DESCRIPTION OF CAPITAL STOCK
The following summary description of our capital stock is based on the provisions of our amended and restated
certificate of incorporation and amended and restated bylaws and the applicable provisions of the Delaware General Corporation Law. This information is qualified entirely by reference to the
applicable provisions of our amended and restated certificate of incorporation, bylaws and the Delaware General Corporation Law. For information on how to obtain copies of our amended and restated
certificate of incorporation and bylaws, which are exhibits to the registration statement of which this prospectus supplement is a part, see the sections titled "Where You Can Find Additional
Information" and "Incorporation of Certain Information by Reference" in this prospectus supplement.
General
Our authorized capital stock consists of (i) 200,000,000 shares of common stock, par value $0.10 per share and (ii) 5,000,000
shares of preferred stock, par value $0.10 per share. As of December 31, 2018, there were 7,141,189 shares of common stock issued and outstanding, and no shares of preferred stock outstanding.
The
following is a summary of the material provisions of the common stock and preferred stock provided for in our amended and restated certificate of incorporation and amended and
restated bylaws.
At
our annual meeting of stockholders to be held on April 23, 2019, our stockholders will be asked to approve an amendment to our amended and restated certificate of incorporation
to decrease our authorized shares of common stock from 200,000,000 to 100,000,000 shares.
Common Stock
Voting
Our common stock is entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, except that
directors will be elected by a plurality of votes cast. Accordingly, the holders of a majority of the shares of common stock entitled to vote in any election of directors are able to elect all of the
directors standing for election, if they so choose.
Dividends
Subject to preferences that may be applicable to any then outstanding preferred stock, the holders of common stock are entitled to receive
dividends, if any, as may be declared from time to time by our board of directors out of legally available funds. We have never paid cash dividends and have no present intention to pay cash dividends.
Liquidation
In the event of a liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in the net assets
legally available for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction of any liquidation preference granted to the holders of any
outstanding shares of preferred stock.
Rights and Preferences
Holders of our common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions
applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to, and may be adversely affected by, the
S-14
Table of Contents
rights
of the holders of shares of any series of our preferred stock that we may designate and issue in the future.
Fully Paid and Nonassessable
All of our outstanding shares of common stock are fully paid and nonassessable.
Preferred Stock
Our board of directors has the authority, without further action by the stockholders, to issue up to 5,000,000 shares of preferred stock in one
or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and
any qualifications, limitations or restrictions thereon and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our
board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the
common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of
delaying, deferring or preventing a change in our control that may otherwise benefit holders of our common stock and may adversely affect the market price of the common stock and the voting and other
rights of the holders of common stock. As of December 31, 2018, there were no shares of preferred stock outstanding and we have no current plans to issue any shares of preferred stock.
Anti-Takeover Effects of Provisions of Our Charter Documents and Delaware Law
Delaware Anti-Takeover Law
We are subject to Section 203 of the DGCL, or Section 203. Section 203 generally prohibits a public Delaware corporation
from engaging in a "business combination" with an "interested stockholder" for a period of three years after the date of the transaction in which the person became an interested stockholder,
unless:
-
•
-
prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction
which resulted in the stockholder becoming an interested stockholder;
-
•
-
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding upon consummation of the transaction,
excluding for purposes of determining the number of shares outstanding (1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in
which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
-
•
-
on or subsequent to the consummation of the transaction, the business combination is approved by the board and authorized at an annual or
special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66
2
/
3
% of the outstanding voting stock which is not owned by
the interested stockholder.
-
•
-
Section 203 defines a business combination to include:
-
•
-
any merger or consolidation involving the corporation and the interested stockholder;
-
•
-
any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation;
S-15
Table of Contents
-
•
-
subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any
class or series of the corporation beneficially owned by the interested stockholder;
-
•
-
subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the
interested stockholder; and
-
•
-
the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or
through the corporation.
In
general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity
or person affiliated with or controlling or controlled by the entity or person.
Certificate of Incorporation and Bylaws
Provisions of our certificate of incorporation and bylaws may delay or discourage transactions involving an actual or potential
change-in-control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares or transactions that our stockholders might otherwise
deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, our certificate of incorporation and
bylaws:
-
•
-
permit our board of directors to issue up to 5,000,000 shares of preferred stock, with any rights, preferences and privileges as they may
designate (including the right to approve an acquisition or other change in control);
-
•
-
provide that the authorized number of directors may be changed only by resolution adopted by a majority of the board of directors;
-
•
-
provide that all vacancies, including newly created directorships, may, except as otherwise required by law or subject to the rights of holders
of preferred stock as designated from time to time, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
-
•
-
require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders or by
action taken by written consent;
-
•
-
provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at
a meeting of stockholders must provide notice in writing in a timely manner and also specify requirements as to the form and content of a stockholder's notice; and
-
•
-
provide that special meetings of our stockholders may be called only by the chairman of the board, the president or by our board of directors
pursuant to a resolution adopted by a majority of the total number of authorized directors (whether or not there exist any vacancies).
