0001580149
false
FY
0001580149
2022-07-01
2023-06-30
0001580149
2022-12-31
0001580149
2023-08-09
0001580149
2023-06-30
0001580149
2022-06-30
0001580149
2021-07-01
2022-06-30
0001580149
us-gaap:CommonStockMember
2021-06-30
0001580149
us-gaap:AdditionalPaidInCapitalMember
2021-06-30
0001580149
bivi:TreasuryStocksMember
2021-06-30
0001580149
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-06-30
0001580149
us-gaap:RetainedEarningsMember
2021-06-30
0001580149
2021-06-30
0001580149
us-gaap:CommonStockMember
2022-06-30
0001580149
us-gaap:AdditionalPaidInCapitalMember
2022-06-30
0001580149
bivi:TreasuryStocksMember
2022-06-30
0001580149
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-06-30
0001580149
us-gaap:RetainedEarningsMember
2022-06-30
0001580149
us-gaap:CommonStockMember
2021-07-01
2022-06-30
0001580149
us-gaap:AdditionalPaidInCapitalMember
2021-07-01
2022-06-30
0001580149
bivi:TreasuryStocksMember
2021-07-01
2022-06-30
0001580149
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2021-07-01
2022-06-30
0001580149
us-gaap:RetainedEarningsMember
2021-07-01
2022-06-30
0001580149
us-gaap:CommonStockMember
2022-07-01
2023-06-30
0001580149
us-gaap:AdditionalPaidInCapitalMember
2022-07-01
2023-06-30
0001580149
bivi:TreasuryStocksMember
2022-07-01
2023-06-30
0001580149
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2022-07-01
2023-06-30
0001580149
us-gaap:RetainedEarningsMember
2022-07-01
2023-06-30
0001580149
us-gaap:CommonStockMember
2023-06-30
0001580149
us-gaap:AdditionalPaidInCapitalMember
2023-06-30
0001580149
bivi:TreasuryStocksMember
2023-06-30
0001580149
us-gaap:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001580149
us-gaap:RetainedEarningsMember
2023-06-30
0001580149
us-gaap:StockOptionMember
2022-07-01
2023-06-30
0001580149
us-gaap:StockOptionMember
2021-07-01
2022-06-30
0001580149
us-gaap:WarrantMember
2022-07-01
2023-06-30
0001580149
us-gaap:WarrantMember
2021-07-01
2022-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2022-07-01
2023-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2021-07-01
2022-06-30
0001580149
us-gaap:USTreasuryBillSecuritiesMember
2023-06-30
0001580149
us-gaap:USTreasuryBillSecuritiesMember
2022-07-01
2023-06-30
0001580149
bivi:AcuitasGroupHoldingsLLCMember
2022-08-15
0001580149
2021-08-31
0001580149
us-gaap:PrimeRateMember
2022-07-01
2023-06-30
0001580149
bivi:AvenueWarrantsMember
2022-07-01
2023-06-30
0001580149
bivi:UnearnedDiscountMember
2023-06-30
0001580149
us-gaap:FinanceReceivablesMember
2023-06-30
0001580149
bivi:LoanAccretionPremiumMember
2023-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel1Member
2023-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel2Member
2023-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel3Member
2023-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
2023-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel1Member
2023-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel2Member
2023-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel3Member
2023-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
2023-06-30
0001580149
us-gaap:FairValueInputsLevel1Member
2023-06-30
0001580149
us-gaap:FairValueInputsLevel2Member
2023-06-30
0001580149
us-gaap:FairValueInputsLevel3Member
2023-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel1Member
2022-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel2Member
2022-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel3Member
2022-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
2022-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel1Member
2022-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel2Member
2022-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel3Member
2022-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
2022-06-30
0001580149
us-gaap:FairValueInputsLevel1Member
2022-06-30
0001580149
us-gaap:FairValueInputsLevel2Member
2022-06-30
0001580149
us-gaap:FairValueInputsLevel3Member
2022-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel3Member
2021-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel3Member
2021-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel3Member
2021-07-01
2022-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel3Member
2021-07-01
2022-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel3Member
2022-07-01
2023-06-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel3Member
2022-07-01
2023-06-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel3Member
2021-11-30
0001580149
bivi:DerivativeLiabilityWarrantsMember
us-gaap:FairValueInputsLevel3Member
2021-11-01
2021-11-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel3Member
2021-11-30
0001580149
bivi:DerivativeLiabilityConversionOptionOnConvertibleDebentureMember
us-gaap:FairValueInputsLevel3Member
2021-11-01
2021-11-30
0001580149
us-gaap:CashMember
us-gaap:FairValueInputsLevel1Member
2023-06-30
0001580149
us-gaap:CashMember
us-gaap:FairValueInputsLevel2Member
2023-06-30
0001580149
us-gaap:CashMember
us-gaap:FairValueInputsLevel3Member
2023-06-30
0001580149
us-gaap:CashMember
2023-06-30
0001580149
us-gaap:USTreasuryBillSecuritiesMember
us-gaap:FairValueInputsLevel1Member
2023-06-30
0001580149
us-gaap:USTreasuryBillSecuritiesMember
us-gaap:FairValueInputsLevel2Member
2023-06-30
0001580149
us-gaap:USTreasuryBillSecuritiesMember
us-gaap:FairValueInputsLevel3Member
2023-06-30
0001580149
bivi:USTreasuryBillSecurities1Member
us-gaap:FairValueInputsLevel1Member
2023-06-30
0001580149
bivi:USTreasuryBillSecurities1Member
us-gaap:FairValueInputsLevel2Member
2023-06-30
0001580149
bivi:USTreasuryBillSecurities1Member
us-gaap:FairValueInputsLevel3Member
2023-06-30
0001580149
bivi:USTreasuryBillSecurities1Member
2023-06-30
0001580149
us-gaap:CashMember
us-gaap:FairValueInputsLevel1Member
2022-06-30
0001580149
us-gaap:CashMember
us-gaap:FairValueInputsLevel2Member
2022-06-30
0001580149
us-gaap:CashMember
us-gaap:FairValueInputsLevel3Member
2022-06-30
0001580149
us-gaap:CashMember
2022-06-30
0001580149
us-gaap:USTreasuryBillSecuritiesMember
us-gaap:FairValueInputsLevel1Member
2022-06-30
0001580149
us-gaap:USTreasuryBillSecuritiesMember
us-gaap:FairValueInputsLevel2Member
2022-06-30
0001580149
us-gaap:USTreasuryBillSecuritiesMember
us-gaap:FairValueInputsLevel3Member
2022-06-30
0001580149
us-gaap:USTreasuryBillSecuritiesMember
2022-06-30
0001580149
bivi:USTreasuryBillSecurities1Member
us-gaap:FairValueInputsLevel1Member
2022-06-30
0001580149
bivi:USTreasuryBillSecurities1Member
us-gaap:FairValueInputsLevel2Member
2022-06-30
0001580149
bivi:USTreasuryBillSecurities1Member
us-gaap:FairValueInputsLevel3Member
2022-06-30
0001580149
bivi:USTreasuryBillSecurities1Member
2022-06-30
0001580149
bivi:StockOptionsMember
2021-06-30
0001580149
bivi:StockOptionsMember
2021-07-01
2022-06-30
0001580149
bivi:StockOptionsMember
2022-06-30
0001580149
bivi:StockOptionsMember
2022-07-01
2023-06-30
0001580149
bivi:StockOptionsMember
2023-06-30
0001580149
bivi:StockOptionAndWarrantsMember
2022-07-01
2023-06-30
0001580149
bivi:StockOptionAndWarrantsMember
2023-06-30
0001580149
bivi:StockOptionAndWarrantsMember
2022-11-01
2022-11-30
0001580149
bivi:StockOptionAndWarrantsMember
2022-11-30
0001580149
bivi:StockOptionAndWarrantsMember
2022-10-01
2022-10-31
0001580149
bivi:StockOptionAndWarrantsMember
2022-10-31
0001580149
bivi:StockOptionAndWarrantsMember
2023-05-01
2023-05-31
0001580149
bivi:StockOptionAndWarrantsMember
2023-05-31
0001580149
2021-07-01
2021-09-30
0001580149
bivi:SalesAgreementMember
2022-07-01
2023-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
bivi:N2019OmnibusIncentiveEquityPlanMember
2021-08-01
2021-08-20
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2021-08-01
2021-08-20
0001580149
us-gaap:RestrictedStockUnitsRSUMember
srt:ChiefExecutiveOfficerMember
2022-07-01
2023-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
bivi:N2019OmnibusIncentiveEquityPlanMember
bivi:PresidentAndCEOMember
2022-06-01
2022-06-21
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2022-07-01
2023-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2021-07-01
2022-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
bivi:N2019OmnibusIncentiveEquityPlanMember
bivi:PresidentAndCEOMember
2022-11-01
2022-11-23
0001580149
us-gaap:StockOptionMember
bivi:NewEmployeeMember
2022-07-01
2023-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2023-02-01
2023-02-16
0001580149
us-gaap:RestrictedStockUnitsRSUMember
bivi:N2019OmnibusIncentiveEquityPlanMember
bivi:FourDirectorsMember
2022-07-01
2023-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
bivi:FourDirectorsMember
2022-07-01
2023-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
bivi:N2019OmnibusIncentiveEquityPlanMember
bivi:ThreeDirectorsMember
2022-11-01
2022-11-23
0001580149
us-gaap:RestrictedStockUnitsRSUMember
bivi:ThreeDirectorsMember
2022-07-01
2023-06-30
0001580149
2022-11-23
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2023-02-01
2023-02-09
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2023-05-01
2023-05-09
0001580149
us-gaap:RestrictedStockUnitsRSUMember
bivi:N2019OmnibusIncentiveEquityPlanMember
bivi:PresidentAndCEOMember
2023-06-01
2023-06-20
0001580149
bivi:VendorMember
2023-04-01
2023-04-06
0001580149
bivi:VendorMember
2023-04-06
0001580149
us-gaap:StockOptionMember
bivi:ExecutiveMember
2021-08-01
2021-08-20
0001580149
us-gaap:StockOptionMember
bivi:NewEmployeeMember
2022-04-01
2022-04-05
0001580149
us-gaap:StockOptionMember
bivi:NewEmployeeMember
srt:MinimumMember
2022-04-01
2022-04-05
0001580149
us-gaap:StockOptionMember
bivi:NewEmployeeMember
srt:MaximumMember
2022-04-01
2022-04-05
0001580149
us-gaap:StockOptionMember
srt:ChiefExecutiveOfficerMember
2022-06-01
2022-06-21
0001580149
us-gaap:StockOptionMember
bivi:NewEmployeeMember
2021-07-01
2022-06-30
0001580149
us-gaap:StockOptionMember
bivi:NewEmployeeMember
srt:MinimumMember
2023-06-01
2023-06-07
0001580149
us-gaap:StockOptionMember
bivi:NewEmployeeMember
2023-06-01
2023-06-07
0001580149
us-gaap:StockOptionMember
srt:ChiefExecutiveOfficerMember
2021-08-01
2021-08-27
0001580149
bivi:StockOption1Member
2023-06-30
0001580149
bivi:StockOption1Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption2Member
2023-06-30
0001580149
bivi:StockOption2Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption3Member
2023-06-30
0001580149
bivi:StockOption3Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption4Member
2023-06-30
0001580149
bivi:StockOption4Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption5Member
2023-06-30
0001580149
bivi:StockOption5Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption6Member
2023-06-30
0001580149
bivi:StockOption6Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption7Member
2023-06-30
0001580149
bivi:StockOption7Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption8Member
2023-06-30
0001580149
bivi:StockOption8Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption9Member
2023-06-30
0001580149
bivi:StockOption9Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption10Member
2023-06-30
0001580149
bivi:StockOption10Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption11Member
2023-06-30
0001580149
bivi:StockOption11Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption12Member
2023-06-30
0001580149
bivi:StockOption12Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption13Member
2023-06-30
0001580149
bivi:StockOption13Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption14Member
2023-06-30
0001580149
bivi:StockOption14Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption15Member
2023-06-30
0001580149
bivi:StockOption15Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption16Member
2023-06-30
0001580149
bivi:StockOption16Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption17Member
2023-06-30
0001580149
bivi:StockOption17Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption18Member
2023-06-30
0001580149
bivi:StockOption18Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption19Member
2023-06-30
0001580149
bivi:StockOption19Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption20Member
2023-06-30
0001580149
bivi:StockOption20Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption21Member
2023-06-30
0001580149
bivi:StockOption21Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption22Member
2023-06-30
0001580149
bivi:StockOption22Member
2022-07-01
2023-06-30
0001580149
bivi:StockOption23Member
2023-06-30
0001580149
bivi:StockOption23Member
2022-07-01
2023-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2021-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2022-06-30
0001580149
us-gaap:RestrictedStockUnitsRSUMember
2023-06-30
0001580149
us-gaap:WarrantMember
2021-06-30
0001580149
us-gaap:WarrantMember
2021-07-01
2022-06-30
0001580149
us-gaap:WarrantMember
2022-06-30
0001580149
us-gaap:WarrantMember
2022-07-01
2023-06-30
0001580149
us-gaap:WarrantMember
2023-06-30
0001580149
us-gaap:SubsequentEventMember
bivi:SalesAgreementMember
bivi:AgentMember
2023-07-01
2023-08-15
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
10-K
(Mark
One)
☒
ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934.
FOR
THE FISCAL YEAR ENDED JUNE 30, 2023
☐
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For
the transition period from ____________to _____________
Commission
File Number: 001-39015
BIOVIE INC.
(Exact
name of registrant as specified in its charter)
| Nevada |
|
46-2510769 |
| (State
or other jurisdiction of |
|
(I.R.S.
Employer Identification Number) |
| incorporation
or organization) |
|
|
| |
680 W Nye Lane Suite 204 |
|
| |
Carson City, NV 89703 |
|
| |
(Address
of principal executive offices, Zip Code) |
|
| |
|
|
| |
(775)-888-3162 |
|
| |
(Registrant’s
telephone number, including area code) |
|
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
Trading
Symbol(s) |
Name
of each exchange on which registered |
| Class
A Common Stock, $.0001 par value per share |
BIVI |
The NASDAQ Stock Market, LLC |
Securities
registered pursuant to Section 12(g) of the Act:
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes
☐
No ☒
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act
Yes
☐
No ☒
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during
the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such
filing requirements for the past 90 days.
Yes
☒
No ☐
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files).
Yes
☒
No ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
Accelerated Filer |
☐ |
Accelerated
Filer |
☐ |
| Non-Accelerated Filer |
☒ |
Smaller
reporting company |
☒ |
| Emerging
growth company |
☐ |
|
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark if the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its
internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7362(b)) by the registered public
accounting firm that prepared or issued its audit report.
Yes
☐
No ☒
If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are
restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes
☐
No ☒
The
aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which
the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s
most recently completed second fiscal quarter was $86,033,369.
There
were 36,803,768
shares of the Registrant’s Class A Common Stock, $0.0001 par value per share, outstanding as of August 9, 2023.
DOCUMENTS
INCORPORATED BY REFERENCE
None.
BIOVIE
INC.
FORM
10-K INDEX
BIOVIE
INC.
FORWARD-LOOKING
STATEMENTS
This
report contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, and Section 27A
of the Securities Act of 1933. Any statements contained in this report that are not statements of historical fact may be forward-looking
statements. When we use the words “intends,” “estimates,” “predicts,” “potential,” “continues,”
“anticipates,” “plans,” “expects,” “believes,” “should,” “could,”
“may,” “will” or the negative of these terms or other comparable terminology, we are identifying forward-looking
statements. Forward-looking statements involve risks and uncertainties, which may cause our actual results, performance or achievements
to be materially different from those expressed or implied by forward-looking statements. These factors include our research and development
activities, distributor channel; compliance with regulatory impositions; and our capital needs. Although we believe that the expectations
reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
Except
as may be required by applicable law, we do not undertake or intend to update or revise our forward-looking statements, and we assume
no obligation to update any forward-looking statements contained in this report as a result of new information or future events or developments.
Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking
statements. You should carefully review and consider the various disclosures we make in this report and our other reports filed with
the Securities and Exchange Commission that attempt to advise interested parties of the risks, uncertainties and other factors that may
affect our business.
All
statements other than statements of historical fact are statements that could be deemed forward-looking statements. The Company assumes
no obligation and does not intend to update these forward-looking statements, except as required by law. When used in this report, the
terms “BioVie”, “Company”, “we”, “our”, and “us” refer to BioVie, Inc.
PART
I
Overview
BioVie
Inc. (the “Company” or “we” or “our”) is a clinical-stage company developing innovative drug therapies
for the treatment of neurological and neurodegenerative disorders and advanced liver disease.
Neurodegenerative
Disease Program
In neurodegenerative disease, the Company’s
drug candidate NE3107 inhibits inflammatory activation of extracellular single-regulated kinase (“ERK”) and Nuclear factor kappa-light-chain-enhancer
of activated B cells (“NFkB”) (e.g., tumor necrosis factor (“TNF”) signaling) that leads to neuroinflammation
and insulin resistance, but not their homeostatic functions (e.g., insulin signaling and neuron growth and survival). Both inflammation
and insulin resistance are drivers of Alzheimer’s disease (“AD”) and Parkinson’s disease (“PD”).
The
Company is conducting a potentially pivotal Phase 3 randomized, double-blind, placebo-controlled, parallel-group, multicenter study to
evaluate NE3107 in patients who have mild to moderate Alzheimer’s disease (NCT04669028). The Company is targeting primary completion
of this study in the fourth quarter of calendar year 2023.
In
December 2022, topline results were released from the Company’s Phase 2 study assessing NE3107’s safety and tolerability
and potential pro-motoric impact in PD patients. The NM201 study (NCT05083260) was a double-blind, placebo-controlled, safety, tolerability,
and pharmacokinetics study in PD participants treated with carbidopa/levodopa and NE3107. Forty-five patients with a defined L-dopa “off
state” were randomized 1:1 to placebo:NE3107 20 mg twice daily for 28 days. The trial was launched with two design objectives:
1) the primary objective was safety and a drug-drug interaction study (as requested by the U.S. Food and Drug Administration (“FDA”)) to demonstrate the absence of adverse interactions of NE3107 with levodopa; and 2) the secondary objective was to determine
if preclinical indications of promotoric activity and apparent enhancement of levodopa activity observed in a Parkinson’s disease
model in monkeys can be seen in humans. Both objectives of the study were met. Patients treated with NE3107 experienced greater motor
control.
The
Company provided the financial support and the use of our NE3107 formulated drug product for an open-label phase 2, Investigator-Initiated
Trial in mild cognitive impairment (“MCI”) and Mild AD, NCT05227820, conducted by (“The Regenesis Project”)
of Dr. Sheldon Jordan. The study received FDA authorization on December 12, 2021 and was designed to measure NE3107’s effect on cognition,
cerebral spinal fluid (“CSF”) and blood biomarkers, and neuro-imagining endpoints. Topline results were released September
7, 2022, and additional data was presented at the Clinical Trial in Alzheimer’s Disease (“CTAD”) annual conference in
December 2022. The data showed that three months of treatment with NE3107 in patients with MCI and mild AD enhanced cognition compared
to baseline, as measured using multiple rating scales, had improvement in daily function and improvements in inflammation correlated with
improved cognition. No drug-related adverse events were observed.
The
Company acquired the biopharmaceutical assets of NeurMedix, Inc. (“NeurMedix”), from a related party privately held clinical-stage
pharmaceutical company, in June 2021. The acquired assets included NE3107, a potentially selective inhibitor of inflammatory ERK signaling that, based on animal studies and Dr. Jordan’s study, is believed to reduce
neuroinflammation. NE3107 is a novel orally administered small molecule that is thought to inhibit inflammation-driven insulin resistance
and major pathological inflammatory cascades with a novel mechanism of action. There is emerging scientific consensus that both inflammation
and insulin resistance may play fundamental roles in the development of AD and PD, and NE3107 could, if approved by the FDA represent
a new medical approach to treating these devastating conditions affecting an estimated 6 million Americans suffering from AD and 1 million
Americans suffering from PD.
Inflammation-driven
insulin resistance is believed to be implicated in a broad range of serious diseases, and we plan to begin exploring these opportunities
in the coming months using NE3107 or related compounds acquired in the NeurMedix asset purchase. NE3107 is patented in the United States
(“U.S.”), Australia, Canada, Europe and South Korea.
Liver
Disease Program
In liver disease, our Orphan Drug candidate BIV201
(continuous infusion terlipressin), with FDA Fast Track status, has been evaluated in a U.S. Phase 2b study (NCT04112199) for the treatment
of refractory ascites due to liver cirrhosis. BIV201 is administered as a patent-pending liquid formulation. The study was closed before
full enrollment, without clinically meaningful adverse effects associated with BIV201 treatment and data that appeared to show that treatment
with BIV201 plus standard-of-care (“SOC”) resulted in a reduction in ascites fluid accumulation during treatment versus pre-treatment.
In June 2023, we requested guidance from the FDA regarding the design and endpoints for definitive clinical testing of BIV201 for the
treatment of ascites due to chronic liver cirrhosis.
While the active agent, terlipressin, is approved
in the U.S. and in about 40 countries for related complications of advanced liver cirrhosis, treatment of ascites is not included in these
authorizations. Patients with refractory ascites suffer from frequent life-threatening complications, generate more than $5 billion in
annual treatment costs, and have an estimated 50% mortality rate within 6 to 12 months. The U.S. FDA has not approved any drug to treat
refractory ascites.
The
BIV201 development program was initiated by LAT Pharma LLC. On April 11, 2016, the Company acquired LAT Pharma LLC and the rights to
its BIV201 development program. The Company currently owns all development and marketing rights to this drug candidate. Pursuant to the
Agreement and Plan of Merger entered into on April 11, 2016, between our predecessor entities, LAT Pharma LLC and NanoAntibiotics, Inc.,
BioVie is obligated to pay a low single digit royalty on net sales of BIV201 (continuous infusion terlipressin) to be shared among LAT
Pharma Members, PharmaIn Corporation, and The Barrett Edge, Inc.
Neurodegenerative
Disease Program
The Company is conducting a potentially pivotal
Phase 3 randomized, double blind, placebo controlled, parallel group, multicenter study to evaluate NE3107 in patients who have mild to
moderate AD (NCT04669028). The study has co-primary endpoints looking at cognition using the Alzheimer’s Disease Assessment Scale-Cognitive
Scale (ADAS-Cog 12) and function using the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change (ADCS-CGIC).
The program is fully enrolled and is targeting primary completion in the fourth quarter of the calendar 2023 year.
The Company supported a Phase 2 exploratory biomarker
study (Investigator-Initiated Trial in MCI and Mild Alzheimer’s Disease, NCT05227820) showing that patients treated with NE3107
experienced improved cognition as measured by a modified ADAS-Cog12 score, reduced TNF- a (i.e.,
inflammation) in a manner that’s correlated to improvements in cognition, reduced CSF p-tau levels and the ratio of p-tau to A b42,
and imaging findings suggestive of improved neuronal health. Despite the open-label nature of the exploratory study, the emerging data
and correlations provide encouraging signs of what we may see in the upcoming Phase 3 data reveal. The Phase 2 Study enrolled a total
of 23 patients – 17 patients with Mini-Mental State Examination (“MMSE”) scores greater than or equal to 20 (i.e., MCI
to mild AD) and 6 patients with MMSE <20 (i.e., moderate AD) – with an average age of 71.1 years. This open-label, single arm
study was designed to measure changes in cognition through verbal and visual test procedures and changes in biomarkers of AD and inflammation
that can be measured in cerebral spinal fluid (“CSF”), blood samples, and functional magnetic resonance imaging in patients
before and after treatment with 20 mg of NE3107 twice daily for 3 months. This data showed the following among patients with MMSE<20
(i.e., mild cognitive impairment and mild AD):
| ● | NE3107
showed the potential to enhance cognition as measured by multiple assessment tools, including
a 2.1 point improvement (p=0.0173) on the modified ADAS-Cog12 scale equating to a 21.1% (p=0.0079)
change compared to baseline, a 0.11 point improvement (p=0.0416) on the Clinical Dementia
Rating scale (CDR), equating to 19.4% (p=0.0416) change from baseline, and a 0.07 point improvement
in the ADCOMS scale, equating to 27.4% improvement (p=0.009). |
| ● | NE3107
reduced CSF phospho-tau levels by -1.66 pg/mL (p=0.0343) and the ratio of p-tau to Ab42
by -0.0024 (p=0.0401) |
| ● | 18
of 22 patients with abnormal baseline scans showed improvement in one or more brain regions
as seen from advanced functional MRI studies. |
| ● | No
drug-related adverse events were observed. |
Other potential NE3107 effects on biomarkers of
aging-related disease states were indicated. Blood samples were taken from the patients who participated in the investigator-initiated
Alzheimer’s Phase 2 trial before and after three months of treatment with NE3107, and these samples were analyzed to assess NE3107’s
potential to alter DNA methylation associated with epigenetic biological clocks. The resulting data for patients
treated with NE3107 for three months showed an average reduction of 3.3 years (p=0.0021) on the Horvath DNA methylation SkinBlood clock.
Furthermore, 19 out of the 22 patients experienced a reduction in the SkinBlood clock score.
In July 2023, the Company presented a poster detailing
the epigenetic basis for how its drug candidate NE3107 may have the potential to regulate methylation of specific genes in a manner that
significantly correlated with observed cognitive and biomarker improvements at the Alzheimer’s Associate’s International Conference
(AAIC) held in Amsterdam from July 16 through July 20, 2023.
The poster presentation titled Treatment-Induced
Epigenetic Modifications in MCI and Probable Alzheimer’s (Reading C, et al.), showed how patients with clinical dementia treated
with NE3107 for three months saw significant reductions in the level of DNA methylation, and that such reductions were, in some cases,
significantly correlated with observed improvements in various cognitive measures (e.g., ADAS-Cog11, CDR, ADCOMS, QDRS) and biomarkers
(including TNFα, CSF p-Tau/Aβ42, precuneus glutathione).
Inflammation has been shown to be associated with
the hypermethylation of our DNA,[1] which in turn has been shown to impact a wide range of diseases, including various forms
of cancers,[2] age-related cognitive impairment and dementia,[3] Parkinson’s disease,[4] cardiovascular
disease,[3,5] COPD and respiratory disease,[6] chronic kidney disease,[7] inflammatory bowel disease,[8]
sepsis,[9] and many others. The new data to be presented details how NE3107 may potentially change or affect the degree of
methylation of specific genes that are correlated with various markers of disease.
About
Inflammation and NE3107’s Mechanism of Action
Neuroinflammation,
insulin resistance, and oxidative stress are common features in the major neurodegenerative diseases, including Alzheimer’s Disease
(AD), Parkinson’s Disease (PD), frontotemporal lobar dementia, and Amyotrophic lateral sclerosis (“ALS”). NE3107 is
an orally bioavailable, blood-brain permeable, small molecule, with potential anti-inflammatory, insulin sensitizing, and ERK-binding
properties that may allow it to selectively inhibit ERK-, NFκB- and TNF-stimulated inflammation. NE3107’s potential to inhibit
neuroinflammation and insulin resistance forms the basis for the Company’s work testing the molecule in AD and PD patients.
Parallels exist between AD and
PD, among them activated microglia driving inflammation, involvement of TNFα, oxidative stress, protein misfolding, mitochondrial
dysfunction, and insulin resistance. In preclinical and clinical studies, NE3107 reduced inflammation and enhanced insulin sensitivity,
both of which are important to PD pathology. Preclinical studies in marmoset monkeys have shown NE3107 administered alone to be as pro-motoric
as levodopa, underscoring the apparently critical role of inflammation in expression of PD motor symptoms. When NE3107 was administered
with levodopa, the combination improved motor control better than either drug alone. Furthermore, in the marmoset study, NE3107 reduced
the severity of levodopa induced dyskinesia (“LID”) concurrent with pro-motoric benefit and decreased neurodegeneration, preserving twice
as many dopaminergic neurons compared to control.
Alzheimer’s
Disease
Alzheimer’s
disease (AD), which affects an estimated 6 million Americans, is a neuroinflammatory and neurodegenerative condition characterized by
progressive deterioration of cognitive function and loss of short-term memory and executive function. Cognitive tests quantifying AD
severity have been exhaustively developed. Formal diagnosis of AD has historically been dependent on the presence of extraneuronal amyloid
beta (Aβ) plaques, which can only be observed at autopsy or with the aid of sophisticated radioimaging techniques. However, diagnostic
methods have recently been approved that quantify Aβ in peripheral blood and correlate well with imaging results. Aβ plaques
can also be found in people without apparent AD symptoms, which has cast doubt about the role of Aβ as the central mediator of disease
pathology.
Scientific
investigations in the past twenty years have provided strong evidence that inflammation, type 2 diabetes (T2D), and inflammation-driven
insulin resistance are drivers of AD. The link between these factors and cognitive impairment are described by relatively new terms,
type 3 diabetes and metabolic-cognitive syndrome.
| 1 | | Stenvinkel
P doi: 10.1111/j.1365-2796.2007.01777.x |
| 2 | | Wang
Z Nucleic Acids Research, 2020, Vol. 48, No. 5 |
| 3 | Sugden K Neurology 2022;99:e1402-e1413 |
| 4 | Tang X DOI: 10.1002/mds.29157 |
| 5 | Tabaeia S Artificial Cells, Nanomedicine,
and Biotechnology, 47:1, 2031-2041 |
| 6 | Qiu W Am J Respir Crit Care Med
Vol 185, Iss. 4, pp 373–381, Feb 15, 2012 |
| 7 | Rysz C Int. J. Mol. Sci. 2022,
23(13), 7108 |
| 8 | Kraiczy J Mucosal Immunology volume
9, pages 647–658 (2016) |
| 9 | Rump K Sci Rep
9, 18511 (2019) |
A large body of evidence supports inflammation
as a primary driver of pathology in AD. The major inflammation signaling node, NFkB, and the cytokine tumor necrosis factor (TNF) are
important initiators of inflammatory signaling in AD pathology. NE3107 is believed to inhibit extracellular signal regulated kinase (ERK)/NFkB
activation and TNF production stimulated by inflammatory mediators, such as lipopolysaccharide. Inhibition of NFkB activation and TNF
production from this type of stimulation has broad potential implications for reduction of pathological peripheral and central nervous
system (CNS) inflammatory signaling in AD, which include reduction of inflammation-driven insulin resistance, decreased inflammatory cell
infiltration into the CNS, and decreased microglia activation. Reduction of systemic inflammation and inflammation-driven insulin resistance
are also predicted to have beneficial effects on hypothalamus-pituitary-adrenal (HPA) axis dysregulation and hippocampal dysregulation
of cortisol secretion that are consequences of adipose inflammation and insulin resistance, and are known to promote cognitive impairment,
and are also forward-feeding for insulin resistance.
Inflammation, insulin resistance, and associated
metabolic dysregulation in the brain contribute to Aβ oligomerization and aggregation, phospho-tau formation, reduced neuron survival
stimulus, and a forward-feeding cycle of neuronal energy deficit and oxidative stress, causing neuronal dysfunction (cognitive impairment)
and neurodegeneration. We believe NE3107’s combination of anti-inflammatory and insulin sensitizing activity has the potential to
disrupt this forward-feeding cycle of AD pathology.
Insulin has a major role in metabolic regulation
and neuron survival, while insulin resistance and T2D are closely linked to AD pathology. Insulin signaling is involved in synaptic plasticity,
learning, and memory. Exogenous insulin enhances cognition in normal and cognitively impaired subjects. Insulin resistance is linked to
cognitive impairment.
The multifactorial influence of insulin signaling
on neuron survival and cognition suggests that correction of insulin signaling deficits with NE3107 in the target population may provide
significant benefits on both cognition and disease progression. Additional rationale for targeting metabolic dysregulation with NE3107
has come from recent work showing that peripheral insulin resistance promotes insulin resistance and senescence in the CNS.
There is also an extensive literature on the complex
role of adipose tissue inflammation in systemic inflammation, insulin resistance, hypothalamus-pituitary-adrenal axis (HPA) dysregulation
and chronic cortisol excess in cognitive impairment in AD. Obesity and inflammation are closely linked in expanding adipose tissue, where
the production of inflammatory cytokines and increased cortisol are driven though up-regulation of 11β-hydroxysteroid dehydrogenase
type 1 and adipocyte mineralocorticoid receptor activation. Inflamed adipose tissue interacts with the HPA axis and hippocampus to increase
systemic cortisol, and promote hippocampal inflammation through chronically elevated cortisol, which freely penetrates the blood-brain
barrier. Hyperglycemia (secondary to insulin resistance) exacerbates adrenal cortisol production and promotes forward feeding of inflammation
and HPA-hippocampal dysregulation.
Systemic inflammation from inflamed adipose and
associated mononuclear cells, promotes CNS inflammation with associated cognitive decline and neurodegeneration. We believe NE3107’s
anti-inflammatory activity against systemic/adipose inflammation and factors that dysregulate cortisol secretion, such as hyperglycemia,
has the potential to decrease cognitive impairment and neurodegenerative mechanisms that have been linked to cortisol excess.
Parkinson’s
Disease
The Company completed its Phase 2 study assessing
NE3107 in Parkinson’s disease patients in the fourth quarter of calendar year 2022. The NM201 study (NCT05083260) was a double-blind,
placebo-controlled, safety, tolerability, and pharmacokinetics study in Parkinson’s disease (PD) participants treated with carbidopa/levodopa
and NE3107. Forty-five patients with a defined L-dopa morning “off state” were randomized 1:1 to placebo:NE3107 20 mg twice
daily for 28 days. The trial was launched with two design objectives: 1) the primary objective was safety and a drug-drug interaction
study (as requested by FDA) to demonstrate the absence of adverse interactions of NE3107 with levodopa; and 2) the secondary objective
was to determine if preclinical indications of promotoric activity and apparent enhancement of levodopa activity observed in a PD model
in monkeys can be seen in humans. Both objectives were met. Highlighted results of the study were:
| ● | Patients
treated with NE3107 + C/L experienced greater improvements in their Motor Disease Society-
Unified Parkinson’s Disease Rating Scale (MDS UPDRS) Part III score than patients treated
with placebo + C/L at the 2- and 3-hour marks after administration of the first daily
dose of C/L. |
| ● | Patients
<70 years old treated with NE3107 + C/L experienced improvements that were ~6 points better
than those who received placebo + C/L. |
| ● | Five
(26%) of the 19 patients treated with NE3107, compared to none of the 19 placebo treated
patients, who had a baseline of morning OFF experienced a morning ON state prior to receiving
their morning medications on day 28; this difference was statistically significant (p=0.046). |
| ● | The
study met its endpoints; investigators concluded that NE3107 + C/L combination treatment
was associated with clinically meaningful and superior improvements (3+ points) on the motor
examination part (Part III) of the MDS UPDRS. |
| ● | NE3107
produced statistically significant improvements in nonmotor symptoms scale assessments (NMSS)
for fatigue (Q4) p=0.02, urge to move legs (Q6) p=0.0036, and saliva dribbling (Q19) p= 0.0395. |
Neuroinflammation and activation of brain microglia,
leading to increased proinflammatory cytokines (particularly TNF) that play a pivotal role in PD, which affects an estimated 1 million
Americans. Multiple daily administrations of levodopa (converted to dopamine in the brain) is the current standard of care treatment for
this movement disorder, but prolonged daily administration leads to side effects of uncontrolled movements called levodopa-induced dyskinesia,
commonly referred to as LID. Recent evidence demonstrates that daily administration of levodopa further increases neuroinflammation, microglia
activation, and TNF inflammatory damage in neurons.
We
have shown in a mouse model of PD that NE3107 decreases inflammation and TNF in the brain and increases neuron survival (Nicoletti, 2012
Parkinson’s Disease 969418.) In this neurotoxin induced model, NE3107 decreased clinical signs of disease and neuronal death compared
to placebo treated mice.
An
unpublished study in a neurotoxin induced marmoset model of Parkinson’s disease reported that administration of NE3107 decreased
movement abnormalities that are the clinical signs of the disease. In the same study, NE3107 in combination with levodopa had a stronger
effect on clinical signs of disease than levodopa or NE3107 alone, while marmosets treated with NE3107 developed less LID. NE3107-treated
monkeys also exhibited neuroprotective activity that promoted the survival of twice as many neurons in the substantia nigra (primary
region of the brain that degenerates to cause parkinsonism) as monkeys treated with placebo. The results from the marmoset study suggest
that NE3107 may decrease clinical signs of disease in humans (improve motor function), which if true could enable a straightforward clinical
development strategy to test NE3107 in PD patients needing promotoric therapy.
If
approved as a promotoric agent, NE3107 would provide a non-dopaminergic alternative to Parkinson’s patients, and an opportunity
to significantly delay the need to start levodopa therapy. This could represent a first step toward supplanting levodopa as the primary
PD therapy, and in addition to delaying the emergence of LID, could also imply a slowing of disease progression, the most important and
still unmet objective of PD drug development.
Liver Cirrhosis Program
BioVie’s orphan drug candidate BIV201 (continuous
infusion terlipressin) represents a novel approach to the treatment of ascites due to chronic liver cirrhosis. Ascites is a common complication
of advanced liver cirrhosis involving the accumulation of large volumes of fluid in the abdomen, often exceeding five liters, due to liver
and kidney dysfunction. The FDA has never approved a drug to treat ascites, and once patients reach the refractory stage the estimated
one-year survival rate is only approximately 50%[10]. BIV201 is a continuous infusion of terlipressin, a drug used in over
40 countries to treat related complications of liver cirrhosis (Type 1 hepatorenal syndrome and bleeding esophageal varices) that was
recently approved in the U.S. but is not approved in Japan. With the novel room temperature stable formulation in a pre-filled syringe,
BIV201 could potentially provide a superior terlipressin drug delivery system throughout the world. The goal of BIV201 therapy is to interrupt
the ascites disease pathway, thereby halting the cycle of accelerated fluid generation in ascites patients.
In a series of interactions between June
2019 and April 2020, representatives of BioVie and the FDA communicated regarding the design and endpoints for the Phase 2 study (NCT04112199).
In June 2021, the Company initiated a Phase 2
study (NCT04112199) designed to evaluate the efficacy of BIV201 (terlipressin, administered by continuous infusion for two 28-day treatment
cycles) combined with standard-of-care (“SOC”), compared to SOC alone, for the treatment of refractory ascites. The primary
endpoints of the study are the incidence of ascites-related complications and change in ascites fluid accumulation during treatment compared
to a pre-treatment period.
In March 2023 the company announced enrolment
was paused and that data from the first 15 patients treated with BIV201 plus SOC appeared to show a 34% reduction in ascites fluid during
the 28 days after treatment initiation compared to the 28 days prior to treatment (p=0.0046). This improvement was significantly different
from those patients receiving SOC treatment. Patients who completed the treatment with BIV201 experienced a 53% reduction in ascites fluid
(p=0.001), which was sustained during the three months after treatment initiation as compared to the three-month pre-treatment period
(43% reduction, p=0.06).
In June 2023, the Company requested guidance from
the FDA regarding the design and endpoints for definitive clinical testing of BIV201 for the treatment of ascites due to chronic liver
cirrhosis. FDA accepted the request and intends to send written comments in the third quarter of calendar year 2023.
Our proprietary novel liquid formulation of terlipressin
in a prefilled syringe is designed to improve convenience for outpatient administration and avoid potential formulation errors when pharmacists
reconstitute the current powder version of terlipressin. To date, analytical testing results have confirmed room temperature stability
of the prefilled syringe in storage for 18 months, with the potential for up two years stability. Room temperature storage presents a
key product differentiation versus terlipressin products in countries where the drug is approved. To the best of the Company’s knowledge,
all other terlipressin products sold globally must be stored under refrigeration and there is no prefilled syringe format of terlipressin
available for treating patients in these countries. BioVie has also filed a Patent Cooperation Treaty (“PCT”) application
covering our novel liquid formulations of terlipressin (international patent application PCT/US2020/034269, published as WO2020/237170)
and we plan to seek patent protection in at least the U.S., Europe, China, Japan and other jurisdictions.
BIV201 (continuous infusion terlipressin) has
the potential to improve the health of thousands of patients suffering from life-threatening complications of liver cirrhosis due to hepatitis,
nonalcoholic steatohepatitis (“NASH”), and alcoholism. The FDA has granted Fast-Track status and Orphan Drug designation for
the most common of these complications, ascites, which represents a significant unmet medical need. Patients with cirrhosis and ascites
account for an estimated 116,000 U.S. hospital discharges annually, with frequent early readmissions. According to the HCUP Nationwide
Readmissions Database 2016, those requiring paracentesis (removal of ascites fluid) experience an average hospital stay lasting eight
days incurring over $86,000 in medical costs. This translates into a total potentially addressable ascites market size for BIV201 therapy
exceeding $650 million based on Company estimates. The FDA has never approved any drug specifically for treating ascites. For patients
with refractory ascites the mean one-year survival rate is only 50% (Bureau et al. 2017). BIV201 has also received Orphan Drug
designation for hepatorenal syndrome (“HRS”). Patients with refractory ascites often progress to HRS which is the onset of
kidney failure and requires emergency hospitalization.
The BIV201 development program began at LAT Pharma
LLC. On April 11, 2016, we acquired LAT Pharma LLC and the rights to its BIV201 development program and currently own all development
and marketing rights to the product candidate. We and PharmaIN, LAT Pharma’s former partner focused on the development of new modified
product candidates in the same therapeutic field but not including BIV201, have agreed to pay royalties equal to less than 1% of future
net sales of each company’s ascites drug development programs, or if such program is licensed to a third party, less than 5% of
each company’s net license revenues. On December 24, 2018, we returned our partial ownership rights to the PharmaIN modified terlipressin
development program and simultaneously paid the remaining balance due on a related debt. PharmaIN’s rights to our program remain
unchanged.
About Ascites and Liver Cirrhosis
Cirrhosis is a leading cause of death in the U.S.
The condition results primarily from hepatitis, alcoholism, and fatty liver disease linked to obesity. Ascites is a common complication
of advanced liver cirrhosis, involving kidney dysfunction and the accumulation of large amounts of fluid in the abdominal cavity.
The Need for an Ascites Therapy
With no medications approved by the FDA specifically
for treating ascites, an estimated 40% of patients die within two years of diagnosis. Certain drugs approved for other uses such as diuretics
may provide initial relief, but patients may fail to respond to treatment as ascites worsens. This represents a critical unmet medical
need, reflected by the Fast Track designation granted to BIV201 by the FDA as a treatment for ascites refractory to or intolerant of diuretic
therapy. U.S. treatment costs for liver cirrhosis, including ascites and other complications, are estimated at more than $5 billion annually.
The
Ascites Development Pathway

Most
experts agree that ascites develops through a sequence of events illustrated by the above diagram. High blood pressure in the vein that
supplies blood to the liver, called “portal hypertension,” occurs as increasing liver damage (fibrosis) impedes blood flow
through the liver. This causes vasodilation and blood pooling in the central or “splanchnic” region of the body and
low blood volume in the arteries. The decrease in effective blood volume activates a signaling pathway (“neurohormonal systems”)
which tells the kidneys to retain large amounts of salt and water in an effort to increase blood volume. Ultimately the retention of
excess sodium and water leads to the formation of ascites as these substances “weep” from the liver and lymph system and
collect in the patient’s abdomen.
The
BIV201 Proposed Mechanism of Action
BIV201
is being developed with the goal of alleviating portal hypertension and correcting splanchnic vasodilation, thereby increasing effective
blood volume and reducing the signals to the kidneys to retain excess salt and water. If successful, BIV201 could halt the
cycle of accelerating fluid generation in ascites patients and reduce the need for the frequent and painful paracentesis procedures many
of these patients currently require.
Future
Possible BIV201 Indications
Based on international investigative studies of
the active agent in BIV201, terlipressin, we believe our drug candidate has potential future applications in other life-threatening conditions
due to liver cirrhosis. Securing marketing approvals for any of these new uses will require well-controlled clinical trials to satisfy
the FDA and/or other countries’ regulatory requirements, none of which have commenced at this time. The Company continues to evaluate
other indications for the use of terlipressin continuous infusion. BioVie will discuss such indications if and when selected for testing.
Intellectual Property
BIV201
BioVie relies on a combination of patent, trade
secret, other intellectual property laws (such as FDA data exclusivity), nondisclosure agreements, and other measures to protect our proposed
products. We require our employees, consultants, and advisors to execute confidentiality agreements and to agree to disclose and assign
to us all inventions conceived during the workday, using our property, or which relate to our business. Despite any measures taken to
protect our intellectual property (IP), unauthorized parties may attempt to copy aspects of our products or to obtain and use information
that we regard as proprietary.
BIV201 was awarded Orphan Drug Designations in
the U.S. for the treatment of hepatorenal syndrome (received November 21, 2018) and treatment of ascites due to all etiologies except
cancer (received September 8, 2016). We also filed a PCT application covering our novel liquid formulations of terlipressin (international
patent application PCT/US2020/034269, published as WO2020/237170) and are seeking patent protection in at least the U.S., Europe, China,
Japan and other jurisdictions. Also, we own U.S. Patent 11,364,277, which is directed to a method of treating ascites with BIV201, and
we are pursuing similar patent coverage in Japan, Europe, and China.
NE3107 and related compounds
As
of August 15, 2023, we have fifteen (15) issued U.S. patents, six (6) pending U.S. patent applications, one (1) pending U.S. PCT application
and six (6) issued foreign patents directed to protecting NE3107 and related compounds and methods of making and using thereof. The U.S.
patents and pending patent applications and their projected expiration dates are provided below.
| Title
|
Patent
Application
Number |
Patent
Number |
Expiration
Date |
| Steroids
Having 7-Oxygen and 17-Heteroaryl Substitution |
13/095,528
14/027,825
14/027,842 |
8,569,275
9,102,702
9,115,168 |
2/14/2024
3/28/2024
3/28/2024 |
| Unsaturated
Steroid Compounds |
13/030,326 |
8,586,770 |
6/2/2026 |
| Solid
State Forms of a Pharmaceutical |
12/418,559 |
8,252,947* |
4/18/2030 |
| Crystalline
Anhydrate Forms of a Pharmaceutical |
14/459,528
15/348,107
16/598,694
17/240,728 |
9,555,046
9,850,271
10,995,112
pending |
4/3/2029
4/3/2029
4/3/2029
— |
| Pharmaceutical
Solid State Forms |
12/370,510 |
8,518,922 |
9/24/2031 |
| Methods
of Preparing Pharmaceutical Solid State Forms |
13/919,593 |
9,314,471 |
6/28/2029 |
| Steroid
Tetrol Solid State Forms |
12/272,767 |
8,486,926 |
1/10/2030 |
| Drug
Identification and Treatment Method |
11/941,936 |
8,354,396 |
7/7/2031 |
| Method
For Preparing Substituted 3,7-Dihydroxy Steroids |
13/664,304
14/886,738 |
9,163,059**
9,994,608 |
6/5/2029
6/5/2029 |
| Treatment
Methods Using Pharmaceutical Solid State Forms |
14/459,493 |
9,877,972 |
4/3/2029 |
| Compositions
for Treatment of Neurodegenerative Conditions |
PCT/US2022/027294 |
pending |
— |
| Methods
for the Treatment of Mild Cognitive Impairment |
63/374631 |
pending |
— |
| Methods
for the Treatment of Mild Cognitive Impairment |
63/498703 |
pending |
— |
| Assay
and Methods for Drug Discovery |
63/479973 |
pending |
— |
| Methods
for the Treatment of Biological Aging |
63/381521 |
pending |
— |
| Methods
for the Treatment of Biological Aging |
63/508856 |
pending |
— |
| |
* |
Foreign
counterparts issued in Australia, Canada, Europe and South Korea expire 4/3/2029. |
| |
** |
Foreign
counterparts issued in Europe and Japan expire 6/5/2029. |
Government
Regulation
Government
authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things,
the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion,
advertising, distribution, post-approval monitoring and reporting, marketing and export and import of products such as those we are developing.
Any pharmaceutical candidate that we develop must be approved by the FDA before it may be legally marketed in the United States and by
the appropriate foreign regulatory agency before it may be legally marketed in foreign countries.
United
States Drug Development Process
In
the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, or FDCA, and implements regulations. Drugs
are also subject to other federal, state and local statutes and regulations. Biologics are subject to regulation by the FDA under the
FDCA, the Public Health Service Act, or the PHSA, and related regulations, and other federal, state and local statutes and regulations.
Biological products include, among other things, viruses, therapeutic serums, vaccines and most protein products. The process of obtaining
regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require
the expenditure of substantial time and financial resources. Failure to comply with the applicable United States requirements at any
time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial
sanctions. FDA sanctions could include refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters,
product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government
contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material
adverse effect on us.
The
process required by the FDA before a drug or biological product may be marketed in the United States generally involves the following:
| |
● |
Completion
of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices or other applicable
regulations; |
| |
● |
Submission
to the FDA of an Investigational New Drug Application, or an IND, which must become effective before human clinical trials may begin; |
| |
● |
Performance
of adequate and well-controlled human clinical trials according to the FDA’s current good clinical practices, or GCPs, to establish
the safety and efficacy of the proposed drug or biologic for its intended use; |
| |
● |
Submission
to the FDA of a New Drug Application, or an NDA, for a new drug product, or a Biologics License Application, or a BLA, for a new
biological product; |
| |
● |
Satisfactory
completion of an FDA inspection of the manufacturing facility or facilities where the drug or biologic is to be produced to assess
compliance with the FDA’s current good manufacturing practice standards, or cGMP, to assure that the facilities, methods and
controls are adequate to preserve the drug’s or biologic’s identity, strength, quality and purity; |
| |
● |
Potential
FDA audit of the nonclinical and clinical trial sites that generated the data in support of the NDA or BLA; and |
| |
● |
FDA
review and approval of the NDA or BLA. |
The
lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require
the expenditure of substantial resources. There can be no certainty that approvals will be granted.
Clinical
trials involve the administration of the drug or biological candidate to healthy volunteers or patients having the disease being studied
under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control. Clinical
trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject
selection and exclusion criteria, and the parameters to be used to monitor subject safety. Each protocol must be submitted to the FDA
as part of the IND. Clinical trials must be conducted in accordance with the FDA’s good clinical practices requirements. Further,
each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution
at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers
such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated
benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative
and must monitor the clinical trial until it is completed.
Human
clinical trials prior to approval are typically conducted in three sequential phases that may overlap or be combined:
| |
● |
Phase 1. The
drug or biologic is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism,
distribution and excretion. In the case of some products for severe or life-threatening diseases, especially when the product may
be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients having
the specific disease. |
| |
● |
Phase 2. The
drug or biologic is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily
evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage and dosing
schedule for patients having the specific disease. |
| |
● |
Phase 3. Clinical
trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically
dispersed clinical trial sites. These clinical trials, which usually involve more subjects than earlier trials, are intended to establish
the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. Generally, at least two adequate
and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA or BLA. |
Post-approval
studies, or Phase 4 clinical trials, may be conducted after initial marketing approval. These studies are used to gain additional
experience from the treatment of patients in the intended therapeutic indication and may be required by the FDA as part of the approval
process.
Progress
reports detailing the results of the clinical trials must be submitted at least annually to the FDA and written IND safety reports must
be submitted to the FDA by the investigators for serious and unexpected adverse events or any finding from tests in laboratory animals
that suggests a significant risk for human subjects. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed
successfully within any specified period, if at all. The FDA or the sponsor or its data safety monitoring board may suspend a clinical
trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable
health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not
being conducted in accordance with the IRB’s requirements or if the drug or biologic has been associated with unexpected serious
harm to patients.
Concurrent
with clinical trials, companies usually complete additional animal studies and develop additional information about the chemistry and
physical characteristics of the drug or biologic as well as finalize a process for manufacturing the product in commercial quantities
in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug
or biological candidate and, among other things, must include methods for testing the identity, strength, quality and purity of the final
drug or biologic. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate
that the drug or biological candidate does not undergo unacceptable deterioration over its shelf life.
U.S.
Review and Approval Processes
The
results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical
tests conducted on the chemistry of the drug or biologic, proposed labeling and other relevant information are submitted to the FDA as
part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA is subject to the payment of substantial
user fees; a waiver of such fees may be obtained under certain limited circumstances.
The
FDA reviews all NDAs and BLAs submitted before it accepts them for filing and may request additional information rather than accepting
an NDA or BLA for filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA or BLA.
