- Company is now funded for and focused on New Drug
Applications (NDAs) for NRX-100 (ketamine) and NRX-101
- Audit of HOPE Therapeutics is now complete, SEC filing of
spinout this quarter
Key Milestones
- Secured $10.8 - $16.3 million in convertible-debt funding from an
institutional investor; funds targeted to support FDA New Drug
Applications for NRX-100 (ketamine) and NRX-101. Replacement
funding entails substantial reduction in interest rate, conversion
discount, and other financial terms compared to prior debt
- Retirement of Streeterville debt and settlement of litigation
at a substantial discount to litigation claims
- NRX-100 NDA for suicidal depression based on data from four
clinical trials in nearly 1000 participants demonstrating highly
significant efficacy compared to placebo, active comparator, and
electroshock therapy
- Ketamine findings have just been confirmed in published 43,000
person cohort study1
- Phase 2b/3 trial of NRX-101 in
suicidal patients with bipolar depression demonstrated depression
efficacy comparable to standard of care and significant reduction
of akathisia (P=0.025) and time to sustained remission from
suicidality (P=.05). Presented at the annual meeting of the
American Society of Clinical Psychopharmacology. Profile
demonstrates possible best in class bipolar depression
medication
- Company plans to file a New Drug Application (NDA) for
Accelerated Approval under Breakthrough Therapy Designation and
Priority Review of NRX-101 in treatment of bipolar depression in
people akathisia or suicidality, based on the Phase 2b/3 and STABIL-B data
- Stability data continues to mature on the three manufacturing
lots required for the NRX-100 (IV ketamine) NDA filing and the
Company announced alignment with FDA on its Pediatric Study Plan
for NRX-100, also a requirement for filing an NDA
- HOPE Therapeutics, the Company's wholly owned subsidiary, is
focused on developing a best-in-class network of clinics that
currently offer ketamine and other lifesaving therapies to patients
with suicidal depression and related disorders. HOPE is planned to
be spun out as a separate company to be owned by NRx, current NRx
shareholders, and new investors. This effort will be funded apart
from NRx.
- Appointed Dr. Dennis McBride, a
Neuroscience, Information Technology and Medical Technology
Veteran, to its Board of Directors
- Management to host a conference call August 14, 2024, at 4:30
PM ET
RADNOR,
Pa., Aug. 14, 2024 /PRNewswire/ -- NRx
Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the
"Company"), a clinical-stage biopharmaceutical company, today
announced its financial results for the quarter and year to date
ended June 30, 2024, and provided a
business update.
"NRx has continued to execute on our plans to file two NDA's
this year. As we near maturation of NRX-100 stability data, we also
achieved an important milestone in aligning with FDA on our
pediatric study plan. Together with strong clinical data from four
clinical trials, we believe this application will be quite robust.
Additionally, the important data generated from two trials
conducted by NRx with NRX-101 in suicidal bipolar depression sets
the stage for a second NDA for Accelerated Approval later this
year. Finally, work continues to spin out Hope Therapeutics and
distribute shares to NRx stockholders. We believe reaching these
important milestones will generate significant value in the company
and reward our shareholders," said Jonathan
Javitt, MD, MPH, Chairman and Chief Scientist of NRx
Pharmaceuticals. "Facilitated by funding to allow us to achieve
these goals and while replacing the prior expensive and toxic debt
on our balance sheet, we are in a position to deliver therapy that
can meet considerable unmet medical need in millions of patients
across the country. We are dedicated to bringing hope to life and I
thank our team and shareholders for their ongoing hard work and
support."
Second Quarter Clinical, Regulatory and Corporate
Highlights
Funding for FDA filings of NRX-100 and NRX-101
The Company has executed a Convertible Debt instrument with
Anson Funds of Toronto for
$10.8 - $16.3
million in funding designed to retire existing debt and to
support FDA New Drug filings of NRX-100 and NRX-101 in the fourth
quarter of 2024. Terms have been disclosed in 8K filings but are at an interest rate and
conversion rate substantially lower than current corporate
indebtedness. The new funding has no provision for "extraordinary
redemptions" triggered by appreciation in NRx share price.
Retirement of current debt and settlement of litigation
Concurrent with the Anson investment, the Company has settled
its outstanding litigation with Streeterville Capital, LLC at a
substantial discount to the amounts claimed in litigation.
Progress towards an NDA for NRX-100 (IV ketamine) in the
treatment of suicidal depression
Intravenous ketamine has now become a standard of care for acute
treatment of suicidal depression, in the absence of an FDA-labeled
product. Intranasal Esketamine is approved by the FDA (SPRAVATO®)
but has not demonstrated a benefit on suicidality and is not
approved for use in patients with bipolar depression. Attempts to
use intranasal racemic ketamine for suicidal depression have
failed.
