false 0001832038 0001832038 2024-08-27 2024-08-27
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): August 27, 2024
Invivyd, Inc.
(Exact Name of Registrant as Specified in its Charter)
|
|
|
|
|
| Delaware |
|
001-40703 |
|
85-1403134 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
| 1601 Trapelo Road, Suite 178 |
|
|
| Waltham, MA |
|
02451 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (781) 819-0080
Not applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common stock, par value $0.0001 per share |
|
IVVD |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01. Other Events.
On August 27, 2024, Invivyd, Inc. (the “Company”) issued a press release entitled “Invivyd Announces PEMGARDA(TM) (pemivibart) Demonstrated 84% Relative Risk Reduction in Symptomatic COVID-19 Compared to Placebo in an Exploratory Analysis from Ongoing CANOPY Phase 3 Clinical Trial.” A copy of the press release is filed herewith as Exhibit 99.1 and is incorporated by reference in this Item 8.01.
On August 27, 2024, the Company posted an updated corporate presentation on its website at www.invivyd.com. A copy of the presentation is filed herewith as Exhibit 99.2 and is incorporated by reference in this Item 8.01.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
INVIVYD, INC. |
|
|
|
|
| Date: August 27, 2024 |
|
|
|
By: |
|
/s/ Jill Andersen |
|
|
|
|
|
|
Jill Andersen |
|
|
|
|
|
|
Chief Legal Officer and Corporate Secretary |
Exhibit 99.1

Invivyd Announces PEMGARDA™ (pemivibart) Demonstrated 84%
Relative Risk Reduction in Symptomatic COVID-19 Compared to Placebo in an Exploratory Analysis from Ongoing CANOPY Phase 3 Clinical Trial
| |
• |
|
In all-comer cohort of immunocompetent individuals at risk of
contracting symptomatic COVID-19 in their everyday social interactions, participants receiving pemivibart experienced a 1.9% rate of confirmed symptomatic COVID-19
compared to an 11.9% rate for participants receiving placebo, an 84% relative risk reduction (nominal p= 0.000061) |
| |
• |
|
In immunocompromised participants, pemivibart demonstrated a rate of 3% of confirmed symptomatic COVID-19, an encouraging potential signal of protection during the assessed time period |
| |
• |
|
CANOPY data from planned exploratory clinical efficacy analyses during the
180-day period that included XBB* and JN.1* virus lineages |
| |
• |
|
Safety profile of pemivibart consistent with previously reported CANOPY clinical trial data
|
| |
• |
|
PEMGARDA (pemivibart) Fact Sheet for Healthcare Providers updated by U.S. Food and Drug Administration (FDA)
including exploratory clinical efficacy data |
| |
• |
|
Conference call today at 8:30AM EDT to discuss CANOPY data analyses |
WALTHAM, Mass., August 27, 2024 – Invivyd, Inc. (Nasdaq: IVVD), a biopharmaceutical company devoted to delivering protection
from serious viral infectious diseases, today announced positive 180-day exploratory clinical efficacy data from the company’s ongoing CANOPY Phase 3 clinical trial of pemivibart, a half-life extended
investigational monoclonal antibody (mAb), for the pre-exposure prophylaxis (PrEP) of COVID-19.
The exploratory clinical efficacy data in Cohort B, a placebo-controlled cohort of all-comer immunocompetent
individuals, showed a relative risk reduction of 84% with pemivibart compared to placebo in the likelihood of trial participants contracting confirmed symptomatic COVID-19, with no hospitalizations or deaths
due to COVID-19 reported. Cohort B participants treated with pemivibart experienced a 1.9% rate of symptomatic COVID-19 across a
180-day time period, whereas Cohort B participants in the placebo arm experienced an 11.9% rate of symptomatic COVID-19, a robust attack rate for the trial. Cases of COVID-19 observed in the pemivibart arm were mild or moderate in severity.
Additionally, in Cohort A, the single-arm immunocompromised cohort of the trial, pemivibart demonstrated a 3% rate of confirmed symptomatic COVID-19 over the 180-day
period. Cases of COVID-19 observed in this cohort were also mild or moderate in severity. These exploratory data support the concept of potential protection observed with pemivibart, aligned with expectations
of a highly active prophylactic monoclonal antibody in this population.
The safety profile of pemivibart in the second half of the assessed 180-day time period remained consistent with previously disclosed CANOPY clinical trial data. In Cohort A, the most common treatment-emergent adverse events (TEAE) were viral infection (7.8%), upper respiratory
tract infection (URTI) (7.5%), influenza like illness (4.2%), infusion related reactions (3.6%), and urinary tract infection (3.6%). Anaphylaxis was observed in 4 participants (0.6%) – 2 participants during the first infusion and 2
participants during the second infusion; two reactions were life-threatening, and all led to permanent discontinuation of pemivibart. Systemic infusion-related reactions and hypersensitivity
reactions were observed within 24 hours of dosing pemivibart in 8.2% and 3.9% of participants after the initial dose and redose, respectively, of this open-label single-arm cohort and were generally mild to
moderate in severity. In Cohort B, the most common TEAEs in the pemivibart arm were URTI (8.2%), viral infection (7.3%), and influenza like illness (5.4%), with similar percentages in the placebo arm. No participants developed anaphylaxis. Systemic
infusion-related reactions and hypersensitivity reactions were observed within 24 hours of dosing pemivibart in 1.3% and 2.5% of participants after the initial dose and redose, respectively, and all were mild or moderate in severity.
CANOPY clinical trial results provide data from mAb administration in a contemporary population that likely had acquired prior immune exposure from either
vaccination or natural infection and overlapped with the height of the September 2023-March 2024 XBB* and JN.1* waves that saw a surge in COVID-19 cases nationwide in the United States. By contrast, ancestral
studies of COVID-19 PrEP candidate mAbs were performed in populations naïve to vaccination or prior infection. CANOPY clinical trial participants received two doses of pemivibart (Cohort A and B) or
placebo (Cohort B) administered via intravenous (IV) infusion three months apart; safety, serum virus neutralizing antibody (sVNA) titers and clinical endpoints were assessed at pre-specified timepoints
over the 180-day period.
The 180-day clinical efficacy exploratory data
announced today complements the initial clinical efficacy exploratory data demonstrating potential signals of clinical protection from symptomatic COVID-19 shared previously. The company expects the full data
set to be provided in an upcoming scientific publication. The PEMGARDA (pemivibart) Fact Sheet for Healthcare Providers was updated by the U.S. Food and Drug Administration (FDA) including 180-day exploratory
clinical efficacy data.
The following table elaborates the principal exploratory clinical efficacy findings based on the full 180-day analyses:
CANOPY Clinical Efficacy Results Over 180 Days
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cohort B |
|
Pemivibart |
|
|
Placebo |
|
|
Standardized
Relative Risk
Reduction
(95% CI) |
|
|
Nominal
p-value |
|
| Modified Full Analysis Set |
|
| N |
|
|
317 |
|
|
|
160 |
|
|
|
|
|
|
|
|
|
| Composite RT-PCR confirmed
COVID-19, COVID-19-related Hospitalization, and All-cause Mortality |
|
|
6 |
(1.9%) |
|
|
19 |
(11.9%) |
|
|
84.1 |
% (60.9, 93.5) |
|
|
0.000061 |
|
| RT-PCR confirmed
COVID-19 |
|
|
6 |
|
|
|
19 |
|
|
|
|
|
|
|
|
|
| COVID-19-related
Hospitalization |
|
|
0 |
|
|
|
0 |
|
|
|
|
|
|
|
|
|
| COVID-19-related
Death |
|
|
0 |
|
|
|
0 |
|
|
|
|
|
|
|
|
|
| All-cause Mortality |
|
|
0 |
|
|
|
0 |
|
|
|
|
|
|
|
|
|
Based on CANOPY 6-month data cutoff (21May2024). Cohort B Modified Full Analysis
Set includes all randomized participants without current SARS-CoV-2 infection at baseline as measured by central lab RT-PCR.
|
|
|
| Cohort A |
|
Pemivibart |
| Full Analysis Set |
| N |
|
298 |
| Composite RT-PCR confirmed
COVID-19, COVID-19-related Hospitalization, and All-cause Mortality |
|
11 (3.7%)* |
| RT-PCR confirmed
COVID-19 |
|
9 (3.0%) |
| COVID-19-related
Hospitalization |
|
0 |
| COVID-19-related
Death |
|
0 |
| All-cause Mortality |
|
2 (0.7%)*
*One death is due
to an unknown
cause and one
due to suicide |
Based on CANOPY 6-month data cutoff (21May2024). Cohort A Full Analysis Set
includes all participants who received a full dose of study drug at the initial dosing.