After
the NDA or BLA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product
is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve
the product’s identity, strength, quality and purity. The FDA reviews a BLA to determine, among other things, whether the product
is safe, pure and potent and the facility in which it is manufactured, processed, packaged or held meets standards designed to assure
the product’s continued safety, purity and potency. In addition to its own review, the FDA may refer applications for novel drug
or biological products or drug or biological products which present difficult questions of safety or efficacy to an advisory committee,
typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application
should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers
such recommendations carefully when making decisions. During the approval process, the FDA also will determine whether a risk evaluation
and mitigation strategy, or REMS, is necessary to assure the safe use of the drug or biologic. If the FDA concludes that a REMS is needed,
the sponsor of the NDA or BLA must submit a proposed REMS; the FDA will not approve the NDA or BLA without a REMS, if required.
Before
approving an NDA or BLA, the FDA will inspect the facilities at which the product is to be manufactured. The FDA will not approve the
product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to
assure consistent production of the product within required specifications. Additionally, before approving an NDA or BLA, the FDA will
typically inspect one or more clinical sites to assure compliance with cGMP. If the FDA determines the application, manufacturing process
or manufacturing facilities are not acceptable it will outline the deficiencies in the submission and often will request additional testing
or information.
The
NDA or BLA review and approval process is lengthy and difficult and the FDA may refuse to approve an NDA or BLA if the applicable regulatory
criteria are not satisfied or may require additional clinical data or other data and information. Even if such data and information is
submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. Data obtained from clinical
trials are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval.
The FDA will issue a “complete response” letter if the agency decides not to approve the NDA or BLA. The complete response
letter usually describes all of the specific deficiencies in the NDA or BLA identified by the FDA. The deficiencies identified may be
minor, for example, requiring labeling changes, or major, for example, requiring additional clinical trials. Additionally, the complete
response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If
a complete response letter is issued, the applicant may either resubmit the NDA or BLA, addressing all of the deficiencies identified
in the letter, or withdraw the application.
If
a product receives regulatory approval, the approval may be limited to specific diseases and dosages or the indications for use may otherwise
be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings
or precautions be included in the product labeling. In addition, the FDA may require Phase 4 testing which involves clinical trials
designed to further assess a product’s safety and effectiveness and may require testing and surveillance programs to monitor the
safety of approved products that have been commercialized.
Orphan
Drug Designation
Under
the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product intended to treat a rare disease or condition,
which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals
in the United States and for which there is no reasonable expectation that the cost of developing and making a drug or biological product
available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan product designation
must be requested before submitting an NDA or BLA. After the FDA grants orphan product designation, the identity of the therapeutic agent
and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten
the duration of the regulatory review and approval process.
If
a product that has Orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such
designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to
market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing
of clinical superiority to the product with orphan exclusivity. Competitors, however, may receive approval of different products for
the indication for which the Orphan product has exclusivity or obtain approval for the same product but for a different indication for
which the Orphan product has exclusivity. Orphan product exclusivity also could block the approval of one of our products for seven years
if a competitor obtains approval of the same drug or biological product as defined by the FDA or if our drug or biological candidate
is determined to be contained within the competitor’s product for the same indication or disease. If a drug or biological product
designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled
to orphan product exclusivity. Orphan Drug status in the European Union has similar but not identical benefits in the European Union.
Expedited
Development and Review Programs
The
FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new drug and biological products that
meet certain criteria. Specifically, new drug and biological products are eligible for Fast Track designation if they are intended to
treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track
designation applies to the combination of the product and the specific indication for which it is being studied. Unique to a Fast Track
product, the FDA may consider for review sections of the NDA or BLA on a rolling basis before the complete application is submitted,
if the sponsor provides a schedule for the submission of the sections of the NDA or BLA, the FDA agrees to accept sections of the NDA
or BLA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section
of the NDA or BLA.
Any
product submitted to the FDA for marketing approval, including those submitted to a Fast Track program, may also be eligible for other
types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible
for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or
a significant improvement in the treatment, diagnosis or prevention of a disease compared with marketed products. The FDA will attempt
to direct additional resources to the evaluation of an application for a new drug or biological product designated for priority review
in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Drug or biological products
studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic
benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and
well-controlled clinical studies establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict
a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition
of approval, the FDA generally requires that a sponsor of a drug or biological product receiving accelerated approval perform adequate
and well-controlled post-marketing clinical studies to establish safety and efficacy for the approved indication. Failure to conduct
such studies or conducting such studies that do not establish the required safety and efficacy may result in revocation of the original
approval. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which
could adversely impact the timing of the commercial launch or subsequent marketing of the product. Fast Track designation, priority review
and accelerated approval do not change the standards for approval but may expedite the development or approval process.
Post-Approval
Requirements
Any
drug or biological products for which we receive FDA approvals are subject to continuing regulation by the FDA, including, among other
things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy
information on an annual basis or as required more frequently for specific events, product sampling and distribution requirements, complying
with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements, which include,
among others, standards for direct-to-consumer advertising, prohibitions against promoting drugs and biologics for uses or in patient
populations that are not described in the drug’s or biologic’s approved labeling (known as “off-label use”),
rules for conducting industry-sponsored scientific and educational activities, and promotional activities involving the internet. Failure
to comply with FDA requirements can have negative consequences, including the immediate discontinuation of noncomplying materials, adverse
publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties.
Although physicians may prescribe legally available drugs and biologics for off-label uses, manufacturers may not market or promote such
off-label uses.
We
will need to rely on third parties for the production of our product candidates. Manufacturers of our product candidates are required
to comply with applicable FDA manufacturing requirements contained in the FDA’s cGMP regulations. cGMP regulations require among
other things, quality control and quality assurance as well as the corresponding maintenance of comprehensive records and documentation.
Drug and biologic manufacturers and other entities involved in the manufacture and distribution of approved drugs and biologics are also
required to register their establishments and list any products made there with the FDA and comply with related requirements in certain
states, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other
laws. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain
cGMP compliance. Discovery of problems with a product after approval may result in serious and extensive restrictions on a product, manufacturer,
or holder of an approved NDA or BLA, including suspension of a product until the FDA is assured that quality standards can be met, continuing
oversight of manufacturing by the FDA under a “consent decree,” which frequently includes the imposition of costs and continuing
inspections over a period of many years, and possible withdrawal of the product from the market. In addition, changes to the manufacturing
process generally require prior FDA approval before being implemented and other types of changes to the approved product, such as adding
new indications and additional labeling claims, are also subject to further FDA review and approval.
The
FDA also may require post-marketing testing, known as Phase 4 testing, risk minimization action plans and surveillance to monitor
the effects of an approved product or place conditions on an approval that could otherwise restrict the distribution or use of the product.
Employees
Our
business is managed by our officers who consist of Mr. Cuong Do, Chief Executive Officer & President; Dr Joseph M Columbo, Executive
Vice President -Chief Medical Officer; and Wendy Kim, our Chief Financial Officer and Corporate Secretary. These individuals devote their
full-time efforts to the Company activities. The Company has 18 employees which are all full time. We also rely on a team of highly experienced
scientific, medical, and regulatory consultants to conduct its product development activities.
Our business, financial condition, operating results
and prospects are subject to the following risks. Additional risks and uncertainties not presently foreseeable to us may also impair our
business operations. If any of the following risks or the risks described elsewhere in this report actually occurs, our business, financial
condition or operating results could be materially adversely affected. In such case, the trading price of our Company’s Class A
Common Stock, par value $0.0001 (“Common Stock”) Common Stock could decline, and our stockholders may lose all or part of
their investment in the shares of our Common Stock.
This
Form 10-K contains forward-looking statements that involve risks and uncertainties. These statements can be identified by the use of
forward-looking terminology such as “believes,” “expects,” “intends,” “plans,” “may,”
“will,” “should,” “predict” or “anticipation” or the negative thereof or other variations
thereon or comparable terminology. Actual results could differ materially from those discussed in the forward- looking statements as
a result of certain factors, including those set forth below and elsewhere in this Form 10-K.
Risks
Relating to Our Business and Industry
We
have no products approved for commercial sale, have never generated any revenues and may never achieve revenues or profitability, which
could cause us to cease operations.
We
have no products approved for commercial sale and, to date, we have not generated any revenue. Our ability to generate revenue depends
heavily on (a) successful completion of one or more development programs demonstrating in human clinical trials that BIV201 and NE3107,
our product candidates, are safe and effective; (b) our ability to seek and obtain regulatory approvals, including, without limitation,
with respect to the indications we are seeking; (c) successful commercialization of our product candidates; and (d) market acceptance
of our products. There are no assurances that we will achieve any of the forgoing objectives. Furthermore, our product candidates are
in the development stage, and have not been fully evaluated in human clinical trials. If we do not successfully develop and commercialize
our product candidates we will not achieve revenues or profitability in the foreseeable future, if at all. If we are unable to generate
revenues or achieve profitability, we may be unable to continue our operations.
We
are a development stage company with a limited operating history, making it difficult for you to evaluate our business and your investment.
BioVie
Inc. was incorporated on April 10, 2013. We are a development stage biopharmaceutical company with potential therapies that have not
been fully evaluated in clinical trials, and our operations are subject to all of the risks inherent in the establishment of a new business
enterprise, including but not limited to the absence of an operating history, the lack of commercialized products, insufficient capital,
expected substantial and continual losses for the foreseeable future, limited experience in dealing with regulatory issues, the lack
of manufacturing experience and limited marketing experience, possible reliance on third parties for the development and commercialization
of our proposed products, a competitive environment characterized by numerous, well-established and well capitalized competitors and
reliance on key personnel.
Since
inception, we have not established any revenues or operations that would provide financial stability in the long term, and there can
be no assurance that we will realize our plans on our projected timetable in order to reach sustainable or profitable operations.
Investors
are subject to all the risks incident to the creation and development of a new business and each investor should be prepared to withstand
a complete loss of his, her or its investment. Furthermore, the accompanying financial statements have been prepared assuming that we
will continue as a going concern. We have not emerged from the development stage, and may be unable to raise further equity. These factors
raise substantial doubt about our ability to continue as a going concern. The financial statements do not include any adjustments that
might result from the outcome of this uncertainty.
Because
we are subject to these risks, you may have a difficult time evaluating our business and your investment in our Company. Our ability
to become profitable depends primarily on our ability to develop drugs, to obtain approval for such drugs, and if approved, to successfully
commercialize our drugs, our research and development (“R&D”) efforts, including the timing and cost of clinical trials;
and our ability to enter into favorable alliances with third-parties who can provide substantial capabilities in clinical development,
regulatory affairs, sales, marketing and distribution.
Even
if we successfully develop and market BIV201 and/or NE3107, we may not generate sufficient or sustainable revenue to achieve or sustain
profitability, which could cause us to cease operations and cause you to lose all of your investment.
If
the FDA or comparable foreign regulatory authorities approve generic versions of any of our product candidates that receive marketing
approval, or such authorities do not grant our products sufficient, or any, periods of exclusivity before approving generic versions
of our products, the sales of our products could be adversely affected.
Once
a new drug application (NDA) is approved, the product covered thereby becomes a “reference listed drug” or
RLD, in the FDA’s publication, “Approved Drug Products with Therapeutic Equivalence Evaluations,” commonly known as
the Orange Book. Other manufacturers may seek approval of generic versions of reference listed drugs through submission of abbreviated
new drug applications (“ANDAs”) in the United States. In support of an ANDA, a generic manufacturer need not conduct clinical
trials. Rather, the applicant generally must show that its product has the same active ingredient(s), dosage form, strength, route of
administration and conditions of use or labeling as the reference listed drug and that the generic version is bioequivalent to the reference
listed drug, meaning it is absorbed in the body at the same rate and to the same extent as the RLD. Generic products may be significantly
less costly to bring to market than the reference listed drug and companies that produce generic products are generally able to offer
them at lower prices. Moreover, generic versions of RLDs are often automatically substituted for the RLD by pharmacies when dispensing
a prescription written for the RLD. Thus, following the introduction of a generic drug, a significant percentage of the sales of any
branded product or reference listed drug is typically lost to the generic product.
The
FDA may not approve an ANDA for a generic product until any applicable period of non-patent exclusivity for the reference listed drug
has expired. The United States Federal Food, Drug, and Cosmetic Act (FDCA) provides a period of five years of non-patent
exclusivity for a new drug containing a new chemical entity (“NCE”). An NCE is an active ingredient that has not previously
been approved by FDA in any other NDA. Specifically, in cases where such exclusivity has been granted, an ANDA may not be submitted to
the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification that a patent covering
the reference listed drug is either invalid or will not be infringed by the generic product, in which case the applicant may submit its
application four years following approval of the reference listed drug. If an ANDA is submitted to FDA with a Paragraph IV Certification,
the generic applicant must also provide a “Paragraph IV Notification” to the holder of the NDA for the RLD and to the owner
of the listed patent(s) being challenged by the ANDA applicant, providing a detailed written statement of the basis for the ANDA applicant’s
position that the relevant patent(s) is invalid or would not be infringed. If the patent owner brings a patent infringement lawsuit against
the ANDA applicant within 45 days of the Paragraph IV Notification, FDA approval of the ANDA will be automatically stayed for 30 months,
or until 7-1/2 years after the NDA approval if the generic application was filed between 4 years and 5 years after the NDA approval.
Any such stay will be terminated earlier if the court rules that the patent is invalid or would not be infringed.
Competition
that our products may face from generic versions of our products could materially and adversely impact our future revenue, profitability
and cash flows and substantially limit our ability to obtain a return on the investments we have made in those product candidates.
If
we fail to obtain or maintain Orphan Drug exclusivity for BIV201, we will have to rely on other potential marketing exclusivity, and
on our intellectual property rights, which may reduce the length of time that we can prevent competitors from selling generic versions
of BIV201.
We
have obtained Orphan Drug Designation for BIV201 (terlipressin) in the U.S. for the treatment of hepatorenal syndrome (received November
21, 2018) and treatment of ascites due to all etiologies except cancer (received September 8, 2016). Under the Orphan Drug Act, the FDA
may designate a product as an Orphan Drug if it is a drug intended to treat a rare disease or condition, defined, in part, as a patient
population of fewer than 200,000 in the U.S. In the EU, Orphan Drug designation may be granted to drugs intended to treat, diagnose or
prevent a life-threatening or chronically debilitating disease having a prevalence of no more than five in 10,000 people in the EU, and
which meet other specified criteria. The company that first obtains FDA approval for a designated Orphan Drug for the associated rare
disease may receive a seven-year period of marketing exclusivity during which time FDA may not approve another application for the same
drug for the same orphan disease or condition. Orphan Drug Exclusivity does not prevent FDA approval of another application for the same
drug for a different disease or condition, or of an application for a different drug for the same rare disease or condition. Orphan Drug
exclusive marketing rights may be lost under several circumstances, including a later determination by the FDA that the request for designation
was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug. Similar regulations are available
in the EU with a ten-year period of market exclusivity.
Even
though BioVie has obtained two Orphan Drug Designations for its lead product candidate, terlipressin, for treatment of ascites and for
treatment of hepatorenal syndrome, and may seek other Orphan Drug Designations for BIV201, and Orphan Drug Designation for other product
candidates, there is no assurance that BioVie will be the first to obtain marketing approval for any particular rare indication. Further,
even though BioVie has obtained Orphan Drug Designations for its lead product candidate, or even if BioVie obtains Orphan Drug Designation
for other potential product candidates, such designation may not effectively protect BioVie from competition because different drugs
can be approved for the same condition and the same drug can be approved for different conditions and potentially used off-label in the
Orphan indication. Even after an Orphan Drug is approved, the FDA can subsequently approve another competing drug with the same active
ingredient for the same condition for several reasons, including, if the FDA concludes that the later drug is clinically superior due
to being safer or more effective or because it makes a major contribution to patient care. Orphan Drug Designation neither shortens the
development time or regulatory review time of a drug, nor gives the drug any advantage in the regulatory review or approval process.
In addition, other companies have received Orphan
Drug designations for terlipressin. Mallinckrodt Hospital Products IP Limited received Orphan Drug designation in 2004 for terlipressin
for the treatment of Hepatorenal Syndrome. Mallinckrodt has already gained FDA approval for its product, lyophilized terlipressin acetate
for bolus intravenous administration for the treatment of hepatorenal syndrome Type 1 in September 2022. PharmaIN Corporation received
Orphan Drug Designation in 2012 for PGC-C12E-terlipressin for treatment of ascites due to all etiologies except cancer. In addition, Ferring
Pharmaceuticals Inc. received Orphan Drug designation in 1986 for terlipressin for the treatment of bleeding esophageal varices. If one
of those or any other company with Orphan Drug Designation for the same drug as ours for the same proposed disease or condition receives
FDA approval and Orphan Drug Exclusivity before our product is approved, approval of our drug(s) for the orphan indication may be blocked
for seven years by the other company’s Orphan Exclusivity and they may obtain a competitive advantage even after the exclusivity
period expires associated with being the first to market.
We
will need to raise substantial additional capital in the future to fund our operations and we may be unable to raise such funds when
needed and on acceptable terms, which could have a materially adverse effect on our business.
Developing
biopharmaceutical products, including conducting pre-clinical studies and clinical trials and establishing manufacturing capabilities,
requires substantial funding. Additional financing will be required to fund the research and development of our product candidates. We
have not generated any product revenues, and do not expect to generate any revenues until, and only if, we develop, and receive approval
to sell our product candidates from the FDA and other regulatory authorities for our product candidates.
We
may not have the resources to complete the development and commercialization of any of our proposed product candidates. We will require
additional financing to further the clinical development of our product candidates. In the event that we cannot obtain the required financing,
we will be unable to complete the development necessary to file an NDA with the FDA for BIV201 or NE3107. This will delay or require
termination of research and development programs, preclinical studies and clinical trials, material characterization studies, regulatory
processes, the establishment of our own laboratory or a search for third party marketing partners to market our products for us, which
could have a materially adverse effect on our business.
The
amount of capital we may need will depend on many factors, including the progress, timing and scope of our research and development programs,
the progress, timing and scope of our preclinical studies and clinical trials, the time and cost necessary to obtain regulatory approvals,
the time and cost necessary to establish our own marketing capabilities or to seek marketing partners, the time and cost necessary to
respond to technological and market developments, changes made or new developments in our existing collaborative, licensing and other
commercial relationships, and new collaborative, licensing and other commercial relationships that we may establish.
Until
we can generate a sufficient amount of product revenue, if ever, we expect to finance future cash needs through public or private equity
offerings, debt financings, or corporate collaboration and licensing arrangements. Additional funds may not be available when we need
them on terms that are acceptable to us, or at all. If adequate funds are not available, we may be required to delay, reduce the scope
of, or eliminate one or more of our research or development programs or our commercialization efforts. In addition, we could be forced
to discontinue product development and reduce or forego attractive business opportunities. To the extent that we raise additional funds
by issuing equity securities, our stockholders may experience additional significant dilution, and debt financing, if available, may
involve restrictive covenants. To the extent that we raise additional funds through collaboration and licensing arrangements, it may
be necessary to relinquish some rights to our technologies or our product candidates or grant licenses on terms that may not be favorable
to us. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate
need for additional capital at that time.
Our
fixed expenses, such as rent and other contractual commitments, will likely increase in the future, as we may enter into leases for new
facilities and capital equipment and/or enter into additional licenses and collaborative agreements. Therefore, if we fail to raise substantial
additional capital to fund these expenses, we could be forced to cease operations, which could cause you to lose all of your investment.
We
have limited experience in drug development and may not be able to successfully develop any drugs, which would cause us to cease operations.
We
have never successfully developed a new drug and brought it to market. Our management and clinical teams have experience in drug development
but they may not be able to successfully develop any drugs. Our ability to achieve revenues and profitability in our business will depend
on, among other things, our ability to develop products internally or to obtain rights to them from others on favorable terms; complete
laboratory testing and human studies; obtain and maintain necessary intellectual property rights to our products; successfully complete
regulatory review to obtain requisite governmental agency approvals; enter into arrangements with third parties to manufacture our
products on our behalf; and enter into arrangements with third parties to provide sales and marketing functions. If we are unable
to achieve these objectives we will be forced to cease operations and you will lose all of your investment.
Development of pharmaceutical products is
a time-consuming process, subject to a number of risks, many of which are outside of our control. Consequently, we can provide no assurance
that our product candidates will obtain regulatory approval, and if we are unsuccessful or fail to timely develop new drugs, we could
be forced to discontinue our operations.
Our drug product candidate NE3107 was cleared
by FDA for use in a Phase 3, randomized, double blind, placebo controlled, parallel group, multicenter study in subjects who have mild
to moderate Alzheimer’s Disease. Enrollment in that trial began in August 2021, with a planned primary completion in late 2022/early
2023. Alzheimer’s Disease is a complex and still poorly understood disease. In June 2021, FDA approved the drug aducanumab for treatment
of Alzheimer’s despite a strong recommendation against approval from an FDA advisory committee. That FDA approval has generated
significant medical and political controversy, including a Congressional investigation, announced on June 25, 2021, into the basis for
FDA’s approval decision. That investigation, other potential investigations, and negative publicity of FDA’s approval decision
could adversely impact the agency’s oversight of our clinical development program, how the agency may view and act upon any NDA
we may file for NE3107, and the commercial viability of NE3107 if it were to be approved and marketed.
Our drug product candidate, BIV201 (continuous
infusion terlipressin), was cleared by the FDA to undergo testing in a mid-stage (Phase 2b) clinical trial for the treatment of refractory
ascites due to cirrhosis. On June 24, 2021, we announced that the first patient has been enrolled in this study. The open-label trial
was stopped after 15 of the planned 30 patients were enrolled and an evaluation of those completed patients assessed. Encouraging data
from these patients appeared to show that treatment with BIV201 plus SOC resulted in a reduction in ascites fluid accumulation during
treatment versus pre-treatment. In June 2023, the Company requested guidance from the FDA regarding the design and endpoints for definitive
clinical testing of BIV201 for the treatment of ascites due to chronic liver cirrhosis. FDA accepted the request and intends to send written
comments in the third quarter of calendar year 2023. BIV201 has also received Orphan Drug designation for HRS. Patients with refractory
ascites often progress to HRS which is the onset of kidney failure and requires emergency hospitalization. As reflected by approved label
for Malllinckrodt’s drug Terlivaz, terlipressin dosed as an intermittent IV bolus (1 or 2 mg every 6 hours) to treat HRS, however,
terlipressin may cause significant toxicity when administered this way in this critically ill patient population. We believe that our
continuous infusion approach to terlipressin treatment may overcome some of those safety concerns, but there can be no assurance that
we will be able to demonstrate an acceptable risk;safety benefit for BIV201 for the treatment of refractory ascites or HRS to the FDA’s
satisfaction. On June 23, 2021, we announced that FDA has provided guidance on our planned Phase 3 clinical trial of BIV201 in (HRS-AKI)
and have since reached agreement on the key elements of the trial design. Although we have deprioritized HRS-AKI program to focus on NE3107,
on April 15, 2022, we received additional FDA comments regarding the trial design in response to a subsequent Type C meeting request related
to BV201 in HRS-AKI.
Further
development and extensive testing will be required to determine the technical feasibility and commercial viability of BIV201 and NE3107.
Our success will depend on our ability to achieve scientific and technological advances and to translate such advances into reliable,
commercially competitive drugs on a timely basis. Drugs that we may develop are not likely to be commercially available, at a minimum,
for several years, if ever. The proposed development schedules for our product candidates may be affected by a variety of factors, including
technological difficulties, proprietary technology of others, and changes in government regulation, many of which will not be within
our control. Any delay in the development, introduction or marketing of our product candidates could result either in such drugs being
marketed at a time when their cost and performance characteristics would not be competitive in the marketplace or in the shortening of
their commercial lives. In light of the long-term nature of our projects and other risk factors described elsewhere in this document,
we may not be able to successfully complete the development or marketing of any drugs, which could cause us to cease operations.
We
may fail to successfully develop and commercialize our product candidate(s) if it is found to be unsafe or ineffective in clinical trials;
does not receive necessary approval from the FDA or foreign regulatory agencies; fails to conform to a changing standard of care for
the disease it seeks to treat; or is less effective or more expensive than current or alternative treatment methods.
Drug development failure can occur at any stage
of clinical trials and as a result of many factors, there can be no assurance that we or our collaborators will reach our anticipated
clinical targets. Even if the trials are successfully completed, clinical data are often susceptible to varying interpretations and analyses,
and we cannot guarantee that the FDA or comparable foreign regulatory authorities will interpret the results as we do, and more trials
could be required before we submit our product candidates for approval. We cannot guarantee that the FDA or comparable foreign regulatory
authorities will view our product candidates as having efficacy even if positive results are observed in clinical trials. In some instances,
there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due
to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations,
changes in and adherence to the clinical trial protocols, and the rate of dropout among clinical trial participants. If the results of
our ongoing or future clinical trials are inconclusive with respect to the efficacy of our product candidates, if we do not meet the clinical
endpoints with statistical and clinically meaningful significance, or if there are safety concerns associated with our product candidates,
we may be delayed in obtaining marketing approval, if at all. Additionally, any safety concerns observed in any one of our clinical trials
in our targeted indications could limit the prospects for regulatory approval of our product candidates in those and other indications.
We also do not know what the long-term effects of exposure to our product candidates will be. Furthermore, our product candidates may
be used in combination with other treatments and there can be no assurance that such use will not lead to unique or unexpected safety
issues.
Failure to complete clinical trials or to prove
that our product candidates are safe and effective would have a material adverse effect on our ability to generate revenue and could require
us to reduce the scope of or discontinue our operations, which could cause you to lose all of your investment.
We rely and will continue to rely on third
parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected
deadlines, we may not be able to obtain regulatory approval of or commercialize our product candidates.
We depend, and will continue to depend, on contract
research organizations (“CROs”), clinical trial sites and clinical trial principal investigators, contract laboratories, and
other third parties to conduct our clinical trials. We rely heavily on these third parties over the course of our clinical trials, and
we control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted
in accordance with the protocol and applicable legal, regulatory, and scientific standards and regulations, and our reliance on third
parties does not relieve us of our regulatory responsibilities. We and these third parties are required to comply with current good clinical
practices (“cGCPs”), which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities
for the conduct of clinical trials on product candidates in clinical development. Regulatory authorities enforce cGCPs through periodic
inspections and for-cause inspections of clinical trial principal investigators and trial sites. If we or any of these third parties fail
to comply with applicable cGCPs or fail to enroll a sufficient number of patients, we may be required to conduct additional clinical trials
to support our marketing applications, which would delay the regulatory approval process. Moreover, our business may be implicated if
any of these third parties violates federal, state, or foreign fraud and abuse or false claims laws and regulations or healthcare privacy
and security laws, or provide us or government agencies with inaccurate, misleading, or incomplete data.
Although we design the clinical trials for our
product candidates, our CROs will facilitate and monitor our clinical trials. As a result, many important aspects of our clinical development
programs, including site and investigator selection, and the conduct and timing and monitoring of the study, will be partly or completely
outside our direct control. Our reliance on third parties to conduct clinical trials will also result in less direct control over the
collection, management, and quality of data developed through clinical trials than would be the case if we were relying entirely upon
our own employees. Communicating with third parties can also be challenging, potentially leading to mistakes as well as difficulties in
coordinating activities.
Any third parties conducting our clinical trials
are not, and will not be, our employees and, except for remedies available to us under our agreements with these third parties, we cannot
control whether they devote sufficient time and resources to our ongoing preclinical, clinical, and nonclinical programs. These third
parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical
trials or other drug development activities, which could affect their performance on our behalf. If these third parties do not successfully
carry out their contractual duties or obligations or meet expected deadlines, if the quality or accuracy of the clinical data they obtain
is compromised due to the failure to adhere to our clinical protocols or regulatory requirements, or if there are other difficulties with
such third parties, such as staffing difficulties, changes in priorities, or financial distress, our clinical trials may be extended,
delayed, or terminated. As a result, we may not be able to complete development of, obtain regulatory approval of, or successfully commercialize
our product candidates. As a result, our financial results and the commercial prospects for our product candidates will be harmed, our
costs could increase, and our ability to generate revenue could be delayed.
If any of our relationships with trial sites, or any CRO that we may
use in the future, terminates, we may not be able to timely enter into arrangements with alternative trial sites or CROs, or do so on
commercially reasonable terms. Switching or adding clinical trial sites or CROs to conduct our clinical trials involves substantial cost
and requires extensive management time, training, and focus. In addition, there is a natural transition lag when a new third party must
learn about our product candidates and protocols, which can result in delays that may materially impact our ability to meet our desired
clinical development timelines.
We
face business disruption and related risks resulting from the outbreak of the novel coronavirus 2019 (COVID-19) pandemic, which could
have a material adverse effect on our business plan.
The
continual widespread health emergencies or pandemics such as the coronavirus (“COVID-19”) pandemic (and its related variants),
has led to continued regional quarantines, business shutdowns, labor shortages, disruptions to supply chains, and overall economic instability,
which could materially and adversely affect the clinical trials, supply chain, financial condition and financial performance of our company.
Although some jurisdictions have relaxed these measures, others have not or have reinstated them as COVID-19 cases surge and its variants
continue to emerge. The duration and spread of the COVID-19 pandemic and the long-term impact of COVID-19 and its variants on the financial
markets and the overall economy are highly uncertain and cannot be predicted at this time. If the financial markets and/or the overall
economy are impacted for an extended period, the Company’s ability to raise funds may be materially adversely affected. In addition,
the COVID-19 pandemic has created a widespread labor shortage, including a shortage of medical professionals, and has impacted and may
continue to impact the potential patient participation in our studies which may adversely impact our ability to continue or complete
our clinical trials in the planned timeline.
We
have no manufacturing experience, and the failure to comply with all applicable manufacturing regulations and requirements could have
a materially adverse effect on our business.
We
have never manufactured products in the highly regulated environment of pharmaceutical manufacturing, and our team has limited experience
in the manufacture of drug therapies. There are numerous regulations and requirements that must be maintained to obtain licensure and
permitting required prior to the commencement of manufacturing, as well as additional requirements to continue manufacturing pharmaceutical
products. We currently do not own or lease facilities that could be used to manufacture any products that might be developed by us, and
have contracted with an experienced Contract Manufacturing Organization (“CMO”) to perform the manufacturing of our new product
candidates BIV201 and NE3107. In addition, we do not have the resources at this time to acquire or lease suitable facilities. If we
or our CMO fail to comply with regulations, to obtain the necessary licenses and knowhow or to obtain the requisite financing in order
to comply with all applicable regulations and to own or lease the required facilities in order to manufacture our products, we could
be forced to cease operations, which would cause you to lose all of your investment.
In
addition, the FDA and other regulatory authorities require that product candidates and drug products be manufactured according to cGMP.
Any failure by our third-party manufacturers to comply with cGMP could lead to a shortage of BIV201 and NE3107. In addition, such failure
could be the basis for action by the FDA to withdraw approval, if granted to us, and for other regulatory enforcement action, including
Warning Letters, product seizure, injunction or other civil or criminal penalties.
BIV201
and NE3107 and any other product candidates that we develop may have to compete with other products and product candidates for access
to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that are both capable
of manufacturing for us and willing to do so. If we need to find another source of drug substance or drug product manufacturing for BIV201
and NE3107, we may not be able to identify, or reach agreement with, commercial-scale manufacturers on commercially reasonably terms,
or at all. If we are unable to do so, we will need to develop our own commercial-scale manufacturing capabilities, which would: impact
commercialization of BIV201 and NE3107 in the U.S. and other countries where it may be approved; require a capital investment by us that
could be quite costly; and increase our operating expenses.
If
our existing third-party manufacturers, or the third parties that we engage in the future to manufacture a product for commercial sale
or for our clinical trials, should cease to continue to do so for any reason, we likely would experience significant delays in obtaining
sufficient quantities of product for us to meet commercial demand or to advance our clinical trials while we identify and qualify replacement
suppliers. If for any reason we are unable to obtain adequate supplies of BIV201 or any other product candidate that we develop, or the
drug substances used to manufacture it, it will be more difficult for us to compete effectively, generate revenue, and further develop
our products. In addition, if we are unable to assure a sufficient quantity of the drug for patients with rare diseases or conditions,
we may lose any Orphan Drug exclusivity to which the product otherwise would be entitled.
We
do not currently have the sales and marketing personnel necessary to sell products, and the failure to hire and retain such staff could
have a materially adverse effect on our business.
We
are an early stage development company with limited resources. Even if we had products available for sale, which we currently do not,
we have not secured sales and marketing staff at this early stage of operations to sell products. We cannot generate sales without sales
or marketing staff and must rely on others to provide any sales or marketing services until such personnel are secured, if ever. If we
fail to hire and retain the requisite expertise in order to market and sell our products or fail to raise sufficient capital in order
to afford to pay such sales or marketing staff, then we could be forced to cease operations and you could lose all of your investment.
Even
if we were to successfully develop approvable drugs, we will not be able to sell these drugs if we or our third-party manufacturers fail
to comply with manufacturing regulations, which could have a materially adverse effect on our business.
If
we were to successfully develop approvable drugs, before we can begin selling these drugs, we must obtain regulatory approval of our
manufacturing facility and process or the manufacturing facility and process of the third party or parties with whom we may outsource
our manufacturing activities. In addition, the manufacture of our products must comply with the FDA’s current Good Manufacturing
Practices regulations, commonly known as GMP regulations. The GMP regulations govern quality control and documentation policies and procedures.
Our manufacturing facilities, if any in the future, and the manufacturing facilities of our third-party manufacturers will be continually
subject to inspection by the FDA and other state, local and foreign regulatory authorities, before and after product approval. We cannot
guarantee that we, or any potential third-party manufacturer of our products, will be able to comply with the GMP regulations or other
applicable manufacturing regulations. The failure to comply with all necessary regulations would have a materially adverse effect on
our business and could force us to cease operations and you could lose all of your investment.
We
must comply with significant and complex government regulations, compliance with which may delay or prevent the commercialization of
our product candidates, which could have a materially adverse effect on our business.
The
R&D, manufacture and marketing of drug product candidates are subject to regulation, primarily by the FDA in the United States and
by comparable authorities in other countries. These national agencies and other federal, state, local and foreign entities regulate,
among other things, R&D activities (including testing in animals and in humans) and the testing, manufacturing, handling, labeling,
storage, record keeping, approval, advertising and promotion of the product that we are developing. Noncompliance with applicable requirements
can result in various adverse consequences, including approval delays or refusals to approve drug licenses or other applications, suspension
or termination of clinical investigations, revocation of approvals previously granted, warning letters, fines, criminal prosecution,
recalls or seizures of products, injunctions against shipping drugs and total or partial suspension of production and/or refusal to allow
a company to enter into governmental supply contracts.
The
process of obtaining FDA approval is costly and time consuming. Current FDA requirements for a new human drug or biological product to
be marketed in the United States include, among other things: (a) the successful conclusion of pre-clinical laboratory and animal tests,
if appropriate, to gain preliminary information on the product’s safety; (b) filing with the FDA of an IND application to conduct
human clinical trials for drugs or biologics; (c) the successful completion of adequate and well-controlled human clinical investigations
to establish the safety and efficacy of the product for its recommended use; and (d) filing by a company and acceptance and approval
by the FDA of a NDA for a drug product or a BLA for a biological product to allow commercial distribution of the drug or biologic. A
delay in one or more of the procedural steps outlined above could be harmful to us in terms of getting our product candidates through
clinical testing and to market, which could have a materially adverse effect on our business.
The
FDA, clinical investigators, Data Safety Monitoring Boards, and Institutional Review Boards review the ongoing conduct of, and emerging
safety information from, clinical trials and may order the temporary or permanent discontinuation of clinical trials at any time if it
believes the product candidate exposes clinical subjects to an unacceptable health risk. Investigational drugs used in clinical studies
must be produced in compliance with cGMP rules pursuant to FDA regulations.
Development,
approval, and sales outside the United States of products that we develop will also be subject to regulatory requirements governing human
clinical trials and marketing for drugs and biological products and devices. The requirements vary widely from country to country, but
typically the registration and approval process takes several years and requires significant resources.
If
we experience delays or discontinuations of our clinical trials by the FDA or comparable authorities in other countries, or if we fail
to obtain registration or other approvals of our products or devices then we could be forced to cease our operations and you will lose
all of your investment.
Even
if we are successful in developing BIV201 and NE3107, our product candidates, we have limited experience in conducting or supervising
clinical trials that must be performed to obtain data to submit in concert with applications for approval by the FDA. The regulatory
process to obtain approval for drugs for commercial sale involves numerous steps. Drugs are subjected to clinical trials that allow development
of case studies to examine safety, efficacy, and other issues to ensure that sale of drugs meets the requirements set forth by various
governmental agencies, including the FDA. In the event that our protocols do not meet standards set forth by the FDA, or that our data
is not sufficient to allow such trials to validate our drugs in the face of such examination, we might not be able to meet the requirements
that allow our drugs to be approved for sale which could have a materially adverse effect on our business.
We may face business disruption
and related risks if there is another surge of COVID-19 or if there is another pandemic caused by other bacteria or viruses, which
could have a material adverse effect on our business plan.
Health emergencies or
pandemics, whether from COVID-19 or other viruses or bacteria, may lead to regional quarantines, business shutdowns, labor shortages,
disruptions to supply chains, and overall economic instability, which could materially and adversely affect the clinical trials, supply
chain, financial condition and financial performance of our company. The duration and spread of a pandemic and its long-term impact on
the financial markets and the overall economy are highly uncertain and cannot be predicted. If the financial markets and/or the overall
economy are impacted for an extended period, the Company’s ability to raise funds may be materially adversely affected. In addition,
such health emergencies or pandemics may create a widespread labor shortage, including a shortage of medical professionals, and may impact
potential patient participation in our studies which may adversely impact our ability to continue or complete our clinical trials in
the planned timeline.
Confidentiality
agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information and disclosure
of our trade secrets or proprietary information could compromise any competitive advantage that we have, which could have a materially
adverse effect on our business.
Our
success depends, in part, on our ability to protect our proprietary rights to the technologies used in our product candidates. We depend
heavily upon confidentiality agreements with our officers, employees, consultants and subcontractors to maintain the proprietary nature
of our technology. These measures may not afford us complete or even sufficient protection, and may not afford an adequate remedy in
the event of an unauthorized disclosure of confidential information. If we fail to protect and/or maintain our intellectual property,
third parties may be able to compete more effectively against us, we may lose our technological or competitive advantage, and/or we may
incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property. In addition, others may independently
develop technology similar to ours, otherwise avoiding the confidentiality agreements, or produce patents that would materially and adversely
affect our business, prospects, financial condition and results of operations, in which event you could lose all of your investment.
We
may be unable to obtain or protect intellectual property rights relating to our product candidates, and we may be liable for infringing
upon the intellectual property rights of others, which could have a materially adverse effect on our business.
Our
ability to compete effectively will depend on our ability to maintain the proprietary nature of our technologies. We cannot assure investors
that we will continue to innovate and file new patent applications, or that if filed any future patent applications will result in granted
patents with respect to the technology owned by us or licensed to us. Further, we cannot predict how long it will take for such patents
to issue, if at all. The patent position of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves
complex legal and factual considerations and, therefore, validity and enforceability cannot be predicted with certainty. Patents may
be challenged, deemed unenforceable, invalidated or circumvented.
The Company has also filed a PCT (“Patent
Cooperation Treaty”) application covering our novel liquid formulations of terlipressin (international patent application PCT/US2020/034269
published as WO2020/237170), and we are seeking patent protection in the United States, Europe, China, Japan and at eight other jurisdictions.
We also have fifteen (15) issued U.S. patents, one (1) pending U.S. application, one (1) pending PCT application and six (6) issued foreign
patents directed to protecting NE3107 and related compounds and methods of making and using thereof. However, there can be no assurance
that our pending patent applications will result in issued patents, or that any issued patent claims from pending or future patent applications
will be sufficiently broad to protect BIV201, NE3107, or any other product candidates or to provide us with competitive advantages.
Any patents we do obtain may be challenged
by re-examination or otherwise invalidated or eventually found unenforceable. Both the patent application process and the process of managing
patent disputes can be time consuming and expensive. If we were to initiate legal proceedings against a third party to enforce a patent
related to one of our products, the defendant in such litigation could counterclaim that our patent is invalid and/or unenforceable. In
patent litigation in the U.S., defendant counterclaims alleging invalidity and/or unenforceability are commonplace, as are validity challenges
by the defendant against the subject patent or other patents before the United States Patent and Trademark Office (the “USPTO”).
Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty,
obviousness or non-enablement, failure to meet the written description requirement, indefiniteness, and/or failure to claim patent eligible
subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent
intentionally withheld material information from the USPTO, or made a misleading statement, during prosecution. Additional grounds for
an unenforceability assertion include an allegation of misuse or anticompetitive use of patent rights, and an allegation of incorrect
inventorship with deceptive intent. Third parties may also raise similar claims before the USPTO even outside the context of litigation.
The outcome is unpredictable following legal assertions of invalidity and unenforceability. With respect to the validity question, for
example, we cannot be certain that no invalidating prior art existed of which we and the patent examiner were unaware during prosecution.
These assertions may also be based on information known to us or the Patent Office. If a defendant or third party were to prevail on a
legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the claims of the challenged patent.
Such a loss of patent protection would or could have a material adverse impact on our business.
The standards that the United States Patent
and Trademark Office (and foreign countries) use to grant patents are not always applied predictably or uniformly and can change. There
is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology
patents. Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be
allowed in any patents issued to us or to others.
Further, we rely on
a combination of trade secrets, know-how, technology and nondisclosure, and other contractual agreements and technical measures to protect
our rights in the technology. If any trade secret, know-how or other technology not protected by a patent were to be disclosed to or
independently developed by a competitor, our business and financial condition could be materially adversely affected. The laws of some
foreign countries do not protect our proprietary rights to the same extent as the laws of the U.S., and we may encounter significant
problems in protecting our proprietary rights in these countries.
We do not believe that either BIV201 or NE3107,
the product candidates we are currently developing, infringe upon the rights of any third parties nor are they infringed upon by third
parties. However, there can be no assurance that our technology will not be found in the future to infringe upon the rights of others
or be infringed upon by others. Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication
of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries
were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications
of which we are unaware that may later result in issued patents that our products or product candidates infringe. For example, pending
applications may exist that provide support or can be amended to provide support for a claim that results in an issued patent that our
product infringes. In such a case, others may assert infringement claims against us, and should we be found to infringe upon their patents,
or otherwise impermissibly utilize their intellectual property, we might be forced to pay damages, potentially including treble damages,
if we are found to have willfully infringed on such parties’ patent rights. In addition to any damages we might have to pay, we
may be required to obtain licenses from the holders of this intellectual property. We may fail to obtain any of these licenses or intellectual
property rights on commercially reasonable terms. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our
competitors access to the same technologies licensed to us. In that event, we may be required to expend significant time and resources
to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected products,
which could materially harm our business and the third parties owning such intellectual property rights could seek either an injunction
prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation. Conversely,
we may not always be able to successfully pursue our claims against others that infringe upon our technology. Thus, the proprietary nature
of our technology or technology licensed by us may not provide adequate protection against competitors.
The pharmaceutical industry is characterized
by extensive litigation regarding patents and other intellectual property rights. Moreover, the cost to us of any litigation or other
proceeding relating to our patents and other intellectual property rights, even if resolved in our favor, could be substantial, and the
litigation would divert our management’s efforts. We may not have sufficient resources to bring any such action to a successful
conclusion. Uncertainties resulting from the initiation and continuation of any litigation could limit our ability to continue our operations
and you could lose all of your investment.
We
depend upon our management and their loss or unavailability could put us at a competitive disadvantage which could have a material adverse
effect on our business.
We currently depend upon the efforts and abilities
of our executive management team of Cuong Do, our Chief Executive Officer - President; Wendy Kim, our Senior Vice President - Chief Financial
Officer; Dr Joseph Palumbo, our Executive Vice President -Chief Medical Officer; Penelope Markham, Senior Vice President – Ascites
Programs & Strategic Initiatives; Chris Reading, our Senior Vice President -Alzheimer’s’ Programs; Mr. Clarence Ahlem,
our Senior Vice President – Operations Development; Steven White, our Senior Vice President – Discovery; and David Morse,
our Senior Vice President – Chief Regulatory Officer; who all serve the Company the full-time. The loss or unavailability of the
services of any of these individuals for any significant period of time could have a material adverse effect on our business, prospects,
financial condition and results of operations which may cause you to lose all of your investment. We have not obtained, do not own, nor
are we the beneficiary of key-person life insurance.
We
may not be able to attract and retain highly skilled personnel, which could have a materially adverse effect on our business.
Our
ability to attract and retain highly skilled personnel is critical to our operations and expansion. We face competition for these types
of personnel from other pharmaceutical companies and more established organizations, many of which have significantly larger operations
and greater financial, technical, human and other resources than us. We may not be successful in attracting and retaining qualified personnel
on a timely basis, on competitive terms, or at all. If we are not successful in attracting and retaining these personnel, our business,
prospects, financial condition and results of operations will be materially and adversely affected.
The
biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition.
We may be unable to compete with enterprises equipped with more substantial resources than us, which could cause us to curtail or cease
operations.
The
biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition
based primarily on scientific and technological factors. These factors include the availability of patent and other protection for technology
and products, the ability to commercialize technological developments and the ability to obtain government approval for testing, manufacturing
and marketing.
We
compete with biopharmaceutical firms in the United States, Europe and elsewhere, as well as a growing number of large pharmaceutical
companies that are applying biotechnology to their operations. Many biopharmaceutical companies have focused their development efforts
in the human therapeutics area. Many major pharmaceutical companies have developed or acquired internal biotechnology capabilities or
made commercial arrangements with other biopharmaceutical companies. These companies, as well as academic institutions, government agencies
and private research organizations, also compete with us in recruiting and retaining highly qualified scientific personnel and consultants.
Our ability to compete successfully with other companies in the pharmaceutical field will also depend to a considerable degree on the
continuing availability of capital to us.
Although
there are not currently any therapies approved by the FDA specifically for the treatment of ascites due to liver cirrhosis, we still
face significant competitive and market risk. Other companies, such as Mallinckrodt Inc., are developing therapies for severe complications
of advanced liver cirrhosis, which may in the future be developed for the treatment of ascites, and these therapies could compete indirectly
or directly with our product candidate. Similarly, other companies, such as Biogen and Eli Lilly, are developing treatments for Alzheimer’s
Disease and Parkinson’s Disease, which could compete indirectly or directly with our product candidate. There may be other competitive
development programs of which we are unaware. Even if our product candidates are ultimately approved by the FDA, there is no guarantee
that once it is on the market doctors will adopt them in favor of current ascites treatment procedures such as diuretics and paracentesis
with respect to BIV201 and Alzheimer’s Disease and Parkinson’s Disease with respect to NE3107. These competitive and market
risks could have a material adverse effect on our business, prospects, financial condition and results of operations which may cause
you to lose all of your investment.
Our
competition will be determined in part by the potential indications for which drugs are developed and ultimately approved by regulatory
authorities. Additionally, the timing of the market introduction of some of our potential product candidate or of competitors’
products may be an important competitive factor. Accordingly, the relative speed with which we can develop drugs, complete pre-clinical
testing, clinical trials, approval processes and supply commercial quantities to market are important competitive factors. We expect
that competition among drugs approved for sale will be based on various factors, including product efficacy, safety, reliability, availability,
price and patent protection.
The
successful development of biopharmaceuticals is highly uncertain. A variety of factors including, pre-clinical study results or regulatory
approvals, could cause us to abandon the development of our product candidates.
Successful
development of biopharmaceuticals is highly uncertain and is dependent on numerous factors, many of which are beyond our control.
Product
candidates that appear promising in the early phases of development may fail to reach the market for several reasons. Pre-clinical study
results may show the product candidate to be less effective than desired (e.g., the study failed to meet its primary endpoints) or to
have harmful or problematic side effects. Product candidates may fail to receive the necessary regulatory approvals or may be delayed
in receiving such approvals. Among other things, such delays may be caused by slow enrollment in clinical studies, length of time to
achieve study endpoints, additional time requirements for data analysis or a IND and later NDA, preparation, discussions with the FDA,
an FDA request for additional pre-clinical or clinical data or unexpected safety or manufacturing issues; manufacturing costs, pricing
or reimbursement issues, or other factors that make the product not economical. Proprietary rights of others and their competing products
and technologies may also prevent the product from being commercialized.
Success
in pre-clinical and early clinical studies does not ensure that large-scale clinical studies will be successful. Clinical results are
frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. The length of time necessary
to complete clinical studies and to submit an application for marketing approval for a final decision by a regulatory authority varies
significantly from one product to the next, and may be difficult to predict. There can be no assurance that any of our products will
develop successfully, and the failure to develop our products will have a materially adverse effect on our business and will cause you
to lose all of your investment.
There
may be conflicts of interest among our officers, directors and stockholders.
Certain of our executive officers and directors
and their affiliates are engaged in other activities and have interests in other entities on their own behalf or on behalf of other persons.
Neither we nor any of our shareholders will have any rights in these ventures or their income or profits. In particular, our executive
officers or directors or their affiliates may have an economic interest in or other business relationship with partner companies that
invest in us or are engaged in competing drug development. Our executive officers or directors may have conflicting fiduciary duties to
us and third parties. The terms of transactions with third parties may not be subject to arm’s length negotiations and therefore
may be on terms less favorable to us than those that could be procured through arm’s length negotiations. Although we have established
an audit committee comprised solely of independent directors to oversee transactions between us and our insiders, we do not have any formal
policies in place to deal with such conflicting fiduciary duties should such a conflict arise.
If we fail to maintain an effective
system of internal controls, we may not be able to accurately report our financial results or detect fraud. Consequently, investors could
lose confidence in our financial reporting and this may decrease the trading price of our Common Stock.
We must maintain effective internal controls to
provide reliable financial reports and detect fraud. We have concluded that our disclosure controls and procedures internal controls,
as well as internal controls over financial reporting, are effective. Failure to implement changes to our internal controls or any others
that we identify as necessary to establish an effective system of internal controls could harm our operating results and cause investors
to lose confidence in our reported financial information. Any such loss of confidence would have a negative effect on the trading price
of our Common Stock.
We indemnify our officers and directors
against liability to us and our security holders, and such indemnification could increase our operating costs.
Our articles of incorporation and bylaws require
us to indemnify our officers and directors against claims associated with carrying out the duties of their offices. We are also required
to advance the costs of certain legal defenses upon the indemnitee undertaking to repay such expenses to the extent it is determined that
such person was not entitled to indemnification of such expenses. Insofar as indemnification for liabilities arising under the Securities
Act may be permitted to our officers, directors, or control persons, the SEC has advised that such indemnification is against public
policy and is therefore unenforceable.
RISKS RELATING TO OUR COMMON STOCK
You may experience future dilution as a
result of future equity offerings or if we issue shares subject to options, warrants, stock awards or other arrangements.
In order to raise additional capital, we may in
the future offer additional shares of our Common Stock or other securities convertible into or exchangeable for our Common Stock, including
under the Controlled Equity Offering Sales Agreement (the “Sales Agreement”), dated as of August 31, 2022, with Cantor Fitzgerald
& Co. and B. (the “Agent”), pursuant to which the Company may issue and sell from time to time shares of Common Stock
through the Agent. We may sell shares or other securities in any other offering at a price per share that is less than the current market
price of our securities, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders.
The sale of additional shares of Common Stock or other securities convertible into or exchangeable for our Common Stock would dilute all
of our stockholders, and if such sales of convertible securities into or exchangeable into our Common Stock occur at a deemed issuance
price that is lower than the current exercise price of our outstanding warrants sold to Acuitas Group Holdings, LLC (“Acuitas”)
in August 2022, the exercise price for those warrants would adjust downward to the deemed issuance price pursuant to price adjustment
protection contained within those warrants.
In addition, as of June 30, 2023, there were
warrants outstanding to purchase an aggregate of 7,770,285 shares of Common Stock at exercise prices ranging from $1.82 to $12.50
per share and 3,952,864 shares issuable upon exercise of outstanding options at exercise prices ranging from $1.69 to $42.09 per
share and restricted stock units totaling 596,457. Our Loan Agreement entered into on November 30, 2021 contains a conversion feature
whereby at the option of lender, up to $5 million of the outstanding loan amount may be converted into shares of Common Stock at a conversion
price of $6.98 per share. We may grant additional options, warrants or equity awards. To the extent such shares are issued, the interest
of holders of our Common Stock will be diluted.
Moreover, we are obligated to issue shares of
Common Stock upon achievement of certain clinical, regulatory and commercial milestones with respect to certain of our drug candidates
(i.e., NE3107, NE3291, NE3413, and NE3789) pursuant to the asset purchase agreement, dated April 27, 2021, by and among the Company, NeurMedix,
Inc. and Acuitas, as amended on May 9, 2021. The achievement of these milestones could result in the issuance of up to 18 million shares
of our Common Stock, further diluting the interest of holders of our Common Stock.
