|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 4 of 33
|
FINANCIAL PERFORMANCE
CONSOLIDATED FINANCIAL STATEMENT FOR THE FIRST NINE MONTHS OF 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PROFIT AND LOSS
|
9M 2020
|
|
9M 2019
|
|
|
|
|
% change 9M 2020 to 9M 2019
|
|
|
(Amounts are in DKK million, except for earnings per share, dividend per share and employees)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales
|
94,808
|
|
89,604
|
|
|
|
|
6
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
79,495
|
|
74,948
|
|
|
|
|
6
|
%
|
|
|
Gross margin
|
83.8
|
%
|
|
83.6
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and distribution costs
|
23,162
|
|
22,287
|
|
|
|
|
4
|
%
|
|
|
Percentage of sales
|
24.4
|
%
|
|
24.9
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development costs
|
10,979
|
|
9,836
|
|
|
|
|
12
|
%
|
|
|
Percentage of sales
|
11.6
|
%
|
|
11.0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Administrative costs
|
2,760
|
|
2,772
|
|
|
|
|
0
|
%
|
|
|
Percentage of sales
|
2.9
|
%
|
|
3.1
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other operating income, net
|
354
|
|
557
|
|
|
|
|
(36
|
%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating profit
|
42,948
|
|
40,610
|
|
|
|
|
6
|
%
|
|
|
Operating margin
|
45.3%
|
|
45.3%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial items (net)
|
(1,820)
|
|
(3,136)
|
|
|
|
|
(42
|
%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income taxes
|
41,128
|
|
37,474
|
|
|
|
|
10
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes
|
8,308
|
|
7,240
|
|
|
|
|
15
|
%
|
|
|
Effective tax rate
|
20.2
|
%
|
|
19.3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net profit
|
32,820
|
|
30,234
|
|
|
|
|
9
|
%
|
|
|
Net profit margin
|
34.6
|
%
|
|
33.7
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER KEY NUMBERS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation, amortisation and impairment losses
|
4,374
|
|
4,263
|
|
|
|
|
3
|
%
|
|
|
Capital expenditure (Purchase of property, plant and equipment)1
|
4,307
|
|
7,025
|
|
|
|
|
(39
|
%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash generated from operating activities
|
51,779
|
|
41,617
|
|
|
|
|
24
|
%
|
|
|
Free cash flow
|
41,559
|
|
32,714
|
|
|
|
|
27
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
139,947
|
|
124,908
|
|
|
|
|
12
|
%
|
|
|
Equity
|
59,573
|
|
52,953
|
|
|
|
|
13
|
%
|
|
|
Equity ratio
|
42.6
|
%
|
|
42.4
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average number of diluted shares outstanding (million)
|
2,345.0
|
|
2,383.8
|
|
|
|
|
(2
|
%)
|
|
|
Diluted earnings per share / ADR (in DKK)
|
14.00
|
|
12.68
|
|
|
|
|
10
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Full-time equivalent employees end of period
|
44,326
|
|
42,158
|
|
|
|
|
5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1) Cash-based capital expenditure.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
These unaudited consolidated financial statements for the first nine months of 2020 have been prepared in
accordance with IAS 34 ‘Interim Financial Reporting’. The accounting policies adopted in the preparation are
consistent with those applied in the Annual Report 2019 of Novo Nordisk. Furthermore, the financial report, including the consolidated financial statements for the first nine months of 2020 and the Management’s review, have been prepared in accordance with additional Danish disclosure requirements for interim reports of listed companies.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 5 of 33
|
GEOGRAPHIC SALES DEVELOPMENT
Sales increased by 6% measured in Danish kroner and by 7% at CER to DKK 94,808 million in the first nine months of 2020. Sales growth was negatively impacted by fewer patients initiating treatment and increasing unemployment in the US, partially offset by COVID-19-related stocking. Sales in International Operations increased by 9% measured in Danish kroner and by 12% at CER. Sales in North America Operations increased by 2% in both Danish kroner and at CER.
As of 1 April 2020, International Operations was reorganised and financial reporting has been divided into: EMEA (covering Europe, the Middle East and Africa), Region China (covering Mainland China, Hong Kong and Taiwan) and Rest of World (covering all other countries except for North America). North America Operations was not impacted by the reorganisation and still includes the US and Canada. Please see appendix 8 for a breakdown of sales per area in 2019.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales split per geographical area
|
Sales 9M 2020
DKK million
|
Growth
as reported
|
Growth
at CER
|
Share of growth
at CER
|
|
|
|
|
|
|
|
|
|
|
|
|
|
International Operations
|
50,399
|
|
9
|
%
|
12
|
%
|
83
|
%
|
|
|
- EMEA
|
26,159
|
|
9
|
%
|
11
|
%
|
39
|
%
|
|
|
- Region China
|
10,836
|
|
10
|
%
|
12
|
%
|
19
|
%
|
|
|
- Rest of World
|
13,404
|
|
8
|
%
|
13
|
%
|
25
|
%
|
|
North America Operations
|
44,409
|
|
2
|
%
|
2
|
%
|
17
|
%
|
|
|
- The US
|
41,947
|
|
2
|
%
|
2
|
%
|
11
|
%
|
|
|
|
|
|
|
|
|
Total sales
|
94,808
|
|
6
|
%
|
7
|
%
|
100
|
%
|
International Operations
Sales in International Operations increased by 9% measured in Danish kroner and by 12% at CER. Sales growth was driven by all geographical areas, with EMEA growing by 11% (CER), Rest of World growing by 13% (CER) and Region China growing by 12% (CER). Sales growth was driven by all therapy areas. Sales growth was negatively impacted by COVID-19 as fewer patients initiated treatment, partially offset by COVID-19-related stocking and timing of shipments.
EMEA
Sales in EMEA increased by 9% measured in Danish kroner and by 11% at CER. Sales growth was driven by Diabetes care growing by 11% (CER) driven by increased GLP-1 and insulin sales. Biopharm sales increased by 10% (CER) and Obesity care increased by 8% (CER).
Region China
Sales in Region China increased by 10% measured in Danish kroner and by 12% at CER. Sales growth was driven by Diabetes care growing by 12% (CER) driven by increased modern insulin sales and Biopharm growing by 19% (CER). Sales growth was impacted by increased distributor stock levels, offset by fewer patients initiating treatment during the COVID-19 pandemic.
Rest of World
Sales in Rest of World increased by 8% measured in Danish kroner and by 13% at CER. Sales growth was driven by Diabetes care growing by 17% (CER) from increased insulin and GLP-1 sales, Biopharm growing by 5% (CER) and Obesity care growing by 7% (CER).
North America Operations
Sales in North America Operations increased by 2% in both Danish kroner and at CER. Sales growth was negatively impacted by COVID-19 as fewer patients initiated treatment and increasing unemployment in the US, partially offset by COVID-19-related stocking in the first quarter.
The sales development reflects GLP-1 sales growing by 27% (CER) and Obesity care growing by 4% (CER). This was offset by insulin sales declining by 22% (CER) due to lower realised prices in the US following unfavourable channel mix, rebate enhancements, affordability programmes and changes in the coverage gap legislation as well as Biopharm sales declining by 2% (CER).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 6 of 33
|
SALES DEVELOPMENT ACROSS THERAPEUTIC AREAS
Sales growth in the first nine months of 2020 was 6% measured in Danish kroner and 7% at CER driven by growth across all therapy areas with Diabetes care sales growth of 8% (CER), Obesity care sales growth of 6% (CER) and Biopharm sales growth of 4% (CER). Sales growth was negatively impacted by fewer patients initiating treatment and increased unemployment in the US, partially offset by COVID-19-related stocking.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales split per therapy
|
Sales 9M 2020
DKK million
|
Sales 9M 2019
DKK million
|
|
Growth
as reported
|
Growth
at CER
|
Share of growth
at CER
|
|
Diabetes and Obesity care segment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-acting insulin
|
13,426
|
|
15,674
|
|
|
(14
|
%)
|
(13
|
%)
|
(33
|
%)
|
|
|
- Tresiba®
|
6,720
|
|
6,948
|
|
|
(3
|
%)
|
(2
|
%)
|
(3
|
%)
|
|
|
- Xultophy®
|
1,842
|
|
1,626
|
|
|
13
|
%
|
15
|
%
|
4
|
%
|
|
|
- Levemir®
|
4,864
|
|
7,100
|
|
|
(31
|
%)
|
(30
|
%)
|
(34
|
%)
|
|
|
|
|
|
|
|
|
|
|
Premix insulin
|
8,220
|
|
7,913
|
|
|
4
|
%
|
6
|
%
|
8
|
%
|
|
|
- Ryzodeg®
|
967
|
|
747
|
|
|
29
|
%
|
33
|
%
|
4
|
%
|
|
|
- NovoMix®
|
7,253
|
|
7,166
|
|
|
1
|
%
|
3
|
%
|
4
|
%
|
|
|
|
|
|
|
|
|
|
|
Fast-acting insulin
|
14,082
|
|
14,367
|
|
|
(2
|
%)
|
(1
|
%)
|
(2
|
%)
|
|
|
- Fiasp®
|
1,021
|
|
804
|
|
|
27
|
%
|
28
|
%
|
3
|
%
|
|
|
- NovoRapid®
|
13,061
|
|
13,563
|
|
|
(4
|
%)
|
(2
|
%)
|
(5
|
%)
|
|
|
|
|
|
|
|
|
|
|
Human insulin
|
7,195
|
|
6,832
|
|
|
5
|
%
|
8
|
%
|
9
|
%
|
|
|
|
|
|
|
|
|
|
|
Total insulin
|
42,923
|
|
44,786
|
|
|
(4
|
%)
|
(3
|
%)
|
(18
|
%)
|
|
|
|
|
|
|
|
|
|
|
Victoza®
|
13,990
|
|
16,507
|
|
|
(15
|
%)
|
(15
|
%)
|
(38
|
%)
|
|
Ozempic®
|
15,023
|
|
6,872
|
|
|
119
|
%
|
120
|
%
|
129
|
%
|
|
Rybelsus®
|
1,038
|
|
—
|
|
|
—
|
—
|
16
|
%
|
|
Total GLP-1
|
30,051
|
|
23,379
|
|
|
29
|
%
|
29
|
%
|
107
|
%
|
|
|
|
|
|
|
|
|
|
|
Other Diabetes care1
|
3,056
|
|
3,230
|
|
|
(5
|
%)
|
(4
|
%)
|
(2
|
%)
|
|
|
|
|
|
|
|
|
|
|
Total Diabetes care
|
76,030
|
|
71,395
|
|
|
6
|
%
|
8
|
%
|
87
|
%
|
|
|
|
|
|
|
|
|
|
|
Obesity care (Saxenda®)
|
4,223
|
|
4,115
|
|
|
3
|
%
|
6
|
%
|
4
|
%
|
|
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care total
|
80,253
|
|
75,510
|
|
|
6
|
%
|
8
|
%
|
91
|
%
|
|
|
|
|
|
|
|
|
|
|
Biopharm segment
|
|
|
|
|
|
|
|
Haemophilia2
|
7,522
|
|
7,727
|
|
|
(3
|
%)
|
(2
|
%)
|
(2
|
%)
|
|
|
- NovoSeven®
|
5,683
|
|
6,160
|
|
|
(8
|
%)
|
(7
|
%)
|
(7
|
%)
|
|
|
- NovoEight®
|
1,115
|
|
1,119
|
|
|
0
|
%
|
1
|
%
|
0
|
%
|
|
Growth disorders (Norditropin®)
|
5,872
|
|
5,199
|
|
|
13
|
%
|
13
|
%
|
11
|
%
|
|
Other Biopharm3
|
1,161
|
|
1,168
|
|
|
(1
|
%)
|
1
|
%
|
0
|
%
|
|
Biopharm total
|
14,555
|
|
14,094
|
|
|
3
|
%
|
4
|
%
|
9
|
%
|
|
|
|
|
|
|
|
|
|
|
Total sales
|
94,808
|
|
89,604
|
|
|
6
|
%
|
7
|
%
|
100
|
%
|
|
|
|
|
|
|
|
|
|
|
1) Primarily NovoNorm®, needles and GlucaGen® HypoKit®.
|
|
|
|
|
|
2) Comprises NovoSeven®, NovoEight®, Refixia®, NovoThirteen® and Esperoct®.
|
|
|
|
3) Primarily Vagifem® and Activelle®.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 7 of 33
|
DIABETES AND OBESITY CARE
Diabetes care, sales development
Sales in Diabetes care increased by 6% measured in Danish kroner and by 8% at CER to DKK 76,030 million driven by GLP-1 growth. Novo Nordisk has improved the global diabetes value market share over the last 12 months from 28.4% to 29.2%, driven by an improved global insulin market share and an increased GLP-1 market share as well as expansion of the GLP-1 segment.
In the following sections, unless otherwise noted, market data are based on moving annual total (MAT) from August 2020 and August 2019 provided by the independent data provider IQVIA.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diabetes care, development per area
|
Novo Nordisk’s share of the total diabetes market (value, MAT)
|
Diabetes care, sales development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aug
|
Aug
|
Sales 9M 2020
DKK million
|
Growth
at CER
|
|
|
|
2020
|
2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Global
|
29.2
|
%
|
28.4
|
%
|
76,030
|
|
8
|
%
|
|
International Operations
|
22.7
|
%
|
22.0
|
%
|
39,768
|
|
13
|
%
|
|
|
- EMEA *
|
27.4
|
%
|
26.8
|
%
|
20,076
|
|
11
|
%
|
|
|
- Region China **
|
28.5
|
%
|
27.8
|
%
|
10,526
|
|
12
|
%
|
|
|
- Rest of World ***
|
13.3
|
%
|
12.7
|
%
|
9,166
|
|
17
|
%
|
|
North America Operations
|
31.6
|
%
|
30.8
|
%
|
36,262
|
|
3
|
%
|
|
|
- The US
|
31.7
|
%
|
31.1
|
%
|
34,396
|
|
2
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Source: IQVIA, August 2020 data. *Data for EMEA available for European markets and seven markets outside Europe representing approximately 90% of Novo Nordisk Diabetes care sales in the area. **Data for mainland China, excluding Hong Kong and Taiwan. *** Data for Rest of World available for seven markets representing approximately 70% of total Novo Nordisk’s Diabetes care sales in the area.
