalents) |
|
$ |
22,825 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
22,825 |
|
Commercial paper (included in cash and cash equivalents) |
|
|
1,997 |
|
|
|
— |
|
|
|
— |
|
|
|
1,997 |
|
Total |
|
$ |
24,822 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
24,822 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
Amortized Cost |
|
|
Gross unrealized gains |
|
|
Gross unrealized losses |
|
|
Fair Value |
|
Money market funds (included in cash and cash equivalents) |
|
$ |
15,476 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
15,476 |
|
Commercial paper (included in cash and cash equivalents) |
|
|
8,482 |
|
|
|
2 |
|
|
|
— |
|
|
|
8,484 |
|
Total |
|
$ |
23,958 |
|
|
$ |
2 |
|
|
$ |
— |
|
|
$ |
23,960 |
|
|
| Schedule of Assumptions Used in Determining Fair Value of Warrants |
Assumptions Used in Determining Fair Value of Warrants The assumptions used in the Black-Scholes option-pricing model for the purchase warrants on the consolidated balance sheets at March 31, 2024 and December 31, 2023 are included below:
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
|
December 31, 2023 |
|
Expected Term |
|
4.5 years |
|
|
4.75 years |
|
Weighted-average, risk free interest rate |
|
|
4.3 |
% |
|
|
3.9 |
% |
Expected volatility |
|
|
98.2 |
% |
|
|
94.0 |
% |
Dividend yield |
|
|
— |
|
|
|
— |
|
Strike price |
|
$ |
3.41 |
|
|
$ |
3.41 |
|
Stock price |
|
$ |
1.79 |
|
|
$ |
3.85 |
|
|
| X |
- DefinitionSchedule Of Option Pricing Model At Issuance Table Text Block
| Name: |
sybx_ScheduleOfOptionPricingModelAtIssuanceTableTextBlock |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSchedule of Fair Value Assets and Liabilities Measured Gross Unrealized Gains and Losses [TableText Block]
| Name: |
sybx_Scheduleoffairvalueassetsandliabilitiesmeasuredgrossunrealizedgainsandlossestabletextblock |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets and liabilities, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Available-for-Sale Securities (Tables)
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Investments, Debt and Equity Securities [Abstract] |
|
| Summary of Available-for-Sale Securities Held |
The following tables summarize the available-for-sale securities held at March 31, 2024 and December 31, 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
|
Amortized cost |
|
|
Gross unrealized
gains |
|
|
Gross unrealized
losses |
|
|
Fair Value |
|
Commercial paper |
|
$ |
7,489 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
7,489 |
|
Total |
|
$ |
7,489 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
7,489 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2023 |
|
Amortized cost |
|
|
Gross unrealized
gains |
|
|
Gross unrealized
losses |
|
|
Fair Value |
|
Commercial paper |
|
$ |
14,338 |
|
|
$ |
4 |
|
|
$ |
— |
|
|
$ |
14,342 |
|
U.S. government agency securities and treasuries |
|
|
9,444 |
|
|
|
1 |
|
|
|
(1 |
) |
|
|
9,444 |
|
Total |
|
$ |
23,782 |
|
|
$ |
5 |
|
|
$ |
(1 |
) |
|
$ |
23,786 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsDebtAndEquitySecuritiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation of available-for-sale securities from cost basis to fair value.
| Name: |
us-gaap_ScheduleOfAvailableForSaleSecuritiesReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Prepaid Expenses and Other Current Assets (Tables)
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Text Block [Abstract] |
|
| Schedule of Prepaid Expenses and Other Current Assets |
Prepaid expenses and other current assets consist of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
2024 |
|
|
2023 |
|
Prepaid insurance |
|
$ |
432 |
|
|
$ |
691 |
|
Prepaid research and development |
|
|
1,384 |
|
|
|
788 |
|
Other prepaid expenses |
|
|
502 |
|
|
|
536 |
|
Other current assets |
|
|
111 |
|
|
|
146 |
|
Total prepaid expenses and other current assets |
|
$ |
2,429 |
|
|
$ |
2,161 |
|
|
| X |
- DefinitionSchedule of prepaid expenses and other current assets.
| Name: |
sybx_ScheduleOfPrepaidExpensesAndOtherCurrentAssetsTableTextBlock |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_TextBlockAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Accrued Expenses (Tables)
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Payables and Accruals [Abstract] |
|
| Schedule of Accrued Expenses |
Accrued expenses consist of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
2024 |
|
|
2023 |
|
Payroll related |
|
$ |
15 |
|
|
$ |
2,556 |
|
Professional fees |
|
|
392 |
|
|
|
290 |
|
Research and development |
|
|
507 |
|
|
|
91 |
|
Restructuring costs |
|
|
2,079 |
|
|
|
— |
|
Other |
|
|
123 |
|
|
|
63 |
|
Total accrued expenses |
|
$ |
3,116 |
|
|
$ |
3,000 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of accrued liabilities.
