AstraZeneca: Koselugo Conditionally Approved in EU for Children With NF1
June 22 2021 - 2:37AM
Dow Jones News
By Ian Walker
AstraZeneca PLC said Tuesday that the European Commission has
conditionally approved Koselugo in the European Union for children
with neurofibromatosis type 1 and plexiform neurofibromas.
The pharmaceutical giant said that Koselugo reduced tumor
volume, pain and improved life quality in a Sprint Phase 2
trial.
Neurofibromatosis type 1 is a rare and debilitating genetic
condition affecting one in 3,000 individuals world-wide.
Koselugo is already approved in the U.S. and several other
countries for the treatment of pediatric patients with NF1 and
symptomatic, inoperable plexiform neurofibromas.
Write to Ian Walker at ian.walker@wsj.com
(END) Dow Jones Newswires
June 22, 2021 02:29 ET (06:29 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
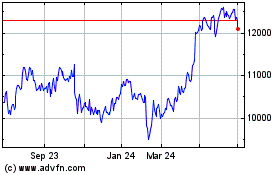
Astrazeneca (LSE:AZN)
Historical Stock Chart
From Mar 2024 to Apr 2024
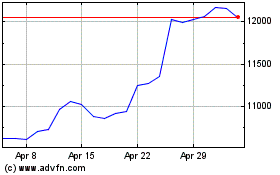
Astrazeneca (LSE:AZN)
Historical Stock Chart
From Apr 2023 to Apr 2024