Insulin
Sales of insulin decreased by 4% measured in Danish kroner and by 3% at CER to DKK 42,923 million. The sales decrease measured at CER was driven by declining sales in the US, partly offset by increased sales in International Operations.
Sales of long-acting insulin decreased by 14% measured in Danish kroner and by 13% at CER to DKK 13,426 million. Novo Nordisk has increased its global volume market share in the long-acting insulin segment from 32.3% to 32.7% in the last 12 months. The sales decline measured at CER was driven by declining Levemir® and Tresiba® sales, partially offset by increased sales of Xultophy®. Tresiba® has been launched in 88 countries, while Xultophy® has now been launched in 42 countries.
Sales of premix insulin increased by 4% measured in Danish kroner and by 6% at CER to DKK 8,220 million. Novo Nordisk is the market leader in the premix insulin segment with a global volume market share of 64.4% compared to 64.0% 12 months ago. The sales increase was driven by increased sales of both Ryzodeg® and NovoMix®. Ryzodeg® has now been launched in 35 countries.
Sales of fast-acting insulin decreased by 2% measured in Danish kroner and by 1% at CER to DKK 14,082 million. Novo Nordisk is the market leader in the fast-acting insulin segment and has increased its global volume market share to 51.4% from 50.5% in the last 12 months. The sales decrease was driven by NovoRapid®, partly offset by increased Fiasp® sales. Fiasp® has now been launched in 40 countries.
Sales of human insulin increased by 5% measured in Danish kroner and by 8% at CER to DKK 7,195 million.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 8 of 33
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Insulin, development per geographical area
|
Novo Nordisk’s share of the total insulin market (volume, MAT)
|
Insulin, sales development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aug
|
Aug
|
Sales 9M 2020
DKK million
|
Growth
at CER
|
|
|
|
2020
|
2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Global
|
46.9
|
%
|
46.5
|
%
|
42,923
|
|
(3
|
%)
|
|
International Operations
|
49.7
|
%
|
49.1
|
%
|
29,431
|
|
10
|
%
|
|
|
- EMEA *
|
47.2
|
%
|
46.5
|
%
|
13,982
|
|
6
|
%
|
|
|
- Region China **
|
49.5
|
%
|
50.2
|
%
|
8,523
|
|
14
|
%
|
|
|
- Rest of World ***
|
57.0
|
%
|
56.0
|
%
|
6,926
|
|
12
|
%
|
|
North America Operations
|
39.6
|
%
|
39.9
|
%
|
13,492
|
|
(22
|
%)
|
|
|
- The US
|
39.5
|
%
|
40.1
|
%
|
12,704
|
|
(23
|
%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Source: IQVIA, Aug 2020 data. *Data for EMEA available for European markets and seven markets outside Europe representing approximately 90% of Novo Nordisk diabetes care sales in the area. **Data for mainland China, excluding Hong Kong and Taiwan. *** Data for Rest of World available for seven markets representing approximately 70% of total Novo Nordisk’s iabetes care sales in the area.
International Operations
Sales of insulin in International Operations increased by 7% measured in Danish kroner and by 10% at CER. Sales growth was driven by all insulin segments.
EMEA
Sales of insulin in EMEA increased by 4% measured in Danish kroner and by 6% at CER. Sales growth was mainly driven by Tresiba® and Xultophy®.
Region China
Sales of insulin in Region China increased by 12% measured in Danish kroner and by 14% at CER. The sales growth was driven by all insulin products. Tresiba® was included on the National Reimbursement Drug List in January 2020 and hospital listings have progressed.
Rest of World
Sales of insulin in Rest of World increased by 7% measured in Danish kroner and by 12% at CER. The sales growth was driven by human insulin and the portfolio of modern and new generation insulin .
North America Operations
Sales of insulin in North America Operations decreased by 22% in both Danish kroner and CER. The sales decline in the US was driven by lower realised prices following rebate enhancements, unfavourable channel mix, changes in the coverage gap legislation and launch of affordability programmes. Novo Nordisk has a volume market share of 39.6% of the total insulin market.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 9 of 33
|
GLP-1 therapy for type 2 diabetes
Sales of GLP-1 products for type 2 diabetes (Victoza®, Ozempic® and Rybelsus®) increased by 29% in both Danish kroner and at CER to DKK 30,051 million. Ozempic® has now been launched in 48 countries with sales of DKK 15,023 million. The GLP-1 segment’s value share of the total diabetes market has increased to 21.0% compared with 17.0% 12 months ago. Novo Nordisk continues to be the global market leader in the GLP-1 segment with a 49.9% value market share, an increase of 3.1 percentage points compared to 12 months ago. Sales growth was negatively impacted by COVID-19 as fewer patients initiated treatment and increased unemployment in the US, partially countered by COVID-19 related stocking.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GLP-1, development per geographical area
|
Novo Nordisk's share of the diabetes GLP-1 market (value, MAT)
|
GLP-1, sales development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aug
|
Aug
|
Sales 9M 2020
DKK million
|
Growth
at CER
|
|
|
|
2020
|
2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Global
|
49.9
|
%
|
46.8
|
%
|
30,051
|
|
29
|
%
|
|
International Operations
|
52.7
|
%
|
50.3
|
%
|
8,087
|
|
37
|
%
|
|
|
- EMEA *
|
54.4
|
%
|
52.9
|
%
|
5,547
|
|
35
|
%
|
|
|
- Region China **
|
92.2
|
%
|
92.4
|
%
|
799
|
|
24
|
%
|
|
|
- Rest of World ***
|
42.4
|
%
|
37.7
|
%
|
1,741
|
|
51
|
%
|
|
North America Operations
|
49.4
|
%
|
46.3
|
%
|
21,964
|
|
27
|
%
|
|
|
- The US
|
48.9
|
%
|
45.8
|
%
|
20,991
|
|
26
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Source: IQVIA, August data. *Data for EMEA available for European markets and seven markets outside Europe representing approximately 90% of Novo Nordisk diabetes sales in the area. **Data for mainland China, excluding Hong Kong and Taiwan. *** Data for Rest of World available for seven markets representing approximately 70% of total Novo Nordisk diabetes care sales in the area.
International Operations
Sales of GLP-1 in International Operations increased by 34% measured in Danish kroner and by 37% at CER. Sales growth is driven by all geographical areas. The value share of the GLP-1 class of the total diabetes market has increased to 10.4% from 8.7% 12 months ago. Novo Nordisk is the market leader with a value market share of 52.7%.
EMEA
Sales in EMEA increased by 35% in both Danish kroner and at CER. The sales growth reflects the uptake of Ozempic® countered by lower sales of Victoza®. Rybelsus® has now been launched in seven countries in EMEA. Novo Nordisk remains the market leader in EMEA with a value market share of 54.4%.
Region China
Sales in Region China increased by 22% measured in Danish kroner and by 24% at CER. The increased sales reflect volume growth, partly offset by lower realised prices. The GLP-1 class' share of the overall diabetes market value increased to 2.6% from 1.9% 12 months ago. Victoza® has a market share of 92.2%.
Rest of World
Sales in Rest of World increased by 39% measured in Danish kroner and by 51% at CER. The sales growth reflects increased Victoza® sales and launches of Ozempic®. Novo Nordisk remains the market leader with a value market share of 42.4%.
North America Operations
Sales of GLP-1 diabetes products in North America Operations increased by 27% in both Danish kroner and at CER. Sales were negatively impacted by fewer patients initiating treatment and increased unemployment in the US, partially off-set by COVID-19-related stocking, Novo Nordisk is the market leader with a 49.4% value market share compared to 46.3% 12 months ago. The value market share of the GLP-1 class of the total North American diabetes market has increased to 24.9%.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 10 of 33
|
Sales growth in the US is driven by a prescription volume growth of the GLP-1 class of around 30%. Rybelsus® market access has progressed and the product is broadly available across commercial and Medicare plans and the weekly new-to-brand market share has reached 13.5%. The combined Novo Nordisk GLP-1 new-to-brand prescription market share is now 60.2%. Novo Nordisk is the market leader measured on total monthly prescriptions for the combined GLP-1 portfolio.
Sales of GLP-1 in the US increased by 26% at CER. The sales increase was driven by continued uptake of Ozempic® and Rybelsus®, partially offset by declining Victoza® sales. GLP-1 sales growth was negatively impacted by rebate enhancements, unfavourable channel and payer mix as well as changes in coverage gap legislation.
Obesity care, sales development
Sales of Saxenda® increased by 3% measured in Danish kroner and by 6% at CER to DKK 4,223 million. Saxenda® sales growth was driven by both International Operations and North America Operations. Sales growth was negatively impacted by COVID-19 as fewer patients initiated treatment. Saxenda® has now been launched in 54 countries. Novo Nordisk currently has a value market share of 63.0% of the global obesity prescription drug market.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Obesity care, development per geographical area
|
Obesity care, sales development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales 9M 2020
DKK million
|
Growth
at CER
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Global
|
4,223
|
|
6
|
%
|
|
International Operations
|
1,611
|
|
8
|
%
|
|
|
- EMEA
|
834
|
|
8
|
%
|
|
|
- Region China
|
6
|
|
(17
|
%)
|
|
|
- Rest of World
|
771
|
|
7
|
%
|
|
North America Operations
|
2,612
|
|
4
|
%
|
|
|
- The US
|
2,420
|
|
4
|
%
|
|
|
|
|
|
|
|
|
|
|
International Operations
Sales of Saxenda® in International Operations remained unchanged in Danish kroner and increased by 8% at CER driven by increased sales in EMEA and Rest of World. Novo Nordisk currently has a value market share of 39.6% in the obesity prescription drug market in International Operations. Sales growth was negatively impacted by fewer patients initiating treatment due to COVID-19.
EMEA
Sales of Saxenda® in EMEA increased by 7% measured in Danish kroner and by 8% at CER. Novo Nordisk currently has a value market share of 65.4% in the obesity prescription drug market in EMEA.
Rest of World
Sales of Saxenda® in Rest of World decreased by 6% measured in Danish kroner and increased by 7% at CER. Saxenda® has been launched in 17 countries in Rest of World. Novo Nordisk currently has a value market share of 31.2% in the obesity prescription drug market in Rest of World.
North America Operations
Sales of Saxenda® in North America Operations increased by 4% in both Danish kroner and at CER and were driven by increased sales in both the US and Canada. Novo Nordisk has a value market share of 77.8% in the obesity prescription drug market in North America Operations. Sales growth was negatively impacted by fewer patients initiating treatment due to COVID-19.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 11 of 33
|
BIOPHARM
Biopharm, sales development
Sales of biopharm products increased by 3% measured in Danish kroner and by 4% at CER to DKK 14,555 million. The sales growth was driven by International Operations. Sales growth was driven by Growth Disorders and the launches of new haemophilia products, offset by declining sales of NovoSeven®. Sales growth was negatively impacted by lower demand due to COVID-19, offset by timing of shipments and COVID-19-related stocking.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biopharm, development per geographical area
|
Biopharm, sales development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales 9M 2020
DKK million
|
Growth
at CER
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Global
|
14,555
|
|
4
|
%
|
|
International Operations
|
9,020
|
|
8
|
%
|
|
|
- EMEA
|
5,249
|
|
10
|
%
|
|
|
- Region China
|
304
|
|
19
|
%
|
|
|
- Rest of World
|
3,467
|
|
5
|
%
|
|
North America Operations
|
5,535
|
|
(2
|
%)
|
|
|
- The US
|
5,131
|
|
(2
|
%)
|
|
|
|
|
|
|
|
|
|
|
Haemophilia
Sales of haemophilia products decreased by 3% measured in Danish kroner and by 2% at CER to DKK 7,522 million. The decreasing sales at CER were driven by NovoSeven® sales, partly offset by the continued global roll-out of Esperoct® and Refixia®.
Sales of NovoSeven® decreased by 8% measured in Danish kroner and by 7% at CER to DKK 5,683 million. The sales development was driven by declining sales in North America Operations and Rest of World, offset by increasing sales in EMEA and Region China. The declining sales partly reflect reduced elective surgeries and bleedings due to COVID-19.
Sales of NovoEight® remained unchanged in Danish kroner and increased by 1% at CER to DKK 1,115 million. The increasing sales at CER were driven by increased sales in Rest of World and the US, partly offset by declining sales in EMEA. NovoEight® has been launched in 59 countries. Esperoct®, the long-acting haemophilia A treatment, has now been launched in 17 countries.
Sales of Refixia® increased to DKK 385 million. Sales growth was driven by continued uptake in the product launches in EMEA and Rest of World as well as continued uptake in North America Operations. Refixia® has been launched in 25 countries.
Growth disorders (Norditropin®)
Sales of Norditropin® increased by 13% in both Danish kroner and at CER to DKK 5,872 million. The sales increase was driven by International Operations and North America Operations increasing by 18% and 7% at CER, respectively. Novo Nordisk is the leading company in the global human growth disorder market with a value market share of 35.3% compared to 32.8% a year ago, driven by new indications and the global roll-out of the next-generation device. In addition to commercial execution, sales growth was positively impacted by changes in inventories and COVID-19-related stocking as well as additional demand, following supply challenges for competing products in select countries.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 12 of 33
|
DEVELOPMENT IN COSTS AND OPERATING PROFIT
The cost of goods sold increased by 4% measured in Danish kroner and by 6% at CER to DKK 15,313 million, resulting in a gross margin of 83.8% measured in Danish kroner, compared with 83.6% in 2019. The increased gross margin reflects a positive product mix driven by increased GLP-1 sales and productivity improvements. This is partly countered by a negative impact from lower realised prices in the US.
Sales and distribution costs increased by 4% measured in Danish kroner and by 5% at CER to DKK 23,162 million. The increase in costs is driven by North America Operations reflecting launch activities for Rybelsus® and continued promotional activities for Ozempic®, partly offset by lower promotional spend related to insulin. In International Operations, promotional spend is related to launch activities for Ozempic® and Rybelsus® as well as the continued roll-out of Saxenda®. COVID-19 resulted in a reduction of the activity level and delay of promotional activities.
Research and development costs increased by 12% in both Danish kroner and at CER to DKK 10,979 million. The cost increase is driven by the amortisation of the priority review voucher for semaglutide in obesity in the third quarter of 2020. Increased activities within Other serious chronic diseases are driving the cost increase following progression of the early pipeline within cardiovascular disease and stem cell projects. This is partly offset by lower spend within Obesity care driven by finalisation of the semaglutide obesity pivotal phase 3a programme and COVID-19 impact on clinical trial activity.
