If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☒
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
BioLargo, Inc. (the “Company,” “we,” or “us”) filed a Registration Statement on Form S-1 with the Securities and Exchange Commission (“SEC”) on June 9, 2017 (the “Registration Statement”), and paid the registration fee. The Registration Statement was declared effective on June 15, 2017. The Company filed post-effective Amendment No. 1 to the Registration Statement on August 28, 2018, and it was declared effective on September 6, 2018, post-effective Amendment No. 2 to the Registration Statement on August 30, 2019, and it was declared effective on September 11, 2019, post-effective Amendment No. 3 to the Registration Statement on April 27, 2020, and it was declared effective on April 28, 2020, and post-effective Amendment No. 4 to the Registration Statement on April 7, 2021, and it was declared effective on April 8, 2021.
The Company is submitting this Post-Effective Amendment No. 5 (“Amendment”) to its Registration Statement for the purpose of providing information from its Annual Report on Form 10-K for the period ended December 31, 2021, filed with the SEC March 31, 2022.
The warrants to purchase 20,159,062 shares of common stock as described in the Registration Statement expired on their terms, unexercised, on June 1, 2020. Therefore, no additional shares will be sold by the Company to the Selling Stockholders pursuant to those warrants.
The contents of the Registration Statement as previously filed which are not modified and revised by this Amendment are hereby incorporated by reference.
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
This prospectus relates to the sale of up to 36,090,857 shares of our common stock by persons who have purchased shares in a series of private placements. The aforementioned persons are sometimes referred to in this prospectus as the selling stockholders. The shares offered under this prospectus by the selling stockholders may be sold on the public market, in negotiated transactions with a broker-dealer or market maker as principal or agent, or in privately negotiated transactions not involving a broker dealer. The prices at which the selling stockholder may sell the shares may be determined by the prevailing market price of the shares at the time of sale, may be different than such prevailing market prices or may be determined through negotiated transactions with third parties. We will not receive proceeds from the sale of our shares by the selling stockholders.
As of the date of this prospectus, the selling stockholders have not exercised any of the warrants to purchase shares registered hereby. The selling shareholders have sold approximately 6,000,000 shares registered for sale in the registration statement of which this prospectus is part.
Each selling stockholder may be considered an “underwriter” within the meaning of the Securities Act of 1933, as amended.
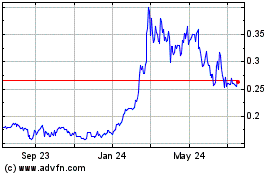
Since January 23, 2008, our common stock has been quoted on the OTC Markets “OTCQB” marketplace (formerly known as the “OTC Bulletin Board”) under the trading symbol “BLGO.” The selling stockholders will sell up the shares at prices established on the OTC Bulletin Board during the term of this offering, at prices different than prevailing market prices or at privately negotiated prices. On March 30, 2022, the last reported sale price of our common stock on the OTC Markets was $0.234.
TABLE OF CONTENTS
Unless otherwise specified, the information in this prospectus is set forth as of April 13, 2022, and we anticipate that changes in our affairs will occur after such date. We have not authorized any person to give any information or to make any representations, other than as contained in this prospectus, in connection with the offer contained in this prospectus. If any person gives you any information or makes representations in connection with this offer, do not rely on it as information we have authorized. This prospectus is not an offer to sell our common stock in any state or other jurisdiction to any person to whom it is unlawful to make such offer.
PROSPECTUS SUMMARY
The following summary highlights selected information from this prospectus and may not contain all the information that is important to you. To understand our business and this registration statement fully, you should read this entire prospectus carefully, including the financial statements and the related notes beginning on page F-1. When we refer in this prospectus to “BioLargo,” the “Company,” “our company,” “we,” “us” and “our,” we mean BioLargo, Inc., a Delaware corporation, and its subsidiaries, BioLargo Life Technologies, Inc., a California corporation, ONM Environmental, Inc., a California corporation, BioLargo Water Investment Group, Inc., a California corporation (and its subsidiary, BioLargo Water, Inc., a Canadian corporation), BioLargo Development Corp., a California corporation, BioLargo Engineering, Science & Technologies, LLC, a Tennessee limited liability company, and partially owned Clyra Medical Technologies, Inc., a California corporation. This prospectus contains forward-looking statements and information relating to BioLargo. See “Cautionary Note Regarding Forward Looking Statements” on page 14.
Our Company
BioLargo, Inc. is a Delaware corporation.
Our principal executive offices are located at 14921 Chestnut St., Westminster, California 92683. Our telephone number is (888) 400-2863.
The Registration Statement
This prospectus covers 36,090,857 shares of stock, all of which are offered for sale by the selling stockholders.
ABOUT THIS REGISTRATION
|
|
|
|
Securities Being Registered
|
This Prospectus covers the following shares, all of which are being sold by the selling stockholders: 20,159,062 shares of common stock of BioLargo issuable upon the exercise of warrants to purchase common stock which have expired since the filing of the initial Registration Statement, and 15,931,795 outstanding shares held by the Selling Stockholders. As of the date hereof, the Selling Stockholders have not exercised any of the warrants and lost their rights to do so, but have sold approximately 6,000,000 shares held by them and offered for sale by this prospectus.
|
|
|
|
|
Initial Offering Price
|
The selling stockholders will sell up to 36,090,857 shares at prices established on the OTC Electronic Bulletin Board during the term of this offering, at prices different than prevailing market prices or at privately negotiated prices.
|
|
|
|
|
Termination of the Offering
|
The offering will conclude when all the 36,090,857 shares of common stock registered hereby have been sold by the selling stockholders.
|
|
|
|
|
Risk Factors
|
An investment in our common stock is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page 3.
|
RISK FACTORS
An investment in our common stock is highly speculative, involves a high degree of risk and should be made only by investors who can afford a complete loss. You should carefully consider the following risk factors, together with the other information in this prospectus, including our financial statements and the related notes, before you decide to buy our common stock. If any of the following risks actually occurs, then our business, financial condition or results of operations could be materially adversely affected, the trading of our common stock could decline, and you may lose all or part of your investment therein.
Risks Relating to our Business
COVID-19
The Covid-19 crisis creates an environment in which no person can be certain about what is next. It continues to evolve even two years after it began. The global reach and impact are far reaching and place extreme pressure on financing, sales, accounts receivable collection cycles, and any growth plan. We believe the Covid-19 virus crisis may have a delaying effect on our plans for growth and expansion. We urge the reader to consider our forward-looking statements in light of the extraordinary circumstances of today’s business, social and economic climate. While our company is mobilizing to be a solutions provider to help inhibit the spread of Covid-19, these business plans are not mature and may be more difficult that we expect. While it may be reasonable to assume that the crisis will subside, we cannot be certain about the timing and a host of impacts that cannot be easily predicted to occur.
Supply Chain Challenges
As we emerge with new products like our AEC and AOS, that find adoption in the commercial markets, we will likely face supply chain challenges, including supply and pricing volatility, that will be beyond our control that might include steel, electrodes, membranes, electronic components (like chips), raw chemicals. We predict that at some level we may face delays and or extended delivery times for systems sold to clients and that could lead to delays in our anticipated growth.
We have a history of losses, and cannot assure you that we will ever become or remain profitable.
We have not yet generated enough revenue or gross profit from operations to fund our expenses, and, accordingly, we have incurred net losses every year since our inception. We have funded the majority of our activities through the issuance of convertible debt or equity securities. Although we are devoting more energy and money to our sales and marketing activities, we continue to anticipate net losses and negative cash flow for the foreseeable future. Our ability to reach positive cash flow depends on many factors, including our ability to fund sales and marketing activities, and the rate of client adoption. There can be no assurance that our revenues will be sufficient for us to become profitable in 2022 or future years, or thereafter maintain profitability. We may also face unforeseen problems, difficulties, expenses or delays in implementing our business plan, including regulatory hurdles.
Our cash requirements are significant. We will continue to require additional financing to sustain our operations and without it we may not be able to continue operations.
Our cash requirements and expenses continue to be significant. Our net cash used in continuing operations for the year ended December 31, 2021, was $3,937,000, approximately $328,000 monthly average. During 2021, we generated $2,531,000 in consolidated gross revenues, approximately $211,000 monthly average. Thus, in order to become profitable, we must significantly increase our revenues. Although our revenues are increasing through sales of our products and from our engineering division, we expect to continue to use cash in 2022 as it becomes available and to continue to sell our securities to fund operations.
At December 31, 2021, we had working capital of $427,000. Our auditor’s report for the year ended December 31, 2021, includes an explanatory paragraph to their audit opinion stating that our recurring losses from operations, negative cash flow from operations, and limited capital resources raise substantial doubt about our ability to continue as a going concern. We do not currently have sufficient financial resources to fund our operations or those of our subsidiaries. Therefore, we need additional financing to continue these operations.
We have relied on private securities offerings, as well as Lincoln Park Capital (see below), to provide cash needed to close the gap between operational revenue and expenses. Our ability to rely on private financing may change if the United States enters a recession, if the Dow Industrial Average or Nasdaq composite decline significantly, if interest rates rise, if real estate values decline, if international events affect the global economy, or many other factors that impact private investors’ willingness to invest in high-risk companies. Thus, while we have been able to rely on private investments in the past, we may not be able to do so in the near future.
In the year ended December 31, 2021, we relied on our financing agreement with Lincoln Park Capital to sell shares and raise capital, as well as other private investors. In total, we received almost $5 million from stock sales, and issued approximately 30 million shares of stock to these investors. These issuances are dilutive to our existing stockholders. We intend to continue these financing activities, and thus intend to continue to dilute the existing stockholders.
These issuances are dilutive to our existing stockholders. We intend to continue these financing activities, and thus intend to continue to dilute the existing stockholders.
We regularly issue stock, or stock options, instead of cash, to pay some of our operating expenses. These issuances are dilutive to our existing stockholders.
We are party to agreements that provide for the payment of, or permit us to pay at our option, securities rather than cash in consideration for services provided to us. We include these provisions in agreements to allow us to preserve cash. We anticipate that we will continue to do so in the future. All such issuances preserve our cash reserves, but are also dilutive to our stockholders because they increase (and will increase in the future) the total number of shares of our common stock issued and outstanding, even though such arrangements assist us with managing our cash flow. These issuances also increase the expense amount recorded.
Our stockholders face further potential dilution in any new financing.
In the year ended December 31, 2021, we issued almost 30 million shares of our common stock in financing activities. Our private securities offerings typically offer convertible securities, including notes and warrants. Those warrants often include provisions that require investors to pay for the underlying shares with cash, which if executed would generate working capital for the company. Any additional capital that we raise would dilute the interest of the current stockholders and any persons who may become stockholders before such financing. Given the price of our common stock, such dilution in any financing of a significant amount could be substantial.
We may be required to seek stockholder approval to amend our charter to increase our authorized number of shares.
We have approximately 262 million common shares outstanding. We have reserved for further issuance approximately 104 million shares: 6 million to Lincoln Park, 42 million in our 2018 Equity Plan, 19 million to “non-plan” option holders, and 37 million to warrant holders. As our Certificate of Incorporation authorizes us to issue 400 million shares, we currently have approximately 34 million shares available for future issuances. In order to continue financing our operations through the sale of our common stock or convertible securities, we will be required to seek stockholder approval to amend our charter to increase the number of shares authorized. If our stockholders do not agree to increase the number of shares our Certificate of Incorporation authorizes us to issue, we may have to cease further financing activities. If we were forced to do so, we would run out of cash and may be forced to significantly curtail our operations.
Our stockholders face further potential adverse effects from the terms of any preferred stock that may be issued in the future.
Our certificate of incorporation authorizes 50 million shares of preferred stock. None are outstanding as of the date hereof. In order to raise capital to meet expenses or to acquire a business, our board of directors may issue additional stock, including preferred stock. Any preferred stock that we may issue may have voting rights, liquidation preferences, redemption rights and other rights, preferences and privileges. The rights of the holders of our common stock will be subject to, and in many respects subordinate to, the rights of the holders of any such preferred stock. Furthermore, such preferred stock may have other rights, including economic rights, senior to our common stock that could have a material adverse effect on the value of our common stock. Preferred stock, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, can also have the effect of making it more difficult for a third party to acquire a majority of our outstanding voting stock, thereby delaying, deferring or preventing a change in control of our company.
Our revenue growth rate may not be indicative of future performance and may slow over time.
Although our revenues have grown over the last several years, our revenue growth rate may slow over time for a number of reasons, including increasing competition, market saturation, slowing demand for our products and services, increasing regulatory costs and challenges, the impact of COVID-19, and failure to capitalize on growth opportunities.
We do not have contracts with customers that require the purchase of a minimum amount of our products.
None of our customers provide us with firm, long-term or short-term volume purchase commitments. As a result, we could have periods during which we have no or limited orders for our products but will continue to have fixed costs. We may not be able to find new customers in a timely manner if we experience no or limited purchase orders. Periods of no or limited purchase orders for our products, particularly from one or more of our four largest customers, could adversely affect our business, financial condition and results of operations.
There are several specific business opportunities we are considering in further development of our business. None of these opportunities is yet the subject of a definitive agreement, and many of these opportunities will require additional funding obligations on our part, for which funding is not currently in place.
In furtherance of our business plan, we are presently considering a number of opportunities to promote our business, to further develop and broaden, and to license, our technology with third parties. While discussions are underway with respect to such opportunities, there are no definitive agreements in place with respect to any of such opportunities at this time. There can be no assurance that any of such opportunities being discussed will result in definitive agreements or, if definitive agreements are entered into, that they will be on terms that are favorable to us.
Moreover, should any of these opportunities result in definitive agreements being executed or consummated, we may be required to expend additional monies above and beyond our current operating budget to promote such endeavors. No such financing is in place at this time for such endeavors, and we cannot assure you that any such financing will be available, or if it is available, whether it will be on terms that are favorable to our company.
We expect to incur future losses and may not be able to achieve profitability.
Although we are generating revenue from the sale of our products and from providing services, and we expect to generate revenue from new products we are introducing, and eventually from other license or supply agreements, we anticipate net losses and negative cash flow to continue for the foreseeable future until our products are expanded in the marketplace and they gain broader acceptance by resellers and customers. Our current level of sales is not sufficient to support the financial needs of our business. We cannot predict when or if sales volumes will be sufficiently large to cover our operating expenses. We intend to expand our marketing efforts of our products as financial resources are available, and we intend to continue to expand our research and development efforts. Consequently, we will need to generate significant additional revenue or seek additional financings to fund our operations. This has put a proportionate corresponding demand on capital. Our ability to achieve profitability is dependent upon our efforts to deliver a viable product and our ability to successfully bring it to market, which we are currently pursuing. Although our management is optimistic that we will succeed in licensing our technology, we cannot be certain as to timing or whether we will generate sufficient revenue to be able to operate profitably. If we cannot achieve or sustain profitability, then we may not be able to fund our expected cash needs or continue our operations. If we are not able to devote adequate resources to promote commercialization of our technology, then our business plans will suffer and may fail.
Because we have limited resources to devote to sales, marketing and licensing efforts with respect to our technology, any delay in such efforts may jeopardize future research and development of technologies and commercialization of our technology. Although our management believes that it can finance commercialization efforts through sales of our securities and possibly other capital sources, if we do not successfully bring our technology to market, our ability to generate revenues will be adversely affected.
Our internal controls are not effective.
We have determined that our disclosure controls and procedures and our internal control over financial reporting are currently not effective. The lack of effective internal controls, has not yet, but could in the future, materially adversely affect our financial condition and ability to carry out our business plan. As more financial resources come available, we need to invest in additional personnel to better manage the financial reporting processes.
If we are not able to manage our anticipated growth effectively, we may not become profitable.
We anticipate that expansion will continue to be required to address potential market opportunities for our technologies and our products. Our existing infrastructure is limited. While we believe our current manufacturing processes as well as our office and warehousing provide the basic resources to expand to sales of more than $2 million per month, our infrastructure will need more staffing to support manufacturing, customer service, administration as well as sales/account executive functions. There can be no assurance that we will have the financial resources to create new infrastructure, or that any such infrastructure will be sufficiently scalable to manage future growth, if any. There also can be no assurance that, if we invest in additional infrastructure, we will be effective in expanding our operations or that our systems, procedures or controls will be adequate to support such expansion. In addition, we will need to provide additional sales and support services to our partners if we achieve our anticipated growth with respect to the sale of our technology for various applications. Failure to properly manage an increase in customer demands could result in a material adverse effect on customer satisfaction, our ability to meet our contractual obligations, and our operating results.
Some of the products incorporating our technology will require regulatory approval.
The products in which our technology may be incorporated have both regulated and non-regulated applications. The regulatory approvals for certain applications may be difficult, impossible, time consuming and/or expensive to obtain. While our management believes such approvals can be obtained for the applications contemplated, until those approvals from the FDA or the EPA or other regulatory bodies, at the federal and state levels, as may be required are obtained, we may not be able to generate commercial revenues for regulated products. Certain specific regulated applications and their use require highly technical analysis and additional third-party validation and will require regulatory approvals from organizations like the FDA. Certain applications may also be subject to additional state and local agency regulations, increasing the cost and time associated with commercial strategies. Additionally, most products incorporating our technology that may be sold in the European Union (“EU”) will require EU and possibly also individual country regulatory approval. All such approvals, including additional testing, are time-consuming, expensive and do not have assured outcomes of ultimate regulatory approval.
We need to outsource and rely on third parties for the manufacture of the chemicals, material components or delivery apparatus used in our technology, and part of our future success will be dependent on the timeliness and effectiveness of the efforts of these third parties.
We do not have the required financial and human resources or capability to manufacture the chemicals necessary to make our odor control products. Our business model calls for the outsourcing of the manufacture of these chemicals in order to reduce our capital and infrastructure costs as a means of potentially improving our financial position and the profitability of our business. Accordingly, we must enter into agreements with other companies that can assist us and provide certain capabilities, including sourcing and manufacturing, which we do not possess. We may not be successful in entering into such alliances on favorable terms or at all. Even if we do succeed in securing such agreements, we may not be able to maintain them. Furthermore, any delay in entering into agreements could delay the development and commercialization of our technology or reduce its competitiveness even if it reaches the market. Any such delay related to such future agreements could adversely affect our business. While we have been able to secure materials and supplies like plastic containers through the COVID-19 crisis, we have not assurances that our ability to purchase in large quantities on a continual basis.
If any party to which we have outsourced certain functions fails to perform its obligations under agreements with us, the commercialization of our technology could be delayed or curtailed.
To the extent that we rely on other companies to manufacture the chemicals used in our technology, or sell or market products incorporating our technology, we will be dependent on the timeliness and effectiveness of their efforts. If any of these parties does not perform its obligations in a timely and effective manner, the commercialization of our technology could be delayed or curtailed because we may not have sufficient financial resources or capabilities to continue such efforts on our own.
We rely on a small number of key supply ingredients in order to manufacture CupriDyne Clean.
The raw ingredients used to manufacture CupriDyne Clean are readily available from multiple suppliers. However, commodity prices for these ingredients can vary significantly, and the margins that we are able to generate could decline if prices rise. If our manufacturing costs rise significantly, we may be forced to raise the prices for our products, which may reduce their acceptance in the marketplace. Given the current delays in supply chain delivery on a global scale, we are anticipating and developing strategies to manage the expected increases in our cost of raw goods and potential supply limitations which could impact our business and results of operations.
If our technology or products incorporating our technology do not gain market acceptance, it is unlikely that we will become profitable.
The potential markets for products into which our technology can be incorporated are rapidly evolving, and we have many successful competitors including some of the largest and most well-established companies in the world. The commercial success of products incorporating our technology will depend on the adoption of our technology by commercial and consumer end users in various fields.
Market acceptance may depend on many factors, including:
| |
●
|
the willingness and ability of consumers and industry partners to adopt new technologies from a company with little or no history in the industry;
|
| |
●
|
our ability to convince potential industry partners and consumers that our technology is an attractive alternative to other competing technologies;
|
| |
●
|
our ability to license our technology in a commercially effective manner;
|
| |
●
|
our ability to continue to fund operations while our products move through the process of gaining acceptance, before the time in which we are able to scale up production to obtain economies of scale; and
|
| |
●
|
our ability to overcome brand loyalties.
|
If products incorporating our technology do not achieve a significant level of market acceptance, then demand for our technology itself may not develop as expected, and, in such event, it is unlikely that we will become profitable.
Any revenues that we may earn in the future are unpredictable, and our operating results are likely to fluctuate from quarter to quarter.
We believe that our future operating results will fluctuate due to a variety of factors, including:
| |
●
|
delays in product development by us or third parties;
|
| |
●
|
market acceptance of products incorporating our technology;
|
| |
●
|
changes in the demand for, and pricing of, products incorporating our technology;
|
| |
●
|
competition and pricing pressure from competitive products; and
|
| |
●
|
expenses related to, and the results of, proceedings relating to our intellectual property.
|
We expect our operating expenses will continue to fluctuate significantly in 2021 and beyond, as we continue our research and development and increase our marketing and licensing activities. Although we expect to generate revenues from licensing our technology in the future, revenues may decline or not grow as anticipated, and our operating results could be substantially harmed for a particular fiscal period. Moreover, our operating results in some quarters may not meet the expectations of stock market analysts and investors. In that case, our stock price most likely would decline.
Some of our revenue may be dependent on the award of new contracts from the U.S. government, which we do not directly control.
Some of our revenue has been generated from sales to the U.S. Defense Logistics Agency through a bid process in response to request for bids. The timing and size of requests for bids is unpredictable and outside of our control. The number of other companies competing for these bids is also unpredictable and outside of our control. In the event of more competition for these awards, we may have to reduce our margins. These variables make it difficult to predict when or if we will sell more products to the U.S. government, which in turns makes it difficult to stock inventory and purchase raw materials.
We have limited product distribution experience, and we rely in part on third parties who may not successfully sell our products.
We have limited product distribution experience and rely in part on product distribution arrangements with third parties. In our future product offerings, we may rely solely on third parties for product sales and distribution. We also plan to license our technology to certain third parties for commercialization of certain applications. We expect to enter into additional distribution agreements and licensing agreements in the future, and we may not be able to enter into these additional agreements on terms that are favorable to us, if at all. In addition, we may have limited or no control over the distribution activities of these third parties. These third parties could sell competing products and may devote insufficient sales efforts to our products. As a result, our future revenues from sales of our products, if any, will depend on the success of the efforts of these third parties.
We may not be able to attract or retain qualified senior personnel.
We believe we are currently able to manage our current business with our existing management team. However, as we expand the scope of our operations, we will need to obtain the full-time services of additional senior management and other personnel. Competition for highly-skilled personnel is intense, and there can be no assurance that we will be able to attract or retain qualified senior personnel. Our failure to do so could have an adverse effect on our ability to implement our business plan. As we add full-time senior personnel, our overhead expenses for salaries and related items will increase from current levels and, depending upon the number of personnel we hire and their compensation packages, these increases could be substantial.
If we lose our key personnel or are unable to attract and retain additional personnel, we may be unable to achieve profitability.
Our future success is substantially dependent on the efforts of our senior management, particularly Dennis P. Calvert, our president and chief executive officer. The loss of the services of Mr. Calvert or other members of our senior management may significantly delay or prevent the achievement of product development and other business objectives. Because of the scientific nature of our business, we depend substantially on our ability to attract and retain qualified marketing, scientific and technical personnel. There is intense competition among specialized and technologically-oriented companies for qualified personnel in the areas of our activities. If we lose the services of, or do not successfully recruit, key marketing, scientific and technical personnel, then the growth of our business could be substantially impaired. At present, we do not maintain key man insurance for any of our senior management, although management is evaluating the potential of securing this type of insurance in the future as may be available.
Nondisclosure agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary technology and processes, we rely in part on nondisclosure agreements with our employees, potential licensing partners, potential manufacturing partners, testing facilities, universities, consultants, agents and other organizations to which we disclose our proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover trade secrets and proprietary information, and in such cases we could not assert any trade secret rights against such parties. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position. Since we rely on trade secrets and nondisclosure agreements, in addition to patents, to protect some of our intellectual property, there is a risk that third parties may obtain and improperly utilize our proprietary information to our competitive disadvantage. We may not be able to detect unauthorized use or take appropriate and timely steps to enforce our intellectual property rights.
We may become subject to product liability claims.
As a business that manufactures and markets products for use by consumers and institutions, we may become liable for any damage caused by our products, whether used in the manner intended or not. Any such claim of liability, whether meritorious or not, could be time-consuming and/or result in costly litigation. Although we maintain general liability insurance, our insurance may not cover potential claims of the types described above and may not be adequate to indemnify for all liabilities that may be imposed. Any imposition of liability that is not covered by insurance or is in excess of insurance coverage could harm our business and operating results, and you may lose some or all of any investment you have made, or may make, in our company.
Litigation or the actions of regulatory authorities may harm our business or otherwise distract our management.
Substantial, complex or extended litigation could cause us to incur major expenditures and distract our management. For example, lawsuits by employees, former employees, investors, stockholders, partners, customers or others, or actions taken by regulatory authorities, could be very costly and substantially disrupt our business. As a result of our financing activities over time, and by virtue of the number of people that have invested in our company, we face increased risk of lawsuits from investors. Such lawsuits or actions could from time to time be filed against our company and/or our executive officers and directors. Such lawsuits and actions are not uncommon, and we cannot assure you that we will always be able to resolve such disputes or actions on terms favorable to our company.
If we suffer negative publicity concerning the safety or efficacy of our products, our sales may be harmed.
If concerns should arise about the safety or efficacy of any of our products that are marketed, regardless of whether or not such concerns have a basis in generally accepted science or peer-reviewed scientific research, such concerns could adversely affect the market for those products. Similarly, negative publicity could result in an increased number of product liability claims, whether or not those claims are supported by applicable law.
The licensing of our technology or the manufacture, use or sale of products incorporating our technology may infringe on the patent rights of others, and we may be forced to litigate if an intellectual property dispute arises.
If we infringe or are alleged to have infringed another party’s patent rights, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, do not successfully defend an infringement action or are unable to have infringed patents declared invalid, we may:
| |
●
|
incur substantial monetary damages;
|
| |
●
|
encounter significant delays in marketing our current and proposed product candidates;
|
| |
●
|
be unable to conduct or participate in the manufacture, use or sale of product candidates or methods of treatment requiring licenses;
|
| |
●
|
lose patent protection for our inventions and products; or
|
| |
●
|
find our patents are unenforceable, invalid or have a reduced scope of protection
|
Parties making such claims may be able to obtain injunctive relief that could effectively block our company’s ability to further develop or commercialize our current and proposed product candidates in the United States and abroad and could result in the award of substantial damages. Defense of any lawsuit or failure to obtain any such license could substantially harm our company. Litigation, regardless of outcome, could result in substantial cost to, and a diversion of efforts by, our company.
Our patents are expensive to maintain, our patent applications are expensive to prosecute, and thus we are unable to file for patent protection in many countries.
Our ability to compete effectively will depend in part on our ability to develop and maintain proprietary aspects of our technology and either to operate without infringing the proprietary rights of others or to obtain rights to technology owned by third parties. Pending patent applications relating to our technology may not result in the issuance of any patents or any issued patents that will offer protection against competitors with similar technology. We must employ patent attorneys to prosecute our patent applications both in the United States and internationally. International patent protection requires the retention of patent counsel and the payment of patent application fees in each foreign country in which we desire patent protection, on or before filing deadlines set forth by the International Patent Cooperation Treaty (“PCT”). We therefore choose to file patent applications only in foreign countries where we believe the commercial opportunities require it, considering our available financial resources and the needs for our technology. This has resulted, and will continue to result, in the irrevocable loss of patent rights in all but a few foreign jurisdictions.
Patents we receive may be challenged, invalidated or circumvented in the future, or the rights created by those patents may not provide a competitive advantage. We also rely on trade secrets, technical know-how and continuing invention to develop and maintain our competitive position. Others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets.
We are subject to risks related to future business outside of the United States.
Over time, we may develop business relationships outside of North America, and as those efforts are pursued, we will face risks related to those relationships such as:
| |
●
|
foreign currency fluctuations;
|
| |
●
|
unstable political, economic, financial and market conditions;
|
| |
●
|
import and export license requirements;
|
| |
●
|
increases in tariffs and taxes;
|
| |
●
|
high levels of inflation;
|
| |
●
|
restrictions on repatriating foreign profits back to the United States;
|
| |
●
|
greater difficulty collecting accounts receivable and longer payment cycles;
|
| |
●
|
less favorable intellectual property laws, and the lack of intellectual property legal protection;
|
| |
●
|
regulatory requirements;
|
| |
●
|
unfamiliarity with foreign laws and regulations; and
|
| |
●
|
changes in labor conditions and difficulties in staffing and managing international operations.
|
The volatility of certain raw material costs may adversely affect operations and competitive price advantages for products that incorporate our technology.
Most of the chemicals and other key materials that we use in our business, such as minerals, fiber materials and packaging materials, are neither generally scarce nor price sensitive, but prices for such chemicals and materials can be cyclical. Supply and demand factors, which are beyond our control, generally affect the price of our raw materials. We try to minimize the effect of price increases through production efficiency and the use of alternative suppliers, but these efforts are limited by the size of our operations. If we are unable to minimize the effects of increased raw material costs, our business, financial condition, results of operations and cash flows may be materially adversely affected.
Certain of our products sales historically have been highly impacted by fluctuations in seasons and weather.
Industrial odor control products have proven highly effective in controlling volatile organic compounds that are released as vapors produced by decomposing waste material. Such vapors are produced with the highest degree of intensity in temperatures between 40 degrees Fahrenheit (5 degrees Celsius) and 140 degrees Fahrenheit (60 degrees Celsius). When weather patterns are cold or in times of precipitation, our clients are less prone to use our odor control products, presumably because such vapors are less noticeable or, in the case of precipitation, can be washed away or altered. This leads to unpredictability in use and sales patterns for, especially, our CupriDyne Clean product line which accounts for over one-half our total sales.
The cost of maintaining our public company reporting obligations is high.
We are obligated to maintain our periodic public filings and public reporting requirements, on a timely basis, under the rules and regulations of the SEC. In order to meet these obligations, we will need to continue to raise capital. If adequate funds are not available, we will be unable to comply with those requirements and could cease to be qualified to have our stock traded in the public market. As a public company, we incur significant legal, accounting and other expenses. In addition, the Sarbanes-Oxley Act of 2002, as well as related rules adopted by the SEC, has imposed substantial requirements on public companies, including certain corporate governance practices and requirements relating to internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations, and those of our contractors and consultants, could be subject to pandemics, earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics, acts of terrorism, acts of war and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely in part on third-party manufacturers to produce and process our products or the raw materials used to make our products. Our ability to obtain supplies of our products or raw materials could be disrupted if the operations of these suppliers are affected by a man-made or natural disaster, pandemics, epidemics, or other business interruption, including the recent novel strain of coronavirus (SARS‑CoV‑2 aka COVID-19) that originally surfaced in Wuhan, China in December 2019. The extent to which COVID‑19 impacts our business will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID‑19 and the actions to contain 2 or treat its impact, among others. Our corporate headquarters and offices of ONM are in Southern California near major earthquake faults and fire zones. Our operations and financial condition could suffer in the event of a major earthquake, fire or other natural disaster.
The COVID-19 coronavirus pandemic is ongoing and may result in significant disruptions to our clients and/or supply chain which could have a material adverse effect on our business and revenues.
The COVID-19 pandemic is still ongoing as of the date of this report, is still evolving and much of its impact remains unknown. It is impossible to predict the impact it may have on the development of our business and on our revenues in 2022.
Our corporate headquarters and offices of our ONM Environmental division are in Southern California. On March 19, 2020, California’s Governor issued an executive order that all residents of the State must stay at home indefinitely except as needed to maintain “essential critical infrastructure”. Although some of these emergency provisions were eliminated or modified in 2021, COVID cases are increasing in certain European and Asian countries, and that may foretell an additional surge of cases in the United States or in California in the next months or longer. The restrictions put in place in March 2020 and thereafter to mitigate the pandemic have affected our clients’ willingness to purchase our products and services. Their continuing affect is impossible for us to predict.
The severity of the coronavirus pandemic could also make access to our existing supply chain difficult or impossible by delaying the delivery of key raw materials used in our product candidates and therefore delay the delivery of our products. Any of these results could materially impact our business and have an adverse effect on our business.
A recession in the United States may affect our business.
If the U.S. economy were to contract into a recession or depression, our existing clients, and potential future clients, may divert their resources to other goods and services, and our business may suffer.
Risks Relating to our Common Stock
The sale or issuance of our common stock to Lincoln Park may cause dilution, and the sale of the shares of common stock acquired by Lincoln Park, or the perception that such sales may occur, could cause the price of our common stock to fall.
On March 30, 2020, we entered into a Purchase Agreement with Lincoln Park, pursuant to which Lincoln Park agreed to purchase from us at our request up to an aggregate of $10,250,000 of our common stock (subject to certain limitations) from time to time over a period of three years, noted above in our Risks Related to our Business. We generally have the right to control the timing and amount of any sales of our shares to Lincoln Park. Sales of our common stock, if any, to Lincoln Park will depend on market conditions and other factors to be determined by us. We may ultimately decide to sell to Lincoln Park all, some or none of the shares of our common stock that may be available for us to sell pursuant to the LPC Agreement. If and when we do sell shares to Lincoln Park, after Lincoln Park has acquired the shares, Lincoln Park may resell all, some or none of those shares at any time or from time to time in its discretion. Therefore, sales to Lincoln Park by us could result in substantial dilution to the interests of other holders of our common stock, as well as sales of our stock by Lincoln Park into the open market causing reductions in the price of our common stock. Additionally, the sale of a substantial number of shares of our common stock to Lincoln Park, or the anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise desire to effect sales.
Our common stock is thinly traded and largely illiquid.
Our stock is currently quoted on the OTC Markets (OTCQB). Being quoted on the OTCQB has made it more difficult to buy or sell our stock and from time to time has led to a significant decline in the frequency of trades and trading volume. Continued trading on the OTCQB will also likely adversely affect our ability to obtain financing in the future due to the decreased liquidity of our shares and other restrictions that certain investors have for investing in OTCQB traded securities. While we intend to seek listing on the Nasdaq Stock Market (“Nasdaq”) or another national stock exchange when our company is eligible, there can be no assurance when or if our common stock will be listed on Nasdaq or another national stock exchange.
The market price of our stock is subject to volatility.
Our stock price has been and is likely to continue to be volatile. As a result of this volatility, investors may not be able to sell their common stock at or above their purchase price. The market price of our common stock and warrants may fluctuate significantly in response to numerous factors, many of which are beyond our control, including:
| |
●
|
Because our stock is thinly traded, its price can change dramatically over short periods, even in a single day. An investment in our stock is subject to such volatility and, consequently, is subject to significant risk. The market price of our common stock could fluctuate widely in response to many factors, including:
|
| |
●
|
developments with respect to patents or proprietary rights;
|
| |
●
|
announcements of technological innovations by us or our competitors;
|
| |
●
|
announcements of new products or new contracts by us or our competitors;
|
| |
●
|
actual or anticipated variations in our operating results due to the level of development expenses and other factors;
|
| |
●
|
changes in financial estimates by securities analysts and whether any future earnings of ours meet or exceed such estimates;
|
| |
●
|
conditions and trends in our industry;
|
| |
●
|
new accounting standards;
|
| |
●
|
the size of our public float;
|
| |
●
|
short sales, hedging, and other derivative transactions involving our common stock;
|
| |
●
|
sales of large blocks of our common stock including sales by our executive officers, directors, and significant stockholders, including Lincoln Park;
|
| |
●
|
general economic, political and market conditions and other factors; and
|
| |
●
|
the occurrence of any of the risks described herein.
|
You may have difficulty selling our shares because they are deemed a “penny stock”.
Because our common stock is not quoted or listed on a national securities exchange, if the trading price of our common stock remains below $5.00 per share, which we expect for the foreseeable future, trading in our common stock will be subject to the requirements of certain rules promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which require additional disclosure by broker-dealers in connection with any trades involving a stock defined as a penny stock (generally, any non-Nasdaq equity security that has a market price of less than $5.00 per share, subject to certain exceptions). Such rules require the delivery, before any penny stock transaction, of a disclosure schedule explaining the penny stock market and the risks associated therewith and impose various sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors (generally defined as an investor with a net worth in excess of $1,000,000 or annual income exceeding $200,000 individually or $300,000 together with a spouse). For these types of transactions, the broker-dealer must make a special suitability determination for the purchaser and have received the purchaser’s written consent to the transaction before the sale. The broker-dealer also must disclose the commissions payable to the broker-dealer and current bid and offer quotations for the penny stock and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Such information must be provided to the customer orally or in writing before or with the written confirmation of trade sent to the customer. Monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. The additional burdens imposed on broker-dealers by such requirements could discourage broker-dealers from effecting transactions in our common stock, which could severely limit the market liquidity of our common stock and the ability of holders of our common stock to sell their shares.
Because our shares are deemed a “penny stock,” rules enacted by FINRA make it difficult to sell previously restricted stock.
Rules put in place by the Financial Industry Regulatory Authority (FINRA) require broker-dealers to perform due diligence before depositing unrestricted common shares of penny stocks, and as such, some broker-dealers, including many large national firms (such as eTrade and Charles Schwab), are refusing to deposit previously restricted common shares of penny stocks. We routinely issued non-registered restricted common shares to investors, vendors and consultants. The issuance of such shares is subjected to the FINRA-enacted rules. As such, it can be difficult for holders of restricted stock, including those issued in our private securities offerings, to deposit the shares with broker-dealers and sell those shares on the open market.
Because we will not pay dividends in the foreseeable future, stockholders will only benefit from owning common stock if it appreciates.