| Name: |
us-gaap_ScheduleOfAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Stockholders' Equity (Tables)
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Equity [Abstract] |
|
| Schedule of Shares of Common Stock Reserved for Future Issuance |
The Company has reserved for future issuance the following shares of common stock related to the potential exercise of Ginkgo pre-funded warrants, exercise of stock options, and the employee stock purchase plan:
|
|
|
|
|
March 31, 2024 |
|
Common stock issuable under pre-funded warrants |
|
552,309 |
|
Common stock issuable under purchase warrants |
|
3,921,928 |
|
Common stock issuable under Ginkgo pre-funded warrants |
|
169,874 |
|
Options exercisable to purchase common stock |
|
334,081 |
|
Employee Stock Purchase Plan |
|
- |
|
Total |
|
4,978,192 |
|
|
| X |
- DefinitionSchedule of shares of common stock reserved for future issuance.
| Name: |
sybx_ScheduleOfSharesOfCommonStockReservedForFutureIssuanceTableTextBlock |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Equity based Compensation (Tables)
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Schedule of Equity-based Compensation Expenses |
The following table summarizes equity‑based compensation expense within the Company’s consolidated statements of operations and comprehensive loss for the three months ended March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Three months ended 31, |
|
|
|
2024 |
|
|
2023 |
|
Research and development |
|
$ |
115 |
|
|
$ |
277 |
|
General and administrative |
|
|
306 |
|
|
|
441 |
|
Restructuring charges: expense acceleration |
|
|
1,800 |
|
|
|
— |
|
|
|
$ |
2,221 |
|
|
$ |
718 |
|
|
| Schedule of Equity-based Compensation Expenses by Award Type |
The following table summarizes equity‑based compensation expense by type of award for the three months ended March 31, 2024 and 2023 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
Three months ended March 31, |
|
|
|
2024 |
|
|
2023 |
|
Stock options |
|
$ |
1,480 |
|
|
$ |
607 |
|
Restricted stock awards |
|
|
763 |
|
|
|
91 |
|
ESPP |
|
|
(22 |
) |
|
|
20 |
|
|
|
$ |
2,221 |
|
|
$ |
718 |
|
|
| X |
- DefinitionTabular disclosure of share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfShareBasedCompensationArrangementsByShareBasedPaymentAwardTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Net Loss per Share (Tables)
|
3 Months Ended |
Mar. 31, 2024 |
|---|
| Earnings Per Share [Abstract] |
|
| Schedule of Potentially Common Shares Excluded from Calculation of Net Loss Per share |
The following potential common shares, presented based on amounts outstanding at each period end, were excluded from the calculation of the diluted net loss per share attributable to common stockholders for the period indicated because including them would have had an anti-dilutive effect.
|
|
|
|
|
|
|
|
|
|
|
As of March 31, |
|
|
|
2024 |
|
|
2023 |
|
Purchase warrants |
|
|
3,921,928 |
|
|
|
— |
|
Unvested restricted common stock awards |
|
|
213,204 |
|
|
|
65,616 |
|
Outstanding options to purchase common stock |
|
|
504,390 |
|
|
|
740,474 |
|
Potential shares issuable under the ESPP |
|
|
— |
|
|
|
— |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Nature of Business - Additional Information (Detail) - USD ($)
$ in Thousands |
3 Months Ended |
|
Mar. 31, 2024 |
Dec. 31, 2023 |
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
|
| Cash, cash equivalents, and short-term marketable securities |
$ 32,300
|
|
| Restricted cash |
1,097
|
$ 1,097
|
| Accumulated deficit |
$ 445,865
|
$ 414,301
|
| Reduction in WorkForce |
90.00%
|
|
| X |
- DefinitionCash includes currency on hand as well as demand deposits with banks or financial institutions. It also includes other kinds of accounts that have the general characteristics of demand deposits in that the customer may deposit additional funds at any time and effectively may withdraw funds at any time without prior notice or penalty. Cash equivalents, excluding items classified as marketable securities, include short-term, highly liquid Investments that are both readily convertible to known amounts of cash, and so near their maturity that they present minimal risk of changes in value because of changes in interest rates. Generally, only investments with original maturities of three months or less qualify under that definition. Original maturity means original maturity to the entity holding the investment. For example, both a three-month US Treasury bill and a three-year Treasury note purchased three months from maturity qualify as cash equivalents. However, a Treasury note purchased three years ago does not become a cash equivalent when its remaining maturity is three months. Short-term investments, exclusive of cash equivalents, generally consist of marketable securities intended to be sold within one year (or the normal operating cycle if longer) and may include trading securities, available-for-sale securities, or held-to-maturity securities (if maturing within one year), as applicable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CashCashEquivalentsAndShortTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash restricted as to withdrawal or usage, classified as noncurrent. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-SubTopic 210
-Topic 954
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480632/954-210-45-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
| Name: |
us-gaap_RestrictedCashNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.1.1.u2
Summary of Significant Accounting Policies (Additional Information) (Details)
|
|
3 Months Ended |
Sep. 27, 2023 |
Mar. 31, 2024 |
| Accounting Policies [Abstract] |
|
|
| Reverse Stock Split ,Decsription |
the Company effected a reverse stock split of its shares of common stock, pursuant to which every fifteen (15) shares of the its issued and outstanding common stock was automatically converted into one (1) issued and outstanding share of common stock without any change in the par value of $0.001 per share.