Certain
stockholders who are also officers and directors of the Company may have significant control over our management.
As of August 9, 2023, our directors and executive
officers and affiliates currently own aggregate 23,587,296 shares of our Common Stock, which currently constitutes 64.0% of our issued
and outstanding Common Stock. As a result, directors and executive officers and affiliates may have a significant influence on our affairs
and management, as well as on all matters requiring member approval, including electing and removing members of our Board of Directors,
causing us to engage in transactions with affiliates entities, causing or restricting our sale or merger, and certain other matters. Our
majority shareholder, Mr. Terren Peizer, may be deemed to beneficially own the 23,166,210 shares of Common Stock held by Acuitas,
which constitutes 63.0% of our issued and outstanding Common Stock Such concentration of ownership and control could have the effect of
delaying, deferring or preventing a change in control of us even when such a change of control would be in the best interests of our stockholders.
We may, in the future, issue additional
Common Stock, which would reduce investors’ percent of ownership and may dilute our share value.
As of June 30, 2023, our Articles of Incorporation,
as amended, authorize the issuance of 800,000,000 shares of Common Stock, and we had 36,451,829 shares of Common Stock issued and
36,428,949 issued and outstanding. Accordingly, we may issue up to an additional 763,548,171 shares of Common Stock. The future issuance
of Common Stock may result in substantial dilution in the percentage of our Common Stock held by our then existing stockholders. We may
value any Common Stock in the future on an arbitrary basis. The issuance of Common Stock for future services or acquisitions or other
corporate actions may have the effect of diluting the value of the shares held by our investors, might have an adverse effect on any trading
market for our Common Stock and could impair our ability to raise capital in the future through the sale of equity securities.
The market price and trading volume of our
Common Stock may be volatile.
The market price and trading volume of our Common
Stock has been volatile. We expect that the market price of our Common Stock will continue to fluctuate significantly for many reasons,
including in response to the risk factors described in this prospectus or for reasons unrelated to our specific performance. In recent
years, the stock market has experienced extreme price and volume fluctuations. This volatility has affected the market prices of securities
issued by many companies for reasons unrelated to their operating performance and may adversely affect the market price and trading volume
of our Common Stock. Prices for our Common Stock may also be influenced by the depth and liquidity of the market for our Common Stock,
investor perceptions about us and our business, our future financial results, the absence of cash dividends on our Common Stock and general
economic and market conditions. In the past, securities class action litigation has often been instituted against companies following
periods of volatility in their stock price. This type of litigation could result in substantial costs and could divert our management
and other resources.
We have a large number of restricted shares
outstanding, a portion of which may be sold under Rule 144 which may reduce the market price of our shares.
As of August 9, 2023, there were 36,803,768 shares
of Common Stock issued and outstanding, of which 13,166,847 shares are held by non-affiliates and 23,587,296 held by affiliates of the
Company, consisting of an affiliate who owns 23,166,210 shares and 421,086 shares owned by our officers and directors or entities controlled
by them. The majority of our Common Stock, including all of the affiliates’ securities are deemed “restricted securities”
within the meaning of Rule 144 as promulgated under the Securities Act.
It is anticipated that all of the “restricted
securities” will be eligible for resale under Rule 144. In general, under Rule 144, subject to the satisfaction of certain other
conditions, a person, who is not an affiliates (and who has not been an affiliates for a period of at least three months immediately preceding
the sale) and who has beneficially owned restricted shares of our Common Stock for at least six months is permitted to sell such shares
without restriction, provided that there is sufficient public information about us as contemplated by Rule 144. An affiliate who has beneficially
owned restricted shares of our Common Stock for a period of at least one year may sell a number of shares equal to one percent of our
issued and outstanding Common Stock approximately every three months.
Any
failure to maintain effective internal control over financial reporting could harm us.
Our
management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over
financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation
of financial statements in accordance with U.S. generally accepted accounting principles (“GAAP”). Under standards established
by the Public Company Accounting Oversight Board (“PCAOB”), a deficiency in internal control over financial reporting exists
when the design or operation of a control does not allow management or personnel, in the normal course of performing their assigned functions,
to prevent or detect misstatements on a timely basis. The PCAOB defines a material weakness as a deficiency, or combination of deficiencies,
in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of annual or interim
financial statements will not be prevented, or detected and corrected, on a timely basis.
If we are unable to assert that our internal
control over financial reporting is effective, or when required in the future, if our independent registered public accounting firm is
unable to express an unqualified opinion as to the effectiveness of our internal control over financial reporting, investors may lose
confidence in the accuracy and completeness of our financial reports, the market price of our Common Stock could be adversely affected
and we could become subject to litigation or investigations by the stock exchange on which our securities are listed, the SEC or other
regulatory authorities, which could require additional financial and management resources.
There is a limited trading market for
our common stock, which could make it difficult to liquidate an investment in our common stock, in a timely manner.
Our common stock is currently traded on the Nasdaq Capital Market.
Because there is a limited public market for our common stock, investors may not be able to liquidate their investment whenever desired.
We cannot assure that there will be an active trading market for our common stock and the lack of an active public trading market could
mean that investors may be exposed to increased risk. In addition, if we failed to meet the criteria set forth in SEC regulations, various
requirements would be imposed by law on broker dealers who sell our securities to persons other than established customers and accredited
investors. Consequently, such regulations may deter broker-dealers from recommending or selling our common stock, which may further affect
its liquidity.
The
lack of public company experience of our management team could adversely impact our ability to comply with the reporting requirements
of U.S. securities laws, which could have a materially adverse effect on our business.
Our
officers have limited public company experience, which could impair our ability to comply with legal and regulatory requirements such
as those imposed by Sarbanes-Oxley Act of 2002. Such responsibilities include complying with federal securities laws and making required
disclosures on a timely basis. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our
ability to comply with the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”),
which is necessary to maintain our public company status. If we were to fail to fulfill those obligations, our ability to continue as
a U.S. public company would be in jeopardy in which event you could lose your entire investment in our Company.
We are considered a smaller reporting company
that is exempt from certain disclosure requirements, which could make our stock less attractive to potential investors.
Rule
12b-2 of the Exchange Act defines a “smaller reporting company” as an issuer that is not an investment company, an asset-backed
issuer, or a majority-owned subsidiary of a parent that is not a smaller reporting company and that:
| |
● |
Had
a public float of less than $250 million as of the last business day of its most recently completed fiscal quarter, computed by multiplying
the aggregate number of worldwide number of shares of its voting and non-voting common equity held by non-affiliates by the price
at which the common equity was last sold, or the average of the bid and asked prices of common equity, in the principle market for
the common equity; or |
| |
● |
In
the case of an initial registration statement under the Securities Act or the Exchange Act for shares of its common equity, had a
public float of less than $250 million as of a date within 30 days of the date of the filing of the registration statement, computed
by multiplying the aggregate worldwide number of such shares held by non-affiliates before the registration plus, in the case of
a Securities Act registration statement, the number of such shares included in the registration statement by the estimated public
offering price of the shares; or |
| |
● |
In
the case of an issuer who had annual revenue of less than $100 million during the most recently completed fiscal year for which audit
financial statements are available, had a public float as calculated under paragraph (1) or (2) of this definition that was either
zero or less than $700 million. |
As
a “smaller reporting company” we are not required and may not include a Compensation Discussion and Analysis (“CD&A”)
section in our proxy statements; we provide only 3 years of business development information; and have other “scaled” disclosure
requirements that are less comprehensive than issuers that are not “smaller reporting companies” which could make our stock
less attractive to potential investors, which could make it more difficult for you to sell your shares.
We
are subject to the periodic reporting requirements of the Exchange Act, which require us to incur audit fees and legal fees in connection
with the preparation of such reports. These additional costs will negatively affect our ability to earn a profit.
We
are required to file periodic reports with the SEC pursuant to the Exchange Act and the rules and regulations thereunder. In order to
comply with such requirements, our independent registered auditors have to review our financial statements on a quarterly basis and audit
our financial statements on an annual basis. Moreover, our legal counsel has to review and assist in the preparation of such reports.
Factors such as the number and type of transactions that we engage in and the complexity of our reports cannot accurately be determined
at this time and may have a major negative effect on the cost and amount of time to be spent by our auditors and attorneys. However,
the incurrence of such costs is an expense to our operations and thus has a negative effect on our ability to meet our overhead requirements
and earn a profit.
Because
we do not intend to pay any cash dividends on our Common Stock, our stockholders will not be able to receive a return on their shares
unless they sell them.
We
intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends
on our Common Stock in the foreseeable future. Unless we pay dividends, our stockholders will not be able to receive a return on their
shares unless they sell them. There is no assurance that stockholders will be able to sell shares when desired.
We
are authorized to issue “blank check” preferred stock without stockholder approval, which could adversely impact the rights
of holders of our securities.
Our articles of incorporation authorize us
to issue up to 10,000,000 shares of blank check preferred stock. Any preferred stock that we issue in the future may rank ahead of our
common stock in terms of dividend priority or liquidation premiums and may have greater voting rights than our common stock. Any preferred
stock issued may contain provisions allowing those shares to be converted into shares of common stock, which could dilute the value of
our common stock to current stockholders and could adversely affect the market price, if any, of our common stock. The preferred stock
could be utilized, under certain circumstances, as a method of discouraging, delaying, or preventing a change in control of our company.
Although we have no present intention to issue any shares of our authorized preferred stock, there can be no assurance that we will not
do so in the future.
Provisions in our articles of incorporation,
our bylaws, and Nevada law might discourage, delay or prevent a change in control of our company or changes in our management and, therefore,
depress the trading price of our common stock.
Provisions of our articles of incorporation,
our bylaws, and Nevada law may have the effect of deterring unsolicited takeovers or delaying or preventing a change in control of our
company or changes in our management, including transactions in which our stockholders might otherwise receive a premium for their shares
over then current market prices. In addition, these provisions may limit the ability of stockholders to approve transactions that they
may deem to be in their best interests. These provisions include:
| |
● |
the inability of stockholders to call special meetings; |
| |
● |
the “business combinations” and “control share acquisitions” provisions of Nevada law, to the extent applicable, could discourage attempts to acquire our stockholders stock even on terms above the prevailing market price; and |
| |
● |
the ability of our board of directors to designate the terms of and issue new series of preferred stock without stockholder approval, which could include the right to approve an acquisition or other change in our control or could be used to institute a rights plan, also known as a poison pill, that would dilute the stock ownership of a potential hostile acquirer, likely preventing acquisitions that have not been approved by our board of directors. |
The existence of the forgoing provisions
and anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock.
They could also deter potential acquirers of our company, thereby reducing the likelihood that you could receive a premium for your common
stock in an acquisition.
| ITEM
1B. |
UNRESOLVED
STAFF COMMENTS |
None.
The
Company paid an annual rent of $2,200 for its headquarters at 680 W Nye Lane, Suite 201, Carson City Nevada 897603. The rental agreement
is for a one-year term and commenced on October 1, 2022.
On
February 26, 2022, the Company’s San Diego office relocated to 5090 Shoreham Place, San Diego, CA 92122. The term for the new office
lease is 38 months and commenced on March 1, 2022. The monthly base rate of $4,175 began June 1, 2022, with annual increases of
three percent.
| ITEM
3. |
LEGAL
PROCEEDINGS |
To
our knowledge, neither the Company nor any of our officers or directors is a party to any material legal proceeding or litigation and
such persons know of no material legal proceeding or contemplated or threatened litigation, other than as described below. There are
no judgments against us or our officers or directors. None of our officers or directors has been convicted of a felony or misdemeanor
relating to securities or performance in corporate office.
| ITEM
4. |
MINE
SAFETY DISCLOSURES |
None.
PART
II
| ITEM
5. |
MARKET
FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Unregistered Sales of Securities
All sales of unregistered securities during the
year ended June 30, 2023 were previously disclosed in a Quarterly Report on Form 10-Q or Current Report on Form 8-K.
Issuer Purchases of Common Stock
During the year ended June 30, 2023, there were
no issuer repurchases of shares of Common Stock.
| ITEM
7. |
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The
following discussion of the Company’s financial condition and the results of operations should be read in conjunction with the
Financial Statements and Notes thereto appearing elsewhere in this report.
Overview
BioVie
Inc. (the “Company” or “we” or “our”) is a clinical-stage company developing innovative drug therapies
to treat chronic debilitating conditions including neurological and neuro-degenerative disorders and liver disease.
The
Company acquired the biopharmaceutical assets of NeurMedix, Inc. (“NeurMedix”), a privately held clinical-stage
pharmaceutical company, in June 2021 (See Note 6 Related Party Transactions). The acquired assets included NE3107, a
potentially selective inhibitor of inflammatory extracellular single-regulated kinase (“ERK”) signaling that, based on
animal studies, is believed to reduce neuroinflammation. NE3107 is a novel orally administered small molecule that is thought to
inhibit inflammation-driven insulin resistance and major pathological inflammatory cascades with a novel mechanism of action. There
is emerging scientific consensus that both inflammation and insulin resistance may play fundamental roles in the development of
Alzheimer’s Disease (AD) and Parkinson’s Disease (PD), and NE3107 could, if approved represent an entirely new medical
approach to treating these devastating conditions affecting an estimated 6 million Americans suffering from AD and 1 million
Americans suffering from PD. In August 2021, the Company initiated the FDA authorized potentially pivotal Phase 3 randomized,
double-blind, placebo-controlled, parallel group, multicenter study to evaluate NE3107 in subjects who have mild to moderate AD
(NCT04669028). The Company is targeting primary completion of this study in the fourth quarter of calendar year 2023.
The Phase 2 study of NE3107 in Parkinson’s
disease (“PD”) (NCT05083260), completed in December 2022 was a double-blind, placebo-controlled, safety, tolerability, and
pharmacokinetics study in PD participants treated with carbidopa/levodopa and NE3107. Forty-five patients with a defined L-dopa “off
state” were randomized 1:1 to placebo:NE3107 20 mg twice daily for 28 days. This trial was launched with two design objectives:
1) the primary objective was safety and a drug-drug interaction study as requested by the FDA to demonstrate the absence of adverse interactions
of NE3107 with levodopa; and 2) the secondary objective is to determine if preclinical indications of promotoric activity and apparent
enhancement of levodopa activity can be seen in humans. Both objectives were met. The Company continues to process its findings from its
completed study as it prepares for the next round of clinical studies in PD.
Neuroinflammation, insulin resistance, and oxidative
stress are common features in the major neurodegenerative diseases, including AD, PD, frontotemporal lobar dementia, and Amyotrophic
lateral sclerosis (ALS). NE3107 is an oral small molecule, blood-brain permeable, compound with potential anti-inflammatory, insulin
sensitizing, and ERK-binding properties that may allow it to selectively inhibit ERK-, NFκB- and TNF-stimulated inflammation. NE3107’s
potential to inhibit neuroinflammation and insulin resistance forms the basis for the Company’s work testing the molecule in AD
and PD patients. NE3107 is patented in the United States, Australia, Canada, Europe and South Korea.
The Company’s Orphan drug candidate BIV201
(continuous infusion terlipressin), with FDA Fast Track status, is being evaluated in a U.S. Phase 2b study (NCT04112199) for the treatment
of refractory ascites due to liver cirrhosis. In March 2023, the Company announced enrollment was paused and that data from the first
15 patients treated with BIV201 plus SOC appeared to result in a 34% reduction in ascites fluid during the 28 days after treatment initiation
compared to the 28 days prior to treatment (p=0.0046). This improvement was significantly different from those treated with SOC only who
experienced a mean increase in ascites fluid of 3.1% (BIV201 vs. SOC p=0.05). Patients who completed the treatment with BIV201 experienced
a 53% reduction in ascites fluid (p=0.001), which was significantly different from those treated with SOC (p=0.007). This improvement
was sustained in this group during the three months after treatment initiation as compared to the three-month pre-treatment period (43%
reduction, p=0.06). There were no unexpected serious adverse events and overall safety was consistent with the patient population. Terlipressin
was administered with a continuous low dose infusion via a portable pump in two 28-day treatment cycles. The primary endpoints are the
incidence of complications of at least Grade 2 severity, and the change in cumulative ascites in the 12-week period following randomization
compared to a 12-week pre-treatment period. The BIV201 trial planned to enroll 30 patients to be treated in the home care setting. The
Company requested and has been granted a meeting with the FDA to discuss the design and endpoints for definitive clinical testing of BIV201
for the treatment of ascites due to chronic liver cirrhosis. The active agent is approved in the U.S. and in about 40 countries for related
complications of advanced liver cirrhosis.
Results
of Operations
Comparison
of the Year Ended June 30, 2023 to the Year Ended June 30, 2022
Net
loss
The net loss for the year ended June 30, 2023
was approximately $50.3 million as compared to net loss of $26.1 million for the year ended June 30, 2022. The increase in net loss of
approximately $24.2 million was primarily due to increased clinical activities of approximately $16.0 million, administrative expenses
of approximately $1.8 million, an increase in other expense of approximately $6.3 million primarily attributed to the change in fair value
of derivative liabilities of approximately $4.7 million.
Total operating expenses for the year ended June
30, 2023, were approximately $45.1 million as compared to $27.3 million for the year ended June 30, 2022. The net increase of approximately
$17.8 million was due to an increase in research and development expenses of approximately $16.0 million due to our increased clinical
activities, and an increase in selling general and administrative expenses of approximately $1.8 million.
Research and Development Expenses
Research and development expenses were approximately
$33.3 million and $17.3 million for the year ended June 30, 2023, and 2022, respectively. The net increase of approximately $16.0 million,
was attributed to increased activities in our clinical studies of approximately $14,3 million; increased expenses in Chemistry, Manufacturing
and Control of approximately $344,000, and increased publications and conferences expenses of approximately $273,000, as we published
our posters for various congresses that we participated; and an increase compensation expense of approximately $1.3 million. During the
year we added for employees including a SVP Discovery, SVP Chief Regulatory Officer, VP of Clinical & Medical Affairs and a VP Safety
& Pharmacovigilance.
$14.1 million of the increase in research
and development expenses of $16.0 million was primarily due to the Neuroscience NE3107 studies, which were significantly more active during
the year ended June 30, 2023 compared to the year ended June 30, 2022. The Parkinson’s Phase 2 study initiated in January 2022,
completed and reported its top-line data results in December 2022, and the Alzheimer Phase 3 study reached full enrollment in November
2022. Our Orphan drug candidate BIV201’s Phase 2b study, which was initiated in June 2021, accounted for approximately $143,000
of the net increase in research and development expenses for the year ended June 30, 2023.
Selling, General and Administrative Expenses
Selling, general and administrative expenses
were approximately $11.6 million and $9.8 million for the year ended June 30, 2023, and 2022, respectively. The net increase of approximately
$1.8 million was primarily attributed to increased stock compensation expense of approximately $1.1 million related to the board of directors’
annual compensation; a net increase in legal, investor relations and other professional fees totaling approximately $405,000, an increase
in management compensation expense of approximately $115,000, an increase in business development and fund raising activities of approximately
$107,000 and increase in insurance expense of approximately $77,000.
Other expense/income, net
Other expense, net was $5.2 million compared
to other income, net of $1.2 million, for the year ended June 30, 2023 and 2022, respectively. The net increase in other expenses of $6.4
million represented an increase in interest expense of approximately $2.1 million and the change in fair value of the related derivative
liabilities of approximately $4.7 million, offset by increase in interest income of approximately $518,000 from investments in U.S. Treasury
Bills.
Capital Resources and Liquidity
As of June 30, 2023 the Company had working capital
of approximately $19.5 million, cash and cash equivalents and U.S. treasury bills totaling of approximately $33.9 million, stockholders’
equity of approximately $15.3 million, and an accumulated deficit of approximately $301 million. In addition, the Company has not generated
any revenues to date and no revenues are expected in the foreseeable future. The Company’s future operations are dependent on the
success of the Company’s ongoing development and commercialization efforts, as well as its ability to secure additional financing
as needed.
During the year ended June 30, 2023, the Company
sold approximately 7.5 million shares of its Common Stock under its Controlled Equity Offering Sales Agreement with Cantor Fitzgerald
& Co for total net proceeds of approximately $49.5 million after 3% commissions and cost totaling approximately $2 million.
The Company has not generated any revenue and
no revenues are expected in the foreseeable future. The Company’s future operations are dependent on the success of the Company’s
ongoing development and commercialization efforts, as well as its ability to secure additional financing. Management expects that
future sources of funding may include sales of equity, obtaining loans, or other strategic transactions.
Although
management continues to pursue the Company’s strategic plans, there is no assurance that the Company will be successful in obtaining
sufficient financing on terms acceptable to the Company, if at all, to fund continuing operations. These circumstances raise substantial
doubt on the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might
result from the outcome of this uncertainty.
Recently
Issued Accounting Pronouncement
In
June 2016, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-13, “Financial Instruments - Credit
Losses (Topic 326), Measurement of Credit Losses on Financial Instruments.” This amendment replaces the incurred loss impairment
methodology in current GAAP with a methodology that reflects expected credit losses on instruments within its scope, including trade
receivables. This update is intended to provide financial statement users with more decision-useful information about the expected credit
losses. In November 2019, the FASB issued No. 2019-10, Financial Instruments --Credit Losses (Topic 326), Derivatives and Hedging (Topic
815), and Leases (Topic 842), which deferred the effective date of ASU 2016-13 for Smaller Reporting Companies for fiscal years beginning
after December 15, 2022, including interim periods within those fiscal years. The Company does not expect a material impact from the
adoption of ASU 2016-13 on the financial statements.
Off-Balance
Sheet Arrangements
The
term “off-balance sheet arrangement” generally means any transaction, agreement or other contractual arrangement to which
an entity unconsolidated with the Company is a party, under which the Company has (i) any obligation arising under a guarantee contract,
derivative instrument or variable interest; or (ii) a retained or contingent interest in assets transferred to such entity or similar
arrangement that serves as credit, liquidity or market risk support for such assets. The Company has no off-balance sheet arrangements
that have or are reasonably likely to have a current or future effect or change on the Company’s financial condition, revenues
or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Critical
Accounting Policies and Estimates
Cash
and cash equivalents
Cash and cash equivalents consisted of cash deposits
and money market funds held at a bank and funds held in a brokerage account which included a U.S. treasury money market fund and U.S.
Treasury Bills with original maturities of three months or less.
Concentration of Credit Risk in the Financial
Service Industry
As
of June 30, 2023, the Company had cash deposited in certain financial institutions in excess of federally insured levels. The Company
regularly monitors the financial stability of these financial institutions and believes that it is not exposed to any significant credit
risk in cash and cash equivalents. However, in March and April 2023, certain U.S. government banking regulators took steps to intervene
in the operations of certain financial institutions due to liquidity concerns, which caused general heightened uncertainties in financial
markets. While these events have not had a material direct impact on the Company’s operations, if further liquidity and financial
stability concerns arise with respect to banks and financial institutions, either nationally or in specific regions, the Company’s
ability to access cash or enter into new financing arrangements may be threatened, which could have a material adverse effect on its
business, financial condition and results of operations.
Investments
in U.S. Treasury Bills
Investments in U.S. Treasury Bills with maturities
greater than three months, are accounted for as available for sale and are recorded at fair value. Unrealized gains were included in other
comprehensive income in the accompanying the statements of operations and comprehensive loss.
Accounting
for Stock-based Compensation
The
Company follows the provision of ASC 718- Stock Compensation, which requires the measurement of compensation expense for all shared –
based payment awards made to employees and non-employee director, including employee stock options. Share-based compensation expense
is based on the grant date fair value estimated in accordance with the provisions of ASC 718 and is generally recognized as an expense
over the requisite service period, net of forfeitures.
Impairment
of Long-Lived Assets
Long-lived
assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not
be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of the assets to the
future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment
to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets and would
be charged to earnings.
Purchase
Accounting for Transactions with Related Party
Purchase
accounting for transactions with related party, entities under common control, are recorded at the historical carrying cost with no step
up in basis to the fair market value of the asset or liability are recognized.
Leases
The Company determines whether an arrangement
contains a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, current portion
of operating lease liabilities, and net of current portion of operating lease liabilities on our balance sheets. ROU assets represent
the Company’s right to use an underlying asset for the lease term and lease liabilities represent an obligation to make lease payments
arising from the lease. Lease ROU assets and lease liabilities are recognized based on the present value of the future minimum lease payments
over the lease term at the commencement date. As the Company’s leases do not provide an implicit rate, an incremental borrowing
rate is used based on the information available at the commencement date in determining the present value of lease payments. The Company
does not include options to extend or terminate the lease term unless it is reasonably certain that the Company will exercise any such
options. Rent expense is recognized under the operating leases on a straight-line basis. The Company does not recognize right of-use assets
or lease liabilities for short-term leases, which have a lease term of 12 months or less, and instead will recognize lease payments as
expense on a straight-line basis over the lease term.
Fair
value measurement of assets and liabilities
We
determine the fair values of our financial instruments based on the fair value hierarchy, which requires an entity to maximize the use
of observable inputs and minimize the use of unobservable inputs when measuring fair value. Fair value is defined as the price that would
be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement
date. The fair value assumes that the transaction to sell the asset or transfer the liability occurs in the principal or most advantageous
market for the asset or liability and establishes that the fair value of an asset or liability shall be determined based on the assumptions
that market participants would use in pricing the asset or liability. The classification of a financial asset or liability within the
hierarchy is based upon the lowest level input that is significant to the fair value measurement. The fair value hierarchy prioritizes
the inputs into three levels that may be used to measure fair value:
Level
1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities.
Level
2 - Inputs are quoted prices for similar assets and liabilities in active markets or inputs that are observable for the asset or liability,
either directly or indirectly through market corroboration, for substantially the full term of the financial instrument.
Level
3 - Inputs are unobservable inputs based on our assumptions.
| ITEM
7A. |
QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Not
applicable.
| ITEM
8. |
FINANCIAL
STATEMENTS |
Our
financial information required to be filed hereunder are indexed under Item 15 of this report and are incorporated herein by reference.
| ITEM
9. |
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES |
Not
applicable.
| ITEM
9A. |
CONTROLS
AND PROCEDURES |
Evaluation
of Disclosure Controls and Procedures
We
have evaluated, with the participation of our principal executive and our principle financial officer, the effectiveness of our disclosure
controls and procedures as defined in Rules 13a-15(e) and 15(d)-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange
Act”) as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our principal executive
officer and our principal financial officer have concluded that our disclosure controls and procedures were effective to ensure that
information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized
and reported, within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management,
including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow
timely decisions regarding required disclosure.
Management’s
Report on Internal Control Over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f)
and 15d-15(f) under the Exchange Act. Because of its inherent limitations, internal control over financial reporting may not prevent
or detect misstatements. Projections of any evaluation of the effectiveness of internal control to future periods are subject to the
risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures
may deteriorate. Under the supervision and with the participation of our management, including our Chief Executive Officer and
Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of June
30, 2023 using the criteria established in Internal Control Integrated Framework (“2013 Framework”) issued by the Committee
of Sponsoring Organization of the Treadway Commission (“COSO”). Based on our evaluation using those criteria, our management
has concluded that, as of June 30, 2023, our internal control over financial reporting was effective to provide reasonable assurance
regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with
generally accepted accounting principles for the reasons discussed above.
Changes
in Internal Control Over Financial Reporting
There
were no changes in our internal controls over financial reporting during quarter ended June 30, 2023, that materially affected, or are
reasonably likely to materially affect our internal controls over financial reporting.
| ITEM
9B. |
OTHER
INFORMATION |
None.
| ITEM
9C. |
DISCLOSURE
REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
Not
applicable.
PART
III.
| ITEM
10. |
DIRECTORS,
EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The following table sets forth certain information
regarding our Board of Directors, our executive officers, and some of our key employees, as of August 9, 2023.
| Name |
|
Age |
|
Director
Since |
|
Position |
| Cuong
Do |
|
|
57 |
|
|
2016 |
|
CEO
& President and Director |
| Joanne
Wendy Kim |
|
|
68 |
|
|
— |
|
CFO |
| Joseph
M. Palumbo, MD |
|
|
63 |
|
|
— |
|
Chief
Medical Officer |
| Jim
Lang |
|
|
58 |
|
|
2016 |
|
Chairman of the Board |
| Michael
Sherman |
|
|
64 |
|
|
2017 |
|
Director
|
| Richard
J. Berman |
|
|
81 |
|
|
2019 |
|
Director
|
| Steve
Gorlin |
|
|
86 |
|
|
2020 |
|
Director
|
| Robert
Hariri, MD, PhD |
|
|
64 |
|
|
2020 |
|
Director
|
| Sigmund
Rogich |
|
|
79 |
|
|
2020 |
|
Director
|
According to our Bylaws, the directors shall
be elected at the annual meeting of the stockholders and each director shall be elected to serve until his successor shall be elected
and shall qualify. A director need not be a stockholder. Directors shall not receive any stated salary for their services as directors
or as members of committees, but by resolution of the Board of Directors a fixed fee and expenses of attendance may be allowed for attendance
at each meeting. The Bylaws shall not be construed to preclude any director from serving the Company in any other capacity as an officer,
agent or otherwise, and receiving compensation therefor.
There are no familial relationships among
any of our directors or officers.
Biographical Information
Mr. Cuong Do, has served on
the Company’s Board of Directors since 2016 and effective April 27, 2021 was appointed the Company’s CEO and President. He
served as the President, Global Strategy Group, at Samsung from February 2015 to December 2020. Mr. Do helped set the strategic direction
for Samsung Group’s diverse business portfolio. He was previously the Chief Strategy Officer for Merck from October 2011 to March
2014, and Tyco Electronics from June 2009 to October 2011, and Lenovo from December 2007 to March 2009. Mr. Do is a former senior partner
at McKinsey & Company, where he spent 17 years and helped build the healthcare, high tech and corporate finance practices. He holds
a BA from Dartmouth College, and an MBA from the Tuck School of Business at Dartmouth.
We believe Mr. Do’s qualifications
to serve on our Board of Directors and as the CEO are primarily based on his decades of experience as an executive in the pharma, biotech,
and other high technology industries and his extensive experience in strategy, corporate finance practice and the development of companies
in all stages.
Ms. Joanne Wendy Kim has served
as the Company’s Chief Financial Officer since October 2018. Ms. Kim previously served as CFO for several companies throughout her
career, previously with Landmark Education Enterprises, and prior to that; other public entities in the entertainment and financial services
industry sectors. She provided interim CFO services to various organizations from 2016 to 2018. In her various roles, Ms. Kim oversaw
corporate finance and operational groups, closed eight acquisitions, secured bank financings, developed and implemented new business strategies,
managed risk and implemented new financial policies and procedures. As a CPA professional, she advised on accounting transactions, SEC
reporting matters and other regulatory matters to clients serving as a Director at BDO USA, LLP’s National Office SEC Department
and served on the U.S. desk in London for BDO LLP UK Firm in 2008-2016 and as a Senior Manager at KPMG in earlier part of her career.
She brings more than 35 years of accounting and finance experience to this position. Ms. Kim earned her BSA in accounting and finance
at California State University, Long Beach.
Wendy
Kim’s qualifications to serve as our Chief Financial Officer are primarily based on her 35 years of accounting and finance experience
both as a CFO and as a CPA in major global accounting and consultancy firms.
Dr.
Joseph M. Palumbo has served as our Chief Medical Officer since November 2021. Formerly he served as the CMO at Zynerba Pharmaceuticals
from July 2019 to October 2021, responsible for clinical operations, development, regulatory, and medical affairs. Prior to his time
at Zynerba, Dr. Palumbo held senior worldwide governance roles at Mitsubishi Tanabe Pharma in both the United States and Japan from April
2012 to June 2019, where he led medical science and translational research across multiple therapeutic areas, and guided successful
registrational programs for Radicava® (edaravone) for the treatment of Amyotrophic Lateral Sclerosis.
From April 2003 to March 2012, Dr. Palumbo was Global Head and Franchise Medical Leader for Psychiatry, and the Interim Head of
Global Neuroscience at Johnson & Johnson, where he led the medical teams who achieved successful global registrations for Risperdal®
(risperidone); Concerta® (methylphenidate HCL); and Invega® (paliperidone). He was Head of Psychiatry and Neurology at Pharmanet
for from April 2002 to April 2003. Dr Palumbo previously held industry positions in European Pharma with Sanofi-Synthelabo from April
1999 to April 2002, Biotech at Cephalon, from April 1997 to April 1998, and from July 1989 to April 2002, he held senior leadership and
hospital administration roles at prestigious academic research institutions including Yale, Cornell, and the University of Pennsylvania.
He holds a Bachelor of Arts at the University of Pennsylvania and received his Doctor of Medicine at the George Washington University
School of Medicine. He was a Biological Sciences Training Program Fellow of the National Institutes of Health and Chief Resident for
the Abraham Ribicoff Clinical Neuroscience Research Unit at Yale University. Dr Palumbo has received Board Certification in Psychiatry
and Addiction Psychiatry.
Dr.
Palumbo’s qualifications to serve as our Chief Medical Officer is based on the decades and depth of experiences in the roles he
has served in his medical profession and commercial experience in the healthcare industry and biopharma industries.
Mr.
Jim Lang has served as the Company’s director since 2016 and as the Chairman of the Board since March 2023. He is currently
CEO of EVERSANA, the leading commercialization services company for the life sciences industry. In five years since he founded EVERSANA,
it is now over $1B in revenue, with >7000 employees across 40 global locations. He formerly served as the CEO of Decision Resources
Group (DRG), which he transformed into a leading healthcare data and analytics firm. Prior to that, Jim was CEO of IHS Cambridge Energy
Research Associates (IHS CERA), a recognized leader in energy industry subscription information products, and formerly the President
of Strategic Decisions Group (SDG), a leading global strategy consultancy. Mr. Lang holds a BS summa cum laude in electrical and computer
engineering from the University of New Hampshire and an MBA with Distinction from the Tuck School of Business. Jim Lang currently also
serves as a Director at OptimizeRX (OPRX), a Nasdaq listed Company.
Jim Lang’s qualifications to serve
on our Board of Directors are primarily based on his decades of experience as a strategy consultant, broad industry expertise, and senior-level
management experience running several healthcare and information technology companies.
Mr. Richard J. Berman has served
as the Company’s director since June 2019. Mr. Berman has over 35 years of venture capital, senior management, and merger &
acquisitions experience. He currently is a director of four public companies including; Cryoport Inc., Genius Group, Context Therapeutics,
and over the last decade served on the boards of six companies that reached a market capitalization over one billion including Cryoport,
Advaxis, EXIDE, Internet Commerce Corporation, Kapitus and Ontrak. From 1998-2000, he was employed by Internet Commerce Corporation (now
Easylink Services) as Chairman and CEO and was a director from 1998-2012. Previously, Mr. Berman was Senior Vice President of Bankers
Trust Company, where he started the M&A and Leveraged Buyout Departments; created the largest battery company in the world in the
1980’s by merging Prestolite, General Battery and Exide and advised on over $4 billion of M&A transactions (completed over 300
deals). He is a past Director of the Stern School of Business of NYU where he obtained his BS and MBA. He also has US and foreign law
degrees from Boston College and The Hague Academy of International Law, respectively.
We believe Richard J. Berman’s qualifications
to serve on our Board of Directors include his experience in the healthcare industry, and his current and past experience in numerous
private and publicly traded companies.
Mr. Steven Gorlin has served
as the Company’s director since June 2020. He has founded many biopharma companies including Hycor Biomedical, Theragenics, Medicis
Pharmaceutical, EntreMed, MRI Interventions, DARA BioSciences, MiMedx, Medivation (sold to Pfizer for $14 billion) and NantKwest. Mr.
Gorlin served for many years on the Business Advisory Council to the Johns Hopkins School of Medicine and on The Johns Hopkins BioMedical
Engineering Advisory Board. He is currently a member of the Research Institute Advisory Committee (RIAC) of Massachusetts General Hospital.
He started The Touch Foundation, a nonprofit organization for the blind, and was a principal contributor to Camp Kudzu for diabetic children.
Steve Gorlin’s qualifications to serve
on our Board of Directors are primarily based on his over 45 years of experience in founding and investing in several biopharma companies,
leading multiple NASDAQ AND NYSE companies to their success.
Dr. Robert Hariri MD, PhD,
has served as the Company’s director since June 2020. Dr Hariri is the Chairman, founder, and CEO of Celularity, Inc., a leading
cellular therapeutics company. He was the founder and CEO of Anthrogenesis Corporation, and after its acquisition served as CEO of Celgene
Cellular Therapeutics. Dr. Hariri co-founded the genomic health intelligence company, Human Longevity, Inc. Dr. Hariri pioneered the use
of stem cells to treat a range of life-threatening human diseases. He is widely acknowledged for his discovery of pluripotent stem cells
and for assisting with discovering the physiological activities of tumor necrosis factor (TNF). He holds over 170 issued and pending patents.
Robert (Bob) Hariri’s qualifications
to serve on our Board of Directors are primarily based on his decades of founding and leading several companies in the cellular therapeutic
space, as well as pioneering in the use of stem cells to treat a range of life-threatening human diseases and discoveries in the physiological
activities of tumor necrosis factor. He has authored over 150 publications and garnered numerous awards for contributions to the fields
of biomedicine and aviation.
Mr. Sigmund (Sig) Rogich has
served as the Company’s director since June 2020. Sig is the CEO and President of The Rogich Communications Group and serves on
the Board of Keep Memory Alive, a philanthropic organization which raises awareness about brain disorders and Alzheimer’s disease. Keep
Memory Alive funds clinical trials to advance new treatments for patients with Alzheimer’s, Huntington’s and Parkinson’s
disease, as well as multiple sclerosis. Mr. Rogich was formerly the U.S. Ambassador to Iceland. He has served as a senior consultant
to Presidents Ronald Reagan and George H.W. Bush. Mr. Rogich serves on multiple boards of directors for charitable causes.
We believe Mr. Rogich’s qualifications
to serve on our Board of Directors are based on his experience in the Communications sector and philanthropic organization raising awareness
about brain disorders. His experience in service as a senior consultant to candidates of the highest office.
Mr. Michael Sherman JD has
served as the Company director since 2017. He retired from his position as a Managing Director at Barclays Plc in 2018, where he had worked
since 2008. Previously he was a Managing Director at Lehman Brothers, Inc. He has worked in investment banking for 30 years. Mr. Sherman
has significant experience in healthcare finance, most recently assisting on a $450 million convertible transaction for Neurocrine Biosciences.
He has worked on successful financial transactions for Teva Pharmaceutical Industries, Amgen Inc., Cubist Pharmaceuticals, Merck &
Co., and Cardinal Health, among other companies. After graduating from the University of Pennsylvania, Michael Sherman received his JD,
cum laude, from the Harvard Law School.
Michael Sherman’s qualifications to
serve on our Board of Directors are primarily based on his decades of finance industry experience and investment banking. Mr. Sherman
has significant experience in healthcare finance including having worked on successful financial transactions for several pharmaceutical
and healthcare focused companies.
Delinquent Section 16(a) Reports
Section 16(a) of the Securities Exchange
Act of 1934, as amended (Exchange Act), requires our directors and executive officers, and persons who own more than 10% of our outstanding
Common Stock, to file with the SEC, initial reports of ownership and reports of changes in ownership of our equity securities. Such persons
are required by SEC regulations to furnish us with copies of all such reports they file.
To our knowledge, based solely on a review
of the copies of such reports furnished to us regarding the filing of required reports, we believe that, except for the reports filed
by Clarence Ahlem (Form 4s filed on January 18, 2023 and February 22, 2023), Richard J. Berman (Form 4s filed on January 18, 2023, April
6, 2023 and June 15, 2023), Cuong Do (Form 4s filed on July 7, 2022, January 18, 2023, February 22, 2023 and June 26, 2023), Steve Gorlin
(Form 4 filed on January 18, 2023), Robert J. Hariri (Form 4 filed on January 18, 2023), Wendy Kim (Form 4s filed on January 8, 2023
and February 22, 2023), James Lang (Form 4 filed on January 18, 2023), Penelope Markham (Form 4s filed on January 18, 2023 and February
22, 2023), Joseph M Palumbo (Form 4s filed on January 18, 2023, February 22, 2023 and July 3, 2023), Terren Peizer (Form 3 filed on August
16, 2022 and Form 4s filed on August 26, 2022 and January 18, 2023), Christopher Reading (Form 4s filed on January 18, 2023 and February
22, 2023), Sigmund Rogich (Form 4 filed on January 18, 2023) and Michael Sherman (Form 4 filed on January 18, 2023), all Section 16(a)
reports applicable to our directors, executive officers and greater-than-ten-percent beneficial owners with respect to fiscal 2023 were
timely filed.
Independence of the Board of Directors
Our Common Stock is traded on the Nasdaq
Capital Market. The Board of Directors has determined that six of the seven members of the Board of Directors qualify as “independent,”
as defined by the listing standards of the Nasdaq. Consistent with these considerations, after review of all relevant transactions and
relationships between each director, or any of the director’s family members, and the Company, its senior management and its independent
auditors, the Board has determined further that Messrs. Lang, Sherman, Berman, Gorlin, Hariri and Rogich are independent under the listing
standards of Nasdaq. In making this determination, the Board of Directors considered that there were no new transactions or relationships
between its current independent directors and the Company, its senior management and its independent auditors since last making this determination.
2023 Meetings and Attendance
During fiscal year 2023, the Board held four
regular Board of Directors meetings and one special meeting of the Board of Directors, four Audit Committee meetings, six Compensation
Committee meetings and one Nominating and Corporate Governance Committee meeting. All Directors attended at least 75% or more of the aggregate
number of meetings of the Board and Board Committees on which they served.
Committees of the Board of Directors
Our Board of Directors has three standing
committees: an audit committee, a compensation committee and a nominating and corporate governance committee. Both our audit committee
and our compensation committee will be composed solely of independent directors. The audit committee is comprised solely of independent
directors, and the compensation committee and the nominating and corporate governance committee are comprised solely of independent directors.
Each committee operates under a charter approved by our Board of Directors and have the composition and responsibilities described below.
The charter of each committee is available on our website.
Audit Committee
We have established an audit committee of
the Board of Directors. The members of our audit committee are Richard Berman, Michael Sherman, Jim Lang and Sigmund Rogich each of whom
is an independent director within the meaning of the Nasdaq rules. Mr. Berman has served as chairman of the audit committee since October
2020 and qualifies as an “audit committee financial expert” as defined by Item 401(h)(2) of Regulation S-K.
We
have adopted an audit committee charter, detailing the principal functions of the audit committee, including:
| ● | assisting
board oversight of (1) the integrity of our financial statements, (2) our compliance
with legal and regulatory requirements, (3) our independent auditor’s qualifications
and independence, and (4) the performance of our internal audit function and independent
auditors; the appointment, compensation, retention, replacement, and oversight of the work
of the independent auditors and any other independent registered public accounting firm engaged
by us; |
| ● | pre-approving
all audit and non-audit services to be provided by the independent auditors or any other
registered public accounting firm engaged by us, and establishing pre-approval policies and
procedures; reviewing and discussing with the independent auditors all relationships the
auditors have with us in order to evaluate their continued independence; |
| ● | setting
clear policies for audit partner rotation in compliance with applicable laws and regulations; |
| ● | obtaining
and reviewing a report, at least annually, from the independent auditors describing (1) the
independent auditor’s internal quality-control procedures and (2) any material issues
raised by the most recent internal quality-control review, or peer review, of the audit firm,
or by any inquiry or investigation by governmental or professional authorities, within the
preceding five years respecting one or more independent audits carried out by the firm and
any steps taken to deal with such issues; |
| ● | meeting
to review and discuss our annual audited financial statements and quarterly financial statements
with management and the independent auditor, including reviewing our specific disclosures
under “Management’s Discussion and Analysis of Financial Condition and Results
of Operations”; reviewing and approving any related party transaction required to be
disclosed pursuant to Item 404 of Regulation S-K promulgated by the SEC prior to us entering
into such transaction; and |
| ● | reviewing
with management, the independent auditors, and our legal advisors, as appropriate, any legal,
regulatory or compliance matters, including any correspondence with regulators or government
agencies and any employee complaints or published reports that raise material issues regarding
our financial statements or accounting policies and any significant changes in accounting
standards or rules promulgated by the Financial Accounting Standards Board, the SEC or other
regulatory authorities. |
Compensation
Committee
We have established a compensation committee
of the Board of Directors. The members of our Compensation Committee are Richard Berman, Michael Sherman and Steve Gorlin. Mr. Sherman
has served as chairman of the compensation committee since October 2020.
We
have adopted a compensation committee charter, which details the principal functions of the compensation committee, including:
| ● | reviewing
and approving on an annual basis the corporate goals and objectives relevant to our Chief
Executive Officer’s compensation, evaluating our Chief Executive Officer’s performance
in light of such goals and objectives and determining and approving the remuneration (if
any) of our Chief Executive Officer based on such evaluation; |
| ● | reviewing
and making recommendations to our Board of Directors with respect to the compensation, and
any incentive-compensation and equity-based plans that are subject to board approval of all
of our other officers; |
| ● | reviewing
our executive compensation policies and plans; |
| ● | implementing
and administering our incentive compensation equity-based remuneration plans; assisting management
in complying with our proxy statement and annual report disclosure requirements; |
| ● | approving
all special perquisites, special cash payments and other special compensation and benefit
arrangements for our officers and employees; and |
| ● | producing
a report on executive compensation to be included in our annual proxy statement; and reviewing,
evaluating and recommending changes, if appropriate, to the remuneration for directors. |
The
charter also provides that the compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant,
independent legal counsel or other adviser and will be directly responsible for the appointment, compensation and oversight of the work
of any such adviser. However, before engaging or receiving advice from a compensation consultant, external legal counsel or any other
adviser, the compensation committee will consider the independence of each such adviser, including the factors required by Nasdaq and
the SEC.
Compensation
Committee Interlocks and Insider Participation
None
of our officers currently serves, or in the past year has served, as a member of the compensation committee of any entity that has one
or more officers serving on our Board of Directors.
Nominating
and Corporate Governance Committee
We
have established a nominating and corporate governance committee of the Board of Directors. The members of our nominating and corporate
governance committee are, Jim Lang, Michael Sherman and Robert Hariri. Mr. Lang has served as chair of the nominating and corporate governance
committee since August 2021.
We
have adopted a nominating and corporate governance committee charter, which details the purpose and responsibilities of the nominating
and corporate governance committee, including:
| ● | identifying,
screening and reviewing individuals qualified to serve as directors, consistent with criteria
approved by the Board of Directors, and recommending to the Board of Directors candidates
for nomination for election at the annual meeting of stockholders or to fill vacancies on
the Board of Directors; |
| ● | developing
and recommending to the Board of Directors and overseeing implementation of our corporate
governance guidelines; |
| ● | coordinating
and overseeing the annual self-evaluation of the Board of Directors, its committees, individual
directors and management in the governance of the company; and |
| ● | reviewing
on a regular basis our overall corporate governance and recommending improvements as and
when necessary. |
The
charter also provides that the nominating and corporate governance committee may, in its sole discretion, retain or obtain the advice
of, and terminate, any search firm to be used to identify director candidates, and will be directly responsible for approving the search
firm’s fees and other retention terms.
We
have not formally established any specific, minimum qualifications that must be met or skills that are necessary for directors to possess.
In general, in identifying and evaluating nominees for director, the Board of Directors considers educational background, diversity of
professional experience, knowledge of our business, integrity, professional reputation, independence, wisdom, and the ability to represent
the best interests of our stockholders. Prior to our initial business combination, holders of our public shares will not have the right
to recommend director candidates for nomination to our Board of Directors.
Set
forth below is information concerning the gender and demographic background of each of our current directors, as self-identified and
reported by each director. This information is being provided in accordance with Nasdaq’s board diversity rules.
Board
Diversity Matrix (As of August 11, 2023)
| Total
Number of Directors: |
8 |
|
| |
|
|
|
|
|
|
|
Did
Not |
|
| |
|
|
|
|
|
Non- |
Disclose |
| |
|
Female |
Male |
|
Binary |
Gender |
| Part
I: Gender Identity |
|
|
|
|
|
|
|
|
| Directors |
0 |
|
7 |
0 |
|
0 |
|
| Part
II: Demographic Background |
|
|
|
|
|
|
|
|
| African
American or Black |
— |
|
— |
— |
|
— |
|
| Alaskan
Native or Native American |
— |
|
— |
— |
|
— |
|
| Asian |
— |
|
1 |
— |
|
— |
|
| Hispanic
or Latinx |
— |
|
— |
— |
|
— |
|
| Native
Hawaiian or Pacific Islander |
— |
|
— |
— |
|
— |
|
| White |
— |
|
3 |
— |
|
— |
|
| Two
or More Races or Ethnicities |
— |
|
— |
— |
|
— |
|
| LGBTQ+ |
— |
|
— |
— |
|
— |
|
| Did
Not Disclose Demographic Background |
— |
|
3 |
— |
|
— |
|
| |
|
|
|
|
|
|
|
| Code
of Ethics |
|
|
|
|
|
|
|
|
We
have adopted a code of conduct and ethics meeting the requirements of Section 406 of the Sarbanes-Oxley Act of 2002. We believe our code
of conduct and ethics is reasonably designed to deter wrongdoing and promote honest and ethical conduct; provide full, fair, accurate,
timely and understandable disclosure in public reports; comply with applicable laws; ensure prompt internal reporting of violations;
and provide accountability for adherence to the provisions of the code of ethic. Our code of conduct and ethics is available on our website.
A
copy of our code of conduct and ethics is filed as an exhibit to this Form 10-K.
Anti-Hedging
Policy
We
have adopted an insider trading policy that includes a provision restricting trading of any interest or provision relating to the future
price of our securities, such as a put, call or short sale.
| ITEM
11. |
EXECUTIVE
COMPENSATION |
Summary
Compensation Table
The
following table sets forth the total compensation paid during the last two fiscal years ended June 30, 2023 and 2022 to the following
executive officers of the Company, who are referred to as our “named executive officers”:
| ● | Cuong
Do, our President and Chief Executive Officer |
| ● | Joanne
Wendy Kim, our Chief Financial Officer and Corporate Secretary |
| ● | Joseph
Palumbo, our Chief Medical Officer |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Name and Principal
Position | |
Year | | |
Salary | | |
Bonus | | |
Stock
Awards (1) | | |
Option
Awards (1) | | |
Non-Equity
Incentive Plan Compensation | | |
Nonqualified
Deferred Compensation Earnings | | |
All
Other Compensation | | |
Total | |
| Cuong
Do (2) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Chief
Executive Officer and President | |
| 2023 | | |
$ | 618,000 | | |
$ | 463,500 | | |
$ | 734,668 | | |
$ | 521,500 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 2,337,668 | |
| | |
| 2022 | | |
$ | 300,000 | | |
$ | 400,000 | | |
$ | 210,439 | | |
$ | 3,632,382 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 4,542,821 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Joanne
Wendy Kim (3) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Chief
Financial Officer, Treasurer and Corporate Secretary | |
| 2023 | | |
$ | 246,750 | | |
$ | 150,625 | | |
$ | 242,499 | | |
$ | 84,000 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 723,874 | |
| | |
| 2022 | | |
$ | 235,000 | | |
$ | 127,656 | | |
$ | — | | |
$ | 582,343 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 944,999 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Joseph
Palumbo (4) | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Chief
Medical officer | |
| 2022 | | |
$ | 525,000 | | |
$ | 197,000 | | |
$ | 242,499 | | |
$ | 126,000 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 1,090,499 | |
| | |
| 2022 | | |
$ | 333,333 | | |
$ | 239,167 | | |
$ | — | | |
$ | 244,465 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 816,965 | |
| |
(1) |
The aggregate grant date fair value of such awards were computed in accordance with Financial Accounting Standards Board ASC Topic 718, Stock Compensation (ASC Topic 718), and do not take into account estimated forfeitures related to service-based vesting conditions, if any. The valuation assumptions used in calculating these values are discussed in Note 10 of our Notes to Financial Statements included in our Annual Report on Form 10-K for the year ended June 30, 2023. These amounts do not represent actual amounts paid or to be realized. Amounts shown are not necessarily indicative of values to be achieved, which may be more or less than the amounts shown as awards may subject to time-based vesting. The stock awards in form of RSUs and Stock Option Awards were awarded pursuant to the 2019 Omnibus Incentive Plan, (the “2019 Plan”). |
| |
(2) |
Mr. Do’s salary from April 27, 2021 (date of his appointment as CEO) through December 31, 2021 was paid through RSUs. The aggregate grant date fair value of the award was $454,794 and the total 58,759 RSUs awarded allows Mr. Do to receive one shares of Common Stock for each RSU. |
| (3) | Ms.