We have never declared or paid a cash dividend to stockholders. We intend to retain any earnings that may be generated in the future to finance operations. Accordingly, any potential investor who anticipates the need for current dividends from his investment should not purchase our common stock, and must rely on the benefit of owning shares, and presumably a rise in share price. We cannot predict the future price of our stock, and due to the factors enumerated herein, can make no assurance of a future increase in the price of our common stock.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
All statements, other than statements of historical fact, included in this prospectus regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects and plans and objectives of management are forward-looking statements. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
We have based these forward-looking statements on our current expectations and projections about future events. Although we believe that the expectations underlying our forward-looking statements are reasonable, these expectations may prove to be incorrect, and all of these statements are subject to risks and uncertainties. Therefore, you should not place undue reliance on our forward-looking statements. We have included important risks and uncertainties in the cautionary statements included in this prospectus, particularly the section titled “Risk Factors” incorporated by reference herein. We believe these risks and uncertainties could cause actual results or events to differ materially from the forward-looking statements that we make. Should one or more of these risks and uncertainties materialize, or should underlying assumptions, projections or expectations prove incorrect, actual results, performance or financial condition may vary materially and adversely from those anticipated, estimated or expected. Our forward-looking statements do not reflect the potential impact of future acquisitions, mergers, dispositions, joint ventures or investments that we may make. We do not assume any obligation to update any of the forward-looking statements contained herein, whether as a result of new information, future events or otherwise, except as required by law. In the light of these risks and uncertainties, the forward-looking events and circumstances discussed in this prospectus may not occur, and actual results could differ materially from those anticipated or implied in the forward-looking statements. Any forward-looking statement made by us in this prospectus is based only on information currently available to us and speaks only as of the date on which it is made.
USE OF PROCEEDS
This prospectus relates to shares of our common stock that may be offered and sold from time to time by the selling stockholders upon exercise of outstanding warrants to purchase common stock. We will receive no proceeds from the sale of shares of common stock by the selling stockholders in this offering. See “Plan of Distribution” elsewhere in this prospectus for more information.
DIVIDEND POLICY
We have never declared or paid a cash dividend to stockholders. We intend to retain any earnings that may be generated in the future to finance operations.
CAPITALIZATION
The following table sets forth our actual cash and cash equivalents and our capitalization as of December 31, 2021 (unaudited), and as adjusted to give effect to the sale of the shares offered hereby and the use of proceeds, as described in the section titled “Use of Proceeds” above.
You should read this information in conjunction with “Managements’ Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes appearing in our Annual Report on Form 10-K for the year ended December 31, 2021.
| |
|
As of December 31, 2021
(in thousands)
|
|
| |
|
Actual
|
|
|
As Adjusted(1)
|
|
|
CASH AND CASH EQUIVALENTS
|
|
$ |
962 |
|
|
$ |
962 |
|
| |
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ DEFICIT:
|
|
|
|
|
|
|
|
|
|
Convertible Preferred Series A, $.00067 Par Value, 50,000,000 Shares Authorized, -0- Shares Issued and Outstanding at December 31, 2021.
|
|
|
— |
|
|
|
— |
|
|
Common stock, $.00067 Par Value, 400,000,000 Shares Authorized, 255,893,726 Shares Issued at December 31, 2021, and 255,893,726 Shares Issued, as adjusted.
|
|
|
171 |
|
|
|
171 |
|
|
Additional paid-in capital
|
|
|
143,718 |
|
|
|
143,718 |
|
|
Accumulated other comprehensive loss
|
|
|
(115 |
) |
|
|
(115 |
) |
|
Accumulated deficit
|
|
|
(139,121 |
) |
|
|
(139,121 |
) |
| |
|
|
|
|
|
|
|
|
|
Total Biolargo and subsidiaries stockholders’ equity
|
|
|
4,653 |
|
|
|
4,653 |
|
|
Non-controlling interest
|
|
|
(3,720 |
) |
|
|
(3,720 |
) |
|
Total stockholders’ equity
|
|
|
933 |
|
|
|
933 |
|
|
Total liabilities and stockholders’ equity
|
|
|
3,023 |
|
|
|
3,023 |
|
| |
(1)
|
The warrants available to the selling stockholders expired unexercised on June 1, 2020. As such, no cash will be received from the Selling Stockholders, and no additional shares will be issued to the Selling Stockholders.
|
DILUTION
The net tangible book value of the Company as of December 31, 2021, was $480,000, or approximately $0.002 per share of common stock. Net tangible book value per share is determined by dividing the net tangible book value of the Company (total tangible assets less total liabilities) by the number of outstanding shares of our common stock.
No additional shares will be issued to the Selling Stockholders, and no proceeds will be received from the Selling Stockholders. As such, we do not expect a change in the net tangible book value from this offering.
MARKET PRICE OF AND DIVIDENDS ON COMMON EQUITY
AND RELATED STOCKHOLDER MATTERS
Market Information
Since January 23, 2008, our common stock has been quoted on the OTC Markets “OTCQB” marketplace (formerly known as the “OTC Bulletin Board”) under the trading symbol “BLGO”.
The table below represents the quarterly high and low closing prices of our common stock for the last three fiscal years as reported by www.otcmarkets.com.
| |
|
|
|
|
2019
|
|
|
2020
|
|
|
2021
|
|
| |
|
High
|
|
|
|
|
|
High
|
|
|
High
|
|
|
|
|
|
Low
|
|
|
High
|
|
|
Low
|
|
|
First Quarter
|
|
|
|
|
|
$ |
0.27 |
|
|
|
|
|
|
$ |
0.16 |
|
|
$ |
0.29 |
|
|
$ |
0.12 |
|
|
$ |
0.25 |
|
|
$ |
0.13 |
|
|
Second Quarter
|
|
|
|
|
|
$ |
0.31 |
|
|
|
|
|
|
$ |
0.16 |
|
|
$ |
0.20 |
|
|
$ |
0.14 |
|
|
$ |
0.24 |
|
|
$ |
0.16 |
|
|
Third Quarter
|
|
|
|
|
|
$ |
0.38 |
|
|
|
|
|
|
$ |
0.22 |
|
|
$ |
0.22 |
|
|
$ |
0.15 |
|
|
$ |
0.22 |
|
|
$ |
0.17 |
|
|
Fourth Quarter
|
|
|
|
|
|
$ |
0.36 |
|
|
|
|
|
|
$ |
0.22 |
|
|
$ |
0.16 |
|
|
$ |
0.12 |
|
|
$ |
0.23 |
|
|
$ |
0.17 |
|
The closing price for our common stock on March 30, 2022, was $0.234 per share.
Holders of our Common Stock
As of March 30, 2022, 262,722,515 shares of our common stock were outstanding and held of record by approximately 650 stockholders of record, and approximately 5,200 beneficial owners.
Dividends
We have never declared or paid a cash dividend to stockholders. We intend to retain any earnings that may be generated in the future to finance operations.
Securities Authorized for Issuance Under Equity Compensation Plans
Equity compensation plan information as of December 31, 2021.
|
(1) Plan Category
|
|
Number of securities to be
issued upon exercise of
outstanding options,
warrants and rights
(a)
|
|
|
Weighted average
exercise price of
outstanding options,
warrants and rights
(b)
|
|
|
Number of securities
remaining available
for future issuance
(c)
|
|
|
Equity compensation plans approved by security holders
|
|
|
26,065,388(1) |
|
|
|
$0.29 |
|
|
|
25,134,475 |
|
|
Equity compensation plans not approved by security holders(2)
|
|
|
20,119,207 |
|
|
|
$0.40 |
|
|
|
n/a |
|
|
Total
|
|
|
45,304,471 |
|
|
|
$0.34 |
|
|
|
25,134,475 |
|
| |
(1)
|
Includes 5,689,363 shares issuable under the 2007 Equity Plan. The 2007 Equity Plan expired September 6, 2017, and 18,865,525 shares issuable under the 2018 Equity Incentive Plan adopted by the Board on March 7, 2018 and subsequently approved by stockholders on May 23, 2018.
|
| |
(2)
|
This includes various issuances to specific individuals either as a conversion of un-paid obligations pursuant to a plan adopted by our board of directors, or as part of their agreement for services
|
2018 Equity Incentive Plan
On June 22, 2018, our stockholders adopted the BioLargo 2018 Equity Incentive Plan (“2018 Plan”) as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years. It is set to expire on its terms on June 22, 2028. Our Board of Director’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. The plan authorizes the following types of awards: (i) incentive and non-qualified stock options, (ii) restricted stock awards, (iii) stock bonus awards, (iv) stock appreciation rights, (v) restricted stock units, and (vi) performance awards. The total number of shares reserved and available for awards pursuant to this Plan as of the date of adoption of this 2018 Plan by the Board is 40 million shares. The number of shares available to be issued under the 2018 Plan increases automatically each January 1st by the lesser of (a) 2 million shares, or (b) such number of shares determined by our Board. As of December 31, 2021, 46,000,000 shares are authorized under the plan.
2007 Equity Incentive Plan
On September 7, 2007, and as amended April 29, 2011, the BioLargo, Inc. 2007 Equity Incentive Plan (“2007 Plan”) was adopted as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years, which expired on September 7, 2017. The Board’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. As of September 2017, the Plan was closed to further stock option grants.
Equity Compensation Plans not approved by stockholders
In addition to the 2018 and 2007 Equity Plans, our board of directors has approved a plan for employees, consultants and vendors by which outstanding amounts owed to them by our company may be converted to common stock or options to purchase common stock. The conversion and exercise price is based on the closing price of our common stock on the date of agreement. If an option is issued, the number of shares purchasable by the option is calculated by dividing the amount owed by the exercise price, times one and one-half.
DESCRIPTION OF BUSINESS
Our Business - Innovator and Solution Provider
BioLargo, Inc. invents, develops, and commercializes innovative platform technologies to solve challenging environmental problems like PFAS contamination, advanced water and wastewater treatment, industrial odor and VOC control, air quality control, infection control, and myriad environmental remediation challenges. Having conducted continual and extensive research and development, BioLargo holds a wide array of issued patents, maintains a robust pipeline of products, and provides full-service environmental engineering. We invent or acquire novel technologies and develop them to maturity through our operating subsidiaries using cutting-edge scientific and engineering methodologies. With a keen emphasis on partnerships with academic, government, and commercial organizations and associations, BioLargo has proven itself by executing on challenging environmental engineering projects, demonstrating its powerful technologies through pilots, trials, and early commercial adoption, publishing high-impact academic and industry publications, and winning over 80 grants. We monetize our innovations through direct sales, recurring service contracts, licensing agreements, strategic joint venture formation and/or the sale of the IP.
The past year held a shift in focus at BioLargo toward the development and commercial execution of several key business initiatives with the potential to generate significant organizational and revenue growth for the company. Three of these projects in particular represent the dominant catalysts for near-term monetization of our core technologies and engineering services. Those are: 1) the advancement of our PFAS removal system, the Aqueous Electrostatic Concentrator (AEC), toward commercial trials with leading customers in the industry (including the federal government) most notably by the in-house piloting of the technology with client-provided water sources in preparation for commercial field trials which are organizing now, 2) the design, manufacture, pre-trial testing, and preparation for field trials with first customers of a novel “minimal liquid discharge” wastewater treatment system in partnership with Garratt-Callahan, the largest privately held water treatment company in America with more than 100 years history, and 3) the manufacture and successful launch of a new pet odor control product based on BioLargo’s intellectual properly launched by our partners at Ikigai Marketing Works, LLC, a venture aimed at building a nationally-branded disruptive new pet odor control brand for ultimate distribution into big-box retailers and sale to a national consumer products company.
Three main factors differentiate 2021 from the years that preceded it for BioLargo. First, we have built our credibility as cleantech technology innovators and environmental engineering service providers to the point where industry stakeholders, clients, and prospective partners rightfully view us as an effective and reliable means to solve their challenges. This has resulted in partners like Garratt-Callahan, Ikigai, and more approaching BioLargo for high-value projects. This “critical mass” of credibility as a cleantech solutions provider is a result of our investments in our talented team of engineers and scientists, our history of developing creative and powerful new technologies, and our track record of executing complex engineering projects. Secondly, in 2021 our core patented water treatment technologies, the BioLargo Advanced Oxidation System (AOS) and Aqueous Electrostatic Concentrator (AEC), were demonstrated in successful pilot projects, either on-site at a prospective client’s facility, or in-house with client-provided contaminated waters. Both of these technologies are now primed for commercialization and monetization. Third, our balance sheet continued to improve throughout 2021, with the near-elimination of debt to the point where only one $50,000 convertible note due in 2023 and $464,000 of covid-related low-interest U.S. Small Business Administration loans are owed, in addition to the elimination of debt owed by our partially owned subsidiary Clyra Medical Technologies.
Formula for Success: Technology, Talent and Purpose
Technology
BioLargo has continually advanced its robust portfolio of technologies since the first acquisition of early iterations of the BioLargo technology in the spring of 2007. Our innovations have primarily been developed through our internal resources, and some through acquisition. These include patents, patents pending, and trade secrets that include solutions for:
| |
●
|
Water decontamination, including:
|
| |
o
|
Removal of per- and poly-fluoroalkyl substances (PFAS) from drinking and ground water
|
| |
o
|
Micropollutant destruction and removal
|
| |
o
|
Legionella detection and water treatment solutions
|
| |
●
|
Air quality controls and systems including odor and VOC control
|
Talent
We have steadily grown our team to 27 team members and numerous other part-time consultants, including highly qualified PhDs, engineers, MDs and medical professionals, construction professionals, field service technicians, innovators, sales marketing specialists, entrepreneurial and executive leadership.
Purpose
Our mission to make life better drives us to serve others with integrity, knowledge, technology, and solutions that protect the environment, improve quality of life, and protect lives. All our technologies were developed from the ground-up to be sustainable, practical solutions to significant global challenges. We are unique in our ability to tailor our offerings to serve our customers with proven expertise, proven technology and, if needed, we often have the ability to develop new technical solutions to meet our customer’s needs.
Combating the PFAS Crisis – the AEC
Our engineers have developed a novel water treatment system, called the AEC (Aqueous Electrostatic Concentrator), that removes per- and poly-fluoroalkyl substances (PFAS) from water at a fraction of the cost of the most commonly used solutions (carbon filtration and reverse osmosis). PFAS chemicals have been linked to cancer, immune disorders, liver dysfunction, and multiple other health problems, are present in a vast range of manufactured goods, common household products (e.g., cleaning products, cookware), and electronics, and contaminate drinking water in unsafe levels all over the globe. The U.S. Geological Survey estimates that 20% of privately-owned water wells and 60% of publicly owned wells in the United States are contaminated with PFAS chemicals. In Orange County, California, where our corporate offices are located, more than 60 drinking water wells have been taken out of service due to PFAS contamination, and county officials estimate that treating the wells using existing technologies will cost more than $200 million in capital costs and more than $400 million in maintenance and operating costs (which include disposal of PFAS-laden filters or media), with a total cost over 30 years of more than $1 billion. Our technology can significantly reduce these costs, as it concentrates the PFAS chemicals onto special membranes, resulting in lower disposal costs because our technology generates approximately 1/1000 the amount of PFAS-laden waste by weight.
Governments and industry are actively seeking less expensive and/or more effective technologies and processes to eliminate PFAS from groundwater and drinking water. The U.S. Environmental Protection Agency (“EPA”) has made finding an economical solution a priority, announcing in February 2021 final regulatory determinations on the safe maximum levels of PFAS in drinking water, paving the way for regulating the chemicals through the Safe Drinking Water Act and creating a regulatory environment where municipalities will be required to install technologies that help remove PFAS from their drinking water supplies prior to distribution. In March 2021, a bipartisan group in the House of Representatives introduced a bill to regulate PFAS titled the PFAS Action Act of 2021; on July 21, 2021, the House passed the bill and sent it to the Senate, where it is still awaiting a final vote. As passed by the House, the proposed legislation would establish a national drinking water standard for select PFAS chemicals under the Safe Drinking Water Act, limit industrial discharges under the Clean Water Act, and provide $200 million annually to assist water utilities and treatment facilities to remove PFAS chemicals from their water. The bill would also restrict incineration of PFAS-containing wastes under the Clean Air Act, which could significantly limit the use of the most common treatment strategy: carbon filtration followed by carbon incineration. When PFAS-laden carbon is incinerated, not only does it produce vast volumes of greenhouse gases such as carbon dioxide, but evidence suggests volatile fluorochemicals like carbon tetrafluoride, hexafluoroethane, and hydrogen fluoride are released into the air which may have serious human health impacts on adjacent communities. Our technology can be disposed of ways other than incineration, and when it is incinerated, it is incinerated in a fraction of the volume of carbon filtration equivalents.
In October 2021, the EPA released its PFAS Strategic Roadmap, outlining the agency’s multifaceted strategy to regulate and remediate PFAS over the next three years and beyond. As part of their roadmap, the EPA announced their intent to regulate certain PFAS compounds as hazardous substances regulated under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), an action that would 1) make PFAS-contaminated sites eligible for Superfund funding, and 2) create more stringent disposal and handling requirements for PFAS compounds and PFAS-laden waste, such as that produced by certain water treatment technologies in the process of treatment. BioLargo anticipates that this regulatory action will have a disproportionately beneficial effect on the relative value proposition of our technology, which produces less PFAS-laden waste than other technologies. Also in October, the EPA began the process of creating rules that designate four of the worst-offending PFAS compounds as hazardous chemicals under the Resource Conservation and Recovery Act (RCRA), an action intended to empower the EPA to mandate the cleanup of sites found to be contaminated with those PFAS compounds.
There are three main markets for PFAS water treatment in the U.S., each of which we intend to address in the coming years. The first is municipal water treatment – that is water which needs treatment for PFAS before it can be distributed to the public or water that must be treated from a wastewater treatment plant. Southern California, Michigan and Wisconsin may be hotbeds for this market, as those states have adopted stringent regulations on PFAS limits in drinking water and wastewater, and municipalities in those states have been moving to adopt new PFAS treatment technologies more quickly than in other states. The second market is military bases, where the use of PFAS-containing fire-fighting foam has contaminated the soil and groundwater across the country. The third market is treatment for water intake or outfalls of industrial facilities that use PFAS compounds in manufacturing and other industrial processes. As our engineers are already providing environmental engineering services on U.S. Air Force bases, we believe that work may lead to sales in that arena. Estimates for the size of the overall PFAS water treatment market vary wildly, due largely to the fact that it is an emerging market, but most estimates place its size over the next ten years in the billions of dollars per year.
Selection and integration of new water and wastewater treatment equipment at a municipal facility, including for PFAS treatment, is often a multi-million-dollar decision with wide-ranging and long-lasting consequences. Our customer acquisition process for the AEC is therefore comprehensive and has the goal of assuring municipal or government decision-makers that the AEC is the right choice for their treatment needs, and that it will perform as-advertised over the course of its lifespan. To do this, our customer onboarding process is as follows: (1) off-site treatment of client-supplied water to quantify PFAS treatment by the AEC with their specific water chemistry, (2) on-site pilot treatment at client location to verify the AEC’s efficacy in real-world conditions, and (3) full-scale operation. The first step involves receiving contaminated water from potential clients, evaluate the properties of the water in order to ensure optimization of our equipment, treat the water with the AEC, and then send the water to an independent laboratory for analysis to confirm the PFAS molecules have been removed in accordance with Federal, State or client specifications. The first phase serves as a “proof of concept” to give the client confidence in moving to the next phase – installation of on-site pilot-level equipment to remove PFAS contaminates in real-world client setting. Once piloting is complete, BioLargo would offer customized commercial-scale systems to each client. Once our systems are operational at a customer location, we would then deliver ongoing maintenance and service as well as a disposal and component replacement program for the collected PFAS.
Testing of water provided by our first prospective PFAS treatment client, a large municipality in Southern California, has proven that BioLargo’s PFAS treatment technology can remove all PFAS compounds of regulatory interest to below their limits of quantification (i.e., “elimination”) while operating under acceptable parameters of flow rate and energy consumption. BioLargo’s engineers are finalizing a techno-economic report for the client that will be integral for establishing in-field trials at the client’s facilities, as well as at other prospective customers’ sites. Testing of water from a second prospective client, an agency of the Federal government, has also been done successfully, although the company is still awaiting final analytical data from the University of Tennessee to validate the results. In both cases, the in-house testing on client-provided waters is a key step in the client onboarding process for this technology, and management is confident that in-field piloting and commercial trials will follow.
We are currently working with a number of potential customers to analyze the cost/benefit of our AEC system as an alternative to competing solutions. We also are negotiating with two channel partners as well as a number of independent manufacturer’s representatives to develop a comprehensive selling and distribution network. We expect these efforts to accelerate as we demonstrate success in our early commercial projects.
ONM Environmental - Industrial Odor and VOC Solutions
ONM Environmental, Inc. is BioLargo’s subsidiary that delivers robust and comprehensive products and services to control and mitigate odor and volatile organic compounds (“VOCs”) emitted from a variety of industrial activities, including landfills and other waste handling facilities. Its flagship product, CupriDyne® Clean, reduces and eliminates tough odors and VOCs in various industrial settings. CupriDyne Clean is delivered through misting systems, sprayers, water trucks and similar water delivery systems designed, manufactured and installed by ONM. We believe the product is the number-one performing odor-control product in the market, and that it offers substantial savings to our customers compared with competing products. In response to customer demand for expanded services, ONM Environmental now holds General, Electrical, Plumbing and Low Voltage contractor licenses issued by the California Contractors State License Board, and offers a menu of services to landfills, transfer stations, wastewater treatment facilities as well as facilities in non-waste related industries. These services include engineering design, construction, installation, ongoing maintenance and on-site support services to assist our clients in the implementation and continued use of the various systems that deliver our liquid products in the field (such as misting systems).
ONM Environmental offers a deodorizing and sanitizing technology, called EcoMist®, that can be installed directly onto waste collection vehicles and automatically sprays odor control products and/or sanitizer into refuse bins or dumpsters during the waste collection process. EcoMist® is an “out-of-the-box” product, allowing customers to install the system themselves, and will thus not require a significant investment in logistics and servicing to support sales. ONM Environmental is focusing on selling the product to its current customers and garbage truck manufacturers.
We have been and expect to continue selling product to the largest solid waste handling companies in the country, with a significant portion of chemistry product sales resulting from national purchasing agreements (NPAs) with large waste handling companies. In the past quarter, ONM Environmental was awarded an exclusive three-year supply contract with a large municipality in Southern California for the delivery of CupriDyne Clean, which will provide a steady source of chemistry supply revenue for the company over the next three years.
In addition to growing its revenues organically through the sale of odor and VOC control chemistry and air quality control systems to its primary market segment (municipal solid waste handling in California), ONM Environmental aims to accelerate its growth through development of new sales and distribution channels. Some of these, including our partnership with Ikigai Marketing Works, LLC (see Consumer Products below) and our joint venture with BKT Co. Ltd. in South Korea (see South Korean Joint Venture) are already actively advancing toward their end-goal, which is to foster new distribution opportunities for our patented odor and VOC control chemistry without being limited by our own sales and distribution infrastructure. Additional new opportunities for distribution channels are presently being developed, including in new vertical market segments such as pulp and paper, wastewater, oil and gas, construction, and the auto industry, as well as in new geographical markets including South and Central America. Company management will provide more information on each of these emerging partnerships as they each become finalized.
Consumer Products
Ikigai Marketing Works, LLC, which was founded by accomplished industry executives from the consumer-packaged goods industry who have executed successful launches of at least five blockbuster products, has licensed from us a CupriDyne-based household pet odor removal product and a laundry additive for pet related odors. After development of television commercials and a successful test marketing campaign, they are beginning a national advertising campaign, and plan to launch the products in major retailers in the United States (e.g., Walmart, Target, etc.).
South Korean Joint Venture
On February 12, 2020, we executed a “Joint Venture Framework Agreement” with a leading wastewater treatment solution provider based in South Korea (BKT Co. Ltd., “BKT”), to create a South Korean entity that would manufacture odor and VOC control products based on our CupriDyne Clean products. We own 40% of the joint venture. Although the joint venture established manufacturing and is marketing the product, the COVID-19 pandemic significantly impacted the expected growth of the company, and continues to do so as the country of South Korea continues implementing measures to reduce infections. Management at Odin reports that while initial sales have required extraordinary efforts, continued testing and trial success has reinforced its confidence in continued expansion of sales for the future.
Full Service Environmental Engineering
Our subsidiary BioLargo Engineering, Science & Technologies, LLC (“BLEST”) offers full service environmental engineering to third parties and provides engineering support services to our internal teams to accelerate the commercialization of our technologies. Its website is found at www.BioLargoEngineering.com.
BLEST focuses its efforts in three areas:
| |
●
|
providing engineering services to third-party clients;
|
| |
●
|
supporting internal product development and business units’ services to customers (e.g., the AOS); and
|
| |
●
|
advancing their own technical innovations such as the AEC PFAS treatment technology
|
The subsidiary is located in Oak Ridge (a suburb of Knoxville, Tennessee), and employs seven scientists and engineers who collectively worked together for almost 30 years and experience in diverse engineering fields. The team is led by Randall Moore, who served as Manager of Operations for Consulting and Engineering for the Knoxville office of CB&I Environmental & Infrastructure and was formerly a leader at The Shaw Group, Inc., a Fortune 500 global engineering firm. The other team members are also former employees of CB&I and Shaw. The team is highly experienced across multiple industries and they are considered experts in their respective fields, including chemical engineering, wastewater treatment (including design, operations, data gathering and data evaluation), process safety, energy efficiency, air pollution, design and control, technology evaluation, technology integration, air quality management & testing, engineering management, permitting, industrial hygiene, applied research and development, air testing, environmental permitting, HAZOP review, chemical processing, thermal design, computational fluid dynamics, mechanical engineering, mechanical design, NEPDES permitting, RCRA/TSCA compliance and permitting, project management, storm water design & permitting, computer assisted design (CAD), bench chemistry, continuous emission monitoring system operator, data handling and evaluation and decommissioning and decontamination of radiological and chemical contaminated facilities. The engineering team also has developed an extended network of trusted engineering subcontractors that assist in serving specific client projects as needed, from time to time.
In association with Garratt-Callahan, a national industrial water treatment company, BLEST is developing a “minimal liquid discharge” wastewater treatment system based on Garratt-Callahan proprietary technology that would industrial wastewater discharge and therefore reduce wastewater discharge fees for customers. Garratt-Callahan plans to begin selling the new systems in early 2022. BLEST will serve as the manufacturing partner and Garratt Callahan will serve as the selling distributor to leverage their national sales force and over one hundred years of providing services and products to customers. In the past quarter, BioLargo’s engineers finished building the first full-scale prototype of this new technology and tested it with Garratt-Callahan client provided water, with Garratt-Callahan technical staff present on-site at BLEST’s facility. In this “factory acceptance” testing, the system removed over 98% of the target contaminants from water provided by a Garratt-Callahan client in continuous operation, in line with results achieved by Garratt-Callahan’s original bench-scale and batch processing tests. This factory acceptance testing was a necessary step before commercial trials with Garratt-Callahan customers can begin. The first customer has already been identified, and the first on-site field trial for that customer is currently being planned, starting with the design of a mobile treatment unit that will be installed at their facility. This work is underway.
In the second quarter of 2021, BLEST was notified of several important contract awards of significance that have the potential to increase the company’s operating cash flow and support the subsidiary’s role of commercializing BioLargo’s patented technologies. The new contract wins are for work with 1) a resin manufacturing facility, 2) a dairy farm, 3) a new project at Picatinny Arsenal, one of the company’s long-time customers, 4) a potato processing plant, and 5) a US Air Force base, adding to the other seven bases already served by the company. Together, the new contracts are worth more than $1.2 million in revenue. To-date, BLEST has completed the majority of these projects, with one of the larger projects having been delayed several months, now expected to complete in mid-2022.
Throughout 2021, BLEST’s revenues from contracts executed for external clients rose significantly, with both the third quarter and fourth quarters setting consecutive revenue records. This is largely a result of the large backlog of service contracts the subsidiary had built since the subsidiary’s inception, and the fact that the company has employed a larger number of 1099 contractors in 2021 to expand its engineering services capacity and execute backlogged projects.
New Technology – Mineral Extraction
BLEST developed a proprietary and patent-pending process to extract valuable minerals from certain types of industrial waste. A substantial long-term project for a client is in the planning stages, and we are working with the client in their efforts to secure project financing. If the project becomes financed, we are contracted to support the project as engineer/operator with a minority equity stake and also a profit sharing arrangement as we serve as the long term project manager. We believe our technology may have application to other similarly situated industrial waste sites which we intend to explore, although at this time, we have not dedicated budget or resources to pursue such opportunities.
BioLargo Water and the Advanced Oxidation System – AOS
BioLargo Water is our wholly owned subsidiary located in Edmonton, Alberta, Canada, that developed and is attempting to commercialize our Advanced Oxidation water treatment system (AOS). The AOS is our patented water treatment device that generates highly oxidative and energetic species of iodine and other molecules which allow it to rapidly and effectively eliminate pathogenic organisms and organic contaminants as water passes through the device. The key value proposition of the AOS is its ability to reduce or eliminate a wide variety of waterborne contaminants with high performance while using very little electricity and input chemicals. This is made possible by the highly oxidative iodine compounds and reactive oxygen species generated within the AOS reactor as well as the unique and proprietary physical constitution and geometry of the reactor. Our proof-of-concept studies and on-site pilot projects have generated results that project the AOS will be more cost- and energy-efficient than commonly used advanced water treatment technologies such as UV, electro-chlorination, and ozonation. Furthermore, our technology has been proven capable of removing hard-to-treat organic micropollutants such as pharmaceuticals from water more quickly and energy-efficiently than other technologies. Together, these characteristics make the AOS an economical and versatile tool to enable wastewater treatment and reuse in the face of emerging water contaminants and increasing regulatory scrutiny on industrial wastewater discharge.
BioLargo’s AOS water treatment technology has completed several pre-commercial demonstration pilots, including one at a poultry farm in Alberta, one at a microbrewery in Southern California, and another in Southern California where stormwater was treated by the AOS. It has an ongoing pilot near Montreal to treat municipal wastewater. It is our belief that once these pre-commercial pilots have concluded with the AOS, our ability to entice major water industry players to partner with BioLargo Water to accelerate market adoption of the AOS will be increased dramatically. Our team in Canada is in discussions with other potential early adopters in the agriculture space, and provincial grant funding which was committed to the Sunworks farm project has been allowed to be allocated toward a different prospective customer site.
Capabilities and characteristics of the BioLargo AOS have been published in peer-reviewed academic papers. In early 2021, the laboratory of Dr. Greg Goss, one of the company’s academic partners, published a paper about the technology’s ability to effectively remove micropollutants from wastewater and abrogate the negative biological effects of those micropollutants on aquatic life. The paper concluded that the AOS is “a promising and environmentally friendly technology for wastewater treatment, remediation, and management”. In June of 2021, the laboratory of Dr. Kimura-Hara published a paper about the safety of water treated by the AOS, specifically with regards to the low levels of disinfection by-products (DBPs) generated by the technology. The paper concluded that the levels of DBPs generated by the AOS were comparable to those found in ordinary tap water. Another recent and important peer-reviewed scientific paper was published confirming that AOS generates highly energetic iodine molecules, establishing the foundational scientific principles about why the AOS is a powerful, efficient, and novel water treatment technology. The company is actively working to publish additional peer-reviewed scientific papers, as these are crucial tools in marketing emerging water treatment technologies and aid decision-makers at customer and partner organizations in recognizing the technical and scientific merits of the technology relative to incumbents in the field.
Municipal Wastewater Treatment Pilot – Montreal
Our commercial-scale AOS demonstration pilot (run in partnership with acclaimed water experts at the Centre des Technologies de L’Eau) at a municipal wastewater treatment plant near Montreal, Quebec, is ongoing and providing important data that shows the AOS is removing five target pharmaceuticals from the wastewater faster and using less electricity than the ultraviolet disinfections system used in the facility. Notably, the pilot project also showed that the AOS was able to also remove total coliforms (bacteria) from the municipal wastewater more effectively than the UV disinfection system currently in use at the facility. Recently, a NSERC grant has extended the project to allow for use of a higher flow-rate AOS system, as well as removal of PFAS chemicals through the installation of a pilot AEC system.
Clyra Medical Technologies
Clyra Medical Technologies, Inc. is our partially owned subsidiary creating medical products based on our technology. It is launching a product to be used by surgeons generally, with a first target market aimed toward orthopedic surgeons for use as a wound irrigation solution and to help manage patient care and outcomes. Clyra has secured its first two hospital customers for the product, established a robust quality control system for FDA compliance, recruited a national director of sales, and is negotiating with three separate channel partners to form a commercial alliance. Its other product designs are on hold until such time as it is able to secure the capital and resources to complete any final development and support additional inventory, technical support and sales for these products. In March 2022, Clyra and BioLargo entered into an agreement with Scion Solutions, LLC, whereby the intellectual property (primarily, the SkinDisc) acquired in September 2018 was returned to Scion, and in exchange Scion forgave the outstanding principal and interest due on the promissory note owed by Clyra with an outstanding principal amount of $1,007,000, returned all its shares of Clyra common stock, and its two principals forgave $305,000 in accounts payable owed to them. In addition to the SkinDisc intellectual property, Scion received 2,000,000 of the 5,000,000 shares it had earned from the September 2018 sale of SkinDisc to Clyra/BioLargo.
Other Developing Relationships
We signed a “memorandum of understanding” which was later modified to a Referral Agreement with Aquaco Resources, Inc., a newly formed water company with a mission to provide agricultural property owners with a property action plan to reduce the risks of drought and climate change by managing water with water reserves and reuse and discharge compliance. The current agreements contemplate the sale of BioLargo products and engineering services to future Aquaco customers. We are presently engaged in project evaluations and are preparing for delivery our first commercial bid to supply a water treatment solution to a client that was developed by Aquaco.
EPA Registration of CupriDyne Plus
CupriDyne Plus has the potential to offer a safer, more environmentally friendly alternative to bleach and other common antimicrobials for applications like hard surface disinfection, sanitization of non-porous non-food contact surfaces, disinfection of air, textiles, and more. Based on our extensive scientific work, we are confident it meets the minimum performance requirements for EPA registration as a disinfectant and/or surface sanitizer.
We have met with and presented data to officials at the EPA for the purpose of refining our product and determining additional data requirements, and have retained a firm specializing in EPA registration work to help us through the process. Recently, we received a communication from the EPA providing clarity on an important factor in the EPA registration process – the number of “active ingredients” in our CupriDyne Plus formula we would be required to claim on our EPA application. Our regulatory consultant has been tasked to help determine the cost and time required to achieve an EPA registration for our CupriDyne Plus product.
Conclusion
BioLargo has advanced its technologies and infrastructure to achieve a critical mass to capitalize on its commercial efforts and have a positive impact around the world with clean water, clean air, and infection control solutions. The company presents a scalable business model that targets high-impact cleantech market opportunities. We leverage our considerable scientific, engineering, and entrepreneurial talent to monetize our technologies and ensure high-quality customer service and increased revenue potential. We seek to unlock the value of our portfolio of disruptive technologies to advance our mission to “make life better” and continue creating shareholder value.
Intellectual Property
We have 21 patents issued, including 19 in the United States, and multiple pending. We believe these patents provide a foundation from which to continue building our patent portfolio, and we believe that our technology is sufficiently useful and novel that we have a reasonable basis upon which to rely on our patent protections. We also rely on trade secrets and technical know-how to establish and maintain additional protection of our intellectual property. As our capital resources permit, we expect to expand our patent protection as we continue to refine our inventions as well as make new discoveries. See the detailed discussion below of our patent portfolio.
We regard our intellectual property as critical to our ultimate success. Our goal is to obtain, maintain and enforce patent protection for our products and technologies in geographic areas of commercial interest and to protect our trade secrets and proprietary information through laws and contractual arrangements.
Our Chief Science Officer, Mr. Kenneth R. Code, has been involved in the research and development of the technology since 1997. He has participated in the Canadian Federal Scientific Research and Experimental Development program, and he was instrumental in the discovery, preparation and filing of the first technology patents. He has worked with manufacturers, distributors and suppliers in a wide variety of industries to gain a full appreciation of the potential applications and the methodologies applicable to our technology for their manufacture and performance. He continues to research methods and applications to continue to expand the potential uses of our technology as well as work to uncover new discoveries that may provide additional commercial applications to help solve real world problems in the field of disinfection.
We incurred approximately $1,367,000 and 1,338,000 in expense related to our research and development activities in the years ended December 31, 2021 and 2020, respectively.
We believe that our suite of intellectual property covers the presently targeted major areas of focus for our licensing strategy. The description of our intellectual property, at present, is as follows:
U.S. Patents
● U.S. Patent 10,654,731, issued on May 19, 2020, 10,238,990, issued on March 26, 2019, and 10,051,866, issued on August 21, 2018, which protect our AOS system.
● U.S. Patent 10,046,078, issued on August 14, 2018, relating to the misting systems that eliminate odors in waste transfer stations, landfills, and other waste handling facilities.
● U.S. Patent 9,883,653 issued on February 8, 2018, which encompasses a litter composition used in the absorption of animal wastes.
● US Patent 9,414,601 granted August 16, 2016, relating to the use of an article for application to a surface to provide antimicrobial and/or anti-odor activity. At least one of the reagents is coated with a water-soluble, water dispersible or water-penetrable covering that prevents ambient conditions of 50% relative humidity at 25ºC from causing more than 10% of the total reagents exposed to the ambient conditions from reacting in a twenty-four hour period.
● U.S. Patent 8,846,067, issued on September 30, 2014, which encompasses a method of treating a wound or burn on tissue to reduce microbe growth about a wound comprising applying an antimicrobial composition to the wound or burn on tissue using a proprietary stable iodine gel or liquid. This patent covers our technology as used in products being developed by our subsidiary, Clyra Medical Technologies.
● U.S. Patent 8,757,253, issued on June 24, 2014, relating to the moderation of oil extraction waste environments.
● U.S. Patent 8,734,559, issued on May 27, 2014, relating to the moderation of animal waste environments.
● U.S. Patent 8,679,515 issued on March 25, 2014, titled “Activated Carbon Associated with Alkaline or Alkali Iodide,” which provides protection for our BioLargo® AOS filter.