“We are thrilled with the clinically meaningful
protection shown by pemivibart in these exploratory analyses during a 180-day period with various SARS-CoV-2 circulating
variants” commented Mark A. Wingertzahn, SVP of Clinical Development and Medical Affairs. “These CANOPY clinical efficacy data provide an important reminder that monoclonal antibodies can provide meaningful protection against COVID-19 when people encounter the virus in indoor settings and unmasked during their everyday lives. Importantly, given the timeframe in which this study was conducted, these CANOPY data suggest that even with a
substantial population-level backdrop of immunologic experience with SARS-CoV-2 from either infection or vaccination, additional protection against symptomatic COVID-19 may be available with monoclonal antibodies, including for certain individuals with moderate-to-severe immunocompromise.”
“Invivyd is devoted to delivering protection from serious viral infectious diseases such as SARS-CoV-2. Today’s data align with and fuel our broader goals of innovating new molecules and educating the clinical community on the possibility of strong protection from monoclonal antibodies so that
we can scale and democratize access to such medicines,” noted Marc Elia, Chairperson of the Board. “COVID-19 remains an unacceptable medical burden in the U.S. and around the world, and the
incremental protection demonstrated by pemivibart in the CANOPY study points the way to the potential for a radically diminished risk of symptomatic disease via antibody prophylaxis. Our mission remains to innovate so that the immunocompromised
community and ultimately much broader populations can experience high rates of protection from symptomatic COVID-19.”
“The positive CANOPY clinical efficacy data through 6 months is encouraging and will be helpful to clinicians in making an informed decision about
PEMGARDA use in their immunocompromised, at-risk patients, “ said Cameron R. Wolfe, M.B.B.S., M.P.H., Professor of Medicine, Transplant Infectious Disease at Duke University School of Medicine. “The
risk of COVID-19 for immunocompromised people remains disproportionate and having a mAb in the toolbox for pre-exposure prophylaxis is extremely beneficial.”
About PEMGARDA
PEMGARDA™ (pemivibart) is a half-life extended investigational monoclonal antibody (mAb). PEMGARDA
was engineered from adintrevimab, Invivyd’s investigational mAb that has a robust safety data package and provided evidence of clinical efficacy in a global Phase 2/3 clinical trial for the prevention and treatment of COVID-19. PEMGARDA has demonstrated in vitro neutralizing activity against major SARS-CoV-2 variants, including JN.1. PEMGARDA targets
the SARS-CoV-2 spike protein receptor binding domain (RBD), thereby inhibiting virus attachment to the human ACE2 receptor on host cells.
PEMGARDA (pemivibart) injection (4500 mg), for intravenous use is an investigational mAb that has not been approved, but has been authorized for emergency use
by the U.S. FDA under an EUA for the pre-exposure prophylaxis (prevention) of COVID-19 in adults and adolescents (12 years of age and older weighing at least 40 kg) who
have moderate-to-severe immune compromise due to certain medical conditions or receipt of certain immunosuppressive medications or treatments and are unlikely to mount
an adequate immune response to COVID-19 vaccination. Recipients should not be currently infected with or have had a known recent exposure to an individual infected with SARS-CoV-2.
PEMGARDA is not authorized for use for treatment of COVID-19
or post-exposure prophylaxis of COVID-19. Anaphylaxis has been observed with PEMGARDA and the PEMGARDA Fact Sheet for Healthcare Providers includes a boxed warning for anaphylaxis. The most common adverse
events (all grades, incidence ≥2%) observed in participants who have moderate-to-severe immune compromise treated with PEMGARDA included systemic and local
infusion-related or hypersensitivity reactions, upper respiratory tract infection, viral infection, influenza-like illness, fatigue, headache, and nausea. For additional information, please see the PEMGARDA
full product Fact Sheet for Healthcare Providers, including important safety information and boxed warning.
To support the EUA for PEMGARDA, an
immunobridging approach was used to determine if PEMGARDA may be effective for pre-exposure prophylaxis of COVID-19. Immunobridging is based on the serum virus
neutralizing titer-efficacy relationships identified with other neutralizing human mAbs against SARS-CoV-2. This includes adintrevimab, the parent mAb of pemivibart, and
other mAbs that were previously authorized for EUA. There are limitations of the data supporting the benefits of PEMGARDA. Evidence of clinical efficacy for other neutralizing human mAbs against SARS-CoV-2 was based on different populations and SARS-CoV-2 variants that are no longer circulating. Further, the variability
associated with cell-based EC50 value determinations, along with limitations related to pharmacokinetic data and efficacy estimates for the mAbs in prior clinical trials, impact the ability to precisely estimate protective titer ranges.
Additionally, certain SARS-CoV-2 viral variants may have substantially reduced susceptibility to PEMGARDA, and PEMGARDA may not be effective at preventing COVID-19 caused by these SARS-CoV-2 viral variants.
The emergency use of PEMGARDA is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency
use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. §
360bbb-3(b)(1), unless the declaration is terminated or authorization revoked sooner. PEMGARDA is authorized for use only when the combined national frequency of variants with substantially reduced
susceptibility to PEMGARDA is less than or equal to 90%, based on available information including variant susceptibility to PEMGARDA and national variant frequencies.
About CANOPY
The ongoing CANOPY Phase 3 clinical trial is designed to evaluate the safety and tolerability of pemivibart and to assess immunobridging from pemivibart to
certain historical data from the company’s previous Phase 2/3 clinical trial of adintrevimab (ADG20) for the prevention of symptomatic COVID-19 (EVADE). Additionally, there are pre-specified exploratory endpoints through three, six and twelve months to evaluate clinical efficacy of pemivibart compared to placebo in the prevention of RT-PCR-confirmed symptomatic COVID-19. The latest analysis from the Phase 3 CANOPY clinical trial includes 180-day data. The
CANOPY clinical trial enrolled participants in two cohorts: Cohort A is a single-arm, open-label trial in adults who have
moderate-to-severe immune compromise including complex underlying medical conditions. Cohort B is a randomized, placebo-controlled cohort that enrolled adults without moderate-to-severe immune compromise who are at risk of acquiring COVID-19 due to regular unmasked face-to-face interactions in indoor settings.
About Pemivibart (VYD222)
Pemivibart is a half-life extended monoclonal antibody (mAb) candidate being investigated for the pre-exposure
prophylaxis (prevention) of COVID-19 and the treatment of mild to moderate symptomatic COVID-19 in certain immunocompromised adults and adolescents. Pemivibart has
demonstrated in vitro neutralizing activity in pseudotyped virus-like particle and authentic virus neutralization assays against various pre-Omicron and Omicron variants. Pemivibart was engineered
from adintrevimab, Invivyd’s investigational mAb that has a robust safety data package and provided evidence of clinical efficacy in global Phase 2/3 clinical trials for both the prevention and treatment of
COVID-19. Pemivibart has not been approved by the U.S. FDA or any other regulatory authority.
About
Invivyd
Invivyd, Inc. (Nasdaq: IVVD) is a biopharmaceutical company devoted to delivering protection from serious viral infectious diseases, beginning
with SARS-CoV-2. The company’s proprietary INVYMAB™ platform approach combines state-of-the-art viral surveillance and predictive modeling with advanced antibody engineering. INVYMAB is designed to facilitate the
rapid, serial generation of new monoclonal antibodies (mAbs) to address evolving viral threats. In March 2024, Invivyd received emergency use authorization (EUA) from the U.S. FDA for its first mAb in a planned series of innovative antibody
candidates. Visit https://invivyd.com/ to learn more.
