Forest Laboratories Announces Termination of Agreement for Faropenem
February 06 2007 - 5:30PM
PR Newswire (US)
NEW YORK, Feb. 6 /PRNewswire-FirstCall/ -- Forest Laboratories,
Inc. (NYSE:FRX) and Replidyne, Inc. announced today that they have
terminated their collaboration agreement for the development of
faropenem medoxomil, a novel oral, community antibiotic. (Logo:
http://www.newscom.com/cgi-bin/prnh/20001011/FORESTLOGO ) The
agreement was announced in February 2006. In October 2006 the U.S.
Food and Drug Administration (FDA) issued a non-approvable letter
for four adult indications: acute bacterial sinusitis (ABS),
community-acquired pneumonia (CAP), acute exacerbation of chronic
bronchitis (AECB) and uncomplicated skin and skin structure
infections (SSSI). There are no payments by Forest associated with
the termination. Howard Solomon, Chairman and Chief Executive
Officer of Forest, stated: "Forest believes the FDA's
non-approvable letter clearly raises regulatory uncertainty. We
have carefully reviewed all the existing data and the FDA's
pronouncements and have determined that we should not continue
further development of the faropenem program." About Forest
Laboratories and Its Products Forest Laboratories
(http://www.frx.com/ ) is a US-based pharmaceutical company
dedicated to identifying, developing, and delivering products that
make a positive difference in peoples' lives. Forest Laboratories'
growing product line includes Lexapro(R) (escitalopram oxalate), an
SSRI indicated for adults for the initial and maintenance treatment
of major depressive disorder and for generalized anxiety disorder;
Namenda(R) (memantine HCl), an N-methyl-D-aspartate (NMDA)-receptor
antagonist indicated for the treatment of moderate to severe
Alzheimer's disease; Benicar(R)* (olmesartan medoxomil), an
angiotensin receptor blocker, and Benicar* HCT(R) (olmesartan
medoxomil-hydrochlorothiazide), an angiotensin receptor blocker and
diuretic combination product, each indicated for the treatment of
hypertension; and Campral(R)* (acamprosate calcium), indicated in
combination with psychosocial support for the maintenance of
abstinence from alcohol in patients with alcohol dependence who are
abstinent at treatment initiation. * Benicar is a registered
trademark of Sankyo Pharma, Inc., and Campral is a registered
trademark of Merck Sante s.a.s., subsidiary of Merck KGaA,
Darmstadt, Germany. Except for the historical information contained
herein, this release contains "forward-looking statements" within
the meaning of the Private Securities Reform Act of 1995. These
statements involve a number of risks and uncertainties, including
the difficulty of predicting FDA approvals, acceptance and demand
for new pharmaceutical products, the impact of competitive products
and pricing, the timely development and launch of new products and
the risk factors listed from time to time in the Companies' SEC
reports, including the Forest Laboratories, Inc.'s Annual Report on
Form 10-K for the fiscal year ended March 31, 2006 and on Form 10-Q
for the periods ended June 30, 2006 and September 30, 2006.
http://www.newscom.com/cgi-bin/prnh/20001011/FORESTLOGO
http://photoarchive.ap.org/ DATASOURCE: Forest Laboratories, Inc.
CONTACT: Charles E. Triano, Vice President - Investor Relations of
Forest Laboratories, Inc., +1-212-224-6714, Web site:
http://www.frx.com/
Copyright
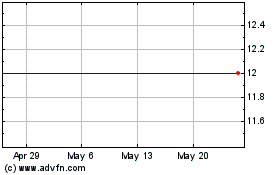
Forest Road Acquisition (NYSE:FRX)
Historical Stock Chart
From Jun 2024 to Jul 2024
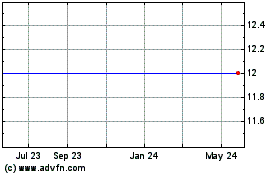
Forest Road Acquisition (NYSE:FRX)
Historical Stock Chart
From Jul 2023 to Jul 2024