Nasdaq Capital Market Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol "VXRT."
WHERE YOU CAN FIND MORE INFORMATION
We filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the shares of common stock offered
hereby. This prospectus supplement and the accompanying prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration
statement or the exhibits filed with the registration statement. For further information about us and the common stock offered hereby, we refer you to the registration statement and the exhibits filed
with the registration statement. Statements contained in this prospectus supplement and the accompanying prospectus regarding the contents of any contract or any other document that is filed as an
exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an
exhibit to the registration statement.
We
are subject to the information and reporting requirements of the Exchange Act and, in accordance with this law, are required to file periodic reports, proxy statements and other
information with the SEC. You can read our SEC filings, including the registration statement, over the internet at the SEC's website at
www.sec.gov
.
We
make available free of charge, on or through the investor relations section of our website, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports
on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such
material with, or furnish it to, the SEC. The information found on our website,
www.vaxart.com
, other than as specifically incorporated by reference in
this prospectus supplement and the accompanying prospectus, is not part of this prospectus supplement and the accompanying prospectus.
S-20
Table of Contents
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to "incorporate by reference" information from other documents that we file with it, which means that we can disclose
important information to you by
referring you to those documents. The information incorporated by reference is considered to be part of this prospectus supplement and the accompanying prospectus. Information in this prospectus
supplement and the accompanying prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus supplement and the accompanying prospectus,
while information that we file later with the SEC will automatically update and supersede the information in this prospectus supplement and the accompanying prospectus. We incorporate by reference
into this prospectus supplement and the accompanying prospectus and the registration statement of which this prospectus supplement and the accompanying prospectus is a part the information or
documents listed below that we have filed with the SEC (Commission File No. 001-35285):
-
•
-
our Annual Report on Form 10-K for the year ended December 31, 2018, filed with the SEC on February 6, 2019;
-
•
-
our definitive proxy statement relating to our 2019 annual meeting of stockholders, filed with the SEC on March 11, 2019;
-
•
-
our Current Reports on Form 8-K filed with the SEC on January 18, 2019 and March 19, 2019; and
-
•
-
the description of our common stock contained in our Registration Statement on Form 10, filed with the SEC on May 4, 1970, as
amended by our Current Report on Form 8-K (File No. 000-04829) filed with the SEC on August 15, 2003.
We
also incorporate by reference any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form
that are related to such items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, until the
termination of the offering of the shares of our common stock made by this prospectus supplement and the accompanying prospectus and will become a part of this prospectus supplement and the
accompanying prospectus from the date that such documents are filed with the SEC. Information in such future filings updates and supplements the information provided in this prospectus supplement and
the accompanying prospectus. Any statements in any such future filings will automatically be deemed to modify and supersede any information in any document we previously filed with the SEC that is
incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements.
You
can request a copy of these filings, at no cost, by writing or telephoning us at the following address or telephone number:
Vaxart, Inc.
290 Utah Ave
Suite 200
South San Francisco, California 94080
Attn: Secretary
(650) 550-3500
Copies
of these filings are also available through the "Investor" section of our website at
www.vaxart.com
. For other ways to obtain a
copy of these filings, please refer to "Where You Can Find More Information" above.
S-21
Table of Contents
PROSPECTUS

$25,000,000
Common Stock
From time to time, we may offer and sell up to an aggregate amount of $25,000,000 of common stock.
We
will provide the specific terms of these offerings in one or more supplements to this prospectus. We may also authorize one or more free writing prospectuses to be provided to you in
connection with these offerings. The prospectus supplement and any related free writing prospectus may also add, update or change information contained in this prospectus. You should carefully read
this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as any documents incorporated by reference, before buying any of the shares of common stock being
offered.
Our
common stock is listed on the Nasdaq Capital Market under the trading symbol "VXRT." On March 13, 2019, the last reported sale price of our common stock was $1.65 per share.
The applicable prospectus supplement will contain information, where applicable, as to other listings, if any, on the Nasdaq Capital Market or other securities exchange of the shares of common stock
covered by the applicable prospectus supplement.
Investing in shares of our common stock involves a high degree of risk. You should review carefully the risks and uncertainties described in the
section titled "Risk Factors" on page 3 of this prospectus and any similar section contained in the applicable prospectus supplement and in any free writing prospectuses we have authorized for
use in connection with a specific offering, and under similar headings in the documents that are incorporated by reference into this prospectus.
