Filed
pursuant to Rule 424(b)(3)
Registration
No. 333-255752
PROSPECTUS

22,222,223
Shares of Common Stock and
22,222,223
Shares of Common Stock Underlying Warrants
This
prospectus relates to the resale, from time to time, by the selling stockholders named herein, or their pledgees, donees, transferees,
or other successors-in-interest, of up to an aggregate of 22,222,223 shares of common stock of ScoutCam Inc., or ScoutCam, and
22,222,223 warrants to purchases shares of common stock of ScoutCam. The selling stockholders under this prospectus, to whom we
refer to as the Selling Stockholders have acquired, pursuant to investments in ScoutCam, an aggregate of 22,222,223 outstanding
shares of common stock that may be resold under this prospectus. The Selling Stockholders may furthermore sell under this prospectus
up to an additional 22,222,223 shares of common stock, in the aggregate, that they may potentially acquire upon exercise of warrants
that we have issued to them pursuant to their investments in ScoutCam.
Our
common stock is quoted on the OTCQB Market, or the OTCQB, under the symbol “SCTC”. On April 30, 2021, the
last reported sale price of our common stock on the OTCQB was $1.35 per share. For additional information on the possible
methods of sale that may be used by the Selling Stockholders, you should refer to the section entitled “Plan of Distribution”
beginning on page 49 of this prospectus. We will not receive any proceeds from the sale of the shares of common stock offered
hereby. All net proceeds from the sale of these shares will go to the Selling Stockholders. However, we will receive cash proceeds
equal to the total exercise price of warrants that are exercised for cash, or up to $25,555,556.45, if all warrants issued to
the Selling Stockholders are exercised. We do not know when or in what amounts the Selling Stockholders may offer the shares of
common stock for sale. The Selling Stockholders may sell any, all or none of the shares of common stock offered by this prospectus.
Investing
in our securities involves a high degree of risk. See “Risk Factors” beginning on page 4 of this prospectus for a
discussion of information that should be considered in connection with an investment in our securities.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or
determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is May 10, 2021
TABLE
OF CONTENTS
You
should rely only on the information contained in this prospectus. Neither we nor the Selling Stockholders have authorized anyone
else to provide you with different information. The shares of common stock offered by this prospectus are being offered only in
jurisdictions where the offer is permitted. You should not assume that the information in this prospectus is accurate as of any
date other than the date on the front of each document. Our business, financial condition, results of operations and prospects
may have changed since that date.
Certain
figures included in this prospectus have been subject to rounding adjustments. Accordingly, figures shown as totals in certain
tables may not be an arithmetic aggregation of the figures that precede them.
Throughout
this prospectus, unless otherwise designated, the terms “we,” “us,” “our,” “ScoutCam,”
“the Company,” and “our Company” refer to ScoutCam Inc. and our consolidated subsidiary, ScoutCam Ltd.,
a private company organized under the laws of the State of Israel. The term “Common Stock” refers to shares of our
common stock, par value $0.001 per share. The terms “dollar,” “US$,” or “$” refer to US dollars,
the lawful currency of the United States.
ABOUT
THIS PROSPECTUS
This
prospectus is part of a registration statement that we have filed with the Securities and Exchange Commission, or the SEC. The
Selling Stockholders named in this prospectus may, from time to time, sell the securities described in this prospectus in one
or more offerings. This prospectus includes important information about us, the shares of Common Stock being offered by the Selling
Stockholders and other information you should know before investing. This prospectus does not contain all of the information provided
in the registration statement that we filed with the SEC. You should read this prospectus together with the additional information
about us described in the section below entitled “Where You Can Find More Information.” You should rely only on information
contained in this prospectus. We have not, and the Selling Stockholders have not, authorized anyone to provide you with information
different from that contained in this prospectus. The information contained in this prospectus is accurate only as of the date
on the front cover of the prospectus. You should not assume that the information contained in this prospectus is accurate as of
any other date.
The
Selling Stockholders may offer and sell the shares of Common Stock covered by this prospectus directly to purchasers, through
agents selected by the Selling Stockholders, or to or through underwriters or dealers. See “Plan of Distribution.”
SPECIAL
NOTE ON FORWARD-LOOKING STATEMENTS
Some
of the statements made in this prospectus may constitute forward-looking statements within the meaning of the United States federal
securities laws. The use of the words “projects,” “expects,” “may,” “plans,” or
“intends,” or words of similar import, identifies a statement as “forward-looking.” The forward-looking
statements contained herein represent our expectations, beliefs, intentions or strategies concerning future events that may affect
our business, financial condition, results of operations and prospects. Many factors could cause our actual performance or results
to differ materially from the performance and results to differ materially from those expressed in or suggested by forward-looking
statements. These factors include, but are not limited to:
|
|
●
|
our
financial performance, including our history of operating losses;
|
|
|
|
|
|
|
●
|
our
ability to obtain additional funding to continue our operations;
|
|
|
|
|
|
|
●
|
our
ability to successfully develop and commercialize our products;
|
|
|
|
|
|
|
●
|
changes
in the regulatory environments of the United States and other countries in which we intend to operate;
|
|
|
|
|
|
|
●
|
our
ability to attract and retain key management and marketing personnel;
|
|
|
|
|
|
|
●
|
competition
from new market entrants; and
|
|
|
|
|
|
|
●
|
our
ability to identify and pursue development of additional products.
|
The
outcome of the events described in forward-looking statements are subject to risks, uncertainties, assumptions and other factors,
including those described in this prospectus under “Risk Factors.” Moreover, we operate in a very competitive and
rapidly changing environment. New risks and uncertainties emerge from time to time, and it is not possible for us to predict all
risks and uncertainties that could have an impact on the forward-looking statements herein.
You
should not rely on forward-looking statements as predictions of future events. Except as required by law, neither we nor any other
person assumes responsibility for the accuracy and completeness of forward-looking statements, and we undertake no obligation
to update any forward-looking statements to reflect events or circumstances after the date of this prospectus.
PROSPECTUS
SUMMARY
You
should read the following summary together with the more detailed information about us, the shares of Common Stock that may be
sold from time to time, and our consolidated financial statements and the notes to them, all of which appear elsewhere in this
prospectus.
Overview
We
are engaged in the development, production and marketing of innovative miniaturized imaging equipment known as our micro ScoutCam™
portfolio for use in medical procedures as well as various industrial applications. As of the date of this prospectus, we derive
a substantial portion of our revenue from applications of our micro ScoutCam™ portfolio within the medical and industrial
fields. We have recently begun examining additional applications for our micro ScoutCam™ portfolio outside of the medical
device industry, including in, among others, the defense, aerospace, automotive, and industrial non-destructing-testing industries.
We plan to further expand the activity in these non-medical spaces.
Our
Corporate History and Background
We
were incorporated under the laws of the State of Nevada on March 22, 2013 under the name Intellisense Solutions Inc., or Intellisense.
We were initially engaged in the business of developing web portals to allow companies and individuals to engage in the purchase
and sale of vegetarian food products over the Internet. However, we were unable to execute our original business plan, develop
significant operations or achieve commercial sales.
On
December 30, 2019, we acquired all of the issued and outstanding share capital of ScoutCam Ltd. Following this transaction, we integrated
and fully adopted ScoutCam Ltd.’s business into our Company as our primary business activity. On December 31, 2019, we changed
our name to ScoutCam Inc.
ScoutCam
Ltd. was formed in the State of Israel on January 3, 2019 as a wholly-owned subsidiary of Medigus Ltd., or Medigus, an Israeli
company traded on the Nasdaq Capital Market and the Tel Aviv Stock Exchange, and commenced operations on March 1, 2019. ScoutCam
Ltd. was incorporated as part of a reorganization of Medigus, which was designed to distinguish ScoutCam Ltd.’s miniaturized
imaging business, or the micro ScoutCam™ portfolio, from Medigus’s other operations and to enable Medigus to form
a separate business unit with dedicated resources focused on the promotion of such technology. In December 2019, Medigus and ScoutCam
Ltd. consummated a certain Amended and Restated Asset Transfer Agreement, which transferred and assigned certain assets and intellectual
property rights related to its miniaturized imaging business.
Risks
Related to Our Business, Operations and Financial Condition
Our
business is subject to a number of risks as discussed more fully in “Risk Factors” beginning on page 4 of this prospectus.
These risks include, but are not limited to, the following:
|
|
●
|
our
reliance on third-party suppliers for most of the components of our products could harm our ability to meet demand for our
products in a timely and cost-effective manner;
|
|
|
|
|
|
|
●
|
because
of our limited operating history, we may not be able to successfully operate our business or execute our business plan;
|
|
|
|
|
|
|
●
|
our
commercial success depends upon the degree of market acceptance by the medical community as well as by other prospect markets
and industries;
|
|
|
|
|
|
|
●
|
we
expect to face significant competition, and if we cannot successfully compete with new or existing technologies or future
developed products, our marketing and sales will suffer and we may never be profitable; and
|
|
|
|
|
|
|
●
|
if
we are unable to establish sales, marketing and distribution capabilities or enter into successful relationships with business
targets and third parties to perform these services, we may not be successful in commercializing our products and technology.
|
Our
Corporate Information
We
were incorporated under the laws of the State of Nevada on March 22, 2013 under the name Intellisense. We changed our name to
ScoutCam Inc. on December 31, 2019. We have one wholly-owned subsidiary, ScoutCam Ltd., a private company organized under the
laws of the State of Israel, which we acquired on December 30, 2019.
Our
principal executive offices are located at Suite 7A, Industrial Park, P.O. Box 3030, Omer, Israel 8496500. Our telephone number
is +972 73 370-4691. Our website address is https://www.scoutcam.com. This website address is included in this prospectus
as an inactive textual reference only. The information and other content appearing on our website are not part of this prospectus.
THE
OFFERING
|
Common
Stock offered by the Selling Stockholders
|
|
22,222,223
shares of Common Stock
Up
to 22,222,223 additional shares of Common Stock potentially issuable to the Selling Stockholders upon exercise of warrants.
|
|
|
|
|
|
OTCQB
symbol
|
|
“SCTC”
|
|
|
|
|
|
Use
of proceeds
|
|
We
will not receive any proceeds from the sale of the shares of Common Stock offered hereby. All net proceeds from the sale of
these shares will go to the Selling Stockholders. However, we will receive cash proceeds equal to the total exercise price
of warrants that are exercised for cash, or up to $25,555,556.45, if all warrants issued to the Selling Stockholders are exercised.
See “Use of Proceeds.”
|
|
|
|
|
|
Common
Stock outstanding as of April 12, 2021 (does not include shares of Common Stock underlying warrants
held by the Selling Stockholders)
|
|
60,295,245
shares of Common Stock
|
|
|
|
|
|
Risk
factors
|
|
Prospective
investors should carefully consider the “Risk Factors” beginning on page 4 of this prospectus for a discussion
of certain factors that should be considered before deciding whether to invest in the shares of Common Stock offered hereby.
|
RISK
FACTORS
You
should carefully consider the risks described below, as well as the financial or other information in this prospectus, including
our consolidated financial statements and the related notes, before deciding whether to invest in our securities. The risks and
uncertainties described below are not the only risks we face. We may face additional risks and uncertainties not currently known
to us or that we currently deem to be immaterial. Any of the risks described below, and any such additional risks, could materially
adversely affect our business, financial condition or results of operations. In such case, you may lose all or part of your original
investment.
Risks
Related to Our Business, Operations and Financial Condition
The
COVID-19 pandemic has adversely affected, and will continue to adversely affect, our business, financial condition, liquidity
and results of operations.
The
COVID-19 pandemic has resulted in a widespread health crisis that has adversely affected businesses, economies and financial markets
worldwide, placed constraints on the operations of businesses, decreased consumer mobility and activity, and caused significant
economic volatility in the United States, Israel and international capital markets. Our business has been affected in various
ways, as discussed below, including in our operations, and we cannot predict the length and severity of the pandemic or its effects
on us and our customers. We have followed guidance by the U.S. and Israeli governments and the other local governments in which
we operate to protect our employees and our operations during the pandemic and have implemented a remote environment for certain
of our employees, and, as a result, may experience inefficiencies in our employees’ ability to collaborate. We have also
experienced difficulty in our efforts to recruit and hire qualified personnel during this time. In addition, the COVID-19 pandemic
has caused an economic recession, high unemployment rates and other disruptions, both in the United States, Israel and the rest
of the world. Any of these impacts, including the prolonged continuation of these impacts, could adversely affect our business.
We
cannot predict the other potential impacts of the COVID-19 pandemic on our business or operations, and there is no guarantee that
any near-term trends in our results of operations will continue, particularly if the COVID-19 pandemic and the adverse consequences
thereof continue for a long period of time. Additional waves of infections, a continuation of the current environment, or any
further adverse impacts caused by the COVID-19 pandemic could further deteriorate employment rates and the economy, detrimentally
affecting our consumer base and divert consumers’ discretionary income to other uses, including for essential items. These
events could adversely impact our cash flows, results of operations and financial conditions and heighten many of the other risks
described in these “Risk Factors.”
Our
reliance on third-party suppliers for most of the components of our products could harm our ability to meet demand for our products
in a timely and cost-effective manner.
Though
we attempt to ensure the availability of more than one supplier for each important component in any product that we commission,
the number of suppliers engaged in the provision of miniature video sensors which are suitable for our Complementary Metal Oxide
Semiconductor (“CMOS”) technology products is very limited, and therefore in some cases we engage with a single supplier,
which may result in our dependency on such supplier. This is the case regarding sensors for the CMOS type technology that is produced
by a single supplier in the United States. As we do not have a contract in place with this supplier, there is no contractual commitment
on the part of such supplier for any set quantity of such sensors. The loss of our sole supplier in providing us with miniature
sensors for our CMOS technology products, and our inability or delay in finding a suitable replacement supplier, could significantly
affect our business, financial condition, results of operations and reputation.
Because
of ScoutCam’s limited operating history, we may not be able to successfully operate our business or execute our business
plan.
Given
the limited operating history of ScoutCam, it is hard to evaluate our proposed business and prospects. Our proposed business operations
will be subject to numerous risks, uncertainties, expenses and difficulties associated with early-stage enterprises. Such risks
include, but are not limited to, the following:
|
●
|
the
absence of a lengthy operating history;
|
|
|
|
|
●
|
insufficient
capital to fully realize our operating plan;
|
|
|
|
|
●
|
expected
continual losses for the foreseeable future;
|
|
|
|
|
●
|
operating
in multiple currencies;
|
|
|
|
|
●
|
our
ability to anticipate and adapt to a developing market(s);
|
|
|
|
|
●
|
acceptance
of our products by the medical community and consumers;
|
|
|
|
|
●
|
acceptance
of our products by the non-medical community and consumers;
|
|
|
|
|
●
|
limited
marketing experience;
|
|
|
|
|
●
|
a
competitive environment characterized by well-established and well-capitalized competitors;
|
|
|
|
|
●
|
the
ability to identify, attract and retain qualified personnel; and
|
|
|
|
|
●
|
operating
in an environment that is highly regulated by a number of agencies.
|
Furthermore,
we have a history of losses, and we may not be able to generate sufficient revenues to achieve and sustain profitability, and
as a result, in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020, we noted that there is substantial
doubt about our ability to continue as a going concern following the fiscal year ended December 31, 2020.
Because
we are subject to these risks, evaluating our business may be difficult, our business strategy may be unsuccessful and we may
be unable to address such risks in a cost-effective manner, if at all. If we are unable to successfully address these risks our
business could be harmed.
Our
commercial success depends upon the degree of market acceptance by the medical community as well as by other prospect markets
and industries.
Our
current business model is that of a business-to-business approach, or B2B, in which we seek to identify target businesses interested
in integrating our technology, or commissioning individual projects using our technology. Any product that we commission or that
is brought to the market may or may not gain market acceptance by prospect customers. The commercial success of our technologies,
commissioned products and any future product that we may develop depends in part on the medical community as well as other industries
for various use cases, depending on the acceptance by such industries of our commissioned products as a useful and cost-effective
solution compared to current technologies. To date, we have not yet commenced proactive market penetration in other industries,
with the exception of the biomedical sector. If our technology or any future product that we may develop does not achieve an adequate
level of acceptance, or does not garner significant commercial appeal, we may not generate significant revenue and may not become
profitable. The degree of market acceptance will depend on a number of factors, including:
|
●
|
the
cost, safety, efficacy/performance, and convenience of our technology and any commissioned product and any future product
that we may develop in relation to alternative products;
|
|
|
|
|
●
|
the
ability of third parties to enter into relationships with us without violating their existing agreements;
|
|
|
|
|
●
|
the
effectiveness of our sales and marketing efforts;
|
|
|
|
|
●
|
the
strength of marketing and distribution support for, and timing of market introduction of, competing technology and products;
and
|
|
|
|
|
●
|
publicity
concerning our technology or commissioned products or competing technology and products.
|
Our
efforts to penetrate industries and educate the marketplace on the benefits of our technology, and reasons to seek the commissioning
of products based on our technology, may require significant resources and may never be successful. Such efforts to educate the
marketplace may require more resources than are required by conventional technologies.
We
expect to face significant competition. If we cannot successfully compete with new or existing technologies or future developed
products, our marketing and sales will suffer and we may never be profitable.
We
expect to compete against existing technologies and proven products in different industries. In addition, some of these competitors,
either alone or together with their collaborative partners, operate larger research and development programs than we do, and may
have substantially greater financial resources than we do, as well as significantly greater experience in obtaining applicable
regulatory approvals applicable to the commercialization of our technologies and future products.
If
we are unable to establish sales, marketing and distribution capabilities or enter into successful relationships with business
targets and third parties to perform these services, we may not be successful in commercializing our products and technology.
Given
that we are currently a B2B company, our business is reliant on our ability to successfully attract potential business targets.
Furthermore, we have a limited sales and marketing infrastructure and have limited experience in the sale, marketing or distribution
of our technologies beyond the B2B model. To achieve commercial success for our technologies or any future developed product,
we will need to establish a sales and marketing infrastructure or to out-license such future products.
In
the future, we may consider building a focused sales and marketing infrastructure to market any future developed products and
potentially other product in the United States or elsewhere in the world. Similarly, we may consider evolving our business model
in the future and adopting a business-to-consumer approach, or B2C. There are risks involved with establishing our own sales,
marketing and distribution capabilities. For example, recruiting and training a sales force could be expensive and time consuming
and could delay any product launch. This may be costly, and our investment would be lost if we cannot retain or reposition our
sales and marketing personnel.
Factors
that may inhibit our efforts to commercialize any future products on our own include:
|
●
|
our
inability to recruit, train and retain adequate numbers of effective sales and marketing personnel;
|
|
|
|
|
●
|
the
inability of sales personnel to obtain access to potential customers;
|
|
|
|
|
●
|
the
lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to
companies with more extensive product lines; and
|
|
|
|
|
●
|
unforeseen
costs and expenses associated with creating an independent sales and marketing organization.
|
If
we are unable to establish our own sales, marketing and distribution capabilities or enter into successful arrangements with third
parties to perform these services, our revenues and our profitability may be materially adversely affected.
In
addition, we may not be successful in entering into arrangements with third parties to sell, market and distribute our products
inside or outside of the United States or may be unable to do so on terms that are favorable to us. We likely will have little
control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our
products effectively. If we do not establish sales, marketing and distribution capabilities successfully, either on our own or
in collaboration with third parties, we will not be successful in commercializing our technologies or any future products we may
develop.
We
depend on the success of micro ScoutCam™ for our revenue, which could impair our ability to achieve profitability.
We
plan to derive most of our future revenue from the development services of our imaging equipment and our flagship micro ScoutCam™
and through the engagement with target businesses that are interested in the commissioning of certain products using our
technology. Our future growth and success is largely dependent on the successful commercialization of the micro ScoutCam™
technology. If we are unable to achieve increased commercial acceptance of the micro ScoutCam™ technology,
or experience a decrease in the utilization of our product line or procedure volume, our revenue would be adversely affected.
We
may be subject to product liability claims, product actions, including product recalls, and other field or regulatory actions
that could be expensive, divert management’s attention and harm our business.
Our
business exposes us to potential liability risks, product actions and other field or regulatory actions that are inherent in the
manufacturing, marketing and sale of medical device products that we may have commissioned for a target business. We may be held
liable if such products cause injury or death or is found otherwise unsuitable or defective during usage. Our products incorporate
mechanical and electrical parts, complex computer software and other sophisticated components, any of which can contain errors
or failures. Complex computer software is particularly vulnerable to errors and failures, especially when first introduced. In
addition, new products or enhancements to our existing products may contain undetected errors or performance problems that, despite
testing, are discovered only after installation.
If
any of our commissioned products are defective, whether due to design or manufacturing defects, improper use of the product, or
other reasons, we may voluntarily or involuntarily undertake an action to remove, repair, or replace the product at our expense.
In some circumstances we will be required to notify regulatory authorities of an action pursuant to a product failure.
We
may require substantial additional funding, which may not be available to us on acceptable terms, or at all.
Our
cash balance as of December 31, 2020 was $3.4 million. We may require additional funding to fund and grow our operations and to
develop certain products. There can be no assurance that financing will be available in amounts or on terms acceptable to us,
if at all. In the event we required additional capital, the inability to obtain additional capital will restrict our ability to
grow and may reduce our ability to continue to conduct business operations. If we require and are unable to obtain additional
financing, we will likely be required to curtail our development plans. In that event, current stockholders would likely experience
a loss of most or all of their investment. Additional funding that we do obtain may be dilutive to the interests of existing stockholders.
Our
failure to effectively manage growth could impair our business.
Our
business strategy contemplates a period of rapid growth which may put a strain on our administrative and operational resources,
and our funding requirements. Our ability to effectively manage growth will require us to successfully expand the capabilities
of our operational and management systems, and to attract, train, manage, and retain qualified personnel. There can be no assurance
that we will be able to do so, particularly if losses continue and we are unable to obtain sufficient financing. If we are unable
to appropriately manage growth, our business, prospects, financial condition, and results of operations could be adversely affected.
We
may not be able to manage our strategic partners effectively.
Our
growth strategy may include strategic partners. The process to bring on, train and assist strategic partners is time-consuming
and costly. We expect to expend significant resources to undertake business, financial and legal due diligence on both existing
and potential partners, and there is no guarantee that these will be successful in ultimately increasing our business.
Failure
to manage our partners effectively may affect our success in executing our business plan and may adversely affect our business,
financial condition and results of operation. We may not realize the anticipated benefits of any or all partnerships, or may not
realize them in the time frame expected.
We
may not have sufficient manufacturing capabilities to satisfy any growing demand for our commissioned products. We may be unable
to control the availability or cost of producing such products.
Our
current manufacturing capabilities may not reach the required production levels necessary in order to meet growing demands for
any products we may commission or future products we may develop. While we do intend to purchase a manufacturing facility in Israel
in the future, such an engagement has not yet materialized and it is not clear at what point the Company will execute such an
acquisition. In the interim, and prior to the purchase of a manufacturing facility by the Company, there can be no assurance that
our commissioned products can be manufactured at our desired commercial quantities, in compliance with our requirements and at
an acceptable cost. Any such failure could delay or prevent us from shipping said products and marketing our technologies in accordance
with our target growth strategies.
Testing
of our technologies potential applications for our products will be required and there is no assurance of regulatory approval.
The
effect of government regulation and the need for approval may delay marketing of our technologies and future potentially developed
products for a considerable period of time, impose costly procedures upon our activities and provide an advantage to larger companies
that compete with us. There can be no assurance that regulatory approval for any products developed by us will be granted on a
timely basis or at all. Any such delay in obtaining, or failure to obtain, such approvals would materially and adversely affect
the marketing of any contemplated products and the ability to earn product revenue. Further, regulation of manufacturing facilities
by state, local, and other authorities is subject to change. Any additional regulation could result in limitations or restrictions
on our ability to utilize any of our technologies, thereby adversely affecting our operations. Various federal and foreign statutes
and regulations also govern or influence the manufacturing, safety, labeling, storage, record keeping and marketing of food products.
The process of obtaining these approvals and the subsequent compliance with appropriate U.S. and foreign statutes and regulations
are time-consuming and require the expenditure of substantial resources. In addition, these requirements and processes vary widely
from country to country.
Our
suppliers may not be able to always supply components or products to us on a timely basis and on favorable terms, and as a result,
our dependency on third party suppliers can adversely affect our revenue.
We
will rely on our third-party suppliers for components and depend on obtaining adequate supplies of quality components on a timely
basis with favorable terms to manufacture our commissioned products. Some of those components that we sell are provided to us
by a limited number of suppliers. We will be subject to disruptions in our operations if our sole or limited supply contract manufacturers
decrease or stop production of components or do not produce components and products of sufficient quantity. Alternative sources
for our components will not always be available. Many of our components are manufactured overseas, so they have long lead times,
and events such as local disruptions, natural disasters or political conflict may cause unexpected interruptions to the supply
of our products or components.
It
is our intention, as mentioned in the use of proceeds, to allocate financial resources to improve our inventory management, including
establishing an inventory buffer of components appropriate to our business. However, we cannot assure that our attempt will be
successful or that product or component shortages will not occur in the future. If we cannot supply commissioned products or future
potentially developed products due to a lack of components, or are unable to utilize other components in a timely manner, our
business will be significantly harmed. If inventory shortages continue, they could be expected to have a material and adverse
effect on our future revenues and ability to effectively project future sales and operating results.
We
rely on highly skilled personnel, and, if we are unable to attract, retain or motivate qualified personnel, we may not be able
to operate our business effectively.
Our
success depends in large part on continued employment of senior management and key personnel who can effectively operate our business,
as well as our ability to attract and retain skilled employees. Competition for highly skilled management, technical, research
and development and other employees is intense and we may not be able to attract or retain highly qualified personnel in the future.
In making employment decisions, particularly in the job candidates often consider the value of the equity awards they would receive
in connection with their employment. Our long-term incentive programs may not be attractive enough or perform sufficiently to
attract or retain qualified personnel.
If
any of our employees leaves us, and we fail to effectively manage a transition to new personnel, or if we fail to attract and
retain qualified and experienced professionals on acceptable terms, our business, financial condition and results of operations
could be adversely affected.
Our
success also depends on our having highly trained financial, technical, recruiting, sales and marketing personnel. We will need
to continue to hire additional personnel as our business grows. A shortage in the number of people with these skills or our failure
to attract them to our company could impede our ability to increase revenues from our existing technology and services, ensure
full compliance with international and federal regulations, or launch new product offerings and would have an adverse effect on
our business and financial results.
We
may have difficulty in entering into and maintaining strategic alliances with third parties.
We
have entered into, and we may continue to enter into, strategic alliances with third parties to gain access to new and innovative
technologies and markets. These parties are often large, established companies. Negotiating and performing under these arrangements
involves significant time and expense, and we may not have sufficient resources to devote to our strategic alliances, particularly
those with companies that have significantly greater financial and other resources than we do. The anticipated benefits of these
arrangements may never materialize, and performing under these arrangements may adversely affect our results of operations.
We
may not be able to obtain patents or other intellectual property rights necessary to protect our proprietary technology and business.
We
may seek to patent concepts, components, processes, designs and methods, and other inventions and technologies that we consider
to have commercial value or that will likely give us a technological advantage. Despite devoting resources to the research and
development of proprietary technology, we may not be able to develop technology that is patentable or protectable. Patents may
not be issued in connection with pending patent applications, and claims allowed may not be sufficient to allow them to use the
inventions that they create exclusively. Furthermore, any patents issued could be challenged, re-examined, held invalid or unenforceable
or circumvented and may not provide sufficient protection or a competitive advantage. In addition, despite efforts to protect
and maintain patents, competitors and other third parties may be able to design around their patents or develop products similar
to our work products that are not within the scope of their patents. Finally, patents provide certain statutory protection only
for a limited period of time that varies depending on the jurisdiction and type of patent.
Prosecution
and protection of the rights sought in patent applications and patents can be costly and uncertain, often involve complex legal
and factual issues and consume significant time and resources. In addition, the breadth of claims allowed in our patents, their
enforceability and our ability to protect and maintain them cannot be predicted with any certainty. The laws of certain countries
may not protect intellectual property rights to the same extent as the laws of the United States. Even if our patents are held
to be valid and enforceable in a certain jurisdiction, any legal proceedings that we may initiate against third parties to enforce
such patents will likely be expensive, take significant time and divert management’s attention from other business matters.
We cannot assure that any of our issued patents or pending patent applications provide any protectable, maintainable or enforceable
rights or competitive advantages to us.
In
addition to patents, we will rely on a combination of copyrights, trademarks, trade secrets and other related laws and confidentiality
procedures and contractual provisions to protect, maintain and enforce our proprietary technology and intellectual property rights
in the United States and other countries. However, our ability to protect our brands by registering certain trademarks may be
limited. In addition, while we will generally enter into confidentiality and nondisclosure agreements with our employees, consultants,
contract manufacturers, distributors and resellers and with others to attempt to limit access to and distribution of our proprietary
and confidential information, it is possible that:
|
●
|
misappropriation
of our proprietary and confidential information, including technology, will nevertheless occur;
|
|
|
|
|
●
|
our
confidentiality agreements will not be honored or may be rendered unenforceable;
|
|
●
|
third
parties will independently develop equivalent, superior or competitive technology or products;
|
|
|
|
|
●
|
disputes
will arise with our current or future strategic licensees, customers or others concerning the ownership, validity, enforceability,
use, patentability or registrability of intellectual property; or
|
|
|
|
|
●
|
unauthorized
disclosure of our know-how, trade secrets or other proprietary or confidential information will occur.
|
We
cannot assure that we will be successful in protecting, maintaining or enforcing our intellectual property rights. If we are unsuccessful
in protecting, maintaining or enforcing our intellectual property rights, then our business, operating results and financial condition
could be materially adversely affected, which could
|
●
|
adversely
affect our reputation with customers;
|
|
|
|
|
●
|
be
time-consuming and expensive to evaluate and defend;
|
|
|
|
|
●
|
cause
product shipment delays or stoppages;
|
|
|
|
|
●
|
divert
management’s attention and resources;
|
|
|
|
|
●
|
subject
us to significant liabilities and damages;
|
|
|
|
|
●
|
require
us to enter into royalty or licensing agreements; or
|
|
|
|
|
●
|
require
us to cease certain activities, including the sale of products.
|
If
it is determined that we have infringed, violated or are infringing or violating a patent or other intellectual property right
of any other person or if we are found liable in respect of any other related claim, then, in addition to being liable for potentially
substantial damages, we may be prohibited from developing, using, distributing, selling or commercializing certain of our technologies
unless we obtain a license from the holder of the patent or other intellectual property right. We cannot assure that we will be
able to obtain any such license on a timely basis or on commercially favorable terms, or that any such licenses will be available,
or that workarounds will be feasible and cost-efficient. If we do not obtain such a license or find a cost-efficient workaround,
our business, operating results and financial condition could be materially adversely affected and we could be required to cease
related business operations in some markets and restructure our business to focus on our continuing operations in other markets.