● U.S. Patent 8,642,057, issued on February 14, 2014, titled “Antimicrobial and Antiodor Solutions and Delivery Systems,” relating to our liquid antimicrobial solutions, including our gels, sprays and liquids imbedded into wipes and other substrates.
● U.S. Patent 8,574,610, issued on November 5, 2013, relating to flowable powder compositions, including our cat litter additive.
● U.S. Patent 8,257,749, issued on September 4, 2012, relating to the use of our technology as protection of against antimicrobial activity in environments that need to be protected or cleansed of microbial or chemical material. These environments include closed and open environments and absorbent sheet materials that exhibit stability until activated by aqueous environments. The field also includes novel particle technology, coating technology or micro-encapsulation technology to control the stability of chemicals that may be used to kill or inhibit the growth of microbes to water vapor or humidity for such applications.
● U.S. Patent 8,226,964, issued on July 24, 2012, relating to use of our technology as a treatment of residue, deposits or coatings within large liquid carrying structures such as pipes, drains, ducts, conduits, run-offs, tunnels and the like, using iodine, delivered in a variety of physical forms and methods, including using its action to physically disrupt coatings. The iodine’s disruptive activity may be combined with other physical removal systems such as pigging, scraping, tunneling, etching or grooving systems or the like.
● U.S. Patent 8,021,610, issued on September 20, 2011, titled “System providing antimicrobial activity to an environment,” relating to the reduction of microbial content in a land mass. Related to this patent are patents held in Canada and the European Union.
● U.S. Patent 7,943,158, issued on May 17, 2011, titled “Absorbent systems providing antimicrobial activity,” relating to the reduction of microbial content by providing molecular iodine to stabilized reagents.
● U.S. Patent 7,867,510, issued on January 11, 2011, titled “Material having antimicrobial activity when wet,” relating to articles for delivering stable iodine-generating compositions.
Pending Patent Applications
Subject to adequate financing, we intend to continue to expand and enhance our suite of intellectual property through ongoing focus on product development, new intellectual property development and patent applications, and further third-party testing and validations for specific areas of focus for commercial exploitation. We currently anticipate that additional patent applications will be filed during the next 12 months with the USPTO and the PCT, although we are uncertain of the cost of such patent filings, which will depend upon the number of such applications prepared and filed. The expense associated with seeking patent rights in multiple foreign countries is expensive and will require substantial ongoing capital resources. However, we cannot give any assurance that adequate capital will be available. Without adequate capital resources, we will be forced to abandon patent applications and irrevocably lose rights to our technologies.
Our Company
BioLargo, Inc. is a corporation organized under the laws of the state of Delaware. Our common stock is quoted on the OTC Markets OTCQB “Venture Marketplace” under the trading symbol “BLGO”.
Our corporate offices are located at 14921 Chestnut St., Westminster, California 92683. We have a research facility and offices at the University of Alberta in Canada, and our engineering team is located at 105 Fordham Road in Oak Ridge, Tennessee. Our telephone number is (888) 400-2863. We operate through multiple wholly-owned subsidiary entities, including: BioLargo Life Technologies, Inc., to hold our intellectual property; ONM Environmental, Inc., to manufacture, market, sell and distribute our odor control products; BioLargo Water Investment Group, Inc., which is the sole owner of a Canadian subsidiary, BioLargo Water, Inc., for our Canadian research and development and AOS commercialization operations; and BioLargo Development Corp., through which our employees are employed. Additionally, we own 89% of BioLargo Engineering, Science & Technologies, LLC, our full service engineering firm in Oak Ridge, Tennessee, and 56% of Clyra Medical Technologies, Inc., formed to develop and market medical products based on our technology.
Our principal corporate website is www.BioLargo.com. We also maintain a blog at www.BioLargo.blogspot.com. Websites concerning our subsidiaries are www.ONMEnvironmental.com, www.CupriDyne.com, www.ClyraMedical.com, www.BioLargoWater.com, and www.BioLargoEngineering.com. The information on our websites and blog are not, and shall not be deemed to be, a part of this Annual Report on Form 10-K.
Executive Officers
As of December 31, 2021, and as the date of this report, our executive officers were:
| |
●
|
Dennis P. Calvert: Chief Executive Officer, President and Chairman of the Board
|
| |
●
|
Charles K. Dargan II: Chief Financial Officer
|
| |
●
|
Joseph L. Provenzano: Corporate Secretary and Vice President of Operations
|
Our operational subsidiaries are led by:
|
Subsidiary
|
President
|
|
ONM Environmental, Inc.
|
Joseph L. Provenzano
|
|
BioLargo Engineering, Science & Technologies, LLC
|
Randall Moore
|
|
BioLargo Water, Inc. (Canada)
|
Richard Smith
|
|
Clyra Medical Technologies, Inc.
|
Steven V. Harrison
|
Employees
As of December 31, 2021, we had 27 full time employees. Our employees including professional engineers, masters of engineering, and PhDs, as well as sales, support and administrative personnel. We also utilize consultants and independent contractors on an as-needed basis who provide certain specified services, such as professional engineers used from time to time by our engineering group in Tennessee.
Competition
We believe that our products contain unique characteristics that distinguish them from competing products. In spite of these unique characteristics, our products face competition from products with similar prices and similar claims. We face stiff competition from companies in all of our market segments, and many of our competitors are larger, better-capitalized, sell under valuable and long-established brands, and have more industry experience.
For example, we would compete with the following leading companies in our respective markets:
| |
●
|
Disinfecting/Sanitizing: Johnson & Johnson, BASF Corporation, Dow Chemical Co., E.I. DuPont De Nemours & Co., Chemical and Mining Company of Chile, Inc., Proctor and Gamble Co., Diversey, Inc., EcoLab, Inc., Steris Corp., Clorox, and Reckitt Benckiser.
|
| |
●
|
Water Treatment: GE Water, Trojan UV, Ecolab, Pentair, Xylem and Siemens AG.
|
| |
●
|
Medical Markets: Smith & Nephew, 3M, ConvaTec and Derma Sciences.
|
| |
●
|
Industrial Odor Control: MCM Odor Control and OMI Industries.
|
Each of these named companies and many other competitors are significantly more capitalized than we are and have many more years of experience in producing and distributing products.
Additionally, our technology and products incorporating our technology must compete with many other applications and long embedded technologies currently on the market (such as, for example, chlorine for disinfection).
In addition to the competition we face for our existing products, we are aware of other companies engaged in research and development of other novel approaches to applications in some or all the markets identified by us as potential fields of application for our products and technologies. Many of our present and potential competitors have substantially greater financial and other resources and larger research and development staffs than we have. Many of these companies also have extensive experience in testing and applying for regulatory approvals.
Finally, colleges, universities, government agencies, and public and private research organizations conduct research and are becoming more active in seeking patent protection and licensing arrangements to collect royalties for the use of technology that they have developed, some of which may be directly competitive with our applications.
Governmental Regulation
We will have products (each, a ‘‘Medical Device”) that will be subject to the Federal Food, Drug, and Cosmetic Act, as amended (including the rules and regulations promulgated thereunder, the “FDCA”), or similar Laws (including Council Directive 93/42/EEC concerning medical devices and its implementing rules and guidance documents) in any foreign jurisdiction (the FDCA and such similar Laws, collectively, the “Regulatory Laws”) that are developed, manufactured, tested, distributed or marketed by our company or its subsidiary Clyra. Each such Medical Device will need to be developed, manufactured, tested, distributed, and marketed in compliance with all applicable requirements under the Regulatory Laws, including those relating to investigational use, premarket clearance or marketing approval to market a medical device, good manufacturing practices, labeling, advertising, record keeping, filing of reports and security, and in compliance with the Advanced Medical Technology Association Code of Ethics on Interactions with Healthcare Professionals.
We believe that no article or part of any Medical Device intended to be manufactured or distributed by our company or any of our subsidiaries will be classified as (i) adulterated within the meaning of Sec. 501 of the FDCA (21 U.S.C. § 351) (or other Regulatory Laws), (ii) misbranded within the meaning of Sec. 502 of the FDCA (21 U.S.C. § 352) (or other Regulatory Laws) or (iii) a product that is in violation of Sec 510 of the FDCA (21 U.S.C. § 360) or Sec. 515 of the FDCA (21 U.S.C. § 360e) (or other Regulatory Laws).
Neither our company nor any of its subsidiaries, nor, to the knowledge of our company, any officer, employee or agent of our company or any of its subsidiaries, has been convicted of any crime or engaged in any conduct for which such Person or entity could be excluded from participating in the federal health care programs under Section 1128 of the Social Security Act of 1935, as amended (the “Social Security Act”), or any similar Law in any foreign jurisdiction.
We may also have products for which we will desire to make claims that come within the purview of the U.S. Environmental Protection, and therefore require their permission.
Description of Property
Our company owns no real property. We currently lease approximately 9,000 square feet of office and industrial space at 14921 Chestnut Street, Westminster, California. In addition to serving as our principal offices, it is also a manufacturing facility where we manufacture our products, including our CupriDyne Clean Industrial Odor control product, and the home of our subsidiary ONM Environmental.
We also lease approximately 13,000 square feet of office and warehouse space at 105 Fordham Road, Oak Ridge, Tennessee, for our professional engineering division, BioLargo Engineering, Science & Technologies, LLC.
We also lease approximately 1,500 square feet of office and lab space from the University of Alberta. These offices serve as our primary research and development facilities and is the home of our subsidiary, BioLargo Water.
Our telephone number is (888) 400-2863.
Legal Proceedings
Our company is not a party to any material legal proceeding.
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This discussion contains forward-looking statements about our business and operations. Our actual results may differ materially from those we currently anticipate as a result of many factors, including those we described under “Risk Factors” and elsewhere in this prospectus. Certain statements contained in this discussion, including, without limitation, statements containing the words “believes,” “anticipates,” “expects” and the like, constitute “forward-looking statements” within the meaning of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). However, as we will issue “penny stock,” as such term is defined in Rule 3a51-1 promulgated under the Exchange Act, we are ineligible to rely on these safe harbor provisions. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any of the future results, performance or achievements expressed or implied by such forward-looking statements. Given these uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements. We disclaim any obligation to update any of such factors or to announce publicly the results of revision of any of the forward-looking statements contained herein to reflect future events or developments. For information regarding risk factors that could have a material adverse effect on our business, refer to the “Risk Factors” section of this prospectus beginning on page 4.
Results of Operations—Comparison of the years ended December 31, 2021 and 2020
We operate our business in distinct business segments:
| |
●
|
ONM Environmental, which manufactures and sells our odor and VOC control products and services, including our flagship product, CupriDyne Clean;
|
| |
●
|
BLEST, our professional engineering services division supporting our internal business units, advancing innovations like the AEC to remove PFAS contaminants from water, and serving outside clients on a fee for service basis;
|
| |
●
|
Clyra Medical, our partially owned subsidiary which develops and sells medical products based on our technology; and
|
| |
●
|
BioLargo Water, our Canadian division that has been historically pure research and development, and is now transitioning to focus on commercializing our AOS system and supporting the work to advance CupriDyne technology-based products through an EPA registration;
|
| |
●
|
Our corporate operations, which support the operating segments with legal, accounting, human resources, and other services.
|
Consolidated revenue for the year ended December 31, 2021 was $2,531,000, which is a 4% increase over the same period in 2020. Our service revenue increased 58%, while revenue from product sales and related services decreased by 14%. Our product revenue includes sales of our CupriDyne Clean industrial odor control product, Clyraguard Personal Protection Spray, and hand sanitizers, which were affected by the COVID-19 pandemic. While we expect overall revenues to continue to increase, given the considerable extended time of the COVID-19 pandemic, we cannot be certain.
ONM Environmental
Our wholly-owned subsidiary ONM Environmental generates revenues through sales of our flagship product CupriDyne Clean, by providing design, installation, and maintenance services on the systems that deliver CupriDyne Clean at its clients’ facilities, and through sales of odor absorption products to the U.S. Government. During 2020, ONM Environmental added two employees to focus on business development, increasing sales and increased levels of construction and maintenance contracts. During 2021, ONM reduced staff in line with the reduction in sales.
Revenue (ONM Environmental)
ONM Environmental’s revenues for the year ended December 31, 2021, were $1,419,000, a decrease of $148,000 or 9% from the same period in 2020. ONM Environmental’s fourth quarter revenue were approximately $360,000, a decrease of 42% over the prior quarter due to the installation of large custom CupriDyne Clean misting systems in that quarter. Of its gross sales in 2021, approximately two-thirds were to the waste handling industry. We expect ONM’s revenues from product sales to increase in the year ended December 31, 2022, due to an anticipated increase in the volume of consumer product sales.
Cost of Goods Sold (ONM Environmental)
ONM Environmental’s cost of goods sold includes costs of raw materials, contract manufacturing, and portions of depreciation, salaries and expenses related to the manufacturing and installation of its products. As a percentage of revenue, ONM Environmental’s costs of goods increased 9% in 2021 to 47%. The increase in cost of goods is due to increase in raw material costs.
Selling, General and Administrative Expense (ONM Environmental)
ONM Environmental’s selling, general and administrative expenses increased by 13% to $1,235,000 during the year ended December 31, 2021. These expenses decreased due to a reduction of sales and support staff. We expect these expenses to remain consistent in the year ending December 31, 2022.
Operating Loss (ONM Environmental)
ONM Environmental generated $1,419,000 in revenue, a gross margin of $724,000, and had total costs and expenses of $1,235,000, resulting in an operating loss of $511,000, compared with $493,000 in 2020.
BLEST (engineering division)
Revenue (BLEST)
Our engineering segment (BLEST) generated $961,000 of revenue in 2021, net of intersegment revenue, compared to $615,000 in 2020, representing a 56% increase from the prior year. The increase is due to an increased number of client contracts, including those as a subcontractor for Bhate pursuant to which BLEST is providing services to U.S. Air Force bases.
In addition to providing service to third party clients, BLEST provides services to BioLargo and its subsidiaries for internal BioLargo projects. These services are billed internally, are considered intersegment revenue, and are eliminated in the consolidation of our financial statements. In the year ended December 31, 2021, it totaled $674,000, primarily used to further engineer and develop our flagship AOS water filtration system and our AEC PFAS treatment system. In addition, BLEST engineers are performing a critical role in the AOS pilot projects, some of which are supported by third-party research grants and has been instrumental in developing and supporting a professional engineered design service for misting systems being sold by our ONM operating unit.
Cost of Goods (Services) Sold (BLEST)
BLEST’s cost of services includes employee labor as well as subcontracted labor costs. In 2021, its cost of services were 69% of its revenues, versus 77% in 2020. This decrease is due to contracts with better margins. We expect the cost of services to remain consistent in 2022 based on the contracts currently in progress.
Selling, General and Administrative Expense (BLEST)
BLEST SG&A expenses were $441,000 in 2021, compared to $413,000 in 2020. We expect these expenses to remain flat in 2022.Increases in engineering staff are included in cost of services.
Operating Loss (BLEST)
BLEST generated $961,000 in revenue from third parties, a gross margin of $300,000, and had total costs and expenses of $929,000, resulting in an operating loss of $629,000, compared with an operating loss of $619,000 in 2020.
BLEST provides substantial support to BioLargo’s other operations, including BioLargo Water and Odor-No-More. While we are unable to record revenues generated from services by the engineering group to other BioLargo operating divisions for important project such as the development of the AOS and AEC technologies, it is important to note that its net loss would be eliminated if it were selling these services to a third party at fair market value.
Because the subsidiary had a net loss, we invested cash during the year to allow it to maintain operations. BLEST’s need for a cash subsidy to support its operations has decreased over time. We expect that in 2022 its sales and thus its gross profit will continue to increase. Our goal for this operation is that it produces a profit and contributes to corporate overhead in a significant way, although predicting when that will happen given the COVID-19 pandemic and other uncertainties in the market, and our limited resources, is difficult.
Other Income
Primarily through our wholly owned Canadian subsidiary, we have been awarded more than 80 research grants over the years from various public and private agencies, including the Canadian National Research Institute – Industrial Research Assistance Program (NRC-IRAP), the National Science and Engineering Research Council of Canada (NSERC), and the Metropolitan Water District of Southern California’s Innovative Conservation Program “ICP”. The research grants received are considered reimbursement grants related to costs we incur and therefore are included as Other Income. The amount of grant income decreased $82,000 in the year ended December 31, 2021, to $55,000. Grant funds paid directly to third parties are not included as income in our financial statements.
Our Canadian subsidiary applied for and received a refund on our income taxes pursuant to the “Scientific Research and Experimental Development (SR&ED) Program”, a Canadian federal tax incentive program designed to encourage Canadian businesses to conduct research and development in Canada. For the years ended December 31, 2021 and 2020, we received a refund of $20,000 and $110,000, respectively.
The U.S. Small Business Administration forgave a Paycheck Protection Program loan in the principal amount of $43,000 granted to our partially owned subsidiary, Clyra Medical.
Although we are continuing to apply for government and industry grants, and indications from the various grant agencies is highly encouraging, we cannot be certain of continuing those successes in the future. We are very active in both the US and Canada, pursuing grant support for various uses of our products that we believe can help in managing the COVID-19 crisis.
Selling, General and Administrative Expense – consolidated
Our Selling, General and Administrative expense (“SG&A”) include both cash (for example, salaries to employees) and non-cash expenses (for example, stock option compensation expense). Our consolidated SG&A decreased in the aggregate by 17% ($1,301,000) in the year ended December 31, 2021, to $6,172,000. Our non-cash expenses (through the issuance of stock and stock options) were relatively flat 2021 compared with 2020 ($2,241,000 compared to $2,232,000). The largest components of our SG&A expenses included (in thousands):
| |
|
Year ended December 31, 2021
|
|
|
Year ended December 31, 2020
|
|
|
Salaries and payroll related
|
|
$ |
2,581 |
|
|
$ |
2,855 |
|
|
Professional fees
|
|
|
662 |
|
|
|
859 |
|
|
Consulting
|
|
|
920 |
|
|
|
1,624 |
|
|
Office expense
|
|
|
1,177 |
|
|
|
1,207 |
|
|
Board of director expense
|
|
|
262 |
|
|
|
259 |
|
|
Sales and marketing
|
|
|
315 |
|
|
|
494 |
|
|
Investor relations
|
|
|
255 |
|
|
|
175 |
|
Our salaries and payroll-related and office-related expenses decreased in the year ended December 31, 2021, due to a reduction of sales personnel and office staff at ONM Environmental. Consulting expense decreased due primarily to a reduction in consultants at Clyra as its business focused shifted to its surgical wash product. There was a decline in professional fees and sales and marketing as Biolargo as Biolargo reduced its efforts in those areas in order to control costs.
Research and Development
In the year ended December 31, 2021, we spent $1,367,000 in the research and development of our technologies and products. This was an increase of 2% ($29,000) compared to 2020.
Interest expense
Our interest expense for the year ended December 31, 2021, was $234,000, a decrease of 88% compared with 2020. The significant decrease in interest expense is related to the significant decrease of our debt obligations and a reduction of debt issued during 2021 versus 2020. Of our total interest expense in 2021, $99,000 was paid in cash, and the remainder, $135,000, was paid by issuing shares of our common stock. Our non-cash interest related expenses were comprised primarily of $119,000 in non-cash debt discounts related to warrants issued in conjunction with debt instruments being amortized over the life of the debt instrument; for the year ended December, 2022, non-cash interest expense totaled $1,618,000.
Our outstanding debt as of December 31, 2021, was lower than as of December 31, 2020. We expect our interest expense in the year ending December 31, 2022, to be in line with the prior year, provided we do not issue debt with attached warrants during the remainder of the year. Additionally, we record the relative fair value of the warrants and the intrinsic value of the beneficial conversion feature sold with the convertible notes payable which typically results in a full discount on the proceeds from the convertible notes. This discount is amortized as interest expense over the term of the convertible notes. We also are currently selling units of common stock and warrants instead of using convertible debt for financing our working capital needs, which if continued, will continue to reduce our ongoing interest expense as compared with prior years.
Net Loss
Net loss for the year ended December 31, 2021, was $6,894,000 a loss of $0.03 per share, compared to a net loss for the year ended December 31, 2020, of $9,700,000 a loss of $0.05 per share. Our net loss this year declined because of reduction in sales, general and administrative costs, and in interest expense.
The net income (loss) per business segment is as follows (in thousands):
|
Net income (loss)
|
|
Year ended December 31, 2021
|
|
|
Year ended December 31, 2020
|
|
|
ONM Environmental
|
|
$ |
(511 |
) |
|
$ |
(483 |
) |
|
BLEST
|
|
|
(629 |
) |
|
|
(619 |
) |
|
Clyra Medical
|
|
|
593 |
|
|
|
(2,139 |
) |
|
BioLargo Water
|
|
|
(566 |
) |
|
|
(466 |
) |
|
BioLargo corporate
|
|
|
(5,781 |
) |
|
|
(5,993 |
) |
|
Consolidated net loss
|
|
$ |
(6,894 |
) |
|
$ |
(9,700 |
) |
In the year ended December 31, 2021, approximately 40% of our net loss was due to non-cash expenses, including $135,000 in interest expense, $1,872,000 of stock option compensation expense, and $367,000 of services paid by the issuance of our common stock. Clyra Medical’s net income of $593,000 during the year ended December 31, 2021, was not due to operating activities, but rather due to the recent transaction with Scion Solutions (see Note 9).
In the year ended December 31, 2020, over half of our net loss is due to non-cash expenses, such as interest and stock/stock options issued to employees and vendors in lieu of cash. Of the net loss of $9,700,000, interest expense was $1,923,000, of which $1,805,000 was non-cash expense. Additionally, we recorded $2,459,000 of stock option compensation expense, an additional $666,000 of services were paid by the issuance of our common stock and we recorded $442,000 loss on extinguishment of debt. The total of these non-cash items account for $5,372,000 of the consolidated loss of $9,700,000.
We believe that ONM and BLEST (engineering) can achieve positive cash flow from operations at some point in the future, although predicting when that will happen given the COVID-19 pandemic and other uncertainties in the market, and our limited capital resources, is difficult. We expect to continue to incur a net loss for the foreseeable future.
Liquidity and Capital Resources
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of our business. For the year ended December 31, 2021, we had a net loss of $6,894,000, used $3,937,000 cash in operations, and at December 31, 2021, we had working capital of $427,000, and current assets of $1,801,000. We are unable to rely on historical results or current contracts that would be sufficient to predict that sales and gross profits in 2022 will be sufficient to fund our current level of operations or pay our debts as they become due during the next 12 months, and therefore we will have to obtain further investment capital to continue to fund operations and seek to refinance our existing debt. We have been, and anticipate that we will continue to be, limited in terms of our capital resources.
During the year ended December 31, 2021, we generated revenues of $2,531,000 through our subsidiaries. (See Note 12.) Our segments did not individually or in the aggregate generate enough revenues or gross profits to fund their operations, or fund our corporate operations or other business segments. Thus, to operate throughout 2021, we continued to sell securities to raise cash (see Notes 3 and 10), and were able to borrow money through programs administered by the Small Business Administration.
As of December 31, 2021, our cash and cash equivalents totaled $962,000. Our liabilities included $50,000 in debt that is due at the March 2023 maturity date, $314,000 due in SBA PPP loans (see Note 14), and $150,000 due to the SBA pursuant to an EIDL loan, payable in monthly installments of $800 beginning July 2023. Additionally, $187,000 owed by our partially owned subsidiary Clyra Medical Technologies, Inc. (“Clyra”) is due in June 2023.
Subsequent to December 30, 2021, we have received approximately $345,000 in gross and net proceeds from sales of our common stock to Lincoln Park. The proceeds we receive from stock sales to Lincoln Park is a function of stock price and volume – a lower stock price and less trading volume results in less money we can receive from Lincoln Park. Our agreement with Lincoln Park precludes us from selling shares to Lincoln Park on a daily basis if our stock price falls below $0.10 per share.
If we are unable to rely on our current arrangement with Lincoln Park to fund our working capital requirements, we will have to rely on other forms of financing, and there is no assurance that we will be able to do so, or if we do so, it will be on favorable terms. To reduce our operational cash burdens, we regularly issue officers and vendors stock or options in lieu of cash, and anticipate that we will continue to be able to do so in the future.
The foregoing factors raise substantial doubt about our ability to continue as a going concern, unless we are able to continue to raise funds through stock sales to Lincoln Park or other private financings, and in the long term, our ability to attain a reasonable threshold of operating efficiencies and achieve profitable operations by licensing or otherwise commercializing products incorporating our technologies. The consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
We operate our business in five distinct business segments. Each of these segments obtains cash to fund operations in unique ways. ONM and BLEST generate cash by selling products and services. Clyra Medical obtains cash from revenues, and third-party investments of sales of its common stock. BioLargo Water generates cash through government research grants and tax credits. Our corporate operations generate cash through private offerings of stock, debt instruments, and warrants. Cash was generated as follows (in thousands):
| |
|
Year ended December 31, 2021
(in thousands)
|
|
|
Year ended December 31, 2020
(in thousands)
|
|
|
SOURCES OF CASH
|
|
|
|
|
|
|
|
|
|
Revenue from operations
|
|
$ |
2,531 |
|
|
$ |
2,432 |
|
|
Grant income
|
|
|
55 |
|
|
|
137 |
|
|
Tax credit income
|
|
|
20 |
|
|
|
111 |
|
|
Stock for cash (BioLargo)
|
|
|
4,882 |
|
|
|
2,783 |
|
|
Stock for cash (Clyra Medical)
|
|
|
50 |
|
|
|
851 |
|
|
Proceeds from warrant exercise (BioLargo)
|
|
|
164 |
|
|
|
-- |
|
|
Debt (BioLargo)
|
|
|
-- |
|
|
|
507 |
|
|
Debt (Clyra Medical)
|
|
|
-- |
|
|
|
260 |
|
|
Total:
|
|
$ |
7,745 |
|
|
$ |
7,081 |
|
Although ONM Environmental, BLEST, and Clyra Medical generated revenues in the year ended December 31, 2021, each incurred an operating loss. We provided cash subsidies to each of these business segments to allow them to continue operations.
Critical Accounting Policies
Our discussion and analysis of our results of operations and liquidity and capital resources are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates and judgments, including those related to revenue recognition, valuation of offerings of debt with equity or derivative features which include the valuation of the warrant component, any beneficial conversion feature and potential derivative treatment, and share-based payments. We base our estimates on anticipated results and trends and on various other assumptions that we believe are reasonable under the circumstances, including assumptions as to future events. These estimates form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. By their nature, estimates are subject to an inherent degree of uncertainty. Actual results that differ from our estimates could have a significant adverse effect on our operating results and financial position. We believe that the following significant accounting policies and assumptions may involve a higher degree of judgment and complexity than others.
The methods, estimates and judgments the Company uses in applying these most critical accounting policies have a significant impact on the results of the Company reports in its financial statements.
Revenue Recognition
We adopted ASU 2014-09, “Revenue from Contracts with Customers”, Topic 606, on January 1, 2018. The guidance focuses on the core principle for revenue recognition.
The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps:
Step 1: Identify the contract(s) with a customer.
Step 2: Identify the performance obligations in the contract.
Step 3: Determine the transaction price.
Step 4: Allocate the transaction price to the performance obligations in the contract.
Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
We have revenue from two subsidiaries, ONM and BLEST. ONM identifies its contract with the customer through a written purchase order, in which the details of the contract are defined including the transaction price and method of shipment. The only performance obligation is to create and ship the product and each product has separate pricing. ONM recognizes revenue at a point in time when the order for its goods are shipped if its agreement with the customer is FOB ONM’s warehouse facility, and when goods are delivered to its customer if its agreement with the customer is FOB destination. Revenue is recognized with a reduction for sales discounts, as appropriate and negotiated in the customer’s purchase order. ONM also installs misting systems for which it bills on a time and materials basis. It identifies its contract with the customer through a written purchase order in which the details of the time to be billed and materials purchased and an estimated completion date. The performance obligation is the completion of the installation. Revenue is recognized in arrears as the work is performed.
BLEST identifies services to be performed in a written contract, which specifies the performance obligations and the rate at which the services will be billed. Each service is separately negotiated and priced. Revenue is recognized as services are performed and completed. BLEST’s contracts typically call for invoicing for time and materials incurred for that contract. A few contracts have called for milestone or fixed cost payments where BLEST bills an agreed-to amount per month for the life of the contract. In these instances, completed work, billed hourly, is recognized as revenue. If the billing amount is greater or lesser than the completed work, a receivable or payable is created. These accounts are adjusted upon additional billings as the work is completed. To date, there have been no discounts or other financing terms for the contracts.
Warrants
Warrants issued with our convertible and non-convertible debt instruments are accounted for under the fair value and relative fair value method.
The warrant is first analyzed per its terms as to whether it has derivative features or not. If the warrant is determined to be a derivative and not qualify for equity treatment, then it is measured at fair value using the Black Scholes option model, and recorded as a liability on the balance sheet. The warrant is re-measured at its then current fair value at each subsequent reporting date (it is “marked-to-market”).
If the warrant is determined to not have derivative features, it is recorded into equity at its fair value using the Black Scholes option model, however, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the convertible note.
Convertible debt instruments are recorded at fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the warrant. Further, the convertible debt instrument is examined for any intrinsic beneficial conversion feature (“BCF”) of which the conversion price is less than the closing common stock price on date of issuance. If the relative fair value method is used to value the convertible debt instrument and there is an intrinsic BCF, a further analysis is undertaken of the BCF using an effective conversion price which assumes the conversion price is the relative fair value divided by the number of shares the convertible debt is converted into by its terms. The BCF value is accounted for as equity.
The warrant and BCF relative fair values are also recorded as a discount to the convertible promissory notes. At present, these equity features of the convertible promissory notes have recorded a discount to the convertible notes that is substantially equal to the proceeds received.
Share-based Payments
It is the Company’s policy to expense share-based payments as of the date of grant or over the term of the vesting period in accordance with Auditing Standards Codification Topic 718 “Share-Based Payment.” Application of this pronouncement requires significant judgment regarding the assumptions used in the selected option pricing model, including stock price volatility and employee exercise behavior. Most of these inputs are either highly dependent on the current economic environment at the date of grant or forward-looking expectations projected over the expected term of the award.
Fair Value Measurement
Generally accepted accounting principles establishes a hierarchy to prioritize the inputs of valuation techniques used to measure fair value. The hierarchy gives the highest ranking to the fair values determined by using unadjusted quoted prices in active markets for identical assets (Level 1) and the lowest ranking to fair values determined using methodologies and models with unobservable inputs (Level 3). Observable inputs are those that market participants would use in pricing the assets based on market data obtained from sources independent of the Company. Unobservable inputs reflect the Company’s assumptions about inputs market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. The Company has determined the appropriate level of the hierarchy and applied it to its financial assets and liabilities.
Management believes the carrying amounts of the Company’s financial instruments as of December 31, 2021 and 2020, approximate their respective fair values because of the short-term nature of these instruments. Such instruments include cash, accounts receivable, prepaid assets, and accounts payable.
Recent Accounting Pronouncements
See Note 2 to the Consolidated Financial Statements, “Summary of Significant Accounting Policies – Recent Accounting Pronouncements”, for the applicable accounting pronouncements affecting the Company.
MANAGEMENT
Executive Officers and Directors
The following table sets forth information about our executive officers and directors as of the date of this prospectus:
|
Name
|
|
Position with Company
|
|
Age
|
|
Director Since
|
|
Dennis P. Calvert
|
|
President, Chief Executive Officer, Chairman, and Director
|
|
59
|
|
June 2002
|
|
Kenneth R. Code
|
|
Chief Science Officer, Director
|
|
75
|
|
April 2007
|
|
Charles K. Dargan II
|
|
Chief Financial Officer
|
|
66
|
|
February 2008
|
|
Dennis E. Marshall(2)(3)(4)
|
|
Director
|
|
79
|
|
April 2006
|
|
Joseph L. Provenzano
|
|
Vice President of Operations, Corporate Secretary and Director
|
|
53
|
|
June 2002
|
|
Kent C. Roberts, II(1)(3)
|
|
Director
|
|
62
|
|
August 2011
|
|
John S. Runyan(1)(5)(6)
|
|
Director
|
|
83
|
|
October 2011
|
|
Jack B. Strommen
|
|
Director
|
|
52
|
|
June 2017
|
|
(1)
|
Member of Audit Committee
|
|
(2)
|
Member of Compensation Committee
|
|
(3)
|
Member of Nominating and Corporate Governance Committee
|
|
(4)
|
Chairman of Audit Committee
|
|
(5)
|
Chairman of Compensation Committee
|
|
(6)
|
Chairman of Nominating and Corporate Governance Committee
|
Dennis P. Calvert is our President, Chief Executive Officer and Chairman of the Board. He also serves in the same positions for BioLargo Life Technologies, Inc. and BioLargo Water Investment Group., Inc., both wholly owned subsidiaries, and chairman of the board of directors of our subsidiaries Odor-No-More, Inc., Clyra Medical Technologies, Inc. and BioLargo Water, Inc. (Canada). Mr. Calvert was appointed a director in June 2002 and has served as President and Chief Executive Officer since June 2002, Corporate Secretary from September 2002 until March 2003 and Chief Financial Officer from March 2003 through January 2008. Mr. Calvert holds a B.A. degree in Economics from Wake Forest University, where he was a varsity basketball player. Mr. Calvert also studied at Columbia University and Harding University. He also serves on the board of directors at The Maximum Impact Foundation, a 501(c)(3), committed to bridging the gap for lifesaving work around the globe for the good of man and in the name of Christ. He serves as a Director of Sustain SoCal, a trade association that seeks to promote economic growth in the Southern California clean technology industry. He also serves on the Board of Directors at TMA Bluetech the leading regional water cluster promoting science-based ocean water industries and also serves on the Board of Directors of Tilly’s Life Center, a nonprofit charitable foundation aimed at empowering teens with a positive mindset and enabling them to effectively cope with crisis, adversity and tough decisions. He serves on the leadership board at Water UCI, which is an interdisciplinary center in the School of Social Ecology at the University of California- Irvine, that facilitates seamless collaboration across schools, departments, and existing research centers around questions of fundamental and applied water science, technology, management, and policy. Mr. Calvert is a scholarship sponsor for the National Water Research Institute. He is also an Eagle Scout. He is married and has two children. Mr. Calvert has an extensive entrepreneurial background as an operator, investor and consultant. Prior to his work with BioLargo, he had participated in more than 300 consulting projects and more than 50 acquisitions as well as various financing transactions and companies that ranged from industrial chemicals, healthcare management, finance, telecommunications and consumer products.
Kenneth R. Code is our Chief Science Officer. He has been a director since April 2007. Mr. Code is our single largest stockholder. He is the founder of IOWC, which is engaged in the research and development of advanced disinfection technology, and from which the Company acquired its core iodine technology in April 2007. Mr. Code has authored several publications and holds several patents, with additional pending, concerning advanced iodine disinfection. Mr. Code graduated from the University of Calgary, Alberta, Canada.
Charles K. Dargan II is our Chief Financial Officer and has served as such since February 2008. Since January 2003, Mr. Dargan has served as founder and President of CFO 911, an organization of senior executives that provides accounting, finance and operational expertise to both small capitalization public and middle market private companies in all phases of their business life cycle. From March 2000 to January 2003, Mr. Dargan was the Chief Financial Officer of Semotus Solutions, Inc., an American Stock Exchange-listed wireless mobility software company. Mr. Dargan also serves as a director of Hiplink Software, Inc. Further, Mr. Dargan began his finance career in investment banking with Drexel Burnham Lambert and later became Managing Director of two other investment banking firms, including Houlihan Lokey Howard & Zukin, where he was responsible for the management of the private placement activities of the firm. Mr. Dargan received his B.A. degree in Government from Dartmouth College, his M.B.A. degree and M.S.B.A. degree in Finance from the University of Southern California. Mr. Dargan is also a CPA (inactive) and CFA.
Dennis E. Marshall has been a director since April 2006. Mr. Marshall has over 45 years of experience in real estate, asset management, management level finance and operations-oriented management. Since 1981, Mr. Marshall has been a real estate investment broker in Orange County, California, representing buyers and sellers in investment acquisitions and dispositions. From March 1977 to January 1981, Mr. Marshall was a real estate syndicator at McCombs Corporation as well as the assistant to the Chairman of the Board. While at McCombs Corporation, Mr. Marshall became the Vice President of Finance, where he financially monitored numerous public real estate syndications. From June 1973 to September 1976, Mr. Marshall served as an equity controller for the Don Koll Company, an investment builder and general contractor firm, at which Mr. Marshall worked closely with institutional equity partners and lenders. Before he began his career in real estate, Mr. Marshall worked at Arthur Young & Co. (now Ernst & Young) from June 1969 to June 1973, where he served as Supervising Senior Auditor and was responsible for numerous independent audits of publicly held corporations. During this period, he obtained Certified Public Accountant certification. Mr. Marshall earned a degree in Accounting from the University of Texas, Austin in 1966 and earned a Master of Science Business Administration from the University of California, Los Angeles in 1969. Mr. Marshall serves as Chairman of the Audit Committee.
Joseph L. Provenzano has been a director since June 2002, assumed the role of Corporate Secretary in March 2003, was appointed Executive Vice President of Operations in January 2008, was elected President of our subsidiary, Odor-No-More, Inc., upon the commencement of its operations in January 2010. He is a co-inventor on several of the company's patents and proprietary manufacturing processes, and has developed over 30 products from our CupriDyne® technology. Mr. Provenzano began his corporate career in 1988 in the marketing field. In 2001 he began work with an investment holding company to manage their mergers and acquisitions department, participating in more than 50 corporate mergers and acquisitions.