This prospectus may not be used to consummate a sale of shares of our common stock unless accompanied by a prospectus
supplement.
As
of March 13, 2019, the aggregate market value of our outstanding common stock held by non-affiliates, or public float, was $9,005,150, which was calculated based on 3,984,580
shares of outstanding common stock held by non-affiliates at a price of $2.26 per share, which was the closing price of our common stock on the Nasdaq Capital Market on February 6, 2019.
Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities registered on the registration statement of which this prospectus is a part in a public primary
offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million. As of the date hereof, we have not
offered any securities pursuant to General Instruction I.B.6 of Form S-3 during the 12 calendar months prior to and including the date of this prospectus.
The
shares of our common stock may be sold directly by us to investors, through agents designated from time to time or to or through underwriters or dealers, on a continuous or delayed
basis. For additional information on the methods of sale, you should refer to the section titled "Plan of Distribution" in this prospectus. If any agents or underwriters are involved in the sale of
any shares of our common stock with respect to which this prospectus is being delivered, the names of such agents or underwriters and any applicable fees, commissions, discounts and over-allotment
options will be set forth in a prospectus supplement. The price to the public of such shares of our common stock and the net proceeds we expect to receive from such sale will also be set forth in a
prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this
prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is March 15, 2019.
Table of Contents
TABLE OF CONTENTS
i
Table of Contents
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC,
using a "shelf" registration process. Under this shelf registration statement, we may, from time to time, offer and sell in one or more offerings, up to a total dollar amount of $25,000,000 of shares
of our common stock as described in this prospectus.
Each
time we offer shares of our common stock under this prospectus, we will provide a prospectus supplement that will contain more specific information about the terms of that offering.
We may also authorize one or more free writing prospectuses to be provided to you that may contain material information relating to these offerings. The prospectus supplement and any related free
writing prospectus that we may authorize to be provided to you may also add, update or change any of the information contained in this prospectus or in the documents that we have incorporated by
reference into this prospectus. We urge you to read carefully this prospectus, any applicable prospectus supplement and any free writing prospectuses we have authorized for use in connection with a
specific
offering, together with the information incorporated herein by reference as described under the heading "Incorporation of Certain Information by Reference," before buying any of the shares of our
common stock being offered.
This prospectus may not be used to consummate a sale of shares of our common stock unless it is accompanied by a prospectus supplement.
You
should rely only on the information contained in, or incorporated by reference into, this prospectus and any applicable prospectus supplement, along with the information contained in
any free writing prospectuses we have authorized for use in connection with a specific offering. We have not authorized anyone to provide you with any information other than that contained or
incorporated by reference in this prospectus and any applicable prospectus supplement, along with the information contained in any free writing prospectuses we have authorized for use in connection
with a specific offering. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus, the accompanying prospectus supplement or in any
related free writing prospectus that we may authorize to be provided to you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may
give you. This prospectus is an offer to sell only the shares of our common stock offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so.
The
information appearing in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate only as of the date on the front of the document and
that any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus, any applicable
prospectus supplement or any related free writing prospectus, or any sale of shares of our common stock. Our business, financial condition, results of operations and prospects may have changed since
those dates.
This
prospectus contains and incorporates by reference market data and industry statistics and forecasts that are based on independent industry publications and other publicly available
information. Although we believe that these sources are reliable, we do not guarantee the accuracy or completeness of this information and we have not independently verified this information. Although
we are not aware of any misstatements regarding the market and industry data presented in this prospectus and the documents incorporated herein by reference, these estimates involve risks and
uncertainties and are subject to change based on various factors, including those discussed in the section titled "Risk Factors" contained in the applicable prospectus supplement and any related free
writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus. Accordingly, investors should not place undue reliance on this
information.
ii
Table of Contents
This
prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All
of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as
exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the section titled "Where You Can Find Additional
Information."
iii
Table of Contents
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus or incorporated by reference in this
prospectus, and does not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire prospectus, the applicable prospectus
supplement and any related free writing prospectus, including the risks of investing in our shares of our common stock discussed in the section titled "Risk Factors" contained in the applicable
prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus. You should also carefully read
the other information incorporated by reference into this prospectus, including our financial statements, and the exhibits to the registration statement of which this prospectus is a
part.
Throughout this prospectus, the terms "we," "us," "our," and "our company" refer to Vaxart, Inc.
Vaxart, Inc.
Overview
We are a clinical-stage biotechnology company focused on the development of oral recombinant vaccines based on our proprietary oral vaccine
platform. Our oral vaccines are designed to generate broad and durable immune responses that protect against a wide range of infectious diseases and may be useful for the treatment of chronic viral
infections and cancer. Our vaccines are administered using a convenient room temperature-stable tablet, rather than by injection.