We
may be unable to keep pace with changes in technology as our business and market strategy evolves.
We
will need to respond to technological advances in a cost-effective and timely manner in order to remain competitive. The need
to respond to technological changes may require us to make substantial, unanticipated expenditures. There can be no assurance
that we will be able to respond successfully to technological change.
Risks
Related to Our Common Stock
Trading
on the OTC Markets is volatile, sporadic and often thin, which could depress the market price of our common stock and make it
difficult for our stockholders to resell their common stock.
Our
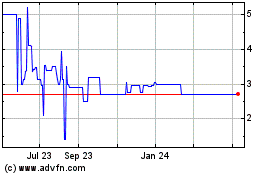
common stock is quoted on the OTCQB. Trading in securities quoted on the OTC Markets is often thin and characterized by wide fluctuations
in trading prices, due to many factors, some of which may have little to do with our operations or business prospects. This volatility
could depress the market price of our common stock for reasons unrelated to operating performance. Moreover, the OTC Markets is
not a stock exchange, and trading of securities on the OTC Markets is often more sporadic than the trading of securities listed
on a stock exchange like NASDAQ or the NYSE. Our common stock has a history of thin trading. During the 52-week period ended December
31, 2020, trades were only reported on 103 trading days. These factors may result in investors having difficulty reselling any
shares of our common stock.
Because
we were a “shell company,” Rule 144 is unavailable until one year has elapsed from the date that we have filed “Form
10 information” with the SEC, including current financial statements.
Rule
144 provides, as indicated above, that sales of securities of a former shell company may only be made once the applicable waiting
period has terminated and only if appropriate current information is available by the company and that it has filed all relevant
periodic reports that it is required to file. Rule 144 will be unavailable to holders of restricted securities until one year
has elapsed from the date that we filed “Form 10 information” (as defined in Rule 144) with the SEC along with audited
financial statements. Once we become current, no assurance can be made that the Company will be able to remain current with its
reports. In addition to the above, because we voluntarily file SEC reports with the SEC, following the one (1) year period discussed
above, holders will not be permitted to rely on Rule 144 for sales of our shares, unless and until such time as we are mandatorily
required under SEC laws, rules and regulations to file periodic reports with the SEC.
The
market price of our Common Stock may be highly volatile and such volatility could cause you to lose some or all of your investment.
The
market price of our common stock, par value $0.001 per share, or Common Stock, may fluctuate significantly in response to numerous
factors, some of which are beyond our control, such as:
|
●
|
the
announcement of new products or product enhancements by us or our competitors;
|
|
|
|
|
●
|
developments
concerning intellectual property rights;
|
|
|
|
|
●
|
changes
in legal, regulatory, and enforcement frameworks impacting our technology or the application of our technology;
|
|
|
|
|
●
|
variations
in our and our competitors’ results of operations;
|
|
|
|
|
●
|
fluctuations
in earnings estimates or recommendations by securities analysts, if our Common Stock is covered by analysts;
|
|
|
|
|
●
|
the
results of product liability or intellectual property lawsuits;
|
|
|
|
|
●
|
future
issuances of Common Stock or other securities;
|
|
|
|
|
●
|
the
addition or departure of key personnel;
|
|
|
|
|
●
|
announcements
by us or our competitors of acquisitions, investments or strategic alliances; and
|
|
|
|
|
●
|
general
market conditions and other factors, including factors unrelated to our operating performance.
|
Further,
the general stock market has recently experienced price and volume fluctuations. The volatility of our Common Stock could be further
exacerbated due to low trading volume. Continued market fluctuations could result in extreme volatility in the price of our Common
Stock, which could cause a decline in the value of our Common Stock and the loss of some or all of our investors’ investment.
Sales of shares of our Common Stock could also depress the then price of our shares.
An
investor’s ability to trade our common stock may be limited by trading volume.
The
Company’s shares are currently quoted on the OTCQB under the symbol “SCTC.” An active trading market for our
common stock has not developed, and may not develop, on the OTCQB. During the period subsequent to our upgrade from the OTC Pink
Market to the OTCQB, which occurred on September 14, 2020 and until December 31, 2020, trades were only reported on 46 trading
days. A limited trading volume may prevent our shareholders from selling shares at such times or in such amounts as they may otherwise
desire.
Because
our Common Stock may be a “penny stock,” it may be more difficult for investors to sell shares of our Common Stock,
and the market price of our Common Stock may be adversely affected.
Our
Common Stock may be a “penny stock” if, among other things, the stock price is below $5.00 per share, it is not listed
on a national securities exchange, or it has not met certain net tangible asset or average revenue requirements. Broker-dealers
who sell penny stocks must provide purchasers of these stocks with a standardized risk-disclosure document prepared by the SEC.
This risk-disclosure document provides information about penny stocks and the nature and level of risks involved in investing
in the penny-stock market. A broker must also give a purchaser, orally or in writing, bid and offer quotations and information
regarding broker and salesperson compensation, make a written determination that the penny stock is a suitable investment for
the purchaser and obtain the purchaser’s written agreement to the purchase. Broker-dealers must also provide customers that
hold penny stock in their accounts with such broker-dealer a monthly statement containing price and market information relating
to the penny stock. If a penny stock is sold to an investor in violation of the penny stock rules, the investor may be able to
cancel its purchase and get their money back.
If
applicable, the penny stock rules may make it difficult for stockholders to sell their shares of our Common Stock. Because of
the rules and restrictions applicable to a penny stock, there is less trading in penny stocks and the market price of our Common
Stock may be adversely affected. Also, many brokers choose not to participate in penny stock transactions. Accordingly, stockholders
may not always be able to resell their shares of our Common Stock publicly at times and prices that they feel are appropriate.
Compliance
with the reporting requirements of federal securities laws can be expensive.
We
are a public reporting company in the United States, and accordingly, subject to the information and reporting requirements of
the Exchange Act and other federal securities laws. The costs of preparing and filing annual and quarterly reports and other information
with the SEC and furnishing audited reports to stockholders are substantial. Failure to comply with the applicable securities
laws could result in private or governmental legal action against us or our officers and directors, which could have a detrimental
impact on our business and financials, the value of our stock, and the ability of stockholders to resell their stock.
Our
investors’ ownership in the Company may be diluted in the future.
In
the future, we may issue additional authorized but previously unissued equity securities, resulting in the dilution of ownership
interests of our present stockholders. For instance, pursuant to that certain Securities Exchange Agreement by and between Intellisense
and Medigus, dated September 16, 2019, if ScoutCam achieves US$33.0 million in sales in the aggregate within the first three years
following December 30, 2019, the consummation date of such agreement, we will issue shares of Common Stock to Medigus representing
10% of our issued and outstanding share capital as of December 30, 2019. Similarly, we may issue a substantial number of shares
of Common Stock or other securities convertible into or exercisable for Common Stock in connection with capital raising activity,
hiring or retaining employees, future acquisitions, raising additional capital in the future to fund our operations, and other
business purposes. We expect to authorize in the future a substantial number of shares of our Common Stock for issuance under
a stock option or similar plan, and may issue equity awards to management, employees and other eligible persons. Additional shares
of Common Stock issued by us in the future will dilute an investor’s investment in the Company. In addition, we may seek
stockholder approval to increase the amount of the Company’s authorized stock, which would create the potential for further
dilution of current investors.
Directors,
executive officers, principal stockholders and affiliated entities own a significant percentage of our capital stock, and they
may make decisions that our stockholders do not consider to be in their best interests.
As
of March 28, 2021, our directors, executive officers, principal stockholders and affiliated entities may be deemed to beneficially
own, in the aggregate, approximately 67.54% of our outstanding voting securities as of the date hereof. As a result, if some or
all of such parties acted together, they would have the ability to exert substantial influence over the election of our board
of directors and the outcome of issues requiring approval by our stockholders. This concentration of ownership may also have the
effect of delaying or preventing a change in control of the Company that may be favored by other stockholders. This could prevent
transactions in which stockholders might otherwise recover a premium for their shares over current market prices. This concentration
of ownership and influence in management and board decision-making could also harm the price of our capital stock by, among other
things, discouraging a potential acquirer from seeking to acquire shares of our capital stock (whether by making a tender offer
or otherwise) or otherwise attempting to obtain control of our Company.
Risks
Related to our Operations in Israel
Political,
economic and military instability in Israel may impede our ability to operate and harm our financial results.
Our
offices and management team are located in Israel. Accordingly, political, economic, and military conditions in Israel and the
surrounding region may directly affect our business and operations. In recent years, Israel has been engaged in sporadic armed
conflicts with Hamas, an Islamist terrorist group that controls the Gaza Strip, with Hezbollah, an Islamist terrorist group that
controls large portions of southern Lebanon, and with Iranian-backed military forces in Syria. In addition, Iran has threatened
to attack Israel and may be developing nuclear weapons. Some of these hostilities were accompanied by missiles being fired from
the Gaza Strip against civilian targets in various parts of Israel, including areas in which our employees and some of our consultants
are located, and negatively affected business conditions in Israel. Any hostilities involving Israel or the interruption or curtailment
of trade between Israel and its trading partners could adversely affect our operations and results of operations.
Our
commercial insurance does not cover losses that may occur as a result of events associated with war and terrorism. Although the
Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of
war, we cannot assure you that this government coverage will be maintained or that it will sufficiently cover our potential damages.
Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability
in the region would likely negatively affect business conditions and could harm our results of operations.
Further,
in the past, the State of Israel and Israeli companies have been subjected to economic boycotts. Several countries still restrict
business with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on
our operating results, financial condition or the expansion of our business. A campaign of boycotts, divestment and sanctions
has been undertaken against Israel, which could also adversely impact our business.
In
addition, many Israeli citizens are obligated to perform several days, and in some cases more, of annual military reserve duty
each year until they reach the age of 40 (or older, for reservists who are military officers or who have certain occupations)
and, in the event of a military conflict, may be called to active duty. In response to increases in terrorist activity, there
have been periods of significant call-ups of military reservists. It is possible that there will be military reserve duty call-ups
in the future. Our operations could be disrupted by such call-ups, which may include the call-up of members of our management.
Such disruption could materially adversely affect our business, prospects, financial condition and results of operations.
Exchange
rate fluctuations between foreign currencies and the U.S. Dollar may negatively affect our earnings.
Our
reporting and functional currency is the U.S. dollar. Our revenues are currently primarily payable in U.S. dollars and we expect
our future revenues to be denominated primarily in U.S. dollars. However, certain amount of our expenses are in NIS and as a result,
we are exposed to the currency fluctuation risks relating to the recording of our expenses in U.S. dollars. We may, in the future,
decide to enter into currency hedging transactions. These measures, however, may not adequately protect us from material adverse
effects.
We
may become subject to claims for remuneration or royalties for assigned service invention rights by our employees, which could
result in litigation and adversely affect our business.
A
significant portion of ScoutCam’s intellectual property has been developed by ScoutCam’s employees in the course of
their employment for us. Under the Israeli Patent Law, 5727-1967, or the Patent Law, inventions conceived by an employee in the
course and as a result of or arising from his or her employment with a company are regarded as “service inventions,”
which belong to the employer, absent a specific agreement between the employee and employer giving the employee service invention
rights. The Patent Law also provides that if there is no such agreement between an employer and an employee, the Israeli Compensation
and Royalties Committee, or the Committee, a body constituted under the Patent Law, will determine whether the employee is entitled
to remuneration for his inventions. Recent case law clarifies that the right to receive consideration for “service inventions”
can be waived by the employee and that in certain circumstances, such waiver does not necessarily have to be explicit. The Committee
will examine, on a case-by-case basis, the general contractual framework between the parties, using interpretation rules of the
general Israeli contract laws. Further, the Committee has not yet determined one specific formula for calculating this remuneration
(but rather uses the criteria specified in the Patent Law). Although we generally enter into assignment-of-invention agreements
with our employees pursuant to which such individuals assign to us all rights to any inventions created in the scope of their
employment or engagement with us, we may face claims demanding remuneration in consideration for assigned inventions. As a consequence
of such claims, we could be required to pay additional remuneration or royalties to our current and/or former employees, or be
forced to litigate such claims, which could negatively affect our business.
USE
OF PROCEEDS
All
of the proceeds from the sale of any shares of Common Stock offered under this prospectus are for the account of the Selling Stockholders.
Accordingly, we will not receive any proceeds from the sales of these securities, although we will receive cash proceeds equal
to the total exercise price of warrants that are exercised for cash, or up to $25,555,556.45, if all warrants issued to the Selling
Stockholders are exercised. We will bear all costs, expenses and fees in connection with the registration of the shares of Common
Stock offered under this prospectus, whereas the Selling Stockholders will bear all brokerage commissions and similar selling
expenses.
MARKET
FOR REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market
Information
Our
Common Stock is quoted on the OTCQB under the symbol “SCTC”. Trading in stocks quoted on the OTCQB is often thin and
is characterized by wide fluctuations in trading prices due to many factors that may be unrelated to a company’s operations
or business prospects. We cannot assure you that there will be a market in the future for our common stock.
OTCQB
securities are not listed or traded on the floor of an organized national or regional stock exchange. Instead, OTCQB securities
transactions are conducted through a telephone and computer network connecting dealers in stocks. OTCQB issuers are traditionally
smaller companies that do not meet the financial and other listing requirements of a regional or national stock exchange.
Holders
As
of April 12, 2021, there were 77 stockholders of record of our Common Stock and 60,295,245 shares of our Common Stock outstanding.
Dividends
We
have never declared or paid any cash dividends on our Common Stock. We currently intend to retain future earnings, if any, to
increase our working capital and do not anticipate paying any cash dividends in the foreseeable future.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND
RESULTS OF OPERATIONS
You
should read the following discussion and analysis of our financial condition and results of operations together with our financial
statements and the related notes appearing elsewhere in this prospectus. In addition to historical information, this discussion
and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. See “Special Note on
Forward-Looking Statements” for a discussion of the uncertainties and assumptions associated with these statements. Our
actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include,
but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included
elsewhere in this prospectus.
Overview
We
were incorporated under the laws of the State of Nevada on March 22, 2013 under the name Intellisense Solutions Inc. We were initially
engaged in the business of developing web portals to allow companies and individuals to engage in the purchase and sale of vegetarian
food products over the Internet. However, were not able to execute our original business plan, develop significant operations
or achieve commercial sales.
On
December 30, 2019, we acquired all of the issued and outstanding share capital of ScoutCam Ltd. (the “Closing Date”).
Following this transaction, we integrated and fully adopted ScoutCam Ltd.’s business into our Company as our primary business
activity. On December 31, 2019, we changed our name to ScoutCam Inc.
Through
ScoutCam Ltd., we are engaged in the development, production and marketing of innovative miniaturized imaging equipment, or our
micro ScoutCam™ portfolio, for use in medical procedures as well as various industrial applications. We derive a substantial
portion of our revenue from applications of our micro ScoutCam™ portfolio within the medical and industrial fields. We have
recently begun examining additional applications for our micro ScoutCam™ portfolio outside of the medical device industry,
including in, among others, the defense, aerospace, automotive, and industrial non-destructing-testing industries. We plan to
further expand the activity in these non-medical spaces.
Going
Concern
The
financial statements of the Company for the fiscal year ended December 31, 2020 were prepared assuming it will continue as a going
concern. As discussed in the notes to the financial statements, the Company has incurred operating losses. These factors, among others,
raise substantial doubt about its ability to continue as a going concern within one year after the date our accompanying consolidated
financial statements are issued. Additionally, our independent registered public accounting firm included an explanatory paragraph in
its report for the years ended December 31, 2020, regarding concerns about Company’s ability to continue as a going concern within
one year after the date our accompanying consolidated financial statements are issued.
Impact
of COVID-19 Pandemic
The
COVID-19 pandemic has had a significant impact on global markets and the global economy, including countries in which the Company
operates. As the extent of the impact on the global economy remains unclear, the Company anticipates that it will have a continuing
impact on global economies in the near and long-term future. In light of the below mentioned factors, the COVID-19 pandemic had
and most likely will continue to have a material effect on the Company’s operations, and the extent to which the COVID-19
pandemic will impact the Company’s operations will depend on future developments. In particular, the continued spread of
COVID-19 globally had and most likely will continue to have material adverse impact on the Company’s operations and workforce,
including its manufacturing activities, product sales, as well as its ability to continue to raise capital. Travel restrictions
had and most likely will continue to have a material adverse impact on our sales and marketing and research and development efforts.
Critical
Accounting Policies and Estimates
Our
management’s discussion and analysis of our financial condition and results of operations is based on our financial statements,
which we have prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP. The preparation
of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities
and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenues
and expenses during the reporting periods. We evaluate these estimates and judgments on an ongoing basis. We base our estimates
on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which
form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other
sources. Our actual results may differ from these estimates under different assumptions or conditions.
While
our significant accounting policies are more fully described in Note 2 to our financial statements appearing elsewhere in our Annual
Report on Form 10-K for the fiscal year ended December 31, 2020, we believe that the following accounting policies are the
most critical for fully understanding and evaluating our financial condition and results of operations.
Significant
Accounting Policies
Basis
of Presentation
We
have prepared the accompanying financial statements in accordance with U.S. GAAP. In our opinion, all adjustments (consisting
of normal recurring accruals) considered necessary for a fair presentation have been included. Operating results for the years
ended December 31, 2020, 2019 and 2018 are not necessarily indicative of the results that may be expected for future years.
The
accompanying financial statements are presented in U.S. dollars in conformity with U.S. GAAP and pursuant to the rules and regulations
of the Securities and Exchange Commission.
The
accompanying comparative consolidated financial statements include the historical accounts of ScoutCam as a “Carve-out Business”,
a division of Medigus. Throughout the comparative periods included in these Financial Statements, the Carve-out Business operated
as part of Medigus. Separate financial statements have not historically been prepared for the Carve-out Business.
These
carve-out comparative financial statements have been prepared on a standalone basis and are derived from Medigus’s consolidated
financial statements and accounting records. The carve-out comparative financial statements reflect ScoutCam’s financial
position, results of operations, changes in net parent deficit and cash flows in accordance with U.S. GAAP.
The
financial position, results of operations, changes in net parent deficit, and cash flows of the Carve-out Business may not be
indicative of its results had it been a separate stand-alone entity during the comparative periods presented.
The
comparative carve-out financial statements of the Company include expenses which were allocated from Medigus for certain functions,
including general corporate expenses related to corporate strategy, procurement, Information Technology (IT), Human Resources
(HR) and legal. These allocation have been made on the basis of direct usage when identifiable, with the remainder allocated on
the basis of headcount. Management believes the expense allocation methodology and results are reasonable and consistently applied
for all comparative periods presented. However, these allocations may not be indicative of the actual expenses that would have
been incurred by an independent company or of the costs to be incurred in the future.
Use
of Estimates
The
preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated
financial statements and the reported amounts of revenue and expenses during the reporting period. The Company evaluates on an
ongoing basis its assumptions, including those related to contingencies, deferred taxes, inventory impairment, as well as in estimates
used in applying the revenue recognition policy. Actual results may differ from those estimates.
Revenue
Recognition
Revenue
Measurement
Commencing
on January 1, 2018, the Company’s revenues are measured according to the ASC 606, “Revenue from Contracts with Customers”
(“ASC 606”). Under ASC 606, revenues are measured according to the amount of consideration that ScoutCam expects to
be entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third
parties, such as sales taxes. Revenues are presented net of VAT.
Revenue
Recognition
The
Company recognizes revenue when a customer obtains control over promised goods or services. For each performance obligation ScoutCam
determines at contract inception whether it satisfies the performance obligation over time or satisfies the performance obligation
at a point in time.
Performance
obligations are satisfied over time if one of the following criteria is met: (a) the customer simultaneously receives and consumes
the benefits provided by ScoutCam’s performance; (b) ScoutCam’s performance creates or enhances an asset that the
customer controls as the asset is created or enhanced; or (c) ScoutCam’s performance does not create an asset with an alternative
use to ScoutCam and ScoutCam has an enforceable right to payment for performance completed to date.
If
a performance obligation is not satisfied over time, a Company satisfies the performance obligation at a point in time.
The
transaction price is allocated to each distinct performance obligations on a relative standalone selling price (“SSP”)
basis and revenue is recognized for each performance obligation when control has passed. In most cases, ScoutCam is able to establish
SSP based on the observable prices of services sold separately in comparable circumstances to similar customers and for products
based on ScoutCam’s best estimates of the price at which ScoutCam would have sold the product regularly on a stand-alone
basis. ScoutCam reassesses the SSP on a periodic basis or when facts and circumstances change.
Product
Revenue
Revenues
from product sales are recognized when the customer obtains control of Company’s product, typically upon shipment to the
customer. Sales taxes collected from customers relating to product sales and remitted to governmental authorities are excluded
from revenues.
Service
Revenue
The
Company also generates revenues from development services. Revenue from development services is recognized over the period of
the applicable service contract. To the extent development services are not distinct from the performance obligation relating
to the subsequent mass production phase of the prototype under development, revenue from these services is deferred until commencement
of the production phase of the project.
There
are no long-term payment terms or significant financing components of the Company’s contracts.
The
Company’s contract payment terms for product and services vary by customer. The Company assesses collectibility based on
several factors, including collection history.
Accounts
Receivable
Accounts
receivable are presented in the Company’s consolidated balance sheets net of allowance for doubtful accounts. The Company
estimates the collectibility of its accounts receivable balances and adjusts its allowance for doubtful accounts accordingly.
When
revenue recognition criteria are not met for a sale transaction that has been billed, the Company does not recognize deferred
revenues or the related account receivable.
Comparison
of the Year Ended December 31, 2020 and the Year Ended December 31, 2019
Overview
The
Company’s primary business activity during 2020 was the completion of R&D and the transition to the production stage
with respect to a contract with a Fortune 500 Multinational Healthcare Corporation, while expanding the R&D team to enable
additional projects in parallel. The main effect of this activity was the increase in the number of employees from 19 at the end
of 2019 to 27 at the end of 2020 to enable the Company to manage the anticipated increased workload.
Other
major activities in 2020 were the following:
|
|
-
|
Expanding
marketing activities, including the recruitment of a Director of Business Development in the US, and launching a multi-platform
digital marketing campaign.
|
|
|
-
|
Extensive
activity around the Company’s IP, including submissions of new patent applications as well as maintenance, defense,
and commercialization efforts of existing patents.
|
|
|
-
|
On
December 30, 2019, upon the completion of the Exchange Agreement (as defined herein), the Company transitioned from a shell
company to an operating company. This turn led to, among other, an increase in professional services (legal counsels, accountants,
SOX consultants, etc.), fees and related costs in connection with ScoutCam Inc.’s post-Closing Date Board of Directors,
increases in D&O insurance, etc.
|
|
|
-
|
Increase
in the operation expenses in order to improve the current Company’s R&D capabilities.
|
|
|
-
|
Investment
in capital expenses to provide the necessary facilities, IT, and lab tools for the newly recruited employees and to upgrade
the Company’s production and quality control capabilities.
|
The
following table summarizes our results of operations for the years ended December 31, 2020 and 2019, together with the changes
in those items in dollars and as a percentage:
|
|
|
2020
|
|
|
2019
|
|
|
% Change
|
|
|
Revenues
|
|
|
491,000
|
|
|
|
309,000
|
|
|
|
59
|
%
|
|
Cost of Revenues
|
|
|
994,000
|
|
|
|
542,000
|
|
|
|
83
|
%
|
|
Gross Loss
|
|
|
(503,000
|
)
|
|
|
(233,000
|
)
|
|
|
116
|
%
|
|
Research and development expenses
|
|
|
725,000
|
|
|
|
274,000
|
|
|
|
165
|
%
|
|
Sales and marketing expense
|
|
|
443,000
|
|
|
|
183,000
|
|
|
|
142
|
%
|
|
General and administrative expenses
|
|
|
3,035,000
|
|
|
|
1,117,000
|
|
|
|
172
|
%
|
|
Operating Loss
|
|
|
(4,706,000
|
)
|
|
|
(1,807,000
|
)
|
|
|
160
|
%
|
Revenues
For
the year ended December 31, 2020, we generated revenues of $491,000, an increase of $182,000 or 59%, from 2019 revenues.
The
increase in revenues was primarily due to the sale of products to A.M. Surgical (see Item 1). Total revenues recorded from A.M.
Surgical during 2020 amounted to approximately $383,000. Total revenues we recorded from A.M. Surgical during 2019 amounted to
approximately $85,000. This increase was partially offset by decrease in revenues to other customers due to:
|
|
a)
|
the
COVID-19 pandemic impact on global markets and the global economy, including countries and industries in which the Company
operates;
|
|
|
b)
|
most
of the revenues for year ended December 31, 2019 were derived from sales of miniature camera and related equipment to occasional
customers. The Company’s management has decided to reduce sales to occasional customers and focus on larger projects.
Our current business model is that of a B2B approach, in which we seek to identify target businesses interested in integrating
our micro ScoutCam™ technology, or commissioning individual projects using our technology.
|
Remaining
Performance Obligations (“RPO”) represents contracted revenue that has not yet been recognized, which includes deferred
revenue and amounts that will be invoiced and recognized as revenue in future periods. As of December 31, 2020, the total RPO
amounted to $2.9 million, which we expect to recognize over the expected manufacturing term of the product under development.
Cost
of Revenues
Cost
of revenues for the year ended December 31, 2020 were $994,000, an increase of $452,000, or 83%, compared to cost of revenues
of $542,000 for the year ended December 31, 2019.
The
increase in cost of revenues was due to:
|
|
a)
|
Increase
in revenues as described above;
|
|
|
|
|
|
|
b)
|
changes
in products and services mix; and
|
|
|
|
|
|
|
c)
|
increase
in payroll expenses as a result of hiring additional employees.
|
Gross
Loss
Gross
loss for the year ended December 31, 2020 was $503,000, an increase of $270,000 compared to a gross loss of $233,000 for the year
ended December 31, 2019. Gross loss is impacted by several factors, including shifts in product mix, sales volume, fluctuations
in manufacturing costs, labor costs, and pricing strategies.
Research
and Development Expenses
Research
and development expenses for the year ended December 31, 2020, were $725,000, an increase of $451,000, or 165%, compared to $274,000
for the year ended December 31, 2019. The increase was primarily due to a $231,000 increase in payroll expenses and a $205,000
increase in materials and subcontractors. The increase in payroll expenses resulted from an increase in share - based compensation
expenses (see note 9 to our financial statements for the year ended December 31, 2020) and hiring additional employees. The increase
in materials and subcontractors was primarily due to an increase in research and development activities as described under “Overview”.
Sales
and Marketing Expenses
Sales
and marketing expenses for the year ended December 31, 2020, were $443,000, an increase of $260,000, or 142%, compared to $183,000
for the year ended December 31, 2019. The increase was primarily due to an increase in marketing activities as described under
“Overview”.
General
and Administrative Expenses
General
and Administrative expenses for the year ended December 31, 2020, were $3,035,000, an increase of $1,918,000, or 172%, compared
to $1,117,000 for the year ended December 31, 2019. The increase was primarily due to a $767,000 increase in payroll expenses,
as a result of an increase in share - based compensation expenses (see note 9 to our financial statements for the year ended December
31, 2020) and hiring additional employees and a $826,000 increase in professional services. The increase in professional services
was primarily due to an increase in share - based compensation expenses, as result from the incorporation of the Subsidiary as
an independent company and in connection with the execution of that certain securities exchange agreement involving the Subsidiary
and increase in patent expenses as described under “Overview”.
Operating
loss
We
incurred an operating loss of $4,706,000 for the year ended December 31, 2020, an increase of $2,899,000, or 160%, compared to
operating loss of $1,807,000 for the year ended December 31, 2019. The increase in operating results was due to an increase of
$270,000 in gross loss, an increase of $451,000 in research and development expenses, an increase of $260,000 in sales and marketing
expenses and increase of $1,918,000 in administrative and general expenses.
Liquidity
and Capital Resources
During
2020, we generated liquidity primarily from fund raising and warrant exercises as described at note 9 to our financial statements
for the year ended December 31, 2020.
During
2020, we received proceeds from fund raising in the aggregate approximate amount of $2.9 million, net of issuance expenses and
$1.7 million from warrants exercise.
As
of December 31, 2020, our total assets were $5,895,000. As of December 31, 2019, our total assets were $4,757,000. The increase
of assets was mainly due to an increase of contract fulfillment assets, increase of property and equipment and increase of other
current assets. As of December 31, 2020, our total liabilities were $1,931,000. As of December 31, 2019, our total liabilities
were $2,235,000. The decrease of liabilities was mainly due to a decrease of loan from Parent Company, decrease of other current
expenses, partially offset by increase of contract liabilities and other accrued compensation expenses.
