Filed Pursuant to Rule 424(b)(3)
Registration No. 333- 254256
PROSPECTUS
THE CORETEC GROUP INC.
163,500,000 Shares of Common Stock Offered by the Selling Stockholders
This prospectus relates to the offering and resale by the selling stockholders identified herein of up to 163,500,000 shares of common stock issued or issuable to such selling stockholders including (i) 23,500,000 shares of our common stock, (ii) 82,500,000 shares of common stock issuable upon the exercise of outstanding warrants and (iii) 51,500,000 shares of common stock issuable upon the exercise of outstanding pre-funded warrants, which were issued by us on March 5, 2021 pursuant to a Securities Purchase Agreement entered into on March 2, 2021 with one institutional investor (the “March 2, 2021 Private Placement”) and (iv) 6,000,000 shares of common stock that may be acquired at an exercise price of $0.10 per share upon the exercise of outstanding unregistered warrants previously issued by us in connection with the March 2, 2021 Private Placement as placement agent consideration. Please see “Private Placement of Shares of Common Stock, Warrants and Pre-Funded Warrants” beginning on page 39 of this prospectus.
We will not receive any proceeds from the sale of shares of common stock by the selling stockholders. Upon the cash exercise of the warrants however, we will receive the exercise price of such warrants, for an aggregate of approximately $7,205,150.
The selling stockholders may sell all or a portion of the shares of common stock beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. Please see the section entitled “Plan of Distribution” on page 41 of this prospectus for more information. For information on the selling stockholders, see the section entitled “Selling Stockholders” on page 40 of this prospectus. We will bear all fees and expenses incident to our obligation to register the shares of common stock.
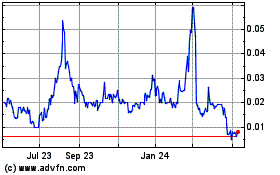
Our common stock is quoted on the OTCQB under the symbol “CRTG.” On March 11, 2021, the last reported sale price per share of our common stock was $0.2147.
The selling stockholders will offer their shares at prevailing market prices or privately negotiated prices.
We may amend or supplement this prospectus from time to time by filing amendments or supplements as required. You should read the entire prospectus and any amendments or supplements carefully before you make your investment decision.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 8 of this prospectus for a discussion of information that you should consider before investing in our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is March 22, 2021
TABLE OF CONTENTS
|
|
Page
|
|
|
|
|
About this Prospectus
|
3
|
|
Prospectus Summary
|
4
|
|
The Offering
|
5
|
|
Risk Factors
|
6
|
|
Special Note Regarding Forward-Looking Statements
|
15
|
|
Use of Proceeds
|
15
|
|
Dividends Policy
|
15
|
|
Our Business
|
16
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
23
|
|
Management
|
28
|
|
Executive and Director Compensation
|
33
|
|
Security Ownership of Certain Beneficial Owners and Management
|
34
|
|
Certain Relationships and Related Transactions
|
36
|
|
Description of Capital Stock
|
36
|
|
Private Placement of Shares of Common Stock, Warrants and Pre-Funded Warrants
|
39
|
|
Selling Stockholders
|
40
|
|
Plan of Distribution
|
41
|
|
Legal Matters
|
43
|
|
Experts
|
43
|
|
Where You Can Find More Information
|
43
|
|
Index to Financial Statements
|
44
|
ABOUT THIS PROSPECTUS
You may only rely on the information contained in this prospectus or that we have referred you to. We have not authorized anyone to provide you with different information. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities other than the common stock offered by this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any common stock in any circumstances in which such offer or solicitation is unlawful. Neither the delivery of this prospectus nor any sale made in connection with this prospectus shall, under any circumstances, create any implication that there has been no change in our affairs since the date of this prospectus. For investors outside the United States: Neither we nor the selling stockholders have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside the United States.
Unless the context otherwise requires, references to “we,” “our,” “us,” the “Group”, or the “Company” in this prospectus mean The Coretec Group, Inc., an Oklahoma corporation and its subsidiary.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus and does not contain all the information that you should consider in making your investment decision. Before investing in our common stock, you should carefully read this entire prospectus, including our financial statements and the related notes and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in each case included elsewhere in this prospectus.
Company Overview –
Coretec’s Technology. Coretec’s underlying technology is based on the production of a high value liquid silicon precursor, cyclohexasilane (“CHS”). A key advantage of CHS is that it remains in liquid form at room temperature and does not convert to a gas until heated above 450°F. CHS is a superior silicon precursor in many ways compared to materials commonly used for manufacturing silicon-based semiconductors and solar cells (monosilane or trichlorosilane) that have much lower boiling points which leads to higher cost handling and shipping. There are several technical advantages of using CHS versus common silicon precursors and one is that the production rate of the silicon-forming step can be increased by a factor of six, and relative to process temperature up to 10X or more, which leads to significant cost savings. We anticipate that CHS will first be used as an alternative to monosilane or trichlorosilane when adding silicon to lithium ion batteries or when used in manufacturing silicon-based semiconductors.
We also see longer term potential in several emerging markets where there are opportunities in the conversion of CHS into nanoparticles and nanowires for use in such emerging, high-growth markets as:
|
|
●
|
Authentication of critical documentation
|
|
|
●
|
Building-integrated solar energy
|
Enhancement of CSpace. A key challenge in the development of CSpace® is the development of the material used for the image chamber. The Company has explored a variety of glass alternatives. While progress has been made, it has been concluded that limitations remain, primarily in the weight and cost of a glass medium.
A key virtue of having our IP portfolio of silicon-based materials is that we use all of the manufacturing infrastructure and knowledge that is available for optical plastics for the CSpace® image chamber. The benefit to CSpace® is that silicon-based optical plastics can be molded into a broad range of shapes and allow the image chamber to be much lighter and much lower in cost than the glass material we worked with before.
Corporate Information
We were incorporated in the State of Oklahoma on August 11, 1995, as First Keating Corporation. Our name was changed to 3DIcon Corporation on August 1, 2003. Our name was changed to The Coretec Group, Inc. on June 22, 2017. Our principal executive offices are located at 333 Jackson Plaza, Suite 1200, Ann Arbor, MI 48103, and our telephone number is (918) 494-0505. Our fiscal year end is December 31.
THE OFFERING
|
Issuer
|
|
The Coretec Group, Inc.
|
|
|
|
|
|
Securities Offered by the Selling Stockholders
|
|
23,500,000 shares of our common stock, including 82,500,000 shares common stock issuable upon the exercise of warrants, 51,500,000 shares of common stock issuable upon the exercise of pre-funded warrants and 6,000,000 shares of common stock issuable upon the exercise of placement agent warrants.
|
|
|
|
|
|
Trading Market
|
|
The common stock offered in this prospectus is quoted on the OTCQB under the symbol “CRTG”.
|
|
|
|
|
|
Common Stock Outstanding Before this Offering
|
|
239,267,102 shares
|
|
|
|
|
|
Common Stock Outstanding After this Offering
|
|
379,267,102 shares1
|
|
|
|
|
|
Use of Proceeds
|
|
We will not receive any of the proceeds from the sale of the shares of our common stock being offered for sale by the selling stockholders. Upon the exercise of the warrants for an aggregate of 82,500,000 shares of common stock by payment of cash however, we will receive the exercise price of the warrants, or an aggregate of approximately $6,000,000 from the investor in the March 2, 2021 Private Placement and $600,000 from the exercise of the placement agent warrants issued to the placement agent in the March 2, 2021 Private Placement.
|
|
|
|
|
|
Plan of Distribution
|
|
The selling stockholders may sell all or a portion of the shares of common stock beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. Registration of the common stock covered by this prospectus does not mean, however, that such shares necessarily will be offered or sold. See “Plan of Distribution.”
|
|
|
|
|
|
Risk Factors
|
|
Please read “Risk Factors” and other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in the securities offered in this prospectus.
|
|
1
|
The number of shares of common stock shown above to be outstanding after this offering is based on 239,267,102 shares outstanding as of March 12, 2021 and assumes the exercise of the warrants into 82,500,000 shares of common stock, pre-funded warrants held by the selling stockholders into 51,500,000 shares of common stock and placement agent warrants issued as consideration into 6,000,000 shares of common stock.
|
RISK FACTORS
An investment in the Company’s common stock involves a high degree of risk. In determining whether to purchase the Company’s common stock, an investor should carefully consider all the material risks described below, together with the other information contained in this prospectus before deciding to purchase the Company’s securities. An investor should only purchase the Company’s securities if he or she can afford to suffer the loss of his or her entire investment.
Risks Relating to Our Businesses
We have a limited operating history, as well as a history of operating losses.
We have a limited operating history. We cannot assure you that we can achieve revenue or sustain revenue growth or profitability in the future. We have a cumulative net loss of $7,339,175 for the period from inception (June 2, 2015) to December 31, 2020. Our operations are subject to the risks and competition inherent in the establishment of a business enterprise. Unanticipated problems, expenses, and delays are frequently encountered in establishing a new business and marketing and developing products. These include, but are not limited to, competition, the need to develop customers and market expertise, market conditions, sales, marketing and governmental regulation. Our failure to meet any of these conditions would have a materially adverse effect upon us and may force us to reduce or curtail our operations. Revenues and profits, if any, will depend upon various factors. We may not achieve our business objectives and the failure to achieve such goals would have an adverse impact on our business.
We may be unable to successfully integrate and develop the vertical synergies anticipated by or complete all obligations under the May 31, 2016 Share Exchange Agreement.
We may not realize all the anticipated benefits from the May 31, 2016 Share Exchange Agreement, such as increased earnings, cost savings and revenue enhancements, for various reasons, including difficulties integrating operations and personnel, higher than expected acquisition and operating costs, unknown liabilities, inaccurate reserve estimates and fluctuations in markets. If these benefits do not meet the expectations of financial or industry analysts, the market price of our shares may decline.
Our research and development efforts with respect to new technologies may not result in customer or market acceptance. Some or all of those technologies may not successfully make the transition from the research and development stage to cost-effective production as a result of technology problems, competitive cost issues, yield problems, and other factors. Even if we successfully complete a research and development effort with respect to a particular technology, our customers may decide not to introduce or may terminate products utilizing the technology for a variety of reasons, including difficulties with other suppliers of components for the products, superior technologies developed by our competitors and unfavorable comparisons of our solutions with these technologies, price considerations and lack of anticipated or actual market demand for the products.
Our business could be harmed if we are unable to develop and utilize new technologies that address the needs of our customers, or our competitors or customers develop and utilize new technologies more effectively or more quickly than we can. Any investments made to enhance or develop new technologies that are not successful could have an adverse effect on our net revenue and operating results.
Fluctuations in direct or indirect raw material costs could have an adverse impact on our business.
The availability and prices of raw material inputs may be influenced by supply and demand, changes in world politics, unstable governments in exporting nations, the COVID-19 pandemic and inflation. The prices of our direct and indirect raw materials have been, and we expect them to continue to be, volatile. If the cost of direct or indirect raw materials increases significantly and we are unable to offset the increased costs with higher selling prices, our profitability will decline. Additionally, we may not be able to obtain lower prices from our suppliers should our sale prices decrease. Increases in prices for our products could also hurt our ability to remain both competitive and profitable in the markets in which we compete.
Future raw material prices may be impacted by new laws or regulations, suppliers’ allocations to other purchasers, changes in our supplier manufacturing processes as some of our products are byproducts of these processes, interruptions in production by suppliers, natural disasters, volatility in the price of crude oil and related petrochemical products and changes in exchange rates.
We operate in industries that are subject to significant fluctuation in supply and demand and ultimately pricing that affects our revenue and profitability.
Many of the markets we intend to serve, such as the LED lighting industry and the Electric Vehicle battery market, are in the relatively early stages of adoption and are characterized by constant and rapid technological change, rapid product obsolescence and price erosion, evolving standards, short product life cycles and fluctuations in product supply and demand. These types of LED industries have experienced significant fluctuations, often in connection with, or in anticipation of, product cycles and changes in general economic conditions. As the markets for our products mature, additional fluctuations may result from variability and consolidations within the industry’s customer base. These fluctuations have been characterized by lower product demand, production overcapacity, higher inventory levels and increased pricing pressure. These fluctuations have also been characterized by higher demand for key components and equipment expected to be used in, or in the manufacture of, our products resulting in longer lead times, supply delays and production disruptions.
We operate in a highly competitive industry.
The silane chemical markets are global, capital intensive and highly competitive. Our competitors may have greater financial resources, as well as other strategic advantages, to maintain, improve and possibly expand their facilities, and as a result, they may be better positioned to adapt to changes in the industry or the global economy. The advantages that our competitors have over us could have a material adverse effect on our business. In addition, new entrants may increase competition in our industry, which could have a material adverse effect on our business. An increase in the use of substitutes for certain of our products could also have a material adverse effect on our financial condition and operations.
Environmental, health and safety regulation—Compliance with extensive environmental, health and safety laws could require material expenditures or changes in our operations.
Our operations are subject to extensive environmental, health and safety laws and regulations at national, international and local levels in numerous jurisdictions. In addition, our production facilities require operating permits that are subject to renewal and, in some circumstances, revocation. The nature of the chemicals industry exposes us to risks of liability under these laws and regulations due to the production, storage, transportation, disposal and sale of chemicals and materials that can cause contamination or personal injury if released into the environment.
A reduction or disruption in our supplies, or an incorrect forecast, could negatively impact our business.
Our production capacity could be affected by manufacturing problems. Difficulties in the production process could reduce yields or interrupt production, and, as a result of such problems, we may not be able to deliver products on time or in a cost-effective, competitive manner. As the complexity of both our products and our fabrication processes has become more advanced, manufacturing tolerances have been reduced and requirements for precision have become more demanding. In the past, we have experienced delays in delivery and product quality. Our failure to adequately manage our capacity or maintain product quality could have a negative impact on net sales and harm our customer relationships.
Furthermore, we may suffer disruptions in our manufacturing operations, either due to production difficulties such as those described above or as a result of external factors beyond our control. We manufacture combustible materials in our manufacturing process and are therefore subject to the risk of explosions and fires, which can cause major disruptions to our operations. If operations at a manufacturing facility are interrupted, we may not be able to shift production to other facilities on a timely basis or at all. In addition, certain of our products are only capable of being produced at a single manufacturing facility due to unique manufacturing requirements and to the extent that any of these facilities fail to produce these products, this risk will be increased. Even if a transfer is possible, transitioning production of a particular material can take between three to six months to accomplish, and in the interim period we would likely suffer extensive or total supply disruption and incur substantial costs. Such an event could have a material negative impact on our business, financial condition and results of operations.
Our ability to meet customer demands also depends on our ability to obtain timely and adequate delivery of materials, parts and components from our suppliers. From time to time, suppliers may extend lead times, limit the amounts supplied to us or increase prices due to capacity constraints or other factors. Supply disruptions may also occur due to shortages in critical resources, such as lithium aluminum hydride, other specialized chemicals or energy or other general supplier disruptions. A reduction or interruption in supplies or a significant increase in the price of one or more supplies could have a material negative impact on our business, financial condition and results of operations.
If we do not keep pace with technological innovations, our future products may not remain competitive and our operating results may suffer.
We operate in rapidly changing highly competitive markets. Technological advances, the introduction of new products and new design techniques could adversely affect our business unless we are able to adapt to changing conditions. Technological advances could render our solutions less competitive or obsolete, and we may not be able to respond effectively to the technological requirements of evolving markets. Therefore, we will be required to expend substantial funds for and commit significant resources to enhancing and developing new technology which may include purchasing advanced design tools and test equipment, hiring additional highly qualified engineering and other technical personnel, and continuing and expanding research and development activities on existing and potential human interface solutions.
We may not be able to achieve the target specifications for the second and third generation CSpace laboratory prototypes.
The process of developing new highly technical products and solutions is inherently complex and uncertain. It requires accurate anticipation of customers’ changing needs and emerging technological trends. We must make long-term investments and commit significant resources before knowing whether these investments will eventually result in products that achieve customer acceptance and generate the revenues required to provide desired returns. If we fail to achieve and meet our target specifications in the development of the second and third generation CSpace laboratory prototypes, we could lose market position and customers to our competitors and that could have a material adverse effect on our results of operations and financial condition.
We may not be able to secure funding necessary to develop our CSpace technology
An important part of our business strategy related to CSpace is the development of a new polymer medium. If we are unable to secure research and development funding or customer funded development contracts to support polymer advancement, we will likely not be able to develop our CSpace technology. Without a new polymer medium for CSpace we will not be able to successfully implement our business strategy for our volumetric 3D Display products, which could cause harm to our competitive position and financial condition.
We may not be able to successfully license the Coretec technology to customers.
A significant portion of our expected future revenues will be generated through licensing our technology to third parties such as Boeing, Lockheed Martin, Siemens, and General Electric. However, there is no guarantee we will be able to successfully license our technology to such companies or to other third parties. If we fail to successfully license our technology, it could negatively impact our revenue stream and financial condition.
We may not be able to compete successfully in the markets applicable to our volumetric 3D display and silicon products technology.
Although the volumetric 3D display and silicon products technology that we are attempting to develop is new, and although at present we are aware of only a limited number of companies that have publicly disclosed their attempts to develop similar technology, we anticipate several companies are or will attempt to develop technologies/products that compete or will compete with our technologies. Further, even if we are the first to market with a technology of this type, and even if the technology is protected by patents or otherwise, because of the vast market and communications potential of such a product, we anticipate the market will be flooded by a variety of competitors (including traditional display companies and silicon companies), many of which will offer a range of products in areas other than those in which we compete, which may make such competitors more attractive to prospective customers. In addition, many if not all of our competitors and potential competitors will initially be larger and have greater financial resources than we do. Some of the companies with which we may now be in competition, or with which we may compete in the future, have or may have more extensive research, marketing and manufacturing capabilities and significantly greater technical and personnel resources than we do, and may be better positioned to continue to improve their technology to compete in an evolving industry. Further, technology in this industry may evolve rapidly once an initially successful product is introduced, making timely product innovations and use of new technologies essential to our success in the marketplace. The introduction by our competitors of products with improved technologies or features may render any product we initially market obsolete and unmarketable. If we or our partners are not able to deliver to market products that respond to industry changes in a timely manner, or if our products do not perform well, our business and financial condition will be adversely affected.
The technologies being developed may not gain market acceptance.
The products that we are currently developing utilize new technologies. As with any new technologies, in order for us to be successful, these technologies must gain market acceptance. Since the technologies that we anticipate introducing to the marketplace will exploit or encroach upon markets that presently utilize or are serviced by products from competing technologies, meaningful commercial markets may not develop for our technologies.
In addition, the development efforts of the Company and the University of Oklahoma (the “University”) on the 3D technology are subject to unanticipated delays, expenses or technical or other problems, as well as the possible insufficiency of funding to complete development. Our success will depend upon the ultimate products and technologies meeting acceptable cost and performance criteria, and upon their timely introduction into the marketplace. The proposed products and technologies may never be successfully developed, and even if developed, they may not satisfactorily perform the functions for which they are designed. Additionally, these may not meet applicable price or performance objectives. Unanticipated technical or other problems may occur which would result in increased costs or material delays in their development or commercialization.
If we are unable to successfully retain existing management and recruit qualified personnel having experience in our business, we may not be able to continue our operations.
Our success depends to a significant extent upon the continued services of our Board of Directors, management officers and other technical advisors. Our success also depends on our ability to attract and retain key executive officers and team members. Currently, we have a full business team covering all functional areas. If we are unable to successfully retain existing management and recruit qualified personnel having experience in our business, we may not be able to continue our operations.
In the past, we have identified conditions and events that raise substantial doubt about our ability to continue as a going concern and it is possible that we may identify conditions and events in the future that raise substantial doubt about our ability to continue as a going concern.
We have identified conditions and events that raise substantial doubt about our ability to continue as a going concern for a year following the balance sheet date of our consolidated financial statements at December 31, 2020. With the completion of the private placement in March 2021, we believe that our existing cash and cash equivalents will enable us to fund our operating expenses and capital expenditure requirements more than one year from the date of this registration statement. Consequently, the substantial doubt about the Company's ability to continue as a going concern has been alleviated. However, we have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. In the future, if we are unable to obtain sufficient funding to support our operations, we could be forced to delay, reduce or eliminate all our research and development programs, product portfolio expansion or commercialization efforts, and our financial condition and results of operations will be materially and adversely affected and we may be unable to continue as a going concern. In the future, reports from our independent registered public accounting firm may also contain statements expressing substantial doubt about our ability to continue as a going concern. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms or at all. See Part II, Item 5, Recent Sales of Unregistered Securities for a description of the private placement.
We will need significant additional capital, which we may be unable to obtain.
Our capital requirements in connection with our development activities and transition to commercial operations have been and will continue to be significant. As of March 12, 2021, we do not expect to require additional funding for more than one year in order to continue research, development and testing of our technologies, to obtain intellectual property protection relating to our technologies when appropriate, and to improve and market our technologies. However, there can be no assurances that we will not need additional funding in the future or that our current cash position will be sufficient to fund any future plans to accelerate our commercialization efforts. In the event additional funding is necessary, there can be no assurance that financing will be available in amounts or on terms acceptable to us, if at all.