Cautionary Note Regarding Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as
“anticipates,” “believes,” “could,” “expects,” “estimates,” “intends,” “potential,” “projects,” and “future” or similar expressions (as well as other words or
expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Forward-looking statements include statements concerning, among other things, the company’s ongoing research and clinical
development activities, as well as future potential research and clinical development efforts; the potential of pemivibart for clinical protection from symptomatic COVID-19 based on the 180-day exploratory clinical efficacy data from the CANOPY clinical trial; the potential for mAbs to provide meaningful protection against COVID-19; the company’s goal to
scale and democratize access to mAbs; the potential for mAbs to provide additional protection against COVID-19; the company’s goals of innovating new molecules and educating the clinical community on the
possibility of strong protection from mAbs; the company’s devotion to delivering protection from serious viral infectious diseases, beginning with SARS-CoV-2; the design of the company’s INVYMAB platform approach to facilitate the rapid, serial generation of new mAbs to address evolving viral threats; the company’s plans for a series of
innovative antibody candidates; the company’s expectation to provide full CANOPY clinical trial data in an upcoming scientific publication; the potential of PEMGARDA as a mAb for pre-exposure prophylaxis
(prevention) of COVID-19 in adults and adolescents who have moderate-to-severe immune compromise; the ongoing in vitro
neutralizing activity of PEMGARDA against major SARS-CoV-2 variants; and other statements that are not historical fact. The company may not actually achieve the plans,
intentions or expectations disclosed in the company’s forward-looking statements and you should not place undue reliance on the company’s forward-looking statements. These forward-looking statements involve risks and uncertainties that
could cause the company’s actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: the timing and progress of the company’s discovery, preclinical and
clinical development activities; the risk that results of nonclinical studies or clinical trials may not be predictive of future results, and interim data are subject to further analysis; unexpected safety or efficacy data observed during
preclinical studies or clinical trials; the predictability of clinical success of the company’s product candidates based on neutralizing activity in nonclinical studies; potential variability in neutralizing activity of product candidates
tested in different assays, such as pseudovirus assays and authentic assays; the company’s reliance on third parties with respect to virus assay creation and product candidate testing and with respect to its clinical trials; variability of
results in models used to predict activity against SARS-CoV-2 variants; whether pemivibart or any other product candidate is able to demonstrate and sustain neutralizing
activity against major SARS-CoV-2 variants, particularly in the face of viral evolution; how long the EUA granted by the FDA for PEMGARDA will remain in effect and
whether the EUA is revoked or revised by the FDA; the company’s ability to build and maintain sales, marketing and distribution capabilities to successfully commercialize PEMGARDA; uncertainties related to the regulatory authorization or