Administration costs remained unchanged in Danish kroner and increased by 1% at CER to DKK 2,760 million, reflecting broadly unchanged spend across administrative areas.
Other operating income (net) was DKK 354 million compared with DKK 557 million in 2019 following reduced royalty income.
Operating profit increased by 6% measured in Danish kroner and by 7% at CER to DKK 42,948 million.
FINANCIAL ITEMS (NET) AND TAX
Financial items (net) showed a net loss of DKK 1,820 million compared with a net loss of DKK 3,136 million in 2019.
In line with Novo Nordisk’s treasury policy, the most significant foreign exchange risks for Novo Nordisk have been hedged, primarily through foreign exchange forward contracts. The foreign exchange result was a net loss of DKK 1,430 million compared with a net loss of DKK 2,649 million in 2019. This mainly reflects losses on non-hedged currencies driven by significant depreciations of several emerging market currencies.
As per the end of September 2020, a positive market value of financial contracts of approximately DKK 1.2 billion has been deferred for recognition later in 2020 and 2021.
The effective tax rate was 20.2% in the first nine months of 2020 compared with an effective tax rate of 19.3% in the first nine months of 2019 as the effective tax rate in 2019 was positively impacted by non-recurring changes to deferred tax assets following the Swiss tax reform.
CAPITAL EXPENDITURE AND FREE CASH FLOW
Capital expenditure for property, plant and equipment was DKK 4.3 billion compared with DKK 7.0 billion in 2019. The lower capital expenditure was mainly related to investments in the new production facility for diabetes active pharmaceutical ingredients in Clayton, North Carolina, US, which is now in the final stages of construction.
Free cash flow was DKK 41.6 billion compared with DKK 32.7 billion in 2019. The increase was driven by the higher net profit and favourable timing of rebate payments in the US. The purchase of intangible assets of DKK 4.9 billion includes upfront consideration for Corvidia Therapeutics acquired in July 2020.
Novo Nordisk’s financial resources, consisting of cash at bank and committed credit facilities, were DKK 36.4 billion by end of September 2020.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 13 of 33
|
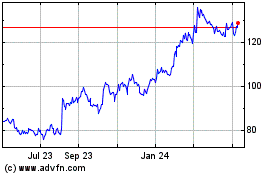
KEY DEVELOPMENTS IN THE THIRD QUARTER OF 2020
Sales in the third quarter of 2020 increased by 2% measured in Danish kroner and by 7% at CER compared to the same period in 2019. Operating profit decreased by 1% measured in Danish kroner and increased by 7% at CER. The sales and operating profit growth rates at CER were announced on 8 October.
Please refer to appendix 1 for an overview of the quarterly numbers in DKK and to appendix 6 for additional details on sales in the third quarter of 2020.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales split per geographical area
|
Sales Q3 2020
DKK million
|
Growth
as reported
|
Growth
at CER
|
Share of growth
at CER
|
|
|
|
|
|
|
|
|
|
|
|
|
|
International Operations
|
15,988
|
|
5
|
%
|
10
|
%
|
70
|
%
|
|
|
- EMEA
|
8,318
|
|
5
|
%
|
9
|
%
|
30
|
%
|
|
|
- Region China
|
3,549
|
|
9
|
%
|
13
|
%
|
19
|
%
|
|
|
- Rest of World
|
4,121
|
|
1
|
%
|
11
|
%
|
21
|
%
|
|
North America Operations
|
14,939
|
|
(1
|
%)
|
5
|
%
|
30
|
%
|
|
|
- The US
|
14,144
|
|
(1
|
%)
|
4
|
%
|
26
|
%
|
|
|
|
|
|
|
|
|
Total sales
|
30,927
|
|
2
|
%
|
7
|
%
|
100
|
%
|
The increased global sales of 7% (CER) was driven by increased Diabetes and Obesity care sales due to increased GLP-1 sales of 31% (CER), offset by insulin sales declining by 2% (CER), as well as unchanged Obesity care sales (CER) and unchanged Biopharm sales (CER). Sales growth was negatively impacted by COVID-19 as fewer patients initiated treatment, unemployment in the US and COVID-19-related destocking, offset by phasing of rebates and inventory changes at wholesaler level in the US.
International Operations
Sales in International Operations increased by 5% measured in Danish kroner and by 10% at CER. Sales growth was driven by all geographical areas and by all therapy areas. COVID-19 negatively impacted sales growth as fewer patients initiated treatment.
Sales growth was driven by Diabetes care growing by 12% (CER) driven by increased GLP-1 sales growing by 37% (CER), insulin sales growing by 8% (CER) and Obesity care increasing by 6% (CER). Biopharm sales increased by 4% (CER) driven by increased Norditropin® sales, partially offset by decreased haemophilia sales.
North America Operations
Sales in North America Operations decreased by 1% measured in Danish kroner and increased by 5% at CER. Sales growth was negatively impacted by COVID-19 as fewer patients initiated treatment, increased unemployment and destocking, offset by phasing of rebates and inventory changes at wholesaler level.
Sales growth was driven by GLP-1 growing by 29% (CER) reflecting the prescription volume growth of the market and the uptake of Ozempic® and the launch of Rybelsus®. Insulin sales decreased by 20% (CER) driven by lower realised prices. Obesity sales decreased by 3% (CER) reflecting fewer patients initiating treatment due to COVID-19, and Biopharm sales decreased by 6% (CER) due to lower NovoSeven® and Norditropin® sales.
The gross margin was 83.3% in the third quarter of 2020 compared with 83.2% in the same period last year. The increase of 0.1 percentage point of the gross margin reflects asset impairments in 2019 as well as positive product mix and productivity, partly countered by a negative impact from lower realised prices in the US and a negative currency impact of 0.3% percentage point.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 14 of 33
|
Sales and distribution costs increased by 5% measured in Danish kroner and by 10% at CER compared with the same period in 2019. The increase was driven by North America Operations reflecting the launch of Rybelsus® and promotional activities related to Ozempic® as well as changes to provisions for legal cases.
Research and development costs increased by 9% measured in Danish kroner and by 10% at CER compared with the same period in 2019. The costs were driven by the amortisation of the priority review voucher for semaglutide in obesity, largely offset by impairments in the third quarter of 2019. The cost increase is driven by patient recruitment to the ongoing cardiovascular outcome trials, SOUL and SELECT, partly offset by lower costs following conclusion of the semaglutide obesity pivotal phase 3a programme.
Administrative costs remained unchanged in Danish kroner and increased by 3% at CER compared with the same period in 2019.
Other operating income (net) was DKK 127 million in the third quarter of 2020 compared with DKK 88 million in the same period last year reflecting increased royalty income.
Operating profit decreased by 1% measured in Danish kroner and increased by 7% at CER compared with the same period in 2019.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 15 of 33
|
EQUITY AND CAPITAL ALLOCATION
Total equity was DKK 59,573 million at the end of the first nine months of 2020, equivalent to 42.6% of total assets, compared with 42.4% at the end of the first nine months of 2019. Please refer to appendix 5 for further elaboration of changes in equity.
2020 share repurchase programme
On 6 August 2020, Novo Nordisk announced a share repurchase programme of up to DKK 2.8 billion to be executed from 6 August to 28 October 2020, as part of an overall programme of up to DKK 17 billion to be executed during a 12-month period beginning 5 February 2020. The purpose of the programme was to reduce the company’s share capital and to meet obligations arising from share-based incentive programmes. Under the programme, Novo Nordisk has repurchased 6,513,843 B shares for an amount of DKK 2.8 billion in the period from 6 August to 28 October 2020. The programme was concluded on 28 October 2020.
As of 28 October 2020, Novo Nordisk A/S has repurchased a total of 26,573,022 B shares equal to a transaction value of DKK 11.351 billion under the DKK 17 billion programme beginning 5 February 2020.
As of 28 October 2020, Novo Nordisk and its wholly-owned affiliates owned 26,561,746 of its own B shares, corresponding to 1.1% of the total share capital.
Share repurchases under the overall programme of up to DKK 17 billion that began 5 February 2020 are expected to be resumed shortly. As announced in February 2020, Novo Nordisk’s majority shareholder Novo Holdings A/S, a holding company fully owned by the Novo Nordisk Foundation, has informed Novo Nordisk that it intends to consider its participation in the Novo Nordisk share repurchase programme on a year-by-year basis. For 2020, Novo Holdings A/S has informed Novo Nordisk that it plans to participate in the share repurchase programme. Novo Holdings A/S has an
ownership of 28.3% of the Novo Nordisk share capital, and Novo Holdings A/S currently intends to maintain its ownership of the Novo Nordisk share capital around 28%.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 16 of 33
|
OUTLOOK
OUTLOOK 2020
The outlook for sales and operating profit growth was raised on 8 October to 5-8% at CER. The current expectations for 2020 are summarised in the table below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expectations are as reported, if not otherwise stated
|
Expectations
30 October 2020
|
Expectations
6 August 2020
|
|
|
|
|
|
|
|
|
|
Sales growth
|
|
|
|
at CER
|
5% to 8%
|
3% to 6%
|
|
as reported
|
Around 3 percentage points lower than at CER
|
Around 2 percentage points lower than at CER
|
|
|
|
|
|
|
|
|
|
Operating profit growth
|
|
|
|
at CER
|
5% to 8%
|
2% to 5%
|
|
as reported
|
Around 4 percentage points lower than at CER
|
Around 3 percentage points lower than at CER
|
|
|
|
|
|
|
|
|
|
Financial items (net)
|
Loss of around DKK 1.4 billion
|
Loss of around DKK 1.2 billion
|
|
|
|
|
|
Effective tax rate
|
20% to 22%
|
20% to 22%
|
|
|
|
|
|
Capital expenditure (PP&E)
|
Around DKK 6.5 billion
|
Around DKK 6.5 billion
|
|
|
|
|
|
Depreciation, amortisation and impairment losses
|
Around DKK 5.5 billion
|
Around DKK 5 billion
|
|
|
|
|
|
Free cash flow
|
DKK 34-39 billion
|
DKK 33-38 billion
|
|
|
|
|
|
|
|
|
The sales growth outlook was updated on 8 October 2020 and is expected to be 5% to 8% at CER. The guidance reflects expectations for continued robust sales performance for the GLP-1 diabetes care products Ozempic®, Victoza® and Rybelsus®, the portfolio of new-generation insulin and the biopharm products. The guidance also reflects intensifying competition both within Diabetes care and Biopharm, especially within the haemophilia inhibitor segment. Furthermore, continued pricing pressure within Diabetes care as well as expansion of affordability initiatives, especially in the US, are expected to impact sales development. Given the current exchange rates versus the Danish krone, growth reported in DKK is now expected to be around 3 percentage points lower than at CER.
The operating profit growth outlook was updated on 8 October 2020 and is expected to be 5% to 8% at CER. The expectation for operating profit growth primarily reflects the sales growth outlook and continued investments in current and future growth drivers across the operating units. Given the current exchange rates versus the Danish krone, growth reported in DKK is now expected to be around 4 percentage points lower than at CER.
The current COVID-19 pandemic causes uncertainty to the outlook regarding the number of patients initiating treatment and societal impacts such as the unemployment rate in the US which is impacting healthcare insurance coverage. The outlook is based on a number of assumptions related to the severity and duration of impacts from COVID-19. Consequently, volatility in quarterly results should be expected.
For 2020, Novo Nordisk now expects financial items (net) to amount to a loss of around DKK 1.4 billion compared to a loss of DKK 1.2 billion in August 2020. This development mainly reflects losses from non-hedged currencies due to depreciations across several emerging market currencies.
The effective tax rate for 2020 is still expected to be in the range of 20-22%.
Capital expenditure is still expected to be around DKK 6.5 billion in 2020, primarily relating to investments in additional capacity for active pharmaceutical ingredient (API) production within Diabetes care and an expansion of the filling capacity within Diabetes care. Depreciation, amortisation and impairment losses are now expected to be around DKK 5.5 billion reflecting the amortisation of the priority review voucher for semaglutide in obesity. The free cash flow is now expected to be DKK 34-39 billion reflecting higher net profit.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 17 of 33
|
All of the above expectations are based on assumptions that the global or regional macroeconomic and political environment will not significantly change business conditions for Novo Nordisk during the remainder of 2020, including the potential implications from major healthcare reforms, and that the currency exchange rates, especially the US dollar, will remain at the current level versus the Danish krone. Neither does the guidance include the financial implications of significant business development transactions during the remainder of 2020.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FX
|
Q3 2020
|
Q3 2019
|
% change
|
9M 2020
|
9M 2019
|
% change
|
Spot rate
26 October 2020
|
|
|
|
|
|
|
|
|
|
|
USD
|
637
|
|
671
|
|
(5
|
%)
|
663
|
|
664
|
|
0
|
%
|
630
|
|
|
CNY
|
92
|
|
96
|
|
(4
|
%)
|
95
|
|
97
|
|
(2
|
%)
|
94
|
|
|
JPY
|
6.00
|
|
6.26
|
|
(4
|
%)
|
6.17
|
|
6.09
|
|
1
|
%
|
5.99
|
|
|
CAD
|
478
|
|
508
|
|
(6
|
%)
|
491
|
|
500
|
|
(2
|
%)
|
478
|
|
|
GBP
|
823
|
|
827
|
|
0
|
%
|
843
|
|
845
|
|
0
|
%
|
820
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Novo Nordisk has hedged expected net cash flows in a number of invoicing currencies and, all other things being equal, movements in key invoicing currencies will impact Novo Nordisk’s operating profit as outlined in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key invoicing currencies
|
Impact on Novo Nordisk's operating profit in the next 12 months of a 5% movement in currency
|
Hedging period (months)
|
|
|
|
|
|
|
|
|
|
USD
|
DKK 1,900 million
|
11
|
|
|
CNY1
|
DKK 450 million
|
6
|
|
JPY
|
DKK 150 million
|
12
|
|
CAD
|
DKK 130 million
|
9
|
|
GBP
|
DKK 100 million
|
10
|
|
|
|
|
|
1) Chinese yuan traded offshore (CNH) used as proxy when hedging Novo Nordisk’s CNY currency exposure.