|
On September 27, 2023, the Company effected a one-for-fifteen reverse stock split of its issued and outstanding common stock, which also adjusted all outstanding warrants. Accordingly, all share and per share amounts for all periods presented in the accompanying consolidated financial statements and notes thereto have been adjusted retroactively, where applicable, to reflect this stock split. All fractional shares resulting from the reverse stock split were paid in cash.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of the reverse stock split arrangement. Also provide the retroactive effect given by the reverse split that occurs after the balance sheet date but before the release of financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 4
-Subparagraph (SAB Topic 4.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-4
| Name: |
us-gaap_StockholdersEquityReverseStockSplit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=sybx_October2023FinancingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Fair Value of Financial Instruments - Schedule of Company's Classified Assets Measured at Fair Value on Recurring Basis (Detail) - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Dec. 31, 2023 |
| Assets: |
|
|
| Estimated Fair Value |
$ 24,822
|
$ 23,960
|
| Liabilities, Fair Value Disclosure [Abstract] |
|
|
| Purchase Warrant Liability |
4,433
|
11,163
|
| Money market funds |
|
|
| Assets: |
|
|
| Estimated Fair Value |
22,825
|
15,476
|
| Cash and Cash Equivalents | Commercial Paper |
|
|
| Assets: |
|
|
| Estimated Fair Value |
1,997
|
8,484
|
| Recurring |
|
|
| Assets: |
|
|
| Total assets |
32,311
|
47,746
|
| Liabilities, Fair Value Disclosure [Abstract] |
|
|
| Purchase Warrant Liability |
4,433
|
11,163
|
| Total liability |
4,433
|
11,163
|
| Recurring | Money market funds |
|
|
| Assets: |
|
|
| Estimated Fair Value |
22,825
|
15,476
|
| Recurring | Commercial Paper |
|
|
| Assets: |
|
|
| Estimated Fair Value |
7,489
|
14,342
|
| Recurring | U.S. government agency securities and treasuries |
|
|
| Assets: |
|
|
| Short-term investments |
|
9,444
|
| Recurring | Cash and Cash Equivalents | Commercial Paper |
|
|
| Assets: |
|
|
| Estimated Fair Value |
1,997
|
8,484
|
| Recurring | Quoted Prices in Active Markets for Identical Assets (Level 1) |
|
|
| Assets: |
|
|
| Total assets |
22,825
|
22,432
|
| Liabilities, Fair Value Disclosure [Abstract] |
|
|
| Purchase Warrant Liability |
0
|
0
|
| Total liability |
0
|
0
|
| Recurring | Quoted Prices in Active Markets for Identical Assets (Level 1) | Money market funds |
|
|
| Assets: |
|
|
| Estimated Fair Value |
22,825
|
15,476
|
| Recurring | Quoted Prices in Active Markets for Identical Assets (Level 1) | Commercial Paper |
|
|
| Assets: |
|
|
| Estimated Fair Value |
0
|
|
| Recurring | Quoted Prices in Active Markets for Identical Assets (Level 1) | U.S. government agency securities and treasuries |
|
|
| Assets: |
|
|
| Short-term investments |
|
6,956
|
| Recurring | Quoted Prices in Active Markets for Identical Assets (Level 1) | Cash and Cash Equivalents | Commercial Paper |
|
|
| Assets: |
|
|
| Estimated Fair Value |
0
|
0
|
| Recurring | Significant Other Observable Inputs (Level 2) |
|
|
| Assets: |
|
|
| Total assets |
9,486
|
25,314
|
| Liabilities, Fair Value Disclosure [Abstract] |
|
|
| Purchase Warrant Liability |
0
|
0
|
| Total liability |
0
|
0
|
| Recurring | Significant Other Observable Inputs (Level 2) | Money market funds |
|
|
| Assets: |
|
|
| Estimated Fair Value |
0
|
0
|
| Recurring | Significant Other Observable Inputs (Level 2) | Commercial Paper |
|
|
| Assets: |
|
|
| Estimated Fair Value |
7,489
|
14,342
|
| Recurring | Significant Other Observable Inputs (Level 2) | U.S. government agency securities and treasuries |
|
|
| Assets: |
|
|
| Short-term investments |
|
2,488
|
| Recurring | Significant Other Observable Inputs (Level 2) | Cash and Cash Equivalents | Commercial Paper |
|
|
| Assets: |
|
|
| Estimated Fair Value |
1,997
|
8,484
|
| Recurring | Significant Unobservable Inputs (Level 3) |
|
|
| Assets: |