approval process, and available development and regulatory pathways for authorization or approval of the company’s product candidates; the ability to maintain a continued acceptable safety, tolerability and efficacy profile of any product
candidate following regulatory authorization or approval; changes in the regulatory environment; changes in expected or existing competition; the complexities of manufacturing mAb therapies; the company’s ability to leverage its INVYMAB
platform approach to facilitate the rapid, serial generation of new mAbs to address evolving viral threats; any legal proceedings or investigations relating to the company; the company’s ability to continue as a going concern; and whether the
company has adequate funding to meet future operating expenses and capital expenditure requirements. Other factors that may cause the company’s actual results to differ materially from those expressed or implied in the forward-looking
statements in this press release are described under the heading “Risk Factors” in the company’s Annual Report on Form 10-K for the year ended December 31, 2023 and the company’s
Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, each filed with the Securities and Exchange Commission (SEC), and in the company’s other filings with the SEC, and in its future
reports to be filed with the SEC and available at www.sec.gov. Forward-looking statements contained in this press release are made as of this date, and Invivyd undertakes no duty to update such information whether as a result of new
information, future events or otherwise, except as required under applicable law.
This press release contains hyperlinks to information that is not deemed to be incorporated by reference in
this press release.
Contacts:
Media Relations
(781) 208-0160
media@invivyd.com
Investor Relations
(781) 208-0160
investors@invivyd.com

Exhibit 99.2 INVIVYD CANOPY DATA UPDATE August 27, 2024 © 2024
Invivyd, Inc. Invivyd , Pemgarda , and the Ribbon logos are trademarks of Invivyd, Inc. All trademarks in this presentation are the property of their respective owners.

CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS This presentation
contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements. Words such as
“may,” “will,” “should,” “expect,” “plan,” “anticipate,” “seek,” “could,” “intend,” “target,” “aim,”
“project,” “designed to,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to
identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements include statements concerning, among other things, the potential of pemivibart for clinical protection from
symptomatic COVID-19 based on the 180-day clinical event exploratory efficacy analysis from the CANOPY clinical trial; our plans to work with the FDA to integrate clinical event findings from the CANOPY clinical trial into future mAb development
work; our expectations regarding the safety profile of pemivibart; our expectations regarding the evolution of the PEMGARDA fact sheet; our belief that mAbs may be critical for managing endemic virus over the long term; our business strategies and
objectives, and ability to execute on them; our future prospects; and other statements that are not historical fact. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not
place undue reliance on our forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause our actual results to differ materially from the results described in or implied by the forward-looking