We
are developing prophylactic vaccine candidates that target a range of infectious diseases. These include norovirus, a widespread cause of acute gastro-intestinal enteritis, for which
two Phase 1 human studies have been completed; seasonal influenza, for which our vaccine protected patients in a recent Phase 2 challenge study; and respiratory syncytial virus, or RSV,
a common cause of respiratory tract infections. In addition, we are developing our first therapeutic immune-oncology vaccine targeting cervical cancer and dysplasia caused by human papillomavirus, or
HPV.
Corporate Background
Vaxart Biosciences, Inc. was originally incorporated in California in March 2004 under the name West Coast Biologicals, Inc. The
Company changed its name to Vaxart, Inc. in July 2007, and reincorporated in the state of Delaware.
On
February 13, 2018, we completed a business combination with Aviragen Therapeutics, Inc., or Aviragen, a publicly-traded company. Under the terms of the agreement and
plan of merger and reorganization dated October 27, 2017, Vaxart, Inc. survived as a wholly owned subsidiary of Aviragen and changed its name to Vaxart Biosciences, Inc. and
Aviragen changed its name to Vaxart, Inc. Our common stock subsequently began trading on the Nasdaq Capital Market under the symbol "VXRT."
The Shares of Common Stock We May Offer
We may offer shares of our common stock up to a total dollar amount of $25,000,000, from time to time under this prospectus, together with the
applicable prospectus supplement and any related free writing prospectus, at prices and on terms to be determined by market conditions at the time of any offering. Each time we offer shares of our
common stock under this prospectus, we will provide a prospectus supplement that will describe the specific amounts, prices and other important terms of the offering.
The
applicable prospectus supplement and any related free writing prospectus that we may authorize to be provided to you may also add, update or change any of the information contained
in
1
Table of Contents
this
prospectus or in the documents we have incorporated by reference. However, no prospectus supplement or free writing prospectus will offer any security other than shares of our common stock.
THIS PROSPECTUS MAY NOT BE USED TO CONSUMMATE A SALE OF SHARES OF OUR COMMON STOCK UNLESS IT IS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
We
may sell the shares of our common stock directly to investors or to or through agents, underwriters or dealers. We and our agents or underwriters, reserve the right to accept or
reject all or part of any proposed purchase of shares of our common stock. If we do offer shares of our common stock to or through agents or underwriters, we will include in the applicable prospectus
supplement:
-
•
-
the names of those agents or underwriters;
-
•
-
applicable fees, discounts and commissions to be paid to them;
-
•
-
details regarding over-allotment options, if any; and
-
•
-
the net proceeds to us.
We
may issue shares of our common stock from time to time. The holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of
stockholders. Subject to preferences that may be applicable to any outstanding shares of preferred stock, the holders of common stock are entitled to receive ratably such dividends as may be declared
by our board of
directors out of legally available funds. Upon our liquidation, dissolution or winding up, holders of our common stock are entitled to share ratably in all assets remaining after payment of
liabilities and the liquidation preferences of any outstanding shares of preferred stock. Holders of common stock have no preemptive rights and no right to convert their common stock into any other
securities. There are no redemption or sinking fund provisions applicable to our common stock. We urge you to read the applicable prospectus supplement (and any related free writing prospectus that we
may authorize to be provided to you) related to any common stock being offered.
Use of Proceeds
Except as described in any applicable prospectus supplement or in any free writing prospectuses we have authorized for use in connection with a
specific offering, we currently intend to use the net proceeds from the sale of the securities offered by us hereunder to support the clinical and preclinical development of our product candidates, to
conduct clinical trials including a Phase I study with our bivalent norovirus vaccines and a Phase II challenge study with our GI.1 monovalent norovirus vaccine, to support the
manufacturing of vaccines for these clinical trials, and to advance our therapeutic HPV vaccine candidate. See the section titled "Use of Proceeds" in this prospectus.
Nasdaq Capital Market Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol "VXRT." The applicable prospectus supplement will contain information,
where applicable, as to other listings, if any, on the Nasdaq Capital Market or other securities exchange of the shares of our common stock covered by the applicable prospectus supplement.