|
|
| Total assets |
0
|
0
|
| Liabilities, Fair Value Disclosure [Abstract] |
|
|
| Purchase Warrant Liability |
4,433
|
11,163
|
| Total liability |
4,433
|
11,163
|
| Recurring | Significant Unobservable Inputs (Level 3) | Money market funds |
|
|
| Assets: |
|
|
| Estimated Fair Value |
0
|
0
|
| Recurring | Significant Unobservable Inputs (Level 3) | Commercial Paper |
|
|
| Assets: |
|
|
| Estimated Fair Value |
0
|
0
|
| Recurring | Significant Unobservable Inputs (Level 3) | U.S. government agency securities and treasuries |
|
|
| Assets: |
|
|
| Short-term investments |
|
0
|
| Recurring | Significant Unobservable Inputs (Level 3) | Cash and Cash Equivalents | Commercial Paper |
|
|
| Assets: |
|
|
| Estimated Fair Value |
$ 0
|
$ 0
|
| X |
- DefinitionPurchase Warrant Liability
| Name: |
sybx_PurchaseWarrantLiability |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value portion of probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_AssetsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsFairValueDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair value portion of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates.
| Name: |
us-gaap_CashAndCashEquivalentsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value portion of investment securities, including, but not limited to, marketable securities, derivative financial instruments, and investments accounted for under the equity method. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_InvestmentsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value of financial and nonfinancial obligations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_LiabilitiesFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesFairValueDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_CashAndCashEquivalentsAxis=us-gaap_MoneyMarketFundsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FairValueByAssetClassAxis=us-gaap_CashAndCashEquivalentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=us-gaap_CommercialPaperMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FairValueByMeasurementFrequencyAxis=us-gaap_FairValueMeasurementsRecurringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=sybx_USTreasuryAndGovernmentLongTermDebtSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Fair Value of Financial Instruments - Schedule of Assets measured at fair value and the gross unrealized holding gains and losses (Details) - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Dec. 31, 2023 |
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
$ 24,822
|
$ 23,958
|
| Gross Unrealized Gain |
0
|
2
|
| Gross Unrealized Loss |
0
|
0
|
| Estimated Fair Value |
24,822
|
23,960
|
| Commercial Paper | Recurring |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Estimated Fair Value |
7,489
|
14,342
|
| Commercial Paper | Cash and Cash Equivalents |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
1,997
|
8,482
|
| Gross Unrealized Gain |
0
|
2
|
| Gross Unrealized Loss |
0
|
0
|
| Estimated Fair Value |
1,997
|
8,484
|
| Commercial Paper | Cash and Cash Equivalents | Recurring |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Estimated Fair Value |
1,997
|
8,484
|
| Money market funds |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Amortized Cost |
22,825
|
15,476
|
| Gross Unrealized Gain |
0
|
0
|
| Gross Unrealized Loss |
0
|
0
|
| Estimated Fair Value |
22,825
|
15,476
|
| Money market funds | Recurring |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Estimated Fair Value |
$ 22,825
|
$ 15,476
|
| X |
- DefinitionAmortized Cost Fair Value
| Name: |
sybx_AmortizedCostFairValue |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCashEquivalentsAccumulatedGrossUnrealizedGain
| Name: |
sybx_Cashequivalentsaccumulatedgrossunrealizedgain |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCashEquivalentsAccumulatedGrossUnrealizedLoss
| Name: |
sybx_Cashequivalentsaccumulatedgrossunrealizedloss |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value portion of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates.