The Company has formed data-sharing partnerships to license
clinical trial data from a French Government-funded trial and two
NIH-funded trials all of which demonstrate efficacy of racemic
Intravenous ketamine against depression and two of which
demonstrate statistically significant benefit vs suicidality. The
Company's role is to reformat these data into the required
presentation required for review by the FDA.
In contrast to nasal ketamine, Intravenous racemic ketamine
demonstrates dramatic and immediate reduction of suicidality in
patients with both Major Depressive Disorder and Bipolar
Depression. Grunebaum and colleagues demonstrated a rapid and
statistically significant reduction in Suicidal Ideation (SSI) at
day 1 (p=0.0003) and in depression (P=0.0234), as measured by the
Profile of Mood States (POMS) among patients randomized to IV
Ketamine compared to those randomized to midazolam. This trial
was published in the American Journal of Psychiatry Grunebaum,
et.al.2. Abbar and colleagues similarly published 84%
remission from suicidality on the Columbia Suicide Severity Rating
Scale (C-SSRS) in patients treated with ketamine, vs. 28% in those
treated with placebo (P<.0001). This trial was published in the
British Medical Journal3. Data are expected to be
transmitted to FDA by in 2024.
In November 2023, the Company
initiated manufacture of ketamine together with Nephron
Pharmaceuticals, Inc. (West Columbia,
SC) to develop a single patient presentation of ketamine.
Nine months of real-time stability is ongoing, the minimum
stability time required for a New Drug Application. This
presentation of ketamine does not contain the preservative included
in multi-dose vials, (Benzanthonium Chloride), that is designed to
preserve sterility in the vial when multiple doses are drawn for
multiple patients. The Company is not aware of any data to
support the safety of this preservative for repeated IV
administration. Data were collected 20 years ago that demonstrated
the toxic effect of this class of preservatives when applied
repeatedly to the surface of the eye, which led to the current
generation of preservative-free eye drops. NRX-100 will therefore
be launched as a preservative-free presentation.
A long-term challenge with ketamine is that the current
formulation (KETALAR®) is highly acidic. While it is suitable for
intravenous use, it cannot be administered subcutaneously. In
March 2024 the Company demonstrated
the formulation of a pH neutral patentable form of IV ketamine that
it anticipates will have widespread applicability both in treatment
of depression and chronic pain.
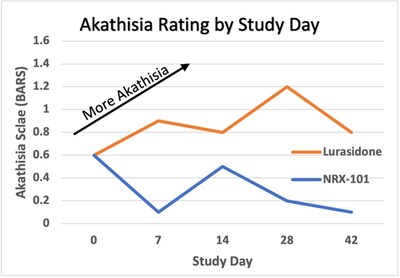
Development of NRX-101 for Suicidal Treatment-Resistant Suicidal
Bipolar Depression
The Company presented final data from the recently completed
phase 2b/3 trial of NRX-101 in
suicidal bipolar depression4 at the American Society of
Clinical Psychopharmacology's annual meeting. These data
demonstrated a significantly improved safety profile versus the
standard of care, as demonstrated by a clinically significant
reduction in akathisia (P=0.025) and time to sustained remission
from suicidality (P=0.05). Akathisia is an adverse event seen
with antidepressant medications considered by many experts to be a
precursor to suicide. Given the vital need for safer medications in
this at-risk population, we plan to submit an NDA for Accelerated
Approval to the US FDA for treatment of bipolar depression patients
with suicidality or akathisia, based on these data as well as
additional data from our STABIL-B5 trial.
Incorporation of HOPE Therapeutics
In February we first presented the contours of HOPE
Therapeutics, a subsidiary that will focus on the delivery of
advance psychiatric treatments, including ketamine-focused
treatment for depression and suicidality. Unlike the core business
of NRx Pharmaceuticals, that is focused on biotechnology Research
and Development, HOPE is organized around consolidating existing
best-in-class clinics into a nationwide network. This has been done
previously and without much success with clinics that are not
necessarily psychiatrist-led. As currently designed, the HOPE
consolidation is likely to be funded as a separate entity, through
bond offerings and, thus, to be non-dilutive to NRx shareholders.
Over the past quarter, HOPE leadership has identified the clinics
that are most likely to participate in the first $100 million consolidation, has completed the
audit required for a public listing of HOPE shares, and has
identified appropriate underwriters for a future bond
offering.
With Hope ownership as an asset of NRx, this will further
strengthen the NRx balance sheet and aims to further enhance NRx
shareholder value.