Kent C. Roberts II has been a director since August 2011. Presently, Mr. Roberts is a senior member of the investment team at Vulcan Capital where he leads the Financials and Fintech practice and has held this role since 2017. He was formerly a partner at Acacia Investment Partners, a management consulting firm serving the asset management industry. He has had a long and successful career in the asset management business as a north American practice leader or at the senior partner level. His investment experience spans 30 years where he served in a leadership role. Mr. Roberts has worked for both large firms as well as boutiques that bring unique investment expertise to investors around the world. Those firms include: Global Evolution USA, First Quadrant and Bankers Trust Company. He has presented at numerous industry conferences and as a guest speaker at numerous industry conferences and events. Prior to entering the financial services industry Mr. Roberts worked in the oil and gas exploration industry. Mr. Roberts received an MBA in Finance from the University of Notre Dame and a BS in Agriculture and Watershed Hydrology from the University of Arizona. Mr. Roberts holds a Series 3 license.
John S. Runyan has been a director since October 2011. He has spent his career in the food industry. He began as a stock clerk at age 12, and ultimately served the Fleming Companies for 38 years, his last 10 years as a Senior Executive Officer in its corporate headquarters where he was Group President of Price Impact Retail Stores with annual sales of over $3 billion. He retired from Fleming in 2001, and established JSR&R Company Executive Advising, with a primary emphasis in the United States and international food business. His clients have included Coca Cola, Food 4 Less Price Impact Stores, IGA, Inc., Golden State Foods, Bozzuto Companies Foodstuffs New Zealand, Metcash Australia and McLane International. In 2005, he joined Associated Grocers in Seattle Washington as President and CEO, overseeing its purchase in 2007 by Unified Grocers, at which time he became Executive Advisor to its CEO and to its President. Mr. Runyan currently serves on the board of directors of Western Association of Food Chains and Retailer Owned Food Distributors of America. Additionally, Mr. Runyan served eight years as a board member of the City of Hope’s Northern California Food Industry Circle, which included two terms as President, and was recognized with the City of Hope “Spirit of Life” award. He was the first wholesale executive to be voted “Man of the Year” by Food People Publication. He is a graduate of Washburn University, which recognized his business accomplishments in 2007 as the honoree from the School of Business “Alumni Fellow Award.” Mr. Runyan serves as Chairman of the Compensation Committee.
Jack B. Strommen is a member of the board of directors of our subsidiary, Clyra Medical Technologies, as the representative of Sanatio Capital LLC. Mr. Strommen is the CEO of PD Instore, a leader in the design, production and installation of retail environments and displays for many Fortune 500 companies including Target, Adidas, Verizon, Disney and Sony. He is also an angel investor in several private companies ranging from bio-tech to med-tech to real estate, and serves on the board of directors of several private and public companies. A relentless force of growth, Mr. Strommen has taken his company, PD Instore, to new and ever increasing levels of success. Mr. Strommen purchased the family owned, local based printing firm, from his grandfather in 1999. With his vision and leadership, it went from a local company with $25M in revenues to a global company with $180M in global sales. Mr. Strommen led the company in a private sale in 2015, remaining as CEO.
CORPORATE GOVERNANCE
Our corporate website, www.biolargo.com, contains the charters for our Audit and Compensation Committees and certain other corporate governance documents and policies, including our Code of Ethics. Any changes to these documents and any waivers granted with respect to our Code of Ethics will be posted at www.biolargo.com. In addition, we will provide a copy of any of these documents without charge to any stockholder upon written request made to Corporate Secretary, BioLargo, Inc., 14921 Chestnut St., Westminster, California 92683. The information at www.biolargo.com is not, and shall not be deemed to be, a part of this prospectus.
Director Independence
The Board has determined that each of Messrs. Marshall, Roberts, Runyan and Strommen is independent as defined under applicable Nasdaq Stock Market, LLC (“Nasdaq”) listing standards. The Board has determined that none of Messrs. Calvert, Code or Provenzano is independent as defined under applicable Nasdaq listing standards. None of Messrs. Calvert, Code or Provenzano serves on any committees of the Board.
Meetings of our Board of Directors
Our board of directors held five meetings during 2021, and acted via unanimous written consent once. Each of the incumbent directors attended all the meetings of our board of directors and committees on which the director served, except for one absence at the August 2021 and November 2021 quarterly board meetings, and one at the March 2021 and August 2021 audit committee meetings. Each of our directors is encouraged to attend our Annual Meeting of Stockholders, when these are held, and to be available to answer any questions posed by stockholders to such director.
Communications with our Board of Directors
The following procedures have been established by our board of directors to facilitate communications between our stockholders and our board of directors:
| |
•
|
Stockholders may send correspondence, which should indicate that the sender is a Stockholder, to our board of directors or to any individual director, by mail to Corporate Secretary, BioLargo, Inc., 14921 Chestnut St., Westminster, California 92683.
|
| |
•
|
Our Corporate Secretary will be responsible for the first review and logging of this correspondence and will forward the communication to the director or directors to whom it is addressed unless it is a type of correspondence which our board of directors has identified as correspondence which may be retained in our files and not sent to directors. Our board of directors has authorized the Corporate Secretary to retain and not send to directors communications that: (a) are advertising or promotional in nature (offering goods or services), (b) solely relate to complaints by clients with respect to ordinary course of business customer service and satisfaction issues or (c) clearly are unrelated to our business, industry, management or Board or committee matters. These types of communications will be logged and filed but not circulated to directors. Except as set forth in the preceding sentence, the Corporate Secretary will not screen communications sent to directors.
|
| |
•
|
The log of stockholder correspondence will be available to members of our board of directors for inspection. At least once each year, the Corporate Secretary will provide to our board of directors a summary of the communications received from stockholders, including the communications not sent to directors in accordance with the procedures set forth above.
|
Our stockholders also may communicate directly with the non-management directors as a group, by mail addressed to Dennis E. Marshall, c/o Corporate Secretary, BioLargo, Inc., 14921 Chestnut St., Westminster, California 92683.
Our Audit Committee has established procedures for the receipt, retention and treatment of complaints regarding questionable accounting, internal controls and financial improprieties or auditing matters. Any of our employees may confidentially communicate concerns about any of these matters by mail addressed to Audit Committee, c/o Corporate Secretary, BioLargo, Inc., 14921 Chestnut St., Westminster, California 92683.
All the reporting mechanisms also are posted on our corporate website, www.biolargo.com. Upon receipt of a complaint or concern, a determination will be made whether it pertains to accounting, internal controls or auditing matters and, if it does, it will be handled in accordance with the procedures established by the Audit Committee.
Committees of our Board of Directors
Our board of directors has established an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee.
The Audit Committee meets with management and our independent registered public accounting firm to review the adequacy of internal controls and other financial reporting matters. Dennis E. Marshall served as Chairman of the Audit Committee during 2021 and continues to serve in that capacity. John S. Runyan and Kent C. Roberts II, current board members, also serve on the Audit Committee. Our board of directors has determined that Mr. Marshall qualifies as an “audit committee financial expert” as defined in Item 401(h) of Regulation S-K of the Securities Exchange Act of 1934, as amended. The Audit Committee met four times in 2021.
The Compensation Committee reviews the compensation for all our officers and directors and affiliates. The Committee also administers our equity incentive option plan. Mr. Runyan served as Chairman of the Compensation Committee during 2021. Mr. Marshall also serves on the Compensation Committee. The Compensation Committee acted by consent once during 2021.
Our board of directors did not modify any action or recommendation made by the Compensation Committee with respect to executive compensation for the 2020 or 2021 fiscal years. It is the opinion of the Compensation Committee that the executive compensation policies and plans provide the necessary total remuneration program to properly align their performance and the interests of our stockholders using competitive and equitable executive compensation in a balanced and reasonable manner, for both the short and long term.
The Nominating and Corporate Governance Committee was established in November 2018. Its responsibilities include to identify and screen individuals qualified to become members of the Board, to make recommendations to the Board regarding to the Board regarding the selection and approval of the nominees for director to be submitted to a stockholder vote at the annual meeting of stockholders, subject to approval by the Board, to development corporate governance guidelines and oversee corporate governance practices, to develop a process for an annual evaluation of the Board and its committees, to review all director compensation and benefits, to review, approve and oversee and related party transaction, to develop and recommend director independent standards, and to develop and recommend a company code of conduct, to investigate any alleged breach and enforce the provisions of the code. This committee did not meet in 2021.
Our board of directors follows the written code of ethics that applies to its principal executive officers, principal financial officer, principal accounting officer or controller, or persons performing similar functions.
Leadership Structure of our Board of Directors
Mr. Calvert serves as both principal executive officer and Chairman of the Board. The Company does not have a lead independent director. Messrs. Marshall, Roberts, Strommen and Runyan serve as independent directors who provide active and effective oversight of our strategic decisions. As of the date of this filing, the Company has determined that the leadership structure of the Board has permitted the Board to fulfill its duties effectively and efficiently and is appropriate given the size and scope of the Company and its financial condition.
Our Board of Directors’ Role in Risk Oversight
As a smaller company, our executive management team, consisting of Messrs. Calvert and Code, are also members of our board of directors. Our board of directors, including our executive management members and independent directors, is responsible for overseeing our executive management team in the execution of its responsibilities and for assessing our company’s approach to risk management. Our board of directors exercises these responsibilities on an ongoing basis as part of its meetings and through its committees. Each member of the management team has direct access to the other Board members, and our committees of our board of directors, to ensure that all risk issues are frequently and openly communicated. Our board of directors closely monitors the information it receives from management and provides oversight and guidance to our executive management team regarding the assessment and management of risk. For example, our board of directors regularly reviews our company’s critical strategic, operational, legal and financial risks with management to set the tone and direction for ensuring appropriate risk taking within the business.
Family Relationships
There are no family relationships among the directors and executive officers of our company.
EXECUTIVE COMPENSATION
The following table sets forth all compensation earned for services rendered to our company in all capacities for the fiscal years ended December 31, 2020 and 2021, by our principal executive officer and our three most highly compensated executive officers other than our principal executive officer, collectively referred to as the “Named Executive Officers.”
Summary Compensation Table
|
Name and
Principal
Positions
|
|
Year
|
|
Salary
|
|
|
Stock
Awards(1)
|
|
|
Option
Awards(1)
|
|
|
All other
Compensation
|
|
|
Total
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dennis P. Calvert,
|
|
2020
|
|
$ |
288,603 |
(2) |
|
$ |
— |
|
|
$ |
388,716 |
(3) |
|
$ |
31,696 |
(4) |
|
$ |
709,016 |
|
| Chairman, Chief Executive Officer and President |
|
2021
|
|
|
288,603 |
|
|
|
— |
|
|
|
335,820 |
|
|
|
27,022 |
|
|
|
651,446 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kenneth R. Code,
|
|
2020
|
|
$ |
288,603 |
(5) |
|
$ |
— |
|
|
$ |
46,176 |
(6) |
|
$ |
12,600 |
(4) |
|
$ |
347,379 |
|
| Chief Science Officer |
|
2021
|
|
|
288,603 |
|
|
|
— |
|
|
|
— |
|
|
|
12,600 |
|
|
|
301,203 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Charles K. Dargan
|
|
2020
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
128,954 |
(7) |
|
$ |
— |
|
|
$ |
128,954 |
|
| Chief Financial Officer |
|
2021
|
|
|
— |
|
|
|
— |
|
|
|
54,250 |
|
|
|
|
|
|
|
54,250 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Joseph Provenzano,
|
|
2020
|
|
$ |
169,772 |
(8) |
|
$ |
— |
|
|
$ |
44,495 |
(9) |
|
$ |
16,789 |
(4) |
|
$ |
231,056 |
|
| Corporate Secretary; President Odor-No-More, Inc |
|
2021
|
|
$ |
169,772 |
(8) |
|
$ |
— |
|
|
$ |
8,600 |
(9) |
|
$ |
12,266 |
(4) |
|
$ |
190,639 |
|
|
(1)
|
Our company recognizes compensation expense for stock option awards on a straight-line basis over the applicable service period of the award, which is the vesting period. Share-based compensation expense is based on the grant date fair value estimated using the Black-Scholes method. The amounts in the “Stock Awards” and “Option Awards” columns reflect the aggregate fair value of awards of stock or options calculated as of the grant date, if the award is fully vested at grant date. These amounts do not represent cash paid to or realized by any of the recipients during the years indicated.
|
|
(2)
|
In 2020 and 2021 the employment agreement for Mr. Calvert provided for a base salary of $288,603, other compensation for health insurance and an automobile allowance. During the year ended December 31, 2020, Mr. Calvert agreed to forego $142,649 of cash compensation due to him and accept 963,282 shares of our common stock in lieu thereof, at prices between $0.12 - $0.17 per share. During the year ended December 31, 2021, Mr. Calvert agreed to forego $14,050 of cash compensation due to him and accept 61,758 shares of our common stock in lieu thereof, at a price of $0.23 per share. The common stock issued to Mr. Calvert is subject to a “lock up agreement” that prohibits Mr. Calvert from selling the shares until the earlier of (i) the sale of the Company; (ii) the successful commercialization of BioLargo products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology; and (iii) the Company’s breach of the employment agreement between the Company and Calvert dated May 2, 2017 and resulting in Calvert’s termination. (See “Employment Agreements—Dennis P. Calvert” and “Outstanding Equity Awards at Fiscal Year-End” below for more details).
|
|
(3)
|
On May 2, 2017, pursuant to his employment agreement, we granted to our president, Dennis P. Calvert, an option to purchase 3,731,322 shares of the Company’s common stock. The option is a non-qualified stock option, exercisable at $0.45 per share, the closing price of our common stock on the grant date, exercisable for ten years from the date of grant, and vesting in equal increments on the anniversary of the option agreement for five years. Any portion of the option which has not yet vested shall immediately vest in the event of, and prior to, a change of control, as defined in the employment agreement. The option cliff vests in 4 equal amounts on each anniversary of the option agreement. The option agreement contains the other terms standard in option agreements issued by the Company, including provisions for a cashless exercise. The fair value of this option totaled $1,679,095 and is being amortized monthly through May 2, 2022. During the year ended December 31, 2020 and 2021, we recorded $335,820 and $335,820, respectively, of selling, general and administrative expense related to this option. Additionally, on May 1, 2020, Mr. Calvert received an option to purchase 393,571 shares of the Company’s common stock. The option is a qualified stock option vested upon issuance, exercisable at $0.13 per share, the closing price of our common stock on the grant date, exercisable for ten years from the date of grant. The fair value of this option totaled $52,896.
|
|
(4)
|
Includes health insurance premiums, automobile allowance, and bonus.
|
|
(5)
|
In 2020 and 2021 the employment agreement for Mr. Code provided for a base salary of $288,603 and other compensation of $12,600. During the year ended December 31, 2020, Mr. Code agreed to forego $157,126 of cash compensation due to him and accept 1,054,646 shares of our common stock in lieu thereof, at $0.12 - $0.17 per share. During the year ended December 31, 2021, Mr. Code agreed to forego $32,100 of cash compensation due to him and accept 152,448 shares of our common stock in lieu thereof, at $0.18 - $0.23 per share. See “Employment Agreements—Kenneth R. Code” and “Outstanding Equity Awards at Fiscal Year-End” below for more details.
|
|
(6)
|
On May 1, 2020, Mr. Code received an option to purchase 343,571 shares of the Company’s common stock. The option is a qualified stock option vested upon issuance, exercisable at $0.13 per share, the closing price of our common stock on the grant date, exercisable for ten years from the date of grant. The fair value of this option totaled $46,176.
|
|
(7)
|
Our Chief Financial Officer, Charles K. Dargan II, did not receive any cash compensation during the years ended December 31, 2020 and 2021. During February 2020, Mr. Dargan received an option to purchase 427,500 shares of our common stock, with 25,000 unvested as of December 31, 2020. Additionally, during 2020, Mr. Dargan received additional options to purchase 311,726 shares of the Company’s common stock. These options are a qualified stock option vested upon issuance, exercisable at a range of $0.12 - $0.15 per share, the closing price of our common stock on the grant date. During March 2021, Mr. Dargan received an option to purchase 332,500 shares of our common stock, with 25,000 unvested as of December 31, 2021. These options are a qualified stock option vested upon issuance, exercisable at a range of $0.18 per share, the closing price of our common stock on the grant date. The value set forth in the table reflects the fair value of the options issued that vested during the 12 months of the years indicated. See “Employment Agreements—Charles K. Dargan II” and “Outstanding Equity Awards at Fiscal Year-End” below for more details.
|
|
(8)
|
In 2020 and 2021, the employment agreement for Mr. Provenzano provided for a base salary of $169,772, and other compensation for health insurance and automobile allowance. See “Employment Agreements – Joseph Provenzano” and “Outstanding Equity Awards at Fiscal Year-End” below for more details.
|
|
(9)
|
During 2020, Mr. Provenzano received additional options to purchase 326,161 shares of the Company’s common stock. These options are a qualified stock option vested upon issuance, exercisable at a range of $0.14 - $0.15 per share, the closing price of our common stock on the grant date. During 2021, Mr. Provenzano received additional options to purchase 50,000 shares of the Company’s common stock. These options are a qualified stock option vested upon issuance, exercisable at a range of $0.18 per share, the closing price of our common stock on the grant date. The value set forth in the table reflects the fair value of the options issued that vested during the 12 months of the years indicated. See “Outstanding Equity Awards at Fiscal Year-End” below for more details.
|
Employment Agreements
Dennis P. Calvert
On May 2, 2017, BioLargo, Inc. (the “Company”) and its President and Chief Executive Officer Dennis P. Calvert entered into an employment agreement (the “Calvert Employment Agreement”), replacing in its entirety the previous employment agreement with Mr. Calvert dated April 30, 2007.
The Calvert Employment Agreement provides that Mr. Calvert will continue to serve as the President and Chief Executive Officer of the Company and receive base compensation equal to his current rate of pay of $288,603 annually. In addition to this base compensation, the agreement provides that he is eligible to participate in incentive plans, stock option plans, and similar arrangements as determined by the Company’s Board of Directors, health insurance premium payments for himself and his immediate family, a car allowance of $800 per month, paid vacation of four weeks per year, and bonuses in such amount as the Compensation Committee may determine from time to time.
The Calvert Employment Agreement provides that Mr. Calvert will be granted an option (the “Option”) to purchase 3,731,322 shares of the Company’s common stock. The Option shall be a non-qualified stock option, exercisable at $0.45 per share, which represents the market price of the Company’s common stock as of the date of the agreement, exercisable for ten years from the date of grant and vesting in equal increments over five years. Notwithstanding the foregoing, any portion of the Option which has not yet vested shall be immediately vested in the event of, and prior to, a change of control, as defined in the Calvert Employment Agreement. The agreement also provides for a grant of 1,500,000 shares of common stock, subject to the execution of a “lock-up agreement” whereby the shares remain unvested unless and until the earlier of (i) a sale of the Company, (ii) the successful commercialization of the Company’s products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology, and (iii) the Company’s breach of the employment agreement resulting in his termination. The Option contains the other terms standard in option agreements issued by the Company, including provisions for a cashless exercise.
The Calvert Employment Agreement has a term of five years, unless earlier terminated in accordance with its terms. The Calvert Employment Agreement provides that Mr. Calvert’s employment may be terminated by the Company due to his death or disability, for cause, or upon a merger, acquisition, bankruptcy or dissolution of the Company. “Disability” as used in the Calvert Employment Agreement means physical or mental incapacity or illness rendering Mr. Calvert unable to perform his duties on a long-term basis (i) as evidenced by his failure or inability to perform his duties for a total of 120 days in any 360-day period, or (ii) as determined by an independent and licensed physician whom Company selects, or (iii) as determined without recourse by the Company’s disability insurance carrier. “Cause” means that Mr. Calvert has (i) engaged in willful misconduct in connection with the Company’s business; or (ii) been convicted of, or plead guilty or nolo contendre in connection with, fraud or any crime that constitutes a felony or that involves moral turpitude or theft. If Mr. Calvert’s employment is terminated due to merger or acquisition, then he will be eligible to receive the greater of (i) one year’s compensation plus an additional one half year for each year of service since the effective date of the employment agreement or (ii) one year’s compensation plus an additional one half year for each year remaining in the term of the agreement. Otherwise, he is only entitled to receive compensation due through the date of termination.
The Calvert Employment Agreement requires Mr. Calvert to keep certain information confidential, not to solicit customers or employees of the Company or interfere with any business relationship of the Company, and to assign all inventions made or created during the term of the Calvert Employment Agreement as “work made for hire”.
Kenneth R. Code
We entered into an employment agreement dated as of April 29, 2007 with Mr. Code, our Chief Science Officer (the “Code Employment Agreement”), which we amended on December 28, 2012 such that his salary will remain at $288,603, the level paid in April 2012, with no further automatic increases. The Code Employment Agreement can automatically renew for one year periods on April 29th of each year but may be terminated “without cause” at any time upon 120 days’ notice, and upon such termination, Mr. Code would not receive the severance originally provided for. All other terms in the 2007 agreement remain the same in the Code Employment Agreement.
In addition, Mr. Code will be eligible to participate in incentive plans, stock option plans, and similar arrangements as determined by our board of directors. When such benefits are made available to our senior employees, Mr. Code is also eligible to receive health insurance premium payments for himself and his immediate family, a car allowance of $800 per month, paid vacation of four weeks per year plus an additional two weeks per year for each full year of service during the term of the agreement up to a maximum of 10 weeks per year, life insurance equal to three times his base salary and disability insurance.
The Code Employment Agreement further requires Mr. Code to keep certain information confidential, not to solicit customers or employees of our company or interfere with any business relationship of our company, and to assign all inventions made or created during the term of the Code Employment Agreement as “work made for hire”.
Charles K. Dargan II
Charles K. Dargan, II has served as our Chief Financial Officer since February 2008 pursuant to an engagement agreement with his company, CFO 911, that has been renewed and extended each year.
On February 25, 2020, we and Mr. Dargan again extended his engagement agreement to expire January 31, 2021. As the sole compensation for the Extended Term, Mr. Dargan was issued an option (“Option”) to purchase 25,000 shares of the Company’s common stock for each month during the term (thus, an option to purchase 400,000 shares reflecting an extended term of 16 months). The Option vests over the period of the agreement, with 75,000 shares having vested as of December 31, 2019, and the remaining shares to vest 25,000 shares monthly beginning January 31, 2020, and each month thereafter, so long as the agreement is in full force and effect. The Option is exercisable at $0.21 per share, the closing price of BioLargo’s common stock on February 25, 2020, expires ten years from the grant date, and was issued pursuant to the Company’s 2018 Equity Incentive Plan. The Option is Mr. Dargan’s sole compensation for the Extended Term. As was the case in all prior terms of his engagement, there is no cash component of his compensation for the Extended Term. Mr. Dargan is eligible to be reimbursed for business expenses he incurs in connection with the performance of his services as the Company’s Chief Financial Officer (although he has made no such requests for reimbursement in the past). All other provisions of the Engagement Agreement not expressly amended pursuant to the Engagement Extension Agreement remain the same, including provisions regarding indemnification and arbitration of disputes.
On March 18, 2021, we and Mr. Dargan again extended his engagement agreement. The Engagement Extension Agreement dated as of March 18, 2021 (the “Engagement Extension Agreement”) provides for an additional one-year term to expire January 31, 2022 (the “Extended Term”).
As the sole compensation for the Extended Term, Mr. Dargan was issued an option (“Option”) to purchase 25,000 shares of the Company’s common stock for each month during the Extended Term (thus, an option to purchase 300,000 shares reflecting an extended term of 12 months). The Option vests over the period of the Extended Term, with 25,000 shares having vested as of March 15, 2021, and the remaining shares to vest 25,000 shares monthly beginning March 31, 2021, and each month thereafter, so long as the agreement is in full force and effect. The Option is exercisable at $0.174 per share, the closing price of BioLargo’s common stock on the March 18, 2021 grant date, expires ten years from the grant date, and was issued pursuant to the Company’s 2018 Equity Incentive Plan.
On March 22, 2022, we and our Chief Financial Officer Charles K. Dargan, II formally agreed to extend the engagement agreement dated February 1, 2008 (the “Engagement Agreement”, which had been previously extended multiple times), pursuant to which Mr. Dargan has been and continues to serve as the Company’s Chief Financial Officer. The Engagement Extension Agreement dated as of March 22, 2022 (the “Engagement Extension Agreement”) provides for an additional one-year term to expire January 31, 2023 (the “Extended Term”).
As the sole compensation for the Extended Term, Mr. Dargan was issued an option (“Option”) to purchase 25,000 shares of the Company’s common stock for each month during the Extended Term (thus, an option to purchase 300,000 shares reflecting an extended term of 12 months). The Option vests over the period of the Extended Term, with 25,000 shares having vested as of March 22, 2022, and the remaining shares to vest 25,000 shares monthly beginning March 22, 2022, and each month thereafter, so long as the agreement is in full force and effect. The Option is exercisable at $0.24 per share, the closing price of BioLargo’s common stock on the March 22, 2022, grant date, expires ten years from the grant date, and was issued pursuant to the Company’s 2018 Equity Incentive Plan.
The Option is Mr. Dargan’s sole compensation for the Extended Term. As was the case in all prior terms of his engagement, there is no cash component of his compensation for the Extended Term. Mr. Dargan is eligible to be reimbursed for business expenses he incurs in connection with the performance of his services as the Company’s Chief Financial Officer (although he has made no such requests for reimbursement in the past). All other provisions of the Engagement Agreement not expressly amended pursuant to the Engagement Extension Agreement remain the same, including provisions regarding indemnification and arbitration of disputes.
Joseph L. Provenzano
Mr. Provenzano has served as Vice President of Operations since January 1, 2008, in addition to continuing to serve as our Corporate Secretary. On May 28, 2019, the Compensation Committee of the Board of Directors approved the terms of a new employment agreement for Mr. Provenzano, and granted to him an incentive stock option (the “Option”) to purchase 1,000,000 shares of the Company’s common stock pursuant to the terms of the Company’s 2018 Equity Incentive Plan (“Plan”). As set forth in the Plan, the exercise price of the Option is equal to the closing price of the Company’s common stock on the May 28 grant date, at $0.17 per share. The shares in the Option vest in five in equal increments over five years, and the Option may be exercised for up to ten years following the grant date. Notwithstanding the foregoing, any portion of the Option which has not yet vested shall be immediately vested in the event of, and prior to, a change of control, as defined in the Provenzano Employment Agreement. The Option contains the other terms standard in option agreements issued by the Company, including provisions for a cashless exercise. On May 28, 2019, the Committee also granted Mr. Provenzano a restricted stock unit of 500,000 shares of common stock, subject to the execution of a “lock-up agreement” whereby the shares remain unvested unless and until the earlier of (i) a sale of the Company, (ii) the successful commercialization of the Company’s products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology, and (iii) the Company’s breach of the employment agreement resulting in his termination. On June 18, 2019, the other terms of his employment agreement were finalized and a document fully executed. Although fully executed on June 18, 2019, the employment agreement is effective as of May 28, 2019, to reflect Option grant date.
The Provenzano Employment Agreement provides that Mr. Provenzano will serve as our Executive Vice President of Operations, as well as the President and Chief Executive Officer of our wholly owned subsidiary Odor-No-More. Mr. Provenzano’s base compensation will remain at his current rate of $170,000 annually. In addition to this base compensation, the agreement provides that he is eligible to participate in incentive plans, stock option plans, and similar arrangements as determined by the our Board of Directors, health insurance premium payments for himself and his immediate family, a car allowance covering the expenses of his personal commercial grade truck which the company uses in company operations on a continual basis, paid vacation of four weeks per year, and bonuses in such amount as the Compensation Committee may determine from time to time.
The Provenzano Employment Agreement has a term of five years, unless earlier terminated in accordance with its terms. The Provenzano Employment Agreement provides that Mr. Provenzano’s employment may be terminated by the Company due to his death or disability, for cause, or upon a merger, acquisition, bankruptcy or dissolution of the Company. “Disability” as used in the Provenzano Employment Agreement means physical or mental incapacity or illness rendering Mr. Provenzano unable to perform his duties on a long-term basis (i) as evidenced by his failure or inability to perform his duties for a total of 120 days in any 360-day period, or (ii) as determined by an independent and licensed physician whom Company selects, or (iii) as determined without recourse by the Company’s disability insurance carrier. “Cause” means that Mr. Provenzano has (i) engaged in willful misconduct in connection with the Company’s business; or (ii) been convicted of, or plead guilty or nolo contendre in connection with, fraud or any crime that constitutes a felony or that involves moral turpitude or theft. If Mr. Provenzano’s employment is terminated due to merger or acquisition, then he will be eligible to receive the greater of (i) one year’s compensation plus an additional one half year for each year of service since the effective date of the employment agreement or (ii) one year’s compensation plus an additional one half year for each year remaining in the term of the agreement. Otherwise, he is only entitled to receive compensation due through the date of termination.
The Provenzano Employment Agreement requires Mr. Provenzano to keep certain information confidential, not to solicit customers or employees of the Company or interfere with any business relationship of the Company, and to assign all inventions made or created during the term of the Provenzano Employment Agreement as “work made for hire”.
Director Compensation
Each director who is not an officer or employee of our company receives an annual retainer of $60,000, paid in cash or shares of our common stock, or options to purchase our common stock (pursuant to a plan put in place by our board of directors), in our sole discretion. Historically, all but one director has received the entirety of his fees in the form of options to purchase stock, rather than cash. In addition, Mr. Marshall and Mr. Runyan each receive an additional $15,000 for their services as the chairman of the Audit Committee and chairman of the Compensation Committee, respectively. The following table sets forth information for the fiscal years ended December 31, 2021 regarding compensation of our non-employee directors. Our employee directors do not receive any additional compensation for serving as a director.
|
Name
|
|
Fees Earned
or Fees Paid
in Cash
|
|
|
Option
Awards
|
|
|
Non-Equity
Incentive Plan
Compensation
|
|
|
All Other
Compensation
|
|
|
Total
|
|
|
Dennis E. Marshall
|
|
$ |
76,117 |
(1) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
77,117 |
|
|
Jack B. Strommen
|
|
$ |
57,118 |
(2) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
57,118 |
|
|
Kent C. Roberts III
|
|
$ |
57,118 |
(3) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
57,118 |
|
|
John S. Runyan
|
|
$ |
71,398 |
(4) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
71,398 |
|
|
(1)
|
In 2021, Mr. Marshall earned director fees of $76,117, which included compensation for serving as Chairman of the Audit Committee of our board of directors. None of these fees was paid in cash. During 2021, Mr. Marshall received options in lieu of cash consisting of (i) on March 31, 2021, an issuance of an option to purchase 82,418 shares of our common stock at $0.22 per share, (ii) on May 21, 2021, an issuance of an option to purchase 27,439 shares of our common stock at $0.17 per share, (iii) on June 30, 2021, an issuance of an option to purchase 110,294 shares of our common stock at $0.16 per share, (iv) on September 30, 2021, an issuance of an option to purchase 98,684 shares of our common stock at $0.18 per share, and (v) on December 31, 2021, an issuance of an option to purchase 89,286 shares of our common stock at $0.20 per share.
|
|
|
|
|
(2)
|
In 2021 Mr. Strommen earned director fees of $57,118. None of these fees was paid in cash. During 2021, Mr. Strommen received options in lieu of cash consisting of (i) on March 31, 2021, an issuance of an option to purchase 65,934 shares of our common stock at $0.22 per share, (ii) on June 30, 2021, an issuance of an option to purchase 88,235 shares of our common stock at $0.16 per share, (iii) on September 30, 2021, an issuance of an option to purchase 78,947 shares of our common stock at $0.18 per share, and (iv) on December 31, 2021, an issuance of an option to purchase 125,000 shares of our common stock at $0.20 per share.
|
|
|
|
|
(3)
|
In 2021 Mr. Roberts earned director fees of $57,118. None of these fees was paid in cash. During 2021, Mr. Roberts received options in lieu of cash consisting of (i) on March 31, 2021, an issuance of an option to purchase 65,934 shares of our common stock at $0.22 per share, (ii) on June 30, 2021, an issuance of an option to purchase 88,235 shares of our common stock at $0.16 per share, (iii) on September 30, 2021, an issuance of an option to purchase 78,947 shares of our common stock at $0.18 per share, and (iv) on December 31, 2021, an issuance of an option to purchase 71,429 shares of our common stock at $0.20 per share.
|
| |
|
|
(4)
|
In 2021, Mr. Runyon earned director fees of $71,398, which included compensation for serving as Chairman of the Audit Committee of our board of directors. None of these fees was paid in cash. During 2021, Mr. Runyon received options in lieu of cash consisting of (i) on March 31, 2021, an issuance of an option to purchase 82,418 shares of our common stock at $0.22 per share, (ii) on June 30, 2021, an issuance of an option to purchase 110,294 shares of our common stock at $0.16 per share, (iii) on September 30, 2021, an issuance of an option to purchase 98,684 shares of our common stock at $0.18 per share, and (iv) on December 31, 2021, an issuance of an option to purchase 89,286 shares of our common stock at $0.20 per share.
|
Limitation of Liability and Indemnification Matters
As permitted by the Delaware general corporation law, we have included a provision in our certificate of incorporation to eliminate the personal liability of our directors for monetary damages for breach of their fiduciary duties as directors, except for liability (i) for any breach of the director’s duty of loyalty to our company, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under section 174 of the Delaware general corporation law or (iv) for any transaction from which the director derived an improper personal benefit. Our certificate of incorporation also provides that our company shall, to the full extent permitted by section 145 of the Delaware general corporation law, as amended from time to time, indemnify all persons whom it may indemnify pursuant thereto.
In addition, our Bylaws provide that we are required to indemnify our officers and directors even when indemnification would otherwise be discretionary, and we are required to advance expenses to our officers and directors as incurred in connection with proceedings against them for which they may be indemnified.
We may enter into indemnification agreements with our officers and directors containing provisions that are in some respects broader than the specific indemnification provisions contained in the Delaware general corporation law. The indemnification agreements would require us to indemnify our officers and directors against liabilities that may arise by reason of their status or service as officers and directors other than for liabilities arising from willful misconduct of a culpable nature, to advance their expenses incurred as a result of any proceeding against them as to which they could be indemnified, and to obtain our directors’ and officers’ insurance if available on reasonable terms. As of the date of this prospectus, our company has not entered into any indemnification agreement with any of its directors or officers, except for Mr. Strommen.
We have obtained directors’ and officers’ liability insurance in amounts comparable to other companies of our size and in our industry.
No pending litigation or proceeding involving a director, officer, employee or other agent of our company currently exists as to which indemnification is being sought. We are not aware of any threatened litigation that may result in claims for indemnification by any director, officer, employee or other agent of our company.
See “Disclosure of SEC Position on Indemnification for Securities Act Liabilities.”