Risks Related to Our Intellectual Property
If we fail to establish, maintain and enforce intellectual property rights with respect to our technology and/or licensed technology, our financial condition, results of operations and business could be negatively impacted.
Our ability to establish, maintain and enforce intellectual property rights with respect to our technology will be a significant factor in determining our future financial and operating performance. We seek to protect our intellectual property rights by relying on a combination of patent, trade secret and copyright laws. We also use confidentiality and other provisions in our agreements that restrict access to and disclosure of its confidential know-how and trade secrets.
Outside the patents and pending patent applications directly granted to us, we seek to protect our technology as trade secrets and technical know-how. However, trade secrets and technical know-how are difficult to maintain and do not provide the same legal protections provided by patents. In particular, only patents will allow us to prohibit others from using independently developed technologies that are similar. If competitors develop knowledge substantially equivalent or superior to our trade secrets and technical know-how or gain access to our knowledge through other means such as observation of our technology that embodies trade secrets at customer sites that we do not control, the value of our trade secrets and technical know-how would be diminished.
While we strive to maintain systems and procedures to protect the confidentiality and security of our trade secrets and technical know-how, these systems and procedures may fail to provide an adequate degree of protection. For example, although we generally enter into agreements with our employees, consultants, advisors, and strategic partners restricting the disclosure and use of trade secrets, technical know-how and confidential information, we cannot provide any assurance that these agreements will be sufficient to prevent unauthorized use or disclosure. In addition, some of the technology deployed at customer sites in the future, which we do not control, may be readily observable by third parties who are not under contractual obligations of non-disclosure, which may limit or compromise our ability to continue to protect such technology as a trade secret.
While we are not currently aware of any infringement or other violation of our intellectual property rights, monitoring and policing unauthorized use and disclosure of intellectual property is difficult. If we learned that a third party was in fact infringing or otherwise violating our intellectual property, we may need to enforce our intellectual property rights through litigation. Litigation relating to our intellectual property may not prove successful and might result in substantial costs and diversion of resources and management attention.
If our technology is licensed to customers at some point in the future, the strength of the intellectual property under which we would grant licenses can be a critical determinant of the value of such potential licenses. If we are unable to secure, protect and enforce our intellectual property now and in the future, it may become more difficult for us to attract such customers. Any such development could have a material adverse effect on our business, prospects, financial condition and results of operations.
We may face claims that we are violating the intellectual property rights of others.
Although we are not aware of any potential violations of others’ intellectual property rights, we may face claims, including from direct competitors, other companies, scientists or research universities, asserting that our technology or the commercial use of such technology infringes or otherwise violates the intellectual property rights of others. We cannot be certain that our technologies and processes do not violate the intellectual property rights of others. If we are successful in developing technologies that allow us to earn revenues and our market profile grows, we could become increasingly subject to such claims.
We may also face infringement claims from the employees, consultants, agents and outside organizations we have engaged to develop our technology. While we have sought to protect ourselves against such claims through contractual means, we cannot provide any assurance that such contractual provisions are adequate, and any of these parties might claim full or partial ownership of the intellectual property in the technology that they were engaged to develop.
If we were found to be infringing or otherwise violating the intellectual property rights of others, we could face significant costs to implement work-around methods, and we cannot provide any assurance that any such work-around would be available or technically equivalent to our potential technology. In such cases, we might need to license a third party’s intellectual property, although any required license might not be available on acceptable terms, or at all. If we are unable to work around such infringement or obtain a license on acceptable terms, we might face substantial monetary judgments against us or an injunction against continuing to use or license such technology, which might cause us to cease operations.
In addition, even if we are not infringing or otherwise violating the intellectual property rights of others, we could nonetheless incur substantial costs in defending ourselves in suits brought against us for alleged infringement. Also, if we are to enter into a license agreement in the future and it provides that we will defend and indemnify our customer licensees for claims against them relating to any alleged infringement of the intellectual property rights of third parties in connection with such customer licensees’ use of such technologies, we may incur substantial costs defending and indemnifying any customer licensees to the extent they are subject to these types of claims. Such suits, even if without merit, would likely require our management team to dedicate substantial time to addressing the issues presented. Any party bringing claims might have greater resources than we do, which could potentially lead to us settling claims against which we might otherwise prevail on the merits.
Any claims brought against us or any customer licensees alleging that we have violated the intellectual property of others could have negative consequences for our financial condition, results of operations and business, each of which could be materially adversely affected as a result.
At this time, we do not own all of the intellectual property in Volumetric Liquid Crystal Display or Light Surface Display for Rendering Three-Dimensional Images, and, apart from the SRA with the University and the exclusive worldwide marketing rights thereto, we have no contracts or agreements pending to acquire the intellectual property. Also, at this time, we do not own all of the intellectual property in silicon precursor uses or poly-silanes and apart from the provisional patents we have filed, which have claims which may or may not be granted, we have no contracts or agreements pending to acquire additional intellectual property in this arena.
Although we have obtained exclusive worldwide marketing rights to “Volumetric Liquid Crystal Display” and “Light Surface Display for Rendering Three-Dimensional Images”, two technologies vital to our business and growth strategy, we do not own all of the intellectual property in these technologies. Although our exclusive worldwide marketing rights to these technologies stand alone and are independent of the SRA, outside of our SRA with the University, we have no pending agreements to obtain or purchase ownership over all intellectual property in these technologies. Should the University lose their rights in such technologies or we are otherwise unable to utilize the rights obtained in such agreements it would be difficult to successfully implement our business strategy going forward and our stock value would likely decrease. In addition, we have filed two provisional patents in the cyclohexasilane (CHS) space, and although we anticipate filing additional provisional patents as we develop applications using CHS, these patents have claims within that may or may not be granted and any such change to these patent applications would make it difficult to successfully implement our business strategy going forward and our stock value would likely decrease.
We do not currently own any patents related to our silicon-based business.
We do not currently own any patents related to our silicon-based businesses; however, The Coretec Group has filed two provisional patents in the silicon-based business.
Risks Relating to Our Current Financing Arrangements:
There are a large number of shares underlying our convertible debt and warrants that may be available for future sale and the sale of these shares may depress the market price of our common stock.
As of March 12, 2021, we had 239,267,102 shares of common stock issued and outstanding and convertible debt outstanding that may be converted into an estimated 49,240,122 shares of common stock and outstanding pre-funded warrants to purchase 51,500,000 shares of common stock at an exercise price of $0.0001. We also have outstanding warrants issued to purchase 2,604,000 shares of common stock at an exercise price of $0.052, outstanding warrants issued to purchase 82,500,000 shares of common stock at an exercise price of $0.08, and outstanding warrants issued to purchase 6,000,000 shares of common stock at an exercise price of $0.010. The sale of the shares underlying the convertible debt and warrants may adversely affect the market price of our common stock.
As of March 12, 2021, we have 1,260,732,898 unissued authorized shares available.
The issuance of shares upon conversion of outstanding Series A Stock, the convertible debt or the exercise of outstanding warrants may cause immediate and substantial dilution to our existing stockholders.
The issuance of shares upon conversion of our outstanding Series A Convertible Preferred Stock, convertible debt and exercise of warrants would result in substantial dilution to the interests of other stockholders since the selling stockholders may ultimately exercise and sell the full amount issuable upon exercise of their warrants.
Risks Relating to Our Common Stock:
The price of our common stock is volatile and fluctuations in our operating results and announcements and developments concerning our business affect our stock price, which may cause investment losses for our stockholders.
The market for our common stock is highly volatile and the trading price of our stock on the OTCQB Marketplace is subject to wide fluctuations in response to, among other things, operating results, the number of stockholders desiring to sell their shares, changes in general economic conditions and the financial markets, the execution of new contracts and the completion of existing agreements and other developments affecting us. In addition, statements or changes in opinions, ratings, or earnings estimates made by brokerage firms or industry analysts relating to our market or relating to us could result in an immediate and adverse effect on the market price of our common stock. The highly volatile nature of our stock price may cause investment losses for our shareholders. In the past, securities class action litigation has often been brought against companies following periods of volatility in the market price of their securities. If securities class action litigation is brought against us, such litigation could result in substantial costs while diverting management’s attention and resources.
Our common stock is subject to the "Penny Stock" rules of the Securities and Exchange Commission and the trading market in our securities is limited, which makes transactions in our stock cumbersome and may reduce the value of an investment in our stock.
The Securities and Exchange Commission (the “SEC”) has adopted Rule 15g-9 which establishes the definition of a "penny stock," for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require:
|
|
●
|
That a broker or dealer approve a person's account for transactions in penny stocks; and
|
|
|
●
|
The broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
|
In order to approve a person's account for transactions in penny stocks, the broker or dealer must:
|
|
●
|
Obtain financial information and investment experience objectives of the person; and
|
|
|
●
|
Make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
|
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the Commission relating to the penny stock market, which, in highlight form:
|
|
●
|
Sets forth the basis on which the broker or dealer made the suitability determination; and
|
|
|
●
|
That the broker or dealer received a signed, written agreement from the investor prior to the transaction.
|
Generally, brokers may be less willing to execute transactions in securities subject to the "penny stock" rules. This may make it more difficult for investors to dispose of our common stock and cause a decline in the market value of our stock.
Disclosure also must be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Financial Industry Regulatory Authority, Inc. (“FINRA”) sales practice requirements may limit a shareholder’s ability to buy and sell our common stock.
In addition to the “penny stock” rules described above, FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
Our stock is thinly traded, so you may be unable to sell your shares at or near the quoted bid prices if you need to sell a significant number of your shares.
The shares of our common stock are thinly traded on the OTCQB Marketplace, meaning that the number of persons interested in purchasing our common stock at or near bid prices at any given time may be relatively small or non-existent. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that current trading levels will be sustained. Due to these conditions, we can give you no assurance that you will be able to sell your shares at or near bid prices or at all if you need money or otherwise desire to liquidate your shares.
Shares eligible for future sale may adversely affect the market.
From time to time, certain of our stockholders may be eligible to sell all or some of their shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144 promulgated under the Securities Act, subject to certain limitations. In general, pursuant to amended Rule 144, non-affiliate stockholders may sell freely after six months subject only to the current public information requirement. Affiliates may sell after six months subject to Rule 144 volume, manner of sale (for equity securities), and current public information and notice requirements. Any substantial sales of our common stock pursuant to Rule 144 may have a material adverse effect on the market price of our common stock.
We could issue additional common stock, which might dilute the book value of our common stock.
Our Board of Directors has authority, without action or vote of our shareholders, to issue all or a part of our authorized but unissued shares. Such stock issuances could be made at a price that reflects a discount or a premium from the then-current trading price of our common stock. In addition, in order to raise capital, we may need to issue securities that are convertible into or exchangeable for a significant amount of our common stock. These issuances would dilute the percentage ownership interest, which would have the effect of reducing your influence on matters on which our shareholders vote and might dilute the book value of our common stock.
Our common stock could be further diluted as a result of the issuance of convertible securities, warrants or options.
In the past, we have issued convertible securities (such as convertible debentures and notes), warrants and options in order to raise money or as compensation for services and incentive compensation for our employees and directors. We have shares of common stock reserved for issuance upon the exercise of certain of these securities and may increase the shares reserved for these purposes in the future. Our issuance of these convertible securities, options and warrants could affect the rights of our stockholders, could reduce the market price of our common stock or could result in adjustments to exercise prices of outstanding warrants (resulting in these securities becoming exercisable for, as the case may be, a greater number of shares of our common stock), or could obligate us to issue additional shares of common stock to certain of our stockholders.
We do not intend to pay dividends.
We do not anticipate paying cash dividends on our common stock in the foreseeable future. We may not have sufficient funds to legally pay dividends. Even if funds are legally available to pay dividends, we may nevertheless decide in our sole discretion not to pay dividends. The declaration, payment and amount of any future dividends will be made at the discretion of our board of directors, and will depend upon, among other things, the results of our operations, cash flows and financial condition, operating and capital requirements, and other factors our board of directors may consider relevant. There is no assurance that we will pay any dividends in the future, and, if dividends are paid, there is no assurance with respect to the amount of any such dividend.
If we fail to maintain effective internal controls over financial reporting, the price of our common stock may be adversely affected.
Our internal control over financial reporting may have weaknesses and conditions that could require correction or remediation, the disclosure of which may have an adverse impact on the price of our common stock. We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely affect our public disclosures regarding our business, prospects, financial condition or results of operations. In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting or disclosure of management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. Such forward-looking statements include those that express plans, anticipation, intent, contingency, goals, targets or future development and/or otherwise are not statements of historical fact. These forward-looking statements are based on our current expectations and projections about future events and they are subject to risks and uncertainties known and unknown that could cause actual results and developments to differ materially from those expressed or implied in such statements.
In some cases, you can identify forward-looking statements by terminology, such as “expects”, “anticipates”, “intends”, “estimates”, “plans”, “potential”, “possible”, “probable”, “believes”, “seeks”, “may”, “will”, “should”, “could” or the negative of such terms or other similar expressions. Accordingly, these statements involve estimates, assumptions and uncertainties that could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified in their entirety by reference to the factors discussed throughout this prospectus.
You should read this prospectus and the documents that we reference herein and have filed as exhibits to the registration statement, of which this prospectus is part, completely and with the understanding that our actual future results may be materially different from what we expect. You should assume that the information appearing in this prospectus is accurate as of the date on the front cover of this prospectus only. Because the risk factors referred to above could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made, and we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of the information presented in this prospectus, and particularly our forward-looking statements, by these cautionary statements.
USE OF PROCEEDS
We will not receive any of the proceeds from the sale of the shares of our common stock being offered for sale by the selling stockholders. Upon the exercise of the warrants for an aggregate of 82,500,000 shares of common stock assuming all payments are made by cash and there is no reliance on cashless exercise provisions, however, we will receive the exercise price of the warrants, or an aggregate of approximately $6,600,000, from the investor in the March 2, 2021 Private Placement and $600,000 from the exercise for cash of the Placement Agent Warrants. We will bear all fees and expenses incident to our obligation to register the shares of common stock. Brokerage fees, commissions and similar expenses, if any, attributable to the sale of shares offered hereby will be borne by the selling stockholder.
There is no assurance the warrants will be exercised for cash. We intend to use such proceeds, if any, for general corporate and working capital purposes.
DIVIDENDS POLICY
We have never declared or paid any cash dividends on our common stock. We do not anticipate paying any cash dividends to stockholders in the foreseeable future. In addition, any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements, and such other factors as our Board of Directors deem relevant. There are no restrictions in our articles of incorporation or bylaws that restrict us from declaring dividends.
OUR BUSINESS
Organizational History
On June 22, 2017, the Group filed an Amended Certificate of Incorporation with the Secretary of State of the State of Oklahoma to change its name from “3DIcon Corporation” to “The Coretec Group Inc.”, which became effective on June 29, 2017.
The Group, formerly known as 3DIcon Corporation, was incorporated on August 11, 1995, under the laws of the State of Oklahoma. Prior to September 30, 2016, the Group’s primary activity had been the raising of capital in order to pursue its goal of becoming a significant participant in the development, commercialization and marketing of next generation 3D display technologies.
On September 30, 2016, Coretec Industries LLC became a wholly owned subsidiary of the Group, and the Group issued an aggregate 15,870 shares of the Group’s Series B Convertible Preferred Stock, which shares were subsequently converted into 30,374,363 shares of common stock.
Overview of the Company.
Coretec’s Technology. Coretec’s underlying technology is based on the production of a high value liquid silicon precursor, cyclohexasilane (“CHS”). A key advantage of CHS is that it remains in liquid form at room temperature and does not convert to a gas until heated above 450°F. CHS is a superior silicon precursor in many ways compared to materials commonly used for manufacturing silicon-based semiconductors and solar cells (monosilane or trichlorosilane) that have much lower boiling points which leads to higher cost handling and shipping. There are several technical advantages of using CHS versus common silicon precursors and one is that the production rate of the silicon-forming step can be increased by a factor of six, and relative to process temperature up to 10X or more, which leads to significant cost savings. We anticipate that CHS will first be used as an alternative to monosilane or trichlorosilane when adding silicon to lithium ion batteries or when used in manufacturing silicon-based semiconductors.
We also see longer term potential in several emerging markets where there are opportunities in the conversion of CHS into nanoparticles and nanowires for use in such emerging, high-growth markets as:
|
|
●
|
Energy storage
|
|
|
●
|
Solid state lighting
|
|
|
●
|
Authentication of critical documentation
|
|
|
●
|
Printable electronics
|
|
|
●
|
Building-integrated solar energy
|
Enhancement of CSpace. A key challenge in the development of CSpace® is the development of the material used for the image chamber. The Company has explored a variety of glass alternatives. While progress has been made, it has been concluded that limitations remain, primarily in the weight and cost of a glass medium.
A key virtue of having our IP portfolio of silicon-based materials is that we use all of the manufacturing infrastructure and knowledge that is available for optical plastics for the CSpace® image chamber. The benefit to CSpace® is that silicon-based optical plastics can be molded into a broad range of shapes and allow the image chamber to be much lighter and much lower in cost than the glass material we worked with before.
Near-Term Revenue Opportunities. Opportunities for near-term revenue continue to be explored in battery and microelectronic markets. Interest in the use of silicon in Li-ion batteries continues to increase driven by the growing demand for electrical vehicles, the exploitation of mobile electronics, and energy storage systems for backup power and improved efficiency of home and commercial wind and solar systems. Discussions are ongoing with suppliers of Li-ion battery anode materials that are seeking next generation materials to further increase performance while improving lifetime, charging time, safety and reliability. We believe these suppliers will be well positioned to take advantage of the benefits provided by CHS when combined as a liquid with other solid-based materials. While we believe the use of CHS in Li-ion batteries will provide near term revenue, we also continue to explore revenue opportunities in microelectronics and especially those early adopter markets where advanced microelectronics are being developed in lower volumes and with less price sensitivity.
Recent Developments.
On October 4, 2019 the Company entered into a credit agreement (the “Credit Agreement”) and related convertible promissory note with Diversified Alpha Fund of Navigator Global Fund Manager Platform SPC, a Grand Cayman entity (the “Lender”). As of December 31, 2020, there was outstanding principal under the Credit Agreement and related convertible promissory note in the amount of $1,275,000.
The securities above were offered and sold pursuant to an exemption from the registration requirements under Section 4(a)(2) of the Securities Act since, among other things, the transactions did not involve a public offering.
On June 30, 2020, the Company accepted the retirement and resignations of Ron Robinson, Chief Financial Officer (CFO) and Judith Keating, Corporate Secretary of the Company. Matthew Hoffman, who joined the Company in May of 2020, was appointed CFO and Corporate Secretary effective June 30, 2020.
On June 30, 2020 the Company moved headquarters and operations from Tulsa, Oklahoma to Ann Arbor, Michigan.
On June 25, 2020, the Company entered into a supply agreement with Evonik Operations GmbH to purchase 500 grams of cyclohexasilane, Si6H12 (CHS) for $185,000. The supply agreement will enable the Company to deliver initial quantities of CHS for sales and R&D evaluation to its customer base. The supply agreement is valid until March 31, 2021. The Company paid Evonik Operations GmbH $92,500 on July 20, 2020, to initiate production of CHS, in accordance with the agreement. Delivery is expected during the months of March and April 2021, at which time the Company will owe the remaining $92,500.
On October 29, 2020, the Company moved the trading of its securities to the OTCQB, also known as the Venture Market, from OTC Pink market. The fee for listing on the OTCQB market is $12,000 per annum, with a one-time application fee of $2,500. The OTCQB market is the middle tier of the OTC Markets and consists of early-stage and developing U.S. and international companies.
On March 2, 2021 (the “Signing Date”), Company entered into a securities purchase agreement (the “Purchase Agreement”) with a single institutional investor (the “Investor”) pursuant to which the Company agreed to sell to the Investor in a private placement (i) 23,500,000 shares of its common stock (the “Shares”), (ii) pre-funded warrants to purchase up to an aggregate of 51,500,000 shares of its common stock (the “Pre-Funded Warrants”), and (iii) warrants (the “Warrants”) to purchase up to an aggregate of 82,500,000 shares of its common stock for gross proceeds of approximately $6,000,000. The combined purchase price for one share of common stock and associated Warrant is $0.08 and for one Pre-Funded Warrant and associated Warrant is $0.0799. The sale of the securities under the Purchase Agreement closed on March 5, 2021.
The Warrants are exercisable for a period of five-and one-half years from the date of issuance and have an exercise price of $0.08 per share, subject to adjustment as set forth in the Warrants for stock splits, stock dividends, recapitalizations and similar events. The Investor may exercise the Warrant on a cashless basis if the shares of common stock underlying the Warrants (the “Warrant Shares”) are not then registered pursuant to an effective registration statement. The Investor has contractually agreed to restrict its ability to exercise the Warrants such that the number of shares of the Company’s common stock held by the Investor and its affiliates after such exercise does not exceed the Beneficial Ownership Limitation set forth in the Warrants which may not exceed initially 4.99% of the Company’s then issued and outstanding shares of common stock.