During
the year ended December 31, 2020, we incurred losses of $4,667,000 and negative cash flow from operating activities of approximately
$4,187,000. Based on the projected cash flows, our management is of the opinion that without further fundraising it will not have
sufficient resources to enable it to continue its operating activities, including the development, manufacturing and marketing
of its products for a period of at least 12 months from the financial statements issuance date. As a result, there is substantial
doubt about our ability to continue as a going concern.
Management’s
plans include continuing commercialization of our products and securing sufficient financing through the sale of additional equity
securities, debt or capital inflows from strategic partnerships and others. There are no assurances, however, that we will be
successful in obtaining the level of financing needed for its operations. If we are unsuccessful in commercializing its products
and securing sufficient financing, it may need to reduce activities, curtail or even cease operations.
Cash
Flows
The
following table sets forth the significant sources and uses of cash for the periods set forth below (in dollars):
|
|
|
2020
|
|
|
2019
|
|
|
Cash used in Operating Activity
|
|
|
(4,187,000
|
)
|
|
|
(1,799,000
|
)
|
|
Cash used in Investing Activity
|
|
|
(276,000
|
)
|
|
|
(55,000
|
)
|
|
Cash provided by Financing Activity
|
|
|
4,506,000
|
|
|
|
5,104,000
|
|
Operating
Activities
For
the fiscal year ended December 31, 2020, net cash flows used in operating activities was $4,187,000, due primarily to a net loss
of $4,667,000, change in operating asset and liabilities of approximately $612,000, partially offset by share based compensation
expenses (non-cash item) of approximately $1,107,000.
Investing
Activities
For
the fiscal year ended December 31, 2020, net cash flows used in investing activities was $276,000, due primarily to purchase of
property and equipment.
Financing
Activities
For
the fiscal year ended December 31, 2020, net cash flows provided by financing activities was $4,506,000, due primarily to proceeds
from issuance of shares and warrants of approximately $2,858,000 and proceeds from exercise from warrants of approximately $1,729,000.
Comparison
of the Year Ended December 31, 2019 and the Year Ended December 31, 2018
Overview
ScoutCam
Ltd. was formed in Israel on January 3, 2019, as a wholly owned subsidiary of Medigus, and commenced operations on March 1, 2019.
ScoutCam was incorporated as part of the Reorganization of Medigus, which was designed to distinguish ScoutCam’s miniaturized
imaging business, or the micro ScoutCam™ portfolio, from Medigus’s other operations and to enable Medigus
to form a separate business unit with dedicated resources focused on the promotion of such technology. In December 2019, Medigus
and ScoutCam consummated an Amended and Restated Asset Transfer Agreement, which transferred and assigned certain assets and intellectual
property rights related to its miniaturized imaging business.
On
March 1, 2019, 12 employees moved from Medigus to ScoutCam. Prior to moving to ScoutCam, the salary costs of those employees were
split among all of Medigus’s activities (including the miniaturized imaging business activity). Hence, in the 2018 data
provided below, most of the salary costs of these employees are not included. The vast majority of these employees were from the
Production and R&D departments. Therefore, their transfer caused large changes in the data of these two line items.
The
following table summarizes our results of operations for the years ended December 31, 2019 and 2018, together with the changes
in those items in dollars and as a percentage:
|
|
|
2019
|
|
|
2018
|
|
|
% Change
|
|
|
Revenues
|
|
|
309,000
|
|
|
|
391,000
|
|
|
|
(21
|
)%
|
|
Cost of Revenues
|
|
|
542,000
|
|
|
|
221,000
|
|
|
|
145
|
%
|
|
Gross Profit (Loss)
|
|
|
(233,000
|
)
|
|
|
170,000
|
|
|
|
(237
|
)%
|
|
Research and development expenses
|
|
|
274,000
|
|
|
|
183,000
|
|
|
|
50
|
%
|
|
Sales and marketing expense
|
|
|
183,000
|
|
|
|
270,000
|
|
|
|
(32
|
)%
|
|
General and administrative expenses
|
|
|
1,117,000
|
|
|
|
240,000
|
|
|
|
365
|
%
|
|
Operating Loss
|
|
|
(1,807,000
|
)
|
|
|
(523,000
|
)
|
|
|
246
|
%
|
Revenues
For
the year ended December 31, 2019, ScoutCam generated revenues of $309,000, a decrease of $82,000 from 2018 revenues.
The
tables below set forth our revenues by product:
|
|
|
2019
|
|
|
2018
|
|
|
U.S. dollars; in thousands
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Services
|
|
|
121
|
|
|
|
39.2
|
%
|
|
|
217
|
|
|
|
55.5
|
%
|
|
Miniature camera and related equipment
|
|
|
188
|
|
|
|
60.8
|
%
|
|
|
174
|
|
|
|
44.5
|
%
|
|
Total
|
|
|
309
|
|
|
|
100
|
%
|
|
|
391
|
|
|
|
100
|
%
|
The
increase in revenues from miniature camera and related equipment was primarily due to an overall increase in the sales of the
Company’s products to occasional customers.
The
decrease in revenues from services was primarily due to:
|
|
(i)
|
during
the year ended December 31, 2018, we recorded revenues for development services provided to a customer in the amount of approximately
$130,000 (see ‘Customer A’ in note 11 to our financial statements for the year ended December 31, 2020). During
year ended December 31, 2019 we recorded revenues for development services provided to this customer in the amount of approximately
$85,000; and
|
|
|
|
|
|
|
(ii)
|
during
the year ended December 31, 2018, we recorded revenues for development services provided to a customer in the amount of approximately
$87,000 (see ‘Customer B’ in note 11 to our financial statements for the year ended December 31, 2020). We did
not receive any revenue from development services from this customer during the year ended December 31, 2019.
|
Cost
of Revenues
Cost
of revenues for the year ended December 31, 2019 were $542,000, an increase of $321,000, or 145%, compared to cost of revenues
of $221,000 for the year ended December 31, 2018.
The
increase in cost of revenues was due to:
|
|
a)
|
changes
in products and services mix; and
|
|
|
|
|
|
|
b)
|
increase
in payroll expenses and allocation of other expenses, as result of the Reorganization (as described under “Overview”)
and allocating employees salaries from research and development line item to the cost of revenues line item due to the nature
of their current work.
|
Gross
Profit (Loss)
Gross
loss for the year ended December 31, 2019 was $233,000, a decrease of $403,000 compared to a gross profit of $170,000 for the
year ended December 31, 2018. The decrease was primarily due to changes in profitability margins of the product and services mix
and due to an increase in payroll expenses as described above.
Research
and Development Expenses
Research
and development expenses for the year ended December 31, 2019, were $274,000, an increase of $91,000, or 50%, compared to $183,000
for the year ended December 31, 2018. The increase was primarily due to increase in payroll expenses, as result of the Reorganization.
In 2018, the salary cost of R&D employees were split among all of Medigus’s activities. Hence, in the 2018 data provided
above, most of the salary costs of these employees are not included.
Sales
and Marketing Expenses
Sales
and marketing expenses for the year ended December 31, 2019, were $183,000, a decrease of $87,000, or 32%, compared to $270,000
for the year ended December 31, 2018. The decrease was primarily due to decrease in payroll expenses, due to the fact that one
of the employees that was classified under sales and marketing in 2018 became the CEO in 2019 and his payroll expenses were not
classified under S&M in 2019.
General
and Administrative Expenses
General
and Administrative expenses for the year ended December 31, 2019, were $1,117,000, an increase of $877,000, or 365%, compared
to $240,000 for the year ended December 31, 2018. The increase was primarily due to an increase in payroll expenses, as result
of the Reorganization (as described under “Overview”) and an increase in professional services. The increase in professional
services is due to establishing ScoutCam Ltd. as an independent company and due to the acquisition of ScoutCam Ltd.
Operating
loss
We
incurred an operating loss of $1,807,000 for the year ended December 31, 2019, an increase of $1,284,000, or 246%, compared to
operating loss of $523,000 for the year ended December 31, 2018. The increase in operating results was due to an increase of $403,000
in gross loss, an increase of $91,000 in research and development expenses, and increase of $877,000 in administrative and general
expenses partially offset by an $87,000 decrease in sales and marketing expenses.
Liquidity
and Capital Resources
We
generated liquidity primarily from Medigus and from fund raising as described at note 9 to our financial statements for the year
ended December 31, 2020.
On
June 3, 2019, Medigus executed a capital contribution into ScoutCam of an aggregate amount of US$720,000.
On
August 27, 2019, Medigus provided ScoutCam with a line of credit in the aggregate amount of US$500,000, and, in exchange, ScoutCam
granted Medigus a capital note that bears an annual interest rate of 4%. The repayment of the credit line amount shall be spread
over one year in monthly payments beginning on the Closing Date. As of the Closing Date, ScoutCam has withdrawn the entire amount
of the line of credit.
On
December 30, 2019, the Company allotted in a private issuance, a total of 3,413,312 units at the price of USD $0.968 per unit.
Each unit was comprised of two shares of Common Stock, one Warrant A (defined below) and two Warrants B (defined below). The immediate
proceeds (gross) from the issuance of the units amounted to approximately USD 3.3 million. Each Warrant A is exercisable into
one share of Common Stock at an exercise price of USD 0.595 per share during the 12 month period following the allotment. Each
Warrant B is exercisable into one share of Common Stock at an exercise price of USD 0.893 per share during the 18 month period
following the allotment. In addition, a consultant of the Company, Shrem Zilberman Group Ltd. (the “Consultant”) will
be entitled to receive the amount representing 3% of any exercise price of each Warrant A or Warrant B that may be exercised in
the future. In the event the total proceeds received as a result of exercise of Warrants A and B will be less than $2 million
at the time of their expiration, the Consultant will be required to invest $250,000 in the Company.
As
of December 31, 2019, our total assets were $4,757,000. As of December 31, 2018, our total assets were $516,000. The increase
of assets was mainly due to an increase of cash and cash equivalents as a result of the private issuance as described above and
increase of inventory. As of December 31, 2019, our total liabilities were $2,235,000. As of December 31, 2018, our total liabilities
were $634,000. The increase of liabilities was mainly due to an increase of contract liabilities, a loan from Medigus, accrued
compensation expenses and other accrued expenses.
During
the year ended December 31, 2019, the Company incurred losses of $1,829,000 and negative cash flow from operating activities of
approximately $1,799,000. Based on the projected cash flows, the Company’s Management is of the opinion that without further
fundraising it will not have sufficient resources to enable it to continue its operating activities, including the development,
manufacturing and marketing of its products for a period of at least 12 months from the financial statements issuance date. As
a result, there is substantial doubt about the Company’s ability to continue as a going concern.
Management’s
plans include continuing commercialization of Company’s products and securing sufficient financing through the sale of additional
equity securities, debt or capital inflows from strategic partnerships and others. There are no assurances, however, that the
Company will be successful in obtaining the level of financing needed for its operations. If the Company is unsuccessful in commercializing
its products and securing sufficient financing, it may need to reduce activities, curtail or even cease operations.
Cash
Flows
The
following table sets forth the significant sources and uses of cash for the periods set forth below (in dollars):
|
|
|
2019
|
|
|
2018
|
|
|
Cash used in Operating Activity
|
|
|
(1,799,000
|
)
|
|
|
(454,000
|
)
|
|
Cash provided by (used in) Investing Activity
|
|
|
(55,000
|
)
|
|
|
4,000
|
|
|
Cash provided by Financing Activity
|
|
|
5,104,000
|
|
|
|
450,000
|
|
Operating
Activities
For
the fiscal year ended December 31, 2019, net cash flows used in operating activities was $1,799,000, compared to net cash flows
used in operating activities of $454,000 for the fiscal year ended December 31, 2018, an increase of $1,345,000. The change was
mainly due to an increase in net loss, increase in inventory, and partially offset by increase in contract liability, increase
in accrued compensation expenses and increase in other current expenses.
Investing
Activities
For
the fiscal year ended December 31, 2019, net cash flows used in investing activities was $55,000, compared to net cash flows provided
in investing activities of $4,000 for the fiscal year ended December 31, 2018. The change was mainly due to purchase of property
and equipment during 2019.
Financing
Activities
For
the fiscal year ended December 31, 2019, net cash flows provided by financing activities was $5,104,000, compared to net cash
flows provided by financing activities of $450,000 for the fiscal year ended December 31, 2018. The change between the two periods
is due to the fact that in 2019 we have transfer of assets to Medigus, capital contribution from Medigus, loan from Medigus and
cash acquired in connection with the reverse merger.
Future
Funding Requirements
We
believe that it will require additional financing in order to provide the capital we need in order to hit our growth targets.
Off-Balance
Sheet Arrangements
None.
BUSINESS
Overview
We
are engaged in the development, production and marketing of innovative miniaturized imaging equipment known as our micro ScoutCam™
technology for use in medical procedures as well as various applications in other industries. Our current business model is that
of a B2B approach, in which we seek to identify target businesses interested in integrating our micro ScoutCam™ technology,
or commissioning individual projects using our technology. As of the date of this prospectus, we derive a substantial portion
of our revenue from applications of our micro ScoutCam™ technology within the medical and industrial fields. We have recently
begun examining additional applications for our micro ScoutCam™ portfolio outside of the medical device industry, including
sectors such as, inter alia, the homeland security and defense, aerospace (including commercial drones, unmanned aerial vehicles
(UAV) and manned airplanes), automotive, industrial non-destructing-testing industries, and predictive maintenance (i.e. Industry
4.0) based on Internet of Things (IoT). We plan to further expand the activity in these non-medical spaces.

Pictured
above (from left to right) are the Company’s micro ScoutCamTM 1.0 Lum and micro ScoutCam™ 1.2.

The
Company’s eye-endoscope, which includes a camera at the distal tip, integrated illumination and embedded irrigation, which
is only 1.2 mm in outer diameter.
Recent
Developments
On
March 22, 2021, the Company undertook to issue to the Selling Stockholders included in this prospectus 22,222,223 units (the “Units”)
in exchange for an aggregate purchase price of approximately $20 million (the “Private Placement”). Each Unit consists
of (i) one share of the Company’s Common Stock and (ii) one warrant to purchase one share of Common Stock with an exercise price
of US$1.15 per share (the “Warrant” and the “Exercise Price”). Each Warrant is exercisable
until the close of business on March 31, 2026. Pursuant to the terms of the Warrants, following April 1, 2024, if the closing
price of the Common Stock equal or exceeds 135% of the Exercise Price (subject to appropriate adjustments for stock splits, stock dividends,
stock combinations and other similar transactions after the issue date of the Warrants) for any thirty (30) consecutive trading days,
the Company may force the exercise of the Warrants, in whole or in part, by delivering to the Selling Stockholders a notice of
forced exercise.
Our
Corporate History and Background
We
were incorporated as a corporation under the laws of the State of Nevada on March 22, 2013 under the name Intellisense Solutions
Inc. We were initially engaged in the business of developing web portals to allow companies and individuals to engage in the purchase
and sale of vegetarian food products over the Internet. However, we were unable to execute our original business plan, develop
significant operations or achieve commercial sales.
We
received initial funding in March 2014 in the aggregate amount of $19,980 through the sale of Common Stock to two of our former
officers and directors, who purchased in the aggregate 1,998,000 shares of our Common Stock at $0.01 per share.
On
January 10, 2019, we formed Canna Patch Ltd., or Canna Patch, an Israeli corporation, of which 90% was initially owned by our
Company, and the remaining 10% owned by Rafael Ezra, Canna Patch’s Chief Technology Officer. Canna Patch did not have any
operations and on December 4, 2019, we sold 100% of our holdings in Canna Patch.
On
September 16, 2019, Intellisense and Medigus entered into that certain Exchange Agreement (as defined herein). For additional
information about the Exchange Agreement, refer to “—Certain Relationships and Related Party Transactions” below.
On
December 30, 2019, we acquired ScoutCam Ltd. As a result of our acquisition of ScoutCam Ltd., we now own all of ScoutCam Ltd.’s
issued and outstanding share capital. Following this transaction, we integrated and fully adopted ScoutCam Ltd.’s business into
our Company as our primary business activity. On December 31, 2019, we changed our name to ScoutCam Inc.
ScoutCam
Ltd. was formed in the State of Israel on January 3, 2019 as a wholly-owned subsidiary of Medigus, an Israeli company traded on
the Nasdaq Capital Market, and commenced operations on March 1, 2019. ScoutCam Ltd. was incorporated as part of a reorganization
of Medigus, which was designed to distinguish ScoutCam Ltd.’s miniaturized imaging business, or the micro ScoutCam™
portfolio, from Medigus’ other operations and to enable Medigus to form a separate business unit with dedicated resources
focused on the promotion of such technology. On December 1, 2019, Medigus and ScoutCam Ltd. consummated a certain Amended and
Restated Asset Transfer Agreement, which transferred and assigned certain assets and intellectual property rights related to its
miniaturized imaging business. For additional information about the Amended and Restated Asset Transfer Agreement, refer to “—Certain
Relationships and Related Party Transactions” below. On May 18, 2020, in connection with the Arkin Transaction (as defined
below), the Company and Medigus entered into a certain Side Letter Agreement (the “Letter Agreement”), whereby the
parties agreed to amend certain terms of the Amended and Restated Asset Transfer Agreement and the License Agreement. For additional
information about the Letter Agreement, refer to “—Certain Relationships and Related Party Transactions” below.
On
April 20, 2020, ScoutCam Ltd. entered into an Amended and Restated Intercompany Services Agreement with Medigus (the “Intercompany
Services Agreement”), which effectively amended and restated an intercompany services agreement dated May 30, 2019. For
additional information about the Intercompany Services Agreement, refer to “—Certain Relationships and Related Party
Transactions” below.
Sales
and Marketing
Our
vision is to improve the performance of organizations by offering prestigious tools that enhance the visual technological capabilities
of companies across a variety of industries. Our mission is to become a global leader providing innovative, custom-tailored visualization
solutions to organizations across a variety of industries based on small and highly resistant cameras and supplementary technologies.
Since we are focused on custom-tailored solutions, we have a very limited offering of off-the-shelf products, which are used mainly
as demonstrators for new prospects of our technology and capabilities, rather than as a major source of revenue. Moreover, as
we focus only on the visualization apparatus and supporting components, including for example a small camera (that consists of
a miniature CMOS video sensor, optics, filters, electronics, housing and cables), illumination, cleaning methods (e.g., irrigation),
and/or a mechanical structure based on the customer’s needs, in most cases our products are components of the customer’s
end-user products rather than independent end-user products.
Certain
illustrative examples of our component parts that have been previously integrated into our clients’ end-user products include:

The
Company’s micro ScoutCam™ 6.5 Lum, pictured above, was integrated into a NASA-commissioned project, and as a result
it became the first micro camera utilized in orbit, and thereafter was successfully operated outside the International Space Station
in May 2015 (see: nexis.gsfc.nasa.gov/rrm_phase2vipir.html and youtu.be/O9bmZJATnJs).

Pictured
above is a single-use visualization solution that was developed and sold to A.M. Surgical, which was designed to replace expensive
and bulky reusable endoscopes used in carpal tunnel surgery by their Stratos surgical device. We prepared both wired and wireless
versions. Wireless device was cleared for marketing by the US Food and Drug Administration (FDA) and it is compliant with FCC
regulations.
Our
business model includes engaging companies seeking to add a video visualization to their existing or new product(s) or looking
into developing new products that include micro video visualization. Accordingly, our customer base is exclusively comprised of
businesses, and therefore we are entirely removed from marketing, manufacturing, selling and distributing end-user products to
consumers. Our engagement with businesses is ordinarily conducted in two phases. During the first phase, we conduct the research
and development that is required in order to specify, design, develop, and product the designated visualization apparatus, all
for an agreed compensation (e.g., a non-recurrent engineering fee). During the second phase, we manufacture the apparatus and
sell it to the customer for an agreed transfer price. In some cases, upon a customer’s request, we offer complete ‘turn-key’
contracts, in which we are responsible for most or all product phases, from the specifications phase to the provision of components
or products that are complete, packaged and ready for sale. In such cases, we may conduct the necessary regulatory tests and handle
the required regulatory approvals. In addition, we may also be responsible, as necessary, for, inter alia, packaging, sterilization,
labeling and shipment.
Our
customers include technology-based companies and organizations of all sizes, from early stage start-ups to large, well-established,
international corporations. However, we prefer engaging the latter business partnership as larger corporations provide financial
stability, large purchased quantities, recurring revenue, and valid forecasts for extended durations. In addition, we engage customers
from various industries, such as biomedical, aerospace, certain sensitive or classified industries, security and defense, and
research.
In
order to locate and secure new customers we employ both active and passive marketing strategies. As part of our active approach,
we employ three business development managers who analyze target industries and assess whether micro visualization components
may add value to companies operating in those industries. Once we have identified a potentially relevant industry, we approach
a variety of target companies and market the benefits of integrating our micro visualization components into their products. As
of the current date, we are in the process of expanding our business development team in order to better and more effectively
implement the foregoing marketing strategy. In addition, in order to assist us in identifying such industries and target companies,
we consult with subject matter experts from various industries.
In
addition to the active marketing strategy described above, we also employ a multitude of available marketing channels in order
to increase the exposure of our services to relevant industries. These marketing channels include advertising, participating in
relevant tradeshows and conferences, web-marketing, which includes a well maintained website, Search Engine Optimization (SEO),
social media presence, frequent distributions of press-releases in target countries, as well as conventional marketing means,
including brochures, presentations, etc. Additionally, we issue industry-specific marketing materials that are tailored to highlight
the relevant features of our technology to a specific target industry.
As
described above, we interact with prospects globally in order to engage in and secure new projects by various business development
and marketing means, specifically by way of active and passive marketing measures in order to gather interest from potential customers.
These efforts may include, but are not limited to, the following:
|
|
●
|
engaging
third party companies as territorial representatives in key markets;
|
|
|
|
|
|
|
●
|
initiating
business engagements based on leads received through our website or via other methods or means;
|
|
|
|
|
|
|
●
|
conducting
initial R&D together with such prospects in order to evaluate the feasibility of their contemplated projects;
|
|
|
|
|
|
|
●
|
maintaining
an updated and detailed website presenting our core competency and proven track record;
|
|
|
|
|
|
|
●
|
promoting
our website in different search engines and other digital forums through SEO campaigning as well as other proactive digital
marketing measures;
|
|
|
|
|
|
|
●
|
employing
certain social media platforms for campaigning and advertising;
|
|
|
|
|
|
|
●
|
reconnecting
with our large database, which includes a multitude of past prospects;
|
|
|
|
|
|
|
●
|
developing
and refining marketing communications materials, including digital and printed brochures; and
|
|
|
|
|
|
|
●
|
participating
in major vision technology exhibitions such as AIA Vision Show (USA) and Vision Show (Germany).
|
In
addition to our business development efforts that are mainly based on currently existing or future customer needs, we aim to identify
new market opportunities. These efforts include systematical analysis of industrial fields as well as medical fields and procedures
in order to identify where miniature visualization solutions might benefit and attract value. To this end, we have contracted
business development executives with expertise in these fields that are using various resources and interviewing potential uses
in identifying the most promising opportunities. When a potential opportunity is identified we protect our rights by establishing
the relevant intellectual property safeguards, develop various prototypes that may be relevant for the specific application and
engage the key opinion leaders of that field to validate the feasibility of our solutions. Given that we are not a B2C company,
our business model does not include commercialization of end-user products; nevertheless, we intend to engage relevant companies
to partner with us in order to convert our innovative prototypes into market-ready products, completing the required regulatory
clearances, and commercializing them based on revenue share models.
We
have certain internal procedures in place for when a potential customer is identified, which when triggered helps provide a roadmap
for the ensuing working relationship with that potential customer. Prior to any formal engagement with a potential customer, two
of our departments – business development and R&D – work in parallel and in accordance with their own internal
procedures. The goal of this work is to define an understanding with the customer that will ordinarily incorporate two phases:
(a) an R&D phase, during which the R&D team develops a custom-tailored visualization component that synthesizes our technology
and skill with the customer’s stated requirements, specifications, and business constraints, and which phase generally includes
a formal agreement with respect to the Non-Recurrent Engineering (NRE) fee that is typically payable according to a pre-defined
set of milestones; and (b) a production phase, during which we manufacture and supply the component part for an agreed upon transfer
price.
Over
the years, we have implemented a pricing scheme that allows us to separately price services rendered during the previously described
first phase. Pricing of this first phase is typically prepared by the engineering team, which provides an assessment of the anticipated
costs associated with the R&D of the project, which price will depend on a given customer’s specifications and project
vision. Such costs may include, inter alia, engineering labor, any contracts with sub-contractors, tooling, off-the-shelf and
newly designed components, materials, prototypes production, testing, management overhead, and travel costs. Once we have completed
our cost estimation for the R&D phase, we issue our quote with a certain margin to the customer.
In
order to price the transfer price that will be issued in connection with the aforementioned second phase, the expected Bill-Of
Material (BOM) and Cost-Of-Good Sold (COGS) are established and we price it accordingly with a certain margin to the customer.
Often times there are certain modifications to the original project outlined and agreed upon in the R&D phase, which might
necessitate an increase or decrease to the pricing of the overall project. For that reason, we tend to include a certain margin
of flexibility in the final target transfer price. In addition, we usually link the end transfer price with both annual and per-order
Minimum Order Quantities (MOQ), in order to reflect the actual production quantity of the COGS as well as to commercially incentivize
the customer to order larger quantities.
Both
the negotiation process and the contract drafting are usually done in collaboration with the customer, such that both sides can
verify throughout the process that the final agreement meets their technological and business expectations. Furthermore, we are
keen to maintain close contact with the customer throughout the two phases of our engagement with the customer, including for
example, by way of teleconferences, virtual and actual meetings, document exchanges, on-site visits, and reporting of any completion
of predefined milestones.
Our
Customers
Currently,
we have three major customers that generate most of our current and forecasted revenue in the near term: (1) a large international
bio-med company that is developing a visualization component for its invasive surgical device, (see ‘Customer B’ in
note 11 to our financial statements for the year ended December 31, 2020) (2) a medical device company that specializes in orthopedic
surgeries and develops and markets minimally invasive surgical devices, (see ‘Customer A’ in note 11 to our financial
statements for the year ended December 31, 2020), and (3) a commercial vehicle manufacturer in the aerospace sphere that is engineering
a prototype for remote diagnostics of jet engines, (see ‘Customer C’ in note 11 to our financial statements for the
year ended December 31, 2020).
In
addition to these three material customers, we are engaged in initial negotiations with multiple potential customers operating
in a variety of sectors, including biomedical, aerospace, industry, military and security, and others. We currently consider the
biomedical and aerospace industries to be our core target industries, and from which we receive the greatest level of interest
and demand. We are pursuing these potential engagements with the goal of securing research and development contracts that may
then materialize into multi-year production contracts. We are in various stages of engagement with a variety of customers in all
the above mentioned industries.
In
the biomedical space, for example, we generally seek to partner with medical device and pharmaceutical companies that develop
endoscopes with or without additional functionality. This variation allows the endoscope to be introduced into anatomical parts
that were previously irrelevant within the video-endoscope space either because of the outer diameter and/or price. To this end,
we focus on single-use products that accommodate the global trend to transition from expensive, multi-use products that require
thorough a cleaning protocol, but which cannot be sterilized, to single-use products.
In
the defense and military space, we have partnered with the research and development apparatus of the Israel Defense Forces, specifically
to assist in the development of a small and lightweight “basket of cameras” that can be mounted on either a military-grade
helmet or a balloon-type device, which would enable the viewer to 360 degrees of vision from the mounted vantage point, in addition
to automatic threat detection.
Lastly,
we have recently mobilized efforts to market the possibility of employing the micro ScoutCam™ technology for the purposes
of monitoring bearings and other sensitive mechanical structures in the IAF helicopters and UAVs. Such an application would complement
the rising global market trends associated with Industry 4.0 and Internet of Things (IoT), in which machines are programmed to
test themselves and their production output, which then automatically alerts the processor of any potential problems at the outset
of the endeavor.
Competition
We
previously operated without competition from other companies; however, today there are several companies that offer small cameras,
including, but not limited to, Opcom, Fujikura-Picoramedic, Awaiba, Fisba and Misumi. We, unlike the aforementioned competitors,
offer customized solutions, which includes additional components as needed. Other companies, such as IntraVu, Medit, and SPI Engineering,
offer complete small diameter off-the shelf endoscopes/borescopes. We, however, focus instead on customizing and integrating our
solutions into a given customer’s device. Certain companies, such as Enable, Myriad Fiber Imaging Tech., Inc., and Precision
Optics, act as direct competitors, since they offer similar services.
Proprietary
Rights and Technology
As
we develop customized components and/or products per specific customer requirements, our various projects are constantly in different
stages of development, including: planning, early R&D for a proof of concept, R&D for a prototype, final product/component
development, engineering necessary for a production-ready version, and production of initial batches.
Our
intellectual property rights include such patents and patent licenses that were granted or transferred by Medigus as part of the
Addendum No. 1 to Amended and Restated Asset Transfer Agreement (the “Addendum”), the License Agreement and the Letter
Agreement and additional patent assets developed by ScoutCam and assigned to us from a third party. For additional information
about the License Agreement refer to “—Certain Relationships and Related Party Transactions” below. Under the
Addendum No. 1 to Amended and Restated Asset Transfer Agreement, Medigus transferred five patent families in exchange for a license
in connection with the marketing and sale of the Medigus Ultrasonic Surgical Endostapler. Under the Addendum, and subject to certain
limitations as further set forth therein, Medigus transferred to us the patent families 33209, 29651, 34802, 11777 and 24994,
each of which is further discussed below.