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information regarding unexercised stock options and equity incentive plan awards for each of the Named Executive Officers outstanding as of December 31, 2021. All stock or options that were granted to the Named Executive Officers during the fiscal year ended December 31, 2021, have fully vested, except as indicated.
|
Name
|
|
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable
|
|
|
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable
|
|
|
Equity
Incentive
Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options
|
|
|
Option
Exercise
Price
|
|
|
Share
Price on
Grant Date
|
|
Option
Expiration
Date
|
|
Dennis P. Calvert
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
3,731,322 |
|
|
|
1,492,530 |
|
|
|
1,492,530 |
|
|
$ |
0.45 |
|
|
$ |
0.45 |
|
May 2, 2027
|
| |
|
|
65,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.22 |
|
|
$ |
0.22 |
|
September 19, 2029
|
| |
|
|
50,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.14 |
|
|
$ |
0.14 |
|
May 1, 2030
|
| |
|
|
343,571 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.14 |
|
|
$ |
0.14 |
|
May 1, 2030
|
|
Charles K. Dargan II
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
10,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.42 |
|
|
$ |
0.42 |
|
January 31, 2021
|
| |
|
|
120,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.41 |
|
|
$ |
0.41 |
|
February 28, 2021
|
| |
|
|
300,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.35 |
|
|
$ |
0.35 |
|
April 10, 2022
|
| |
|
|
410,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.30 |
|
|
$ |
0.30 |
|
December 28, 2022
|
| |
|
|
300,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.30 |
|
|
$ |
0.30 |
|
July 17, 2023
|
| |
|
|
300,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.30 |
|
|
$ |
0.30 |
|
June 23, 2024
|
| |
|
|
300,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.57 |
|
|
$ |
0.57 |
|
September 30, 2025
|
| |
|
|
300,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.69 |
|
|
$ |
0.69 |
|
February 10, 2027
|
| |
|
|
300,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.39 |
|
|
$ |
0.39 |
|
December 31, 2027
|
| |
|
|
300,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.22 |
|
|
$ |
0.22 |
|
January 16, 2029
|
| |
|
|
79,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.22 |
|
|
$ |
0.22 |
|
September 19, 2029
|
| |
|
|
400,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.21 |
|
|
$ |
0.21 |
|
February 25, 2030
|
| |
|
|
27,500 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.21 |
|
|
$ |
0.21 |
|
February 25, 2030
|
| |
|
|
25,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.14 |
|
|
$ |
0.14 |
|
May 01, 2030
|
| |
|
|
214,286 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.14 |
|
|
$ |
0.14 |
|
May 01, 2030
|
| |
|
|
5,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.16 |
|
|
$ |
0.16 |
|
June 30, 2030
|
| |
|
|
5,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.15 |
|
|
$ |
0.15 |
|
September 30, 2030
|
| |
|
|
2,500 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.15 |
|
|
$ |
0.15 |
|
September 30, 2030
|
| |
|
|
50,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.15 |
|
|
$ |
0.15 |
|
September 30, 2030
|
| |
|
|
7,500 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.12 |
|
|
$ |
0.12 |
|
December 31, 2030
|
| |
|
|
300,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.23 |
|
|
$ |
0.23 |
|
March 17, 2031
|
| |
|
|
32,500 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.18 |
|
|
$ |
0.18 |
|
May 21, 2031
|
|
Kenneth R. Code
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
200,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
1.03 |
|
|
$ |
0.94 |
|
July 17, 2023
|
| |
|
|
65,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.22 |
|
|
$ |
0.22 |
|
September 19, 2029
|
| |
|
|
343,571 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.14 |
|
|
$ |
0.14 |
|
May 01, 2030
|
|
Joseph Provenzano
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
200,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.41 |
|
|
$ |
0.41 |
|
March 21, 2021
|
| |
|
|
78,947 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.45 |
|
|
$ |
0.45 |
|
October 23, 2027
|
| |
|
|
1,000,000 |
|
|
|
800,000 |
|
|
|
800,000 |
|
|
$ |
0.17 |
|
|
$ |
0.17 |
|
May 28, 2029
|
| |
|
|
32,500 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.22 |
|
|
$ |
0.22 |
|
September 18, 2029
|
| |
|
|
50,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.14 |
|
|
$ |
0.14 |
|
May 01, 2030
|
| |
|
|
202,110 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.14 |
|
|
$ |
0.14 |
|
May 01, 2030
|
| |
|
|
74,051 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.15 |
|
|
$ |
0.15 |
|
September 30, 2030
|
| |
|
|
50,000 |
|
|
|
-- |
|
|
|
-- |
|
|
$ |
0.18 |
|
|
$ |
0.18 |
|
May 21, 2031
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information regarding the beneficial ownership of shares of our common stock as of April 11, 2022, including rights to acquire beneficial ownership of shares of our common stock within 60 days of April 11, 2022, by (a) all stockholders known to the Company to be beneficial owners of more than 5% of the outstanding Common stock; (b) each director, (c) each Named Executive Officer, and (d) all directors and executive officers of the Company as a group:
|
Name and Address of Beneficial Owner(1)
|
|
Amount of
Beneficial
Ownership
|
|
|
Percent of
Class(2)
|
|
|
Kenneth R. Code(3)
|
|
|
25,004,666 |
|
|
|
8.9 |
% |
|
Dennis P. Calvert(4)
|
|
|
13,348,596 |
|
|
|
4.7 |
% |
|
Jack B. Strommen(5)
|
|
|
5,911,249 |
|
|
|
2.1 |
% |
|
Charles K. Dargan II(6)
|
|
|
4,051,744 |
|
|
|
1.4 |
% |
|
Dennis E. Marshall(7)
|
|
|
3,665,878 |
|
|
|
1.3 |
% |
|
Joseph L. Provenzano(8)
|
|
|
3,042,039 |
|
|
|
1.1 |
% |
|
Kent C. Roberts II(9)
|
|
|
2,846,870 |
|
|
|
1.0 |
% |
|
John S. Runyan(10)
|
|
|
2,970,737 |
|
|
|
1.1 |
% |
|
All directors and officers as a group (8 persons)
|
|
|
60,841,779 |
|
|
|
21.6 |
% |
Except as noted in any footnotes below, each person has sole voting power and sole dispositive power as to all of the shares shown as beneficially owned by them. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities.
| |
(1)
|
The address for all directors and the Named Executive Officers is: c/o BioLargo, Inc., 14921 Chestnut St., Westminster, CA 92683, except for: Kent C. Roberts II’s address is 1146 Oxford Road, San Marino, CA 91108; Charles K. Dargan II’s address is 18841 NE 29th Avenue, Suite 700, Aventura, FL 33180; and John S. Runyan’s address is 30001 Hillside Terrace, San Juan Capistrano, CA 92675
|
| |
(2)
|
Our company has only one class of stock outstanding. The sum of 262,722,515 shares of common stock outstanding as of the date hereof, and 19,333,453 shares of common stock subject to options currently exercisable or exercisable within 60 days by the directors and officers, are deemed outstanding for determining the number of shares beneficially owned by the directors and officers, and the directors and officers as a group, and for computing the percentage ownership of the person holding such options, but are not deemed outstanding for computing the percentage ownership of any other person.
|
| |
(3)
|
Includes 22,139,012 shares owned indirectly by Mr. Code issued on April 29, 2007 to IOWC Technologies, Inc. in connection with the acquisition by our company of certain intellectual property and other assets on that date. Includes 408,571 shares issuable to Mr. Code upon exercise of options.
|
| |
(4)
|
Includes 1,528,695 shares of common stock held by New Millennium Capital Partners, LLC, which is wholly owned and controlled by Mr. Calvert. Includes 3,846,322 shares issuable to Mr. Calvert upon exercise of other options granted from time to time by our company.
|
| |
(5)
|
Includes 1,262,371 shares issuable to Mr. Strommen upon exercise of options; includes 333,334 shares issuable to Mr. Strommen upon the exercise of warrants.
|
| |
(6)
|
Includes 3,861,500 shares issuable to Mr. Dargan upon exercise of options.
|
| |
(7)
|
Includes 3,405,846 shares issuable to Mr. Marshall upon exercise of options.
|
| |
(8)
|
Includes 1,271,296 shares issuable to Mr. Provenzano upon exercise of options.
|
| |
(9)
|
Includes 2,320,645 shares issuable to Mr. Roberts upon exercise of options.
|
| |
(10)
|
Includes 2,623,568 shares issuable to Mr. Runyan upon exercise of options.
|
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Our company has adopted a policy that all transactions between our company and its executive officers, directors and other affiliates must be approved by a majority of the members of our board of directors and by a majority of the disinterested members of our board of directors, and must be on terms no less favorable to our company than could be obtained from unaffiliated third parties.
From time to time, our company is unable to pay in full amounts due to its officers for salary and business expenses, and those amounts are recorded as liabilities in our financial statements. These amounts are then paid in the future as our company’s cash position allows, or through the issuance of our common stock, or an option to purchase common stock, pursuant to a plan adopted by our board for the payment of outstanding payables.
Our officers and board members routinely forego cash compensation in lieu of receiving common stock or options to purchase common stock, pursuant to a plan adopted by our board for the payment of outstanding payables.
During the year ended December 31, 2021, certain of our officers agreed to convert an aggregate $46,000 of accrued and unpaid salary into 214,206 shares of our common stock. The unpaid salary is converted on the last day of each quarter as follows: December 31, 2021, we issued 15,000 shares of our common stock at $0.21 per share; on September 30, 2021, we issued 61,842 shares of our common stock at $0.19; on March 31, 2021, we issued 137,364 shares of our common stock at $0.23 per share.
On March 31, 2021, we issued options to purchase 296,704 shares of our common stock at an exercise price of $0.22 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 82,418 to Mr. Marshall in exchange for $18,750 in fees due; 65,934 to Mr. Strommen in exchange for $15,000 in fees due; 65,934 to Mr. Roberts in exchange for $15,000 in fees due; and 65,934 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On June 30, 2021, we issued options to purchase 397,058 shares of our common stock at an exercise price of $0.16 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 110,924 to Mr. Marshall in exchange for $18,750 in fees due; 88,235 to Mr. Strommen in exchange for $15,000 in fees due; 88,235 to Mr. Roberts in exchange for $15,000 in fees due; and 110,924 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On September 30, 2021, we issued options to purchase 355,262 shares of our common stock at an exercise price of $0.18 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 71,429 to Mr. Marshall in exchange for $18,750 in fees due; 78,947 to Mr. Strommen in exchange for $15,000 in fees due; 78,947 to Mr. Roberts in exchange for $15,000 in fees due; and 71,429 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On December 31, 2021, we issued options to purchase 321,430 shares of our common stock at an exercise price of $0.22 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 89,286 to Mr. Marshall in exchange for $18,750 in fees due; 71,429 to Mr. Strommen in exchange for $15,000 in fees due; 71,429 to Mr. Roberts in exchange for $15,000 in fees due; and 89,286 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On March 31, 2020, we issued options to purchase 397,058 shares of our common stock at an exercise price of $0.17 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 110,294 to Mr. Marshall in exchange for $18,750 in fees due; 88,235 to Mr. Strommen in exchange for $15,000 in fees due; 88,235 to Mr. Roberts in exchange for $15,000 in fees due; and 110,294 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On June 30, 2020, we issued options to purchase 421,876 shares of our common stock at an exercise price of $0.16 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 117,188 to Mr. Marshall in exchange for $18,750 in fees due; 93,750 to Mr. Strommen in exchange for $15,000 in fees due; 93,750 to Mr. Roberts in exchange for $15,000 in fees due; and 117,188 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On September 30, 2020, we issued options to purchase 450,000 shares of our common stock at an exercise price of $0.15 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 125,000 to Mr. Marshall in exchange for $18,750 in fees due; 100,000 to Mr. Strommen in exchange for $15,000 in fees due; 100,000 to Mr. Roberts in exchange for $15,000 in fees due; and 125,000 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
On December 31, 2020, we issued options to purchase 562,500 shares of our common stock at an exercise price of $0.15 per share to four members of our board of directors, in lieu of $67,500 in fees, as follows: 156,250 to Mr. Marshall in exchange for $18,750 in fees due; 125,000 to Mr. Strommen in exchange for $15,000 in fees due; 125,000 to Mr. Roberts in exchange for $15,000 in fees due; and 156,250 to Mr. Runyan in exchange for $18,750 in fees due. The options expire 10 years from the date of grant.
DESCRIPTION OF CAPITAL STOCK
As reflected in the Certificate of Incorporation as amended May 25, 2018, our authorized capital stock consists of 400,000,000 shares of common stock, par value $0.00067 per share, and 50,000,000 shares of preferred stock, par value $0.00067 per share.
|
Authorized and Issued Stock
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Number of shares at April 11, 2022
|
|
|
Title of Class
|
|
Authorized
|
|
|
Outstanding
|
|
|
Reserved
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock, par value $0.00067 per share
|
|
|
400,000,000 |
|
|
|
262,722,515 |
|
|
|
104,000,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred stock, $0.00067 par value per share
|
|
|
50,000,000 |
|
|
|
-0- |
|
|
|
-0- |
|
Common Stock
Dividends. Each share of our common stock is entitled to receive an equal dividend, if one is declared. We cannot provide any assurance that we will declare or pay cash dividends on our common stock in the future. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to applicable laws, and will depend on our financial condition, results of operations, capital requirements, general business conditions and other factors that our board of directors may deem relevant. Our board of directors may determine it to be necessary to retain future earnings (if any) to finance operations. See “Risk Factors” and “Dividend Policy.”
Liquidation. If our company is liquidated, then assets that remain (if any) after the creditors are paid and the owners of preferred stock receive liquidation preferences (as applicable) will be distributed to the owners of our common stock pro rata.
Voting Rights. Each share of our common stock entitles the owner to one vote. There is no cumulative voting. A simple majority can elect all of the directors at a given meeting, and the minority would not be able to elect any director at that meeting.
Preemptive Rights. Owners of our common stock have no preemptive rights. We may sell shares of our common stock to third parties without first offering such shares to current stockholders.
Redemption Rights. We do not have the right to buy back shares of our common stock except in extraordinary transactions, such as mergers and court approved bankruptcy reorganizations. Owners of our common stock do not ordinarily have the right to require us to buy their common stock. We do not have a sinking fund to provide assets for any buy back.
Conversion Rights. Shares of our common stock cannot be converted into any other kind of stock except in extraordinary transactions, such as mergers and court approved bankruptcy reorganizations.
Nonassessability. All outstanding shares of our common stock are fully paid and nonassessable.
Preferred Stock
Our certificate of incorporation authorizes our board of directors to issue “blank check” preferred stock. Our board of directors may divide this preferred stock into series and establish the rights, preferences and privileges thereof. Our board of directors may, without prior stockholder approval, issue any or all of the shares of this preferred stock with dividend, liquidation, conversion, voting or other rights that could adversely affect the relative voting power or other rights of our common stock. Preferred stock could be used as a method of discouraging, delaying or preventing a takeover or other change in control of our company. Issuances of preferred stock in the future could have a dilutive effect on our common stock.
As of the date of this prospectus, there are no shares of our preferred stock outstanding.
DESCRIPTION OF THE OFFERING
This is a registration of shares that were previously sold by the Company in a series of private placements. This prospectus relates to the sale of up to 36,090,857 shares of our common stock by selling stockholders. Of the 36,090,857 shares, 20,159,062 are for expired warrants, that would have allowed the Selling Stockholders to purchase additional shares from the company and sell those shares pursuant to the Registration Statement.
SELLING STOCKHOLDERS
The following table presents information regarding the selling stockholders and the shares of our common stock that may be sold by them pursuant to this prospectus.
Each of the selling shareholders acquired their shares from the company for cash (or as payment of accrued interest) in a private placement transaction which was exempt from registration pursuant Regulation D promulgated under the Securities Act of 1933, except Freedom Investors Corp., Sexton Equities LLC, and Randall A. Heller, each of whom received their warrants to purchase shares as broker-dealer commissions.
Except for Jack Strommen, who is on our board of directors, none of the selling stockholders has had within the past three years any position, office or other material relationship with our company or any of its predecessors or affiliates. Other than Freedom Investors Corp., Sexton Equities LLC, and Randall A. Heller, no selling stockholder is a broker-dealer or an affiliate of a broker-dealer. Freedom Investors Corp. is a registered broker-dealer and purchased the shares being offered under this prospectus in the ordinary course of its business. Sexton Equities LLC, and Randall A. Heller are affiliated persons of Freedom Investor Corp. At the time of purchase, Freedom Investors Corp., Sexton Equities LLC, and Randall A. Heller did not have any agreement or understanding, directly or indirectly, with any person to distribute the shares. Freedom Investor Corp. is deemed to be an underwriter with respect to the shares of stock it offers for sale under this prospectus. Shares beneficially owned prior to the offering includes shares that may be issued to the selling stockholders pursuant to the exercise of stock purchase warrants and conversion of convertible promissory notes.
Our company issued the shares being offered for resale pursuant to this prospectus to the selling stockholders in private placements that we effected in 2013, 2015, and 2016 in exchange for consideration that we received from the selling stockholders. The following information is current as of April 11, 2022.
|
Name
|
Number of
Shares of
Common stock
Beneficially
Owned Prior to
Offering(1)
|
Number of
Shares of
Common Stock
Being Offered
|
Shares of
Common Stock
Beneficially
Owned After
the Offering
|
Percentage
Beneficially
Owned After
Registration
|
|
Andrea Kochensparger
|
986,992
|
986,992
|
–
|
*
|
|
Anthony J. Jacobson
|
876,596
|
585,559
|
291,037
|
*
|
|
Austin N. Heberger
|
149,883
|
149,883
|
–
|
*
|
|
Best Home Choices, LLC(2)
|
179,858
|
179,858
|
–
|
*
|
|
Black Mountain Equities, Inc.(3)
|
640,000
|
640,000
|
–
|
*
|
|
BMS Endeavor, LLP(4)
|
612,117
|
212,117
|
400,000
|
*
|
|
Brandan Adams and Dr. Shelley Thompson
|
202,771
|
202,771
|
–
|
*
|
|
Brandan M Adams
|
191,429
|
191,429
|
–
|
*
|
|
Brian Griffith
|
201,502
|
201,502
|
–
|
*
|
|
Brian Griffith and Lorelei Griffith
|
107,135
|
107,135
|
–
|
*
|
|
Bruce Evans
|
500,000
|
500,000
|
–
|
*
|
|
Bruce Kelber
|
92,857
|
40,000
|
52,857
|
*
|
|
C1P Solutions Inc.(5)
|
431,221
|
345,507
|
85,714
|
*
|
|
California Clock Co.(6)
|
210,568
|
210,568
|
–
|
*
|
|
Carl Soderlund
|
161,224
|
161,224
|
–
|
*
|
|
Chris T. Washburn
|
71,429
|
71,429
|
–
|
*
|
|
Christopher A. Herr
|
691,050
|
691,050
|
–
|
*
|
|
Coastal Real Estate Investments Inc.(7)
|
273,817
|
273,817
|
–
|
*
|
|
Daniel J. Conger
|
485,233
|
485,233
|
–
|
*
|
|
David Azari
|
1,054,335
|
1,054,335
|
–
|
*
|
|
Demosthenes Dionis
|
147,819
|
147,819
|
–
|
*
|
|
Dennis DeSmith
|
124,000
|
60,000
|
64,000
|
*
|
|
Don and Bryn Oates
|
40,000
|
40,000
|
–
|
*
|
|
Douglas Goularte
|
207,440
|
207,440
|
–
|
*
|
|
Douglas J. Morgan
|
333,981
|
333,981
|
–
|
*
|
|
Duane Fitzgerald
|
209,471
|
209,471
|
–
|
*
|
|
Ermelinda Arriola
|
745,283
|
745,283
|
–
|
*
|
|
Eva Wald
|
150,117
|
150,117
|
–
|
*
|
|
Freedom Investor Corp.
|
128,800
|
128,800
|
–
|
*
|
|
G. Scott McComb
|
133,652
|
133,652
|
–
|
*
|
|
Gemini Master Fund, Ltd.(8)
|
480,000
|
480,000
|
–
|
*
|
|
Golfbully Venture Capital, LLC(9)
|
211,592
|
211,592
|
–
|
*
|
|
Harvey Bibicoff
|
502,095
|
502,095
|
–
|
*
|
|
Irving Cantor
|
150,140
|
150,140
|
–
|
*
|
|
Jack Strommen
|
6,854,154
|
6,854,154
|
–
|
*
|
|
James C. Hilbert
|
798,255
|
798,255
|
–
|
*
|
|
Jeanne M. Stratta
|
290,473
|
290,473
|
–
|
*
|
|
Jeffrey Jackson
|
200,000
|
200,000
|
–
|
*
|
|
Jeffrey Jeremiah McCarty
|
186,802
|
186,802
|
–
|
–*
|
|
Jennifer Blake
|
207,769
|
207,769
|
–
|
*
|
|
John J. Dombroski
|
50,000
|
50,000
|
–
|
*
|
|
John L. Martino
|
484,777
|
484,777
|
–
|
*
|
|
Johnathan Rubic
|
180,601
|
180,601
|
–
|
*
|
|
Jonathan Phillips
|
50,000
|
50,000
|
–
|
*
|
|
Joseph A. Martino
|
765,248
|
765,248
|
–
|
*
|
|
Julius Argumedo
|
102,307
|
102,307
|
–
|
*
|
|
Kent Shuster
|
465,514
|
465,514
|
–
|
*
|
|
Larry Backus
|
267,688
|
80,000
|
187,688
|
*
|
|
Larry Levine
|
1,259,931
|
1,259,931
|
–
|
*
|
|
Mark Sherman
|
397,517
|
397,517
|
–
|
*
|
|
Matthew B. Madden
|
134,272
|
134,272
|
–
|
*
|
|
Michael A. Krever
|
127,964
|
127,964
|
–
|
*
|
|
Michael B. Greenberg
|
206,541
|
206,541
|
–
|
*
|
|
Michael Rivkind
|
108,364
|
108,364
|
–
|
*
|
|
Moshe and Gabriel Azari
|
1,644,219
|
1,644,219
|
–
|
*
|
|
Neta Phillips
|
50,000
|
50,000
|
–
|
*
|
|
Nicholas H. Nguyen
|
466,543
|
466,543
|
–
|
*
|
|
Nicholas Steele
|
93,350
|
93,350
|
–
|
*
|
|
Partner Ship Inc.
|
697,157
|
622,157
|
75,000
|
*
|
|
Patricia Jonikaitis
|
212,301
|
212,301
|
–
|
*
|
|
Paul McDermott
|
74,666
|
74,666
|
–
|
*
|
|
Pedro Arriola
|
278,099
|
102,672
|
175,427
|
*
|
|
Peter Jonikaitis
|
212,406
|
212,406
|
–
|
*
|
|
Peter K Nitz
|
257,762
|
257,762
|
–
|
*
|
|
Phillip Harris
|
100,000
|
100,000
|
–
|
*
|
|
R. Jonathan Robinson
|
499,114
|
499,114
|
–
|
*
|
|
Ralph C. Jenney and Joanne M. Jenney
|
241,919
|
241,919
|
–
|
*
|
|
Randall A. Heller
|
40,000
|
40,000
|
–
|
*
|
|
Raymond A. Pronto
|
1,307,901
|
1,307,901
|
–
|
*
|
|
Robert A. Commandeur
|
504,094
|
504,094
|
–
|
*
|
|
Robert G Szewc
|
273,852
|
273,852
|
–
|
*
|
|
Rona K. Krenik and Mark Krenik
|
148,601
|
148,601
|
–
|
*
|
|
Scott Garver
|
147,661
|
147,661
|
–
|
*
|
|
Sean M. Tabor
|
249,357
|
249,357
|
–
|
*
|
|
Sexton Equities LLC(10)
|
12,000
|
12,000
|
–
|
*
|
|
Stephen E. Harmon
|
293,046
|
293,046
|
–
|
*
|
|
Stephen W. Prough and Kathleen R. Prough
|
210,489
|
210,489
|
–
|
*
|
|
Susan Carlisle
|
93,276
|
93,276
|
–
|
*
|
|
Teri Nowe Myers and Scott Garver
|
295,446
|
295,446
|
–
|
*
|
|
Terry Hartshorn and Sharon Hartshorn
|
631,598
|
631,598
|
–
|
*
|
|
Tim C. Wachter
|
169,930
|
169,930
|
–
|
*
|
|
Timothy C. Larsen
|
220,629
|
46,000
|
174,629
|
*
|
|
Timothy J. Ertmer and Jodi L. Ertmer
|
416,912
|
416,912
|
–
|
*
|
|
Timothy Romanow
|
250,000
|
150,000
|
100,000
|
*
|
|
Tom and Yolanda Talbot
|
548,004
|
488,004
|
60,000
|
*
|
|
Vince J. Severino
|
1,831,364
|
1,831,364
|
–
|
*
|
|
Wesley Larsen
|
280,996
|
250,996
|
30,000
|
*
|
|
William Waligora
|
211,933
|
211,933
|
–
|
*
|
|
TOTAL
|
37,787,209
|
36,090,857
|
1,696,352
|
0.9%
|
| |
(1)
|
Except as noted in any footnotes below, each person has sole voting power and sole dispositive power as to all of the shares shown as beneficially owned by them. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Our company has only one class of stock outstanding. The sum of 99,040,328 shares of common stock was outstanding on June 5, 2017, and 58,547,926 shares of common stock subject to options, warrants and convertible notes currently exercisable or convertible, or exercisable or convertible within 60 days, are deemed outstanding for determining the number of shares beneficially owned by the stockholders.
|
| |
(2)
|
Julius Argumedo is the manager of the limited liability company and therefore has dispositive power but disclaims any direct beneficial ownership.
|
| |
(3)
|
Black mountain Equities, Inc. is managed by Adam Baker who has dispositive power but disclaims beneficial ownership.
|
| |
(4)
|
Dispositive power held by Mark Stangret who disclaims beneficial ownership.
|
| |
(5)
|
Dispositive power held by Julius Argumedo, Gerarld Argumedo, and Nicole Argumedo, each of whom disclaims beneficial ownership.
|
| |
(6)
|
Dispositive power held by Woodrow Young who disclaims beneficial ownership.
|
| |
(7)
|
Dispositive power held by Robert Capetz who disclaims beneficial ownership.
|
| |
(8)
|
Gemini Strategies Inc. is the investment manager for Gemini Master Fund, Ltd. Gemini Strategies Inc. is managed by Steven Winters who has dispositive power but disclaims beneficial ownership.
|
| |
(9)
|
Dispositive power held by Kyle Berger who disclaims beneficial ownership.
|
| |
(10)
|
Dispositive power held by AJ Sexton who disclaims beneficial ownership.
|
* Less than one percent (1%)
PLAN OF DISTRIBUTION
This prospectus covers the sale of up to 36,090,857 shares of our common stock by the selling stockholders. See “Description of Offering.”
The Selling Stockholders are “underwriters,” and may be deemed to be an “underwriter,” within the meaning of the Securities Act. The Selling Stockholders and any of their respective pledgees, donees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock being offered under this prospectus on any stock exchange, market or trading facility on which shares of our common stock are traded or in private transactions. These sales may be at fixed or negotiated prices. The Selling Stockholders may use any one or more of the following methods when disposing of shares:
| |
☐ |
ordinary brokerage transactions and transactions in which the broker-dealer solicits investors;
|
| |
☐ |
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
| |
☐ |
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
| |
☐ |
an exchange distribution in accordance with the rules of the applicable exchange;
|
| |
☐ |
privately negotiated transactions;
|
| |
☐ |
to cover short sales made after the date that this Registration Statement is declared effective by the Commission;
|
| |
☐ |
broker-dealers may agree with the Selling Stockholder to sell a specified number of such shares at a stipulated price per share;
|
| |
☐ |
a combination of any such methods of sale; and
|
| |
☐ |
any other method permitted pursuant to applicable law; provided, however, that Selling Stockholders who are executive officers or directors of the Company have agreed to sell the shares they hold personally through their individual broker (not a broker that may be engaged on behalf of the Company, if applicable) and not as principal acting for their own accounts.
|
The Selling Stockholder may also sell shares under an exemption from the registration requirements under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholder (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated.
The Selling Stockholders may from time to time pledge or grant a security interest in some or all of the Shares owned by it and, if it defaults in the performance of their secured obligations, the pledgees or secured parties may offer and sell shares of common stock from time to time under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act of 1933 amending the list of Selling Stockholders to include the pledgee, transferee or other successors in interest as Selling Stockholders under this prospectus.
The Selling Stockholders also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
Any broker-dealers or agents that are involved in selling the shares offered under this prospectus may be deemed to be "underwriters," within the meaning of the Securities Act in connection with these sales. Commissions received by these broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Any broker-dealers or agents that are deemed to be underwriters may not sell shares offered under this prospectus unless and until we set forth the names of the underwriters and the material details of their underwriting arrangements in a supplement to this prospectus or, if required, in a replacement prospectus included in a post-effective amendment to the registration statement of which this prospectus is a part.
The Company has advised the Selling Stockholders that it may not use shares registered on this Registration Statement to cover short sales of common stock made prior to the date on which this Registration Statement shall have been declared effective by the Commission. If the Selling Stockholder uses this prospectus for any sale of the common stock, it will be subject to the prospectus delivery requirements of the Securities Act. The Selling Stockholder will be responsible to comply with the applicable provisions of the Securities Act and Exchange Act, and the rules and regulations thereunder promulgated, including, without limitation, Regulation M, as applicable to such Selling Stockholder in connection with resales of their respective shares under this Registration Statement. The Equity Purchaser has agreed not to engage in any direct or indirect short selling of our common stock during the term of the Purchase Agreement.
The company is required to pay all fees and expenses incident to the registration of the shares, but the company will not receive any proceeds from the sale of the common stock by Selling Stockholders.
Blue Sky Restrictions on Resale
If the selling stockholder desires to sell shares of our common stock under this prospectus in the United States, then the selling stockholder will also need to comply with state securities laws, also known as “Blue Sky laws,” with regard to secondary sales. All states offer a variety of exemptions from registration for secondary sales. Many states, for example, have an exemption for secondary trading of securities registered under Section 12(g) of the Exchange Act or for securities of issuers that publish continuous disclosure of financial and non-financial information in a recognized securities manual, such as Standard & Poor’s.
Any person who purchases shares of our common stock from the selling stockholder under this prospectus who then desires to sell such shares also will have to comply with Blue Sky laws regarding secondary sales.
DISCLOSURE OF SEC POSITION ON
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
As permitted by the Delaware general corporation law, we have included a provision in our certificate of incorporation to eliminate the personal liability of our directors for monetary damages for breach of their fiduciary duties as directors, except for liability (i) for any breach of the director’s duty of loyalty to our company, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under section 174 of the Delaware general corporation law or (iv) for any transaction from which the director derived an improper personal benefit. Our certificate of incorporation also provides that our company shall, to the full extent permitted by section 145 of the Delaware general corporation law, as amended from time to time, indemnify all persons whom it may indemnify pursuant thereto.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to the directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by our company of expenses incurred or paid by such director, officer or controlling person of our company in the successful defense of any action, suit or proceeding) is asserted by any director, officer or controlling person of our company in connection with the securities being registered in the registration statement of which this prospectus is a part, the registrant will, unless in the opinion of counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by our company is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
LEGAL OPINION
The validity of the shares covered by the registration statement of which this prospectus is a part has been passed upon for us by Wilson Bradshaw, LLP.
EXPERTS
The consolidated financial statements included in this prospectus for the years ended December 31, 2021 and 2020 have been audited by Haskell & White LLP, an independent registered public accounting firm, to the extent and for the periods set forth in their report appearing elsewhere herein (which expressed an unqualified opinion and includes an explanatory paragraph referring to conditions that raise substantial doubt about BioLargo, Inc. and subsidiaries’ ability to continue as a going concern) and are included in reliance upon such report given upon the authority of said firm as experts in auditing and accounting.
ADDITIONAL INFORMATION
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, and file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information may be inspected and copied at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549 and at the SEC’s regional offices located at the Northwestern Atrium Center, 500 West Madison Street, Suite 1400, Chicago, Illinois 60661 and 233 Broadway, New York, New York 10279. You can obtain copies of these materials from the Public Reference Section of the SEC upon payment of fees prescribed by the SEC. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC’s website (www.SEC.gov) contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC.
We have filed a registration statement on Form S-1 with the SEC under the Securities Act of 1933, as amended, with respect to the securities offered in this prospectus. This prospectus, which is filed as part of a registration statement, does not contain all of the information set forth in the registration statement, some portions of which have been omitted in accordance with the SEC’s rules and regulations. Statements made in this prospectus as to the contents of any contract, agreement or other document referred to in this prospectus are not necessarily complete and are qualified in their entirety by reference to each such contract, agreement or other document that is filed as an exhibit to the registration statement. The registration statement may be inspected without charge at the public reference facilities maintained by the SEC, and copies of such materials can be obtained from the Public Reference Section of the SEC at prescribed rates. You may obtain additional information regarding our company on our website, located at www.BioLargo.com.
INDEX TO FINANCIAL STATEMENTS
Index to Audited Consolidated Financial Statements of BioLargo, Inc. as of December 31, 2020 and 2019
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
BioLargo, Inc. and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of BioLargo, Inc. and Subsidiaries (the “Company”) as of December 31, 2021 and 2020, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the two years in the period ended December 31, 2021, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2021 and 2020, and the consolidated results of its operations and its cash flows for each of the two years in the period ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has experienced recurring losses, negative cash flows from operations, significant debt due in the near term, and has limited capital resources. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Fair Value of Stock Options—Refer to Notes 2, 5 and 10 to the Consolidated Financial Statements
Critical Audit Matter Description
The Company issues options from both BioLargo, Inc. as well as its partially-owned subsidiary, Clyra Medical. Management uses the Black-Scholes option-pricing model to estimate the fair value of its stock options. The Black-Scholes option-pricing model involves the use of significant estimates, including the following:
☐ Risk-free interest rate;
☐ Expected share price volatility;
☐ Expected dividend yield; and
☐ Expected life of the award.
In addition, management discounts the estimated fair value of the Clyra Medical stock options because the partially-owned subsidiary is a private company with no secondary market for its common stock. Given the significant estimates involved in estimating the fair value of stock options, the related audit effort in evaluating management’s estimates in determining the fair value of stock options was extensive and required a high degree of auditor judgment.
How the Critical Audit Matter was Addressed in the Audit
We obtained an understanding over the Company’s process to estimate the fair value of stock options, including how the Company develops each of the estimates required to utilize the Black-Scholes option-pricing model. We applied the following audit procedures related to testing the Company’s estimates utilized in the Black-Scholes option-pricing model:
☐ We compared the Company’s risk-free interest rate used to the comparable United States Treasury yield for a term comparable to the stock options’ expected term.
☐ We recalculated the Company’s historical share price volatility for a term comparable to the stock options’ expected term. For Clyra Medical, we recalculated a comparable public company’s historical share price volatility for a term comparable to the stock options’ expected term.
☐ We performed a look-back at the Company’s previously issued dividends, noting there were none. We inquired with management of the Company who informed us that no future dividends were currently anticipated.
☐ We agreed the expected term of stock options granted to employees and non-employees to the original contractual term of the option as management deems it likely they will remain outstanding for the entire original term. We further noted that this was consistent with historical options granted.
In addition, we reviewed management’s analysis over the discount used on the estimated fair value of the Clyra Medical stock options. Management concluded that both the illiquidity and lack of marketability warranted a discount to the estimated fair value calculated using the Black-Scholes option-pricing model. We noted that Clyra Medical is a private company and therefore its stock is not actively traded. We also reviewed the stock sales history of Clyra Medical noting the infrequent stock sales supports management’s assertions of both illiquidity and lack of marketability. We further researched published articles on valuation discounts and noted that the liquidity and lack of marketability discount used by management was within a reasonable range.
Impairment of Long-lived Asset – Refer to Notes 2 and 9 to the Consolidated Financial Statements
Critical Audit Matter Description
As reflected in the Company’s consolidated financial statements at December 31, 2021, the Company’s net carrying amount of Clyra Medical prepaid marketing is $591,000. As disclosed in Note 2 to the consolidated financial statements, long-lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. As a result of these assessments, management concluded that there was an impairment to the Company’s prepaid marketing asset during the year ended December 31, 2021.
Auditing management’s impairment test of its prepaid marketing asset was complex and highly judgmental due to the significant measurement uncertainty in determining the fair value of the prepaid marketing asset. In particular, the fair value estimate of the prepaid marketing asset was sensitive to changes in significant assumptions such as discount rates and revenue growth rates. These assumptions are affected by expected future market or economic conditions.
How the Critical Audit Matter was Addressed in the Audit
We obtained an understanding of the Company’s process to evaluate long-lived assets for impairment and related controls. We then obtained the projected revenues of Clyra Medical as well as the Company’s calculation of the expected present value of the prepaid marketing asset that were used by management to determine the fair value of the prepaid marketing asset, in accordance with ASC 350 (Intangibles – Goodwill and Other).
We applied the following audit procedures related to testing the fair value of the prepaid marketing asset:
☐ We assessed the valuation methodologies and tested the reasonableness of significant assumptions and underlying data used by management, including forecasted revenue and discount rates.
☐ We agreed the triggering start date and term used in the present value calculation to the forecasted revenue and the original contractual term.
☐ We compared management’s summary of the fair value of the prepaid marketing asset to its carrying value, noting that the carrying value exceeded the fair value of the prepaid marketing asset. As such, we concurred with management that there was an impairment of its prepaid marketing asset.
/s/ HASKELL & WHITE LLP
We have served as the Company’s auditor since 2011.