The Pre-Funded Warrants have an exercise price of $0.0001 per share, subject to adjustment as set forth in the Pre-Funded Warrants for stock splits, stock dividends, recapitalizations and similar events. The Pre-Funded Warrants will be exercisable immediately and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full.
In connection with the Purchase Agreement, the Company entered into a registration rights agreement (the “Registration Rights Agreement”) with the Investor. Pursuant to the Registration Rights Agreement, the Company will be required to file a resale registration statement (the "Registration Statement") with the Securities and Exchange Commission (the “SEC”) to register for resale of the Shares, Warrant Shares and shares issuable upon exercise of the Pre-Funded Warrants, within 20 days of the Signing Date, and to have such Registration Statement declared effective within 45 days after the Signing Date in the event the Registration Statement is not reviewed by the SEC, or 90 days of the Signing Date in the event the Registration Statement is reviewed by the SEC.
In support of the Purchase Agreement, the Company entered into an engagement with H.C. Wainwright & Co. (HCW) to act as exclusive agent, advisor or underwriter in any offering of securities by the Company. Compensation to HCW includes 8.0% cash fee of gross proceeds and warrant coverage equal to 8% of the aggregate number of shares of common stock placed in each offering at an exercise price equal to 125% of the offering price per share available over a 5-year term. The Company will also pay HCW (a) a management fee equal to 1.0% of the gross proceeds raised in each Offering; (b) $35,000 for non-accountable expenses; (c) up to $50,000 for fees and expenses of legal counsel and other out-of-pocket expenses. The initial term of the agreement is for one month.
Cyclohexasilane Business
The Company’s business model is to identify and commercialize disruptive technologies in silicon serving advanced technology markets. Sources of disruptive technology are licensed technology created by major universities, institutes, national laboratories and other research centers. Where technology does not already exist, research is to be sponsored and jointly developed with our customers. The initial candidates for commercialization center around CHS, and a source of this technology includes silicon technologies.
Coretec is developing, testing, and providing new and/or improved technologies and resulting product solutions for energy-related industries including, but not limited to oil/gas, renewable energy, energy conservation, and distributed energy industries. Many of these technologies and resulting product solutions also have application to the broader markets of anti-counterfeit packaging, medical devices, electronics, photonics, and displays. The initial technologies and product solutions are based on new innovations in cyclohexasilane (Si6H12), Si QDs, “stacked” polysilane ((R2Si)n), their alloys with various dopants, and in the future, high refractive index siloxane polymers (HRISP). Early adoption of these technologies and resulting product solutions is anticipated in markets for energy storage (Li-ion batteries), solid-state lighting (LEDs), solar energy (BIPV) and printable electronics (Asset Monitoring).
Coretec’s management leverages years of expertise and experience in equipment and services for the oil/gas industries, procuring and managing investments and financial services, and in R&D and commercialization of material and chemical technologies.
CHS Business Model
Coretec’s business model includes monitoring the ever-growing catalogue of new technologies and valuable IP for licensing opportunities that could lead to incremental improvements and/or additional features in resulting products or lead to next generation products for use by energy-related industries and is created and held within universities and other parties that may lack financial resources and/or interest to further develop and commercialize them.
Additionally, where needs exist, but new technologies and resulting products are not currently available, conduct research and development (“R&D”) activities through sponsored projects performed at major universities, institutes, national laboratories and other research centers. Coretec will leverage existing, world-class expertise, experience, and laboratory facilities that reside in these non-profit, R&D entities for R&D, testing, and “proof of concept” studies up to and including at the device level that may be required to create commercialization opportunities.
Following these “proof of concept studies”, commercialization opportunities (e.g., manufacturing, marketing, sales) created for its technologies and IP will include, but are not limited to:
|
|
●
|
joint ventures or other business collaborations with Coretec’s joint development partners who can manufacture, market and sell new or improved products (based upon Coretec’s technologies and IP) into existing or new supply chains (that the partner company/companies already have an established, significant presence or can capture and grow market share); or
|
|
|
●
|
manufacturing, marketing and selling its own products; or
|
|
|
●
|
creating “exit strategies” such as:
|
|
|
o
|
sale of one or more technologies and IP to the private sector;
|
|
|
o
|
license and/or sublicense one or more technologies and IP to the private sector; or
|
|
|
o
|
other business transactions, e.g., merger, acquisition, spinoffs.
|
CHS Research & Development
Coretec’s priorities for R&D and commercialization are customer/market-driven and guided by the needs and specifications of the energy-related industries served. Identified customer/market-driven opportunities include:
|
|
●
|
New and novel silicon-based materials that facilitate “greener” more eco-friendly energy production, including:
|
|
|
o
|
lower cost, longer life, higher capacity battery energy storage systems, e.g., Li-ion batteries (LiBs), for use in transportation and distributed power generation systems
|
|
|
o
|
more aesthetically appealing, lower cost building integrated photovoltaics (BIPV); and
|
|
|
o
|
flexible and/or printable electronics for use in monitoring the condition of distributed or remote assets, e.g., wind power and embedded, wireless sensors to detect corrosion and other changes in pipelines.
|
|
|
●
|
New and novel silicon-based materials that facilitate “greener” more energy efficient products, including encapsulation of high brightness LEDs to improve light extraction and solar cells to improve full spectrum light collection;
|
|
|
●
|
New and novel silicon-based materials that facilitate more efficient and eco-friendly exploration and monitoring of distributed energy industries, including imaging materials for visualizing oil and gas exploration and distribution data using volumetric 3D displays; and
|
|
|
●
|
New and novel silicon-based materials that prevent illegal imitation or reproduction of a product or service used within energy-related industries, including trusted supply (anti-counterfeit packaging) products for supply chain assurance, currency, identity documents, lottery tickets, etc.
|
Future CHS Revenue
In the future, we foresee revenue coming from one or more business transactions such as:
|
|
●
|
sale of Coretec’s novel silicon-based materials that improve or otherwise enhance performance of various products, e.g., Li-ion batteries, electronics, PV/solar cells, and displays and/or other optical-based devices;
|
|
|
●
|
a share of the revenue coming from the sale of jointly developed product(s) and/or from one or more joint ventures with strategic partners; and/or
|
|
|
●
|
sale or licensing of technology/technologies and associated IP to joint development partners or other companies.
|
CHS Competition
Based on our market research and competitive analysis, we have concluded that our CHS technology is unique and provides an advantage in that it should allow 1) production at high yields at low cost using readily available raw materials, 2) storage, transport and use as a liquid at room temperature 3) processing of the liquid into fibers, particles, and films that when heated forms silicon, and 4) the simple addition of dopants to the liquid at an atomic level that when heated forms doped silicon. Competing silanes provided by numerous manufacturers exist as a gas at room temperature and are explosive resulting in greater cost during storage, handling, transportation and use. Our closest competitor is cyclopentasilane which exists as a gas at room temperature and has proven costly and difficult to manufacture. Other competitors exist in specific applications. For example, graphene and carbon nanotubes are potential competitors in printable electronics but are only now emerging and require purification that is proving costly.
Coretec’s business and commercialization model is based in part upon establishing joint development partnerships with companies that are commercially successful and financially sound as well as deeply embedded in the supply chains for the aforementioned energy-related products. For example, Coretec is developing a strategic partnership with a domestic supplier of silicon-based materials that will facilitate further development and scale-up of Si6H12 plus chemical derivatives and other materials based on Si6H12. This strategic partnership will enable Coretec to supply large quantities of these novel silicon materials to those companies interested in producing prototype batteries, electronics, and PV/solar cells for testing and commercial evaluation. Coretec will continue to seek other such strategic partnerships within the private sector.
Volumetric 3D Display Business
The Company owns the rights to a patented volumetric 3D display technology that was developed by and with the University under a Sponsored Research Agreement (“SRA”). The development to date has resulted in multiple technologies, two working laboratory prototypes (Lab Proto 1 and Lab Proto 2), and eight provisional patents; five of the eight provisional patents have been combined and converted to five utility patents. Under the SRA, the Company has obtained the exclusive worldwide marketing rights to these 3D display technologies.
On May 26, 2009, the United States Patent and Trademark Office ("USPTO") approved the patent called "Volumetric Liquid Crystal Display" for rendering a three-dimensional image and converted it to U.S. patent No. 7,537,345. On December 28, 2010, USPTO approved the patent called “Light Surface Display for Rendering a Three-Dimensional Image,” and issued the United States Patent No. 7,858,913. On August 21, 2012, the USPTO approved a continuation patent called “3D Volumetric Display” and issued the US Patent No. 8,247,755. These patents describe the foundation of what is called CSpace® technology (“CSpace”).
Overview of Volumetric 3D Display Technology
Commercialization Strategy and Target Applications
The Company plans to commercialize the CSpace volumetric 3D technology through customer funded research and development contracts and technology licensing agreements for high value applications like air traffic control, design visualization, and medical imaging. The Company plans to develop products for contract engineering and with joint development customers. At this time the Company does not have any commercialized products and does not plan to develop its own products based on the CSpace technology due to the high value / low volume nature of the best-fit initial applications for this technology. These applications include but are not limited to the following:
|
|
●
|
Healthcare (diagnostics, surgical planning, training, telemedicine, bio surveillance);
|
|
|
●
|
Cyber security data visualization;
|
|
|
●
|
Military (operational planning, training, modeling and simulation, battlespace awareness, damage assessment, autonomous piloting);
|
|
|
●
|
Physical security (passenger, luggage & cargo screening);
|
|
|
●
|
Mining, oil & gas exploration; or
|
|
|
●
|
Meteorological and oceanographic data visualization.
|
CSpace Competition
Based on our market research and competitive analysis to date, we have concluded that the CSpace volumetric technology is unique and advantaged versus other 3D technologies in that it can deliver both 1) a true 360 degree viewing experience for multiple simultaneous users, and 2) high image quality, high reliability and large image size. Rear projection 3D displays such as those from Zecotek, Setred, and EuroLCDs (formerly LC Tech LightSpace) do not provide a 360-degree viewing experience and are typically limited to one or two users. Early proof of concept work done on infrared active phosphor displays by 3D Display Laboratories proved to not be scalable due to limited phosphor persistence and vector scanning limitations. While holographic and light field displays show promise, they do not deliver a true 360-degree viewing experience and cost-effective multiple user systems do not appear feasible due to current and expected pixel density, data bandwidth and compute power limitations.
History of 3D Technology Research and Development at the University of Oklahoma
Beginning in 2007 the University, under an SRA with the Company, undertook the development of high potential 3D display technologies. It is anticipated that Coretec’s technology will play a key role in the continued development of an image space material for CSpace.
3D Technology - Intellectual Property History, Status and Rights
The USPTO approved the pending patent called "Volumetric Liquid Crystal Display" for rendering a three-dimensional image and converted it to US patent No. 7,537,345. On July 16, 2013, USPTO approved the pending patent called “Computer System with Digital Micromirror Device,” and issued US patent No. 8,487,865.
CSpace Patents are as follows: On December 28, 2010, USPTO approved the pending patent called “Light Surface Display for Rendering a Three-Dimensional Image,” and issued the US Patent No. 7,858,913. On August 21, 2012, the USPTO approved a continuation patent called “3D Volumetric Display” and issued the US Patent No. 8,247,755. On December 13, 2011, USPTO approved a continuation patent called “3D Light Surface Display,” and issued the US Patent No. 8,075,139.
Through an SRA with the University, we have obtained the exclusive worldwide marketing rights to certain 3D display technologies under development by the University. The development to date has resulted in the University filing eight provisional patents; five of the eight provisional patents have been combined and converted to five utility US patents, one Japanese patent, and one pending European patent.
In addition, the Company owns exclusively two U.S. patents as noted below.
Key Patents Exclusively Licensed to the Company from the University:
United States Patents Granted
|
|
●
|
“3D Volumetric Display” - 8,247,755, August 21, 2012
|
|
|
●
|
“3DLight Surface Display” - 8,075,139, December 13, 2011
|
|
|
●
|
“Light Surface Display for Rendering a Three-Dimensional Image” - 7,858,913, December 28, 2010
|
|
|
●
|
“Volumetric Liquid Crystal Display”- 7,537,345, May 26, 2009
|
|
|
●
|
“Computer System with Digital Micromirror Device” – 8,487,865, July 16, 2014
|
International Patents Granted-Japan
|
|
●
|
“Light Surface Display for Rendering a Three-Dimensional Image” - Japanese Patent Number 5,594,718, August 15, 2014
|
International Patents Pending-Europe
|
|
●
|
“Light Surface Display for Rendering a Three-Dimensional Image” - European Application Number EP07755984, filed April 25, 2007
|
Key Patents Exclusively Owned by the Company:
|
|
●
|
“Ultra High-Resolution Volumetric Three-Dimensional Display” - 9,423,682, August 23, 2016
|
|
|
●
|
“Hloform 3D Projection Display” - 2014/02680162A1, September 18, 2014
|
Employees
We have built out a full business team including Michael Kraft, Chief Executive Officer, Ramez Elgammal, PhD, Vice-President of Technology, Matthew Hoffman, Chief Financial Officer, Michelle Tokarz, Business Development Consultant, Lindsay McCarthy, Ann Arbor Office Manager, Allison Gabrys, Chief Marketing Officer Consultant and additional part time supporting staff. None of our employees are covered by a collective bargaining agreement. We consider relations with our employees to be good.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following description of our financial condition and results of operations in conjunction with the consolidated financial statements and accompanying notes included in this prospectus.
Overview
Coretec’s Technology. Coretec’s underlying technology is based on the production of a high value liquid silicon precursor, cyclohexasilane (“CHS”). A key advantage of CHS is that it remains in liquid form at room temperature and does not convert to a gas until heated above 450°F. CHS is a superior silicon precursor in many ways compared to materials commonly used for manufacturing silicon-based semiconductors and solar cells (monosilane or trichlorosilane) that have much lower boiling points which leads to higher cost handling and shipping. There are several technical advantages of using CHS versus common silicon precursors and one is that the production rate of the silicon-forming step can be increased by a factor of six, and relative to process temperature up to 10X or more, which leads to significant cost savings. We anticipate that CHS will first be used as an alternative to monosilane or trichlorosilane when adding silicon to lithium-ion batteries or when used in manufacturing silicon-based semiconductors.
We also see longer term potential in several emerging markets where there are opportunities in the conversion of CHS into nanoparticles and nanowires for use in such emerging, high-growth markets as:
|
|
●
|
Energy storage
|
|
|
●
|
Solid state lighting
|
|
|
●
|
Authentication of critical documentation
|
|
|
●
|
Printable electronics
|
|
|
●
|
Building-integrated solar energy
|
Enhancement of CSpace. A key challenge in the development of CSpace® is the development of the material used for the image chamber. The Company has explored a variety of glass alternatives. While progress has been made, it has been concluded that limitations remain, primarily in the weight and cost of a glass medium.
A key virtue of having access to the Coretec IP portfolio of silicon-based materials is that we can now use all of the manufacturing infrastructure and knowledge that is available for optical plastics for the CSpace® image chamber. The benefit to CSpace® is that silicon-based optical plastics can be molded into a broad range of shapes and allow the image chamber to be much lighter and much lower in cost than the glass material we worked with before.
Near-Term Revenue Opportunities. Opportunities for near-term revenue continue to be explored in battery and microelectronic markets. Interest in the use of silicon in Li-ion batteries continues to increase driven by the growing demand for electrical vehicles, the exploitation of mobile electronics, and energy storage systems for backup power and improved efficiency of home and commercial wind and solar systems. Discussions are ongoing with suppliers of Li-ion battery anode materials that are seeking next generation materials to further increase performance while improving lifetime, charging time, safety and reliability. We believe these suppliers will be well positioned to take advantage of the benefits provided by CHS when combined as a liquid with other solid-based materials. While we believe the use of CHS in Li-ion batteries will provide near term revenue, we also continue to explore revenue opportunities in microelectronics and especially those early adopter markets where advanced microelectronics are being developed in lower volumes and with less price sensitivity.
Recent Developments.
On October 4, 2019 the Company entered into a credit agreement (the “Credit Agreement”) and related convertible promissory note with Diversified Alpha Fund of Navigator Global Fund Manager Platform SPC, a Grand Cayman entity (the “Lender”). As of December 31, 2020, there was outstanding principal under the Credit Agreement and related convertible promissory note in the amount of $1,275,000.
On March 2, 2021, the Company entered into the Purchase Agreement with the Investor pursuant to which the Company agreed to sell to the Investor in a private placement (i) 23,500,000 Shares, (ii) Pre-Funded Warrants to purchase up to an aggregate of 51,500,000 shares of its common stock, and (iii) the Warrants to purchase up to an aggregate of 82,500,000 shares of its common stock for gross proceeds of approximately $6,000,000. The combined purchase price for one share of common stock and associated Warrant is $0.08 and for one Pre-Funded Warrant and associated Warrant is $0.0799. The sale of the securities under the Purchase Agreement closed on March 5, 2021.
The Warrants are exercisable for a period of five- and one-half years from the date of issuance and have an exercise price of $0.08 per share, subject to adjustment as set forth in the Warrants for stock splits, stock dividends, recapitalizations and similar events. The Investor may exercise the Warrant on a cashless basis if the Warrant Shares are not then registered pursuant to an effective registration statement. The Investor has contractually agreed to restrict its ability to exercise the Warrant such that the number of shares of the Company’s common stock held by the Investor and its affiliates after such exercise does not exceed the Beneficial Ownership Limitation set forth in the Warrant which may not exceed initially 4.99% of the Company’s then issued and outstanding shares of common stock.
The Pre-Funded Warrants have an exercise price of $0.0001 per share, subject to adjustment as set forth in the Pre-Funded Warrants for stock splits, stock dividends, recapitalizations and similar events. The Pre-Funded Warrants will be exercisable immediately and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full.
In connection with the Purchase Agreement, the Company entered into the Registration Rights Agreement with the Investor. Pursuant to the Registration Rights Agreement, the Company will be required to file the Registration Statement with the SEC to register for resale of the Shares, Warrant Shares and shares issuable upon exercise of the Pre-Funded Warrants, within 20 days of the Signing Date, and to have such Registration Statement declared effective within 45 days after the Signing Date in the event the Registration Statement is not reviewed by the SEC, or 90 days of the Signing Date in the event the Registration Statement is reviewed by the SEC.
In support of the Purchase Agreement, the Company entered into an engagement with HCW to act as exclusive agent, advisor or underwriter in any offering of securities by the Company. Compensation to HCW includes 8.0% cash fee of gross proceeds and warrant coverage equal to 8% of the aggregate number of shares of common stock placed in each offering at an exercise price equal to 125% of the offering price per share available over a 5-year term. The Company will also pay HCW (a) a management fee equal to 1.0% of the gross proceeds raised in each Offering; (b) $35,000 for non-accountable expenses (c) up to $50,000 for fees and expenses of legal counsel and other out-of-pocket expenses. The initial term of the agreement is for one month.
The securities above were offered and sold pursuant to an exemption from the registration requirements under Section 4(a)(2) of the Securities Act since, among other things, the transactions did not involve a public offering.
On June 30, 2020, the Company accepted the retirement and resignations of Ron Robinson, Chief Financial Officer (CFO) and Judith Keating, Corporate Secretary of the Company. Matthew Hoffman, who joined the Company in May of 2020, was appointed CFO and Corporate Secretary effective June 30, 2020.
On June 30, 2020 the Company moved headquarters and operations from Tulsa, Oklahoma to Ann Arbor, Michigan.
On June 25, 2020, the Company entered into a supply agreement with Evonik Operations GmbH to purchase 500 grams of cyclohexasilane, Si6H12 (CHS) for $185,000. The supply agreement will enable the Company to deliver initial quantities of CHS for sales and R&D evaluation to its customer base. The supply agreement is valid until March 31, 2021. The Company paid Evonik Operations GmbH $92,500 on July 20, 2020, to initiate production of CHS, in accordance with the agreement. Delivery is expected during the March and April of 2021, at which time the Company will owe the remaining $92,500.
On October 29, 2020, the Company moved the trading of its securities to the OTCQB, also known as the Venture Market, from OTC Pink market. The fee for listing on the OTCQB market is $12,000 per annum, with a one-time application fee of $2,500. The OTCQB market is the middle tier of the OTC Markets and consists of early-stage and developing U.S. and international companies.
RESULTS OF OPERATIONS FOR THE YEAR ENDED DECEMBER 31, 2020 COMPARED TO THE YEAR ENDED DECEMBER 31, 2019
Revenue
We did not have revenues for the years ended December 31, 2020 and 2019.
Research and Development Expenses
The research and development expenses were $151,864 for the year ended December 31, 2020, as compared to $148,875 for the year ended December 31, 2019. The approximately $3,000 increase was a result of the approximately $13,000 increase in legal fees related to CHS intellectual property and approximately $10,000 decrease in consultant compensation cost in 2020.
General and Administrative Expenses
Our general and administrative expenses were $1,029,136 for the year ended December 31, 2020, as compared to $1,418,203 for the year ended December 31, 2019.
