Feasibility arrangement to test GreenLight Bioscience Inc’s
COVID-19 messenger RNA vaccine candidate as a shelf-stable dry
powder formulation using TFF Pharmaceuticals' Thin-Film Freezing
technology.
An easily reconstituted and shelf-stable dry powder formulation
of messenger RNA COVID-19 vaccine could overcome the extreme low
temperature cold chain requirements for current RNA vaccines.
Eliminating extreme cold from the supply chain simplifies global
distribution and opens vaccine availability to the large
populations in regions and countries with limited refrigeration
infrastructure
Should the study prove successful, the next phase may include
non-needle administration of mRNA vaccines, including nasal spray
and lung inhalation form.
GreenLight Biosciences, Inc., a privately-held RNA vaccine
developer and manufacturer, and TFF Pharmaceuticals, Inc. (NASDAQ:
TFFP), a clinical-stage biopharmaceutical company focused on
developing and commercializing innovative drug products based on
its patented Thin Film Freezing (TFF) technology platform, today
announced that the two biotech companies have partnered for
feasibility studies aimed at opening broader global vaccine
distribution through production of a shelf-stable powder form of
messenger RNA COVID-19 vaccine that would be easily reconstituted
prior to injection and not require the extreme cold chain of
current RNA vaccines.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20210309005642/en/
While messenger RNA COVID-19 vaccines have proved among the
fastest to develop, produce and adapt to new variants of concern,
maintaining stability has required supply chain temperatures for
some vaccines as low as -80°C (-112°F).
This requirement for extreme cold increases distribution
complexity, cost and also constrains vaccine distribution to
regions and countries with limited cold chain infrastructure.
To address this challenge, GreenLight Biosciences and TFF
Pharmaceuticals have entered into a feasibility and material
transfer agreement to evaluate a shelf-stable dry powder
formulation of GreenLight’s COVID-19 messenger RNA vaccine
candidate.
“We are excited to partner with GreenLight Biosciences on their
unique messenger RNA production platform,” said Glenn Mattes,
President & CEO of TFF Pharmaceuticals. “Their platform
technology represents a breakthrough in efficient production of
messenger RNA vaccines, and by combining both of our technologies,
this collaboration could be a real game changer for people around
the world suffering through this pandemic.”
Should the feasibility study prove successful, a further stage
of work will include non-needle administration methods for the
GreenLight mRNA vaccine candidate in a dry powder form that could
be administered via nasal spray or lung inhalation.
Under the Feasibility Agreement, GreenLight Biosciences is
delivering its COVID-19 messenger RNA product candidate materials
to TFF in order to perform feasibility formulation work and
testing.
The goal of this feasibility work is to formulate and identify
an optimal formulation of the GreenLight Biosciences messenger RNA
product candidate in a dry powder form, which has superior
stability, maintains particle size of the encapsulated messenger
RNA as well as high encapsulation efficiency and has rapid
reconstitution characteristics for injection.
If successful, this should make messenger RNA vaccines available
to the whole world, simplifying cold-chain supply challenges. Thin
film technology potentially allows vaccines to be transported at
fridge, or even room temperatures as a powder. It can then be
reconstituted by a health care worker at the point of use.
This agreement is part of GreenLight Biosciences goal of using
its unique manufacturing platform to produce vaccines in volumes
that can serve the world’s need for billions of doses. This
partnership offers the hope of speeding these doses into use.
“Overcoming the COVID-19 pandemic requires a large volume of
second generation vaccines that adapt rapidly and can be delivered
to all parts of the world, regardless of local cold chain
infrastructure,” said Andrey J. Zarur, Ph.D, CEO of GreenLight
Biosciences. “Thin Film Freezing has the potential to deliver on
this promise by reformulating the complex messenger RNA molecules
of our vaccine candidate into a shelf-stable powder readily
reconstituted by a healthcare worker just prior to injection.”
TFF has two drug candidates in phase one clinical trials,
Voriconazole Inhalation Powder and Tacrolimus Inhalation Powder.
TFF requires approximately six weeks from receipt of materials to
prepare an initial dry powder form of GreenLight’s vaccine
candidate to test for reconstitutability and viability.
About GreenLight Biosciences, Inc.
GreenLight Biosciences has several messenger RNA Covid vaccine
candidates in development built off of GreenLight’s manufacturing
platform, which delivers high-quality RNA at a lower cost and
higher speed than comparable processes.
