GNBT – GENEREX BIOTECHNOLOGY
ASSEMBLING MASSIVE GROWTH MACHINE
September 18, 2019 -- InvestorsHub NewsWire -- via BioResearch
Alert
Sep. 18, 2019 8:57 AM ET|About: Generex Biotechnology Corp.
(GNBT)

- A37 Breast Cancer
Vaccine demonstrates high efficacy with low adverse side
effects against many common cancers.
- Began enrollment of A37 Combination Phase II
trial with Merck blockbuster Keytruda for Breast
Cancer.
- Upfront license
payments for AE37 by Shenzhen BioScien Pharmaceuticals for
Prostate Cancer in China, Taiwan, Hong Kong and Macau.
- Plans underway for the spin-out of NuGenerex Immuno-Oncology
that includes A37.
- Proprietary buccal oral insulin delivery for $27 billion insulin
market.
- LOI to acquire majority interest
in ALTuCELL and their Cellular Therapy Product Altsulin®
with Patented Microencapsulation Technology for the Treatment of
Type I Diabetes.
- FDA approved Advanced Wound Care products.
- Broad product line of FDA approved orthopedic implants.
- Aggressive acquisition strategy building Generex rapidly.
- 9-month 2019 revenues increased 2,800
times versus same 2018 period.
- Upcoming 1 for 1 dividend ONLY
to float shareholders.
- Recently retired 20,375,900 shares
- Upcoming Nasdaq up-list announced.
Summary
Generex Biotechnology Corp. (GNBT:
OTC) shares have been in a strong uptrend and steadily climbing
during the short time since Joe Moscoto took over the helm of the
company. Since then, the company has amassed an almost unbelievable
number of acquisitions and LOI’s where each one has
multi-blockbuster potential to transform Generex from a minnow into
a whale. Revenues are already showing substantial gains with 9
month ending April 30, 2019 reported at $6,217,039 versus $2,218
for the same period in 2018. With expected high growth from the
accumulated earning of all divisions, it would not be surprising to
see shares trade over $12 in the next 12 months and there is also
the potential for soaring prices if the news is good on A37 and the
new diabetes delivery system and the new treatment for Type 1
diabetes and other indications.
Generex Biotechnology - GNBT - 1 year chart
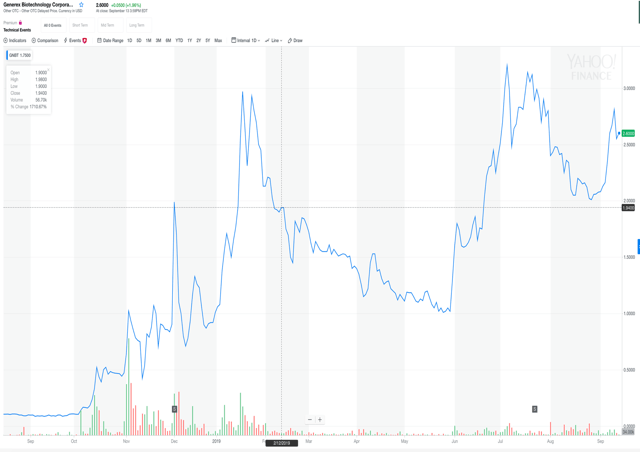
In addition to large revenue growth expectations, the company
has announced a spin-out of the NuGenerex Immuno-Oncologythat owns
the rights to the exciting new cancer treatment drug, A37, a 1 for
1 share dividend pay able only to float shareholders, the
retirement of over 20 million shares, and an impending up-listing
to NASDAQ.
With breakthrough products that are either already approved for
sale by regulatory agency or in the pipeline for approvals, Generex
is strongly positioned to enjoy extraordinary financial performance
in the not too distant future.
With a market cap of well under $200 million, Generex shares
appear very undervalued.