2
Table of Contents
RISK FACTORS
Investing in shares of our common stock involves a high degree of risk. Before deciding whether to invest in shares of our common stock, you
should consider carefully the risks and uncertainties described in the section titled "Risk Factors" contained in the applicable prospectus supplement and any related free writing prospectus, and
discussed under the section titled "Risk Factors" contained in our Annual Report on Form 10-K for the year ended December 31, 2018, as may be updated by our Quarterly Reports on
Form 10-Q, as well as any amendments thereto reflected in subsequent filings with the SEC, which are incorporated by reference into this prospectus in their entirety, together with other
information in this prospectus, the documents incorporated by reference and any free writing prospectus that we may authorize for use in connection with this offering. The risks described in these
documents are not the only ones we face, but those that we consider to be material. There may be other unknown or unpredictable economic, business, competitive, regulatory or other factors that could
have material adverse effects on our future results. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to anticipate results or
trends in future periods. If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could be harmed. This could cause the trading price of our
common stock to decline, resulting in a loss of all or part of your investment. Please also read carefully the section below titled "Special Note Regarding Forward-Looking Statements."
3
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents we have filed with the SEC that are incorporated by reference contain "forward-looking statements" within the
meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, or the Exchange Act. These statements
relate to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or
achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. Forward-looking statements may include, but are
not limited to, statements about:
-
•
-
our ability to fund our working capital requirements;
-
•
-
the amount and timing of royalties received on sales of Relenza and Inavir;
-
•
-
the timing and costs of our planned clinical trials for our product candidates, both tablet vaccines and small-molecule antiviral drugs;
-
•
-
our ability to obtain and maintain regulatory approval of our product candidates;
-
•
-
our ability to establish and scale commercial manufacturing capabilities;
-
•
-
the rate and degree of market acceptance of our products, if any, that are approved;
-
•
-
our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional
financing;
-
•
-
our ability to obtain and maintain intellectual property protection for our product candidates;
-
•
-
our ability to identify and develop new product candidates and the number and characteristics of product candidates that we pursue;
-
•
-
our ability to retain and recruit key personnel;
-
•
-
our financial performance;
-
•
-
our ability to become profitable and generate consistent cash flows to remain profitable;
-
•
-
developments and projections relating to our competitors or our industry; and
-
•
-
our planned use of the proceeds from this offering.
In
some cases, you can identify forward-looking statements by terms such as "anticipates," "believes," "could," "estimates," "intends," "may," "plans," "potential," "will," "would," or
the negative of these terms or other similar expressions. These statements reflect our current views with respect to future events and are based on assumptions and are subject to risks and
uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We discuss in greater detail many of these risks in the section titled "Risk Factors"
contained in the applicable prospectus supplement, in any free writing prospectuses we may authorize for use in connection with a specific offering, and in our Annual Report on Form 10-K for
the year ended December 31, 2018 as may be updated by our Quarterly Reports on Form 10-Q, as well as any amendments thereto reflected in subsequent filings with the SEC, which are
incorporated by reference into this prospectus in their entirety. Also, these forward-looking statements represent our estimates and assumptions only as of the date of the document containing the
applicable statement. Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments.
In
addition, statements that "we believe" and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as
of the date of this
4
Table of Contents
prospectus,
and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate
that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly
rely upon these statements.
You
should read this prospectus, any applicable prospectus supplement, together with the documents we have filed with the SEC that are incorporated by reference and any free writing
prospectus that we may authorize for use in connection with this offering completely and with the understanding that our actual future results may be materially different from what we expect. We
qualify all of the forward-looking statements in the foregoing documents by these cautionary statements.
5
Table of Contents
USE OF PROCEEDS
Except as described in any applicable prospectus supplement or in any free writing prospectuses we have authorized for use in connection with a
specific offering, we currently intend to use the net proceeds from the sale of the securities offered by us hereunder to support the clinical and preclinical development of our product candidates, to
conduct clinical trials, including a Phase I study with our bivalent norovirus vaccines and a Phase II challenge study with our GI.1 monovalent norovirus vaccine, to support the
manufacturing of vaccines for these clinical trials and to advance our therapeutic HPV vaccine candidate, and for general corporate and working capital purposes.
6
Table of Contents
DESCRIPTION OF CAPITAL STOCK
The following summary description of our capital stock is based on the provisions of our amended and restated certificate of incorporation and
amended and restated bylaws and the applicable provisions of the Delaware General Corporation Law. This information is qualified entirely by reference to the applicable provisions of our amended and
restated certificate of incorporation, bylaws and the Delaware General Corporation Law. For information on how to obtain copies of our amended and restated certificate of incorporation and bylaws,
which are exhibits to the registration statement of which this prospectus is a part, see the sections titled "Where You Can Find Additional Information" and "Incorporation of Certain Information by
Reference" in this prospectus.
General
Our authorized capital stock consists of (i) 200,000,000 shares of common stock, par value $0.10 per share and (ii) 5,000,000
shares of preferred stock, par value $0.10 per share. As of December 31, 2018, there were 7,141,189 shares of common stock issued and outstanding, and no shares of preferred stock outstanding.