The approximately $389,000 net decrease was largely a result of a reduction in stock option expense of approximately $599,000. Stock option expense included in the year ended December 31, 2020 was approximately $125,000 compared with the approximately $724,000 of option expense incurred for the year ended December 31, 2019. Other significant decreases during the year ended December 31, 2020 include approximately $33,000 in labor costs, approximately $7,000 in accounting and audit fees and approximately $6,000 in travel expenses.
These reductions were offset for the year ended December 31, 2020 by increases of approximately $67,000 in sales consultant fees and approximately $53,000 in public relations and marketing consultants both of which were driven by the Company’s focus on initial CHS production and sales in 2021. Additional increases for the year ended December 31, 2020 include finance and administrative consultant expenses of approximately $48,000 and increases in legal fees of approximately $31,000 both of which resulted from activities for potential reverse mergers, funding opportunities, and chief financial officer transition. The Company also incurred increases of approximately $22,000 related to the market elevation from OTC Pink to OTCQB and increased transfer agent fees driven by the volume of stock activity. The Company also recognized increases of approximately $18,000 for insurance expenses as a result of Director and Officer premium increases and approximately $9,000 in IT, software and office supplies due to the staff increases and overall business activity.
Interest Expense
Interest expense was $665,232 for the year ended December 31, 2020, as compared to $287,307 for the year ended December 31, 2019. The increase of approximately $378,000 was a net result of the approximate $647,000 increase in interest expense and amortization of warrant debt cost, beneficial conversion feature and deferred costs of the Diversified Alpha Fund debt activity during 2020 and the decrease, absence, of approximately $269,000 interest expense of various notes fully paid during the year ended December 31, 2019.
Financial Condition, Liquidity and Capital Resources
Management remains focused on controlling cash expenses. We recognize our limited cash resources and plan our expenses accordingly. We intend to leverage stock-for-services wherever possible. The 2021 fiscal year operating budget consists of the following expenses:
|
●
|
Initial production of CHS for sale and evaluation by customer base and research institutes
|
|
●
|
Research and development costs for CHS sponsored research activities, Chief Technological consultant and costs related to strengthening our patent portfolio
|
|
●
|
Sales consulting staff to support CHS customer relationships
|
|
●
|
Chief Marketing consultant, marketing outreach and public relations firm to bolster the Company’s message and digital platform
|
|
●
|
General and administrative expenses: Chief Executive and Chief Financial officer expenses, salaries, insurance, investor related expenses, rent, travel, website, etc.
|
|
●
|
Professional fees for accounting and audit; legal services for securities and financing
|
As of December 31, 2020, we had net cash of $22,219 and a negative working capital of $240,417.
During the year ended December 31, 2020, we used $1,352,902 of cash for operating activities, an increase of $740,443 or 121% compared to the year ended December 31, 2019.
The increase in the use of cash for operating activities was a net result of the decrease in the loss from operations of $9,403, the increase in amortization of debt discount of $516,252, the increase in common stock issued for services of $22,171, the decrease in options issued for services of $673,135, the decrease in common stock issued for interest of $225,664, the increase in the change in prepaid expenses of $38,298, the increase in the change in deposits of $15,371 and a decrease in the change in accounts payable of $335,171.
During the year ended December 31, 2020, there was $1,316,972 of cash provided by financing activities, an increase of $650,365 or 98% compared to the year ended December 31, 2019. The increase was the net result of an increase in proceeds from debt and warrants issued of $580,178 and the $70,187 decrease in payments of notes payable.
As a result of the March 2, 2021, private placement stock purchase agreement, we will fund the ongoing operations through the existing financing in place. Raising additional funds for future activities could be achieved through a potential reverse merger arrangement or an additional financing partnership. Our ability to fund the future operations of the Company is highly dependent on the underlying stock price of the Company.
Off Balance Sheet Arrangements
The Company does not engage in any off-balance sheet arrangements that are reasonably likely to have a current or future effect on our consolidated financial condition, revenues, and results of operations, liquidity or capital expenditures.
Significant Accounting Policies
See Note 1 to our Consolidated Financial Statements included in this prospectus.
Recently Issued AccountingPronouncements
See the Recent Accounting Pronouncements section of Note 1 to our Consolidated Financial Statements included in this prospectus.
MANAGEMENT
The following table sets forth the names and ages of the members of our Board of Directors and our executive officers and the positions held by each. There are no family relationships among any of our Directors and Executive Officers.
|
Name
|
|
Age
|
|
Position
|
|
Victor Keen
|
|
79
|
|
Director, Co-Chairman
|
|
Simon Calton
|
|
40
|
|
Director, Co-Chairman
|
|
Michael A. Kraft
|
|
58
|
|
Chief Executive Officer
|
|
Matthew Hoffman
|
|
44
|
|
Chief Financial Officer
|
|
Ron Dombrowski
|
|
56
|
|
Director
|
Victor Keen – Director, Co-Chairman
Mr. Keen is a significant shareholder in the Company and has been a member of the Board of Directors since November 2007. Mr. Keen is a graduate of Harvard Law School and Trinity College. Until November 2010 he was the chair of the Tax Practice Group at Duane Morris LLP, an international law firm and one of the 100 largest law firms in the world. In November 2010, Mr. Keen became Of Counsel to the firm and has since devoted the majority of his time to charitable board memberships, as well as real estate investments and other ventures. For more than ten years Mr. Keen served on the board of Research Frontiers (NASDAQ: REFR), a developer of “Smart Glass” through licensees around the world. Mr. Keen has been an active investor in a number of private companies, both start up and later stage, including: Lending Tree, acquired by IAC Interactive Corp. (NASDAQ: IACI); Circle Lending, Inc., now part of Richard Branson’s Virgin Group; and Rollover Systems, Inc., a privately held company involved in the matching of individual IRA/pension accounts with appropriate managers. Mr. Keen is a co-founder and co-owner of Bantam Pharmaceutical LLC, a privately held biotechnology company founded in 2015 focusing on the discovery and development of innovative cancer therapies.
Simon Calton – Director, Co-Chairman
Simon Calton has over 13 years of experience in financing and company structuring and utilizes his experience to find opportunities in different sectors. Since 2008, Mr. Calton has structured a number of Alternative Investment Products geared around Construction and Development in the United States and United Kingdom. In 2012 he co-founded Carlton James Ltd., which specializes in funding specific projects and developments throughout the United States. In 2007 Mr. Calton co-founded Carlton James Private and Commercial, a project investment, pension administration service and global financing firm which helps to fund projects around the globe.
We believe Mr. Calton is qualified to serve on our board of directors because of his extensive business and management experience.
Michael A. Kraft – Chief Executive Officer
In addition to his role as CEO, Michael is a Mentor-in-Residence at the University of Michigan Technology Transfer Office and he is Founder/Managing Director of MKT Partners, LLC, an Executive Advisory and Interim C-Level firm focused on business development for materials science, performance materials and technology systems. Michael has served as an Executive in Residence for a large European PE Firm, CEO of Covaron Advanced Materials, Executive Officer and Vice President of Ceradyne Inc. (previously NASDAQ: CRDN), and GM at both Kulicke & Soffa and General Electric. As CEO/GM he has managed P&L’s from start-up to $300M and as a member of two executive teams grew shareholder value to >$1.4B by identifying critical business drivers, growing revenues and market share organically, building strategic partnerships, and completing accretive acquisitions. Michael completed the General Electric Crotonville Management Program with a Master of Management and Business from Penn State University. He holds a Bachelor of Science in Electrical Engineering & Systems Science from Michigan State University and recently extended his executive education by completing courses in Strategic Planning and Technology Marketing at CalTech.
Matthew Hoffman - Chief Financial Officer
Mr. Hoffman was appointed Chief Financial Officer of the Company on June 30, 2020. Since May 2020, he has served as Director of Finance of the Company. Prior to joining the Company, Mr. Hoffman was the Executive Director of Finance at Covance, Inc., from March 2019 through February 2020. From 2014 through 2019, he was Chief Financial Officer of MI Bioresearch. In these prior roles, Mr. Hoffman was responsible for all financial aspects of early stage company growth through acquisition, business unit financial reporting and forecasting, system integration and guidance to ERP platform, budgeting and business structure development. His leadership skills enabled strong financial performance at his former companies by managing cash flow and scaling the organizations while achieving 30-40% compounded annual growth. In his most recent year with Covance, the company achieved a 20 percent growth in revenue and 57 percent growth in profit over the prior year. From November 2012 through April 2014, Mr. Hoffman served as a partner of Onset CFO, LLC, and, from January 2011 through October 2012, he served as Vice President of Finance & Administration at Ultra Electronics, AMI.
Ron Dombrowski – Director
Since August 2015, Mr. Dombrowski served as a member of Coretec’s Board of Directors. Between April 2015 and November 2015, he was Director of Sales and Marketing at Lifting Solutions Automation Inc. From August 2010 to April 2015, Mr. Dombrowski served as the Vice President of Sales and Marketing at Limited Solutions Automation Inc. He is a graduate of Southern Illinois University with degrees in Electrical Engineering and Management. Mr. Dombrowski has also attended executive education programs at University of Phoenix and Marquette University. Mr. Dombrowski has over 25 years of global sales and operations experience, growing and scaling both startups and Fortune 500 technology companies.
We believe that Mr. Dombrowski is qualified to serve on our board of directors because of his background in sales and operations experience.
Audit Committee
On February 25, 2008, the Board of Directors created an Audit Committee comprised of Mr. Victor Keen. We intend to continue to evaluate the composition of our Audit Committee.
Compensation Committee
On February 25, 2008, the Board of Directors created a Compensation Committee comprised of Mr. Victor Keen. We intend to continue to evaluate the composition of our Compensation Committee.
Nomination and Corporate Governance Committee
On February 25, 2008, the Board of Directors created Nominations and Corporate Governance Committee comprising of Mr. Victor Keen. We intend to continue to evaluate the composition of our Nominations and Corporate Governance Committee.
Director or Officer Involvement in Certain Legal Proceedings
Our directors and executive officers were not involved in any legal proceedings as described in Item 401(f) of Regulation S-K in the past ten years.
Board Leadership Structure and Role in Risk Oversight
Although we have not adopted a formal policy on whether the Chairman and Chief Executive Officer positions should be separate or combined, in the past we determined that it was in the best interests of the Company and its shareholders to keep these two roles separate.
Our Board of Directors receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding our Company's assessment of risks. Our Board of Directors focuses on the most significant risks facing our Company and our Company's general risk management strategy and ensures that risks undertaken by us are consistent with the Board of Directors' appetite for risk. While the Board of Directors oversees our Company's risk management, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing our Company and that our board leadership structure and role in risk oversight is effective.
Involvement in Certain Legal Proceedings
To our knowledge, our directors and executive officers have not been involved in any of the following events during the past ten years:
|
|
1.
|
any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
|
|
|
|
|
|
|
2.
|
any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses);
|
|
|
|
|
|
|
3.
|
being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities;
|
|
|
|
|
|
|
4.
|
being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated;
|
|
|
|
|
|
|
5.
|
being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
|
|
|
|
|
|
|
6.
|
being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
|
Code of Ethics
We have not adopted a Code of Ethics and Business Conduct for Officers, Directors and Employees that applies to all of our officers, directors and employees.
Employment Agreements
The Company entered into a consulting agreement dated March 20, 2017 with Mr. Michael A. Kraft, who became the Company’s CEO. Under the terms of the agreement the Company agreed to compensate Mr. Kraft, $1,500 per day for his commitment to allocate seven days a month (subsequently amended to ten day a month) to the Company and a $25,000 bonus payable in the Company’s restricted stock upon occurrence of certain events. Mr. Kraft was issued ten million options during August 2019 for (1) as compensation for the $25,000 bonus in the consulting agreement, (2) approximately $91,000 as payment for unpaid consulting fees and, (3) approximately $294,000 as additional compensation for his consulting services. During the years ended December 31, 2020 and 2019, the Company recognized $180,000 and $144,000 of expense respectively, under the terms of the agreement. Mr. Kraft was owed $51,720 and $95,966 in unpaid consulting fees and out of pocket expenses, which is included in accounts payable and accrued expenses as of December 31, 2020 and 2019 respectively.
On November 13, 2017, the Company hired Ramez Elgammal as Vice President of Technology. Elgammal leads research and development and intellectual property initiatives for the Company’s proprietary liquid silicon precursor, CHS. The one-year consulting agreement was effective as of November 15, 2017 and continued in full force and effect through November 14, 2018 whereupon the agreement was renewed on a month to month term. Under the terms of the agreement. Mr. Elgammal is compensated at the rate of $125 per hour. On February 3, 2020 Ramez Elgammal agreed to an addendum to his November 15, 2017 consulting agreement providing for the payment of certain amounts owed him in common shares of the Company. The addendum was related to the balance that was owed to Mr. Elgammal for consulting services on August 31, 2019 totaling $46,054. Under the terms of the addendum the $46,054 is to be paid one-half in cash, $23,027 and one-half in S8 stock $23,027, at a discount to the market of 30% of the average closing price of the previous fifteen (15) days prior to August 31, 2019, or 534,022 shares of S8 stock at a conversion price of $0.0431 per share. The Company recognized expenses of $54,003 for the year ended December 31, 2020.
The Company entered into a one-year consulting agreement with Michelle Tokarz effective February 10, 2020 and expiring February 9, 2021. Under the terms of the agreement, Tokarz will have the position of Business Development Consultant. Tokarz will be paid an hourly fee of $115 with a maximum of $1,000 per day and shall make up to ten days available to the Company each month. The Company recognized expenses of approximately $52,000 during the year ended December 31, 2020.
The Company entered into a one-year consulting agreement with Matthew Hoffman, doing business as Integrate Growth, LLC, effective May 18, 2020 and expiring May 19, 2021. Under the terms of the agreement, Hoffman had the position of Director of Finance. On June 30, 2020 Ron Robinson, Chief Financial Officer and Judith Keating, Corporate Secretary, both retired from the Company. As part of the management transition plan Hoffman was elevated to Chief Financial Officer and Corporate Secretary on June 30, 2020. Hoffman will be paid a monthly fee of $6,000 and shall make up to twenty hours per week available to the Company for each week of each month. The Company recognized $42,000 of consultant expense to Hoffman for the year ended December 31, 2020.
Director Compensation
Our directors have not received monetary compensation for their service on the Board of Directors. Directors may receive compensation for their services and reimbursement for their expenses as shall be determined from time to time by resolution of the Board of Directors.
Except as below, none of the following parties has, since our date of incorporation, had any material interest, direct or indirect, in any transaction with us or in any presently proposed transaction that has or will materially affect us:
|
|
●
|
Any of our directors or officers;
|
|
|
●
|
Any person proposed as a nominee for election as a director;
|
|
|
●
|
Any person who beneficially owns, directly or indirectly, shares carrying more than 10% of the voting rights attached to our outstanding shares of common stock;
|
|
|
●
|
Any member of the immediate family of any of the foregoing persons.
|
On December 27, 2019, the Group issued 123,330,807 shares of common stock (“Common Stock”) of the Company upon the conversion of debt held by certain holders (the “Legacy Holders”), which Legacy Holders consists substantially of the Company’s Co-Chairmen, Victor Keen and Simon Calton. The total outstanding debt converted was $2,711,359 (“Legacy Debt”), which consisted of $2,017,434 in outstanding principal and $693,925 in accrued interest.
Risk Management
The Company does not believe risks arising from its compensation policies and practices for its employees are reasonably likely to have a material adverse effect on the Company.
Director Independence
Because the Company’s Common Stock is not currently listed on a national securities exchange, the Company has used the definition of “independence” of The NASDAQ Stock Market to make this determination. NASDAQ Listing Rule 5605(a)(2) provides that an “independent director” is a person other than an officer or employee of the company or any other individual having a relationship which, in the opinion of the company’s board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The NASDAQ listing rules provide that a director cannot be considered independent if:
|
|
●
|
the director is, or at any time during the past three years was, an employee of the company;
|
|
|
●
|
the director or a family member of the director accepted any compensation from the company in excess of $120,000 during any period of 12 consecutive months within the three years preceding the independence determination (subject to certain exclusions, including, among other things, compensation for board of director or board committee service);
|
|
|
●
|
a family member of the director is, or at any time during the past three years was, an executive officer of the company;
|
|
|
●
|
the director or a family member of the director is a partner in, controlling stockholder of, or an executive officer of an entity to which the company made, or from which the company received, payments in the current or any of the past three fiscal years that exceed 5% of the recipient’s consolidated gross revenue for that year or $200,000, whichever is greater (subject to certain exclusions);
|
|
|
●
|
the director or a family member of the director is employed as an executive officer of an entity where, at any time during the past three years, any of the executive officers of the company served on the compensation committee of such other entity; or
|
|
|
●
|
the director or a family member of the director is a current partner of the company’s outside auditor, or at any time during the past three years was a partner or employee of the company’s outside auditor, and who worked on the company’s audit.
|
Based on this review of the Company’s Board of Directors, none of the members are considered to be independent under the listing standards of the Rules of NASDAQ set forth in the NASDAQ Manual.
EXECUTIVE AND DIRECTOR COMPENSATION
The following information is furnished for the years ended December 31, 2020, 2019 and 2018 for our Co-Chairman, Chief Executive Officer and our Chief Financial Officers.
|
Name and
Principal
Position
|
|
Year
|
|
Salary
($)
|
|
|
Bonus
($)
|
|
|
Stock Awards
($)
|
|
|
Option Awards
($)
|
|
|
Non-Equity
Incentive Plan
Compensation
($)
|
|
|
Change in
Pension
Value
and Non-
Qualified
Deferred
Compensation
($)
|
|
|
Earnings
All Other
Compensation
($)
|
|
|
Total
($)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Simon Calton Co-Chairman
|
|
2020
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
2019
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
2018
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Victor Keen Co-Chairman
|
|
2020
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
2019
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
2018
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael A. Kraft CEO*
|
|
2020
|
|
$
|
180,000
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
180,000
|
|
|
|
|
2019
|
|
$
|
144,000
|
|
|
$
|
25,000
|
|
|
$
|
294,132
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
463,132
|
|
|
|
|
2018
|
|
$
|
126,000
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
126,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Matthew Hoffman CFO**
|
|
2020
|
|
$
|
42,000
|
|
|
$
|
-
|
|
|
$
|
37,446
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
79,446
|
|
|
|
|
2019
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
2018
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ronald Robinson Former CFO***
|
|
2020
|
|
$
|
40,200
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
40,200
|
|
|
|
|
2019
|
|
$
|
72,000
|
|
|
$
|
-
|
|
|
$
|
20,500
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
92,500
|
|
|
|
|
2018
|
|
$
|
72,000
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
72,000
|
|
|
*
|
Michael A. Kraft was appointed CEO in March 2017.
|
|
**
|
Matthew Hoffman was appointed CFO in June 2020 and was a consultant to the Company between May 2020 and June 2020.
|
|
***
|
Ron Robinson resigned from his position as CFO of the Company in June 2020.
|
Outstanding Equity Awards at Fiscal Year End
The following table sets forth with respect to grants of options to purchase our common stock to the executive officers as of December 31, 2020:
|
Name
|
|
Number of
Securities
Underlying
Unexercised
Options
#
Exercisable
|
|
|
Number of
Securities
Underlying
Unexercised
Options
#
Un-exercisable
|
|
|
Equity
Incentive
Plan Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options
#
|
|
|
Option
Exercise
Price
$
|
|
|
Option
Expiration
Date
|
|
|
Number
of
Shares
or Units
of Stock
That
Have Not
Vested
#
|
|
|
Market
Value
of
Shares
or Units
of Stock
That
have
not
vested
$
|
|
|
Equity
Incentive
Plan
Awards:
Number of
Unearned
Shares Units
or Other
Rights That
Have Not
Vested #
|
|
|
Equity
Incentive
Plan
Awards
Market or
Payout
Value of
Unearned
Shares Units
or Other
Rights That
have not
Vested
$
|
|
|
Victor Keen, former CEO
|
|
|
1,338
|
|
|
|
-
|
|
|
|
-
|
|
|
|
$70
|
to
|
$420
|
|
|
|
2021
|
-
|
2022
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Michael A. Kraft, CEO
|
|
|
10,208,160
|
|
|
|
-
|
|
|
|
-
|
|
|
|
$.04
|
to
|
$0.24
|
|
|
|
2024
|
-
|
2027
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Matthew L. Hoffman, CFO
|
|
|
250,000
|
|
|
|
750,000
|
|
|
|
-
|
|
|
|
|
|
$.065
|
|
|
|
|
|
2025
|
|
|
|
750,000
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
Director Compensation 2020
|
Name
|
|
Fees
Earned or
Paid in
Cash ($)
|
|
|
Stock
Awards
($)
|
|
|
Option
Awards
($)
|
|
|
Non-Equity
Incentive Plan
Compensation
($)
|
|
|
Change in
Pension
Value and
Nonqualified
Deferred
Compensation
Earnings ($)
|
|
|
All Other
Compensation
($)
|
|
|
Total ($)
|
|
|
Victor Keen
|
|
$
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
Simon Calton
|
|
$
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
|
Ron Dombrowski
|
|
$
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
$
|
-
|
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table provides information about shares of common stock beneficially owned as of March 12, 2021 by:
|
|
●
|
each director;
|
|
|
|
|
|
|
●
|
each officer named in the summary compensation table;
|
|
|
|
|
|
|
●
|
each person owning of record or known by us, based on information provided to us by the persons named below, to own beneficially at least 5% of our common stock; and
|
|
|
|
|
|
|
●
|
all directors and executive officers as a group.