We
currently have rights to a total of five (5) patent families, four of which we consider material to our business and operating
success, and which include the following:
|
|
●
|
Patent
Family 29651 (Integrated Endoscope Irrigation): this patent relates to our ability to develop visualization components and
endoscopes, which include irrigation with a smaller outer diameter by saving the space of the tube that is required to lead
the fluids in the conventional manner. This patent has been granted in Canada, Europe (validated in Germany, Spain, France,
Great Britain, Italy and Ireland), Israel, Japan and the United States, and has a pending continuation in part patent application
in the United States. The expiration date for this patent in the United States patent is December 3rd, 2033 and
in each of the other aforementioned jurisdictions, is February 28, 2033;
|
|
|
|
|
|
|
●
|
Patent
Family 11777 (Multiple View Endoscopes): this patent relates to our ability to develop visualization components and endoscopes,
which include multiple cameras, especially ones that provide side views, and thereby improve the field of view of the visualization
components or endoscopes and provide more information to the user. This patent has been granted and in force in Japan, Mexico,
New Zealand and the United States. The expiration date for this patent in the United States is October 12th, 2021,
and in each of the other aforementioned jurisdictions, is September 6, 2021;
|
|
|
|
|
|
|
●
|
Patent
Family 24994 (Small Diameter Medical Devices Containing Visualization Means): this patent relates to our ability to develop
cameras, visualization components, and endoscopes with a smaller total outer diameter, thus enabling the insertion of the
camera into smaller cavities or leaving more space in the device for the use and application of other functions, such as a
working channel. This patent has been granted in Japan, Korea, Israel and the United States, Europe (validated in Germany,
France, Great Britain and Italy) and also has patent assets pending an opposition appeal in Europe. The expiration dates for
this patent in the United States are April 5, 2032 and March 10, 2031, respectively, and in each of the other aforementioned
jurisdictions, is September 16, 2030;
|
|
|
|
|
|
|
●
|
Patent
Family 33209 (Camera Head): this patent relates to our ability to develop cameras, visualization components, and endoscopes
with a smaller total outer diameter, by reducing the outer diameter of the electronic board on which the sensor is mounted,
thus enabling the insertion of the camera into smaller cavities or leaving more space in the device for the use and application
of other functions, such as a working channel. This patent has been granted in Israel and the United States, and is pending
approval in Canada, Europe, Japan and a continuation in part patent application in the United States. The expiration date
for this patent in Israel is June 11, 2035, and in each of the other aforementioned jurisdictions it is June 9, 2036; and
|
|
|
|
|
|
|
●
|
Patent
Family 34802 (Endoscope-Like Devices Comprising Sensors that Provide Positional Information): this patent relates to our ability
to develop visualization components and endoscopes, which would provide the user with information concerning the spatial position
and angulation of the device when the user is not maintaining eye contact with the device (or, at least, with its distal tip)
due to its presence inside the cavity. Furthermore, this patent allows us to maintain the image in the same direction (e.g.
“north-up”) despite the maneuvers of the device (as performed in cellular phones, for example). This patent is
pending approval in Canada and Japan. The expiration date for this patent, in each of the aforementioned jurisdictions, is
June 1, 2037.
|
Employment
We
currently have 32 full-time (or near full-time) employees. This number is expected to grow. We may recruit additional engineers
to the R&D team, and recruit additional production employees to support an anticipated increase in production commitments
to our customers.
Regulation
Our
approach to regulation is generally determined based on a given project. In our engagements with customers operating in the biomedical
sector, we comply with the medical device standards in that corresponding territory, such as the FDA or International Organization
for Standardization (ISO), among others. Compliance with these regulations is achieved through our QA department and the support
we receive from highly experienced quality assurance and regulatory affairs consultants. In addition, we are being audited annually
by MEDCERT GmbH, a German Notified Body.
For
instance, ISO 13485:2016 is a regulatory benchmark that we comply with while working on our medical device projects. ISO 13845:2016
is similar to ISO 9001 in terms of its quality management system (QMS) requirements, however, ISO 13485:2016 is generally considered
more rigorous and comprehensive.
Given
that we do not manufacture or distribute end-user products, and instead service businesses pursuant to a B2B model, we are subject
to far fewer regulatory standards commonly associated with medical device manufacturers or distributors. We develop components
for other companies that thereafter develop, manufacture and distribute our components, and therefore our involvement in the production
chain demands comparatively less regulatory compliance. This notwithstanding, we are careful to communicate with the business
customer in order to identify certain regulatory dimensions inherent to the project, to which we should pay additional attention.
For example, when a component of ours is integrated into a business’s end-user product, such as for the purpose of touching
human tissue, we develop and manufacture our parts and components while taking into account certain applicable regulatory standards.
These standards might include, inter alia, relevant FDA regulations (e.g. CFR 21 part 820, the medical device reporting
requirements (MDR), among others) as well as ISO regulations (e.g. ISO 14644-1, specifically in connection with cleanrooms and
associated controlled environments, among other items, or ISO 10993, in connection with the biological evaluation of medical devices).
Furthermore, we prioritize our team’s compliance with the Restriction of Hazardous Substances Directives (RoHS) and REACH
(EC 1907/2006).
Similarly,
if a component part of ours is incorporated into an electronic device for the purpose of being used inside a human body, we ensure
compliance with certain FDA requirements as well as IEC 60601 for safety and Electrostatic discharge, including the heating of
parts at more than 42 degrees Celsius, as well as a variety of additional technical standards designed for the safety and essential
performance of medical electrical equipment. Moreover, we perform risk management assessments in accordance with EN ISO 14971:2019
and ISO/TR 24971:2013.
In
certain instances, our customers prefer that we conduct the testing of its products in internationally certified labs in order
to further guarantee our component parts satisfy the applicable regulatory standards. In this scenario, we perform the required
tests as a service to the customer and provide the customer with the official test results, specifically in accordance with ISO/IEC
17025:2017, which the customer can later use in order to apply for the required marketing clearance of its end-user product.
Properties
We
do not own property and currently lease our principal corporate office, which is located at Suite 7A, Industrial Park, P.O. Box
3030, Omer, Israel 8496500. We believe our leased office sufficiently meets our current needs.
MANAGEMENT
Current
Management
The
following table sets forth the names and ages of our directors and executive officers:
|
Name
|
|
Age
|
|
Position
|
|
Prof.
Benad Goldwasser†
|
|
70
|
|
Chairman
of the Board
|
|
Shmuel
Donnerstein†
|
|
68
|
|
Director
|
|
Ronen
Rosenbloom
|
|
49
|
|
Director
|
|
Issac
Zilberman
|
|
69
|
|
Director
|
|
Lior
Amit
|
|
54
|
|
Director
|
|
Moshe
(Mori) Arkin
|
|
68
|
|
Director
|
|
Inbal
Kreiss†
|
|
54
|
|
Director
|
|
Yovav
Sameah*
|
|
48
|
|
Chief
Executive Officer
|
|
Tanya
Yosef*
|
|
38
|
|
Chief
Financial Officer
|
|
Amir
Govrin*
|
|
54
|
|
Chief
Technology Officer
|
|
Katrin
Dlugach*
|
|
38
|
|
VP
of Research and Development
|
|
*
|
Executive
Officer
|
|
†
|
Independent
Director
|
Directors
Prof.
Benad Goldwasser has served as chairman of our board of directors since December 26, 2019, and has served as chairman of ScoutCam
Ltd.’s board of directors since its inception. Prof. Goldwasser is a serial entrepreneur and retired urology medical doctor.
In 2016, Prof. Goldwasser launched a venture capital fund partnered with SAIL, a Shanghai Government investment company. Prof.
Goldwasser has served as a member of the board of directors of Innoventric Ltd. since 2017. From 2013-2016 Prof. Goldwasser served
as an external director of BioCanCell Ltd. (TASE: BICL). Prof. Goldwasser was the co-founder of Vidamed Inc., Medinol Ltd., Rita
Medical Inc., Optonol Ltd. and GI View Ltd. Prof. Goldwasser served as managing director of Biomedical Investments Ltd., an Israeli
Venture Capital firm. During his medical career, he served as Chairman of Urology at the Chaim Sheba Medical Center and Professor
of Surgery at Tel-Aviv University. Prof. Goldwasser holds an MD and MBA from Tel-Aviv University.
Shmuel
Donnerstein has served on our board of directors since December 26, 2019. Mr. Donnerstein is the chairman and owner of the
RB Group. Prior to that, in 1995 Mr. Donnerstein established Open Gallery Door Company, and in 1998 led its merger with Carmiel
Timber Plants, which Mr. Donnerstein had acquired prior to the merger. Mr. Donnerstein managed the combined company until 2006.
Earlier in his career, Mr. Donnerstein was owner and CEO of Motti Sweets from 1975 until it was acquired by the Strauss Group
in 1983. In 2014, Mr. Donnerstein was awarded the Industry Prize from the Manufacturers’ Association of Israel.
Ronen
Rosenbloom has served as a member of our board since December 26, 2019. Mr. Rosenbloom is an independent lawyer working out
of a self-owned law firm specializing in white collar offences. Mr. Rosenbloom serves as chairman of the Israeli Money Laundering
Prohibition committee and the Prohibition of Money Laundering Committee of the Tel Aviv District, both of the Israel Bar Association.
Mr. Rosenbloom previously served as a police prosecutor in the Tel Aviv District. Mr. Rosenbloom holds an LLB from the Ono Academic
College, an Israeli branch of University of Manchester.
Issac
Zilberman has served as a member of our board since December 26, 2019. From 2007 through the end of 2016, Mr. Zilberman also
served as a special investment advisor at Sullam Holdings L.R. Ltd., a financial services corporation in the Lenny Recanati Group,
focusing primarily on investments in high-tech, biotechnology and real estate companies. Mr. Zilberman also serves as a director
in other private Israeli companies, and has over 20 years of prior experience as an executive officer of various public and private
companies. Mr. Zilberman holds a BA in economics and accounting from Tel Aviv University in Tel Aviv, Israel, and he is a certified
public accountant in Israel.
Lior
Amit has served on our board of directors since December 26, 2019. Since 2014, Mr. Amit has served as a financial consultant
to multiple companies on matters related to, inter alia, mergers and acquisitions. Mr. Amit currently serves as a member of the
board of directors for multiple Israeli public and private companies, including in the role of an external or independent director.
Mr. Amit holds both a BA in economics and accounting and an MBA from Tel-Aviv University. Mr. Amit is a certified public accountant
in Israel.
Mr.
Moshe (Mori) Arkin has served on our board of directors since February 15, 2021. Mr. Arkin is a leading life science and pharmaceutical
entrepreneur and serves as the chairman of Arkin Holdings Ltd., which he founded in 2009. Mr. Arkin has served as chairman of
the board of directors of Sol Gel Technologies Ltd. (NASDAQ: SLGL) since 2014 and sits on the board of directors of several private
pharmaceutical and medical device companies, including SoniVie Ltd., a company developing systems for the treatment of pulmonary
arterial hypertension, Digma Medical, a company developing systems to treat insulin resistance present in type 2 diabetes and
other metabolic syndrome diseases, and Valcare Medical, a company developing heart valve devices. From 2005 to 2008, Mr. Arkin
served as the head of generics at Perrigo Company, and from 2005 until 2011, as a member of its board of directors. Prior to joining
Sol Gel Technologies Ltd., Mr. Arkin served as a director of cCAM Biotherapeutics Ltd., a company focused on the discovery and
development of novel immunotherapies to treat cancer from 2012 until its acquisition in 2015 by Merck & Co., Inc. Mr. Arkin
served as chairman of Agis Industries Ltd. from 1972 until its acquisition by Perrigo Company in 2005. Mr. Arkin holds a B.A.
in psychology from the Tel Aviv University, Israel.
Ms.
Inbal Kreiss has served on our board of directors since April 9, 2021. Ms. Kreiss is currently the Head of Innovation at the Systems,
Missiles and Space Division of the Israeli Aerospace Industries Ltd. (IAI) and Chairwoman of RAKIA, Israel’s 2nd Scientific and
Technological Mission to the International Space Station. Since 2013, Ms. Kreiss has served as Deputy Director of the Space Division
at IAI, leading the development, construction, launch and operation of observation and communication satellites for both Israeli and
foreign users. Prior to that, Ms. Kreiss held various leadership positions within IAI, including chief engineer of Israel’s Arrow
2 anti-ballistic missile defense system from 2000 to 2006, and project manager of the Arrow 3 exo-atmospheric interceptor from 2007 to
2013. Ms. Kreiss holds a B.Sc in chemical engineering from the Technion, Israeli Institute of Technology, an Executive Masters in Business
Administration from Tel Aviv University, and completed a visiting research fellowship at the Aeronautics & Astronautics Department
of the Massachusetts Institute of Technology (MIT).
Executive
Officers
Mr.
Yovav Sameah has served as Chief Executive Officer of the Company since April 15, 2021. Prior to his position with the Company,
Mr. Sameah was the Chief Executive Officer of Frontline PCB Solutions, a non-public worldwide leading provider of Pre-Production
and Industry 4.0 SW solutions in the PCB industry, and the subsidiary of KLA-Tencor Corp. (Nasdaq: KLAC). From September 2013
until July of 2015, Mr. Sameah was the Corporate Vice President and Chief Products Officer at Orbotech Ltd. (acquired by KLA-Tencor
in February of 2019). Prior to that, Mr. Sameah held a variety of roles at Orbotech, including Vice President of Electronic Components
Manufacturers Business (PCB Division) from September 2012 until September 2013, and Vice President AOI & Repair Product Line
(PCB Division) from March 2008 until March 2012. Mr. Sameah holds both a BSc in chemical engineering and an MBA from Ben-Gurion
University, Israel.
Tanya
Yosef has served as our Chief Financial Officer since December 27, 2019. Ms. Yosef is a certified public accountant with many
years of experience, and held various positions with Medigus Ltd. (Nasdaq:MDGS) since December of 2009, including most recently
as chief financial officer and prior thereto as financial controller. During 2008-2009 Ms. Yosef worked in the audit department
at Kesselman & Kesselman, a member firm of PricewaterhouseCoopers International Limited. Ms. Yosef holds a BA in Economics
and Accounting from the Ben-Gurion University, Israel.
Mr.
Amir Govrin has served as our Chief Technology Officer since May 1, 2019. Prior to his position with ScoutCam, Mr. Govrin
held various positions at Medigus Ltd. (Nasdaq: MDGS) beginning in 2003, including VP R&D, R&D manager and GERD project
manager. Prior to his tenure at Medigus, Mr. Govrin was project manager at Aran R&D from 1997 until 2003, and an R&D engineer
at Netafim Ltd. from 1992 until 1997. Mr. Govrin holds a B.Sc in mechanical engineering from Tel Aviv University, Israel.
Ms.
Katrin Dlugach has served as our VP of Research and Development since July 1, 2019. Prior to her position with ScoutCam, Ms.
Dlugach was a system engineer and project manager at Nanofabrica Ltd. from August 2018 to June 2019. Before that, Ms. Dlugach
served in a number of roles, including chief of development and chief executive officer, at Nitinotes Ltd. from 2014 until 2018.
Earlier in her career, Ms. Dlugach held a variety of R&D positions at Medigus Ltd. (Nasdaq: MDGS). Ms. Dlugach holds a B.Sc.,
M.Sc. and MBA from Ben-Gurion University, Israel.
Director
Independence
We
currently have three independent directors on our board of directors, Professor Benad Goldwasser, Mr. Shmuel Donnerstein and Ms.
Inbal Kreiss. We are not currently subject to listing requirements of any national securities exchange, which generally stipulates
certain requirements that a majority of a company’s board of directors be classified as “independent”. As a
result, we are not at this time required to have our board of directors comprised of a majority of “independent directors”.
Notwithstanding the foregoing, we have voluntarily adopted the definition of “independent” as defined under Nasdaq
Rule 5605(a)(2), and believe both Professor Goldwasser, Mr. Donnerstein and Ms. Kreiss qualify accordingly.
Board
Leadership Structure and Role in Risk Oversight
Our
board of directors is primarily responsible for overseeing our risk management processes on behalf of the Company. The board of
directors intends going forward to receive and review periodic reports from management, auditors, legal counsel, and others, as
considered appropriate regarding our assessment of risks. The board of directors intends to focus on the most significant risks
facing the Company and our general risk management strategy, and also will attempt to ensure that risks undertaken by the Company
are consistent with our board of directors’ appetite for risk. While the board of directors oversees our risk management,
management is responsible for day-to-day risk management processes.
Board
Committees
Currently,
our board of directors does not have any audit, nominating or compensation committees, or committees performing similar functions.
Director
Relationships
There
are no family relationships between or among any of our directors or executive officers.
EXECUTIVE
COMPENSATION
Summary
Compensation Table
The
following table sets out the compensation paid, for the year ended December 31, 2020, to the following Named Executive Officers:
|
●
|
Dr.
Yaron Silberman, the former Chief Executive Officer of ScoutCam Inc. and the former Chief Executive Officer of our wholly-owned
subsidiary, ScoutCam Ltd.;
|
|
|
|
|
●
|
Amir
Govrin, the Chief Technology Officer of ScoutCam Inc. and of our wholly-owned subsidiary, ScoutCam Ltd.; and
|
|
|
|
|
●
|
Katrin
Dlugach, VP R&D of ScoutCam Inc. and of our wholly-owned subsidiary, ScoutCam Ltd.
|
|
Name and Principal Position
|
|
Year
|
|
|
Salary
|
|
|
Bonus
|
|
|
Stock Awards
|
|
|
Option Awards (*)
|
|
|
All Other Compensation
|
|
|
Total
|
|
|
|
|
$ in thousands
|
|
|
Dr. Yaron Silberman,
Former Chief Executive Officer(1)
|
|
|
2020
|
|
|
$
|
198
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
167
|
|
|
$
|
20
|
|
|
$
|
385
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amir Govrin,
Chief Technology Officer (2)
|
|
|
2020
|
|
|
$
|
168
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
111
|
|
|
$
|
21
|
|
|
$
|
300
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ms. Katrin Dlugach, VP R&D of ScoutCam Ltd. (3)
|
|
|
2020
|
|
|
$
|
156
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
51
|
|
|
$
|
-
|
|
|
$
|
207
|
|
|
(1)
|
Consists
of Dr. Silberman’s compensation earned in his capacity as the Chief Executive Officer of wholly-owned subsidiary, ScoutCam
Ltd. Dr. Silberman did not earn any compensation in his capacity as the Chief Executive Officer of ScoutCam Inc.
|
|
|
|
|
(2)
|
Consists
of Mr. Govrin’s compensation earned in his capacity as the Chief Technology Officer of our wholly-owned subsidiary,
ScoutCam Ltd. Mr. Govrin did not earn any compensation in his capacity as the Chief Technology Officer of ScoutCam Inc.
|
|
|
|
|
(3)
|
Consists
of Ms. Katrin Dlugach compensation earned in his capacity as the Chief Technology Officer of our wholly-owned subsidiary,
ScoutCam Ltd. Ms. Dlugach did not earn any compensation in her capacity as the VP R&D of ScoutCam Inc.
|
|
|
|
|
(*)
|
Represents
the equity-based compensation expenses recorded in the Company’s consolidated financial statements for the year ended
December 31, 2020, based on the option’s fair value, calculated in accordance with accounting guidance for equity-based
compensation.
|
Employment
Agreements
We,
and through our Israeli subsidiary, have entered into written employment agreements with each of our executive officers. All of
these agreements contain customary provisions regarding noncompetition, confidentiality of information and assignment of inventions.
However, the enforceability of the noncompetition provisions may be limited under applicable law. In addition, we have entered
into agreements with each executive officer and director pursuant to which we have agreed to indemnify each of them to the fullest
extent permitted by law to the extent that these liabilities are not covered by directors and officers insurance.
Outstanding
Equity Awards
The
following table provides information concerning unexercised options for each of our named executive officers, as that term is
defined in Item 402(m)(2) of Regulation S-K as of our fiscal year end of December 31, 2020.
|
Name
and Position
|
|
No.
of Securities Underlying Unexercised Options (#) Exercisable
|
|
|
No.
of Securities Underlying Unexercised Options (#) Unexercisable
|
|
|
Option
Exercise
Price ($)
|
|
|
Vesting
Schedule
|
|
Option
Expiration
Date
|
|
Dr.
Yaron Silberman,
|
|
|
291,460
|
|
|
|
374,735
|
|
|
|
0.29
|
|
|
(*)
|
|
February
12, 2027
|
|
Former
Chief Executive Officer (***)
|
|
|
-
|
|
|
|
314,081
|
|
|
|
0.29
|
|
|
(**)
|
|
June
22, 2027
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ms.
Tanya Yosef, Chief Financial Officer
|
|
|
116,584
|
|
|
|
149,894
|
|
|
|
0.29
|
|
|
(*)
|
|
February
12, 2027
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mr.
Amir Govrin, Chief Technology Officer
|
|
|
233,168
|
|
|
|
299,788
|
|
|
|
0.29
|
|
|
(*)
|
|
February
12, 2027
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ms.
Katrin Dlugach, VP R&D
|
|
|
83,274
|
|
|
|
183,204
|
|
|
|
0.29
|
|
|
(*)
|
|
February
12, 2027
|
(*)
25% of the options granted will vest on the first anniversary, and 6.25% of the options will vest at the end of each subsequent
three-month period thereafter over the course of the following three (3) years; and (iii) an acceleration mechanism pursuant to
which any outstanding and unvested option shall immediately accelerate and vest upon the occurrence of certain events, including,
inter alia, a merger or sale of all assets of the Company.
(**)
33.33% of the options granted will vest on the first, and 8.33% of the options will vest at the end of each subsequent three-month
period thereafter over the course of the following two (2) years; and (iii) an acceleration mechanism pursuant to which any outstanding
and unvested option shall immediately accelerate and vest upon the occurrence of certain events, including, inter alia, a merger
or sale of all assets of the Company.
(***)
As a result of the termination of Dr. Silberman’s employment with the Company on March 31, 2021, 647,179 options to purchase
our common stock previously granted to Dr. Silberman terminated concurrently therewith.
Retirement
or Similar Benefit Plans
We
do not have any arrangements or plans that provide for the payment of retirement or similar benefits to our directors or executive
officers.
Resignation,
Retirement, Other Termination, or Change in Control Arrangements
We
have no contract, agreement, plan or arrangement, whether written or unwritten, that provides for payments to our directors or
executive officers at, following, or in connection with the resignation, retirement or other termination of our directors or executive
officers, or a change in control of our Company or a change in our directors’ or executive officers’ responsibilities
following a change in control.
Director
Compensation
The
following table sets out the compensation paid to directors for services rendered during the year ended December 31, 2020.
|
Name
|
|
Fees Earned or
Paid in Cash
|
|
|
Stock Awards
|
|
|
Option Awards (*)
|
|
|
All Other
Compensation
|
|
|
Total
|
|
|
|
|
$ in thousands
|
|
|
Prof. Benad Goldwasser(1)(2)
|
|
$
|
110
|
|
|
$
|
-
|
|
|
$
|
541
|
|
|
$
|
-
|
|
|
$
|
651
|
|
|
Shmuel Donnerstein(3)
|
|
$
|
15
|
|
|
$
|
-
|
|
|
$
|
49
|
|
|
$
|
-
|
|
|
$
|
64
|
|
|
Ronen Rosenbloom(3)
|
|
$
|
15
|
|
|
$
|
-
|
|
|
$
|
20
|
|
|
$
|
-
|
|
|
$
|
35
|
|
|
Issac Zilberman(3)
|
|
$
|
15
|
|
|
$
|
-
|
|
|
$
|
20
|
|
|
$
|
-
|
|
|
$
|
35
|
|
|
Lior Amit(3)
|
|
$
|
15
|
|
|
$
|
-
|
|
|
$
|
27
|
|
|
$
|
-
|
|
|
$
|
35
|
|
|
Irit Yaniv(4) (5)
|
|
$
|
10
|
|
|
$
|
-
|
|
|
$
|
13
|
|
|
$
|
-
|
|
|
$
|
23
|
|
|
(1)
|
Appointed
as a director of ScoutCam Inc. on December 26, 2019, and served as Chairman of the Board of Directors of our wholly-owned
subsidiary, ScoutCam Ltd., since its inception.
|
|
|
|
|
(2)
|
On
July 31, 2019, ScoutCam Ltd. and Prof. Benad Goldwasser entered into a consulting agreement, whereby Prof. Goldwasser agreed
to serve as chairman of the board of directors of ScoutCam Ltd., effective retroactively to March 1, 2019, in consideration
for, inter alia, a monthly fee of $10,000 and options representing 5% of our fully-diluted share capital as of the
Closing Date.
|
|
|
|
|
(3)
|
Appointed
as a director of ScoutCam Inc. on December 26, 2019.
|
|
|
|
|
(4)
|
Appointed
as a director of ScoutCam Inc. on May 18, 2020.
|
|
|
|
|
(5)
|
On
February 14, 2021, Dr. Irit Yaniv tendered her resignation as a member of the Board of Directors and our wholly-owned subsidiary,
ScoutCam Ltd. On February 15, 2021, the Board of Directors appointed Mr. Moshe (Mori)
Arkin to serve as a member of the Board of Directors and to fill the vacancy immediately following the resignation of Dr.
Irit Yaniv.
|
|
|
|
|
(*)
|
Represents
the equity-based compensation expenses recorded in the Company’s consolidated financial statements for the year ended
December 31, 2020, based on the option’s fair value, calculated in accordance with accounting guidance for equity-based
compensation.
|
Equity
Compensation Plan Information
2020
Share Incentive Plan
We
have adopted the 2020 Plan under which we may grant equity-based incentive awards to attract, motivate and retain the talent for
which we compete.
Authorized
Shares. The maximum number of ordinary shares available for issuance under the 2020 Plan is equal to the sum of 9,422,440
shares, or such number as our board of directors may determine from time to time.
Administration.
Our board of directors, or a duly authorized committee of our board of directors, will administer the 2020 Plan. Under the
2020 Plan, the administrator has the authority, subject to applicable law, to interpret the terms of the 2020 Plan and any award
agreements or awards granted thereunder, designate recipients of awards, determine and amend the terms of awards, including the
exercise price of an option award, the fair market value of an ordinary share, the time and vesting schedule applicable to an
award or the method of payment for an award, accelerate or amend the vesting schedule applicable to an award, prescribe the forms
of agreement for use under the 2020 Plan and take all other actions and make all other determinations necessary for the administration
of the 2020 Plan.
The
administrator also has the authority to amend and rescind rules and regulations relating to the 2020 Plan or terminate the 2020
Plan at any time before the date of expiration of its ten year term.
Eligibility.
The 2020 Plan provides for granting awards under various tax regimes, including, without limitation, in compliance with Section
102 of the Israeli Income Tax Ordinance (New Version), 5721-1961 (the “Ordinance”), and Section 3(i) of the Ordinance
and for awards granted to our United States employees or service providers, including those who are deemed to be residents of
the United States for tax purposes, Section 422 of the Code and Section 409A of the Code.
Section
102 of the Ordinance allows employees, directors and officers who are not controlling shareholders and are considered Israeli
residents to receive favorable tax treatment for compensation in the form of shares or options. Our non-employee service providers
and controlling shareholders may only be granted options under section 3(i) of the Ordinance, which does not provide for similar
tax benefits.
Grant.
All awards granted pursuant to the 2020 Plan will be evidenced by an award agreement, in a form approved, from time to time,
by the administrator in its sole discretion. The award agreement will set forth the terms and conditions of the award, including
the type of award, number of shares subject to such award, vesting schedule and conditions (including performance goals or measures)
and the exercise price, if applicable. Certain awards under the 2020 Plan may constitute or provide for a deferral of compensation,
subject to Section 409A of the Code, which may impose additional requirements on the terms and conditions of such awards.
Each
award will expire seven years from the date of the grant thereof, unless such shorter term of expiration is otherwise designated
by the administrator.
Awards.
The 2020 Plan provides for the grant of stock options (including incentive stock options and nonqualified stock options),
shares of common stock, restricted shares, restricted share units and other share-based awards.
Options
granted under the 2020 Plan to our employees who are U.S. residents may qualify as “incentive stock options” within
the meaning of Section 422 of the Code, or may be non-qualified stock options. The exercise price of a stock option may not be
less than 100% of the fair market value of the underlying share on the date of grant (or 110% in the case of ISOs granted to certain
significant stockholders).
Exercise.
An award under the 2020 Plan may be exercised by providing the company with a written or electronic notice of exercise and
full payment of the exercise price for such shares underlying the award, if applicable, in such form and method as may be determined
by the administrator and permitted by applicable law. An award may not be exercised for a fraction of a share. With regard to
tax withholding, exercise price and purchase price obligations arising in connection with awards under the 2020 Plan, the administrator
may, in its discretion, accept cash, provide for net withholding of shares in a cashless exercise mechanism or direct a securities
broker to sell shares and deliver all or a part of the proceeds to the Company or the trustee.
Transferability.
Other than by will, the laws of descent and distribution or as otherwise provided under the 2020 Plan, neither the options
nor any right in connection with such options are assignable or transferable.
Termination
of Employment. In the event of termination of a grantee’s employment or service with the company or any of its affiliates,
all vested and exercisable awards held by such grantee as of the date of termination may be exercised within three months after
such date of termination, unless otherwise determined by the administrator. After such three month period, all such unexercised
awards will terminate and the shares covered by such awards shall again be available for issuance under the 2020 Plan.
In
the event of termination of a grantee’s employment or service with the company or any of its affiliates due to such grantee’s
death, permanent disability or retirement, all vested and exercisable awards held by such grantee as of the date of termination
may be exercised by the grantee or the grantee’s legal guardian, estate, or by a person who acquired the right to exercise
the award by bequest or inheritance, as applicable, within twelve months after such date of termination, unless otherwise provided
by the administrator. Any awards which are unvested as of the date of such termination or which are vested but not then exercised
within the twelve month period following such date, will terminate and the shares covered by such awards shall again be available
for issuance under the 2020 Plan.
Notwithstanding
any of the foregoing, if a grantee’s employment or services with the company or any of its affiliates is terminated for
“cause” (as defined in the 2020 Plan), all outstanding awards held by such grantee (whether vested or unvested) will
terminate on the date of such termination and the shares covered by such awards shall again be available for issuance under the
2020 Plan.
Transactions.