The
following is a summary of the material provisions of the common stock and preferred stock provided for in our amended and restated certificate of incorporation and amended and
restated bylaws.
Common Stock
Voting
Our common stock is entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders, except that
directors will be elected by a plurality of votes cast. Accordingly, the holders of a majority of the shares of common stock entitled to vote in any election of directors are able to elect all of the
directors standing for election, if they so choose.
Dividends
Subject to preferences that may be applicable to any then outstanding preferred stock, the holders of common stock are entitled to receive
dividends, if any, as may be declared from time to time by our board of directors out of legally available funds. We have never paid cash dividends and have no present intention to pay cash dividends.
Liquidation
In the event of a liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in the net assets
legally available for distribution to stockholders after the payment of all of our debts and other liabilities, subject to the satisfaction of any liquidation preference granted to the holders of any
outstanding shares of preferred stock.
Rights and Preferences
Holders of our common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions
applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any
series of our preferred stock that we may designate and issue in the future.
7
Table of Contents
Fully Paid and Nonassessable
All of our outstanding shares of common stock are fully paid and nonassessable.
Preferred Stock
Our board of directors has the authority, without further action by the stockholders, to issue up to 5,000,000 shares of preferred stock in one
or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and
any qualifications, limitations or restrictions thereon and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our
board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the
common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of
delaying, deferring or preventing a change in our control that may otherwise benefit holders of our common stock and may adversely affect the market price of the common stock and the voting and other
rights of the holders of common stock. As of December 31, 2018, there were no shares of preferred stock outstanding and we have no current plans to issue any shares of preferred stock.
Anti-Takeover Effects of Provisions of Our Charter Documents and Delaware Law
Delaware Anti-Takeover Law
We are subject to Section 203 of the DGCL, or Section 203. Section 203 generally prohibits a public Delaware corporation
from engaging in a "business combination" with an "interested stockholder" for a period of three years after the date of the transaction in which the person became an interested stockholder,
unless:
-
•
-
prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction
which resulted in the stockholder becoming an interested stockholder;
-
•
-
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding upon consummation of the transaction,
excluding for purposes of determining the number of shares outstanding (1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in
which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
-
•
-
on or subsequent to the consummation of the transaction, the business combination is approved by the board and authorized at an annual or
special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66
2
/
3
% of the outstanding voting stock which is not owned by the interested stockholder.
Section 203
defines a business combination to include:
-
•
-
any merger or consolidation involving the corporation and the interested stockholder;
-
•
-
any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation;
-
•
-
subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any
class or series of the corporation beneficially owned by the interested stockholder;
8
Table of Contents
-
•
-
subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the
interested stockholder; and
-
•
-
the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or
through the corporation.
In
general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity
or person affiliated with or controlling or controlled by the entity or person.
Certificate of Incorporation and Bylaws
Provisions of our certificate of incorporation and bylaws may delay or discourage transactions involving an actual or potential
change-in-control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares or transactions that our stockholders might otherwise
deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, our certificate of incorporation and
bylaws:
-
•
-
permit our board of directors to issue up to 5,000,000 shares of preferred stock, with any rights, preferences and privileges as they may
designate (including the right to approve an acquisition or other change in control);
-
•
-
provide that the authorized number of directors may be changed only by resolution adopted by a majority of the board of directors;
-
•
-
provide that all vacancies, including newly created directorships, may, except as otherwise required by law or subject to the rights of holders
of preferred stock as designated from time to time, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
-
•
-
require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders or by
action taken by written consent;
-
•
-
provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at
a meeting of stockholders must provide notice in writing in a timely manner and also specify requirements as to the form and content of a stockholder's notice; and
-
•
-
provide that special meetings of our stockholders may be called only by the chairman of the board, the president or by our board of directors
pursuant to a resolution adopted by a majority of the total number of authorized directors (whether or not there exist any vacancies).
Nasdaq Capital Market Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol "VXRT."
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC. The transfer agent and
registrar's address is 6201 15th Avenue, Brooklyn, New York 11219.
9
Table of Contents
PLAN OF DISTRIBUTION
We may sell the shares of our common stock from time to time pursuant to underwritten public offerings, direct sales to the public, negotiated
transactions, block trades or a combination of these methods. We may sell the shares of our common stock to or through underwriters or dealers, through agents, or directly to one or more purchasers.
We may distribute the shares from time to time in one or more transactions:
-
•
-
at a fixed price or prices, which may be changed;
-
•
-
at market prices prevailing at the time of sale;
-
•
-
at prices related to such prevailing market prices; or
-
•
-
at negotiated prices.