statements, including, without limitation: the timing, progress and results of our discovery, preclinical and clinical development activities; unexpected safety or efficacy data observed during preclinical studies or clinical trials; the ability to
maintain a continued acceptable safety, tolerability and efficacy profile of any product candidate following regulatory authorization or approval; the predictability of clinical success of our product candidates based on neutralizing activity in
nonclinical studies; the risk that results of nonclinical studies or clinical trials may not be predictive of future results, and interim data are subject to further analysis; our reliance on third parties with respect to virus assay creation and
product candidate testing and with respect to our clinical trials; potential variability in neutralizing activity of product candidates tested in different assays, such as pseudovirus assays and authentic assays; variability of results in models
used to predict activity against SARS-CoV-2 variants; whether pemivibart or any other product candidate is able to demonstrate and sustain neutralizing activity against major SARS-CoV-2 variants, particularly in the face of viral evolution; the
complexities of manufacturing mAb therapies; our ability to leverage our INVYMAB platform approach to facilitate the rapid, serial generation of new mAbs to address evolving viral threats; our dependence on third parties to manufacture, label,
package, store and distribute clinical and commercial supplies of our product candidates; whether we can obtain and maintain third-party coverage and adequate reimbursement for pemivibart or any other product candidate; whether we are able to
achieve improved clinical and commercial profiles with our product pipeline; any legal proceedings or investigations relating to the company; our ability to continue as a going concern; and whether we have adequate funding to meet future operating
expenses and capital expenditure requirements. Other factors that may cause our actual results to differ materially from those expressed or implied in the forward-looking statements in this presentation are described under the heading “Risk
Factors” in our Annual Report on Form 10-K for the year ended December 31, 2023 and our Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, each filed with the Securities and Exchange Commission (SEC), and in our other filings
with the SEC, and in our future reports to be filed with the SEC and available at www.sec.gov. Forward-looking statements contained in this presentation are made as of this date, and we undertake no duty to update such information whether as a
result of new information, future events or otherwise, except as required under applicable law. 2
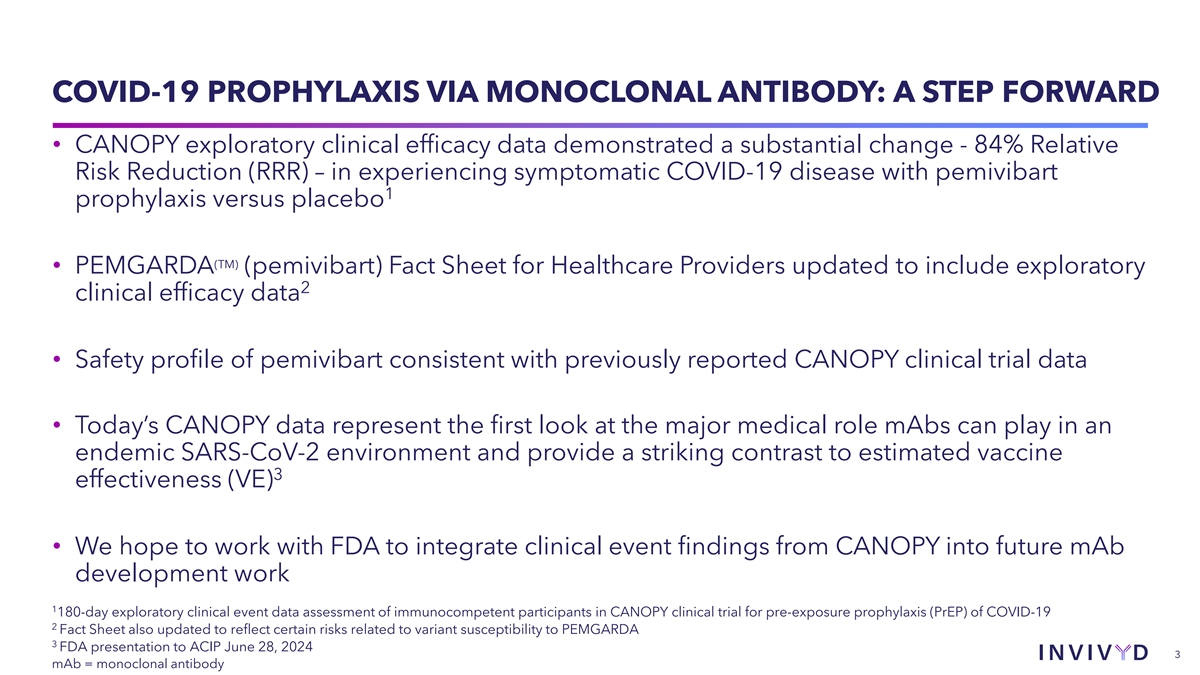
COVID-19 PROPHYLAXIS VIA MONOCLONAL ANTIBODY: A STEP FORWARD •
CANOPY exploratory clinical efficacy data demonstrated a substantial change - 84% Relative Risk Reduction (RRR) – in experiencing symptomatic COVID-19 disease with pemivibart 1 prophylaxis versus placebo (TM) • PEMGARDA (pemivibart) Fact
Sheet for Healthcare Providers updated to include exploratory 2 clinical efficacy data • Safety profile of pemivibart consistent with previously reported CANOPY clinical trial data • Today’s CANOPY data represent the first look at