In the event of a share split, reverse share split, share dividend, recapitalization, combination or reclassification of our
shares, or any other increase or decrease in the number of issued shares effected without receipt of consideration by the company
(but not including the conversion of any convertible securities of the company), the administrator in its sole discretion shall
make an appropriate adjustment in the number of shares related to each outstanding award and to the number of shares reserved
for issuance under the 2020 Plan, to the class and kind of shares subject to the 2020 Plan, as well as the exercise price per
share of each outstanding award, as applicable, the terms and conditions concerning vesting and exercisability and the term and
duration of outstanding awards, or any other terms that the administrator adjusts in its discretion, or the type or class of security,
asset or right underlying the award (which need not be only that of the Company, and may be that of the surviving corporation
or any affiliate thereof or such other entity party to any of the above transactions); provided that any fractional shares resulting
from such adjustment shall be rounded down to the nearest whole share unless otherwise determined by the administrator. In the
event of a distribution of a cash dividend to all shareholders, the administrator may determine, without the consent of any holder
of an award, that the exercise price of an outstanding and unexercised award shall be reduced by an amount equal to the per share
gross dividend amount distributed by the Company, subject to applicable law.
In
the event of a merger or consolidation of our company, or a sale of all, or substantially all, of the Company’s shares or
assets or other transaction having a similar effect on the Company, or change in the composition of the board of directors, or
liquidation or dissolution, or such other transaction or circumstances that the board of directors determines to be a relevant
transaction, then without the consent of the grantee, the administrator may but is not required to (i) cause any outstanding award
to be assumed or substituted by such successor corporation, or (ii) regardless of whether or not the successor corporation assumes
or substitutes the award (a) provide the grantee with the option to exercise the award as to all or part of the shares, and may
provide for an acceleration of vesting of unvested awards, or (b) cancel the award and pay in cash, shares of the company, the
acquirer or other corporation which is a party to such transaction or other property as determined by the administrator as fair
in the circumstances. Notwithstanding the foregoing, the administrator may upon such event amend, modify or terminate the terms
of any award as it shall deem, in good faith, appropriate.
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Related
Party Transactions
On
June 3, 2019, Medigus executed a capital contribution into ScoutCam Ltd. for an aggregate amount of $720,000.
On
July 31, 2019, ScoutCam Ltd. and Prof. Benad Goldwasser entered into a consulting agreement, whereby Prof. Goldwasser agreed to
serve as chairman of the board of directors of ScoutCam Ltd., effective retroactively to March 1, 2019, in consideration for,
inter alia, a monthly fee of $10,000 and options representing 5% of our fully-diluted share capital as of the Closing Date.
On
August 27, 2019, Medigus provided ScoutCam Ltd. with a line of credit in the aggregate amount of US$500,000, and, in exchange,
ScoutCam Ltd. granted Medigus a capital note that bears an annual interest rate of 4%. The repayment of the credit line amount
is spread over one year in monthly payments beginning on the Closing Date, being January 2020. As of December 31, 2019, ScoutCam
Ltd. withdrew the entire amount of the line of credit.
On
September 3, 2019, a certain Asset Transfer Agreement by and between ScoutCam Ltd. and Medigus dated May 28, 2019 became effective,
whereby, inter alia, ScoutCam Ltd. transferred certain assets to Medigus representing an aggregate amount of $168,000.
Under the terms of the Amended and Restated Asset Transfer Agreement, Medigus transferred certain intellectual property rights
and licenses, collectively representing an aggregate of $9.8 million.
On
September 16, 2019, Intellisense and Medigus entered into the Exchange Agreement, pursuant to which Medigus assigned, transferred
and delivered 100% of its holdings in ScoutCam Ltd. to Intellisense, in exchange for consideration consisting of shares of the
Company’s common stock representing 60% of the issued and outstanding share capital of the Company immediately upon the
Closing Date. The Exchange Agreement was conditioned on certain obligations by the respective parties, including, but not limited
to, the Company having no less than $3 million in cash on hand upon the Closing Date, and that the Company bear the costs and
expenses in connection with the execution of the Exchange Agreement. The Exchange Agreement provided that if ScoutCam Ltd. achieves
an aggregate amount of $33 million in sales within the first three years immediately after the Closing Date, the Company will
issue to Medigus 2,688,492 shares of the Company’s common stock, which represents 10% of the Company’s issued and
outstanding share capital as of the Closing Date.
On
December 1, 2019, Medigus and ScoutCam Ltd. entered into that certain Amended and Restated Asset Transfer Agreement, which transferred
and assigned certain assets and intellectual property rights related to its miniaturized imaging business. Under the Amended and
Restated Asset Transfer Agreement, Medigus transferred two patent families to ScoutCam Ltd. in exchange for a perpetual, transferable,
worldwide, royalty free, sub licensable license, to access and use the transferred patent families in connection with the development,
marketing and sale of the Medigus Ultrasonic Surgical Endostapler. In addition, Medigus granted us a non-exclusive license to
access, use, improve, develop, market and sell licensed intellectual property, including the right to any future versions, enhancements,
improvements and derivative works of such licensed intellectual property in connection with the development and commercialization
of the ScoutCam miniature video technology.
As
a condition of the aforementioned license, Medigus is prohibited from selling, offering to sell or grant any ownership right in
the licensed intellectual property to any potential direct competitor of ScoutCam Ltd. In addition, ScoutCam Ltd. is obligated
to provide Medigus with consultancy and support services for no consideration, on matters relating to the management, development,
maintenance and commercialization of Medigus’ patent portfolio. The Amended and Restated Asset Transfer Agreement is for
an indefinite term and it was contractually permissible to terminate the agreement pursuant to the mutual written consent of the
parties prior to closing.
Also
on December 1, 2019, ScoutCam Ltd. and Medigus entered into that certain License Agreement granting ScoutCam Ltd. a perpetual,
non-exclusive, transferable solely upon an M&A Event (as defined therein), royalty free, license to access, use, improve,
develop either by or on behalf of ScoutCam Ltd., market and sell the licensed patent family, including the right to any future
versions, enhancements, improvements and derivative works of the licensed intellectual property for the purpose of developing
and commercializing the ScoutCam miniature video technology. As a condition to the agreement, Medigus is prohibited from selling,
offering to sell or grant any ownership right in the licensed intellectual property to any potential direct competitor of ScoutCam
Ltd.
The
patent family licensed under the License Agreement includes know-how which was funded through benefits and incentives provided
by the IIA. As a result of such funding, the patent family is subject to certain restrictions and obligations pursuant to the
Innovation Law. The restrictions applicable to patent family licensed pursuant to the License Agreement require approval of the
IIA prior to manufacturing products resulting from IIA funded know-how outside of Israel, prior to the transfer of IIA funded
know-how out of Israel and prior to a grant of the license out of Israel in connection with the IIA funded know-how. In addition,
ScoutCam Ltd. is obligated to notify the IIA of any change of control and of any non-Israeli entity which becomes an “Interested
Party” as defined in the Israeli Companies Law, 5759-1999, as amended. An Interested Party includes a shareholder holdings
5% or more of a company’s issued and outstanding share capital, an entity entitled to appoint a director or the chief executive
officer of a company as well as the directors and chief executive officer of a company.
On
December 10, 2019, ScoutCam Ltd. and Shrem Zilberman Group Ltd. (the “Consultant”) entered into a consulting agreement
whereby in exchange for certain consulting services, the Consultant received, among other things, an aggregate flat fee of $165,000
and an amount representing 3% of any exercise price related to those warrants issued as part of that certain Securities Purchase
Agreement executed by and between the Company and those investors listed therein (the “Purchase Agreement”). Additionally,
in the event the total proceeds received as a result of exercise of warrants issued in connection with the Purchase Agreement
will be less than $2 million at the time of their expiration, the Consultant will be required to invest $250,000 in the Company.
On
March 15, 2020, the Company’s Board of Directors approved, among other things, a quarterly fee of $4,000 payable to each
of the Company’s currently serving directors, excluding Professor Benad Goldwasser.
On
April 20, 2020, Medigus and ScoutCam Ltd. entered into that certain Intercompany Services Agreement, which amended and restated
the intercompany services agreement executed between the parties on May 30, 2019. The agreement has an initial term of one year,
and renews automatically for additional one-year periods, unless either party provides 60 (sixty) days written notice of non renewal.
Either Medigus or ScoutCam Ltd. may terminate the agreement for convenience upon providing 60 (sixty) days prior written notice.
The services to be provided by ScoutCam Ltd. include, inter alia, the provision of office space, utilities, car services, insurance
and chief financial officer services. In consideration for the foregoing services, ScoutCam Ltd. is entitled to arm’s length
service fees based on the most recent transfer pricing analysis as performed by an external expert, which may be adjusted from
time to time.
On
May 18, 2020, the Company entered into and consummated a securities purchase agreement with M. Arkin (1999) Ltd. (“Arkin
Ltd.”) in connection with the sale and issuance of 2,066,116 units (“Arkin Units”), at a purchase price of US$0.968
per Arkin Unit, and for an aggregate purchase price of US$2,000,000 (the “Arkin Transaction”). Each Arkin Unit consists
of: (i) two shares of Common Stock and (ii) (a) one warrant to purchase one share of Common Stock with an exercise price of US$0.595
(“Arkin Warrant A”) and (b) two warrants, each to purchase one share of Common Stock with an exercise price of US$0.893
(“Arkin Warrant B”, and together with Arkin Warrant A, the “Arkin Warrants”). The shares of Common Stock
and Arkin Warrants were issued to Arkin Ltd. pursuant to Regulation S of the Securities Act of 1933, as amended.
On
May 18, 2020, and in connection with the Arkin Transaction, the Company, Medigus and Arkin Ltd. entered into a Voting Agreement,
pursuant to which Arkin Ltd. and Medigus each agreed to vote their respective shares of Common Stock in favor of the election
of the opposite party’s designated representative(s), as applicable, to the Board. Each of Arkin Ltd.’s and Medigus’
rights under the Voting Agreement are contingent upon, inter alia, such party maintaining a certain beneficial ownership threshold
in the Company, as defined therein.
Also
on May 18, 2020, in connection with the Arkin Transaction, the Company, Medigus and Arkin, entered into the Letter Agreement, whereby, provided the Company
obtains certain regulatory approvals described therein, Medigus and the Company agreed to amend certain terms of the Amended and
Restated Asset Transfer Agreement and the License Agreement, thereby transferring outright certain patent assets from Medigus
to the Company; provided, however, that in the event the Company neglects the foregoing patent assets, the Company must transfer
back ownership of the patent assets to Medigus for no additional consideration and absent any additional contingencies.
On
June 23, 2020, the Company and Medigus entered into a certain Conversion Side Letter, pursuant to which the Company converted
US$381,136 worth of outstanding credit previously extended by Medigus to the Company, which amount, as of the date thereof, included
interest accrued thereon. In accordance with the terms of the Conversion Side Letter, the Company issued to Medigus, at a purchase
price of US$0.968, (a) 787,471 shares of Common Stock, (b) warrants to purchase 393,736 shares of Common Stock at an exercise
price of US$0.595, and (c) warrants to purchase 787,471 shares of Common Stock at an exercise price of US$0.893.
In
November 2020, the Company and certain of warrant holders, including Professor Benad Goldwasser and Arkin Ltd., executed
an amendment in connection with previously issued warrants to purchase shares of Common Stock, pursuant to which
the parties agreed to remove the restrictions on transferability originally imposed on said warrants. As of December 31, 2020,
warrants to purchase 902,271 shares of Common Stock were transferred in accordance with the foregoing amendment.
During
2020, the Company’s Board of Directors authorized the allotment of options to purchase 2,863,854 shares of Common Stock
to Prof. Benad Goldwasser, our Chairman of the Board, and an aggregate of 3,625,318 options to purchase shares of Common Stock
to additional directors and certain officers of the Company. See also note 9 to our financial statements for year ended December
31, 2020.
On
March 22, 2021, the Company undertook to issue to the Selling Stockholders 22,222,223 units (the “Units”) in
exchange for an aggregate purchase price of approximately $20 million (the “Private Placement”). Each Unit
consists of (i) one share of Common Stock and (ii) one warrant to purchase one share of Common Stock with an exercise price of
US$1.15 per share (the “Warrant” and the “Exercise Price”). Each Warrant is exercisable
until the close of business on March 31, 2026. Pursuant to the terms of the Warrants, following April 1, 2024, if the closing
price of the Common Stock equal or exceeds 135% of the Exercise Price (subject to appropriate adjustments for stock splits, stock
dividends, stock combinations and other similar transactions after the issue date of the Warrants) for any thirty (30) consecutive
trading days, the Company may force the exercise of the Warrants, in whole or in part, by delivering to the Investors a notice of
forced exercise. The shares of Common Stock and the Warrants were issued to the Investors pursuant to Regulation S of the Securities
Act of 1933, as amended. The securities issued in connection with the Private Placement are being registered for resale under this
registration statement on Form S-1.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Security
Ownership of Certain Beneficial Owners and Management
The
table below provides information regarding the beneficial ownership of our Common Stock as of April 13, 2021, of (i) each of our
current directors, (ii) each of the Named Executive Officers, (iii) all of our current directors and officers as a group, and
(iv) each person or entity known to us who owns more than 5% of our Common Stock.
The
percentage of Common Stock beneficially owned is based on 60,295,245 shares of Common Stock outstanding as of April 13, 2021.
The number and percentage of shares beneficially owned by a person or entity also include shares of Common Stock issuable upon
exercise of warrants that are currently exercisable or will become exercisable within 60 days of April 13, 2021. However, these
shares are not deemed to be outstanding for the purpose of computing the percentage of shares beneficially owned of any other
person or entity.
Unless
otherwise indicated below, the address for each beneficial owner listed in the table below is c/o ScoutCam Inc., Suite 7A, Industrial
Park, P.O. Box 3030, Omer, Israel 8496500.
|
Name and Address of Beneficial Owner
|
|
Title of Class
|
|
Amount and Nature
of Beneficial
Ownership(1)
|
|
|
Percent of Class
|
|
|
Prof. Benad Goldwasser(2)
|
|
Common Stock
|
|
|
2,092,359
|
|
|
|
3.38
|
%
|
|
Shmuel Donnerstein(3)
|
|
Common Stock
|
|
|
1,082,104
|
|
|
|
1.78
|
%
|
|
Ronen Rosenbloom(4)
|
|
Common Stock
|
|
|
48,069
|
|
|
|
*
|
|
|
Isaac Zilberman(5)
|
|
Common Stock
|
|
|
48,069
|
|
|
|
*
|
|
|
Lior Amit(6)
|
|
Common Stock
|
|
|
48,069
|
|
|
|
*
|
|
|
Inbal Kreiss
|
|
Common Stock
|
|
|
-
|
|
|
|
-
|
|
|
Moshe (Mori) Arkin(7)
|
|
Common Stock
|
|
|
14,330,580
|
|
|
|
21.03
|
%
|
|
Yovav Sameah
|
|
Common Stock
|
|
|
-
|
|
|
|
-
|
|
|
Tanya Yosef(8)
|
|
Common Stock
|
|
|
149,893
|
|
|
|
*
|
|
|
Amir Govrin(9)
|
|
Common Stock
|
|
|
299,787
|
|
|
|
*
|
|
|
Katrin Dlugach(10)
|
|
Common Stock
|
|
|
116,584
|
|
|
|
*
|
|
|
Directors and officers as a group (11 individuals)
|
|
|
|
|
18,215,514
|
|
|
|
25.65
|
%
|
|
Medigus Ltd. (11)
|
|
Common Stock
|
|
|
18,099,630
|
|
|
|
29.44
|
%
|
|
M. Arkin (1999) Ltd. (12)
|
|
Common Stock
|
|
|
14,330,580
|
|
|
|
21.03
|
%
|
|
The More Group (13)
|
|
Common Stock
|
|
|
7,777,778
|
|
|
|
12.12
|
%
|
|
The Phoenix Group (14)
|
|
Common Stock
|
|
|
12,222,224
|
|
|
|
18.41
|
%
|
|
The Psagot Group (15)
|
|
Common Stock
|
|
|
11,111,112
|
|
|
|
16.87
|
%
|
|
Noked Long Limited Partnership (16)
|
|
Common Stock
|
|
|
3,333,334
|
|
|
|
5.38
|
%
|
*
Less than 1%
|
(1)
|
Beneficial
ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect
to securities. Each of the beneficial owners named in the table have, to our knowledge, direct ownership of and sole voting
and investment power with respect to the shares of Common Stock beneficially owned by them.
|
|
|
|
|
(2)
|
Consists
of 395,464 shares of Common Stock, options to purchase 1,490,088 shares of Common Stock and warrants to purchase 206,807 shares
of Common Stock, which are currently exercisable or will become exercisable within 60 days of April 13, 2021.
|
|
|
|
|
(3)
|
Consists
of 620,421 shares of Common Stock, options to purchase 48,069 shares of Common Stock and warrants to purchase 413,614 shares
of Common Stock, which are currently exercisable or will become exercisable within 60 days of April 13, 2021.
|
|
|
|
|
(4)
|
Consists
of 48,069 options to purchase shares of Common Stock, which are currently exercisable or will become exercisable within 60
days of April 13, 2021.
|
|
|
|
|
(5)
|
Consists
of 48,069 options to purchase shares of Common Stock, which are currently exercisable or will become exercisable within 60
days of April 13, 2021.
|
|
|
|
|
(6)
|
Consists
of 48,069 options to purchase shares of Common Stock, which are currently exercisable or will become exercisable within 60
days of April 13, 2021.
|
|
(7)
|
Mr.
Moshe Arkin is the sole shareholder and sole director of M. Arkin (1999) Ltd. and may therefore be deemed to be the indirect
beneficial owner of the shares of Common Stock and warrants to purchase shares of Common Stock owned directly by M. Arkin
(1999) Ltd.
|
|
|
|
|
(8)
|
Consists
of 149,893 options to purchase shares of Common Stock, which are currently exercisable or will become exercisable within 60
days of April 13, 2021.
|
|
|
|
|
(9)
|
Consists
of 299,787 options to purchase shares of Common Stock, which are currently exercisable or will become exercisable within 60
days of April 13, 2021.
|
|
|
|
|
(10)
|
Consists
of 116,584 options to purchase shares of Common Stock, which are currently exercisable or will become exercisable within 60
days of April 13, 2021.
|
|
|
|
|
(11)
|
Consists
of 16,918,423 shares of Common Stock and warrants to purchase 1,181,207 shares of Common Stock, which are currently exercisable
or will become exercisable within 60 days of April 13, 2021.
|
|
|
|
|
(12)
|
Consists
of 6,468,367 shares of Common Stock and 7,862,213 warrants to purchase shares of Common Stock, which are currently exercisable
or will become exercisable within 60 days of April 13, 2021.
|
|
|
|
|
(13)
|
The
More Group consists of (i) More Provident Funds LTD – Alfa More Provident Funds – Bond 25%, (ii) More Provident
Funds – Advanced Study Fund – Bond 25%, (iii) More Provident Funds LTD – Investment Fund – Bond
25%, (iv) More Provident Funds LTD – Alfa More Provident Age 50, (v) More Provident Funds LTD – Alfa More
Provident Over Age 60, (vi) More Provident Funds LTD – Alfa More Provident Fund 50 – 60, (vii) More Provident
Funds LTD – Alfa More Provident Fund Stock, (viii) More Provident Funds LTD – Investment Fund – Stocks,
(ix) More Provident Funds LTD – Investment Fund – General, (x) More Provident Funds – Advanced Study
Fund – Stocks, and (xi) More Provident Funds LTD – Advanced Study Fund – General, of which hold 3,888,889
shares of Common Stock and warrants to purchase 3,888,889 shares of Common Stock, which are currently exercisable or will
become exercisable within 60 days of April 13, 2021.
|
|
|
|
|
(14)
|
The
Phoenix Group consists of (i) The Phoenix insurance LTD and (ii) Shotfut Menayot Israel Phoenix Amitim, of which hold 6,111,112
shares of Common Stock and 6,111,112 warrants to purchase shares of Common Stock, which are currently exercisable or will
become exercisable within 60 days of April 13, 2021.
|
|
|
|
|
(15)
|
The
Psagot Group consists of (i) Kranot Hishtalmut Le Morim ve Gananot Hevra Menahelet LTD, (ii) Gal Provident Fund Management
for Teachers LTD, (iii) Gal Provident Fund Management for Teachers LTD - for Kupat Gemel Kalanit, (iv) OS – Hevra Lenihul
Kupot Gemel LTD, (v) Yahav P.R.H Provident Funds Management Company LTD, (vi) K.S.M - Keren Hishtalmut le Biochimaim LTD,
(vii) Provident Fund of the Employees of Haifa Municipality, (viii) Aram Gmulim Provident Fund Management LTD, (ix) Kav Habriut
- Provident Fund Management LTD Israel, (x) Mishpetanim Education Fund for Lawyers Management Company LTD, (xi) Management
Company of the Israeli Judges Study Fund LTD, (xii) PARETO HF, (xiii) Reut Management Company of Provident Fund LTD, (xiv)
Further Education Found for Liberal Arts and Social Scince LTD, (xv) WESURE INSURANCE, (xvi) Yahav Physicians Management Company
for Provident Funds LTD, (xvii) Kelah - Social Workers Study Fund Management Company LTD, (xviii) The Management Company of
I.E.C. Workers Education Fund LTD, and (xix) Gal Provident Fund Management for Teachers LTD - for Kupat Gemel Hagomel, of
which hold 5,555,556 shares of Common Stock and warrant to purchase 5,555,556 shares of Common Stock which are currently exercisable
or will become exercisable within 60 days of April 13, 2021.
|
|
|
|
|
(16)
|
Consists
of 1,666,667 shares of Common Stock and 1,666,667 warrants to purchase shares of Common Stock, which are currently exercisable
or will become exercisable within 60 days of April 13, 2021.
|
DESCRIPTION
OF CAPITAL STOCK
Our
authorized capital stock consists of 300,000,000 shares of Common Stock, par value $0.001 per share.
Common
Stock
Of
the authorized Common Stock, 60,295,245 shares are outstanding as of April 13, 2021. The holders of our Common Stock are entitled
to receive dividends from our funds legally available therefor only when, as and if declared by our board of directors, and are
entitled to share ratably in all of our assets available for distribution to holders of our Common Stock upon the liquidation,
dissolution or winding-up of our affairs. Holders of our Common Stock do not have any preemptive, subscription, redemption or
conversion rights. Holders of our Common Stock are entitled to one vote per share on all matters which they are entitled to vote
upon at meetings of stockholders or upon actions taken by written consent pursuant to Nevada corporate law. The holders of our
Common Stock do not have cumulative voting rights, which mean that the holders of a plurality of the outstanding shares can elect
all of our directors. All of the shares of our Common Stock currently issued and outstanding are fully-paid and nonassessable.
No dividends have been paid to holders of our Common Stock since our incorporation, and no cash dividends are anticipated to be
declared or paid in the reasonably foreseeable future.
Anti-Takeover
Effects of Nevada Law
Business
Combination
The
“business combination” provisions of Sections 78.411 to 78.444, inclusive, of the Nevada Revised Statutes, or the
NRS, generally prohibit a Nevada corporation with at least 200 stockholders from engaging in various “combination”
transactions with any interested stockholder for a period of three years after the date of the transaction in which the person
became an interested stockholder, unless the transaction is approved by the board of directors prior to the date the interested
stockholder obtained such status; and extends beyond the expiration of the three-year period, unless:
|
|
●
|
the
transaction was approved by the board of directors prior to the person becoming an interested stockholder or is later approved
by a majority of the voting power held by disinterested stockholders, or
|
|
|
|
|
|
|
●
|
if
the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per
share paid by the interested stockholder within the three years immediately preceding the date of the announcement of the
combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value
per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired
the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock,
if it is higher.
|
A
“combination” is generally defined to include mergers or consolidations or any sale, lease exchange, mortgage, pledge,
transfer or other disposition, in one transaction or a series of transactions, with an “interested stockholder” having:
(a) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation, (b) an aggregate
market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation, (c) 10% or more of
the earning power or net income of the corporation, and (d) certain other transactions with an interested stockholder or an affiliate
or associate of an interested stockholder.
In
general, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within three
years, did own) 10% or more of a corporation’s voting stock. The statute could prohibit or delay mergers or other takeover
or change in control attempts and, accordingly, may discourage attempts to acquire the Company even though such a transaction
may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Control
Share Acquisition
The
“control share” provisions of Sections 78.378 to 78.3793, inclusive, of the NRS apply to “issuing corporations,”
which are Nevada corporations with at least 200 stockholders, including at least 100 stockholders of record who are Nevada residents,
and which conduct business directly or indirectly in Nevada. The control share statute prohibits an acquirer, under certain circumstances,
from voting its shares of a target corporation’s stock after crossing certain ownership threshold percentages, unless the
acquirer obtains approval of the target corporation’s disinterested stockholders. The statute specifies three thresholds:
one-fifth or more but less than one-third, one-third but less than a majority, and a majority or more, of the outstanding voting
power. Generally, once an acquirer crosses one of the above thresholds, those shares in an offer or acquisition and acquired within
90 days thereof become “control shares” and such control shares are deprived of the right to vote until disinterested
stockholders restore the right. These provisions also provide that if control shares are accorded full voting rights and the acquiring
person has acquired a majority or more of all voting power, all other stockholders who do not vote in favor of authorizing voting
rights to the control shares are entitled to demand payment for the fair value of their shares in accordance with statutory procedures
established for dissenters’ rights.
A
corporation may elect to not be governed by, or “opt out” of, the control share provisions by making an election in
its articles of incorporation or bylaws, provided that the opt-out election must be in place on the tenth day following the date
an acquiring person has acquired a controlling interest, that is, crossing any of the three thresholds described above. We have
not opted out of the control share statutes, and will be subject to these statutes if we are an “issuing corporation”
as defined in such statute.
At
this time, we do not have 100 stockholders of record resident in Nevada. Therefore, the provisions of the control share acquisition
act do not apply to acquisitions of our shares and will not until such time as these requirements have been met. At such time
as they may apply to us, the provisions of the control share acquisition act may discourage companies or persons interested in
acquiring a significant interest in or control of the Company, regardless of whether such acquisition may be in the interest of
our stockholders.
Shares
Eligible for Future Sale
Rule
144
|
|
●
|
Pursuant
to Rule 144 of the Securities Act, a person who has beneficially owned restricted shares of our common stock or warrants for
at least six months (or longer in the case of former shell companies as described below) would be entitled to sell their securities
provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the three
months preceding, a sale, (ii) we are subject to the Exchange Act reporting requirements for at least 90 days before the sale
and (iii) if the sale occurs prior to satisfaction of a one-year holding period, we provide current information at the time
of sale.
|
|
|
|
|
|
|
●
|
Persons
who have beneficially owned restricted shares of our common stock or warrants for at least six months but who are our affiliates
at the time of, or at any time during the three months preceding, a sale, would be subject to additional restrictions, by
which such person would be entitled to sell within any three-month period only a number of securities that does not exceed
the greater of: 1% of total shares outstanding and the average weekly trading volume of such securities during the four calendar
weeks preceding the filing of a 144 notice with respect to such sale (which average volume criteria only applies if the company’s
securities become listed on Nasdaq or an exchange).
|
These
provisions are, in each case, dependent on the Company being subject to the Exchange Act periodic reporting requirements for at
least three months before the sale. However, since our shares are quoted on the OTC Markets, which is not an “automated
quotation system”, our stockholders will not be able to rely on the market-based volume limitation described in the second
bullet above. If, in the future, our securities are listed on an exchange or quoted on Nasdaq, then our stockholders would be
able to rely on the market-based volume limitation. Unless and until our stock is so listed or quoted, our stockholders can only
rely on the percentage based volume limitation described in the first bullet above.
Such
sales by affiliates must also comply with the manner of sale, current public information and notice provisions of Rule 144. The
selling stockholders will not be governed by the foregoing restrictions when selling their shares pursuant to this prospectus.
Restrictions
on the Use of Rule 144 by Shell Companies or Former Shell Companies
Our
Company was a shell company prior to December 30, 2019. The SEC has prohibited the use of Rule 144 for resale of securities issued
by any shell companies (other than business combination related shell companies) or any issuer that has been at any time previously
a shell company. The SEC has provided an exception to this prohibition, however, if the following conditions are met:
|
|
●
|
the
issuer of the securities that was formerly a shell company has ceased to be a shell company;
|
|
|
|
|
|
|
●
|
the
issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act;
|
|
|
|
|
|
|
●
|
the
issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding
12 months (or such shorter period that the issuer was required to file such reports and materials), other than Current Reports
on Form 8-K; and
|
|
|
|
|
|
|
●
|
at
least one year has elapsed from the time that the issuer filed current comprehensive disclosure with the SEC reflecting its
status as an entity that is not a shell company.
|
SELLING
STOCKHOLDERS
Beneficial
Ownership and Other Information
We
are registering the resale of up to 22,222,223 outstanding shares of Common Stock, which are held by, or may be issued to, the
Selling Stockholders, and an additional aggregate of 22,222,223 shares of Common Stock that the Selling Stockholders may acquire
upon exercise of warrants that we have issued to them pursuant to equity financings.
In
this prospectus, the term “Selling Stockholders” includes (i) the entities identified in the table below (as such
table may be amended from time to time by means of an amendment to the registration statement of which this prospectus forms a
part) and (ii) any donees, pledgees, transferees or other successors-in-interest that acquire any of the shares of Common Stock
covered by this prospectus after the date of this prospectus from the Selling Stockholders as a gift, pledge, partnership distribution
or other non-sale related transfer.
The
registration of the resale of the shares of Common Stock covered by this prospectus does not necessarily mean that the Selling
Stockholders will acquire (if not already held by them) or resell any or all of those shares.