|
|
Name of Beneficial Owner
|
|
Common
Stock
Beneficial
Ownership(1)
|
|
|
Percent
of
Class(2)
|
|
|
Series A
Preferred
Beneficial
Ownership
|
|
|
Percent of
Class(11)
|
|
|
Named Executive Officers and Directors:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Victor Keen (3)
|
|
|
98,577,083
|
|
|
|
41.18
|
%
|
|
|
265,000
|
|
|
|
76.81
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael A. Kraft (4)
|
|
|
10,208,160
|
|
|
|
4.09
|
%
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Matthew Hoffman (5)
|
|
|
250,000
|
|
|
|
*
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Simon Calton (6)
|
|
|
16,818,760
|
|
|
|
7.03
|
%
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ronald Dombrowski (7)
|
|
|
3,644,920
|
|
|
|
1.80
|
%
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All directors and executive officers as a group (5 person)
|
|
|
129,498,923
|
|
|
|
51.84
|
%
|
|
|
265,000
|
|
|
|
76.81
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other 5% Stockholders:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carlton James Ltd. (8)
|
|
|
27,017,391
|
|
|
|
11.29
|
%
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diversified Alpha Fund (9)
|
|
|
24,699,105
|
|
|
|
9.99
|
%
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Armistice Capital Master Fund Ltd. (10)
|
|
|
23,500,000
|
|
|
|
9.82
|
%
|
|
|
-
|
|
|
|
-
|
|
*less than 1%
|
|
(1)
|
Number of Shares Beneficially Owned include the conversion of all Series B Preferred shares into common stock.
|
|
|
(2)
|
Percentage ownership is determined based on shares owned together with securities exercisable or convertible into shares of common stock within 60 days of the date of this registration statement, for each stockholder. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Furthermore, the percentages set forth in this column are based on the 239,267,102 issued and outstanding shares of common stock on March 12, 2021.
|
|
|
(3)
|
Represents 94,528,679 shares owned by Mr. Keen and (i) 3,958,733 shares, representing his pecuniary interest in shares held by Carlton James Ltd., (ii) 1,338 shares issuable upon exercise of the options held by Mr. Keen, and (iii) 88,333 shares issuable upon the conversion of 265,000 shares of Series A preferred stock held by Mr. Keen. Victor Keen is a Co-Chairman of the Company’s Board of Directors.
|
|
|
(4)
|
Represents 10,208,160 shares issuable upon exercise of vested options held by Mr. Kraft. Michael A. Kraft is the Company’s Chief Executive Officer.
|
|
|
(5)
|
Represents 250,000 shares issuable upon exercise of vested options held by Mr. Hoffman but excludes 750,000 shares issuable upon exercise of options that do not vest within 60 days. Matthew Hoffman is the Company’s Chief Financial Officer.
|
|
|
(6)
|
Represents 5,771,131 shares owned by Mr. Calton and 11,047,629 shares, representing his pecuniary interest in shares held by Carlton James Ltd. Simon Calton is the Co-Chairman of the Company’s Board of Directors.
|
|
|
(7)
|
Represents 3,644,920 owned by Mr. Dombrowski. Ronald Dombrowski is a Director on the Company’s Board of Directors.
|
|
|
(8)
|
Shares held by Carlton James Ltd., are controlled by Simon Calton, the Co-Chairman of the Company’s Board of Directors.
|
|
|
(9)
|
Represents 16,727,920 shares owned by Diversified Alpha Fund (DAF) and 7,971,185 shares issuable upon conversions, which conversions are subject to a 9.99% ownership limitation, of outstanding balances under a Credit Agreement and related Promissory Note entered into by the Company and DAF. DAF is managed and controlled by Mollitium Investment Management.
|
|
|
|
|
|
|
(10)
|
The shares are directly held by Armistice Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”), and may be deemed to be indirectly beneficially owned by: (i) Armistice Capital, LLC (“Armistice Capital”), as the investment manager of the Master Fund; and (ii) Steven Boyd, as the Managing Member of Armistice Capital. The number of shares excludes 134,000,000 shares of common stock issuable upon exercise of the pre-funded warrants and the warrants, both of which are subject to certain beneficial ownership limitations. Armistice Capital and Steven Boyd disclaim beneficial ownership of the securities except to the extent of their respective pecuniary interests therein. The business address for the Master Fund is c/o Armistice Capital, LLC, 510 Madison Avenue 7th Floor, New York 10022.
|
|
|
(11)
|
Calculated on the basis of 345,000 issued and outstanding shares of Series A Convertible Preferred Stock as of March 12, 2021.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Related Party Transactions
The Company entered into a consulting agreement dated March 20, 2017 with Mr. Michael A. Kraft, who became the Company’s CEO. Under the terms of the agreement the Company agreed to compensate Mr. Kraft, $1,500 per day for his commitment to allocate seven days a month (subsequently amended to ten days a month) to the Company and a $25,000 bonus payable in the Company’s restricted stock upon occurrence of certain events. Mr. Kraft was issued ten million options during August 2019 for (1) as compensation for the $25,000 bonus in the consulting agreement, (2) approximately $91,000 as payment for unpaid consulting fees and, (3) approximately $294,000 as additional compensation for his consulting services. During the years ended December 31, 2020 and 2019, the Company recognized $180,000 and $144,000 of expense respectively, under the terms of the agreement. Mr. Kraft was owed $51,720 and $95,966 in unpaid consulting fees and out of pocket expenses, which is included in accounts payable and accrued expenses as of December 31, 2020 and 2019 respectively.
At December 31, 2018 the Company had an aggregate balance of $971,500 of advances due to Mr. Victor Keen, Co-Chairman of the Board of Directors. During the year ended December 31, 2019 Mr. Keen advanced the Company an additional $135,000, such that an aggregate amount of $1,106,500 was due to Mr. Keen under the terms of certain promissory notes and convertible debentures (“the Notes”) which were included in notes payable – related party (see Note 6 of the consolidated financial statements). The Notes along with accrued interest of $342,292, were converted to common stock on December 27, 2019. Interest expense related to the Notes was $112,969 for the year ended December 31, 2019.
At December 31, 2018, the Company had an aggregate balance of $775,934 of advances due to Carlton James Ltd., a company owned by Mr. Simon Calton, a director of the Company. During the years ended December 31, 2019, Carlton James Ltd., advanced an additional $135,000 such that as of November 30, 2019, an aggregate amount of $910,934 was due to Carlton James Ltd. under the terms of two loans (“Loans”), which were included in notes payable-related parties (see Note 6 of the notes to financial statements). The Loans along with accrued interest of $351,633 were converted to common stock on December 27, 2019. Interest expense related to the Loans was $112,695 for the year ended December 31, 2019.
Director Independence
As discussed above, none of the members are considered to be independent under the listing standards of the Rules of NASDAQ set forth in the NASDAQ Manual.
DESCRIPTION OF CAPITAL STOCK
Authorized Capital Stock
Our authorized capital stock consists of 1,500,000,000 shares of common stock, par value $0.0002 per share, and 25,000,000 shares of preferred stock, par value $0.0002 per share. The number of shares of our common stock issued and outstanding as of a recent date is set forth on the cover page of our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission. As of the same date, we had preferred stock designated as follows: 500,000 shares designated as Series A Preferred Stock (of which 345,000 were outstanding).
Common Stock
Voting Rights. Each holder of our common stock is entitled to one vote per share on all matters on which stockholders are generally entitled to vote. Except as otherwise required by law, the holders of shares of Series A Preferred Stock do not have voting rights or powers. When a quorum is present at any meeting, the vote of the holders of a majority of the stock having voting power present in person or represented by proxy shall decide any question brought before such meeting, unless the question is one upon which, by express provision of the statutes or of the Certificate of Incorporation, a different vote is required, in which case such express provision shall govern and control the decision of such question. Our Certificate of Incorporation does not provide for cumulative voting in the election of directors.
Dividends. We have not declared any dividends to date. We have no present intention of paying any cash dividends on our common stock in the foreseeable future, as we intend to use earnings, if any, to generate growth. The payment of dividends, if any, in the future, rests within the discretion of our Board of Directors and will depend, among other things, upon our earnings, capital requirements and our financial condition, as well as other relevant factors. There are no restrictions in our Certificate of Incorporation or Bylaws that restrict us from declaring dividends.
Other Rights. The holders of our common stock have no preemptive rights and no rights to convert their common stock into any other securities, and our common stock is not subject to any redemption or sinking fund provisions.
Preferred Stock
Under our Certificate of Incorporation and subject to the limitations prescribed by law, our Board of Directors, without stockholder approval, may issue our preferred stock in one or more series, and may establish from time to time the number of shares to be included in such series and may fix the designation, powers, privileges, preferences and relative participating, optional or other rights, if any, of the shares of each such series and any qualifications, limitations or restrictions thereof.
When and if we issue additional shares of preferred stock, we will establish the applicable preemptive rights, dividend rights, voting rights, conversion privileges, redemption rights, sinking fund rights, rights upon voluntary or involuntary liquidation, dissolution or winding up and any other relative rights, preferences and limitations for the particular preferred stock series.
Anti-Takeover Effects of Provisions of Oklahoma Law, Our Certificate of Incorporation and Bylaws
Oklahoma statutory law and our Certificate of Incorporation and Bylaws contain provisions that could make acquisition of our Company by means of a tender offer, a proxy contest or otherwise more difficult. These provisions are intended to discourage certain types of coercive takeover practices and takeover bids that our Board of Directors may consider inadequate and to encourage persons seeking to acquire control of us to first negotiate with our Board of Directors. We believe that the benefits of increased protection of our ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging takeover or acquisition proposals because, among other things, negotiation of these proposals could result in an improvement of their terms. The description of our Certificate of Incorporation and Bylaws set forth below is only a summary and is qualified in its entirety by reference to our Certificate of Incorporation and Bylaws, which have been filed as exhibits to our most recent Annual Report on Form 10-K.
Blank Check Preferred Stock. Our Certificate of Incorporation permits us to issue, without any further vote or action by the stockholders, up to 25,000,000 shares of preferred stock in one or more series and, with respect to each such series, to fix the number of shares constituting the series and the designation of the series, the voting powers (if any) of the shares of the series, and the preferences and relative, participating, optional and other rights, if any, and any qualifications, limitations or restrictions, of the shares of such series. The ability to issue such preferred stock could discourage potential acquisition proposals and could delay or prevent a change in control.
Number of Directors; Filling Vacancies; Removal. Our Certificate of Incorporation and Bylaws provide that the Board of Directors will consist of not less than one nor more than seven members, with the exact number of directors determined by resolution of the Board of Directors or by the Shareholders at an annual or special meeting. In addition, our certificate of incorporation and by-laws provide that a board vacancy resulting from the death, resignation, disqualification or removal of a director or other cause, as well as a vacancy resulting from an increase in the number of directors, may be filled by a majority ofthe Directors then in office, though less than a quorum, or by a sole remaining Director, and the Directors so chosen shall hold office until the next annual election or until their successors are duly elected and qualified, unless sooner displaced. If there are no Directors in office, then an election of Directors may be held in the manner provided by law.
Special Meetings. Our Certificate of Incorporation and by-laws provide that special meetings of the board may be called by the president on three days' notice to each Director, either personally or by mail or by telegram. Special meetings shall be called by the Chairman of the Board, any Vice Chairman of the Board, the President, any Vice-president or the Secretary in like manner and on like notice on the written request of two "Directors unless the board consists of less than three Directors in which case special meetings shall be called by the Chairman of the Board, any Vice Chairman of the Board, the President, any Vice-President or the Secretary in like manner and on like notice on the written request of only one Director. Notice of such meetings shall state the place, date, hour and business to be conducted at such meeting.
Section 1090.3 of the Oklahoma General Corporation Act. Section 1090.3 of the Oklahoma General Corporation Act provides that, subject to certain specified exceptions, a corporation cannot engage in any “business combination” with any “interested stockholder” for a three-year period following the time that such stockholder becomes an interested stockholder unless (1) before that time, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder, (2) upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85 percent of the voting stock of the corporation outstanding at the time the transaction commenced (excluding certain shares) or (3) on or after such time, both the board of directors of the corporation and at least 66 percent of the outstanding voting stock which is not owned by the interested stockholder approves the business combination. Section 1090.3 generally defines an "interested stockholder" to include any person that owns 15 percent or more of the outstanding voting stock of the corporation, or is an affiliate or associate of the corporation and owned 15 percent or more of the outstanding voting stock of the corporation within a three-year period before the person was determined to be an interested shareholder.
Section 1090.3 of the Oklahoma General Corporation Act generally defines a "business combination" to include (1) mergers and sales or other dispositions of 10 percent or more of the corporation's assets with or to an interested stockholder, (2) certain transactions resulting in the issuance or transfer to the interested stockholder of any stock of the corporation or its subsidiaries, (3) certain transactions which would increase the proportionate share of the stock of the corporation or its subsidiaries owned by the interested stockholder and (4) receipt by the interested stockholder of the benefit (except proportionately as a stockholder) of any loans, advances, guarantees, pledges, or other financial benefits.
Under certain circumstances, 1090.3 of the Oklahoma General Corporation Act makes it more difficult for a person who would be an “interested stockholder” to effect various business combinations with a corporation for a three-year period, although the certificate of incorporation or stockholder-adopted by-laws may exclude a corporation from the restrictions imposed under Section 203. Neither our Certificate of Incorporation nor our Bylaws exclude our Company from the restrictions imposed under Section 1090. We anticipate that Section 1090.3 may encourage companies interested in acquiring our Company to negotiate in advance with our Board of Directors since the statute’s supermajority stockholder approval requirement would not be applicable if our Board of Directors approves, prior to the time the stockholder becomes an interested stockholder, either the business combination or the transaction which results in the stockholder becoming an interested stockholder.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company, One State Street Plaza, 30th Floor, New York, NY 10004.
Stock Quotation
Our common stock is currently quoted on OTCQB and under the symbols “CRTG”.
Indemnification of Directors and Officers
Our bylaws provide that the Company may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, other than an action by or in the right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorney’s fees, judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with such action, suit or proceeding if he acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 (the "Act") may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the small business issuer has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities, other than the payment by us of expenses incurred or paid by our directors, officers or controlling persons in the successful defense of any action, suit or proceedings, is asserted by such director, officer, or controlling person in connection with any securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Act and will be governed by the final adjudication of such issues.
Disclosure of Commission Position on Indemnification for Securities Act Liabilities
Insofar as indemnification for liabilities under the Securities Act may be permitted to officers, directors or persons controlling the Company pursuant to the foregoing provisions, the Company has been informed that is it is the opinion of the SEC that such indemnification is against public policy as expressed in such Securities Act and is, therefore, unenforceable.
PRIVATE PLACEMENT OF SHARES OF COMMON STOCK, WARRANTS AND PRE-FUNDED WARRANTS
On March 2, 2021 (the “Signing Date”), we entered into a securities purchase agreement (the “Purchase Agreement”) with a single institutional and accredited investor pursuant to which we sold to the investor in a private placement an aggregate of (i) 23,500,000 shares of common stock (the “Shares”), (ii) pre-funded warrants to purchase up to an aggregate of 51,500,000 shares of common stock (the “Pre-Funded Warrants”) and (iii) warrants to purchase up to an aggregate of 82,500,000 shares of common stock for gross proceeds to the Company of approximately $6,000,000 (the “Warrants”). The combined purchase price for one share of common stock and a warrant to purchase one share of common stock is $0.08 and the combined purchase price for one pre-funded warrant to purchase one share of common stock and a warrant to purchase one share of common stock is 0.0799. The closing for the sale of the Shares, Pre-Funded Warrants and Warrants occurred on March 5, 2021.
We intend to use the net proceeds primarily for working capital purposes and shall not use such proceeds: (a) for the satisfaction of any portion of the Company’s debt (other than payment of trade payables in the ordinary course of the Company’s business and prior practices), (b) for the redemption of any Common Stock or Common Stock Equivalents, (c) for the settlement of any outstanding litigation or (d) in violation of FCPA or OFAC regulations.
The Pre-Funded Warrants have an exercise price of $0.0001 per share, subject to adjustment and no expiration date. The Pre-Funded Warrants are exercisable immediately and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full.
The Warrants are exercisable for a period of five and one-half years from the date of issuance and have an exercise price of $0.08 per share, subject to adjustment as set forth in the Warrants for stock splits, stock dividends, recapitalizations and similar customary adjustments. The investor may exercise the Warrants on a cashless basis if the shares of common stock underlying the Warrants (the “Warrant Shares”) are not then registered pursuant to an effective registration statement.
Under the terms of the Warrants and Pre-Funded Warrants, the investor may not exercise the Warrants to the extent such exercise would cause such investor, together with its affiliates and attribution parties, to beneficially own a number of shares of common stock which would exceed 4.99% of our then outstanding common stock following such exercise, excluding for purposes of such determination shares of common stock issuable upon exercise of the Warrants which have not been exercised.
In connection with the Purchase Agreement, we entered into a registration rights agreement (the “Registration Rights Agreement”) with the investor. Pursuant to the Registration Rights Agreement, we will be required to file a resale registration statement (the “Registration Statement”) with the Securities and Exchange Commission (the “SEC”) to register for resale of the Shares, the shares issuable upon exercise of the Pre-Funded Warrants and the Warrant Shares, within 15 days of the Signing Date, and to have such Registration Statement declared effective within 45 days after the Signing Date, or 90 days of the Signing Date in the event the Registration Statement is “fully” reviewed by the SEC. We will be obligated to pay certain liquidated damages to the investor if we fail to file the resale registration statement when required, fail to cause the Registration Statement to be declared effective by the SEC when required, or if we fail to maintain the effectiveness of the Registration Statement.
Pursuant to an engagement letter (the “Engagement Letter”), dated as of February 26, 2021, by and between the Company and H.C. Wainwright & Co., LLC, we engaged HCW to act as our exclusive placement agent in connection with the offering. Pursuant to the engagement agreement, we agreed to pay HCW a cash fee of 8.0% of the gross proceeds we received under the Purchase Agreement. We also agreed to pay HCW (i) a management fee equal to 1.0% of the gross proceeds raised in the offering; and (ii) $85,000 for non-accountable and legal expenses. In addition, we agreed to issue to HCW (or its designees) placement agent warrants (the “Placement Agent Warrants”) to purchase a number of shares equal to 8.0% of the aggregate number of Shares sold under the Purchase Agreement, or warrants to purchase up to an aggregate of 600,000 shares. The Placement Agent Warrants generally will have the same terms as the Warrants, except they will have an exercise price of $0.10 per share.
SELLING STOCKHOLDERS
The common stock being offered by the selling stockholders are those previously issued to the investor in the March 2, 2021 Private Placement and those issuable to the investor upon exercise of the Warrants, Pre-Funded Warrants as well as the Placement Agent Warrants. For additional information regarding the issuances of those shares of common stock, Warrants, Pre-Funded Warrants and Placement Agent Warrants see “Private Placement of Shares of Common Stock Warrants and Pre-Funded Warrants” above. We are registering the shares of common stock in order to permit the selling stockholders to offer the shares for resale from time to time. Except for the ownership of the shares of common stock, Warrants and the Pre-Funded Warrants, the selling stockholders have not had any material relationship with us within the past three years.
The table below lists the selling stockholders and other information regarding the beneficial ownership of the shares of common stock by the selling stockholders. The second column lists the number of shares of common stock beneficially owned each of the by the selling stockholders, based on its ownership of the shares of common stock and warrants, as of March 2, 2021, assuming exercise of the Warrants and Pre-Funded Warrants held by the selling stockholders on that date, without regard to any limitations on exercises. As of March 12, 2021, 239,267,102 shares of the Company’s common stock were issued and outstanding.
The third column lists the shares of common stock being offered by this prospectus by the selling stockholders.
This prospectus generally covers the resale of the sum of (i) the number of shares of common stock issued to the investor in the March 2, 2021 Private Placement as described above and (ii) the maximum number of shares of common stock issuable upon exercise of the related warrants and the Placement Agent Warrants, determined as if the outstanding warrants were exercised in full as of the trading day immediately preceding the date this registration statement was initially filed with the SEC, each as of the trading day immediately preceding the applicable date of determination and all subject to adjustment as provided in the registration right agreement, without regard to any limitations on the exercise of the warrants. The fourth column assumes the sale of all of the shares offered by the selling stockholders pursuant to this prospectus.