the major medical role mAbs can play in an endemic SARS-CoV-2 environment and provide a striking contrast to estimated vaccine 3 effectiveness (VE) • We hope to work with FDA to integrate clinical event findings from CANOPY into future mAb
development work 1 180-day exploratory clinical event data assessment of immunocompetent participants in CANOPY clinical trial for pre-exposure prophylaxis (PrEP) of COVID-19 2 Fact Sheet also updated to reflect certain risks related to variant
susceptibility to PEMGARDA 3 FDA presentation to ACIP June 28, 2024 3 mAb = monoclonal antibody
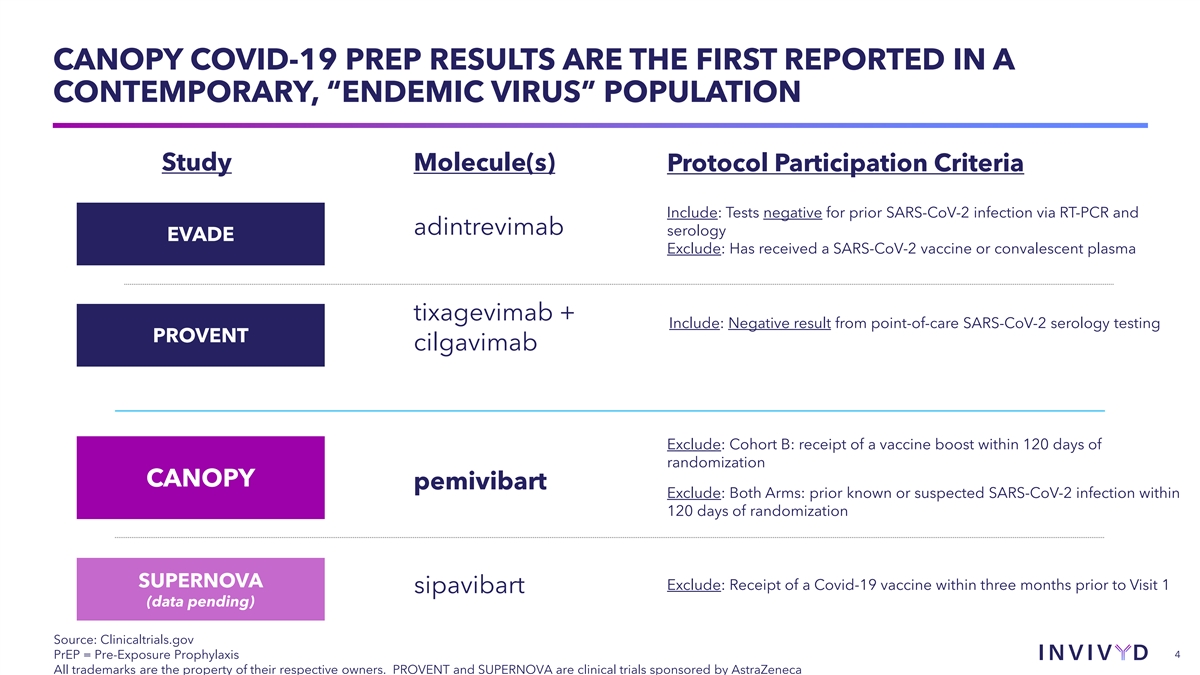
CANOPY COVID-19 PREP RESULTS ARE THE FIRST REPORTED IN A CONTEMPORARY,
“ENDEMIC VIRUS” POPULATION Study Molecule(s) Protocol Participation Criteria Include: Tests negative for prior SARS-CoV-2 infection via RT-PCR and adintrevimab serology EVADE Exclude: Has received a SARS-CoV-2 vaccine or convalescent
plasma tixagevimab + Include: Negative result from point-of-care SARS-CoV-2 serology testing PROVENT cilgavimab Exclude: Cohort B: receipt of a vaccine boost within 120 days of randomization CANOPY pemivibart Exclude: Both Arms: prior known or
suspected SARS-CoV-2 infection within 120 days of randomization SUPERNOVA Exclude: Receipt of a Covid-19 vaccine within three months prior to Visit 1 sipavibart (data pending) Source: Clinicaltrials.gov PrEP = Pre-Exposure Prophylaxis 4 All
trademarks are the property of their respective owners. PROVENT and SUPERNOVA are clinical trials sponsored by AstraZeneca

AGENDA u CANOPY Clinical Trial Results Q&A 5
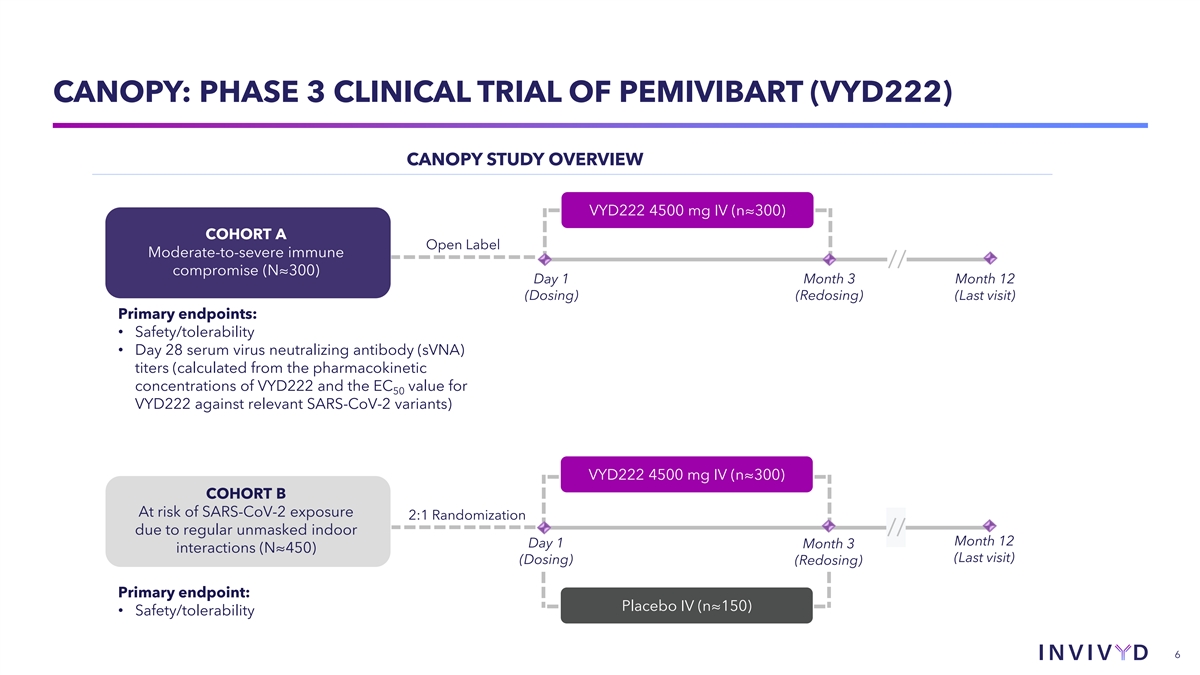
CANOPY: PHASE 3 CLINICAL TRIAL OF PEMIVIBART (VYD222) CANOPY STUDY
OVERVIEW VYD222 4500 mg IV (n≈300) COHORT A Open Label Moderate-to-severe immune // compromise (N≈300) Day 1 Month 3 Month 12 (Dosing) (Redosing) (Last visit) Primary endpoints: • Safety/tolerability • Day 28 serum virus
neutralizing antibody (sVNA) titers (calculated from the pharmacokinetic concentrations of VYD222 and the EC value for 50 VYD222 against relevant SARS-CoV-2 variants) VYD222 4500 mg IV (n≈300) COHORT B At risk of SARS-CoV-2 exposure 2:1
Randomization due to regular unmasked indoor // Month 12 Day 1 Month 3 interactions (N≈450) (Last visit) (Dosing) (Redosing) Primary endpoint: Placebo IV (n≈150) • Safety/tolerability 6
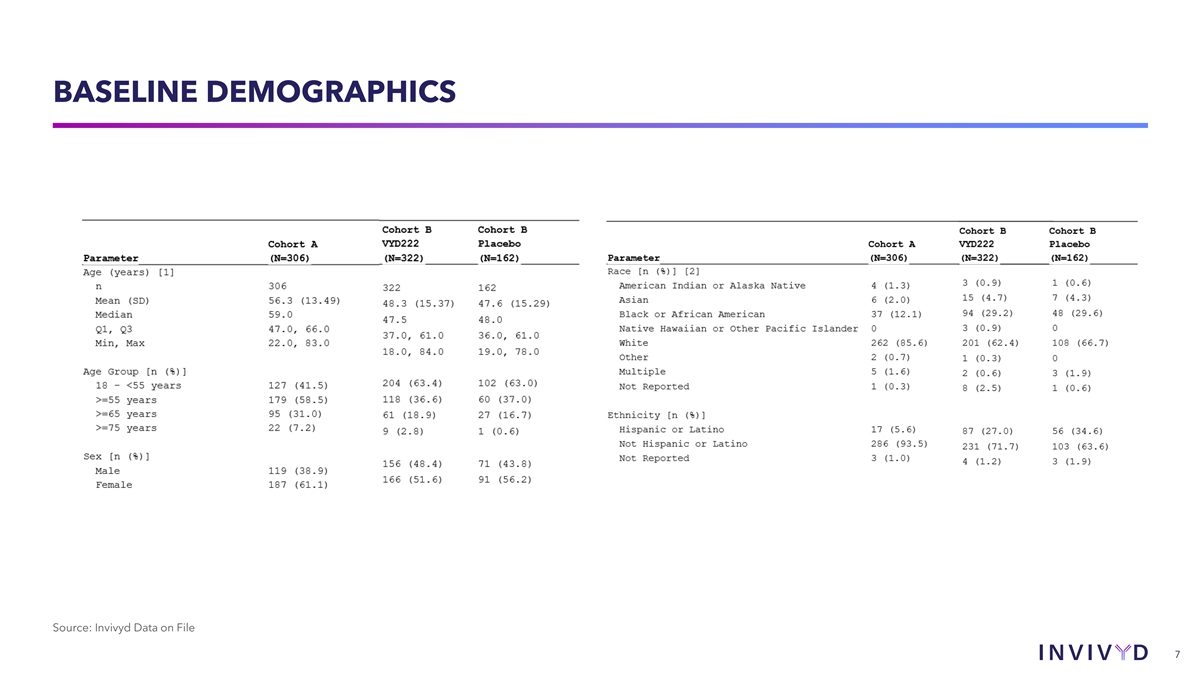
BASELINE DEMOGRAPHICS Source: Invivyd Data on File 7
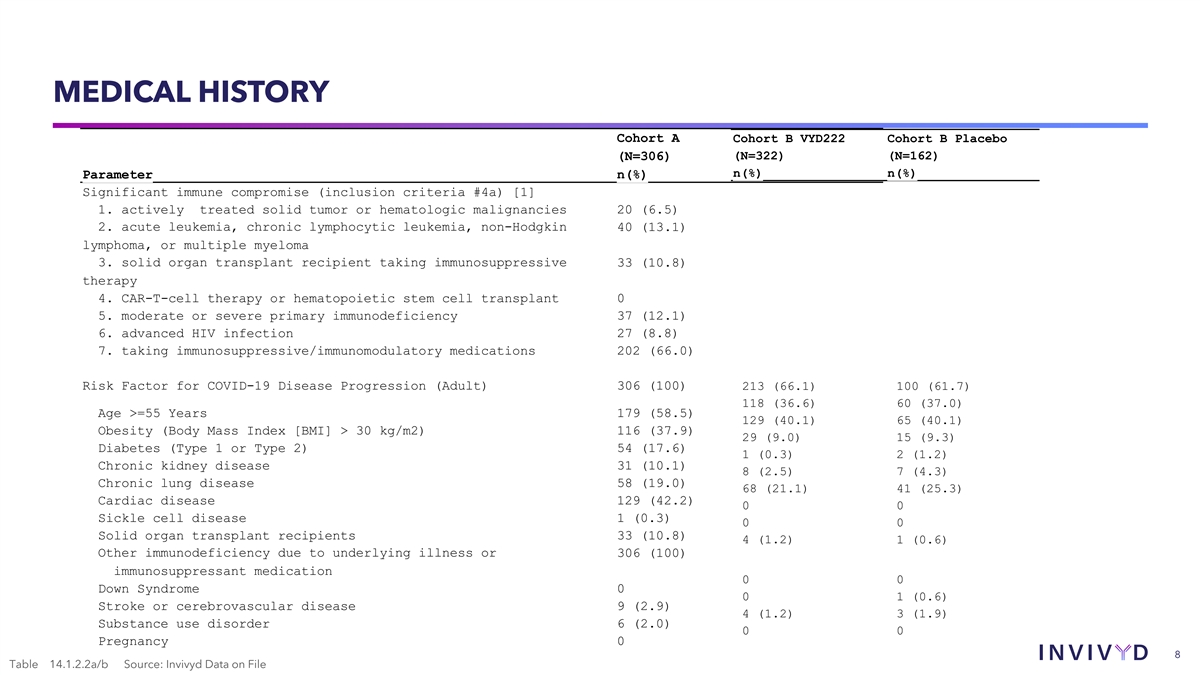
MEDICAL HISTORY Cohort A Cohort B VYD222 Cohort B Placebo (N=306)
(N=322) (N=162) n(%) n(%) Parameter n(%) Significant immune compromise (inclusion criteria #4a) [1] 1. actively treated solid tumor or hematologic malignancies 20 (6.5) 2. acute leukemia, chronic lymphocytic leukemia, non-Hodgkin 40 (13.1) lymphoma,
or multiple myeloma 3. solid organ transplant recipient taking immunosuppressive 33 (10.8) therapy 4. CAR-T-cell therapy or hematopoietic stem cell transplant 0 5. moderate or severe primary immunodeficiency 37 (12.1) 6. advanced HIV infection 27
(8.8) 7. taking immunosuppressive/immunomodulatory medications 202 (66.0) Risk Factor for COVID-19 Disease Progression (Adult) 306 (100) 213 (66.1) 100 (61.7) 118 (36.6) 60 (37.0) Age >=55 Years 179 (58.5) 129 (40.1) 65 (40.1) Obesity (Body Mass
Index [BMI] > 30 kg/m2) 116 (37.9) 29 (9.0) 15 (9.3) Diabetes (Type 1 or Type 2) 54 (17.6) 1 (0.3) 2 (1.2) Chronic kidney disease 31 (10.1) 8 (2.5) 7 (4.3) Chronic lung disease 58 (19.0) 68 (21.1) 41 (25.3) Cardiac disease 129 (42.2) 0 0 Sickle