The following recent press release tells more of their exciting
story:
Generex Biotechnology Announces First Patient Enrolled in the
Phase II Clinical Trial of AE37 in Combination with Pembrolizumab
(KeytrudaÒ) for the Treatment of Triple Negative Breast Cancer
MIRAMAR, Fla., Sept. 17, 2019 (GLOBE NEWSWIRE) -- Generex
Biotechnology Corporation (www.generex.com) (GNBT)
(http://www.otcmarkets.com/stock/GNBT/quote)
today announced that the NSABP Foundation, Inc. (NSABP) has
enrolled the first patient in the study: A Phase II
Clinical Trial ofPembrolizumab in Combination with the
AE37 Peptide Vaccine in Patients with Metastatic Triple Negative
Breast Cancer (NSABP FB-14). The trial, conducted in
conjunction with research partners Merck and the NSABP Foundation,
is designed to evaluate the safety and tolerability of AE37 given
in combination with KEYTRUDAÒ (pembrolizumab) as well as the
objective response rate.
Eric von Hofe, President of NuGenerex-ImmunoOncology commented,
“This is an exciting milestone for NuGenerex Immuno-Oncology, as we
have now revitalized our AE37 development program with a focus on
combination therapy with checkpoint inhibitors, which are now
becoming standard of care for a broad array of cancers. Combining
our promising immunotherapeutic with Merck’s checkpoint inhibitor,
KEYTRUDA for the treatment of triple negative breast cancer
represents a novel treatment strategy for a cancer of high unmet
need. Prior studies with each agent alone showed promising signs of
efficacy. By combining the mechanisms of checkpoint inhibition by
KEYTRUDA with the robust, long-lasting and tumor-specific T-cell
activation by AE37, we hope to enhance the clinical effectiveness
of the immunotherapy strategies.”
Jason Terrell, MD, Chief Scientific and Medical Officer of
Generex Biotechnology commented, “The start of patient enrollment
represents the culmination of a committed effort by the team at
NuGenerex Immuno-Oncology (formerly Antigen Express) and our
research partners at the NSABP Foundation and Merck. NuGenerex
Immuno-Oncology has been a pioneer on the forefront of
immunotherapy for over a decade with our Ii-Key platform that
ensures CD4 T-cell activation against any tumor antigen to which
the Ii-Key is attached. The Ii-Key platform holds great promise,
and we plan to initiate additional trials using the combination of
checkpoint inhibitors with AE37 immune system activation in the
field of personalized immuno-oncology to help patients in need of
treatment options.”
About Generex Biotechnology Corp.Generex
Biotechnology is an integrated healthcare holding company with
end-to-end solutions for patient centric care from rapid diagnosis
through delivery of personalized therapies. Generex is building a
new kind of healthcare company that extends beyond traditional
models providing support to physicians in an MSO network, and
ongoing relationships with patients to improve the patient
experience and access to optimal care.
In addition to advancing a legacy portfolio of immune-oncology
assets, medical devices, and diagnostics, the Company is focused on
an acquisition strategy of strategic businesses that complement
existing assets and provide immediate sources of revenue and
working capital. Recent acquisitions include a management services
organization, a network of pharmacies, clinical laboratory, and
medical device companies with new and approved products.
Our newly formed, wholly-owned subsidiary, NuGenerex
Distribution Solutions (NDS), integrates our MSO network with a
pharmacy network, clinical diagnostic lab, durable medical
equipment company (DME-IQ) and dedicated call center.
About Olaregen TherapeuticxOlaregen
Therapeutix, Inc. is a regenerative medicine company focused on the
development, manufacturing and commercialization of products that
fill unmet needs in the current wound care market. Generex aims to
provide advanced healing solutions that substantially improve
medical outcomes while lowering the overall cost of care.
