AstraZeneca: AZD7442 Trial for Prevention of Symptomatic Covid-19 Didn't Meet Primary Goal
June 15 2021 - 2:51AM
Dow Jones News
By Adria Calatayud
AstraZeneca PLC said Tuesday that a Phase 3 trial to assess the
safety and efficacy of a long-acting antibody combination called
AZD7442 for the prevention of symptomatic Covid-19 after exposure
didn't meet the primary goal.
The Cambridge, U.K.-based pharmaceutical company said AZD7442
reduced the risk of developing symptomatic Covid-19 in unvaccinated
adults with confirmed exposure to a person with a case of the
SARS-CoV-2 virus within the past eight days by 33% compared with
placebo, which wasn't statistically significant.
However, in patients who were polymerase chain reaction, or PCR,
negative at the time of dosing, AZD7442 reduced the risk of
developing symptomatic Covid-19 by 92% compared with placebo more
than seven days following dosing, which suggests that the drug may
be useful in preventing symptoms in individuals not already
infected, AstraZeneca said.
"While this trial did not meet the primary endpoint against
symptomatic illness, we are encouraged by the protection seen in
the PCR negative participants following treatment with AZD7442,"
AstraZeneca's BioPharmaceuticals R&D Executive Vice President
Mene Pangalos said.
The company in March said it had extended an agreement with the
U.S. government to supply up to 500,000 additional doses of AZD7442
for $205 million, contingent on AZD7442 receiving emergency-use
authorization from the U.S. Food and Drug Administration.
Discussions with the U.S. government regarding next steps are
continuing, AstraZeneca said.
Write to Adria Calatayud at adria.calatayud@dowjones.com
(END) Dow Jones Newswires
June 15, 2021 02:39 ET (06:39 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
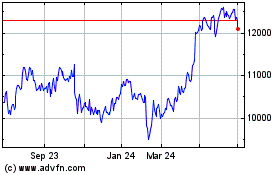
Astrazeneca (LSE:AZN)
Historical Stock Chart
From Mar 2024 to Apr 2024
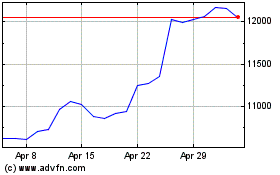
Astrazeneca (LSE:AZN)
Historical Stock Chart
From Apr 2023 to Apr 2024