| Name: |
us-gaap_CashAndCashEquivalentsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=us-gaap_CommercialPaperMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FairValueByMeasurementFrequencyAxis=us-gaap_FairValueMeasurementsRecurringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FairValueByAssetClassAxis=us-gaap_CashAndCashEquivalentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CashAndCashEquivalentsAxis=us-gaap_MoneyMarketFundsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionShare Based Compensation Arrangement By ShareBased PaymentAward Fair Value Assumptions Stock Price
| Name: |
sybx_ShareBasedCompensationArrangementBySharebasedPaymentawardFairValueAssumptionsStockPrice |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionShare Based Compensation Arrangement By Shares Based Payment Award Fair Value Assumptions Strike Price
| Name: |
sybx_ShareBasedCompensationArrangementBySharesBasedPaymentAwardFairValueAssumptionsStrikePrice |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Available-for-Sale Securities - Summary of Available-for-Sale Securities Held (Detail) - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Dec. 31, 2023 |
| Schedule of Available-for-sale Securities [Line Items] |
|
|
| Amortized cost |
$ 7,489
|
$ 23,782
|
| Gross unrealized gains |
0
|
5
|
| Gross unrealized losses |
0
|
(1)
|
| Fair Value |
7,489
|
23,786
|
| Commercial Paper |
|
|
| Schedule of Available-for-sale Securities [Line Items] |
|
|
| Amortized cost |
7,489
|
14,338
|
| Gross unrealized gains |
0
|
4
|
| Gross unrealized losses |
0
|
0
|
| Fair Value |
$ 7,489
|
14,342
|
| U.S. government agency securities and treasuries |
|
|
| Schedule of Available-for-sale Securities [Line Items] |
|
|
| Amortized cost |
|
9,444
|
| Gross unrealized gains |
|
1
|
| Gross unrealized losses |
|
(1)
|
| Fair Value |
|
$ 9,444
|
| X |
- DefinitionAmount, before tax, of unrealized gain in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedGainBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before tax, of unrealized loss in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmortized cost of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleDebtSecuritiesAmortizedCostBasis |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481830/320-10-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479130/326-30-45-1
| Name: |
us-gaap_AvailableForSaleSecuritiesDebtSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (aaa)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-3
| Name: |
us-gaap_ScheduleOfAvailableForSaleSecuritiesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_CommercialPaperMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=sybx_USGovernmentAgenciesDebtSecuritiesAndTreasuriesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionOther than temporary impairment losses investments number debt securities available for sale.
| Name: |
sybx_OtherThanTemporaryImpairmentLossesInvestmentsNumberDebtSecuritiesAvailableForSale |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of investments in debt securities measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for 12 months or longer, without an allowance for credit loss. Includes beneficial interest in securitized financial asset. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-4
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLongerNumberOfPositions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in unrealized loss position without allowance for credit loss. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479081/326-30-55-8
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479106/326-30-50-4
| Name: |
us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPosition |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_InvestmentsDebtAndEquitySecuritiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_LongLivedAssetsHeldForSaleLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses associated with exit or disposal activities pursuant to an authorized plan. Excludes expenses related to a discontinued operation or an asset retirement obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482047/420-10-45-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 5.P.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-1
| Name: |
us-gaap_RestructuringCharges |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_LongLivedAssetsHeldForSaleByAssetTypeAxis=sybx_LabEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.1.1.u2
Prepaid Expenses and Other Current Assets (Details) - USD ($)
$ in Thousands |
Mar. 31, 2024 |
Dec. 31, 2023 |
| Deferred Costs, Capitalized, Prepaid, and Other Assets Disclosure [Abstract] |
|
|
| Prepaid insurance |
$ 432
|
$ 691
|
| Prepaid Research And Development Current |
1,384
|
788
|
| Other Prepaid Expense, Current |
502
|
536
|
| Other current assets |
111
|
146
|
| Prepaid Expense and Other Assets, Current |
$ 2,429
|
$ 2,161
|
| X |
- DefinitionPrepaid research and development, current.
| Name: |
sybx_PrepaidResearchAndDevelopmentCurrent |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_DeferredCostsCapitalizedPrepaidAndOtherAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of current assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for other costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
| Name: |
us-gaap_OtherPrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for insurance that provides economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
| Name: |
us-gaap_PrepaidInsurance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.1.1.u2
| X |
- DefinitionAccrued research and development, current.