Kim served as the Chief Financial Officer and Corporate Secretary and Treasurer on a full
time basis effective July 1, 2021. |
| (4) | Dr.
Palumbo joined the Company on November 1, 2021 and served as the Chief Medical Officer. |
Employment
Agreements
All
employment arrangements are “at will” agreements.
Outstanding
Equity Awards at Fiscal Year-End
The
following table sets forth all outstanding equity awards held by our named executive officers as of June 30, 2023:
| | |
| |
Options | | |
Stock
Awards | |
| Name | |
Grant
Date | |
Number
of securities underlying unexercised options exercisable | | |
Number
of securities underlying unexercised options unexercisable | | |
Equity
incentive plan awards: number of securities underlying unexercised unearned options | | |
Option
exercise price | | |
Option
expiration date | |
Number
of shares or units of stock that have not vested | | |
Market
value of shares or units of stock that have to vested | | |
Equity
incentive plan awards: number of unearned shares, units or other rights that have not vested | | |
Equity
incentive plan awards: market or payout value of unearned shares, units or other right that have not vested | |
| | |
| |
| | |
| | |
| | |
| | |
| |
| | |
| | |
| | |
| |
| Cuong
Do, CEO | |
01-19-19 | |
| 800 | | |
| — | | |
| — | | |
$ | 3.75 | | |
01-19-24 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
01-19-20 | |
| 800 | | |
| — | | |
| — | | |
$ | 2.80 | | |
01-19-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
12-18-20 | |
| 24,375 | | |
| — | | |
| — | | |
$ | 13.91 | | |
12-18-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
08-20-21 | |
| 387,400 | | |
| — | | |
| 357,600 | | |
$ | 7.74 | | |
08-20-31 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
06-21-22 | |
| 41,506 | | |
| — | | |
| 83,014 | | |
$ | 1.69 | | |
06-21-32 | |
| — | | |
| — | | |
| 83,014 | | |
$ | 357,790 | |
| | |
11-23-23 | |
| — | | |
| — | | |
| — | | |
$ | — | | |
| |
| — | | |
| — | | |
| 59,436 | | |
$ | 256,169 | |
| | |
06-29-23 | |
| — | | |
| — | | |
| 175,000 | | |
$ | 4.09 | | |
06-29-33 | |
| — | | |
| — | | |
| 149,500 | | |
$ | 644,345 | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Joanne
W. Kim, CFO | |
10-01-18 | |
| 800 | | |
| — | | |
| — | | |
$ | 8.75 | | |
10-01-23 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
10-01-19 | |
| 800 | | |
| — | | |
| — | | |
$ | 8.75 | | |
10-01-24 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
10-01-20 | |
| 800 | | |
| — | | |
| — | | |
$ | 9.54 | | |
10-01-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
08-20-21 | |
| 40,726 | | |
| — | | |
| 83,441 | | |
$ | 7.74 | | |
08-20-31 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
11-23-22 | |
| — | | |
| — | | |
| — | | |
$ | — | | |
| |
| — | | |
| — | | |
| 29,718 | | |
$ | 128,085 | |
| | |
06-07-23 | |
| 5,000 | | |
| — | | |
| 15,000 | | |
$ | 5.78 | | |
06-07-33 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Joseph
M. Palumbo, CMO | |
02-01-22 | |
| 24,833 | | |
| — | | |
| 99,334 | | |
$ | 3.20 | | |
02-01-32 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
11-23-22 | |
| — | | |
| — | | |
| — | | |
$ | — | | |
| |
| — | | |
| — | | |
| 29,718 | | |
$ | 128,085 | |
| | |
06-07-23 | |
| 7,500 | | |
| — | | |
| 22,500 | | |
$ | 5.78 | | |
06-07-33 | |
| — | | |
| — | | |
| — | | |
$ | — | |
Named executive officers held stock options
to purchase a total of 1,371,729 shares of Common Stock as of June 30, 2023, with an aggregate grant date fair value of approximately
$5.4 million, the last of which vests in 2027. Stock options granted prior to August 20, 2021, vested on the grant date; the stock options
granted on August 20, 2021 vested 20% on the grant date, with the remaining stock options vesting in five equal annual installments beginning
on the first grant date anniversary; the stock options granted on June 7, 2023, vested 25% on the grant date, with the remaining stock
options vesting in four equal annual installments beginning on the first grant date anniversary; and the stock options and stock awards
in the form RSUs granted to the CEO on June 21, 2022 and June 29, 2023 vests in three equal annual installments beginning on the first
grant date anniversary. The RSU awarded on November 23, 2022 vested 25% on the grant date with the remaining RSU vesting in three equal
annual installments beginning on the first grant date anniversary. The total RSUs outstanding awarded to the named executive officers
totaled 351,386 with a market value totaling approximately $1.5 million as of June 30, 2023.
Potential Payments Upon Termination or Change
of Control
There are no arrangements with the named
executive officers or our equity incentive plan or individual award agreements thereunder providing for certain payments to our named
executive officers at or following or in connection with a termination of their employment or a change of control of the Company.
Director
Compensation
There
are no arrangements pursuant to which our directors are or will be compensated in the future for any services provided to the Company.
The
following table provides information regarding compensation that was earned or paid to the individuals who served as non-employee directors
during the year ended June 30, 2023. Except as set forth in the table, during the fiscal year 2023, directors did not earn nor receive
cash compensation or compensation in the form of stock awards, options awards or any other form.
| Name | |
Stock awards (1) | | |
Option awards(1) | | |
Non-equity incentive plan compensation | | |
Change in pension value and nonqualified deferred compensation | | |
All other compensation | | |
Total | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Jim Lang | |
$ | 266,697 | | |
| — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 266,697 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Michael Sherman | |
| — | | |
| 304,500 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 304,500 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Richard Berman | |
$ | 266,697 | | |
| — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 266,697 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Steve Gorlin | |
$ | 209,549 | | |
| — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 209,549 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Robert Hariri MD, Phd | |
$ | 209,549 | | |
| — | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 209,549 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Sigmund Rogich | |
$ | — | | |
| 223,300 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 223,300 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Terren Piezer (2) | |
$ | — | | |
| 263,900 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 263,900 | |
| (1) | The
aggregate grant date fair value of such awards were computed in accordance with Financial
Accounting Standards Board ASC Topic 718, Stock Compensation (ASC Topic 718), and do not
take into account estimated forfeitures related to service-based vesting conditions, if any.
The valuation assumptions used in calculating these values are discussed in Note 10 of our
Notes to Financial Statements included in our Annual Report on Form 10-K for the year ended
June 30, 2023. These amounts do not represent actual amounts paid or to be realized. Amounts
shown are not necessarily indicative of values to be achieved, which may be more or less
than the amounts shown as awards may subject to time-based vesting. |
| (2) | Mr.
Piezer resigned from the Board of Directors effective March 2, 2023. |
Our directors are eligible to participate
in our equity incentive plans, which are administered by our Compensation Committee under authority delegated by our Board of Directors.
The terms and conditions of the option grants to our non-employee directors under our equity incentive plans are and will be determined
in the discretion of our Compensation Committee, consistent with the terms of the applicable plan. The fiscal year 2023 annual compensation
granted to existing board members consisted of either an award of RSUs at one unit per share of Common Stock, a total of 155,636 RSU at
a grant date market value of $952,492 or stock options to purchase a total of 195,000 shares of commons stock with a grant date fair value
totaling $791,700. The former chairman of the Board of Directors, the chairman of the compensation committee and a member of the audit
committee received stock options to purchase 65,000, 75,000 and 55,000 shares of Common Stock, respectively. The chairmen of the audit
committee and the corporate governance and nominating committee each received 43,578 RSUs and the members of those committees each received
34,240 RSUs.
The
following tables sets forth the outstanding equity awards held by non-employee directors as of June 30, 2023:
| | |
| |
Options
(1) | | |
Stock
Awards (2) | |
| Name | |
Grant
Date | |
Number
of securities underlying unexercised options exercisable | | |
Number
of securities underlying unexercised options unexercisable | | |
Equity
incentive plan awards: number of securities underlying unexercised unearned options | | |
Option
exercise price | | |
Option
expiration date | |
Number
of shares or units of stock that have not vested | | |
Market
value of shares or units of stock that have to vested | | |
Equity
incentive plan awards: number of unearned shares, units or other rights that have not vested | | |
Equity
incentive plan awards: market or payout value of unearned shares, units or other right that have not vested | |
| | |
| |
| | |
| | |
| | |
| | |
| |
| | |
| | |
| | |
| |
| James
Lang | |
01-19-19 | |
| 800 | | |
| — | | |
| — | | |
$ | 3.13 | | |
01-19-24 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
01-19-20 | |
| 800 | | |
| — | | |
| — | | |
$ | 2.80 | | |
01-19-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
12-18-20 | |
| 74,250 | | |
| — | | |
| 24,750 | | |
$ | 13.91 | | |
12-18-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
04-05-22 | |
| 63,950 | | |
| — | | |
| 63,950 | | |
$ | 7.74 | | |
04-05-32 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
11-23-22 | |
| — | | |
| — | | |
| — | | |
$ | — | | |
| |
| — | | |
| — | | |
| 21,789 | | |
$ | 93,911 | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Richard
J. Berman | |
01-19-20 | |
| 800 | | |
| — | | |
| — | | |
$ | 2.80 | | |
01-19-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
12-18-20 | |
| 76,875 | | |
| — | | |
| 25,625 | | |
$ | 13.91 | | |
12-18-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
04-05-22 | |
| 64,525 | | |
| — | | |
| 64,525 | | |
$ | 5.04 | | |
04-05-27 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
11-23-22 | |
| | | |
| — | | |
| | | |
$ | — | | |
| |
| — | | |
| — | | |
| 21,789 | | |
$ | 93,911 | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Steve
Gorlin | |
12-18-20 | |
| 72,225 | | |
| — | | |
| 24,075 | | |
$ | 13.91 | | |
12-18-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
04-05-22 | |
| 61,125 | | |
| — | | |
| 61,125 | | |
$ | 5.04 | | |
04-05-27 | |
| | | |
| | | |
| | | |
| | |
| | |
11-23-22 | |
| — | | |
| — | | |
| — | | |
$ | — | | |
| |
| — | | |
| — | | |
| 17,120 | | |
$ | 73,787 | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Robert
Hariri | |
12-18-20 | |
| 71,925 | | |
| — | | |
| 23,975 | | |
$ | 13.91 | | |
12-18-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
04-05-22 | |
| 61,125 | | |
| | | |
| 61,125 | | |
$ | 5.04 | | |
04-05-27 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
11-23-22 | |
| — | | |
| — | | |
| — | | |
$ | — | | |
| |
| | | |
| | | |
| 17,120 | | |
$ | 73,787 | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Sigmund
Rogich | |
12-18-20 | |
| 72,975 | | |
| — | | |
| 24,325 | | |
$ | 13.91 | | |
12-18-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
04-05-22 | |
| 61,700 | | |
| — | | |
| 61,700 | | |
$ | 5.04 | | |
04-05-27 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
11-23-22 | |
| 27,500 | | |
| — | | |
| 27,500 | | |
$ | 6.12 | | |
11-23-27 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Michael
Sherman | |
10-13-18 | |
| 800 | | |
| — | | |
| — | | |
$ | 6.25 | | |
10-13-23 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
10-13-19 | |
| 800 | | |
| — | | |
| — | | |
$ | 7.13 | | |
10-13-24 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
10-13-20 | |
| 800 | | |
| — | | |
| — | | |
$ | 9.90 | | |
10-13-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
12-18-20 | |
| 77,325 | | |
| — | | |
| 25,775 | | |
$ | 13.91 | | |
12-18-25 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
04-05-22 | |
| 65,075 | | |
| — | | |
| 65,075 | | |
$ | 5.04 | | |
04-05-27 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | |
11-23-22 | |
| 37,500 | | |
| — | | |
| 37,500 | | |
$ | 6.12 | | |
11-23-27 | |
| — | | |
| — | | |
| — | | |
$ | — | |
| |
(1) |
There was a total of 1,483,300 stock options outstanding to directors as of June 30, 2023, with an aggregate grant date fair value of approximately $13.2 million, the last of which vest in 2027. Stock options granted on December 18, 2020 and April 5, 2022 vest 25% on grant date with the remaining stock options vesting in three annual equal installments beginning on the first grant date anniversary. Stock options granted on November 23, 2022 vest in four equal quarterly installments beginning February 9, 2023. |
| |
(2) |
Equity awards granted the Board of Directors on November 23, 2022 were in the form of RSUs, one unit for one share of Common Stock, vest in four equal quarterly installments beginning February 9, 2023. There were 77,460 RSUs outstanding as of June 30, 2023, with an aggregate market value of approximately $335,000. |
Long-Term
Incentive Plans and Awards
Other
than the options granted and RSU awards as described above, we do not currently have any long-term incentive plans that provide compensation
intended to serve as incentive for performance. Since prior to such grants, no individual grants or agreements regarding future payouts
under non-stock price-based plans had been made to any executive officer or any director or any employee or consultant since our inception,
no future payouts under non-stock price-based plans or agreements had been granted or entered into or exercised by our officer or director
or employees or consultants.
2019
Omnibus Equity Incentive Plan
On April 20, 2019, our Board of Directors
and our stockholders approved and adopted the 2019 Plan. The 2019 Plan allows us, under the direction of our Board of Directors or a committee
thereof, to make grants of stock options, restricted and unrestricted stock and other stock-based awards to employees, including our executive
officers, consultants and directors. The 2019 Plan allows for the issuance of up to 6,540,000 shares of common pursuant to new awards
granted under the 2019 Plan and as of June 30, 2023, there were 2,269,952 shares of Common Stock available for new awards granted under
the 2019 Plan.
Equity
Compensation Plan Information[1]
The
following table provides certain aggregate information with respect to all of the Company’s equity compensation plans in effect
as of June 30, 2023:
| | |
(a) | | |
(b) | | |
(c) | |
| Plan Category | |
Number of securities to be issued upon exercise of outstanding
options, warrants and rights | | |
Weighted-average exercise price of outstanding options,
warrants and rights | | |
Number of securities remaining available for future
issuance under equity compensation pans (excluding securities reflected in column (a)) | |
| Equity compensation plans approved by security holders | |
| 4,530,121 | | |
$ | 6.71 | | |
| 2,269,952 | |
| Equity compensation not approved by security holders | |
| — | | |
$ | — | | |
| — | |
| Total | |
| 4,530,121 | | |
$ | 6.71 | | |
| 2,269,952 | |
PAY
VERSUS PERFORMANCE
As
required by Item 402(v) of Regulation S-K, we are providing the following information regarding the relationship between executive compensation
and our financial performance for each of the last two completed calendar years. In determining the “compensation actually paid”
to our named executive officers (“NEOs”), we are required to make various adjustments to amounts that have been previously
reported in the Summary Compensation Table in previous years, as the SEC’s valuation methods for this section differ from those
required in the Summary Compensation Table.
Pay
Versus Performance Table
The
table below summarizes compensation values both previously reported in our Summary Compensation Table, as well as the adjusted values
required in this section for fiscal years 2022 and 2023. Note that for our NEOs other than our principal executive officer (the “PEO”),
compensation is reported as an average.
| Year |
Summary
Compensation Table Total for PEO
($)(1)(2) |
Compensation
Actually Paid to PEO
($)(1)(3) |
Average
Summary Compensation Table Total for Non-PEO Named Executive Officers
($)(1)(4) |
Average
Compensation Actually Paid to Non-PEO Named Executive Officers
($)(1)(5) |
Value
of Initial Fixed $100 Investment Based on Total Shareholder Return
($)(6) |
Net
Loss
($)(7)
(in
thousands) |
| 2023 |
$2,337,668 |
$3,434,517 |
$1,185,289 |
$1,185,289 |
$25.43 |
$(50,256) |
| 2022 |
$4,542,821 |
$916,050 |
$605,653 |
$605,653 |
$8.55 |
$(25,084) |
| (1) | During fiscal years 2023 and 2022, the PEO was Cuong Do. During fiscal years 2023 and 2022, the non-PEO NEOs were Joanne W Kim and Joseph M Palumbo M.D. |
| (2) | The
dollar amounts reported are the amounts of total compensation reported for Mr. Do and the
average total compensation reported for Non-PEO Named Executive Officers for the applicable
fiscal year in the “Total” column of the Summary Compensation Table (SCT). |
| (3) | The
following table sets forth the adjustments made to the SCT total for each year represented
in the pay versus performance table to arrive at “compensation actually paid”
to our PEO, as computed in accordance with Item 402(v) of Regulation S-K: |
| |
2023 |
2022 |
| SCT
Total for PEO |
$2,337,668 |
$4,542,821 |
| Less:
Amount reported under the “Stock Awards” column in the SCT |
$(1,256,168) |
$(3,842,821) |
| Add:
Fair value as of fiscal year-end of awards granted during the fiscal year that are outstanding and unvested as of the end of the
fiscal year |
$2,770,583 |
$- |
| Add:
Change in fair value as of fiscal year-end, compared to prior fiscal year-end, of awards granted in any prior fiscal year that are
outstanding and unvested as of the end of the fiscal year |
$(1,545,275) |
$- |
| Add:
Fair value as of vest date of awards granted and vested in the fiscal year |
$1,186,095 |
$1,153,260 |
| Add:
Change in fair value as of vesting date, compared to prior fiscal year-end, of awards granted in any prior fiscal year for which
all vesting conditions were satisfied at fiscal year-end or during the fiscal year |
$(58,386) |
$(937,210) |
| Less:
Forfeitures during fiscal year equal to prior fiscal year-end value |
$- |
$- |
| Total
Adjustments |
$1,096,849 |
$(3,626,771) |
| Compensation
Actually Paid to PEO |
$3,434,517 |
$916,050 |
| (4) | The
following table sets forth the adjustments made to the SCT total for each year represented
in the pay versus performance table to arrive at “compensation actually paid”
to our PEO, as computed in accordance with Item 402(v) of Regulation S-K: |
| |
2023 |
2022 |
| Average
SCT Total for Non-PEO NEOs |
$907,186 |
$880,982 |
| Less:
Amount reported under the “Stock Awards” column in the SCT |
$(347,499) |
$(413,404) |
| Add:
Fair value as of fiscal year-end of awards granted during the fiscal year that are outstanding and unvested as of the end of the
fiscal year |
$551,399 |
$144,034 |
| Add:
Fair value as of vest date of awards granted and vested in the fiscal year |
$96,750 |
$135,839 |
| Add: change in fair value as of vesting date, compared to prior fiscal year-end of awards granted in any prior fiscal year for which all vesting conditions were satisfied at fiscal year-end or during the fiscal year |
$(22,548) |
$(141,798) |
| Less: Forfeitures during fiscal year equal to prior fiscal year-end value |
$- |
$- |
| Total
Adjustments |
$278,102 |
$(275,329) |
| Average
Compensation Actually Paid to Non-PEO NEOs |
$1,185,289 |
$605,653 |
| (5) | The
amounts reported represent the measurement period value of an investment of $100 in our stock
on June 30, 2021 (the last trading day before the 2022 fiscal year), and then valued again
on each of June 30, 2022 (the last trading day of the 2022 fiscal year) and June 30, 2023
(the last trading day of the 2023 fiscal year), based on the closing price per share of the
Company’s common stock as of such dates and assuming the reinvestment of dividends. |
| (6) | The
amounts reported represent net loss for the applicable fiscal year calculated in accordance
with generally accepted accounting principles in the United States. |
Relationship
Between CAP Amounts and Performance Measures
The
following charts show graphically the relationships over the past two years of the CAP Amounts for the PEO and the Other NEOs as compared
to our (i) cumulative total shareholder return and (ii) net loss.
While
the Compensation Committee makes executive compensation decisions in consideration of a variety of factors, including corporate and individual
performance, the decisions of the Compensation Committee and Board of Directors in 2022 and 2023 were made independently of these disclosure
requirements.
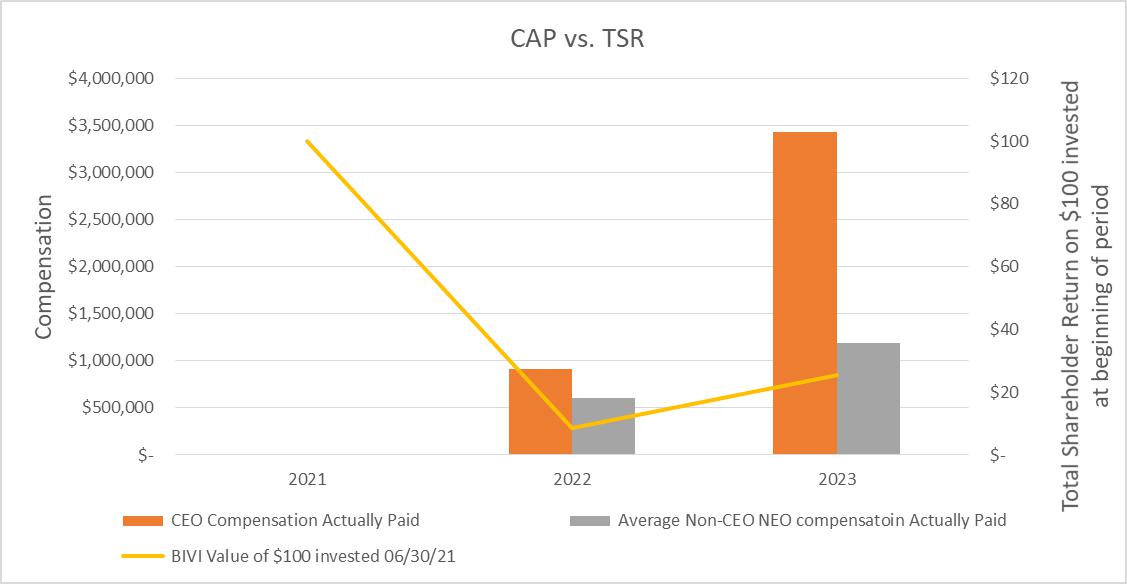

| ITEM
12. |
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Based solely upon information made available
to us, the following table sets forth information as of August 9, 2023 regarding the beneficial ownership of our Common Stock by:
| ● | each
person known by us to be the beneficial owner of more than 5% of our outstanding shares of
Common Stock; |
| ● | each
of our named executive officers and directors; and |
| ● | all
our executive officers and directors as a group. |
The percentage ownership information shown in
the table is based upon 36,765,035 shares of Common Stock outstanding as of August 9, 2023.
Beneficial
ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities.
Except as otherwise indicated, each person or entity named in the table has sole voting and investment power with respect to all shares
of our capital shown as beneficially owned, subject to applicable community property laws.
In
computing the number and percentage of shares beneficially owned by a person as of a particular date, shares that may be acquired by
such person (for example, upon the exercise of options or warrants) within 60 days of such date are counted as outstanding, while these
shares are not counted as outstanding for computing the percentage ownership of any other person.
The
address of each holder listed below, except as otherwise indicated, is c/o BioVie Inc., 680 W Nye Lane, Suite 201, Carson City, Nevada
89703.
| Name and Address of Beneficial Owner | |
Number of Common Shares of Beneficial Ownership | | |
Percentage of Beneficial Ownership | |
Named
executive officers and directors: | |
| | |
| |
| James Lang (1) | |
| 170,570 | | |
| * | |
| Richard Berman (2) | |
| 110,738 | | |
| * | |
| Steve Gorlin (3) | |
| 178,468 | | |
| * | |
| Robert Hariri (4) | |
| 119,895 | | |
| * | |
| Sigmund Rogich (5) | |
| 145,075 | | |
| * | |
| Michael Sherman (6) | |
| 202,525 | | |
| * | |
| Cuong Do (7) | |
| 738,312 | | |
| 2.0 | % |
| Joanne Wendy Kim (8) | |
| 80,397 | | |
| * | |
| Joseph Palumbo (9) | |
| 38,263 | | |
| * | |
| All directors and executive officers as a group (9) | |
| 1,784,243 | | |
| 4.8 | % |
| 5% Stockholders | |
| | | |
| | |
| Acuitas Group Holdings (10) | |
| 30,503,938 | | |
| 69.1 | % |
* Less than 1%
| (1) | | Includes warrants
to purchase 17,333 shares of Common Stock and options to purchase 134,636 shares of Common Stock, all of which are exercisable
within 60 days of August 9, 2023. |
| (2) | | Includes options
to purchase 109,138 shares of Common Stock, all of which are exercisable within 60 days of August 9, 2023. |
| (3) | | Includes options
to purchase 102,788 shares of Common Stock, all of which are exercisable within 60 days of August 9, 2023. 50,000 shares of
common stock is held by Mr. Gorlin’s wife. |
| (4) | | Includes options to
purchase 102,775 shares of Common Stock, all of which are exercisable within 60 days of August 9, 2023. |
| (5) | | Includes options
to purchase 145,175 shares of Common Stock, all of which are exercisable within 60 days of August 9, 2023. |
| (6) | | Includes warrants
to purchase 13,333 shares of Common Stock and options to purchase 168,513 shares of Common Stock, all of which are exercisable
within 60 days of August 9, 2023. Common stock held of record by Sherman Children’s Trust Brian Krisber, Trustee. All shares
of common stock, warrants and options are deemed to be beneficially owned or controlled by Michael Sherman. |
| (7) | | Includes warrants to
purchase 70,666 shares of Common Stock and options to purchase 455,681 shares of Common Stock, all of which are exercisable within
60 days of August 9, 2023. 211,965 shares of Common Stock and warrants are held of record by Do & Rickles
Investments, LLC, a limited liability company 100% owned by Cuong Do and his wife, and as such, Mr. Do may be deemed to beneficially
own or control. |
| (8) | | Include options
to purchase shares 70,967of Common Stock, all of which are exercisable within 60 days of August 9, 2023. |
| (9) | | Includes options
to purchase 30,833 shares of Common Stock, all of which are exercisable within 60 days of August 9, 2023. |
| (10) | | Includes warrants
to purchase 7,272,728 shares of Common Stock and options to purchase 65,000 shares of Common Stock, all of which are exercisable
within 60 days of August 9, 2023. All shares held of record by Acuitas Group Holdings, LLC, a limited liability company 100%
owned by Terren Peizer, and as which Mr. Peizer may be deemed to beneficially own or control. Mr. Peizer disclaims beneficial
of any such securities. |
| ITEM
13. |
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The
following includes a summary of transactions since June 30, 2022, to which we have been a party in which the amount involved exceeded
or will exceed the lesser of (i) $120,000 and (ii) one percent (1%) of the average of our total assets at year-end for the prior two
fiscal years, and in which any of our directors, executive officers or beneficial owners of more than 5% of our capital stock or any
member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest.
On July 15, 2022, the Company, entered into
a Securities Purchase Agreement (the “Purchase Agreement”) with Acuitas, pursuant to which Acuitas agreed to purchase from
the Company, in a private placement (the “Private Placement”), (i) an aggregate of 3,636,364 shares of the Company’s
Class A Common Stock, par value $0.0001 per share at a price of $1.65 per share, and (ii) a warrant to purchase 7,272,728 shares of Common
Stock, at an exercise price of $1.82, with a term of exercise of five years; (collectively, the “Securities”). The aggregate
purchase price for the Securities sold in the Private Placement was $6 million. The Private Placement closed on August 15, 2022.
Review and Approval of Transactions with
Related Persons
Either the audit committee or the Board of
Directors approves all related party transactions. The procedure for the review, approval or ratification of related party transactions
involves discussing the proposed transaction with management, discussing the proposed transaction with the external auditors, reviewing
financial statements and related disclosures, and reviewing the details of major deals and transactions to ensure that they do not involve
related party transactions. Members of management have been informed and understand that they are to bring related party transactions
to the audit committee or the Board of Directors for pre-approval. These policies and procedures are evidenced in the audit committee
charter and our code of ethics.
| ITEM
14. |
PRINCIPAL
ACCOUNTANT FEES AND SERVICES |
The
following table shows what the auditor billed for the audit and other services for the years ended June 30, 2023 and 2022.
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Audit Fees | |
$ | 317,772 | | |
$ | 223,102 | |
| Audit - Related Fees | |
| — | | |
| — | |
| Tax Fees | |
| — | | |
| — | |
| All other Fees | |
| — | | |
| — | |
| | |
| | | |
| | |
| Total | |
$ | 317,772 | | |
$ | 223,102 | |
Audit
Fees—This category includes the audit of the Company’s annual financial statements, review of financial statements included
in the Company’s Form 10-Q Quarterly Reports and services that are normally provided by the independent auditors in connection
with engagements for those years.
Audit-Related
Fees—N/A
Tax
Fees—N/A
All Other
Fees—N/A
Policy
on Audit Committee Pre-Approval of Audit and Permissible Non-audit Services of Independent Public Accountant
Consistent
with SEC policies regarding auditor independence, the Audit Committee has responsibility for appointing, setting compensation and overseeing
the work of our independent registered public accounting firm. In recognition of this responsibility, the Audit Committee has established
a policy to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm.
Prior
to engagement of an independent registered public accounting firm for the next year’s audit, management will submit an aggregate
of services expected to be rendered during that year for each of four categories of services to the Audit Committee for approval.
| 1. | Audit
services include audit work performed in the preparation of financial statements,
as well as work that generally only an independent registered public accounting firm can
reasonably be expected to provide, including comfort letters, statutory audits, and attest
services and consultation regarding financial accounting and/or reporting standards. |
| 2. | Audit-Related
services are for assurance and related services that are traditionally performed
by an independent registered public accounting firm, including due diligence related to mergers
and acquisitions, employee benefit plan audits, and special procedures required to meet certain
regulatory requirements. |
| 3. | Tax
services include all services performed by an independent registered public accounting
firm’s tax personnel except those services specifically related to the audit of the
financial statements, and includes fees in the areas of tax compliance, tax planning, and
tax advice. |
| 4. | Other
Fees are those associated with services not captured in the other categories. The
Company generally does not request such services from our independent registered public accounting
firm. |
Prior
to engagement, the Audit Committee pre-approves these services by category of service. The fees are budgeted and the Audit Committee
requires our independent registered public accounting firm and management to report actual fees versus the budget periodically throughout
the year by category of service. During the year, circumstances may arise when it may become necessary to engage our independent registered
public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the Audit Committee
requires specific pre-approval before engaging our independent registered public accounting firm.
The
Audit Committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must
report, for informational purposes only, any pre-approval decisions to the Audit Committee at its next scheduled meeting.
PART
IV
| ITEM
15. |
EXHIBITS
AND FINANCIAL STATEMENT SCHEDULES |
(a)(1),(2) Financial Statements
The
Financial Statements listed on page F-1 of this document are filed as part of this filing.
(a)(3)
Exhibits
The
following is a list of exhibits filed as a part of this report:
Exhibit
Number |
|
Description of Document |
| 2.1 |
|
Agreement and Plan of Merger, dated April 11, 2016, among the Company, LAT Acquisition Corp and LAT Pharma, LLC (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed on April 15, 2016). |
| 3.1 |
|
Articles of Incorporation of the Company as filed with the Secretary of State of Nevada (incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form S-1 filed on August 15, 2013, File No. 333-190635). |
| 3.2 |
|
Certificate of Amendment to Articles of Incorporation (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on July 22, 2016). |
| 3.3 |
|
Certificate of Amendment to Articles of Incorporation (incorporated by reference to Appendix A to the Company’s Information Statement on Schedule 14C filed on July 13, 2018). |
| 3.4 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on July 3, 2018). |
| 3.5 |
|
Certificate of Amendment to Articles of Incorporation (incorporated by reference to Exhibit 3.6 to the Company’s Registration Statement on Form S-1 filed on November 22, 2019, File No. 333-231136). |
| 3.6 |
|
Amended and Restated Bylaws of the Company, dated June 16, 2020 (incorporated by reference to Exhibit 3.5 to the Company’s Quarterly Report on Form 10-Q filed on November 10, 2021). |
| 3.7 |
|
First Amendment to the Amended and Restated Bylaws of the Company, dated March 12, 2023 (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on March 13, 2023). |
| 4.1 |
|
Specimen Certificate representing shares of Class A Common Stock (incorporated by reference to Exhibit 4.1 to the Company’s Registration Statement on Form S-1 filed on April 26, 2019, File No. 333-231136). |
| 4.2 |
|
Form of Warrant (incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on September 25, 2019). |
| 4.3 |
|
Form of 10% OID Convertible Delayed Draw Debenture (incorporated by reference to Exhibit 4.1 the Company’s Current Report on Form 8-K filed on September 25, 2019). |
| 4.4 |
|
Description of Securities (incorporated by reference to Exhibit 4.4 to the Company’s Annual Report on Form 10-K filed on August 30, 2021). |
| 4.5 |
|
Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K/A filed on July 18, 2022). |
| 4.6 |
|
Form of Warrant to Purchase Shares of Class A Common Stock of the Company (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on December 1, 2021). |
| 10.1# |
|
BioVie Inc. 2019 Omnibus Equity Incentive Plan (incorporated by reference to Appendix D to the Definitive Information Statement on Schedule 14C, filed on May 8, 2019). |
| 10.2 |
|
Asset Purchase Agreement, dated April 27, 2021, among the Company, NeurMedix, Inc. and Acuitas Group Holdings, LLC (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed on April 27, 2021). |
| 10.3 |
|
Amendment No. 1 of the Asset Purchase Agreement, dated May 9, 2021, among the Company, NeurMedix, Inc. and Acuitas Group Holdings, LLC (incorporated by reference to Exhibit 2.2 to the Company’s Current Report on Form 8-K filed on May 10, 2021). |
| 10.4 |
|
Amendment
No. 2 to the Asset Purchase Agreement, dated January 13, 2023, among the Company, Acuitas Group Holdings, LLC and Acuitas Group
Holdings, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on May 12,
2023). |
| 10.5# |
|
Employment Offer & Agreement, between Chris Reading and the Company, dated June 18, 2021 (incorporated by reference to Exhibit 10.14 to the Company’s Quarterly Report on Form 10-Q filed on November 10, 2021). |
| 10.6# |
|
Employment Offer & Agreement, between Clarence Ahlem and the Company, dated June 18, 2021 (incorporated by reference to Exhibit 10.15 to the Company’s Quarterly Report on Form 10-Q filed on November 10, 2021). |
| 10.7# |
|
Employment Offer & Agreement, between Joanne Wendy Kim and the Company, dated June 26, 2021 (incorporated by reference to Exhibit 10.16 to the Company’s Quarterly Report on Form 10-Q filed on November 10, 2021). |
| 10.8# |
|
Employment Offer & Agreement, between Penelope Markham and the Company, dated September 7, 2021 (incorporated by reference to Exhibit 10.18 to the Company’s Quarterly Report on Form 10-Q filed on November 10, 2021). |
| 10.9# |
|
Employment Offer & Agreement, between Joseph Palumbo and the Company, dated September 3, 2021 (incorporated by reference to Exhibit 10.19 to the Company’s Quarterly Report on Form 10-Q filed on November 10, 2021). |
| 10.10 |
|
Loan and Security Agreement, dated November 30, 2021, among the Company, Avenue Venture Opportunities Fund II, L.P. and Avenue Venture Opportunities Fund, L.P. (incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on December 1, 2021). |
| 10.11 |
|
Supplement to Loan and Security Agreement, dated November 30, 2021, among the Company, Avenue Venture Opportunities Fund II, L.P. and Avenue Venture Opportunities Fund, L.P. (incorporated by reference to Exhibit 10.2 to the Company’s Form 8-K filed on December 1, 2021). |
| 10.12 |
|
Securities Purchase Agreement, dated July 15, 2022, by and between the Company and Acuitas Group Holdings, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed on July 18, 2022). |
| 10.13 |
|
Controlled
Equity OfferingSM Sales Agreement, dated August 31, 2022, among the Company, Cantor Fitzgerald & Co. and B. Riley
Securities, Inc. (incorporated by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on August 31,
2022). |
| 10.14 |
|
Amended and Restated Registration Rights Agreement, dated August 15, 2022, by and between BioVie Inc. and Acuitas Group Holdings, LLC (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on November 4, 2022). |
| 14.1 |
|
Code of Conduct and Ethics of BioVie Inc. (incorporated by reference to Exhibit 14.1 to the Company’s Registration Statement on Form S-1, File No. 333-231136). |
| 23.1 |
|
Consent of Independent Registered Public Accounting Firm - EisnerAmper LLP |
| 31.1 |
|
Rule 13a-14(a) Certification |
| 31.2 |
|
Rule 13a-14(a) Certification |
| 32.1 |
|
Certification Pursuant to 18 U.S.C Section 1350, as Adopted Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 |
| 32.2 |
|
Certification Pursuant to 18 U.S.C Section 1350, as Adopted Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 |
| 101.INS |
|
XBRL Instance Document |
| 101.SCH |
|
XBRL Taxonomy Extension Schema Document |
| 101.CAL |
|
XBRL Taxonomy Calculation Linkbase Document |
| 101.LAB |
|
XBRL Taxonomy Label Linkbase Document |
| 101.PRE |
|
XBRL Taxonomy Presentation Linkbase Document |
| 101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document |
#
Indicates a management contract or compensatory plan or arrangement
Signatures
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed
on its behalf by the undersigned, thereunto duly authorized.
| |
BIOVIE
INC. |
| |
|
|
| |
By: |
/s/ Cuong Do |
| |
|
Name: |
Cuong
Do |
| |
|
Title: |
Chief
Executive Officer
(Principal Executive Officer) |
| |
|
|
|
Pursuant
to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons in the capacities and
on the dates indicated.
| Person |
|
Capacity |
|
Date |
| |
|
|
|
|
| /s/
Cuong Do |
|
Chief
Executive Officer |
|
August
16, 2023 |
| Cuong
Do |
|
(Principal
Executive Officer) |
|
|
| |
|
|
|
|
| /s/
Joanne Wendy Kim |
|
Chief
Financial Officer |
|
August
16, 2023 |
| Joanne
Wendy Kim |
|
(Principal
Financial Officer) |
|
|
| |
|
|
|
|
| /s/
Jim Lang |
|
Director |
|
August
16, 2023 |
| Jim
Lang |
|
|
|
|
| |
|
|
|
|
| /s/
Michael Sherman |
|
Director |
|
August
16, 2023 |
| Michael
Sherman |
|
|
|
|
| |
|
|
|
|
| /s/
Richard J. Berman |
|
Director |
|
August
16, 2023 |
| Richard
J. Berman |
|
|
|
|
| |
|
|
|
|
| /s/
Steve Gorlin |
|
Director |
|
August
16, 2023 |
| Steve
Gorlin |
|
|
|
|
| |
|
|
|
|
| /s/
Robert Hariri |
|
Director |
|
August
16, 2023 |
| Robert
Hariri |
|
|
|
|
| |
|
|
|
|
| /s/
Sigmund Rogich |
|
Director |
|
August
16, 2023 |
| Sigmund
Rogich |
|
|
|
|
| |
|
|
|
|
BioVie,
Inc.
Index
to Financial Statements
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Board of Directors and Stockholders of
BioVie,
Inc.
Opinion
on the Financial Statements
We have audited the accompanying balance sheets of
BioVie, Inc. (the “Company”) as of June 30, 2023 and 2022, and the related statements of operations and comprehensive loss,
changes in stockholders’ equity, and cash flows for each of the years then ended, and the related notes (collectively referred to
as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial
position of the Company as of June 30, 2023 and 2022, and the results of its operations and its cash flows for each of the years then
ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared
assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company’s recurring
losses from operations and negative cash flows from operating activities raise substantial doubt about its ability to continue as a going
concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any
adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”)
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards
of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements
are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform,
an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal
control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal
control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical
Audit Matter
The critical audit matter communicated below is a
matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit
committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially
challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on
the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion
on the critical audit matter or on the accounts or disclosures to which it relates.
Research and development expenses and related accruals
As described in Note 3 to the accompanying financial
statements, research and development expenses consists primarily of costs associated with the preclinical and/or clinical trials of drug
candidates, compensation and other expenses for research and development, supplies and development materials, costs for consultants and
related contract research and third-party facility costs. The amounts recorded for clinical trial expenses represent the Company’s
estimates of clinical trial expenses based on facts and circumstances known to the Company at that time, and are dependent upon the timely
and accurate reporting of contract research organizations and other third-party vendors.
We identified the accounting for the research
and development expenses and related accruals to be a critical audit matter due to the degree of management judgement in ensuring they
are complete and accurate, their significance, their increase from the prior year, and the risk of material misstatement due to the nature
and timing of these costs and accruals. This in turn led to a high degree of auditor judgment, subjectivity, and effort in applying the
procedures related to their accounting.
Addressing the matter involved performing procedures
and evaluating audit evidence in connection with forming our overall opinion on the financial statements. These procedures included, obtaining
an understanding of management’s process and evaluating the design of controls over research and development expenses and the completeness
and accuracy of related accruals, reading the terms of the master service agreements and statements of work for significant vendors and
making selections of transactions to determine the adequacy of the support, their mathematical accuracy and their recording as research
and development expenses. We also made inquiries of management and reviewed subsequent payments of major research and development expenses
to ensure that accruals were complete as of June 30, 2023.
/s/
EisnerAmper LLP
We
have served as the Company’s auditor since 2019.
EISNERAMPER
LLP
Iselin,
New Jersey
August
16, 2023
BioVie
Inc.
Balance
Sheets
| | |
| | |
| |
| | |
June 30 | | |
June 30, | |
| | |
2023 | | |
2022 | |
| ASSETS | |
| | |
| |
| | |
| | |
| |
| CURRENT ASSETS: | |
| | | |
| | |
| Cash and
cash equivalents | |
$ | 19,460,883 | | |
$ | 18,641,716 | |
| Investments in U.S.
Treasury Bills | |
| 14,477,726 | | |
| — | |
| Prepaids
and other assets | |
| 102,526 | | |
| 137,879 | |
| Total
current assets | |
| 34,041,135 | | |
| 18,779,595 | |
| | |
| | | |
| | |
| Operating lease right-of-use
assets | |
| 80,789 | | |
| 118,254 | |
| Intangible assets,
net | |
| 637,095 | | |
| 866,472 | |
| Goodwill | |
| 345,711 | | |
| 345,711 | |
| Other assets, non-current | |
| — | | |
| 4,562 | |
| | |
| | | |
| | |
| TOTAL
ASSETS | |
$ | 35,104,730 | | |
$ | 20,114,594 | |
| | |
| | | |
| | |
| LIABILITIES AND
STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| | |
| | | |
| | |
| CURRENT LIABILITIES: | |
| | | |
| | |
| Accounts payable and
accrued expenses | |
$ | 3,476,259 | | |
$ | 2,442,804 | |
| Current portion of
other liabilities | |
| 48,385 | | |
| 1,304,925 | |
| Current portion of
operating lease liabilities | |
| 44,909 | | |
| 38,884 | |
| Current portion of
Note payable, net of financing cost, unearned premium and discount of $894,926 at June 30, 2023 | |
| 9,105,074 | | |
| — | |
| Warrant liabilities | |
| 894,280 | | |
| 194,531 | |
| Embedded
derivative liability | |
| 925,762 | | |
| 188,030 | |
| Total
current liabilities | |
| 14,494,669 | | |
| 4,169,174 | |
| | |
| | | |
| | |
| Other liabilities,
net of current portion | |
| — | | |
| 48,385 | |
| Operating lease liabilities,
net of current portion | |
| 42,505 | | |
| 87,414 | |
| Note
payable, net of current portion, financing cost, unearned premium and discount of $227,268 at June 30, 2023 and $2,861,314
at June 30, 2022 | |
| 5,227,270 | | |
| 12,138,686 | |
| TOTAL
LIABILITIES | |
| 19,764,444 | | |
| 16,443,659 | |
| | |
| | | |
| | |
| Commitments and contingencies
(Note 12) | |
| | | |
| | |
| | |
| | | |
| | |
| STOCKHOLDERS’
EQUITY : | |
| | | |
| | |
| | |
| | | |
| | |
| Preferred stock; $0.001
par value; 10,000,000 shares authorized; 0 shares issued and outstanding | |
| — | | |
| — | |
| Common stock, $0.0001
par value; 800,000,000 shares authorized at June 30, 2023 and June 30, 2022, respectively; 36,451,829 shares issued of which 36,428,949
shares outstanding at June 30, 2023 and 24,984,083 issued and outstanding at June 30, 2022; | |
| 3,643 | | |
| 2,496 | |
| Additional paid in capital | |
| 316,385,759 | | |
| 254,638,329 | |
| Accumulated other comprehensive
income | |
| 176,591 | | |
| | |
| Accumulated deficit | |
| (301,225,705 | ) | |
| (250,969,890 | ) |
| Treasury
stock | |
| (2 | ) | |
| — | |
| Total
stockholders’ equity | |
| 15,340,286 | | |
| 3,670,935 | |
| | |
| | | |
| | |
| TOTAL
LIABILITIES AND STOCKHOLDERS’ EQUITY | |
$ | 35,104,730 | | |
$ | 20,114,594 | |
The
accompanying notes are an integral part of the financial statements.
BioVie
Inc.
Statements
of Operations and Comprehensive Loss
| | |
| | |
| |
| | |
Year ended | | |
Year ended | |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| OPERATING EXPENSES: | |
| | | |
| | |
| Amortization | |
$ | 229,377 | | |
$ | 229,377 | |
| Research and development expenses | |
| 33,299,503 | | |
| 17,258,341 | |
| Selling, general and administrative expenses | |
| 11,551,568 | | |
| 9,765,259 | |
| TOTAL OPERATING EXPENSES | |
| 45,080,448 | | |
| 27,252,977 | |
| | |
| | | |
| | |
| LOSS FROM OPERATIONS | |
| (45,080,448 | ) | |
| (27,252,977 | ) |
| | |
| | | |
| | |
| OTHER EXPENSE (INCOME): | |
| | | |
| | |
| Change in fair value of derivative liabilities | |
| 1,437,481 | | |
| (3,287,418 | ) |
| Interest expense | |
| 4,300,150 | | |
| 2,162,989 | |
| Interest income | |
| (562,264 | ) | |
| (44,080 | ) |
| TOTAL OTHER EXPENSE (INCOME), NET | |
| 5,175,367 | | |
| (1,168,509 | ) |
| | |
| | | |
| | |
| NET LOSS | |
$ | (50,255,815 | ) | |
$ | (26,084,468 | ) |
| | |
| | | |
| | |
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS | |
$ | (50,255,815 | ) | |
$ | (26,084,468 | ) |
| | |
| | | |
| | |
| NET LOSS PER COMMON SHARE | |
| | | |
| | |
| - Basic | |
$ | (1.55 | ) | |
$ | (1.06 | ) |
| - Diluted | |
$ | (1.55 | ) | |
$ | (1.06 | ) |
| | |
| | | |
| | |
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING | |
| | | |
| | |
| - Basic | |
| 32,483,489 | | |
| 24,662,557 | |
| - Diluted | |
| 32,483,489 | | |
| 24,662,557 | |
| | |
| | | |
| | |
| NET LOSS | |
$ | (50,255,815 | ) | |
$ | (26,084,468 | ) |
| | |
| | | |
| | |
| Other comprehensive income | |
| | | |
| | |
| Unrealized gain on investments for available-for-sale | |
| 176,591 | | |
| — | |
| Other comprehensive income | |
| 176,591 | | |
| — | |
| Comprehensive loss | |
$ | (50,079,224 | ) | |
$ | (26,084,468 | ) |
The
accompanying notes are an integral part of the financial statements.
BioVie
Inc.
Statements
of Changes in Stockholders’ Equity
For the Years Ended June 30, 2023 and 2022
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | |
| |
| |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
| | |
| |
| |
Additional | | |
| | |
| | |
Other | | |
| | |
Total | |
| | |
Common
Stock | | |
Common
Stock | |
| |
Paid
in | | |
Treasury
Stock | | |
Treasury
Stock | | |
Comprehensive | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amount | |
| |
Capital | | |
Shares | | |
Amount | | |
Income | | |
Deficit | | |
Equity | |
| | |
| | |
| |
| |
| | |
| | |
| | |
| | |
| | |
| |
| Balance,
June 30, 2021 | |
| 22,333,324 | | |
$ | 2,232 | |
| | |
$ | 229,933,505 | | |
| — | | |
$ | — | | |
$ | — | | |
$ | (224,885,422 | ) | |
$ | 5,050,315 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock
option-based compensation | |
| — | | |
| — | |
| | |
| 5,807,871 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 5,807,871 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Proceeds
from issuance of common stock, net costs of $2,224,992 | |
| 2,592,000 | | |
| 259 | |
| | |
| 18,510,750 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 18,511,009 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock
based compensation – restricted stock | |
| 58,759 | | |
| 5 | |
| | |
| 386,203 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 386,208 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net
loss | |
| — | | |
| — | |
| | |
| — | | |
| — | | |
| — | | |
| — | | |
| (26,084,468 | ) | |
| (26,084,468 | ) |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance,
June 30, 2022 | |
| 24,984,083 | | |
| 2,496 | |
| | |
| 254,638,329 | | |
| — | | |
| — | | |
| — | | |
| (250,969,890 | ) | |
| 3,670,935 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock
option-based compensation | |
| — | | |
| — | |
| | |
| 4,222,845 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 4,222,845 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based
compensation – restricted stock units | |
| 215,175 | | |
| 21 | |
| | |
| 1,780,028 | | |
| (22,880 | ) | |
| (2 | ) | |
| — | | |
| — | | |
| 1,780,047 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock-based
compensation – issuance of common stock | |
| 50,000 | | |
| 5 | |
| | |
| 372,495 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 372,500 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| — | |
| Cashless
exercise of options | |
| 22,563 | | |
| 3 | |
| | |
| (3 | ) | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| — | |
| Cashless
exercise of warrants | |
| 3,590 | | |
| — | |
| | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| — | |
| Proceeds
from exercise of options | |
| 800 | | |
| — | |
| | |
| 2,240 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 2,240 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Proceeds
from issuance of common stock, net costs of $2,008,898 | |
| 7,539,254 | | |
| 754 | |
| | |
| 49,464,349 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 49,465,103 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Proceeds
from issuance of common stock, net of costs of $94,160 – Related Party | |
| 3,636,364 | | |
| 364 | |
| | |
| 5,905,476 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 5,905,840 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Unrealized
gain on available-for-sale securities | |
| — | | |
| — | |
| | |
| — | | |
| — | | |
| — | | |
| 176,591 | | |
| — | | |
| 176,591 | |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net
loss | |
| — | | |
| — | |
| | |
| — | | |
| — | | |
| — | | |
| — | | |
| (50,255,815 | ) | |
| (50,255,815 | ) |
| | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance,
June 30, 2023 | |
| 36,451,829 | | |
$ | 3,643 | |
| | |
$ | 316,385,759 | | |
| (22,880 | ) | |
$ | (2 | ) | |
$ | 176,591 | | |
$ | (301,225,705 | ) | |
$ | 15,340,286 | |
The
accompanying notes are an integral part of the financial statements.
BioVie
Inc.