The financial impact from foreign exchange hedging is included in Financial items (net).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 18 of 33
|
RESEARCH & DEVELOPMENT UPDATE
Diabetes care
Xultophy® marketing authorisation application submitted in China
In August 2020, Novo Nordisk submitted a new drug application to the China Centre of Drug Evaluation for Xultophy® for the treatment of people with insufficiently controlled type 2 diabetes.
Phase 3b trial initiated with Ozempic® for peripheral artery disease
During the third quarter of 2020, Novo Nordisk initiated a phase 3b trial investigating the effects of Ozempic® (once-weekly subcutaneous semaglutide) in around 800 people with type 2 diabetes and peripheral artery disease. The trial is a randomised, double-blinded and placebo-controlled trial, investigating semaglutide versus standard of care.
Phase 1 trial initiated with glucose-sensitive insulin (NN1845)
During the third quarter of 2020, Novo Nordisk initiated the first human dose trial for a glucose-sensitive insulin. The trial is investigating safety, tolerability, pharmacokinetics and pharmacodynamics of subcutaneously administrated glucose-sensitive insulin.
Phase 1 trial initiated with oral semaglutide at higher doses with different formulations
During the third quarter of 2020, Novo Nordisk initiated a phase 1 trial with higher doses of oral semaglutide for type 2 diabetes. The trial is a randomised and open-label trial designed to assess oral semaglutide at higher doses, including investigation of different formulations in healthy volunteers. The primary objective of the trial is to investigate the steady state exposure of 25 and 50 mg of oral semaglutide in different formulations.
Development of anti-IL-21 in combination with liraglutide discontinued
In 2019, Novo Nordisk successfully completed a phase 2 trial evaluating the effects of the combination of anti-IL-21 and liraglutide as therapy for patients recently diagnosed with type 1 diabetes. Following an evaluation of the regulatory path forward, Novo Nordisk has decided to discontinue the development of anti-IL-21 in combination with liraglutide.
Obesity care
Priority review voucher to be applied for semaglutide in obesity
During the third quarter of 2020, Novo Nordisk notified the US Food and Drug Administration (FDA) that Novo Nordisk will request priority review for the file of semaglutide in obesity using a priority review voucher. Novo Nordisk still expects to file semaglutide in obesity around the turn of the year.
Biopharm
Sogroya® approved in the US in adults with growth hormone deficiency
On 28 August 2020, once-weekly somapacitan was approved by the FDA under the brand name Sogroya® for substitution of endogenous growth hormone in adults with growth hormone deficiency (AGHD). The approval is based on results from the REAL programme where the global pivotal trial enrolled 301 treatment-naïve adults with growth hormone deficiency. Sogroya® administered once-weekly demonstrated superiority over placebo on the primary endpoint, change in truncal fat percentage. Sogroya® for AGHD is currently under regulatory review in the EU, Switzerland and Japan.
Concizumab phase 3 trial reinitiated
On 13 August 2020, Novo Nordisk announced that the clinical trials in the concizumab phase 3 programme (explorer6, 7 and 8) would be reinitiated. The trials are investigating subcutaneous concizumab prophylaxis treatment in haemophilia A and B patients regardless of inhibitor status. This follows pausing of the trials in March 2020 due to the occurrence of non-fatal thrombotic events in three patients enrolled. Novo Nordisk has together with international experts and relevant authorities identified a path forward for concizumab. New safety measures and guidelines, based on analysis of all available data, have been agreed with the US FDA and the clinical hold has been lifted. The trials were reinitiated in September 2020.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 19 of 33
|
Other serious chronic diseases
Semaglutide in NASH granted Breakthrough Therapy Designation in the US
In August 2020, the US FDA granted Breakthrough Therapy designation for semaglutide for non-alcoholic steatohepatitis (NASH). The designation means amongst others that the FDA will work closely with Novo Nordisk to develop semaglutide expeditiously for this indication. Novo Nordisk expects to initiate phase 3 clinical development with semaglutide in NASH in 2021.
Ziltivekimab phase 2 trial successfully completed
In September 2020, Novo Nordisk successfully completed the RESCUE phase 2 trial with once-monthly subcutaneous ziltivekimab. The 24-week blinded phase 2 trial investigated the efficacy of ziltivekimab compared to placebo on markers of inflammation measured by high-sensitivity C-reactive protein (hsCRP) in a chronic kidney disease patient population with atherosclerotic cardiovascular disease and inflammation. 264 patients were randomised to treatment with three different monthly doses of ziltivekimab (7.5, 15 and 30 mg) or placebo. The study met its primary objective, demonstrating a decrease in hsCRP after 12 weeks of treatment of up to 92% compared to a decrease of 5% with placebo. The treatment difference was statistically significant for all dose levels compared to placebo. In the trial, ziltivekimab appeared to have a safe and well-tolerated profile. Following the completion of the phase 2 trial, a cardiovascular outcomes trial is expected to be initiated in 2021.
SUSTAINABILITY UPDATE
Social responsibility
As part of the social responsibility strategy 'Defeat Diabetes' and the focus on providing affordable insulin to vulnerable patients in every country, Novo Nordisk has as of August 2020 lowered the ceiling price of human insulin to 3 USD from 4 USD per vial, and letters have been sent out to ministries of health in 76 low- and middle-income countries in scope for the company’s Access to Insulin Commitment.
Environment
In September 2020, Novo Nordisk set a target to ensure all direct suppliers supply the company based on 100% renewable power by 2030. Novo Nordisk will work with all existing and new suppliers to meet the target. The commitment is the next step in Novo Nordisk’s ‘Circular for Zero’ environmental strategy, which was launched in April 2019 and set the ambition for the company to have zero environmental impact. The majority of Novo Nordisk’s total carbon emissions originate in the supply chain, making this commitment with suppliers on renewable power integral to achieving Novo Nordisk’s environmental strategy. By achieving this target, Novo Nordisk would therefore be eliminating at least 300,000 tons of greenhouse gases from the supply chain. Novo Nordisk has already committed to achieve zero CO2 emissions from the global operations and transport by 2030, and in 2020 the target of using 100% renewable power across all production sites was achieved.
Employees
The number of full-time employees at the end of the first nine months of 2020 increased by 5.1% compared to 12 months ago. The total number of employees was 44,893 corresponding to 44,326 full-time positions. The increase in employees in Novo Nordisk is mainly driven by International Operations. Novo Nordisk also continues to increase the number of employees in Global Business Services in India.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial Performance
|
Equity
|
Outlook
|
Strategic aspirations
|
R&D
|
Sustainability
|
Corporate governance
|
Legal
|
Financial Information
|
|
|
|
|
|
|
Company announcement No XX / 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial Performance
|
Equity
|
Outlook
|
Strategic aspirations
|
R&D
|
Sustainability
|
Corporate governance
|
Legal
|
Financial Information
|
|
|
|
|
|
|
Company announcement No XX / 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial Performance
|
Equity
|
Outlook
|
Strategic aspirations
|
R&D
|
Sustainability
|
Corporate governance
|
Legal
|
Financial Information
|
|
|
|
|
|
|
Company announcement No XX / 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial Performance
|
Equity
|
Outlook
|
Strategic aspirations
|
R&D
|
Sustainability
|
Corporate governance
|
Legal
|
Financial Information
|
|
|
|
|
|
|
Company announcement No XX / 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial Performance
|
Equity
|
Outlook
|
Strategic aspirations
|
R&D
|
Sustainability
|
Corporate governance
|
Legal
|
Financial Information
|
|
|
|
|
|
|
Company announcement No XX / 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial Performance
|
Equity
|
Outlook
|
Strategic aspirations
|
R&D
|
Sustainability
|
|
Legal
|
Financial Information
|
|
|
|
|
|
|
Company announcement No XX / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 20 of 33
|
CORPORATE GOVERNANCE
Communication to shareholders
As part of Novo Nordisk's ambition to reduce the environmental footprint, shareholders in Novo Nordisk A/S will as of 1 January 2021 only receive formal shareholder communication from the company by e-mail. This also applies to notices to convene general meetings that will no longer be sent to shareholders as hardcopy by regular mail.
Shareholders are encouraged to sign up and register their email addresses via Novo Nordisk's InvestorPortal on the website:
https://www.novonordisk.com/investors/investor-portal-and-SHARE-magazine.html (the contents of the company’s website do not form a part of this Form 6-K).
Shareholders who wish to receive communication from Novo Nordisk are responsible for ensuring that the company is in possession of the correct email addresses.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 21 of 33
|
MANAGEMENT STATEMENT
The Board of Directors and Executive Management have reviewed and approved the financial report of Novo Nordisk A/S for the first nine months of 2020. The financial report has not been audited or reviewed by the company’s
independent auditors.
The financial report for the first nine months of 2020 has been prepared in accordance with IAS 34 'Interim Financial Reporting'. The accounting policies adopted in the preparation are consistent with those applied in the Annual Report 2019 of Novo Nordisk. Furthermore, the financial report for the first nine months of 2020 and Management’s Review are prepared in accordance with additional Danish disclosure requirements for interim reports of listed companies.
In our opinion, the accounting policies used are appropriate and the overall presentation of the financial report for the first nine months of 2020 is adequate. Furthermore, in our opinion, Management’s Review includes a true and fair account of the development in the operations and financial circumstances of the results for the period and of the financial position of the Group as well as a description of the most significant risks and elements of uncertainty facing the Group in accordance with Danish disclosure requirements for listed companies.
Besides what has been disclosed in the quarterly financial report, no changes in the Group’s most significant risks and uncertainties have occurred relative to what was disclosed in the consolidated Annual Report 2019.
Bagsværd, 30 October 2020
|
|
|
|
|
|
|
|
|
|
|
Executive Management:
|
|
|
|
|
|
|
|
Lars Fruergaard Jørgensen
President and CEO
|
Karsten Munk Knudsen
CFO
|
Monique Carter
|
|
|
|
|
|
Camilla Sylvest
|
Mads Krogsgaard Thomsen
|
Henrik Wulff
|
|
|
|
|
|
|
|
|
|
Board of Directors:
|
|
|
|
|
|
|
|
Helge Lund
Chair
|
Jeppe Christiansen
Vice chair
|
Brian Daniels
|
|
|
|
|
|
Laurence Debroux
|
Andreas Fibig
|
Sylvie Grégoire
|
|
|
|
|
|
Liz Hewitt
|
Mette Bøjer Jensen
|
Kasim Kutay
|
|
|
|
|
|
Anne Marie Kverneland
|
Martin Mackay
|
Thomas Rantzau
|
|
|
|
|
|
Stig Strøbæk
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 22 of 33
|
About Novo Nordisk
Novo Nordisk is a leading global healthcare company, founded in 1923 and headquartered in Denmark. Our purpose is to drive change to defeat diabetes and other serious chronic diseases such as obesity and rare blood and endocrine disorders. We do so by pioneering scientific breakthroughs, expanding access to our medicines and working to prevent and ultimately cure disease. Novo Nordisk employs about 44,000 people in 80 countries and markets its products in around 170 countries. Novo Nordisk's B shares are listed on Nasdaq Copenhagen (Novo-B). Its ADRs are listed on the New York Stock Exchange (NVO). For more information, visit novonordisk.com, Facebook, Twitter, LinkedIn, YouTube.
|
|
|
|
|
|
|
|
Financial calendar
|
|
|
03 February 2021
|
Financial statement for 2020
|
|
25 March 2021
|
Annual General Meeting
|
|
5 May 2021
|
Financial statement for the first three month of 2021
|
|
5 August 2021
|
Financial statement for the first six month of 2021
|
|
3 November 2021
|
Financial statement for the first nine month of 2021
|
|
|
|
|
|
|
|
|
|
|
|
Contacts for further information
|
|
|
Media:
|
|
|
|
Mette Kruse Danielsen
|
+45 3079 3883
|
mkd@novonordisk.com
|
|
Ken Inchausti (US)
|
+1 609 240 9429
|
kiau@novonordisk.com
|
|
|
|
|
|
Investors:
|
|
|
|
Daniel Muusmann Bohsen
|
+45 3075 2175
|
dabo@novonordisk.com
|
|
Valdemar Borum Svarrer
|
+45 3079 0301
|
jvls@novonordisk.com
|
|
Ann Søndermølle Rendbæk
|
+45 3075 2253
|
arnd@novonordisk.com
|
|
Mark Joseph Root
|
+45 3079 4211
|
mjhr@novonordisk.com
|
|
Kristoffer Due Berg (US)
|
+1 609 235 2989
|
krdb@novonordisk.com
|
Further information about Novo Nordisk is available on novonordisk.com.
Forward-looking statements
Novo Nordisk’s reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this document as well as the company’s statutory Annual Report 2019 and Form 20-F both filed with the SEC in February 2020 in continuation of the publication of the Annual Report 2019, and written information released, or oral statements made, to the public in the future by or on behalf of Novo Nordisk, may contain forward-looking statements. Words such as ‘believe’, ‘expect’, ‘may’, ‘will’, ‘plan’, ‘strategy’, ‘prospect’, ‘foresee’, ‘estimate’, ‘project’, ‘anticipate’, ‘can’, ‘intend’, ‘target’ and other words and terms of similar meaning in connection with any discussion of future operating or financial performance identify forward-looking statements. Examples of such forward-looking statements include, but are not limited to:
•statements of targets, plans, objectives or goals for future operations, including those related to Novo Nordisk’s products, product research, product development, product introductions and product approvals as well as cooperation in relation thereto,
•statements containing projections of or targets for revenues, costs, income (or loss), earnings per share, capital expenditures, dividends, capital structure, net financials and other financial measures,
•statements regarding future economic performance, future actions and outcome of contingencies such as legal proceedings, and
•statements regarding the assumptions underlying or relating to such statements.
In this document, examples of forward-looking statements can be found under the headings ‘Outlook’, ‘Research and Development update’ and 'Equity’.
These statements are based on current plans, estimates and projections. By their very nature, forward-looking statements involve inherent risks and uncertainties, both general and specific. Novo Nordisk cautions that a number of important factors, including those described in this document, could cause actual results to differ materially from those contemplated in any forward-looking statements.
