Novo Nordisk Files For EU Approval of Obesity Drug
December 18 2020 - 5:06AM
Dow Jones News
By Dominic Chopping
Danish pharmaceutical company Novo Nordisk AS said Friday that
it has filed for European Union regulatory approval of its
semaglutide weight-loss drug.
The company has submitted a Marketing Authorisation Application
to the European Medicines Agency for semaglutide as a treatment for
obese or overweight adults in conjunction with a reduced-calorie
diet and increased physical activity.
Novo Nordisk earlier this month submitted a new drug application
to the U.S. Food and Drug Administration for the drug.
"It is a milestone for Novo Nordisk but more importantly it
represents a new treatment option with the potential to transform
the medical management for people living with obesity in Europe,"
Novo Nordisk Chief Scientific Officer Mads Krogsgaard Thomsen
said.
Semaglutide is a glucagon-like peptide-1 drug, or GLP-1, that
helps enhance the production of insulin and regulate appetite.
Write to Dominic Chopping at dominic.chopping@wsj.com
(END) Dow Jones Newswires
December 18, 2020 04:51 ET (09:51 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
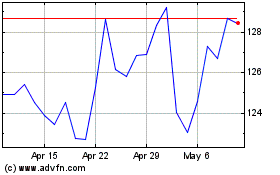
Novo Nordisk (NYSE:NVO)
Historical Stock Chart
From Mar 2024 to Apr 2024
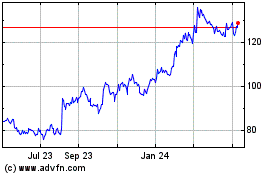
Novo Nordisk (NYSE:NVO)
Historical Stock Chart
From Apr 2023 to Apr 2024