Statements
of Cash Flows
| | |
| | |
| |
| | |
Year ended | | |
Year ended | |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| CASH FLOWS FROM OPERATING ACTIVITIES: | |
| | | |
| | |
| Net loss | |
$ | (50,255,815 | ) | |
$ | (26,084,468 | ) |
| Adjustments to reconcile net loss to net cash used in operating
activities: | |
| | | |
| | |
| Amortization of intangible assets | |
| 229,377 | | |
| 229,377 | |
| Stock based compensation – restricted stock units | |
| 1,780,047 | | |
| 386,208 | |
| Stock based compensation expense – stock options | |
| 4,222,845 | | |
| 5,807,871 | |
| Stock based compensation expense – stock issued | |
| 372,500 | | |
| — | |
| Amortization of financing costs | |
| 170,219 | | |
| 99,295 | |
| Accretion of unearned loan discount | |
| 1,601,445 | | |
| 934,177 | |
| Accretion of loan premium | |
| 421,994 | | |
| 165,278 | |
| Change in operating lease right-of-use assets | |
| 37,465 | | |
| 8,044 | |
| Change in fair value of derivative liabilities | |
| 1,437,481 | | |
| (3,287,418 | ) |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Prepaids and other assets | |
| 39,915 | | |
| (48,954 | ) |
| Accounts payable and accrued expenses | |
| 1,033,455 | | |
| 1,446,430 | |
| Operating lease liabilities | |
| (38,884 | ) | |
| — | |
| Other liabilities | |
| (1,304,925 | ) | |
| 1,353,310 | |
| Net cash used in operating activities | |
| (40,252,881 | ) | |
| (18,990,850 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | |
| | | |
| | |
| Purchases of U.S. Treasury Bills | |
| (14,301,135 | ) | |
| — | |
| Net cash used in investing activities | |
| (14,301,135 | ) | |
| — | |
| | |
| | | |
| | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | |
| | | |
| | |
| Net proceeds from issuance of common stock | |
| 49,465,103 | | |
| 18,511,009 | |
| Proceeds from note payable net of financing costs | |
| — | | |
| 14,609,915 | |
| Proceeds from exercise of stock options | |
| 2,240 | | |
| — | |
| Net proceeds from issuance of common
stock – Related Party | |
| 5,905,840 | | |
| — | |
| Net cash provided by financing activities | |
| 55,373,183 | | |
| 33,120,924 | |
| | |
| | | |
| | |
| Net increase in cash and cash equivalents | |
| 819,167 | | |
| 14,130,074 | |
| | |
| | | |
| | |
| Cash and cash equivalents,
beginning of period | |
| 18,641,716 | | |
| 4,511,642 | |
| | |
| | | |
| | |
| Cash and cash equivalents,
end of period | |
$ | 19,460,883 | | |
$ | 18,641,716 | |
| | |
| | | |
| | |
| SUPPLEMENTAL CASH FLOW INFORMATION: | |
| | | |
| | |
| Cash paid for interest | |
$ | 2,106,491 | | |
$ | 964,241 | |
| | |
| | | |
| | |
| SCHEDULE OF NON-CASH
FINANCING AND INVESTING ACTIVITIES: | |
| | | |
| | |
| Right of use assets
obtained in exchange for lease obligations | |
$ | — | | |
$ | 130,039 | |
| Unrealized gain on
U.S. Treasury Bills | |
$ | 176,591 | | |
$ | — | |
The
accompanying notes are an integral part of the financial statements.
BioVie
Inc.
Notes
to Financial Statements
For
the Years Ended June 30, 2023 and 2022
| 1. |
Background
Information |
BioVie
Inc. (the “Company” or “we” or “our”) is a clinical-stage company developing innovative drug therapies
to treat chronic debilitating conditions including neurological and neuro-degenerative disorders and liver disease.
The
Company acquired the biopharmaceutical assets of NeurMedix, Inc. (“NeurMedix”), from a related party privately held clinical-stage
pharmaceutical company, in June 2021. The acquired assets included NE3107, a potentially selective inhibitor of inflammatory extracellular
single-regulated kinase(“ERK”) signaling that, based on animal studies and is believed to reduce neuroinflammation. NE3107
is a novel orally administered small molecule that is thought to inhibit inflammation-driven insulin resistance and major pathological
inflammatory cascades with a novel mechanism of action. There is emerging scientific consensus that both inflammation and insulin resistance
may play fundamental roles in the development of Alzheimer’s Disease (AD) and Parkinson’s Disease (PD), and NE3107 could,
if approved represent an entirely new medical approach to treating these devastating conditions affecting an estimated 6 million Americans
suffering from AD and 1 million Americans suffering from PD.
The
Company is conducting a potentially pivotal Phase 3 randomized, double-blind, placebo-controlled, parallel-group, multicenter study to
evaluate NE3107 in patients who have mild to moderate Alzheimer’s disease (NCT04669028). The Company is targeting primary completion
of this study in the fourth quarter of calendar year 2023.
The
Company completed its Phase 2 study assessing NE3107 in Parkinson’s disease patients in the fourth quarter of calendar year 2022.
The NM201 study (NCT05083260) was a double-blind, placebo-controlled, safety, tolerability, and pharmacokinetics study in Parkinson’s
disease (PD) participants treated with carbidopa/levodopa and NE3107. The study was primarily designed to assess safety (general safety
in the patient population and potential for drug-drug interactions of NE3107 with levodopa); and secondary, to look for indications of
promotoric activity akin to promotoric activity and apparent enhancement of levodopa activity observed in preclinical models. Both the
safety and efficacy objectives of the study were met.
Neuroinflammation,
insulin resistance, and oxidative stress are common features in the major neurodegenerative diseases, including Alzheimer’s Disease
(AD), Parkinson’s Disease (PD), frontotemporal lobar dementia, and Amyotrophic lateral sclerosis (ALS). NE3107 is
an orally bioavailable, blood-brain permeable, small molecule, with potential anti-inflammatory, insulin sensitizing, and ERK-binding
properties that may allow it to selectively inhibit ERK-, NFκB- and TNF-stimulated inflammation. NE3107’s potential to inhibit
neuroinflammation and insulin resistance forms the basis for the Company’s work testing the molecule in AD and PD patients. NE3107
is patented in the United States, Australia, Canada, Europe and South Korea.
The Company’s Orphan Drug candidate BIV201
(continuous infusion terlipressin), with FDA Fast Track status, is being evaluated in a U.S. Phase 2b study (NCT04112199) for the treatment
of refractory ascites due to liver cirrhosis. BIV201 is administered as a patent-pending liquid formulation. The study was closed before
full enrollment, without clinically meaningful adverse effects associated with BIV201 treatment. While the active agent is approved in
the U.S. and in about 40 countries for related complications of advanced liver cirrhosis, treatment of ascites is not included in these
authorizations. Patients with refractory ascites suffer from frequent life-threatening complications, generate more than $5 billion in
annual treatment costs, and have an estimated 50% mortality rate within 6 to 12 months. The U.S. Food and Drug Administration (“FDA”)
has not approved any drug to treat refractory ascites.
The BIV201 development program was initiated by
LAT Pharma LLC (LAT Pharma). On April 11, 2016, the Company acquired LAT Pharma and the rights to its BIV201 development
program. The Company currently owns all development and marketing rights to this drug candidate. Pursuant to the Agreement and Plan of
Merger entered into on April 11, 2016, between our predecessor entities, LAT Pharma and NanoAntibiotics, Inc., the Company is obligated
to pay a low single digit royalty on net sales of BIV201 (continuous infusion terlipressin), if approved, to be shared by the members
of LAT Pharma, PharmaIn Corporation and The Barrett Edge, Inc.
BioVie
Inc.
Notes
to Financial Statements
For
the Years Ended June 30, 2023 and 2022
| 2. |
Liquidity
and Going Concern |
The Company’s operations are subject to
a number of factors that can affect its operating results and financial conditions. Such factors include, but are not limited to: the
results of clinical testing and trial activities of the Company’s products, the Company’s ability to obtain regulatory approval
to market its products; competition from products manufactured and sold or being developed by other companies; the price of, and demand
for, Company products; the Company’s ability to negotiate favorable licensing or other manufacturing and marketing agreements for
its products; and the Company’s ability to raise capital. The Company’s financial statements have been prepared assuming the
Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal
course of business. As of June 30, 2023 the Company had working capital of approximately $19.5 million, cash and cash equivalents and
US treasury bills totaling of approximately $33.9 million, stockholders’ equity of approximately $15.3 million, and an accumulated
deficit of approximately $301 million. The Company is in the pre-revenue stage and no revenues are expected in the foreseeable future.
The Company’s future operations are dependent on the success of the Company’s ongoing development and commercialization efforts,
as well as its ability to secure additional financing as needed. Although our cash balance may possibly sustain operations over the next
12 months from the balance sheet date if measures are taken to delay planned expenditures in our research protocols and slow the progress
in the Company’s development of next phase clinical programs, the Company’s current planned operations to meet certain goals
and objectives, project cash flows to be depleted within that period of time.
The
future viability of the Company is largely dependent upon its ability to raise additional capital to finance its operations. Management
expects that future sources of funding may include sales of equity, obtaining loans, or other strategic transactions.
The Impact of COVID-19 pandemic created a widespread
labor shortage, including a shortage of medical professionals, and has impacted and may continue to impact the potential patient participation
in our studies, which may adversely impact our ability to continue or complete our clinical trials in the planned timeline.
Although
management continues to pursue the Company’s strategic plans, there is no assurance that the Company will be successful in obtaining
sufficient financing on terms acceptable to the Company, if at all, to fund continuing operations. These circumstances raise substantial
doubt on the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might
result from the outcome of this uncertainty.
| 3. |
Significant
Accounting Policies |
Basis
of Presentation
The
Company’s financial statements have been prepared in accordance with accounting principles generally accepted in the United States
(“GAAP”) and include all adjustments necessary for the fair presentation of the Company’s financial position for the
periods presented.
Use
of Estimates
The
preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts
reported in the financial statements and accompanying notes. The Company bases its estimates on historical experience and on various
assumptions that are believed to be reasonable under the circumstances. The amounts of assets and liabilities reported in the Company’s
balance sheet and the amounts of expenses reported for each of the periods presented are affected by estimates and assumptions, which
are used for, but not limited to, accounting for clinical accruals, share-based compensation, accounting for derivatives, assumptions used in leases and recoverability of intangible assets, the inputs used in the valuation of goodwill and intangible assets in connection with impairment testing and accounting for income taxes.
Actual results could differ from those estimates.
Cash
and cash equivalents
Cash and cash equivalents consisted of cash deposits
and money market funds held at a bank and funds held in a brokerage account which included a U.S. treasury money market fund and U.S.
Treasury Bills with original maturities of three months or less.
BioVie
Inc.
Notes
to Financial Statements
For
the Years Ended June 30, 2023 and 2022
| 3. |
Significant
Accounting Policies (continued) |
Investments
in U.S. Treasury Bills
Investments in U.S. Treasury Bills with maturities
greater than three months, are accounted for as available for sale and are recorded at fair value. Unrealized gains were included in other
comprehensive income in the accompanying statements of operations and comprehensive loss.
Concentration of Credit Risk in the Financial
Service Industry
As of June 30, 2023, the Company had cash deposited
in certain financial institutions in excess of federally insured levels. The Company regularly monitors the financial stability of these
financial institutions and believes that it is not exposed to any significant credit risk in cash and cash equivalents. However, in March
and April 2023, certain U.S. government banking regulators took steps to intervene in the operations of certain financial institutions
due to liquidity concerns, which caused general heightened uncertainties in financial markets. While these events have not had a material
direct impact on the Company’s operations, if further liquidity and financial stability concerns arise with respect to banks and
financial institutions, either nationally or in specific regions, the Company’s ability to access cash or enter into new financing
arrangements may be threatened, which could have a material adverse effect on its business, financial condition and results of operations.
Fair
value measurement of assets and liabilities
We
determine the fair values of our financial instruments based on the fair value hierarchy, which requires an entity to maximize the use
of observable inputs and minimize the use of unobservable inputs when measuring fair value. Fair value is defined as the price that would
be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement
date. The fair value assumes that the transaction to sell the asset or transfer the liability occurs in the principal or most advantageous
market for the asset or liability and establishes that the fair value of an asset or liability shall be determined based on the assumptions
that market participants would use in pricing the asset or liability. The classification of a financial asset or liability within the
hierarchy is based upon the lowest level input that is significant to the fair value measurement. The fair value hierarchy prioritizes
the inputs into three levels that may be used to measure fair value:
Level
1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities.
Level
2 - Inputs are quoted prices for similar assets and liabilities in active markets or inputs that are observable for the asset or liability,
either directly or indirectly through market corroboration, for substantially the full term of the financial instrument.
Level
3 - Inputs are unobservable inputs based on our assumptions.
The Company’s financial instruments include
cash, accounts payable, the carrying value of the operating lease liabilities and notes payable. The carrying amounts of cash and accounts
payable approximate their fair value, due to the short-term nature of these items. The carrying amounts of notes payable and operating
lease liabilities approximate their fair values since they bear interest at rates which approximate market rates for similar debt instruments.
Prepaid
and other Assets
Prepaid
and other assets consist of prepayments of certain expenses and direct costs related to capital raise which will offset proceeds upon
the close.
Other
Assets, non-current
Other
assets consist of a security deposit for an office lease.
BioVie
Inc.
Notes
to Financial Statements
For
the Years Ended June 30, 2023 and 2022
| 3. |
Significant
Accounting Policies (continued) |
Leases
The
Company determines whether an arrangement contains a lease at inception. Operating leases are included in operating lease right-of-use
(“ROU”) assets, current portion of operating lease liabilities, and operating lease liabilities, net of current portion on
our balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities
represent an obligation to make lease payments arising from the lease. Lease ROU assets and lease liabilities are recognized based on
the present value of the future minimum lease payments over the lease term at the commencement date. As the Company’s leases do
not provide an implicit rate, an incremental borrowing rate is used based on the information available at the commencement date in determining
the present value of lease payments. The Company does not include options to extend or terminate the lease term in its calculation unless
it is reasonably certain that the Company will exercise any such options. Rent expense is recognized under the operating leases on a
straight-line basis. The Company does not recognize right of-use assets or lease liabilities for short-term leases, which have a lease
term of 12 months or less at inception, and instead will recognize lease payments as expense on a straight-line basis over the lease term.
Research
and Development
Research
and development expenses consist primarily of costs associated with the preclinical and/ or clinical trials of drug candidates, compensation
and other expenses for research and development, personnel, supplies and development materials, costs for consultants and related contract
research and facility costs.
Income
Taxes
The
Company uses the asset and liability method of accounting for deferred income taxes. Deferred income taxes are measured by applying enacted
statutory rates to net operating loss carryforwards and to the differences between the financial reporting and tax bases of assets and
liabilities. Deferred tax assets are reduced, if necessary, by a valuation allowance if it is more likely than not that some portion
or all of the deferred tax assets will not be realized.
The
Company recognizes uncertainty in income taxes in the financial statements using a recognition threshold and measurement attribute
of a tax position taken or expected to be taken in a tax return. The Company applies the “more-likely-than-not”
recognition threshold to all tax positions, commencing at the adoption date of the applicable accounting guidance, which resulted in
no unrecognized tax benefits as of such date. Additionally, there have been no unrecognized tax benefits subsequent to adoption. The
Company has opted to classify interest and penalties that would accrue, if any, according to the provisions of relevant tax law as
general and administrative expenses, in the Statements of Operations and Comprehensive Loss. For the years ended June 30, 2023 and 2022, there was no
such interest or penalty.
Net
Loss per Common Share
Basic net loss per common share is computed by
dividing the net loss attributable to Common Stockholders by the weighted average number of shares of Common Stock outstanding during
the period. Diluted net loss per common share is computed by dividing the net loss attributable to Common Stockholders by the weighted
average number of shares of Common Stock outstanding and potentially outstanding shares of Common Stock during the period to reflect the
potential dilution that could occur from common shares issuable through stock options, warrants, and convertible debentures. For the years
ended June 30, 2023 and 2022, such amounts were excluded from the diluted loss since their effect was considered anti-dilutive due to
the net loss for the periods.
The
table below shows the number of outstanding stock options, warrants and restricted stock units as of June 30:
| Schedule of Dilutive securities were excluded from the computation of diluted loss per share | |
| | | |
| | |
| | |
| | |
| |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
Number of Shares | | |
Number of Shares | |
| Stock Options | |
| 3,952,864 | | |
| 3,398,764 | |
| Warrants | |
| 7,770,285 | | |
| 510,372 | |
| Restricted Stock Units | |
| 596,457 | | |
| 124,520 | |
| Total | |
| 12,319,606 | | |
| 4,033,656 | |
BioVie
Inc.
Notes
to Financial Statements
For
the Years Ended June 30, 2023 and 2022
| 3. |
Significant
Accounting Policies (continued) |
Stock-based
Compensation
The
Company has accounted for stock-based compensation under the provisions of FASB ASC 718 – “Stock Compensation” which
requires the use of the fair-value based method to determine compensation for all arrangements under which employees and others receive
shares of stock or equity instruments (stock options and Common Stock purchase warrants). For employee awards, the fair value of each
stock option award is estimated on the date of grant using the Black-Scholes valuation model that uses assumptions for expected volatility,
expected dividends, expected term, and the risk-free interest rate. For non-employees, the fair value of each stock option award is estimated
on the measurement date using the Black-Scholes valuation model that uses assumptions for expected volatility, expected dividends, expected
term, and the risk-free interest rate. For non-employees, the Company utilizes the graded vesting attribution method under which the
entity treats each separately vesting portion (tranche) as a separate award and recognizes compensation cost for each tranche over its
separate vesting schedule. Expected volatilities are based on historical volatility of peer companies and other factors estimated over
the expected term of the stock options. For employee awards, the expected term of options granted is derived using the “simplified
method” which computes expected term as the average of the sum of the vesting term plus the contract term. The risk-free rate is
based on the U.S. Treasury yield curve in effect at the time of grant for the period of the expected term. The Company recognizes forfeitures
as they occur.
Goodwill
Goodwill
is recorded when the purchase price paid for an acquisition exceeds the fair value of net identified tangible and intangible assets acquired.
The Company performs an annual impairment test of goodwill and further periodic tests to the extent indicators of impairment develop
between annual impairment tests. The Company’s impairment review process compares the fair value of the reporting unit to its carrying
value, including the goodwill related to the reporting unit. To determine the fair value of the reporting unit, the Company may use various
approaches including an asset or cost approach, market approach or income approach or any combination thereof. These approaches may require
the Company to make certain estimates and assumptions including future cash flows, revenue and expenses. These estimates and assumptions
are reviewed each time the Company tests goodwill for impairment and are typically developed as part of the Company’s routine business
planning and forecasting process. While the Company believes its estimates and assumptions are reasonable, variations from those estimates
could produce materially different results. The Company did not recognize any goodwill impairments for the years ended June 30, 2023
and 2022.
Impairment
of Long-Lived Assets
Long-lived
assets, including intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying
amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount
of an asset to estimated undiscounted future cash flows expected to be generated by the asset.
If
the carrying amount of an asset exceeds its undiscounted estimated future cash flows, an impairment review is performed. An impairment
charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Generally, fair value
is determined using valuation techniques such as expected discounted cash flows or appraisals, as appropriate. Assets to be disposed
of would be separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell,
and are no longer depreciated or amortized. The assets and liabilities of a disposed group classified as held for sale would be presented
separately in the appropriate asset and liability sections of the balance sheets.
Recent
Accounting Pronouncements
The
Company considers the applicability and impact of all Accounting Standards Updates (“ASU’s”). There were no recent
ASU’s that are expected to have a material impact on our balance sheets or statements of operations and comprehensive loss.
In June 2016, the Financial Accounting Standards
Board (“FASB”) issued ASU No. 2016-13, “Financial Instruments - Credit Losses (Topic 326), Measurement of Credit Losses
on Financial Instruments.” This amendment replaces the incurred loss impairment methodology in current GAAP with a methodology
that reflects expected credit losses on instruments within its scope, including trade receivables. This update is intended to provide
financial statement users with more decision-useful information about the expected credit losses. In November 2019, the FASB issued No.
2019-10, Financial Instruments --Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842), which deferred
the effective date of ASU 2016-13 for Smaller Reporting Companies for fiscal years beginning after December 15, 2022, including interim
periods within those fiscal years. The Company does not expect a material impact from the adoption of ASU 2016-13 on the financial statements.
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
4.
Investments in U.S. Treasury Bills available for sale
The
following is a summary of the U.S. Treasury Bills held at June 30, 2023:
| Schedule of U.S. treasury bills held | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Amortized
Cost Basis | | |
Gross
Unrealized Gain | | |
Gross
Unrealized loss | | |
Fair
Value | | |
Total
Accumulated Other Comprehensive Income | |
| U.S.
Treasury Bills due is 3 - 6 months | |
$ | 14,301,136 | | |
$ | 176,591 | | |
$ | — | | |
$ | 14,477,726 | | |
$ | 176,591 | |
The Company purchased a total of approximately $46 million of U.S.
Treasury Bills during the year ended June 30, 2023. The U.S Treasury Bills that matured were approximately $18 million and none were
sold before maturity.
The
Company’s intangible assets consist of intellectual property acquired from LAT Pharma, Inc. and are amortized over their estimated
useful lives. The following is a summary of the intangible assets as of June 30, 2023 and 2022:
| Schedule of intangible assets | |
| | | |
| | |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| Intellectual Property | |
$ | 2,293,770 | | |
$ | 2,293,770 | |
| Less Accumulated Amortization | |
| (1,656,675 | ) | |
| (1,427,298 | ) |
| Intellectual Property, Net | |
$ | 637,095 | | |
$ | 866,472 | |
Amortization
expense amounted to $229,377 for each of the years ended June 30, 2023 and 2022, respectively. The Company amortizes intellectual property
over the expected original useful lives of 10 years.
Estimated
future amortization expense is as follows:
| | Schedule of future amortization expense | | |
| | |
| Year ending June 30, | | |
| |
| | 2024 | | |
$ | 229,377 | |
| 2025 | | |
| 229,377 | |
| | 2026 | | |
| 178,341 | |
| | | | |
$ | 637,095 | |
| 6. |
Related
Party Transactions |
Equity
Transactions with Acuitas
On
July 15, 2022, the Company entered into a securities purchase agreement with Acuitas Group Holdings, LLC (Acuitas), the Company’s
majority stockholder, pursuant to which Acuitas agreed to purchase from the Company, in a private placement, (i) an aggregate of 3,636,364
shares of the Company’s Common Stock, at a price of $1.65 per share (the “PIPE Shares”), and (ii) a warrant to purchase
7,272,728 shares of Common Stock (“PIPE Warrant Shares”), at an exercise price of $1.82, with a term of exercise of five
years. The warrant has a down round feature that reduces the exercise price of the warrant if the Company sells stock at a price lower
than the exercise price of the warrant. On August 15, 2022, the Company received net proceeds of approximately $5.9 million, net of costs
of approximately $94,000, and entered into an amended and restated registration agreement with Acuitas, which amended and restated that
certain registration rights agreement, dated as of June 10, 2021, by and between the Company and Acuitas (the “Existing Registration
Rights Agreement”), to amend the definition of “Registrable Securities” in the Existing Registration Rights Agreement
to include the PIPE Shares and the PIPE Warrant Shares as Registrable Securities thereunder.
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
| 6. |
Related
Party Transactions (continued) |
Asset
Acquisition with NeurMedix
On April 27, 2021, the Company entered into an
Asset Purchase Agreement (“APA”) with NeurMedix and Acuitas, which are related party affiliates, pursuant to which the Company
acquired certain assets from NeurMedix and assumed certain liabilities of NeurMedix. The acquired assets include, among others, certain
assets related to the drug candidates then being developed by NeurMedix, including NE3107. On June 10, 2021, and pursuant to the terms
of the APA, the Company issued to Acuitas (as NeurMedix’s assignee) 8,361,308 shares of the Company’s Common Stock and made
a cash payment to Acuitas of approximately $2.3 million. Since the transaction was between entities under common control, there were no
fair value adjustments of the purchased assets, and the historical cost basis of the purchased assets was zero. The total consideration
paid was expensed as in process research and development expense in the year ended June 30, 2021.
Subject to the terms and conditions of APA, as
amended on May 9, 2021, the Company may be obligated to deliver contingent stock consideration to NeurMedix (or its successor) consisting
of up to 18 million shares of the Company’s Common Stock, with 4.5 million shares issuable upon the achievement of each of the
four milestones related to certain clinical, regulatory and commercial milestones set forth in the APA, subject to a cap limiting the
issuance of shares if such issuance would result in the beneficial ownership of NeurMedix and its affiliates exceeding 87.5% of the
Company’s issued and outstanding Common Stock.
The
current portion of other liabilities at June 30, 2023 and June 30, 2022 were approximately $48,400 and $1.3 million, and included $48,400 and $580,614,
respectively, of a retention bonus payable for arrangements with certain employees. The payment terms of the total retention bonus arrangements
of $1,161,000 recognized in August 2021 provided for equal monthly installments over a 24-month period and began in August 2021.
On
November 30, 2021 (the “Closing Date”), the Company entered into a Loan and Security Agreement and the Supplement to the
Loan and Security Agreement and Promissory Notes (together, the “Loan Agreement”) with Avenue Venture Opportunities Fund,
L.P. (“AVOPI”) and Avenue Venture Opportunities Fund II, L.P. (“AVOPII,” and together with AVOPI, “Avenue”)
for growth capital loans in an aggregate commitment amount of up to $20 million (the “Loan”). On the Closing Date, $15 million
of the Loan was funded (“Tranche 1”). The Loan provided for an additional $5 million to be available to the Company on or
prior to September 15, 2022, subject to the Company’s achievement of certain milestones with respect to certain of its ongoing
clinical trials, which were not achieved. The Loan bears interest at an annual rate equal to the greater of (a) the sum of 7.00% plus
the prime rate as reported in The Wall Street Journal and (b) 10.75%. The prime rate at June 30, 2023 was 8.25%. The Loan is secured
by a lien upon and security interest in all of the Company’s assets, including intellectual property, subject to agreed exceptions.
The maturity date of the Loan is December 1, 2024.
The
Loan Agreement requires monthly interest-only payments during the first eighteen months of the term of the Loan. Following the interest-only
period, the Company will make equal monthly payments of principal, plus accrued interest, until the Loan’s maturity date when all
remaining principal and accrued interest is due. If the Company prepays the Loan, it will be required to pay (a) a prepayment fee in
an amount equal to 3.0% of the principal amount of the Loan that is prepaid during the interest-only period; and (b) a prepayment fee
in an amount equal to 1.0% of the principal amount of the Loan that is prepaid after the interest-only period. At the Loan’s maturity
date, or on the date of the prepayment of the Loan, the Company will be obligated to pay a final payment equal to 4.25% of the Loan commitment
amount, the sum of Tranche 1 and Tranche 2.
The
Loan Agreement includes a conversion option to convert up to $5.0 million of the principal amount of the Loan outstanding at the option
of Avenue, into shares of the Company’s Common Stock at a conversion price of $6.98 per share.
On
the Closing Date, the Company issued to Avenue warrants to purchase 361,002 shares of Common Stock of the Company (the “Avenue
Warrants”) at an exercise price per share equal to $5.82. The Avenue Warrants are exercisable until November 30, 2026.
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
| 8. |
Notes
Payable (continued) |
The
amount of the carrying value of the notes payable was determined by allocating portions of the outstanding principal of the notes; approximately
$1.4 million to the fair value of the Avenue Warrants and approximately $2.2 million to the fair value of the embedded conversion option.
Accordingly, the total amount of unearned discount of approximately $3.7 million, the total direct financing cost of approximately $390,000
and premium of $850,000 are recognized on an effective interest method over the term of the Loan. The adjusted effective interest rate
is 25%. The total interest expense of approximately $4.3 million for the year ended June 30, 2023, was recognized in the accompanying
statements of operations and comprehensive loss and included the interest only payments totaling approximately $2.1 million, the amortization of financing costs
of approximately $170,000, unearned discount of approximately $1.6 million and the accretion of loan premium of approximately $422,000.
The
total interest expense of approximately $2.2 million for the year ended June 30, 2022; was recognized in the accompanying statements
of operations and comprehensive loss and included the interest only payments totaling approximately $952,000, the amortization of financing costs of approximately
$99,000, unearned discount of approximately $934,000 and the accretion of loan premium totaled of approximately $165,000.
As
of June 30, 2023, the remaining principal balance of $15 million under the Loan is payable in 18 monthly equal installments beginning
July 1, 2023; for a total of $10.0 million and $5.0 million in the fiscal years ended June 30, 2024 and 2025 respectively.
The
following is a summary of the Note Payable as of June 30, 2023 and June 30, 2022:
Current
portion of Notes Payable
| Schedule of note payable | |
| | | |
| | |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| Current portion of Notes Payable | |
$ | 10,000,000 | | |
$ | — | |
| Less debt financing costs | |
| (108,751 | ) | |
| — | |
| Less unearned discount | |
| (1,023,145 | ) | |
| — | |
| Plus accretion of loan premium | |
| 236,970 | | |
| — | |
| Current portion of Notes Payable, net of financing costs, unearned premiums and
discount | |
$ | 9,105,074 | | |
$ | — | |
Non-current
portion of Notes Payable
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| Notes Payable | |
$ | 5,000,000 | | |
$ | 15,000,000 | |
| Less debt financing costs | |
| (11,820 | ) | |
| (290,790 | ) |
| Less unearned discount | |
| (111,212 | ) | |
| (2,735,802 | ) |
| Plus accretion of loan premium | |
| 350,302 | | |
| 165,278 | |
| Notes Payable, net of the current portion financing costs, unearned premiums
and discount | |
$ | 5,227,270 | | |
$ | 12,138,686 | |
Estimated
future amortization expense and accretion of premium is as follows:
| | Schedule of Estimated future amortization expense and accretion of premium | | |
| | | |
| | | |
| | |
| | | |
Unearned Discount | | |
Debt Financing Costs | | |
Loan accretion Premium | |
| | | | |
| | | |
| | | |
| | |
| | Year ending June 30, | | |
| | | |
| | | |
| | |
| | 2024 | | |
$ | 1,023,145 | | |
$ | 108,751 | | |
$ | 236,970 | |
| | 2025 | | |
| 111,212 | | |
| 11,820 | | |
| 25,758 | |
| | Total | | |
$ | 1,134,357 | | |
$ | 120,571 | | |
$ | 262,728 | |
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
| 9. |
Fair
Value Measurements |
At
June 30, 2023 and 2022, the estimated fair value of derivative liabilities measured on a recurring basis are as follows:
| Schedule of derivative liabilities at fair value | |
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at | |
| | |
June 30, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Derivative liability - Warrants | |
$ | — | | |
$ | — | | |
$ | 894,280 | | |
$ | 894,280 | |
| Derivative liability - Conversion option on notes payable | |
| — | | |
| — | | |
| 925,762 | | |
| 925,762 | |
| Total derivatives | |
$ | — | | |
$ | — | | |
$ | 1,820,042 | | |
$ | 1,820,042 | |
| | |
Fair Value Measurements at | |
| | |
June 30, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Derivative liability - Warrants | |
$ | — | | |
$ | — | | |
$ | 194,531 | | |
$ | 194,531 | |
| Derivative liability - Conversion option on note payable | |
| — | | |
| — | | |
| 188,030 | | |
| 188,030 | |
| Total derivatives | |
$ | — | | |
$ | — | | |
$ | 382,561 | | |
$ | 382,561 | |
The
following table presents the activity for liabilities measured at fair value using unobservable inputs for the years ended June 30, 2023
and 2022:
| Fair value, liabilities measured on recurring basis | |
| | | |
| | |
| | |
Derivative liabilities - Warrants | | |
Derivative liability - Conversion Option on Convertible Debenture | |
| | |
| | |
| |
| Balance at July 1, 2021 | |
$ | — | | |
$ | — | |
| Additions to level 3 liabilities | |
| 1,456,513 | | |
| 2,213,466 | |
| Change in fair value of level 3 liability | |
| (1,261,982 | ) | |
| (2,025,436 | ) |
| Transfer in and/or out of Level 3 | |
| — | | |
| — | |
| Balance at June 30, 2022 | |
$ | 194,531 | | |
$ | 188,030 | |
| Additions to level 3 liabilities | |
| — | | |
| — | |
| Change in in fair value of level 3 liability | |
| 699,749 | | |
| 737,732 | |
| Transfer in and/or out of Level 3 | |
| — | | |
| — | |
| Balance at June 30, 2023 | |
$ | 894,280 | | |
$ | 925,762 | |
The
fair values of derivative liabilities for the Avenue Warrants and conversion option at June 30, 2023 in the accompanying balance sheet,
were approximately $894,000 and approximately $926,000, respectively. The total change in the fair value of the derivative liabilities
totaled approximately $1.4 million and $3.3 million for the year ended June 30, 2023, and 2022, respectively; and accordingly, was recorded
in the accompanying statements of operations and comprehensive loss. The assumptions used in the Black Scholes model to value the derivative liabilities at June
30, 2023 included the closing stock price of $4.31 per share; for the Avenue Warrants, the exercise price of $5.82, remaining term 3.4
year, risk free rate of 4.4% and volatility of 92.0%; and for the embedded derivative liability of the conversion option, the conversion
price of $6.98; remaining term 1.4 years, risk free rate of 5.18% and volatility of 92.0%.
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
| 9. |
Fair
Value Measurements (continued) |
Derivative
liability – Avenue Warrants
The
Company accounts for stock purchase warrants as either equity instruments or derivative liabilities depending on the specific terms
of the warrant agreements. Under applicable accounting guidance, stock warrants that are precluded from being indexed to the
Company’s own stock because of full-rachet and anti-dilution provisions or adjustments to the strike price due to an occurrence
of a future event are accounted for as derivative financial instruments. The Avenue Warrants were not considered to be indexed to
the Company’s own stock, and accordingly, were recorded as a derivative liability at fair value in the accompany balance sheets at June 30, 2023 and 2022.
The
Black Scholes model was used to calculate the fair value of the warrant derivative to bifurcate the warrant derivative amount from the
Avenue Loan amount funded. The Avenue Warrants are recorded at their fair values at the date of issuance and remeasured at June 30, 2023.
The assumptions used for the fair value calculation at November 30, 2021 included: the closing stock price of $6.44 per share; the exercise
price of $5.82; 5 year term; a risk free rate of 1.14% and volatility of 74.4%.
Embedded
derivative liability – Conversion Option
The
embedded derivative liability represents the optional conversion feature of up to $5.0 million of the outstanding Loan, which meets the
definition of a derivative and requires bifurcation from the loan amount.
The
Black Scholes model was used to calculate the fair value of the embedded derivative to bifurcate the embedded derivative amount representing
the conversion option from the Loan amount funded. The assumption used for the fair value calculation at November 30, 2021 included:
the closing stock price of $6.44 per share; the conversion price of $6.98; 3 year term; risk free rate of 0.81% and volatility of 76.85%.
Financial
assets
As
of June 30, 2023, investments in U.S. Treasury Bills were valued through use of quoted prices and are classified as Level 1. The following
table presents information about our assets that are measured at fair value on a recurring basis using the above input categories.
| Measured at fair value on a recurring basis | |
| | | |
| | | |
| | | |
| | |
| | |
Fair Value Measurements at | |
| | |
June 30, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Cash | |
$ | 6,304,543 | | |
$ | — | | |
$ | — | | |
$ | 6,304,543 | |
| U.S. Treasury Bills due in 3 months or less | |
| 13,156,340 | | |
| — | | |
| — | | |
| 13,156,340 | |
| U.S. Treasury Bills due in 3 - 6 months | |
| 14,477,726 | | |
| — | | |
| — | | |
| 14,477,726 | |
| | |
| | | |
| | | |
| | | |
| | |
| Total | |
$ | 33,938,609 | | |
$ | — | | |
$ | — | | |
$ | 33,938,609 | |
| | |
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at | |
| | |
June 30, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Cash | |
$ | 18,641,716 | | |
$ | — | | |
$ | — | | |
$ | 18,641,716 | |
| U.S. Treasury Bills due in 3 months or less | |
| — | | |
| — | | |
| — | | |
| — | |
| U.S. Treasury Bills due in 3 - 6 months | |
| — | | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | | |
| | | |
| | |
| Total | |
$ | 18,641,716 | | |
$ | — | | |
$ | — | | |
$ | 18,641,716 | |
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
Stock
Options
The
following table summarizes the activity relating to the Company’s stock options for the years ended June 30, 2023 and 2022:
| Schedule of summarizes the activity relating to the Company’s stock options | |
| | |
| | |
| | |
| |
| | |
Options | | |
Weighted-Average
Exercise Price | | |
Weighted
Remaining Average Contractual Term | | |
Aggregate
Intrinsic Value | |
| Outstanding
at June 30, 2021 | |
| 755,200 | | |
$ | 4.34 | | |
| 4.4 | | |
$ | 2,569,232 | |
| Granted | |
| 2,724,689 | | |
| 5.86 | | |
| 7.7 | | |
| — | |
| Options
Expired | |
| (8,000 | ) | |
| 29.17 | | |
| — | | |
| — | |
| Options
Forfeited | |
| (73,125 | ) | |
| (13.91 | ) | |
| — | | |
| — | |
| Outstanding
at June 30, 2022 | |
| 3,398,764 | | |
| 7.42 | | |
| 5.5 | | |
| — | |
| Granted | |
| 714,667 | | |
| 5.90 | | |
| 8.6 | | |
| 38,610 | |
| Options
Expired | |
| (10,000 | ) | |
| 28.69 | | |
| — | | |
| — | |
| Options
Canceled | |
| (49,667 | ) | |
| 7.74 | | |
| — | | |
| — | |
| Options
Exercised | |
| (100,900 | ) | |
| 8.12 | | |
| — | | |
| — | |
| Outstanding
at June 30, 2023 | |
| 3,952,864 | | |
$ | 7.10 | | |
| 6.3 | | |
$ | 1,067,966 | |
| Exercisable
at June 30, 2023 | |
| 1,473,413 | | |
$ | 7.68 | | |
| 5.4 | | |
$ | 315,206 | |
The
fair value of each option grant on the date of grant is estimated using the Black-Scholes option. The pricing model reflects the following
weighted-average assumptions for the years ended June 30, 2023 and 2022:
| Schedule of assumptions used |
|
|
|
| |
June 30, 2023 |
|
June 30, 2022 |
| Expected life of options (In years) |
6 |
|
5 |
| Expected volatility |
81.65% |
|
76.47% |
| Risk free interest rate |
3.82% |
|
1.56% |
| Dividend Yield |
0% |
|
0% |
Expected
volatility is based on the historical volatilities of three comparable companies of the daily closing price of their respective Common
Stock and the expected life of options is based on historical data with respect to employee exercise periods. The Company accounts for
forfeitures as they are incurred.
The
Company recorded stock option-based compensation expense of approximately $4.2 million and $5.8 million for the years ended June 30,
2023 and 2022, respectively.
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
| 10. |
Equity
Transactions (continued) |
The
following is a summary of stock options outstanding and exercisable by exercise price as of June 30, 2023:
| | Schedule of summary of stock options outstanding and exercisable | | |
| | | |
| | | |
| | |
| Exercise
Price | | |
Outstanding | | |
Weighted
Average Contract Life | | |
Exercisable | |
| $ | 1.69 | | |
| 124,520 | | |
| 4.0 | | |
| 41,507 | |
| $ | 1.81 | | |
| 10,000 | | |
| 3.9 | | |
| 2,000 | |
| $ | 1.98 | | |
| 72,000 | | |
| 3.9 | | |
| 16,000 | |
| $ | 2.74 | | |
| 124,167 | | |
| 8.6 | | |
| 35,180 | |
| $ | 2.80 | | |
| 5,600 | | |
| 1.6 | | |
| 5,600 | |
| $ | 3.13 | | |
| 4,000 | | |
| 0.6 | | |
| 4,000 | |
| $ | 3.20 | | |
| 248,167 | | |
| 8.6 | | |
| 79,834 | |
| $ | 3.24 | | |
| 25,000 | | |
| 8.7 | | |
| 6,667 | |
| $ | 4.09 | | |
| 175,500 | | |
| 10.0 | | |
| — | |
| $ | 5.04 | | |
| 755,000 | | |
| 3.8 | | |
| 377,500 | |
| $ | 5.21 | | |
| 10,000 | | |
| 9.4 | | |
| — | |
| $ | 5.78 | | |
| 148,000 | | |
| 9.9 | | |
| 29,600 | |
| $ | 6.12 | | |
| 195,000 | | |
| 4.4 | | |
| 97,500 | |
| $ | 6.25 | | |
| 1,600 | | |
| 0.3 | | |
| 1,600 | |
| $ | 7.36 | | |
| 124,167 | | |
| 9.8 | | |
| — | |
| $ | 7.50 | | |
| 800 | | |
| 1.3 | | |
| 800 | |
| $ | 7.74 | | |
| 1,241,668 | | |
| 8.1 | | |
| 447,000 | |
| $ | 7.81 | | |
| 62,000 | | |
| 9.8 | | |
| — | |
| $ | 8.75 | | |
| 1,600 | | |
| 0.8 | | |
| 1,600 | |
| $ | 9.54 | | |
| 800 | | |
| 2.3 | | |
| 800 | |
| $ | 9.90 | | |
| 800 | | |
| 2.3 | | |
| 800 | |
| $ | 13.91 | | |
| 618,475 | | |
| 2.5 | | |
| 321,425 | |
| $ | 42.09 | | |
| 4,000 | | |
| 2.6 | | |
| 4,000 | |
| | | | |
| 3,952,864 | | |
| | | |
| 1,473,413 | |
Issuance
of Common Stock through exercise of Stock Options and Warrants
In December 2022, the Company issued 22,082 shares of Common Stock pursuant to a cashless exercise of stock options
to purchase 99,300 shares at an average exercise price of $7.64.
In
November 2022, the Company issued 800 shares of Common Stock pursuant to a cash exercise of stock options to purchase 800 shares at an
average exercise price of $2.80 per share.
In
October 2022, the Company issued 3,590 shares of Common Stock pursuant to a cashless exercise of warrants to purchase 8,000 shares at
an average exercise price of $2.25.
In
May 2023, the Company issued 481 shares of Common Stock pursuant to a cashless exercise of stock options to purchase 800 shares at an
average exercise price of $3.13.
Issuance of common stock for cash
During the three months ended September 30, 2021,
the Company issued 2,592,000 of its Class A common stock at $8.00 per share in connection with its registered public offering of approximately
$18.5 million, net of issuance costs of approximately $2.2 million.
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
| 10. |
Equity
Transactions (continued) |
On August 31, 2022, the Company entered into a
Controlled Equity Offering Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. and B. Riley Securities,
Inc. (collectively, the “Agents”), pursuant to which the Company may issue and sell from time-to-time shares of the Company’s
common stock through the Agents, subject to the terms and conditions of the Sales Agreement. On April 6, 2023, the Company and B. Riley
Securities, Inc. mutually agreed to terminate B. Riley Securities, Inc.’s role as a sales agent under the Sales Agreement. During
the year ended June 30, 2023, the Company sold 7,539,254 shares of common stock under the Sales Agreement for total net proceeds of $49.5
million after 3% commissions and expenses of approximately $2.0 million.
Issuance
of restricted stock units for services
On
August 20, 2021, the Company awarded 58,759 RSUs to the Company’s President and CEO under
the Company’s 2019 Omnibus Incentive Equity Plan (the “2019 Omnibus Plan”) as his salary for the period from April
27, 2021, the date of his appointment, through December 31, 2021. The number of RSUs awarded was based on a prorated annual base salary
of $600,000 at a 10% discount to the grant date fair value of $7.74 per share of the Company’s Common Stock. Each RSU awarded to
the CEO entitled him to receive one share of Common Stock upon vesting. A total of 15,339 RSUs (representing the pro rata portion of
the RSU award for the period from April 27, 2021 to June 30, 2021) vested at the grant date, 21,710 vested at September 30, 2021 and
the remaining 21,710 vested at December 31, 2021. Accordingly, the CEO was issued an aggregate of 58,759 shares of Common Stock over
the vesting period of the RSUs. The stock-based compensation expense related to these RSUs was $384,456 for the year ended June 30, 2022.
On
June 21, 2022, the Company awarded 124,520 RSUs to the President and CEO under the Company’s 2019 Omnibus Plan. Each RSU awarded
to the CEO entitles him to receive one share of Common Stock upon vesting. The RSUs vest in three equal annual installments beginning on the first grant anniversary date. 41,506 RSUs vested in June 2023 at a fair value of $5.90 per share of the Company’s Common
Stock. The stock-based compensation expense related to these RSUs was approximately $243,000 and $1,754 for the years ended June 30, 2023, and 2022, respectively.
On
November 23, 2022, the Company awarded 381,976 RSUs to certain employees and a consultant, with a grant date fair value of $6.12 per
share. 25% of these RSUs vested on the grant date and the remaining RSUs vest in three equal installments over three years
beginning on the first anniversary of the grant date. For the year ended June 30, 2023, the stock-based compensation expense
related to these RSUs was $584,424. On February 16, 2023, the Company delivered the vested portion of the RSU’s and issued 72,612
shares of Common Stock net of 25% withholding. 22,880 shares issued to employees were withheld in Treasury stock in exchange for payment
of withholding tax on behalf of the employees.
On
November 23, 2022, the Company issued equity awards for the Board of Directors’ annual compensation. Four directors received RSUs
to purchase a total of 155,636 shares of Common Stock at the grant date fair value of $6.12 per share, a total cost of $952,492 recognized
as stock compensation in the year ended June 30, 2023. Three directors received stock options to purchase 195,000 shares
of Common Stock at an exercise price of $6.12 per share, the grant date fair value. The total stock compensation cost of stock options
of $791,700 was recognized in the year ended June 30, 2023. The equity awards vest every three months beginning from the
last annual shareholders’ meeting on November 9, 2022, on February 9, 2023, May 9, 2023, August 9, 2023 and earlier of November
9, 2023 or the next annual shareholders’ meeting. While the agreements contain certain contractual vesting terms, there are circumstances
where the vesting can be accelerated that is not within the Company’s control and as a result, for accounting purposes, the awards
are assumed to have been fully vested on the grant date, accordingly, the Company recognized the total compensation cost of $1,744,192
on November 23, 2022. On February 9, 2023, the Company delivered the vested portion and issued 39,088 shares of Common Stock. On May 9, 2023, the Company delivered the vested portion and issued 39,088 shares of Common Stock.
On
June 20, 2023, the Company awarded 149,500 RSUs to the President and CEO under the Company’s 2019 Omnibus Plan. Each RSU awarded
to the CEO entitles him to receive one share of Common Stock upon vesting. The RSUs vest in three equal annual installments beginning on the first grant date anniversary.
Compensation expense related to vested RSUs for
the year ended June 30, 2023 was approximately $1.8 million.
The
following table summarizes vesting of restricted common stock:
| Schedule of vesting of restricted common stock | |
| | | |
| | |
| | |
Number of Shares | | |
Weighted Average Grant Date Fair Value Per Share | |
| | |
| | |
| |
| Unvested at June 30, 2021 | |
| — | | |
$ | — | |
| Granted | |
| 124,520 | | |
| 1.69 | |
| Unvested at June 30, 2022 | |
| 124,520 | | |
| 1.69 | |
| Granted | |
| 687,112 | | |
| 5.89 | |
| Vested | |
| (215,175 | ) | |
| 5.27 | |
| Unvested at June 30, 2023 | |
| 596,457 | | |
$ | 5.24 | |
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
| 10. |
Equity
Transactions (continued) |
Issuance
of Shares for Services
On
April 6, 2023, the Company awarded 50,000 shares of Common Stock to a vendor as part of their fees in exchange for services. The fair
value of the Common Stock at the date of issuance was $7.45 per share. The stock-based compensation expense related to this Common Stock
issuance was $372,500.
Issuance
of Stock Options under the 2019 Omnibus Plan.
On
August 20, 2021, the Company granted stock options to purchase 1,365,835 shares of Common Stock to the executive management team. 20% of the shares underlying the options awarded vested on the grant date, and the remaining 80% will vest equally over a 5-year
period, on the first, second, third, fourth and fifth anniversary of the grant date. The exercise price of the options is $7.74 per share,
the grant date fair value of the stock, and the options terminate on the earlier of the tenth anniversary of the grant date or the date
on which the options have been fully exercised.
On
April 5, 2022, the Company granted stock options to purchase 755,000 shares of Common Stock to the independent directors of the board
as compensation for services at an exercise price of $5.04
per share, the grant date fair value. 25%
of the shares underlying the options awarded
vested on the grant date, and the remaining 75%
vest ratably over three 3
years on the first, second, and third anniversary
of the grant date. The options terminate on the earlier of the fifth anniversary of the grant date or the date as of Xwhich the options
are fully exercised.
Pursuant
to a former employee Separation Agreement, dated April 11, 2022, the Company modified a former employee’s stock option award granted
on August 20, 2021, pursuant to the 2019 Omnibus Plan (“2021 Options Grant”). Pursuant to the terms of the Separation Agreement,
effective on July 8, 2022 (“the Separation Date”), the Company accelerated the vesting of options scheduled to vest on the
first and second anniversary of the grant date as deemed vested (“Accelerated Options”) and after giving effect to the Accelerated
Options, extended the exercise period of the total vested outstanding and unexercised options (totaling 74,500 options) to one year following
the Separation Date. The unvested portion of the 2021 Option Grant (totaling 49,667 options) was canceled. The modification was remeasured
as of July 8, 2022, and the incremental difference totaled $181,154, net credit, due to the original exercise price of $7.74 being greater
than the stock price of $1.80 on the remeasurement date, and accordingly was recognized on July 8, 2022.
On
June 21, 2022, the Company granted stock options to purchase 124,520
shares of Common Stock to the CEO. The options vest in three equal annual installments beginning on the first grant date anniversary. The exercise price is $1.69
per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
During
the fiscal year ended June 30, 2022, the Company granted stock options to purchase a total of 479,334 shares of Common Stock in connection
with compensation packages of seven new employees. The exercise prices were based on each of respective the grant date fair values with
vesting terms over a five years period and the options terminate on the earlier of tenth grant date anniversary or the date of which
the options are fully exercised.
On
June 7, 2023, the Company granted stock options to purchase 148,000 shares of Common Stock to the certain employees. 20% of
the shares underlying the options awarded vested on the grant date, and the remaining 80% will vest in four equal annual installments
beginning, on the first grant date anniversary. The exercise price of the options is $5.78 per share, the grant date fair value of the
stock, and the options terminate on the earlier of the tenth grant date anniversary or the date of which the options are fully exercised.
During
the fiscal year ended June 30, 2023, the Company granted stock options to purchase a total of 286,167 share of Common Stock in connection
with compensation packages of three new employees. The exercise prices were as of each respective grant date fair value with vesting
terms over five year period and the options terminate on the earlier of tenth grant date anniversary or the date of which the options
are fully exercised.
Forfeiture
of Stock Options
On
August 27, 2021, the Chief Executive Officer forfeited unvested stock options to purchase up to 73,125 shares of Common Stock that were
previously granted to him as compensation as an independent director of the Board of Directors.
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
| 10. |
Equity
Transactions (continued) |
Stock
Warrants
The
following table summarizes the warrants activity during the years ended June 30, 2023 and 2022:
| Summary of warrants activity | |
| | | |
| | | |
| | | |
| | |
| | |
Number of Shares | | |
Weighted Average Exercise Price | | |
Weighted Average Remaining Life (Years) | | |
Aggregate Intrinsic Value | |
| Outstanding and exercisable at June 30, 2021 | |
| 158,761 | | |
$ | 10.37 | | |
| 3.1 | | |
$ | 1,765,437 | |
| Granted | |
| 361,002 | | |
| 5.82 | | |
| 5.0 | | |
| — | |
| Expired | |
| (9,391 | ) | |
| 12.29 | | |
| — | | |
| — | |
| Exercised | |
| — | | |
| — | | |
| — | | |
| — | |
| Outstanding and exercisable at June 30, 2022 | |
| 510,372 | | |
$ | 6.17 | | |
| 3.8 | | |
$ | — | |
| Granted | |
| 7,272,728 | | |
| 1.82 | | |
| 5.0 | | |
| — | |
| Expired | |
| (4,815 | ) | |
| 75.00 | | |
| — | | |
| — | |
| Exercised | |
| (8,000 | ) | |
| 2.25 | | |
| — | | |
| — | |
| Outstanding and exercisable at June 30, 2023 | |
| 7,770,285 | | |
$ | 2.06 | | |
| 4.0 | | |
$ | 18,318,954 | |
The
total warrants outstanding at June 30, 2023 expire in the following fiscal years ending June 30 as follows: 101,380 in 2025; 35,175 expire
in 2026; and 7,633,730 in 2027.
11.
Leases
Office
Leases
The
Company paid an annual rent of $2,200 for its headquarters at 680 W Nye Lane, Suite 201, Carson City Nevada 897603. The rental agreement
is for a one-year term and commenced on October 1, 2022.
On
February 26, 2022, the Company’s San Diego office relocated to 5090 Shoreham Place, San Diego, CA 92122. The term for the new office
lease is 38 months and commenced on March 1, 2022. The monthly base rate of $4,175 began June 1, 2022, with annual increases of
three percent.
Total
operating lease expense of approximately $52,000 and $89,000 for the year ended June 30, 2023 and 2022, respectively; were included in
the accompanying statements of operations and comprehensive loss.