Under the terms of the Warrants and Pre-Funded Warrants, the investor (Armistice Capital Master Fund Ltd.) may not exercise the Warrants to the extent such exercise would cause such investor, together with its affiliates and attribution parties, to beneficially own a number of shares of common stock which would exceed 4.99% of our then outstanding common stock following such exercise, excluding for purposes of such determination shares of common stock issuable upon exercise of the Warrants which have not been exercised (the “Beneficial Ownership Limitation”). The Placement Agent Warrants are also subject to a Beneficial Ownership Limitation. The number of shares in the second column does not reflect this limitation. The selling stockholder may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
Each of Michael Vasinkevich, Noam Rubinstein, Craig Schwabe and Charles Worthman, are affiliated with H.C. Wainwright & Co., LLC, a registered broker-dealer. H.C. Wainwright & Co., LLC and/or any of its affiliates previously served as our exclusive placement agent for the March 2, 2021 Private Placement pursuant to the Engagement Letter and as financial advisor from time to time in the ordinary course of their business, for which they have received customary fees and commissions.
|
Name of Selling Stockholder
|
|
Number of
shares of
Common
Stock
Owned
Prior to
Offering
|
|
|
Maximum
Number of
shares of
Common
Stock to be
Sold
Pursuant to
this
Prospectus
|
|
|
Number
of shares
of
Common
Stock
Owned
After
Offering
|
|
|
Armistice Capital Master Fund Ltd.
|
|
|
157,500,000
|
|
|
|
157,500,000
|
(1)
|
|
|
-
|
|
|
Michael Vasinkevich(2)
|
|
|
3,847,500
|
|
|
|
3,847,500
|
|
|
|
-
|
|
|
Noam Rubinstein(2)
|
|
|
1,890,000
|
|
|
|
1,890,000
|
|
|
|
-
|
|
|
Craig Schwabe(2)
|
|
|
202,500
|
|
|
|
202,500
|
|
|
|
-
|
|
|
Charles Worthman(2)
|
|
|
60,000
|
|
|
|
60,000
|
|
|
|
-
|
|
|
(1)
|
The shares are directly held by Armistice Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”), and may be deemed to be indirectly beneficially owned by: (i) Armistice Capital, LLC (“Armistice Capital”), as the investment manager of the Master Fund; and (ii) Steven Boyd, as the Managing Member of Armistice Capital. The number of shares includes 134,000,000 shares of common stock issuable upon exercise of the Pre-Funded Warrants and the Warrants, both of which are subject to certain beneficial ownership limitations. Armistice Capital and Steven Boyd disclaim beneficial ownership of the securities except to the extent of their respective pecuniary interests therein. The business address for the Master Fund is c/o Armistice Capital, LLC, 510 Madison Avenue 7th Floor, New York 10022.
|
|
(2)
|
Each of Michael Vasinkevich, Noam Rubinstein, Craig Schwabe and Charles Worthman have a registered address of 430 Park Ave, 3rd Floor, New York, NY 10022.
|
PLAN OF DISTRIBUTION
The Selling Stockholders (the “Selling Stockholders”) of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities covered hereby on the OTCQB or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. Each Selling Stockholder may use any one or more of the following methods when selling securities: The selling stockholders will offer their shares at prevailing market prices or privately negotiated prices.
|
|
●
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
●
|
block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
|
●
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
●
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
●
|
privately negotiated transactions;
|
|
|
●
|
settlement of short sales;
|
|
|
●
|
in transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated price per security;
|
|
|
●
|
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
|
|
|
●
|
a combination of any such methods of sale; or
|
|
|
●
|
any other method permitted pursuant to applicable law.
|
The Selling Stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933, as amended (the “Securities Act”), if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2440.
In connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each of the Selling Stockholders have informed the Company that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities.
The Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for the Company to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the common stock by the Selling Stockholder or any other person. We will make copies of this prospectus available to the Selling Stockholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
LEGAL MATTERS
The validity of the securities being offered by this prospectus has been passed upon for us by Sichenzia Ross Ference LLP, New York, New York. Sichenzia Ross Ference LLP or certain members or employees of Sichenzia Ross Ference LLP have been issued common stock of the Company.
EXPERTS
The consolidated financial statements of The Coretec Group, Inc. as of and for the years ended December 31, 2020 and 2019 appearing in this prospectus, have been audited by HoganTaylor LLP, as set forth in its report thereon, included herein. Such consolidated financial statements are included herein in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
Federal securities laws require us to file information with the SEC concerning our business and operations. Accordingly, we file annual, quarterly, and special reports, and other information with the Commission. The SEC maintains a web site (http://www.sec.gov) at which you can read or download our reports and other information.
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities being offered hereby. As permitted by the rules and regulations of the SEC, this prospectus does not contain all the information set forth in the registration statement and the exhibits and schedules thereto. For further information with respect to the Company and the securities offered hereby, reference is made to the registration statement, and such exhibits and schedules. The registration statement may be accessed at the SEC’s web site.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
Consolidated Balance Sheets As of December 31, 2020 and 2019
|
F-3
|
|
Consolidated Statements of Operations For the Years Ended December 31, 2020 and 2019
|
F-4
|
|
Consolidated Statements of Changes in Stockholders' Equity (Deficiency) For the Years Ended December 31, 2020 and 2019
|
F-5
|
|
Consolidated Statements of Cash Flows For the Years Ended December 31, 2020 and 2019
|
F-6
|
|
Notes to Consolidated Financial Statements For the Years Ended December 31, 2020 and 2019
|
F-7
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of The Coretec Group Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of The Coretec Group Inc. and its subsidiary (the Company) as of December 31, 2020 and 2019, the related consolidated statements of operations, stockholders' equity (deficiency) and cash flows for the years then ended, and the related notes to the consolidated financial statements (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Patent Impairment Assessment
As described further in Note 1 to the consolidated financial statements, the Company reviews patents for potential impairment whenever events or changes in circumstances indicate that the carrying amount of the asset or asset group may not be recoverable. In performing the review for impairment, management makes significant estimates and assumptions related to the use of the patents and forecasts of future undiscounted cash flows. The net balance of the patents was approximately $1,100,000 as of December 31, 2020.
Given the significant assumptions made by management regarding the Company's discretionary strategic decisions to use the patent technology in the future and the uncertainty of the outcome of the use of the technology, performing audit procedures required a high degree of auditor judgment and was impacted by the nature of audit evidence regarding the matter.
Our audit procedures related to the patent impairment assessment included the following, among others:
|
|
●
|
We compared the remaining useful life of the patents to the Company's underlying register and to the remaining legal life under patent law.
|
|
|
●
|
To identify any potential unidentified impairment triggers, we made specific inquiries to management regarding the Company's plans for the use of the technology and considered if any audit evidence obtained in other areas supported or contradicted the assertions made by management.
|
|
|
●
|
We evaluated the reasonableness of significant assumptions used in management's assessment of whether impairment indicators exist.
|
Going Concern Assessment
As described further in Note 2 to the consolidated financial statements, the Company has realized net losses each year from inception through December 31, 2020, and has negative working capital as of December 31, 2020. The Company determined these, and other factors, raised substantial doubt as to the Company's ability to continue as a going concern one year from the issuance date of the consolidated financial statements. The Company believes that the capital raise after December 31, 2020, is sufficient to fund development of its planned products and pay operating expenses for at least one year following the issuance of these consolidated financial statements, which alleviates any substantial doubt about the Company's ability to continue as a going concern. In making this determination, management prepared a short-term cash flow projection. Management used significant assumptions in preparing the short-term cash flow projection, which included expected operating costs and financing obligations.
The principal considerations for our determination that the evaluation of management's going concern analysis was a critical audit matter are the significant judgment and subjectivity from management when evaluating the uncertainty related to the Company's future cash flow projection and a high degree of auditor judgment in evaluating management's forecasts for at least the next 12 months.
Our audit procedures related to the evaluation of management's forecasted expenditures and going concern analysis included the following, among others:
|
|
●
|
Obtaining evidence of the capital raised after December 31, 2020.
|
|
|
●
|
Evaluation of the reasonableness of key assumptions and estimates used by the management in the short-term cash flow projection in the light of its existing operating requirements and plans.
|
|
|
●
|
Testing the completeness, accuracy, and relevance of underlying data in the short-term cash flow projection.
|
|
|
●
|
Evaluation of the reasonableness of management's plans on the cash flow requirements of the operations.
|
|
|
●
|
Evaluation of the adequacy of the Company's disclosure of management's plans in the notes to the consolidated financial statements.
|
/s/ HOGANTAYLOR LLP
We have served as the Company's auditor since 2016.
Tulsa, Oklahoma
March 12, 2021
THE CORETEC GROUP INC.
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2020 AND 2019
|
|
|
2020
|
|
|
2019
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
22,219
|
|
|
$
|
58,149
|
|
|
Prepaid expenses
|
|
|
179,963
|
|
|
|
88,821
|
|
|
Total current assets
|
|
|
202,182
|
|
|
|
146,970
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
|
-
|
|
|
|
126
|
|
|
|
|
|
|
|
|
|
|
|
|
Other assets:
|
|
|
|
|
|
|
|
|
|
Patents, net
|
|
|
1,059,026
|
|
|
|
1,139,255
|
|
|
Goodwill
|
|
|
166,000
|
|
|
|
166,000
|
|
|
Deposits-other
|
|
|
18,946
|
|
|
|
3,575
|
|
|
Total other assets
|
|
|
1,243,972
|
|
|
|
1,308,830
|
|
|
Total Assets
|
|
$
|
1,446,154
|
|
|
$
|
1,455,926
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders' Equity
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Notes payable
|
|
$
|
46,580
|
|
|
$
|
39,138
|
|
|
Accounts payable and accrued expenses
|
|
|
396,019
|
|
|
|
615,815
|
|
|
Total current liabilities
|
|
|
442,599
|
|
|
|
654,953
|
|
|
|
|
|
|
|
|
|
|
|
|
Long term debt, net
|
|
|
266,598
|
|
|
|
120,508
|
|
|
Total Liabilities
|
|
|
709,197
|
|
|
|
775,461
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' equity:
|
|
|
|
|
|
|
|
|
|
Preferred stock, Series A convertible, $0.0002 par value, 500,000 shares authorized; 345,000 shares issued and outstanding at December 31, 2020 and 2019
|
|
|
69
|
|
|
|
69
|
|
|
Common stock $0.0002 par value, 1,500,000,000 shares authorized; 213,751,145 and 193,521,506 shares issued and outstanding at December 31, 2020 and 2019, respectively
|
|
|
42,750
|
|
|
|
38,704
|
|
|
Additional paid-in capital
|
|
|
8,033,313
|
|
|
|
6,135,885
|
|
|
Accumulated deficit
|
|
|
(7,339,175
|
)
|
|
|
(5,494,193
|
)
|
|
Total Stockholders' Equity
|
|
|
736,957
|
|
|
|
680,465
|
|
|
Total Liabilities and Stockholders' Equity
|
|
$
|
1,446,154
|
|
|
$
|
1,455,926
|
|
|
See notes to consolidated financial statements
|
THE CORETEC GROUP INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
YEARS ENDED DECEMBER 31, 2020 AND 2019
|
|
|
2020
|
|
|
2019
|
|
|
Income:
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Expenses:
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
151,864
|
|
|
|
148,875
|
|
|
General and administrative
|
|
|
1,029,136
|
|
|
|
1,418,203
|
|
|
Interest
|
|
|
665,232
|
|
|
|
287,307
|
|
|
Total expenses
|
|
|
1,846,232
|
|
|
|
1,854,385
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income
|
|
|
1,250
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(1,844,982
|
)
|
|
$
|
(1,854,385
|
)
|
|
Loss per share:
|
|
|
|
|
|
|
|
|
|
Basic and diluted
|
|
$
|
(0.009
|
)
|
|
$
|
(0.026
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding, basic and diluted
|
|
|
202,680,171
|
|
|
|
70,919,722
|
|
|
See notes to consolidated financial statements
|
THE CORETEC GROUP INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIENCY)
YEARS ENDED DECEMBER 31, 2020 and 2019
|
|
|
Series A
Preferred
Stock
|
|
|
Common Stock
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Par
|
|
|
|
|
|
|
Par
|
|
|
Paid-In
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
Shares
|
|
|
Value
|
|
|
Shares
|
|
|
Value
|
|
|
Capital
|
|
|
Deficit
|
|
|
Total
|
|
|
Balance December 31, 2018
|
|
|
345,000
|
|
|
$
|
69
|
|
|
|
68,474,520
|
|
|
$
|
13,695
|
|
|
$
|
2,166,745
|
|
|
$
|
(3,639,808
|
)
|
|
$
|
(1,459,299
|
)
|
|
Debentures converted to common stock
|
|
|
-
|
|
|
|
-
|
|
|
|
91,788,776
|
|
|
|
18,358
|
|
|
|
1,999,077
|
|
|
|
-
|
|
|
|
2,017,435
|
|
|
Common stock issued for liabilities
|
|
|
-
|
|
|
|
-
|
|
|
|
472,486
|
|
|
|
94
|
|
|
|
18,960
|
|
|
|
-
|
|
|
|
19,054
|
|
|
Common stock issued for interest and accrued interest
|
|
|
-
|
|
|
|
-
|
|
|
|
31,542,031
|
|
|
|
6,308
|
|
|
|
687,616
|
|
|
|
-
|
|
|
|
693,924
|
|
|
Common stock issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
1,243,693
|
|
|
|
249
|
|
|
|
39,557
|
|
|
|
-
|
|
|
|
39,806
|
|
|
Beneficial conversion feature of notes payable
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
281,837
|
|
|
|
-
|
|
|
|
281,837
|
|
|
Warrants issued
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
60,593
|
|
|
|
-
|
|
|
|
60,593
|
|
|
Options issued for compensation and services
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
881,500
|
|
|
|
-
|
|
|
|
881,500
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,854,385
|
)
|
|
|
(1,854,385
|
)
|
|
Balance December 31, 2019
|
|
|
345,000
|
|
|
$
|
69
|
|
|
|
193,521,506
|
|
|
$
|
38,704
|
|
|
$
|
6,135,885
|
|
|
$
|
(5,494,193
|
)
|
|
$
|
680,465
|
|
|
Warrants issued
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
135,705
|
|
|
|
-
|
|
|
|
135,705
|
|
|
Beneficial conversion feature of notes payable
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,049,826
|
|
|
|
-
|
|
|
|
1,049,826
|
|
|
Common stock issued for liabilities
|
|
|
-
|
|
|
|
-
|
|
|
|
337,353
|
|
|
|
67
|
|
|
|
11,403
|
|
|
|
-
|
|
|
|
11,470
|
|
|
Common stock issued for services
|
|
|
-
|
|
|
|
-
|
|
|
|
1,364,366
|
|
|
|
273
|
|
|
|
61,704
|
|
|
|
-
|
|
|
|
61,977
|
|
|
Options issued for compensation and services
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
92,496
|
|
|
|
-
|
|
|
|
92,496
|
|
|
Notes payable converted to common stock
|
|
|
-
|
|
|
|
-
|
|
|
|
16,727,920
|
|
|
|
3,346
|
|
|
|
546,654
|
|
|
|
-
|
|
|
|
550,000
|
|
|
Exchange of stock options for common stock
|
|
|
-
|
|
|
|
-
|
|
|
|
1,800,000
|
|
|
|
360
|
|
|
|
(360
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,844,982
|
)
|
|
|
(1,844,982
|
)
|
|
Balance December 31, 2020
|
|
|
345,000
|
|
|
$
|
69
|
|
|
|
213,751,145
|
|
|
$
|
42,750
|
|
|
$
|
8,033,313
|
|
|
$
|
(7,339,175
|
)
|
|
$
|
736,957
|
|
|
See notes to consolidated financial statements
|
THE CORETEC GROUP INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
YEARS ENDED DECEMBER 31, 2020 and 2019
|
|
|
2020
|
|
|
2019
|
|
|
Cash Flows from Operating Activities
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(1,844,982
|
)
|
|
$
|
(1,854,385
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
126
|
|
|
|
756
|
|
|
Amortization - patents
|
|
|
80,229
|
|
|
|
80,229
|
|
|
Amortization - debt discount
|
|
|
572,091
|
|
|
|
55,839
|
|
|
Options issued for services
|
|
|
92,496
|
|
|
|
765,631
|
|
|
Common stock issued for services
|
|
|
61,977
|
|
|
|
39,806
|
|
|
Common stock issued for interest
|
|
|
-
|
|
|
|
225,664
|
|
|
Change in:
|
|
|
|
|
|
|
|
|
|
Prepaid expenses
|
|
|
(91,142
|
)
|
|
|
(52,844
|
)
|
|
Deposits
|
|
|
(15,371
|
)
|
|
|
-
|
|
|
Accounts payable and accrued liabilities
|
|
|
(208,326
|
)
|
|
|
126,845
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(1,352,902
|
)
|
|
|
(612,459
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows from Financing Activities
|
|
|
|
|
|
|
|
|
|
Payments on notes payable
|
|
|
(69,709
|
)
|
|
|
(117,129
|
)
|
|
Proceeds from debt and warrants issued
|
|
|
1,386,681
|
|
|
|
806,503
|
|
|
Payments on debt issue cost
|
|
|
-
|
|
|
|
(22,767
|
)
|
|
Net cash provided by financing activities
|
|
|
1,316,972
|
|
|
|
666,607
|
|
|
|
|
|
|
|
|
|
|
|
|
Net change in cash
|
|
|
(35,930
|
)
|
|
|
54,148
|
|
|
Cash, beginning of period
|
|
|
58,149
|
|
|
|
4,001
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, end of period
|
|
$
|
22,219
|
|
|
$
|
58,149
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Disclosure of Cash flow Information
|
|
|
|
|
|
|
|
|
|
Cash paid during the period for interest
|
|
$
|
84,366
|
|
|
$
|
50,237
|
|
|
Non-Cash Financing Activities
|
|
|
|
|
|
|
|
|
|
Notes payable converted to common stock
|
|
$
|
550,000
|
|
|
$
|
2,017,434
|
|
|
Stock options exchanged for common stock
|
|
$
|
360
|
|
|
$
|
-
|
|
|
Options issued for accounts payable
|
|
$
|
-
|
|
|
$
|
115,869
|
|
|
Common stock issued to satisfy liabilities
|
|
$
|
11,470
|
|
|
$
|
487,315
|
|
|
Recognition of beneficial conversion feature
|
|
$
|
1,049,826
|
|
|
$
|
281,837
|
|
|
See notes to consolidated financial statements
|
THE CORETEC GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – Business and Summary of Significant Accounting Policies
Nature of Business
The Coretec Group Inc. (the “Group”) (formerly 3DIcon Corporation) (“3DIcon”) was incorporated on August 11, 1995, under the laws of the State of Oklahoma as First Keating Corporation. The articles of incorporation were amended August 1, 2003 to change the name to 3DIcon Corporation. During 2001, First Keating Corporation began to focus on the development of 360-degree holographic technology. From January 1, 2001, 3DIcon’s primary activity has been the raising of capital in order to pursue its goal of becoming a significant participant in the development, commercialization and marketing of next generation 3D display technologies.
Coretec Industries, LLC (“Coretec”), is a wholly owned subsidiary of the Group (collectively the “Company”). The Company is currently developing, testing, and providing new and/or improved technologies, products, and service solutions for energy-related industries including, but not limited to oil/gas, renewable energy, and distributed energy industries. Many of these technologies and products also have application for medical, electronic, photonic, display, and lighting markets among others. Early adoption of these technologies and products is anticipated in markets for energy storage (Li-ion batteries), renewable energy (BIPV), and electronics (Asset Monitoring).
Reverse Acquisition
On May 31, 2016, the Group entered into a Share Exchange Agreement (the “Share Exchange Agreement”) with Coretec and four Coretec members (the “Members”), which Members held all outstanding membership interests in Coretec. On September 30, 2016 (the “Closing Date”), the Group closed the transaction contemplated by the Share Exchange Agreement. Pursuant to the Share Exchange Agreement, the Members agreed to sell all their membership interests in Coretec to the Group in exchange for the Group’s issuance of an aggregate 4,760,872 shares of the Group’s Series B Convertible Preferred Stock to the Members (the “Exchange”). Coretec became a wholly owned subsidiary of the Group and the former Members beneficially owned approximately 65% of the Group’s common stock on a fully diluted basis on the Closing Date. Upon the closing of the Share Exchange Agreement, two of the Group’s Directors resigned and three new Directors associated with Coretec were nominated and elected, giving control of the board of directors to former Coretec Members.
Basis of Presentation
Under accounting principles generally accepted in the United States of America (“U.S. GAAP”), the acquisition is treated as a “reverse acquisition” under the purchase method of accounting. The consolidated statements of operations herein reflect the historical results of Coretec prior to the completion of the reverse acquisition since it was determined to be the accounting acquirer, and do not include the historical results of operations for 3DIcon prior to the completion of the acquisition. 3DIcon’s assets and liabilities were consolidated with the assets and liabilities of Coretec as of the September 30, 2016 consummation of the acquisition.