The
information in the table below is based upon information provided by the Selling Stockholders. The percentage of Common Stock owned by
each of the Selling Stockholders (including shares underlying warrants that we have issued to them pursuant to equity financings)
is based on 60,295,245 shares of Common Stock outstanding as of April 13, 2021. To the best of our knowledge, the Selling Stockholders
do not have an agreement or understanding, directly or indirectly, with any person to distribute the shares of Common Stock at the time
that they entered into the equity financing agreement under which they have been issued or may be issued such shares.
|
|
|
Shares of Common Stock Owned Prior to this Offering(1)
|
|
|
Maximum Number of Shares of Common Stock to be Sold Pursuant to this Prospectus(2)
|
|
|
Name of Selling Stockholder(3)
|
|
Number
|
|
|
Percentage
|
|
|
|
|
|
M. Arkin (1999) Ltd.
|
|
|
14,330,580
|
(4)
|
|
|
21.03
|
%
|
|
|
4,000,000
|
|
|
Amatrine L.P.
|
|
|
1,111,110
|
(5)
|
|
|
1.83
|
%
|
|
|
1,111,110
|
|
|
Klirmark Opportunity Fund III, L.P.
|
|
|
2,222,222
|
(6)
|
|
|
3.62
|
%
|
|
|
2,222,222
|
|
|
Neopharm Investments LTD
|
|
|
2,145,147
|
(7)
|
|
|
3.50
|
%
|
|
|
1,111,112
|
|
|
Noked Long Limited Partnership
|
|
|
3,333,334
|
(8)
|
|
|
5.38
|
%
|
|
|
3,333,334
|
|
|
Sphera Small Cap Fund L.P.
|
|
|
1,111,110
|
(9)
|
|
|
1.83
|
%
|
|
|
1,111,110
|
|
|
The Phoenix insurance LTD
|
|
|
1,833,334
|
(10)
|
|
|
3.00
|
%
|
|
|
1,833,334
|
|
|
Shotfut Menayot Israel Phoenix Amitim
|
|
|
10,388,890
|
(11)
|
|
|
15.86
|
%
|
|
|
10,388,890
|
|
|
Lior Prosor
|
|
|
444,444
|
(12)
|
|
|
0.73
|
%
|
|
|
444,444
|
|
|
More Provident Funds LTD - Alfa More Provident Fund - Bond 25%
|
|
|
40,000
|
(13)
|
|
|
0.07
|
%
|
|
|
40,000
|
|
|
More Provident Funds LTD - Advanced Study Fund - Bond 25%
|
|
|
40,000
|
(14)
|
|
|
0.07
|
%
|
|
|
40,000
|
|
|
More Provident Funds LTD - Investment Fund - Bond 25%
|
|
|
20,000
|
(15)
|
|
|
0.03
|
%
|
|
|
20,000
|
|
|
More Provident Funds LTD - Alfa More Provident Age 50
|
|
|
700,000
|
(16)
|
|
|
1.15
|
%
|
|
|
700,000
|
|
|
More Provident Funds LTD - Alfa More Provident Over Age 60
|
|
|
600,000
|
(17)
|
|
|
0.99
|
%
|
|
|
600,000
|
|
|
More Provident Funds LTD - Alfa More Provident Fund 50-60
|
|
|
1,800,000
|
(18)
|
|
|
2.94
|
%
|
|
|
1,800,000
|
|
|
More Provident Funds LTD - Alfa More Provident Fund Stock
|
|
|
960,000
|
(19)
|
|
|
1.58
|
%
|
|
|
960,000
|
|
|
More Provident Funds LTD - Investment Fund - Stocks
|
|
|
680,000
|
(20)
|
|
|
1.12
|
%
|
|
|
680,000
|
|
|
More Provident Funds LTD - Investment Fund - General
|
|
|
380,000
|
(21)
|
|
|
0.63
|
%
|
|
|
380,000
|
|
|
More Provident Funds LTD - Advanced Study Fund – Stocks
|
|
|
1,157,778
|
(22)
|
|
|
1.90
|
%
|
|
|
1,157,778
|
|
|
More Provident Funds LTD - Advanced Study Fund – General
|
|
|
1,400,000
|
(23)
|
|
|
2.30
|
%
|
|
|
1,400,000
|
|
|
Kranot Hishtalmut Le Morim ve Gananot Hevra Menahelet LTD
|
|
|
5,611,256
|
(24)
|
|
|
8.89
|
%
|
|
|
5,611,256
|
|
|
Gal Provident Fund Management for Teachers LTD
|
|
|
591,204
|
(25)
|
|
|
0.98
|
%
|
|
|
591,204
|
|
|
Gal Provident Fund Management for Teachers LTD - for Kupat Gemel Kalanit
|
|
|
556,642
|
(26)
|
|
|
0.92
|
%
|
|
|
556,642
|
|
|
OS – Hevra Lenihul Kupot Gemel LTD
|
|
|
66,558
|
(27)
|
|
|
0.11
|
%
|
|
|
66,558
|
|
|
Yahav P.R.H Provident Funds Management Company LTD
|
|
|
53,732
|
(28)
|
|
|
0.09
|
%
|
|
|
53,732
|
|
|
K.S.M - Keren Hishtalmut le Biochimaim LTD
|
|
|
95,356
|
(29)
|
|
|
0.16
|
%
|
|
|
95,356
|
|
|
Provident Fund of the Employees of Haifa Municipality
|
|
|
76,060
|
(30)
|
|
|
0.13
|
%
|
|
|
76,060
|
|
|
Aram Gmulim Provident Fund Management LTD
|
|
|
358,444
|
(31)
|
|
|
0.59
|
%
|
|
|
358,444
|
|
|
Kav Habriut - Provident Fund Management LTD Israel
|
|
|
506,242
|
(32)
|
|
|
0.84
|
%
|
|
|
506,242
|
|
|
Mishpetanim Education Fund for Lawyers Management Company LTD
|
|
|
120,180
|
(33)
|
|
|
0.20
|
%
|
|
|
120,180
|
|
|
Management Company of the Israeli Judges Study Fund LTD
|
|
|
86,074
|
(34)
|
|
|
0.14
|
%
|
|
|
86,074
|
|
|
PARETO HF
|
|
|
1,203,634
|
(35)
|
|
|
1.98
|
%
|
|
|
1,203,634
|
|
|
Reut Management Company of Provident Fund LTD
|
|
|
532,794
|
(36)
|
|
|
0.88
|
%
|
|
|
532,794
|
|
|
Further Education Found for Liberal Arts and Social Science LTD
|
|
|
273,308
|
(37)
|
|
|
0.45
|
%
|
|
|
273,308
|
|
|
WESURE INSURANCE
|
|
|
63,550
|
(38)
|
|
|
0.11
|
%
|
|
|
63,550
|
|
|
Yahav Physicians Management Company for Provident Funds LTD
|
|
|
395,596
|
(39)
|
|
|
0.65
|
%
|
|
|
395,596
|
|
|
Kelah - Social Workers Study Fund Management Company LTD
|
|
|
167,730
|
(40)
|
|
|
0.28
|
%
|
|
|
167,730
|
|
|
The Management Company of I.E.C. Workers Education Fund LTD
|
|
|
178,734
|
(41)
|
|
|
0.30
|
%
|
|
|
178,734
|
|
|
Gal Provident Fund Management for Teachers LTD - for Kupat Gemel Hagomel
|
|
|
174,018
|
(42)
|
|
|
0.29
|
%
|
|
|
174,018
|
|
|
(1)
|
Includes
shares of Common Stock that the Selling Stockholders may acquire upon exercise of warrants that we have issued to them pursuant
to equity financings. The percentage of shares of Common Stock owned is based on 60,295,245 shares of Common Stock outstanding
as of April 13, 2021.
|
|
|
|
|
(2)
|
Includes
all shares of Common Stock issuable upon exercise of outstanding warrants, all of which shares may be sold in the offering
under this prospectus.
|
|
|
|
|
(3)
|
We
have assumed for purposes of the above table that all shares of Common Stock being registered for resale hereunder are sold
by the relevant Selling Stockholders. There is no guarantee that any of those shares will actually be sold by the Selling
Stockholders.
|
|
|
|
|
(4)
|
Consists
of (i) 4,468,367 outstanding shares of Common Stock and 5,862,213 shares of Common Stock issuable upon exercise of outstanding
warrants owned by the Selling Stockholder prior to the Private Placement, (ii) and 2,000,000 shares of Common Stock and 2,000,000
shares of Common Stock issuable upon exercise of outstanding warrants purchased by the Selling Stockholder in the Private
Placement. Only the securities purchased in the Private Placement 2,000,000 shares of Common Stock and 2,000,000 shares of
Common Stock issuable upon exercise of outstanding warrants) are being registered for resale under this registration statement
on Form S-1. Mr. Moshe (Mori) Arkin, a director of the Company, may be deemed to share voting and investment power with respect
to the Common Stock and Common Stock underlying the warrants held by the Selling Stockholder.
|
|
|
|
|
(5)
|
Consists
of 555,555 outstanding shares of Common Stock and 555,555 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Eyal Bakshi.
|
|
|
|
|
(6)
|
Consists
of 1,111,111 outstanding shares of Common Stock and 1,111,111 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Yaniv Zalel.
|
|
|
|
|
(7)
|
Consists
of (i) 620,421 outstanding shares of Common Stock and 413,614 shares of Common Stock issuable upon exercise of outstanding
warrants owned by the Selling Stockholder prior to the Private Placement, (ii) and 555,556 shares of Common Stock and 555,556
shares of Common Stock issuable upon exercise of outstanding warrants purchased by the Selling Stockholder in the Private
Placement. Only the securities purchased in the Private Placement 555,556 shares of Common Stock and 555,556 shares of Common
Stock issuable upon exercise of outstanding warrants) are being registered for resale under this registration statement on
Form S-1. David Fuhrer may be deemed to share voting and investment power with respect to the Common Stock and Common
Stock underlying the warrants held by the Selling Stockholder.
|
|
|
|
|
(8)
|
Consists
of 1,666,667 outstanding shares of Common Stock and 1,666,667 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to each of Roy Vemus, Shlomi Bracha and Shay Itzkahki.
|
|
|
|
|
(9)
|
Consists
of 555,555 outstanding shares of Common Stock and 555,555 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ron Senator.
|
|
|
|
|
(10)
|
Consists
of 916,667 outstanding shares of Common Stock and 916,667 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Dan Kerner.
|
|
|
|
|
(11)
|
Consists
of 5,194,445 outstanding shares of Common Stock and 5,194,445 shares of Common Stock issuable upon exercise of outstanding
warrants, the voting and investment control of which belongs to Yaron Katz.
|
|
|
|
|
(12)
|
Consists
of 222,222 outstanding shares of Common Stock and 222,222 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Lior Prosor.
|
|
|
|
|
(13)
|
Consists
of 20,000 outstanding shares of Common Stock and 20,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
(14)
|
Consists
of 20,000 outstanding shares of Common Stock and 20,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(15)
|
Consists
of 10,000 outstanding shares of Common Stock and 10,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(16)
|
Consists
of 350,000 outstanding shares of Common Stock and 350,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(17)
|
Consists
of 300,000 outstanding shares of Common Stock and 300,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(18)
|
Consists
of 900,000 outstanding shares of Common Stock and 900,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(19)
|
Consists
of 480,000 outstanding shares of Common Stock and 480,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(20)
|
Consists
of 340,000 outstanding shares of Common Stock and 340,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(21)
|
Consists
of 190,000 outstanding shares of Common Stock and 190,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(22)
|
Consists
of 578,889 outstanding shares of Common Stock and 578,889 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(23)
|
Consists
of 700,000 outstanding shares of Common Stock and 700,000 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Ori Keren.
|
|
|
|
|
(24)
|
Consists
of 2,805,628 outstanding shares of Common Stock and 2,805,628 shares of Common Stock issuable upon exercise of outstanding
warrants, the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(25)
|
Consists
of 295,602 outstanding shares of Common Stock and 295,602 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(26)
|
Consists
of 278,321 outstanding shares of Common Stock and 278,321 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(27)
|
Consists
of 33,279 outstanding shares of Common Stock and 33,279 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(28)
|
Consists
of 26,866 outstanding shares of Common Stock and 26,866 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(29)
|
Consists
of 47,678 outstanding shares of Common Stock and 47,678 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(30)
|
Consists
of 38,030 outstanding shares of Common Stock and 38,030 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(31)
|
Consists
of 179,222 outstanding shares of Common Stock and 179,222 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(32)
|
Consists
of 253,121 outstanding shares of Common Stock and 253,121 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
(33)
|
Consists
of 60,090 outstanding shares of Common Stock and 60,090 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(34)
|
Consists
of 43,037 outstanding shares of Common Stock and 43,037 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(35)
|
Consists
of 601,817 outstanding shares of Common Stock and 601,817 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Daniel Alon.
|
|
|
|
|
(36)
|
Consists
of 266,397 outstanding shares of Common Stock and 266,397 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(37)
|
Consists
of 136,654 outstanding shares of Common Stock and 136,654 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(38)
|
Consists
of 31,775 outstanding shares of Common Stock and 31,775 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(39)
|
Consists
of 197,798 outstanding shares of Common Stock and 197,798 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(40)
|
Consists
of 83,865 outstanding shares of Common Stock and 83,865 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(41)
|
Consists
of 89,367 outstanding shares of Common Stock and 89,367 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
|
|
|
|
(42)
|
Consists
of 87,009 outstanding shares of Common Stock and 87,009 shares of Common Stock issuable upon exercise of outstanding warrants,
the voting and investment control of which belongs to Michael Banvolgi.
|
PLAN
OF DISTRIBUTION
The
Selling Stockholders, which as used herein includes donees, pledgees, transferees or other successors-in-interest selling shares
of Common Stock or interests in shares of Common Stock received after the date of this prospectus from a Selling Stockholder as
a gift, pledge, partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any
or all of their shares of Common Stock or interests in shares of Common Stock on any stock exchange, market or trading facility
on which the shares are traded or in private transactions.
The
Selling Stockholders may use any one or more of the following methods when disposing of shares or interests therein:
|
|
●
|
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
|
|
|
|
●
|
block
trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a portion of the block
as principal to facilitate the transaction;
|
|
|
|
|
|
|
●
|
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
|
|
|
|
●
|
an
exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
|
|
|
|
●
|
privately
negotiated transactions;
|
|
|
|
|
|
|
●
|
short
sales effected after the date the registration statement of which this prospectus is a part is declared effective by the SEC;
|
|
|
|
|
|
|
●
|
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
|
|
|
|
|
|
|
●
|
broker-dealers
may agree with the Selling Stockholders to sell a specified number of such shares at a stipulated price per share;
|
|
|
|
|
|
|
●
|
a
combination of any such methods of sale; and
|
|
|
|
|
|
|
●
|
any
other method permitted by applicable law.
|
The
Selling Stockholders may, from time to time, pledge or grant a security interest in some or all of the shares of Common Stock
owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer
and sell the shares of Common Stock, from time to time, under this prospectus, or under an amendment to this prospectus under
Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of Selling Stockholders to include the pledgee,
transferee or other successors in interest as Selling Stockholders under this prospectus. The Selling Stockholders also may transfer
the shares of Common Stock in other circumstances, in which case the transferees, pledgees or other successors in interest will
be the selling beneficial owners for purposes of this prospectus.
In
connection with the sale of our Common Stock or interests therein, the Selling Stockholders may enter into hedging transactions
with broker-dealers or other financial institutions, which may in turn engage in short sales of the Common Stock in the course
of hedging the positions they assume. The Selling Stockholders may also sell shares of our Common Stock short and deliver these
securities to close out their short positions, or loan or pledge the Common Stock to broker-dealers that in turn may sell these
securities. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial institutions
or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution
of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this
prospectus (as supplemented or amended to reflect such transaction).
The
aggregate proceeds to the Selling Stockholders from the sale of the Common Stock offered by them will be the purchase price of
the Common Stock less discounts or commissions, if any. Each of the Selling Stockholders reserves the right to accept and, together
with their agents from time to time, to reject, in whole or in part, any proposed purchase of Common Stock to be made directly
or through agents. We will not receive any of the proceeds from this offering.
The
Selling Stockholders also may resell all or a portion of the shares in open market transactions in reliance upon Rule 144 under
the Securities Act of 1933, provided that they meet the criteria and conform to the requirements of that rule.
The
Selling Stockholders and any underwriters, broker-dealers or agents that participate in the sale of the Common Stock or interests
therein may be “underwriters” within the meaning of Section 2(11) of the Securities Act. Any discounts, commissions,
concessions or profit they earn on any resale of the shares may be underwriting discounts and commissions under the Securities
Act. Selling Stockholders who are “underwriters” within the meaning of Section 2(11) of the Securities Act will be
subject to the prospectus delivery requirements of the Securities Act.
To
the extent required, the shares of our Common Stock to be sold, the names of the Selling Stockholders, the respective purchase
prices and public offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with
respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective
amendment to the registration statement that includes this prospectus.
In
order to comply with the securities laws of some states, if applicable, the Common Stock may be sold in these jurisdictions only
through registered or licensed brokers or dealers. In addition, in some states the Common Stock may not be sold unless it has
been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied
with.
We
have advised the Selling Stockholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales
of shares in the market and to the activities of the Selling Stockholders and their affiliates. In addition, to the extent applicable
we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the Selling Stockholders
for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The Selling Stockholders may indemnify
any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities
arising under the Securities Act.
We
have agreed to indemnify the Selling Stockholders against liabilities, including liabilities under the Securities Act and state
securities laws, relating to the registration of the shares offered by this prospectus.
We
have agreed with the Selling Stockholders to keep the registration statement of which this prospectus constitutes a part effective
until the earlier of (i) the date that such securities become eligible for resale without volume or manner-of-sale restrictions
and without current public information pursuant to Rule 144 and certain other conditions have been satisfied, or (ii) all of the
securities have been sold or otherwise disposed of pursuant to the registration statement of which this prospectus forms a part
or in a transaction in which the transferee receives freely tradable shares.
LEGAL
MATTERS
The
validity of the shares of Common Stock offered by this prospectus will be passed upon for us by The Crone Law Group, P.C.
EXPERTS
The financial statements
as of December 31, 2020 and for the year then ended included in this Prospectus have been so included in reliance on the reports
(which contains an explanatory paragraph relating to the Company’s ability to continue as a going concern as described in
Note 1b to the financial statements) of Brightman Almagor Zohar & Co., a firm in the Deloitte global network, given on the
authority of said firm as experts in auditing and accounting.
The
financial statements as of December 31, 2019 and for each of the two years in the period ended December 31, 2019 included in
this Prospectus have been so included in reliance on the report (which contains an explanatory paragraph relating to the
Company’s ability to continue as a going concern as described in Note 1b to the financial statements) of Kesselman
& Kesselman Certified Public Accountant (Isr.), a member of PricewaterhouseCoopers International Limited, an independent registered public accounting firm,
given on the authority of said firm as experts in auditing and accounting.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form S-1 under the Securities Act relating to the offering of these securities.
The registration statement, including the attached exhibits and schedules, contains additional relevant information about us and
the securities. This prospectus does not contain all of the information set forth in the registration statement and the exhibits
and schedules thereto. For further information in respect of our Company and the securities offered by this prospectus, you should
refer to the registration statement, including the exhibits and schedules thereto.
We
file annual, quarterly and other reports, proxy statements and other information with the SEC. The SEC maintains an Internet site
that contains reports, proxy and information statements, and other information regarding issuers that file electronically with
the SEC. You can read our SEC filings, including the registration statement, at the SEC’s website at http://www.sec.gov.
You
may also obtain information about us by visiting our website at https://www.scoutcam.com. Information contained in our
website is not part of this prospectus.
You
should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with different
information. You should not rely on any other representations. Our affairs may change after this prospectus is distributed. You
should not assume that the information in this prospectus is accurate as of any date other than the date on the front of those
documents.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
CONSOLIDATED
FINANCIAL STATEMENTS
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
TABLE
OF CONTENTS

REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Shareholders and the Board of Directors of ScoutCam Inc.
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheets of ScoutCam Inc. and its subsidiary (the “Company”) as of
December 31, 2020 and the related consolidated statements of operations, shareholders’ equity (capital deficiency), and
cash flows for the year ended December 31, 2020, and the related notes (collectively referred to as the “consolidated financial
statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial
position of the Company as of December 31, 2020, and the results of its operations and its cash flows for the year ended December
31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going
Concern
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed
in Note 1B to the financial statements, the Company’s accumulated losses and the additional funds needed to maintain its
operations raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these
matters are also described in Note 1B. The financial statements do not include any adjustments that might result from the outcome
of this uncertainty.
Basis
for Opinion
These
consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an
opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered
with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to
the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and
Exchange Commission and the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit
to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether
due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over
financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting
but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting.
Accordingly, we express no such opinion.
Our
audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether
due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis,
evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the
accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the
consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical
Audit Matter
The
critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was
communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material
to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of
critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by
communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or
disclosures to which it relates.
Development
Services Revenue and Contract Liabilities – Refer to Note 2J. and Note 10 to the Consolidated Financial Statements
Critical
Audit Matter Description
The
Company generates revenues from development services. The Company determines at contract inception whether development services
are distinct from the performance obligation to manufacture the product under development. Revenues from development services
that are determined as not distinct from the performance obligation to manufacture the product under development are deferred
until commencement of manufacturing and are recognized over the manufacturing term. During 2020, all development services revenues
billed have been deferred and recorded as contract liabilities (representing the majority of the contract liabilities balance
of $848,000 as of December 31, 2020) and the respective service costs have been deferred and recorded as contract fulfillment
assets ($1,130,000 as of December 31, 2020), as the development services were determined as not distinct from the performance
obligation to manufacture the product under development.
We
identified the assessment of whether development services were a distinct performance obligation and the impact on the timing
of revenue recognition as a critical audit matter. Evaluating whether development services should be accounted for separately
required judgment and increased audit effort in comparison to our audit as a whole, because of the complexity of the technical
accounting analysis and due to the magnitude of the related contract liabilities as of December 31, 2020.
How
the Critical Audit Matter Was Addressed in the Audit
Our
audit procedures related to the Company’s determination of the performance obligations and the timing of revenue recognition
for development service contracts included the following, among others:
|
|
●
|
We
read the agreements and analyzed the terms of the Company’s development service contracts.
|
|
|
●
|
We
read communications between the Company and its clients relating to development services contracts.
|
|
|
●
|
We
inquired of Company research and development personnel to understand the commercial facts and circumstances relating to development
services contracts.
|
|
|
●
|
We
evaluated the Company’s interpretation and application of the relevant requirements of generally accepted accounting
principles in relation to the development services contracts and the related contract liabilities.
|
/s/
Brightman Almagor Zohar & Co.
Certified
Public Accountants
A
Firm in the Deloitte Global Network
Tel
Aviv, Israel
March
31, 2021
We
have served as the Company’s auditor since 2020.

Report
of Independent Registered Public Accounting Firm
To
the Shareholders and Board of Directors of ScoutCam Inc.
Opinion
on the Financial Statements
We
have audited the accompanying consolidated balance sheet of ScoutCam Inc. and its subsidiary (the “Company”) as of
December 31, 2019, and the related consolidated statements of operations, of changes in shareholders' equity (capital deficiency)
and of cash flows for each of the two years in the period ended December 31, 2019, including the related notes (collectively referred
to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly,
in all material respects, the financial position of the Company as of December 31, 2019, and the results of its operations and
its cash flows for each of the two years in the period ended December 31, 2019 in conformity with accounting principles generally
accepted in the United States of America.
Substantial
Doubt about the Company’s Ability to Continue as a Going Concern
The
accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As discussed
in Note 1(b) to the consolidated financial statements, the Company has suffered recurring losses from operations and cash outflows
from operating activities that raise substantial doubt about its ability to continue as a going concern. Management's plans in
regard to these matters are also described in Note 1(b). The consolidated financial statements do not include any adjustments
that might result from the outcome of this uncertainty.
Basis
for Opinion
These
consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an
opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered
with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to
the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and
Exchange Commission and the PCAOB.
We
conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards
require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements
are free of material misstatement, whether due to error or fraud.
Our
audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether
due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis,
evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the
accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the
consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
|
/s/
Kesselman & Kesselman
|
|
|
Certified
Public Accountants (Isr.)