The
right-of-use asset, net and current and non current portion of the operating lease liabilities included in the accompany balance sheets
at June 30 follows:
| Schedule of balance sheet information related to leases | |
| | | |
| | |
| | |
June
30, 2023 | | |
June
30, 2022 | |
| Assets | |
| | |
| |
| Operating
lease, right-of-use asset, net | |
$ | 80,789 | | |
$ | 118,254 | |
| | |
| | | |
| | |
| Liabilities | |
| | | |
| | |
| Current
portion of operating lease liabilities | |
$ | 44,909 | | |
$ | 38,884 | |
| Operating
lease liabilities, net of current portion | |
| 42,505 | | |
| 87,414 | |
| Total
operating lease liabilities | |
$ | 87,414 | | |
$ | 126,298 | |
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
At
June 30, 2023, the future estimated minimum lease payments under non-cancelable operating leases are as follows:
| Schedule of future estimated minimum lease payments under non-cancelable operating leases | |
| | |
| Year
ending June 30, 2023 | |
| |
| 2024 | |
$ | 52,156 | |
| 2025 | |
| 44,636 | |
| Total
minimum lease payments | |
| 96,792 | |
| Less
amount representing interest | |
| (9,378 | ) |
| Present
value of future minimum lease payments | |
| 87,414 | |
| Less
current portion of operating lease liabilities | |
| (44,909 | ) |
| Operating
lease liabilities, net of current portion | |
$ | 42,505 | |
Total
cash paid for amounts included in the measurement of lease liabilities were $50,600 and $4,175 for the years ended June 30, 2023 and
2022, respectively.
The
weighted average remaining lease term and discount rate as of June 30, 2023, and 2022 were as follows:
| Schedule of weighted average remaining lease term and discount rate | |
| | | |
| | |
| | |
June
30, 2023 | | |
June
30, 2022 | |
| | |
| | |
| |
| Weighted
average remaining lease term (Years) | |
| | | |
| | |
| Operating
leases | |
| 1.8 | | |
| 2.8 | |
| Weighted
average discount rate | |
| | | |
| | |
| Operating
leases | |
| 10.75 | % | |
| 10.75 | % |
| 12. |
Commitments
and Contingencies |
Royalty
Agreements
Pursuant
to the Agreement and Plan of Merger entered into on April 11, 2016, by and between our predecessor entities, LAT Pharma and NanoAntibiotics,
Inc., the Company is obligated to pay a low single digit royalty on net sales of BIV201 (continuous infusion terlipressin) to be shared
by the members of LAT Pharma Members, PharmaIn Corporation, and The Barrett Edge, Inc.
Pursuant
to the Technology Transfer Agreement entered into on July 25, 2016, by and between the Company and the University of Padova (Italy),
the Company is obligated to pay a low single digit royalty on net sales of all terlipressin products covered by U.S. patent no. 9,655,645
and any future foreign issuances, capped at a maximum of $200,000 per year.
| 13. |
Employee
Benefit Plan |
On
August 1, 2021, the Company began sponsoring an employee benefit plan subject to Section 401(K) of the Internal Revenue Service Code
(the “401K Plan”) pursuant to which, all employees meeting eligibility requirements are able to participate.
Subject
to certain limitations in the Internal Revenue Code, eligible employees are permitted to make contributions to the 401K Plan on a pre-tax
salary reduction basis and the Company will match 5% of the first 5% of an employee’s contributions to the 401K Plan., The Company
made contributions of approximately $171,900 and $121,000, for the years ended June 30, 2023 and 2022, respectively.
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2023 and 2022
Significant
components of the Company’s deferred tax assets (liabilities) are as follows:
| Schedule of deferred tax assets | |
| | | |
| | |
| | |
June
30, 2023 | | |
June
30, 2022 | |
| Deferred
tax assets (liabilities): | |
| | | |
| | |
| Tax
loss carryforward | |
$ | 4,018,817 | | |
$ | 6,410,653 | |
| Intangible
assets | |
| (189,854 | ) | |
| (258,209 | ) |
| Stock
based compensation | |
| 1,788,862 | | |
| 1,845,836 | |
| R&D
capitalized | |
| 7,938,602 | | |
| — | |
| Valuation
Allowance | |
| (13,556,427 | ) | |
| (7,998,280 | ) |
| Net
deferred tax assets | |
$ | — | | |
$ | — | |
At
June 30, 2023 and 2022, the Company has recorded a full valuation against its net deferred tax assets of approximately $13.6 million
and $8.0 million, respectively, since in the judgement of management, these assets are not more than likely than not to be realized.
The increase in the valuation allowance during the years ended June 30, 2023 and 2022 were approximately, $5.6 million and $6.0 million, respectively.
At
June 30, 2023, the Company had a Net Operating Loss (“NOL”) carryforward of approximately $168 million. NOL’s generated
prior to 2018 have expiration dates ranging from 2032 to 2037.
The Company has no current tax expense due to its net losses and a
full valuation allowance.
Reconciliation
of the differences between income tax benefit computed at the federal and state statutory tax rates and the provision for income tax
benefit for the years ended June 30, 2023 and 2022 is as follows:
| Schedule of effective income tax rate reconciliation | |
| | |
| |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Income tax expense at federal statutory rate | |
| 21 | % | |
| 21 | % |
| State taxes, net of federal benefit | |
| 9 | % | |
| 9 | % |
| Change in valuation allowance | |
| (30 | )% | |
| (30 | )% |
| Effective tax rate | |
| — | | |
| — | |
Subsequent to June 30, 2023 the Company sold
336,089 shares of common stock for net proceeds of $1.6 million net of 3% commission and expenses totaling approximately $50,000 under
the Sales Agreement with the Agent.
CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We
consent to the incorporation by reference in the Registration Statement of BioVie Inc. on Form S-3 (No. 333-252386), of our report dated
August 16, 2023, on our audits of the financial statements as of June 30, 2023 and 2022, and for each of the years then ended, which
report is included in this Annual Report on Form 10-K to be filed on or about August 16, 2023. Our report for each of the years then
ended, includes an explanatory paragraph about the existence of substantial doubt concerning the Company’s ability to continue
as a going concern.
/s/
EisnerAmper LLP
EISNERAMPER
LLP
Iselin,
New Jersey
August
16, 2023
Exhibit 31.1
CERTIFICATION PURSUANT TO
RULE 13-a-14(a) and 15d-14(a)
AS ADOPTED PURSUANT TO
SECTION 302 OF THE SARBANES OXLEY ACT OF
2002
| I, Cuong Do, certify that: |
| |
|
|
| 1. |
I have reviewed this annual report on Form 10-K of Biovie Inc.; |
| |
|
|
| 2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| |
|
|
| 3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| |
|
|
| 4. |
The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a – 15(f) and 15d – 15(f)) for the registrant and have: |
| |
|
| |
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| |
|
|
| |
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| |
|
|
| |
c) |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| |
|
|
| |
d) |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of the annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| |
|
|
| 5. |
|
The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| |
|
|
| |
a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| |
|
|
| |
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: August 16, 2023
| |
|
|
|
|
|
|
|
/s/
Cuong Do |
| |
|
|
|
|
|
|
|
Cuong Do
Chief Executive Officer
(Principal Executive Officer) |
Exhibit 31.2
CERTIFICATION PURSUANT TO
RULE 13-a-14(a) and 15d-14(a)
AS ADOPTED PURSUANT TO
SECTION 302 OF THE SARBANES OXLEY ACT OF
2002
| I, Joanne Wendy Kim, certify that: |
| |
|
|
| 1. |
I have reviewed this annual report on Form 10-K of Biovie Inc.; |
| |
|
|
| 2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| |
|
|
| 3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| |
|
|
| 4. |
The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a – 15(f) and 15d – 15(f)) for the registrant and have: |
| |
|
| |
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| |
|
|
| |
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| |
|
|
| |
c) |
Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| |
|
|
| |
d) |
Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of the annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| |
|
|
| 5. |
|
The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| |
|
|
| |
a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| |
|
|
| |
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: August 16, 2023
| |
|
|
|
|
|
|
|
/s/Joanne Wendy Kim |
| |
|
|
|
|
|
|
|
Joanne Wendy Kim
Chief Financial Officer
(Principal Financial and Accounting Officer) |
Exhibit 32.1
CERTIFICATION PURSUANT TO 18 U.S. C. SECTION
1350 AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF
2002
In connection with the Annual Report of Biovie
Inc., (the “Company”) on Form 10-K for the year ended June 30, 2023, as filed with the Securities and Exchange Commission
on the date hereof (the “Report”), I, Cuong Do, Chief Executive Officer and Chairman of the Board of the Company,
certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes Oxley Act of 2002, that:
| (1) |
The Report fully complies with the requirements of Section 13 (a) or 15 (d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m (a) or 78o(d)); and |
| |
|
| (2) |
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Date: August 16, 2023
| |
|
|
|
|
|
|
|
/s/
Cuong Do |
| |
|
|
|
|
|
|
|
Cuong Do
Chief Executive Officer
(Principal Executive Officer) |
Exhibit 32.2
CERTIFICATION PURSUANT TO 18 U.S. C. SECTION
1350 AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF
2002
In connection with the Annual Report of Biovie
Inc., (the “Company”) on Form 10-K for the year ended June 30, 2023, as filed with the Securities and Exchange Commission
on the date hereof (the “Report”), I, Joanne Wendy Kim, Chief Financial Officer of the Company, certify, pursuant to
18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes Oxley Act of 2002, that, to my knowledge:
| (1) |
The Report fully complies with the requirements of Section 13 (a) or 15 (d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m (a) or 78o(d)); and |
| |
|
| (2) |
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Date: August 16, 2023
| |
|
|
|
|
|
|
|
/s/
Joanne Wendy Kim |
| |
|
|
|
|
|
|
|
Joanne Wendy Kim
Chief Financial Officer
(Principal Financial and Accounting Officer) |
v3.23.2
Cover - USD ($)
|
12 Months Ended |
|
|
Jun. 30, 2023 |
Aug. 09, 2023 |
Dec. 31, 2022 |
| Cover [Abstract] |
|
|
|
| Document Type |
10-K
|
|
|
| Amendment Flag |
false
|
|
|
| Document Annual Report |
true
|
|
|
| Document Transition Report |
false
|
|
|
| Document Period End Date |
Jun. 30, 2023
|
|
|
| Document Fiscal Period Focus |
FY
|
|
|
| Document Fiscal Year Focus |
2023
|
|
|
| Current Fiscal Year End Date |
--06-30
|
|
|
| Entity File Number |
001-39015
|
|
|
| Entity Registrant Name |
BIOVIE INC.
|
|
|
| Entity Central Index Key |
0001580149
|
|
|
| Entity Tax Identification Number |
46-2510769
|
|
|
| Entity Incorporation, State or Country Code |
NV
|
|
|
| Entity Address, Address Line One |
680 W Nye Lane Suite 204
|
|
|
| Entity Address, City or Town |
Carson City
|
|
|
| Entity Address, State or Province |
NV
|
|
|
| Entity Address, Postal Zip Code |
89703
|
|
|
| City Area Code |
775
|
|
|
| Local Phone Number |
888-3162
|
|
|
| Title of 12(b) Security |
Class
A Common Stock, $.0001 par value per share
|
|
|
| Trading Symbol |
BIVI
|
|
|
| Security Exchange Name |
NASDAQ
|
|
|
| Entity Well-known Seasoned Issuer |
No
|
|
|
| Entity Voluntary Filers |
No
|
|
|
| Entity Current Reporting Status |
Yes
|
|
|
| Entity Interactive Data Current |
Yes
|
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
|
| Entity Small Business |
true
|
|
|
| Entity Emerging Growth Company |
false
|
|
|
| Entity Shell Company |
false
|
|
|
| Entity Public Float |
|
|
$ 86,033,369
|
| Entity Common Stock, Shares Outstanding |
|
36,803,768
|
|
| ICFR Auditor Attestation Flag |
false
|
|
|
| Document Financial Statement Error Correction [Flag] |
false
|
|
|
| Auditor Name |
EisnerAmper LLP
|
|
|
| Auditor Firm ID |
274
|
|
|
| Auditor Location |
Iselin,
New Jersey
|
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPCAOB issued Audit Firm Identifier Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorFirmId |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:nonemptySequenceNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorLocation |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an annual report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentAnnualReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates whether any of the financial statement period in the filing include a restatement due to error correction. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection w
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 4: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentFinStmtErrorCorrectionFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
| Name: |
dei_EntityPublicFloat |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
| Name: |
dei_EntityVoluntaryFilers |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 405
| Name: |
dei_EntityWellKnownSeasonedIssuer |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_IcfrAuditorAttestationFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Balance Sheets - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
| CURRENT ASSETS: |
|
|
| Cash and cash equivalents |
$ 19,460,883
|
$ 18,641,716
|
| Investments in U.S. Treasury Bills |
14,477,726
|
|
| Prepaids and other assets |
102,526
|
137,879
|
| Total current assets |
34,041,135
|
18,779,595
|
| Operating lease right-of-use assets |
80,789
|
118,254
|
| Intangible assets, net |
637,095
|
866,472
|
| Goodwill |
345,711
|
345,711
|
| Other assets, non-current |
|
4,562
|
| TOTAL ASSETS |
35,104,730
|
20,114,594
|
| CURRENT LIABILITIES: |
|
|
| Accounts payable and accrued expenses |
3,476,259
|
2,442,804
|
| Current portion of other liabilities |
48,385
|
1,304,925
|
| Current portion of operating lease liabilities |
44,909
|
38,884
|
| Current portion of Note payable, net of financing cost, unearned premium and discount of $894,926 at June 30, 2023 |
9,105,074
|
|
| Warrant liabilities |
894,280
|
194,531
|
| Embedded derivative liability |
925,762
|
188,030
|
| Total current liabilities |
14,494,669
|
4,169,174
|
| Other liabilities, net of current portion |
|
48,385
|
| Operating lease liabilities, net of current portion |
42,505
|
87,414
|
| Note payable, net of current portion, financing cost, unearned premium and discount of $227,268 at June 30, 2023 and $2,861,314 at June 30, 2022 |
5,227,270
|
12,138,686
|
| TOTAL LIABILITIES |
19,764,444
|
16,443,659
|
| STOCKHOLDERS’ EQUITY : |
|
|
| Preferred stock; $0.001 par value; 10,000,000 shares authorized; 0 shares issued and outstanding |
|
|
| Common stock, $0.0001 par value; 800,000,000 shares authorized at June 30, 2023 and June 30, 2022, respectively; 36,451,829 shares issued of which 36,428,949 shares outstanding at June 30, 2023 and 24,984,083 issued and outstanding at June 30, 2022; |
3,643
|
2,496
|
| Additional paid in capital |
316,385,759
|
254,638,329
|
| Accumulated other comprehensive income |
176,591
|
|
| Accumulated deficit |
(301,225,705)
|
(250,969,890)
|
| Treasury stock |
(2)
|
|
| Total stockholders’ equity |
15,340,286
|
3,670,935
|
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
$ 35,104,730
|
$ 20,114,594
|
| X |
- References
+ Details
| Name: |
bivi_InvestmentsInU.s.TreasuryBills |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_WarrantLiabilities |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionValue received from shareholders in common stock-related transactions that are in excess of par value or stated value and amounts received from other stock-related transactions. Includes only common stock transactions (excludes preferred stock transactions). May be called contributed capital, capital in excess of par, capital surplus, or paid-in capital. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapitalCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFair value as of the balance sheet date of the embedded derivative or group of embedded derivatives classified as a liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 815
-SubTopic 10
-Section 50
-Paragraph 4B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480434/815-10-50-4B
| Name: |
us-gaap_EmbeddedDerivativeFairValueOfEmbeddedDerivativeLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated impairment loss of an asset representing future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognized. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482548/350-20-55-24
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482598/350-20-45-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Goodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph ((a)(1),(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-1
| Name: |
us-gaap_IntangibleAssetsNetExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of notes payable (with maturities initially due after one year or beyond the operating cycle if longer), excluding current portion. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of the portions of long-term notes payable due within one year or the operating cycle if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_NotesPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of noncurrent assets classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other, due after one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.24)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherLiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets.
| Name: |
us-gaap_PrepaidExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount allocated to treasury stock. Treasury stock is common and preferred shares of an entity that were issued, repurchased by the entity, and are held in its treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481520/505-30-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481549/505-30-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29,30)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_TreasuryStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
Balance Sheets (Parenthetical) - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
| Statement of Financial Position [Abstract] |
|
|
| Unearned premium and discount current |
$ 894,926
|
|
| Unearned premium and discount |
$ 227,268
|
$ 2,861,314
|
| Preferred Stock, Par Value |
$ 0.001
|
$ 0.001
|
| Preferred Stock, Shares Authorized |
10,000,000
|
10,000,000
|
| Preferred Stock, Shares Issued |
0
|
0
|
| Preferred Stock, Shares Outstanding |
0
|
0
|
| Common Stock, Par Value |
$ 0.0001
|
$ 0.0001
|
| Common Stock, Shares Authorized |
800,000,000
|
800,000,000
|
| Common Stock, Shares Issued |
36,451,829
|
24,984,083
|
| Common Stock, Shares Outstanding |
36,428,949
|
24,984,083
|
| X |
- References
+ Details
| Name: |
bivi_UnearnedPremiumAndDiscount |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_UnearnedPremiumAnddiscountCurrent |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Statements of Operations - USD ($)
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| OPERATING EXPENSES: |
|
|
| Amortization |
$ 229,377
|
$ 229,377
|
| Research and development expenses |
33,299,503
|
17,258,341
|
| Selling, general and administrative expenses |
11,551,568
|
9,765,259
|
| TOTAL OPERATING EXPENSES |
45,080,448
|
27,252,977
|
| LOSS FROM OPERATIONS |
(45,080,448)
|
(27,252,977)
|
| OTHER EXPENSE (INCOME): |
|
|
| Change in fair value of derivative liabilities |
1,437,481
|
(3,287,418)
|
| Interest expense |
4,300,150
|
2,162,989
|
| Interest income |
(562,264)
|
(44,080)
|
| TOTAL OTHER EXPENSE (INCOME), NET |
5,175,367
|
(1,168,509)
|
| NET LOSS |
(50,255,815)
|
(26,084,468)
|
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS |
$ (50,255,815)
|
$ (26,084,468)
|
| NET LOSS PER COMMON SHARE |
|
|
| - Basic |
$ (1.55)
|
$ (1.06)
|
| - Diluted |
$ (1.55)
|
$ (1.06)
|
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING |
|
|
| - Basic |
32,483,489
|
24,662,557
|
| - Diluted |
32,483,489
|
24,662,557
|
| NET LOSS |
$ (50,255,815)
|
$ (26,084,468)
|
| Other comprehensive income |
|
|
| Unrealized gain on investments for available-for-sale |
176,591
|
|
| Other comprehensive income |
176,591
|
|
| Comprehensive loss |
$ (50,079,224)
|
$ (26,084,468)
|
| X |
- References
+ Details
| Name: |
bivi_TotalOtherExpenseIncome |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_UnrealizedGainOnInvestmentsForAvailableforsale |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AmortizationOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe difference between the book value and the sale price of options, swaps, futures, forward contracts, and other derivative instruments. This element refers to the gain (loss) included in earnings. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(a)(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(a)(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.13(h))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_GainLossOnSaleOfDerivatives |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of income (loss) attributable to parent. Includes, but is not limited to, income (loss) from continuing operations, discontinued operations and equity method investments. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_IncomeLossAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04.9)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (210.5-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest income earned from interest bearing assets classified as other.
| Name: |
us-gaap_InterestIncomeOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of tax, noncontrolling interests, dividends on preferred stock and participating securities; of income (loss) available to common shareholders. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 6.B)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-5
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-11
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
| Name: |
us-gaap_NetIncomeLossAvailableToCommonStockholdersBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherComprehensiveIncomeDefinedBenefitPlansTaxAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of other comprehensive income (loss). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-4
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(21))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482739/220-10-55-15
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total costs related to selling a firm's product and services, as well as all other general and administrative expenses. Direct selling expenses (for example, credit, warranty, and advertising) are expenses that can be directly linked to the sale of specific products. Indirect selling expenses are expenses that cannot be directly linked to the sale of specific products, for example telephone expenses, Internet, and postal charges. General and administrative expenses include salaries of non-sales personnel, rent, utilities, communication, etc. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_SellingGeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Statements of Changes in Stockholders' Equity - USD ($)
|
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Treasury Stocks [Member] |
AOCI Attributable to Parent [Member] |
Retained Earnings [Member] |
Total |
| Beginning balance, value at Jun. 30, 2021 |
$ 2,232
|
$ 229,933,505
|
|
|
$ (224,885,422)
|
$ 5,050,315
|
| Beginning balance, shares at Jun. 30, 2021 |
22,333,324
|
|
|
|
|
|
| Stock option-based compensation |
|
5,807,871
|
|
|
|
5,807,871
|
| Proceeds from issuance of common stock, net costs of $2,008,898 |
$ 259
|
18,510,750
|
|
|
|
18,511,009
|
| Proceeds from issuance of common stock, shares |
2,592,000
|
|
|
|
|
|
| Stock-based compensation – restricted stock units |
$ 5
|
386,203
|
|
|
|
386,208
|
| Stock-based compensation - restricted stock units, Shares |
58,759
|
|
|
|
|
|
| Net loss |
|
|
|
|
(26,084,468)
|
(26,084,468)
|
| Ending balance, value at Jun. 30, 2022 |
$ 2,496
|
254,638,329
|
|
|
(250,969,890)
|
3,670,935
|
| Ending balance, shares at Jun. 30, 2022 |
24,984,083
|
|
|
|
|
|
| Stock option-based compensation |
|
4,222,845
|
|
|
|
4,222,845
|
| Stock-based compensation – issuance of common stock |
$ 5
|
372,495
|
|
|
|
372,500
|
| Stock-based compensation - issuance of common stock, shares |
50,000
|
|
|
|
|
|
| Cashless exercise of options |
$ 3
|
(3)
|
|
|
|
|
| Cashless exercise of options, shares |
22,563
|
|
|
|
|
|
| Cashless exercise of warrants |
|
|
|
|
|
|
| Cashless exercise of warrants, shares |
3,590
|
|
|
|
|
|
| Proceeds from exercise of options |
|
2,240
|
|
|
|
2,240
|
| Proceeds from exercise of options, shares |
800
|
|
|
|
|
|
| Proceeds from issuance of common stock, net costs of $2,008,898 |
$ 754
|
49,464,349
|
|
|
|
49,465,103
|
| Proceeds from issuance of common stock, shares |
7,539,254
|
|
|
|
|
|
| Stock-based compensation – restricted stock units |
$ 21
|
1,780,028
|
$ (2)
|
|
|
1,780,047
|
| Stock-based compensation - restricted stock units, Shares |
215,175
|
|
(22,880)
|
|
|
|
| Proceeds from issuance of common stock, net of costs of $94,160 – Related Party |
$ 364
|
5,905,476
|
|
|
|
5,905,840
|
| Proceeds from issuance of common stock related party, shares |
3,636,364
|
|
|
|
|
|
| Unrealized gain on available-for-sale securities |
|
|
|
176,591
|
|
176,591
|
| Net loss |
|
|
|
|
(50,255,815)
|
(50,255,815)
|
| Ending balance, value at Jun. 30, 2023 |
$ 3,643
|
$ 316,385,759
|
$ (2)
|
$ 176,591
|
$ (301,225,705)
|
$ 15,340,286
|
| Ending balance, shares at Jun. 30, 2023 |
36,451,829
|
|
(22,880)
|
|
|
|
| X |
- References
+ Details
| Name: |
bivi_CashlessExerciseOfOptions |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_CashlessExerciseOfOptionsShares |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_CashlessExerciseOfWarrants |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_CashlessExerciseOfWarrantsShares |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_StockbasedCompensationIssuanceOfCommonStock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_StockbasedCompensationIssuanceOfCommonStockShares |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_UnrealizedGainOnAvailableforsaleSecurities |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTotal number of shares issued during the period, including shares forfeited, as a result of Restricted Stock Awards. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesRestrictedStockAwardGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate value of stock related to Restricted Stock Awards issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueRestrictedStockAwardGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue, after forfeiture, of shares issued under share-based payment arrangement. Excludes employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_StockIssuedDuringPeriodValueShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued as a result of the exercise of stock options. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.29-31)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
Statements of Cash Flows - USD ($)
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
| Net loss |
$ (50,255,815)
|
$ (26,084,468)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Amortization of intangible assets |
229,377
|
229,377
|
| Stock based compensation – restricted stock units |
1,780,047
|
386,208
|
| Stock based compensation expense – stock options |
4,222,845
|
5,807,871
|
| Stock based compensation expense – stock issued |
372,500
|
|
| Amortization of financing costs |
170,219
|
99,295
|
| Accretion of unearned loan discount |
1,601,445
|
934,177
|
| Accretion of loan premium |
421,994
|
165,278
|
| Change in operating lease right-of-use assets |
37,465
|
8,044
|
| Change in fair value of derivative liabilities |
1,437,481
|
(3,287,418)
|
| Changes in operating assets and liabilities: |
|
|
| Prepaids and other assets |
39,915
|
(48,954)
|
| Accounts payable and accrued expenses |
1,033,455
|
1,446,430
|
| Operating lease liabilities |
(38,884)
|
|
| Other liabilities |
(1,304,925)
|
1,353,310
|
| Net cash used in operating activities |
(40,252,881)
|
(18,990,850)
|
| CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
| Purchases of U.S. Treasury Bills |
(14,301,135)
|
|
| Net cash used in investing activities |
(14,301,135)
|
|
| CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
| Net proceeds from issuance of common stock |
49,465,103
|
18,511,009
|
| Proceeds from note payable net of financing costs |
|
14,609,915
|
| Proceeds from exercise of stock options |
2,240
|
|
| Net proceeds from issuance of common stock – Related Party |
5,905,840
|
|
| Net cash provided by financing activities |
55,373,183
|
33,120,924
|
| Net increase in cash and cash equivalents |
819,167
|
14,130,074
|
| Cash and cash equivalents, beginning of period |
18,641,716
|
4,511,642
|
| Cash and cash equivalents, end of period |
19,460,883
|
18,641,716
|
| SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
| Cash paid for interest |
2,106,491
|
964,241
|
| SCHEDULE OF NON-CASH FINANCING AND INVESTING ACTIVITIES: |
|
|
| Right of use assets obtained in exchange for lease obligations |
|
130,039
|
| Unrealized gain on U.S. Treasury Bills |
$ 176,591
|
|
| X |
- References
+ Details
| Name: |
bivi_AccretionOfLoanPremium |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ChangeInOperatingLeaseRightofuseAssets |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_InterestExpenseFromConvertibleDebenture |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_PurchasesOfU.s.TreasuryBills |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ScheduleOfNoncashFinancingAndInvestingActivitiesAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_StockBasedCompensationExpenseStockIssued |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_StockBasedCompensationRestrictedStock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_UnrealizedGainOnU.s.TreasuryBill |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense included in interest expense to amortize debt discount and premium associated with the related debt instruments. Excludes amortization of financing costs. Alternate captions include noncash interest expense. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 1A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-1A
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.8)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_AmortizationOfDebtDiscountPremium |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AmortizationOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; excluding effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseExcludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in the fair value of derivatives recognized in the income statement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 815
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4A
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480434/815-10-50-4A
| Name: |
us-gaap_DerivativeGainLossOnDerivativeNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in operating liabilities classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherOperatingLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from a borrowing supported by a written promise to pay an obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromNotesPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from exercise of option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in right-of-use asset obtained in exchange for finance lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_RightOfUseAssetObtainedInExchangeForFinanceLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
Background Information
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Background Information |
| 1. |
Background
Information |
BioVie
Inc. (the “Company” or “we” or “our”) is a clinical-stage company developing innovative drug therapies
to treat chronic debilitating conditions including neurological and neuro-degenerative disorders and liver disease.
The
Company acquired the biopharmaceutical assets of NeurMedix, Inc. (“NeurMedix”), from a related party privately held clinical-stage
pharmaceutical company, in June 2021. The acquired assets included NE3107, a potentially selective inhibitor of inflammatory extracellular
single-regulated kinase(“ERK”) signaling that, based on animal studies and is believed to reduce neuroinflammation. NE3107
is a novel orally administered small molecule that is thought to inhibit inflammation-driven insulin resistance and major pathological
inflammatory cascades with a novel mechanism of action. There is emerging scientific consensus that both inflammation and insulin resistance
may play fundamental roles in the development of Alzheimer’s Disease (AD) and Parkinson’s Disease (PD), and NE3107 could,
if approved represent an entirely new medical approach to treating these devastating conditions affecting an estimated 6 million Americans
suffering from AD and 1 million Americans suffering from PD.
The
Company is conducting a potentially pivotal Phase 3 randomized, double-blind, placebo-controlled, parallel-group, multicenter study to
evaluate NE3107 in patients who have mild to moderate Alzheimer’s disease (NCT04669028). The Company is targeting primary completion
of this study in the fourth quarter of calendar year 2023.
The
Company completed its Phase 2 study assessing NE3107 in Parkinson’s disease patients in the fourth quarter of calendar year 2022.
The NM201 study (NCT05083260) was a double-blind, placebo-controlled, safety, tolerability, and pharmacokinetics study in Parkinson’s
disease (PD) participants treated with carbidopa/levodopa and NE3107. The study was primarily designed to assess safety (general safety
in the patient population and potential for drug-drug interactions of NE3107 with levodopa); and secondary, to look for indications of
promotoric activity akin to promotoric activity and apparent enhancement of levodopa activity observed in preclinical models. Both the
safety and efficacy objectives of the study were met.
Neuroinflammation,
insulin resistance, and oxidative stress are common features in the major neurodegenerative diseases, including Alzheimer’s Disease
(AD), Parkinson’s Disease (PD), frontotemporal lobar dementia, and Amyotrophic lateral sclerosis (ALS). NE3107 is
an orally bioavailable, blood-brain permeable, small molecule, with potential anti-inflammatory, insulin sensitizing, and ERK-binding
properties that may allow it to selectively inhibit ERK-, NFκB- and TNF-stimulated inflammation. NE3107’s potential to inhibit
neuroinflammation and insulin resistance forms the basis for the Company’s work testing the molecule in AD and PD patients. NE3107
is patented in the United States, Australia, Canada, Europe and South Korea.
The Company’s Orphan Drug candidate BIV201
(continuous infusion terlipressin), with FDA Fast Track status, is being evaluated in a U.S. Phase 2b study (NCT04112199) for the treatment
of refractory ascites due to liver cirrhosis. BIV201 is administered as a patent-pending liquid formulation. The study was closed before
full enrollment, without clinically meaningful adverse effects associated with BIV201 treatment. While the active agent is approved in
the U.S. and in about 40 countries for related complications of advanced liver cirrhosis, treatment of ascites is not included in these
authorizations. Patients with refractory ascites suffer from frequent life-threatening complications, generate more than $5 billion in
annual treatment costs, and have an estimated 50% mortality rate within 6 to 12 months. The U.S. Food and Drug Administration (“FDA”)
has not approved any drug to treat refractory ascites.
The BIV201 development program was initiated by
LAT Pharma LLC (LAT Pharma). On April 11, 2016, the Company acquired LAT Pharma and the rights to its BIV201 development
program. The Company currently owns all development and marketing rights to this drug candidate. Pursuant to the Agreement and Plan of
Merger entered into on April 11, 2016, between our predecessor entities, LAT Pharma and NanoAntibiotics, Inc., the Company is obligated
to pay a low single digit royalty on net sales of BIV201 (continuous infusion terlipressin), if approved, to be shared by the members
of LAT Pharma, PharmaIn Corporation and The Barrett Edge, Inc.
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Liquidity and Going Concern
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Liquidity and Going Concern |
| 2. |
Liquidity
and Going Concern |
The Company’s operations are subject to
a number of factors that can affect its operating results and financial conditions. Such factors include, but are not limited to: the
results of clinical testing and trial activities of the Company’s products, the Company’s ability to obtain regulatory approval
to market its products; competition from products manufactured and sold or being developed by other companies; the price of, and demand
for, Company products; the Company’s ability to negotiate favorable licensing or other manufacturing and marketing agreements for
its products; and the Company’s ability to raise capital. The Company’s financial statements have been prepared assuming the
Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal
course of business. As of June 30, 2023 the Company had working capital of approximately $19.5 million, cash and cash equivalents and
US treasury bills totaling of approximately $33.9 million, stockholders’ equity of approximately $15.3 million, and an accumulated
deficit of approximately $301 million. The Company is in the pre-revenue stage and no revenues are expected in the foreseeable future.
The Company’s future operations are dependent on the success of the Company’s ongoing development and commercialization efforts,
as well as its ability to secure additional financing as needed. Although our cash balance may possibly sustain operations over the next
12 months from the balance sheet date if measures are taken to delay planned expenditures in our research protocols and slow the progress
in the Company’s development of next phase clinical programs, the Company’s current planned operations to meet certain goals
and objectives, project cash flows to be depleted within that period of time.
The
future viability of the Company is largely dependent upon its ability to raise additional capital to finance its operations. Management
expects that future sources of funding may include sales of equity, obtaining loans, or other strategic transactions.
The Impact of COVID-19 pandemic created a widespread
labor shortage, including a shortage of medical professionals, and has impacted and may continue to impact the potential patient participation
in our studies, which may adversely impact our ability to continue or complete our clinical trials in the planned timeline.
Although
management continues to pursue the Company’s strategic plans, there is no assurance that the Company will be successful in obtaining
sufficient financing on terms acceptable to the Company, if at all, to fund continuing operations. These circumstances raise substantial
doubt on the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might
result from the outcome of this uncertainty.
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure when substantial doubt is raised about the ability to continue as a going concern. Includes, but is not limited to, principal conditions or events that raised substantial doubt about the ability to continue as a going concern, management's evaluation of the significance of those conditions or events in relation to the ability to meet its obligations, and management's plans that alleviated or are intended to mitigate the conditions or events that raise substantial doubt about the ability to continue as a going concern. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-SubTopic 40
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205-40/tableOfContent
| Name: |
us-gaap_SubstantialDoubtAboutGoingConcernTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Significant Accounting Policies
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Significant Accounting Policies |
| 3. |
Significant
Accounting Policies |
Basis
of Presentation
The
Company’s financial statements have been prepared in accordance with accounting principles generally accepted in the United States
(“GAAP”) and include all adjustments necessary for the fair presentation of the Company’s financial position for the
periods presented.
Use
of Estimates
The
preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts
reported in the financial statements and accompanying notes. The Company bases its estimates on historical experience and on various
assumptions that are believed to be reasonable under the circumstances. The amounts of assets and liabilities reported in the Company’s
balance sheet and the amounts of expenses reported for each of the periods presented are affected by estimates and assumptions, which
are used for, but not limited to, accounting for clinical accruals, share-based compensation, accounting for derivatives, assumptions used in leases and recoverability of intangible assets, the inputs used in the valuation of goodwill and intangible assets in connection with impairment testing and accounting for income taxes.
Actual results could differ from those estimates.
Cash
and cash equivalents
Cash and cash equivalents consisted of cash deposits
and money market funds held at a bank and funds held in a brokerage account which included a U.S. treasury money market fund and U.S.
Treasury Bills with original maturities of three months or less.
Investments
in U.S. Treasury Bills
Investments in U.S. Treasury Bills with maturities
greater than three months, are accounted for as available for sale and are recorded at fair value. Unrealized gains were included in other
comprehensive income in the accompanying statements of operations and comprehensive loss.
Concentration of Credit Risk in the Financial
Service Industry
As of June 30, 2023, the Company had cash deposited
in certain financial institutions in excess of federally insured levels. The Company regularly monitors the financial stability of these
financial institutions and believes that it is not exposed to any significant credit risk in cash and cash equivalents. However, in March
and April 2023, certain U.S. government banking regulators took steps to intervene in the operations of certain financial institutions
due to liquidity concerns, which caused general heightened uncertainties in financial markets. While these events have not had a material
direct impact on the Company’s operations, if further liquidity and financial stability concerns arise with respect to banks and
financial institutions, either nationally or in specific regions, the Company’s ability to access cash or enter into new financing
arrangements may be threatened, which could have a material adverse effect on its business, financial condition and results of operations.
Fair
value measurement of assets and liabilities
We
determine the fair values of our financial instruments based on the fair value hierarchy, which requires an entity to maximize the use
of observable inputs and minimize the use of unobservable inputs when measuring fair value. Fair value is defined as the price that would
be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement
date. The fair value assumes that the transaction to sell the asset or transfer the liability occurs in the principal or most advantageous
market for the asset or liability and establishes that the fair value of an asset or liability shall be determined based on the assumptions
that market participants would use in pricing the asset or liability. The classification of a financial asset or liability within the
hierarchy is based upon the lowest level input that is significant to the fair value measurement. The fair value hierarchy prioritizes
the inputs into three levels that may be used to measure fair value:
Level
1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities.
Level
2 - Inputs are quoted prices for similar assets and liabilities in active markets or inputs that are observable for the asset or liability,
either directly or indirectly through market corroboration, for substantially the full term of the financial instrument.
Level
3 - Inputs are unobservable inputs based on our assumptions.
The Company’s financial instruments include
cash, accounts payable, the carrying value of the operating lease liabilities and notes payable. The carrying amounts of cash and accounts
payable approximate their fair value, due to the short-term nature of these items. The carrying amounts of notes payable and operating
lease liabilities approximate their fair values since they bear interest at rates which approximate market rates for similar debt instruments.
Prepaid
and other Assets
Prepaid
and other assets consist of prepayments of certain expenses and direct costs related to capital raise which will offset proceeds upon
the close.
Other
Assets, non-current
Other
assets consist of a security deposit for an office lease.
Leases
The
Company determines whether an arrangement contains a lease at inception. Operating leases are included in operating lease right-of-use
(“ROU”) assets, current portion of operating lease liabilities, and operating lease liabilities, net of current portion on
our balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities
represent an obligation to make lease payments arising from the lease. Lease ROU assets and lease liabilities are recognized based on
the present value of the future minimum lease payments over the lease term at the commencement date. As the Company’s leases do
not provide an implicit rate, an incremental borrowing rate is used based on the information available at the commencement date in determining
the present value of lease payments. The Company does not include options to extend or terminate the lease term in its calculation unless
it is reasonably certain that the Company will exercise any such options. Rent expense is recognized under the operating leases on a
straight-line basis. The Company does not recognize right of-use assets or lease liabilities for short-term leases, which have a lease
term of 12 months or less at inception, and instead will recognize lease payments as expense on a straight-line basis over the lease term.
Research
and Development
Research
and development expenses consist primarily of costs associated with the preclinical and/ or clinical trials of drug candidates, compensation
and other expenses for research and development, personnel, supplies and development materials, costs for consultants and related contract
research and facility costs.
Income
Taxes
The
Company uses the asset and liability method of accounting for deferred income taxes. Deferred income taxes are measured by applying enacted
statutory rates to net operating loss carryforwards and to the differences between the financial reporting and tax bases of assets and
liabilities. Deferred tax assets are reduced, if necessary, by a valuation allowance if it is more likely than not that some portion
or all of the deferred tax assets will not be realized.
The
Company recognizes uncertainty in income taxes in the financial statements using a recognition threshold and measurement attribute
of a tax position taken or expected to be taken in a tax return. The Company applies the “more-likely-than-not”
recognition threshold to all tax positions, commencing at the adoption date of the applicable accounting guidance, which resulted in
no unrecognized tax benefits as of such date. Additionally, there have been no unrecognized tax benefits subsequent to adoption. The
Company has opted to classify interest and penalties that would accrue, if any, according to the provisions of relevant tax law as
general and administrative expenses, in the Statements of Operations and Comprehensive Loss. For the years ended June 30, 2023 and 2022, there was no
such interest or penalty.
Net
Loss per Common Share
Basic net loss per common share is computed by
dividing the net loss attributable to Common Stockholders by the weighted average number of shares of Common Stock outstanding during
the period. Diluted net loss per common share is computed by dividing the net loss attributable to Common Stockholders by the weighted
average number of shares of Common Stock outstanding and potentially outstanding shares of Common Stock during the period to reflect the
potential dilution that could occur from common shares issuable through stock options, warrants, and convertible debentures. For the years
ended June 30, 2023 and 2022, such amounts were excluded from the diluted loss since their effect was considered anti-dilutive due to
the net loss for the periods.
The
table below shows the number of outstanding stock options, warrants and restricted stock units as of June 30:
| Schedule of Dilutive securities were excluded from the computation of diluted loss per share | |
| | | |
| | |
| | |
| | |
| |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
Number of Shares | | |
Number of Shares | |
| Stock Options | |
| 3,952,864 | | |
| 3,398,764 | |
| Warrants | |
| 7,770,285 | | |
| 510,372 | |
| Restricted Stock Units | |
| 596,457 | | |
| 124,520 | |
| Total | |
| 12,319,606 | | |
| 4,033,656 | |
Stock-based
Compensation
The
Company has accounted for stock-based compensation under the provisions of FASB ASC 718 – “Stock Compensation” which
requires the use of the fair-value based method to determine compensation for all arrangements under which employees and others receive
shares of stock or equity instruments (stock options and Common Stock purchase warrants). For employee awards, the fair value of each
stock option award is estimated on the date of grant using the Black-Scholes valuation model that uses assumptions for expected volatility,
expected dividends, expected term, and the risk-free interest rate. For non-employees, the fair value of each stock option award is estimated
on the measurement date using the Black-Scholes valuation model that uses assumptions for expected volatility, expected dividends, expected
term, and the risk-free interest rate. For non-employees, the Company utilizes the graded vesting attribution method under which the
entity treats each separately vesting portion (tranche) as a separate award and recognizes compensation cost for each tranche over its
separate vesting schedule. Expected volatilities are based on historical volatility of peer companies and other factors estimated over
the expected term of the stock options. For employee awards, the expected term of options granted is derived using the “simplified
method” which computes expected term as the average of the sum of the vesting term plus the contract term. The risk-free rate is
based on the U.S. Treasury yield curve in effect at the time of grant for the period of the expected term. The Company recognizes forfeitures
as they occur.
Goodwill
Goodwill
is recorded when the purchase price paid for an acquisition exceeds the fair value of net identified tangible and intangible assets acquired.
The Company performs an annual impairment test of goodwill and further periodic tests to the extent indicators of impairment develop
between annual impairment tests. The Company’s impairment review process compares the fair value of the reporting unit to its carrying
value, including the goodwill related to the reporting unit. To determine the fair value of the reporting unit, the Company may use various
approaches including an asset or cost approach, market approach or income approach or any combination thereof. These approaches may require
the Company to make certain estimates and assumptions including future cash flows, revenue and expenses. These estimates and assumptions
are reviewed each time the Company tests goodwill for impairment and are typically developed as part of the Company’s routine business
planning and forecasting process. While the Company believes its estimates and assumptions are reasonable, variations from those estimates
could produce materially different results. The Company did not recognize any goodwill impairments for the years ended June 30, 2023
and 2022.
Impairment
of Long-Lived Assets
Long-lived
assets, including intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying
amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount
of an asset to estimated undiscounted future cash flows expected to be generated by the asset.
If
the carrying amount of an asset exceeds its undiscounted estimated future cash flows, an impairment review is performed. An impairment
charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Generally, fair value
is determined using valuation techniques such as expected discounted cash flows or appraisals, as appropriate. Assets to be disposed
of would be separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell,
and are no longer depreciated or amortized. The assets and liabilities of a disposed group classified as held for sale would be presented
separately in the appropriate asset and liability sections of the balance sheets.
Recent
Accounting Pronouncements
The
Company considers the applicability and impact of all Accounting Standards Updates (“ASU’s”). There were no recent
ASU’s that are expected to have a material impact on our balance sheets or statements of operations and comprehensive loss.
In June 2016, the Financial Accounting Standards
Board (“FASB”) issued ASU No. 2016-13, “Financial Instruments - Credit Losses (Topic 326), Measurement of Credit Losses
on Financial Instruments.” This amendment replaces the incurred loss impairment methodology in current GAAP with a methodology
that reflects expected credit losses on instruments within its scope, including trade receivables. This update is intended to provide
financial statement users with more decision-useful information about the expected credit losses. In November 2019, the FASB issued No.
2019-10, Financial Instruments --Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842), which deferred
the effective date of ASU 2016-13 for Smaller Reporting Companies for fiscal years beginning after December 15, 2022, including interim
periods within those fiscal years. The Company does not expect a material impact from the adoption of ASU 2016-13 on the financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the basis of presentation and significant accounting policies concepts. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Accounting policies describe all significant accounting policies of the reporting entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_BasisOfPresentationAndSignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Investments in U.S. Treasury Bills available for sale
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Investments In U.s. Treasury Bills Available For Sale |
|
| Investments in U.S. Treasury Bills available for sale |
4.
Investments in U.S. Treasury Bills available for sale
The
following is a summary of the U.S. Treasury Bills held at June 30, 2023:
| Schedule of U.S. treasury bills held | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Amortized
Cost Basis | | |
Gross
Unrealized Gain | | |
Gross
Unrealized loss | | |
Fair
Value | | |
Total
Accumulated Other Comprehensive Income | |
| U.S.
Treasury Bills due is 3 - 6 months | |
$ | 14,301,136 | | |
$ | 176,591 | | |
$ | — | | |
$ | 14,477,726 | | |
$ | 176,591 | |
The Company purchased a total of approximately $46 million of U.S.
Treasury Bills during the year ended June 30, 2023. The U.S Treasury Bills that matured were approximately $18 million and none were
sold before maturity.
|
| X |
- References
+ Details
| Name: |
bivi_DisclosureInvestmentsInU.s.TreasuryBillsAvailableForSaleAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_InvestmentsInUSTreasuryBillsAvailableForSaleTextBlock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Intangible Assets
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Goodwill and Intangible Assets Disclosure [Abstract] |
|
| Intangible Assets |
The
Company’s intangible assets consist of intellectual property acquired from LAT Pharma, Inc. and are amortized over their estimated
useful lives. The following is a summary of the intangible assets as of June 30, 2023 and 2022:
| Schedule of intangible assets | |
| | | |
| | |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| Intellectual Property | |
$ | 2,293,770 | | |
$ | 2,293,770 | |
| Less Accumulated Amortization | |
| (1,656,675 | ) | |
| (1,427,298 | ) |
| Intellectual Property, Net | |
$ | 637,095 | | |
$ | 866,472 | |
Amortization
expense amounted to $229,377 for each of the years ended June 30, 2023 and 2022, respectively. The Company amortizes intellectual property
over the expected original useful lives of 10 years.
Estimated
future amortization expense is as follows:
| | Schedule of future amortization expense | | |
| | |
| Year ending June 30, | | |
| |
| | 2024 | | |
$ | 229,377 | |
| 2025 | | |
| 229,377 | |
| | 2026 | | |
| 178,341 | |
| | | | |
$ | 637,095 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all or part of the information related to intangible assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//350-30/tableOfContent
| Name: |
us-gaap_IntangibleAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Related Party Transactions
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Related Party Transactions [Abstract] |
|
| Related Party Transactions |
| 6. |
Related
Party Transactions |
Equity
Transactions with Acuitas
On
July 15, 2022, the Company entered into a securities purchase agreement with Acuitas Group Holdings, LLC (Acuitas), the Company’s
majority stockholder, pursuant to which Acuitas agreed to purchase from the Company, in a private placement, (i) an aggregate of 3,636,364
shares of the Company’s Common Stock, at a price of $1.65 per share (the “PIPE Shares”), and (ii) a warrant to purchase
7,272,728 shares of Common Stock (“PIPE Warrant Shares”), at an exercise price of $1.82, with a term of exercise of five
years. The warrant has a down round feature that reduces the exercise price of the warrant if the Company sells stock at a price lower
than the exercise price of the warrant. On August 15, 2022, the Company received net proceeds of approximately $5.9 million, net of costs
of approximately $94,000, and entered into an amended and restated registration agreement with Acuitas, which amended and restated that
certain registration rights agreement, dated as of June 10, 2021, by and between the Company and Acuitas (the “Existing Registration
Rights Agreement”), to amend the definition of “Registrable Securities” in the Existing Registration Rights Agreement
to include the PIPE Shares and the PIPE Warrant Shares as Registrable Securities thereunder.
Asset
Acquisition with NeurMedix
On April 27, 2021, the Company entered into an
Asset Purchase Agreement (“APA”) with NeurMedix and Acuitas, which are related party affiliates, pursuant to which the Company
acquired certain assets from NeurMedix and assumed certain liabilities of NeurMedix. The acquired assets include, among others, certain
assets related to the drug candidates then being developed by NeurMedix, including NE3107. On June 10, 2021, and pursuant to the terms
of the APA, the Company issued to Acuitas (as NeurMedix’s assignee) 8,361,308 shares of the Company’s Common Stock and made
a cash payment to Acuitas of approximately $2.3 million. Since the transaction was between entities under common control, there were no
fair value adjustments of the purchased assets, and the historical cost basis of the purchased assets was zero. The total consideration
paid was expensed as in process research and development expense in the year ended June 30, 2021.
Subject to the terms and conditions of APA, as
amended on May 9, 2021, the Company may be obligated to deliver contingent stock consideration to NeurMedix (or its successor) consisting
of up to 18 million shares of the Company’s Common Stock, with 4.5 million shares issuable upon the achievement of each of the
four milestones related to certain clinical, regulatory and commercial milestones set forth in the APA, subject to a cap limiting the
issuance of shares if such issuance would result in the beneficial ownership of NeurMedix and its affiliates exceeding 87.5% of the
Company’s issued and outstanding Common Stock.
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Other Liabilities
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Other Liabilities Disclosure [Abstract] |
|
| Other Liabilities |
The
current portion of other liabilities at June 30, 2023 and June 30, 2022 were approximately $48,400 and $1.3 million, and included $48,400 and $580,614,
respectively, of a retention bonus payable for arrangements with certain employees. The payment terms of the total retention bonus arrangements
of $1,161,000 recognized in August 2021 provided for equal monthly installments over a 24-month period and began in August 2021.
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherLiabilitiesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for other liabilities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20,24)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherLiabilitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Notes Payable
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Debt Disclosure [Abstract] |
|
| Notes Payable |
On
November 30, 2021 (the “Closing Date”), the Company entered into a Loan and Security Agreement and the Supplement to the
Loan and Security Agreement and Promissory Notes (together, the “Loan Agreement”) with Avenue Venture Opportunities Fund,
L.P. (“AVOPI”) and Avenue Venture Opportunities Fund II, L.P. (“AVOPII,” and together with AVOPI, “Avenue”)
for growth capital loans in an aggregate commitment amount of up to $20 million (the “Loan”). On the Closing Date, $15 million
of the Loan was funded (“Tranche 1”). The Loan provided for an additional $5 million to be available to the Company on or
prior to September 15, 2022, subject to the Company’s achievement of certain milestones with respect to certain of its ongoing
clinical trials, which were not achieved. The Loan bears interest at an annual rate equal to the greater of (a) the sum of 7.00% plus
the prime rate as reported in The Wall Street Journal and (b) 10.75%. The prime rate at June 30, 2023 was 8.25%. The Loan is secured
by a lien upon and security interest in all of the Company’s assets, including intellectual property, subject to agreed exceptions.
The maturity date of the Loan is December 1, 2024.
The
Loan Agreement requires monthly interest-only payments during the first eighteen months of the term of the Loan. Following the interest-only
period, the Company will make equal monthly payments of principal, plus accrued interest, until the Loan’s maturity date when all
remaining principal and accrued interest is due. If the Company prepays the Loan, it will be required to pay (a) a prepayment fee in
an amount equal to 3.0% of the principal amount of the Loan that is prepaid during the interest-only period; and (b) a prepayment fee
in an amount equal to 1.0% of the principal amount of the Loan that is prepaid after the interest-only period. At the Loan’s maturity
date, or on the date of the prepayment of the Loan, the Company will be obligated to pay a final payment equal to 4.25% of the Loan commitment
amount, the sum of Tranche 1 and Tranche 2.
The
Loan Agreement includes a conversion option to convert up to $5.0 million of the principal amount of the Loan outstanding at the option
of Avenue, into shares of the Company’s Common Stock at a conversion price of $6.98 per share.
On
the Closing Date, the Company issued to Avenue warrants to purchase 361,002 shares of Common Stock of the Company (the “Avenue
Warrants”) at an exercise price per share equal to $5.82. The Avenue Warrants are exercisable until November 30, 2026.
The
amount of the carrying value of the notes payable was determined by allocating portions of the outstanding principal of the notes; approximately
$1.4 million to the fair value of the Avenue Warrants and approximately $2.2 million to the fair value of the embedded conversion option.
Accordingly, the total amount of unearned discount of approximately $3.7 million, the total direct financing cost of approximately $390,000
and premium of $850,000 are recognized on an effective interest method over the term of the Loan. The adjusted effective interest rate
is 25%. The total interest expense of approximately $4.3 million for the year ended June 30, 2023, was recognized in the accompanying
statements of operations and comprehensive loss and included the interest only payments totaling approximately $2.1 million, the amortization of financing costs
of approximately $170,000, unearned discount of approximately $1.6 million and the accretion of loan premium of approximately $422,000.