Principles of Consolidation
The consolidated balance sheets as of December 31, 2020 and 2019 and the consolidated statements of operations and cash flows for the years then ended include the accounts of the Group and its wholly owned subsidiary, Coretec. Intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and the disclosure of contingent assets and liabilities. Actual results could differ from the estimates and assumptions used.
Reclassification
Certain amounts in the prior period year balance sheet and statement of operations have been reclassified to conform to the presentation of the current year. These reclassifications were immaterial and had no effect on the previously reported net loss.
Property and Equipment
Property and equipment is recorded at cost. Depreciation is recorded over the estimated useful lives using the straight-line method. Maintenance and repairs are expensed as incurred; major improvements and betterments are capitalized.
Patents
The Company acquired patents valued at $1,400,000 in conjunction with the reverse acquisition discussed in Note 1. As these intangible assets have finite lives based on the patents' expiration dates, they are amortized on a straight-line basis over their useful lives.
Goodwill
Goodwill was acquired with the reverse acquisition discussed in Note 1. The Company evaluates the carrying value of goodwill on an annual basis and between annual evaluations if events occur or circumstances change that would more likely than not reduce the fair value of goodwill below its carrying amount. When assessing whether goodwill is impaired, management considers first a qualitative approach to evaluate whether it is more likely than not the fair value of the goodwill is below its carrying amount; if so, management considers a quantitative approach by analyzing changes in performance and market-based metrics as compared to those used at the time of the initial acquisition. For the periods presented, no impairment charges were recognized.
Impairment of Long-Lived Assets
Long-lived assets, such as property and equipment and patents, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If circumstances require a long-lived asset or asset group be tested for possible impairment, the Company first compares undiscounted cash flows expected to be generated by that asset or asset group to its carrying value. If the carrying value of the long-lived asset or asset group is not recoverable on an undiscounted cash flow basis, impairment is recognized to the extent that the carrying value exceeds its fair value. Fair value is determined through various valuation techniques including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary.
Fair Value of Financial Instruments
The following methods and assumptions were used to estimate the fair value of each class of financial instrument held by the Company:
Current assets and current liabilities - The carrying value approximates fair value due to the short maturity of these items.
Notes payable - The fair value of the Company's notes payable has been estimated by the Company based upon the liability's characteristics, including interest rates, embedded instruments and conversion discounts. The carrying value approximates fair value after taking into consideration the liability’s characteristics.
Basic and Diluted Loss Per Common Share
Basic loss per common share is computed by dividing net loss by the weighted average number of vested common shares outstanding during the period. Diluted earnings per share reflects the potential dilution that could occur if securities or other instruments to issue common stock were exercised or converted into common stock. The following securities are excluded from the calculation of weighted average dilutive common shares because their inclusion would have been anti-dilutive:
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Options
|
|
|
20,212,174
|
|
|
|
20,716,557
|
|
|
Warrants
|
|
|
2,190,000
|
|
|
|
570,000
|
|
|
Series A convertible preferred stock
|
|
|
115,000
|
|
|
|
115,000
|
|
|
Convertible debt
|
|
|
38,753,799
|
|
|
|
14,448,285
|
|
|
Total potentially dilutive shares
|
|
|
61,270,973
|
|
|
|
35,849,842
|
|
Research and Development
Research and development costs are expensed as incurred. Research and development costs amounted to approximately $152,000 and $149,000 for the years ended December 31, 2020 and 2019, respectively.
Income Taxes
The Company accounts for income taxes under an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the Company’s consolidated financial statements or tax returns. In estimating future tax consequences, the Company generally considers all expected future events other than enactments of changes in tax laws or rates. The effect on deferred tax assets and liabilities of a change in tax rates will be recognized as income or expense in the period that includes the enactment date. A valuation allowance is established when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company’s tax benefits are fully offset by a valuation allowance due to the uncertainty that the deferred tax assets would be realized. Management considers the likelihood of changes by taxing authorities in its filed income tax returns and recognizes a liability for or discloses potential changes that management believes are more likely than not to occur upon examination by tax authorities. Management has not identified any uncertain tax positions in filed income tax returns that require recognition or disclosure in the accompanying consolidated financial statements.
Recent Accounting Pronouncements
The following is a summary of recent accounting pronouncements that are relevant to the Company:
In January 2017, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2017-04, Intangibles – Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. This ASU simplifies the subsequent measurement of goodwill by eliminating Step 2 from the goodwill test. Under Step 2, an entity had to perform procedures to determine the fair value at the impairment testing date of its assets and liabilities following the procedure that would be required in determining the fair value of assets acquired and liabilities assumed in a business combination. Instead, an entity should measure goodwill impairment and test by comparing the fair value of a reporting unit with its carrying amount. The Company adopted this standard effective January 1, 2020 and will apply the standard on a prospective basis. The adoption of this standard did not have a material impact on its consolidated financial position and results of operations.
In August 2020, the FASB issued ASU 2020-06, Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40). This ASU simplifies the accounting for convertible instruments. The guidance removes certain accounting models which separate the embedded conversion features from the host contract for convertible instruments. As a result, after adopting ASU 2020-06, the Company will no longer separately present the embedded conversion feature of its convertible debt within stockholders’ equity and interest expense is expected to decrease due to the elimination of the related debt discount amortization. ASU 2020-06 is effective for the Company in the first quarter of 2024, with early adoption permitted in the first quarter of 2021 and may be adopted using either a full or modified retrospective approach. The Company intends to adopt ASU 2020-06 under the modified retrospective approach for the 2021 fiscal year effective January 1, 2021. Adoption is expected to result in an approximate $989,000 decrease in additional paid in capital from the derecognition of the beneficial conversion feature, $863,000 increase in long term debt from the derecognition of the discount associated with the beneficial conversion feature and $126,000 decrease to the opening balance of accumulated deficit, representing the cumulative interest expense recognized related to the amortization of the beneficial conversion feature. The adoption and financial adjustments will be included in the Company’s next reporting period.
Note 2 – Recent Capital Financing and Management’s Plans
The Company has realized a cumulative net loss of $7,339,175 for the period from inception (June 2, 2015) to December 31, 2020, negative working capital of $240,417 and no revenues. As of December 31, 2020, these conditions raised substantial doubt about the Company’s ability to continue as a going concern for a year following the balance sheet date of these consolidated financial statements. As of December 31, 2020, the Company had insufficient revenue and capital commitments to fund the development of its planned products and to pay operating expenses. The ability of the Company to continue as a going concern depended on the Company’s capital raising efforts to fund the development of its planned products.
As a result of the securities purchase agreement and capital raise of $6,000,000 subsequent event to December 31, 2020 (see Note 9), management believes that the Company has sufficient capital commitments to fund the development of its planned products and to pay operating expenses for a period of more than one year following the issuance of these consolidated financial statements. Consequently, the substantial doubt about the Company's ability to continue as a going concern has been alleviated. Management is committed to utilizing this capital to expand and accelerate the development of its CHS technology, while scaling business functions and appropriately adding resources necessary for future growth.
Note 3 – Property and Equipment
Property and equipment consist of the following:
|
|
|
December 31,
2020
|
|
|
December 31,
2019
|
|
|
Furniture and Fixtures
|
|
$
|
-
|
|
|
$
|
13,286
|
|
|
Less: Accumulated Depreciation
|
|
|
-
|
|
|
|
13,160
|
|
|
Totals
|
|
$
|
-
|
|
|
$
|
126
|
|
Depreciation expense amounted to $126 and $756 for the years ended December 31, 2020 and 2019, respectively. The Company moved headquarters from Tulsa, OK to Ann Arbor, MI during June 2020. At that time, the Company sold all furniture and reported a gain on sale of assets of $1,250.
Note 4 – Patents
The following table sets forth patents:
|
Patents
|
|
December 31,
2020
|
|
|
December 31,
2019
|
|
|
Gross Carrying Amount
|
|
$
|
1,400,000
|
|
|
$
|
1,400,000
|
|
|
Accumulated Amortization
|
|
|
(340,974
|
)
|
|
|
(260,745
|
)
|
|
Net Book Value
|
|
$
|
1,059,026
|
|
|
$
|
1,139,255
|
|
The patents were acquired with the September 30, 2016 reverse acquisition. Amortization expense for the next five fiscal years and thereafter is expected to be approximately $80,000 annually through the year ended December 31, 2034.
Note 5 – Debt
Notes payable and long-term debt consists of the following:
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2020
|
|
|
2019
|
|
|
Notes payable:
|
|
|
|
|
|
|
|
|
|
6.3% Insurance premium finance agreement due July 2020
|
|
$
|
-
|
|
|
$
|
39,138
|
|
|
3.8% Insurance premium finance agreement due June 2021
|
|
|
46,580
|
|
|
|
-
|
|
|
Total notes payable
|
|
$
|
46,580
|
|
|
$
|
39,138
|
|
|
|
|
|
|
|
|
|
|
|
|
Long term debt:
|
|
|
|
|
|
|
|
|
|
10% Promissory note due January 2024
|
|
|
1,275,000
|
|
|
|
475,000
|
|
|
Less:
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature
|
|
|
(862,775
|
)
|
|
|
(273,422
|
)
|
|
Warrants issued
|
|
|
(106,167
|
)
|
|
|
(59,108
|
)
|
|
Debt issue costs
|
|
|
(39,460
|
)
|
|
|
(21,962
|
)
|
|
Net long term debt
|
|
$
|
266,598
|
|
|
$
|
120,508
|
|
3.8% Insurance premium finance agreement, due June 2021
The Company entered into an insurance financing agreement in August 2020 totaling $77,151. The monthly payments under the agreement are due in ten installments of $7,849. The Company made the first installment payment in September 2020.
6.3% Insurance premium finance agreement due July 2020
The Company entered into an insurance financing agreement in September 2019 totaling $61,503. The agreement was due in eleven installments of $5,591 through July 2020. The Company paid the balance due of $39,138 during the year ended December 31, 2020.
4.75% Convertible debenture due September 2019
On November 3, 2006, the Company issued to Golden State a 4.75% convertible debenture in a principal amount of $100,000, due December 31, 2014, subsequently extended to December 31, 2018 and most recently extended to September 30, 2019 and warrants to buy 61 post-split equivalent shares of common stock at a post-split exercise price of $114,450 per share. On January 8, 2018, Golden State converted $225 of the 4.75% convertible debenture into 244,618 shares of common stock at $0.0009 per share and exercised 0.2143 warrants at $114,450 per share for $24,525. On May 24, 2018, Golden State converted $225 of the 4.75% convertible debenture into 396,635 shares of common stock at $0.0006 per share and exercised 0.2143 warrants at $114,450 per share and advanced $23,766 cash for the exercise. On October 15,2019, the Company paid the balance due on the debenture of $63,675 along with the accrued interest due of $26,065.
10% Promissory note due January 2024, net
On October 4, 2019, the Company entered into a Credit Agreement and related Promissory Note with Diversified Alpha Fund of Navigator Global Fund Manager Platform SPC (DAF), the Lender. DAF is a segregated portfolio fund of Navigator Global Fund Manager Platform SPC. DAF is managed and controlled by Mollitium Investment Management (Mollitium). Mollitium utilizes Diversified Global Investment Advisors Ltd. (DGIA) to act in an advisory role. DGIA maintains an Investment Committee to support the services to Mollitium. Simon Calton serves as part of this five-member investment committee and in accordance with the investment committee’s guidelines, Mr. Calton does not participate in matters or voting that pertain to the Company due to his conflict of interest. Investment advice provided by DGIA to Mollitium are recommendations only and the final decision on actions are the responsibility of Mollitium. Carlton James Global Management, Ltd (CJGM) serves as a distributer of investments by introducing funds available to the market of which DAF is included in CJGM’s group of funds. Compensation to CJGM occurs when investments are made into funds that they introduce. CJGM is part of the Carlton James Group of which Mr. Calton is CEO.
The 10% Promissory Note, in a principal amount of $2,500,000, is due February 15, 2024 and has attached warrants to subscribe for and purchase 3,000,000 shares of common stock at an exercise price of $0.052 per share. Under the terms of the Credit Agreement, DAF will fund the Promissory Note in sixteen (16) tranches in amounts of $125,000 and $175,000 per month beginning in October 2019. The funding of the Promissory Note is at the discretion of DAF and may differ from the planned schedule. As of December 31, 2020, DAF has advanced $1,825,000 with the remaining $675,000 to be funded in 2021. Interest is accrued monthly and paid in advance for the first 12 months and thereafter principal and interest payments shall be paid monthly in equal amounts, amortized over a 36-month period.
Under the terms of the Credit Agreement, DAF has the right to elect to convert all or part of the Promissory Note at a price equal to seventy percent (70%) of the average closing price of the Company’s common stock as reported on the over-the-counter quotation system on the OTC Markets during the fifteen (15) calendar days prior to the loan closing date of October 4, 2019, which calculates to $0.0329 per share.
The embedded conversion option was deemed to be a beneficial conversion feature because the active conversion price was less than the commitment date market price of the common stock. Given the terms and related-party nature of the agreement, the commitment date was determined to be the date the funds are advanced to the Company and is limited to the funding value less other debt discounts (see below). A debt discount of $1,049,825 and $281,837 was recorded, with a corresponding credit to additional paid-in capital, for the beneficial conversion feature for the years ended December 31, 2020 and 2019, respectively. The debt discount is amortized over the life of the debt relative to unconverted debt. A debt discount of $175,506 and $8,415 was amortized to interest expense during the years ended December 31, 2020 and 2019.
Under the terms of the Credit Agreement, warrants to subscribe for and purchase 3,000,000 shares of common stock at an exercise price of $0.052 per share were issued to DAF. The warrants will be issued in amounts of 150,000 and 210,000 per month as the advance is received during the funding period. In the event that funding advances deviate from the planned schedule then warrants will be issued pro-rata at 1.2 warrants for every $1 of funding. Warrants granted under the terms of the DAF Credit Agreement as of December 31, 2020 and 2019 were 2,190,000 and 570,000, respectively. The estimated value of the warrants granted monthly, with each advance, is calculated using the Black-Scholes option pricing model. The resulting estimated value of the warrant is used to proportionally allocate the fair value of the debt advance and the fair value of the warrants. The allocated cost of the warrants amounted to $135,706 and $60,593 for the years ended December 31, 2020 and 2019, respectively, and is being amortized over the life of the debt with $28,216 and $1,485 of allocated costs amortized during the years ended December 31, 2020 and 2019, respectively.
Additionally, under the terms of the Credit Agreement, the Company agreed to pay a commitment fee of 3% of each advance and reimburse DAF for certain expenses in connection with the preparation, interpretation, performance and enforcement of the Credit Agreement. Those costs amounted to $42,000 and $22,767 during the years ended December 31, 2020 and 2019, respectively, and are being amortized over the life of the debt with $9,034 and $805 amortized during the years ended December 31, 2020 and 2019, respectively.
On March 31, 2020, under the terms of the Credit Agreement, DAF converted $300,000 of the principle of the Promissory Note into 9,129,136 shares of common stock at $0.0329 per share. A related charge of $130,370 of the beneficial conversion feature was made to interest expense along with debt issue related charges of $25,523 for the warrants and $8,123 for the deferred cost at the time of the conversion.
On October 30, 2020, under the terms of the Credit Agreement, DAF converted $250,000 of the principle of the Promissory Note into 7,598,784 shares of common stock at $0.0329 per share. A related charge of $156,265 of the beneficial conversion feature was made to interest expense along with debt issue related charges of $34,912 for the warrants and $5,796 for the deferred cost at the time of the conversion.
Conversion of related party loans and convertible debentures to common stock
On December 27, 2019, the Company issued 123,330,807 shares of Common Stock of the Company upon the conversion of debt held by certain Legacy Holders, which consists substantially of the Company’s Co-Chairmen, Victor Keen and Simon Calton. The total outstanding Legacy Debt converted was $2,711,359, which consisted of $2,017,435 in outstanding principal and $693,924 in accrued interest. The Legacy Debt was converted at conversion prices of $0.022 per share.
14% Term loan due December 2019, related party
On April 18, 2016, the Company entered into an unsecured loan agreement whereby Carlton James Ltd ("CJL”), a company owned by Mr. Simon Calton, a director of the Company, agreed to provide the Company a loan facility of up to $100,000. Under the terms of the agreement, the Company accrued interest on the outstanding unpaid balance at the rate of 1.167% per month. The interest was due quarterly, and the principal was due September 30, 2019 and subsequently extended to December 31, 2019. CJL had advanced $374,993 ($274,993 in excess of the facility) on the loan as of September 30, 2019. During 2017, CJL agreed that the excess amount funded and any future funding under the loan would be done on the same terms and conditions as the original note. The loan and accrued interest were a part of the Legacy Debt and effective November 30, 2019, the loan and accrued interest were retired December 27, 2019.
14% Term loan due December 2019, related party
On February 24, 2016, the Company entered into an unsecured loan agreement whereby Victor Keen, Co-Chairman of the Company (“Keen”) agreed to provide the Company a loan facility of up to $300,000. Under the terms of the agreement, the Company accrued interest on the outstanding unpaid balance at the rate of 1.167% per month. The interest was due quarterly, and the principal was due September 30, 2019 and subsequently extended to December 31, 2019. Keen had advanced $756,500 ($456,500 in excess of the facility) on the loan through November 30, 2019. During 2017, Keen agreed that the excess amount funded and any future funding under the loan will be done on the same terms and conditions as the original note. The loan and accrued interest were a part of the Legacy Debt and effective November 30, 2019, the loan and accrued interest were retired on December 27, 2019.
14% Term loan due December 2019, related party
On June 1, 2015, Coretec obtained a $500,000 revolving note agreement with CJL. Coretec accrued the interest on the outstanding balance at the rate of 1.167% per month. CJL had advanced $535,941 on the loan ($35,941 in excess of the facility) through November 30, 2019. During 2019, CJL agreed that the excess amount funded and any future funding under the loan would be done on the same terms and conditions as the original note. Outstanding borrowings were secured by substantially all assets of the Company. The note was due on September 30, 2019 and subsequently extended to December 31, 2019. The loan and accrued interest were a part of the Legacy Debt and effective November 30, 2019, the note and accrued interest were retired on December 27, 2019.
7% Convertible promissory note due December 2019, related party
On March 30, 2017, the Company issued to Mr. Victor Keen, Co-Chairman of the Board of Directors, a 7% convertible promissory note in a principal amount of $250,000, due March 1, 2019, subsequently extended to December 31, 2019. The promissory note automatically converted into eight percent (8%) of the fully diluted outstanding shares of common stock of the Company. The embedded conversion option was deemed to be a beneficial conversion feature because the active conversion price was less than the commitment date market price of the common stock. The dollar amount of the beneficial conversion feature was limited to the carrying value of the promissory note, so a $250,000 debt discount was recorded, with a corresponding credit to additional paid-in capital for the beneficial conversion feature. The debt discount was amortized over the original life of the debt and $21,373 was amortized during the year ended December 31, 2019. The note and accrued interest were a part of the Legacy Debt retired on December 27, 2019.
7% Convertible promissory note due December 2019, related party
On June 21, 2017, the Company issued to Mr. Victor Keen, Co-Chairman of the Board of Directors, a 7% convertible promissory note in a principal amount of $100,000, due June 21, 2019 and subsequently extended to December 31, 2019. The promissory note automatically converted into four percent (4%) of the fully diluted outstanding shares of common stock of the Company. The embedded conversion option was deemed to be a beneficial conversion feature because the active conversion price was less than the commitment date market price of the common stock. The dollar amount of the beneficial conversion feature is limited to the carrying value of the promissory note, so a $100,000 debt discount was recorded, with a corresponding credit to additional paid-in capital for the beneficial conversion feature. The debt discount was amortized over the original life of the debt and $23,761was amortized during the year ended December 31, 2019. The note and accrued interest were a part of the Legacy Debt retired December 27, 2019.
Note 6 – Common Stock, Preferred Stock, Warrants and Options
Common Stock
On December 27, 2019, the Company issued 123,330,807 shares of Common Stock of the Company upon the conversion of debt held by certain Legacy Holders (see Note 5).
On June 8, 2020, the Board of Directors consented to a share exchange agreement with holders of 21,500,000 options awarded on August 7, 2019. The agreement allows for holders to exchange their options for rule 144 common stock at an exchange rate of 0.6 shares per 1 option. Since the execution of the option exchange agreement 3,000,000 options have been exchanged for 1,800,000 shares of common stock.
On October 22, 2020, the Board of Directors consented to satisfying accrued liabilities of vendors by issuing S8 common stock from the 2018 Equity Incentive Plan from August 26, 2020 through September 1, 2021. The number of shares issued to satisfy a liability was determined by the average closing price for the fifteen (15) days prior to conversion at a discount rate of 50% to that fifteen (15) day average. The stock issuance, in lieu of cash payment, requires written approval of the Chief Executive Officer. During the year ended December 31, 2020, 1,701,719 shares were issued to satisfy $73,107 of vendor accrued liabilities and services.