Olaregen's first product
introduction, Excellagen (flowable dermal
matrix) is a topically applied product for dermal wounds and other
indications. Excellagen is a FDA 510K cleared
device for a broad array of dermal wounds, including partial and
full thickness wounds, pressure ulcers, venous ulcers, diabetic
ulcers, chronic vascular ulcers, tunneled/undermined wounds,
surgical wounds (donor sites/ grafts, post-Mohs surgery, post-laser
surgery, podiatric, wound dehiscence), trauma wounds (abrasions,
lacerations, second-degree burns and skin tears) and draining
wounds, enabling Olaregen to
market Excellagen in multiple vertical markets.
Additionally, Excellagen can serve as an
Enabling Delivery Platform for pluripotent stem cells,
antimicrobial agents, small molecule drugs, DNA-Based Biologics,
conditioned cell media and peptides. Olaregen's initial focus will
be in advanced wound care including diabetic foot ulcers (DFU),
venous leg ulcers and pressure ulcers. Future products focusing on
innovative therapies in bone and joint regeneration comprise the
current pipeline. Generex's mission is to become a significant
force in regenerative medicine and advance the science of
healing.
About Service-Disabled Veteran-Owned Small Business
(SDVOSB)This a Service-Disabled Veteran-Owned Small
Business (SDVOSB) that specializes in the sale, marketing, and
distribution of innovative medical products through a nationwide
network of veteran owned distribution services.
About Pantheon MedicalPantheon Medical is a
manufacturer of a physician friendly, “all-in-one”, integrated kit
that includes plates, screws, and tools required for orthopedic
surgeons and podiatrists conducting foot and ankle surgeries.
Generex is developing and submitting several new product lines to
the FDA which will include cannulated surgical screws, plates, and
implants.
About MediSource PartnersMediSource Partners is
a 10-year-old private company, currently contracted with over 25
vendors (including Pantheon Medical) for nationwide distribution of
implants and devices for spine, hips, knees, foot, ankle, hand, and
wrist surgeries. Additional product lines include biologics (blood,
bone, tissue, stem cells), durable medical equipment, and soft
goods. Generex also supplies kits to process bone marrow aspirates
and platelet rich plasma biologics at the time of surgery.
Conclusion
Generex Biotechnology appears strongly positioned to enjoy
substantially higher price multiples of today’s $2.70 per share.
GNBT share price has been continually rising as the company is
positioning for several major achievements in 2019 and beyond.
There are risks associated with all drug development, but
navigating any drug through the challenging FDA approval, can
create remarkably high valuations and share prices. Generex offers
the additional advantage of diversification of potentially large
revenues from several different profit centers other than drug
development. Generex Biotechnology is showing early signs of very
strong revenue reversal and of being the new rising star of
biotech. Generex warrants a close look and early positioning before
revenues and developments advance further. An interesting note is
that small investors have the advantage of being able to purchase
shares now while larger institutional investors typically cannot
buy any shares that are under $5.00.
Legal Disclaimer:
Except for the historical information presented herein, matters
discussed in this release contain forward-looking statements that
are subject to certain risks and uncertainties that could cause
actual results to differ materially from any future results,
performance or achievements expressed or implied by such
statements. Emerging MicroCaps or BioResearchAlert or its
principals may have been compensated for its services in the form
of cash-based compensation. The Information contains
forward-looking statements, i.e. statements or discussions that
constitute predictions, expectations, beliefs, plans, estimates, or
projections as indicated by such words as ''expects,'' ''will,''
''anticipates,'' and ''estimates''; therefore, you should proceed
with extreme caution in relying upon such statements and conduct a
full investigation of the Information and the Profiled Issuer as
well as any such forward-looking statements.
Disclosure: I/we have no positions in any
stocks mentioned, and no plans to initiate any positions within the
next 72 hours.
Orignal Article - Seeking Alpha/GNBT
SOURCE: BioResearch Alert
Generex Biotechnology (CE) (USOTC:GNBT)
Historical Stock Chart
From Mar 2024 to Apr 2024
Generex Biotechnology (CE) (USOTC:GNBT)
Historical Stock Chart
From Apr 2023 to Apr 2024