The
total interest expense of approximately $2.2 million for the year ended June 30, 2022; was recognized in the accompanying statements
of operations and comprehensive loss and included the interest only payments totaling approximately $952,000, the amortization of financing costs of approximately
$99,000, unearned discount of approximately $934,000 and the accretion of loan premium totaled of approximately $165,000.
As
of June 30, 2023, the remaining principal balance of $15 million under the Loan is payable in 18 monthly equal installments beginning
July 1, 2023; for a total of $10.0 million and $5.0 million in the fiscal years ended June 30, 2024 and 2025 respectively.
The
following is a summary of the Note Payable as of June 30, 2023 and June 30, 2022:
Current
portion of Notes Payable
| Schedule of note payable | |
| | | |
| | |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| Current portion of Notes Payable | |
$ | 10,000,000 | | |
$ | — | |
| Less debt financing costs | |
| (108,751 | ) | |
| — | |
| Less unearned discount | |
| (1,023,145 | ) | |
| — | |
| Plus accretion of loan premium | |
| 236,970 | | |
| — | |
| Current portion of Notes Payable, net of financing costs, unearned premiums and
discount | |
$ | 9,105,074 | | |
$ | — | |
Non-current
portion of Notes Payable
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| Notes Payable | |
$ | 5,000,000 | | |
$ | 15,000,000 | |
| Less debt financing costs | |
| (11,820 | ) | |
| (290,790 | ) |
| Less unearned discount | |
| (111,212 | ) | |
| (2,735,802 | ) |
| Plus accretion of loan premium | |
| 350,302 | | |
| 165,278 | |
| Notes Payable, net of the current portion financing costs, unearned premiums
and discount | |
$ | 5,227,270 | | |
$ | 12,138,686 | |
Estimated
future amortization expense and accretion of premium is as follows:
| | Schedule of Estimated future amortization expense and accretion of premium | | |
| | | |
| | | |
| | |
| | | |
Unearned Discount | | |
Debt Financing Costs | | |
Loan accretion Premium | |
| | | | |
| | | |
| | | |
| | |
| | Year ending June 30, | | |
| | | |
| | | |
| | |
| | 2024 | | |
$ | 1,023,145 | | |
$ | 108,751 | | |
$ | 236,970 | |
| | 2025 | | |
| 111,212 | | |
| 11,820 | | |
| 25,758 | |
| | Total | | |
$ | 1,134,357 | | |
$ | 120,571 | | |
$ | 262,728 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_DebtDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for information about short-term and long-term debt arrangements, which includes amounts of borrowings under each line of credit, note payable, commercial paper issue, bonds indenture, debenture issue, own-share lending arrangements and any other contractual agreement to repay funds, and about the underlying arrangements, rationale for a classification as long-term, including repayment terms, interest rates, collateral provided, restrictions on use of assets and activities, whether or not in compliance with debt covenants, and other matters important to users of the financial statements, such as the effects of refinancing and noncompliance with debt covenants. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//470/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1C
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1C
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1C
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
| Name: |
us-gaap_DebtDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Fair Value Measurements
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Fair Value Measurements |
| 9. |
Fair
Value Measurements |
At
June 30, 2023 and 2022, the estimated fair value of derivative liabilities measured on a recurring basis are as follows:
| Schedule of derivative liabilities at fair value | |
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at | |
| | |
June 30, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Derivative liability - Warrants | |
$ | — | | |
$ | — | | |
$ | 894,280 | | |
$ | 894,280 | |
| Derivative liability - Conversion option on notes payable | |
| — | | |
| — | | |
| 925,762 | | |
| 925,762 | |
| Total derivatives | |
$ | — | | |
$ | — | | |
$ | 1,820,042 | | |
$ | 1,820,042 | |
| | |
Fair Value Measurements at | |
| | |
June 30, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Derivative liability - Warrants | |
$ | — | | |
$ | — | | |
$ | 194,531 | | |
$ | 194,531 | |
| Derivative liability - Conversion option on note payable | |
| — | | |
| — | | |
| 188,030 | | |
| 188,030 | |
| Total derivatives | |
$ | — | | |
$ | — | | |
$ | 382,561 | | |
$ | 382,561 | |
The
following table presents the activity for liabilities measured at fair value using unobservable inputs for the years ended June 30, 2023
and 2022:
| Fair value, liabilities measured on recurring basis | |
| | | |
| | |
| | |
Derivative liabilities - Warrants | | |
Derivative liability - Conversion Option on Convertible Debenture | |
| | |
| | |
| |
| Balance at July 1, 2021 | |
$ | — | | |
$ | — | |
| Additions to level 3 liabilities | |
| 1,456,513 | | |
| 2,213,466 | |
| Change in fair value of level 3 liability | |
| (1,261,982 | ) | |
| (2,025,436 | ) |
| Transfer in and/or out of Level 3 | |
| — | | |
| — | |
| Balance at June 30, 2022 | |
$ | 194,531 | | |
$ | 188,030 | |
| Additions to level 3 liabilities | |
| — | | |
| — | |
| Change in in fair value of level 3 liability | |
| 699,749 | | |
| 737,732 | |
| Transfer in and/or out of Level 3 | |
| — | | |
| — | |
| Balance at June 30, 2023 | |
$ | 894,280 | | |
$ | 925,762 | |
The
fair values of derivative liabilities for the Avenue Warrants and conversion option at June 30, 2023 in the accompanying balance sheet,
were approximately $894,000 and approximately $926,000, respectively. The total change in the fair value of the derivative liabilities
totaled approximately $1.4 million and $3.3 million for the year ended June 30, 2023, and 2022, respectively; and accordingly, was recorded
in the accompanying statements of operations and comprehensive loss. The assumptions used in the Black Scholes model to value the derivative liabilities at June
30, 2023 included the closing stock price of $4.31 per share; for the Avenue Warrants, the exercise price of $5.82, remaining term 3.4
year, risk free rate of 4.4% and volatility of 92.0%; and for the embedded derivative liability of the conversion option, the conversion
price of $6.98; remaining term 1.4 years, risk free rate of 5.18% and volatility of 92.0%.
Derivative
liability – Avenue Warrants
The
Company accounts for stock purchase warrants as either equity instruments or derivative liabilities depending on the specific terms
of the warrant agreements. Under applicable accounting guidance, stock warrants that are precluded from being indexed to the
Company’s own stock because of full-rachet and anti-dilution provisions or adjustments to the strike price due to an occurrence
of a future event are accounted for as derivative financial instruments. The Avenue Warrants were not considered to be indexed to
the Company’s own stock, and accordingly, were recorded as a derivative liability at fair value in the accompany balance sheets at June 30, 2023 and 2022.
The
Black Scholes model was used to calculate the fair value of the warrant derivative to bifurcate the warrant derivative amount from the
Avenue Loan amount funded. The Avenue Warrants are recorded at their fair values at the date of issuance and remeasured at June 30, 2023.
The assumptions used for the fair value calculation at November 30, 2021 included: the closing stock price of $6.44 per share; the exercise
price of $5.82; 5 year term; a risk free rate of 1.14% and volatility of 74.4%.
Embedded
derivative liability – Conversion Option
The
embedded derivative liability represents the optional conversion feature of up to $5.0 million of the outstanding Loan, which meets the
definition of a derivative and requires bifurcation from the loan amount.
The
Black Scholes model was used to calculate the fair value of the embedded derivative to bifurcate the embedded derivative amount representing
the conversion option from the Loan amount funded. The assumption used for the fair value calculation at November 30, 2021 included:
the closing stock price of $6.44 per share; the conversion price of $6.98; 3 year term; risk free rate of 0.81% and volatility of 76.85%.
Financial
assets
As
of June 30, 2023, investments in U.S. Treasury Bills were valued through use of quoted prices and are classified as Level 1. The following
table presents information about our assets that are measured at fair value on a recurring basis using the above input categories.
| Measured at fair value on a recurring basis | |
| | | |
| | | |
| | | |
| | |
| | |
Fair Value Measurements at | |
| | |
June 30, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Cash | |
$ | 6,304,543 | | |
$ | — | | |
$ | — | | |
$ | 6,304,543 | |
| U.S. Treasury Bills due in 3 months or less | |
| 13,156,340 | | |
| — | | |
| — | | |
| 13,156,340 | |
| U.S. Treasury Bills due in 3 - 6 months | |
| 14,477,726 | | |
| — | | |
| — | | |
| 14,477,726 | |
| | |
| | | |
| | | |
| | | |
| | |
| Total | |
$ | 33,938,609 | | |
$ | — | | |
$ | — | | |
$ | 33,938,609 | |
| | |
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at | |
| | |
June 30, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Cash | |
$ | 18,641,716 | | |
$ | — | | |
$ | — | | |
$ | 18,641,716 | |
| U.S. Treasury Bills due in 3 months or less | |
| — | | |
| — | | |
| — | | |
| — | |
| U.S. Treasury Bills due in 3 - 6 months | |
| — | | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | | |
| | | |
| | |
| Total | |
$ | 18,641,716 | | |
$ | — | | |
$ | — | | |
$ | 18,641,716 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Equity Transactions
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Equity [Abstract] |
|
| Equity Transactions |
Stock
Options
The
following table summarizes the activity relating to the Company’s stock options for the years ended June 30, 2023 and 2022:
| Schedule of summarizes the activity relating to the Company’s stock options | |
| | |
| | |
| | |
| |
| | |
Options | | |
Weighted-Average
Exercise Price | | |
Weighted
Remaining Average Contractual Term | | |
Aggregate
Intrinsic Value | |
| Outstanding
at June 30, 2021 | |
| 755,200 | | |
$ | 4.34 | | |
| 4.4 | | |
$ | 2,569,232 | |
| Granted | |
| 2,724,689 | | |
| 5.86 | | |
| 7.7 | | |
| — | |
| Options
Expired | |
| (8,000 | ) | |
| 29.17 | | |
| — | | |
| — | |
| Options
Forfeited | |
| (73,125 | ) | |
| (13.91 | ) | |
| — | | |
| — | |
| Outstanding
at June 30, 2022 | |
| 3,398,764 | | |
| 7.42 | | |
| 5.5 | | |
| — | |
| Granted | |
| 714,667 | | |
| 5.90 | | |
| 8.6 | | |
| 38,610 | |
| Options
Expired | |
| (10,000 | ) | |
| 28.69 | | |
| — | | |
| — | |
| Options
Canceled | |
| (49,667 | ) | |
| 7.74 | | |
| — | | |
| — | |
| Options
Exercised | |
| (100,900 | ) | |
| 8.12 | | |
| — | | |
| — | |
| Outstanding
at June 30, 2023 | |
| 3,952,864 | | |
$ | 7.10 | | |
| 6.3 | | |
$ | 1,067,966 | |
| Exercisable
at June 30, 2023 | |
| 1,473,413 | | |
$ | 7.68 | | |
| 5.4 | | |
$ | 315,206 | |
The
fair value of each option grant on the date of grant is estimated using the Black-Scholes option. The pricing model reflects the following
weighted-average assumptions for the years ended June 30, 2023 and 2022:
| Schedule of assumptions used |
|
|
|
| |
June 30, 2023 |
|
June 30, 2022 |
| Expected life of options (In years) |
6 |
|
5 |
| Expected volatility |
81.65% |
|
76.47% |
| Risk free interest rate |
3.82% |
|
1.56% |
| Dividend Yield |
0% |
|
0% |
Expected
volatility is based on the historical volatilities of three comparable companies of the daily closing price of their respective Common
Stock and the expected life of options is based on historical data with respect to employee exercise periods. The Company accounts for
forfeitures as they are incurred.
The
Company recorded stock option-based compensation expense of approximately $4.2 million and $5.8 million for the years ended June 30,
2023 and 2022, respectively.
The
following is a summary of stock options outstanding and exercisable by exercise price as of June 30, 2023:
| | Schedule of summary of stock options outstanding and exercisable | | |
| | | |
| | | |
| | |
| Exercise
Price | | |
Outstanding | | |
Weighted
Average Contract Life | | |
Exercisable | |
| $ | 1.69 | | |
| 124,520 | | |
| 4.0 | | |
| 41,507 | |
| $ | 1.81 | | |
| 10,000 | | |
| 3.9 | | |
| 2,000 | |
| $ | 1.98 | | |
| 72,000 | | |
| 3.9 | | |
| 16,000 | |
| $ | 2.74 | | |
| 124,167 | | |
| 8.6 | | |
| 35,180 | |
| $ | 2.80 | | |
| 5,600 | | |
| 1.6 | | |
| 5,600 | |
| $ | 3.13 | | |
| 4,000 | | |
| 0.6 | | |
| 4,000 | |
| $ | 3.20 | | |
| 248,167 | | |
| 8.6 | | |
| 79,834 | |
| $ | 3.24 | | |
| 25,000 | | |
| 8.7 | | |
| 6,667 | |
| $ | 4.09 | | |
| 175,500 | | |
| 10.0 | | |
| — | |
| $ | 5.04 | | |
| 755,000 | | |
| 3.8 | | |
| 377,500 | |
| $ | 5.21 | | |
| 10,000 | | |
| 9.4 | | |
| — | |
| $ | 5.78 | | |
| 148,000 | | |
| 9.9 | | |
| 29,600 | |
| $ | 6.12 | | |
| 195,000 | | |
| 4.4 | | |
| 97,500 | |
| $ | 6.25 | | |
| 1,600 | | |
| 0.3 | | |
| 1,600 | |
| $ | 7.36 | | |
| 124,167 | | |
| 9.8 | | |
| — | |
| $ | 7.50 | | |
| 800 | | |
| 1.3 | | |
| 800 | |
| $ | 7.74 | | |
| 1,241,668 | | |
| 8.1 | | |
| 447,000 | |
| $ | 7.81 | | |
| 62,000 | | |
| 9.8 | | |
| — | |
| $ | 8.75 | | |
| 1,600 | | |
| 0.8 | | |
| 1,600 | |
| $ | 9.54 | | |
| 800 | | |
| 2.3 | | |
| 800 | |
| $ | 9.90 | | |
| 800 | | |
| 2.3 | | |
| 800 | |
| $ | 13.91 | | |
| 618,475 | | |
| 2.5 | | |
| 321,425 | |
| $ | 42.09 | | |
| 4,000 | | |
| 2.6 | | |
| 4,000 | |
| | | | |
| 3,952,864 | | |
| | | |
| 1,473,413 | |
Issuance
of Common Stock through exercise of Stock Options and Warrants
In December 2022, the Company issued 22,082 shares of Common Stock pursuant to a cashless exercise of stock options
to purchase 99,300 shares at an average exercise price of $7.64.
In
November 2022, the Company issued 800 shares of Common Stock pursuant to a cash exercise of stock options to purchase 800 shares at an
average exercise price of $2.80 per share.
In
October 2022, the Company issued 3,590 shares of Common Stock pursuant to a cashless exercise of warrants to purchase 8,000 shares at
an average exercise price of $2.25.
In
May 2023, the Company issued 481 shares of Common Stock pursuant to a cashless exercise of stock options to purchase 800 shares at an
average exercise price of $3.13.
Issuance of common stock for cash
During the three months ended September 30, 2021,
the Company issued 2,592,000 of its Class A common stock at $8.00 per share in connection with its registered public offering of approximately
$18.5 million, net of issuance costs of approximately $2.2 million.
On August 31, 2022, the Company entered into a
Controlled Equity Offering Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. and B. Riley Securities,
Inc. (collectively, the “Agents”), pursuant to which the Company may issue and sell from time-to-time shares of the Company’s
common stock through the Agents, subject to the terms and conditions of the Sales Agreement. On April 6, 2023, the Company and B. Riley
Securities, Inc. mutually agreed to terminate B. Riley Securities, Inc.’s role as a sales agent under the Sales Agreement. During
the year ended June 30, 2023, the Company sold 7,539,254 shares of common stock under the Sales Agreement for total net proceeds of $49.5
million after 3% commissions and expenses of approximately $2.0 million.
Issuance
of restricted stock units for services
On
August 20, 2021, the Company awarded 58,759 RSUs to the Company’s President and CEO under
the Company’s 2019 Omnibus Incentive Equity Plan (the “2019 Omnibus Plan”) as his salary for the period from April
27, 2021, the date of his appointment, through December 31, 2021. The number of RSUs awarded was based on a prorated annual base salary
of $600,000 at a 10% discount to the grant date fair value of $7.74 per share of the Company’s Common Stock. Each RSU awarded to
the CEO entitled him to receive one share of Common Stock upon vesting. A total of 15,339 RSUs (representing the pro rata portion of
the RSU award for the period from April 27, 2021 to June 30, 2021) vested at the grant date, 21,710 vested at September 30, 2021 and
the remaining 21,710 vested at December 31, 2021. Accordingly, the CEO was issued an aggregate of 58,759 shares of Common Stock over
the vesting period of the RSUs. The stock-based compensation expense related to these RSUs was $384,456 for the year ended June 30, 2022.
On
June 21, 2022, the Company awarded 124,520 RSUs to the President and CEO under the Company’s 2019 Omnibus Plan. Each RSU awarded
to the CEO entitles him to receive one share of Common Stock upon vesting. The RSUs vest in three equal annual installments beginning on the first grant anniversary date. 41,506 RSUs vested in June 2023 at a fair value of $5.90 per share of the Company’s Common
Stock. The stock-based compensation expense related to these RSUs was approximately $243,000 and $1,754 for the years ended June 30, 2023, and 2022, respectively.
On
November 23, 2022, the Company awarded 381,976 RSUs to certain employees and a consultant, with a grant date fair value of $6.12 per
share. 25% of these RSUs vested on the grant date and the remaining RSUs vest in three equal installments over three years
beginning on the first anniversary of the grant date. For the year ended June 30, 2023, the stock-based compensation expense
related to these RSUs was $584,424. On February 16, 2023, the Company delivered the vested portion of the RSU’s and issued 72,612
shares of Common Stock net of 25% withholding. 22,880 shares issued to employees were withheld in Treasury stock in exchange for payment
of withholding tax on behalf of the employees.
On
November 23, 2022, the Company issued equity awards for the Board of Directors’ annual compensation. Four directors received RSUs
to purchase a total of 155,636 shares of Common Stock at the grant date fair value of $6.12 per share, a total cost of $952,492 recognized
as stock compensation in the year ended June 30, 2023. Three directors received stock options to purchase 195,000 shares
of Common Stock at an exercise price of $6.12 per share, the grant date fair value. The total stock compensation cost of stock options
of $791,700 was recognized in the year ended June 30, 2023. The equity awards vest every three months beginning from the
last annual shareholders’ meeting on November 9, 2022, on February 9, 2023, May 9, 2023, August 9, 2023 and earlier of November
9, 2023 or the next annual shareholders’ meeting. While the agreements contain certain contractual vesting terms, there are circumstances
where the vesting can be accelerated that is not within the Company’s control and as a result, for accounting purposes, the awards
are assumed to have been fully vested on the grant date, accordingly, the Company recognized the total compensation cost of $1,744,192
on November 23, 2022. On February 9, 2023, the Company delivered the vested portion and issued 39,088 shares of Common Stock. On May 9, 2023, the Company delivered the vested portion and issued 39,088 shares of Common Stock.
On
June 20, 2023, the Company awarded 149,500 RSUs to the President and CEO under the Company’s 2019 Omnibus Plan. Each RSU awarded
to the CEO entitles him to receive one share of Common Stock upon vesting. The RSUs vest in three equal annual installments beginning on the first grant date anniversary.
Compensation expense related to vested RSUs for
the year ended June 30, 2023 was approximately $1.8 million.
The
following table summarizes vesting of restricted common stock:
| Schedule of vesting of restricted common stock | |
| | | |
| | |
| | |
Number of Shares | | |
Weighted Average Grant Date Fair Value Per Share | |
| | |
| | |
| |
| Unvested at June 30, 2021 | |
| — | | |
$ | — | |
| Granted | |
| 124,520 | | |
| 1.69 | |
| Unvested at June 30, 2022 | |
| 124,520 | | |
| 1.69 | |
| Granted | |
| 687,112 | | |
| 5.89 | |
| Vested | |
| (215,175 | ) | |
| 5.27 | |
| Unvested at June 30, 2023 | |
| 596,457 | | |
$ | 5.24 | |
Issuance
of Shares for Services
On
April 6, 2023, the Company awarded 50,000 shares of Common Stock to a vendor as part of their fees in exchange for services. The fair
value of the Common Stock at the date of issuance was $7.45 per share. The stock-based compensation expense related to this Common Stock
issuance was $372,500.
Issuance
of Stock Options under the 2019 Omnibus Plan.
On
August 20, 2021, the Company granted stock options to purchase 1,365,835 shares of Common Stock to the executive management team. 20% of the shares underlying the options awarded vested on the grant date, and the remaining 80% will vest equally over a 5-year
period, on the first, second, third, fourth and fifth anniversary of the grant date. The exercise price of the options is $7.74 per share,
the grant date fair value of the stock, and the options terminate on the earlier of the tenth anniversary of the grant date or the date
on which the options have been fully exercised.
On
April 5, 2022, the Company granted stock options to purchase 755,000 shares of Common Stock to the independent directors of the board
as compensation for services at an exercise price of $5.04
per share, the grant date fair value. 25%
of the shares underlying the options awarded
vested on the grant date, and the remaining 75%
vest ratably over three 3
years on the first, second, and third anniversary
of the grant date. The options terminate on the earlier of the fifth anniversary of the grant date or the date as of Xwhich the options
are fully exercised.
Pursuant
to a former employee Separation Agreement, dated April 11, 2022, the Company modified a former employee’s stock option award granted
on August 20, 2021, pursuant to the 2019 Omnibus Plan (“2021 Options Grant”). Pursuant to the terms of the Separation Agreement,
effective on July 8, 2022 (“the Separation Date”), the Company accelerated the vesting of options scheduled to vest on the
first and second anniversary of the grant date as deemed vested (“Accelerated Options”) and after giving effect to the Accelerated
Options, extended the exercise period of the total vested outstanding and unexercised options (totaling 74,500 options) to one year following
the Separation Date. The unvested portion of the 2021 Option Grant (totaling 49,667 options) was canceled. The modification was remeasured
as of July 8, 2022, and the incremental difference totaled $181,154, net credit, due to the original exercise price of $7.74 being greater
than the stock price of $1.80 on the remeasurement date, and accordingly was recognized on July 8, 2022.
On
June 21, 2022, the Company granted stock options to purchase 124,520
shares of Common Stock to the CEO. The options vest in three equal annual installments beginning on the first grant date anniversary. The exercise price is $1.69
per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
During
the fiscal year ended June 30, 2022, the Company granted stock options to purchase a total of 479,334 shares of Common Stock in connection
with compensation packages of seven new employees. The exercise prices were based on each of respective the grant date fair values with
vesting terms over a five years period and the options terminate on the earlier of tenth grant date anniversary or the date of which
the options are fully exercised.
On
June 7, 2023, the Company granted stock options to purchase 148,000 shares of Common Stock to the certain employees. 20% of
the shares underlying the options awarded vested on the grant date, and the remaining 80% will vest in four equal annual installments
beginning, on the first grant date anniversary. The exercise price of the options is $5.78 per share, the grant date fair value of the
stock, and the options terminate on the earlier of the tenth grant date anniversary or the date of which the options are fully exercised.
During
the fiscal year ended June 30, 2023, the Company granted stock options to purchase a total of 286,167 share of Common Stock in connection
with compensation packages of three new employees. The exercise prices were as of each respective grant date fair value with vesting
terms over five year period and the options terminate on the earlier of tenth grant date anniversary or the date of which the options
are fully exercised.
Forfeiture
of Stock Options
On
August 27, 2021, the Chief Executive Officer forfeited unvested stock options to purchase up to 73,125 shares of Common Stock that were
previously granted to him as compensation as an independent director of the Board of Directors.
Stock
Warrants
The
following table summarizes the warrants activity during the years ended June 30, 2023 and 2022:
| Summary of warrants activity | |
| | | |
| | | |
| | | |
| | |
| | |
Number of Shares | | |
Weighted Average Exercise Price | | |
Weighted Average Remaining Life (Years) | | |
Aggregate Intrinsic Value | |
| Outstanding and exercisable at June 30, 2021 | |
| 158,761 | | |
$ | 10.37 | | |
| 3.1 | | |
$ | 1,765,437 | |
| Granted | |
| 361,002 | | |
| 5.82 | | |
| 5.0 | | |
| — | |
| Expired | |
| (9,391 | ) | |
| 12.29 | | |
| — | | |
| — | |
| Exercised | |
| — | | |
| — | | |
| — | | |
| — | |
| Outstanding and exercisable at June 30, 2022 | |
| 510,372 | | |
$ | 6.17 | | |
| 3.8 | | |
$ | — | |
| Granted | |
| 7,272,728 | | |
| 1.82 | | |
| 5.0 | | |
| — | |
| Expired | |
| (4,815 | ) | |
| 75.00 | | |
| — | | |
| — | |
| Exercised | |
| (8,000 | ) | |
| 2.25 | | |
| — | | |
| — | |
| Outstanding and exercisable at June 30, 2023 | |
| 7,770,285 | | |
$ | 2.06 | | |
| 4.0 | | |
$ | 18,318,954 | |
The
total warrants outstanding at June 30, 2023 expire in the following fiscal years ending June 30 as follows: 101,380 in 2025; 35,175 expire
in 2026; and 7,633,730 in 2027.
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Leases
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Leases |
|
| Leases |
11.
Leases
Office
Leases
The
Company paid an annual rent of $2,200 for its headquarters at 680 W Nye Lane, Suite 201, Carson City Nevada 897603. The rental agreement
is for a one-year term and commenced on October 1, 2022.
On
February 26, 2022, the Company’s San Diego office relocated to 5090 Shoreham Place, San Diego, CA 92122. The term for the new office
lease is 38 months and commenced on March 1, 2022. The monthly base rate of $4,175 began June 1, 2022, with annual increases of
three percent.
Total
operating lease expense of approximately $52,000 and $89,000 for the year ended June 30, 2023 and 2022, respectively; were included in
the accompanying statements of operations and comprehensive loss.
The
right-of-use asset, net and current and non current portion of the operating lease liabilities included in the accompany balance sheets
at June 30 follows:
| Schedule of balance sheet information related to leases | |
| | | |
| | |
| | |
June
30, 2023 | | |
June
30, 2022 | |
| Assets | |
| | |
| |
| Operating
lease, right-of-use asset, net | |
$ | 80,789 | | |
$ | 118,254 | |
| | |
| | | |
| | |
| Liabilities | |
| | | |
| | |
| Current
portion of operating lease liabilities | |
$ | 44,909 | | |
$ | 38,884 | |
| Operating
lease liabilities, net of current portion | |
| 42,505 | | |
| 87,414 | |
| Total
operating lease liabilities | |
$ | 87,414 | | |
$ | 126,298 | |
At
June 30, 2023, the future estimated minimum lease payments under non-cancelable operating leases are as follows:
| Schedule of future estimated minimum lease payments under non-cancelable operating leases | |
| | |
| Year
ending June 30, 2023 | |
| |
| 2024 | |
$ | 52,156 | |
| 2025 | |
| 44,636 | |
| Total
minimum lease payments | |
| 96,792 | |
| Less
amount representing interest | |
| (9,378 | ) |
| Present
value of future minimum lease payments | |
| 87,414 | |
| Less
current portion of operating lease liabilities | |
| (44,909 | ) |
| Operating
lease liabilities, net of current portion | |
$ | 42,505 | |
Total
cash paid for amounts included in the measurement of lease liabilities were $50,600 and $4,175 for the years ended June 30, 2023 and
2022, respectively.
The
weighted average remaining lease term and discount rate as of June 30, 2023, and 2022 were as follows:
| Schedule of weighted average remaining lease term and discount rate | |
| | | |
| | |
| | |
June
30, 2023 | | |
June
30, 2022 | |
| | |
| | |
| |
| Weighted
average remaining lease term (Years) | |
| | | |
| | |
| Operating
leases | |
| 1.8 | | |
| 2.8 | |
| Weighted
average discount rate | |
| | | |
| | |
| Operating
leases | |
| 10.75 | % | |
| 10.75 | % |
|
| X |
- References
+ Details
| Name: |
bivi_DisclosureLeasesAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Commitments and Contingencies
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
| 12. |
Commitments
and Contingencies |
Royalty
Agreements
Pursuant
to the Agreement and Plan of Merger entered into on April 11, 2016, by and between our predecessor entities, LAT Pharma and NanoAntibiotics,
Inc., the Company is obligated to pay a low single digit royalty on net sales of BIV201 (continuous infusion terlipressin) to be shared
by the members of LAT Pharma Members, PharmaIn Corporation, and The Barrett Edge, Inc.
Pursuant
to the Technology Transfer Agreement entered into on July 25, 2016, by and between the Company and the University of Padova (Italy),
the Company is obligated to pay a low single digit royalty on net sales of all terlipressin products covered by U.S. patent no. 9,655,645
and any future foreign issuances, capped at a maximum of $200,000 per year.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Employee Benefit Plan
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Employee Benefit Plan |
|
| Employee Benefit Plan |
| 13. |
Employee
Benefit Plan |
On
August 1, 2021, the Company began sponsoring an employee benefit plan subject to Section 401(K) of the Internal Revenue Service Code
(the “401K Plan”) pursuant to which, all employees meeting eligibility requirements are able to participate.
Subject
to certain limitations in the Internal Revenue Code, eligible employees are permitted to make contributions to the 401K Plan on a pre-tax
salary reduction basis and the Company will match 5% of the first 5% of an employee’s contributions to the 401K Plan., The Company
made contributions of approximately $171,900 and $121,000, for the years ended June 30, 2023 and 2022, respectively.
|
| X |
- References
+ Details
| Name: |
bivi_DisclosureEmployeeBenefitPlanAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_EmployeeBenefitPlanTextBlock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Income Taxes
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Income Taxes |
Significant
components of the Company’s deferred tax assets (liabilities) are as follows:
| Schedule of deferred tax assets | |
| | | |
| | |
| | |
June
30, 2023 | | |
June
30, 2022 | |
| Deferred
tax assets (liabilities): | |
| | | |
| | |
| Tax
loss carryforward | |
$ | 4,018,817 | | |
$ | 6,410,653 | |
| Intangible
assets | |
| (189,854 | ) | |
| (258,209 | ) |
| Stock
based compensation | |
| 1,788,862 | | |
| 1,845,836 | |
| R&D
capitalized | |
| 7,938,602 | | |
| — | |
| Valuation
Allowance | |
| (13,556,427 | ) | |
| (7,998,280 | ) |
| Net
deferred tax assets | |
$ | — | | |
$ | — | |
At
June 30, 2023 and 2022, the Company has recorded a full valuation against its net deferred tax assets of approximately $13.6 million
and $8.0 million, respectively, since in the judgement of management, these assets are not more than likely than not to be realized.
The increase in the valuation allowance during the years ended June 30, 2023 and 2022 were approximately, $5.6 million and $6.0 million, respectively.
At
June 30, 2023, the Company had a Net Operating Loss (“NOL”) carryforward of approximately $168 million. NOL’s generated
prior to 2018 have expiration dates ranging from 2032 to 2037.
The Company has no current tax expense due to its net losses and a
full valuation allowance.
Reconciliation
of the differences between income tax benefit computed at the federal and state statutory tax rates and the provision for income tax
benefit for the years ended June 30, 2023 and 2022 is as follows:
| Schedule of effective income tax rate reconciliation | |
| | |
| |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Income tax expense at federal statutory rate | |
| 21 | % | |
| 21 | % |
| State taxes, net of federal benefit | |
| 9 | % | |
| 9 | % |
| Change in valuation allowance | |
| (30 | )% | |
| (30 | )% |
| Effective tax rate | |
| — | | |
| — | |
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Subsequent Events
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Subsequent Events [Abstract] |
|
| Subsequent Events |
Subsequent to June 30, 2023 the Company sold
336,089 shares of common stock for net proceeds of $1.6 million net of 3% commission and expenses totaling approximately $50,000 under
the Sales Agreement with the Agent.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Significant Accounting Policies (Policies)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of Presentation |
Basis
of Presentation
The
Company’s financial statements have been prepared in accordance with accounting principles generally accepted in the United States
(“GAAP”) and include all adjustments necessary for the fair presentation of the Company’s financial position for the
periods presented.
|
| Use of Estimates |
Use
of Estimates
The
preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts
reported in the financial statements and accompanying notes. The Company bases its estimates on historical experience and on various
assumptions that are believed to be reasonable under the circumstances. The amounts of assets and liabilities reported in the Company’s
balance sheet and the amounts of expenses reported for each of the periods presented are affected by estimates and assumptions, which
are used for, but not limited to, accounting for clinical accruals, share-based compensation, accounting for derivatives, assumptions used in leases and recoverability of intangible assets, the inputs used in the valuation of goodwill and intangible assets in connection with impairment testing and accounting for income taxes.
Actual results could differ from those estimates.
|
| Cash and cash equivalents |
Cash
and cash equivalents
Cash and cash equivalents consisted of cash deposits
and money market funds held at a bank and funds held in a brokerage account which included a U.S. treasury money market fund and U.S.
Treasury Bills with original maturities of three months or less.
|
| Investments in U.S. Treasury Bills |
Investments
in U.S. Treasury Bills
Investments in U.S. Treasury Bills with maturities
greater than three months, are accounted for as available for sale and are recorded at fair value. Unrealized gains were included in other
comprehensive income in the accompanying statements of operations and comprehensive loss.
|
| Concentration of Credit Risk in the Financial Service Industry |
Concentration of Credit Risk in the Financial
Service Industry
As of June 30, 2023, the Company had cash deposited
in certain financial institutions in excess of federally insured levels. The Company regularly monitors the financial stability of these
financial institutions and believes that it is not exposed to any significant credit risk in cash and cash equivalents. However, in March
and April 2023, certain U.S. government banking regulators took steps to intervene in the operations of certain financial institutions
due to liquidity concerns, which caused general heightened uncertainties in financial markets. While these events have not had a material
direct impact on the Company’s operations, if further liquidity and financial stability concerns arise with respect to banks and
financial institutions, either nationally or in specific regions, the Company’s ability to access cash or enter into new financing
arrangements may be threatened, which could have a material adverse effect on its business, financial condition and results of operations.
|
| Fair value measurement of assets and liabilities |
Fair
value measurement of assets and liabilities
We
determine the fair values of our financial instruments based on the fair value hierarchy, which requires an entity to maximize the use
of observable inputs and minimize the use of unobservable inputs when measuring fair value. Fair value is defined as the price that would
be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement
date. The fair value assumes that the transaction to sell the asset or transfer the liability occurs in the principal or most advantageous
market for the asset or liability and establishes that the fair value of an asset or liability shall be determined based on the assumptions
that market participants would use in pricing the asset or liability. The classification of a financial asset or liability within the
hierarchy is based upon the lowest level input that is significant to the fair value measurement. The fair value hierarchy prioritizes
the inputs into three levels that may be used to measure fair value:
Level
1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities.
Level
2 - Inputs are quoted prices for similar assets and liabilities in active markets or inputs that are observable for the asset or liability,
either directly or indirectly through market corroboration, for substantially the full term of the financial instrument.
Level
3 - Inputs are unobservable inputs based on our assumptions.
The Company’s financial instruments include
cash, accounts payable, the carrying value of the operating lease liabilities and notes payable. The carrying amounts of cash and accounts
payable approximate their fair value, due to the short-term nature of these items. The carrying amounts of notes payable and operating
lease liabilities approximate their fair values since they bear interest at rates which approximate market rates for similar debt instruments.
|
| Prepaid and other Assets |
Prepaid
and other Assets
Prepaid
and other assets consist of prepayments of certain expenses and direct costs related to capital raise which will offset proceeds upon
the close.
|
| Other Assets, non-current |
Other
Assets, non-current
Other
assets consist of a security deposit for an office lease.
|
| Leases |
Leases
The
Company determines whether an arrangement contains a lease at inception. Operating leases are included in operating lease right-of-use
(“ROU”) assets, current portion of operating lease liabilities, and operating lease liabilities, net of current portion on
our balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities
represent an obligation to make lease payments arising from the lease. Lease ROU assets and lease liabilities are recognized based on
the present value of the future minimum lease payments over the lease term at the commencement date. As the Company’s leases do
not provide an implicit rate, an incremental borrowing rate is used based on the information available at the commencement date in determining
the present value of lease payments. The Company does not include options to extend or terminate the lease term in its calculation unless
it is reasonably certain that the Company will exercise any such options. Rent expense is recognized under the operating leases on a
straight-line basis. The Company does not recognize right of-use assets or lease liabilities for short-term leases, which have a lease
term of 12 months or less at inception, and instead will recognize lease payments as expense on a straight-line basis over the lease term.
|
| Research and Development |
Research
and Development
Research
and development expenses consist primarily of costs associated with the preclinical and/ or clinical trials of drug candidates, compensation
and other expenses for research and development, personnel, supplies and development materials, costs for consultants and related contract
research and facility costs.
|
| Income Taxes |
Income
Taxes
The
Company uses the asset and liability method of accounting for deferred income taxes. Deferred income taxes are measured by applying enacted
statutory rates to net operating loss carryforwards and to the differences between the financial reporting and tax bases of assets and
liabilities. Deferred tax assets are reduced, if necessary, by a valuation allowance if it is more likely than not that some portion
or all of the deferred tax assets will not be realized.
The
Company recognizes uncertainty in income taxes in the financial statements using a recognition threshold and measurement attribute
of a tax position taken or expected to be taken in a tax return. The Company applies the “more-likely-than-not”
recognition threshold to all tax positions, commencing at the adoption date of the applicable accounting guidance, which resulted in
no unrecognized tax benefits as of such date. Additionally, there have been no unrecognized tax benefits subsequent to adoption. The
Company has opted to classify interest and penalties that would accrue, if any, according to the provisions of relevant tax law as
general and administrative expenses, in the Statements of Operations and Comprehensive Loss. For the years ended June 30, 2023 and 2022, there was no
such interest or penalty.
|
| Net Loss per Common Share |
Net
Loss per Common Share
Basic net loss per common share is computed by
dividing the net loss attributable to Common Stockholders by the weighted average number of shares of Common Stock outstanding during
the period. Diluted net loss per common share is computed by dividing the net loss attributable to Common Stockholders by the weighted
average number of shares of Common Stock outstanding and potentially outstanding shares of Common Stock during the period to reflect the
potential dilution that could occur from common shares issuable through stock options, warrants, and convertible debentures. For the years
ended June 30, 2023 and 2022, such amounts were excluded from the diluted loss since their effect was considered anti-dilutive due to
the net loss for the periods.
The
table below shows the number of outstanding stock options, warrants and restricted stock units as of June 30:
| Schedule of Dilutive securities were excluded from the computation of diluted loss per share | |
| | | |
| | |
| | |
| | |
| |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
Number of Shares | | |
Number of Shares | |
| Stock Options | |
| 3,952,864 | | |
| 3,398,764 | |
| Warrants | |
| 7,770,285 | | |
| 510,372 | |
| Restricted Stock Units | |
| 596,457 | | |
| 124,520 | |
| Total | |
| 12,319,606 | | |
| 4,033,656 | |
|
| Stock-based Compensation |
Stock-based
Compensation
The
Company has accounted for stock-based compensation under the provisions of FASB ASC 718 – “Stock Compensation” which
requires the use of the fair-value based method to determine compensation for all arrangements under which employees and others receive
shares of stock or equity instruments (stock options and Common Stock purchase warrants). For employee awards, the fair value of each
stock option award is estimated on the date of grant using the Black-Scholes valuation model that uses assumptions for expected volatility,
expected dividends, expected term, and the risk-free interest rate. For non-employees, the fair value of each stock option award is estimated
on the measurement date using the Black-Scholes valuation model that uses assumptions for expected volatility, expected dividends, expected
term, and the risk-free interest rate. For non-employees, the Company utilizes the graded vesting attribution method under which the
entity treats each separately vesting portion (tranche) as a separate award and recognizes compensation cost for each tranche over its
separate vesting schedule. Expected volatilities are based on historical volatility of peer companies and other factors estimated over
the expected term of the stock options. For employee awards, the expected term of options granted is derived using the “simplified
method” which computes expected term as the average of the sum of the vesting term plus the contract term. The risk-free rate is
based on the U.S. Treasury yield curve in effect at the time of grant for the period of the expected term. The Company recognizes forfeitures
as they occur.
|
| Goodwill |
Goodwill
Goodwill
is recorded when the purchase price paid for an acquisition exceeds the fair value of net identified tangible and intangible assets acquired.
The Company performs an annual impairment test of goodwill and further periodic tests to the extent indicators of impairment develop
between annual impairment tests. The Company’s impairment review process compares the fair value of the reporting unit to its carrying
value, including the goodwill related to the reporting unit. To determine the fair value of the reporting unit, the Company may use various
approaches including an asset or cost approach, market approach or income approach or any combination thereof. These approaches may require
the Company to make certain estimates and assumptions including future cash flows, revenue and expenses. These estimates and assumptions
are reviewed each time the Company tests goodwill for impairment and are typically developed as part of the Company’s routine business
planning and forecasting process. While the Company believes its estimates and assumptions are reasonable, variations from those estimates
could produce materially different results. The Company did not recognize any goodwill impairments for the years ended June 30, 2023
and 2022.
|
| Impairment of Long-Lived Assets |
Impairment
of Long-Lived Assets
Long-lived
assets, including intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying
amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount
of an asset to estimated undiscounted future cash flows expected to be generated by the asset.
If
the carrying amount of an asset exceeds its undiscounted estimated future cash flows, an impairment review is performed. An impairment
charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Generally, fair value
is determined using valuation techniques such as expected discounted cash flows or appraisals, as appropriate. Assets to be disposed
of would be separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell,
and are no longer depreciated or amortized. The assets and liabilities of a disposed group classified as held for sale would be presented
separately in the appropriate asset and liability sections of the balance sheets.
|
| Recent Accounting Pronouncements |
Recent
Accounting Pronouncements
The
Company considers the applicability and impact of all Accounting Standards Updates (“ASU’s”). There were no recent
ASU’s that are expected to have a material impact on our balance sheets or statements of operations and comprehensive loss.
In June 2016, the Financial Accounting Standards
Board (“FASB”) issued ASU No. 2016-13, “Financial Instruments - Credit Losses (Topic 326), Measurement of Credit Losses
on Financial Instruments.” This amendment replaces the incurred loss impairment methodology in current GAAP with a methodology
that reflects expected credit losses on instruments within its scope, including trade receivables. This update is intended to provide
financial statement users with more decision-useful information about the expected credit losses. In November 2019, the FASB issued No.
2019-10, Financial Instruments --Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842), which deferred
the effective date of ASU 2016-13 for Smaller Reporting Companies for fiscal years beginning after December 15, 2022, including interim
periods within those fiscal years. The Company does not expect a material impact from the adoption of ASU 2016-13 on the financial statements.
|
| X |
- References
+ Details
| Name: |
bivi_ConcentrationOfCreditRiskinTheFinancialServiceIndustryPolicyTextBlock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_InvestmentsUSTreasuryBillsPolicyTextBlock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_OtherAssetsnoncurrentPolicyTextBlock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_PrepaidAndOtherAssetsPolicyTextBlock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for salaries, bonuses, incentive awards, postretirement and postemployment benefits granted to employees, including equity-based arrangements; discloses methodologies for measurement, and the bases for recognizing related assets and liabilities and recognizing and reporting compensation expense. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (b),(f(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_CompensationRelatedCostsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for fair value measurements of financial and non-financial assets, liabilities and instruments classified in shareholders' equity. Disclosures include, but are not limited to, how an entity that manages a group of financial assets and liabilities on the basis of its net exposure measures the fair value of those assets and liabilities.
| Name: |
us-gaap_FairValueMeasurementPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for goodwill. This accounting policy also may address how an entity assesses and measures impairment of goodwill, how reporting units are determined, how goodwill is allocated to such units, and how the fair values of the reporting units are determined. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482548/350-20-55-24
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//350-20/tableOfContent
| Name: |
us-gaap_GoodwillAndIntangibleAssetsGoodwillPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing and measuring the impairment of long-lived assets. An entity also may disclose its accounting policy for long-lived assets to be sold. This policy excludes goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.CC)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480091/360-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 05
-Paragraph 4
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482338/360-10-05-4
| Name: |
us-gaap_ImpairmentOrDisposalOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Significant Accounting Policies (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Schedule of Dilutive securities were excluded from the computation of diluted loss per share |
| Schedule of Dilutive securities were excluded from the computation of diluted loss per share | |
| | | |
| | |
| | |
| | |
| |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
Number of Shares | | |
Number of Shares | |
| Stock Options | |
| 3,952,864 | | |
| 3,398,764 | |
| Warrants | |
| 7,770,285 | | |
| 510,372 | |
| Restricted Stock Units | |
| 596,457 | | |
| 124,520 | |
| Total | |
| 12,319,606 | | |
| 4,033,656 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
bivi_DisclosureInvestmentsInU.s.TreasuryBillsAvailableForSaleAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ScheduleOfUSTreasuryBillsHeldTableTextBlock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Intangible Assets (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Goodwill and Intangible Assets Disclosure [Abstract] |
|
| Schedule of intangible assets |
| Schedule of intangible assets | |
| | | |
| | |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| Intellectual Property | |
$ | 2,293,770 | | |
$ | 2,293,770 | |
| Less Accumulated Amortization | |
| (1,656,675 | ) | |
| (1,427,298 | ) |
| Intellectual Property, Net | |
$ | 637,095 | | |
$ | 866,472 | |
|
| Schedule of future amortization expense |
| | Schedule of future amortization expense | | |
| | |
| Year ending June 30, | | |
| |
| | 2024 | | |
$ | 229,377 | |
| 2025 | | |
| 229,377 | |
| | 2026 | | |
| 178,341 | |
| | | | |
$ | 637,095 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets, excluding financial assets and goodwill, lacking physical substance with a finite life, by either major class or business segment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_ScheduleOfFiniteLivedIntangibleAssetsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the amount of amortization expense expected to be recorded in succeeding fiscal years for finite-lived intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_ScheduleofFiniteLivedIntangibleAssetsFutureAmortizationExpenseTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Notes Payable (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Debt Disclosure [Abstract] |
|
| Schedule of note payable |
| Schedule of note payable | |
| | | |
| | |
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| Current portion of Notes Payable | |
$ | 10,000,000 | | |
$ | — | |
| Less debt financing costs | |
| (108,751 | ) | |
| — | |
| Less unearned discount | |
| (1,023,145 | ) | |
| — | |
| Plus accretion of loan premium | |
| 236,970 | | |
| — | |
| Current portion of Notes Payable, net of financing costs, unearned premiums and
discount | |
$ | 9,105,074 | | |
$ | — | |
Non-current
portion of Notes Payable
| | |
June 30, 2023 | | |
June 30, 2022 | |
| | |
| | |
| |
| Notes Payable | |
$ | 5,000,000 | | |
$ | 15,000,000 | |
| Less debt financing costs | |
| (11,820 | ) | |
| (290,790 | ) |
| Less unearned discount | |
| (111,212 | ) | |
| (2,735,802 | ) |
| Plus accretion of loan premium | |
| 350,302 | | |
| 165,278 | |
| Notes Payable, net of the current portion financing costs, unearned premiums
and discount | |
$ | 5,227,270 | | |
$ | 12,138,686 | |
|
| Schedule of Estimated future amortization expense and accretion of premium |
| | Schedule of Estimated future amortization expense and accretion of premium | | |
| | | |
| | | |
| | |
| | | |
Unearned Discount | | |
Debt Financing Costs | | |
Loan accretion Premium | |
| | | | |
| | | |
| | | |
| | |
| | Year ending June 30, | | |
| | | |
| | | |
| | |
| | 2024 | | |
$ | 1,023,145 | | |
$ | 108,751 | | |
$ | 236,970 | |
| | 2025 | | |
| 111,212 | | |
| 11,820 | | |
| 25,758 | |
| | Total | | |
$ | 1,134,357 | | |
$ | 120,571 | | |
$ | 262,728 | |
|
| X |
- References
+ Details
| Name: |
bivi_ScheduleofFutureAmortizationExpenseTableTextBlock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_DebtDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of information pertaining to short-term and long-debt instruments or arrangements, including but not limited to identification of terms, features, collateral requirements and other information necessary to a fair presentation.
| Name: |
us-gaap_ScheduleOfDebtTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Fair Value Measurements (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Schedule of derivative liabilities at fair value |
| Schedule of derivative liabilities at fair value | |
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at | |
| | |
June 30, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Derivative liability - Warrants | |
$ | — | | |
$ | — | | |
$ | 894,280 | | |
$ | 894,280 | |
| Derivative liability - Conversion option on notes payable | |
| — | | |
| — | | |
| 925,762 | | |
| 925,762 | |
| Total derivatives | |
$ | — | | |
$ | — | | |
$ | 1,820,042 | | |
$ | 1,820,042 | |
| | |
Fair Value Measurements at | |
| | |
June 30, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Derivative liability - Warrants | |
$ | — | | |
$ | — | | |
$ | 194,531 | | |
$ | 194,531 | |
| Derivative liability - Conversion option on note payable | |
| — | | |
| — | | |
| 188,030 | | |
| 188,030 | |
| Total derivatives | |
$ | — | | |
$ | — | | |
$ | 382,561 | | |
$ | 382,561 | |
|
| Fair value, liabilities measured on recurring basis |
| Fair value, liabilities measured on recurring basis | |
| | | |
| | |
| | |
Derivative liabilities - Warrants | | |
Derivative liability - Conversion Option on Convertible Debenture | |
| | |
| | |
| |
| Balance at July 1, 2021 | |
$ | — | | |
$ | — | |
| Additions to level 3 liabilities | |
| 1,456,513 | | |
| 2,213,466 | |
| Change in fair value of level 3 liability | |
| (1,261,982 | ) | |
| (2,025,436 | ) |
| Transfer in and/or out of Level 3 | |
| — | | |
| — | |
| Balance at June 30, 2022 | |
$ | 194,531 | | |
$ | 188,030 | |
| Additions to level 3 liabilities | |
| — | | |
| — | |
| Change in in fair value of level 3 liability | |
| 699,749 | | |
| 737,732 | |
| Transfer in and/or out of Level 3 | |
| — | | |
| — | |
| Balance at June 30, 2023 | |
$ | 894,280 | | |
$ | 925,762 | |
|
| Measured at fair value on a recurring basis |
| Measured at fair value on a recurring basis | |
| | | |
| | | |
| | | |
| | |
| | |
Fair Value Measurements at | |
| | |
June 30, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Cash | |
$ | 6,304,543 | | |
$ | — | | |
$ | — | | |
$ | 6,304,543 | |
| U.S. Treasury Bills due in 3 months or less | |
| 13,156,340 | | |
| — | | |
| — | | |
| 13,156,340 | |
| U.S. Treasury Bills due in 3 - 6 months | |
| 14,477,726 | | |
| — | | |
| — | | |
| 14,477,726 | |
| | |
| | | |
| | | |
| | | |
| | |
| Total | |
$ | 33,938,609 | | |
$ | — | | |
$ | — | | |
$ | 33,938,609 | |
| | |
| | |
| | |
| | |
| |
| | |
Fair Value Measurements at | |
| | |
June 30, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| | |
| | |
| | |
| | |
| |
| Cash | |
$ | 18,641,716 | | |
$ | — | | |
$ | — | | |
$ | 18,641,716 | |
| U.S. Treasury Bills due in 3 months or less | |
| — | | |
| — | | |
| — | | |
| — | |
| U.S. Treasury Bills due in 3 - 6 months | |
| — | | |
| — | | |
| — | | |
| — | |
| | |
| | | |
| | | |
| | | |
| | |
| Total | |
$ | 18,641,716 | | |
$ | — | | |
$ | — | | |
$ | 18,641,716 | |
|
| X |
- DefinitionTabular disclosure of financial instruments measured at fair value, including those classified in shareholders' equity measured on a recurring or nonrecurring basis. Disclosures include, but are not limited to, fair value measurements recorded and the reasons for the measurements, level within the fair value hierarchy in which the fair value measurements are categorized and transfers between levels 1 and 2. Nonrecurring fair value measurements are those that are required or permitted in the statement of financial position in particular circumstances. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2C
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2C
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the fair value measurement of liabilities using significant unobservable inputs (Level 3), a reconciliation of the beginning and ending balances, separately presenting changes attributable to the following: (1) total gains or losses for the period (realized and unrealized), segregating those gains or losses included in earnings (or changes in net assets), and gains or losses recognized in other comprehensive income (loss) and a description of where those gains or losses included in earnings (or changes in net assets) are reported in the statement of income (or activities); (2) purchases, sales, issues, and settlements (each type disclosed separately); and (3) transfers in and transfers out of Level 3 (for example, transfers due to changes in the observability of significant inputs) by class of liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-SubTopic 10
-Topic 820
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 820
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueLiabilitiesMeasuredOnRecurringBasisUnobservableInputReconciliationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of derivative liabilities at fair value.