Series A Convertible Preferred Stock
A total of 500,000 shares of Series A Convertible Preferred Stock (the “Series A Preferred Stock”) have been authorized for issuance under the Certificate of Designation of Preferences, Rights and Limitation of Series A Convertible Preferred Stock of the Company (the “Certificate of Designation”), which Certificate of Designation was filed with the Secretary of State of the State of Oklahoma on December 11, 2013. The shares of Series A Preferred Stock have a par value of $0.0002 per share and a stated value of $1.00 per share (the “Stated Value”) and shall receive a dividend of 6% of their Stated Value per annum payable or upon conversion or redemption of Series A Preferred at the option of the Company We have not paid any cash or stock dividends to the holders of our Series A Preferred Stock. Dividends in arrears totaled approximately $148,000 and $128,000 for the years ended December 31, 2020 and 2019, respectively. Under the Certificate of Designation, the holders of the Series A Preferred Stock have the following rights, preferences and privileges:
The Series A Preferred Stock may, at the option of the Investor, be converted at any time after the first anniversary of the issuance of the Series A Preferred Stock or from time to time thereafter into 166,667 post-split shares of Common Stock that such investor is entitled to in proportion to the 500,000 shares of Series A Preferred so designated in the Certificate of Designation.
The Series A Preferred Stock will automatically be converted into Common Stock anytime the post-split 5-day Volume-Weighted Average Price (VWAP) of the Company’s Common Stock prior to such conversion is equal to $15.00 or more. Such mandatory conversion would be converted by the same method described above for discretionary conversions.
Except as otherwise required by law, the holders of shares of Series A Preferred Stock shall not have voting rights or powers.
In the event of any (i) liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, or ii) sale, merger, consolidation, reorganization or other transaction that results in a change of control of the Company, each holder of a share of Series A Preferred shall be entitled to receive, subject to prior preferences and other rights of any class or series of stock of the Company senior to the Series A Preferred, but prior and in preference to any distribution of any of the assets or surplus funds of the Company to holders of Common Stock, or any other class or series of stock of the Company junior to the Series A Preferred, an amount equal to the Stated Value plus accrued and unpaid dividends (as adjusted for any stock dividends, combinations or splits with respect to such shares) (the “Preference Amount”). After such payment has been made to the holders of Series A Preferred of the full Preference Amount to which such holders shall be entitled, the remaining net assets of the Company available for distribution, if any, shall be distributed pro rata among the holders of Common Stock. In the event the funds or assets legally available for distribution to the holders of Series A Preferred are insufficient to pay the Preference Amount, then all funds or assets available for distribution to the holders of capital stock shall be paid to the holders of Series A Preferred pro rata based on the full Preference Amount to which they are entitled.
The Company may not declare, pay or set aside any dividends on shares of any class or series of capital stock of the Company (other than dividends on shares of Common Stock payable in shares of Common Stock) unless the holders of the Series A Preferred Stock shall first receive, or simultaneously receive, a dividend on each outstanding share of Series A Preferred in an amount equal to the dividend per share that such holders would have received had they converted their shares of Series A Preferred into shares of Common Stock immediately prior to the record date for the declaration of the Common Stock dividend in an amount equal to the average VWAP during the 5 trading days prior to the date such dividend is due.
Warrants
Warrants to subscribe for and purchase up to 3,000,000 shares of common stock at an exercise price of $0.052 per share were included under the terms of the DAF Credit Agreement. The warrants will be issued in amounts of 150,000 and 210,000 per month during the funding period. In the event that funding advances deviate from the planned schedule then warrants will be issued pro-rata at 1.2 warrants for every $1 of funding. Warrants granted under the terms of the DAF Credit Agreement as of December 31, 2020 and 2019 total 2,190,000 and 570,000, respectively. The estimated value of the warrants granted monthly, with each advance, is calculated using the Black-Scholes option pricing model. The expected dividend yield is based on the average annual dividend yield as of the grant date. Expected volatility is based on the historical volatility of our stock. The risk-free interest rate is based on the U.S. Treasury Constant Maturity rates as of the grant date. The expected life of the warrant is based on historical exercise behavior and expected future experience. The resulting estimated value of the warrant is used to proportionally allocate the fair value of the debt advance and the fair value of the warrants.
As of December 31, 2018, Golden State had warrants outstanding to purchase 61 shares of common stock at a price of $114,450 per share which expired December 31, 2018, subsequently extended to September 30, 2019 and cancelled in October 2019 with the retirement of the Golden State convertible debenture. Global Capital had warrants outstanding to purchase 1,000 shares of common stock at a price of $0.96 per shares which expired on March 31, 2019.
Warrants Summary
The following table summarizes the Company’s warrant activity during the year ended December 31, 2020:
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Remaining
|
|
|
Aggregate
|
|
|
|
|
Number of
|
|
|
Exercise
|
|
|
Life
|
|
|
Intrinsic
|
|
|
|
|
Warrants
|
|
|
Price
|
|
|
In Years
|
|
|
Value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2019
|
|
|
570,000
|
|
|
$
|
0.052
|
|
|
|
|
|
|
$
|
-
|
|
|
Granted
|
|
|
1,620,000
|
|
|
|
0.052
|
|
|
|
|
|
|
|
-
|
|
|
Outstanding, December 31, 2020
|
|
|
2,190,000
|
|
|
|
0.052
|
|
|
|
4.30
|
|
|
|
-
|
|
Options
Stock options for employees, directors or consultants, are valued at the date of award, which does not precede the approval date, and compensation cost is recognized in the period the options are vested. The Company recognizes compensation expense for awards subject to graded vesting on a straight-line basis. Stock options generally become exercisable on the date of grant and expire based on the terms of each grant.
The estimated fair value of options for common stock granted was determined using the Black-Scholes option pricing model. The expected dividend yield is based on the average annual dividend yield as of the grant date. Expected volatility is based on the historical volatility of our stock. The risk-free interest rate is based on the U.S. Treasury Constant Maturity rates as of the grant date. The expected life of the option is based on historical exercise behavior and expected future experience.
On August 7, 2019 the Company issued 21,500,000 five (5) year options to purchase common stock of the Company at an exercise price of $0.041 per share. The estimated fair value of the options was $881,500. Michael Kraft, CEO was issued 10,000,000 options, Concordia Financial Group was issued 10,000,000 options, Ramez Elgammal, CTO was issued 1,000,000 options and Ronald Robinson, former CFO, was issued 500,000 options. Mr. Kraft’s options were issued for $90,869 in accrued compensation due him, $25,000 under the terms of his employment agreement and $294,132 as additional compensation for his services as CEO. Concordia’s, Mr. Elgammal’s and Mr. Robinson’s options were issued for additional compensation for services during the year ended December 31, 2019. The $881,500 estimated fair value of options to purchase common stock issued in August 2019 was determined using the Black-Scholes option pricing model. The expected dividend yield of $0 is based on the average annual dividend yield at the date issued. Expected volatility of 337.19% is based on the historical volatility of the stock. The risk-free interest rate of 1.52% is based on the U.S. Treasury Constant Maturity rates as of the issue date. The expected life of the options of five years is based on historical exercise behavior and expected future experience.
On June 8, 2020, the Board of Directors consented to a share exchange agreement with holders of 21,500,000 options awarded on August 7, 2019. The agreement allows for holders to exchange their options for rule 144 common stock at an exchange rate of 0.6 shares per 1 option. The modification of these options did not result in any additional compensation because there was no change in the fair value. As of December 31, 2020, 3,000,000 options have been exchanged for 1,800,000 shares that were issued under the executed exchange agreement.
The Company granted 1,500,000 options during the year ended December 31, 2020 at an average grant date fair value of $0.057 determined using the Black-Scholes option pricing model, with 500,000 options vesting immediately and 1,000,000 options vesting over a two-year time frame in four equal six-month periods. The Company recognized $92,496 of stock option expense related to the options during the year ended December 31, 2020. The remaining expense of $112,554 at December 31, 2020, will be recognized on a straight-line basis over the remaining vesting period of 18 months.
Options Summary
The following table summarizes the Company’s option activity during the year ended December 31, 2020:
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Remaining
|
|
|
Aggregate
|
|
|
|
|
Number of
|
|
|
Exercise
|
|
|
Life
|
|
|
Intrinsic
|
|
|
|
|
Options
|
|
|
Price
|
|
|
In Years
|
|
|
Value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2019
|
|
|
21,716,557
|
|
|
$
|
0.076
|
|
|
|
|
|
|
$
|
-
|
|
|
Expired
|
|
|
(4,383
|
)
|
|
|
52.50
|
|
|
|
|
|
|
|
-
|
|
|
Exchanged for common stock
|
|
|
(3,000,000
|
)
|
|
|
0.041
|
|
|
|
|
|
|
|
-
|
|
|
Granted
|
|
|
1,500,000
|
|
|
|
0.057
|
|
|
|
|
|
|
|
-
|
|
|
Outstanding, December 31, 2020
|
|
|
20,212,174
|
|
|
|
0.068
|
|
|
|
3.69
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, December 31, 2020
|
|
|
19,462,174
|
|
|
$
|
0.068
|
|
|
|
3.66
|
|
|
$
|
-
|
|
The following table summarizes the Company’s options as of December 31, 2020:
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
Outstanding
|
|
|
Remaining
|
|
|
Exercisable
|
|
|
Exercise
|
|
|
Number of
|
|
|
Life
|
|
|
Number of
|
|
|
Price
|
|
|
Options
|
|
|
In Years
|
|
|
Options
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
0.041
|
|
|
|
19,000,000
|
|
|
|
3.62
|
|
|
|
19,000,000
|
|
|
$
|
0.065
|
|
|
|
1,000,000
|
|
|
|
4.50
|
|
|
|
250,000
|
|
|
$
|
0.240
|
|
|
|
208,160
|
|
|
|
6.21
|
|
|
|
208,160
|
|
|
$
|
70.260
|
|
|
|
3,449
|
|
|
|
1.50
|
|
|
|
3,449
|
|
|
$
|
420.000
|
|
|
|
565
|
|
|
|
0.37
|
|
|
|
565
|
|
|
Total
|
|
|
|
20,212,174
|
|
|
|
3.69
|
|
|
|
19,462,174
|
|
Incentive Stock Plan
In January 2018, the Company established its 2018 Equity Incentive Plan (the “2018 EIP”). The total number of shares of stock which may be purchased or granted directly by options, stock awards or restricted stock purchase offers, or purchased indirectly through exercise of options granted under the 2018 EIP shall not exceed fifteen million (15,000,000) shares. The shares are included in a registration statement filed January 2018. There are 10,534,263 shares available for issuance under the 2018 EIP as of December 31, 2020.
Note 7 – Commitments
Consulting Agreements
The Company entered into a one-year consulting agreement with Michelle Tokarz (“Tokarz”) effective February 10, 2020 and expiring February 9, 2021. Under the terms of the agreement, Tokarz will have the position of Business Development Consultant. Tokarz will be paid an hourly fee of $115 with a maximum of $1,000 per day and shall make up to ten days available to the Company each month. The Company recognized expenses of approximately $52,000 during the year ended December 31, 2020.
North Dakota State University Sponsored Research Agreement
The Company entered into a Sponsored Research Agreement (“SRA”) dated August 14, 2015 with North Dakota State University Research Foundation (“NDSU/RF”). With the proposed research for this project, NDSU/RF planned to make prototypical compounds and materials from CHS and CHS derivatives with the potential; 1) to act as efficient photoactive materials for solar cells, 2) to serve in electro active devices for optimization of current and voltage performance, 3) to perform at high levels of efficiency as silicon anodes in lightweight batteries (silicon has more than 11 times the capacity of carbon in the ubiquitous carbon based batteries), and, 4) to be incorporated into specialty inks for printed electronics applications. The research was conducted August 14, 2015 through August 31, 2016. The Company agreed to reimburse NDSU/RF for all costs incurred in performing the research up to a maximum amount of $70,000. On June 7, 2016 the Company and NDSU/RF mutually agreed to amend the SRA. Under the terms of the amendment the term was extended to June 30, 2017 and the consideration was increased by $120,000 to a maximum amount of $190,000.
As of December 31, 2020, the remaining balance of the SRA to be paid under the terms of the agreement is $93,578. As of December 31, 2020, and pursuant to the SRA, Coretec was in arrears on the payment of that obligation. Accordingly, as of December 31, 2020, Coretec would be considered in default under the SRA because of the unpaid obligations, which could allow NDSU/RF to exercise various options under the SRA, including an option to terminate the SRA if Coretec does not cure the default within 10 business days after receiving written notice by NDSU/RF. Due to Coretec’s belief that certain obligations of NDSU/RF were unsatisfied, Coretec has actively communicated with NDSU/RF in order to determine what obligations are owed and what actions all parties are required to take, and will agree to take, in furtherance of the SRA. In connection with such objective, Coretec has sent NDSU/RF a detailed communication setting forth, among other things, the basis for its belief that (i) the payment obligation was not due to NDSU/RF; and (ii) NDSU/RF does not have the right to enforce a default. Coretec did not attempt communication or receive communication from NDSU/RF during 2020.
As of the date of this report, there have been no legal proceedings initiated in connection with the SRA. However, no assurances can be made that the active communications between the parties will result in a resolution or that legal proceedings will not be initiated in the future.
Office Lease
On June 30, 2020 the Company moved headquarters from Tulsa, Oklahoma to Ann Arbor, Michigan at which time the Company terminated the lease agreement in Tulsa. The Company continued to occupy the office space in Ann Arbor under the lease agreement that was executed on December 3, 2019. The Company signed a one-year lease in Ann Arbor, Michigan commencing January 1, 2020 with an annual rent obligation of $15,120 ($1,260 per month). Rent expense for the office operating leases was $25,592 and $23,760 and for the years ended December 31, 2020 and 2019, respectively. The Company has renewed the Ann Arbor lease for 2021 under the same terms.
Supply Agreement
During June 2020, the Company entered into a supply agreement with Evonik Operations GmbH to purchase 500 grams of cyclohexasilane, Si6H12 (CHS) for $185,000. The supply agreement is valid until March 31, 2021. The Company paid Evonik Operations GmbH $92,500 on July 20, 2020, to initiate production of CHS, in accordance with the agreement. Delivery is expected during the months of March and April of 2021, at which time the Company will owe the remaining $92,500.
Note 8 – Related Party Transactions
The Company entered into a consulting agreement dated March 20, 2017 with Mr. Michael A. Kraft, who became the Company’s CEO. Under the terms of the agreement the Company agreed to compensate Mr. Kraft, $1,500 per day for his commitment to allocate seven days a month (subsequently amended to ten day a month) to the Company and a $25,000 bonus payable in the Company’s restricted stock upon occurrence of certain events. Mr. Kraft was issued ten million options during August 2019 for (1) as compensation for the $25,000 bonus in the consulting agreement, (2) approximately $91,000 as payment for unpaid consulting fees and, (3) approximately $294,000 as additional compensation for his consulting services. During the years ended December 31, 2020 and 2019, the Company recognized $180,000 and $144,000 of expense respectively, under the terms of the agreement. Mr. Kraft was owed $51,720 and $95,966 in unpaid consulting fees and out of pocket expenses, which is included in accounts payable and accrued expenses the years ended December 31, 2020 and 2019 respectively.
At December 31, 2018 the Company had an aggregate balance of $971,500 of advances due to Mr. Victor Keen, Co-Chairman of the Board of Directors. During the year ended December 31, 2019 Mr. Keen advanced the Company an additional $135,000, such that as of November 30, 2019, an aggregate amount of $1,106,500 was due to Mr. Keen under the terms of certain promissory notes and convertible debentures (“the Notes”) which were included in notes payable – related party (see Note 5). The Notes along with accrued interest of $342,292, were converted to common stock on December 27, 2019. Interest expense related to the Notes was $112,969 for the year ended December 31, 2019.
At December 31, 2018 the Company had an aggregate balance of $775,934 of advances due to CJL, a company owned by Mr. Simon Calton, a director of the Company, During the years ended December 31, 2019, CJL, advanced an additional $135,000 such that as of November 30, 2019, an aggregate amount of $910,934 was due to CJL under the terms of two loans (“Loans”), which were included in notes payable-related parties (see Note 5). The Loans along with accrued interest of $351,633 were converted to common stock on December 27, 2019. Interest expense related to the Loans was $112,695 for the year ended December 31, 2019.
The Company entered into a one-year consulting agreement with Matthew Hoffman (“Hoffman”), doing business as, Integrate Growth, LLC, effective May 21, 2020 and expiring May 19, 2021. Under the terms of the agreement, Hoffman held the position of Director of Finance. On June 30, 2020 Ron Robinson, Chief Financial Officer and Judith Keating, Corporate Secretary both retired from the Company. As part of the management transition plan, Hoffman was elevated to Chief Financial Officer and Corporate Secretary on June 30, 2020. Under the terms of the agreement, Hoffman will be paid a monthly fee of $6,000 and shall make up to twenty hours per week available to the Company for each week of each month. The Company recognized $42,000 of consultant expense to Hoffman for the year ended December 31, 2020.
Note 9 – Subsequent Events
In January 2021, the Company agreed to settle accrued liabilities in the amount of $41,800 due to multiple vendors for 1,115,961 common shares. This transaction was pursuant to the October 22, 2020 Board of Directors consent to issue S8 common stock from the 2018 Equity Incentive Plan. The number of shares issued to satisfy the liabilities was determined by the average closing price for the fifteen (15) days prior to conversion at a discount rate of 50% to that fifteen (15) day average.
In January 2021, Ken Evans exchanged 1,500,000 options for 900,000 shares of rule 144 common stock. This transaction was pursuant to the June 8, 2020 consent by the Board of Directors for a share exchange agreement with holders of 21,500,000 options awarded on August 7, 2019. The agreement allows for holders to exchange their options for rule 144 common stock at an exchange rate of 0.6 shares per 1 option.
In January 2021, the Company entered into a one-year consulting agreement with Allison Gabrys (“Gabrys”), doing business as, Mears Advisory, LLC, effective February 8, 2021 and expiring February 8, 2022. Under the terms of the agreement, Gabrys will perform services as Chief Marketing Officer Consultant. Gabrys will make 20 hours per week available to the Company with an hourly bill rate of $125.
In February 2021, the Company received a gross advance of $225,000 as part of the credit agreement with DAF, bringing the total advances to $2,170,000. The Company and DAF investment manager also agreed to a revised funding schedule of $110,000 tranches to be received in March, April and May of 2021 to complete the $2,500,000 credit agreement.
On March 2, 2021, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with a single institutional investor in a private placement to sell (i) 23,500,000 shares of its common stock, (ii) pre-funded warrants to purchase up to an aggregate of 51,500,000 shares of its common stock, and (iii) warrants to purchase up to an aggregate of 82,500,000 shares of its common stock for gross proceeds of approximately $6,000,000. The combined purchase price for one share of common stock and associated Warrant is $0.08 and for one Pre-Funded Warrant and associated Warrant is $0.0799. The sale of the securities under the Purchase Agreement closed on March 5, 2021.
The Warrants are exercisable for a period of five and one half years from the date of issuance and have an exercise price of $0.08 per share, subject to adjustment as set forth in the warrants for stock splits, stock dividends, recapitalizations and similar events. The Investor may exercise the warrant on a cashless basis if the shares of common stock underlying the warrant are not then registered pursuant to an effective registration statement. The investor has contractually agreed to restrict its ability to exercise the warrant such that the number of shares of the Company’s common stock held by the investor and its affiliates after such exercise does not exceed the beneficial ownership limitation set forth in the warrant which may not exceed initially 4.99% of the Company’s then issued and outstanding shares of common stock.
The pre-funded warrants have an exercise price of $0.0001 per share, subject to adjustment as set forth in the pre-funded warrants for stock splits, stock dividends, recapitalizations and similar events. The pre-funded warrants will be exercisable immediately and may be exercised at any time until all of the pre-funded warrants are exercised in full.
Pursuant to an engagement letter, dated as of February 26, 2021, by and between the Company and H.C. Wainwright & Co., LLC (“Wainwright”), the Company engaged Wainwright to act as the Company’s exclusive placement agent in connection with the private placement. The Company agreed to pay Wainwright a cash fee of 8.0% of the gross proceeds raised by the Company in the private placement. The Company also agreed to pay Wainwright (i) a management fee equal to 1.0% of the gross proceeds raised in the private placement; and (ii) $85,000 for non-accountable expenses. In addition, the Company agreed to issue to Wainwright (or its designees) placement agent warrants to purchase a number of shares equal to 8.0% of the aggregate number of shares and pre-funded warrant shares sold under the Purchase Agreement, or warrants to purchase an aggregate of up to 6,000,000 shares. The placement agent warrants generally will have the same terms as the warrants, except they will have an exercise price of $0.10.
THE CORETEC GROUP INC.
163,500,000 Shares of Common Stock
PROSPECTUS
March 22, 2021
Coretec (QB) (USOTC:CRTG)
Historical Stock Chart
From Mar 2024 to Apr 2024
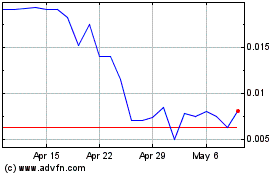
Coretec (QB) (USOTC:CRTG)
Historical Stock Chart
From Apr 2023 to Apr 2024