Factors that may affect future results include, but are not limited to, global as well as local political and economic conditions, including interest rate and currency exchange rate fluctuations, delay or failure of projects related to research and/or development, unplanned loss of patents, interruptions of supplies and production, product recalls, unexpected contract breaches or terminations, government-mandated or market-driven price decreases for Novo Nordisk’s products, introduction of competing products, reliance on information technology, Novo Nordisk’s ability to successfully market current and new products, exposure to product liability and legal proceedings and investigations, changes in governmental laws and related interpretation thereof, including on reimbursement, intellectual property protection and regulatory controls on testing, approval, manufacturing and marketing, perceived or actual failure to adhere to ethical marketing practices, investments in and divestitures of domestic and foreign companies, unexpected growth in costs and expenses, failure to recruit and retain the right employees, and failure to maintain a culture of compliance.
For an overview of some, but not all, of the risks that could adversely affect Novo Nordisk’s results or the accuracy of forward-looking statements in this document, reference is made to the overview of risk factors in ‘Managing risks to protect value’ of the Annual Report 2019.
Unless required by law, Novo Nordisk is under no duty and undertakes no obligation to update or revise any forward-looking statement after the distribution of this document, whether as a result of new information, future events or otherwise.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 23 of 33
|
APPENDIX 1: QUARTERLY NUMBERS IN DKK
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Amounts in DKK million, except number of full-time equivalent employees, earnings per share and number of shares outstanding).
|
|
|
|
|
|
|
|
|
|
|
|
|
% change
|
|
|
|
2020
|
|
2019
|
|
Q3 2020 vs.
|
|
|
|
Q3
|
Q2
|
Q1
|
|
Q4
|
Q3
|
Q2
|
Q1
|
|
Q3 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales
|
|
30,927
|
30,006
|
33,875
|
|
32,417
|
30,277
|
30,036
|
29,291
|
|
2
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
25,772
|
25,234
|
28,489
|
|
26,985
|
25,202
|
25,187
|
24,559
|
|
2
|
%
|
|
Gross margin
|
|
83.3%
|
84.1%
|
84.1%
|
|
83.2%
|
83.2%
|
83.9%
|
83.8%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and distribution costs
|
|
8,174
|
7,398
|
7,590
|
|
9,536
|
7,761
|
7,580
|
6,946
|
|
5
|
%
|
|
Percentage of sales
|
|
26.4%
|
24.7%
|
22.4%
|
|
29.4%
|
25.6%
|
25.2%
|
23.7%
|
|
|
|
Research and development costs
|
|
3,911
|
3,291
|
3,777
|
|
4,384
|
3,601
|
3,557
|
2,678
|
|
9
|
%
|
|
Percentage of sales
|
|
12.6%
|
11.0%
|
11.1%
|
|
13.5%
|
11.9%
|
11.8%
|
9.1%
|
|
|
|
Administrative costs
|
|
1,006
|
827
|
927
|
|
1,235
|
1,009
|
852
|
911
|
|
0
|
%
|
|
Percentage of sales
|
|
3.3%
|
2.8%
|
2.7%
|
|
3.8%
|
3.3%
|
2.8%
|
3.1%
|
|
|
|
Other operating income, net
|
|
127
|
120
|
107
|
|
43
|
88
|
254
|
215
|
|
44
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating profit
|
|
12,808
|
13,838
|
16,302
|
|
11,873
|
12,919
|
13,452
|
14,239
|
|
(1
|
%)
|
|
Operating margin
|
|
41.4%
|
46.1%
|
48.1%
|
|
36.6%
|
42.7%
|
44.8%
|
48.6%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial income
|
|
162
|
97
|
17
|
|
20
|
17
|
15
|
13
|
|
N/A
|
|
Financial expenses
|
|
279
|
519
|
1,298
|
|
814
|
829
|
1,322
|
1,030
|
|
(66
|
%)
|
|
Financial items (net)
|
|
(117)
|
(422)
|
(1,281)
|
|
(794)
|
(812)
|
(1,307)
|
(1,017)
|
|
(86
|
%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income taxes
|
|
12,691
|
13,416
|
15,021
|
|
11,079
|
12,107
|
12,145
|
13,222
|
|
5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes
|
|
2,393
|
2,791
|
3,124
|
|
2,362
|
1,913
|
2,550
|
2,777
|
|
25
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net profit
|
|
10,298
|
10,625
|
11,897
|
|
8,717
|
10,194
|
9,595
|
10,445
|
|
1
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation, amortisation and
impairment losses
|
|
2,130
|
1,158
|
1,086
|
|
1,398
|
2,095
|
1,110
|
1,058
|
|
2
|
%
|
|
Capital expenditure1
|
|
1,401
|
1,239
|
1,667
|
|
1,907
|
2,234
|
2,147
|
2,644
|
|
(37
|
%)
|
|
Net cash generated from operating activities
|
|
17,506
|
24,261
|
10,012
|
|
5,165
|
16,688
|
15,039
|
9,890
|
|
5
|
%
|
|
Free cash flow
|
|
11,224
|
22,666
|
7,669
|
|
1,737
|
14,039
|
12,020
|
6,655
|
|
(20
|
%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
139,947
|
136,121
|
126,256
|
|
125,612
|
124,908
|
117,909
|
110,135
|
|
12
|
%
|
|
Total equity
|
|
59,573
|
60,054
|
54,399
|
|
57,593
|
52,953
|
53,085
|
47,319
|
|
13
|
%
|
|
Equity ratio
|
|
42.6%
|
44.1%
|
43.1%
|
|
45.8%
|
42.4%
|
45.0%
|
43.0%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Full-time equivalent employees end of period
|
|
44,326
|
43,526
|
43,158
|
|
42,703
|
42,158
|
41,611
|
42,453
|
|
5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings per share/ADR (in DKK)
|
|
4.42
|
4.54
|
5.07
|
|
3.71
|
4.3
|
4.03
|
4.37
|
|
3
|
%
|
|
Diluted earnings per share/ADR (in DKK)
|
|
4.42
|
4.53
|
5.05
|
|
3.70
|
4.29
|
4.03
|
4.36
|
|
3
|
%
|
|
Average number of shares outstanding (million)
|
|
2,329.0
|
2,338.8
|
2,348.8
|
|
2,357.9
|
2,368.8
|
2,380.2
|
2,390.3
|
|
(2
|
%)
|
|
Average number of diluted shares
|
|
|
|
|
|
|
|
|
|
|
|
|
outstanding (million)
|
|
2,335.1
|
2,344.9
|
2,354.8
|
|
2,363.3
|
2,373.2
|
2,383.5
|
2,394.6
|
|
(2
|
%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales by business segment:
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-acting insulin
|
|
4,048
|
4,220
|
5,158
|
|
5,102
|
5,019
|
5,411
|
5,244
|
|
(19
|
%)
|
|
Premix insulin
|
|
2,572
|
2,693
|
2,955
|
|
2,665
|
2,596
|
2,560
|
2,757
|
|
(1
|
%)
|
|
Fast-acting insulin
|
|
4,589
|
4,379
|
5,114
|
|
4,936
|
4,632
|
4,758
|
4,977
|
|
(1
|
%)
|
|
Human insulin
|
|
2,194
|
2,314
|
2,687
|
|
2,204
|
2,237
|
2,180
|
2,415
|
|
(2
|
%)
|
|
Total insulin
|
|
13,403
|
13,606
|
15,914
|
|
14,907
|
14,484
|
14,909
|
15,393
|
|
(7
|
%)
|
|
Total GLP-1
|
|
10,651
|
9,425
|
9,975
|
|
9,842
|
8,492
|
7,740
|
7,147
|
|
25
|
%
|
|
Other Diabetes care
|
|
954
|
977
|
1,125
|
|
1,017
|
1,038
|
1,125
|
1,067
|
|
(8
|
%)
|
|
Total Diabetes care
|
|
25,008
|
24,008
|
27,014
|
|
25,766
|
24,014
|
23,774
|
23,607
|
|
4
|
%
|
|
Obesity care (Saxenda®)
|
|
1,338
|
1,308
|
1,577
|
|
1,564
|
1,442
|
1,462
|
1,211
|
|
(7
|
%)
|
|
Diabetes and Obesity care total
|
|
26,346
|
25,316
|
28,591
|
|
27,330
|
25,456
|
25,236
|
24,818
|
|
3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Haemophilia
|
|
2,311
|
2,401
|
2,810
|
|
2,554
|
2,524
|
2,670
|
2,533
|
|
(8
|
%)
|
|
Growth disorders (Norditropin®)
|
|
1,890
|
1,952
|
2,030
|
|
2,076
|
1,886
|
1,758
|
1,555
|
|
0
|
%
|
|
Other Biopharm
|
|
380
|
337
|
444
|
|
457
|
411
|
372
|
385
|
|
(8
|
%)
|
|
Biopharm total
|
|
4,581
|
4,690
|
5,284
|
|
5,087
|
4,821
|
4,800
|
4,473
|
|
(5
|
%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales by geographic segment:
|
|
|
|
|
|
|
|
|
|
|
|
|
International Operations2
|
|
15,988
|
16,115
|
18,296
|
|
15,351
|
15,261
|
15,565
|
15,387
|
|
5
|
%
|
|
- EMEA
|
|
8,318
|
8,167
|
9,674
|
|
8,231
|
7,913
|
8,120
|
7,944
|
|
5
|
%
|
|
- Region China
|
|
3,549
|
3,474
|
3,813
|
|
3,019
|
3,258
|
3,192
|
3,375
|
|
9
|
%
|
|
- Rest of World
|
|
4,121
|
4,474
|
4,809
|
|
4,101
|
4,090
|
4,253
|
4,068
|
|
1
|
%
|
|
North America Operations
|
|
14,939
|
13,891
|
15,579
|
|
17,066
|
15,016
|
14,471
|
13,904
|
|
(1
|
%)
|
|
- The US
|
|
14,144
|
13,028
|
14,775
|
|
16,252
|
14,256
|
13,767
|
13,211
|
|
(1
|
%)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment operating profit:
|
|
|
|
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care
|
|
9,748
|
11,434
|
13,456
|
|
9,013
|
10,403
|
11,393
|
11,828
|
|
(6
|
%)
|
|
Biopharm
|
|
3,060
|
2,404
|
2,846
|
|
2,860
|
2,516
|
2,059
|
2,411
|
|
22
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1) Cash-based capital expenditure (Purchase of property, plant and equipment).
