Talaris Shares Slide Premarket After Patient Death in Phase 3 Study
October 20 2022 - 8:17AM
Dow Jones News
By Colin Kellaher
Shares of Talaris Therapeutics Inc. fell sharply in premarket
trading Thursday after the cell-therapy company said it had
temporarily halted a Phase 3 study of its lead drug candidate
FCR001 in living donor kidney transplant recipients following the
death of a patient.
Talaris said it received the report of a patient death on
Wednesday, triggering a stopping requirement and review by the
study's data-monitoring committee, which determined that trial
enrollment and dosing may continue.
Talaris said it has reported the patient's death and the
committee's recommendation to the U.S. Food and Drug
Administration.
Talaris in June reported that three study subjects had been
diagnosed with acute graft-vs-host disease. The company said the
deceased patient was one of those subjects, and that while the
patient was responding to treatment at the time of the June update,
the subject was recently hospitalized with a more severe case of
graft-vs-host disease.
Talaris said the data-monitoring committee has concluded that
study protocol modifications implemented in June should be
sufficient to mitigate the risk of graft-vs-host disease and
recommended continuation of the study without further
modifications.
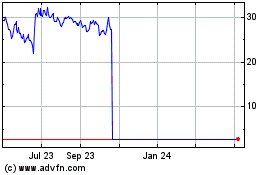
Talaris shares, which closed Wednesday at $2.42, were recently
down nearly 27% to $1.77 in premarket trading.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
October 20, 2022 08:02 ET (12:02 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Talaris Therapeutics (NASDAQ:TALS)
Historical Stock Chart
From Mar 2024 to Apr 2024
Talaris Therapeutics (NASDAQ:TALS)
Historical Stock Chart
From Apr 2023 to Apr 2024