| Name: |
sybx_AccruedResearchAndDevelopmentCurrent |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAccured Restructuring cost
| Name: |
sybx_AccuredRestructuringCost |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for professional fees, such as for legal and accounting services received. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedProfessionalFeesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_PayablesAndAccrualsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.1.1.u2
Restructuring and Other Charges (Additional Information) (Details) - USD ($)
$ in Thousands |
3 Months Ended |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Restructuring and Related Activities [Abstract] |
|
|
| Restructuring charge |
$ 28,289
|
$ 0
|
| Severance and related costs |
5,600
|
|
| Compensation included in restructuring charges |
$ 1,800
|
|
| Reduction in WorkForce |
90.00%
|
|
| impairment of the right-of-use assets |
$ 9,571
|
$ 0
|
| Existing Lease Obligations |
9,600
|
|
| Leasehold Improvements, Gross |
5,300
|
|
| Impairment for in-process research and development |
5,200
|
|
| Restructuring including legal fees |
800
|
|
| Accrued restructuring charges. |
$ 2,100
|
|
| X |
- DefinitionAccrued restructuring charges.
| Name: |
sybx_AccruedRestructuringCharges |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCompensation included in restructuring charges
| Name: |
sybx_CompensationIncludedInRestructuringCharges |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionExisting lease obligations
| Name: |
sybx_ExistingLeaseObligations |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionRestructuring including legal fees
| Name: |
sybx_RestructuringIncludingLegalFees |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accumulated depreciation of additions or improvements to assets held under a lease arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_LeaseholdImprovementsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of loss from impairment of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 25
-Paragraph 6
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479365/842-20-25-6
| Name: |
us-gaap_OperatingLeaseImpairmentLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses associated with exit or disposal activities pursuant to an authorized plan. Excludes expenses related to a discontinued operation or an asset retirement obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482047/420-10-45-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 5.P.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-1
| Name: |
us-gaap_RestructuringCharges |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of restructuring charges, remediation cost, and asset impairment loss. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_RestructuringSettlementAndImpairmentProvisions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses for special or contractual termination benefits provided to current employees involuntarily terminated under a benefit arrangement associated exit or disposal activities pursuant to an authorized plan. Excludes expenses related to one-time termination benefits, a discontinued operation or an asset retirement obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_SeveranceCosts1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.1.1.u2
Stockholders Equity - Additional Information (Detail) - USD ($)
$ / shares in Units, $ in Thousands |
|
|
1 Months Ended |
3 Months Ended |
|
|
Oct. 03, 2023 |
Sep. 27, 2023 |
Jul. 31, 2021 |
Jun. 30, 2019 |
Mar. 31, 2024 |
Mar. 31, 2023 |
Dec. 31, 2023 |
Sep. 30, 2023 |
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Common stock, par value |
|
$ 0.001
|
|
|
$ 0.001
|
|
$ 0.001
|
|
| Reverse Stock Split Approval Date |
|
Sep. 21, 2023
|
|
|
|
|
|
|
| Reverse Stock Split ,Decsription |
|
the Company effected a reverse stock split of its shares of common stock, pursuant to which every fifteen (15) shares of the its issued and outstanding common stock was automatically converted into one (1) issued and outstanding share of common stock without any change in the par value of $0.001 per share.
|
|
|
On September 27, 2023, the Company effected a one-for-fifteen reverse stock split of its issued and outstanding common stock, which also adjusted all outstanding warrants. Accordingly, all share and per share amounts for all periods presented in the accompanying consolidated financial statements and notes thereto have been adjusted retroactively, where applicable, to reflect this stock split. All fractional shares resulting from the reverse stock split were paid in cash.