2) Comparatives numbers have been restated following the re-organisation of International Operations in 2020.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 24 of 33
|
APPENDIX 2: INCOME STATEMENT AND STATEMENT OF COMPREHENSIVE INCOME
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DKK million
|
9M 2020
|
|
9M 2019
|
|
Q3 2020
|
|
Q3 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income statement
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales
|
94,808
|
|
89,604
|
|
30,927
|
|
30,277
|
|
Cost of goods sold
|
15,313
|
|
14,656
|
|
5,155
|
|
5,075
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
79,495
|
|
74,948
|
|
25,772
|
|
25,202
|
|
|
|
|
|
|
|
|
|
|
Sales and distribution costs
|
23,162
|
|
22,287
|
|
8,174
|
|
7,761
|
|
Research and development costs
|
10,979
|
|
9,836
|
|
3,911
|
|
3,601
|
|
Administrative costs
|
2,760
|
|
2,772
|
|
1,006
|
|
1,009
|
|
Other operating income, net
|
354
|
|
557
|
|
127
|
|
88
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating profit
|
42,948
|
|
40,610
|
|
12,808
|
|
12,919
|
|
|
|
|
|
|
|
|
|
|
Financial income
|
276
|
|
45
|
|
162
|
|
17
|
|
Financial expenses
|
2,096
|
|
3,181
|
|
279
|
|
829
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income taxes
|
41,128
|
|
37,474
|
|
12,691
|
|
12,107
|
|
|
|
|
|
|
|
|
|
|
Income taxes
|
8,308
|
|
7,240
|
|
2,393
|
|
1,913
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET PROFIT
|
32,820
|
|
30,234
|
|
10,298
|
|
10,194
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings per share (DKK)
|
14.03
|
|
12.70
|
|
4.42
|
|
4.30
|
|
Diluted earnings per share (DKK)
|
14.00
|
|
12.68
|
|
4.42
|
|
4.29
|
|
|
|
|
|
|
|
|
|
|
Segment Information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment sales:
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care
|
80,253
|
|
75,510
|
|
26,346
|
|
25,456
|
|
Biopharm
|
14,555
|
|
14,094
|
|
4,581
|
|
4,821
|
|
|
|
|
|
|
|
|
|
|
Segment operating profit:
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care
|
34,638
|
|
33,624
|
|
9,748
|
|
10,403
|
|
Operating margin
|
43.2%
|
|
44.5%
|
|
37.0%
|
|
40.9%
|
|
|
|
|
|
|
|
|
|
|
Biopharm
|
8,310
|
|
6,986
|
|
3,060
|
|
2,516
|
|
Operating margin
|
57.1%
|
|
49.6%
|
|
66.8%
|
|
52.2%
|
|
|
|
|
|
|
|
|
|
|
Total segment operating profit
|
42,948
|
|
40,610
|
|
12,808
|
|
12,919
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Statement of comprehensive income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net profit for the period
|
32,820
|
|
30,234
|
|
10,298
|
|
10,194
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income
|
|
|
|
|
|
|
|
Items that will not subsequently be reclassified to the Income
statement
|
|
|
|
|
|
|
|
|
Remeasurements on defined benefit plans
|
27
|
|
(369)
|
|
(77)
|
|
(167)
|
|
|
|
|
|
|
|
|
|
Items that will be reclassified subsequently to the Income
statement
|
|
|
|
|
|
|
|
|
Exchange rate adjustments of investments in subsidiaries
|
(1,002)
|
|
489
|
|
(788)
|
|
305
|
|
Cash flow hedges, realisation of previously deferred (gains)/losses
|
343
|
|
1,686
|
|
(65)
|
|
202
|
|
Cash flow hedges, deferred gains/(losses) incurred during the period
|
1,019
|
|
(1,405)
|
|
606
|
|
(1,006)
|
|
Other items
|
4
|
|
10
|
|
1
|
|
(6)
|
|
Tax on other comprehensive income, income/(expense)
|
(512)
|
|
25
|
|
(169)
|
|
212
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income for the period, net of tax
|
(121)
|
|
436
|
|
(492)
|
|
(460)
|
|
TOTAL COMPREHENSIVE INCOME FOR THE PERIOD
|
32,699
|
|
30,670
|
|
9,806
|
|
9,734
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 25 of 33
|
APPENDIX 3: CASH FLOW STATEMENT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DKK million
|
9M 2020
|
|
9M 2019
|
|
|
|
|
|
|
|
|
|
|
|
Net profit
|
32,820
|
|
|
30,234
|
|
|
|
|
|
|
|
Adjustment for non-cash items:
|
|
|
|
|
Income taxes in the Income Statement
|
8,308
|
|
|
7,240
|
|
|
Depreciation, amortisation and impairment losses
|
4,374
|
|
|
4,263
|
|
|
Other non-cash items
|
13,508
|
|
|
7,139
|
|
|
Change in working capital
|
(384)
|
|
|
(1,304)
|
|
|
Interest received
|
50
|
|
|
45
|
|
|
Interest paid
|
(182)
|
|
|
(148)
|
|
|
Income taxes paid
|
(6,715)
|
|
|
(5,852)
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash generated from operating activities
|
51,779
|
|
|
41,617
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of intangible assets
|
(4,869)
|
|
|
(1,238)
|
|
|
Proceeds from sale of property, plant and equipment
|
3
|
|
|
2
|
|
|
Purchase of property, plant and equipment
|
(4,307)
|
|
|
(7,025)
|
|
|
Proceeds from other financial assets
|
11
|
|
|
—
|
|
|
Purchase of other financial assets
|
—
|
|
|
(6)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investment in associated company
|
(392)
|
|
|
(50)
|
|
|
Proceeds from the divestment of Group and associated companies
|
—
|
|
|
(3)
|
|
|
Dividend received from associated company
|
18
|
|
|
20
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
(9,536)
|
|
|
(8,300)
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of treasury shares
|
(11,195)
|
|
|
(10,404)
|
|
|
Dividends paid
|
(20,121)
|
|
|
(19,409)
|
|
|
Repayment of borrowings, net
|
(568)
|
|
|
(470)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in financing activities
|
(31,884)
|
|
|
(30,283)
|
|
|
|
|
|
|
|
|
|
|
|
|
NET CASH GENERATED FROM ACTIVITIES
|
10,359
|
|
|
3,034
|
|
|
|
|
|
|
|
Cash and cash equivalents at the beginning of the year
|
15,411
|
|
|
15,629
|
|
|
|
|
|
|
|
Exchange gain/(loss) on cash and cash equivalents
|
(305)
|
|
|
93
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at the end of the period
|
25,465
|
|
|
18,756
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 26 of 33
|
APPENDIX 4: BALANCE SHEET
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DKK million
|
30 Sep 2020
|
|
31 Dec 2019
|
|
|
|
|
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
Intangible assets
|
9,415
|
|
|
5,835
|
|
|
Property, plant and equipment
|
49,925
|
|
|
50,551
|
|
|
Investments in associated companies
|
601
|
|
|
474
|
|
|
Deferred income tax assets
|
4,745
|
|
|
4,121
|
|
|
Other receivables and prepayments
|
562
|
|
|
841
|
|
|
Other financial assets
|
1,032
|
|
|
1,334
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL NON-CURRENT ASSETS
|
66,280
|
|
|
63,156
|
|
|
|
|
|
|
|
Inventories
|
18,105
|
|
|
17,641
|
|
|
Trade receivables
|
24,358
|
|
|
24,912
|
|
|
Tax receivables
|
423
|
|
|
806
|
|
|
Other receivables and prepayments
|
3,613
|
|
|
3,434
|
|
|
Derivative financial instruments
|
1,688
|
|
|
188
|
|
|
Cash at bank
|
25,480
|
|
|
15,475
|
|
|
|
|
|
|
|
TOTAL CURRENT ASSETS
|
73,667
|
|
|
62,456
|
|
|
TOTAL ASSETS
|
139,947
|
|
|
125,612
|
|
|
|
|
|
|
|
EQUITY AND LIABILITIES
|
|
|
|
|
|
|
|
|
|
Share capital
|
470
|
|
|
480
|
|
|
Treasury shares
|
(5)
|
|
|
(10)
|
|
|
Retained earnings
|
59,850
|
|
|
57,817
|
|
|
Other reserves
|
(742)
|
|
|
(694)
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL EQUITY
|
59,573
|
|
|
57,593
|
|
|
|
|
|
|
|
Borrowings
|
2,636
|
|
|
3,009
|
|
|
Deferred income tax liabilities
|
991
|
|
|
80
|
|
|
Retirement benefit obligations
|
1,324
|
|
|
1,334
|
|
|
Provisions
|
4,475
|
|
|
4,613
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current liabilities
|
9,426
|
|
|
9,036
|
|
|
|
|
|
|
|
Borrowings
|
1,468
|
|
|
1,474
|
|
|
Trade payables
|
6,123
|
|
|
6,358
|
|
|
Tax payables
|
5,019
|
|
|
4,212
|
|
|
Other liabilities
|
15,298
|
|
|
15,085
|
|
|
Derivative financial instruments
|
1,002
|
|
|
734
|
|
|
Provisions
|
42,038
|
|
|
31,120
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
70,948
|
|
|
58,983
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
|
80,374
|
|
|
68,019
|
|
|
|
|
|
|
|
TOTAL EQUITY AND LIABILITIES
|
139,947
|
|
|
125,612
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 27 of 33
|
APPENDIX 5: EQUITY STATEMENT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other reserves
|
|
|
DKK million
|
Share
capital
|
Treasury
shares
|
Retained
earnings
|
Exchange
rate
adjust-ments
|
Cash
flow
hedges
|
Tax and
other
adjust-ments
|
Total
other
reserves
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9M 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at the beginning of the period
|
480
|
|
(10)
|
|
57,817
|
|
(839)
|
|
(329)
|
|
474
|
|
(694)
|
|
|
57,593
|
|
|
Net profit for the period
|
|
|
32,820
|
|
|
|
|
|
|
32,820
|
|
|
Other comprehensive income for the period
|
|
|
27
|
|
(1,002)
|
|
1,362
|
|
(508)
|
|
(148)
|
|
|
(121)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the period
|
|
|
32,847
|
|
(1,002)
|
|
1,362
|
|
(508)
|
|
(148)
|
|
|
32,699
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transfer of cash flow hedge reserve to intangible assets
|
|
|
|
|
128
|
|
(28)
|
|
100
|
|
|
100
|
|
|
Transactions with owners:
|
|
|
|
|
|
|
|
|
|
|
Dividends
|
|
|
(20,121)
|
|
|
|
|
|
|
(20,121)
|
|
|
Share-based payments
|
|
|
468
|
|
|
|
|
|
|
468
|
|
|
Tax related to restricted stock units
|
|
|
29
|
|
|
|
|
|
|
29
|
|
|
Purchase of treasury shares
|
|
(5)
|
|
(11,190)
|
|
|
|
|
|
|
(11,195)
|
|
|
Reduction of the B share capital
|
(10)
|
|
10
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at the end of the period
|
470
|
|
(5)
|
|
59,850
|
|
(1,841)
|
|
1,161
|
|
(62)
|
|
(742)
|
|
|
59,573
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other reserves
|
|
|
DKK million
|
Share
capital
|
Treasury
shares
|
Retained
earnings
|
Exchange
rate
adjust-ments
|
Cash
flow
hedges
|
Tax and
other
adjust-ments
|
Total
other
reserves
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9M 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at the beginning of the period
|
490
|
|
(11)
|
|
53,406
|
|
(1,065)
|
|
(1,677)
|
|
696
|
|
(2,046)
|
|
|
51,839
|
|
|
Net profit for the period
|
|
|
30,234
|
|
|
|
|
|
|
30,234
|
|
|
Other comprehensive income for the period
|
|
|
(369)
|
|
489
|
|
281
|
|
35
|
|
805
|
|
|
436
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the period
|
|
|
29,865
|
|
489
|
|
281
|
|
35
|
|
805
|
|
|
30,670
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transactions with owners:
|
|
|
|
|
|
|
|
|
|
|
Dividends
|
|
|
(19,409)
|
|
|
|
|
|
|
(19,409)
|
|
|
Share-based payments
|
|
|
245
|
|
|
|
|
|
|
245
|
|
|
Tax related to restricted stock units
|
|
|
12
|
|
|
|
|
|
|
12
|
|
|
Purchase of treasury shares
|
|
(6)
|
|
(10,398)
|
|
|
|
|
|
|
(10,404)
|
|
|
Reduction of the B share capital
|
(10)
|
|
10
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at the end of the period
|
480
|
|
(7)
|
|
53,721
|
|
(576)
|
|
(1,396)
|
|
731
|
|
(1,241)
|
|
|
52,953
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 28 of 33
|
APPENDIX 6: SALES SPLIT PER AREA
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q3 2020 sales split per area
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DKK million
|
Total
|
International
Operations
|
EMEA
|
Region
China
|
Rest of
World
|
North America
Operations
|
The US
|
|
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care segment
|
|
|
|
|
|
|
|
|
|
Long-acting insulin
|
4,048
|
2,460
|
1,577
|
399
|
484
|
1,588
|
1,467
|
|
|
% change at CER
|
(15%)
|
14%
|
14%
|
43%
|
(1%)
|
(38%)
|
(40%)
|
|
|
|
Tresiba®
|
2,102
|
1,126
|
652
|
139
|
335
|
976
|
887
|
|
|
|
% change at CER
|
(4%)
|
33%
|
37%
|
—
|
(1%)
|
(27%)
|
(30%)
|
|
|
|
Xultophy®
|
604
|
443
|
405
|
—
|
38
|
161
|
158
|
|
|
|
% change at CER
|
9%
|
24%
|
20%
|
—
|
70%
|
(17%)
|
(18%)
|
|
|
|
Levemir®
|
1,342
|
891
|
520
|
260
|
111
|
451
|
422
|
|
|
|
% change at CER
|
(33%)
|
(6%)
|
(9%)
|
3%
|
(14%)
|
(57%)
|
(58%)
|
|
|
Premix insulin
|
2,572
|
2,447
|
645
|
1,201
|
601
|
125
|
119
|
|
|
% change at CER
|
4%
|
4%
|
(11%)
|
14%
|
6%
|
12%
|
15%
|
|
|
|
Ryzodeg®
|
296
|
296
|
79
|
13
|
204
|
—
|
—
|
|
|
|
% change at CER
|
15%
|
15%
|
30%
|
—
|
3%
|
—
|
—
|
|
|
|
NovoMix®
|
2,276
|
2,151
|
566
|
1,188
|
397
|
125
|
119
|
|
|
|
% change at CER
|
3%
|
3%
|
(15%)
|
13%
|
7%
|
12%
|
15%
|
|
|
Fast-acting insulin
|
4,589
|
2,611
|
1,562
|
508
|
541
|
1,978
|
1,883
|
|
|
% change at CER
|
4%
|
8%
|
7%
|
14%
|
6%
|
(1%)
|
(1%)
|
|
|
|
Fiasp®
|
345
|
201
|
186
|
—
|
15
|
144
|
136
|
|
|
|
% change at CER
|
18%
|
24%
|
25%
|
—
|
21%
|
10%
|
11%
|
|
|
|
NovoRapid®
|
4,244
|
2,410
|
1,376
|
508
|
526
|
1,834
|
1,747
|
|
|
|
% change at CER
|
3%
|
7%
|
5%
|
14%
|
6%
|
(2%)
|
(1%)
|
|
|
Human insulin
|
2,194
|
1,774
|
551
|
696
|
527
|
420
|
397
|
|
|
% change at CER
|
5%
|
5%
|
(7%)
|
2%
|
25%
|
1%
|
3%
|
|
|
Total insulin
|
13,403
|
9,292
|
4,335
|
2,804
|
2,153
|
4,111
|
3,866
|
|
|
% change at CER
|
(2%)
|
8%
|
4%
|
14%
|
8%
|
(20%)
|
(20%)
|
|
|
Victoza®
|
4,765
|
1,817
|
1,057
|
294
|
466
|
2,948
|
2,863
|
|
|
% change at CER
|
(7%)
|
8%
|
(7%)
|
33%
|
43%
|
(15%)
|
(15%)
|
|
|
Ozempic®
|
5,432
|
892
|
765
|
2
|
125
|
4,540
|
4,295
|
|
|
% change at CER
|
82%
|
188%
|
171%
|
—
|
302%
|
70%
|
69%
|
|
|
Rybelsus®
|
454
|
16
|
16
|
—
|
—
|
438
|
437
|
|
|
% change at CER
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
|
|
Total GLP-1
|
10,651
|
2,725
|
1,838
|
296
|
591
|
7,926
|
7,595
|
|
|
% change at CER
|
31%
|
37%
|
29%
|
35%
|
70%
|
29%
|
29%
|
|
|
Other Diabetes care1
|
954
|
730
|
179
|
389
|
162
|
224
|
190
|
|
|
% change at CER
|
(4%)
|
(7%)
|
(28%)
|
3%
|
2%
|
9%
|
13%
|
|
|
Total Diabetes care
|
25,008
|
12,747
|
6,352
|
3,489
|
2,906
|
12,261
|
11,651
|
|
|
% change at CER
|
9%
|
12%
|
9%
|
14%
|
17%
|
7%
|
6%
|
|
|
Obesity care (Saxenda®)
|
1,338
|
485
|
258
|
2
|
225
|
853
|
794
|
|
|
% change at CER
|
0%
|
6%
|
14%
|
(50%)
|
0%
|
(3%)
|
(3%)
|
|
|
Diabetes and Obesity care total
|
26,346
|
13,232
|
6,610
|
3,491
|
3,131
|
13,114
|
12,445
|
|
|
% change at CER
|
9%
|
12%
|
9%
|
14%
|
15%
|
6%
|
6%
|
|
|
|
|
|
|
|
|
|
|
|
|
Biopharm segment
|
|
|
|
|
|
|
|
|
|
Haemophilia2
|
2,311
|
1,345
|
974
|
44
|
327
|
966
|
910
|
|
|
% change at CER
|
(4%)
|
(2%)
|
8%
|
(40%)
|
(17%)
|
(6%)
|
(6%)
|
|
|
|
NovoSeven®
|
1,674
|
891
|
627
|
40
|
224
|
783
|
763
|
|
|
|
% change at CER
|
(11%)
|
(13%)
|
(3%)
|
(42%)
|
(27%)
|
(8%)
|
(9%)
|
|
|
|
NovoEight®
|
375
|
287
|
213
|
4
|
70
|
88
|
83
|
|
|
|
% change at CER
|
10%
|
12%
|
6%
|
0%
|
33%
|
3%
|
6%
|
|
|
Growth disorders (Norditropin®)
|
1,890
|
1,143
|
519
|
13
|
611
|
747
|
741
|
|
|
% change at CER
|
5%
|
14%
|
16%
|
40%
|
12%
|
(6%)
|
(7%)
|
|
|
Other Biopharm3
|
380
|
268
|
215
|
1
|
52
|
112
|
48
|
|
|
% change at CER
|
(5%)
|
(6%)
|
(6%)
|
(100%)
|
(4%)
|
(2%)
|
(15%)
|
|
|
Biopharm total
|
4,581
|
2,756
|
1,708
|
58
|
990
|
1,825
|
1,699
|
|
|
% change at CER
|
0%
|
4%
|
8%
|
(31%)
|
0%
|
(6%)
|
(7%)
|
|
|
|
|
|
|
|
|
|
|
|
|
Total sales
|
30,927
|
15,988
|
8,318
|
3,549
|
4,121
|
14,939
|
14,144
|
|
|
% change at CER
|
7%
|
10%
|
9%
|
13%
|
11%
|
5%
|
4%
|
|
|
% change as reported
|
2%
|
5%
|
5%
|
9%
|
1%
|
(1%)
|
(1%)
|
|
|
Share of growth
|
100%
|
70%
|
30%
|
19%
|
21%
|
30%
|
26%
|
1) Primarily NovoNorm®, needles and GlucaGen® HypoKit®.