cell disease 1 (0.3) 0 0 Solid organ transplant recipients 33 (10.8) 4 (1.2) 1 (0.6) Other immunodeficiency due to underlying illness or 306 (100) immunosuppressant medication 0 0 Down Syndrome 0 0 1 (0.6) Stroke or cerebrovascular disease 9 (2.9) 4
(1.2) 3 (1.9) Substance use disorder 6 (2.0) 0 0 Pregnancy 0 8 Table 14.1.2.2a/b Source: Invivyd Data on File
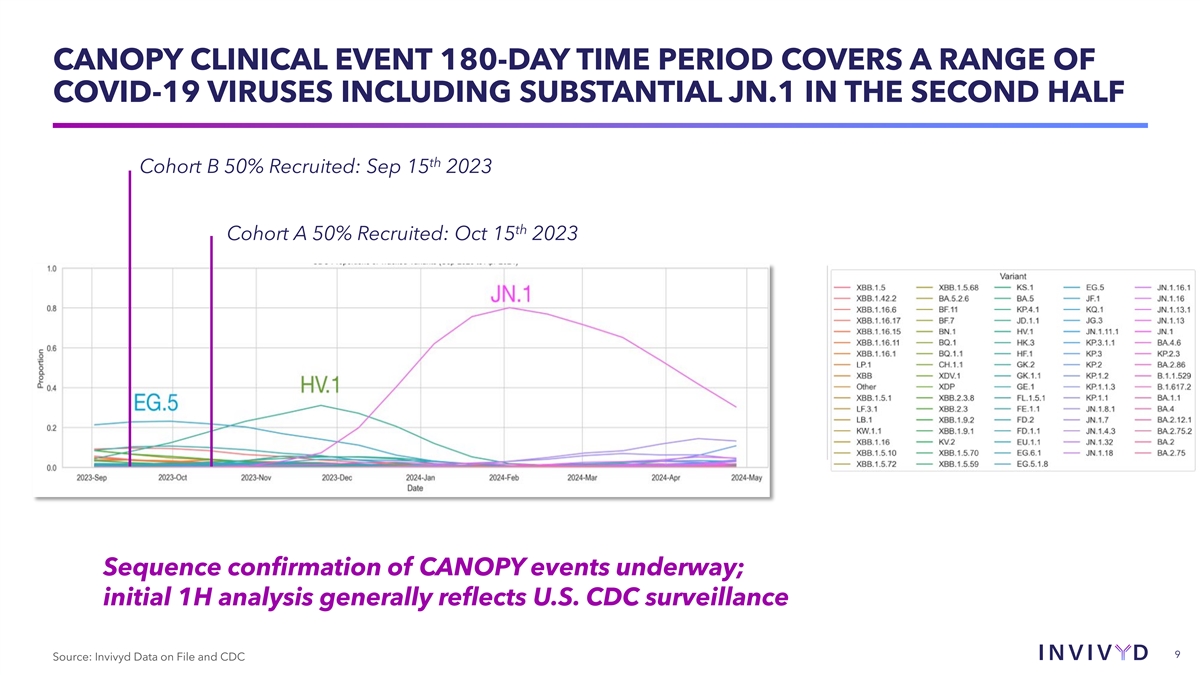
CANOPY CLINICAL EVENT 180-DAY TIME PERIOD COVERS A RANGE OF COVID-19
VIRUSES INCLUDING SUBSTANTIAL JN.1 IN THE SECOND HALF th Cohort B 50% Recruited: Sep 15 2023 th Cohort A 50% Recruited: Oct 15 2023 Sequence confirmation of CANOPY events underway; initial 1H analysis generally reflects U.S. CDC surveillance 9
Source: Invivyd Data on File and CDC
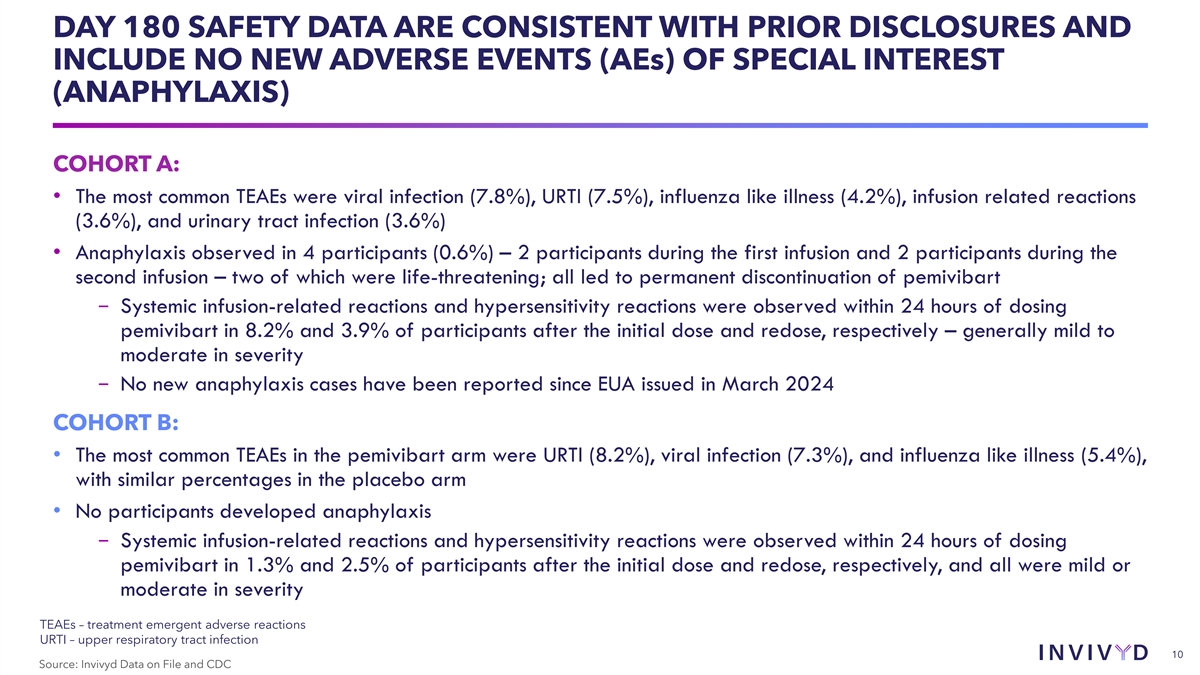
DAY 180 SAFETY DATA ARE CONSISTENT WITH PRIOR DISCLOSURES AND INCLUDE
NO NEW ADVERSE EVENTS (AEs) OF SPECIAL INTEREST (ANAPHYLAXIS) COHORT A: • The most common TEAEs were viral infection (7.8%), URTI (7.5%), influenza like illness (4.2%), infusion related reactions (3.6%), and urinary tract infection (3.6%)
• Anaphylaxis observed in 4 participants (0.6%) – 2 participants during the first infusion and 2 participants during the second infusion – two of which were life-threatening; all led to permanent discontinuation of pemivibart
− Systemic infusion-related reactions and hypersensitivity reactions were observed within 24 hours of dosing pemivibart in 8.2% and 3.9% of participants after the initial dose and redose, respectively – generally mild to moderate in
severity − No new anaphylaxis cases have been reported since EUA issued in March 2024 COHORT B: • The most common TEAEs in the pemivibart arm were URTI (8.2%), viral infection (7.3%), and influenza like illness (5.4%), with similar
percentages in the placebo arm • No participants developed anaphylaxis − Systemic infusion-related reactions and hypersensitivity reactions were observed within 24 hours of dosing pemivibart in 1.3% and 2.5% of participants after the
initial dose and redose, respectively, and all were mild or moderate in severity TEAEs – treatment emergent adverse reactions URTI – upper respiratory tract infection 10 Source: Invivyd Data on File and CDC
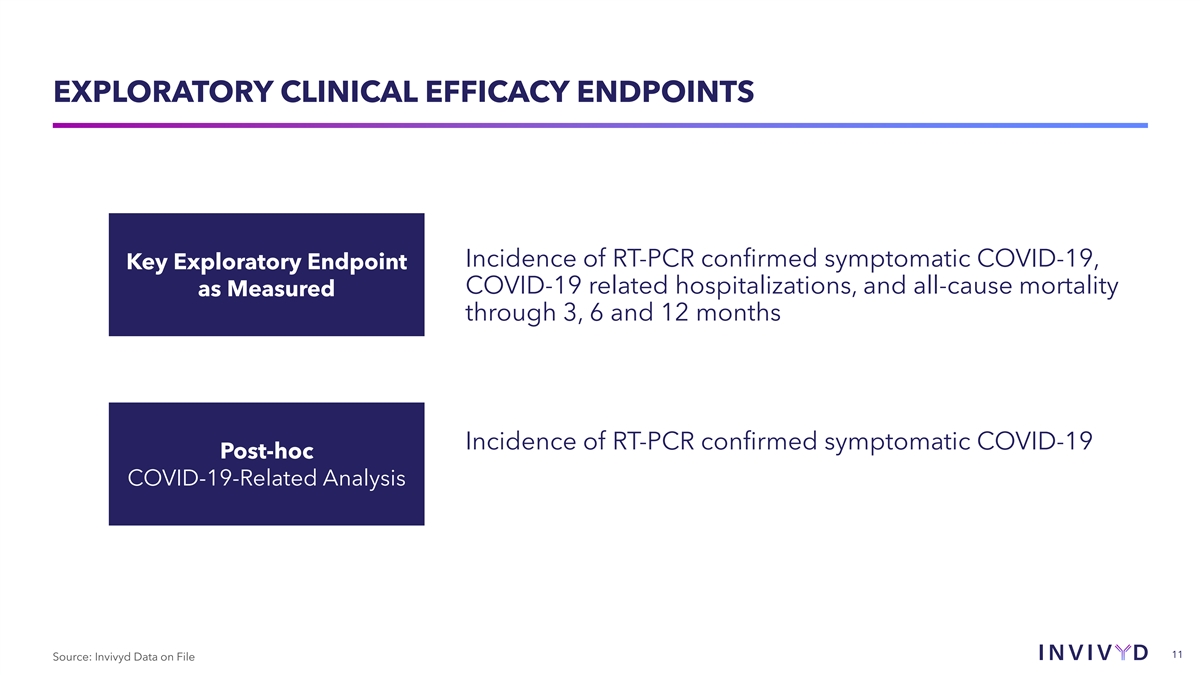
EXPLORATORY CLINICAL EFFICACY ENDPOINTS Incidence of RT-PCR confirmed
symptomatic COVID-19, Key Exploratory Endpoint COVID-19 related hospitalizations, and all-cause mortality as Measured through 3, 6 and 12 months Incidence of RT-PCR confirmed symptomatic COVID-19 Post-hoc COVID-19-Related Analysis 11 Source: Invivyd
Data on File
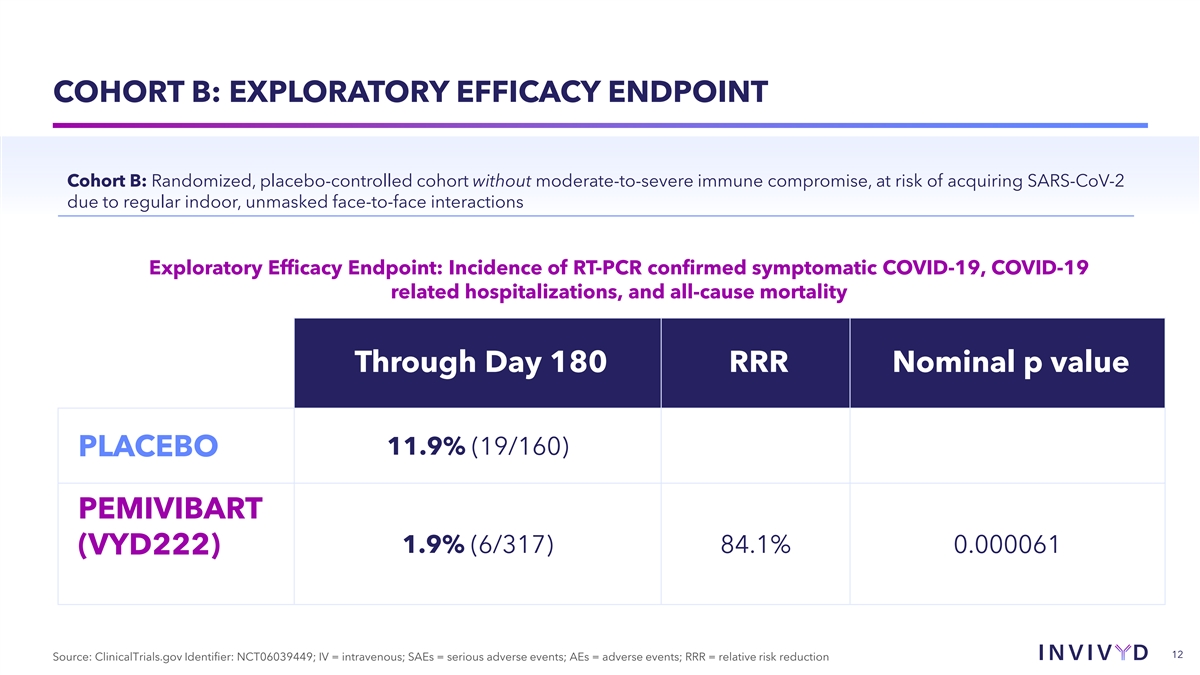
COHORT B: EXPLORATORY EFFICACY ENDPOINT Cohort B: Randomized,
placebo-controlled cohort without moderate-to-severe immune compromise, at risk of acquiring SARS-CoV-2 due to regular indoor, unmasked face-to-face interactions Exploratory Efficacy Endpoint: Incidence of RT-PCR confirmed symptomatic COVID-19,
COVID-19 related hospitalizations, and all-cause mortality Through Day 180 RRR Nominal p value 11.9% (19/160) PLACEBO PEMIVIBART 1.9% (6/317) 84.1% 0.000061 (VYD222) 12 Source: ClinicalTrials.gov Identifier: NCT06039449; IV = intravenous; SAEs =
serious adverse events; AEs = adverse events; RRR = relative risk reduction
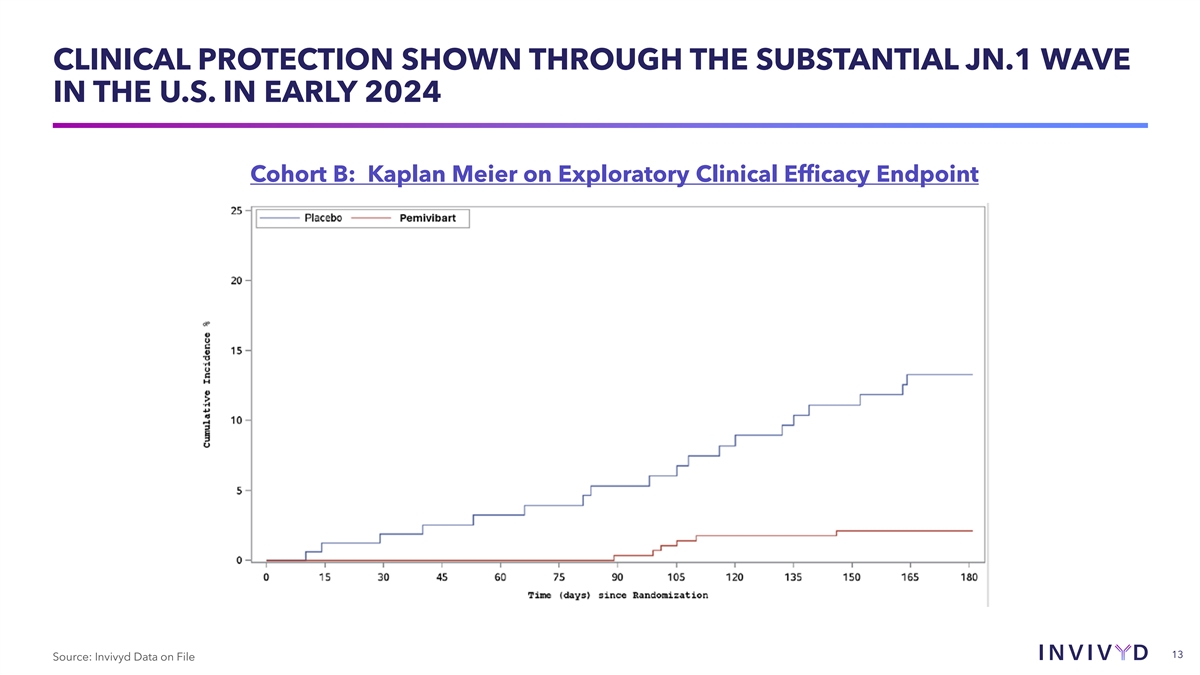
CLINICAL PROTECTION SHOWN THROUGH THE SUBSTANTIAL JN.1 WAVE IN THE U.S.