GreenLight is a bio-performance company with a unique, cell-free
production platform that delivers high-performing RNA solutions to
human, plant and animal challenges. GreenLight develops RNA
products for plant and life science applications, and collaborates
with industry leaders to advance vaccine development, pandemic
preparation, crop management, and plant protection. The GreenLight
team is committed to social justice, diversity, inclusion, and
equality, and promises to use collaboration to remain
scientifically imaginative and passionately focused on making a
difference in the world. For more information, visit
https://www.greenlightbiosciences.com/.
About TFF Pharmaceuticals’ Thin Film Freezing technology
platform
TFF Pharmaceuticals’ Thin Film Freezing (TFF) platform was
designed to improve the solubility and absorption of poorly
water-soluble drugs and is particularly suited to generate dry
powder particles with properties targeted for inhalation delivery,
especially to the deep lung, an area of extreme interest in
respiratory medicine. The TFF process results in a “Brittle Matrix
Particle,” which possesses low bulk density, high surface area, and
typically an amorphous morphology, allowing the particles to
supersaturate when contacting the target site, such as lung tissue.
Based upon laboratory experiments the aerodynamic properties of the
particles are such that the portion of a drug deposited to the deep
lung has the potential to reach as high as 75 percent.
About TFF Pharmaceuticals
TFF Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical
company focused on developing and commercializing innovative drug
products based on its patented Thin Film Freezing, or TFF,
technology platform. Early testing confirms that the TFF platform
can significantly improve the solubility and absorption of poorly
water-soluble drugs, a class of drugs that comprises approximately
one-third of the major pharmaceuticals worldwide, thereby improving
their pharmacokinetics. TFF Pharmaceuticals has two lead drug
candidates: Voriconazole Inhalation Powder and Tacrolimus
Inhalation Powder. The Company plans to add to this pipeline by
collaborating with large pharmaceutical partners. The TFF Platform
is protected by 42 patents issued or pending in the US and
internationally. To learn more about TFF Pharmaceuticals and its
product candidates, visit the Company’s website at
https://tffpharma.com/.
Safe Harbor
This press release contains forward-looking statements regarding
TFF Pharmaceuticals, Inc., including the benefits of the Company’s
TFF platform and its dry powder versions of GreenLight Bioscience
Inc’s COVID-19 messenger RNA vaccine candidate. Those
forward-looking statements involve known and unknown risks,
uncertainties and other factors that could cause actual results to
differ materially. Among those factors are: (i) the risk that
GreenLight Bioscience may not be able to successfully conclude
clinical testing or obtain pre-market approval of its COVID-19
messenger RNA vaccine candidate, (ii) the risk that GreenLight
Bioscience and the Company may not be able to produce a dry powder
version GreenLight Bioscience Inc’s COVID-19 messenger RNA vaccine
candidate, (iii) the risk that GreenLight Bioscience and the
Company may not be able to successfully conclude clinical testing
or obtain pre-market approval of a dry powder version GreenLight
Bioscience Inc’s COVID-19 messenger RNA vaccine candidate, (iv) no
drug product incorporating the TFF platform has received FDA
pre-market approval or otherwise been incorporated into a
commercial drug product, and (iv) those other risks disclosed in
the section “Risk Factors” included in the Company’s prospectus
supplement filed with the SEC on December 8, 2020. TFF
Pharmaceuticals cautions readers not to place undue reliance on any
forward-looking statements. TFF Pharmaceuticals does not undertake,
and specifically disclaims, any obligation to update or revise such
statements to reflect new circumstances or unanticipated events as
they occur, except as required by law.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20210309005642/en/
GreenLight: Thomas Crampton Head of Corporate Affairs
tcrampton@greenlightbio.com +1-914-202-2762 +44-7826-995794
TFF: Glenn Mattes President and CEO TFF Pharmaceuticals,
Inc. gmattes@tffpharma.com 737-802-1973
Kirk Coleman Chief Financial Officer TFF Pharmaceuticals, Inc.
kcoleman@tffpharma.com 817-989-6358
TFF Investor Relations and Media: Paul Sagan
LaVoieHealthScience psagan@lavoiehealthscience.com 617-865-0041
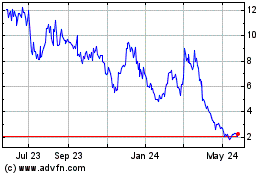
TFF Pharmaceuticals (NASDAQ:TFFP)
Historical Stock Chart
From Mar 2024 to Apr 2024
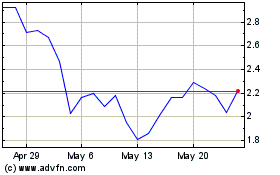
TFF Pharmaceuticals (NASDAQ:TFFP)
Historical Stock Chart
From Apr 2023 to Apr 2024