2) Comprises NovoSeven®, NovoEight®, Refixia®, NovoThirteen® and Esperoct®.
3) Primarily Vagifem® and Activelle®.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 29 of 33
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9M 2020 sales split per area
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DKK million
|
Total
|
International
Operations
|
EMEA
|
Region
China
|
Rest of
World
|
North America
Operations
|
The US
|
|
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care segment
|
|
|
|
|
|
|
|
|
|
Long-acting insulin
|
13,426
|
7,590
|
4,916
|
1,120
|
1,554
|
5,836
|
5,452
|
|
|
% change at CER
|
(13%)
|
14%
|
12%
|
39%
|
8%
|
(34%)
|
(36%)
|
|
|
|
Tresiba®
|
6,720
|
3,345
|
1,957
|
300
|
1,088
|
3,375
|
3,101
|
|
|
|
% change at CER
|
(2%)
|
31%
|
32%
|
—
|
8%
|
(22%)
|
(26%)
|
|
|
|
Xultophy®
|
1,842
|
1,326
|
1,209
|
1
|
116
|
516
|
508
|
|
|
|
% change at CER
|
15%
|
25%
|
19%
|
—
|
125%
|
(5%)
|
(6%)
|
|
|
|
Levemir®
|
4,864
|
2,919
|
1,750
|
819
|
350
|
1,945
|
1,843
|
|
|
|
% change at CER
|
(30%)
|
(4%)
|
(8%)
|
9%
|
(9%)
|
(51%)
|
(52%)
|
|
|
Premix insulin
|
8,220
|
7,799
|
2,275
|
3,662
|
1,862
|
421
|
401
|
|
|
% change at CER
|
6%
|
8%
|
(1%)
|
13%
|
11%
|
(22%)
|
(22%)
|
|
|
|
Ryzodeg®
|
967
|
967
|
252
|
26
|
689
|
—
|
—
|
|
|
|
% change at CER
|
33%
|
33%
|
47%
|
—
|
25%
|
—
|
—
|
|
|
|
NovoMix®
|
7,253
|
6,832
|
2,023
|
3,636
|
1,173
|
421
|
401
|
|
|
|
% change at CER
|
3%
|
5%
|
(5%)
|
13%
|
5%
|
(22%)
|
(22%)
|
|
|
Fast-acting insulin
|
14,082
|
8,170
|
4,968
|
1,563
|
1,639
|
5,912
|
5,604
|
|
|
% change at CER
|
(1%)
|
8%
|
6%
|
18%
|
5%
|
(11%)
|
(12%)
|
|
|
|
Fiasp®
|
1,021
|
591
|
547
|
—
|
44
|
430
|
404
|
|
|
|
% change at CER
|
28%
|
34%
|
32%
|
—
|
68%
|
19%
|
19%
|
|
|
|
NovoRapid®
|
13,061
|
7,579
|
4,421
|
1,563
|
1,595
|
5,482
|
5,200
|
|
|
|
% change at CER
|
(2%)
|
6%
|
3%
|
18%
|
4%
|
(13%)
|
(13%)
|
|
|
Human insulin
|
7,195
|
5,872
|
1,823
|
2,178
|
1,871
|
1,323
|
1,247
|
|
|
% change at CER
|
8%
|
8%
|
0%
|
2%
|
25%
|
10%
|
12%
|
|
|
Total insulin
|
42,923
|
29,431
|
13,982
|
8,523
|
6,926
|
13,492
|
12,704
|
|
|
% change at CER
|
(3%)
|
10%
|
6%
|
14%
|
12%
|
(22%)
|
(23%)
|
|
|
Victoza®
|
13,990
|
5,578
|
3,371
|
795
|
1,412
|
8,412
|
8,137
|
|
|
% change at CER
|
(15%)
|
5%
|
(6%)
|
23%
|
29%
|
(24%)
|
(24%)
|
|
|
Ozempic®
|
15,023
|
2,493
|
2,160
|
4
|
329
|
12,530
|
11,839
|
|
|
% change at CER
|
120%
|
303%
|
304%
|
—
|
292%
|
101%
|
101%
|
|
|
Rybelsus®
|
1,038
|
16
|
16
|
—
|
—
|
1,022
|
1,015
|
|
|
% change at CER
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
|
|
Total GLP-1
|
30,051
|
8,087
|
5,547
|
799
|
1,741
|
21,964
|
20,991
|
|
|
% change at CER
|
29%
|
37%
|
35%
|
24%
|
51%
|
27%
|
26%
|
|
|
Other Diabetes care1
|
3,056
|
2,250
|
547
|
1,204
|
499
|
806
|
701
|
|
|
% change at CER
|
(4%)
|
(12%)
|
(31%)
|
(5%)
|
(2%)
|
31%
|
38%
|
|
|
Total Diabetes care
|
76,030
|
39,768
|
20,076
|
10,526
|
9,166
|
36,262
|
34,396
|
|
|
% change at CER
|
8%
|
13%
|
11%
|
12%
|
17%
|
3%
|
2%
|
|
|
Obesity care (Saxenda®)
|
4,223
|
1,611
|
834
|
6
|
771
|
2,612
|
2,420
|
|
|
% change at CER
|
6%
|
8%
|
8%
|
(17%)
|
7%
|
4%
|
4%
|
|
|
Diabetes and Obesity care total
|
80,253
|
41,379
|
20,910
|
10,532
|
9,937
|
38,874
|
36,816
|
|
|
% change at CER
|
8%
|
12%
|
11%
|
12%
|
16%
|
3%
|
2%
|
|
|
|
|
|
|
|
|
|
|
|
|
Biopharm segment
|
|
|
|
|
|
|
|
|
|
Haemophilia2
|
7,522
|
4,513
|
2,864
|
257
|
1,392
|
3,009
|
2,794
|
|
|
% change at CER
|
(2%)
|
3%
|
8%
|
14%
|
(8%)
|
(7%)
|
(7%)
|
|
|
|
NovoSeven®
|
5,683
|
3,222
|
1,920
|
247
|
1,055
|
2,461
|
2,364
|
|
|
|
% change at CER
|
(7%)
|
(4%)
|
3%
|
17%
|
(17%)
|
(10%)
|
(9%)
|
|
|
|
NovoEight®
|
1,115
|
856
|
616
|
10
|
230
|
259
|
242
|
|
|
|
% change at CER
|
1%
|
2%
|
(4%)
|
(23%)
|
20%
|
1%
|
1%
|
|
|
Growth disorders (Norditropin®)
|
5,872
|
3,676
|
1,705
|
42
|
1,929
|
2,196
|
2,183
|
|
|
% change at CER
|
13%
|
18%
|
17%
|
72%
|
18%
|
7%
|
7%
|
|
|
Other Biopharm3
|
1,161
|
831
|
680
|
5
|
146
|
330
|
154
|
|
|
% change at CER
|
1%
|
1%
|
2%
|
(20%)
|
(3%)
|
1%
|
2%
|
|
|
Biopharm total
|
14,555
|
9,020
|
5,249
|
304
|
3,467
|
5,535
|
5,131
|
|
|
% change at CER
|
4%
|
8%
|
10%
|
19%
|
5%
|
(2%)
|
(2%)
|
|
|
|
|
|
|
|
|
|
|
|
|
Total sales
|
94,808
|
50,399
|
26,159
|
10,836
|
13,404
|
44,409
|
41,947
|
|
|
% change at CER
|
7%
|
12%
|
11%
|
12%
|
13%
|
2%
|
2%
|
|
|
% change as reported
|
6%
|
9%
|
9%
|
10%
|
8%
|
2%
|
2%
|
|
|
Share of growth
|
100%
|
83%
|
39%
|
19%
|
25%
|
17%
|
11%
|
|
|
|
|
|
|
|
|
|
|
|
1) Primarily NovoNorm®, needles and GlucaGen® HypoKit®.
2) Comprises NovoSeven®, NovoEight®, Refixia®, NovoThirteen® and Esperoct®.
3) Primarily Vagifem® and Activelle®.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 30 of 33
|
APPENDIX 7: NON-IFRS FINANCIAL MEASURES (ADDITIONAL INFORMATION)
In this Company Announcement, Novo Nordisk discloses certain financial measures of the Group’s financial performance, financial position and cash flows that reflect adjustments to the directly comparable measures calculated and presented in accordance with IFRS. These non-IFRS financial measures may not be defined and calculated by other companies in the same manner and may thus not be comparable with such measures. The non-IFRS financial measures presented in the Company Announcement are Sales and operating profit at CER, Free cash flow and Financial resources.
Sales and operating profit growth at CER
'Growth at CER’ means that the effect of changes in exchange rates is excluded. It is defined as Net sales/Operating profit for the period measured at the average exchange rates for the same period prior year compared with Net sales/Operating profit for the same period prior year. Price adjustments within hyperinflation countries as defined in IAS 29 ‘Financial reporting in hyperinflation economies’ are excluded from the calculation to avoid growth at CER being artificially inflated.
Growth at CER is considered to be relevant information for investors in order to understand the underlying development in sales and operating profit by adjusting for the impact of currency fluctuations.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales at CER
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DKK million
|
9M 2020
|
9M 2019
|
% change
9M 2020 to
9M 2019
|
|
Q3 2020
|
Q3 2019
|
% change
Q3 2020 to
Q3 2019
|
|
|
|
|
|
|
|
|
|
|
Net sales
|
94,808
|
|
89,604
|
|
6
|
%
|
|
30,927
|
|
30,277
|
|
2
|
%
|
|
Effect of exchange rates
|
1,209
|
|
—
|
|
|
|
1,591
|
|
—
|
|
|
|
Sales at CER
|
96,017
|
|
89,604
|
|
7
|
%
|
|
32,518
|
|
30,277
|
|
7
|
%
|
|
|
|
|
|
|
|
|
|
|
Operating profit at CER
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DKK million
|
9M 2020
|
9M 2019
|
% change
9M 2020 to
9M 2019
|
|
Q3 2020
|
Q3 2019
|
% change
Q3 2020 to
Q3 2019
|
|
|
|
|
|
|
|
|
|
|
Operating profit
|
42,948
|
|
40,610
|
|
6
|
%
|
|
12,808
|
|
12,919
|
|
(1
|
%)
|
|
Effect of exchange rates
|
680
|
|
—
|
|
|
|
983
|
|
—
|
|
|
|
Operating profit at CER
|
43,628
|
|
40,610
|
|
7
|
%
|
|
13,791
|
|
12,919
|
|
7
|
%
|
Free cash flow
Novo Nordisk defines free cash flow as ’net cash generated from operating activities’, less ‘net cash used in investing activities’, less repayment on lease liabilities and excluding net change of marketable securities. Free cash flow is a measure of the amount of cash generated in the period which is available for the Board to allocate between Novo Nordisk's capital providers, through eg dividends, share repurchases and repayment of debt (excluding lease liability repayments) or for retaining in the business to fund future growth.