Irvine, California
March 30, 2022
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands, except for per share data)
| |
|
DECEMBER 31,
|
|
| |
|
2021
|
|
|
2020
|
|
|
Assets
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$ |
962 |
|
|
$ |
716 |
|
|
Accounts receivable, net of allowance
|
|
|
513 |
|
|
|
484 |
|
|
Inventories, net of allowance
|
|
|
241 |
|
|
|
277 |
|
|
Prepaid expenses and other current assets
|
|
|
85 |
|
|
|
28 |
|
|
Total current assets
|
|
|
1,801 |
|
|
|
1,505 |
|
| |
|
|
|
|
|
|
|
|
|
In-process research and development (Note 9)
|
|
|
— |
|
|
|
2,150 |
|
|
Equipment, net of depreciation
|
|
|
61 |
|
|
|
60 |
|
|
Other non-current assets
|
|
|
69 |
|
|
|
35 |
|
|
Investment in South Korean joint venture
|
|
|
48 |
|
|
|
63 |
|
|
Right of use, operating lease, net of amortization
|
|
|
453 |
|
|
|
341 |
|
|
Clyra Medical prepaid marketing (Note 10)
|
|
|
591 |
|
|
|
788 |
|
|
Total assets
|
|
$ |
3,023 |
|
|
$ |
4,942 |
|
| |
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity (deficit)
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses
|
|
$ |
559 |
|
|
$ |
513 |
|
|
Clyra Medical accounts payable and accrued expenses
|
|
|
230 |
|
|
|
536 |
|
|
Debt obligations (Note 4)
|
|
|
314 |
|
|
|
1,102 |
|
|
Clyra Medical debt obligations (Note 10)
|
|
|
— |
|
|
|
1,231 |
|
|
Deferred revenue
|
|
|
89 |
|
|
|
48 |
|
|
Lease liability, current
|
|
|
103 |
|
|
|
114 |
|
|
Deposits
|
|
|
79 |
|
|
|
— |
|
|
Total current liabilities
|
|
|
1,374 |
|
|
|
3,544 |
|
| |
|
|
|
|
|
|
|
|
|
Long-term liabilities:
|
|
|
|
|
|
|
|
|
|
Debt obligations (Note 4)
|
|
|
180 |
|
|
|
507 |
|
|
Lease liability
|
|
|
349 |
|
|
|
226 |
|
|
Clyra Medical debt obligations (Note 10)
|
|
|
187 |
|
|
|
— |
|
|
Common stock held for redemption (Note 9)
|
|
|
— |
|
|
|
900 |
|
|
Total long-term liabilities
|
|
|
716 |
|
|
|
1,633 |
|
|
Total liabilities
|
|
|
2,090 |
|
|
|
5,177 |
|
| |
|
|
|
|
|
|
|
|
|
COMMITMENTS AND CONTINGENCIES (Note 13)
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY (DEFICIT):
|
|
|
|
|
|
|
|
|
|
Preferred Series A, $0.00067 Par Value, 50,000,000 Shares Authorized, -0- Shares Issued and Outstanding, at December 31, 2021 and December 31, 2020
|
|
|
— |
|
|
|
— |
|
|
Common stock, $0.00067 Par Value, 400,000,000 Shares Authorized, 255,893,726 and 225,885,682 Shares Issued, at December 31, 2021 and December 31, 2020
|
|
|
171 |
|
|
|
151 |
|
|
Additional paid-in capital
|
|
|
143,718 |
|
|
|
135,849 |
|
|
Accumulated deficit
|
|
|
(139,121 |
) |
|
|
(132,041 |
) |
|
Accumulated other comprehensive loss
|
|
|
(115 |
) |
|
|
(101 |
) |
|
Total BioLargo Inc. and subsidiaries stockholders’ equity
|
|
|
4,653 |
|
|
|
3,858 |
|
|
Non-controlling interest (Note 10)
|
|
|
(3,720 |
) |
|
|
(4,093 |
) |
|
Total stockholders’ equity (deficit)
|
|
|
933 |
|
|
|
(235 |
) |
|
Total liabilities and stockholders’ equity
|
|
$ |
3,023 |
|
|
$ |
4,942 |
|
See accompanying notes to consolidated financial statements and report of Independent Registered Public Accounting Firm.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except for per share data)
| |
|
Year ended December 31,
|
|
| |
|
2021
|
|
|
2020
|
|
| |
|
|
|
|
|
|
|
|
|
Revenue
|
|
|
|
|
|
|
|
|
|
Product revenue
|
|
$ |
1,572 |
|
|
$ |
1,825 |
|
|
Service revenue
|
|
|
959 |
|
|
|
607 |
|
|
Total revenue
|
|
|
2,531 |
|
|
|
2,432 |
|
| |
|
|
|
|
|
|
|
|
|
Cost of revenue
|
|
|
|
|
|
|
|
|
|
Cost of goods sold
|
|
|
(781 |
) |
|
|
(743 |
) |
|
Cost of service
|
|
|
(647 |
) |
|
|
(461 |
) |
|
Total cost of revenue
|
|
|
(1,428 |
) |
|
|
(1,204 |
) |
|
Gross profit
|
|
|
1,103 |
|
|
|
1,228 |
|
| |
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative expenses
|
|
|
6,172 |
|
|
|
7,473 |
|
|
Research and development
|
|
|
1,367 |
|
|
|
1,338 |
|
|
Total operating expenses
|
|
|
7,539 |
|
|
|
8,811 |
|
| |
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
(6,436 |
) |
|
|
(7,583 |
) |
| |
|
|
|
|
|
|
|
|
|
Other income (expense):
|
|
|
|
|
|
|
|
|
|
Grant income
|
|
|
55 |
|
|
|
137 |
|
|
Tax credit income
|
|
|
63 |
|
|
|
111 |
|
|
Interest expense
|
|
|
(234 |
) |
|
|
(1,923 |
) |
|
Impairment expense
|
|
|
(342 |
) |
|
|
— |
|
|
Loss on extinguishment of debt
|
|
|
— |
|
|
|
(442 |
) |
|
Total other (expense) income
|
|
|
(458 |
) |
|
|
(2,117 |
) |
| |
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
(6,894 |
) |
|
|
(9,700 |
) |
| |
|
|
|
|
|
|
|
|
|
Net income (loss) attributable to noncontrolling interest
|
|
|
186 |
|
|
|
(1,268 |
) |
|
Net loss attributable to common stockholders
|
|
$ |
(7,080 |
) |
|
$ |
(8,432 |
) |
| |
|
|
|
|
|
|
|
|
|
Net loss per share attributable to common stockholders:
|
|
|
|
|
|
|
|
|
|
Loss per share attributable to stockholders – basic and diluted
|
|
$ |
(0.03 |
) |
|
$ |
(0.05 |
) |
|
Weighted average number of common shares outstanding:
|
|
|
247,203,625 |
|
|
|
195,993,575 |
|
| |
|
|
|
|
|
|
|
|
|
Comprehensive loss attributable to common stockholders
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Net loss
|
|
$ |
(6,894 |
) |
|
$ |
(9,700 |
) |
|
Foreign currency translation adjustment
|
|
|
(14 |
) |
|
|
(2 |
) |
|
Comprehensive loss
|
|
|
(6,908 |
) |
|
|
(9,702 |
) |
| |
|
|
|
|
|
|
|
|
|
Comprehensive income (loss) attributable to noncontrolling interest
|
|
|
186 |
|
|
|
(1,268 |
) |
|
Comprehensive loss attributable to stockholders
|
|
$ |
(7,094 |
) |
|
$ |
(8,434 |
) |
See accompanying notes to consolidated financial statements and report of Independent Registered Public Accounting Firm.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands, except for share data)
| |
|
Common stock
|
|
|
Additional
paid-in
|
|
|
Accumulated
|
|
|
Accumulated
other comprehensive
|
|
|
Non-
controlling
|
|
|
Total stockholders’
|
|
| |
|
Shares
|
|
|
Amount
|
|
|
capital
|
|
|
deficit
|
|
|
Loss
|
|
|
interest
|
|
|
equity (deficit)
|
|
|
Balance, December 31, 2019
|
|
|
166,256,024 |
|
|
$ |
111 |
|
|
$ |
121,327 |
|
|
$ |
(123,492 |
) |
|
$ |
(99 |
) |
|
$ |
(27 |
) |
|
$ |
(2,180 |
) |
|
Conversion of notes
|
|
|
33,157,961 |
|
|
|
22 |
|
|
|
3,504 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,526 |
|
|
Issuance of common stock for service
|
|
|
4,458,731 |
|
|
|
3 |
|
|
|
663 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
666 |
|
|
Issuance of common stock for interest
|
|
|
1,728,331 |
|
|
|
1 |
|
|
|
183 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
184 |
|
|
Sale of stock for cash
|
|
|
17,356,064 |
|
|
|
12 |
|
|
|
2,771 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,783 |
|
|
Stock issued as a commitment fee
|
|
|
2,928,571 |
|
|
|
2 |
|
|
|
(124 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(122 |
) |
|
Stock option compensation expense
|
|
|
— |
|
|
|
— |
|
|
|
1,821 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,821 |
|
|
Loss on extinguishment
|
|
|
— |
|
|
|
— |
|
|
|
442 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
442 |
|
|
Noncontrolling interest allocation
|
|
|
— |
|
|
|
— |
|
|
|
3,157 |
|
|
|
— |
|
|
|
— |
|
|
|
(3,157 |
) |
|
|
— |
|
|
Clyra stock options issued for service
|
|
|
— |
|
|
|
— |
|
|
|
638 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
638 |
|
|
Clyra stock issued for consulting agreement
|
|
|
— |
|
|
|
— |
|
|
|
788 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
788 |
|
|
Clyra stock issued as line of credit commitment fee
|
|
|
— |
|
|
|
— |
|
|
|
70 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
70 |
|
|
Issuance of Clyra common stock for cash
|
|
|
— |
|
|
|
— |
|
|
|
492 |
|
|
|
— |
|
|
|
— |
|
|
|
359 |
|
|
|
851 |
|
|
Deemed dividend
|
|
|
— |
|
|
|
— |
|
|
|
117 |
|
|
|
(117 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Net loss
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(8,432 |
) |
|
|
— |
|
|
|
(1,268 |
) |
|
|
(9,700 |
) |
|
Foreign currency translation
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2 |
) |
|
|
— |
|
|
|
(2 |
) |
|
Balance, December 31, 2020
|
|
|
225,885,682 |
|
|
$ |
151 |
|
|
$ |
135,849 |
|
|
$ |
(132,041 |
) |
|
$ |
(101 |
) |
|
$ |
(4,093 |
) |
|
$ |
(235 |
) |
|
Conversion of notes
|
|
|
1,966,439 |
|
|
|
1 |
|
|
|
327 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
328 |
|
|
Issuance of common stock for service
|
|
|
2,127,467 |
|
|
|
1 |
|
|
|
366 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
367 |
|
|
Issuance of common stock for interest
|
|
|
81,777 |
|
|
|
— |
|
|
|
16 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
16 |
|
|
Sale of stock for cash
|
|
|
29,691,886 |
|
|
|
20 |
|
|
|
4,862 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,882 |
|
|
Warrant exercise
|
|
|
1,283,333 |
|
|
|
1 |
|
|
|
163 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
164 |
|
| Return of shares held by Clyra Medical (re Scion) |
|
|
(5,142,858 |
) |
|
|
(3 |
) |
|
|
(921 |
) |
|
|
— |
|
|
|
— |
|
|
|
1,286 |
|
|
|
362 |
|
|
Stock option compensation expense
|
|
|
— |
|
|
|
— |
|
|
|
1,308 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,308 |
|
|
Fair value of warrant recorded as debt discount
|
|
|
— |
|
|
|
— |
|
|
|
35 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
35 |
|
|
Noncontrolling interest allocation
|
|
|
— |
|
|
|
— |
|
|
|
1,149 |
|
|
|
— |
|
|
|
— |
|
|
|
(1,149 |
) |
|
|
— |
|
|
Clyra stock options issued for service
|
|
|
— |
|
|
|
— |
|
|
|
564 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
564 |
|
|
Issuance of Clyra common stock for cash
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
50 |
|
|
|
50 |
|
|
Net (loss) gain
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(7,080 |
) |
|
|
— |
|
|
|
186 |
|
|
|
(6,894 |
) |
|
Foreign currency translation
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(14 |
) |
|
|
— |
|
|
|
(14 |
) |
|
Balance, December 31, 2021
|
|
|
255,893,726 |
|
|
$ |
171 |
|
|
$ |
143,718 |
|
|
$ |
(139,121 |
) |
|
$ |
(115 |
) |
|
$ |
(3,720 |
) |
|
$ |
933 |
|
See accompanying notes to consolidated financial statements and report of Independent Registered Public Accounting Firm.
BIOLARGO, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands, except for per share data)
| |
|
DECEMBER
31, 2021
|
|
|
DECEMBER
31, 2020
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$ |
(6,894 |
) |
|
$ |
(9,700 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Stock option compensation expense
|
|
|
1,872 |
|
|
|
2,459 |
|
|
Common stock issued in lieu of salary to officers and fees for services from vendors
|
|
|
367 |
|
|
|
666 |
|
|
Impairment expense
|
|
|
342 |
|
|
|
— |
|
|
Common stock issued for interest
|
|
|
16 |
|
|
|
184 |
|
|
Interest expense related to amortization of the discount on convertible notes payable, line of credit and deferred financing costs
|
|
|
119 |
|
|
|
1,618 |
|
|
Loss on extinguishment of debt
|
|
|
— |
|
|
|
442 |
|
|
Loss on investment in South Korean joint venture
|
|
|
15 |
|
|
|
37 |
|
|
PPP forgiveness
|
|
|
(43 |
) |
|
|
— |
|
|
Amortization and depreciation expense
|
|
|
20 |
|
|
|
58 |
|
|
Bad debt expense
|
|
|
— |
|
|
|
13 |
|
|
Changes in assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
|
(29 |
) |
|
|
(142 |
) |
|
Inventories
|
|
|
36 |
|
|
|
(261 |
) |
|
Accounts payable and accrued expenses
|
|
|
47 |
|
|
|
122 |
|
|
Clyra accounts payable and accrued expenses
|
|
|
132 |
|
|
|
327 |
|
|
Deferred revenue
|
|
|
41 |
|
|
|
14 |
|
|
Prepaid expenses and other assets
|
|
|
(57 |
) |
|
|
9 |
|
|
Deposit
|
|
|
79 |
|
|
|
— |
|
|
Net cash used in operating activities
|
|
|
(3,937 |
) |
|
|
(4,154 |
) |
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
Equipment purchases
|
|
|
(21 |
) |
|
|
(23 |
) |
|
Patent purchase
|
|
|
(13 |
) |
|
|
— |
|
|
Investment in South Korean joint venture
|
|
|
— |
|
|
|
(100 |
) |
|
Net cash used in investing activities
|
|
|
(34 |
) |
|
|
(123 |
) |
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
Proceeds from sale of common stock
|
|
|
4,882 |
|
|
|
2,783 |
|
|
Proceeds from SBA loans
|
|
|
— |
|
|
|
507 |
|
|
Proceeds from warrant exercise
|
|
|
164 |
|
|
|
— |
|
|
Repayment of note payable and line of credit
|
|
|
(828 |
) |
|
|
(25 |
) |
|
Proceeds received by Clyra from inventory line of credit
|
|
|
— |
|
|
|
260 |
|
|
Repayment by Clyra on inventory line of credit
|
|
|
(37 |
) |
|
|
(36 |
) |
|
Proceeds from sale of stock in Clyra Medical
|
|
|
50 |
|
|
|
851 |
|
|
Net cash provided by financing activities
|
|
|
4,231 |
|
|
|
4,340 |
|
|
Net effect of foreign currency translation
|
|
|
(14 |
) |
|
|
(2 |
) |
|
Net change in cash
|
|
|
246 |
|
|
|
61 |
|
|
Cash at beginning of year
|
|
|
716 |
|
|
|
655 |
|
|
Cash at end of year
|
|
$ |
962 |
|
|
$ |
716 |
|
|
Supplemental disclosures of cash flow information
|
|
|
|
|
|
|
|
|
|
Cash paid during the year for:
|
|
|
|
|
|
|
|
|
|
Interest
|
|
$ |
99 |
|
|
$ |
118 |
|
|
Income taxes
|
|
$ |
2 |
|
|
$ |
2 |
|
| Short-term lease payments not included in lease liability |
|
$ |
228 |
|
|
$ |
228 |
|
|
Non-cash investing and financing activities
|
|
|
|
|
|
|
|
|
| Return of in-process research and development (Scion) |
|
$ |
1,804 |
|
|
$ |
— |
|
| Cancellation of Clyra debt obligations and accounts payable (Scion) |
|
$ |
(1,465 |
) |
|
$ |
— |
|
| Liability to Scion shareholders |
|
$ |
(540 |
) |
|
$ |
— |
|
|
Fair value of warrants issued with convertible notes and letter of credit
|
|
$ |
35 |
|
|
$ |
— |
|
|
Conversion of convertible notes payable into common stock
|
|
$ |
328 |
|
|
$ |
3,526 |
|
|
Present value of Right of use and lease liability
|
|
$ |
186 |
|
|
$ |
— |
|
|
Deemed dividend
|
|
$ |
— |
|
|
$ |
117 |
|
|
Deferred offering costs recorded as additional paid in capital
|
|
$ |
— |
|
|
$ |
(122 |
) |
|
Fair value of shares issued for In Process research and development
|
|
$ |
— |
|
|
$ |
257 |
|
|
Exchange of consulting services for Clyra common shares
|
|
$ |
— |
|
|
$ |
788 |
|
|
Fair value of Clyra shares issued as commitment fee
|
|
$ |
— |
|
|
$ |
70 |
|
|
Allocation of stock option expense within noncontrolling interest
|
|
$ |
1,149 |
|
|
$ |
3,157 |
|
See accompanying notes to consolidated financial statements and report of Independent Registered Public Accounting Firm
F-8
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Business and Organization
Description of Business
BioLargo, Inc. (“BioLargo”, or the “Company”) is an innovative technology developer and environmental engineering company driven by a mission to “make life better” by delivering robust, sustainable solutions for a broad range of industries and applications, with a focus on clean water, clean air. The Company also owns a minority interest in an advanced wound care subsidiary that has licensed BioLargo’s technologies. Our business strategy is straightforward: we invent or acquire technologies that we believe have the potential to be disruptive in large commercial markets; we develop and validate these technologies to advance and promote their commercial success as we leverage our considerable scientific, engineering, and entrepreneurial talent; we then monetize these technical assets through a variety of business structures that may include licensure, joint venture, sale, spin off, or by deploying direct to market strategies.
Liquidity / Going concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of our business. For the year ended December 31, 2021, we had a net loss of $6,894,000, used $3,937,000 cash in operations, and at December 31, 2021, we had working capital of $427,000, and current assets of $1,801,000. We do not believe gross profits in 2022 will be sufficient to fund our current level of operations or pay our debts as they become due during the next 12 months, and therefore we will have to obtain further investment capital to continue to fund operations and seek to refinance our existing debt. We have been, and anticipate that we will continue to be, limited in terms of our capital resources.
During the year ended December 31, 2021, we generated revenues of $2,531,000 through our subsidiaries. (See Note 12.) Our subsidiaries did not individually or in the aggregate generate enough revenues or gross profits to fund their operations, or fund our corporate operations or other business segments. To meet our cash obligations during the year ended December 31, 2021, we (i) sold 24,255,920 shares of our common stock to Lincoln Park Capital (“Lincoln Park”) for $4,018,000 (see Note 3), and (ii) sold 5,435,966 shares of common stock, and issued warrants to purchase 10,876,932 shares of common stock, to private investors for $864,000 (see Notes 3 and 6).
As of December 31, 2021, our cash and cash equivalents totaled $962,000, and our total liabilities included $50,000 in debt that is due at the March 2023 maturity date; $187,000 owed by our partially owned subsidiary Clyra Medical Technologies, Inc. (“Clyra”) due in June 2023; $314,000 due in SBA loans issued pursuant to the Paycheck Protection Program (see note 14); and $150,000 due to the SBA issued pursuant to the Economic Injury Disaster program (EIDL) over 30 years, with $800 monthly payments scheduled to in begin January 2023.
Subsequent to December 31, 2021, we continue to sell common stock to Lincoln Park for working capital (see Note 14).
If we are unable to rely on our current arrangement with Lincoln Park to fund our working capital requirements, we will have to rely on other forms of financing, and there is no assurance that we will be able to do so, or if we do so, it will be on favorable terms.
The foregoing factors raise substantial doubt about our ability to continue as a going concern, unless we are able to continue to raise funds through stock sales to Lincoln Park or other private financings, and in the long term, our ability to attain a reasonable threshold of operating efficiencies and achieve profitable operations by licensing or otherwise commercializing products incorporating our technologies. The consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
Organization
We are a Delaware corporation formed in 1991. We have four wholly-owned subsidiaries: BioLargo Life Technologies, Inc., organized under the laws of the State of California in 2006; ONM Environmental, Inc. (formerly, Odor-No-More, Inc.), organized under the laws of the State of California in 2009; BioLargo Water Investment Group Inc. organized under the laws of the State of California in 2019, which wholly owns BioLargo Water, Inc., organized under the laws of Canada in 2014; and BioLargo Development Corp., organized under the laws of the State of California in 2016. Additionally, we own 89% (see Note 11) of BioLargo Engineering Science and Technologies, LLC (“BLEST”), organized under the laws of the State of Tennessee in 2017. We also own 56% of Clyra Medical Technologies, Inc. (“Clyra” or “Clyra Medical”), organized under the laws of the State of California in 2012, and consolidate its financial statements (see Note 2, subheading “Principles of Consolidation,” and Note 10).
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 2. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its wholly-owned subsidiaries, and partially-owned subsidiaries BLEST and Clyra Medical.
All intercompany accounts and transactions have been eliminated.
Foreign Currency
The Company has designated the functional currency of BioLargo Water, Inc., our Canadian subsidiary, to be the Canadian dollar. Therefore, translation gains and losses resulting from differences in exchange rates are recorded in accumulated other comprehensive income.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less when acquired to be cash equivalents. Substantially all cash equivalents are held in short-term money market accounts at one of the largest financial institutions in the United States. From time to time, our cash account balances are greater than the Federal Deposit Insurance Corporation insurance limit of $250,000 per owner per bank, and during such times, we are exposed to credit loss for amounts in excess of insured limits in the event of non-performance by the financial institution. We do not anticipate non-performance by our financial institution.
As of December 31, 2021 and 2020, our cash balances were made up of the following (in thousands):
| |
|
December 31,
2021
|
|
|
December 31,
2020
|
|
| |
|
|
|
|
|
|
|
|
|
BioLargo, Inc. and subsidiaries
|
|
$ |
941 |
|
|
$ |
637 |
|
| |
|
|
|
|
|
|
|
|
|
Clyra Medical Technologies, Inc.
|
|
|
21 |
|
|
|
79 |
|
|
Total
|
|
$ |
962 |
|
|
$ |
716 |
|
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Accounts Receivable
Trade accounts receivable are recorded net of allowances for doubtful accounts. Estimates for allowances for doubtful accounts are determined based on payment history and individual customer circumstances. The allowance for doubtful accounts as of December 31, 2021 and 2020 was $12,000 and $13,000, respectively.
Credit Concentration
We have a limited number of customers that account for significant portions of our revenue. During the year ended December 31, 2021, there were three customers that each accounted for more than 10% of consolidated revenues, and during the year ended December 31, 2020, there were two customers that each accounted for more than 10% of consolidated revenues, as follows:
| |
|
December 31,
2021
|
|
|
December 31,
2020
|
|
|
Customer A
|
|
|
14 |
% |
|
<10
|
% |
|
Customer B
|
|
|
11 |
% |
|
|
11 |
% |
|
Customer C
|
|
|
11 |
% |
|
<10
|
% |
|
Customer L
|
|
<10
|
% |
|
|
13 |
% |
We had two customers that each accounted for more than 10% of consolidated accounts receivable at December 31, 2021, and at December 31, 2020, as follows:
| |
|
December 31,
2021
|
|
|
December 31,
2020
|
|
|
Customer M
|
|
|
32 |
% |
|
<10
|
% |
|
Customer N
|
|
|
12 |
% |
|
<10
|
% |
|
Customer O
|
|
<10
|
% |
|
|
32 |
% |
|
Customer P
|
|
<10
|
% |
|
|
10 |
% |
Inventory
Inventories are stated at the lower of cost or net realizable value using the average cost method. The allowance for obsolete inventory as of December 31, 2021 and 2020 was $3,000. As of December 31, 2021, and 2020, inventories consisted of (in thousands):
| |
|
December 31,
2021
|
|
|
December 31,
2020
|
|
|
Raw material
|
|
$ |
108 |
|
|
$ |
111 |
|
|
Finished goods
|
|
|
133 |
|
|
|
166 |
|
|
Total
|
|
$ |
241 |
|
|
$ |
277 |
|
Other Non-Current Assets
Other non-current assets consisted of (i) security deposits of $35,000 related to our business offices, and (ii) three patents acquired on October 22, 2021, for $34,000, of which $13,000 was paid in cash and the remaining $21,000 was paid through the issuance of 125,000 shares of common stock at $0.17 per share.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Equity Method of Accounting
On March 20, 2020, we invested $100,000 into a South Korean entity (Odin Co. Ltd., “Odin”) pursuant to a Joint Venture agreement we had entered into with BKT Co. Ltd. and its U.S. based subsidiary, Tomorrow Water. We received a 40% non-dilutive equity interest, and BKT and Tomorrow Water each received 30% equity interests for an aggregate $150,000 investment.
We account for our investment in the joint venture under the equity method of accounting. We have determined that while we have significant influence over the joint venture through our technology license and our position on the Board of Directors, we do not control the joint venture or are otherwise involved in managing the entity and we own less than a majority of the equity. Therefore, we record the asset on our consolidated balance sheet and record an increase or decrease the recorded balance by our percentage ownership of the profits or losses in the joint venture. During the years ended December 31, 2021 and 2020, the joint venture incurred a loss and our 40% ownership share reduced our investment interest by $15,000 and $37,000, respectively.
Impairment
Long-lived and definite lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the sum of the expected future undiscounted cash flows from the use of the asset and its eventual disposition is less than the carrying amount of the asset, then an impairment loss is recognized. The impairment loss is measured based on the fair value of the asset. Any resulting impairment is recorded as a reduction in the carrying value of the related asset in excess of fair value and a charge to operating results. For the year ended December 31, 2021, management determined that there was an impairment expense related to the sale back to Scion Solutions, LLC (“Scion’) of certain intellectual property, recorded on our balance sheet as “In-Process Research and Development” (see Note 9), and an impairment of Clyra’s prepaid marketing asset (see Note 9). Total impairment expense for 2021 is $342,000; there was no impairment during the year ended December 31, 2020.
Earnings (Loss) Per Share
We report basic and diluted earnings (loss) per share (“EPS”) for common and common share equivalents. Basic EPS is computed by dividing reported earnings by the weighted average shares outstanding. Diluted EPS is computed by adding to the weighted average shares the dilutive effect if convertible notes payable, stock options and warrants were exercised into common stock. For the years ended December 31, 2021 and 2020, the denominator in the diluted EPS computation is the same as the denominator for basic EPS due to the Company’s net loss which creates an anti-dilutive effect of the convertible notes payable, warrants and stock options.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and revenues and expenses during the period reported. Actual results could differ from those estimates. Estimates are used when accounting for stock-based transactions, debt transactions, derivative liabilities, allowance for bad debt, asset depreciation and amortization, impairment expense, among others.
The methods, estimates and judgments we use in applying these most critical accounting policies have a significant impact on the results of our financial statements.
Share-Based Compensation Expense
We recognize compensation expense for stock option awards on a straight-line basis over the applicable service period of the award, which is the vesting period. Fair value is determined on the grant date. Share-based compensation expense is based on the grant date fair value estimated using the Black-Scholes Option Pricing Model.
For stock and stock options issued to consultants and other non-employees for services, the Company measures and records an expense as of the earlier of the date at which either: a commitment for performance by the non-employee has been reached or the non-employee’s performance is complete. The equity instruments are measured at the current fair value, and for stock options, the instruments are measured at fair value using the Black Scholes option model.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The following methodology and assumptions were used to calculate share-based compensation for the years ended December 31, 2021 and 2020:
| |
|
2021
|
|
|
2020
|
|
| |
|
Non Plan
|
|
|
2018 Plan
|
|
|
Non Plan
|
|
|
2018 Plan
|
|
|
Risk free interest rate
|
|
|
1.49 |
– |
1.73 |
%
|
|
|
0.93 |
– |
1.73 |
%
|
|
|
0.66 |
– |
1.02 |
%
|
|
|
0.64 |
– |
1.90 |
%
|
|
Expected volatility
|
|
|
118 |
– |
124 |
%
|
|
|
118 |
– |
124 |
%
|
|
|
125 |
– |
131 |
%
|
|
|
126 |
– |
133 |
%
|
|
Expected dividend yield
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
Forfeiture rate
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
Life in years
|
|
|
|
|
10 |
|
|
|
|
|
10 |
|
|
|
|
|
10 |
|
|
|
|
|
10 |
|
Expected price volatility is the measure by which our stock price is expected to fluctuate during the expected term of an option. Expected volatility is derived from the historical daily change in the market price of our common stock, as we believe that historical volatility is the best indicator of future volatility.
The risk-free interest rate used in the Black-Scholes calculation is based on the prevailing U.S. Treasury yield as determined by the U.S. Federal Reserve. We have never paid any cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future.
Warrants
Warrants issued with our convertible and non-convertible debt instruments are accounted for under the fair value and relative fair value method.
The warrant is first analyzed per its terms as to whether it has derivative features or not. If the warrant is determined to be a derivative and not qualify for equity treatment, then it is measured at fair value using the Black Scholes option model, and recorded as a liability on the balance sheet. The warrant is re-measured at its then current fair value at each subsequent reporting date (it is “marked-to-market”).
If the warrant is determined to not have derivative features, it is recorded into equity at its fair value using the Black Scholes option model, however, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the convertible note.
Convertible debt instruments are recorded at fair value, limited to a relative fair value based upon the percentage of its fair value to the total fair value including the fair value of the warrant. Further, the convertible debt instrument is examined for any intrinsic beneficial conversion feature (“BCF”) of which the conversion price is less than the closing common stock price on date of issuance. If the relative fair value method is used to value the convertible debt instrument and there is an intrinsic BCF, a further analysis is undertaken of the BCF using an effective conversion price which assumes the conversion price is the relative fair value divided by the number of shares the convertible debt is converted into by its terms. The BCF value is accounted for as equity.
The warrant and BCF relative fair values are also recorded as a discount to the convertible promissory notes. As present, these equity features of the convertible promissory notes have recorded a discount to the convertible notes that is substantially equal to the proceeds received.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Non-Cash Transactions
We have established a policy relative to the methodology to determine the value assigned to each intangible we acquire, and/or services or products received for non-cash consideration of our common stock. The value is based on the market price of our common stock issued as consideration, at the date of the agreement of each transaction or when the service is rendered or product is received.
Revenue Recognition
We account for revenue in accordance with ASC 606, “Revenue from Contacts with Customers”. The guidance focuses on the core principle for revenue recognition, which is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, the guidance provides that an entity should apply the following steps:
Step 1: Identify the contract(s) with a customer.
Step 2: Identify the performance obligations in the contract.
Step 3: Determine the transaction price.
Step 4: Allocate the transaction price to the performance obligations in the contract.
Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
The Company’s products are sold a through a contract with the customer and a written purchase order, in which the details of the contract are defined including the transaction price and method of shipment. The only performance obligation is to create and ship the product, and each product has separate pricing. Revenue is recognized at a point in time when the goods are shipped if the agreement is FOB manufacturer, and when goods are delivered if FOB destination. Revenue is recognized with a reduction for sales discounts, as appropriate and negotiated in the customer’s purchase order.
Service contracts are performed through a written contract, which specifies the performance obligations and the rate at which the services will be billed, typically by time and materials. Each service is separately negotiated and priced. Revenue is recognized as services are performed and completed, or, for services related to product installations, at the completion of the installation. A few contracts have called for milestone or fixed cost payments, where we invoice an agreed-to amount per month for the life of the contract. In these instances, completed work, billed hourly, is recognized as revenue. If the billing amount is greater or lesser than the completed work, a receivable or payable is created. These accounts are adjusted upon additional billings as the work is completed. To date, there have been no discounts or other financing terms for the contracts.
In the event that we generate revenues from royalties or license fees from our intellectual property, we anticipate a licensee would pay a license fee in one or more installments and ongoing royalties based on their sales of products incorporating or using our licensed intellectual property. Upon entering into a licensing agreement, we will determine the appropriate method of recognizing the royalty and license fees.
Clyra also has certain distribution agreements that call for consigned inventory. Although the product is shipped to a third party, it is not revenue until that consigned inventory is sold to end user customer.
Government Grants
We have been awarded multiple research grants from the private and public Canadian research programs. Income we receive directly from grants is recorded as other income. We have been awarded over 80 grants since our first in 2015. Some of the funds from these grants are given directly to third parties (such as the University of Alberta or a third-party research scientist) to support research on our technology. The grants have terms generally ranging between six and eighteen months and support a majority, but not all, of the related research budget costs. This cooperative research allows us to utilize (i) a depth of resources and talent to accomplish highly skilled work, (ii) financial aid to support research and development costs, (iii) independent and credible validation of our technical claims.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The grants typically provide for (i) recurring monthly amounts, (ii) reimbursement of costs for research talent for which we invoice to request payment, and (iii) ancillary cost reimbursement for research talent travel related costs. All awarded grants have specific requirements on how the money is spent, typically to employ researchers. None of the funds may be used for general administrative expenses or overhead in the United States. These grants have substantially increased our level of research and development activities in Canada. We continue to apply for Canadian government and agency grants to fund research and development activities. Not all of our grant applications have been awarded, and no assurance can be made that any pending grant application, or any future grant applications, will be awarded.
Income Taxes
The asset and liability approach is used to recognize deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of asset and liabilities. Deferred tax assets and liabilities are determined based on the differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect on deferred tax asset and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
We account for uncertainties in income tax law under a comprehensive model for the financial statement recognition, measurement, presentation and disclosure of uncertain tax positions taken or expected to be taken in income tax returns as prescribed by generally accepted accounting principles (“GAAP”). Under GAAP, the tax effects of a position are recognized only if it is “more-likely-than-not” to be sustained by the taxing authority as of the reporting date. If the tax position is not considered “more-likely-than-not” to be sustained, then no benefits of the position are recognized. Management believes there are no unrecognized tax benefits or uncertain tax positions as of December 31, 2021 and 2020.
The Company assessed its earnings history, trends and estimates of future earnings and determined that the deferred tax asset could not be realized as of December 31, 2021. Accordingly, a valuation allowance was recorded against the net deferred tax asset.
The Company recognizes interest and penalties on income taxes as a component of income tax expense, should such an expense be realized.
Fair Value of Financial Instruments
Management believes the carrying amounts of the Company’s financial instruments (excluding debt and equity instruments) as of December 31, 2021 and 2020 approximate their respective fair values because of the short-term nature of these instruments. Such instruments consist of cash, accounts receivable, prepaid assets, accounts payable, lines of credit, and other assets and liabilities.
Tax Credits
Our research and development activities in Canada may entitle our Canadian subsidiary to claim benefits under the “Scientific Research and Experimental Development Program”, a Canadian federal tax incentive program designed to encourage Canadian businesses of all sizes and in all sectors to conduct research and development in Canada. Benefits under the program include credits to taxable income. If our Canadian subsidiary does not have taxable income in a reporting period, we instead receive a tax refund from the Canadian Revenue Authority. Those refunds are classified in Other Income on our Consolidated Statement of Operations and Comprehensive Loss.
Leases
In accordance with ASC 842, the Company elects to use hindsight as a practical expedient with respect to determining the lease terms (as we considered our updated expectations of acceptance of the Westminster California facility lease renewal) and in assessing any impairment of right-of-use assets for existing leases. No impairment is expected at this time. The Company has also elected the short-term leases recognition exemption for all leases that qualify. This means that the Company will not recognize right-of-use assets or lease liabilities, and this includes not recognizing right-of-use assets and lease liabilities, for existing short-term leases of those assets in transition. As of December 31, 2021, the right-of-use assets and the lease liability on our balance sheet related to our operating leases totals $453,000.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Recent Accounting Pronouncements
In August 2020, the FASB issued Accounting Standards Update No. 2020-06, “Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging— Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity”. For convertible instruments, the FASB decided to reduce the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models results in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP. Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. The FASB decided to amend the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. The FASB observed that the application of the derivatives scope exception guidance results in accounting for some contracts as derivatives while accounting for economically similar contracts as equity. The FASB also decided to improve and amend the related EPS guidance. The amendments in this Update are effective for public business entities that meet the definition of a Securities and Exchange Commission (SEC) filer, excluding entities eligible to be smaller reporting companies as defined by the SEC, for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Management is currently evaluating the effect on the Company’s financials if and when future convertible securities are issued. This Update does not affect the Company’s current financial statements.
Note 3. Sale of Stock for Cash
Lincoln Park Financing
On March 30, 2020, we entered into a stock purchase agreement (the “2020 LPC Purchase Agreement”) with Lincoln Park, pursuant to which Lincoln Park agreed to purchase from us at our request up to an aggregate of $10,250,000 of our common stock (subject to certain limitations) from time to time over a period of three years. The agreement allows us, at our sole discretion, to direct Lincoln Park to purchase shares of our common stock, subject to limitations in both volume and dollar amount. The purchase price of the shares that may be sold to Lincoln Park under the agreement is the lower of (i) the lowest sale price on the date of purchase, or (ii) the average of the three lowest closing prices in the prior 12 business days. There are no restrictions on future financings, rights of first refusal, participation rights, penalties or liquidated damages other than a prohibition on entering into a “Variable Rate Transaction,” as defined in the agreement. This agreement replaced the August 2017 agreement with Lincoln Park. Concurrently with the 2020 LPC Purchase Agreement, we entered into a Registration Rights Agreement, pursuant to which we filed a registration statement on Form S-1 with the SEC on April 10, 2020. This registration statement was declared effective on April 21, 2020, and as of April 29, 2020, we commenced regular purchases under the agreement.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Pursuant to the 2020 LPC Purchase Agreement, we issued 2,928,571 shares to Lincoln Park as a commitment fee, valued at $527,000 and recorded as additional paid in capital on our consolidated balance sheet.
During the years ended December 31, 2021 and 2020, we sold 24,255,920 and 13,388,642 shares to Lincoln Park, and received $4,018,000 and $2,058,000 in gross and net proceeds. Subsequent to December 31, 2021, we continue to draw on the 2020 LPC Purchase Agreement for working capital (see Note 14).
2020 Unit Offering
During the years ended December 31, 2021 and 2020, pursuant to an offering commenced in March 2020, we sold 5,435,966 and 2,374,335 shares, respectively, of our common stock and received $864,000 and $367,000, respectively, in gross and net proceeds, from a total of nine accredited investors. In addition to the shares, we issued each shareholder a six-month and a five-year warrant to purchase additional shares (see Note 6, “Warrants Issued in 2020 Unit Offering”).
BKT Joint Venture
On February 12, 2020, we executed a “Joint Venture Framework Agreement” with a leading wastewater treatment solution provider based in South Korea (BKT Co. Ltd., “BKT”), to create a South Korean entity that would manufacture odor and VOC control products based on our CupriDyne Clean products. We received a $350,000 investment from BKT and issued 1,593,087 shares of our common stock, and invested $100,000 into the joint venture for a 40% ownership share.
Note 4. Debt Obligations
The following table summarizes our debt obligations outstanding as of December 31, 2021 and 2020 (in thousands). The table does not include debt obligations of our partially owned subsidiary Clyra Medical (see Note 10, “Debt Obligations of Clyra Medical”).
| |
|
December 31,
|
|
| |
|
2021
|
|
|
2020
|
|
|
Current portion of debt:
|
|
|
|
|
|
|
|
|
|
Note payable, matures on demand 60 days’ notice
|
|
$ |
— |
|
|
$ |
50 |
|
|
SBA Paycheck Protection Program loans, mature April 2022
|
|
|
314 |
|
|
|
— |
|
|
Line of credit, matures on 30-day demand
|
|
|
— |
|
|
|
50 |
|
|
Total notes payable and line of credit
|
|
$ |
314 |
|
|
$ |
100 |
|
| |
|
|
|
|
|
|
|
|
|
Convertible notes payable:
|
|
|
|
|
|
|
|
|
|
Convertible note payable, matures April 20, 2021
|
|
|
— |
|
|
|
100 |
|
|
Convertible note payable, matures August 9, 2021
|
|
|
— |
|
|
|
600 |
|
|
Convertible notes, mature August 12 and 16, 2021
|
|
|
— |
|
|
|
406 |
|
|
Total convertible notes payable
|
|
|
— |
|
|
|
1,106 |
|
|
Total current liabilities
|
|
$ |
314 |
|
|
$ |
1,206 |
|
| |
|
|
|
|
|
|
|
|
|
Long-term debt:
|
|
|
|
|
|
|
|
|
|
Convertible note payable, matures March 1, 2023
|
|
$ |
50 |
|
|
$ |
— |
|
|
Debt discount, net of amortization
|
|
|
(20 |
) |
|
|
— |
|
| SBA Paycheck Protection Program loans, mature April 2022 |
|
|
— |
|
|
|
357 |
|
|
SBA EIDL Loan, matures July 2053
|
|
|
150 |
|
|
|
150 |
|
|
Total long-term liabilities
|
|
$ |
180 |
|
|
$ |
507 |
|
| |
|
|
|
|
|
|
|
|
|
Total
|
|
$ |
494 |
|
|
$ |
1,713 |
|
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the years ended December 31, 2021 and 2020, we recorded $234,000 and $1,923,000 of interest expense related to the amortization of discounts on convertible notes payable and coupon interest from our convertible notes and lines of credit.