|
|
|
|
| Commissions and Offering Expenses Payable |
$ 19,600
|
|
|
|
|
|
|
|
| Fair Value Adjustment of Warrants |
|
|
|
|
$ 6,730
|
$ 0
|
|
|
| Common Stock [Member] |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Sale of common stock, shares |
|
|
|
422,718
|
|
68,893
|
|
|
| Common Stock [Member] | Underwritten Public Offering |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Sale of common stock, shares |
3,921,928
|
|
|
|
|
|
|
|
| Public offering price |
$ 2.84
|
|
|
|
|
|
|
|
| Common Stock And Warrants [Member] | Underwritten Public Offering |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Warrants To Purchase Shares Of Common Stock |
3,472,435
|
|
|
|
|
|
|
|
| Sale of stock, price per share |
$ 2.839
|
|
|
|
|
|
|
|
| Pre-Funded Warrants |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Proceeds from exercise of warrants |
|
|
|
|
$ 2
|
$ 0
|
|
|
| Warrants to purchase shares of common stock |
|
|
|
169,874
|
|
|
|
|
| Warrants exercise price per share |
|
|
|
$ 135
|
|
|
|
|
| Warrants exercise price per share paid at closing of offering |
|
|
|
134.85
|
|
|
|
|
| Pre-Funded Warrants | Common Stock [Member] |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Sale of common stock, shares |
|
|
|
|
7,839
|
|
|
|
| October 2023 Financing [Member] |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| The purchase warrants were recorded as a liability |
$ 7,100
|
|
|
|
|
|
|
|
| Proceeds from exercise of warrants |
|
|
|
|
$ 4,400
|
|
|
|
| Fair Value Adjustment of Warrants |
|
|
|
|
$ 6,700
|
|
|
|
| October 2023 Financing [Member] | Maximum Member |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Percentage Of Common Stock Ownership To Exercise Warrant |
9.99%
|
|
|
|
|
|
|
|
| October 2023 Financing [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Percentage Of Common Stock Ownership To Exercise Warrant |
4.99%
|
|
|
|
|
|
|
|
| October 2023 Financing [Member] | Warrant [Member] |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Warrants exercised |
|
|
|
|
2,920,126
|
|
|
|
| October 2023 Financing [Member] | Common Stock And Warrants [Member] | Underwritten Public Offering |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Warrants To Purchase Shares Of Common Stock |
7,394,363
|
|
|
|
|
|
|
|
| Sale of stock, price per share |
$ 3.408
|
|
|
|
|
|
|
|
| Ginkgo Bioworks, Inc. |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Sale of stock, price per share |
|
|
|
$ 135
|
|
|
|
|
| Proceeds from issuance of common stock and pre-funded warrants |
|
|
|
$ 79,900
|
|
|
|
|
| Ginkgo Bioworks, Inc. | Common Stock [Member] |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Sale of common stock, shares |
|
|
|
422,718
|
|
|
|
|
| Ginkgo Bioworks, Inc. | Pre-Funded Warrants |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Warrants exercise price per share |
|
|
|
$ 135
|
|
|
|
|
| Warrants exercise price per share paid at closing of offering |
|
|
|
$ 134.85
|
|
|
|
|
| Warrants exercised |
|
|
|
|
|
|
|
0
|
| Ginkgo Bioworks, Inc. | Pre-Funded Warrants | Maximum Member |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Warrants to purchase shares of common stock |
|
|
|
169,874
|
|
|
|
|
| Jefferies, LLC | ATM |
|
|
|
|
|
|
|
|
| Class Of Stock [Line Items] |
|
|
|
|
|
|
|
|
| Sale of common stock, shares |
|
|
|
|
7,839
|
|
|
|
| Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs |
|
|
$ 50,000
|
|
$ 10
|
|
|
|
| X |
- DefinitionClass of warrant or right exercise price of warrants paid at closing of offering.
| Name: |
sybx_ClassOfWarrantOrRightExercisePriceOfWarrantsPaidAtClosingOfOffering |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionCommissions and Offering Expenses Payable
| Name: |
sybx_CommissionsAndOfferingExpensesPayable |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage Of Common Stock Ownership To Exercise Warrant
| Name: |
sybx_PercentageOfCommonStockOwnershipToExerciseWarrant |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionProceeds from issuance of common stock and pre funded warrants.
| Name: |
sybx_ProceedsFromIssuanceOfCommonStockAndPreFundedWarrants |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionReverse Stock Split Approval Date
| Name: |
sybx_ReverseStockSplitApprovalDate |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWarrants To Purchase Shares Of Common Stock
| Name: |
sybx_WarrantsToPurchaseSharesOfCommonStock |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in additional paid in capital (APIC) resulting from the issuance of warrants. Includes allocation of proceeds of debt securities issued with detachable stock purchase warrants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 20
-Section 25
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481284/470-20-25-2
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalWarrantIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which the class of warrant or right may be converted. For example, but not limited to, 500,000 warrants may be converted into 1,000,000 shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByWarrantsOrRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDescription of the reverse stock split arrangement. Also provide the retroactive effect given by the reverse split that occurs after the balance sheet date but before the release of financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 4
-Subparagraph (SAB Topic 4.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-4
| Name: |
us-gaap_StockholdersEquityReverseStockSplit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=sybx_UnderwrittenPublicOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=sybx_CommonStockAndWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=sybx_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=sybx_October2023FinancingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=sybx_AtTheMarketMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=sybx_PurchaseWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=sybx_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_OptionIndexedToIssuersEquityEquityAxis=sybx_OptionsExercisableToPurchaseCommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_EmployeeStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Equity based Compensation - Additional Information (Detail) - USD ($)