|
|
|
A
member firm of PricewaterhouseCoopers International Limited
|
|
Tel-Aviv,
Israel
March
16, 2020
We
served as the Company's auditor from 2019 to 2020.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
CONSOLIDATED
BALANCE SHEETS
|
|
|
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
|
|
USD
in thousands
|
|
|
|
|
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT ASSETS:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
|
3,373
|
|
|
|
3,245
|
|
|
Accounts receivable
|
|
|
17
|
|
|
|
22
|
|
|
Inventory
|
|
|
244
|
|
|
|
900
|
|
|
Receivable from
Parent Company
|
|
|
47
|
|
|
|
73
|
|
|
Other current
assets
|
|
|
348
|
|
|
|
78
|
|
|
Total current
assets
|
|
|
4,029
|
|
|
|
4,318
|
|
|
|
|
|
|
|
|
|
|
|
|
NON-CURRENT ASSETS:
|
|
|
|
|
|
|
|
|
|
Contract
fulfillment assets
|
|
|
1,130
|
|
|
|
-
|
|
|
Property and equipment, net
|
|
|
269
|
|
|
|
59
|
|
|
Operating lease right-of-use assets
|
|
|
107
|
|
|
|
53
|
|
|
Severance pay
asset
|
|
|
360
|
|
|
|
327
|
|
|
Total non-current
assets
|
|
|
1,866
|
|
|
|
439
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS
|
|
|
5,895
|
|
|
|
4,757
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and
shareholders’ equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
|
79
|
|
|
|
35
|
|
|
Contract liabilities
|
|
|
69
|
|
|
|
502
|
|
|
Operating lease liabilities - short term
|
|
|
60
|
|
|
|
24
|
|
|
Accrued compensation expenses
|
|
|
369
|
|
|
|
297
|
|
|
Loan from Parent Company
|
|
|
-
|
|
|
|
500
|
|
|
Other accrued
expenses
|
|
|
195
|
|
|
|
552
|
|
|
Total current
liabilities
|
|
|
772
|
|
|
|
1,910
|
|
|
|
|
|
|
|
|
|
|
|
NON-CURRENT LIABILITIES:
|
|
|
|
|
|
|
|
|
|
Contract
liabilities
|
|
|
779
|
|
|
|
-
|
|
|
Operating lease liabilities - long term
|
|
|
47
|
|
|
|
29
|
|
|
Liability for
severance pay
|
|
|
333
|
|
|
|
296
|
|
|
Total non-current
liabilities
|
|
|
1,159
|
|
|
|
325
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
|
|
|
1,931
|
|
|
|
2,235
|
|
|
|
|
|
|
|
|
|
|
|
|
SHAREHOLDERS’ EQUITY:
|
|
|
|
|
|
|
|
|
|
Ordinary
shares Common stock, $0.001 par value; 75,000,000 shares authorized, 36,756,983 and
26,884,921 shares issued and outstanding as of December 31, 2020 and 2019, respectively
|
|
|
37
|
|
|
|
27
|
|
|
Additional paid-in capital
|
|
|
10,234
|
|
|
|
4,135
|
|
|
Accumulated deficit
|
|
|
(6,307
|
)
|
|
|
(1,640
|
)
|
|
TOTAL SHAREHOLDERS’ EQUITY
|
|
|
3,964
|
|
|
|
2,522
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
AND SHAREHOLDERS’ EQUITY
|
|
|
5,895
|
|
|
|
4,757
|
|
The
accompanying notes are an integral part of these consolidated financial statements.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
CONSOLIDATED
STATEMENTS OF OPERATIONS
|
|
|
Year
ended December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
2018
|
|
|
|
|
USD
in thousands
(except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
REVENUES (*):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PRODUCTS
|
|
|
491
|
|
|
|
188
|
|
|
|
174
|
|
|
SERVICES
|
|
|
-
|
|
|
|
121
|
|
|
|
217
|
|
|
|
|
|
491
|
|
|
|
309
|
|
|
|
391
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF REVENUES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PRODUCTS
|
|
|
994
|
|
|
|
421
|
|
|
|
104
|
|
|
SERVICES
|
|
|
-
|
|
|
|
121
|
|
|
|
117
|
|
|
|
|
|
994
|
|
|
|
542
|
|
|
|
221
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT (LOSS)
|
|
|
(503
|
)
|
|
|
(233
|
)
|
|
|
170
|
|
|
RESEARCH AND DEVELOPMENT EXPENSES
|
|
|
725
|
|
|
|
274
|
|
|
|
183
|
|
|
SALES AND MARKETING EXPENSES
|
|
|
443
|
|
|
|
183
|
|
|
|
270
|
|
|
GENERAL AND
ADMINISTRATIVE EXPENSES
|
|
|
3,035
|
|
|
|
1,117
|
|
|
|
240
|
|
|
OPERATING LOSS
|
|
|
(4,706
|
)
|
|
|
(1,807
|
)
|
|
|
(523
|
)
|
|
FINANCING INCOME
(EXPENSES), NET
|
|
|
41
|
|
|
|
(20
|
)
|
|
|
**
|
|
|
LOSS BEFORE TAXES ON INCOME
|
|
|
(4,665
|
)
|
|
|
(1,827
|
)
|
|
|
(523
|
)
|
|
TAXES ON INCOME
|
|
|
(2
|
)
|
|
|
(2
|
)
|
|
|
(1
|
)
|
|
NET LOSS
|
|
|
(4,667
|
)
|
|
|
(1,829
|
)
|
|
|
(524
|
)
|
|
Net loss per ordinary share (basic
and diluted, in USD)
|
|
|
(0.15
|
)
|
|
|
(0.11
|
)
|
|
|
(0.03
|
)
|
|
Weighted average ordinary shares (basic
and diluted, in thousands)
|
|
|
31,753
|
|
|
|
16,190
|
|
|
|
16,131
|
|
|
|
*
|
As
for revenues related to transaction with the Parent Company – see Note 11
|
|
|
**
|
Less than
1 thousand
|
The
accompanying notes are an integral part of these consolidated financial statements.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
CONSOLIDATED
STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY (CAPITAL DEFICIENCY)
|
|
|
Ordinary
shares
|
|
|
Additional
paid-in capital
|
|
|
Accumulated
deficit
|
|
|
Total
Shareholders’ equity (Capital deficiency)
|
|
|
|
|
Shares
in
thousands
|
|
|
amount
|
|
|
USD
in thousands
|
|
|
Balance at January 1, 2020
|
|
|
26,885
|
|
|
$
|
27
|
|
|
|
4,135
|
|
|
|
(1,640
|
)
|
|
|
2,522
|
|
|
Issuance of shares and warrants
|
|
|
6,092
|
|
|
$
|
6
|
|
|
|
2,852
|
|
|
|
-
|
|
|
|
2,858
|
|
|
Exercise of warrants
|
|
|
2,993
|
|
|
$
|
3
|
|
|
|
1,726
|
|
|
|
-
|
|
|
|
1,729
|
|
|
Stock based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
1,141
|
|
|
|
-
|
|
|
|
1,141
|
|
|
Conversion of loan from Parent Company
|
|
|
787
|
|
|
$
|
1
|
|
|
|
380
|
|
|
|
-
|
|
|
|
381
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,667
|
)
|
|
|
(4,667
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December
31, 2020
|
|
|
36,757
|
|
|
$
|
37
|
|
|
|
10,234
|
|
|
|
(6,307
|
)
|
|
|
3,964
|
|
|
|
|
Ordinary
shares
|
|
|
Additional
paid-in capital
|
|
|
Parent
Company deficit
|
|
|
Accumulated
deficit
|
|
|
Total
Shareholders’ equity (Capital deficiency)
|
|
|
|
|
Shares
in
thousands
|
|
|
USD
in thousands
|
|
|
Balance at January 1, 2019
|
|
|
16,131
|
|
|
|
16
|
|
|
|
(16
|
)
|
|
|
(118
|
)
|
|
|
-
|
|
|
|
(118
|
)
|
|
Net transfer from Parent Company
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
514
|
|
|
|
-
|
|
|
|
514
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(189
|
)
|
|
|
(1,640
|
)
|
|
|
(1,829
|
)
|
|
Consummation of the carve-out
|
|
|
-
|
|
|
|
-
|
|
|
|
207
|
|
|
|
(207
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Capital contribution from Parent Company
|
|
|
-
|
|
|
|
-
|
|
|
|
720
|
|
|
|
-
|
|
|
|
-
|
|
|
|
720
|
|
|
Sale of assets to Parent Company
|
|
|
-
|
|
|
|
-
|
|
|
|
168
|
|
|
|
-
|
|
|
|
-
|
|
|
|
168
|
|
|
Effect of reverse recapitalization
|
|
|
10,754
|
|
|
|
11
|
|
|
|
3,029
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,040
|
|
|
Share based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
27
|
|
|
|
-
|
|
|
|
-
|
|
|
|
27
|
|
|
Balance at December
31, 2019
|
|
|
26,885
|
|
|
|
27
|
|
|
|
4,135
|
|
|
|
-
|
|
|
|
(1,640
|
)
|
|
|
2,522
|
|
|
|
|
Ordinary
shares
|
|
|
Additional
paid-in capital
|
|
|
Parent
Company deficit
|
|
|
Total
Shareholders’ equity (Capital deficiency)
|
|
|
|
|
Shares
in
thousands
|
|
|
USD
in thousands
|
|
|
Balance at January 1,
2018
|
|
|
16,131
|
|
|
|
16
|
|
|
|
(16
|
)
|
|
|
(117
|
)
|
|
|
(117
|
)
|
|
Net transfer from Parent Company
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
523
|
|
|
|
523
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(524
|
)
|
|
|
(524
|
)
|
|
Balance at
December 31, 2018
|
|
|
16,131
|
|
|
|
16
|
|
|
|
(16
|
)
|
|
|
(118
|
)
|
|
|
(118
|
)
|
The
accompanying notes are an integral part of these consolidated financial statements.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
CONSOLIDATED
STATEMENTS OF CASH FLOWS
|
|
|
Year
ended December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
2018
|
|
|
|
|
USD
in thousands
|
|
|
CASH FLOWS FROM OPERATING
ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(4,667
|
)
|
|
|
(1,829
|
)
|
|
|
(524
|
)
|
|
Adjustments to reconcile
net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
66
|
|
|
|
6
|
|
|
|
5
|
|
|
Share based compensation
|
|
|
1,107
|
|
|
|
27
|
|
|
|
25
|
|
|
Loss (profit) from exchange differences
on cash and cash equivalents
|
|
|
(85
|
)
|
|
|
5
|
|
|
|
-
|
|
|
Other non-cash items
|
|
|
4
|
|
|
|
(10
|
)
|
|
|
1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CHANGES IN OPERATING
ASSET AND LIABILITY:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
5
|
|
|
|
68
|
|
|
|
(85
|
)
|
|
Decrease (increase)
in inventory
|
|
|
693
|
|
|
|
(819
|
)
|
|
|
(25
|
)
|
|
Other current assets
|
|
|
(270
|
)
|
|
|
(16
|
)
|
|
|
(62
|
)
|
|
Account payables
|
|
|
44
|
|
|
|
16
|
|
|
|
-
|
|
|
Contract fulfillment assets
|
|
|
(1,130
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Contract liability
|
|
|
346
|
|
|
|
302
|
|
|
|
192
|
|
|
Accrued compensation expenses
|
|
|
72
|
|
|
|
166
|
|
|
|
(13
|
)
|
|
Receivable
from Parent Company
|
|
|
(15
|
)
|
|
|
(73
|
)
|
|
|
-
|
|
|
Other accrued
expenses
|
|
|
(357
|
)
|
|
|
358
|
|
|
|
32
|
|
|
Net cash flows used in operating activities
|
|
|
(4,187
|
)
|
|
|
(1,799
|
)
|
|
|
(454
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING
ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment
|
|
|
(276
|
)
|
|
|
(52
|
)
|
|
|
-
|
|
|
Change in severance
pay asset
|
|
|
-
|
|
|
|
(3
|
)
|
|
|
4
|
|
|
Net cash flows generated from (used
in) investing activities
|
|
|
(276
|
)
|
|
|
(55
|
)
|
|
|
4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING
ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of shares and
warrants
|
|
|
2,858
|
|
|
|
-
|
|
|
|
-
|
|
|
Proceeds from exercise of warrants
|
|
|
1,729
|
|
|
|
-
|
|
|
|
-
|
|
|
Repayment of loan from Parent Company
|
|
|
(81
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Transfer from Parent Company
|
|
|
-
|
|
|
|
514
|
|
|
|
450
|
|
|
Sale of assets to Parent Company
|
|
|
-
|
|
|
|
168
|
|
|
|
-
|
|
|
Capital contribution from Parent Company
|
|
|
-
|
|
|
|
720
|
|
|
|
-
|
|
|
Loan from Parent Company
|
|
|
-
|
|
|
|
500
|
|
|
|
-
|
|
|
Cash obtained
in connection with Recapitalization Transaction
|
|
|
-
|
|
|
|
3,202
|
|
|
|
-
|
|
|
Net cash flows
provided by financing activities
|
|
|
4,506
|
|
|
|
5,104
|
|
|
|
450
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCREASE IN CASH
AND CASH EQUIVALENTS
|
|
|
43
|
|
|
|
3,250
|
|
|
|
-
|
|
|
BALANCE
OF CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR
|
|
|
3,245
|
|
|
|
-
|
|
|
|
-
|
|
|
PRPFITS
(LOSSES) FROM EXCHANGE DIFFERENCES ON CASH AND CASH EQUIVALENTS
|
|
|
85
|
|
|
|
(5
|
)
|
|
|
-
|
|
|
BALANCE
OF CASH AND CASH EQUIVALENTS AT END OF YEAR
|
|
|
3,373
|
|
|
|
3,245
|
|
|
|
-
|
|
Non
cash activities -
|
|
|
Year
ended December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
2018
|
|
|
|
|
USD
in thousands
|
|
|
Loan
from Parent Company settled
against receivable from Parent Company
|
|
|
41
|
|
|
|
-
|
|
|
|
-
|
|
|
Conversion
of a loan from Parent Company
|
|
|
381
|
|
|
|
-
|
|
|
|
-
|
|
SUPPLEMENTAL
INFORMATION FOR CASH FLOW:
|
|
|
As
of
December
30, 2019
|
|
|
|
|
|
|
|
Assets
acquired (liabilities assumed):
|
|
|
|
|
|
|
|
|
|
|
|
Current assets excluding cash and
cash equivalents
|
|
$
|
-
|
|
|
Current liabilities
|
|
|
(73
|
)
|
|
Recapitalization Transaction costs
|
|
|
(89
|
)
|
|
Reverse recapitalization
effect on equity
|
|
|
(3,040
|
)
|
|
|
|
|
|
|
|
Cash
obtained in connection with Recapitalization Transaction
|
|
$
|
3,202
|
|
The
accompanying notes are an integral part of these consolidated financial statements.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
1 – GENERAL:
|
|
a.
|
ScoutCam
Inc. (the “Company”), formerly known as Intellisense Solutions Inc.
(“Intellisense”), was incorporated under the laws of the State of Nevada
on March 22, 2013. The Company was initially engaged in the business of developing web
portals to allow companies and individuals to engage in the purchase and sale of vegetarian
food products over the Internet. The Company was unable to execute its original business
plan, develop significant operations or achieve commercial sales. Prior to the closing
of the Securities Exchange Agreement (as defined below), the Company was a “shell
company”.
ScoutCam
Ltd. (the “Subsidiary”, “ScoutCam”), was formed in the State of Israel on January 3, 2019 as a wholly-owned
subsidiary of Medigus Ltd. (the “Parent Company”, “Medigus”), an Israeli company traded both on the Nasdaq
Capital Market and the Tel Aviv Stock Exchange, and commenced operations on March 1, 2019. Upon incorporation, the Subsidiary
issued to Medigus 1,000,000 ordinary shares with no par value. On March 2019, the Subsidiary issued to Medigus an additional
1,000,000 ordinary shares with no par value.
The
Subsidiary was incorporated as part of a reorganization of Medigus, which was designed to distinguish the Subsidiary’s
miniaturized imaging business, or the micro ScoutCam™ portfolio, from Medigus’s other operations and to enable Medigus
to form a separate business unit with dedicated resources focused on the promotion of such miniaturized imaging business.
In December 2019, Medigus and the Subsidiary consummated a certain Amended and Restated Asset Transfer Agreement, under which
Medigus transferred and assigned certain assets and intellectual property rights related to its miniaturized imaging business
to the Subsidiary.
On
September 16, 2019, Intellisense entered into a Securities Exchange Agreement (the “Exchange Agreement”), with Medigus, pursuant
to which Medigus assigned, transferred and delivered 100% of its holdings in the Subsidiary to Intellisense, in exchange for consideration
consisting of shares of Intellisense’s common stock representing 60% of the issued and outstanding share capital of Intellisense
immediately upon the closing of the Exchange Agreement (the “Closing”). In addition, the Exchange Agreement provides that
if ScoutCam achieves an aggregated amount of USD 33 million in sales within the first three years immediately after the Closing, the
Company will issue to Medigus 2,688,492 additional shares of Company’s common stock. The Closing occurred on December
30, 2019 (the “Closing Date”). On December 31, 2019, Intellisense changed its name to ScoutCam Inc.
Although
the transaction resulted in the Subsidiary becoming a wholly owned subsidiary of Intellisense, the transaction constituted a reverse
recapitalization since Medigus, the only shareholder of the Subsidiary prior to the Exchange Agreement, was issued a majority
of the outstanding capital stock of Intellisense upon consummation of the Exchange Agreement, and also taking into account that
prior to the Closing Date, Intellisense was considered as a shell corporation. Accordingly, the Subsidiary is considered the accounting
acquirer of the merged company.
“Group”
- the Company together with ScoutCam.
The
Subsidiary has developed a range of micro CMOS (complementary metal-oxide semiconductor) and CCD (charge-coupled device) video
cameras, including micro ScoutCam™ 1.2. These innovative cameras are suitable for both medical and industrial applications.
Based on its proprietary technology, the Subsidiary designs and manufactures endoscopy and micro camera systems for partner companies.
|
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
1 – GENERAL (continued):
|
|
b.
|
During the year ended December 31, 2020, the Company
incurred a loss of USD 4,667 thousand and negative cash flows from operating activities of approximately USD 4,187 thousand. Based on
the projected cash flows, the Company’s Management is of the opinion that without further fundraising it will not have sufficient
resources to enable it to continue its operating activities including the development, manufacturing and marketing of its products within
one year after the issuance date of these consolidated financial statements. As a result, there is a substantial doubt about the Company’s
ability to continue as a going concern within one year after the issuance date of these financial statements.
Management did not take into account the proceeds
from the private placement (see note 13c), because the closing of the private placement didn’t occur as of the date of issuance of these
financial statements.
Management’s plans include continuing commercialization
of the Company’s products and securing sufficient financing through the sale of additional equity securities, debt or capital inflows
from strategic partnerships and other opportunities. There are no assurances however, that the Company will be successful in obtaining
the level of financing needed for its operations. If the Company is unsuccessful in commercializing its products and securing sufficient
financing, it may need to reduce activities, curtail or even cease operations.
These consolidated financial statements have been
prepared assuming the Company will continue as a going concern, which assumes the realization of assets and the satisfaction of liabilities
and commitments in the normal course of business. Accordingly, the consolidated financial statements do not include any adjustments relating
to the recoverability and classification of recorded assets and the amounts and classification of liabilities that might be necessary
should the Company be unable to continue as a going concern.
|
|
|
c.
|
The
COVID-19 pandemic has had a significant impact on global markets and the global economy, including countries in which the
Company operates. As the extent of the impact on the global economy remains unclear, the Company anticipates that it will
have a continuing impact on global economies in the near and long-term future. In light of the below mentioned factors, the
COVID-19 pandemic had and most likely will continue to have a material effect on the Company’s operations, and the extent
to which the COVID-19 pandemic will impact the Company’s operations will depend on future developments. In particular,
the continued spread of COVID-19 globally had and most likely will continue to have material adverse impact on the Company’s
operations and workforce, including its manufacturing activities, product sales, as well as its ability to continue to raise
capital. Travel restrictions had and most likely will continue to have a material adverse impact on Company’s
sales and marketing and research and development efforts.
|
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
2 – SIGNIFICANT ACCOUNTING POLICIES:
The accounting treatment for
the Exchange Agreement was as a reverse recapitalization of ScoutCam, for financial accounting and reporting purposes.
As such, ScoutCam Ltd. is treated as the acquirer for accounting and financial reporting purposes while the Company is treated
as the acquired entity for accounting and financial reporting purposes. As a result, the comparative figures that are reflected
in the Company’s financial statements are those of ScoutCam and from the Closing Date, the Company’s assets, liabilities
and results of operations are consolidated with the assets, liabilities and results of operations of ScoutCam.
The
consolidated financial statements reflect the Company’s financial position, results of operations, changes in shareholders
equity (capital deficiency) and cash flows in accordance with generally accepted accounting principles in the United States
(“U.S. GAAP”).
The
accompanying comparative financial statements include the historical accounts of ScoutCam as a “Carve-out Business”,
a division of Medigus. Throughout the comparative periods included in these financial statements, the Carve-out Business
operated as part of Medigus. Separate financial statements have not historically been prepared for the Carve-out Business.
These
comparative carve-out financial statements have been prepared on a standalone basis and are derived from Medigus’s consolidated
financial statements and accounting records. The carve-out comparative financial statements reflect ScoutCam’s financial
position, results of operations, changes in net Parent Company deficit and cash flows in accordance with U.S. GAAP.
The
financial position, results of operations, changes in net parent deficit, and cash flows of the Carve-out Business may not be
indicative of its results had it been a separate stand-alone entity during the comparative periods presented.
The
comparative carve-out financial statements of the Company include expenses which were allocated from Medigus for certain functions,
including general corporate expenses related to corporate strategy, procurement, Information Technology (“IT”), Human
Resources (“HR”) and legal. These allocation have been made on the basis of direct usage when identifiable, with the
remainder allocated on the basis of headcount. Management believes the expense allocation methodology and results are reasonable
and consistently applied for all comparative periods presented. However, these allocations may not be indicative of the actual
expenses that would have been incurred by an independent company or of the costs to be incurred in the future.
The
carve-out comparative financial statements include assets and liabilities specifically attributable to the Carve-out Business.
Transfers of cash between Carve-out Business and Medigus are included within “Transfers from Parent
Company” on the Statements of Cash Flows and the Statements of changes in shareholder’s equity (capital deficiency).
As
the carve-out comparative financial statements have been prepared on a carve-out basis, the amounts reflected in Parent Company deficit
in the comparative statement of changes in shareholder’s equity (capital deficiency) refer to net loss for the period attributed
to ScoutCam.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
2 - SIGNIFICANT ACCOUNTING POLICIES (continued):
The
preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated
financial statements and the reported amounts of revenue and expenses during the reporting period. The Company evaluates on an
ongoing basis its assumptions, including those related to contingencies, deferred taxes, inventory impairment, as well as in estimates
used in applying the revenue recognition policy. Actual results may differ from those estimates.
A
majority of ScoutCam’s revenues are generated in U.S. dollars. The substantial majority of ScoutCam costs are incurred in
U.S. dollars and New Israeli Shekels (“NIS”). ScoutCam management believes that the U.S. dollar is the currency of
the primary economic environment in which ScoutCam operates. Thus, the functional currency of ScoutCam is the U.S. dollar.
Transactions
and balances originally denominated in U.S. dollars are presented at their original amounts. Balances in non U.S.
dollar currencies are translated into U.S. dollars using historical and current exchange rates for non-monetary and monetary
balances, respectively. For non-U.S. dollar transactions and other items in the statements of operations (indicated below),
the following exchange rates are used: (i) for transactions exchange rates at transaction dates and (ii) for other items
(derived from non-monetary balance sheet items such as depreciation and amortization) historical exchange rates. Currency
transaction gains and losses are presented in financial income or expenses, as appropriate.
|
|
d.
|
Cash
and Cash Equivalents
|
The
Company considers as cash equivalents all short-term, highly liquid investments, which include short-term bank deposits with original
maturities of three months or less from the date of purchase that are not restricted as to withdrawal or use and are readily convertible
to known amounts of cash.
Accounts
receivable are presented in the Company’s consolidated balance sheets net of allowance for doubtful accounts. The
Company estimates the collectibility of its accounts receivable balances and adjusts its allowance for doubtful accounts accordingly.
When
revenue recognition criteria are not met for a sale transaction that has been billed, the Company does not recognize deferred
revenues or the related account receivable.
As
of December 31, 2020 and 2019, no allowance for doubtful accounts was recorded.
|
|
f.
|
Property
and equipment
|
Property
and equipment is stated at cost, net of accumulated depreciation and amortization. Depreciation is calculated on a straight-line basis
over the estimated useful lives.
The
annual depreciation rates are as follows:
|
|
|
%
|
|
Machinery
and laboratory equipment
|
|
10%-15%
|
|
Office
furniture and equipment
|
|
10%
|
|
Computers
and computer software
|
|
33%
|
|
Leasehold
improvements
|
|
Over
the shorter of the lease term (including options if any) or useful life
|
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
2 - SIGNIFICANT ACCOUNTING POLICIES (continued):
Israeli
labor law generally requires payment of severance pay upon dismissal of an employee or upon termination of employment in certain other
circumstances. Pursuant to Section 14 of the Severance Compensation Act, 1963 (“Section 14”), all of the Company’s
employees in Israel are entitled a monthly contribution, at a rate of 8.33% of their monthly salary, made in their name
with insurance companies. Contributions under Section 14 relieve the Company from any future severance payment obligation with
respect to those employees. The aforementioned contributions are not recorded as an asset on the Company’s balance sheet, and
there is no liability recorded as the Company does not have a future obligation to make any additional payments.
The
asset and the liability for severance pay presented in the balance sheets reflects employees that began employment prior to automatic
application of Section 14.
The
severance pay liability of the Company to its employees that began employment prior to automatic application of Section 14 based
upon the number of years of service and the latest monthly salary and is partly covered by regular deposits with recognized pension funds
and deposits with severance pay funds. Under labor laws, these deposits are in the employees’ names and, subject to certain
limitations, are the property of the employees. The Company records the obligation as if it were payable at each balance sheet date on
an undiscounted basis.
|
|
h.
|
Stock-Based
Compensation
|
The
Company measures and recognizes compensation expense for its equity classified stock-based awards, including option awards exercisable
into shares of common stock of the Parent Company under its plan based on estimated fair values on the grant
date. The Company calculates the fair value of option awards on the grant date using the Black-Scholes option pricing model. The
Black-Scholes option-pricing model requires a number of assumptions, of which the most significant are the stock price
volatility and the expected option term. For the years ended December 31, 2019, and 2018, the volatility was based on the historical
stock volatility of the Parent Company. The Company’s expected dividend rate is zero since the Company does not currently
pay cash dividends on its stocks and does not anticipate doing so in the foreseeable future. Each of the above factors requires
the Company to use judgment and make estimates in determining the percentages and time periods used for the calculation. If the
Company were to use different percentages or time periods, the fair value of option awards could be materially different. The
Company recognizes stock-based compensation cost for option awards on a accelerated basis over the employee’s requisite
service period, net of estimated forfeitures.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
2 - SIGNIFICANT ACCOUNTING POLICIES (continued):
Inventories
include raw materials, inventory in process and finished products and are valued at the lower of cost or net realizable value.
The
cost is determined on the basis of “first in-first out” basis. Cost of purchased raw materials and inventory in process
includes costs of design, raw materials, direct labor, other direct costs and fixed production overheads. Materials and other
supplies held for use in the production of inventories are not written down if the finished products in which they
will be incorporated are expected to be sold at or above cost.
The
Company regularly evaluates its ability to realize the value of inventory based on a combination of factors including the following:
forecasted sales or usage, estimated current and future market values.
Commencing
January 1, 2018, the Company’s revenues are measured according to the ASC 606, “Revenue from Contracts with Customers”
(“ASC 606”). Under ASC 606, revenues are measured according to the amount of consideration that the Company expects to be
entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties,
such as VAT taxes. Revenues are presented net of VAT.
The
Company recognizes revenue when a customer obtains control over promised goods or services. For each performance obligation,
the Company determines at contract inception whether it satisfies the performance obligation over time or satisfies the performance
obligation at a point in time.
Performance
obligations are satisfied over time if one of the following criteria is met:
(a)
the customer simultaneously receives and consumes the benefits provided by the Company’s performance; (b) the Company’s
performance creates or enhances an asset that the customer controls as the asset is created or enhanced; or (c) the Company’s
performance does not create an asset with an alternative use to the Company and the Company has an enforceable right to payment
for performance completed to date.
If
a performance obligation is not satisfied over time, a Company satisfies the performance obligation at a point in time.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
2 — SIGNIFICANT ACCOUNTING POLICIES (continued):
The
transaction price is allocated to each distinct performance obligations on a relative standalone selling price (“SSP”)
basis and revenue is recognized for each performance obligation when control has passed. In most cases, the Company is able to
establish SSP based on the observable prices of services sold separately in comparable circumstances to similar customers and
for products based on the Company’s best estimates of the price at which the Company would have sold the product regularly
on a stand-alone basis. The Company reassesses the SSP on a periodic basis or when facts and circumstances change.
Product
Revenue
Revenues
from product sales are recognized at a point in time when the customer obtains control of the Company’s product,
typically upon shipment to the customer. Sales taxes collected from customers relating to product sales and remitted to
governmental authorities are excluded from revenues.
Service
Revenue
The
Company also generates revenues from development services. Revenue from development services is recognized over the period of
the applicable service contract. To the extent development services are not distinct from the performance obligation relating
to the subsequent mass production phase of the prototype under development, revenue from these services is deferred until commencement
of the production phase of the project.
There
are no long-term payment terms or significant financing components of the Company’s contracts.
The
Company’s contract payment terms for product and services vary by customer. The Company assesses collectibility based on
several factors, including collection history.
Cost of revenue consists of products
purchased from sub-contractors, raw materials for in-house assembly line, shipping and handling costs to customers, salary, employee-related
expenses, depreciation and overhead expenses.
Cost of revenues are expensed commensurate
with the recognition of the respective revenues. Costs deferred in respect of deferral of revenues are recorded as contract fulfilment
assets on the Company’s balance sheet, and are written down to the extent the contract is expect to incur losses.
|
|
l.
|
Research
and development costs
|
Research
and development costs are expensed as incurred and includes salaries and employee-related expenses, overhead expenses, material
and third-party contractor’s charges.
Income
taxes are accounted for using the asset and liability approach under ASC-740, “Income Taxes” (“ASC-740”).
The asset and liability approach require the recognition of taxes payable or refundable for the current year and deferred tax
liabilities and assets for the future tax consequences of events that have been recognized in the Company’s financial statements
or tax returns.
The
measurement of current and deferred tax liabilities and assets is based on provisions of the relevant tax law. The measurement
of deferred tax assets is reduced, if necessary, by the amount of any tax benefits that, based on available evidence, are not
expected to be realized.
Uncertain tax positions are accounted
for in accordance with the provisions of ASC 740-10, under which a company may recognize the tax benefit from an uncertain tax position
claimed or expected to be claimed on a tax return only if it is more likely than not that the tax position will be sustained on examination
by the taxation authorities, based on the technical merits of the position, at the largest benefit that has a greater than fifty percent
likelihood of being realized upon ultimate settlement. Interest and penalties, if any, related to unrecognized tax benefits, are
recognized in tax expense.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
2 — SIGNIFICANT ACCOUNTING POLICIES (continued):
From
time to time, the Company becomes involved in legal proceedings or is subject to claims arising in its ordinary course of business.
Such matters are generally subject to many uncertainties and outcomes are not predictable with assurance. The Company accrues
for contingencies when the loss is probable, and it can reasonably estimate the amount of any such loss.
Basic
loss per share is computed by dividing net loss, by the weighted average number of ordinary shares as described below.
In
computing the Company’s diluted earnings per share, the numerator used in the basic earnings per share computation is adjusted
for the dilutive effect, if any, of the Company’s potential common stock. The denominator for diluted earnings per share
is a computation of the weighted-average number of ordinary shares and the potential dilutive shares common stock outstanding
during the period.
The
loss per share information in these consolidated financial statements is reflected and calculated as if the Company had existed
since January 1, 2018. Accordingly, loss per share for all periods was calculated based on the number of ordinary shares
retroactively adjusted for the exchange ratio determined in the reverse recapitalization (see also note 3).
The
Company determines if an arrangement contains a lease at inception. Company’s leases do not contain any residual
value guarantees or material restrictive covenants.
The
rate implicit is most of Company’s leases are
not reasonably determinable, therefore we use our incremental borrowing rate based on the information available at the commencement date
to determine the present value of the future lease payments.
Certain
of Company’s leases include variable costs. Variable costs include non-lease components that were incurred based
upon actual terms rather than contractually fixed amounts. In addition, variable costs are incurred for lease payments that are
indexed to a change in rate or index. Because the ROU asset recorded on the balance sheet was determined based upon factors considered
at the commencement date, subsequent changes in the rate or index that were not contemplated in the ROU asset balances recorded
on the balance sheets result in variable expenses being incurred when paid during the lease term. See Note 12.
The
Company has elected not to recognize on the balance sheet leases with terms of 12 months or less.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
3 - REVERSE RECAPITALIZATION
On
December 30, 2019, Intellisense and Medigus completed the Exchange Agreement. The accounting treatment for the Exchange Agreement
was as a reverse recapitalization transaction. Pursuant to the Exchange Agreement, Intellisense issued to Medigus 16,130,952
shares. Upon such issuance, ScoutCam became a wholly-owned subsidiary of Intellisense. On December 31,
2019, Intellisense Solutions Inc. changed its name to ScoutCam Inc.
Immediately
prior to the Closing Date the Company’s outstanding common stock was comprised of 3,927,346 shares of common stock $0.001 par value, of which 1,352,666 shares were issued immediately prior to the Closing Date as part of the conversion
of promissory notes to related parties and the exercise of warrants by related parties, employees and service providers.
Also,
on the Closing Date, 3,413,312 units, each comprised of two shares
of common stock par value USD 0.001 per share, one Warrant A (as defined below) and two Warrants B (as defined below), were issued
to investors as part of the financing transaction that the Company was obligated to secure prior to the Closing. The immediate
gross proceeds from the issuance of the units amounted to approximately USD 3.3 million.
Each
Warrant A was exercisable into one share of common stock of the Company at an exercise price of USD 0.595 per share during
the12 month period from the date of issuance. Each Warrant B is exercisable into one share of common stock of the Company
at an exercise price of USD 0.893 per share during the 18 month period from the date of issuance.
During
2020, 2,992,855 Warrants A were exercised. 420,457 unexercised Warrants A expired on December 30,2020.
While
ScoutCam Inc. was the legal acquirer, ScoutCam was treated as the acquiring company for accounting purposes as the Exchange Agreement
was accounted for as a reverse recapitalization which is equivalent to the issuance of 10,753,969 shares by ScoutCam for the net
monetary assets of ScoutCam Inc. As a result, the financial statements of the Company prior to the Closing Date are the historical financial
statements of ScoutCam Ltd. The financial statements of the Company after the Closing Date reflect the results of the operations of ScoutCam
Ltd. and ScoutCam Inc. on a combined basis. The net acquired assets of the Company as of the Closing Date was $3,040 thousands. There
were no fair value adjustments necessary to perform as the carrying values of the net acquired assets approximated fair value. Further,
given the nature of the operations of ScoutCam Inc. prior to the Closing Date, there were no intangible assets, including goodwill, established
as a result of the Exchange Agreement.