A
prospectus supplement or supplements (and any related free writing prospectus that we may authorize to be provided to you) will describe the terms of the offering of the shares of our
common stock, including, to the extent applicable:
-
•
-
the name or names of the underwriters, if any;
-
•
-
the purchase price of the shares of our common stock or other consideration therefor, and the proceeds, if any, we will receive from the sale;
-
•
-
any over-allotment options under which underwriters may purchase additional shares of our common stock from us;
-
•
-
any agency fees or underwriting discounts and other items constituting agents' or underwriters' compensation;
-
•
-
any public offering price;
-
•
-
any discounts or concessions allowed or reallowed or paid to dealers; and
-
•
-
any securities exchange or market on which the shares of our common stock may be listed.
Only
underwriters named in the prospectus supplement will be underwriters of the shares of our common stock offered by the prospectus supplement.
If
underwriters are used in the sale, they will acquire the shares of our common stock for their own account and may resell the shares of our common stock from time to time in one or
more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the shares of our common stock will be subject
to the conditions set forth in the applicable underwriting agreement. We may offer the shares of our common stock to the public through underwriting syndicates represented by managing underwriters or
by underwriters without a syndicate. Subject to certain conditions, the underwriters will be obligated to purchase all of the shares of our common stock offered by the prospectus supplement, other
than shares of our common stock covered by any over-allotment option. Any public offering price and any discounts or concessions allowed or reallowed or paid to dealers may change from time to time.
We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, the nature of any such relationship.
We
may sell shares of our common stock directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of shares of our common stock
and we will describe any commissions we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of
its appointment.
10
Table of Contents
We
may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase shares of our common stock from us at the public offering price set
forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and
the commissions we must pay for solicitation of these contracts in the prospectus supplement.
We
may provide agents and underwriters with indemnification against civil liabilities, including liabilities under the Securities Act, or contribution with respect to payments that the
agents or underwriters may make with respect to these liabilities. Agents and underwriters may engage in transactions with, or perform services for us in the ordinary course of business.
Any
underwriter may engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the Exchange Act.
Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do
not exceed a specified maximum price. Syndicate-covering or other short-covering transactions involve purchases of the shares of our common stock, either through exercise of the over-allotment option
or in the open market after the distribution is completed, to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the shares of our common
stock originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of the shares of our common stock to be higher
than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
Any
underwriters or agents that are qualified market makers on the Nasdaq Capital Market may engage in passive market making transactions in the common stock on the Nasdaq Capital Market
in accordance with Regulation M under the Exchange Act, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the common stock. Passive
market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess
of the highest independent bid for such security; if all independent bids are lowered below the passive market maker's bid, however, the passive market maker's bid must then be lowered when certain
purchase limits are exceeded. Passive market making may stabilize the market price of the shares of our common stock at a level above that which might otherwise prevail in the open market and, if
commenced, may be discontinued at any time.
11
Table of Contents
LEGAL MATTERS
Cooley LLP, Palo Alto, California, will pass upon the validity of the shares of common stock offered hereby. Additional legal matters may
be passed upon for us or any underwriters, dealers or agents, by counsel that we name in the applicable prospectus supplement.
EXPERTS
The consolidated financial statements of Vaxart, Inc. as of December 31, 2018 and 2017, and for each of the years in the two year
period ended December 31, 2018, have been incorporated by reference herein in reliance upon the report of KPMG LLP, independent registered public accounting firm, incorporated by
reference herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering the December 31, 2018 consolidated financial statements contains an
explanatory paragraph that states that the Company has experienced losses and negative cash flows from operations since its inception, has an accumulated deficit, and has debt obligations which raise
substantial doubt about its ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of that uncertainty.
On
February 13, 2018, privately-held Vaxart, Inc., or Private Vaxart, and Aviragen Therapeutics, Inc., or Aviragen, completed a business combination in accordance
with the terms an agreement and plan of merger and reorganization, dated October 27, 2017, by and among Aviragen, Agora Merger Sub, Inc., or Merger Sub, and Private Vaxart, pursuant to
which Merger Sub merged with and into Private Vaxart, with Private Vaxart surviving as a wholly-owned subsidiary of Aviragen, or the Merger. Aviragen changed its name at the closing of the Merger to
Vaxart, Inc., or the Combined Company, and Private Vaxart changed its name to Vaxart Biosciences, Inc. For accounting purposes, Aviragen was deemed to be the acquired entity in the
Merger, and the financial statements of Private Vaxart became the historical financial statements of the Combined Company following the Merger.