Cash payment of Debt Obligations
On August 13, 2021, we paid $178,000 in cash to Vernal Bay Investments, LLC, as payment of one-half the outstanding principal on the convertible note scheduled to mature on August 12, 2021. In addition, we issued 1,272,321 shares of our common stock to pay the remaining $228,000 principal and interest due on the note.
On March 1, 2021, we paid in cash the outstanding principal of $600,000 on the promissory note issued August 9, 2019, and scheduled to mature on August 9, 2021.
On March 1, 2021, we paid in cash the outstanding principal of $50,000 on the remaining amount due on a line of credit in which was due on demand at any time after September 1, 2019. There is no remaining balance on this line of credit, and we no longer have the ability to draw on the line of credit.
Conversion of Debt Obligations
On its maturity date of April 20, 2021, we converted to equity a promissory note in the principal balance of $100,000 into 400,000 shares of our common stock, and $9,994 of accrued interest into 48,706 shares of our common stock.
Note payable, matures on demand 60 day’s notice (or March 8, 2023)
On March 8, 2018, we received $50,000 and entered into a note payable. The note is due on upon demand from the noteholder, with sixty days’ notice. On March 1, 2021, we and the holder of a $50,000 note payable modified the note to set a specific maturity date of March 1, 2023, and allow the investor to convert the note to our common stock at a price of $0.16 per share. In lieu of interest during the extended period of the note, we issued the investor a stock purchase warrant (see Note 6).
SBA Program Loans
In April 2020, our subsidiaries ONM, BLEST and Clyra Medical received $218,000, $96,000 and $43,000, respectively, in loans pursuant to the SBA Paycheck Protection Program. The loans mature in two years and incur interest at 1%. Management believes that it has fully complied with the terms of forgiveness as set forth by the SBA, and has filed forgiveness applications. The Clyra Medical PPP loan was forgiven on March 19, 2021.
Our subsidiary ONM Environmental received an Economic Injury Disaster loan from the U.S. Small Business Administration in the amount of $150,000. The term of the loan is 30 years and has a 3.75% interest rate. Monthly payments of $800 begin January 2023.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 5. Share-Based Compensation
Issuance of Common Stock in exchange for payment of payables
Payment of Officer Salaries
During the year ended December 31, 2021, certain of our officers agreed to convert an aggregate $46,000 of accrued and unpaid salary into 214,206 shares of our common stock. The unpaid salary is converted on the last day of each quarter as follows: December 31, 2021, we issued 15,000 shares of our common stock at $0.21 per share; on September 30, 2021, we issued 61,842 shares of our common stock at $0.19; on March 31, 2021, we issued 137,364 shares of our common stock at $0.23 per share.
During the year ended December 31, 2020, certain of our officers agreed to convert an aggregate $299,000 of accrued and unpaid salary into 2,017,928 shares of our common stock. The unpaid salary is converted on the last day of each quarter as follows: December 31, 2020, we issued 652,100 shares of our common stock at $0.12 per share; on September 30, 2020, we issued 349,670 shares of our common stock at $0.15; on June 30, 2020, we issued 367,403 shares of our common stock at $0.16; on March 31, 2020, we issued 648,755 shares of our common stock at $0.17 per share.
Shares issued to Officers are unvested at the date of grant and subject to a lock-up agreement restricting vesting and sale until the earlier of (i) the consummation of a sale (in a single transaction or in a series of related transactions) of BioLargo by means of a sale of (a) a majority of the then outstanding common stock of BioLargo (whether by merger, consolidation, sale or transfer of common stock, reorganization, recapitalization or otherwise) or (b) all or substantially all of the assets of BioLargo; and (ii) the successful commercialization of BioLargo’s products or technologies as demonstrated by its receipt of at least $3,000,000 in cash, or the recognition of $3,000,000 in revenue, over a 12-month period from the sale of products and/or the license of technology; and (iii) the Company’s breach of the employment agreement between the Company and Officer and resulting in Officer’s termination.
Payment of Consultant Fees
During 2021, certain of our consultants agreed to convert an aggregate $282,000 accrued and unpaid obligations into 1,913,261 shares of our common stock. The unpaid obligations were converted on the last day of each quarter as follows: December 31, 2021, we issued 348,772 shares of our common stock at $0.21 per share; September 30, 2021, we issued 586,963 shares of our common stock at $0.19 per share; June 30, 2020, we issued 367,403 shares of our common stock at $0.16 per share; March 31, 2021, we issued 610,123 shares of our common stock at $0.23 per share.
During 2020, certain of our consultants agreed to convert an aggregate $366,000 accrued and unpaid obligations into 2,440,803 shares of our common stock. The unpaid obligations are converted on the last day of each quarter as follows: December 31, 2020, we issued 373,438 shares of our common stock at $0.12 per share; September 30, 2020, we issued 270,000 shares of our common stock at $0.15 per share; June 30, 2020, we issued 1,406,630 shares of our common stock at $0.16 per share; March 31, 2020, we issued 390,735 shares of our common stock at $0.17 per share.
All of these offerings and sales were made in reliance on the exemption from registration contained in Section 4(2) of the Securities Exchange Act and/or Regulation D promulgated thereunder as not involving a public offering of securities.
Payment of Interest
During June 2021, pursuant to terms included in our debt agreements, we converted an aggregate $16,000 accrued interest into 81,777 shares of our common stock at $0.19 per share.
During 2020, pursuant to terms included in our debt agreements, we converted an aggregate $184,000 accrued interest into 1,728,331 shares of our common stock as follows: September 30, 2020, we issued 1,412,052 shares of our common stock at $0.11 per share; June 30, 2020, we issued 297,001 shares of our common stock at $0.16 per share; March 31, 2020, we issued 19,278 shares of our common stock at $0.17 per share.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
All of these offerings and sales were made in reliance on the exemption from registration contained in Section 4(2) of the Securities Exchange Act and/or Regulation D promulgated thereunder as not involving a public offering of securities.
Stock Option Expense
During the years ended December 31, 2021 and 2020, we recorded an aggregate $1,308,000 and $1,821,000, in selling general and administrative expense related to the issuance of stock options. We issued options through our 2018 Equity Incentive Plan, and outside of this plan. See Note 10 for information on stock option expense for options issued by subsidiary Clyra Medical.
2018 Equity Incentive Plan
On June 22, 2018, our stockholders adopted the BioLargo 2018 Equity Incentive Plan (“2018 Plan”) as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years. It is set to expire on its terms on June 22, 2028. Our Board of Director’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. The plan authorizes the following types of awards: (i) incentive and non-qualified stock options, (ii) restricted stock awards, (iii) stock bonus awards, (iv) stock appreciation rights, (v) restricted stock units, and (vi) performance awards. The total number of shares reserved and available for awards pursuant to this Plan as of the date of adoption of this 2018 Plan by the Board is 40 million shares. The number of shares available to be issued under the 2018 Plan increases automatically each January 1st by the lesser of (a) 2 million shares, or (b) such number of shares determined by our Board. As of December 31, 2021, 46,000,000 shares are authorized under the plan.
Activity for our stock options under the 2018 Plan during the year ended December 31, 2021, and the year ended December 31, 2020, is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Aggregate
|
|
| |
|
Options
|
|
|
Exercise
|
|
|
Price per |
|
|
intrinsic
|
|
| |
|
Outstanding
|
|
|
Price per share
|
|
|
share
|
|
|
Value(1) |
|
|
Balance, December 31, 2019
|
|
|
9,214,356 |
|
|
$ |
0.16 |
– |
0.43 |
|
|
$ |
0.25 |
|
|
|
|
|
|
Granted
|
|
|
11,197,687 |
|
|
$ |
0.12 |
|
0.40 |
|
|
$ |
0.15 |
|
|
|
|
|
|
Expired
|
|
|
(1,546,518 |
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2020
|
|
|
18,865,525 |
|
|
$ |
0.12 |
– |
0.43 |
|
|
$ |
0.19 |
|
|
|
|
|
|
Granted
|
|
|
4,320,617 |
|
|
$ |
0.13 |
– |
0.23 |
|
|
$ |
0.19 |
|
|
|
|
|
|
Balance, December 31, 2021
|
|
|
23,186,142 |
|
|
$ |
0.12 |
– |
0.43 |
|
|
$ |
0.19 |
|
|
|
|
|
|
Non-vested
|
|
|
(4,541,241 |
)
|
|
$ |
0.12 |
– |
0.40 |
|
|
$ |
0.19 |
|
|
|
|
|
|
Vested, December 31, 2021
|
|
|
18,644,901 |
|
|
$ |
0.12 |
– |
0.43 |
|
|
$ |
0.19 |
|
|
$ |
725,000 |
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.21 at December 31, 2021.
The options granted under the 2018 Plan to purchase 4,320,617 shares during 2021 were issued to officers, board of directors, employees and consultants: (i) we issued options to purchase 300,000 shares of our common stock at an exercise price on the respective grant date of $0.17 per share to our CFO as described below; (ii) we issued options to purchase 1,370,454 shares of our common stock at an exercise price on the respective grant dates between $0.17 and $0.23 per share to members of our board of directors for services performed, in lieu of cash; the fair value of these options totaled $257,000; (iii) we issued options to purchase 2,214,594 shares of our common stock to employees as part of an employee retention and expiring options plan at exercises price on the respective date ranging between $0.17 and $0.23 per share; the fair value of employee retention plan options totaled $410,000 and will vest quarterly over four years as long as they are retained as employees; and (iv) we issued options to purchase 435,569 shares of our common stock to consultants and employees in lieu of cash for unpaid obligations totaling $77,000. All stock option expense is recorded on our consolidated statement of operations as selling, general and administrative expense.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The options granted under the 2018 Plan to purchase 11,197,687 shares during 2020 were issued to officers, board of directors, employees and consultants: (i) we issued options to purchase 4,880,945 shares of our common stock at an exercise price of $0.14 per share to employees and consultants as a bonus during the COVID-19 pandemic. These options vest quarterly over one year and the fair value totaled $616,000; (ii) we issued options to purchase 517,500 shares of our common stock at an exercise price range of $0.14 – $0.21 per share to our CFO, with 492,500 shares having vested during 2020, and the remaining shares to vest 25,000 in January 31, 2021, the fair value of the options issued to our CFO totals $100,000; (iii) we issued options to purchase 1,746,434 shares of our common stock at an exercise price on the respective grant date of $0.17 ,$0.16, $0.15 and $0.12 per share to members of our board of directors for services performed, all options vested at issuance and the fair value of these options totaled $250,000; (iv) we issued options to purchase 2,019,556 shares of our common stock to employees as part of an employee retention plan at an exercise price on the respective date of $0.17, $0.16, $0.15 and $0.12 per share; the fair value of employee retention plan options totaled $277,000 and vest quarterly over four years as long as they are retained as employees; (v) we issued options to purchase 531,298 shares of our common stock to consultants in lieu of cash for unpaid obligations totaling $74,000; and (vi) we issued options to purchase 1,501,954 shares of common stock at an exercise price ranging between $0.14 – $0.17 per share to employees to convert accrued and unpaid obligations and for previously issued options that expire. All of these options vested at issuance and the fair value totaled $198,000. All stock option expense is recorded on our consolidated statement of operations as selling, general and administrative expense.
Chief Financial Officer Contract Extension
On March 17, 2021, we and our Chief Financial Officer Charles K. Dargan, II, formally agreed to extend the engagement agreement dated February 1, 2008 (the “Engagement Agreement”, which had been previously extended multiple times), pursuant to which Mr. Dargan has been and continues to serve as the Company’s Chief Financial Officer. The extension agreement provides for an additional one-year term to expire January 31, 2022 (the “Extended Term”).
As the sole compensation for the Extended Term, Mr. Dargan was issued an option (“Option”) to purchase 300,000 shares of our common stock. The Option vests over the period of the Extended Term, with 275,000 shares having vested as of March 15, 2021, and the remaining shares to vest 25,000 shares monthly beginning March 31, 2021, and each month thereafter, so long as the agreement is in full force and effect. The Option is exercisable at $0.174 per share, the closing price of BioLargo’s common stock on the March 18, 2021 grant date, expires ten years from the grant date, and was issued pursuant to the Company’s 2018 Equity Incentive Plan. The fair value of these options totaled $49,000, which expense is recorded ratably over the twelve-month agreement term.
The Option is Mr. Dargan’s sole compensation for the Extended Term. As was the case in all prior terms of his engagement, there is no cash component of his compensation for the Extended Term. Mr. Dargan is eligible to be reimbursed for business expenses he incurs in connection with the performance of his services as the Company’s Chief Financial Officer (although he has made no such requests for reimbursement in the past). All other provisions of the Engagement Agreement not expressly amended pursuant to the Engagement Extension Agreement remain the same, including provisions regarding indemnification and arbitration of disputes.
See also Note 14, Subsequent Events.
2007 Equity Incentive Plan
On September 7, 2007, and as amended April 29, 2011, the BioLargo, Inc. 2007 Equity Incentive Plan (“2007 Plan”) was adopted as a means of providing our directors, key employees and consultants additional incentive to provide services. Both stock options and stock grants may be made under this plan for a period of 10 years, which expired on September 7, 2017. The Board’s Compensation Committee administers this plan. As plan administrator, the Compensation Committee has sole discretion to set the price of the options. As of September 2017, the Plan was closed to further stock option grants.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Activity for our stock options under the 2007 Plan for the years ended December 31, 2021 and 2020 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Aggregate
|
|
|
|
|
Options |
|
|
Exercise
|
|
|
Price per
|
|
|
intrinsic
|
|
|
|
|
Outstanding |
|
|
price per share
|
|
|
share
|
|
|
Value(1)
|
|
|
Balance, December 31, 2019
|
|
|
8,769,451 |
|
|
$ |
0.22 |
– |
0.94 |
|
|
$ |
0.43 |
|
|
|
|
|
|
Expired
|
|
|
(3,080,088 |
)
|
|
|
0.22 |
– |
0.58 |
|
|
|
0.38 |
|
|
|
|
|
|
Balance, December 31, 2020
|
|
|
5,689,363 |
|
|
$ |
0.23 |
– |
0.94 |
|
|
$ |
0.44 |
|
|
|
|
|
|
Expired
|
|
|
(2,810,117 |
)
|
|
|
0.34 |
– |
0.51 |
|
|
|
0.38 |
|
|
|
|
|
|
Balance, December 31, 2021
|
|
|
2,879,246 |
|
|
$ |
0.23 |
– |
0.94 |
|
|
$ |
0.49 |
|
|
$ |
— |
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.21 at December 31, 2021.
Non-Plan Options issued
Activity of our non-plan stock options issued for the years ended December 31, 2021 and 2020 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
| |
|
Non-plan
|
|
|
|
|
|
|
|
|
average
|
|
|
Aggregate
|
|
| |
|
Options
|
|
|
Exercise
|
|
|
price per
|
|
|
intrinsic
|
|
| |
|
outstanding
|
|
|
price per share |
|
|
share
|
|
|
value(1)
|
|
|
Balance, December 31, 2019
|
|
|
19,604,107 |
|
|
$ |
0.16 |
– |
1.00 |
|
|
$ |
0.43 |
|
|
|
|
|
|
Granted
|
|
|
1,145,476 |
|
|
|
0.12 |
– |
0.21 |
|
|
|
0.15 |
|
|
|
|
|
|
Balance, December 31, 2020
|
|
|
20,749,583 |
|
|
$ |
0.12 |
– |
1.00 |
|
|
$ |
0.41 |
|
|
|
|
|
|
Granted
|
|
|
169,624 |
|
|
|
0.17 |
– |
0.23 |
|
|
|
0.20 |
|
|
|
|
|
|
Expired
|
|
|
(800,000 |
)
|
|
|
|
1.00 |
|
|
|
|
1.00 |
|
|
|
|
|
|
Balance, December 31, 2021
|
|
|
20,119,207 |
|
|
$ |
0.12 |
– |
0.83 |
|
|
$ |
0.39 |
|
|
|
|
|
|
Unvested
|
|
|
(1,360,944 |
)
|
|
|
|
0.45 |
|
|
|
|
0.45 |
|
|
|
|
|
|
Vested and outstanding, December 31, 2021
|
|
|
18,758,263 |
|
|
$ |
0.12 |
– |
0.83 |
|
|
$ |
0.38 |
|
|
$ |
93,000 |
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.21 at December 31, 2021.
During the year ended December 31, 2021, we issued options to purchase an aggregate 169,624 shares of our common stock at exercise prices ranging between $0.17 – $0.23 per share to vendors for fees for services. The fair value of the options issued totaled an aggregate $34,000 and is recorded in our selling, general and administrative expense.
During the year ended December 31, 2020, we issued options to purchase an aggregate 1,145,476 shares of our common stock at exercise prices ranging between $0.12 – $0.21 per share to vendors for fees for services. The fair value of the options issued totaled an aggregate $167,000 and is recorded in our selling, general and administrative expense.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 6. Warrants
We have certain warrants outstanding to purchase our common stock, at various prices, as described in the following table:
| |
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
average
|
|
|
Aggregate
|
|
|
|
|
|
Warrants |
|
|
Exercise
|
|
|
price per |
|
|
intrinsic
|
|
|
|
|
|
outstanding |
|
|
price per share |
|
|
share |
|
|
value(1)
|
|
|
Balance, December 31, 2019
|
|
|
43,231,161 |
|
|
$ |
0.25 |
– |
1.00 |
|
|
$ |
0.35 |
|
|
|
|
|
|
Granted
|
|
|
5,594,314 |
|
|
|
0.13 |
– |
0.27 |
|
|
|
0.20 |
|
|
|
|
|
|
Expired
|
|
|
(15,844,486 |
)
|
|
|
0.18 |
– |
0.70 |
|
|
|
0.43 |
|
|
|
|
|
|
Balance, December 31, 2020
|
|
|
32,980,989 |
|
|
$ |
0.13 |
– |
1.00 |
|
|
$ |
0.29 |
|
|
|
|
|
|
Granted
|
|
|
11,096,992 |
|
|
|
0.12 |
– |
0.14 |
|
|
|
0.21 |
|
|
|
|
|
|
Exercised
|
|
|
(1,283,333 |
)
|
|
|
0.12 |
– |
0.14 |
|
|
|
0.13 |
|
|
|
|
|
|
Expired
|
|
|
(6,029,086 |
)
|
|
|
0.12 |
– |
0.70 |
|
|
|
0.30 |
|
|
|
|
|
|
Balance, December 31, 2021
|
|
|
36,765,562 |
|
|
$ |
0.13 |
– |
1.00 |
|
|
$ |
0.27 |
|
|
$ |
280,000 |
|
(1) – Aggregate intrinsic value based on closing common stock price of $0.21 at December 31, 2021.
Warrants issued in 2020 Unit Offering
During the years ended December 31, 2021 and 2020, pursuant to our 2020 Unit Offering (see Note 3), we issued six-month stock purchase warrants to purchase an aggregate 5,435,996 shares of our common stock at prices from $0.14 - $0.23 per share, and five-year stock purchase warrants to purchase an aggregate 5,435,996 shares of our common stock at prices from $0.16 - $0.29 per share.
Warrant issued in conjunction with amendment to note payable
On March 1, 2021, we and the holder of a $50,000 note payable modified the note (see Note 4). In lieu of interest during the extended period of the note, we issued the investor a warrant to purchase 225,000 shares of our common stock at $0.16 per share for a period of five years. The fair value of these warrants totaled $35,000 and is recorded as a debt discount on our consolidated balance sheets, of which amount will be amortized to interest expense over the two-year term of the debt.
Exercise of Warrants
During the year ended December 31, 2021, we issued an aggregate 1,283,333 shares of our common stock from the exercise of outstanding stock purchase warrants and in exchange we received proceeds totaling an aggregate $164,000.
Fair Value – Interest Expense
To determine interest expense related to our outstanding warrants issued in conjunction with debt offerings, the fair value of each award grant is estimated on the date of grant using the Black-Scholes option pricing model and the relative fair values are amortized over the life of the warrant. For the determination of expense of warrants issued for services, extinguishment of debt and settlement management also uses the option-pricing model. The principal assumptions we used in applying this model were as follows:
| |
|
2021
|
|
|
2020
|
|
|
Risk free interest rate
|
|
|
|
|
0.71 |
%
|
|
|
0.10 |
– |
0.23 |
%
|
|
Expected volatility
|
|
|
|
|
100 |
%
|
|
|
100 |
– |
112 |
%
|
|
Expected dividend yield
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
Forfeiture rate
|
|
|
|
|
— |
|
|
|
|
|
— |
|
|
Expected life in years
|
|
|
.5 |
– |
5 |
|
|
|
2 |
– |
5 |
|
The risk-free interest rate is based on U.S. Treasury yields in effect at the time of grant. Expected volatilities are based on historical volatility of our common stock. The expected life in years is based on the contract term of the warrant.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 7. Accounts Payable and Accrued Expenses
As of December 31, 2021, accounts payable and accrued expenses included the following (in thousands):
|
Category
|
|
BioLargo
|
|
|
ONM
|
|
|
BLEST
|
|
|
Water
|
|
|
Elim
|
|
|
Totals
|
|
|
Accounts payable
|
|
$ |
156 |
|
|
$ |
72 |
|
|
$ |
73 |
|
|
$ |
96 |
|
|
$ |
(47 |
) |
|
$ |
350 |
|
|
Accrued payroll
|
|
|
37 |
|
|
|
53 |
|
|
|
94 |
|
|
|
— |
|
|
|
— |
|
|
|
184 |
|
|
Accrued interest
|
|
|
25 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
25 |
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
559 |
|
As of December 31, 2020, accounts payable and accrued expenses included the following (in thousands):
|
Category
|
|
BioLargo
|
|
|
ONM
|
|
|
BLEST
|
|
|
Water
|
|
|
Elim
|
|
|
Totals
|
|
|
Accounts payable
|
|
$ |
125 |
|
|
$ |
73 |
|
|
$ |
56 |
|
|
$ |
103 |
|
|
$ |
(42 |
) |
|
$ |
315 |
|
|
Accrued payroll
|
|
|
23 |
|
|
|
42 |
|
|
|
91 |
|
|
|
— |
|
|
|
— |
|
|
|
156 |
|
|
Accrued interest
|
|
|
42 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
42 |
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
513 |
|
See Note 10, “Accounts Payable and Accrued Expenses”, for the accounts payable and accrued expenses of Clyra Medical.
Note 8. Provision for Income Taxes
Given our historical losses from operations, income tax obligations have been limited to the minimum franchise tax assessed by the State of California. Since 2016, we have not consolidated for tax purposes, our subsidiary, Clyra Medical, as our ownership interest was less than 80%. Our subsidiary BLEST is a Tennessee limited liability company and as such, is not consolidated in our corporate tax return. As a pass-through entity, it does not pay federal taxes. However, the state of Tennessee charges franchise and excise taxes for limited liability companies, and thus BLEST will incur a nominal franchise tax and will not pay an excise tax unless and until it is profitable.
At December 31, 2021, we had federal and California tax net operating loss carry-forwards (“NOLs”) of approximately $109,000,000 and $52,000,000 respectively. Due to changes in our ownership through common stock issuances throughout the year, the utilization of NOLs may be subject to annual limitations and discounts under provisions of the Internal Revenue Code. We have not conducted a complete analysis to determine the extent of these limitations or any future limitation. Such limitations could result in the permanent loss of a significant portion of the NOLs. Under the Tax Cuts and Jobs Act (“TCJA”) signed into law on December 22, 2018, post‑2018 NOLs may be carried forward indefinitely, and pre‑2018 NOLs have a 20-year limitation on carryforwards; however, the NOLs are limited to the lesser of (1) the aggregate of the NOL carryovers to such year, plus the NOL carry-backs to such year, or (2) 80% of taxable income (determined without regard to the deduction) (Internal Revenue Code Sec. 172(a)). Generally, NOLs can no longer be carried back but are allowed to be carried forward indefinitely (Sec. 172(b)(1)(A), which applies to 2018 and later NOLs only). Nevertheless, for California purposes, the additional taxable income limitations on NOL carryforwards as well as the indefinite time to use the NOLs have not been adopted. Therefore, for California, NOLs expire after 20 years. As such, ours will begin to expire in for the tax period ending December 31, 2021. On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security (CARES) Act was signed into law, under which federal NOLs could be carried back for five years against taxable income. Since BioLargo does not have any taxable income, this provision of the CARES Act will not affect any tax position. Realization of our deferred tax assets, which relate to operating loss carryforwards and timing differences, is dependent on future earnings. The timing and amount of future earnings are uncertain and therefore we have established a 100% valuation allowance.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 9. In-Process Research and Development; Impairment expense
Scion Solutions Transaction dated March 1, 2022
On September 26, 2018, BioLargo and Clyra Medical entered into a transaction (the “Scion Transaction”) whereby BioLargo would acquire, and then license back to Clyra, the intangible assets of Scion Solutions, LLC (“Scion”), and in particular its in-process research and development of the “SkinDisc,” a method for treating advanced hard-to-treat wounds including diabetic ulcers. In addition to a pending patent application, the assets included the technical know-how and data developed by the Scion team.
The consideration provided to Scion, which was subject to an escrow agreement dated September 26, 2018 (“Escrow Agreement”) and earn out provisions, included: (i) 21,000 shares of the Clyra Medical common stock; (ii) 10,000 shares of Clyra Medical common stock redeemable for 7,142,858 BioLargo common shares (detailed below); and (iii) a promissory note in the principal amount of $1,250,000 owed by Clyra Medical (“Clyra-Scion Note”). The Clyra-Scion note accrued interest at an annual rate of 5%. As of December 31, 2021, $243,000 had be paid in reduction of principal owed on the Clyra-Scion note.
Immediately following Clyra Medical’s purchase of Scion’s intangible assets, Clyra Medical sold to BioLargo the assets, along with 12,755 Clyra Medical common shares. In exchange, BioLargo issued Clyra Medical 7,142,858 shares of BioLargo common stock. Concurrently, BioLargo licensed back to Clyra Medical the Scion assets. Scion may exchange its 10,000 Clyra Medical common shares for the 7,142,858 shares of BioLargo common stock issued to Clyra Medical, subject to the escrow and earn-out provisions described above. The fair value of the 7,142,858 BioLargo shares at December 31, 2020, was $2,150,000.
During the year ended December 31, 2020, Clyra Medical’s gross revenue exceeded $200,000, and thus the first and second performance metrics in the Escrow Agreement were met. As a result, Scion vested 6,200 Clyra Medical common shares, of which 2,200 are redeemable for 1,428,571 BioLargo shares. The fair value of the newly vested shares total was $257,000 at December 31, 2020. On our balance sheet, the In-Process Research and Development asset, and Common Stock Held for Redemption liability, each increased by that amount as of December 31, 2020.
By written agreement dated March 1, 2022, fully executed on March 3, 2022, Clyra Medical and BioLargo agreed to sell back to Scion the Scion IP purchased in the 2018 Scion Transaction. In exchange, Scion agreed to (i) accept 2,000,000 (of the 5,000,000) BioLargo common shares it had earned, (ii) forgive the outstanding principal (of $1,007,000) and interest (of $133,000) due on the Scion Promissory Note, and (iii) return all shares of Clyra common stock owned by Scion. Additionally, Scion members Spencer Brown, Tanya Rhodes, and Dr. Brock Liden each forgave all amounts due to them pursuant to their consulting agreements with Clyra Medical, which, in the aggregate, represented $305,000 on Clyra Medical’s accounts payable, and Spencer Brown resigned from Clyra Medical’s board of directors. The agreement further provided that Clyra Medical and BioLargo indemnify Scion and related parties from any claims related to the SkinDisc, and for mutual releases of any claims between the parties.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Spencer Brown and Tanya Rhodes each entered into non-disclosure agreements whereby they agreed not to disclose Clyra Medical or BioLargo’s proprietary information, and five-year non-compete provisions whereby they agreed not to engage in competition with copper-iodine complex technologies that are the same or substantially similar to Clyra Medical’s intellectual property. In exchange, Clyra Medical, BioLargo, and certain agents of Clyra Medical and BioLargo agreed not to engage in competition with Scion’s SkinDisc technology.
Separately, BioLargo and Clyra Medical entered into an agreement dated March 3, 2022, whereby (i) BioLargo agreed to transfer to Scion the Scion IP in accordance with the March 1, 2022 agreement listed on its balance sheet as In-process Research and Development, which was valued at $2,150,000, and (ii) Clyra Medical transferred to BioLargo for its return to treasury 5,142,858 shares of BioLargo common stock.
Although these agreements were not fully executed until March 3, 2022, the essential terms of the agreement between Scion and Clyra Medical/BioLargo that would have a material impact on BioLargo’s financial statements remained unchanged since the first draft of the transaction document prepared and agreed to by both parties in December 2021, subject to document finalization and execution, and therefore, management considered the guidance in Accounting Standard Codification 855, whereby since the condition existed as of the balance sheet date, with further evidence arising subsequent to the balance sheet date, the Company recognizes the financial statement effects of this transaction as of December 31, 2021.
Impairment of Other Asset, Prepaid Marketing
On December 30, 2015, Clyra entered into a consulting agreement with Beach House Consulting, LLC, through which Jack B. Strommen is obligated to provide consulting services to Clyra Medical related to its sales and marketing activities, in exchange for $23,000 per month for a period of four years. On June 30, 2020, at Clyra’s request, Beach House Consulting agreed to accept 3,639 shares of Clyra common stock valued at $788,000, in lieu of cash, as full prepayment of the consulting fee. The obligation to provide the consulting services is dependent on Clyra generating an average of $250,000 in monthly sales over three consecutive months, which has not been met. The value of the shares issued to Beach House totaled $788,000, and the obligation is recorded as a non-current asset on our balance sheet. In light of Clyra Medical’s revenues for the year ended December 31, 2021, and its shift of focus to a surgical wash product which it began selling in the three months ending March 31, 2022, Management determined as of December 31, 2021, to impair the asset by 25% ($197,000). The impairment amount was charged to our selling, general and administrative expenses.
The following table summarizes the expenses related to the foregoing transactions as of December 31, 2021.
| |
|
Biolargo
Corporate
|
|
|
Clyra
|
|
|
Total
|
|
|
In-Process Research and Development
|
|
$ |
(2,150,000 |
) |
|
$ |
— |
|
|
$ |
(2,150,000 |
) |
|
Clyra debt obligations (Clyra-Scion note)
|
|
|
— |
|
|
|
1,007,000 |
|
|
|
1,007,000 |
|
|
Accounts payable and accrued interest
|
|
|
— |
|
|
|
458,000 |
|
|
|
458,000 |
|
|
Liability to Scion shareholders
|
|
|
— |
|
|
|
540,000 |
|
|
|
540,000 |
|
|
Other asset, prepaid marketing
|
|
|
— |
|
|
|
(197,000 |
) |
|
|
(197,000 |
) |
|
Total
|
|
$ |
(2,150,000 |
) |
|
$ |
1,808,000 |
|
|
$ |
(342,000 |
) |
Note 10. Noncontrolling Interest – Clyra Medical
As discussed in Note 2, above, we consolidate the operations of our partially owned subsidiary Clyra Medical, of which we owned 56% of its outstanding shares as of December 31, 2021. The increase in BioLargo’s ownership is due to the reduction in the shares outstanding through the Scion transaction (see Note 9, “ In-process Research and Development”).
Debt Obligations of Clyra Medical
Line of Credit
On June 30, 2020, Clyra Medical entered into a Revolving Line of Credit Agreement whereby Vernal Bay Capital Group, LLC committed to provide a $1,000,000 inventory line of credit. Clyra Medical received $260,000 in draws and made repayments totaling $73,000. As of December 31, 2021, the balance outstanding on this line of credit totals $187,000. Funds from the line of credit must be used to produce inventory. Additional draws are conditional upon the presentation of invoices or purchase orders to the lender equal to the greater of one-half of principal outstanding on the line of credit, and $200,000. The line of credit note earns interest at 15%, matures in one year, and requires Clyra pay interest and principal from gross product sales. For the first 180 days, on a monthly basis, Clyra is required to pay 30% of gross product sales to reduce amounts owed, and thereafter 60% of gross sales. Clyra issued Vernal Bay 323 shares of its common stock as a commitment fee for the line of credit, valued at $70,000. A security agreement of the same date grants Vernal Bay a security interest in Clyra’s inventory, as that term is defined in the Uniform Commercial Code. Clyra may prepay the note at any time.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Consulting Agreement
On December 30, 2015, Clyra entered into a consulting agreement with Beach House Consulting, LLC, through which Jack B. Strommen will be providing consulting services to Clyra related to its sales and marketing activities, and in exchange receive $23,000 per month for a period of four years. On June 30, 2020, at Clyra’s request, Beach House Consulting agreed to accept 3,639 shares of Clyra common stock, in lieu of cash, as full prepayment of the consulting fee. The obligation to provide the consulting services is dependent on Clyra generating an average of $250,000 in monthly sales over three consecutive months, which has not been met. The value of the shares issued to Beach House totaled $591,000 and is recorded as a non-current asset on our balance sheet. (See Note 9, “Other Asset, Prepaid Marketing”.)
Equity Transactions
As of December 31, 2021, Clyra had the following common shares outstanding:
|
Shareholder
|
|
Shares
|
|
|
Percent
|
|
|
BioLargo, Inc.
|
|
|
49,207 |
|
|
|
56 |
% |
|
Sanatio Capital
|
|
|
18,704 |
|
|
|
22 |
% |
|
Other
|
|
|
19,280 |
|
|
|
22 |
% |
|
Total
|
|
|
87,191 |
|
|
|
|
|
Sales of Common Shares
During the year ended December 31, 2021, Clyra sold 161 shares of its common stock for $50,000 to private investors at $310 per Clyra share.
In June 2020, BioLargo increased its investment in Clyra by 23,004 shares. Of this amount, 22,513 shares were issued to BioLargo pursuant to an amendment to the BioLargo/Clyra license agreement whereby BioLargo has granted Clyra rights to commercialize its technology in certain medical fields. The amendment provided, among other things, for the payment of the “initial license fee” through the issuance of 22,513 shares of Clyra common stock. Additionally, BioLargo acquired 490 shares of Clyra common stock by making vendor payments on Clyra’s behalf in exchange for the equity, at a price of $310 per share.
During the year ended December 31, 2020, Clyra sold 2,742 shares of its common stock for $851,000 at $200 per share.
Stock Options
Clyra issues options to its employees and consultants in lieu of compensation owed on a regular basis. As of December 31, 2021, the Company had issued options to purchase 11,411 shares of Clyra stock. During the years ended December 31, 2021 and 2020, Clyra issued options to purchase 2,594 and 3,943 shares of its common stock, respectively. Each option issued has an exercise price of $1.00 per share, are vested upon issuance and an expiration date 10 years from the date of grant. The fair value of the options issued in in the nine months ended September 30, 2021 and 2020 totaled $564,000 and $788,000, respectively. We used the Black-Scholes model to calculate the initial fair value, assuming a stock price on date of grant of $310 per share. Because Clyra is a private company with no secondary market for its common stock, the resulting fair value was discounted by 30%.
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Accounts Payable and Accrued Expenses
At December 31, 2021 and 2020, Clyra had the following accounts payable and accrued expenses: Amount (in thousands)
|
Category
|
|
December 31, 2021
|
|
|
December 31, 2020
|
|
|
Accounts payable
|
|
$ |
149 |
|
|
$ |
402 |
|
|
Accrued payroll
|
|
|
30 |
|
|
|
32 |
|
|
Accrued interest
|
|
|
51 |
|
|
|
102 |
|
|
Total
|
|
$ |
230 |
|
|
$ |
536 |
|
Note 11. BioLargo Engineering, Science and Technologies, LLC
In September 2017, we commenced a full-service environmental engineering firm and formed a Tennessee entity named BioLargo Engineering, Science & Technologies, LLC (“BLEST”). In conjunction with the start of this subsidiary, we entered into a three-year office lease in the Knoxville, Tennessee area, and entered into employment agreements with six scientists and engineers. (See Note 12 “Business Segment Information”.) BLEST was capitalized with two classes of membership units: Class A, 100% owned by BioLargo, and Class B, held by management of BLEST, and which initially have no “profit interest,” as that term is defined in Tennessee law. However, over the succeeding five years, the Class B members can earn up to a 30% profit interest. They also have been granted options to purchase up to an aggregate 1,750,000 shares of BioLargo, Inc. common stock. The profit interest and option shares are subject to a five year vesting schedule tied to the performance of the subsidiary, including gross revenue targets that increase over time, obtaining positive cash flow by March 31, 2018 (which was not met), collecting 90% of its account receivables, obtaining a profit of 10% in its first year (and increasing in subsequent years), making progress in the scale-up and commercialization of our AOS system, and using BioLargo research scientists (such as our Canadian team) for billable work on client projects. These criteria are to be evaluated annually by BLEST’s compensation committee (which includes BioLargo’s president, CFO, and BLEST’s president), beginning September 2018. Given the significant performance criteria, the Class B units and the stock options will only be recognized in compensation expense if or when the criteria are satisfied.
The BLEST Compensation Committee has met regularly since the subsidiary commenced operations. In 2018, it reviewed the operating performance and determined that the performance metrics were not met and as a result, did not award any Class B units or stock options. In November 2019, it determined that a portion of the performance metrics were met, and that one-half of the eligible profits interests would be vested (2.5% in the aggregate), and therefore one-half of the option interests (10%) would be vested (175,000 options shares in the aggregate). The vesting of option shares resulted in a fair value totaling $44,000, recorded on our consolidated statement of operations as selling, general and administrative expense. The fair value of the profit interest was nominal and not booked. In January 2021, the committee again reviewed the operating performance and determined that a portion of the performance metrics were met. It was agreed that one-half and one-quarter of the eligible profit interests would be vested (3.75% in the aggregate), and therefore one-half of the option interests (15%) would be vested (262,500 options shares in the aggregate). The vesting of option shares resulted in a fair value totaling $65,000, recorded on our consolidated statement of operations as selling, general and administrative expense for the year ended December 31, 2020. In January 2022, the committee again reviewed the operating performance and determined that a portion of the performance metrics were met. It was agreed that an additional one-half and one-quarter of the eligible profits interests would be vested (6.50% in the aggregate), and therefore an additional half of the option interests would be vested (525,000 options shares in the aggregate). The vesting of option shares resulted in a fair value totaling $130,000; $65,000 is recorded on our consolidated statement of operations as selling, general and administrative expense for each of the years ended December 31, 2021 and December 31, 2020.