The following table shows a reconciliation of Free cash flow with Net cash generated from operating activities, the most directly comparable IFRS financial measure:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Free cash flow
|
|
|
|
|
|
|
|
|
|
|
|
DKK million
|
9M 2020
|
9M 2019
|
Q3 2020
|
Q3 2019
|
|
|
|
|
|
|
|
Net cash generated from operating activities
|
51,779
|
|
41,617
|
|
17,506
|
|
16,688
|
|
|
Net cash used in investing activities
|
(9,536)
|
|
(8,300)
|
|
(6,063)
|
|
(2,441)
|
|
|
Repayment on lease liabilities
|
(684)
|
|
(603)
|
|
(219)
|
|
(208)
|
|
|
Free cash flow
|
41,559
|
|
32,714
|
|
11,224
|
|
14,039
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 31 of 33
|
Financial resources
Financial resources is defined as the sum of cash and cash equivalents at the end of the year, undrawn committed credit facilities less bank overdrafts classified as liabilities arising from financing activities (part of borrowings). The following table reconciles total financial resources with cash and cash equivalents, the most directly comparable IFRS financial measure:
|
|
|
|
|
|
|
|
|
|
|
Financial resources
|
|
|
|
|
|
|
|
DKK million
|
9M 2020
|
Q4 2019
|
|
|
|
|
|
Cash and cash equivalents
|
25,465
|
|
15,411
|
|
|
Undrawn committed credit facilities
|
11,542
|
|
11,578
|
|
|
Borrowings (bank overdrafts)
|
(595)
|
|
(595)
|
|
|
Financial resources
|
36,412
|
|
26,394
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 32 of 33
|
APPENDIX 8: NEW SALES SPLIT PER AREA (ADDITIONAL INFORMATION)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q3 2019 sales split per area - DKK million
|
Total
|
International
Operations
|
EMEA
|
Region
China
|
Rest of
World
|
North America
Operations
|
The US
|
|
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care segment
|
|
|
|
|
|
|
|
|
|
Long-acting insulin
|
5,019
|
|
2,266
|
|
1,434
|
|
289
|
|
543
|
|
2,753
|
|
2,630
|
|
|
|
|
Tresiba®
|
2,306
|
|
888
|
|
486
|
|
27
|
|
375
|
|
1,418
|
|
1,343
|
|
|
|
|
Xultophy®
|
575
|
|
370
|
|
343
|
|
—
|
|
27
|
|
205
|
|
202
|
|
|
|
|
Levemir®
|
2,138
|
|
1,008
|
|
605
|
|
262
|
|
141
|
|
1,130
|
|
1,085
|
|
|
|
Premix insulin
|
2,596
|
|
2,479
|
|
781
|
|
1,095
|
|
603
|
|
117
|
|
109
|
|
|
|
|
Ryzodeg®
|
278
|
|
278
|
|
69
|
|
—
|
|
209
|
|
—
|
|
—
|
|
|
|
|
NovoMix®
|
2,318
|
|
2,201
|
|
712
|
|
1,095
|
|
394
|
|
117
|
|
109
|
|
|
|
Fast-acting insulin
|
4,632
|
|
2,536
|
|
1,524
|
|
463
|
|
549
|
|
2,096
|
|
1,989
|
|
|
|
|
Fiasp®
|
301
|
|
164
|
|
150
|
|
—
|
|
14
|
|
137
|
|
129
|
|
|
|
|
NovoRapid®
|
4,331
|
|
2,372
|
|
1,374
|
|
463
|
|
535
|
|
1,959
|
|
1,860
|
|
|
|
Human insulin
|
2,237
|
|
1,799
|
|
610
|
|
706
|
|
483
|
|
438
|
|
406
|
|
|
|
Total insulin
|
14,484
|
|
9,080
|
|
4,349
|
|
2,553
|
|
2,178
|
|
5,404
|
|
5,134
|
|
|
|
Victoza®
|
5,370
|
|
1,741
|
|
1,157
|
|
227
|
|
357
|
|
3,629
|
|
3,507
|
|
|
|
Ozempic®
|
3,122
|
|
326
|
|
285
|
|
—
|
|
41
|
|
2,796
|
|
2,659
|
|
|
|
Rybelsus®
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
|
Total GLP-1
|
8,492
|
|
2,067
|
|
1,442
|
|
227
|
|
398
|
|
6,425
|
|
6,166
|
|
|
|
Other Diabetes care
|
1,038
|
|
819
|
|
262
|
|
387
|
|
170
|
|
219
|
|
178
|
|
|
|
Total Diabetes care
|
24,014
|
|
11,966
|
|
6,053
|
|
3,167
|
|
2,746
|
|
12,048
|
|
11,478
|
|
|
|
Obesity care (Saxenda®)
|
1,442
|
|
514
|
|
239
|
|
2
|
|
273
|
|
928
|
|
865
|
|
|
|
Diabetes and Obesity care total
|
25,456
|
|
12,480
|
|
6,292
|
|
3,169
|
|
3,019
|
|
12,976
|
|
12,343
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biopharm segment
|
|
|
|
|
|
|
|
|
|
Haemophilia
|
2,524
|
|
1,441
|
|
928
|
|
78
|
|
435
|
|
1,083
|
|
1,020
|
|
|
|
|
NovoSeven®
|
1,985
|
|
1,083
|
|
667
|
|
74
|
|
342
|
|
902
|
|
880
|
|
|
|
|
NovoEight®
|
357
|
|
268
|
|
204
|
|
4
|
|
60
|
|
89
|
|
82
|
|
|
|
Growth disorders (Norditropin®)
|
1,886
|
|
1,051
|
|
461
|
|
10
|
|
580
|
|
835
|
|
833
|
|
|
|
Other Biopharm
|
411
|
|
289
|
|
232
|
|
1
|
|
56
|
|
122
|
|
60
|
|
|
|
Biopharm total
|
4,821
|
|
2,781
|
|
1,621
|
|
89
|
|
1,071
|
|
2,040
|
|
1,913
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total sales
|
30,277
|
|
15,261
|
|
7,913
|
|
3,258
|
|
4,090
|
|
15,016
|
|
14,256
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9M 2019 sales split per area - DKK million
|
Total
|
International
Operations
|
EMEA
|
Region
China
|
Rest of
World
|
North America
Operations
|
The US
|
|
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care segment
|
|
|
|
|
|
|
|
|
|
Long-acting insulin
|
15,674
|
|
6,783
|
|
4,454
|
|
820
|
|
1,509
|
|
8,891
|
|
8,556
|
|
|
|
|
Tresiba®
|
6,948
|
|
2,593
|
|
1,485
|
|
58
|
|
1,050
|
|
4,355
|
|
4,164
|
|
|
|
|
Xultophy®
|
1,626
|
|
1,080
|
|
1,021
|
|
—
|
|
59
|
|
546
|
|
540
|
|
|
|
|
Levemir®
|
7,100
|
|
3,110
|
|
1,948
|
|
762
|
|
400
|
|
3,990
|
|
3,852
|
|
|
|
Premix insulin
|
7,913
|
|
7,376
|
|
2,381
|
|
3,286
|
|
1,709
|
|
537
|
|
513
|
|
|
|
|
Ryzodeg®
|
747
|
|
747
|
|
184
|
|
1
|
|
562
|
|
—
|
|
—
|
|
|
|
|
NovoMix®
|
7,166
|
|
6,629
|
|
2,197
|
|
3,285
|
|
1,147
|
|
537
|
|
513
|
|
|
|
Fast-acting insulin
|
14,367
|
|
7,732
|
|
4,773
|
|
1,353
|
|
1,606
|
|
6,635
|
|
6,334
|
|
|
|
|
Fiasp®
|
804
|
|
444
|
|
416
|
|
—
|
|
28
|
|
360
|
|
339
|
|
|
|
|
NovoRapid®
|
13,563
|
|
7,288
|
|
4,357
|
|
1,353
|
|
1,578
|
|
6,275
|
|
5,995
|
|
|
|
Human insulin
|
6,832
|
|
5,628
|
|
1,834
|
|
2,170
|
|
1,624
|
|
1,204
|
|
1,110
|
|
|
|
Total insulin
|
44,786
|
|
27,519
|
|
13,442
|
|
7,629
|
|
6,448
|
|
17,267
|
|
16,513
|
|
|
|
Victoza®
|
16,507
|
|
5,378
|
|
3,579
|
|
655
|
|
1,144
|
|
11,129
|
|
10,776
|
|
|
|
Ozempic®
|
6,872
|
|
641
|
|
536
|
|
—
|
|
105
|
|
6,231
|
|
5,900
|
|
|
|
Rybelsus®
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
|
Total GLP-1
|
23,379
|
|
6,019
|
|
4,115
|
|
655
|
|
1,249
|
|
17,360
|
|
16,676
|
|
|
|
Other Diabetes care
|
3,230
|
|
2,611
|
|
808
|
|
1,280
|
|
523
|
|
619
|
|
509
|
|
|
|
Total Diabetes care
|
71,395
|
|
36,149
|
|
18,365
|
|
9,564
|
|
8,220
|
|
35,246
|
|
33,698
|
|
|
|
Obesity care (Saxenda®)
|
4,115
|
|
1,611
|
|
781
|
|
6
|
|
824
|
|
2,504
|
|
2,323
|
|
|
|
Diabetes and Obesity care total
|
75,510
|
|
37,760
|
|
19,146
|
|
9,570
|
|
9,044
|
|
37,750
|
|
36,021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biopharm segment
|
|
|
|
|
|
|
|
|
|
Haemophilia
|
7,727
|
|
4,475
|
|
2,683
|
|
225
|
|
1,567
|
|
3,252
|
|
3,012
|
|
|
|
|
NovoSeven®
|
6,160
|
|
3,413
|
|
1,895
|
|
212
|
|
1,306
|
|
2,747
|
|
2,610
|
|
|
|
|
NovoEight®
|
1,119
|
|
863
|
|
645
|
|
13
|
|
205
|
|
256
|
|
239
|
|
|
|
Growth disorders (Norditropin®)
|
5,199
|
|
3,140
|
|
1,467
|
|
25
|
|
1,648
|
|
2,059
|
|
2,049
|
|
|
|
Other Biopharm
|
1,168
|
|
838
|
|
681
|
|
5
|
|
152
|
|
330
|
|
152
|
|
|
|
Biopharm total
|
14,094
|
|
8,453
|
|
4,831
|
|
255
|
|
3,367
|
|
5,641
|
|
5,213
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total sales
|
89,604
|
|
46,213
|
|
23,977
|
|
9,825
|
|
12,411
|
|
43,391
|
|
41,234
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
|
|
|
|
|
|
|
|
Financial report for the period 1 January 2020 to 30 September 2020
|
Page 33 of 33
|
APPENDIX 8: NEW SALES SPLIT PER AREA - CONTINUED (ADDITIONAL INFORMATION)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2019 sales split per area - DKK million
|
Total
|
International
Operations
|
EMEA
|
Region
China
|
Rest of
World
|
North America
Operations
|
The US
|
|
|
|
|
|
|
|
|
|
|
Diabetes and Obesity care segment
|
|
|
|
|
|
|
|
|
|
Long-acting insulin
|
20,776
|
|
9,035
|
|
5,955
|
|
1,059
|
|
2,021
|
|
11,741
|
|
11,271
|
|
|
|
|
Tresiba®
|
9,259
|
|
3,477
|
|
1,983
|
|
87
|
|
1,407
|
|
5,782
|
|
5,500
|
|
|
|
|
Xultophy®
|
2,210
|
|
1,493
|
|
1,407
|
|
—
|
|
86
|
|
717
|
|
708
|
|
|
|
|
Levemir®
|
9,307
|
|
4,065
|
|
2,565
|
|
972
|
|
528
|
|
5,242
|
|
5,063
|
|
|
|
Premix insulin
|
10,578
|
|
9,707
|
|
3,160
|
|
4,306
|
|
2,241
|
|
871
|
|
839
|
|
|
|
|
Ryzodeg®
|
993
|
|
993
|
|
237
|
|
4
|
|
752
|
|
—
|
|
—
|
|
|
|
|
NovoMix®
|
9,585
|
|
8,714
|
|
2,923
|
|
4,302
|
|
1,489
|
|
871
|
|
839
|
|
|
|
Fast-acting insulin
|
19,303
|
|
10,304
|
|
6,422
|
|
1,753
|
|
2,129
|
|
8,999
|
|
8,592
|
|
|
|
|
Fiasp®
|
1,243
|
|
617
|
|
585
|
|
—
|
|
32
|
|
626
|
|
597
|
|
|
|
|
NovoRapid®
|
18,060
|
|
9,687
|
|
5,837
|
|
1,753
|
|
2,097
|
|
8,373
|
|
7,995
|
|
|
|
Human insulin
|
9,036
|
|
7,361
|
|
2,438
|
|
2,847
|
|
2,076
|
|
1,675
|
|
1,552
|
|
|
|
Total insulin
|
59,693
|
|
36,407
|
|
17,975
|
|
9,965
|
|
8,467
|
|
23,286
|
|
22,254
|
|
|
|
Victoza®
|
21,934
|
|
7,249
|
|
4,713
|
|
898
|
|
1,638
|
|
14,685
|
|
14,217
|
|
|
|
Ozempic®
|
11,237
|
|
1,143
|
|
969
|
|
—
|
|
174
|
|
10,094
|
|
9,599
|
|
|
|
Rybelsus®
|
50
|
|
—
|
|
—
|
|
—
|
|
—
|
|
50
|
|
50
|
|
|
|
Total GLP-1
|
33,221
|
|
8,392
|
|
5,682
|
|
898
|
|
1,812
|
|
24,829
|
|
23,866
|
|
|
|
Other Diabetes care
|
4,247
|
|
3,389
|
|
1,052
|
|
1,647
|
|
690
|
|
858
|
|
705
|
|
|
|
Total Diabetes care
|
97,161
|
|
48,188
|
|
24,709
|
|
12,510
|
|
10,969
|
|
48,973
|
|
46,825
|
|
|
|
Obesity care (Saxenda®)
|
5,679
|
|
2,083
|
|
981
|
|
9
|
|
1,093
|
|
3,596
|
|
3,348
|
|
|
|
Diabetes and Obesity care total
|
102,840
|
|
50,271
|
|
25,690
|
|
12,519
|
|
12,062
|
|
52,569
|
|
50,173
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Biopharm segment
|
|
|
|
|
|
|
|
|
|
Haemophilia
|
10,281
|
|
5,946
|
|
3,646
|
|
284
|
|
2,016
|
|
4,335
|
|
4,031
|
|
|
|
|
NovoSeven®
|
8,119
|
|
4,502
|
|
2,577
|
|
269
|
|
1,656
|
|
3,617
|
|
3,454
|
|
|
|
|
NovoEight®
|
1,525
|
|
1,143
|
|
844
|
|
15
|
|
284
|
|
382
|
|
358
|
|
|
|
Growth disorders (Norditropin®)
|
7,275
|
|
4,225
|
|
1,960
|
|
36
|
|
2,229
|
|
3,050
|
|
3,035
|
|
|
|
Other Biopharm
|
1,625
|
|
1,122
|
|
912
|
|
5
|
|
205
|
|
503
|
|
247
|
|
|
|
Biopharm total
|
19,181
|
|
11,293
|
|
6,518
|
|
325
|
|
4,450
|
|
7,888
|
|
7,313
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total sales
|
122,021
|
|
61,564
|
|
32,208
|
|
12,844
|
|
16,512
|
|
60,457
|
|
57,486
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COVID-19
|
Strategic aspirations
|
Financial
Performance
|
Equity and capital allocation
|
Outlook
|
R&D
|
Sustainability
|
Corporate governance
|
|
Financial Information
|
|
|
|
|
|
|
Company announcement No 63 / 2020
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf of the undersigned, thereunto duly authorized.
Date: October 30, 2020
Novo Nordisk A/S
Lars Fruergaard Jørgensen
Chief Executive Officer
Novo Nordisk (NYSE:NVO)
Historical Stock Chart
From Mar 2024 to Apr 2024
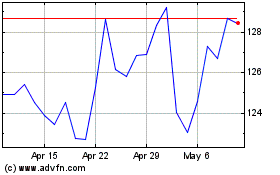
Novo Nordisk (NYSE:NVO)
Historical Stock Chart
From Apr 2023 to Apr 2024