NRX-101 for Treatment of Chronic Pain:
In 2023, the Company licensed US Patent 8,653,120 for the
use of DCS in chronic pain and filed a now-accepted Investigational
New Drug (IND) application with the FDA to initiate commercial drug
development of NRX-101 in chronic pain. Data lock has now
been achieved in a 200-person randomized prospective trial funded
by the US DOD (NCT 03535688) in which patients with chronic pain
were randomly assigned to DCS 400mg/day vs. placebo. Should
these results support efficacy of DCS in the treatment of chronic
low back pain, they are expected to provide a Breakthrough Therapy
path towards treatment of chronic pain with DCS and DCS-containing
medicines.
Treatment of Urinary Tract Infection (UTI) and Urosepsis:
Although treatment of UTI is quite different from use of NRX-101
to treat Central Nervous System disorders, D-cycloserine was
originally developed as an antibiotic because of its role in
disrupting the cell wall of certain pathogens. During Q3
2023, NRx tested NRX-101 and its components against resistant
pathogens that appear on the Congressionally mandated Qualified
Infectious Disease Product (QIDP) list and proved in vitro
effectiveness against antibiotic-resistant E. coli, Pseudomonas,
and Acinetobacter. Accordingly, NRx was granted QIDP
designation, Fast Track Designation, and Priority Review by the US
FDA in January 2024.
In recent years, increased antibiotic resistance to common
pathogens that cause urinary tract infections and urosepsis (i.e.,
sepsis originating in the urinary tract) has resulted in a marked
increase in cUTI, hospitalization, and death from urosepsis. The US
Center for Disease Control and Prevention reports that more than
1.7 million Americans contract sepsis each year, of whom at
least 350,000 die during their hospitalization or are
discharged to hospice (CDC Sepsis Ref.)6. There
are approximately 3 million patients per year who contract cUTI in
the US annually (Lodise, et. al.)7. Additionally, should
NRX-101 succeed in clinical trials, the Company will consider
developing a follow-on product that is anticipated to achieve
another 20 years of patent exclusivity.
A key challenge in the treatment of cUTI is the tendency of
advanced antibiotics to cause C. difficile infection, which
is fatal in 10% of those who contract it over the age of 65 and
results in prolonged hospitalization in many more. The Company
recently announced data demonstrating that NRX-101 does not
compromise the intestinal microbiome, unlike common antibiotics
including Clindamycin and Ciprofloxacin. Should these findings be
documented in human patients, NRX-101 would represent the only
treatment for cUTI that does not cause C. Difficile infection.
In The Company does not anticipate funding this initiative with
core NRx assets and is exploring structures for partnership
opportunities. Should the Company or its partners succeed in
serving 10% of the cUTI market, the Company believes that the
revenue from NRX-101 has the potential to be hundreds of million
annually, based on 3 million cases per year in the US and potential
pricing of over $3,500/course of
therapy.
Financial Results for the Quarter and Year to Date
2024
For the three months ended June 30, 2024, we at NRx
Pharmaceuticals reduced our net loss from $8.7 million in
the second quarter of 2023 to $7.9 million in 2024,
representing nearly a 10% improvement year over year. For that same
period, we reduced research and development expenses from $3.9
million in 2023 to $2.8 million in 2024.
The $1.1 million decrease is related primarily to a
decrease of $2.4 million in clinical trial and
development expenses, offset by an increase of $1.3m related to the Alvogen warrants. Also
in that 3 month period we recorded an increase in general and
administrative expenses, from $4.1 million in 2023
to $4.2 million in 2024. The increase of $0.1
million is related primarily to an increase in consultants and
legal fees partially offset by lower insurance
expenses.
For the six months ended June 30, 2024, NRx Pharmaceuticals
reduced its net loss to $14.4 million compared
to $19.8 million in the prior year. These efficiencies
represent an improvement in net loss of $5.4 million year
over and a $1.32, or 47%, improvement in net loss per share
year over year. Over that six-month period we
recorded $4.6 million of research and development
expenses compared to $7.5 million for the same period in
2023 representing a 39% decrease year over year. The decrease
of $2.9 million is related primarily to a decrease
of $4.1 million in clinical trial and development
expenses, $0.3 million related to fees paid to regulatory
and process development consultants while offset by $1.3 million related to the Alvogen warrants
and $0.4 million related to fees paid
to regulatory and development consultants. Also in that
six-month period, we decreased G&A by $1.4 million,
from $9.9 million in 2023 to $8.5 million in
2024, nearly a 15% decrease year over year.
As of June 30, 2024, we had $1.9 million in cash
and cash equivalents. As previously stated, we recently announced
we secured up to $16.3 Million Senior
Secured Debt Financing from Anson Funds. This financing is expected
to support 2024 filing of New Drug Applications for NRX-100
(ketamine) and NRX-101 and to support launch of HOPE Therapeutics
as well as retire historical debt with more favorable terms, and a
lower annual interest rate.
NRx continues to implement operational efficiencies to extend
cash runway and maintain focus on our path to generating revenue
and value for our shareholders.
Please see detailed financials on our Form 10-Q, filed with the
SEC and available on our website.