| Name: |
us-gaap_ScheduleOfDerivativeLiabilitiesAtFairValueTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Equity Transactions (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Equity [Abstract] |
|
| Schedule of summarizes the activity relating to the Company’s stock options |
| Schedule of summarizes the activity relating to the Company’s stock options | |
| | |
| | |
| | |
| |
| | |
Options | | |
Weighted-Average
Exercise Price | | |
Weighted
Remaining Average Contractual Term | | |
Aggregate
Intrinsic Value | |
| Outstanding
at June 30, 2021 | |
| 755,200 | | |
$ | 4.34 | | |
| 4.4 | | |
$ | 2,569,232 | |
| Granted | |
| 2,724,689 | | |
| 5.86 | | |
| 7.7 | | |
| — | |
| Options
Expired | |
| (8,000 | ) | |
| 29.17 | | |
| — | | |
| — | |
| Options
Forfeited | |
| (73,125 | ) | |
| (13.91 | ) | |
| — | | |
| — | |
| Outstanding
at June 30, 2022 | |
| 3,398,764 | | |
| 7.42 | | |
| 5.5 | | |
| — | |
| Granted | |
| 714,667 | | |
| 5.90 | | |
| 8.6 | | |
| 38,610 | |
| Options
Expired | |
| (10,000 | ) | |
| 28.69 | | |
| — | | |
| — | |
| Options
Canceled | |
| (49,667 | ) | |
| 7.74 | | |
| — | | |
| — | |
| Options
Exercised | |
| (100,900 | ) | |
| 8.12 | | |
| — | | |
| — | |
| Outstanding
at June 30, 2023 | |
| 3,952,864 | | |
$ | 7.10 | | |
| 6.3 | | |
$ | 1,067,966 | |
| Exercisable
at June 30, 2023 | |
| 1,473,413 | | |
$ | 7.68 | | |
| 5.4 | | |
$ | 315,206 | |
|
| Schedule of assumptions used |
| Schedule of assumptions used |
|
|
|
| |
June 30, 2023 |
|
June 30, 2022 |
| Expected life of options (In years) |
6 |
|
5 |
| Expected volatility |
81.65% |
|
76.47% |
| Risk free interest rate |
3.82% |
|
1.56% |
| Dividend Yield |
0% |
|
0% |
|
| Schedule of summary of stock options outstanding and exercisable |
| | Schedule of summary of stock options outstanding and exercisable | | |
| | | |
| | | |
| | |
| Exercise
Price | | |
Outstanding | | |
Weighted
Average Contract Life | | |
Exercisable | |
| $ | 1.69 | | |
| 124,520 | | |
| 4.0 | | |
| 41,507 | |
| $ | 1.81 | | |
| 10,000 | | |
| 3.9 | | |
| 2,000 | |
| $ | 1.98 | | |
| 72,000 | | |
| 3.9 | | |
| 16,000 | |
| $ | 2.74 | | |
| 124,167 | | |
| 8.6 | | |
| 35,180 | |
| $ | 2.80 | | |
| 5,600 | | |
| 1.6 | | |
| 5,600 | |
| $ | 3.13 | | |
| 4,000 | | |
| 0.6 | | |
| 4,000 | |
| $ | 3.20 | | |
| 248,167 | | |
| 8.6 | | |
| 79,834 | |
| $ | 3.24 | | |
| 25,000 | | |
| 8.7 | | |
| 6,667 | |
| $ | 4.09 | | |
| 175,500 | | |
| 10.0 | | |
| — | |
| $ | 5.04 | | |
| 755,000 | | |
| 3.8 | | |
| 377,500 | |
| $ | 5.21 | | |
| 10,000 | | |
| 9.4 | | |
| — | |
| $ | 5.78 | | |
| 148,000 | | |
| 9.9 | | |
| 29,600 | |
| $ | 6.12 | | |
| 195,000 | | |
| 4.4 | | |
| 97,500 | |
| $ | 6.25 | | |
| 1,600 | | |
| 0.3 | | |
| 1,600 | |
| $ | 7.36 | | |
| 124,167 | | |
| 9.8 | | |
| — | |
| $ | 7.50 | | |
| 800 | | |
| 1.3 | | |
| 800 | |
| $ | 7.74 | | |
| 1,241,668 | | |
| 8.1 | | |
| 447,000 | |
| $ | 7.81 | | |
| 62,000 | | |
| 9.8 | | |
| — | |
| $ | 8.75 | | |
| 1,600 | | |
| 0.8 | | |
| 1,600 | |
| $ | 9.54 | | |
| 800 | | |
| 2.3 | | |
| 800 | |
| $ | 9.90 | | |
| 800 | | |
| 2.3 | | |
| 800 | |
| $ | 13.91 | | |
| 618,475 | | |
| 2.5 | | |
| 321,425 | |
| $ | 42.09 | | |
| 4,000 | | |
| 2.6 | | |
| 4,000 | |
| | | | |
| 3,952,864 | | |
| | | |
| 1,473,413 | |
|
| Schedule of vesting of restricted common stock |
| Schedule of vesting of restricted common stock | |
| | | |
| | |
| | |
Number of Shares | | |
Weighted Average Grant Date Fair Value Per Share | |
| | |
| | |
| |
| Unvested at June 30, 2021 | |
| — | | |
$ | — | |
| Granted | |
| 124,520 | | |
| 1.69 | |
| Unvested at June 30, 2022 | |
| 124,520 | | |
| 1.69 | |
| Granted | |
| 687,112 | | |
| 5.89 | |
| Vested | |
| (215,175 | ) | |
| 5.27 | |
| Unvested at June 30, 2023 | |
| 596,457 | | |
$ | 5.24 | |
|
| Summary of warrants activity |
| Summary of warrants activity | |
| | | |
| | | |
| | | |
| | |
| | |
Number of Shares | | |
Weighted Average Exercise Price | | |
Weighted Average Remaining Life (Years) | | |
Aggregate Intrinsic Value | |
| Outstanding and exercisable at June 30, 2021 | |
| 158,761 | | |
$ | 10.37 | | |
| 3.1 | | |
$ | 1,765,437 | |
| Granted | |
| 361,002 | | |
| 5.82 | | |
| 5.0 | | |
| — | |
| Expired | |
| (9,391 | ) | |
| 12.29 | | |
| — | | |
| — | |
| Exercised | |
| — | | |
| — | | |
| — | | |
| — | |
| Outstanding and exercisable at June 30, 2022 | |
| 510,372 | | |
$ | 6.17 | | |
| 3.8 | | |
$ | — | |
| Granted | |
| 7,272,728 | | |
| 1.82 | | |
| 5.0 | | |
| — | |
| Expired | |
| (4,815 | ) | |
| 75.00 | | |
| — | | |
| — | |
| Exercised | |
| (8,000 | ) | |
| 2.25 | | |
| — | | |
| — | |
| Outstanding and exercisable at June 30, 2023 | |
| 7,770,285 | | |
$ | 2.06 | | |
| 4.0 | | |
$ | 18,318,954 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the change in common stock outstanding.
| Name: |
us-gaap_ScheduleOfCommonStockOutstandingRollForwardTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of warrants or rights issued. Warrants and rights outstanding are derivative securities that give the holder the right to purchase securities (usually equity) from the issuer at a specific price within a certain time frame. Warrants are often included in a new debt issue to entice investors by a higher return potential. The main difference between warrants and call options is that warrants are issued and guaranteed by the company, whereas options are exchange instruments and are not issued by the company. Also, the lifetime of a warrant is often measured in years, while the lifetime of a typical option is measured in months. Disclose the title of issue of securities called for by warrants and rights outstanding, the aggregate amount of securities called for by warrants and rights outstanding, the date from which the warrants or rights are exercisable, and the price at which the warrant or right is exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-1
| Name: |
us-gaap_ScheduleOfStockholdersEquityNoteWarrantsOrRightsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the change in restricted stock units (RSUs).
| Name: |
us-gaap_ScheduleOfUnvestedRestrictedStockUnitsRollForwardTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Leases (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Leases |
|
| Schedule of balance sheet information related to leases |
| Schedule of balance sheet information related to leases | |
| | | |
| | |
| | |
June
30, 2023 | | |
June
30, 2022 | |
| Assets | |
| | |
| |
| Operating
lease, right-of-use asset, net | |
$ | 80,789 | | |
$ | 118,254 | |
| | |
| | | |
| | |
| Liabilities | |
| | | |
| | |
| Current
portion of operating lease liabilities | |
$ | 44,909 | | |
$ | 38,884 | |
| Operating
lease liabilities, net of current portion | |
| 42,505 | | |
| 87,414 | |
| Total
operating lease liabilities | |
$ | 87,414 | | |
$ | 126,298 | |
|
| Schedule of future estimated minimum lease payments under non-cancelable operating leases |
| Schedule of future estimated minimum lease payments under non-cancelable operating leases | |
| | |
| Year
ending June 30, 2023 | |
| |
| 2024 | |
$ | 52,156 | |
| 2025 | |
| 44,636 | |
| Total
minimum lease payments | |
| 96,792 | |
| Less
amount representing interest | |
| (9,378 | ) |
| Present
value of future minimum lease payments | |
| 87,414 | |
| Less
current portion of operating lease liabilities | |
| (44,909 | ) |
| Operating
lease liabilities, net of current portion | |
$ | 42,505 | |
|
| Schedule of weighted average remaining lease term and discount rate |
| Schedule of weighted average remaining lease term and discount rate | |
| | | |
| | |
| | |
June
30, 2023 | | |
June
30, 2022 | |
| | |
| | |
| |
| Weighted
average remaining lease term (Years) | |
| | | |
| | |
| Operating
leases | |
| 1.8 | | |
| 2.8 | |
| Weighted
average discount rate | |
| | | |
| | |
| Operating
leases | |
| 10.75 | % | |
| 10.75 | % |
|
| X |
- References
+ Details
| Name: |
bivi_DisclosureLeasesAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ScheduleOfFutureEstimatedMinimumLeasePaymentsUnderNonCancelableOperatingLeases |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ScheduleOfWeightedAverageRemainingLeaseTermAndDiscountRateTableTextBlock |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Income Taxes (Tables)
|
12 Months Ended |
Jun. 30, 2023 |
|---|
| Income Tax Disclosure [Abstract] |
|
| Schedule of deferred tax assets |
| Schedule of deferred tax assets | |
| | | |
| | |
| | |
June
30, 2023 | | |
June
30, 2022 | |
| Deferred
tax assets (liabilities): | |
| | | |
| | |
| Tax
loss carryforward | |
$ | 4,018,817 | | |
$ | 6,410,653 | |
| Intangible
assets | |
| (189,854 | ) | |
| (258,209 | ) |
| Stock
based compensation | |
| 1,788,862 | | |
| 1,845,836 | |
| R&D
capitalized | |
| 7,938,602 | | |
| — | |
| Valuation
Allowance | |
| (13,556,427 | ) | |
| (7,998,280 | ) |
| Net
deferred tax assets | |
$ | — | | |
$ | — | |
|
| Schedule of effective income tax rate reconciliation |
| Schedule of effective income tax rate reconciliation | |
| | |
| |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Income tax expense at federal statutory rate | |
| 21 | % | |
| 21 | % |
| State taxes, net of federal benefit | |
| 9 | % | |
| 9 | % |
| Change in valuation allowance | |
| (30 | )% | |
| (30 | )% |
| Effective tax rate | |
| — | | |
| — | |
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 12
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-12
| Name: |
us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Liquidity and Going Concern (Details Narrative)
|
Jun. 30, 2023
USD ($)
|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Working capital |
$ 19,500,000
|
| Cash and cash equivalent |
33,900,000
|
| Stockholders' equity |
15,300,000
|
| Accumulated deficit |
$ 301,000,000
|
| X |
- References
+ Details
| Name: |
bivi_AccumulatedDeficit |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_StockholderEquities |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_WorkingCapital |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Significant Accounting Policies (Details) - shares
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
| Total |
12,319,606
|
4,033,656
|
| Equity Option [Member] |
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
| Total |
3,952,864
|
3,398,764
|
| Warrant [Member] |
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
| Total |
7,770,285
|
510,372
|
| Restricted Stock Units (RSUs) [Member] |
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
| Total |
596,457
|
124,520
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_StockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTotal loss recognized during the period from the impairment of goodwill plus the loss recognized in the period resulting from the impairment of the carrying amount of intangible assets, other than goodwill.
| Name: |
us-gaap_GoodwillAndIntangibleAssetImpairment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
bivi_AmortizedCostBasis |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_GrossUnrealizedGain |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_GrossUnrealizedLoss |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThis element represents the excess of the cost (face amount, notional amount) of an investment (security, contract) over its fair value which deficiency has not been recognized in earnings of the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-22
| Name: |
us-gaap_InvestmentOwnedUnrecognizedUnrealizedDepreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column B)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column D))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 1)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 1)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column B)(Footnote 10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 40: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 41: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 42: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 43: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 44: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 45: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 46: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 47: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 48: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 49: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 50: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 51: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 52: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 53: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 54: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 55: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 56: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 57: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 58: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 59: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 60: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 5)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 61: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 62: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 63: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 64: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-19
Reference 65: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-19
| Name: |
us-gaap_ScheduleOfInvestmentsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=us-gaap_USTreasuryBillSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- References
+ Details
| Name: |
bivi_NumberOfStockPurchased |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of consideration received by subsidiary or equity investee in exchange for shares of stock issued or sold. Includes amount of cash received, fair value of noncash assets received, and fair value of liabilities assumed by the investor.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedPerTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column B)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 8)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column C)(Footnote 10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column D))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 1)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 1)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column B)(Footnote 10))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 40: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 41: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 42: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 43: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 44: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 11)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 45: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 46: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column C)(Footnote 13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 47: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 48: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 49: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 50: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 51: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 52: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 53: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 54: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 55: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 56: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 57: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 1)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 58: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 59: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 60: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 5)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 61: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 62: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 63: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column F)(Footnote 9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 64: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-19
Reference 65: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 19
-Subparagraph (3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-19
| Name: |
us-gaap_ScheduleOfInvestmentsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=us-gaap_USTreasuryBillSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Intangible Assets (Details) - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
| Goodwill and Intangible Assets Disclosure [Abstract] |
|
|
| Intellectual Property |
$ 2,293,770
|
$ 2,293,770
|
| Less Accumulated Amortization |
(1,656,675)
|
(1,427,298)
|
| Intellectual Property, Net |
$ 637,095
|
$ 866,472
|
| X |
- DefinitionAccumulated amount of amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAccumulatedAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 928
-SubTopic 340
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483147/928-340-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the next rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseNextRollingTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the third rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization expense for assets, excluding financial assets and goodwill, lacking physical substance with a finite life expected to be recognized in the second rolling twelve months following the latest balance sheet. For interim and annual periods when interim periods are reported on a rolling approach, from latest balance sheet date.
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseRollingYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionThe aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AmortizationOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionUseful life of finite-lived intangible assets, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.
| Name: |
us-gaap_FiniteLivedIntangibleAssetUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Related Party Transactions (Details Narrative) - shares
|
Jun. 30, 2023 |
Aug. 15, 2022 |
Jun. 30, 2022 |
| Related Party Transaction [Line Items] |
|
|
|
| Common Stock, shares issued |
36,451,829
|
|
24,984,083
|
| Acuitas Group Holdings L L C [Member] |
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
| Common Stock, shares issued |
|
3,636,364
|
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.2
Other Liabilities (Details Narrative) - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
Aug. 31, 2021 |
| Other Liabilities Disclosure [Abstract] |
|
|
|
| Other liabilities |
$ 48,400
|
$ 1,300,000
|
|
| Accrued Bonuses, Current |
$ 48,400
|
$ 580,614
|
$ 1,161,000
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable for incentive compensation awarded to employees and directors or earned by them based on the terms of one or more relevant arrangements. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedBonusesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.15)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_OtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherLiabilitiesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Notes Payable (Details) - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
| Debt Disclosure [Abstract] |
|
|
| Current portion of Notes Payable |
$ 10,000,000
|
|
| Less debt financing costs |
(108,751)
|
|
| Less unearned discount |
(1,023,145)
|
|
| Plus accretion of loan premium |
236,970
|
|
| Current portion of Notes Payable, net of financing costs, unearned premiums and discount |
9,105,074
|
|
| Notes Payable |
5,000,000
|
15,000,000
|
| Less debt financing costs |
(11,820)
|
(290,790)
|
| Less unearned discount |
(111,212)
|
(2,735,802)
|
| Plus accretion of loan premium |
350,302
|
165,278
|
| Notes Payable, net of the current portion financing costs, unearned premiums and discount |
$ 5,227,270
|
$ 12,138,686
|
| X |
- References
+ Details
| Name: |
bivi_AccretionOfLoanPremiums |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_CurrentPortionOfNotesPayable |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_DebtFinancingCosts |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_LongTermAccretionOfLoanPremiums |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_LongTermDeferredDiscountsFinanceChargesAndInterestIncludedInReceivables |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_LongTermNotesPayableNetOfFinancingCostsUnearnedPremiumsAndDiscount |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_LongtermDebtFinancingCosts |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_NotesPayableNetOfCurrentPortionFinancingCostsUnearnedPremiumsAndDiscount |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_NotesPayableNoncurrent |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_DebtDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUnearned discounts (other than cash or quantity discounts and the like), finance charges, and interest included in the face amount of receivables, that are shown as a deduction from the related receivables. For example, 1) finance charges booked as a receivable when a loan is made and recognized as income at a later date; and 2) interest charges deducted from the face loan amount, resulting in a discounted amount actually advanced to the borrower (wherein the receivable includes the amount actually advanced to the borrower and the as yet unearned interest income). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 310
-SubTopic 10
-Section 45
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-8
| Name: |
us-gaap_DeferredDiscountsFinanceChargesAndInterestIncludedInReceivables |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
Notes Payable (Details 1)
|
Jun. 30, 2023
USD ($)
|
| Unearned Discount [Member] |
|
| Accounts, Notes, Loans and Financing Receivable [Line Items] |
|
| 2024 |
$ 1,023,145
|
| 2025 |
111,212
|
| Total |
1,134,357
|
| Financing Receivable [Member] |
|
| Accounts, Notes, Loans and Financing Receivable [Line Items] |
|
| 2024 |
108,751
|
| 2025 |
11,820
|
| Total |
120,571
|
| Loan Accretion Premium [Member] |
|
| Accounts, Notes, Loans and Financing Receivable [Line Items] |
|
| 2024 |
236,970
|
| 2025 |
25,758
|
| Total |
$ 262,728
|
| X |
- References
+ Details
| Name: |
bivi_AmortizationExpenseNextRollingThreeYears |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_AmortizationExpenseNextRollingTwoYears |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_AmortizationExpenseTotal |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AccountsNotesAndLoansReceivableLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AccountsNotesLoansAndFinancingReceivableByReceivableTypeAxis=bivi_UnearnedDiscountMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AccountsNotesLoansAndFinancingReceivableByReceivableTypeAxis=us-gaap_FinanceReceivablesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AccountsNotesLoansAndFinancingReceivableByReceivableTypeAxis=bivi_LoanAccretionPremiumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Notes Payable (Details Narrative) - USD ($)
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Debt Instrument [Line Items] |
|
|
| Interest rate |
8.25%
|
|
| Unearned discount |
$ 1,600,000
|
|
| Direct financing cost |
390,000
|
|
| Unamortized premium recognized |
850,000
|
|
| Interest expense |
4,300,000
|
$ 2,200,000
|
| Interest payment |
2,100,000
|
952,000
|
| Amortization of financing costs |
170,000
|
99,000
|
| Accretion of loan premium |
422,000
|
$ 165,000
|
| Avenue Warrants [Member] |
|
|
| Debt Instrument [Line Items] |
|
|
| Fair value of warrants |
1,400,000
|
|
| Fair value of embedded conversion option |
2,200,000
|
|
| Unearned discount |
$ 3,700,000
|
|
| Prime Rate [Member] |
|
|
| Debt Instrument [Line Items] |
|
|
| Interest rate |
7.00%
|
|
| X |
- References
+ Details
| Name: |
bivi_AccretionsOfLoanPremium |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_DirectFinancingCost |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_FairValueOfEmbeddedConversionOption |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_UnearnedDiscount |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of amortization expense attributable to debt issuance costs. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-3
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_AmortizationOfFinancingCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average effective interest rate during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentInterestRateDuringPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482900/835-30-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(f))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69B
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69C
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69E
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69E
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69F
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69F
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 11: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1E
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1I
| Name: |
us-gaap_DebtInstrumentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the required periodic payments applied to interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentPeriodicPaymentInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after accumulated amortization, of debt premium. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 45
-Paragraph 1A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482925/835-30-45-1A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 835
-SubTopic 30
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482949/835-30-55-8
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1D
| Name: |
us-gaap_DebtInstrumentUnamortizedPremium |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of the cost of borrowed funds accounted for as interest expense for debt. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69E
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69E
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69F
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481568/470-20-55-69F
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-1F
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.8)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481139/470-20-50-6
| Name: |
us-gaap_InterestExpenseDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=bivi_AvenueWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VariableRateAxis=us-gaap_PrimeRateMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Fair Value Measurements (Details) - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
Jun. 30, 2021 |
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
$ 1,820,042
|
$ 382,561
|
|
| Fair Value, Inputs, Level 1 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
|
|
|
| Fair Value, Inputs, Level 2 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
|
|
|
| Fair Value, Inputs, Level 3 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
1,820,042
|
382,561
|
|
| Derivative Liability Warrants [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
894,280
|
194,531
|
|
| Derivative Liability Warrants [Member] | Fair Value, Inputs, Level 1 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
|
|
|
| Derivative Liability Warrants [Member] | Fair Value, Inputs, Level 2 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
|
|
|
| Derivative Liability Warrants [Member] | Fair Value, Inputs, Level 3 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
894,280
|
194,531
|
|
| Derivative Liability Conversion Option On Convertible Debenture [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
925,762
|
188,030
|
|
| Derivative Liability Conversion Option On Convertible Debenture [Member] | Fair Value, Inputs, Level 1 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
|
|
|
| Derivative Liability Conversion Option On Convertible Debenture [Member] | Fair Value, Inputs, Level 2 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
|
|
|
| Derivative Liability Conversion Option On Convertible Debenture [Member] | Fair Value, Inputs, Level 3 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Total derivatives |
$ 925,762
|
$ 188,030
|
|
| X |
- DefinitionFair value, after the effects of master netting arrangements, of a financial liability or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset. Includes liabilities not subject to a master netting arrangement and not elected to be offset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(9)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5C
-Subparagraph (SX 210.12-13C(Column H)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5C
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(9)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(9)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SX 210.12-13(Column G)(Footnote 8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5C
-Subparagraph (SX 210.12-13C(Column H))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5C
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SX 210.12-13(Column G))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5A
-Subparagraph (SX 210.12-13A(Column E))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5B
-Subparagraph (SX 210.12-13B(Column E))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5B
-Subparagraph (SX 210.12-13B(Column E)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483466/210-20-50-3
Reference 22: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483444/210-20-55-22
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483444/210-20-55-10
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-10
| Name: |
us-gaap_DerivativeLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=bivi_DerivativeLiabilityWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=bivi_DerivativeLiabilityConversionOptionOnConvertibleDebentureMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Fair Value Measurements (Details 1) - USD ($)
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Balance at beginning |
$ 382,561
|
|
| Balance at ending |
1,820,042
|
$ 382,561
|
| Fair Value, Inputs, Level 3 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Balance at beginning |
382,561
|
|
| Balance at ending |
1,820,042
|
382,561
|
| Derivative Liability Warrants [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Balance at beginning |
194,531
|
|
| Balance at ending |
894,280
|
194,531
|
| Derivative Liability Warrants [Member] | Fair Value, Inputs, Level 3 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Balance at beginning |
194,531
|
|
| Additions to level 3 liabilities |
|
1,456,513
|
| Change in in fair value of level 3 liability |
699,749
|
(1,261,982)
|
| Transfer in and/or out of Level 3 |
|
|
| Balance at ending |
894,280
|
194,531
|
| Derivative Liability Conversion Option On Convertible Debenture [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Balance at beginning |
188,030
|
|
| Balance at ending |
925,762
|
188,030
|
| Derivative Liability Conversion Option On Convertible Debenture [Member] | Fair Value, Inputs, Level 3 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Balance at beginning |
188,030
|
|
| Additions to level 3 liabilities |
|
2,213,466
|
| Change in in fair value of level 3 liability |
737,732
|
(2,025,436)
|
| Transfer in and/or out of Level 3 |
|
|
| Balance at ending |
$ 925,762
|
$ 188,030
|
| X |
- DefinitionFair value, after the effects of master netting arrangements, of a financial liability or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset. Includes liabilities not subject to a master netting arrangement and not elected to be offset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480524/946-210-50-6
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(9)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5C
-Subparagraph (SX 210.12-13C(Column H)(Footnote 7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5C
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(9)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(9)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SX 210.12-13(Column G)(Footnote 8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5C
-Subparagraph (SX 210.12-13C(Column H))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5C
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SX 210.12-13(Column G))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5A
-Subparagraph (SX 210.12-13A(Column E))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5B
-Subparagraph (SX 210.12-13B(Column E))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 5B
-Subparagraph (SX 210.12-13B(Column E)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-5B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483466/210-20-50-3
Reference 22: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483444/210-20-55-22
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483444/210-20-55-10
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-10
| Name: |
us-gaap_DerivativeLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=bivi_DerivativeLiabilityWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=bivi_DerivativeLiabilityConversionOptionOnConvertibleDebentureMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Fair Value Measurements (Details 2) - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
$ 33,938,609
|
$ 18,641,716
|
| US Treasury Bill Securities [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
13,156,340
|
|
| U S Treasury Bill Securities 1 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
14,477,726
|
|
| Fair Value, Inputs, Level 1 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
33,938,609
|
18,641,716
|
| Fair Value, Inputs, Level 1 [Member] | US Treasury Bill Securities [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
13,156,340
|
|
| Fair Value, Inputs, Level 1 [Member] | U S Treasury Bill Securities 1 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
14,477,726
|
|
| Fair Value, Inputs, Level 2 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
|
|
| Fair Value, Inputs, Level 2 [Member] | US Treasury Bill Securities [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
|
|
| Fair Value, Inputs, Level 2 [Member] | U S Treasury Bill Securities 1 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
|
|
| Fair Value, Inputs, Level 3 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
|
|
| Fair Value, Inputs, Level 3 [Member] | US Treasury Bill Securities [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
|
|
| Fair Value, Inputs, Level 3 [Member] | U S Treasury Bill Securities 1 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
|
|
| Cash [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
6,304,543
|
18,641,716
|
| Cash [Member] | Fair Value, Inputs, Level 1 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
6,304,543
|
18,641,716
|
| Cash [Member] | Fair Value, Inputs, Level 2 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
|
|
| Cash [Member] | Fair Value, Inputs, Level 3 [Member] |
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
| Total |
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=us-gaap_USTreasuryBillSecuritiesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=bivi_USTreasuryBillSecurities1Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CashAndCashEquivalentsAxis=us-gaap_CashMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Fair Value Measurements (Details Narrative) - $ / shares
|
1 Months Ended |
12 Months Ended |
Nov. 30, 2021 |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Term |
|
6 years
|
5 years
|
| Risk Free Interest Rate |
|
3.82%
|
1.56%
|
| Volatility Rate |
|
81.65%
|
76.47%
|
| Derivative Liability Warrants [Member] | Fair Value, Inputs, Level 3 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Share Price |
$ 6.44
|
$ 4.31
|
|
| Exercise Price |
$ 5.82
|
$ 5.82
|
|
| Term |
5 years
|
3 years 4 months 24 days
|
|
| Risk Free Interest Rate |
1.14%
|
4.40%
|
|
| Volatility Rate |
74.40%
|
92.00%
|
|
| Derivative Liability Conversion Option On Convertible Debenture [Member] | Fair Value, Inputs, Level 3 [Member] |
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
| Share Price |
$ 6.44
|
$ 6.98
|
|
| Exercise Price |
$ 6.98
|
|
|
| Term |
3 years
|
1 year 4 months 24 days
|
|
| Risk Free Interest Rate |
0.81%
|
5.18%
|
|
| Volatility Rate |
76.85%
|
92.00%
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAgreed-upon price for the exchange of the underlying asset relating to the share-based payment award.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=bivi_DerivativeLiabilityWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ConcentrationRiskByBenchmarkAxis=bivi_DerivativeLiabilityConversionOptionOnConvertibleDebentureMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Equity Transactions (Details) - USD ($)
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Offsetting Assets [Line Items] |
|
|
| Options outstanding at ending |
3,952,864
|
|
| Options Exercisable |
1,473,413
|
|
| Stock Options [Member] |
|
|
| Offsetting Assets [Line Items] |
|
|
| Options outstanding at beginning |
3,398,764
|
755,200
|
| Weighted average exercise price, Options outstanding at beginning |
$ 7.42
|
$ 4.34
|
| Weighted Average Remaining Contractual Term, Options outstanding at beginning |
5 years 6 months
|
4 years 4 months 24 days
|
| Aggregate Intrinsic Value, Outstanding at beginning of period |
|
$ 2,569,232
|
| Options Granted |
714,667
|
2,724,689
|
| Weighted Average Exercise Price, Options Granted |
$ 5.90
|
$ 5.86
|
| Weighted Average Remaining Contractual Term, Granted |
8 years 7 months 6 days
|
7 years 8 months 12 days
|
| Aggregate Intrinsic Value, Options Granted |
$ 38,610
|
|
| Options Expired |
(10,000)
|
(8,000)
|
| Weighted Average Exercise Price, Options Expired |
$ 28.69
|
$ 29.17
|
| Aggregate Intrinsic Value, Options Expired |
|
|
| Options Forfeited |
|
(73,125)
|
| Weighted Average Exercise Price, Options Forfeited |
|
$ (13.91)
|
| Aggregate Intrinsic Value, Options Forfeited |
|
|
| Aggregate Intrinsic Value, Outstanding at beginning of period |
$ 1,067,966
|
|
| Options Canceled |
(49,667)
|
|
| Weighted Average Exercise Price, Options Canceled |
$ 7.74
|
|
| Aggregate Intrinsic Value, Options Canceled |
|
|
| Options Exercised |
(100,900)
|
|
| Weighted Average Exercise Price, Options Exercised |
$ 8.12
|
|
| Aggregate Intrinsic Value, Options Exercised |
|
|
| Options outstanding at ending |
3,952,864
|
3,398,764
|
| Weighted Average Exercise Price, Options outstanding at ending |
$ 7.10
|
$ 7.42
|
| Weighted Remaining Average Contractual Term, Ending Balance |
6 years 3 months 18 days
|
|
| Aggregate Intrinsic Value, Outstanding at end of period |
$ 1,067,966
|
|
| Options Exercisable |
1,473,413
|
|
| Weighted Average Exercise Price, Options Exercisable |
$ 7.68
|
|
| Weighted Average Remaining Contractual Term, Options Exercisable |
5 years 4 months 24 days
|
|
| Aggregate Intrinsic Value, Options Exercisable |
$ 315,206
|
|
| X |
- References
+ Details
| Name: |
bivi_AggregateIntrinsicValueOptionsCanceled |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_AggregateIntrinsicValueOptionsExercised |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_AggregateIntrinsicValueOptionsExpired |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_AggregateIntrinsicValueOptionsForfeited |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_OptionsCanceled |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm3 |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageRemainingContractualTerm4 |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_WeightedAverageExercisePriceOptionsCanceled |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OffsettingAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of non-option equity instruments exercised by participants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(2)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options or other stock instruments for which the right to exercise has lapsed under the terms of the plan agreements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of fully vested and expected to vest exercisable options that may be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest exercisable or convertible options. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options of the plan that expired. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExpirationsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIntrinsic value of outstanding award under share-based payment arrangement. Excludes share and unit options.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardEquityInstrumentsOtherThanOptionsAggregateIntrinsicValueOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionIntrinsic value of vested award under share-based payment arrangement. Excludes share and unit options.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardEquityInstrumentsOtherThanOptionsAggregateIntrinsicValueVested |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for fully vested and expected to vest exercisable or convertible options, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOptionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Equity Transactions (Details 2)
|
12 Months Ended |
|
Jun. 30, 2023
$ / shares
shares
|
|---|
| Offsetting Assets [Line Items] |
|
| Outstanding |
3,952,864
|
| Exercisable |
1,473,413
|
| Stock Option 1 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 1.69
|
| Outstanding |
124,520
|
| Weighted Average Contractual Life |
4 years
|
| Exercisable |
41,507
|
| Stock Option 2 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 1.81
|
| Outstanding |
10,000
|
| Weighted Average Contractual Life |
3 years 10 months 24 days
|
| Exercisable |
2,000
|
| Stock Option 3 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 1.98
|
| Outstanding |
72,000
|
| Weighted Average Contractual Life |
3 years 10 months 24 days
|
| Exercisable |
16,000
|
| Stock Option 4 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 2.74
|
| Outstanding |
124,167
|
| Weighted Average Contractual Life |
8 years 7 months 6 days
|
| Exercisable |
35,180
|
| Stock Option 5 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 2.80
|
| Outstanding |
5,600
|
| Weighted Average Contractual Life |
1 year 7 months 6 days
|
| Exercisable |
5,600
|
| Stock Option 6 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 3.13
|
| Outstanding |
4,000
|
| Weighted Average Contractual Life |
7 months 6 days
|
| Exercisable |
4,000
|
| Stock Option 7 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 3.20
|
| Outstanding |
248,167
|
| Weighted Average Contractual Life |
8 years 7 months 6 days
|
| Exercisable |
79,834
|
| Stock Option 8 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 3.24
|
| Outstanding |
25,000
|
| Weighted Average Contractual Life |
8 years 8 months 12 days
|
| Exercisable |
6,667
|
| Stock Option 9 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 4.09
|
| Outstanding |
175,500
|
| Weighted Average Contractual Life |
10 years
|
| Exercisable |
|
| Stock Option 10 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 5.04
|
| Outstanding |
755,000
|
| Weighted Average Contractual Life |
3 years 9 months 18 days
|
| Exercisable |
377,500
|
| Stock Option 11 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 5.21
|
| Outstanding |
10,000
|
| Weighted Average Contractual Life |
9 years 4 months 24 days
|
| Exercisable |
|
| Stock Option 12 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 5.78
|
| Outstanding |
148,000
|
| Weighted Average Contractual Life |
9 years 10 months 24 days
|
| Exercisable |
29,600
|
| Stock Option 13 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 6.12
|
| Outstanding |
195,000
|
| Weighted Average Contractual Life |
4 years 4 months 24 days
|
| Exercisable |
97,500
|
| Stock Option 14 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 6.25
|
| Outstanding |
1,600
|
| Weighted Average Contractual Life |
3 months 18 days
|
| Exercisable |
1,600
|
| Stock Option 15 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 7.36
|
| Outstanding |
124,167
|
| Weighted Average Contractual Life |
9 years 9 months 18 days
|
| Exercisable |
|
| Stock Option 16 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 7.50
|
| Outstanding |
800
|
| Weighted Average Contractual Life |
1 year 3 months 18 days
|
| Exercisable |
800
|
| Stock Option 17 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 7.74
|
| Outstanding |
1,241,668
|
| Weighted Average Contractual Life |
8 years 1 month 6 days
|
| Exercisable |
447,000
|
| Stock Option 18 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 7.81
|
| Outstanding |
62,000
|
| Weighted Average Contractual Life |
9 years 9 months 18 days
|
| Exercisable |
|
| Stock Option 19 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 8.75
|
| Outstanding |
1,600
|
| Weighted Average Contractual Life |
9 months 18 days
|
| Exercisable |
1,600
|
| Stock Option 20 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 9.54
|
| Outstanding |
800
|
| Weighted Average Contractual Life |
2 years 3 months 18 days
|
| Exercisable |
800
|
| Stock Option 21 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 9.90
|
| Outstanding |
800
|
| Weighted Average Contractual Life |
2 years 3 months 18 days
|
| Exercisable |
800
|
| Stock Option 22 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 13.91
|
| Outstanding |
618,475
|
| Weighted Average Contractual Life |
2 years 6 months
|
| Exercisable |
321,425
|
| Stock Option 23 [Member] |
|
| Offsetting Assets [Line Items] |
|
| Exercise Price | $ / shares |
$ 42.09
|
| Outstanding |
4,000
|
| Weighted Average Contractual Life |
2 years 7 months 6 days
|
| Exercisable |
4,000
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OffsettingAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of fully vested and expected to vest exercisable options that may be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term for fully vested and expected to vest options outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption1Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption2Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption3Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption4Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption5Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption6Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption7Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption8Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption9Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption10Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption11Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption12Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption13Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption14Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption15Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption16Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption17Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption18Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption19Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption20Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption21Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption22Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOption23Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Equity Transactions (Details 3) - Restricted Stock Units (RSUs) [Member] - $ / shares
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
| Number of Shares Unvested at beginning |
124,520
|
|
| Weighted Average Grant Date Fair Value Per Share at beginning |
$ 1.69
|
|
| Number of Shares Unvested, Granted |
687,112
|
124,520
|
| Weighted Average Grant Date Fair Value Per Share, Granted |
$ 5.89
|
$ 1.69
|
| Number of Shares Unvested, Vested |
(215,175)
|
|
| Weighted Average Grant Date Fair Value Per Share, Vested |
$ 5.27
|
|
| Number of Shares Unvested at ending |
596,457
|
124,520
|
| Weighted Average Grant Date Fair Value Per Share at ending |
$ 5.24
|
$ 1.69
|
| X |
- DefinitionThe number of non-vested equity-based payment instruments, excluding stock (or unit) options, that validly exist and are outstanding as of the balance sheet date. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPer share or unit weighted-average fair value of nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of equity-based payment instruments, excluding stock (or unit) options, that vested during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value as of grant date pertaining to an equity-based award plan other than a stock (or unit) option plan for which the grantee gained the right during the reporting period, by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash in accordance with the terms of the arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Equity Transactions (Details 4) - Warrant [Member] - USD ($)
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Accumulated Other Comprehensive Income (Loss) [Line Items] |
|
|
| Number of Shares Unvested at beginning |
510,372
|
158,761
|
| Weighted Average Exercise Price, at the beginning of the period |
$ 6.17
|
$ 10.37
|
| Weighted Average Remaining Life, at the beginning of the period |
3 years 9 months 18 days
|
3 years 1 month 6 days
|
| Aggregate Intrinsic Value, Outstanding at beginning of period |
|
$ 1,765,437
|
| Warrant Outstanding, granted |
7,272,728
|
361,002
|
| Weighted Average Exercise Price, granted |
$ 1.82
|
$ 5.82
|
| Weighted Average Remaining Life, Granted |
5 years
|
5 years
|
| Warrant Expired |
(4,815)
|
(9,391)
|
| Weighted Average Exercise Price, Expired |
$ 75.00
|
$ 12.29
|
| Warrant Exercised |
(8,000)
|
|
| Weighted Average Exercise Price, Exercised |
$ 2.25
|
|
| Number of Shares Unvested at ending |
7,770,285
|
510,372
|
| Weighted Average Exercise Price, at the end of period |
$ 2.06
|
$ 6.17
|
| Weighted Average Remaining Life, at the end of period |
4 years
|
|
| Aggregate Intrinsic Value, Outstanding at end of period |
$ 18,318,954
|
|
| X |
- References
+ Details
| Name: |
bivi_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsExcersiedIntrinsicValue1 |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsExercisePrice |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsExpired |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsExpiredInPeriod |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsOutstandingWeightedAverageRemainingContractualTerms1 |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsOutstandingWeightedAverageRemainingContractualTerms3 |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsPeriodIncreaseDecrease1 |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-4
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-17
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481694/830-30-45-20
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or unit weighted-average intrinsic value of nonvested award under share-based payment arrangement. Excludes share and unit options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of non-vested equity-based payment instruments, excluding stock (or unit) options, that validly exist and are outstanding as of the balance sheet date. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term for equity-based awards excluding options, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsOutstandingWeightedAverageRemainingContractualTerms |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIntrinsic value of outstanding award under share-based payment arrangement. Excludes share and unit options.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardEquityInstrumentsOtherThanOptionsAggregateIntrinsicValueOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Equity Transactions (Details Narrative) - USD ($)
|
|
|
|
|
|
1 Months Ended |
3 Months Ended |
12 Months Ended |
Jun. 07, 2023 |
May 09, 2023 |
Apr. 06, 2023 |
Feb. 09, 2023 |
Apr. 05, 2022 |
Jun. 20, 2023 |
May 31, 2023 |
Feb. 16, 2023 |
Nov. 30, 2022 |
Nov. 23, 2022 |
Oct. 31, 2022 |
Jun. 21, 2022 |
Aug. 27, 2021 |
Aug. 20, 2021 |
Sep. 30, 2021 |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 4,222,845
|
$ 5,807,871
|
| Proceeds from issuance of common stock, shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,592,000
|
|
|
| Proceeds from issuance of common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 18,500,000
|
$ 49,465,103
|
18,511,009
|
| Issuance cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 2,200,000
|
|
|
| Total compensation cost |
|
|
|
|
|
|
|
|
|
$ 1,744,192
|
|
|
|
|
|
|
|
| Vendor [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation expense |
|
|
$ 372,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock issued during period for service |
|
|
50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Share Price |
|
|
$ 7.45
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sales Agreement [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Number of common stock sold |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,539,254
|
|
| Value of common stock sold |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 49,500,000
|
|
| Commission and expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 2,000,000.0
|
|
| Stock Option And Warrants [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shares issued for stock option |
|
|
|
|
|
|
481
|
|
800
|
|
3,590
|
|
|
|
|
22,082
|
|
| Stock option to purchase |
|
|
|
|
|
|
800
|
|
800
|
|
8,000
|
|
|
|
|
99,300
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
|
|
|
$ 3.13
|
|
$ 2.80
|
|
$ 2.25
|
|
|
|
|
$ 7.64
|
|
| Restricted Stock Units (RSUs) [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 243,000
|
$ 1,754
|
| Rsu granted |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
41,506
|
|
| Rsu granted, grant date fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 5.90
|
|
| Shares issued over the vesting period |
|
39,088
|
|
39,088
|
|
|
|
72,612
|
|
|
|
|
|
58,759
|
|
|
|
| Awarded Vested rights, percentage |
|
|
|
|
|
|
|
25.00%
|
|
|
|
|
|
|
|
|
|
| Shares issued to employees |
|
|
|
|
|
|
|
22,880
|
|
|
|
|
|
|
|
|
|
| Options Grants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
687,112
|
124,520
|
| Weighted Average Exercise Price, Options Grants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 5.89
|
$ 1.69
|
| Restricted Stock Units (RSUs) [Member] | Chief Executive Officer [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 384,456
|
|
| Restricted Stock Units (RSUs) [Member] | Four Directors [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
952,492
|
|
| Restricted Stock Units (RSUs) [Member] | Three Directors [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 791,700
|
|
| Restricted Stock Units (RSUs) [Member] | N 2019 Omnibus Incentive Equity Plan [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Rsu granted |
|
|
|
|
|
|
|
|
|
|
|
|
|
58,759
|
|
|
|
| Rsu granted, grant date fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 7.74
|
|
|
|
| Restricted Stock Units (RSUs) [Member] | N 2019 Omnibus Incentive Equity Plan [Member] | President And C E O [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Rsu granted |
|
|
|
|
|
|
|
|
|
381,976
|
|
124,520
|
|
|
|
|
|
| Rsu granted, grant date fair value |
|
|
|
|
|
|
|
|
|
$ 6.12
|
|
|
|
|
|
|
|
| Rsu awarded |
|
|
|
|
|
149,500
|
|
|
|
|
|
|
|
|
|
|
|
| Restricted Stock Units (RSUs) [Member] | N 2019 Omnibus Incentive Equity Plan [Member] | Four Directors [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Rsu granted |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
155,636
|
|
| Rsu granted, grant date fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 6.12
|
|
| Restricted Stock Units (RSUs) [Member] | N 2019 Omnibus Incentive Equity Plan [Member] | Three Directors [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Rsu granted |
|
|
|
|
|
|
|
|
|
195,000
|
|
|
|
|
|
|
|
| Rsu granted, grant date fair value |
|
|
|
|
|
|
|
|
|
$ 6.12
|
|
|
|
|
|
|
|
| Stock Options [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 4,200,000
|
$ 5,800,000
|
| Options Grants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
714,667
|
2,724,689
|
| Weighted Average Exercise Price, Options Grants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 5.90
|
$ 5.86
|
| Equity Option [Member] | Chief Executive Officer [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock option to purchase |
|
|
|
|
|
|
|
|
|
|
|
|
73,125
|
|
|
|
|
| Options Grants |
|
|
|
|
|
|
|
|
|
|
|
124,520
|
|
|
|
|
|
| Weighted Average Exercise Price, Options Grants |
|
|
|
|
|
|
|
|
|
|
|
$ 1.69
|
|
|
|
|
|
| Equity Option [Member] | New Employee [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation expense |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 584,424
|
|
| Stock option to purchase |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
286,167
|
479,334
|
| Awarded Vested rights, percentage |
80.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted Average Contractual Term, Granted (in Years) |
|
|
|
|
3 years
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted Average Exercise Price, Options Grants |
|
|
|
|
$ 5.04
|
|
|
|
|
|
|
|
|
|
|
|
|
| Equity Option [Member] | New Employee [Member] | Minimum [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Stock option to purchase |
148,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Awarded Vested rights, percentage |
|
|
|
|
25.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted Average Exercise Price, Options Grants |
$ 5.78
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Equity Option [Member] | New Employee [Member] | Maximum [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Awarded Vested rights, percentage |
|
|
|
|
75.00%
|
|
|
|
|
|
|
|
|
|
|
|
|
| Equity Option [Member] | Executive [Member] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Offsetting Assets [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Options Grants |
|
|
|
|
|
|
|
|
|
|
|
|
|
1,365,835
|
|
|
|
| Weighted Average Contractual Term, Granted (in Years) |
|
|
|
|
|
|
|
|
|
|
|
|
|
5 years
|
|
|
|
| Weighted Average Exercise Price, Options Grants |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ 7.74
|
|
|
|
| X |
- References
+ Details
| Name: |
bivi_RsuGranted |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsExcersiedIntrinsicValue1 |
| Namespace Prefix: |
bivi_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageGrantedContractualTerm1 |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_SharesIssuedOverVestingPeriod |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_TotalCompensationCost |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_OffsettingAssetsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of cash paid for commissions during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForCommissions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for cost incurred directly with the issuance of an equity security. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfStockIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of consideration received by subsidiary or equity investee in exchange for shares of stock issued or sold. Includes amount of cash received, fair value of noncash assets received, and fair value of liabilities assumed by the investor.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedPerTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued or sold by the subsidiary or equity method investee per stock transaction.
| Name: |
us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrice of a single share of a number of saleable stocks of a company.
| Name: |
us-gaap_SharePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPercentage of vesting of award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardAwardVestingRightsPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued during the period to an employee benefit plan, such as a defined contribution or defined benefit plan.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesEmployeeBenefitPlan |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued in lieu of cash for services contributed to the entity. Number of shares includes, but is not limited to, shares issued for services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares related to Restricted Stock Award forfeited during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesRestrictedStockAwardForfeited |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares that have been repurchased during the period and have not been retired and are not held in treasury. Some state laws may govern the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockRepurchasedDuringPeriodShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=bivi_VendorMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=bivi_SalesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=bivi_StockOptionAndWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_RestrictedStockUnitsRSUMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=srt_ChiefExecutiveOfficerMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=bivi_FourDirectorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=bivi_ThreeDirectorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=bivi_N2019OmnibusIncentiveEquityPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=bivi_PresidentAndCEOMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=bivi_StockOptionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DerivativeInstrumentRiskAxis=us-gaap_StockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=bivi_NewEmployeeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=bivi_ExecutiveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Leases (Details) - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
| Leases |
|
|
| Operating lease, right-of-use asset, net |
$ 80,789
|
$ 118,254
|
| Current portion of operating lease liabilities |
44,909
|
38,884
|
| Operating lease liabilities, net of current portion |
42,505
|
87,414
|
| Total operating lease liabilities |
$ 87,414
|
$ 126,298
|
| X |
- References
+ Details
| Name: |
bivi_DisclosureLeasesAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
Leases (Details 1) - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
| Leases |
|
|
| 2024 |
$ 52,156
|
|
| 2025 |
44,636
|
|
| Total minimum lease payments |
96,792
|
|
| Less amount representing interest |
(9,378)
|
|
| Present value of future minimum lease payments |
87,414
|
|
| Less current portion of operating lease liabilities |
(44,909)
|
$ (38,884)
|
| Operating lease liabilities, net of current portion |
$ 42,505
|
|
| X |
- References
+ Details
| Name: |
bivi_DisclosureLeasesAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_OperatingLeaseLiabilitiesNetOfCurrentPortion |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
bivi_PresentValueOfFutureMinimumLeasePayments |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
| X |
- References
+ Details
| Name: |
bivi_DisclosureLeasesAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.2
| X |
- References
+ Details
| Name: |
bivi_CashPaidForAmountsIncludedInMeasurementOfLeaseLiabilities |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
bivi_DisclosureLeasesAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
bivi_DisclosureEmployeeBenefitPlanAbstract |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of contribution received by defined benefit plan from employer which increases plan assets. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480482/715-20-55-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480482/715-20-55-18
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 715
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480506/715-20-50-1
| Name: |
us-gaap_DefinedBenefitPlanContributionsByEmployer |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
Income Taxes (Details) - USD ($)
|
Jun. 30, 2023 |
Jun. 30, 2022 |
| Deferred tax assets (liabilities): |
|
|
| Tax loss carryforward |
$ 4,018,817
|
$ 6,410,653
|
| Intangible assets |
(189,854)
|
(258,209)
|
| Stock based compensation |
1,788,862
|
1,845,836
|
| R&D capitalized |
7,938,602
|
|
| Valuation Allowance |
(13,556,427)
|
(7,998,280)
|
| Net deferred tax assets |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_ComponentsOfDeferredTaxAssetsAndLiabilitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible capital loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsCapitalLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from intangible assets including goodwill.
| Name: |
us-gaap_DeferredTaxAssetsGoodwillAndIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from in-process research and development costs expensed in connection with a business combination. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsInProcessResearchAndDevelopment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from share-based compensation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsShareBasedCompensationCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
v3.23.2
Income Taxes (Details Narrative) - USD ($)
|
12 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Income Tax Disclosure [Abstract] |
|
|
| Net deferred tax assets |
$ 13,600,000
|
$ 8,000,000.0
|
| Increase in valuation allowance |
5,600,000
|
$ 6,000,000.0
|
| Net Operating Loss carryforward |
$ 168,000,000
|
|
| Expirations dates |
2032 to 2037
|
|
| X |
- References
+ Details
| Name: |
bivi_ExpirationsDates |
| Namespace Prefix: |
bivi_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after allocation of valuation allowances and deferred tax liability, of deferred tax asset attributable to deductible differences and carryforwards, without jurisdictional netting. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsLiabilitiesNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in the valuation allowance for a specified deferred tax asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ValuationAllowanceDeferredTaxAssetChangeInAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.2
Subsequent Events (Details Narrative) - Sales Agreement [Member] - USD ($)
|
1 Months Ended |
12 Months Ended |
Aug. 15, 2023 |
Jun. 30, 2023 |
| Subsequent Event [Line Items] |
|
|
| Number of common stock sold |
|
7,539,254
|
| Value of common stock sold |
|
$ 49,500,000
|
| Commission and expenses |
|
$ 2,000,000.0
|
| Subsequent Event [Member] | Agent [Member] |
|
|
| Subsequent Event [Line Items] |
|
|
| Number of common stock sold |
336,089
|
|
| Value of common stock sold |
$ 1,600,000
|
|
| Commission and expenses |
$ 50,000
|
|
| X |
- DefinitionThe amount of cash paid for commissions during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForCommissions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of consideration received by subsidiary or equity investee in exchange for shares of stock issued or sold. Includes amount of cash received, fair value of noncash assets received, and fair value of liabilities assumed by the investor.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedPerTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued or sold by the subsidiary or equity method investee per stock transaction.
| Name: |
us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDetail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TransactionTypeAxis=bivi_SalesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=bivi_AgentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
BioVie (NASDAQ:BIVI)
Historical Stock Chart
From Jun 2024 to Jul 2024
BioVie (NASDAQ:BIVI)
Historical Stock Chart
From Jul 2023 to Jul 2024