IN EARLY 2024 Cohort B: Kaplan Meier on Exploratory Clinical Efficacy Endpoint 13 Source: Invivyd Data on File
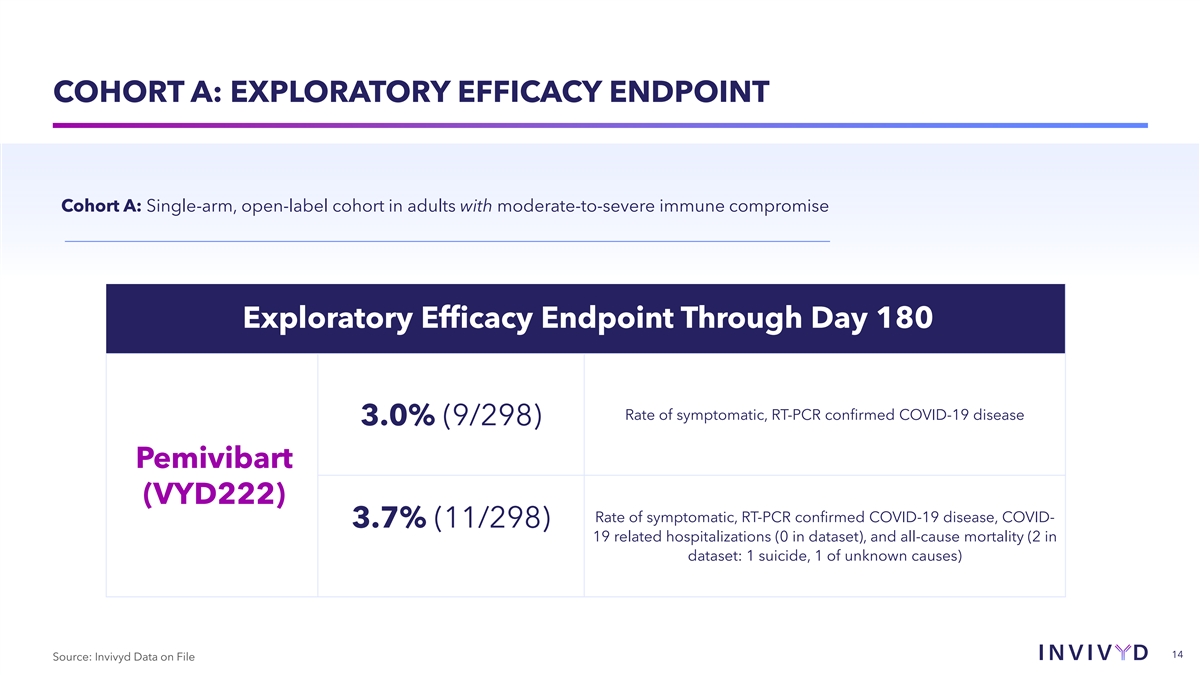
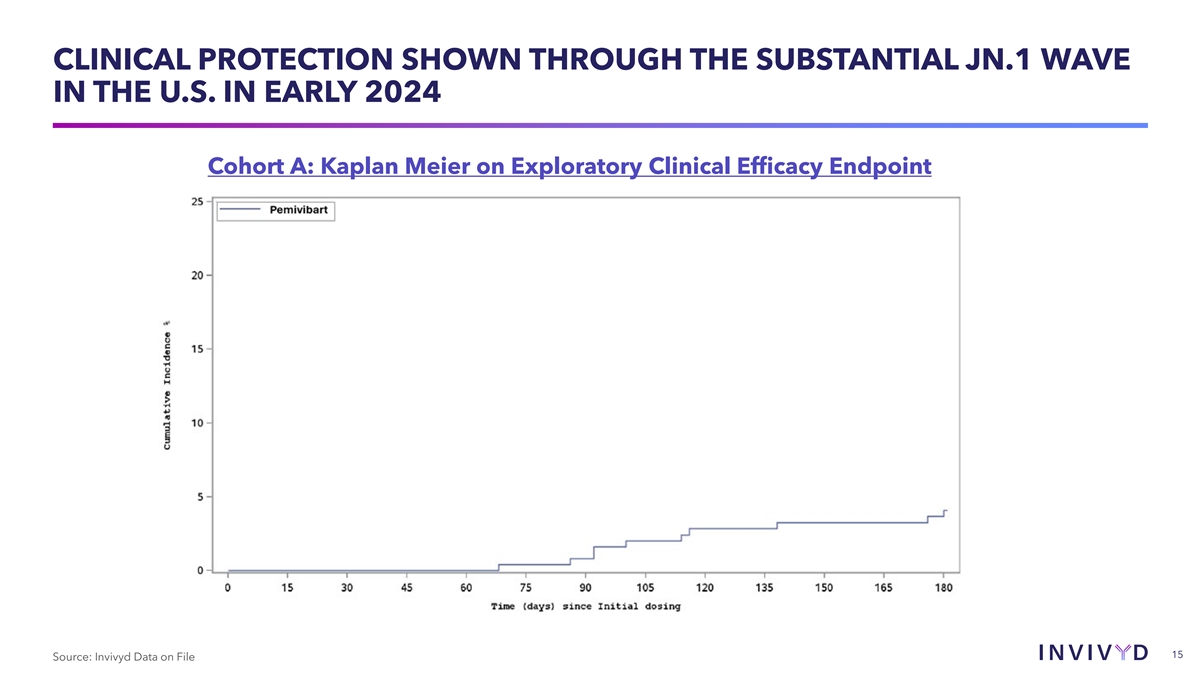
COHORT A: EXPLORATORY EFFICACY ENDPOINT Cohort A: Single-arm,
open-label cohort in adults with moderate-to-severe immune compromise Exploratory Efficacy Endpoint Through Day 180 Rate of symptomatic, RT-PCR confirmed COVID-19 disease 3.0% (9/298) Pemivibart (VYD222) Rate of symptomatic, RT-PCR confirmed
COVID-19 disease, COVID- 3.7% (11/298) 19 related hospitalizations (0 in dataset), and all-cause mortality (2 in dataset: 1 suicide, 1 of unknown causes) 14 Source: Invivyd Data on File
CLINICAL PROTECTION SHOWN THROUGH THE SUBSTANTIAL JN.1 WAVE IN THE U.S.
IN EARLY 2024 Cohort A: Kaplan Meier on Exploratory Clinical Efficacy Endpoint 15 Source: Invivyd Data on File
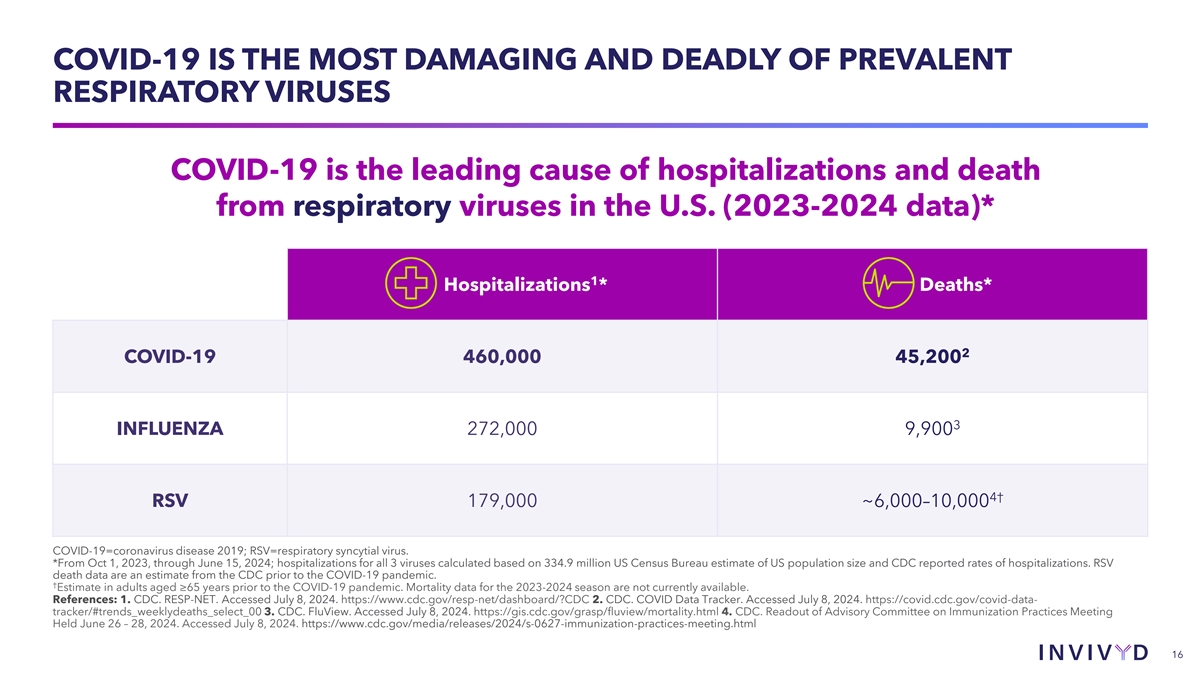
COVID-19 IS THE MOST DAMAGING AND DEADLY OF PREVALENT RESPIRATORY
VIRUSES COVID-19 is the leading cause of hospitalizations and death from respiratory viruses in the U.S. (2023-2024 data)* 1 Hospitalizations * Deaths* 2 COVID-19 460,000 45,200 3 INFLUENZA 272,000 9,900 4† RSV 179,000 ~6,000–10,000
COVID-19=coronavirus disease 2019; RSV=respiratory syncytial virus. *From Oct 1, 2023, through June 15, 2024; hospitalizations for all 3 viruses calculated based on 334.9 million US Census Bureau estimate of US population size and CDC reported rates
of hospitalizations. RSV death data are an estimate from the CDC prior to the COVID-19 pandemic. † Estimate in adults aged ≥65 years prior to the COVID-19 pandemic. Mortality data for the 2023-2024 season are not currently available.
References: 1. CDC. RESP-NET. Accessed July 8, 2024. https://www.cdc.gov/resp-net/dashboard/?CDC 2. CDC. COVID Data Tracker. Accessed July 8, 2024. https://covid.cdc.gov/covid-data- tracker/#trends_weeklydeaths_select_00 3. CDC. FluView. Accessed
July 8, 2024. https://gis.cdc.gov/grasp/fluview/mortality.html 4. CDC. Readout of Advisory Committee on Immunization Practices Meeting Held June 26 – 28, 2024. Accessed July 8, 2024.
https://www.cdc.gov/media/releases/2024/s-0627-immunization-practices-meeting.html 16
AGENDA u CANOPY Results u Q&A 17
v3.24.2.u1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Invivyd (NASDAQ:IVVD)
Historical Stock Chart
From Jul 2024 to Aug 2024
Invivyd (NASDAQ:IVVD)
Historical Stock Chart
From Aug 2023 to Aug 2024