Conference Call and Webcast Details
A live webcast of the conference call will be available on the
Company's website at 4:30 p.m. ET
today, at https://ir.nrxpharma.com/events. An archive of the
webcast will be available on the Company's website for 30
days. Participants that are unable to join the webcast can
access the conference call via telephone by dialing domestically
1-800-717-1738 or internationally 1-646-307-1865.
About NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals is a clinical-stage biopharmaceutical
company developing therapeutics based on its NMDA platform for the
treatment of central nervous system disorders, specifically
suicidal bipolar depression, chronic pain, and PTSD. The Company is
developing NRX-101, an FDA-designated investigational Breakthrough
Therapy for suicidal treatment-resistant bipolar depression and
chronic pain. NRx plans to file an NDA for Accelerated Approval for
NRX-101 in patients with bipolar depression and suicidality or
akathisia. NRX-101 additionally has potential to act as a
non-opioid treatment for chronic pain, as well as a treatment for
complicated UTI.
NRx has recently announced plans to submit a New Drug
Application for NRX-100 (IV ketamine) for the treatment of suicidal
depression, based on results of well-controlled clinical trials
conducted under the auspices of the US National Institutes of
Health and newly obtained data from French health authorities,
licensed under a data sharing agreement. NRx was awarded Fast Track
Designation for development of ketamine (NRX-100) by the US FDA as
part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a care
delivery company developing a best-in-class network of clinics that
currently offer ketamine and other lifesaving therapies to patients
with suicidal depression and related disorders, together with a
digital therapeutic-enabled platform designed to augment and
preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking
statements within the meaning of Section 21E of the Securities
Exchange Act of 1934, as amended, and Section 27A of the Securities
Act of 1933, as amended. These statements include, among others,
statements regarding the proposed public offering and the timing
and the use of the proceeds from the offering. Forward-looking
statements generally include statements that are predictive in
nature and depend upon or refer to future events or conditions, and
include words such as "may," "will," "should," "would," "expect,"
"plan," "believe," "intend," "look forward," and other similar
expressions among others. These statements relate to future events
or to the Company's future financial performance, and involve known
and unknown risks, uncertainties and other factors that may cause
the Company's actual results to be materially different from any
future results, levels of activity, performance or achievements
expressed or implied by these forward-looking statements. You
should not place undue reliance on forward-looking statements since
they involve known and unknown risks, uncertainties and other
factors which are, in some cases, beyond the Company's control and
which could, and likely will, materially affect actual results,
levels of activity, performance or achievements. Any
forward-looking statement reflects the Company's current views with
respect to future events and is subject to these and other risks,
uncertainties and assumptions relating to the Company's operations,
results of operations, growth strategy and liquidity. More detailed
information about the Company and the risk factors that may affect
the realization of forward-looking statements is set forth in the
Company's most recent Annual Report on Form 10-K and other filings
with the Securities and Exchange Commission. Investors and security
holders are urged to read these documents free of charge on the
SEC's website at http://www.sec.gov. Except as may be required
by applicable law, The Company assumes no obligation to publicly
update or revise these forward-looking statements for any reason,
or to update the reasons actual results could differ materially
from those anticipated in these forward-looking statements, whether
as a result of new information, future events or otherwise.
1 Pan, Y., Gorenflo, M.P., Davis, P.B. et
al. Suicidal ideation following ketamine prescription in
patients with recurrent major depressive disorder: a nation-wide
cohort study. Transl Psychiatry 14, 327
(2024). https://doi.org/10.1038/s41398-024-03033-4
2 Grunebaum, et. al., Ketamine for Rapid Reduction of
Suicidal Thoughts. Am J Psychiatry. 2018 Apr
1: 175(4): 327-335
3 Abbar, et. al. Ketamine for Acute Treatment of Severe
Suicidal Ideation, BMJ 2022; 376
4 Nierenberg, et. al., A Randomized, Double-Blind
Controlled Comparison of NRX-101 (D-cycloserine/ lurasidone) to
Lurasidone for Adults with Bipolar Depression and Subacute Suicidal
Ideation or Behavior. Am Soc Clin Psych Annual Meeting 2024.
5 Nierenberg et al. International Journal of Bipolar
Disorders (2023) 11:28
https://doi.org/10.1186/s40345-023-00
6 https://www.cdc.gov/sepsis/what-is-sepsis.html
7 Open Forum Infectious Diseases, Volume 9, Issue 7,
July 2022, ofac315,
https://doi.org/10.1093/ofid/ofac315
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-reports-second-quarter-and-year-to-date-2024-financial-results-and-provides-business-update-302222664.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-reports-second-quarter-and-year-to-date-2024-financial-results-and-provides-business-update-302222664.html
SOURCE NRx Pharmaceuticals, Inc.