$ / shares in Units, $ in Millions |
|
3 Months Ended |
Jan. 01, 2024 |
Mar. 31, 2024 |
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Number of stock options granted |
|
44,701
|
| Weighted average exercise price |
|
$ 4.2
|
| Employee unrecognized compensation expense |
|
$ 0.7
|
| Employee unrecognized compensation cost, period of recognition |
|
1 year 11 months 4 days
|
| Restricted Common Stock |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Employee unrecognized compensation expense |
|
$ 0.5
|
| Employee unrecognized compensation cost, period of recognition |
|
1 year 9 months 18 days
|
| Number of restricted stock awards granted |
|
362,700
|
| Weighted average grant date fair value |
|
$ 4.44
|
| ESPP |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Increase in number of shares available for issuance (in shares) |
91,878
|
|
| Shares available for future grants |
|
187,012
|
| Plan 2015 |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Increase in number of shares available for issuance (in shares) |
459,392
|
|
| 2017 Plan and 2015 Plan |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
| Shares available for future grants |
|
604,072
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cost to be recognized for option under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value at grant date for nonvested equity-based awards issued during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of additional shares authorized for issuance under share-based payment arrangement.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfAdditionalSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_EmployeeStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=sybx_LongTermIncentivePlan2015Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=sybx_TwoThousandSeventeenAndTwoThousandFifteenEquityIncentiveAwardPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=sybx_RestructuringChargesExpenseAccelerationMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_EmployeeStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of amounts paid in advance for expenses which will be charged against earnings in periods after one year or beyond the operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of revenue recognized from goods sold, services rendered, insurance premiums, or other activities that constitute an earning process. Includes, but is not limited to, investment and interest income before deduction of interest expense when recognized as a component of revenue, and sales and trading gain (loss). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-42
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-40
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-41
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
| Name: |
us-gaap_Revenues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=sybx_RocheCollaborationAndOptionAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
Net Loss per Share - Additional Information (Detail) - $ / shares
|
1 Months Ended |
3 Months Ended |
Jun. 30, 2019 |
Mar. 31, 2024 |
Mar. 31, 2023 |
| Common Stock $0.001 Par Value |
|
|
|
| Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs, Shares |
422,718
|
|
68,893
|
| Pre-Funded Warrants |
|
|
|
| Warrants to purchase shares of common stock |
169,874
|
|
|
| Warrants exercise price per share |
$ 135
|
|
|
| Warrants exercise price per share paid at closing of offering |
$ 134.85
|
|
|
| Pre-Funded Warrants | Common Stock $0.001 Par Value |
|
|
|
| Proceeds from issuance of common stock in connection with at-the-market offering, net of issuance costs, Shares |
|
7,839
|
|
| X |
- DefinitionClass of warrant or right exercise price of warrants paid at closing of offering.
| Name: |
sybx_ClassOfWarrantOrRightExercisePriceOfWarrantsPaidAtClosingOfOffering |
| Namespace Prefix: |
sybx_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which the class of warrant or right may be converted. For example, but not limited to, 500,000 warrants may be converted into 1,000,000 shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByWarrantsOrRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=sybx_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=sybx_UnvestedRestrictedStockAwardsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=sybx_PurchaseWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_StockCompensationPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.1.1.u2
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483049/450-30-50-1
| Name: |
us-gaap_GainContingenciesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.1.1.u2
Related-Party Transactions - Additional Information (Detail) - USD ($)
$ in Millions |
1 Months Ended |
|
|
Jun. 30, 2019 |
Mar. 31, 2024 |
Dec. 31, 2023 |
| Related Party Transaction [Line Items] |
|
|
|
| Common stock, outstanding |
|
11,627,212
|
9,186,157
|
| Ginkgo Bioworks, Inc. |
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
| Common stock, outstanding |
|
422,718
|
|
| Prepayment to related party for collaboration agreement |
$ 30.0
|
|
|
| Related party transaction collaboration agreement, initial term |
5 years
|
|
|
| Current Pre-Paid Research and Development |
|
$ 5.2
|
|
| X |
- DefinitionCurrent Pre-Paid Research and Development.
| Name: |
sybx_CurrentPrePaidResearchAndDevelopment |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.1.1.u2
| X |
- DefinitionCommissions and Offering Expenses Payable
| Name: |
sybx_CommissionsAndOfferingExpensesPayable |
| Namespace Prefix: |
sybx_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDetail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Synlogic (NASDAQ:SYBX)
Historical Stock Chart
From Apr 2024 to May 2024
Synlogic (NASDAQ:SYBX)
Historical Stock Chart
From May 2023 to May 2024