Note 12. Business Segment Information
BioLargo currently has four operating business segments, plus its corporate entity which is responsible for general corporate operations, including administrative functions, finance, human resources, marketing, legal, etc. The four operational business segments are:
| |
1.
|
ONM Environmental -- which sells odor and volatile organic control products and services (located in Westminster, California);
|
| |
2.
|
Clyra Medical Technologies (“Clyra Medical”) -- which develops and sells medical products based on our technologies;
|
| |
3.
|
BLEST -- which provides professional engineering services on a time and materials basis for outside clients and supports our internal operations as needed (located in Oak Ridge, Tennessee); and
|
| |
4.
|
BioLargo Water (“Water”) -- which historically focused entirely on R&D, and has now shifted its focus to commercializing the AOS technology (located in Edmonton, Alberta Canada).
|
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Historically, none of our operating business units have operated at a profit and therefore each required additional cash to meet its monthly expenses. The additional sources of the cash to fund the shortfall from operations of ONM, BLEST and BioLargo Water have been provided by BioLargo’s sales of debt or equity, research grants, and tax credits. Clyra Medical has been funded by third party investors who invest directly in Clyra Medical in exchange for equity ownership in that entity.
The segment information for the years December 31, 2021 and 2020, is as follows (in thousands):
| |
|
2021
|
|
|
2020
|
|
| |
|
|
|
|
|
|
|
|
|
Revenues
|
|
|
|
|
|
|
|
|
|
BioLargo corporate
|
|
$ |
7 |
|
|
$ |
14 |
|
|
ONM Environmental
|
|
|
1,419 |
|
|
|
1,568 |
|
|
Clyra Medical
|
|
|
139 |
|
|
|
240 |
|
|
BLEST
|
|
|
1,635 |
|
|
|
1,050 |
|
|
BioLargo Water
|
|
|
12 |
|
|
|
37 |
|
|
Intersegment revenue
|
|
|
(681 |
) |
|
|
(477 |
) |
|
Total
|
|
$ |
2,531 |
|
|
$ |
2,432 |
|
| |
|
|
|
|
|
|
|
|
|
Operating loss
|
|
|
|
|
|
|
|
|
|
BioLargo corporate
|
|
$ |
(3,538 |
) |
|
$ |
(3,947 |
) |
|
ONM Environmental
|
|
|
(511 |
) |
|
|
(493 |
) |
|
BLEST
|
|
|
(629 |
) |
|
|
(619 |
) |
|
Clyra Medical
|
|
|
(1,142 |
) |
|
|
(1,827 |
) |
|
BioLargo Water
|
|
|
(616 |
) |
|
|
(697 |
) |
|
Total
|
|
$ |
(6,436 |
) |
|
$ |
(7,583 |
) |
| |
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
|
|
|
|
|
|
|
BioLargo corporate
|
|
$ |
(1,001 |
) |
|
$ |
(754 |
) |
|
BLEST
|
|
|
(488 |
) |
|
|
(351 |
) |
|
Clyra Medical
|
|
|
(66 |
) |
|
|
(164 |
) |
|
BioLargo Water
|
|
|
(486 |
) |
|
|
(505 |
) |
|
BioLargo corporate - intersegment
|
|
|
674 |
|
|
|
436 |
|
|
Total
|
|
$ |
(1,367 |
) |
|
$ |
(1,338 |
) |
| |
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
|
|
|
|
|
|
|
BioLargo corporate
|
|
$ |
(118 |
) |
|
$ |
(1,823 |
) |
|
ONM Environmental
|
|
|
— |
|
|
|
— |
|
|
Clyra Medical
|
|
|
(116 |
) |
|
|
(100 |
) |
|
Total
|
|
$ |
(234 |
) |
|
$ |
(1,923 |
) |
| |
|
|
|
|
|
|
|
|
|
Net loss
|
|
|
|
|
|
|
|
|
|
ONM Environmental
|
|
$ |
(511 |
) |
|
$ |
(483 |
) |
|
BLEST
|
|
|
(629 |
) |
|
|
(619 |
) |
|
Clyra Medical
|
|
|
593 |
|
|
|
(2,139 |
) |
|
BioLargo Water
|
|
|
(566 |
) |
|
|
(466 |
) |
|
BioLargo corporate
|
|
|
(5,781 |
) |
|
|
(5,993 |
) |
|
Consolidated net loss
|
|
$ |
(6,894 |
) |
|
$ |
(9,700 |
) |
|
As of December 31, 2021
|
|
BioLargo
|
|
|
ONM
|
|
|
Clyra
|
|
|
BLEST
|
|
|
Water
|
|
|
Elimination
|
|
|
Total
|
|
|
Tangible assets
|
|
$ |
555 |
|
|
$ |
451 |
|
|
$ |
816 |
|
|
$ |
595 |
|
|
$ |
152 |
|
|
$ |
(47 |
) |
|
$ |
2,522 |
|
|
Right of use
|
|
|
222 |
|
|
|
— |
|
|
|
— |
|
|
|
231 |
|
|
|
— |
|
|
|
— |
|
|
|
453 |
|
|
Investment in South Korean joint venture
|
|
|
48 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
48 |
|
| Total |
|
$ |
753 |
|
|
$ |
451 |
|
|
$ |
816 |
|
|
$ |
433 |
|
|
$ |
152 |
|
|
$ |
(47 |
) |
|
$ |
3,023 |
|
|
As of December 31, 2020
|
|
BioLargo
|
|
|
ONM
|
|
|
Clyra
|
|
|
BLEST
|
|
|
Water
|
|
|
Elimination
|
|
|
Total
|
|
|
Tangible assets
|
|
$ |
388 |
|
|
$ |
624 |
|
|
$ |
1,125 |
|
|
$ |
188 |
|
|
$ |
105 |
|
|
$ |
(42 |
) |
|
$ |
2,388 |
|
|
Right of use
|
|
|
215 |
|
|
|
— |
|
|
|
— |
|
|
|
126 |
|
|
|
— |
|
|
|
— |
|
|
|
341 |
|
|
Investment in South Korean joint venture
|
|
|
63 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
63 |
|
|
Intangible assets
|
|
|
2,150 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,150 |
|
| Total |
|
$ |
2,816 |
|
|
$ |
624 |
|
|
$ |
1,125 |
|
|
$ |
314 |
|
|
$ |
105 |
|
|
$ |
(42 |
) |
|
$ |
4,942 |
|
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 13. Commitments and Contingencies
Office Leases
We have long-term operating leases for office, industrial and laboratory space in Westminster, California, Oak Ridge, Tennessee, and Alberta, Canada. Payments made under operating leases are charged to the Consolidated Statement of Operations and Comprehensive Loss on a straight-line basis over the term of the operating lease agreement. For the years ended December 31, 2021 and 2020, rental expense was $228,000 and $228,000, respectively. On January 1, 2019, we adopted ASC 842 which resulted in a right-of-use asset and lease liability. Short-term leases are not included in our analysis. The adoption resulted in an immaterial cumulative effect of an accounting change that was not recorded. The lease of our Westminster facility qualifies for the new treatment; it originated in August 2016, was originally scheduled to expire August 2020, contains a yearly escalation of 3%, and includes a four-year renewal option whereby the base rent is adjusted to then market value. During 2020, we exercised our option to extend the lease for four years. It is too early for management to determine if it will extend another four years, therefore the additional four-year extension is not included in the analysis. The lease of our Oak Ridge, Tennessee facility also qualifies, and it had one three-year extension to September 2022, and has one renewal option for another five years where the rental rate would adjust to greater of the current price and fair market value. During 2021 management determined that it will exercise the five-year renewal option for the Oak Ridge facility. The lease of our Canadian facility is less than one year. None of our leases have additional terms related to the payments or mechanics of the lease. The leases have no additional payment terms such as common area maintenance payments, tax sharing payments or other allocable expenses. Likewise, the leases do not contain other terms and conditions of use, such as variable lease payments, residual value guaranties or other restrictive financial terms. Since there is no explicit interest rate in our leases, management used its incremental borrowing rate, which is estimated to be 18% to determine lease liability.
As of December 31, 2021, our weighted average remaining lease term is four years and the total remaining operating lease payments is $670,000. Our minimum lease payments over the next five years are as follows:
|
Years ending
|
|
BioLargo
Corp / ONM
|
|
|
BLEST
|
|
|
Total
|
|
|
December 31, 2022
|
|
$ |
115,000 |
|
|
$ |
65,000 |
|
|
$ |
180,000 |
|
|
December 31, 2023
|
|
|
118,000 |
|
|
|
65,000 |
|
|
|
183,000 |
|
|
December 31, 2024
|
|
|
70,000 |
|
|
|
65,000 |
|
|
|
135,000 |
|
|
December 31, 2025
|
|
|
-- |
|
|
|
65,000 |
|
|
|
65,000 |
|
|
December 31, 2026
|
|
|
-- |
|
|
|
107,000 |
|
|
|
107,000 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total minimum lease payments
|
|
$ |
303,000 |
|
|
$ |
367,000 |
|
|
$ |
670,000 |
|
Note 14. Subsequent Events.
Management has evaluated subsequent events through the date of the filing of this Annual Report and management noted the following for disclosure.
Lincoln Park Capital Purchase of Shares
From January 1, 2022, through March 30, 2022, we sold 1,506,821 shares of common stock to Lincoln Park pursuant to our the 2020 LPC Purchase Agreement (see Note 3), and received $345,000 in gross and net proceeds. These sales were registered with the SEC on Form S-1 (file number 333-237651).
BIOLARGO, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Chief Financial Officer Contract Extension
On March 22, 2022, we and our Chief Financial Officer Charles K. Dargan, II formally agreed to extend the engagement agreement dated February 1, 2008 (the “Engagement Agreement”, which had been previously extended multiple times), pursuant to which Mr. Dargan has been and continues to serve as the Company’s Chief Financial Officer. The Engagement Extension Agreement dated as of March 22, 2022 (the “Engagement Extension Agreement”) provides for an additional one-year term to expire January 31, 2023 (the “Extended Term”).
As the sole compensation for the Extended Term, Mr. Dargan was issued an option (“Option”) to purchase 25,000 shares of the Company’s common stock for each month during the Extended Term (thus, an option to purchase 300,000 shares reflecting an extended term of 12 months). The Option vests over the period of the Extended Term, with 25,000 shares having vested as of March 22, 2022, and the remaining shares to vest 25,000 shares monthly beginning March 22, 2022, and each month thereafter, so long as the agreement is in full force and effect. The Option is exercisable at $0.24 per share, the closing price of BioLargo’s common stock on the March 22, 2022, grant date, expires ten years from the grant date, and was issued pursuant to the Company’s 2018 Equity Incentive Plan.
The Option is Mr. Dargan’s sole compensation for the Extended Term. As was the case in all prior terms of his engagement, there is no cash component of his compensation for the Extended Term. Mr. Dargan is eligible to be reimbursed for business expenses he incurs in connection with the performance of his services as the Company’s Chief Financial Officer (although he has made no such requests for reimbursement in the past). All other provisions of the Engagement Agreement not expressly amended pursuant to the Engagement Extension Agreement remain the same, including provisions regarding indemnification and arbitration of disputes.
SBA Loan Forgiveness
We received a notice dated February 7, 2022, that the Small Business Administration had partially approved our application for forgiveness of Paycheck Protection Act loan to ONM Environmental in the amount of $174,000. This leaves a balance on the loan of $35,000.
Clyra Medical – Scion Transaction
BioLargo and its partially owned subsidiary Clyra Medical entered into an agreement dated March 3, 2022, whereby BioLargo agreed to convert $633,091 in working capital advances, made to or on behalf of Clyra Medical, into 2,042.23 shares of Clyra Medical common stock at a rate of $310 per share. See also Note 9 “Impairment Expense”.
Unit Offering
During the three months ended March 31, 2022, pursuant to an offering commenced in March 2020, we sold 4,196,968 shares of our common stock and received $692,000 in gross and net proceeds from ten accredited investors. In addition to the shares, we issued the investors six-month warrants that allow the investors to purchase an aggregate 4,196,968 shares at 120% of the share purchase price, and five-year warrants that allow the investors to purchase an aggregate 4,196,968 shares at 150% of the share purchase price.
PART II — INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the expenses expected to be incurred by us in connection with the issuance and distribution of the securities being registered.
|
SEC Registration
|
|
$ |
2,092 |
(1) |
|
Legal Fees and Expenses*
|
|
$ |
65,000 |
|
|
Accounting Fees*
|
|
$ |
15,000 |
|
|
Miscellaneous*
|
|
$ |
2,098 |
|
|
Total
|
|
$ |
85,000 |
|
(1) paid upon filing of Form S-1 on September 15, 2017.
* Estimated.
Item 14. Indemnification of Directors and Officers.
As permitted by the Delaware general corporation law, we have included a provision in our certificate of incorporation to eliminate the personal liability of our directors for monetary damages for breach of their fiduciary duties as directors, except for liability (i) for any breach of the director’s duty of loyalty to our company, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under section 174 of the Delaware general corporation law or (iv) for any transaction from which the director derived an improper personal benefit. Our certificate of incorporation also provides that our company shall, to the full extent permitted by section 145 of the Delaware general corporation law, as amended from time to time, indemnify all persons whom it may indemnify pursuant thereto.
Item 15. Recent Sales of Unregistered Securities
Stock issued as payment for amounts owed
During the year ended December 31, 2021, certain of our officers agreed to convert an aggregate $46,000 of accrued and unpaid salary into 214,206 shares of our common stock. The unpaid salary is converted on the last day of each quarter as follows: December 31, 2021, we issued 15,000 shares of our common stock at $0.21 per share; on September 30, 2021, we issued 61,842 shares of our common stock at $0.19; on March 31, 2021, we issued 137,364 shares of our common stock at $0.23 per share.
During the year ended December 31, 2020, certain of our officers agreed to convert an aggregate $299,000 of accrued and unpaid salary into 2,017,928 shares of our common stock. The unpaid salary is converted on the last day of each quarter as follows: December 31, 2020, we issued 652,100 shares of our common stock at $0.12 per share; on September 30, 2020, we issued 349,670 shares of our common stock at $0.15; on June 30, 2020, we issued 367,403 shares of our common stock at $0.16; on March 31, 2020, we issued 648,755 shares of our common stock at $0.17 per share.
During 2021, certain of our consultants agreed to convert an aggregate $282,000 accrued and unpaid obligations into 1,913,261 shares of our common stock. The unpaid obligations were converted on the last day of each quarter as follows: December 31, 2021, we issued 348,772 shares of our common stock at $0.21 per share; September 30, 2021, we issued 586,963 shares of our common stock at $0.19 per share; June 30, 2020, we issued 367,403 shares of our common stock at $0.16 per share; March 31, 2021, we issued 610,123 shares of our common stock at $0.23 per share.
On December 31, 2020, we issued 150,000 shares of our common stock to a vendor to reduce amounts owed to the vendor in the aggregate amount of $18,000.
In the three months ended September 30, 2020, we issued 189,762 shares of our common stock to vendors in exchange for reduction of $28,000 of amounts owed.
In the three months ended June 30, 2020, we issued 876,079 shares of our common stock to vendors in exchange for reduction of $132,000 of amounts owed.
On March 11 and March 29, 2019, we issued 100,000 and 138,252 shares, respectively, of our common stock pursuant to consulting agreements totaling $47,000 for services to our company.
On December 31, 2019, we issued 255,225 shares of our common stock to vendors to reduce amounts owed to the vendors in the aggregate amount of $69,000.
During the three months ended September 30, 2019, we issued 513,285 shares of our common stock in exchange for a reduction of approximately $130,000 owed to vendors and consultants.
During the three months ended June 30, 2019, we issued options to purchase 330,434 shares of our common stock for $0.23 per share in exchange for a reduction of $38,000 owed to vendors and consultants.
During the three months ended June 30, 2019, a noteholder elected to convert $296,000 of principal of a note due August 31, 2019, into 2,767,833 shares of our common stock.
During the three months ended June 30, 2019, we issued 87,748 shares of our common stock in lieu of accrued and unpaid interest totaling $15,000.
On January 15 and March 20, 2019, we issued 44,288 and 54,305 shares, respectively, of our common stock for $20,000 of interest due to our note and line of credit holders.
On March 11 and March 29, 2019, we issued 100,000 and 138,252 shares, respectively, of our common stock pursuant to consulting agreements totaling $47,000 for services to our company.
On January 24, February 13, March 6 and March 26, 2019, we issued a total of 1,679,248 shares of our common stock to Vista Capital upon its election to convert $215,000 of the Vista 2017 Note. Of that amount, 1,638,479 shares were issued as payment of principal, and 40,769 shares as payment of interest.
During the three-months ended December 31, 2019, we issued 98,496 shares of common stock to two consultants. The common stock was issued for services provided by consultants and is recorded in selling general and administrative expense in our consolidated statement of operations.
Stock issued as payment of principal and interest
On its maturity date of April 20, 2021, we converted to equity a promissory note in the principal balance of $100,000 into 400,000 shares of our common stock, and $9,994 of accrued interest into 48,706 shares of our common stock.
In the three months ended September 30, 2020, we issued 24,776,554 shares of our common stock in conversion of principal and interest due on convertible promissory notes in the principal amount of $2,286,000.
In the three months ended June 30, 2020, we issued 6,567,133 shares of our common stock in conversion of principal and interest due on convertible promissory notes in the principal amount of $531,000.
During the three months ended March 31, 2020, Vista Capital elected to convert the remaining balance of $269,600 of the outstanding principal and interest due on its promissory note dated October 7, 2019, and we issued 2,417,059 shares of our common stock.
During the three months ended March 31, 2020, noteholders elected to convert $165,000 of the outstanding principal of 12-Month OID Notes and we issued 970,590 shares of our common stock.
On January 15 and March 20, 2019, we issued 44,288 and 54,305 shares, respectively, of our common stock for $20,000 of interest due to our note and line of credit holders.
On January 24, February 13, March 6 and March 26, 2019, we issued a total of 1,679,248 shares of our common stock to Vista Capital upon its election to convert $215,000 of the Vista 2017 Note. Of that amount, 1,638,479 shares were issued as payment of principal, and 40,769 shares as payment of interest.
During the three months ended December 31, 2019, we issued 5,362,471 shares of our common stock in satisfaction of $875,943 in principal and interest due on promissory notes.
During the three months ended September 30, 2019, we issued 19,586 shares of our common stock in lieu of accrued and unpaid interest totaling $15,000.
On November 21, 2018, we issued 340,848 shares of our common stock to an investor who elected to convert $100,000 principal amount of convertible notes. Of that amount, 333,334 shares were issued as payment of principal, and 7,514 shares as payment of outstanding interest.
Non-Plan Options
The following describes non-plan options issued over time, which are not part of our 2018 Equity Incentive Plan.
On March 30, 2021, we issued options to purchase 43,956 shares of our common stock at $0.2275 per share in exchange for a reduction of $5,000 owed to a consultant.
During the three months ended March 31, 2019, we issued options to purchase 731,250 shares of our common stock at exercise prices ranging between $0.16 – $0.25 per share to vendors for fees for service, and an aggregate 138,252 shares of our common stock to a consultant for fees for service at $0.22 per share.
On September 30, 2019, we issued options to purchase 139,682 shares of our common stock at $0.315 per share in exchange for a reduction of $22,000 owed to vendors and consultants.
On June 30, 2019, we issued options to purchase 330,435 shares of our common stock at $0.23 per share in exchange for a reduction of $38,000 owed to vendors and consultants.
On April 29, 2019, we issued options to purchase 481,250 shares of our common stock at $0.16 per share in exchange for a reduction of $38,500 owed to vendors and consultants.
BKT
On March 13, 2020, we issued 1,593,807 shares of our common stock to BKT Co. Ltd., pursuant to the Joint Venture Agreement, and in exchange received $350,000 from BKT.
2020 Unit Offering
During the years ended December 31, 2021 and 2020, pursuant to an offering commenced in March 2020, we sold 5,435,966 and 2,374,335 shares, respectively, of our common stock and received $864,000 and $367,000, respectively, in gross and net proceeds, from a total of nine accredited investors. In addition to the shares, we issued each shareholder a six-month and a five-year warrant to purchase additional shares.
Twelve-month OID Notes
From June 7, 2019 through September 30, 2019, we received $2,235,000 and issued convertible promissory notes (each, a “12-Month OID Note”) in the aggregate principal amount of $2,794,000, with a 25% original issue discount, to 34 accredited investors. Each OID note matures 12 months from the date of issuance, and accrues interest at an annual rate of 5%. It may be converted by the investor at any time at $0.17 per share, and automatically converts to equity at maturity at the lower of the fixed conversion rate and seventy percent (70%) of the lowest daily volume weighted average price during the 25 trading days immediately preceding the conversion. It must be prepaid upon conclusion of a securities offering in which we raise at least $3.5 million in a single financing. The $0.17 initial conversion rate may be adjusted downward in the event the Company issues a fixed-price convertible note at a lower conversion rate, or conducts an equity offering at a per-share price less than the conversion price. The Company may prepay the notes at any time upon 10 days’ notice to the investor, during which time they may convert the note to stock. In addition to the note, each investor received a warrant to purchase common stock for $0.25 per share, expiring 5 years from the date of issuance. The number of shares purchasable under the warrant is equal to the 75% of the principal balance of the note divided by .17. In the aggregate, we issued warrants to purchase 12.3 million shares.
Two-year Convertible Note
On August 9, 2019, we received $600,000 from one accredited investor and issued a promissory note in the principal amount of $600,000, maturing in two years, accruing interest at 15% to be paid in cash monthly, and which converts to common stock at the holder’s option at $0.30 per share. This investor also received a warrant to purchase 1,200,000 shares of our common stock at $0.30 per share, expiring five years from the grant date.
Crossover Capital Investment
On May 14, 2019, we received $95,000 and issued a convertible note to Crossover Capital Fund I, LP (“Crossover Capital”) in the principal amount of $110,000, representing a 10% original issue discount, and a deduction of $5,000 for legal fees and due diligence. The note was due nine months from the date of issuance, on February 14, 2020. On June 17, 2019, we received a second investment from Crossover Capital in the amount of $77,000 and issued a convertible note in the principal amount of $90,000, representing a 10% original issue discount, and a deduction of $5,000 for legal fees and due diligence. The notes are convertible at the option of Crossover Capital at a conversion price equal to 70% of the lowest closing bid price of our common stock during the 25 trading days prior to the conversion date. These notes have been satisfied in full.
Tangiers Global
On January 31, 2019, we issued a 12% Convertible Promissory Note to Tangiers Global, LLC (“Tangiers”) in the aggregate principal amount of up to $495,000 (the “Tangiers Note”). The note allows for two payments, each due in nine months after receipt, and incurs a guaranteed interest of 12% at inception. The initial payment of $300,000 was received on February 5, 2019, representing a $330,000 principal amount and 10% original issue discount. It was due November 5, 2019. We received the second payment, in the amount of $150,000, on March 7, 2019, increasing the principal amount due under the note to $495,000. This second amount, plus guaranteed interest, was due December 7, 2019. In the aggregate, the principal amount of the note, plus guaranteed interest, totals $554,000. On July 29, 2019, Tangiers Global, LLC, elected to convert $369,000 principal amount due on its promissory note issued January 31, 2019, into 2,640,000 shares of common stock. On October 2, 2019, Tangiers Global, LLC, elected to convert the remaining $184,000 principal amount due on its promissory note issued January 31, 2019, into 1,200,000 shares of common stock.
Convertible Note, matures April 7, 2020 (Vista Capital)
On January 7, 2019, Vista Capital Investments, LLC (“Vista Capital”) invested $300,000 and we issued a convertible promissory note (the “Vista 2019 Note”) in the principal amount of $330,000, maturing nine months from the date of issuance (October 7, 2019). The Vista 2019 Note earned a one-time interest charge of 12%, recorded as a discount on convertible notes and will be amortized over the term of the note. The Vista 2019 Note allows Vista Capital to convert the note to our common stock at any time at a price equal to 65% of the lowest closing bid price of the Company’s common stock during the 25 consecutive trading days immediately preceding the conversion date. We and Vista Capital have since amended the note extending the maturity date to May 7, 2020.
With respect to the above transactions with Vista Capital, Lincoln Park Capital Fund, LLC agreed to waive the provisions of the Purchase Agreement dated August 25, 2017, prohibiting variable rate transactions. As consideration for this waiver, we issued to Lincoln Park a warrant to purchase 250,000 shares of our common stock at $0.25 per share, expiring five years from the date of grant.
On November 22, 2019 and December 17, 2019, Vista Capital elected to convert $50,000, totaling $100,000, into 690,530 shares of common stock. Subsequent to December 31, 2019, Vista Capital converted the remaining amount of its note that had been scheduled to mature on April 7, 2020, and we issued an aggregate 2,079,359 shares of common stock in full payment thereof.
All of these offerings and sales were made in reliance on the exemption from registration contained in Section 4(2) of the Securities Exchange Act and/or Regulation D promulgated thereunder as not involving a public offering of securities.
Item 16. Exhibits.
| |
|
Incorporated by
Reference Herein
|
|
Exhibit
Number
|
Exhibit Description
|
Form
|
File Date
|
|
3.1
|
Bylaws of BioLargo, Inc., as amended and restated
|
Form 10-KSB
|
5/23/2003
|
|
3.2
|
Amended and Restated Certificate of Incorporation for BioLargo, Inc. filed March 16, 2007
|
Form 10-KSB
|
5/4/2007
|
|
3.3
|
Certificate of Amendment to Certificate of Incorporation, filed May 25, 2018
|
Pos Am
|
6/22/2018
|
|
3.4
|
Amended and Restated Articles of Incorporation of Clyra Medical Technologies, Inc.
|
Form 8-K
|
1/6/2016
|
|
4.1
|
BioLargo, Inc. 2007 Equity Incentive Plan
|
Form 10-QSB
|
11/19/2007
|
|
4.2
|
Amendment No. 1 to BioLargo 2007 Equity Incentive Plan
|
Def 14C (Exhibit A)
|
5/2/2011
|
|
4.3
|
2018 Equity Incentive Plan
|
Form S-8
|
6/22/2018
|
|
4.4
|
Stock Option Award Agreement under 2018 Equity Incentive Plan
|
Form S-8
|
6/22/2018
|
|
4.5
|
Notice of Stock Option Grant under 2018 Equity Incentive Plan
|
Form S-8
|
6/22/2018
|
|
4.6
|
Restricted Stock Unit Award Agreement under 2018 Equity Incentive Plan
|
Form S-8
|
6/22/2018
|
|
4.7
|
Notice of Restricted Stock Unit Award under 2018 Equity Incentive Plan
|
Form S-8
|
6/22/2018
|
|
4.8
|
Form of Stock Options issued in exchange for reduction in accounts payable.
|
Form 10-K
|
3/31/2015
|
|
4.9
|
Promissory note issued by Clyra Medical to Scion Solutions dated September 26, 2018
|
Form 8-K
|
10/2/2018
|
|
4.10
|
Promissory Note issued to Vernal Bay Investments, LLC on September 19, 2018
|
Form 8-K
|
9/24/2018
|
|
4.11
|
Stock Purchase Warrant issued to Vernal Bay Investments, LLC on September 19, 2018
|
Form 8-K
|
9/24/2018
|
|
4.12
|
Promissory Note issued to Chappy Bean, LLC on September 19, 2018
|
Form 8-K
|
9/24/2018
|
|
4.13
|
Stock Purchase Warrant issued to Chappy Bean, LLC on September 19, 2018
|
Form 8-K
|
9/24/2018
|
|
4.14
|
Warrant issued with Line of credit that matures September 1, 2019
|
Form 10-Q
|
5/14/2018
|
|
4.15
|
Amendment dated March 5, 2019 to Promissory Note issued to Vernal Bay Investments, LLC on September 19, 2018
|
Form 8-K
|
3/8/2019
|
|
4.16
|
Amendment dated March 5, 2019 to Promissory Note issued to Chappy Bean, LLC on September 19, 2018
|
Form 8-K
|
3/8/2019
|
|
4.17
|
$50,000 convertible note, matures March 8, 2020
|
Form 10-Q
|
5/14/2018
|
|
4.18
|
Amendment dated August 12, 2019 to Promissory Note issued to Vernal Bay Investments, LLC on September 19, 2018
|
Form 10-Q
|
8/14/2019
|
|
4.19
|
Form of convertible notes that mature April 20, 2021 (Spring 2018 Offering)
|
Form 10-Q
|
5/14/2018
|
|
4.20
|
Warrant issued to Vernal August 12, 2019
|
Form 10-Q
|
8/14/2019
|
|
4.21
|
Revolving Line of Credit Agreement dated June 30, 2020, between Clyra Medical and Vernal Bay
|
Form 8-K
|
7/7/2020
|
|
4.22
|
Form of warrant issued with convertible notes that mature April 20, 2021 (Spring 2018 Offering)
|
Form 10-Q
|
5/14/2018
|
|
4.23
|
Security Agreement dated June 30, 2020, between Clyra Medical and Vernal Bay
|
Form 8-K
|
7/7/2020
|
|
4.24
|
Revolving Line of Credit Note issued by Clyra Medical to Vernal Bay on June 30, 2020
|
Form 8-K
|
7/7/2020
|
|
4.25
|
Stock Purchase Agreement and Plan of Reorganziation dated September 26, 2018, with Scion Solutions, LLC
|
Form 8-K
|
10/2/2018
|
|
4.26
|
OID twelve-month promissory note
|
Form 8-K
|
8/2/2019
|
|
4.27
|
Stock purchase warrant issued to OID twelve-month investors
|
Form 8-K
|
8/2/2019
|
|
4.28
|
$600,000 Promissory note dated August 9, 2019
|
Form 10-Q
|
8/14/2019
|
|
4.29
|
Warrant to purchase 1.2 million shares issued August 9, 2019
|
Form 10-Q
|
8/14/2019
|
|
4.30
|
Amendment to $440,000 convertible notes that matures July 20, 2019
|
Form 10-Q
|
5/14/2018
|
|
4.31
|
Warrant issued March 2018, expiring March 2023
|
S-1
|
8/29/2019
|
|
4.32
|
Form of warrant issued January 2019 to Lincoln Park, expiring January 2024
|
S-1
|
8/29/2019
|
|
4.33
|
Registration Rights Agreement, dated as of March 30, 2020, by and between BioLargo, Inc. and Lincoln Park Capital Fund, LLC
|
Form 8-K
|
3/31/2020
|
|
4.34
|
Warrant issued in 2020 Unit Offering
|
Form 10-Q
|
8/14/2020
|
|
4.35
|
Amendment to $50,000 Convertible Note dated March 8, 2018
|
Form 10-K
|
3/30/2021
|
|
4.36
|
Warrant issued to $50,000 Convertible Noteholder on March 1, 2020
|
Form 10-K
|
3/30/2021
|
| 4.37 |
Second Amendment dated August 12, 2019, to Promissory Note issued to Chappy Bean, LLC dated September 19, 2018 |
Form 8-K |
5/19/2021 |
| 4.38 |
Amended and restated note issued to Chappy Bean on August 12, 2019 |
Form 8-K |
5/19/2021 |
| 4.39 |
Third Amendment dated August 10, 2020 to Promissory Note issued to Vernal Bay Investments, LLC on September 19, 2018 |
Form 8-K |
5/19/2021 |
| 4.40 |
Third Amendment dated August 10, 2020, to Promissory Note issued to Chappy Bean, LLC dated September 19, 2018 |
Form 8-K |
5/19/2021 |
| 4.41 |
Final Payoff Agreement dated May 17, 2021 to Promissory Note issued to Vernal Bay Investments, LLC on September 19, 2018 |
Form 8-K |
5/19/2021 |
| 4.42 |
Final Payoff Agreement dated May 18, 2021 to Promissory Note issued to Chappy Bean, LLC dated September 19, 2018 |
Form 8-K |
5/19/2021 |
| 4.43 |
Amended and restated note issued to Vernal August 12, 2019 |
Form 10-Q |
8/14/2019 |
|
5.1
|
Opinion of counsel
|
Form S-1
|
1/25/2017
|
|
10.1
|
License Agreement to Clyra Medical Technologies, Inc., dated December 17, 2012
|
Form 8-K
|
1/6/2016
|
|
10.2
|
December 30, 2015 amendment to License Agreement with Clyra Medical Technologies, Inc.
|
Form 8-K
|
1/6/2016
|
|
10.3
|
Escrow Agreement dated September 26, 2018 regarding Clyra/Scion transaction
|
Form 8-K
|
10/2/2018
|
|
10.4
|
Closing Agreement dated December 17, 2018 between Clyra Medical and Scion Solutions
|
Form 8-K
|
12/19/2018
|
|
10.5
|
Amendment dated June 30, 2020 to License Agreement with Clyra Medical Technologies, Inc.
|
Form 8-K
|
7/7/2020
|
|
10.6
|
Amendment dated June 30, 2020 to Consulting Agreement dated December 30, 2015 between Clyra Medical and Beach House Consulting LLC
|
Form 8-K
|
7/7/2020 |
|
10.7
|
Consulting Agreement dated December 30, 2015 with Beach House Consulting LLC
|
Form 8-K
|
1/6/2016
|
|
10.8
|
Commercial Office Lease Agreement for 14921 Chestnut St., Westminster, CA 92683
|
Form 8-K
|
8/24/2016
|
|
10.9
|
Commercial Office Lease Agreement for Oak Ridge Tennessee
|
Form 8-K
|
9/8/2017
|
|
10.10
|
Form of Employment Agreement for Engineering Subsidiary
|
Form 8-K
|
9/8/2017
|
|
10.11
|
Form of Option issued to founding employees of Engineering subsidiary (BLEST)
|
Form 8-K
|
9/8/2017
|
|
10.12†
|
2020 Engagement Extension Agreement with CFO
|
Form 8-K
|
2/27/2020
|
|
10.13
|
Purchase Agreement, dated as of March 30, 2020 by and between BioLargo, Inc. and Lincoln Park Capital Fund, LLC.
|
Form 8-K
|
3/31/2020
|
|
10.14†
|
2021 Engagement Extension Agreement with CFO
|
Form 8-K
|
3/19/2021
|
| 10.15 |
Agreement dated March 1, 2022, by and between Scion Solutions, LLC, Clyra Medical Technologies, Inc., and BioLargo, Inc. |
Form 8-K |
3/3/2022 |
| 10.16 |
Agreement dated March 1, 2022, by and between Clyra Medical Technologies, Inc., and BioLargo, Inc. |
Form 8-K |
3/3/2022 |
| 10.17† |
2022 Engagement Extension Agreement with CFO |
Form 8-K |
3/24/2022 |
|
14.1
|
Code of Ethics
|
Form 10-KSB
|
11/16/2004
|
|
21.1
|
List of Subsidiaries of the Registrant.
|
Form 10-K
|
3/30/2021
|
|
23.1*
|
Consent of Haskell & White LLP
|
|
|
|
24.1
|
Power of Attorney - see signature page
|
|
|
* Filed herewith.
† Management contract or compensatory plan, contract or arrangement.
Item 17. Undertakings.
The undersigned hereby undertakes:
|
(1)
|
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
|
| |
(i)
|
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
|
| |
|
|
| |
(ii)
|
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
|
| |
|
|
| |
(iii)
|
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
|
|
(2)
|
That, for the purpose of determining liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
| |
|
|
(3)
|
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
|
| |
|
|
(4)
|
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
|
|
(5)
|
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
|
| |
(i)
|
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
|
| |
|
|
| |
(ii)
|
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
|
| |
|
|
| |
(iii)
|
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
|
| |
|
|
| |
(iv)
|
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
|
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in City of Westminster, State of California, on April 13, 2022.
|
|
BioLargo, Inc.
|
|
|
|
|
|
By: /s/ Dennis P. Calvert
|
|
|
|
Dennis P. Calvert
|
|
|
Chief Executive Officer (Principal Executive Officer)
|
| |
|
| |
|
POWER OF ATTORNEY AND SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Dennis P. Calvert
|
|
Chief Executive Officer, President, Chairman, Director, Principal Executive Officer
|
|
April 13, 2022
|
|
Dennis P. Calvert
|
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
|
/s/ Charles K. Dargan II
|
|
Chief Financial Officer (principal financial officer and principal accounting officer)
|
|
April 13, 2022 |
|
Charles K. Dargan II
|
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
|
/s/ Kenneth R. Code *
|
|
Chief Science Officer, Director
|
|
April 13, 2022
|
|
Kenneth R. Code
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
/s/ Joseph L. Provenzano *
|
|
Executive Vice President, Corporate Secretary, Director
|
|
April 13, 2022
|
|
Joseph L. Provenzano
|
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
|
/s/ Jack B. Strommen *
|
|
Director
|
|
April 13, 2022
|
|
Jack B. Strommen
|
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
|
/s/ Dennis E. Marshall *
|
|
Director
|
|
April 13, 2022
|
|
Dennis E. Marshall
|
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
|
/s/ Kent C. Roberts III *
|
|
Director
|
|
April 13, 2022
|
|
Kent C. Roberts III
|
|
|
|
|
| |
|
|
|
|
| |
|
|
|
|
|
/s/ John S. Runyan *
|
|
Director
|
|
April 13, 2022
|
|
John S. Runyan
|
|
|
|
|
* By Dennis P. Calvert, Attorney-in-fact
BioLargo (QB) (USOTC:BLGO)
Historical Stock Chart
From Mar 2024 to Apr 2024
BioLargo (QB) (USOTC:BLGO)
Historical Stock Chart
From Apr 2023 to Apr 2024