In
connection with the closing of the Merger on February 13, 2018, the board of directors of Vaxart, Inc. dismissed Ernst & Young LLP as its independent
registered public accounting firm, effective immediately. The reports of Ernst & Young LLP on Aviragen Therapeutics, Inc.'s consolidated financial statements for the fiscal years
ended June 30, 2017 and 2016 did not contain an adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles. During
the fiscal years ended June 30, 2017 and 2016, and the subsequent interim period through February 13, 2018 there were no: (1) disagreements (as defined in
Item 304(a)(1)(iv) of Regulation S-K and the related instructions) with Ernst & Young LLP on any matter of accounting principles or practices, financial statement
disclosure, or auditing scope or procedures, which disagreement if not resolved to the satisfaction of Ernst & Young LLP would have caused Ernst &
Young LLP to make reference thereto in its reports on the consolidated financial statements for such years, or (2) reportable events (as described in Item 304(a)(1)(v) of
Regulation S-K).
On
February 13, 2018, the board of directors of Vaxart, Inc., in connection with the Merger and the dismissal of Ernst & Young LLP, approved the engagement of
KPMG LLP as the Combined Company's independent registered public accounting firm for the year ending December 31, 2017.
12
Table of Contents
WHERE YOU CAN FIND ADDITIONAL INFORMATION
This prospectus is part of the registration statement on Form S-3 we filed with the SEC under the Securities Act and does not contain all
the information set forth in the registration statement. Whenever a reference is made in this prospectus to any of our contracts, agreements or other documents, the reference may not be complete and
you should refer to the exhibits that are a part of the registration statement or the exhibits to the reports or other documents incorporated by reference into this prospectus for a copy of such
contract, agreement or other document. Because we are subject to the information and reporting requirements of the Exchange Act, we file annual, quarterly and current reports, proxy statements and
other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC's website at http://www.sec.gov.
We
make available free of charge, on or through the investor relations section of our website, Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports
on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such
material with, or furnish it to, the SEC. We maintain a website at www.vaxart.com. Information contained in or accessible through our website does not constitute a part of this prospectus.
13
Table of Contents
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to "incorporate by reference" information from other documents that we file with it, which means that we can disclose
important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes
information incorporated by reference that we filed with the SEC prior to the date of this prospectus, while information that we file later with the SEC will automatically update and supersede the
information in this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we
have filed with the SEC (Commission File No. 001-35285):
-
•
-
Our Annual Report on Form 10-K, for the year ended December 31, 2018, filed with the SEC on February 6, 2019;
-
•
-
Our definitive proxy statement relating to our 2019 annual meeting of stockholders, filed with the SEC on March 11, 2019;
-
•
-
Our Current Report on Form 8-K filed with the SEC on January 18, 2019; and
-
•
-
The description of our common stock contained in our Registration Statement on Form 10, filed with the SEC on May 4, 1970, as
amended by our Current Report on Form 8-K (File No. 000-04829) filed with the SEC on August 15, 2003.
All
filings filed by us pursuant to the Exchange Act after the date of the initial filing of the registration statement of which this prospectus is a part and prior to effectiveness of
the registration statement shall be deemed to be incorporated by reference into this prospectus.
We
also incorporate by reference any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form
that are related to such items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including
those made after the date of the initial filing of the registration statement of which this prospectus is a part and prior to effectiveness of such registration statement, until we file a
post-effective amendment that indicates the termination of the offering of the shares of our common stock made by this prospectus and will become a part of this prospectus from the date that such
documents are filed with the SEC. Information in such future filings updates and supplements the information provided in this prospectus. Any statements in any such future filings will automatically
be deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein by reference to the extent that statements
in the later filed document modify or replace such earlier statements.
You
can request a copy of these filings, at no cost, by writing or telephoning us at the following address or telephone number:
Vaxart, Inc.
290 Utah Ave
Suite 200
South San Francisco, CA 94080
Attn: Secretary
(650) 550-3500
Copies
of these filings are also available through the "Investor" section of our website at www.vaxart.com. For other ways to obtain a copy of these filings, please refer to "Where You
Can Find More Information" above.
14
Table of Contents
1,200,000 Shares

Common Stock
Prospectus Supplement
Exclusive Lead Placement Agent
H.C. Wainwright & Co.
Co-Placement Agent
Brookline Capital Markets
March 19, 2019
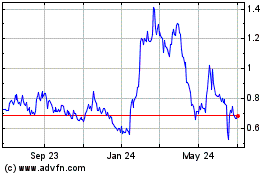
Vaxart (NASDAQ:VXRT)
Historical Stock Chart
From Mar 2024 to Apr 2024
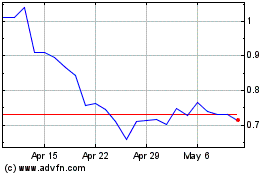
Vaxart (NASDAQ:VXRT)
Historical Stock Chart
From Apr 2023 to Apr 2024