Under the Exchange Agreement, the number
of shares of common stock and USD amount for common stock is based on the nominal value and the shares of common stock
issued by ScoutCam Inc. (reflecting the legal structure of ScoutCam Inc. as the legal acquirer) on the Closing Date plus shares of
common stock issued by ScoutCam Inc. as part of the Exchange Agreement as described above. Historical stockholders’ equity
reflects the accounting acquirer, except for share number and USD amount adjusted for the shares exchange ratio pursuant to the Exchange
Agreement amounting to 8.065.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
4 - INVENTORY:
Composed
as follows:
|
|
|
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
|
|
USD
in thousands
|
|
|
Raw materials and supplies
|
|
|
45
|
|
|
|
24
|
|
|
Work in progress
|
|
|
-
|
|
|
|
316
|
|
|
Finished goods
|
|
|
278
|
|
|
|
560
|
|
|
Inventory write downs
|
|
|
(79
|
)
|
|
|
-
|
|
|
|
|
|
244
|
|
|
|
900
|
|
During
the year ended 2019, no impairment occurred.
NOTE
5 - PROPERTY AND EQUIPMENT, NET:
Property,
plant and equipment, net consisted of the following:
|
|
|
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
|
|
USD
in thousands
|
|
|
Cost:
|
|
|
|
|
|
|
|
|
|
Machinery and laboratory equipment
|
|
|
285
|
|
|
|
87
|
|
|
Leasehold improvements, office furniture and equipment
|
|
|
36
|
|
|
|
25
|
|
|
Computers and computer software
|
|
|
87
|
|
|
|
20
|
|
|
|
|
|
408
|
|
|
|
132
|
|
|
Less: accumulated
deprecation
|
|
|
(139
|
)
|
|
|
(73
|
)
|
|
Total property
and equipment, net
|
|
|
269
|
|
|
|
59
|
|
Depreciation
expenses were USD 66 thousand, USD 6 thousand and USD 5 thousand in the years ended December 31, 2020,
2019 and 2018, respectively.
NOTE
6 – OTHER ACCRUED EXPENSES:
|
|
|
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
|
|
USD
in thousands
|
|
|
Unpaid recapitalization
transaction costs
|
|
|
-
|
|
|
|
89
|
|
|
IRS (see note 7b)
|
|
|
73
|
|
|
|
73
|
|
|
Accrued expenses
|
|
|
122
|
|
|
|
390
|
|
|
|
|
|
195
|
|
|
|
552
|
|
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
7 - INCOME TAXES:
The
Company and its subsidiary are taxed under the domestic tax laws of the jurisdiction of incorporation of each entity (United States
and Israel).
Income
from Israel was taxed at the corporate tax rate of 23%.
ScoutCam
Inc. was incorporated in the United States and is subject to the Federal and State tax laws established in the United States.
On
December 22, 2017, the Tax Cuts and Jobs Act (the “Act”) was signed into law. The Act reduces the corporate tax rate
to 21 percent from 35 percent, among other things.
|
|
b.
|
ScoutCam
Inc. did not timely file its tax return for 2013-2014 and therefore the IRS imposed penalties in the amount of $60
thousand (approximately $73 thousands including interest).
|
|
|
c.
|
Israel
tax loss carry forwards
|
As
of December 31, 2020, the Company has accumulated losses for tax purposes that were generated in Israel. These losses may
be carried forward and offset against taxable income in the future for an indefinite period. A full valuation allowance was created
against the Company’s deferred tax assets generated in Israel. Management currently believes that it is more likely than
not that the deferred taxes generated in Israel will not be realized in the foreseeable future.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
8 – RELATED PARTIES:
|
|
a.
|
On
May 30, 2019, ScoutCam entered into an intercompany agreement with Medigus (the “Intercompany
Agreement”) according to which ScoutCam agreed to hire and retain certain services
from Medigus. The agreed upon services provided under the Intercompany Agreement included:
(1) lease of office space and clean room based on actual space utilized by ScoutCam and
in shared spaces according to employee ratio; (2) utilities such as electricity water,
IT and communication services based on employee ratio; (3) car services, including car
rental, gas usage, payment for toll roads based on 100% of expense incurred from a ScoutCam
employee car; (4) external accountant services at a price of USD 6,000 per annum; (5)
directors and officers insurance at a sum of 1/3 of Parent Company cost; (6) CFO
services at a sum of 50% of Parent Company CFO employer cost; (7) every direct
expense of ScoutCam that is paid by the Parent Company in its entirety subject
to approval of such direct expenses in advance; and (8) any other mutual expense that
is borne by the parties according to the respective portion of the Mutual Expense.
|
The
total expenses for year ended December 31, 2019 amounted to USD 329 thousand. As of December 31, 2019, the balance with
Medigus amounted to USD 73 thousand.
On
April 20, 2020, the Subsidiary entered into an amended and restated intercompany services agreement with Medigus. The agreed upon
services provided under the amended and restated Intercompany Agreement included:
1)
lease of office space based on actual space utilized by the Parent Company and in shared spaces according to employee ratio; (2)
utilities such as electricity water, IT and communication services based on employee ratio; (3) car services, including car rental,
gas usage, payment for toll roads based on 100% of expense incurred from a Subsidiary employee car; (5) directors and officers
insurance the Parent Company shall pay $150,000 of the annual premium.; (6) CFO services at a sum of 50% of Parent Company CFO
employer cost; (7) every direct expense of the Subsidiary that is paid by the Parent Company in its entirety subject to approval
of such direct expenses in advance; and (7) any other mutual expense that is borne by the parties according to the respective
portion of the mutual expense.
The
total net expenses for year ended December 31, 2020 amounted to USD 143 thousand. As of December 31, 2020, the balance with Medigus
amounted to USD 47 thousand.
In
addition, ScoutCam’s employees provide support services to Medigus. For additional information see note 11b.
|
|
b.
|
On
June 3, 2019, the Parent Company executed a capital contribution with ScoutCam
whereby it paid an aggregate amount of USD 720 thousand.
|
|
|
|
|
|
|
c.
|
On
July 31, 2019, ScoutCam and Prof. Benad Goldwasser entered into a consulting agreement,
whereby Prof. Goldwasser agreed to serve as chairman of the Board of Directors
of ScoutCam. The consulting agreement effective retroactively to March 1, 2019,
in consideration for, inter alia, a monthly fee of $10,000 and options representing
5% of Company’s fully-diluted share capital as of the Closing Date.
|
|
|
|
|
|
|
d.
|
On
August 27, 2019, the Parent Company provided ScoutCam with a line of credit in the aggregate
amount of USD 500 thousand and, in exchange, ScoutCam agreed to grant the Parent Company
a capital note that will bear an annual interest rate of 4%. The repayment of the credit
line amount shall be spread over one year in monthly payments beginning January 2020.
The said note is presented in the consolidated balance sheets within “Loan
from Parent Company”.
|
|
|
|
|
|
|
|
On June 23, 2020, the Company and Medigus entered into a certain
Conversion Side Letter, pursuant to which the Company converted US$381,136 worth of outstanding credit previously extended by Medigus
to the Company, which amount, as of the date thereof, included interest accrued thereon. In accordance with the terms of the Conversion
Side Letter, the Company issued to Medigus, at a purchase price of US$0.968, (a) 787,471 shares of common stock, (b) warrants
to purchase 393,736 shares of common stock at an exercise price of US$0.595, and (c) warrants to purchase 787,471 shares of
common stock at an exercise price of US$0.893.
|
|
|
|
|
|
|
e.
|
On
September 3, 2019, a certain Asset Transfer Agreement, by and between ScoutCam and the Parent Company dated May 28, 2019,
became effective. According to the Asset Transfer Agreement, the Company transferred certain assets (property and equipment)
with a nil carrying amount to the Parent Company in consideration of USD 168 thousand. The assets were then sold to a third
party. The excess of the said consideration over the carrying amount was directly recorded to shareholders’ equity.
|
|
|
|
|
|
|
f.
|
During
December 2019, the Company entered into a consulting agreement with Shrem Zilberman Group (the “Consultant”) in
the amount of USD 165 thousand (see also note 9b). A director of the Company is related to one of the Consultant’s shareholders.
|
|
|
|
|
|
|
g.
|
On
February 12, 2020, the Company’s Board of Directors authorized the grant of options to purchase 2,235,691 shares
of common stock of the Company to Professor Benad Goldwasser, the Company’s Chairman of the Board, and options
to purchase 1,865,346 shares of common stock of the Company to certain officers of the Company. Each option is exercisable
into one share of common stock of the Company of $0.001 par value at an exercise price of $0.29. See also note
13b.
|
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
8 – RELATED PARTIES (continued):
|
|
h.
|
On
March 15, 2020, the Company’s Board of Directors approved, among other things,
a quarterly fee of $4,000 payable to each of the Company’s directors, excluding
Professor Benad Goldwasser; and a grant of options to purchase 576,888 shares
of common stock of the Company to each of the Company’s currently serving
directors, excluding Professor Benad Goldwasser. The terms of the options granted to
the Company’s currently serving directors include (i) an exercise price of $0.29
(ii) a vesting schedule whereby 33.33% of the options granted will vest on the first
anniversary of March 15, 2020, and 8.33% of the options will vest at the end of each
subsequent three-month period thereafter over the course of the following two (2) years;
and (iii) an acceleration mechanism pursuant to which any outstanding and unvested option
shall immediately accelerate and vest upon the occurrence of certain events, including,
inter alia, a merger or sale of all assets of the Company.
|
|
|
|
|
|
|
i.
|
On
April 20, 2020, Medigus and ScoutCam entered into that certain Intercompany Services
Agreement, which amended and restated the intercompany services agreement executed between
the parties on May 30, 2019. The agreement has an initial term of one year, and renews
automatically for additional one-year periods, unless either party provides 60 (sixty)
days written notice of non renewal. Either Medigus or ScoutCam may terminate the agreement
for convenience upon providing 60 (sixty) days prior written notice. The services to
be provided by ScoutCam include, inter alia, the provision of office space, utilities,
car services, insurance and chief financial officer services. In consideration for the
foregoing services, ScoutCam is entitled to arm’s length service fees based on
the most recent transfer pricing analysis as performed by an external expert, which may
be adjusted from time to time.
|
|
|
|
|
|
|
j.
|
On
May 18, 2020, in connection with the Arkin Transaction (as defined below), the Company, Medigus
and Arkin (as defined below), entered into the Letter Agreement, whereby, provided the Company
obtains certain regulatory approvals described therein, Medigus and the Company agreed to
amend certain terms of the Amended and Restated Asset Transfer Agreement and the License
Agreement, thereby transferring outright certain patent assets from Medigus to the Company;
provided, however, that in the event the Company abandons the foregoing patent assets,
the Company must transfer back ownership of the patent assets to Medigus for no additional
consideration and absent any additional contingencies.
Also,
on May 18, 2020, and in connection with the Arkin Transaction, the
Company, Medigus and Arkin entered into a Voting Agreement, pursuant to which Arkin and Medigus each agreed to vote their respective
shares of common stock in favor of the election of the opposite party’s designated representative(s), as applicable, to
the Board. Each of Arkin’s and Medigus’ rights under the Voting Agreement are contingent upon, inter alia, such party maintaining
a certain beneficial ownership threshold in the Company, as follows:
at
each annual or special meeting of stockholders at which an election of directors is held or pursuant to any written consent of
the stockholders, (a) one person designated by Arkin shall be elected to the Board, for so long as Arkin, together with its Affiliates,
continues to own beneficially at least eight (8%) of the issued and outstanding capital stock of the Company (“Arkin
Director”), and (b) (i) three persons designated by Medigus shall be elected to the Board, for so long as Medigus, together
with its Affiliates, continues to own beneficially at least thirty five (35%) of the issued and outstanding capital stock of the
Company, or (ii) two persons designated by Medigus for so long as Medigus, together with its Affiliates, continues to own beneficially
less than thirty five (35%) and more than twenty (20%) of the issued and outstanding capital stock of the Company, or (iii) one
person designated by Medigus for so long as Medigus, together with its Affiliates, continues to own beneficially less than twenty
(20%) and more than eight (8%) of the issued and outstanding capital stock of the Company.
|
|
|
|
|
|
|
k.
|
On
June 22, 2020, the Company’s Board of Directors authorized the grant of options
to purchase 628,163 shares of common stock to Prof. Benad Goldwasser, Chairman of
the Board, and 628,162 options to purchase shares of common stock to CEO and director
of the Company. Each option is exercisable into one share of common stock at
an exercise price of $0.29.
|
|
|
|
|
|
|
l.
|
On
November 11, 2020, the Company’s Board of Directors authorized the grant
of options to purchase 144,222 shares of common stock to director of the Company.
Each option is exercisable into one share of common stock at an
exercise price of $0.35.
|
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
9 - EQUITY:
Reverse
Recapitalization:
|
|
|
As
discussed in note 3, the Recapitalization is accounted for as a reverse recapitalization with ScoutCam Inc. as the legal acquirer
and ScoutCam Ltd. as the accounting acquirer. Under the Recapitalization, the USD amount for shares of common stock
is based on the nominal value and the shares of common stock issued by ScoutCam Inc. (reflecting the legal structure
of ScoutCam Inc. as the legal acquirer) on the Recapitalization Date plus shares of common stock issued by the Company
as part of the Recapitalization as described above. Historical stockholders’ equity reflects the accounting acquirer’s
share number and USD amount adjusted for the exchange ratio determined in the Recapitalization.
|
Private
placement:
|
|
a.
|
In
December 2019, the Company allocated in a private issuance, a total of 3,413,312 units at a purchase price of USD $0.968
per unit. Each unit was comprised of two shares of common stock par value US$0.001 per share, one Warrant A (defined
below) and two Warrants B (defined below). The immediate proceeds (gross) from the issuance of the units amounted to approximately
USD 3.3 million.
|
Each Warrant A was exercisable
into one share of common stock of the Company at an exercise price of USD 0.595 per share during the 12 month period
following the allocation. Each Warrant B is exercisable into one share of common stock of the Company at an exercise
price of USD 0.893 per share during the 18 month period following the allocation.
In addition, Shrem Zilberman Group
Ltd. (the “Consultant”) will be entitled to receive the amount representing 3% of any exercise price of each Warrant
A or Warrant B that may be exercised in the future. In the event the total proceeds received as a result of exercise of Warrants
will be less than $2 million at the time of their expiration, the Consultant will be required to invest $250,000 in the Company
in return for shares of common stock of Company.
During
2020, 2,992,855 Warrants A were exercised. 420,457 unexercised Warrants A expired on December 30, 2020.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
9 – EQUITY (continued):
|
|
b.
|
On
March 3, 2020, the Company issued in a private issuance a total of 979,754 units at a purchase price of USD $0.968 per unit.
|
Each
unit was comprised of two shares of common stock par value US$0.001 per share, one Warrant A (defined below) and two
Warrants B (defined below).
Each
Warrant A was exercisable into one share of common stock of the Company at an exercise price of USD 0.595 per
share during the 12 month period following the allocation.
Each
Warrant B is exercisable into one share of common stock of the Company at an exercise price of USD 0.893 per share during the
18 month period following the allocation.
The
gross proceeds from the issuance of all securities offered amounted to approximately USD 948 thousands. After deducting
issuance costs, the Company received proceeds of approximately USD 909 thousand.
During
2021, 979,784 Warrants A were exercised.
|
|
c.
|
On
May 18, 2020, the Company allocated in a private issuance a total of 2,066,116 units at a purchase price of USD $0.968
per unit.
|
Each
unit was comprised of two shares of common stock par value US$0.001 per share, one Warrant A (defined below) and two Warrants
B (defined below).
Each
Warrant A is exercisable into one share of common stock of the Company at an exercise price of USD 0.595 per share during
the 18 month period following the allocation.
Each
Warrant B is exercisable into one share of common stock of the Company at an exercise price of USD 0.893 per share during
the 24 month period following the allocation.
The
gross proceeds from the issuance of all securities offered amounted to approximately USD 2 million. After deducting issuance
costs, the Company received proceeds of approximately USD 1.9 million.
During
February 2021, 336,135 Warrants A were exercised.
|
|
d.
|
On
June 23, 2020, (the “Conversion Date”), the Company entered into and consummated
a Side Letter Agreement with Medigus, whereby the parties agreed to convert, at a conversion
price of $0.484, an outstanding line of credit previously extended by Medigus to the Subsidiary,
which as of the Conversion Date was $381,136, into (a) 787,471 shares of the Company’s
common stock, (b) warrants to purchase 393,736 shares of common stock with
an exercise price of $0.595 (Warrant A), and (c) warrants to purchase 787,471 shares of common
stock with an exercise price of $0.893 (Warrant B). As the conversion price represented
the same unit price as in the March 2020 and May 2020 private placements, no finance expenses
have been recorded in statement of operations as a result of the conversion.
Each
Warrant A is exercisable into one share of common stock of the Company at an exercise price of USD 0.595 per
share during the 12 months period following the allocation.
Each
Warrant B is exercisable into one share of common stock of the Company at an exercise price of USD 0.893 per
share during the 18 months period following the allocation.
|
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
9 – EQUITY (continued):
As
of December 31, 2020, the Company had the following outstanding warrants to purchase common stock:
|
Warrant
|
|
Issuance
Date
|
|
Expiration
Date
|
|
Exercise
Price
Per Share ($)
|
|
|
Number
of Shares
of common stock
Underlying
Warrants
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant Medigus
|
|
December 30, 2019
|
|
December 30, 2022
|
|
(*)
|
|
|
2,688,492
|
|
|
Warrant B
|
|
December 30, 2019
|
|
June 30, 2021
|
|
|
0.893
|
|
|
|
6,826,623
|
|
|
Warrant A
|
|
March 3, 2020
|
|
March 3, 2021
|
|
|
0.595
|
|
|
|
979,754
|
|
|
Warrant B
|
|
March 3, 2020
|
|
September 3, 2021
|
|
|
0.893
|
|
|
|
1,959,504
|
|
|
Warrant A
|
|
May 18, 2020
|
|
November 18, 2021
|
|
|
0.595
|
|
|
|
2,066,116
|
|
|
Warrant B
|
|
May 18 2020
|
|
May 18, 2022
|
|
|
0.893
|
|
|
|
4,132,232
|
|
|
Warrant A
|
|
June 23, 2020
|
|
June 23, 2021
|
|
|
0.595
|
|
|
|
393,736
|
|
|
Warrant B
|
|
June 23,2020
|
|
December 23, 2021
|
|
|
0.893
|
|
|
|
787,471
|
|
|
|
|
|
|
|
|
|
|
|
|
|
19,833,928
|
|
|
(*)
|
If ScoutCam. achieves an aggregate
amount of $33 million in sales within the first three years immediately after the Exchange Agreement, the Company will issue
to Medigus 2,688,492 shares of the Company’s common stock, which represents 10% of the Company’s issued and outstanding
share capital as of the Exchange Agreement.
|
Stock
based compensation:
2020
Equity Incentive Plan
In February 2020, the Company’s
Board of Directors approved the 2020 Share Incentive Plan (the “Plan”). The Plan initially included an option
pool of 5,228,007 shares of common stock for grant to Company employees, consultants, directors, and other service providers.
On March 15, 2020, the Company’s Board of Directors approved an increase to the Company’s option pool pursuant to the Plan
by an additional 576,888 shares of common stock. On June 22, 2020, the Company’s Board of Directors approved an increase
to the Company’s option pool pursuant to the Plan by an additional 3,617,545 shares of common stock.
The Plan is designed to enable the
Company to grant options to purchase ordinary shares and RSUs under various and different tax regimes including, without limitation:
(i) pursuant and subject to Section 102 of the Israeli Tax Ordinance or any provision which may amend or replace it and any regulations,
rules, orders or procedures promulgated thereunder and to designate them as either grants made through a trustee or not through
a trustee; and (ii) pursuant and subject to Section 3(i) of the Israeli Tax Ordinance.
On February 12, 2020, the Company granted
4,367,515 options pursuant to the Plan. Each option is exercisable into one share of common stock of the Company of $0.001
par value at the exercise price of $0.29.
On March 15, 2020, the Company granted
576,888 options pursuant to the Plan to each of the Company’s then serving directors, excluding Professor Benad Goldwasser. Each
option is exercisable into one share of common stock of the Company of $0.001 par value at the exercise price of $0.29.
On June 22, 2020, the Company granted
1,544,769 options pursuant to the Plan to Company employees, consultants, directors. Each option is exercisable into one
share of common stock of the Company of $0.001 par value at the exercise price of $0.29.
On November 11, 2020, the Company
granted 144,222 options pursuant to the Plan to Company director. Each option is exercisable into one share of common stock of the Company of $0.001 par value at the exercise price of $0.35.
Options
granted generally have a contractual term of 7 years and vest over a period of 3 up to 4 years.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
9 – EQUITY (continued):
Stock
Option Activity
The
following summarizes stock option activity:
|
|
|
Amount
of options
|
|
|
Weighted
average exercise price
|
|
|
Weighted
Average Remaining Contractual Term (years)
|
|
|
Aggregate
Intrinsic Value (in thousands)
|
|
|
|
|
|
|
|
$
|
|
|
|
|
|
$
in thousands
|
|
|
Outstanding - December 31, 2019
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
Granted
|
|
|
6,633,394
|
|
|
|
0.29
|
|
|
|
|
|
|
|
|
|
|
Outstanding - December 31, 2020
|
|
|
6,633,394
|
|
|
|
0.29
|
|
|
|
6.23
|
|
|
|
2,446
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Exercisable - December
31, 2020
|
|
|
1,941,701
|
|
|
|
0.29
|
|
|
|
6.12
|
|
|
|
718
|
|
At
December 31, 2020, the aggregate intrinsic value of options granted is calculated as the difference between the exercise price
and the closing price on the same date.
The
Company estimates the fair value of stock option awards on the grant date using the Black-Scholes option pricing model.
The weighted-average grant date fair value per option granted during the years ended December 31, 2020 was $0.27. The fair value of each
award is estimated using Black-Scholes option pricing model based on the following assumptions:
|
|
|
Year
ended
December
31, 2020
|
|
|
Underlying value of ordinary shares
($)
|
|
|
0.446-0.800
|
|
|
Exercise price ($)
|
|
|
0.29-0.35
|
|
|
Expected volatility (%)
|
|
|
43.35%-45.00
|
%
|
|
Term of the options (years)
|
|
|
7
|
|
|
Risk-free interest rate (%)
|
|
|
0.54%-1.55
|
%
|
Volatility
is derived from the historical volatility of publicly traded set of peer companies. The risk-free interest rates used in the Black-Scholes
calculations are based on the prevailing U.S. Treasury yield as determined by the U.S. Federal Reserve. The Company has not paid
dividends does not anticipate paying dividends in the foreseeable future. Accordingly, no dividend yield was assumed for purposes
of estimating the fair value of the Company's share-based compensation. The weighted average expected life of options was estimated
individually in respect of each grant.
The
unrecognized compensation expense calculated under the fair-value method for stock options expected to vest as of December 31,
2020 is approximately $0.6 million and is expected to be recognized over a weighted-average period of 1.2 years.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
10 - REVENUES:
|
|
a.
|
Contract
fulfillment assets:
|
The
Company’s contract fulfillment assets as of December 31, 2020:
|
|
|
December
31,
|
|
|
|
|
2020
|
|
|
|
|
USD
in thousands
|
|
|
Contract
fulfillment assets from contract with Customer B (see note 11b)
|
|
|
1,130
|
|
The
Company’s contract liabilities were as follows:
|
|
|
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
2018
|
|
|
|
|
USD
in thousands
|
|
|
The change in deferred revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at beginning of
year
|
|
|
502
|
|
|
|
200
|
|
|
|
8
|
|
|
Deferred revenue
relating to new sales
|
|
|
735
|
|
|
|
387
|
|
|
|
200
|
|
|
Revenue
recognition during the period
|
|
|
(389
|
)
|
|
|
(85
|
)
|
|
|
(8
|
)
|
|
Balance at end of year
|
|
|
848
|
|
|
|
502
|
|
|
|
200
|
|
Contract
liabilities include advance payments, which are primarily related to advanced billings for development services.
Revenue
recognized in 2020 that was included in deferred revenue balance as of December 31, 2019 was USD 389 thousand.
There
was no revenue recognized in 2019 that was included in deferred revenue balance as of December 31, 2018.
Revenue
recognized in 2018 that was included in deferred revenue balance as of January 1, 2018 was USD 8 thousand.
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
10 – REVENUES (continued):
Remaining
Performance Obligations
Remaining
Performance Obligations (“RPO”) represents contracted revenue that has not yet been recognized, which includes contract
liability and amounts that will be invoiced and recognized as revenue in future periods. As of December 31, 2020, the total
RPO amounted to USD 2.9 million, Which the Company expects to recognize over the expected manufacturing
term of the product under development.
NOTE
11 - ENTITY WIDE DISCLOSURES:
ASC
280, “Segment Reporting,” establishes standards for reporting information about operating segments. The Company manages
its business based on one operating segment and derives revenues from sales of products and services developing minimally invasive
endosurgical tools and highly innovative imaging solutions.
|
|
a.
|
Revenues
by geographical area (based on the location of customers)
|
The
following is a summary of revenues within geographic areas:
|
|
|
Year
ended on
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
2018
|
|
|
|
|
USD
in thousands
|
|
|
United States
|
|
|
418
|
|
|
|
142
|
|
|
|
300
|
|
|
United Kingdom
|
|
|
41
|
|
|
|
33
|
|
|
|
24
|
|
|
South Korea
|
|
|
-
|
|
|
|
-
|
|
|
|
7
|
|
|
Israel
|
|
|
5
|
|
|
|
67
|
|
|
|
12
|
|
|
Other
|
|
|
27
|
|
|
|
67
|
|
|
|
48
|
|
|
|
|
|
491
|
|
|
|
309
|
|
|
|
391
|
|
Set
forth below is a breakdown of Company’s revenue by major customers (major customer –revenues from these customers
constituted at least 10% of total revenues in a certain year):
|
|
|
Year
ended on
|
|
|
|
|
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
2018
|
|
|
|
|
USD
in thousands
|
|
|
Customer
A
|
|
|
383
|
|
|
|
85
|
|
|
|
134
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer B
|
|
|
-
|
|
|
|
30
|
|
|
|
92
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customers C
|
|
|
41
|
|
|
|
33
|
|
|
|
21
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer D –
Parent Company
|
|
|
5
|
|
|
|
36
|
|
|
|
-
|
|
SCOUTCAM
INC. (Formerly known as Intellisense Solutions Inc.)
NOTES
TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE
12 - LEASES
The
Company’s leases relate to vehicles leases and to short term lease of Company’s offices.
The
components of lease expenses during the periods presented were as follows:
|
|
|
Year
ended
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
|
|
USD
in thousands
|
|
|
Operating lease expenses
|
|
|
45
|
|
|
|
29
|
|
|
Short-term lease
expenses
|
|
|
88
|
|
|
|
60
|
|
|
Total lease expenses
|
|
|
133
|
|
|
|
89
|
|
Supplemental
cash flow information related to operating leases during the period presented was as follows:
|
|
|
Year
ended December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
|
|
USD
in thousands
|
|
|
Cash
paid for amounts included in the measurement of lease liabilities:
|
|
|
|
|
|
|
|
|
|
Operating
cash flows from operating leases
|
|
|
45
|
|
|
|
29
|
|
Lease
term and discount rate related to operating leases as of the period presented were as follows:
|
|
|
December
31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
|
|
USD
in thousands
|
|
|
Weighted-average remaining
lease term (in years)
|
|
|
1.85
|
|
|
|
1.4
|
|
|
Weighted-average
discount rate
|
|
|
10
|
%
|
|
|
10
|
%
|
The
maturities of lease liabilities under operating leases as of December 31, 2020 are as follows:
|
|
|
USD
in thousands
|
|
|
2021
|
|
|
63
|
|
|
2022
|
|
|
47
|
|
|
2023
|
|
|
8
|
|
|
Total undiscounted lease payments
|
|
|
118
|
|
|
Less: Imputed
interest
|
|
|
(11
|
)
|
|
Total lease liabilities
|
|
|
107
|
|
NOTE
13 - SUBSEQUENT EVENTS:
|
|
a.
|
On
January 20, 2021, the Company’s Board of Directors approved an increase of the authorized share capital of the Company
by an additional 225,000,000 ordinary shares par value $0.001 per share, such that the authorized share capital of the Company
following such increase shall be consisting of 300,000,000 ordinary shares.
|
|
|
|
|
|
|
b.
|
Refer
to Note 9b-c regarding exercising of warrants.
|
|
|
|
|
|
|
c.
|
On March 22, 2021, the Company undertook to issue to certain investors
(the “Investors”) 22,222,223 units (the “Units”) in exchange for an aggregate purchase price of $20 million.
Each Unit consists of (i) one share of the Company’s common stock and (ii) one warrant to purchase one share of common stock with an exercise price of US$1.15 per share (the “Warrant” and the “Exercise Price”). Each Warrant
is exercisable until the close of business on March 31, 2026.
|
|
|
|
|
|
|
|
Pursuant
to the terms of the Warrants, following April 1, 2024, if the closing price of the common stock equal or exceeds 135% of the Exercise
Price (subject to appropriate adjustments for stock splits, stock dividends, stock combinations and other similar transactions
after the issue date of the Warrants) for any thirty (30) consecutive trading days, the Company may force the exercise of the
Warrants, in whole or in part, by delivering to the Investors a notice of forced exercise.
|

22,222,223
Shares of Common Stock and
22,222,223
Shares of Common Stock Underlying Warrants
PROSPECTUS
May
10, 2021
Scoutcam (QB) (USOTC:SCTC)
Historical Stock Chart
From Mar 2024 to Apr 2024
Scoutcam (QB) (USOTC:SCTC)
Historical Stock Chart
From Apr 2023 to Apr 2024